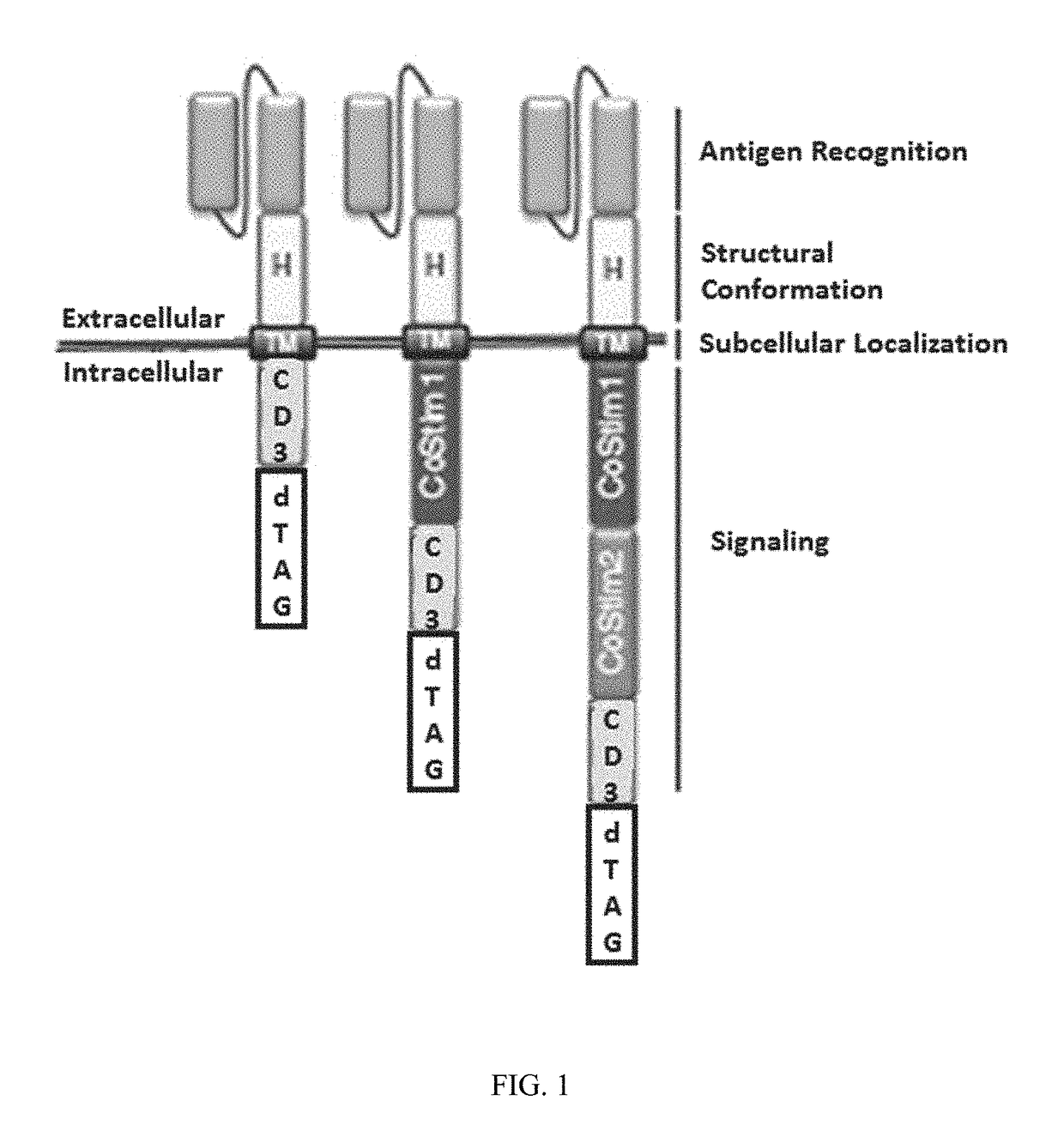
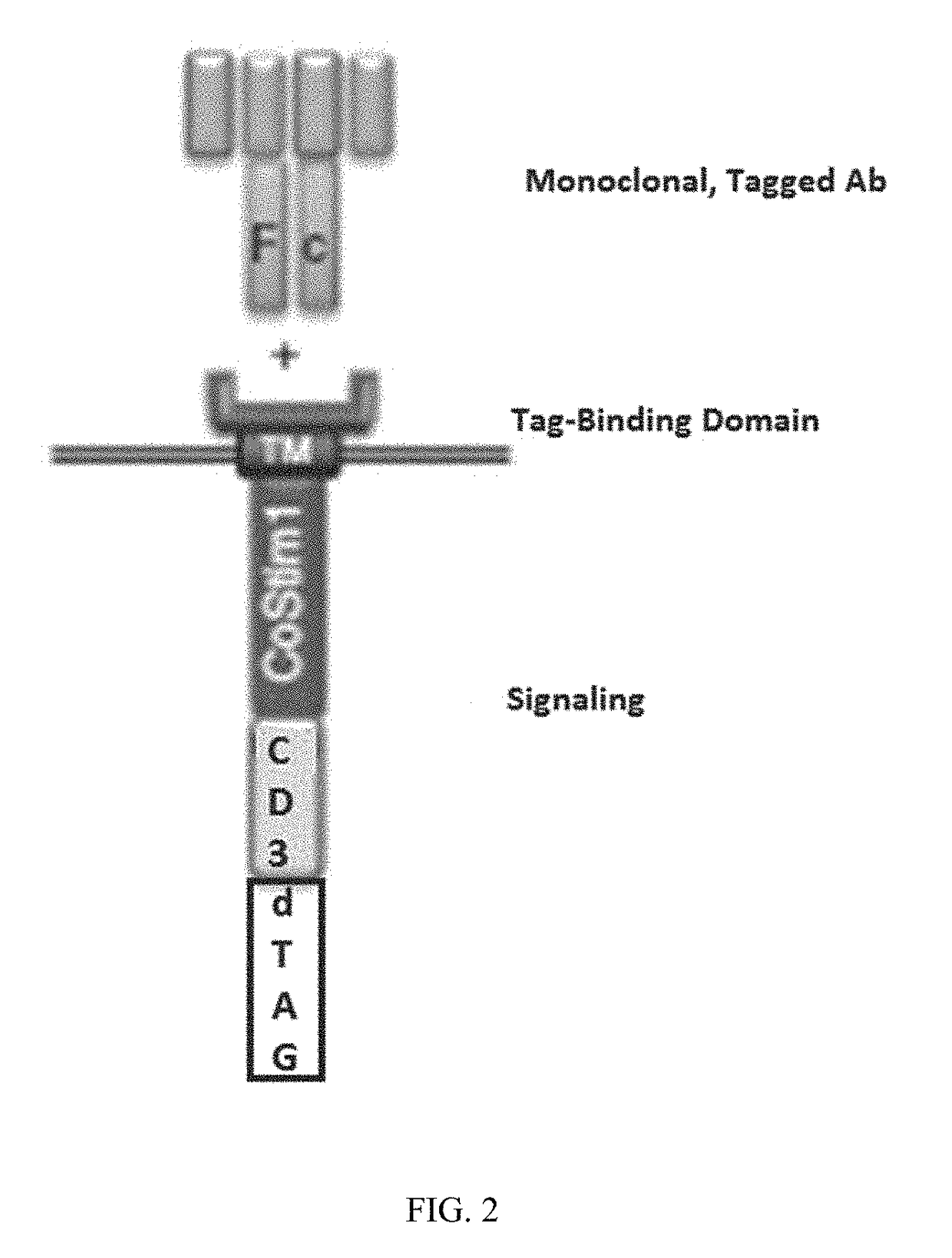
Patents
Literature
68 results about "Cytokine release syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytokine release syndrome, also known as an infusion reaction, is a form of systemic inflammatory response syndrome that arises as a complication of some diseases or infections, and is also an adverse effect of some monoclonal antibody drugs, as well as adoptive T-cell therapies. Severe cases have been called cytokine storms.
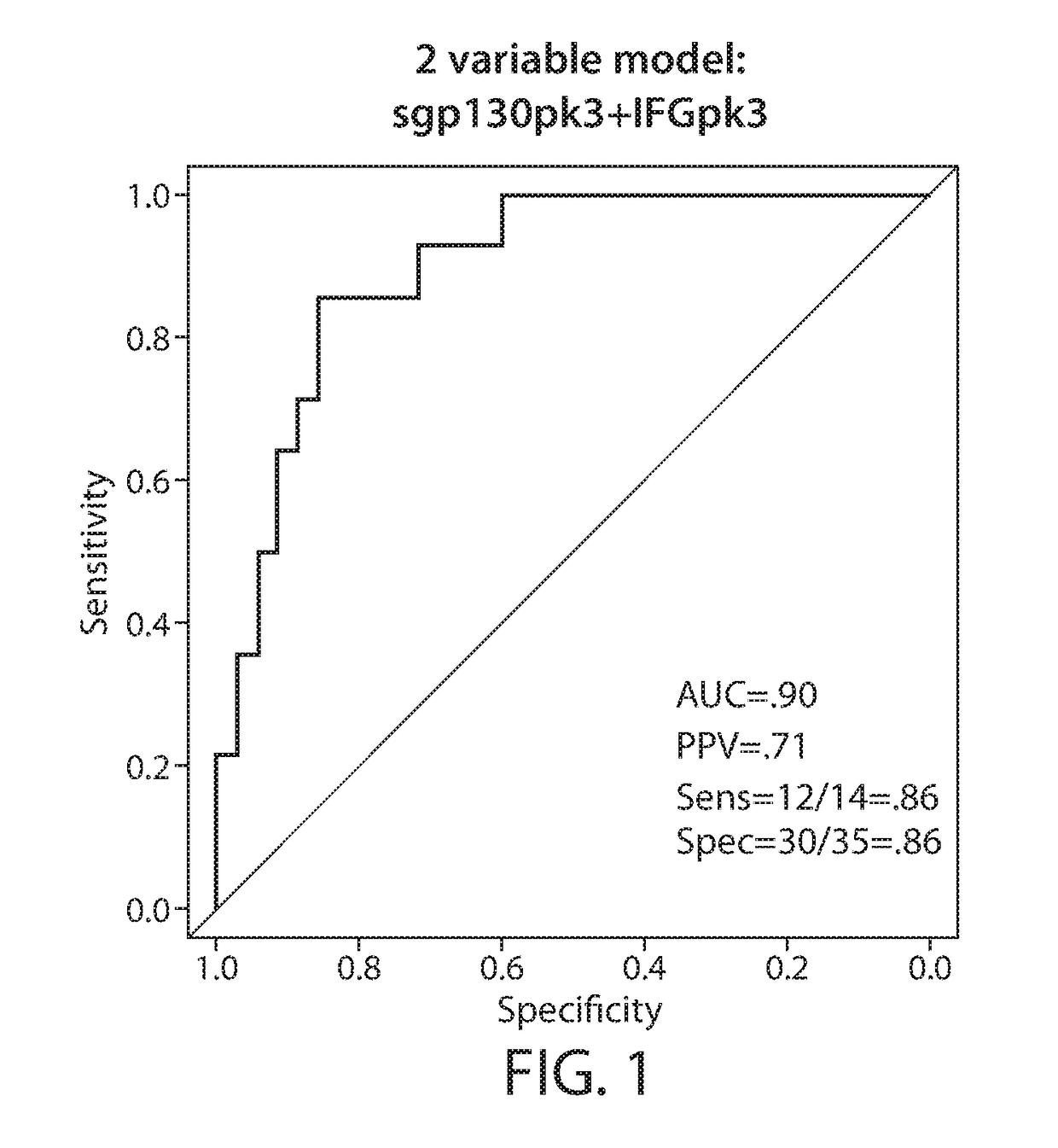
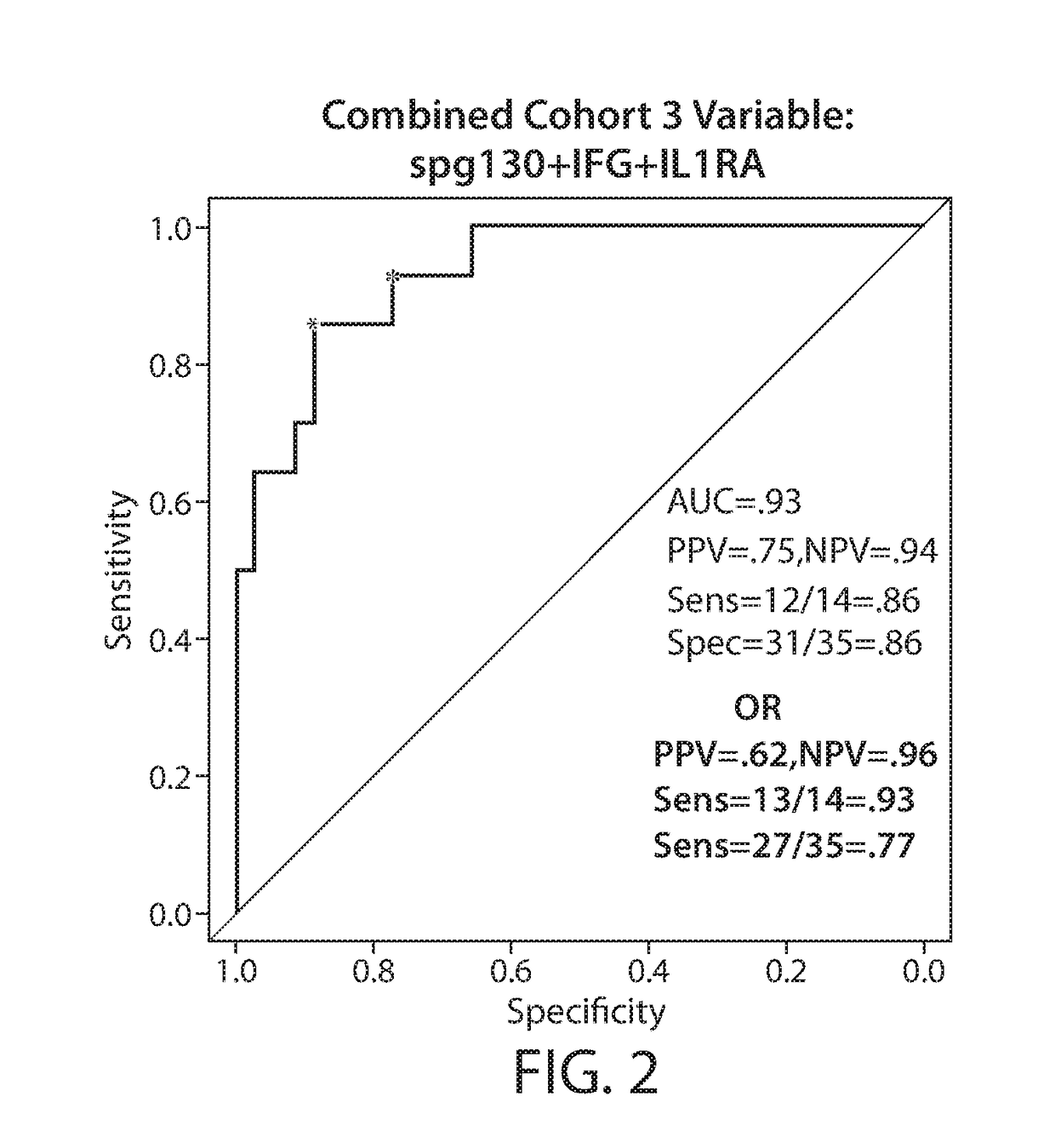
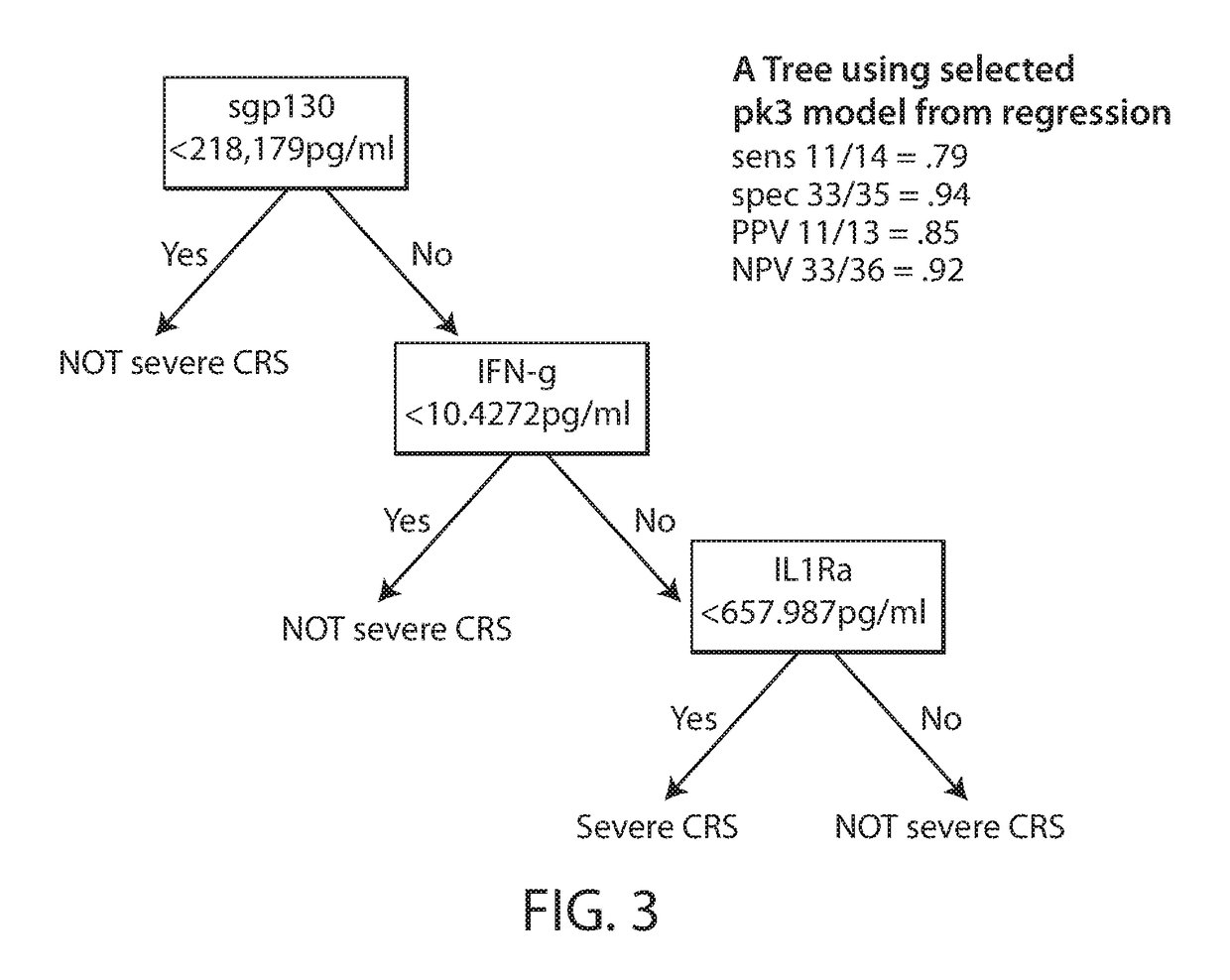
Biomarkers predictive of cytokine release syndrome
ActiveUS20180252727A1Reduce riskHigh activityOrganic active ingredientsAntipyreticAnalyteProtein expression profile
Owner:NOVARTIS AG +1
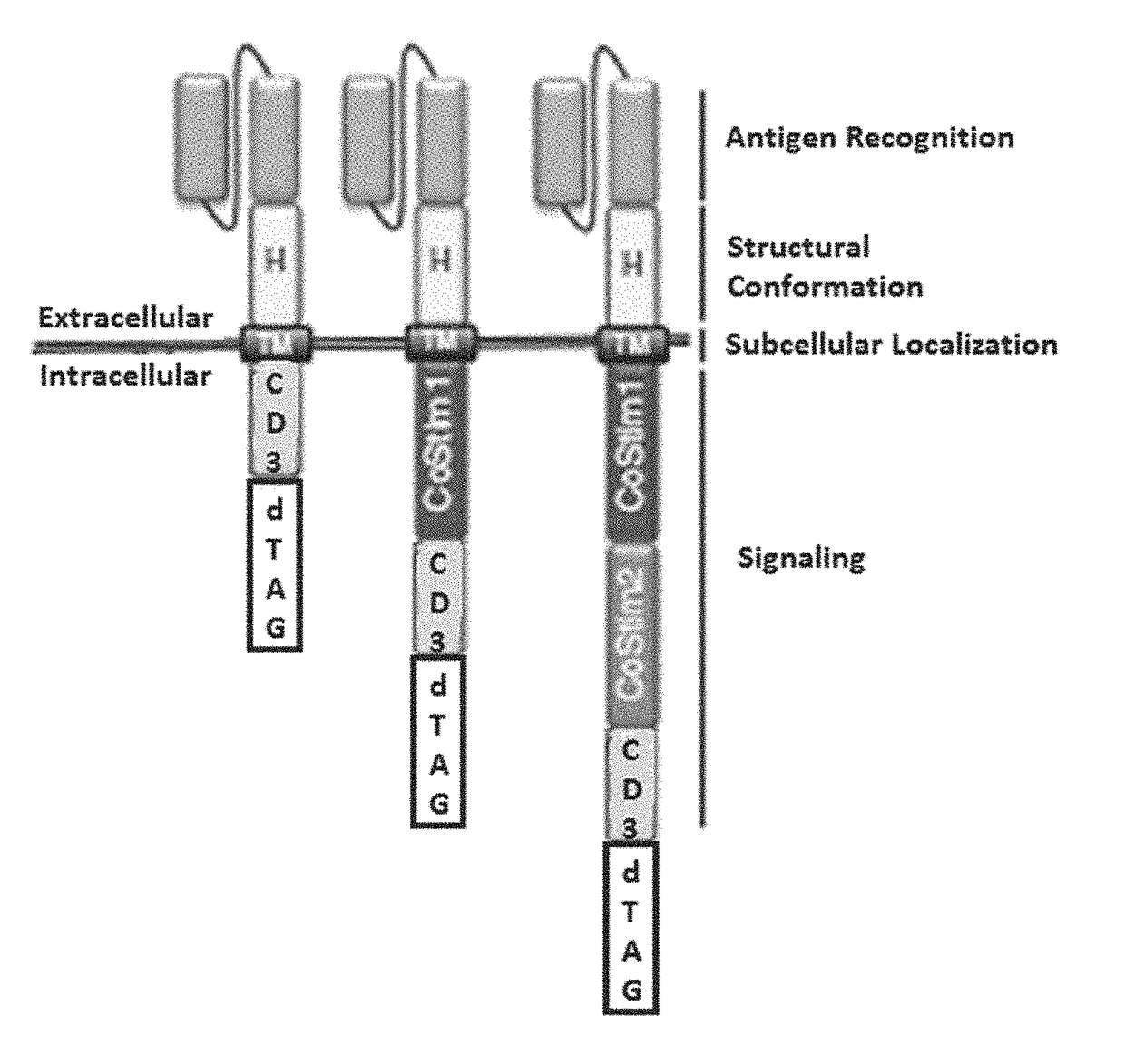
Targeted protein degradation to attenuate adoptive t-cell therapy associated adverse inflammatory responses
ActiveUS20180169109A1Rapid cell deathOrganic active ingredientsFusion with degradation motifTumor lysis syndromeTumor Syndrome
This invention is in the area of compositions and methods for regulating chimeric antigen receptor immune effector cell, for example T-cell (CAR-T), therapy to modulate associated adverse inflammatory responses, for example, cytokine release syndrome and tumor lysis syndrome, using targeted protein degradation.
