Human interleukin 2-polyethylene glycol conjugate as well as preparation method and application thereof
A technology of interleukin and polyethylene glycol, which is applied in the field of human interleukin 2-polyethylene glycol conjugates and its preparation, can solve the problems of weakened binding ability, complicated mechanism of action, and complex molecular structure, and achieve The effect of reducing immunogenicity, reducing affinity, and high product uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Construction of expression strains expressing recombinant human IL-2 (rhIL-2) with site-specific insertion of unnatural amino acids
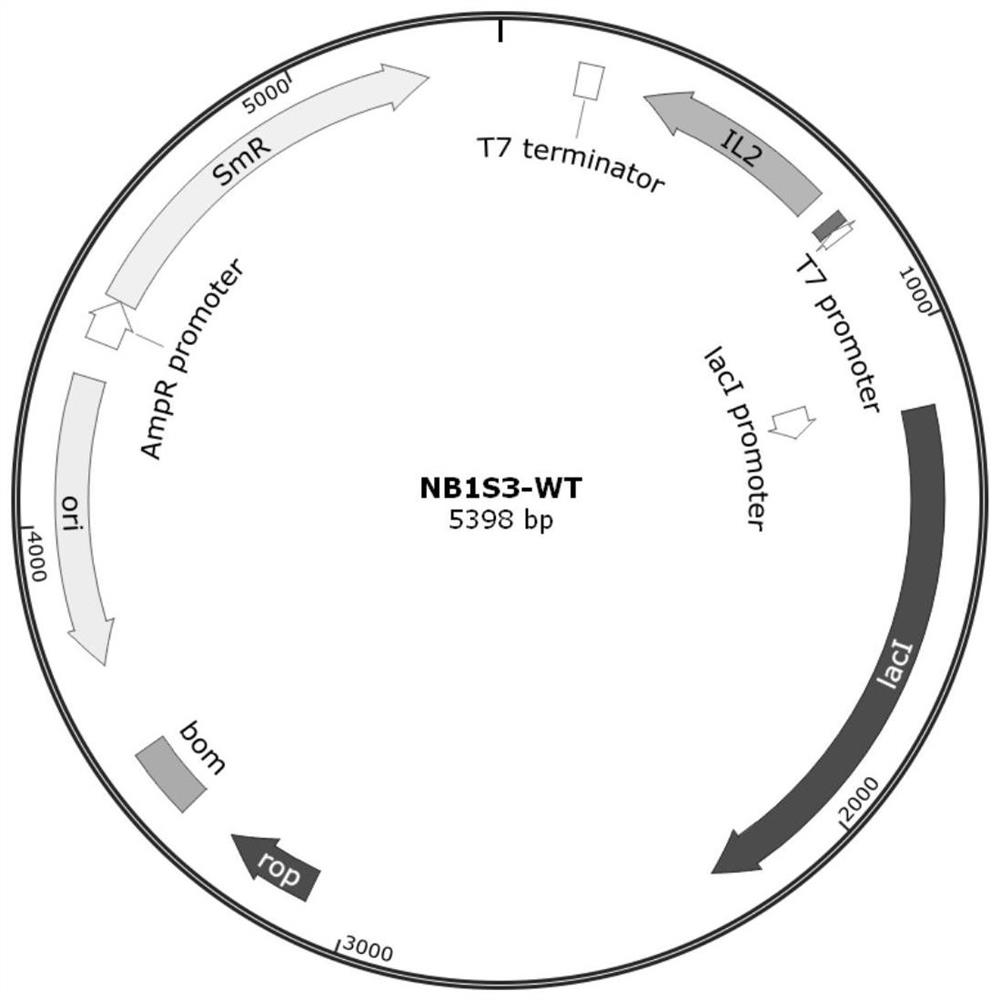
[0067] 1. Obtaining the expression plasmid NB1S3-WT of wild-type recombinant human IL-2
[0068] The precursor protein sequence of Homo sapiens IL-2 (GenBankID: CAA25292.1) was obtained from the National Center for Biotechnology Information (NCBI), USA, as shown in SEQ ID NO:1. The N-terminus of the precursor sequence contains a signal peptide sequence consisting of 20 amino acids, which will be excised during the process of IL-2 protein molecule processing and maturation, so after removing the signal peptide sequence, the mature Homo sapiens IL-2 can be obtained Protein sequence (SEQ ID NO: 2). According to literature reports (Liang S.M et al. Journal of Biological Chemistry, 261 (1): 334-337, 1986) the protein sequence of mature Homo sapiens IL-2 contains 3 cysteine Cys, wherein the 58th and 105th The two Cys at the posit...
