Patents
Literature
60 results about "CD117" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mast/stem cell growth factor receptor (SCFR), also known as proto-oncogene c-KIT or tyrosine-protein kinase KIT or CD117, is a receptor tyrosine kinase protein that in humans is encoded by the KIT gene. Multiple transcript variants encoding different isoforms have been found for this gene. KIT was first described by the German biochemist Axel Ullrich in 1987 as the cellular homolog of the feline sarcoma viral oncogene v-kit.
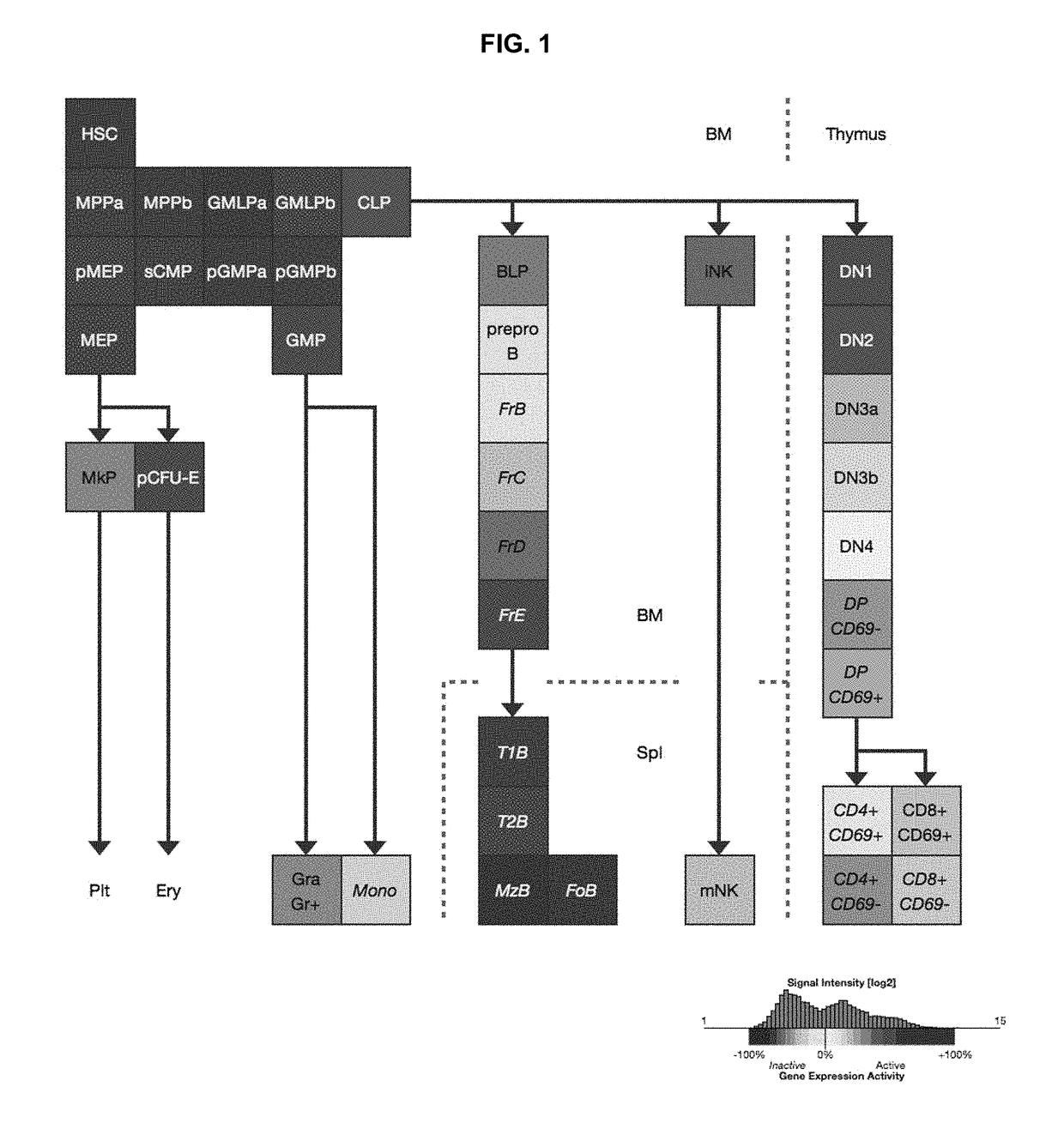
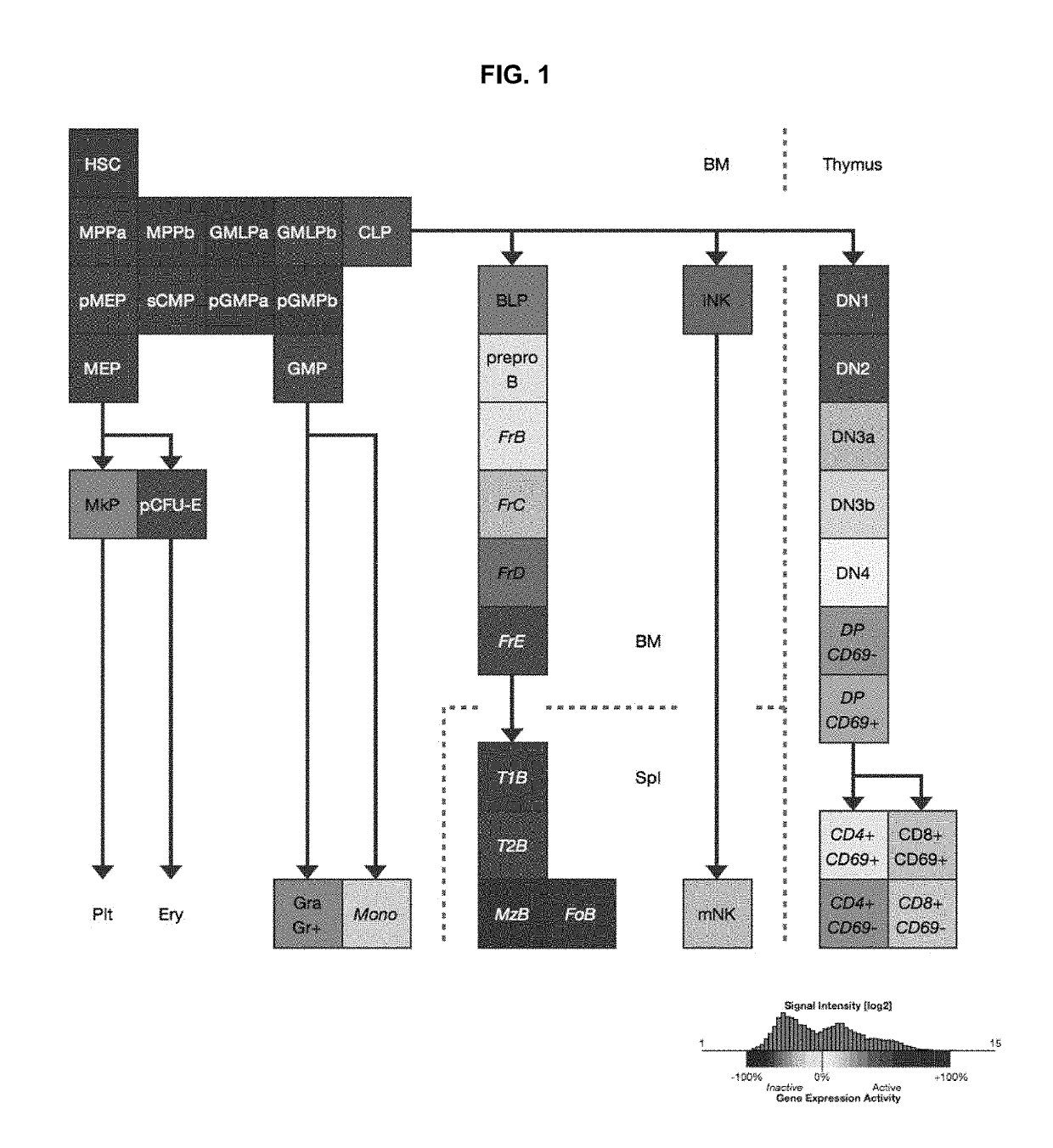
Mammalian common lymphoid progenitor cell
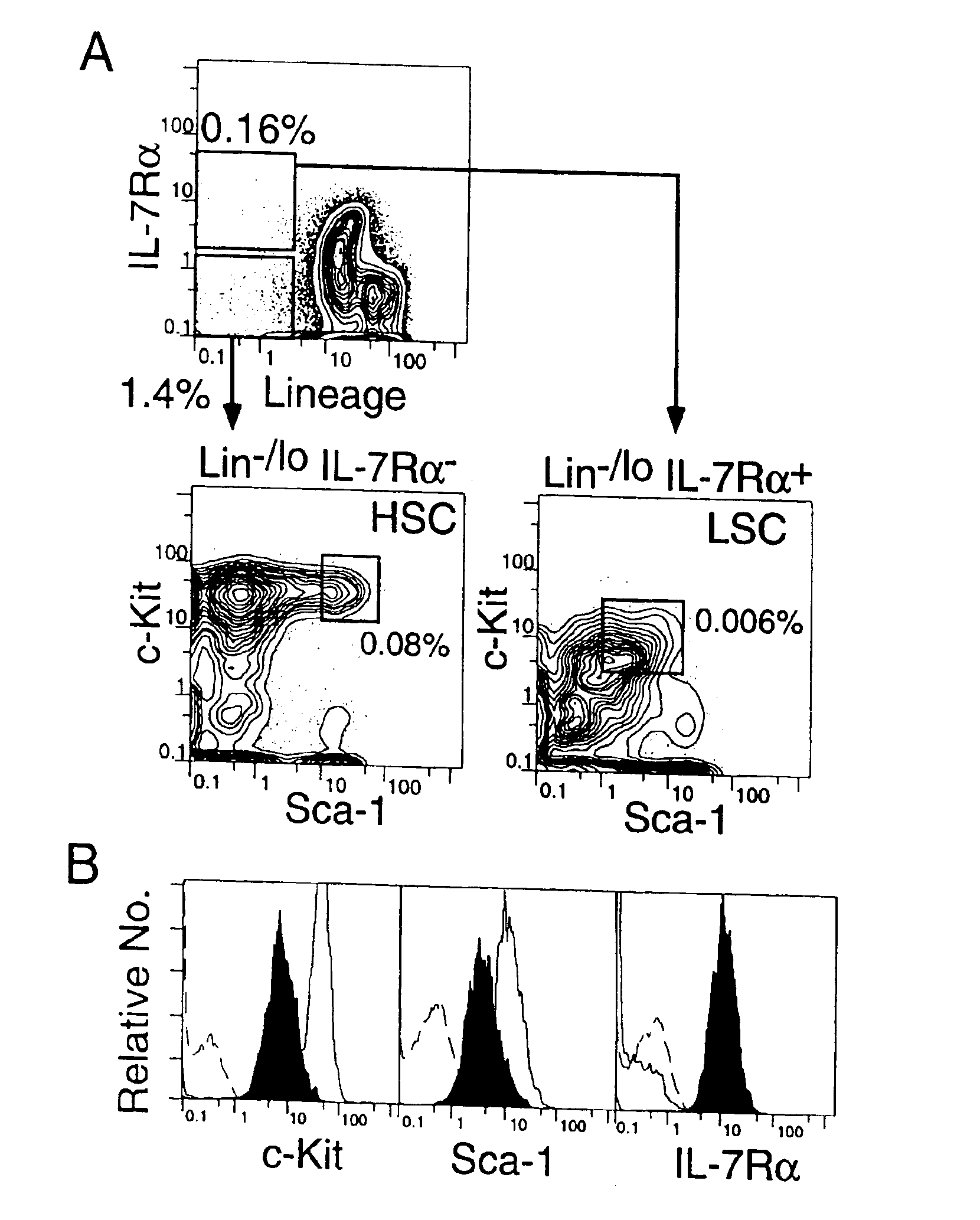
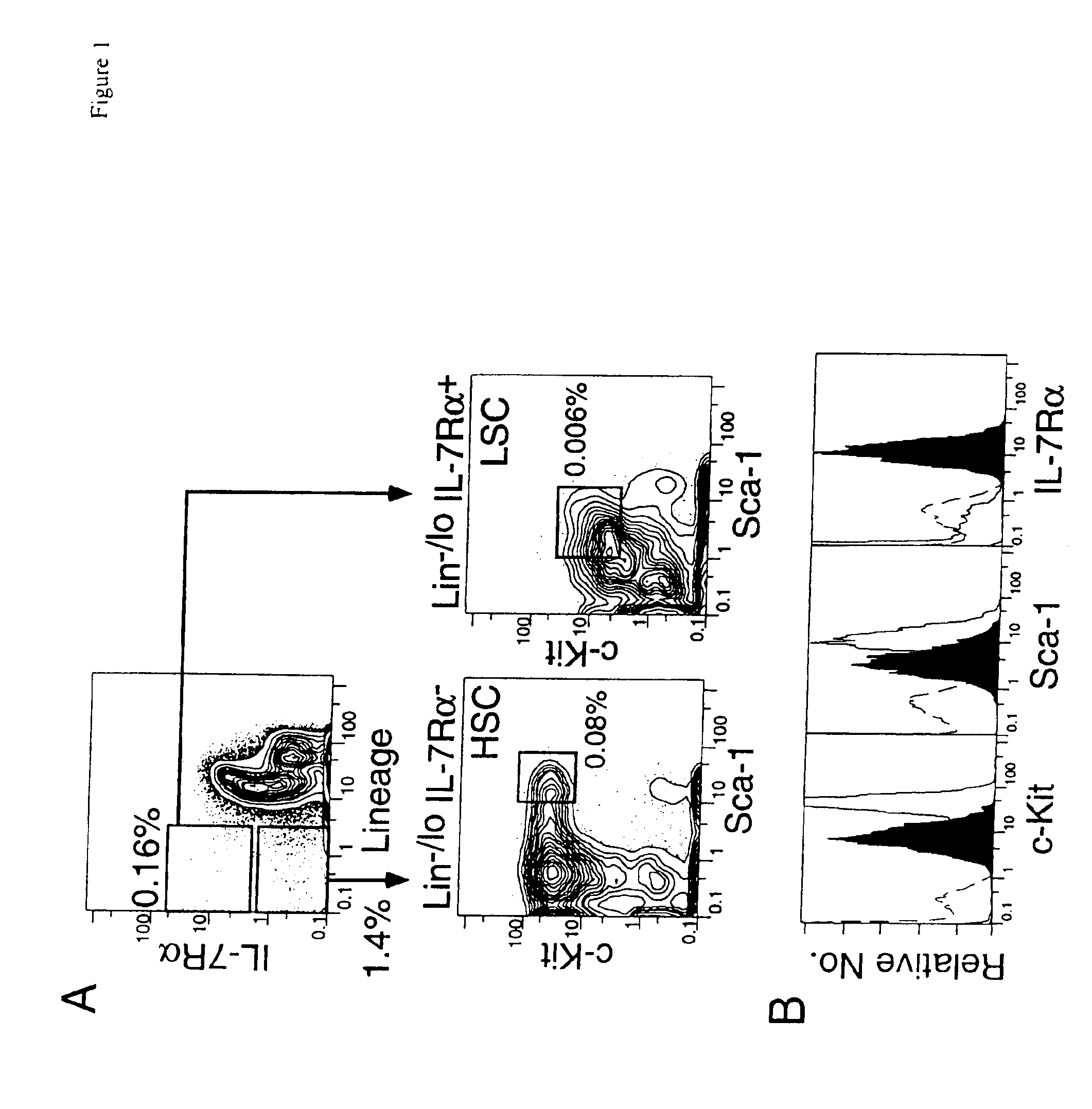
A substantially enriched mammalian hematopoietic cell subpopulation is provided, which is characterized by progenitor cell activity for lymphoid lineages, but lacking the potential to differentiate into myeloid and erythroid lineages. Methods are provided for the isolation and culture of this common lymphoid progenitor cell (CLP). The cell enrichment