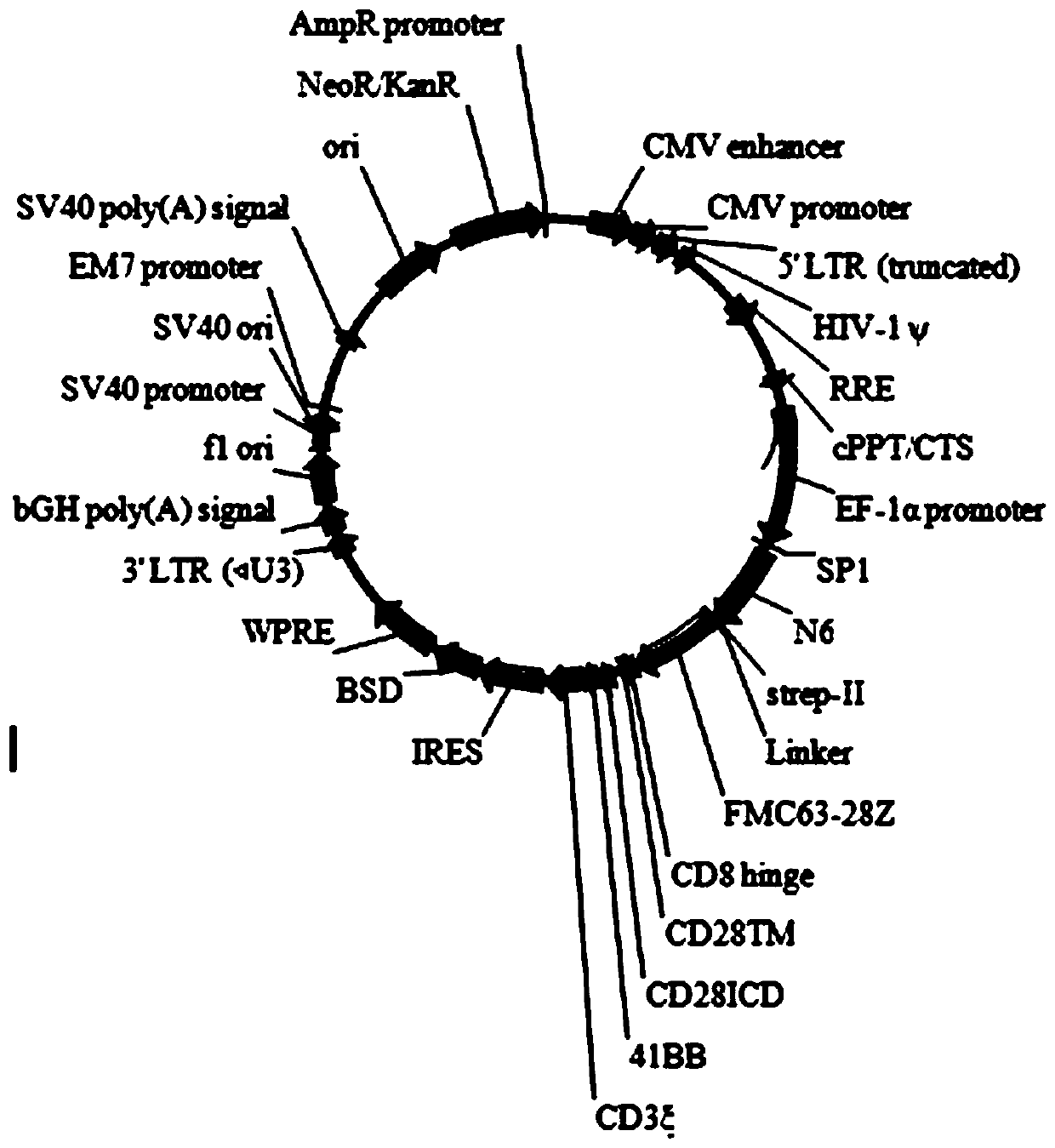
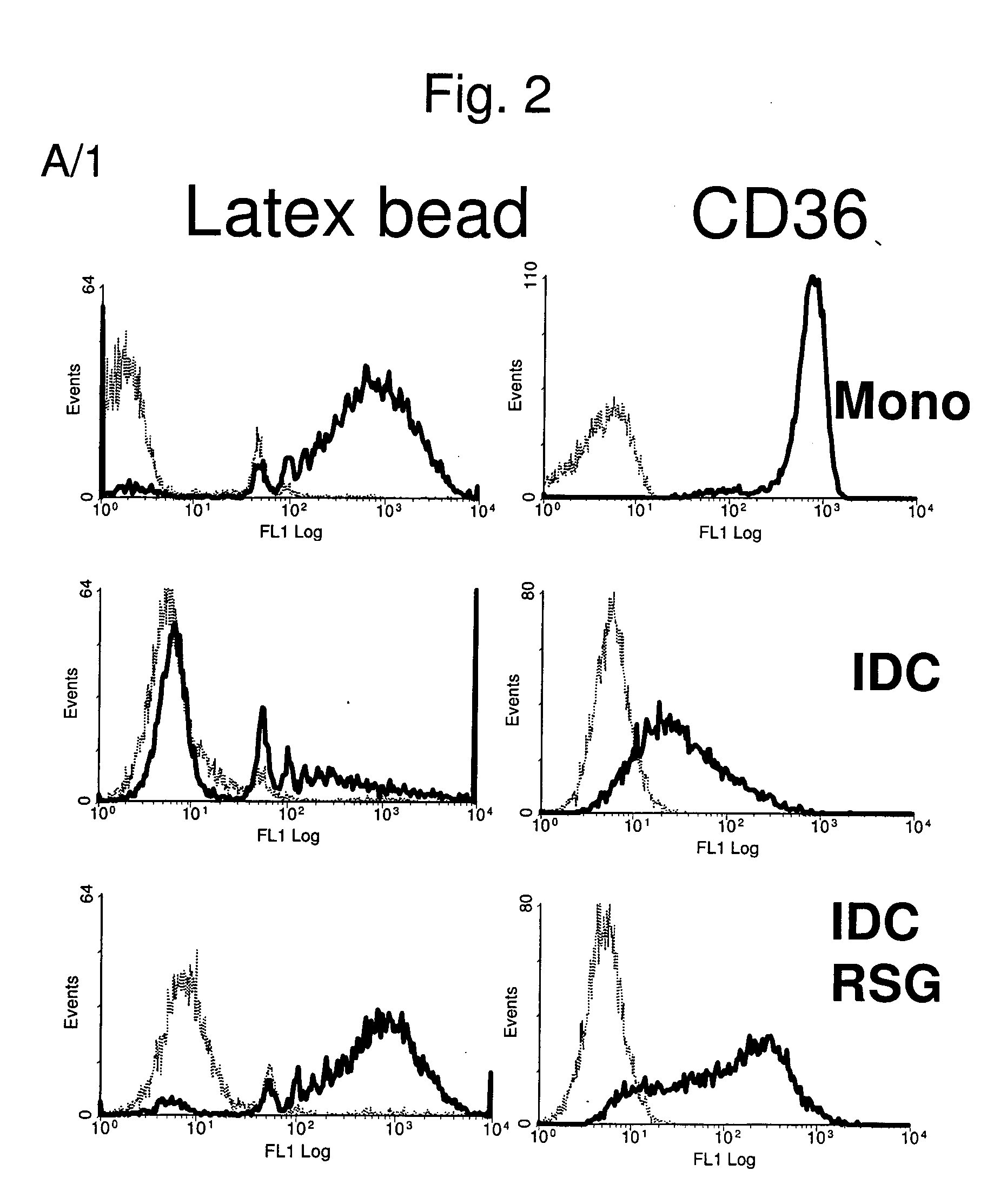
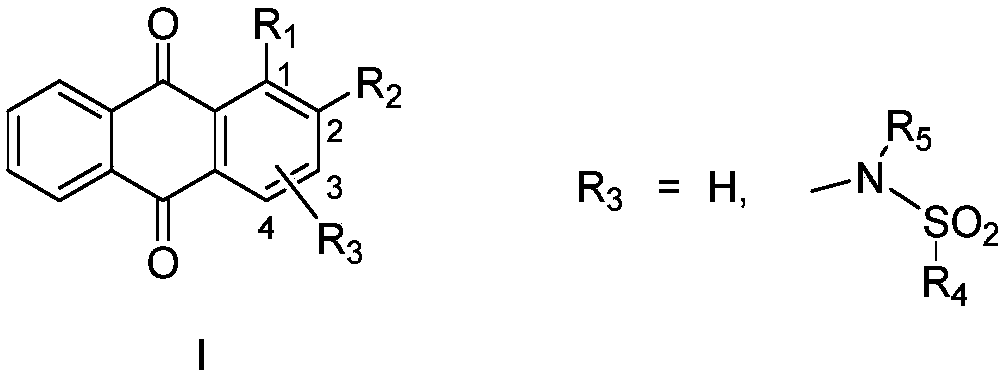Patents
Literature
116 results about "Hematological Tumor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
Novel immunotherapy against several tumors of the blood, such as acute myeloid leukemia (AML)
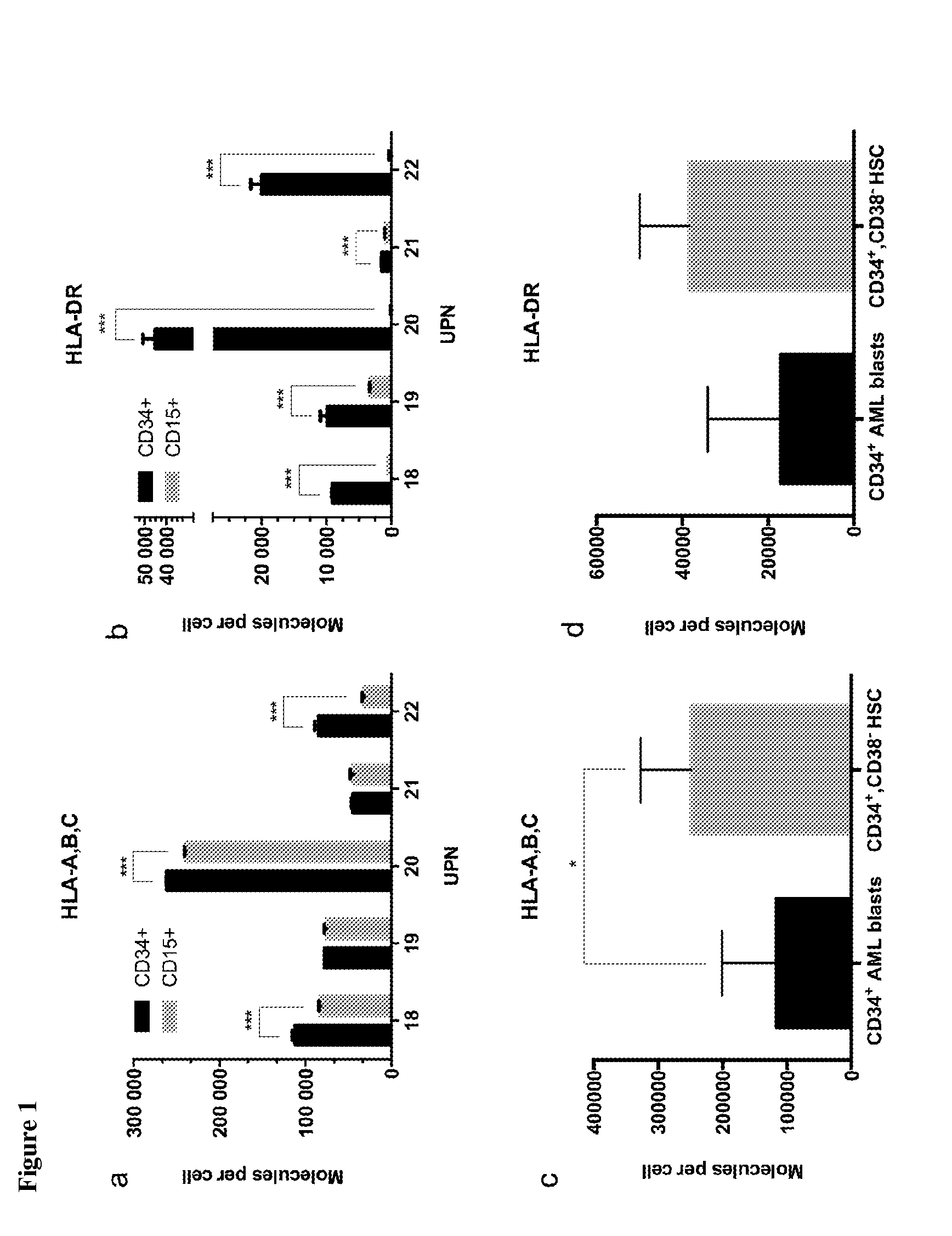
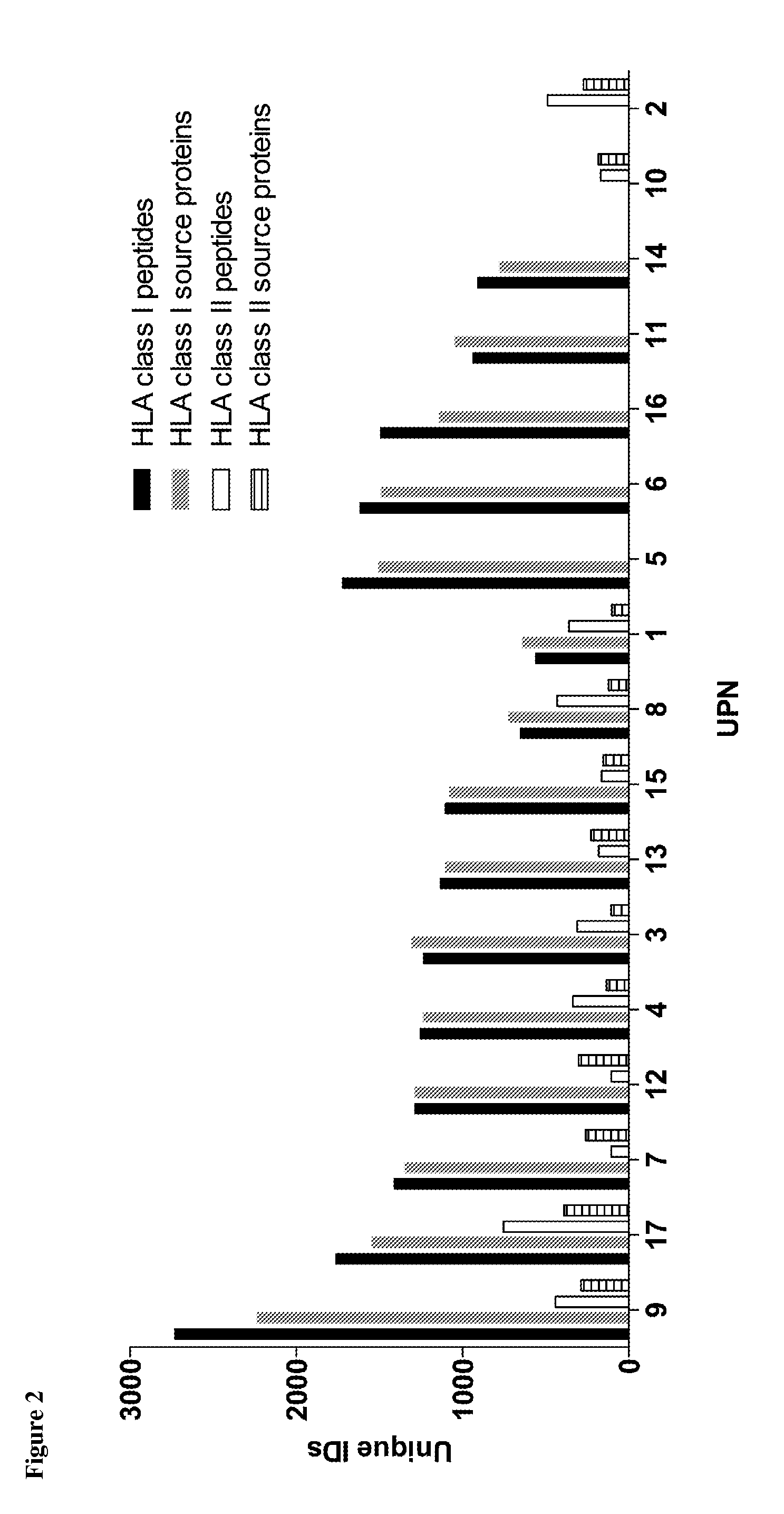
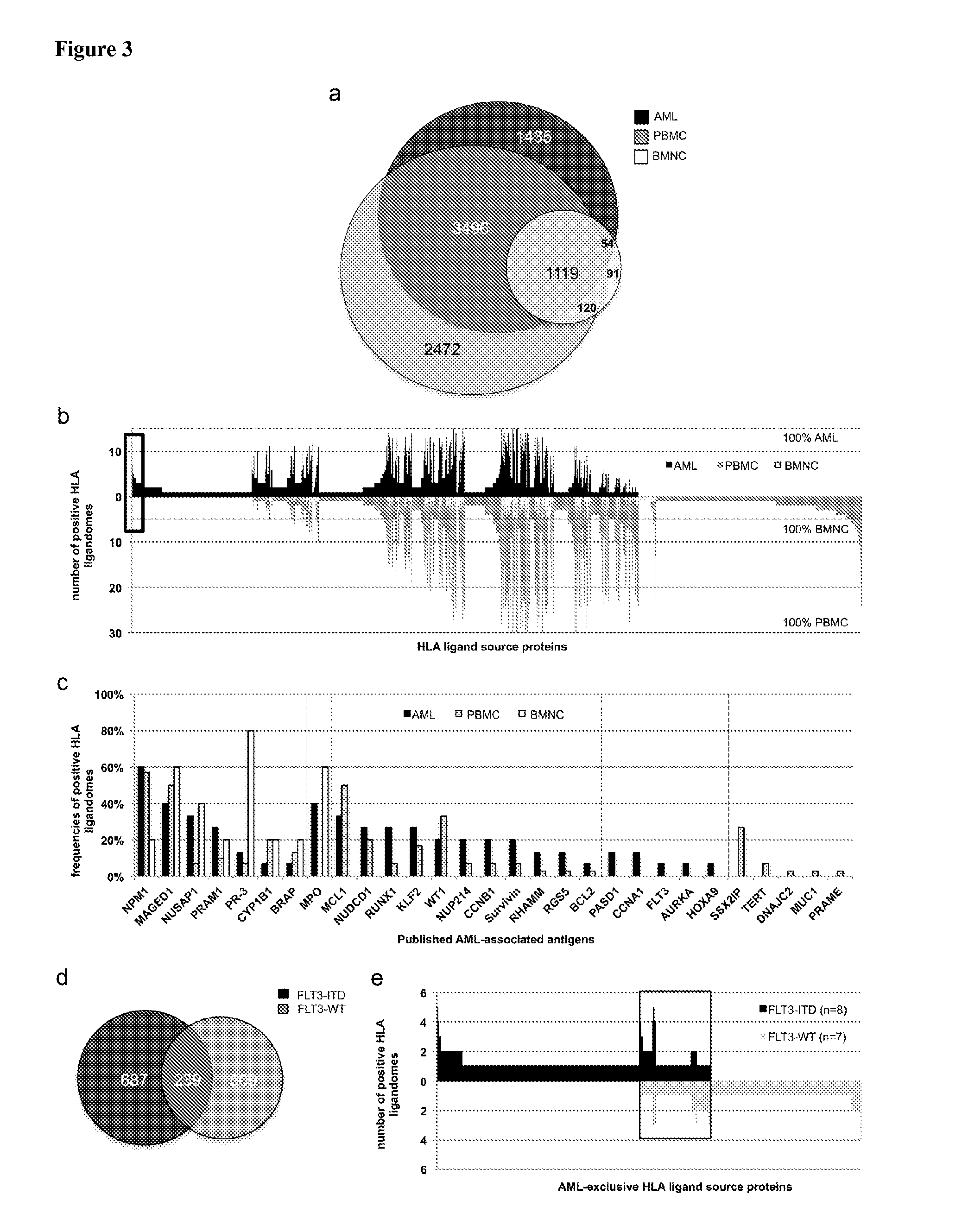
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated cytotoxic T cell (CTL) peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. The present invention relates to several novel peptide sequences and their variants derived from HLA class I and HLA class II molecules of human tumor cells that can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
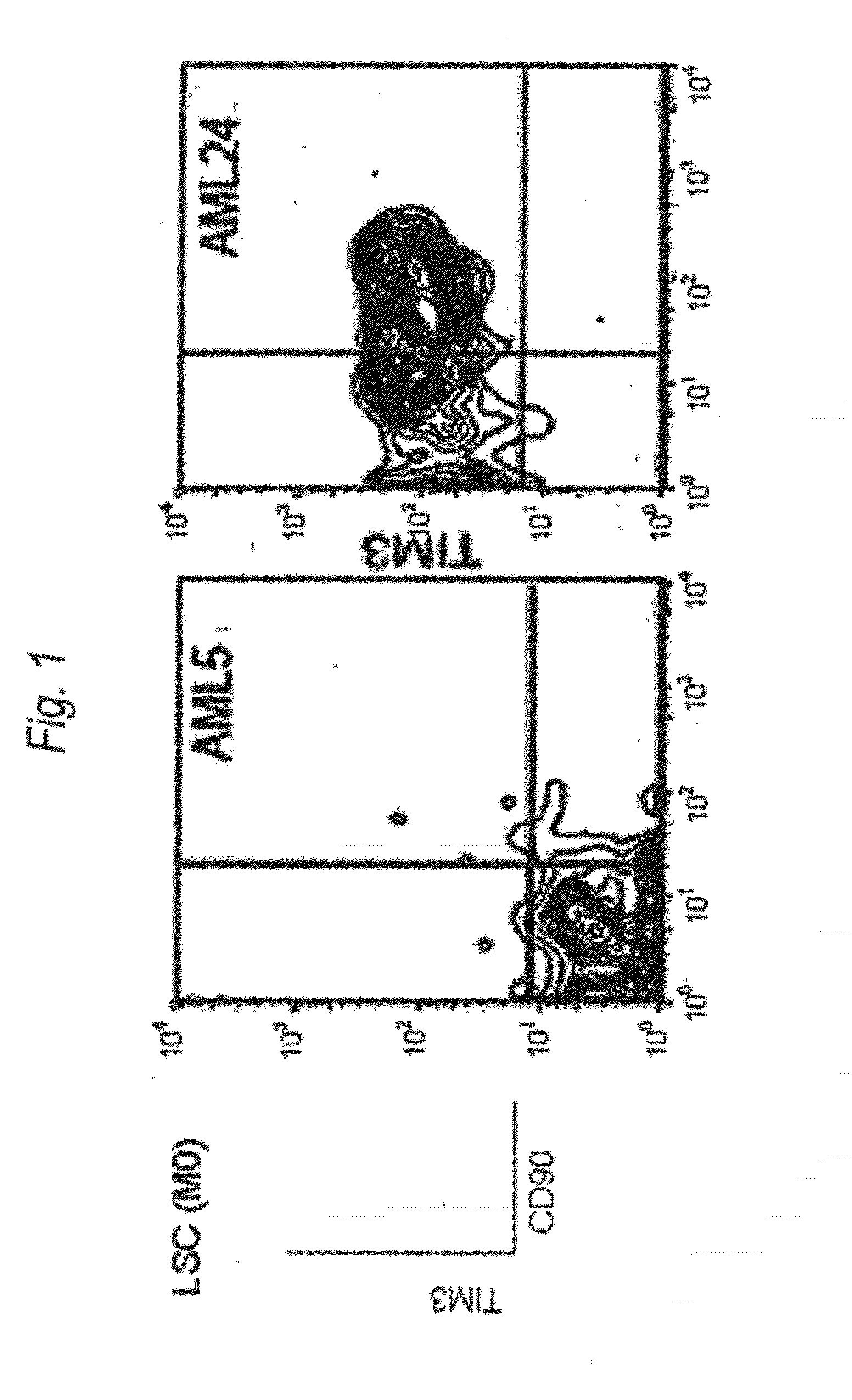
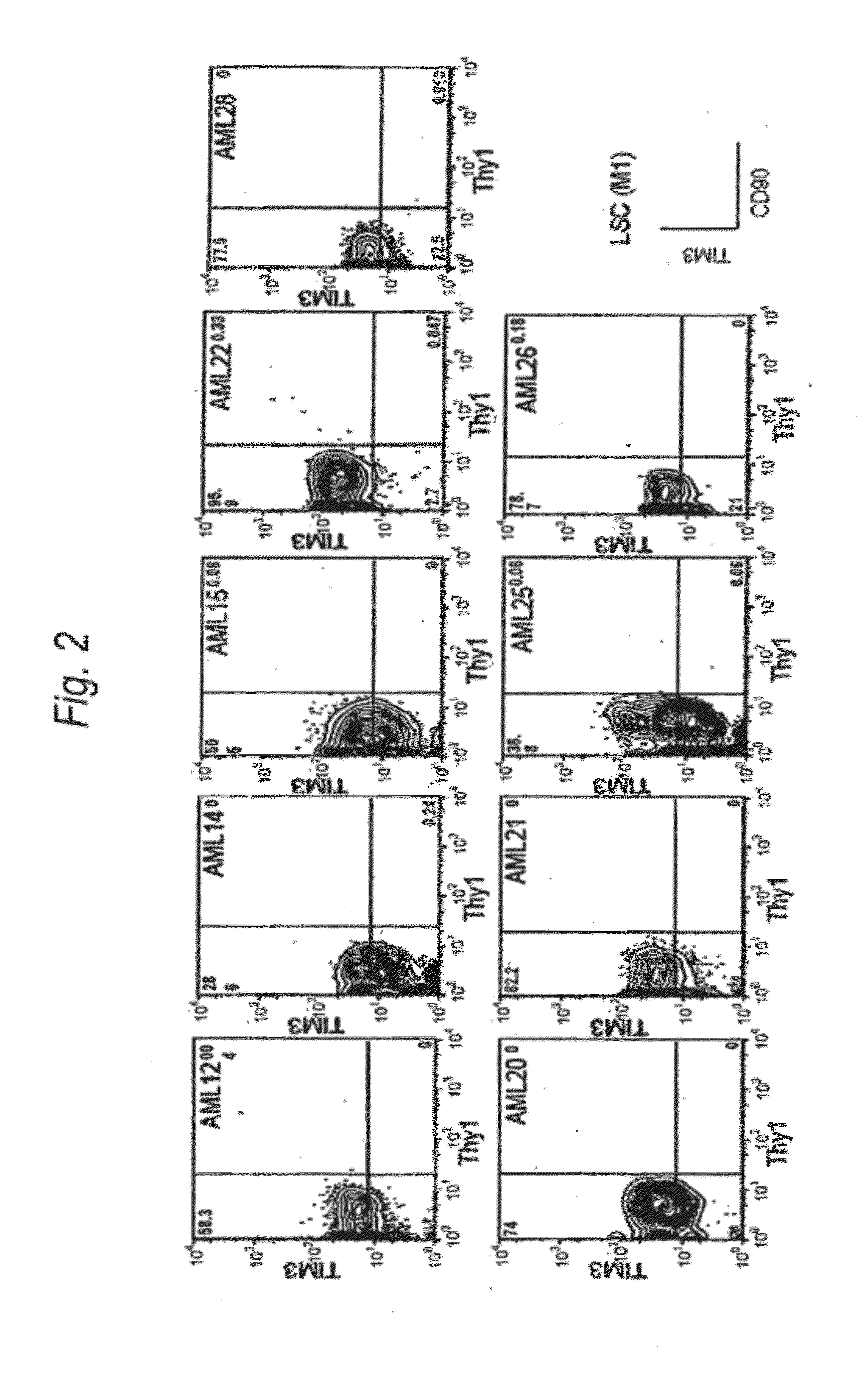
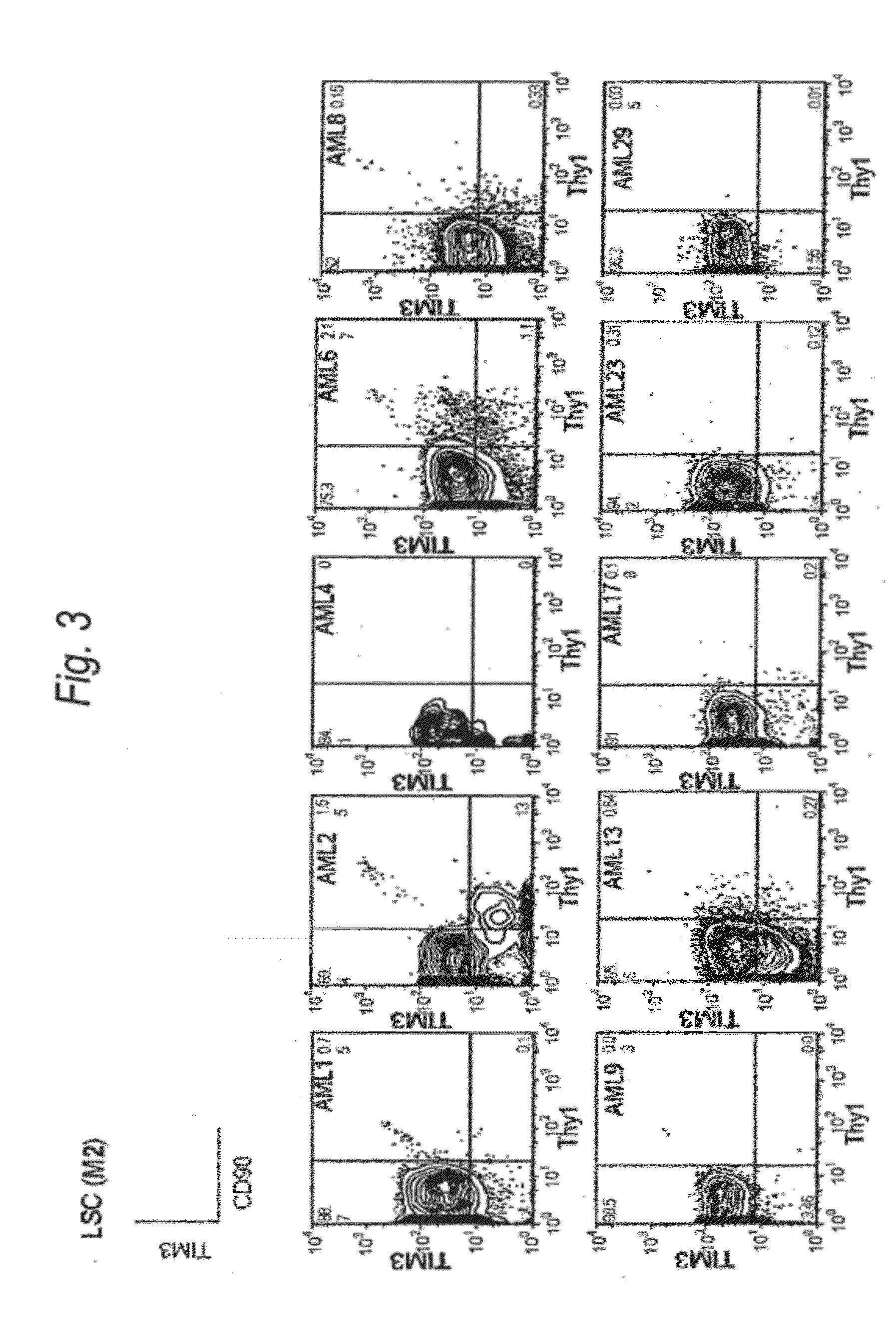
Method for treatment of blood tumor using Anti-tim-3 antibody
ActiveUS20120100131A1Use to establishBiological material analysisAntibody ingredientsDiseaseCell Fraction
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
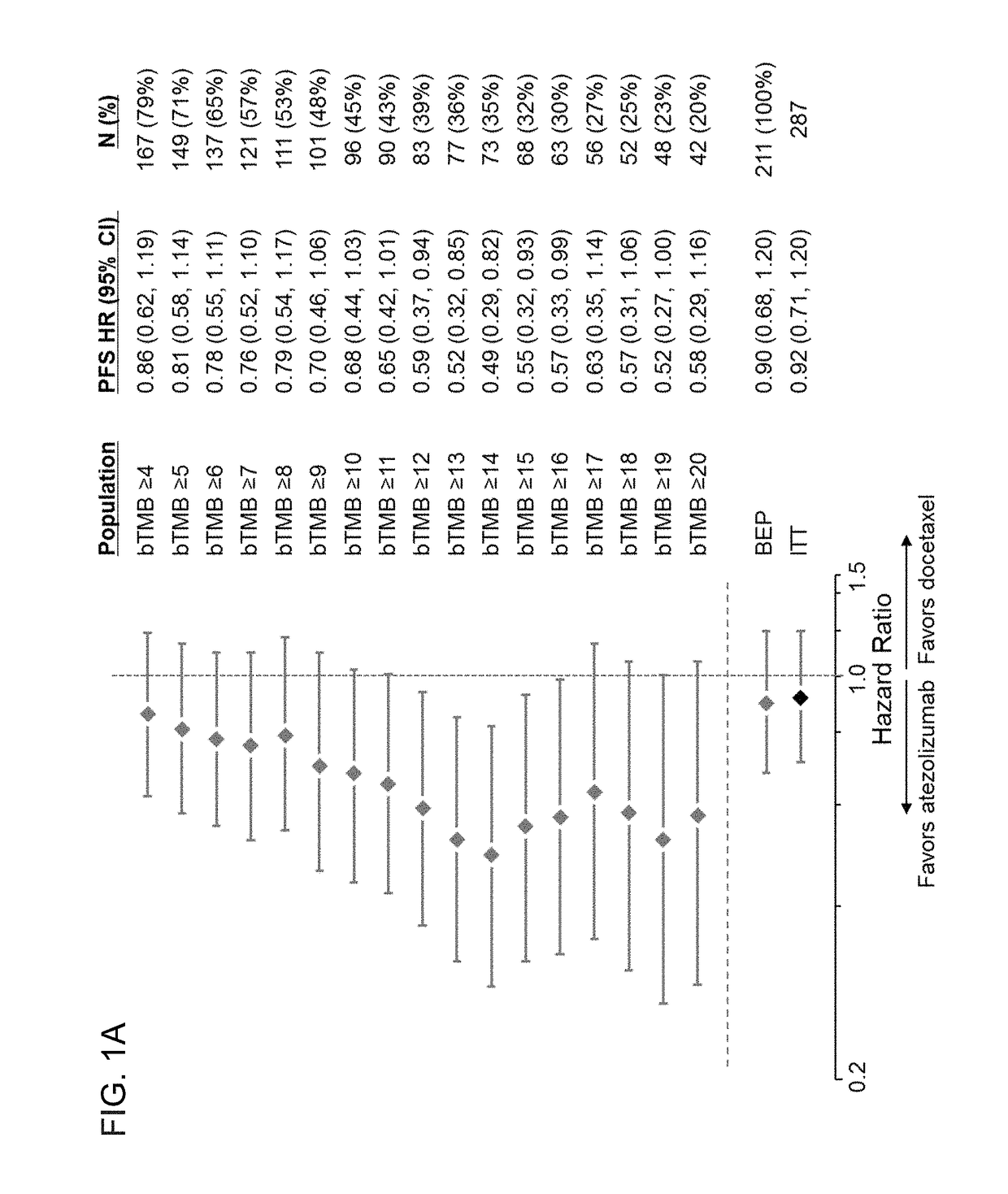
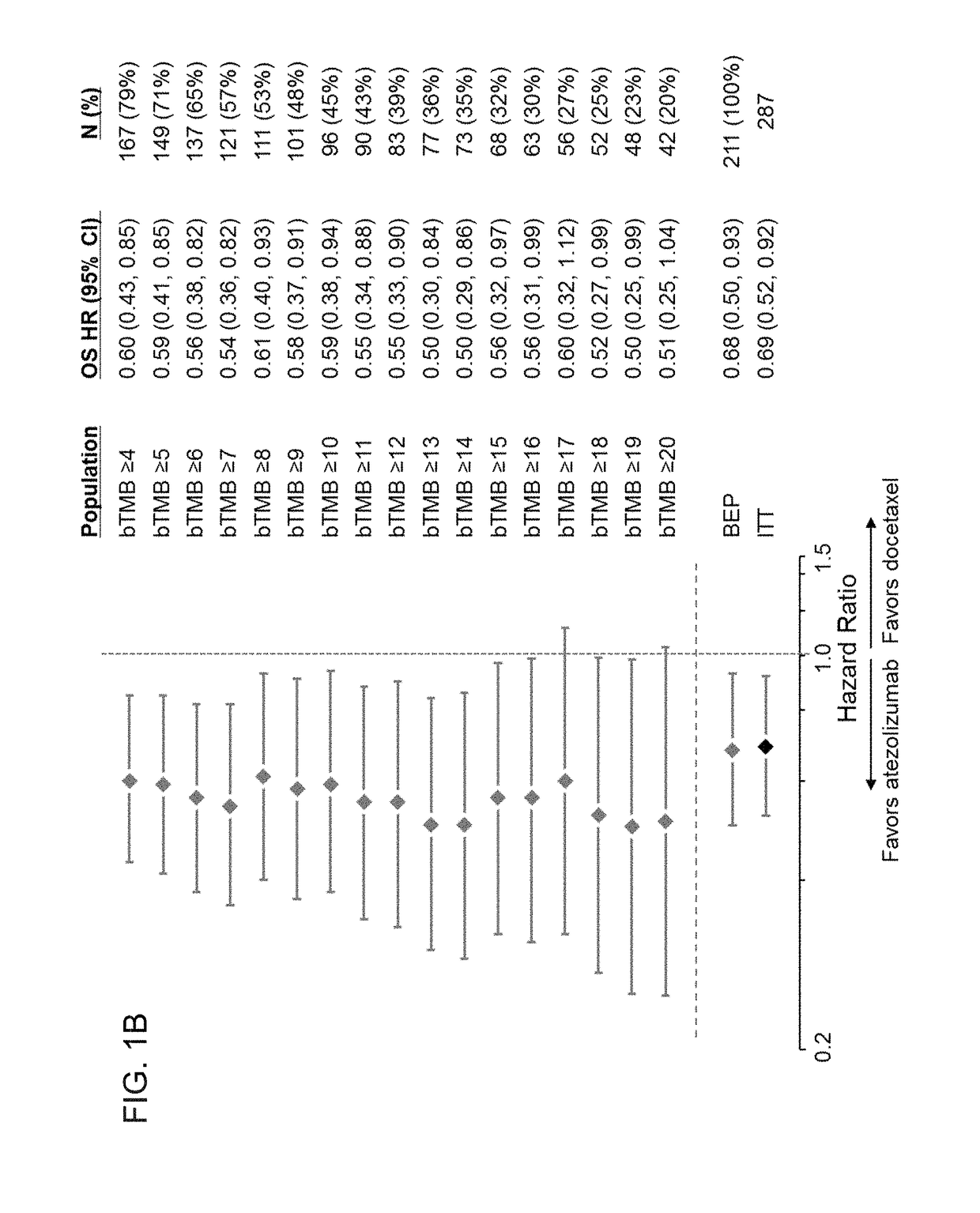
Therapeutic and diagnostic methods for cancer
PendingUS20190025308A1Prolonged progression-free survivalImprove survivalImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisImmune checkpoint inhibitorsPD-L1
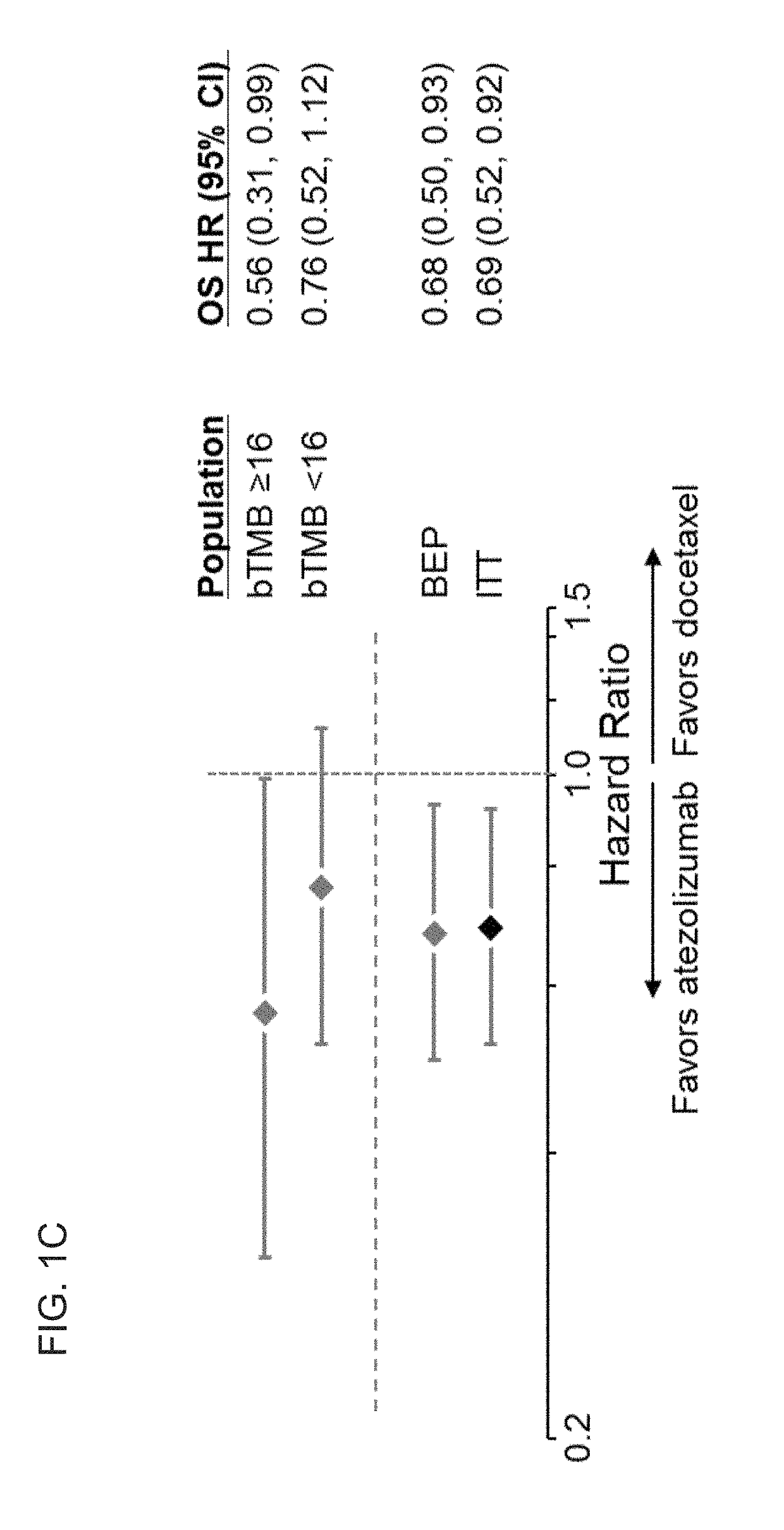
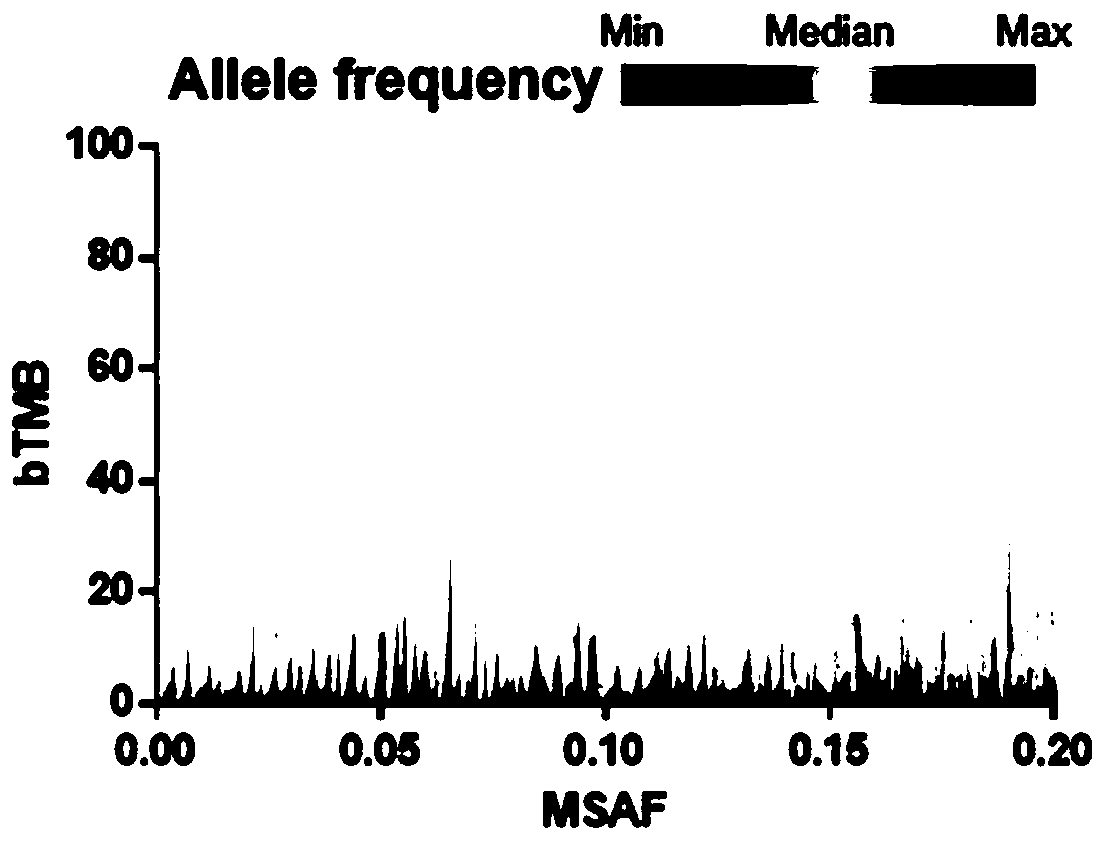
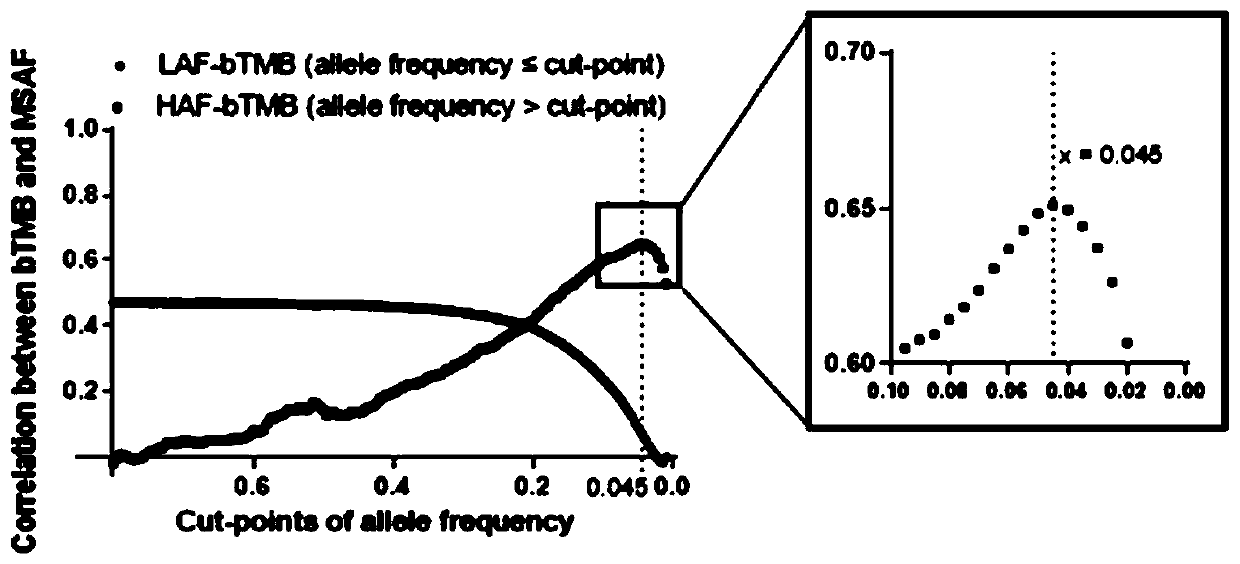
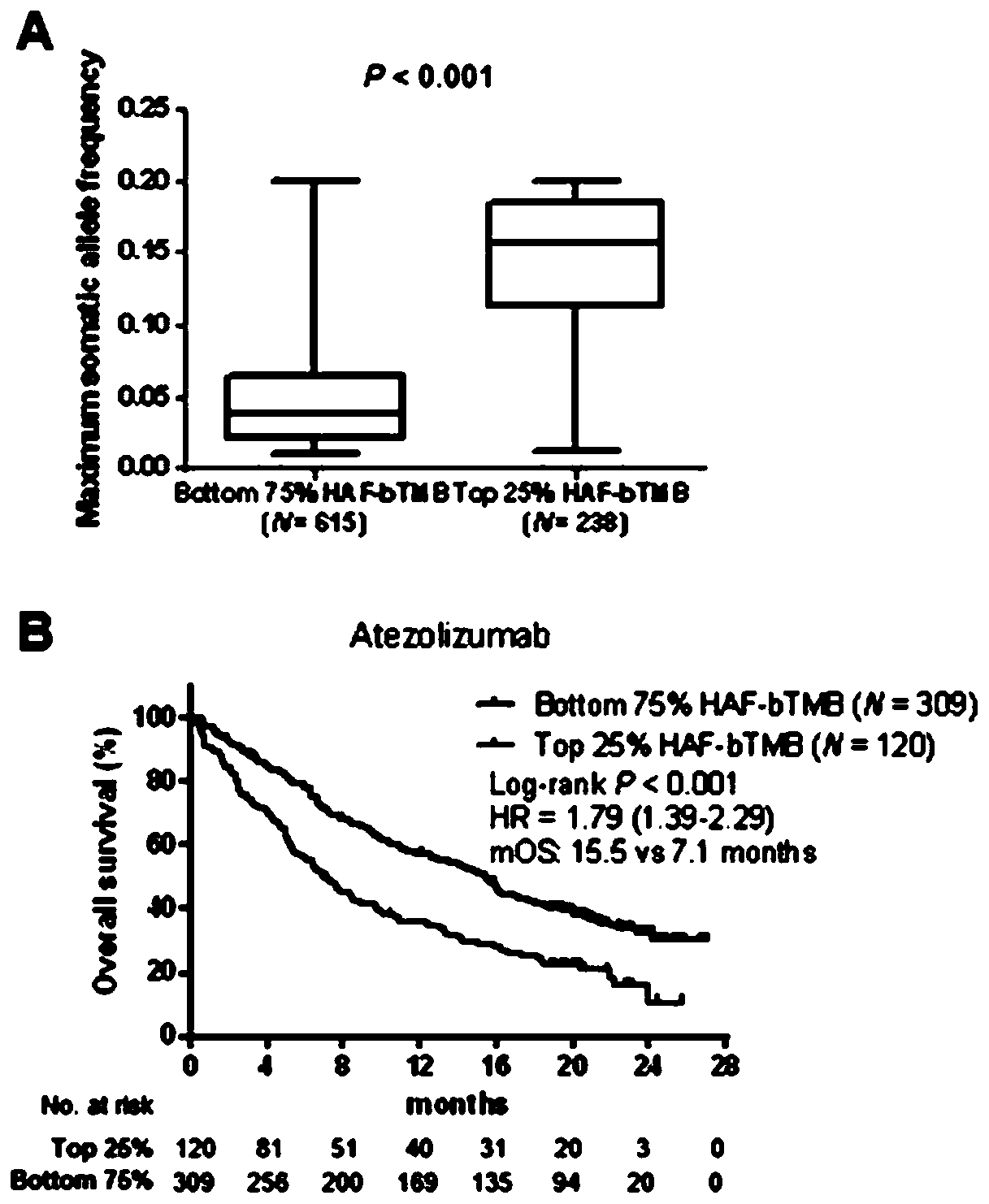
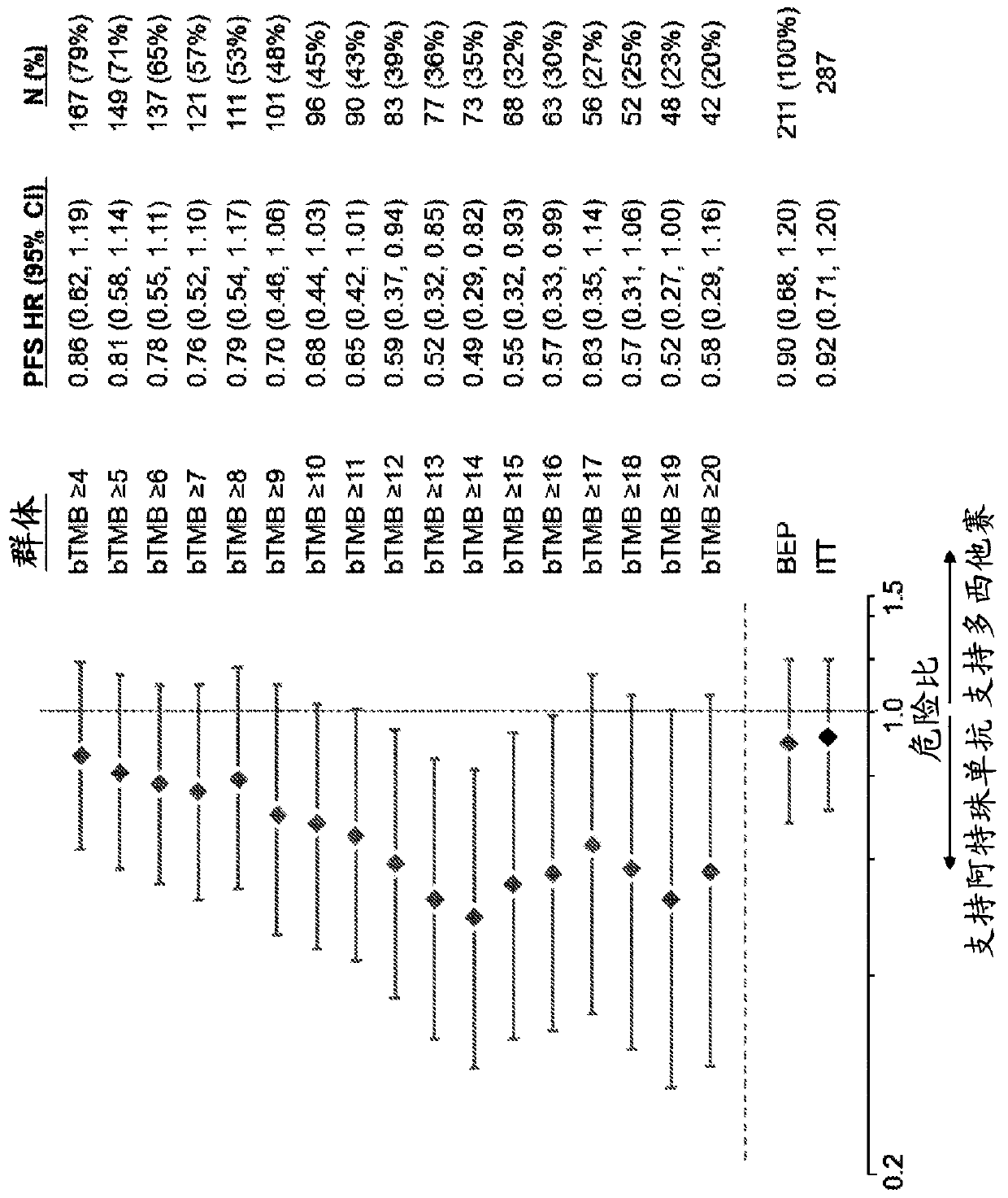
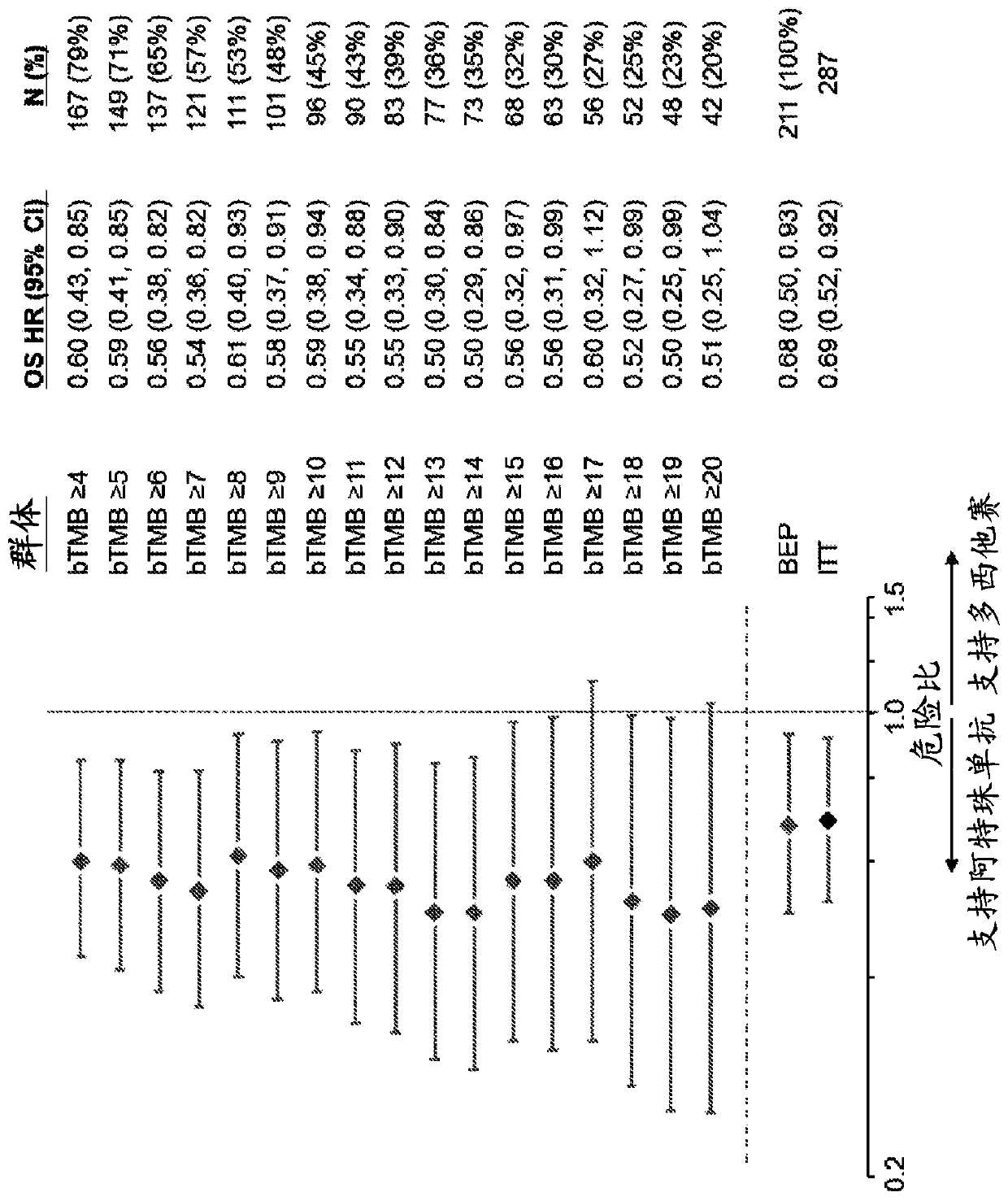
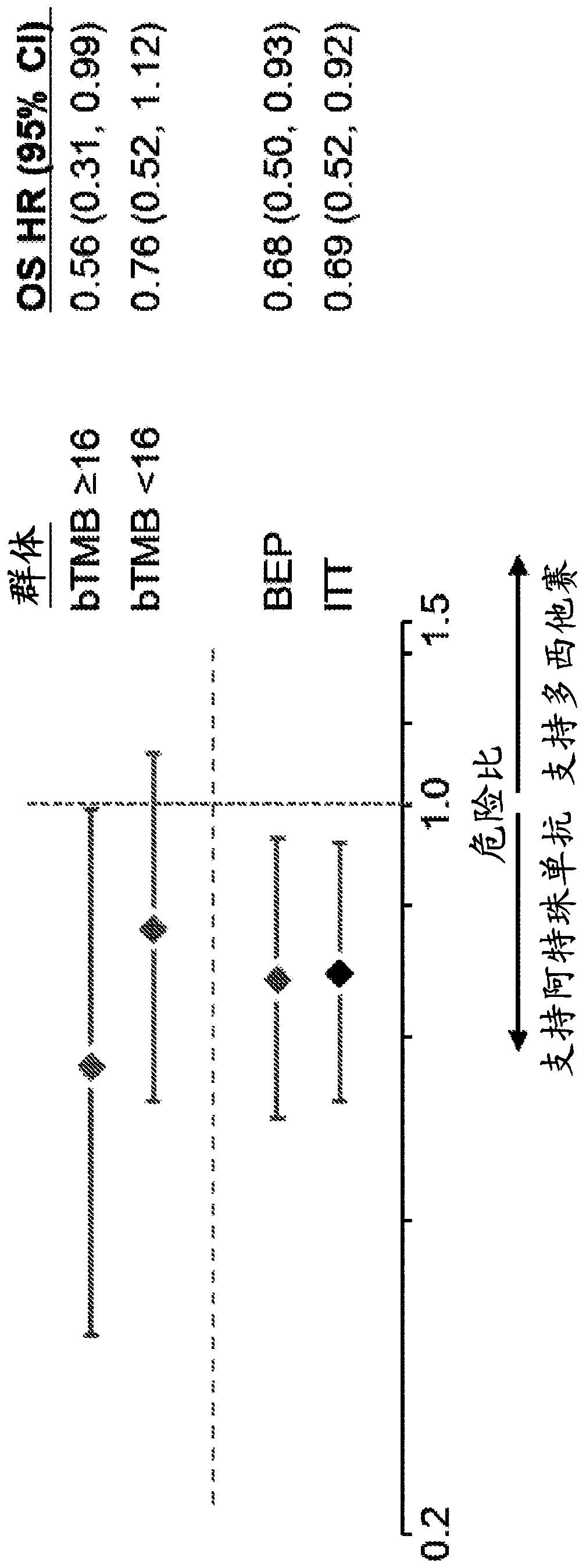
