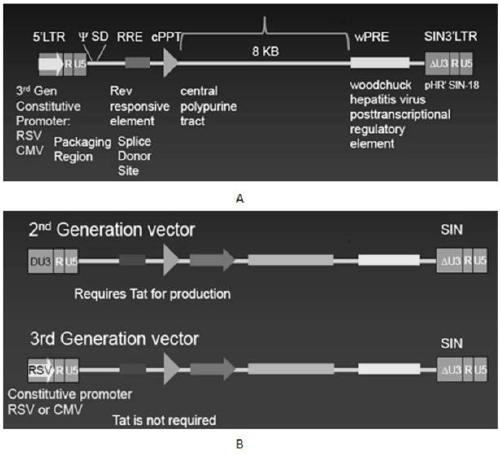
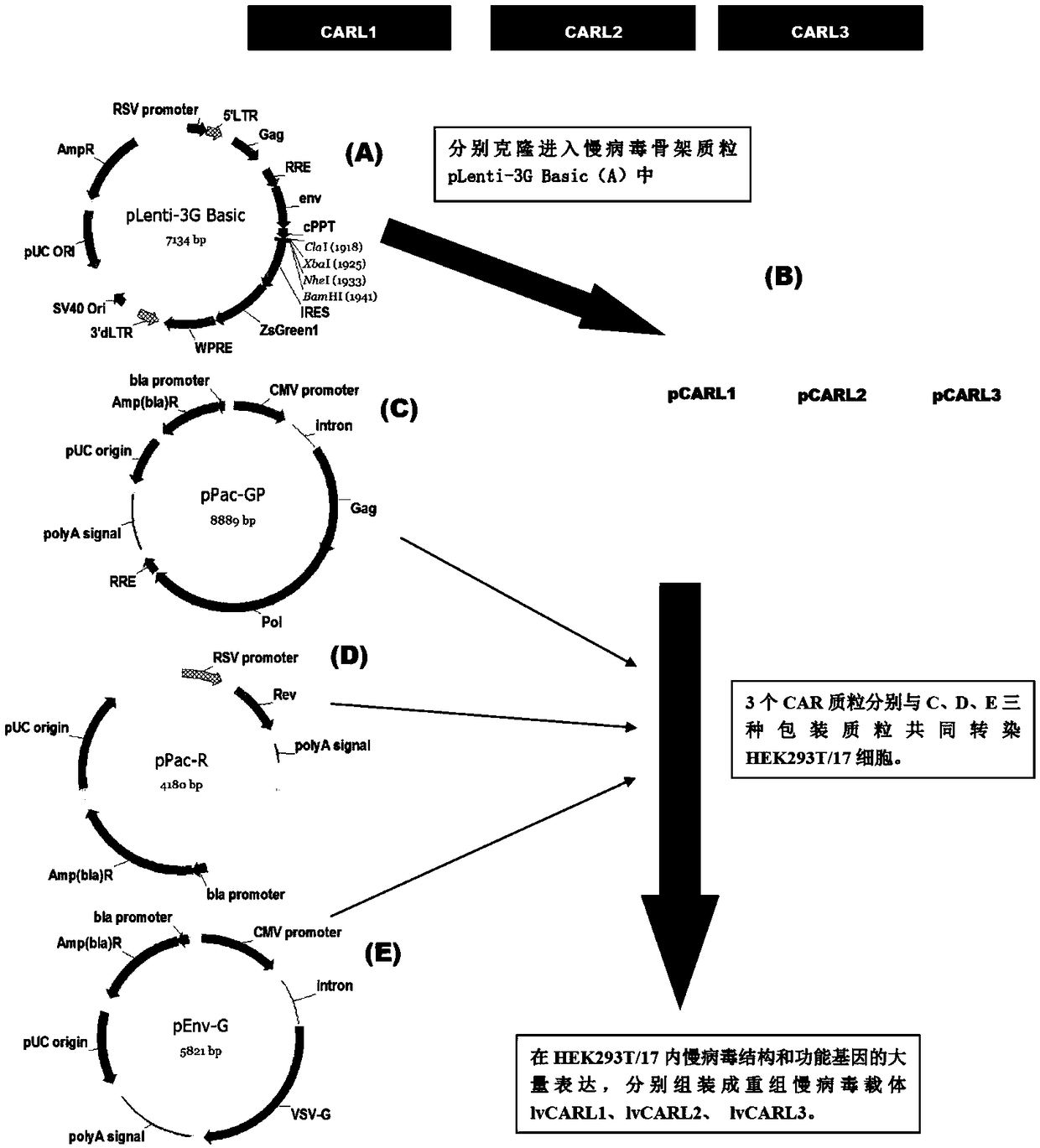
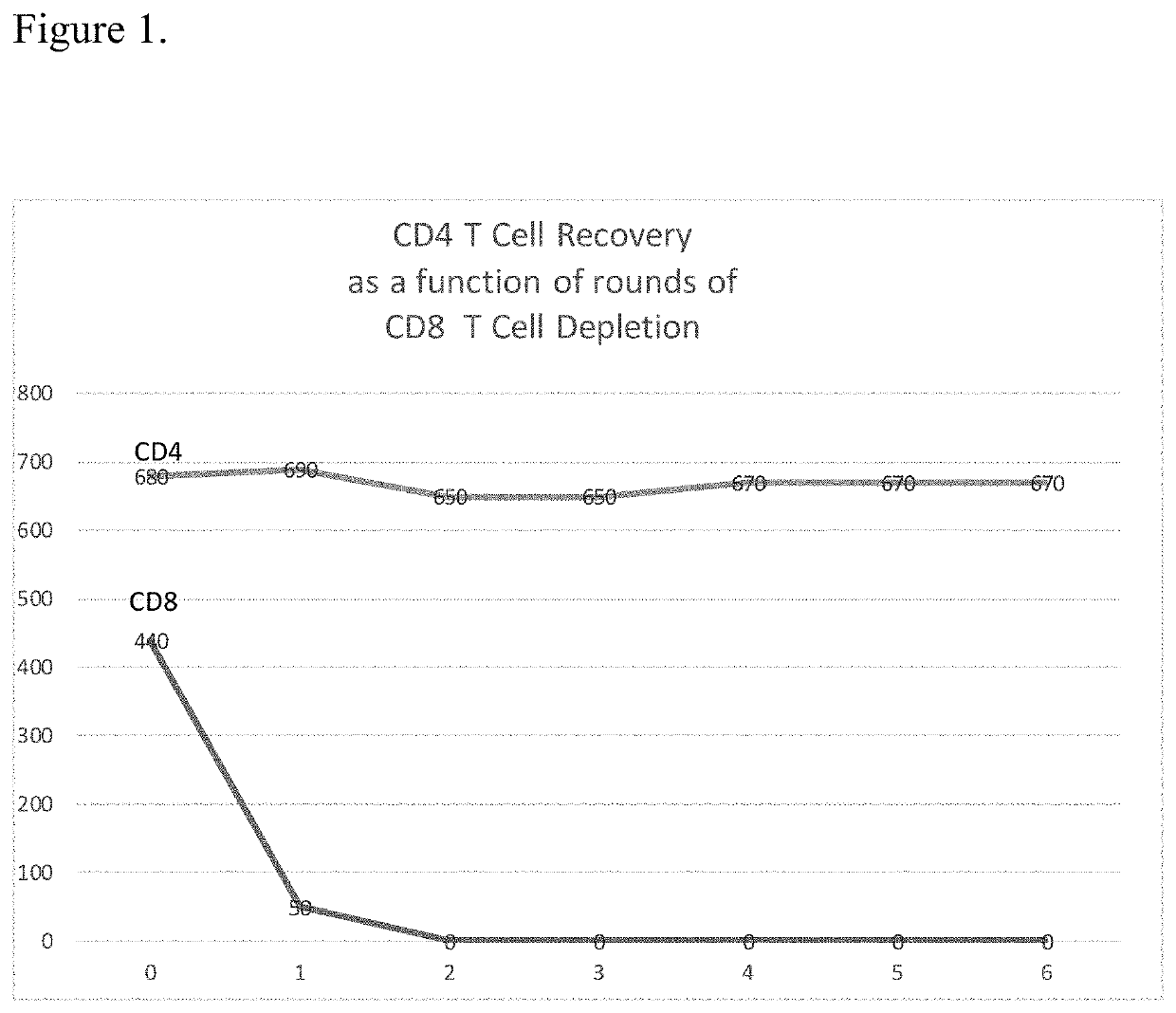
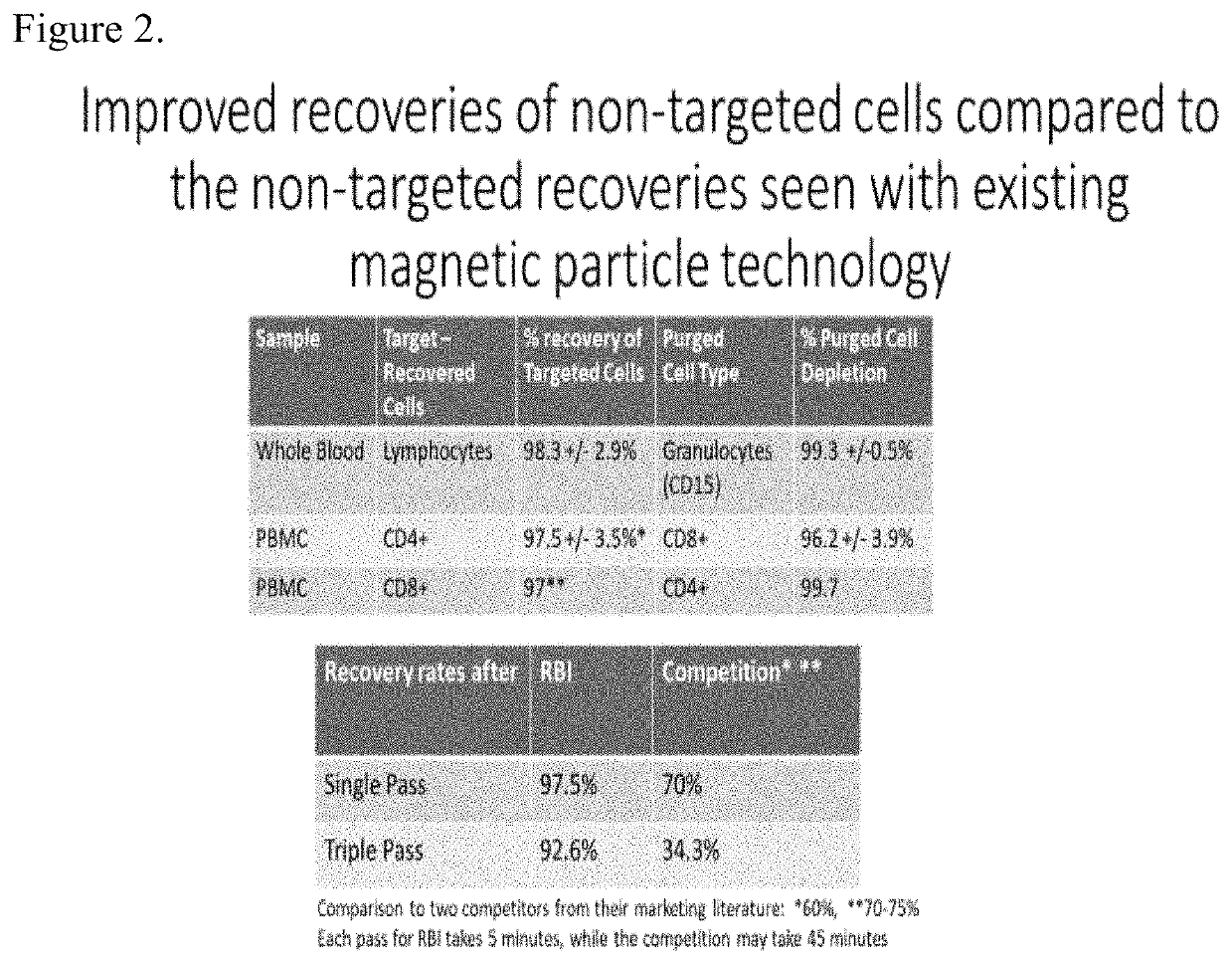
Patents
Literature
34 results about "CAR T-cell therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The progress made with CAR T-cell therapy in children with ALL “has been fantastic,” said Terry Fry, M.D., a lead investigator on several POB trials of CAR T cells. CD19-targeted CAR T cells were initially tested in adults.
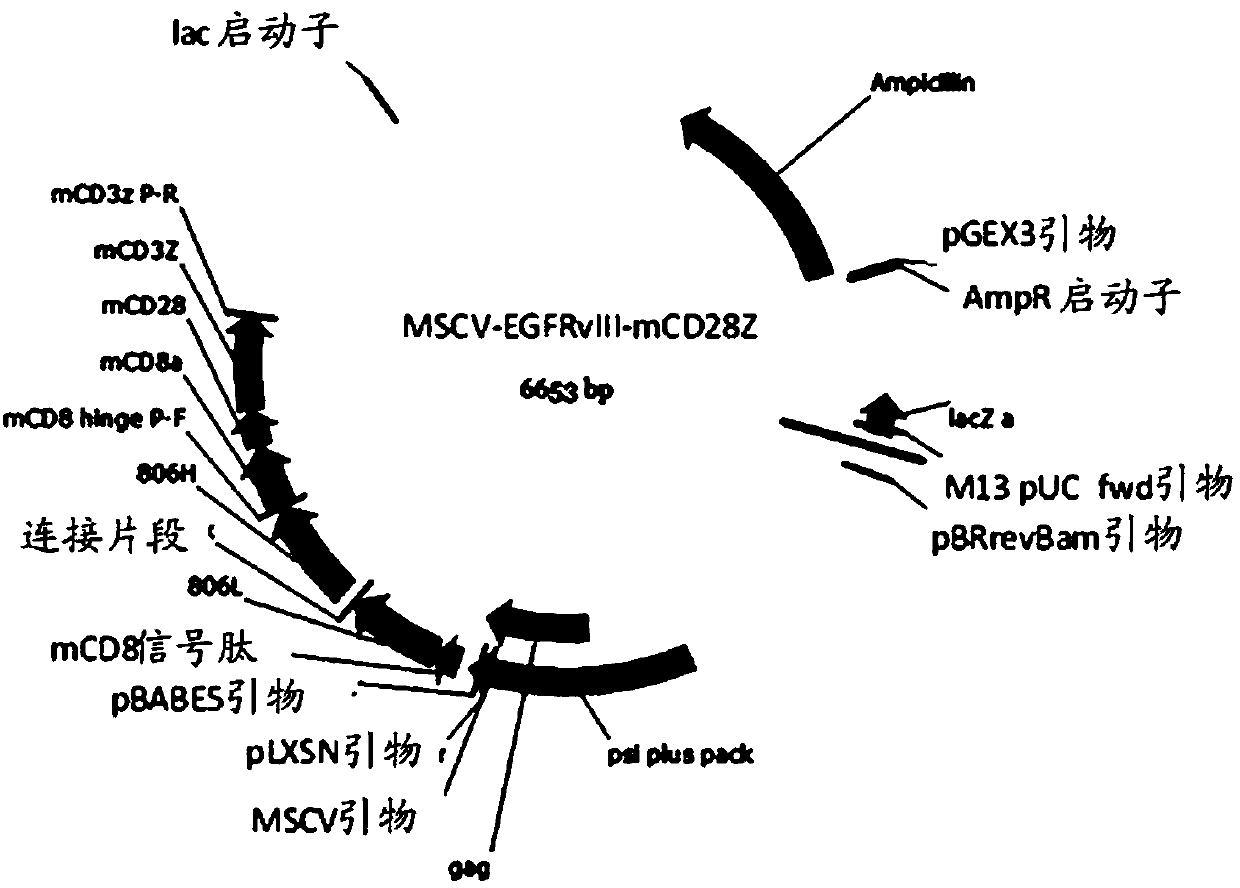
Chimeric antigen receptors (CAR) and methods for making and using the same
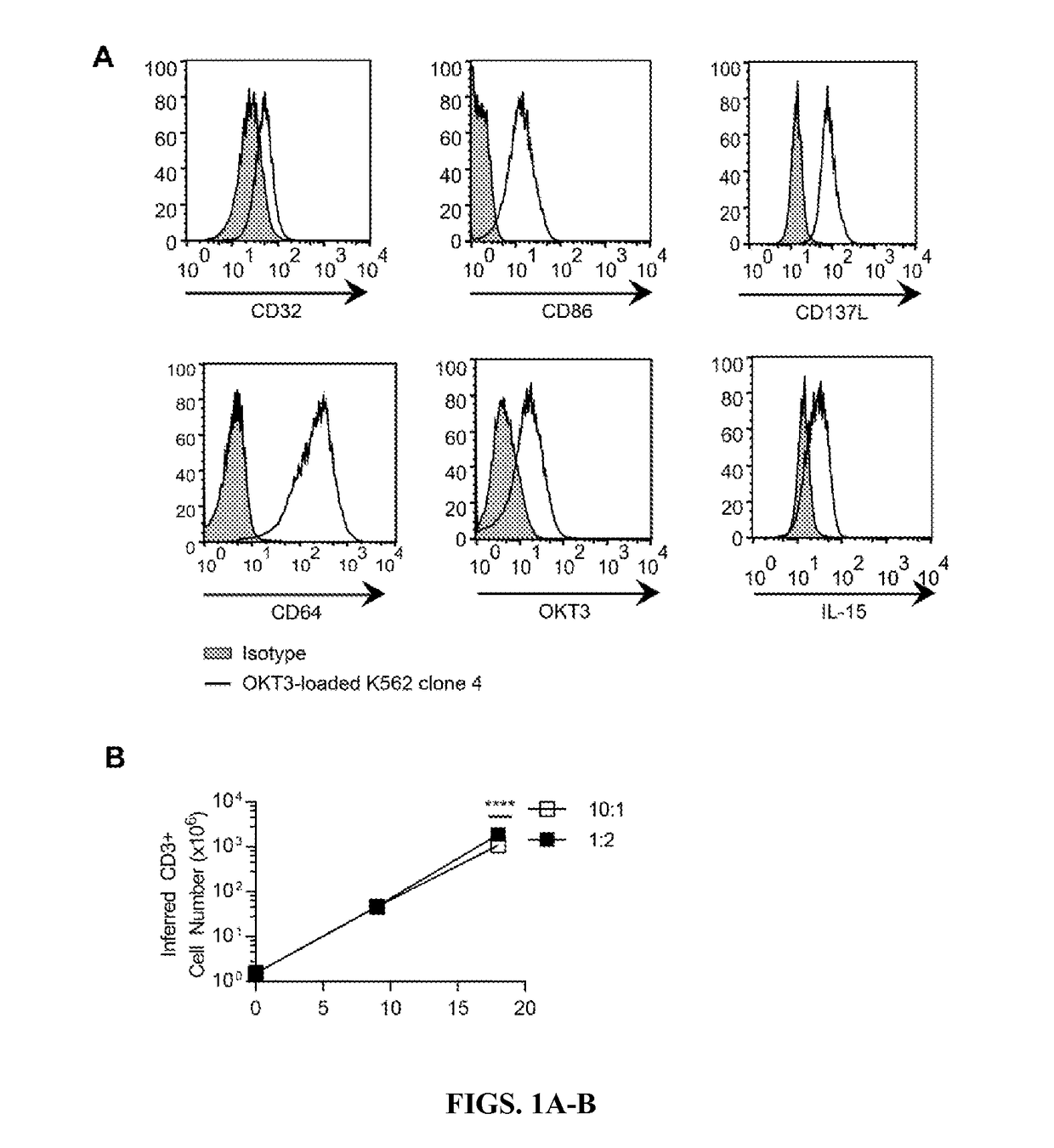
InactiveUS20170158749A1Facilitate cell targetingReduce off-target cytotoxicity of cellAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenCAR T-cell therapy
Chimeric antigen receptors (CARs) and CAR-expressing T cells are provided that can specifically target cells that express an elevated level of a target antigen. Likewise, methods for specifically targeting cells that express elevated levels of antigen (e.g., cancer cells) with CAR T-cell therapies are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
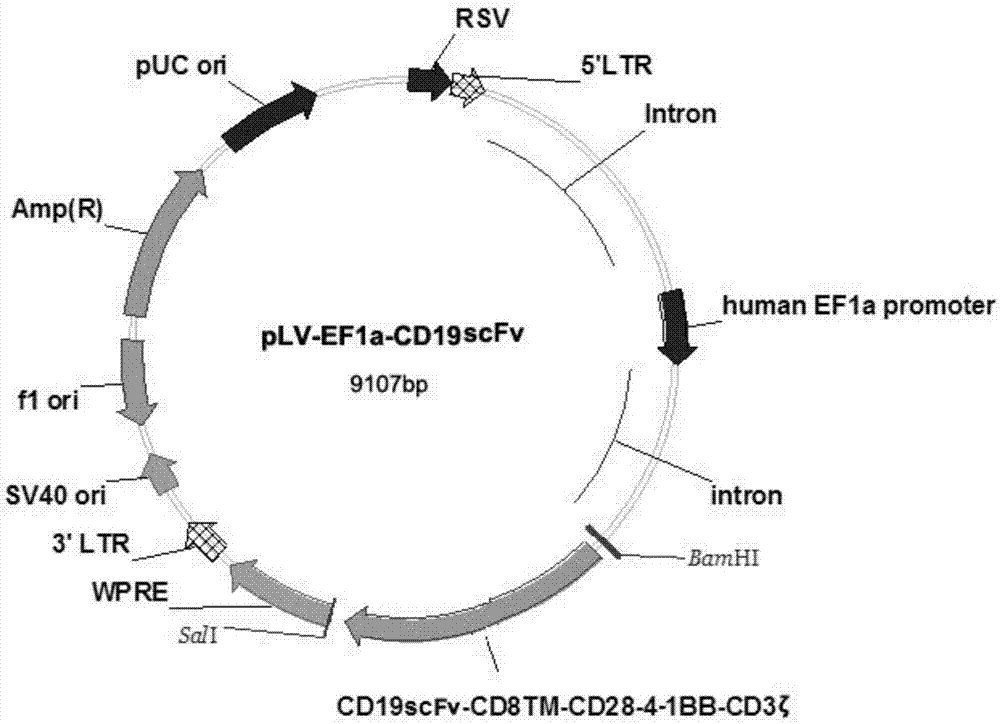
CD19 targeted CAR (chimeric antigen receptor)-T cell, preparation method and application
InactiveCN107287164AIncrease lethalityHigh transduction efficiencyGenetically modified cellsMammal material medical ingredientsCAR T-cell therapySingle-Chain Antibodies
The invention provides a CD19 targeted CAR (chimeric antigen receptor)-T cell, a preparation method and an application and relates to the field of immune cells. The activation capacity of T cells in CAR-T cell therapy can be improved, the problem of insufficient transfection efficiency can be solved, and the CAR-T cell has a high killing capacity for CD19. The CD19 targeted CAR-T cell expresses a CD19 targeted CAR gene on surface, and the CD19 targeted CAR gene is formed by connecting a single-chain antibody CD19ScFv of CD19, a hinge region and a transmembrane region of CD8, an intracellular signal structure of CD28, an intracellular signal structure of 4-1BB and an intracellular signal structural domain of CD3zeta in series, and the base sequence of the CD19 targeted CAR gene is shown as SEQ ID NO:2.
