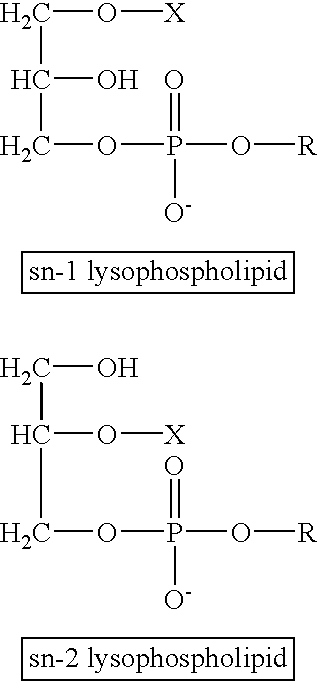Patents
Literature
78 results about "Eclamptic seizure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
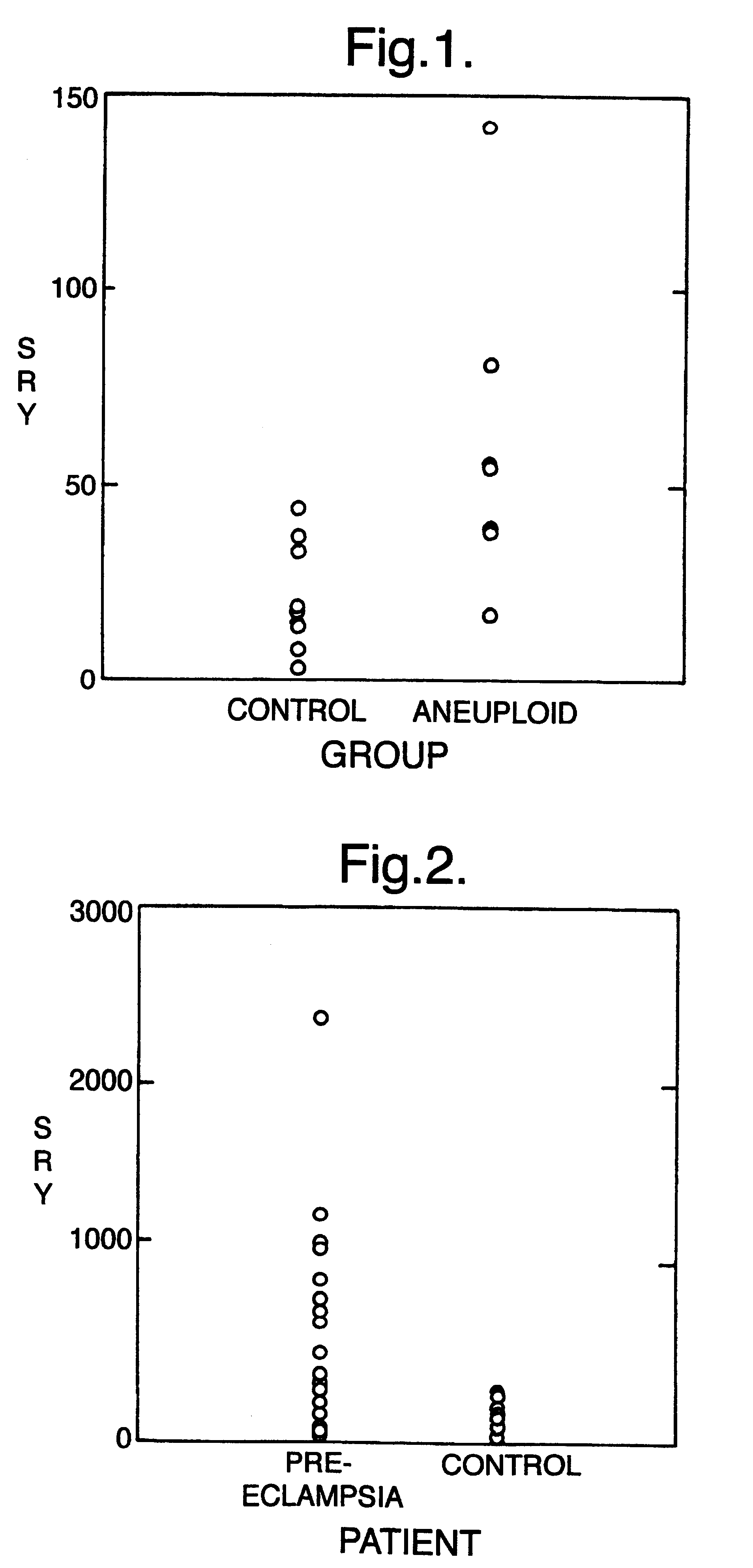
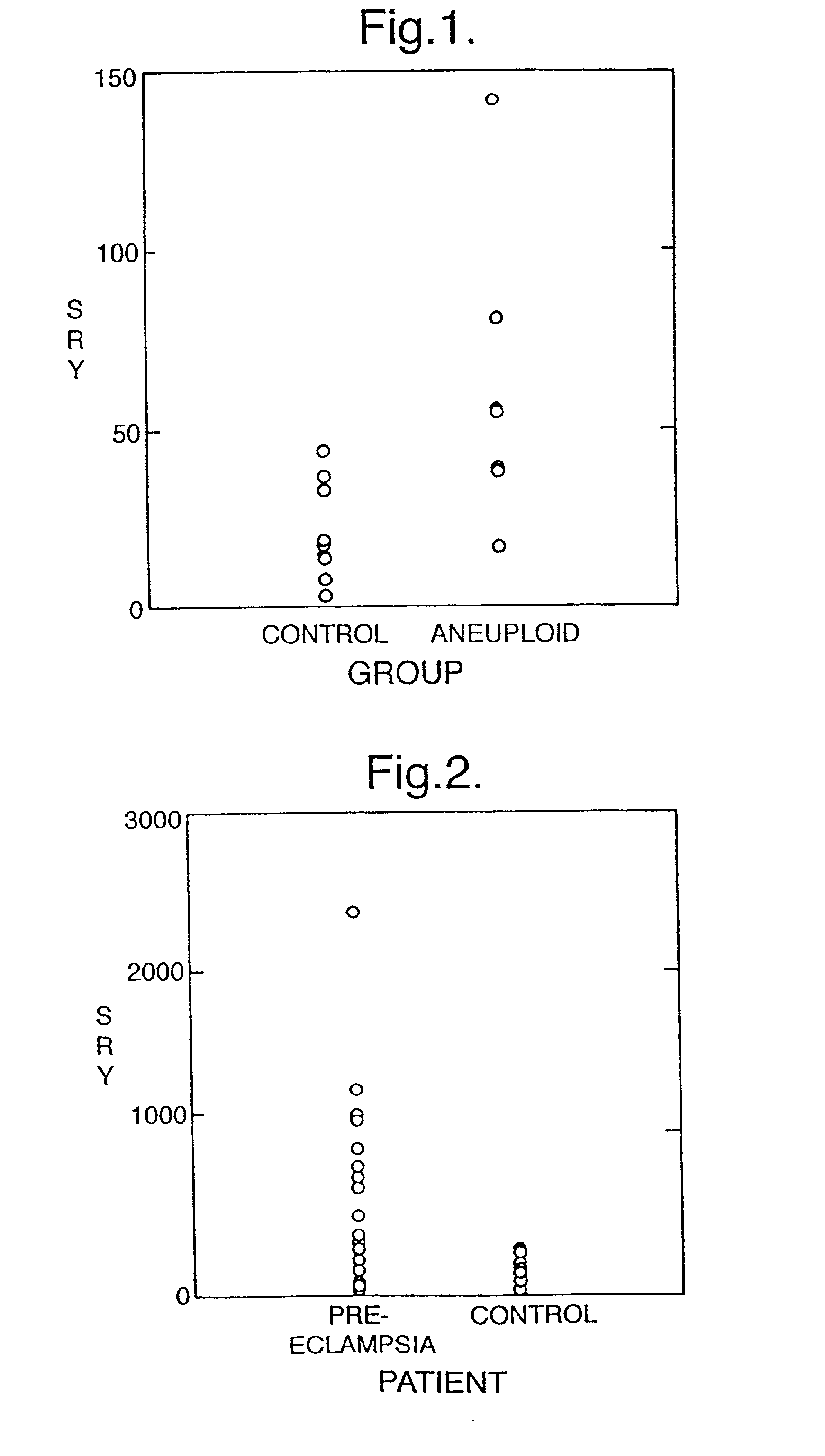
Eclampsia is the onset of seizures (convulsions) in a woman with pre-eclampsia. Pre-eclampsia is a disorder of pregnancy in which there is high blood pressure and either large amounts of protein in the urine or other organ dysfunction. Onset may be before, during, or after delivery.
Non-invasive prenatal diagnosis
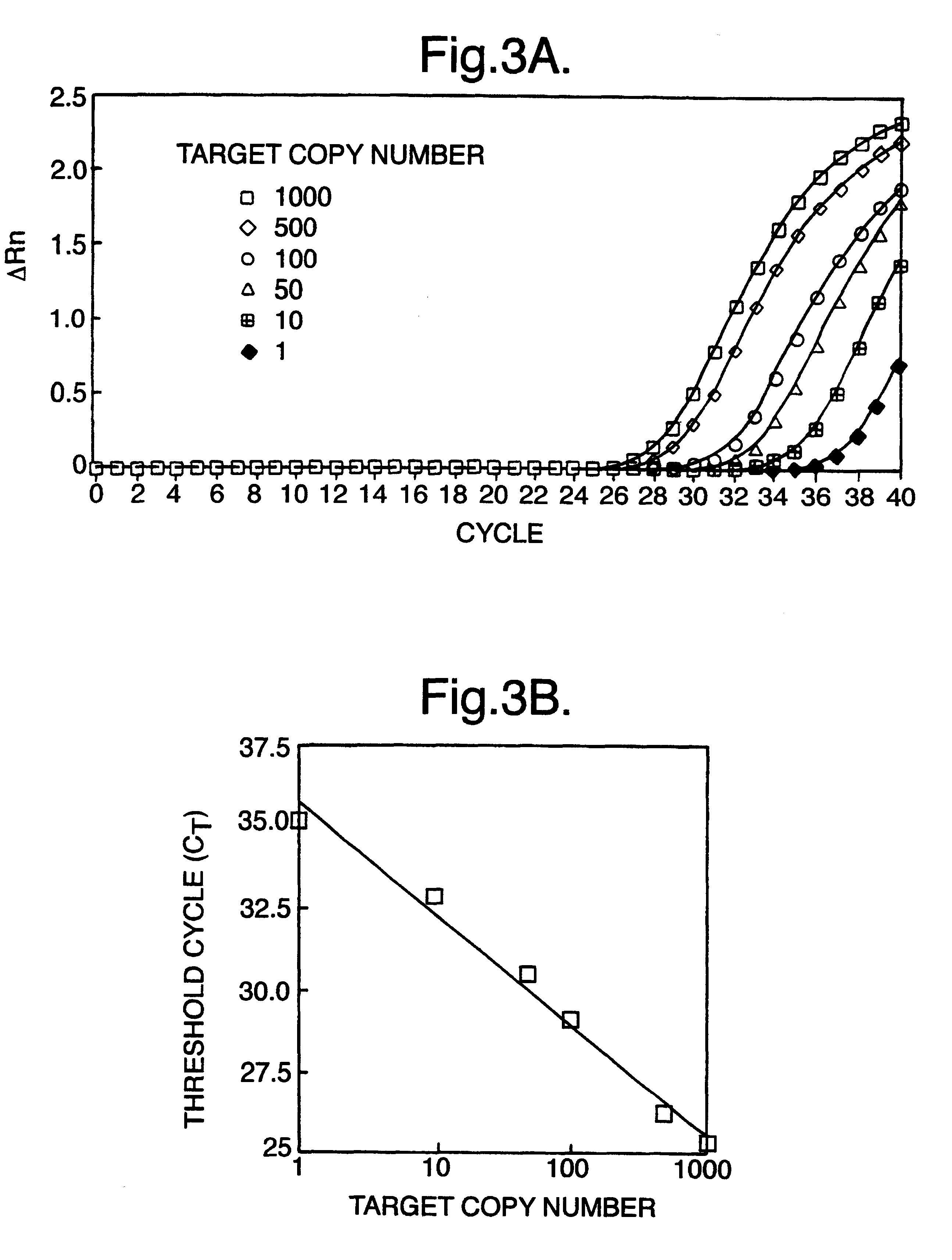
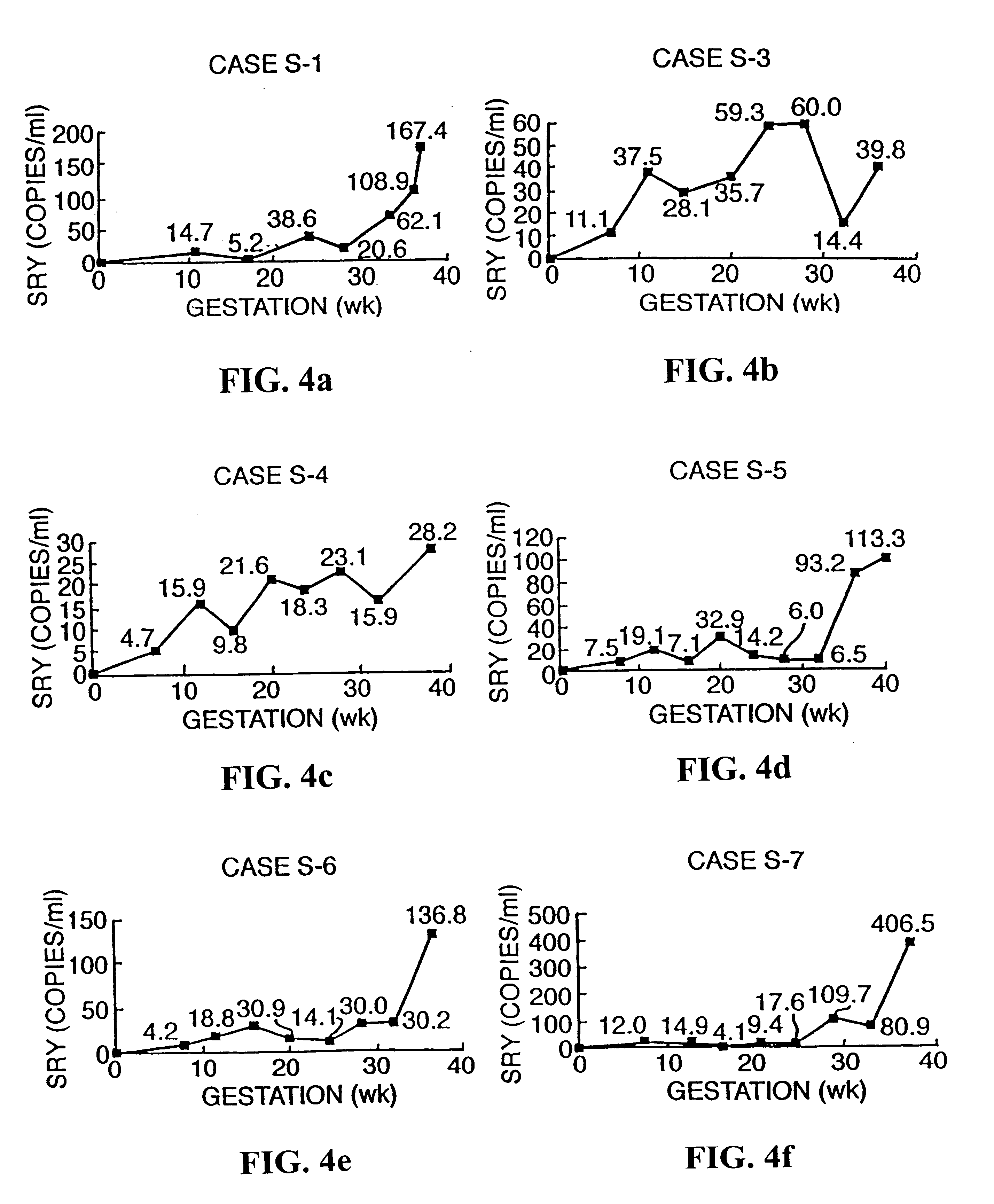
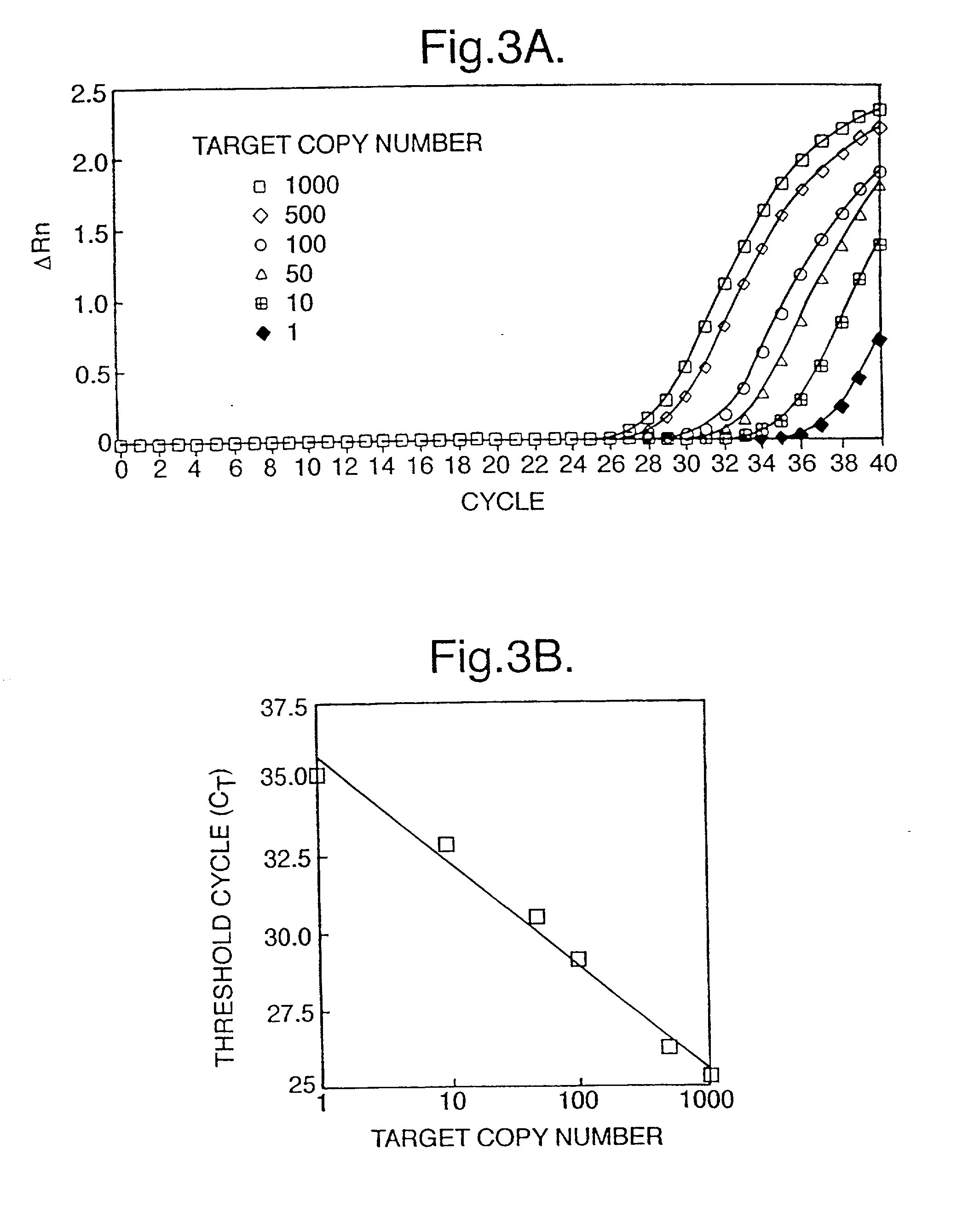
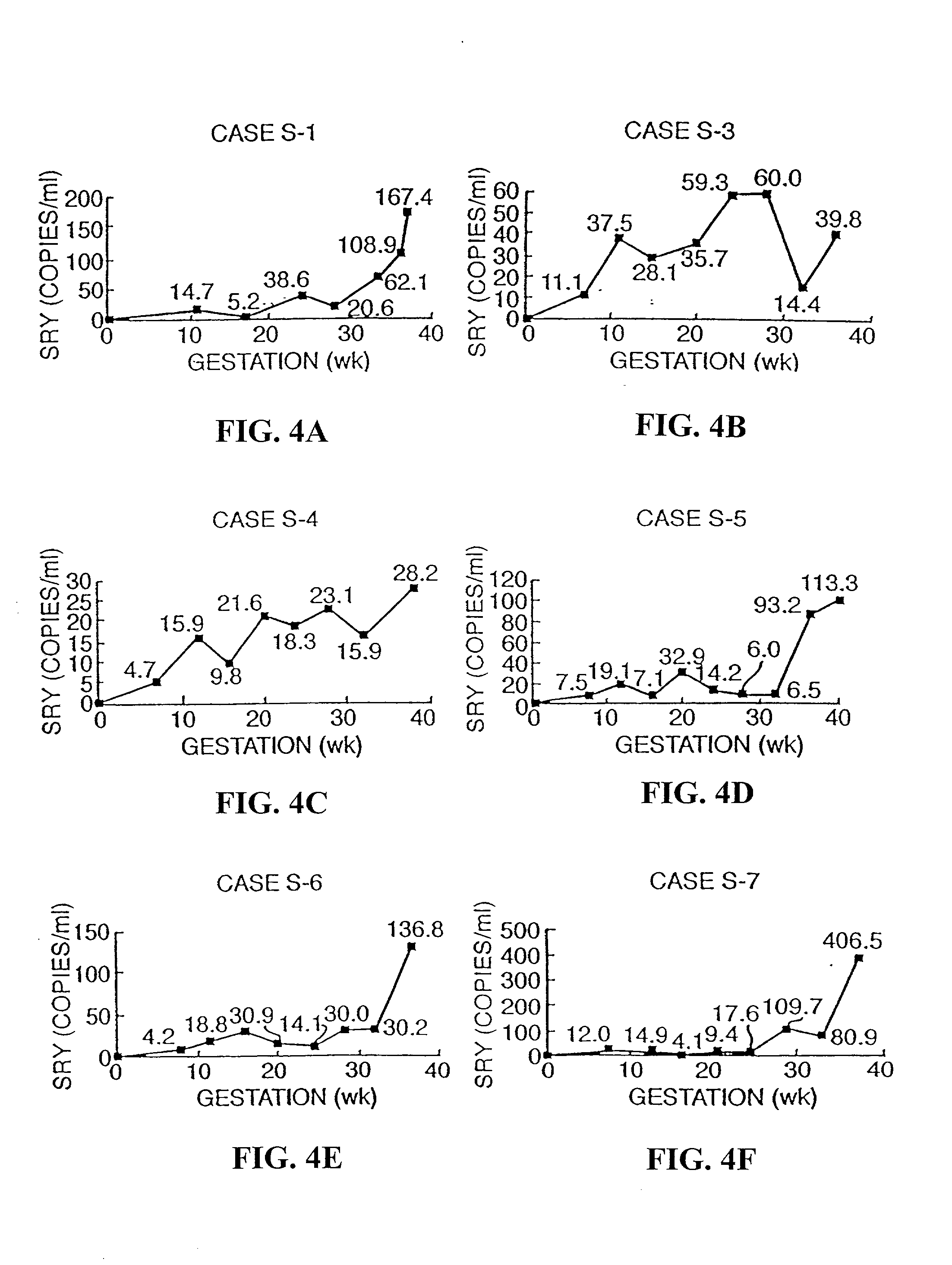
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
Non-invasive prenatal diagnosis
InactiveUS20010051341A1Improve accuracy100% accurate detection rateMicrobiological testing/measurementFermentationPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma from a pregnant female, which method comprises the presence of a nucleic acid of fetal origin in the sample. The invention enables non-invasive prenatal diagnosis including, for example, sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:ISIS INNOVATION LTD
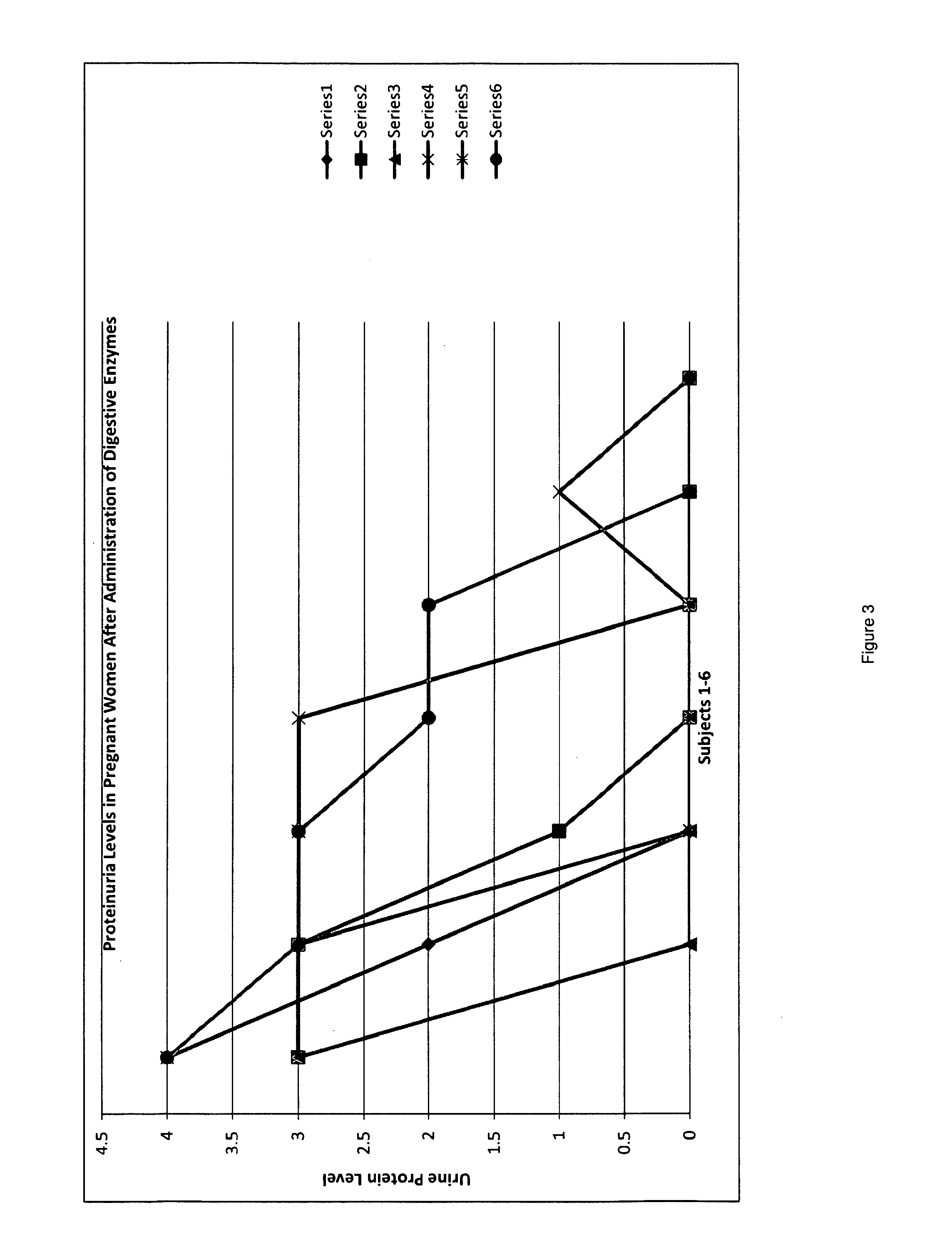
Novel pharmaceutical preparation for preeclampsia, eclampsia, and toxemia, and their related symptoms and related disorders of pregnancy
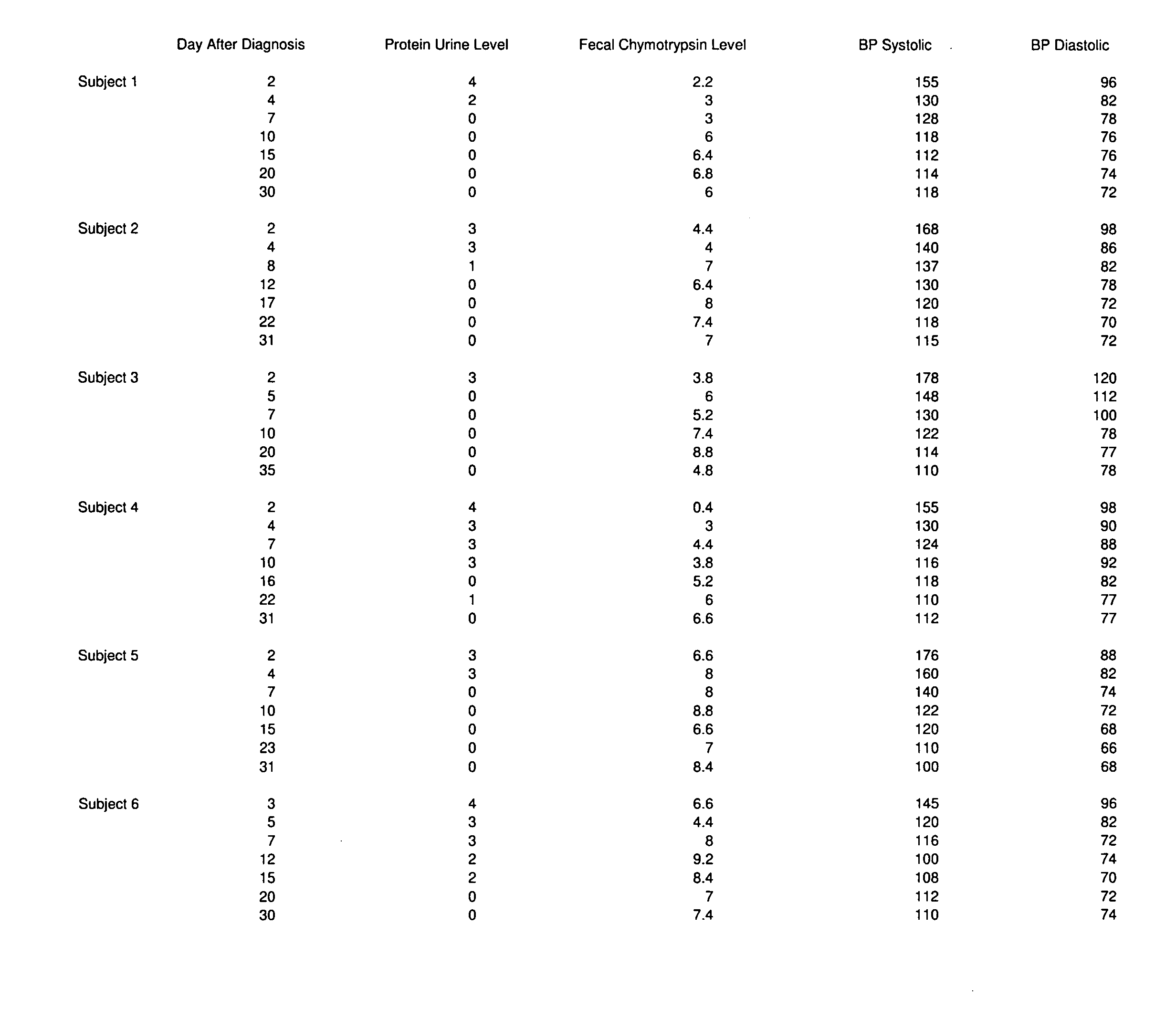
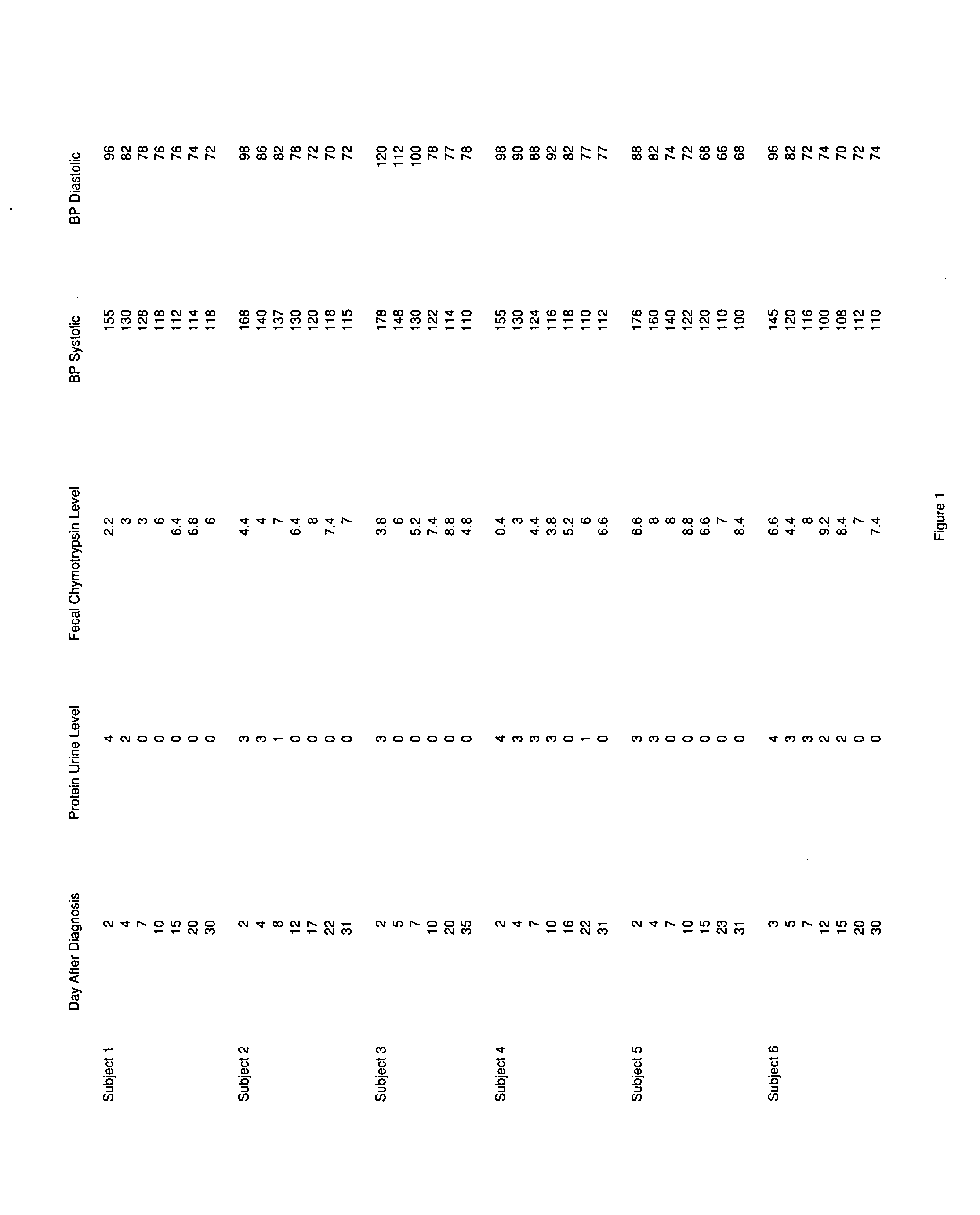
A therapeutic agent for the treatment of toxemia, preeclampsia and eclampsia and the method for preparing the therapeutic agents is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the maternal GI tract to determine the likelihood of developing preeclampsia, pregnancy induced hypertension, and eclampsia / toxemia is disclosed.
Owner:CUREMARK
Methods of diagnosing and treating complications of pregnancy
InactiveUS20060067937A1Decrease elevated levelLevelOrganic active ingredientsBiocideDiseaseObstetrics
