Test kit for prediction or early diagnosis of hypertension of pregnancy
A technology for gestational hypertension and early diagnosis, applied in disease diagnosis, biological testing, measuring devices, etc., can solve problems such as sensitivity that does not meet clinical requirements and cannot effectively distinguish obstetric diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
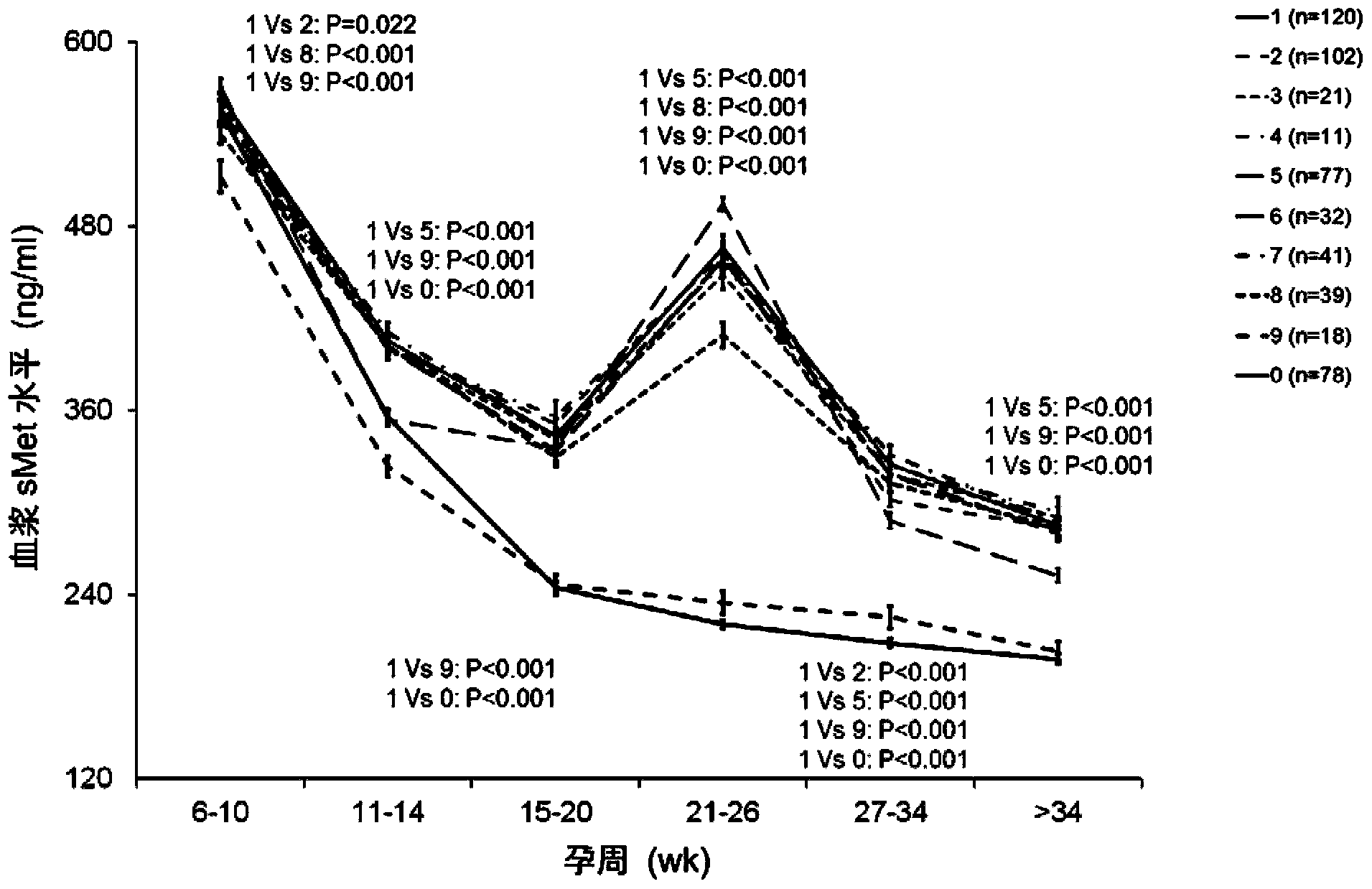
[0044] Example 1 Application of sMet in Prediction or Early Diagnosis of Hypertensive Disorders in Pregnancy
[0045] 1. Experimental design
[0046] The experiment can be divided into two parts: training-validation. The cohort for the training study included 3336 pregnant Chinese Han women. The plasma sMet levels of these pregnant women were measured at 6-10, 11-14, 15-20, 21-26, 27-34 weeks and >34 weeks of pregnancy. The validation study cohort included 62 Chinese Han women with normal pregnancy and 62 Chinese Han women with preeclampsia. The plasma sMet levels of these pregnant women were measured at 14-20 weeks of gestation.
