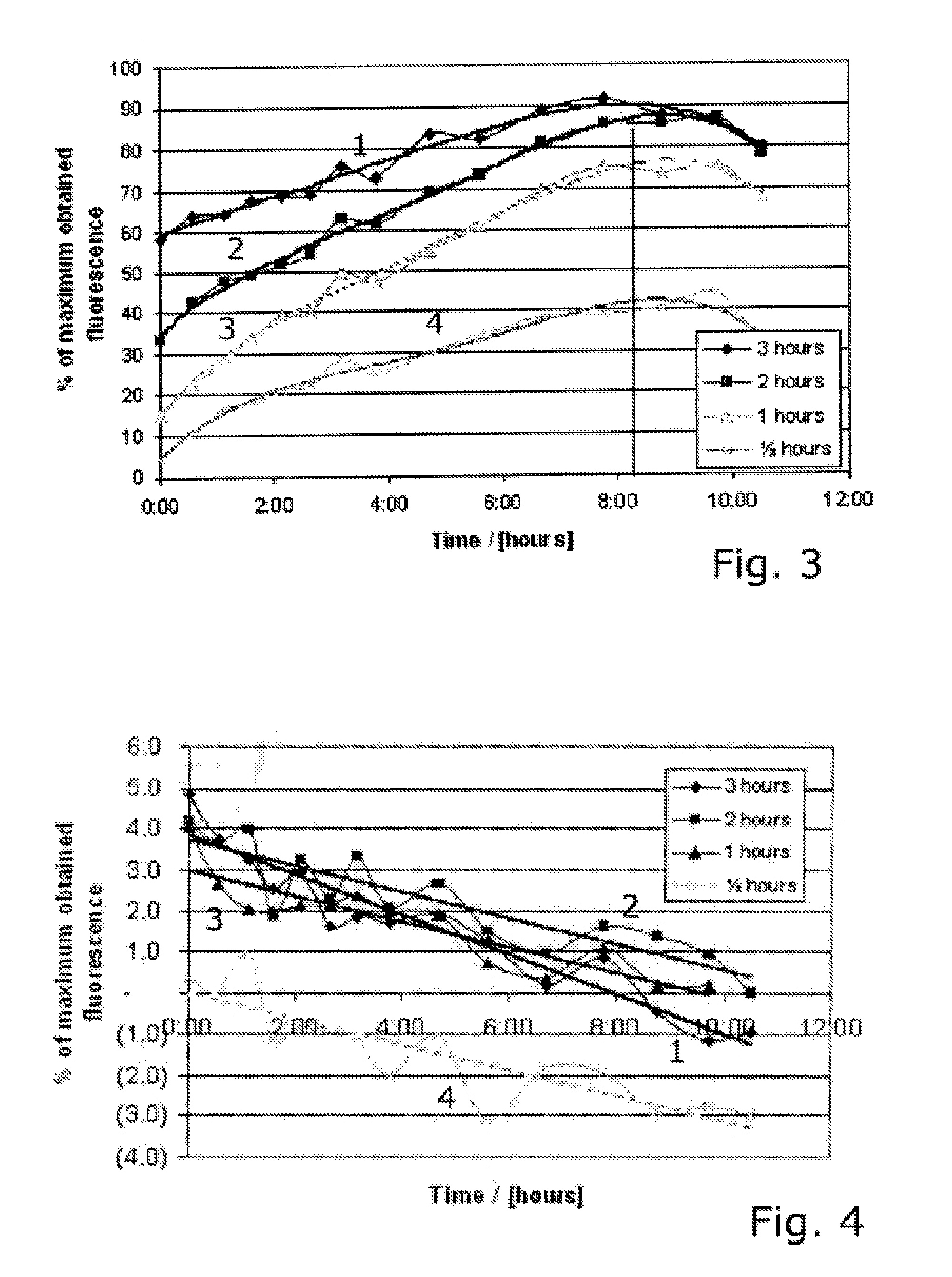
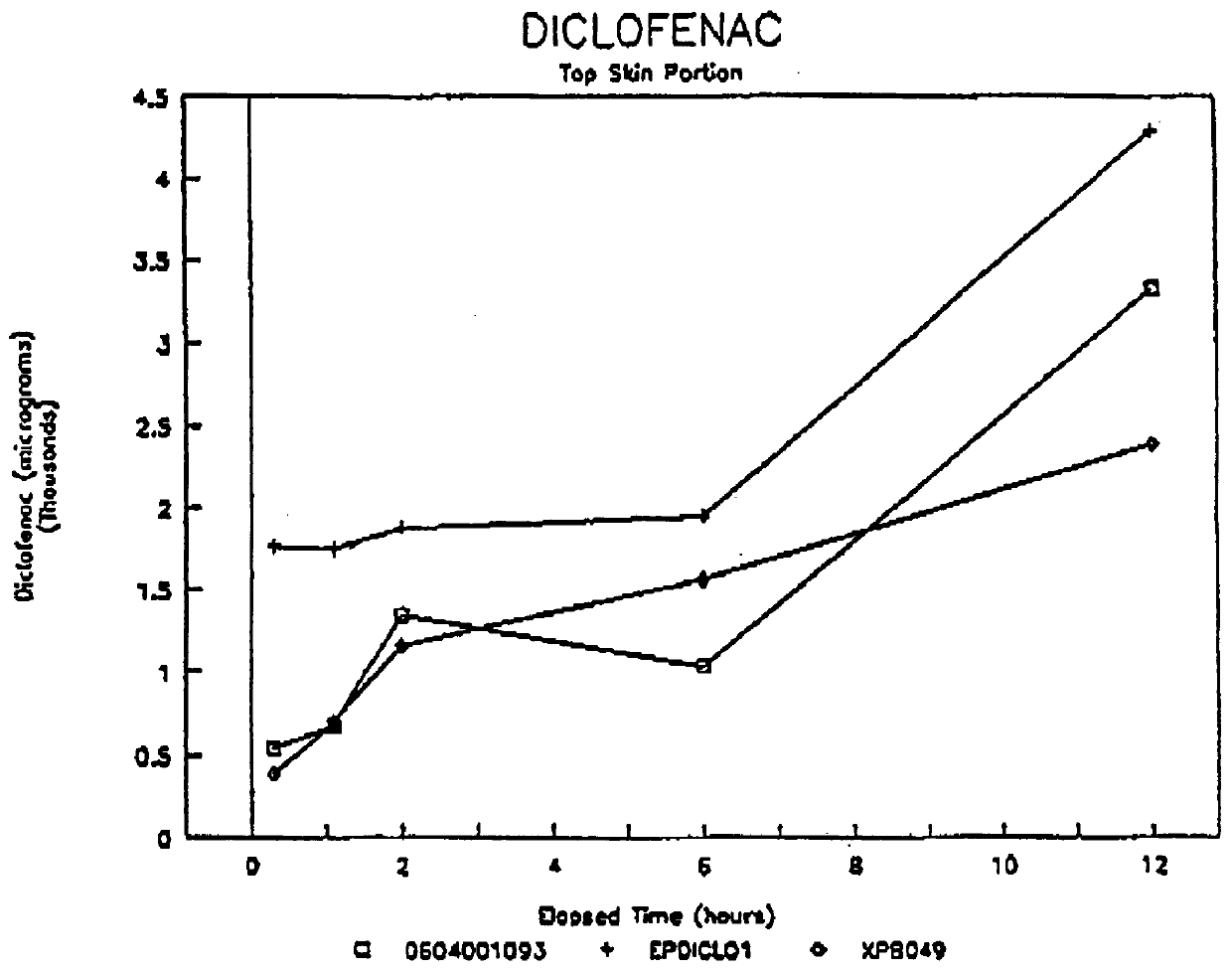
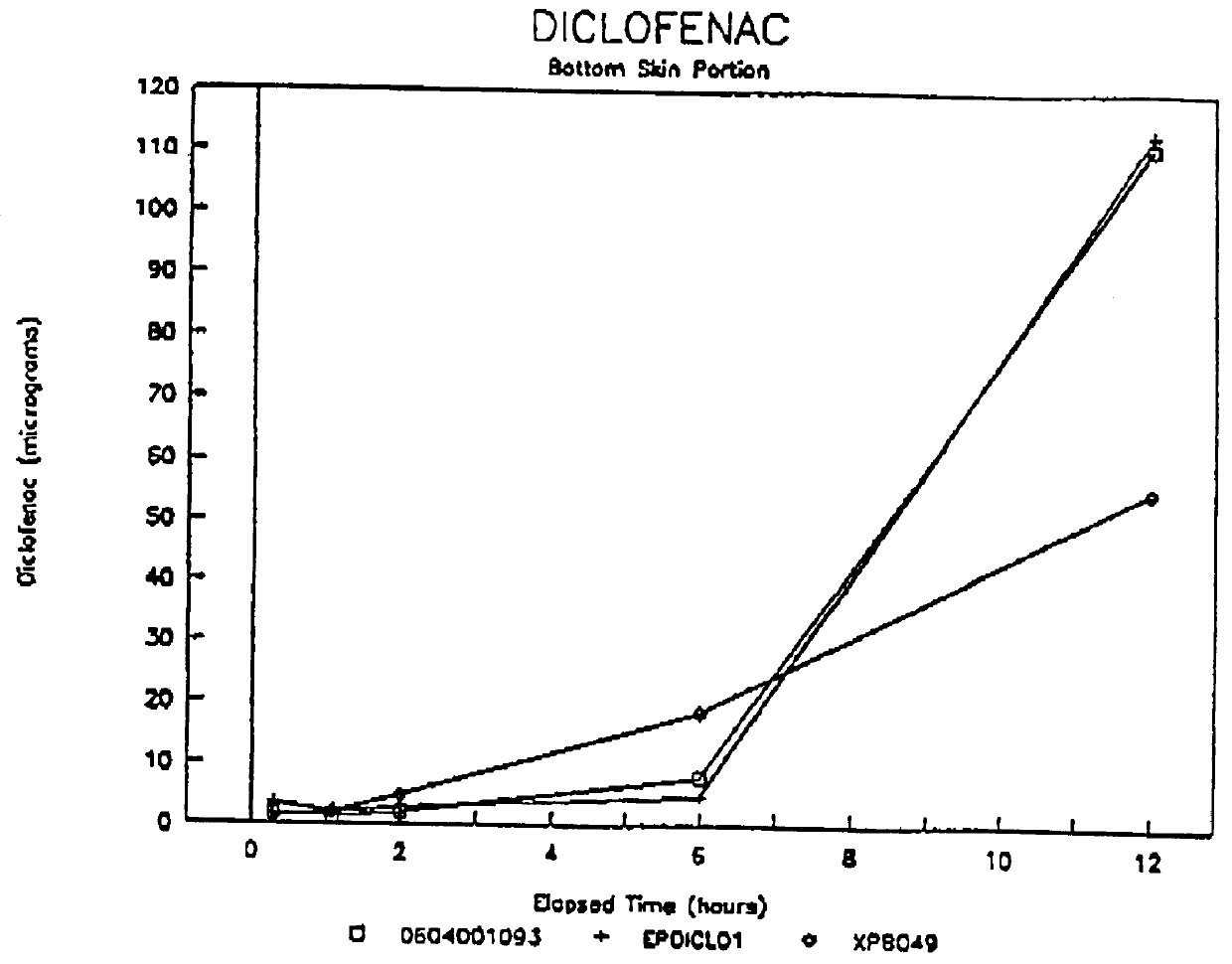
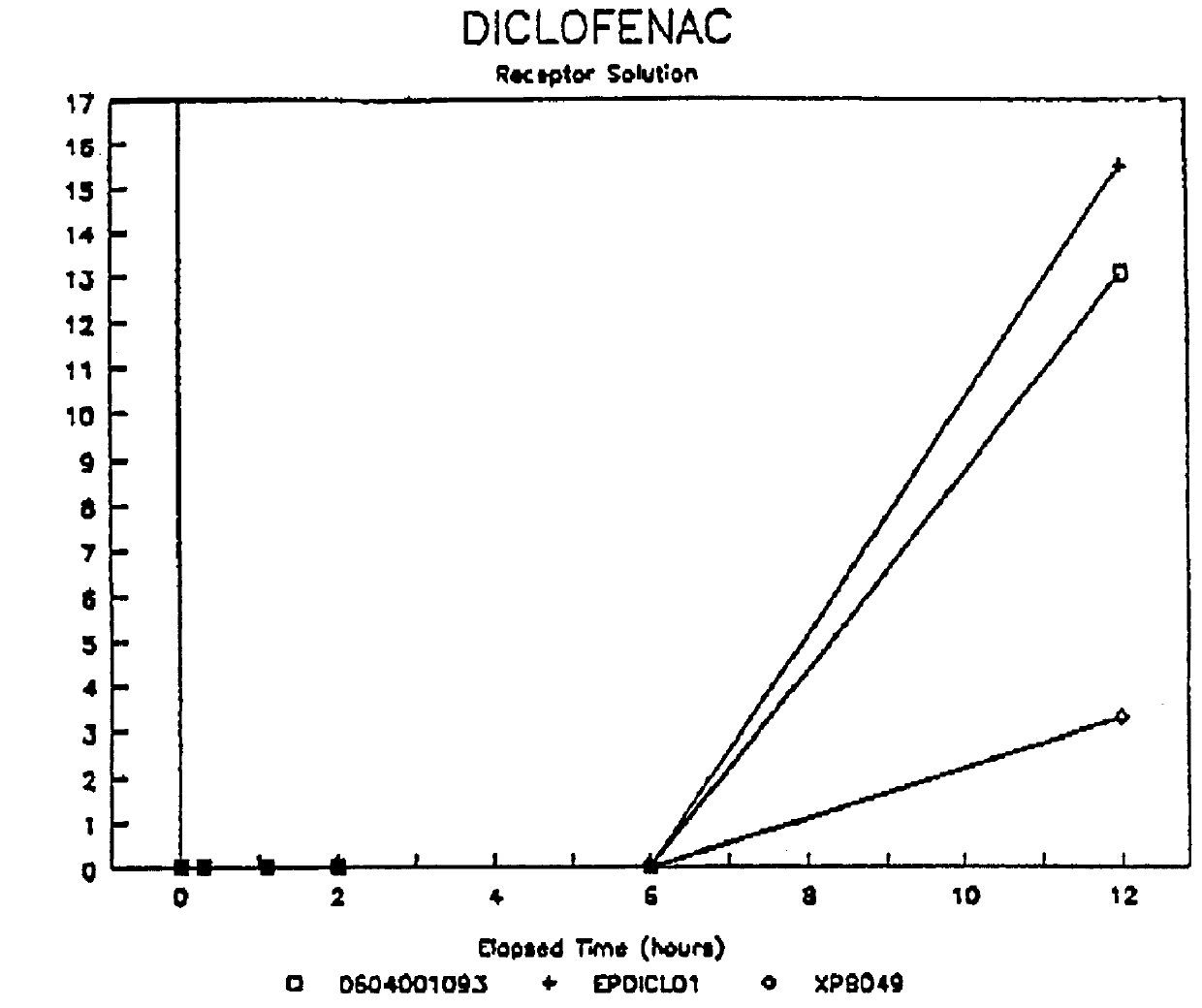
Patents
Literature
105 results about "Basal cell carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A type of skin cancer which develops in basal cells.
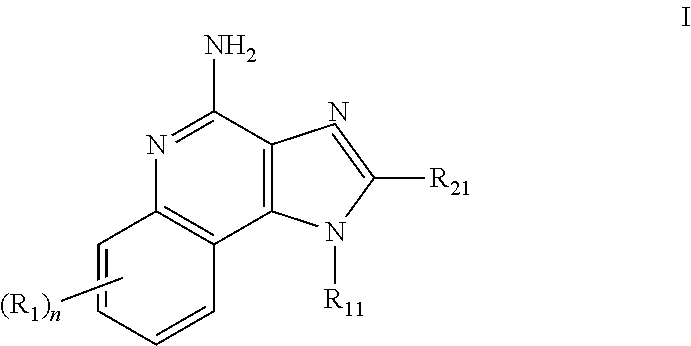
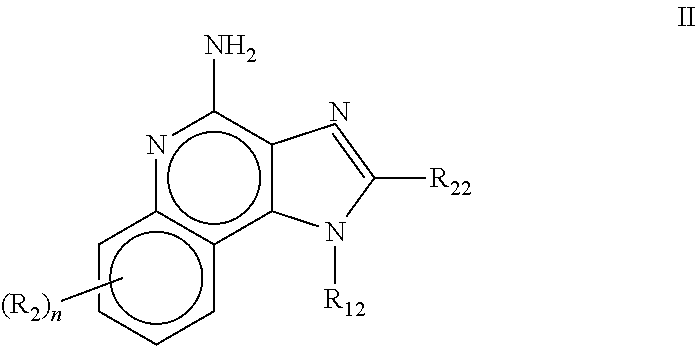
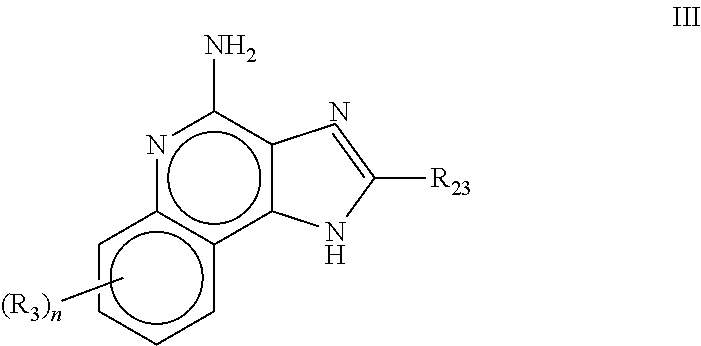
Pharmaceutical formulations comprising an immune response modifier
Pharmaceutical formulations comprising an immune response modifier (IRM) chosen from imidazoquinoline amines, imidazotetrahydroquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, thiazolo-quinolineamines, oxazolo-quinolinamines, thiazolo-pyridinamines, oxazolo-pyridinamines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, and thiazolonaphthyridine amines; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid are useful for the treatment of dermal associated conditions. Novel topical formulations are provided. In one embodiment, the topical formulations are advantageous for treatment of actinic keratosis, postsurgical scars, basal cell carcinoma, atopic dermatitis, and warts.
Owner:3M INNOVATIVE PROPERTIES CO
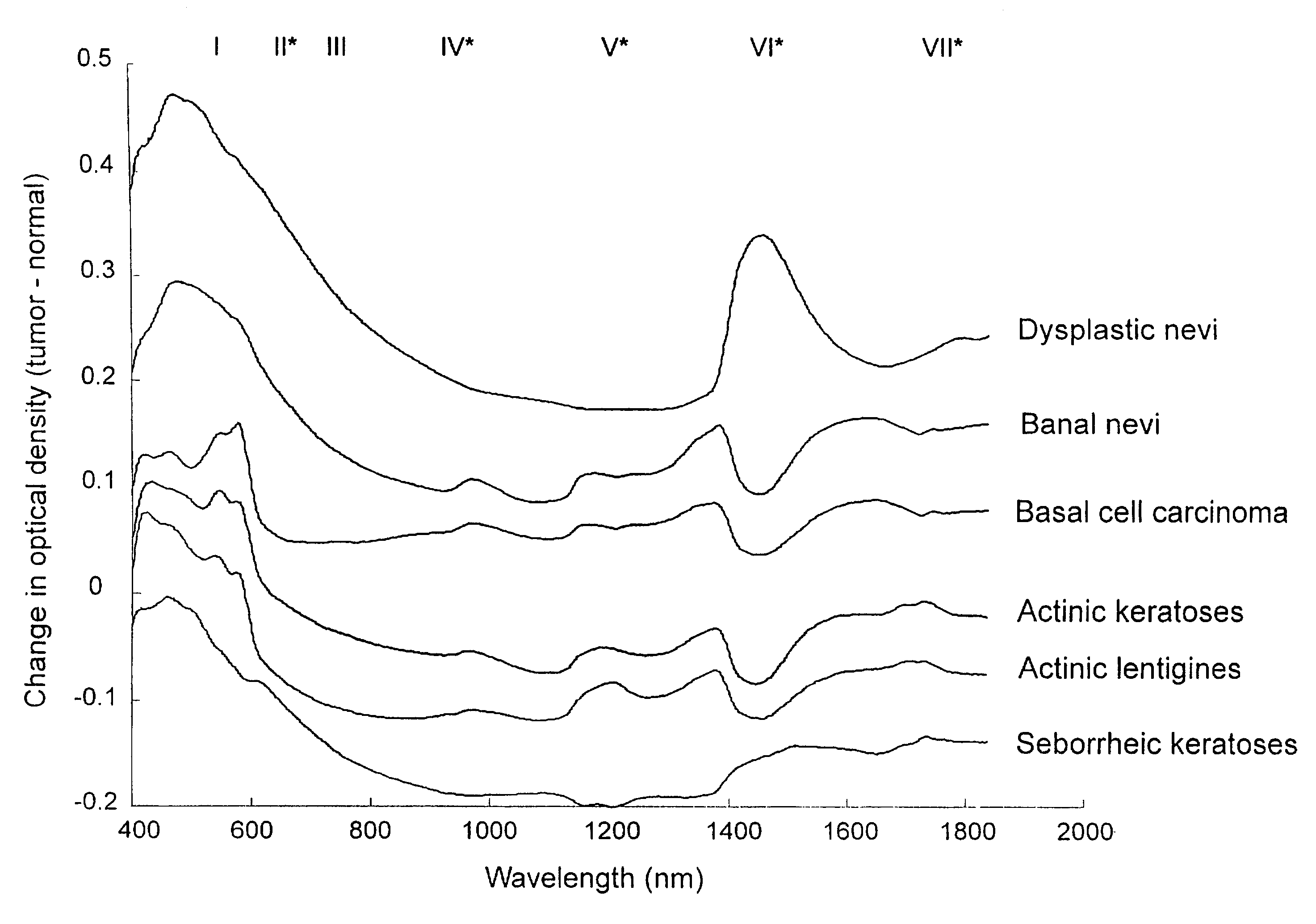
Non-invasive screening of skin diseases by visible/near-infrared spectroscopy
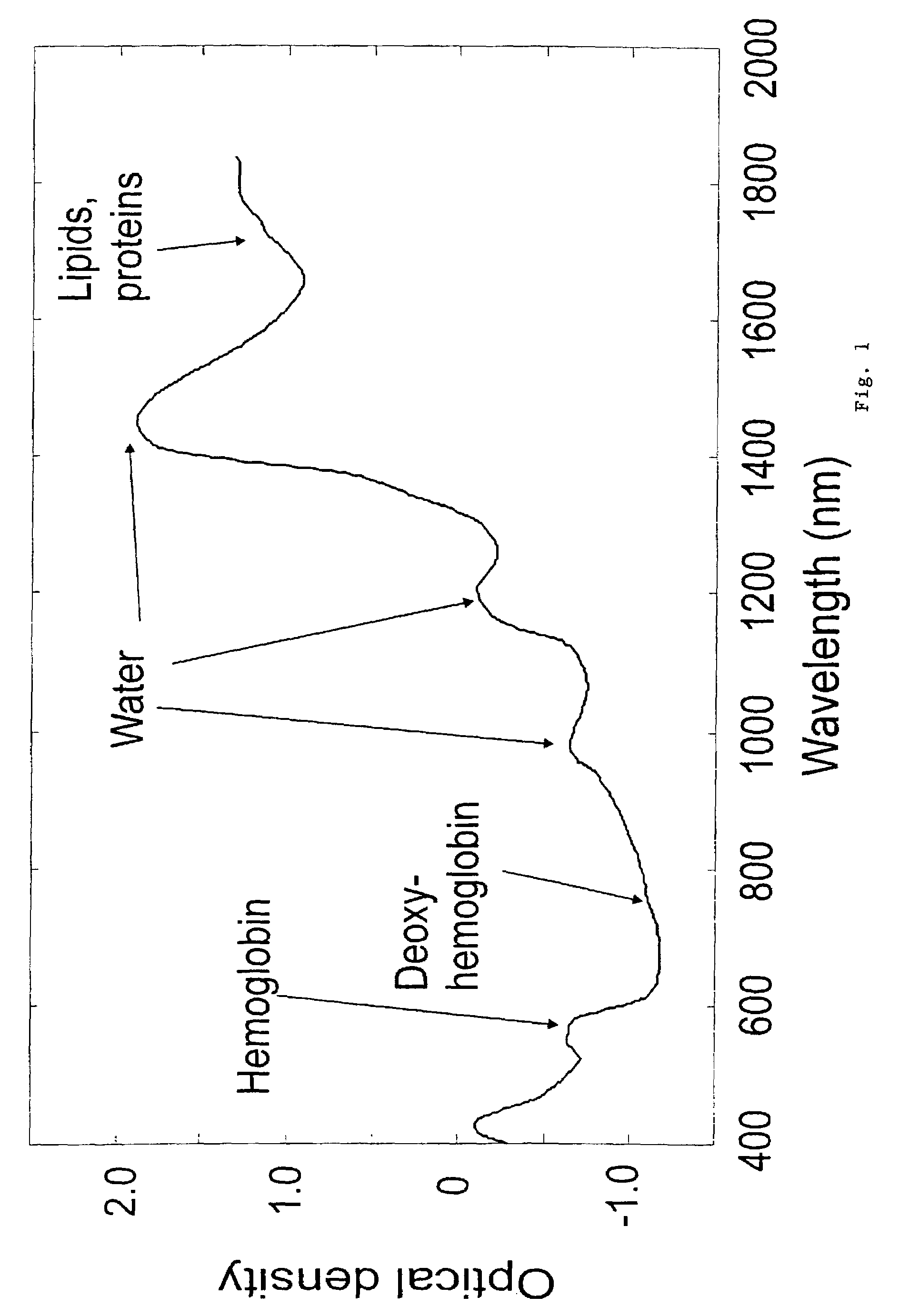
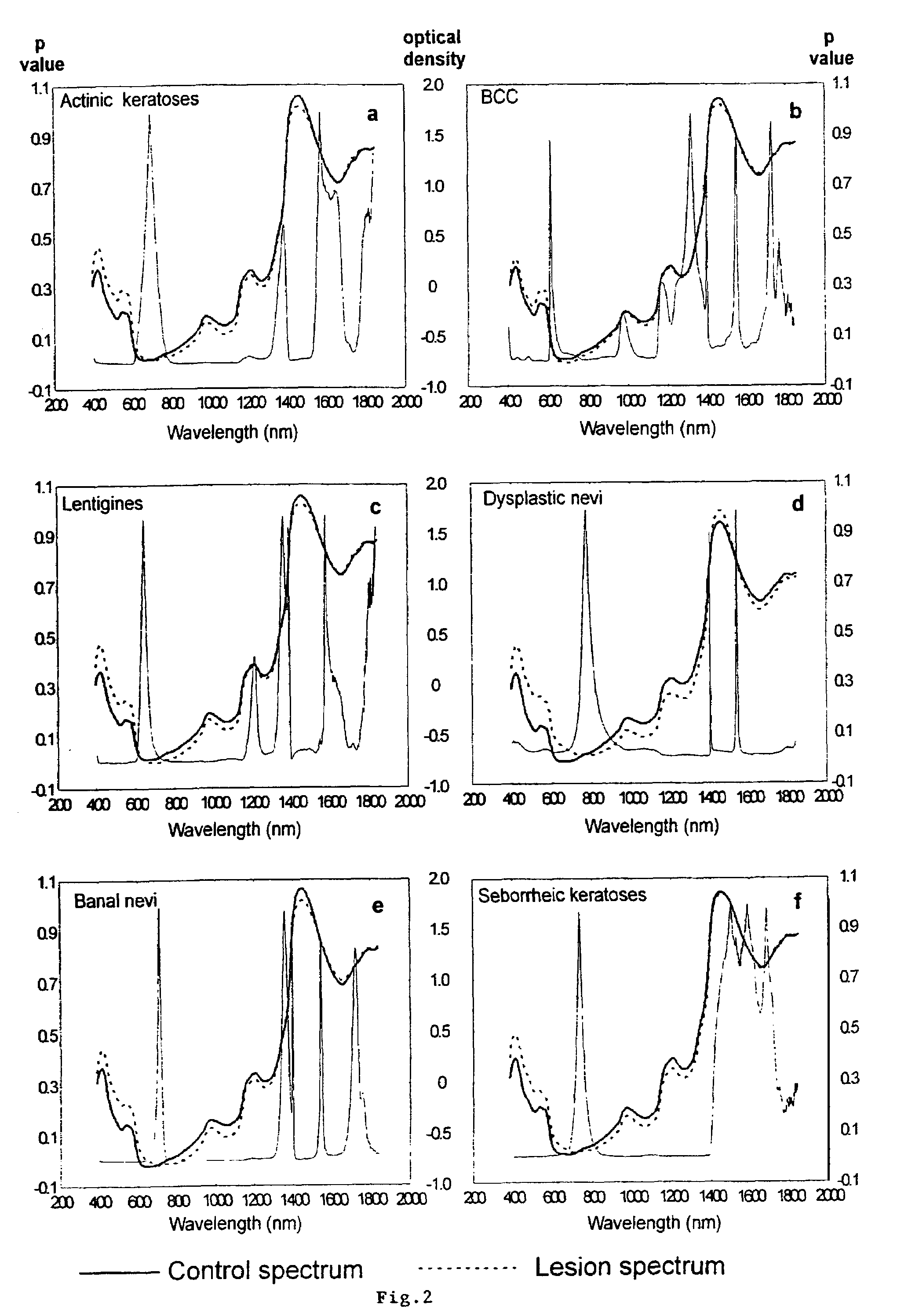
A non-invasive tool for skin disease diagnosis would be a useful clinical adjunct. The purpose of this study was to determine whether visible / near-infrared spectroscopy can be used to non-invasively characterize skin diseases. In-vivo visible- and near-infrared spectra (400-2500 nm) of skin neoplasms (actinic keratoses, basal cell carcinomata, banal common acquired melanocytic nevi, dysplastic melanocytic nevi, actinic lentigines and seborrheic keratoses) were collected by placing a fiber optic probe on the skin. Paired t-tests, repeated measures analysis of variance and linear discriminant analysis were used to determine whether significant spectral differences existed and whether spectra could be classified according to lesion type. Paired t-tests showed significant differences (p<0.05) between normal skin and skin lesions in several areas of the visible / near-infrared spectrum. In addition, significant differences were found between the lesion groups by analysis of variance. Linear discriminant analysis classified spectra from benign lesions compared to pre-malignant or malignant lesions with high accuracy. Visible / near-infrared spectroscopy is a promising non-invasive technique for the screening of skin diseases.