Disclosed herein are methods for diagnosing a pregnancy related hypertensive disorder or a predisposition to a pregnancy related hypertensive disorder by measuring the level or biological activity of soluble endoglin. Also disclosed herein are methods for treating a a pregnancy related hypertensive disorder, such as pre-eclampsia and eclampsia, using compounds that alter soluble endoglin levels or biological activity.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Methods of diagnosing and treating pre-eclampsia or eclampsia
InactiveUS20050170444A1Lower Level RequirementsGreat molecular weightCompound screeningApoptosis detectionObstetricsPlacental growth factor
Disclosed herein are methods for diagnosing pre-eclampsia and eclampsia or a propensity to develop pre-eclampsia or eclampsia by detecting the levels of placental growth factor in a subject.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Maternal serum biomarkers for detection of pre-eclampsia
InactiveUS20100016173A1Microbiological testing/measurementLibrary screeningProteomics methodsPlacental insufficiency
The present invention concerns the identification and detection of maternal serum biomarkers of pre-eclampsia and associated complications, gestational hypertension and placental insufficiency using global proteomic approaches. The invention further concerns the identification of maternal serum biomarkers for detection of pre-eclampsia and associated complications, gestational hypertension and placental insufficiency during early gestation.
Owner:HOLOGIC INC
Nitrosated and nitrosylated diuretic compounds, compositions and methods of use
The invention describes novel nitrosated and / or nitrosylated diuretic compounds or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitrosated and / or nitrosylated diuretic compound, and, optionally, at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides novel compositions and kits comprising at least one diuretic compound of the invention, that is optionally nitrosated and / or nitrosylated, and, optionally, at least one nitric oxide donor compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating conditions resulting from excessive water and / or electrolyte retention; (b) treating