Patents
Literature
396 results about "Plasma samples" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
Methods for prenatal diagnosis of chromosomal abnormalities
ActiveUS20070059707A1Rapid productionAccurate detectionMicrobiological testing/measurementFermentationDiseaseNon invasive
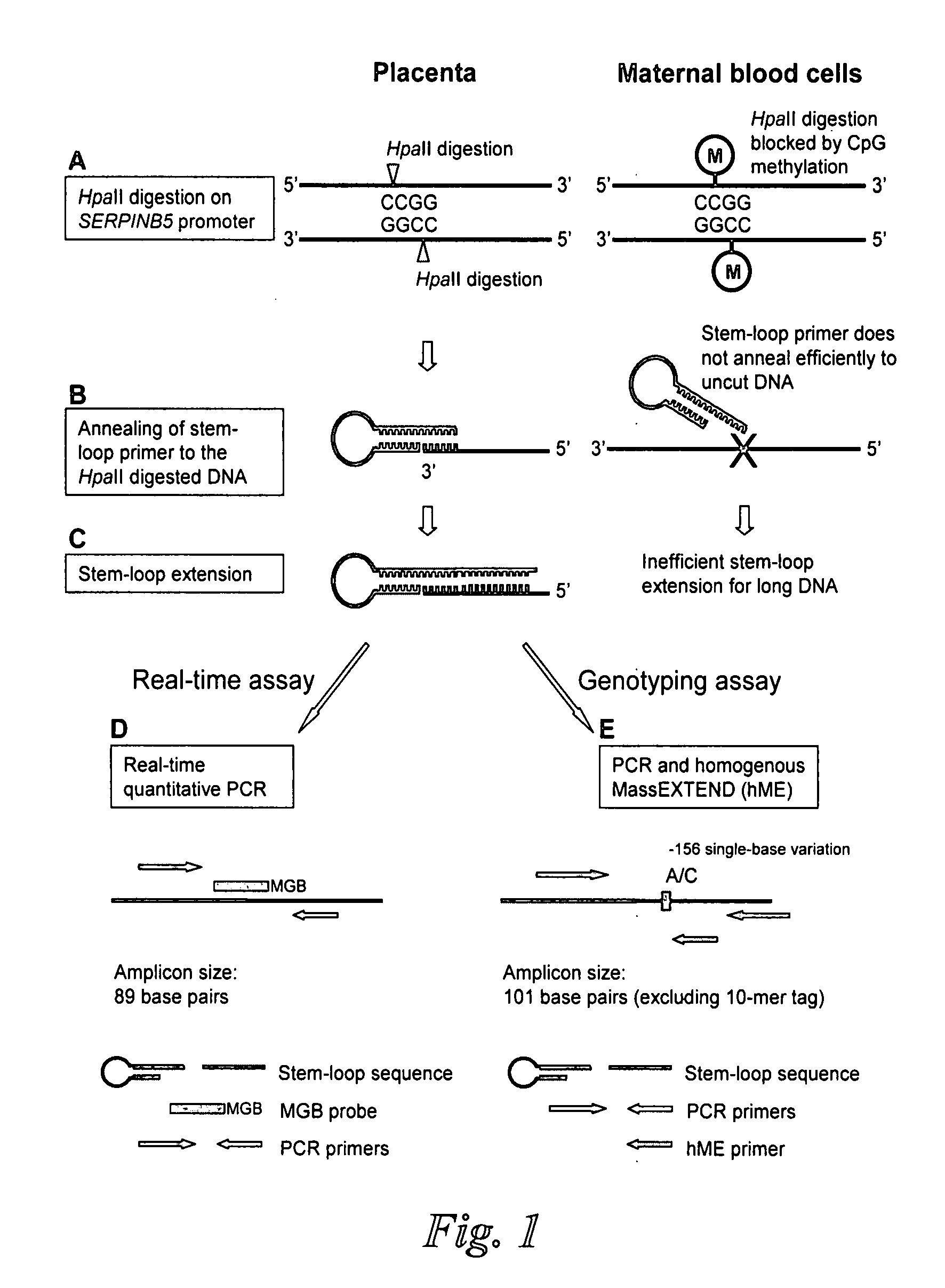
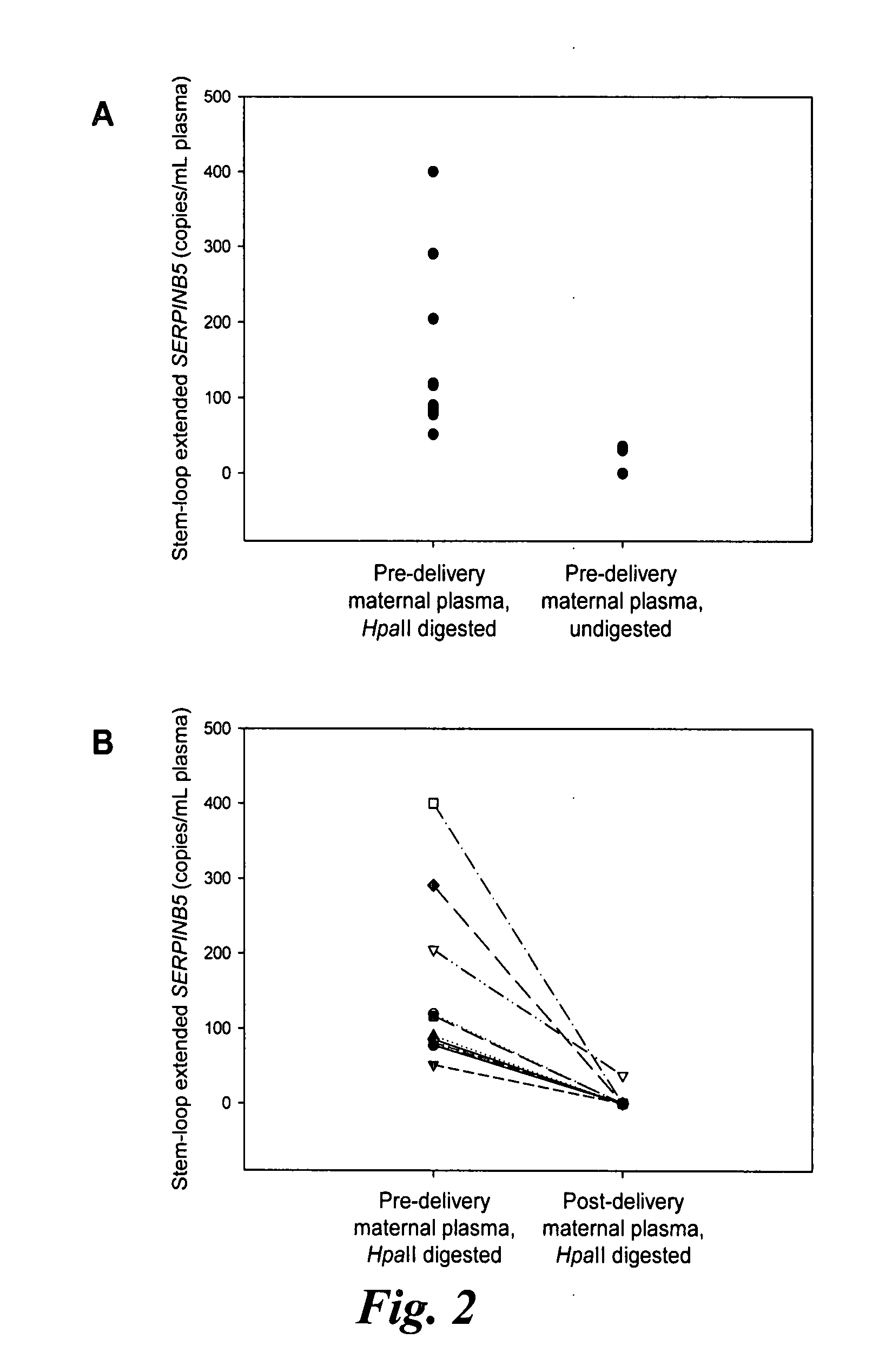
Chromosomal abnormalities are responsible for a significant number of birth defects, including mental retardation. The present invention is related to methods for non-invasive and rapid, prenatal diagnosis of chromosomal abnormalities based on analysis of a maternal blood sample. The invention exploits the differences in DNA between the mother and fetus, for instance differences in their methylation states, as a means to enrich for fetal DNA in maternal plasma sample. The methods described herein can be used to detect chromosomal DNA deletions and duplications. In a preferred embodiment, the methods are used to diagnose chromosomal aneuploidy and related disorders, such as Down's and Turner's Syndrome.
Owner:TRUSTEES OF BOSTON UNIV
Methods for prenatal diagnosis of chromosomal abnormalities
ActiveUS7655399B2Rapid productionAccurate detectionMicrobiological testing/measurementPlasma samplesNon invasive
Chromosomal abnormalities are responsible for a significant number of birth defects, including mental retardation. The present invention is related to methods for non-invasive and rapid, prenatal diagnosis of chromosomal abnormalities based on analysis of a maternal blood sample. The invention exploits the differences in DNA between the mother and fetus, for instance differences in their methylation states, as a means to enrich for fetal DNA in maternal plasma sample. The methods described herein can be used to detect chromosomal DNA deletions and duplications. In a preferred embodiment, the methods are used to diagnose chromosomal aneuploidy and related disorders, such as Down's and Turner's Syndrome.
Owner:TRUSTEES OF BOSTON UNIV
Methods and kits for selectively amplifying, detecting or quantifying target DNA with specific end sequences
ActiveUS20090061425A1Microbiological testing/measurementFermentationPlasma samplesRestriction enzyme digestion
Disclosed herein are methods and kits for selectively amplifying, detecting or quantifying a DNA fragment with a specific end sequence, especially generated following restriction enzyme digestion. This method can be used, for example, to detect a hypomethylated DNA fragment. This methods and kits are especially useful in detecting or quantifying a hypomethylated fetal DNA fragment in a maternal plasma sample containing a corresponding hypermethylated maternal DNA fragment.
Owner:THE CHINESE UNIVERSITY OF HONG KONG +1
Method for calibrating spectrophotometric apparatus with synthetic fluids to measure plasma and serum analytes
InactiveUS6470279B1Testing/calibration apparatusScattering properties measurementsSample MeasureAnalyte
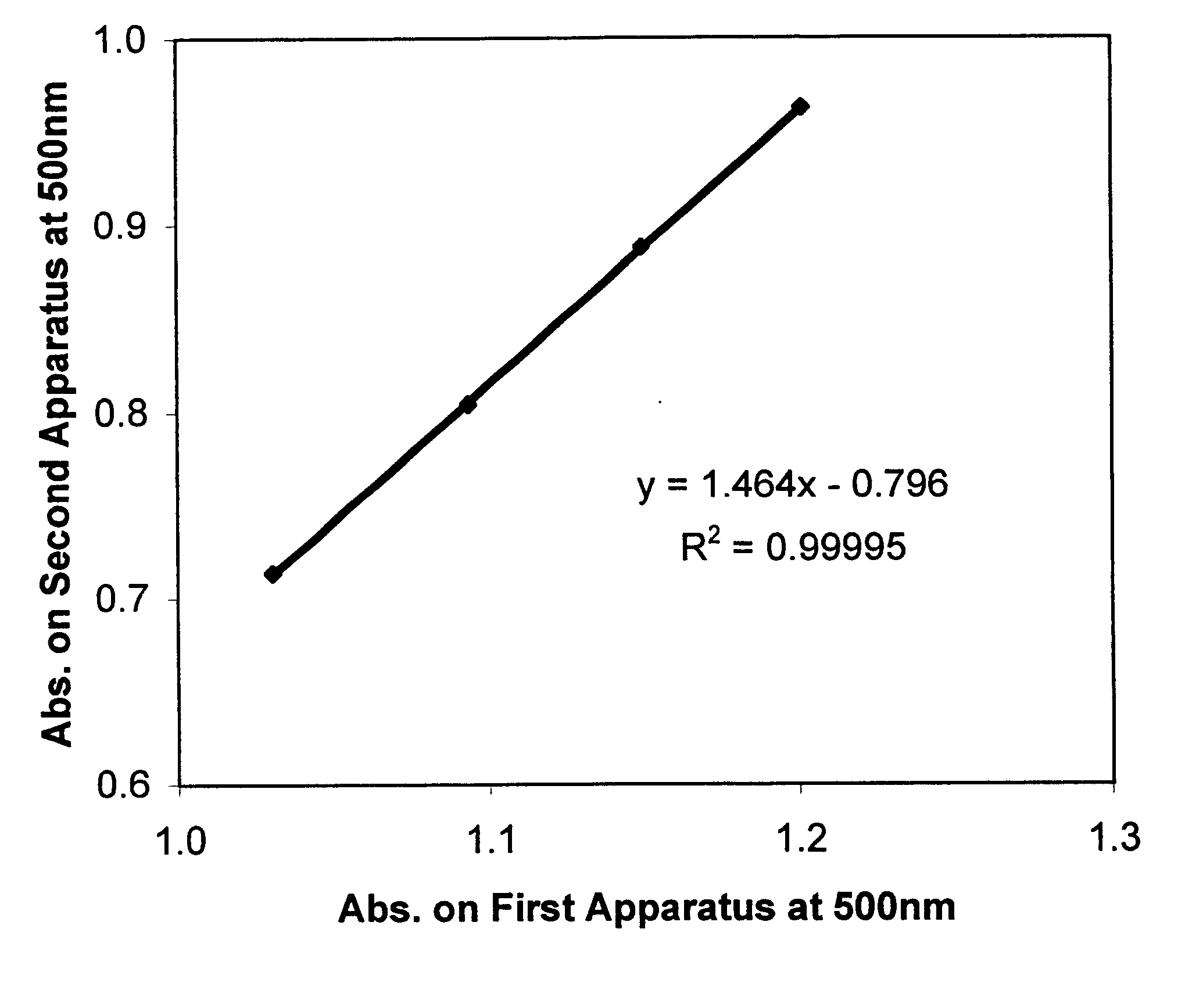
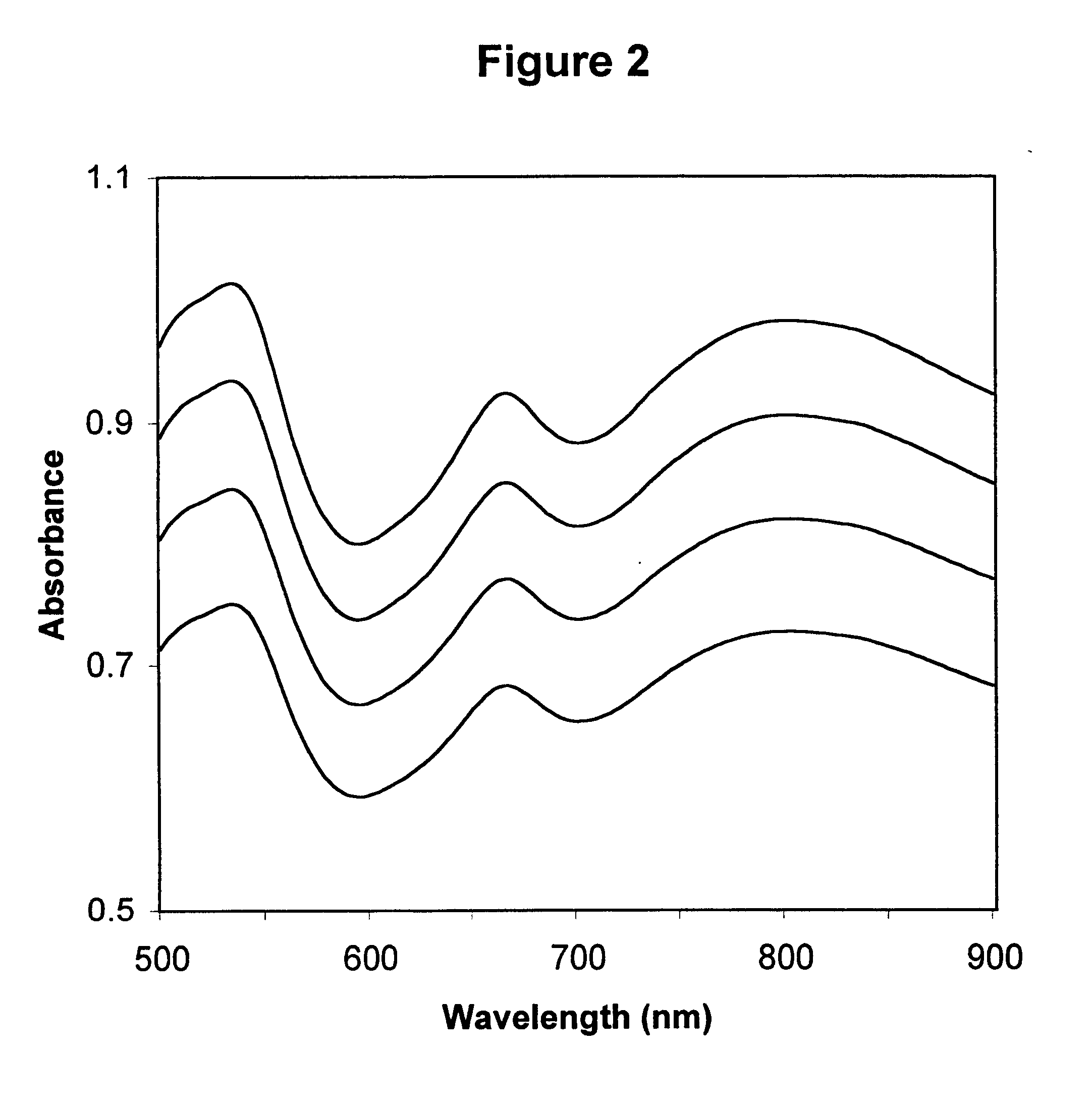
Described is a method for calibrating a spectrophotometric apparatus which is used to measure analytes in plasma and / or serum samples based on the calibration of a First Apparatus, and for recalibrating such apparatus, including recalibration of the First Apparatus all using synthetic calibrators. These apparatus use absorption of radiation to measure analytes in serum or plasma samples. The method described includes using synthetic calibrators which are submitted to the First Apparatus for measurement and compared with measurements of similar calibrators in a Second Apparatus and using the comparison to derive concentrations of analytes in samples measured on the Second Apparatus. As an alternative to making all apparatus identical, in terms of wavelength calibration, the absorbances of all apparatus should be mapped onto a standard set of wavelengths.
