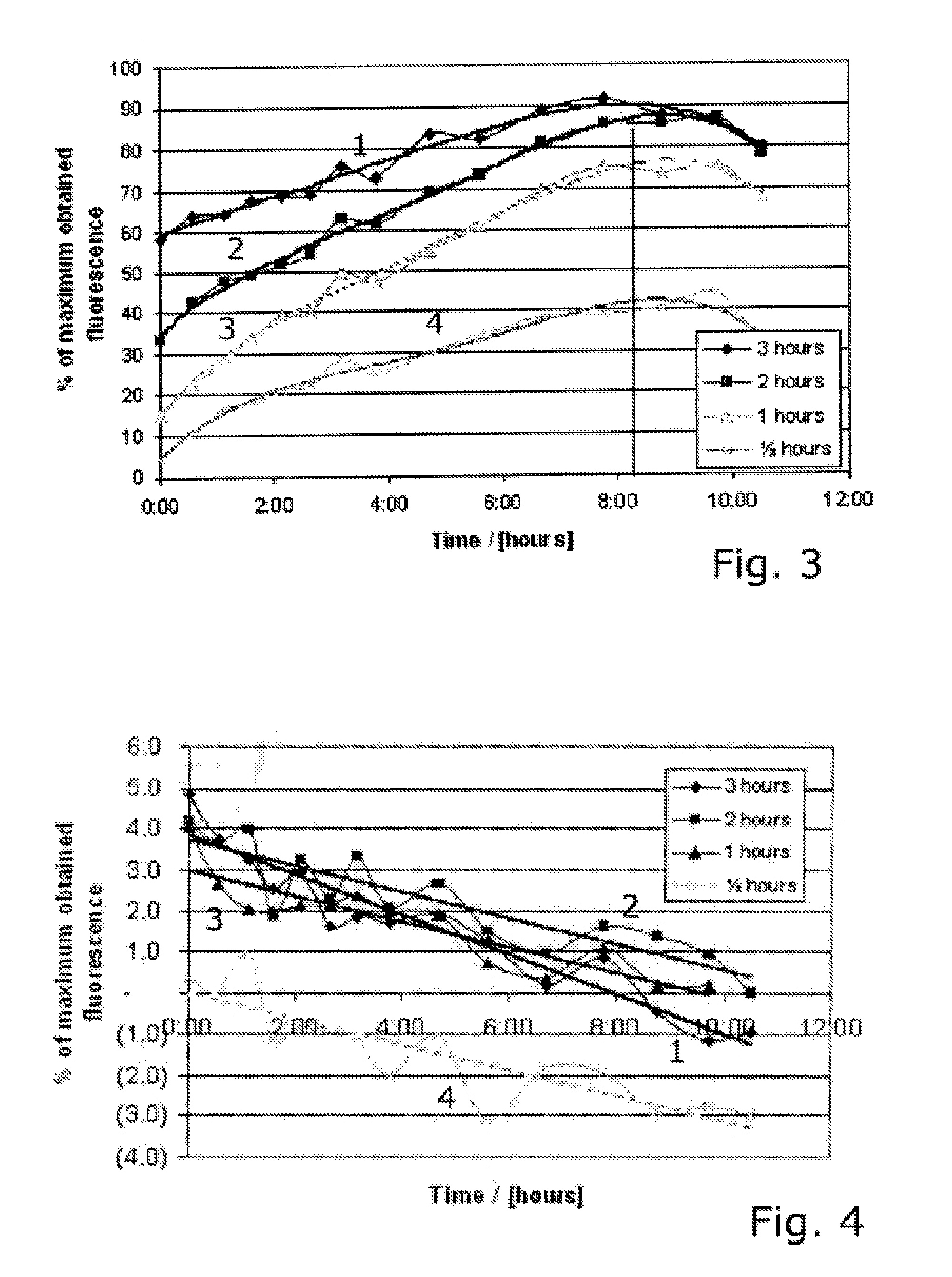
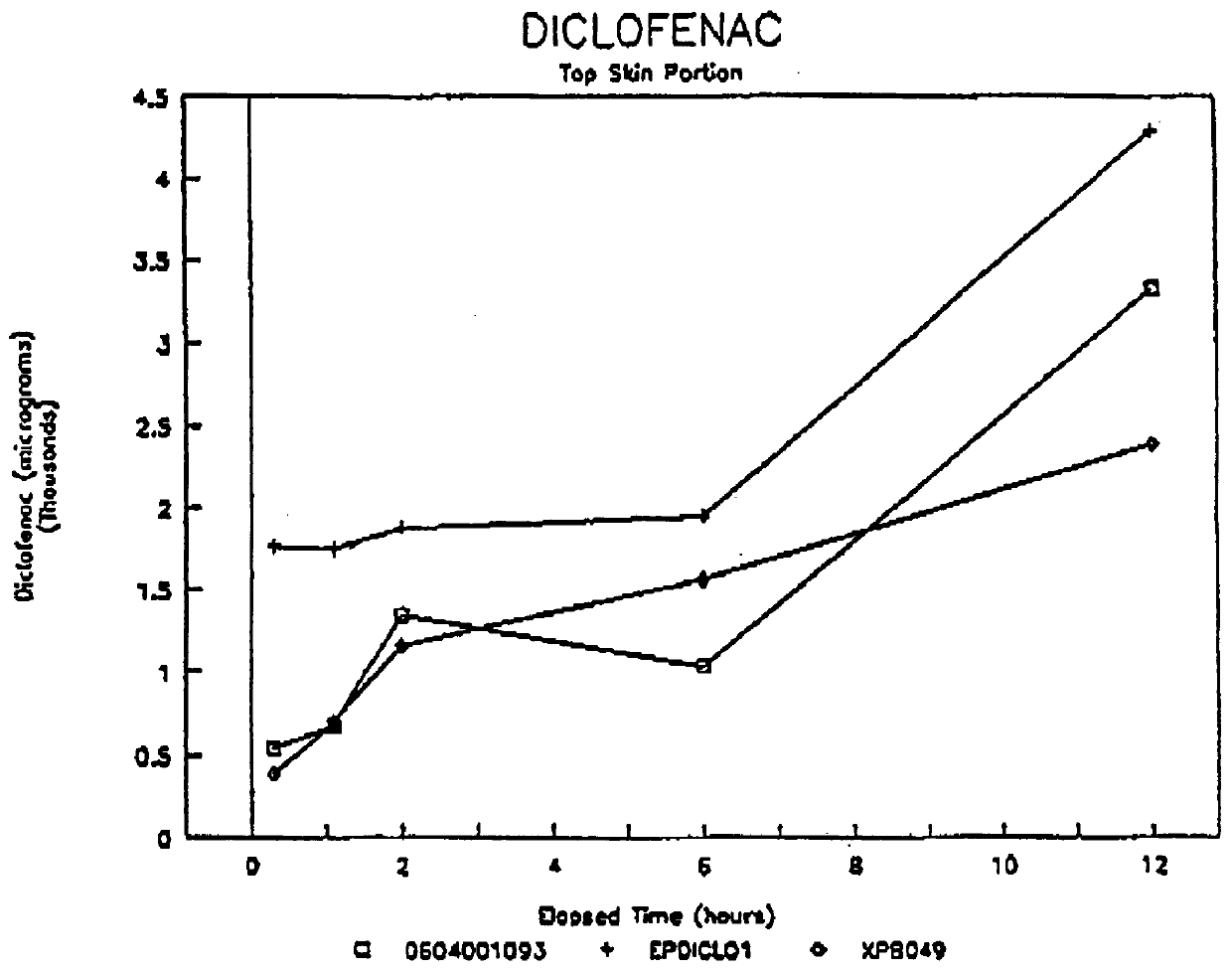
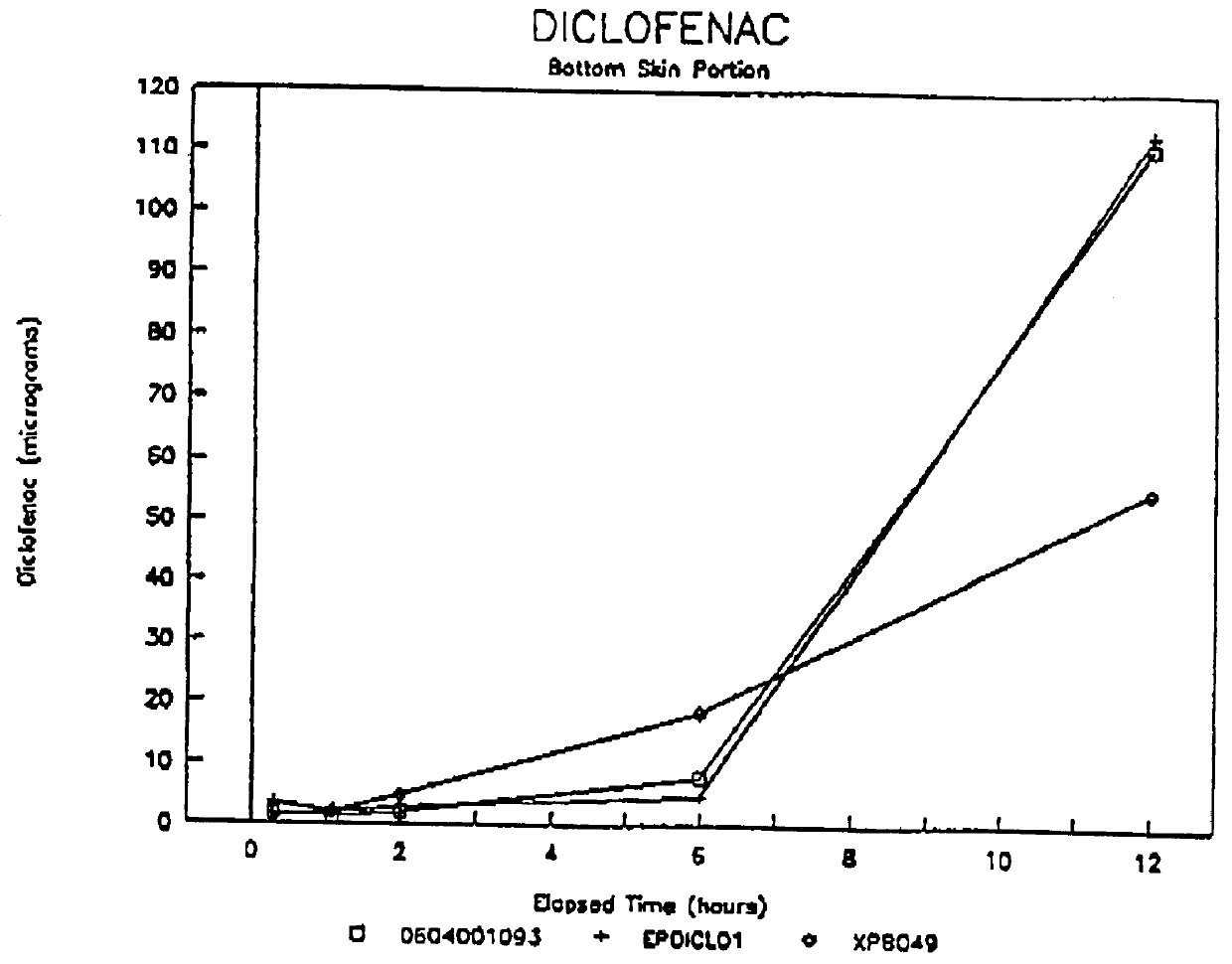
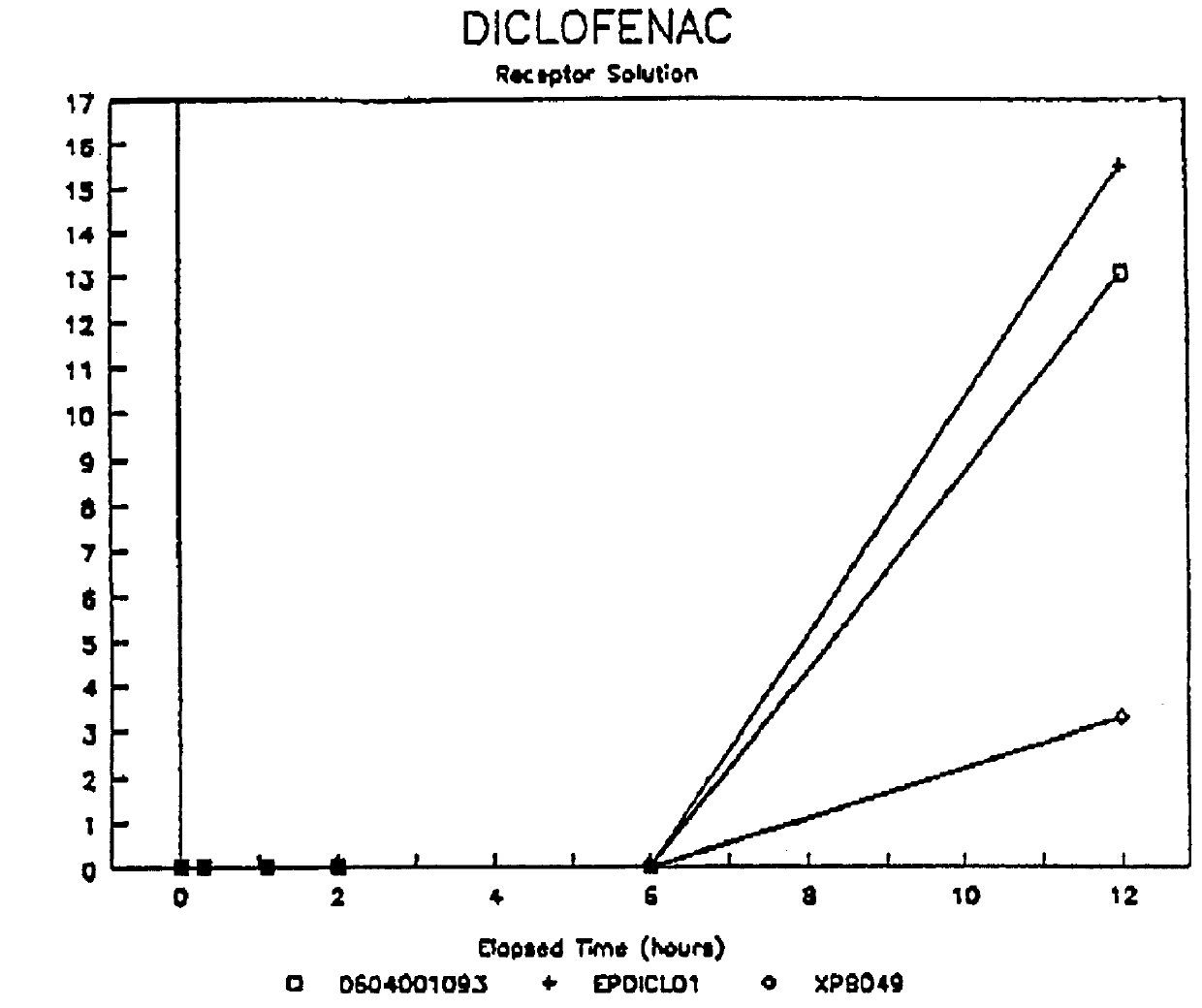
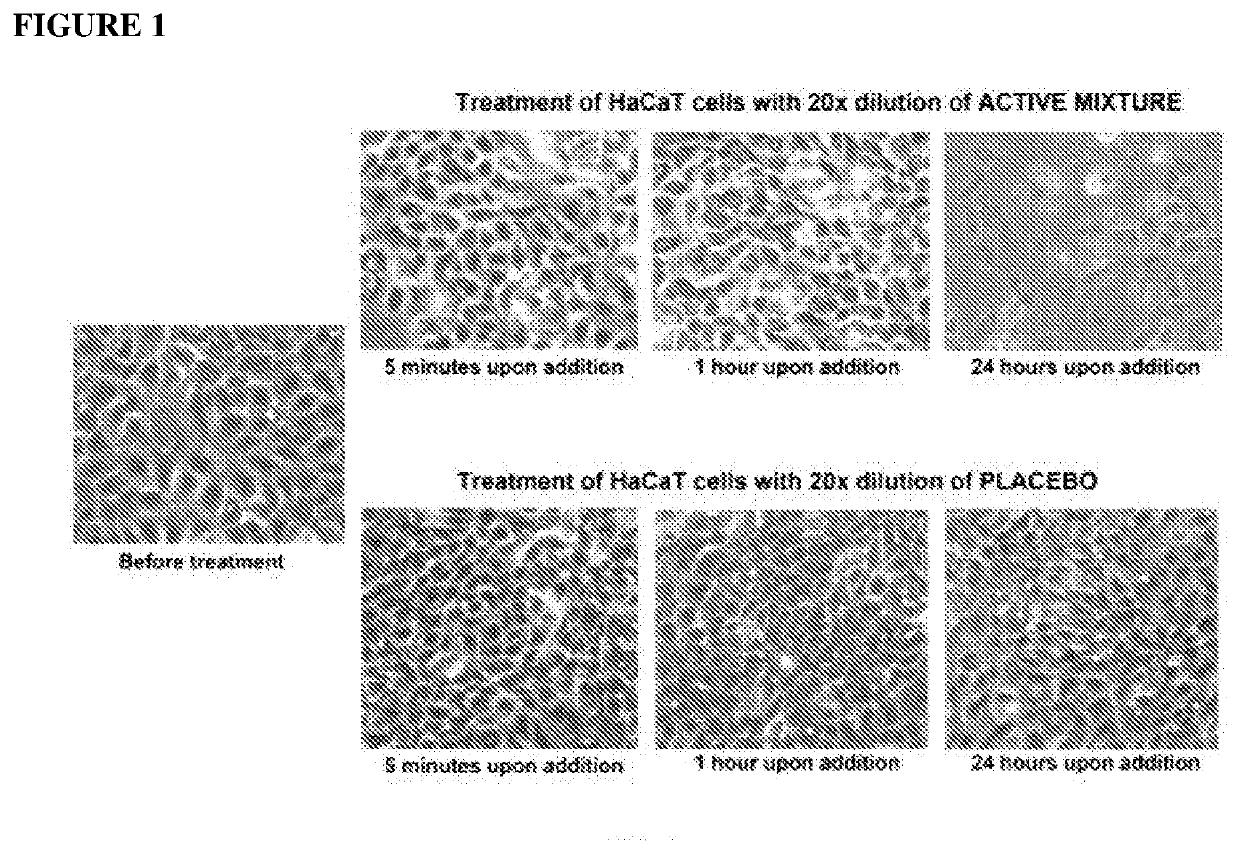
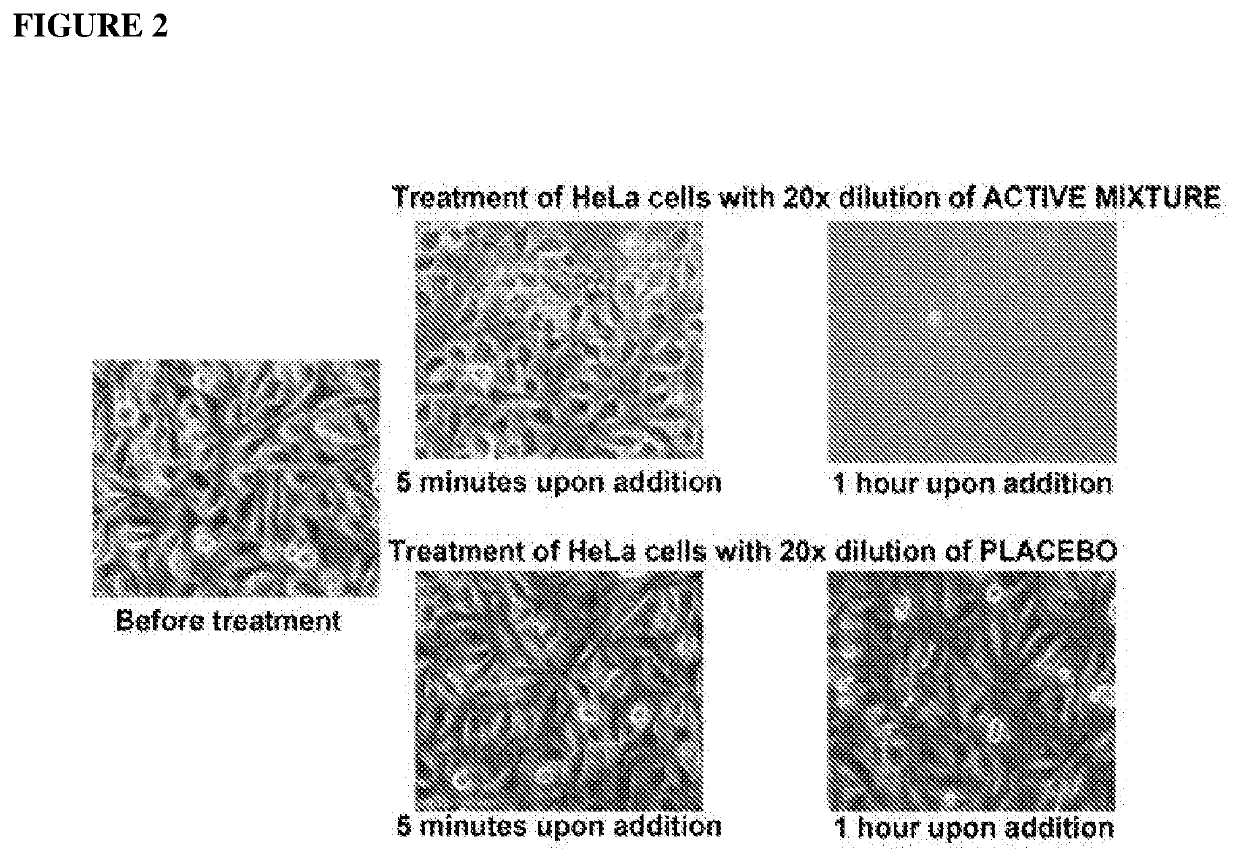
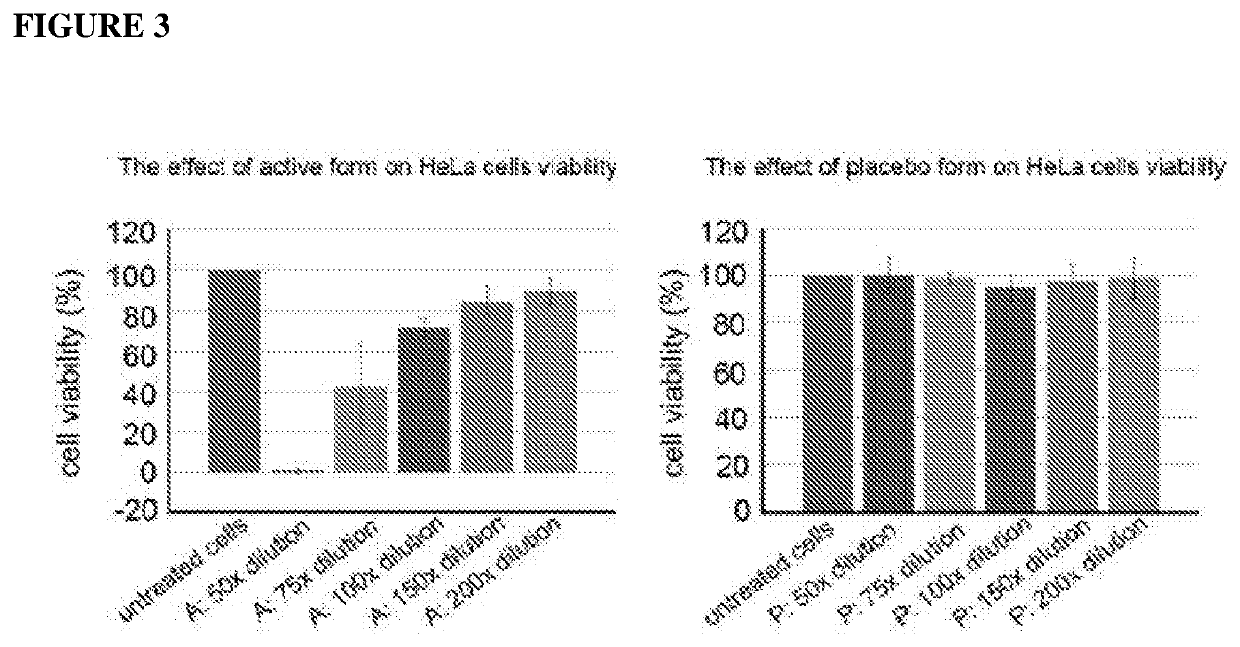
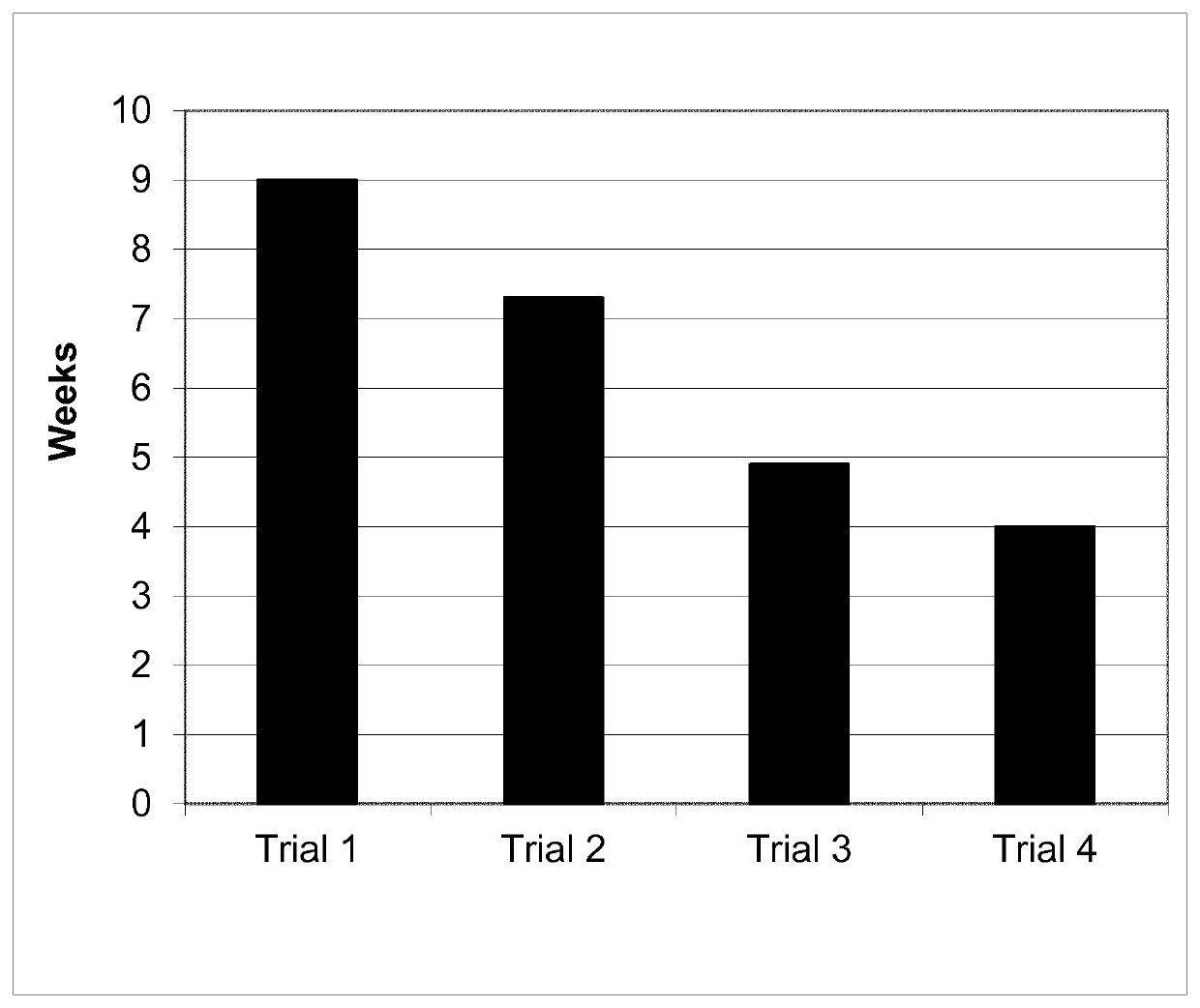
Patents
Literature
38 results about "Actinic keratoses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dermal delivery
InactiveUS20110212157A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentOrganic active ingredientsPowder deliveryHypopigmentationChromhidrosis
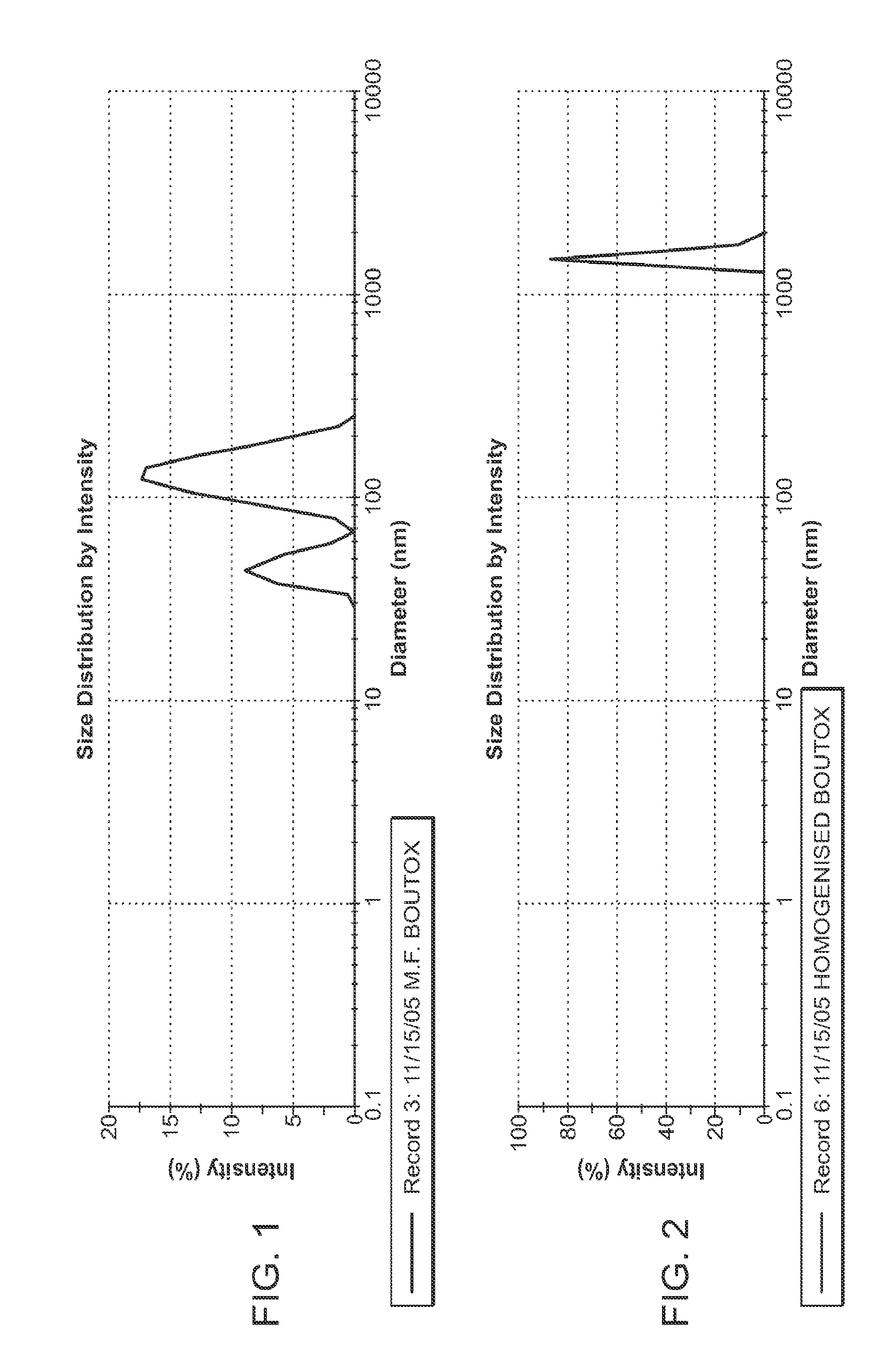
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Non-invasive screening of skin diseases by visible/near-infrared spectroscopy
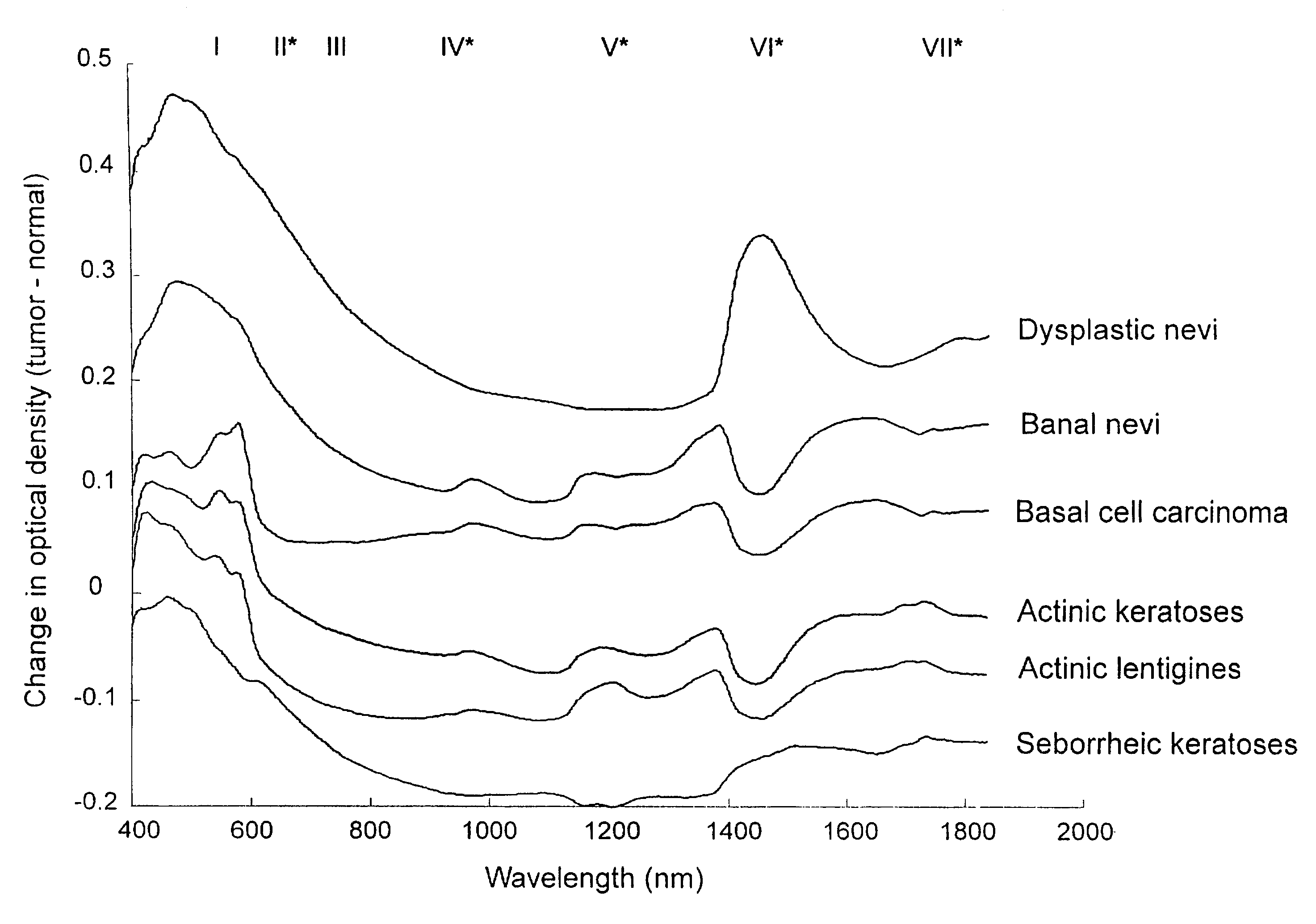
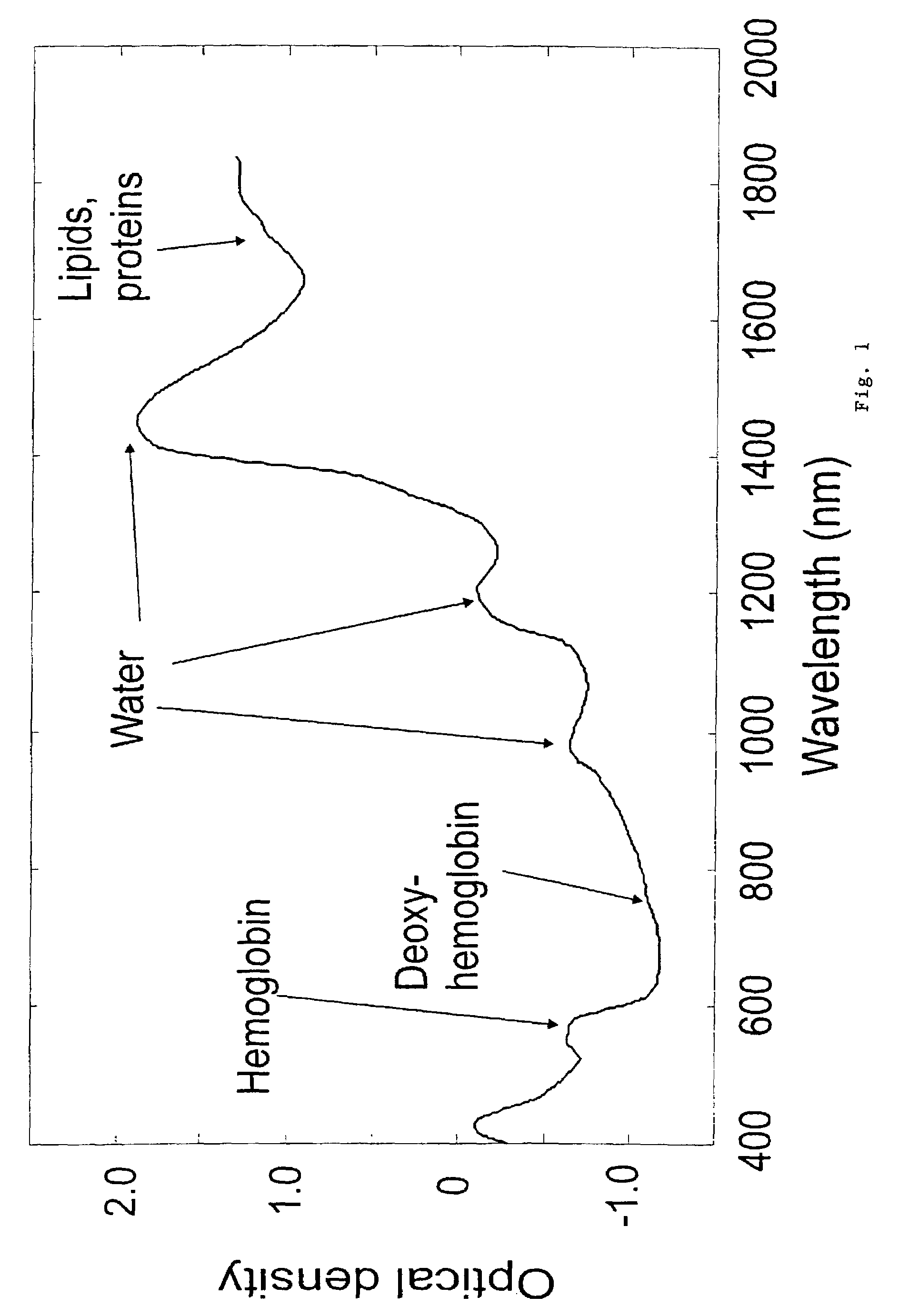
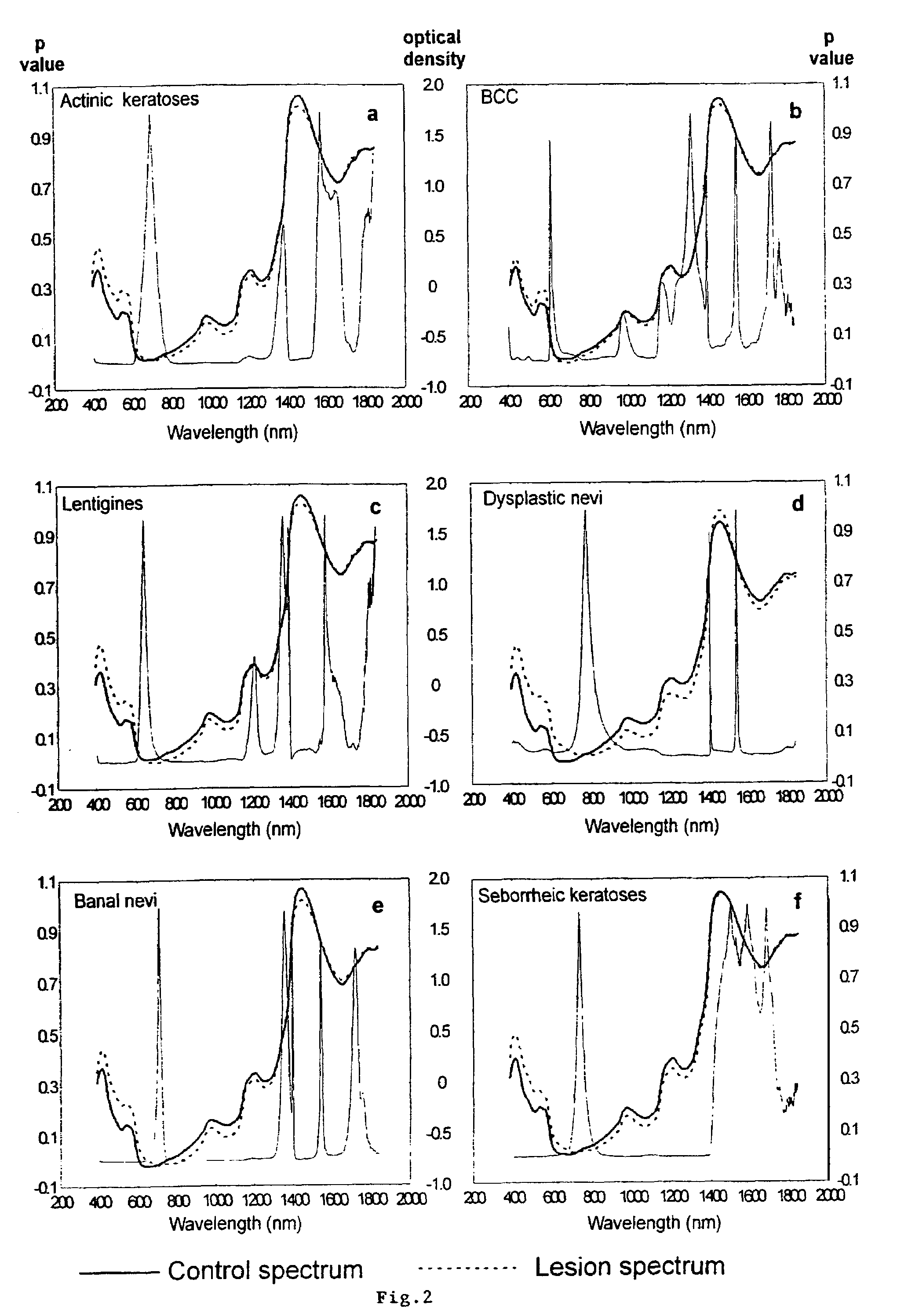
A non-invasive tool for skin disease diagnosis would be a useful clinical adjunct. The purpose of this study was to determine whether visible / near-infrared spectroscopy can be used to non-invasively characterize skin diseases. In-vivo visible- and near-infrared spectra (400-2500 nm) of skin neoplasms (actinic keratoses, basal cell carcinomata, banal common acquired melanocytic nevi, dysplastic melanocytic nevi, actinic lentigines and seborrheic keratoses) were collected by placing a fiber optic probe on the skin. Paired t-tests, repeated measures analysis of variance and linear discriminant analysis were used to determine whether significant spectral differences existed and whether spectra could be classified according to lesion type. Paired t-tests showed significant differences (p<0.05) between normal skin and skin lesions in several areas of the visible / near-infrared spectrum. In addition, significant differences were found between the lesion groups by analysis of variance. Linear discriminant analysis classified spectra from benign lesions compared to pre-malignant or malignant lesions with high accuracy. Visible / near-infrared spectroscopy is a promising non-invasive technique for the screening of skin diseases.
Owner:NAT RES COUNCIL OF CANADA
Method for non-therapeutic or therapeutic photodynamic skin treatment
InactiveUS20100298758A1Reduce inconvenienceEasy to practiceOrganic active ingredientsCosmetic preparationsActinic keratosesWrinkle skin
In a method for non-therapeutic or therapeutic photodynamic treatment of a skin target area, a photosensitizer fluid incorporating a photosensitizing agent or a precursor for a photosensitizing agent is delivered to the target area, the photosensitizing agent having at least one absorption wavelength in a wavelength range higher than 400 nm. The target area is then exposed to light energy in a range of wavelengths comprising at least one absorption wavelength of the photosensitizing agent, the light energy being supplied to the target area in the form of at least one light pulse producing in the target area an accumulated energy density sufficient for photodynamic activation of the photosensitizing agent. The photosensitizing agent or precursor is incorporated in the photosensitizer fluid with a concentration in the range from 