Owner:DANA FARBER CANCER INST INC
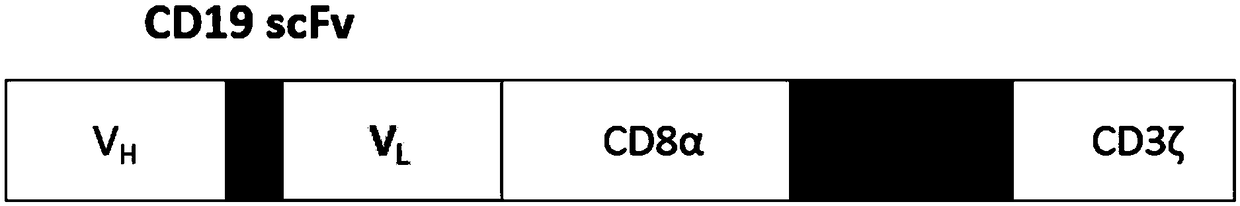
Specific chimeric antigen receptor T cells targeting CD19 and preparation and clinical application thereof
PendingCN110272493AEffective targeted attackHigh kill rateVirusesAntipyreticSide effectCD19-specific chimeric antigen receptor
The invention relates to specific chimeric antigen receptor T cells targeting CD19, and a preparation method and clinical application thereof. The present invention constructs a specific chimeric antigen receptor targeting CD19 and immune response cells modified by the chimeric antigen receptor based on a targeted human CD19 single-chain antibody sequence. The novel modified immune response cells can effectively target and attack a plurality of tumor cells, especially tumor cells with positive CD19 expressiion, and can be used to prepare a preparation for the treatment of tumors. The method for preparing the modified immune response cells targeting CD19 is simple, and the obtained modified immune response cells targeting CD19 have a high killing rate on tumor cells. Clinical verification shows that: after a million-grade low-dose back transfusion, patients with recurrent and refractory advanced CD19-positive lymphoma get a significant clinical symptom relief after two weeks of treatment, almost complete relief curative effect is obtained on the 77th day, and no fever caused by cytokine release syndrome and any neurotoxic side effect occur.
Owner:NANJING KAEDI BIOTECH INC
Regulating chimeric antigen receptors
PendingCN111386263APeptide/protein ingredientsAntibody mimetics/scaffoldsTumor lysis syndromeProtein target
This invention is in the area of compositions and methods for regulating chimeric antigen receptor immune effector cell, for example T-cell (CAR-T), therapy to modulate associated adverse inflammatoryresponses, for example, cytokine release syndrome and tumor lysis syndrome, using targeted protein degradation.
Owner:DANA FARBER CANCER INST INC
Defibrotide for the prevention and treatment of cytokine release syndrome and neurotoxicity associated with immunodepletion
ActiveUS20210187004A1Reduce the impactPolypeptide with localisation/targeting motifOrganic active ingredientsNeurotoxicityImmunotherapy
The present disclosure provides method of preventing, lessening the effects, or treating cytokine release syndrome (CRS) or related disorders, and / or neurotoxicity associated with immunotherapy comprising administering defibrotide. The defibrotide can be administered after the immunotherapy begins or be administered prophylactically before immunotherapy begins or before the patient develops CRS and / or neurotoxicity.
Owner:JAZZ PHARMA IRELAND LTD
Defibrotide for the prevention and treatment of cytokine release syndrome and neurotoxicity associated with immunodepletion
PendingCN112236149APolypeptide with localisation/targeting motifOrganic active ingredientsNeurotoxicityImmunotherapy
The present disclosure provides method of preventing, lessening the effects, or treating cytokine release syndrome (CRS) or related disorders, and / or neurotoxicity associated with immunotherapy comprising administering defibrotide. The defibrotide can be administered after the immunotherapy begins or be administered prophylactically before immunotherapy begins or before the patient develops CRS and / or neurotoxicity.
Owner:JAZZ PHARMA
Use Of Humanized Mice To Determine Toxicity
InactiveUS20150007357A1Enhance immune responseImprove fidelityCompounds screening/testingBiological material analysisPhysiologyCytokine
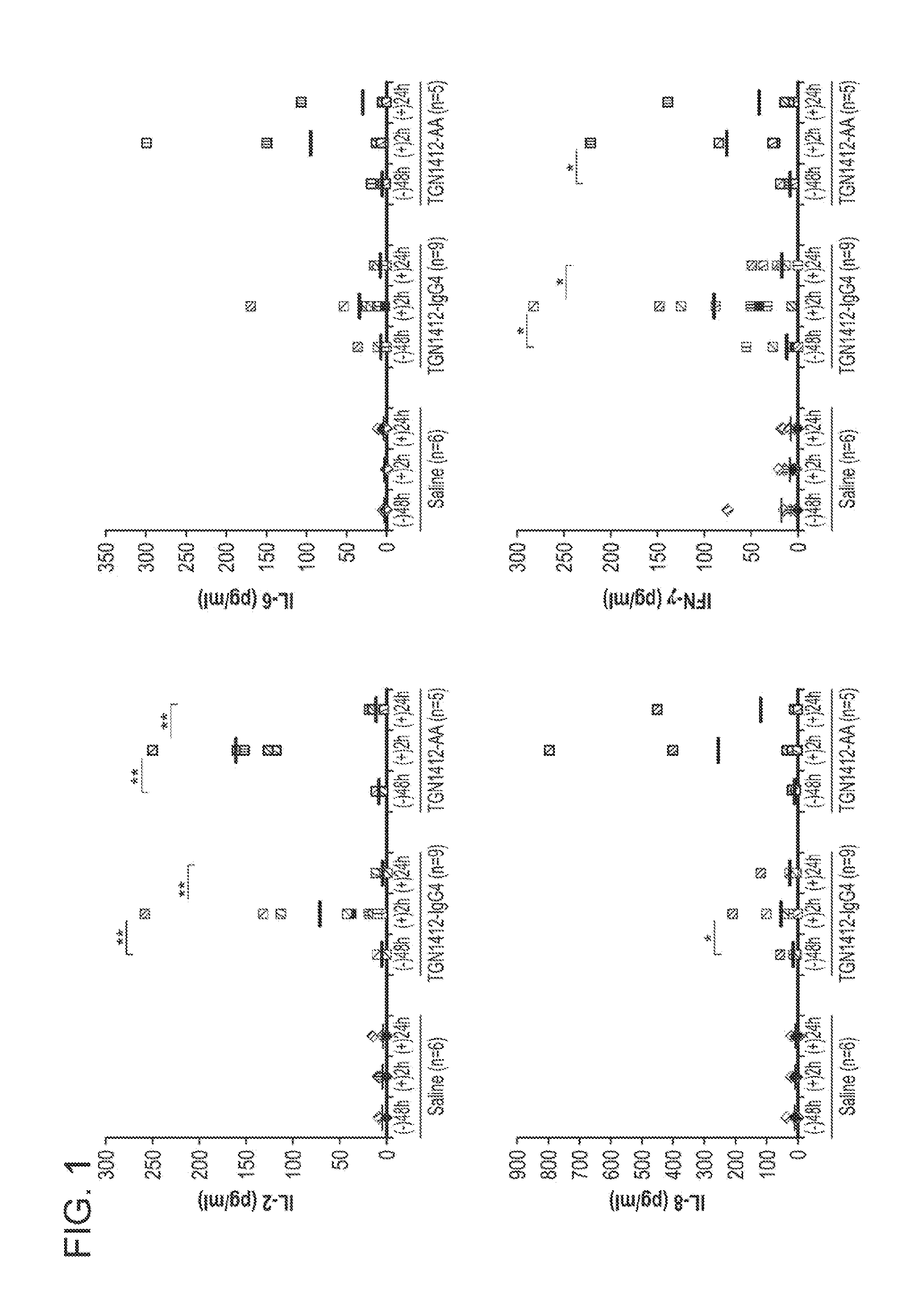
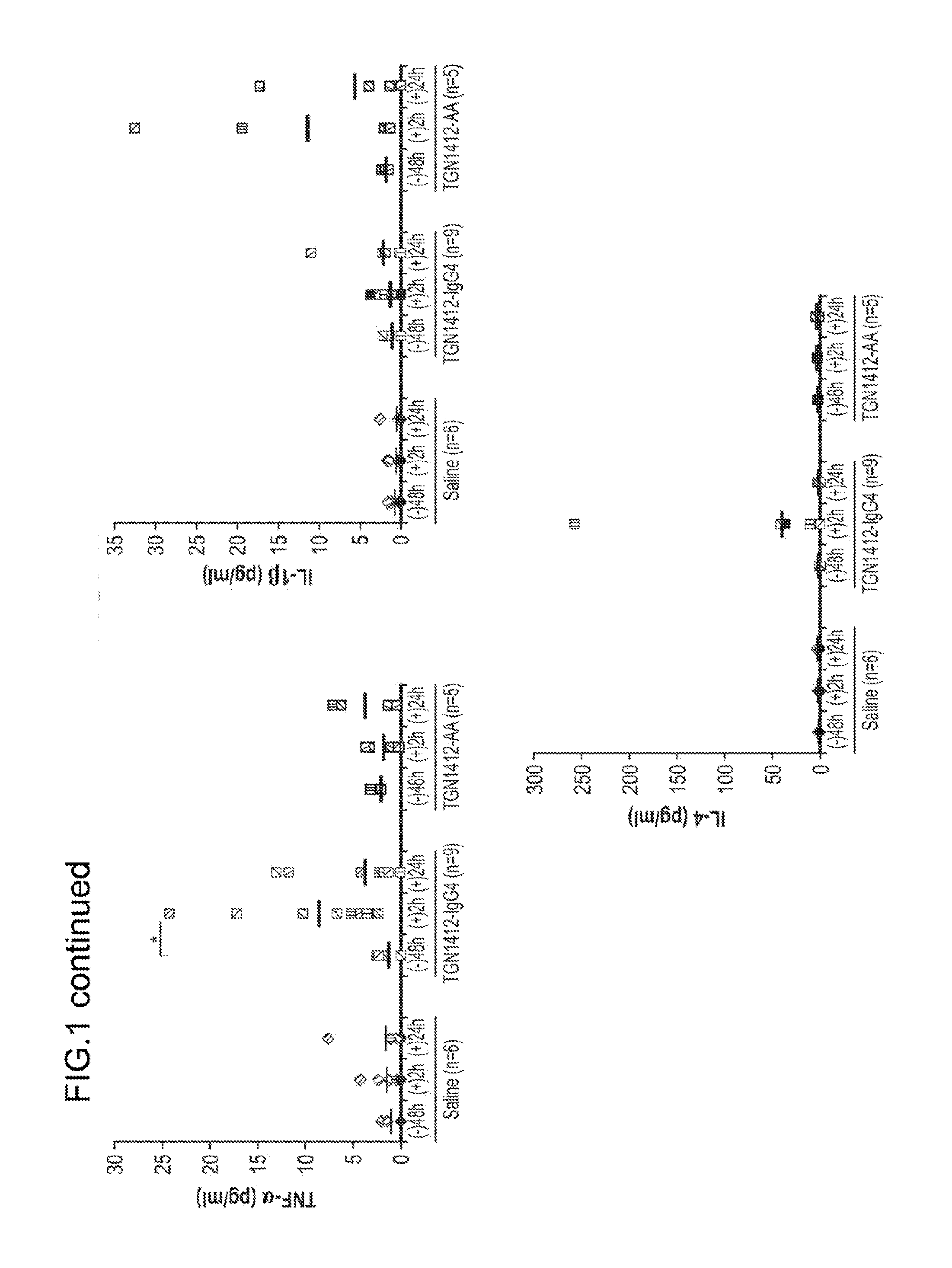
The invention is directed to a method of determining whether an agent causes immune toxicity in a human comprising administering the agent to a non-human mammal that has been engrafted with human hematopoietic stem cells (HSCs) and administered one or more human cytokines; and determining whether the agent causes immune toxicity in the non-human mammal. If the agent causes immune toxicity in the non-human mammal then the agent causes toxicity in a human. The invention is also directed to a method of determining whether administration of an agent causes cytokine release syndrome in an individual in need thereof comprising administering the agent to a non-human mammal that has been engrafted with HSCs and administered one or more human cytokines; and determining whether the agent causes cytokine release syndrome in the non-human mammal. If the agent causes cytokine release syndrome in the non-human mammal then the agent will cause cytokine release syndrome in the human.
Owner:MASSACHUSETTS INST OF TECH
Novel target in the treatment of cytokine release syndrome
InactiveUS20090175879A1AntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseViral infection
A novel therapeutic and diagnostic target in the treatment of Cytokine Release Syndrome is provided. More specifically, a novel target is disclosed in the treatment of CRS occurring with diseases such as bacterial and viral infection as well as adverse reaction in drug therapy.
Owner:CELLACT PHARMA
Compositions
InactiveUS20180228866A1Improve the effect of immunotherapyControlling the riskOrganic active ingredientsAntipyreticMedicineUlcerative colitis
The present invention relates to a formulation comprising an inhibitor of NFAT activation for use in treating or preventing undesirable effects, more particularly undesirable effects occurring in conjunction with T cell-mediated therapies. The undesirable effects may be cytokine release syndrome (CRS) or symptoms associated with gastrointestinal (GI) inflammation, for example associated with inflammatory bowel diseases, such as ulcerative colitis, optionally caused by activated T cell activity. In addition to ameliorating undesirable effects, the invention is aimed at also maintaining the therapeutic effects of the T-cell mediated therapy.