Owner:NAT RES COUNCIL OF CANADA
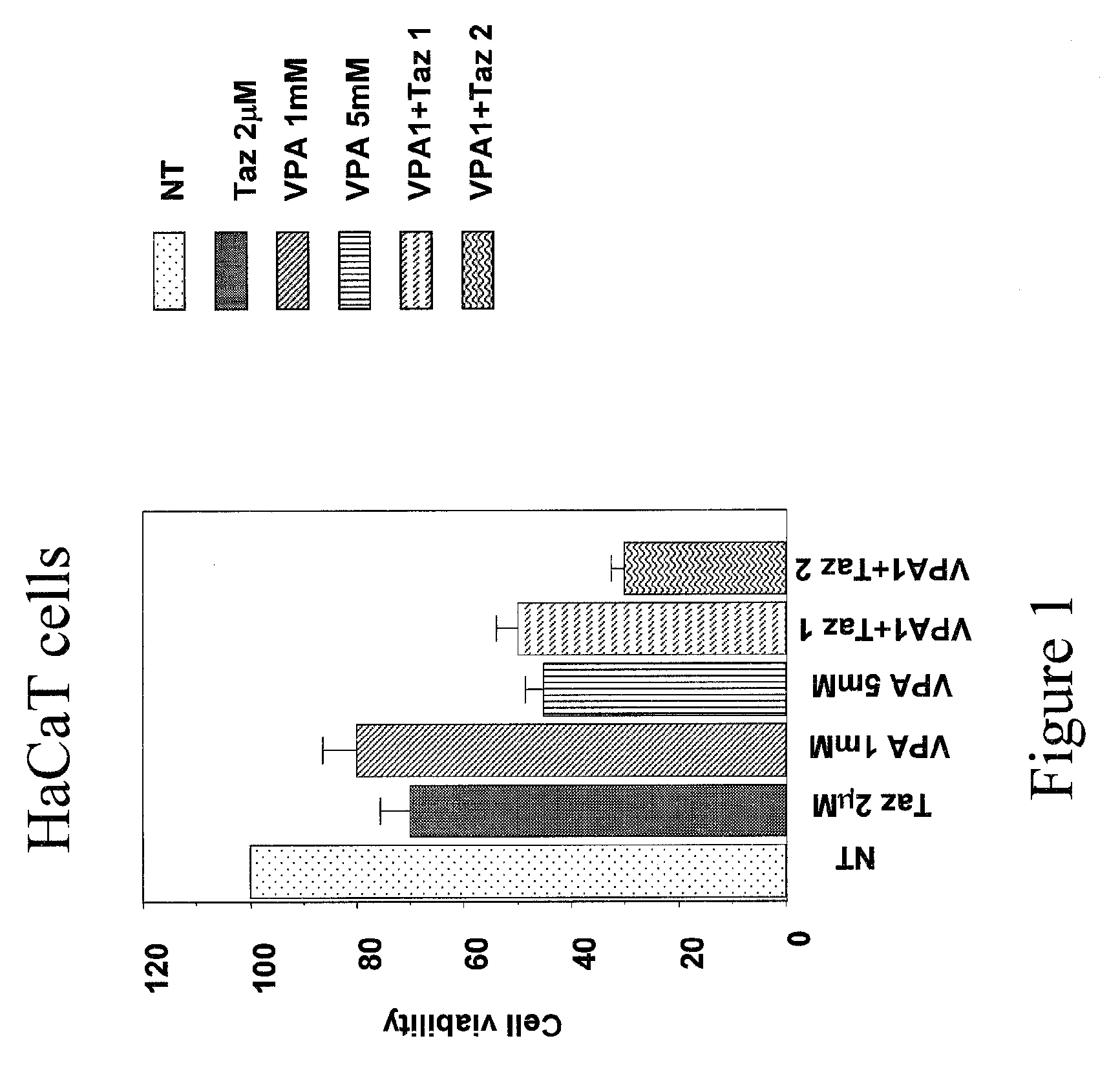
Topical Use of Valproic Acid for the Prevention or Treatment of Skin Disorders
The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell Lymphoma and also for the topical treatment of pre-malignant lesions, and of inflammations of the skin and / or mucosa. The invention also relates to the use of this topically applicable formulation for the protection from UV light and for the treatment of sun burn. The invention includes the use of VPA for the manufacture of a clinically used medicament for the topical treatment of the human diseases listed above.
Owner:TOPOTARGET GERMANY AG
Use of cyclopamine in the treatment of basal cell carcinoma and other tumors
InactiveUS7893078B2Avoid problemsUseful or even desirableCosmetic preparationsBiocideApoptosisRadical radiotherapy
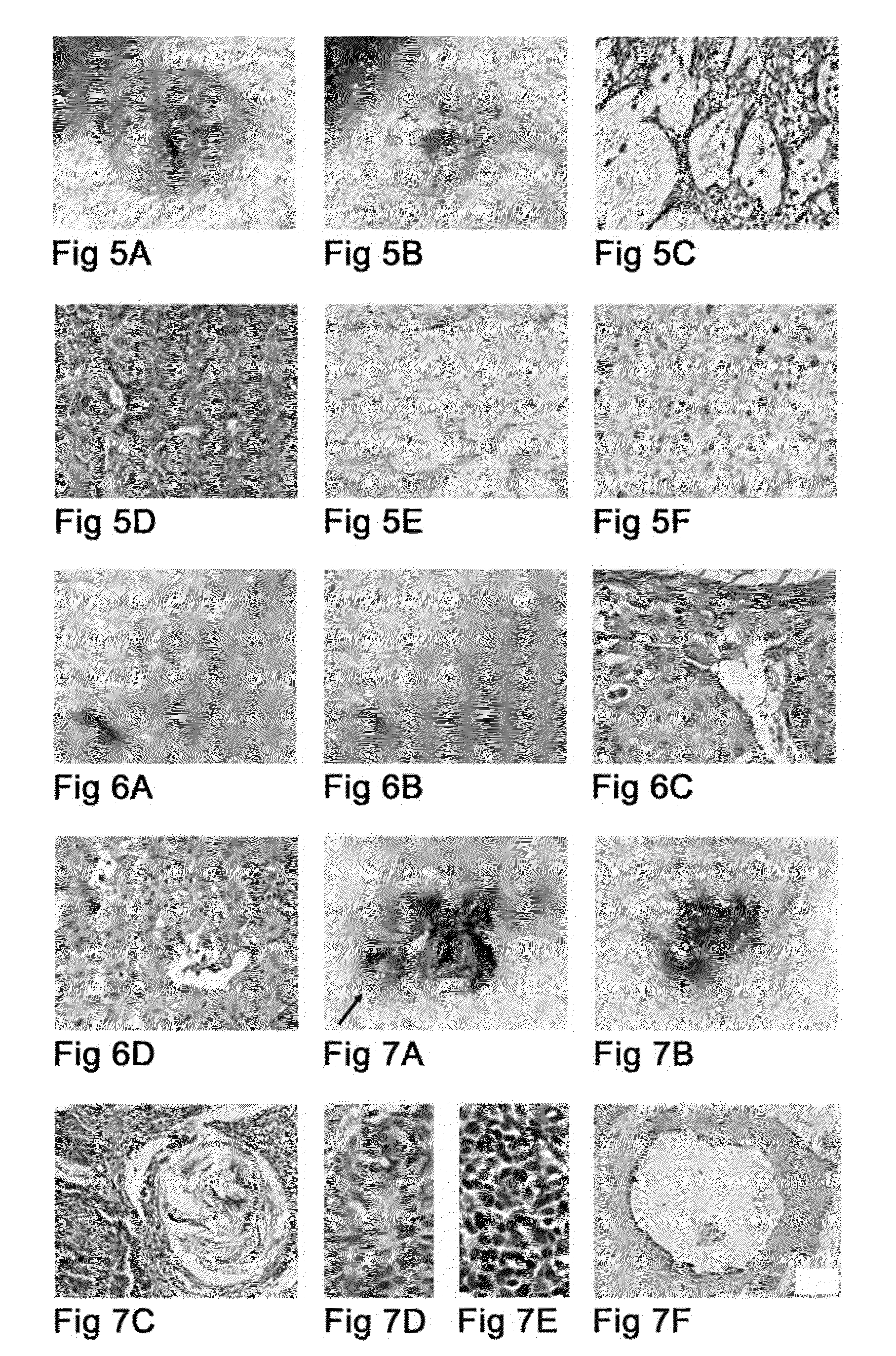
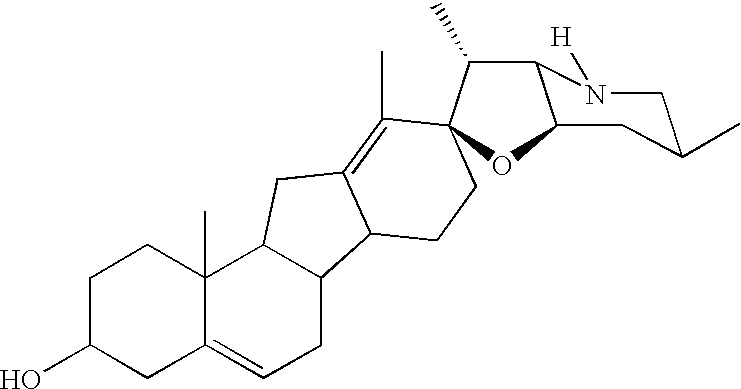
This invention concerns the use of cyclopamine in vivo on basal cell carcinomas (BCC's) to achieve therapeutic effect by causing differentiation of the tumor cells and, at the same time, apoptotic death and removal of these tumor cells while preserving the normal tissue cells, including the undifferentiated cells of the normal epidermal basal layer and hair follicles. Causation of apoptosis by cyclopamine is by a non-genotoxic mechanism and thus unlike the radiation therapy and most of the currently used cancer chemotherapeutics which act by causing DNA-damage. These novel effects, previously unachieved by a cancer chemotherapeutic, make the use of cyclopamine highly desirable in cancer therapy, in the treatment of BCC's and other tumors that use the hedgehog / smoothened signal transduction pathway for proliferation and prevention of apoptosis.
Owner:TAS SINAN
Method for non-therapeutic or therapeutic photodynamic skin treatment
InactiveUS20100298758A1Reduce inconvenienceEasy to practiceOrganic active ingredientsCosmetic preparationsActinic keratosesWrinkle skin
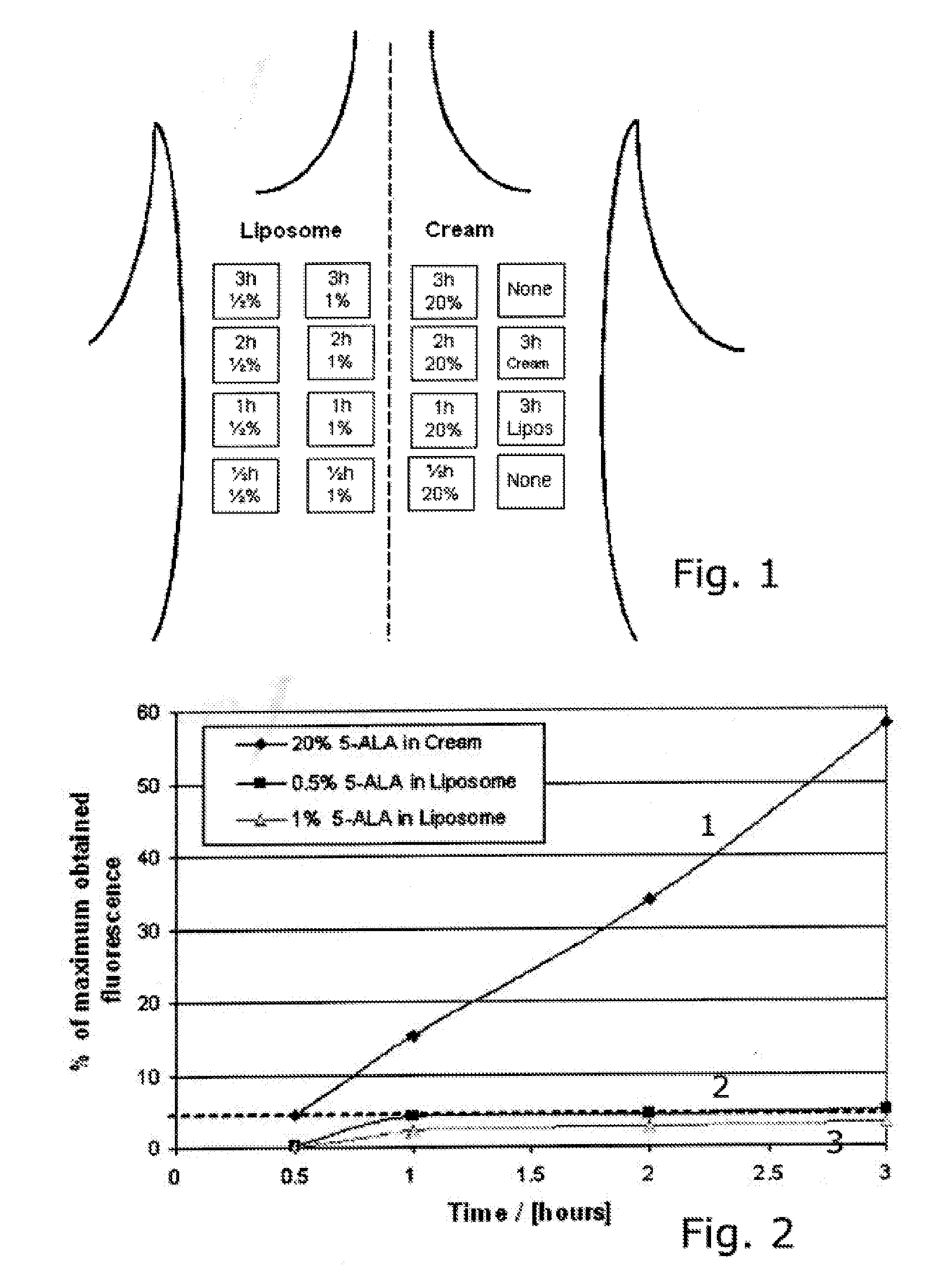
In a method for non-therapeutic or therapeutic photodynamic treatment of a skin target area, a photosensitizer fluid incorporating a photosensitizing agent or a precursor for a photosensitizing agent is delivered to the target area, the photosensitizing agent having at least one absorption wavelength in a wavelength range higher than 400 nm. The target area is then exposed to light energy in a range of wavelengths comprising at least one absorption wavelength of the photosensitizing agent, the light energy being supplied to the target area in the form of at least one light pulse producing in the target area an accumulated energy density sufficient for photodynamic activation of the photosensitizing agent. The photosensitizing agent or precursor is incorporated in the photosensitizer fluid with a concentration in the range from 0.1 to 2.0% preferably from 0.1 to 0.9%, and the photosensitizer fluid is delivered to the target area in the form of a number of repeated spray doses during an overall delivery time ranging from 0.25 to 8 hours. The method can be applied to cosmetic treatments for skin rejuvenation, reduction of wrinkles, treatment of telangiectasia or removal of hair as wells a medical treatment of dermatologic diseases such as acne vulgaris, acne scars, rosacea, psorieasis, actinic keratoses, basal cell carcinoma, Bowen's disease or eczema.
Owner:CHRISTIANSEN +2
Formulations containing hyaluronic acid
InactiveUS6114314AInhibit synthesisQuick to penetrate into skinBiocideSugar derivativesDiseaseActinic keratoses
Topically applied transdermally quick penetrating (best targeting the epidermis and subsequently remaining there for a prolonged period of time) systemic independent acting, combinations and formulations which employ, combine, or incorporate a therapeutically effective non-toxic (to the patient) amount of a drug which inhibits prostaglandin synthesis together with an amount of hyaluronic acid and / or salts thereof (for example the sodium salt) and / or homologues, analogues, derivatives, complexes, esters, fragments, and / or sub units of hyaluronic acid to treat a disease and condition of the skin and exposed tissue for example, basal cell carcinoma, the precancerous, often recurrent, actinic keratoses lesions, fungal lesions, "liver" spots and like lesions (found for the most part in the epidermis), squamous cell tumours, metastatic cancer of the breast to the skin, primary and metastatic melanoma in the skin, genital warts cervical cancer, and HPV (Human Papilloma Virus) including HPV of the cervix, psoriasis (both plaque-type psoriasis and nail bed psoriasis), corns on the feet and hair loss on the head of pregnant women and remain in the skin for a prolonged period of time.
Owner:JAGOTEC AG +1
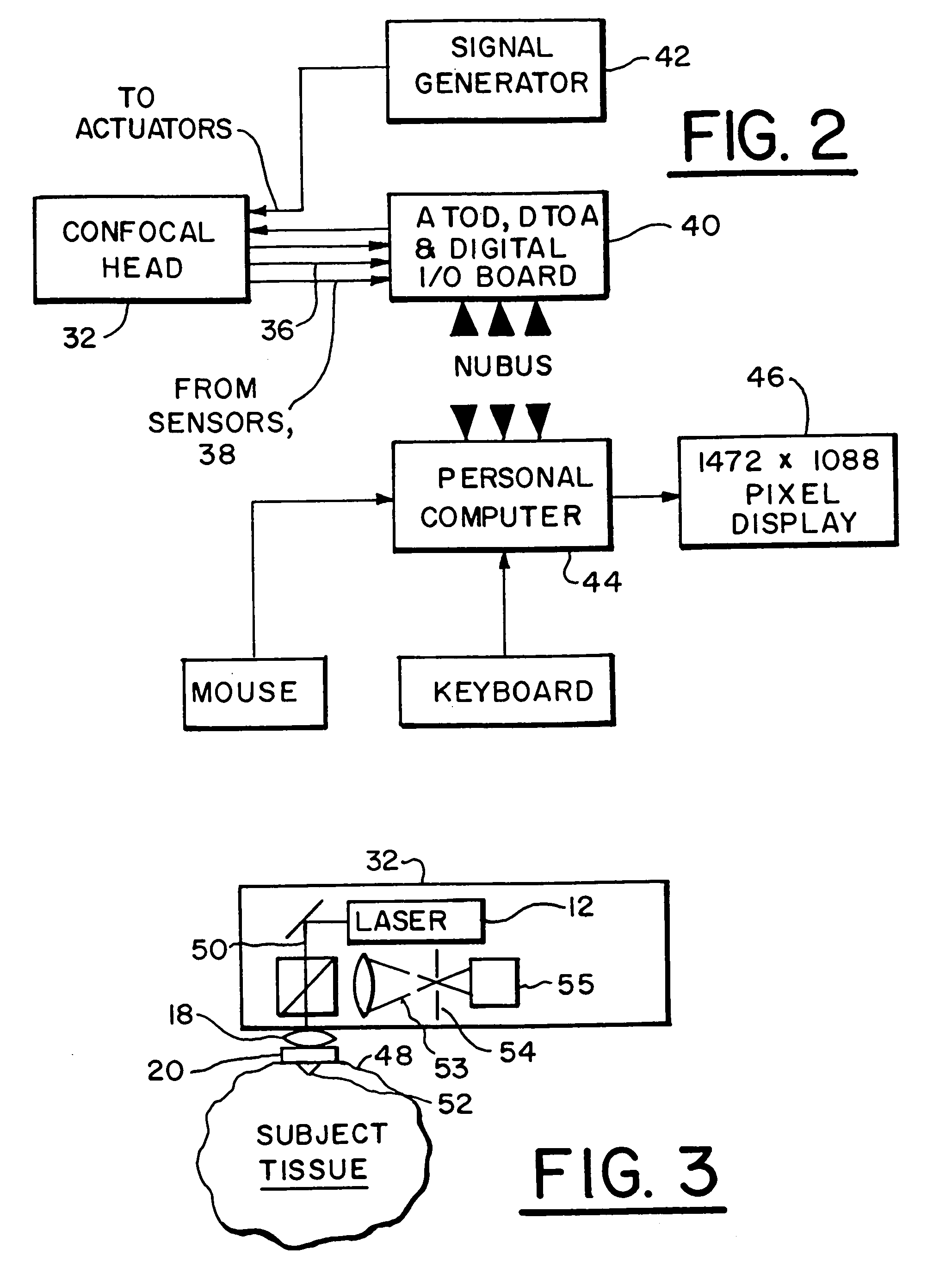
Contrast enhancing solution for use in confocal microscopy
InactiveUS7128894B1Improve featuresIncrease contrastIn-vivo testing preparationsRadiologyContrast enhancement
A method of optically detecting a tumor during surgery. The method includes imaging at least one test point defined on the tumor using a first optical imaging system to provide a first tumor image. The method further includes excising a first predetermined layer of the tumor for forming an in-vivo defect area. A predetermined contrast enhancing solution is disposed on the in-vivo defect area, which is adapted to interact with at least one cell anomaly, such as basal cell carcinoma, located on the in-vivo defect area for optically enhancing the cell anomaly. Thereafter the defect area can be optically imaged to provide a clear and bright representation of the cell anomaly to aid a surgeon while surgically removing the cell anomaly.
