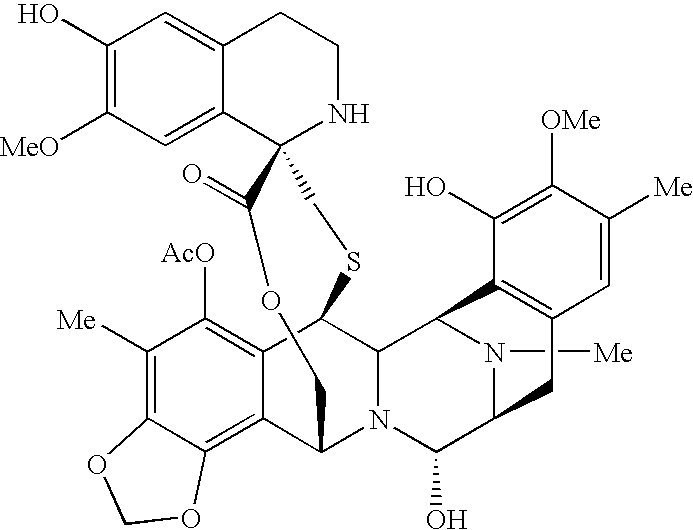
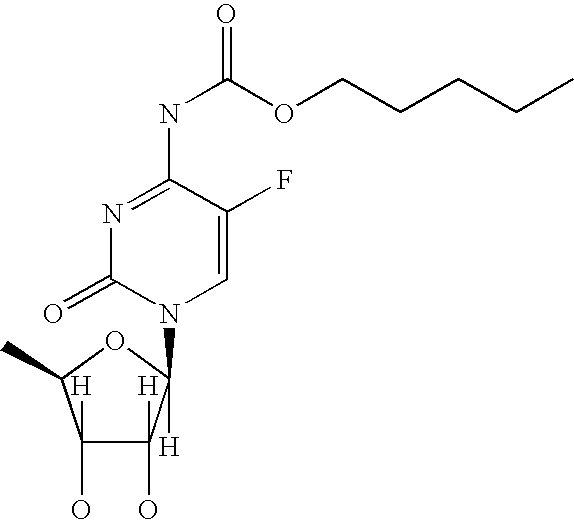
Patents
Literature
415 results about "5-Fluoracil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a medication used to treat cancer. By injection into a vein it is used for colon cancer, esophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis and basal cell carcinoma.
Medicinal compositions for concomitant use as anticancer agent
InactiveUS20030215523A1Good synergyEliminate side effectsHeavy metal active ingredientsBiocideCarboplatinAnticarcinogen
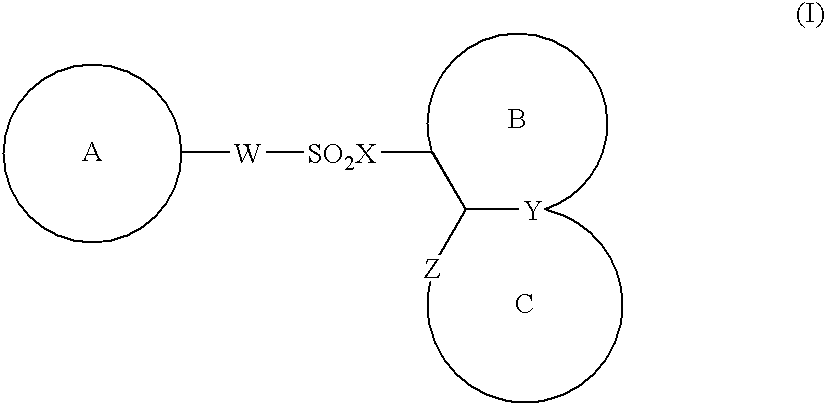
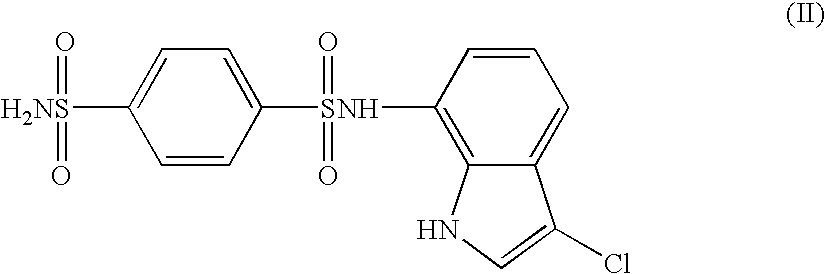
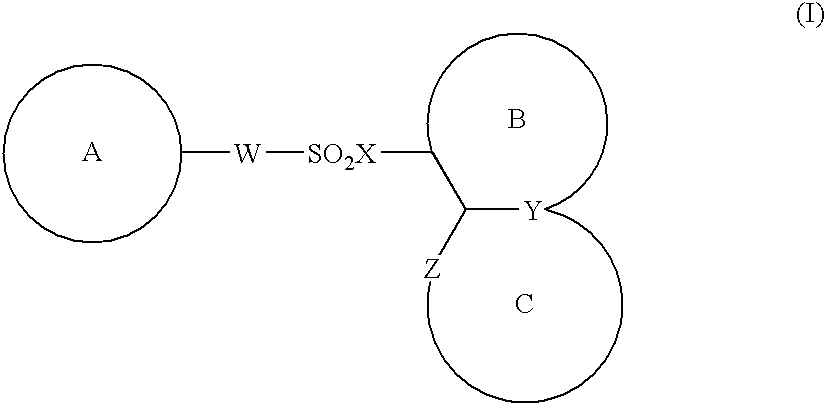
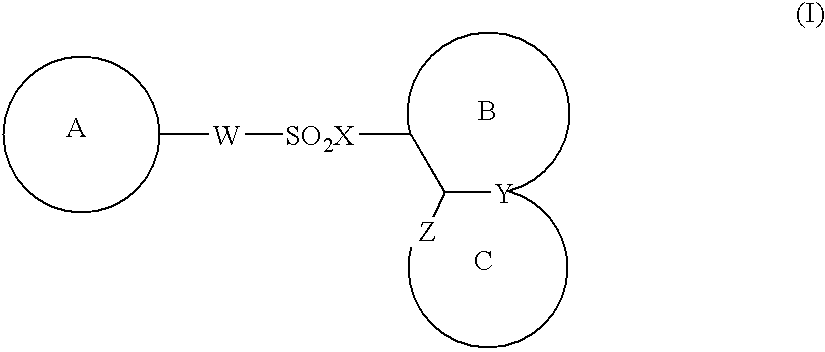
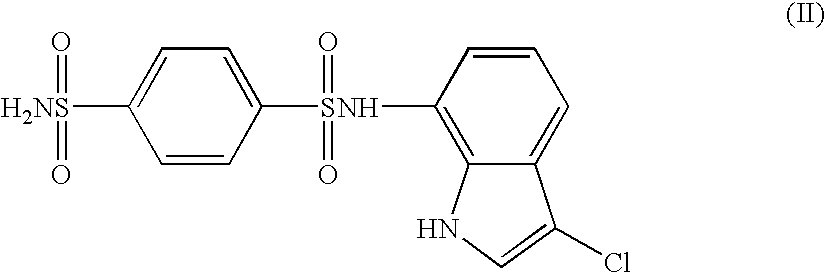
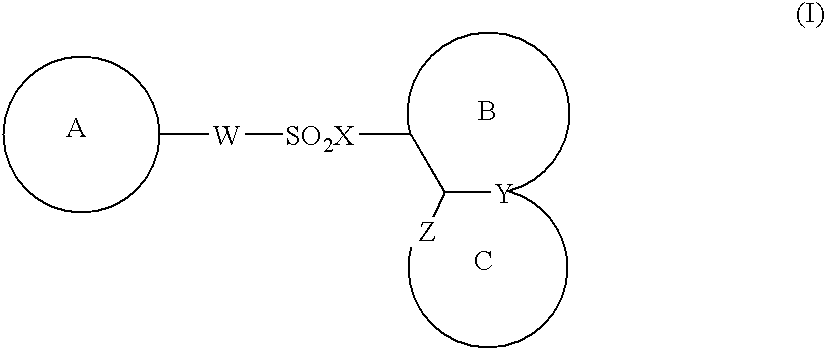
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Biodegradable fluorourcacil polyester medicine-carried nanospheres and its preparation method
InactiveCN101053553AHigh drug loadingSmall particle sizeOrganic active ingredientsPowder deliveryPolyesterPolymer science
The invention relates to biodegradable fluorouracil(Fu) polyester drug-bearing manoparticles with a coating material of polylactic acid, polylactic acid-glycolic acid, polylactic acid-polyethylene glycol block copolymer or polylactic acid-glycolic acid-polyethylene glycol block copolymer and the producing method including: firstly, fully dissolving the copolymer in the dichloromethane, under the ultrasonic shock, injecting the fluorouracil NaOH solution in the dichloromethane solution, dispersing uniformly, forming W / O primary latex, and beating up the primary latex and injecting into the fluorouracil saturated water solution containing 5 wt% of polyvinylalcohol (PVA), and storing in the refrigeratory after freeze-dry. The drug-bearing manoparticle has a drug content which is 10-25% of the microparticle mass, and has a smooth surface, an even diameter distribution, a remarkable slow release function and not adhesive. The micropartical size is 100-1000nm.
Owner:JILIN UNIV +1
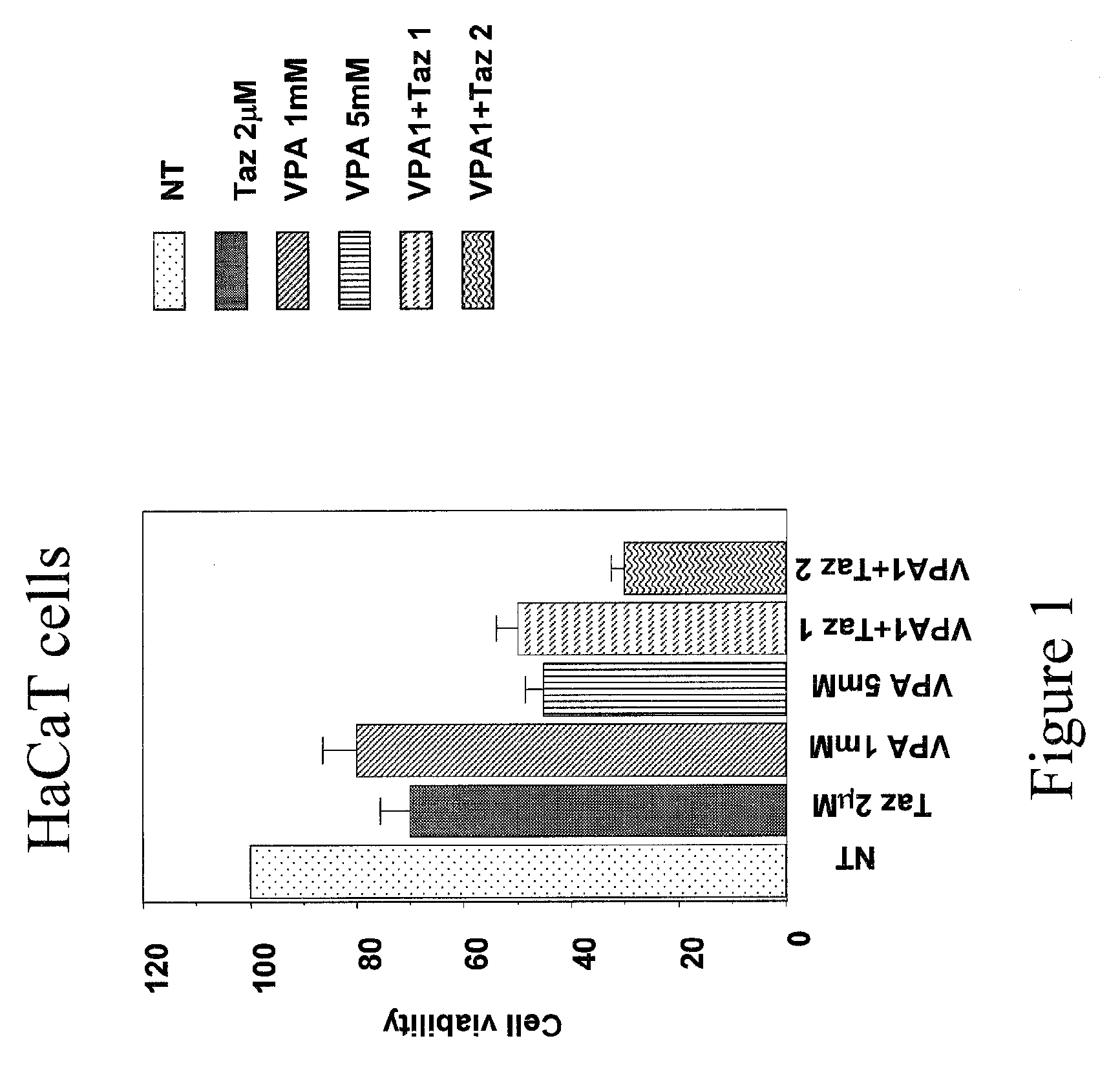
Topical Use of Valproic Acid for the Prevention or Treatment of Skin Disorders
The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell Lymphoma and also for the topical treatment of pre-malignant lesions, and of inflammations of the skin and / or mucosa. The invention also relates to the use of this topically applicable formulation for the protection from UV light and for the treatment of sun burn. The invention includes the use of VPA for the manufacture of a clinically used medicament for the topical treatment of the human diseases listed above.
Owner:TOPOTARGET GERMANY AG
Methods for treating or preventing colorectal cancer
InactiveUS20110104256A1Organic active ingredientsPeptide/protein ingredientsFormyltetrahydrofolic AcidsSunitinib
The present invention provides, for example, methods for treating or preventing colorectal cancer with an anti-IGF1R antibody in association with sunitinib or a combination of leucovorin and 5-fluorouracil.