Owner:TYCO HEALTHCARE GRP LP
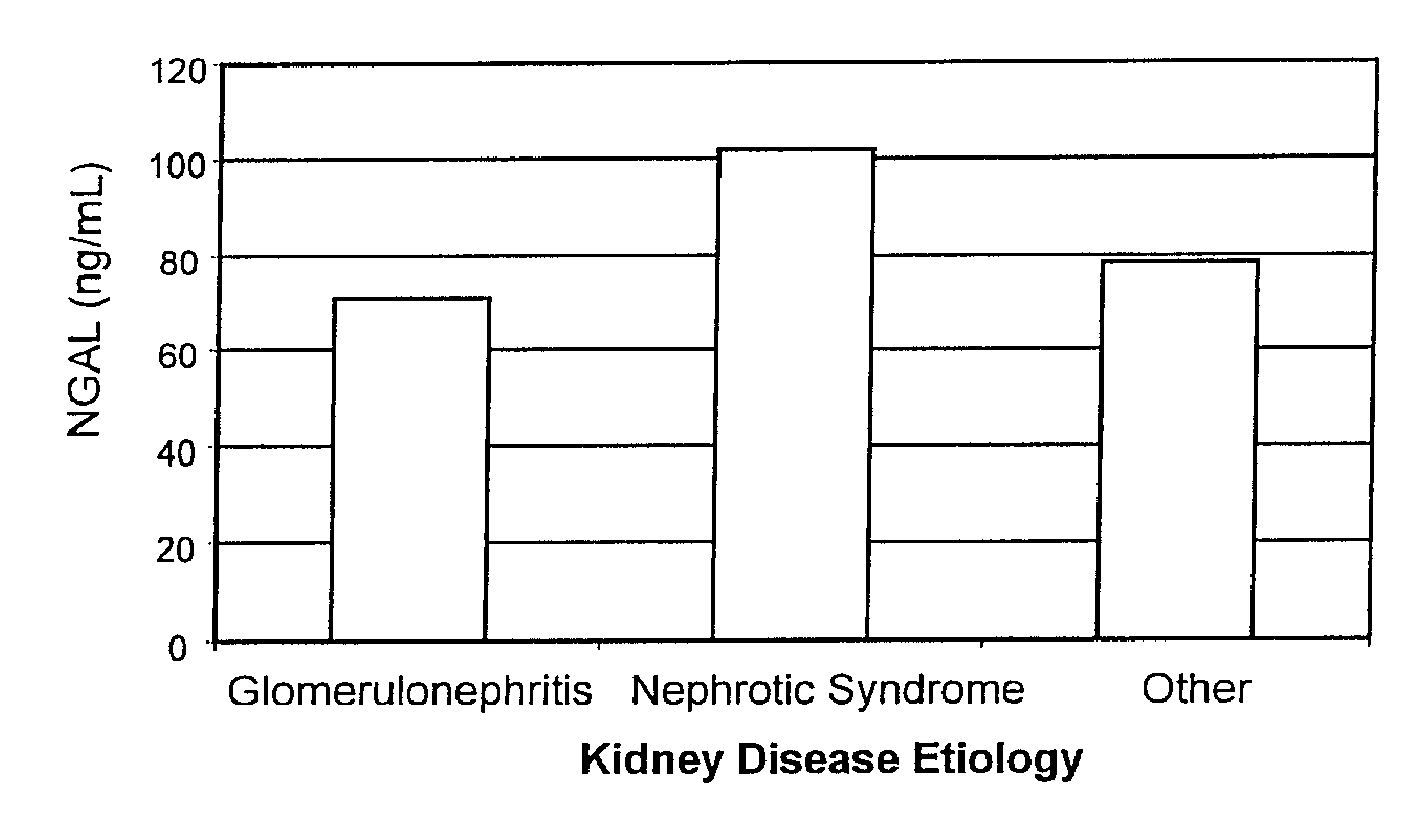
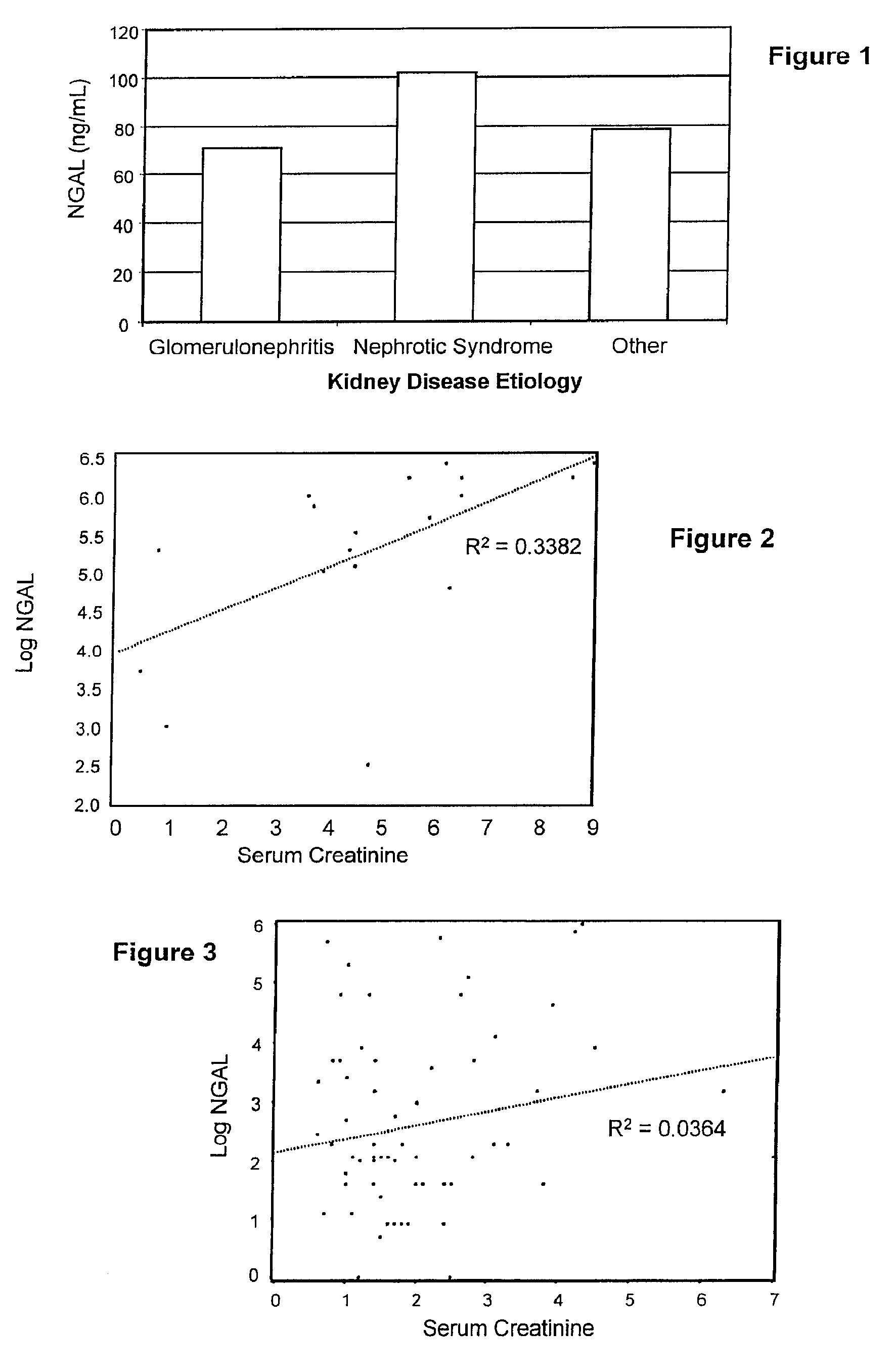
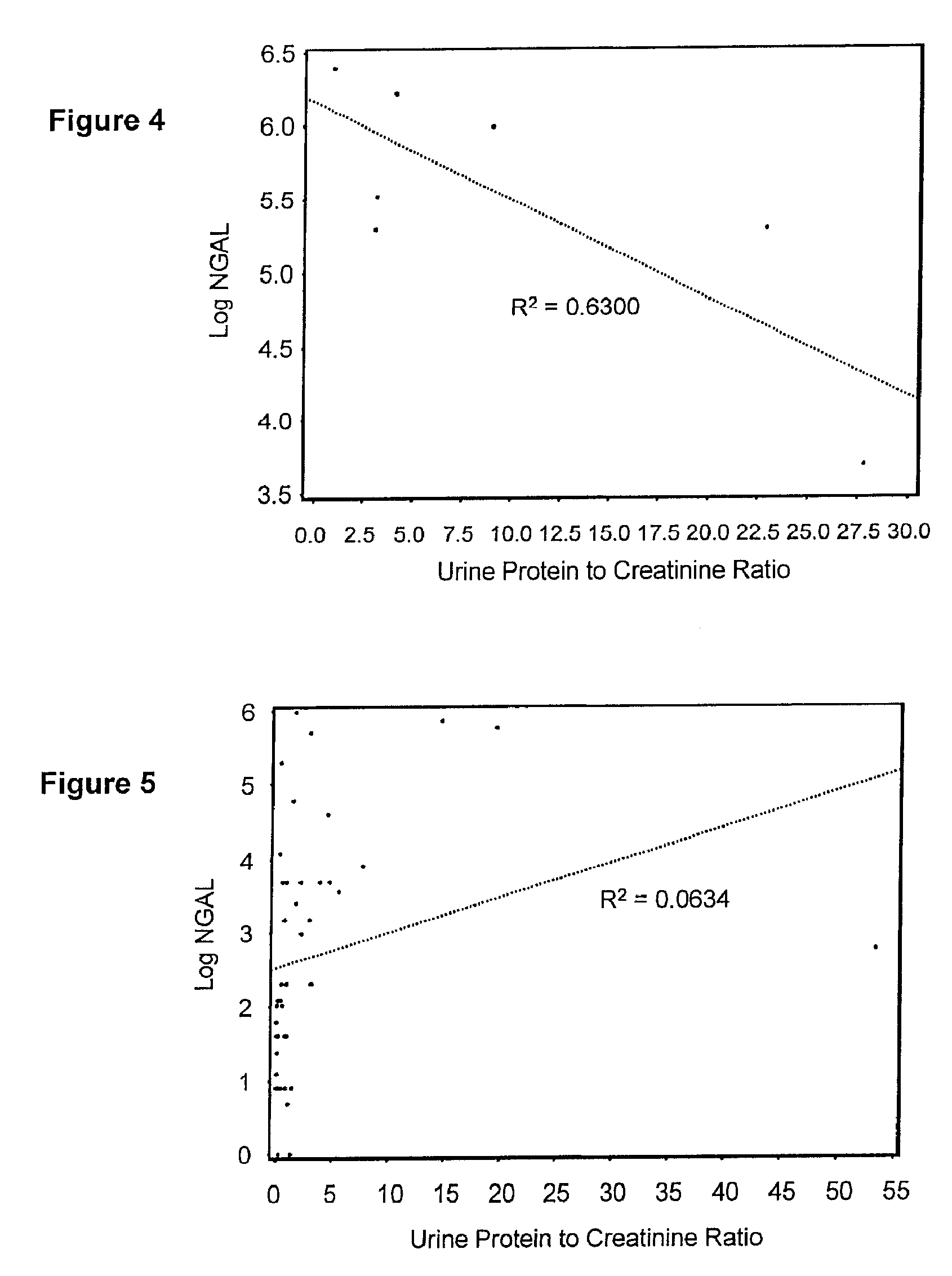
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20080090304A1Difficult to levelImprove the level ofDisease diagnosisBiological testingRegimenProper treatment
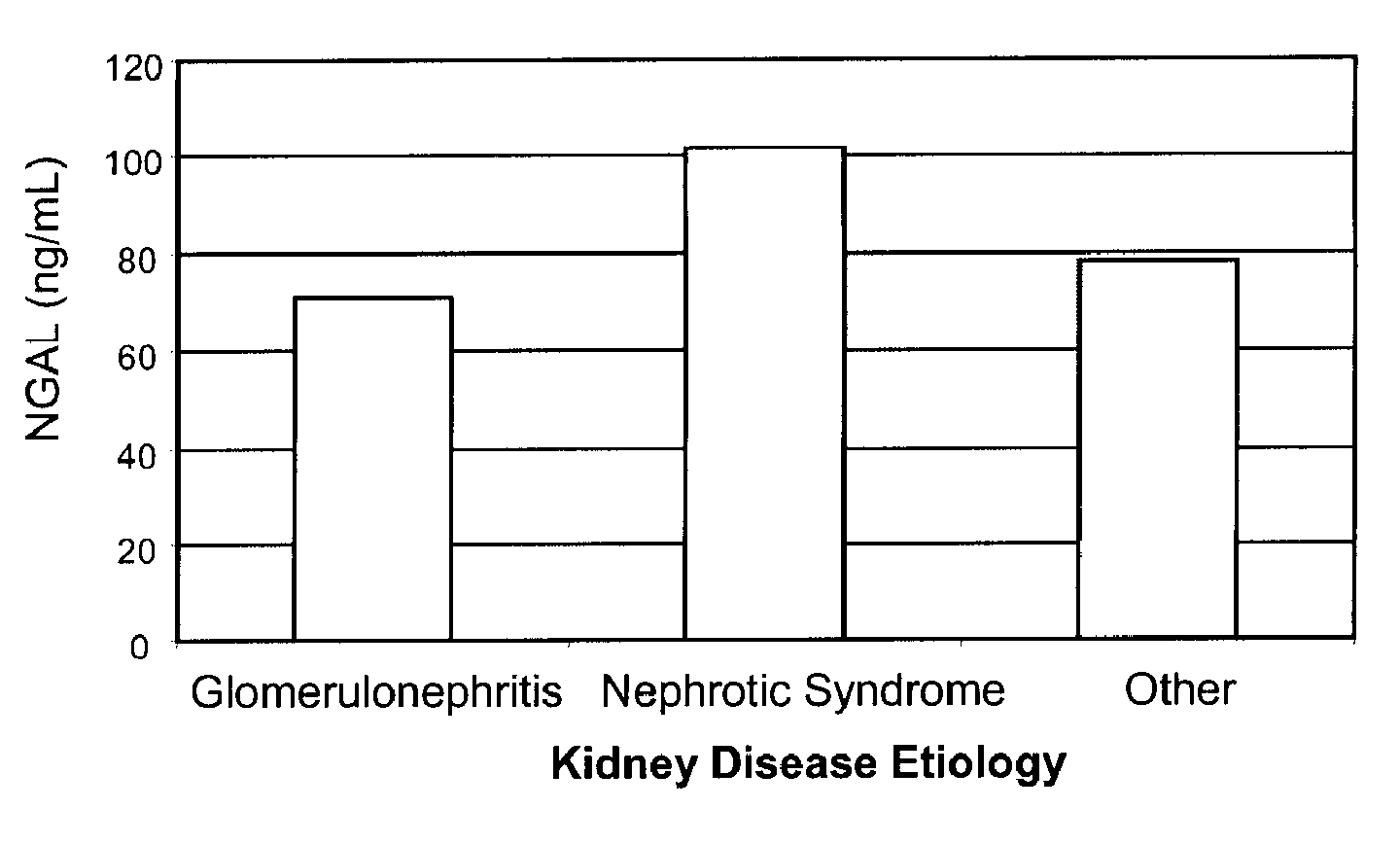
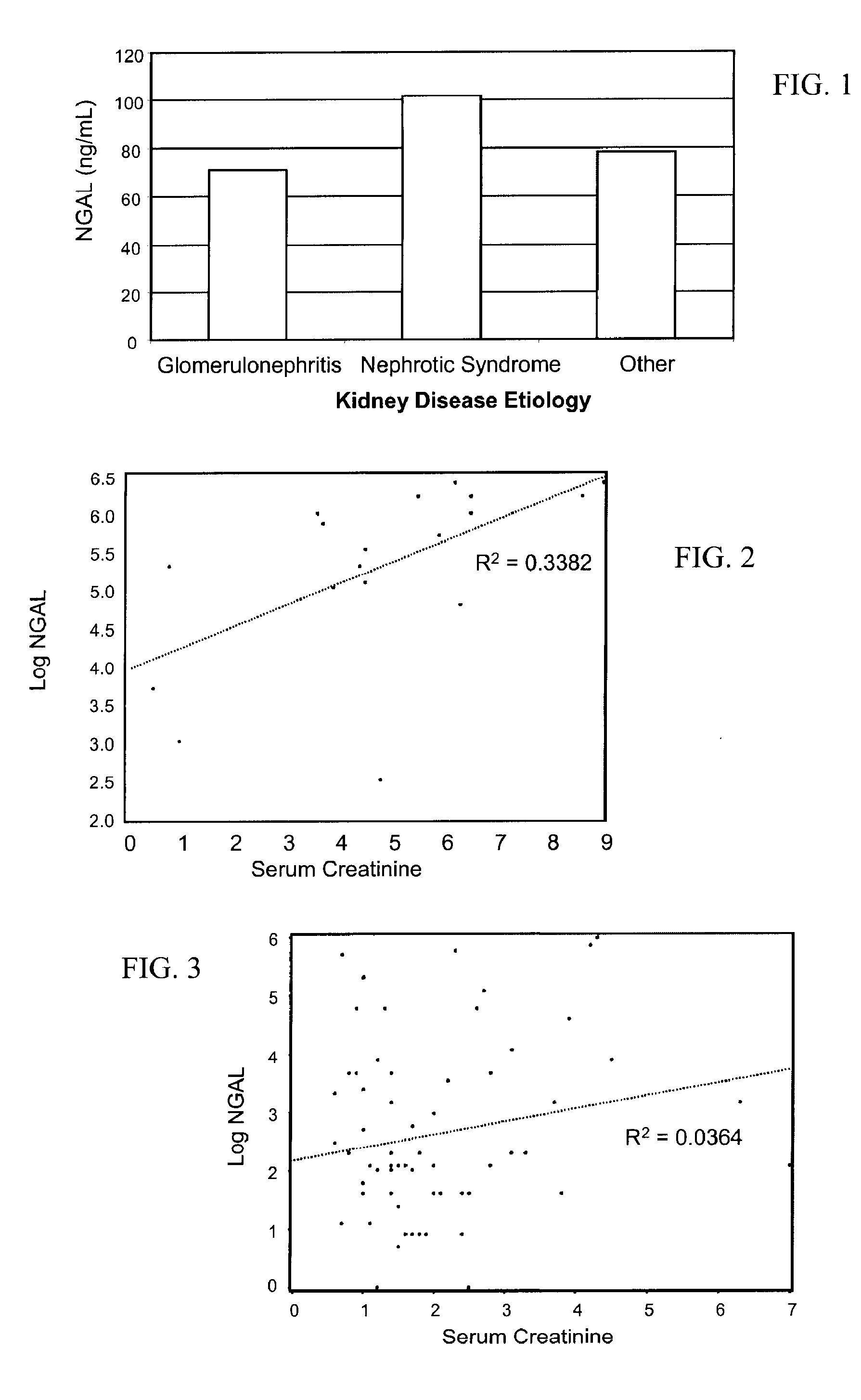
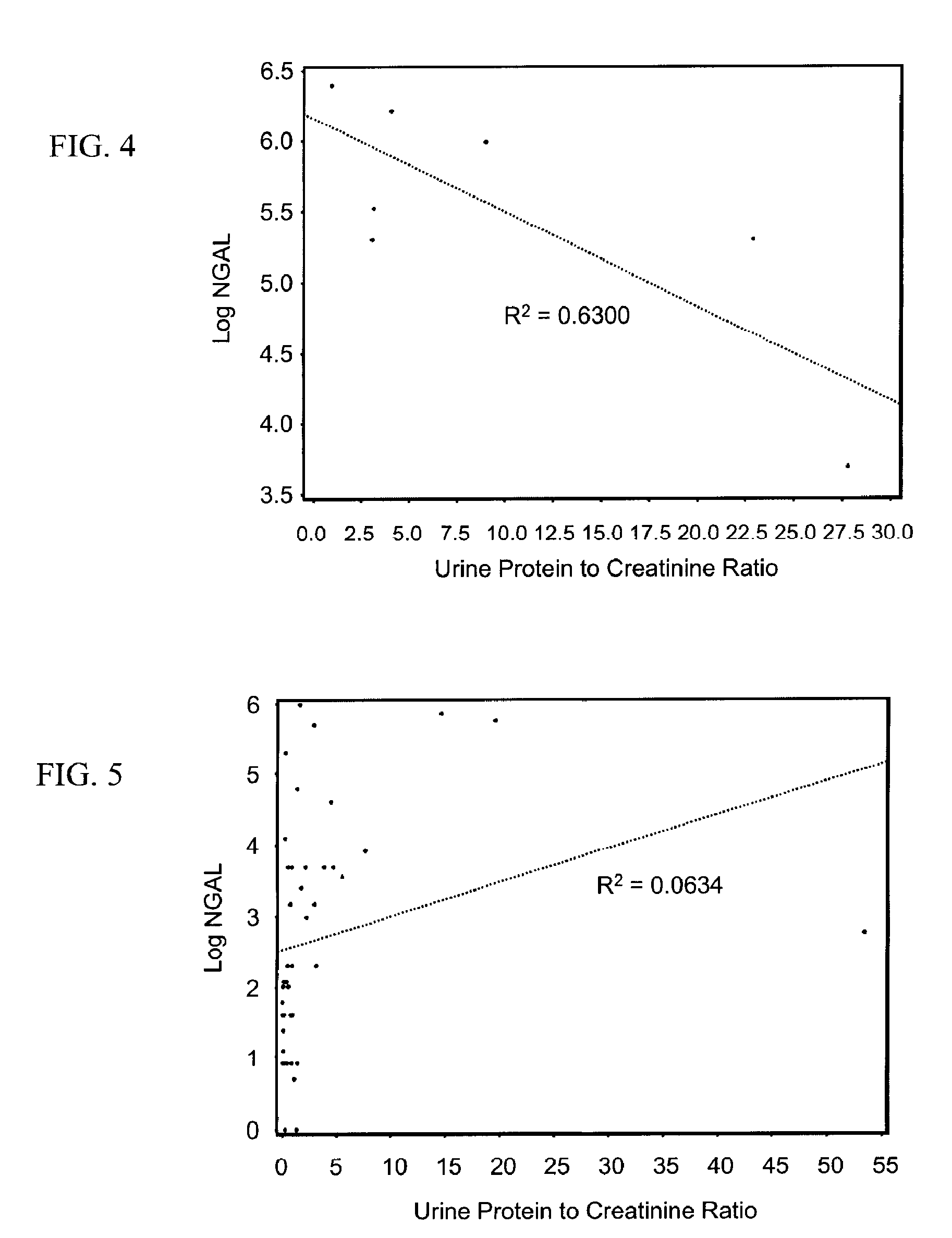
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
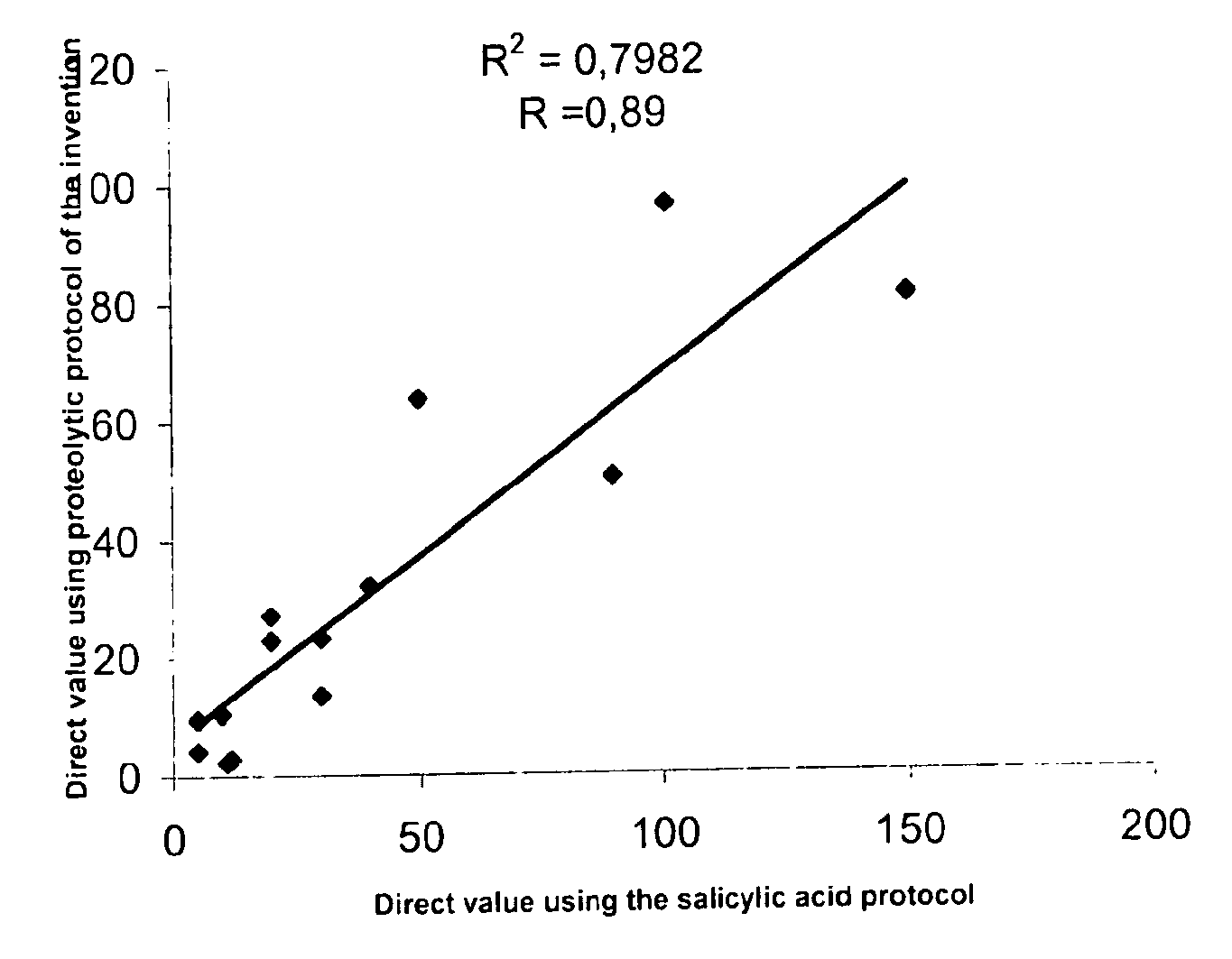
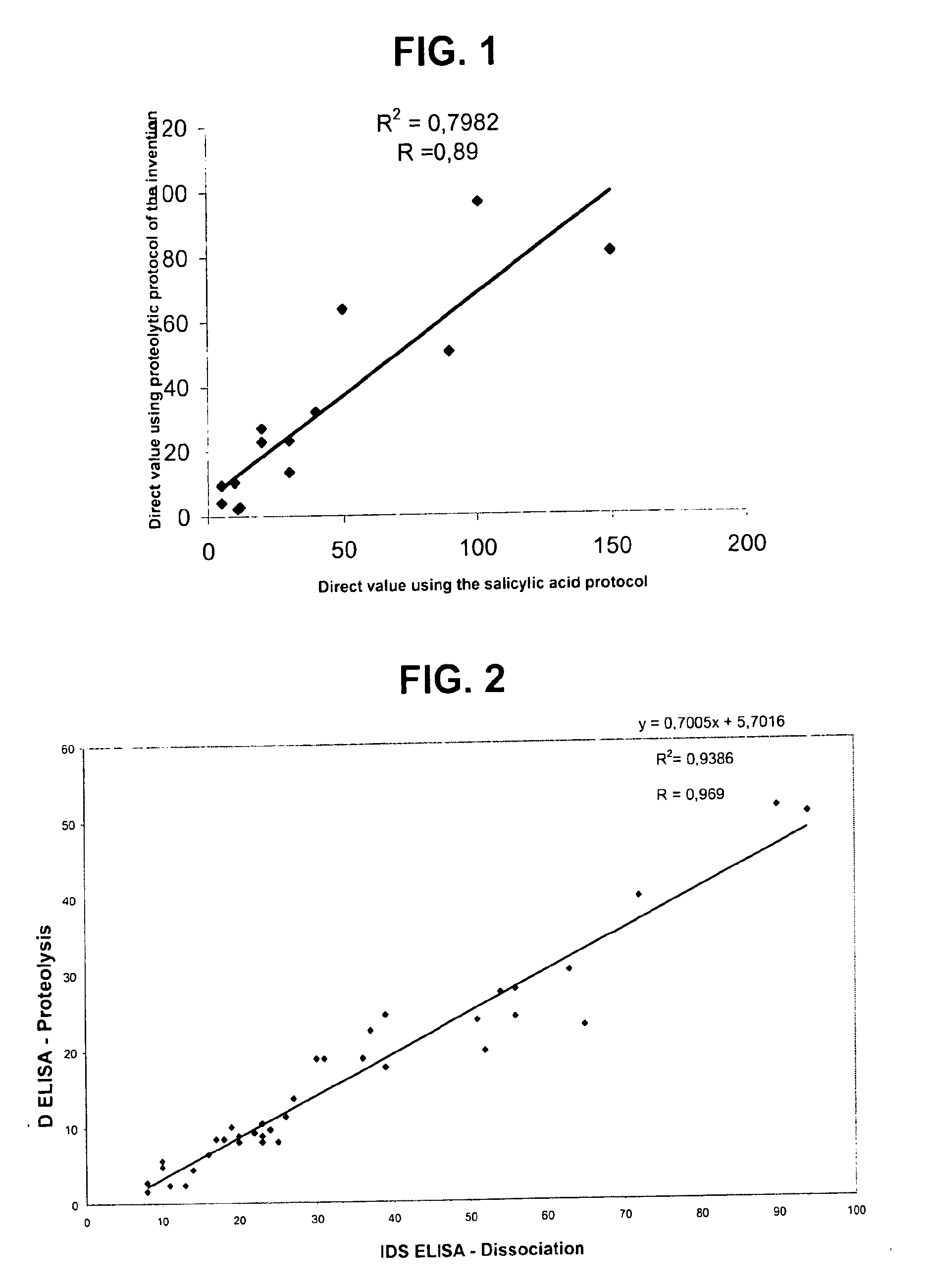
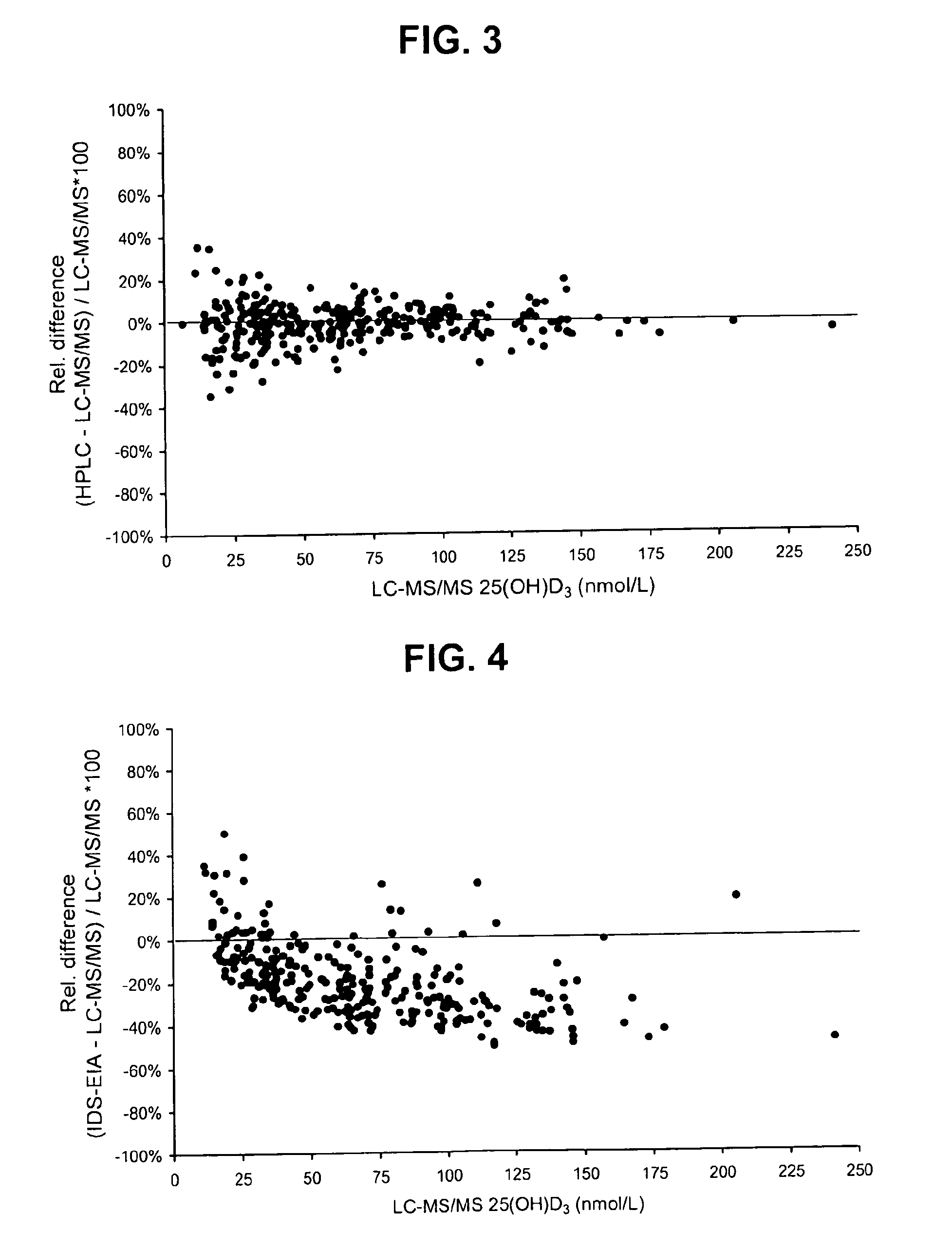
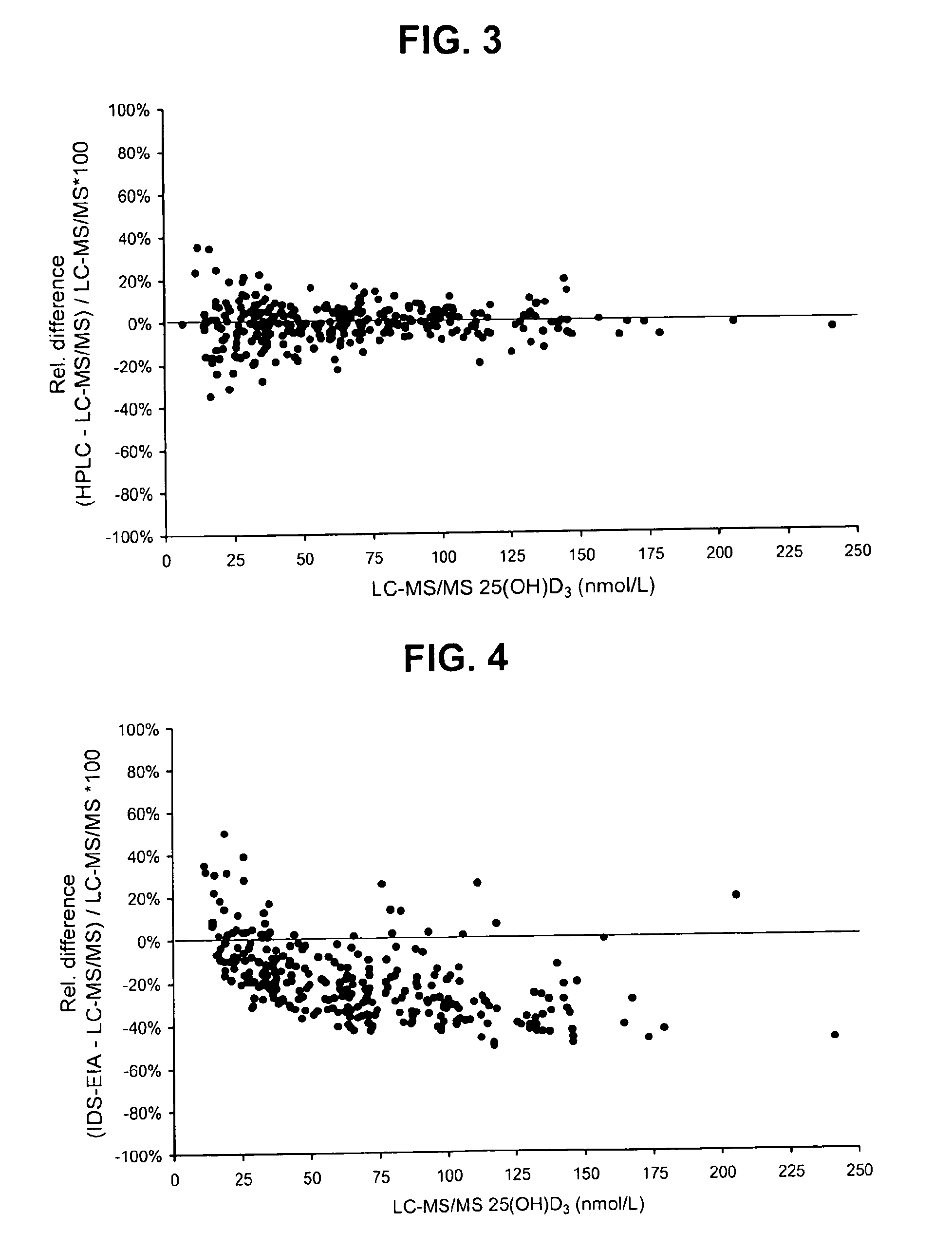
Direct determination of vitamin d in serum or plasma
ActiveUS20100068725A1Easy to controlPromote digestionBioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeCompetitive binding
A method for quantitating vitamin D metabolites directly in blood plasma or serum, without the need for prior purification of the vitamin D metabolites, comprising a digestion of the serum proteins with a serine protease such as proteinase K and sequence of steps for inhibiting the proteinase K activity in the competitive binding analysis. The advantages of this method are its high accuracy over the whole range of physiologically relevant values and that it can be easily adapted for a fully automated analysis of serum and plasma samples.
Owner:IMMUNDIAGNOSTIK
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20080014644A1Monitor effectivenessEarly detectionDisease diagnosisBiological testingRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
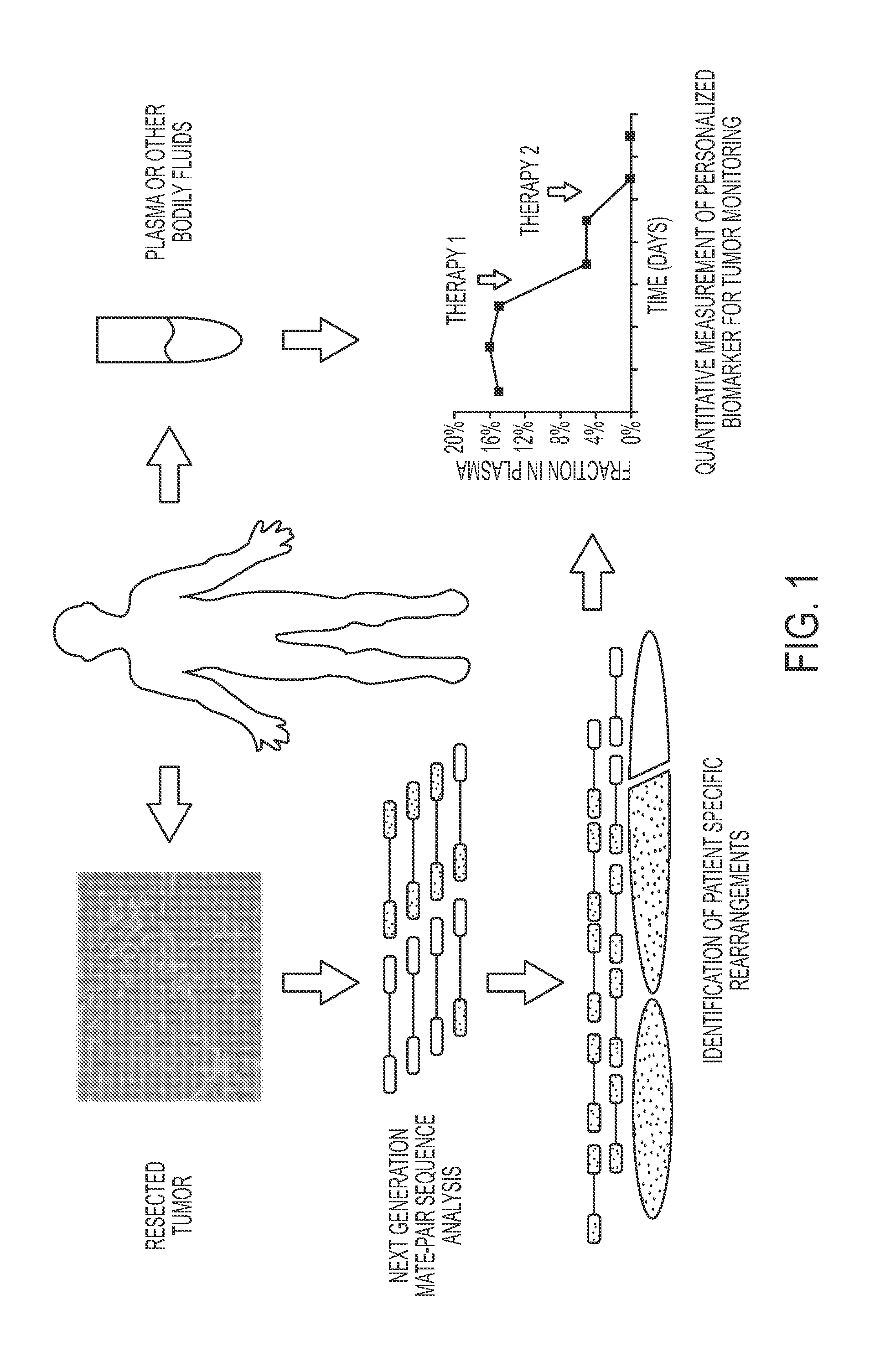
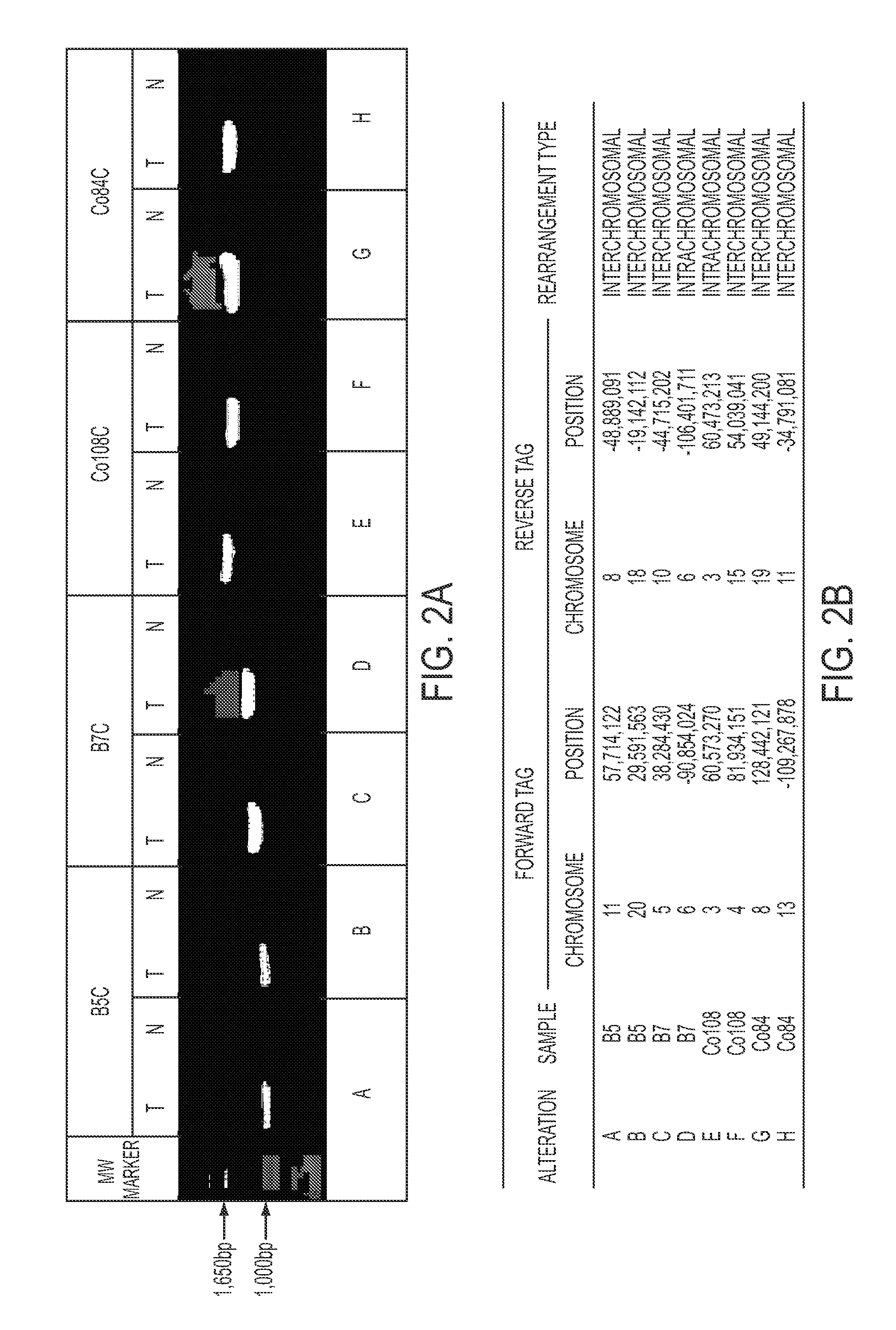
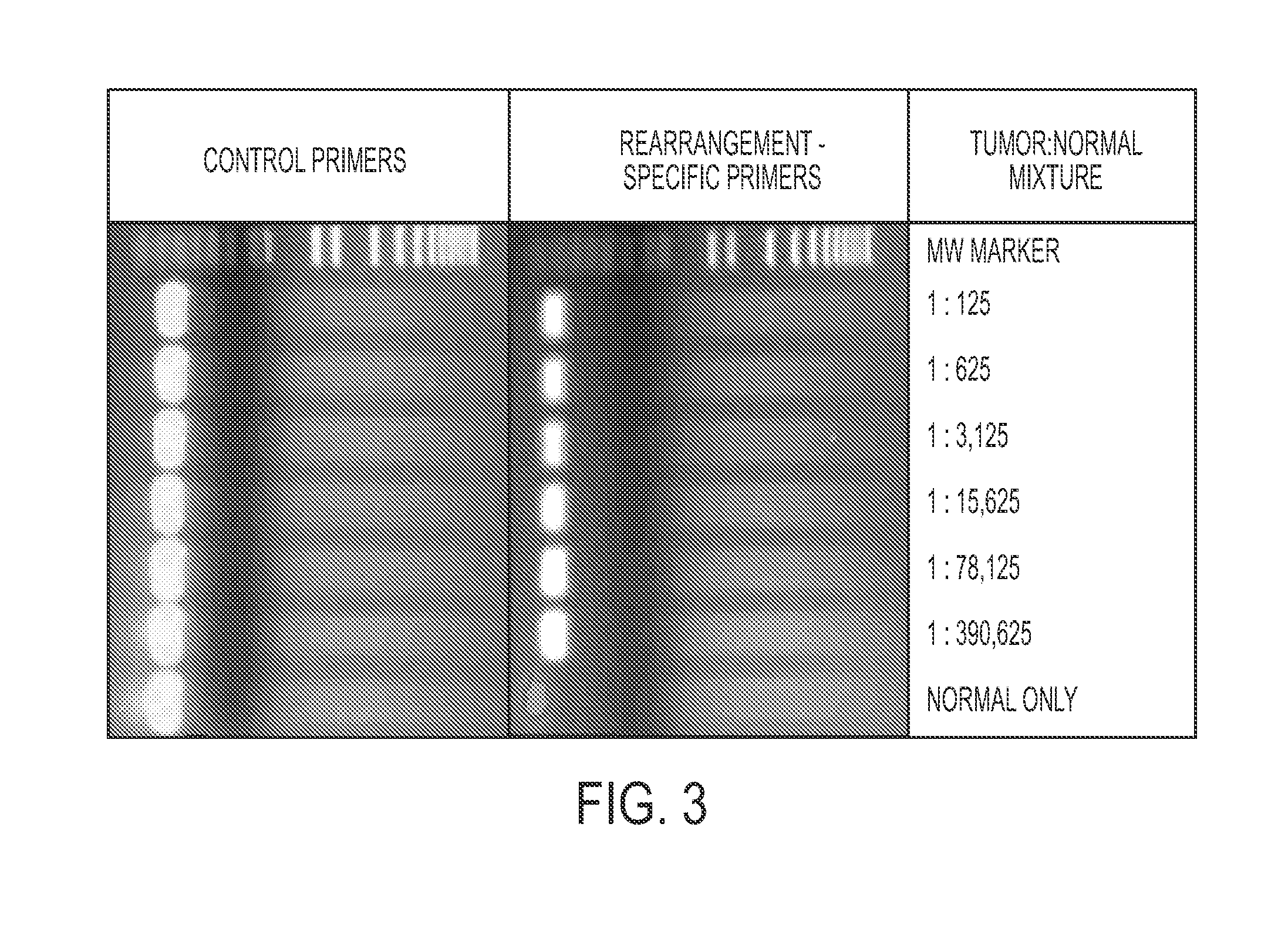
Personalized tumor biomarkers
