Patents
Literature
45 results about "Conventional chemotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
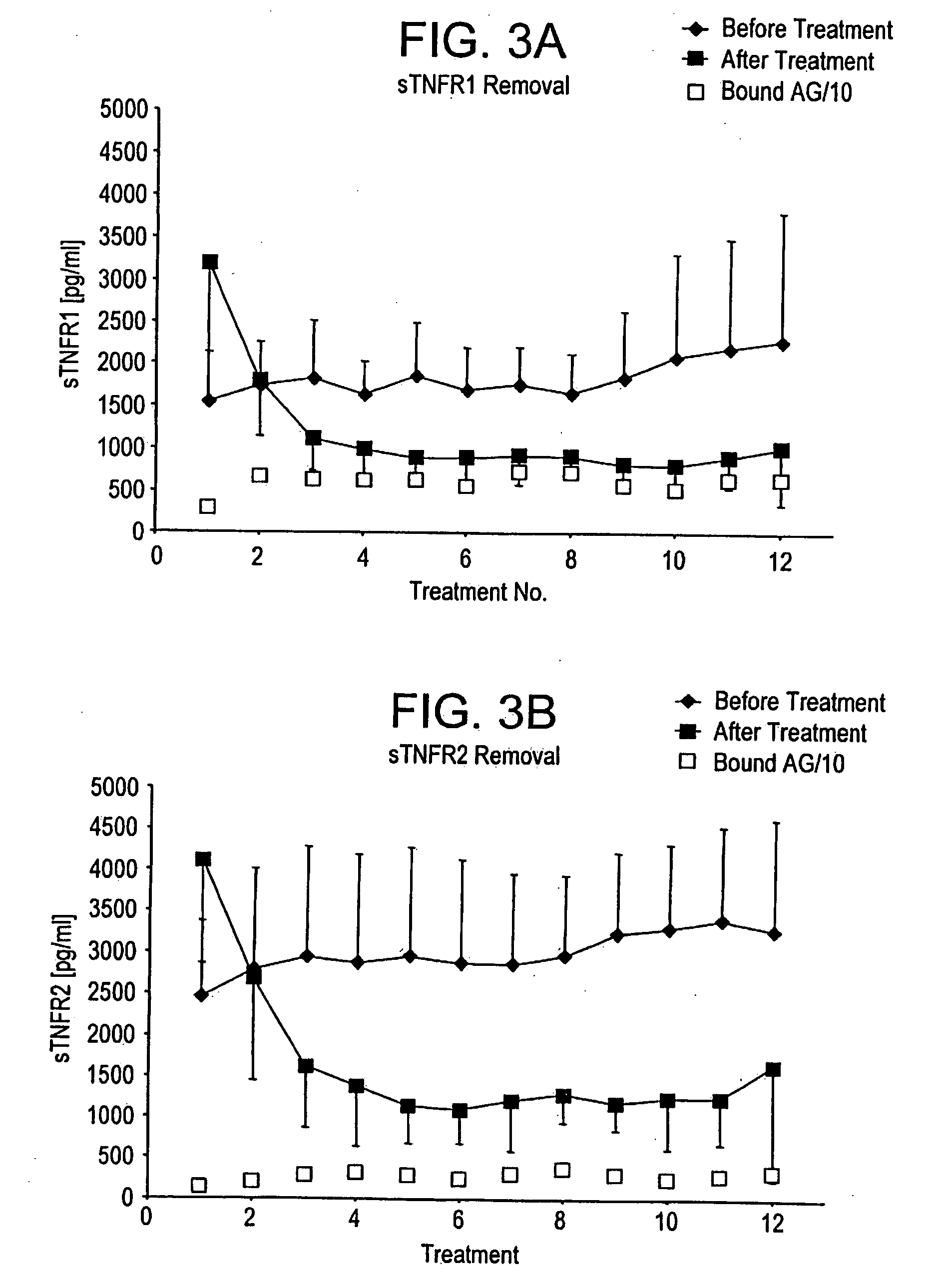
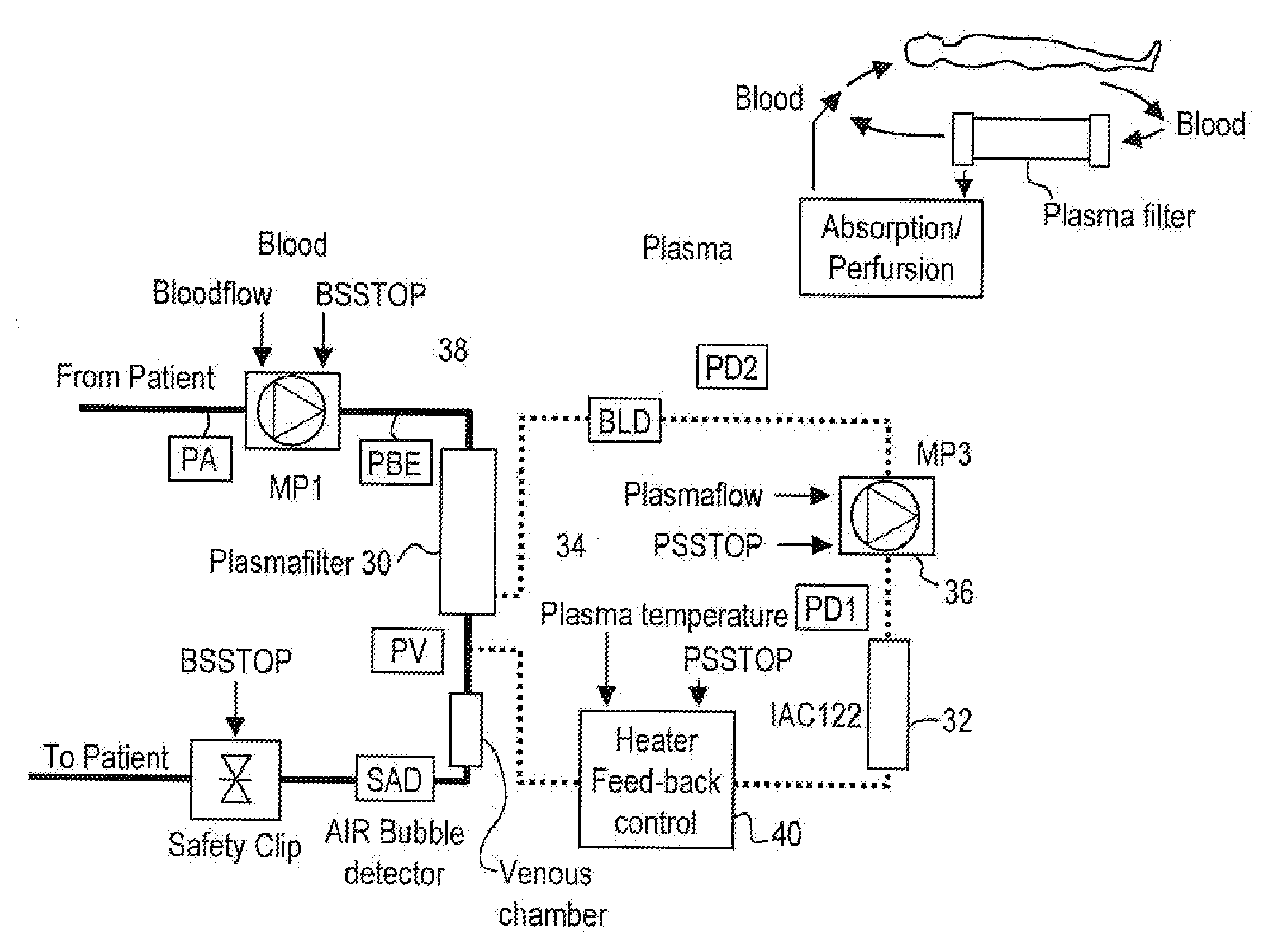
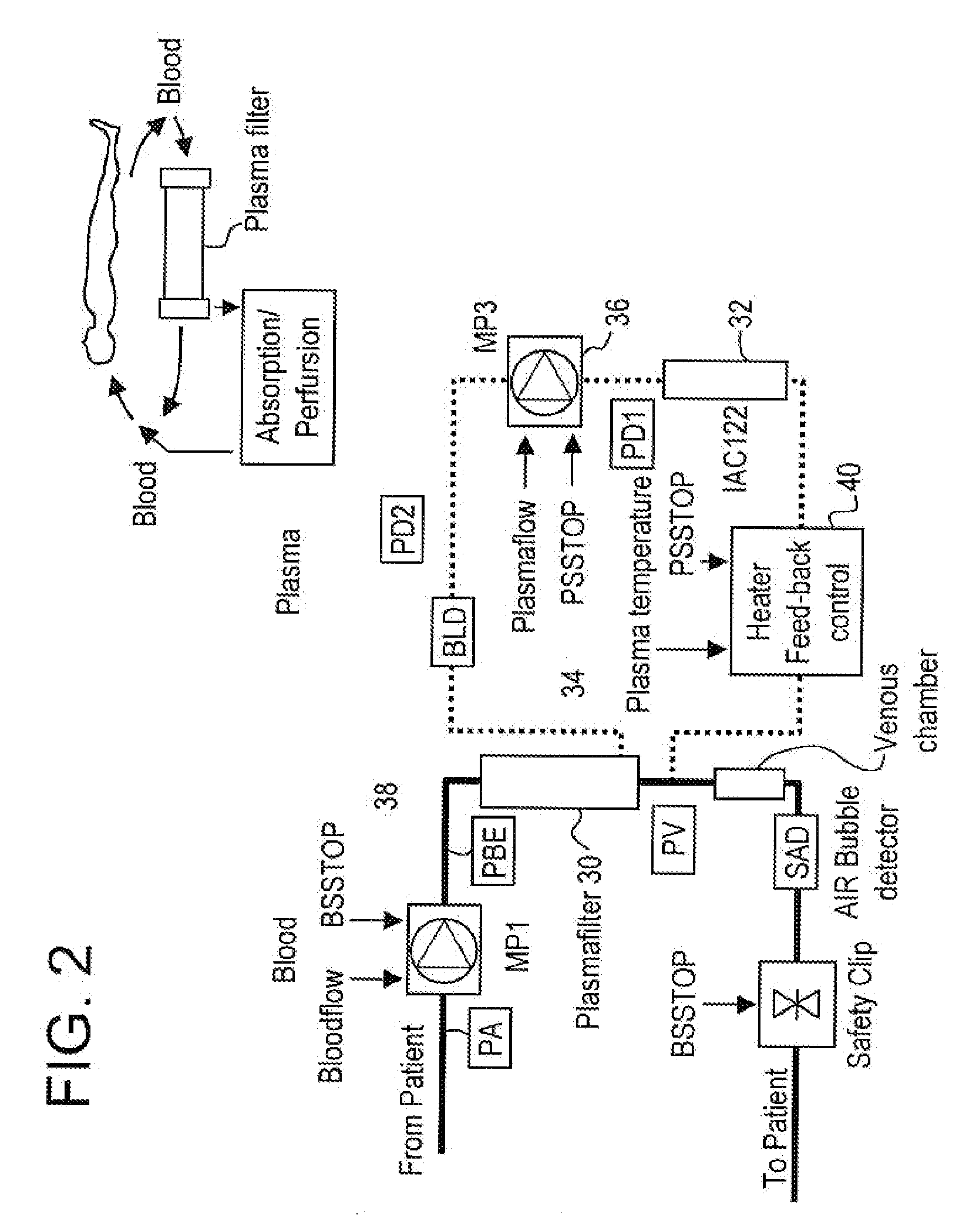
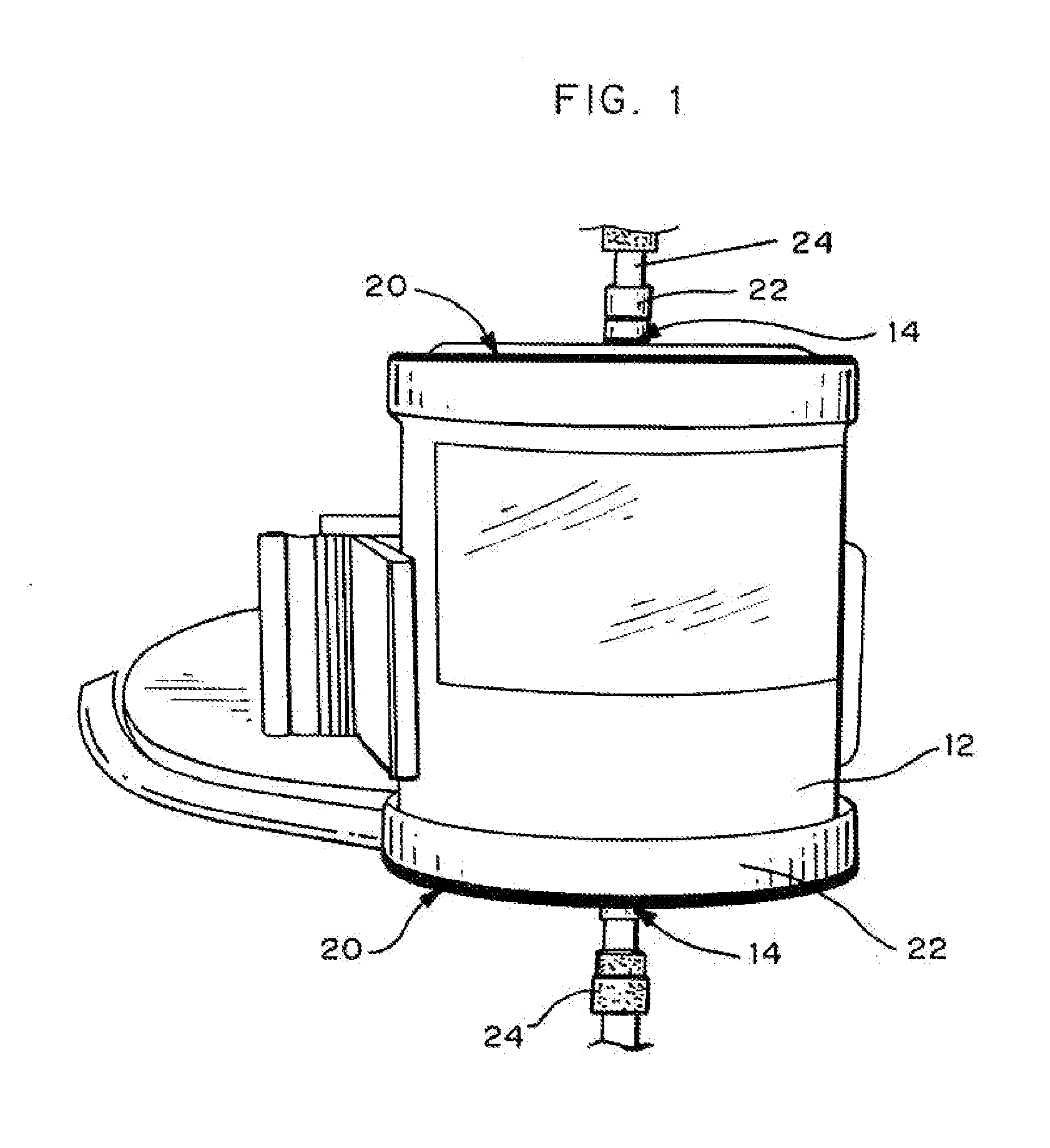
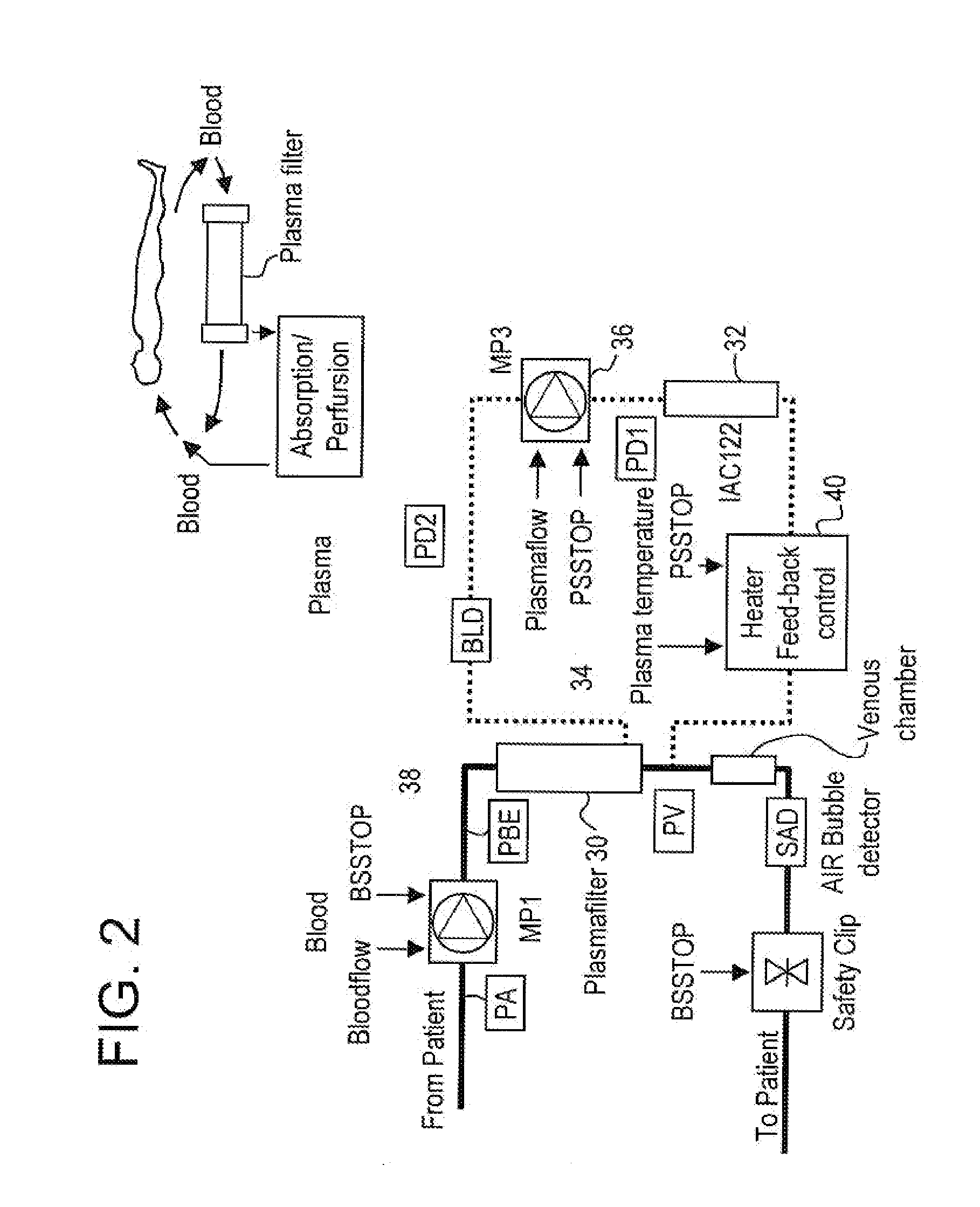
Method and system to remove soluble TNFR1, TNFR2, and IL2 in patients
InactiveUS20050265996A1Induce remissionPeptide/protein ingredientsHaemofiltrationDiseaseAntiendomysial antibodies
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Betulinic acid and derivatives thereof useful for the treatment of neuroectodermal tumor
The present invention is, generally, directed to the use of betulinic acid and derivatives thereof for the treatment of neuroectodermal tumors. The present invention is based on the discovery that betulinic acid and its derivatives are potent anti-neuroectodermal agents. As disclosed herein, betulinic acid and its derivatives are useful for the treatment of neuroectodermal tumors, including, due to its distinct mechanism of action, neuroectodermal tumors that are resistant to conventional chemotherapeutical agents. In addition to the new use of known compounds, the invention discloses novel compounds and pharmaceutical compositions for the treatment of neuroectodermal tumors.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
InactiveUS20110129441A1Peptide/protein ingredientsIon-exchanger regenerationDiseaseConventional chemotherapy
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Methods for assessing cisplatin resistance, disease progression, and treatment efficacy in ovarian cancer
ActiveUS7700280B2Organic active ingredientsPeptide/protein ingredientsAbnormal tissue growthChemotherapeutic drugs
The invention provides methods for predicting whether an ovarian cancer patient's tumor will be resistant to chemotherapy. The invention also provides methods for monitoring the effectiveness of treatment, particularly a chemotherapeutic treatment, in a patient treated for ovarian cancer. The invention further provides methods for treating ovarian cancer, by reducing chemotherapeutic drug resistance in said cells. In addition, the invention provides methods of screening compounds to identify tumor cell growth inhibitors in tumor cells resistant to conventional chemotherapeutic treatment regimes.