Owner:MERCK SHARP & DOHME CORP
Molecular chemotherapy enhancement of radiotherapy
InactiveUS6552005B1Tumour growth inhibitionCurrent is limitedBiocidePeptide/protein ingredientsWhole bodyCytotoxicity
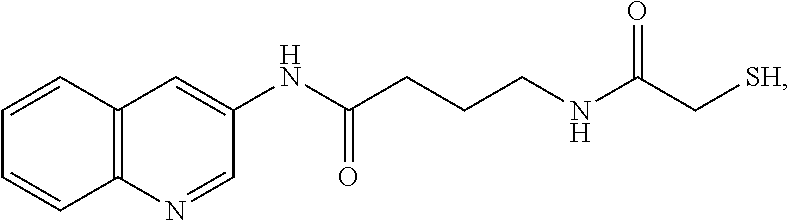
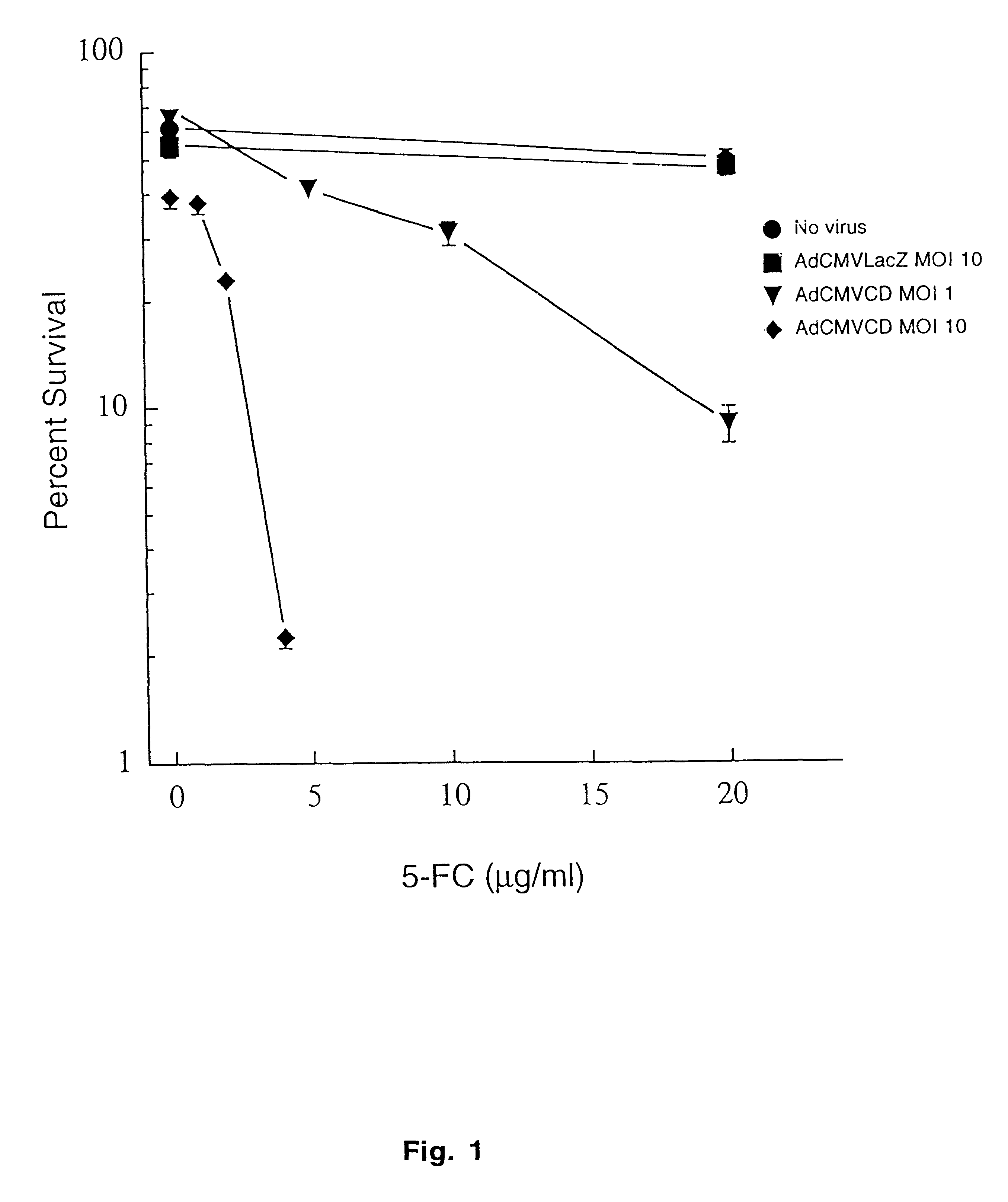
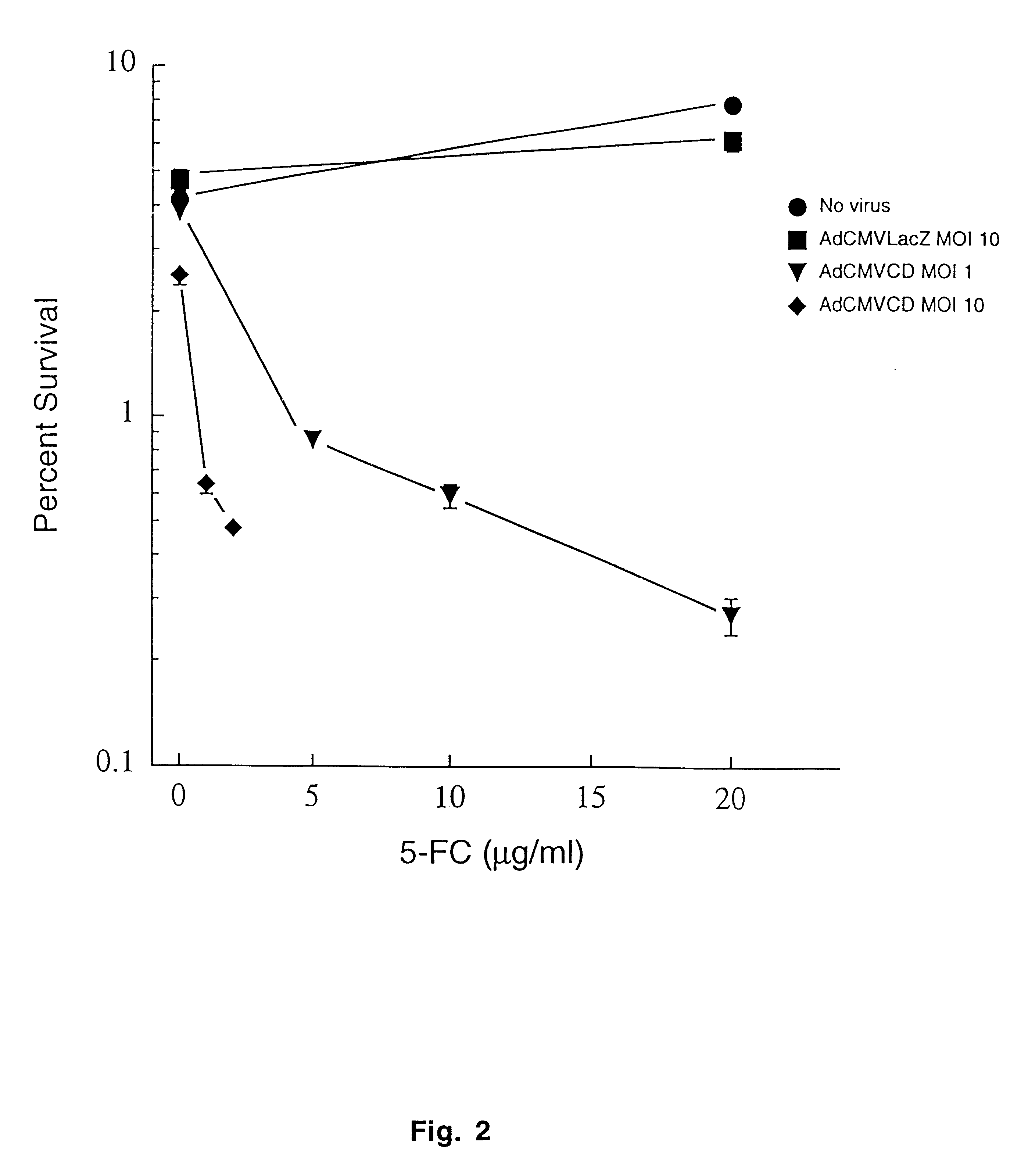
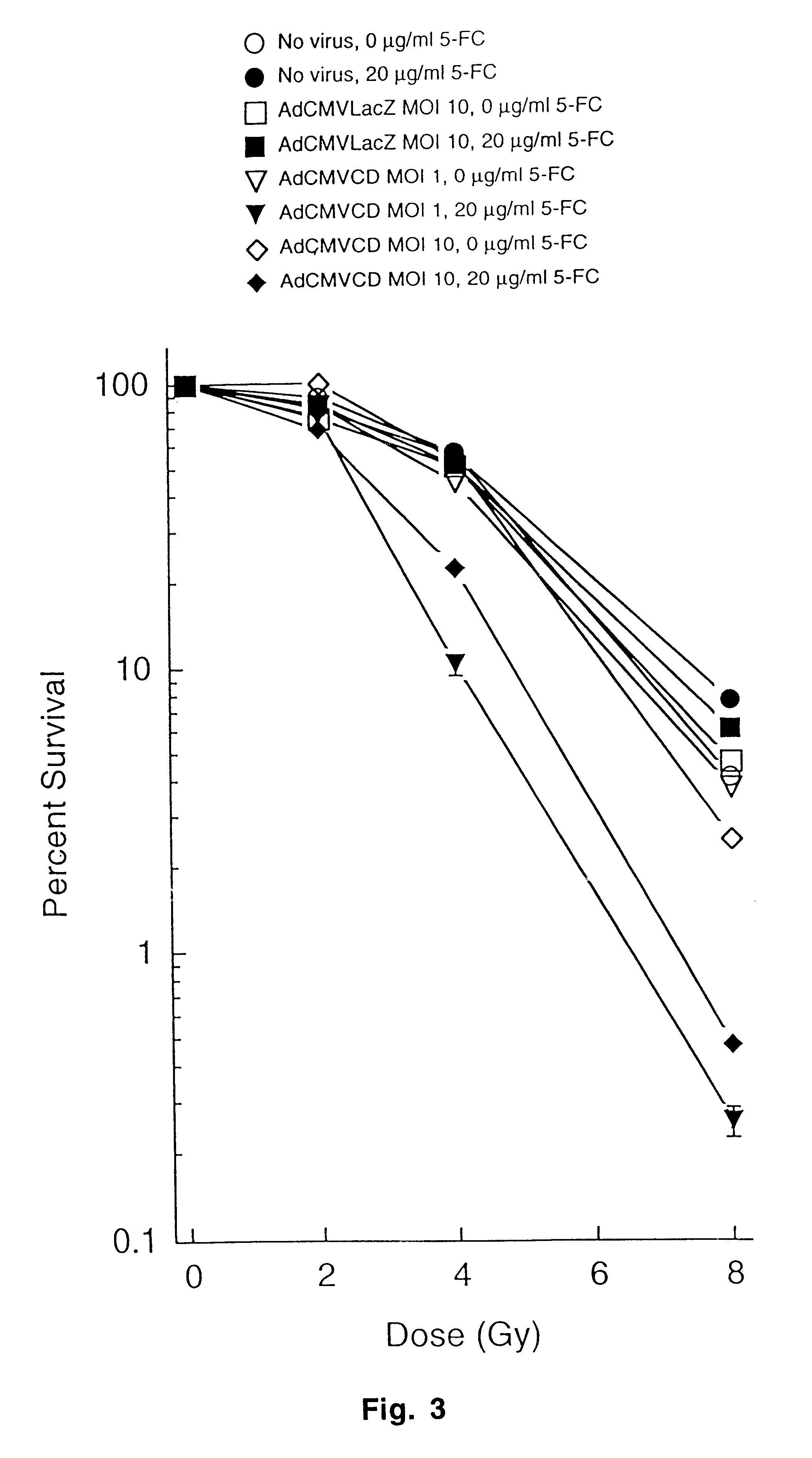
The present invention provides a new approach for cancer treatment by utilizing gene therapy combined with radiation therapy to enhance cytotoxicity in malignant cells. Specifically, the present invention demonstrates that molecular chemotherapy with the cytosine deaminase gene and 5-fluorocytosine is an effective radiosensitizing strategy which may lead to substantial improvement in tumor control, with less normal tissue toxicity than conventional systemic administration of 5-fluorouracil, that would translate into improved cure rates and better survival. A noninvasive method is described for continuous in vivo monitoring of 5-fluorouracil production via magnetic resonance spectroscopy. An adenovirus encoding cytosine deaminase gene which selectively replicates in tumor cells with a defective p53 pathway was constructed. Also provided is an adenovirus which encodes a fusion protein of cytosine deaminase and uracil phosphoribosyltransferase.
Owner:CDEPT
Treatment method against side-effects of chemotherapy
InactiveUS6979688B2Preserving anti-cancer systemic efficacyAdverse side effectBiocideKetone active ingredientsSide effectWhole body
A method and composition is provided for organ rescue wherein a specific counter-measure is applied locally to a tissue at risk for or exhibiting an adverse side effect of a cancer treatment. More particularly, the method and composition is directed at controlling Hand-Foot Syndrome, a painful redness and cracking of the skin of the hands and feet which can occur with systemic treatment with 5-fluorouracil or a precursor thereof. Uracil ointment is applied to the skin of the hands and feet to prevent Hand-Foot Syndrome which can occur from systemic administration of 5-fluorouracil (or precursor thereof) as cancer treatment.
Owner:ASYMMETRIC THERAPEUTICS
Combination of ET-743 and a 5-fluorouracil pro-drug for cancer treatment
Methods of treating a human body for cancer are provided. In one aspect, a therapeutic amount of capecitabine is administered in combination with ET-743 in a dose range between 0.75 and 1.4 mg / m2 for Et-743. In a related aspect, an effective therapeutic amount of ET-743 is administered in combination with capecitabine in a dose range between 1500 to 2500 mg / m / day for capecitabine.
Owner:PHARMA MAR U
Nanometer fluorouracil coat artificial crystalloid and the preparing method
InactiveCN101036804ASuppress turbidityLow toxicityCoatingsIntraocular lensPosterior capsule opacificationChitosan nanoparticles
The invention relates to a intraocular lens. At present the Poly-Methyl Methacrylate (PMMA) intraocular lens conventional for clinical treatment of cataract always causes inflammatory treaction after implantation. Posterior capsule opacification is also a major compalication after cataract surgery. The invention selects fluorouracil to solve the above problems. Based on weak penetrating force of chitosan nanoparticles in eyes and the relation between the phagocytosis amount of conjunctival epithelial cells to nanoparticles and the particle size of nanoparticles, the fluorouracil nanoparticles preparation is prepared using chitosan-poly(acrylic acid) as carrier, composite coated on the surface of PMMA intraocular lens. The invantion also provides a preparation method thereof. The said intraocular lens not only increases the biocompatibility of the intraocular lens, but also inhibites posterior capsule opacification pafter cataract surgery. The said intraocular lens can also prevent anterior membrane after implantation of intraocular lens and after-cataract.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Medicinal composition for treating non-small cell lung cancer and application thereof
InactiveCN103948689AReduce dosageLow toxicityAntineoplastic agentsHeavy metal compound active ingredientsSalvia miltiorrhizaCarboplatin
The invention discloses a medicinal composition for treating non-small cell lung cancer. The medicinal composition comprises a target medicament, a chemotherapeutic medicament and a traditional Chinese medicament, wherein the target medicament is one or more of bortezomib, imatinib, gefitinib and sunitinib; the chemotherapeutic medicament is one or more of 5-fluorouracil, carboplatin, epirubicin, adriamycin and fludarabine; and the traditional Chinese medicament is one or more of salvia miltiorrhiza, astragalus membranaceus, sappanwood, Chinese pulsatilla root and portulaca oleracea. The medicinal composition has a remarkable synergetic treatment effect when being used for treating the non-small cell lung cancer, and can be used for remarkably strengthening the cancer-inhibition effect compared with treatment of a single medicament, so that the medicament dosage can be reduced, and the toxic and side effects of chemotherapeutic medicaments can be reduced.
Owner:NORTHWEST A & F UNIV
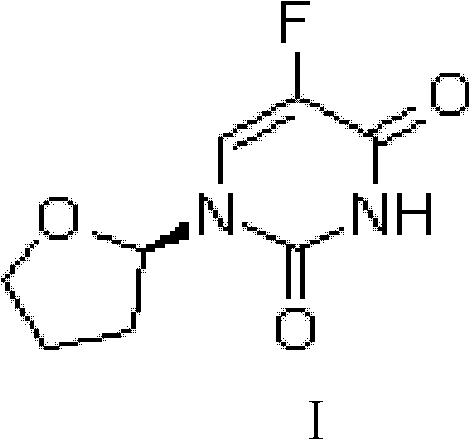
Industrial tegafur synthesizing method
The invention belongs to the technical field of medicine, and specifically relates to an industrial tegafur synthesizing method comprising the steps that: under inert gas pressure control and the effects of a Lewis acid catalyst, 5-fluorouracil and 2,3-dihydrofuran are subjected to a substitution reaction in an aprotic polar solvent; and acidification and refining are carried out, such that tegafur is obtained. The method has the advantages of simple reaction process, high yield, less side reactions, mild reaction conditions, and the like. Pharmacopoeia standards can be satisfied with simple refining, and purity is higher than 99.7%.