InactiveUS20130210645A1Sugar derivativesMicrobiological testing/measurementBlood plasmaRecurrent Tumor
Clinical management of human cancer is dependent on the accurate monitoring of residual and recurrent tumors. We have developed a method, called personalized analysis of rearranged ends (PARE), which can identify translocations in solid tumors. Analysis of four colorectal and two breast cancers revealed an average of nine rearranged sequences (range 4 to 15) per tumor. Polymerase chain reaction with primers spanning the breakpoints were able to detect mutant DNA molecules present at levels lower than 0.001% and readily identified mutated circulating DNA in patient plasma samples. This approach provides an exquisitely sensitive and broadly applicable approach for the development of personalized biomarkers to enhance the clinical management of cancer patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Automated method and system for testing blood samples
InactiveUSH1960H1Safely and cost-effectively collectedEfficient and cost-effectiveSamplingLaboratory glasswaresPlasma samplesAutosampler
An automated system and process is provided for pooling samples from a multiplicity of blood or plasma donations for subsequent testing to uniquely identify any such blood or plasma donations which may be infected with a particular virus. The system includes an autosampler needle which is directly inserted into a blood or plasma sample aliquot container in order to withdraw an aliquot portion of the contents for forming a sample pool with aliquot portions of other blood or plasma samples. The autosampler comprises a tubular piercing member having a hollow interior bore and a tubular shroud surrounding the piercing member.
