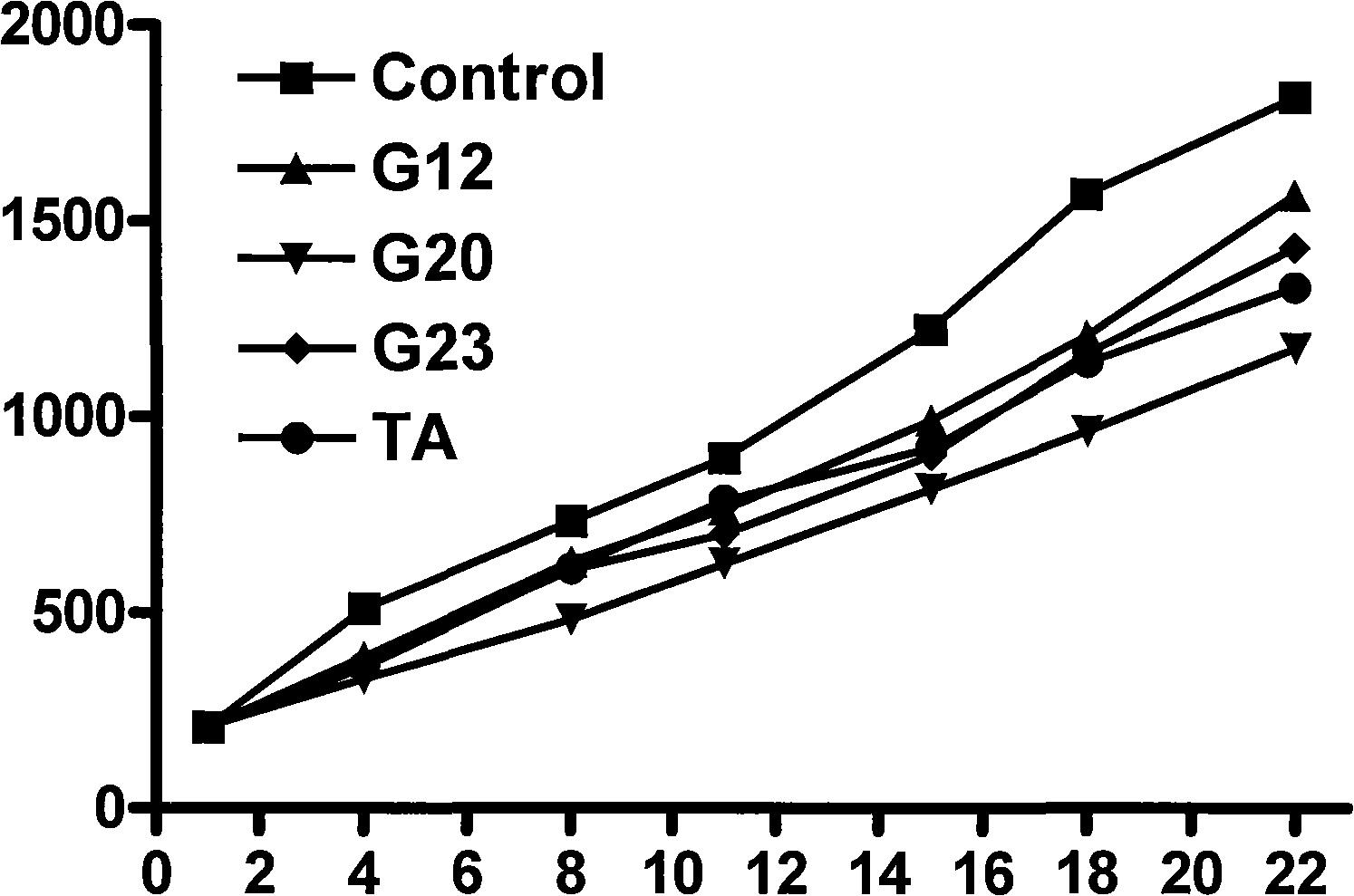
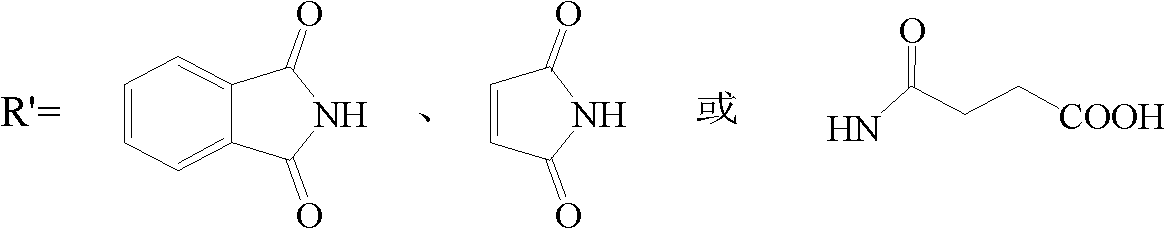
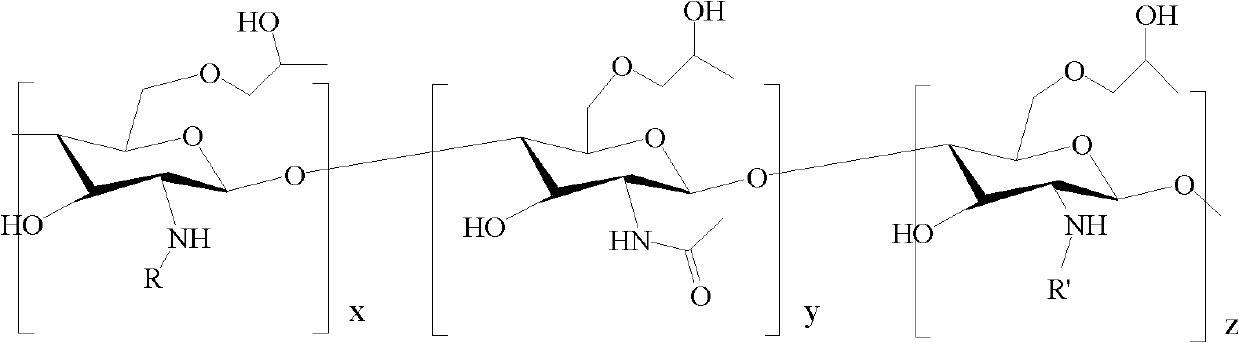
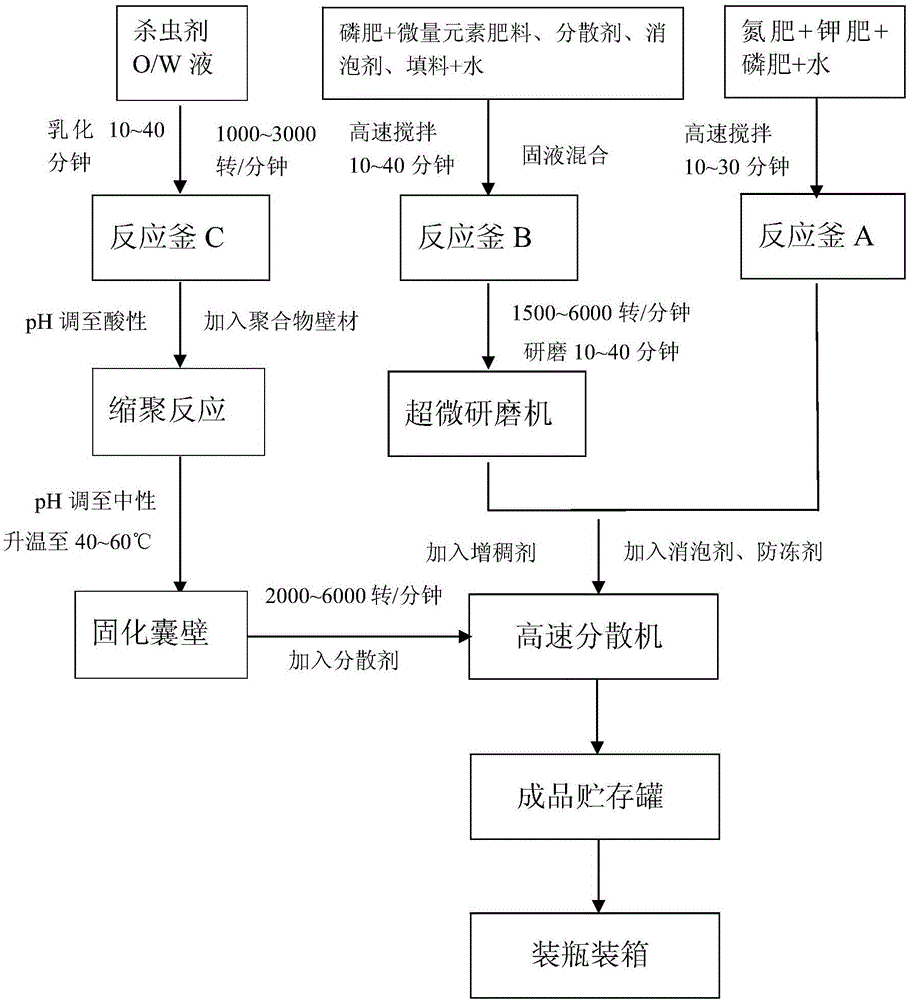
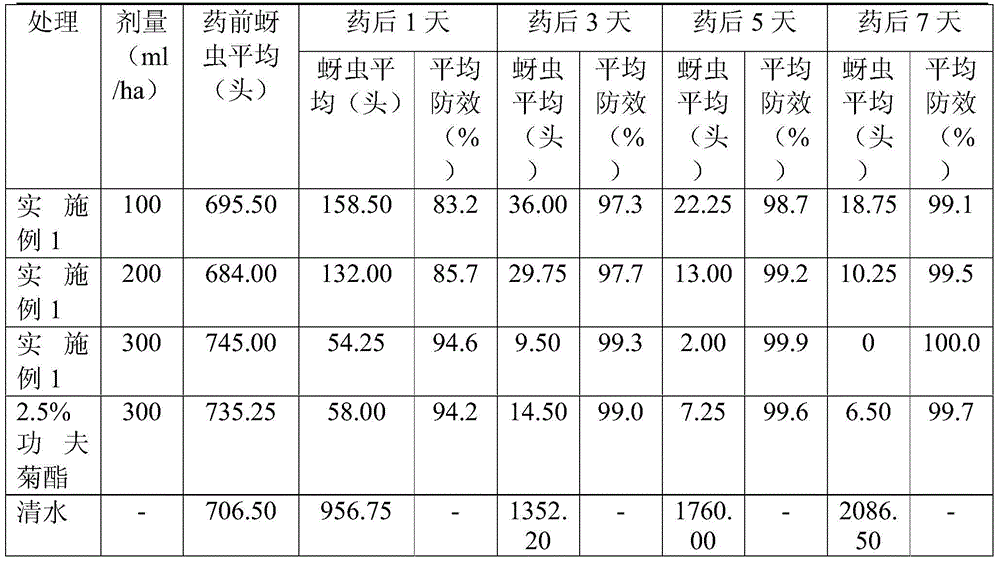
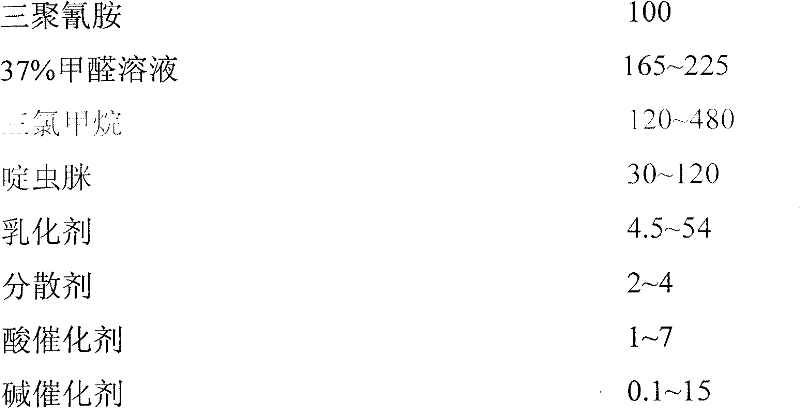Patents
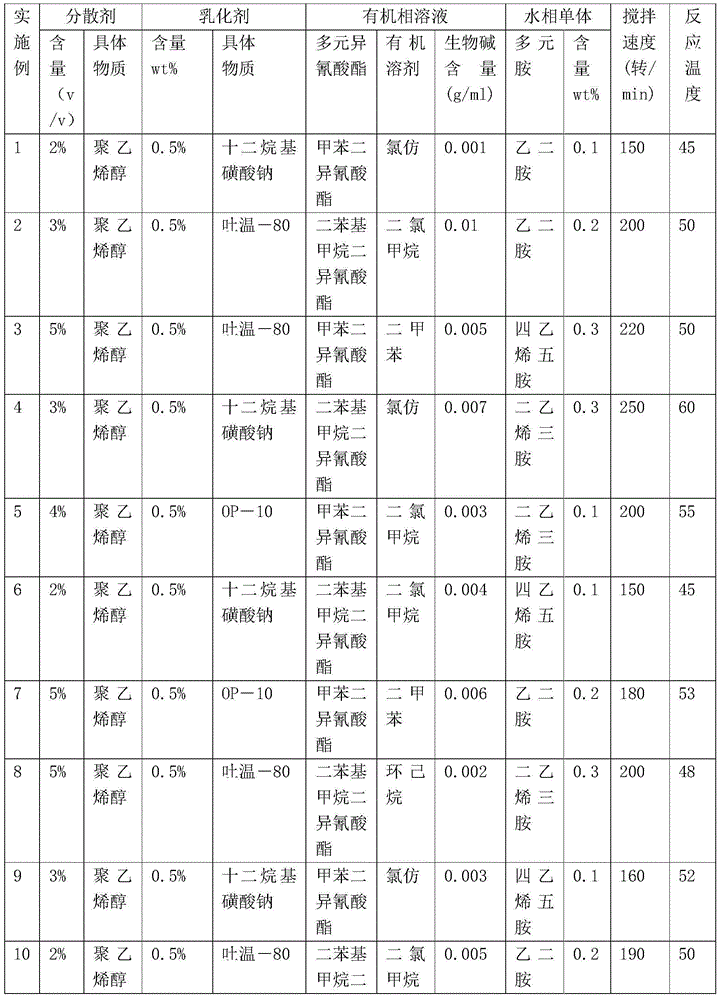
Literature
142results about How to "Low acute toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
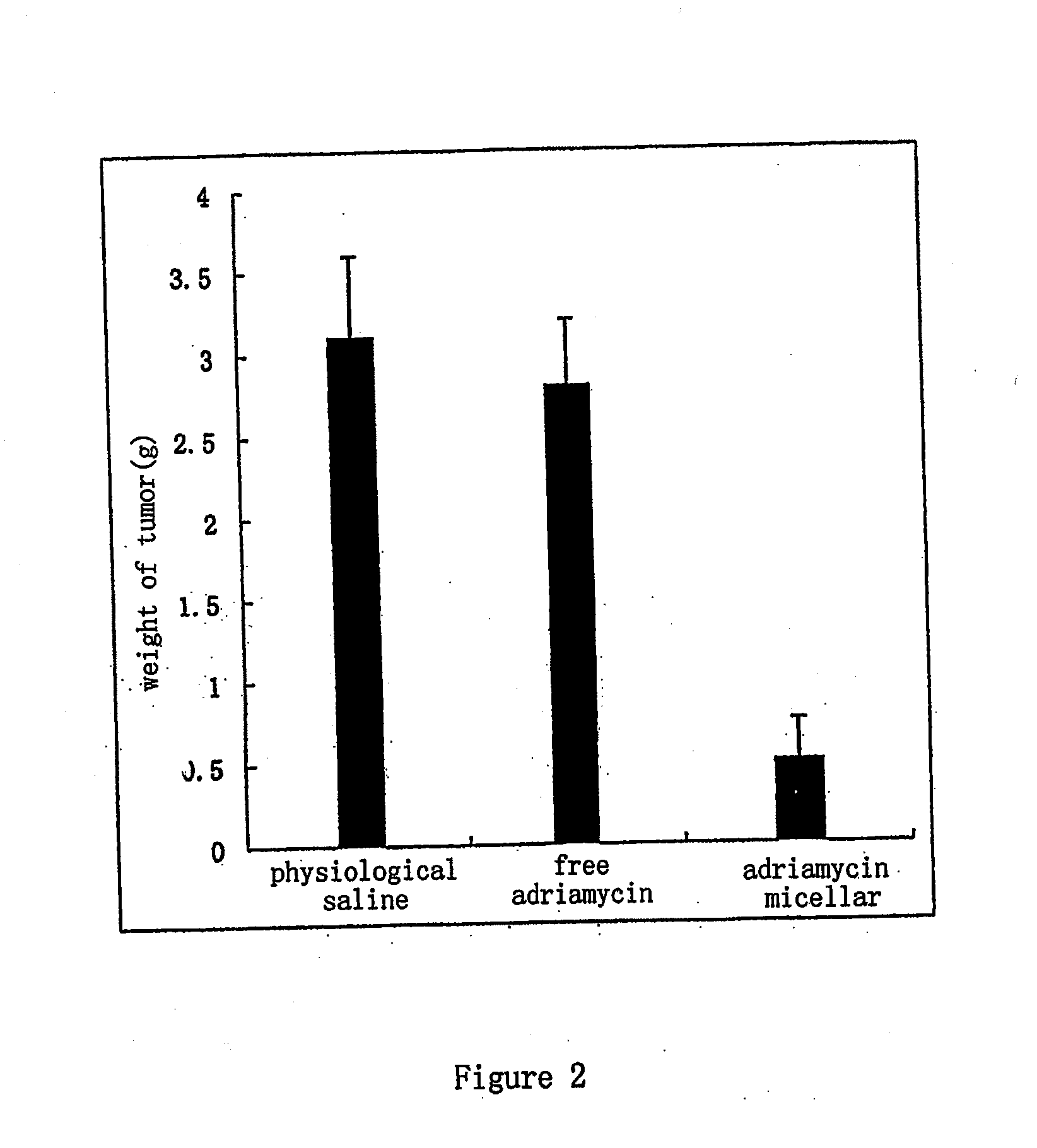
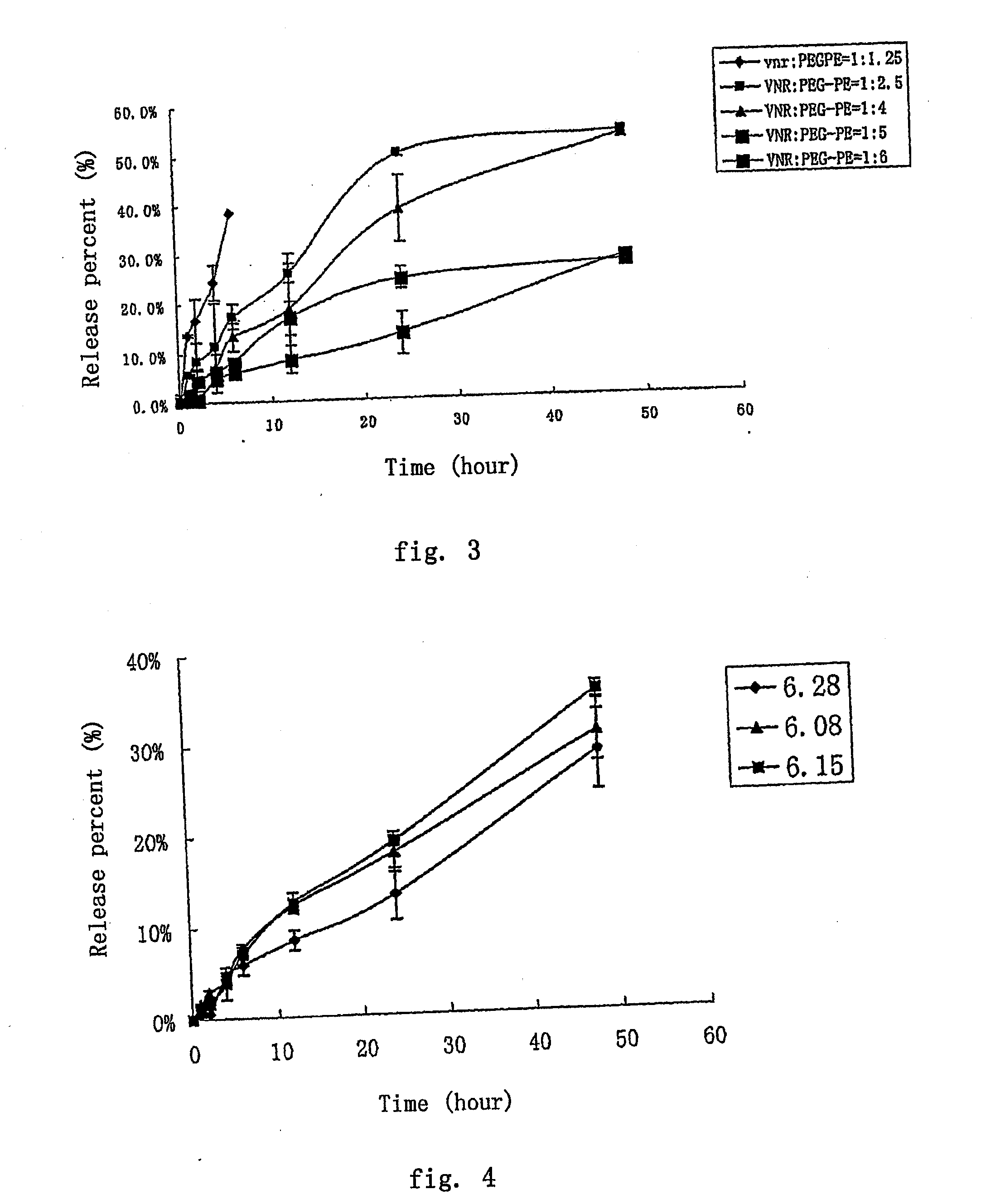
Nano Anticancer Micelles of Vinca Alkaloids Entrapped in Polyethylene Glycolylated Phospholipids
ActiveUS20090053293A1Good stabilityImprove drug distributionBiocidePharmaceutical non-active ingredientsVinorelbineAdjuvant
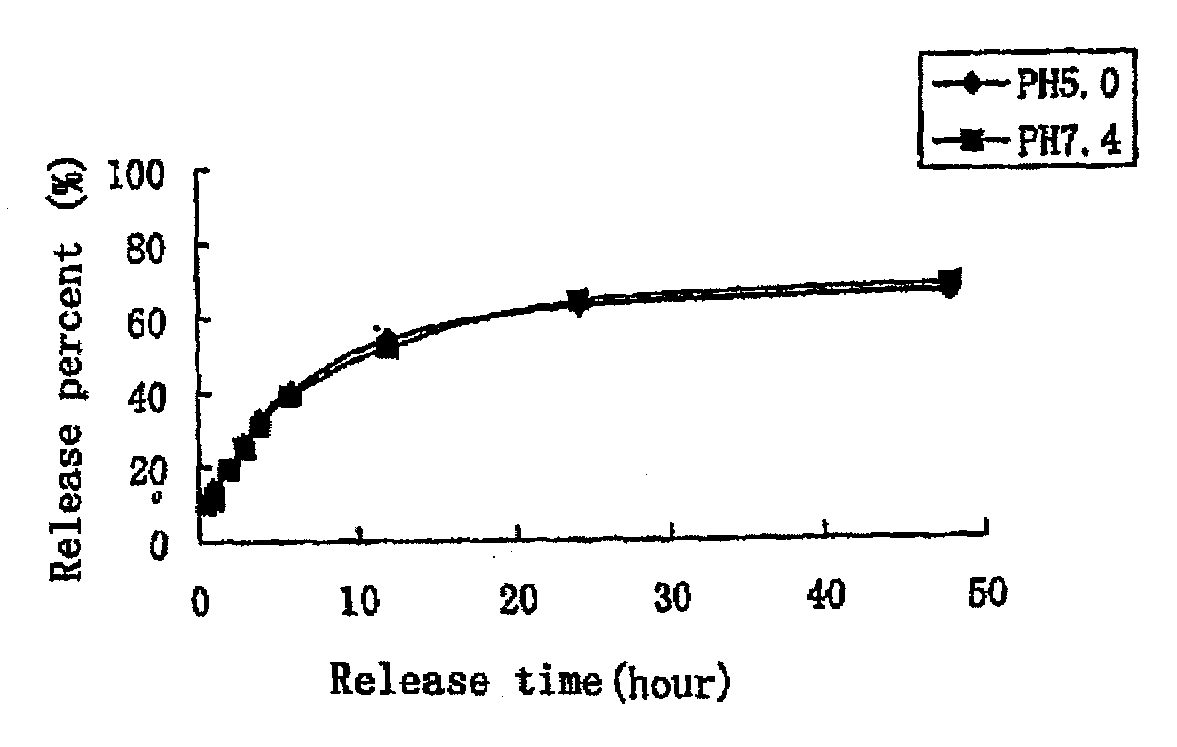
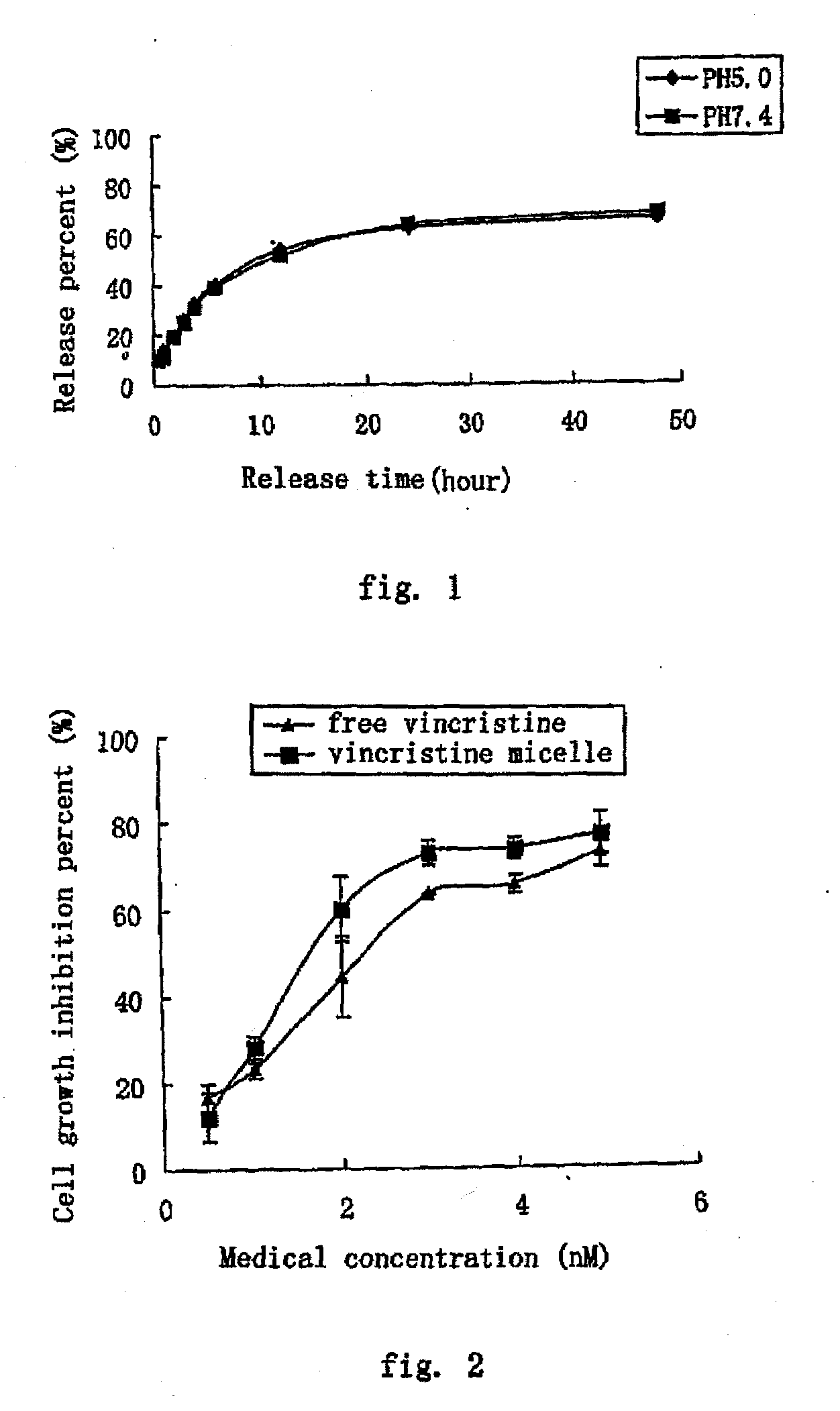
The present invention provides a nano-micellar preparation containing vinca alkaloids antitumor agent for intravenous injection, which cincludes a therapeutically effective amount of vinca alkaloids antitumor agent (vinblastine, vincristine, vindesine and vinorelbine), a phosphatide derivatized with polyethylene glycol, together with pharmaceutically acceptable adjuvants. The preparation is prepared by encapsulating the medicament with a nano-micelle to obtain the nano-micellar preparation containing vinca alkaloids antitumor agent for injection. The vinca alkaloids antitumor agent and the phosphatide derivatized with polyethylene glycol form a nano-micelle with a highly uniform particle size. In the