Owner:THE GENERAL HOSPITAL CORP
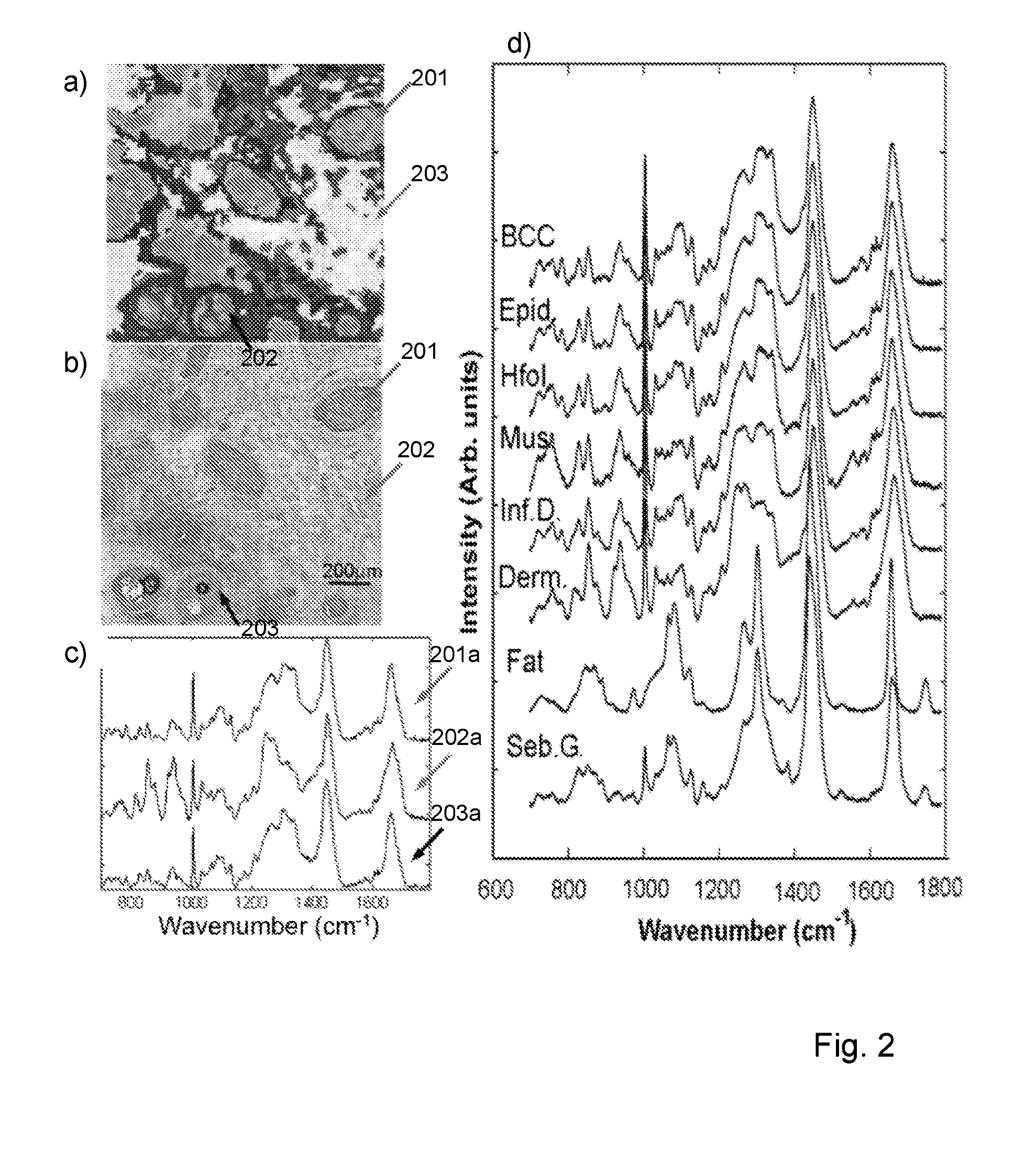
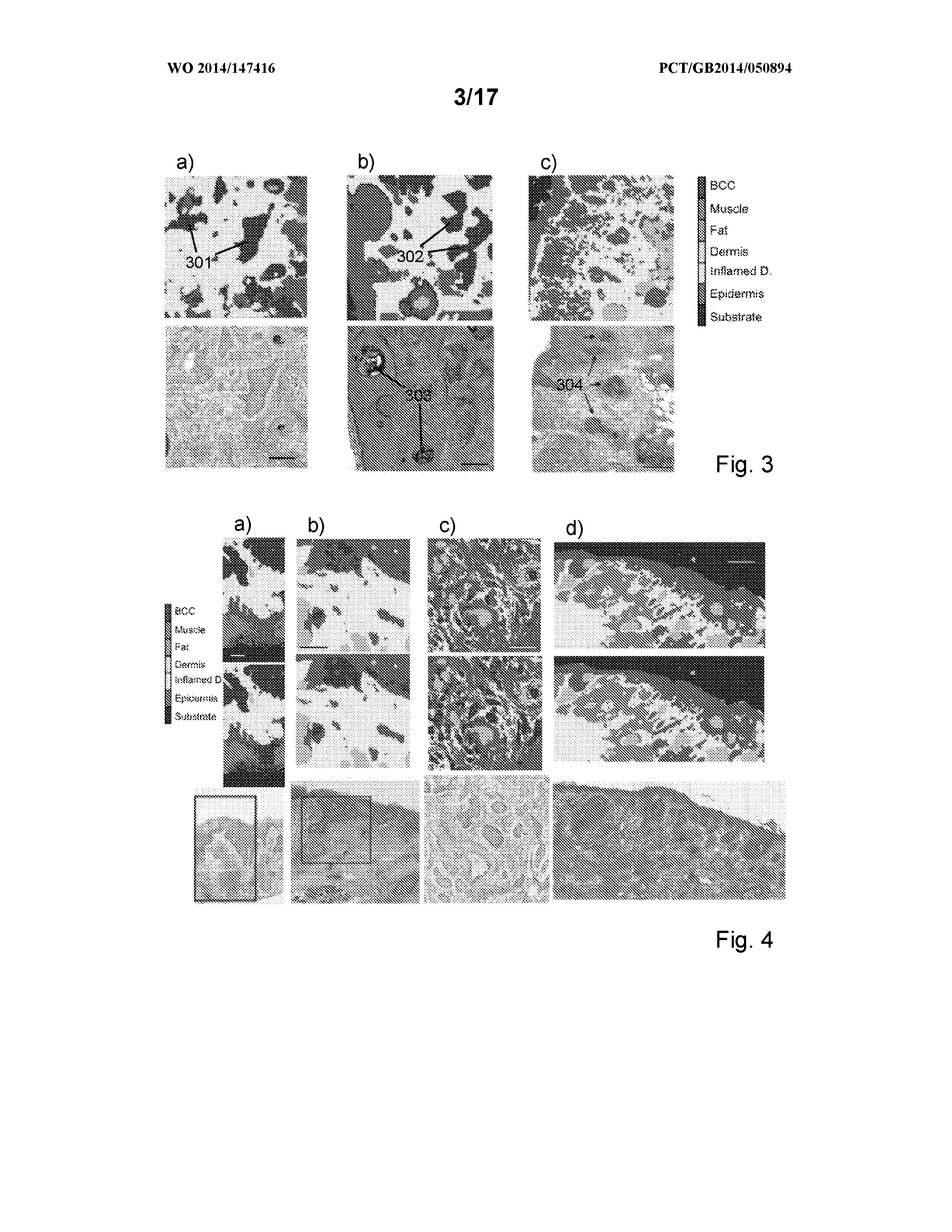
Measurement of tissue structures
ActiveUS20160290926A1Reduce the possibilityMaintain precisionSpectrum investigationRaman scatteringTissue sampleSpectroscopy
The disclosure relates to measurement and classification of tissue structures in samples using a combination of light imaging and spectroscopy, in particular although not necessarily exclusively for detection of tumours such as basal cell carcinoma or breast tumours in tissue samples. Embodiments disclosed include a method of automatically identifying tissue structures in a sample, the method comprising the steps of: measuring (1702, 1703) a response of an area of the sample to illumination with light; identifying (1704) regions within the area having a measured response within a predetermined range; determining (1705) locations within the identified regions; performing (1706) spectroscopic analysis of the sample at the determined locations; and identifying (1707) a tissue structure for each region from the spectroscopic analysis performed on one or more locations therein.
Owner:ROYAL HOLLOWAY UNIV OF LONDON +1
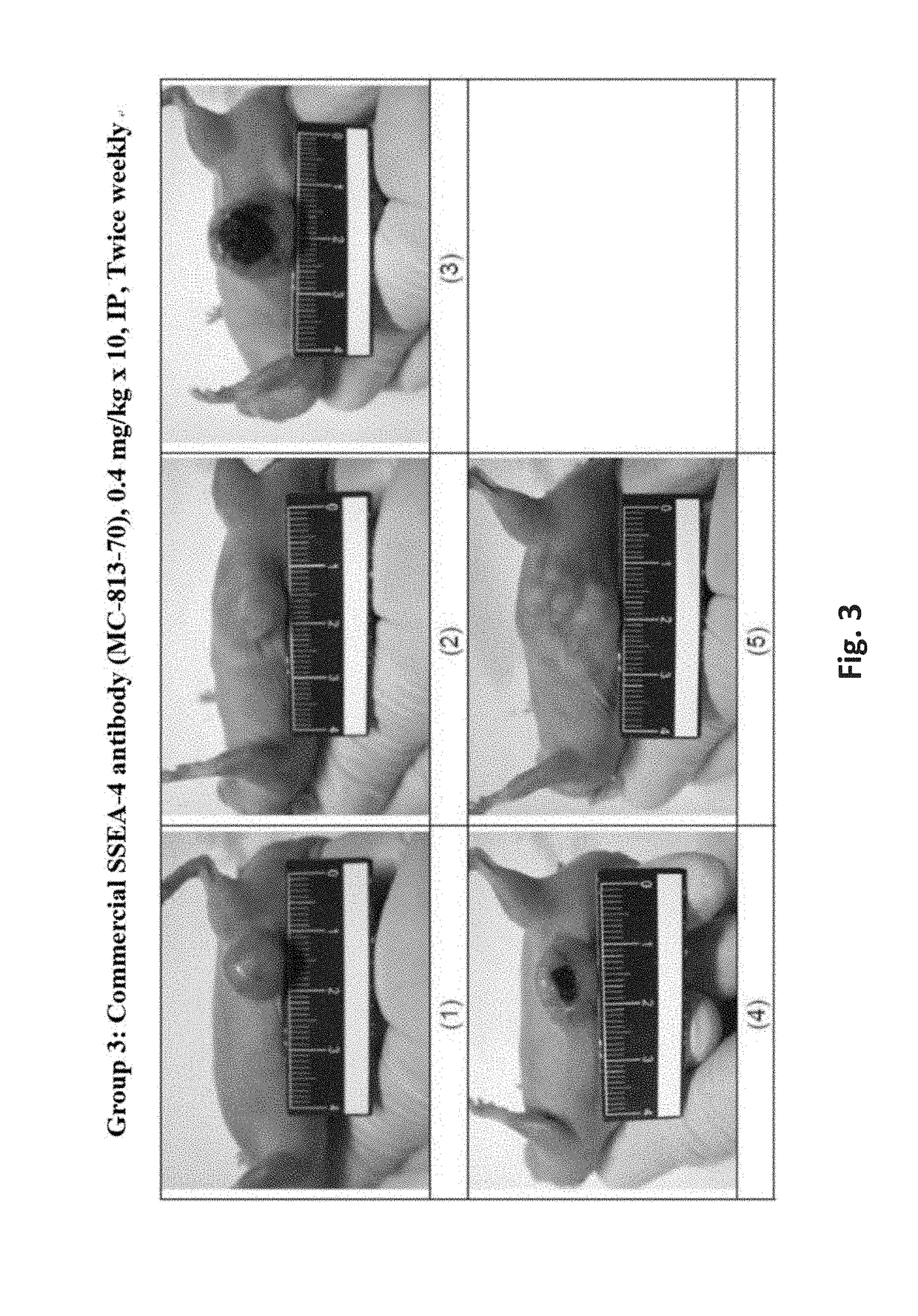
Antibodies, pharmaceutical compositions and methods
ActiveUS20170283488A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsURINARY BLADDER CARCINOMASquamous Carcinomas
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA INC
Treatment of hyperproliferative, inflammatory and related mucocutaneous disorders using inhibitors of mevalonate synthesis and metabolism
InactiveUS20020010128A1Inhibit cell proliferationInhibit expressionCosmetic preparationsBiocideSquamous CarcinomasAntiinflammatory drug
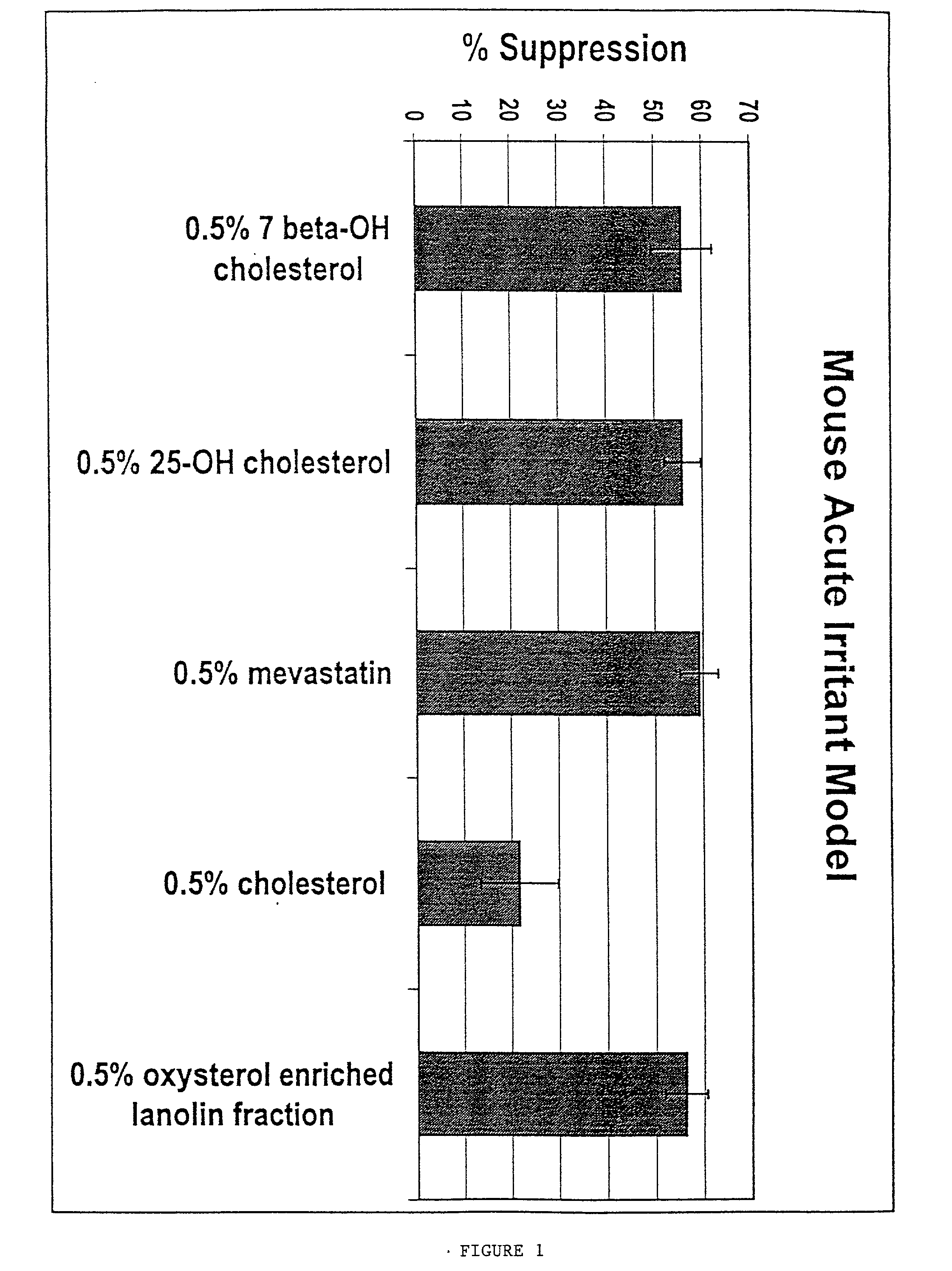
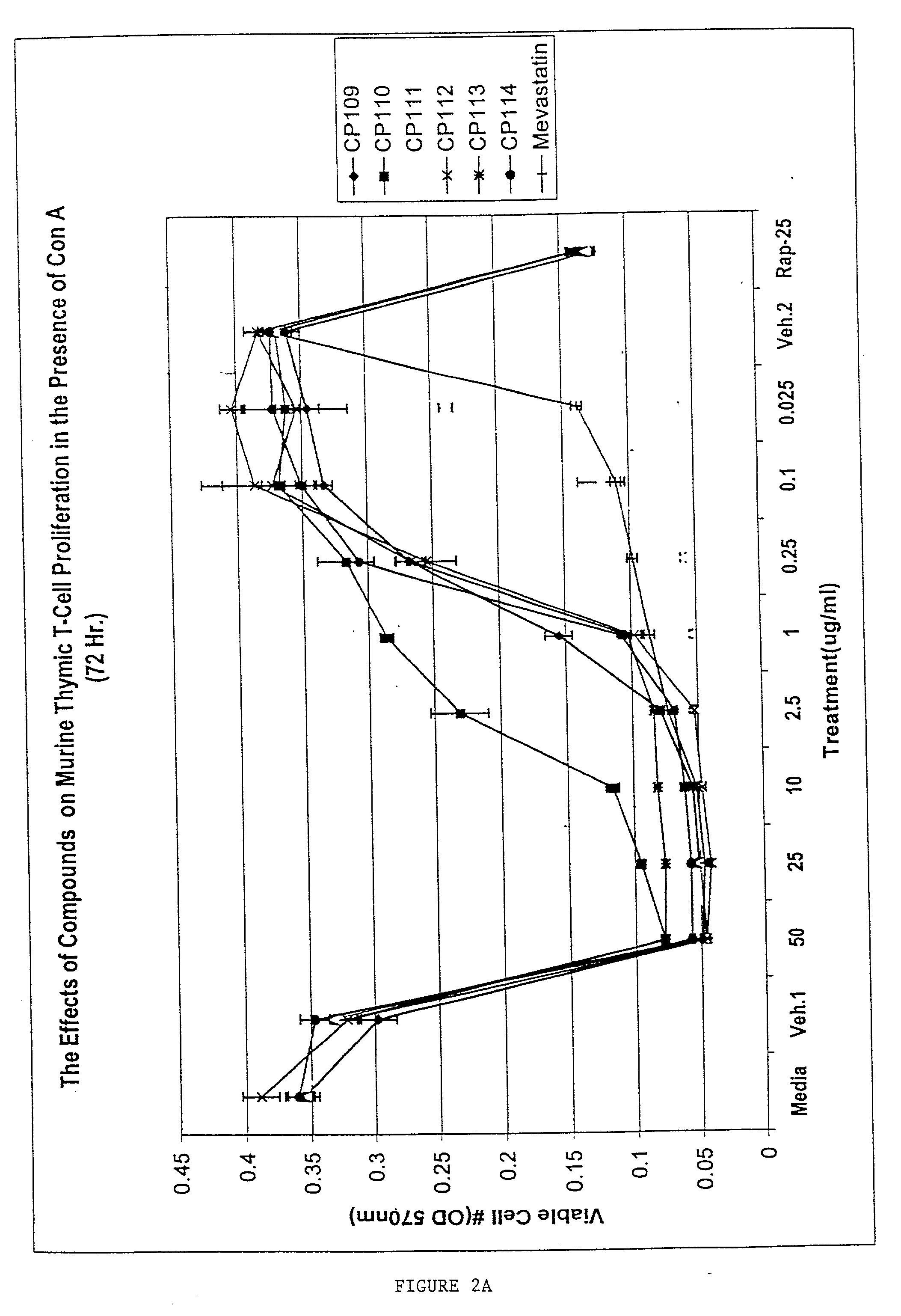
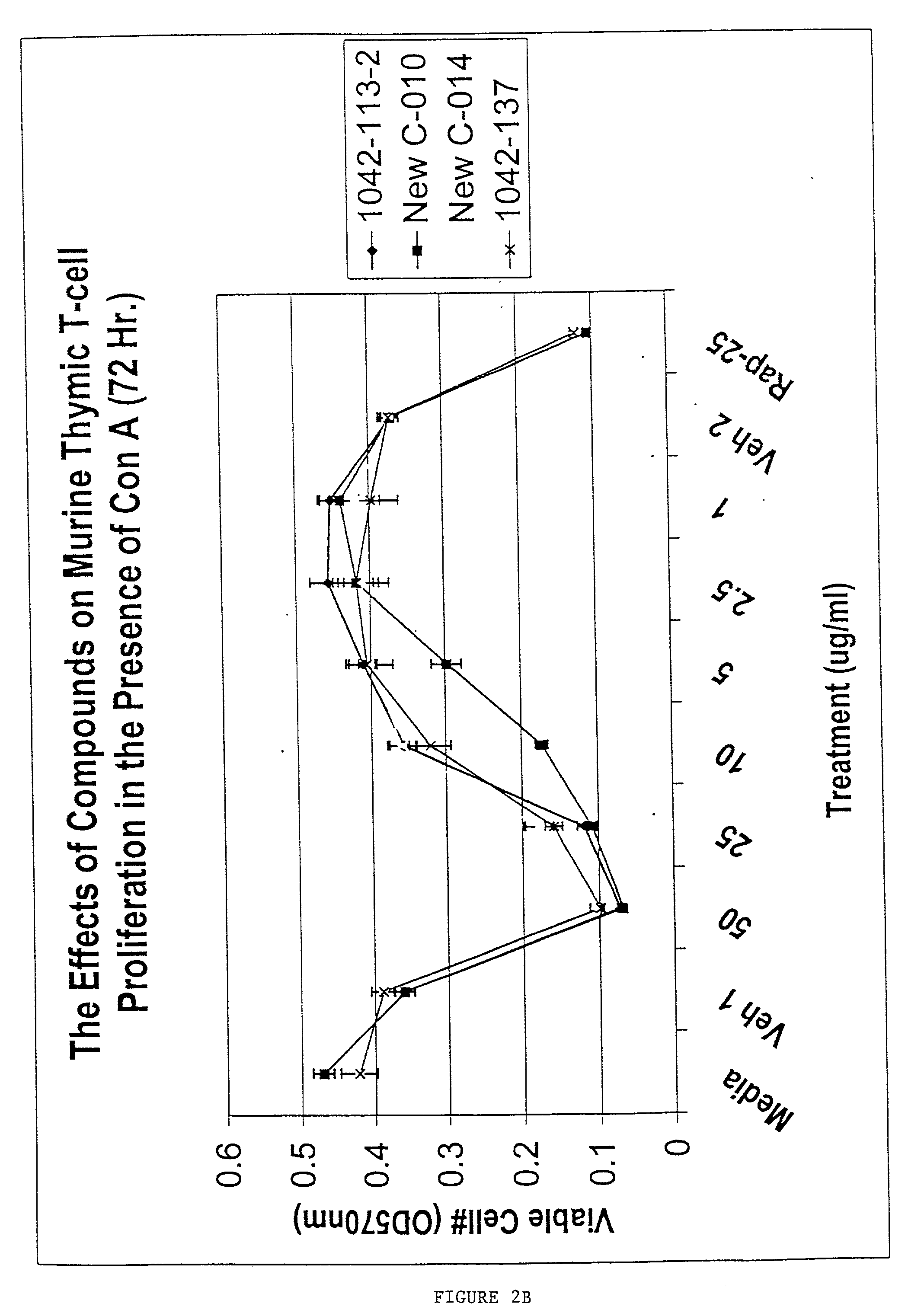
The present invention provides methods for treating a variety of hyperproliferative and inflammatory mucocutaneous disorders, including, basal cell carcinoma, squamous cell carcinoma, psoriasis and atopic dermatitis, as well as skin irritation and disorders associated with skin aging and skin photodamage using inhibitors of cholesterol metabolism. The present invention further relates to the discovery that the combined use of several inhibitors of cholesterol metabolism produces synergistic effects. Furthermore, the present invention is directed to the use of inhibitors of cholesterol metabolism as excipients to enhance the effects of antiinflammatory drugs.