0.1 to 2.0% preferably from 0.1 to 0.9%, and the photosensitizer fluid is delivered to the target area in the form of a number of repeated spray doses during an overall delivery time ranging from 0.25 to 8 hours. The method can be applied to cosmetic treatments for skin rejuvenation, reduction of wrinkles, treatment of telangiectasia or removal of hair as wells a medical treatment of dermatologic diseases such as acne vulgaris, acne scars, rosacea, psorieasis, actinic keratoses, basal cell carcinoma, Bowen's disease or eczema.
Owner:CHRISTIANSEN +2
Formulations containing hyaluronic acid
InactiveUS6114314AInhibit synthesisQuick to penetrate into skinBiocideSugar derivativesDiseaseActinic keratoses
Topically applied transdermally quick penetrating (best targeting the epidermis and subsequently remaining there for a prolonged period of time) systemic independent acting, combinations and formulations which employ, combine, or incorporate a therapeutically effective non-toxic (to the patient) amount of a drug which inhibits prostaglandin synthesis together with an amount of hyaluronic acid and / or salts thereof (for example the sodium salt) and / or homologues, analogues, derivatives, complexes, esters, fragments, and / or sub units of hyaluronic acid to treat a disease and condition of the skin and exposed tissue for example, basal cell carcinoma, the precancerous, often recurrent, actinic keratoses lesions, fungal lesions, "liver" spots and like lesions (found for the most part in the epidermis), squamous cell tumours, metastatic cancer of the breast to the skin, primary and metastatic melanoma in the skin, genital warts cervical cancer, and HPV (Human Papilloma Virus) including HPV of the cervix, psoriasis (both plaque-type psoriasis and nail bed psoriasis), corns on the feet and hair loss on the head of pregnant women and remain in the skin for a prolonged period of time.
Owner:JAGOTEC AG +1
Cosmetic compositions for the inhibition of reactive oxygen species
InactiveUS20090246153A1Good effectInhibit arNOXBiocideCosmetic preparationsWrinkle skinActinic keratoses
A composition and method having anti-aging properties is provided. The composition comprises an arNOX inhibitory agent present in a natural plant extract and is useful topically as a cosmetic. The symptoms of aging including lines, wrinkles, hyperpigmentation, dehydration, loss of elasticity, angioma, dryness, itching, telangietasias, actinic purpura, seborrheic keratoses and actinic keratoses. The invention may be used multiple times a day without deleterious effects.
Owner:NU SKIN INT
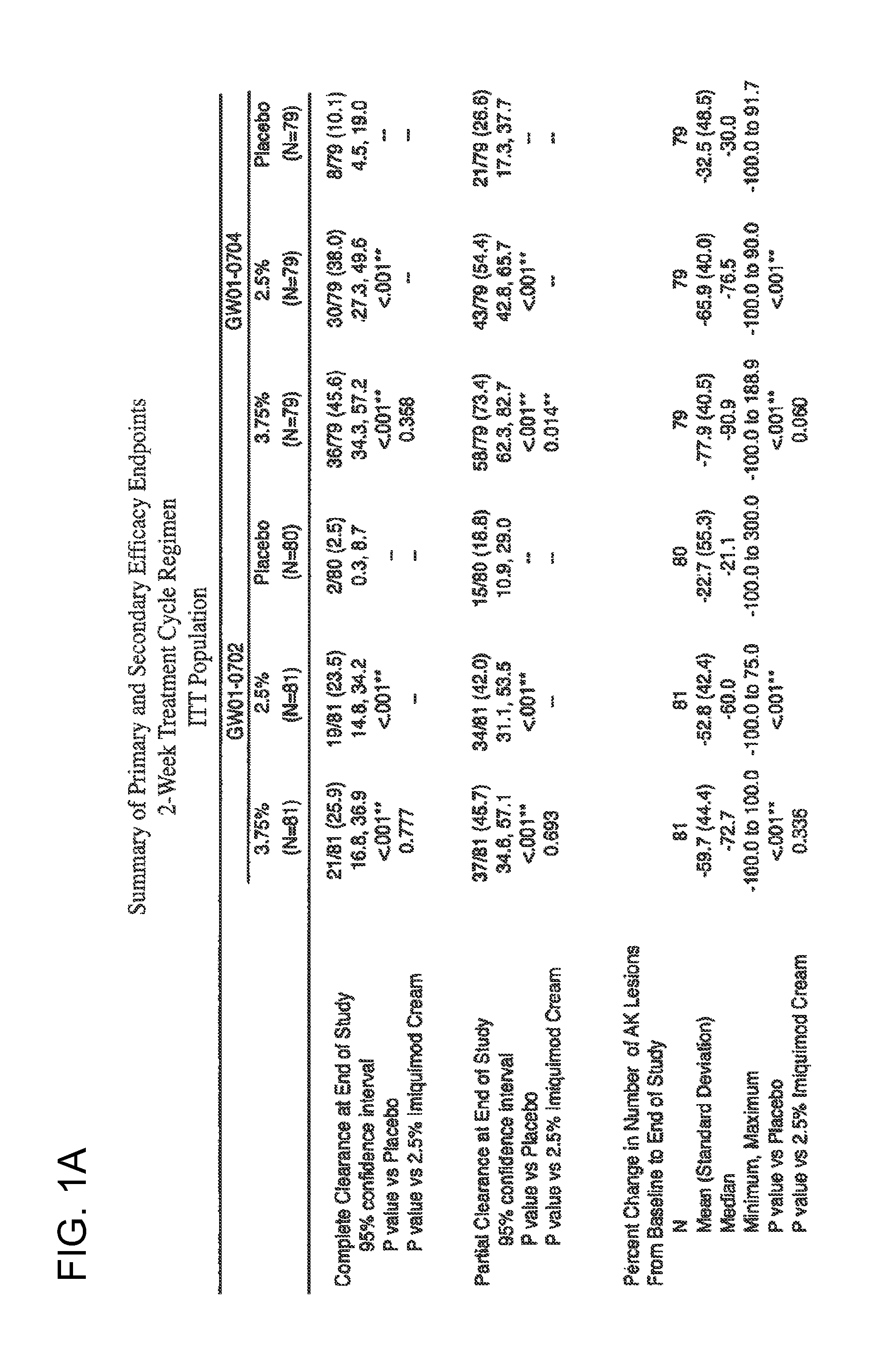
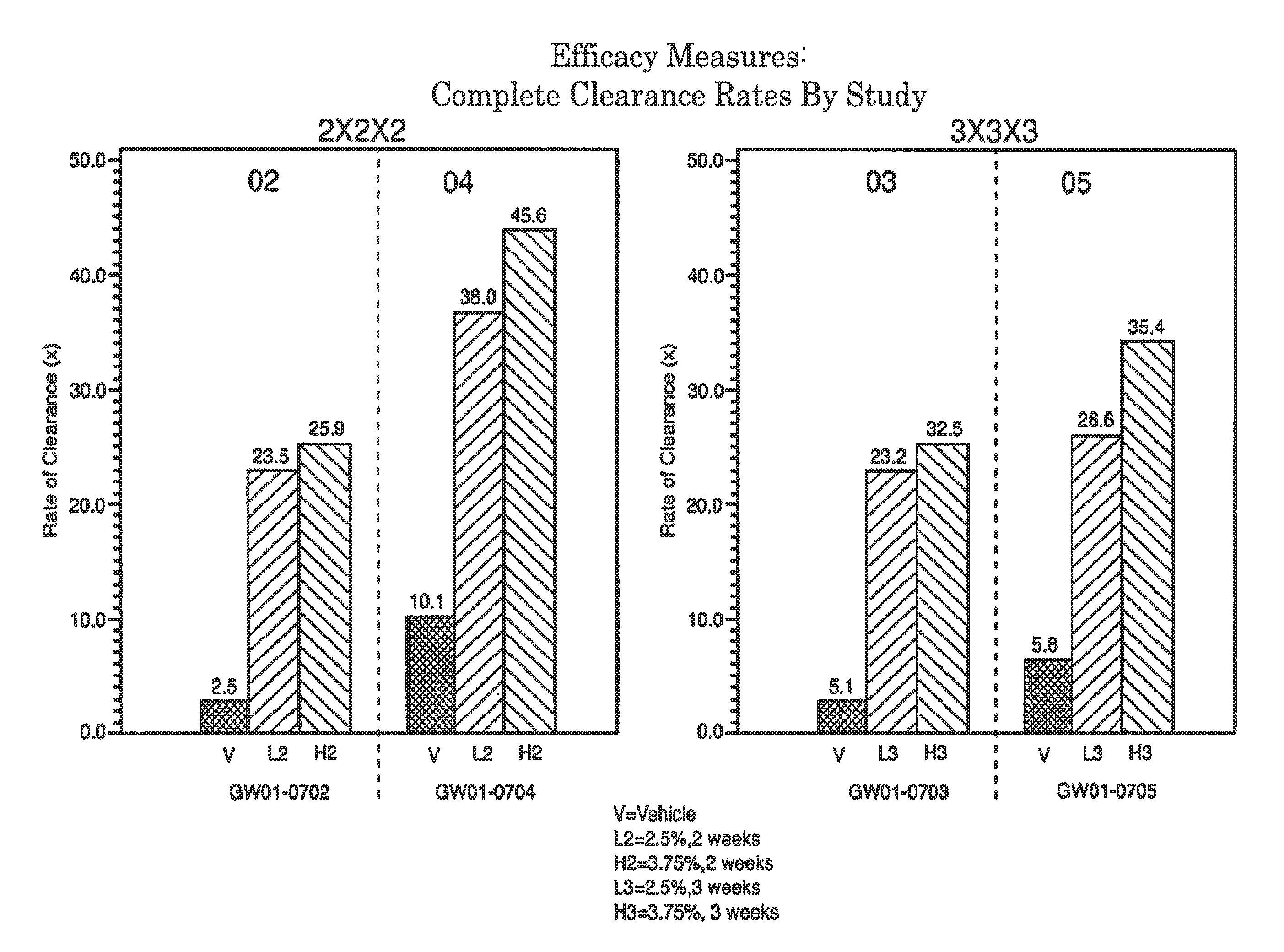
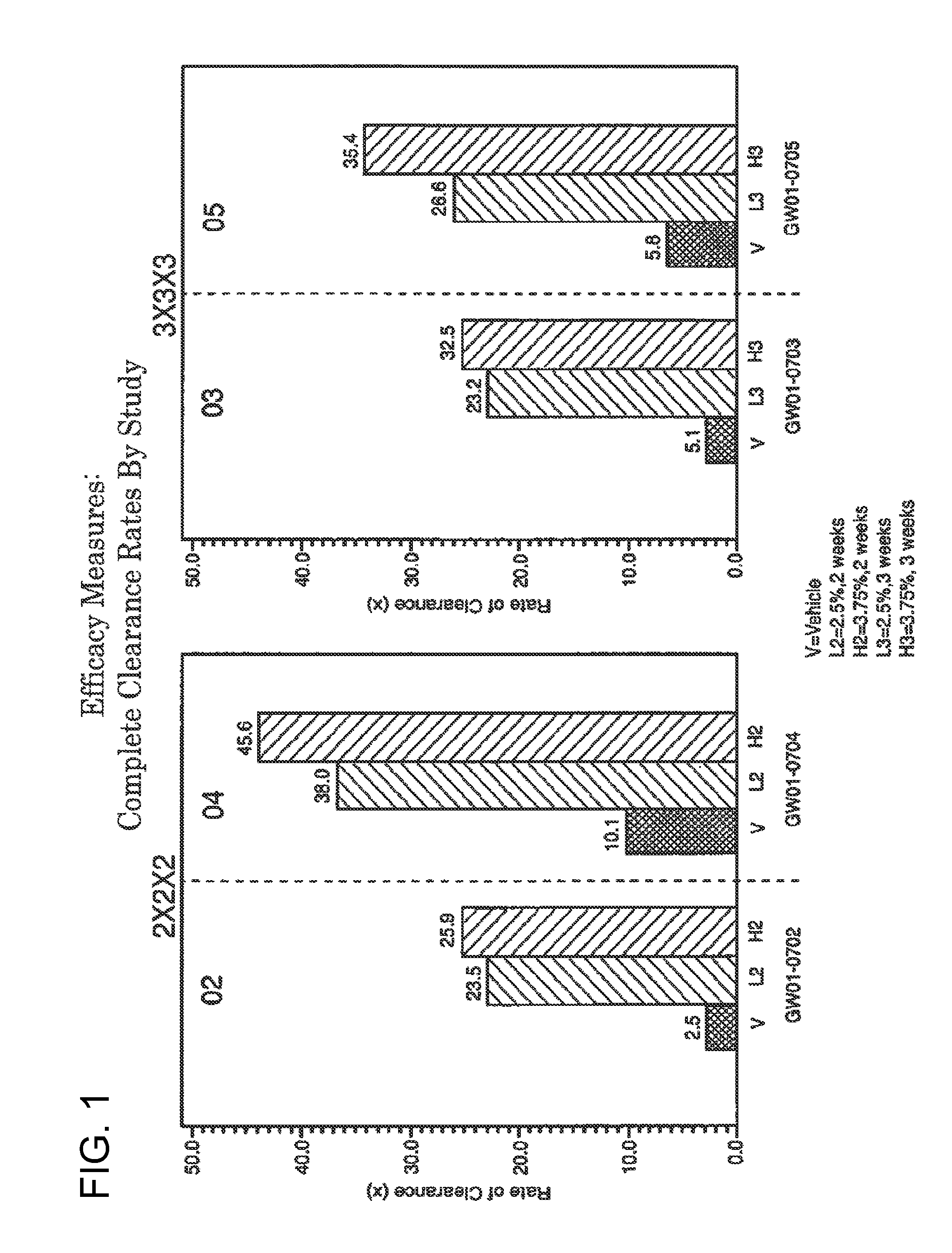
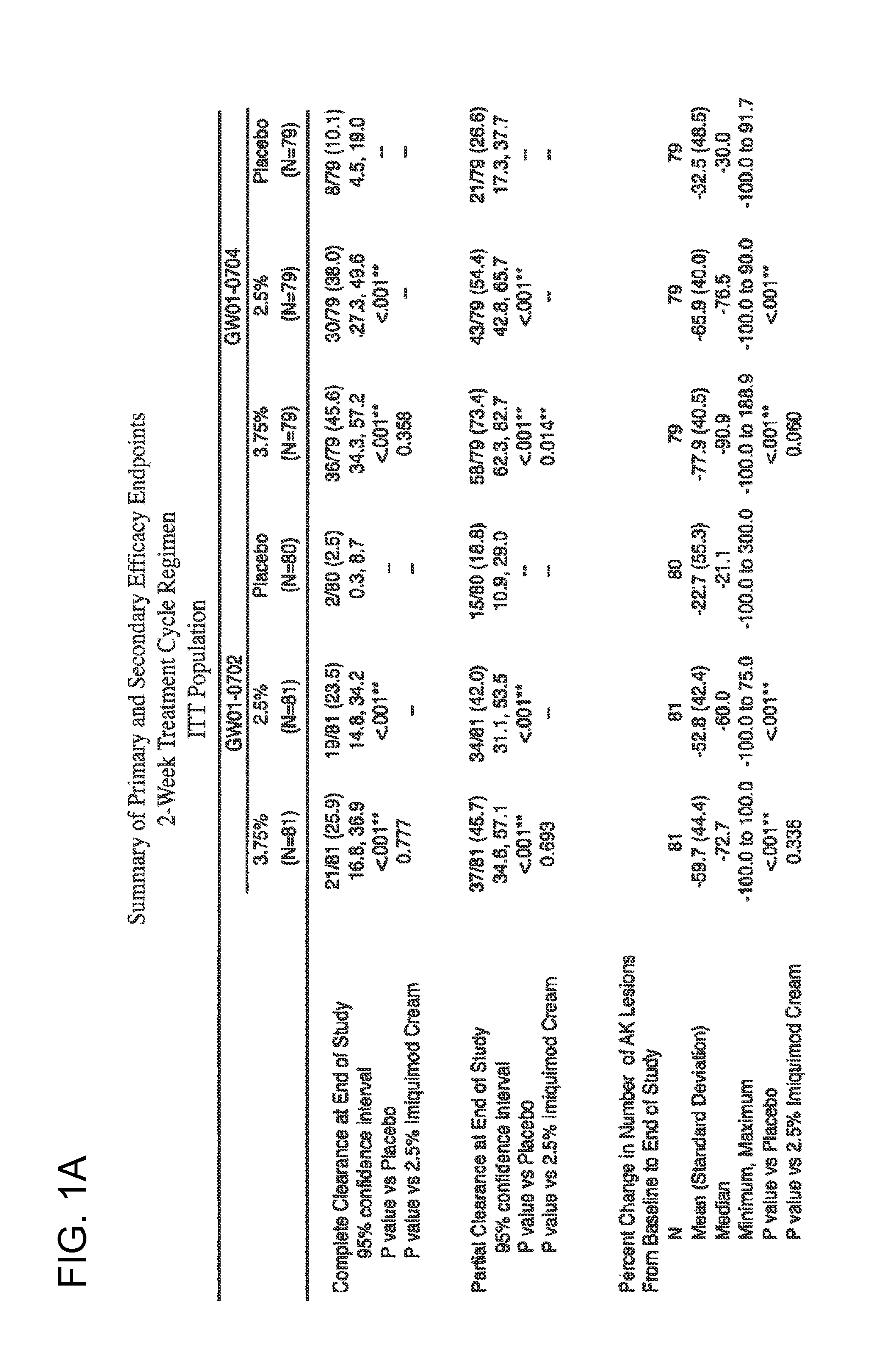
2 x 2 x 2 WEEK TREATMENT REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 2.5% IMIQUIMOD
ActiveUS20110257216A1Improved imiquimodReduced strengthBiocideOintment deliveryActinic keratosesDosing regimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Treatment of actinic keratoses with calcium channel blockers
The present invention relates to methods and compositions for the treatment and / or prevention of actinic keratoses comprising a calcium channel blocking compound.