Owner:BAXALTA INC
Diagnostic composition and its use in the determination of coagulation characteristics of a test liquid
InactiveUS20100190193A1Improve stabilityEasy to handleBioreactor/fermenter combinationsBiological substance pretreatmentsPlasma samplesMedicine
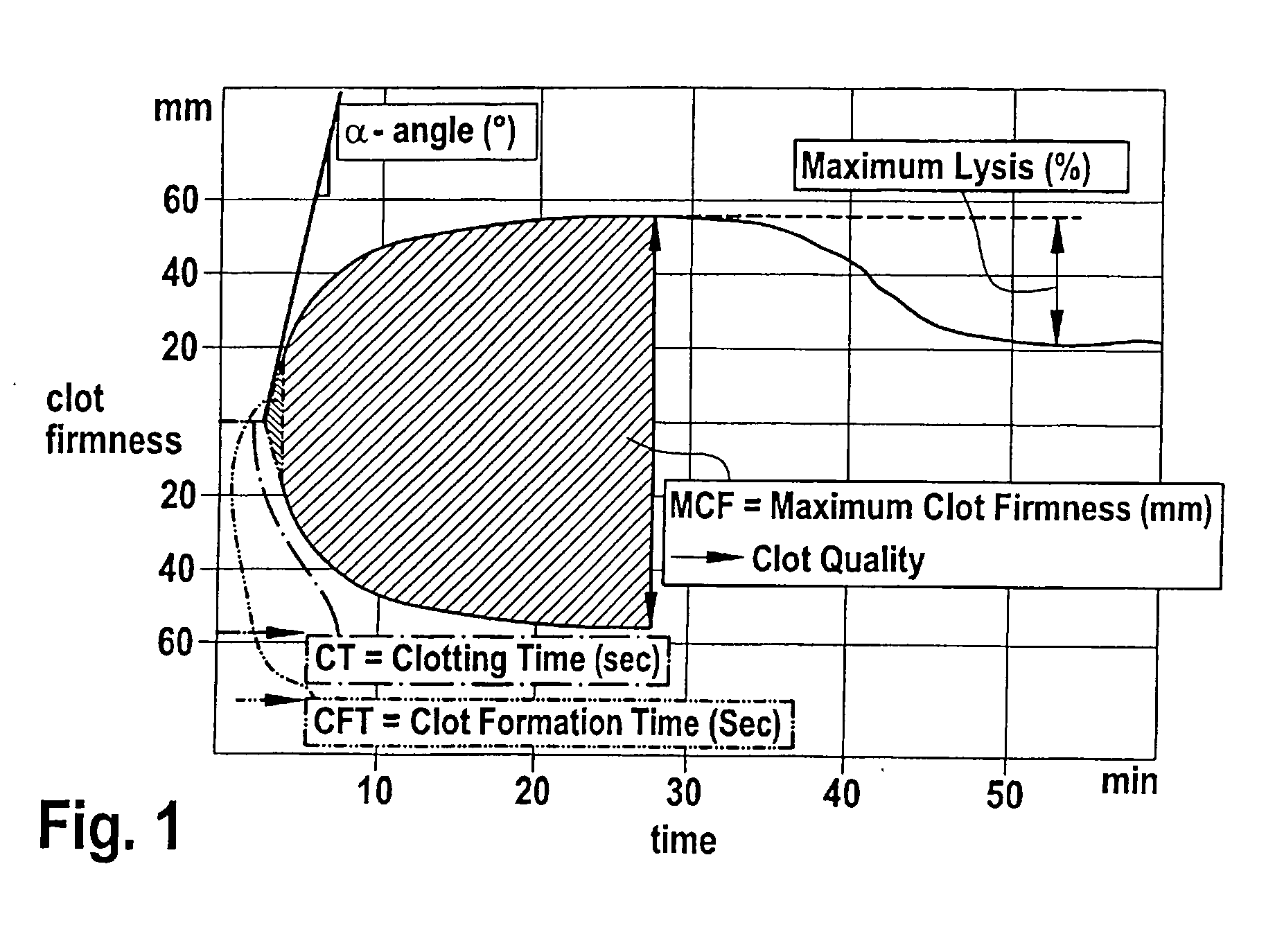
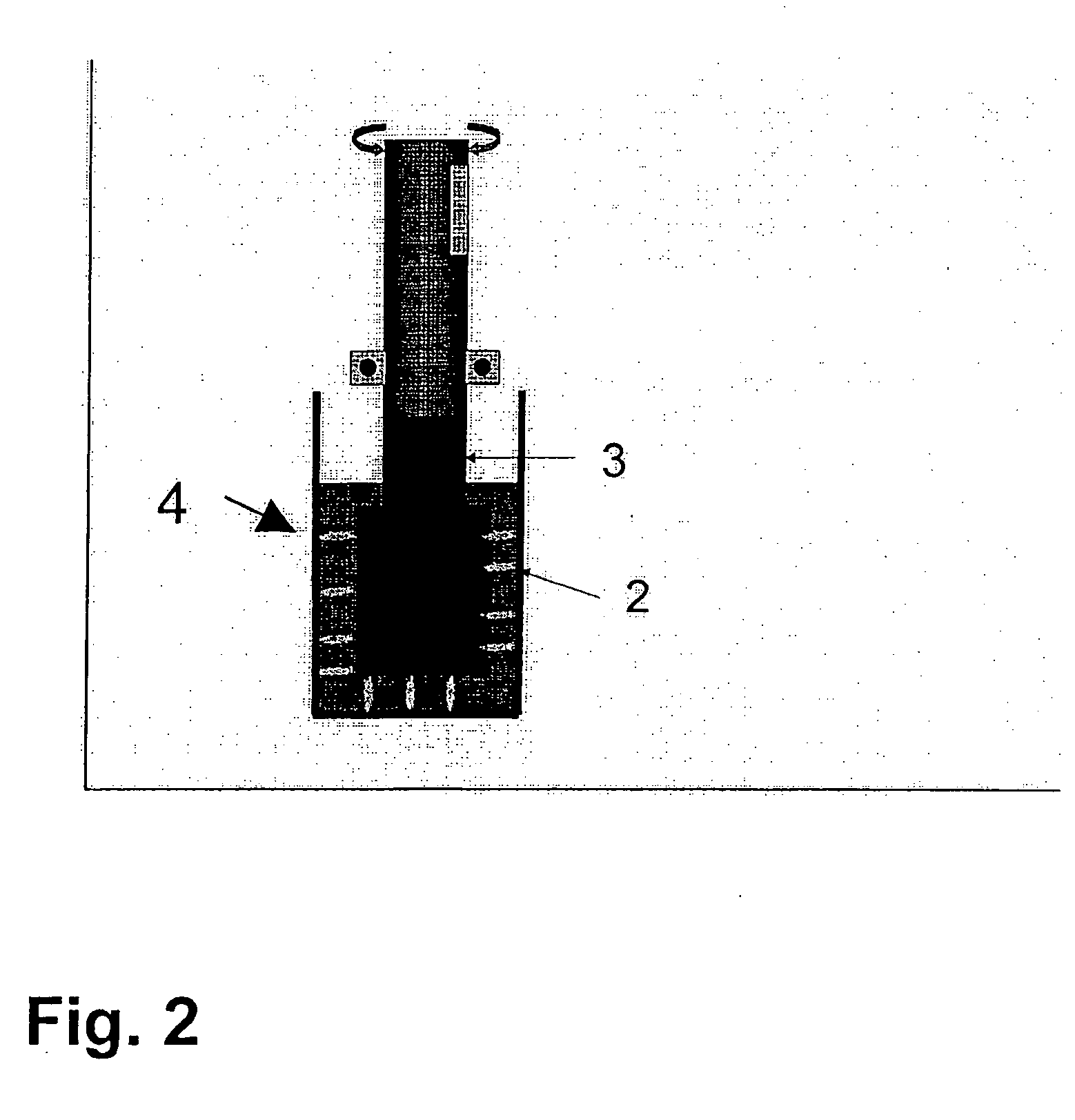
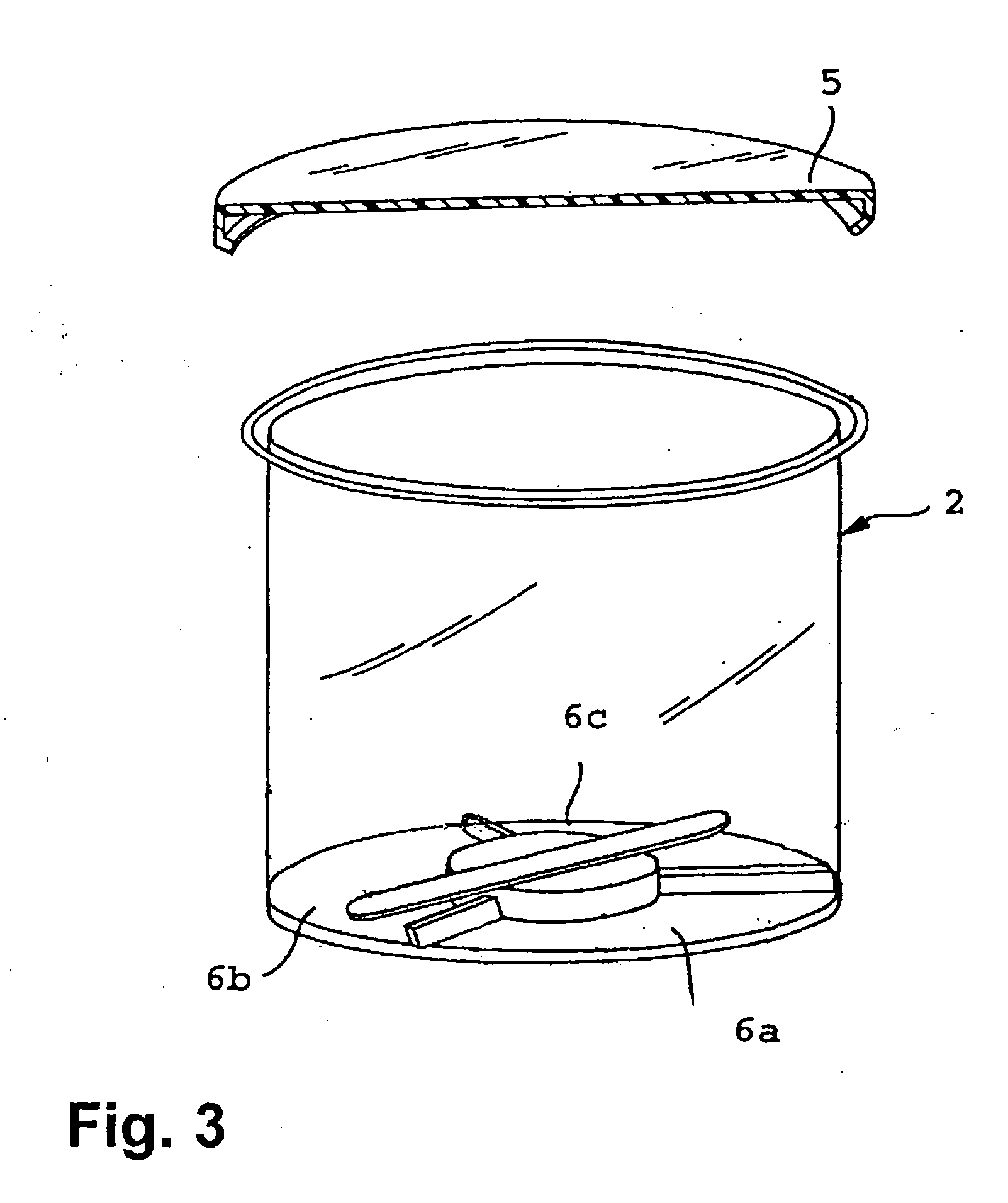
The present invention is directed to a diagnostic composition for use in the viscoelastic analysis of a test liquid, and to a container (1) comprising same. The composition comprises at least an activator of coagulation, and, optionally CaCl2 an optionally, one or more inhibitors and / or coagulation components, wherein the composition is present as an essentially dry mixture of all constituents and in an amount sufficient for performing one single viscoelastic analysis of a specified blood or plasma sample. The present invention is further directed to a method of performing a viscoelastic analysis on a test liquid, and to the use of the diagnostic composition in such a method.
Owner:DSM IP ASSETS BV
Osteopontin as Novel Prognostic Biomarker for Heart Failure
InactiveUS20100267062A1Improve non invasive monitoringReduce functionMicrobiological testing/measurementDisease diagnosisPlasma samplesOsteopontin Gene
The present invention relates to methods for providing a diagnosis, prognosis and / or risk stratification of a subject with heart failure, comprising determining the concentration of osteopontin (OPN) in the biological sample, preferably in a plasma sample. An OPN cut-off value is discloses as a valuable reference value. The present invention furthermore relates to the use of osteopontin as marker for diagnosis, prognosis and / or risk stratification of a subject with heart failure, the use of the determination of the osteopontin plasma concentration in a biological sample of a subject for diagnosis, prognosis and / or risk stratification of heart failure as well as kits for performing the methods and uses of the invention. The present invention allows particularly for risk stratification of patients with heart failure, such as mortality prediction and prognosis of heart failure severity.
Owner:UNIVERSITATSKLINIKUM HEIDELBERG
Method and apparatus for ratio fluorometry
A method and apparatus for determining the concentration of molecules or atoms by measuring the ratio of two fluorescence signals. A sample having fluorescent molecules is exposed to radiation or excitation energy from a first source, which can be a broadband light source. The fluorescence of the sample is detected at two different wavelengths. The concentration of specific molecules or atoms within the sample is determined using the ratio of the two fluorescence signals. Fluorescent molecules can be bound to a human serum or plasma sample to allow determination of the concentration of unbound free fatty acids in the sample.
Owner:KLEINFELD ALAN
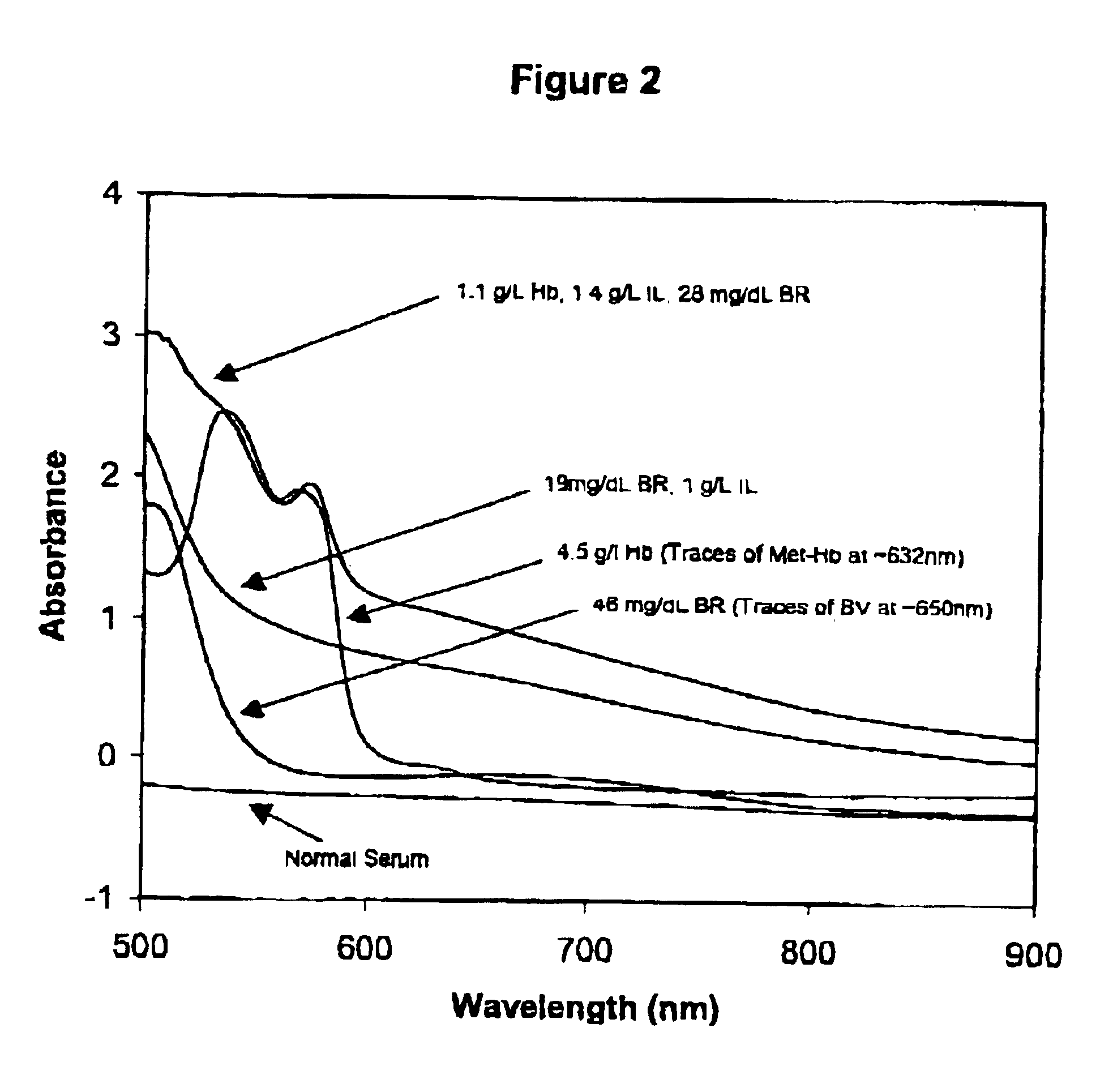
Calibrator material for instruments which measure interferents in serum and plasma specimens
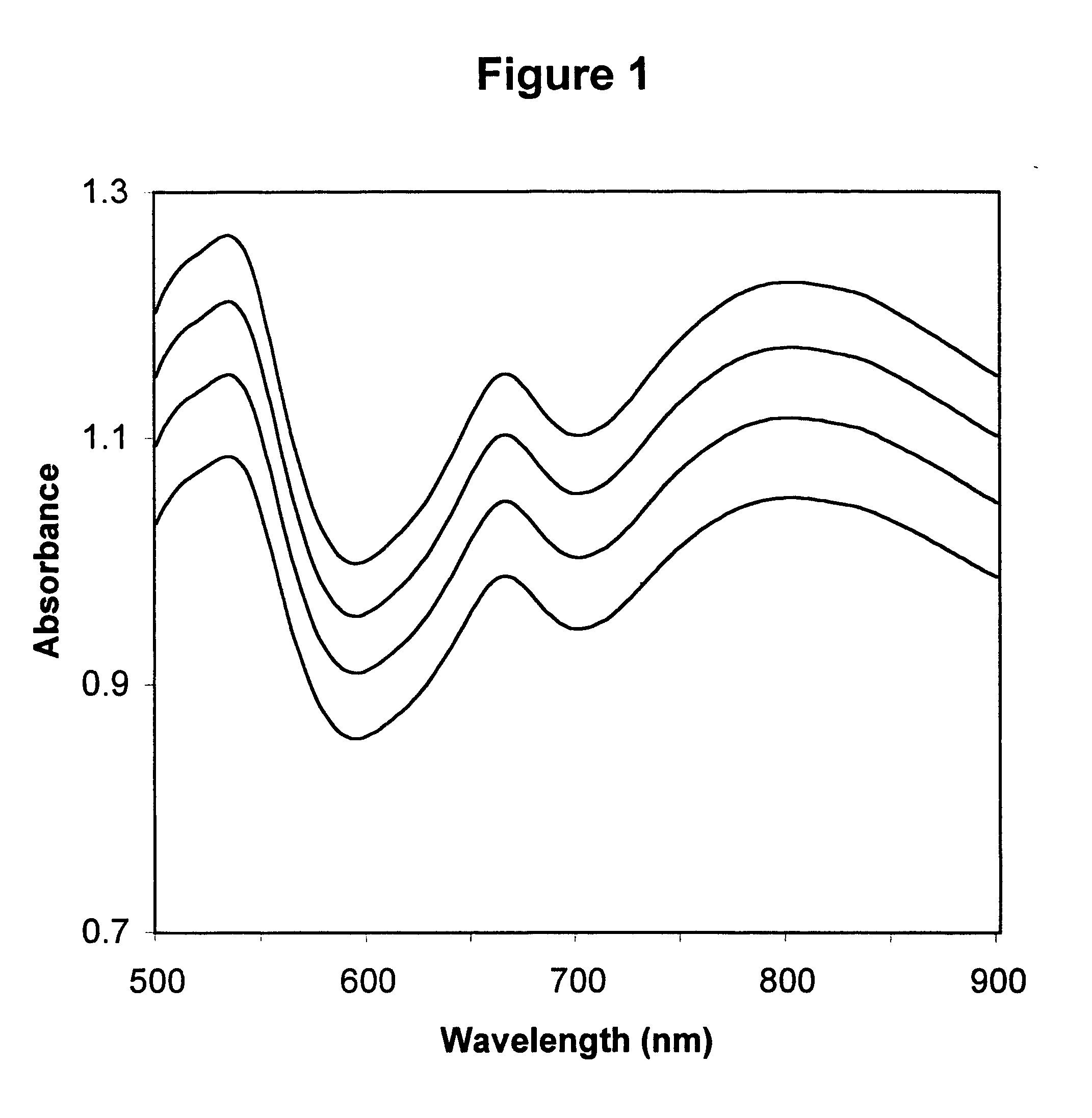