Owner:CELLEGY PHARMACEUTICALS INC
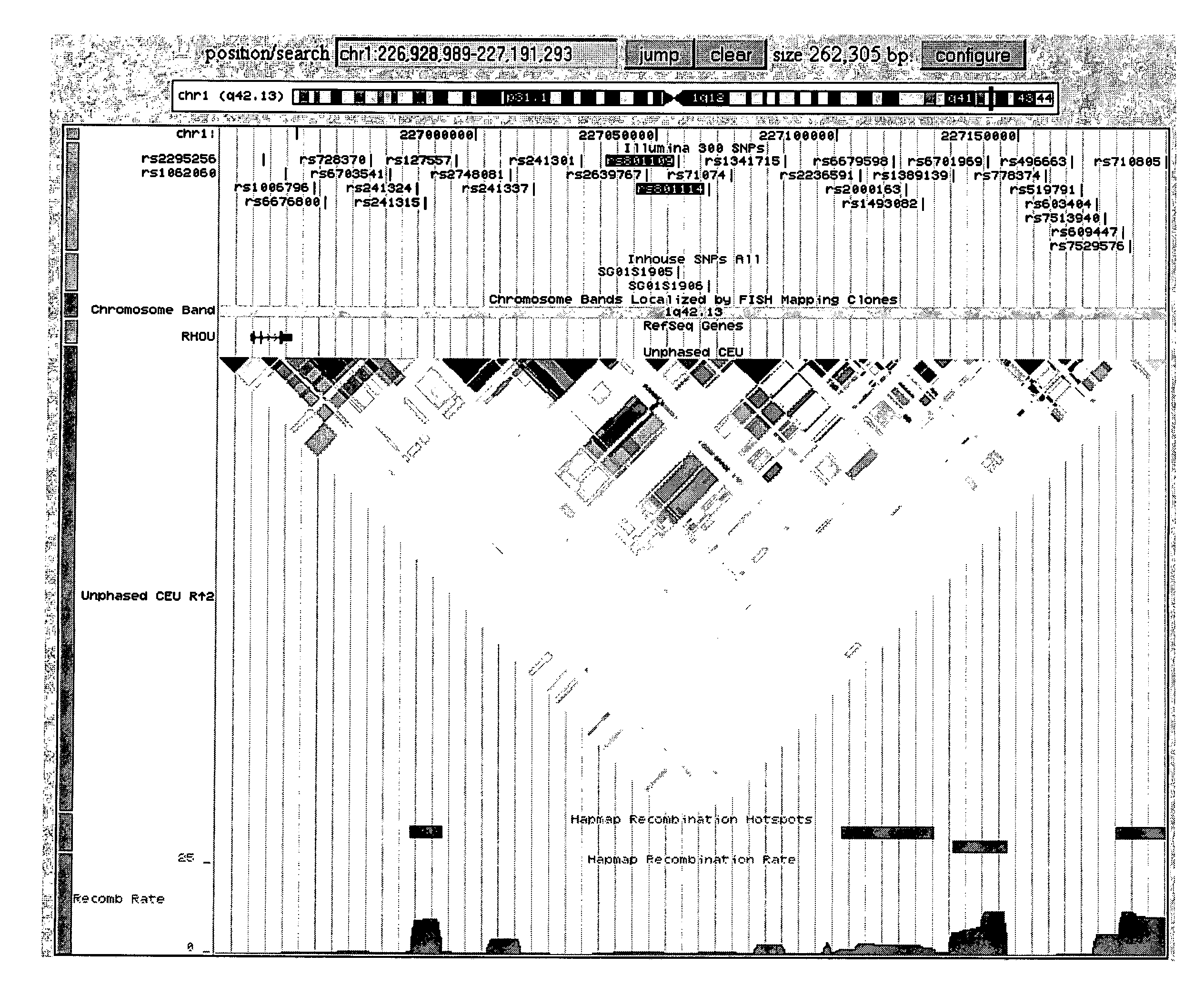
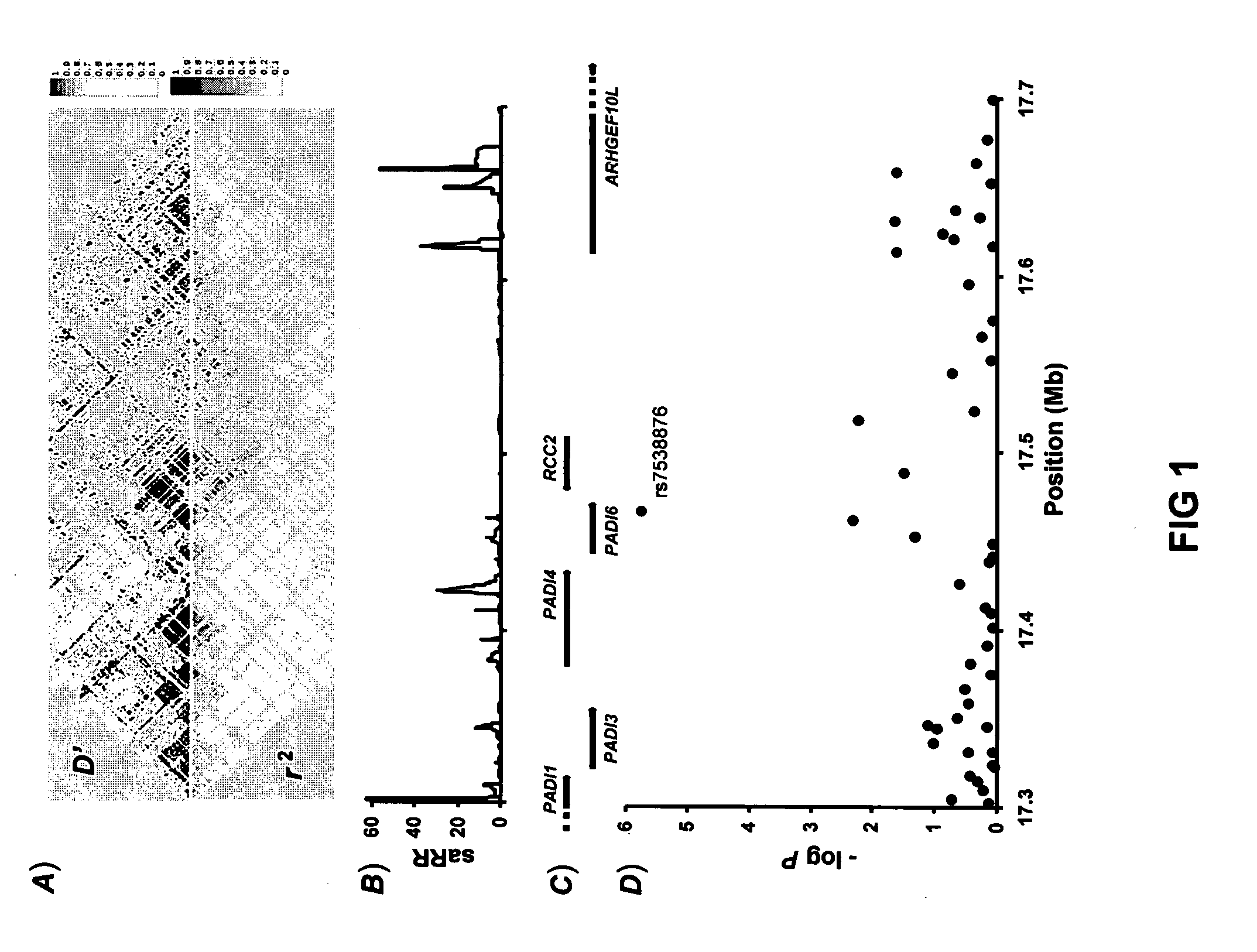
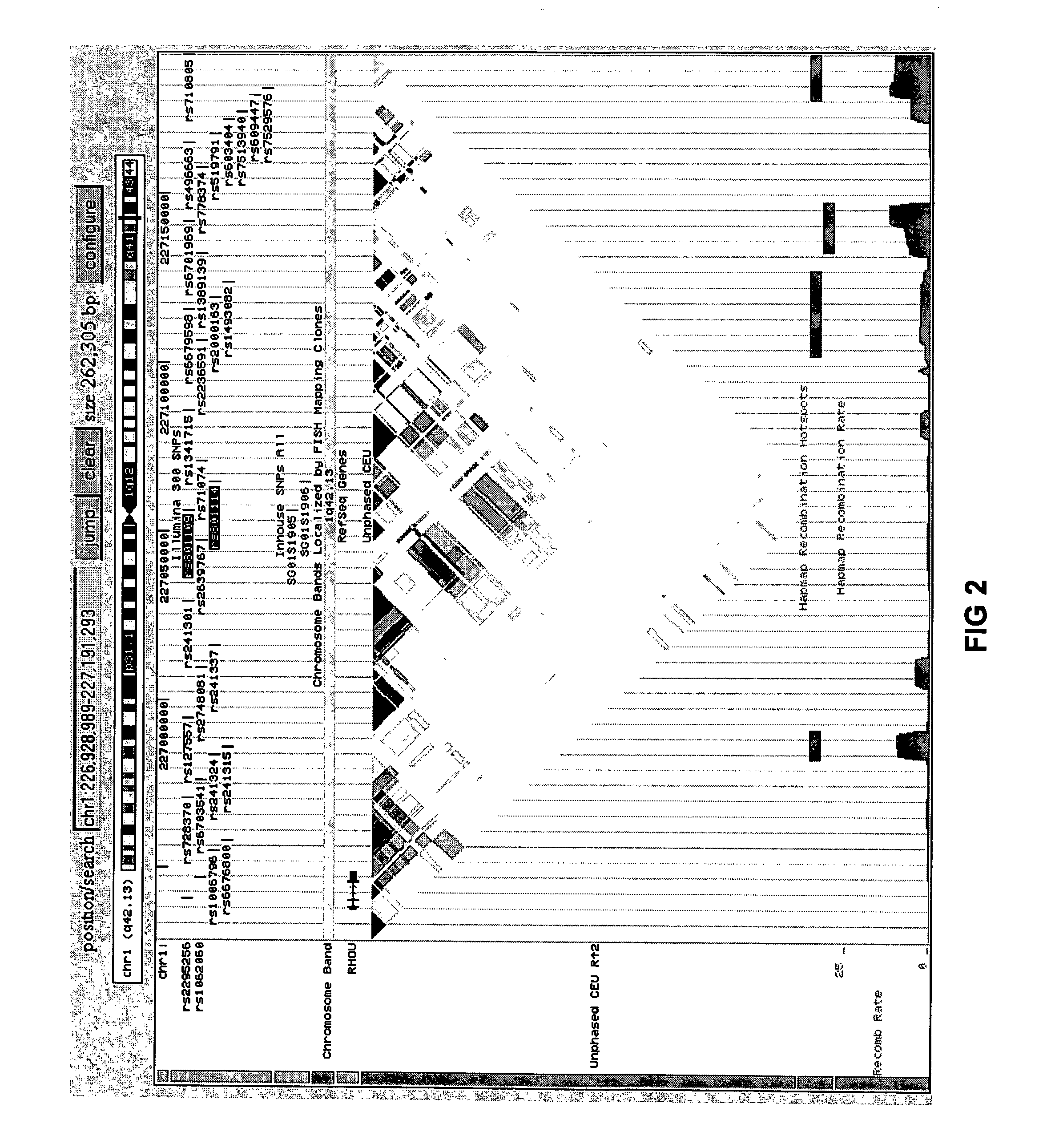
Genetic Variants Predictive of Cancer Risk in Humans
InactiveUS20120122698A1Reduce sensitivityMicrobiological testing/measurementLibrary member identificationComputerized systemBasal cell carcinoma
The present invention discloses genetic variants that have been found to be predictive of risk of particular forms of cancer, in particular basal cell carcinoma and cutaneous melanoma. The invention provides methods of predicting risk of developing such cancers, and other methods pertaining to risk management of cancer utilizing such risk variants. The invention furthermore provides kits and computer systems for use in such methods.
Owner:DECODE GENETICS EHF
Microscopic imaging apparatus and method
InactiveUS7047064B1Avoid the needDiagnostics using lightSurgical instrument detailsMicroscopic imageTissue sample
An imaging apparatus is provided for imaging tissue samples substantially beneath the surface of the tissue sample. The apparatus includes an objective lens and a window defining a tissue contacting surface in pressure contacting relationship with the surface of the tissue sample when the tissue sample is imaged by the objective lens to view tissue structures for pathological applications. The objective lens focuses an illumination beam through the window to the tissue sample and receives returned reflected light of the beam representative of one or more sections of the tissue sample. The apparatus enables a method for in vivo observation of tissue for diagnosis of conditions substantially beneath the surface of the tissue sample. Both two and three-dimensional imaging may be provided for diagnosis and location of basal cell carcinomas and melanomas, and so as to enable visualization of tumor borders prior to excision.
Owner:CALIBER IMAGING & DIAGNOSTICS
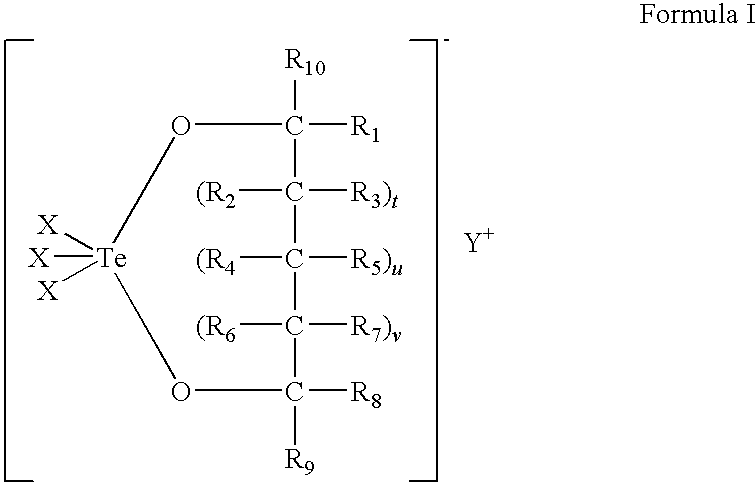
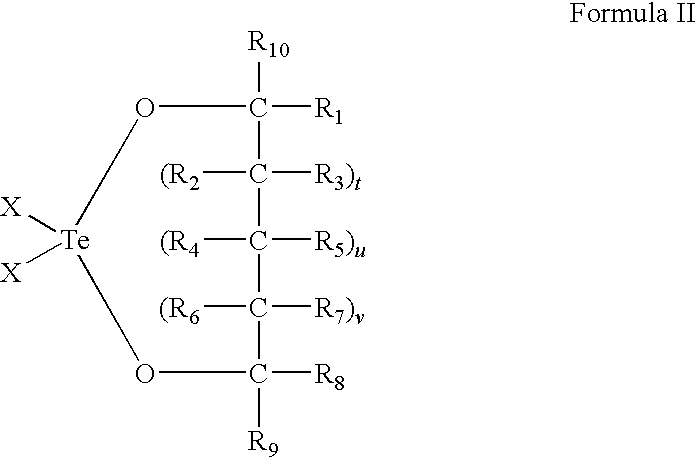
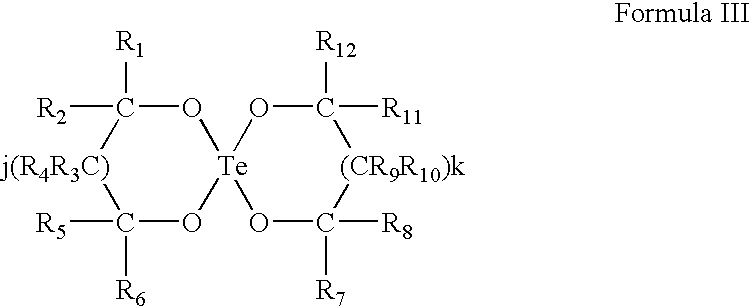
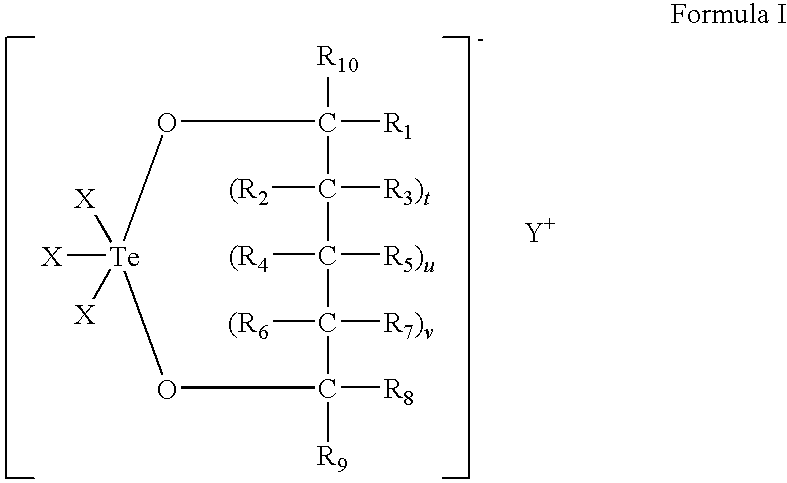
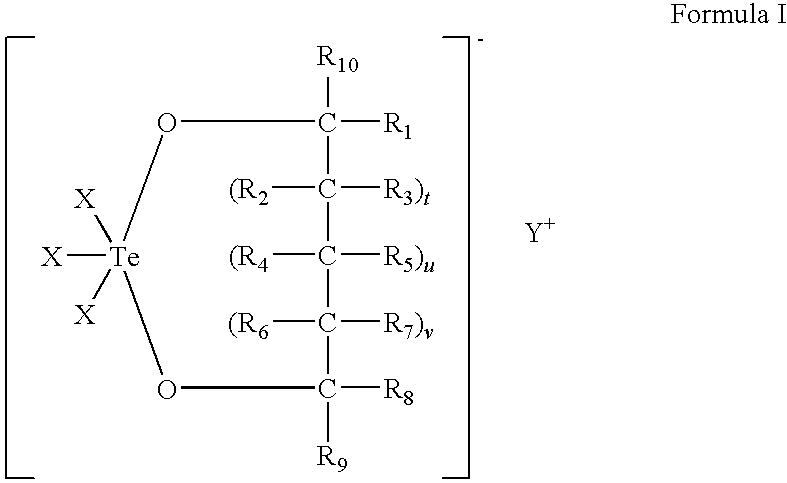
Use of tellurium compounds for treatment of basal cell carcinoma and/or actinic keratosis
Methods for treating skin conditions such as basal cell carcinoma and / or actinic keratosis are provided, which are effected by administering a pharmaceutically effective amount of a tellurium-containing compound. A pharmaceutical composition for treatment of skin conditions such as basal cell carcinoma an / or actinic keratosis is also provided.
Owner:CASSIOPEA SPA
Pharmaceutical formulations comprising an immune response modifier
Pharmaceutical formulations comprising an immune response modifier (IRM) chosen from imidazoquinoline amines, imidazotetrahydroquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, thiazolo-quinolineamines, oxazolo-quinolinamines, thiazolo-pyridinamines, oxazolo-pyridinamines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, and thiazolonaphthyridine amines; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid are useful for the treatment of dermal associated conditions. Novel topical formulations are provided. In one embodiment, the topical formulations are advantageous for treatment of actinic keratosis, postsurgical scars, basal cell carcinoma, atopic dermatitis, and warts.