cardiovascular diseases; (c) treating renovascular diseases; (d) treating diabetes; (e) treating diseases resulting from oxidative stress; (f) treating endothelial dysfunctions; (g) treating diseases caused by endothelial dysfunctions; (h) treating cirrhosis; (j) treating pre-eclampsia; (k) treating osteoporosis; and (l) treating nephropathy.
Owner:NICOX SA
Methods of treating pre-eclampsia or eclampsia
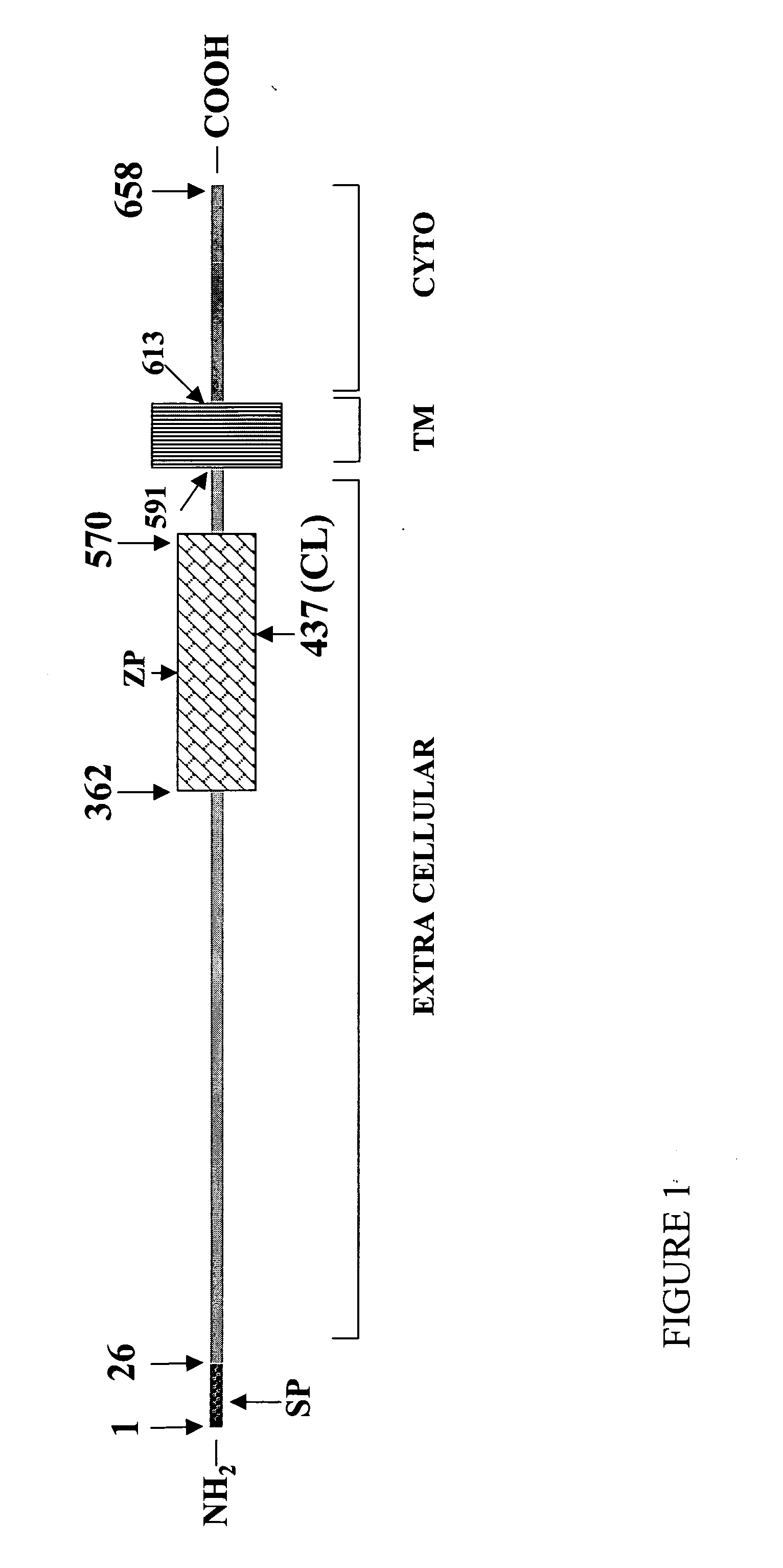
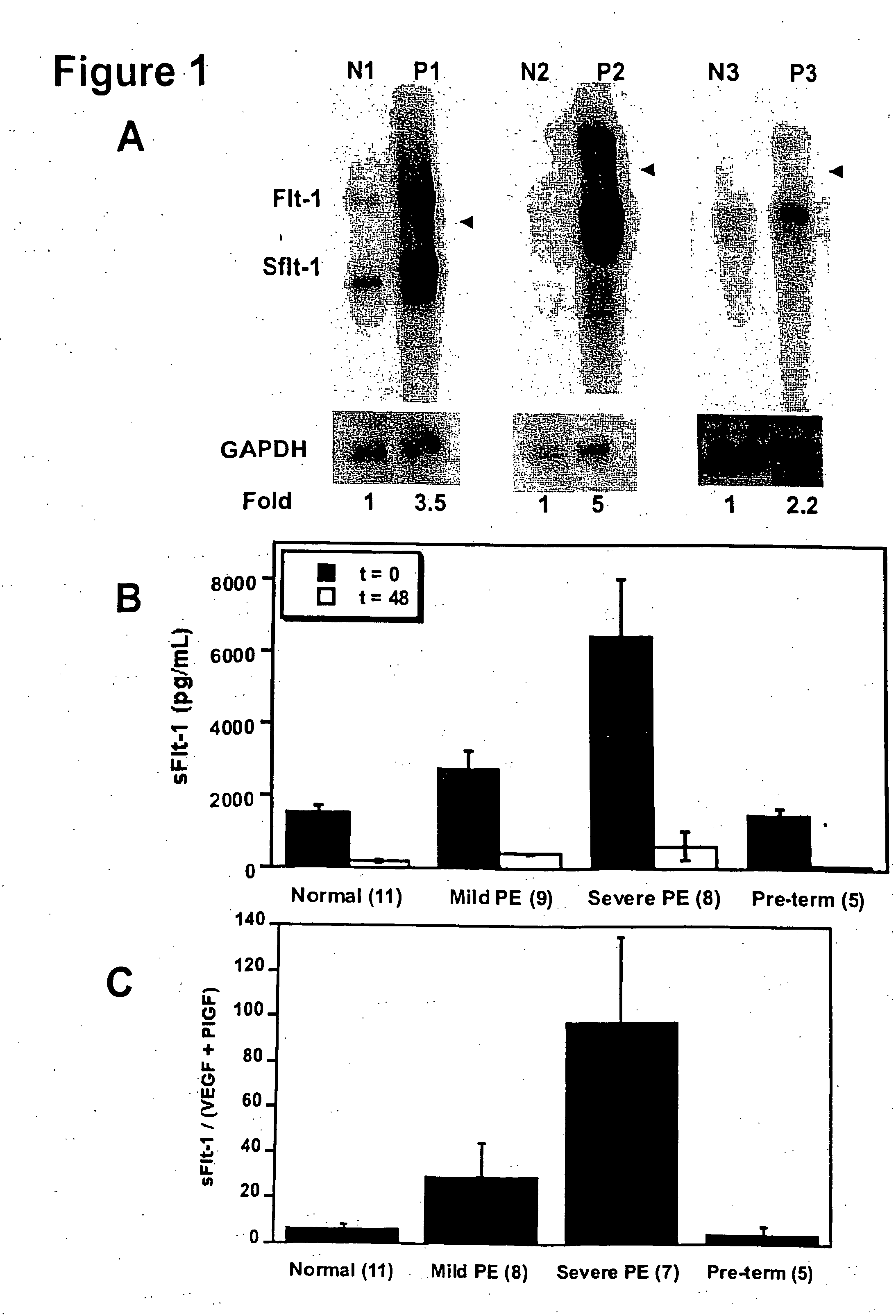
InactiveUS7335362B2Treating or preventing pre-eclampsia or eclampsia in a subjectImprove the level ofOrganic active ingredientsPeptide/protein ingredientsObstetricsEclamptic seizure
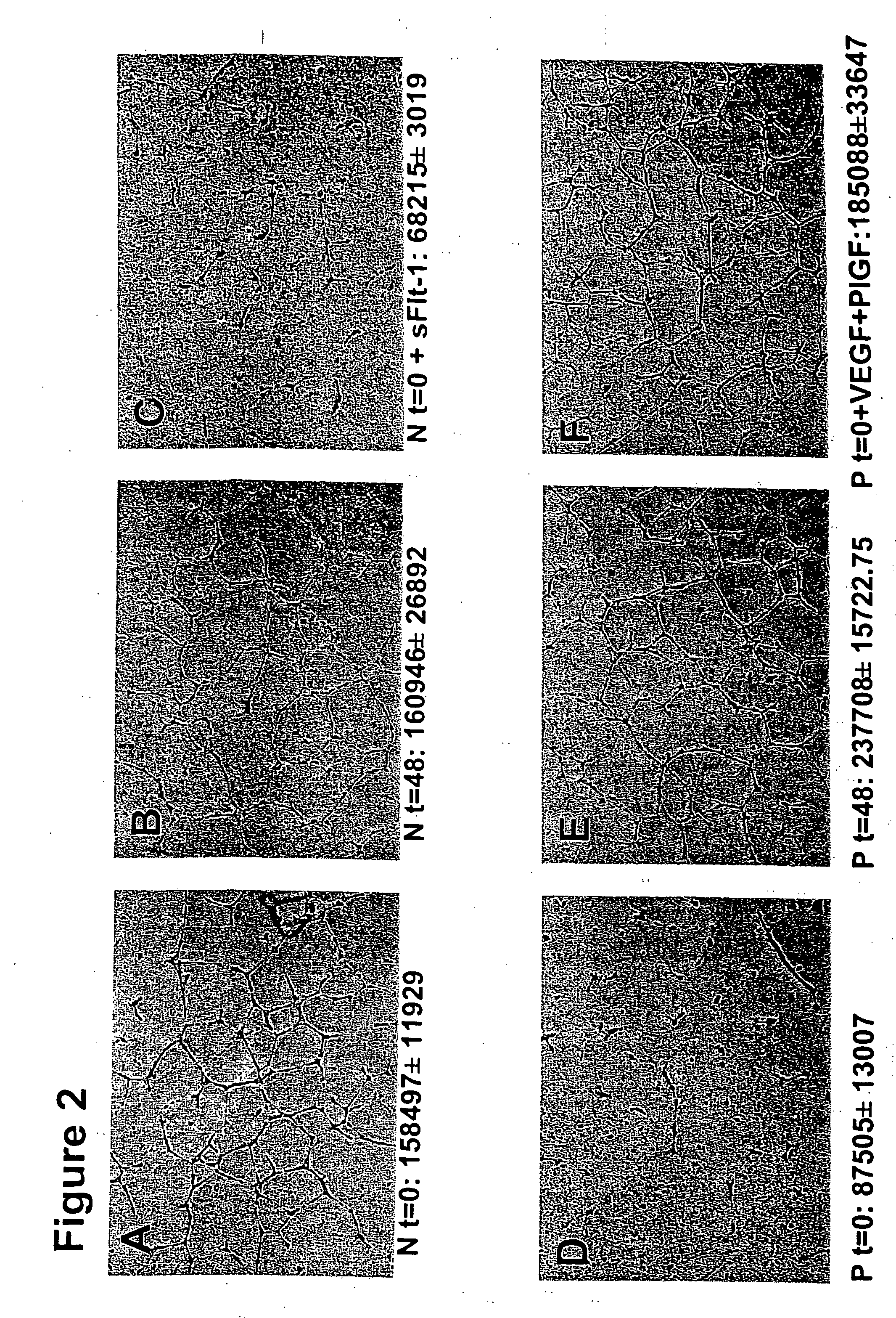
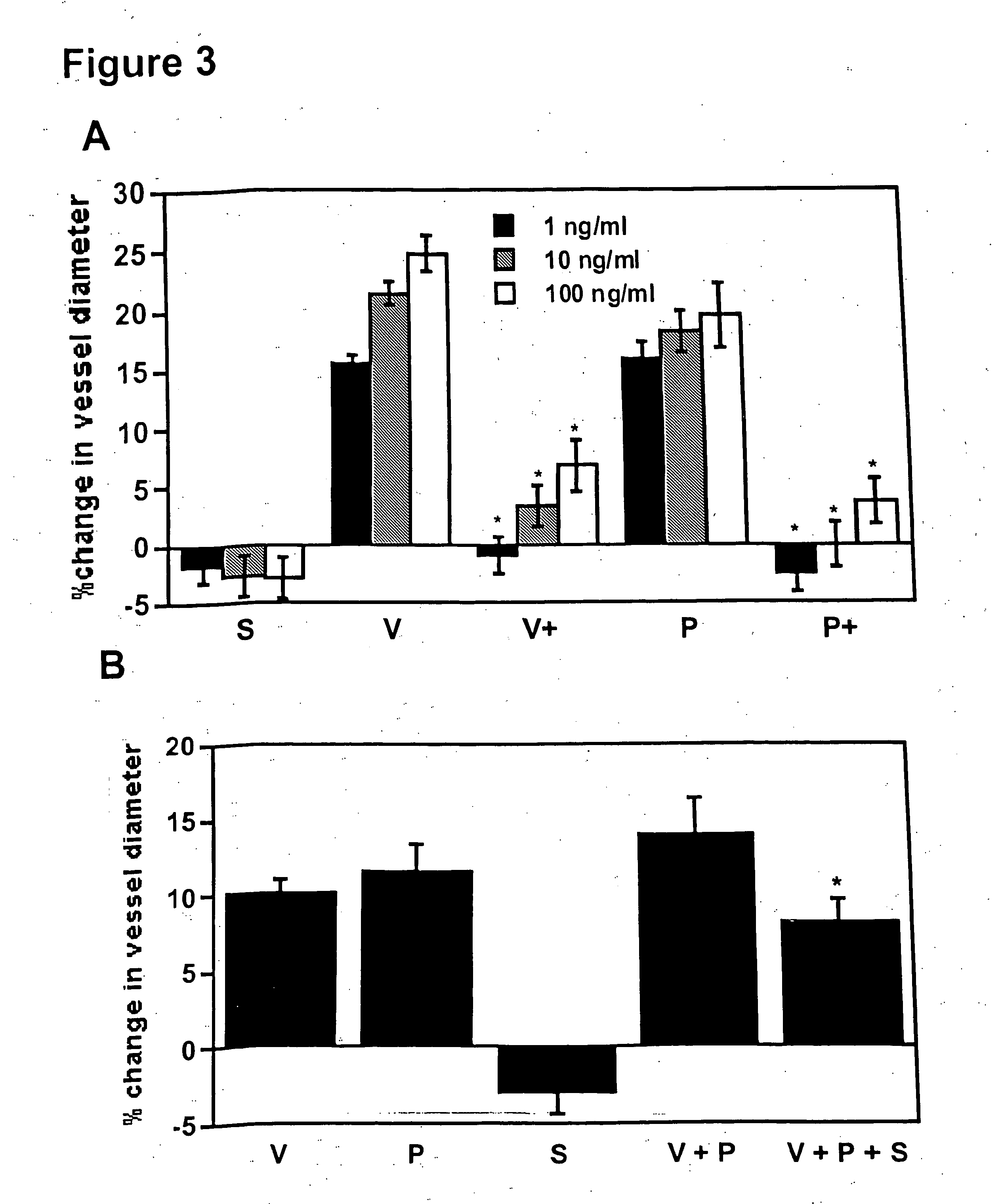
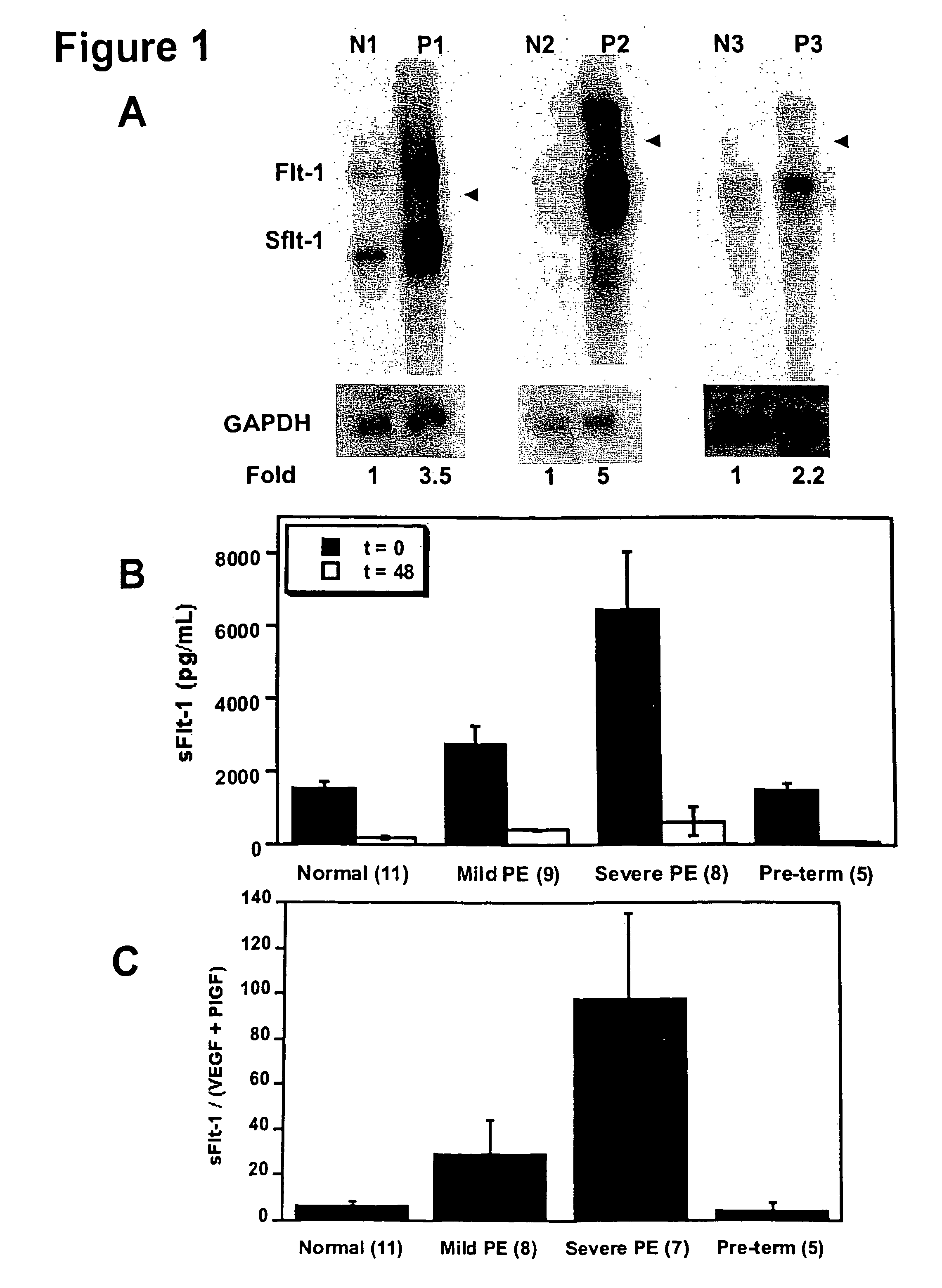
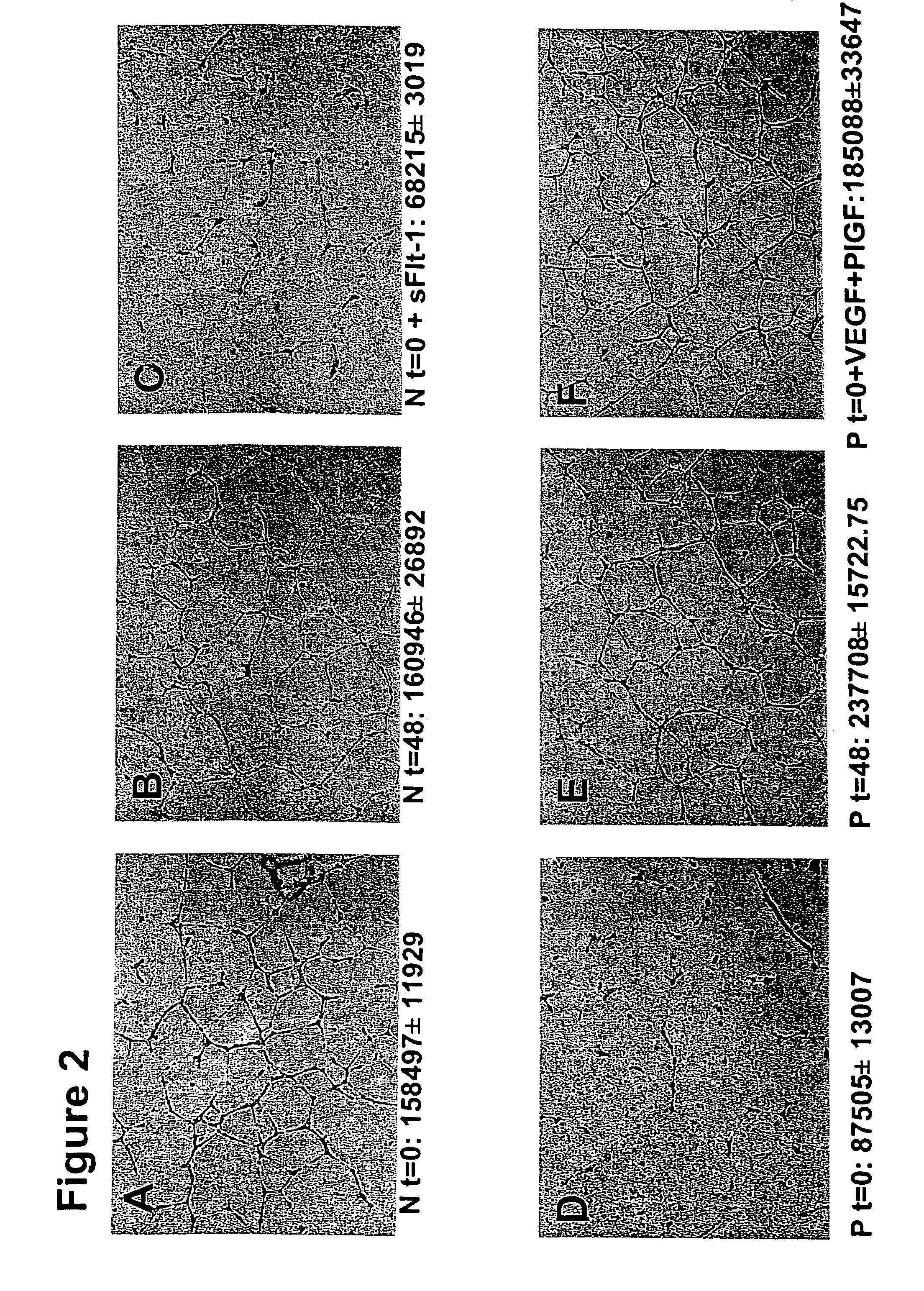
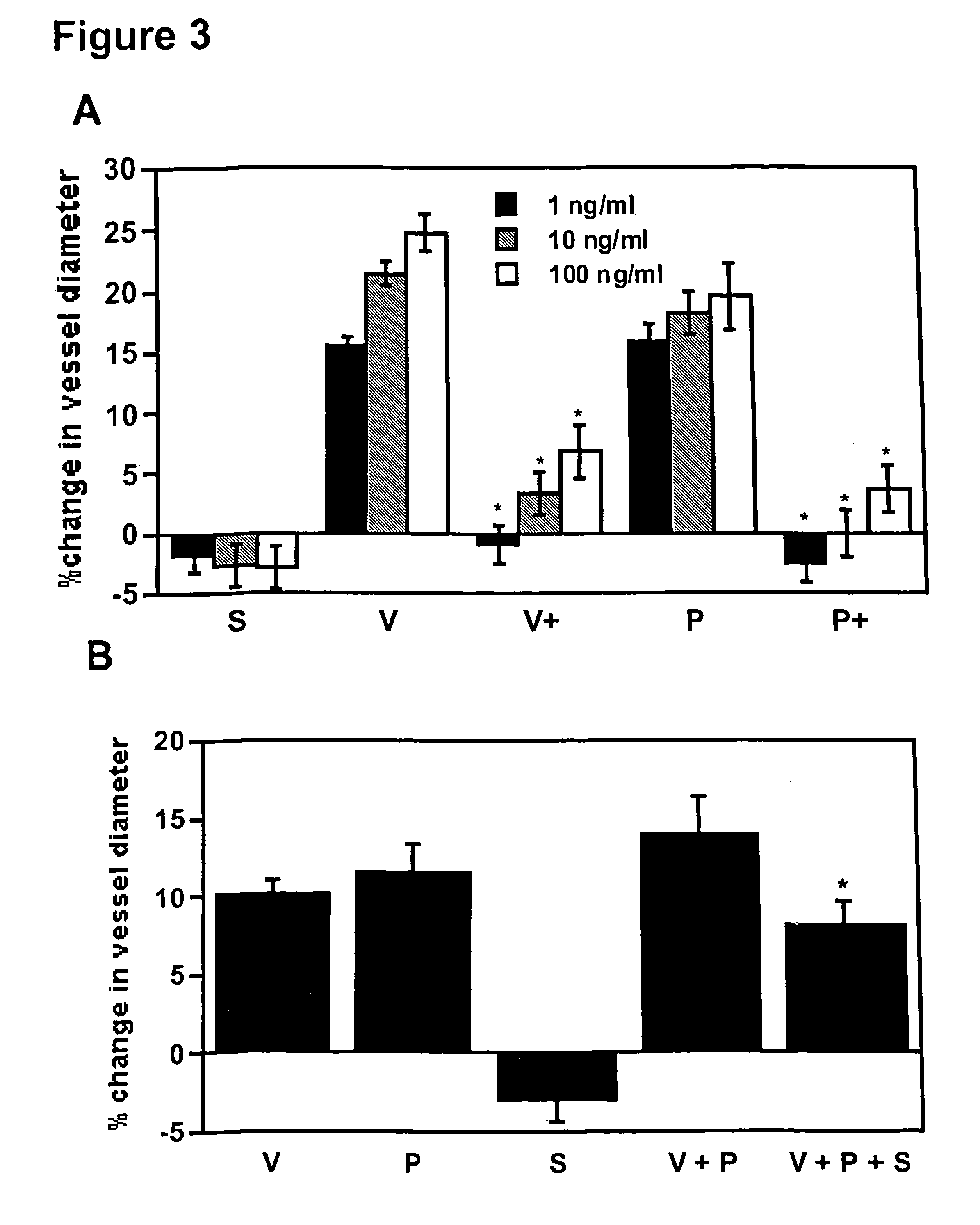
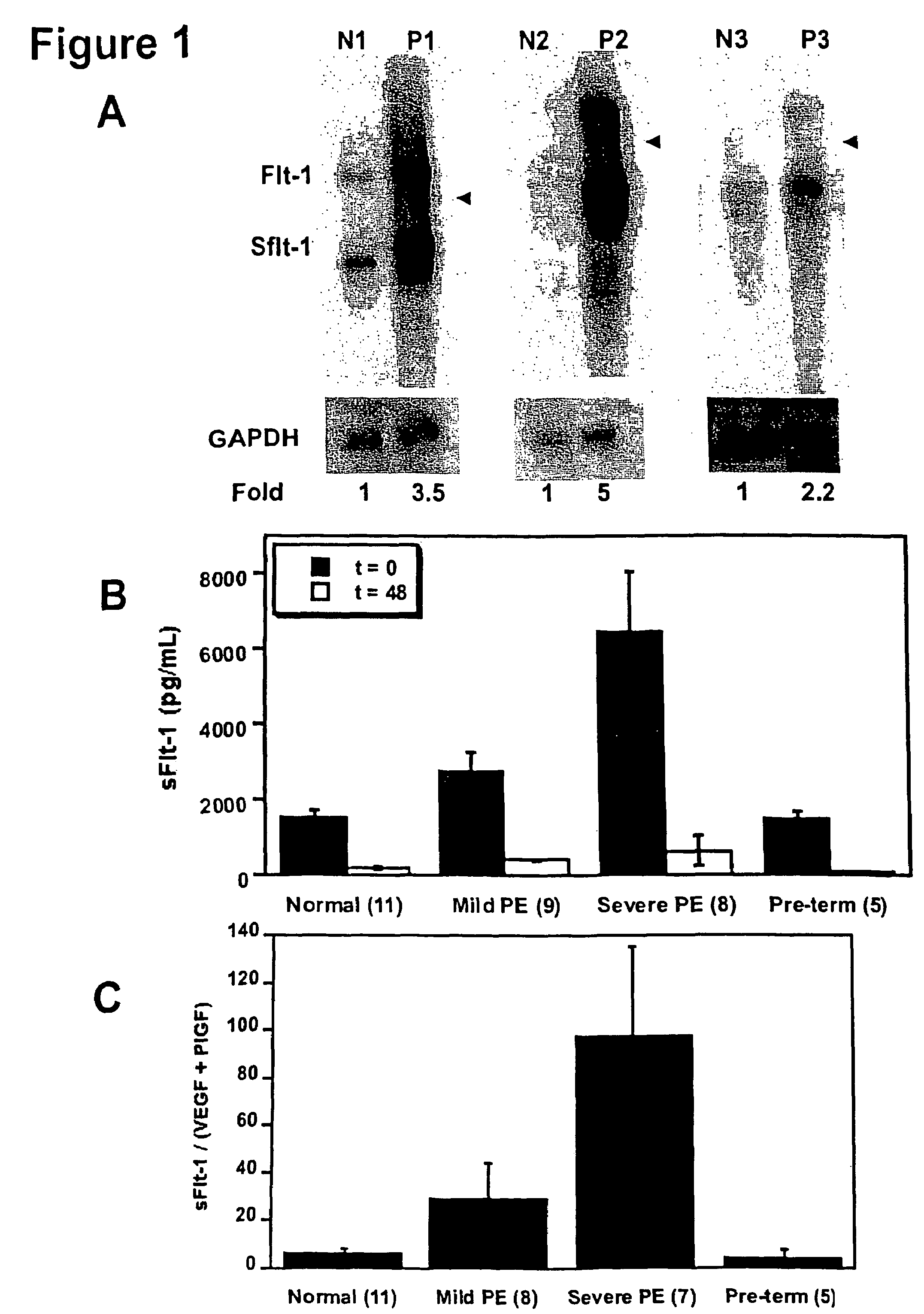
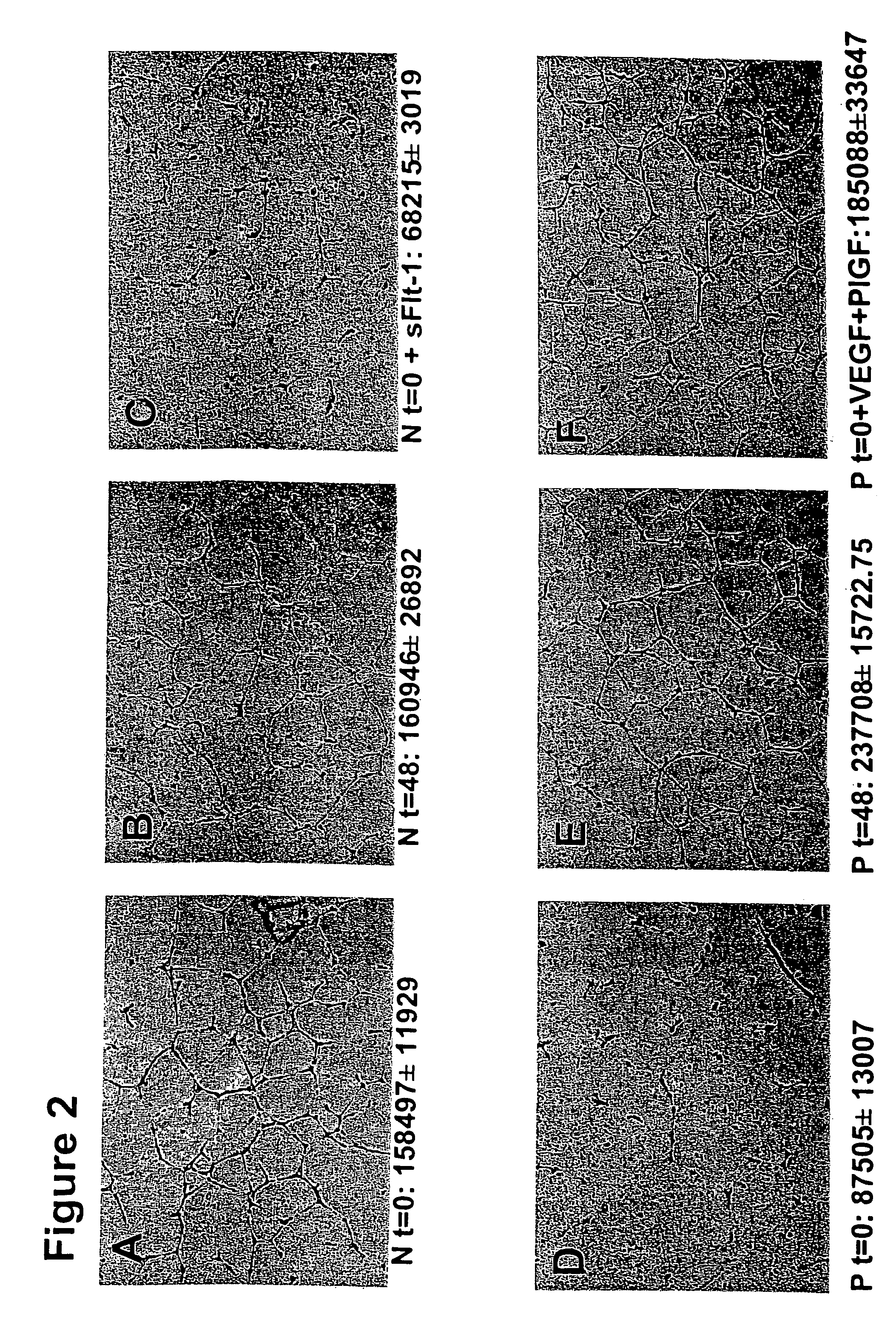
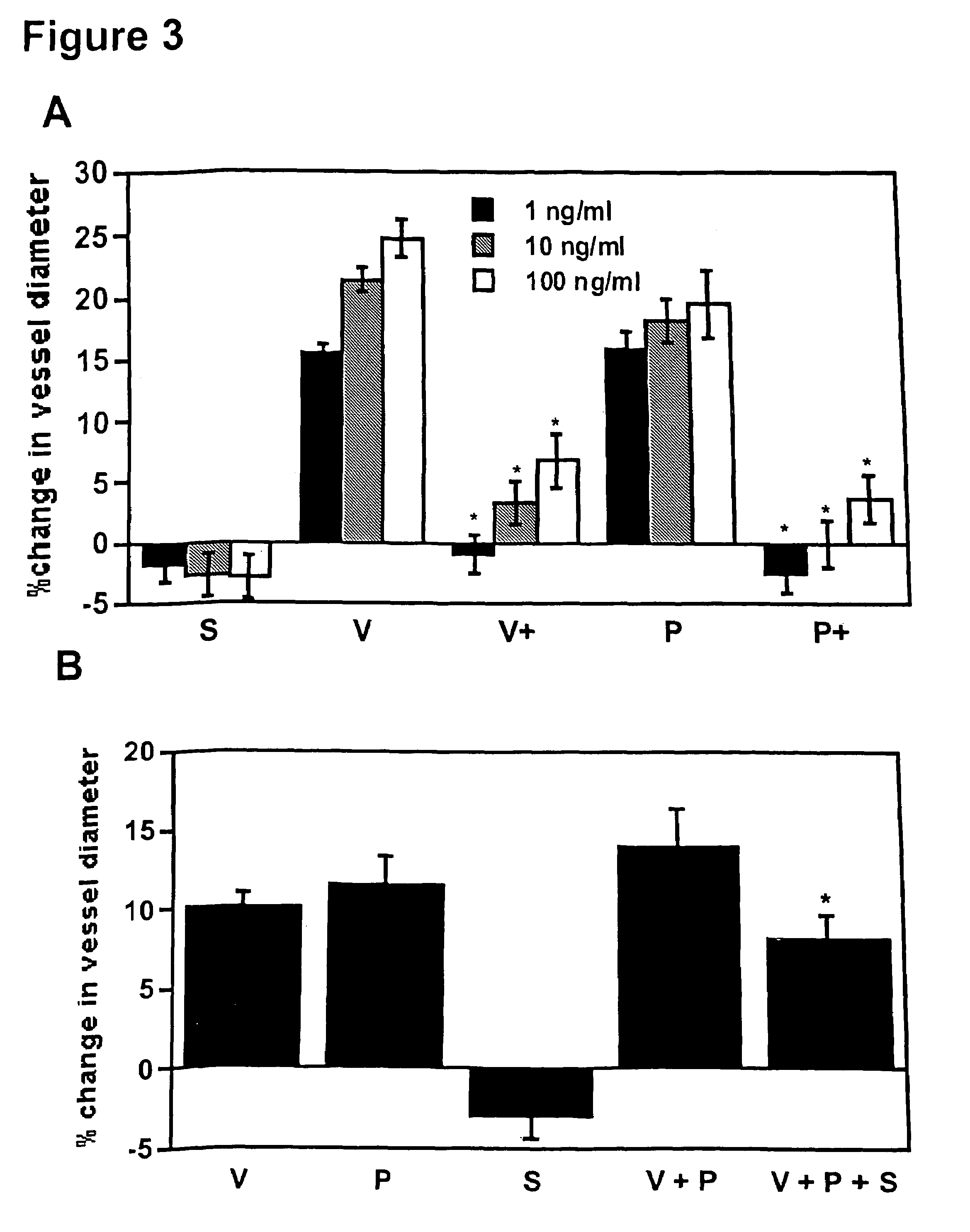
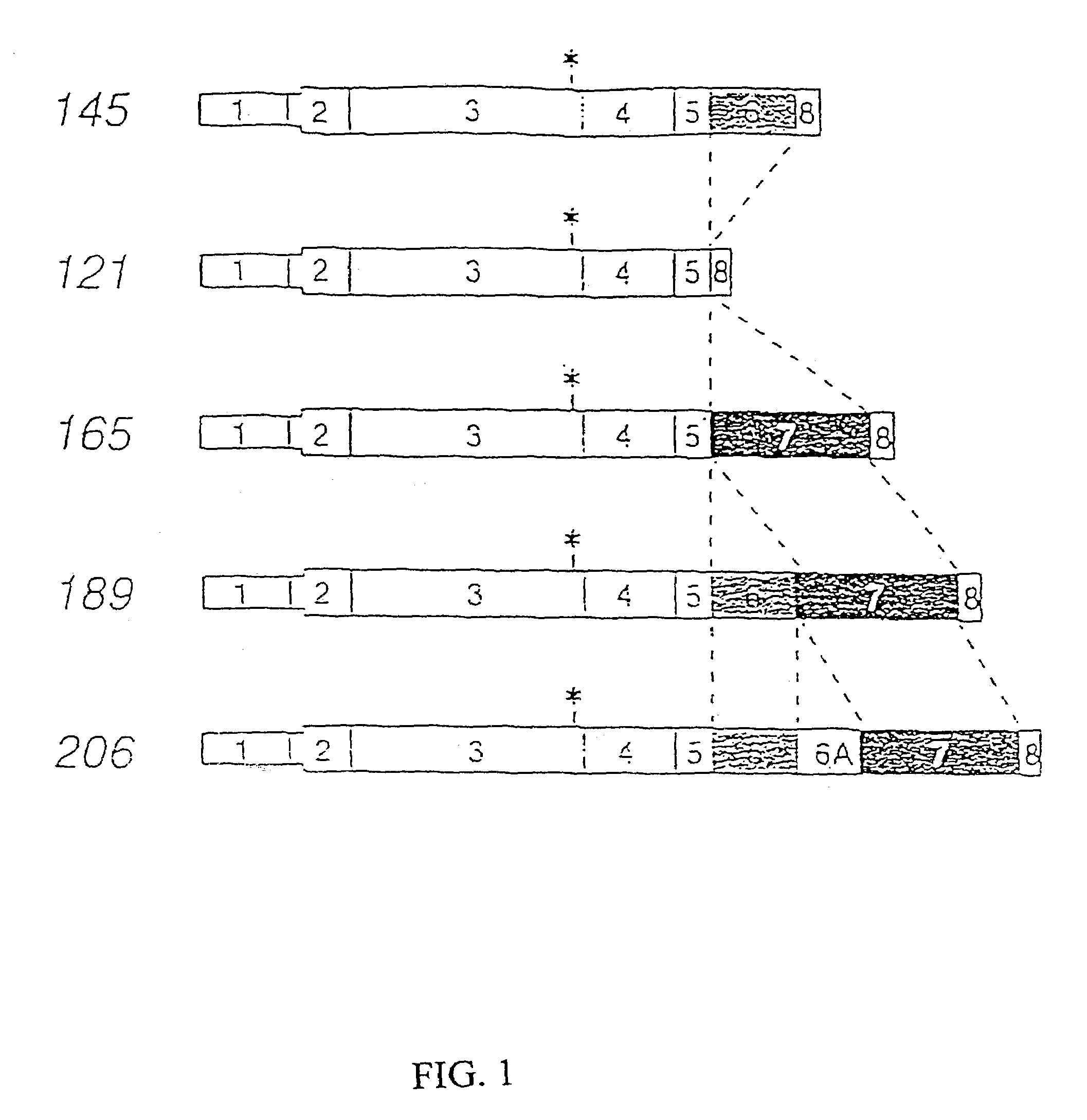
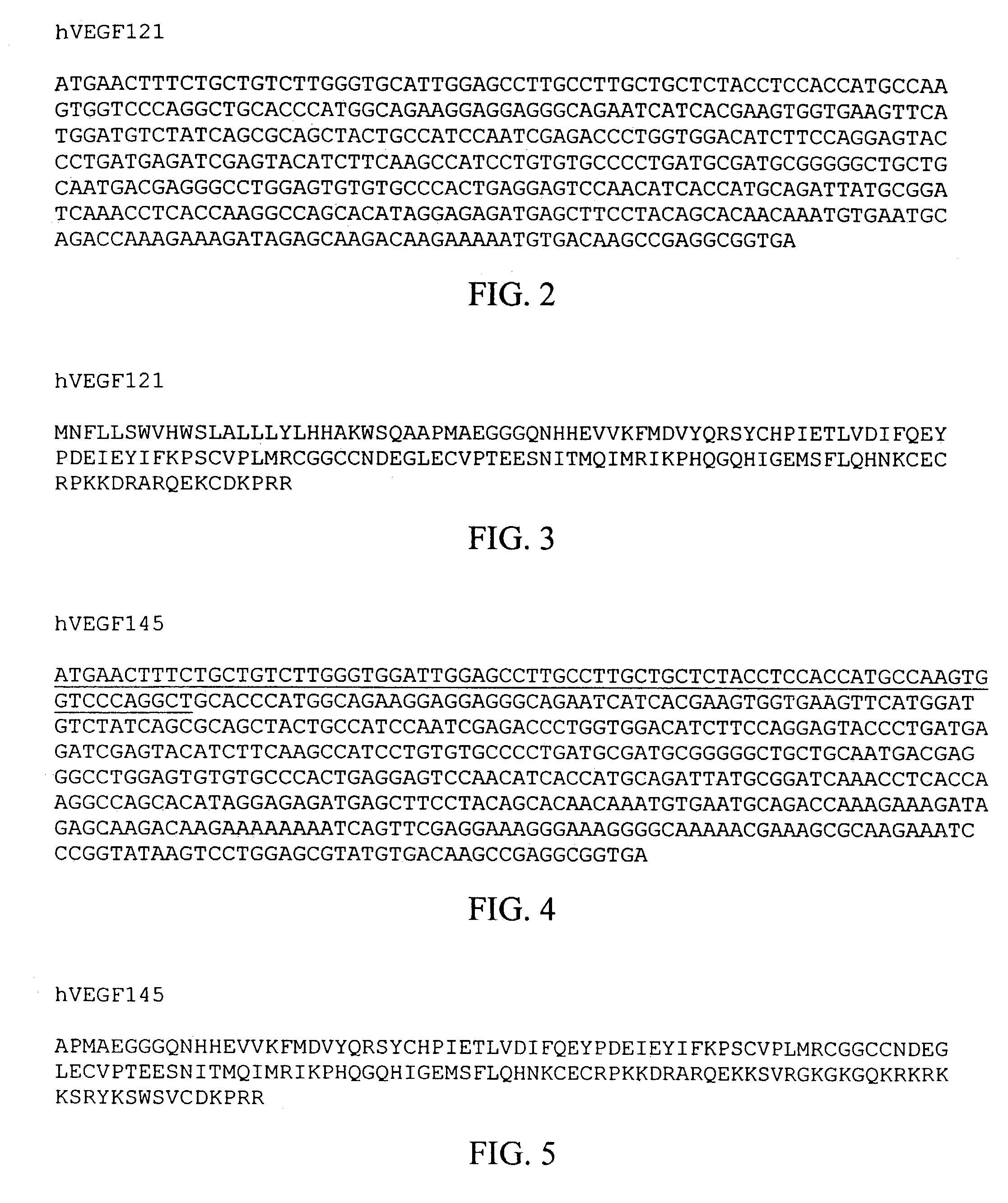
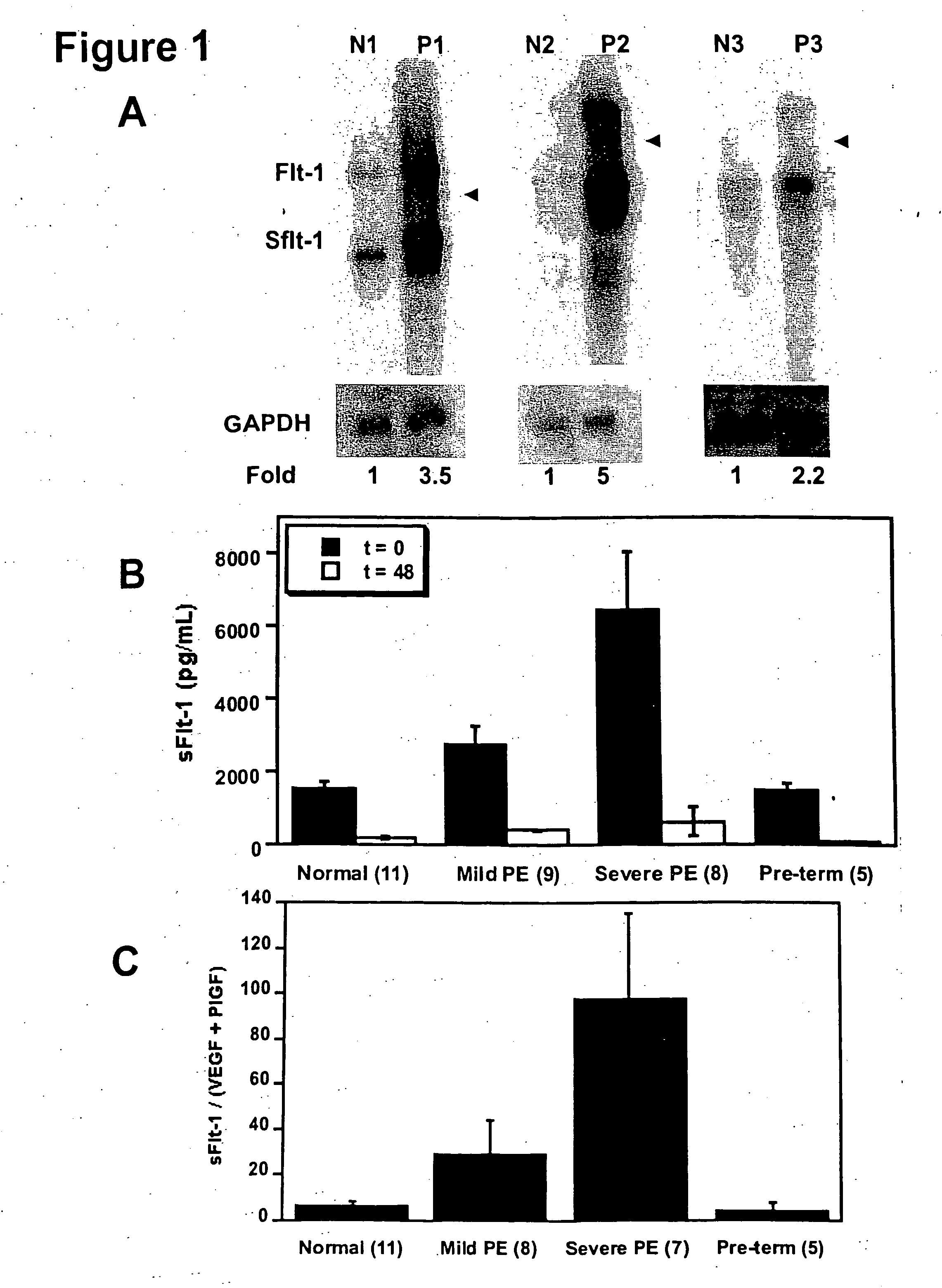
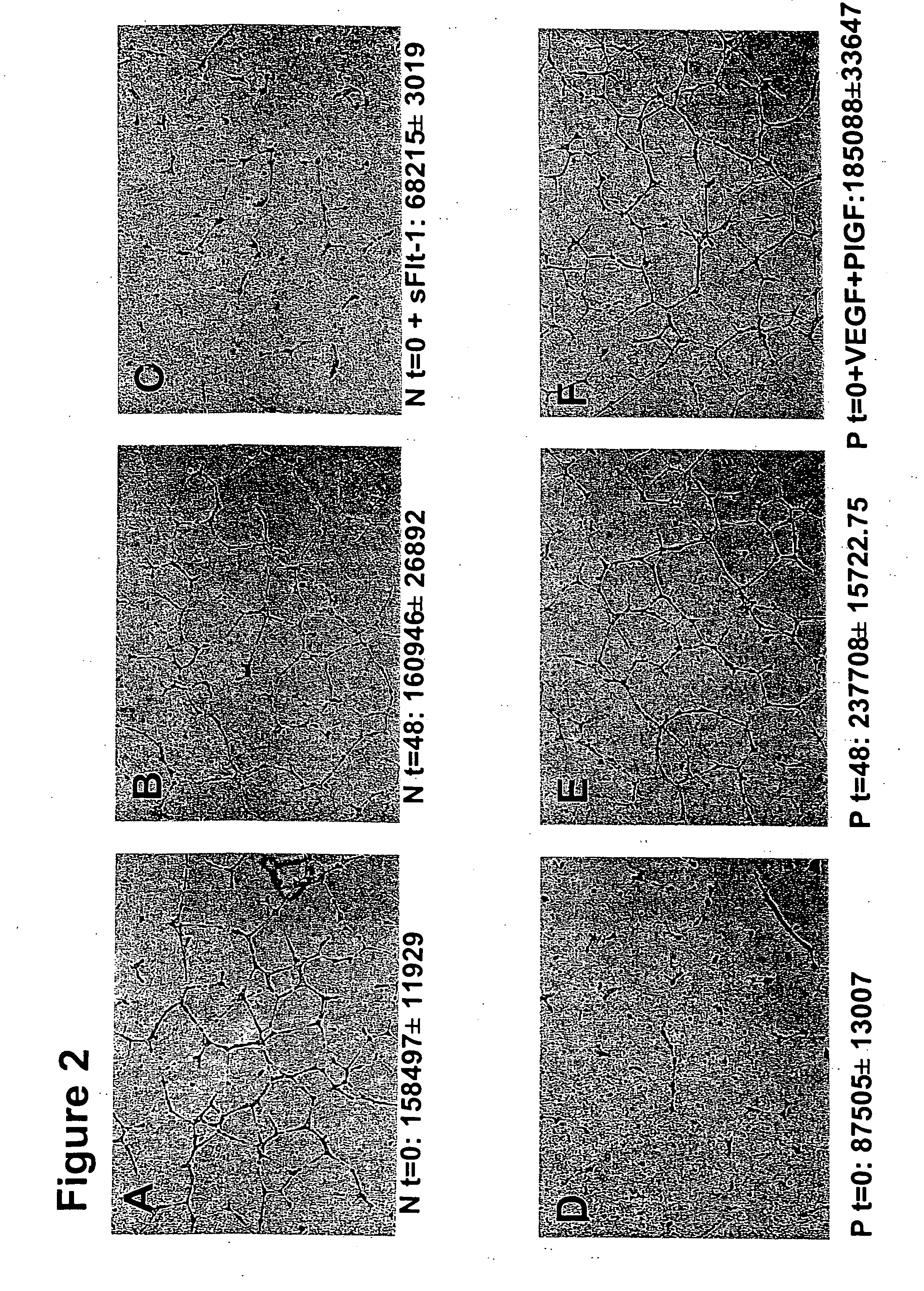
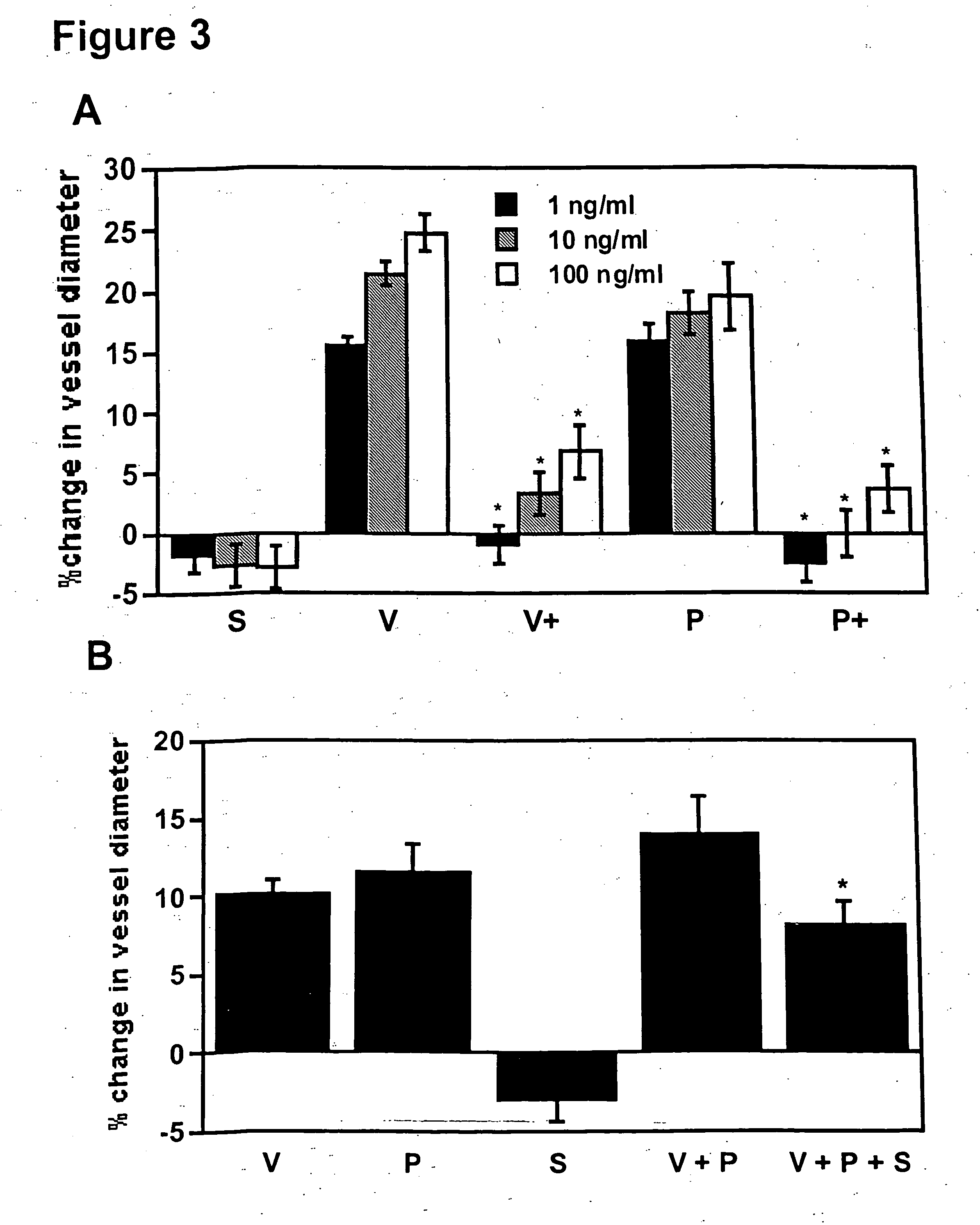
Disclosed herein are methods for diagnosing pre-eclampsia and eclampsia. Also disclosed herein are methods for treating pre-eclampsia and eclampsia using compounds that increase VEGF or PlGF levels or compounds that decrease sFlt-1 levels. Compounds that inhibit the binding of VEGF or PlGF to sFlt1- are also disclosed herein for the treatment of pre-eclampsia or eclampsia.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Methods of diagnosing and treating pre-eclampsia or eclampsia
InactiveUS7435419B2Lower Level RequirementsWeight optimizationCompound screeningApoptosis detectionObstetricsPlacental growth factor
Disclosed herein are methods for diagnosing pre-eclampsia and eclampsia or a propensity to develop pre-eclampsia or eclampsia by detecting the levels of placental growth factor in a subject.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Treatment of eclampsia and preeclampsia