micelle, the hydrophobic core of encapsulated medicament is surrounded by polyethylene glycol molecules to form a hydrophilic protective layer, so that the medicament is prevented from contacting with the enzymes and other protein molecules in blood and being recognized and phagocytozed by reticuloendothelial system in body, and the circulation time in vivo of the micelle is prolonged.
Owner:BEIJING DIACRID MEDICAL TECH
Disposable controlled completely-degradable plastic packing bag and preparation method thereof
The invention discloses a disposable controlled completely-degradable plastic packing bag, which is formed through melting, blending, extruding and film-blowing of components comprising in parts by weight: 20 to 45 parts of polyvinyl alcohol, 72 to 87 parts of starch, 10 to 20 parts of micromolecule plasticizer, 0.2 to 2 parts of modified inorganic nanometer additive, 0.1 to 1.5 parts of antioxidant, 0.5 to 3 parts of compatibilizer, 8 to 15 parts of inorganic filler, 0.1 to 1 part of ultraviolet absorber, and 0.1 to 1 part of pigment. The invention further discloses a preparation method of the plastic packing bag. The nanometer composite modifier in the packing bag adopts a micromolecule plasticizer easy to form a hydrogen bond with the starch, a certain amount of inorganic nanometer additives subject to surface treatment are added in the packing bag, so that after PVA (polyvinyl acetate) is modified by the nanometer composite modifier, the use amount of