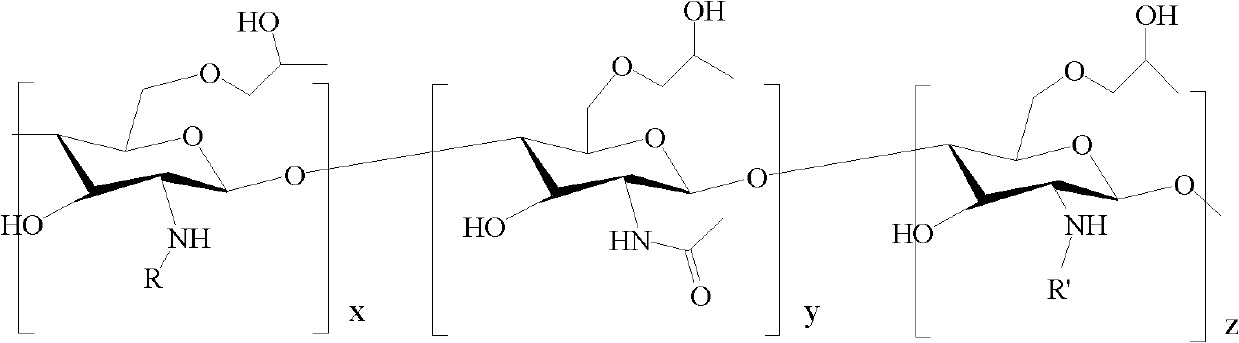
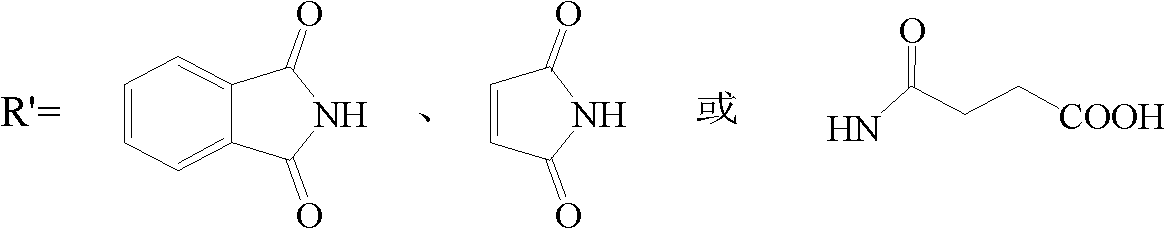
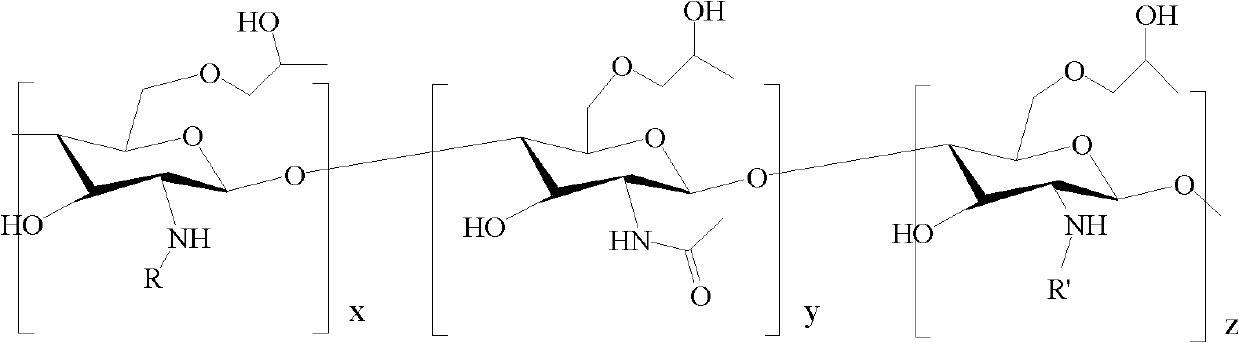
Amphiphilic chitosan derivative, its preparation method and its application in medicinal preparation
A technology for chitosan derivatives and drugs, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and liquid delivery, etc., can solve problems such as insolubility in organic solvents, achieve low toxicity, improve therapeutic effects, and high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1, the preparation of N-octyl chitosan (referring to CN 03112981.1)
[0048] Chitosan (1.0g) was suspended in 50mL of methanol and stirred at room temperature, octanal (1.02g) was added, and after 24 hours of reaction, KBH was added 4 (0.5g) in 5mL aqueous solution, continue to stir overnight, the reaction solution is neutralized with 2N hydrochloric acid solution, precipitated with methanol, filtered, and the filter residue is repeatedly washed with methanol and water for 3 times, and dried overnight at 60°C in a vacuum oven to obtain shallow Yellow powder N-octyl chitosan 1.0g.