InactiveUS7030083B2Prevention and reduction of endothelial cell injuryPeptide/protein ingredientsGenetic material ingredientsVascular endotheliumAngiogenesis Factor
The invention concerns the prevention and treatment of endothelial injury and the injury of tissues containing injured blood vessels by administration of angiogenic factors, such as vascular endothelial cell growth factor (VEGF).
Owner:UNIV OF WASHINGTON
Methods of diagnosing and treating pre-eclampsia or eclampsia
ActiveUS20050025762A1Treating or preventing pre-eclampsia or eclampsia in a subjectImprove the level ofOrganic active ingredientsPeptide/protein ingredientsObstetricsEclamptic seizure
Disclosed herein are methods for diagnosing pre-eclampsia and eclampsia. Also disclosed herein are methods for treating pre-eclampsia and eclampsia using compounds that increase VEGF or PlGF levels or compounds that decrease sFlt-1 levels. Compounds that inhibit the binding of VEGF or PlGF to sFlt1- are also disclosed herein for the treatment of pre-eclampsia or eclampsia.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Assay for the detection of biomarkers associated with pregnancy related conditions
The invention relates to screening methods for determination of the risk of preterm delivery and / or pregnancy associated conditions. The methods involve detection of the level of one or more biomarkers in a biological sample from the patient. In particular, in embodiments of the invention there are provided methods for determining the risk of pre-eclampsia and other hypertensive disorders, and intrauterine growth retardation (IGUR).
Owner:NEWCASTE INNOVATION LTD
Novel Pharmaceutical Preparation for Preeclampsia, Eclampsia, and Toxemia and Their Related Symptoms and Related Disorders of Pregnancy
A therapeutic agent for the treatment of toxemia, preeclampsia and eclampsia and the method for preparing the therapeutic agents is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the maternal GI tract to determine the likelihood of developing preeclampsia, pregnancy induced hypertension, and eclampsia / toxemia is disclosed.