The present invention provides therapeutic, diagnostic, and prognostic methods for cancer. The invention provides methods of treating a cancer, methods of determining whether an individual having a cancer is likely to respond to a treatment including an immune checkpoint inhibitor (e.g., a PD-L1 axis binding antagonist), methods of predicting responsiveness of an individual having a cancer to a treatment including an immune checkpoint inhibitor (e.g., a PD-L1 axis binding antagonist), methods of selecting a therapy for an individual having a cancer, methods of providing a prognosis for an individual having a cancer, and methods of monitoring a response of an individual having a cancer, based on a blood tumor mutational burden (bTMB) score or a maximum somatic allele frequency (MSAF) from a sample (e.g., a whole blood sample, a plasma sample, a serum sample, or a combination thereof) from the individual.
Owner:FOUND MEDICINE INC
Application of novel CAR (Chimeric Antigen Receptor) modified T cells for treating cancer
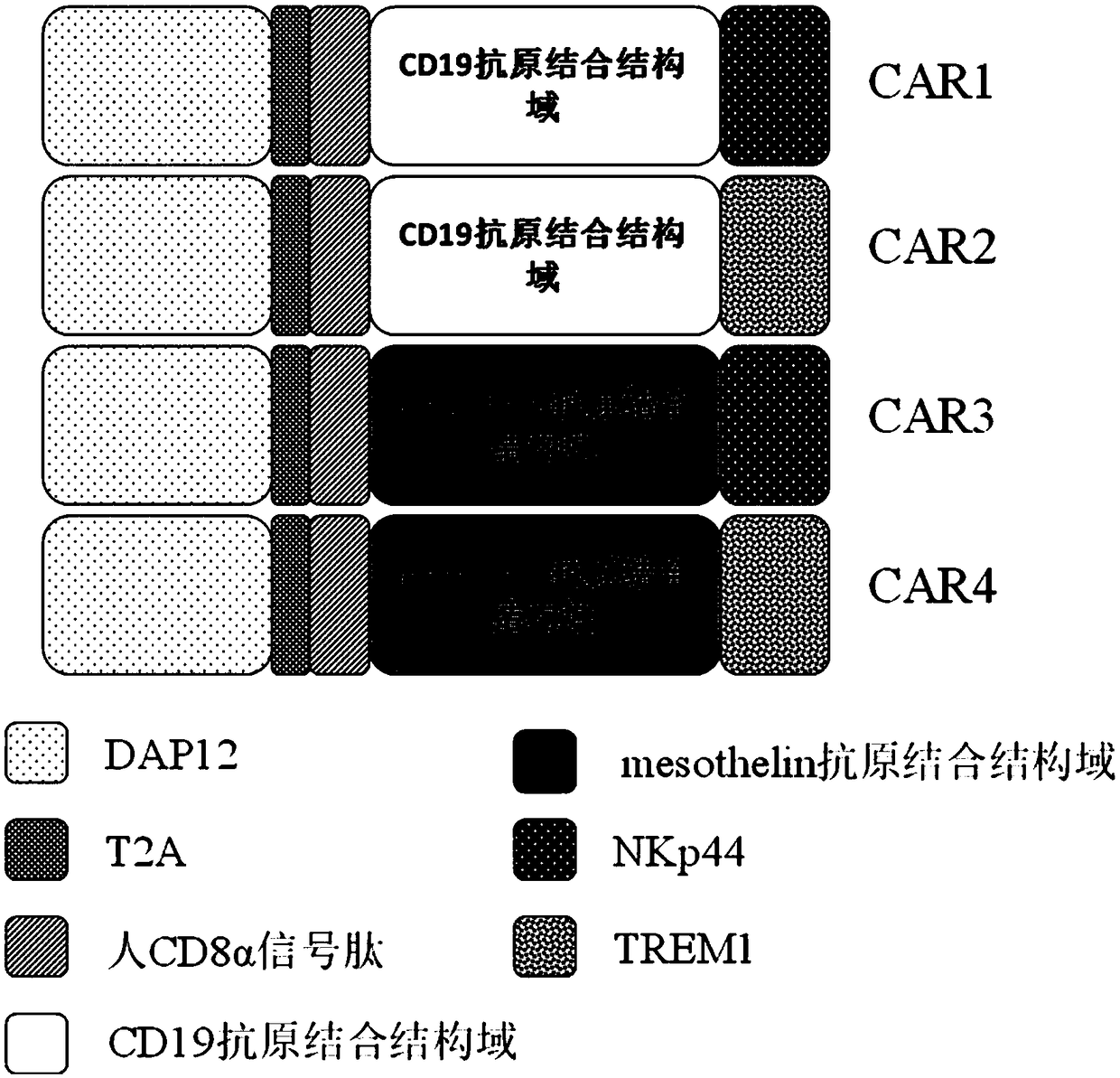
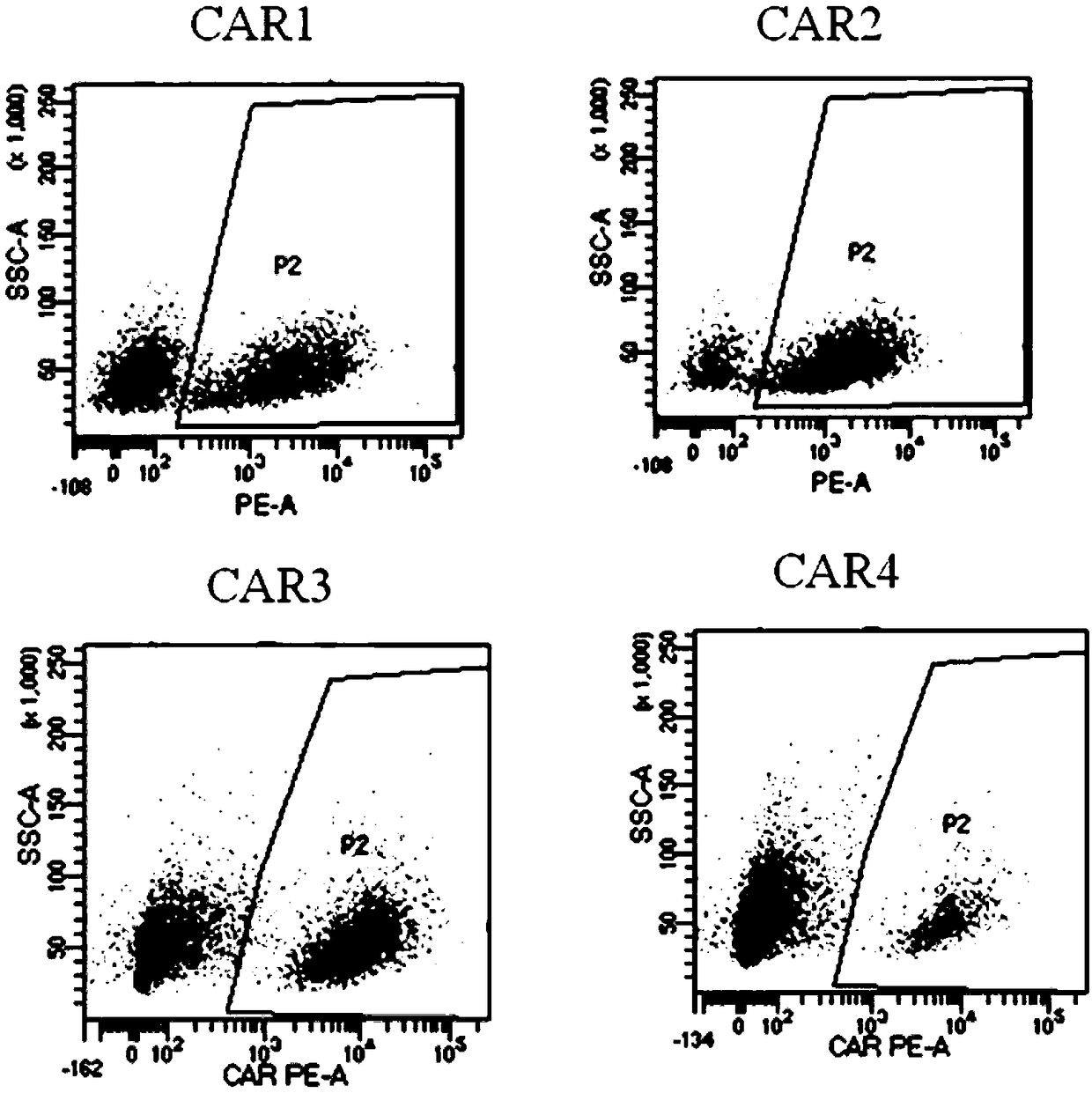
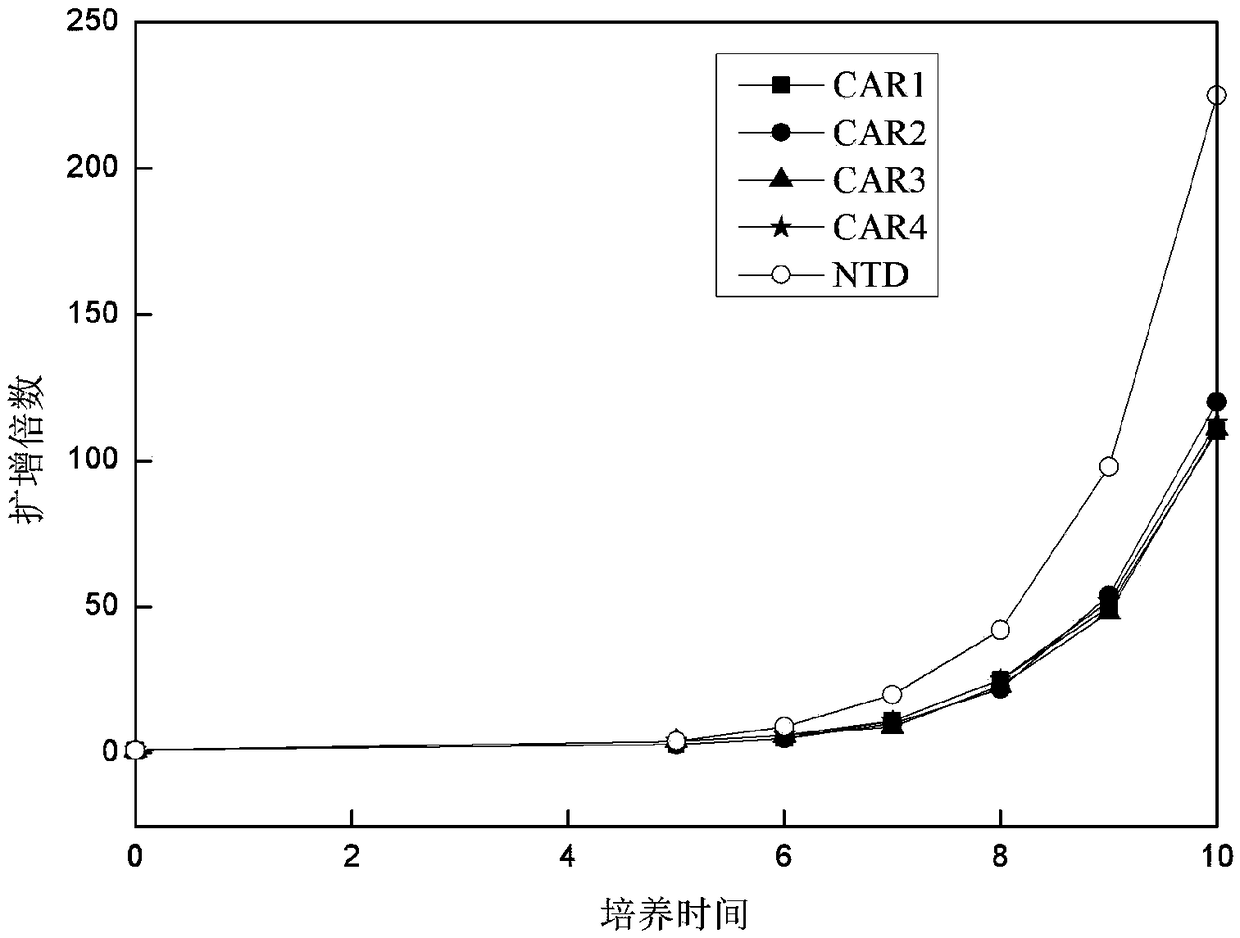
ActiveCN109400713AMild release responseSignificant effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyExtracellular signalAbnormal tissue growth
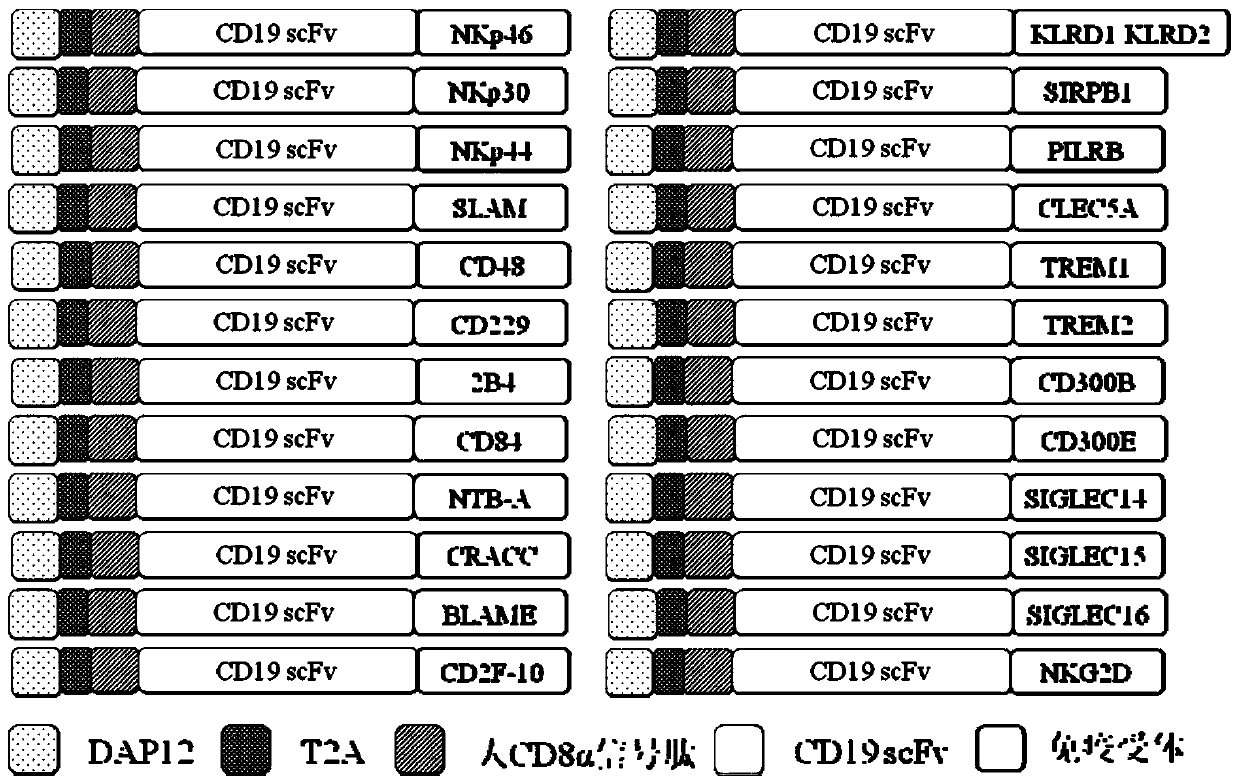
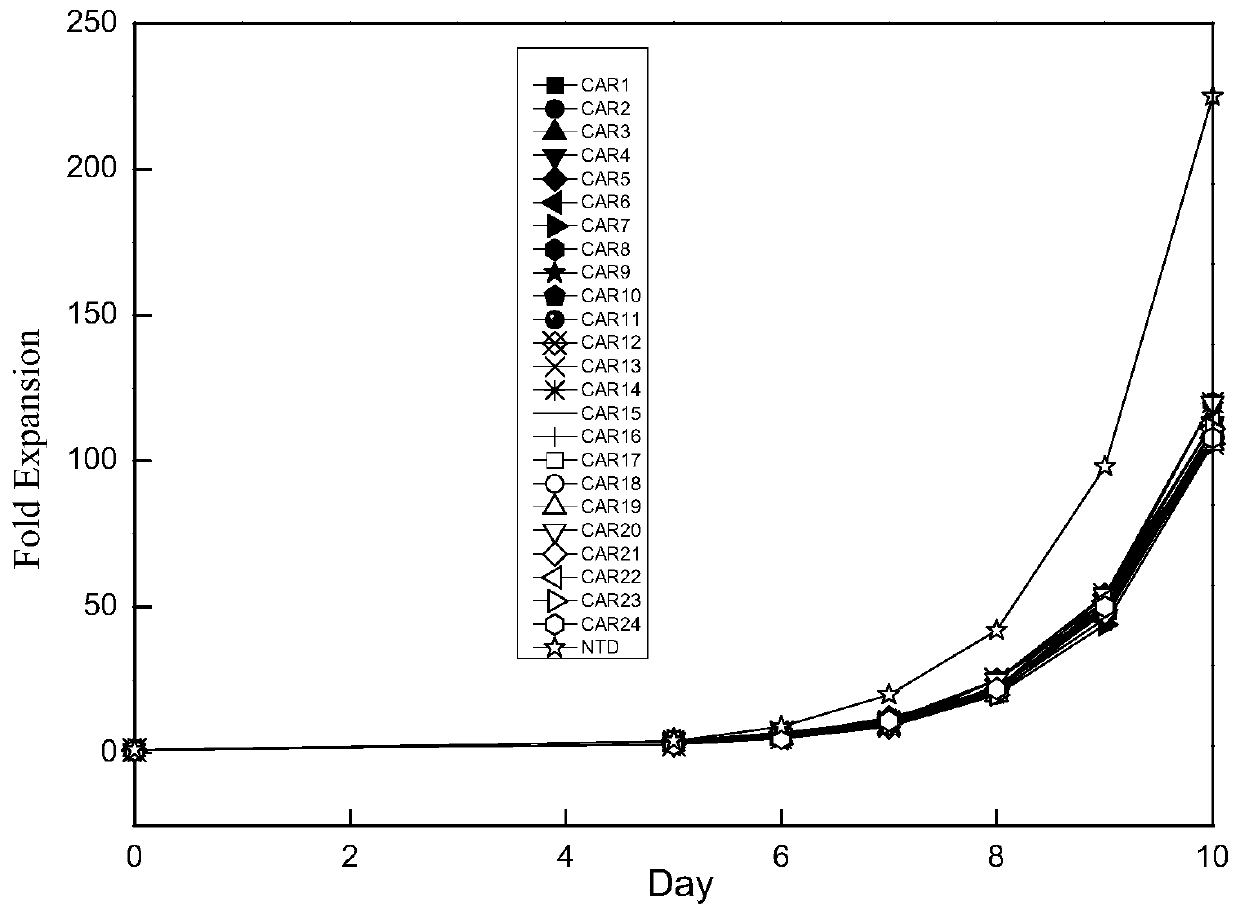
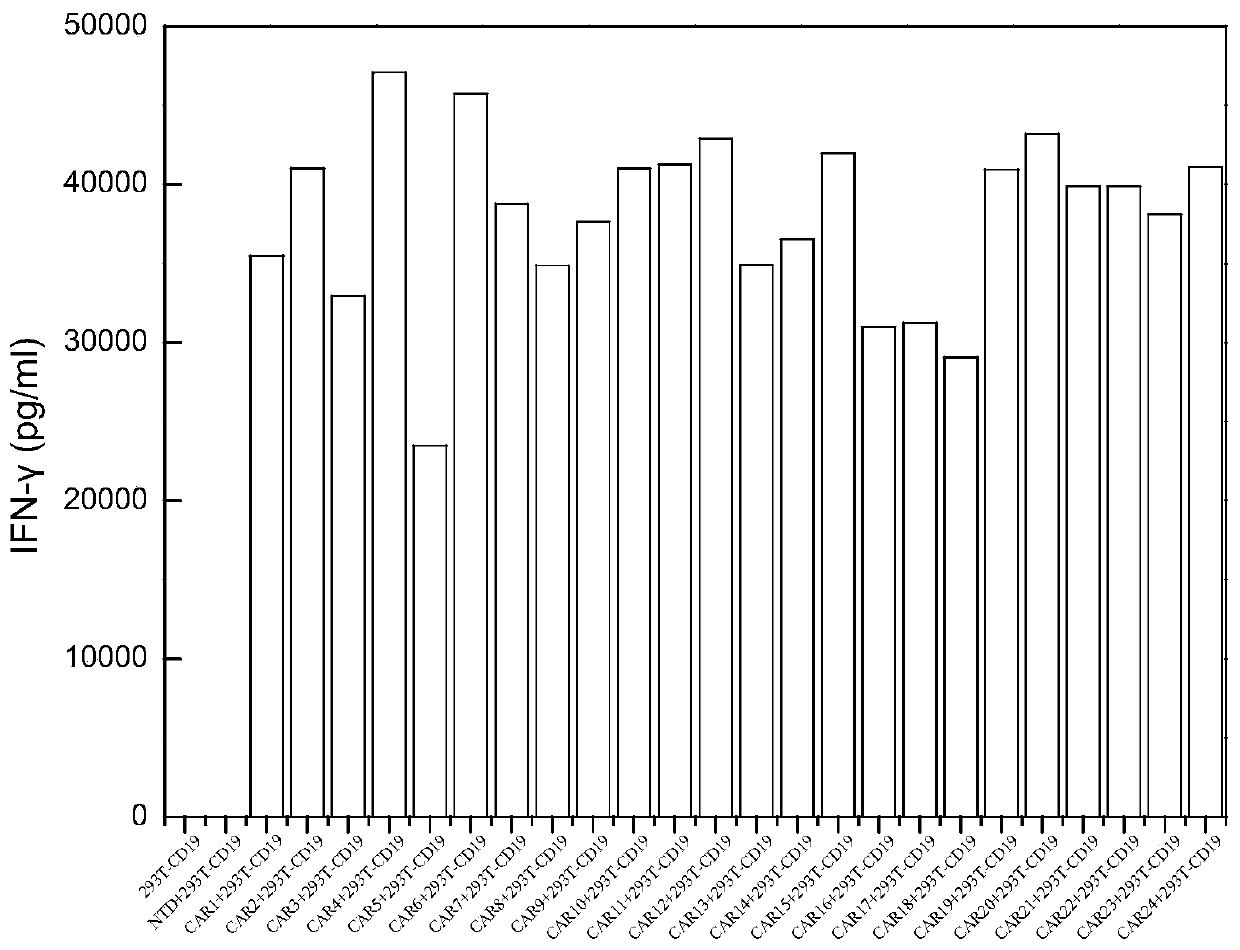
The invention discloses a novel CAR (Chimeric Antigen Receptor) and application of novel CAR modified T cells for treating cancer. The novel CAR is formed by an extracellular signal peptide, an antigen binding structural domain, an intracellular first conduction structural domain and an intracellular second conduction structural domain, and contains an intracellular first conduction structural domain