Owner:PENN STATE RES FOUND
Exosome-nucleic acid aptamer liposome composite drug carrier system as well as preparation method and application thereof
InactiveCN107913408AGood biocompatibilityImprove targetingPharmaceutical non-active ingredientsAntineoplastic agentsDiseaseAptamer
The invention discloses an exosome-nucleic acid aptamer liposome composite drug carrier system which comprises an anti-tumor medicine, wherein the surface of the anti-tumor medicine is wrapped by nano-liposomes, a nucleic acid aptamer is connected with the surfaces of the nano-liposomes through chemical bonds to form a nucleic acid aptamer liposome on which the anti-tumor medicine is carried; thenucleic acid aptamer liposome on which the anti-tumor medicine is carried is compounded with exosome. The invention further discloses a preparation method and an application of the composite drug carrier system. The composite drug carrier system is good in targeting property, good in immune evasion property, free of cytotoxicity and not liable to be degraded by macrophage, can be applied to anti-tumor medicine chemotherapy of cancer patients, is capable of greatly improving the treatment effect of anti-tumor medicines, reducing the situations of chemotherapy effect degradation and disease reoccurrence caused by drug resistance, and alleviating the bottle neck problems of conventional chemotherapy of cancer patients, and has great application values and application prospects in intensifyingcancer treatment effects.
Owner:CENT SOUTH UNIV
Application of paris saponin I and its derivatives
ActiveCN101143148APromote growthGood anticancer effectOrganic active ingredientsAntineoplastic agentsFuranSide effect
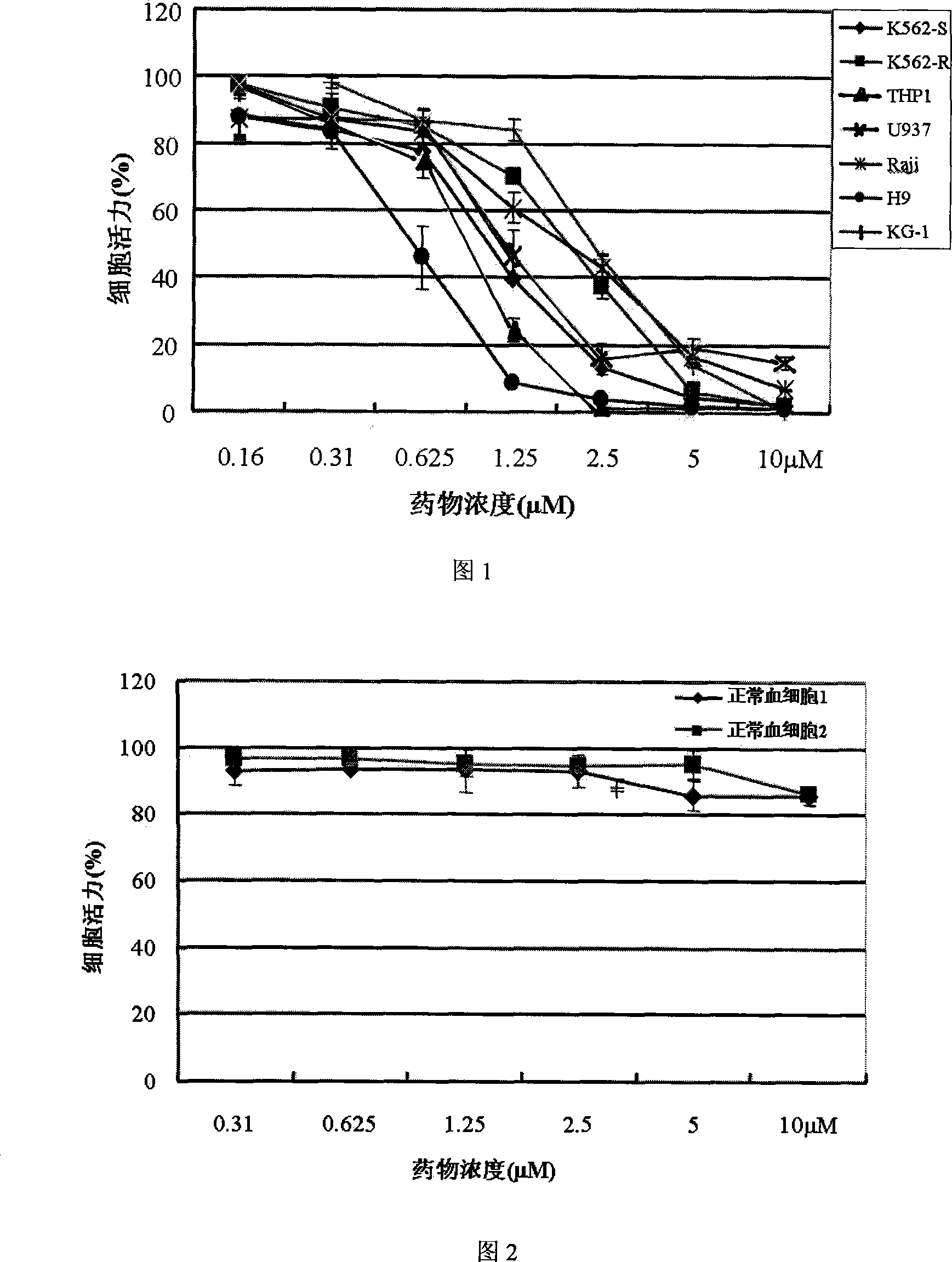
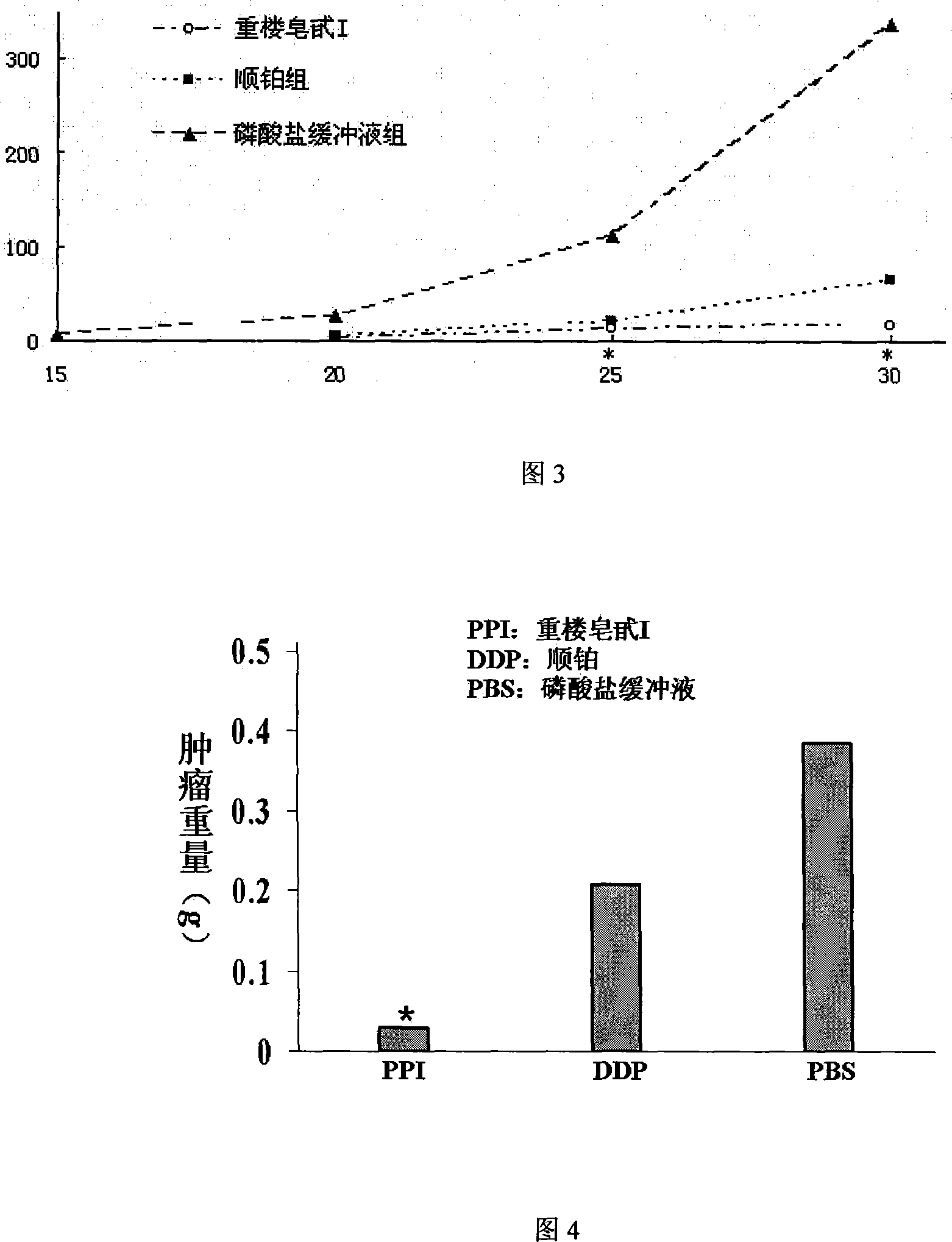
The invention provides the application of a paris saponin (I) and a ramification of the paris saponin (I) in the preparation of a drug for remedying the tumor. The chemical name of the paris saponin (I) is 4-O-furan Arabian glycosyl-2-O-pyran buckthorn glycosyl-beta-D-pyran glucosyl dioscorea saponin, and the molecular formula of the paris saponin (I) is C44H70O16. The drug, which is prepared by the invention, also contains a drug excipient or carrier which is allowed by the preparation. The invention is mainly applied to prepare the drugs for remedying the lung cancer and the leukaemia and has strong anticancer effect, and the animal experiment testifies that the effect of resisting the growth of the cancer cells of the paris saponin (I) is obviously better than a chemotherapeutic drug of cisplatin which is in common clinical use at present. The toxic and side effect of the paris saponin (I) is light, and the experiment of cell culture in vitro testifies that the paris saponin (I) and the ramification of the paris saponin (I) do not have obvious toxic and side effect towards the growth of the hematopoietic cells of the normal person under the quantity of an antineoplastic and are better than the normal chemotherapeutic drug.
Owner:HANGZHOU BENSHENG PHARMA
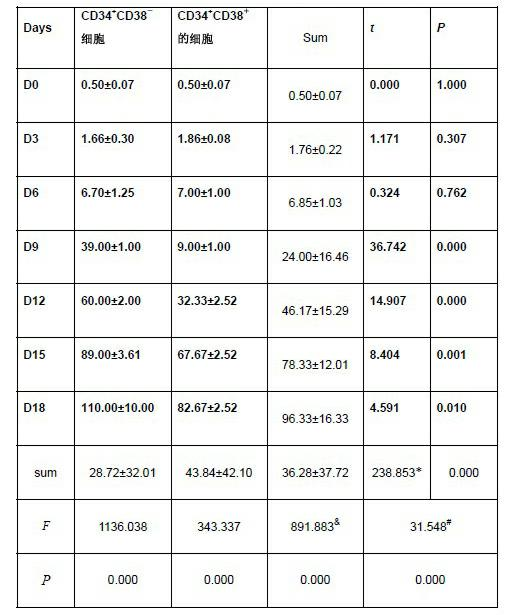
Method for separating leukemia stem cells from leukemia cell strain
InactiveCN102220282AHigh purityEasy to operateTumor/cancer cellsConventional chemotherapyCell strain
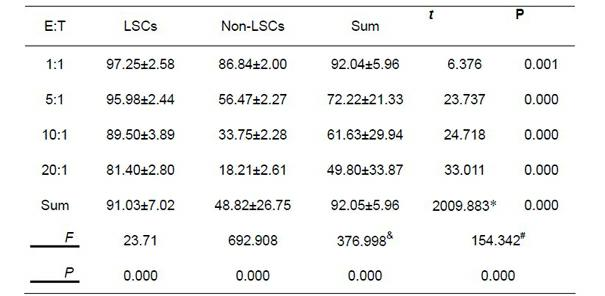
The invention provides a method for separating leukemia stem cells from a leukemia cell strain, which comprises the following steps: firstly, subculturing human acute myelogenous leukemia cell strain KD1a cells; and secondly, separating CD34+CD38-cells by an immunomagnetic bead separation method to obtain the leukemia stem cells, wherein the leukemia stem cells can resist conventional chemotherapy and NK immunization therapy. The separation method disclosed by the invention is simple to operate, can separate a large quantity of high-purity leukemia stem cells from KG1a cells, and has an important significance for the research of the leukemia stem cells.
Owner:GUANGDONG GENERAL HOSPITAL
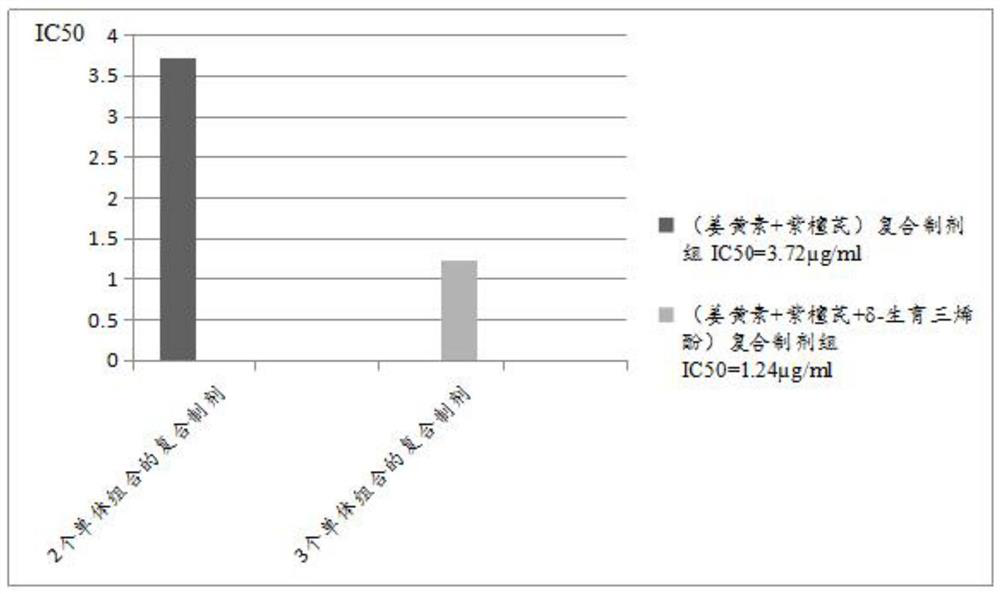
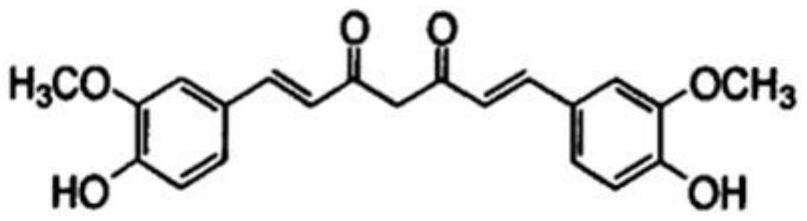
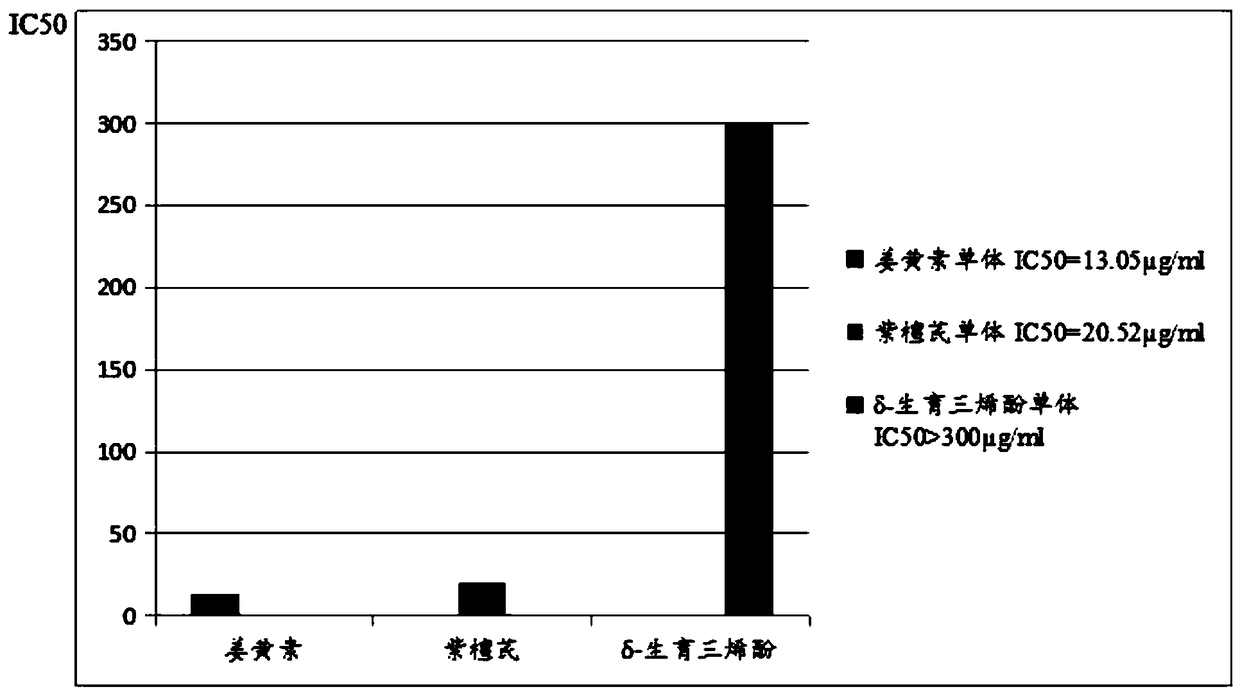
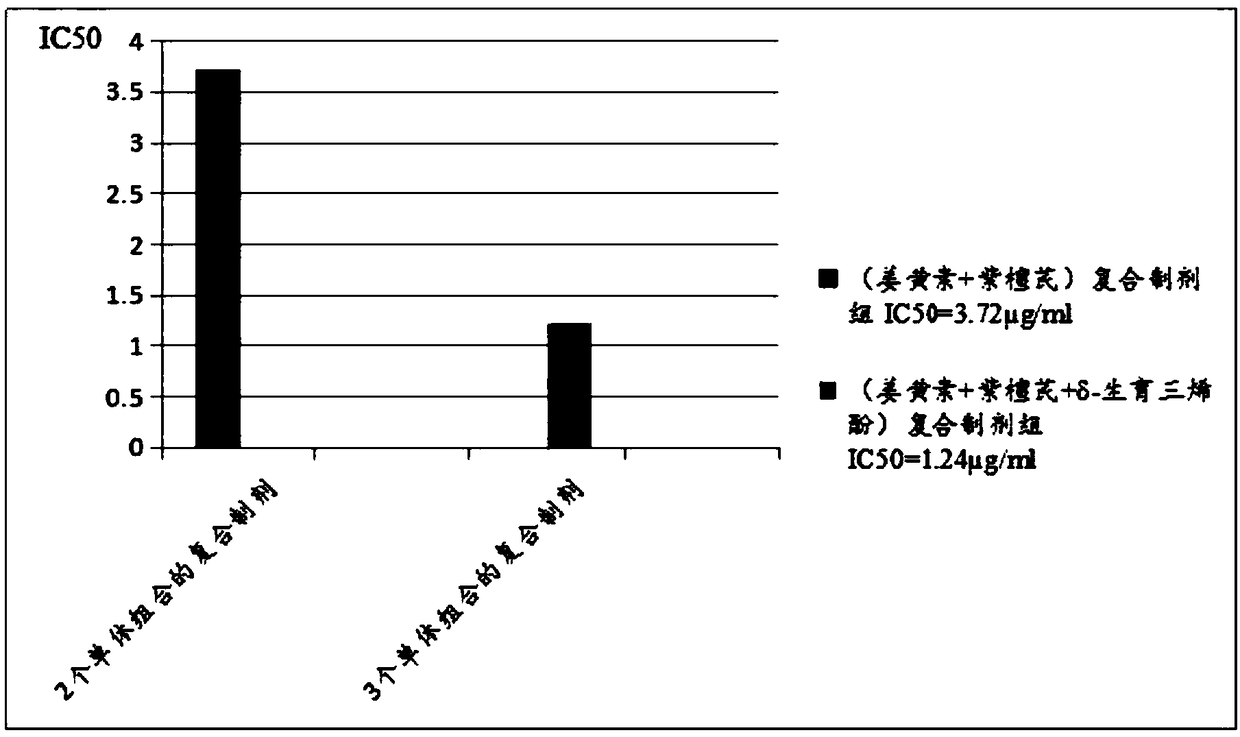
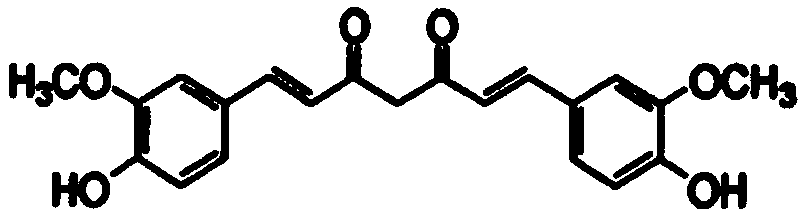
Cancer treatment curcumin compound preparation
ActiveCN109350613AImprove securityNo side effectsEther/acetal active ingredientsKetone active ingredientsChemotherapeutic drugsCompounded preparations
The invention relates to a cancer treatment curcumin compound preparation. According to the cancer treatment curcumin compound preparation, the three adverse effect-free components of curcumin, pterostilbene and delta-tocotrienol 3 serve as raw materials and are compounded at an appropriate ratio to achieve synergistic effects, thereby combining the number of anti-cancer target points and anticancer spectrum and significantly increasing the anticancer effects. Due to the fact that the curcumin can kill cancer stem cells, compared with normal chemotherapy which cannot kill the cancer stem cells, the cancer treatment curcumin compound preparation can significantly reduce cancer recurrence. The cancer treatment curcumin compound preparation is non-toxic to human body and can avoid adverse effects when applied with large dose. Meanwhile, the cancer treatment curcumin compound preparation can reduce the estimated treatment expense by a half or more compared with conventional chemotherapeutic drugs and is far lower in cost than CAR-T (chimeric antigen receptor T-cell immunotherapy, thereby having a broad application prospect in the field of cancer treatment.