Owner:SIGMOID PHARM LIMITED
Combination immune therapy and cytokine control therapy for cancer treatment
PendingCN107530376AGenetically modified cellsMammal material medical ingredientsCAR T-cell therapyImmune therapy
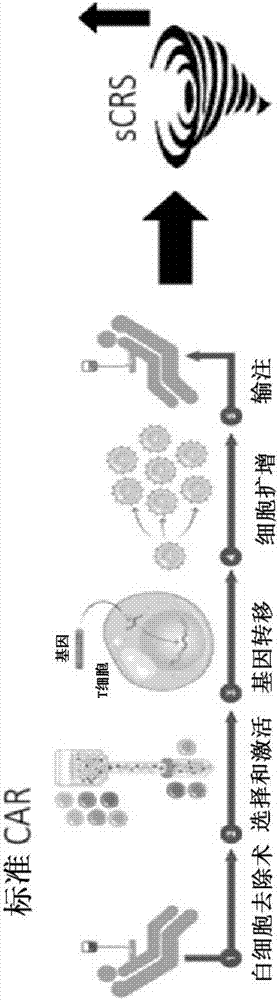
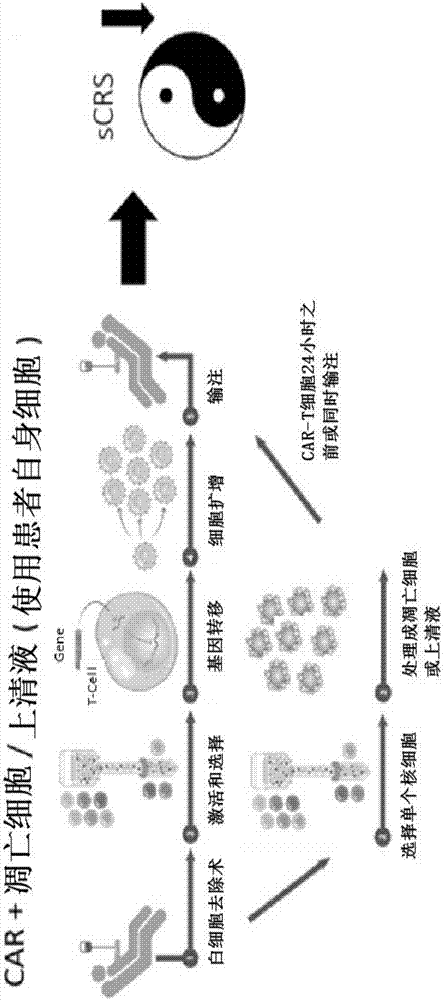
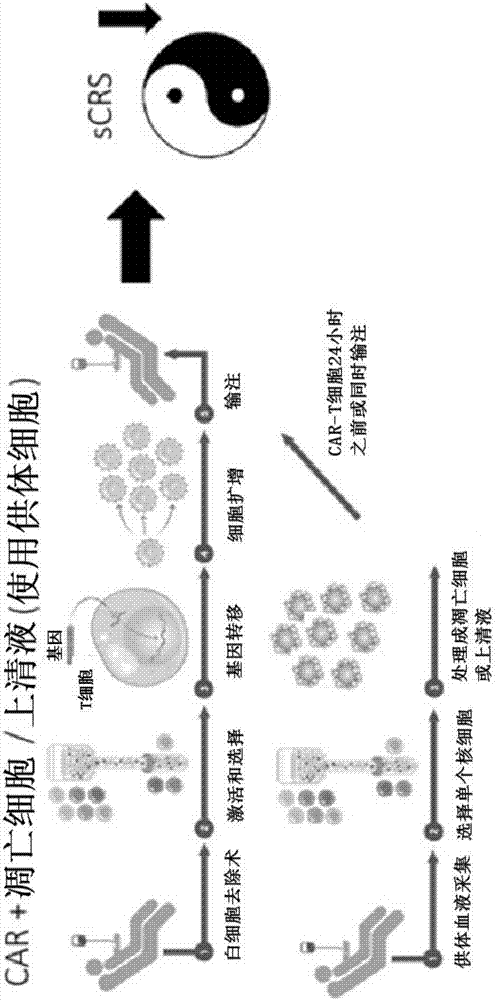
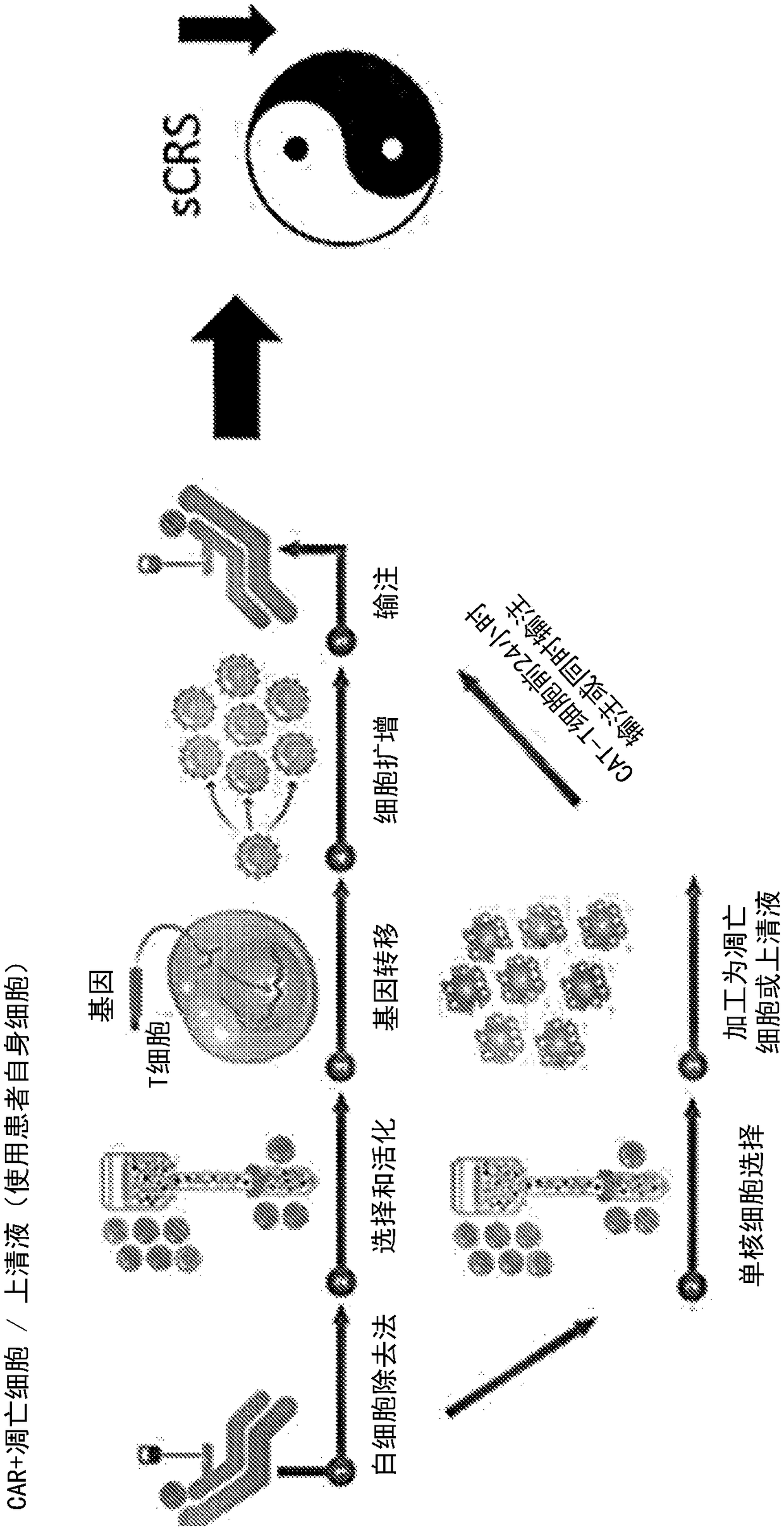
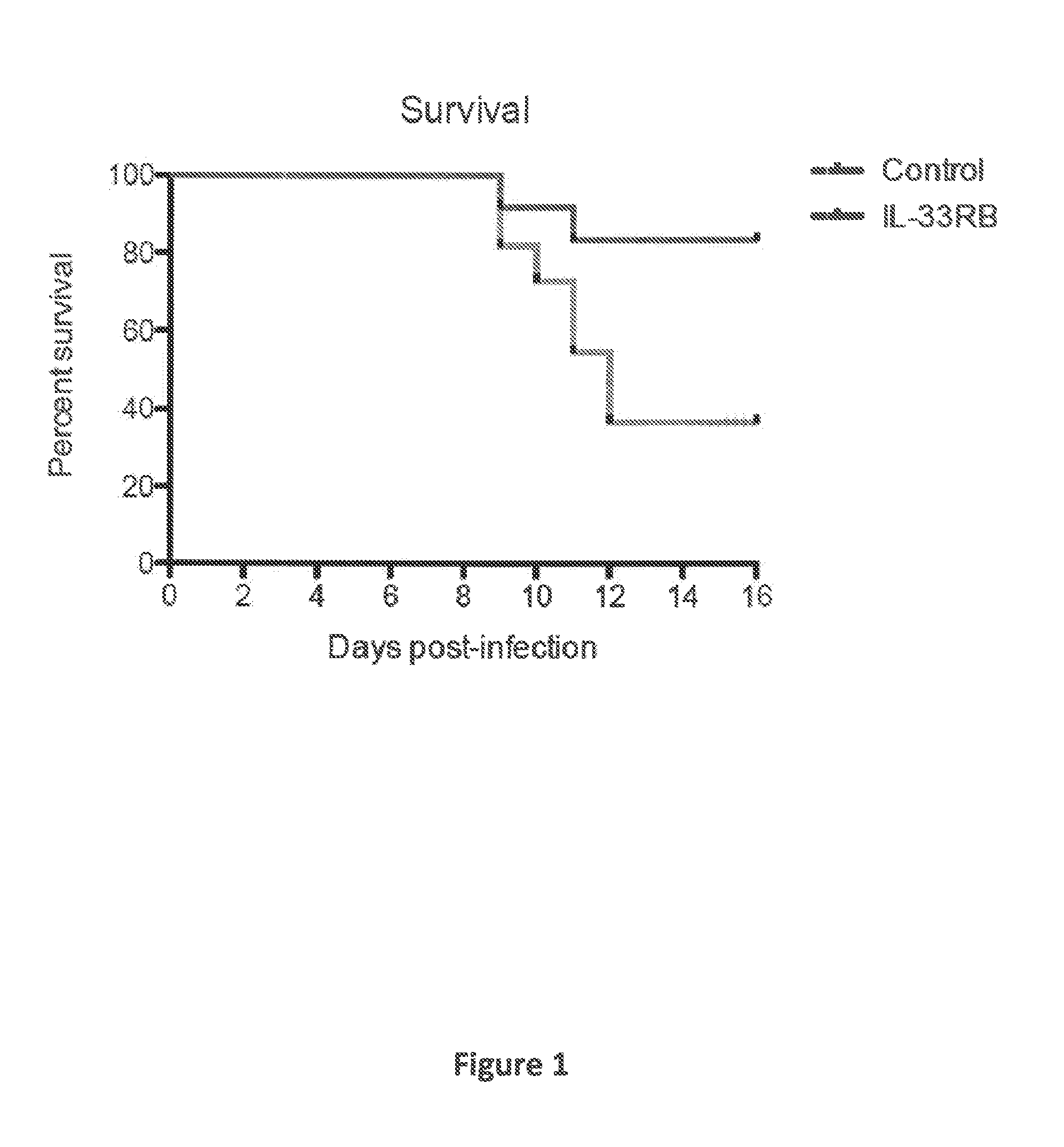
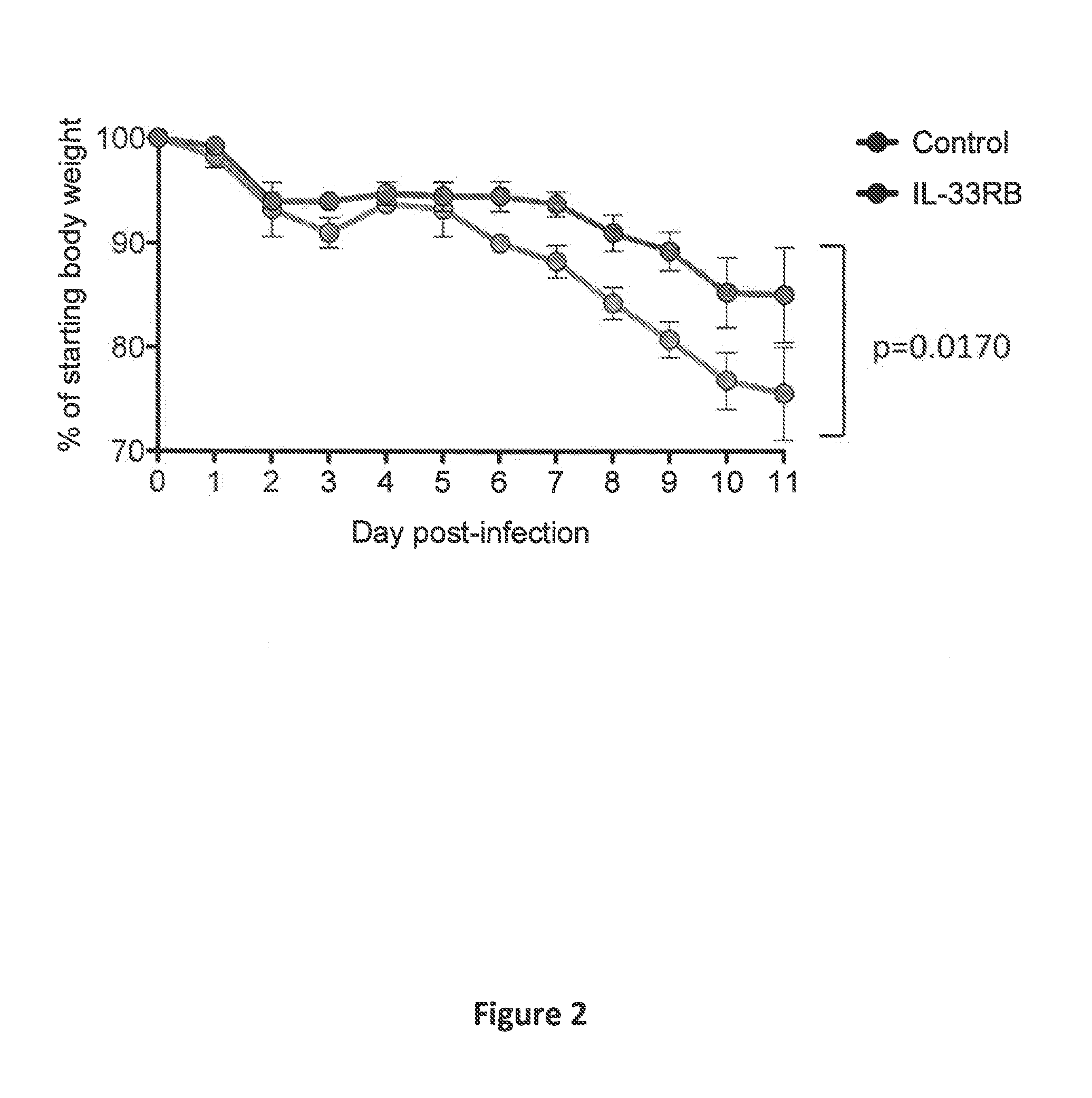
Compositions disclosed herein, and methods of use thereof include those for inhibiting or reducing the incidence of cytokine release syndrome or cytokine storm in a subject undergoing CAR T-cell therapy, wherein the subjects are administered compositions including apoptotic cells or apoptotic cell supernatants. In certain instances compositions and methods of use thereof disclosed herein do not reduce the efficacy of the CAR T-cell cancer therapy. Disclosed herein are also compositions and methods of use thereof for decreasing or inhibiting cytokine production in a subject experiencing cytokine release syndrome or cytokine storm including administration of a composition including apoptotic cells or an apoptotic cell supernatant.
Owner:ENLIVEX THERAPEUTICS LTD
Powdered formulations of cromolyn sodium and alpha-lactose
The present disclosure is directed to a composition comprising micronized cromolyn sodium, α-lactose, and a salt of fatty acid, wherein the α-lactose has a particle size distribution of D90 of 45-70 μm, D50 of 10-35 μm, and D10 of 2-13 μm. The present disclosure is also directed to a method of treating Alzheimer's disease, amyloidosis-associated condition (AAC), traumatic brain injury, Huntington's disease, atherosclerosis, cytokine release syndrome (CRS), dementia, head injury, infection, neuroinflammation, prion disease, stroke, amyotrophic lateral sclerosis (ALS), Parkinson's disease, or asthma using the composition.
Owner:THE GENERAL HOSPITAL CORP +1
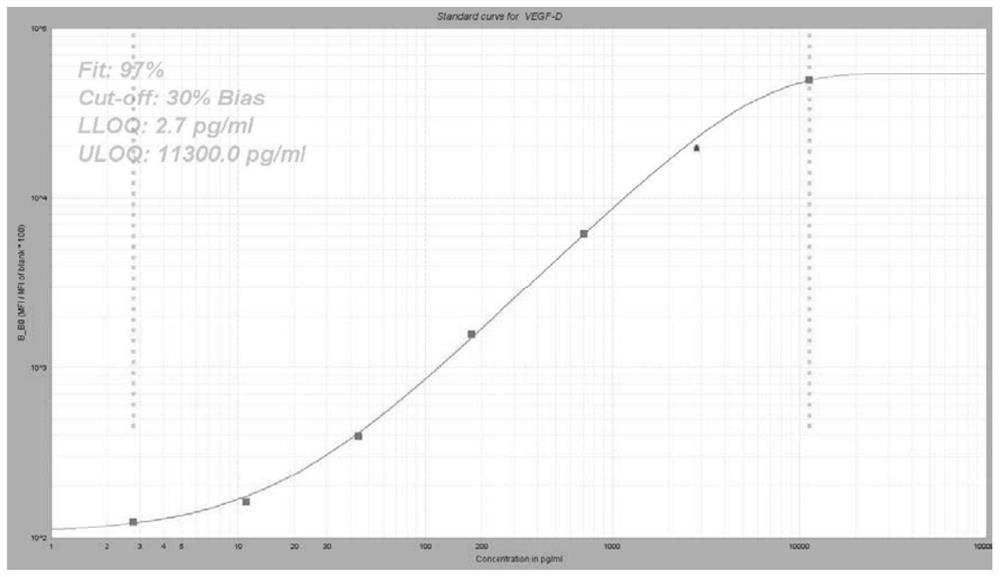
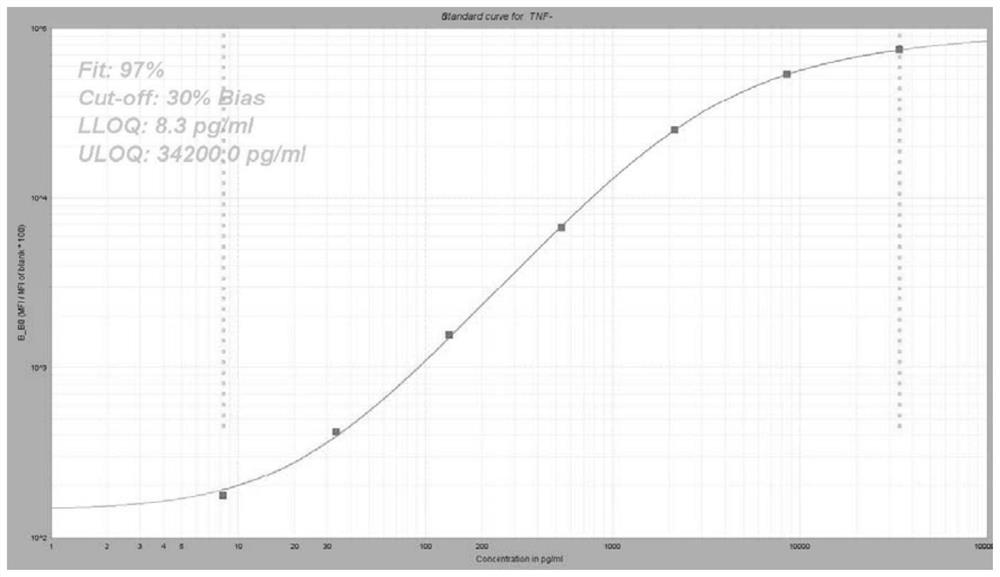
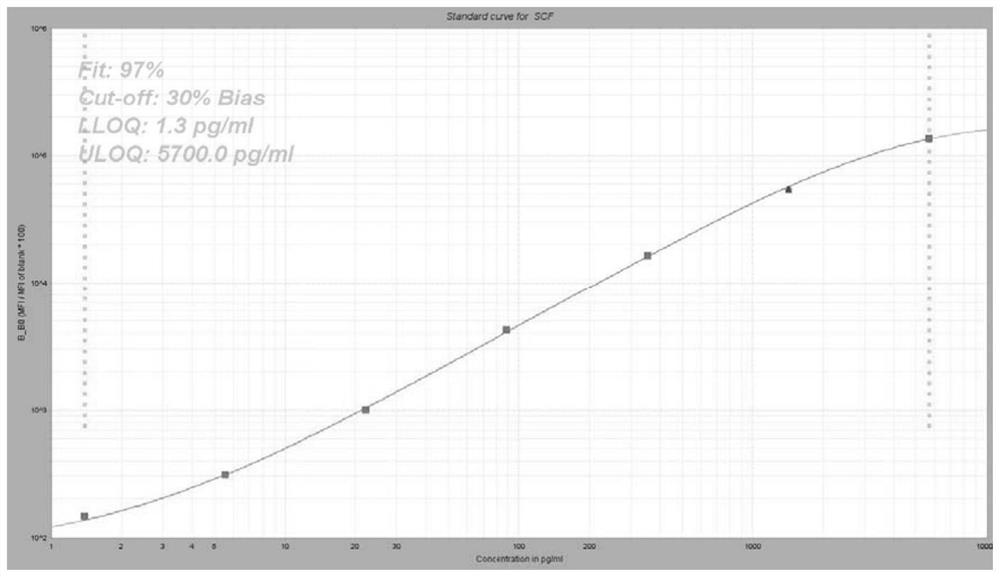
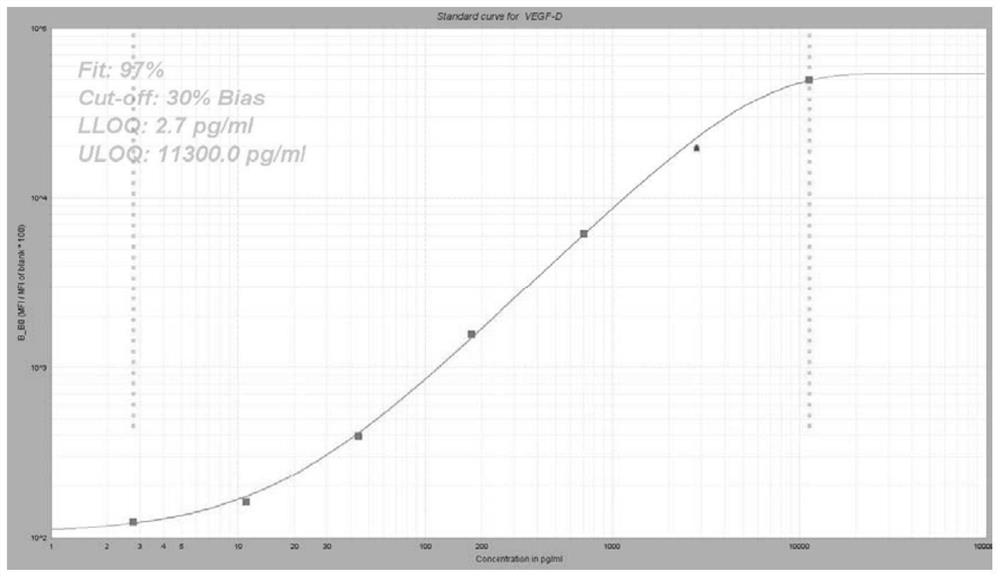
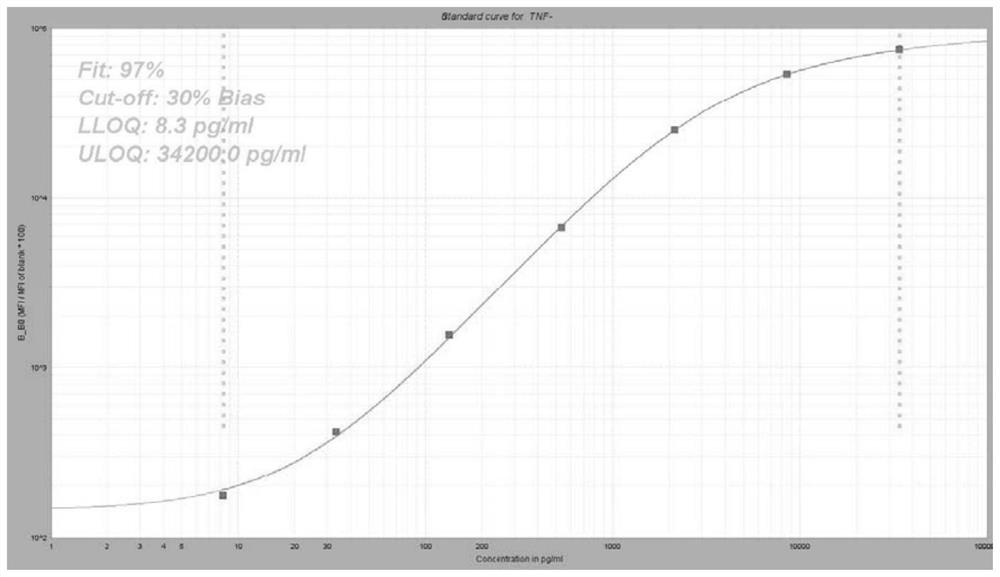
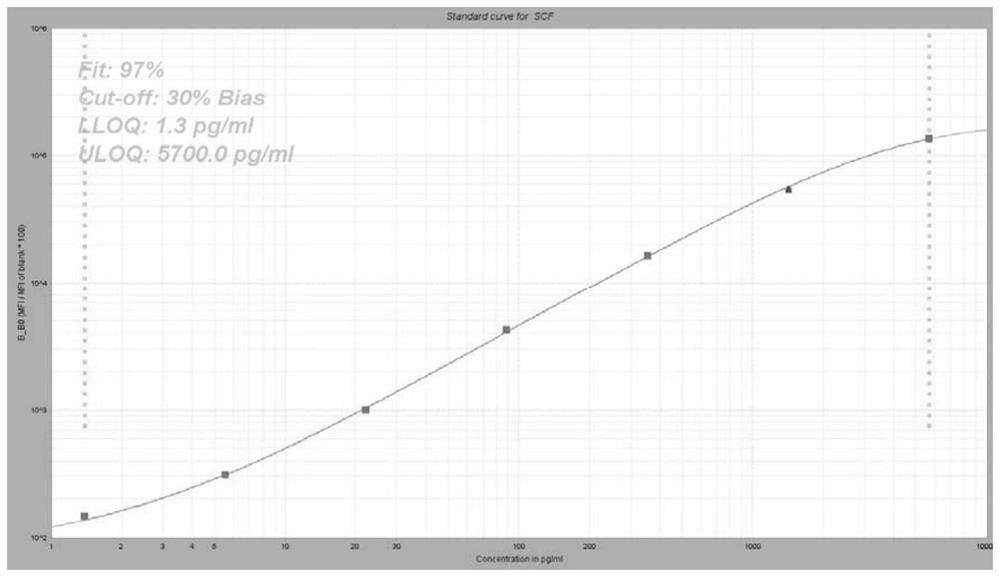
Group of markers for predicting cytokines and thrombostorms of 2019 coronavirus, application and kit
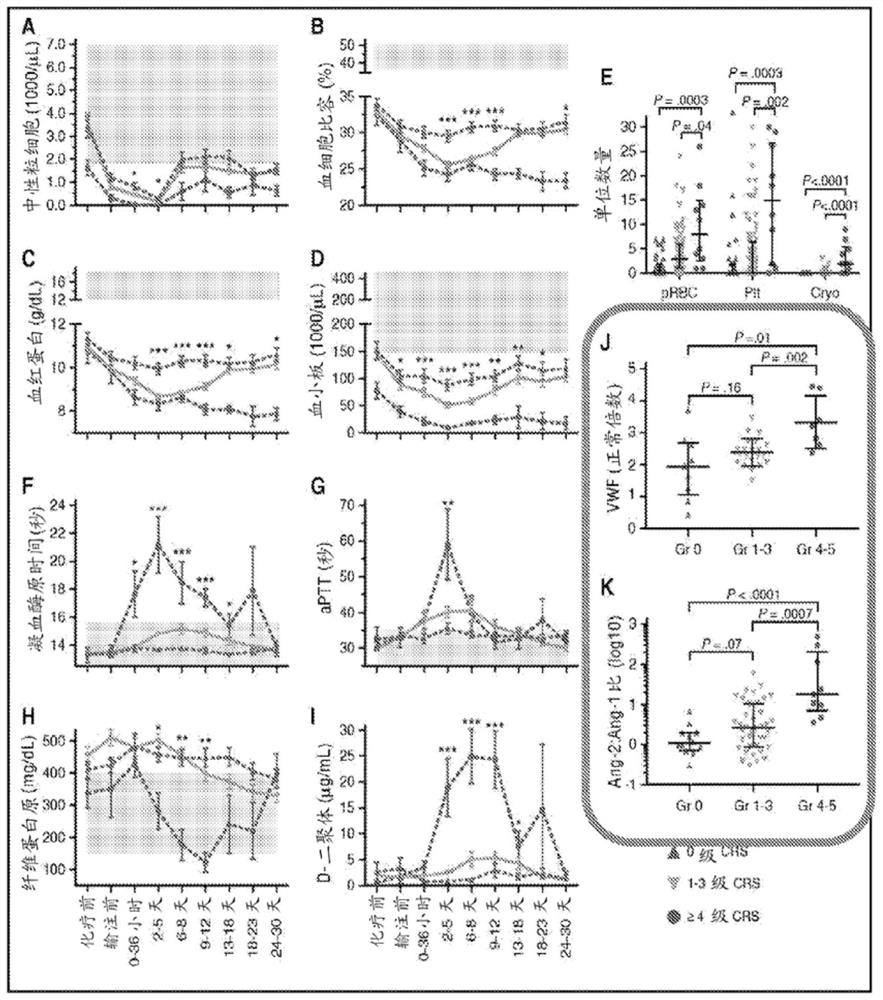
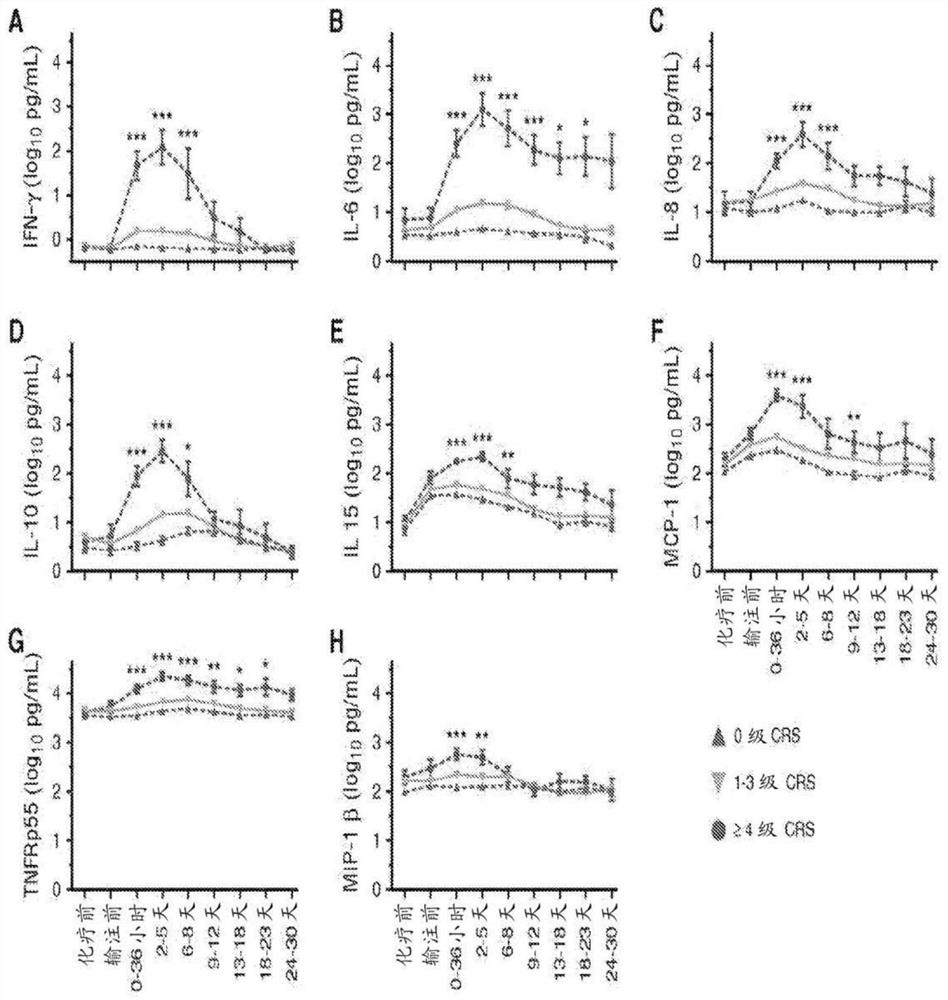
The invention provides a group of markers for predicting cytokine release syndrome and thrombostorm of 2019 coronavirus, application of the markers, a kit and a preparation method of the kit, and belongs to the technical field of biological medicines. The markers provided by the invention are respectively VEGFD, TNF-alpha, SCF, LIF, IL-2, IL-4, IL-6, IL-8, IL-10, IL-15 , IL-17A, IL-18, IL-1beta and IFN-gamma. The kit for predicting the cytokine release syndrome and thrombus storm of the 2019 coronavirus disease (COVED19) by utilizing the group of markers of VEGFD, TNF-alpha, SCF, LIF, IL-2, IL-4, IL-6, IL-8, IL-10, IL-15 , IL-17A, IL-18, IL-1beta and IFN-gamma or the kit prepared from the markers has the advantages of rapid and accurate detection, low cost and the like.
Owner:BEIJING IMMUPEUTICS MEDICINE TECH LTD
Combination immune therapy and cytokine control therapy for cancer treatment
PendingCN109069539APolypeptide with localisation/targeting motifGenetically modified cellsCAR T-cell therapyImmune therapy
Compositions disclosed herein, and methods of use thereof included those for inhibiting or reducing the incidence of cytokine release syndrome or cytokine storm in a subject undergoing CAR T-cell therapy, wherein the subjects are administered compositions comprising apoptotic cells or apoptotic cell supernatants. In certain instances compositions and methods of use thereof disclosed herein do notreduce the efficacy of the CAR T-cell cancer therapy. Disclosed herein are also compositions and methods of use thereof for decreasing or inhibiting cytokine production in a subject experiencing cytokine release syndrome or cytokine storm comprising administration of a composition comprising apoptotic cells or an apoptotic cell supernatant.
