CD117-based chimeric antigen receptor (CAR) and application thereof
A chimeric antigen receptor and antigen technology, which is applied in the field of tumor cell immunotherapy, can solve the problems of reduced expression, poor curative effect, and CD19 easily mutated expression, and achieves the effect of enhanced immune effect and high expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
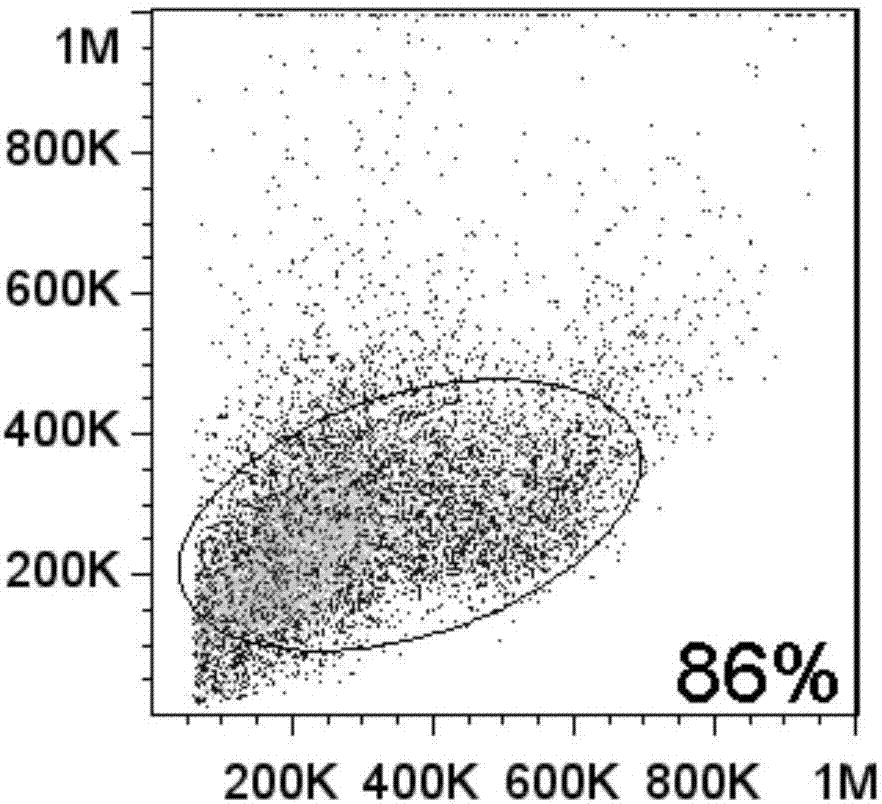
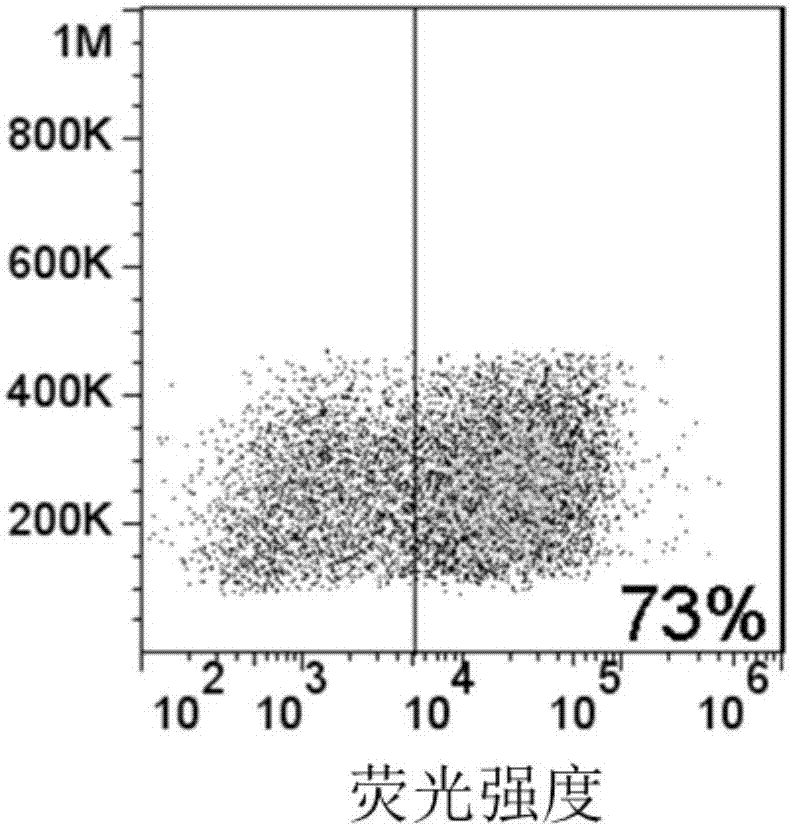
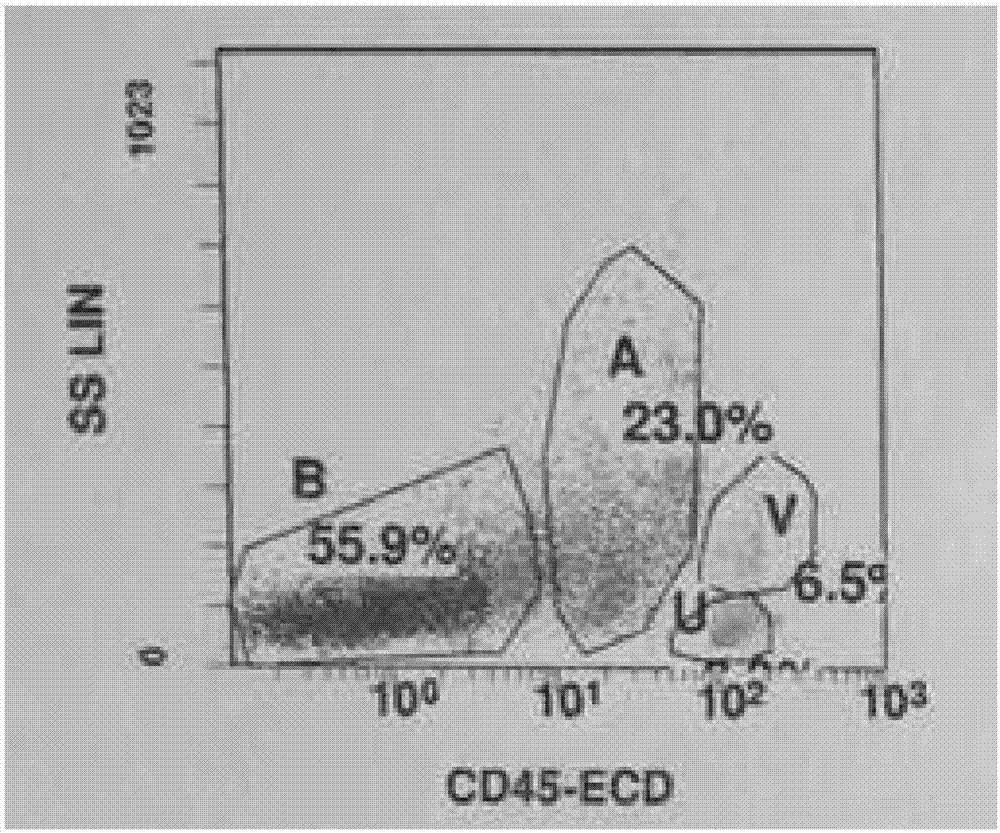
Image
Examples
Embodiment 1
[0050] Example 1: Construction of Chimeric Antigen Receptor
[0051] (1) Synthesize Secretory signal peptide, CD117 antigen binding domain, CD8α and / or CD28 transmembrane domain, CD28 signaling domain, CD137 signaling domain and / or CD27 signaling domain, CD3ζ signaling domain through whole gene synthesis Domain, 2A sequence and caspase 9 domain, namely Secretory-CD117-CD28-CD137-CD27-CD3ζ-2A-iCasp9;
[0052] The nucleotide sequence SEQ ID NO.3 of the chimeric antigen receptor is as follows:
[0053] ATGCTGCTGCTGGTGACCAGCCTGCTGCTGTGCGAGCTGCCCCACCCCGCCTTCCTGCTGATCCCCCAGGTGCAGCTGGTGCAGAGCGGCGCCGCCGTGAAGAAGCCCGGCGAGAGCCTGAAGATCAGCTGCAAGGGCAGCGGCTACAGATTCACCAGCTACTGGATCGGCTGGGTGAGACAGATGCCCGGCAAGGGCCTGGAGTGGATGGGCATCATCTACCCCGGCGACAGCGACACCAGATACAGCCCCAGCTTCCAGGGCCAGGTGACCATCAGCGCCGGCAAGAGCATCAGCACCGCCTACCTGCAGTGGAGCAGCCTGAAGGCCAGCGACACCGCCATGTACTACTGCGCCAGACACGGCAGAGGCTACAACGGCTACGAGGGCGCCTTCGACATCTGGGGCCAGGGCACCATGGTGACCGTGAGCAGCGGCAGCACCAGCGGCAGCGGCAAGCCCGGCAGCAGCGAGGGCAGCACCAAG...
Embodiment 2
[0054] Example 2: Lentiviral packaging
[0055] (1) Culture 293T cells in a six-well plate, 1×10 6 cells / well, cultivated for 17-18 hours;
[0056] (2) Add 600 μL / well of fresh DMEM containing 10% FBS;