Owner:朱理查德澄朗
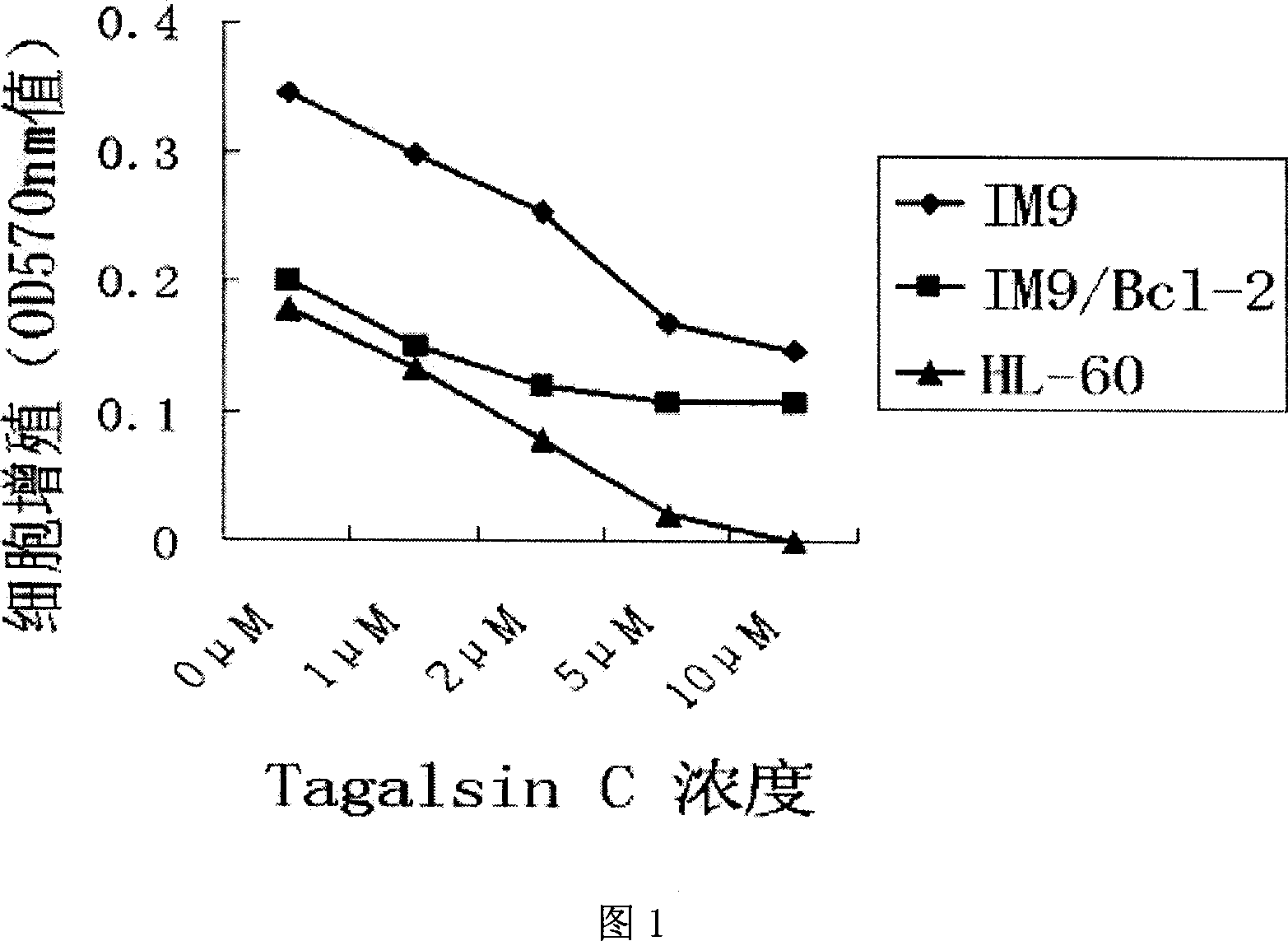
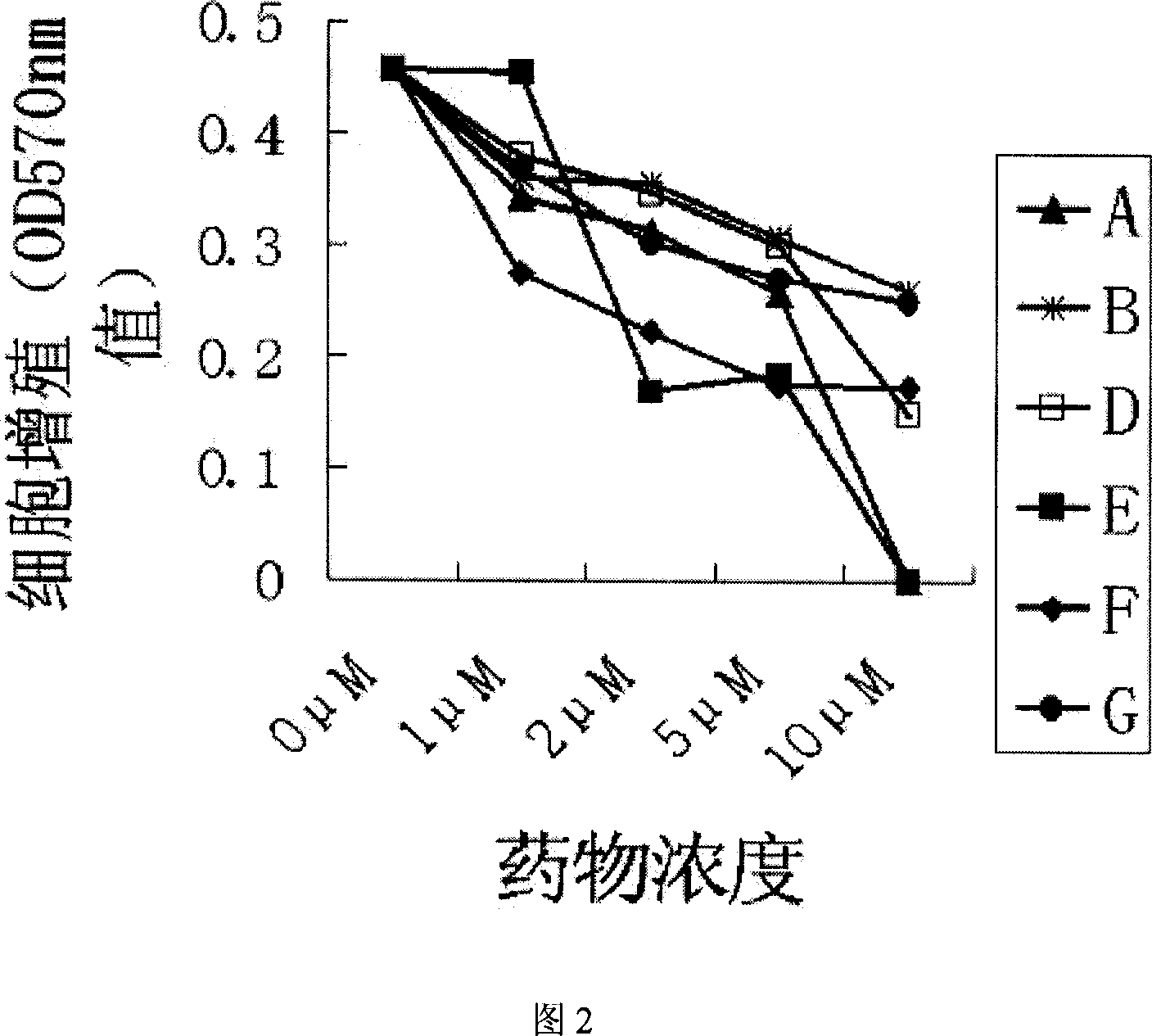
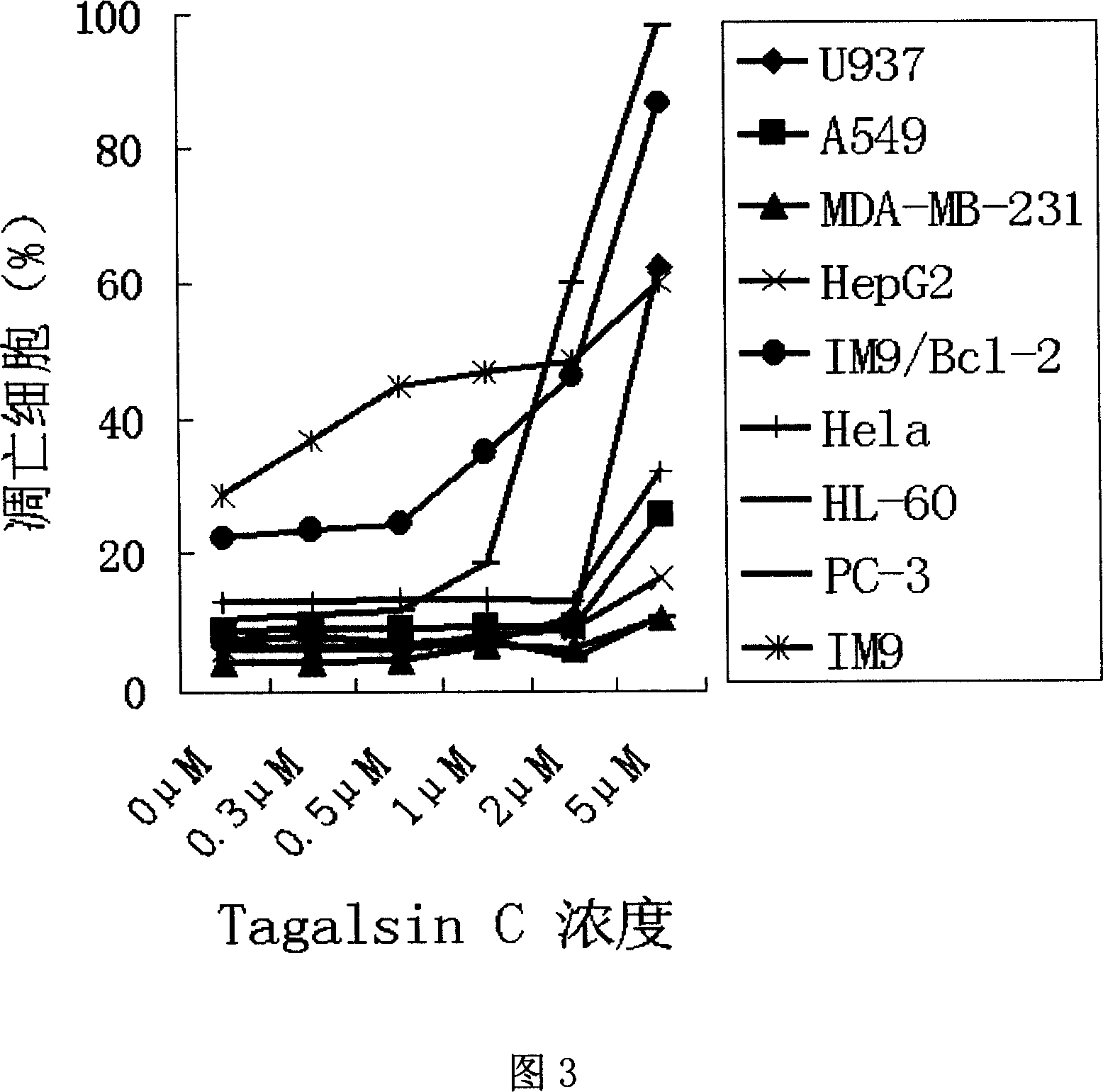
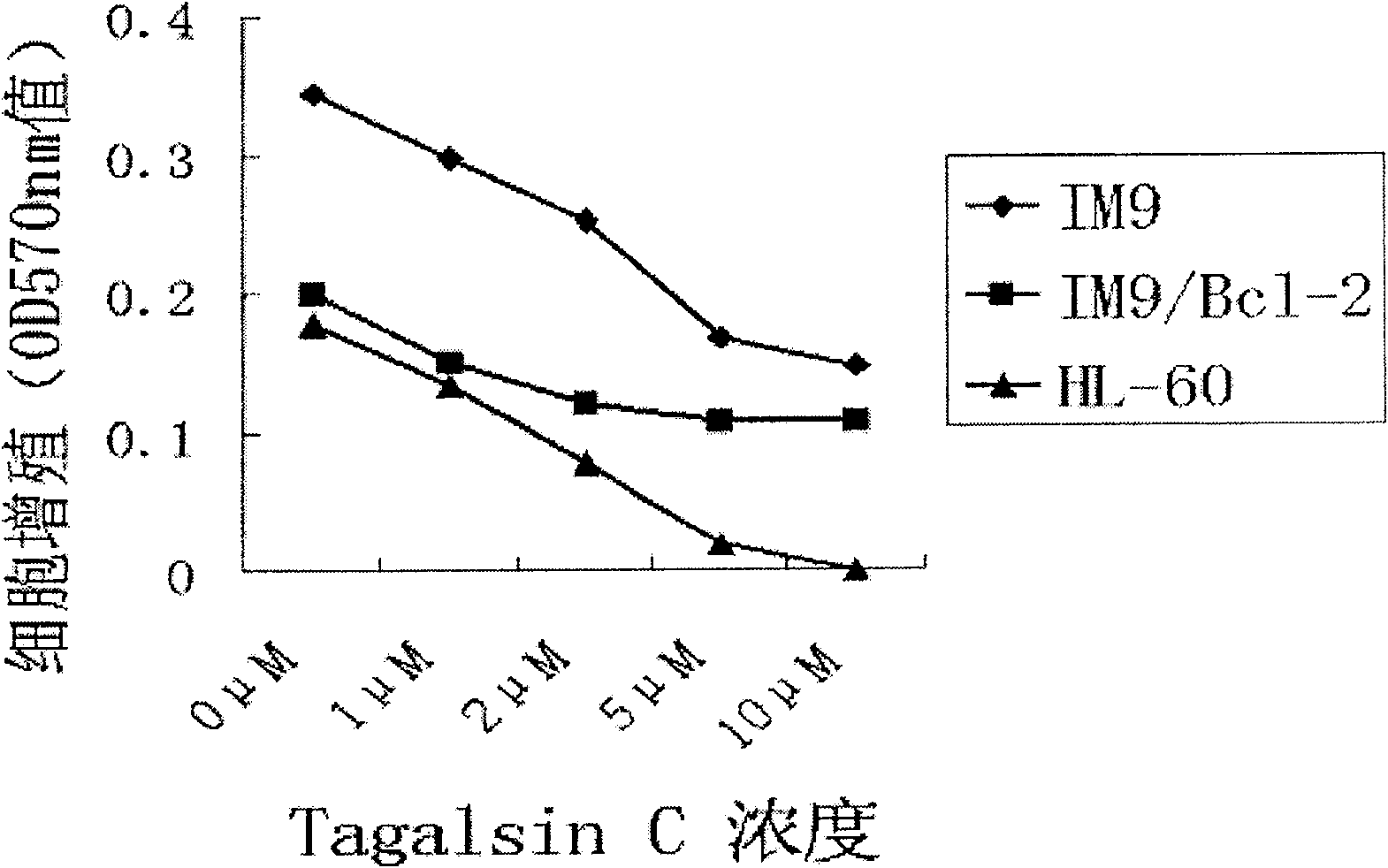
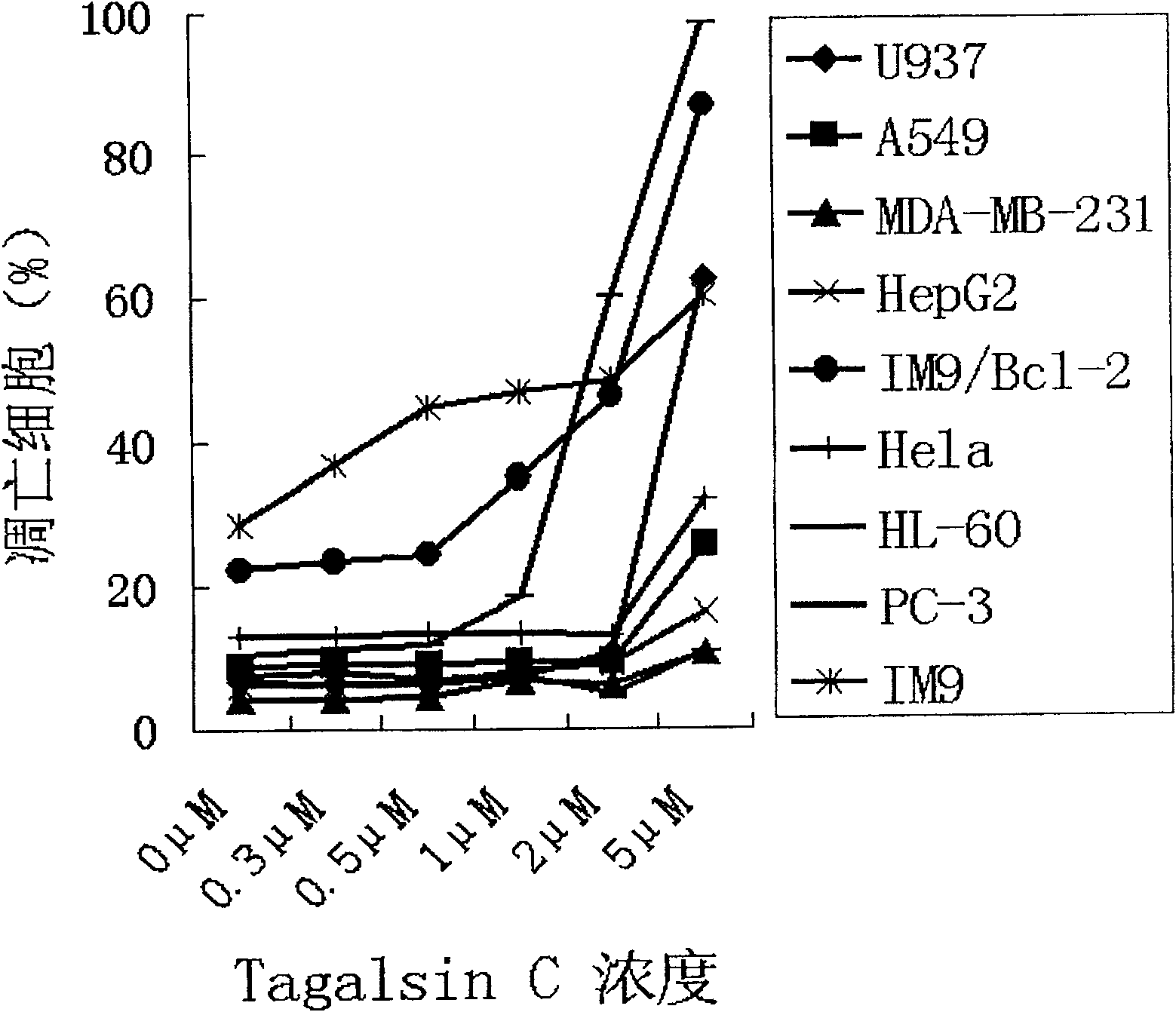
Application of Tagalsin C and its homologous compound in preparing anti-tumor medicine
The present invention relates to the application of the metabolite Tagalsin C of marine life and its homologs Tagalsins A, B, D, E, F and G in preparing medicine for treating tumor. The series of compounds has high membrane permeability and broad spectrum antitumor activity of killing human promyelocytic acute leukemia cell HL-60, human myeloma cell IM9, human acute T-cell leukemia cell Jurkat, human macrophage lymphoma cell U937, human lung cancer cell A549, etc, and no obvious injury on health human nephridium cell 293aa human liver diploid cell L02. The series of compounds may be used in preparing medicine for treating tumor, medicine with high clinical Bcl-2 expression and conventional chemotherapy resistance, and medicine for treating cancer recurrence.