Owner:恩立夫克治疗RDO有限责任公司
Compositions and Methods for Treating Cytokine-Related Disorders
ActiveUS20170037134A1Reduce morbidityReduce mortalityPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHemophagocytic lymphohistiocytosisCytokine
Compositions and methods for treating a cytokine release syndrome such as chimeric antigen receptor T-cell cytokine release syndrome or hemophagocytic lymphohistiocytosis are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
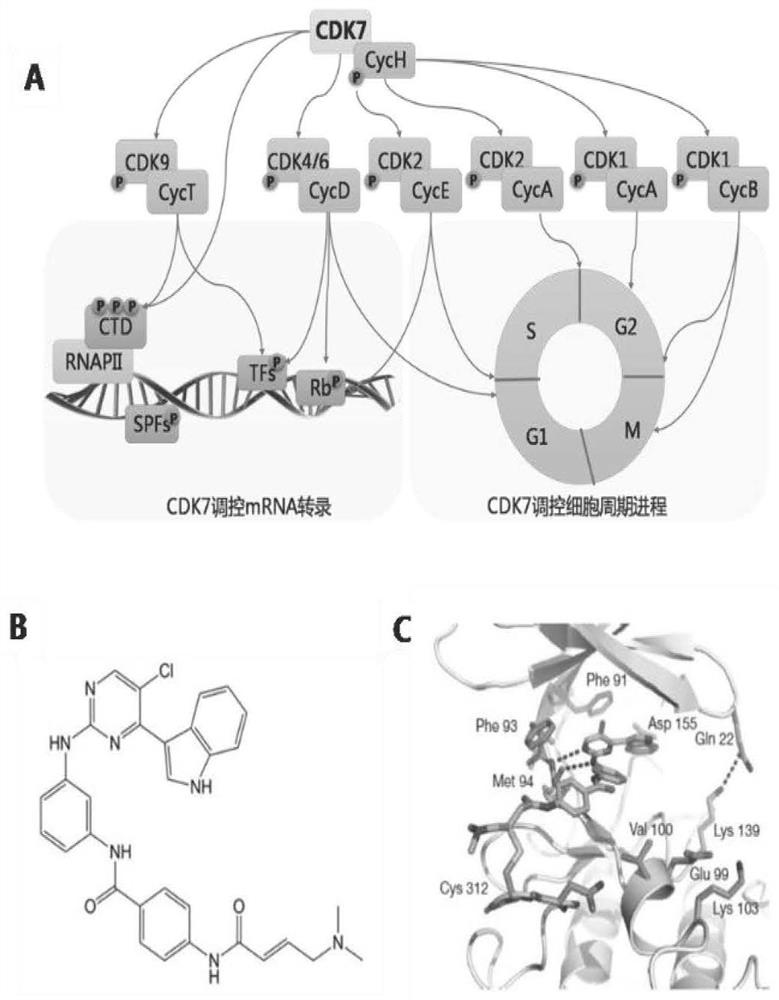
The invention also discloses application of CDK7 targeting inhibitor in preparation of drugs for treating cytokine release syndrome
ActiveCN112656798AGood effectReduce deathAntibacterial agentsOrganic active ingredientsBacterial virusInflammatory factors
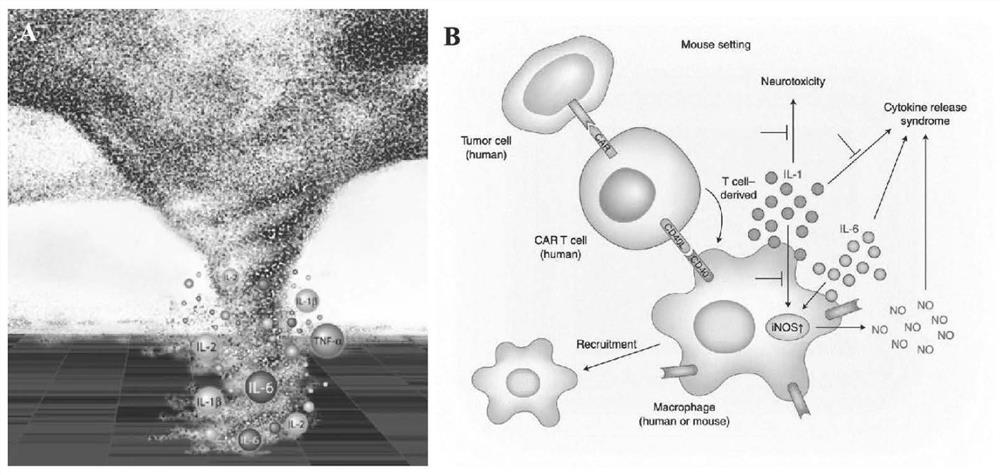
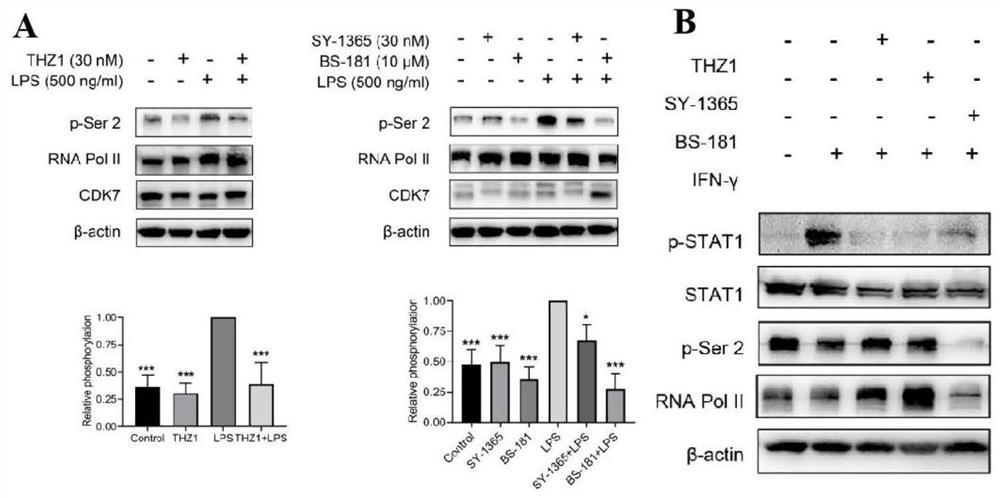
The invention discloses an application of a CDK7 targeting inhibitor in preparation of a medicine for treating cytokine release syndrome, which is verified by biochemical and animal model experiments as follows: (1) under proper concentration, the CDK7 targeting inhibitor inhibits the expression of super enhancers and transcription factors related to inflammatory factors such as stat1, IL-1 and IL-6, and thereby inflammatory signal pathways of immune cells such as macrophages, T cells and endothelial cells can be specifically regulated and controlled; (2) mouse death caused by acute inflammatory storm caused by bacterial or viral infection can be remarkably reduced, functional failure caused by inflammation of related organs is reduced, and obvious toxic and side effects are not found in the period. According to the results, the polypeptide has great significance in treatment of cytokine release syndrome caused by bacteria, viruses or immunotherapy.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
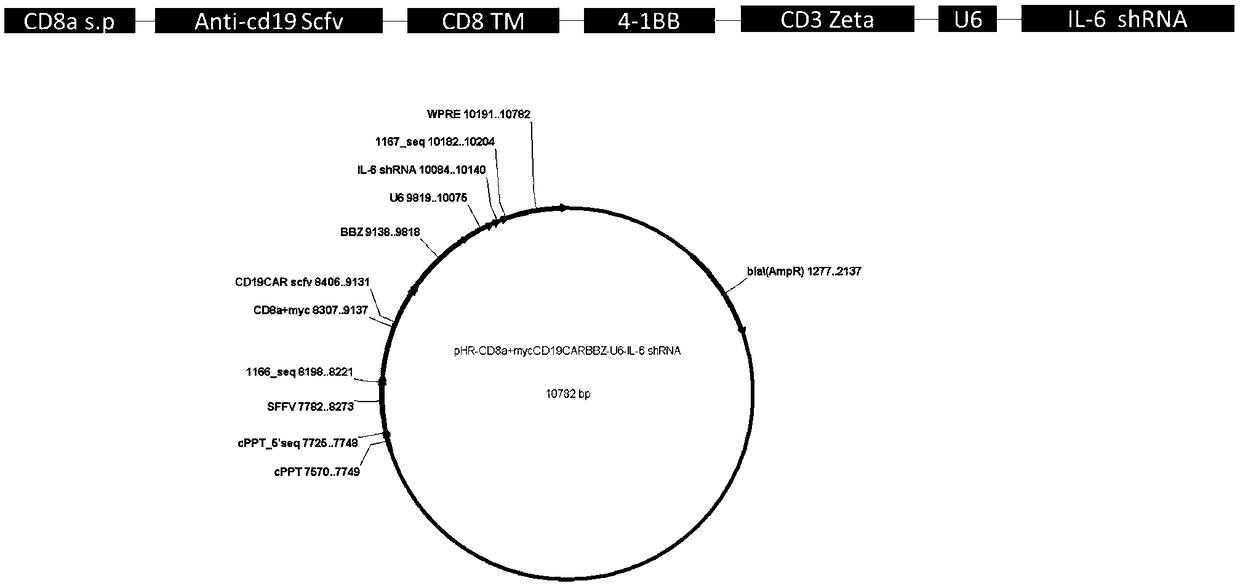
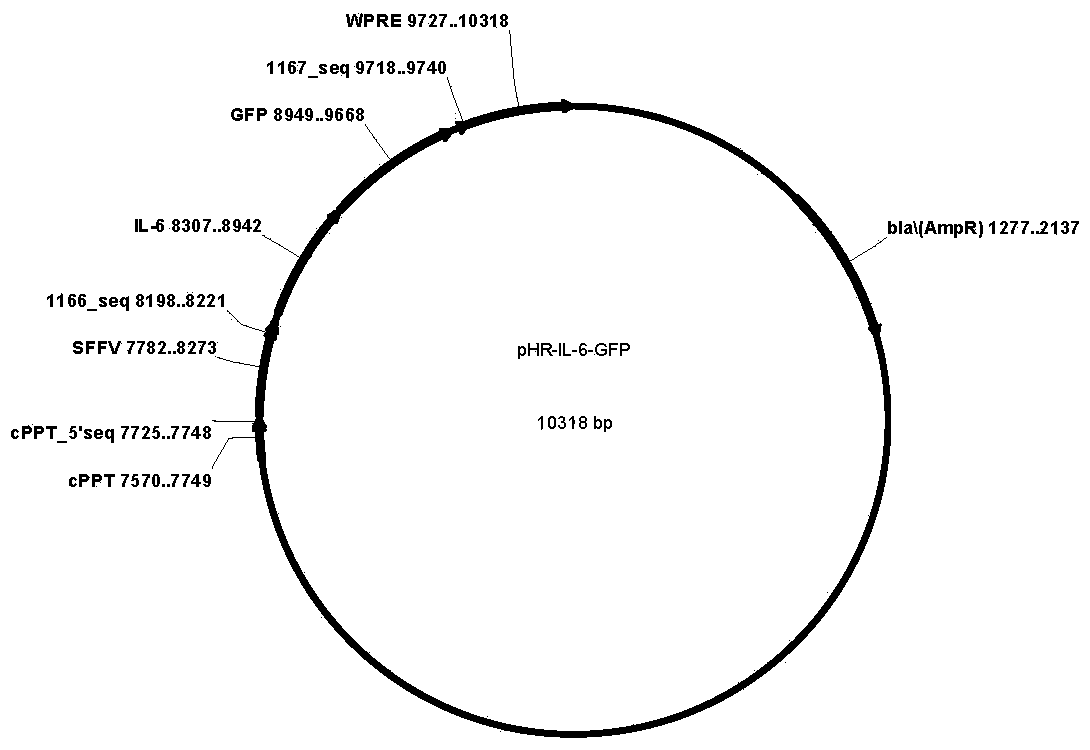
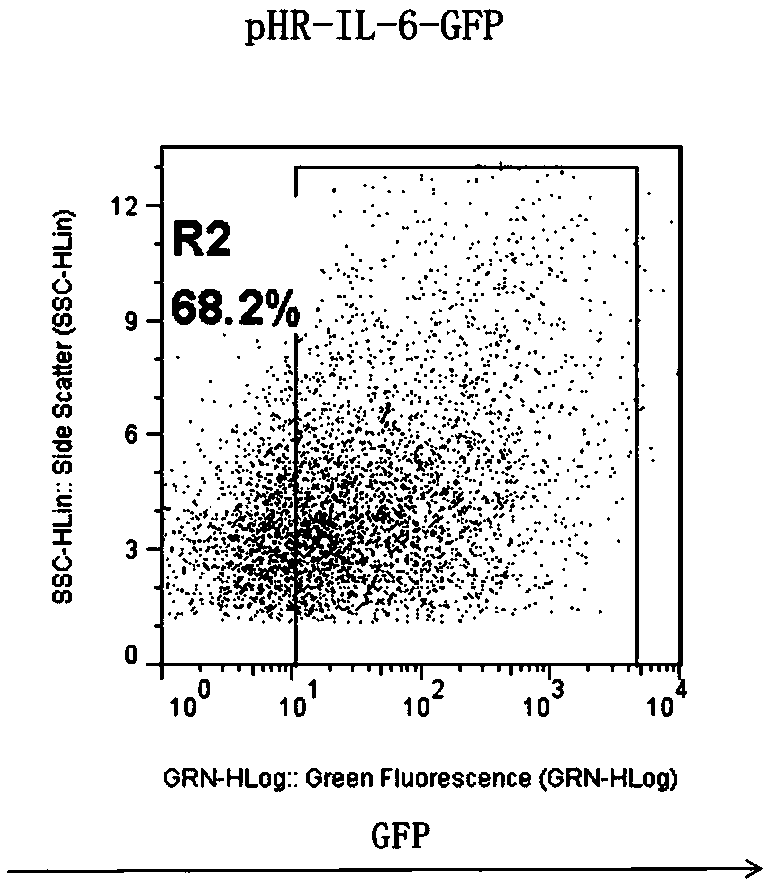
CD19-CAR-T (Chimeric Antigen Receptor T) cell interfering with IL (interleukin)-6 expression and application thereof
ActiveCN108753774AEffective silenceGenetically modified cellsMammal material medical ingredientsAdjuvantNucleotide
The invention discloses two shRNAs (short hairpin RNA) interfering with human IL (interleukin)-6 gene expression shown as SEQ ID NO: 1 and SEQ ID NO: 2. The invention further discloses a lentiviral expression vector. The lentiviral expression vector contains the shRNAs interfering with the human IL-6 gene expression and a chimeric antigen receptor encoding nucleotide fragment shown as SEQ ID NO: 4. The invention further discloses a CD19-CAR-T cell interfering with the human IL-6 gene expression. The CD19-CAR-T cell is a T lymphocyte containing the lentiviral expression vector, or a T lymphocyte which is integrated with the shRNAs interfering with the human IL-6 gene expression and the chimeric antigen receptor encoding nucleotide fragment in a chromosome. The IL-6 shRNAs are introduced into the CAR-T cell, the shRNAs of IL-6 are coexpressed while CAR is expressed, and release of the IL-6 is inhibited from the source by a gene silencing technology, so that influence of CRS (cytokine release syndrome) is reduced and the safety of CAR-T treatment is improved. Important application to preparation of medicines for prevention, treatment and adjuvant treatment of malignant tumors can be expected.
