Application of Tagalsin C and its homologous compound in preparing anti-tumor medicine
A technology of anti-tumor drugs and homologues, applied in the field of drugs, can solve problems such as drug resistance and cancer recurrence, and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
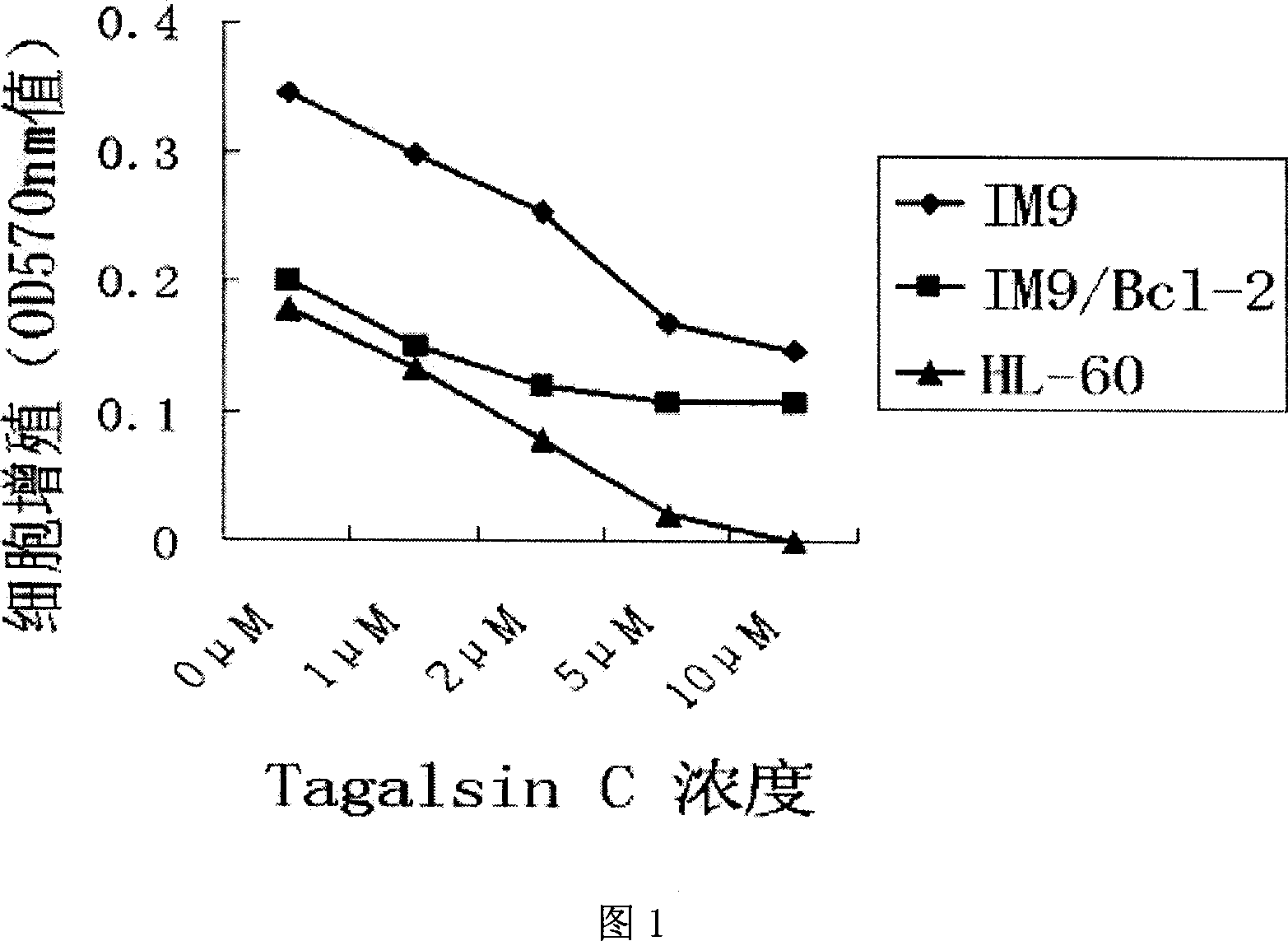
[0023] Example 1, MTT method to detect Tagalsin C and its homologues inhibiting the growth of IM9, IM9 / Bcl-2 and HL-60 cells
[0024] Human myeloma IM9 cells, human myeloma IM9 cells with high expression of Bcl-2 (stable expression cell line IM9 / BcL-2 transfected with human Bcl-2 cDNA), and human leukemia cells HL-60 were mixed at 10 per well. 5 Cells, 100μl / well spread in a 96-well culture plate, add Tagalsin C and its homologues to make three duplicate holes for each compound, at 37°C, 5% CO 2 Incubate in an incubator for 24 hours, add MTT reagent (product of Sigma Company) and mix well, continue to incubate for 3 to 5 hours, and analyze the growth inhibition of different concentrations of drugs on cells by an ELISA plate reader at a wavelength of 570nm.