Owner:CUREMARK
Methods
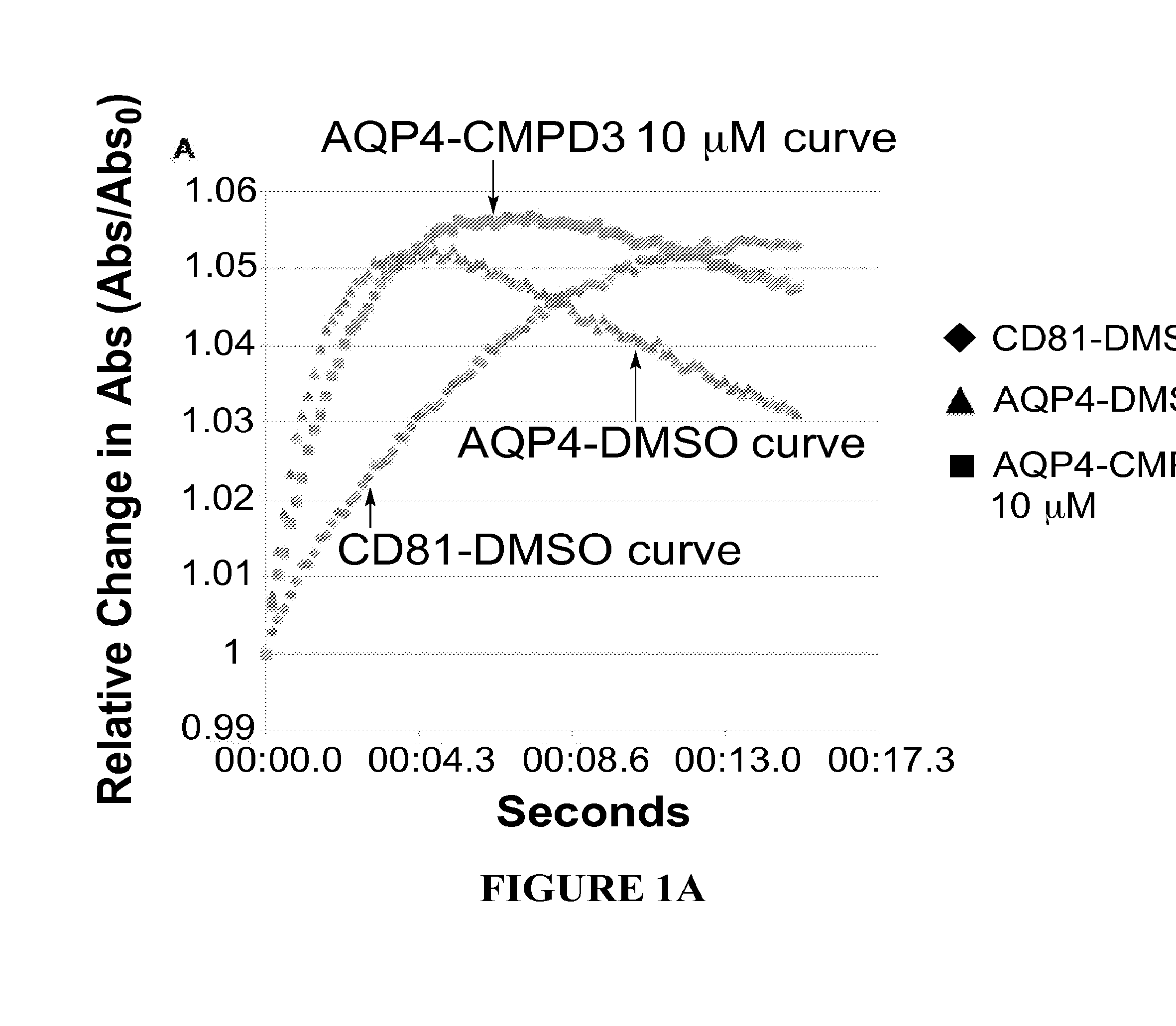
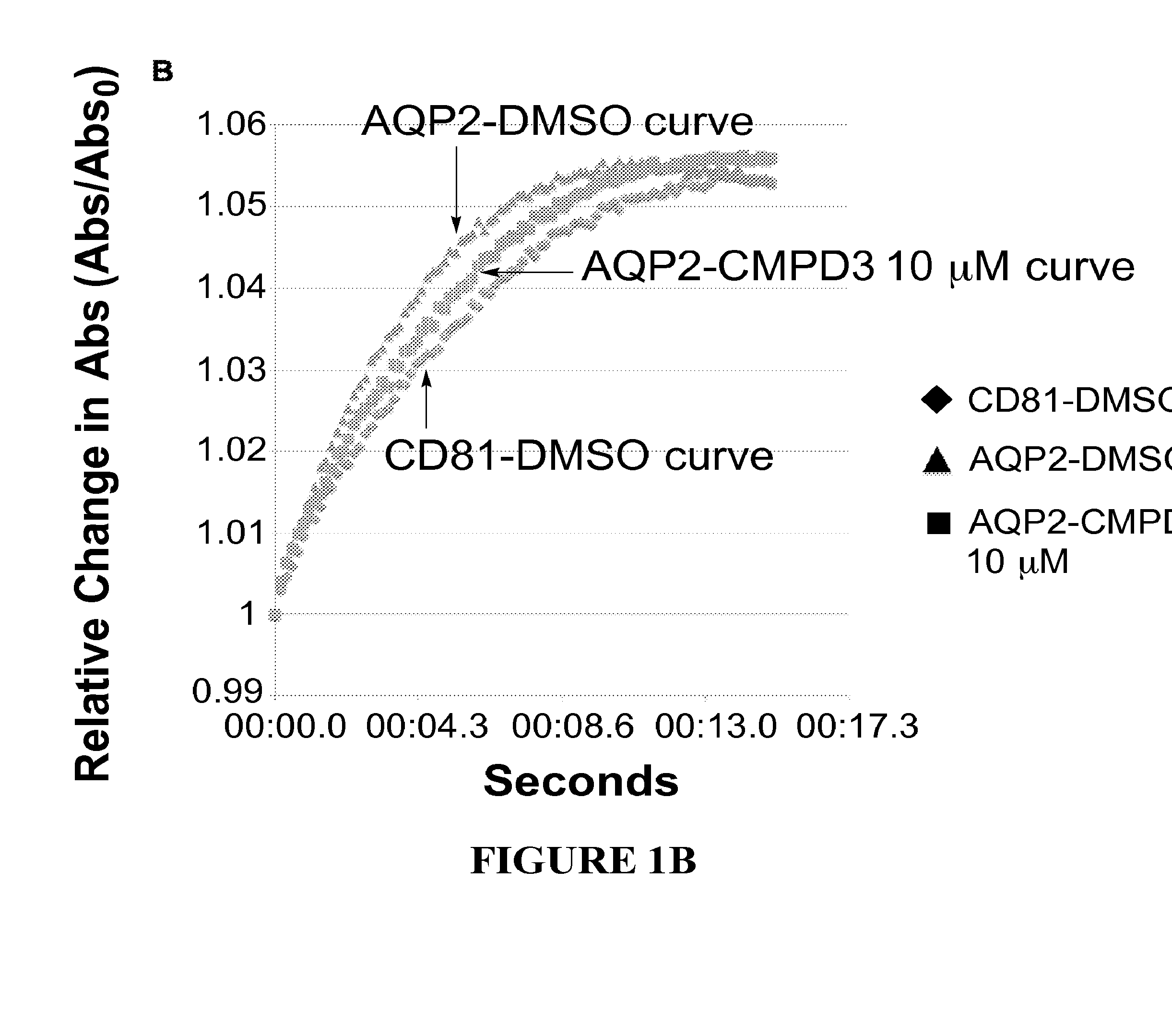
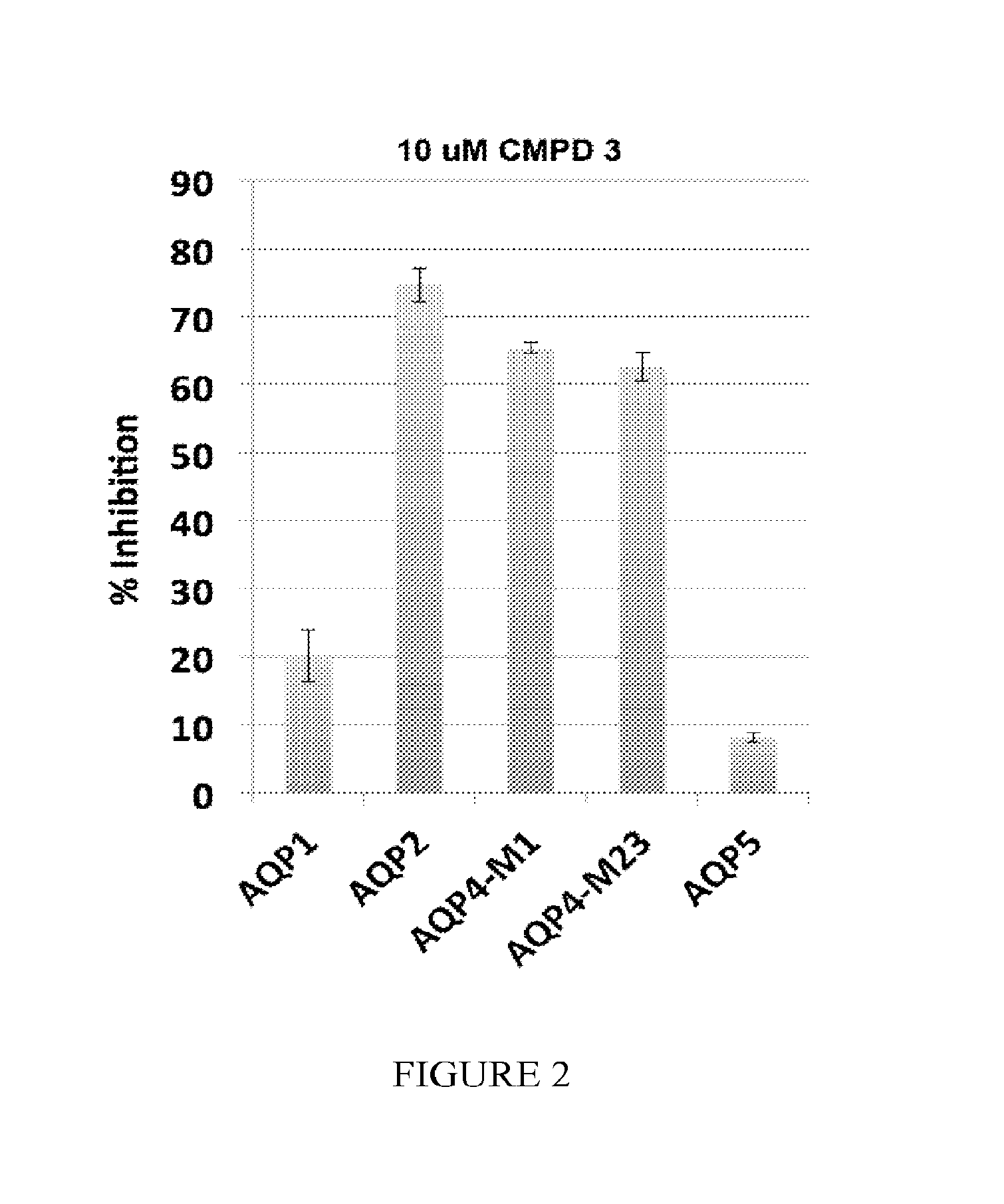
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
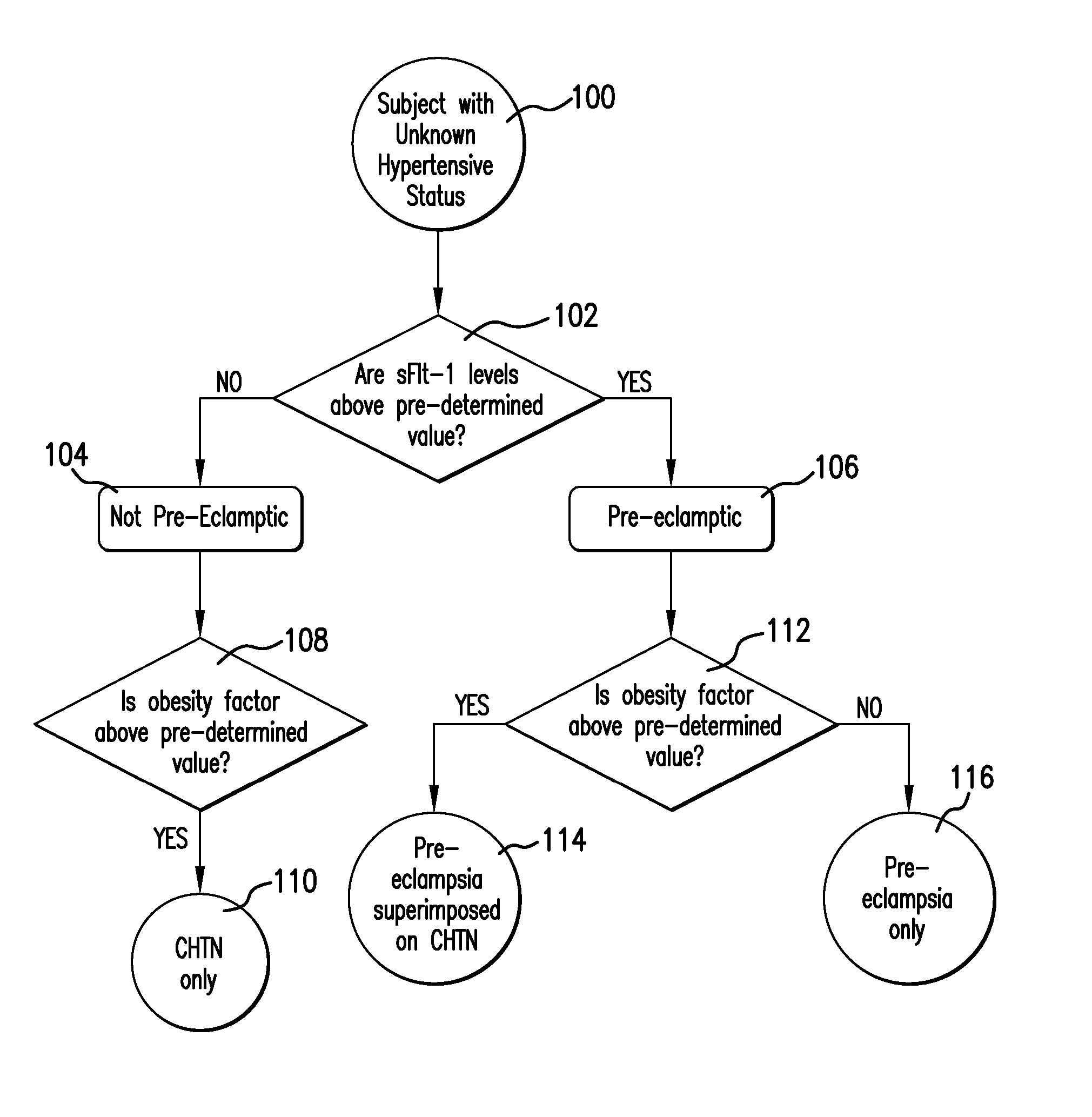
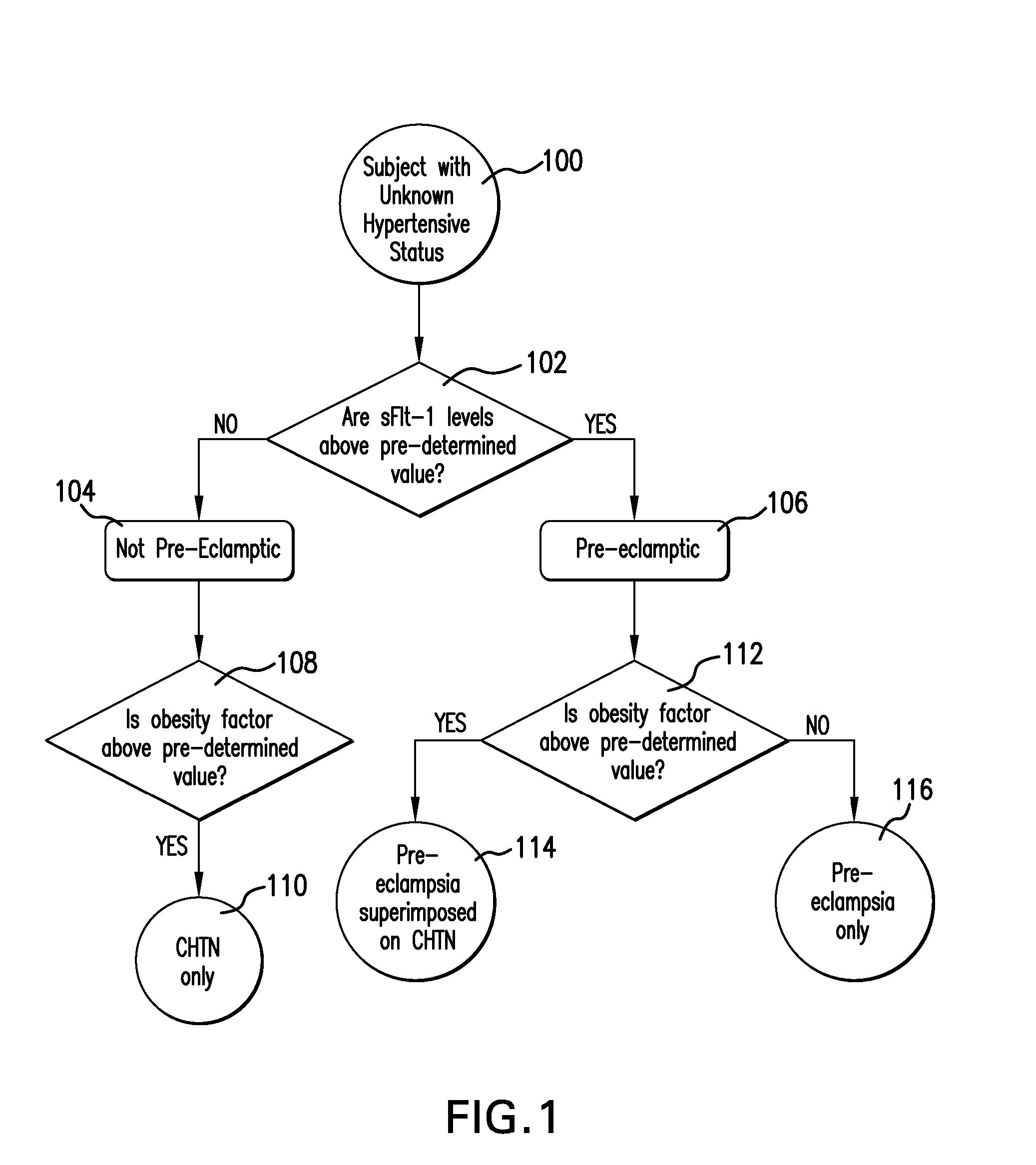
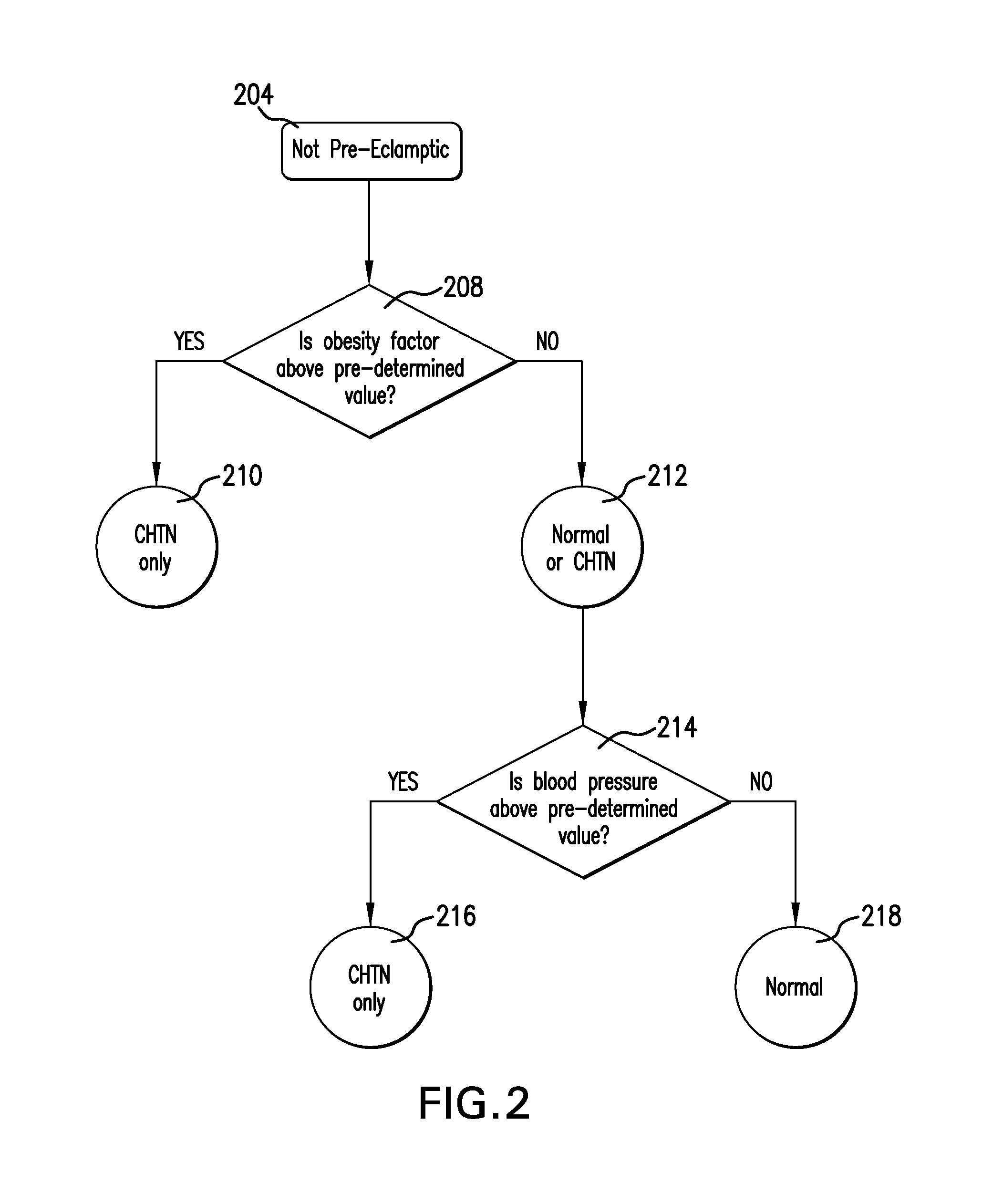
Method and Apparatus for Diagnosing Pre-eclampsia
InactiveUS20080071151A1Microbiological testing/measurementEvaluation of blood vesselsAnti-angiogenic drugsObesity Factor
A method is provided that allows a subject to be diagnosed as having one of a variety of hypertensive states, including pre-eclampsia, based on the measurement of a plurality of factors including the level of soluble fms-like tyrosine kinase 1 (sFlt-1), an obesity factor and optionally one or more additional factors, which may be physiological parameters or biomarkers. The method can be used to determine hypertensive states associated with pregnancy, or associated with anti-angiogenic drug therapy. The method is thus useful for diagnosing the hypertensive status of pregnant women, as well as patients undergoing anti-angiogenic treatment (e.g., chemotherapy).
Owner:ABBOTT LAB INC +1
Methods of diagnosing and treating complications of pregnancy
ActiveUS20070104707A1Increasing expression level and biological activityIncreases dephosphorylationOrganic active ingredientsDisease diagnosisDiseaseComplicated pregnancy
Disclosed herein are methods for treating a pregnancy related hypertensive disorder, such as pre-eclampsia and eclampsia, using combinations of compounds that alter soluble endoglin and sF1t-1 expression levels or biological activity. Also disclosed are methods for treating a pregnancy related hypertensive disorder, such as pre-eclampsia and eclampsia, using compounds that increase endothelial nitric oxide synthase levels or biological activity.
Owner:HOSPITAL FOR SICK CHILDREN +1
Nitric oxide enhancing angiotensin II antagonist compounds, compositions and methods of use
The invention describes compositions and kits comprising at least one nitric oxide enhancing angiotensin II antagonist compound, or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitric oxide enhancing angiotensin II antagonist compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension (o) treating central nervous system disorders; (p) treating metabolic syndrome; and (q) treating hyperlipidemia. The nitric oxide enhancing angiotensin II antagonist compounds comprise at least one nitric oxide enhancing group linked to the angiotensin II antagonist compound through one or more sites such as carbon, oxygen and / or nitrogen via a bond or moiety that cannot be hydrolyzed.
Owner:NICOX SA
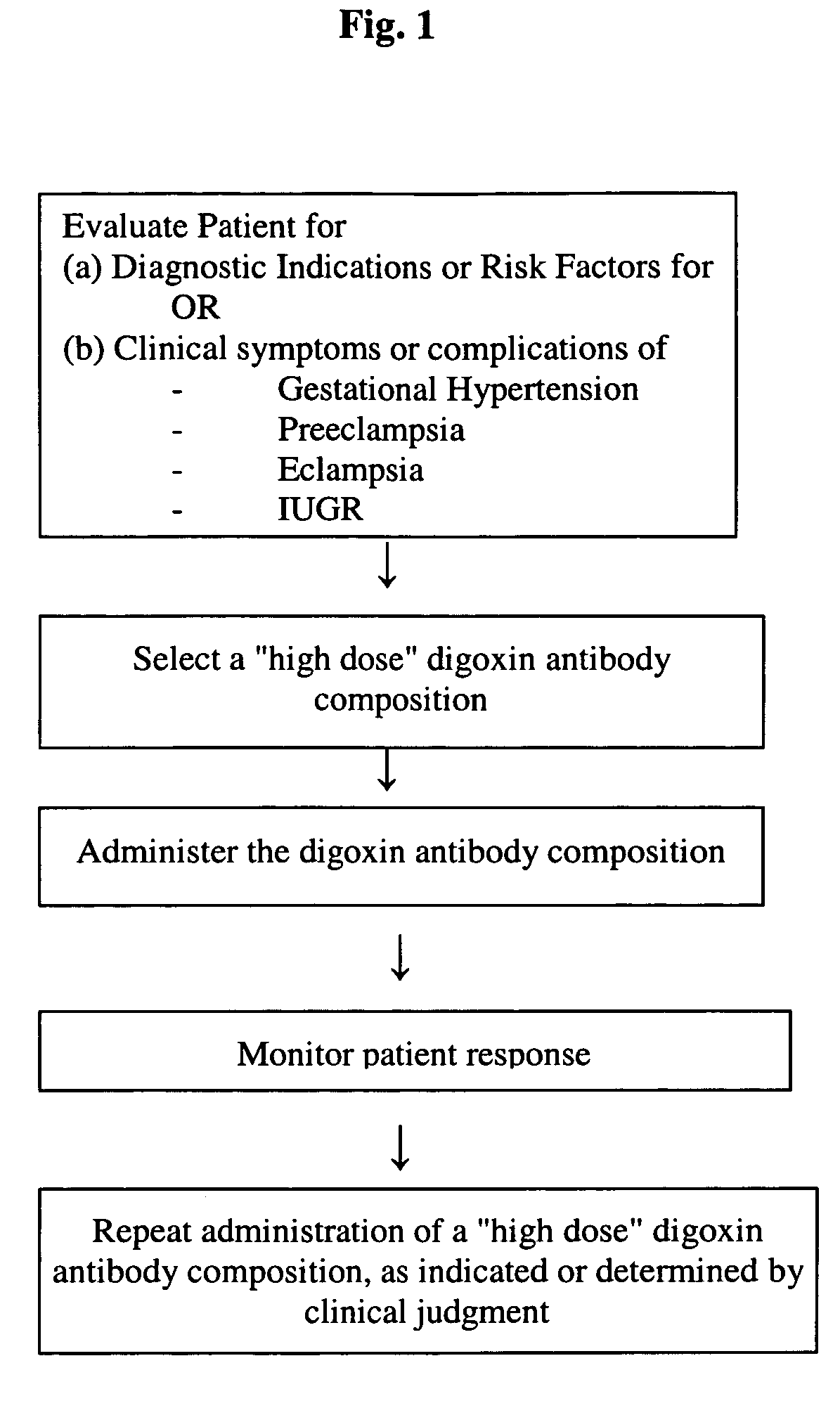
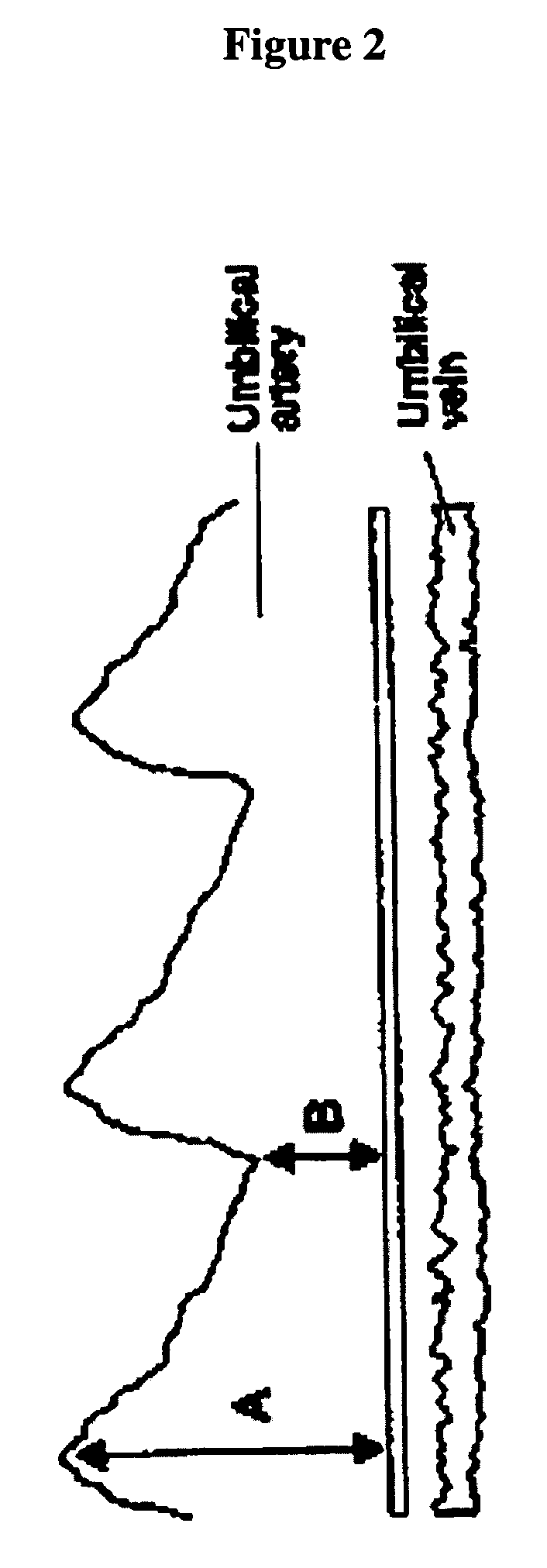
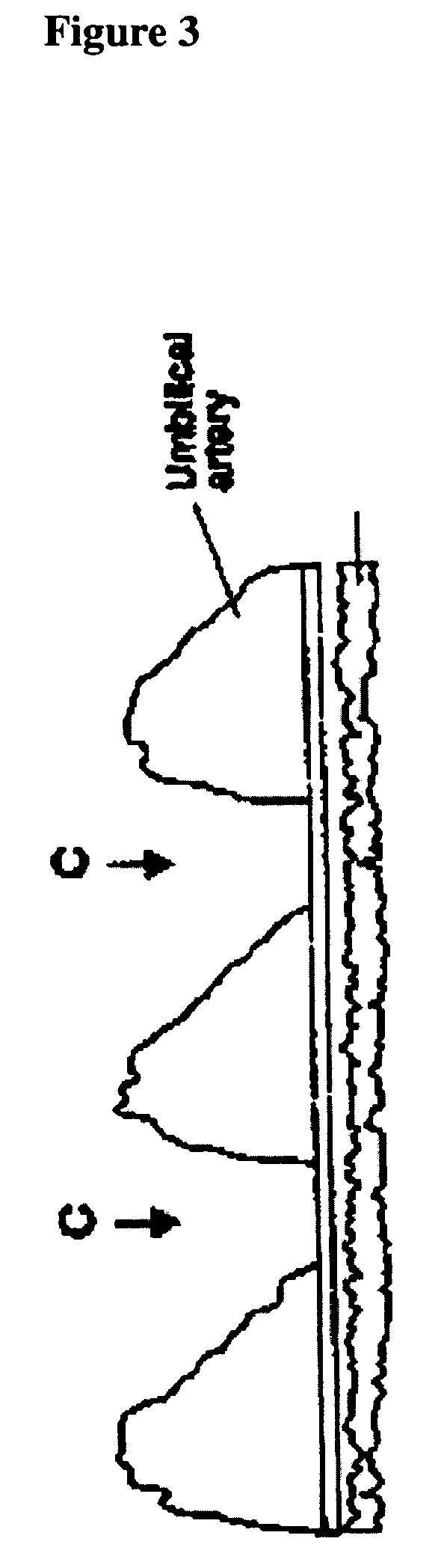
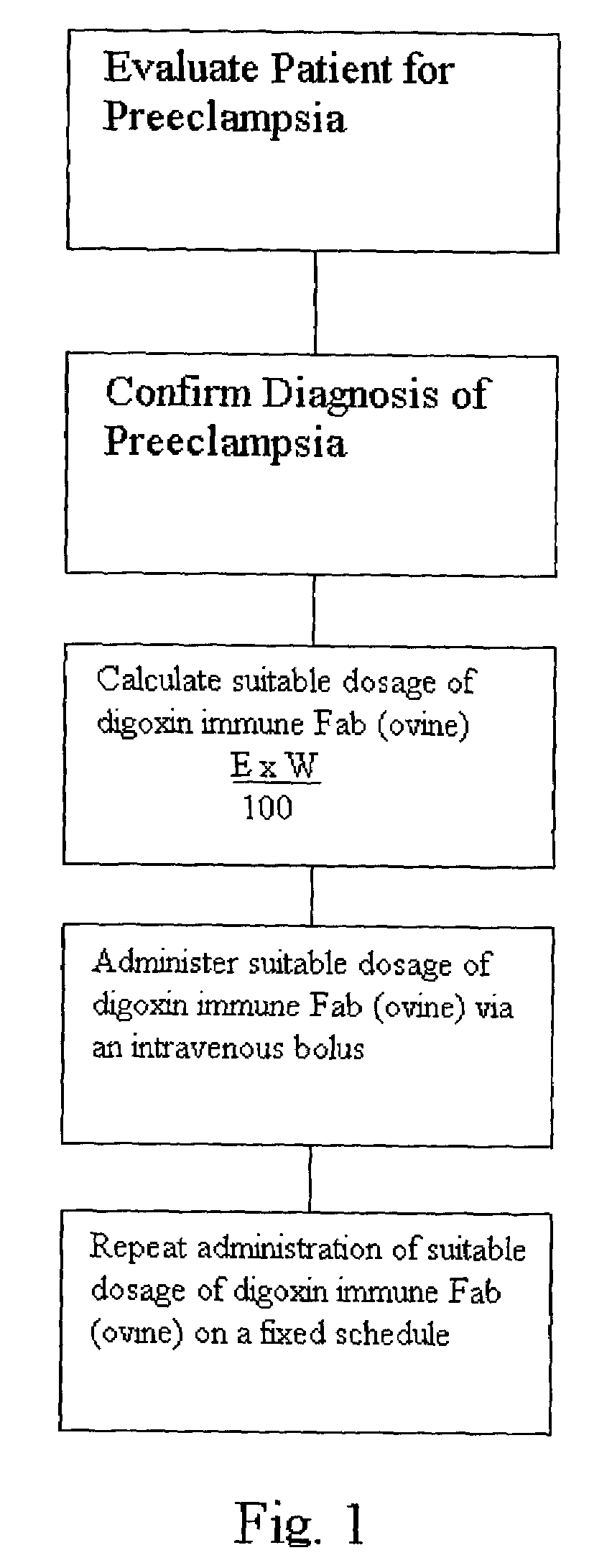
Antibody composition and passive immunization against pregnancy-induced hypertension
A composition is provided to prevent, limit the effects of, delay the onset of, or treat one or more of the causes, symptoms or complications of gestational hypertension, preeclampsia, eclampsia and / or intrauterine growth restriction. The composition comprises a therapeutically effective amount of an antibody that reacts immunologically with or binds digoxin and has a high dose of digoxin binding capacity as the active ingredient. There is also provided a method of preventing, limiting the effects of, delaying the onset of, or treating a cause, symptom or complication of gestational hypertension, preeclampsia, eclampsia or intrauterine growth restriction, comprising the step of administering to a mammal a composition comprising a therapeutically effective amount of an antibody that reacts immunologically with or binds digoxin and has a high dose of digoxin binding capacity.