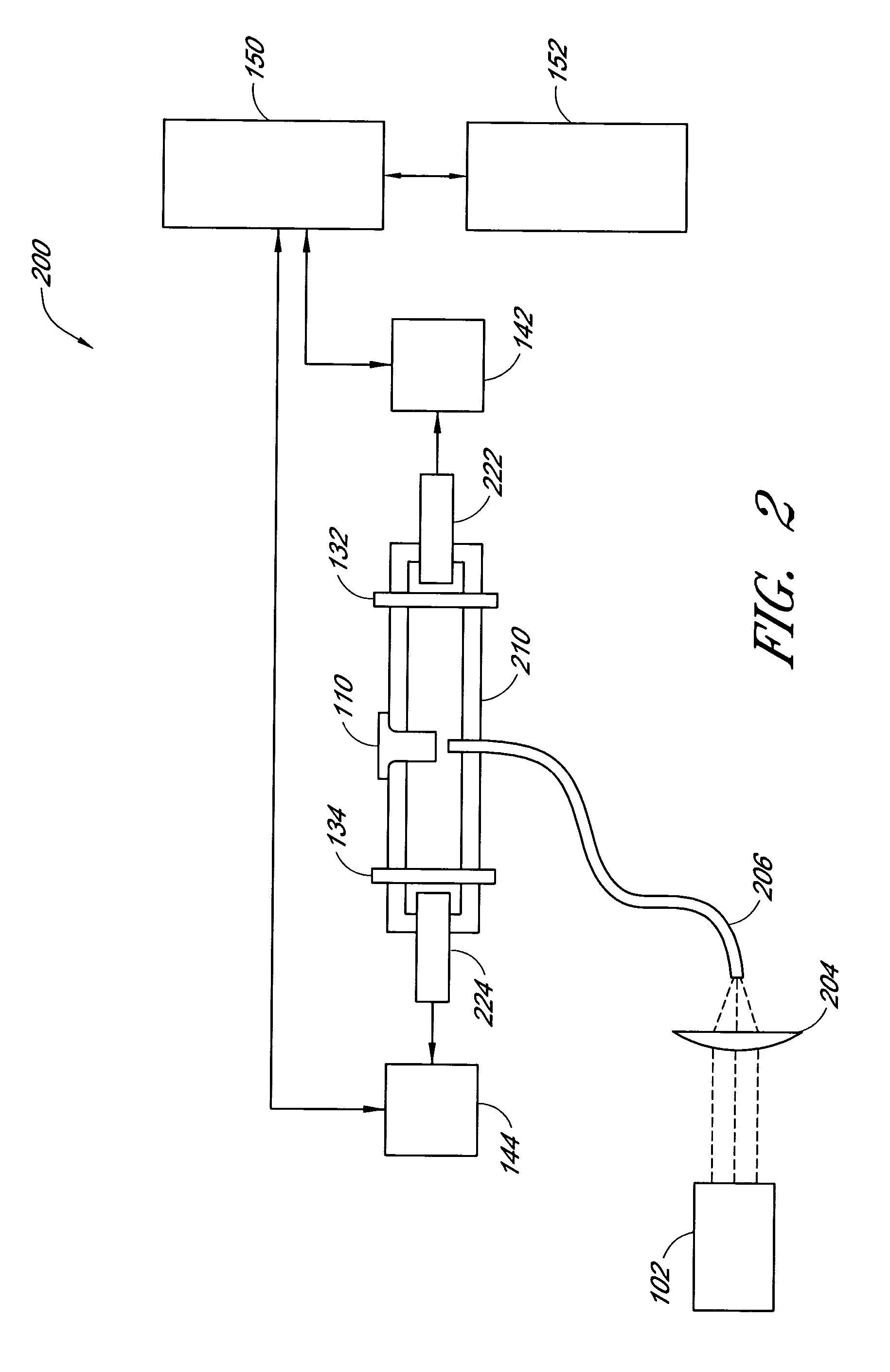
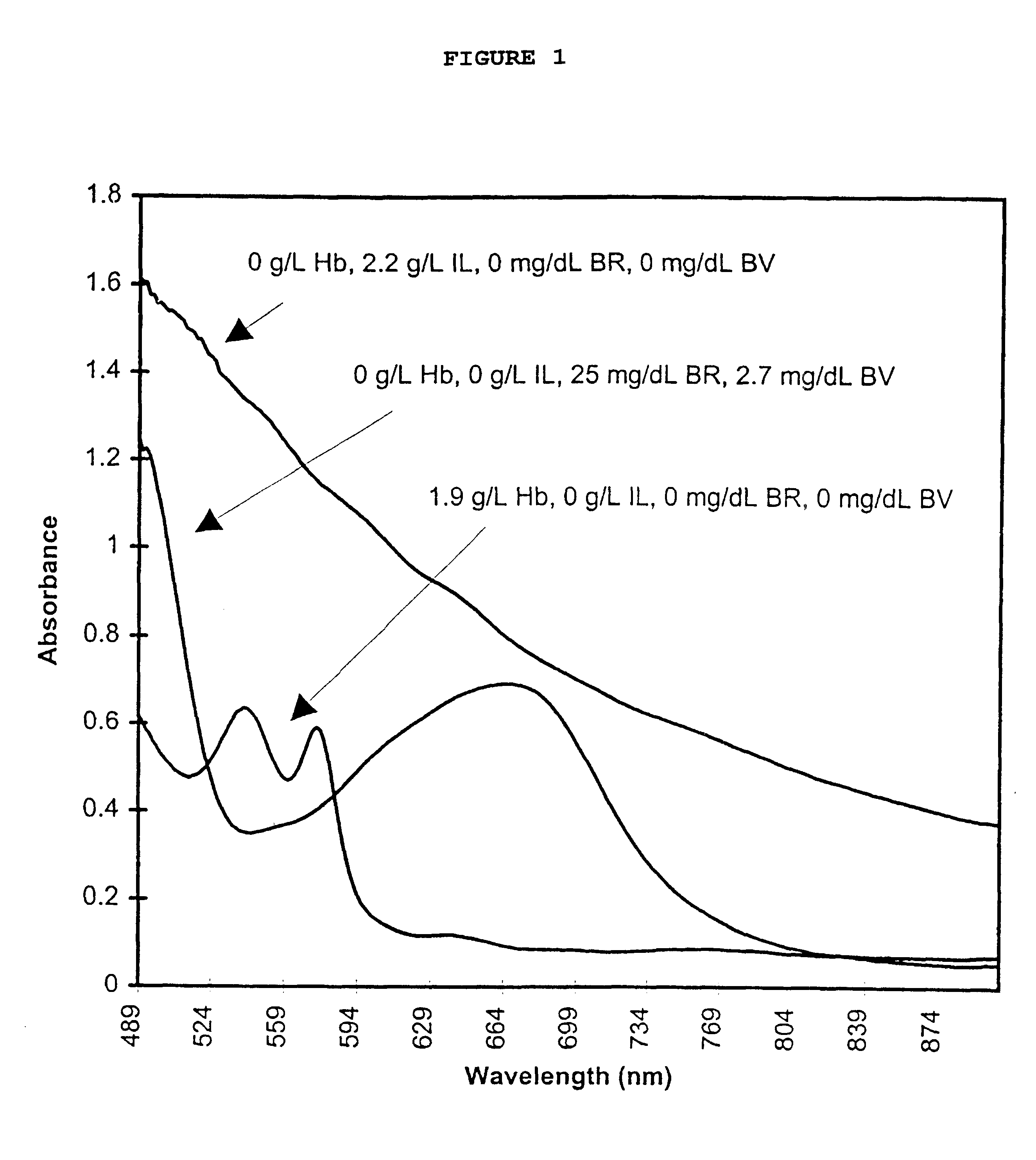
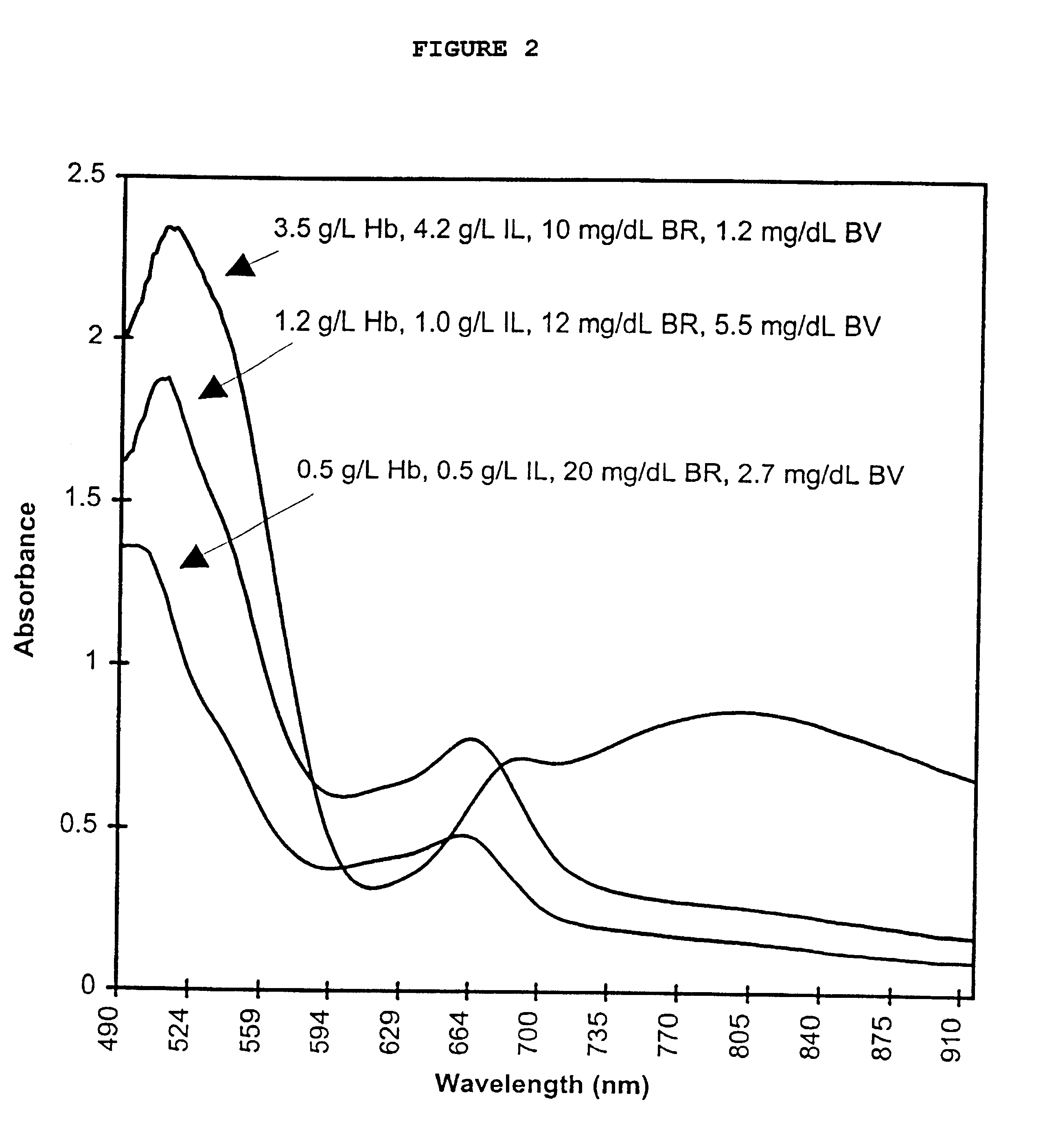
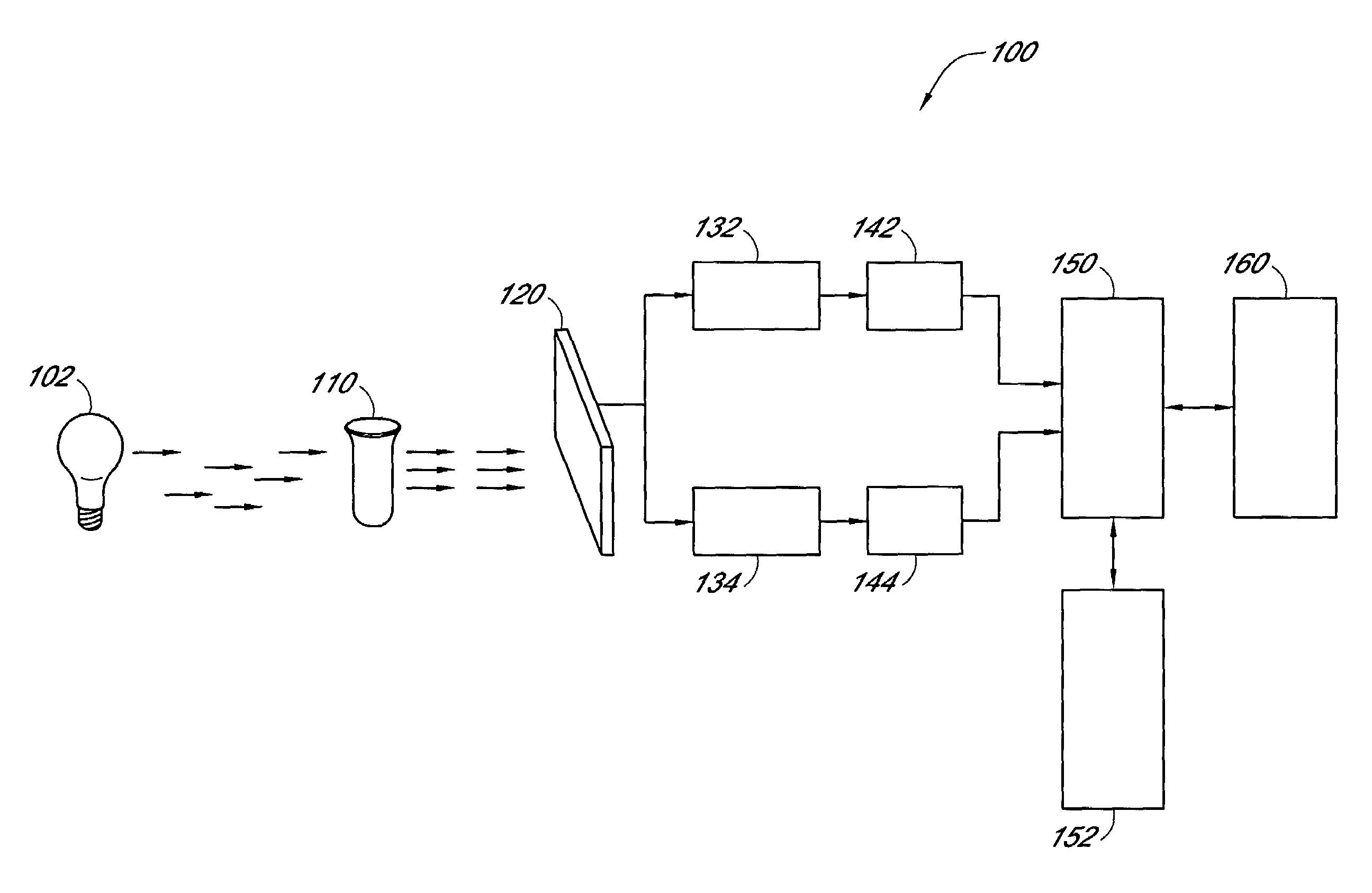
InactiveUS6372503B1Increase absorbanceLeft outBiological testingSpecial data processing applicationsBILIRUBINAEMIAHemolysis
A quality control material is disclosed which is used to monitor the calibration or used for recalibration of instruments used to screen for interferents in serum or plasma specimens. In particular, the quality control material disclosed is used to monitor instrument calibrations or used for recalibration for instruments which assess the amount of hemolysis, turbidity, bilirubinemia and biliverdinemia, either separately, or any two, or any three, or all four simultaneously, in plasma or serum samples. The quality control material does not contain any blood products such as plasma lipids, bile pigments, or hemoglobin, is stable at room temperature, and is ready for use with up to four constituents.
Owner:TYCO HEALTHCARE GRP LP
Method and apparatus for ratio fluorometry
A method and apparatus for determining the concentration of molecules or atoms by measuring the ratio of two fluorescence signals. A sample having fluorescent molecules is exposed to radiation or excitation energy from a first source, which can be a broadband light source. The fluorescence of the sample is detected at two different wavelengths. The concentration of specific molecules or atoms within the sample is determined using the ratio of the two fluorescence signals. Fluorescent molecules can be bound to a human serum or plasma sample to allow determination of the concentration of unbound free fatty acids in the sample.
Owner:KLEINFELD ALAN
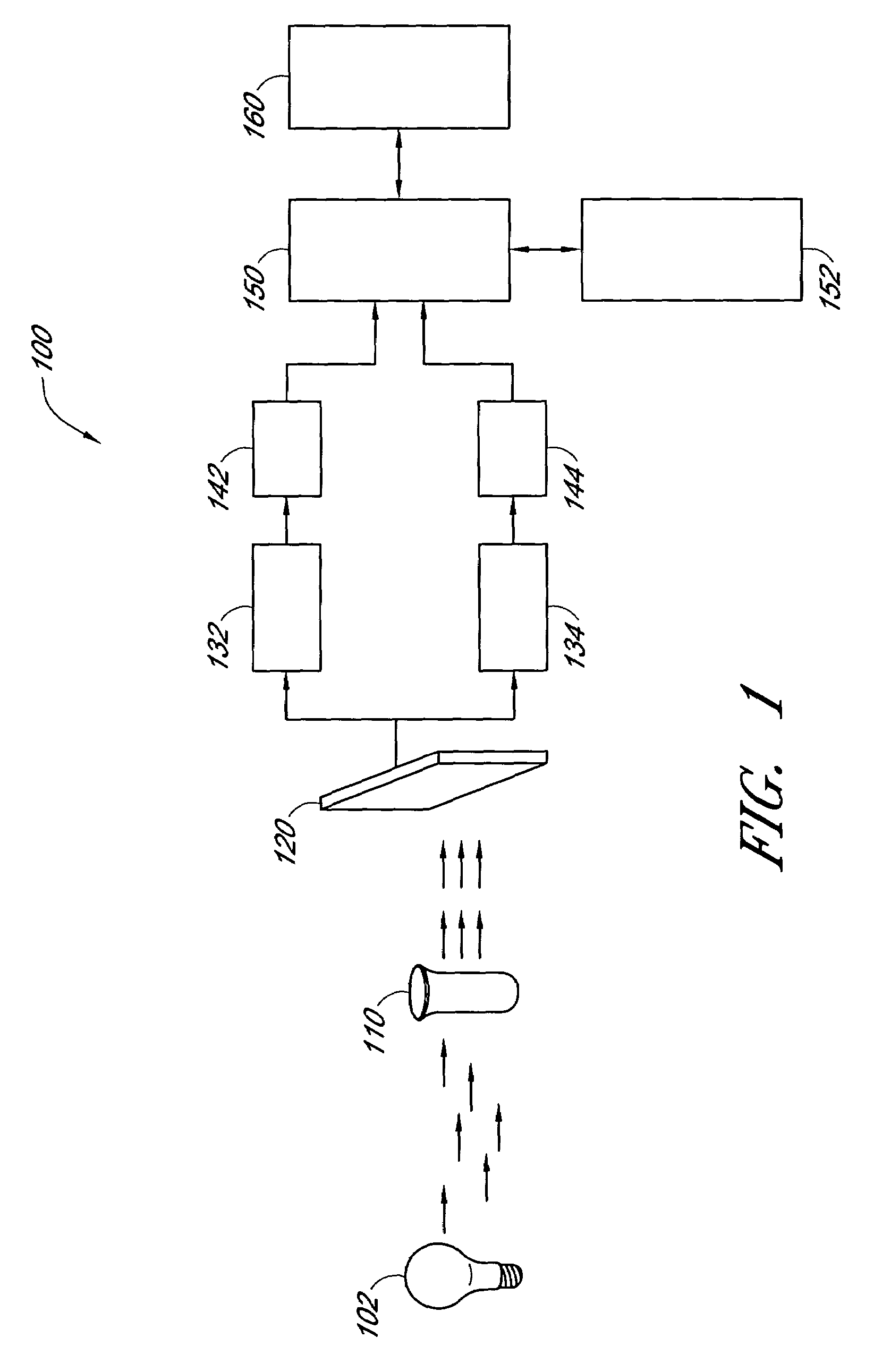
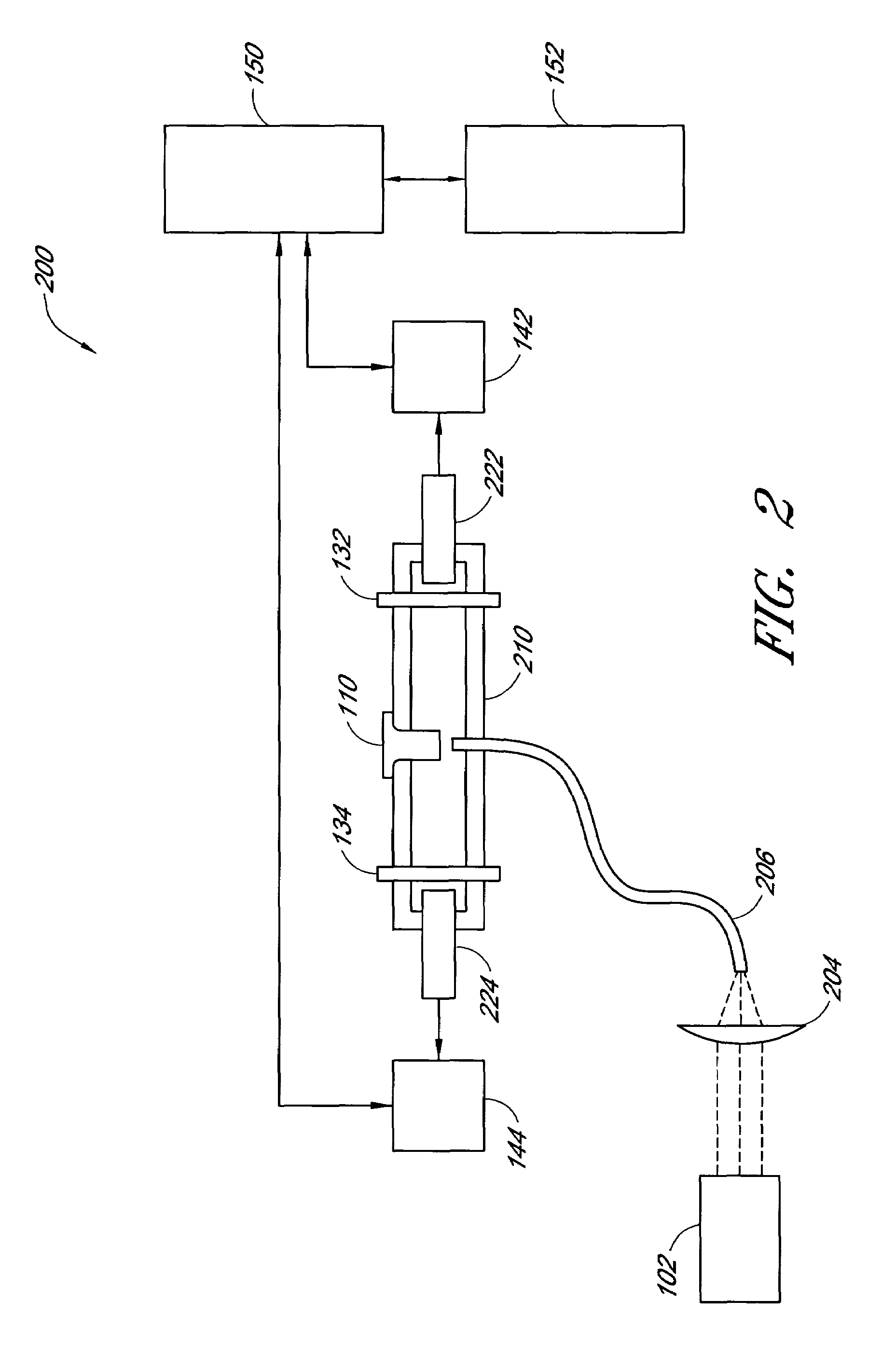
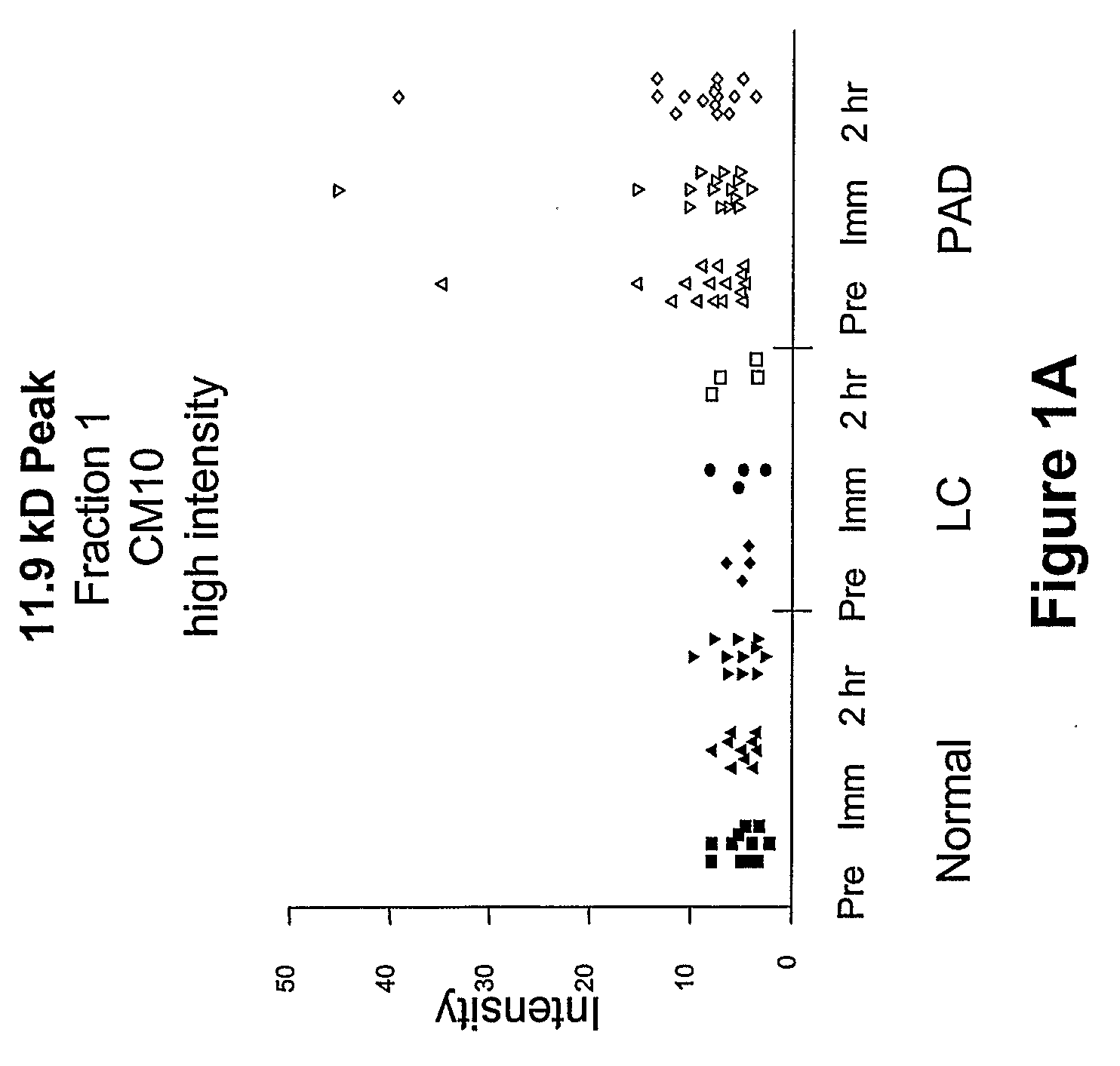
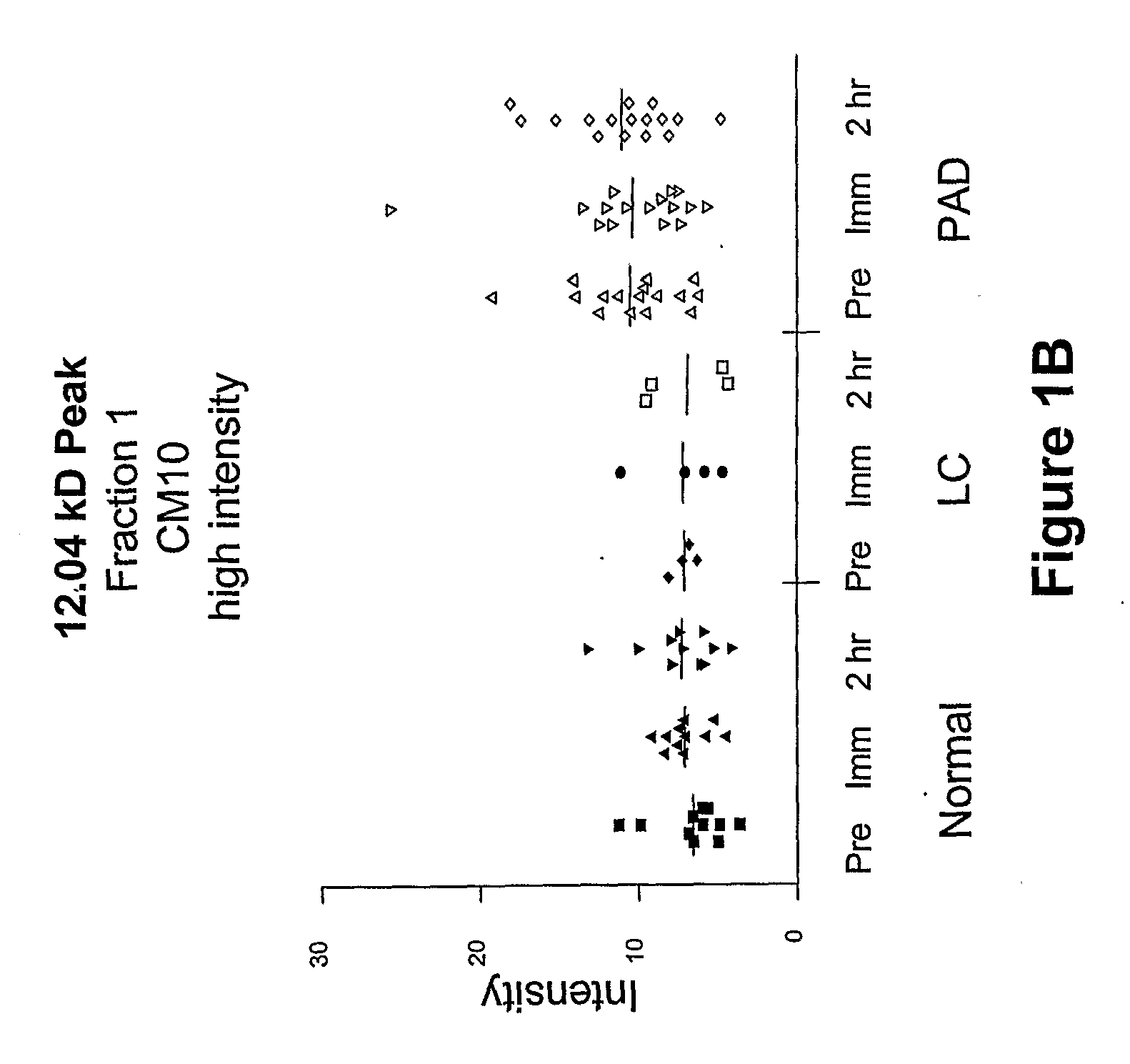
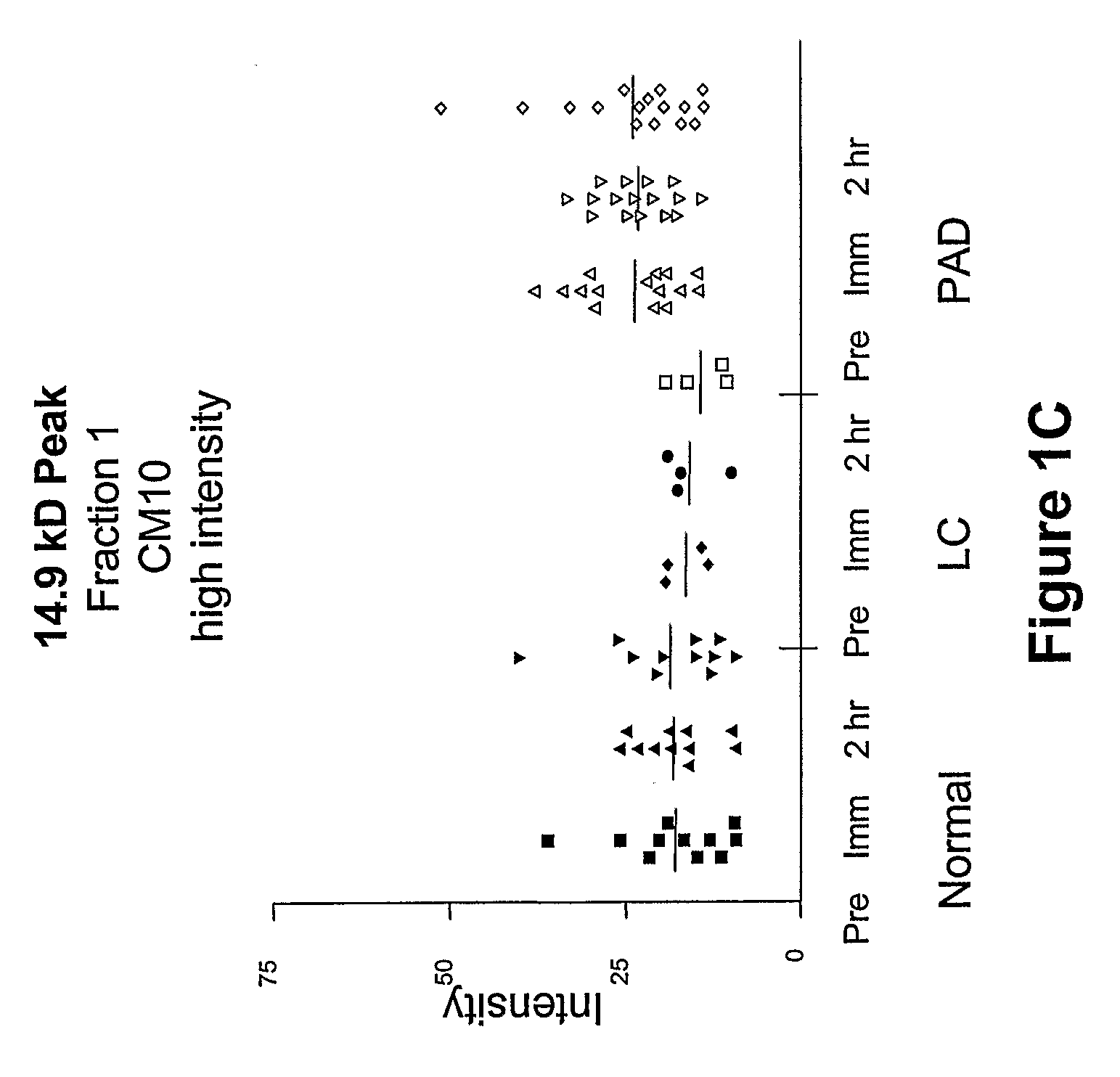
Biomarkers for peripheral artery disease
This invention provides biomarkers whose concentrations in blood plasma are associated with the presence or absence of PAD in the patient from whom the plasma sample is taken. The invention also provides biomarkers for distinguishing between PAD patients who are long claudicators and PAD patients who are not. In addition, the invention provides methods for identifying additional biomarkers, methods for detecting the biomarkers in patients, and methods for identifying agents, including pharmaceutical agents, which interact with the biomarkers and are useful for preventing or treating PAD in patients.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
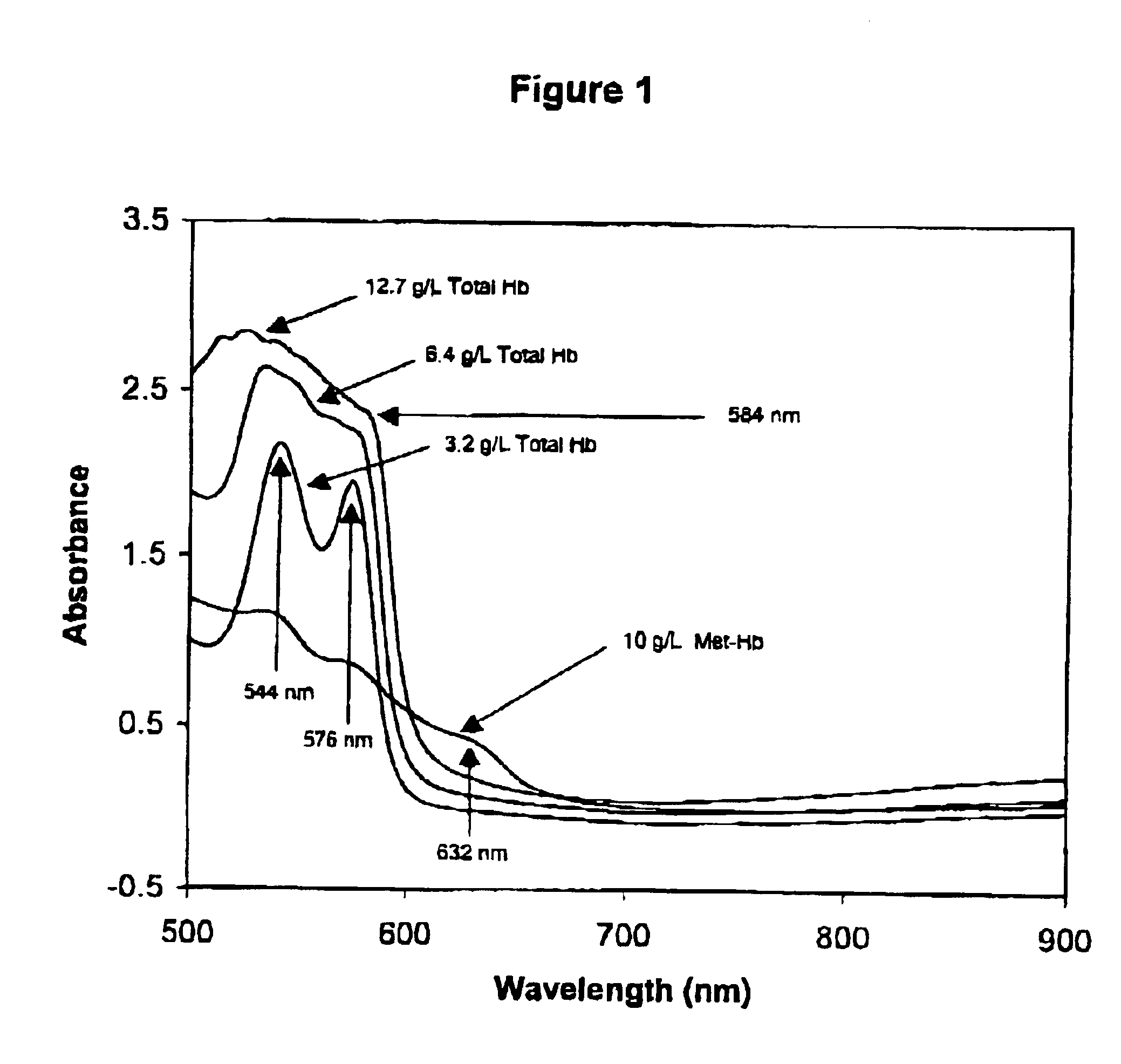
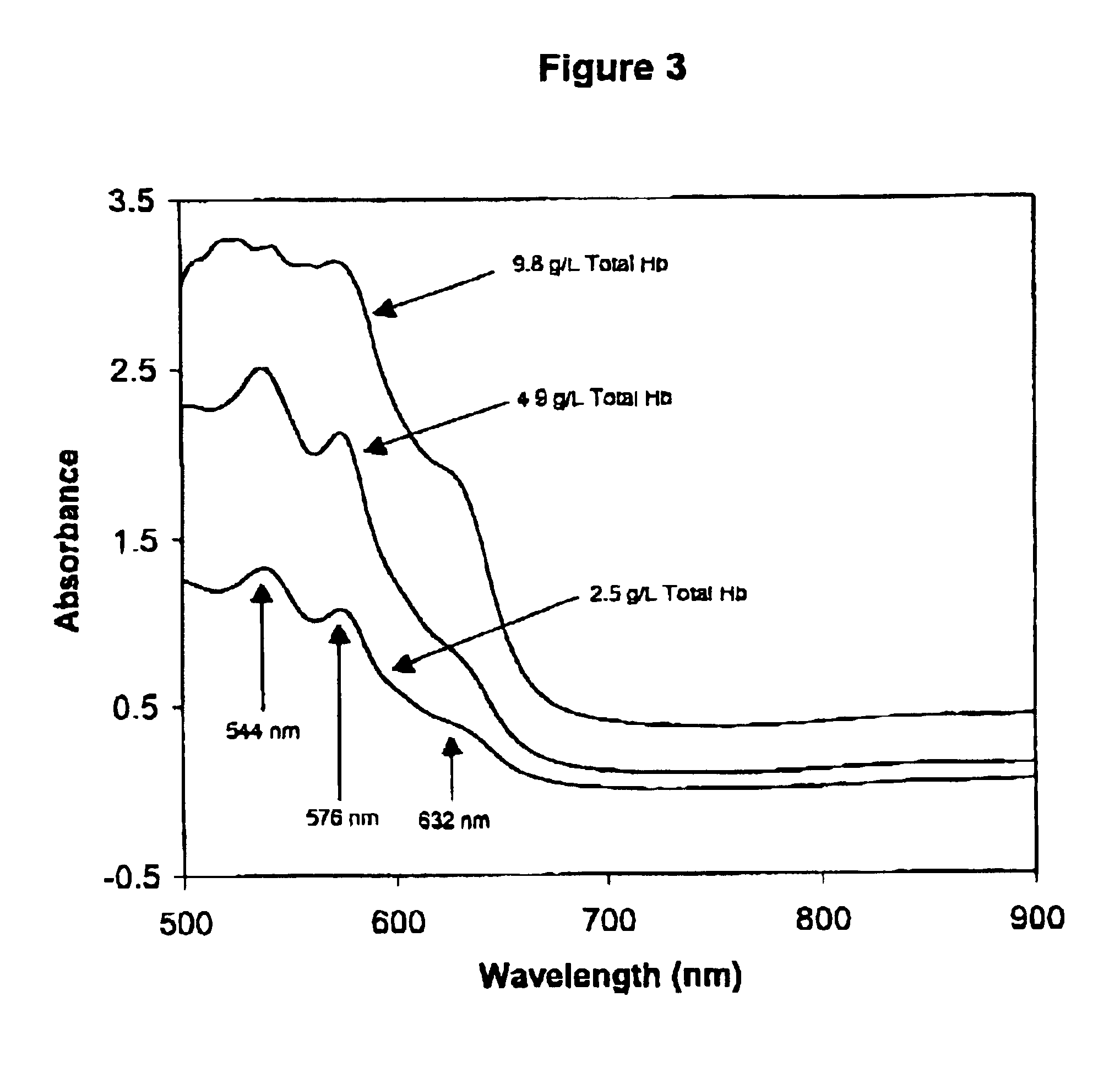
Method for monitoring degradation of Hb-based blood substitutes
InactiveUS6949384B2Analysis by material excitationAnalysis by subjecting material to chemical reactionPlasma samplesSerum samples