NKp44 or an intracellular first conduction structural domain TREM1. According to the novel CAR disclosed by the invention, multiple CAR nucleotide sequences are separated and purified, and the CARand CAR-T cells which specifically aims at CD19 malignant hematologic tumor and mesothelin malignant solid tumor antigens. In a hematologic tumor and solid tumor killing test, the killing capacity ofthe CAR-T cells to tumor cells is obviously enhanced, and good safety and good anti-tumor activity in clinic application are expressed.
Owner:NANJING CART MEDICAL TECH LTD
ROR1 specific chimeric antigen receptor and application thereof
ActiveCN105924533AConfirmed specific killing effectDoes not affect the functionAntibody mimetics/scaffoldsMammal material medical ingredientsAntigen receptorHinge region
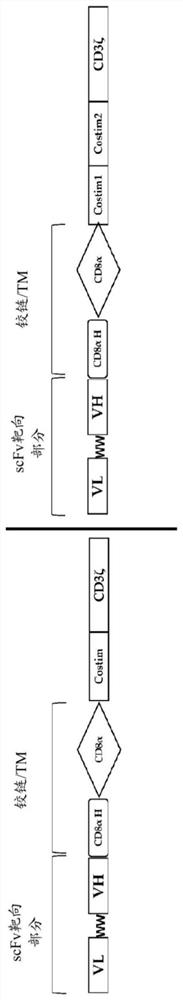
The invention discloses an ROR1 specific chimeric antigen receptor and an application thereof. The ROR1 specific chimeric antigen receptor is a protein formed through serially connecting scFv of anti-human ROR1, the hinge region and the transmembrane region of CD8alpha, the transmembrane region and the intracellular region of CD28 and the intracellular region of CD3zeta from the amino end to the carboxyl end. T cells modified with the chimeric antigen receptor have a specific killing effect on tumor cells, and are expected to be in widely used in treatment of all kinds of solid tumor and blood tumors.
Owner:BEIJING BIOHEALTHCARE BIOTECH
Method of treating hematologic tumors and cancers
InactiveUS7070797B2Improved treatment of cancerImprove treatmentBiocidePharmaceutical delivery mechanismHematological TumorApoptosis