[0049] N-alkylated chitosan such as N-decyl chitosan, N-lauryl chitosan, N-octadecyl, N-cholesteryl chitosan can be prepared by the same method.
[0050] 2. Preparation of N-octyl-N'-phthaloyl chitosan
[0051] N-octyl chitosan (1.5g) was suspended in DMF (40mL) and mechanically stirred overnight, N 2 Under protection and stirring, 1.9 g of phthalic anhydride was slowly added, the temperat...
Embodiment 2
[0060] 1. Preparation of N-decyl chitosan
[0061] Using decanal and chitosan to react, the preparation method is the same as that of N-octyl chitosan.
[0062] 2, Preparation of N-decyl-N'-phthaloyl chitosan
[0063] It is prepared by reacting N-decyl chitosan with phthalic anhydride, and the method is the same as that of N-octyl-N'-phthaloyl chitosan. The same method can prepare N-decyl-N'-maleyl chitosan, N-decyl-N'-(3-formyl propionic acid) chitosan, N-decyl-N'- (4-formylbutyric acid) chitosan and other acid anhydride chitosan.
[0064] 3, Preparation of N-decyl-N'-phthaloyl-O-hydroxypropylated chitosan
[0065] React with N-decyl-N'-phthaloyl chitosan and polyphosphoric acid, and the preparation method is the same as OPHPC.
[0066] FT IR: 2860, 1386, 1287, 908, 718cm -1 (long chain alkyl), 1121cm -1 (hydroxypropyl), 1718cm -1 (phthaloyl).
[0067] 'H-NMR (D 2 O): δ (ppm): 0.6-1.8 (m, 15H) (-NH-CH 2 -(C H 2 ) 6 -C H 3 ); 2.5-2.8(m, 2H)(-NH-C H 2 -(CH 2 )...
Embodiment 3
[0072] 1, Preparation of N-octadecyl chitosan
[0073] The reaction between octadecanal and chitosan is the same as that of N-octyl chitosan.
[0074] 2, Preparation of N-octadecyl-N'-phthaloyl chitosan
[0075] It is prepared by reacting N-octadecyl chitosan with phthalic anhydride, and the method is the same as that of N-octyl-N'-phthaloyl chitosan. The same method can prepare N-octadecyl-N'-maleyl chitosan, N-octadecyl-N'-(3-formyl propionic acid) chitosan, N-octadecyl Anhydrous chitosan such as alkyl-N'-(4-formylbutyric acid) chitosan.
[0076] 3, Preparation of N-octadecyl-N'-phthaloyl-O-hydroxypropylated chitosan
[0077] React with N-octadecyl-N'-phthaloyl chitosan and polyphosphoric acid, and the preparation method is the same as OPHPC.
[0078] FT IR: 2860, 1386, 1287, 908, 718cm -1 (long chain alkyl), 1121cm -1 (hydroxypropyl), 1718cm -1 (phthaloyl).
[0079] 'H-NMR (D 2 O): δ (ppm): 0.6-1.8 (m, 15H) (-NH-CH 2 -(C H 2 ) 6 -C H 3 ); 2.5-2.8(m, 2H)(-NH-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com