Owner:PEKING UNIV
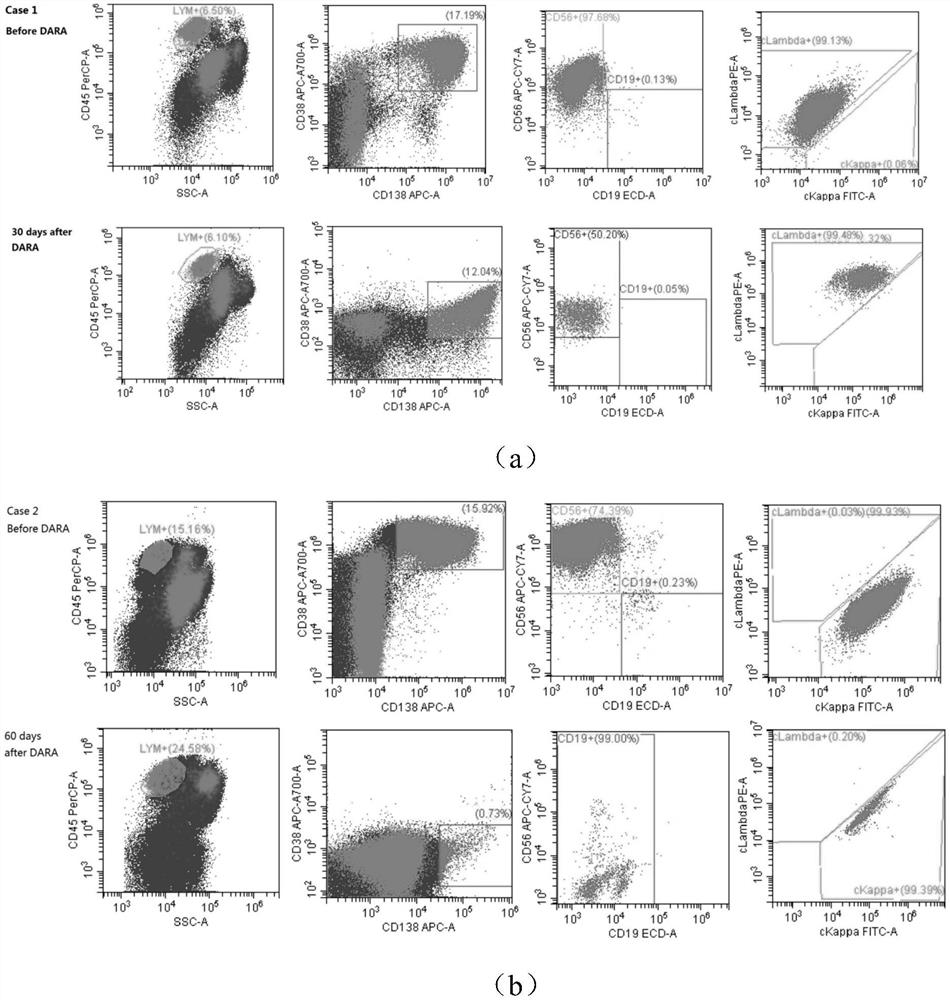
Antibody composition for detecting minimal residual lesions of multiple myeloma, kit and application
InactiveCN111912983ACombination worksThe combination is accurateDisease diagnosisBiological testingTreatment effectConventional chemotherapy
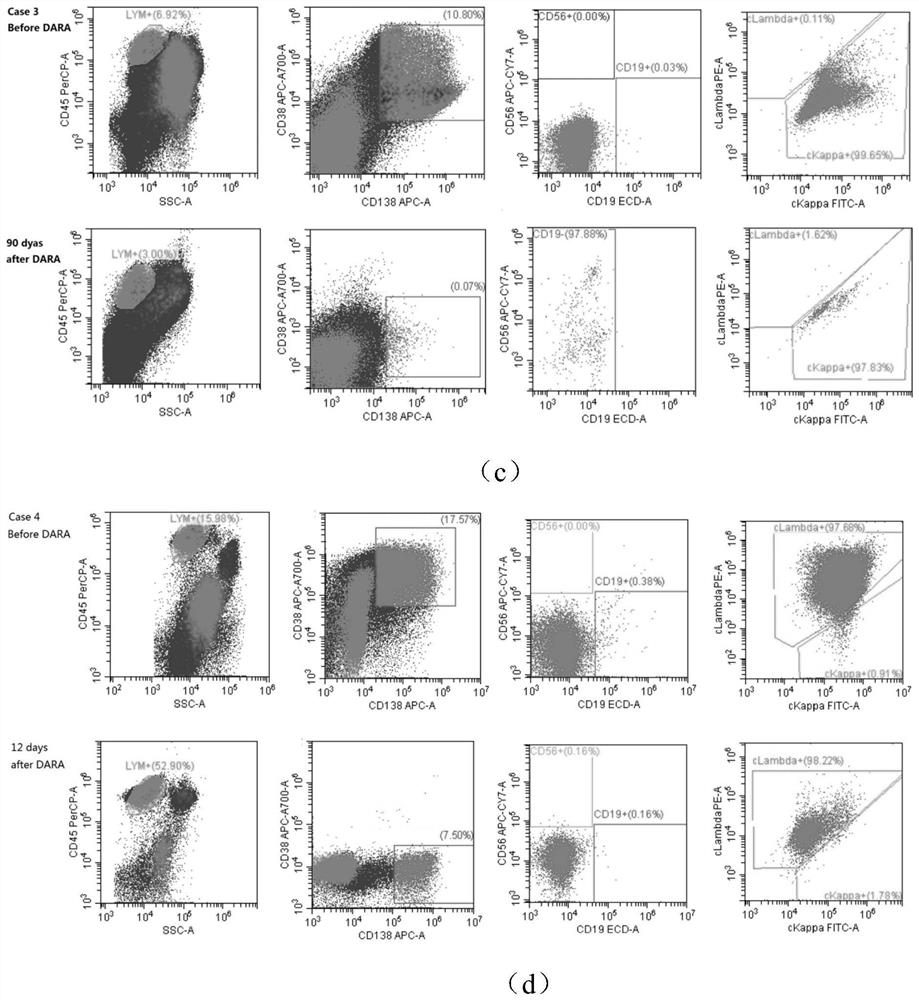
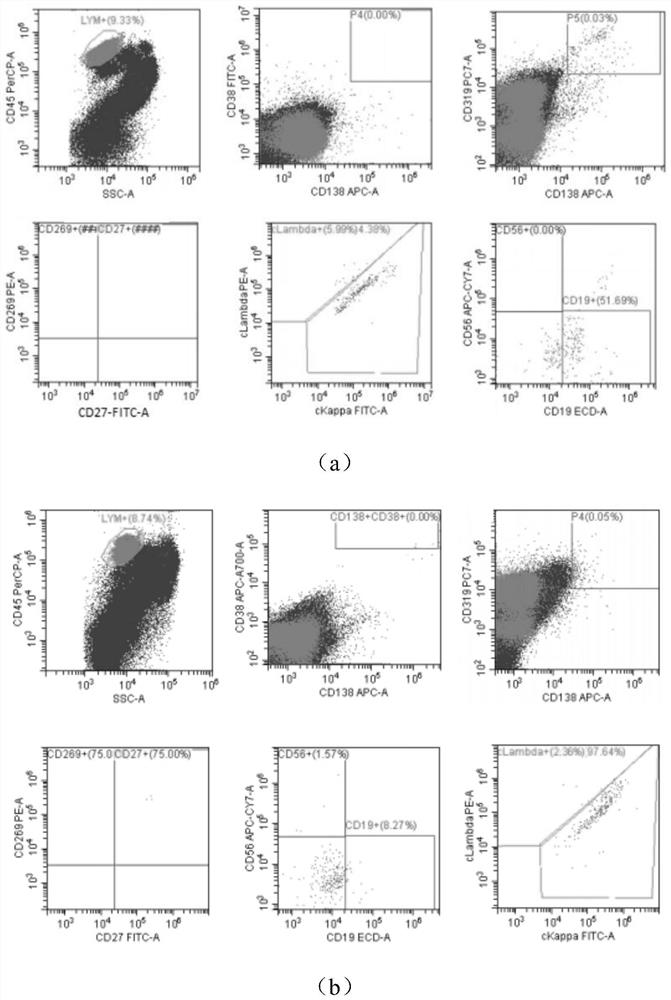
The invention discloses an antibody composition for detecting multiple myeloma minimal residual diseases, a kit and application, and belongs to the technical field of blood tumor treatment effect monitoring. According to the novel flow antibody combination scheme capable of being used for MRD monitoring of myeloma patients disclosed by the invention, CD319 or CD269 and other seven antibodies (CD138 / CD38 / CD184 / CD27 / CD19 / CD56 / ckappa / clamda) are combined to form a fixed combination of two groups of antibodies; through verification of 303 cases of myeloma patients, the antibody combination is feasible, accurate, convenient and fast, can be used for MRD monitoring after conventional chemotherapeutic drug treatment, and provides a good solution for the problem that MRD monitoring is difficult after DARA treatment.
Owner:BEIJING JISHUITAN HOSPITAL
Immune profiling of lymph nodes associated with cancer
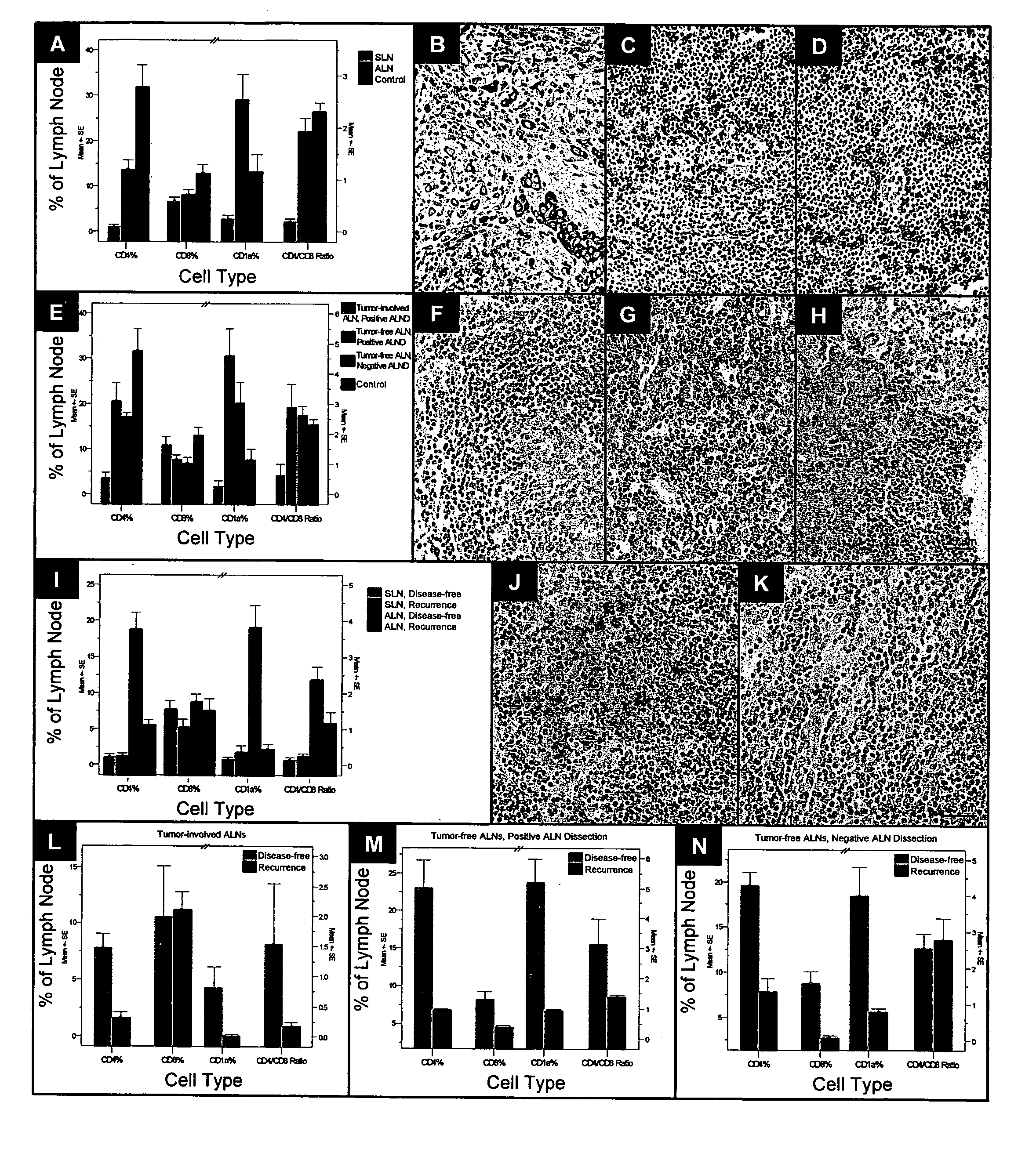
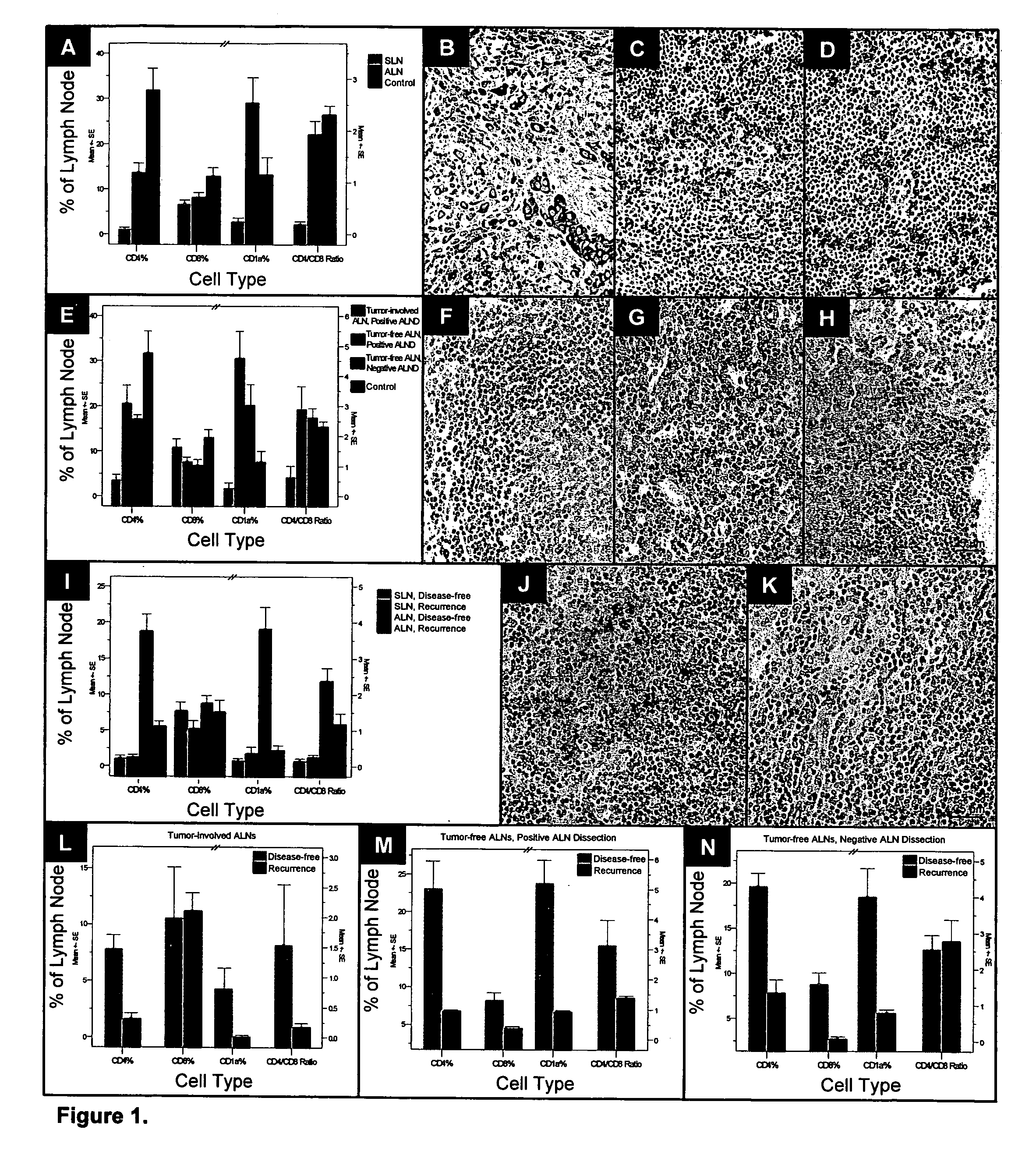
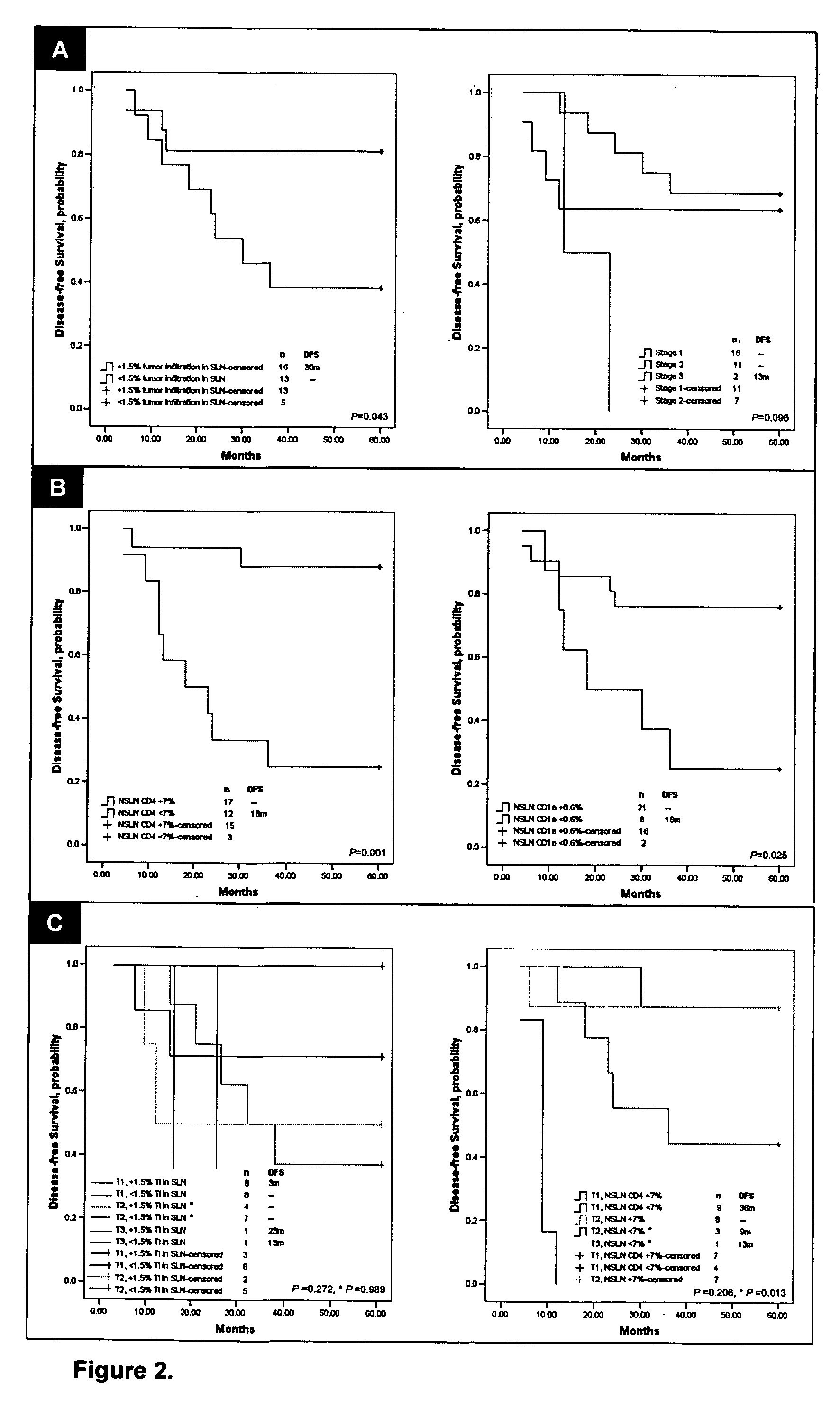
InactiveUS20070048803A1Improved patient careDetermination of patient prognosisBiological testingImmune profilingRegion lymph node
