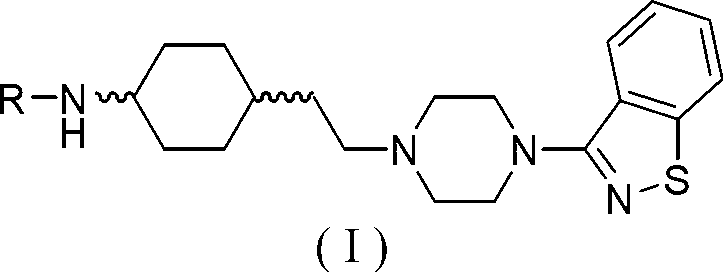
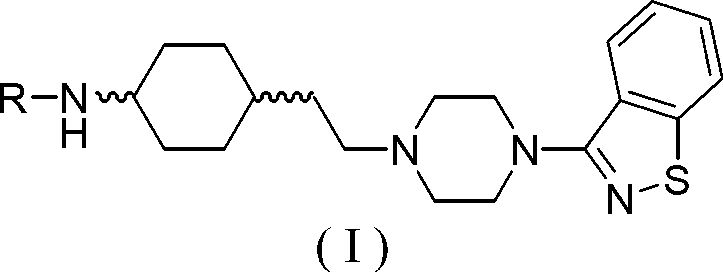
Cyclohexane amine compound and application of cyclohexane amine compound as anti-schizophrenia medicine
A kind of technology of cyclohexaneamine and compound, applied in the field of cyclohexaneamine compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Preparation of trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)acetamide (I-1) ( 1), the preparation of trans-N-tert-butoxycarbonyl-4-aminocyclohexylethanol (2)
[0145] Trans-N-tert-butoxycarbonyl-4-aminocyclohexylacetic acid (25.7g, 0.1mol) and 200mL of dichloromethane were added to a 1000mL four-necked flask, cooled to 0°C in an ice bath, and triethylamine ( 0.25mol), slowly add isopropyl chloroformate (0.15mol) dropwise, control the temperature not to exceed 10 degrees Celsius, after the addition, stir at room temperature for 2 h, cool to 5 °C, add 200 mL of cold water, stir, and separate the organic layer with saturated brine Washed (200mL×1), evaporated to dryness, N 2 Under the protection, 200 mL of anhydrous tetrahydrofuran was added to the residue, the temperature was lowered to 0 °C, and KBH was slowly added in batches. 4 (0.1mol), the temperature should not exceed 5 °C, the addition was completed, stirred at room temperature for 2 ...
Embodiment 2
[0175] Preparation of trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)n-pentanamide (I-2)
[0176] Using intermediate 5 (10 mmol) and n-valeryl chloride (12 mmol) as raw materials, according to the preparation method of compound I-1, the target compound I-23.72 g was obtained with a yield of 87%.
[0177] 1 H NMR (CDCl 3 ,δ:ppm):0.93(t,3H,J=7.2Hz,CH 3 ),1.07-1.11(m,4H,A-H),1.28-1.33(m,3H,A-H),1.39-1.43(m,2H,A-H),1.50-1.55(m,2H,A-H),1.88-1.93( m,2H,A-H),2.10-2.21(m,4H,A-H),2.44(t,2H,J=7.6Hz,N-CH 2 ),2.69-2.73(m,4H,piperazine-CH 2 ),3.52-3.56(m,4H,piperazine-CH 2 ),4.07-4.09(m,1H,A-H),7.38(d×t,1H,J=8.4Hz,0.8Hz,Ar-H),7.49(d×t,1H,J=8.4Hz,0.8Hz, Ar-H),7.85(d,1H,J=8.4Hz,Ar-H),7.89(d,1H,J=8.0Hz,Ar-H).
[0178] ESI-MS: 429[M+H + ]
[0179] Preparation of compound I-2 hydrochloride
[0180] Using compound I-2 (5 mmol) and 5% (5 mmol) hydrochloric acid as starting materials, and adopting the synthesis method of compound I-1 hydrochloride, 2.0 g of whi...
Embodiment 3
[0192] trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)-2,2,2-trifluoroethyl Preparation of amide (I-3)
[0193] Using intermediate 5 (10 mmol) and trifluoroacetic anhydride (12 mmol) as raw materials, according to the preparation method of compound I-1, the target compound I-33.82 g was obtained with a yield of 86.7%.
[0194] 1 H NMR (CDCl 3 ,δ:ppm):1.06-1.19(m,3H,A-H),1.22-1.30(m,2H,A-H),1.42-1.58(m,2H,A-H),1.85-1.89(m,2H,A-H), 2.15-2.18(m,2H,A-H),2.43(t,2H,J=7.6Hz,N-CH 2 ),2.63-2.65(m,4H,piperazine-CH 2 ),3.34-3.49(m,1H,A-H),3.54-3.58(m,4H,piperazine-CH 2 ),4.08(m,1H,A-H),7.30-7.35(m,1H,Ar-H),7.42-7.46(m,1H,Ar-H),7.79(d,1H,J=8.0Hz),7.88 (d,1H,J=8.0Hz).
[0195] ESI-MS:441[M+H + ]
[0196] Preparation of compound I-3 hydrochloride
[0197] Using compound I-3 (5 mmol) and 5% hydrochloric acid (5 mmol) as starting materials, and adopting the synthesis method of compound I-1 hydrochloride, 1.96 g of white solid was obtained with a yield of 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com