Owner:SHANDONG NEWTIME PHARMA
Medicinal compositions for concomitant use as anticancer agent
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
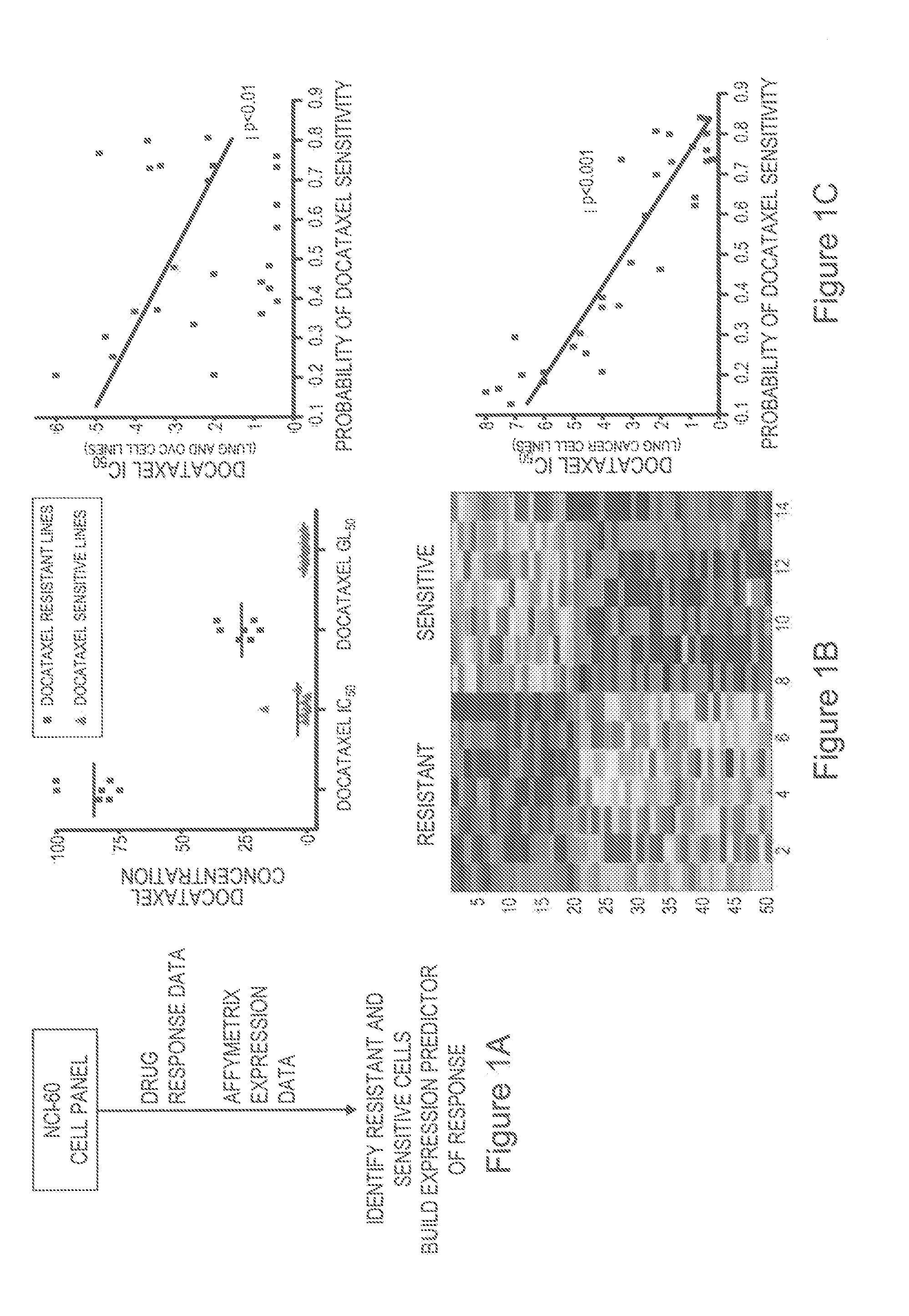
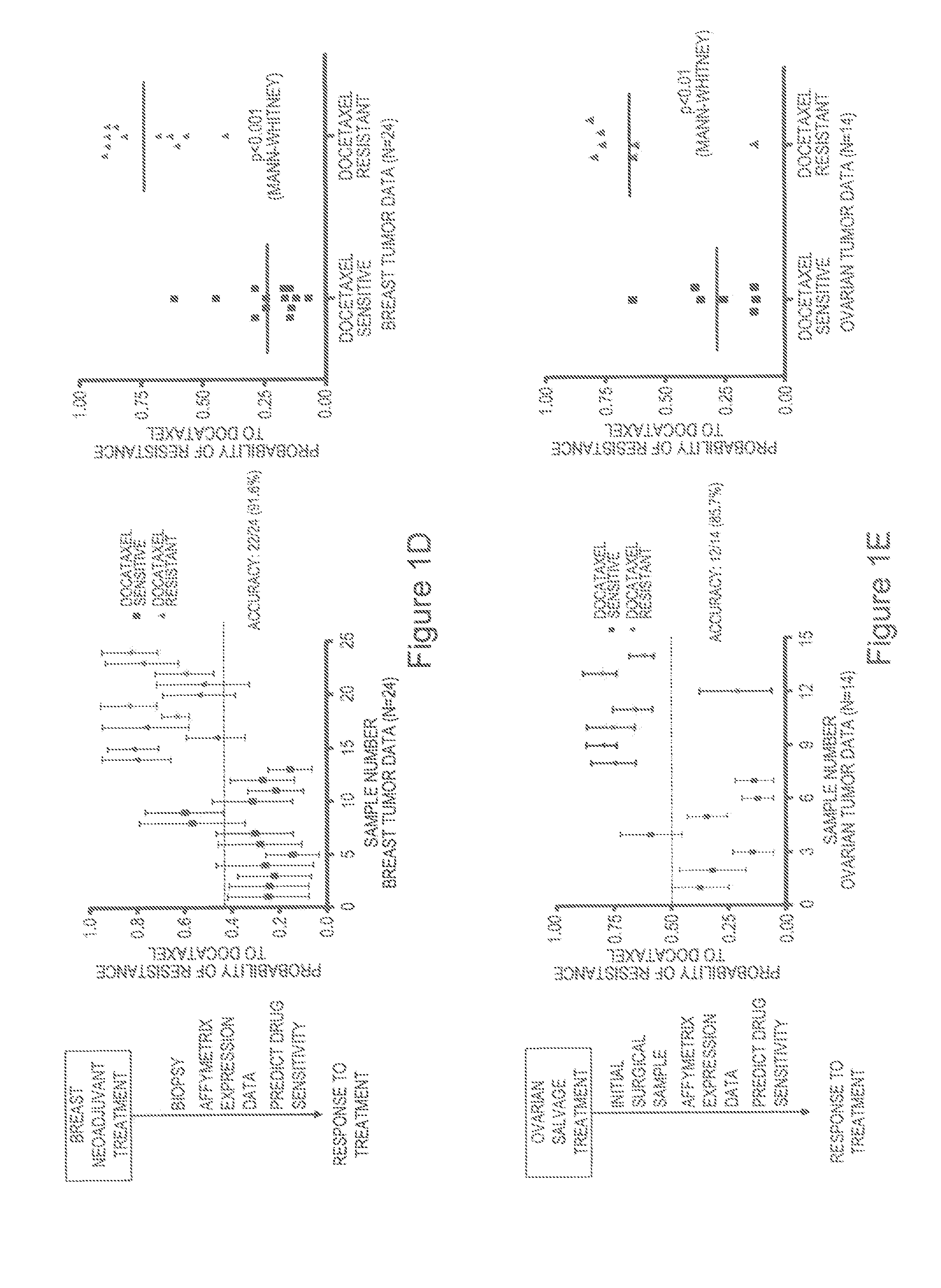
Predicting responsiveness to cancer therapeutics
Owner:UNIV OF SOUTH FLORIDA +1
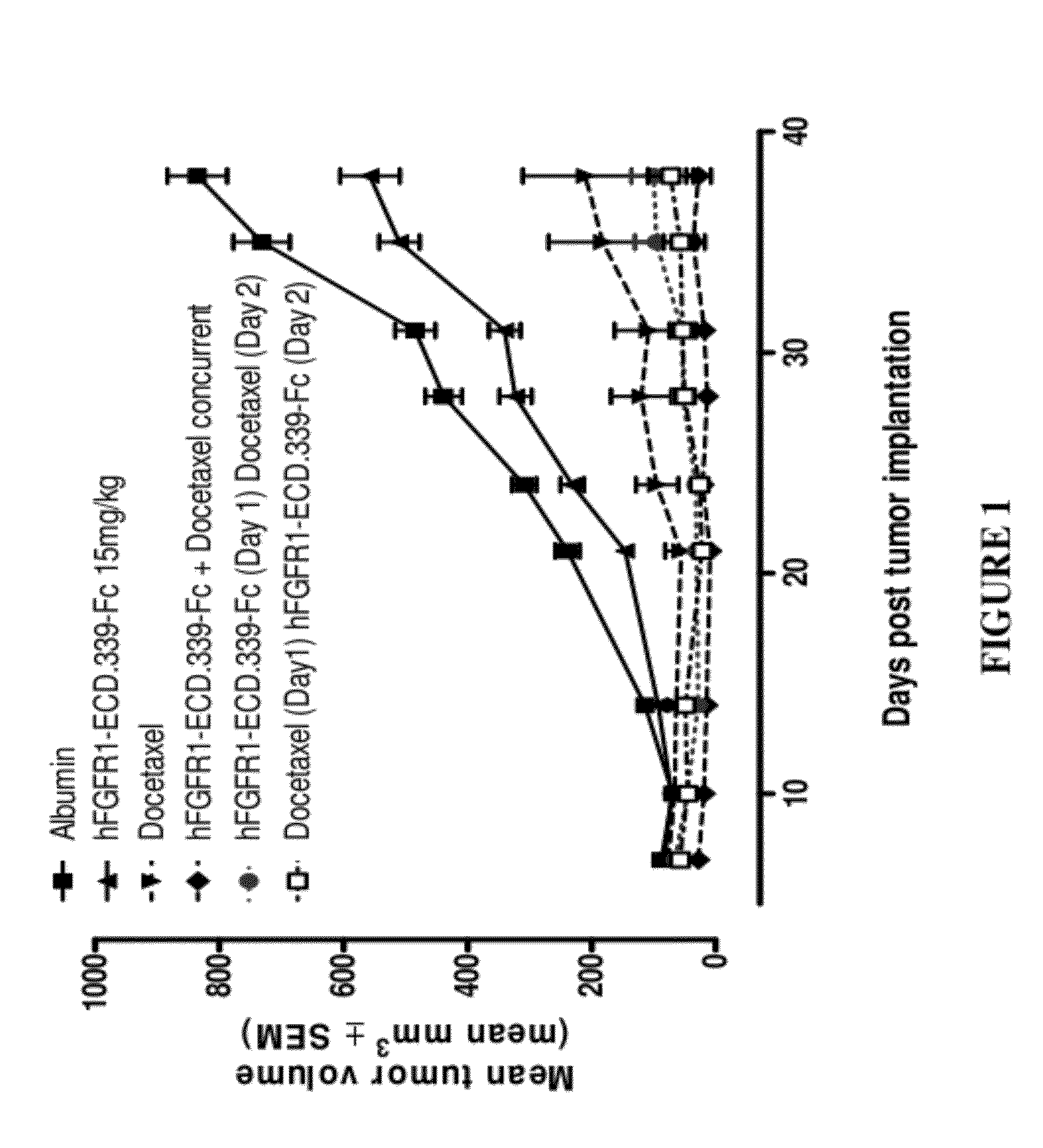
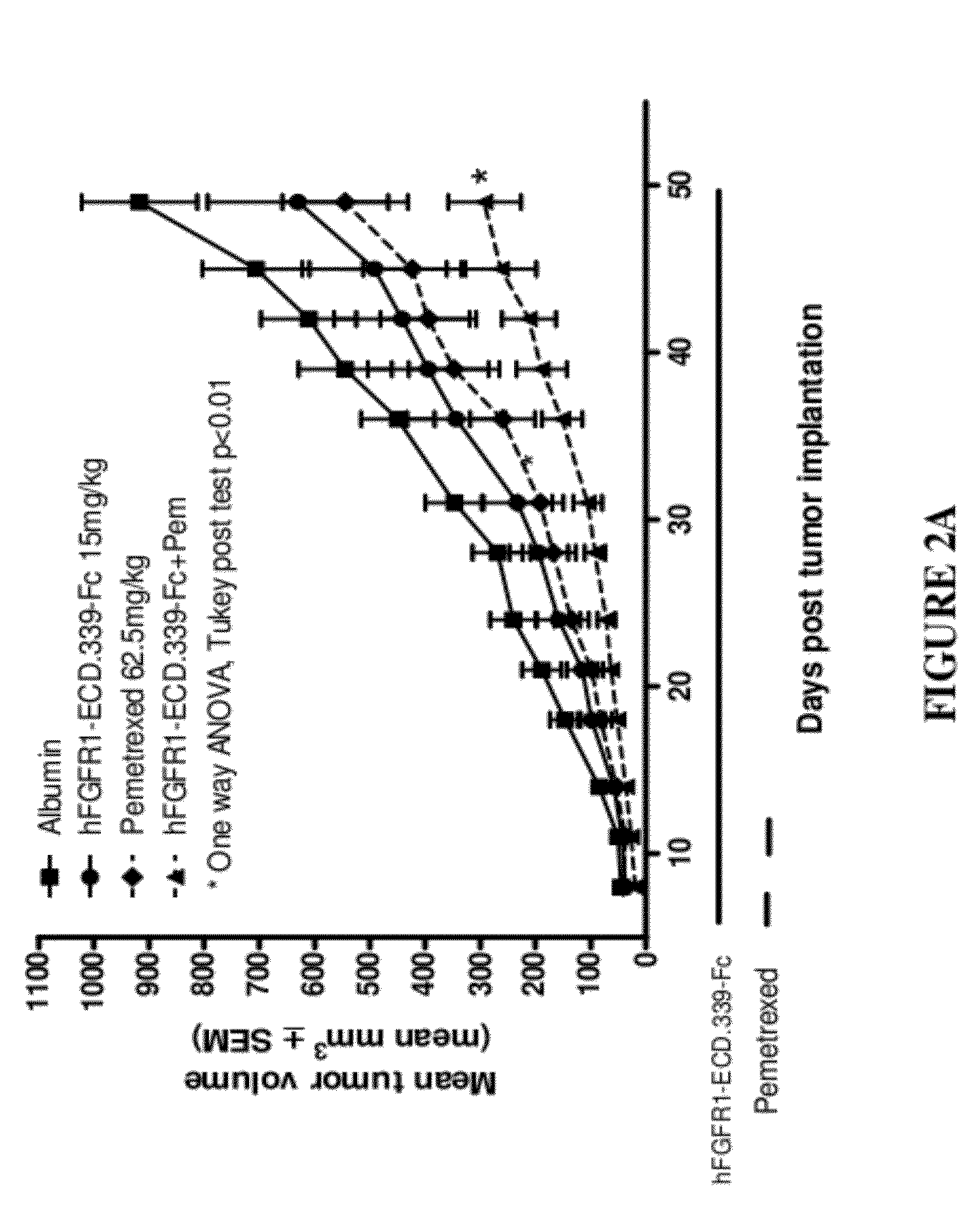
Fgfr1 extracellular domain combination therapies
InactiveUS20120237511A1Inhibit tumor cell growthOrganic active ingredientsHeavy metal active ingredientsCarboplatinDocetaxel
Methods of treating cancer comprising administering a fibroblast growth factor receptor 1 (FGFR1) extracellular domain (ECD) and / or an FGFR1 ECD fusion molecule in combination with at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are provided. Dosage packs comprising an FGFR1 ECD and / or an FGFR1 ECD fusion molecule and / or at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are also provided. In some embodiments, a dosage pack comprises instructions for administering FGFR1 ECD and / or FGFR1 ECD fusion molecule with at least one additional therapeutic agent.
Owner:FIVE PRIME THERAPEUTICS
Preparation method of long-circulating nanoparticle
InactiveCN102451160AEasy to operateImprove targetingPowder deliveryOrganic active ingredientsFreeze-dryingPolyethylene glycol
The invention which belongs to the medicine processing field concretely relates to a preparation method of a long-circulating nanoparticle. The preparation method of the long-circulating nanoparticle provided in the invention has the advantages of simple operation, and good targeting and good in vitro release of products. The method comprises the following steps: 1, dissolving 5-Fu (5-fluorouracil), PEG-PHDCA (polyethylene glycol-poly(hexadecyl cyanoacrylate)) and a phosphatide in a mixed organic solvent of tetrahydrofuran and ethanol to form an organic phase; 2, slowly adding the organic phase to a water phase of a surfactant in a dropwise manner under magnetic stirring, and fully diffusing the organic phase by continuously stirring for 1h after finishing the dropwise addition; 3, carrying out reduced pressure evaporation to remove the organic solvent to obtain a nanoparticle colloidal suspension with a blue opalescence; 4, carrying out ultracentrifugation separation deposition on the nanoparticle, and washing; and 5, carrying out ultrasonic dispersion with a 4% mannitol solution, and freeze-drying.
Owner:夏落
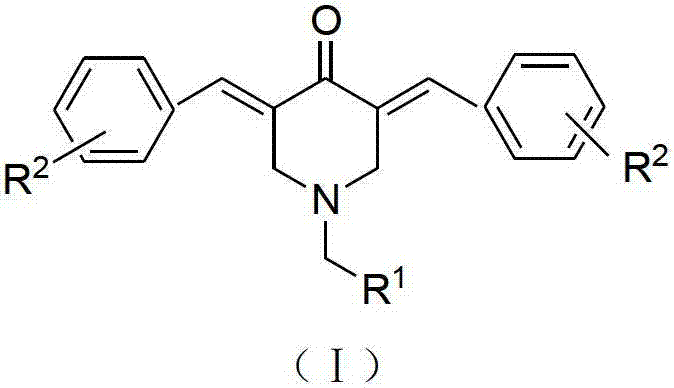
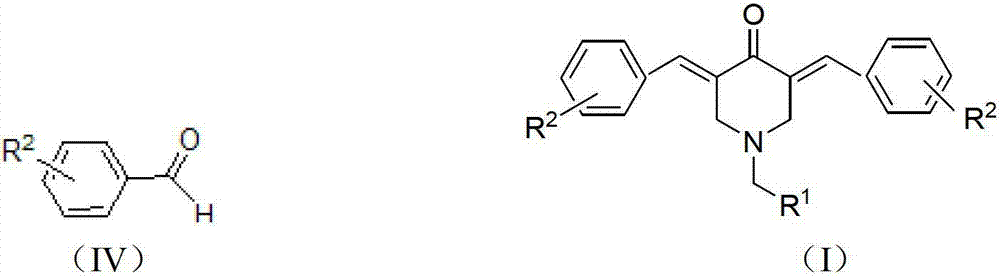
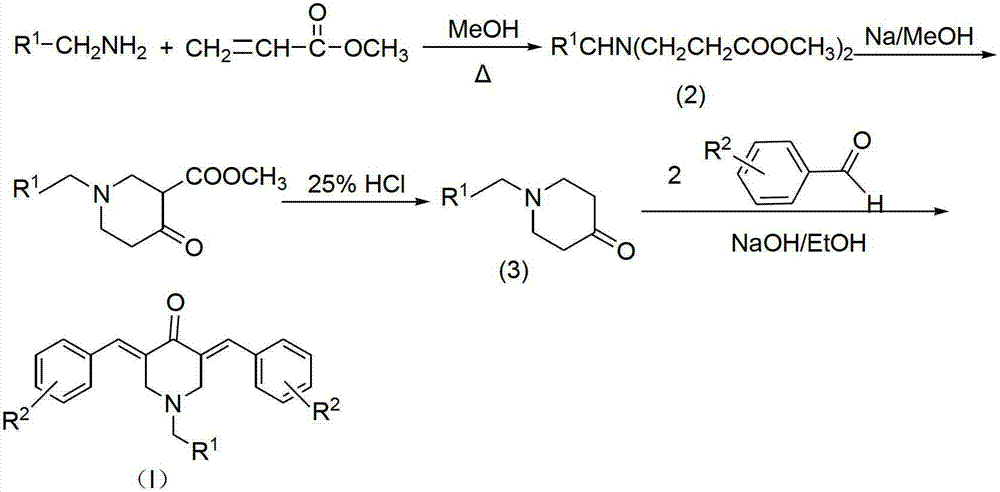
N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and preparation method and application thereof
InactiveCN102863376AInhibit biological activitySmall side effectsOrganic chemistryAntineoplastic agentsCarcinoma cell lineCancer cell
The invention relates to the field of organic synthesis and medicine, and discloses a preparation method for N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and biological activity for efficiently inhibiting cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer. The method includes: starting from various substituted methylamine and methyl acrylate, sequentially going through Michael addition, Dieckmann condensation, acidolysis and decarboxylation to obtain N-substituted methyl-4-piperidone, and subjecting the N-substituted methyl-4-piperidone to aldol reaction with substituted benzaldehyde to obtain a target compound N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone. The target compound can selectively and efficiently inhibit cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer, and activity of inhibiting carcinoma cell line proliferation is obviously higher than conventional chemotherapeutic 5-fluorouracil.