Owner:PRESCRIPTION DISPENSING LAB
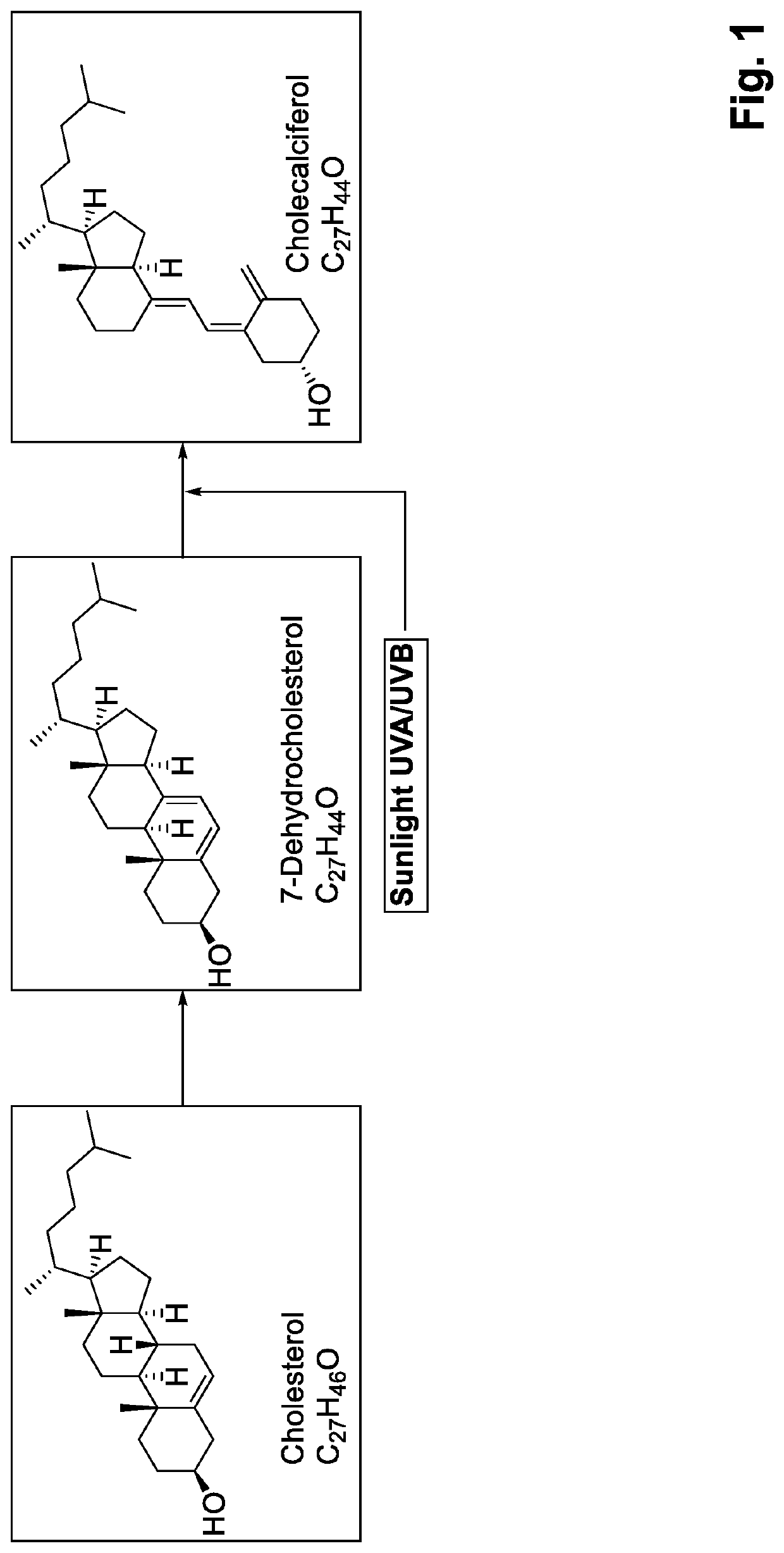
B complex vitamin compositions that protect against cellular damage caused by ultraviolet light
InactiveUS7018623B2Reduces UV damageReduce riskBiocideCosmetic preparationsVitamin K2Immune depression
The present invention relates generally to the use of vitamin B12 (cobalamin or cyanocobalamin) alone or in combination with other photoprotective agents, including specifically other vitamins such as vitamin B9 (folic acid or folate) and vitamin B3 (niacin or niacinamide), or any chemical derivative of these vitamins and their salts, as a filter to protect cells against the damaging effects of ultraviolet (UV) light. The invention is, in one aspect, a method of reducing the rate of UV damage to cells exposed to a UV light source, by treating the cells with the vitamin composition, either alone or in combination with other photoprotective agents. Other aspects of the invention are compositions comprising effective amounts of vitamin B12 alone or in combination with other photoprotective agents including vitamin B9 and vitamin B3 and a pharmaceutically-acceptable carrier, that are useful in protecting cells, particularly skin cells, against the burning, genotoxic (mutagenic and carcinogenic), immunosuppressive and photoaging effects of UV light, especially sunlight. The invention has application as a UV light filter in oral preparations including tablets and drinks, topical creams, lotions, sprays, wipes and cosmetics. The invention also has application as a medicinal treatment for dermatological conditions caused by exposure to sunlight, such as actinic keratoses, photodermatitis, photo-induced (discoid) lupus erythematosus and the photosensitizing effects of a variety of drugs used commonly in clinical practice (e.g. certain antihistamines, ACE inhibitors, and antibiotics such as tetracycline).
Owner:BARCLAY BARRY J
Fluorouracil-containing formulation
Oil-in-water emulsion formulations contain both free fluorouracil and fluorouracil impregnated in porous microparticles. The formulations are suitable for topical administration, and are useful for the treatment of solar keratoses, actinic keratoses, and superficial basal cell carcinomas.
Owner:BAUSCH HEALTH IRELAND LTD
Metal substituted non-centrosimmetrical phthalocyanine analogues, their preparation and use in photodynamic therapy and in vivo diagnostic
Phthalocyanine analogues having an active group able to link the phthalocyanine t carriers molecules and phthalocyanine to carriers molecules and phthalocyanine analogues as phthalocyanine-carrier conjugates showing enhanced photodynamic properties, red shifted absorption characteristic, all useful for photodynamic therapy, are described.Photosensitizers themselves or the photosensitizers-carriers conjugates are useful compounds either for treatment of various infectious diseases, the in vivo eradication of micro-organisms as well as diseases characterized by cellular hyperproliferation, in particular tumours psoriasis, actinic keratosis, atheromas, endoarterial hyperplasia and prostate hyperplasia.The above compounds can be also useful for blood and blood derivatives sterilization and in vivo / vitro diagnostics.
