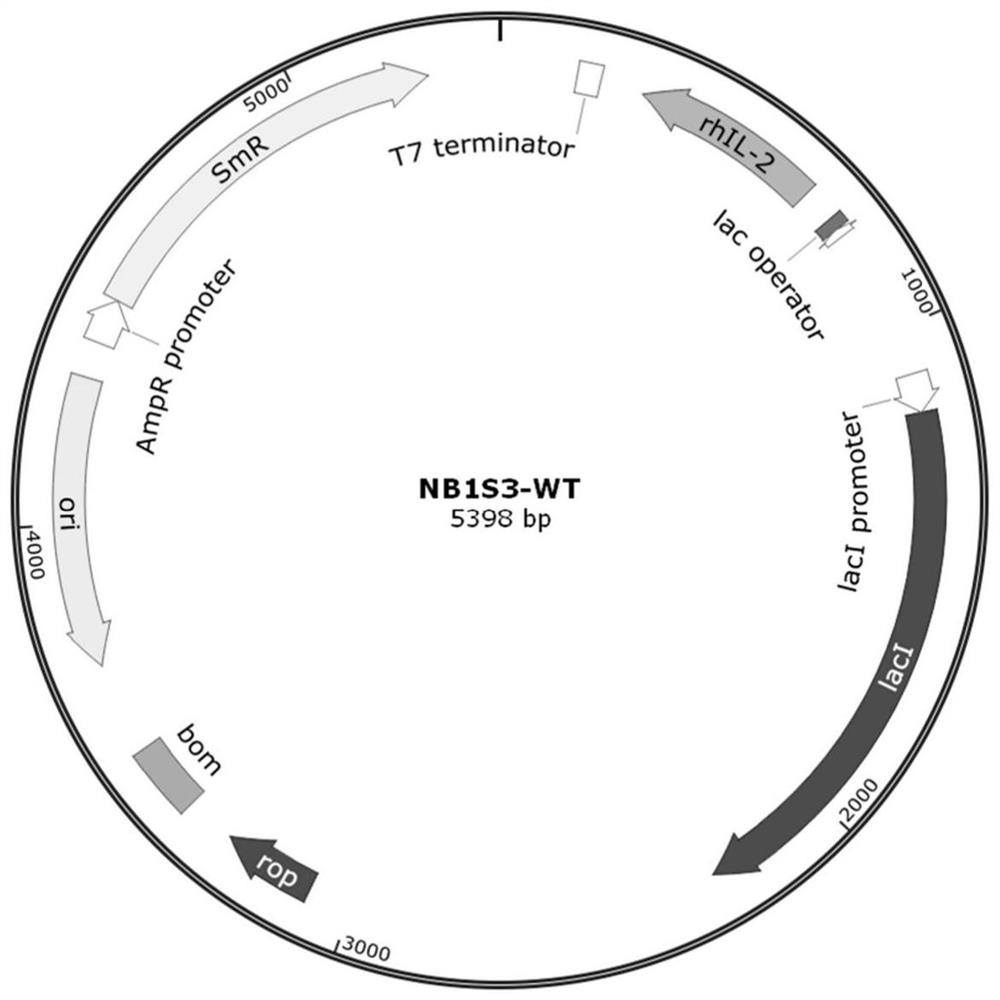
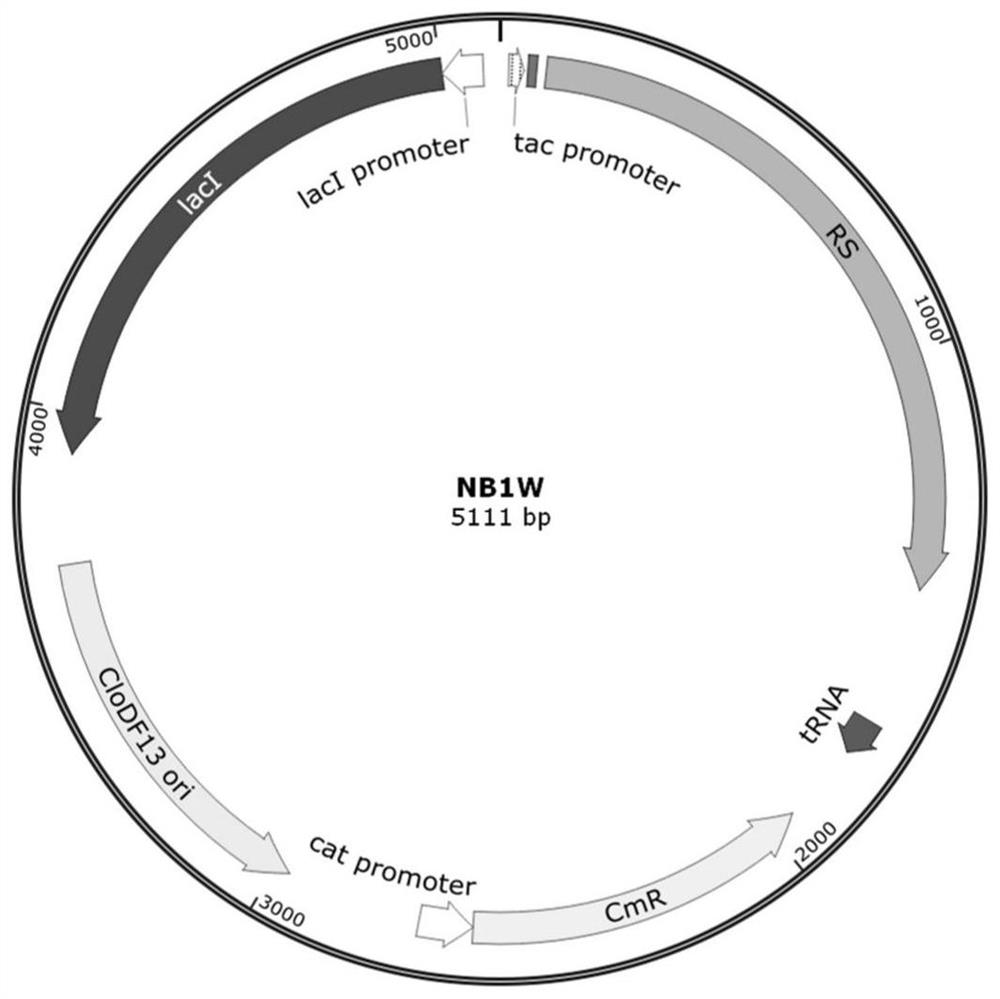
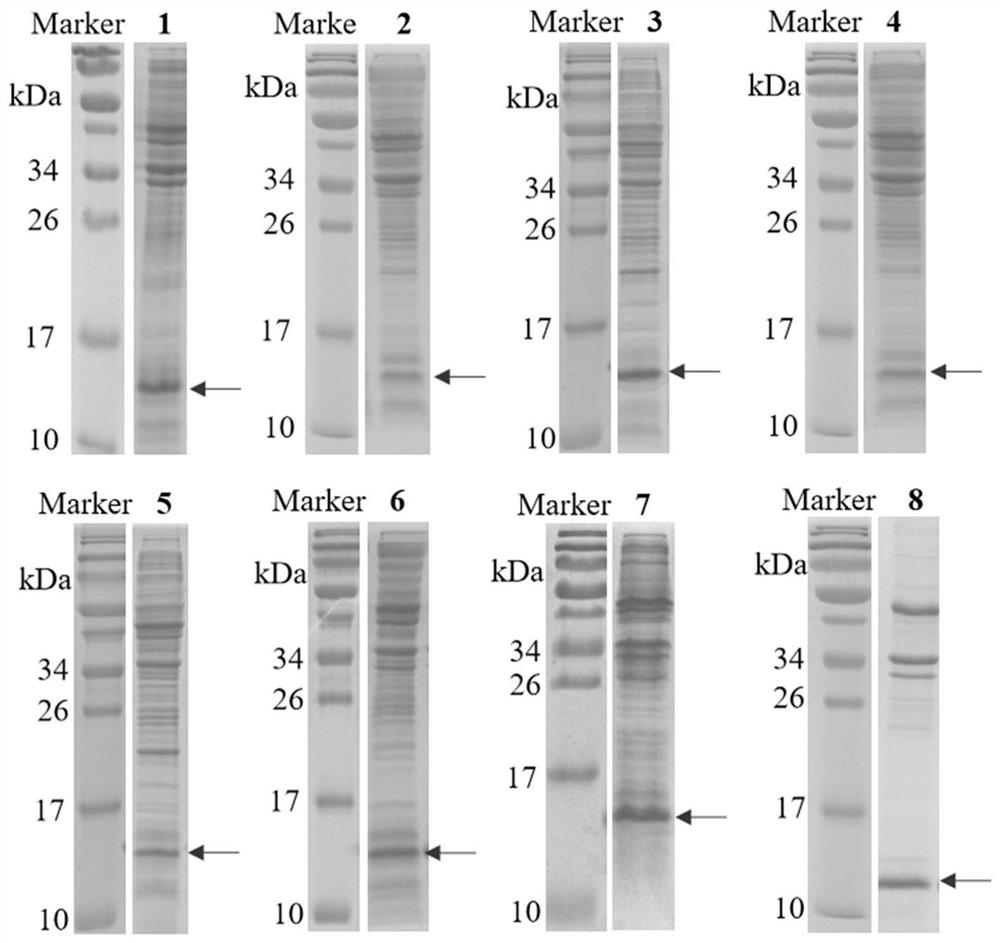
Human interleukin 2-polyethylene glycol conjugate and application thereof
A technology of interleukin and polyethylene glycol, which is applied in the field of human interleukin 2-polyethylene glycol conjugates, can solve the problems of weakened binding ability, complicated mechanism of action, and no performance, so as to reduce the binding activity , high product uniformity, and the effect of reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The reagents or raw materials used in the preparation examples and examples of the present invention are all commercially available products unless otherwise specified; the experimental methods used are all conventional methods in the art unless otherwise specified.
[0080] Human YT cells in the literature "Yodoi, J. et al. (1985). TCGF(IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YTcells). Journal of Immunology, 134(3), 1623-1630", the public can obtain from Zhejiang Xinma Biomedical Co., Ltd.