Owner:SHANGHAI NORMAL UNIVERSITY
Methods for facilitating recovery of functions of endogenous or implanted or transplanted stem cells using hyaluronic acid
InactiveUS20060069064A1Lower Level RequirementsImproving overall in vivo microenvironmental nicheOrganic active ingredientsBiocideAdjuvantCell-Extracellular Matrix
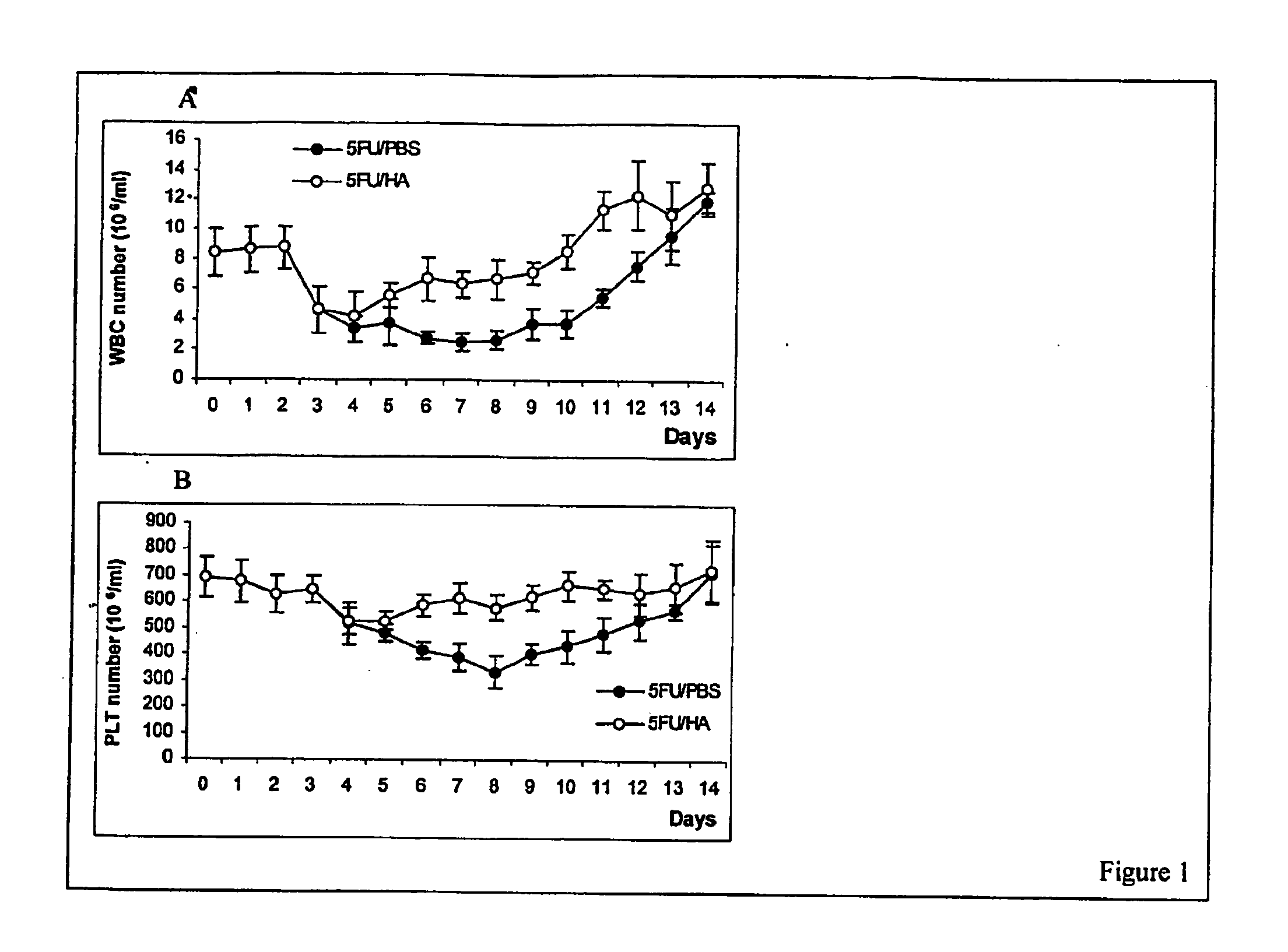
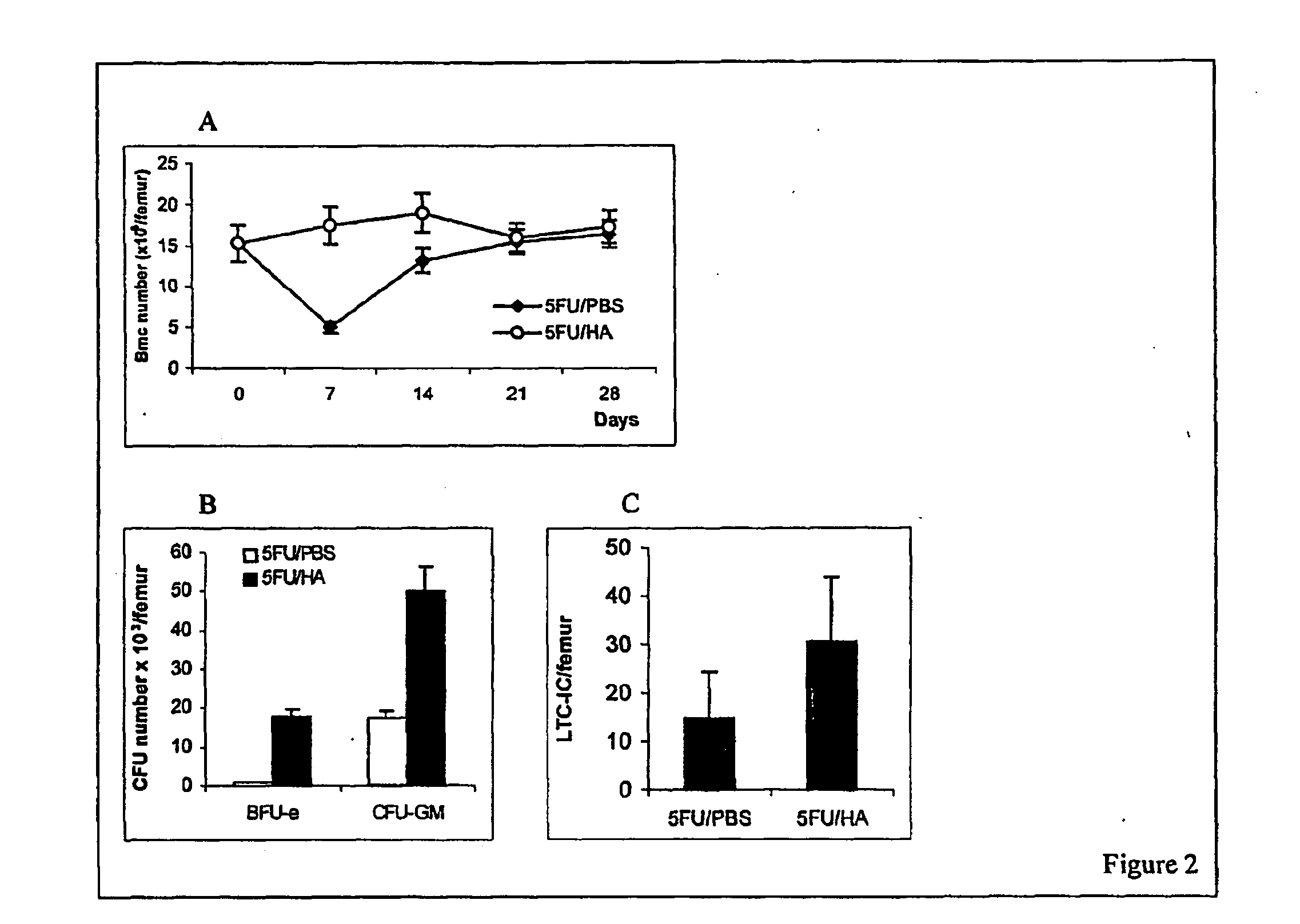
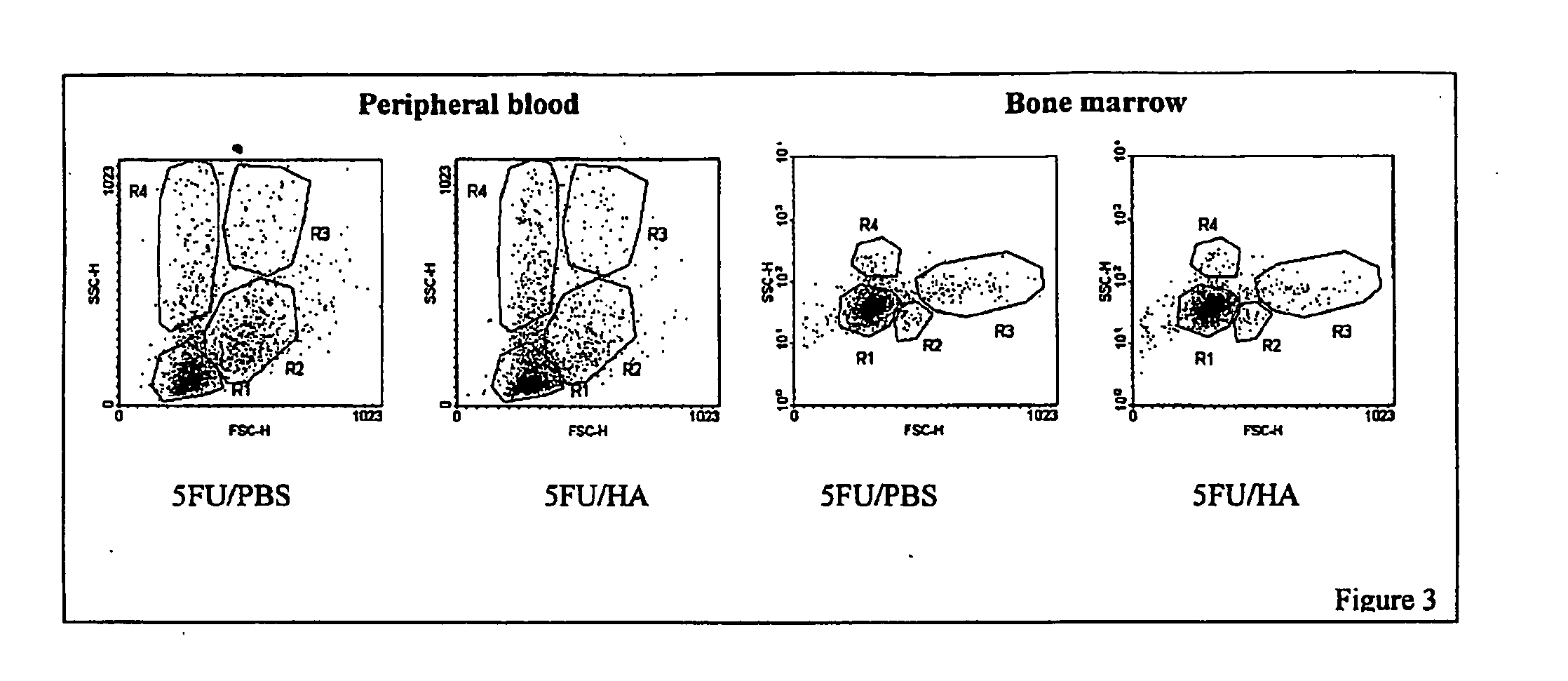
Hyaluronic Acid (HA) is an essential component of tissue extracellular matrices that contributes to the architecture of stem cell niches, which determine the fate of stem cells. Decreased levels of HA are found in subjects experiencing a variety of pathological conditions, as well as in subjects receiving a variety of therapeutic interventions, for example, chemotherapy or radiotherapy, to treat pathological conditions. The use of HA to reconstitute a tissue extracellular matrix partially or completely depleted of HA is described. More particularly, described herein is the use of exogenous forms of HA as an adjuvant in the restoration of the local tissue specific stem cell microenvironment to enhance stem cell recovery or engraftment and thus tissue recovery and remodeling following stem cell transplantation or other therapies. The effect of HA on hematopoietic stem cells is illustrative of the invention. Mice having severe bone marrow hypoplasia, and pancytopenia resulting from treatment with 5-fluorouracil recovered more rapidly if treated with HA. Similarly, mice transplanted with hematopoietic stem cells following lethal irradiation exhibited enhanced recovery of peripheral blood cell counts when treated with HA as an adjuvant therapy compared to control mice transplanted with hematopoietic stem cells without adjuvant therapy.
Owner:LA JOLLA INST FOR MOLECULAR MEDICINE
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
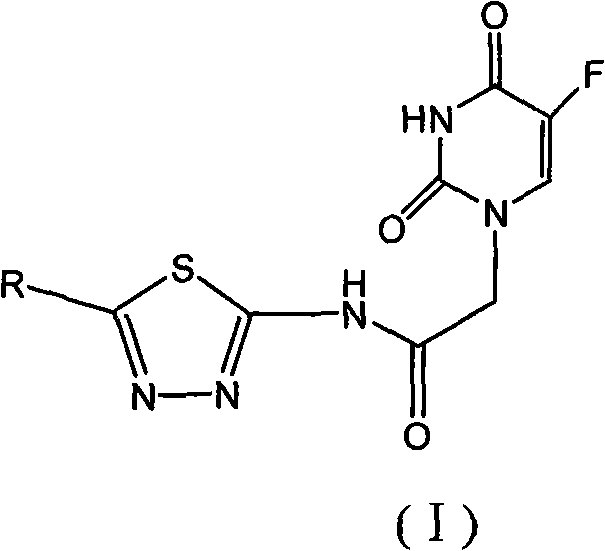
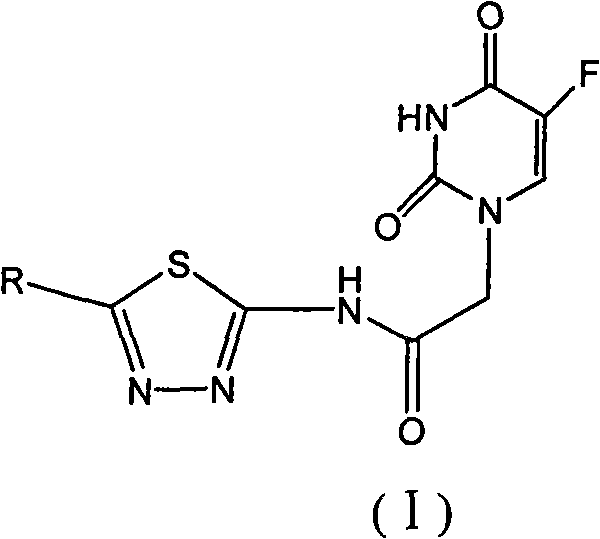
1,3,4-thiadiazole fluorouracil compound as well as preparation method and application thereof
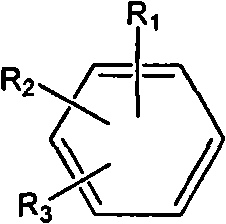
The invention discloses a 1,3,4-thiadiazole fluorouracil compound as well as a preparation method and application thereof. The compound has a general formula I, wherein R is substituted phenyl, aromatic heterocycle or aryloxy alkyl; the substituent group R1 in tri-substituted phenyl is hydrogen, nitryl, alkoxyl, halogen atom, alkyl, substituted alkyl or phenoxyl, R2 is hydrogen, nitryl, alkoxyl, halogen atom, alkyl, substituted alkyl or phenoxyl, and R3 is hydrogen, nitryl, alkoxyl, halogen atom, alkyl, substituted alkyl or phenoxyl; and the aromatic heterocycle is pyridine, thiofuran, furan, indole or isoindole. The invention has less compound consumption, good insecticidal effect, simple process method, low cost and wide market prospect.
