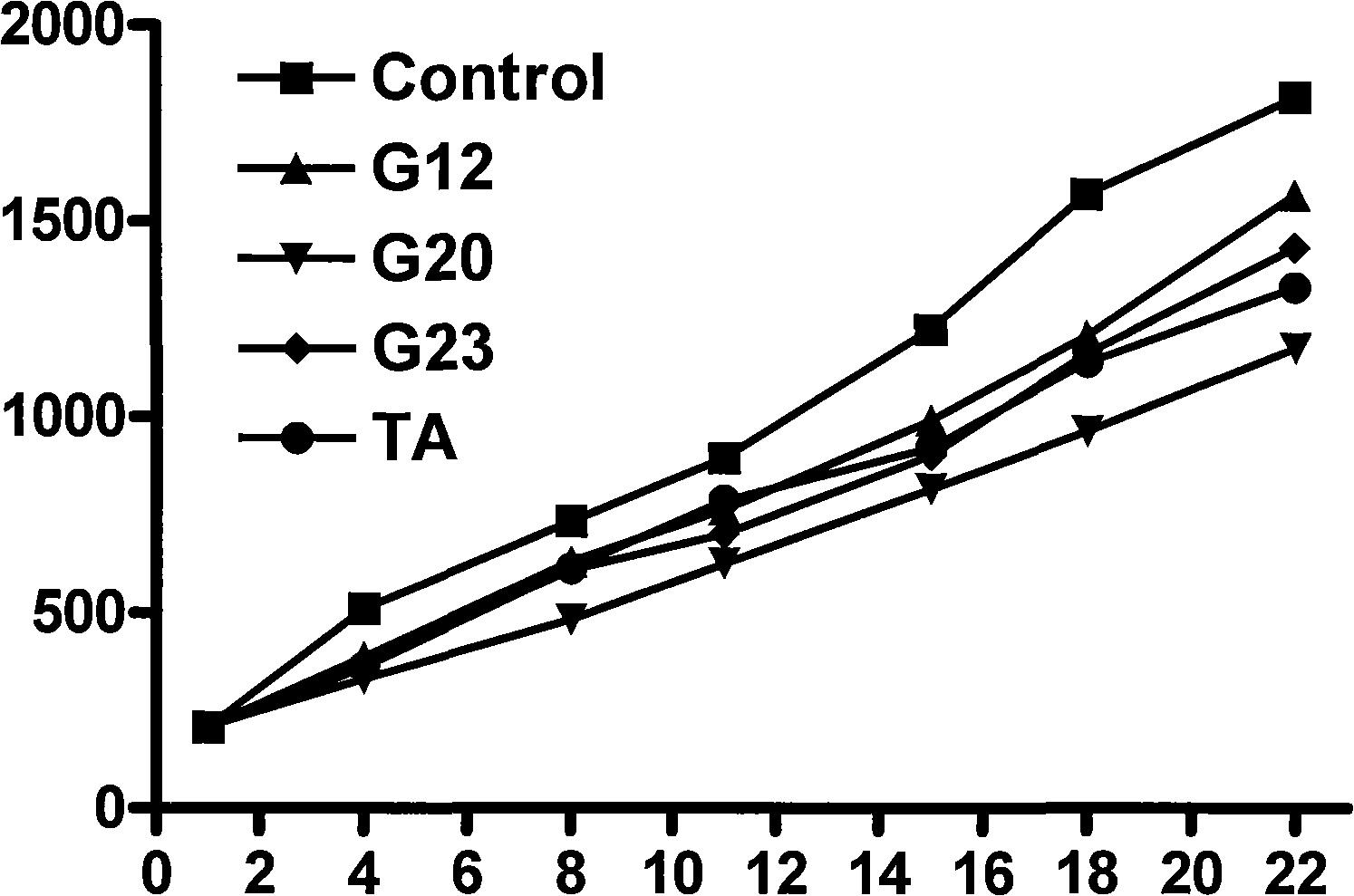
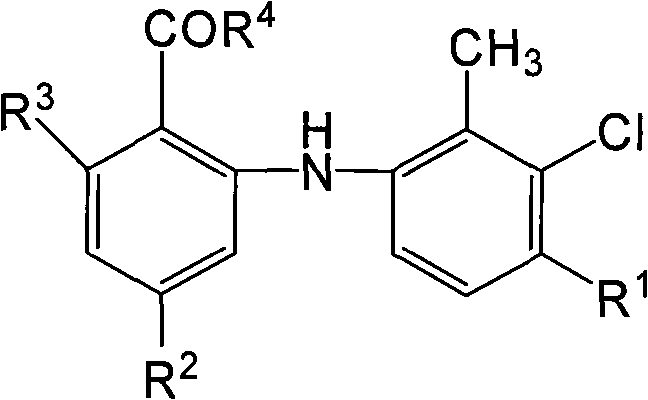
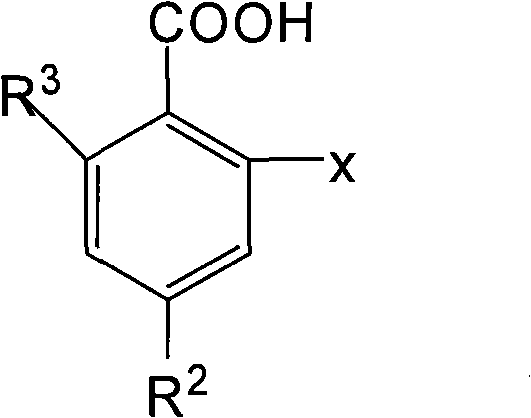
O-anilino benzoic acid derivatives or pharmaceutically acceptable salts thereof as well as preparation method and application thereof
A technology of o-anilino and benzoic acid, applied to o-anilino benzoic acid derivatives or their pharmaceutically acceptable salts, their preparation and their fields of use, can solve problems such as no examples, and achieve easy operation and pain suppression. , the preparation method is simple and effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] [Example 1] Preparation of 2-[(2-chloro-3-methyl-4-nitrophenyl)-amino]benzoic acid (G-1)
[0059] In a 250 ml three-neck flask equipped with an electric stirrer, a thermometer, and a reflux tube, add 8.8 g (43.5 mmol) of o-bromobenzoic acid, 80 ml of n-butanol and 10.4 g of anhydrous potassium carbonate, and stir for 20 minutes; gram of copper powder, 8.8 grams (47.5 mmol) of 4-nitro-3-chloro-2-methylaniline, heating, 90 ° C insulation reaction for 24 hours; cooling to 60 ° C, adding 44 ml of cold water under strong stirring, vacuum filtration , the filtrate was poured into a 250 ml beaker, cooled to 10°C, 15% concentrated hydrochloric acid was added dropwise, and the pH value was adjusted to 3.0; a light yellow solid was precipitated, suction filtered, washed with cold water, and the filter cake was dried; the sample was added to 145 ml Ethanol, heated in a water bath to dissolve, 1.6 g of activated carbon; filtered while hot, cooled the filtrate, and dried with suctio...
Embodiment 2
[0061] [Example 2] Preparation of 2-[(2-chloro-3-methyl-4-aminophenyl)-amino]benzoic acid (G-2)
