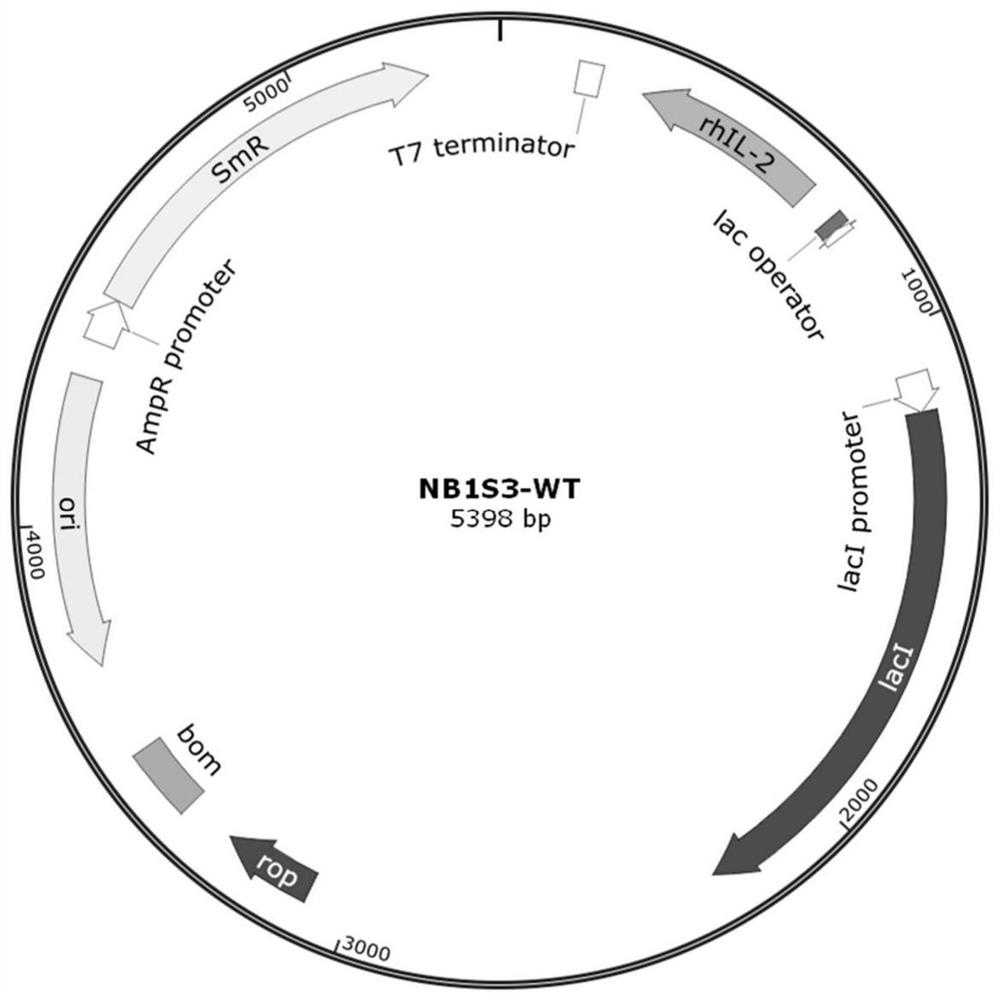
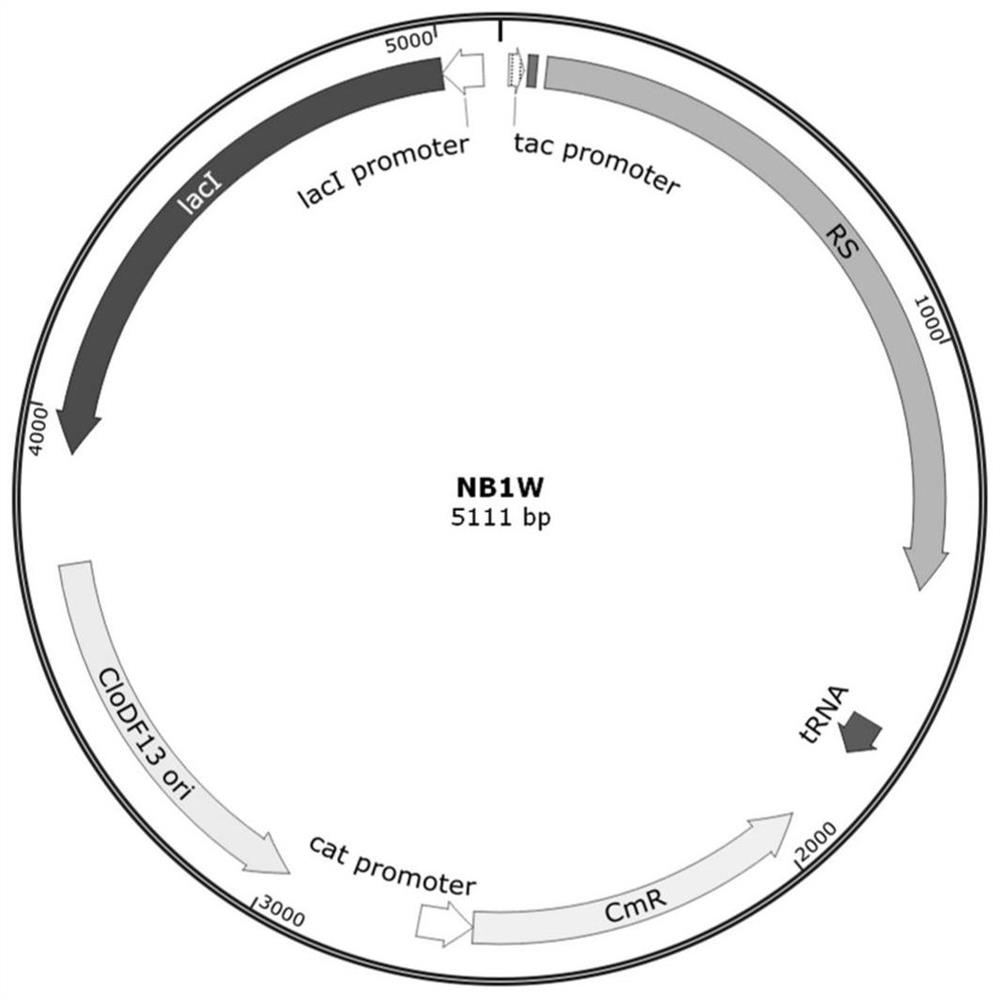
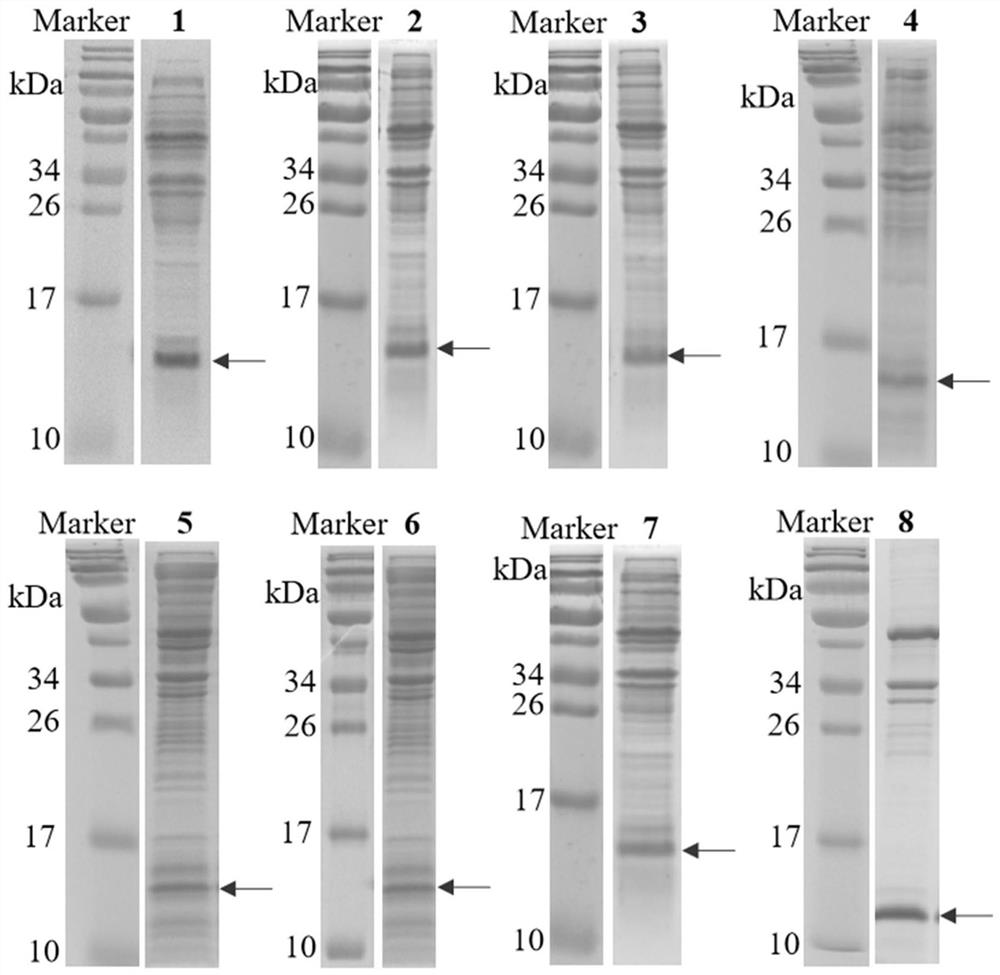
Human interleukin 2-polyethylene glycol conjugate and application thereof
A technology of interleukin and polyethylene glycol, which is applied in the field of human interleukin 2-polyethylene glycol conjugates, can solve the problems of weakened binding ability, complicated mechanism of action, and no performance, so as to reduce the binding activity , high product uniformity, and the effect of reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The reagents or raw materials used in the preparation examples and examples of the present invention are all commercially available products unless otherwise specified; the experimental methods used are all conventional methods in the art unless otherwise specified.
[0079] Human YT cells in the literature "Yodoi, J. et al. (1985). TCGF(IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YTcells). Journal of Immunology, 134(3), 1623-1630", the public can obtain from Zhejiang Xinma Biomedical Co., Ltd.