Owner:VELO BIO
Determining existence of complications in pregnancies by measuring levels of bioactive lipids
InactiveUS20030068653A1Microbiological testing/measurementBiological material analysisLipidomePlacental abnormality
The present invention relates generally to methods for detecting complications, or abnormal conditions occurring during pregnancy, including placental abnormalities, eclampsia, or preelcampsia. The present invention comprises the steps of obtaining a sample from a patient, assaying the specimen to determine the level of bioactive lipids and comparing levels in the sample to control values or levels in normal samples, and correlating alterations to disease. The invention includes measuring panels of bioactive lipids to screen patients for disease and to monitor the progress of disease for diagnostic or therapeutic purposes.
Owner:LPL TECH
Methods of diagnosing and treating complications of pregnancy
The present invention discloses methods for treating a pregnancy related hypertensive disorder, such as pre-eclampsia and eclampsia, using combinations of compounds that alter soluble endoglin, endothelial nitric oxide synthase, PGI2, TGF-betal, TGF-beta3, activin A, BMP2, BMP7, and sFlt-1 expression levels or biological activity. Also disclosed are methods of diagnosing a pregnancy related hypertensive disorder, such as pre-eclampsia and eclampsia, that include the measurement of any one or more of the following: soluble endoglin, endothelial nitric oxide synthase, PGI2, TGF-betal, TGF-beta3, activin A, BMP2, BMP7, and sFlt-1 expression levels or biological activity.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
System and method for monitoring pre-eclamptic patients
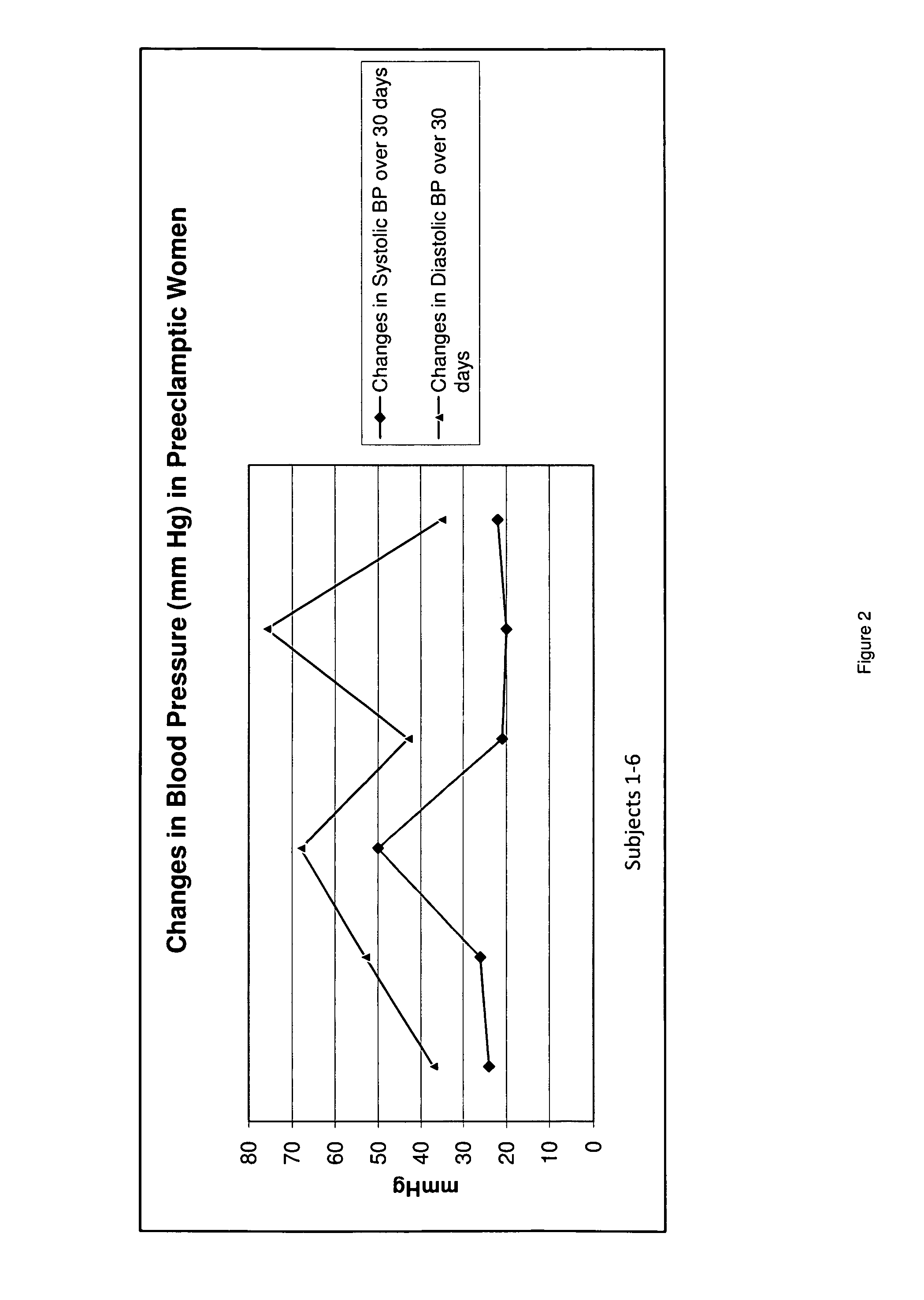
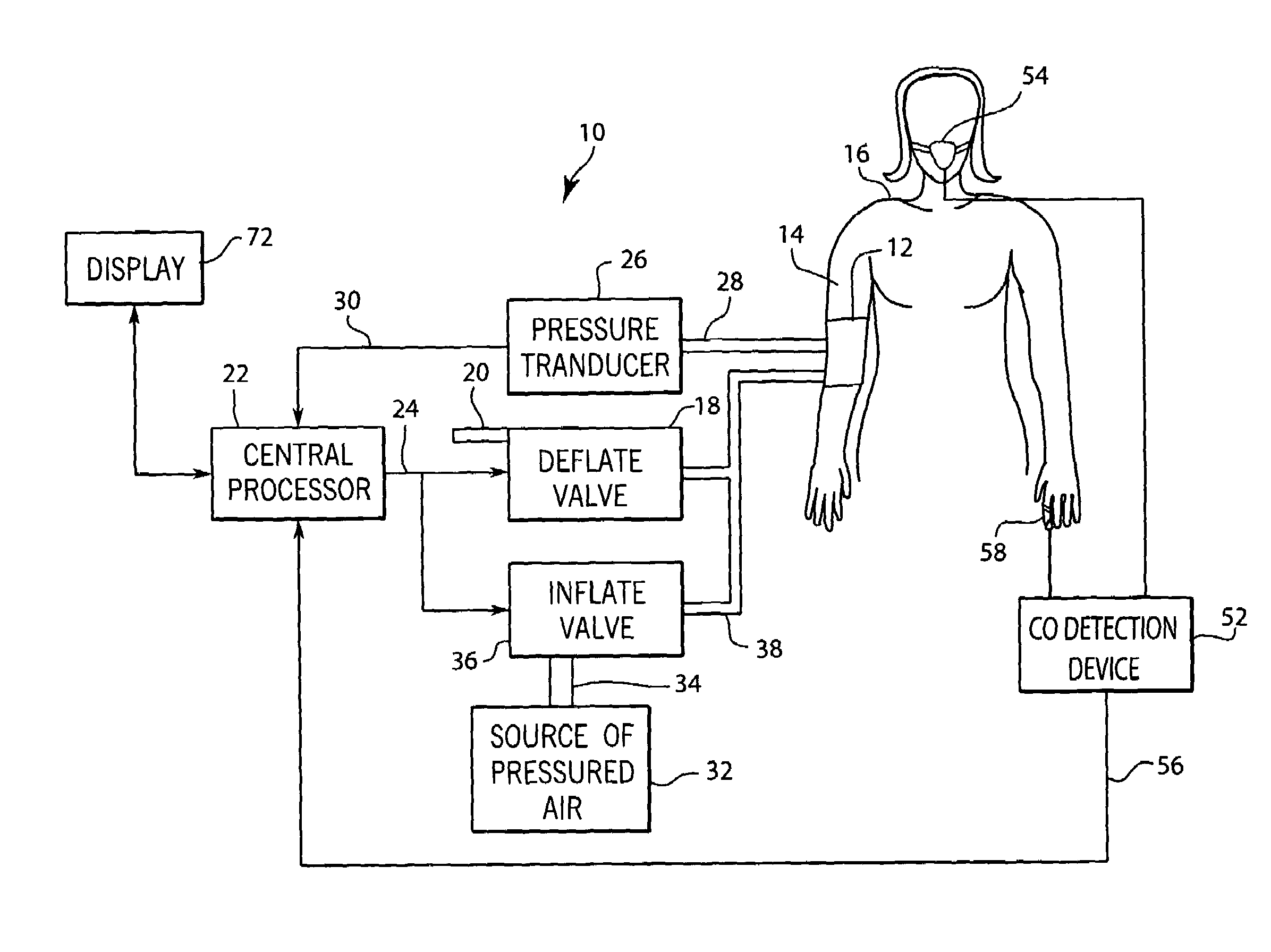
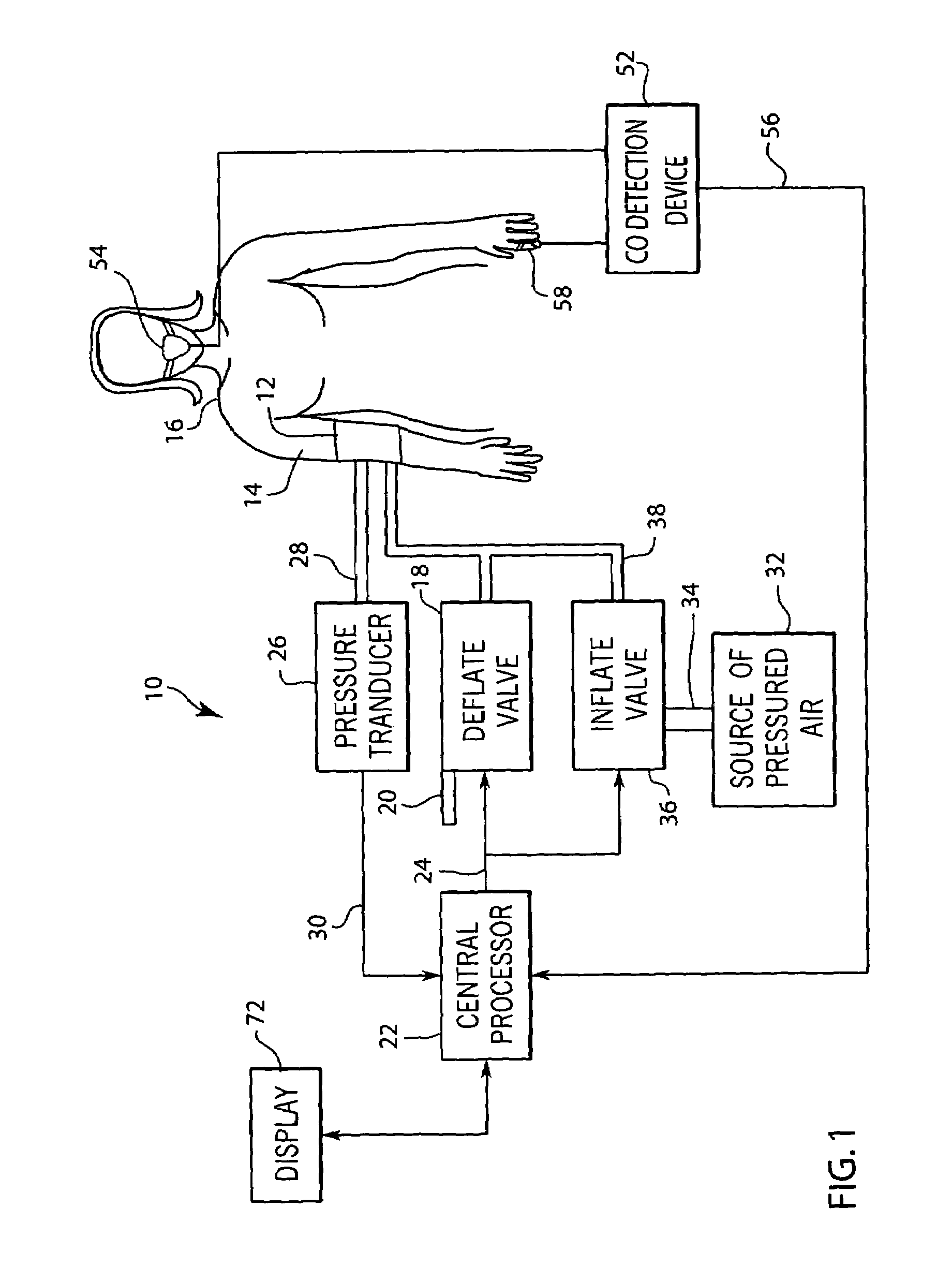
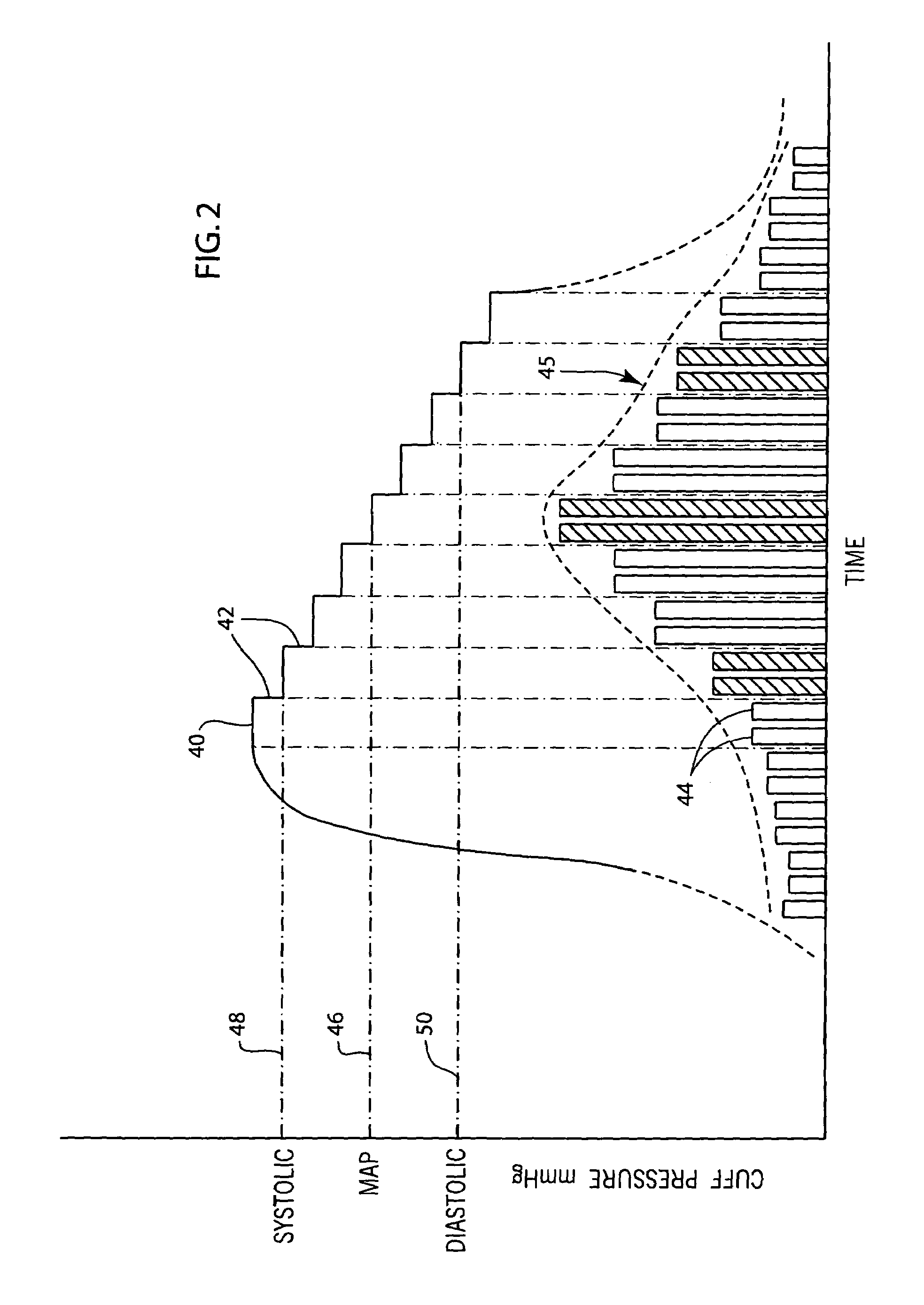
A system and method for monitoring and estimating the blood pressure of a pregnant patient that modifies the blood pressure estimating algorithm when the patient is pre-eclamptic. The level of carbon monoxide within a patient's bloodstream or exhaled breath can be analyzed to determine whether a pregnant patient is pre-eclamptic. After the patient has been diagnosed as pre-eclamptic, the NIBP monitoring system adjusts its algorithm for estimating the patient's blood pressure to compensate for the physical changes that occur in the patient during pre-eclampsia. The adjusted blood pressure estimates calculated by the NIBP monitoring system can be calculated using different adjustment techniques and methods and are displayed on the NIBP monitor.