Owner:MOLTENI THERAPEUTICS CO LTD
Pump systems and methods for storing and dispensing a plurality of precisely measured unit-doses of imiquimod cream
ActiveUS9072876B2Effective treatmentEasy to storeOrganic active ingredientsAerosol deliveryActinic keratosesSemi solid
The present invention is directed to airless storage and dispensing systems that include a pump or dispensing package pre-filled with a topical semi-solid imiquimod pharmaceutical formulation (“pump systems”) and methods for storing and dispensing from the pump systems a plurality of precisely measured and uniform unit doses of a topical semi-solid imiquimod pharmaceutical formulation, and more particularly to pump systems, pre-filled with a topical imiquimod pharmaceutical cream and methods for delivering multiple precisely measured unit doses of a topical imiquimod pharmaceutical cream, and methods for using a controlled delivery pump system to store and dispense a plurality of consistent and precisely measured unit doses of a topical imiquimod pharmaceutical cream for use in topically treating a dermal and mucosal-associated condition, such as, external genital warts and / or perianal warts (EGWs), actinic keratosis or actinic keratoses (AK or AKs) and superficial basal cell carcinoma (sBCC).
Owner:MEDICIS PHARMA CORP
Treatment of actinic keratoses with calcium channel blockers
The present invention relates to methods and compositions for the treatment and / or prevention of actinic keratoses comprising a calcium channel blocking compound.
Owner:PRESCRIPTION DISPENSING LAB
Lower dosage strength pharmaceutical compositions forumlated with 3.75% imiquimod
InactiveUS20110257219A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Novel treatments for actinic keratoses
InactiveUS20140256667A1Effective treatmentPromote resultsBiocideAerosol deliveryActinic keratosesMedicine
A composition of gentamicin and colchicine in a dermatologically acceptable cream base provided improved clearance of actinic keratoses following topical application for 1 to 3 months. The composition provides improved clearance of recalcitrant actinic keratoses. Furthermore, the treatment causes less pain and irritation than other topical treatments for actinic keratoses.
Owner:MOY LAWRENCE
Method for treating cancerous and pre-cancerous skin
The present disclosure provides a method for treating clinical or pre-clinical skin damage in a skin field of a subject, wherein the skin field has been allocated a skin cancerization field index (SCFI) score of at least 1 as determined by a process comprising the steps of: (i) assessing the number of keratoses in the skin field; (ii) assessing the thickness of the thickest keratosis in the skin field; and (iii) assessing the proportion of the field affected by clinical or subclinical skin damage. Based on the assessments made in (i), (ii) and (iii) the subject is optionally treated by at least one of (a) freezing one or more lesions, (b) shaving, curetting or surgically removing one or more lesions, (c) applying a topical treatment for actinic keratosis, basal cell carcinoma or squamous cell carcinoma, and (d) radiation therapy.