methods employ reagents that specifically recognize CDw127 (IL-7 receptor α); CD117 (c-kit) protein, in conjunction with other markers expressed on lineage committed cells. The murine cells are also characterized as expressing low levels of sca-1 (Ly-6E and Ly-6A). The CLPs are predominantly cycling, blast cells. These cells give rise to B cells, T cells and natural killer cells, as evidenced by their growth and differentiation in vitro and in vivo.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
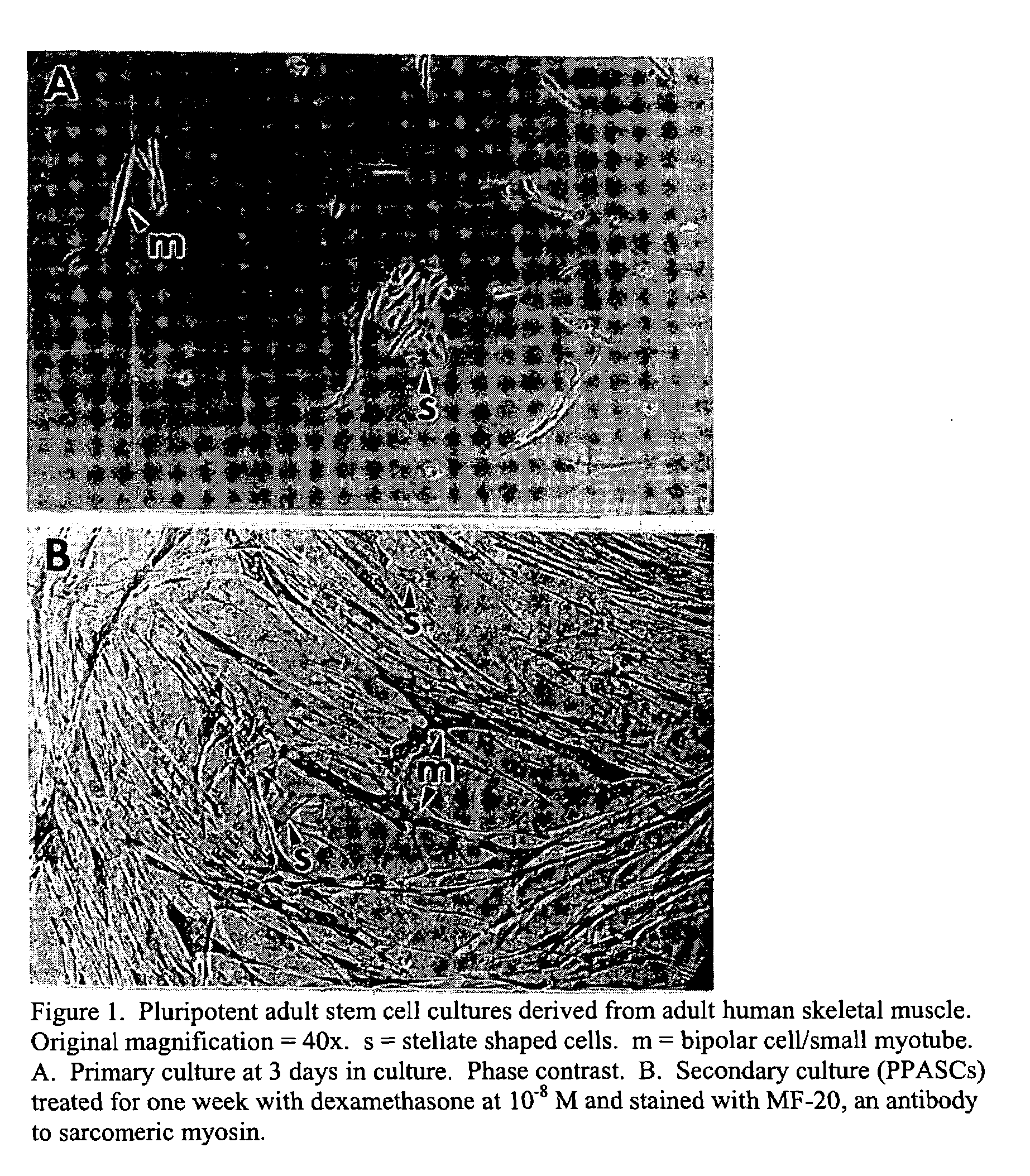
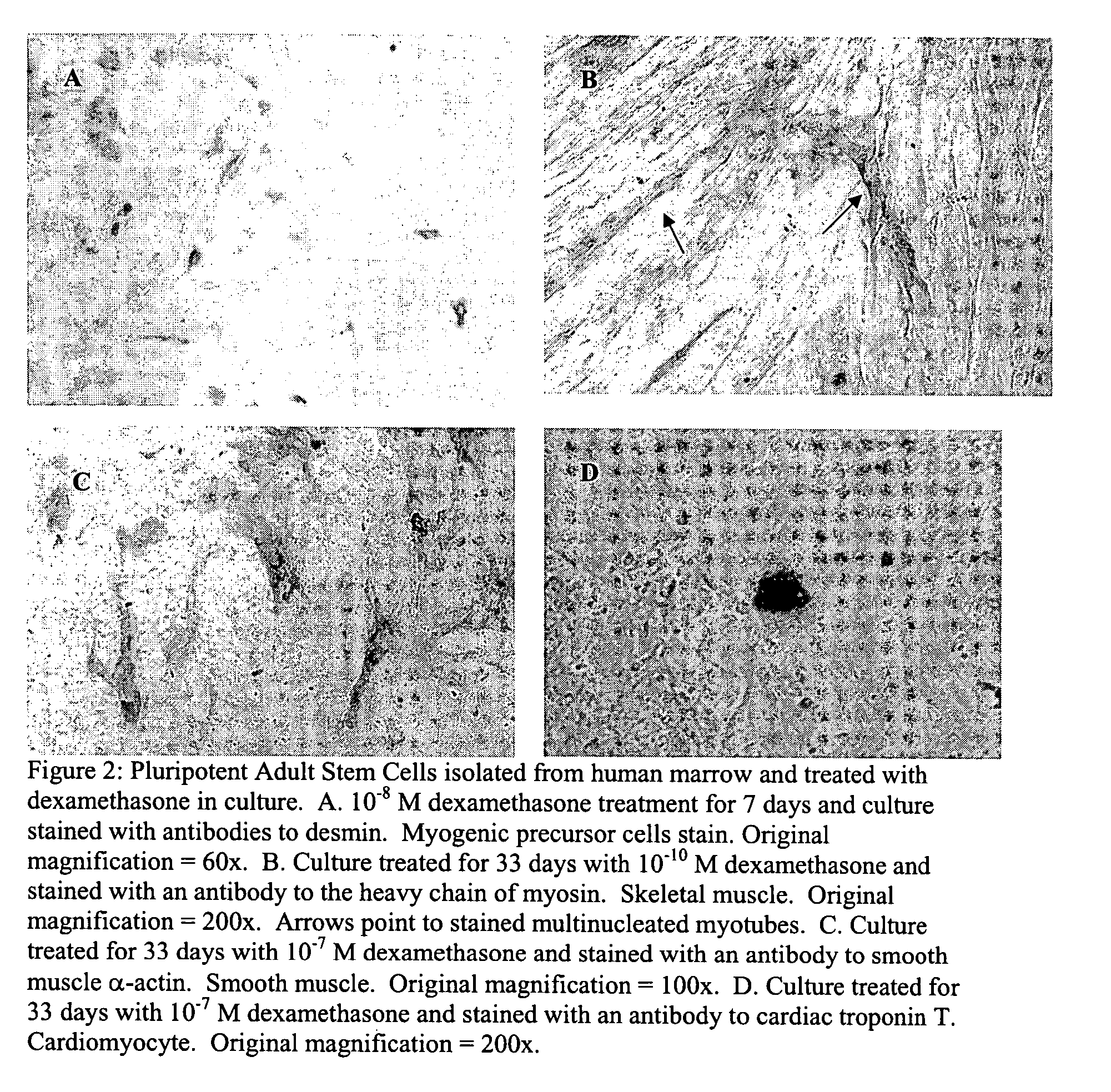
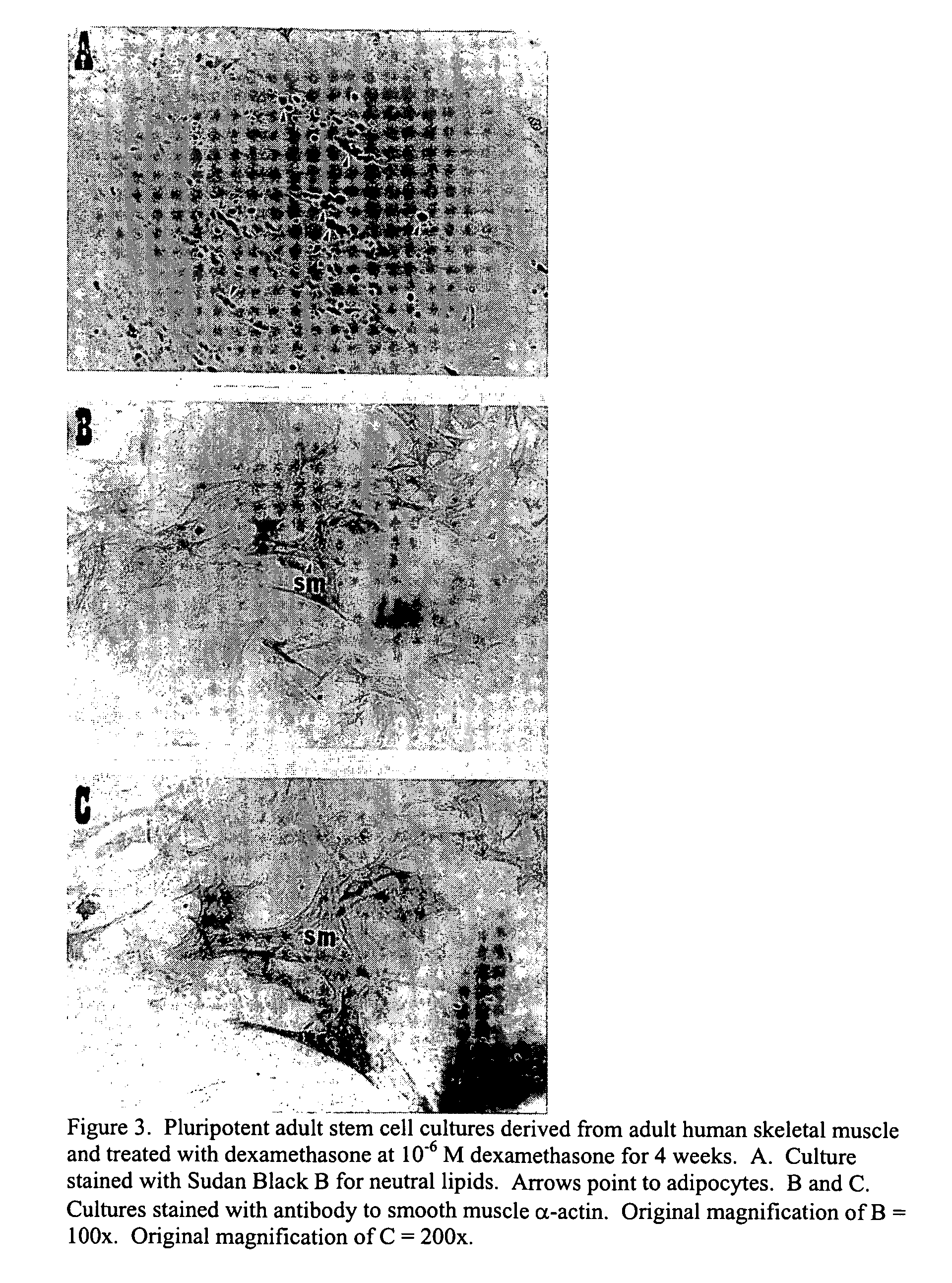
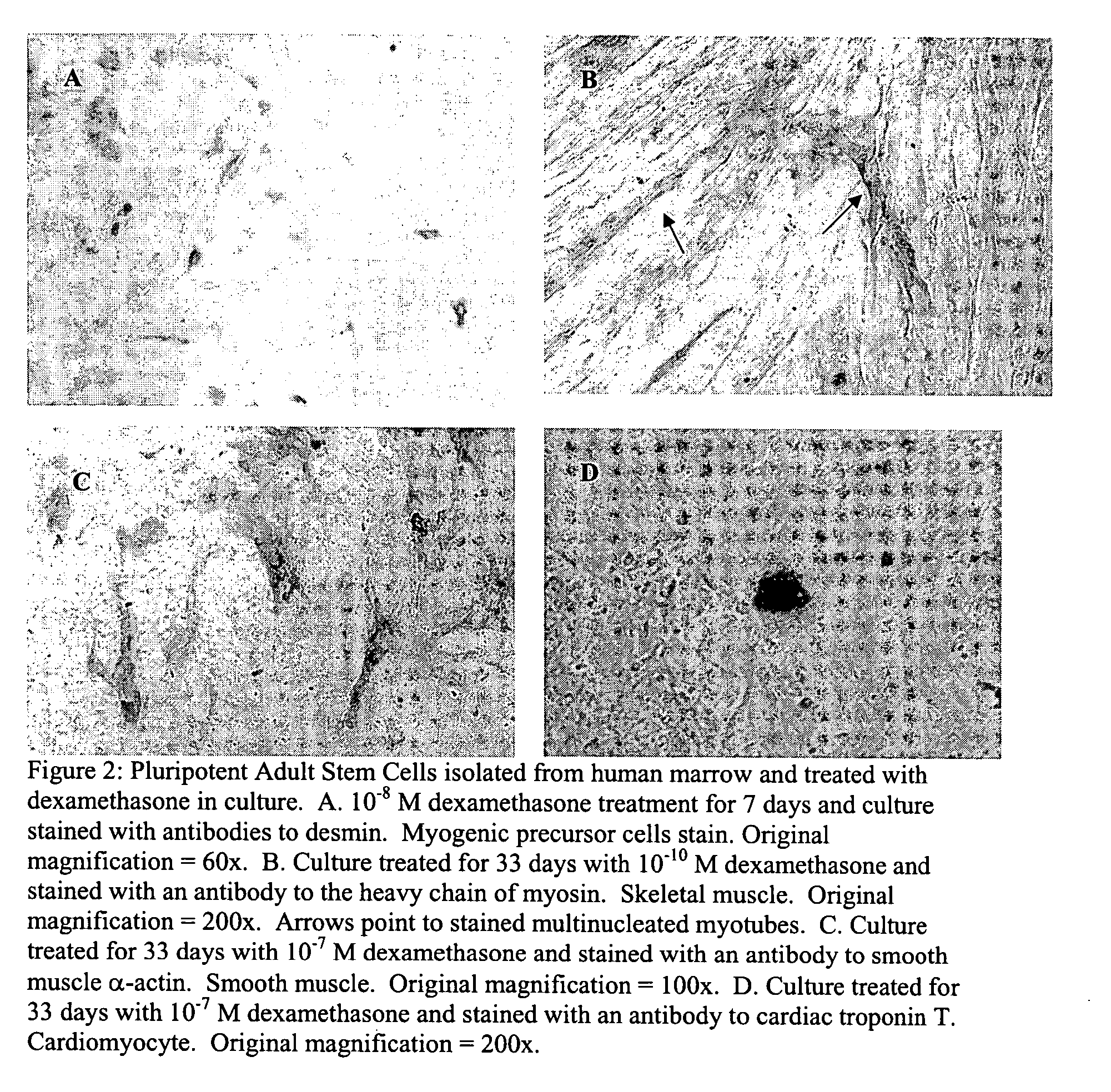
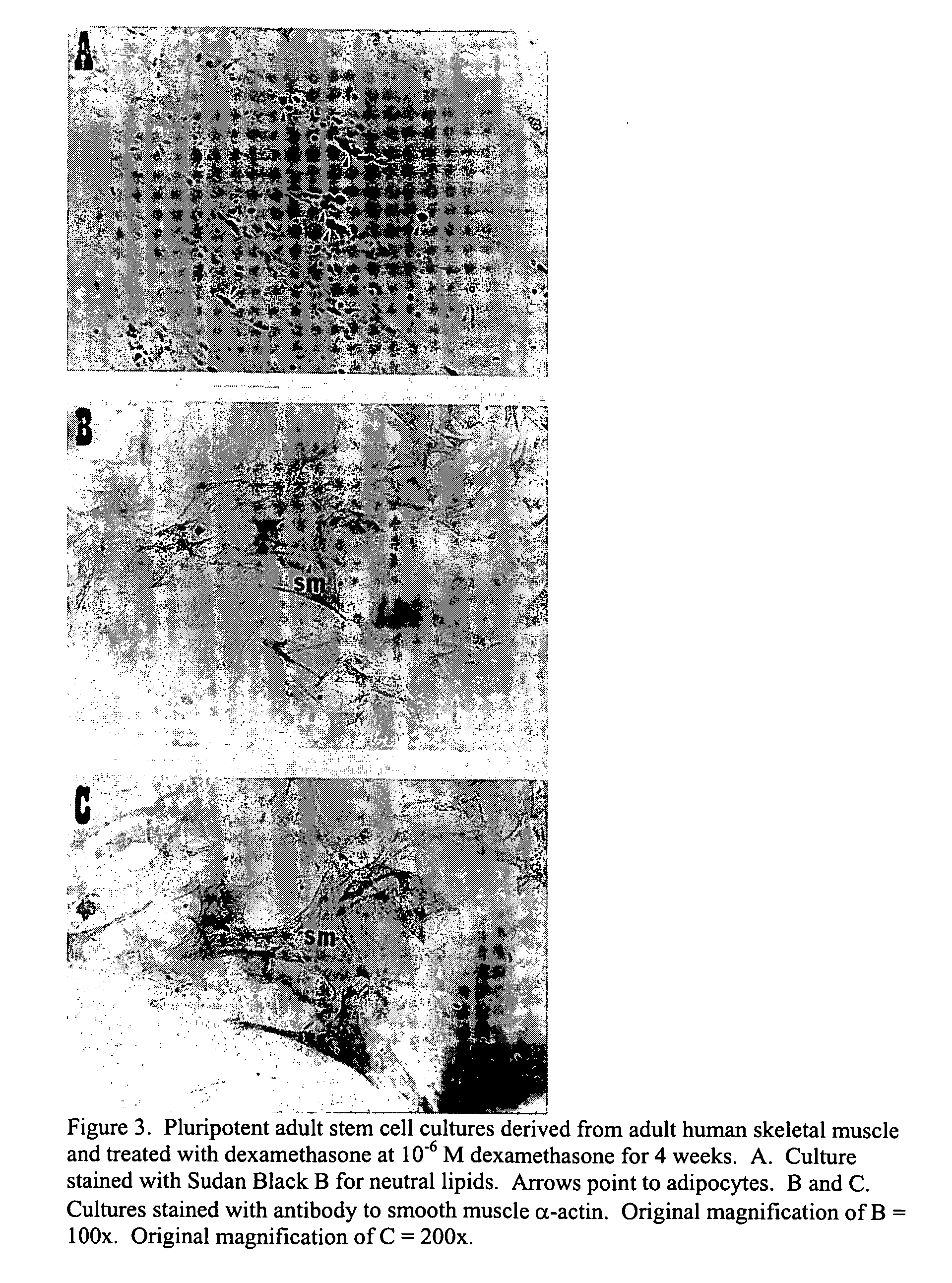
Pluripotent adult stem cells
Owner:NEW YORK MEDICAL COLLEGE
Stem Cell Populations and Methods of Use
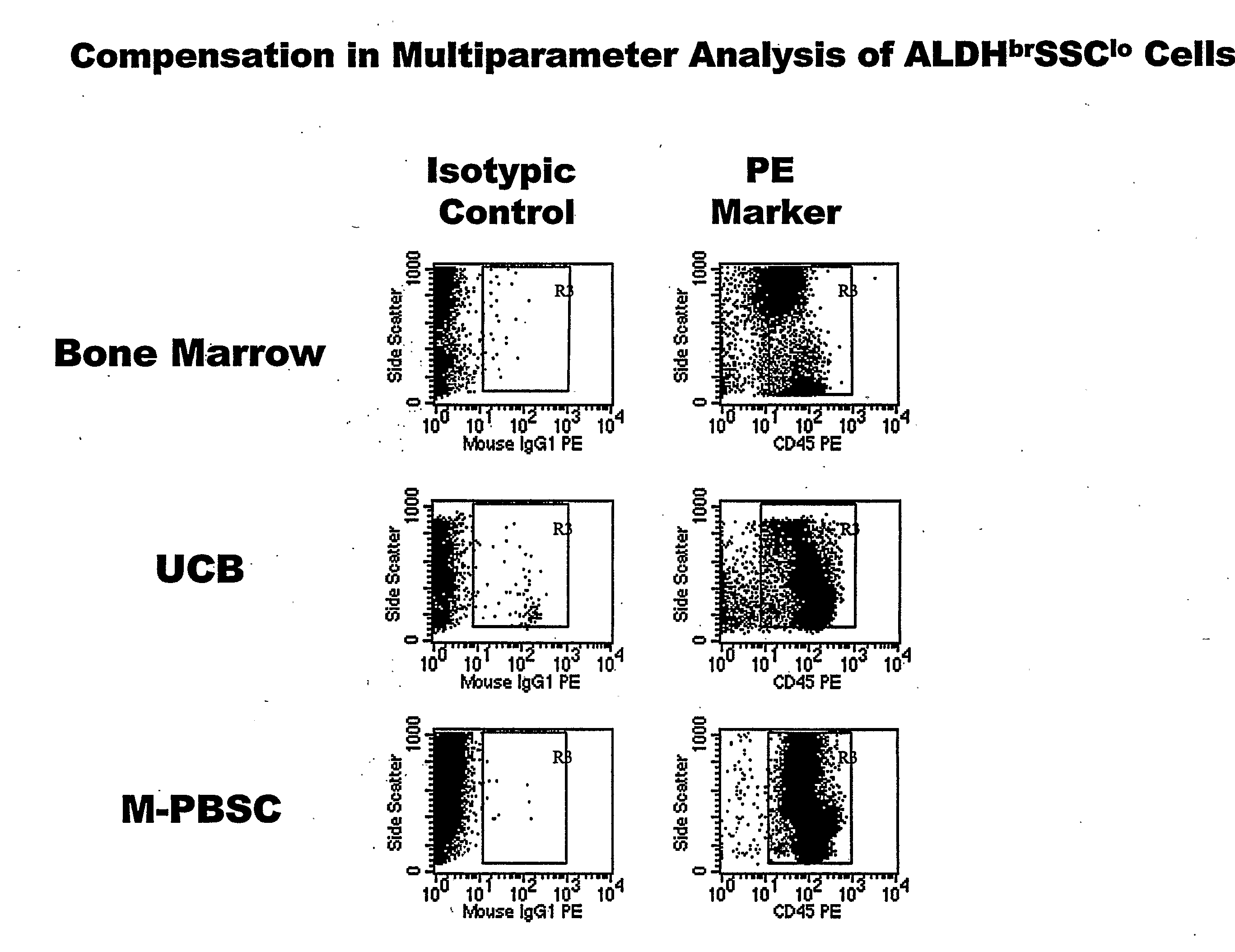
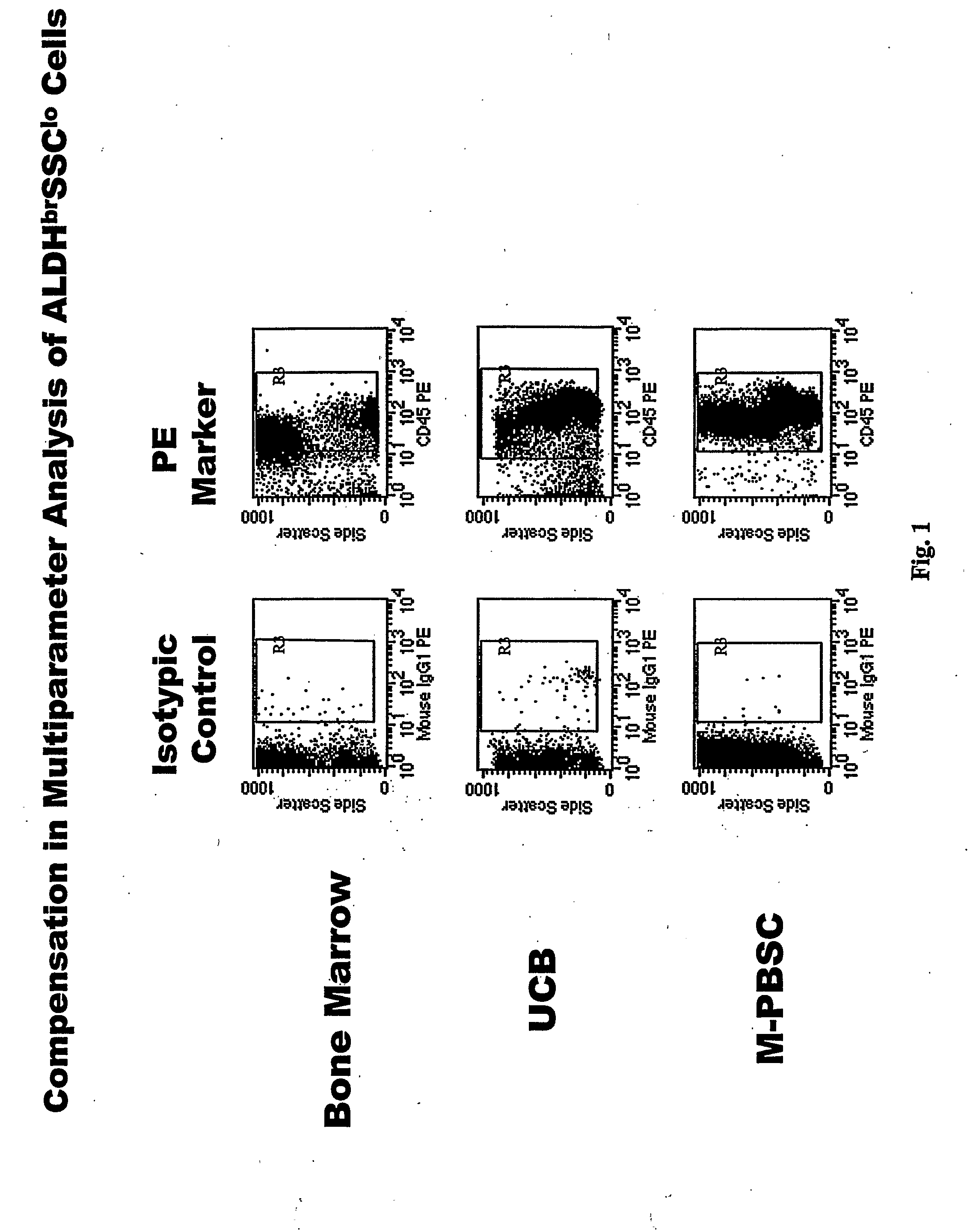
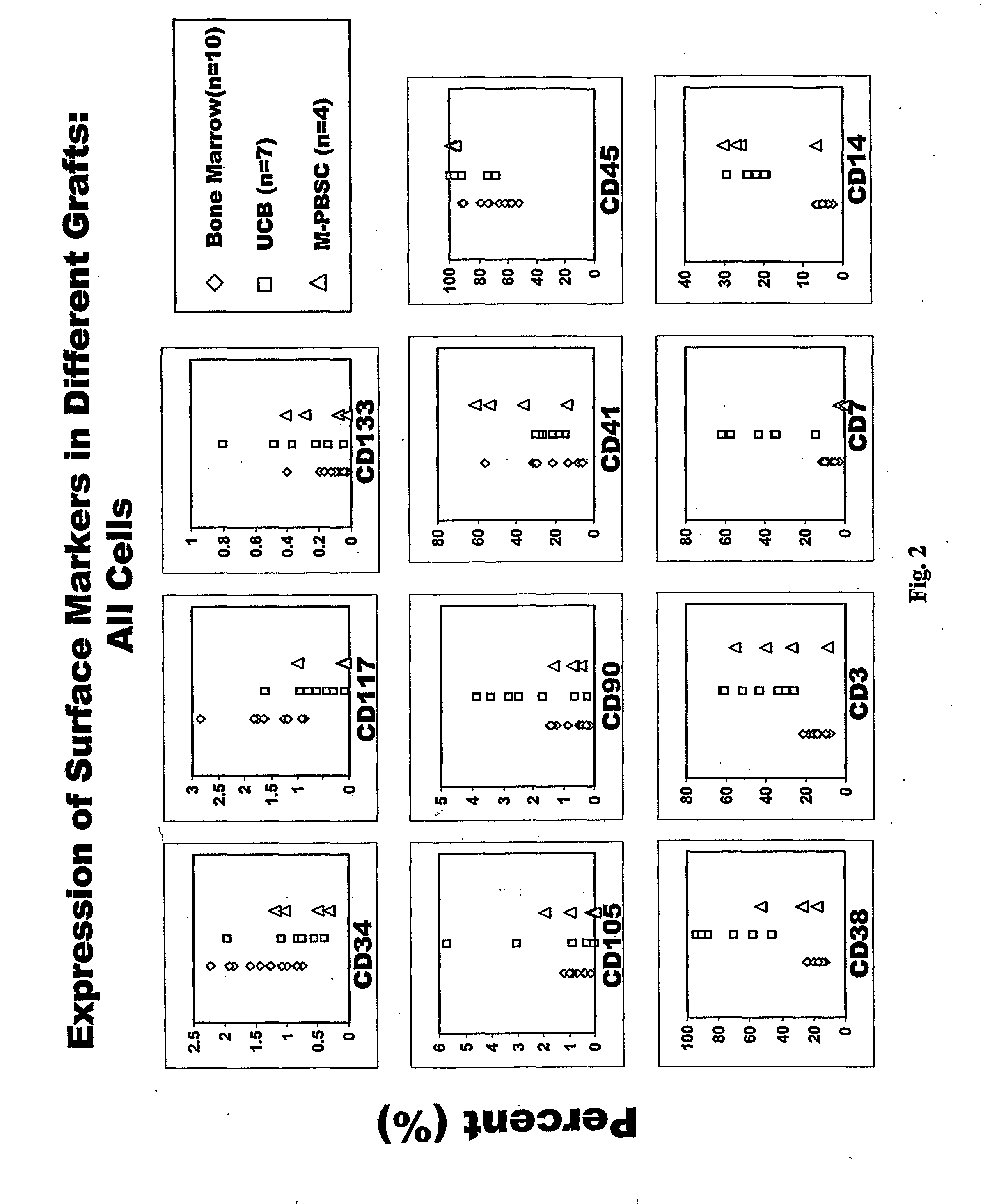
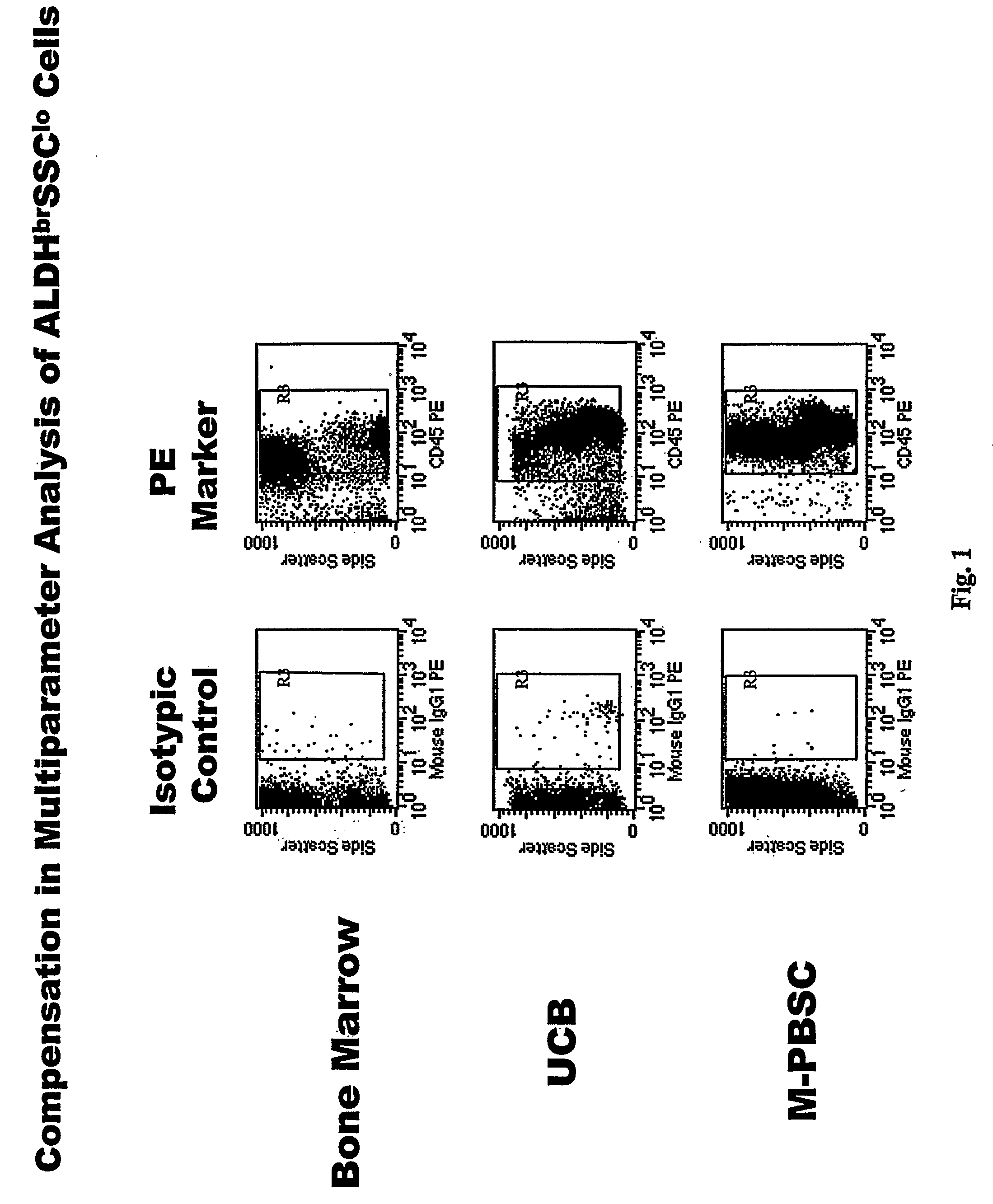
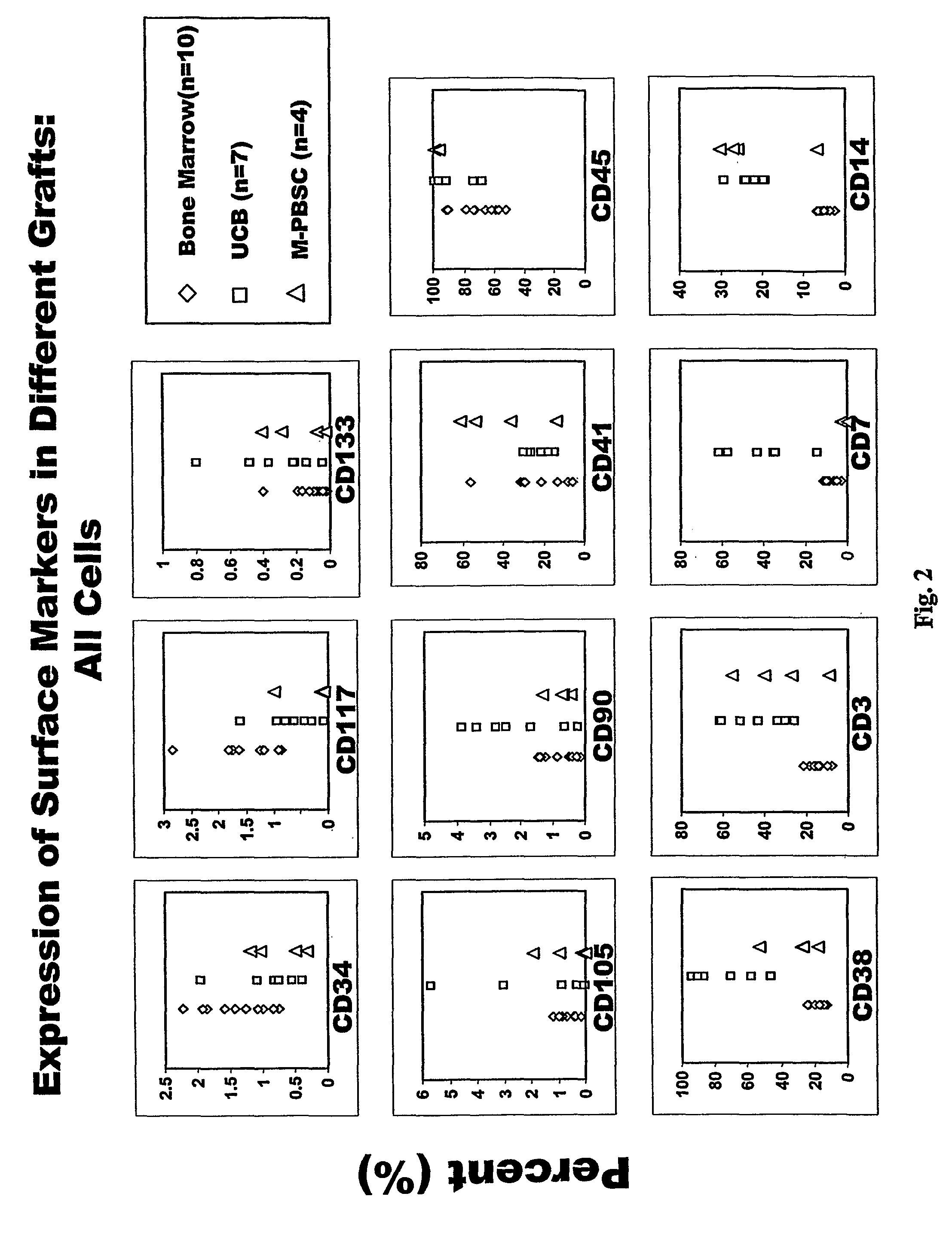
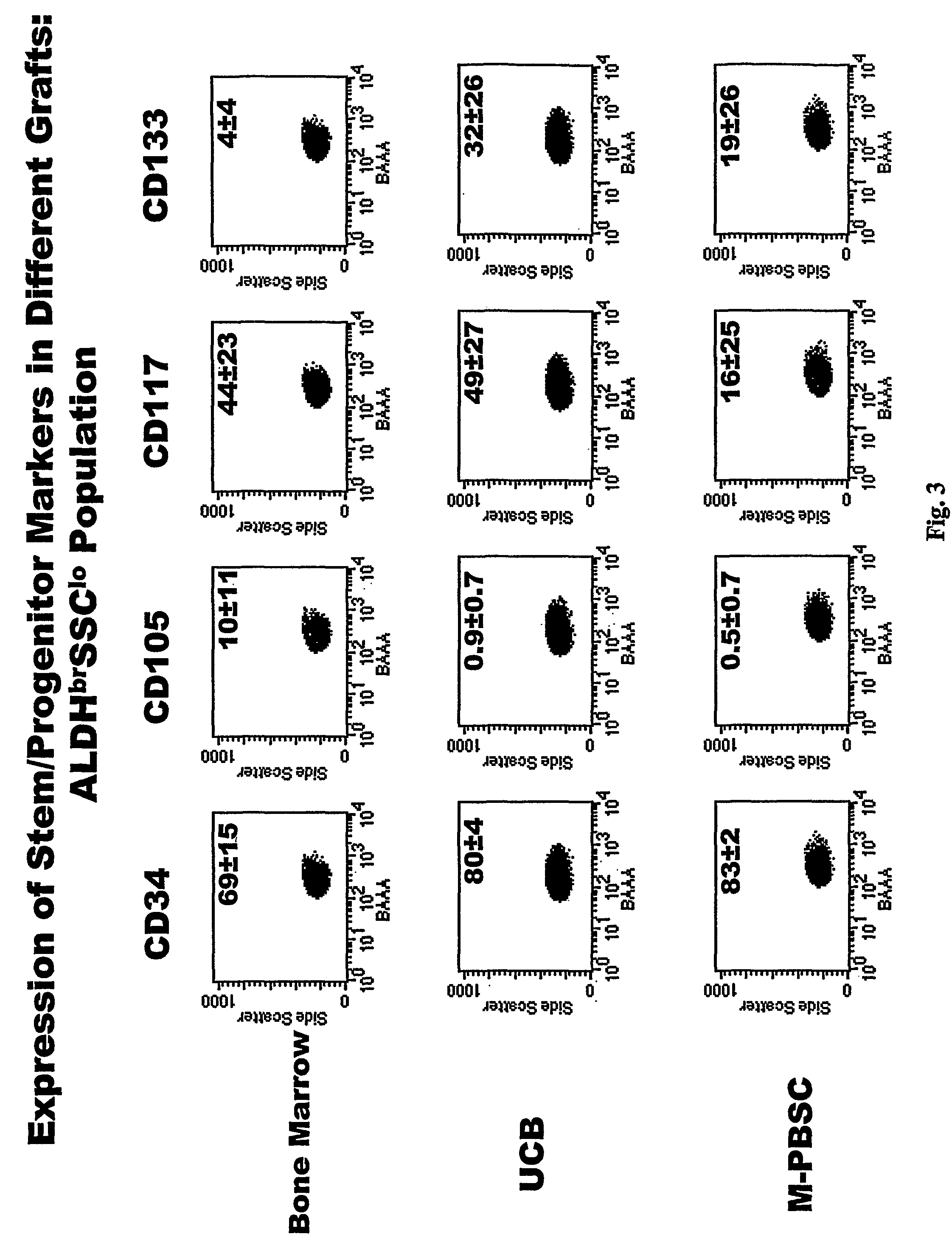