Multiple myeloma and other hematologic tumors and / or malignancies can be treated by administration of a G1 and / or S phase drug, which is preferably β-lapachone, or a derivative or analog thereof, combined with a G2 / M phase drug such as a taxane derivative, which is advantageously paclitaxel. This combination of the G1 and / or S phase drug with the G2 / M phase drug results in an unexpectedly greater than additive (i.e., synergistic) apoptosis in multiple myeloma cells. The invention includes methods of treating multiple myeloma by administering the combination of the G1 and / or S phase drug and the G2 / M phase drug, pharmaceutical compositions comprising the combination of drugs used in these methods, as well as pharmaceutical kits.
Owner:DANA FARBER CANCER INST INC
Human interleukin 2-polyethylene glycol conjugate as well as preparation method and application thereof
ActiveCN112279906AReduced binding activityReduced stabilityPeptide/protein ingredientsDepsipeptidesWhite blood cellPolyethylene glycol
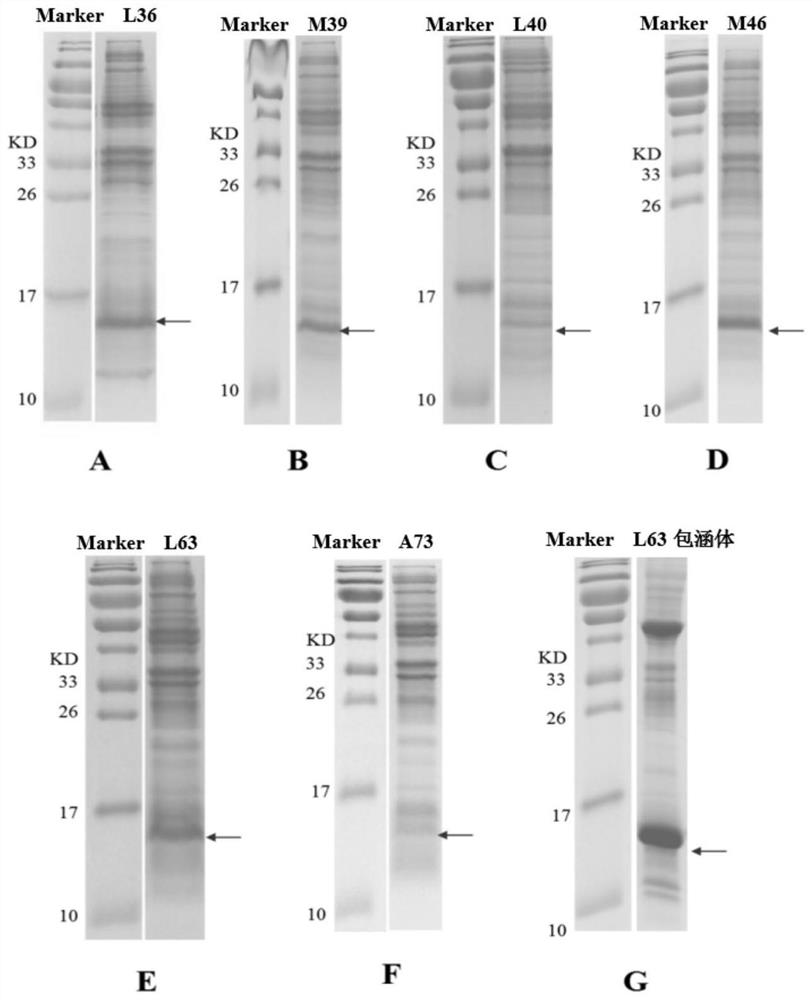
The invention discloses a human interleukin 2-polyethylene glycol conjugate as well as a preparation method and an application thereof. The human interleukin 2-polyethylene glycol conjugate comprisesrecombinant human interleukin 2 of at least one non-natural amino acid and PEG coupled to the at least one non-natural amino acid, the position of the at least one non-natural amino acid is selected from one or more sites of L36, M39, L40, M46, P47, L63, L66, E67, L70 and A73 corresponding to SEQ ID NO: 2, wherein the recombinant human interleukin 2 is a protein as shown in SEQ ID NO: 3 or a functional active fragment thereof. The human interleukin 2-polyethylene glycol conjugate provided by the invention can be used as a single drug or combined with an anti-tumor drug, and is used for treating solid tumors or blood tumors.