Owner:青岛见康华美医学检验有限公司
Macrophages capable of targeting tumor cells and preparation method thereof
ActiveCN109266618AActivate phagocytosisEasy accessPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyAntigen receptors
Owner:赛元生物科技(杭州)有限公司
Joint use of immunologic effector cells and radiation in tumor treatment
PendingCN109908176AImprove anti-cancer effectAvoid damageEnergy modified materialsImmunoglobulins against cell receptors/antigens/surface-determinantsLocal radiotherapyCAR T-cell therapy
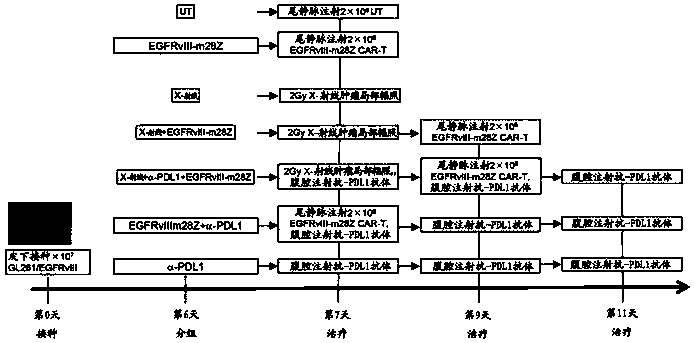
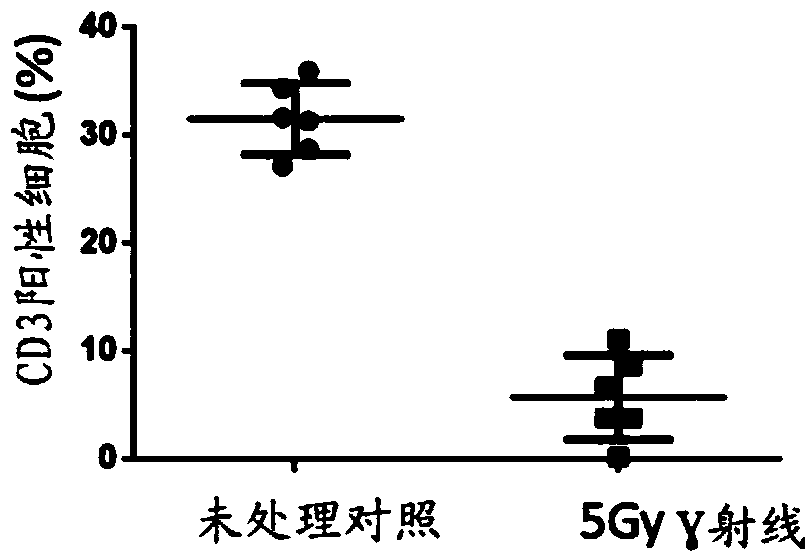
The invention relates to a tumor treatment method and particularly provides joint use of immunologic effector cells and radiation in tumor treatment, and a method of treating an individual with tumorsby joint use of immunologic effector cells and local radiation. The method provides no lymphocyte removal for the individual; the immunologic effector cells comprise a receptor to recognize tumor antigens to tumors. Tests prove that the method provided herein has significant antitumor effect for solid tumors; at the premise of pretreatment without removing lymphocytes, the joint treatment of CAR-T cell therapy and local radiotherapy with no lymphocyte removal helps attain better treatment effect than CAR-T treatment with lymphocyte removal; antitumor treatment effect is improved greatly.
Owner:CRAGE MEDICAL CO LTD
CAR-T cell capable of efficiently and stably expressing inhibiting antibody and application thereof
PendingCN107523547AAntibacterial agentsGenetically modified cellsCAR T-cell therapyAntigen receptors
The invention provides a CAR-T cell capable of efficiently and stably expressing an inhibiting antibody and application thereof. Specifically, the invention provides a transgenic T cell, and the genome of the transgenic T cell is stably integrated with expression cassettes containing nucleic acid sequences coding chimeric antigen receptors and immune checkpoint inhibiting antibodies, wherein two ends of the expression cassettes contain inverted terminal repeats of transposons. In the genome of the pluripotent CAR-T cell, the expression cassettes of the inhibiting antibodies are stably integrated via a transposon system, so the CAR-T cell has the activity of stably and efficiently expressing the inhibiting antibodies on the premise that original killing activity of the T cell is maintained, transfer-back CAR-T cells are prevented from inhibition by a tumor microenvironment, and residual tumor-specific T cells can be activated in situ. Meanwhile, a molecular brake system is introduced to ensure the security of CAR-T cell therapy, and transfer-back pluripotent CAR-T cells can be timely removed if necessary.
Owner:SHANGHAI CELL THERAPY RES INST +1
Reducing immune tolerance induced by PD-L1
ActiveUS9572837B2Less effectAntibody mimetics/scaffoldsGenetic material ingredientsCAR T-cell therapyAntigen receptors
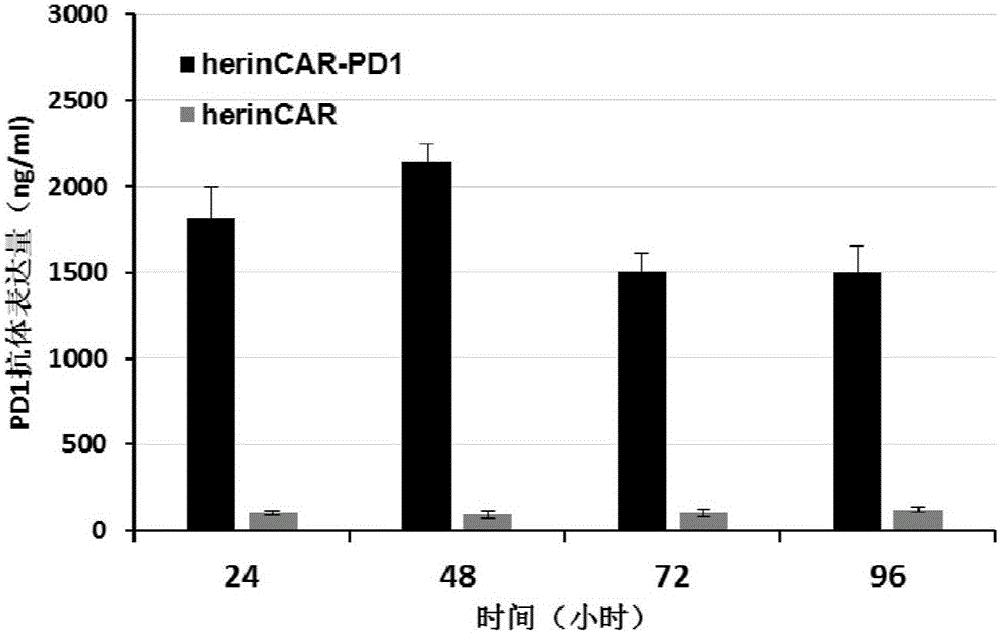
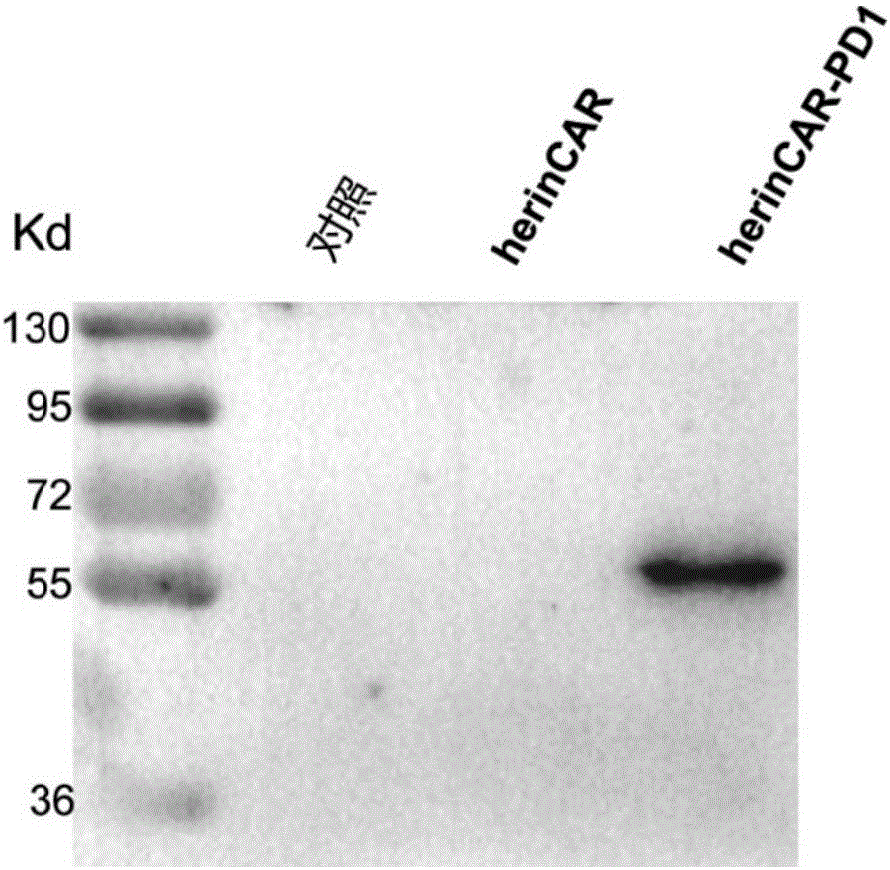
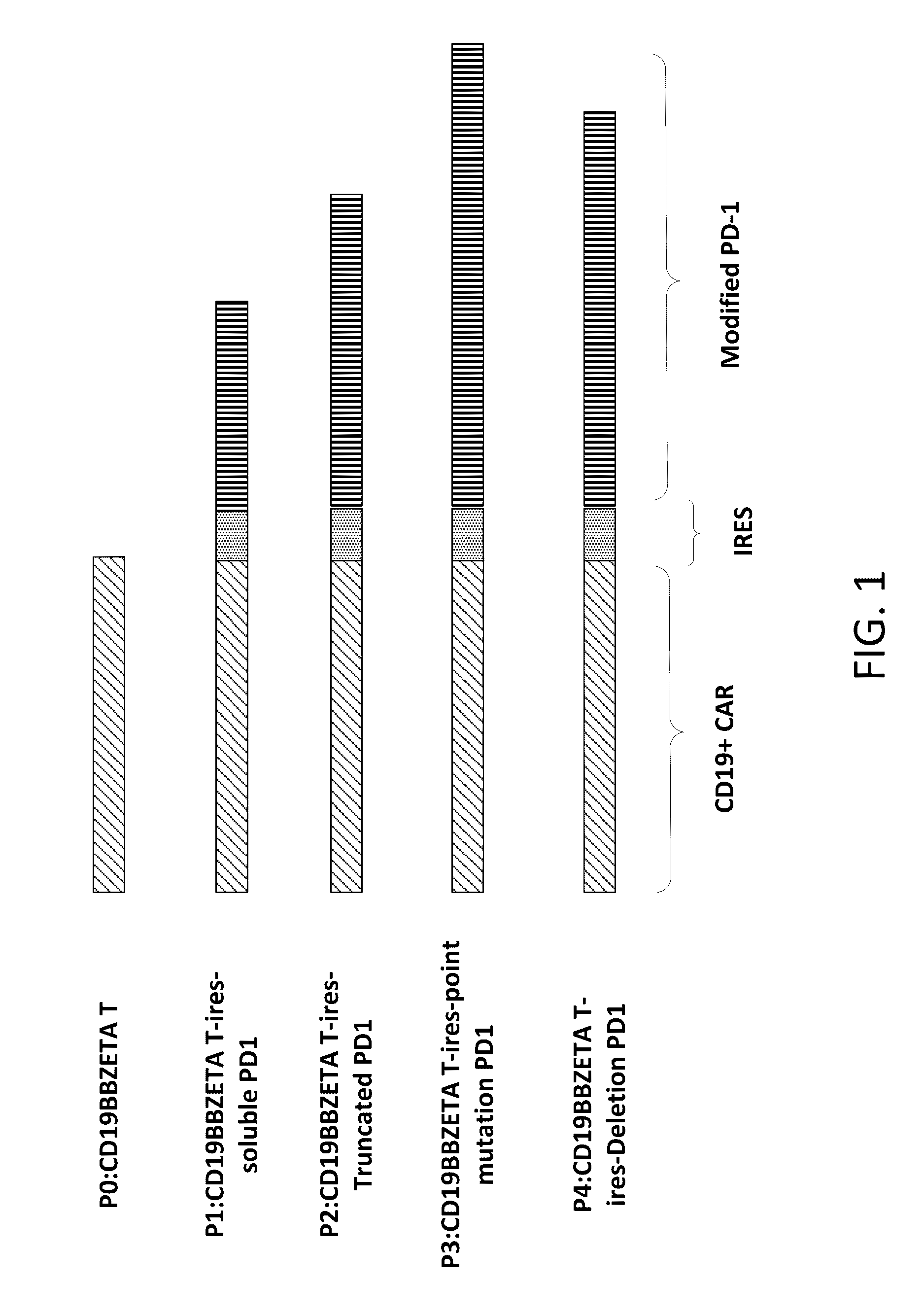
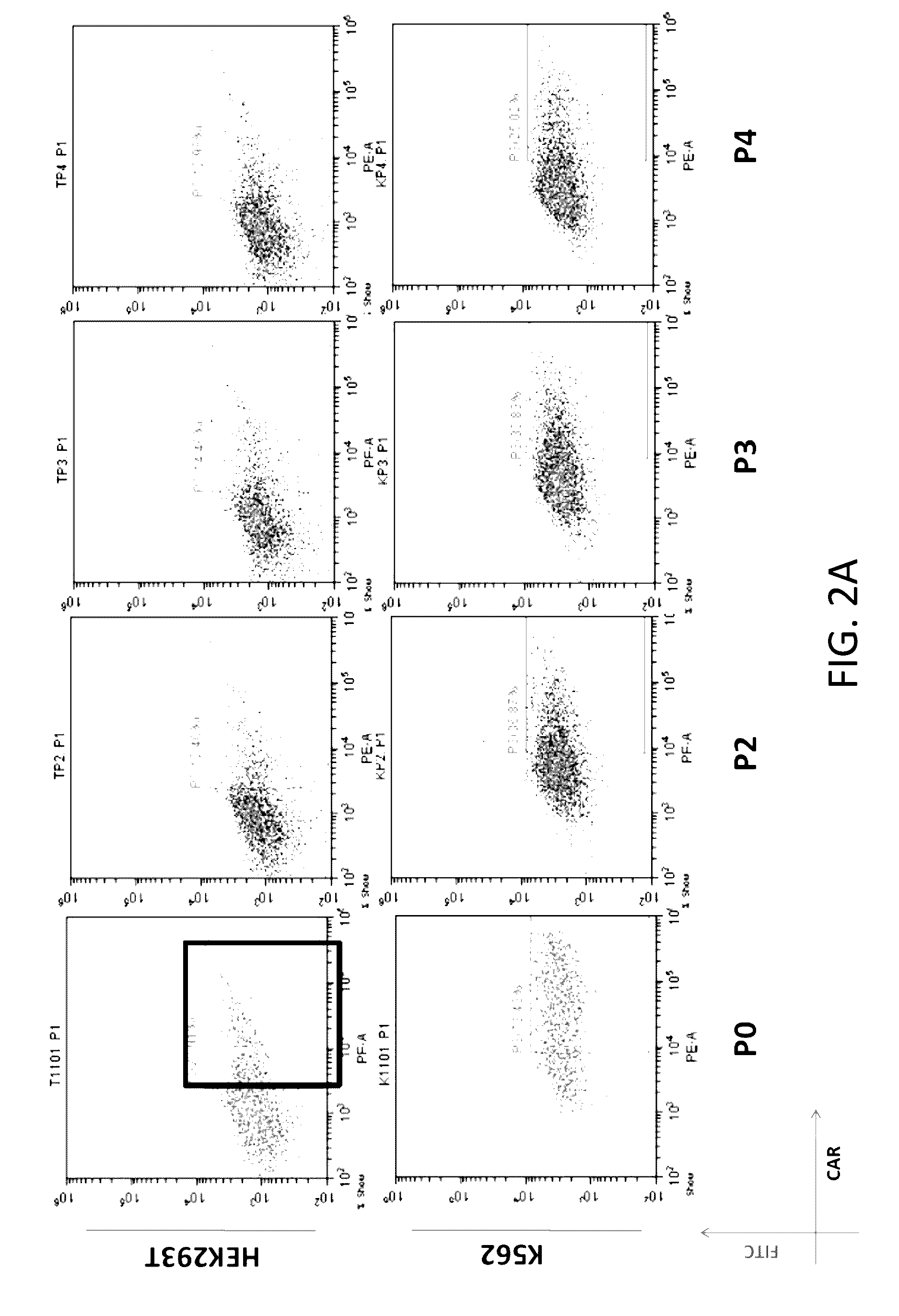
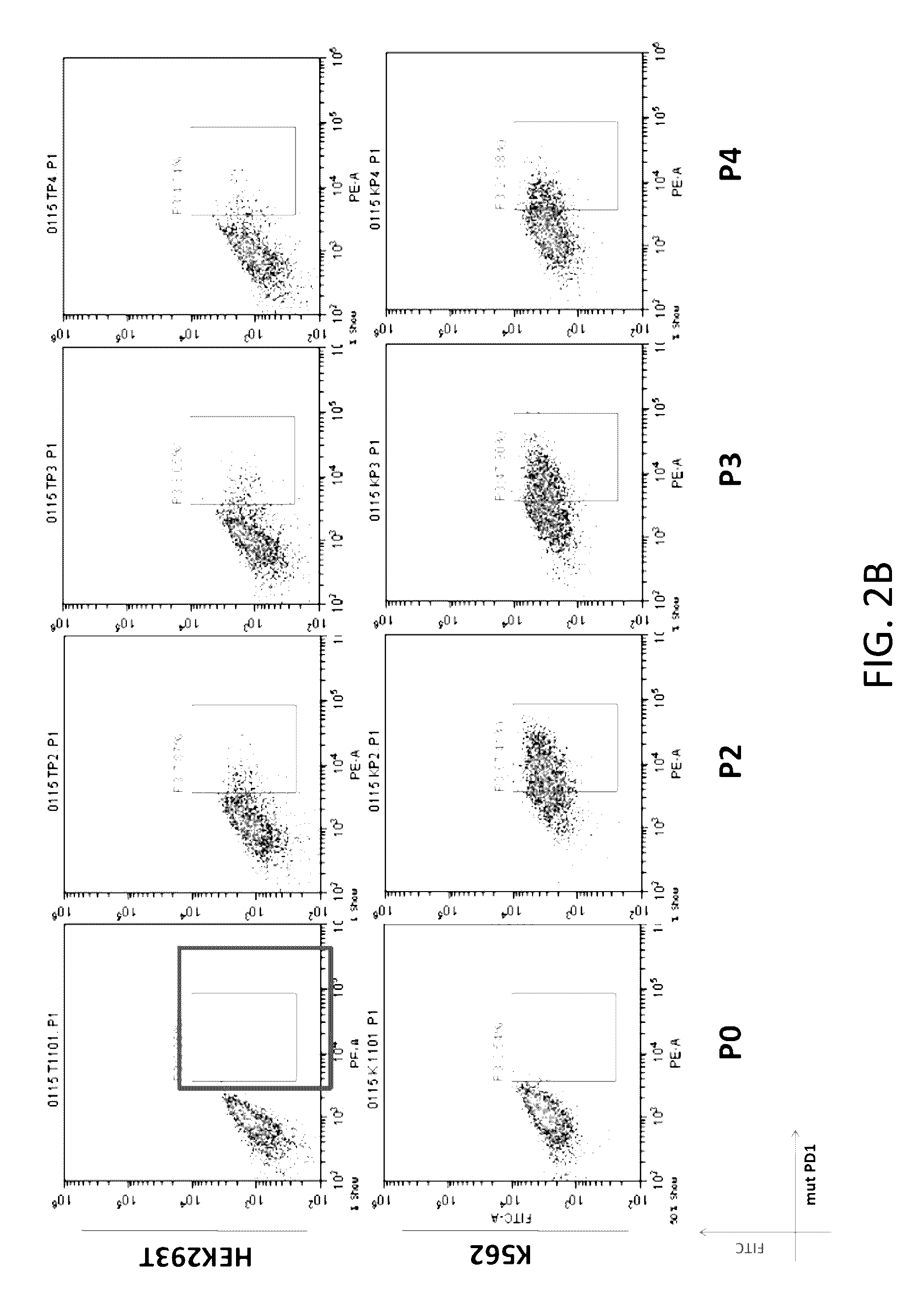
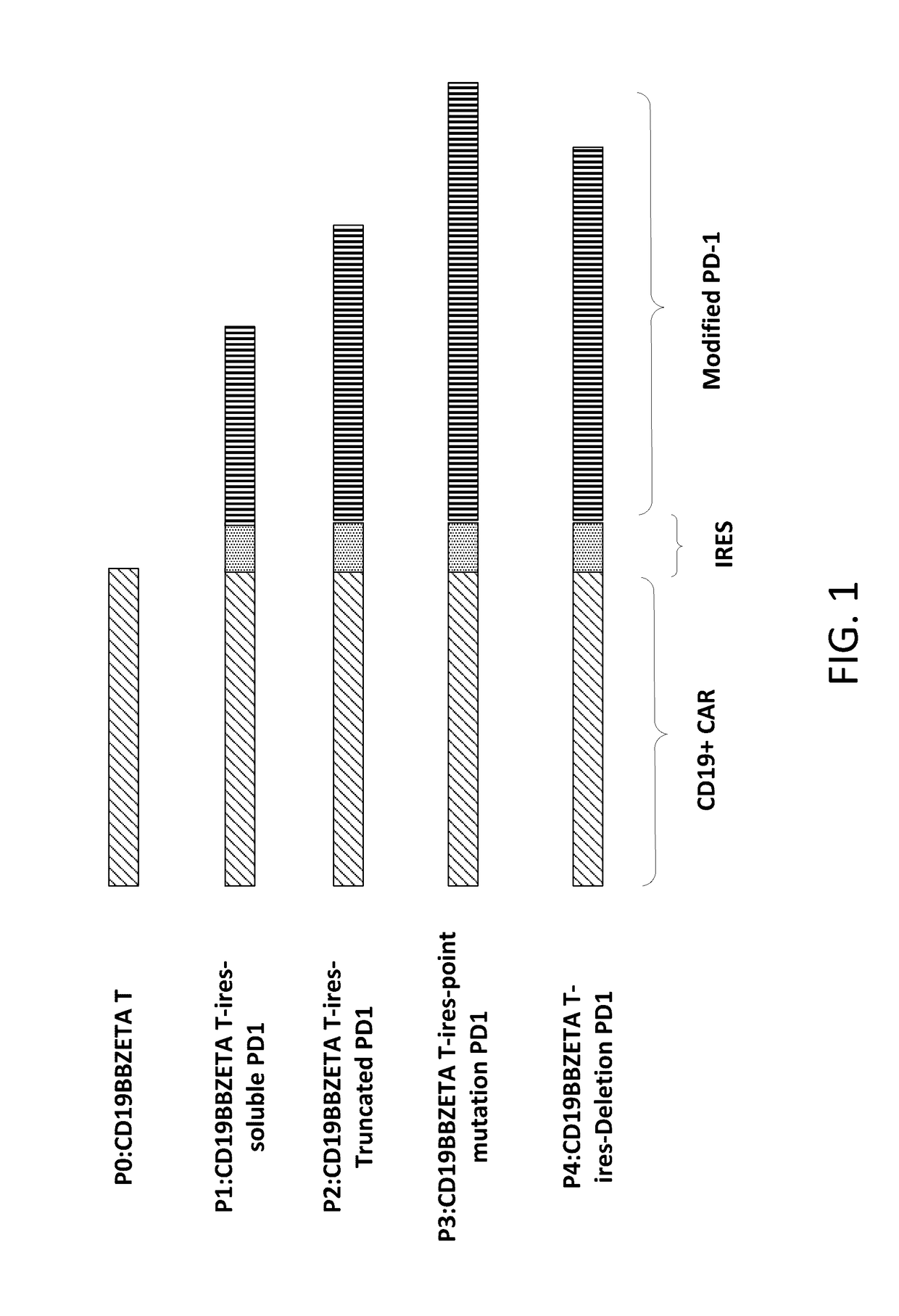
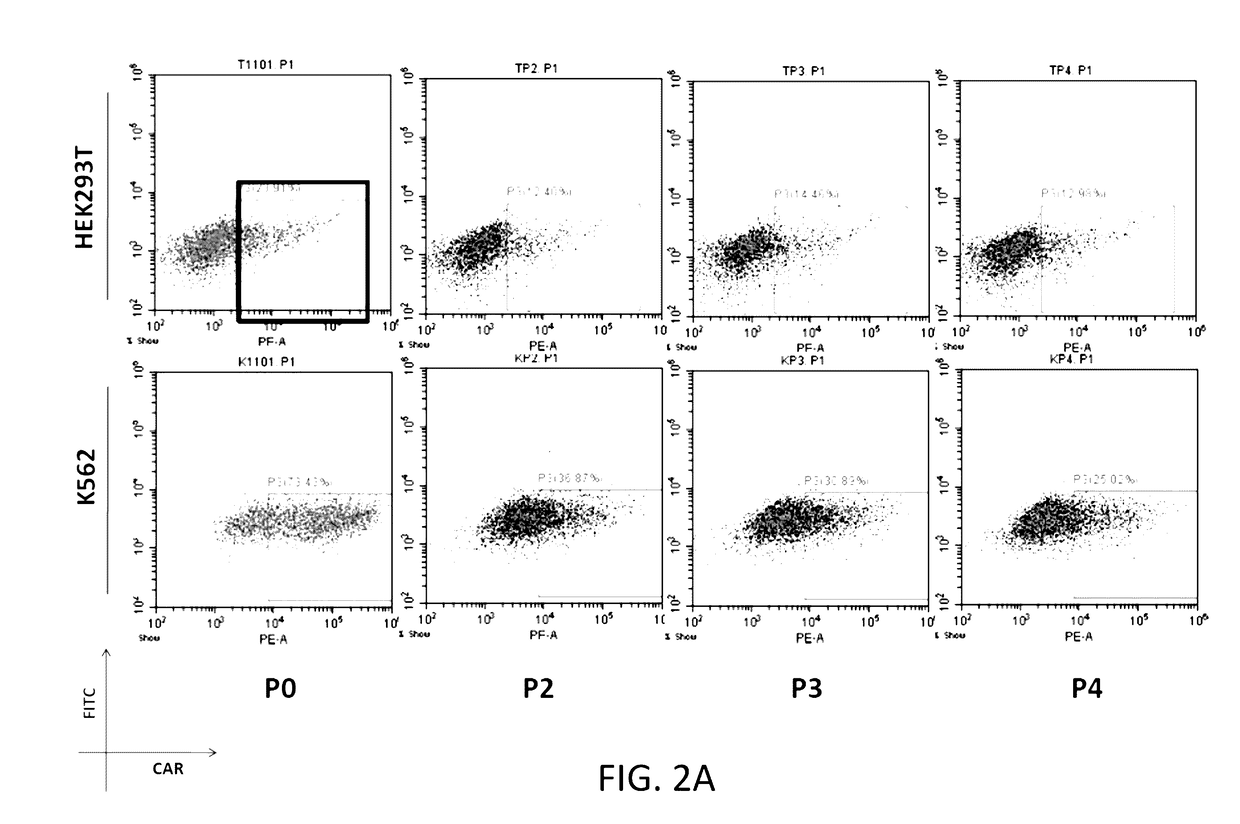
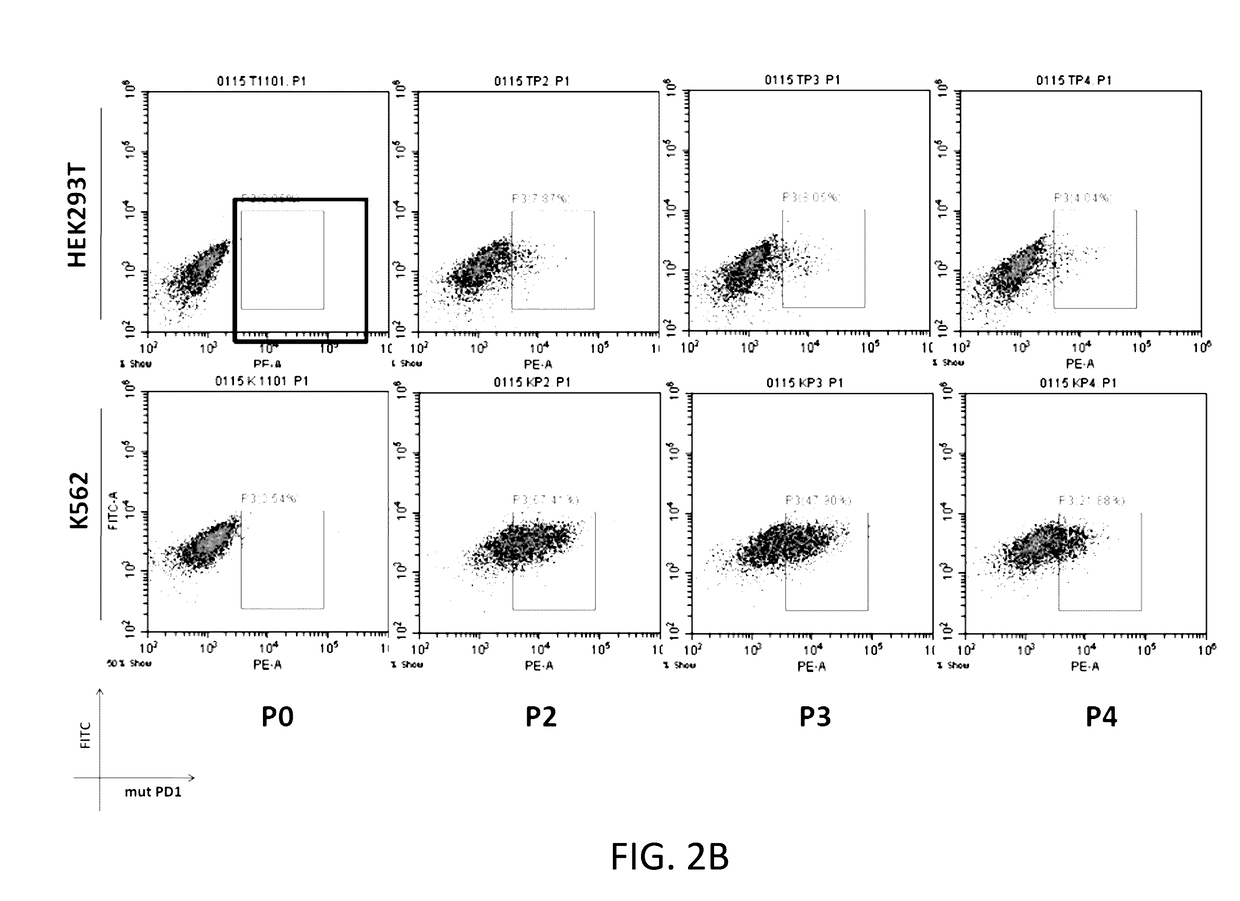
The present disclosure relates to compositions and methods for reducing immune tolerance associated with CAR T cell therapy. Embodiments of the present disclosure include isolated nucleic acid sequence comprising a nucleic acid sequence that encodes modified programmed cell death protein 1 (PD-1) and a nucleic acid sequence that encodes chimeric antigen receptor (CAR).
Owner:INNOVATIVE CELLULAR THERAPEUTICS HLDG LTD
Reducing Immune Tolerance Induced by PD-L1
ActiveUS20170096638A1Antibody mimetics/scaffoldsGenetic material ingredientsCAR T-cell therapyNucleic acid sequencing
The present disclosure relates to compositions and methods for reducing immune tolerance associated with CAR T cell therapy. Embodiments of the present disclosure include isolated nucleic acid sequence comprising a nucleic acid sequence that encodes modified programmed cell death protein 1 (PD-1) and a nucleic acid sequence that encodes chimeric antigen receptor (CAR).