Owner:3M INNOVATIVE PROPERTIES CO
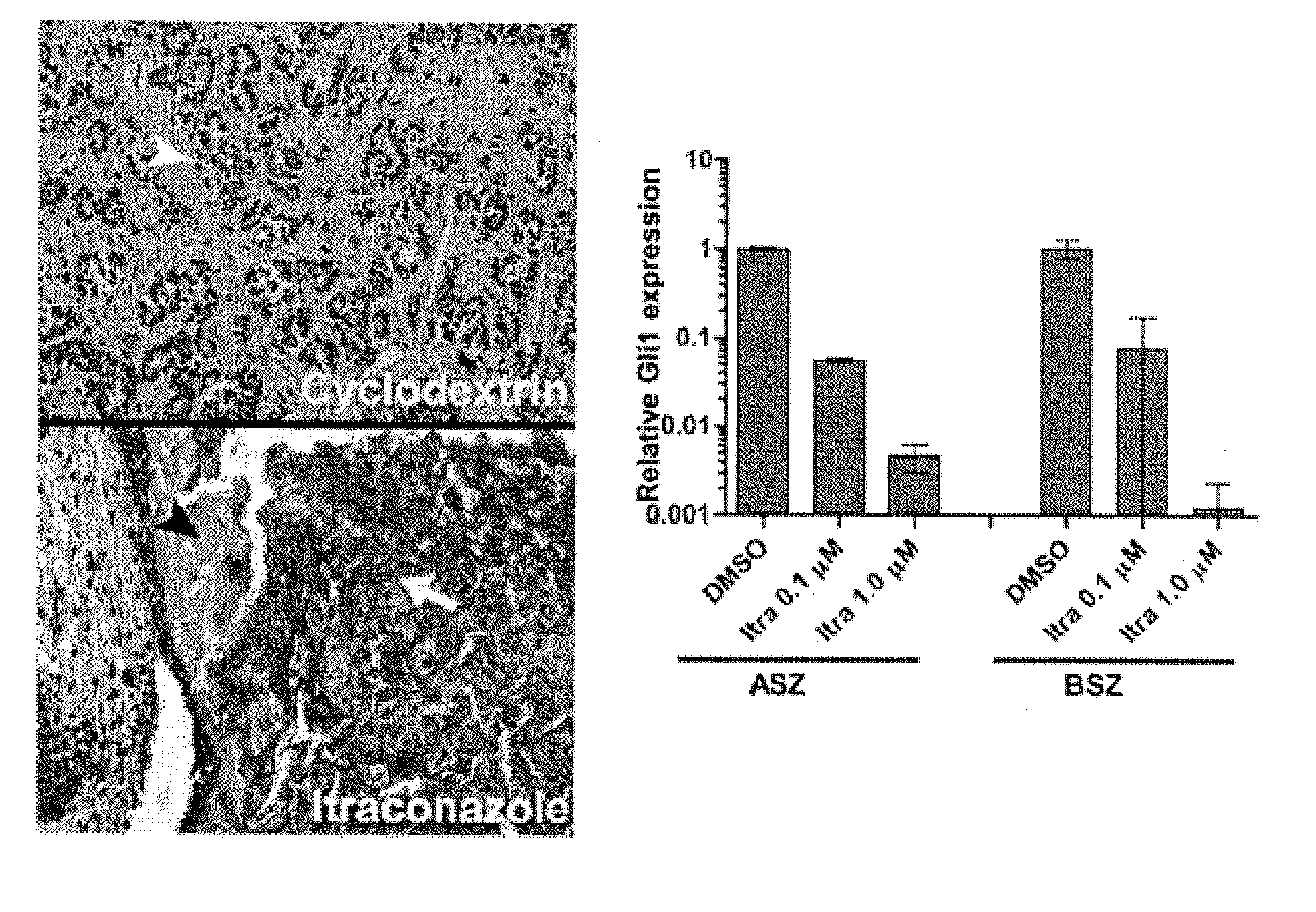
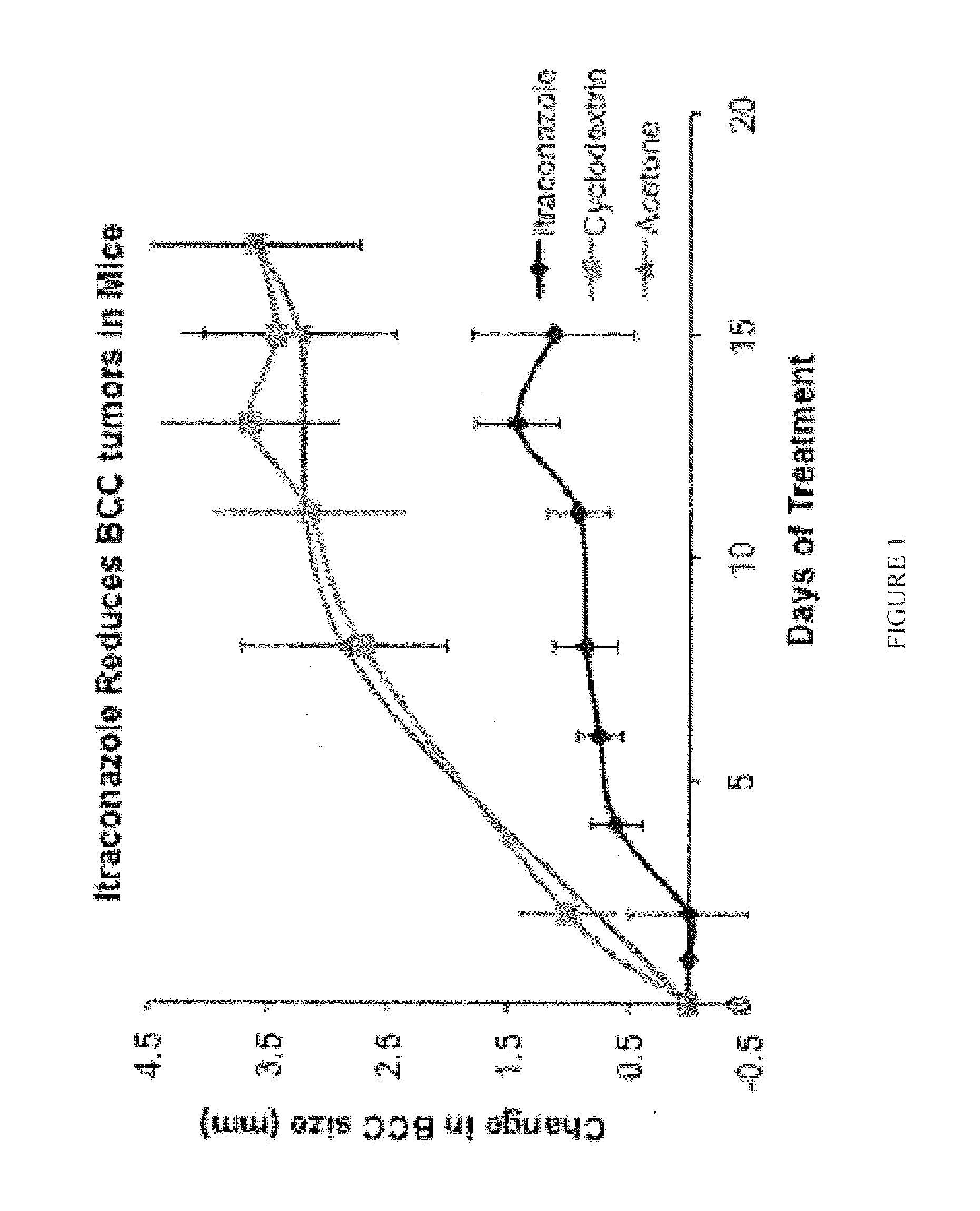
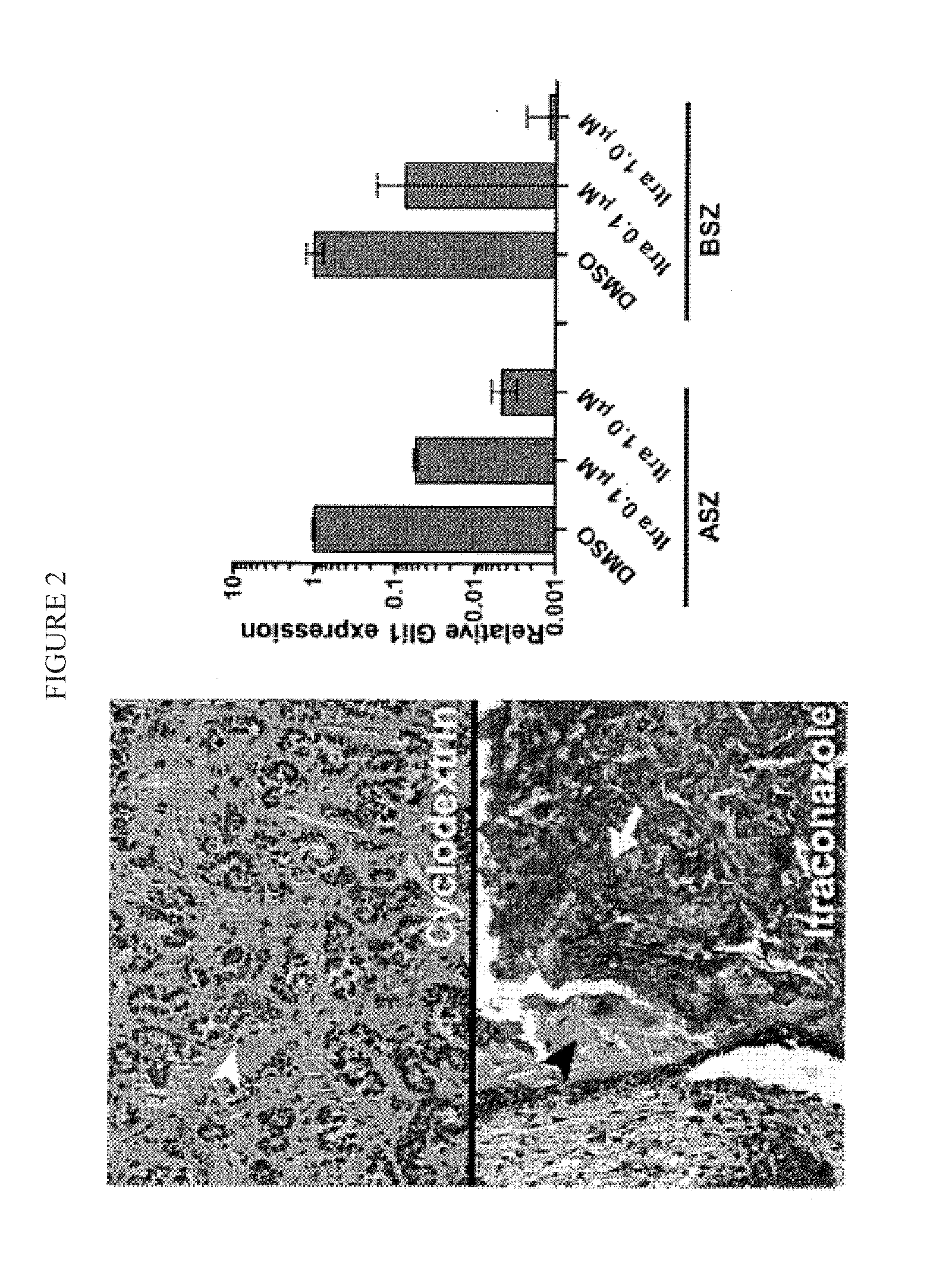
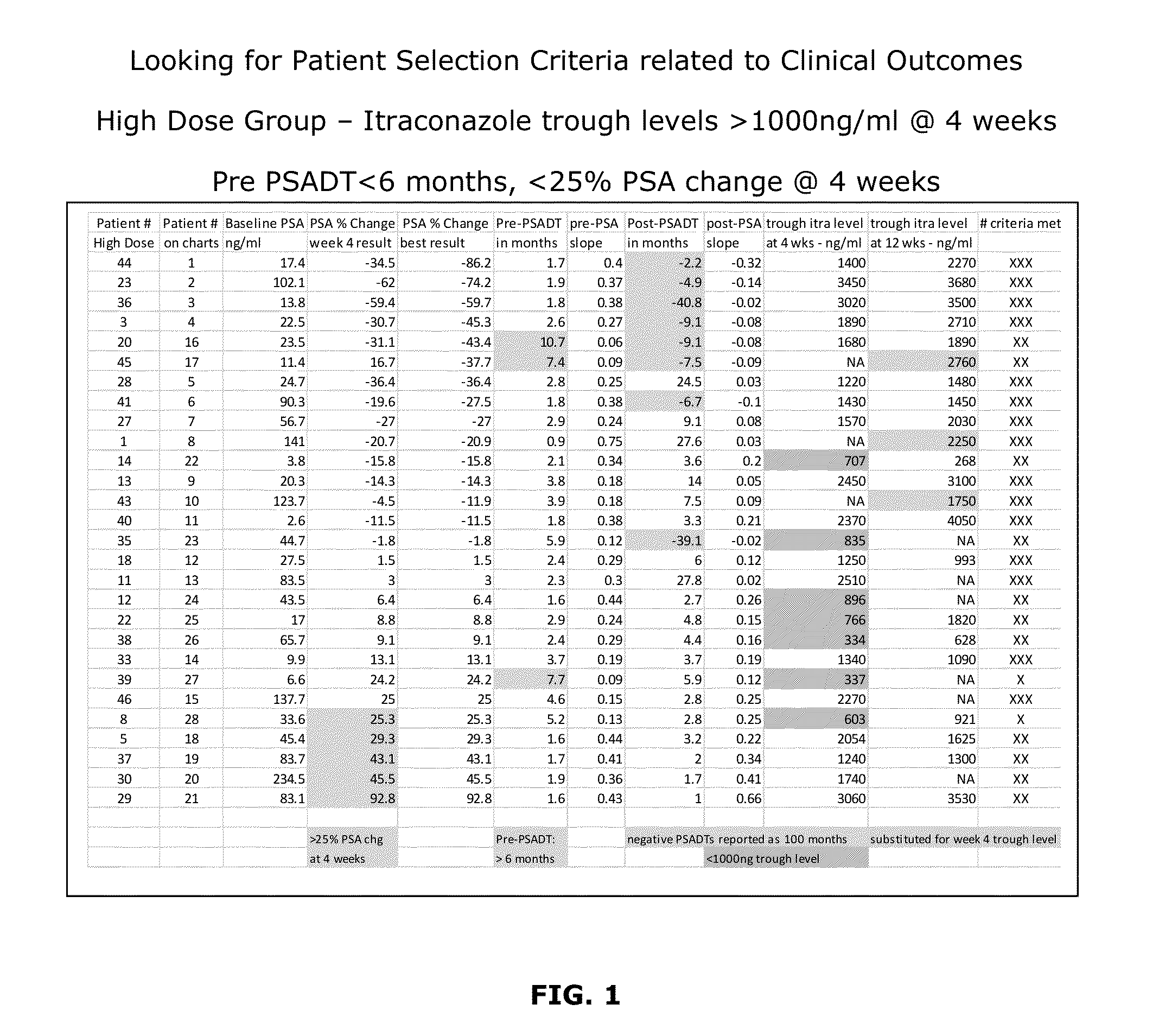
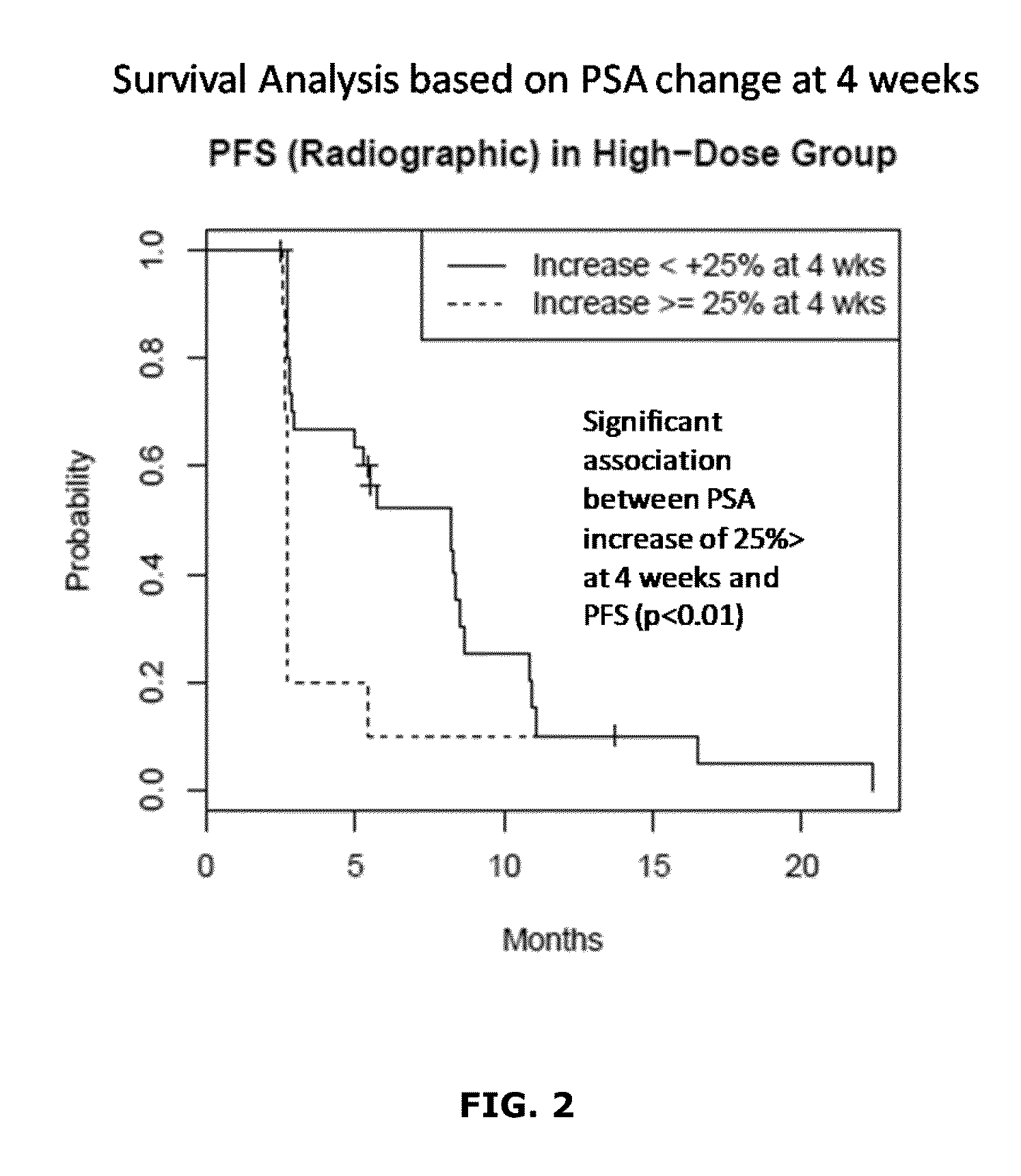
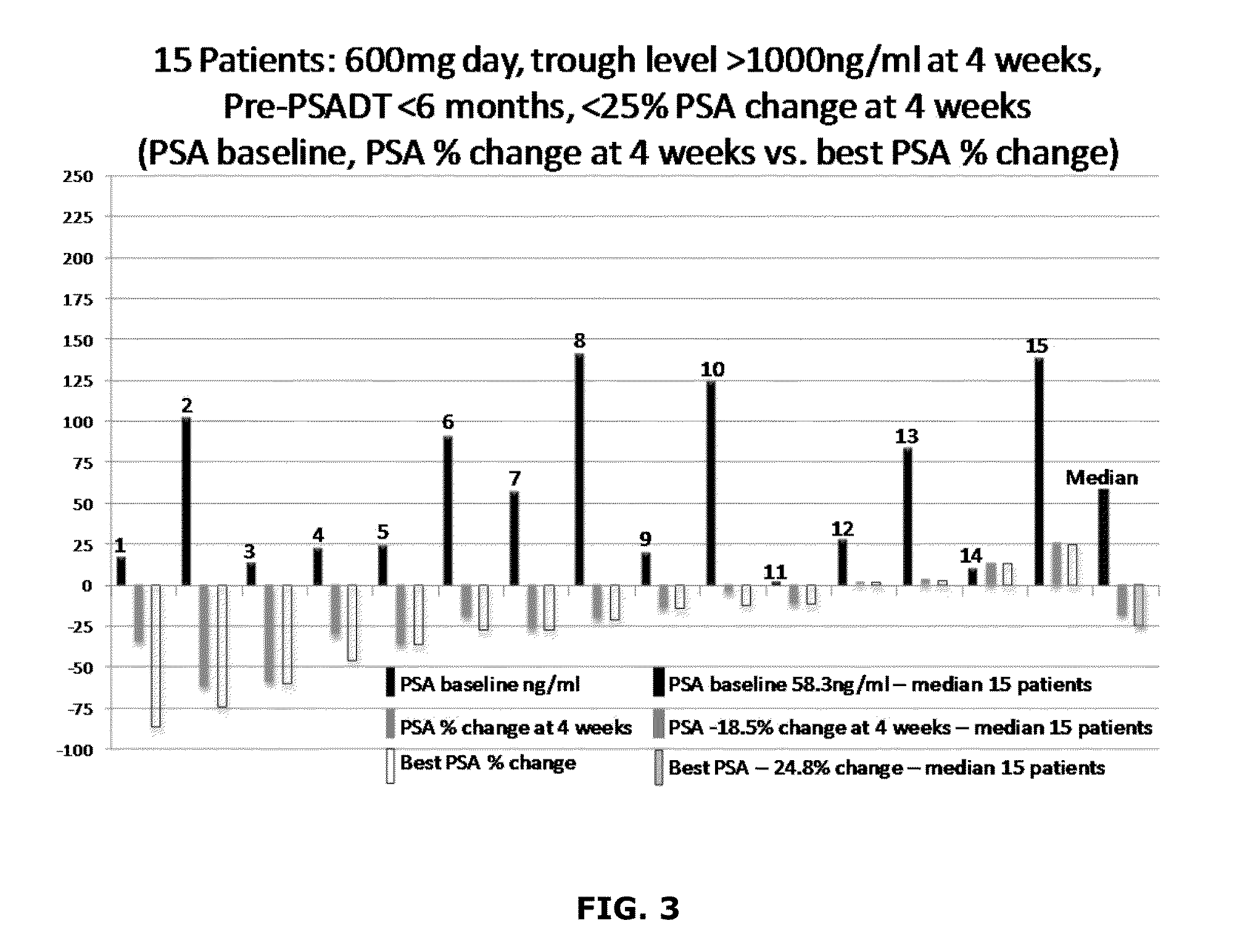
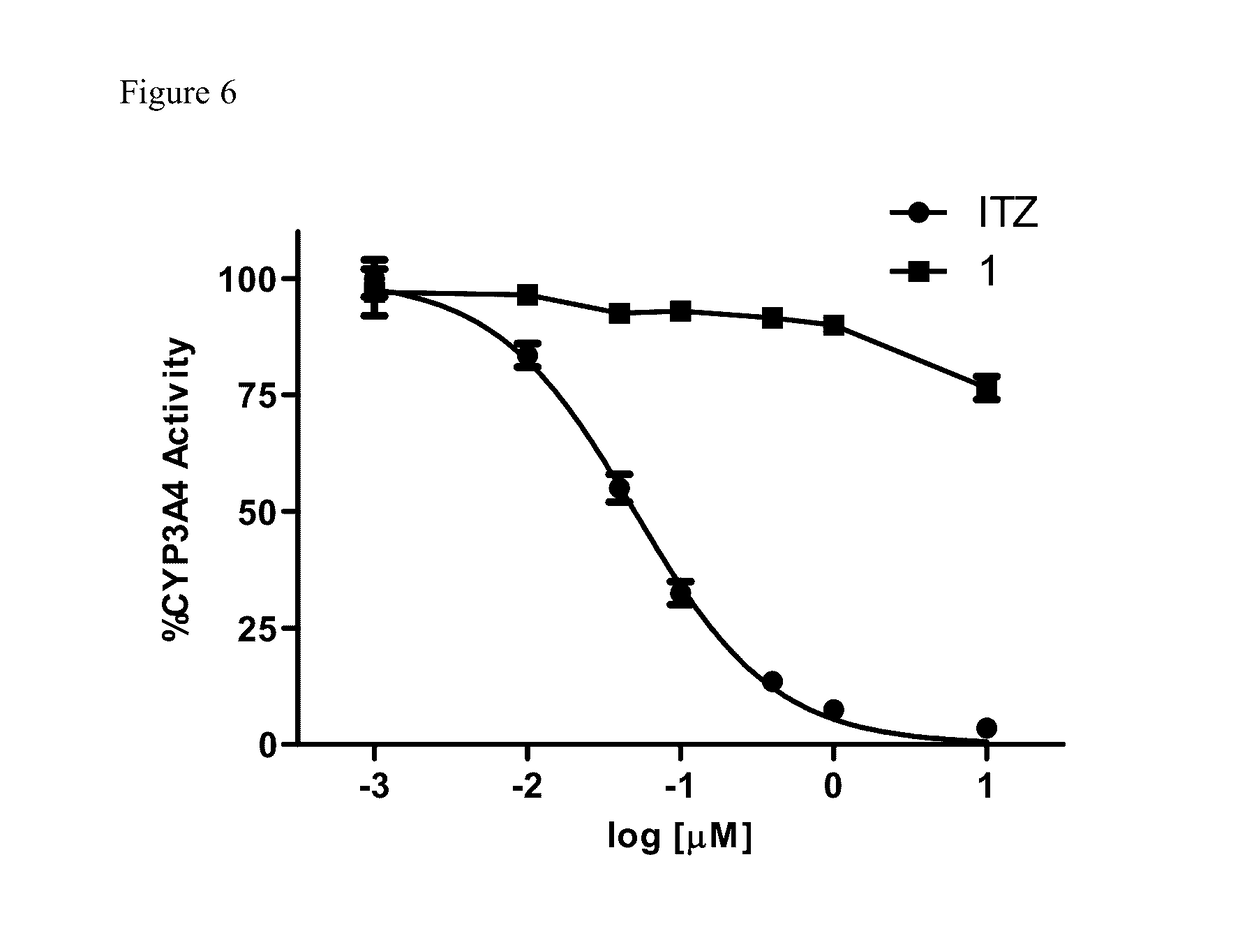
Topical Itraconazole Formulations and Uses Thereof
Methods of transdermally delivering a therapeutic amount of a triazole-triazolone compound are provided, e.g. for the prevention or treatment of basal cell carcinoma (BCC) in a subject. A therapeutic level of a triazole-triazolone compound such as itraconazole is delivered transdermally to a subject. Also provided are topical triazole-triazolone compositions that find use in practicing the subject methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Antibodies, pharmaceutical compositions and methods
ActiveUS20180339061A1Effective preventionEffective treatmentOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSquamous CarcinomasProstate cancer
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA
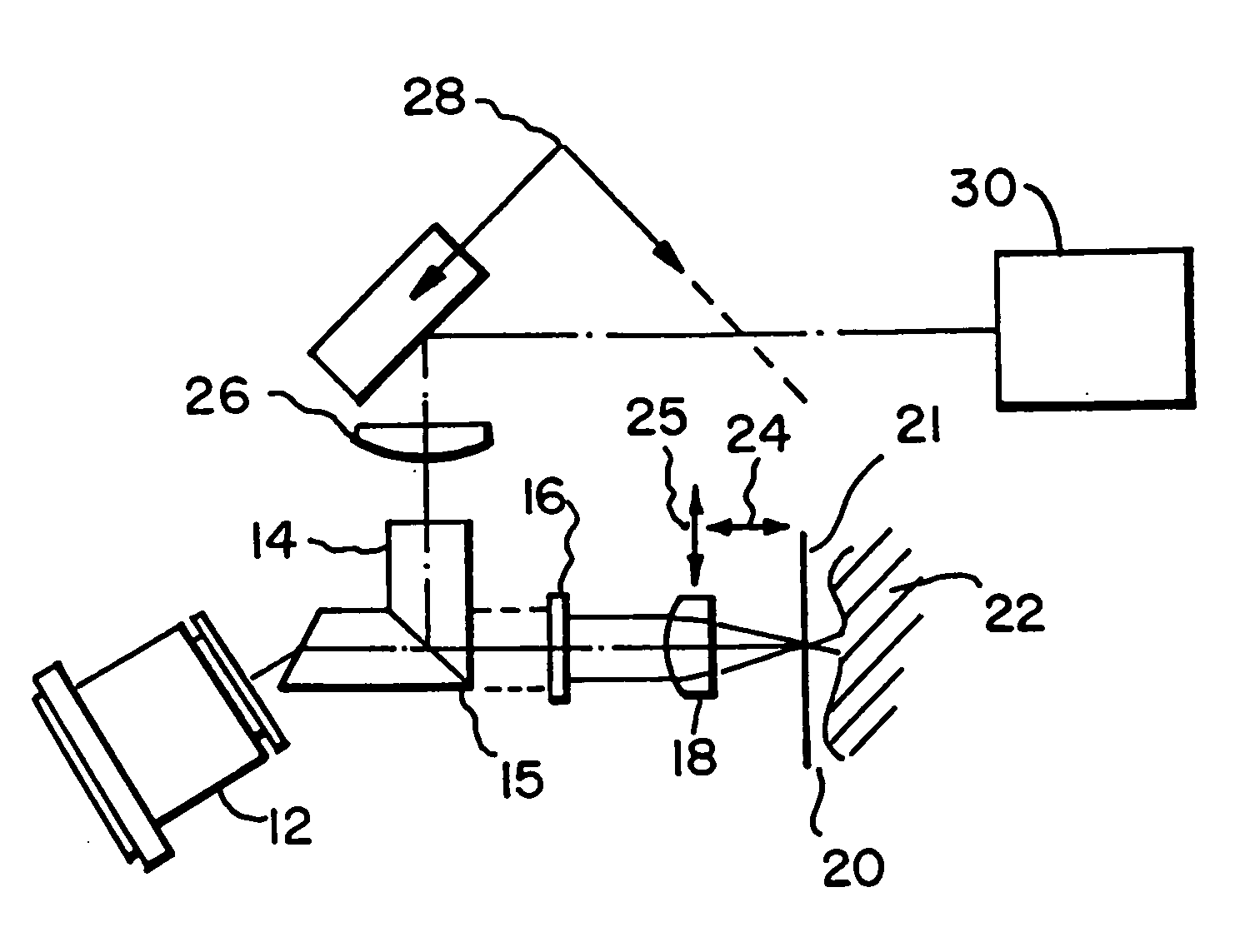
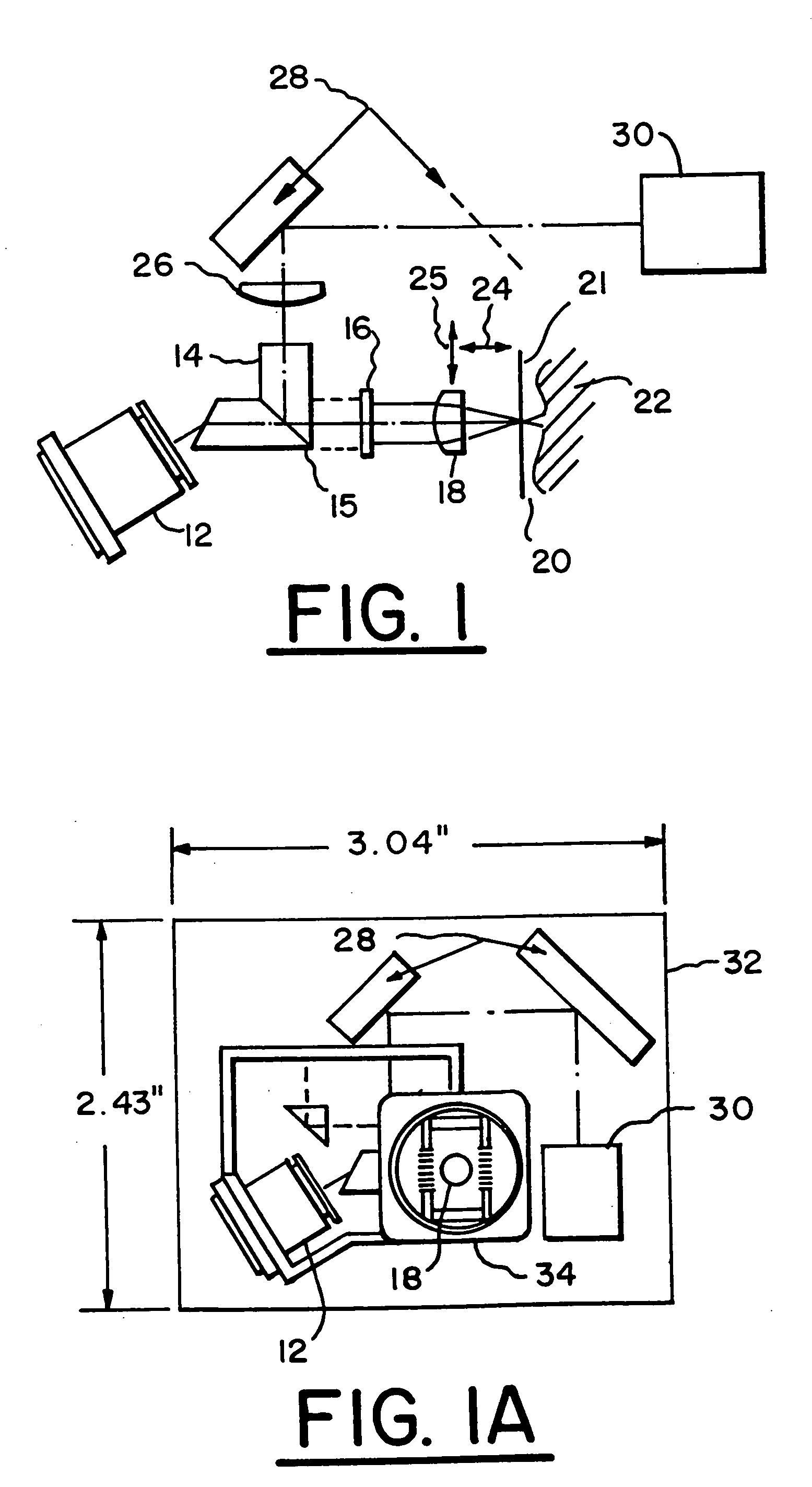
System and method for enhancing confocal reflectance images of tissue specimens
InactiveUS7139122B1Easy to observeImprove cell structureLuminescence/biological staining preparationMicroscopesMohs surgeryCross polarization
A system using cross polarization effects and an enhancement agent having citric or other similar alpha hydroxy acid to enhance confocal microscope reflectance images and particularly images of the nuclei of BCCs (basal cell carcinomas) and SCCs (squamous cell carcinomas) in the confocal reflectance images of excised tumor slices obtained during Mohs surgery by illuminating the tissue being imaged (a tumor slice) using polarized light. The reflected illumination is passed to a polarization analyzer, which passes the polarization component which is crossed with respect to the polarization of the illuminating light. The light from the analyzer is passed through the confocal aperture and detected. The section of the tissue either at the surface or within the tissue is scanned and the reflectance image is produced with enhanced visualization of the cellular or nuclear structure thereof thereby enabling determination of the extent of the tumor (cancerous cells) in the section. A method is also provided using the system for diagnosing cancerous cells in skin tissue.