Cancers are phenotyped by analysis of the presence of immune cells, particularly T cells and dendritic cells, present in regional lymph nodes. When non-sentinel regional lymph nodes of a cancer patient are compared to normal lymph nodes, it is found that increased number of dendritic cells and decreased numbers of T cells, particularly T helper cells, correlate with a positive prognosis and disease-free survival following conventional chemotherapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
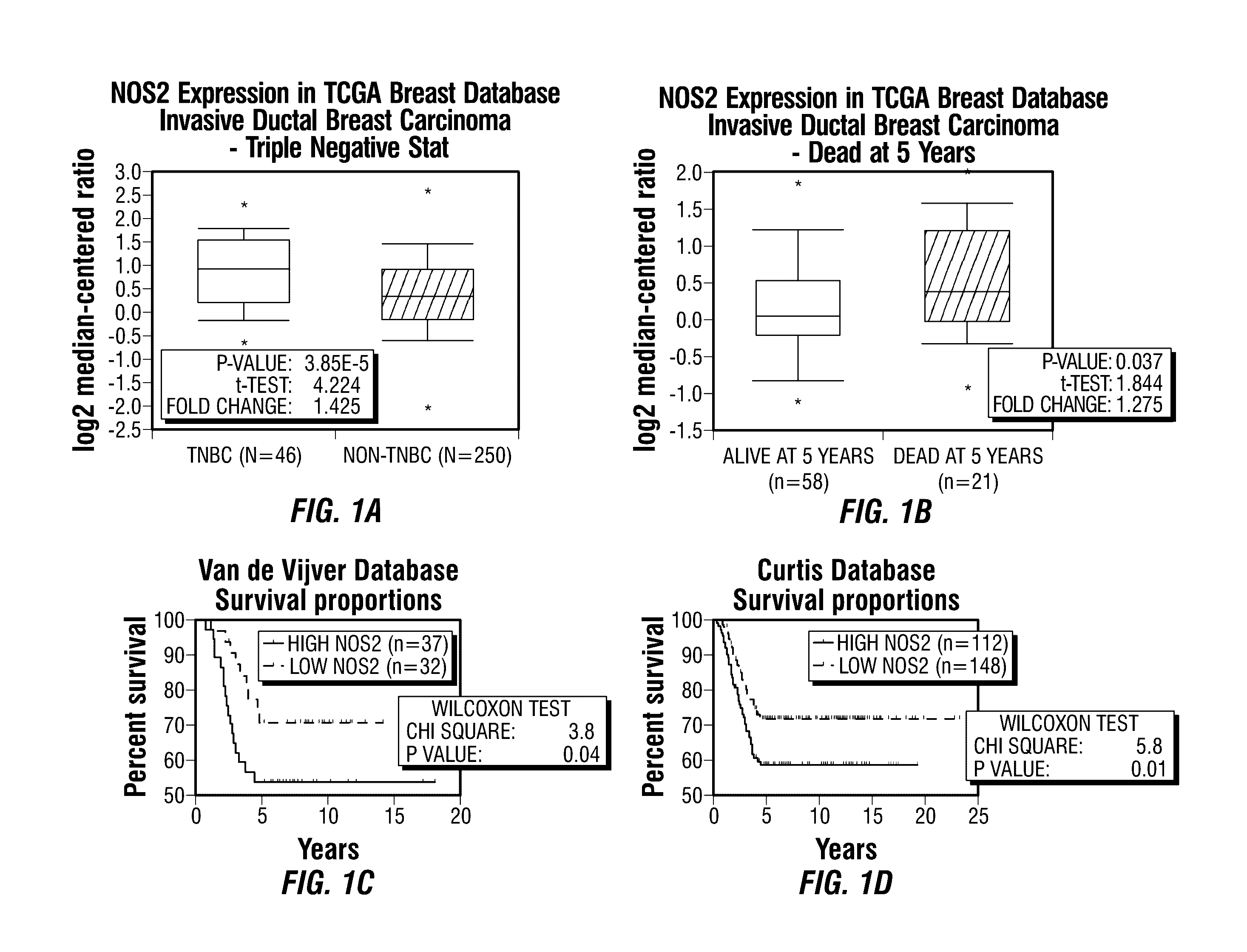
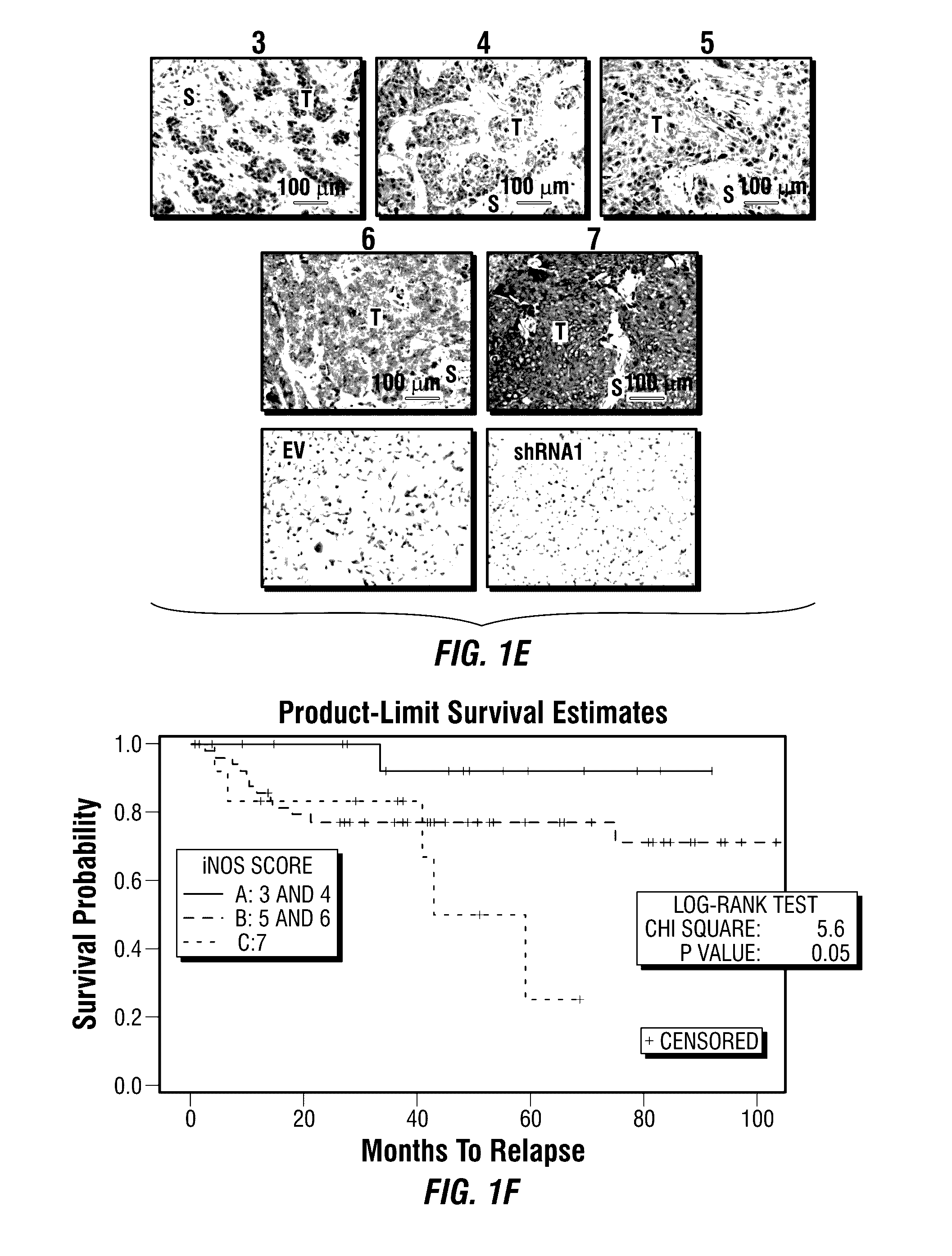
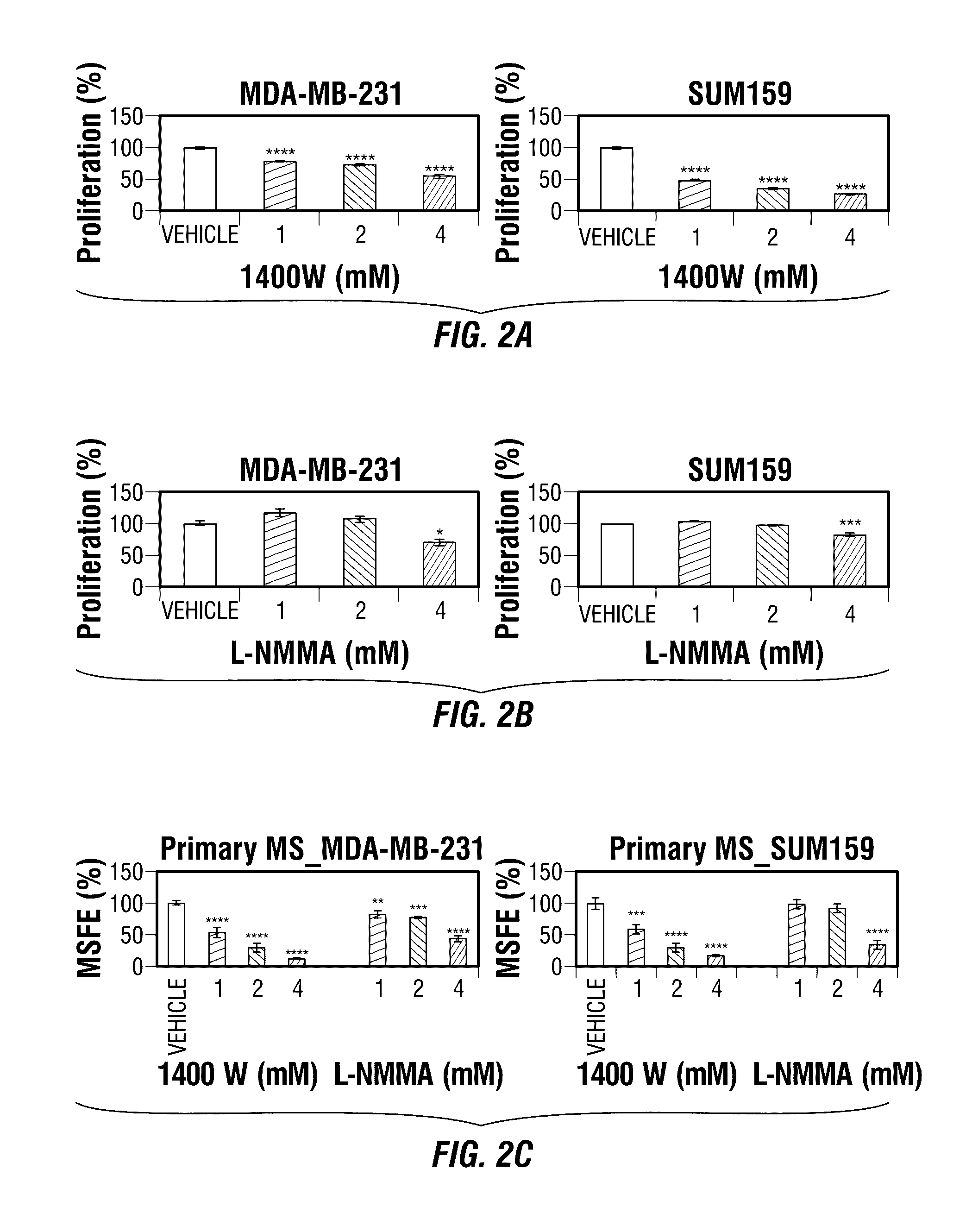
Inos-inhibitory compositions and their use as breast cancer therapeutics
InactiveUS20170020835A1Unexpected benefitEasy to useInorganic active ingredientsAntibody ingredientsRefractoryHuman breast
Disclosed are methods for treating one or more mammalian cancers, and in particular, methods for treating human breast cancer employing one or more iNOS pathway-inhibitory compounds, either alone, or in combination with one or more selected antihypertensive agents, including calcium channel antagonists, either alone, and further in combination with one or more conventional chemotherapeutic or anti-cancer regimens. Also disclosed are particular therapeutic formulations including these compositions, and methods for their use in treating refractory, metastatic, and relapsed cancers, and for managing or reversing treatment resistance in human triple-negative breast cancers in particular.