Owner:INNOVATIVE CELLULAR THERAPEUTICS HLDG LTD
CAR-T cell capable of efficiently and stably expressing activated antibody and application thereof
InactiveCN107523549AAntibacterial agentsGenetic material ingredientsCAR T-cell therapyAntigen receptors
The invention provides a CAR-T cell capable of efficiently and stably expressing an activated antibody and application thereof. Specifically, the genome of the transgenic T cell in the invention is stably integrated with expression cassettes containing nucleic acid sequences coding activated antibodies of chimeric antigen receptors and immune costimulatory molecules or receptors of the immune costimulatory molecules, wherein two ends of the expression cassettes contain inverted terminal repeats of transposons. In the genome of the pluripotent CAR-T cell, the expression cassettes of the activated antibodies are stably integrated via a transposon system, so the CAR-T cell has the activity of stably and efficiently expressing the activated antibodies on the premise that original killing activity of the CAR-T cell is maintained, and transfer-back cells are allowed to rapidly propagate at a tumor site. Meanwhile, a molecular brake system is introduced to ensure the security of CAR-T cell therapy, and transfer-back pluripotent CAR-T cells can be timely removed if necessary.
Owner:SHANGHAI CELL THERAPY RES INST +1
Marker used for in-vivo tracing and manual removal of CAR-T cells and application thereof
ActiveCN107287207AActivate killingGuaranteed normal treatmentMammal material medical ingredientsImmunoglobulinsCAR T-cell therapyT lymphocyte
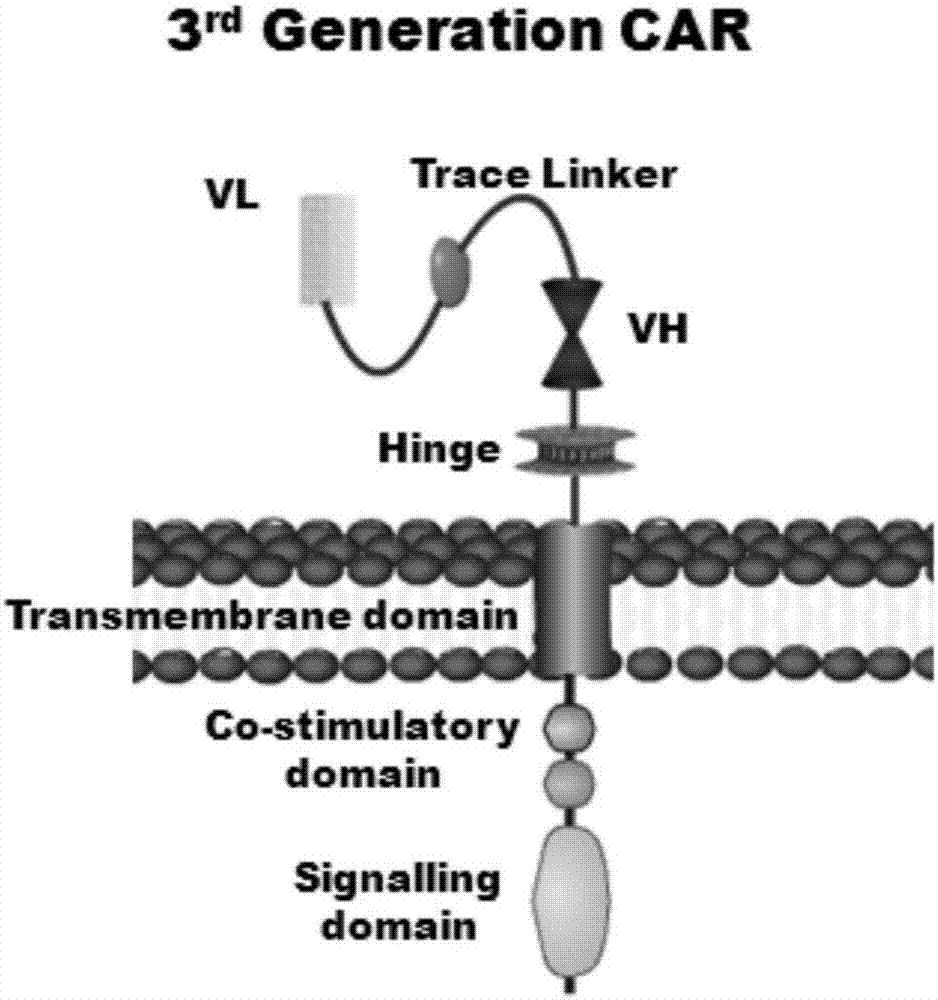
The invention discloses a marker used for in-vivo tracing and manual removal of CAR-T cells. The marker comprises a trace-linker 1 with a sequence as shown in SEQ ID No. 18, a trace-linker 2 with a sequence as shown in SEQ ID No. 19 and a trace-linker 3 with a sequence as shown in SEQ ID No. 19. The invention also discloses a CAR-T therapy vector including the marker and a construction method thereof. Moreover, the invention further discloses application of the marker to preparation of the CAR-T therapy vector and drugs used for treating triple-negative breast cancer. The marker provided by the invention can realize controllable in-vivo tracing, in-vitro separation and manual removal of CAR-T cells without influence on the tumor killing effect of the CAR-T cells, so the security of CAR-T cell therapy is greatly improved, and a powerful pool can be provided for deeper analysis of the process of CAR-T cell therapy. The vector provided by the invention can express a targeting chimeric antigen receptor of CD117 in human T lymphocytes, guides and activates killing effect of T lymphocytes on CD117 positive cells, and is applicable to treatment of triple-negative breast cancer in clinical practice.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Combination immune therapy and cytokine control therapy for cancer treatment
PendingCN107530376AGenetically modified cellsMammal material medical ingredientsCAR T-cell therapyImmune therapy
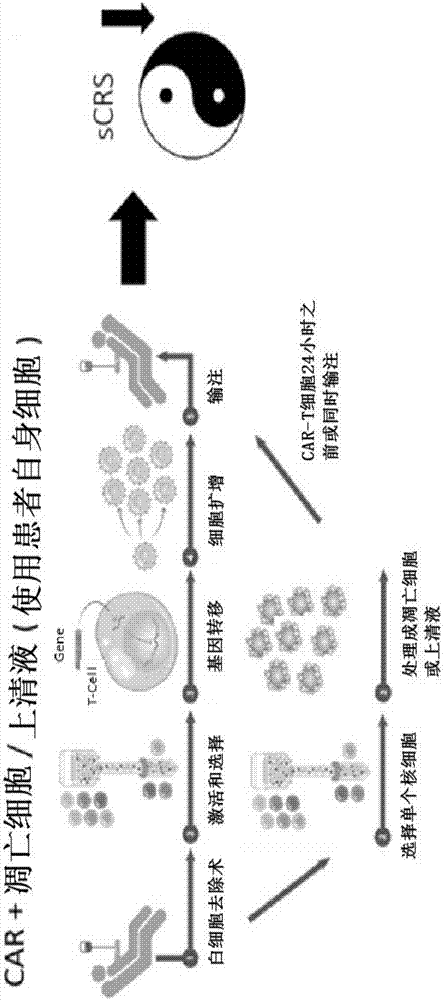
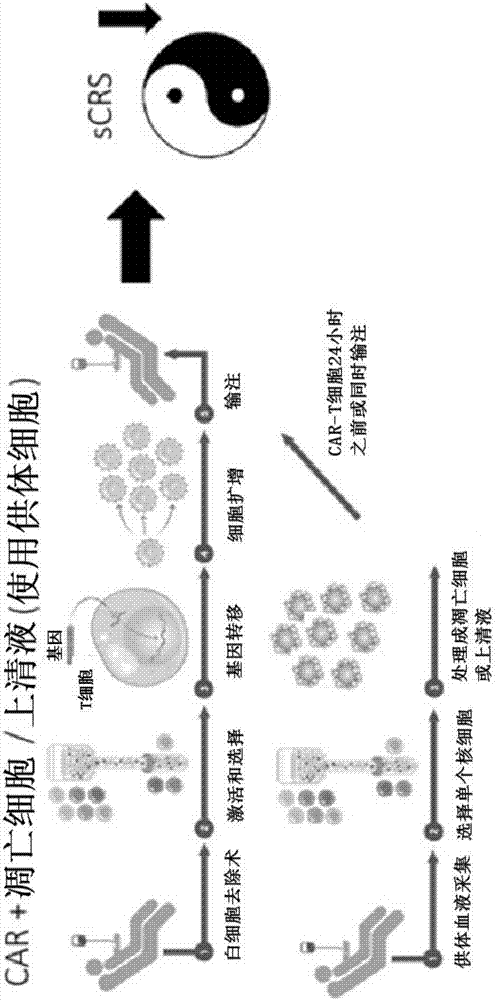
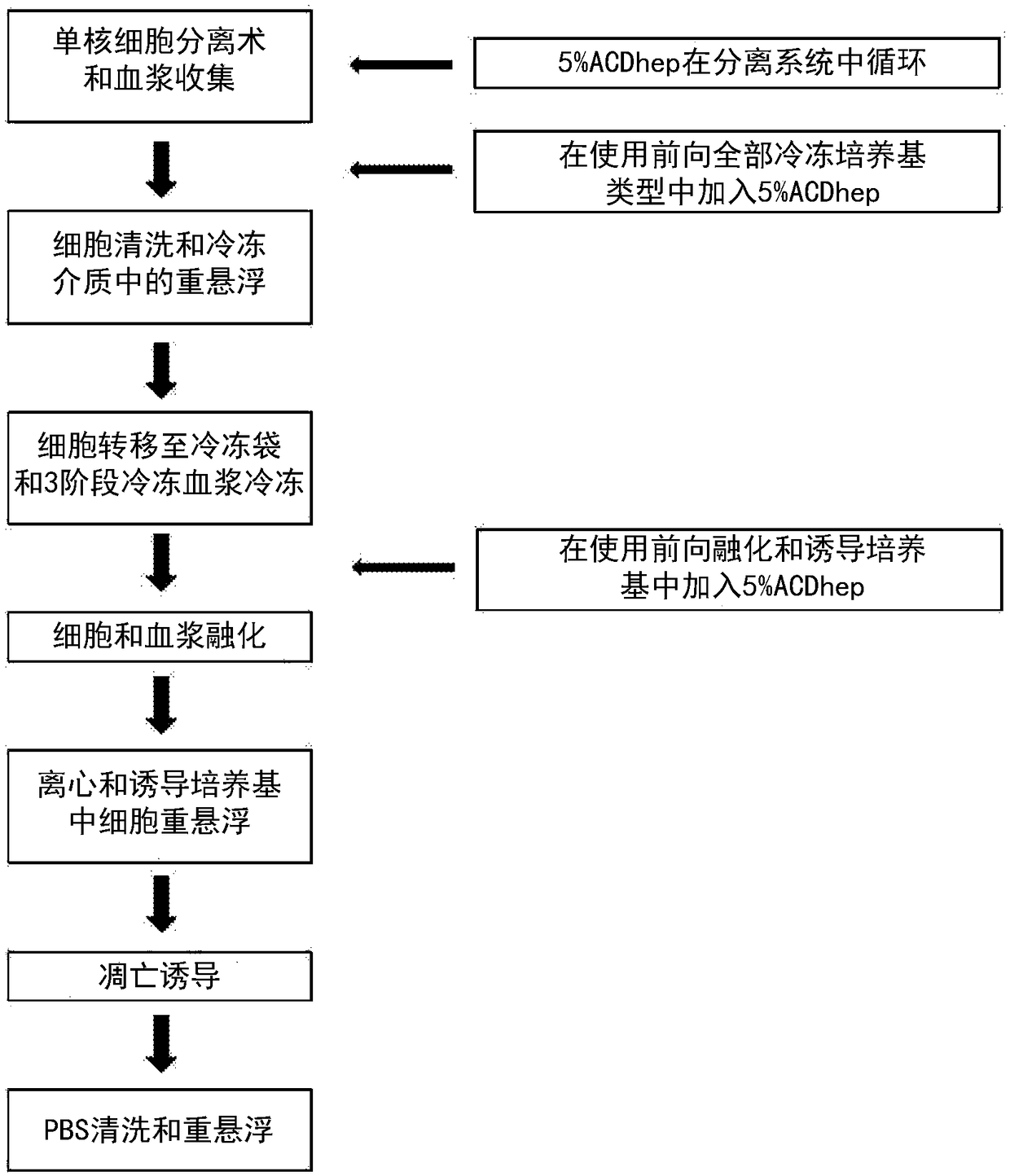
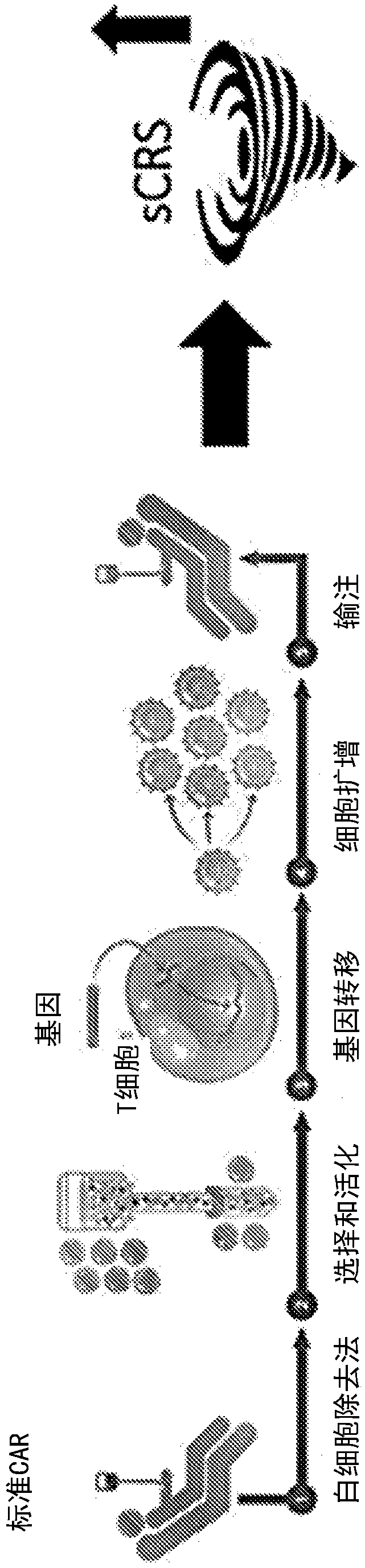
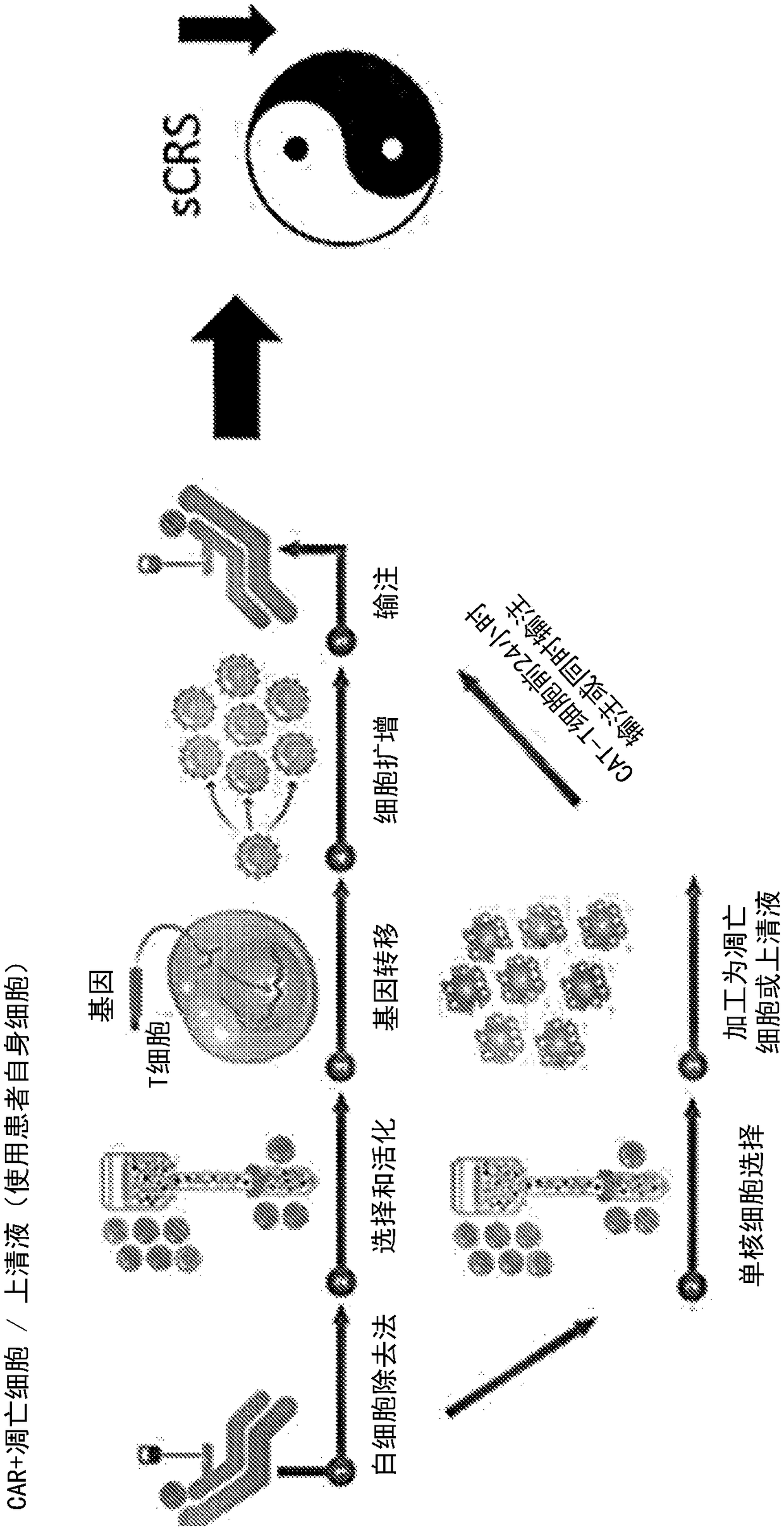
Compositions disclosed herein, and methods of use thereof include those for inhibiting or reducing the incidence of cytokine release syndrome or cytokine storm in a subject undergoing CAR T-cell therapy, wherein the subjects are administered compositions including apoptotic cells or apoptotic cell supernatants. In certain instances compositions and methods of use thereof disclosed herein do not reduce the efficacy of the CAR T-cell cancer therapy. Disclosed herein are also compositions and methods of use thereof for decreasing or inhibiting cytokine production in a subject experiencing cytokine release syndrome or cytokine storm including administration of a composition including apoptotic cells or an apoptotic cell supernatant.