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
Treatment of stroke and other acute neural degenerative disorders via intranasal administration of umbilical cord-derived cells
Owner:DEPUY SYNTHES PROD INC
Human multipotent embryonic stem cell-like progenitor cells
InactiveUS20120094380A1Microbiological testing/measurementBiological particle analysisAntigenProgenitor
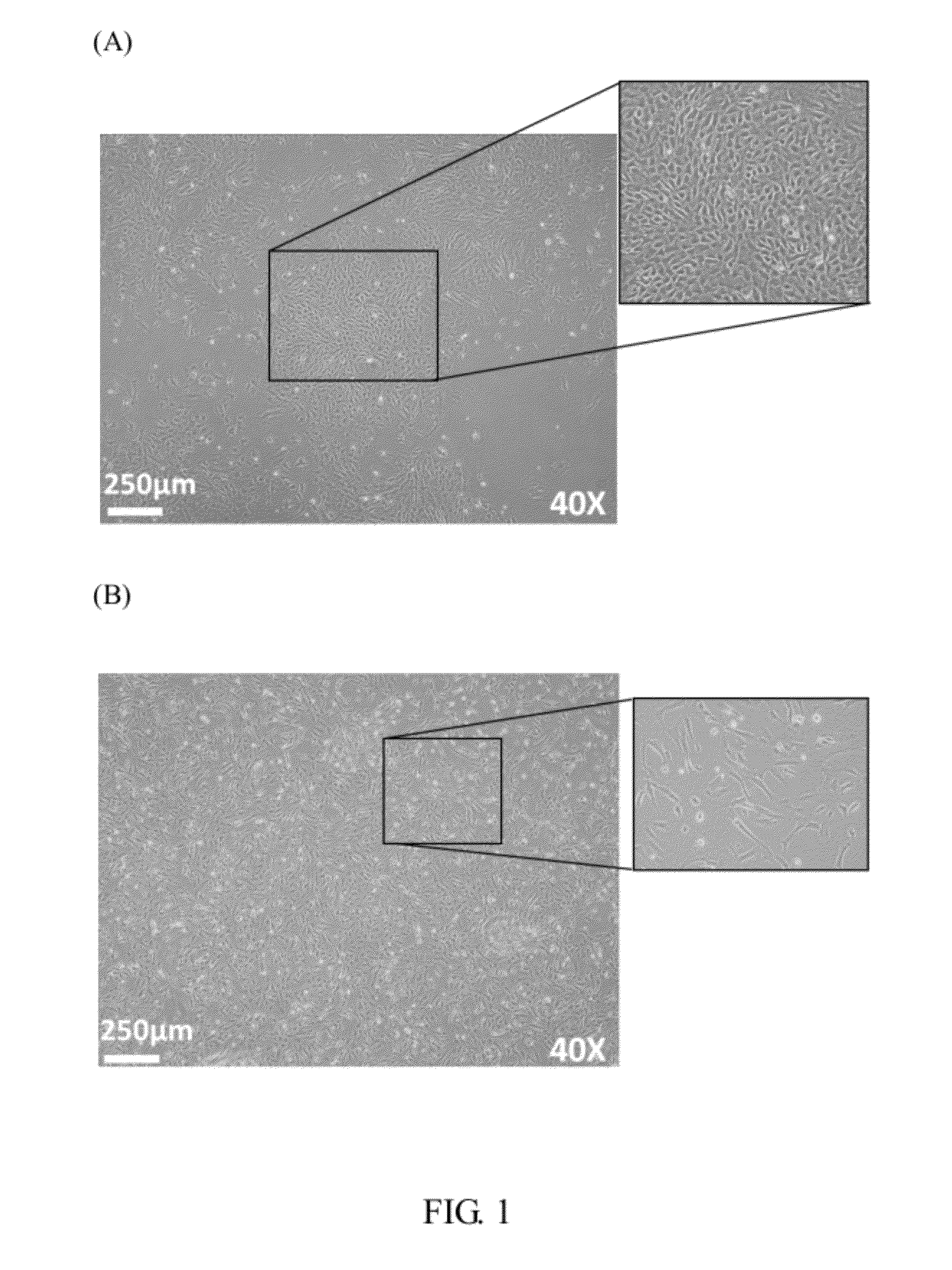
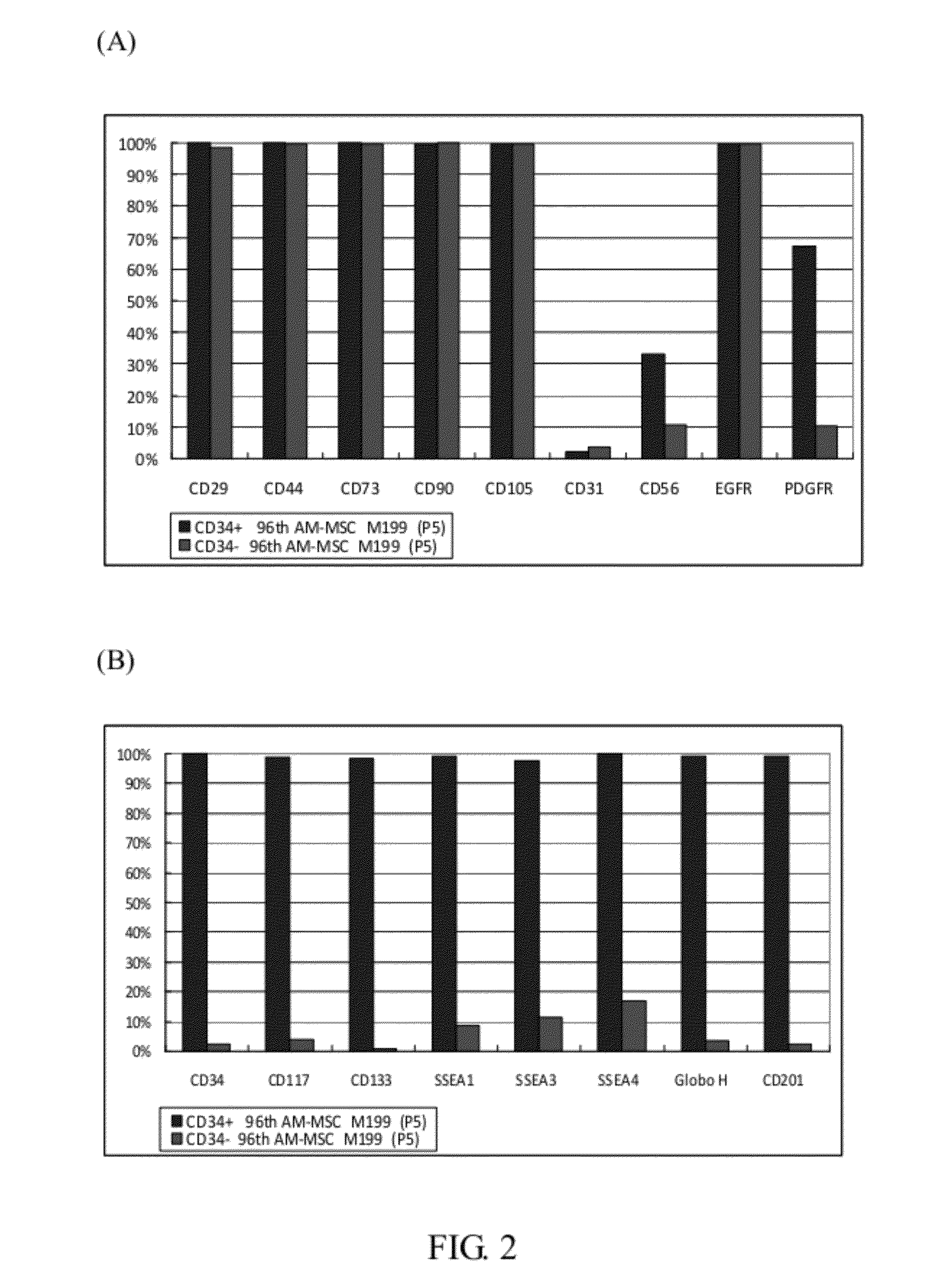
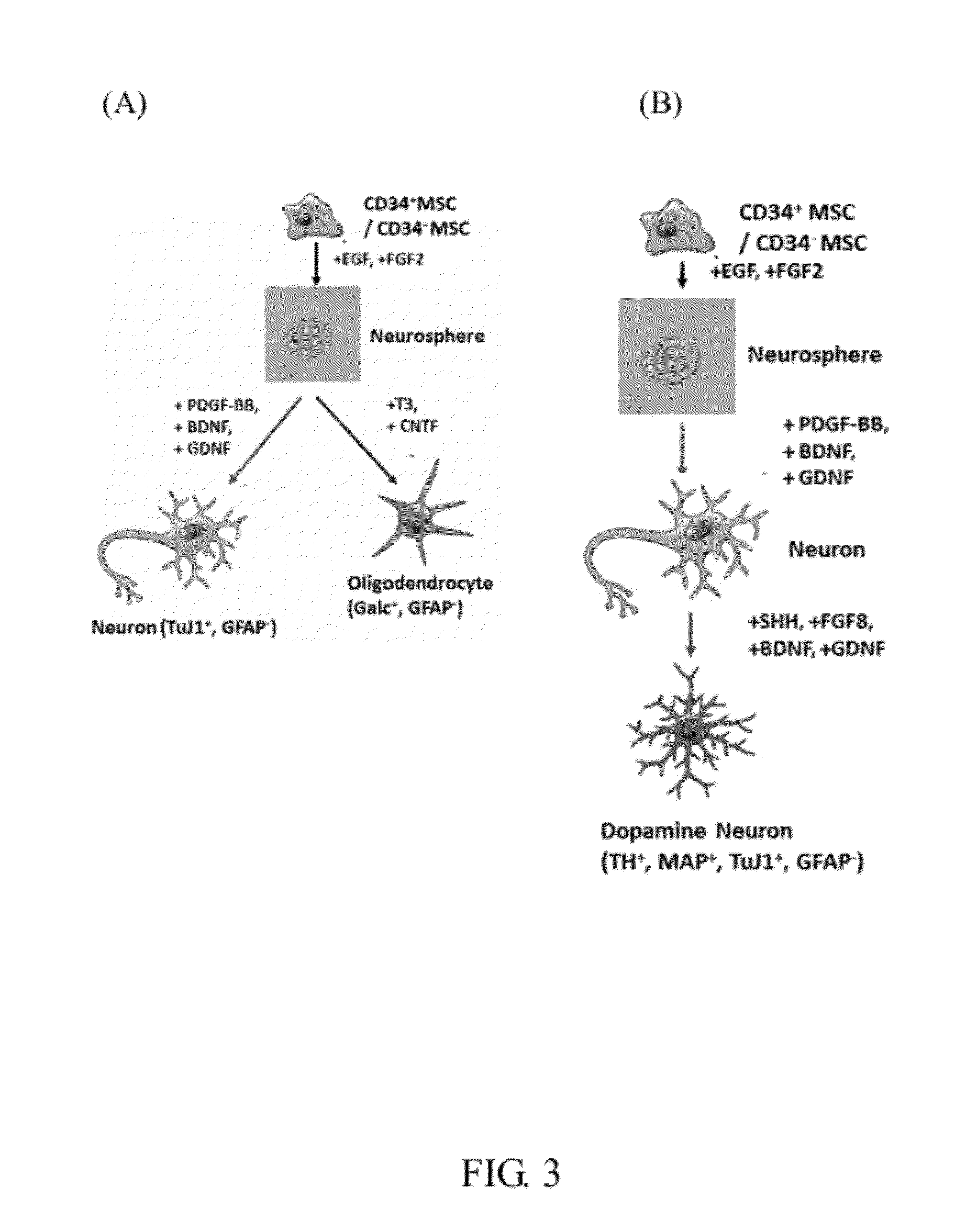
The invention provides a plurality of embryonic stem cell-like progenitor cells, which are isolated from a human tissue by a systemic screening of human mesenchymal stromal stem / progenitor cells and a cell sorting by a cell antigen selected from the group consisting of CD34, CD117, CD133, CD201, GloboH and combination thereof, and cultured in a medium supplemented with at least one or more steroids and one or more growth factors. The cells of the invention express CD34 and exhibit sphere-like clonogenicity in early passages and express multipotent embryonic stem cells (ESCs) like characteristics.