Owner:GENERAL ELECTRIC CO
Serious preeclampsia/eclampsia illness state evaluation system
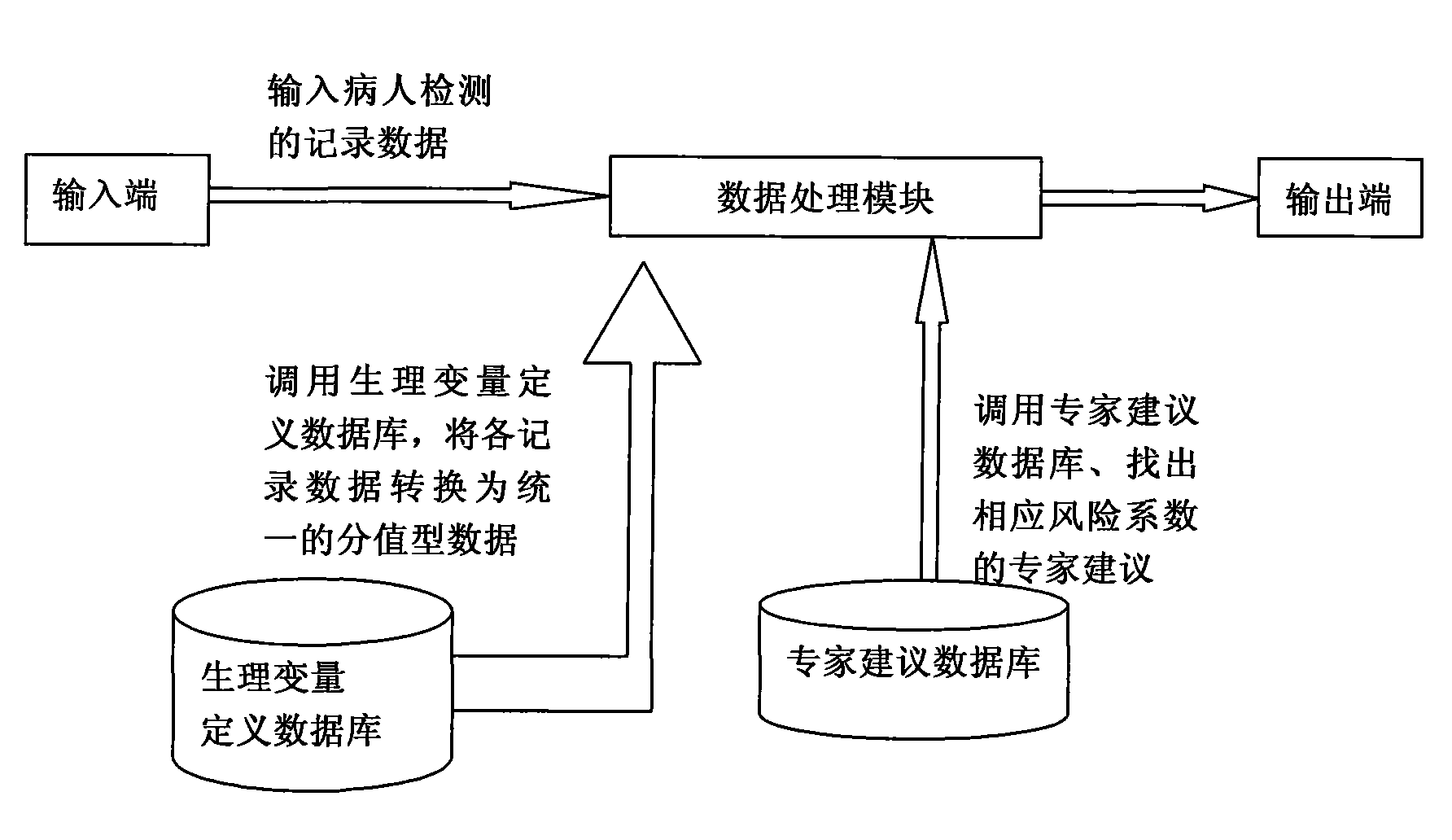
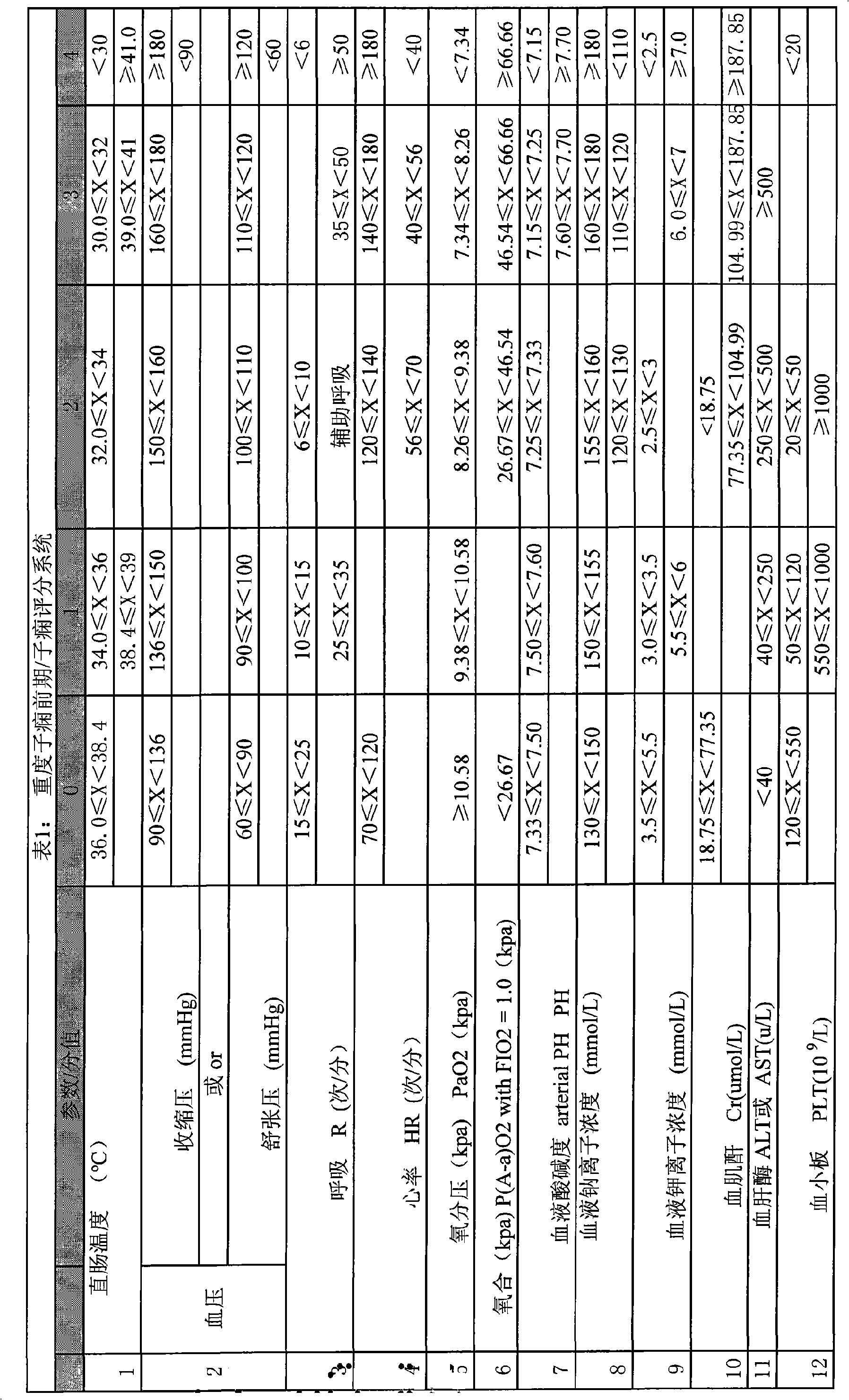
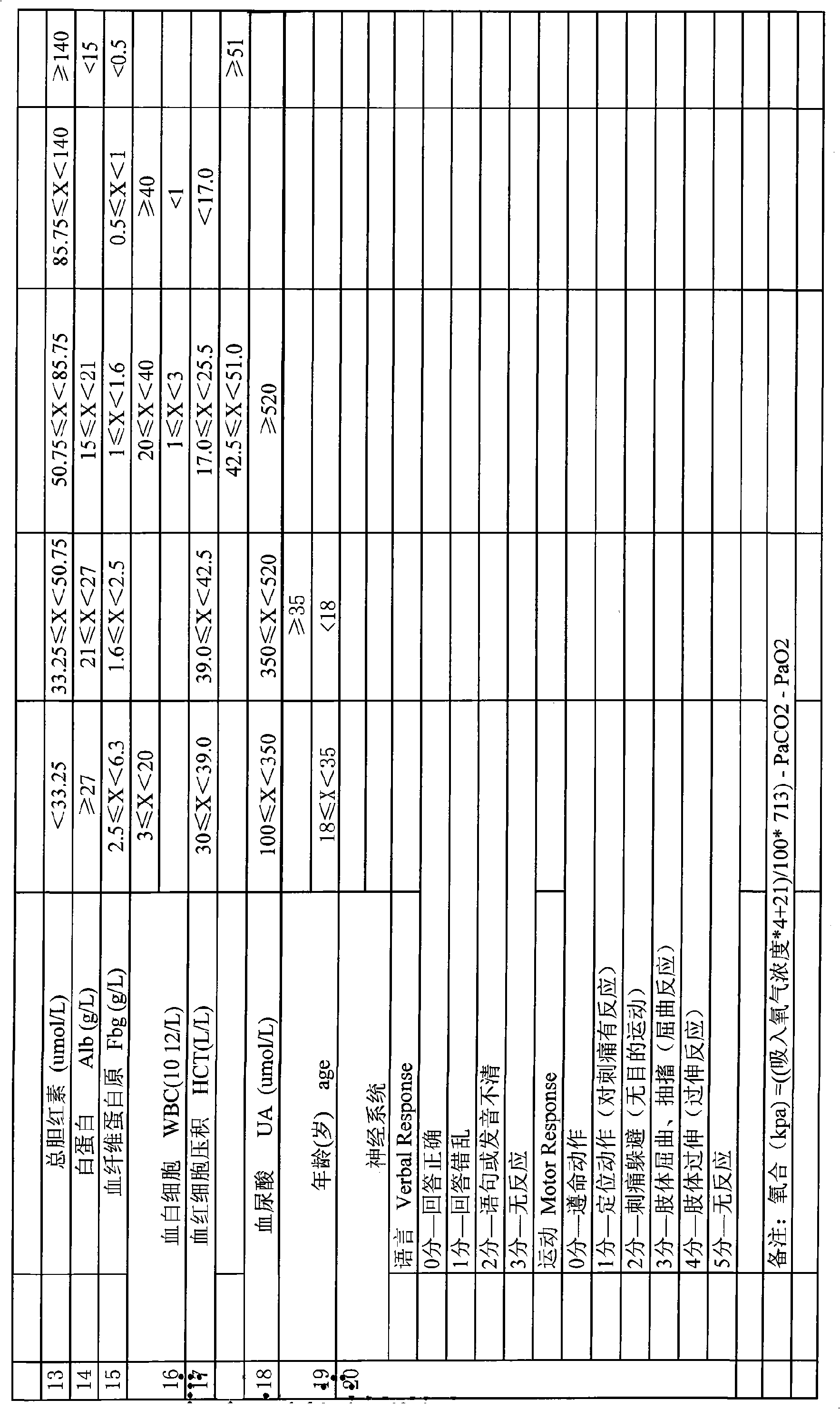
InactiveCN101548876AReduce mortalityReduce inappropriateness of criticality assessmentSurgeryDiagnostic recording/measuringNervous systemCreatinine rise
The invention discloses a serious preeclampsia / eclampsia illness state evaluation system which comprises an input end, an output end and a data processing module. Firstly, the measured heart rate, the blood pressure, the body temperature, the breathing rate, the pH, the oxygen partial pressure, the oxygenation, the sodium ion concentration, the hematokrit, the white cell count, the platelet count, the fibrinogen, the blood liver enzyme, the albumin, the bilirubin, the creatinine, the blood uric acid and an age scoring and nervous system scoring data input end of a patient are input to the serious preeclampsia / eclampsia illness state evaluation system; then the data processing module works out death risk factor; and finally, the output end directly reflects results of the patient and expert suggestions. The invention can quantificationally evaluate the illness state critical degree of a serious preeclampsia / eclampsia patient, dynamically evaluate the serious preeclampsia / eclampsia of the patient, predict death risks and provide clinical processing reference proposals and is beneficial to enhance the consistency and the comparability of a selected contrast and a clinical case, thereby lowering the mortality rate of newborn babies and pregnant women.
Owner:刘慧姝 +1
Detection of risk of pre-eclampsia
ActiveUS20130073212A1Improve predictive performanceBiostatisticsDisease diagnosisDiseaseHELLP syndrome
A method for the early prediction of risk of hypertensive disorders in pregnant women, including for example eclampsia, mild pre-eclampsia, chronic hypertension, EPH gestosis, gestational hypertension, superimposed pre-eclampsia, HELLP syndrome, or nephropathy.
Owner:UNIV COLLEGE CORK NAT UNIV OF IRELAND CORK
Extracorporeal devices and methods of treating complications of pregnancy
The invention features extracorporeal methods for the treatment of a subject having a pregnancy related hypertensive disorder, such as pre-eclampsia or eclampsia. The invention also features devices used for the extracorporeal treatment of subjects have a pregnancy related hypertensive disorder, such as pre-eclampsia or eclampsia.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
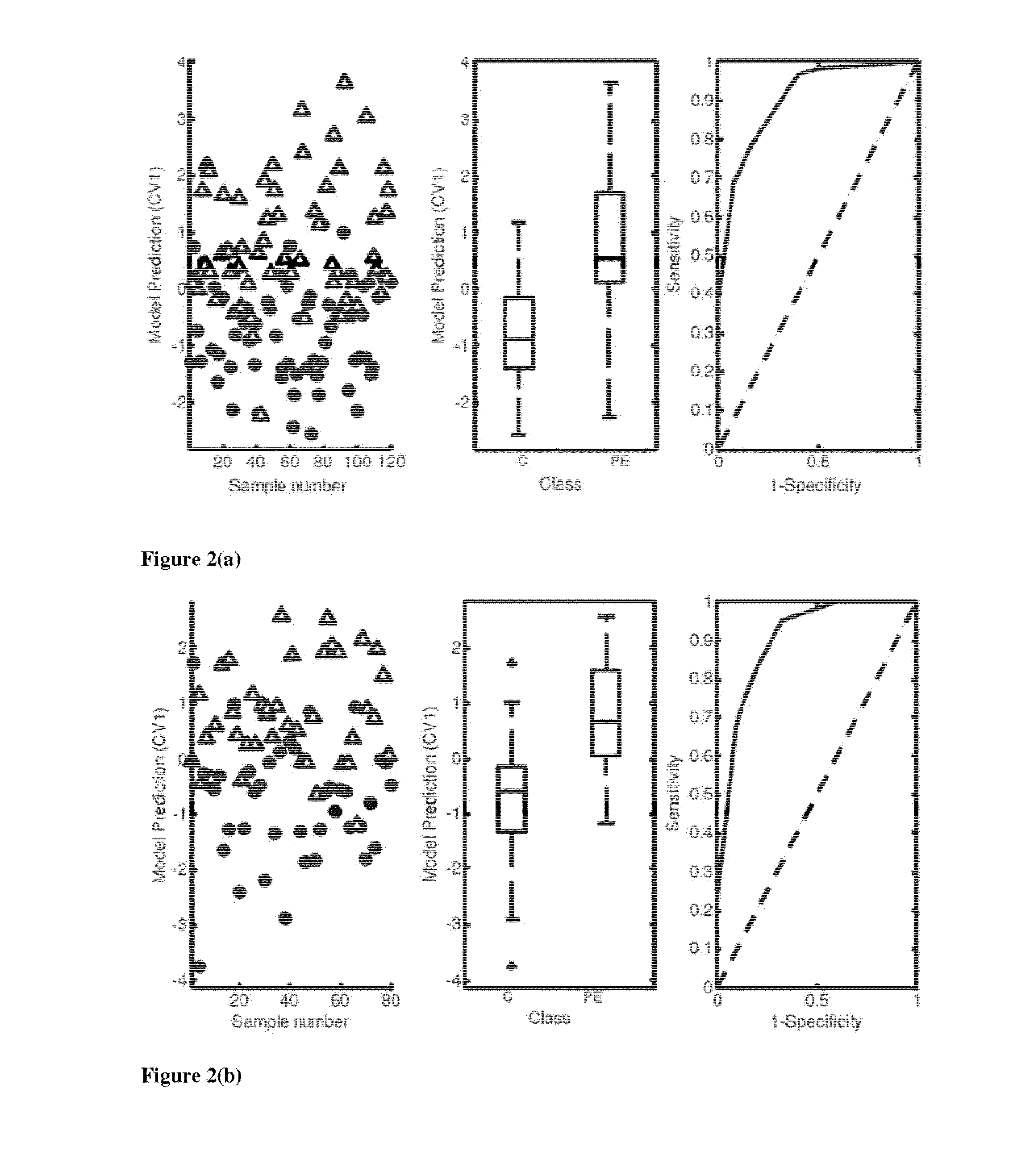
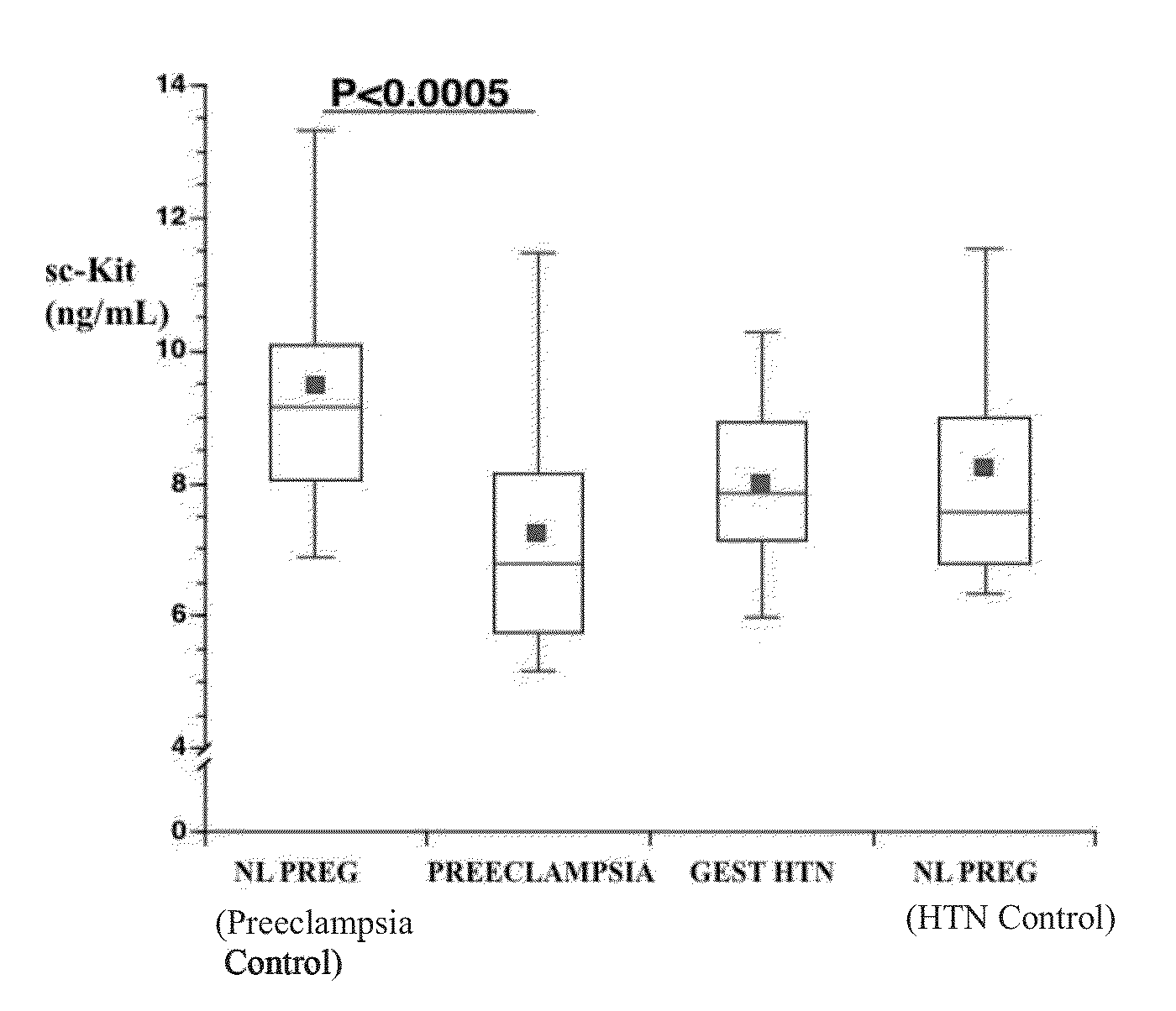
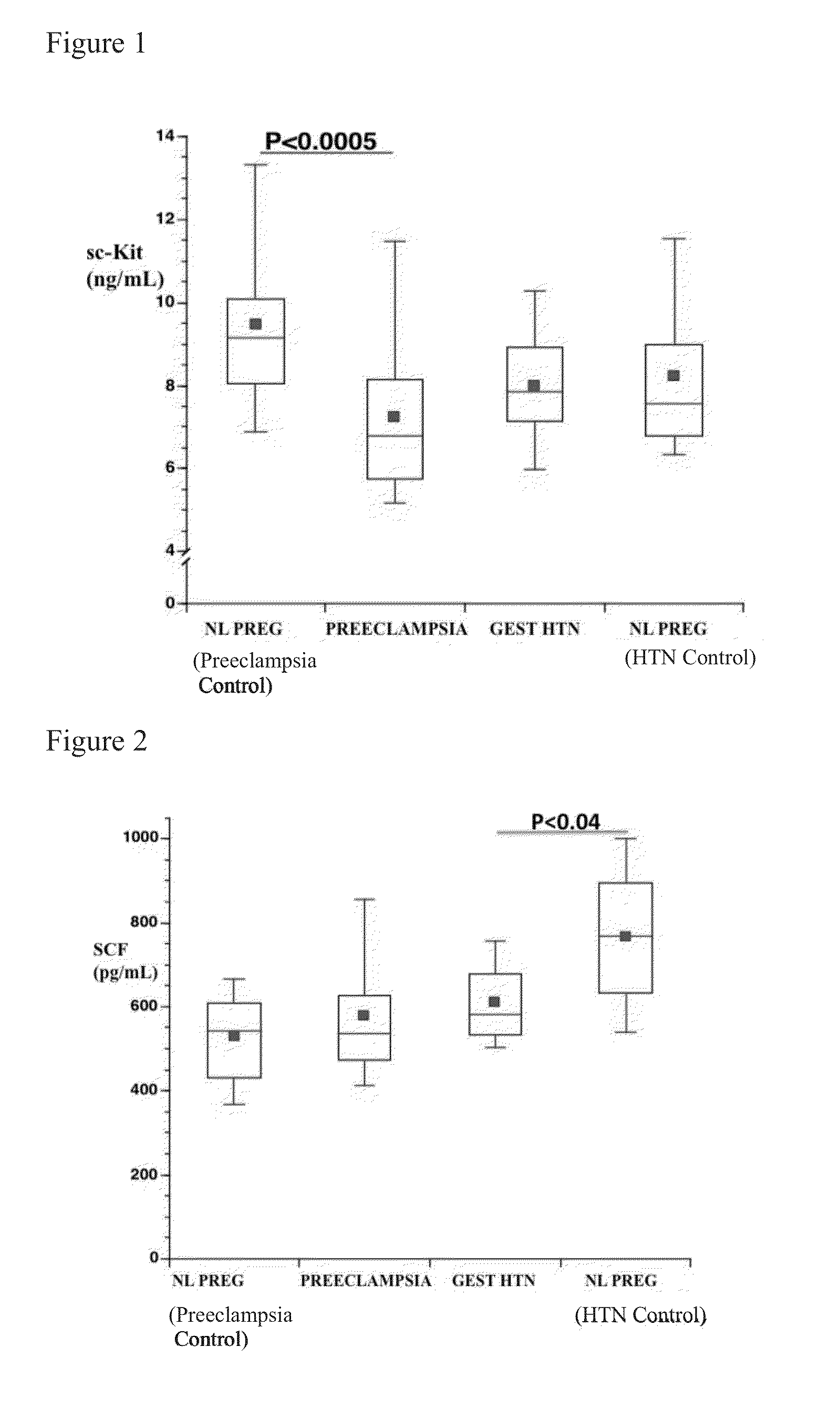
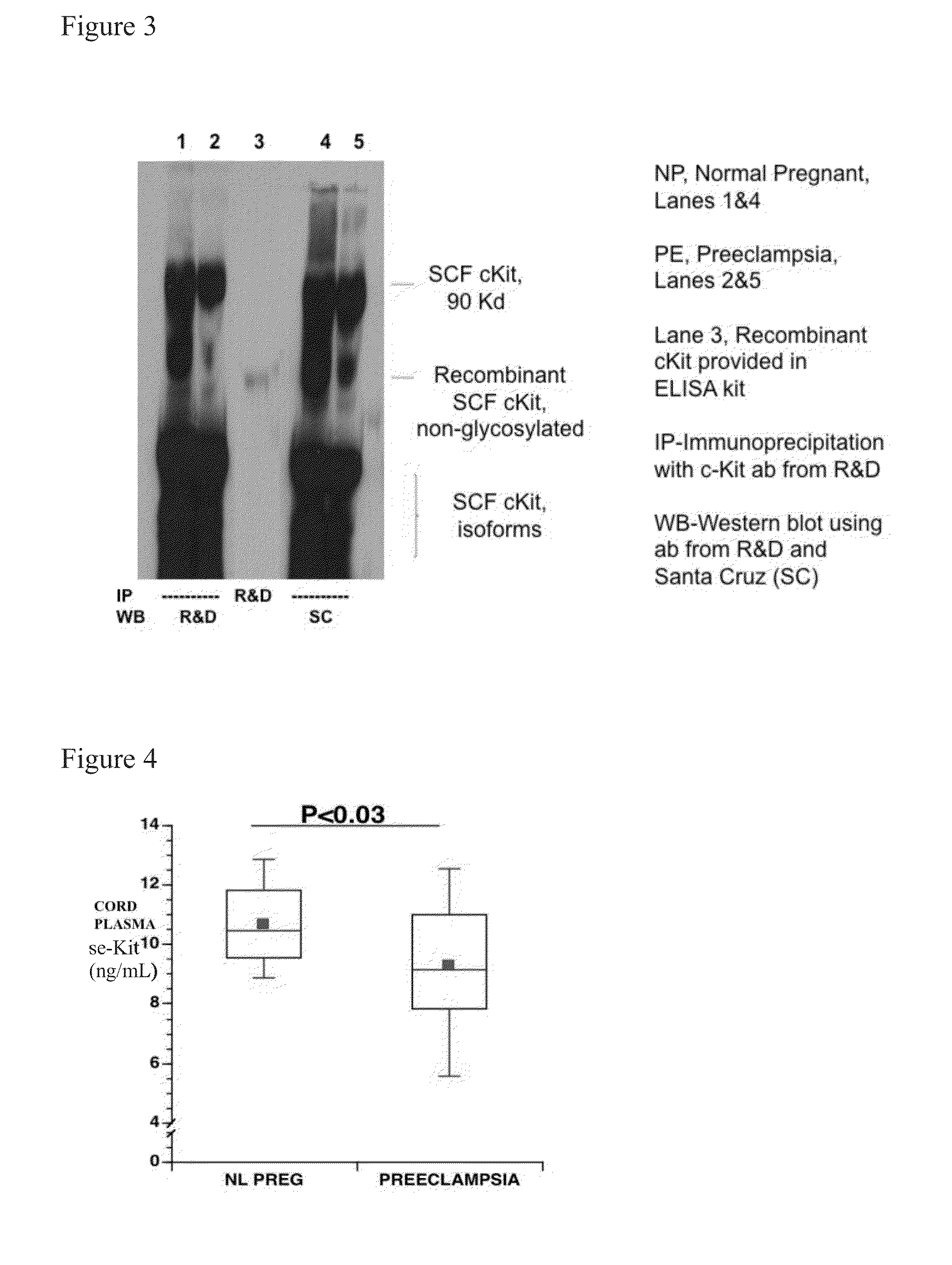
Soluble CD117 (sc-kit) for diagnosis of preeclampsia and eclampsia
Owner:UNIVERSITY OF PITTSBURGH
Method for controlling preeclampsia and eclampsia
Owner:VELO BIO
Pre-eclampsia screening methods
The present invention relates generally to methods for treating early and late onset pre-eclampsia, as well as to methods of screening for and predicting the likelihood that a pregnant female patient will develop early and / or late pre-eclampsia, by assessing specific combinations of factors. In the methods of the invention, the a priori risk of developing eariy preeclampsia may be calculated utilizing coefficients for each of the maternal factors (binary variables), the coefficients being generated utilizing logistic regression analysis. The a posteriori risk of developing eariy pre-eclampsia may be calculated utilizing coefficients for each of the patient-specific factors, the coefficients being generated utilizing logistic regression analysis.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Biomarkers for preeclampsia
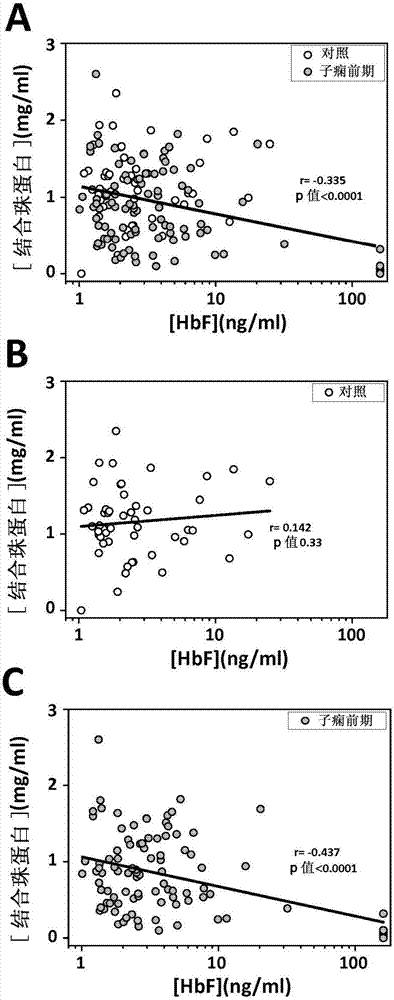
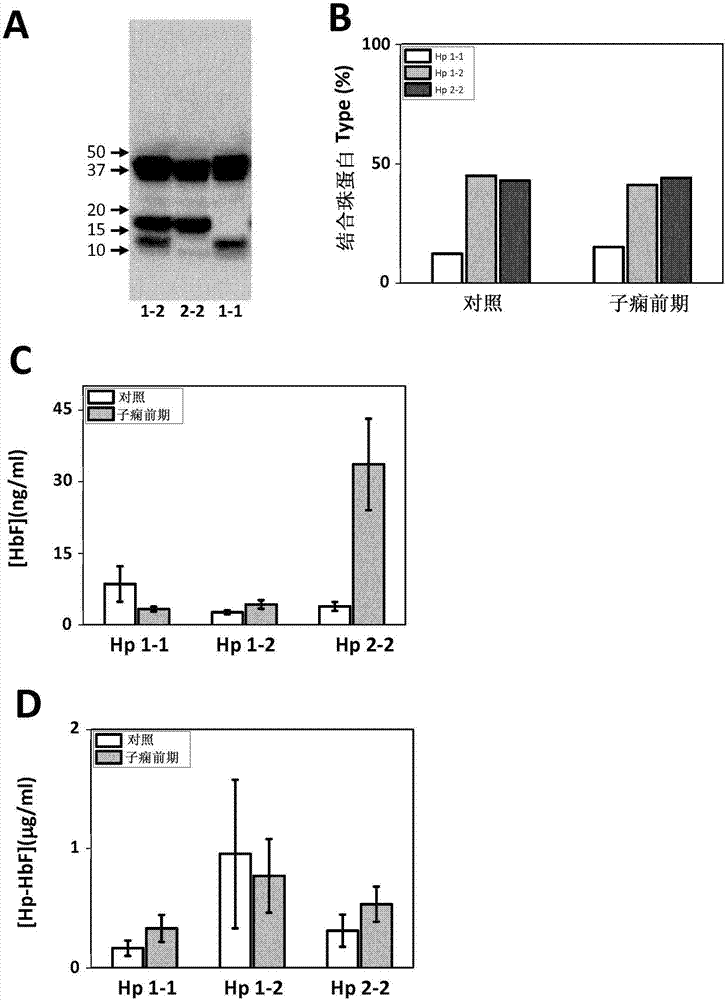
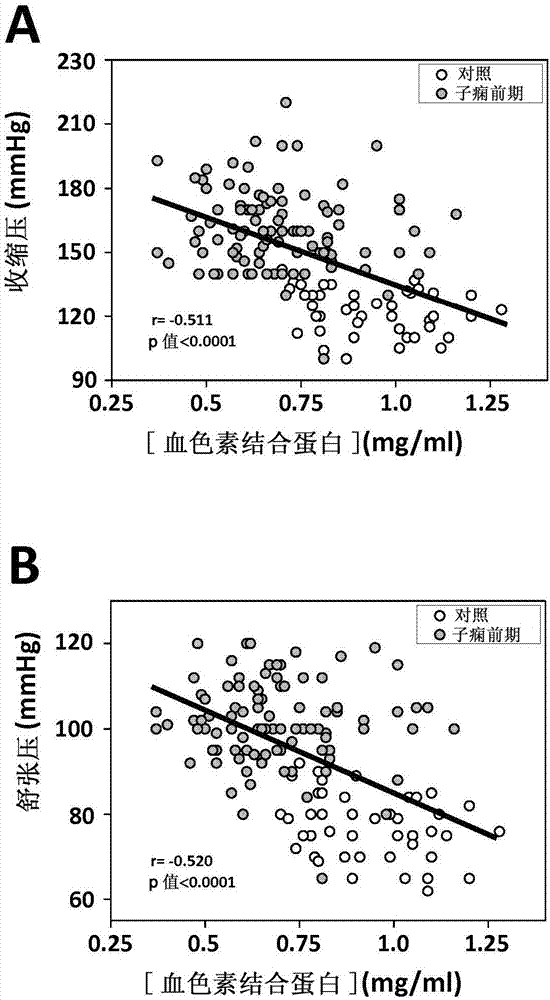
InactiveCN107430134ALess quantityAmount to preventMicrobiological testing/measurementDisease diagnosisObstetricsHemoglobin F
The present invention relates to the use of hemopexin, free, non-cell bound fetal hemoglobin and alpha-1-microglobulin as markers for preeclampsia.