Owner:GENESISCARE VENTURES PTY LTD
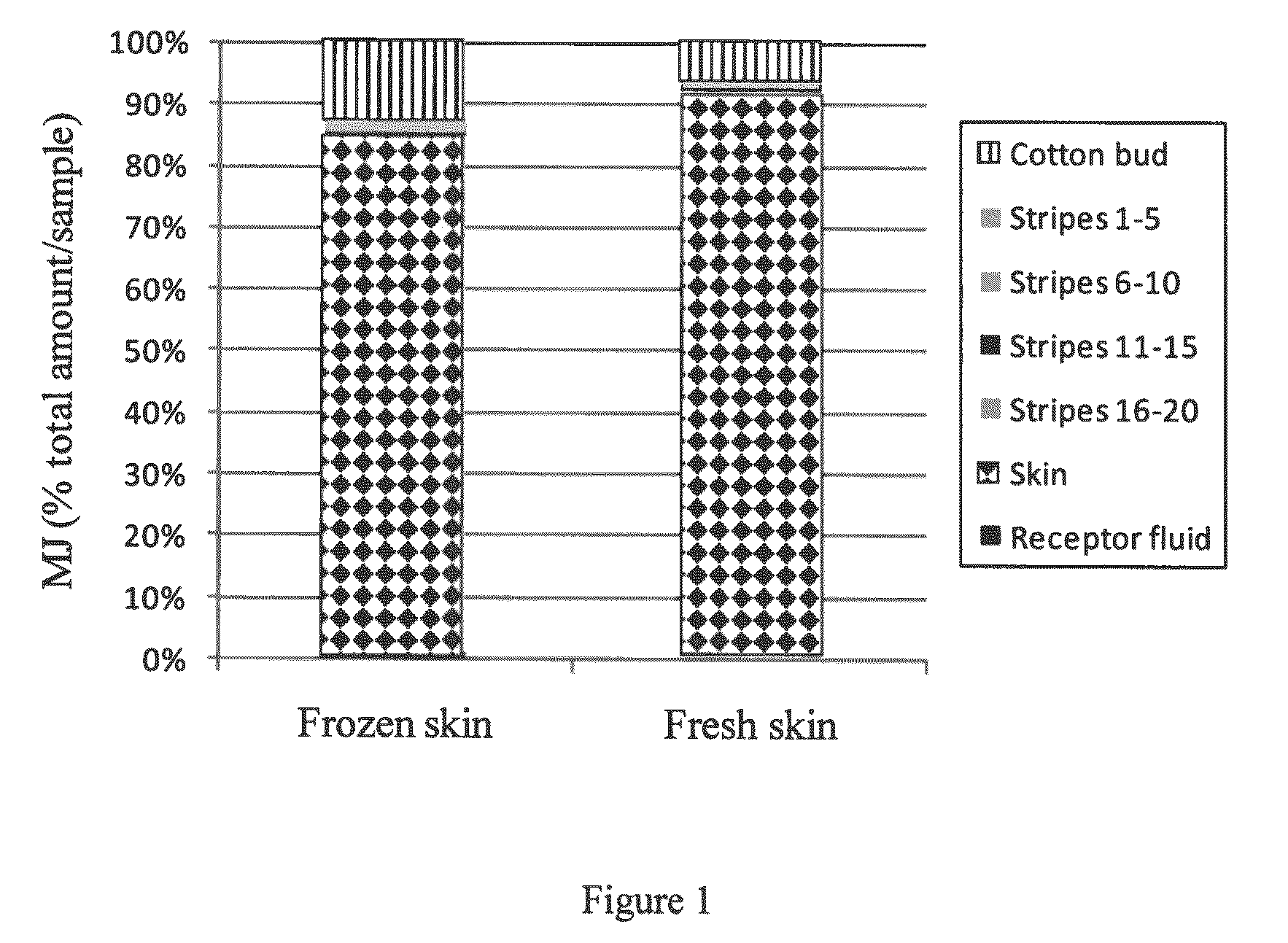
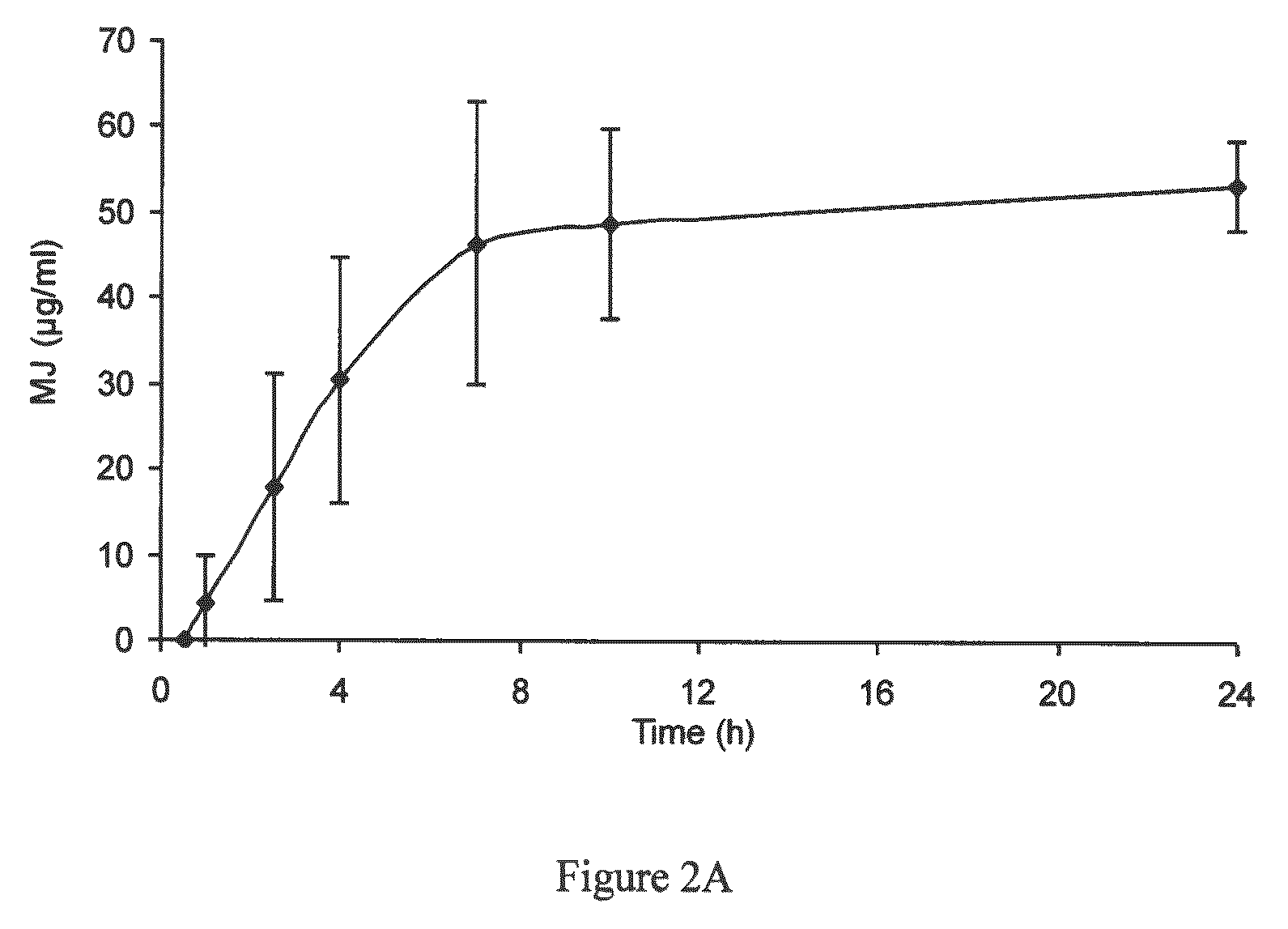
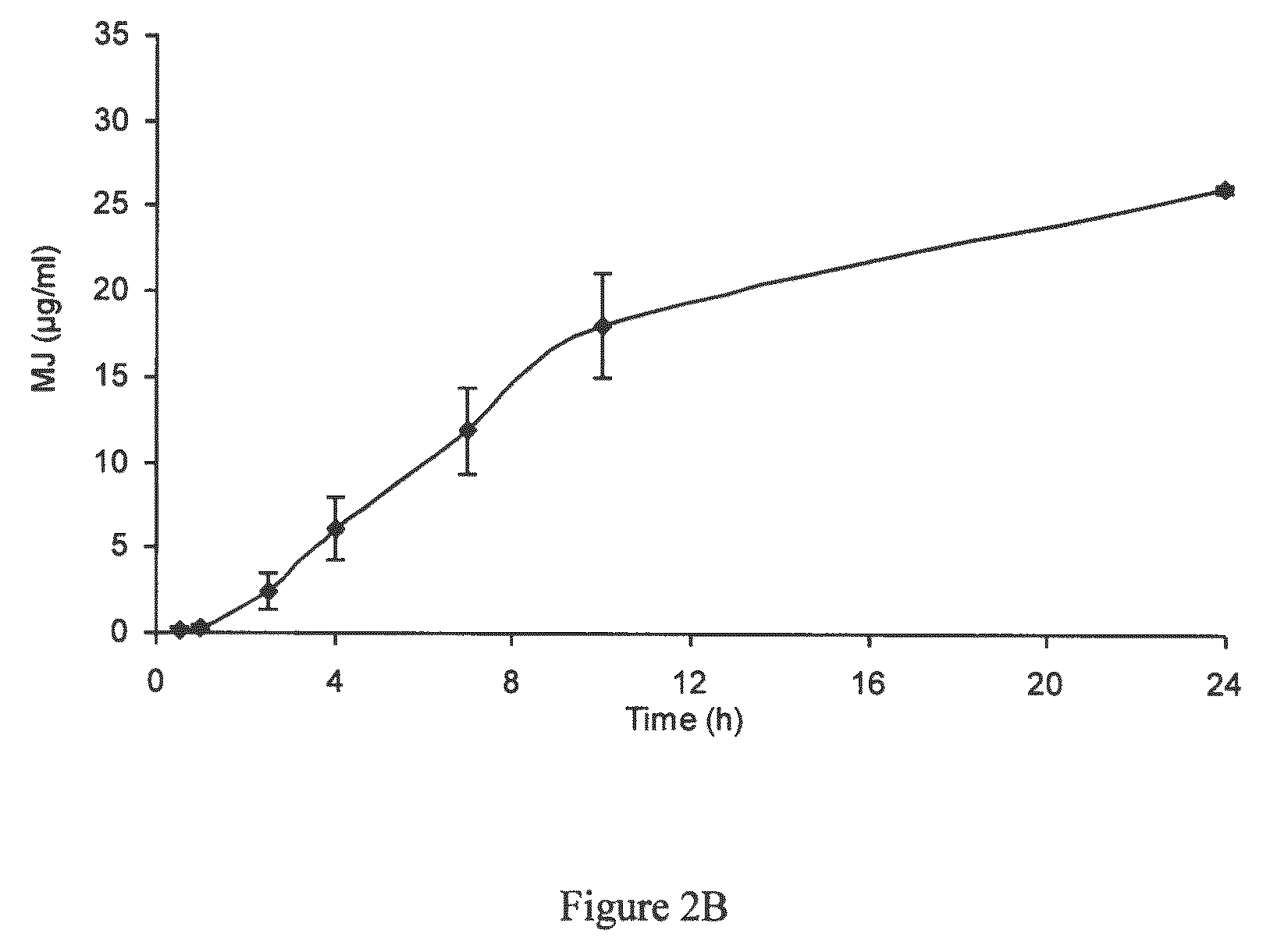
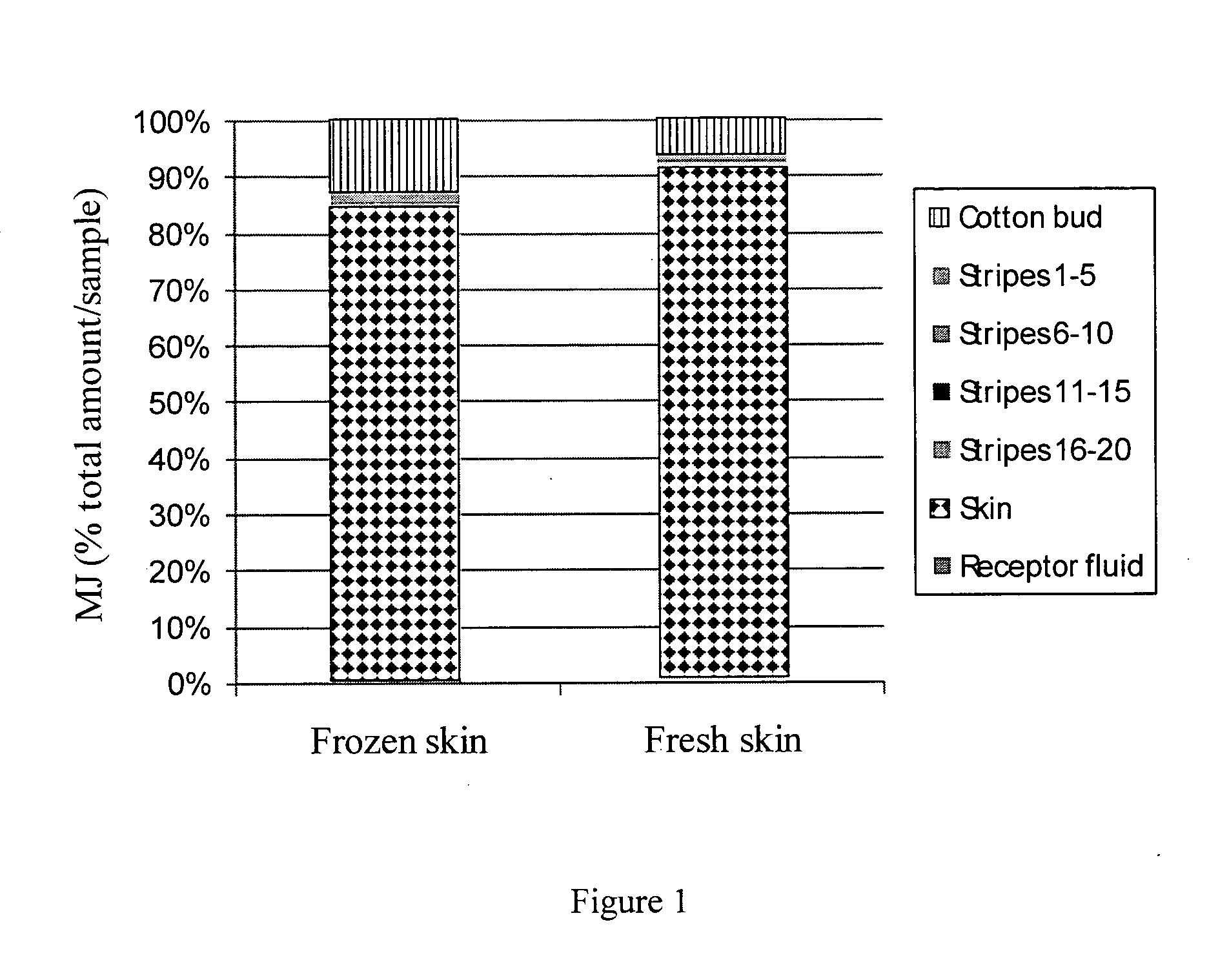
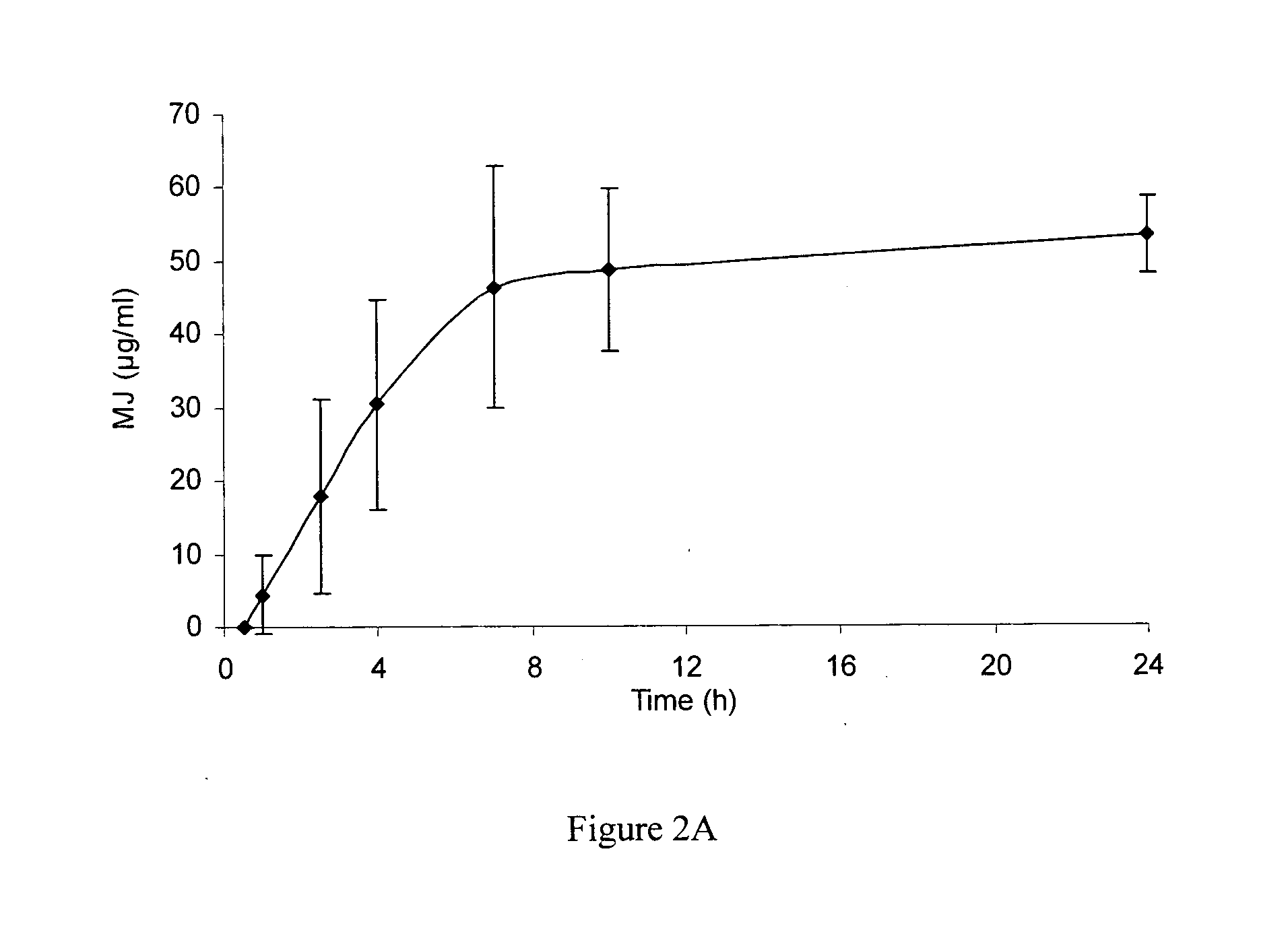
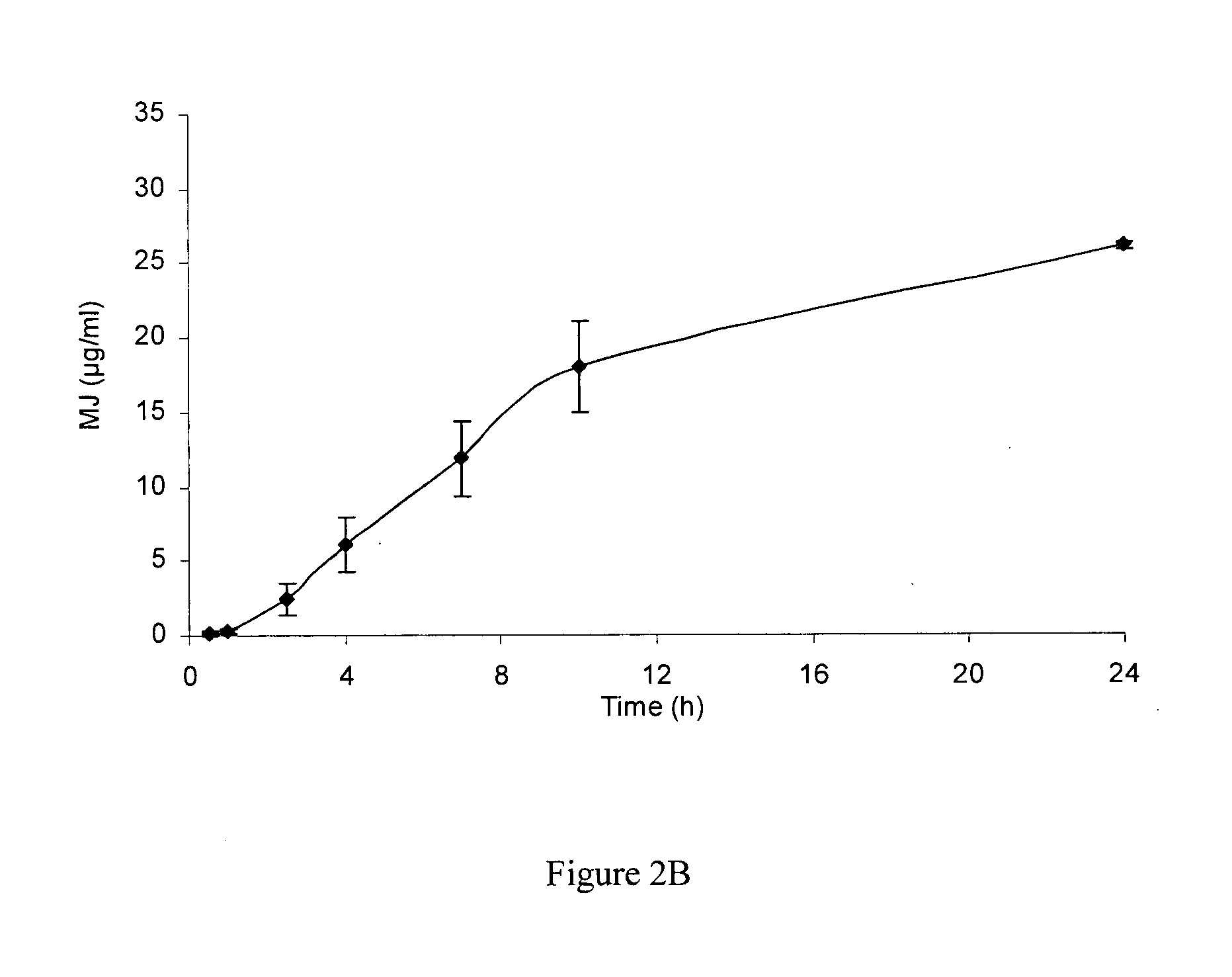
Use of jasmonate ester derivatives for treating benign hyperproliferative skin disorders
The present invention relates to methods of treating benign hyperproliferative diseases of the epidermis by administering a composition comprising at least one jasmonate ester derivative. In particular, the present invention provides jasmonate ester derivatives as potent compounds useful for the treatment of disorders such as actinic keratoses with reduced side effects.