Owner:SUNSHINE LIFE SCI & TECH CORP
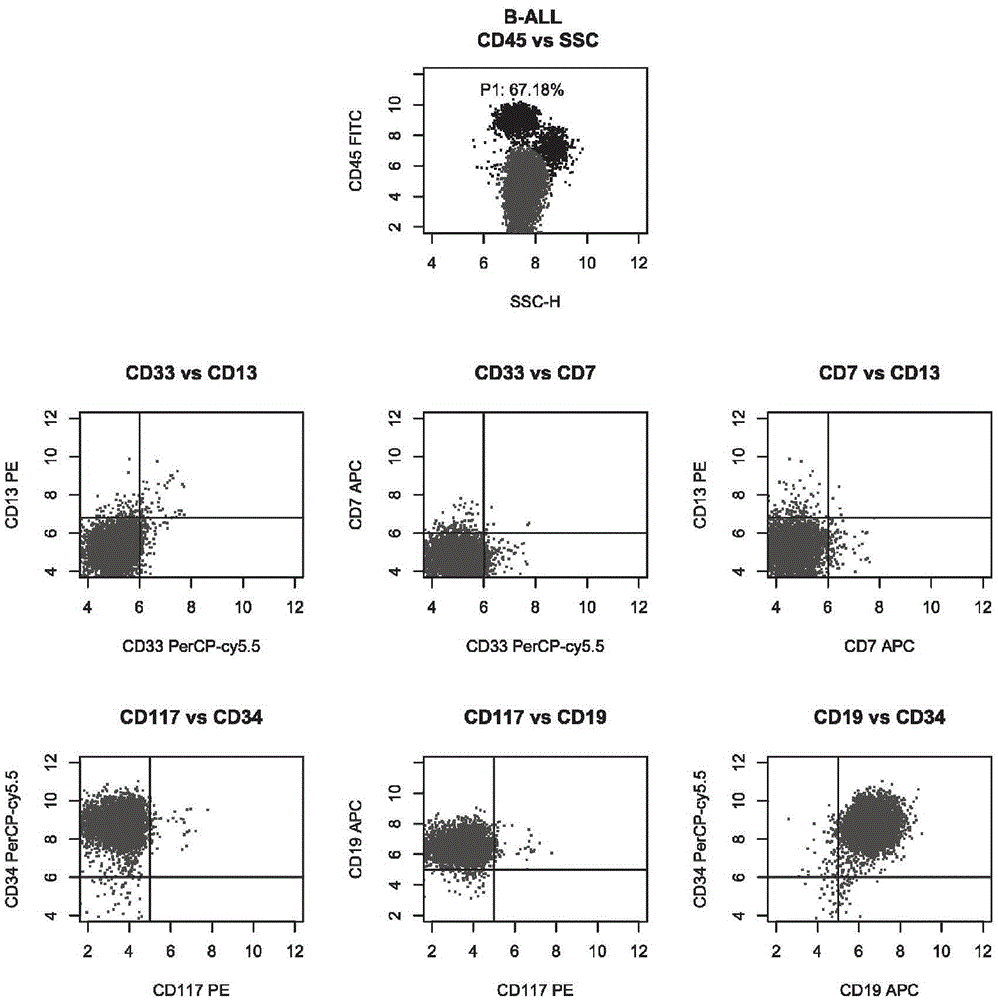
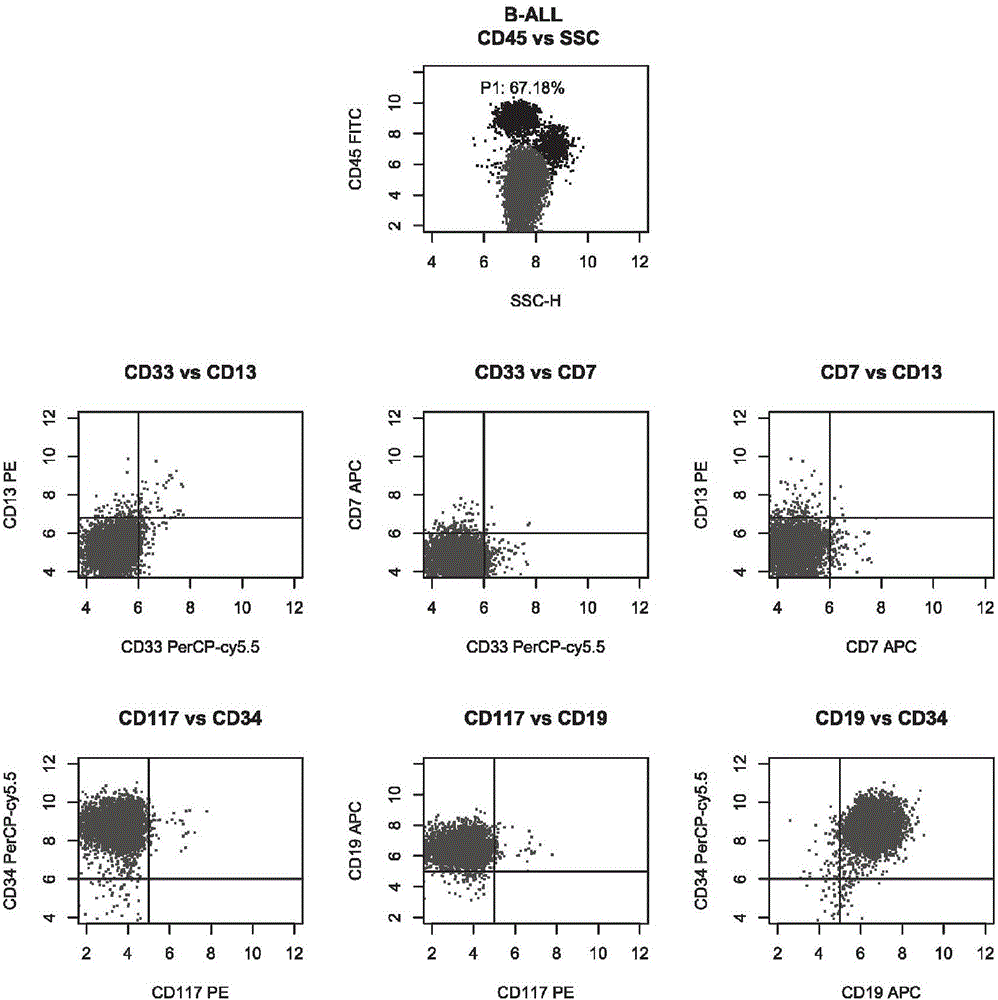
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
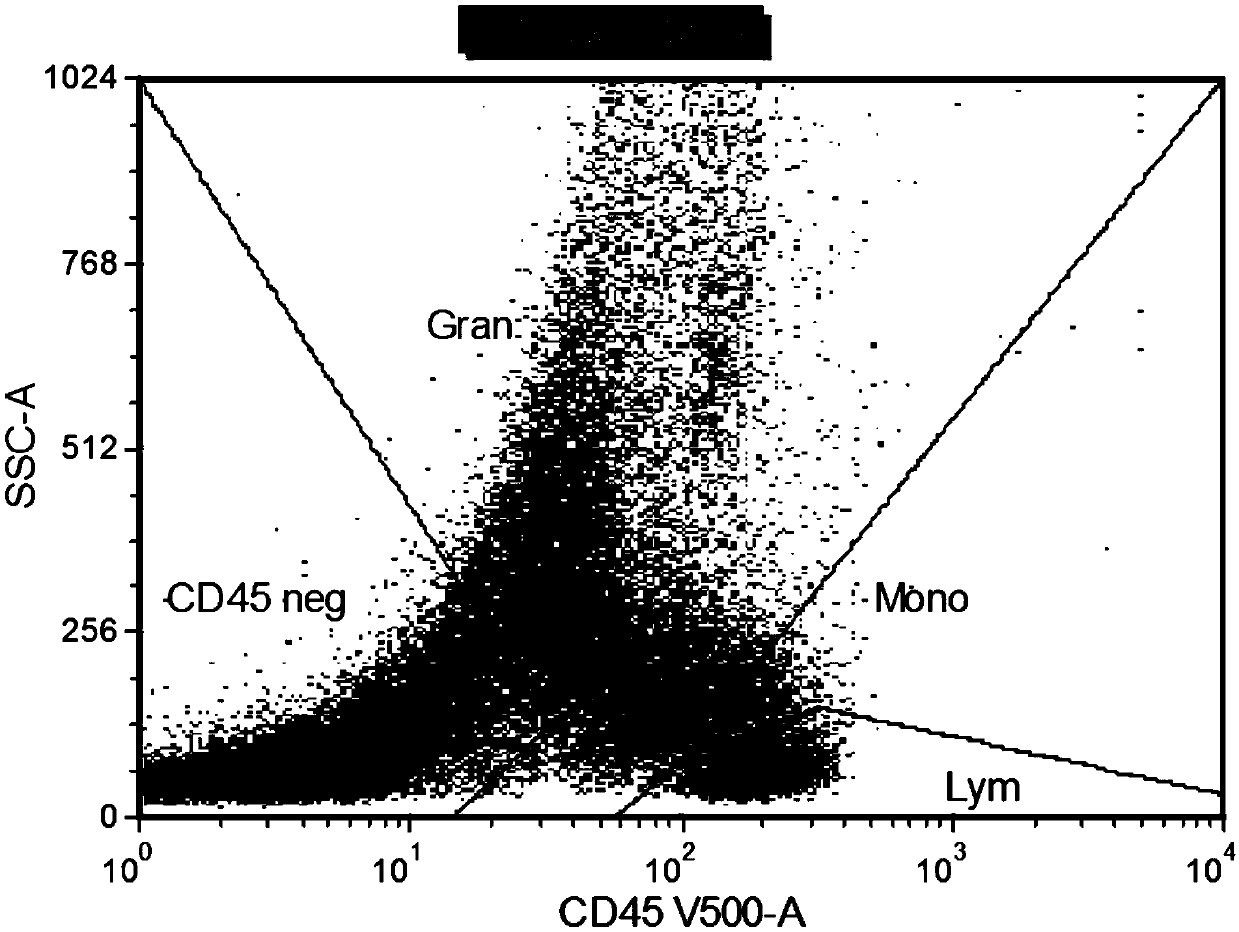
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
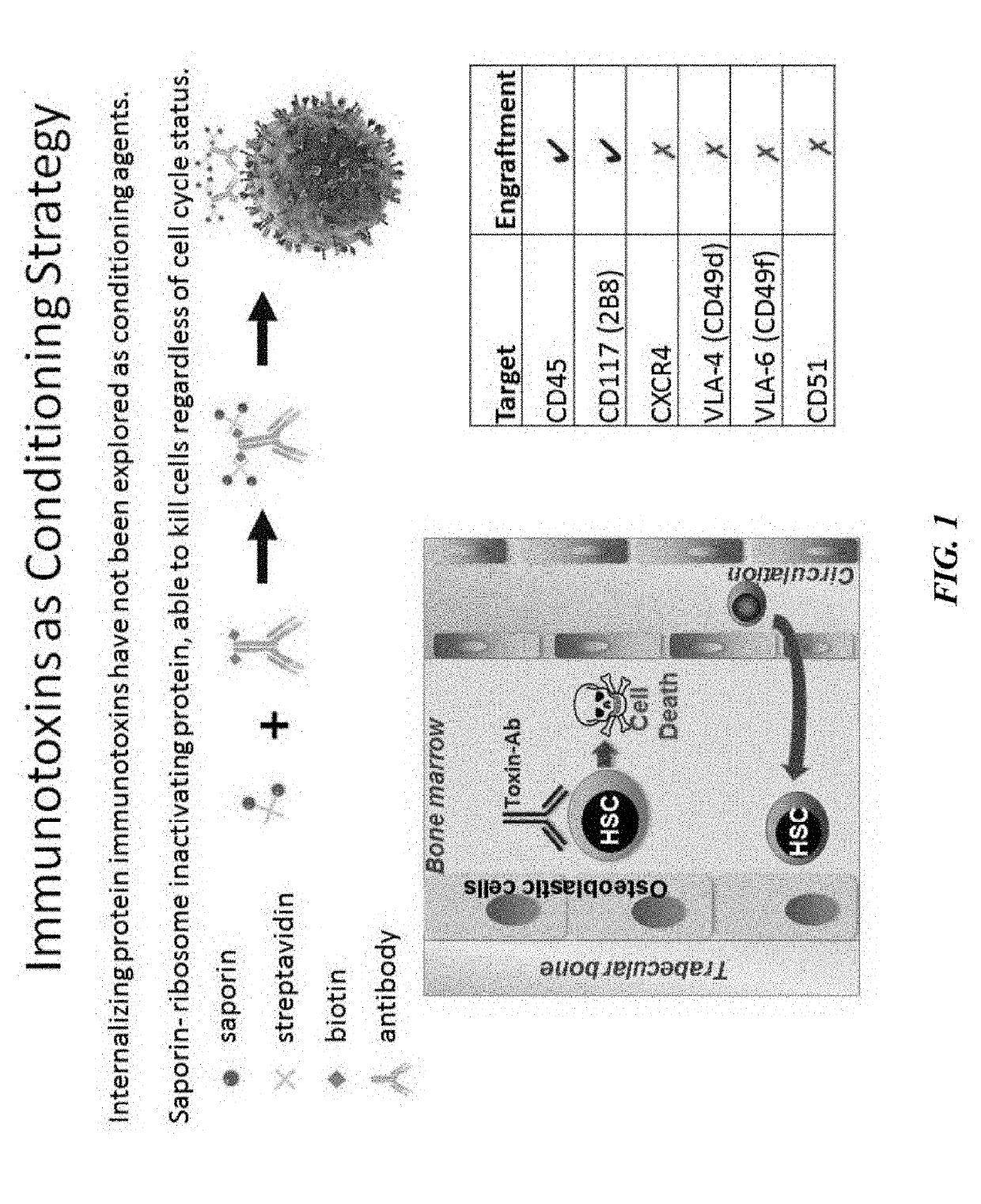
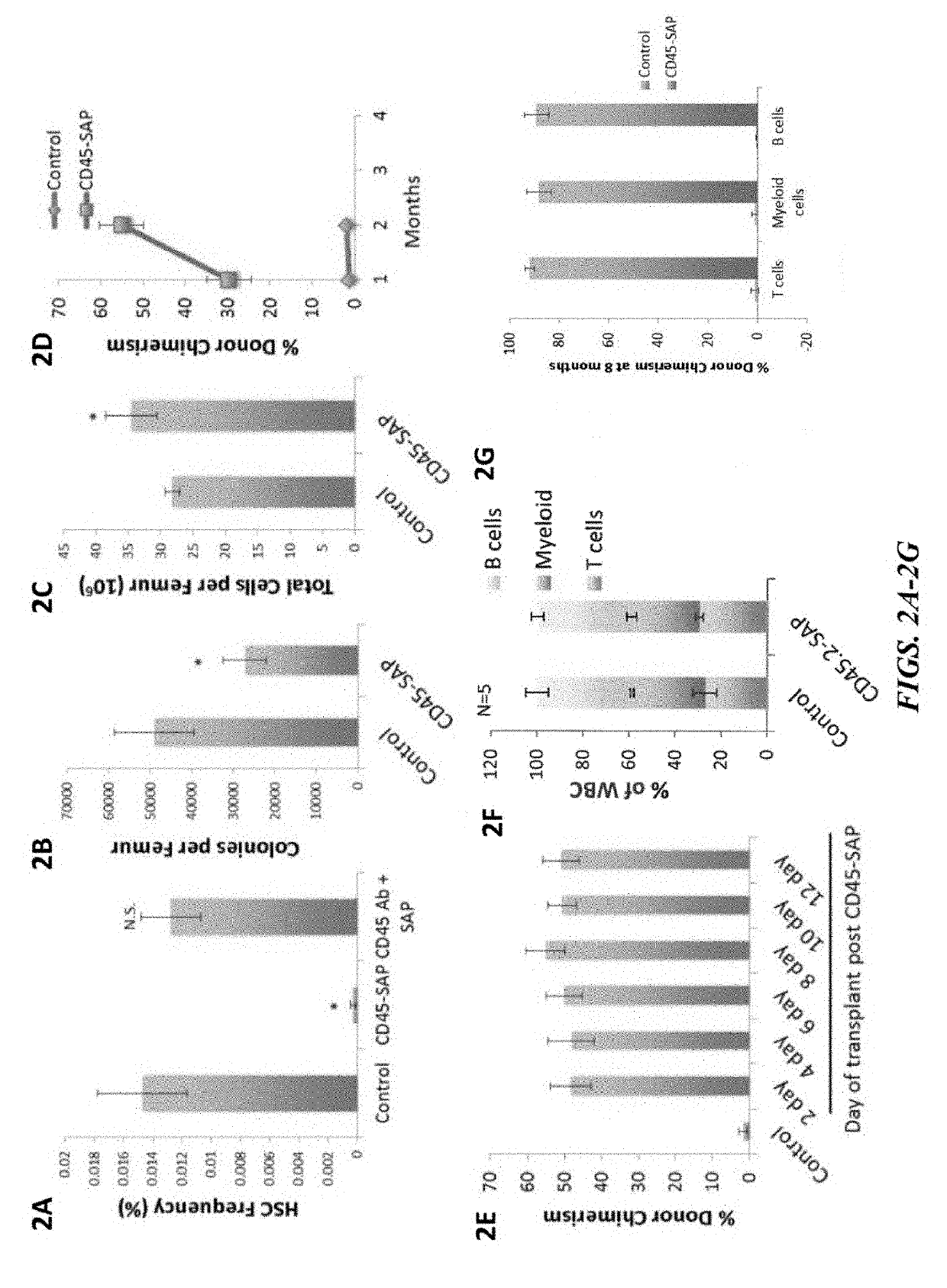
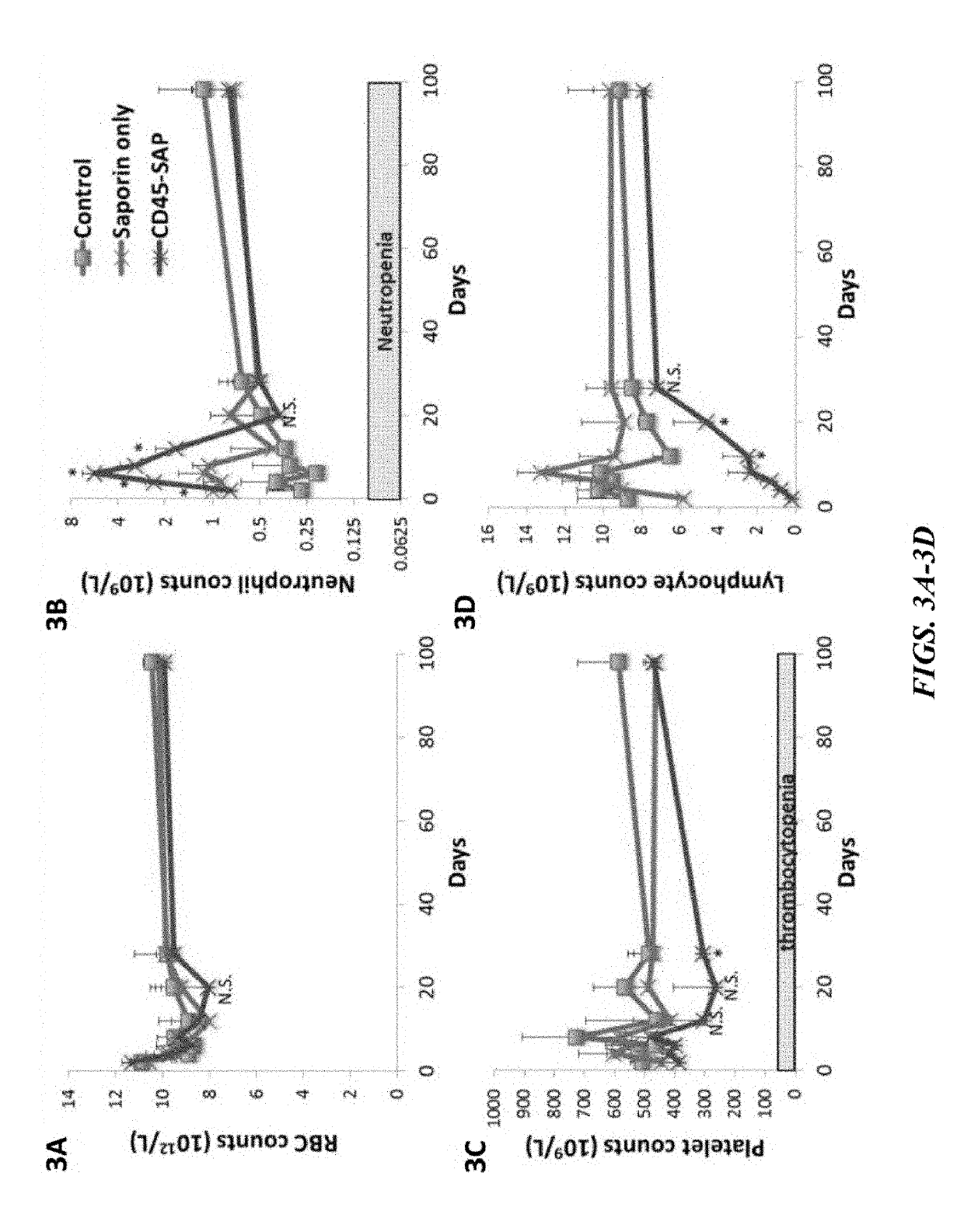
Compositions and methods for the depletion of cd117+ cells
ActiveUS20190144558A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCancer cell
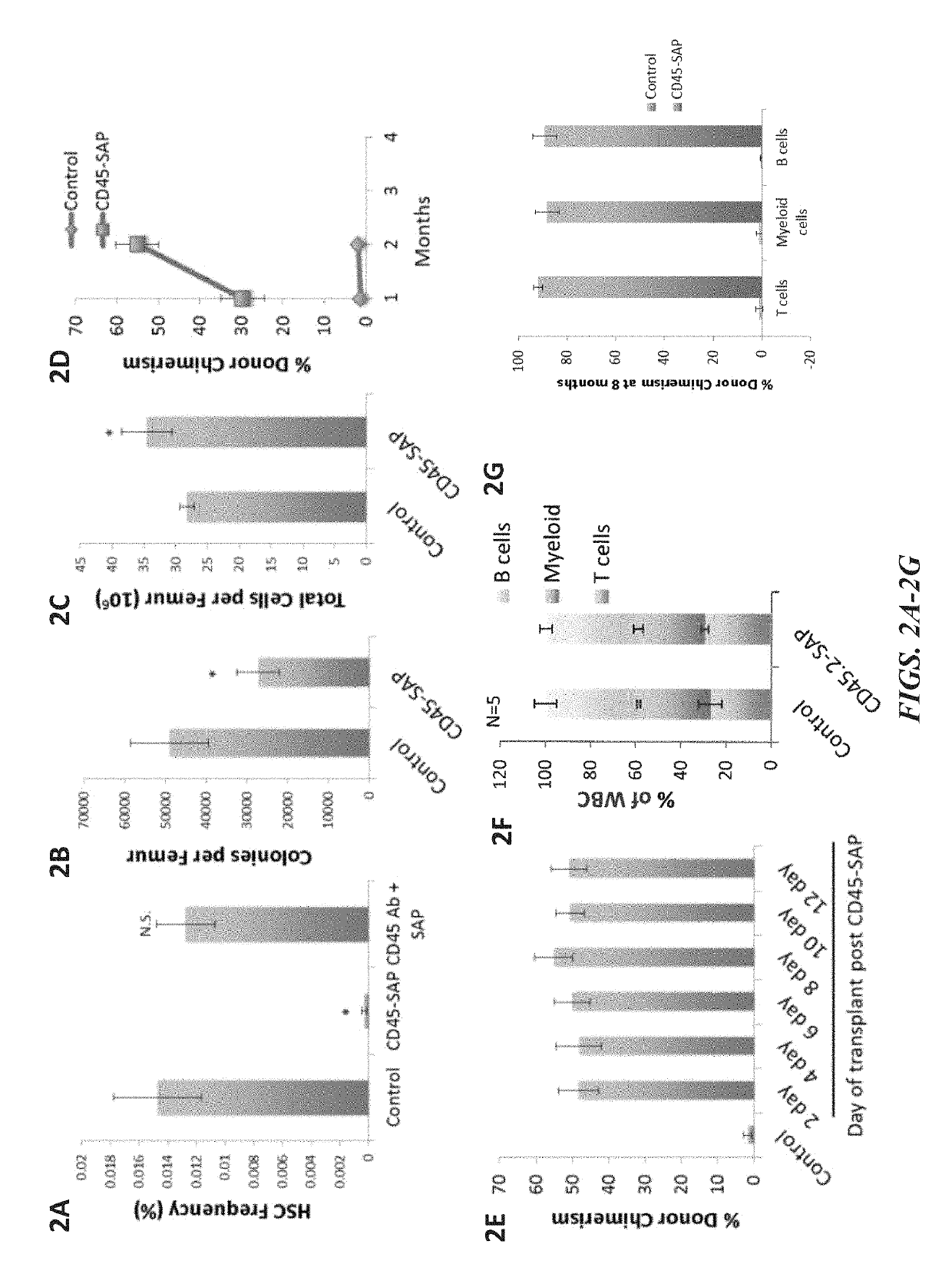
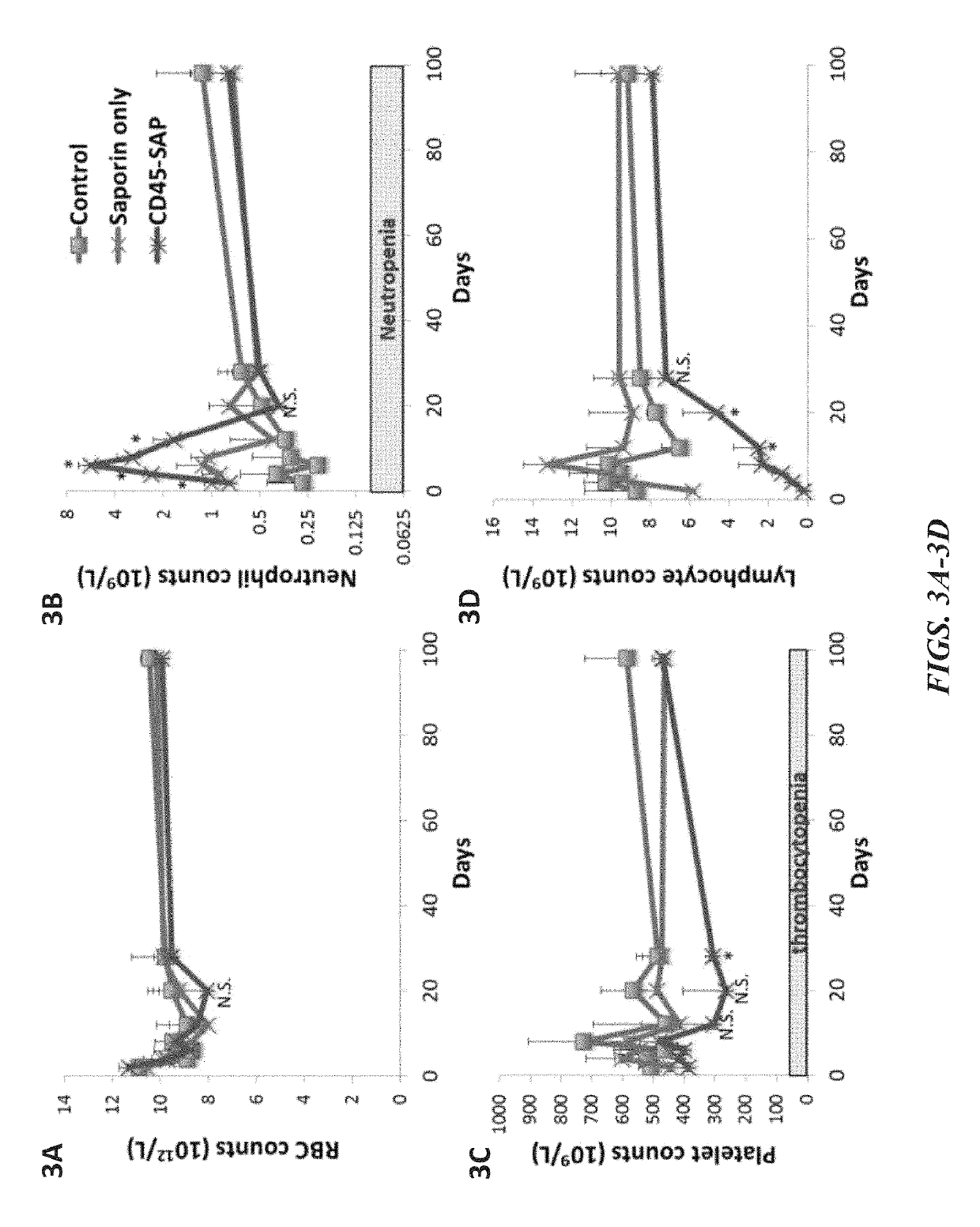
The invention provides compositions and methods useful for the depletion of CD117+ cells and for the treatment of various hematopoietic diseases, metabolic disorders, cancers, and autoimmune diseases, among others. Described herein are antibodies, antigen-binding fragments, and conjugates thereof that can be applied to effect the treatment of these conditions, for instance, by depleting a population of CD117+ cells in a patient, such as a human. The compositions and methods described herein can be used to treat a disorder directly, for instance, by depleting a population of CD117+ cancer cells or autoimmune cells. The compositions and methods described herein can also be used to prepare a patient for hematopoietic stem cell transplant therapy and to improve the engraftment of hematopoietic stem cell transplants by selectively depleting endogenous hematopoietic stem cells prior to the transplant procedure.