Owner:THE METHODIST HOSPITAL
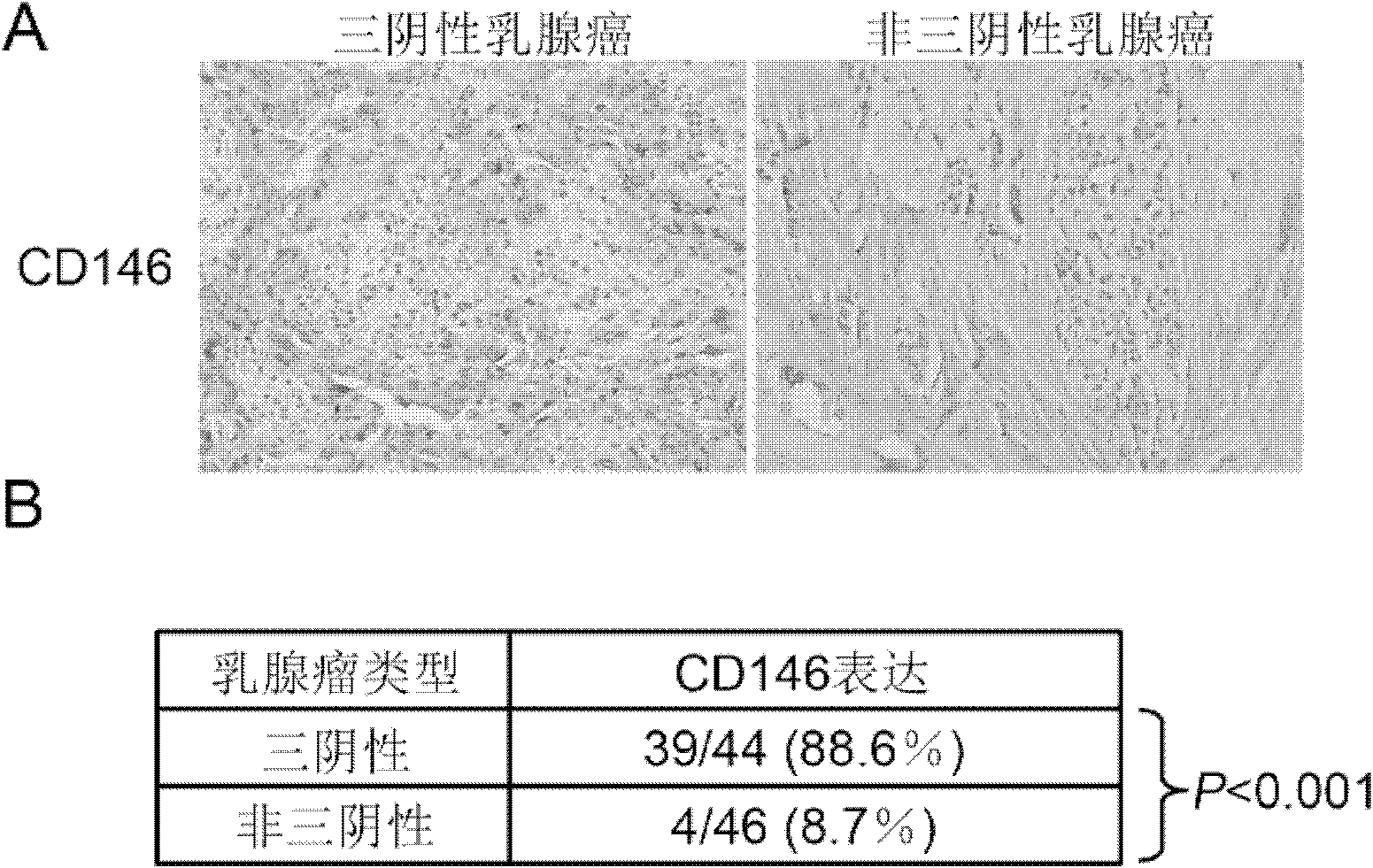
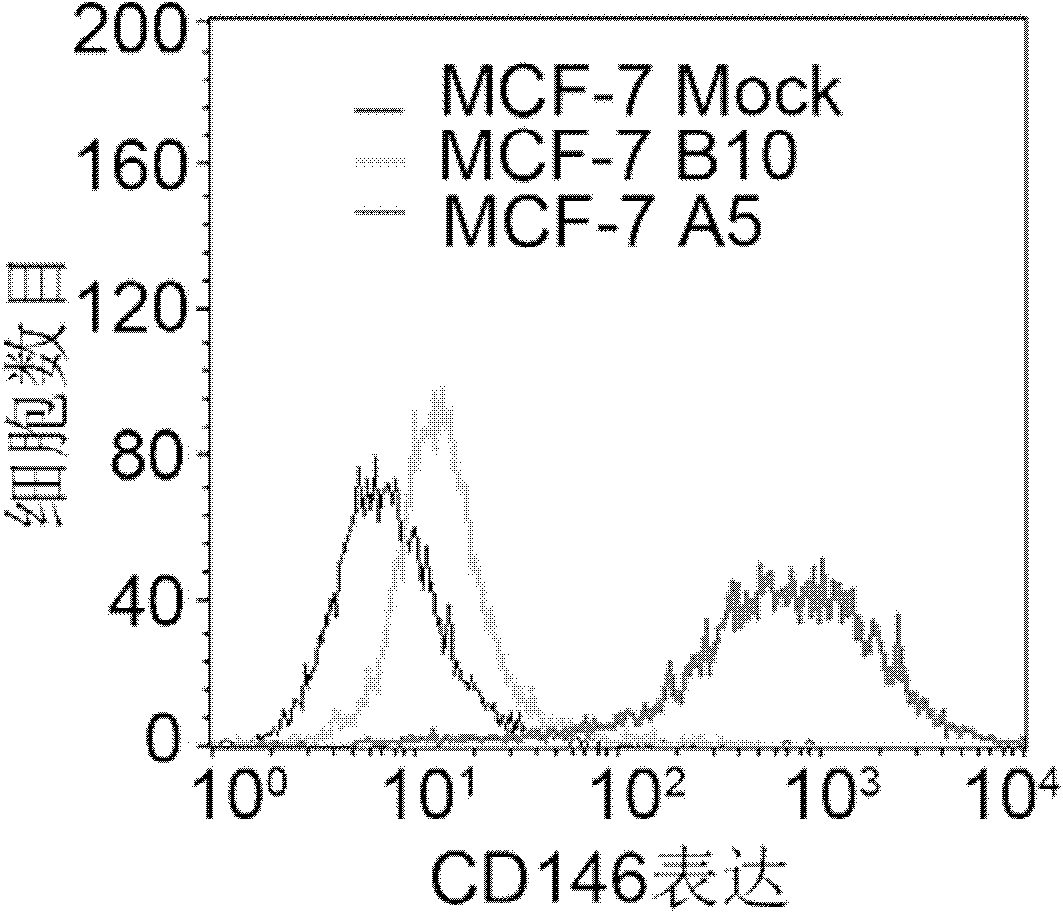
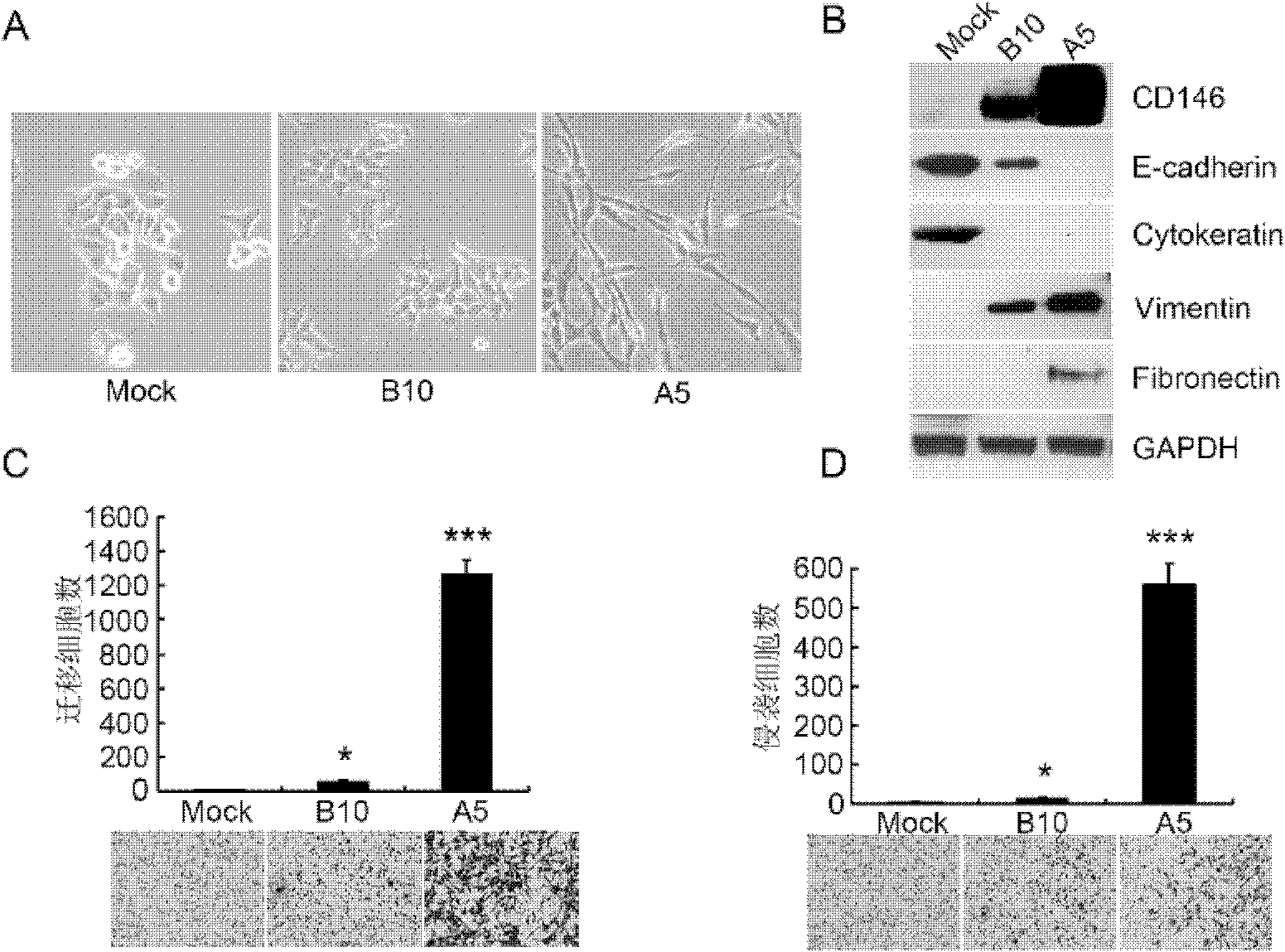
CD146 and antibody diagnosis thereof, and application in treating triple negative breast cancer
The invention relates to CD146 and antibody diagnosis thereof, and an application thereof in treating triple negative breast cancers. For a first time, the invention provides that CD146 is a novel target of triple negative breast cancer, and an anti-CD146 antibody might become a novel targeting medicine for treating the disease. Therefore, the invention provides an application of CD146 or anti-CD146 antibody or a functional form of the antibody in preparing medicines used for diagnosing and / or treating triple negative breast cancers. CD146 molecules are subjected to specific high expression in triple negative breast cancer tissues, and induce the occurrences of transformation of epithelial cell to mesenchymal cell in tumor cells. Therefore, tumor invasion migration is promoted. Therefore, the mechanism for CD146 antibody to treat triple negative breast cancer is mainly that the CD146 antibody inhibits the mesenchymal cell characteristics of triple negative breast cancer, and reduces the metastasis invasion capacity thereof. Compared with common chemotherapy medicines, the anti-CD146 antibody has the advantages of low side effects and clear target. The anti-CD146 antibody does not cause whole-body side effect.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
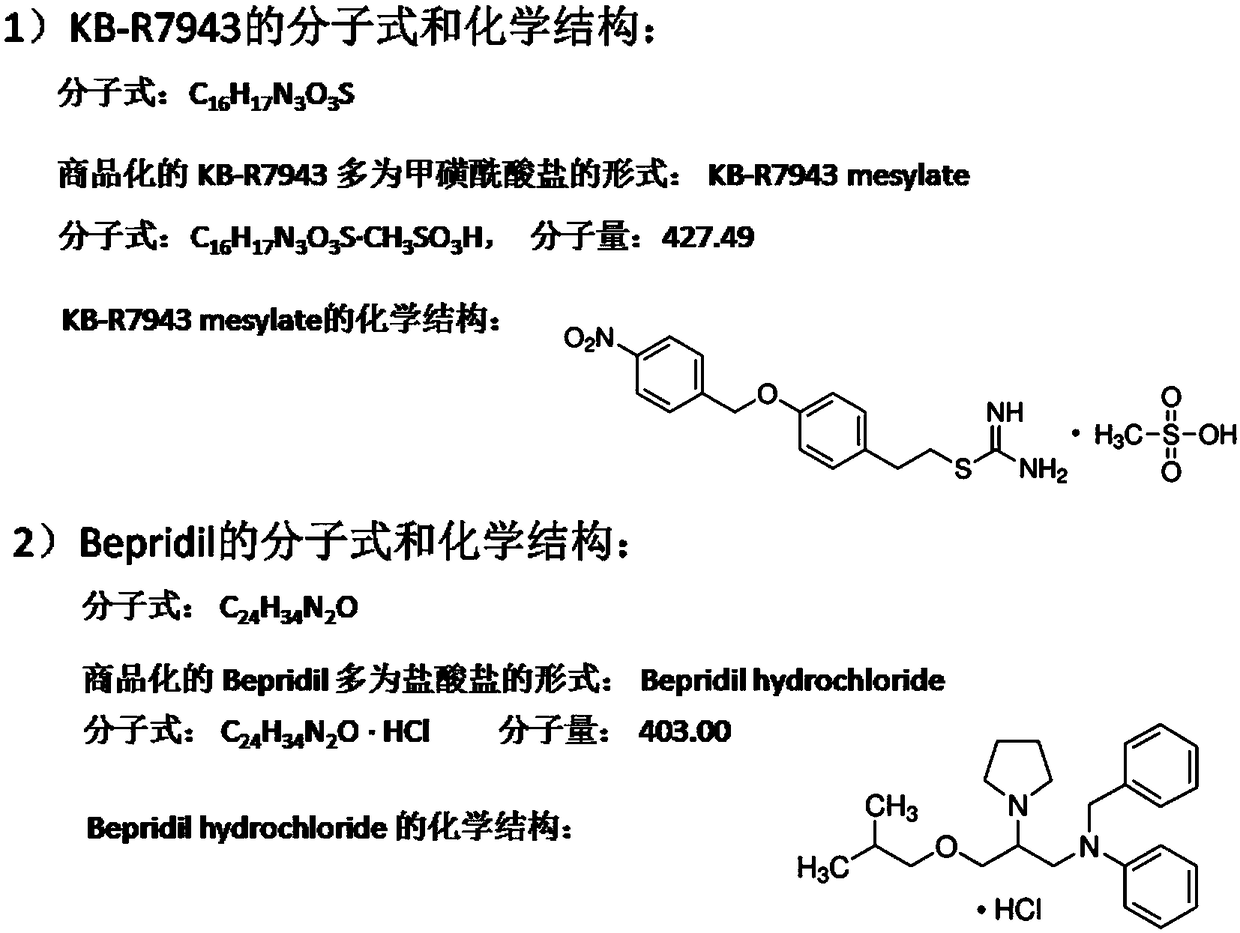
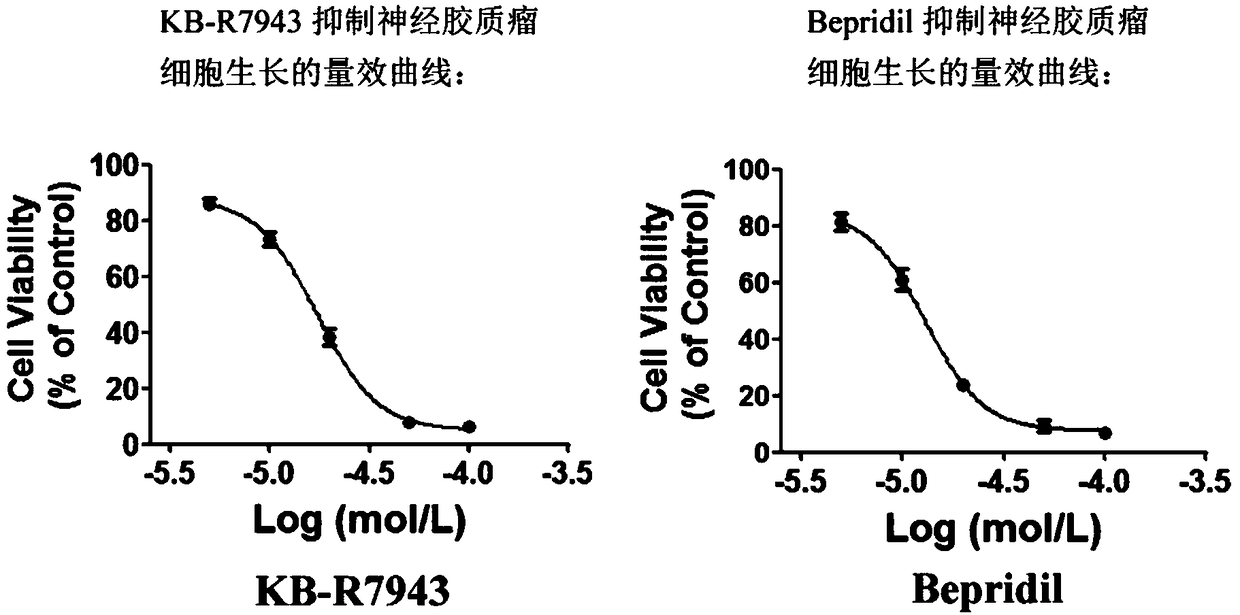
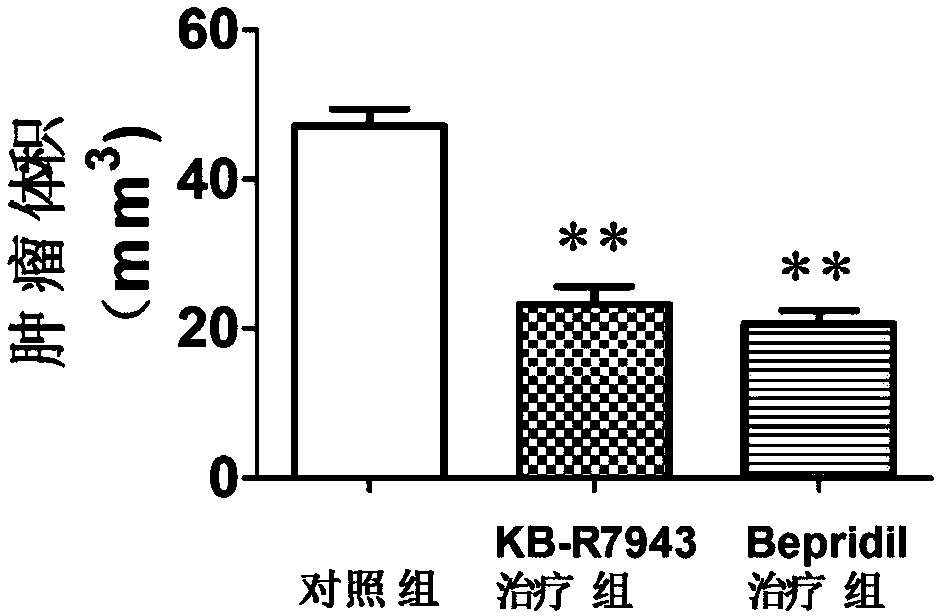
Application of KB-R7943 or Bepridil in preparing medicine for treating gliomas
ActiveCN108066337AEfficient killingBright future for treatmentAntineoplastic agentsHeterocyclic compound active ingredientsWilms' tumorTumor cells
The invention discloses application of KB-R7943 or Bepridil in preparing a medicine for treating gliomas. The invention discloses a novel strategy in treating gliomas, that is, gliomas are treated byusing a sodium-calcium permutoid inhibitor KB-R7943 or Bepridil, and an old medicine has new application. KB-R7943 and Bepridil have tumor cell killing mechanisms which are completely different from those of conventional medicines, and have no drug resistance as well; in addition, both KB-R7943 and Bepridil are capable of improving sensitivity of gliomas to other common chemotherapeutic drugs; dueto adoption of the two novel medicines, prognosis of patients suffering from gliomas can be improved, the survival rate of the patients can be increased, the social burden can be alleviated, and goodprospects can be achieved for treatment on gliomas.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Erianin nano preparation, and preparation method and application thereof
The invention provides an Erianin nano preparation and a preparation method and application thereof. The Erianin nano preparation comprises the following raw materials by weight: 15 to 25 parts of Erianin monomers, 20 to 30 parts of a W / O type emulsifier, 70 to 80 parts of lecithin, 10 to 20 parts of ethanol, and 45 to 55 parts of an O / W type emulsifier. The Erianin nano preparation of the invention breaks through the bottleneck of poor water solubility of the Erianin and cannot be applied clinically, focuses on preparing the poorly soluble monomeric Erianin into related nano preparations, andpushes the Erianin to a clinical application stage. The Erianin nano preparation can solve the defects of poor targetability and many adverse reactions of conventional chemotherapeutic drugs. The nano preparation prepared by the invention has good targeting to the liver of mice, and is expected to be a related targeted preparation for treating primary liver cancer.
Owner:中国人民解放军西部战区总医院
Combination of compounds derived from gallic acid for the treatment of cancer
ActiveUS20150313925A1Reduces clonogenic capacitySmall sizeBiocideCarbohydrate active ingredientsGallic acid esterConventional chemotherapy
The invention relates to a combination of compounds derived from gallic acid, with an antitumoral and antimetastatic activity via a mechanism that involves the induction of apoptosis and the immunogenic death of the tumour cells and the subsequent activation of the specific immune response. The invention also relates to a composition containing a combination of derivatives of gallic acid and pharmaceutically acceptable excipients for the production of useful medicaments in the treatment of cancer. The invention further relates to the use of said composition in a coadjuvant in conventional chemotherapy, reducing the doses of chemptherapeutic agents used in the treatment of cancer.
