Combination immune therapy and cytokine control therapy for cancer treatment
A pro-inflammatory cytokine and cell technology, applied in the fields of proliferation rate composition and efficacy composition, can solve problems such as tissue damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0554] Example 1: Apoptotic cell generation process
[0555] Purpose: To generate early apoptotic cells for use in the methods described herein.
[0556] method:
[0557] Methods for preparing early apoptotic cell populations are described in detail in WO2014 / 087408 International Publication and US2015 / 0275175-A1 US Application Publication, see, for example, the previous examples of "ApoCell Preparation" and "Apoptotic Cell Generation" The Methods section (paragraphs [0223] to [0288]), as well as Examples 11, 12, 13 and 14, are incorporated herein in their entirety.
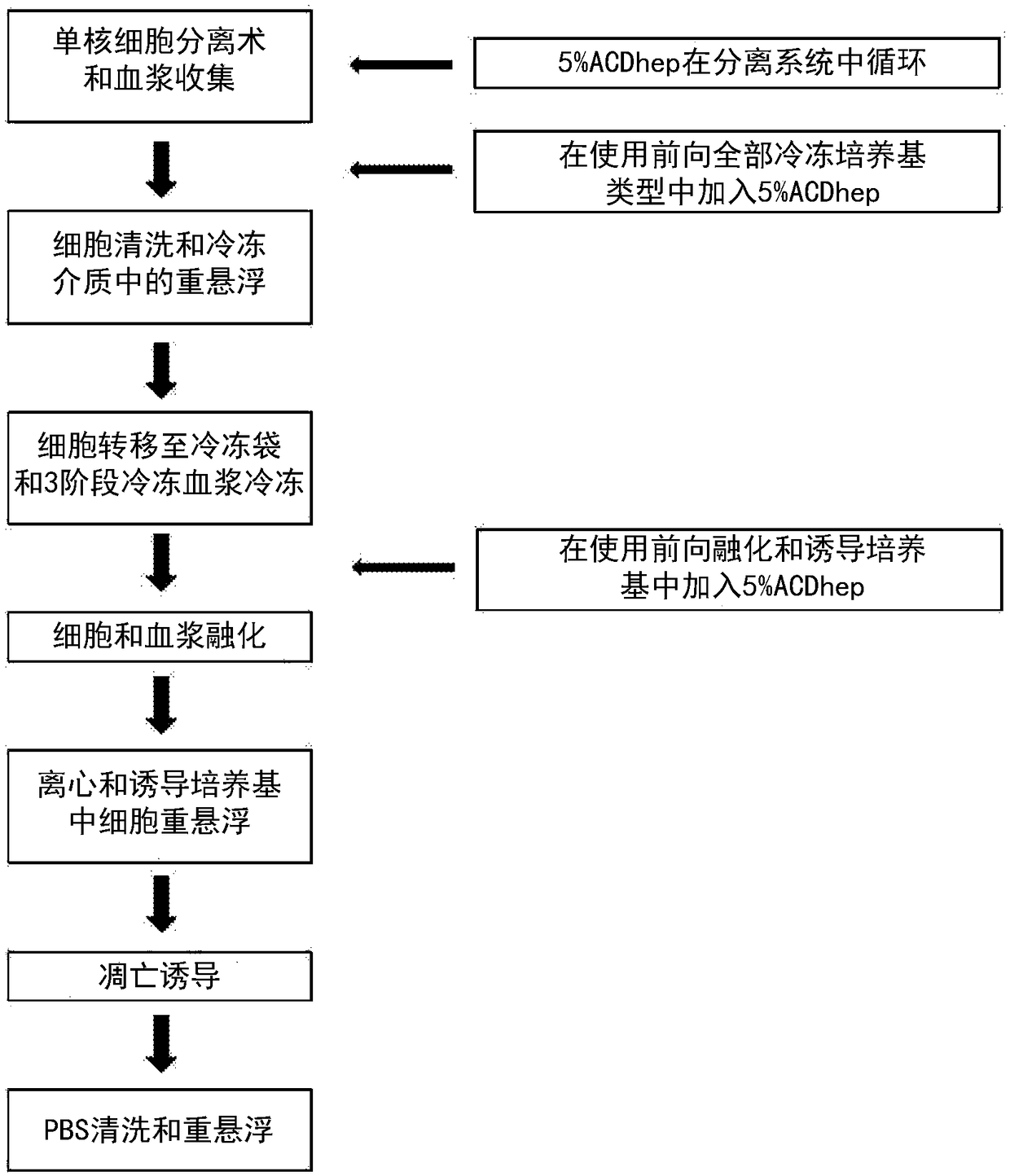
[0558] figure 1 The flow chart shown provides an overview of one embodiment of the steps used in the production of an early apoptotic cell population, where an anticoagulant is included in the preparation step. The time point of adding anticoagulant during the production process is indicated in the flowchart. As described in detail in Example 14 of International Publication No. WO2014 / 087408 and U.S. Applicat...