Owner:CRISPR THERAPEUTICS AG
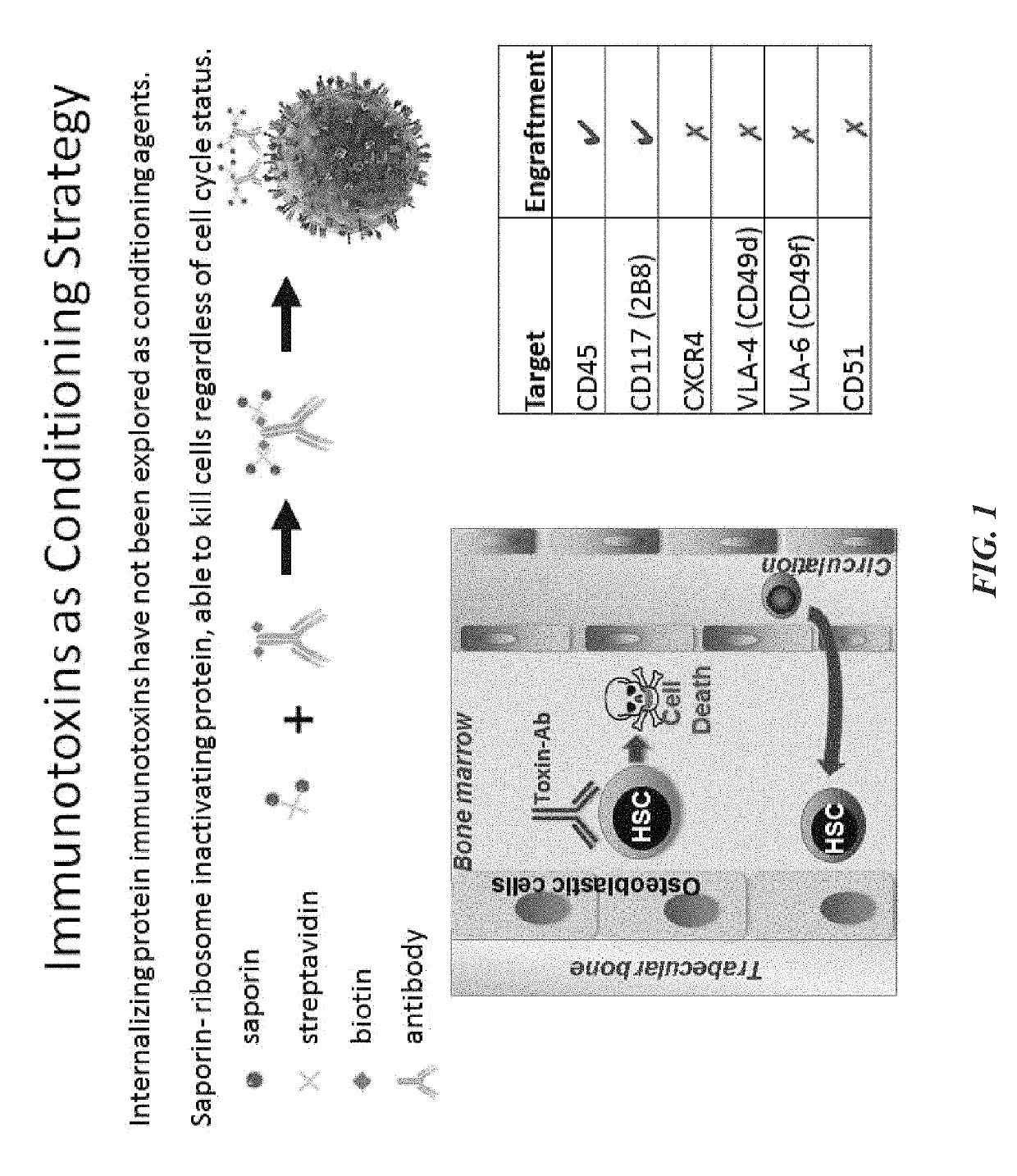
Compositions and methods for non-myeloablative conditioning
ActiveUS20160324982A1InduceImprove efficiencyNervous disorderPeptide/protein ingredientsSurface markerNon myeloablative
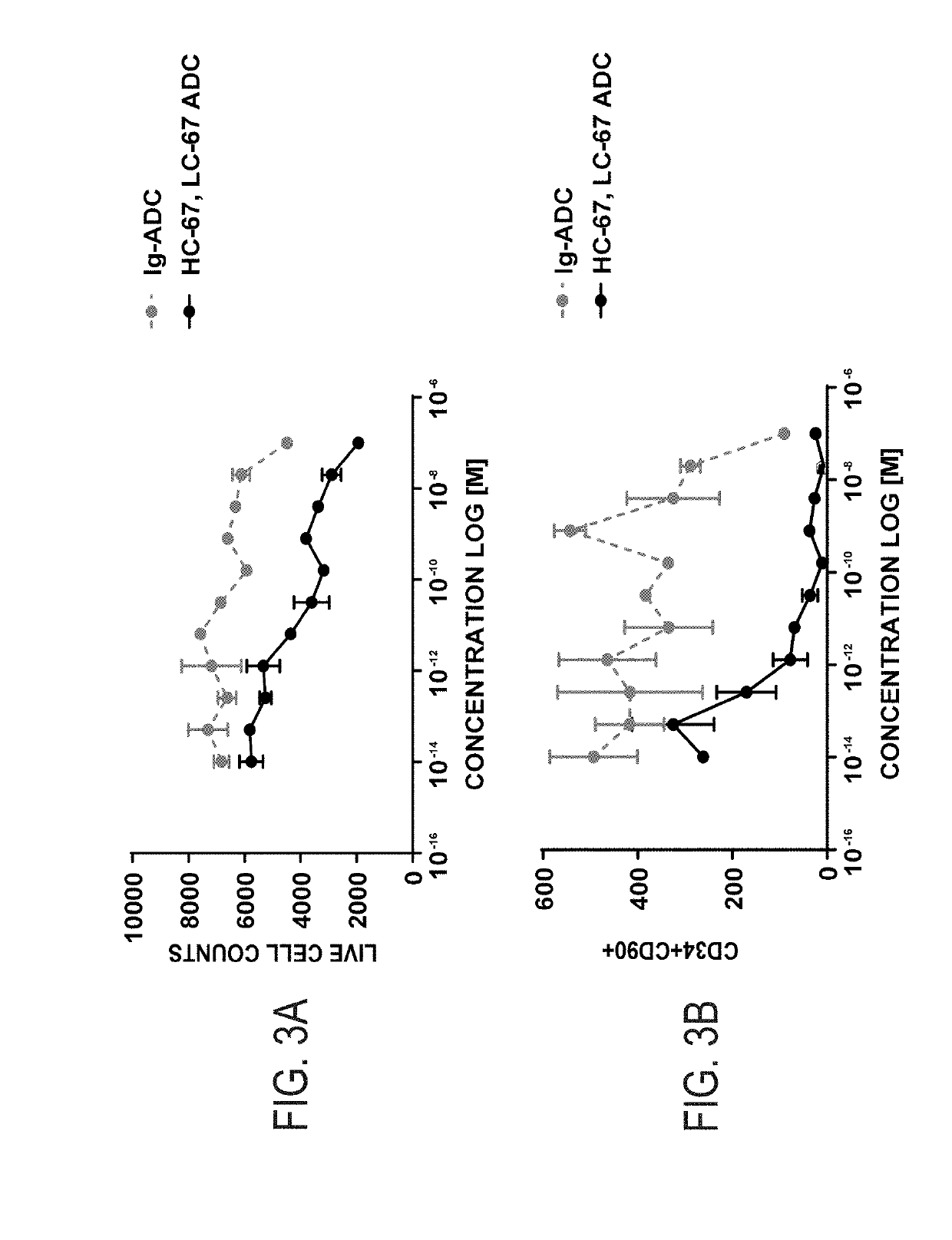
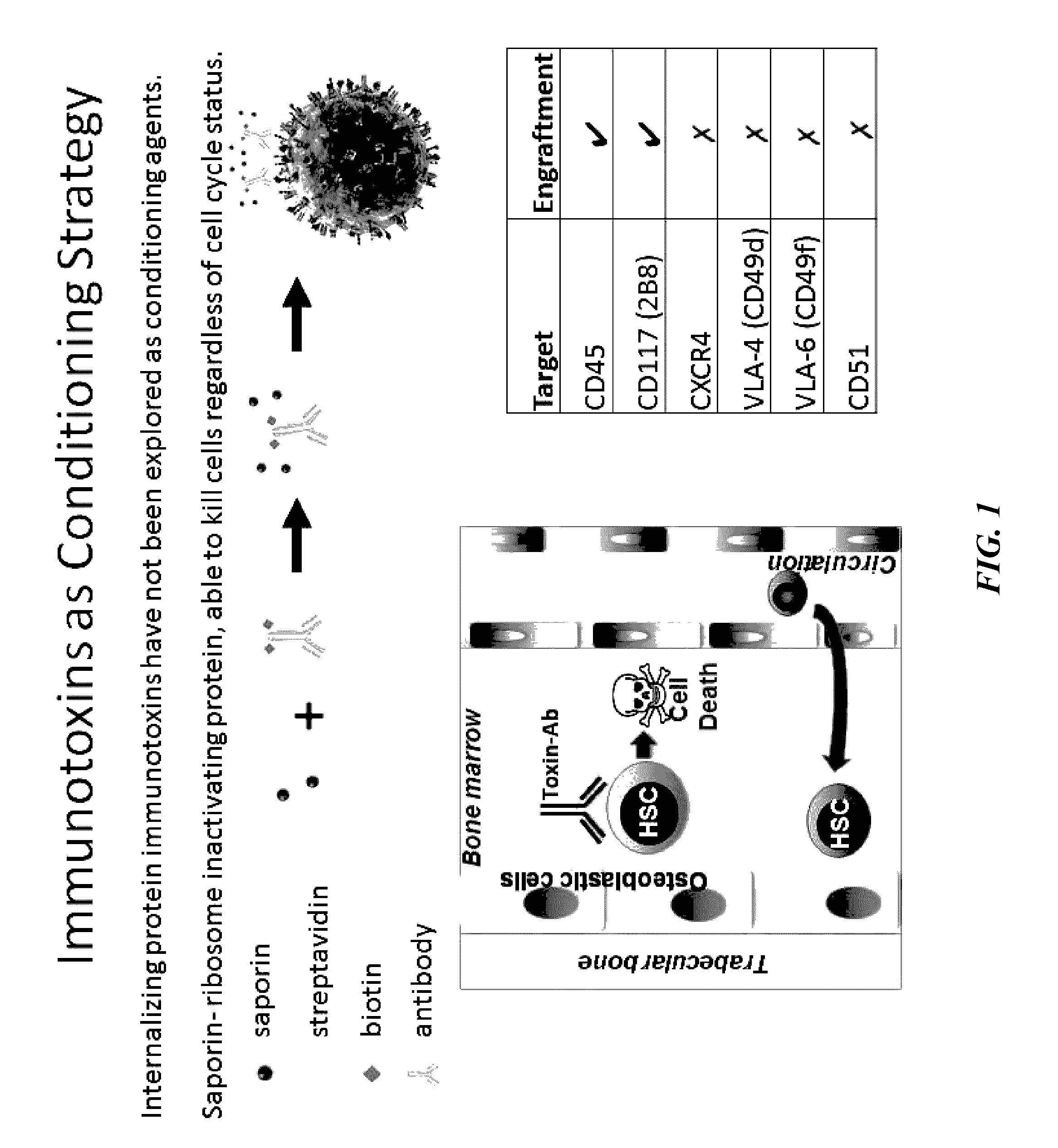
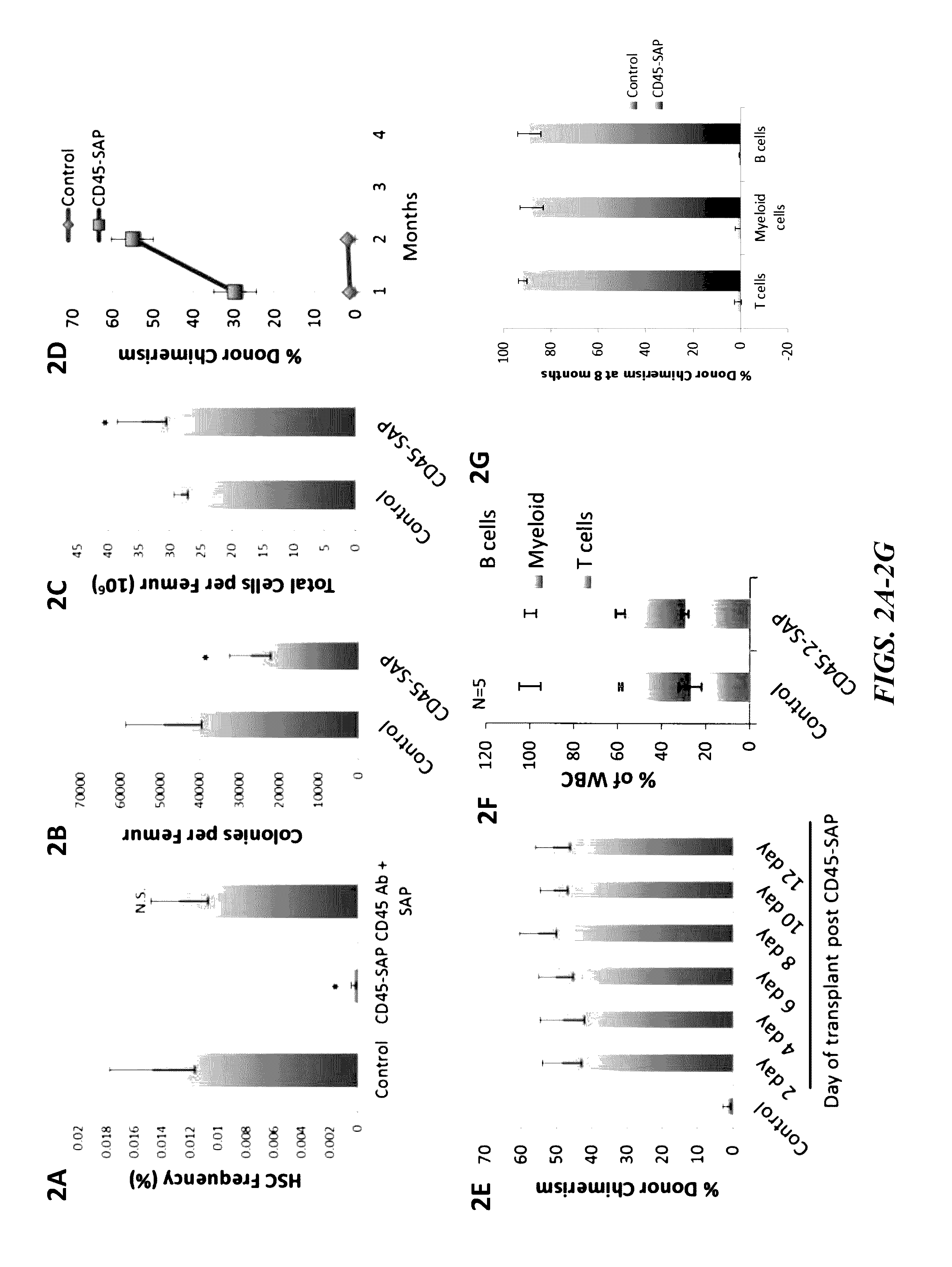
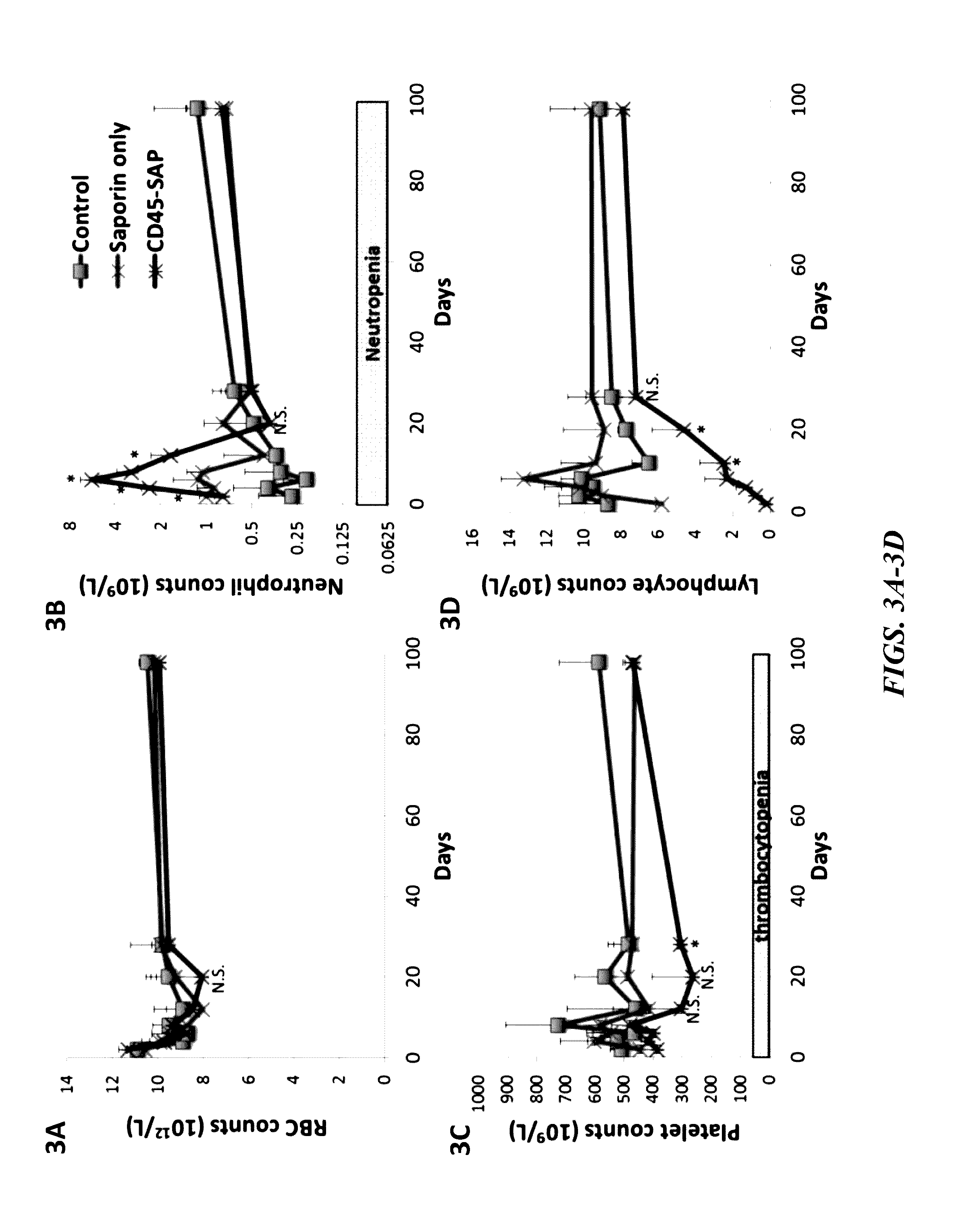
Disclosed herein are non-myeloablative antibody-toxin conjugates and compositions that target cell surface markers, such as the CD34, CD45 or CD117 receptors, and related methods of their use to effectively conditioning a subject's tissues (e.g., bone marrow tissue) prior to engraftment or transplant. The compositions and methods disclosed herein may be used to condition a subject's tissues in advance of, for example, hematopoietic stem cell transplant and advantageously such compositions and methods do not cause the toxicities that are commonly associated with traditional conditioning methods.
Owner:THE GENERAL HOSPITAL CORP +2
Treatment of stroke and other acute neural degenerative disorders via intranasal administration of umbilical cord-derived cells
ActiveUS8518390B2Convenient treatmentHigh expressionBiocideGenetic material ingredientsTelomeraseMammal
This invention relates to methods of treating stroke by intranasal administration of umbilical cord tissue-derived cells, which are isolated from mammalian umbilical cord tissue substantially free of blood or expanded in culture from a cell isolated from mammalian umbilical cord tissue substantially free of blood, are capable of self-renewal and expansion in culture, and do not produce CD117 and / or telomerase. The methods of the invention regenerate, repair and improve neural tissue and improve behavior and neurological function in stroke patients.
Owner:DEPUY SYNTHES PROD INC
Human hepatic progenitor cells and methods of use thereof
Liver progenitor cells immunoreactive for CD117, as well as for CD34 capable of proliferating in a culture; and differentiating in vivo into a hepatocyte, a cholangiocyte or a sinusoidal cell are provided. The cultures can be expanded over a large number of passages and integrate well after transplantation into adult liver.
Owner:NOVAHEP
Antibody composition and application of antibody composition in leukemia and lymphoma typing
ActiveCN105606797AOptimizing Fluorescent Labeling CombinationsSimple methodMaterial analysisTypingCD33
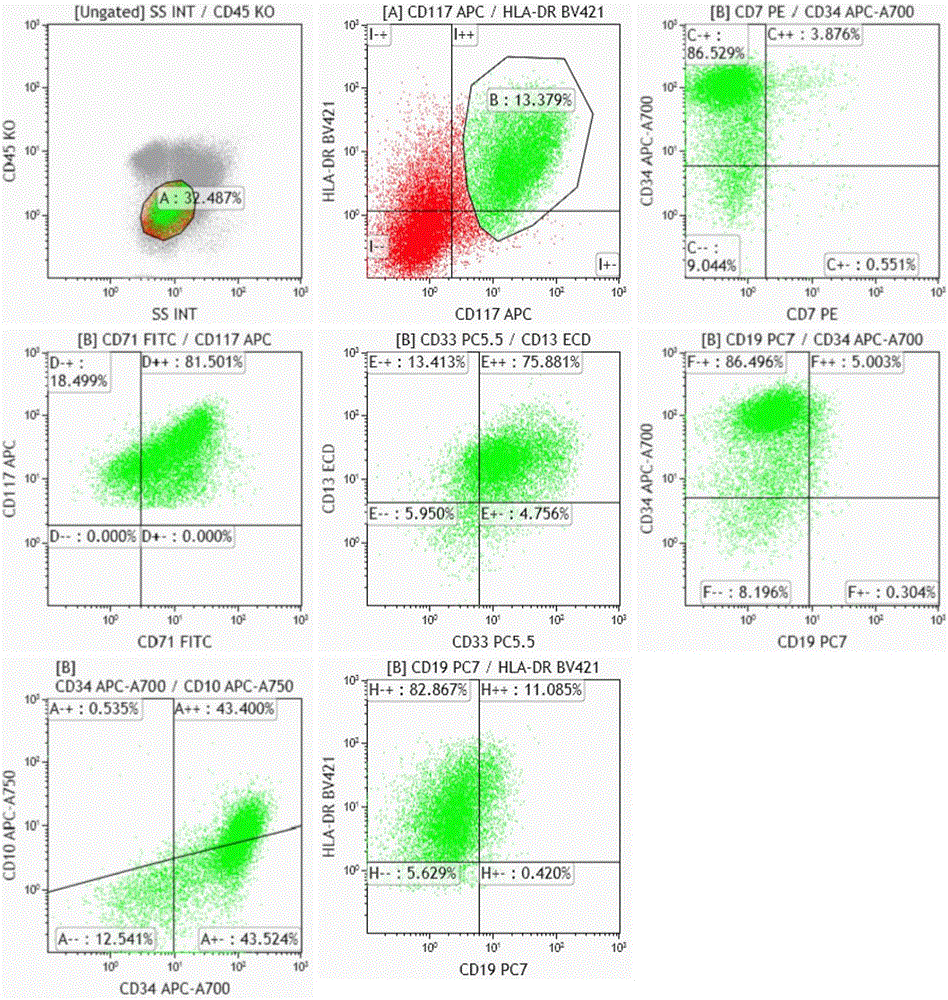
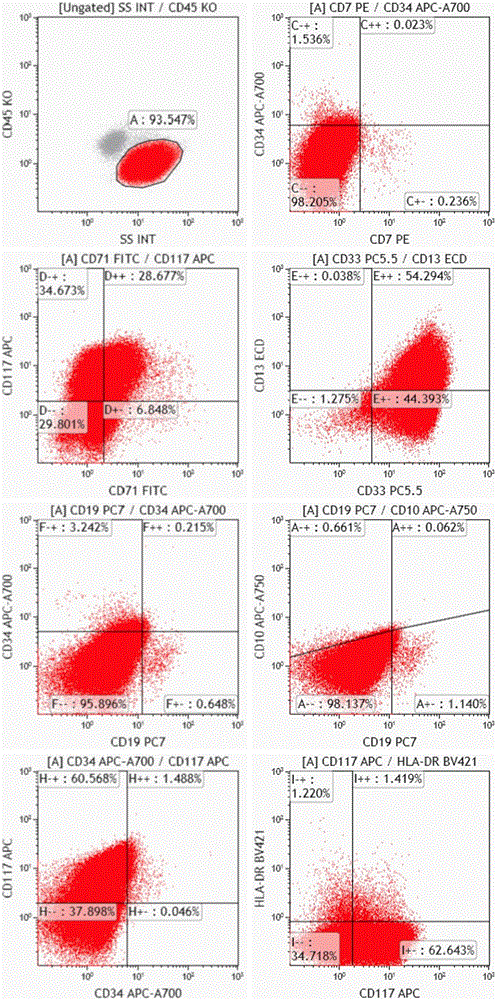
The invention belongs to the technical field of antibody medicines and provides two types of antibody compositions including CD45 antibody, CD13 antibody, CD33 antibody and CD7 antibody or including CD45 antibody, CD117 antibody, CD34 antibody and CD19 antibody. The invention further provides a leukemia and lymphoma typing kit comprising the two types of antibody compositions and capable of being used with a flow cytometry to achieve typing of various hematological neoplasms.
Owner:ZHEJIANG BOZHEN BIOTECH CO LTD
Compositions and methods for non-myeloablative conditioning
ActiveUS20190100593A1Improve efficiencyPreserve thymic integrityNervous disorderPeptide/protein ingredientsSurface markerNon myeloablative
Disclosed herein are non-myeloablative antibody-toxin conjugates and compositions that target cell surface markers, such as the CD34, CD45 or CD117 receptors, and related methods of their use to effectively conditioning a subject's tissues (e.g., bone marrow tissue) prior to engraftment or transplant. The compositions and methods disclosed herein may be used to condition a subject's tissues in advance of, for example, hematopoietic stem cell transplant and advantageously such compositions and methods do not cause the toxicities that are commonly associated with traditional conditioning methods.