Owner:山东省齐鲁细胞治疗工程技术有限公司
In-vitro cytokine storm model and construction method and application thereof
ActiveCN114032208AReduce cell viabilityIncrease apoptosis rateCompound screeningApoptosis detectionCell activityVascular endothelium
The invention belongs to the technical field of biology, and particularly relates to an in-vitro cytokine storm model and a construction method and application thereof. The construction system of the in-vitro cytokine storm model contains a TNF-alpha factor, an IL-1[beta] factor, an IL-6 factor and vascular endothelial cells. The in-vitro cytokine storm model is formed by the body. According to the in-vitro cytokine storm model construction method, vascular endothelial cells, TNF-alpha factors, IL-1[beta] factors and IL-6 factors are incubated together. The invention discloses application of an in-vitro cytokine storm model in screening of anti-cytokine release syndrome drugs. The invention relates to an application of an in-vitro cytokine storm model in preparing and screening anti-cytokine release syndrome drugs. The in-vitro cytokine storm model disclosed by the invention is obtained by jointly treating vascular endothelial cells through three factors, namely TNF-alpha, IL-1[beta] and IL-6, the in-vitro cytokine storm model with high cell apoptosis rate and obviously reduced cell activity can be prepared, and a better basis is provided for research on inflammatory response related to multiple factors.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Novel target in the treatment of cytokine release syndrome
A novel therapeutic and diagnostic target in the treatment of Cytokine Release Syndrome is provided. More specifically, a novel target is disclosed in the treatment of CRS occurring with diseases such as bacterial and viral infection. as well as adverse reaction in drug therapy.
Owner:CELLACT PHARMA
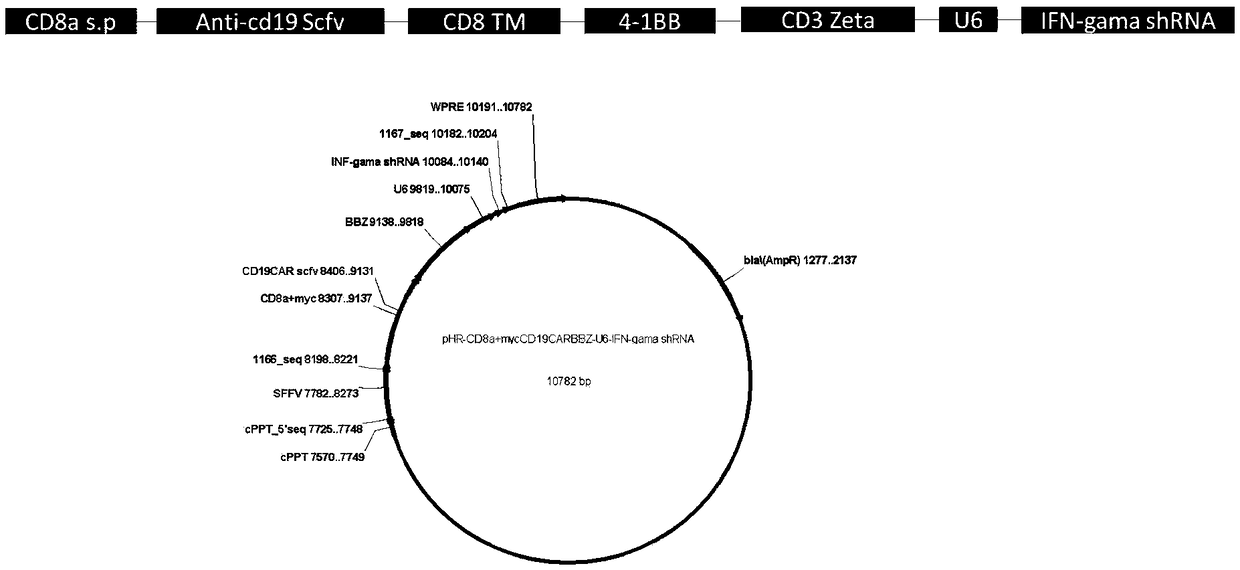
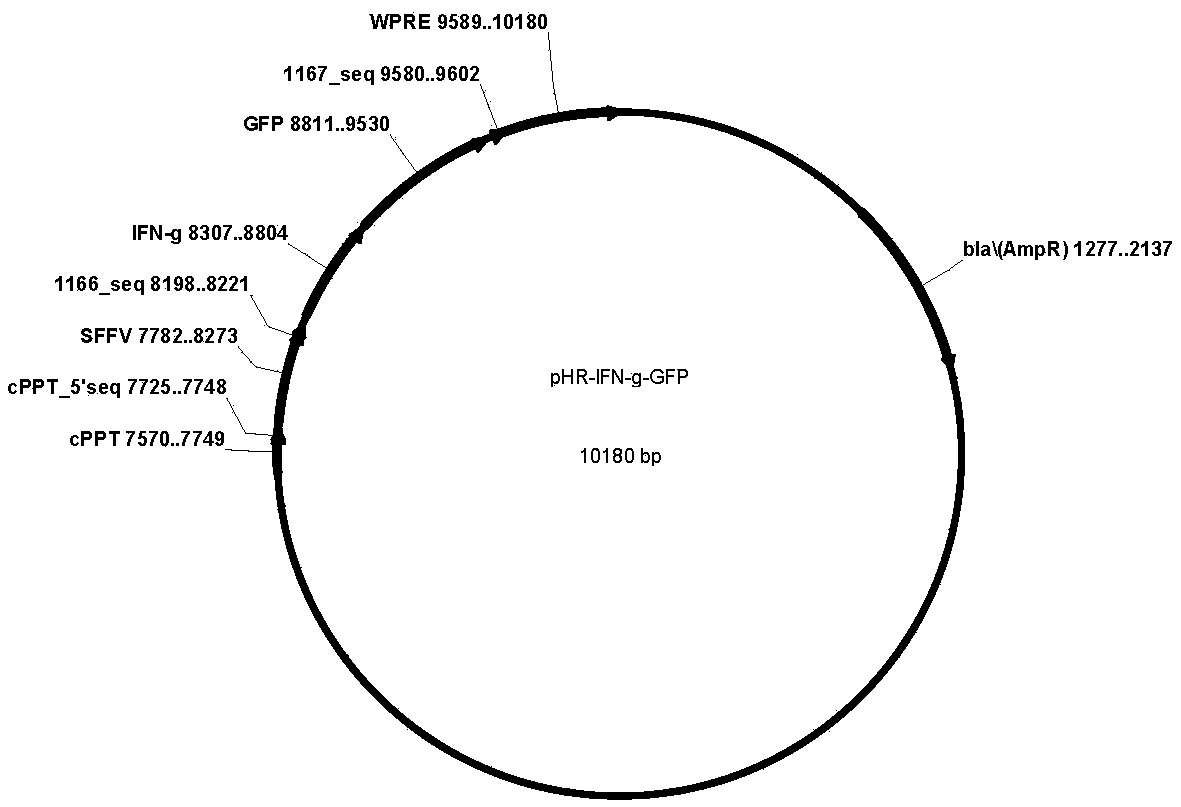
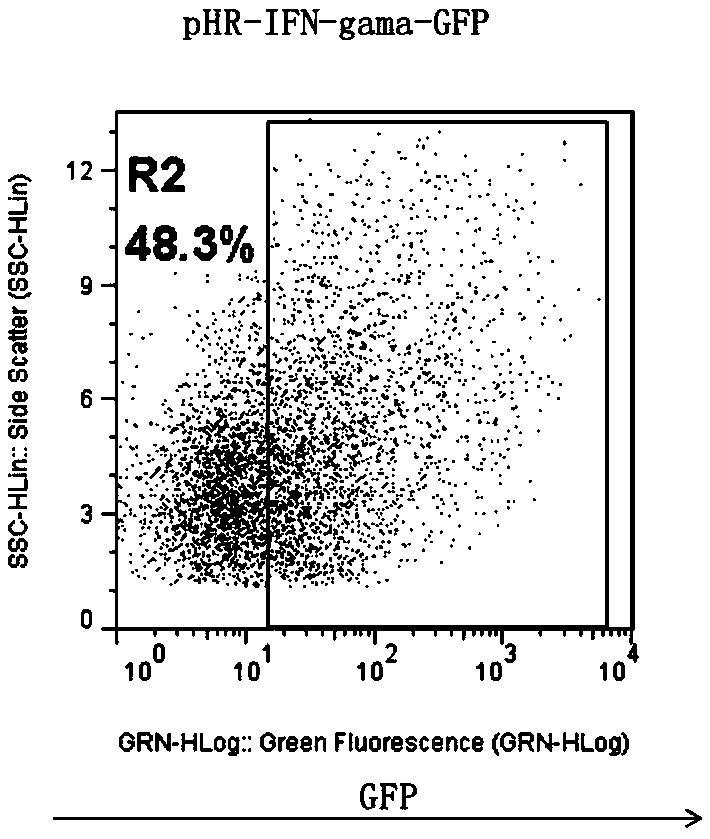
CD19-CAR-T (Chimeric Antigen Receptor T) cell interfering with IFN (interferon)-gama expression and application thereof
ActiveCN108753773AEffective silenceGenetically modified cellsMammal material medical ingredientsAdjuvantNucleotide
The invention discloses two shRNAs (short hairpin RNA) interfering with human IFN (interferon)-gama gene expression shown as SEQ ID NO: 1 and SEQ ID NO: 2. The invention further discloses a lentiviralexpression vector. The lentiviral expression vector contains the shRNAs interfering with the human IFN-gama gene expression and a chimeric antigen receptor encoding nucleotide fragment shown as SEQ ID NO: 4. The invention further discloses a CD19-CAR-T cell interfering with the human IFN-gama gene expression. The CD19-CAR-T cell is a T lymphocyte containing the lentiviral expression vector, or aT lymphocyte which is integrated with the shRNAs interfering with the human IFN-gama gene expression and the chimeric antigen receptor encoding nucleotide fragment in a chromosome. The IFN-gama shRNAsare introduced into the CAR-T cell, the shRNAs of IFN-gama are coexpressed while CAR is expressed, and release of the IFN-gama is inhibited from the source by a gene silencing technology, so that influence of CRS (cytokine release syndrome) is reduced and the safety of CAR-T treatment is improved. Important application to preparation of medicines for prevention, treatment and adjuvant treatment of malignant tumors can be expected.