Owner:ENLIVEX THERAPEUTICS LTD
Combination immune therapy and cytokine control therapy for cancer treatment
PendingCN109069539APolypeptide with localisation/targeting motifGenetically modified cellsCAR T-cell therapyImmune therapy
Compositions disclosed herein, and methods of use thereof included those for inhibiting or reducing the incidence of cytokine release syndrome or cytokine storm in a subject undergoing CAR T-cell therapy, wherein the subjects are administered compositions comprising apoptotic cells or apoptotic cell supernatants. In certain instances compositions and methods of use thereof disclosed herein do notreduce the efficacy of the CAR T-cell cancer therapy. Disclosed herein are also compositions and methods of use thereof for decreasing or inhibiting cytokine production in a subject experiencing cytokine release syndrome or cytokine storm comprising administration of a composition comprising apoptotic cells or an apoptotic cell supernatant.
Owner:恩立夫克治疗RDO有限责任公司
Compositions and methods for improved car-t cell therapies
ActiveUS20170354724A1Reduce spreadPeptide/protein ingredientsAntibody mimetics/scaffoldsCAR T-cell therapyEffector cell
The present invention relates to the preparation and use in recipients of CAR-T cell-derived effector cells which are modified to limit their proliferation within the recipient. This is accomplished through the introduction of adducts into the nucleic acids of CAR-T cell-derived effector cells following expansion in vitro to provide expanded and activated CAR-T cell-derived effector cells that retain immunologic function, including the expression of one ore more cytokines.
Owner:CERUS CORP
Bcma binding molecules and methods of use thereof
ActiveCN109414455AOrganic active ingredientsPeptide/protein ingredientsCAR T-cell therapyAntigen Binding Fragment
The invention provides antibodies, antigen binding fragments thereof, chimeric antigen receptors (CARs), and engineered T cell receptors, polynucleotides encoding the same, and in vitro cells comprising the same. The polynucleotides, polypeptides, and in vitro cells described herein can be used in an engineered CAR T cell therapy for the treatment of a patient suffering from a cancer. In one embodiment, the polynucleotides, polypeptides, and in vitro cells described herein can be used for the treatment of multiple myeloma.
Owner:KITE PHARMA INC
Preparation method of cell for expressing anti-CD22 chimeric antigen receptor and PD-L1 blocking protein, expression vector and application
ActiveCN111944850AProlong the action timeAlleviate immune tolerance problemsAntibody mimetics/scaffoldsNucleic acid vectorCAR T-cell therapyT cell
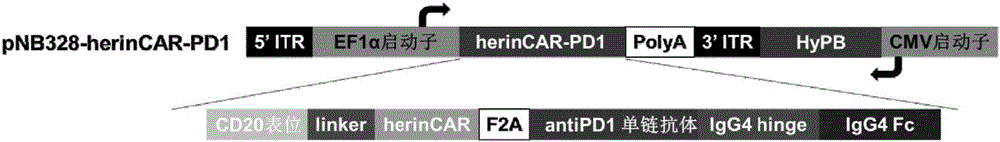
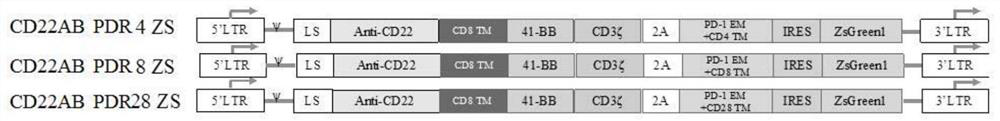
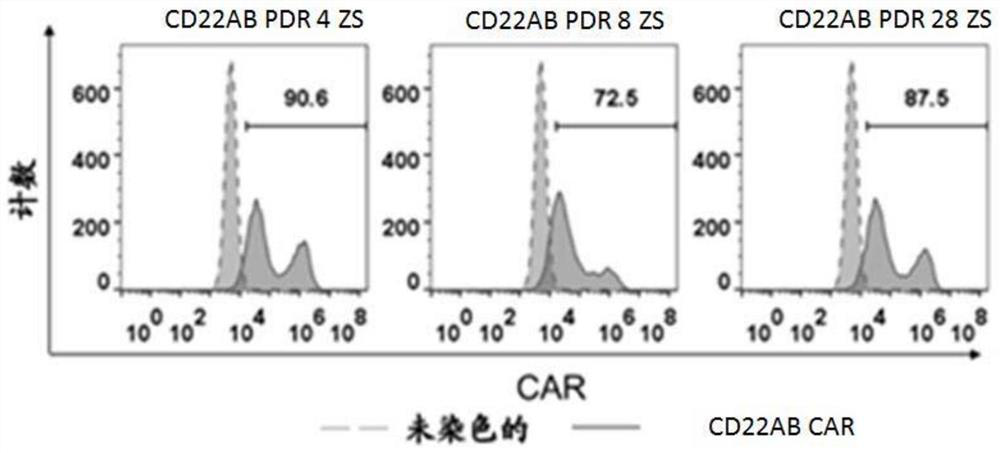
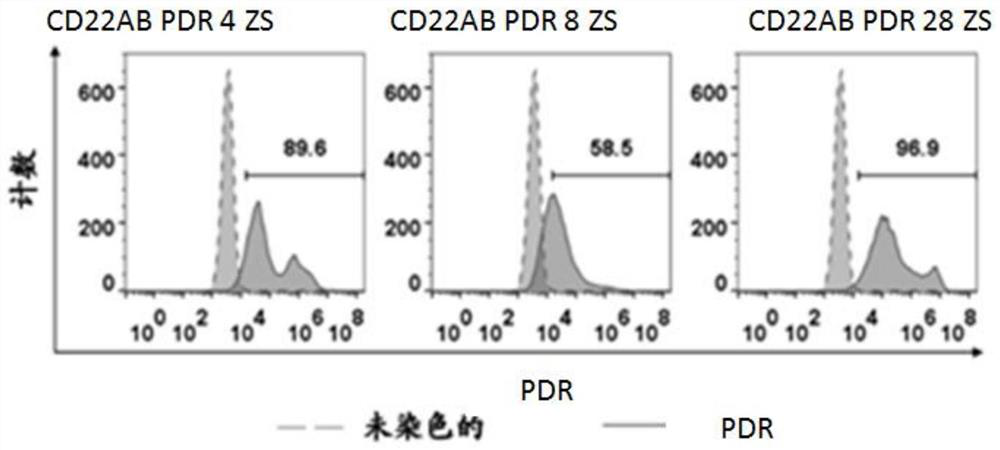
The invention discloses a preparation method of a cell for expressing an anti-CD22 chimeric antigen receptor and a PD-L1 blocking protein, an expression vector and application, and relates to the technical field of medical biology. A first nucleic acid encoding the anti-CD22 chimeric antigen receptor and a second nucleic acid encoding the PD-L1 blocking protein are inserted into the expression vector. By utilizing a CD22 target and a PD-L1 target and targeting an antibody or a fragment thereof of CD22, the T cell can efficiently and specifically target a tumor cell for expressing an antigen CD22; and the PD-L1 blocking protein can compete with endogenously expressed PD-1 to be combined with PD-L1 on a target cell, so that a PD-1 / PD-L1 signal path is blocked, the action time of the T cell is prolonged, the tumor killing effect is improved, and the problem of immune tolerance of solid tumors to CAR T cell therapy is relieved.
Owner:UNIVERSITY OF MACAU
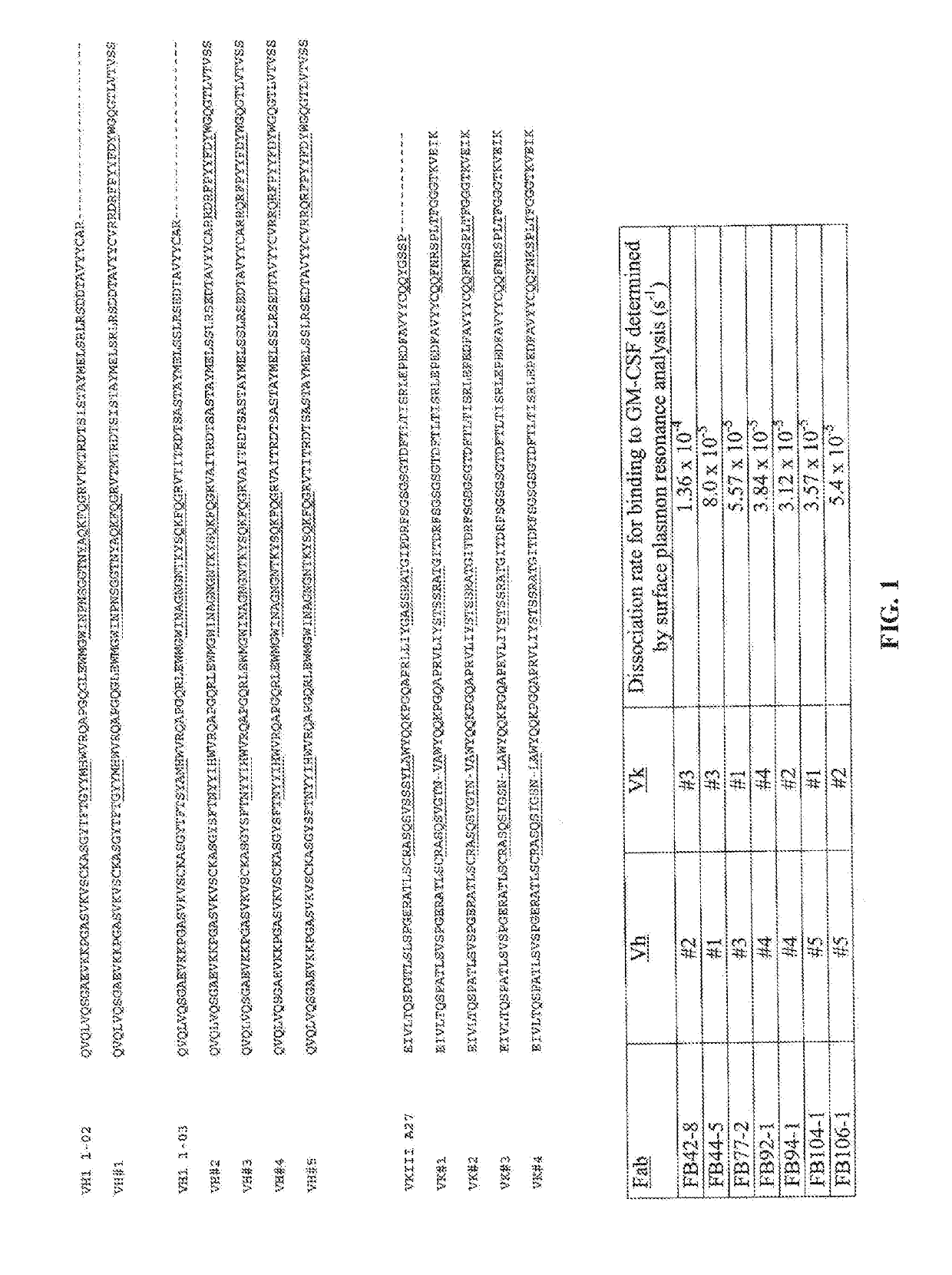
Methods of treating immunotherapy-related toxicity using a gm-csf antagonist
ActiveUS20190322735A1Reduce serum concentrationProlong survival timePolypeptide with localisation/targeting motifNervous disorderCAR T-cell therapyRecombinant hGM-CSF
Methods for reducing blood-brain barrier disruption in a subject treated with immunotherapy, the method comprising administering a recombinant GM-CSF antagonist to the subject. Methods for preserving blood-brain barrier integrity in a subject treated with immunotherapy, the method comprising administering a recombinant hGM-CSF antagonist to the subject. Methods for decreasing or preventing CAR-T cell therapy-induced neuroinflammation in a subject in need thereof, the method comprising administering a recombinant GM-CSF antagonist to the subject. Methods for preventing or reducing blood-brain barrier disruption in a subject treated with immunotherapy, the method comprising administering CAR-T cells having a GM-CSF gene knockout (GM-CSFk / o CAR-T cells) to the subject.