Owner:THE GENERAL HOSPITAL CORP +2
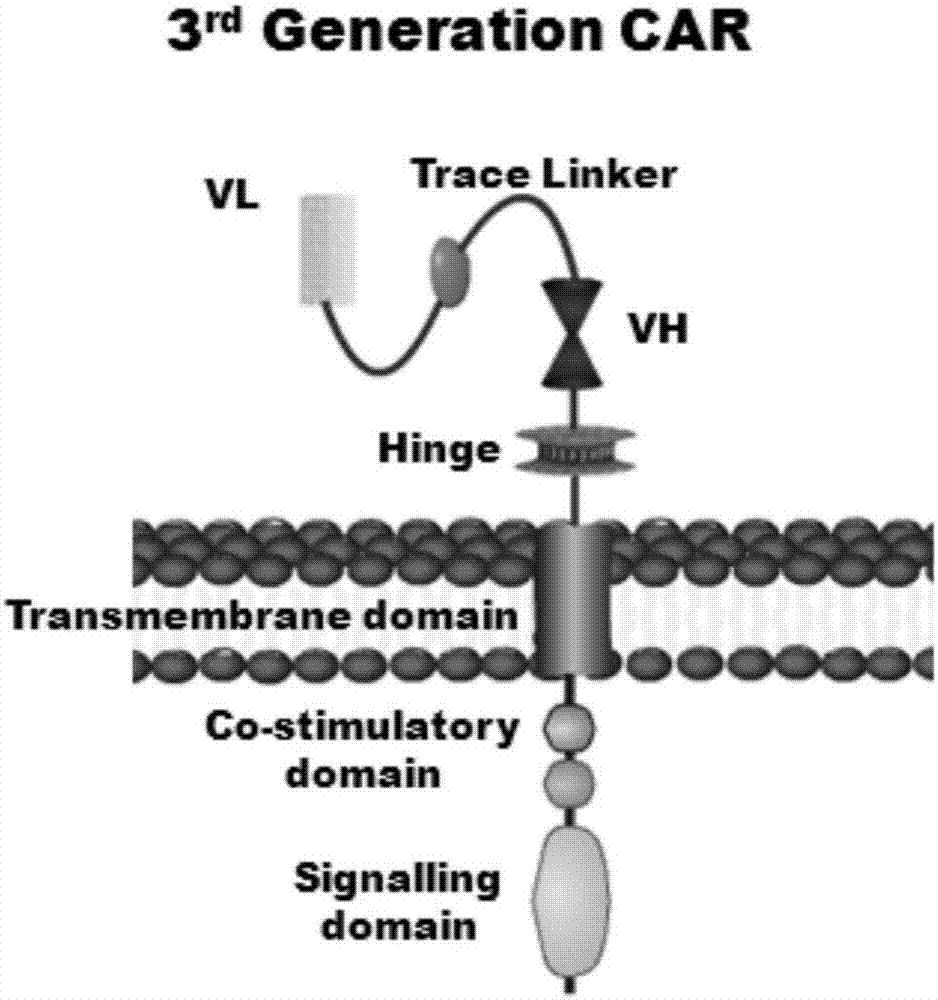
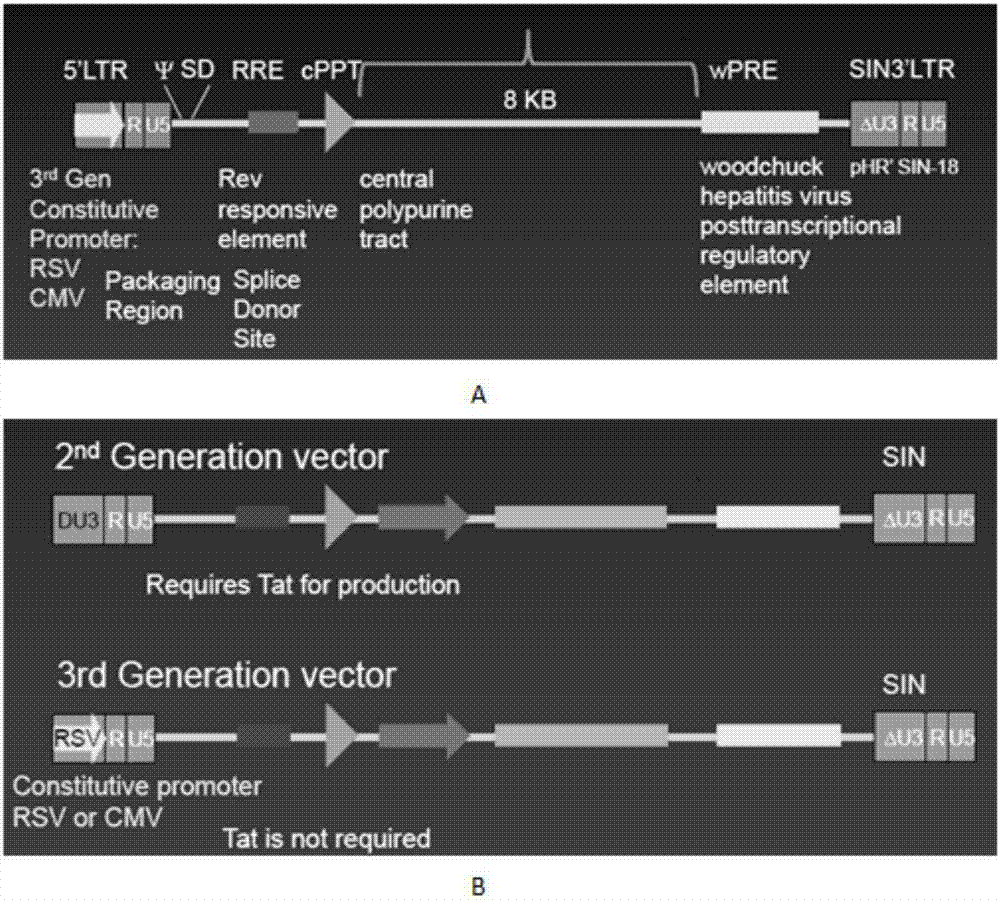
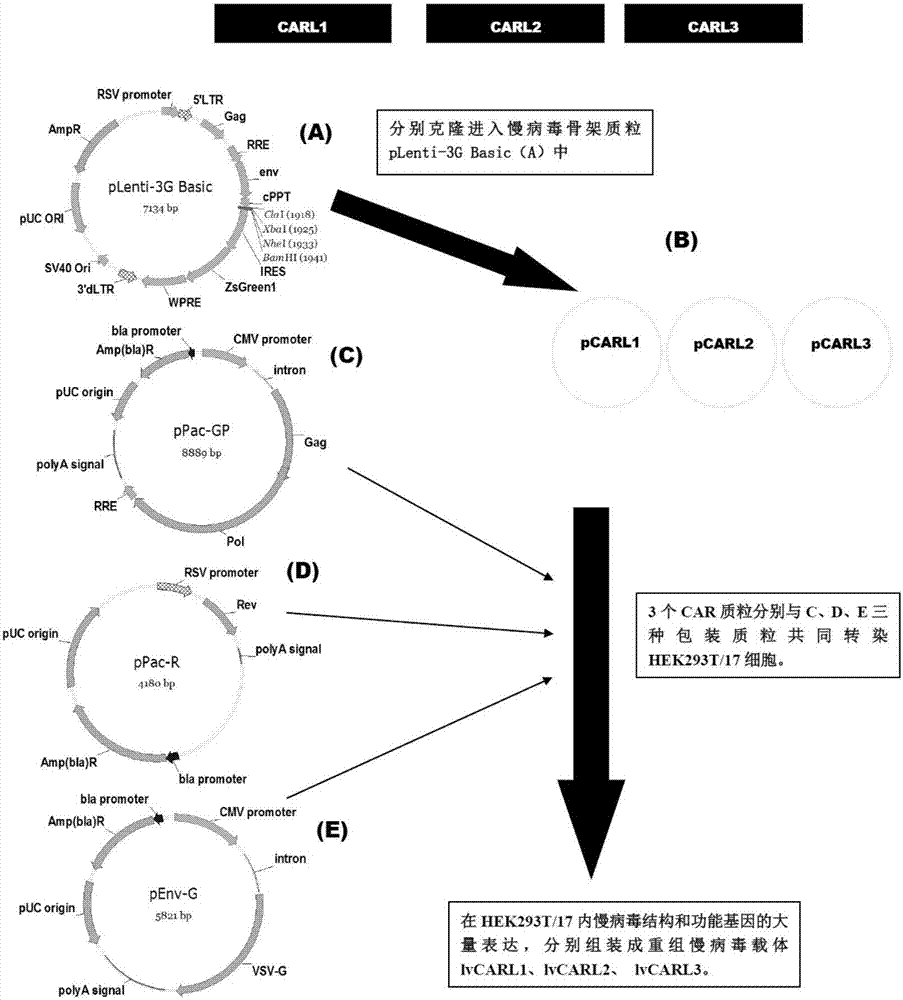
Marker used for in-vivo tracing and manual removal of CAR-T cells and application thereof
ActiveCN107287207AActivate killingGuaranteed normal treatmentMammal material medical ingredientsImmunoglobulinsCAR T-cell therapyT lymphocyte
The invention discloses a marker used for in-vivo tracing and manual removal of CAR-T cells. The marker comprises a trace-linker 1 with a sequence as shown in SEQ ID No. 18, a trace-linker 2 with a sequence as shown in SEQ ID No. 19 and a trace-linker 3 with a sequence as shown in SEQ ID No. 19. The invention also discloses a CAR-T therapy vector including the marker and a construction method thereof. Moreover, the invention further discloses application of the marker to preparation of the CAR-T therapy vector and drugs used for treating triple-negative breast cancer. The marker provided by the invention can realize controllable in-vivo tracing, in-vitro separation and manual removal of CAR-T cells without influence on the tumor killing effect of the CAR-T cells, so the security of CAR-T cell therapy is greatly improved, and a powerful pool can be provided for deeper analysis of the process of CAR-T cell therapy. The vector provided by the invention can express a targeting chimeric antigen receptor of CD117 in human T lymphocytes, guides and activates killing effect of T lymphocytes on CD117 positive cells, and is applicable to treatment of triple-negative breast cancer in clinical practice.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Compositions and methods for non-myeloablative conditioning
ActiveUS10280225B2Improve efficiencyPreserve thymic integrityNervous disorderPeptide/protein ingredientsSurface markerNon myeloablative
Disclosed herein are non-myeloablative antibody-toxin conjugates and compositions that target cell surface markers, such as the CD34, CD45 or CD117 receptors, and related methods of their use to effectively conditioning a subject's tissues (e.g., bone marrow tissue) prior to engraftment or transplant. The compositions and methods disclosed herein may be used to condition a subject's tissues in advance of, for example, hematopoietic stem cell transplant and advantageously such compositions and methods do not cause the toxicities that are commonly associated with traditional conditioning methods.
Owner:THE GENERAL HOSPITAL CORP +2
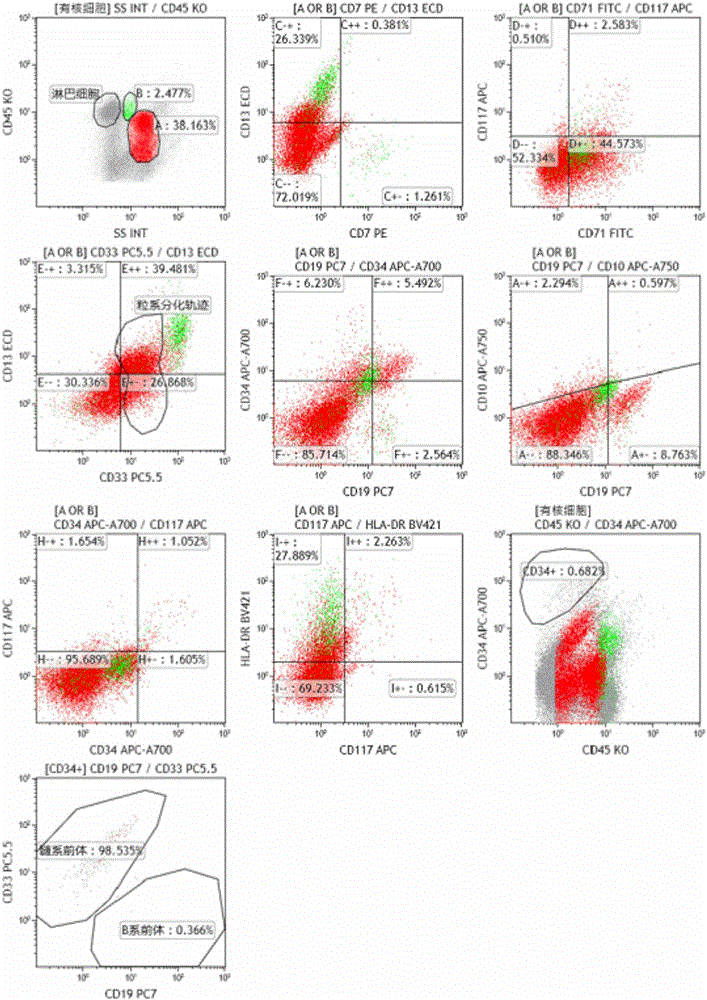
Ten-color antibody composition and application thereof in leukemia-lymphomas subtype
The invention belongs to the technical field of antibodies, and provides an antibody composition composed of ten antibodies, wherein the tan antibodies comprises CD71 resistant antibody, CD7 resistant antibody, CD13 resistant antibody, CD33 resistant antibody, CD19 resistant antibody, CD117 resistant antibody, CD34 resistant antibody, CD10 resistant antibody, HLA-DR resistant antibody, and CD45 resistant antibody. The invention provides a leukemia-lymphomas primary screening kit containing the antibodies, and the application thereof.
Owner:ZHEJIANG BOZHEN BIOTECH CO LTD
Pluripotent adult stem cells
Owner:NEW YORK MEDICAL COLLEGE
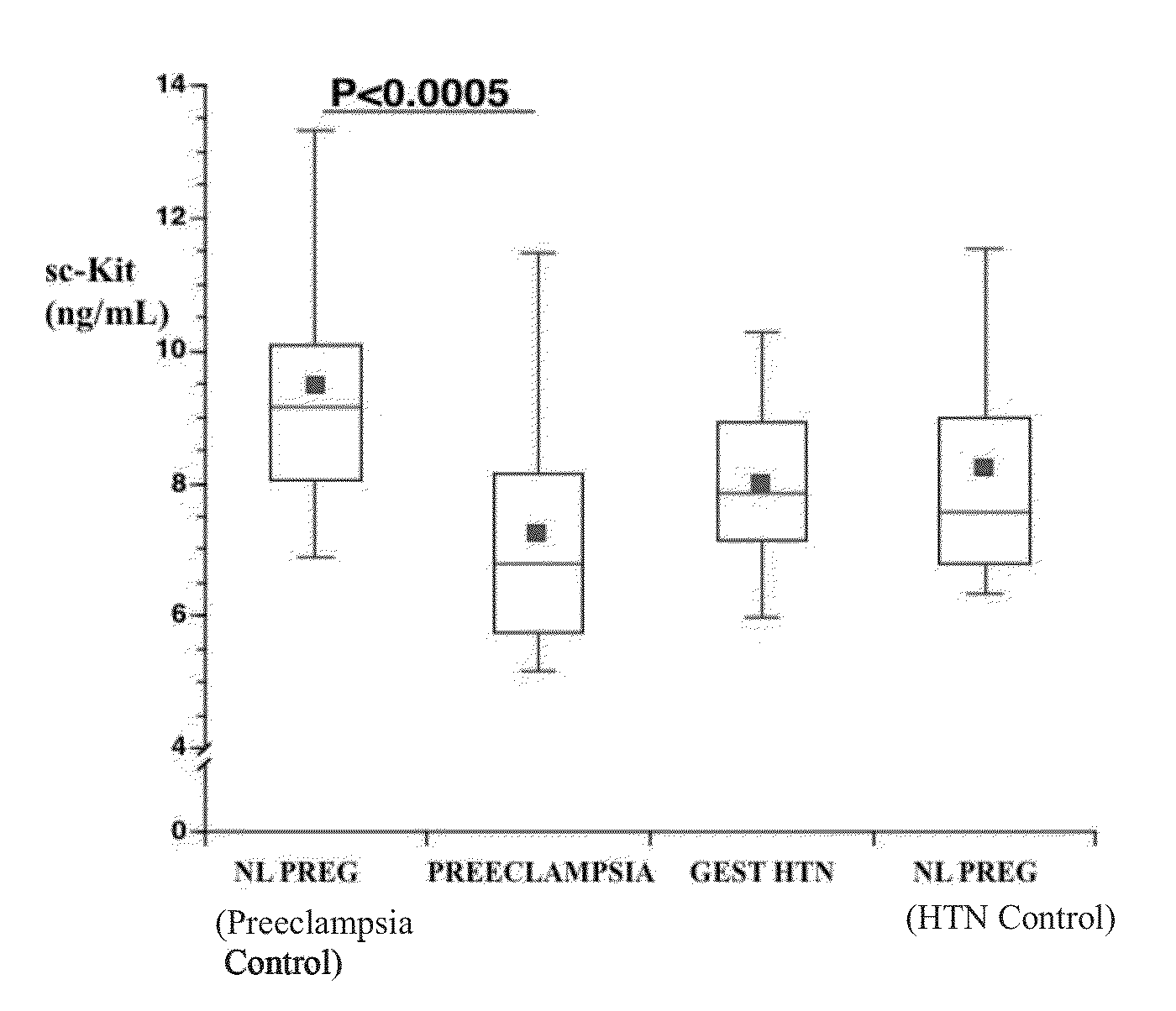
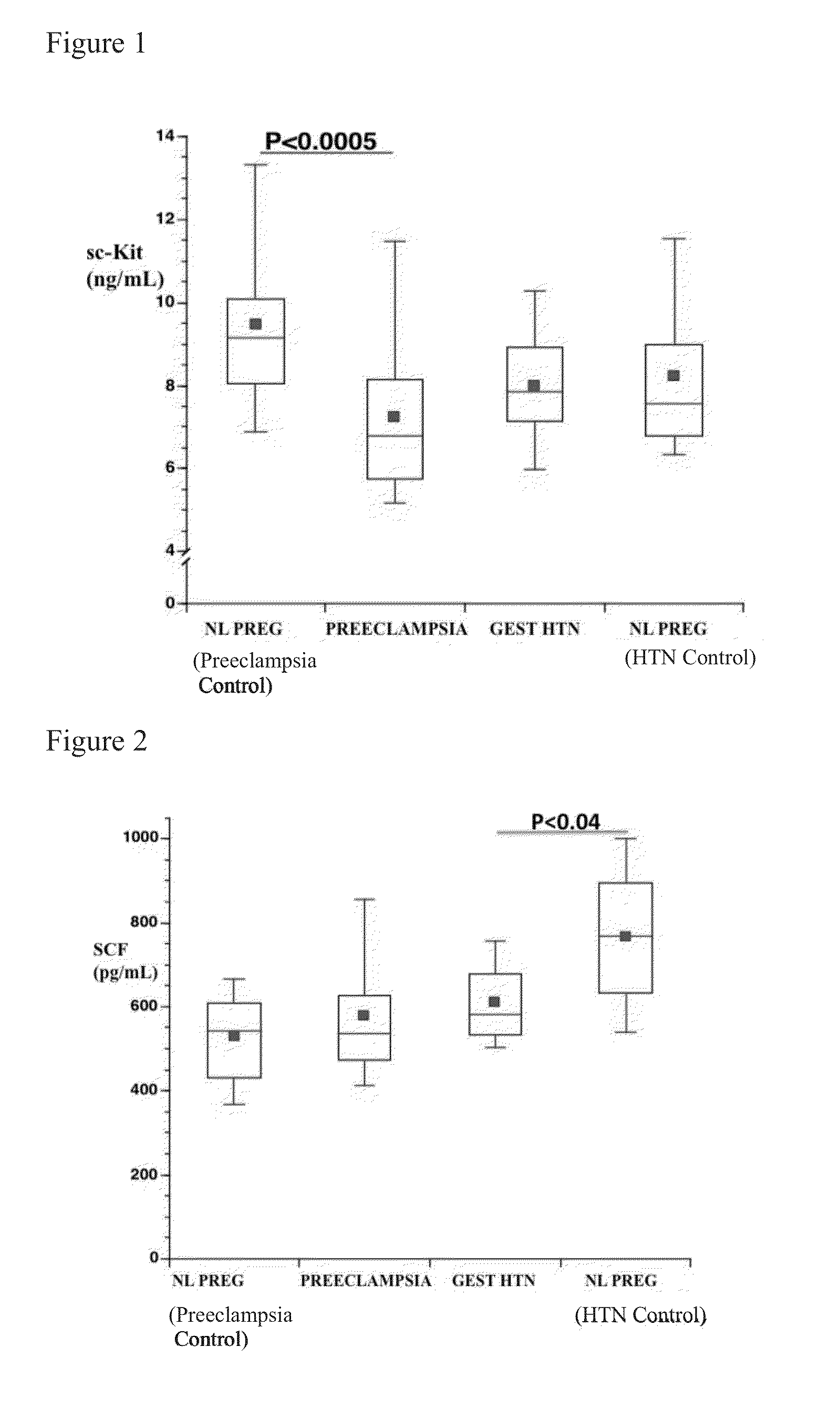
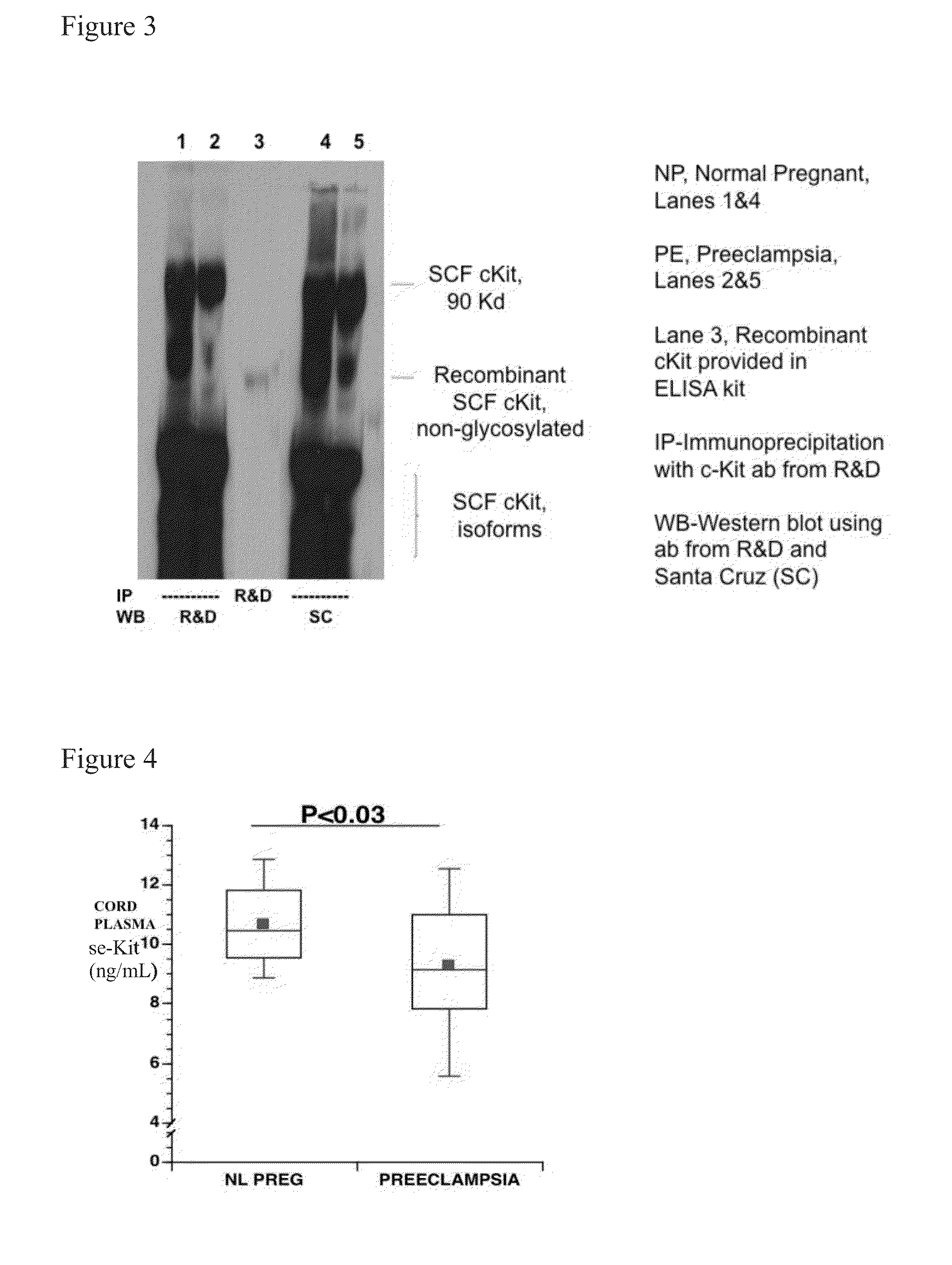
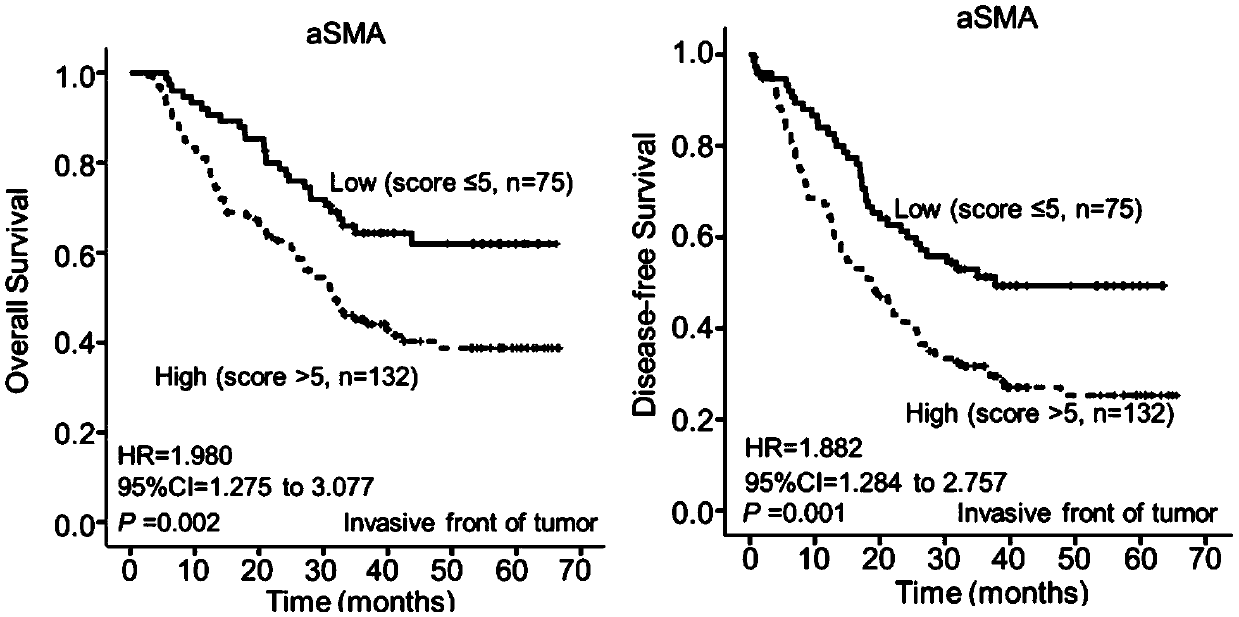
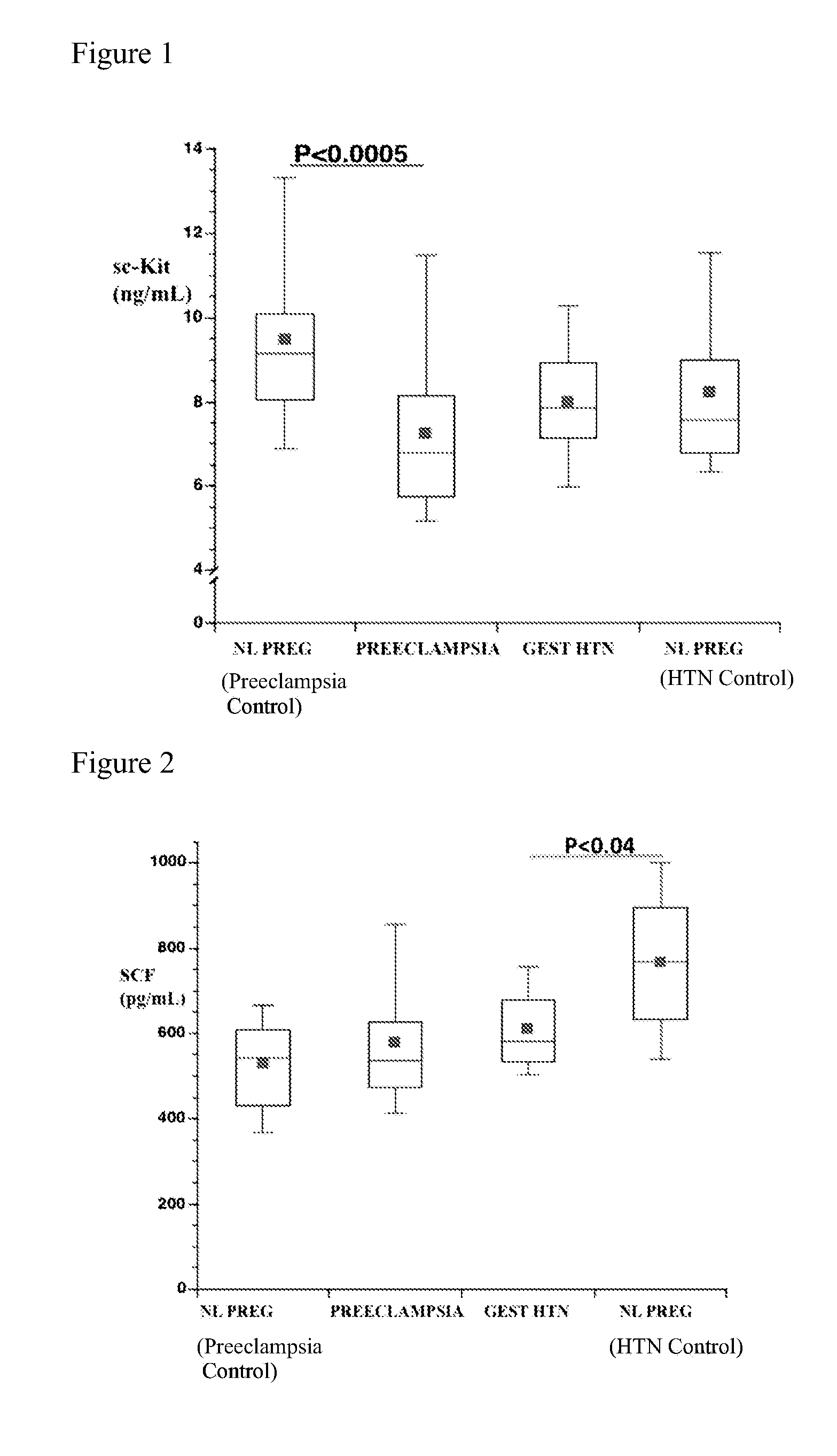