Owner:山东省齐鲁细胞治疗工程技术有限公司
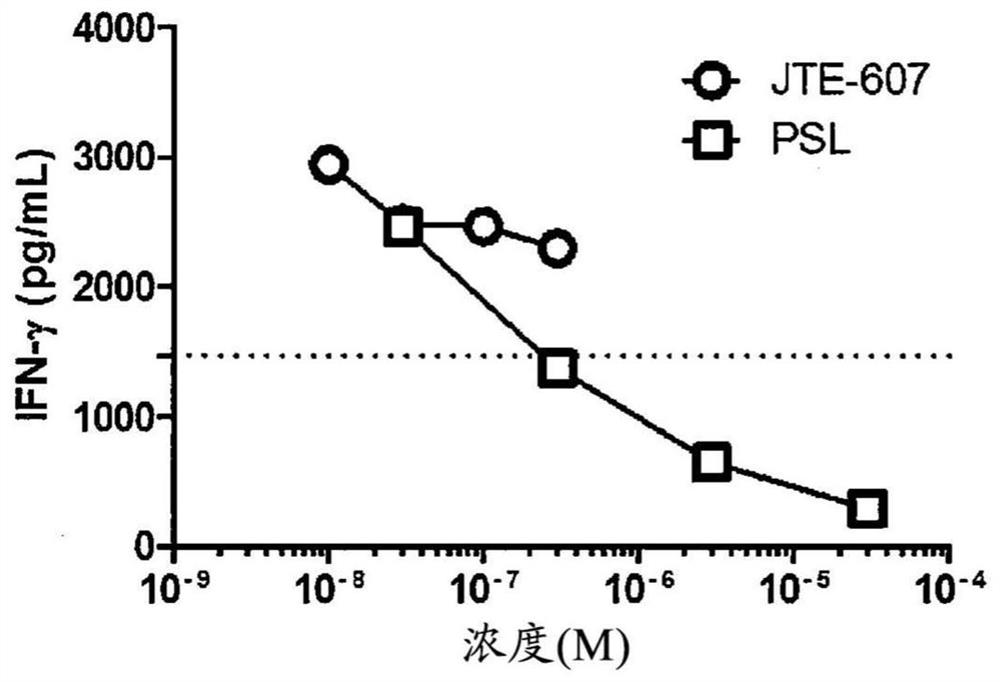
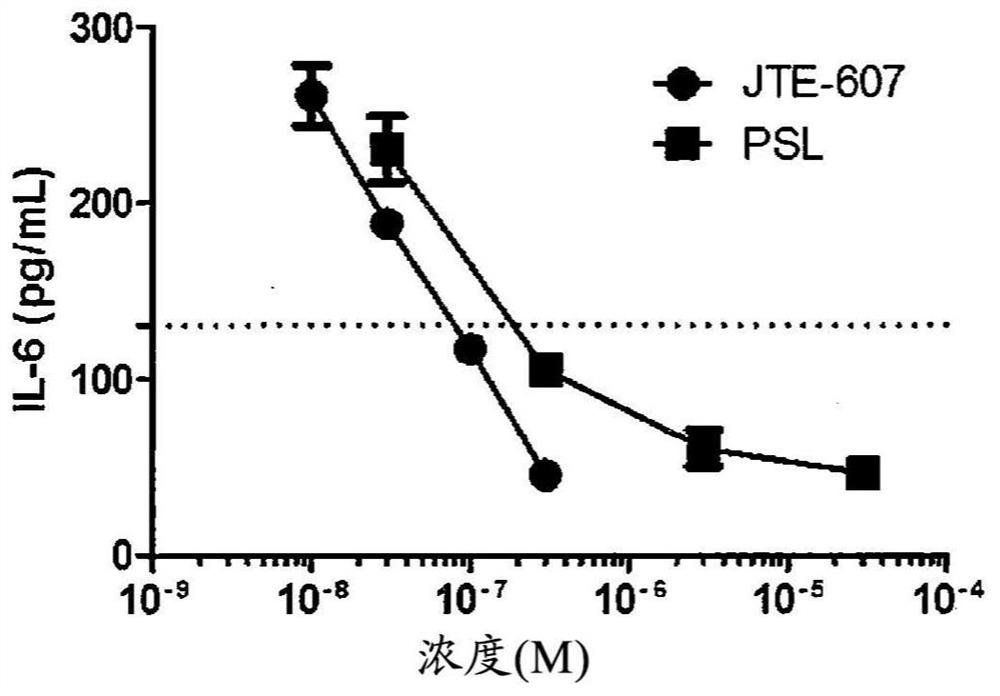
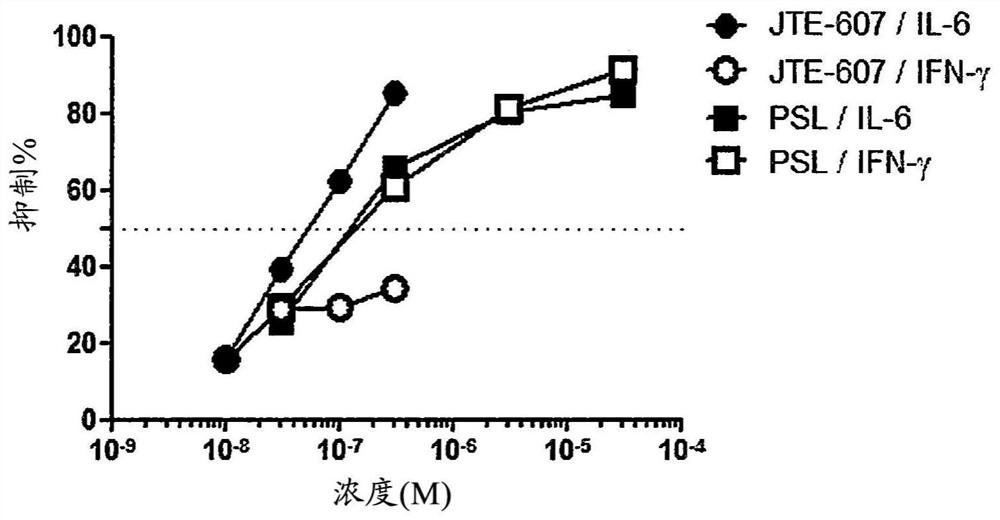
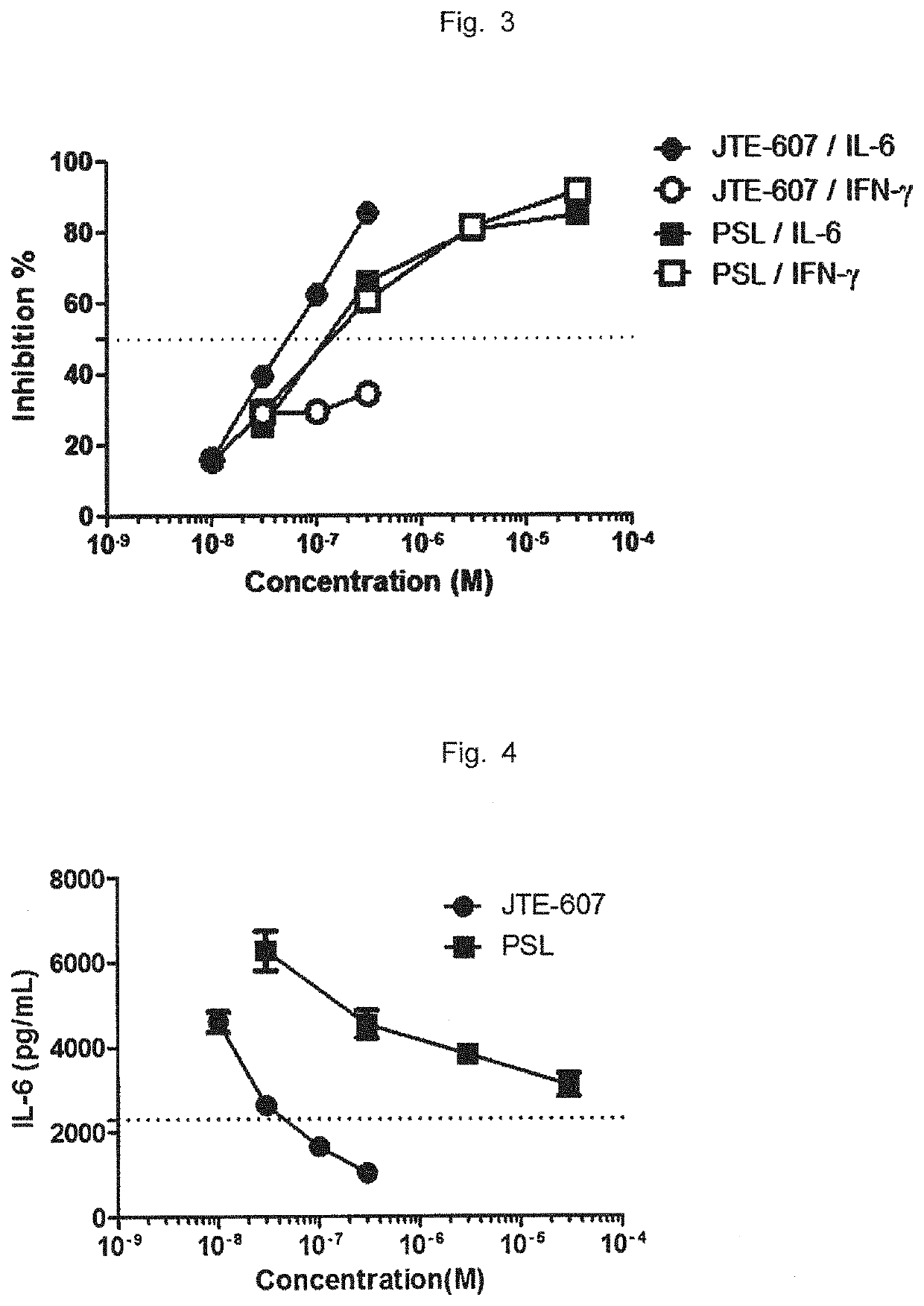
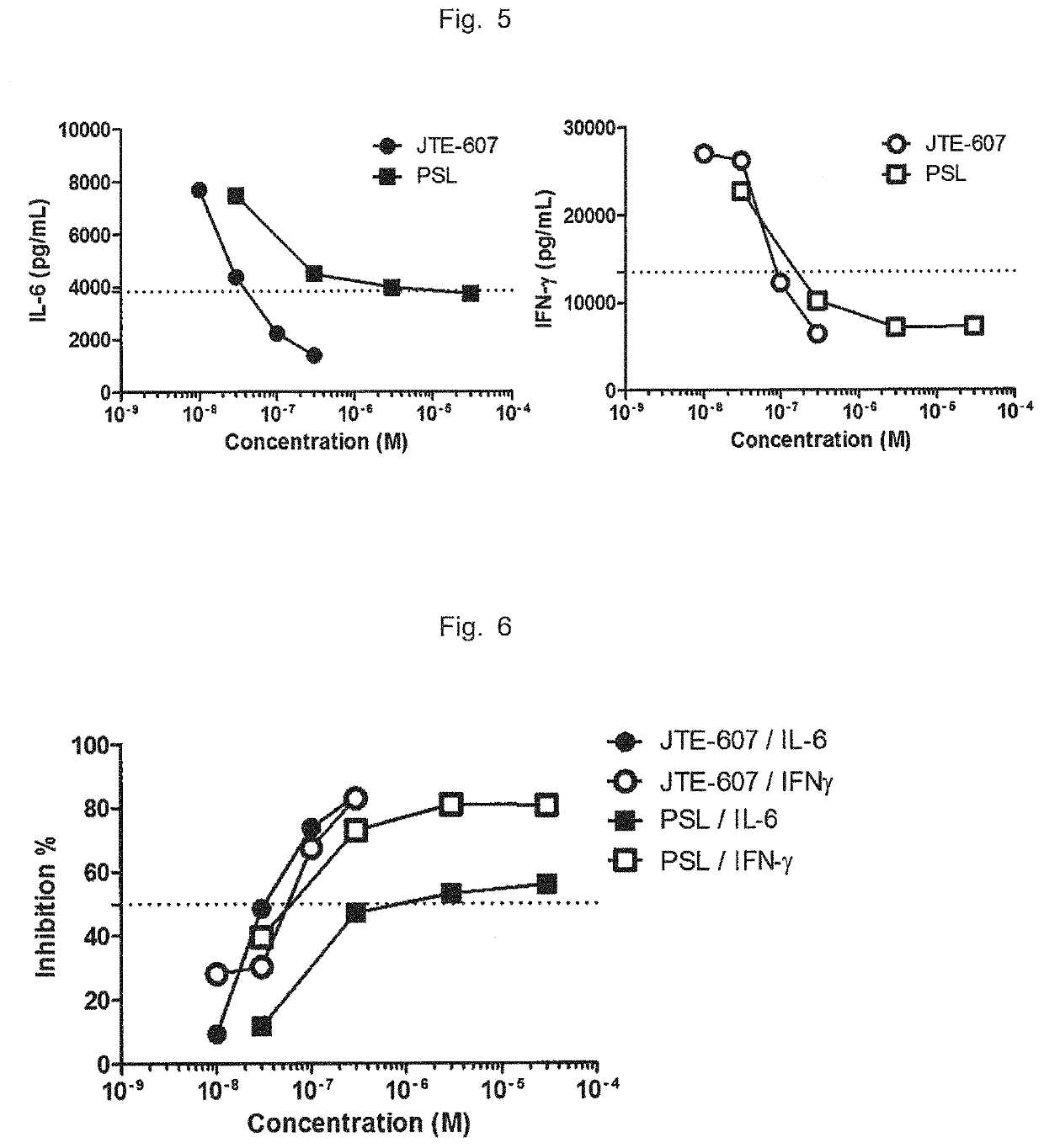
Agent for improving cytokine release syndrome, etc.
PendingCN112105362AInhibition releasePossibility of enhanced effectOrganic active ingredientsAntipyreticLangerhan cellMacrophage activation syndrome
To provide a drug for cytokine release syndrome, autoimmune-disease-related side effects, macrophage activation syndrome, hemophagocytic lymphohistiocytosis, or Langerhans cell histiocytosis. A drug for at least one selected from the group consisting of cytokine release syndrome, autoimmune-disease-related side effects, macrophage activation syndrome, hemophagocytic lymphohistiocytosis, and Langerhans cell histiocytosis, the drug containing a compound represented by formula I or a pharmacologically acceptable salt thereof.
Owner:TORII PHARMA
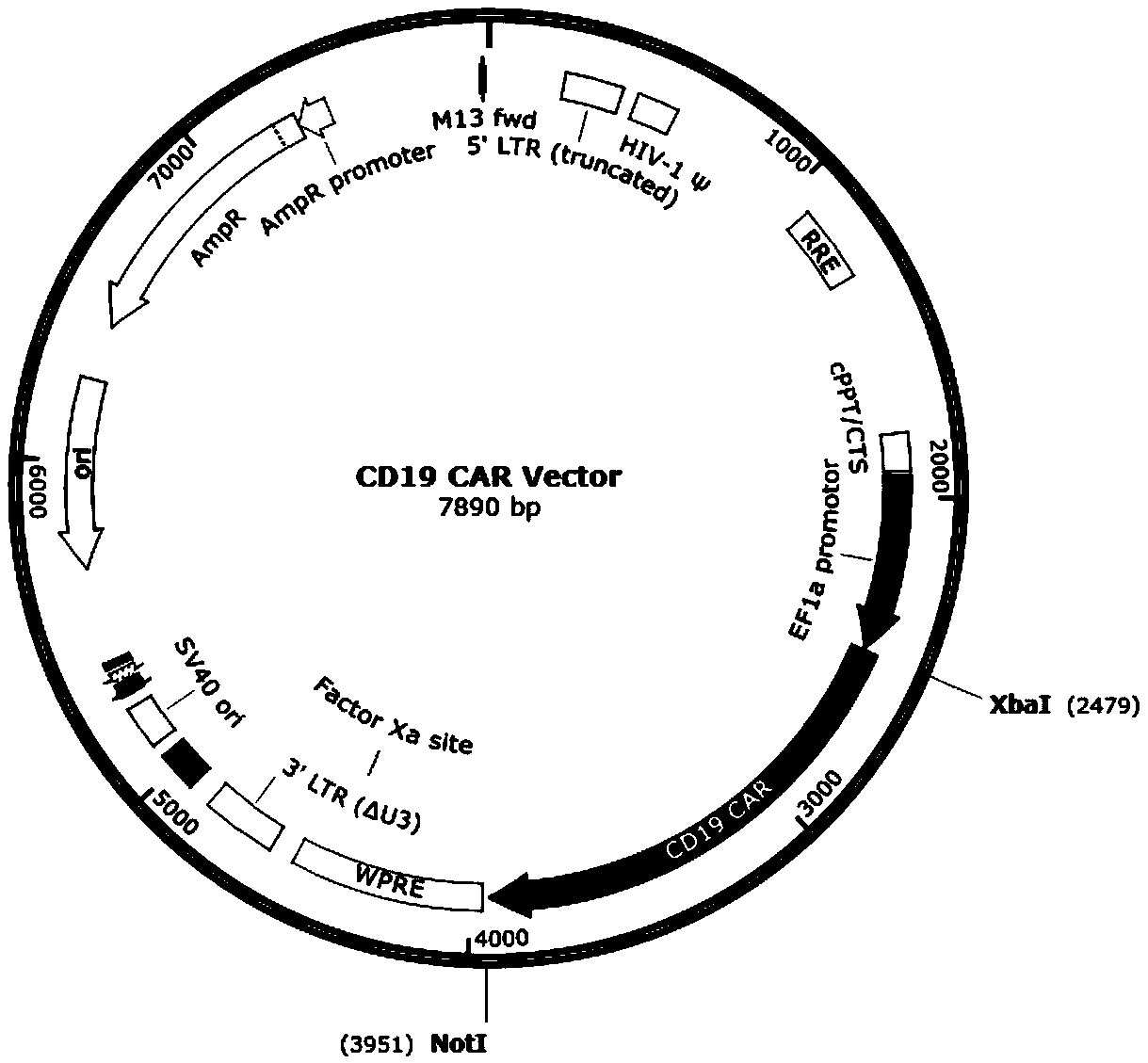
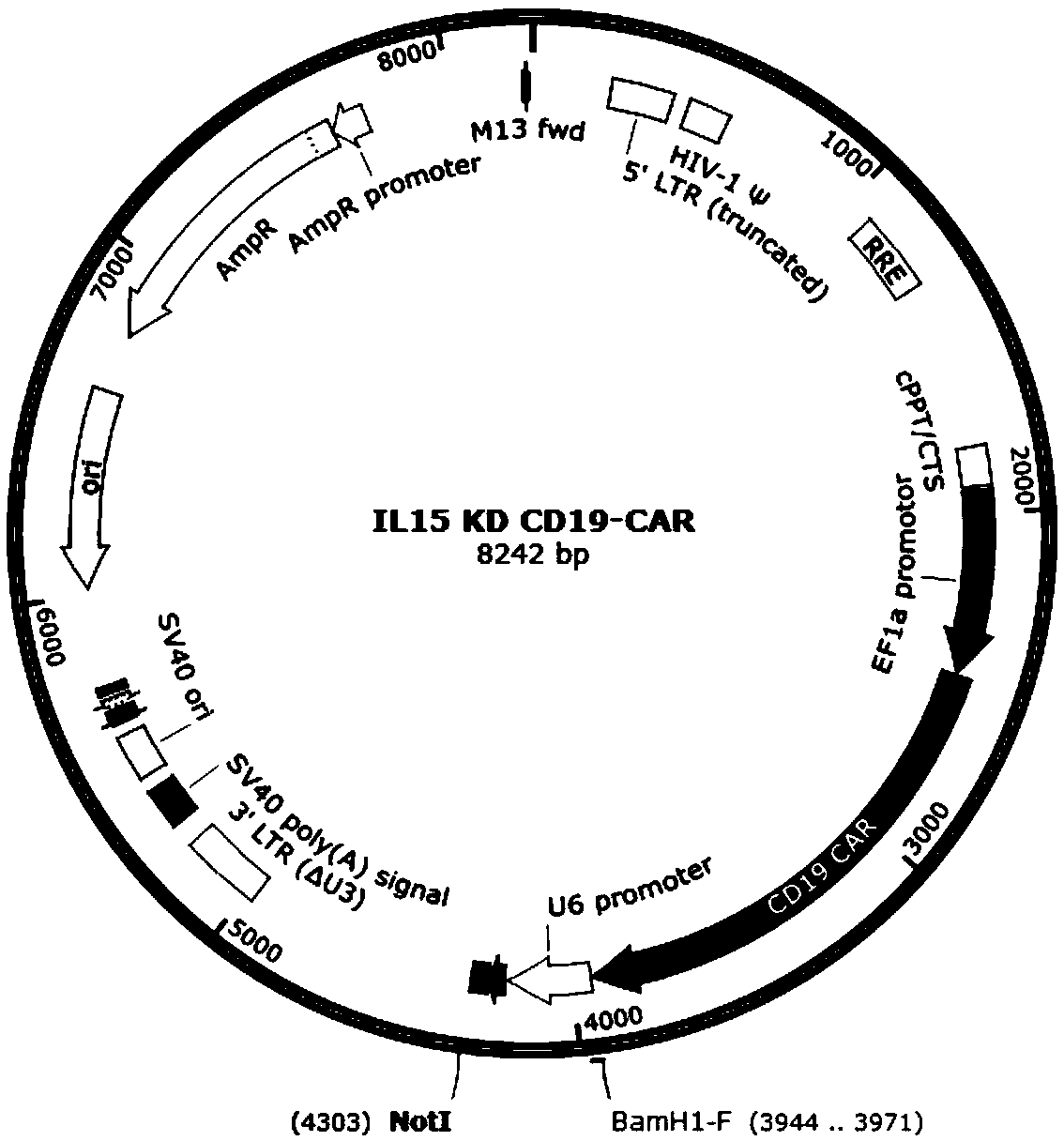
SiRNA for knocking down human IL-15, CD19 CAR expression vector, CAR-T cell and construction method and application of siRNA
ActiveCN108949759AAchieving a knockdown effectReduce secretionAntibody mimetics/scaffoldsNucleic acid vectorVirus typeT cell
The invention discloses siRNA for knocking down human IL-15, a CD19 CAR expression vector, a CD19 CAR-T cell and application of siRNA based on the problem that IL-15 released by the CD19 CAR-T cell through self-activating proliferation cannot be controlled by a method, relating to the technical field of tumor immunotherapy. The invention discloses siRNA for knocking down human IL-15, a lentiviralexpression vector of siRNA and the CD19 CAR-T cell for knocking down IL-15. The siRNA has the beneficial effects that the knock-down effect of IL-15 in the CD19 CAR-T cell is realized, and the secretion volume of IL-15 is greatly reduced, so that the risks of cell factor release syndrome and encephaledema are reduced.
Owner:苏州一兮生物技术有限公司
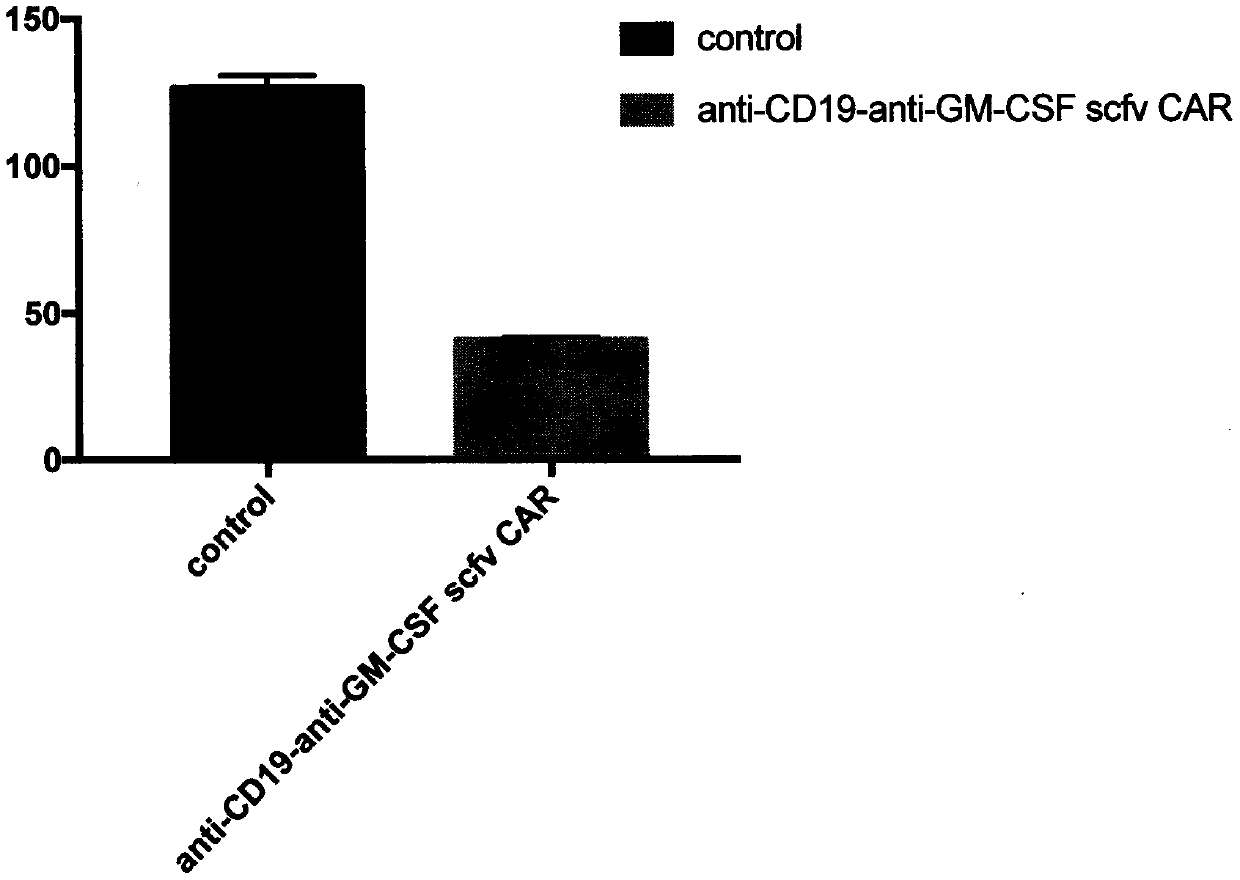
Construction and application of chimeric antigen receptor T cell having GM-CSF knockdown and secreting single chain antibody for neutralizing GM-CSF
ActiveCN110760001AImprove securityAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsSingle-Chain AntibodiesNucleotide
The invention discloses construction and application of a fourth-generation chimeric antigen receptor (CAR)-T cell secreting anti-GM-CSF scFv for neutralizing GM-CSF and having GM-CSF gene knockdown.The integral safety of CAR-T therapy can be improved by secreting anti-GM-CSF scFv neutralizing the GM-CSF, combining the GM-CSF gene knockdown in the CAR-T cell, preventing or retarding occurrence and development of the cytokine release syndrome and the neurotoxicity. Furthermore, a nucleotide sequence encoding microRNA is inserted into an EF1 alpha promoter, and the package titer of a chronic virus carrier is not influenced.
