Antibody composition and application of antibody composition in leukemia and lymphoma typing
An antibody composition and composition technology, applied in the direction of analytical materials, measuring devices, instruments, etc., can solve the problems of promotion restrictions, increase the difficulty of operation, increase the cost of typing, etc., and achieve broad application prospects, simple operation, and cost saving Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 reagent
[0041] In the following examples, monoclonal antibodies against the following two groups of cell surface antigens are used, and different monoclonal antibodies in each group are labeled with different fluorescent labels selected from FITC, PE, PerCP-cy5.5 and APC:
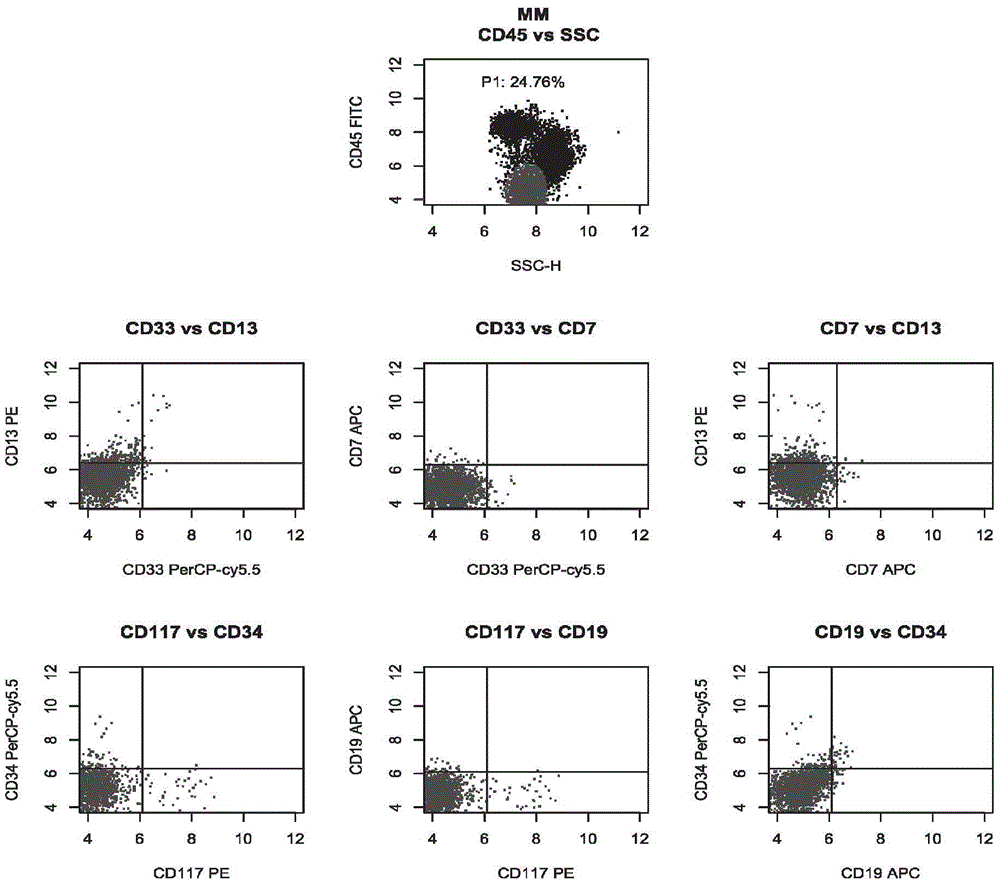
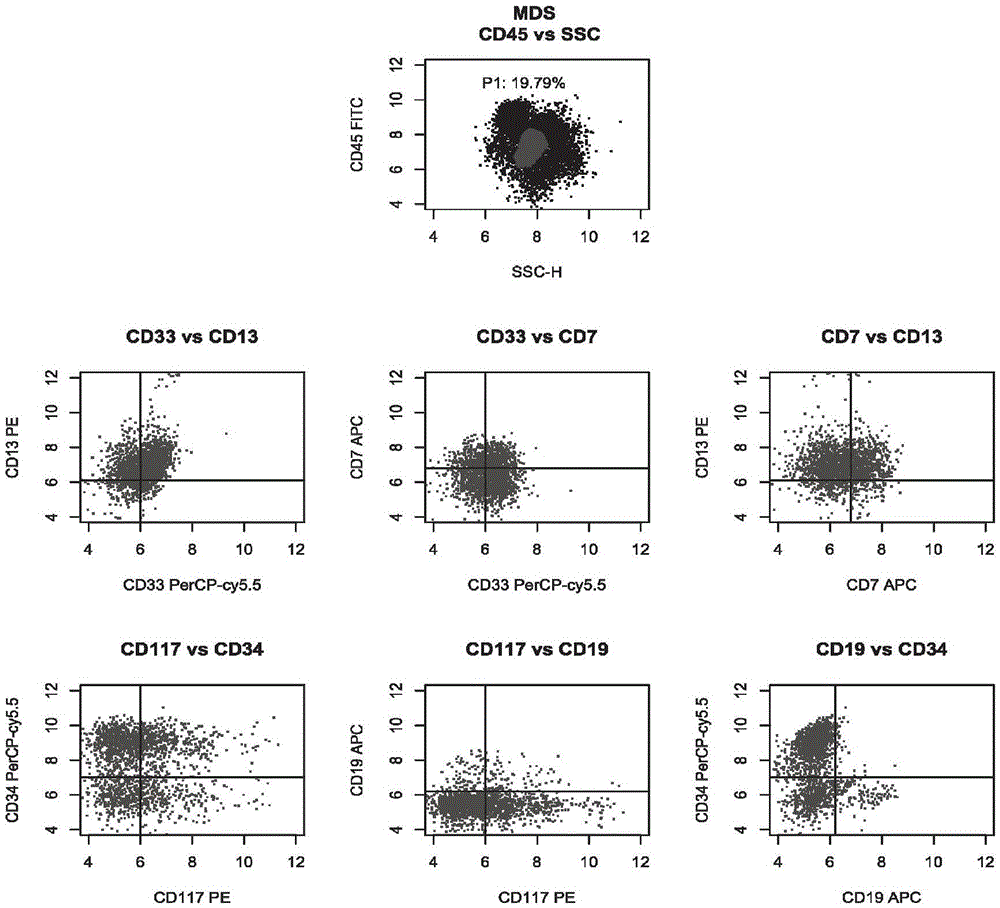
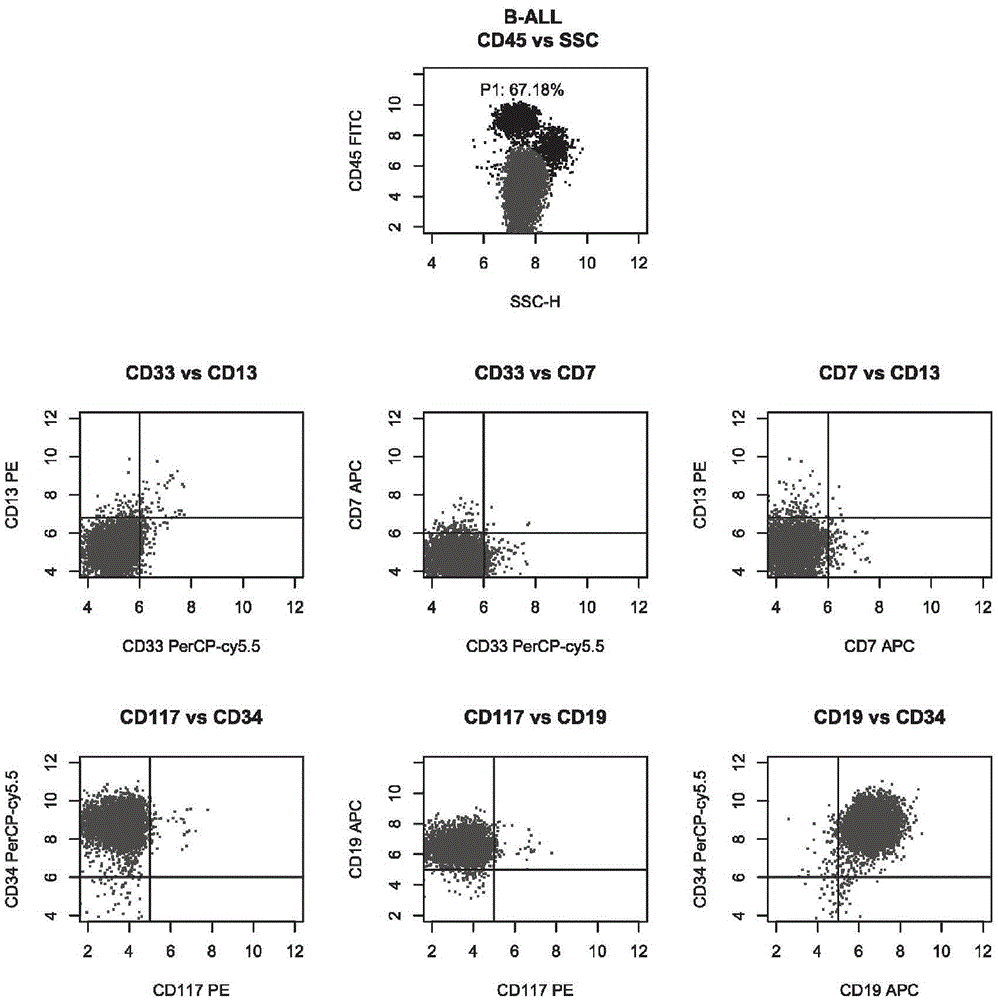
[0042] Group 1: CD45, CD13, CD33 and CD7;
[0043] Group 2: CD45, CD117, CD34 and CD19.