[0047] The study was approved by the Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences, the Ethics Committee of the Third Affiliated Hospital of Peking University, the Ethics Committee of the First Affiliated Hospital of Peking University, and the Ethics Committee of Beijing Miyun Hospital. Patients participating in the s...
Embodiment 2
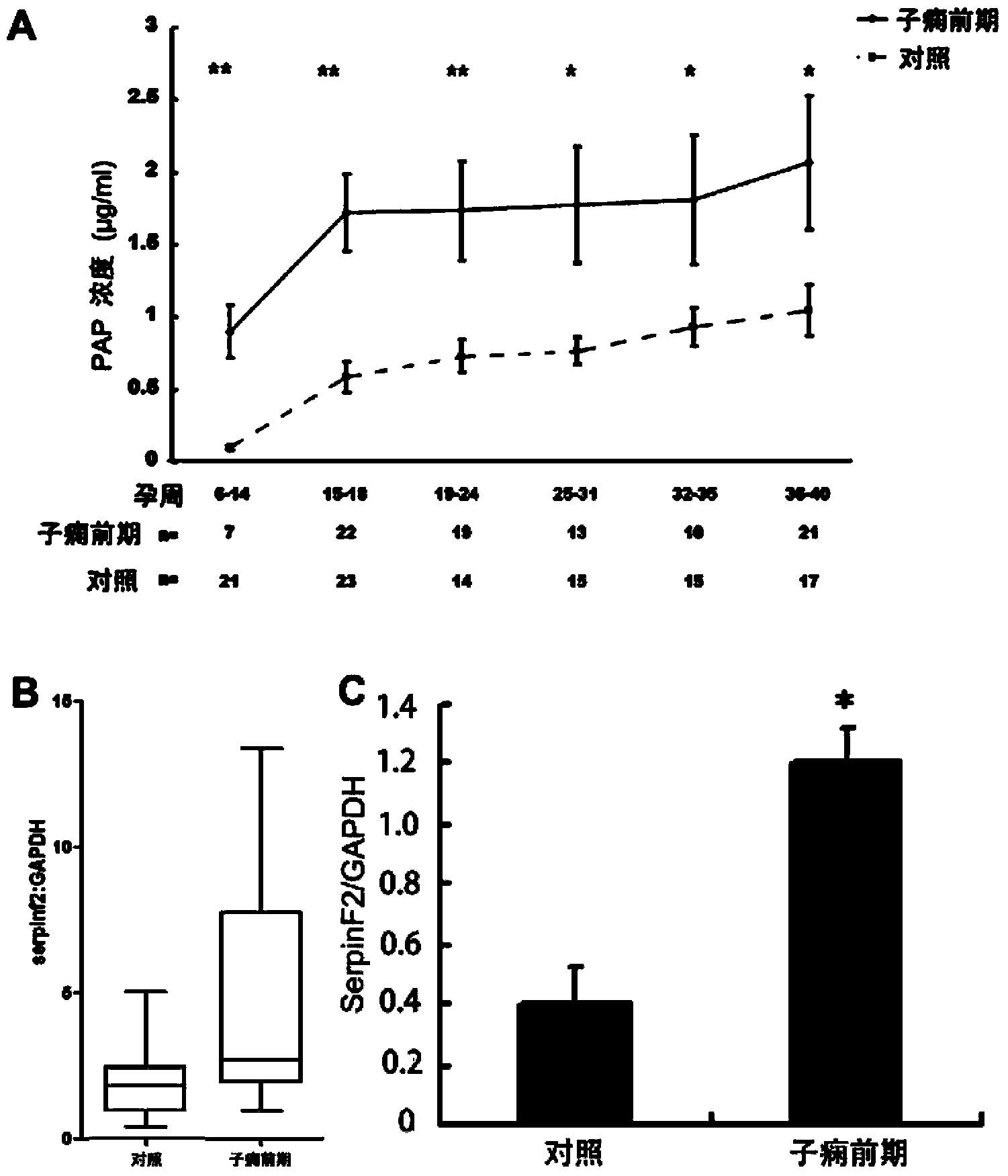
[0069] Example 2 Application of SerpinF2 in Prediction or Early Diagnosis of Hypertensive Disorders in Pregnancy
[0070] 1. Case selection and plasma sample collection
[0071] This study was a prospective nested case-control study, and the research protocol was approved by the Institute of Zoology, Chinese Academy of Sciences and the Ethics Committee of Peking University First Hospital. The research subjects were pregnant women who received prenatal care in Peking University First Hospital from November 2005 to December 2007. The peripheral blood of pregnant women was collected at different stages of pregnancy. After delivery, the subjects were divided into normal pregnancy group according to the pregnancy outcome. and severe preeclampsia group. The selected research objects excluded chronic hypertension, diabetes, history of kidney disease, twins, hepatitis, tumor and other diseases and medical history; the definition standard of normal pregnancy was: all indicators before...