The present invention provides a method for monitoring degradation of Hb-based blood substitutes, in a sample. This method involves determining a concentration of met-Hb within the sample, by applying a calibration algorithm for met-Hb to an absorbance obtained from the sample at one or more than one wavelengths, and using the concentration of met-Hb, as a measurement of degradation of the Hb-based blood substitutes. Using this assay, a concentration of met-Hb that is equal to or greater than 3% may be used as an indicator of degradation of Hb. Alternatively, by obtaining samples over a period of time, the concentration of met-Hb and the concentration of Hb-based blood substitute may be determined in each of these samples, and an increase in the concentration of met-Hb over the period of time is an indicator of degradation of Hb. The sample may be a whole blood sample, a serum sample, or a plasma sample obtained from a patient transfused with one or more than one Hb-based blood substitutes, a stock Hb-based blood substitute sample, or a body part of a patient.
Owner:COVIDIEN LP
Detection device for instability of genome copy number
InactiveCN104560697ABioreactor/fermenter combinationsBiological substance pretreatmentsGenomic sequencingInformation analysis
The invention relates to the technical field of medical detection and discloses a detection device for the instability of the genome copy number. The detection device comprises a sequencing unit, a calculation unit and a statistical analysis unit, wherein the sequencing unit is used for sequencing of free DNAs in a plasma sample of a person to be detected and obtaining the sequencing number of each genome window of the sample to be detected; the calculating unit is used for calculating the variance value of the copy number of each genome window; and the statistical analysis unit is used for carrying out statistics on the variance value of the copy number of each genome window and analyzing whether the instability of the genome copy number exists. The detected instability of the genome copy number can be further used for judging the risk of the person to be detected suffered from the cancers. The detection device disclosed by the invention has the advantages that a molecular biological sequencing technology and a biological information analysis technology are combined, the recognition for the abnormal condition of the copy number of chromosome is achieved by detecting free DNAs in blood, so that whether the person to be detected is suffered from cancers is judged, and further early-stage non-invasive screening for the cancers is realized.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Direct determination of vitamin D in serum or plasma
A method for quantitating vitamin D metabolites directly in blood plasma or serum, without the need for prior purification of the vitamin D metabolites, comprising a digestion of the serum proteins with a serine protease such as proteinase K and sequence of steps for inhibiting the proteinase K activity in the competitive binding analysis. The advantages of this method are its high accuracy over the whole range of physiologically relevant values and that it can be easily adapted for a fully automated analysis of serum and plasma samples.