Owner:CALIBER IMAGING & DIAGNOSTICS +1
Fluorouracil-containing formulation
Oil-in-water emulsion formulations contain both free fluorouracil and fluorouracil impregnated in porous microparticles. The formulations are suitable for topical administration, and are useful for the treatment of solar keratoses, actinic keratoses, and superficial basal cell carcinomas.
Owner:BAUSCH HEALTH IRELAND LTD
Topical formulations for delivery of hedgehog inhibitor compounds and use thereof
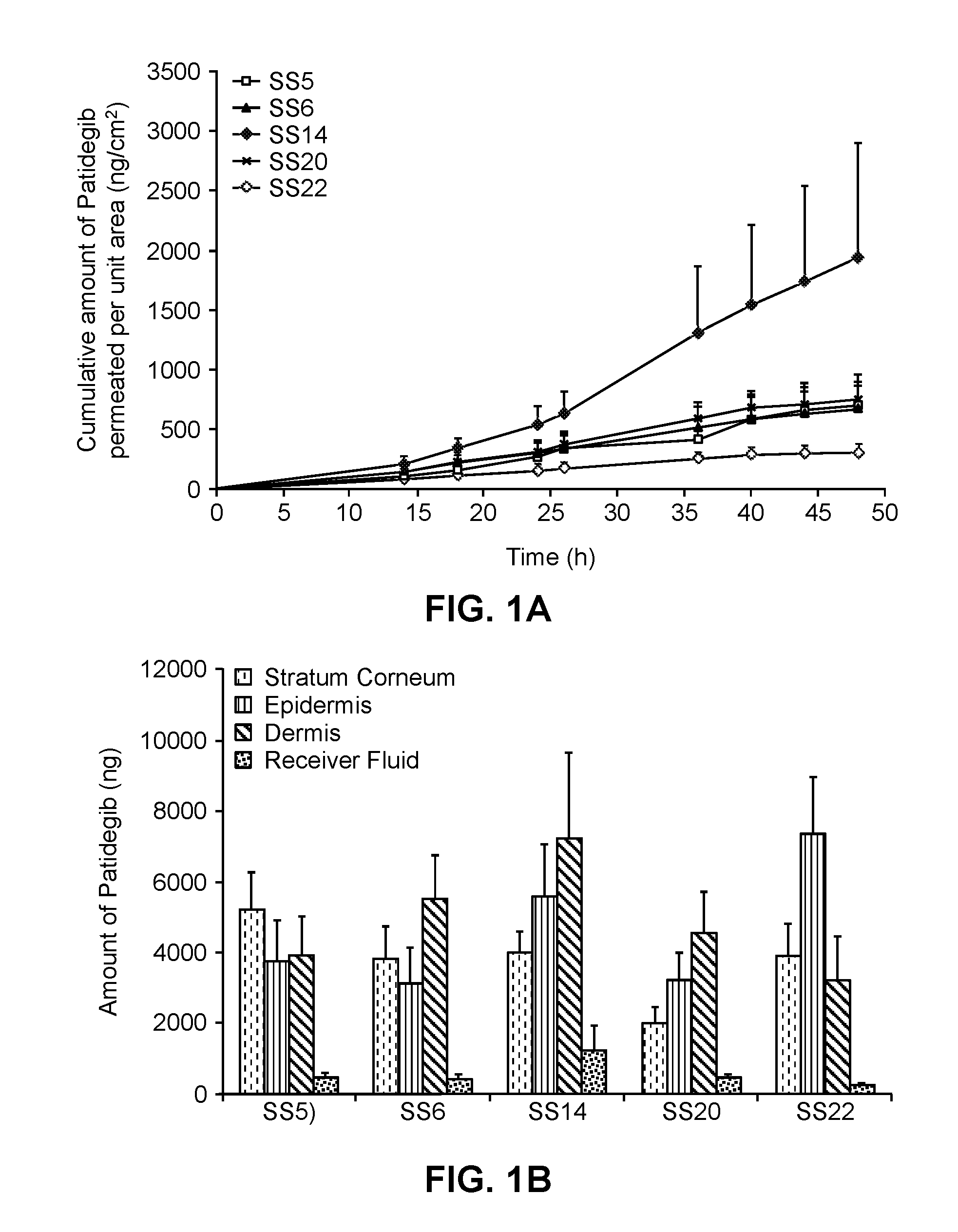
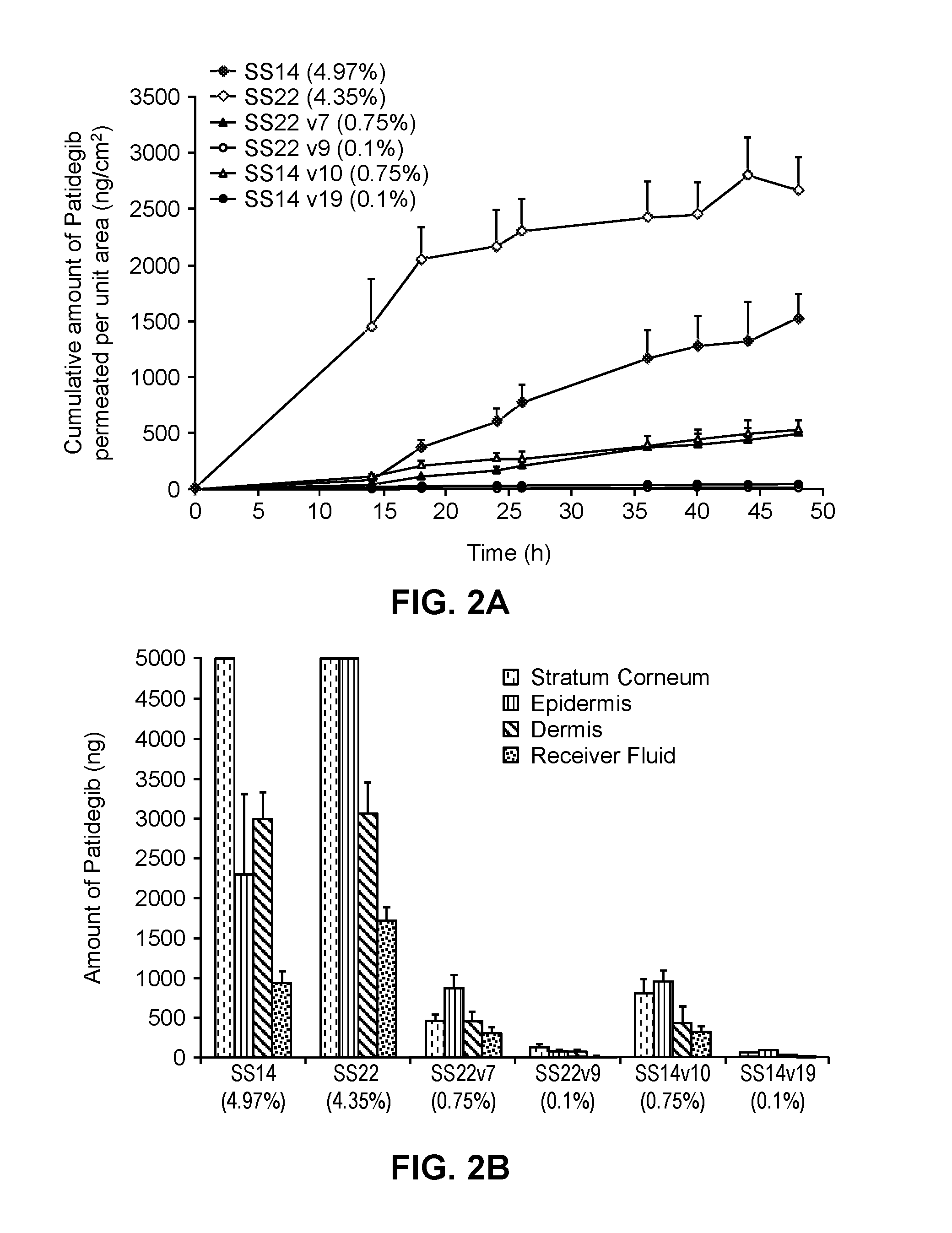
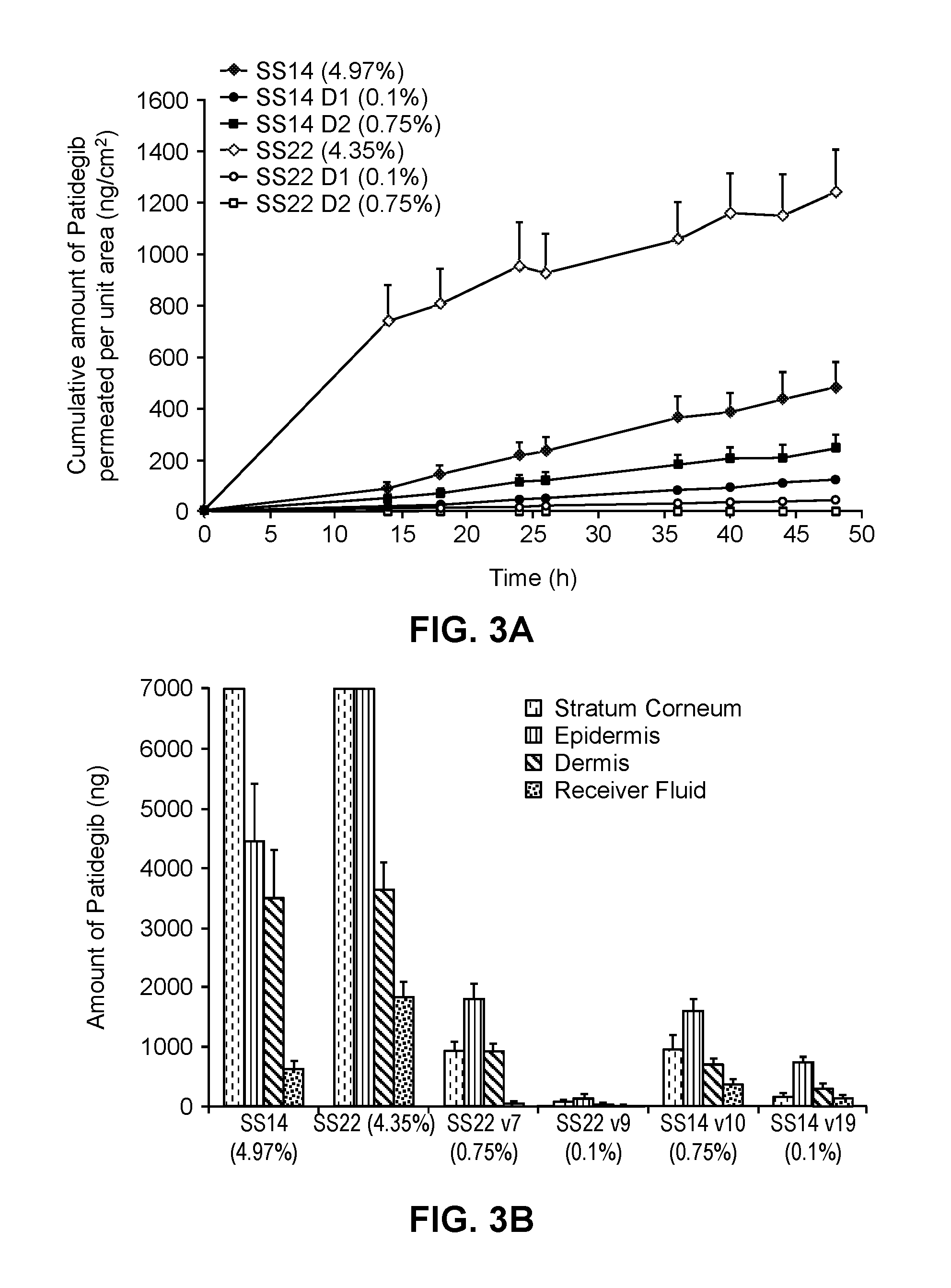
Compositions for topical administration of a hedgehog inhibitor compound are described. In one embodiment, the hedgehog inhibitor compound is patidegib and the topical composition comprises the compound in a solvent system of a monohydric primary alcohol and a polyol in a w / w ratio of between about 0.9-1.8. In another embodiment, the hedgehog inhibitor is itraconazole and the topical composition comprises the compound in a solvent system comprising a monohydric primary alcohol and an optionally lower alkyl end-capped oligomeric alkylene glycol in a w / w ratio of between about 0.8 and 2.6 and a fused bicyclic ether. Method of using the compositions are also described, where in one embodiment, the compositions are topically applied for treating or preventing basal cell carcinoma.
Owner:SOL GEL TECH
Use of Tellurium Compounds for Protection from Ultra-Violet Radiation
Methods for treating skin conditions such as basal cell carcinoma and / or actinic keratosis are provided, which are effected by administering a pharmaceutically effective amount of a tellurium-containing compound. A pharmaceutical composition for treatment of skin conditions such as basal cell carcinoma an / or actinic keratosis is also provided.
Owner:SREDNI BENJAMIN
Use of tellurium compounds for protection from ultra-violet radiation
Methods for treating skin conditions such as basal cell carcinoma and / or actinic keratosis are provided, which are effected by administering a pharmaceutically effective amount of a tellurium-containing compound. A pharmaceutical composition for treatment of skin conditions such as basal cell carcinoma an / or actinic keratosis is also provided.
Owner:SREDNI BENJAMIN
Treatment and prognostic monitoring of proliferation disorders using hedgehog pathway inhibitors
ActiveUS20140315920A1Lower testosterone levelsSpeed upHeavy metal active ingredientsOrganic active ingredientsProstate cancerBasal cell carcinoma
The present invention concerns methods for treating a proliferation disorder, such as prostate cancer, basal cell carcinoma, lung cancer, and other cancers, using an inhibitor of the Hedgehog pathway (HhP); and methods for monitoring subjects undergoing such treatments based on biomarkers and other criteria predictive of efficacy.
Owner:HEDGEPATH PHARMA
Medical apparatus for determination of biological conditions using impedance measurements
ActiveUS9636035B2Accurate and reliable wayDiagnostic recording/measuringSensorsMelanomaSquamous Carcinomas
Medical apparatus for diagnosing of a diseased condition of the skin of a subject. The apparatus comprises an electrically conducting probe including a plurality of electrodes, each electrode comprising a plurality of micro-needles, wherein each electrode comprises a base substrate, said micro-needles being integrally formed with said substrate and arranged in a laterally spaced relationship apart from each other and having a length being sufficient to penetrate the stratum corneum, said micro-needles being arranged with an at least partially oblique shape. Furthermore, the present invention relates to an electrode for use in the device, arrays of micro-needle and method for the diagnostics of biological conditions using impedance measurements. The diagnostics is in particular related to cancer, and preferably skin cancer, wherein skin cancer is a basal cell carcinoma, a malignant melanoma, a squamous cell carcinoma, or precursors of such lesions.
Owner:SCIBASE
Methods of modulating the activity of the MC1 receptor and treatment of conditions related to this receptor
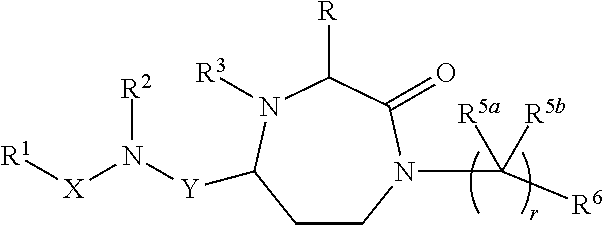
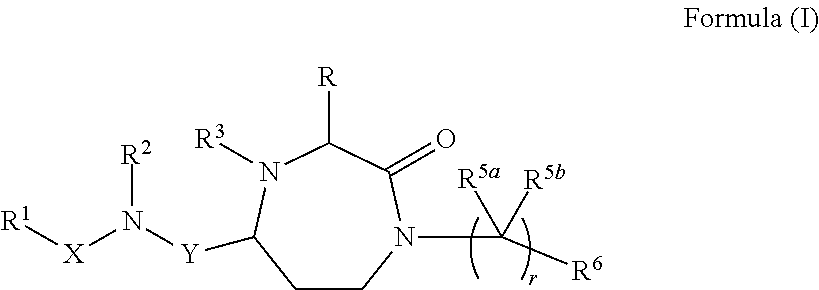
InactiveUS20140128380A1Reduce formation rateBiocideCosmetic preparationsSolar urticariaHypopigmentation
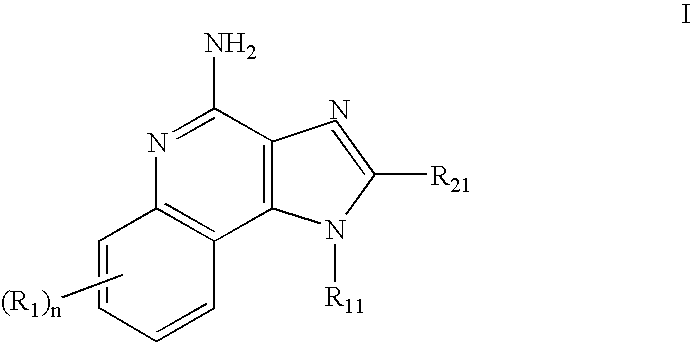
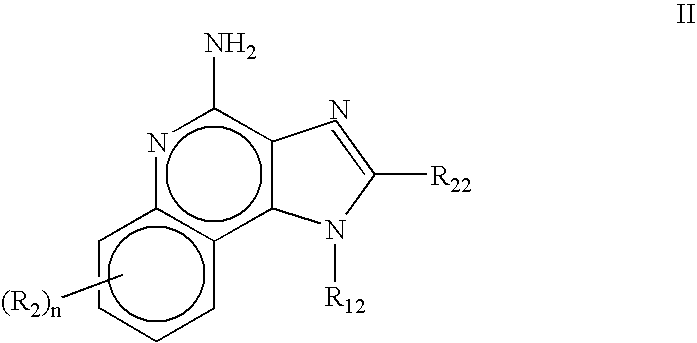
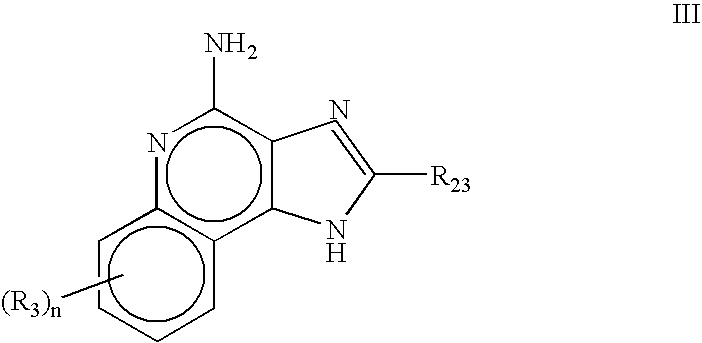
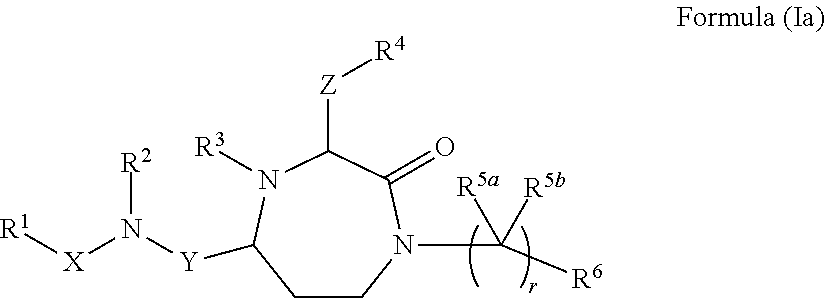
The present invention provides compounds of Formula (I) that are useful for binding and / or modulating the biological activity of the melanocortin-1 receptor (MC1R). Compounds of this invention can be used to treat diseases and / or conditions in which modulation of MC1R is beneficial. Such diseases and / or conditions include, but are not limited to, hyperpigmentation (including melasma), hypopigmentation (including vitiligo), melanoma, basal cell carcinoma, squamous cell carcinoma, erythropoietic protoporphyria, polymorphous light eruption, solar urticaria, photosensitivity, sunburn, inflammatory diseases, aberrant fibroblast activity and pain.