Owner:NOVOCODEX BIOPHARMACEUTICALS CO LTD
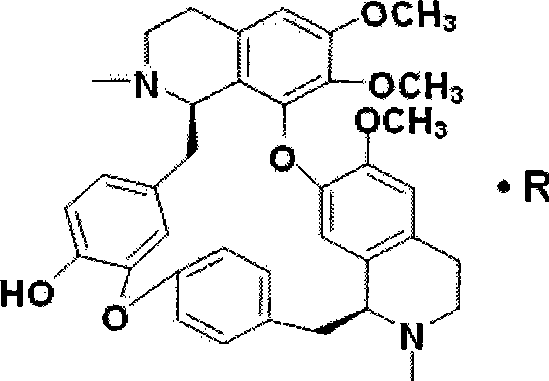
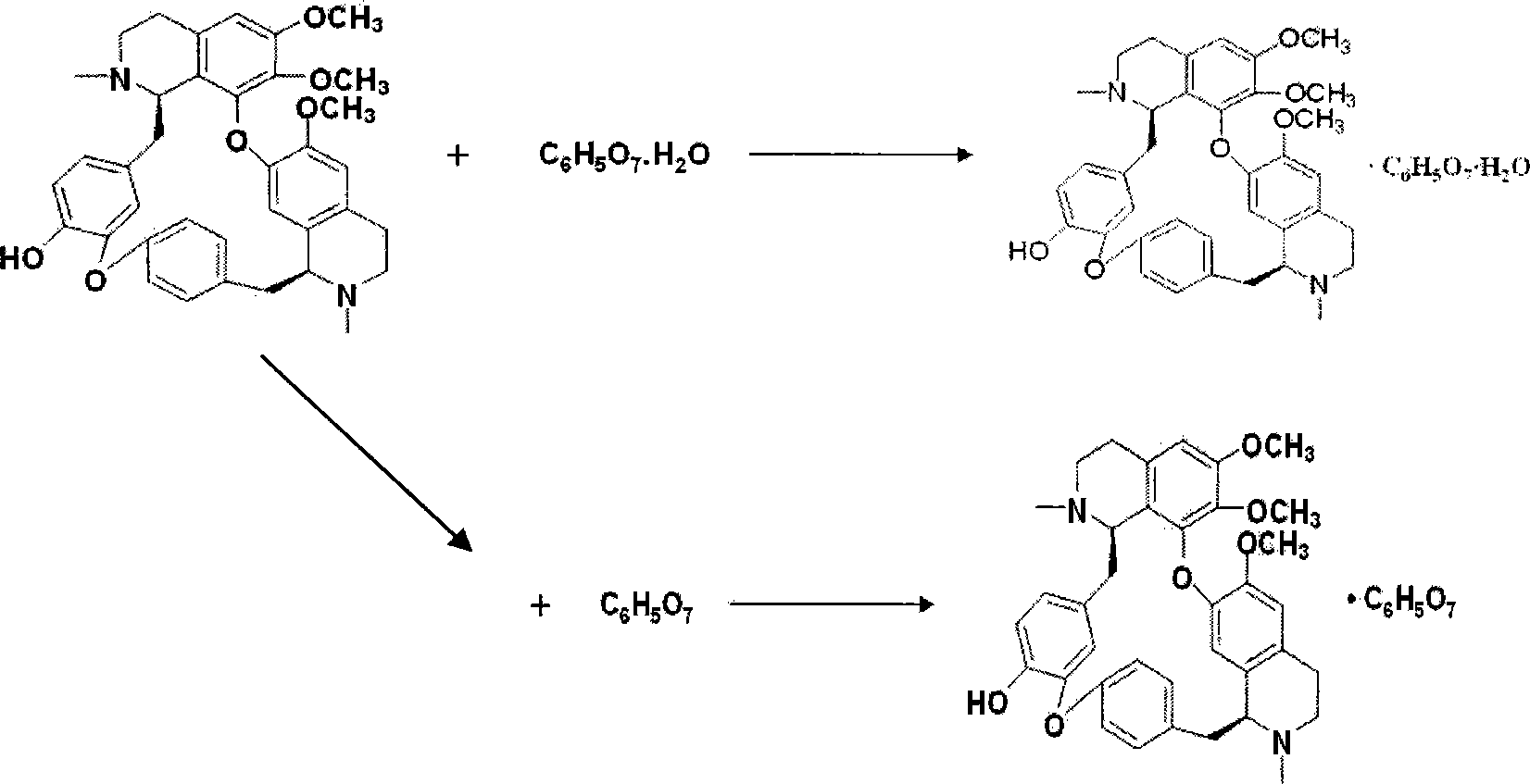
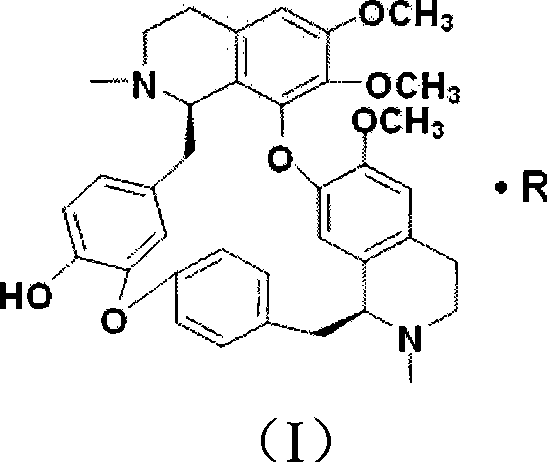
Lemon acid berbamine salt, preparation method and application thereof
InactiveCN101429201AImprove securityGood water solubilityOrganic active ingredientsOrganic chemistryDiseaseHuman leukemia
The invention provides berbamine citrate. Berbamine free alkali is taken as a raw material, and edible strong organic acid is applied to substitute the prior commonly-used inorganic acid to perform a salt-forming reaction according to certain proportion at room temperature to obtain the berbamine citrate. The berbamine citrate has remarkable killing effect on various hematological tumor cells cultured in vitro such as human leukemia, multiple myeloma, lymphoma and so on, and also has remarkable inhibition effect on the growth of human hematological tumor cells in the bodies of nude mice, but the berbamine citrate with the same concentration has no remarkable effect on the cell growth of normal persons and has no remarkable toxic side effect on the nude rice; and the berbamine citrate can form a pharmaceutical composition with other optional known anti-tumor medicines with one or more therapeutically effective amounts to achieve the synergistic effect. Therefore, the berbamine citrate can be applied to the preparation of medicines for treating hematological tumor diseases.
Owner:ZHEJIANG UNIV
Application of engineered T cell with immune receptor for treatment of cancer
InactiveCN109734814AMild release responseEnhance killing activityMammal material medical ingredientsGenetic engineeringAntigenExtracellular signal
The invention discloses application of an engineered T cell with an immune receptor for treatment of cancer. A chimeric antigen receptor consists of an extracellular signal peptide, an antigen bindingstructural domain, a first intracellular conduction structural domain and a second intracellular conduction structural domain and comprises the first intracellular conduction structural domain in theform of the immune receptor. The invention provides a plurality of amino acid sequences of the chimeric antigen receptor and provides the chimeric antigen receptor specific for CD19 malignant hematological tumors and CAR-T cells. In a blood tumor killing test, the ability of the CAR-T cells to kill tumor cells is significantly improved, and high safety and high antitumor activity are shown in clinical application.
Owner:NANJING CART MEDICAL TECH LTD
Anti-il-3ra antibody for use in treatment of blood tumor
ActiveUS20120070448A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsBULK ACTIVE INGREDIENTActive ingredient
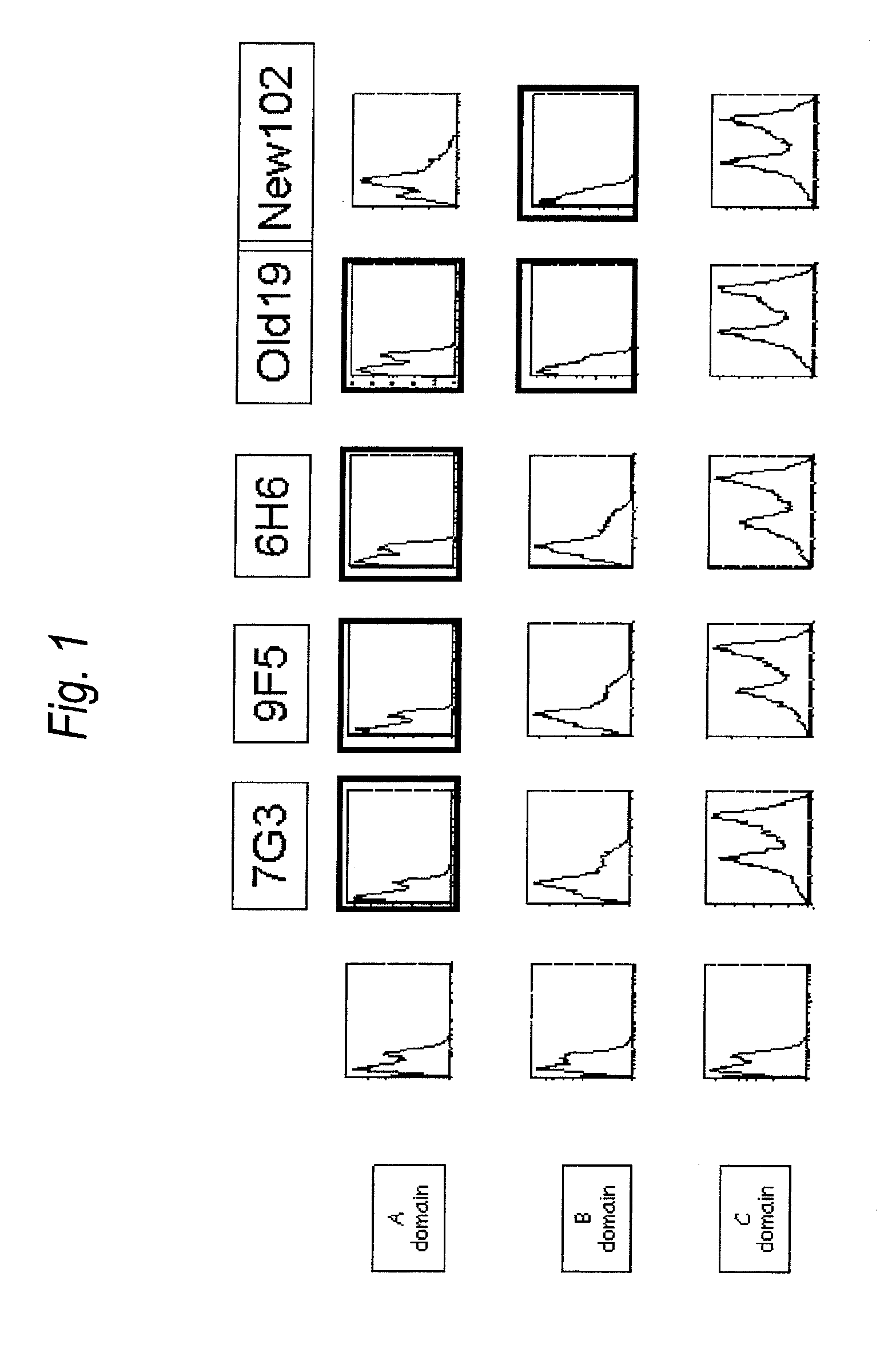
The present invention provides an antibody to human IL-3Rα chain, which does not inhibit IL-3 signaling and binds to B domain of the human IL-3Rα chain but does not bind to C domain of the human IL-3Rα chain; a composition for preventing or treating a blood tumor in which a cell expressing IL-3Rα is found in bone marrow or peripheral blood of a subject, which comprises the antibody to human IL-3Rα as an active ingredient; and a method for treating a blood tumor in which a cell expressing IL-3Rα is found in bone marrow or peripheral blood, which comprises administering, to a subject, a composition comprising the IL-3Rα antibody as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
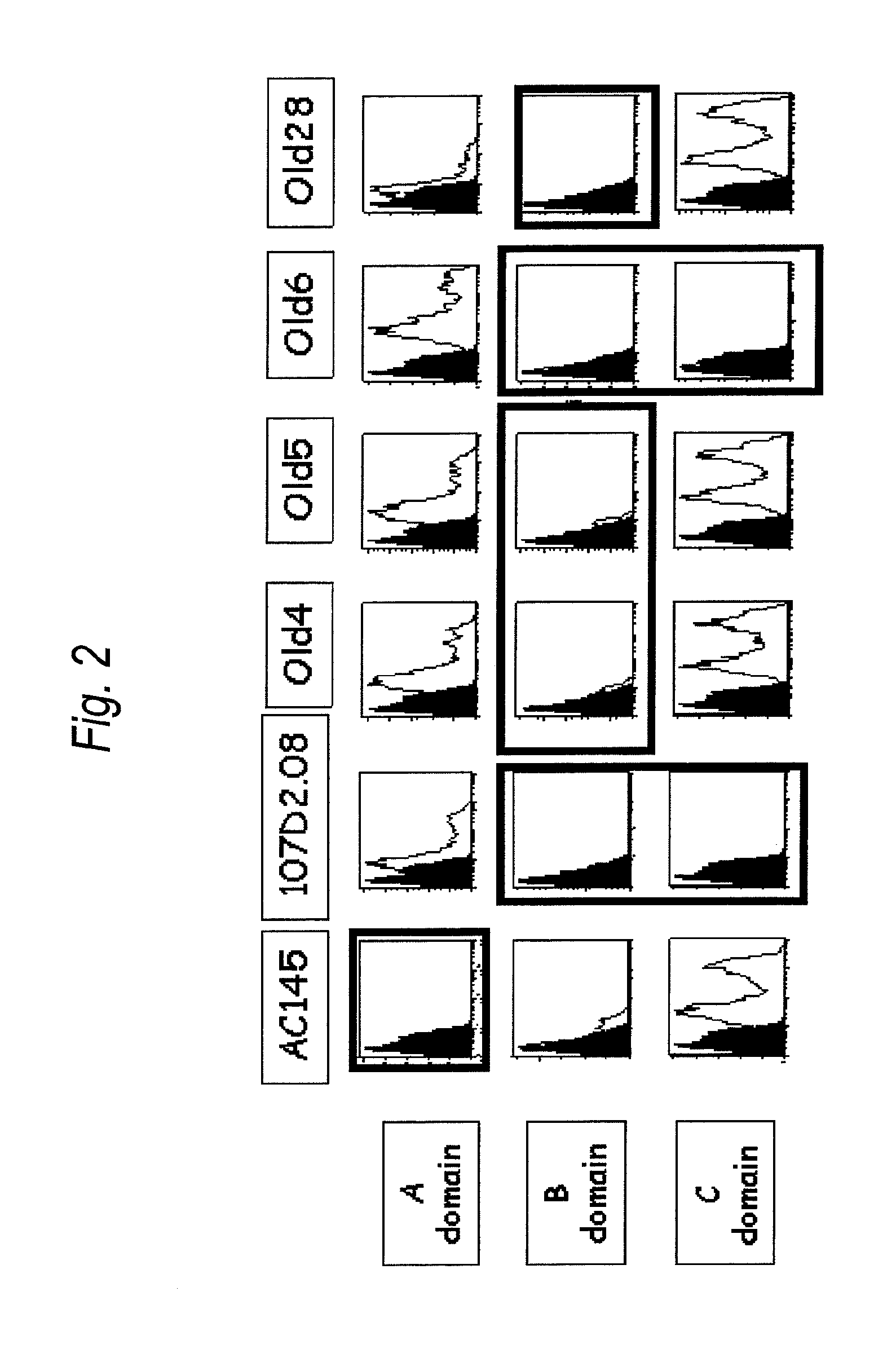
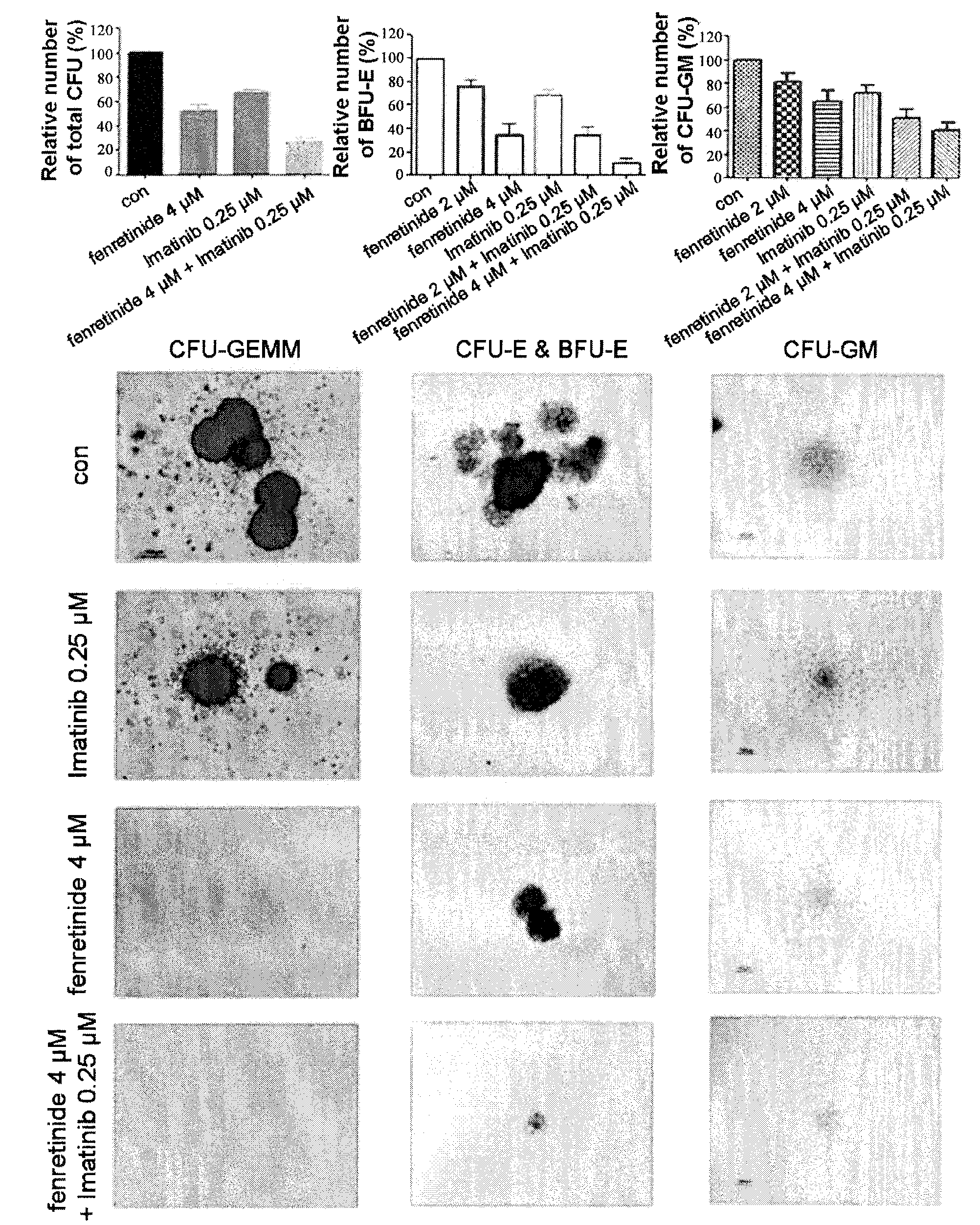
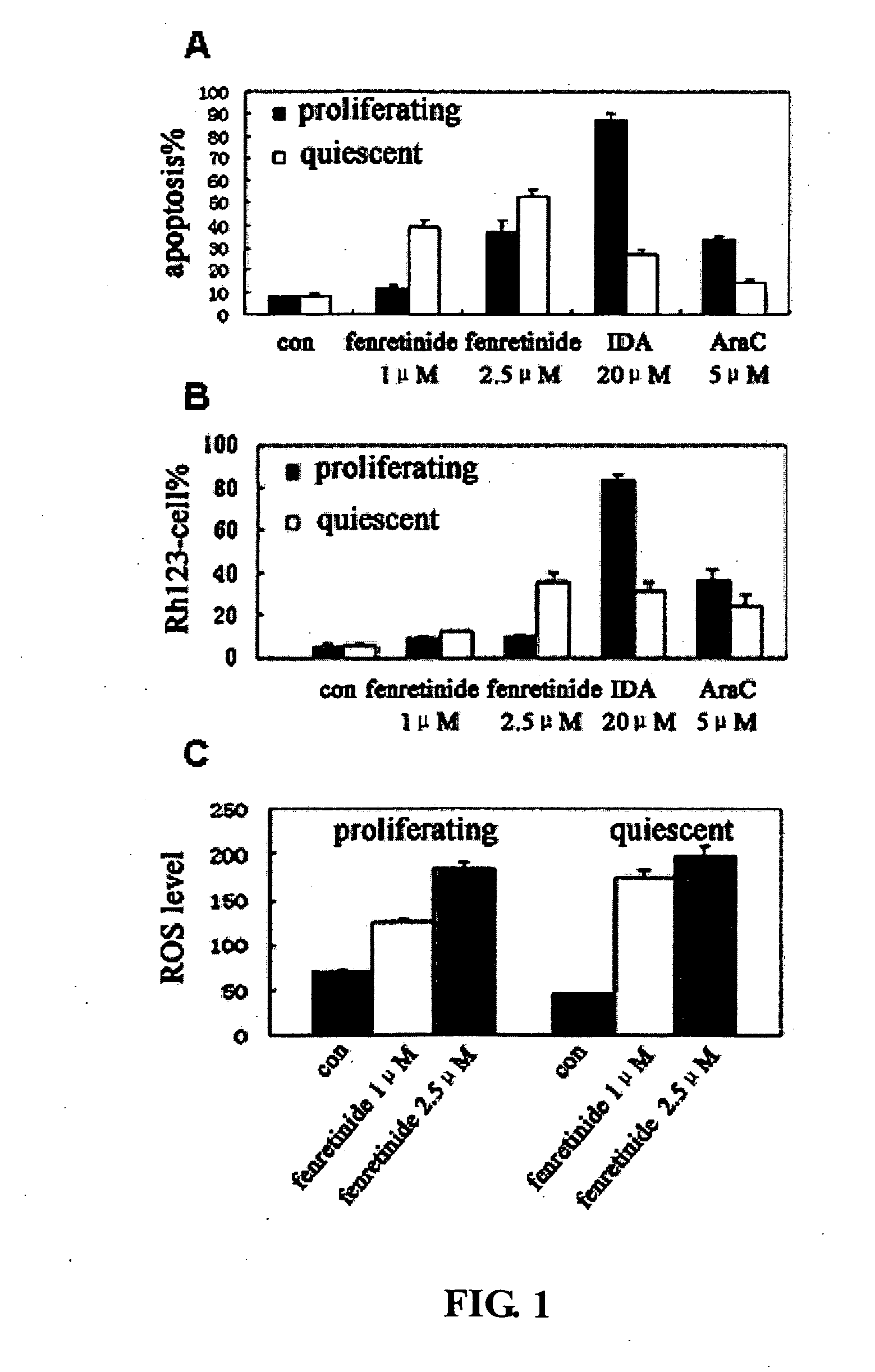
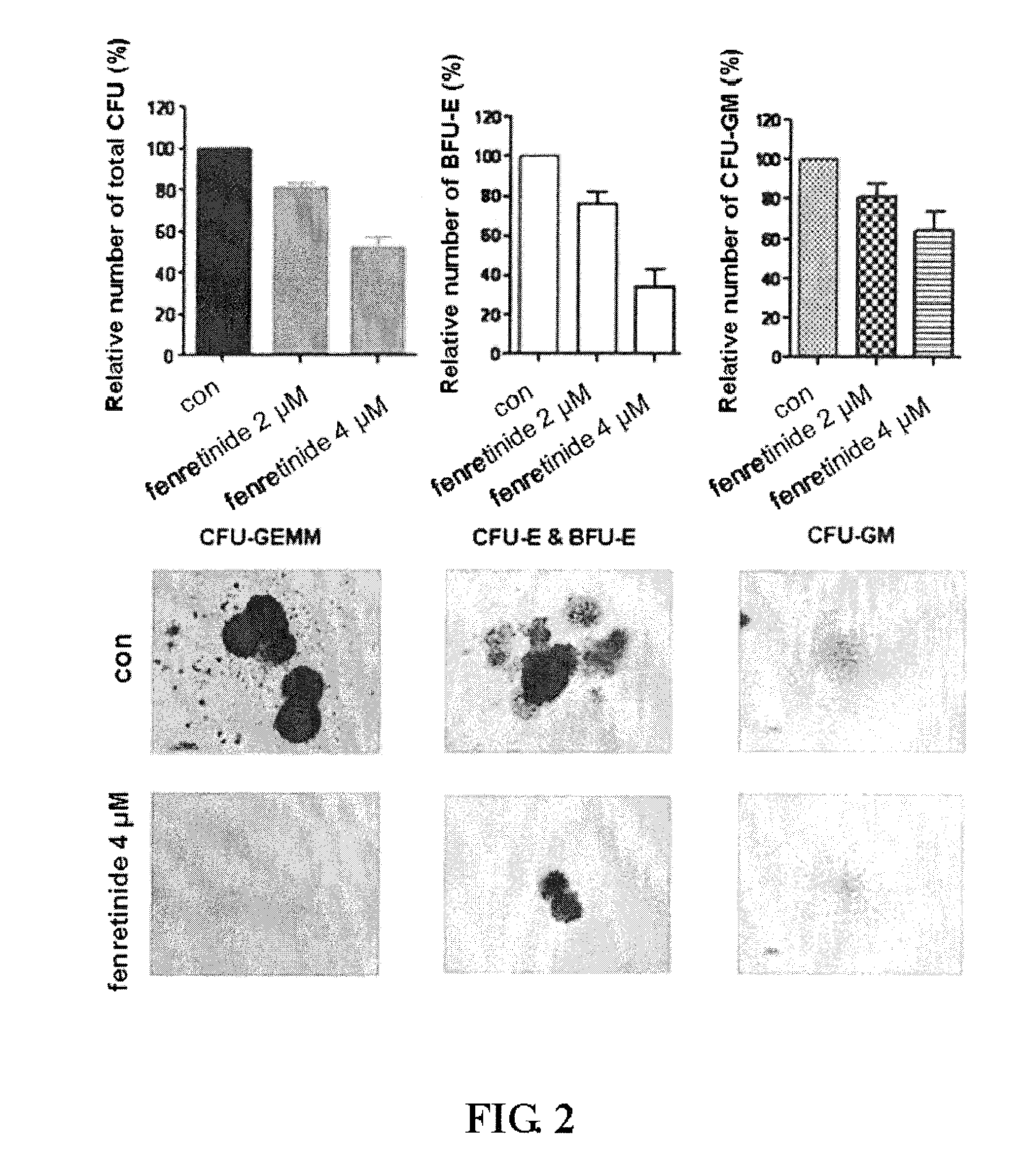
Use of Fenretinide or Bioactive Derivatives Thereof and Pharmaceutical Compositions Comprising the Same
ActiveUS20100008896A1Altering redox balanceIncreased oxygen levelsBiocideHeavy metal active ingredientsDiseaseMedicine
The present invention relates to a new medical use of fenretinide or bioactive derivatives thereof, particularly to the use of fenretinide or bioactive derivatives thereof in the preparation of a medicament for eliminating or killing tumor stem cells in a subject or for treating and / or preventing a tumor disease originating from tumor stem cells in a subject. The invention further relates to a new use of fenretinide or bioactive derivatives thereof in combination with other anti-tumor agents, a pharmaceutical composition comprising said fenretinide or bioactive derivatives thereof and at least one additional anti-tumor agent, a method of screening said other anti-tumor agent, a method of eliminating or killing tumor stem cells or particularly hematologic tumor stem cells in a subject by administrating said fenretinide or bioactive derivatives thereof, as well as a method of eliminating or killing tumor stem cells and tumor cells derived from tumor stem cells, particularly hematologic tumor stem cells and hematologic tumor cells derived from hematologic tumor stem cells in a subject by administrating said fenretinide or bioactive derivatives thereof in combination with other anti-tumor agent(s).