[0062]50 milliliters of there-necked flasks with electromagnetic stirring, thermometer, reflux tube, add 1.6 grams (5.2 millimoles) G-1 (the sample prepared by the method of embodiment 1), 20 milliliters of water and 0.4 gram of iron powder, stir, add dropwise concentrated Hydrochloric acid 0.4 ml, heating, 90 ° C heat preservation reaction for 24 hours; cooling to 30 ° C, adding 2.2 grams of potassium carbonate under strong stirring, vacuum filtration, pouring the filtrate into a 100 ml beaker, cooling to 10 ° C, adding 15% of Concentrated hydrochloric acid, adjust the pH value to 4.0; Precipitate pale yellow solid, filter with suction, wash with cold water, dry the filter cake; add 13 ml of ethanol, dissolve with water bath heating, 0.7 g of activated carbon; filter while hot, cool the filtrate, and dry with suction 0.8 g of white product was obtained with a yield of 55.6%. The...
Embodiment 3
[0064] [Example 3] Preparation of 4-fluoro-2-[N-(3-chloro--2 methylphenyl)amino]benzoic acid (G-3)
[0065] Add 33 grams (151 mmol) of 4-fluoro-o-bromobenzoic acid, 250 milliliters of n-butanol and 45 grams of anhydrous potassium carbonate to a 500-milliliter three-neck flask equipped with electric stirring, a thermometer, and a reflux tube, stir and heat to 60°C Insulate for 30 minutes; cool to 40°C, pass N2 protection, add 6 grams of copper powder, 40 grams (283 mmol) 3-chloro-2-methylaniline, heat, keep warm at 90°C for 24 hours; cool to 60°C Add 150 ml of cold water while stirring, vacuum filter, pour the filtrate into a 500 ml beaker, cool to 10°C, add 15% concentrated hydrochloric acid dropwise, the pH value reaches 3, a white solid precipitates, filter with suction, wash with cold water, filter cake Drying; 300 ml of ethanol was heated to dissolve, 5 g of activated carbon was hot filtered, the filtrate was cooled with ice water, and 30.3 g of white powder crystals were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com