Owner:RAMOT AT TEL AVIV UNIV LTD
Treatment for actinic keratoses
InactiveUS9585962B1Inhibit cell divisionEnhance colchicine effectivenessOrganic active ingredientsAerosol deliveryActinic keratosesCream base
A composition of cyclodextrin and colchicine in a dermatologically acceptable cream base provided improved clearance of actinic keratoses following topical application for 1 to 3 months. The composition provides improved clearance of recalcitrant actinic keratoses. Furthermore, the treatment causes much less pain and irritation than other topical treatments for actinic keratoses.
Owner:MOY LAWRENCE
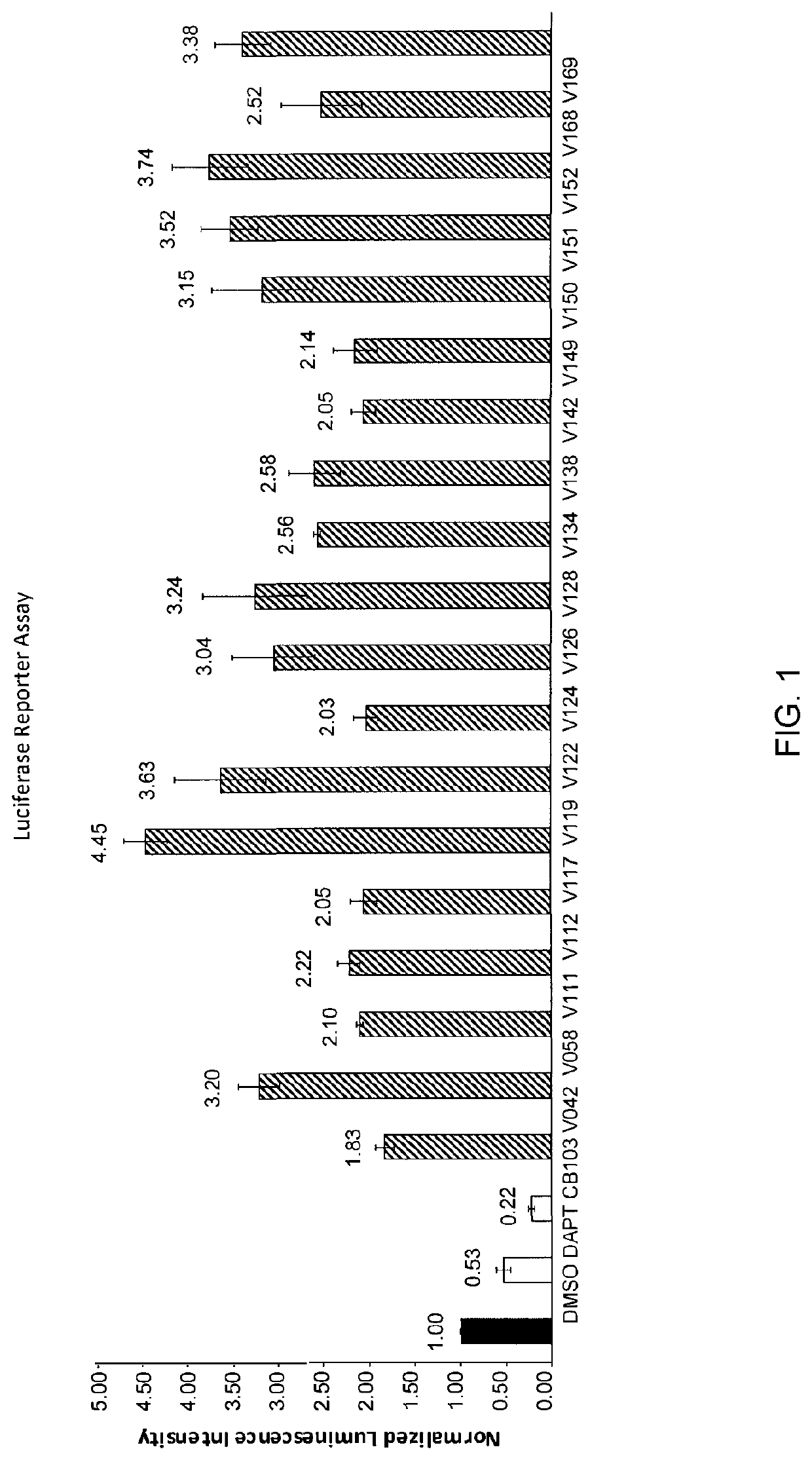
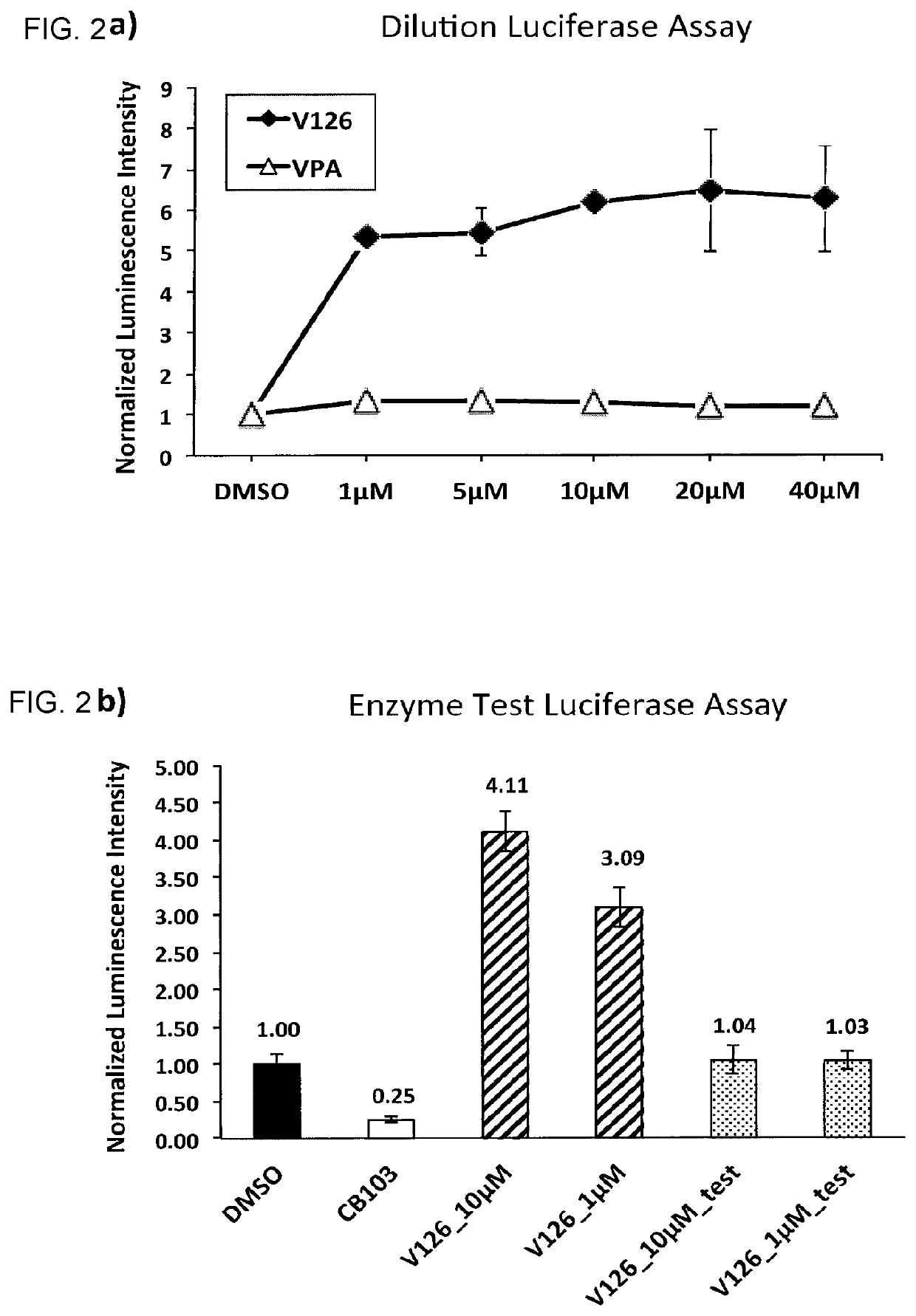
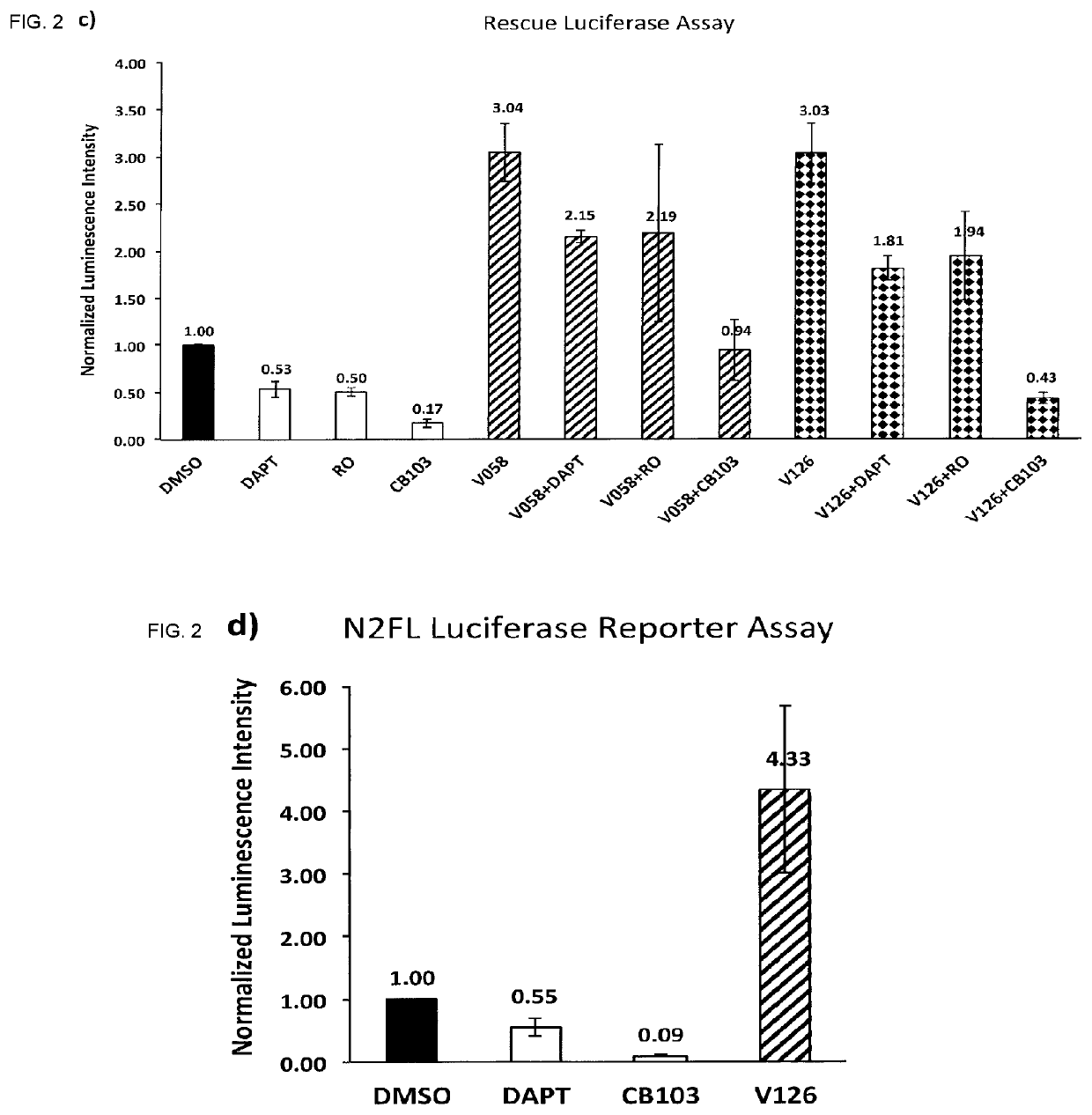
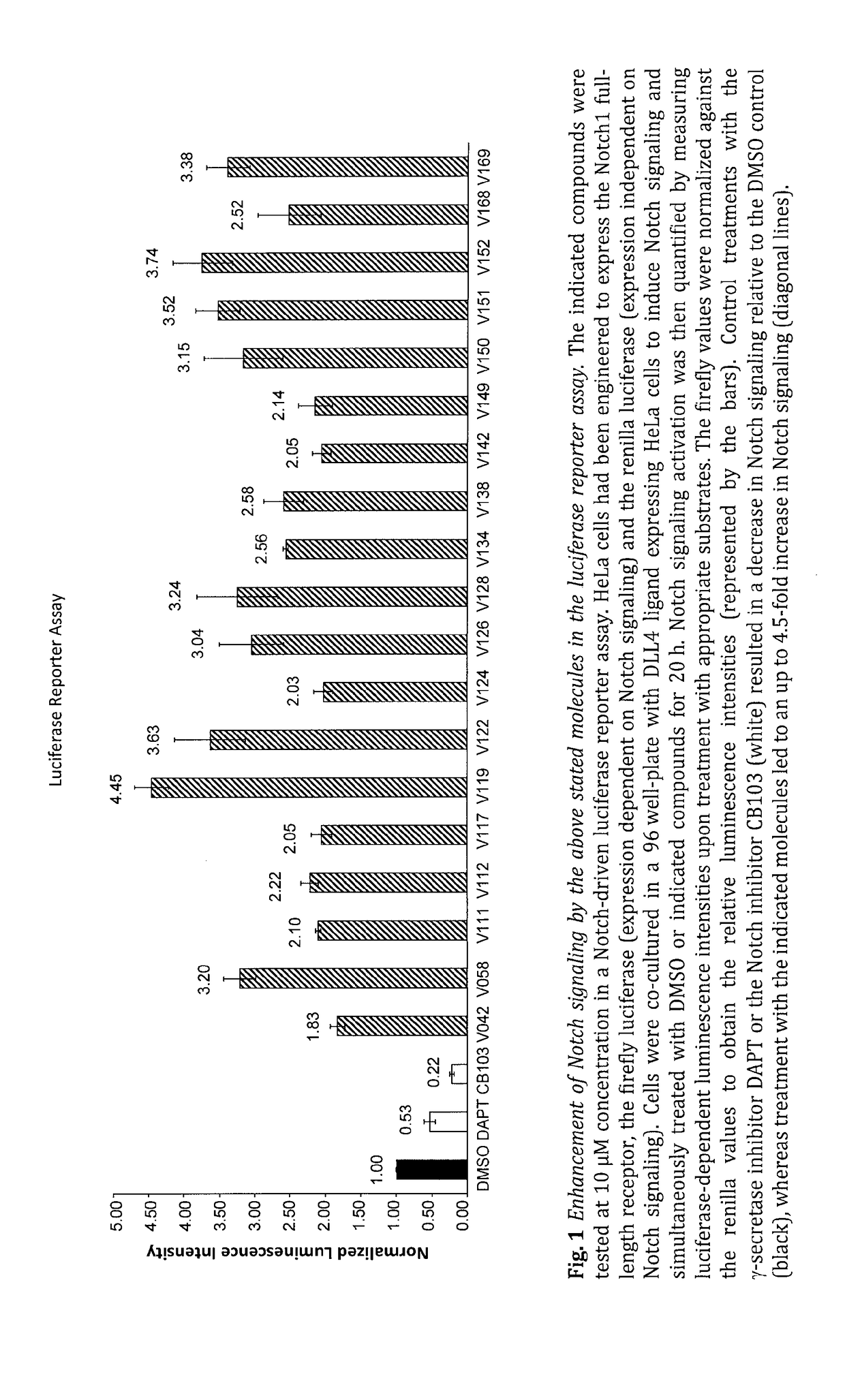
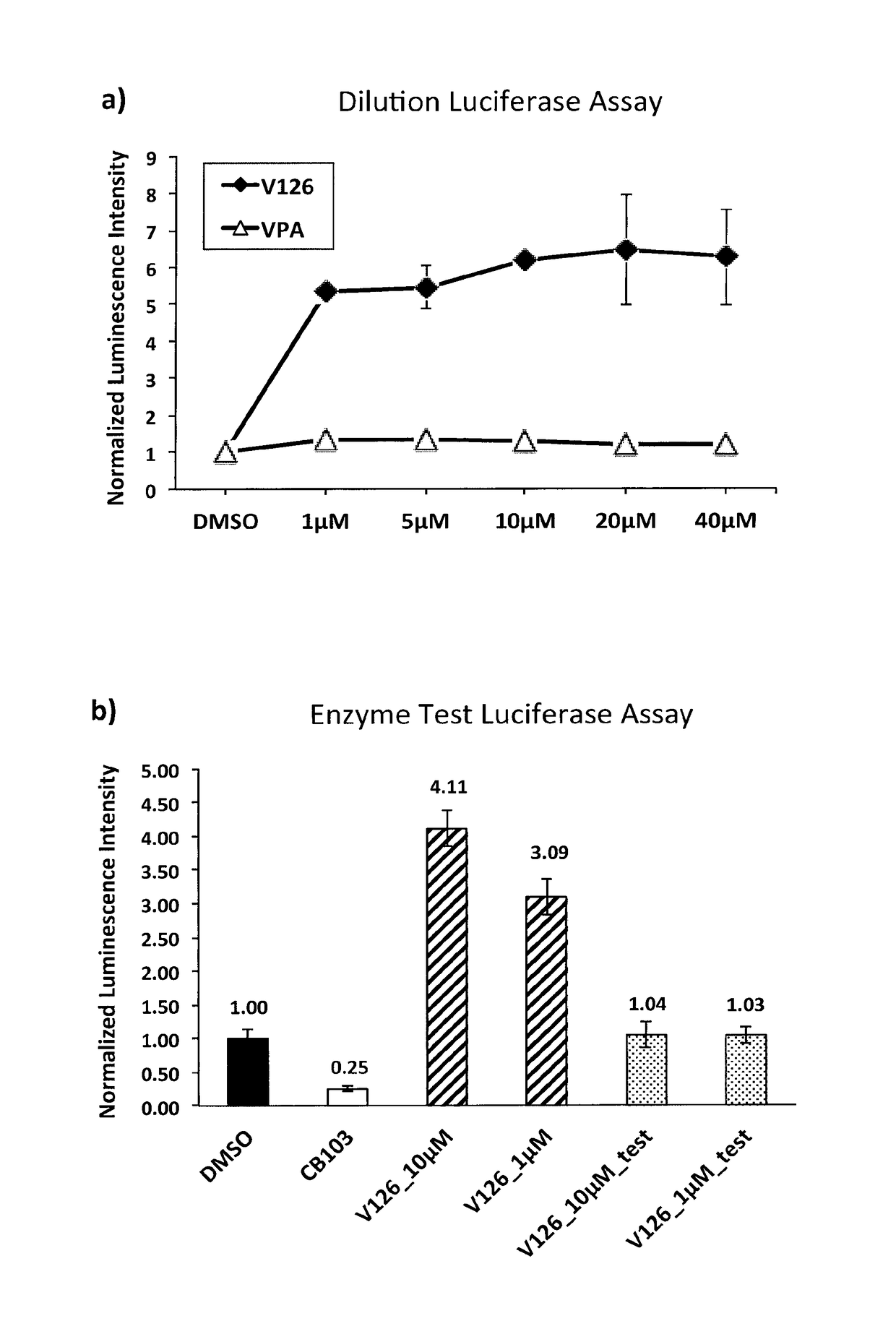
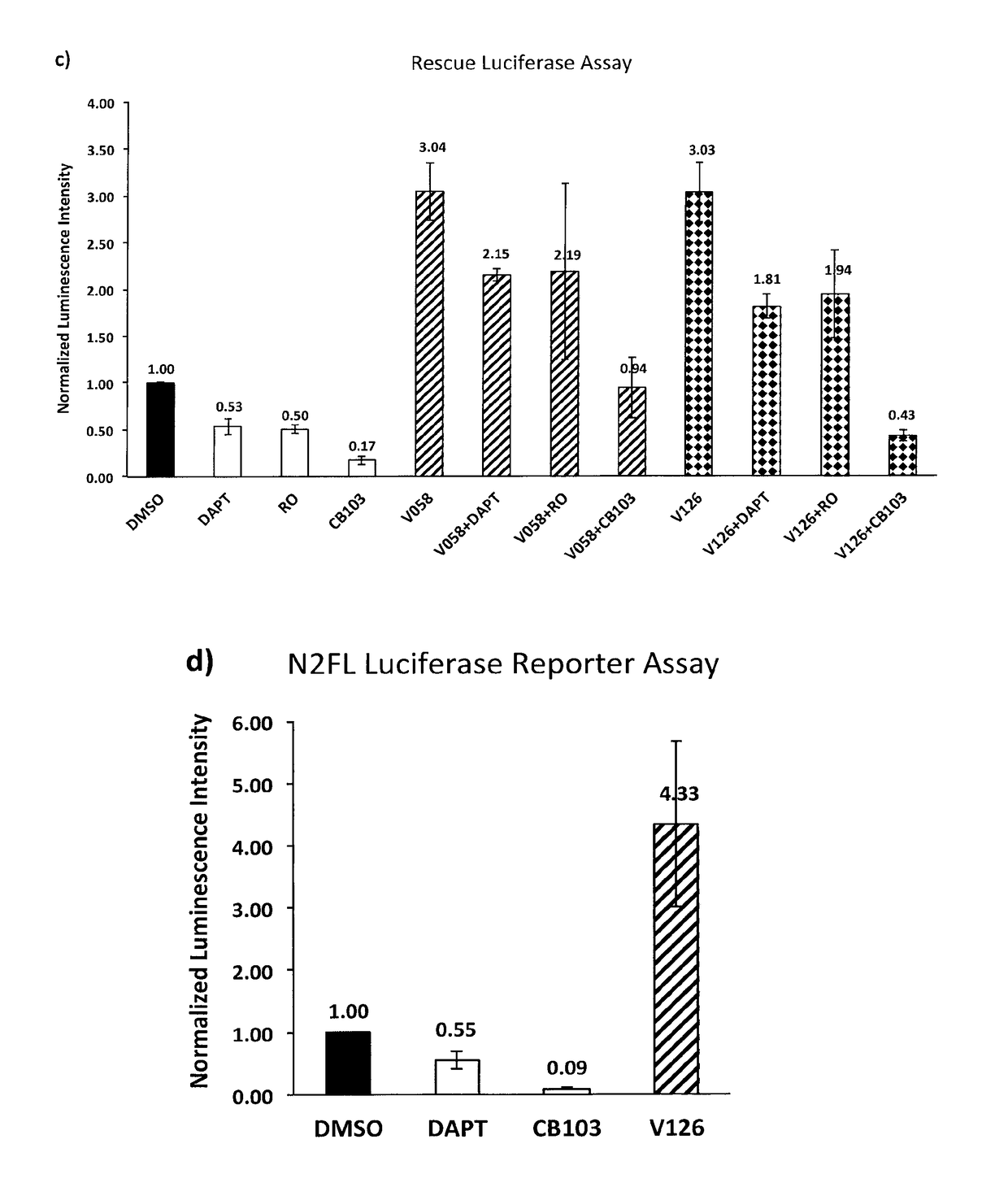
Enhancers of Notch signaling and the use thereof in the treatment of cancers and malignancies medicable by upregulation of Notch
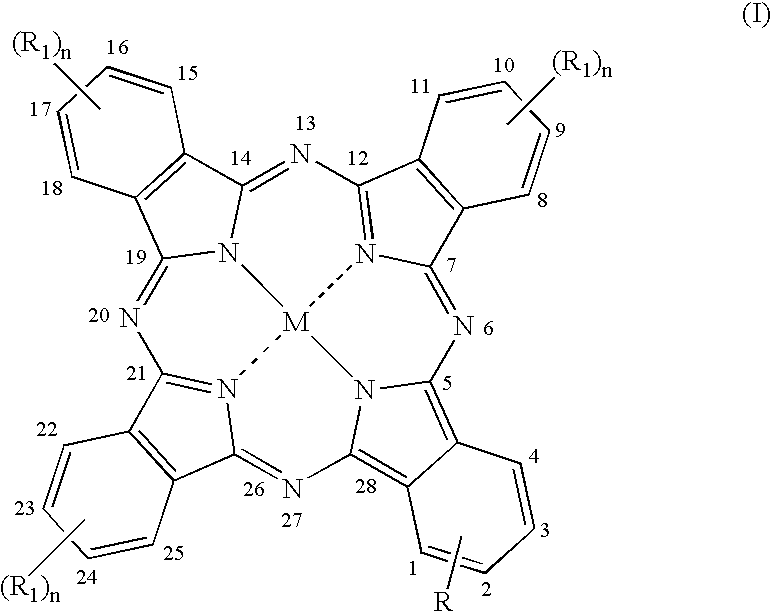
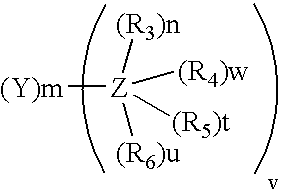
The present invention relates to the use for enhancing Notch signaling in an individual, of a compound showing the general formula (I) and / or a pharmaceutically acceptable salt or ester thereof, for the treatment of a disease selected from the group of dermatological disorders including atopic dermatitis, psoriasis, immune related disorders, cancer, squamous cell carcinoma, cutaneous and lung squamous cell carcinoma, head and neck cancer, non-melanoma skin cancer, basal cell carcinoma and actinic keratosis, neuroendocrine tumors, neuroendocrine small cell carcinoma and carcinoid tumors, thyroid carcinomas, muscular disorders muscular dystrophy and impaired regeneration capacity after injury; use in immunotherapy for cancer.