Owner:ZHANG JI +1
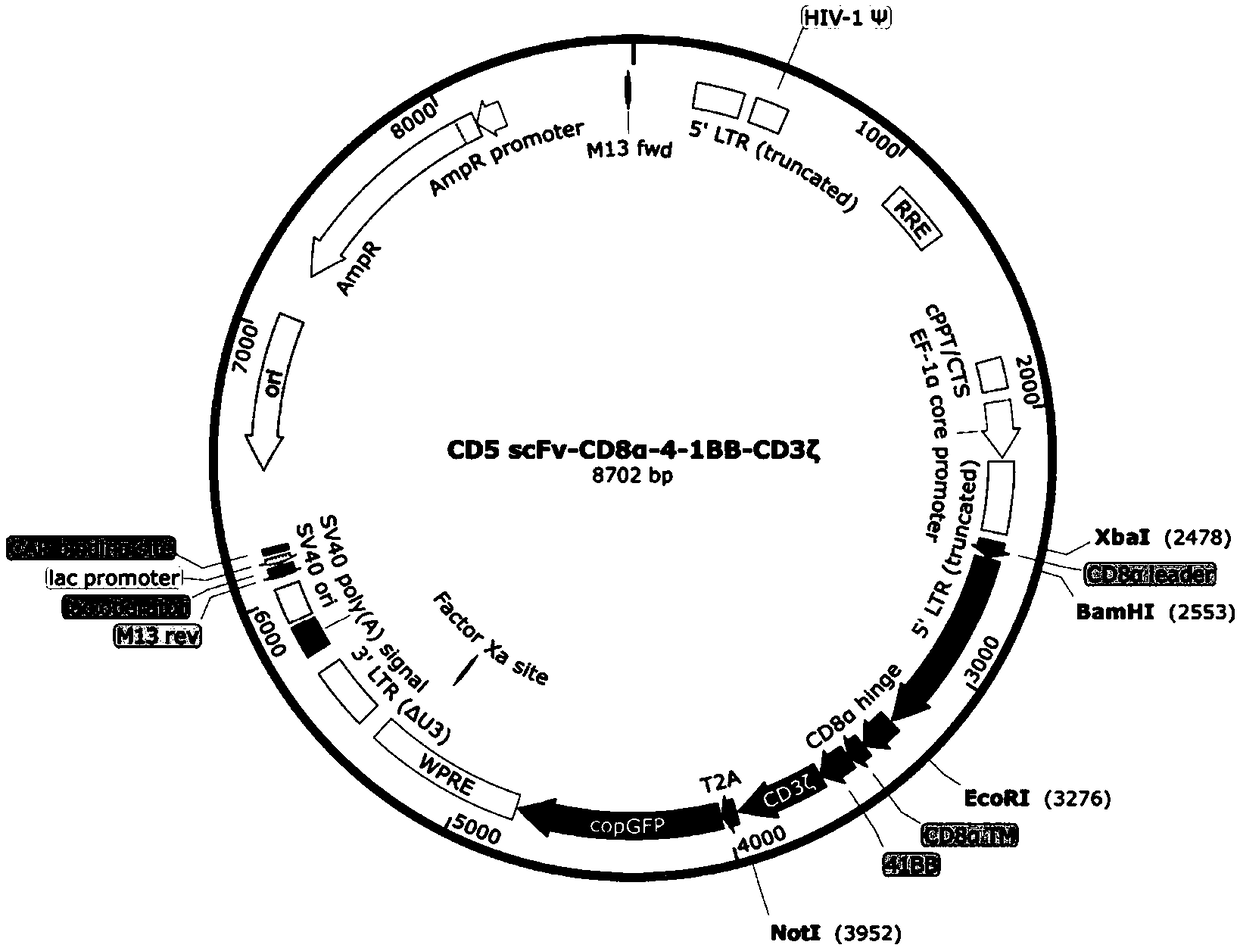
Chimeric antigen receptor targeting CD5 and application thereof
ActiveCN109266667AGood killing effectNo lethal effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD8
The present invention provides a nucleic acid molecule encoding a chimeric antigen receptor targeting CD5, the chimeric antigen receptor comprises an extracellular domain, a transmembrane domain and an intracellular signal transduction domain, and the extracellular domain encoded by the chimeric antigen receptor comprises a CD5 binding domain which is an amino acid sequence as shown in SEQ ID NO:3. The chimeric antigen receptor comprises an extracellular domain, a transmembrane domain and an intracellular signal transduction domain, and the extracellular domain encoded by the chimeric antigenreceptor comprises a CD5 binding domain which is an amino acid sequence shown in SEQ ID NO:3. The cytokines secreted by NK cells were detected by flow cytometry, degranulation assay and ELISA. The results showed that the CD5 CAR NK cells had strong cytotoxic effect on hematological tumor cells expressing CD5, but had weak cytotoxic effect on cells not expressing CD5. The cytokines secreted by CD5CAR NK cells could effectively prevent the missed target effect. A chimeric antigen receptor CD5 scFv-CD8[alpha]-4-1BB-CD 3 Xi can be used in the treatment of lymphocytic hematologic tumors with positive CD 5.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
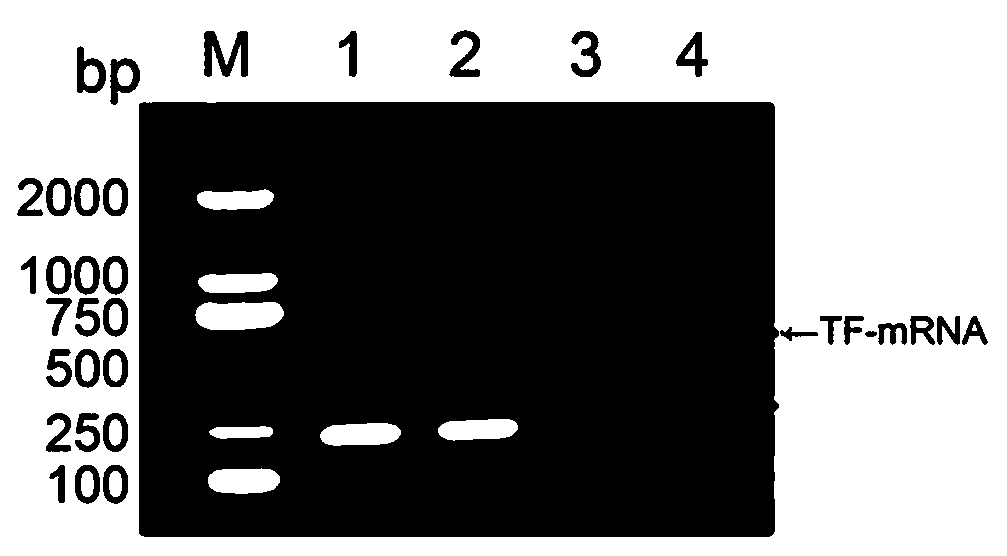
Method and Device for the in Vitro Analysis for MRNA of Genes Involved in Haematological Neoplasias
InactiveUS20120157329A1Accurate classificationSugar derivativesNucleotide librariesHematological TumorIn vitro analysis
Method and device for the in vitro analysis of mRNA of genes involved in hematological neoplasias. The device, composed of probes which specifically hybridize with genes involved in hematological neoplasias, designed so that its behaviour in the hybridization is similar, permits the evaluation of the mRNA level in biological samples taken from subjects suspected to be suffering from hematological neoplasia and facilitating the comparison between the different samples and their grouping by similarity in the gene expression patterns, especially when the probes are disposed in the form of microarray. The application of the method of the invention to obtain and process data of gene expression differences from the device of the invention permits the identification of genes significant for distinguishing samples associated to hematological neoplasias, facilitates the diagnosis of neoplasias as CLL and permits making a prognosis of the evolution thereof.
Owner:GIRALDO CASTELLANO PILAR +2
Blood-based tumor mutation burden (bTMB) biomarker, and measuring method and uses thereof
ActiveCN110643703AEffectively predict the efficacy of immunotherapyMicrobiological testing/measurementBase JHematological Tumor
The invention relates to a blood-based tumor mutation burden (bTMB) biomarker, and an measuring method and uses thereof. The bTMB biomarker is obtained from the following steps that cell-free DNA (cfDNA) is obtained from a blood sample of a subject, the number of somatic mutations on sequencing bases is measured, the number of somatic mutations of low allele abundance is taken as the bTMB biomarker and expressed as LAF-bTMB, wherein the low allele abundance means that the allele frequency is less than, for example, 25%, 24%, 23%, 22%, 21%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%,9%, 8%, 7%, 6%, 5%, 4 %, 3%, 2%, 1%, for example, between 0.3% to 25%, preferably less than, for example, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, for example, between 0.5% to 13%, andparticularly preferably less than 6.5%, 6%, 5.5%, 5%, 4.5%, 4 %, 3.5%, 3%, 2.5%, and the LAF-bTMB is calculated according to the total number of mutations in a sample measuring area.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
Hetero ring derivative
Disclosed is a novel excellent prophylactic or therapeutic method for rejections in various types of organ transplantation, allergic diseases, autoimmune diseases, hematologic tumors and others, which relies on a selective inhibitory activity on PI3Kd and / or an inhibitory activity on the production of IL-2 and / or an inhibitory activity on the proliferation of B cells (including an inhibitory activity on the activation of B cells). It is found that a tri-substituted triazine derivative or a tri-substituted pyrimidine derivative has a selective inhibitory activity on PI3Kd and / or an inhibitory activity on the production of IL-2 and / or an inhibitory activity on the proliferation of B cells (including an inhibitory activity on the activation of B cells) and can be used as a prophylactic or therapeutic agent for rejections in various types of organ transplantation, allergic diseases (e.g., asthma, atopic dermatitis), autoimmune diseases (e.g., rheumatoid arthritis, psoriasis, ulcerative colitis, Crohn diseases, systemic lupus erythematosus), hematologic tumors (e.g., leukemia) and others.
Owner:ASTELLAS PHARMA INC
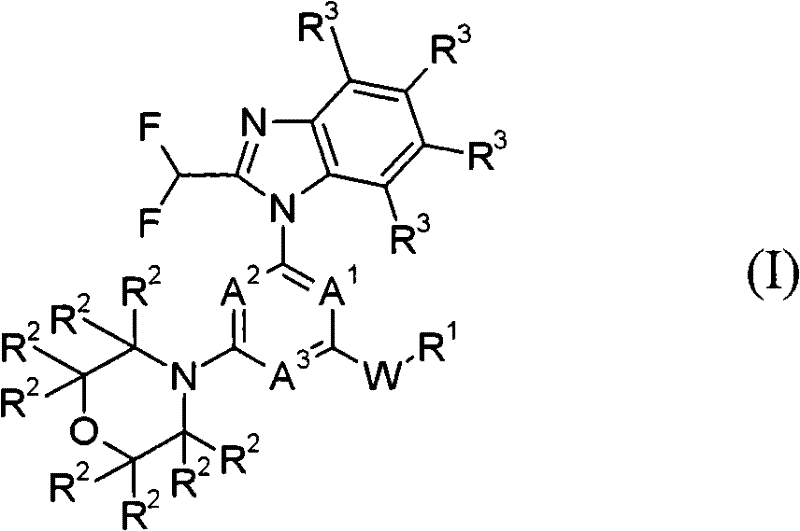
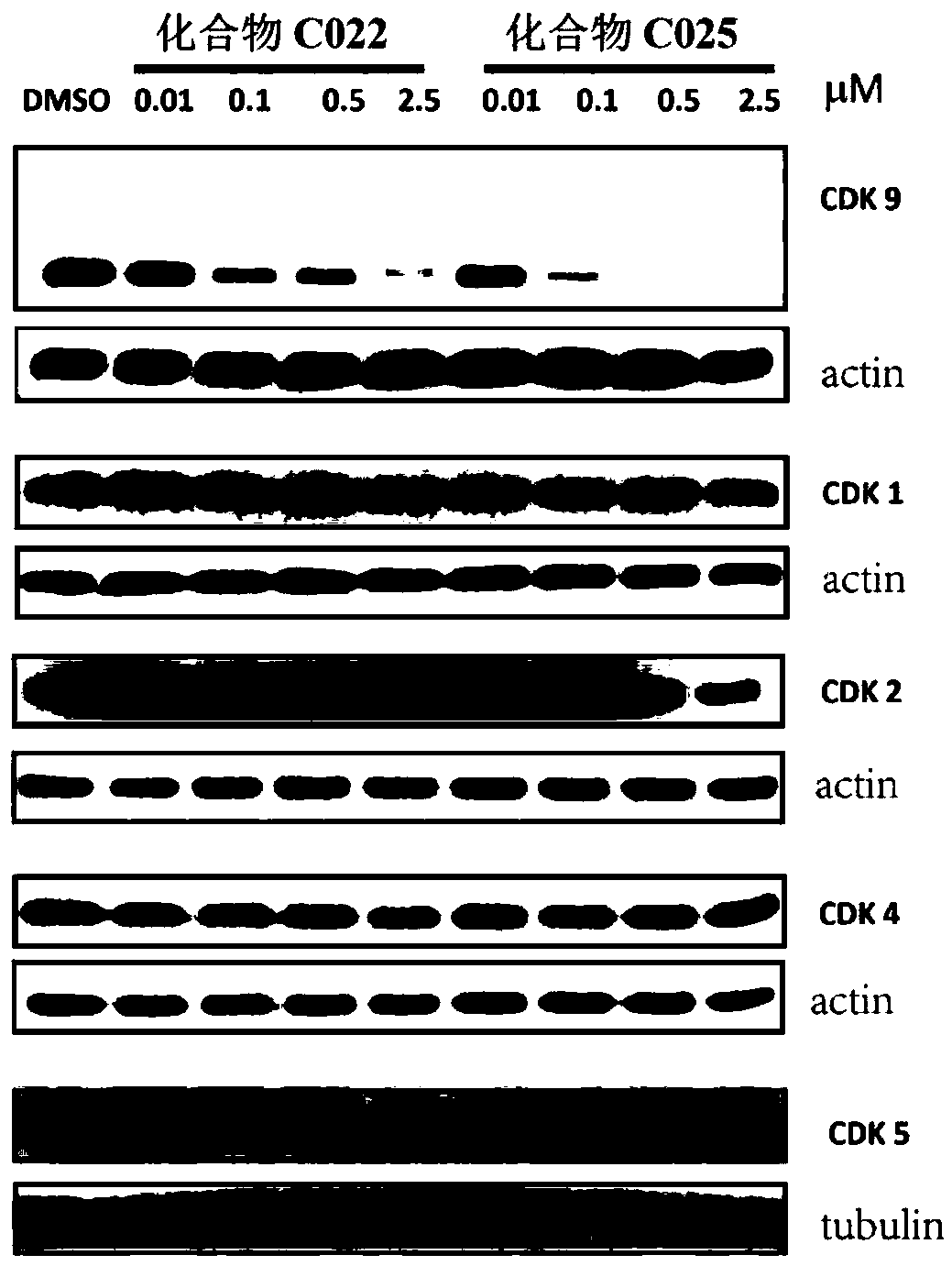
Degradation agents for cell cycle-dependent kinases, preparation method thereof, pharmaceutical compositions and use thereof
The present invention relates to cell cycle-dependent kinase (CDKs) degradation agents shown as a general formula (I), and / or pharmaceutically acceptable salts thereof, pharmaceutical compositions thereof, and use of the derivatives, as pharmaceutically active agents, in medicines for preventing and / or treating diseases related to abnormal activity of CDKs. The compounds have good cell proliferation inhibiting effect on both solid tumors and hematological tumors, indicating that the compounds have potentials to treat related cancers and autoimmune diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Bispecific chimeric antigen receptor for treating hematologic tumor complicated with HIV infection, gene thereof, and construction method and application of gene
ActiveCN111196858AAchieve the effect of treating two diseases at the same timeEasy to removeVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesAntiendomysial antibodies
The invention discloses a construction method and an application of a recombinant gene of a bispecific chimeric antigen receptor for treating HIV infection complicated with blood tumor. The chimeric antigen receptor is formed by sequentially connecting a signal peptide, an HIV gp120 antigen specific single-chain antibody and an anti-CD19 single-chain antibody and then sequentially connecting witha CD28 transmembrane region, a CD28 intracellular structural domain (ICD), a 4-1BB costimulatory structural domain and a CD3zeta intracellular signal transduction structural domain in series, or the chimeric antigen receptor comprises: first CAR composed of the signal peptide, the chimeric antigen receptor, the HIV gp120 antigen specific single-chain antibody, the CD28 transmembrane region, the CD28-ICD, the anti-CD19 single-chain antibody, the CD8 transmembrane region, the CD28-ICD, the 4-1BB costimulatory structural domain and the CD3zeta intracellular signal transduction domain; and secondCAR composed of and the signal peptide, the anti-CD19 single-chain antibody, the CD8 transmembrane region in parallel, connecting the CD28-ICD, the 4-1BB costimulatory structural domain and the CD3zeta intracellular signal transduction domain, wherein the first CAR and the second CAR are sequentially connected in parallel.
Owner:WUHAN UNIV OF SCI & TECH
Application of zkscan3 gene or protein inhibitor thereof in tumor therapy
ActiveCN109970849AImprove immunityAchieve anti-tumor effectImmunoglobulinsBlood/immune system cellsHematological TumorTumor therapy
The present invention provides an application of a zkscan3 gene or a protein modifier thereof in tumor therapy and particularly relates to a use of an inhibitor of the ZKSCAN3. The inhibitor of the ZKSCAN3 is used for preparation of a pharmaceutical composition promoting plasmocyte differentiation and proliferation, and / or treating tumors. The application provides a theoretical reference for a treatment strategy of selective knocking-out of the ZKSCAN3 in hematological tumors clinically, and has great significance for the clinical treatment of the hematological tumors targeting the ZKSCAN3.
Owner:PERSONGEN ANKE CELLULAR THERAPEUTICS CO LTD
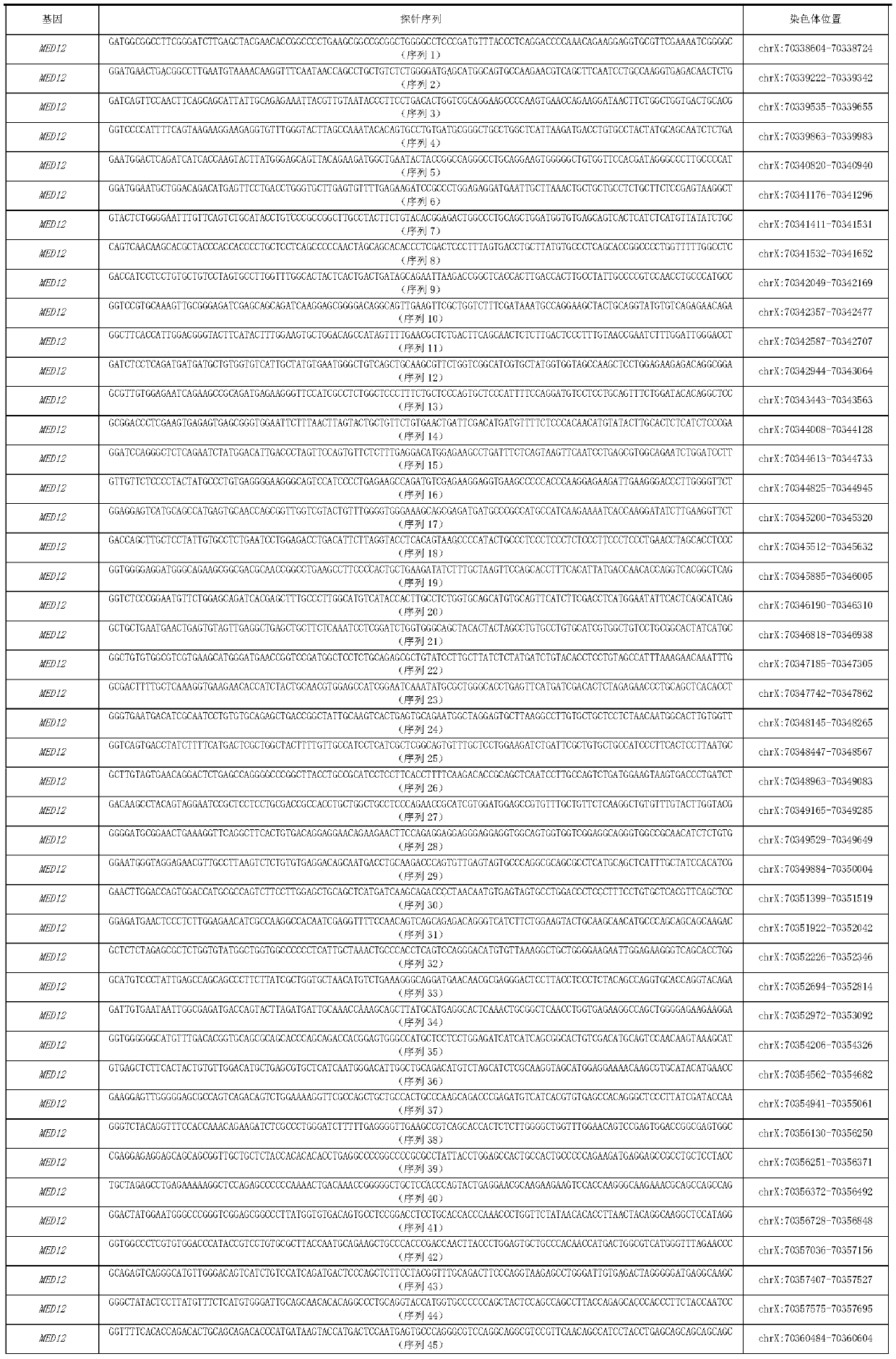
MED12 gene mutation detecting kit and application thereof
ActiveCN110229897AThe detection method is simpleSensitive detection methodMicrobiological testing/measurementDNA/RNA fragmentationMRD NegativeHematological Tumor
The present invention discloses a MED12 gene mutation detecting kit and an application thereof. The kit comprises probe sets for detecting MED12 gene mutations and the probe sets consist of a probe 1to a probe 48. The kit can be used for detecting MED12 somatic mutations and germline mutations at a tumor genome level, can also be used for assistant diagnosis and minimal residual disease monitoring and prognosis evaluation, etc. of patients with hematological tumors, especially patients with acute myeloid leukemia. Besides, the kit is simple, sensitive and accurate in detection method and plays an important role in the field of medical detection
Owner:PEOPLES HOSPITAL PEKING UNIV
Novel uses of PPAR modulators and professional APCs manipulated by the same
InactiveUS20060159669A1Ameliorate any conditionAltered phenotypeBiocideGenetic material ingredientsAntigenAutoimmune condition
The invention relates to a manipulated professional antigen presenting cell (APC) having increased expression of a CD1 type II molecule, preferably at least a CD1d molecule, relative to a control non manipulated cell. The invention further relates to the use of PPARg modulators in the preparation of a pharmaceutical composition or kit for the treatment of a disease treatable by activation of CD1d restricted T-cells, e.g. in autoimmune diseases, allergies, post-transplant conditions or infectious diseases, or in the treatment of a neoplastic disease, e.g. skin cancer, hematological tumors, colorectal carcinoma, and therapeutic compositions and kits therefor. Furthermore, the invention relates to methods of manipulating professional APCs and kits therefor.