Owner:A1M PHARMA
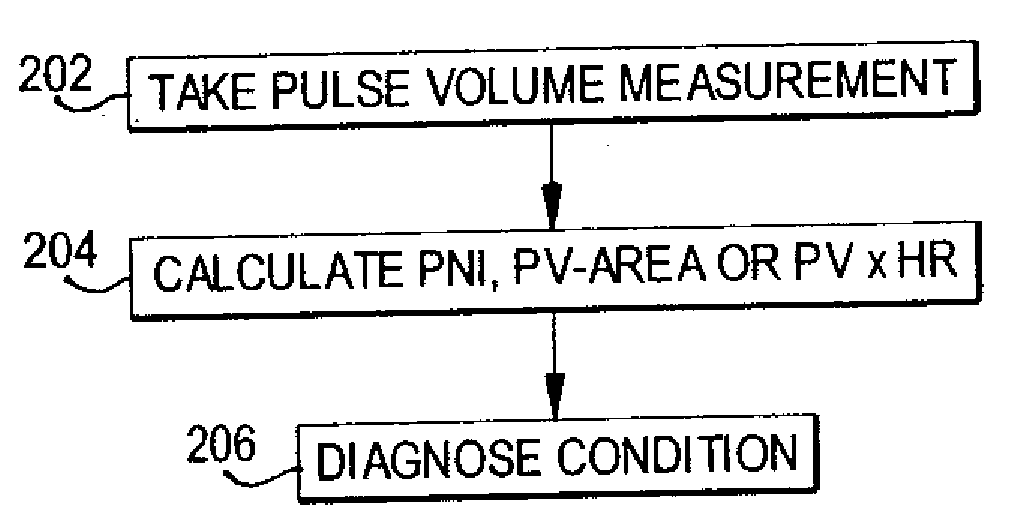
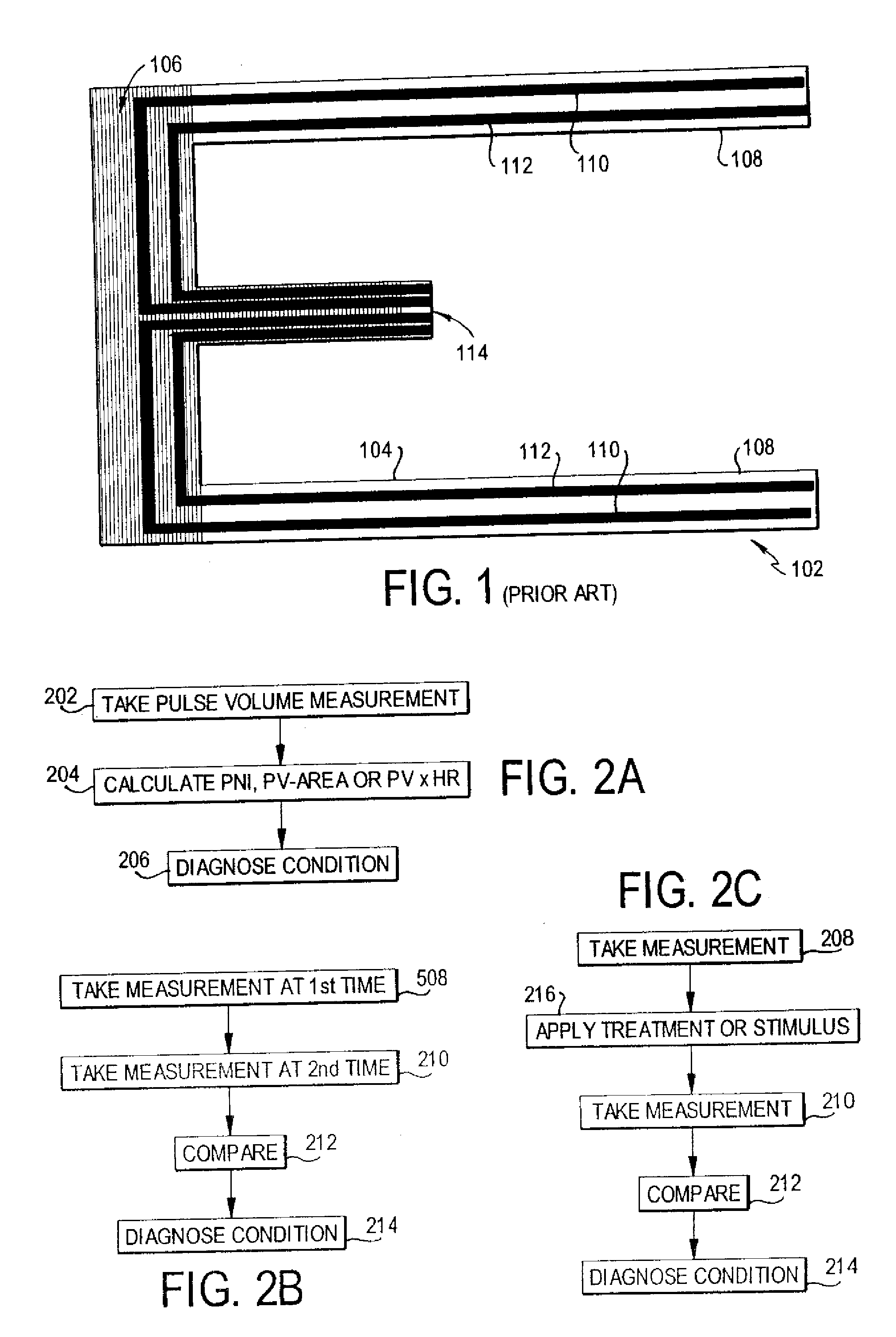
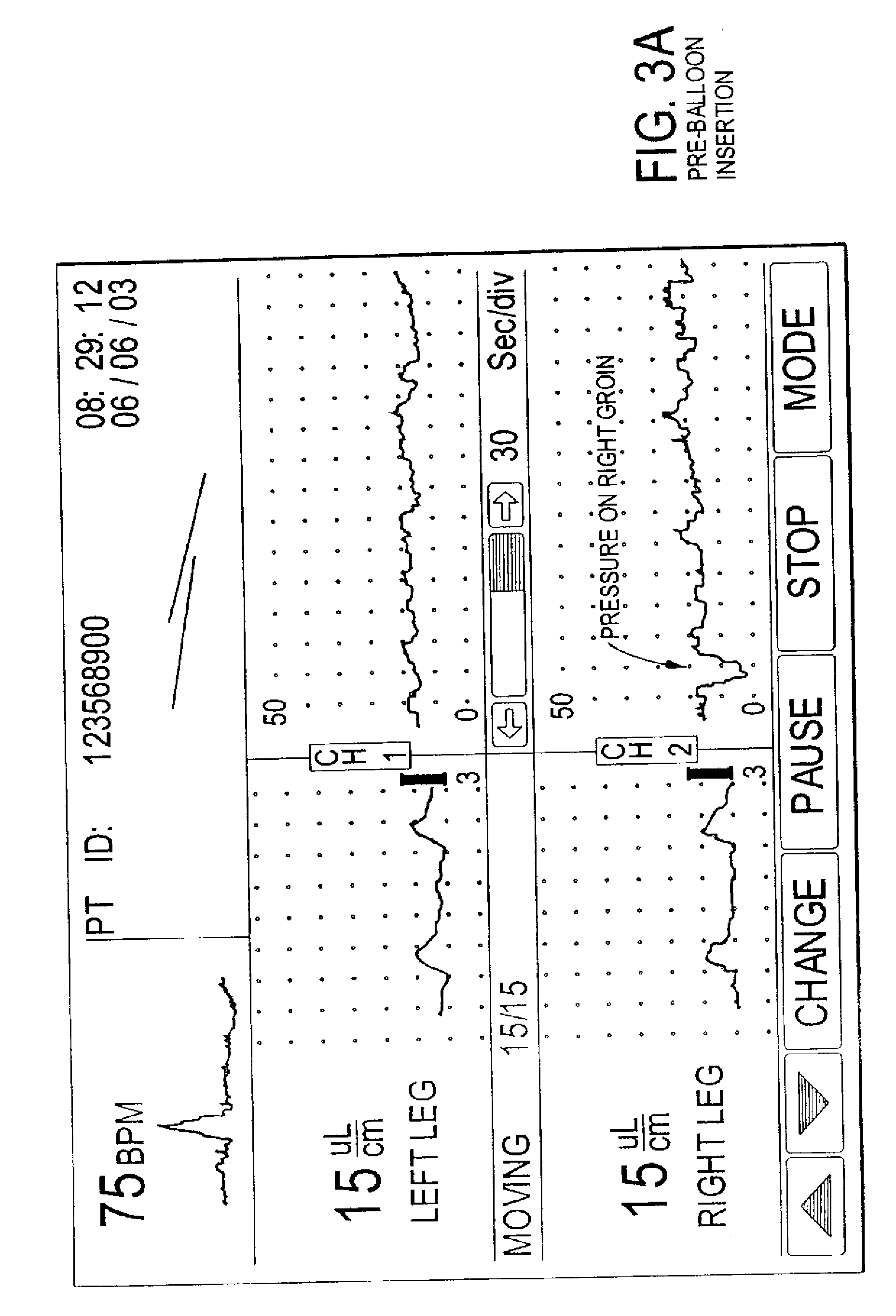
Methods of diagnosis using pulse volume measurement
The utility of pulse volume measurement is expanded to detection of many conditions which have previously not been detected or have been detected using more complicated techniques. Such conditions include blood loss, septic shock, cardiogenic shock, neonatal sepsis, patent ductus arteriosus, limb ischemia, intra-aortic balloon pump performance, peripheral vascular disease, congestive heart failure, the effectiveness of vasoactive medications, syncope, dehydration, pre-eclampsia, deep vein thrombosis, thermal injuries, vascular instability due to renal dialysis, compromising of circulation to the hand caused by radial artery harvesting, changes in cardiac output, and hypertension. According to the present invention, such diagnoses can be performed by taking one measurement, by taking measurements over time to detect a change or by taking measurements before and after application of a treatment or stimulus.
Owner:SMITHMARKS INC
Test kit for prediction or early diagnosis of hypertension of pregnancy
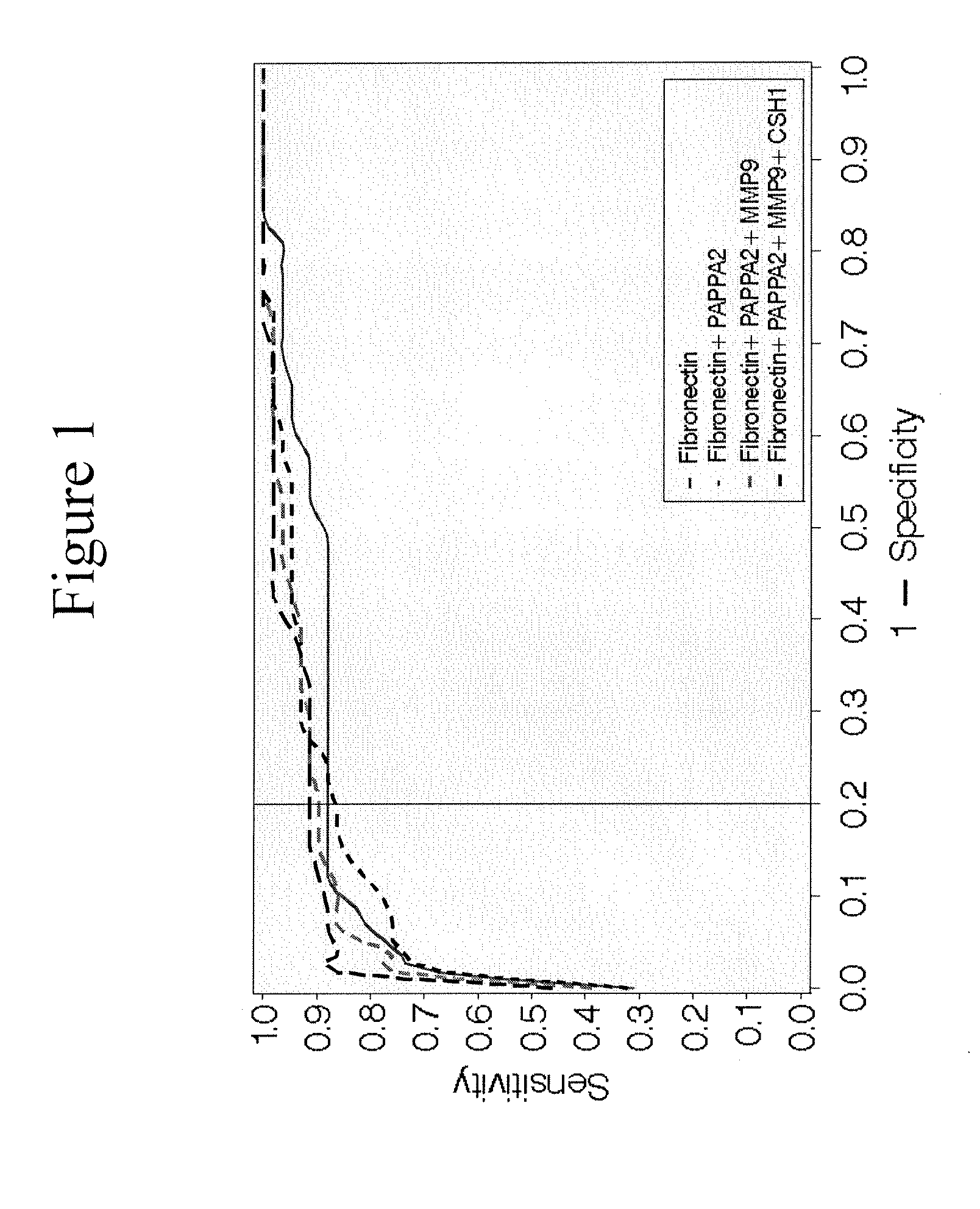
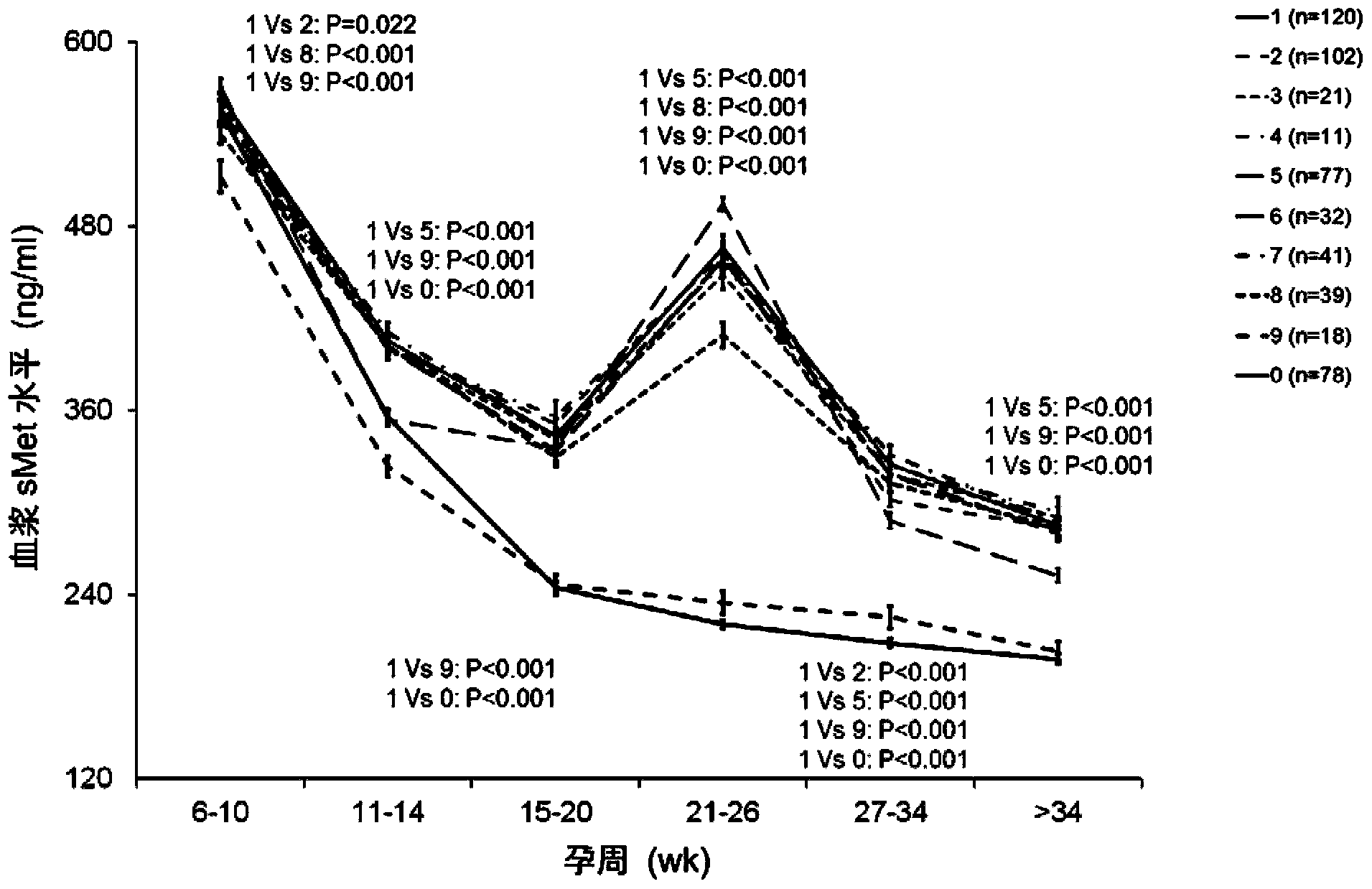
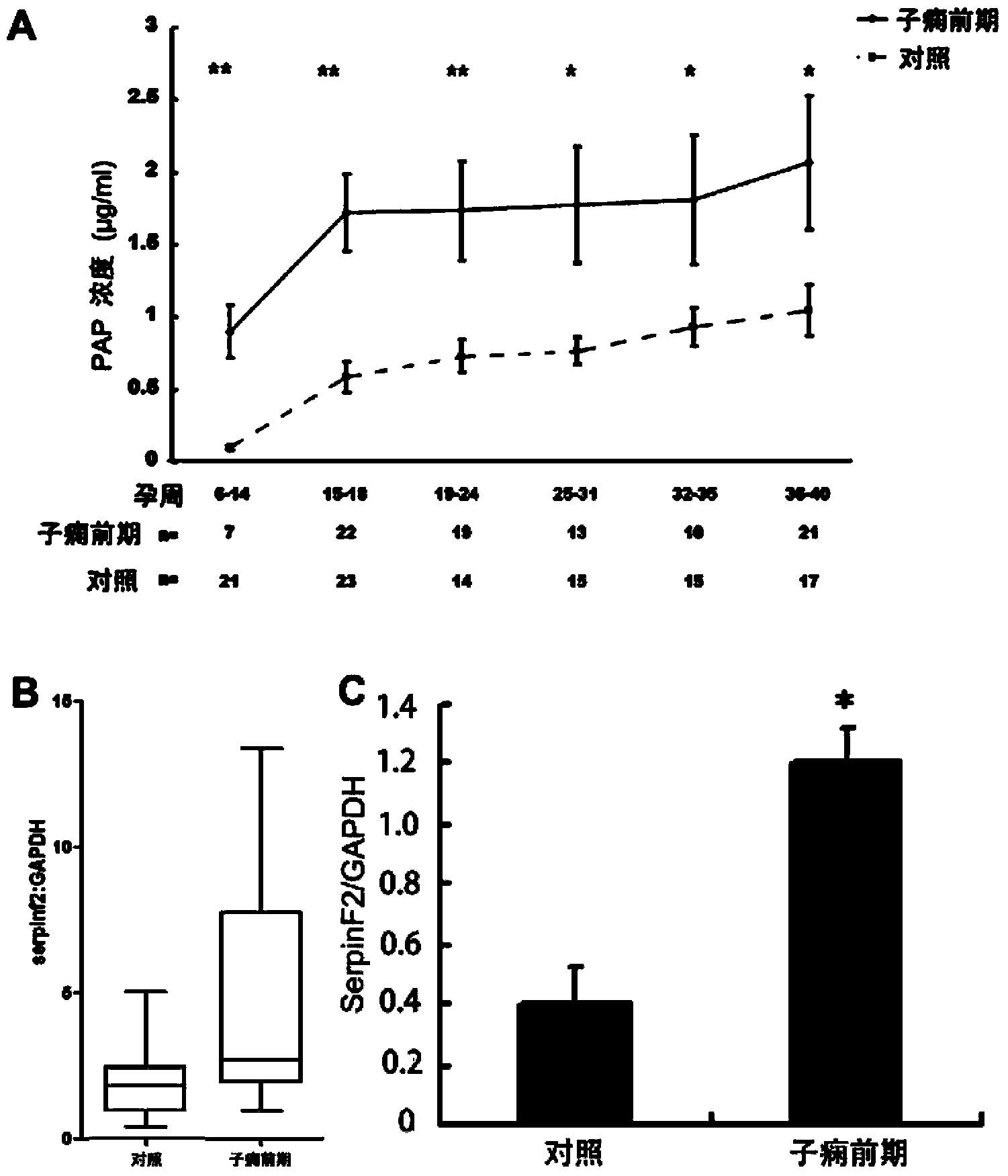
The invention provides a test kit for prediction or early diagnosis of hypertension of pregnancy. The test kit is used for detecting markers in the plasma, serum, urine or placenta of a pregnant woman after 6-week pregnancy, and the markers are at least one of sMet, SerpinF2, testosterone and estradiol. Through large sample size validation, the level of sMet in the plasma of a pregnant woman suffered from preeclampsia or having high preeclampsia susceptibility after 14-week pregnancy is significantly lower than that of a normal pregnant woman, and the level of SerpinF2 or To / E2 in the plasma of a pregnant woman suffered from preeclampsia or having high preeclampsia susceptibility is significantly higher than that of a normal pregnant woman. The result is far better than the results of angiogenesis factor (such as VEGF, P1GF) and anti-angiogenic factor (such as sFlt-1 and sEndoglin) which are regarded as the currently best factors for predicting preeclampsia or preeclampsia susceptibility. The test kit provided by the invention is helpful for prediction and diagnosis of preeclampsia.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com