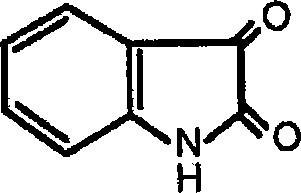
Application of indole-2, 3-diketone in preparing antiphlogistic medicament
The technology of an anti-inflammatory drug and indole is applied in the application field of indole-2,3-dione in the preparation of anti-inflammatory drugs, and can solve the problem that the drug is difficult to meet social needs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of tablet
[0019] Mix ultrapure water and absolute ethanol at a ratio of 1:5, add artificially synthesized indole-2,3-dione, and slowly heat to dissolve at 60°C with a magnetic stirrer, then gradually cool to form needles. Form orange-red crystals, repeat the above steps, and repeat the cleaning 3 times to reduce heavy metal ions. Grind the indole-2,3-dione crystals into powders passing through an 80-mesh sieve, weigh out 100 mg of indole-2,3-dione, 100 mg of lactose, and 245 mg of starch, mix them uniformly, granulate by wet method, and dry. After granulation, it is mixed with 5 mg of magnesium stearate, and compressed into tablets to make tablets with a weight of 500 mg each.
Embodiment 2
[0020] Embodiment 2: the preparation of capsule
[0021] Mix ultrapure water and absolute ethanol at a ratio of 1:5, add artificially synthesized indole-2,3-dione, and slowly heat to dissolve at 60°C with a magnetic stirrer, then gradually cool to form needles. Form orange-red crystals, repeat the above steps, and repeat the cleaning 3 times to reduce heavy metal ions. Grind indole-2,3-dione crystals into powders passing through a 80-mesh sieve, weigh 100 mg of indole-2,3-dione, 345 mg of starch, and 5 mg of stearic acid, mix well, fill capsules, and prepare into capsules with a pure weight of 500mg each.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com