Owner:HUMANIGEN INC
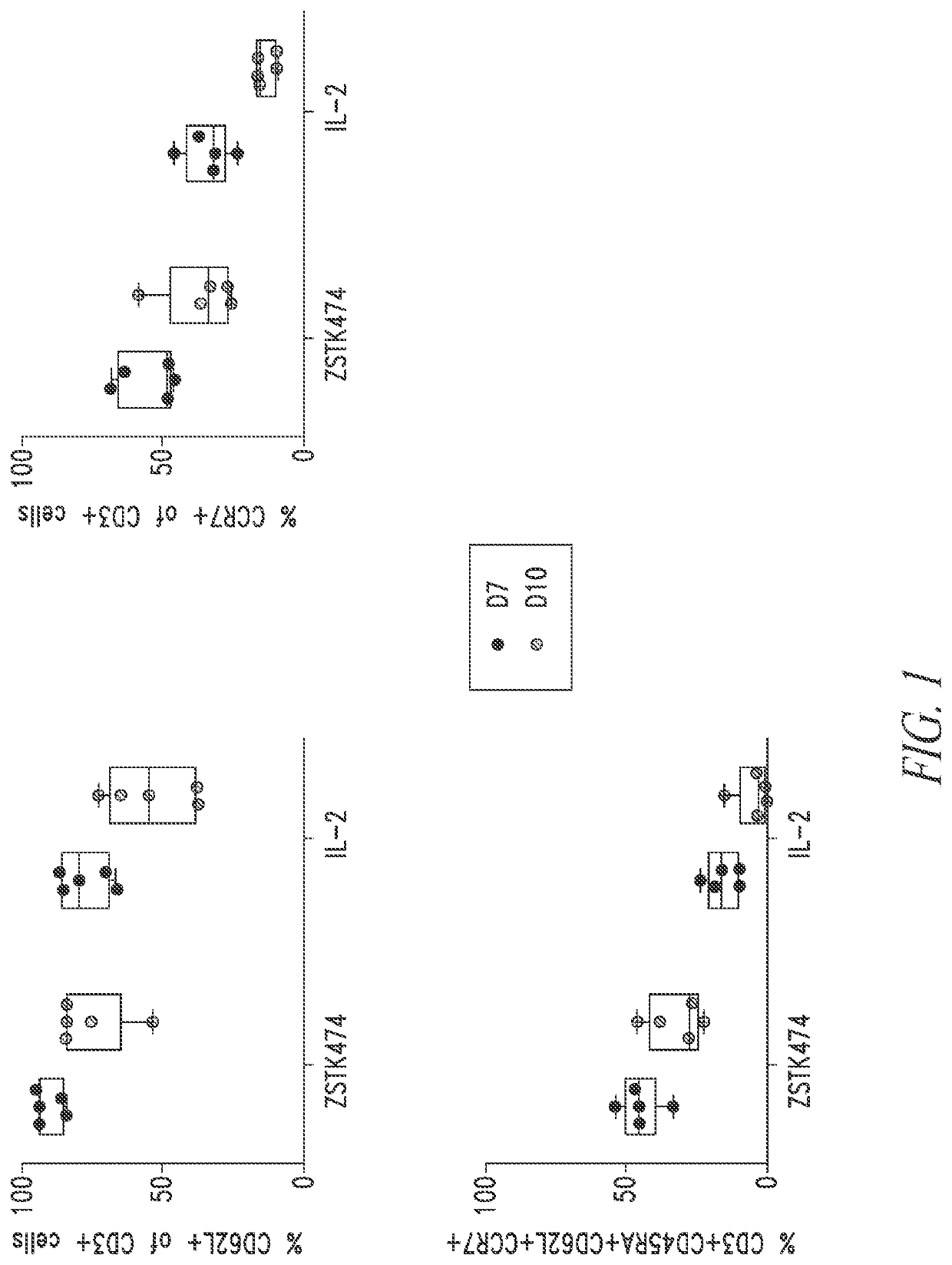
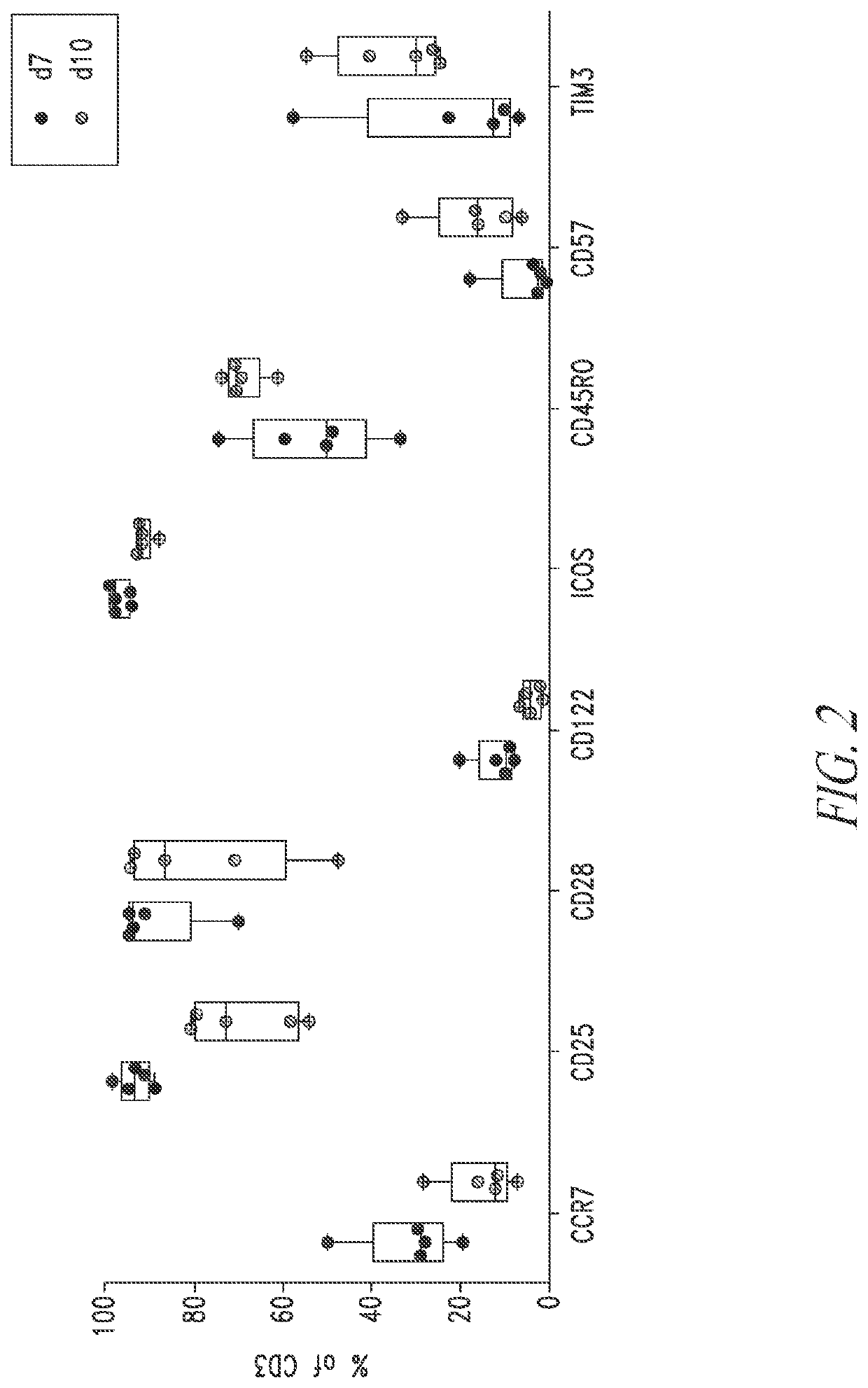
Method for promoting in-vitro amplification of umbilical cord blood T cell and maintaining high-proportion TSCM subpopulation
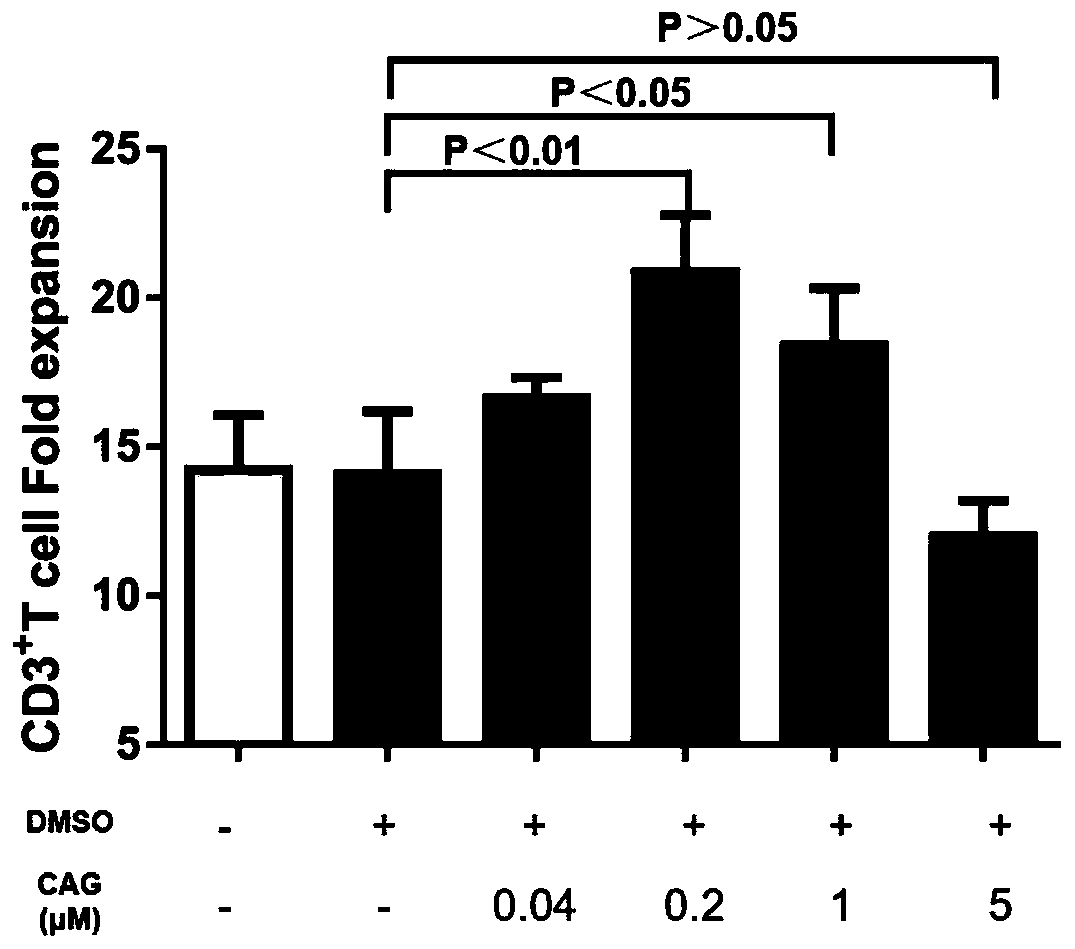
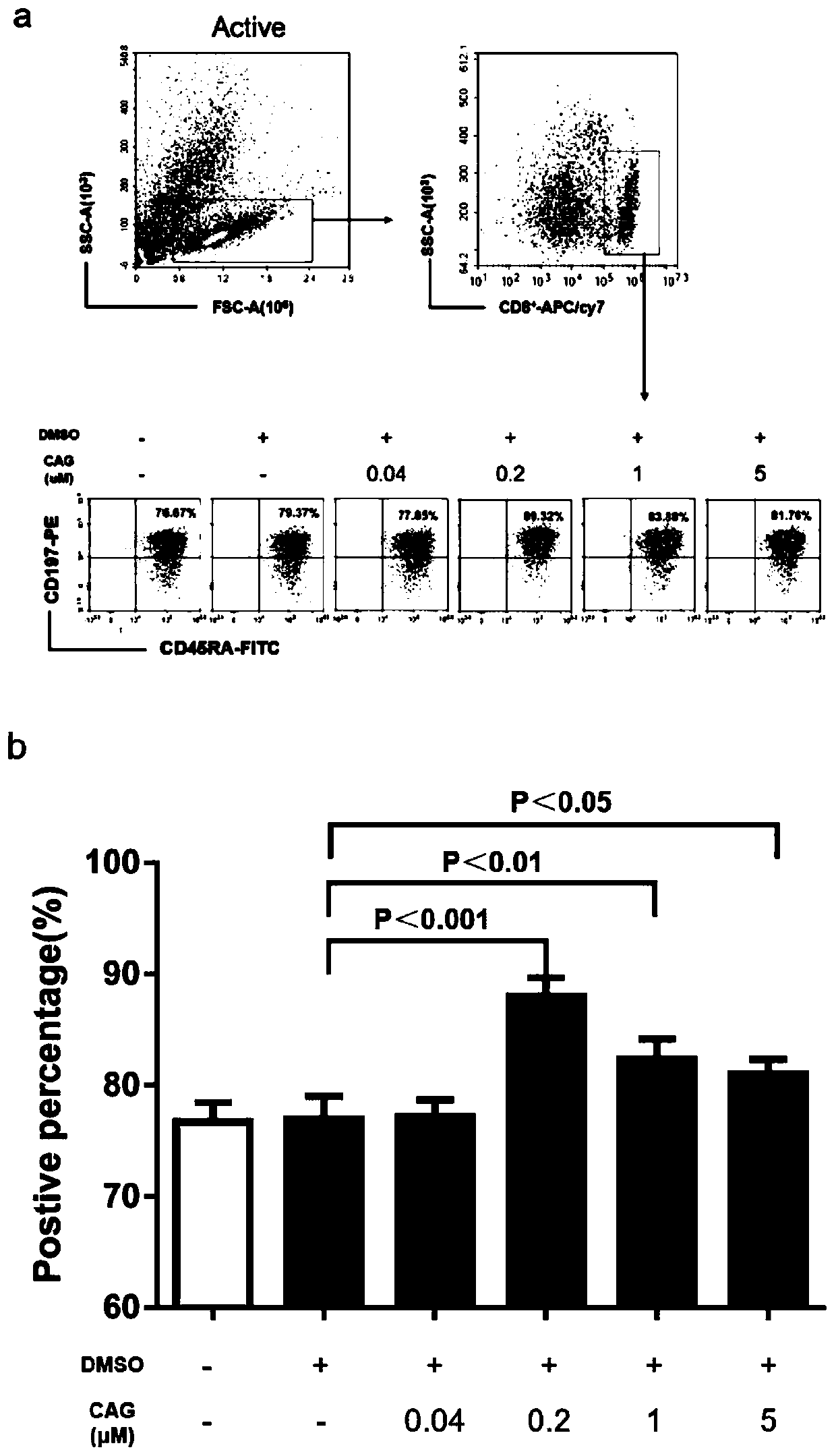
InactiveCN109943527APromote amplificationRaise the ratioBlood/immune system cellsCAR T-cell therapyCulture fluid
The invention discloses a method for promoting in-vitro amplification of an umbilical cord blood T cell and maintaining a high-proportion TSCM subpopulation. The method comprises the steps that cycloastragenol, a cell factor IL-7 and a cell factor IL-15 are combined and added into an in-vitro culture solution of the T cell to promote the amplification of the T cell and maintain the high-proportionTSCM subpopulation. The method for promoting the in-vitro amplification of the umbilical cord blood T cell and maintaining the high-proportion TSCM subpopulation has the advantages that the high-proportion TSCM cell subpopulation can be maintained while the low-concentration CAG combined with the IL-7 and the IL-15 to promote the amplification of the umbilical cord blood derived T cell is successively explored; the novel in-vitro culture method lays an experimental foundation for the in-vitro long-term culture of the T cell and an immunization therapy containing a CAR-T cell therapy.
Owner:四川药智联恒科技有限公司
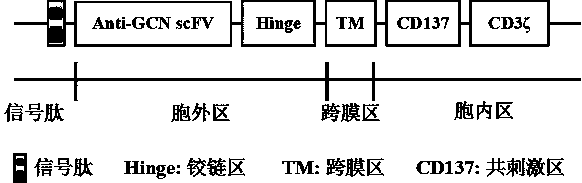
Universal chimeric antigen receptor-T (CAR-T) cell as well as preparation method and application thereof
InactiveCN108795876AShort manufacturing timeReduce manufacturing costPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyImmune effects
The invention provides a universal chimeric antigen receptor-T (CAR-T) cell as well as a preparation method and application thereof. The universal CAR-T cell can recognize polypeptide GCN and GCN-autoantibody affinity peptide fusion polypeptides, and can recognize and kill different self-reactive b cells in a targeted way under the guidance and regulation of different GCN-autoantibody affinity peptide fusion polypeptide guiders. The intensity or complete closure of an in vivo immune effect of the CAR-T cell can be very conveniently controlled, so that the reduction of other risks or side effects such as cytokine storms in general CAR-T cell therapy is facilitated; the universal CAR-T cell is different from conventional CAR-T cells need to be designed with different CARs aiming at differenttargets; therefore, time and cost are greatly reduced, and the success rate is increased.
Owner:武汉圣惠康生物医药科技有限责任公司
Protection of tcr signaling chains in cancer patients and enhancement of car-t cell therapy
InactiveUS20160231323A1Organic active ingredientsBiological material analysisAbnormal tissue growthCAR T-cell therapy
Disclosed are means of protecting signaling integrity of T cells in cancer patients through reduction of neutrophil and other cellular induced oxidative stress. In one embodiment the FDA approved drug Mucomyst is administered at a concentration of 50-150 mg / kg to increase expression of T cell receptor (TCR)-zeta chain in patients with cancer. In other embodiments enhancement of CAR-T cell therapy is performed through modulation of inflammatory and oxidative stress in a tumor bearing patient.
Owner:BATU BIOLOGICS
Compositions and Methods for Regulating CAR T Cells
InactiveUS20190367610A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCAR T-cell therapyT cell
The present invention provides compositions and methods for inhibiting the depletion of healthy tissue during CAR T cell therapy. In another embodiment, the invention includes a drug-molecule conjugate which is administered to a subject receiving CAR T cell therapy, where the conjugate binds to the CAR resulting in internalization of the conjugate and inhibition of T cell activity and / or death of the T cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for car t cell therapy
ActiveUS20200405760A1Reduce cytokine release syndromeBody weight loss due to CRS is reduced or preventedPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyMoiety
Owner:PURDUE RES FOUND INC +1
Method for treating side effect of chimeric antigen receptor (CAR) T cell therapy
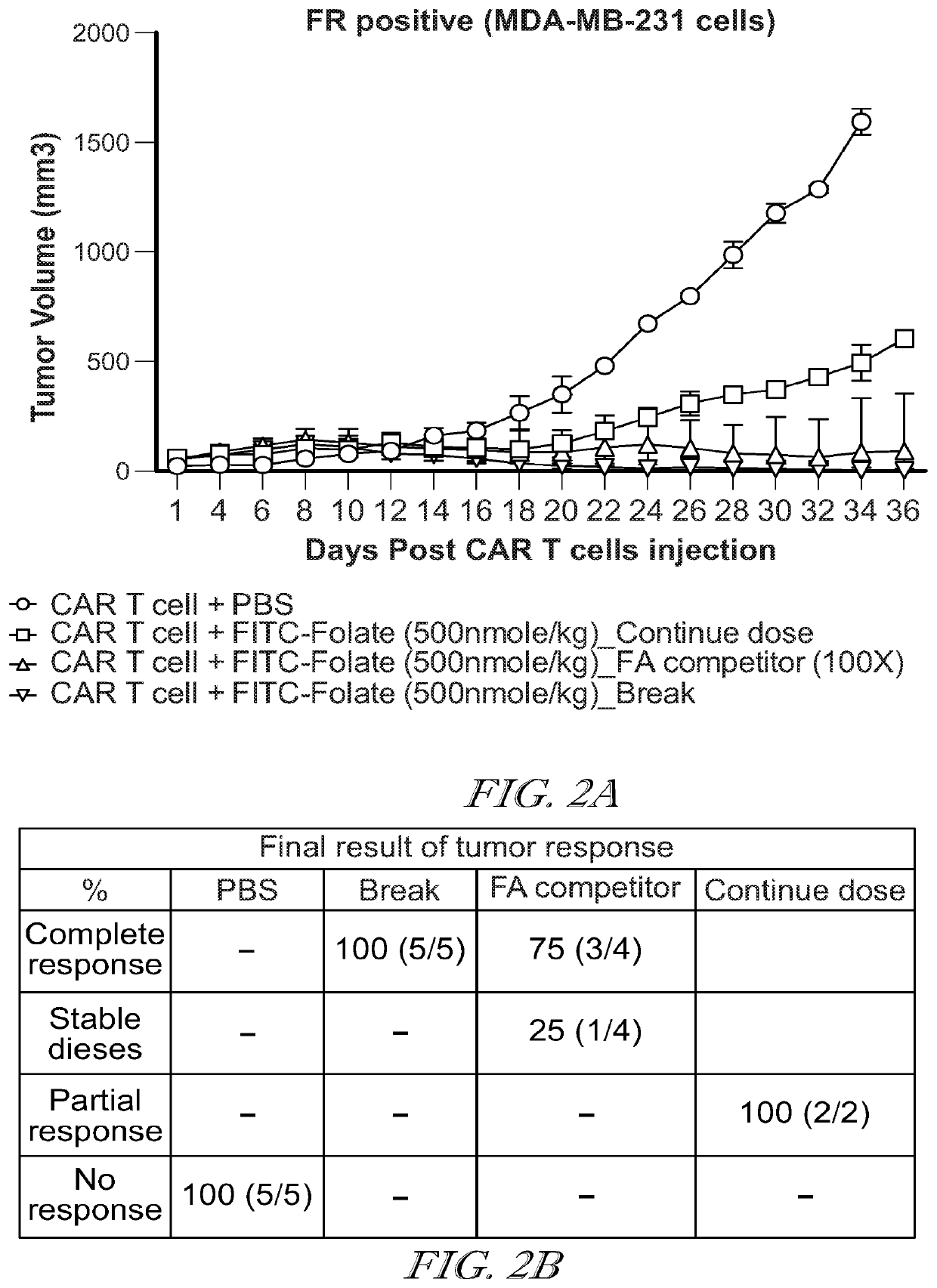
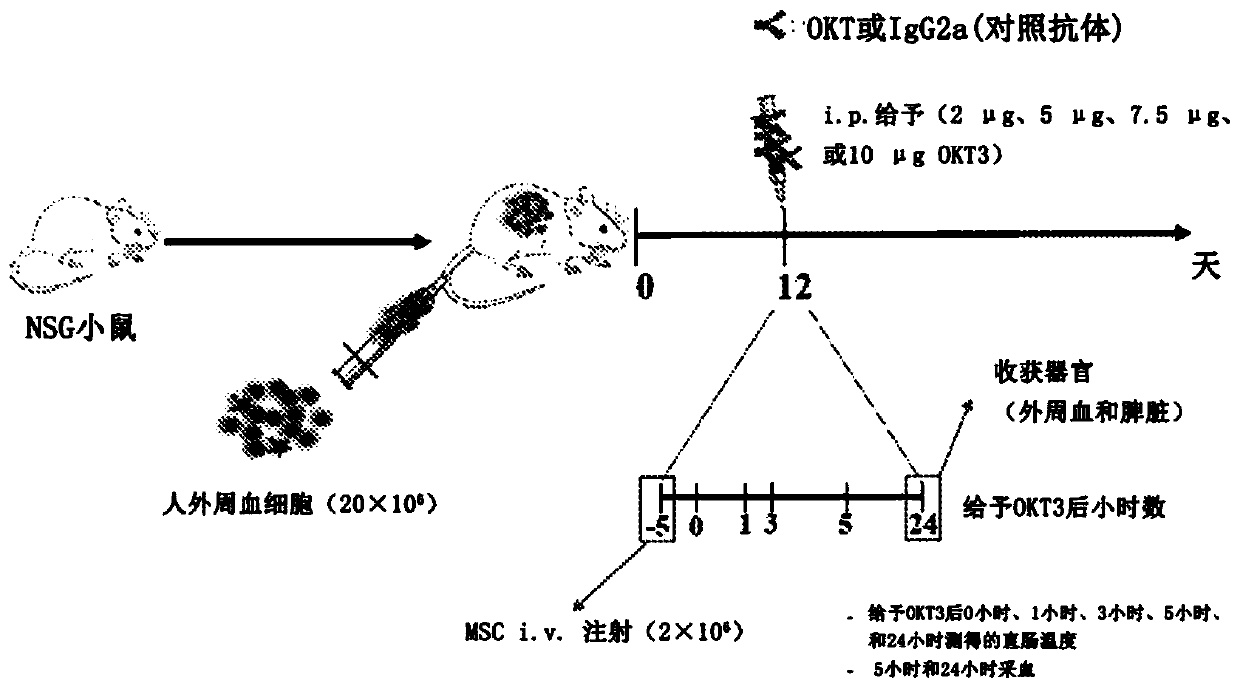
InactiveCN110678191AAntibody mimetics/scaffoldsMammal material medical ingredientsCAR T-cell therapySide effect
The invention relates to a method for treating a side effect of chimeric antigen receptor (CAR) T cell therapy, the method comprising administering a mesenchymal stem cell (MSC) to a subject who has been or is being administered CAR T cell therapy.
Owner:CYNATA THERAPEUTICS LTD
Manufacturing Anti-bcma car t cells
PendingUS20220195060A1High potencyIncreased persistencePeptide/protein ingredientsAntibody mimetics/scaffoldsCAR T-cell therapyOncology
The invention provides improved anti-BCMA CAR T cell compositions and methods for manufacturing anti-BCMA CAR T cell therapies. More particularly, the invention relates to improved methods of for manufacturing anti-BCMA CAR T cells that result in more potent, persistence, and efficacious adoptive T cell immunotherapies. In certain embodiments, the cells were manufactured from a subject that has a multiple myeloma or a lymphoma.