Owner:MIMETICA PTY LTD
System and method for enhancing confocal reflectance images of tissue specimens
InactiveUS7110114B2Easy to observeEnhance the imagePolarisation-affecting propertiesInvestigating moving sheetsAbnormal tissue growthPolarizer
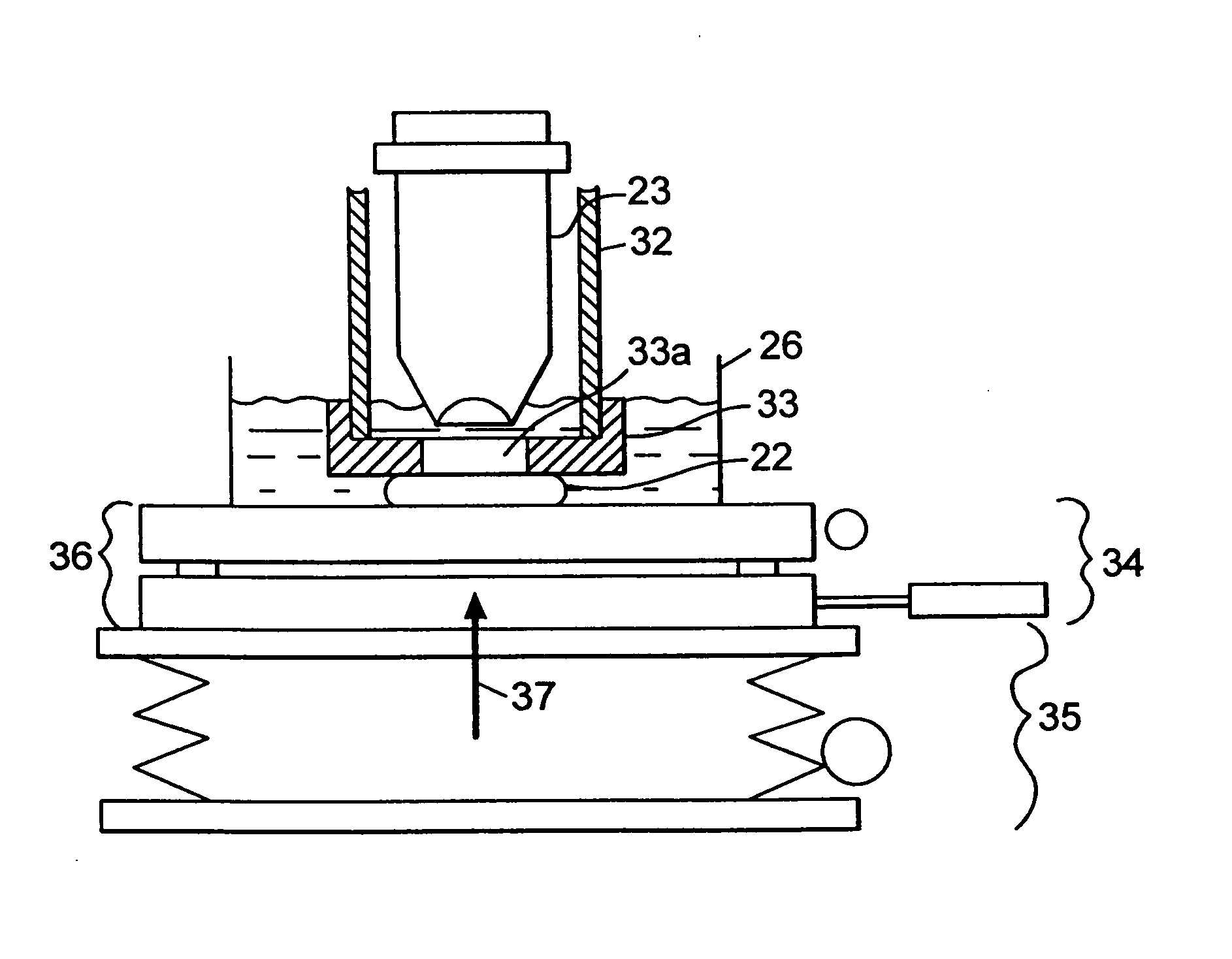
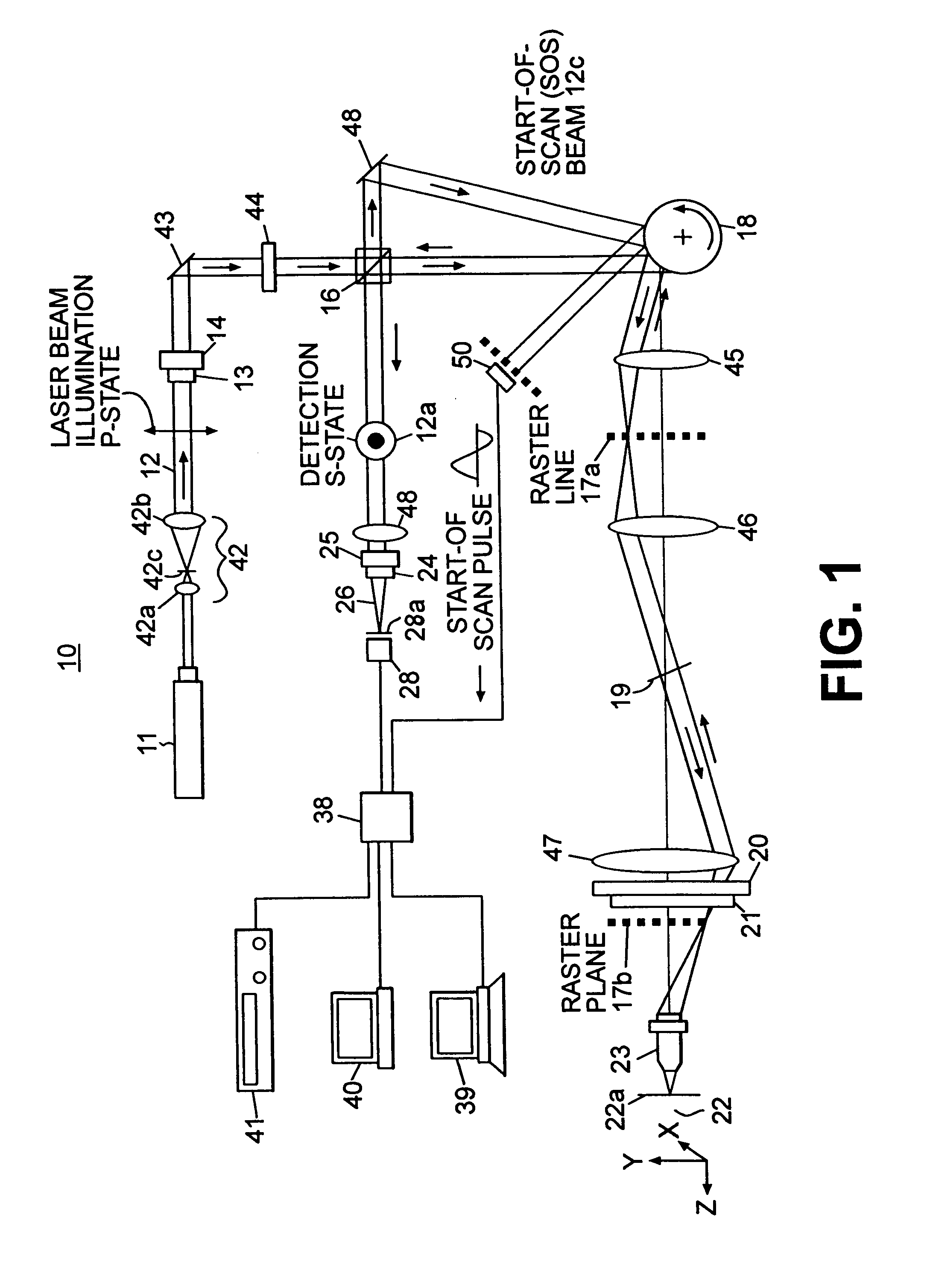
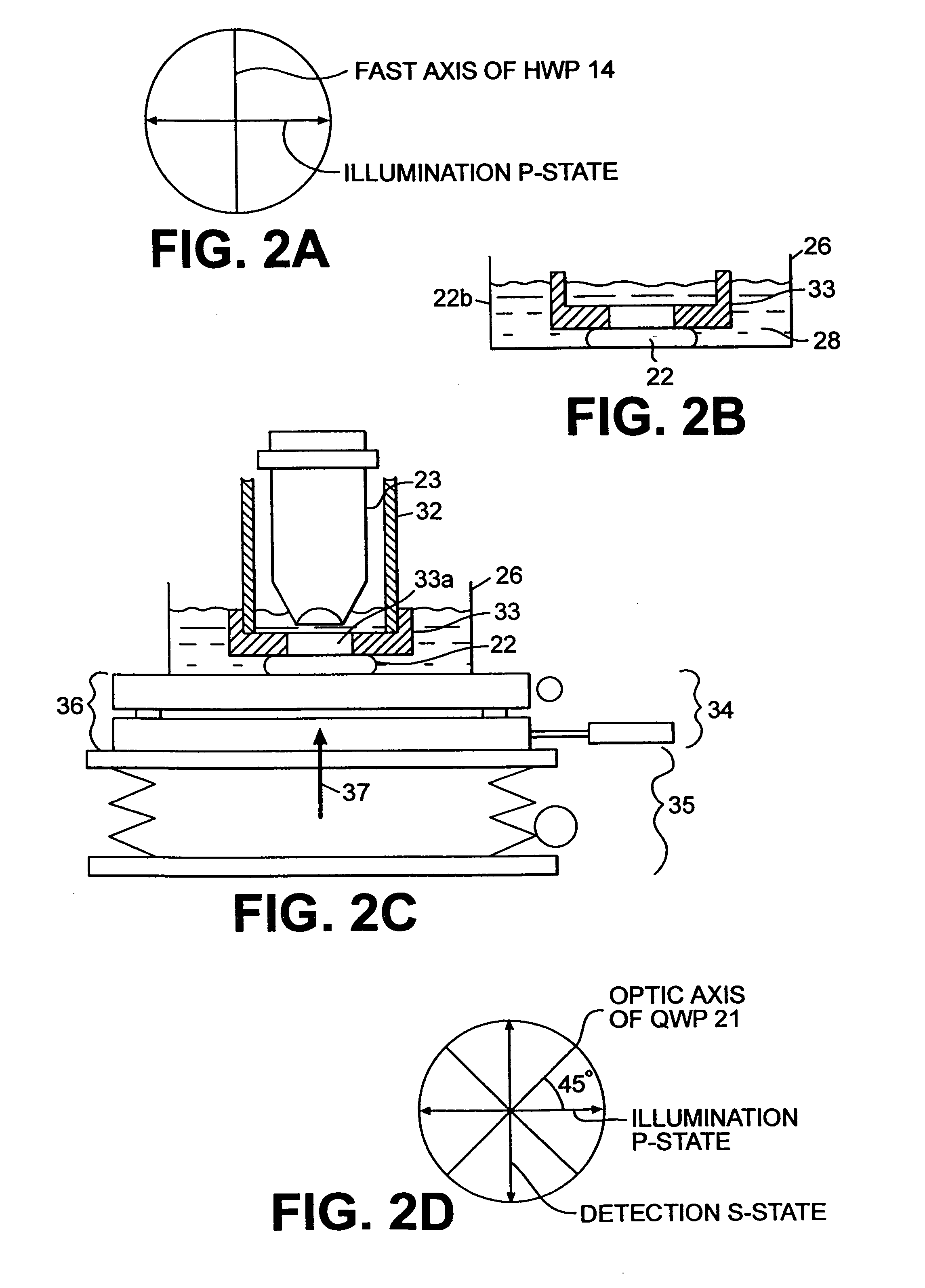
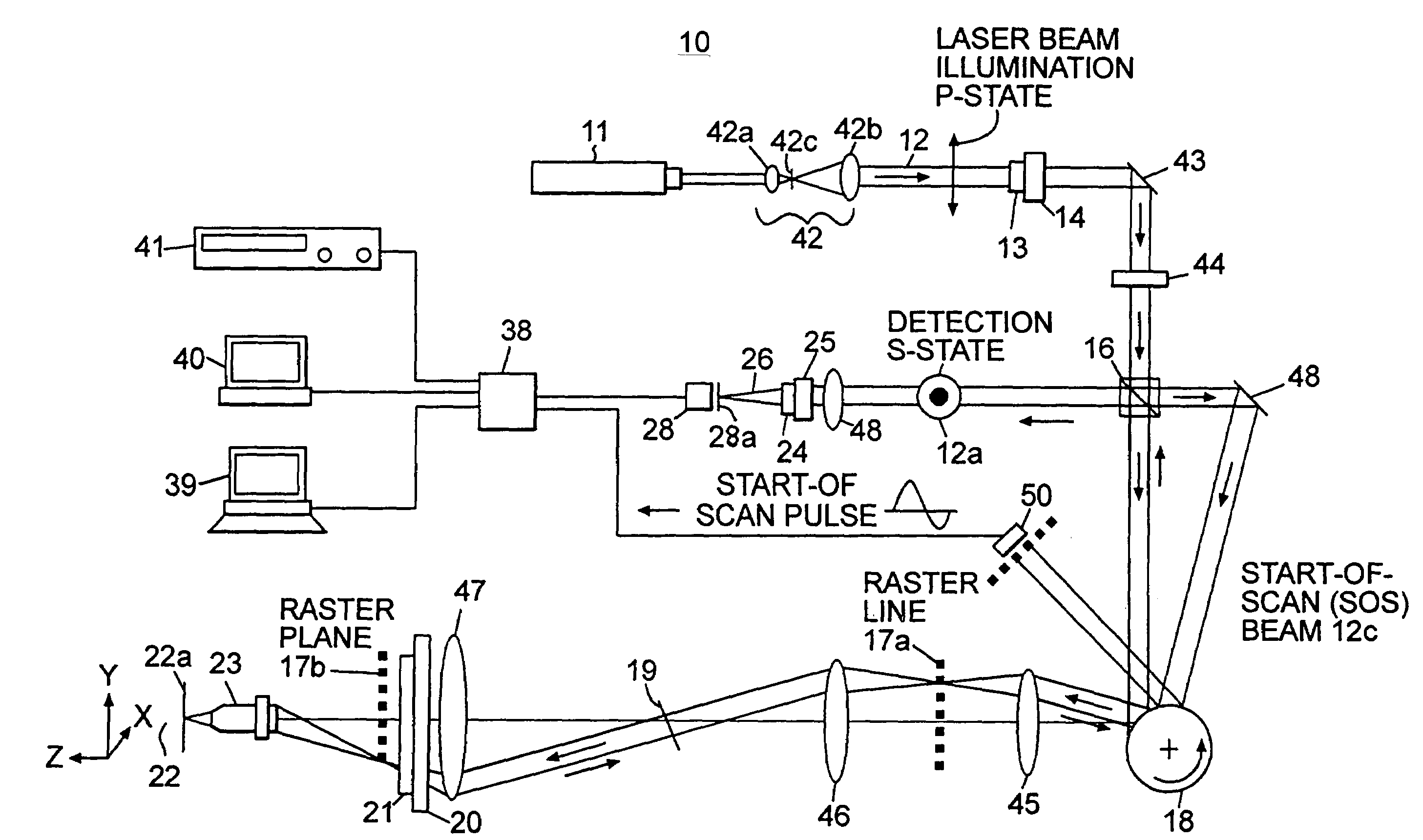
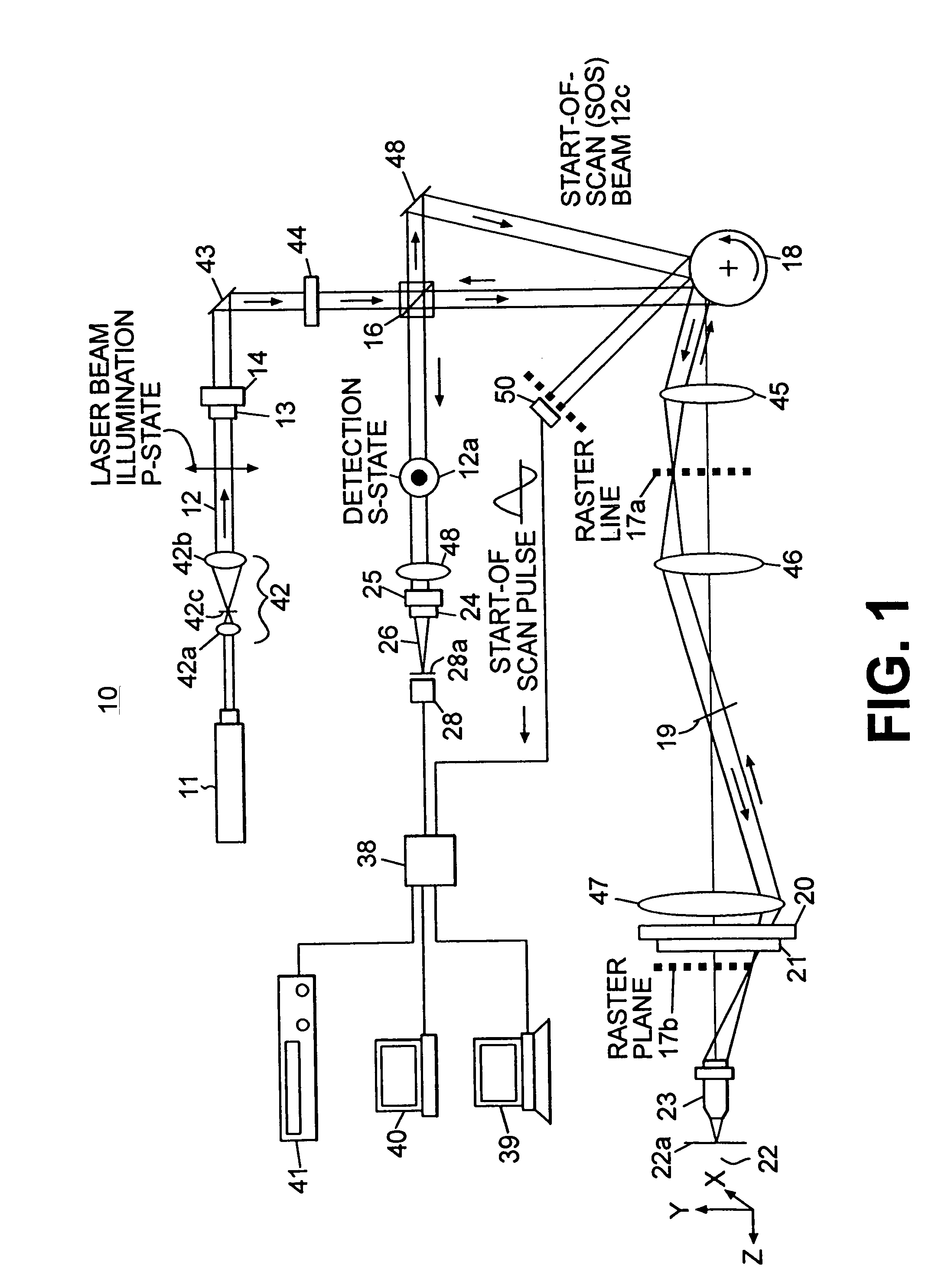
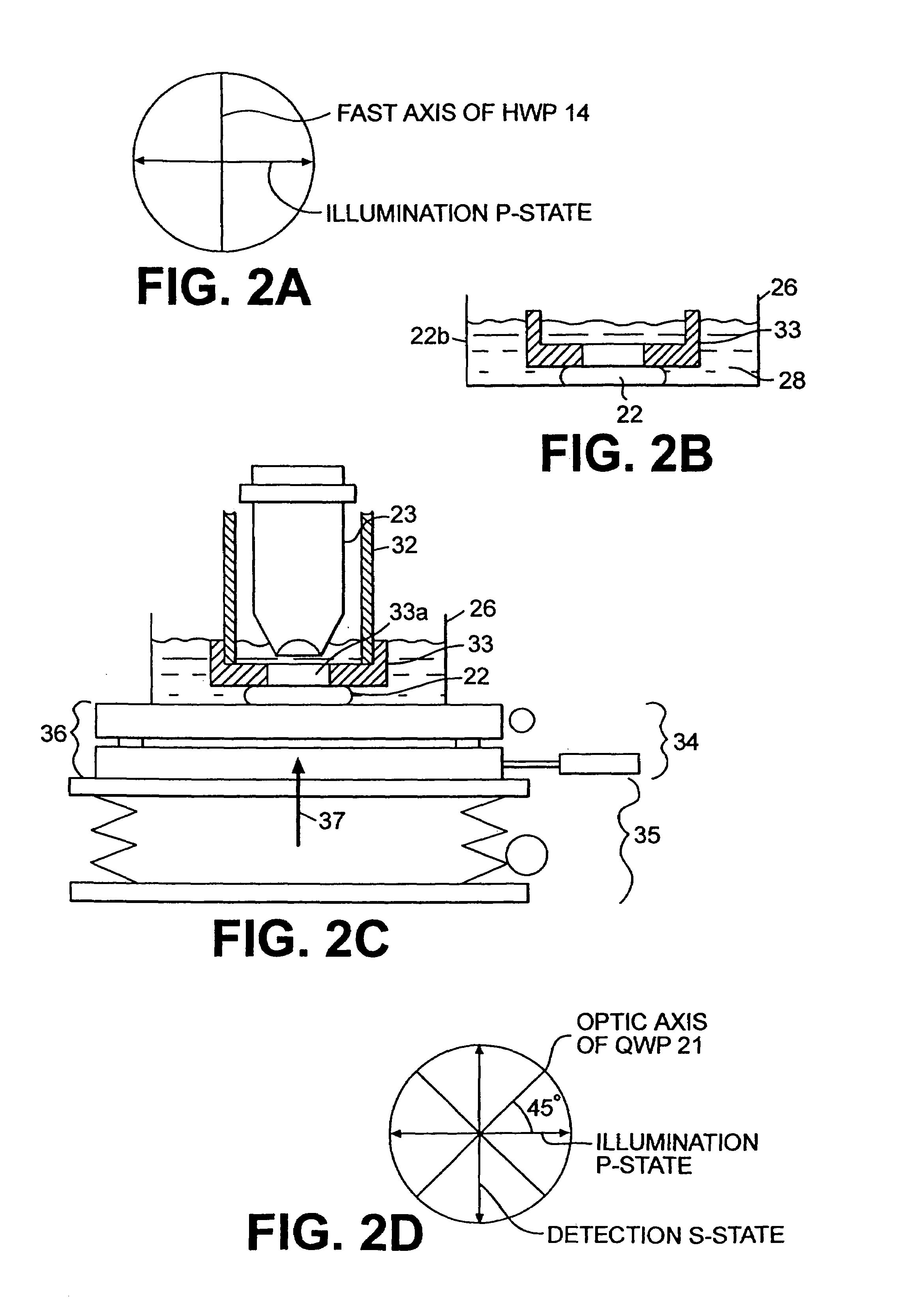
A confocal scanning microscope system (10) using cross polarization effects and an enhancement agent (acetic acid) to enhance confocal microscope reflectance images of the nuclei of BCCs (basal cell carcinomas) and SCCs (squamous call carcinomas) in the confocal reflectance images of excised tumor slices. The confocal scanning microscope system having a laser (11) for generating an illumination beam (12), a polygon mirror (18) for scanning the beam to a tissue sample (22) and for receiving a return beam from the tissue sample and detector (28) for detecting the returned beam to form an image. The system further includes a half-waveplate (13) having a rotatable stage (14) and a quarter-wave plate (21) having a rotatable stage (20) disposed in the optical path of the illumination beam and at least a linear polarizer (24) having a rotatable stage (25) disposed in the optical pat of the returned beam from the tissue sample.