Owner:UNIVERSITY OF DEBRECEN
Abdominal cavity drainage tube indwelling equipment for hematologic tumor caring
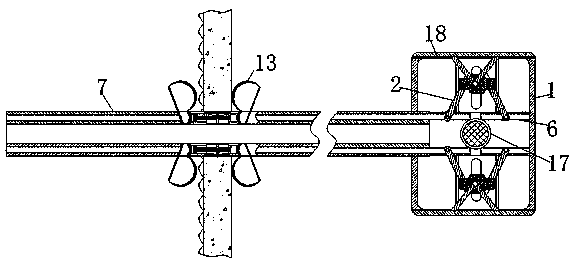
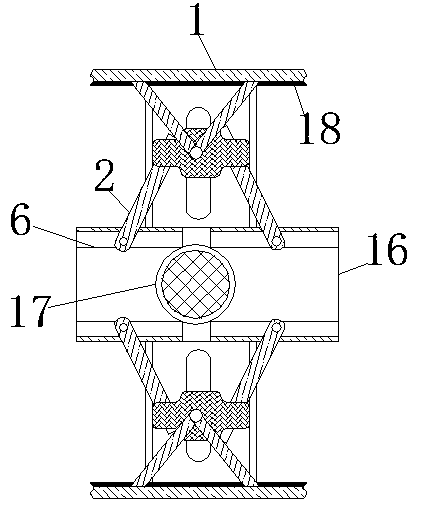
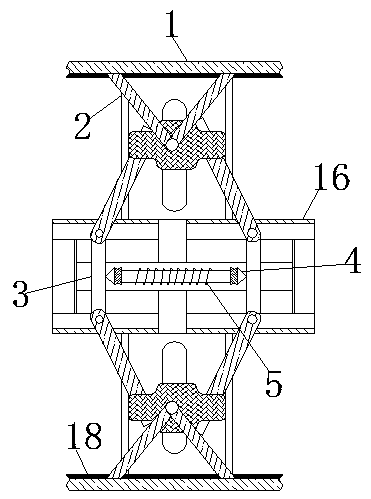
InactiveCN110743087AThe deformation volume is not largeTo achieve the effect of internal and external clampingBalloon catheterMedical devicesHematological TumorAbdominal cavity
The invention relates to the technical field of abdominal cavity drainage tube indwelling, and discloses abdominal cavity drainage tube indwelling equipment for hematologic tumor caring. The abdominalcavity drainage tube indwelling equipment comprises a box body, clamping pieces are movably connected to the two side walls in the box body correspondingly, pushing rods are slidably connected to theend, away from the box body, of the clamping pieces, piston blocks are slidably connected to the surfaces of the pushing rods, spring rods are movably connected to the ends, away from the pushing rods, of the piston blocks, an airflow groove is formed in the box body, and a drainage tube is fixedly connected to the end, away from the box body, of the airflow groove. When liquid flow exists on thesurface of a load bearing net, the generated pressure is the same as elastic force for overcoming shrinkage of the spring rods; when the drainage tube is blocked, the liquid flow cannot reach the surface of the load bearing net, thus the load bearing net does not provide force for overcoming shrinkage of the spring rods any more, the spring rods pull the piston blocks to shrink inwards, an air block belt is attracted to move under clamping of a limiting piece in the direction away from a fixing bag, released gas directly enters the drainage tube, and thus the effect that dredging can be conducted immediately when blockage occurs is achieved.
Owner:JILIN UNIV
Antibody composition for detecting minimal residual lesions of multiple myeloma, kit and application
InactiveCN111912983ACombination worksThe combination is accurateDisease diagnosisBiological testingTreatment effectConventional chemotherapy
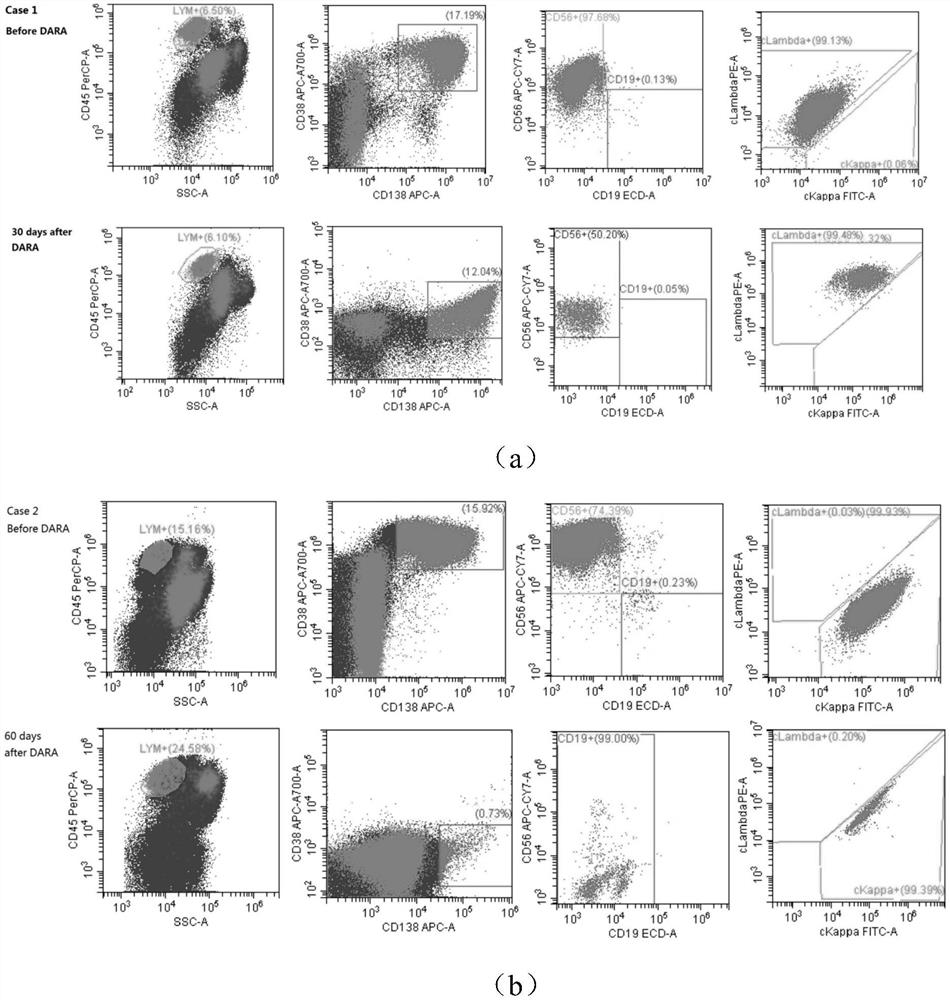
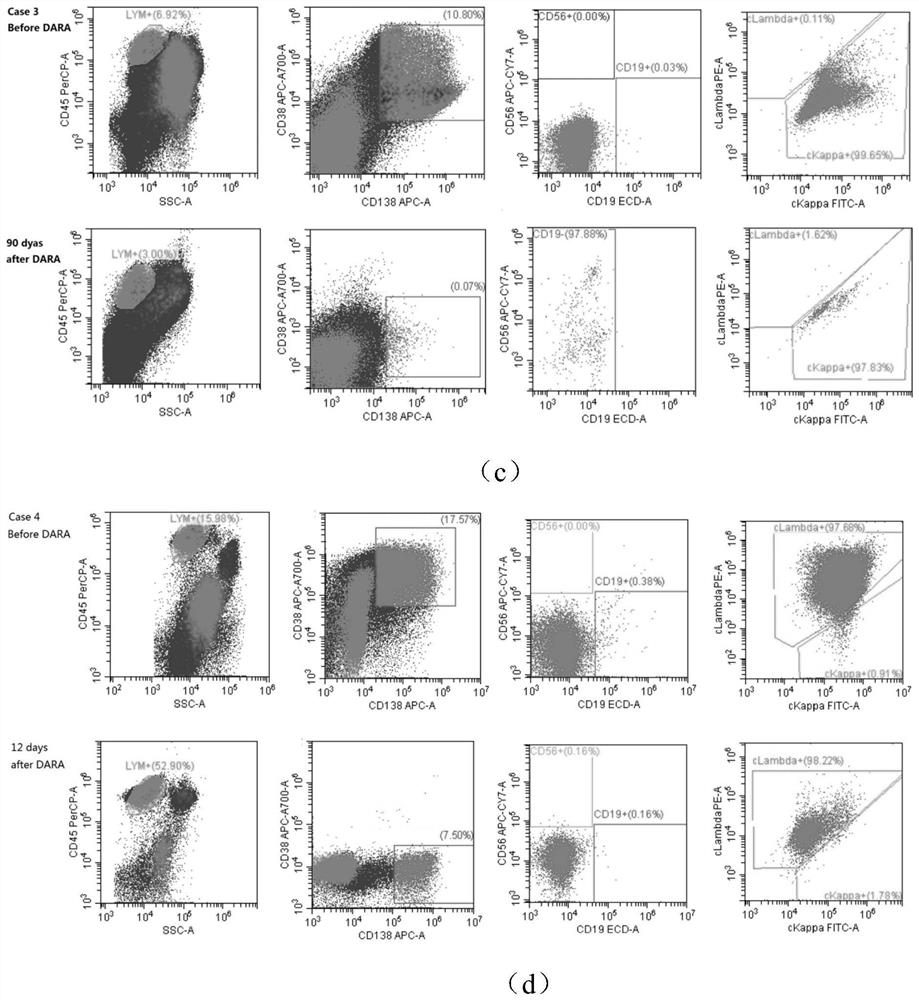
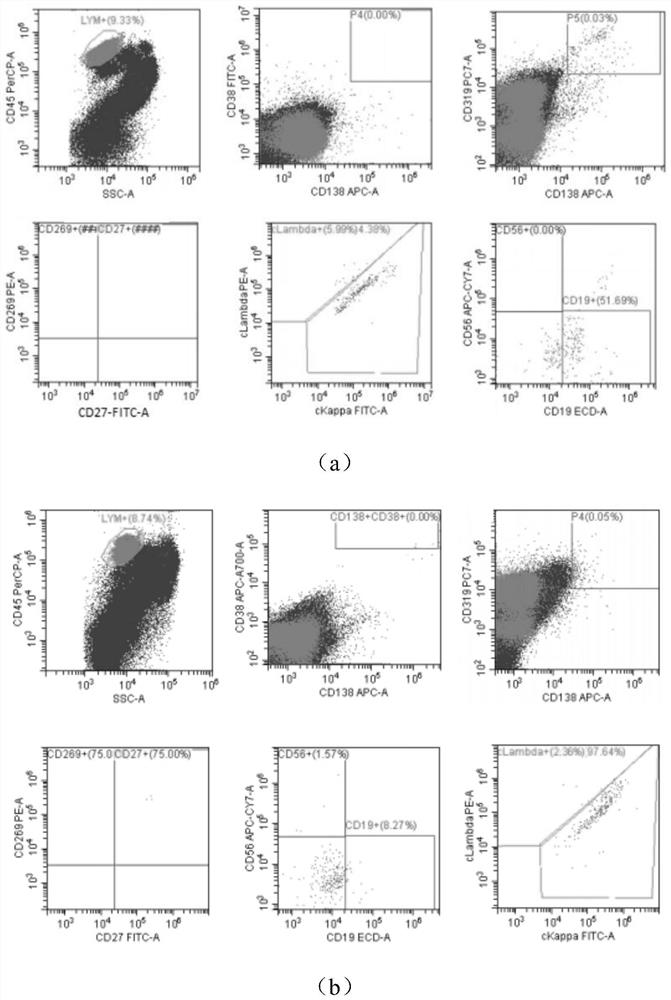
The invention discloses an antibody composition for detecting multiple myeloma minimal residual diseases, a kit and application, and belongs to the technical field of blood tumor treatment effect monitoring. According to the novel flow antibody combination scheme capable of being used for MRD monitoring of myeloma patients disclosed by the invention, CD319 or CD269 and other seven antibodies (CD138 / CD38 / CD184 / CD27 / CD19 / CD56 / ckappa / clamda) are combined to form a fixed combination of two groups of antibodies; through verification of 303 cases of myeloma patients, the antibody combination is feasible, accurate, convenient and fast, can be used for MRD monitoring after conventional chemotherapeutic drug treatment, and provides a good solution for the problem that MRD monitoring is difficult after DARA treatment.
Owner:BEIJING JISHUITAN HOSPITAL
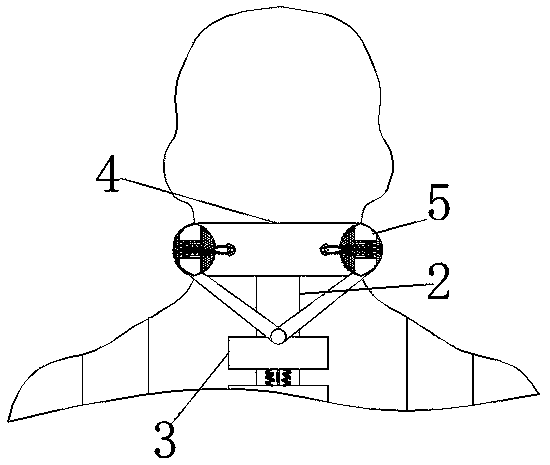
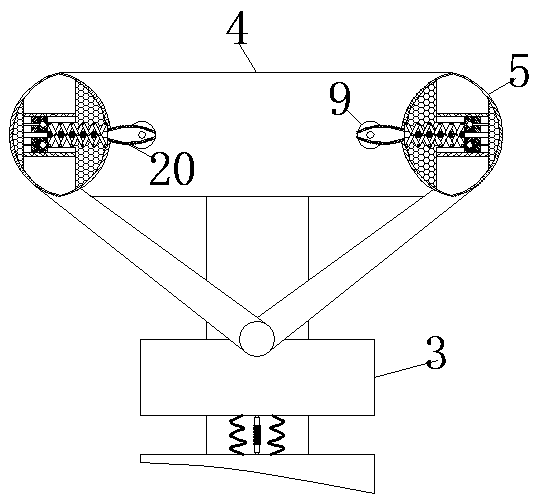
Cerebrospinal fluid extraction device for hematologic tumor patients
ActiveCN110742655AAccurate puncture positioningSurgical needlesVaccination/ovulation diagnosticsHematological TumorMechanical engineering
The invention relates to the technical field of cerebrospinal fluid extraction for hematologic tumor patients, and discloses a cerebrospinal fluid extraction device for hematologic tumor patients. Thedevice comprises a strap, a flexible strap is movably connected to the surface of the strap, a positioning block is movably connected to the surface of the flexible strap, a clamping box is movably connected to the end, away from the strap, of the flexible strap through a connecting rod, a clamping ring is movably connected to the interior of the clamping box, an air bag is fixedly connected to the interior of the clamping ring, and a vent pipe is fixedly connected to the surface of the air bag. The step of moving an exhaust plate to a placing table is perforation positioning coarse adjustment, and a corrugated pipe is extruded to generate gas. As the placing table is only fixedly connected to the fourth from last positioning block, only the gas generated by the corrugated pipe connectedto the placing table can enter the exhaust plate through a telescopic rod. The gas flows to a chute to push out a push rod to push the placing table to move. In addition, perforation positioning fineadjustment and two-step adjustment are combined, so that the effect of improving puncture positioning accuracy is achieved.
Owner:JILIN UNIV
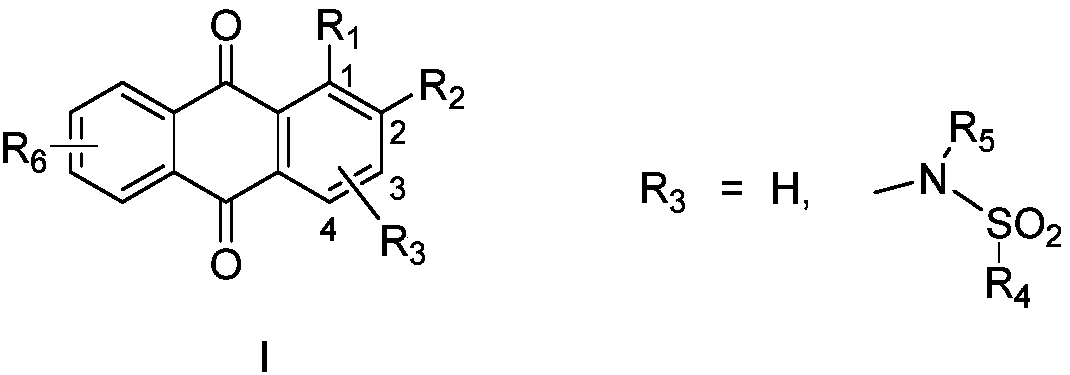
9,10-anthraquinone compound, pharmaceutically-acceptable salts and pharmaceutical application thereof
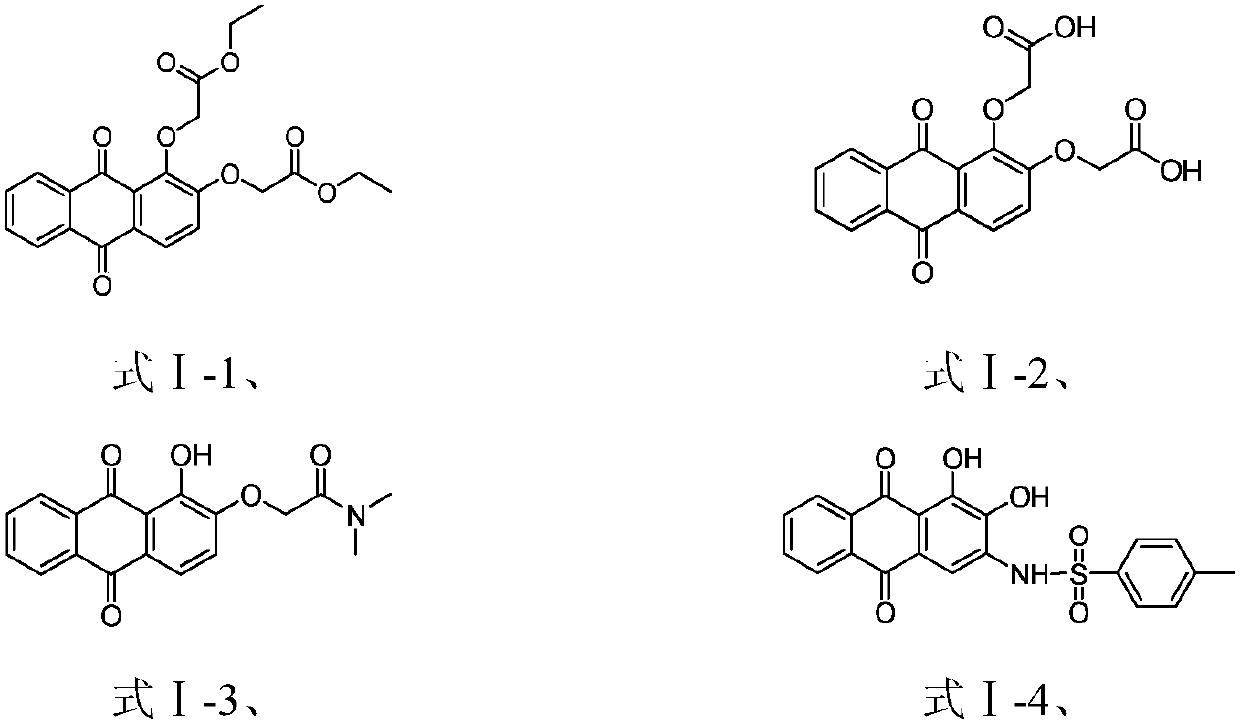
ActiveCN110317137ANovel structureMolecular inhibitory activityOrganic chemistryAntineoplastic agentsMelanomaPhosphoglycerate mutase activity
The invention belongs to the field of pharmaceutical chemistry and relates to a 9,10-anthraquinone compound and a pharmaceutical application thereof, in particular to the 9,10-anthraquinone compound and the application thereof in the preparation of a phosphoglycerate mutase inhibitor and in the preparation of drugs for treating cancer. In particular, the invention discloses the application of the9,10-anthraquinone compound shown in the structure of a formula I, pharmaceutically-acceptable salts thereof, or a pharmaceutical composition taking the 9,10-anthraquinone compound as an effective active ingredient in preparing drugs for preventing and treating tumors. The compound can inhibit phosphoglycerate mutase activity and reduce cell metabolism level, and can be used for treating diseasessuch as solid tumors and blood tumors, and the tumors involved are pancreatic cancer, lung cancer, liver cancer, gastric cancer, esophageal cancer, intestinal cancer, breast cancer, cervical cancer, leukemia and melanoma.