Owner:PONTIFICIA UNIV JAVERIANA +1
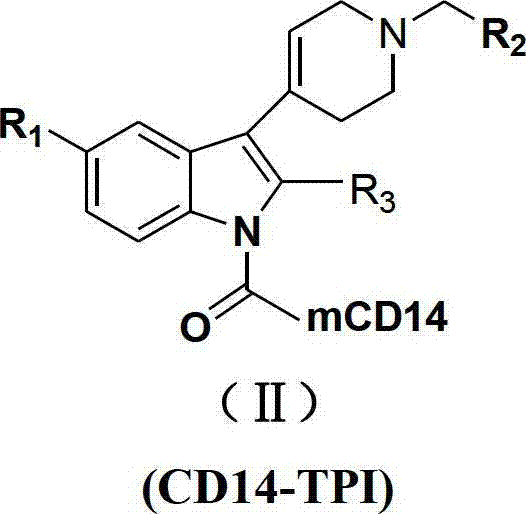
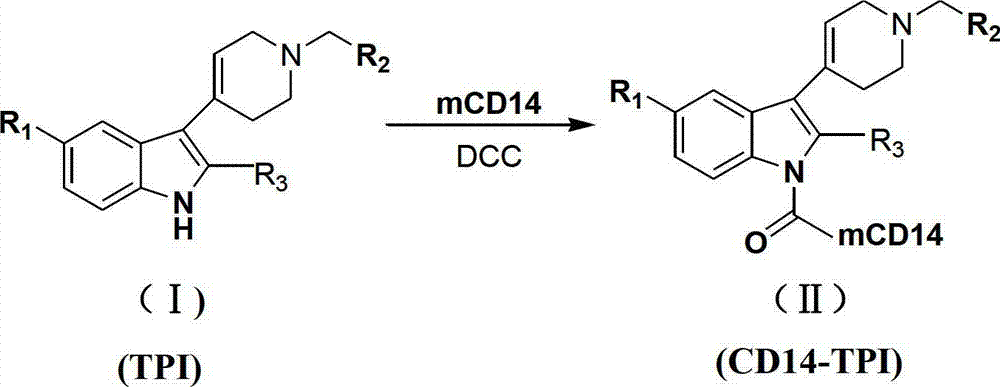
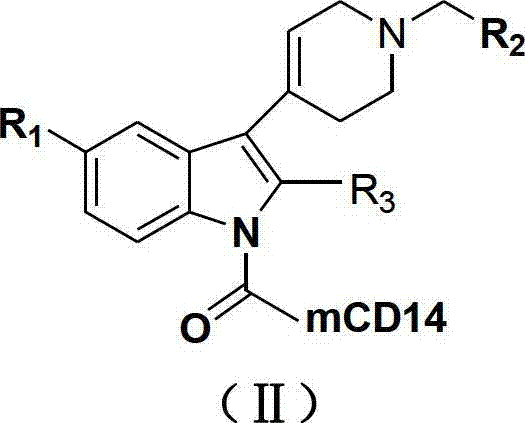
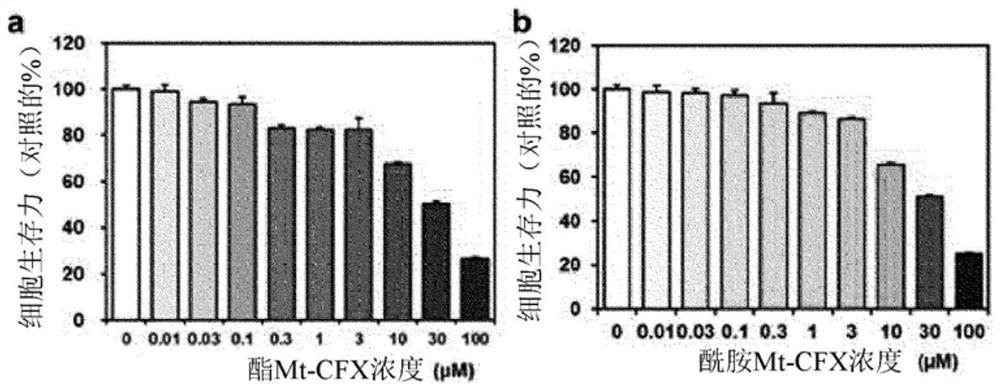
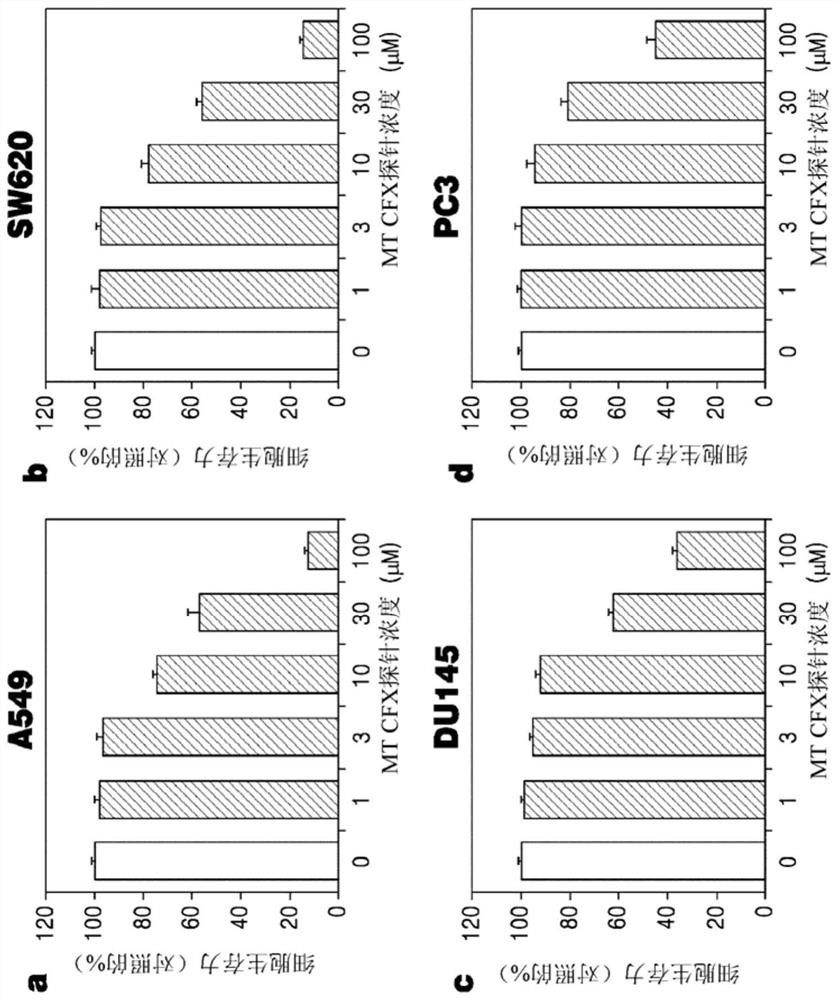
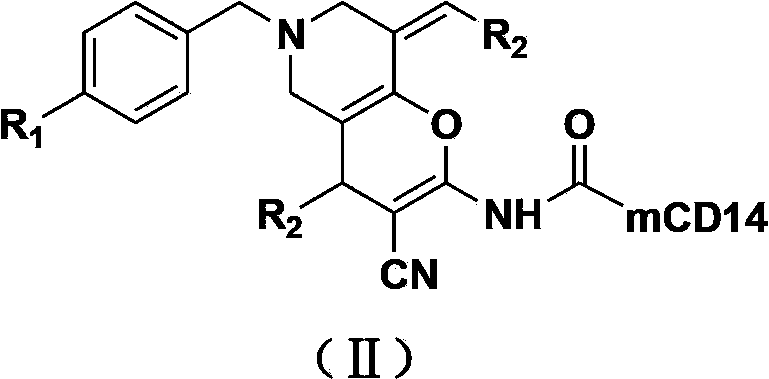
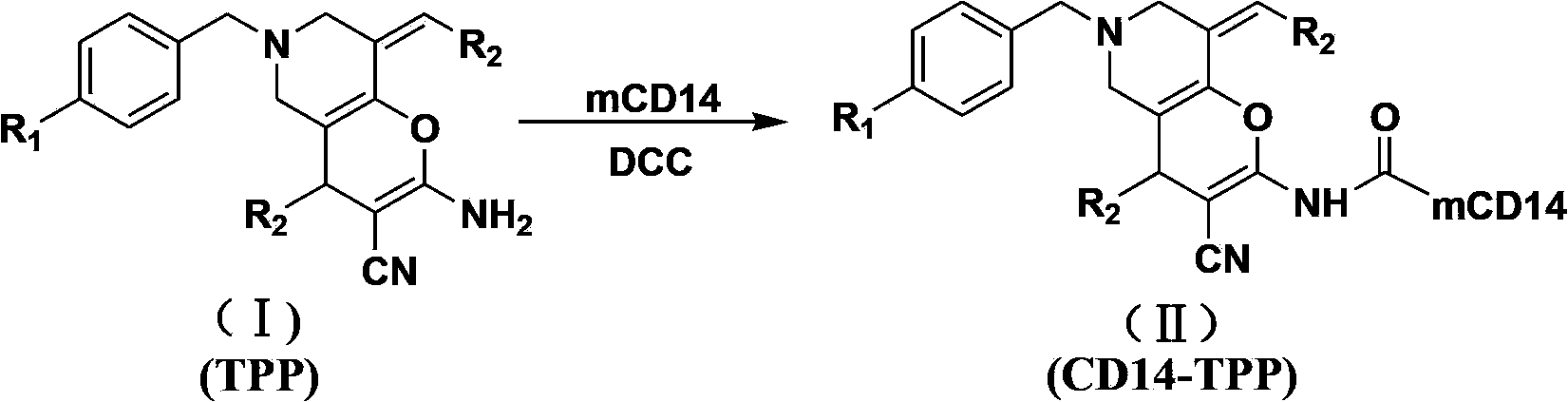
N-substituted-tetrahydropyridylindole-monoclonal antibody CD14 conjugates, and preparation method and application thereof
InactiveCN102898523AInhibit biological activityLow acute toxicityOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesConventional chemotherapy
The invention discloses N-substituted-tetrahydropyridylindole-monoclonal antibody CD14 conjugates, and a preparation method and application thereof, belonging to the field of chemical biomedicine. The structure of the conjugates is disclosed as Formula (II): under the action of a condensing agent DCC, the amino acid residue at the C terminal in the CD14 monoclonal antibody molecule is combined with sec-amino group on the indole ring in N-substituted-tetrahydropyridylindole compounds (I) to obtain the monoclonal antibody CD14 conjugates with General Formula (II). The conjugates disclosed as Formula (II) can efficiently inhibit leukaemia K562 cell proliferation, and the IC50 value for inhibiting leukaemia K562 cell proliferation is obviously lower than that of the conventional chemotherapeutic drug 5-fluorouracil (5Fu); and the conjugates disclosed as Formula (II) have low toxicity for mouse normal marrow cells, and can be used for preparing drugs for treating leukaemia.
Owner:SHANGHAI NORMAL UNIVERSITY
Recombinant DNA, plasmid, transformed microorganism and vaccine protein for prevention and therapy of urinary tract infection
Disclosed is a novel vaccine against Escherichia coli (E. coli) responsible for urinary tract infections. The vaccine is a recombinant chimeric protein which is prepared by linking by genetic recombination a gene encoding an antigenic determinant of uropathogenic E. coli to a CTXA2B gene encoding nontoxic A2 and B subunits of Vibrio cholerae cholera toxin (CTX) or a LTXA2B gene encoding nontoxic A2 and B subunits of E. coli heat-labile enterotoxin, wherein a translation product of the CTXA2B or LTXA2B gene serves as an immunogenic adjuvant stimulating mucosal immune responses, expressing the resulting recombinant gene in E. coli, and isolating and purifying an expressed recombinant fusion protein. The recombinant chimeric protein is useful as an oral vaccine with mild side effects and excellent vaccination efficiency against uropathogenic E. coli. Thus, the chimeric vaccine protein can remarkably reduce recurrence of urinary tract infections, prevent occurrence of antibiotic-resistant bacteria, and replace the conventional chemotherapy for urinary tract infections. Also, the chimeric vaccine protein has other advantages of being capable of being produced and commercialized in a short period with relatively low costs, and being easily modified by replacing its genetic constituents with other genes to provide various vaccines.
Owner:SUNGKYUNKWAN UNIVERSITY
Treatment of aml
InactiveCN101076338AOrganic active ingredientsAntineoplastic agentsConventional chemotherapyAntitumor Antibiotics
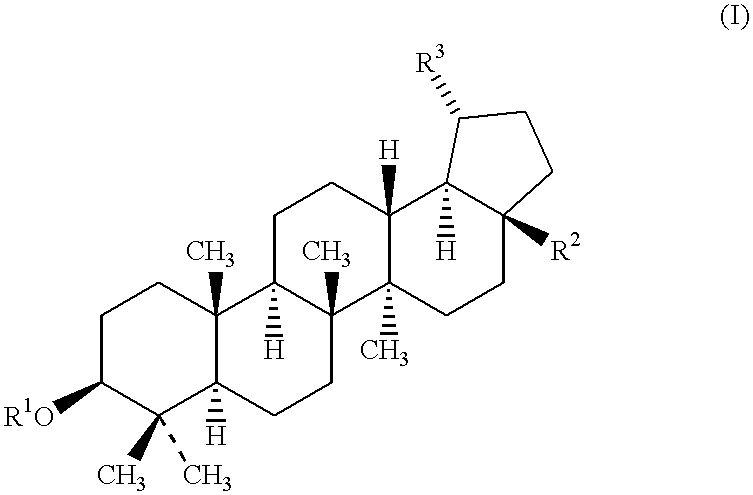
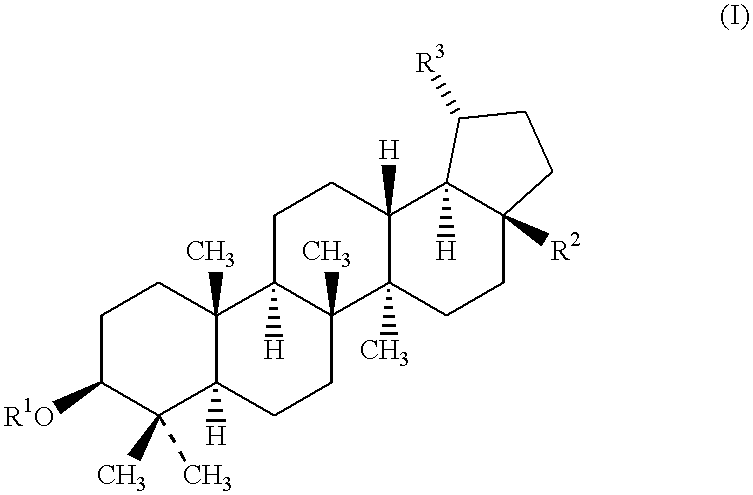
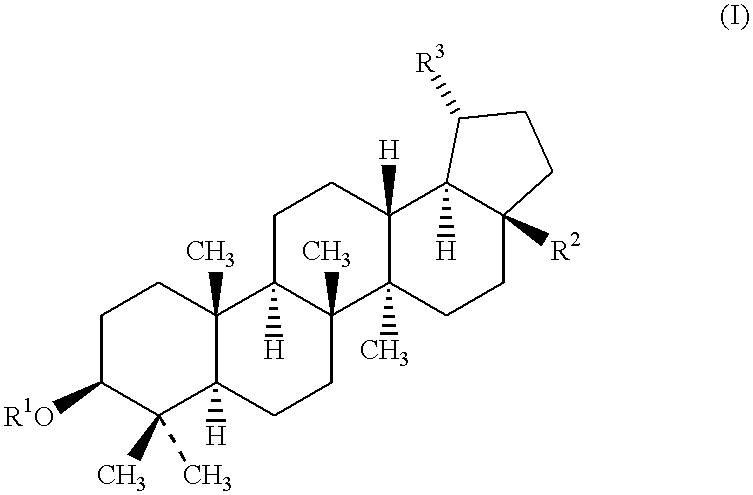
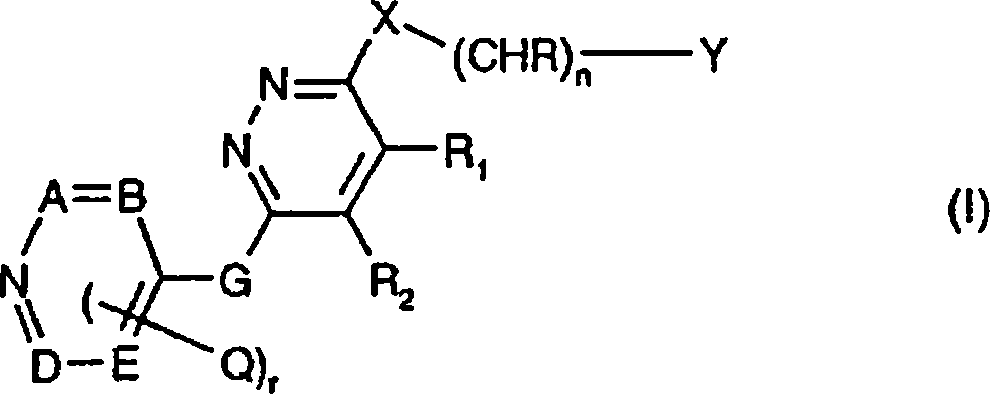
The present Invention relates to a method of treating a warm-blooded animal having acute myeloid leukemia (AML) which is resistant to conventional chemotherapy, comprising administering to said animal a therapeutically effective amount of a compound of formula (I), wherein the radicals and symbols have the meanings as defined in the specification, together or in combination with a conventional compound or compound mixture useful in AML treatment, in particular a topoisomerase II inhibitor, an antimetabolite, or an antitumor antibiotic, for simultaneous, separate or sequential use; and to a pharmaceutical composition and a commercial package comprising said combination.
Owner:齐肯胡伊斯-格罗宁根学院(UMCG)
Combination of compounds derived from gallic acid for the treatment of cancer
ActiveUS9931355B2Organic active ingredientsAntineoplastic agentsGallic acid esterConventional chemotherapy
The invention relates to a combination of compounds derived from gallic acid, with an antitumoral and antimetastatic activity via a mechanism that involves the induction of apoptosis and the immunogenic death of the tumor cells and the subsequent activation of the specific immune response. The invention also relates to a composition containing a combination of derivatives of gallic acid and pharmaceutically acceptable excipients for the production of useful medicaments in the treatment of cancer. The invention further relates to the use of said composition in a coadjuvant in conventional chemotherapy, reducing the doses of chemotherapeutic agents used in the treatment of cancer.
Owner:PONTIFICIA UNIV JAVERIANA +1
Method for predicting survival in children with acute lymphoblastic leukemia
InactiveUS20210139993A1Good effectMicrobiological testing/measurementGlucocorticoidConventional chemotherapy
The invention relates to methods for predicting the clinical outcome of cancer patients, and in particular of acute lymphoblastic leukaemia (ALL) patients, in response to a therapy against ALL, preferably conventional chemotherapy, more preferably based on glucocorticoids, said methods based on the presence of particular polymorphism in genes coding for drug-metabolizing enzymes and apoptotic proteins. The invention relates as well to method for predicting the efficacy of a therapy based on conventional glucocorticoids as well as to method for personalized medicine in patients carrying said polymorphisms.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA
Repurposed antibiotics for non-nuclear genotoxic chemotherapy and pharmaceutical composition for Anti-cancer containing the same
PendingCN113853380APrevent relapseGroup 5/15 element organic compoundsPhosphorous compound active ingredientsCancer preventionCancer cell
The present invention relates to a repurposed antibiotic compound for the treatment of cancer with minimal nuclear gene damage and an anticancer pharmaceutical composition comprising same. Since the repurposed antibiotic compound has a therapeutic effect in a manner that targets only the mitochondria of cancer cells, the modified antibiotic compound does not cause gene degeneration unlike conventional chemotherapy which damages nuclear DNAs to kill cancer cells,, thereby preventing the recurrence of cancer. In addition, a mitochondria targeted therapy using the compound according to the present invention can effectively treat malignant tumors that are difficult to treat due to acquiring drug resistance by general anticancer treatment.
Owner:诊疗化学公司
Anti-avian influenza traditional Chinese medicine composition
InactiveCN106924351APreserve the combination effectDefinite curative effectAntiviralsAluminium/calcium/magnesium active ingredientsDiseaseConventional chemotherapy
The present invention relates to an anti-avian flu traditional Chinese medicine composition, the constituent components and parts by weight are 35-45 parts of Folium Folium; 25-35 parts of Forsythia; 25-35 parts of Burdock; 15-25 parts of capillary ; Goose does not eat grass 15-25 parts; Guizhi 15-25 parts. The present invention overcomes the disability and teratogenic defects of existing chemotherapeutic drugs, and the main drugs Folium Folium, Forsythia, Arctium Fructus, capillary, goose herbivore, and cassia twig act synergistically, and have strong inhibitory effect on avian influenza virus The prescription has the effects of mediating the body's immunity and relieving symptoms, and has a significant preventive effect on bird flu diseases, and is a new type of traditional Chinese medicine for preventing and treating bird flu.