Embodiment 3
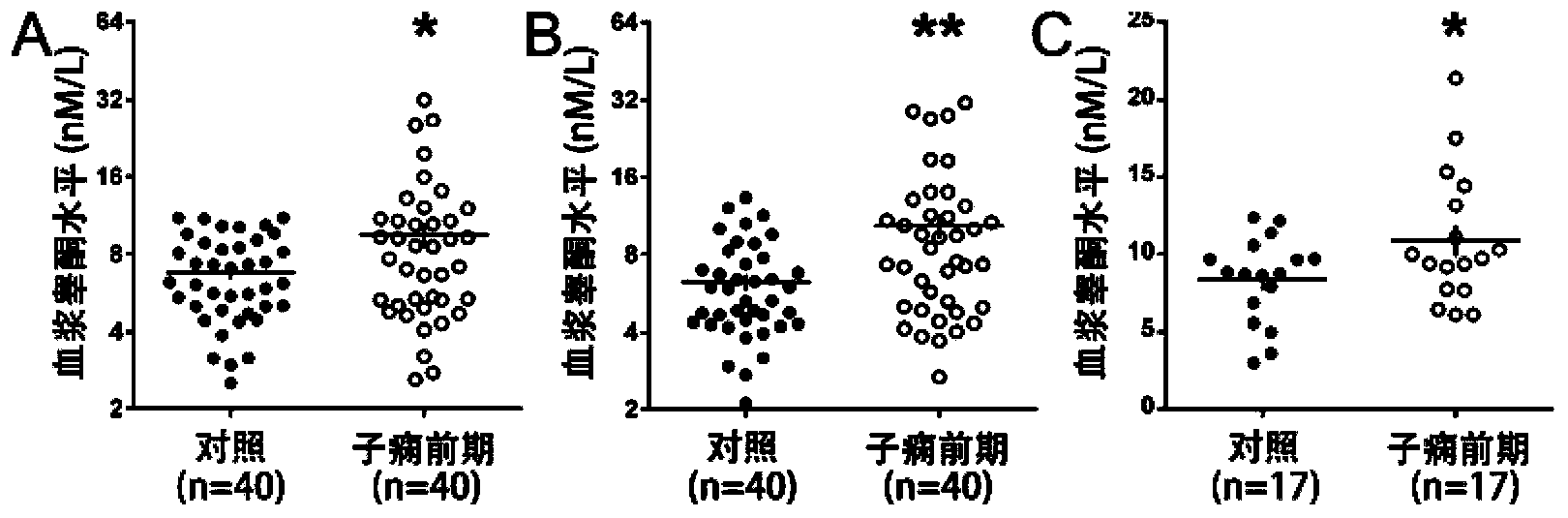
[0079] Example 3 The application of quorone and estradiol in prediction or early diagnosis of hypertensive disorders in pregnancy
[0080] 1. Cases and samples involved in the experiment
[0081]The prospective cohort involved in the study came from the regular antenatal care population of Peking University Third Hospital from June 2009 to December 2010. The population included 3336 pregnant women, including 194 multiple pregnancies, 5 cases were younger than 18 years old or older than 45 years old, 23 cases gave birth before 28 weeks of gestation, and 784 cases had other diseases not related to hypertension. All the above cases were excluded from this study. Of the remaining 2330 cases, 1911 were normal pregnancies, 78 were severe preeclampsia, and the rest were other pregnancy complications. All cases in this population collected 2mL of peripheral blood and obtained plasma at 6-10, 11-14, 15-20, 21-26, 27-34 weeks and >34 weeks of pregnancy. The collected plasma was store...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com