Embodiment 2
[0567] Example 2: Effect of apoptotic cells on cytokine release in an in vitro cytokine storm model
[0568]Objective: To detect the cytokine storm markers (cytokines IL-6, IL-10, MIP-1α, IL-8, TNF-α, MIP-1β, MCP-1 and IL-9) levels.
[0569] method:
[0570] Cell Lines and Culture Reagents
[0571] Human lymphoma cell line Raji (eCACC, UK, accession number 85011429), human cervical cancer cell line HeLa (ATCC, US, number CCL-2) and HeLa-CD19 (ProMab, US, catalog number PM-Hela-CD19) Be cultured in supplemented with 10% FBS (Gibco, Thermo Fisher Scientific Company, South America, catalog number 12657-029), 2mM GlutaMAX (Gibco, Thermo Fisher Scientific Company, USA, catalog number 35050-038) And in RPMI 1640 (Gibco, Thermo Fisher Scientific, USA, catalog number 31870-025) of 100U / ml penicillin+100U / ml streptomycin (Gibco, ThermoFisher Scientific, USA, catalog number 15140-122), This RPMI 1640 is hereinafter referred to as "complete medium". The HeLa-CD19 medium was further ...
Embodiment 3
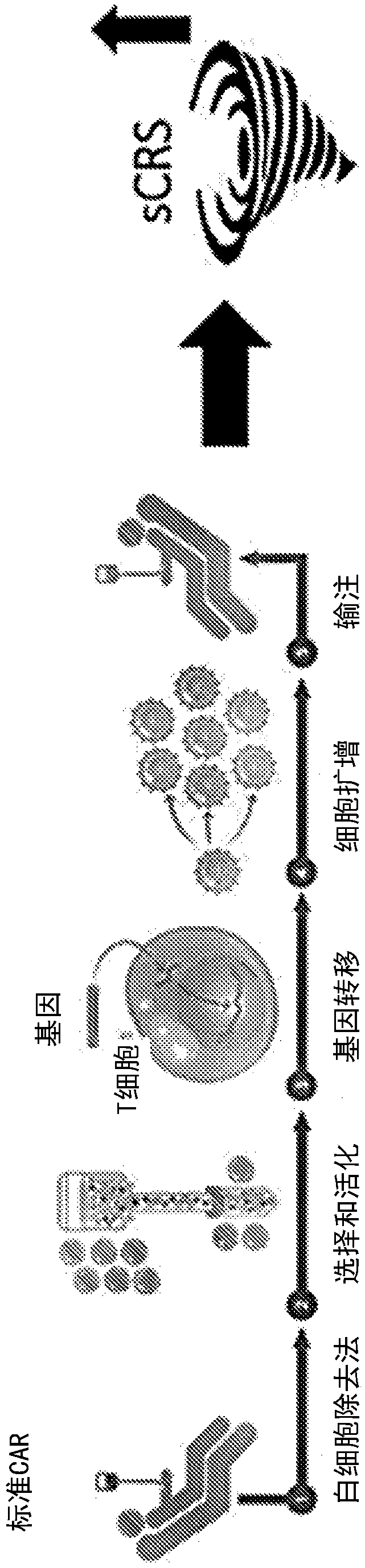