Owner:浙江启新生物技术有限公司
Powdered formulations of cromolyn sodium and alpha-lactose
Owner:THE GENERAL HOSPITAL CORP +1
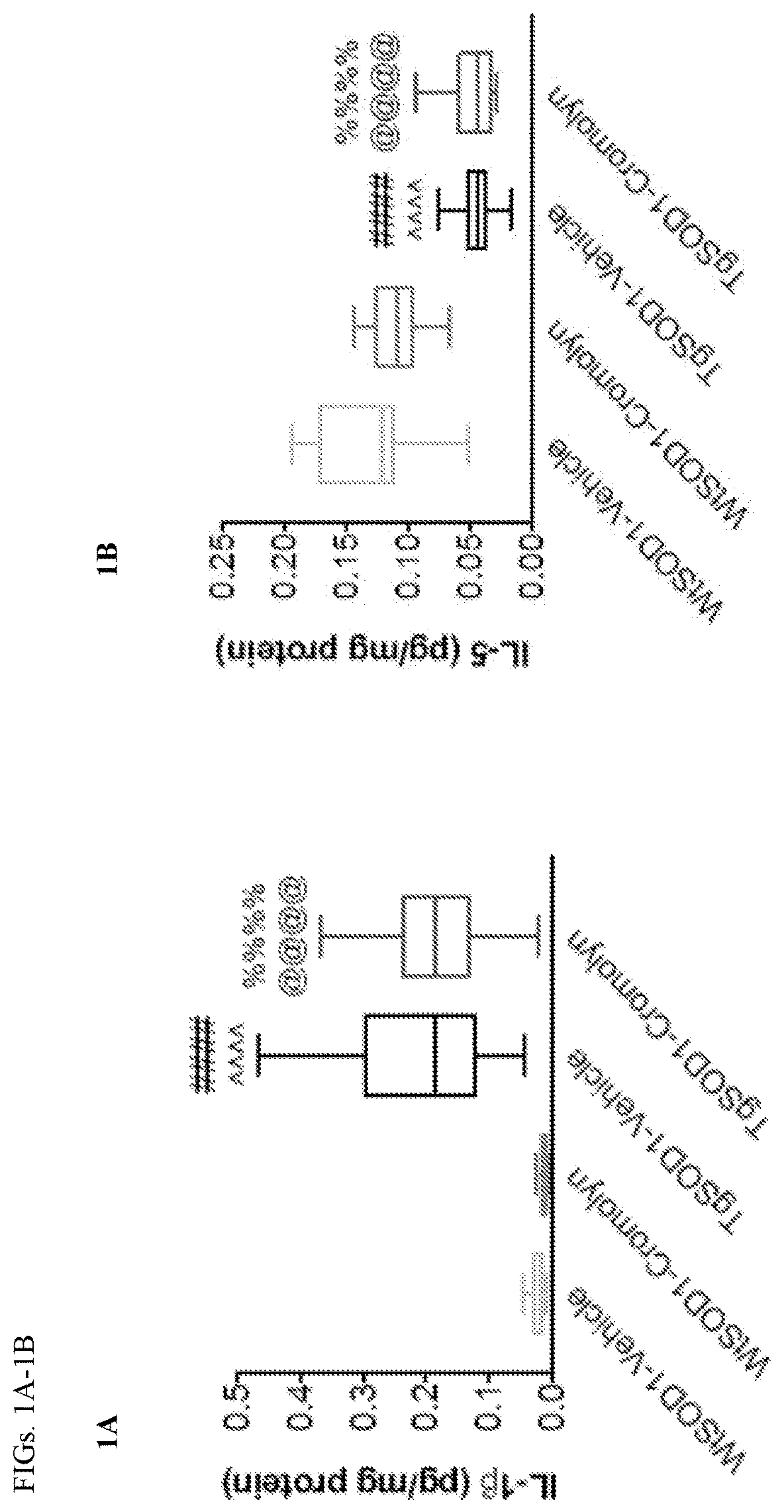
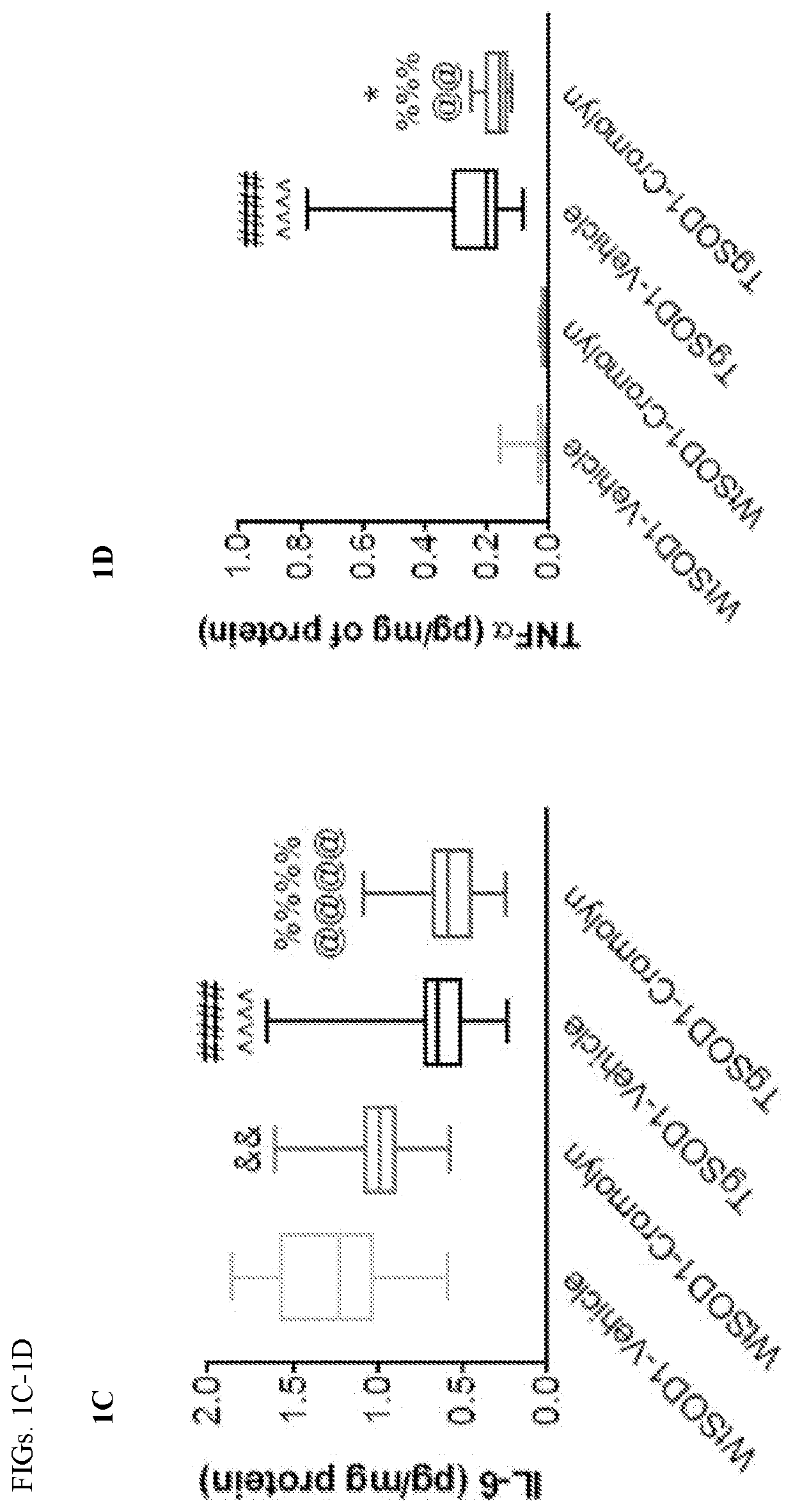
Methods and compositions for alleviating cytokine release syndrome
PendingUS20200268793A1Reduce the burden onImprove survivalPeptide/protein ingredientsNGF/TNF-superfamilyAntigen receptorsCytokine
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
A group of markers and applications and kits for predicting cytokines and thrombosis storm of 2019 coronavirus disease
The invention provides a group of markers for predicting cytokine release syndrome and thrombosis of 2019 coronavirus disease and its application, kit and preparation method of the kit, belonging to the technical field of biomedicine. The markers of the present invention are VEGF-D, TNF-α, SCF, LIF, IL-2, IL-4, IL-6, IL-8, IL-10, IL-15, IL-17A, IL-17A, IL-8, respectively 18. IL-1β and IFN-γ. VEGF-D, TNF-α, SCF, LIF, IL-2, IL-4, IL-6, IL-8, IL-10, IL-15, IL-17A, IL-18, IL-18 provided by the present invention A group of ‑1β, IFN‑γ markers or the prepared kits can be used to predict the cytokine release syndrome and thrombosis storm of coronavirus disease 2019 (COVID‑19), which has the advantages of rapid detection, accuracy, and low cost. bright future.
Owner:BEIJING IMMUPEUTICS MEDICINE TECH LTD
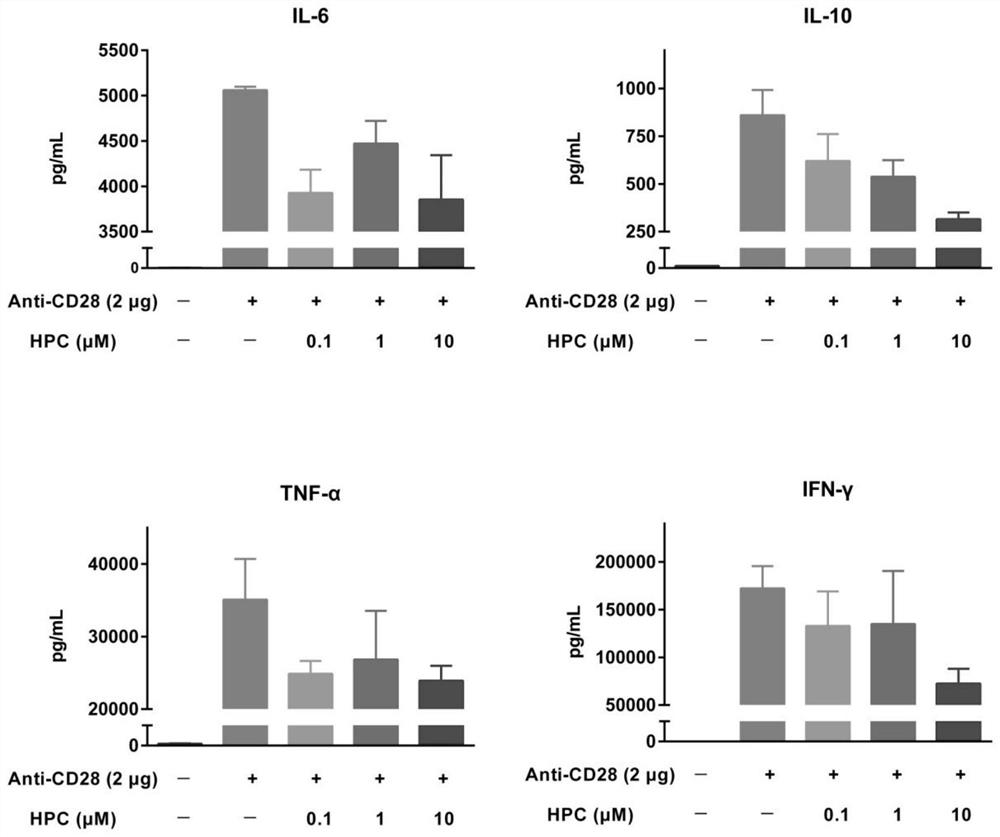
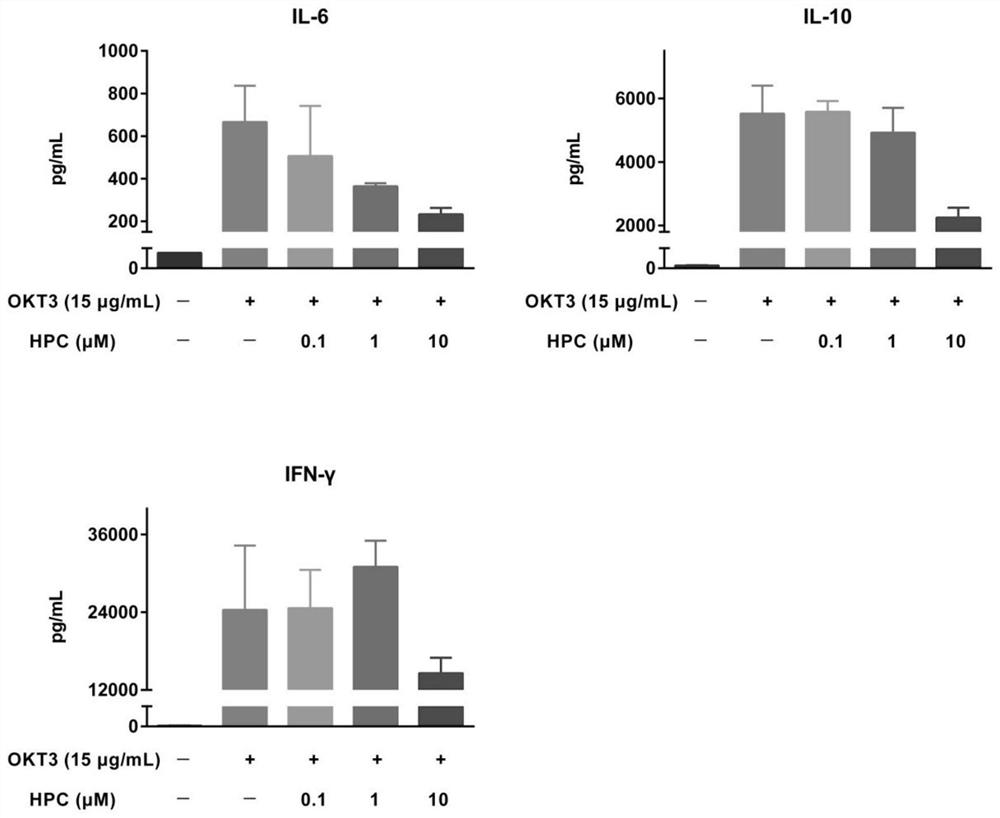
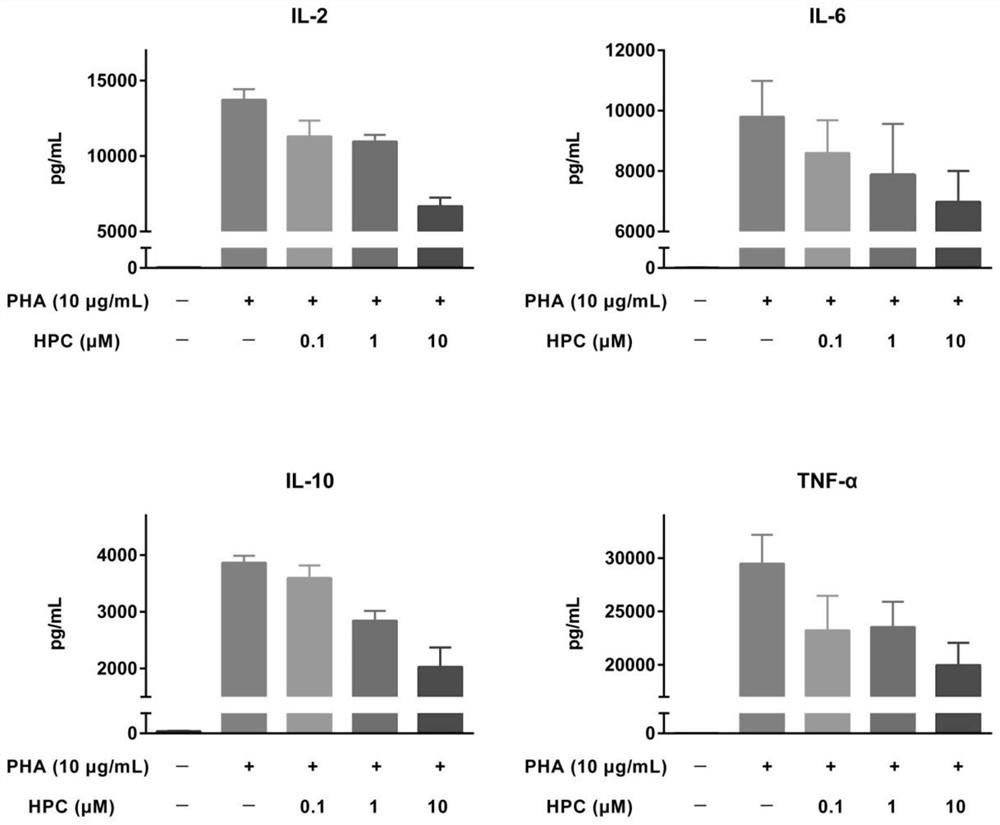
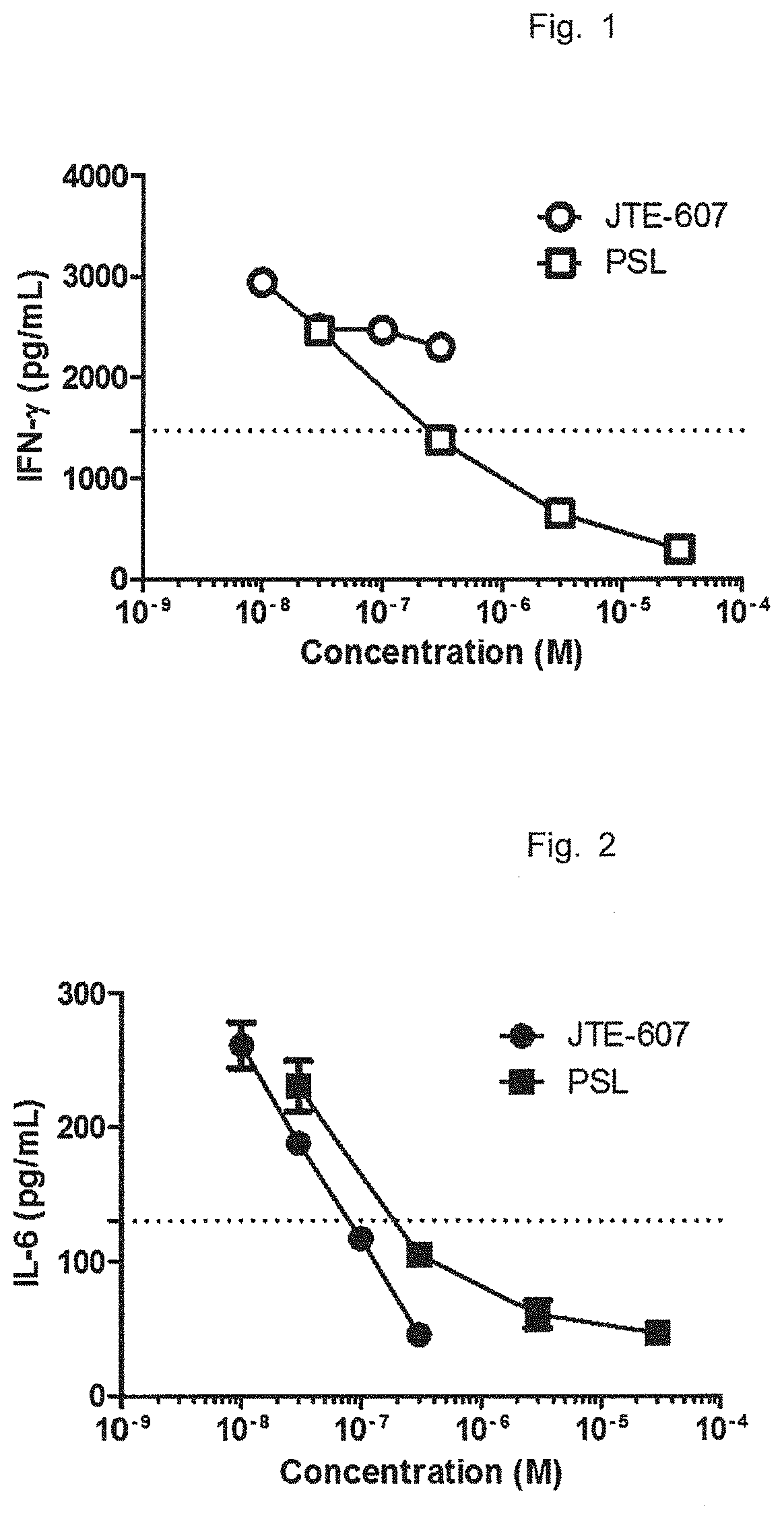
Application of progestational hormone in treatment of cytokine release syndrome
ActiveCN112656801AOrganic active ingredientsAntibody ingredientsAntiendomysial antibodiesCD3 Antibody
The invention provides an application of hydroxyprogesterone hexanoate in preparation of a drug for inhibiting cytokine storm, especially PBMC cytokine release caused by a CD28 antibody and / or a CD3 antibody. Experimental results show that hydroxyprogesterone hexanoate can inhibit release of multiple PBMC cytokines caused by the CD28 antibody and / or the CD3 antibody in a concentration-dependent manner, and is a potential drug for treating cytokine storm.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
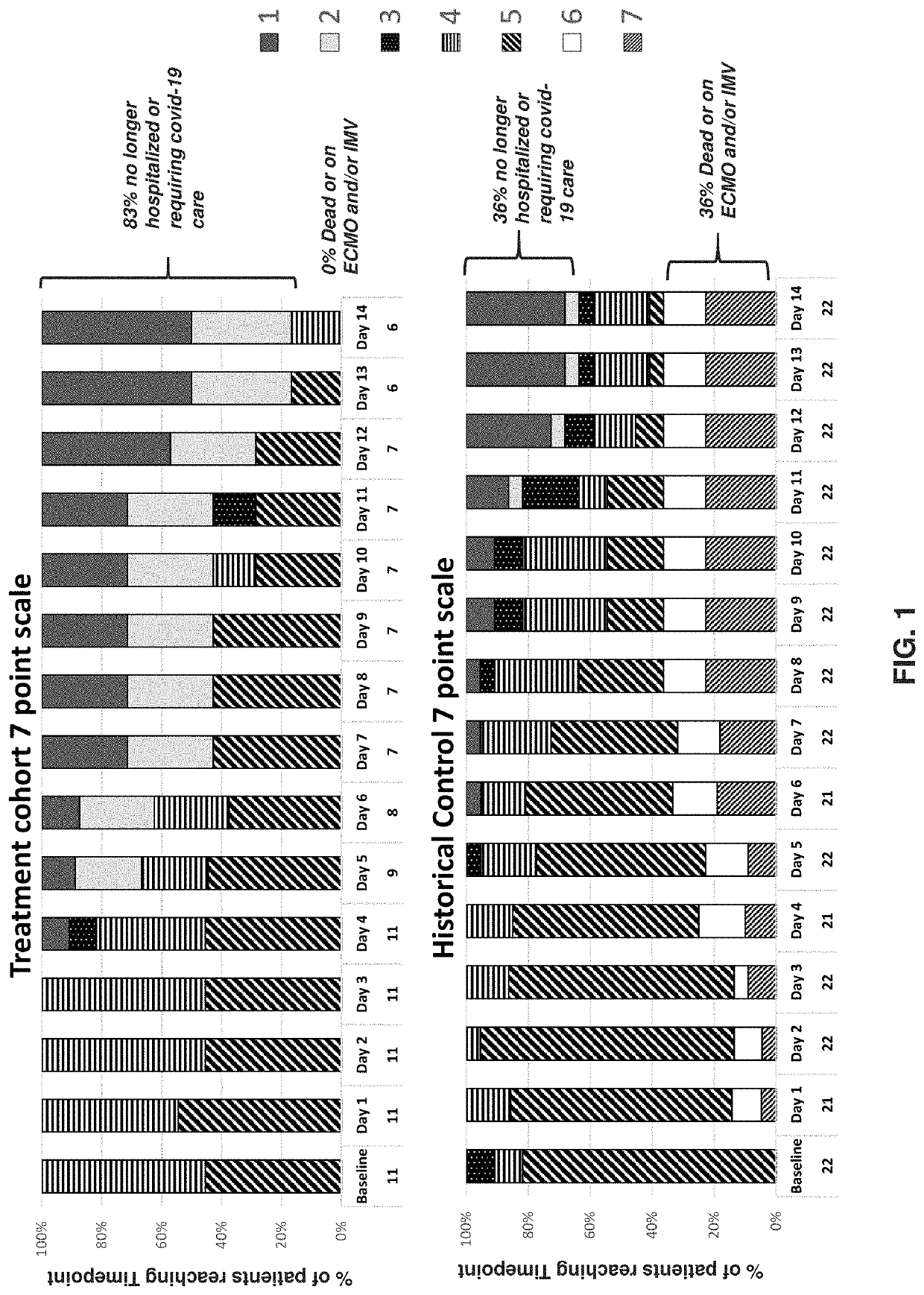
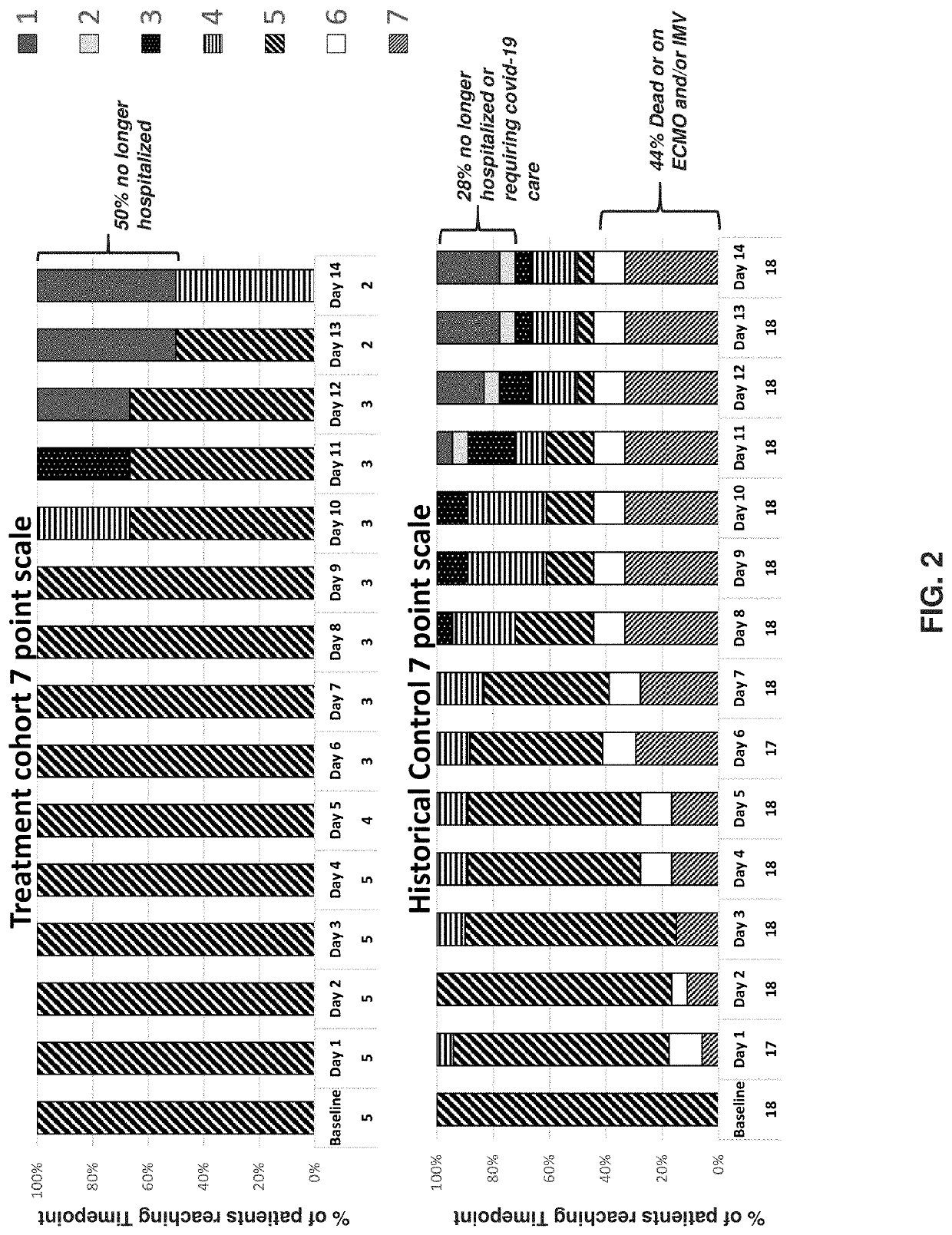
Methods of treating cytokine release syndrome
PendingUS20220218652A1Improve throughputLimit deliveryNervous disorderOrganic chemistryTumor SyndromePharmaceutical medicine