the molecule plasticizers is reduced greatly, the cost is lowed, the reduction of the mechanical property of a film is avoided, and the controlled completely-degradable film with excellent mechanical property and the use performance can be obtained.
Owner:NINGXIA GREEN BIODEGRADABLE PROD +1
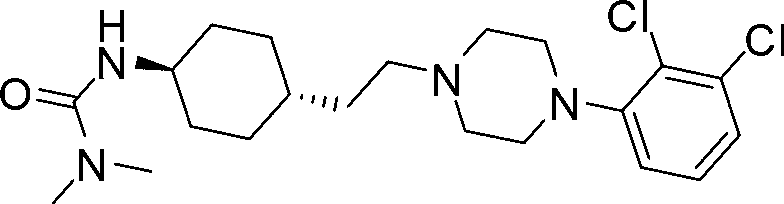
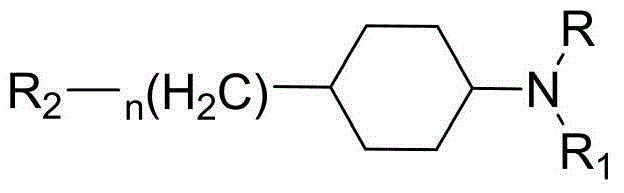
Cyclohexane amine compound and application of cyclohexane amine compound as anti-schizophrenia medicine
InactiveCN103130737ASignificant anti-schizophrenia effectPromote oral absorptionOrganic active ingredientsNervous disorderDiseaseIn vivo tests
The invention discloses a cyclohexane amine compound and application of the cyclohexane amine compound as anti-schizophrenia medicine. The cyclohexane amine compound has high affinity to a dopamine D3 receptor and a 5-HT1A, and representative compounds have a high selectivity of D3 / D2 receptors. According to in vivo tests, the representative compounds such as I-1 compound can remarkably improve relative symptoms of apomorphine model mice and MK-801 model mice. The cyclohexane amine compound has the advantages of being good in anti-schizophrenia effect, low in acute toxicity and good in safety, and having development value of serving as novel high-efficiency and low-toxicity medicine for anti-neurological disorder diseases. The cyclohexane amine compound is a compound with a structural general formula (I) or stereomer, free alkali, aquo-complex or salt.