Owner:IMMUNDIAGNOSTIK AG
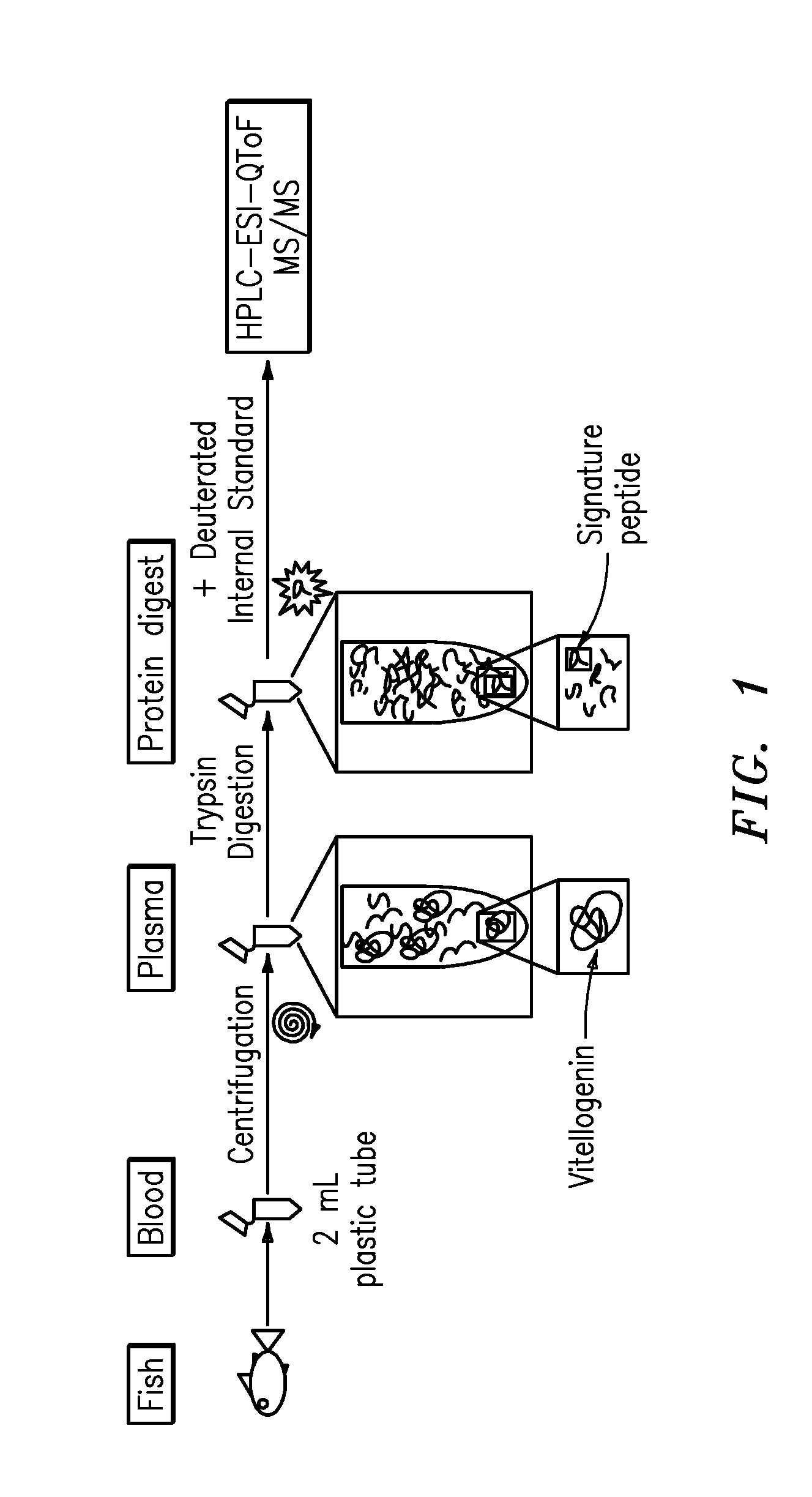
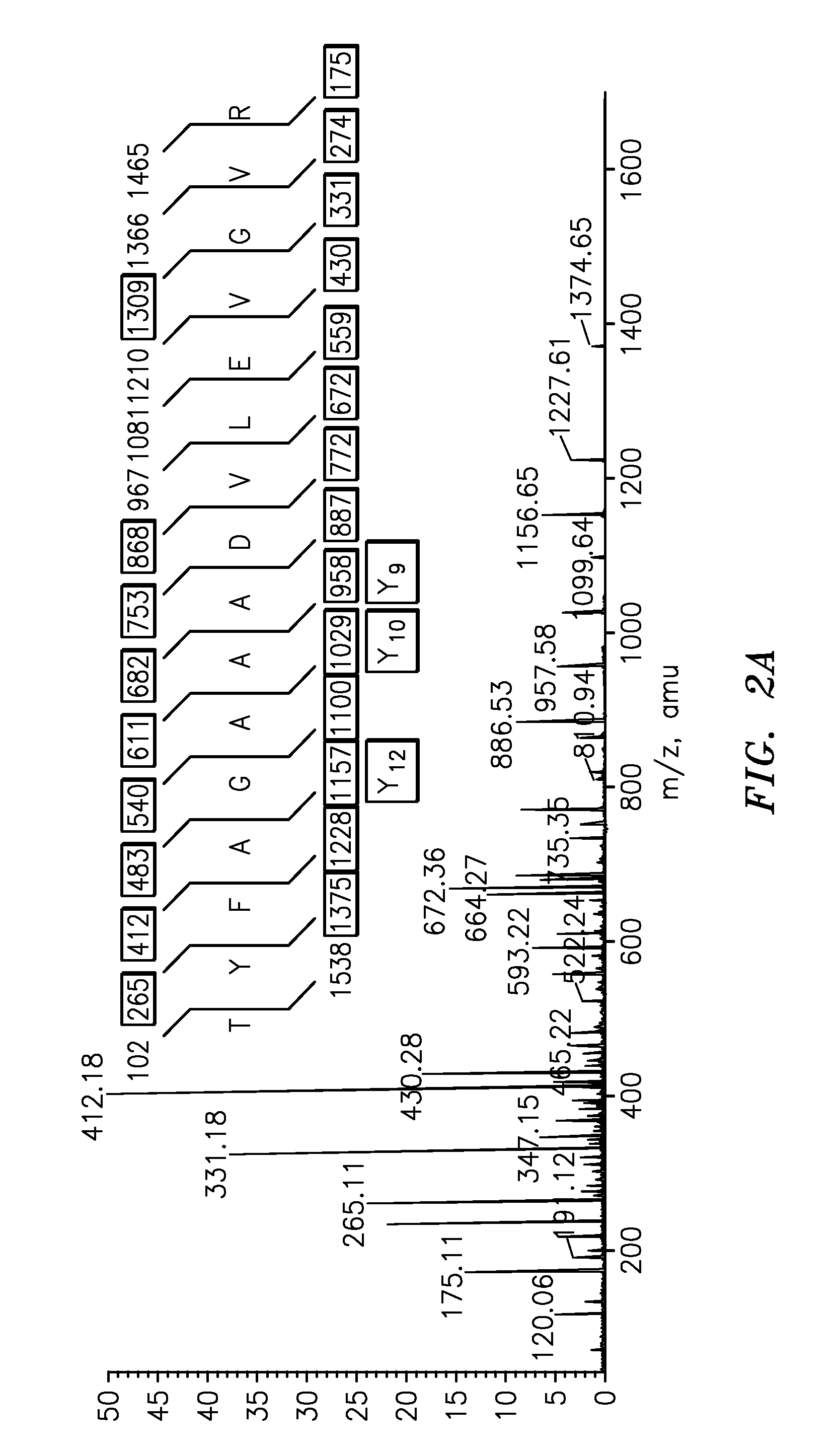
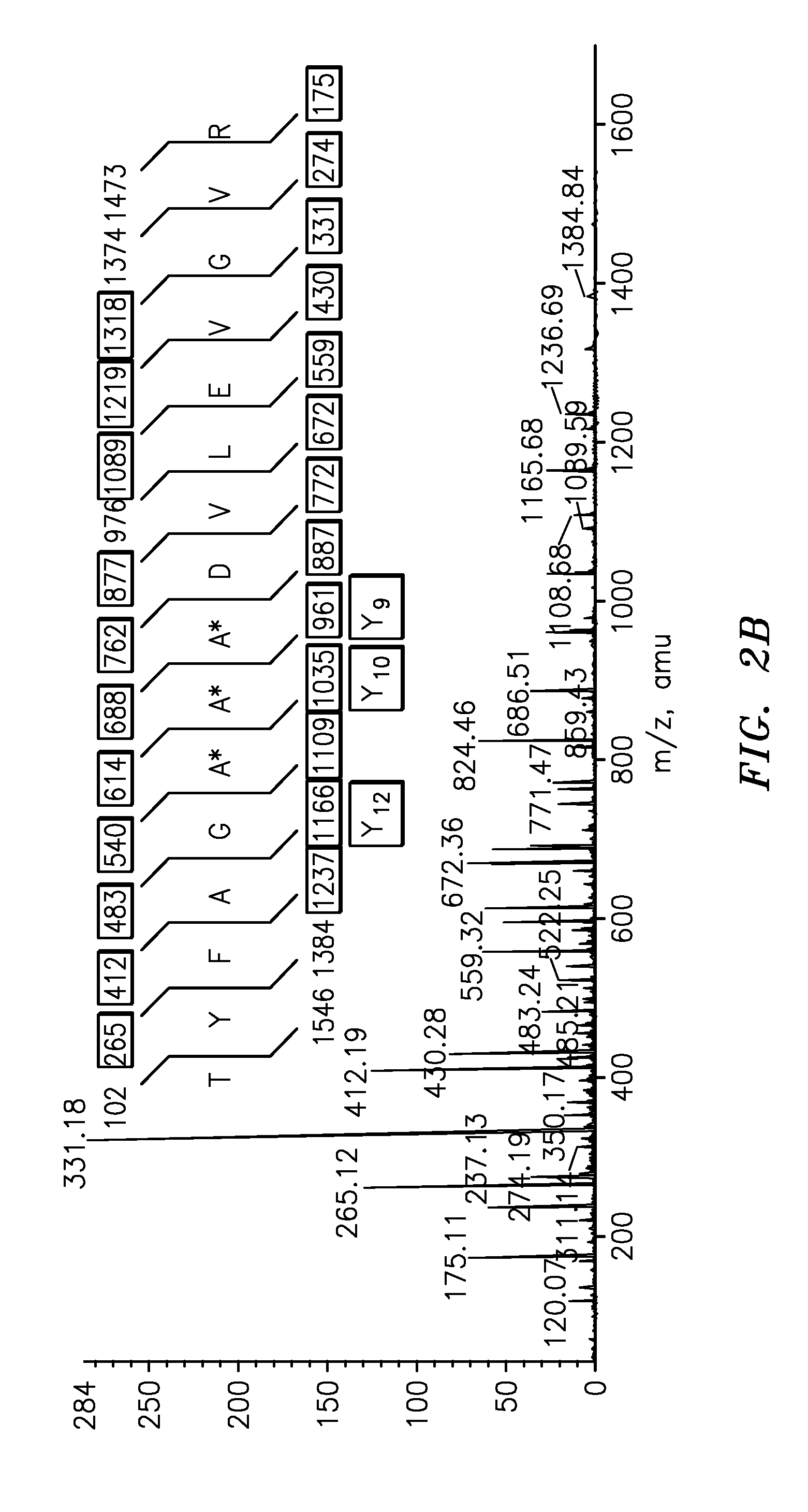
Quantification of vitellogenin
InactiveUS20090011447A1Simple methodMicrobiological testing/measurementPeptidesVitellogeninsGlu-Val-Gly
The present invention is directed to a simple method for absolute quantification of plasma vitellogenin from two or more different fish species such as Rainbow trout and Atlantic salmon, or Atlantic cod and haddock. In the case of Rainbow trout and Atlantic salmon, plasma samples obtained from control and β-estradiol induced fish were digested with trypsin. A characteristic ‘signature peptide’ was selected and analyzed by high performance liquid chromatography coupled to an electrospray quadrupole-time-of-flight tandem mass spectrometer, using a deuterated homologue peptide as an internal standard. The hybrid tandem mass spectrometer was operated in a ‘pseudo’ selected reaction monitoring mode by which three diagnostic product ions were monitored for identification and quantification purposes. The reproducibility (coefficient of variation ˜5%) and sensitivity (limit of quantification of 0.009 mg / mL) achieved by this simple assay allow it to be considered as an alternative to immunological assays. In the case of Atlantic cod and haddock, the amino acid sequence of the vitellogenin protein has not yet been determined, but, the Atlantic cod vitellogenin has been characterized using a ‘bottom-up’ mass spectrometric approach. Vitellogenin synthesis was induced ‘in vivo’ with β-Estradiol, and subjected to trypsin digestion for characterization by matrix-assisted laser desorption / ionization-Quadrupole-Time-of-flight tandem mass spectrometry. A peptide mass fingerprint was obtained and ‘de novo’ sequencing of the most abundant tryptic peptides was performed by low energy collision induced dissociation-tandem mass spectrometry. Thus, the sequences of various tryptic peptides have been elucidated. It has also been determined that Atlantic cod vitellogenin shares a series of common peptides with the two different known vitellogenin sequences of Haddock, a closely related species. There are also disclosed novel isolated signature peptides, namely Thr-Tyr-Phe-Ala-Gly-Ala-Ala-Ala-Asp-Val-Leu-Glu-Val-Gly-Val-Arg, Asp Leu Gly Leu Ala Tyr Thr Glu Lys, Phe Phe Gly Gln Glu Ile Ala Asn Ile Asp Lys, Glu Ile Val Leu Leu Gly Tyr Gly Thr Met Ile Ser Lys and Tyr Glu Ser Phe Ala Val Ala Arg.
Owner:BANOUB JOSEPH H +2
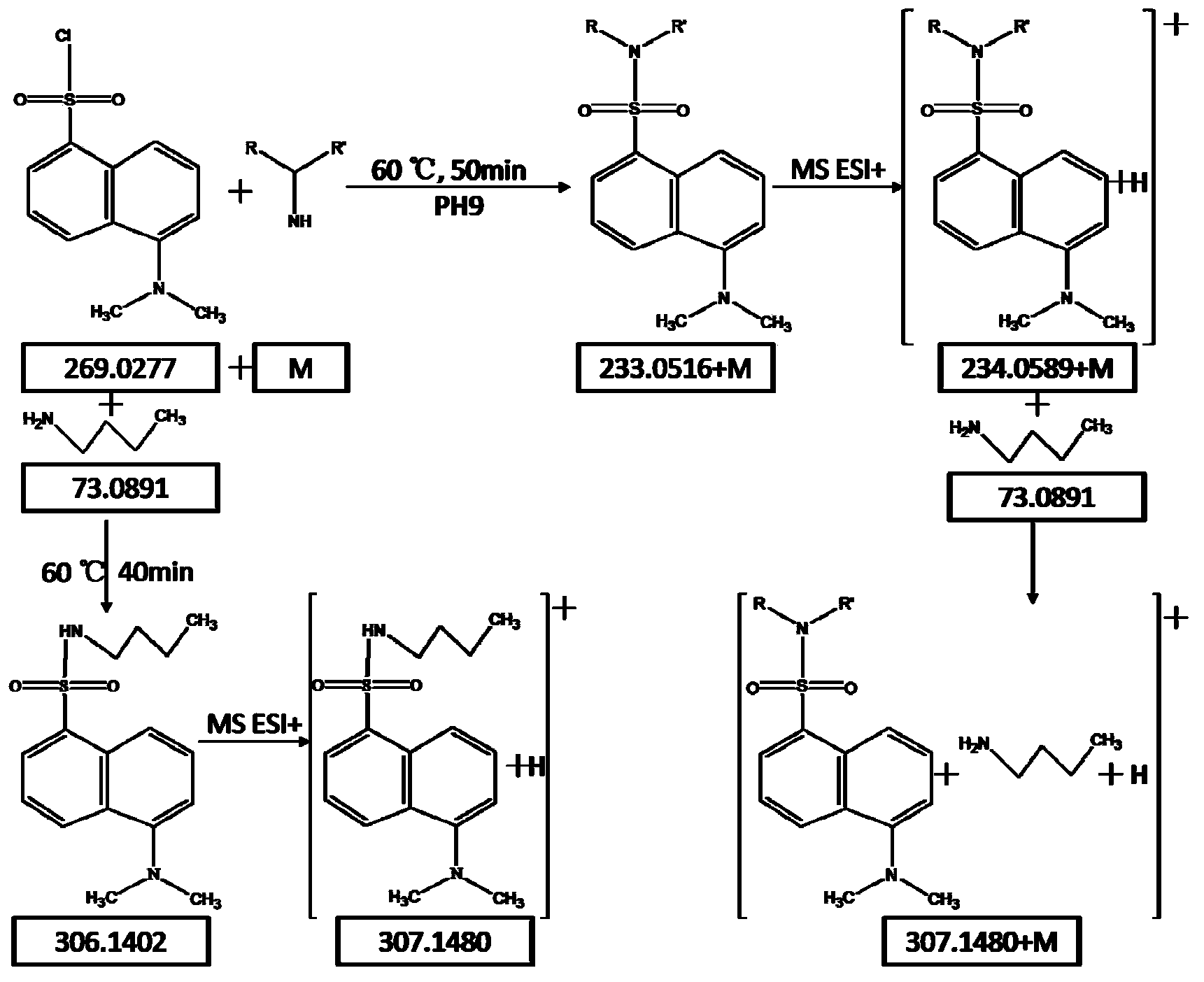
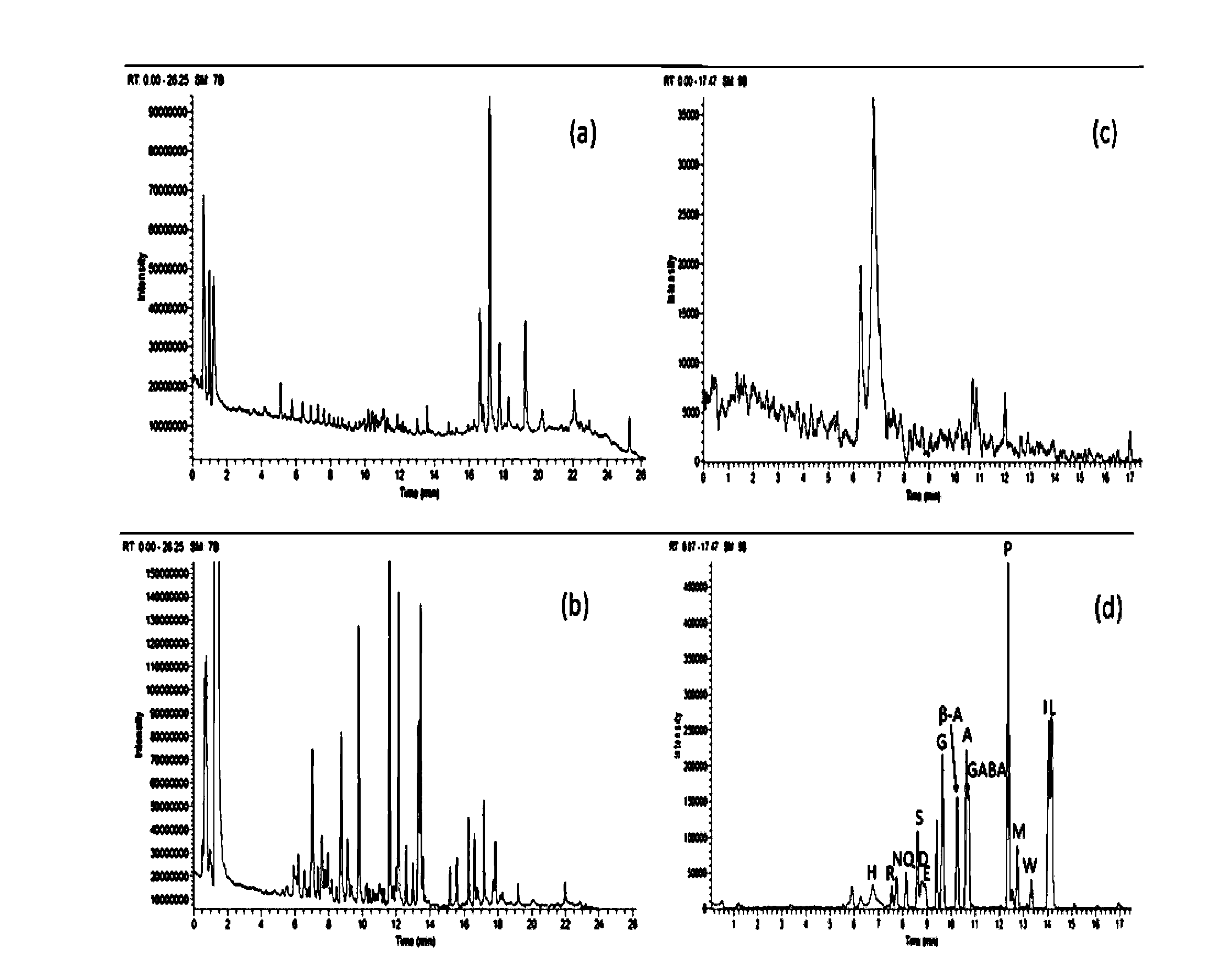
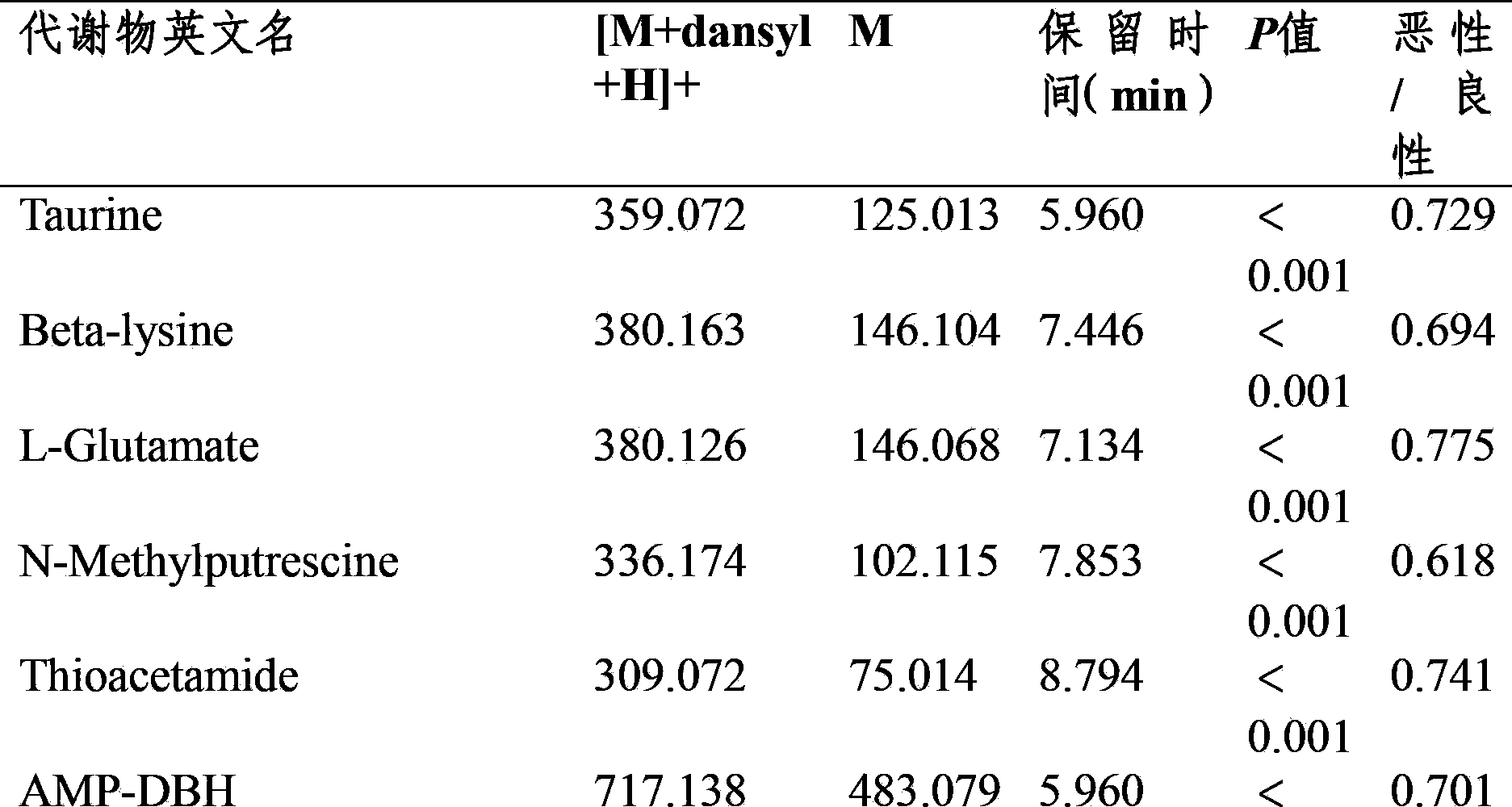
Method for analyzing amine substances in dansyl chloride derived-plasma based on liquid chromatography mass spectrometry
InactiveCN103822998AReduce consumptionStrong specificityComponent separationDipeptideGas chromatography–mass spectrometry
The invention discloses a method for analyzing amine substances in dansyl chloride derived-plasma based on liquid chromatography mass spectrometry. The method is characterized in that dansyl chloride is used as a derivatization reagent to perform derivatization on a metabolite containing primary amine and secondary amine, therefore the retention capability of the amine substances in reversion phase chromatography is increased, the ionization efficiency in mass spectrometric detection is increased, and the purpose of increasing the quantitative accuracy and detection sensitivity of the amine substances is achieved. The method has wide coverage for amine metabolites (comprising amino acid, methylation products, acetylation products, dipeptide and the like), can utilize 100 [mu]L of plasma samples to detect 113 amine substances, supplies the outline information of metabolome of the amine substances, and has the characteristics of good specificity, high accuracy and repeatability, less reagent consumption and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
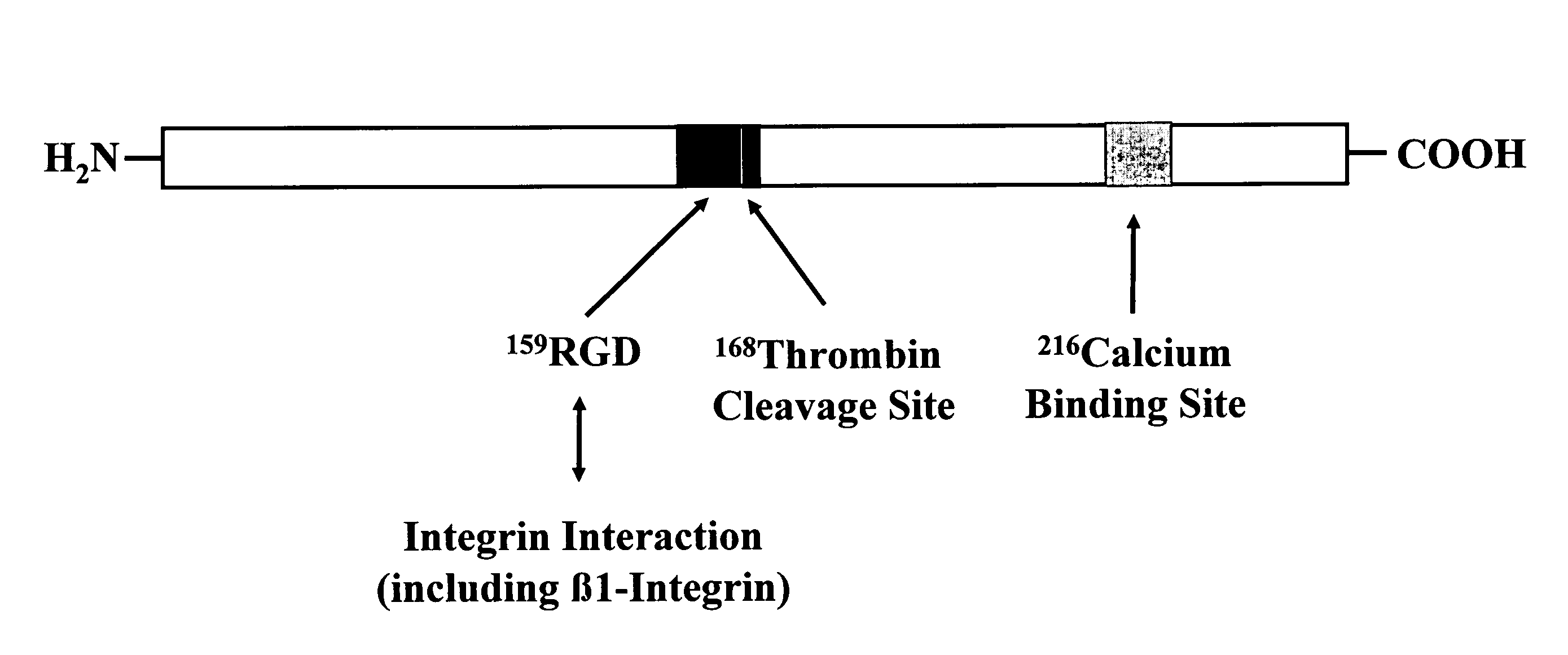
Antibodies to lipoproteins and apolipoproteins and methods of use thereof
InactiveUS7098036B2Rapid determinationImmunoglobulins against animals/humansEnzymologyLipid formationBlood lipoprotein
Compositions and methods using antibodies which are immunoreactive with specific apolipoproteins to determine the concentrations of lipoproteins such as HDL and LDL, and / or apolipoproteins in human blood, serum or plasma sample, are described. Monoclonal antibodies (MAbs) are described that specifically bind to epitopes present in apolipoproteins and lipoproteins, enabling rapid and reliable determinations of levels of specific blood lipoprotein and / or apolipoprotein levels, including Apo B-100, Apo A-I, Apo A-II, Apo C-III, and Apo E, and thereby determination of relative ratios of HDL and LDL and LpaI and LpaII. In a preferred embodiment, the compositions are strips of a solid phase material coated with one or more of the antibodies and are referred to herein as “dipsticks”. The dipsticks specifically bind a lipoprotein or apolipoprotein when dipped into a protein sample. The amount of lipid associated with a bound lipoprotein or the amount of apolipoprotein bound on the dipstick is quantitated using an appropriate method, for example, by staining with a lipid stain or reaction with a second labelled antibody. The intensity of the stain on the dipstick is proportional to the concentration of the lipoprotein lipid or apolipoprotein circulating in the blood and can be quantitated by comparison with standards containing known amounts of lipid.
