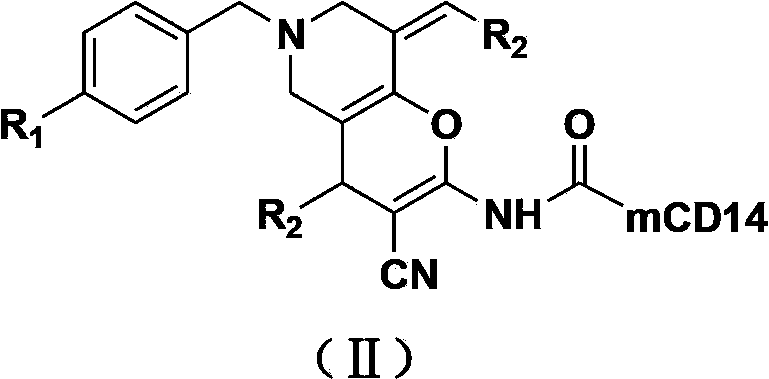
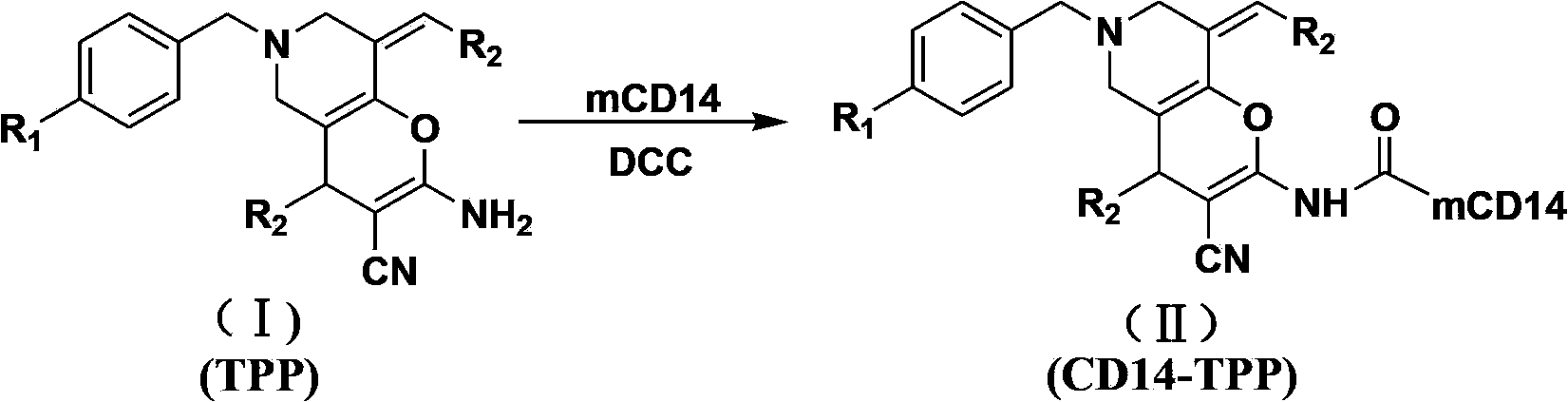
Tetrahydropyridinopyran-monoclonal antibody CD14 conjugate with anti-tumor activity and preparation method and use thereof
A technology of monoclonal antibody and anti-tumor activity, which is applied in the field of the lead compound of monoclonal antibody drug, tetrahydropyridopyran-monoclonal antibody CD14 conjugate, which can solve the problems of low selectivity and toxicity, and achieve toxic and side effects Minimal, low acute toxicity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Conjugates (IIa)~(IIh)
[0040] For the preparation methods of compounds (Ia)~(Ih), please refer to the patent with publication number: CN102285993A.
[0041] Take 10 mg of compounds (Ia)~(Ih), dissolve them in 0.1 mL DMSO respectively, add 0.1 mL of DCC solution with a concentration of 0.1 mol / L and 0.1 mL of CD14 with a concentration of 2 μg / mL to the above solutions, and then use Physiological saline was used to dilute the above mixed solution to 1 mL. After reacting at 37°C for 30 minutes, the conjugates (Ⅱa)~(Ⅱh) were obtained.
[0042] The killing effects of the compound of formula (I) and the conjugate of formula (II) on human leukemia K562 cell line were determined by MTT method; The lethal effect, and set up multiple control groups at the same time. The control group included: blank group, CD14+DMSO+DCC, CD14, compound (Ia)~(Ih), CD14+compound (Ia)~(Ih).