Owner:JIANGSU HENGYI PHARMA +1
Pesticide water suspending nano capsule prepn and its preparing method
The present invention discloses water suspended nano pesticide capsule preparation prepared via emulsion polymerization process. It has capsule shell of styrene, isocyanate, acrylate and methacrylate copolymer, and core comprising ididacloprid, high effect cypermethrin or diflubenzuron as effective component; contains pesticide 0.2-5 wt%, capsule shell 5-30 wt%, additive 10-35 wt% and water in proper proportion. The nano capsule is disersive spherical particle of size smaller than 100 nm. Compared with traditional pesticide preparation forms, the present invention has the features of water based solvent, nano level dispersive effective component, high stability, etc. and may be used in killing various pests of fruit tree, vegetable, flower, cotton, tobacco, etc.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
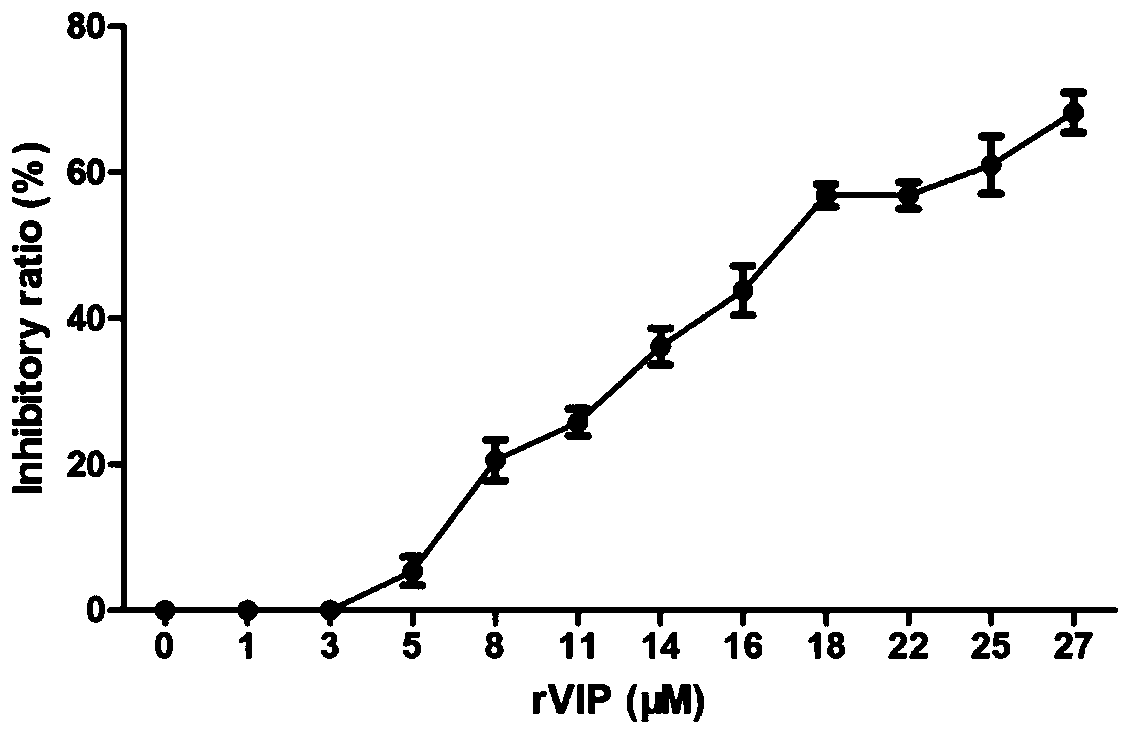
Multifunctional compound microecological preparation nano-selenium-recombination expression VIP (vasoactive intestinal peptide)-lactococcus lactis and preparation method
InactiveCN108753670AMany functionsLow acute toxicityBacteriaMicroorganism based processesStaphylococcus lactisVasoactive intestinal peptide
The invention relates to multifunctional compound microecological preparation nano-selenium-recombination expression VIP (vasoactive intestinal peptide)-lactococcus lactis and a preparation method. Toxic sodium selenite can be converted into non-toxic red simple-substance nano-selenium, and synthesized nano-selenium is enriched in lactococcus lactis NZ9000 in cells. The invention also discloses amethod for preparing a multifunctional compound microecological preparation SENPs-rVIP-L.lactis NZ9000 based on lactococcus lactis NZ9000. The problems that existing selenium supplementing additives have high toxicity action and low bioavailability and easily cause environment pollution, existing production technical procedures are complicated, the cycle is long and the production cost is high andthe problems about remaining of antibiotics, drug resistance and the like are solved.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Chlorpyrifos microcapsule suspension and preparation method thereof
InactiveCN102057897ASatisfy the appropriate anti-efficacy requirementsLong durationBiocideAnimal repellantsChlorpyrifosAcute toxicity testing
The invention discloses a chlorpyrifos microcapsule suspension. The chlorpyrifos microcapsule suspension mainly comprises the following components in percentage by weight: 10 to 45 percent of chlorpyrifos, 5 to 20 percent of solvent, 1 to 10 percent of capsule wall material, 0.1 to 3 percent of lacquer, 2 to 5 percent of emulsifier, 3 to 5 percent of dispersant, 0 to 14 percent of auxiliary agentand 10 to 80 percent of water. The invention also discloses a preparation method of the chlorpyrifos microcapsule suspension. The chlorpyrifos microcapsule suspension has the advantages of reducing acute toxicity of raw medicaments, reducing environmental pollution and stimulation to people and livestock, improving prevention and control effect on insect damages such as a citrus tree scale insectand the like, greatly prolonging duration of a pesticide and reducing pesticide application frequency and agricultural cost. Since microcapsule technology is adopted and water is used as a dispersingmedium in the chlorpyrifos microcapsule suspension, the chlorpyrifos microcapsule suspension is difficult to flame and explode, and can be safely and reliably used and transported.
Owner:联合国南通农药剂型开发中心 +1
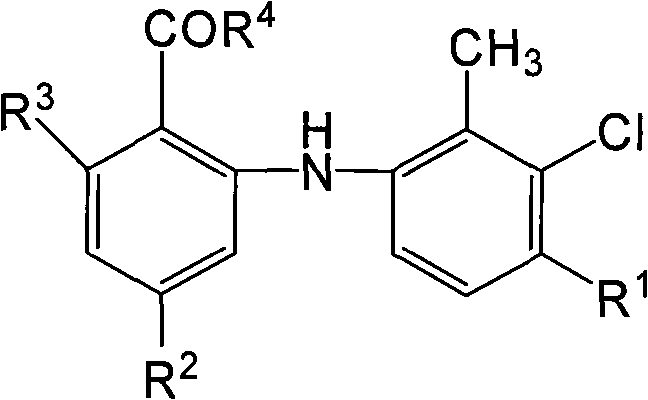
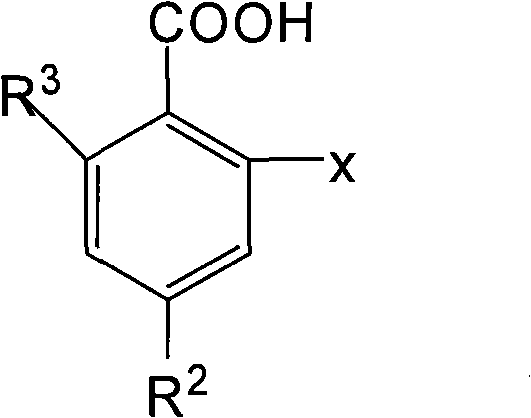
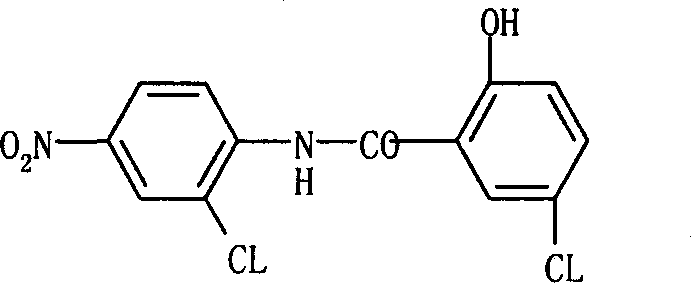
O-anilino benzoic acid derivatives or pharmaceutically acceptable salts thereof as well as preparation method and application thereof
ActiveCN101985428ALow acute toxicityEasy to prepareOrganic active ingredientsOrganic compound preparationDiseaseBenzoic acid
The invention provides o-anilino benzoic acid derivatives or pharmaceutically acceptable salts thereof as well as a preparation method and applications thereof in preparing medicines or functional food for preventing and / or treating tumors and other abnormal proliferation diseases of organisms and in preparing medicines or functional food for preventing and / or treating inflammations, pains and other diseases of organisms.
Owner:HANGZHOU MINSHENG PHARM CO LTD
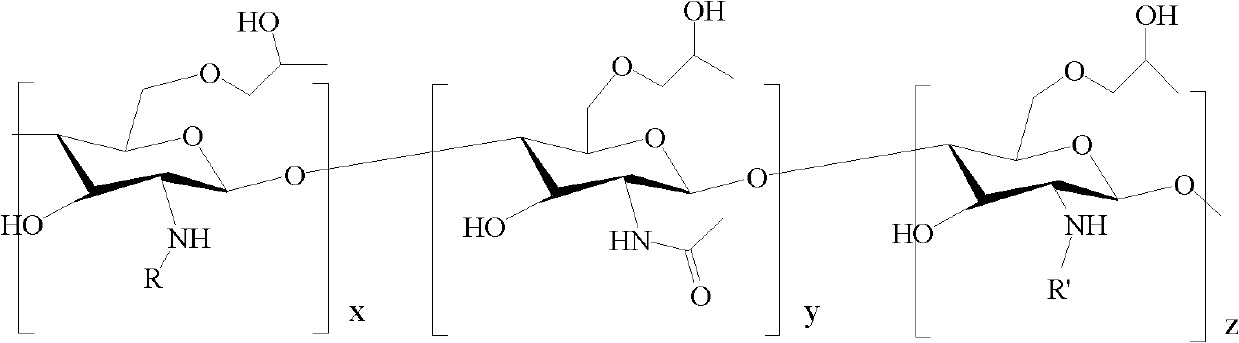
Amphiphilic chitosan derivative, its preparation method and its application in medicinal preparation
InactiveCN102585036ADoes not cause hemolytic reactionLow acute toxicitySolution deliveryPharmaceutical non-active ingredientsDrug carrierIn vivo
The invention relates to the medical auxiliary material chitosan derivative field, and more specifically relates to an amphiphilic chitosan derivative (I), its preparation method and its application in a medicinal preparation. The chitosan derivative of the present invention is a polymer capable of carrying out biodegradation in vivo possessing amphiphilic polymer molecules, which is characterized in that one end contains hydrophilic group, the other end contains hydrophobic group, and is suitable for medicines, matching with medicines or being as a medicine carrier, and especially can be the medicine carrier with small toxicity and no hemolytic reaction capable of intravenous injection administration.