Owner:2SEVENTY BIO INC
Sequencing method for car t cell therapy
PendingUS20210346431A1Easy to optimizeBody weight loss due to CRS is reduced or preventedPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyMoiety
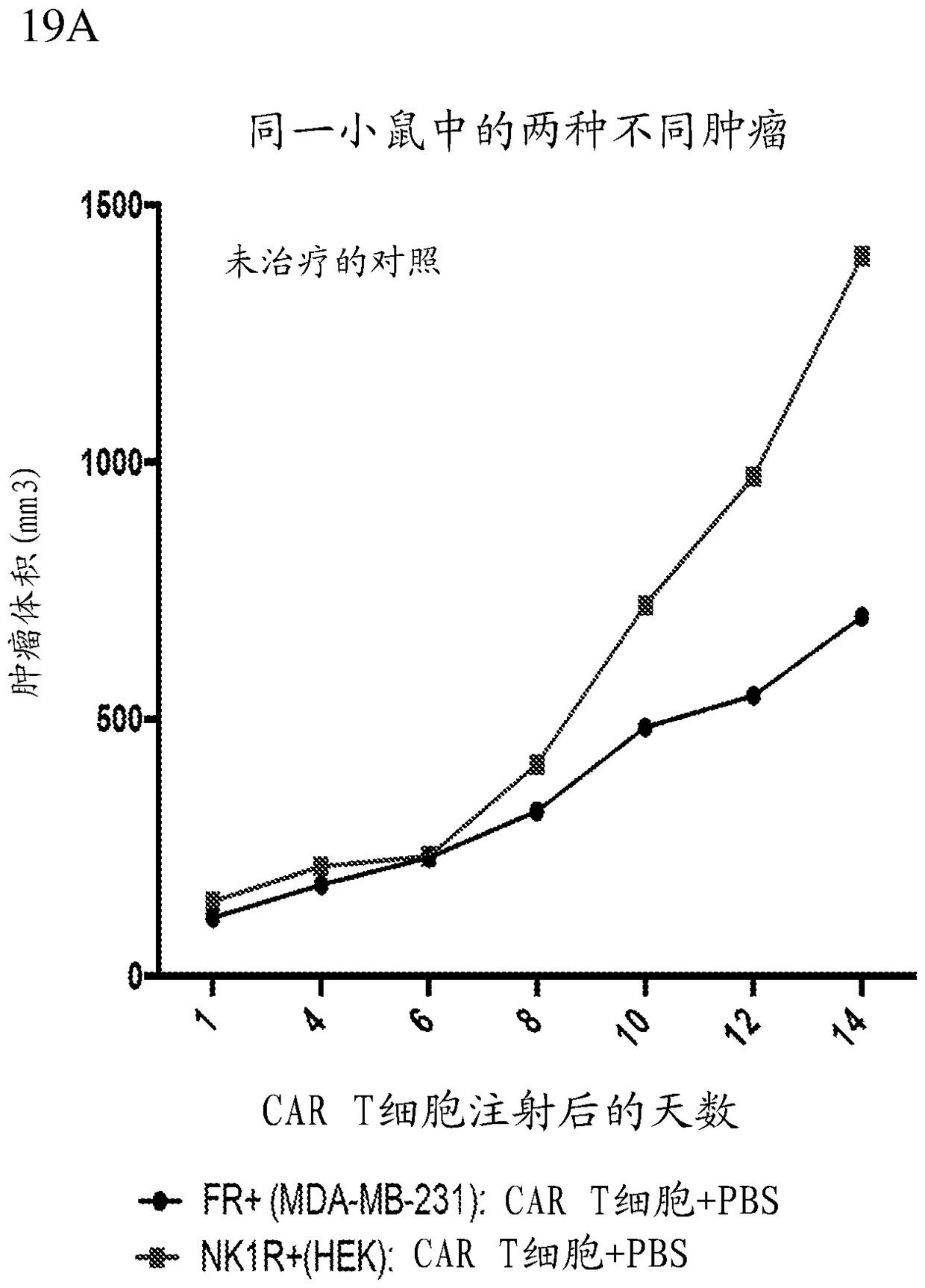
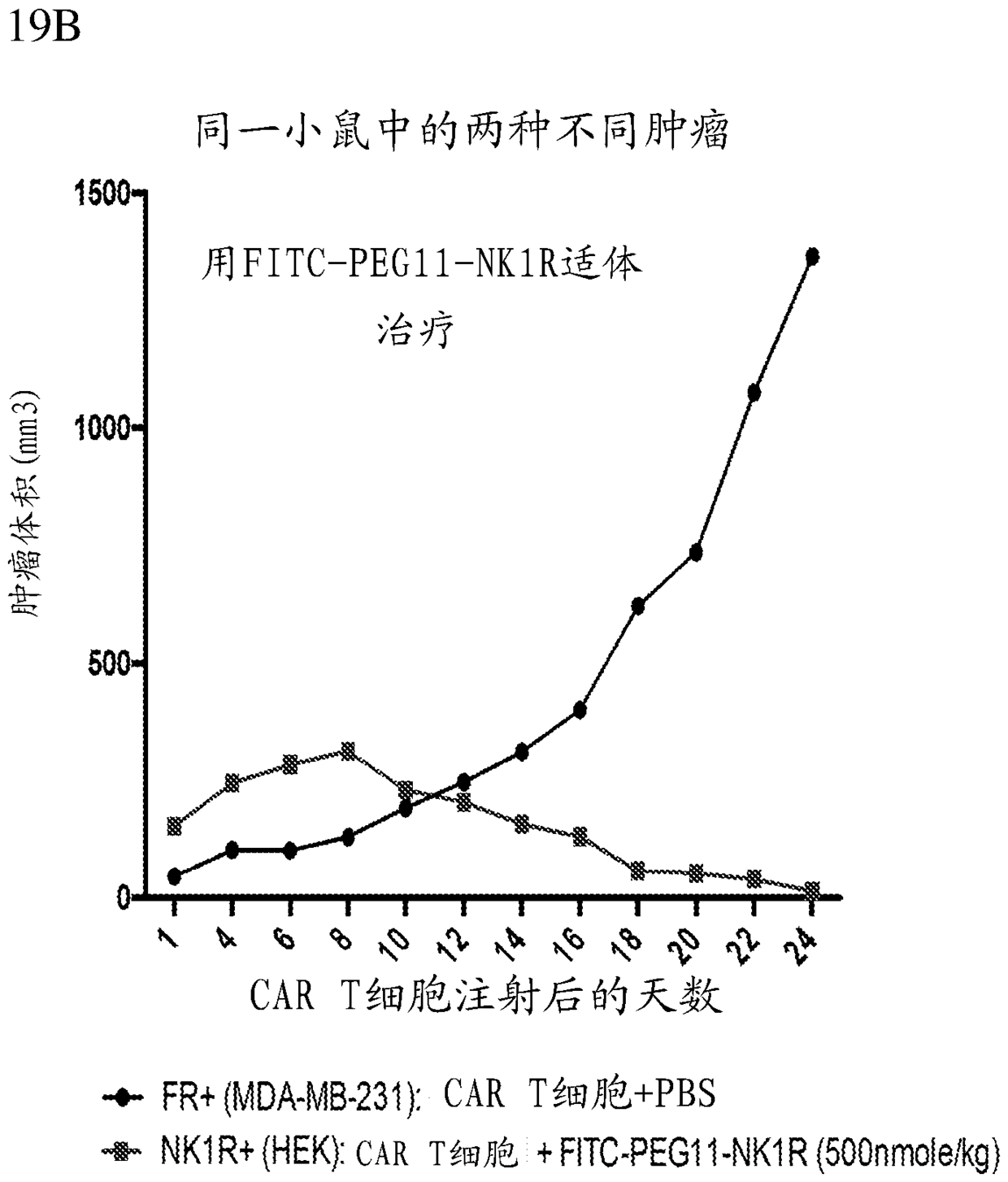
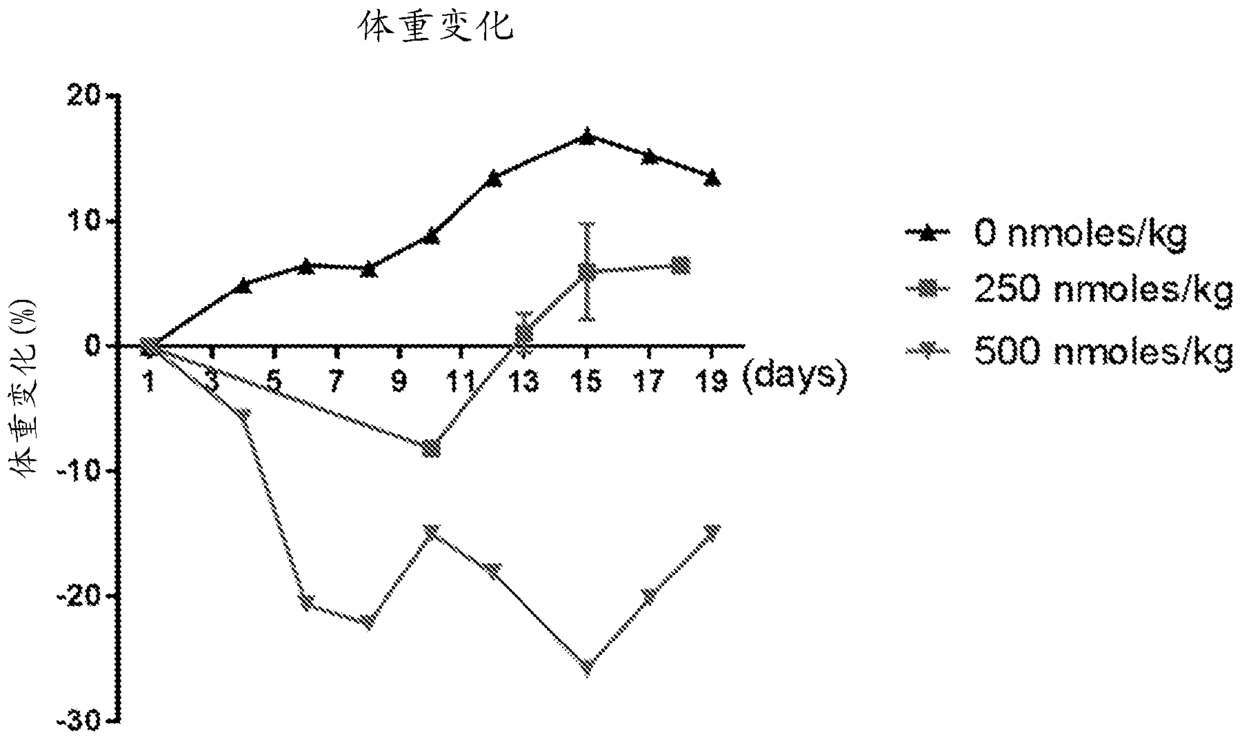
The present disclosure relates to methods of treating a patient with a cancer by administering to the patient a composition comprising CAR T cells and administering to the patient a small molecule linked to a targeting moiety by a linker. The disclosure also relates to compositions for use in such methods.
Owner:PURDUE RES FOUND INC
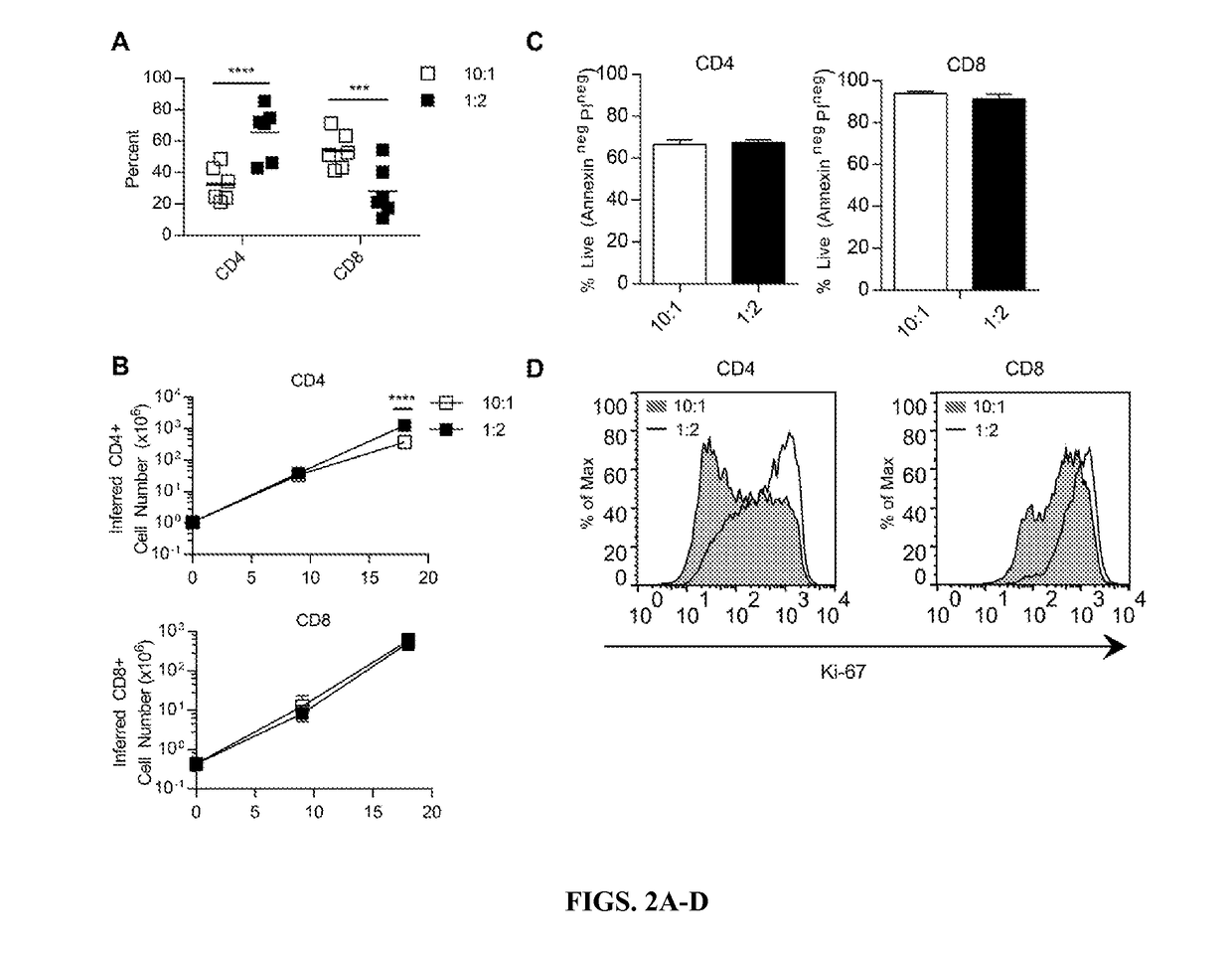
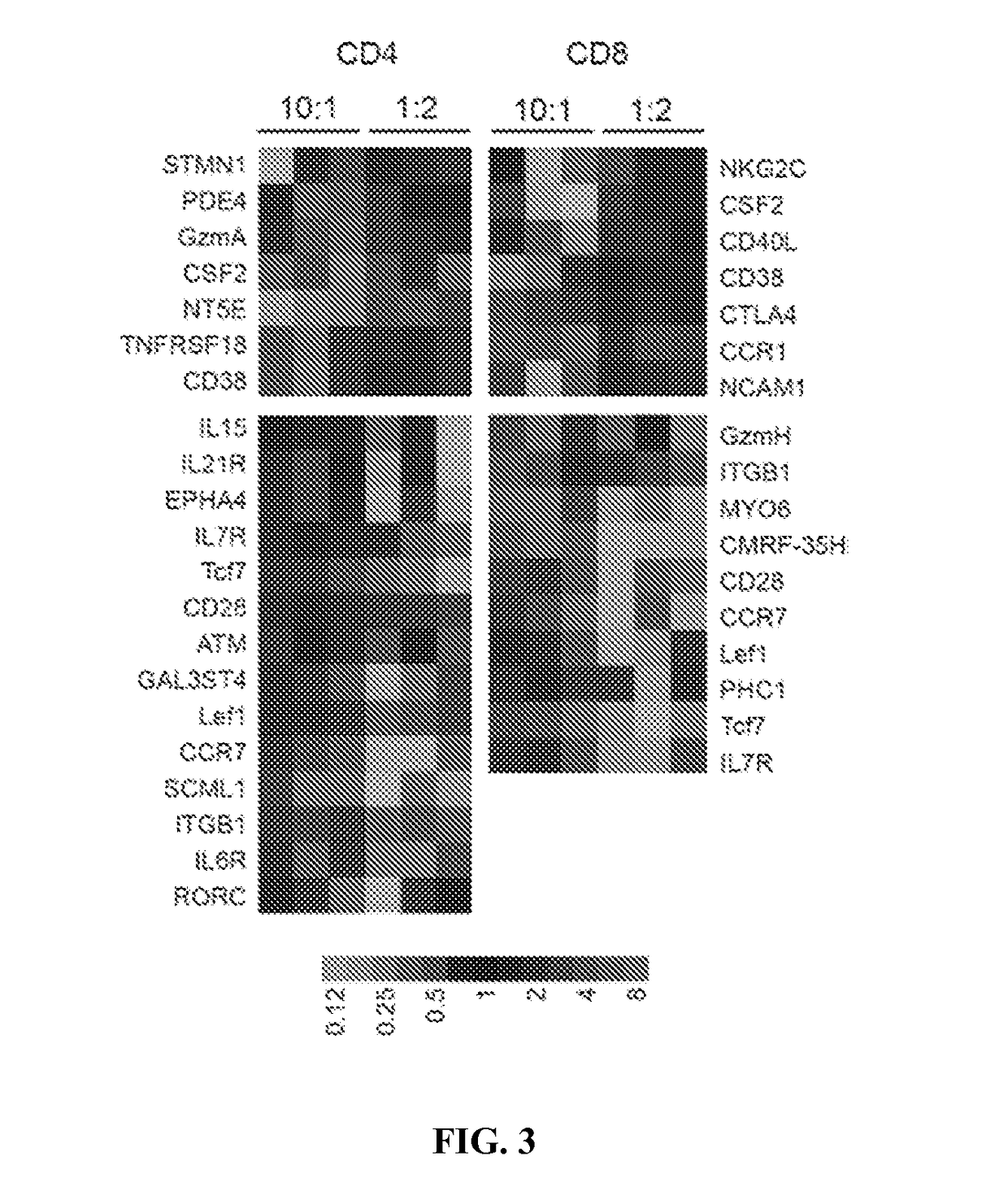
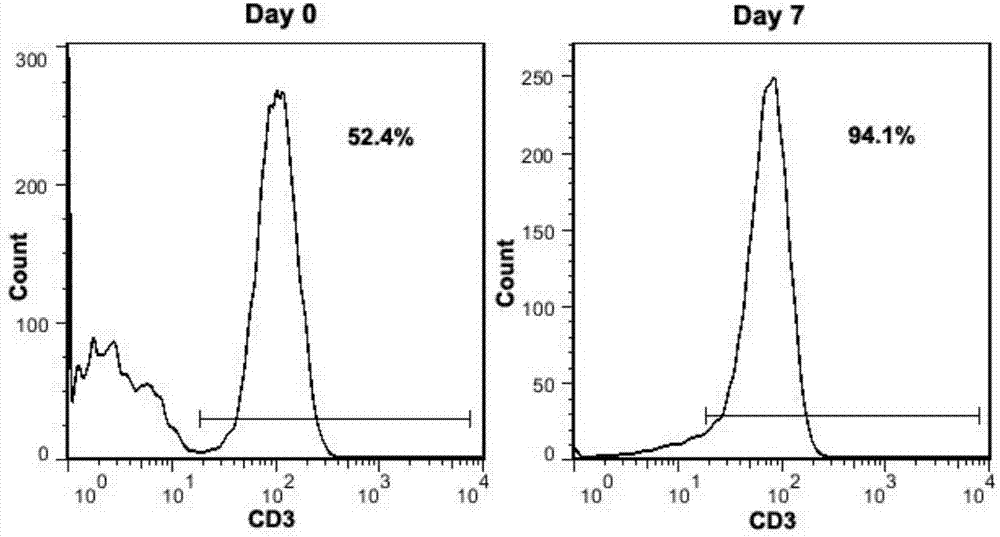
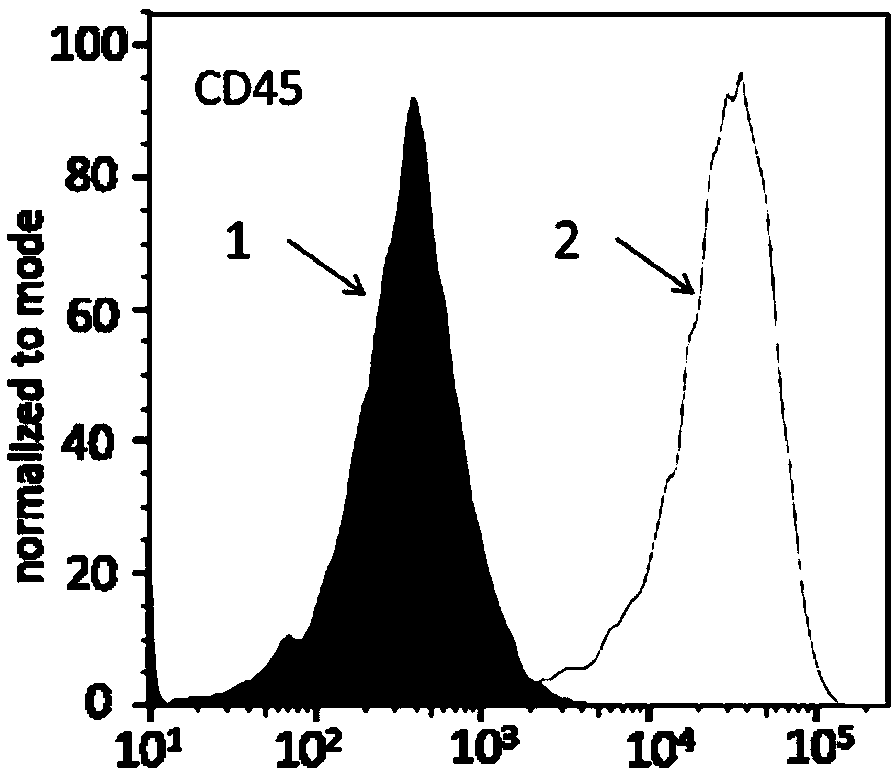
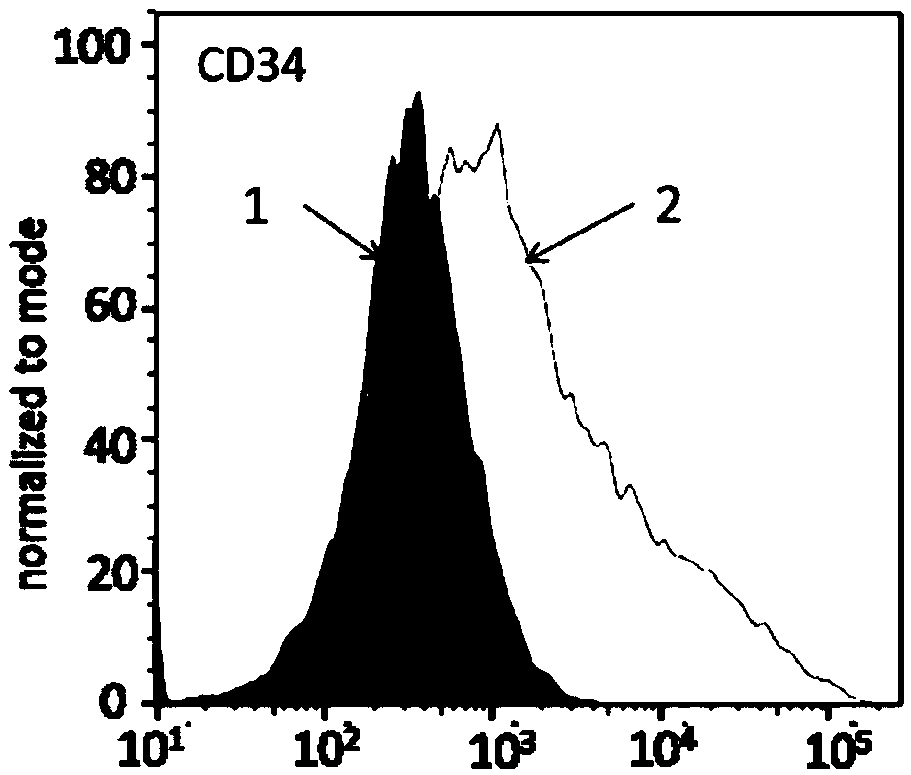
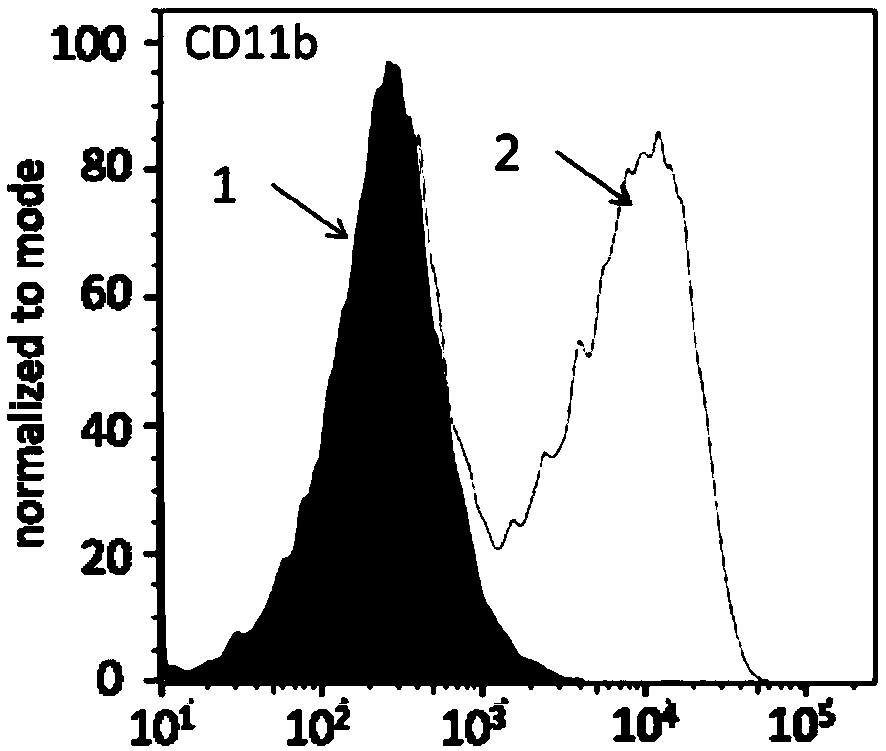
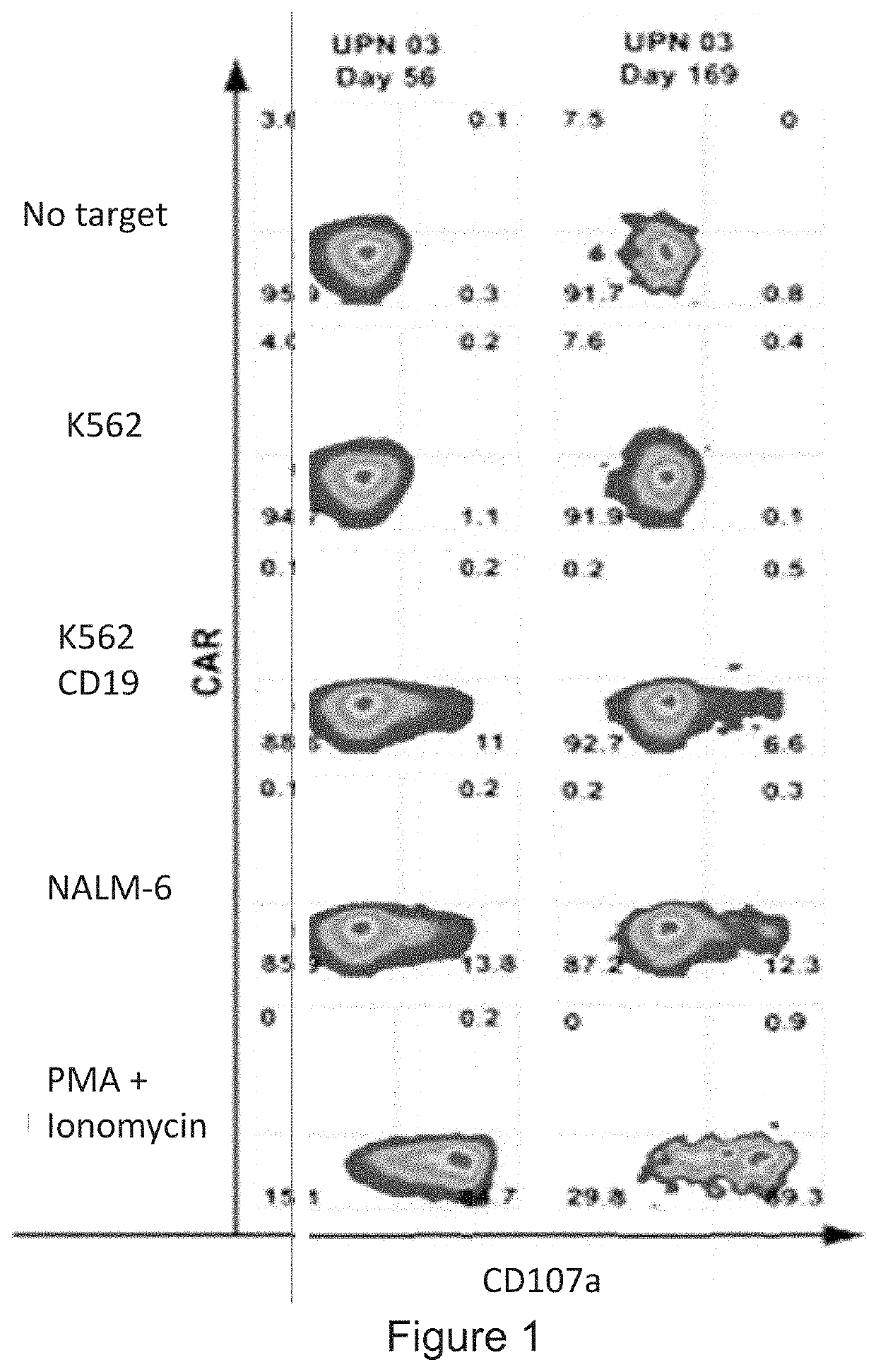
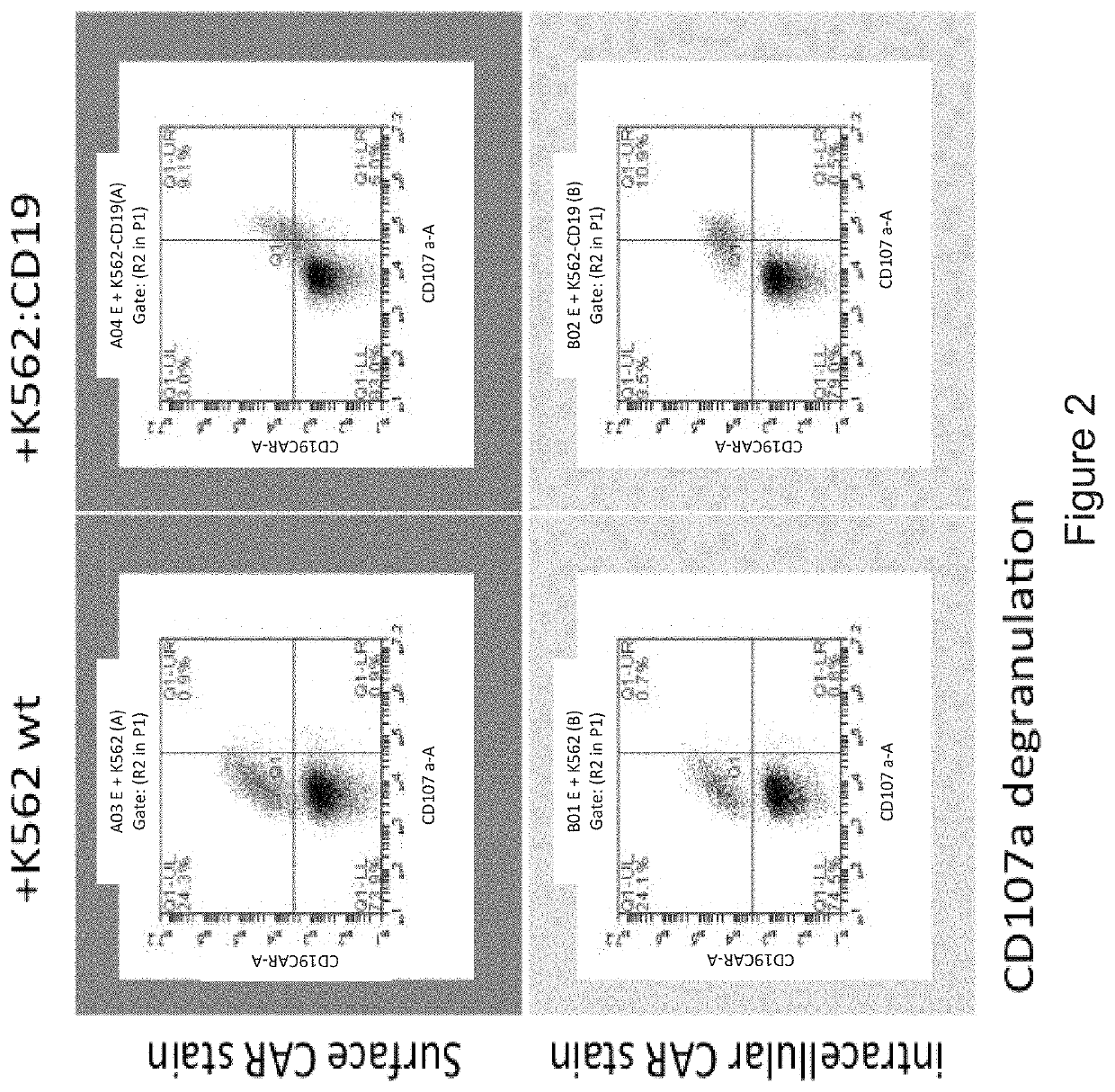
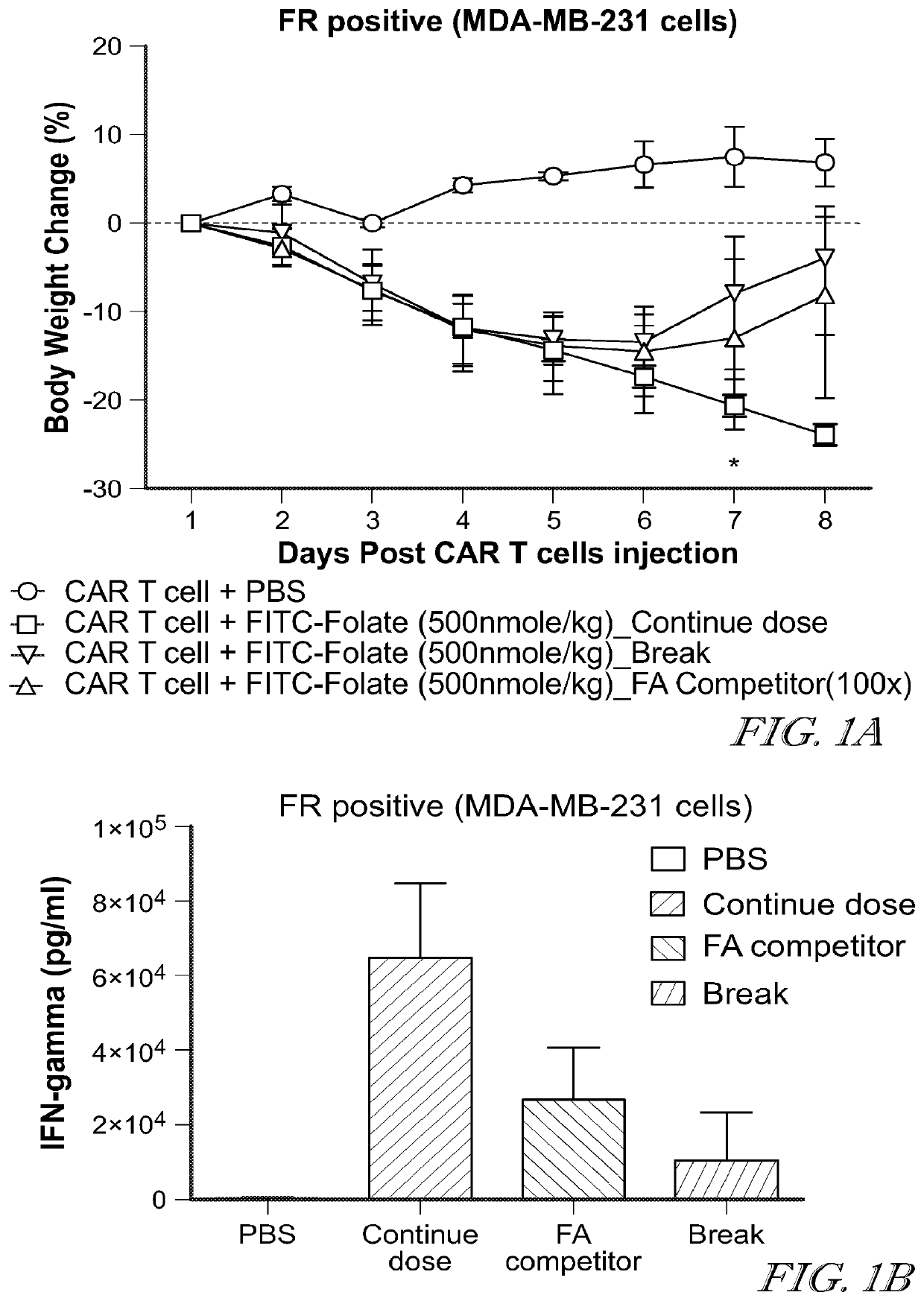
METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY
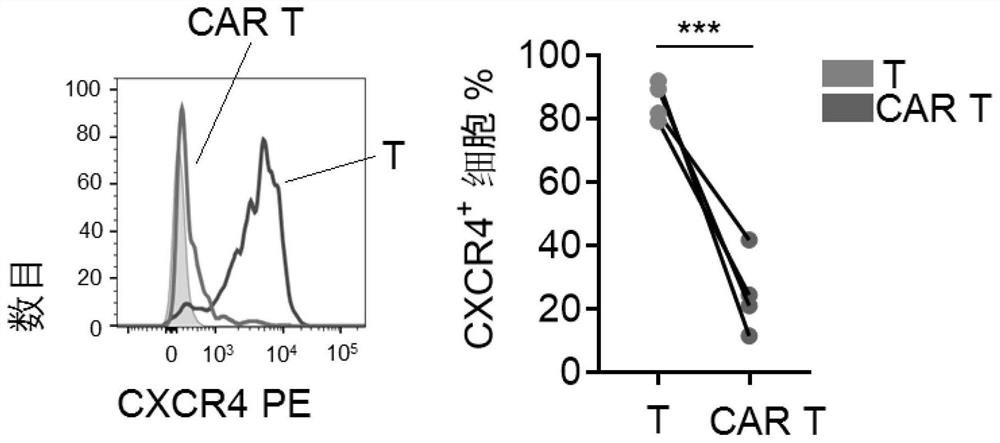
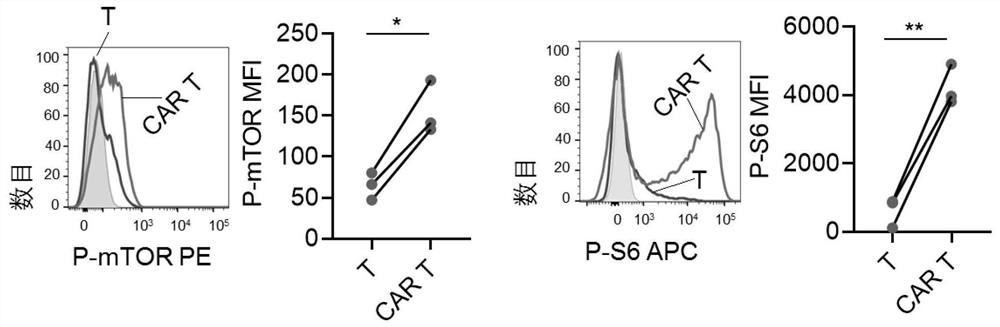
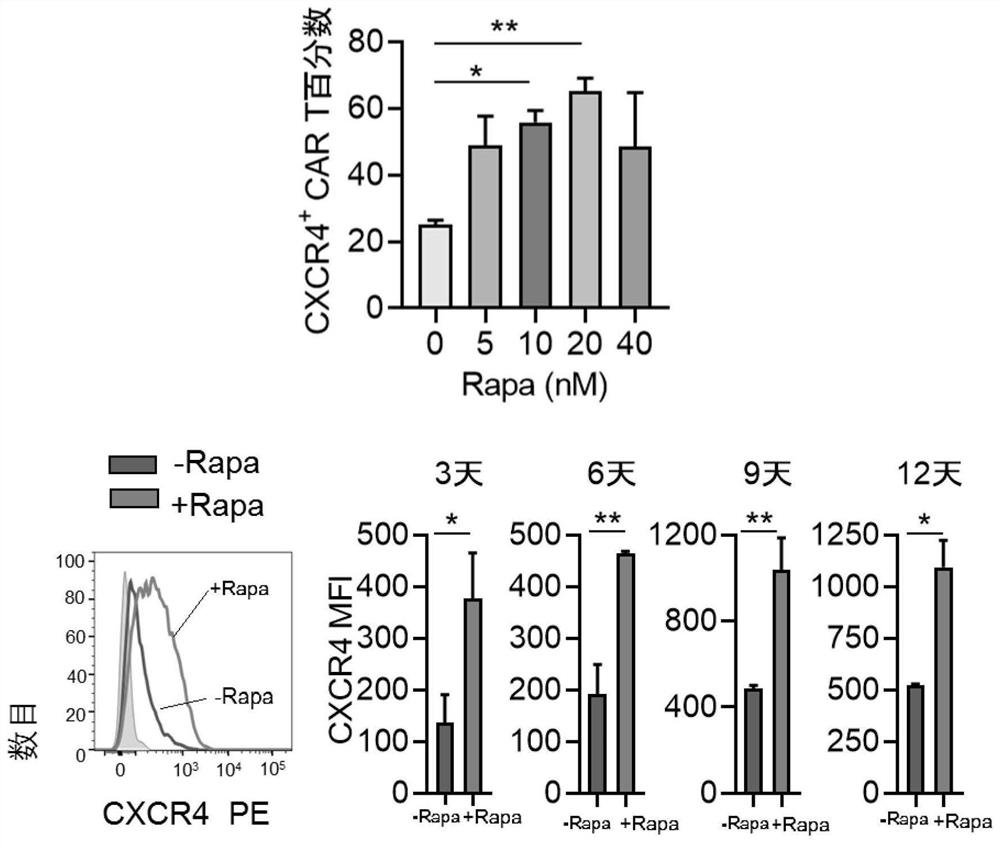
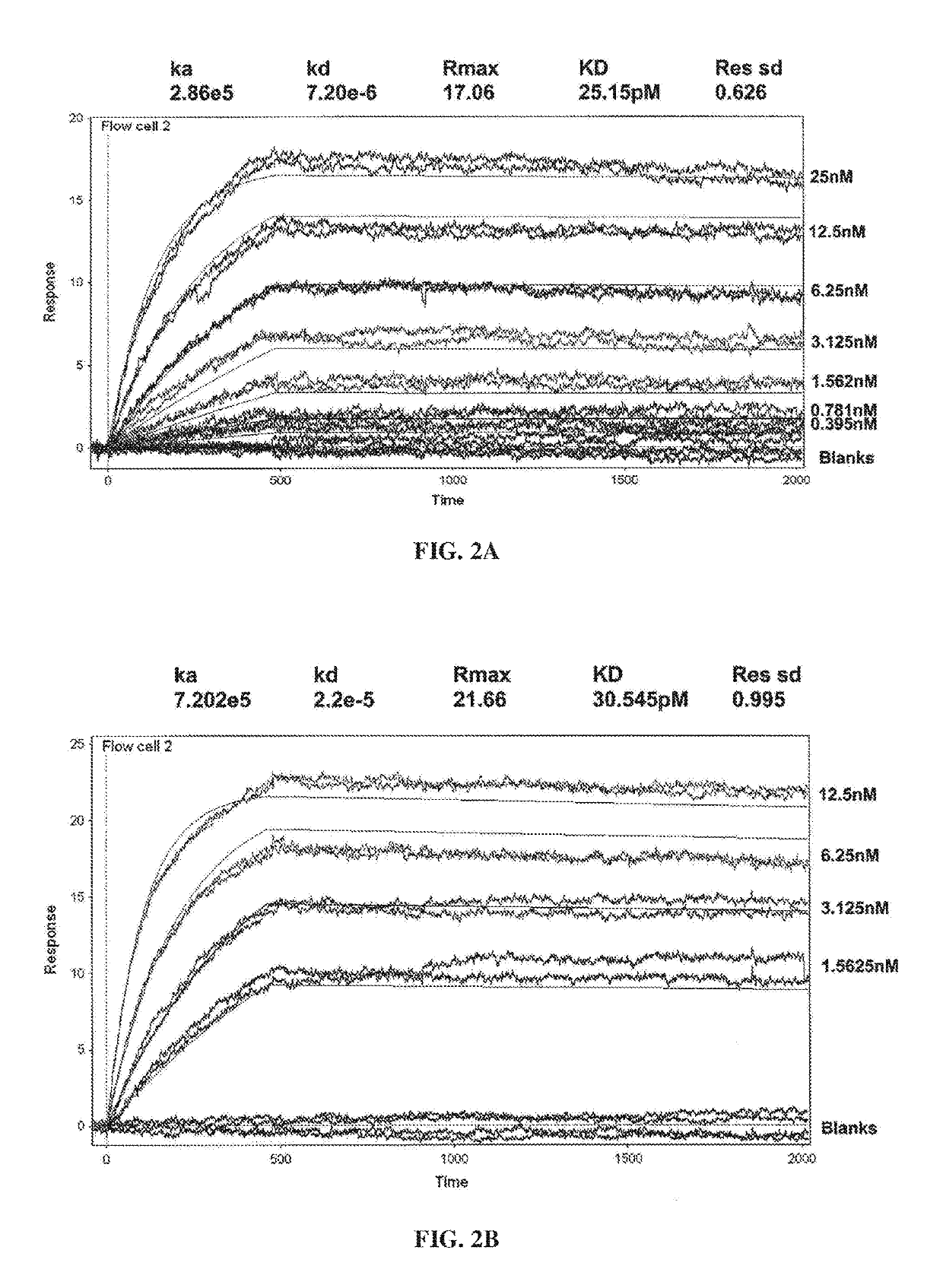
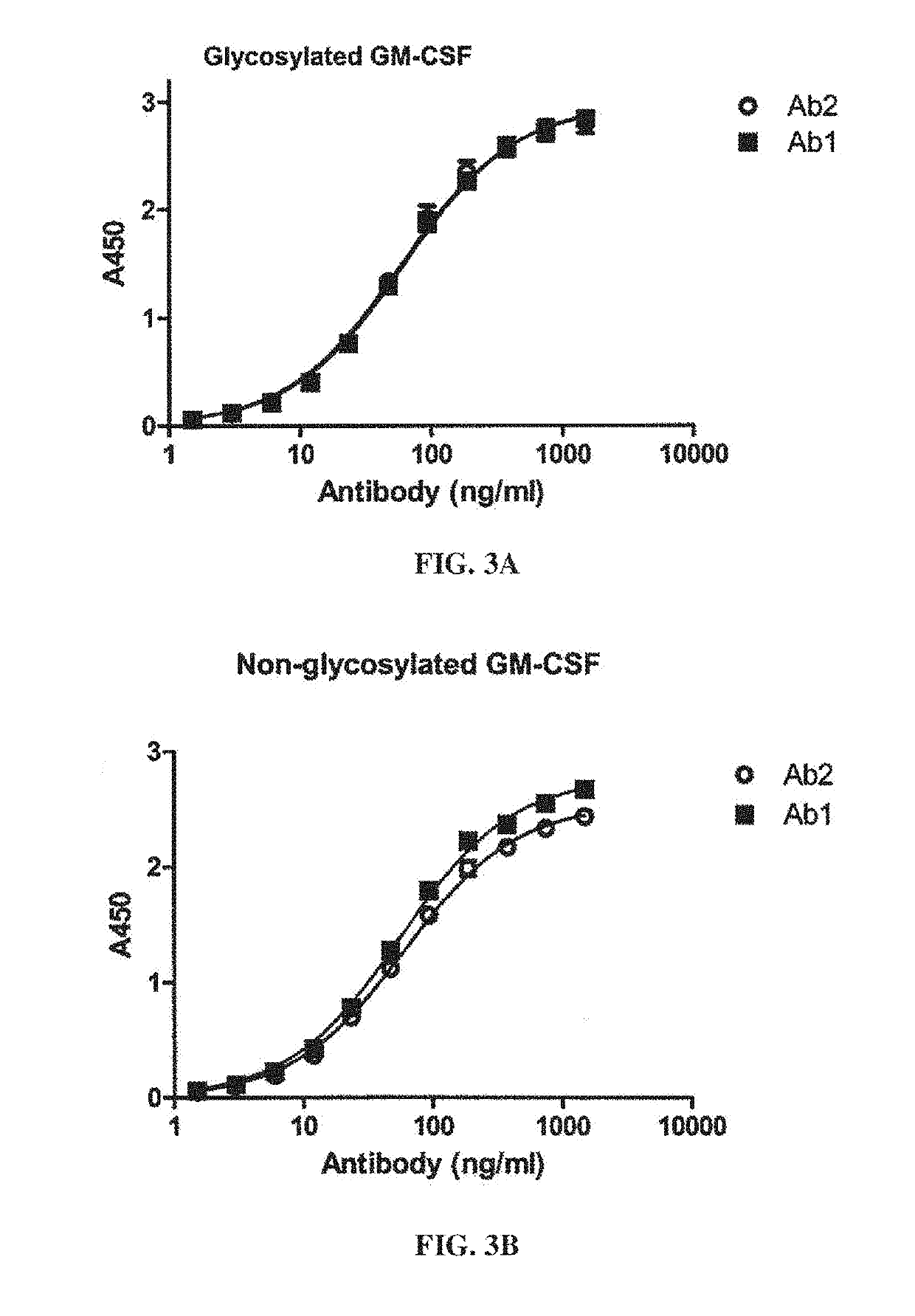
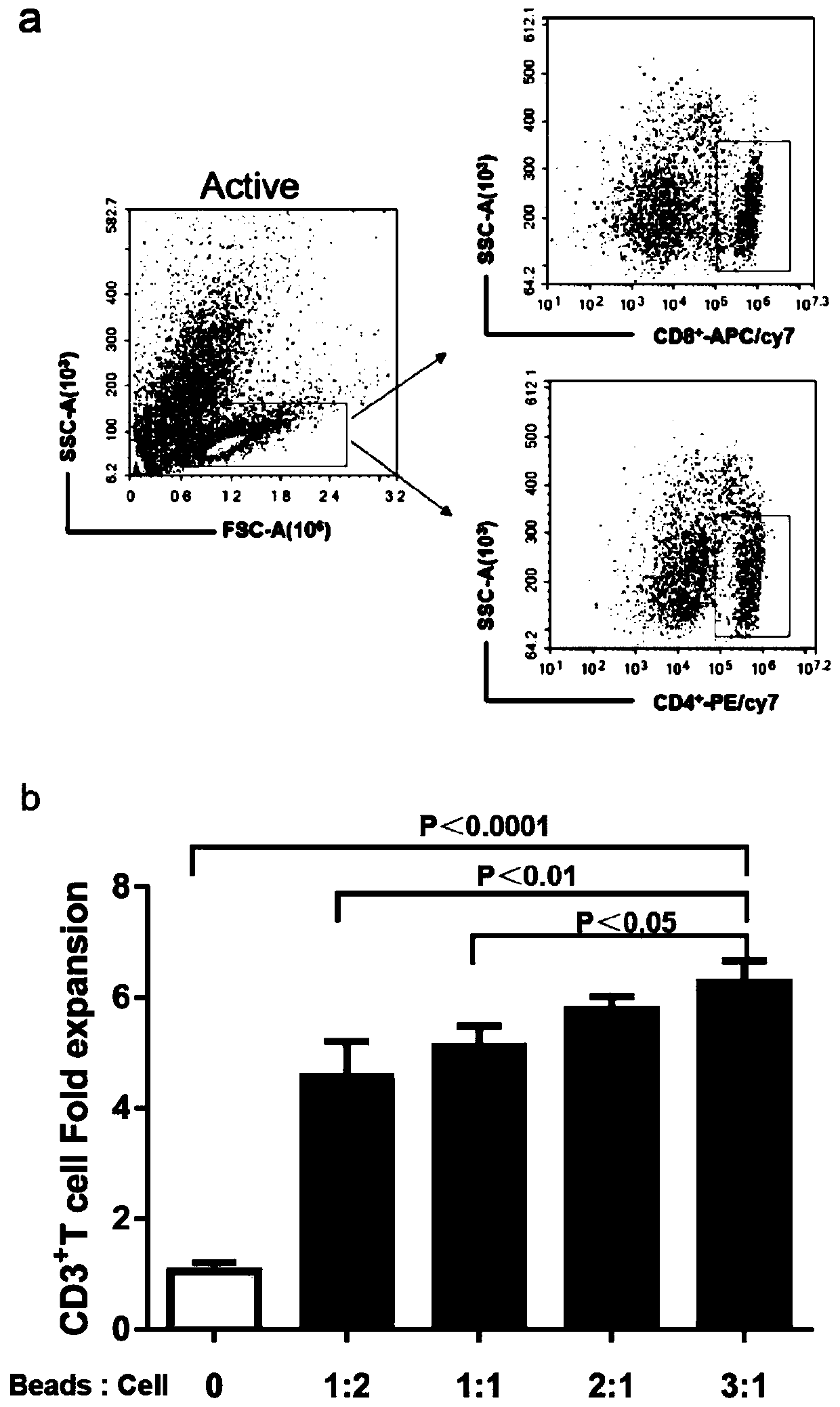
PendingCN113490502AGenetic material ingredientsStable introduction of DNAAllogeneic cellCAR T-cell therapy
Provided herein are improved methods for robust TCR+ cell depletion and production of populations of TCR- cells, which can be beneficial to minimize the GvHD risk in patients receiving allogeneic CAR T cell therapy. Provided herein are methods that increase the efficiency of depleting TCR+ cells from a population of cells in order to significantly reduce any residual levels of TCR+ cells present in cell populations in which expression of endogenous TCR has been reduced or eliminated. Associated kits and cell populations are also provided.
Owner:ALLOGENE THERAPEUTICS INC
Car t cell therapy to target t cell specific cancers
PendingUS20210283180A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyIntracellular signalling
Disclosed are chimeric antigen receptor (CAR) polypeptides comprising a T cell receptor (TCR) antigen binding domain, a transmembrane domain, and an intracellular signaling domain. Disclosed are methods of making a CAR T cell comprising obtaining a cell from a subject diagnosed with T cell lymphoma; determining the sequence of the TCR on the cell; and transducing a T cell with a vector comprising a nucleic acid sequence that encodes a CAR polypeptide, wherein the CAR polypeptide comprises a TCR antigen binding domain, a transmembrane domain, and an intracellular signaling domain, wherein the TCR antigen binding domain is specific to a subsequence of the sequence of the TCR on the cell identified in the step of determining the sequence of the TCR