[0044] These fluorescently labeled monoclonal antibodies can be directly purchased through public channels, and the specific sources are shown in Table 1.
[0045] Table 1 Product-related monoclonal antibody information
[0046] raw material
Antibody clone number
manufacturer
supplier
APC-CD7 monoclonal antibody
CD7-6B7
American Biolegend
Dakowei Biotechnology Co., Ltd.
PE-CD13 Monoclonal Antibody
WM15
American Biolegend
Dakowei Biotechnology Co., Ltd.
APC-CD19 monoclonal antibody
HIB19
American Bioleg...
Embodiment 2
[0051] The processing of embodiment 2 sample
[0052] Adjust the bone marrow or peripheral blood samples stored in heparin or EDTA anticoagulant tubes to a cell density of 1-5×10 6 / ml, and then filtered through a 200-mesh industrial sieve to remove agglomerate-like substances in the sample to ensure a single cell suspension state, and store at 2-8°C to obtain the processed sample.
Embodiment 3
[0053] The typing detection of embodiment 3 samples
[0054] Take two flow tubes, mark ① and ② respectively, and then add the antibody mixture in tubes A and B of the kit in Example 1, respectively, in an amount of 3 μL. Then, take 100 μL of the sample treated in Example 2, add 50 μL to the flow tubes ① and ② after aliquoting, shake and mix with a vortex shaker, and incubate at room temperature (25° C.) in the dark for 15 minutes.
[0055] Dilute 10× PBS buffer solution to 1× PBS buffer solution with distilled water, then dilute 10× red blood cell lysate solution into 1× red blood cell lysate solution with 1× PBS buffer solution, and then pour into flow tubes ① and ② after incubation. Add 1ml of 1× red blood cell lysate, oscillate evenly with a vortex shaker, and let stand at room temperature (25°C) in the dark for 10 minutes.
[0056] Then, centrifuge flow tubes ① and ② at 1500rpm for 5 minutes, remove the supernatant, add 200 μL 1×PBS to resuspend, and use four-color flow c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com