Owner:CHINA PHARM UNIV
Drug targeting slow-release carrier material and preparing process thereof
InactiveCN1528461AWide variety of sourcesRich reservesInorganic non-active ingredientsHuman bodyControlled release
The present invention discloses a new type medicine target slowly-releasing carrier material and its preparation process. Said medicine target slowly-releasing carrier material is made up by utilizing attapulgite and Fe3O4. Its principle lies in that it utilizes the large specific surface area and adsorbability of ultrafine high-purity attapulgite and uses it as carrier material of medicine to prolong action time of medicine in interior of human body, raise concentration of local medicine, raise therapeutic effect of medicine and reduce toxic side effect of the medicine, and utilizes magnetization treatment so as to attain the goal of making target administration and controlling release of medicine.
Owner:甘肃省中心实验室
Insecticide microcapsule suspension type liquid medicine fertilizer and preparation method and application thereof
InactiveCN105732199AImprove stabilityReduce degradation rateBiocideAlkali orthophosphate fertiliserMaterial resourcesDefoaming Agents
The invention discloses an insecticide microcapsule suspension type liquid medicine fertilizer and a preparation method and application thereof.The insecticide microcapsule suspension type liquid medicine fertilizer is prepared from insecticide, an organic solvent, a cyst wall material, a pH adjusting agent I for preparing microcapsules, a nitrogen fertilizer, a phosphate fertilizer, a potash fertilizer, a micronutrient fertilizer, an emulsifying agent, a filler, a dispersing agent, a defoaming agent, a thickening agent, an antifreezing agent and water, wherein the pH adjusting agent I is acid.The raw materials further include a pH adjusting agent II for adjusting the pH value of the liquid medicine fertilizer, wherein the pH adjusting agent II is alkali.The average control effect of the insecticide microcapsule suspension type liquid medicine fertilizer on wheat aphid is 80.0% or above, meanwhile, the yield of eggplants can be increased, and the N, P and K unit nutrient partial productivity is improved.The insecticide microcapsule suspension type liquid medicine fertilizer is simple and reasonable in technology, reduces the material grinding size as much as possible, is low in energy consumption, suitable for industrial production and suitable for being adopted in a small area, and saves material resources, financial resources and labor force.
Owner:CHINA AGRI UNIV
Ivermectin water suspension nano capsule prepn and its preparing method
The present invention relates to water suspended nano Ivermectin capsule preparation prepared via emulsion polymerization process. It has capsule shell of styrene, isocyanate, acrylate and methacrylate copolymer, and core comprising biologically originated pesticide and animal remedy Ivermectin as effective component; contains Ivermectin 0.2-2 wt%, capsule shell 5-30 wt%, additive 10-35 wt% and water in proper proportion. The nano capsule is disersive spherical particle of size smaller than 100 nm. Compared with traditional pesticide preparation forms, the present invention has the features of water based solvent, nano level dispersive effective component, high stability, etc. and may be used in killing animalí»s parasite and various pests of fruit tree, vegetable, flower, cotton, tobacco, etc.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
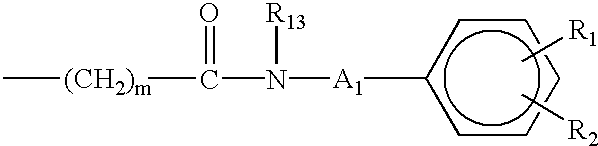
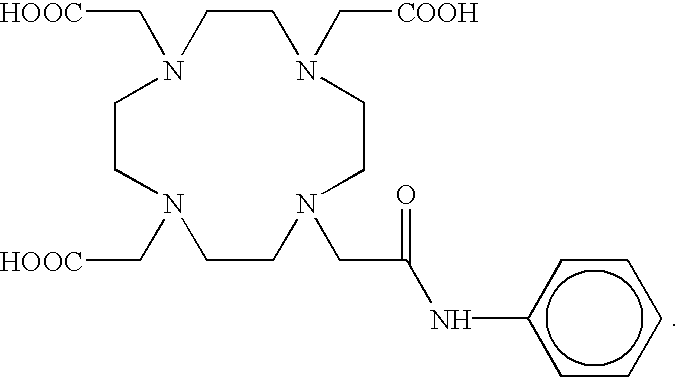
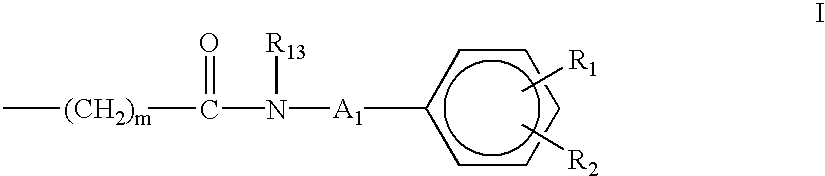
Aminocarboxylate ligands having substituted aromatic amide moieties
InactiveUS6875864B2Relaxivity be increaseThermodynamically stableRadioactive preparation carriersGroup 3/13 element organic compoundsMetal chelateCoordination complex
Owner:BRISTOL MYERS SQUIBB CO +1
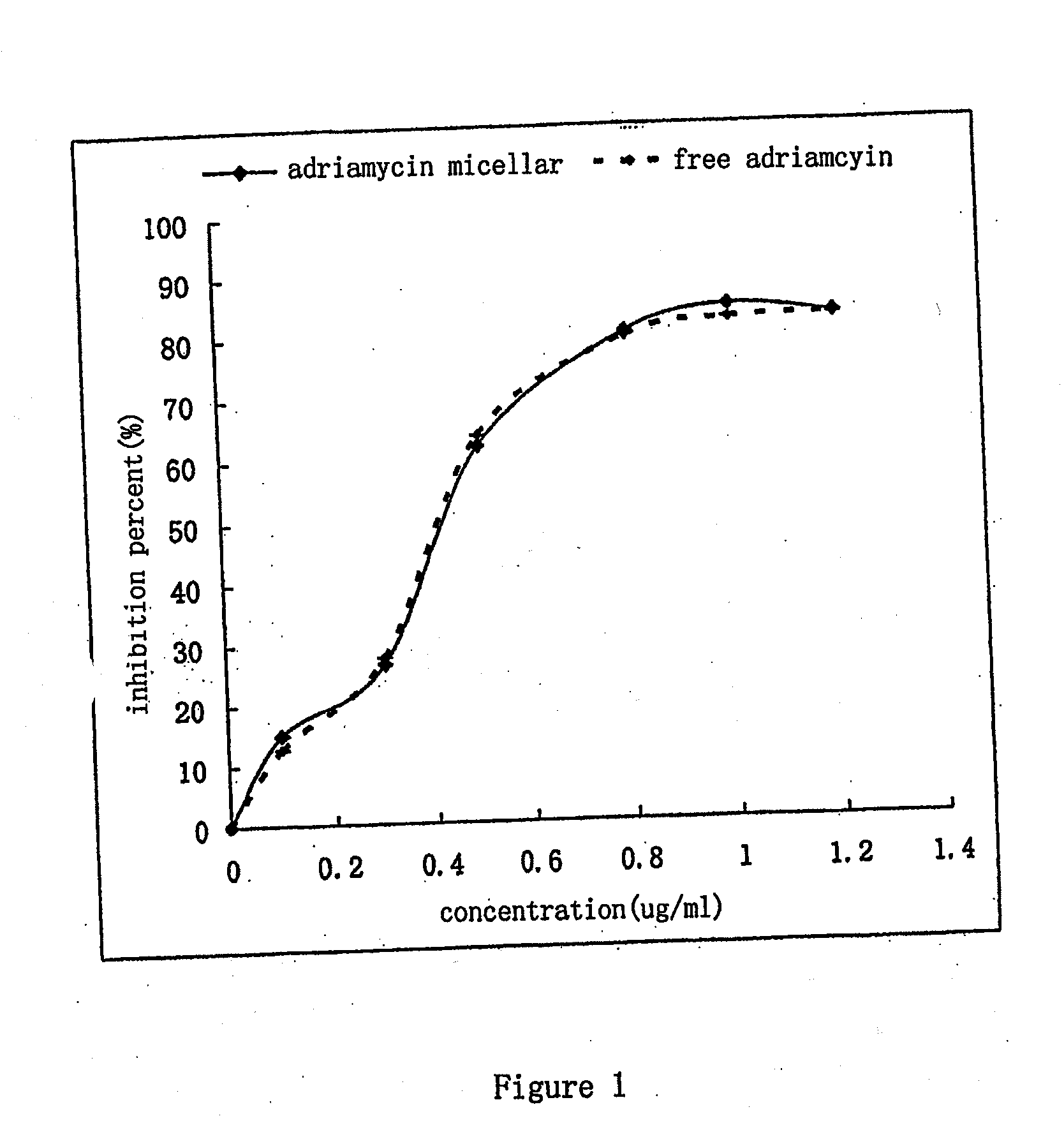
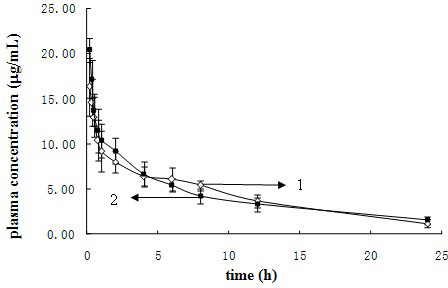
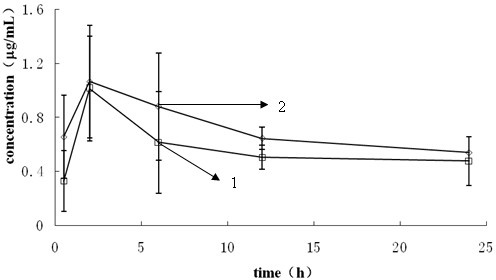
Nano-micellar preparation of anthracylcline antitumor antibiotics encapsulated by the phosphatide derivatized with polyethylene glycol
InactiveUS20090232900A1Improve stabilityEnhanced drug distributionBiocideCarbohydrate active ingredientsAdjuvantProtein molecules
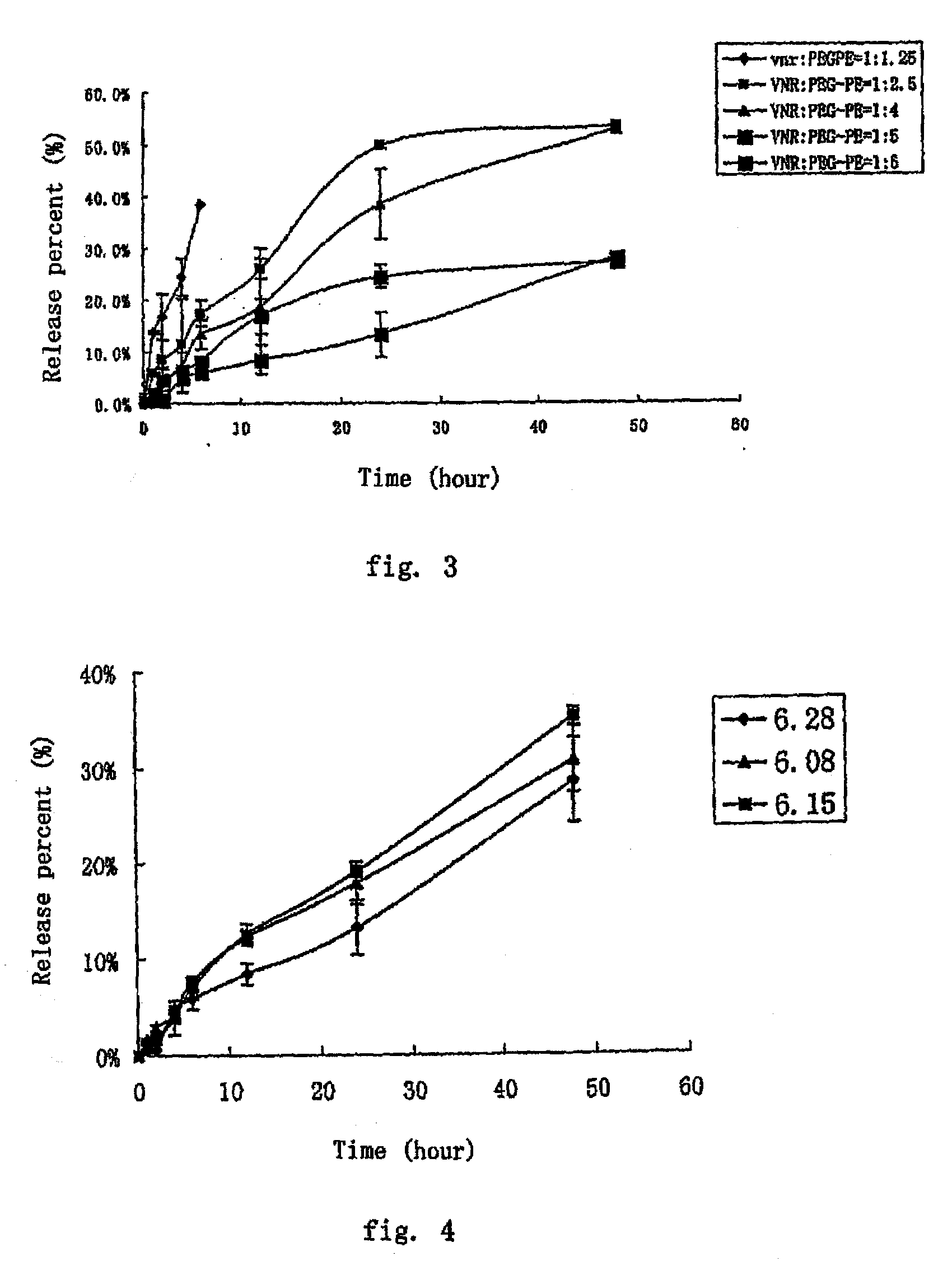
The present invention provides a nano-micellar preparation of anthracycline antitumor antibiotics for intravenous injection, which comprises a therapeutically effective amount of anthracycline antitumor antibiotics, a phosphatide derivatized with polyethylene glycol, together with pharmaceutically acceptable adjuvants. The preparation is prepared by encapsulating the medicament with a nano-micelle to obtain the nano-micellar preparation of anthracycline antitumor antibiotics for injection. The anthracycline antitumor antibiotics and the phosphatide derivatized with polyethylene glycol form a nano-micelle with a highly homogeneous particle size. In the micelle, the hydrophobic core of encapsulated medicament is surrounded by polyethylene glycol molecules to form a hydrophilic protective layer, so that the medicament is prevented from contacting with the enzymes and other protein molecules in blood and being recognized and phagocytozed by reticuloendothelial system in the body, and the circulation time in vivo of the micelle is prolonged.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Preparation of avermectin medicament sustained-release nano microsphere preparations and uses thereof