Owner:FUDAN UNIV
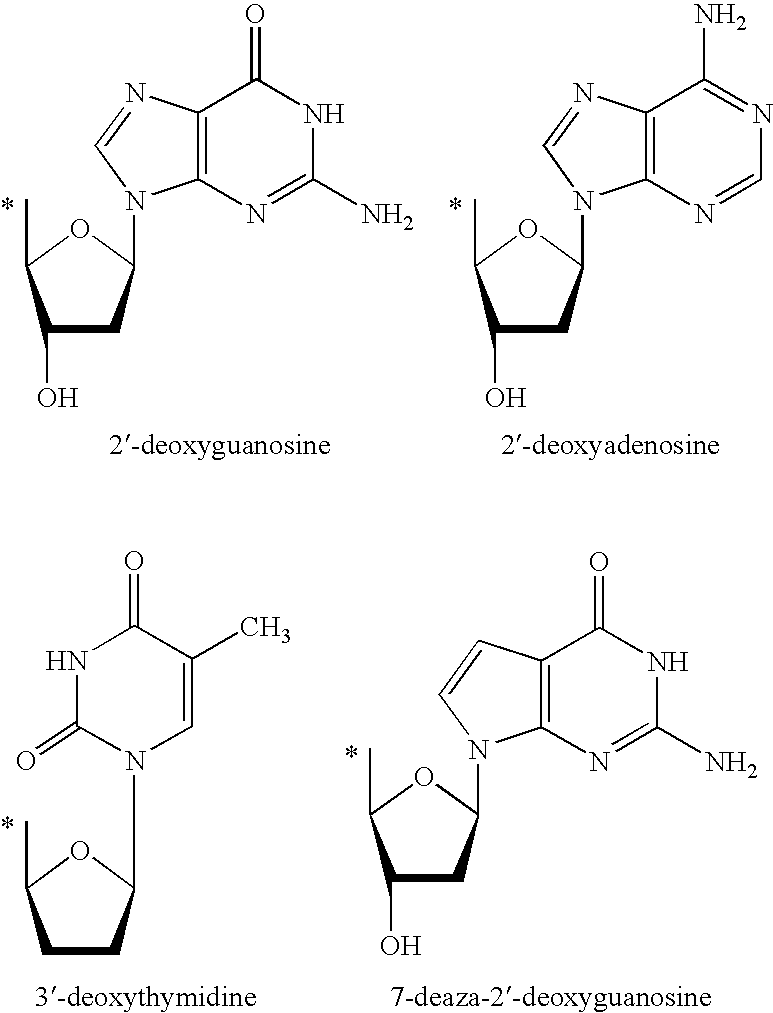
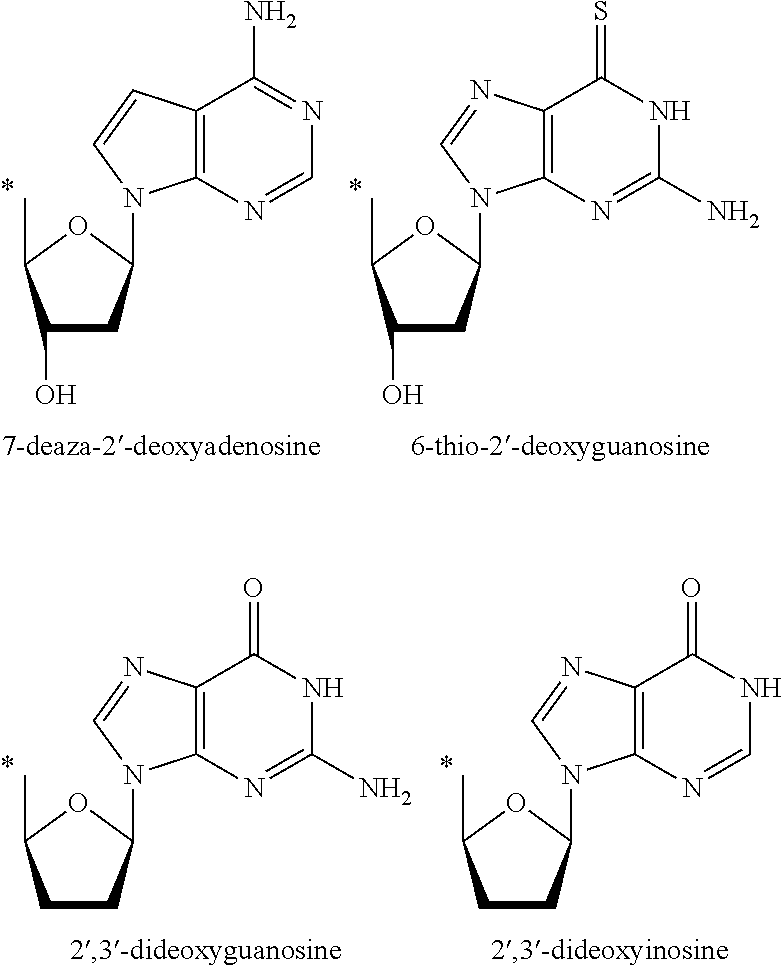
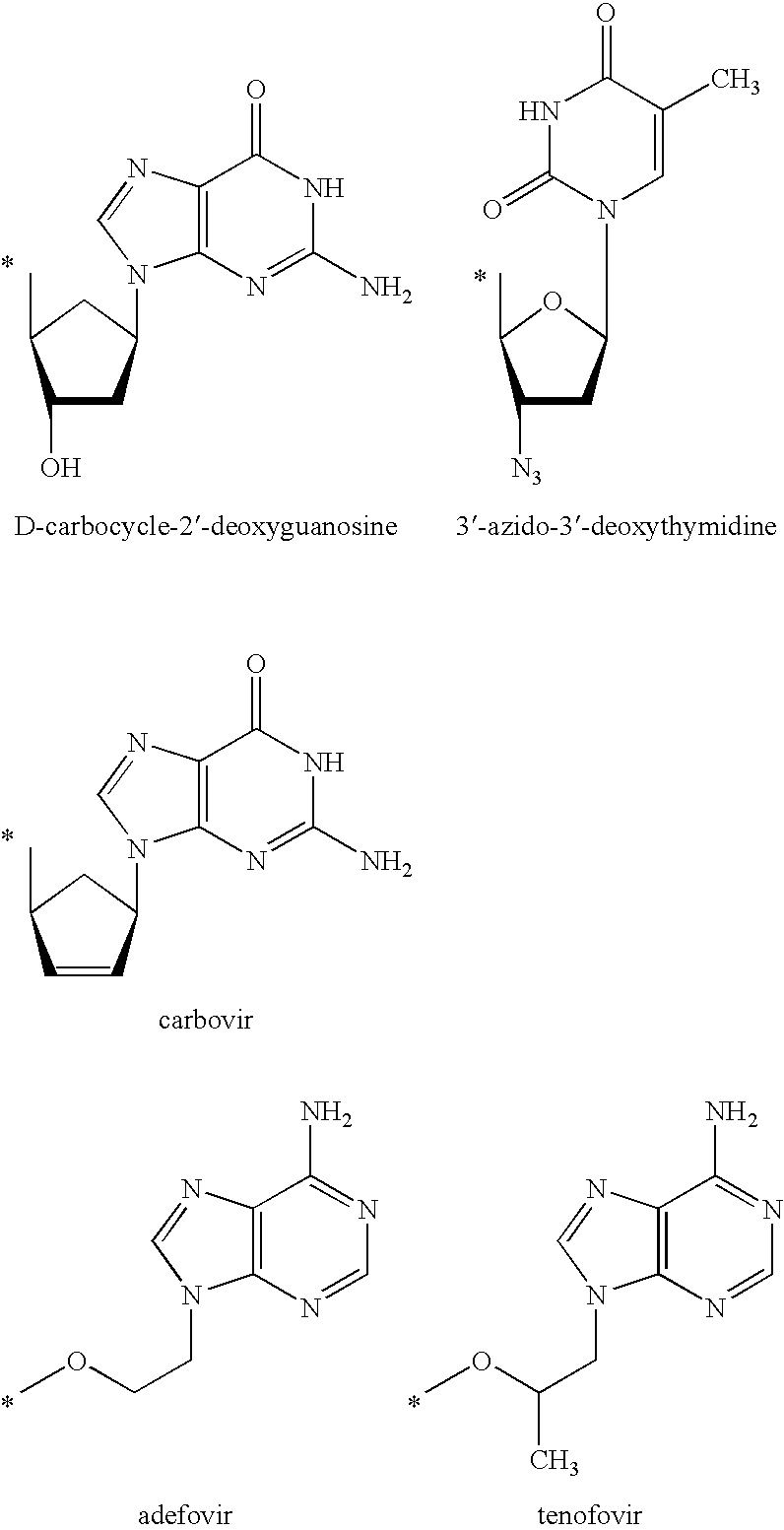
Prodrugs activated by RNA-dependent DNA-polymerases
The invention provides prodrugs activated by RNA-dependent DNA-polymerases, and methods for treating hematological tumors, blood, and blood derivatives from patients affected by retroviral infections by administering the prodrugs. The invention also provides methods for the preparation of pharmaceutical compositions containing the prodrugs.
Owner:PROTERA SRL
Therapeutic and diagnostic methods for cancer
The present invention provides therapeutic, diagnostic, and prognostic methods for cancer. The invention provides methods of treating a cancer, methods of determining whether an individual having a cancer is likely to respond to a treatment including an immune checkpoint inhibitor (e.g., a PD-L1 axis binding antagonist), methods of predicting responsiveness of an individual having a cancer to a treatment including an immune checkpoint inhibitor (e.g., a PD-L1 axis binding antagonist), methods of selecting a therapy for an individual having a cancer, methods of providing a prognosis for an individual having a cancer, and methods of monitoring a response of an individual having a cancer, based on a blood tumor mutational burden (bTMB) score or a maximum somatic allele frequency (MSAF) from asample (e.g., a whole blood sample, a plasma sample, a serum sample, or a combination thereof) from the individual.
Owner:GENENTECH INC +1
Application of platelet microparticles in elevated expression of cell tissue factors
InactiveCN110068682AHigh specificityStrong specificityPeptide/protein ingredientsIndividual particle analysisDrugLeukaemia cell
The application of platelet microparticles in complications of elevated expression of leukemia cell tissue factors belongs to the field of biomedicine. In particular, the present invention discloses an application of platelet microparticles in preparation of agents for diagnosing or predicting elevated expression of cell tissue factors in a leukemia patient, and an application of an annexin V in preparation or screening of drugs for preventing or treating complications caused by elevated expression of cell tissue factors in a leukemia patient. The invention has an important value for assessment and timely treatment of malignant tumor patient patients, in particular to hematologic tumor patients in critical condition when applied in clinical practices.
Owner:SHENZHEN HUISONG TECH DEV
Peripheral blood miRNA colon cancer diagnostic marker combination and detection kit thereof
ActiveCN112609002AStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseCancers diagnosis
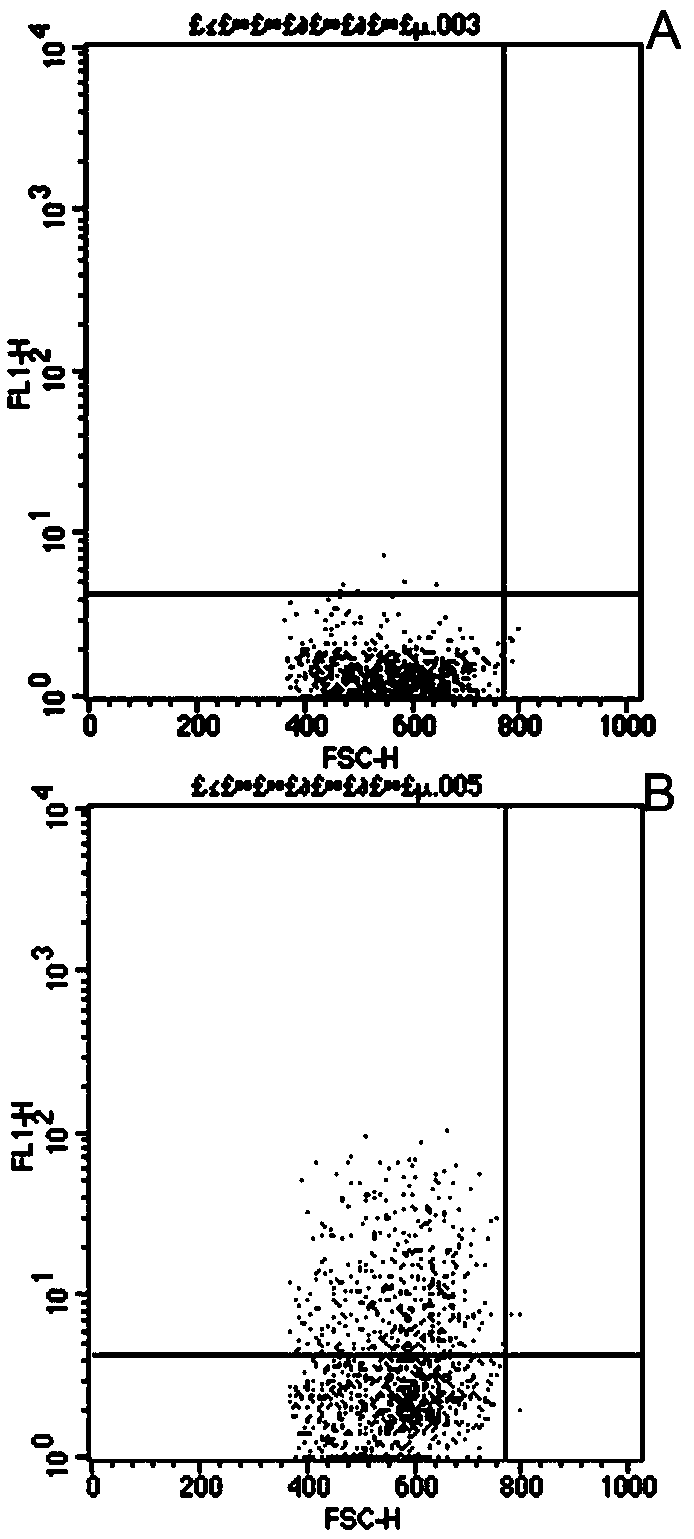
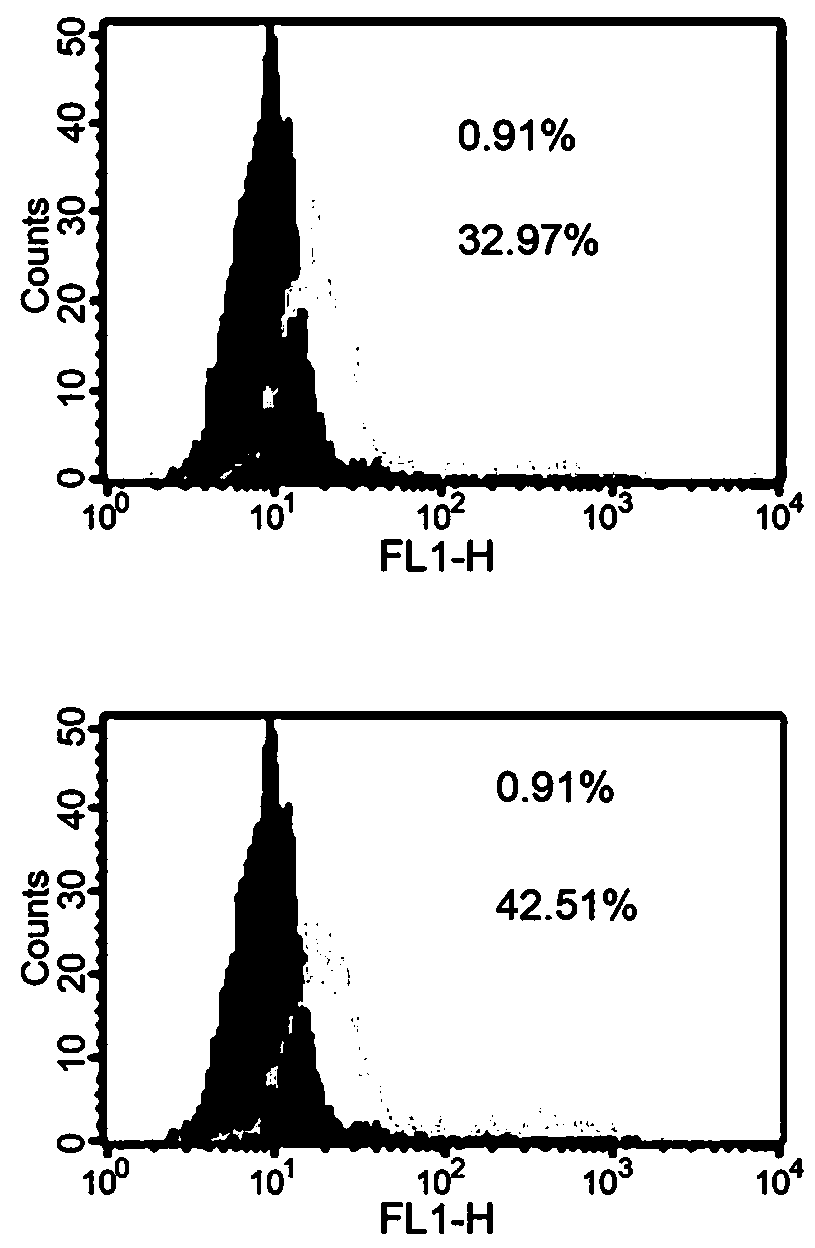
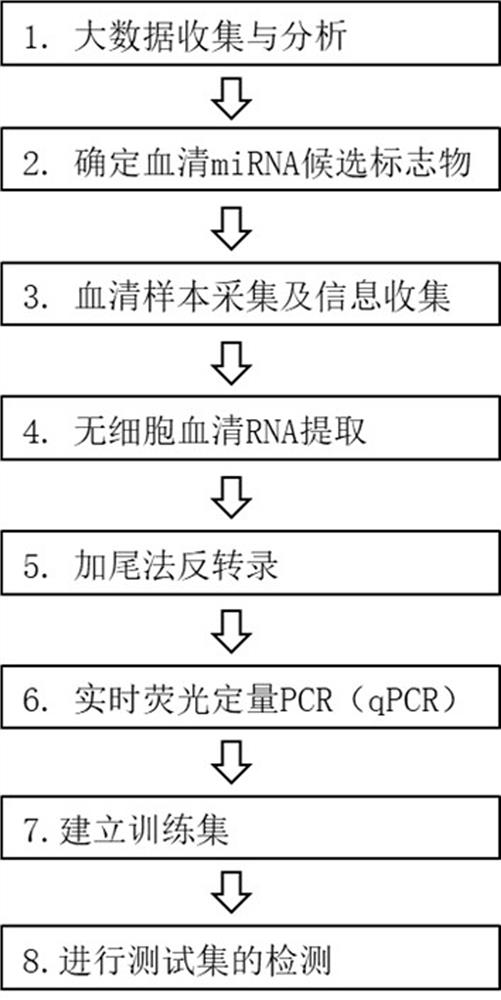
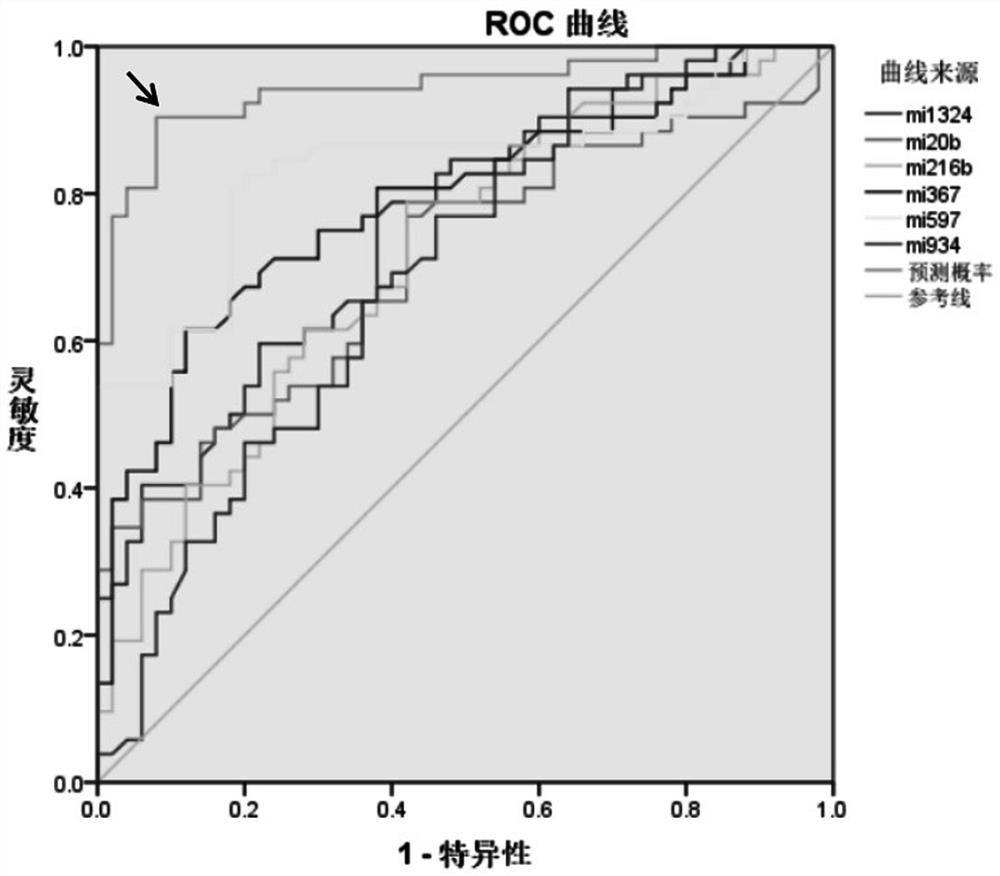
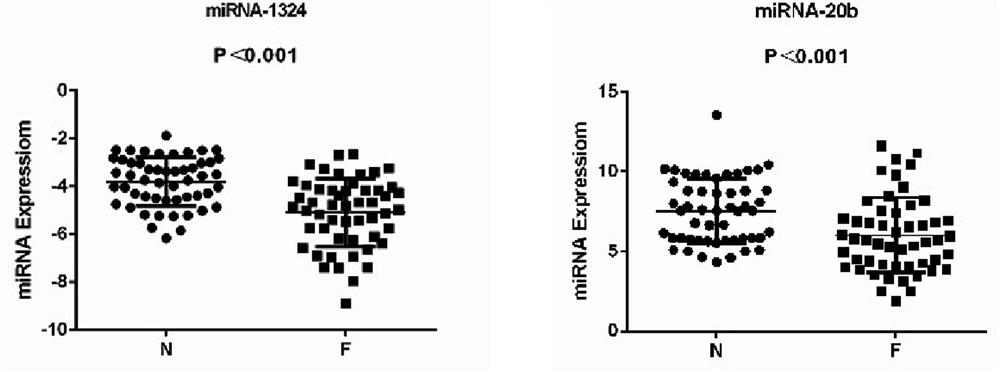
The invention relates to the field of biomedical technologies and molecular biology, in particular to a peripheral blood miRNA marker combination and kit for diagnosing colon cancer and application of the peripheral blood miRNA marker combination and kit. The invention discloses a peripheral blood miRNA marker combination for colon cancer diagnosis. The miRNA marker combination comprises one or a combination of at least two of the following miRNAs: hsa-miR-1324, hsa-miR-20b-5p, hsa-miR-216b-5p, hsa-miR-367-5p, hsa-miR-597-5p and hsa-miR-934. The peripheral blood miRNA marker combination for colon cancer diagnosis is applied to preparation of a colon cancer detection reagent or tool. The peripheral blood miRNA tumor marker detection provided by the invention belongs to the field of liquid biopsy, has the characteristics of high specificity, earlier discovery, high sensitivity and minimal invasion compared with clinically conventional blood tumor markers, and is a novel marker for diagnosing various diseases including tumors. The detection result of the training set established by the invention on the test set shows that the diagnosis efficiency is as high as 95%, and the method has important significance for clinical colon cancer diagnosis in the future.
Owner:中国医科大学
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com