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Sequencing method for car t cell therapy
PendingCN112105382APolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyMoiety
Owner:ENDOCTYE INC +2
A label and application for in vivo tracking and artificial removal of CAR-T cells
ActiveCN107287207BWide recognitionEliminates the possibility of self-replicationMammal material medical ingredientsImmunoglobulinsAbnormal tissue growthCAR T-cell therapy
The invention discloses a marker used for in-vivo tracing and manual removal of CAR-T cells. The marker comprises a trace-linker 1 with a sequence as shown in SEQ ID No. 18, a trace-linker 2 with a sequence as shown in SEQ ID No. 19 and a trace-linker 3 with a sequence as shown in SEQ ID No. 19. The invention also discloses a CAR-T therapy vector including the marker and a construction method thereof. Moreover, the invention further discloses application of the marker to preparation of the CAR-T therapy vector and drugs used for treating triple-negative breast cancer. The marker provided by the invention can realize controllable in-vivo tracing, in-vitro separation and manual removal of CAR-T cells without influence on the tumor killing effect of the CAR-T cells, so the security of CAR-T cell therapy is greatly improved, and a powerful pool can be provided for deeper analysis of the process of CAR-T cell therapy. The vector provided by the invention can express a targeting chimeric antigen receptor of CD117 in human T lymphocytes, guides and activates killing effect of T lymphocytes on CD117 positive cells, and is applicable to treatment of triple-negative breast cancer in clinical practice.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Improved Manufacturing Procedures for Cell Based Therapies
PendingUS20220195414A1Easy to manufactureAdvanced technologyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapySeparation technology
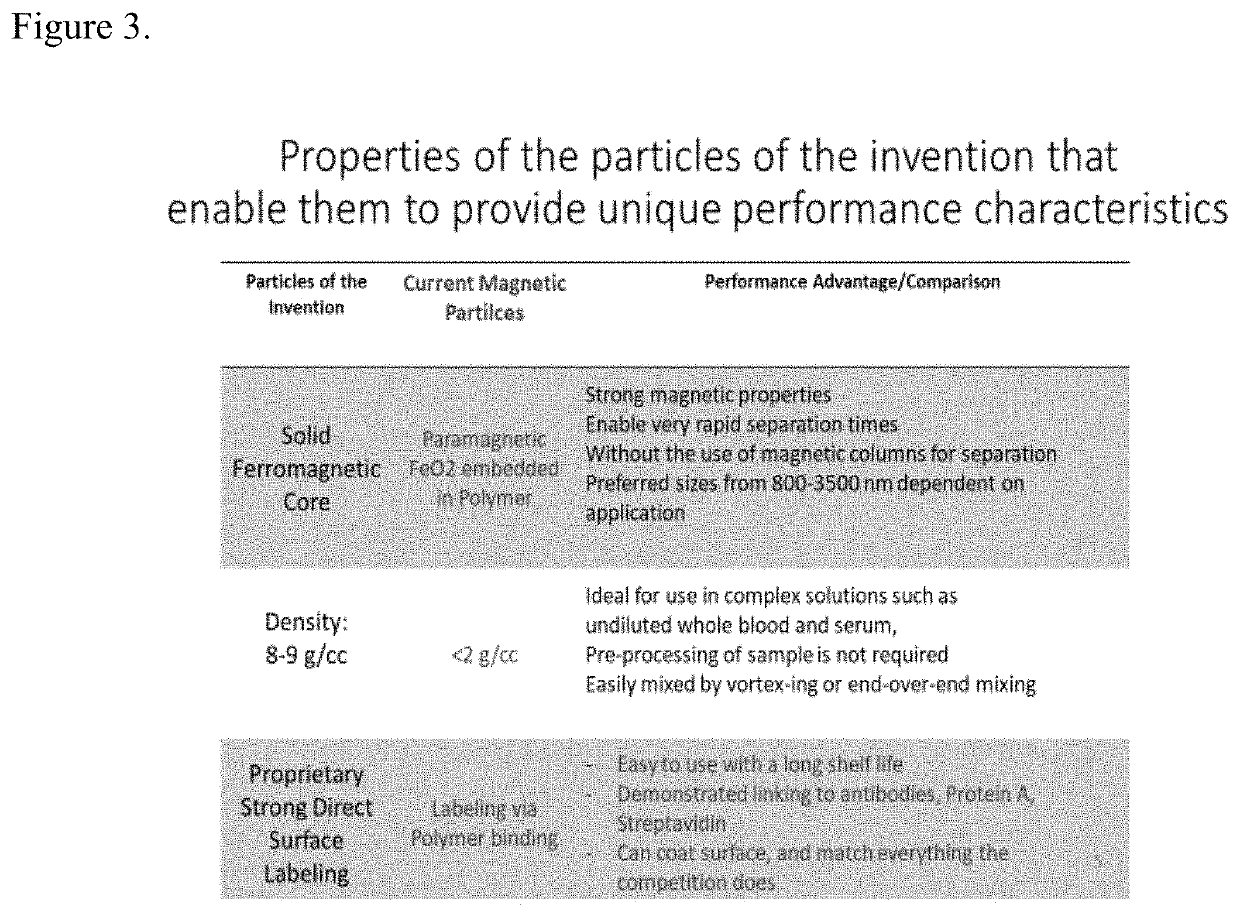
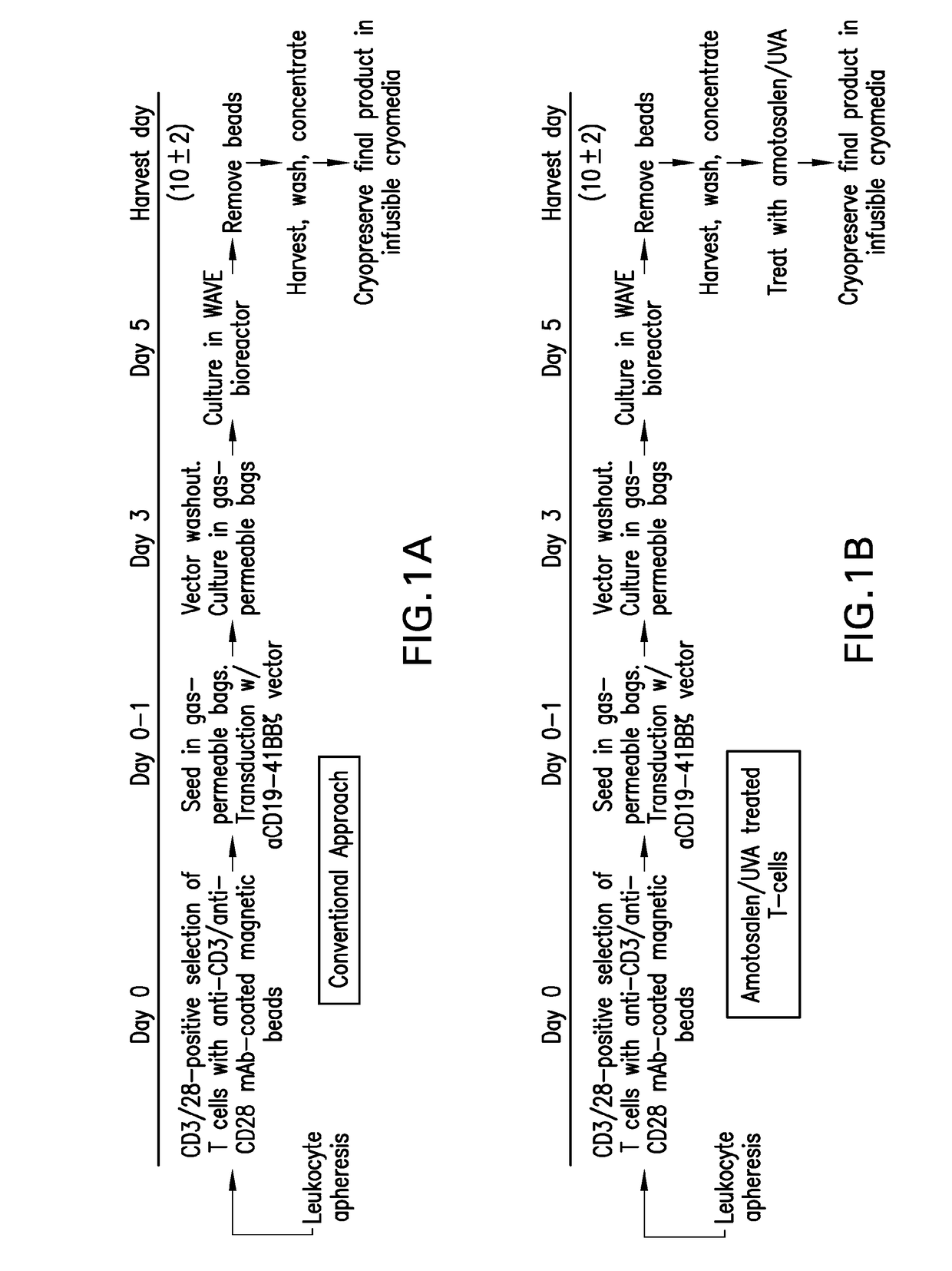
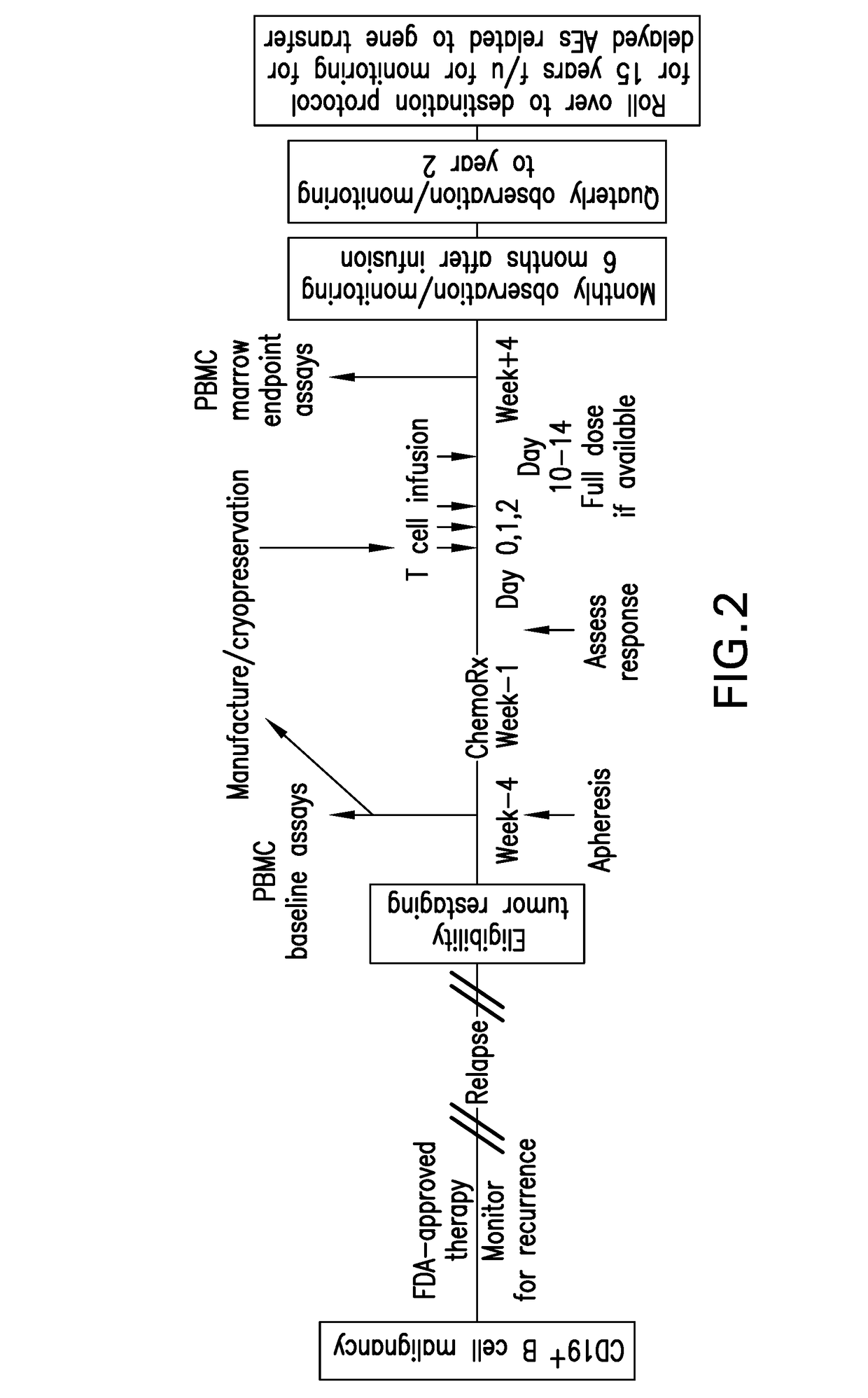