InactiveCN101194616AGood dispersionSmall particle sizeOrganic active ingredientsBiocideCross-linkGlycidyl methacrylate
The invention relates to a process for preparing avermectin and derivant dimethylamino avermectin, ivermectin, and doramectin slow release nanometer micro-balloon preparation. The technical procedures comprise suspending the suspending solution of 1%-40% unequal avermectin-type medicine, 0%-4% divinylbenzene (cross linking agent), (metyl group) methyl acrylate of 1% BPO (initiating agent) or solvent of methacrylic acid glycidyl ester in an aqueous phase of 40 DEG C with 1% sodium dodecyl benzene sulfonate and 0.5% gelatine or 0.5% PVA (the size ratio of an oil phase and the aqueous phase is 1:3) in a three-mouth bottle with an agitator, a reflux condensing tube and a thermometer, adding numerous ml of 0.5% methylene blue, regulating ultrasonic homogenization power, heating-up to 61 DEG C at 5 DEG C / 10mins after the grain size is even, aggregating for two hours, heating-up to 85 DEG C with the same speed, aggregating for 8 hours and preparing 50nm-100nm avermectin-type medicinal nanometer micro-balloon preparation with different medicine-loading amount, and the packaging and loading rate reaches more than 91%. The invention solves the problem of long-acting controlled release of biological medicament, reduces the toxic and side effect simultaneously, improves the biological availability, reduces the cost and is friendly to the environment.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Lipid microsphere injection containing sodium demethyl cantharidate-phosphatide complex and preparation method thereof
The invention discloses a lipid microsphere injection containing sodium demethyl cantharidate-phosphatide complex and preparation method thereof. The injection comprises the sodium demethyl cantharidate-phosphatide complex, fat-soluble medium, surfactant and other ingredients. In the sodium demethyl cantharidate-phosphatide complex, the molar ratio of sodium demethyl cantharidate to phosphatide is 1-1:10. The prepared sodium demethyl cantharidate-phosphatide complex improves the distribution of sodium demethyl cantharidate in oil-water 2-phase interfacial film and oil phase greatly, increasesthe entrapment efficiency of the medicine in preparation, realizes the interfacial film loading medicine, reduces the toxicity, and can carry out targeting drug release in vivo. The prepared lipid microsphere injection reduces the vascular stimulation of sodium demethyl cantharidate, improves the curative effect, and reduces toxic and side effect.
Owner:SHENYANG PHARMA UNIVERSITY
Prochloraz microcapsule suspending agent and compound preparation thereof
InactiveCN104365651ASolve technical problems that are not suitable for making suspensionsRealize recombinationBiocideFungicidesBakanaeSuspending Agents
The invention relates to the field of pesticide preparations, and in particular relates to a prochloraz microcapsule suspending agent and a compound preparation thereof. The prochloraz microcapsule suspending agent is prepared from prochloraz, a solvent, an emulsifier, a wet dispersing agent, a capsule wall material, an initiator and water. According to the prochloraz microcapsule suspending agent, the technical problem that prochloraz is not suitable for being made into a suspending agent is solved; meanwhile, the microcapsule suspending-suspending seed coating agent provided by the invention can be used for effectively preventing and treating pests and diseases such as rice bakanae disease, rice black-streaked dwarf disease, thrips and rice fulgorid, and has an extremely good slow release effect.
Owner:LIANBAO CROP TECH
Use of derivatives of succinate esters for the treatment of dementia
ActiveUS20060281692A1Reduce in quantityEffectively ameliorate scopolamine-induced impaired acquisition of learningBiocideNervous disorderHigh activityDisease cause
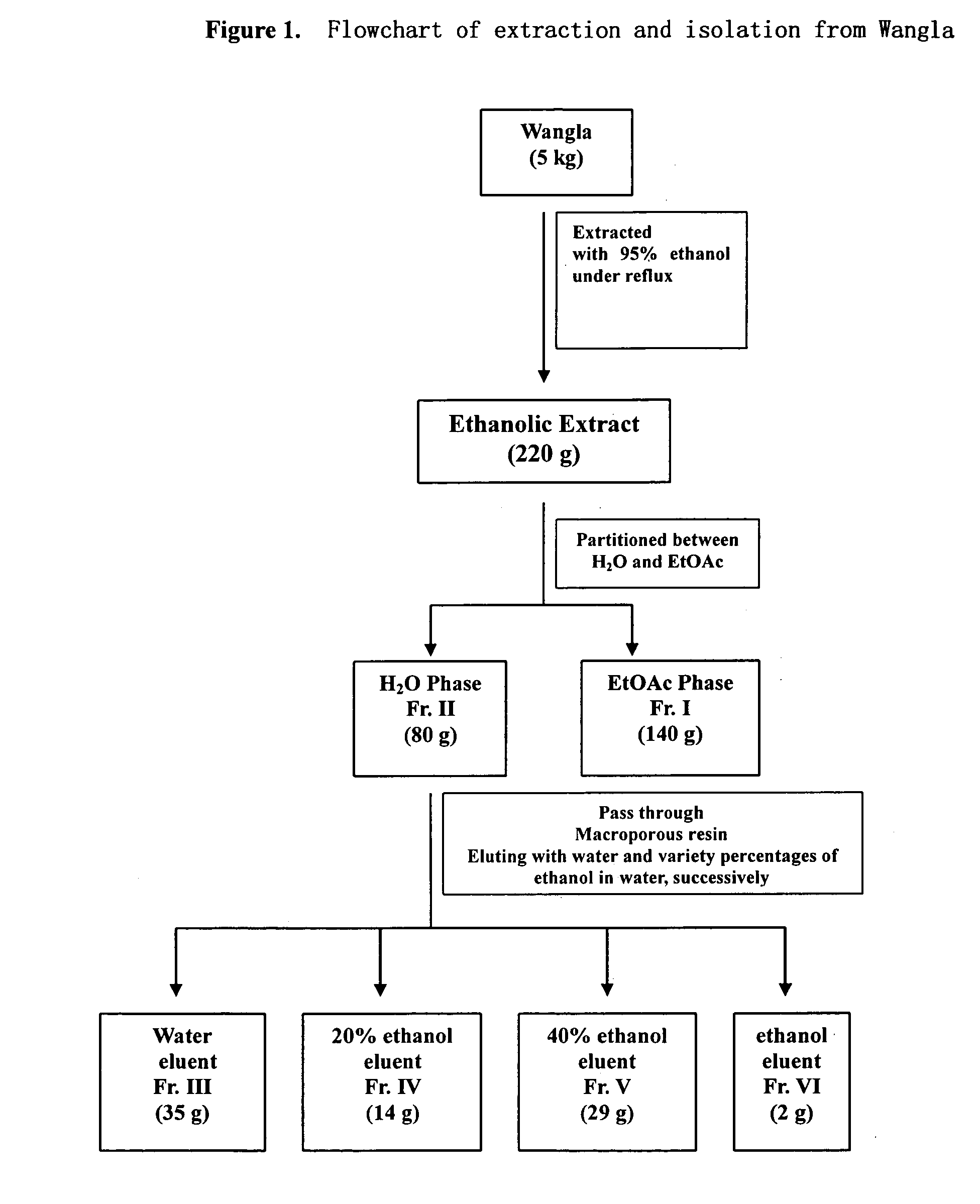
The use of extract form Wangla (coeloglossum viride (L) Hartm. Var. Bracteatum (Willd.) Richter), succinate derivative esters, and a derivative and pharmaceutical acceptable salts thereof, for the manufacture of a pharmaceutical preparation for the treatment of dementia, particularly for the treatment of Alzheimer' disease and Vascular dementia. Through Animal experiment, it has been demonstrated that, succinate derivative esters can improve learning and memory ability in dementia rats induced by scopolamine and cyclohexenyl imine; improve learning and memory ability in dementia rats induced by β-amyloid; improve learning and memory ability in dementia rats induced by permanent ligation of bilateral carotid; and improve memory ability of normal animals. It has the advantage of high activity, low toxicity and no inhibition to cholinesterase.