Owner:XENIOPRO GMBH
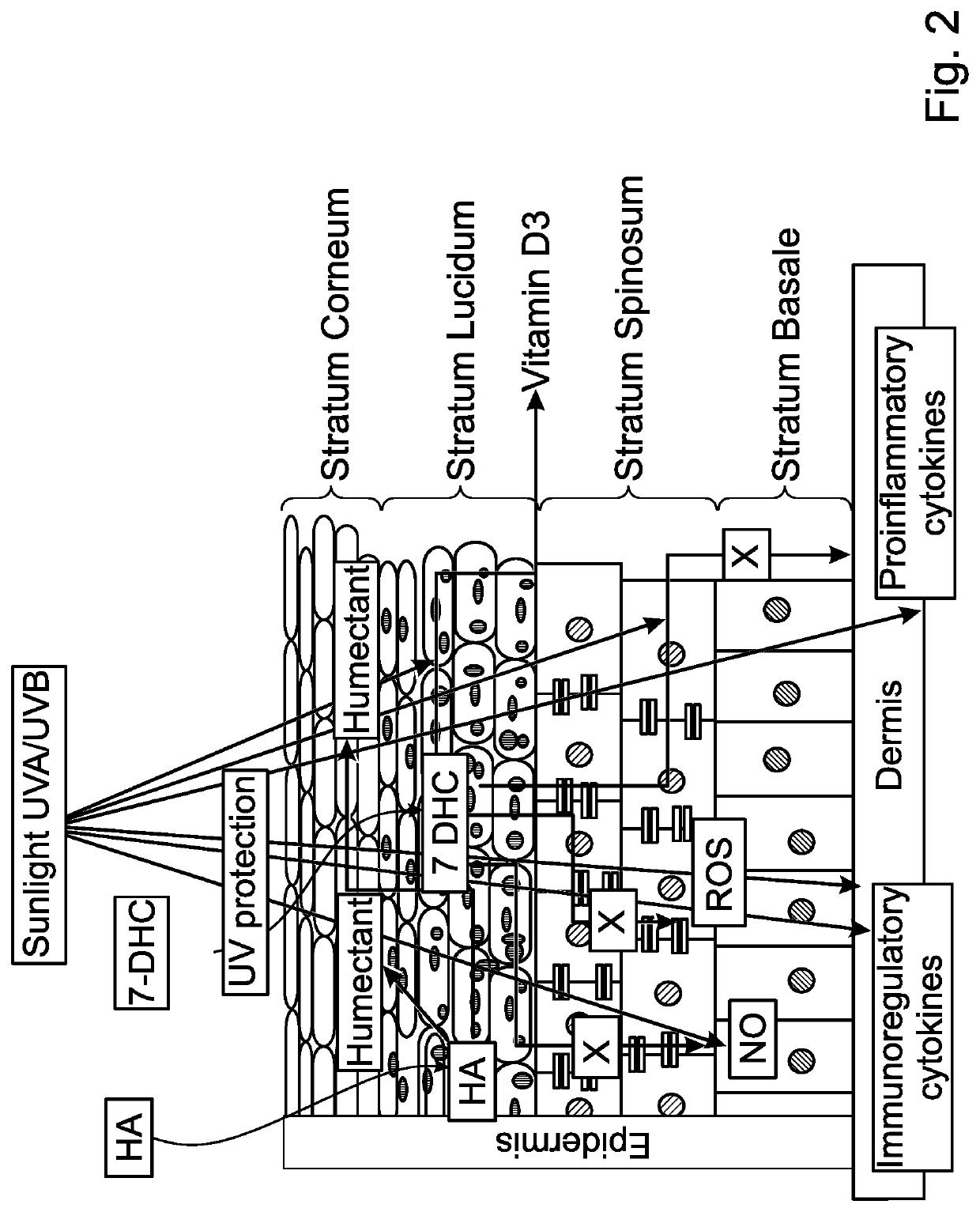
Pharmaceutical compositions
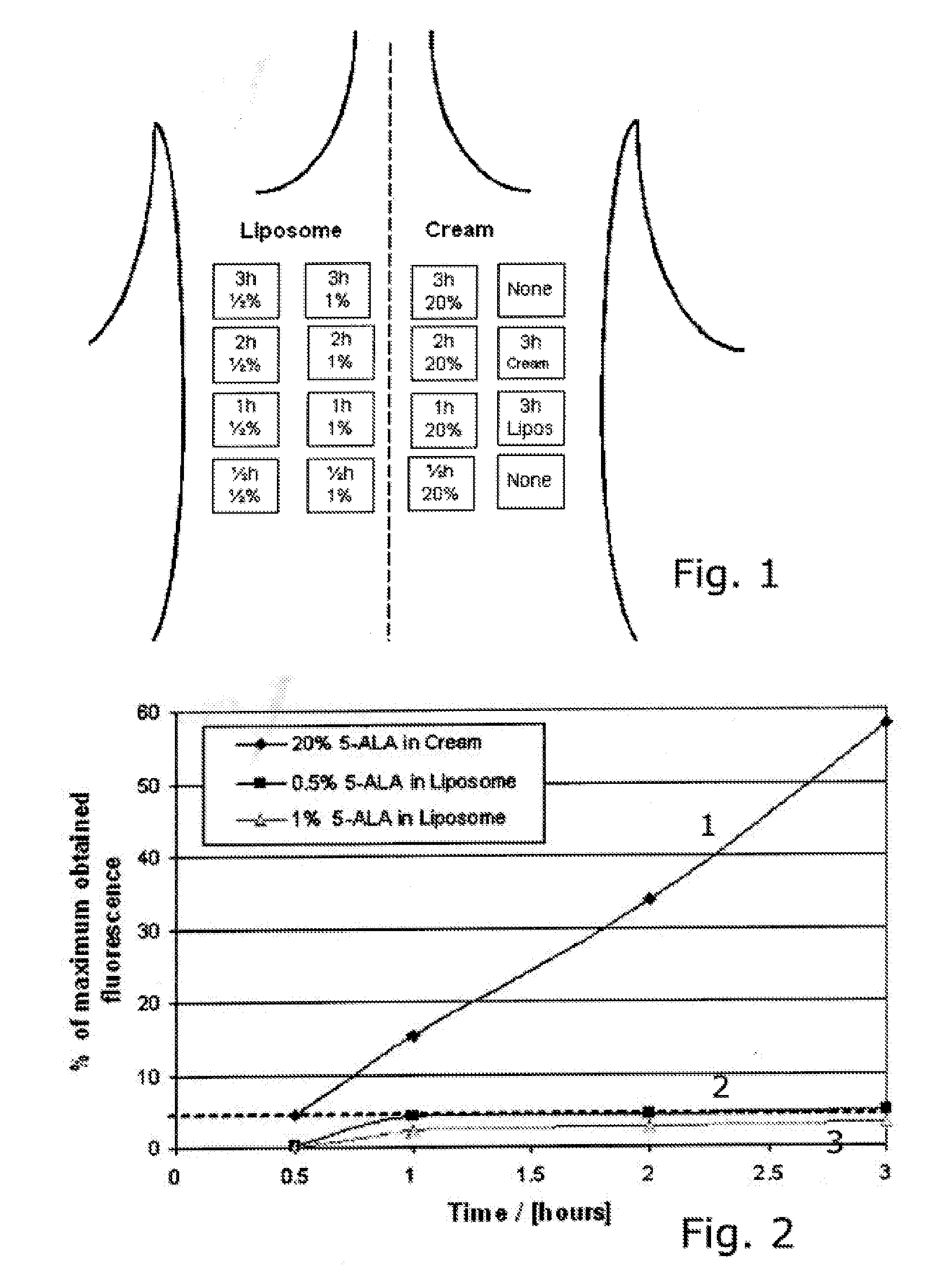
InactiveUS20200046627A1Improve skin penetrationImprove stabilityCosmetic preparationsHydroxy compound active ingredientsActinic keratosesLiposome
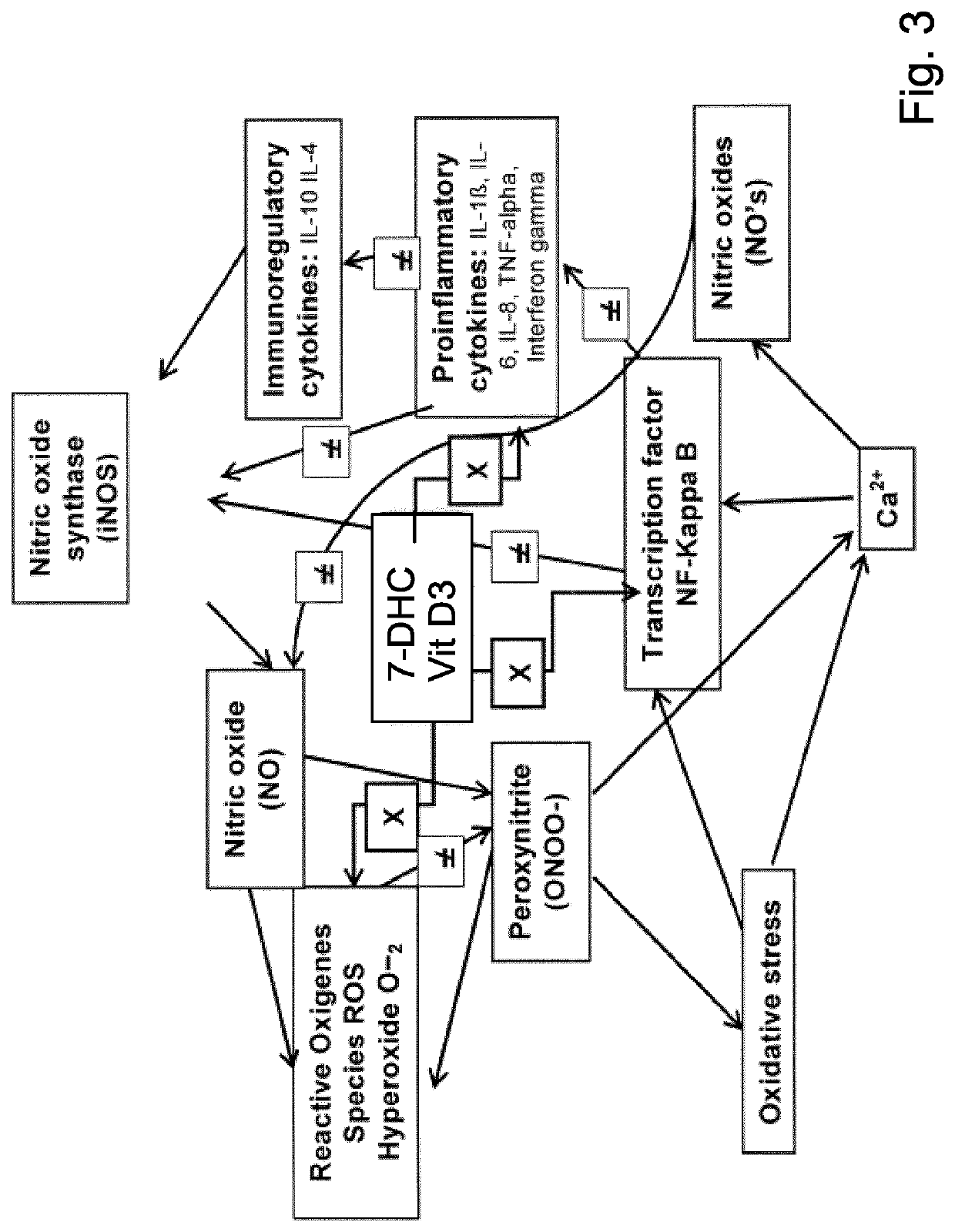
A composition including a synergistic combination of a vitamin D3 or a derivative or precursor thereof and hyaluronic acid or derivate thereof encapsulated in a lipid based colloidal carrier system (preferably lipid based vesicles such as liposomes, niosomes, tranferosomes) and topical formulations thereof, as well as their use in the prevention and / or treatment of inflamed skin and mucous membrane, especially in the prevention and / or treatment of skin photodamage, in particular in the prevention and / or treatment of skin erythema (skin inflammation) and actinic keratosis, as well non melanoma skin cancer.
Owner:JOYDERMA AG
Use of jasmonate ester derivatives for treating benign hyperproliferative skin disorders
InactiveUS20120083529A1Good effectInduce inhibitionOrganic active ingredientsBiocideDiseaseActinic keratoses
The present invention relates to methods of treating benign hyperproliferative diseases of the epidermis by administering a composition comprising at least one jasmonate ester derivative, preferably methyl jasmonate. In particular, the present invention provides jasmonate ester derivatives as potent compounds useful for the treatment of disorders such as actinic keratoses with reduced side effects.