Embodiment 2
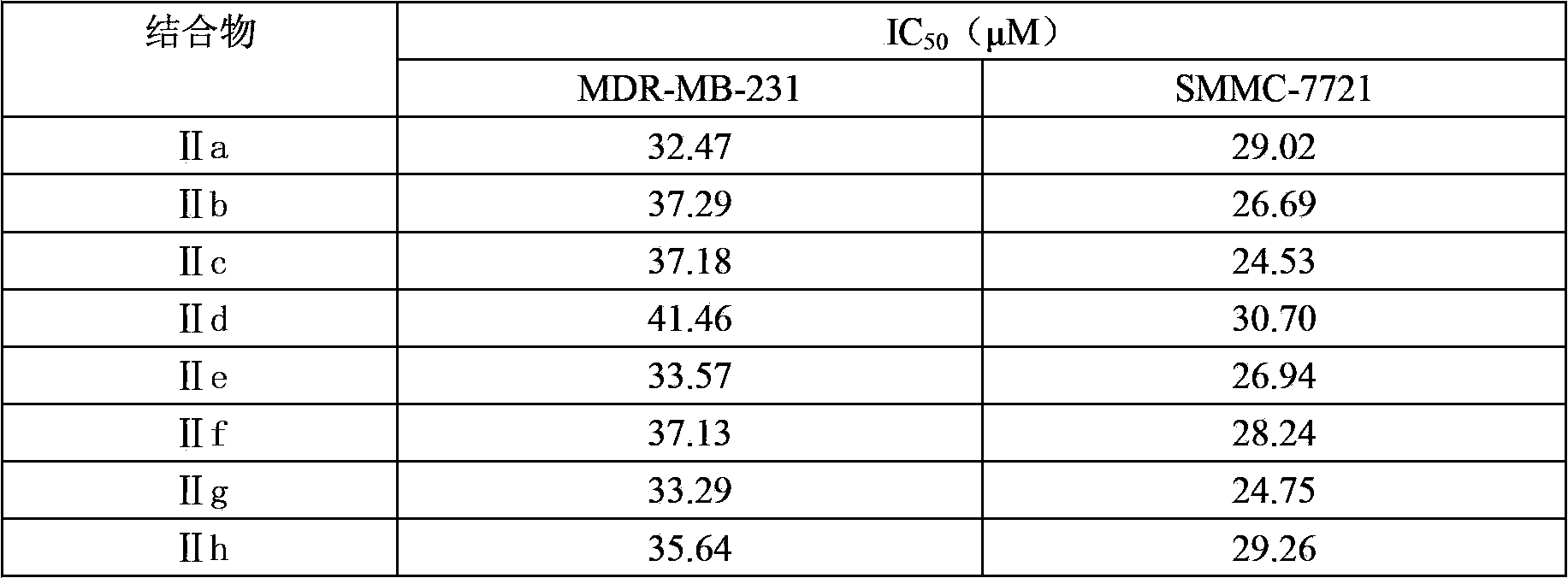
[0043] Example 2: Conjugates (IIa)~(IIh) and compounds (Ia)~(Ih) inhibit human leukemia K562 cell line, and conjugates (IIa)~(IIh) inhibit ovarian cancer MDR-MB-231 cell line and liver cancer IC of proliferation of SMMC-7721 cell line 50 the test
[0044] 1. Test reagents and equipment
[0045] Experimental drugs and reagents: self-made compounds (Ⅰa)~(Ⅰh) and conjugates (Ⅱa)~(Ⅱh), dissolved in DMSO, added distilled water to the required concentration (DMSO concentration ≤ 1‰), sterilized and stored at 4°C. MTT (tetramethylazolazolium blue) reagent was purchased from Sigma. Human leukemia cells K562 ovarian cancer MDR-MB-231 cell line and liver cancer SMMC-7721 cell line were purchased from Shanghai Chinese Academy of Sciences Cell Bank. 10% SDS reagent (Sino-American Biotechnology product), RPMI-1640 (GiBCo, USA) culture medium containing 20% fetal bovine serum (FBS) was used, and other reagents were commercially available analytically pure. At 37°C, 5% CO 2 Subculture...
example 3
[0058] Example 3 Compounds (Ia)~(Ih) and conjugates (IIa)~(IIh) inhibit the proliferation of mouse normal bone marrow cells IC 50 the test
[0059] 1. Test method (colony counting method)
[0060] The mice were sacrificed by vertebral dislocation, the femur was taken out under aseptic conditions, and the bone marrow cells were washed out with TMEM to make a single cell suspension, and 10 6 Mouse bone marrow cells were planted in 2ml Endo M culture system containing 20% fetal bovine serum, placed in a 6-well plate, and drug treatment groups were set up for compound (Ⅰa)~(Ⅰh), conjugates (Ⅱa)~(Ⅱh) and negative control group (DMSO), at 37°C, 5% CO 2 1. Count after 3 days of culture in saturated humidity, take the aggregation of ≥50 cells as a colony, record the number of colonies in each well, and use EXCEL as a trend line to calculate the IC 50 value.
[0061] 2. Outcome Survey
[0062] The ICs of compounds (Ia)~(Ih) and conjugates (IIa)~(IIh) inhibiting the proliferation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com