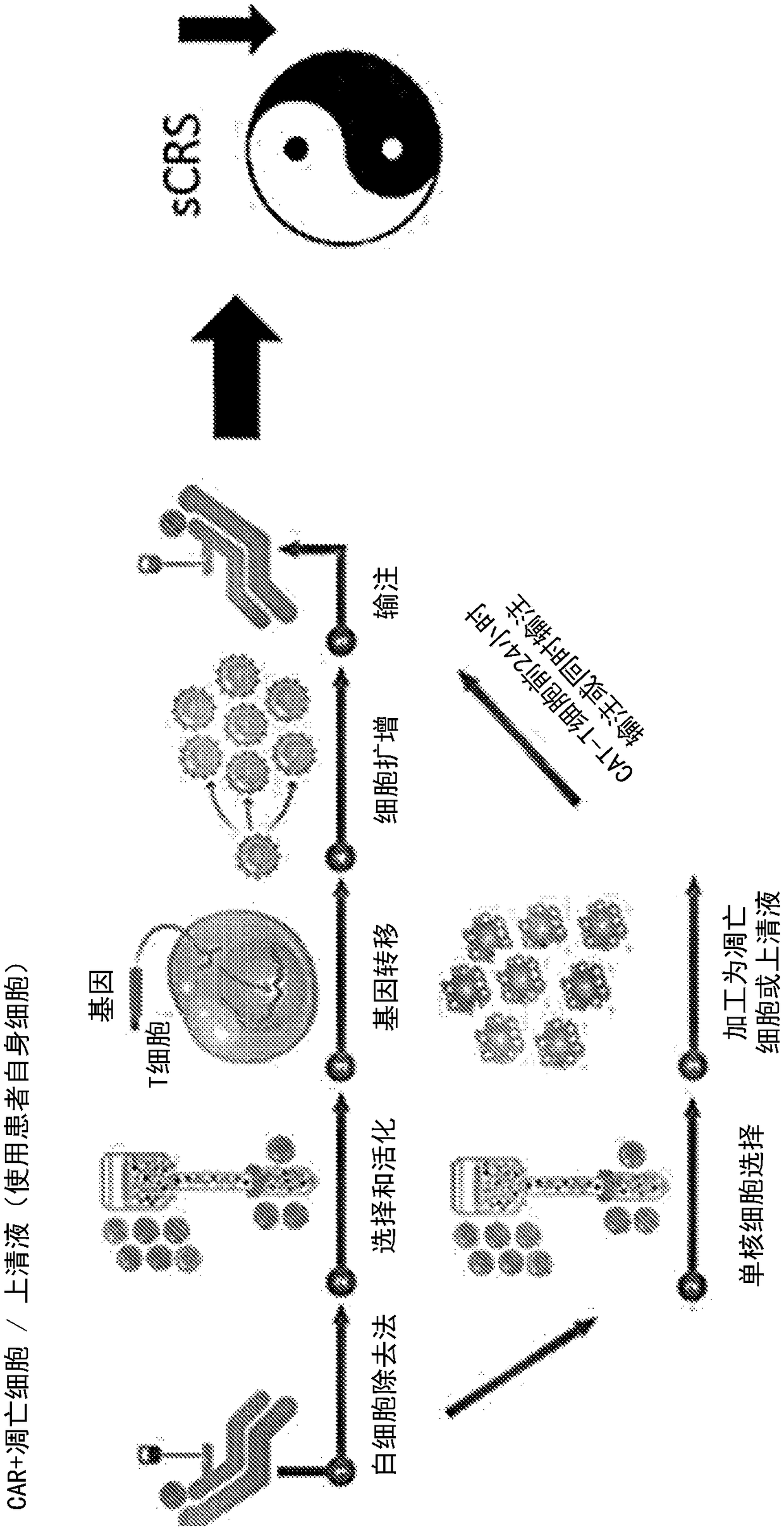
[0581] Example 3: Effect of apoptotic cells on cytokine storm without negative impact on CAR T cell efficacy
[0582] Objective: To detect the effect of apoptotic cells or supernatants derived from apoptotic cells on cytokine storm marker cytokines and the efficacy of CAR T cells on tumor cells.
[0583] method:
[0584] T4+CAR T cells
[0585] A solid tumor model reported to induce a cytokine storm in mice was utilized (van der Stegen et al., 2013 supra). In this model, T cells (T4 + CAR-T cells), these ErbB dimers are often highly upregulated in specific solid tumors such as head and neck tumors and ovarian cancer. T cells were isolated from PBMC isolated from peripheral blood using CD3 microbeads. Construct containing chimeric T4 + recipient vector and transduce it into isolated T cells to obtain T4 + CAR T cells. For the experiments carried out in this paper, T4 + CAR T cells were purchased from Creative Biolabs (New York, USA) or Promab Biotechnologies (California...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com