Owner:NANJING UNIV OF TECH
Method of orally treating inflammatory skin conditions with prodrugs of 5-fluorouracil
Owner:NEW YORK UNIV
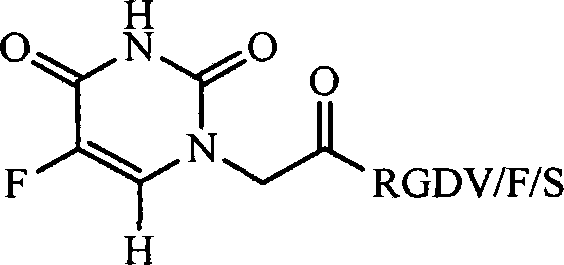
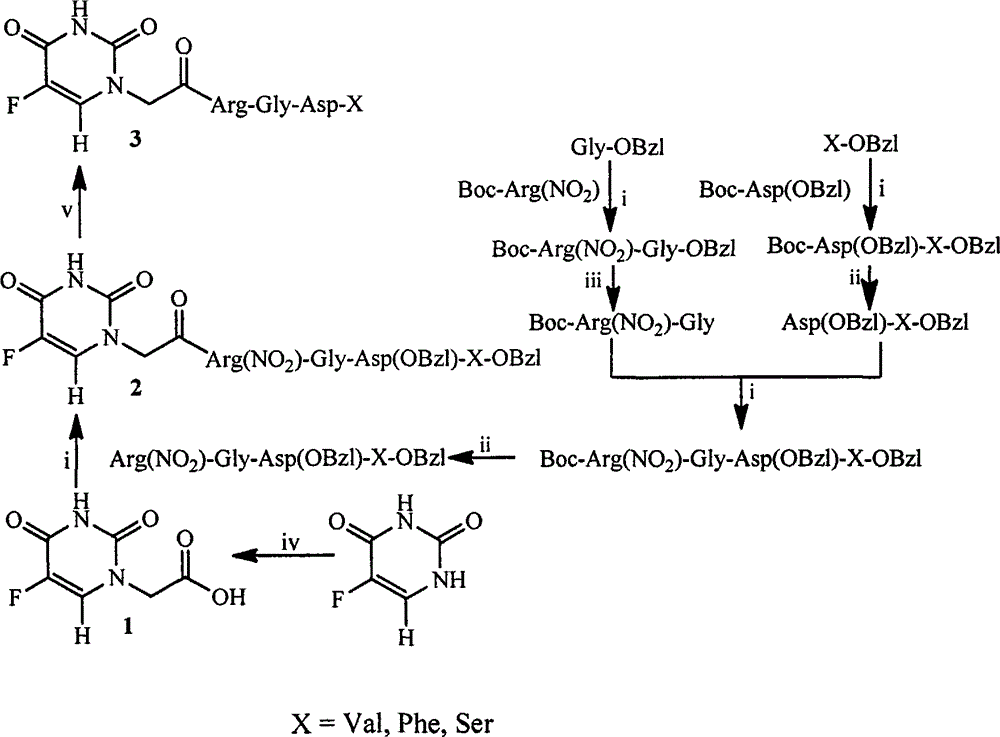
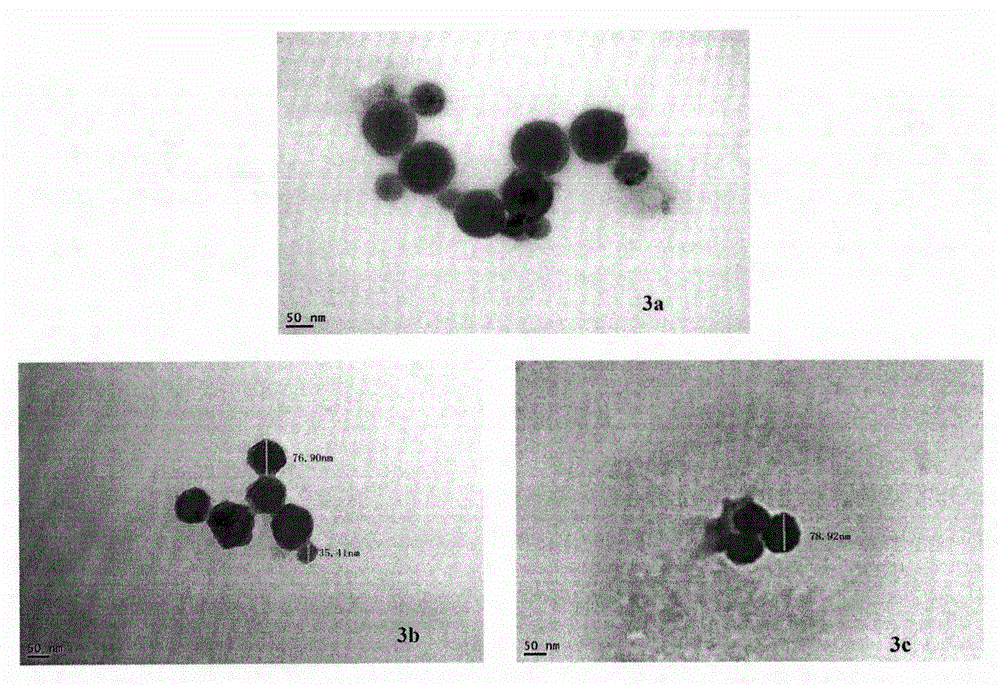
RGD modified 5-fluorouracil and preparation method, nanostructure, activity and application thereof
The invention discloses a compound being 5-fluorouracil-1-acetyl-Arg-Gly-Asp tetrapeptide, a preparation method, nanostructure, anti-tumor effect and tumor cell adhesion, invasion and migration resisting effect thereof, and application thereof in medical science.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Combination of a chemotherapeutic agent and an inhibitor of the TGF-beta system
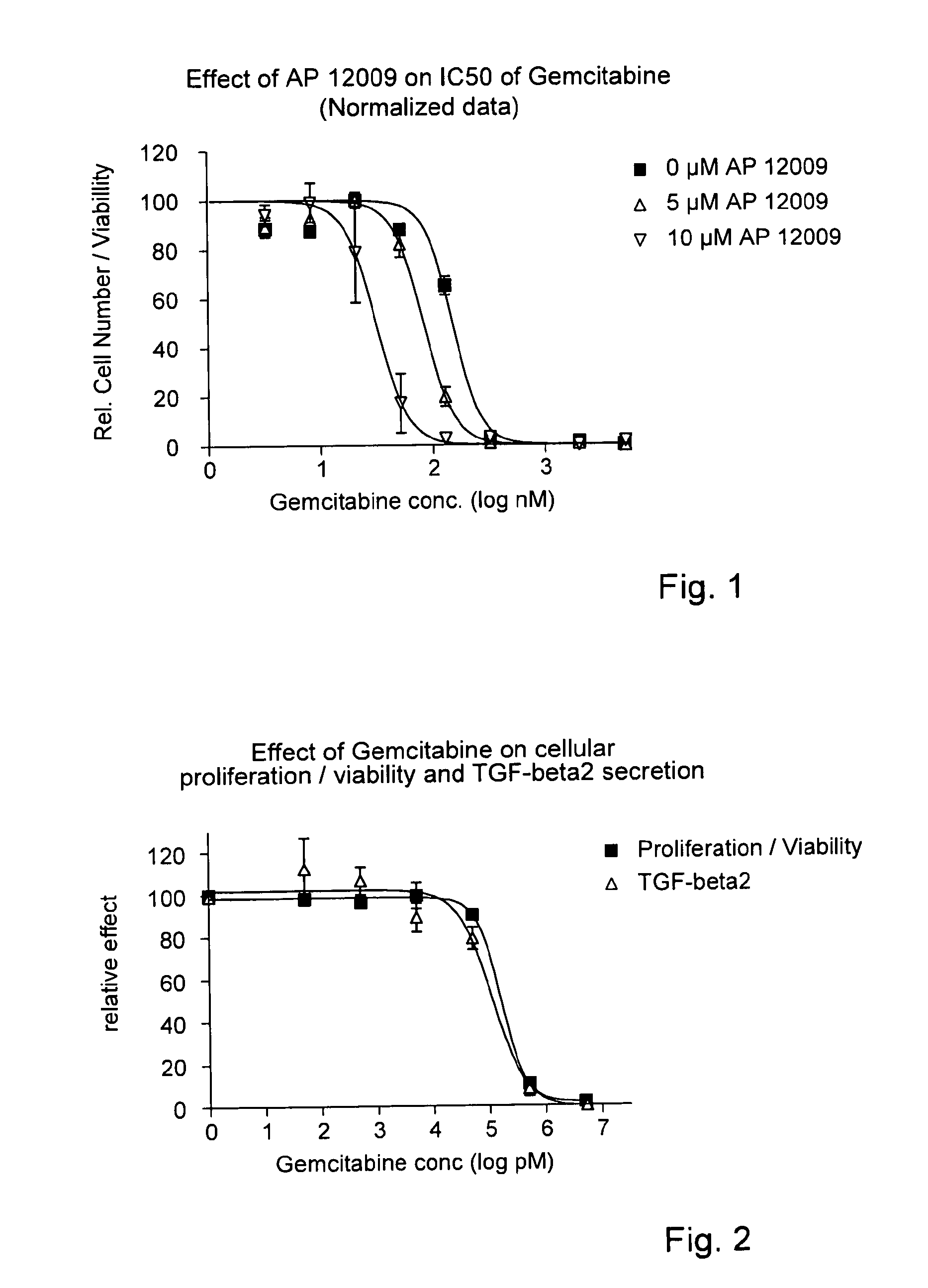
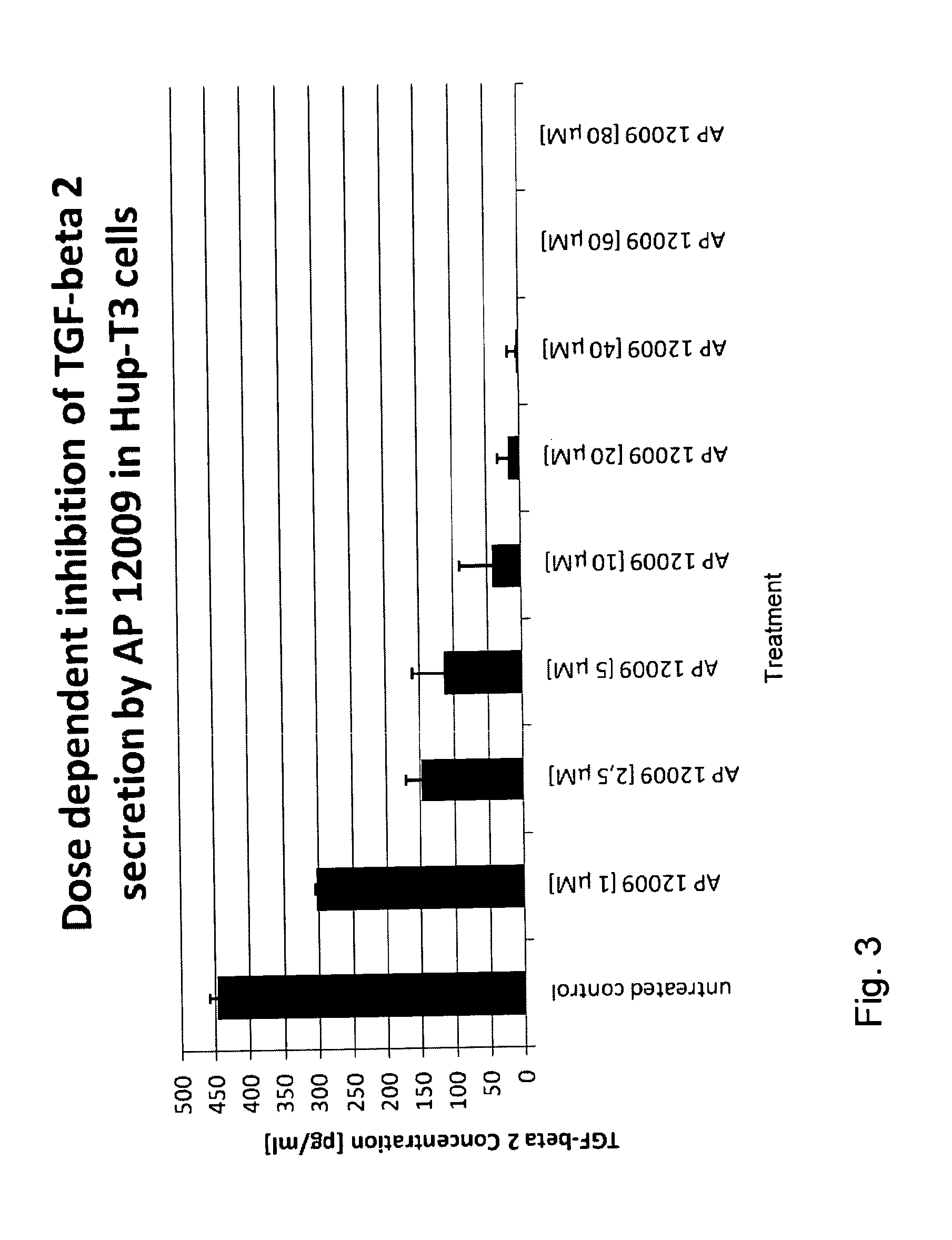
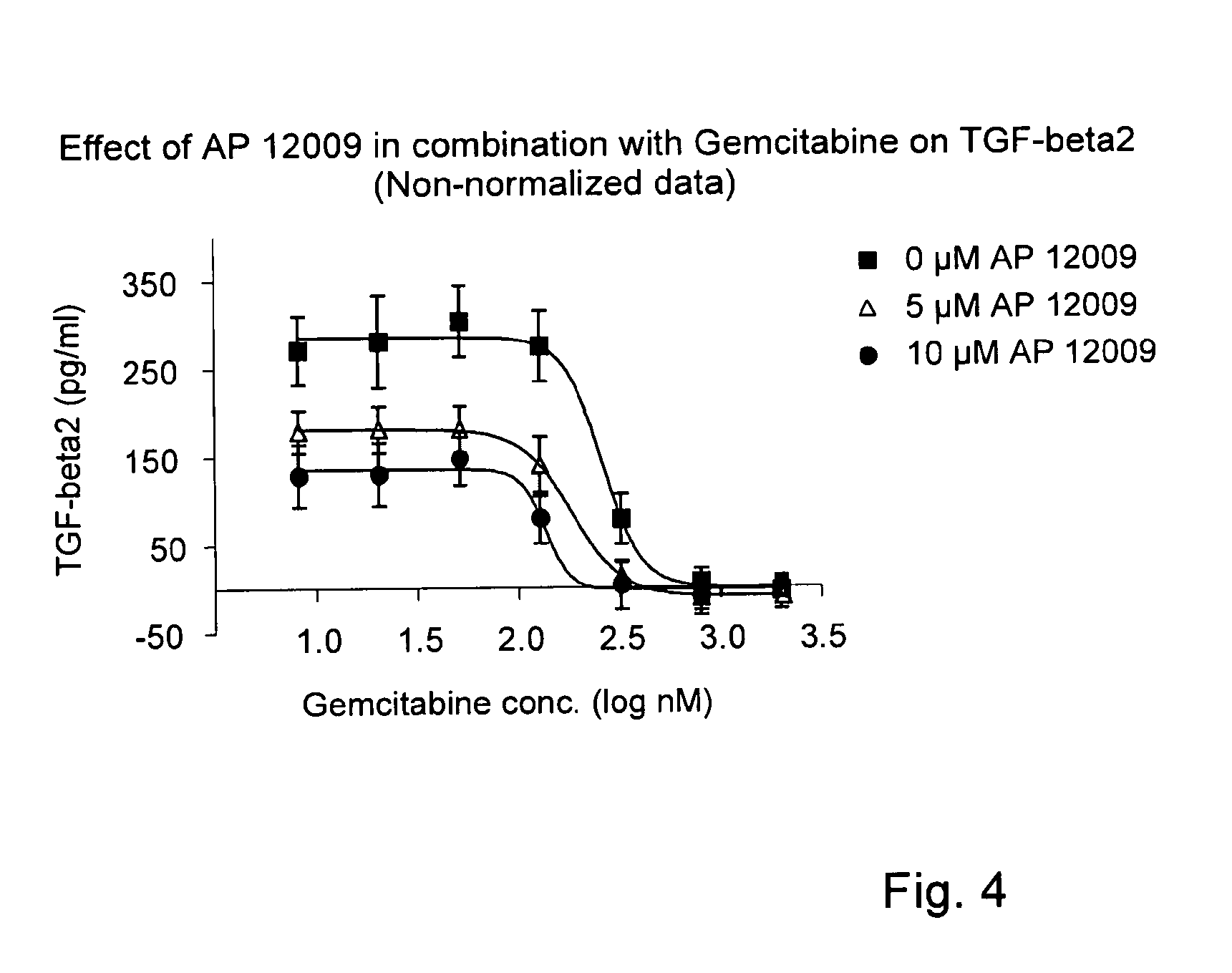
ActiveUS8476246B2Reduce IC50Improve efficiencyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
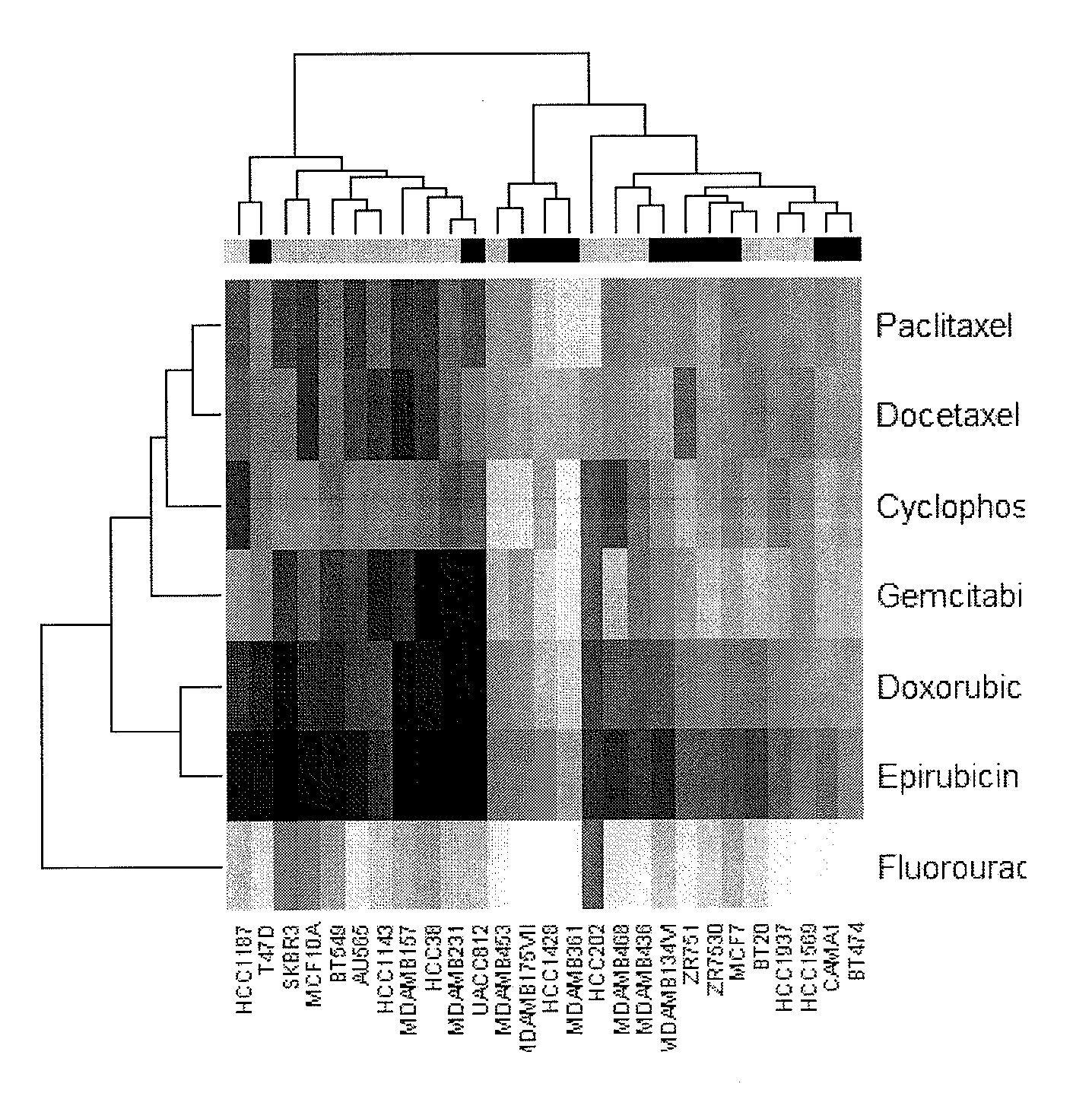