Embodiment 2
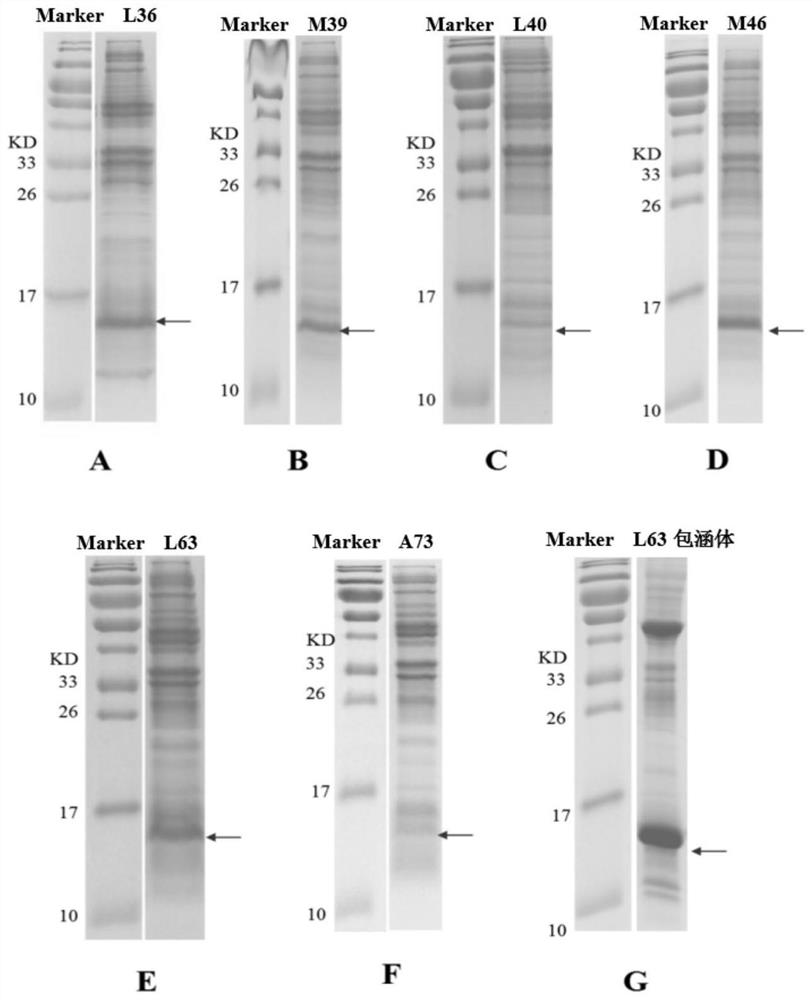
[0090] Example 2: Expression and purification of rhIL-2 inserted with unnatural amino acid by site-directed mutation
[0091] 1. Unnatural amino acid incorporation and expression of mutant rhIL-2
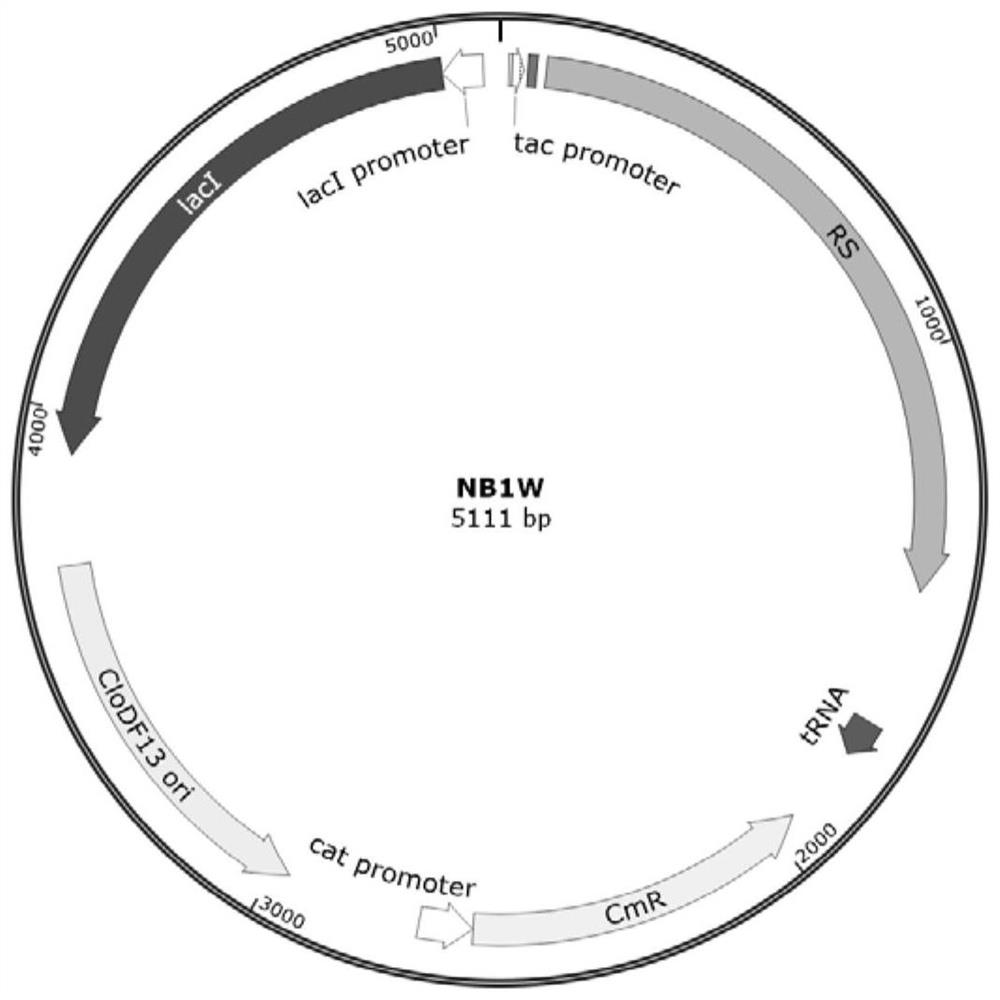
[0092] The 10 expression strains obtained in Example 1, rhIL2-L36-BL21, rhIL2-M39-BL21, rhIL2-L40-BL21, rhIL2-M46-BL21, rhIL2-P47-BL21, rhIL2-L63-BL21, rhIL2-L66- BL21, rhIL2-E67-BL21, rhIL2-L70-BL21 and rhIL2-A73-BL21 were inoculated into LB medium (yeast extract 5g / L, tryptone 10g / L, NaCl 10g / L, containing 100mg / L spectinomyces and 37.5mg / L chloramphenicol), cultured at 37°C for 5-8 hours, and then carried out secondary expansion (the composition of the medium was the same as before) to the OD of the bacterial solution 600 2.0 ± 0.2, to obtain the secondary seed liquid.
[0093] The above-mentioned secondary seed liquid is inoculated in the fermentation medium to carry out fermentation culture, implement in 5L fermentor, culture volume is 2L, and medium is 2 * YT medium (yeast e...
Embodiment 3
[0099] Example 3: Preparation of PEG-linker
[0100]
[0101] As shown in formula 1, a certain amount of general formula is BCN-PEG N -NHS (succinimide-activated-polyethylene glycol-eight-membered cycloalkene) cycloalkyne and 30KD PEG are mixed in a ratio of 1:1.2 equivalents, dissolved in dichloromethane until clear, and then 10 times equivalent of cycloalkyne is added TEA is used as an acid-binding agent, and the reaction is carried out with stirring at 25°C. The reaction time is 18-24h. After the raw materials are completely reacted by TLC, the reaction solution is concentrated under reduced pressure, and then methyl tert-butyl ether is added. The product BCN-PEG is white in color. A solid was separated out which was filtered and dried for later use. The reaction yield is above 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com