Owner:BEIJING LIANXIN PHARMA CO LTD
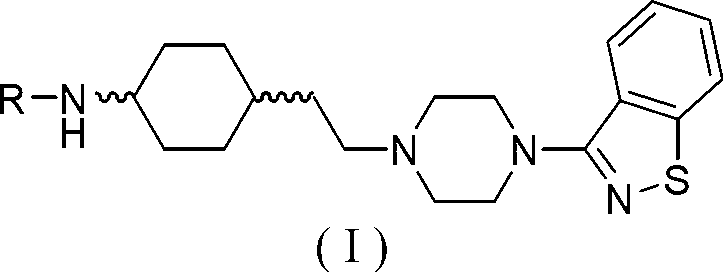
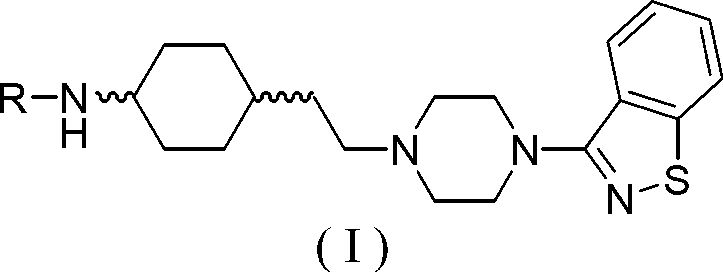
Piperazine (piperidine) cyclohexyl derivative and applications of piperazine (piperidine) cyclohexyl derivative in treatment of neuropsychiatric diseases
ActiveCN105367565AGood receptor selectivityImproving the effect of cognitive impairmentOrganic active ingredientsNervous disorderLow affinityAcute toxicity testing
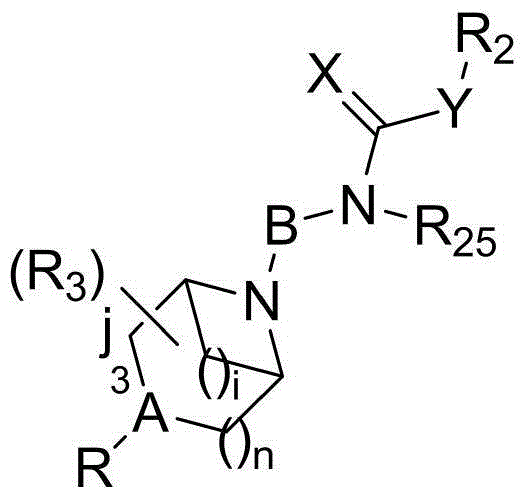
The present invention discloses a piperazine (piperidine) cyclohexyl derivative and applications of the piperazine (piperidine) cyclohexyl derivative in treatment of neuropsychiatric diseases. According to the present invention, the pharmacological experiment results show that the piperazine (piperidine) cyclohexyl derivative provides high affinity with a dopamine D2 receptor, a dopamine D3 receptor, a serotonin 5-HT1A receptor and a serotonin 5-HT2A receptor, provides good D3 / D2 receptor selectivity and good 5-HT1A / 5-HT2A receptor selectivity, and provides low affinity with alpha receptor; the in vivo test results show that the preferred compound has characteristics of good anti-schizophrenia effect, good pharmacokinetic property, low side effect, low acute toxicity and high safety, and has the development value of being adopted as the novel efficient and low-toxic anti-neuropsychiatric disease; and the piperazine (piperidine) cyclohexyl derivative is a compound represented by the structural general formula (I), or a geometric isomer, an optical isomer, a salt, a hydrate or a solvate thereof. The formula (I) is defined in the specification.
Owner:SHANGHAI INST OF PHARMA IND +1
Methyl-amino-ivermectin water suspension nano capsule prepn and its preparing method
The present invention discloses a water suspended nano methyl-amino-Ivermectin capsule preparation prepared via emulsion polymerization process. It has capsule shell of styrene, isocyanate, acrylate and methacrylate copolymer, and core comprising biologically originated pesticide and animal remedy methyl-amino-Ivermectin as effective component; contains methyl-amino-Ivermectin 0.2-2 wt%, capsule shell 5-30 wt%, additive 10-35 wt% and water in proper proportion. The nano capsule is disersive spherical particle of size smaller than 100 nm. Compared with traditional pesticide preparation forms, the present invention has the features of water based solvent, nano level dispersive effective component, high stability, etc. and may be used in killing animalí»s parasite and various pests of fruit tree, vegetable, flower, cotton, tobacco, etc.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Sterilization compound containing benzothiostrobin and difenoconazole and application thereof
ActiveCN103548840AGood control effectDelaying Antimicrobial ResistanceBiocideFungicidesAcute toxicity testingBULK ACTIVE INGREDIENT
The invention relates to a sterilization compound containing benzothiostrobin and difenoconazole. The sterilization compound comprises active ingredients and pesticide preparation auxiliary components, wherein the active ingredients comprise benzothiostrobin and difenoconazole. The benzothiostrobin and the difenoconazole in the sterilization compound provided by the invention belong to two bactericides with different mechanisms, and can be compounded within a certain proportion range to generate a remarkable synergistic role. The sterilization compound is remarkable in prevention and control effect, is safe to crops without damage risk, is reduced in acute toxicity to mammals, and is improved in safety.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
A snail-killing medicine-nano suspension concentrate of niclosamide ethanolamine and method for preparing same
InactiveCN1883265AGood dispersionImprove bioavailabilityBiocideMolluscicidesOncomelaniaWater insoluble
The invention relates to niclosamide ethanolamine salt nano suspending agent for killing oncomelania and its preparation method, belongs to oncomelania-killing drug and insoluble drug nano preparation technology field. The method comprises steps of adding dimethyl sulphoxide to stablizer 1%-10% in mass content and niclosamide ethanolamine salt 5%-25% in mass content and mixing ,confecting solution, obtaining niclosamide ethanolamine salt nano suspending agent stock solution, adding it to aqueous solution when stirring niclosamide ethanolamine salt content being 0.01%-1%, niclosamide ethanolamine salt grain size being 50nm-1 mum, obtaining niclosamide ethanolamine salt nano suspending agent. The inventive method can make water-insoluble niclosamide ethanolamine salt disperse well in waterbody. The solution can be used while being prepared. The inventive niclosamide ethanolamine salt nano suspending agent is simply prepared, and promote bioavailability while meet the need of killing snail and reduce the toxicity to fish.
Owner:JIANGSU INST OF PARASITIC DISEASES +1
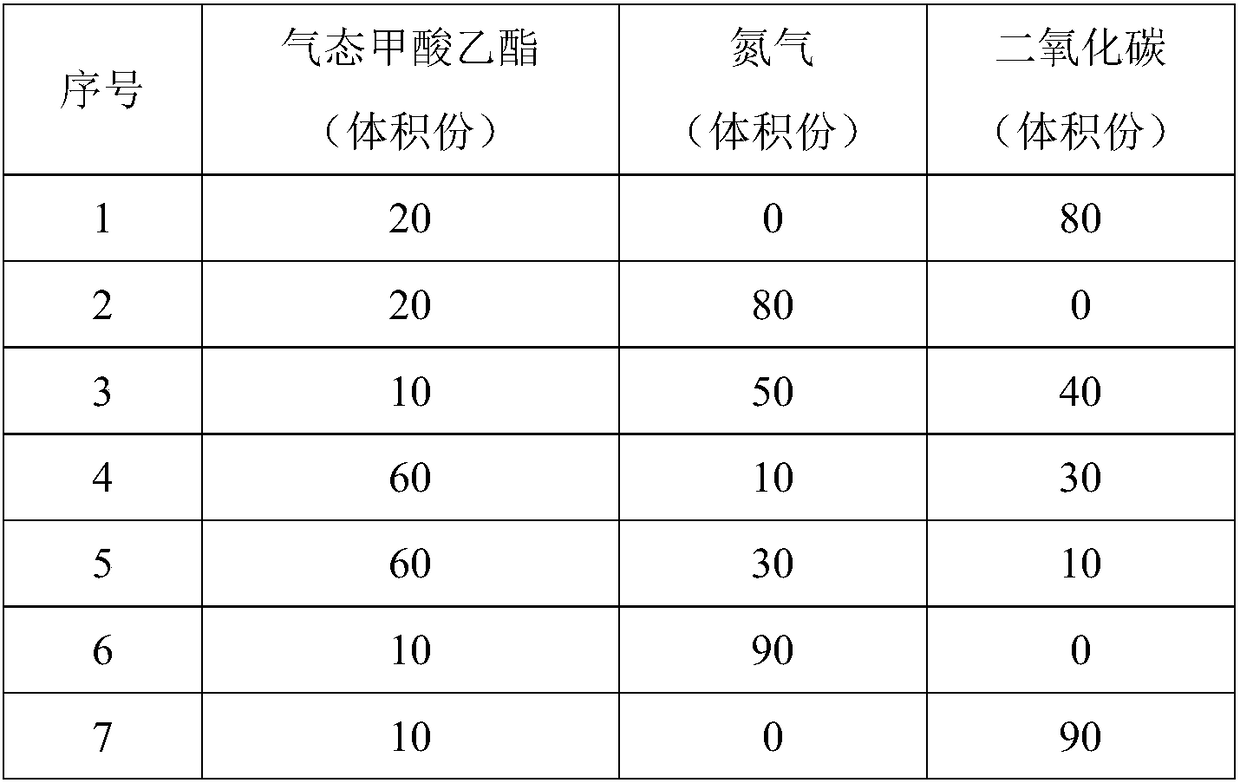
Composition for pest control and its preparation method and application
The invention discloses a composition for pest control and its preparation method. The composition can solve the problems that pests cannot be rapidly killed by the prior art and main quarantine pestscan generally resist hydrogen phosphide and is poor in pest killing effect. The composition is prepared from, by volume, 10-60 parts of gaseous ethyl formate and 40-90 parts of inert gas. The invention further provides an application of the composition in preparing pesticide for pest control. The composition can rapidly kill pest and is environment-friendly. The preparation method is simple and convenient to operate, and suitable for popularization and application.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
Melamino-formaldehyde resin encapsulated acetamiprid microcapsule, and preparation method thereof
InactiveCN102388865AFacilitated releaseReduce decompositionBiocideAnimal repellantsMelamine formaldehydeRelease time
The invention relates to a melamino-formaldehyde resin encapsulated acetamiprid microcapsule, and a preparation method thereof. The microcapsule is technically characterized by comprising the following components: melamine, a formaldehyde solution, acetamiprid, chloroform, an emulsifying agent, a dispersing agent, water, an acid catalyst and a base catalyst. The preparation method comprises the following steps of: putting melamine and the formaldehyde solution in a three-neck flask, adding the base catalyst and regulating, and heating to obtain a melamino-formaldehyde prepolymer; putting the acetamiprid, chloroform, the emulsifying agent, and water in a reaction flask, and stirring to obtain a pre-emulsification solution; mixing the pre-emulsification solution and the prepolymer, adding acid and catalyzing, and adding an appropriate amount of dispersing agent, raising temperature to 60 DEG C, reacting for three hours and stopping reaction; and washing a product by using water for multiple times, and suction-filtering and drying to obtain a microcapsule product. The encapsulation rate of the microcapsule is relatively high, sustained release time is long, pollution to environment is reduced, the preparation method is simple, wall materials are low in cost and the microcapsule is suitable for large-scale production and application.