[0057] (3) Add the following reagents to a sterile centrifuge tube: Take 75 μL of DMEM supernatant per well, 2.7 μg of auxiliary DNA mixture (1.8 μg pNHP, 0.5 μg pHEF-VSV-G, 0.2 μg pHEF-GFP) And 0.8 μg of pTYF DNA vector, vortex;
[0058] (4) Pipette 7 μL of Superfect from the center of each well plate into the centrifuge tube for 5 times, and let stand at room temperature for 7-10 minutes;
[0059] (5) Add the DNA-Superfect mixture in the centrifuge tube to each culture well drop by drop, and vortex to mix well;
[0060] (6) 37°C 3% CO 2 Cultivate in the incubator for 4-5 hours;
[0061](7) Aspirate the culture medium of the medium, wash the medium with 1.5mL AIM-V, and add 1.5mL of AIM-V to continue culturing;
[0062] (8) Return the culture medium to 3% CO 2 Cultiva...
Embodiment 3
[0063] Example 3: Purification and concentration of lentivirus
[0064] 1) Virus purification
[0065] Cell debris was removed by centrifugation (1000g, 5 minutes) to obtain virus supernatant, which was filtered through a 0.45 micron low protein binding filter, virus was aliquoted and stored at -80°C;
[0066] Typically, transfected cells can produce 10 6 to 10 7 Lentiviral vectors for titration of transducing units.
[0067] 2) Concentrate lentiviral vectors with Centricon filters
[0068] (1) In a biological safety cabinet, take a Centricon tube, disinfect it once with 70% alcohol, and then wash it three times with sterile PBS;
[0069] (2) Add 18ml of virus supernatant to each Centricon P-20 filter tube, then centrifuge at 2500g for 30 minutes or until the virus volume is reduced to 0.5ml;
[0070] (3) Shake the filter tube, then centrifuge at 400g for 2 minutes to collect the concentrated virus into the collection cup. Finally, pool the virus in all tubes into one ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com