Owner:RAMOT AT TEL AVIV UNIV LTD
Space, luminous ceiling system and method for conducting photodynamic therapy
A space for conducting photodynamic therapy (PDT) for treatment of skin diseases, especially for treatment of superficial skin tumors such as actinic keratoses, basal cell carcinomas, and initial carcinomas, wherein an active ingredient is applied to a skin area to be treated in order to form porphyrins and irradiation with light is conducted to form singlet oxygen based on the porphyrins in order to destroy diseased skin cells, wherein the space has side walls, a ceiling, and a floor, as well as at least two lighting fixtures, which are separated from one another by at least 50 cm and illuminate at least a subregion of the space, wherein there is an illuminance of at least 8,000 lux at every point in the subregion and the subregion has horizontal dimensions of at least 1 m2 and a vertical height of at least 40 cm. This invention also relates to a luminous ceiling system and method for conducting PDT.
Owner:SWISS RED
Novel pyropheophorbide a derivative, preparation method thereof and use thereof
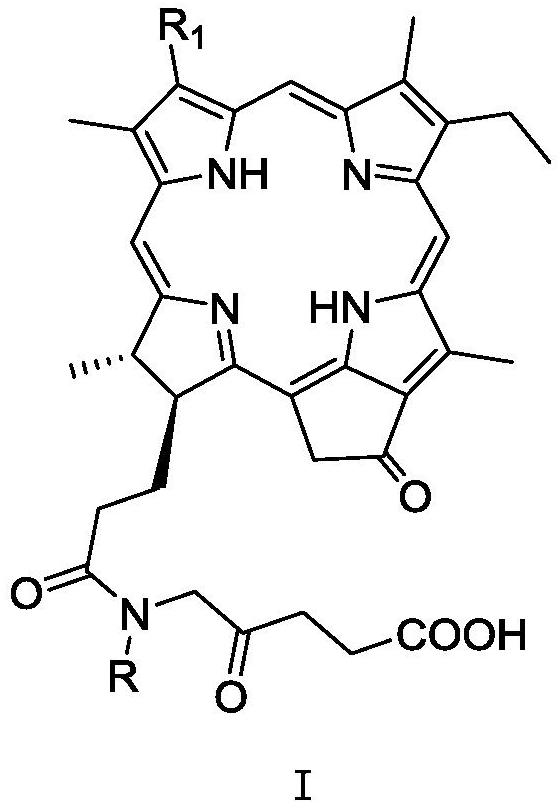
ActiveCN109265465AChemically stableSingle structureSenses disorderOrganic chemistryDiseaseActinic keratosis
The invention relates to a novel pyropheophorbide a derivative, a preparation method thereof and medical use. More specifically, the invention relates to a compound with a formula I as shown below, which is defined by the specification. The invention designs and prepares the pyropheophorbide a derivative which is single and stable in structure and simple and convenient in preparation method. The pyropheophorbide a derivative has very strong photodynamic activity, and can be used as a photodynamic drug for diagnosis and treatment of diseases such as tumor, macular degeneration, actinic keratosis, nevus flammeus and condyloma acuminata.
Owner:陈志龙
Surfactants for the treatment of conditions through targeted necrosis
InactiveUS20200188328A1Improve securityConvenient treatmentAerosol deliverySulfur/selenium/tellurium active ingredientsActinic keratosesActive agent
The present invention relates to a pharmaceutical composition for use in the treatment of pre-cancerous conditions of the cervix / anogenital region, non-melanoma skin cancers (NMSCs), or actinic keratosis wherein said composition comprises a surfactant, preferably an anionic or amphoteric surfactant.
Owner:IMUNEKS FARMA ILAC SAN VE TIC AS
Composition for dressing cutaneous lesions and manufacturing method thereof
Owner:BECKER DIETER DR
Compositions and methods for treating or preventing dermal disorders
ActiveUS10695326B2Treating or preventing an age-related dermal disorderExtend your lifeOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsActinic keratosesDisease
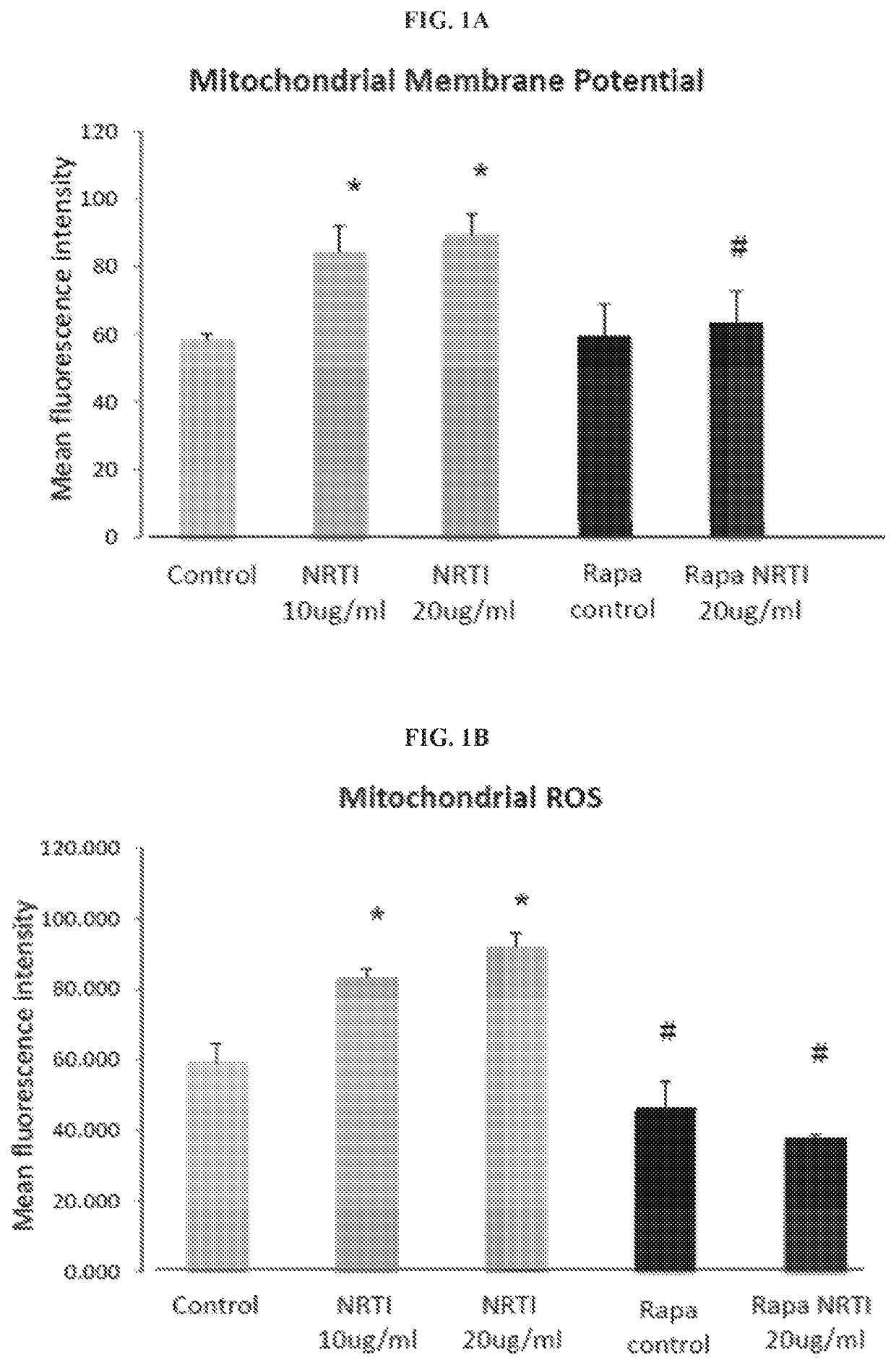
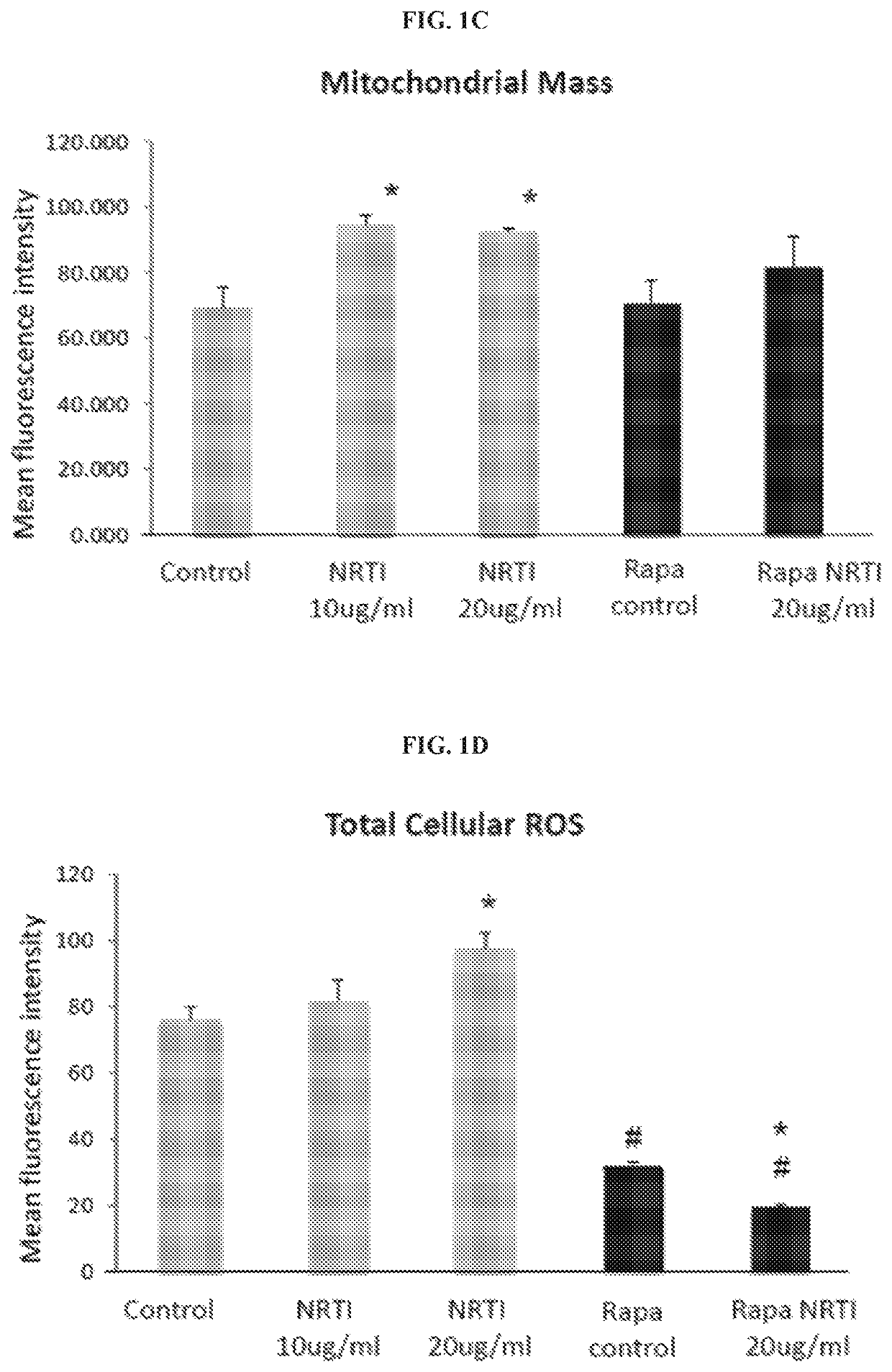
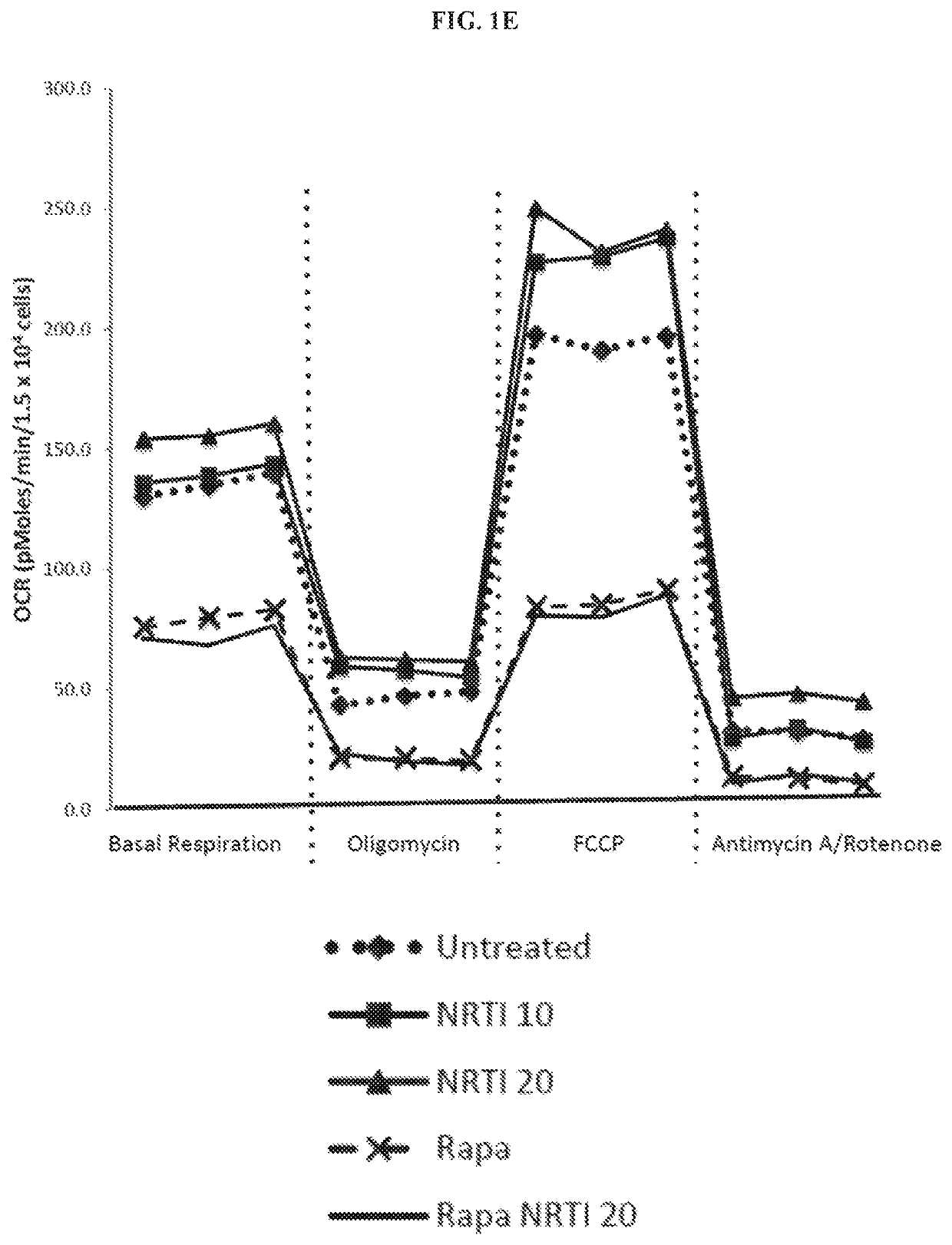
The present invention includes compositions and methods for treating or preventing certain dermal disorders including dermal atrophy, pseudoscars, actinic keratosis, seborrheic or actinic keratoses, lentigines, focal areas of dermal thickening, and coarse wrinkles. In certain embodiments, the compositions useful within the invention comprise a therapeutically effective amount of a mTORC1 inhibitor and a dermatologically acceptable carrier.
Owner:DREXEL UNIV
A Novel Pyropheophorbide A Derivative and Its Preparation Method and Application
ActiveCN109265465BChemically stableSingle structureSenses disorderOrganic chemistryActinic keratosesDisease
The invention relates to a class of novel pyropheophorbide a derivatives, a preparation method and a medical application thereof. More specifically, the present invention relates to the compound of formula I shown in the following structure, whose definition is described in the description. The present invention designs and prepares a class of pyropheophorbide a derivatives with single structure, stability and convenient preparation method, and has strong photodynamic activity, and can be used as a diagnosing and treating tumor, retinal macular degeneration, actinic keratin Photodynamic drugs for cancer, port wine stains, condyloma acuminatum and other diseases.
Owner:陈志龙
Treatments for actinic keratoses
InactiveUS8741857B1Effective treatmentPromote resultsBiocideOintment deliveryCream baseActinic keratoses
A composition of gentamicin and colchicine in a dermatologically acceptable cream base provided improved clearance of actinic keratoses following topical application for 1 to 3 months. The composition provides improved clearance of recalcitrant actinic keratoses. Furthermore, the treatment causes less pain and irritation than other topical treatments for actinic keratoses.
Owner:MOY LAWRENCE
Treatment of skin disorders with topical tapinalov-EGFR inhibitor compositions
Disclosed are topical compositions and methods of treating a skin or mucosal disease selected from the group consisting of palmoplantar psoriasis, hereditary palmoplantar keratosis, hereditary palmoplantar keratosis, or the like by topically administering to a subject a composition comprising a therapeutically effective amount of tapinalov, at least one EGFR inhibitor, or a combination of tapinalov-EGFR inhibitor as an active agent, once or twice a day. Acquired palmoplantar keratosis, purulent edema, dermatitis, ichthyosis vulgaris, hereditary ichthyosis, acquired ichthyosis, actinic keratosis, keratodermatosis, keratomucosal disease, golin syndrome, psoriasis on nails, psoriasis curvilinear / reverse type, non-melanoma skin cancer and precancerous skin, mucosal and nail lesions.
Owner:SOL GEL TECH
Method for Treating Cancerous and Pre-Cancerous Skin
ActiveUS20220001201A1Avoid developmentReduces pre-clinical skin damageOrganic active ingredientsImage enhancementActinic keratosesSquamous Carcinomas
The present disclosure provides a method for treating clinical or pre-clinical skin damage in a skin field of a subject, wherein the skin field has been allocated a skin cancerization field index (SCFI) score of at least 1 as determined by a process comprising the steps of: (i) assessing the number of keratoses in the skin field; (ii) assessing the thickness of the thickest keratosis in the skin field; and (iii) assessing the proportion of the field affected by clinical or subclinical skin damage. Based on the assessments made in (i), (ii) and (iii) the subject is optionally treated by at least one of (a) freezing one or more lesions, (b) shaving, curetting or surgically removing one or more lesions, (c) applying a topical treatment for actinic keratosis, basal cell carcinoma or squamous cell carcinoma, and (d) radiation therapy.
Owner:GENESISCARE VENTURES PTY LTD
Enhancers of notch signaling and the use thereof in the treatment of cancers and malignancies medicable by upregulation of notch
The present invention relates to the use for enhancing Notch signaling in an individual, of a compound showing the general formula (I) and / or a pharmaceutically acceptable salt or ester thereof, for the treatment of a disease selected from the group of dermatological disorders including atopic dermatitis, psoriasis, immune related disorders, cancer, squamous cell carcinoma, cutaneous and lung squamous cell carcinoma, head and neck cancer, non-melanoma skin cancer, basal cell carcinoma and actinic keratosis, neuroendocrine tumors, neuroendocrine small cell carcinoma and carcinoid tumors, thyroid carcinomas, muscular disorders muscular dystrophy and impaired regeneration capacity after injury; use in immunotherapy for cancer.
Owner:XENIOPRO GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com