Owner:OKLAHOMA MEDICAL RES FOUND
Method and device for detecting microorganisms in blood
PendingCN105525033ARelieve painAdjust in timeBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismPlasma samples
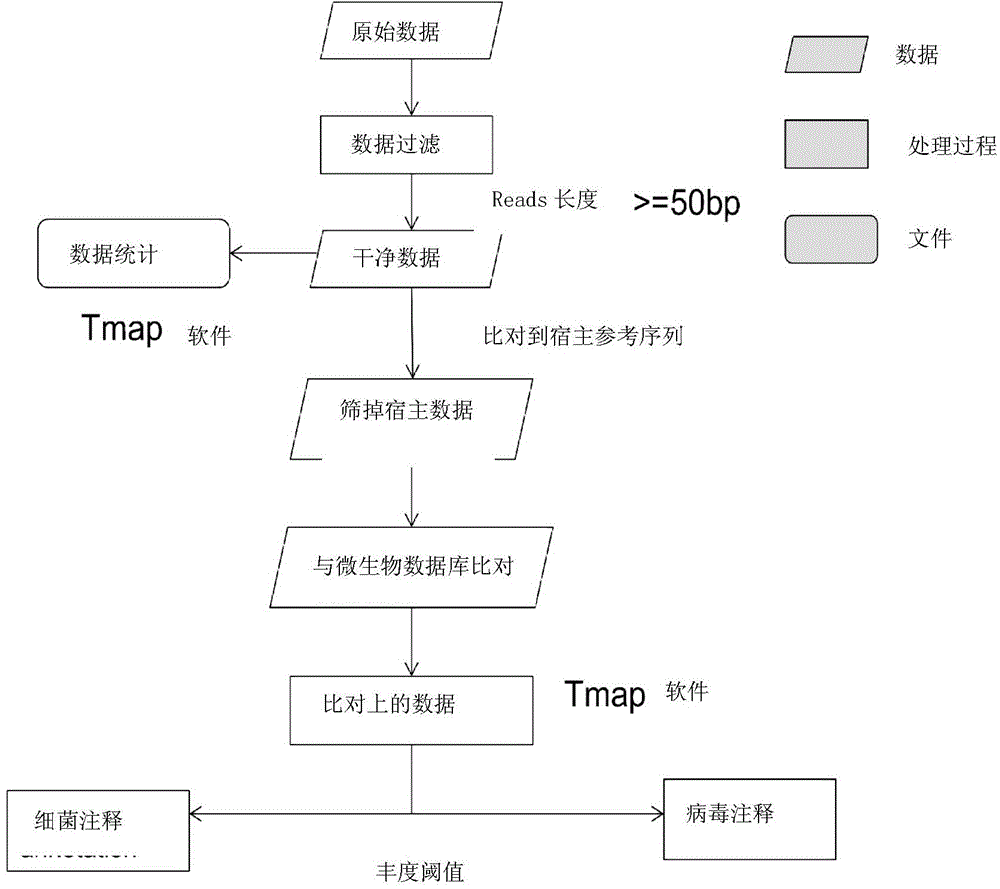
The invention provides a method for detecting microorganisms in plasma as well as a method and a device for detecting host infection pathogens. The method for detecting the microorganisms in the plasma includes extracting host plasma sample nucleic acid and subjecting at least part of the extracted nucleic acid to sequencing so as to obtain sequencing data; removing host sequencing data from the sequencing data; comparing the rest of sequencing data to a microorganism reference database so as to obtain comparison results, wherein the microorganism reference database includes reference sequences of multiple kinds of bacteria and multiple kinds of viruses; determining microorganism species in the plasma and richness of the microorganism species according to the comparison results. By the methods or the device, the microorganisms in the plasma can be detected rapidly. The methods and the device can be used for assisting clinical diagnosis.
Owner:深圳华大因源医药科技有限公司
Method for screening specific circular RNA (ribonucleic acid) of triple-negative breast cancer
The invention discloses a method for screening specific circular RNA (ribonucleic acid) of triple-negative breast cancer. The method includes comparing circular gene expression of triple-negative breast cancer plasma samples and normal comparison groups by the aid of 70 parts of specific circular RNA of breast cancer; screening the specific circular RNA to obtain the circular RNA with functions of specifically obviously expressing the triple-negative breast cancer. The specific circular RNA is used as primers. The method has the advantage that results obtained by the aid of the method have important significance on triple-negative breast cancer diagnosis and treatment.
Owner:重庆鼎晶医学检验所有限公司
Application of inductively coupled plasma mass spectrometry in drug testing of hemin
The invention relates to an application of inductively coupled plasma mass spectrometry in drug testing of hemin. A method for measuring concentration of stable isotope iron in animal plasma is carried out by an ICP-MS (Inductively Coupled Plasma Mass Spectrometry) method and comprises the following steps of: providing an inductively coupled plasma source mass spectrometer; determining operating conditions of the ICP-MS; providing stable isotope iron powder, marked hemin, internal standard elements and the like; preparing standard solution and internal standard solution; preparing ICP-MS diluent; establishing a standard curve; measuring the stable isotope iron concentration in the plasma; and adding the ICP-MS diluent into a sample tube containing animal plasma sample containing iron, mixing uniformly and putting the sample tube into a refrigerator to be refrigerated, and determining the concentration of the iron in the plasma by the obtained standard curve. The application of the inductively coupled plasma mass spectrometry in the drug testing of the hemin has well performances, e.g., the method has well quantification lower limit, accuracy, absolute recovery test, sample stability, medium effect and quality control requirement, and can be taken as a content measurement method for analyzing the content of the stable isotope iron in a biological sample such as the plasma.
Owner:新疆科丽生物技术有限公司
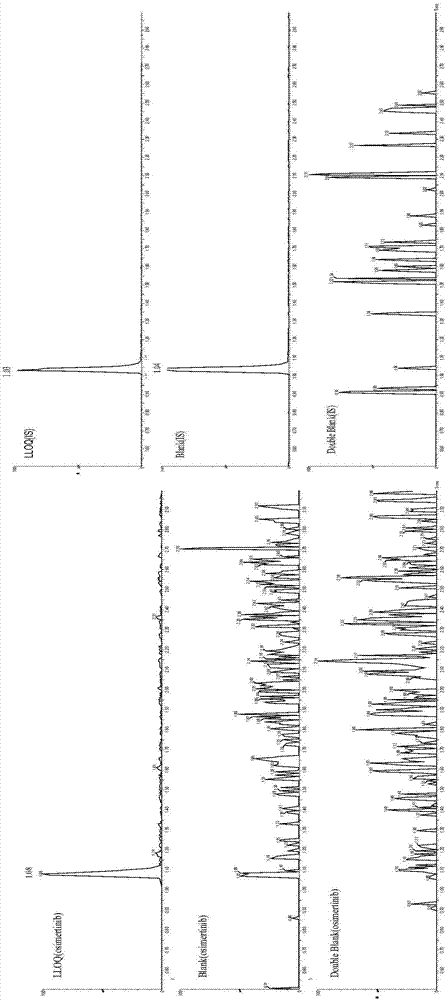
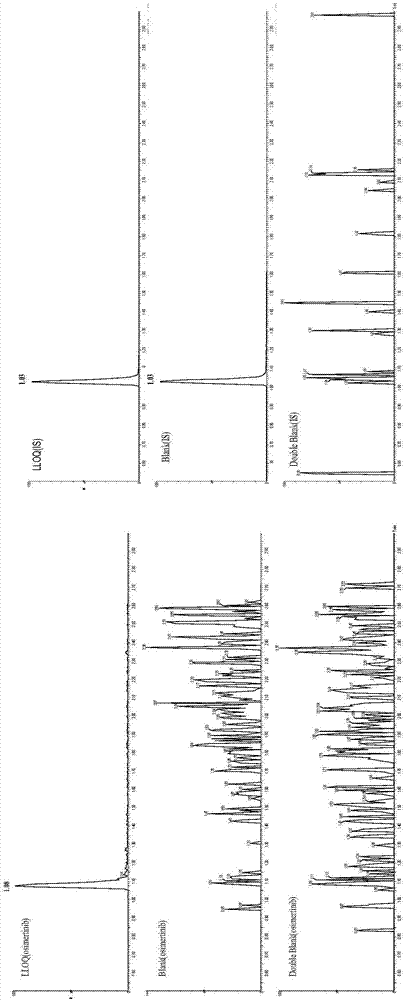
Detection of drug concentration of osimertinib in human plasma and/or cerebrospinal fluid by combining UPLC-MS/MS
The invention relates to detection of the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid by combining UPLC-MS / MS. According to the present invention, the method for determining the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid through an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS / MS) is provided, wherein formic acid-containing ammonium formate water-formic acid acetonitrile is used as a mobile phase so as to perform the method; and the method has advantages of good specificity and high sensitivity, and is used for the detection of clinical pharmacokinetic samples, wherein the linearity is good when the osimertinib concentration is 2-500 ng.ml<-1> in the plasma sample and the osimertinib concentration is 0.5-20 ng.ml<-1> in the cerebrospinal fluid sample.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Quick test kit of schistosomiasis and preparation method thereof
The invention discloses a quick test kit of schistosomiasis and a preparation method thereof. A water absorbing pad, a pyroxylin film, an aerosol bonding pad and a sample pad are and sequentially connected in horizontal direction and fixed on a baseplate which is arranged in a kit body, and the kit body is provided with an observation window corresponding to the pyroxylin film and a sample loading hole corresponding to the sample pad. Only an one-step operation is needed when the quick test kit is used, and the quick test kit not only can detect serum and plasma samples, but also can detect awhole blood sample, thereby avoiding the step of obtaining the serum sample which can be obtained only through a centrifugation step from a blood sample, and greatly improving the screening speed. The quick test kit can also detect whether animals such as cows, horses, pigs, rabbits, and the like have the schistosomiasis or not so as to overcome the defect that different kits are needed to be prepared for detecting different species of other kits.
Owner:SICHUAN MACCURA BIOTECH CO LTD
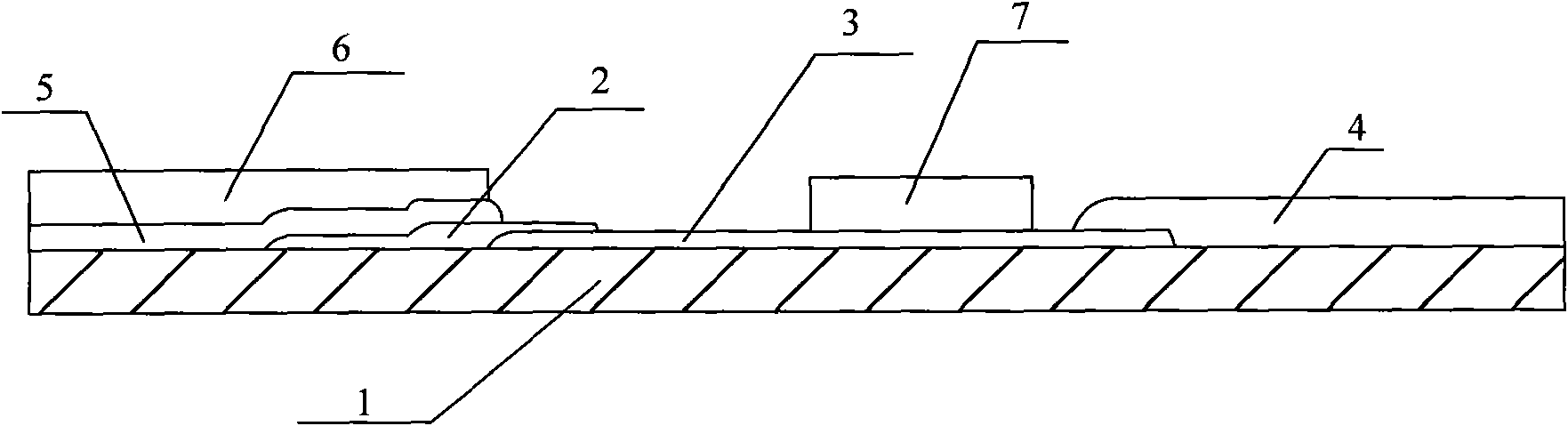
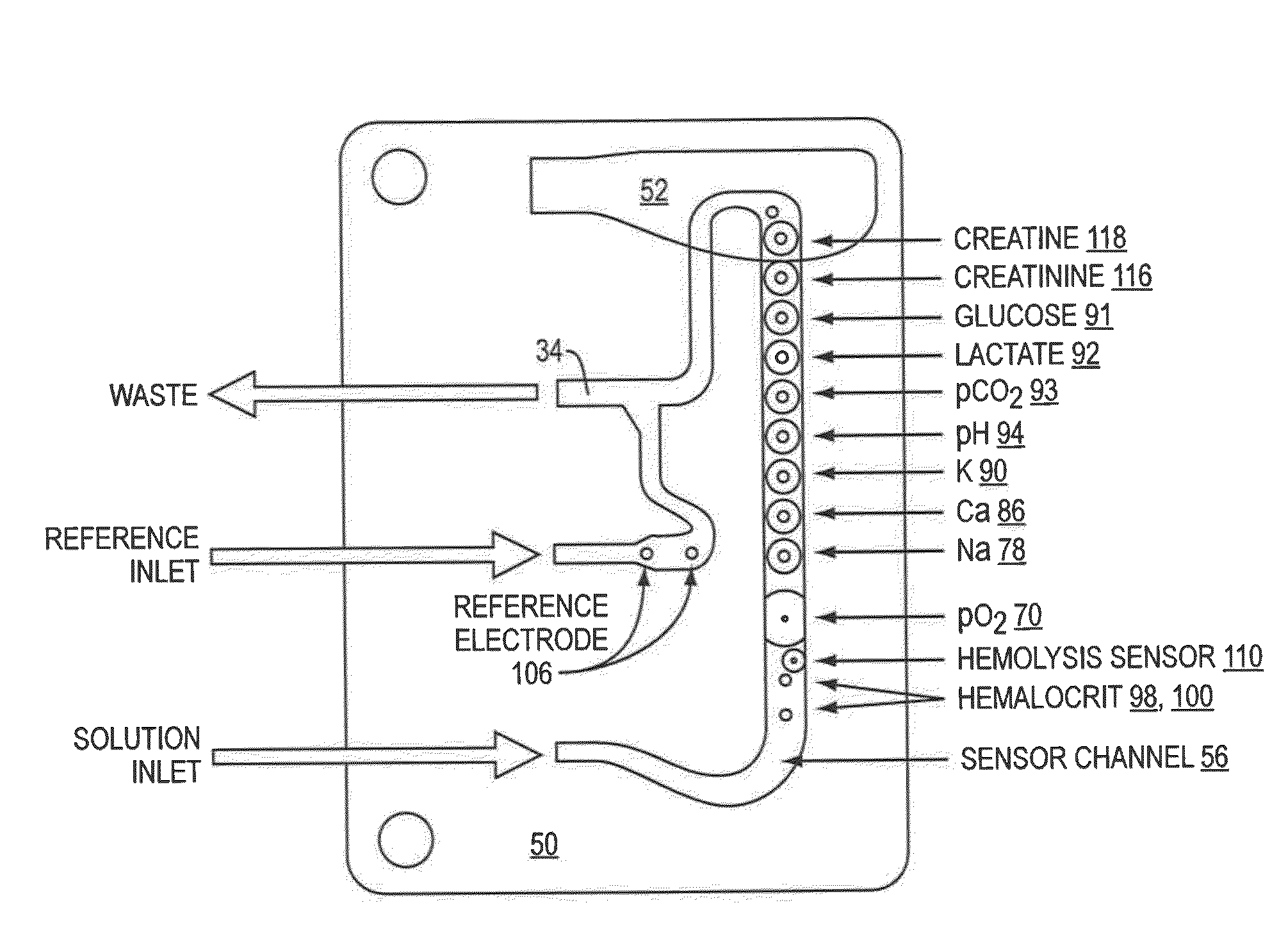
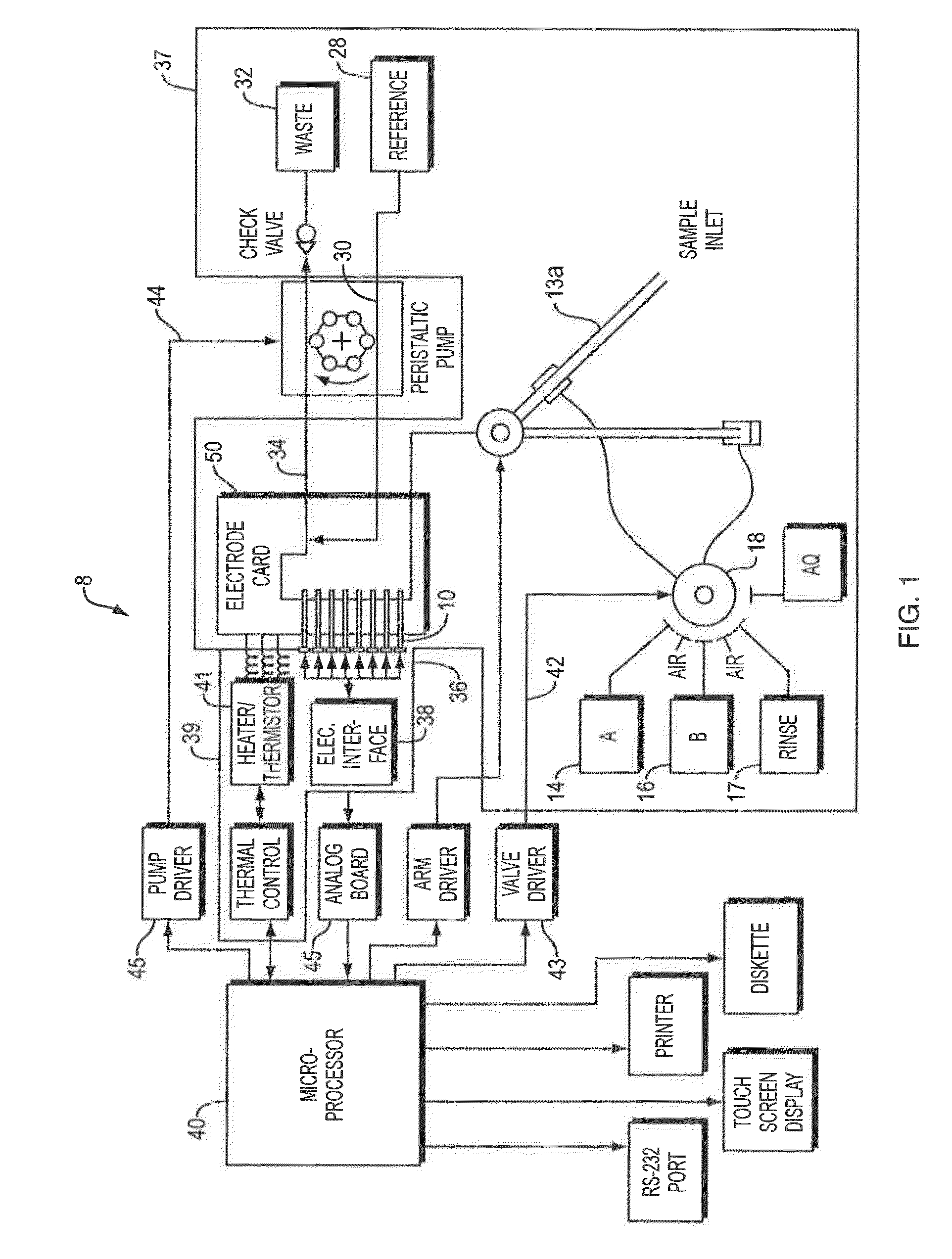
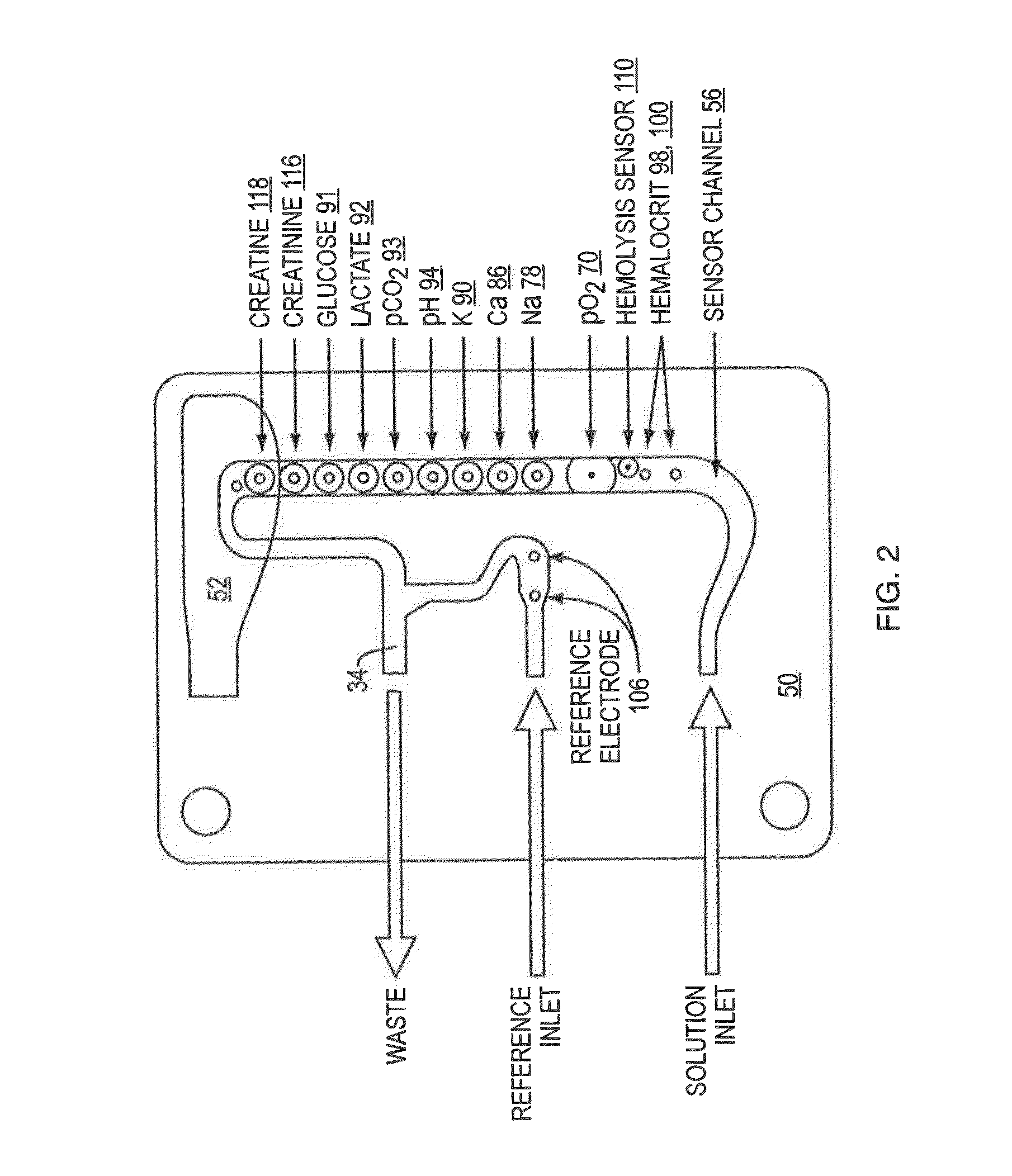
Whole blood hemolysis sensor
ActiveUS20140262831A1Enhancing effluxEnhance effluxImmobilised enzymesBioreactor/fermenter combinationsExtracellularHemolysis
The present invention pertains to a hemolysis sensor, a hemolysis sensor system and methods of utilizing the hemolysis sensor or hemolysis sensor system to monitor or detect hemolysis in a sample, such as a whole blood sample, a plasma sample, a serum sample or hemolyzed blood. The hemolysis sensor responds to extracellular hemoglobin levels, for example, extracellular hemoglobin in a whole blood sample as a method for detecting hemolysis in whole blood.
Owner:INSTR LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com