preparation example 1
[0081] Preparation Example 1 The preparation of unnatural amino acid NBOK
[0082] The structural formula of NBOK is as follows:
[0083]
[0084] The reaction process is shown in the figure below:
[0085]
[0086] The preparation process includes the following steps:
[0087] a) In the reaction flask, add p-methylacetophenone (4.0mL, 30.0mmol), add solvent DCE (50.0mL), add NBS (6.41g, 36.0mmol) and BPO (0.05g, 0.3mmol), the mixture After reflux at 80°C for 24 hours, the container was cooled in ice water, and a solid precipitated, which was removed by filtration and washed with saturated Na 2 CO 3 Washed 3 times, extracted 3 times with DCM, combined the organic phases, added anhydrous sodium sulfate to dry, filtered, and concentrated under reduced pressure to obtain the crude product 1-1 (5.46 g, yield 85%), which was directly used in the next step without purification .
[0088] b) In a reaction flask, add product 1-1 (2.73g, 12.80mmol), add solvent dioxane (40mL...
preparation example 2
[0093] Preparation Example 2 The preparation of unnatural amino acid NPAK
[0094] The structural formula of NPAK is as follows:
[0095]
[0096] The reaction process is shown in the figure below:
[0097]
[0098] The preparation process includes the following steps:
[0099] a) In the reaction flask, add p-chloroacetophenone (1.00g, 6.47mmol), under nitrogen atmosphere, add diethyl malonate (6.84g, 47.70mmol), KHCO 3 (0.97g, 9.70mmol) and K 2 CO 3 (1.34g, 9.70mmol), then add Pd(dba) 2 (0.019g, 0.030mmol) and P(t-Bu) 3 HBF 4 (0.021g, 0.071mmol), after the addition was completed, the nitrogen was replaced, and the temperature was raised to 160°C for 40h. After the reaction was complete as detected by TLC, water (30 mL) was added to the reaction solution, extracted 3 times with EA, the organic phases were combined, washed 2 times with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure at 0-5°C to obtain a colorless The t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com