The present disclosure relates to a method of treating at least one Cell-Associated Neurotoxicity Syndrome (ICANS), cancer-related cognitive impairment, Infusion Reaction Syndrome (IRS), Capillary Leak Syndrome (CLS), Tumor Lysis Syndrome (TLS), Macrophage Activation Syndrome (MAS), Systemic Inflammatory Response Syndrome (SIRS), Immune Reconstitution Inflammatory Syndrome (IRIS), Graft-Versus-Host Disease (GVHD), Acute Respiratory Distress Syndrome (ARDS), sepsis, Ebola, avian influenza, smallpox, Systemic Inflammatory Response Syndrome (SIRS), and Immune-related Adverse Events Syndrome (IrAES) in a subject in need thereof, comprising administering a mast cell stabilizer or a compound of Formula I or Formula II: Formula I, Formula II, wherein R1 is halogen, OH, or —OC(O)C1-5alkyl R2 and R3 are each independently selected from CO2R4 or CH2OR5; R4 is i L, Na, K, H, C1-5alkyl, or —CH2CO(C1-5alkyl); and R5 is H or C(O)(C1-5alkyl), or a pharmaceutically acceptable salt thereof.
Owner:AZTHERAPIES INC +1
Uses of il-41
InactiveUS20190262425A1Antibacterial agentsPeptide/protein ingredientsAutoimmune diseaseBlood plasma
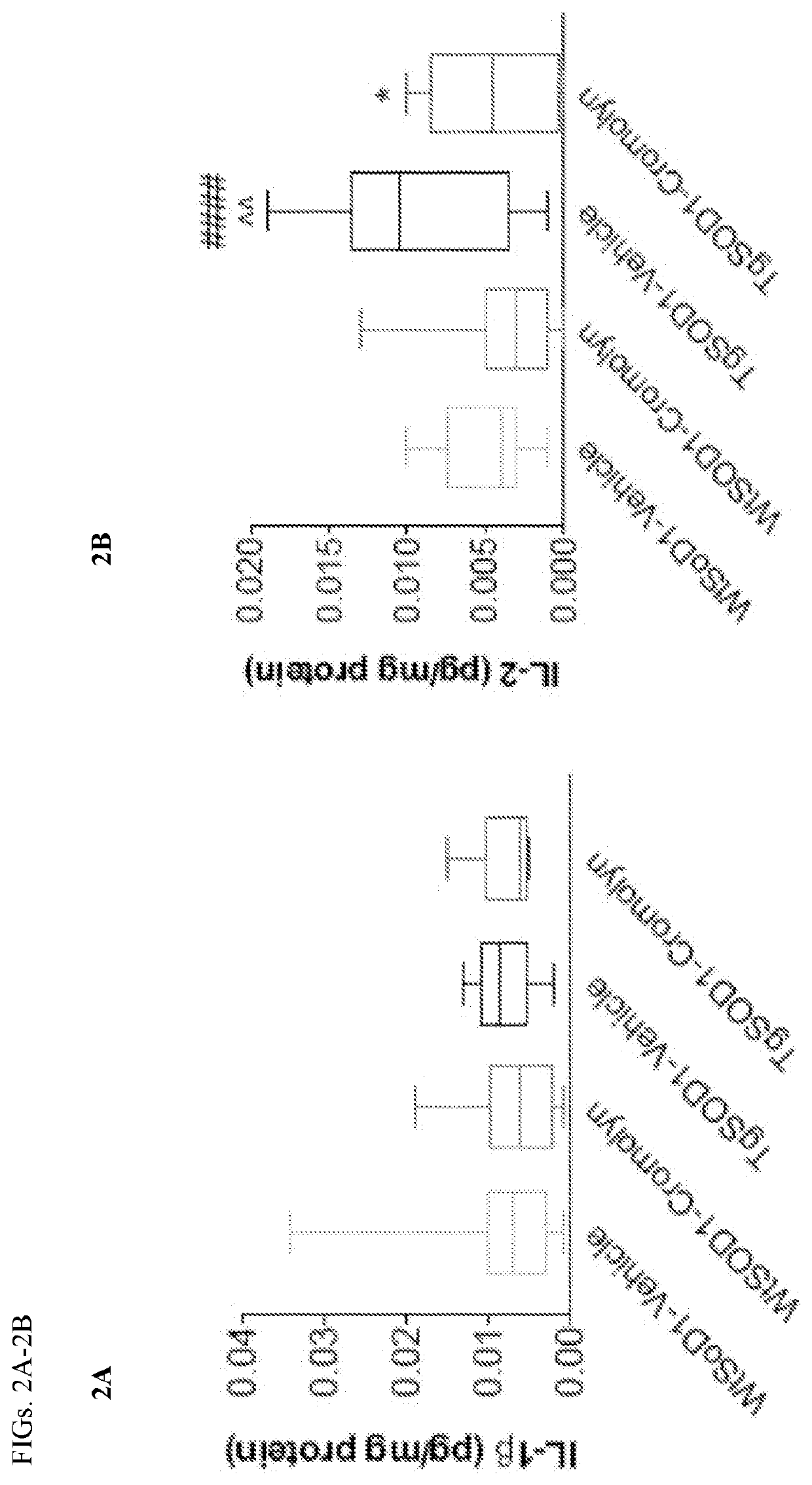
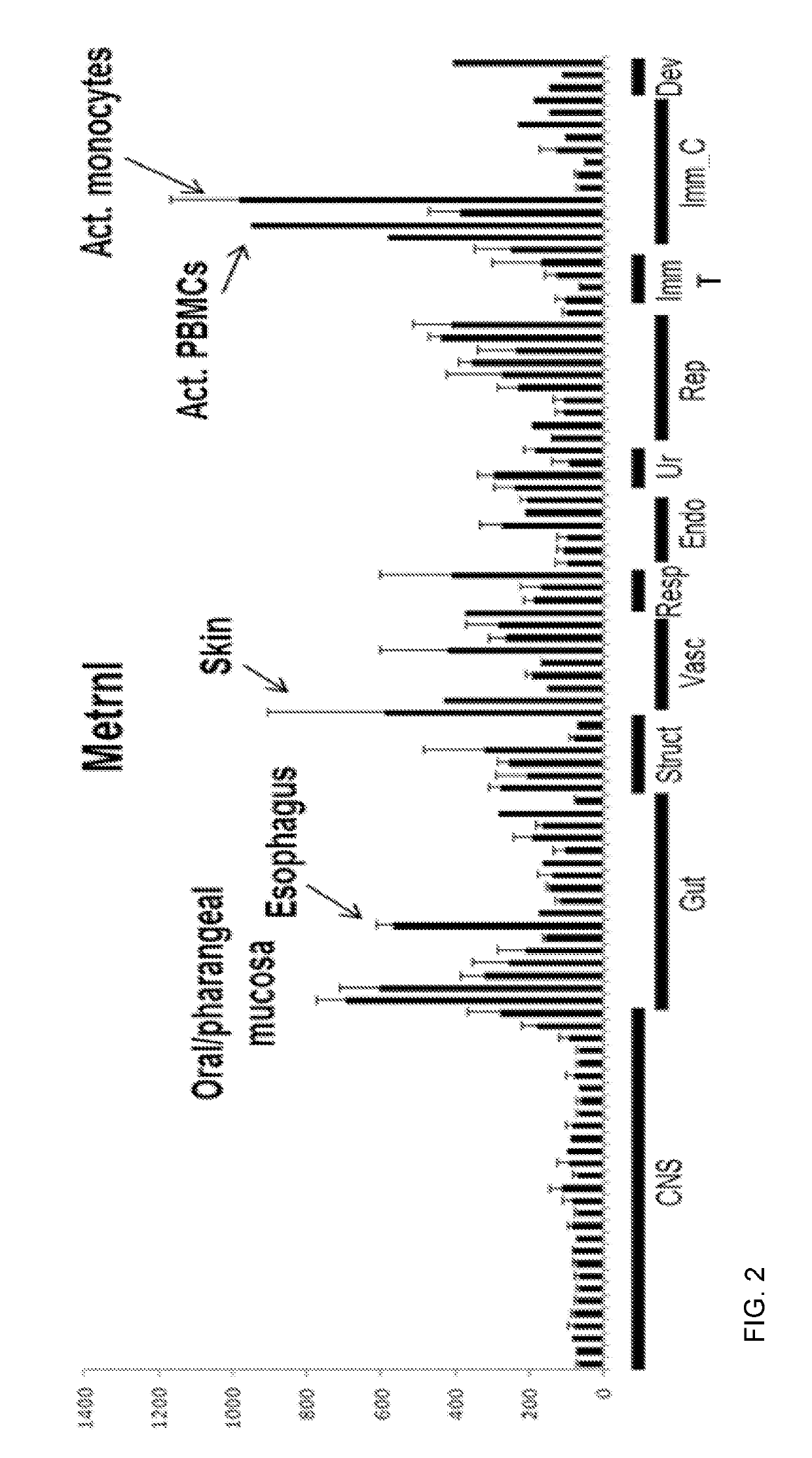
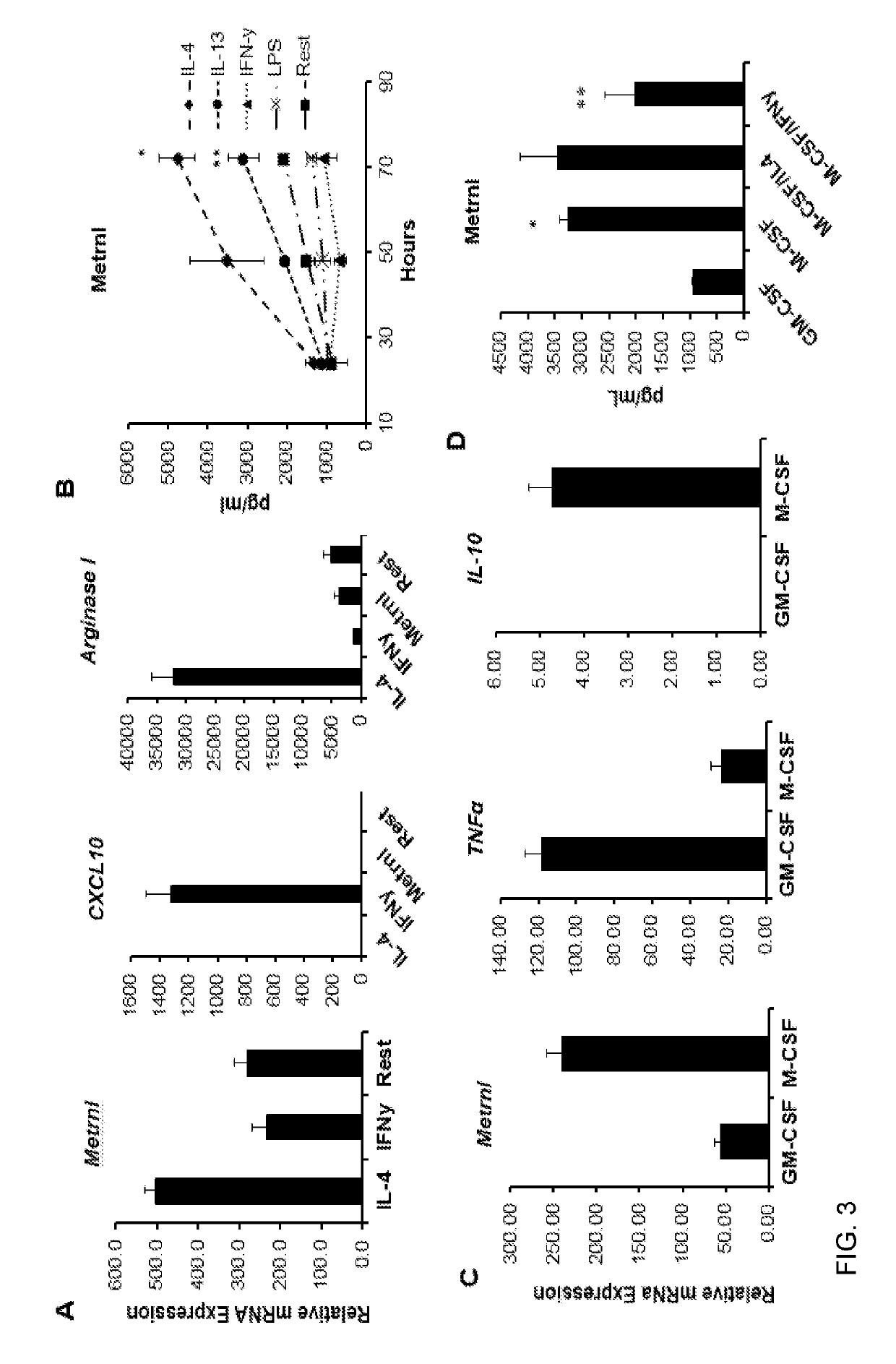
Methods of using Metrnl / IL-41 to identify inflammation, inflammatory and autoimmune diseases, infection and cancer in a subject are provided. The methods include determining the serum or plasma levels of Metrnl / IL-41 in the subject. Methods of modulating a condition selected from cytokine release syndrome, systemic immune response syndrome, cytokine storm, or a combination thereof, using Metrnl / IL-41 or antibodies against Metrnl / I-41 are also provided.
Owner:RGT UNIV OF CALIFORNIA
Ameliorative agent for cytokine release syndrome and so on
ActiveUS20210128550A1Inhibition releaseGood effectOrganic active ingredientsAntipyreticLangerhan cellMacrophage activation syndrome
[Problem to be Solved] The present invention provides a medicament for cytokine release syndrome, autoimmune-related adverse events, macrophage activation syndrome, hemophagocytic lymphohistiocytosis or Langerhans cell histiocytosis.[Means to Solve the Problem] The present invention provides a medicament for at least one selected from the group consisting of cytokine release syndrome, autoimmune-related adverse events, macrophage activation syndrome, hemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis, wherein the medicament comprises a compound represented by the following formula I:or a pharmaceutically acceptable salt thereof.
Owner:TORII PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com