Multi drug response markers for breast cancer cells
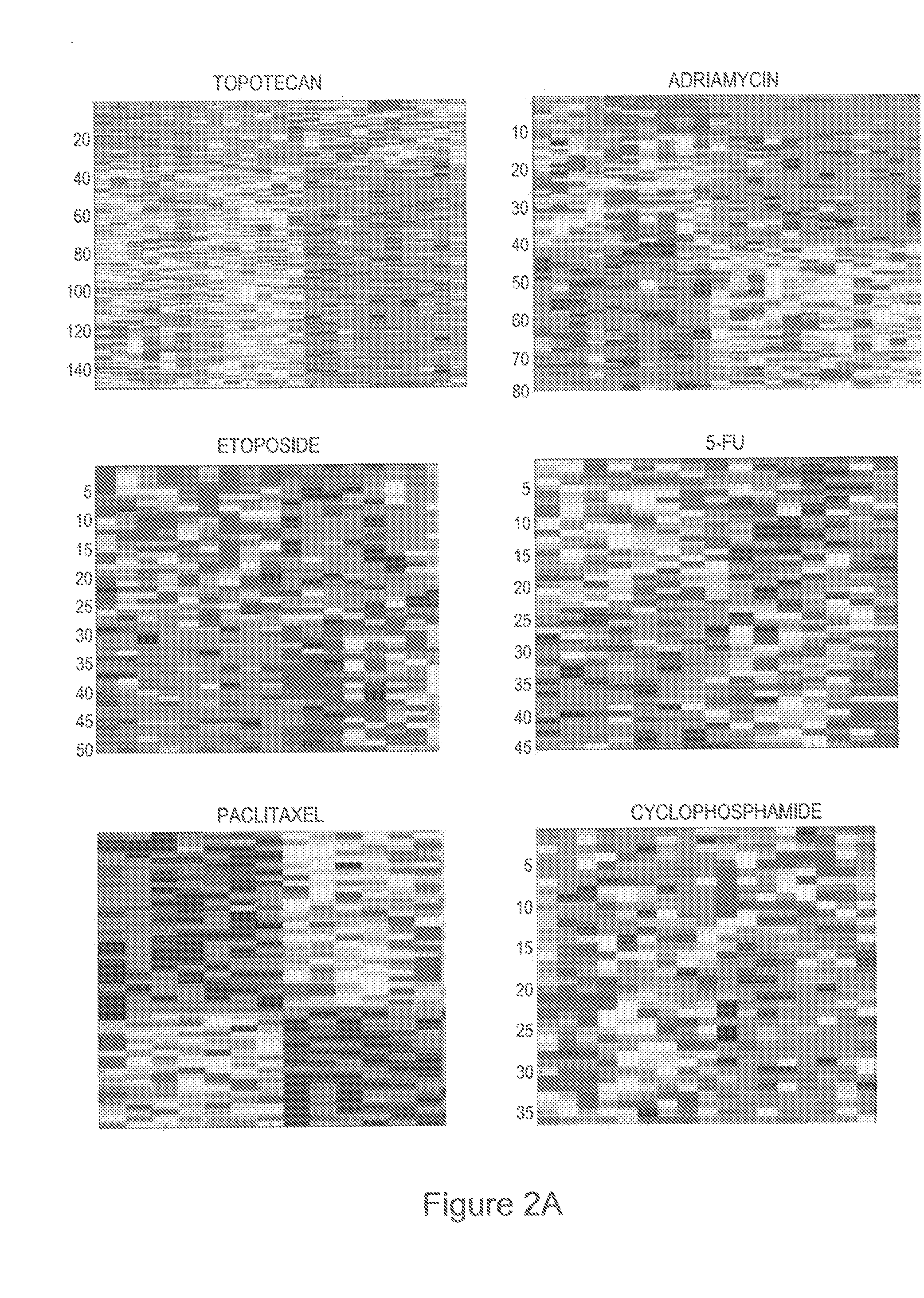
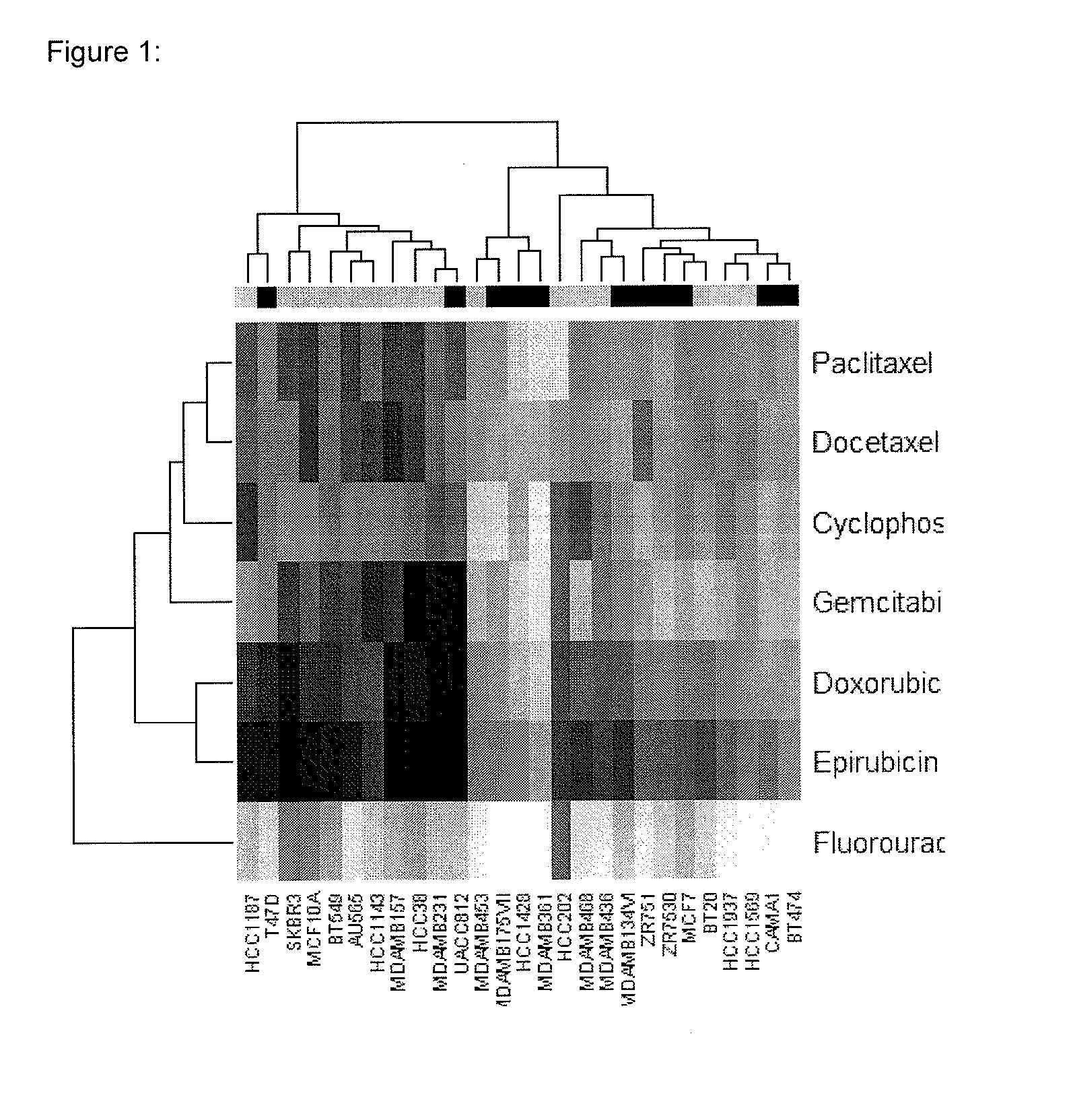
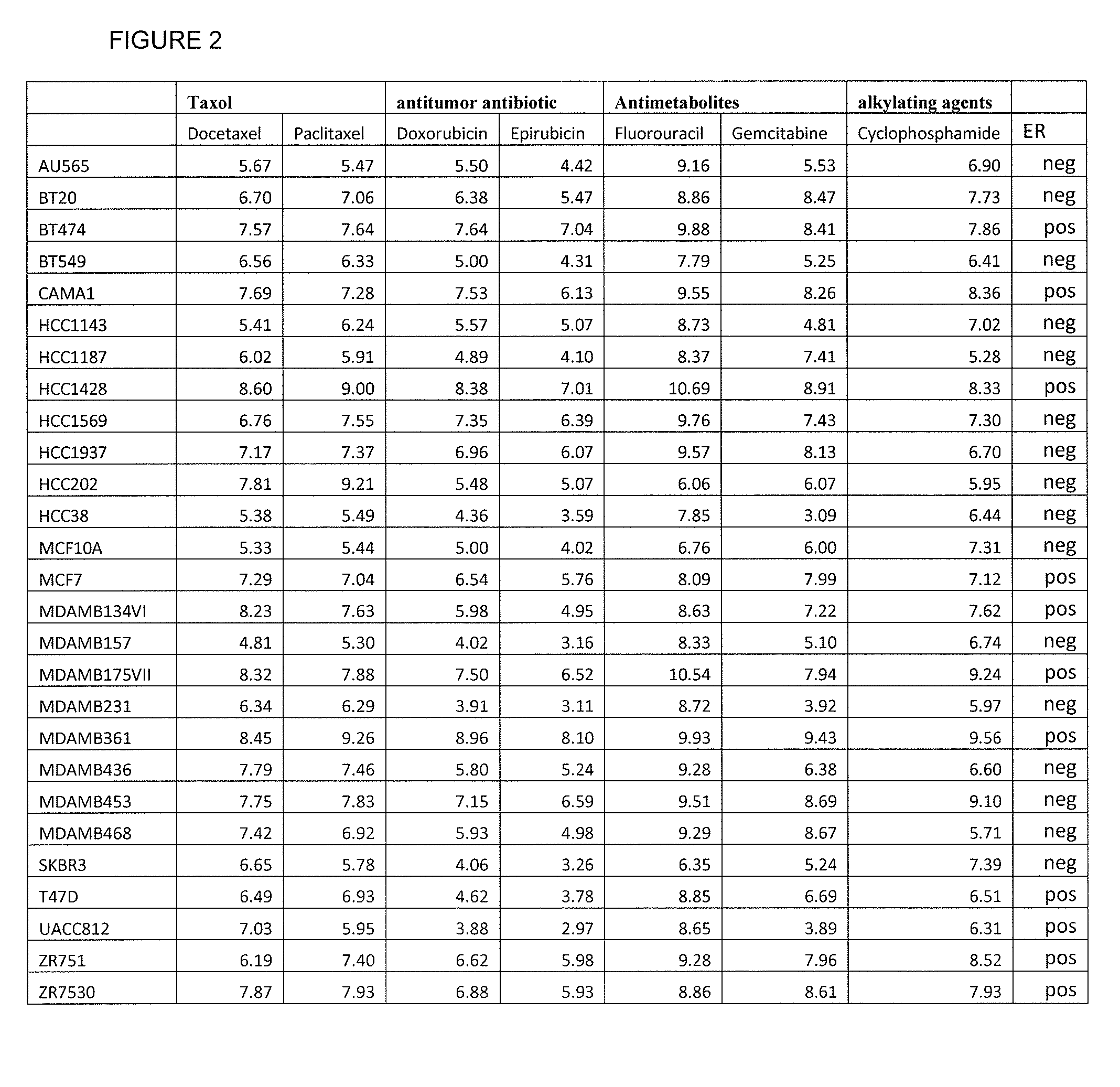
The present invention provides methods for preparing a gene expression profile of a breast cancer cell, tumor, or cell line, where the gene expression profile may be evaluated for one or more gene expression signatures indicative of multidrug resistance. The signature may be indicative of resistance to one or more chemotherapeutic agents selected from a Taxol (e.g., Docetaxel or Paclitaxel), an antibiotic (e.g., Doxorubicin or Epirubicin), an antimetabolite (e.g., Fluorouracil and / or Gemcitabine), and an alkylating agent (e.g., Cyclophosphamide). Generally, the gene expression profile contains the level of expression for a plurality of genes listed in FIGS. 3, 4, and / or 5. Gene expression profiles for evaluating multidrug resistance for ER positive and ER negative breast cancers are also provided.