CAR T cell therapies have shown promise in treating human blood cell cancer. The preparation of CAR T cells involves many complex, time consuming steps prior to infusion of the CAR T cells into a cancer patient. One step in the process to create CAR T cells often involves using magnetic separation technologies to isolate specific subsets of T cells prior to creating the CAR T cells. When using current magnetic separation technologies to remove undesired cell populations the recovery of the desired cell population can be as low as 50-70% or even lower and the procedures often take 30-60 minutes. In the case of autologous CAR T cell therapies such cell loss is often not acceptable. The present invention offers means to improve the recovery of desired cells to close to 100% very rapidly thus significantly improving a step in the manufacture of CART cells and in many cases will make such therapy possible for a larger patient population.
Owner:RUSSELL BIOTECH
Methods and compositions for CAR T cell therapy
PendingCN109195611APharmaceutical delivery mechanismMammal material medical ingredientsCAR T-cell therapyMedicine
The present disclosure relates to methods of treating a patient with a cancer by administering to the patient a composition comprising CAR T cells and a small molecule linked to a targeting moiety bya linker. The disclosure also relates to compositions for use in such methods.
Owner:PURDUE RES FOUND INC +1
T cell synergist for CAR T cell therapy of leukemia and method for obtaining synergistic T cells
PendingCN114426952APolypeptide with localisation/targeting motifOrganic active ingredientsCAR T-cell therapyHematological Tumor
The present invention relates to T cell potentiators for use in CAR T cell therapy against hematologic tumors, such as acute myelogenous leukemia, and methods of using the same to enhance the effect of CAR T cell therapy. The method comprises treating T cells used in CAR T therapy in an in vitro culture process using a T cell potentiator. The T cell synergist provided by the invention can enhance migration of CAR T / NK cells to bone marrow, and improve the effect of CAR T cell therapy in removing tumors.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY](https://images-eureka.patsnap.com/patent_img/6b528952-6df4-4456-86ee-3eae8b26d331/HDA0003241518250000011.png)
![METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY](https://images-eureka.patsnap.com/patent_img/6b528952-6df4-4456-86ee-3eae8b26d331/HDA0003241518250000021.png)
![METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY METHODS FOR ENHANCING TCR[alpha][beta]+ CELL DEPLETION EFFICIENCY](https://images-eureka.patsnap.com/patent_img/6b528952-6df4-4456-86ee-3eae8b26d331/HDA0003241518250000031.png)