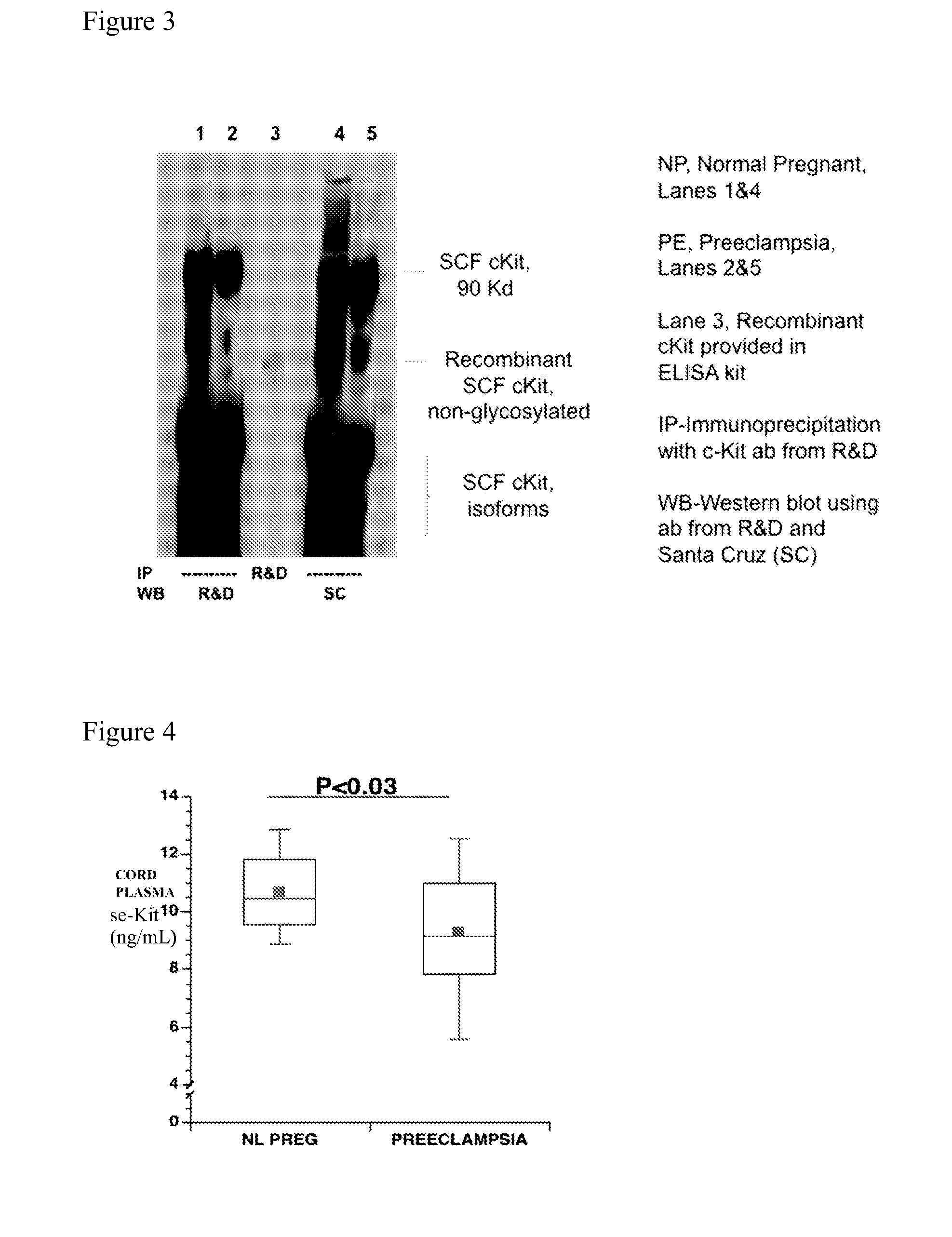
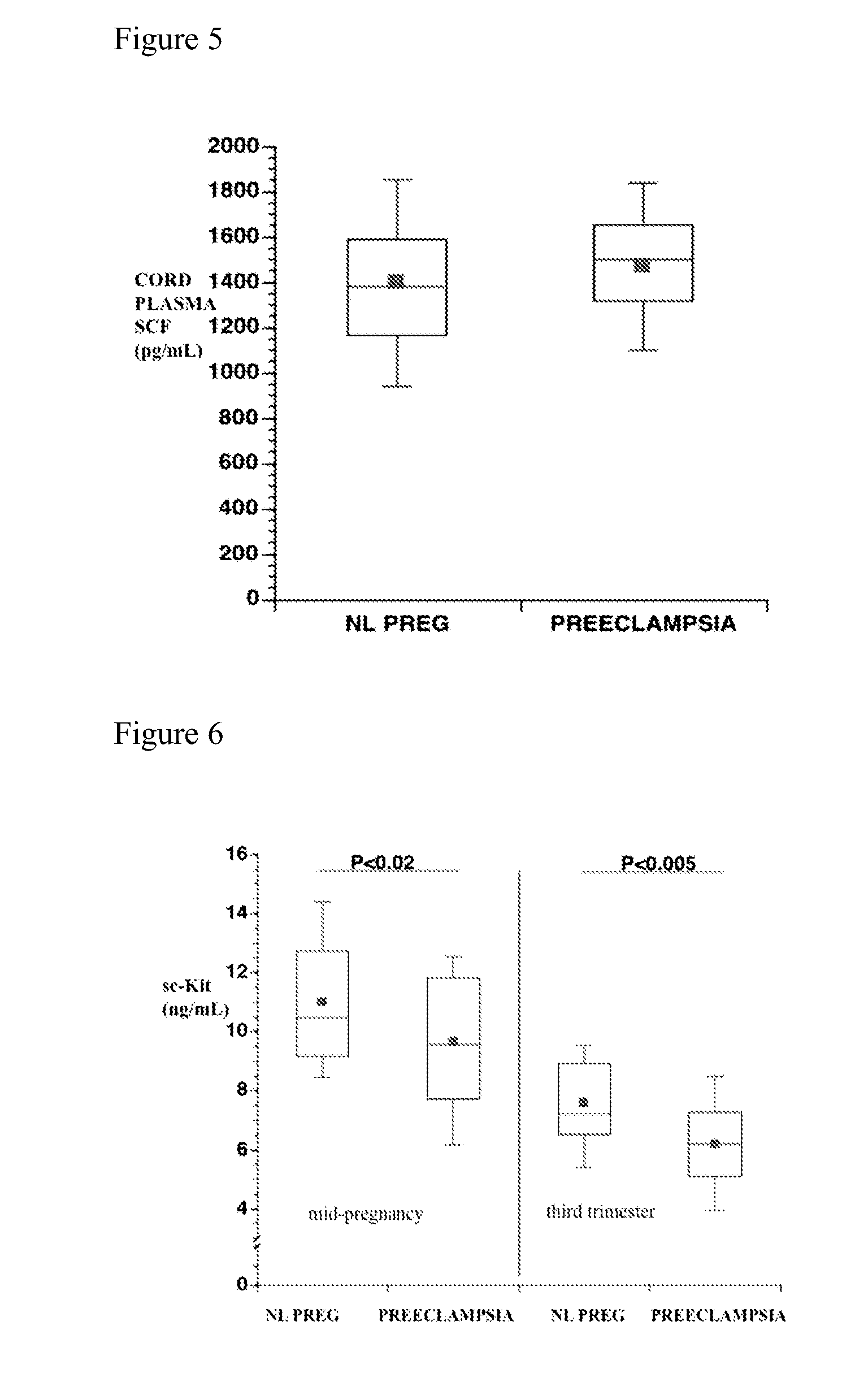
Soluble CD117 (sc-kit) for diagnosis of preeclampsia and eclampsia
Owner:UNIVERSITY OF PITTSBURGH
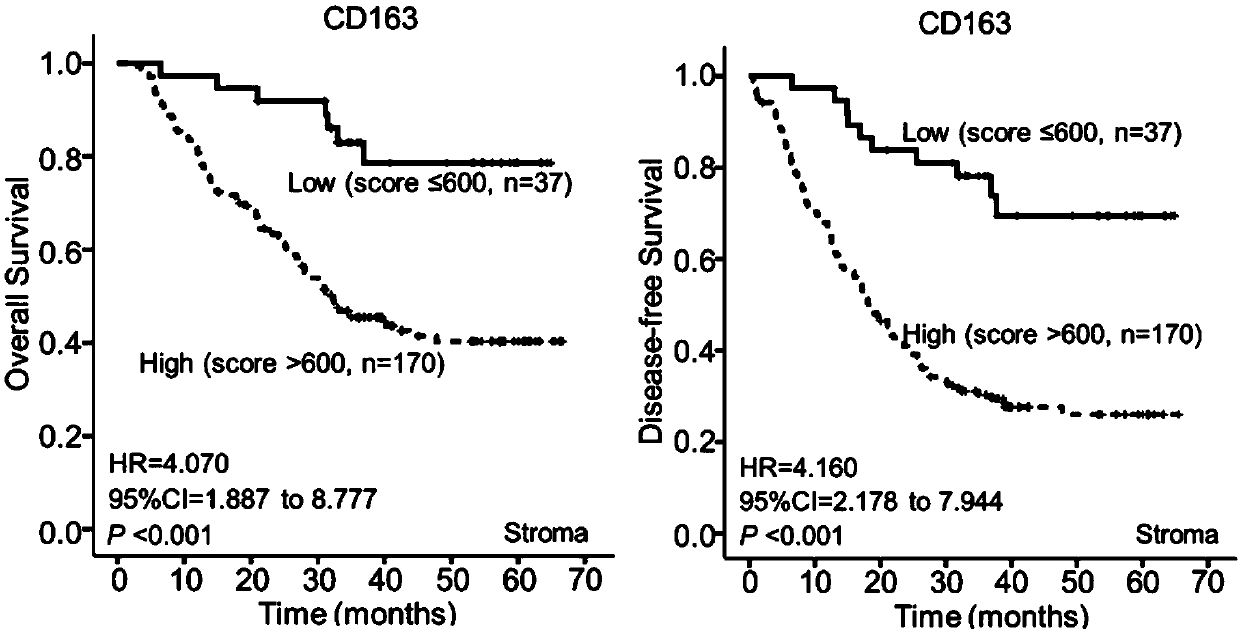
Esophageal squamous cell carcinoma microenvironmental cell marker molecular model and application thereof
ActiveCN109541209ASensitiveWith characteristicsMaterial analysisAdjuvantStage I Esophageal Squamous Cell Carcinoma
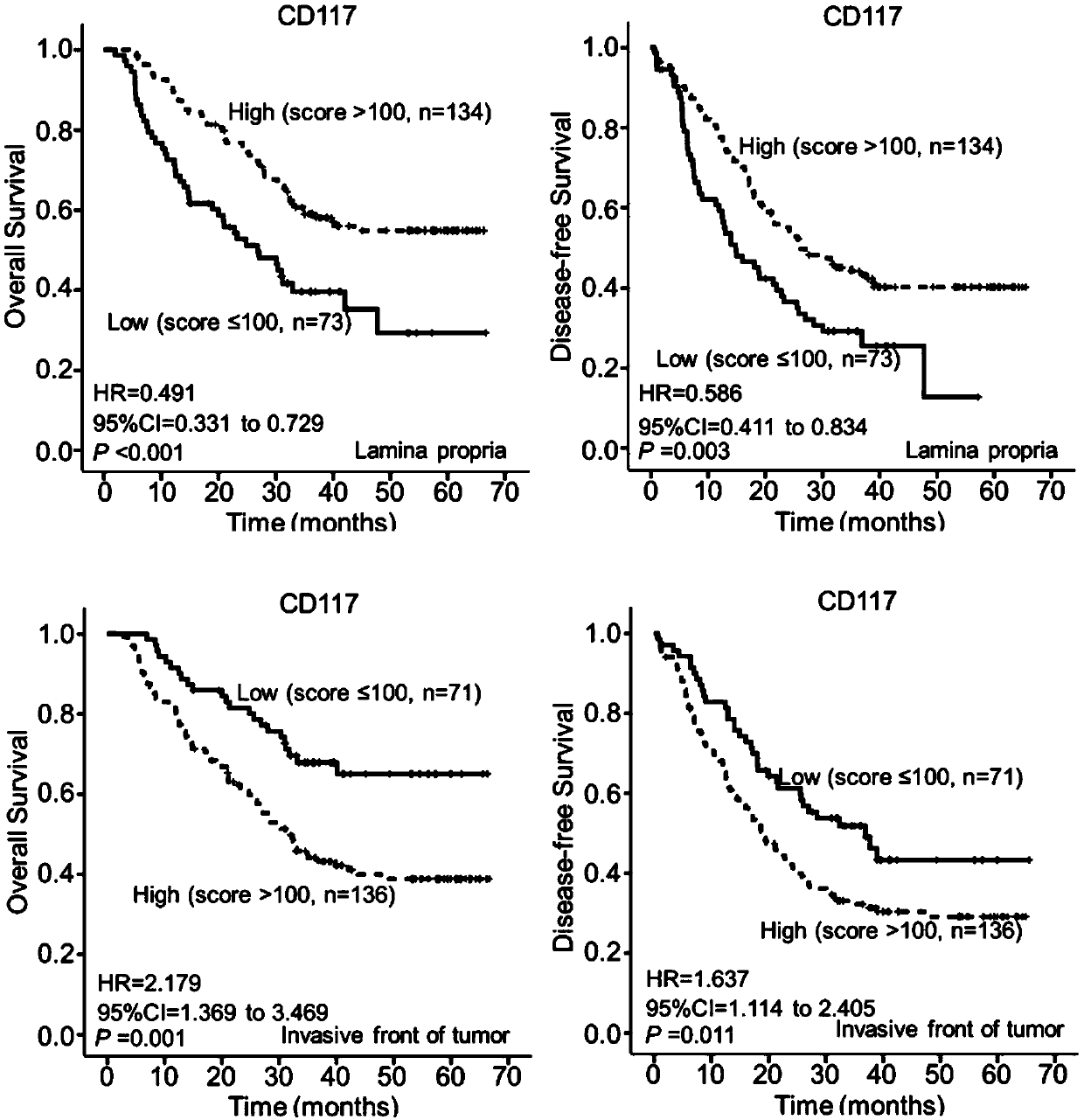
The invention relates to an esophageal squamous cell carcinoma microenvironmental cell marker molecular model and application thereof. The molecular model mainly comprises a-SMA+ (invasive frontier) fibroblasts, CD163+ (interstitial) macrophages, CD117+ (protogenic layer) mastocytes and CD117+ (invasion frontier) mastocytes. The molecular model can be used to predict the prognosis of patients withesophageal squamous carcinoma and group adjuvant treatment sensitivity groups. A kit mainly includes an anti-CD117 protein antibody, an anti-CD163 protein antibody and an anti-a-SMA protein antibody.According to the invention, the combination of three molecules combined with histopathological features predicts patients' prognosis, which has a higher predictive effect than single index detectionand clearly distinguishes patients with esophageal squamous carcinoma adjuvant treatment sensitivity; an immune tissue chemical hypersensitivity two-step method on which the molecular model is based is a mature and reliable method which can be widely used in primary hospitals; and compared with international TNM staging, the molecular model has a higher predictive effect, and is simpler, easier, more sensitive and more specific.
Owner:SHANTOU UNIV MEDICAL COLLEGE
CD117-based chimeric antigen receptor (CAR) and application thereof
ActiveCN107400168AHigh expressionImprove immunityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsTumor therapy
The invention relates to a CD117-based chimeric antigen receptor (CAR) and application thereof, in particular to a method for constructing a CRA-T cell technology based on a tumor specific target CD117 and application of the method in anti-tumor therapy. The CAR is formed by connecting an antigen binding domain, a transmembrane domain, a costimulatory signal transduction zone and a CD3 zeta signal transduction domain in series, wherein the antigen binding domain binds to a tumor surface antigen, and the tumor surface antigen is CD117. The CAR provided by the invention is obtained by carrying out specific genetic modification on the tumor surface antigen CD117, and the modified antibody can enable the antigen-antibody binding force to be stronger and is not easy to mutate. Compared with other CARs and other tumor antigens, the CD117-based CRA has better effects and high target expression quantity, thus enhancing the immune effect of CAR-T cells and improving the therapeutic effect of the CAR-T cells.
Owner:SHENZHEN GENO IMMUNE MEDICAL INST
Soluble cd117 (sc-kit) for diagnosis of preeclampsia and eclampsia
This disclosure relates to methods of predicting and diagnosing preeclampsia and eclampsia in pregnant subjects. These methods include detecting a decrease of soluble c-kit in a sample obtained from the pregnant subject. A significantly reduced concentration of soluble c-kit in the sample as compared to a gestational age-adjusted control indicates that the pregnant subject will develop or has preeclampsia or eclampsia.