Owner:PRECISION THERAPEUTICS
Kit for detecting 5 fluorouracil medicine insensitive gene chip
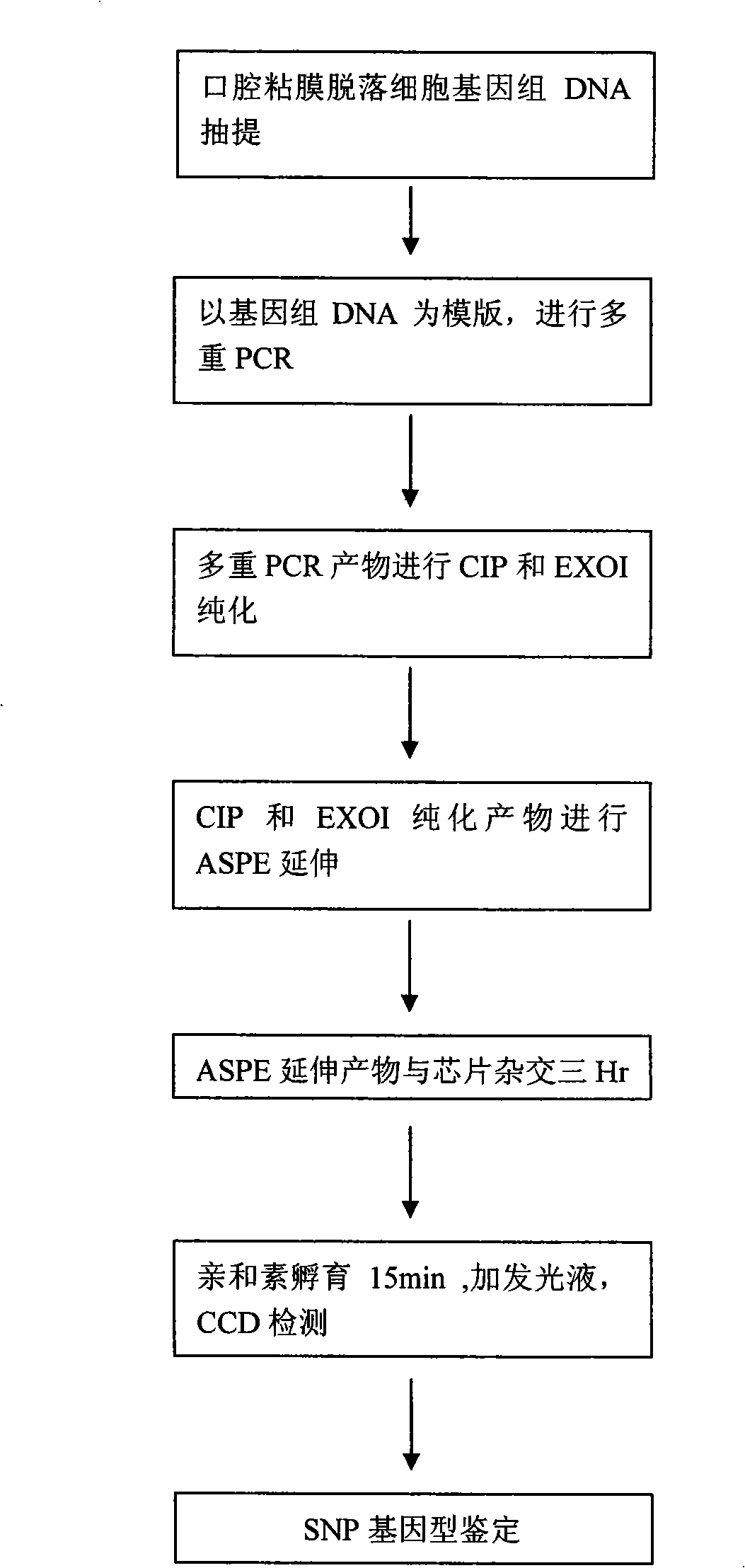
InactiveCN101333558ATo achieve the purpose of parallel detectionThe result is accurateMicrobiological testing/measurementXRCC1 GeneWilms' tumor
The invention provides a 5-fluorouracil drug sensitivity gene chip detection kit, which can be used to parallelly and economically detect genes related to 5-fluorouracil drug sensitivity. The kit comprises an extraction solution, a hybridization buffer solution, a washing liquor, an amplifying solution, Taq enzyme and a gene chip, the amplifying solution contains a primer for amplifying four genes containing mutant sites, and the four genes include a DPD gene containing IVS14 A / G mutant sites, a GSTPi gene containing Ile105Val mutant sites, an MTHFR gene containing C677T mutant sites, and an XRCC1 gene containing Arg399Glu mutant sites. The 5 fluorouracil is a first-line anti-tumor chemotherapeutic drug, and the detection kit can be used for detecting the four gene mutation conditions sensitive to the 5 fluorouracil drug.
Owner:上海裕隆生物科技有限公司
Drug eluting stent coating with extended duration of drug release
A stent having a drug eluting formulation has three components: 1) Anti-neointimal hyperplasia or anti-restenosis agent 2) Main polymer 3) Additive polymer The anti-neointimal hyperplasia or anti-restenosis agent includes, but not limited to, Paclitaxel, Taxol, Rapamycin, Tacrolimus, Actinomycin D, Methotrexate, Doxorubicin, cyclophosphamide, and 5-fluorouracil, 6-mercapatopurine, 6-thioguanine, cytoxan, cyclosporine, cytarabinoside, cis-platin, chlorambucil, busulfan, and any other drug that can inhibit cell proliferation, and combinations thereof. The main polymer includes, but not limited to, polystyrene, parylene and polyurethane. The additive polymer includes, but not limited to, polyethylene glycol capped with diisocyanate moiety (NCO-PEG). TABLERatio between three components without solvent%ComponentformulationAgent1-10%Main polymer80-98% Additive1-19%polymer9.0 g of parylene, 0.6 g of tacrolimus, 0.4 g of NCO-PEG and 0.01 g of triethylene amine were dissolved in 90 g of tetrahydrofuran. The resulting mixture was heated at 40° C. for 30 minutes and cooled to room temperature. To the solution was added 0.1 g of pH 8.0 aqueous solution and mixed thoroughly. The resulting solution is applied to bare metal stents for coating.
Owner:HAHN SOONKAP
Amphiphile prodrugs
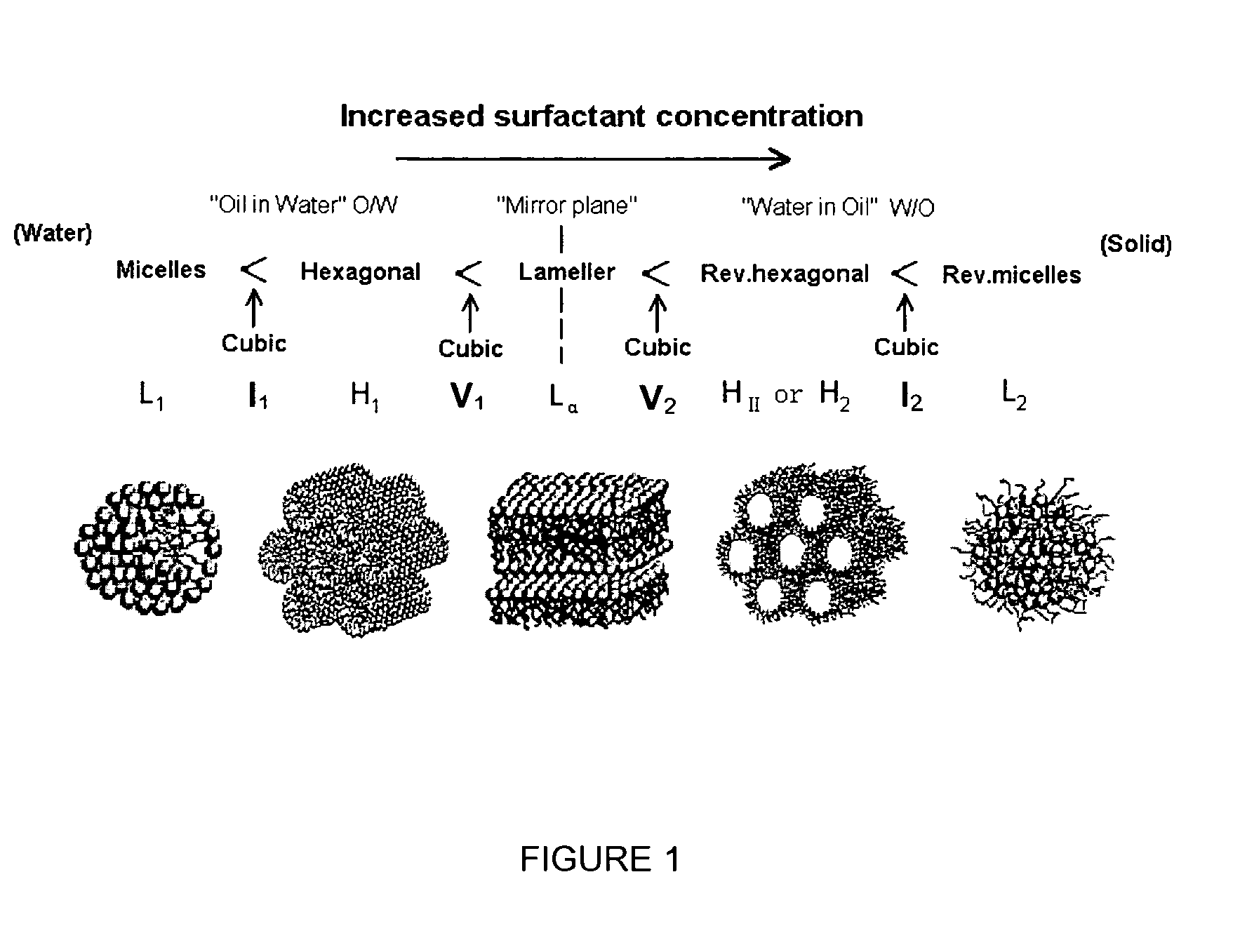
Amphiphilic prodrugs of general formula A-X are disclosed, wherein A is a biologically active agent or may be metabolised to a biologically active agent; and X is selected from the group consisting of R, or up to three R moieties attached to a linker, Y1, Y2 or Y3, wherein R is selected from a group consisting of alkyl, alkenyl, alkynyl, branched alkyl, branched alkenyl, branched alkynyl, substituted alkyl, substituted alkenyl and substituted alkynyl groups and their analogues; Y1 is a linker group which covalently attached to an R group at one site and is attached to A at a further independent site; Y2 is a linker group which is covalently attached to two R groups at two independent sites and is attached to A at a further independent site; and Y3 is a linker group which is covalently attached to three R groups at three independent sites and is attached to A at a further independent site. Self-assembly of the amphiphilic prodrugs into reverse lyotropic phases, particularly hexagonal, cubic and sponge, is disclosed. In preferred embodiments A is dopamine or a 5-fluorouracil prodrug.
Owner:NANOMED HLDG PTY LTD
Compound with antitumor activity and preparation method and application of compound
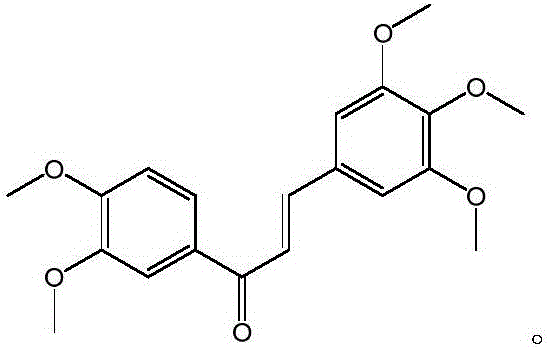
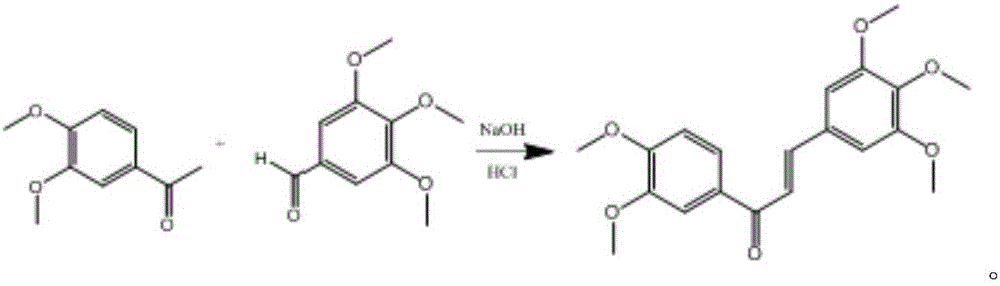
ActiveCN105967991AReduce dosageAvoid destructionOrganic compound preparationCarbonyl compound preparationChalconeTumor cells
The invention discloses a compound with antitumor activity and a preparation method and application of the compound and belongs to the technical fields of new compound synthesis and medicine application. According to the compound, the preparation and the application thereof disclosed by the invention, aromatic aldehyde and aromatic ketone are catalyzed by sodium hydroxide to synthesize 3,4,5-triethoxy-3',4'-dimethoxy chalcone for the first time, and the test of in vitro tumor cell inhibitory activity on the 3,4,5-triethoxy-3',4'-dimethoxy chalcone is realized; the result shows that the compound has higher inhibitory activity for human lung cancer cell A549, human colon carcinoma cell SW620 and human liver cancer cell HepG2. In addition, the antitumor activity of the 3,4,5-triethoxy-3',4'-dimethoxy chalcone for the human colon carcinoma cell SW620 and the human liver cancer cell HepG2 is superior to that of a reference drug 5-fluorouracil. The invention provides a new effective treatment means for tumor treatment and has a broad application prospect.
Owner:HARBIN MEDICAL UNIVERSITY
Fluorouracil-containing formulation
Oil-in-water emulsion formulations contain both free fluorouracil and fluorouracil impregnated in porous microparticles. The formulations are suitable for topical administration, and are useful for the treatment of solar keratoses, actinic keratoses, and superficial basal cell carcinomas.
