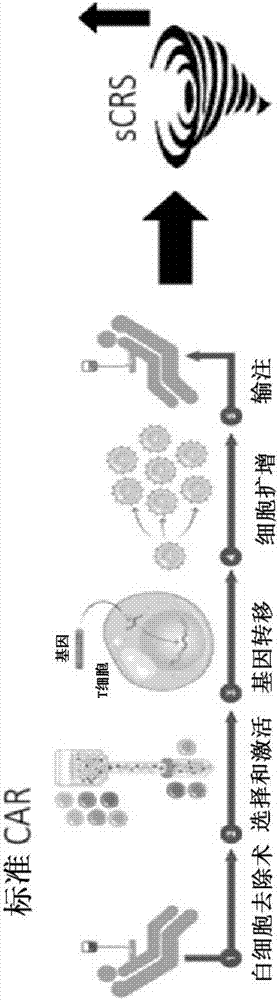
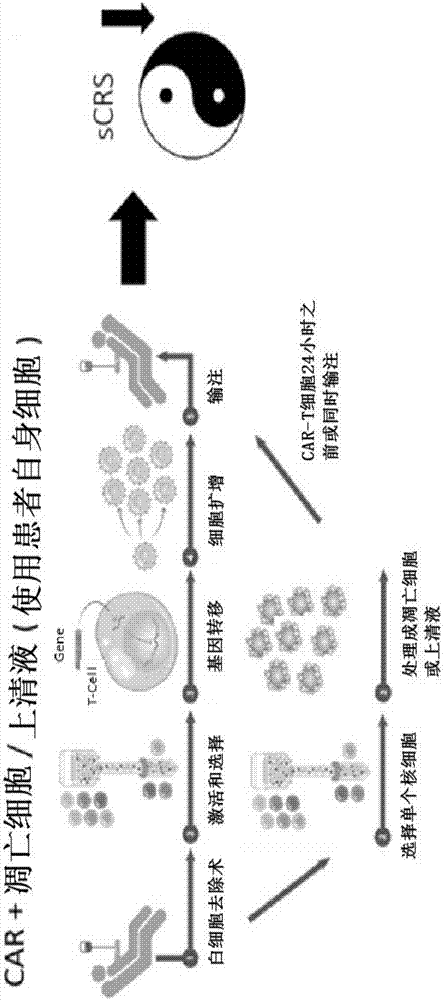
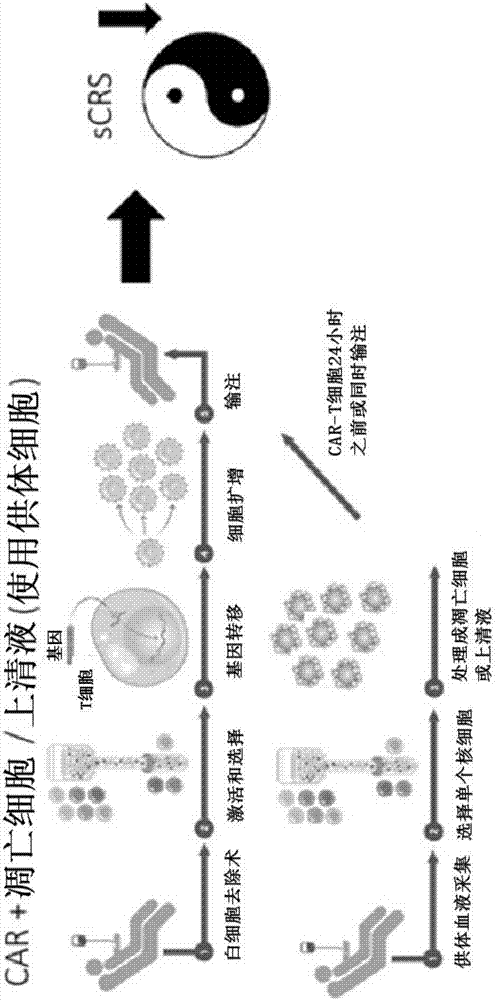
Combination immune therapy and cytokine control therapy for cancer treatment
A technology of cytokines and cells, which is applied in the field of compositions that reduce or inhibit the production of cytokines, and can solve the problems of affecting the activity and proliferation of CART-cells, the inability to effectively control cytokine release syndrome, and the limited ability to inject anti-inflammatory drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0471] Example 1: Apoptotic cell therapy prevents cytokine storm in subjects administered CAR T-cell therapy
[0472] Materials and methods
[0473] recombinant DNA construct
[0474] Develop CARs that retarget T-cells specific to specific tumor-associated antigens. Control CARs were developed that directed T-cells to unrelated tumor-associated antigens. CARs used for background were armed CAR T or 4th passage CAR T-cells. In one embodiment, cells are also engineered to express the extracellular structure of the IL-4 receptor alpha subunit linked to the transmembrane and intracellular domains of the beta-chain used by the IL-2 and IL-15 receptors domain, allowing the expansion of T-cells by adding IL-4.
[0475] Retroviral transduction and culture of T4+ T-cells
[0476] Blood samples were obtained from healthy volunteers and cancer patients. T-cells can be activated prior to gene transfer using CD3 / CD28 coated paramagnetic beads (1:1 bead / cell ratio; Life Technologies) or...
Embodiment 2
[0496] Example 2: Effect of apoptotic cells on cytokine storm without negative impact on CAR-T cell potency
[0497] Objective: To test the effect of apoptotic cells or supernatants derived from apoptotic cells on cytokine storm marker cytokines and CAR T-cell potency against tumor and cancer cells.
[0498] method:
[0499] A solid tumor model reported to induce a cytokine storm in mice was utilized (van der Stegen et al., 2013ibid). In this model, T cells are engineered with chimeric antigen receptors (CARs) targeting certain ErbB dimers (T4 + CAR-T cells), which are often highly upregulated in certain solid tumors such as head and neck tumors and ovarian cancer. T-cells were isolated from PBMC isolated from peripheral blood using CD3 microbeads. Vectors containing chimeric T4+ receptors were constructed and transduced into isolated T-cells, resulting in T4+ CAR T-cells. For the experiments performed here, T4+ CAR T-cells were purchased (Creative Biolabs (NY USA) or Prom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com