[0025] The relationship between the growth of tumor cells and the concentration of Tagalsin C is shown in Figure 1, indicating that Tagalsin C has cell membrane permeability and can effectively inhibit the growth of myelom...
Embodiment 2
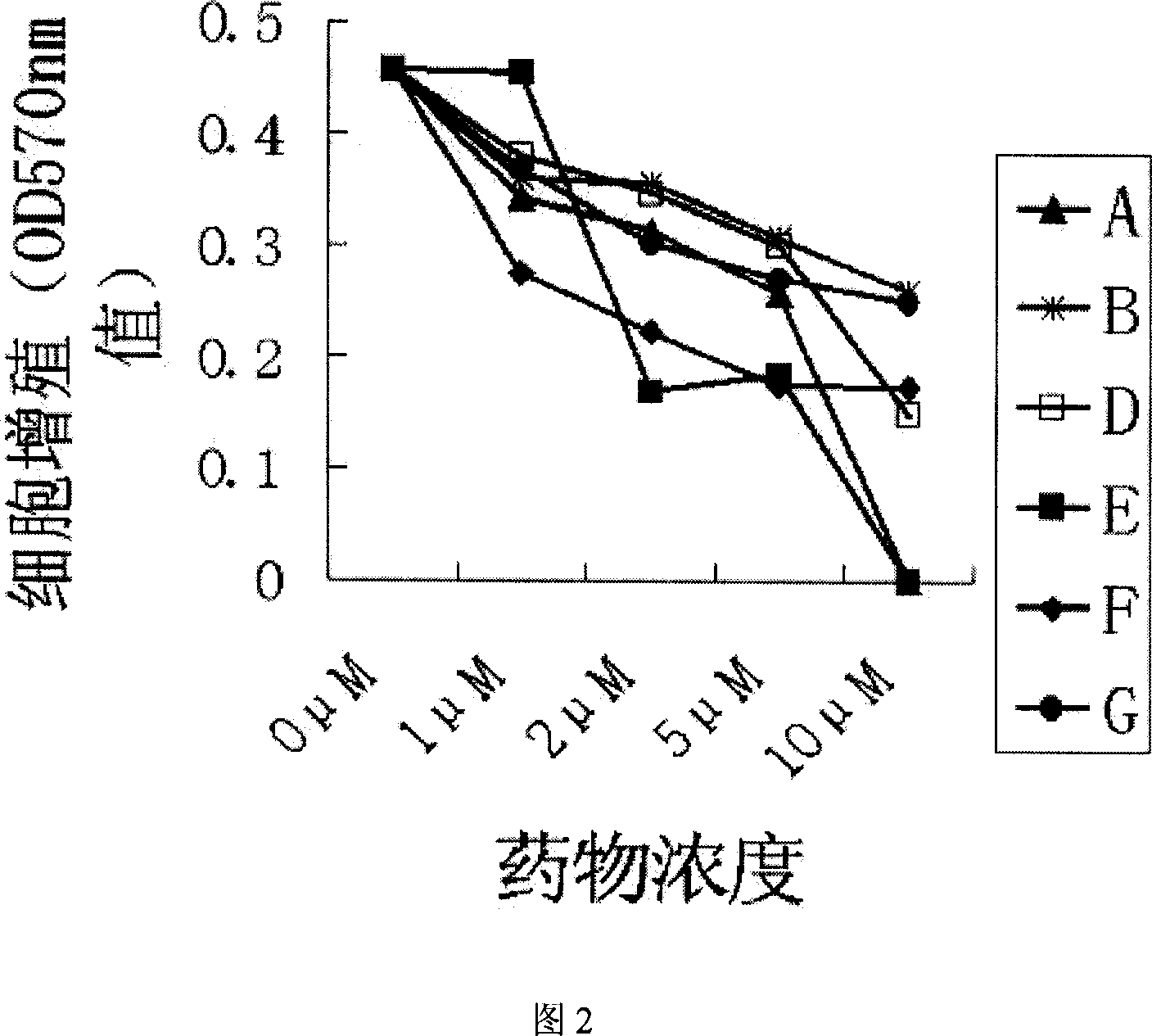
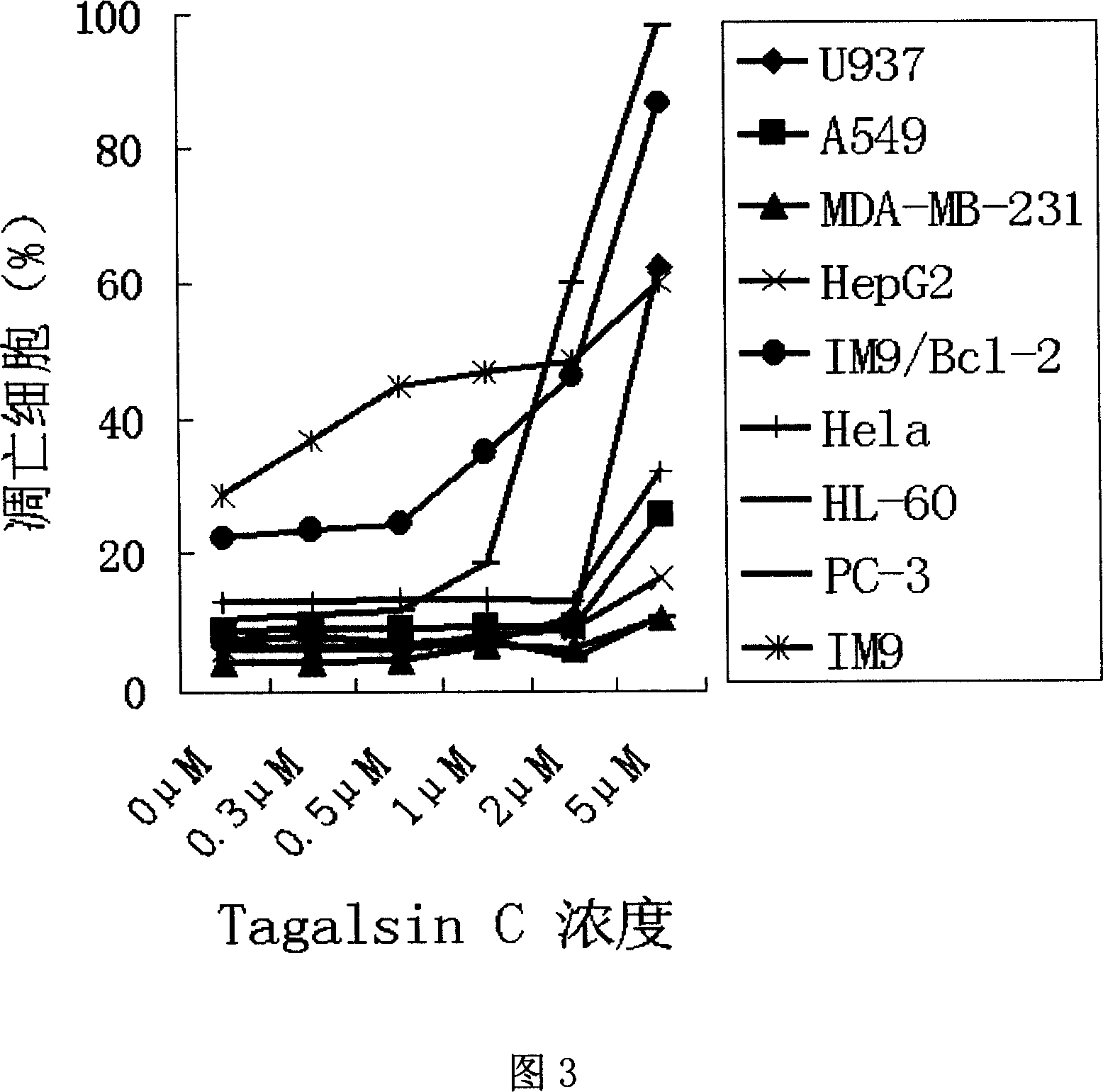
[0028] Example 2, Tagalsin C and its homologues kill human myeloma cells and other tumor cells with high expression of Bcl-2
[0029] Then we studied whether Tagalsin C and its homologues Tagalsin A, B, D, E, F, G could induce the apoptosis of human myeloma cells and other tumor cells with high expression of Bcl-2. We applied flow cytometry, which is commonly used at home and abroad, combined with FITC-AnnexinV staining, an indicator of apoptosis, to quantitatively detect the apoptosis induced by Tagalsin C and its homologues. Inoculate different types of tumor cells in 24-well plates, then add different concentrations of Tagalsin C and its homologues Tagalsin A, B, D, E, F, G for 24 hours, wash twice with PBS, add FITC-labeled AnnexinV (The kit is provided by Beijing Baosai Biotechnology Co., Ltd.), and the killing effect of the drug on tumor cells is detected by flow cytometry (FACSCalibur, a product of BD Company of the United States).
[0030] Table 1 shows the experiment...
Embodiment 3
[0039] Example 3, Experiment of Tagalsin C Inducing Tumor Cell Nuclear DNA Fragmentation
[0040] Various tumor cells that were normally cultured and treated with Tagalsin C for 24 hours were washed twice with PBS, incubated with PBS containing 0.2% Tween20 at 37°C for 15 minutes, and then specifically mixed with DNA at a final concentration of 0.25 μg / ml (prepared in PBS). The permanent dye DAPI (product of Sigma Company) was reacted at room temperature in the dark for 30 minutes, and the changes of DAPI-stained nuclei during apoptosis were observed under an Olympus fluorescence microscope.
[0041] As shown in Figure 5A, the changes of DAPI-stained nuclei in human lung cancer cell A549 and human cervical cancer epithelial HeLa cells during the apoptosis process and in Figure 5B are the changes of DAPI-stained nuclei in human liver cancer cells HePG2, human macrophage lymphocytes The changes in the apoptosis process of tumor cell U937, human prostate cancer cell PC-3 and huma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com