Owner:BAUSCH HEALTH IRELAND LTD
Novel anti-cancer medicaments using NGR(NO2) as targeting carrier, preparation thereof and use thereof
ActiveCN101948507AInhibition of anti-tumor effectImprove targetingTripeptide ingredientsPeptidesTumor targetCytarabine
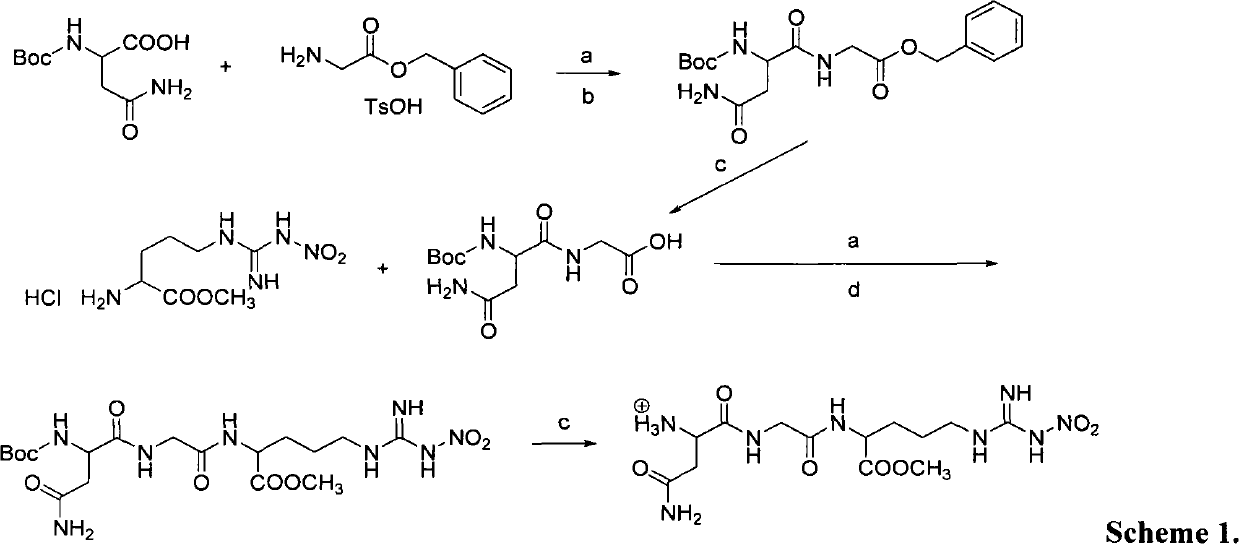
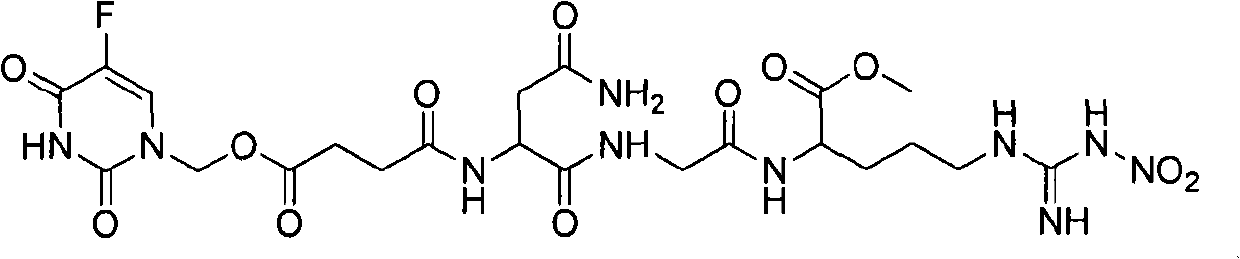
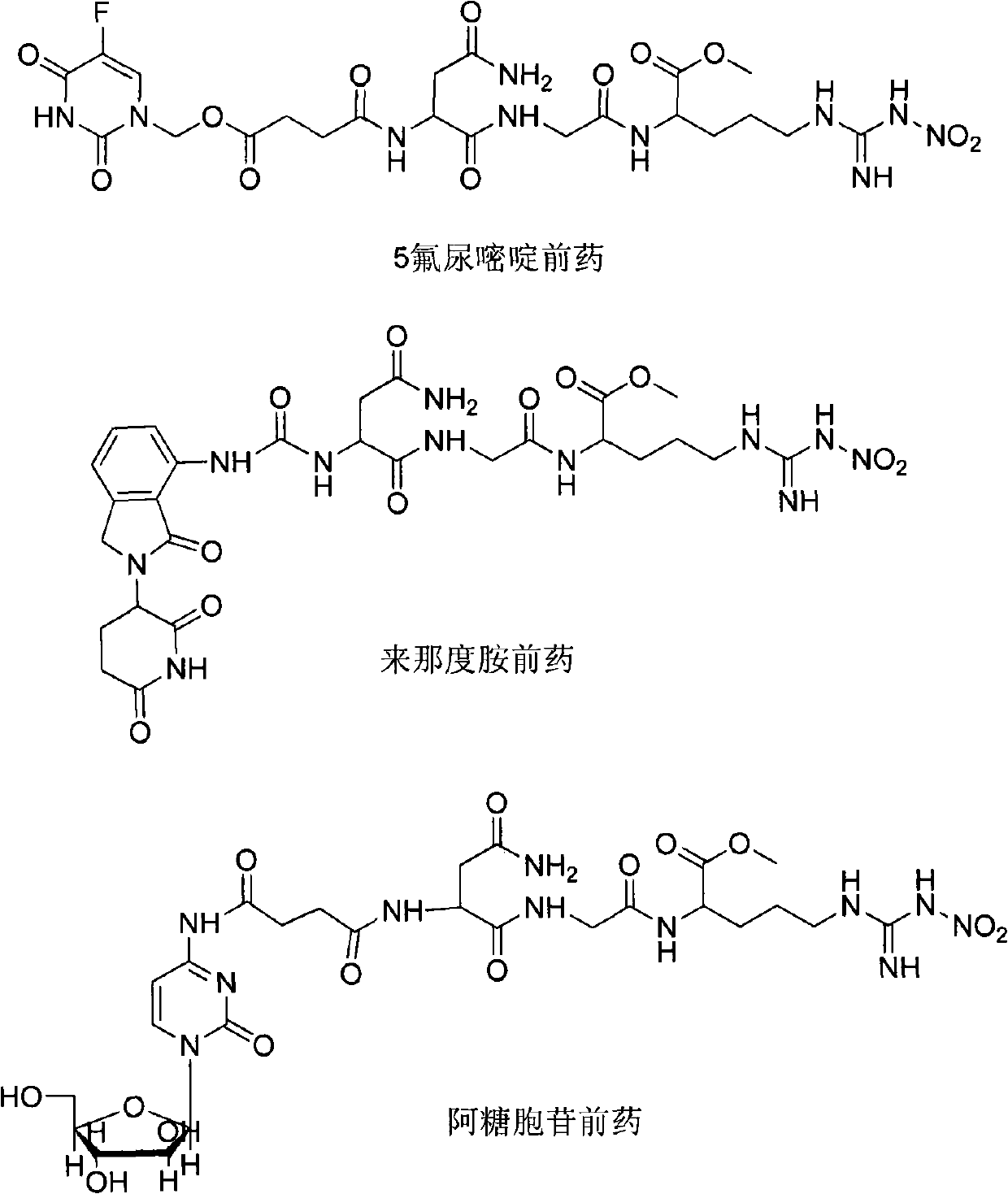
The invention provides novel anti-cancer precursor medicaments using NGR(NO2) as a targeting carrier. The novel anti-cancer precursor medicaments are prepared by designing and synthesizing a 5-fluorouracil precursor medicament, a lenalidomide precursor medicament, a cytarabine precursor medicament, an epirubicin precursor medicament and a dasatinib precursor medicament. According to the initial research on the anti-tumor activity of the 5-fluorouracil precursor medicament, the 5-fluorouracil precursor medicament can inhibit the invasion and metastasis of tumor cells and the growth of solid tumors. The 5-fluorouracil precursor medicament is modified in both effectiveness and preparation compared with the 5-fluorouracil serving as a parent medicament and is widely applicable. Concretely, the invention mainly relates to three aspects: (1) design and antiangiogenic effect of a novel tumor-targeted tripeptide NGR(NO2); (2) preparation of anti-cancer precursor medicaments by coupling the tumor-targeted tripeptide NGR(NO2) and 5 anti-cancer medicaments through covalent bonds; and (3) antitumor and antiangiogenic medical use of the novel 5-fluorouracil precursor medicament.
Owner:廖年生 +1
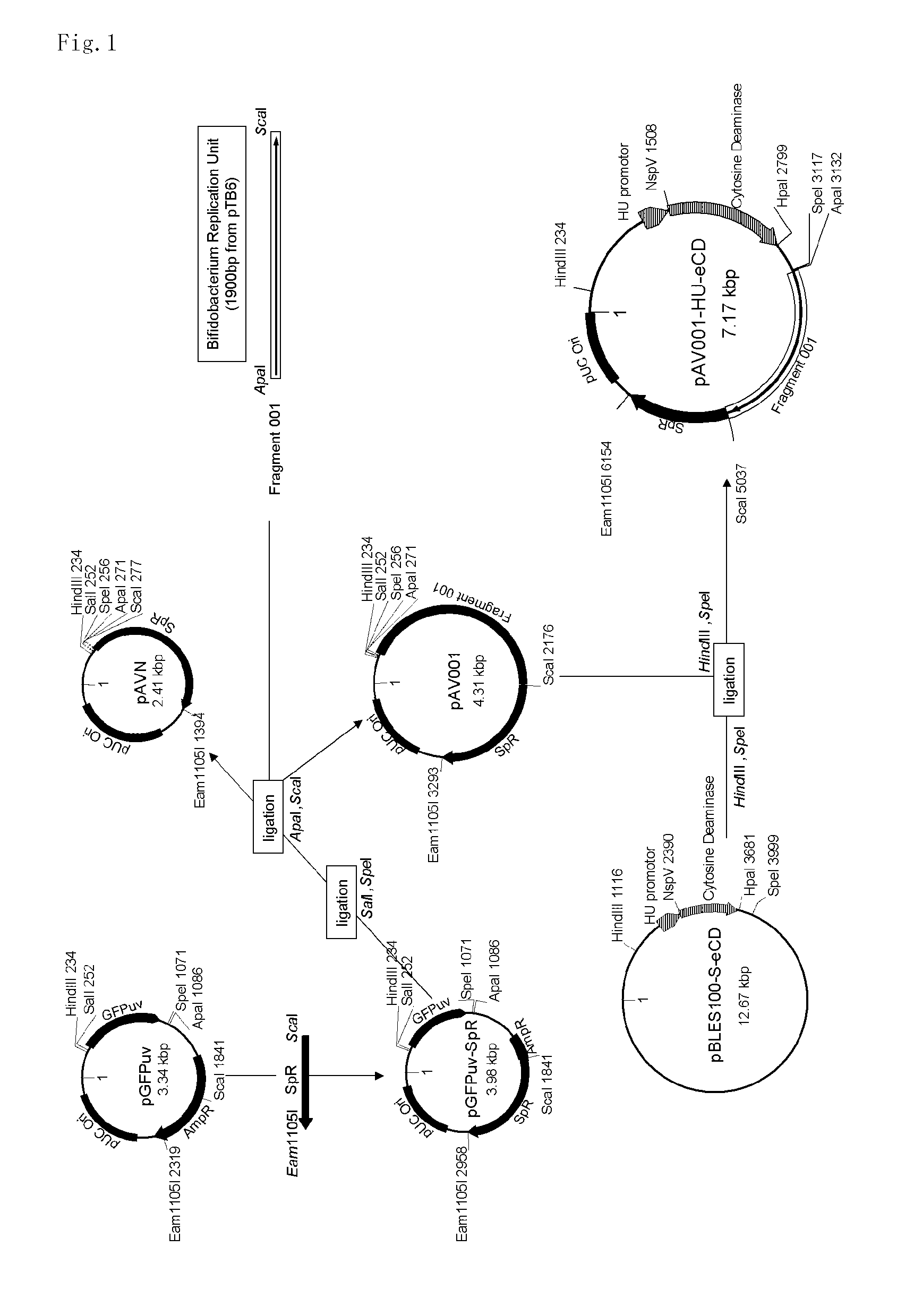
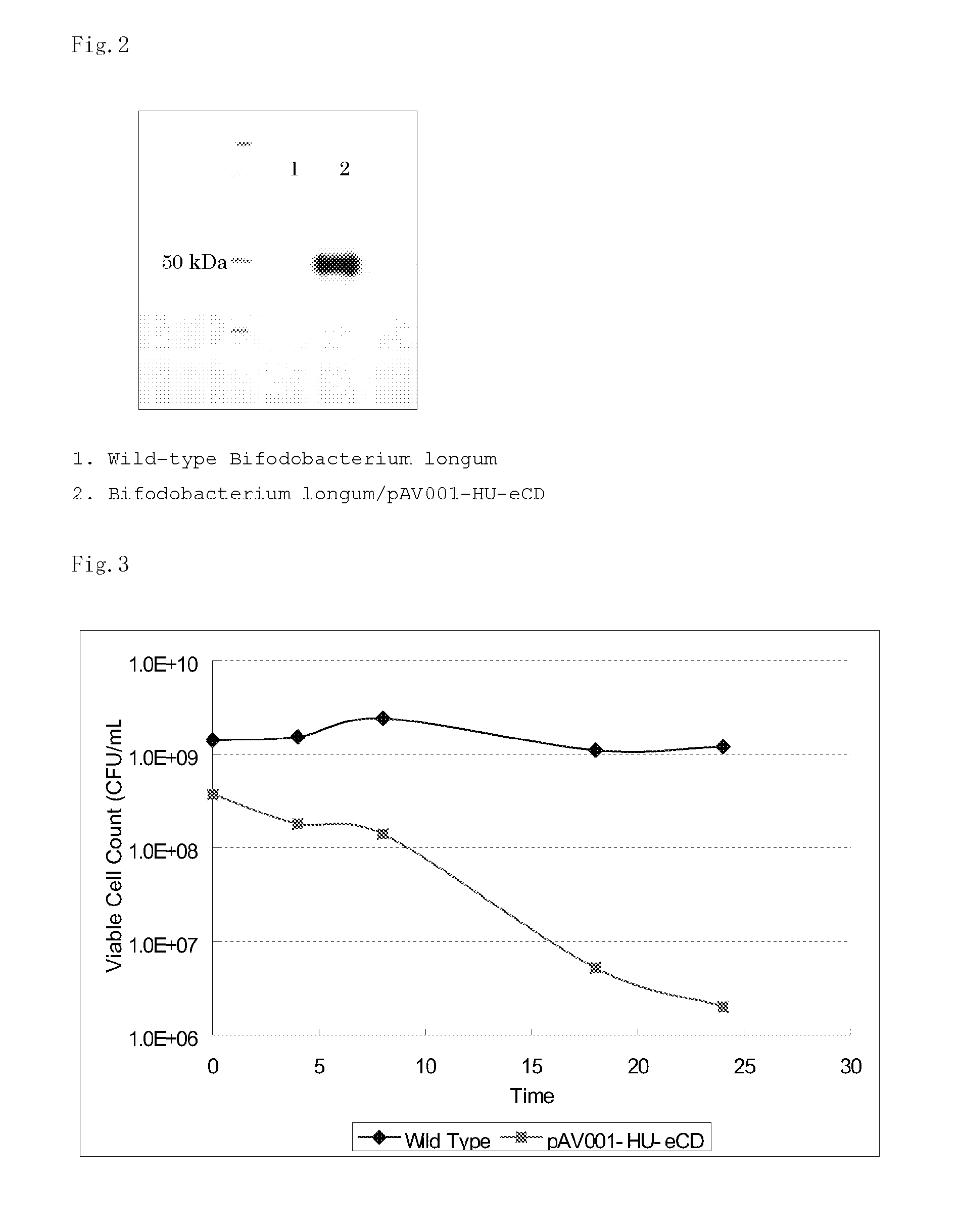
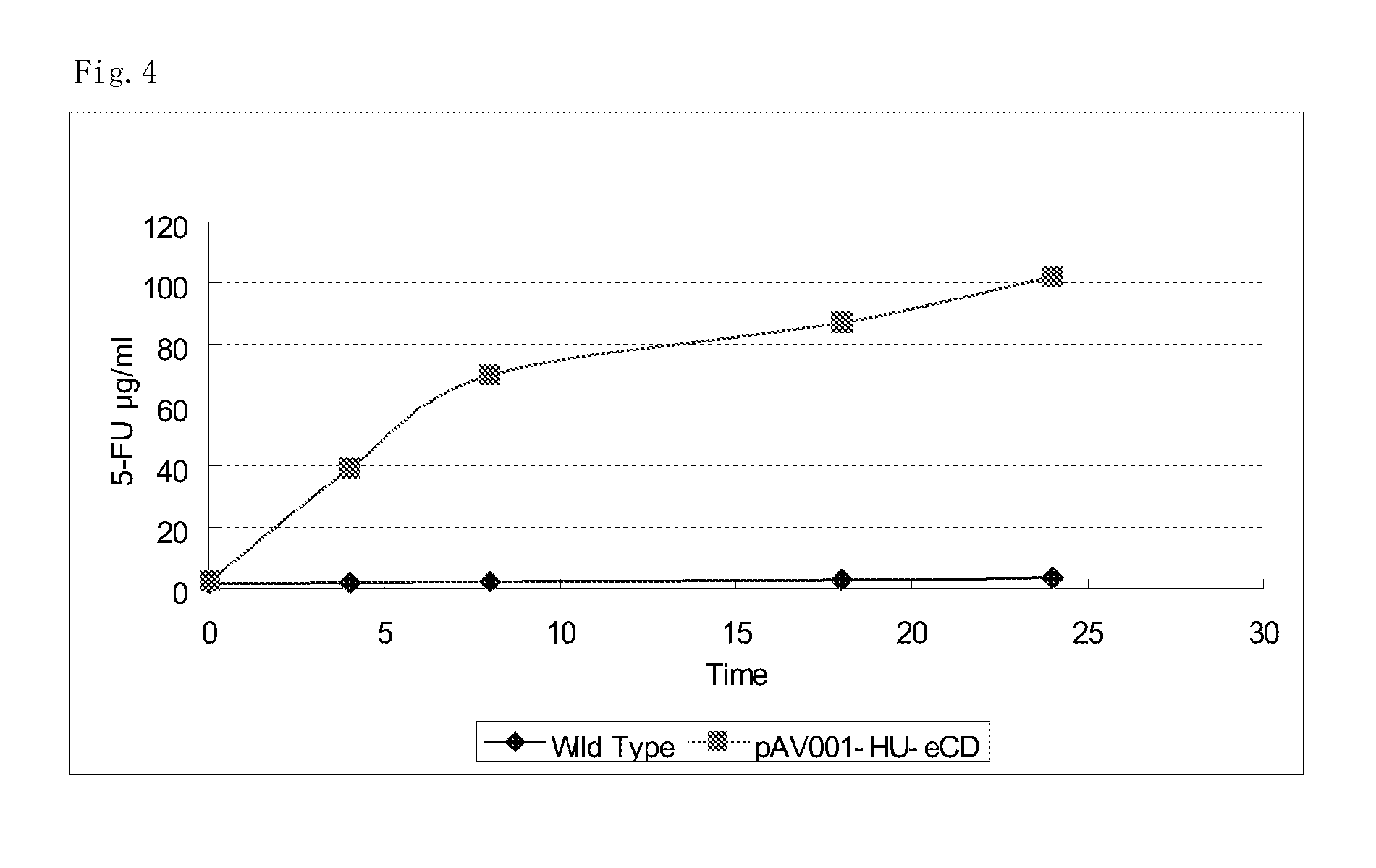
5-fluorouracil-resistant bacteria and method for production thereof
The present invention provides a method for producing a cytosine deaminase (CD)-expressing, 5-fluorouracil (5-FU)-resistant microorganism which can grow in anaerobic tumor tissues, can express CD, and has a resistance to 5-FU at a concentration that is at least effective for antitumor activity. More specifically, the method is a method (A) comprising performing subculture or acclimation culture of a CD-expressing microorganism which can grow in anaerobic tumor tissues, in the presence of 5-fluorocytosine (5-FC), or a method (B) comprising (1) performing subculture or acclimation culture of a microorganism which can grow in anaerobic tumor tissues and does not express CD, in the presence of 5-FU to produce a 5-FU-resistant microorganism and (2) transforming the 5-FU-resistant microorganism by introducing a CD gene. The present invention also provides the CD-expressing, 5-FU-resistant microorganism and a pharmaceutical composition comprising the microorganism.
Owner:ANAEROPHARMA SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com