Owner:UNIVERSITY OF PITTSBURGH
Engraftment of stem cells with a combination of an agent that targets stem cells and modulation of immunoregulatory signaling
ActiveUS20170224737A1Good curative effectEffective stem cell engraftmentPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsImmunocompetenceSignalling pathways
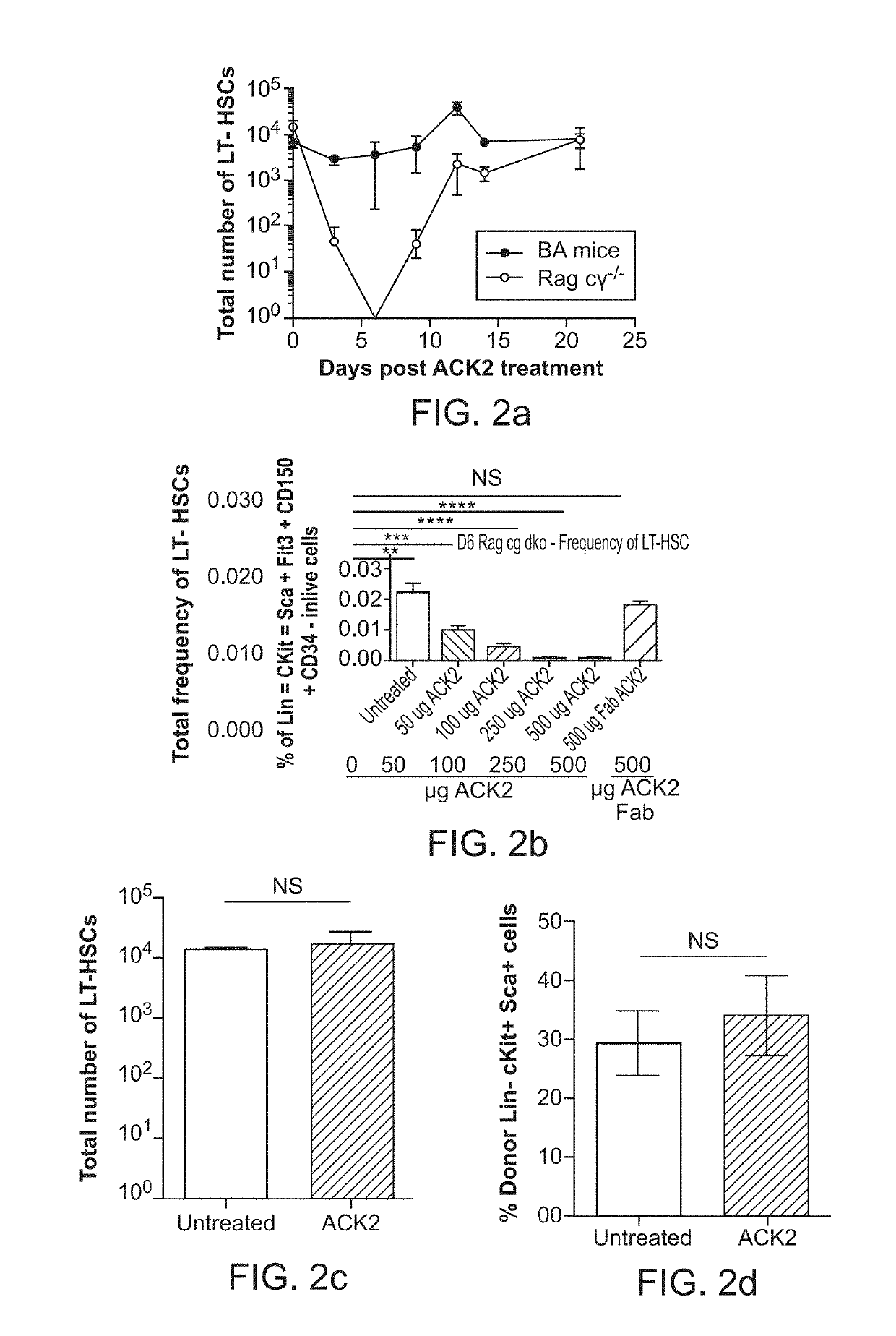
The present invention provides a clinically applicable method of stem cell transplantation that facilitates engraftment and reconstitutes immunocompetence of the recipient without requiring radiotherapy or chemotherapy, and without development of GVHD or graft rejection. Aspects of the present invention are based on the discovery that the depletion of the endogenous stem cell niche facilitates efficient engraftment of stem cells into that niche. In particular, the present invention combines the use of selective ablation of endogenous stem cells with a combination of antibodies specific for CD117, and agents that modulate immunoregulatory signaling pathways, e.g. agonists of immune costimulatory molecules, in combination with the administration to the recipient of exogenous stem cells, resulting in efficient, long-term engraftment, even in immunocompetent recipients.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Stem cell populations and methods of use
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
Ovarian cancer stem cell vaccine and preparation method thereof
InactiveCN105219731AHigh activityHigh tumorigenicityTumor/cancer cellsAntibody medical ingredientsCancer cellNatural Killer Cell Inhibitory Receptors
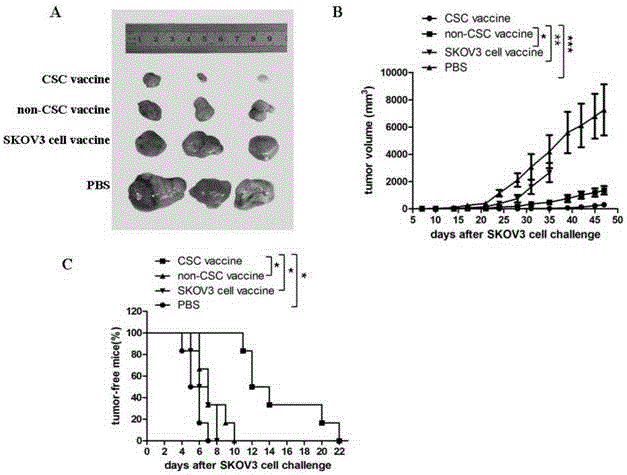
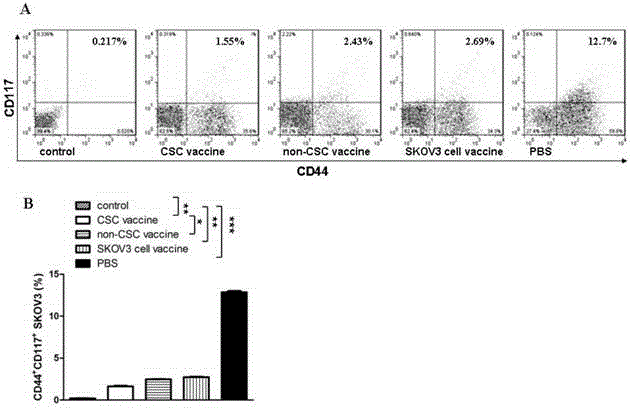
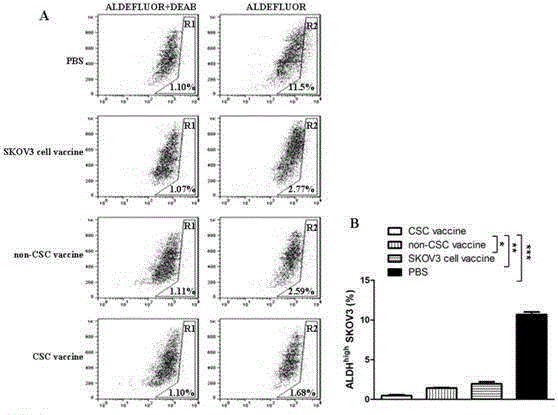
The invention relates to the field of molecular biology and discloses an ovarian cancer stem cell vaccine and a preparation method and application thereof. Cancer stem cells are insensitive to radiotherapy and chemotherapy, which is the root of cancer metastasis and recurrence. Aiming at active immunotherapy of the cancer stem cells, satisfactory therapeutic effect can be gained only on the condition that cancer 'seed' cells, namely the cancer stem cells are eliminated. The ovarian cancer stem cell CD117+CD44+ vaccine is capable of improving blood serum IFN (interferon)-gamma level, lowering TGF (transforming growth factor)-beta expression, enhancing NK cell activity, decreasing the number of ovarian cancer stem cells CD117+CD44+ remarkably and inhibiting cancer growth in animal experiments.
Owner:SOUTHEAST UNIV +1
Engraftment of stem cells with a combination of an agent that targets stem cells and modulation of immunoregulatory signaling
ActiveUS10406179B2Effective stem cell engraftmentIncreased riskPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesImmunocompetence
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Isolation, expansion and characterization of precursor/stem cells from dental tissues
A method for isolating and proliferating at least one type of precursor cell from dental origin from a single donor includes the following isolating the precursor cells from dental origin of a single donor in a sample collection media and preparing a primary stock culture. The primary stock culture is proliferated sequentially to obtain first, second and third sub-cultured stocks with cell counts ranging between 5×106 cells and 10×106 cells, 20×106 cells and 400×106 cells, 150×106 and 300×106 cells respectively. The precursor cells from the third subculture are harvested and cryo-preserved to obtain a precursor cell population for cell transplantation. The dental origin of the precursor cells is from pulp, apical papilla or periodontal ligament. The precursor cell originates from mesenchymal stem cells, ecto-mesenchymal cells, neural stem cells, dental progenitor cells or CD117+ cells.
Owner:HYGIEIA INNOVATION
Antibody composition and its application in leukemia and lymphoma typing
The invention belongs to the technical field of antibody medicines and provides two types of antibody compositions including CD45 antibody, CD13 antibody, CD33 antibody and CD7 antibody or including CD45 antibody, CD117 antibody, CD34 antibody and CD19 antibody. The invention further provides a leukemia and lymphoma typing kit comprising the two types of antibody compositions and capable of being used with a flow cytometry to achieve typing of various hematological neoplasms.
Owner:ZHEJIANG BOZHEN BIOTECH CO LTD
Method for inducing differentiation of pluripotent stem cells into germ cells
ActiveUS20180187147A1Improve efficiencyGood reproducibilityGenetically modified cellsCulture processSurface markerInduced pluripotent stem cell
The invention provides a method for inducing human primordial germ cell-like (PGC-like) cells from human pluripotent stem cells, with high efficiency and high reproducibility, and a cell surface marker for identifying human PGC-like cells. In particular, the invention provides a method for producing a human PGC-like cell from a human pluripotent stem cell, includes a step of producing a mesoderm-like cell by culturing a human pluripotent stem cell in a culture medium comprising activin A and a GSK3β inhibitor, and a step of culturing the mesoderm-like cell in a culture medium containing BMP. The invention also provides a method for producing an isolated human PGC-like cell, which includes the aforementioned two steps and the additional step of selecting a cell positive to at least one cell surface marker selected from the group consisting of PECAM (CD31), INTEGRINα6 (CD49f), INTEGRINβ3 (CD61), KIT (CD117), EpCAM, PODOPLANIN and TRA1-81.
Owner:KYOTO UNIV
Compositions and methods for the depletion of CD117+ cells
ActiveUS10882915B2Peptide/protein ingredientsMammal material medical ingredientsAutoimmune conditionCancer cell
The invention provides compositions and methods useful for the depletion of CD117+ cells and for the treatment of various hematopoietic diseases, metabolic disorders, cancers, and autoimmune diseases, among others. Described herein are antibodies, antigen-binding fragments, and conjugates thereof that can be applied to effect the treatment of these conditions, for instance, by depleting a population of CD117+ cells in a patient, such as a human. The compositions and methods described herein can be used to treat a disorder directly, for instance, by depleting a population of CD117+ cancer cells or autoimmune cells. The compositions and methods described herein can also be used to prepare a patient for hematopoietic stem cell transplant therapy and to improve the engraftment of hematopoietic stem cell transplants by selectively depleting endogenous hematopoietic stem cells prior to the transplant procedure.
Owner:CRISPR THERAPEUTICS AG
Spontaneously immortalized multiponent mesenchymal cell-line derived from mouse subcutaneous adipose tissue: tool for regenerative medicine and bioactive molecules and/or drugs screening
InactiveUS8835165B2Microbiological testing/measurementDrug screeningSubcutaneous adipose tissueMedicine
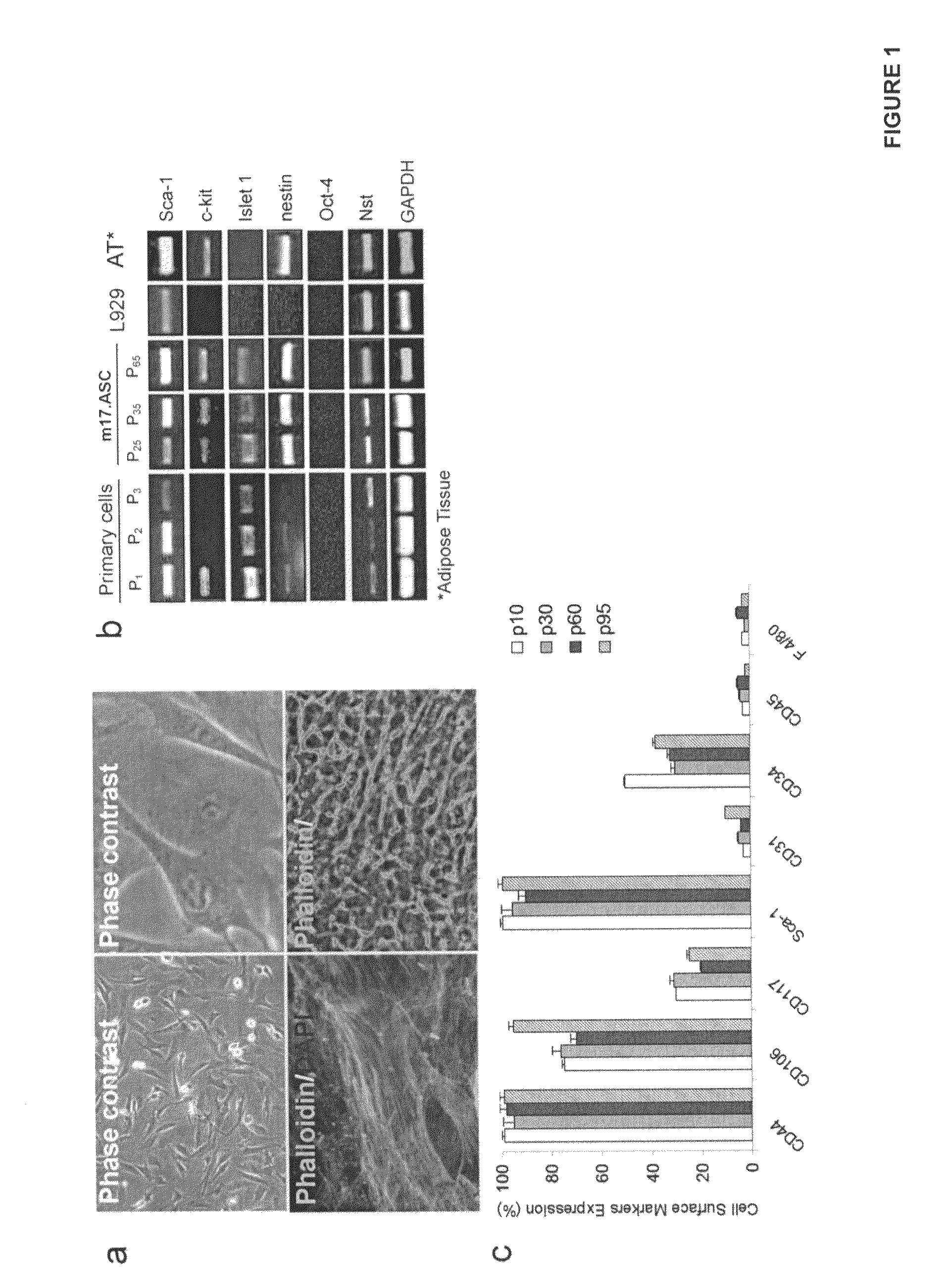
Disclosed is a spontaneously immortalized multipotent mesenchymal cell-line, wherein the cell-line has been isolated from mouse subcutaneous adipose tissue, and wherein the cell-line presents fibroblastoid morphology and expresses Sca-1, c-Kit / CD117, nestin, nucleostemin, CD44 and CD106 markers.
Owner:UNIV DEGLI STUDI DEL PIEMONTE ORIENTALEAMEDEO AVOGADRO
Method for the generation of a cell composition ventral midbrain dopaminergic progenitor cells
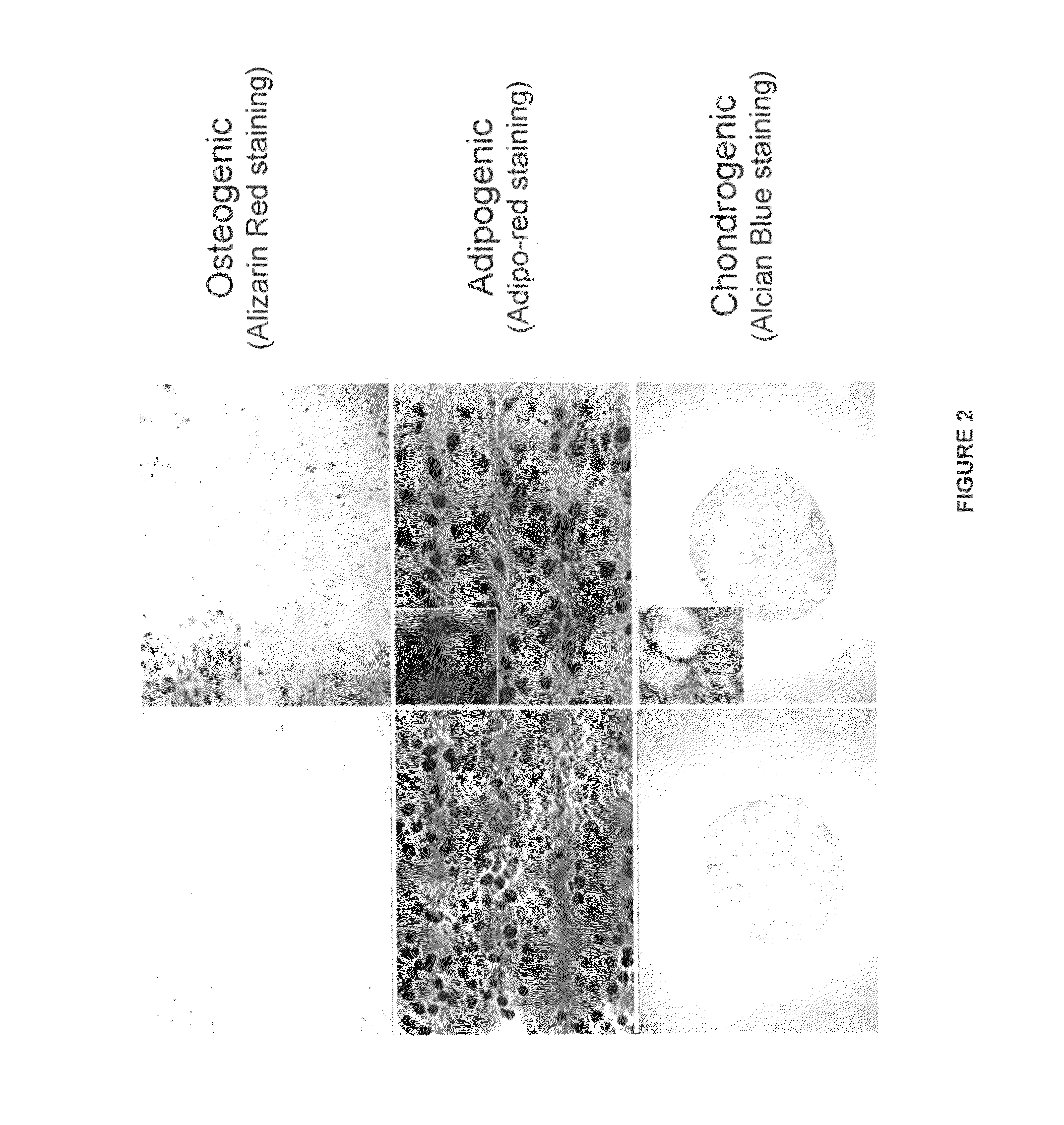
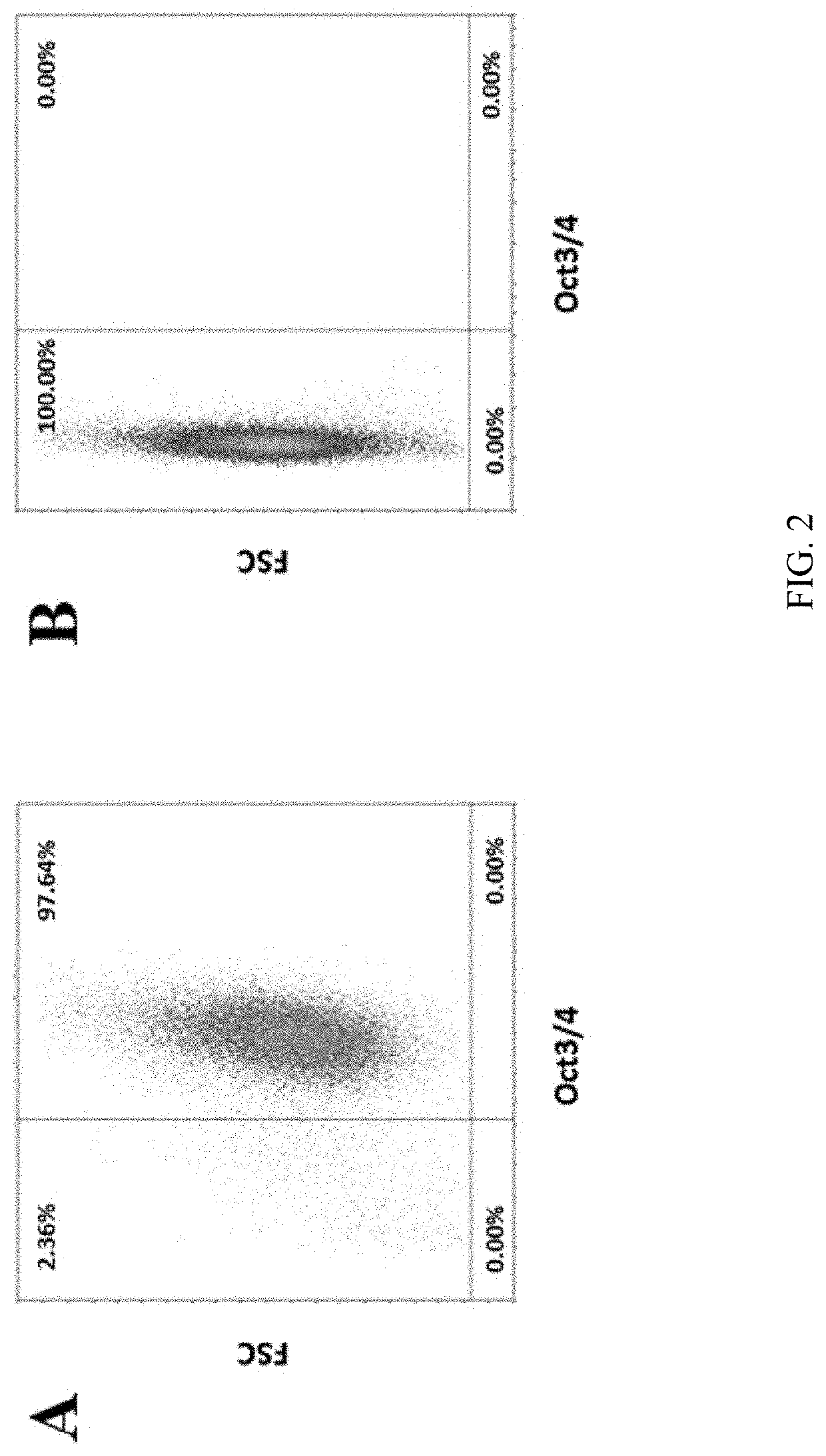
PendingUS20210079344A1Ameliorate and reverse symptomCulture processNervous system cellsMidbrainDopaminergic
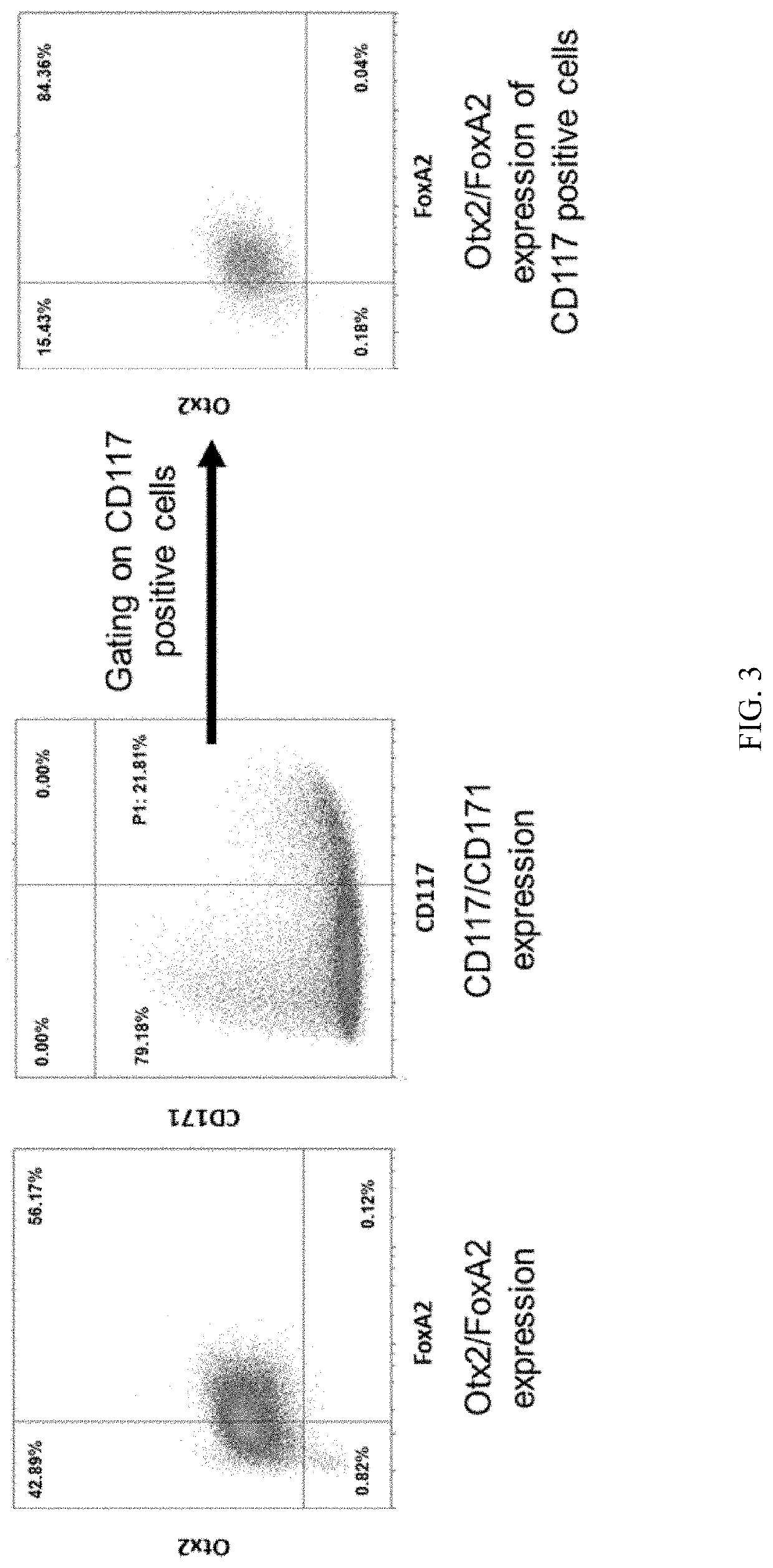
The present invention discloses an in vitro method for the generation of a cell composition comprising or consisting of ventral midbrain dopaminergic progenitor cells from a cell composition comprising pluripotent and / or multipotent stem cells, the method comprising the steps of A) differentiating said pluripotent and / or multipotent stem cells into ventral dopaminergic progenitor cells, thereby generating a cell composition comprising ventral dopaminergic progenitor cells comprising ventral midbrain dopaminergic progenitor cells and ventral hindbrain dopaminergic progenitor cells, and B) Enriching CD117 positive cells from said cell composition comprising ventral dopaminergic progenitor cells by using an antigen binding molecule specific for the CD117 antigen, thereby generating said cell composition comprising or consisting of ventral midbrain dopaminergic progenitor cells. Cell compositions obtainable by said method are also disclosed.
Owner:MILTENYI BIOTEC B V & CO KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com