Owner:TIANJIN JIHE TECH
Tetrahydropyridinopyran-monoclonal antibody CD14 conjugate with anti-tumor activity and preparation method and use thereof
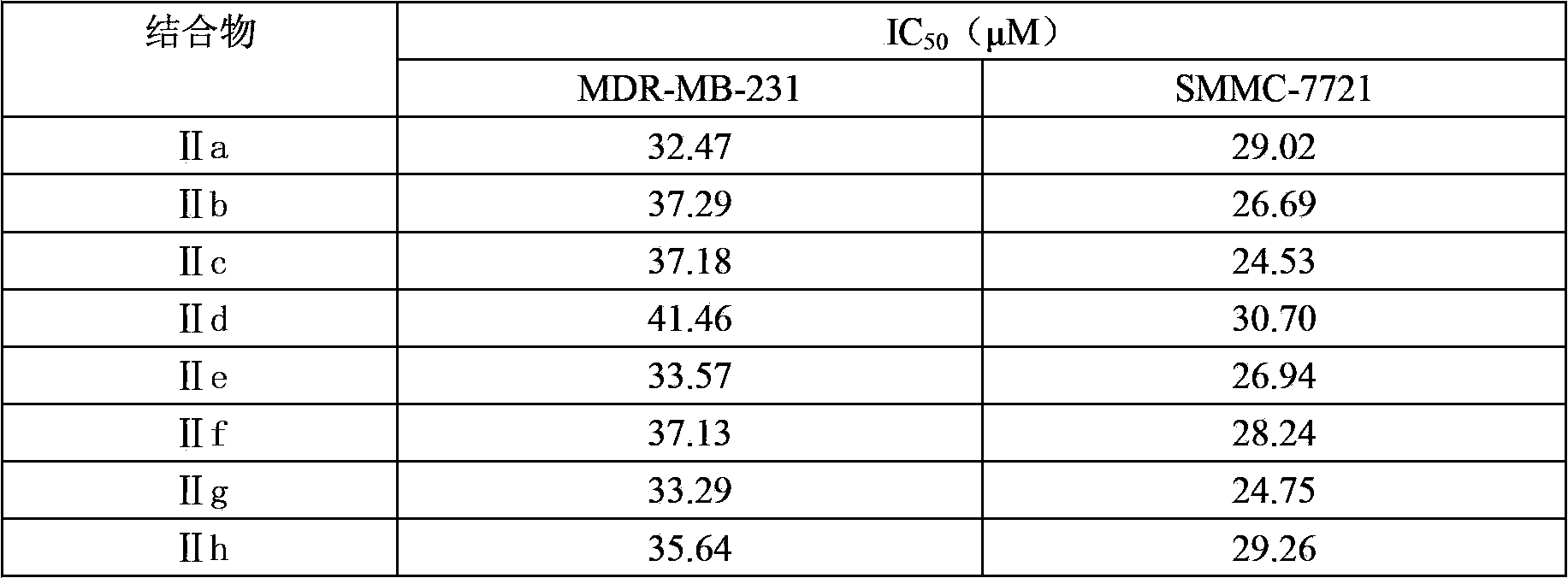
InactiveCN102898522BLow toxicityLow acute toxicityImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical non-active ingredientsAbnormal tissue growthNormal bone
The invention discloses tetrahydropyridinopyran-monoclonal antibody CD14 conjugates with anti-tumor activity and a preparation method and use thereof. The structure of the conjugate is represented by a formula (II). Under the action of dicyclohexylcarbodiimide (DCC) serving as a condensing agent, an amino acid residue on the carbon terminal of a CD14 monoclonal molecule is bonded with an amino on a pyran ring in a tetrahydropyridinopyran derivative to form a monoclonal antibody conjugate CD14-TPP of the formula (II). CD14-TPP can effectively inhibit the proliferation of a human erythroleukemia cell line K562, an ovarian cancer cell line MDR-MB-231 and a liver cancer cell line SMMC-7721 with an IC50 value much lower than 5-fluorouracil (5Fu) which is a conventional chemotherapy medicine. The conjugate of the formula (II) with low toxic effect on normal bone narrow cells of mouse can be used for preparing medicines for treating leukemia, ovarian cancer and liver cancer.
Owner:SHANGHAI NORMAL UNIVERSITY
Application of Tagalsin C and its homologous compound in preparing anti-tumor medicine
The invention relates to the application of marine biological metabolite Tagalsin C and its homologues Tagalsins A, B, D, E, F, G in the preparation of antitumor drugs. This series of compounds has membrane permeability and broad-spectrum anti-tumor activity, and can kill human promyelocytic acute leukemia cells HL-60, human myeloma cells IM9, human acute T-cell leukemia cells Jurkat, and human macrophage lymphoma cells U937, human lung cancer cell A549, human breast cancer cell MCF-7 and MDA-MB-231, human liver cancer cell HepG2, human prostate cancer cell PC-3 and human cervical cancer epithelial cell Hela, while normal cell human kidney blast 293 And the human liver diploid cell L02 has no obvious killing ability, and can be applied to the preparation of drugs for treating tumors, drugs for treating clinical high expression of Bcl-2, resistant to conventional chemotherapy, and drugs for treating recurrent cancer.
Owner:PEKING UNIV
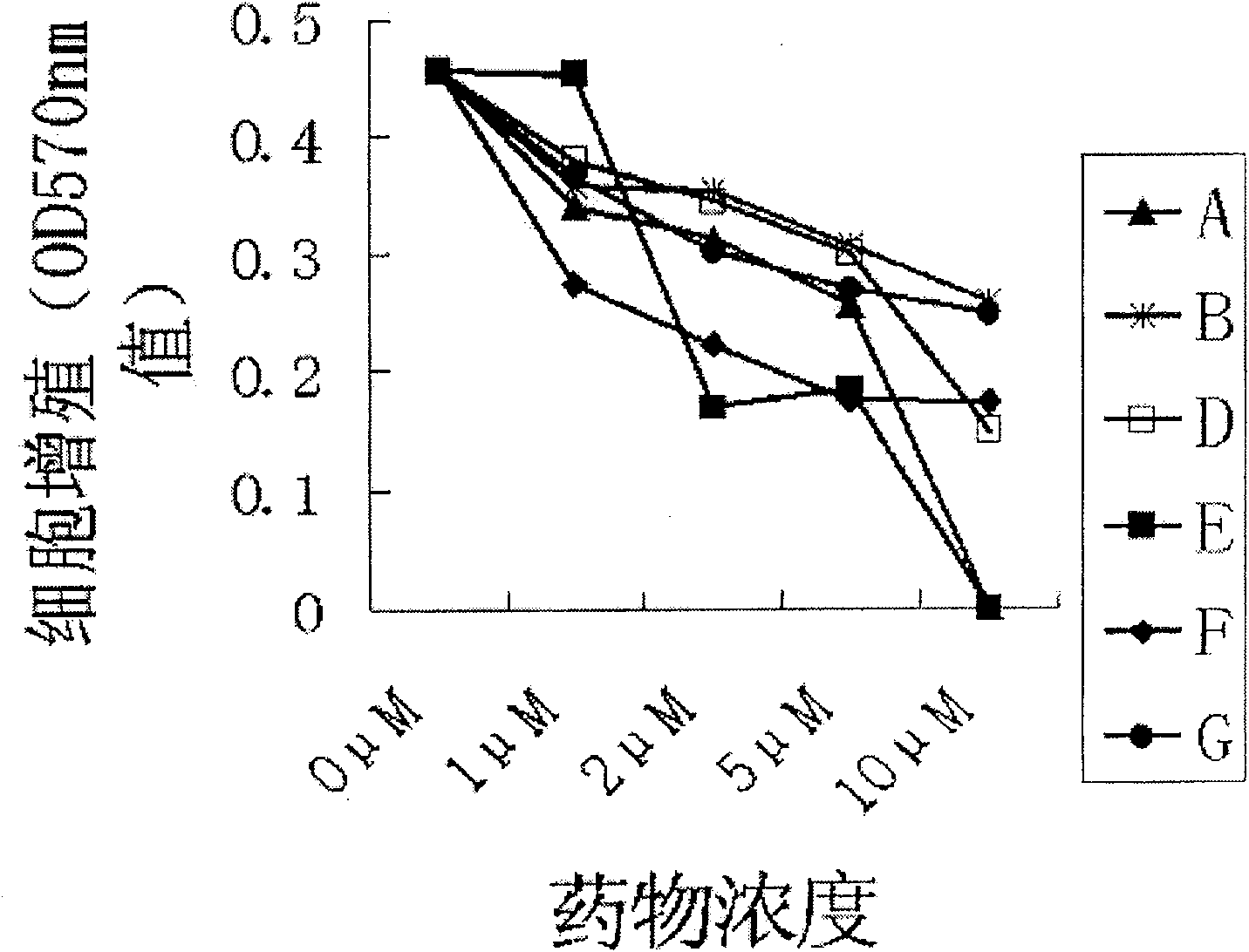
Application of PGC-1[alpha] specific RNA interfering adenovirus
ActiveCN107929305AGood treatment effectRaise the ratioOrganic active ingredientsUnknown materialsDiseaseConventional chemotherapy
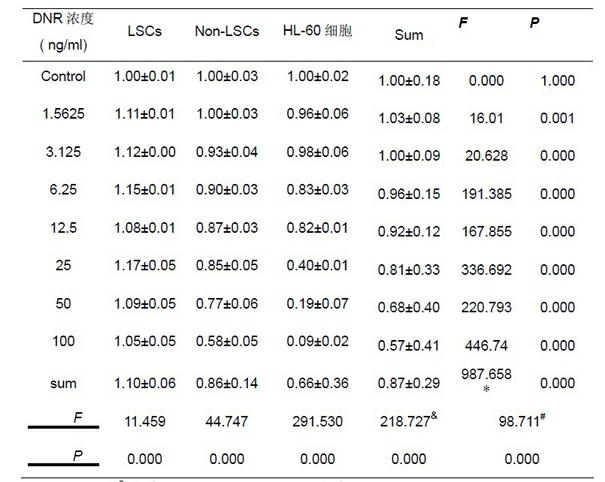
The invention discloses an application of PGC-1[alpha] specific RNA interfering adenovirus in reducing leukemia cell drug resistance. The PGC-1[alpha] specific RNA interfering adenovirus provided by the invention can enhance the sensitivity of acute myeloid leukemia cells to conventional chemotherapeutic drugs (an apoptotic cell proportion is increased from a range of 22.7-27.7% to a range from 40.3-50.5%), so that the survival time of a disease animal model can be obviously prolonged; taking a leukemia mouse model, which is prepared by virtue of OCI-AML3 cells, as an example, the average survival time of a leukemia mouse, under the circumstance of accepting conventional chemotherapy, is 37.9 days; and on the basis of the conventional chemotherapy, and in combination with PGC-1[alpha] specific RNA interfering adenovirus treatment, the average survival time of the mouse exceeds 45 days, which is prolonged by more than 20%.
Owner:ZHEJIANG UNIV
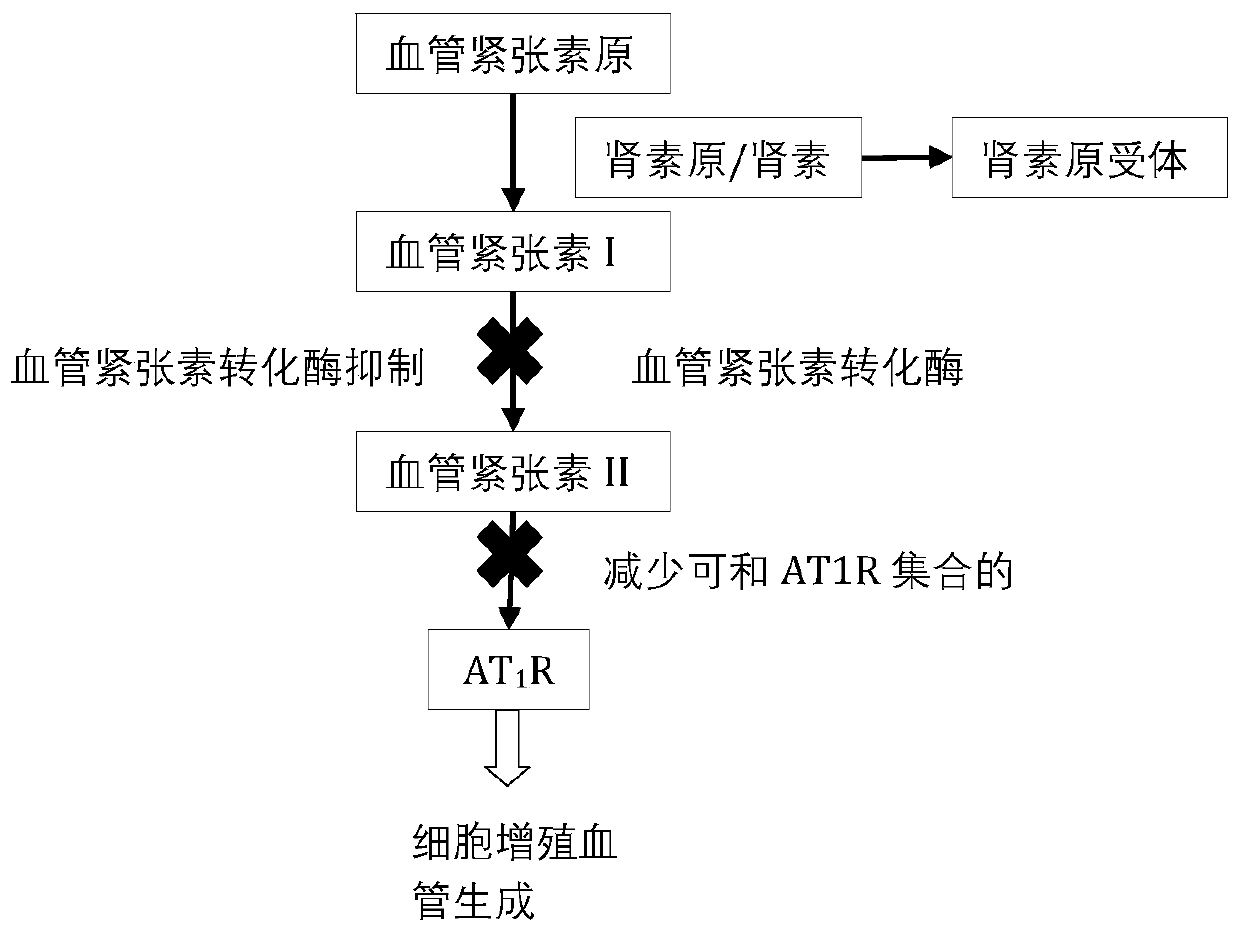
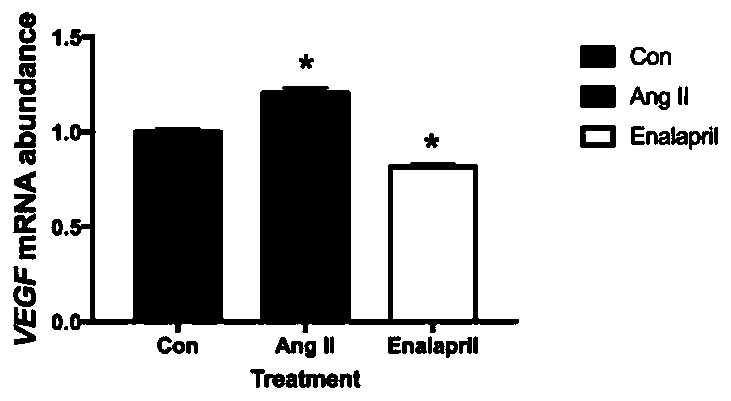
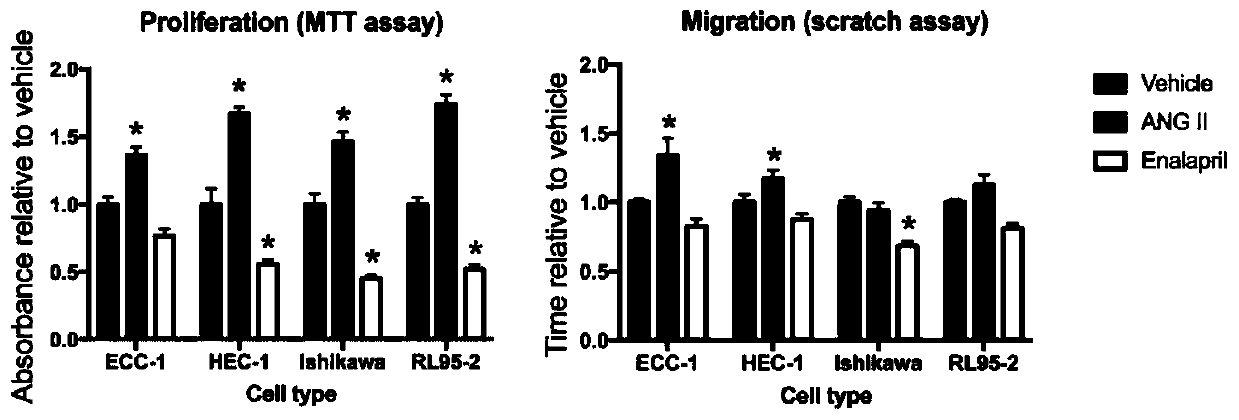
Application of enalapril to preparation of antitumor medicines
PendingCN110772511ALittle impact on quality of lifePrevent relapseAntineoplastic agentsHeterocyclic compound active ingredientsCell invasionCancer prevention
The invention provides a new application of enalapril to preparation of antitumor medicines. The enalapril can act on a renin angiotensin system (a regulator of a VEGF mediated angiogenesis system), and an ACE / AngII / AT1R route participating in adjusting cell proliferation and cell invasion. Treatment medicines can act on an ACE route participating in adjusting cell proliferation and invasion. Thetreatment manner not only can be used in the whole treatment period but also can be used for preventing cancer recurrence after treatment is successful. The use of the enalapril as an anticancer medicine also contributes to alleviating cardiovascular system side effects of conventional chemotherapy.
Owner:天津贝罗尼生物科技有限公司
Cancerous mucus dissolution function and novel anti-tumor application of standardized myrtol
The invention discloses a mucus dissolution function of standardized myrtol and an anti-tumor function of pharmaceutical composition of the standardized myrtol. The standardized myrtol can effectivelydissolve cancerous mucus, has the anti-tumor function and can improve chemosensitivity in combination with conventional chemotherapy. The standardized myrtol has good safety and has no obvious adverse effects by long-term application.
Owner:李雁
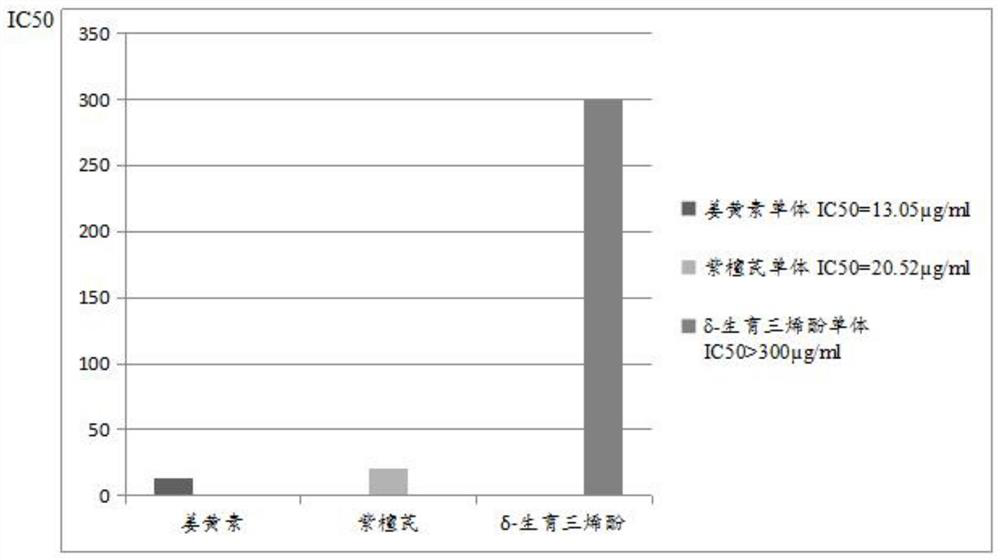
A curcumin compound preparation for treating cancer
ActiveCN109350613BLow recurrence rateImprove anti-cancer effectEther/acetal active ingredientsKetone active ingredientsConventional chemotherapyOncology
The invention relates to a curcumin compound preparation for treating cancer. By using curcumin, pterostilbene and δ-tocotrienol as raw materials and carrying out a suitable weight ratio, the three components are synergistically The number of anti-cancer targets and the anti-cancer spectrum are superimposed, and the anti-cancer effect is significantly multiplied. The curcumin in the curcumin compound preparation of the present invention can kill cancer stem cells, and can significantly reduce the recurrence rate of cancer compared with conventional chemotherapy methods that cannot kill cancer stem cells. The invention is non-toxic to human body and will not produce adverse reactions when applied in large doses. And the treatment cost is expected to be less than half or lower than that of conventional chemotherapy drugs, and far lower than the high treatment cost of cellular immunotherapy CAR‑T, which has broad application prospects in the field of cancer treatment.
Owner:朱理查德澄朗
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Application of PGC-1[alpha] specific RNA interfering adenovirus Application of PGC-1[alpha] specific RNA interfering adenovirus](https://images-eureka.patsnap.com/patent_img/4429e9a1-c7fa-4766-b2bc-d439af584322/HDA0001446640140000012.png)
![Application of PGC-1[alpha] specific RNA interfering adenovirus Application of PGC-1[alpha] specific RNA interfering adenovirus](https://images-eureka.patsnap.com/patent_img/4429e9a1-c7fa-4766-b2bc-d439af584322/DEST_PATH_HDA0001580100780000011.png)