Owner:BEIJING UNIV OF CHEM TECH
Surface active agent containing selenous anions and preparation method and application thereof
ActiveCN107998980AImprove surface activityInterfacial Tension Reduction ActivityCosmetic preparationsHair cosmeticsChemical industryAcute toxicity testing
The invention discloses a surface active agent containing selenous anions and a preparation method and application thereof, and belongs to the technical field of the daily chemical industry. The surface active agent is benzyl selenium-alkyl sulphate, the structural formula is C6H5CH2Se(CH2)nSe(CH2)nSO4-M+, wherein n in the formula is an integer from 2 to 4, and M+ is one of a sodion or a potassiumion or an ammonium ion. The surface active agent containing selenous anions is mild in synthesis condition, no special equipment requirement is needed, the energy consumption is low, and raw materials are easy to obtain; the liquid interface activity of the surface active agent containing selenous anions is better than the gas-liquid surface activity, the excellent dynamic foaming performance isachieved, the surface active agent containing selenous anions has the better oxidation resistance and free radical removal capability compared with a surface active agent containing single selenium atoms, and the cytotoxicity, the aquatic organism acute toxicity and the skin irritation are low.
Owner:JIANGNAN UNIV
Avilamycin water-suspension nano capsule preparation and method for preparing same
The present invention discloses an abamectin water suspension nano capsule preparation made up by enulsion polymerization process. Its capsule skin is the styrene, isocyanate, acrylic and methyl acrylic monomer copolymer, and the capsule core contains effective component biological source pesticide and abamectic new dosage form, in which abamectin content is 0.2%-5% (w / w), capsule content is 5%-30% (w / w), additive content is 10%-35% (w / w) and proper quantity of water. Said nano capsule is monodisperse spherical granules, and its average grain size less than 100 nano. It can be extensively used for controlling several pests of fruit tree, vegetable, flowers, cotton and tobacco and other crops.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
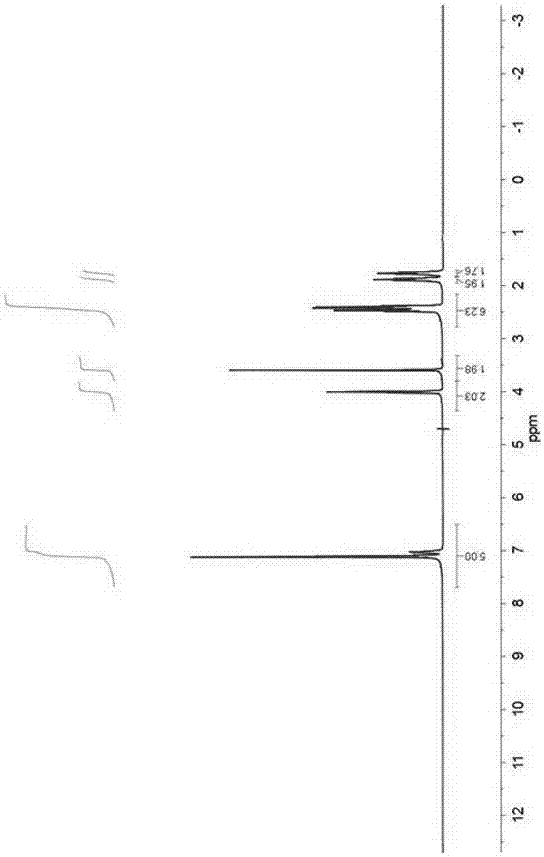
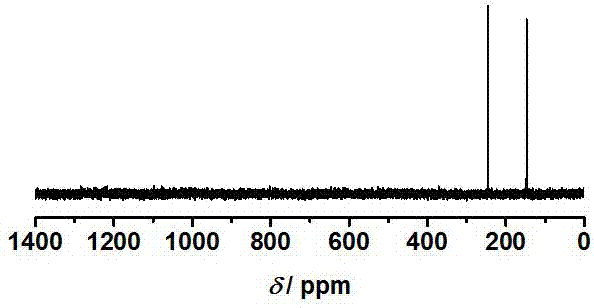
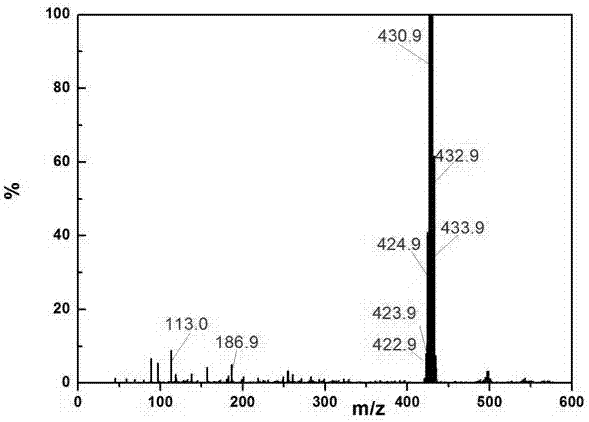
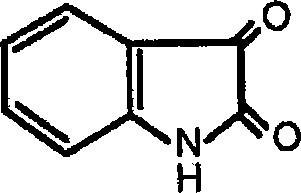
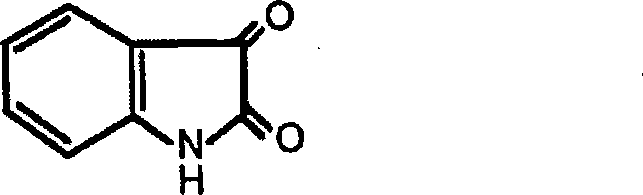
Application of indole-2, 3-diketone in preparing antiphlogistic medicament
InactiveCN101214240AEffective anti-inflammatory effectEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryDiketoneSide effect
The present invention relates to an application of indole-2, 3-dione for preparing for an anti-inflammation drug. The indole-2, 3-dione is natural product or synthetic product with low toxicity, little side effect and good anti-inflammation and diminishing inflammation effects.
Owner:QINGDAO UNIV
Layer feed containing NANO-Se
InactiveCN103005207AImprove bioavailabilityEasy to produceAnimal feeding stuffAcute toxicity testingBioavailability
The invention relates to a layer feed containing NANO-Se. The selenium-rich layer feed is prepared by breaking and smashing 60 parts of corn and 23 parts of soybean meal into 40-mesh particles, adding 4.6 parts of wheat bran, 12 parts of mixtures and 0.4 parts of NANO-Se, and mixing in a mixer for 20min. The NANO-Se (red elemental selenium) is high in bioavailability and very low in acute toxicity. As the use amount of NANO-Se (red elemental selenium) is small and no toxicity is caused to hens, the egg yield is not affected, the nutritional level of amino acid in the hens is raised, and the egg production is facilitated.
Owner:安徽旭超富硒农业科技股份有限公司
Water suspension acetamiprid nano capsule prepn and its preparing method
The present invention discloses a water suspended nano acetamiprid capsule preparation prepared via emulsion polymerization process. It has capsule shell of styrene, isocyanate, acrylate and methacrylate copolymer, and core comprising biologically originated pesticide and animal remedy acetamiprid as effective component; contains acetamiprid 0.5-5 wt%, capsule shell 5-30 wt%, additive 10-35 wt% and water in proper proportion. The nano capsule is disersive spherical particle of size smaller than 100 nm. Compared with traditional pesticide preparation forms, the present invention has the features of water based solvent, nano level dispersive effective component, high stability, etc. and may be used in killing animalí»s parasite and various pests of fruit tree, vegetable, flower, cotton, tobacco, etc.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Alkaloid microcapsule and preparation method thereof
InactiveCN104472479AImprove stabilityLong duration of actionBiocideAnimal repellantsPesticideAconitum pendulum
The invention relates to an alkaloid microcapsule and a preparation method thereof. The alkaloid microcapsule comprises a capsule core and a capsule wall, wherein the capsule core comprises one or more alkaloids extracted from aconite, aconitum pendulum, mandala, black false hellebore, radix sophorae flavescentis and semen strychni; the capsule wall comprises polyurea which is prepared from polyamine and polyisocyanate. The bitter taste of the alkaloid is covered by microcapsule coating, and the stability of the alkaloid is certainly improved. The alkaloid inside the microcapsule can be slowly released for a long time by the effect of the external environment, so the medicinal effect time is prolonged; the acute toxicity of the alkaloid is certainly alleviated and the alkaloid is chronic in toxicity; on one hand, the vigilance of insects can be reduced; on the other hand, harms to beneficial organisms and humans can be reduced, so the alkaloid microcapsule belongs to an environment-friendly pesticide.
Owner:LANZHOU UNIVERSITY
Application of indole-2, 3-diketone in preparing antimycotics medicament
InactiveCN101214241AInhibition is effectiveEfficient killingOrganic active ingredientsAntimycoticsDiketoneSide effect
The present invention relates to an application of indole-2, 3-dione for preparing for an antifungal drug. The indole-2, 3-dione is natural product or manual synthetical product with low toxicity, little side effect and good antifungal effect.
Owner:QINGDAO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com