preparation example 1
[0080] Preparation Example 1 The preparation of unnatural amino acid NOBK
[0081] The structural formula of NOBK is as follows:
[0082]
[0083] The reaction process is as follows:
[0084]
[0085] The preparation process includes the following steps:
[0086] a) In the reaction flask, add 3-buten-1-ol (2.00g, 27.74mmol), add solvent DCM (15.0mL), add CDI (4.50g, 27.74mmol) at 0C °, the mixture is at room temperature After reacting for 18 hours, 10 mL of water was added, extracted three times with DCM, the organic phases were combined, dried by adding anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain product 1-1 (4.50 g, yield 98%).
[0087] b) In a reaction flask, add product 1-1 (0.55g, 3.31mmol) and Fmoc-lysine hydrochloride (1.12g, 2.76 mmol), add solvent dioxane 9mL and water 3mL (v / v =3:1), then added triethylamine (0.70g, 6.9mmol), and reacted the mixture at room temperature for 24 hours, then added 20mL of 1M HCl solution...
preparation example 2
[0091] Preparation Example 2 The preparation of unnatural amino acid NOHK
[0092] The structural formula of NOHK is as follows:
[0093]
[0094] The reaction process is as follows:
[0095]
[0096] The preparation process includes the following steps:
[0097] a) In a reaction flask, add 5-oxohexanoic acid (2.60g, 20.0mmol), N-hydroxysuccinimide (2.30g, 20.0mmol), EDCI (3.83g, 20.0mmol), add solvent DCM (100 mL), the mixture was reacted at room temperature for 18 hours, washed with water three times, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain product 2-1 (4.62 g, yield 102%), which was directly used in the next step without purification.
[0098] b) In a reaction flask, add product 2-1 (4.62g, 20.35mmol) and Boc-lysine (4.42g, 16.96mmol), then add DIPEA (4.07mL, 42.4mmol), add solvent DMF (50mL) , the mixture was reacted at 40°C for 24 hours, washed with water 5 times, dried by adding anhydrous sodium sulfate, filtered,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com