Owner:CALIBER IMAGING & DIAGNOSTICS +1
Treatment method and apparatus for basal cell carcinoma
InactiveUS20050251231A1Effective treatment responseIncrease supplyBiocideOrganic active ingredientsMedicineMonochromatic color
A method and device for treatment of basal cell carcinoma utilizing controllable and substantially monochromatic electromagnetic radiation, such as emitted by a pulsed ray laser. The wavelength may range from 585 nm to 1560 nm.
Owner:GOLDBERG LEONARD H
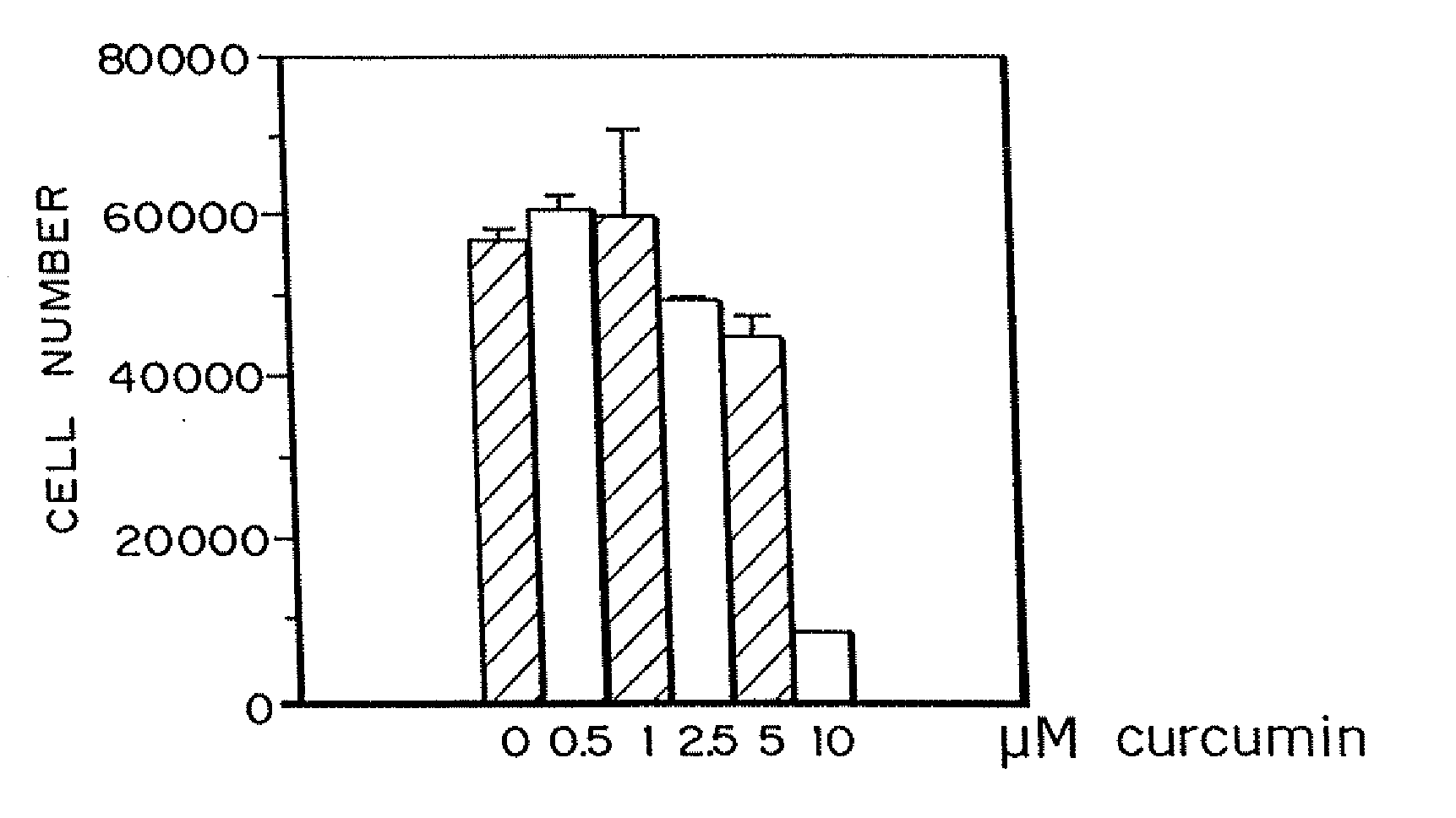
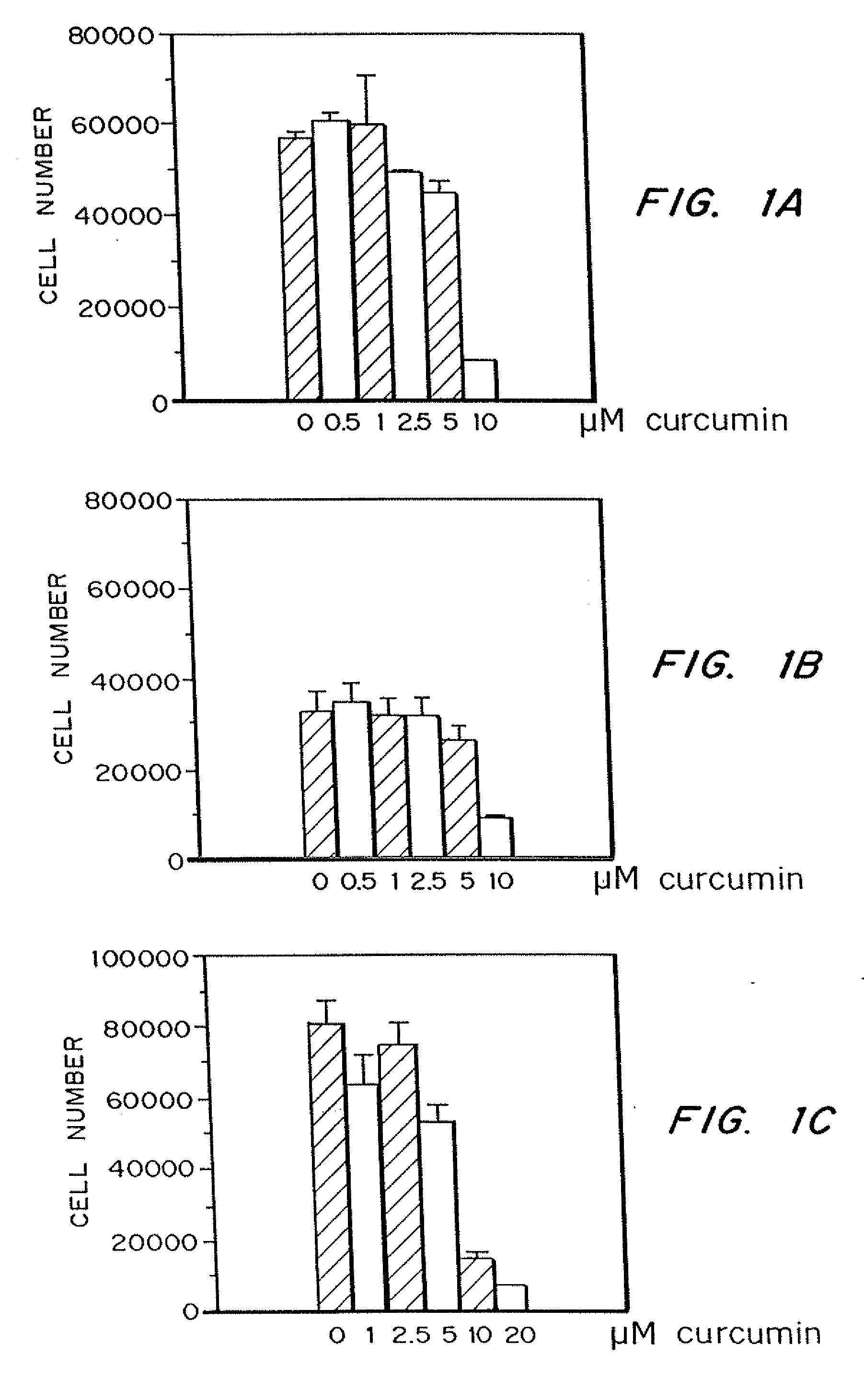
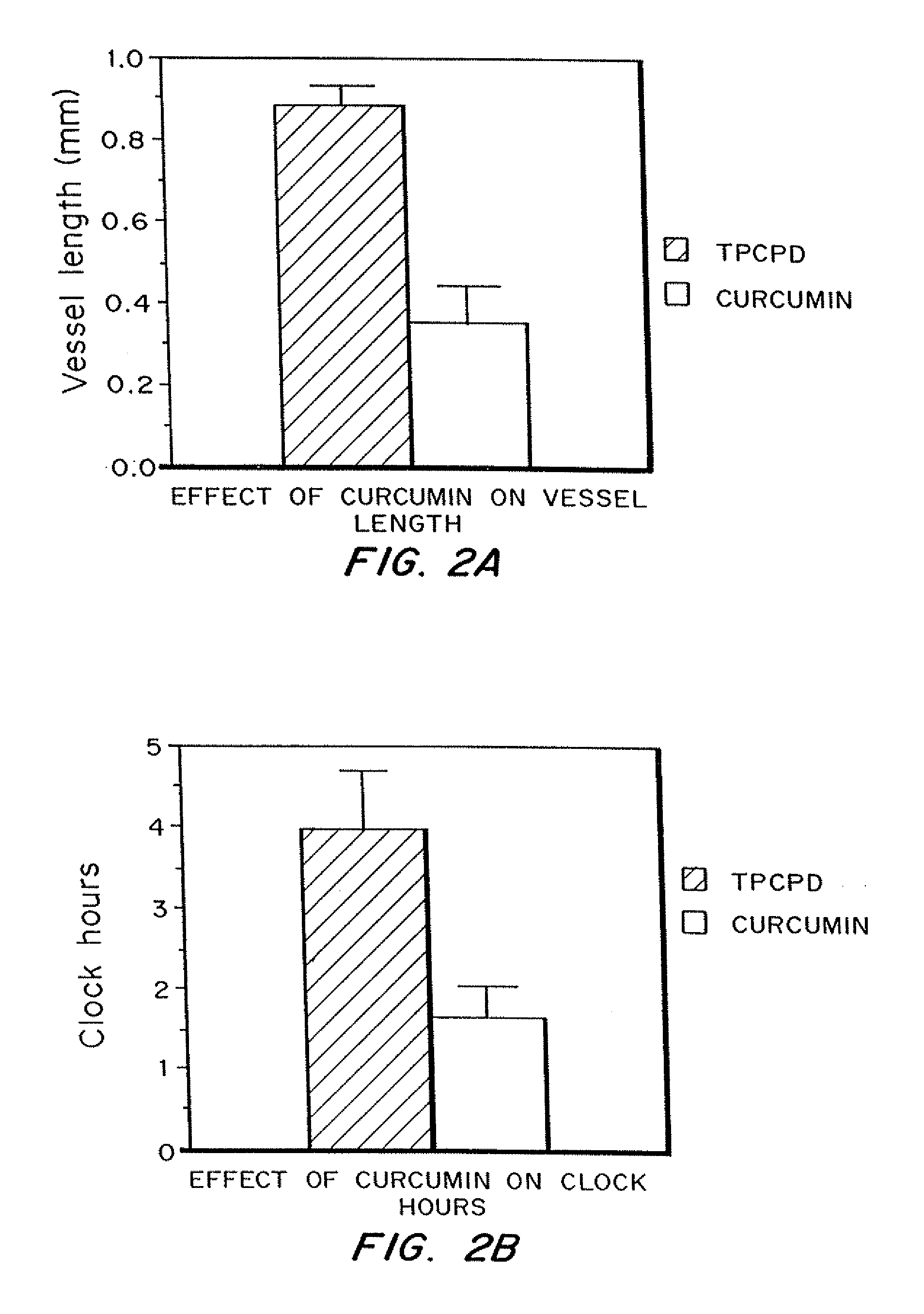
Curcumin and curcuminoid inhibition of angiogenesis
InactiveUS20090018209A1Inhibit angiogenesisImprove the level ofBiocideDigestive systemRecessive dystrophic epidermolysis bullosaSturge–Weber syndrome
Methods for treating diseases or disorders of the skin which are characterized by angiogenesis have been developed using curcumin and curcumin analogs. Based on the results obtained with curcumin, it has been determined that other angiogenesis inhibitors can also be used to treat these skin disorders. It has further been discovered that curcumin acts to inhibit angiogenesis in part by inhibition of basic fibroblast growth factor (bFGF), and thereby provides a means for treating other disorders characterized by elevated levels of bFGF, such as bladder cancer, using curcumin and other analogues which also inhibit bFGF. Representative skin disorders to be treated include the malignant diseases angiosarcoma, hemangioendothelioma, basal cell carcinoma, squamous cell carcinoma, malignant melanoma and Karposi's sarcoma, and the non-malignant diseases or conditions including psoriasis, lymphangiogenesis, hemangioma of childhood, Sturge-Weber syndrome, verruca vulgaris, neurofibromatosis, tuberous sclerosis, pyogenic granulomas, recessive dystrophic epidermolysis bullosa, venous ulcers, acne, rosacea, eczema, molluscum contagious, seborrheic keratosis, and actinic keratosis.
Owner:EMORY UNIVERSITY
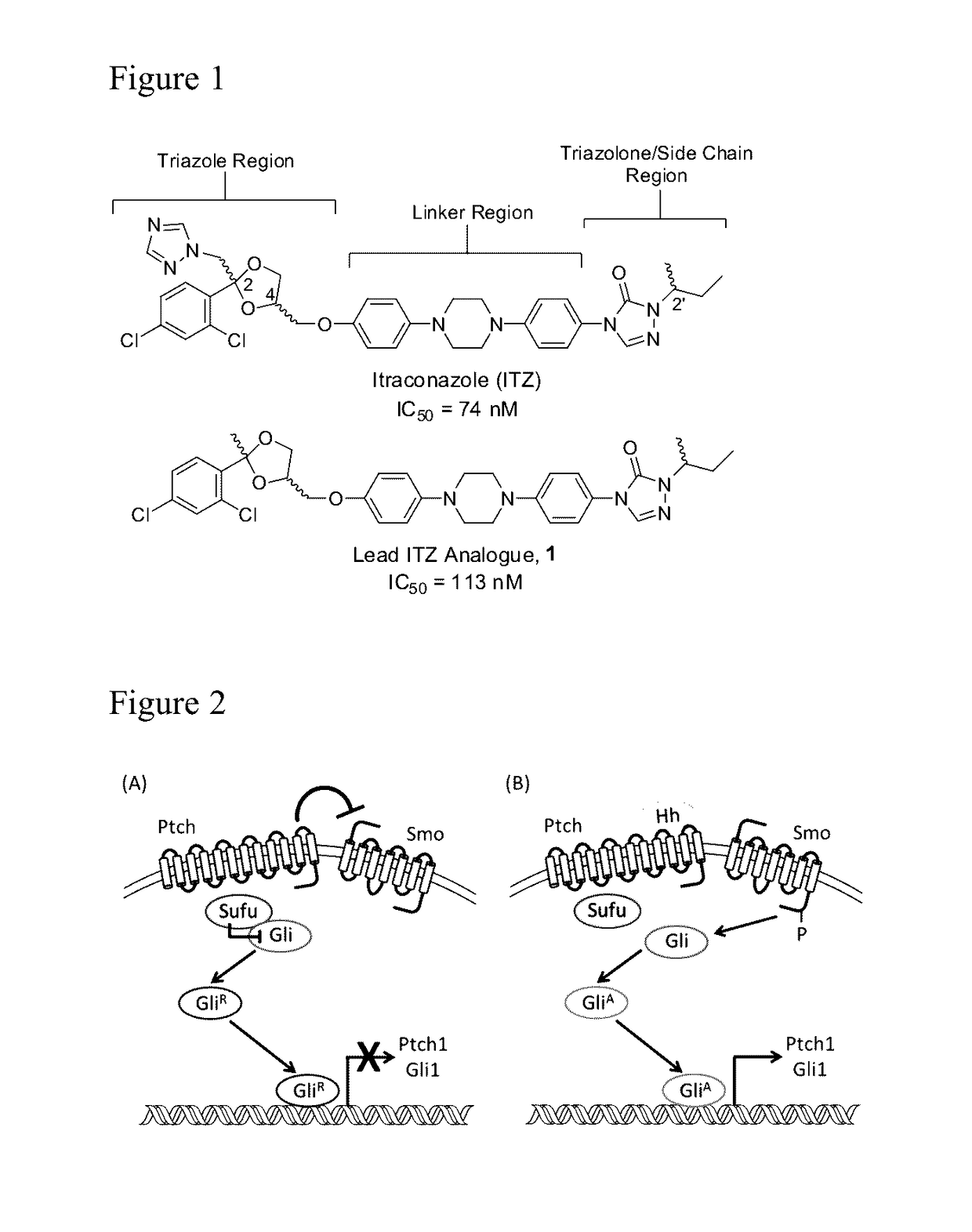
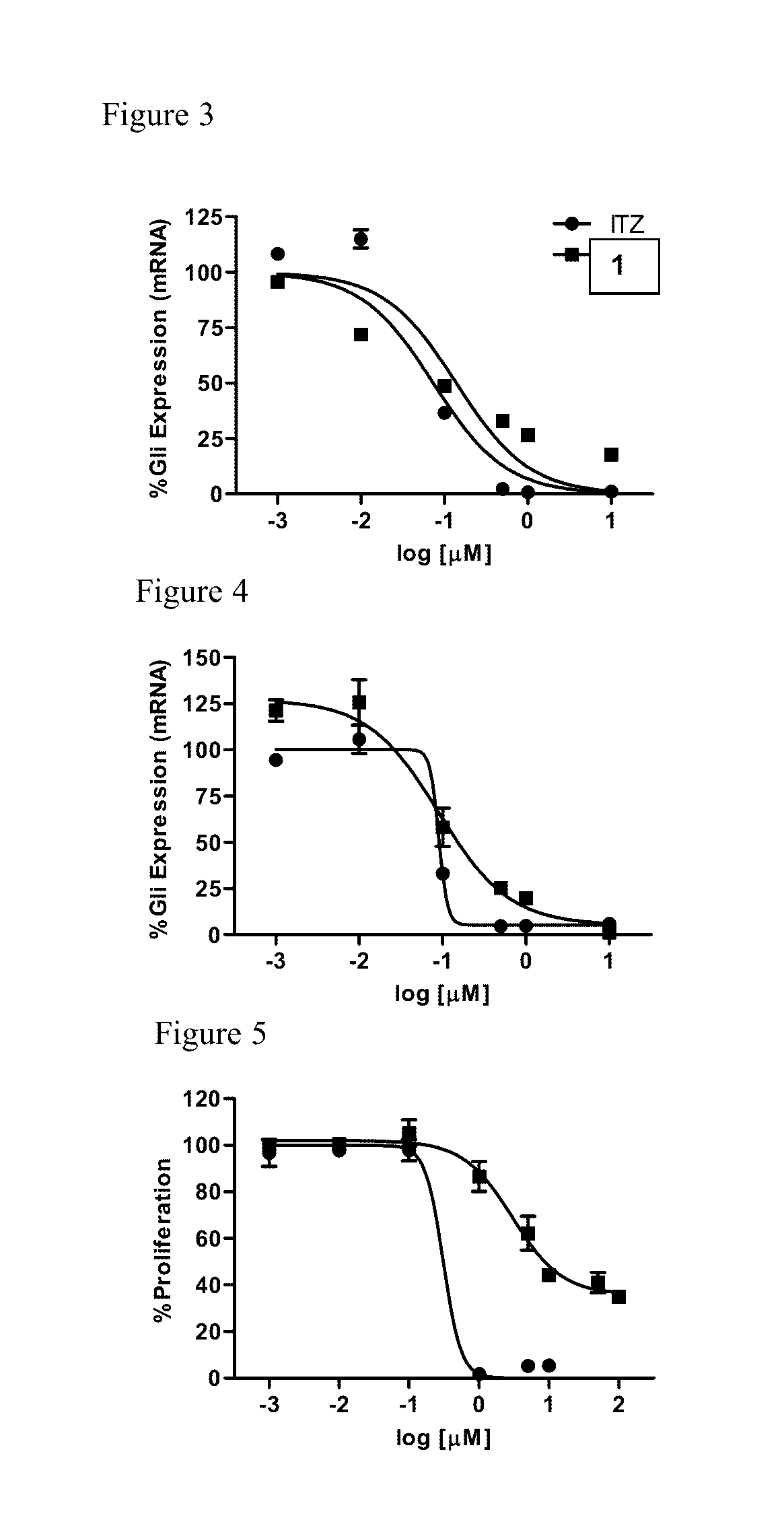
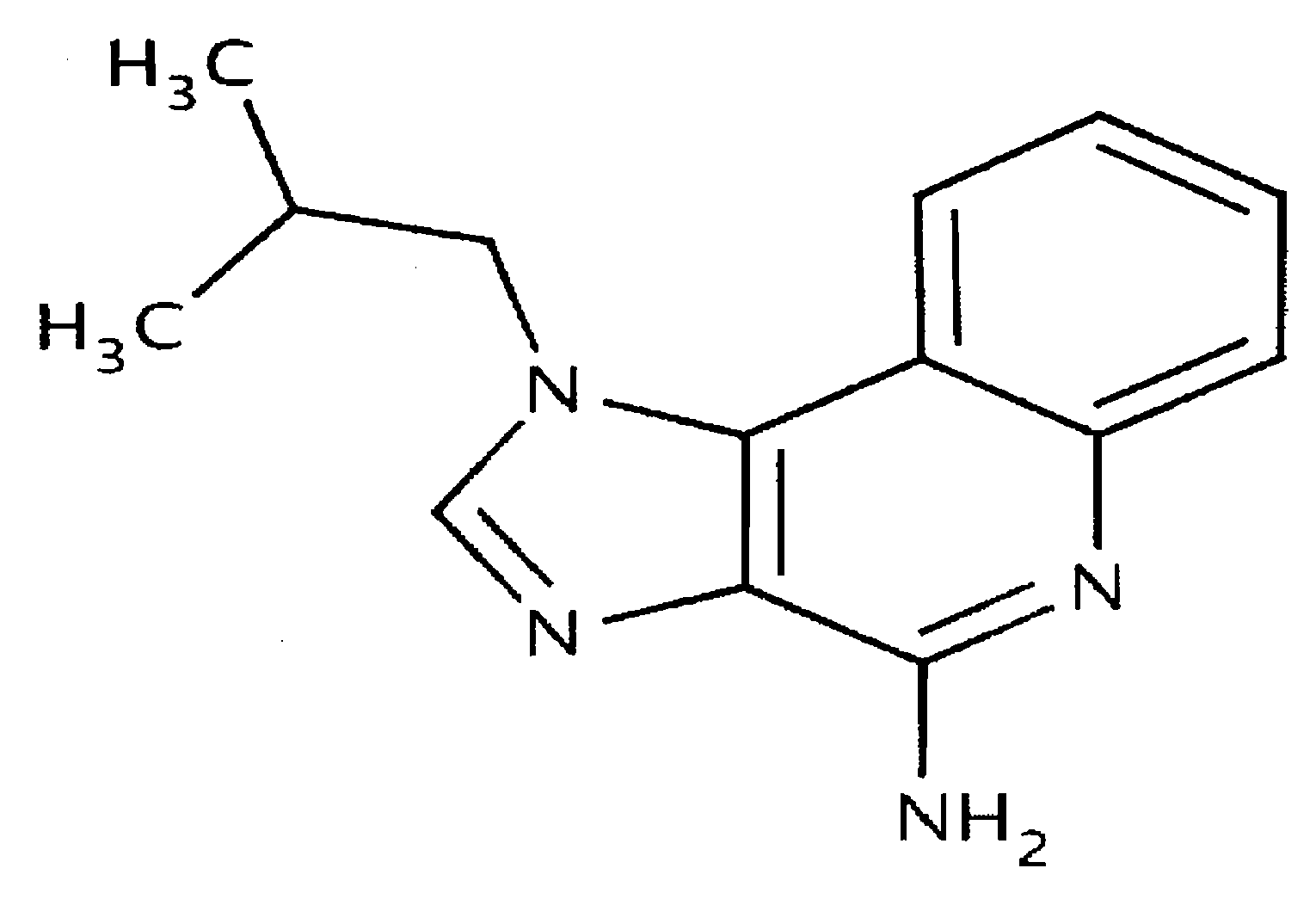
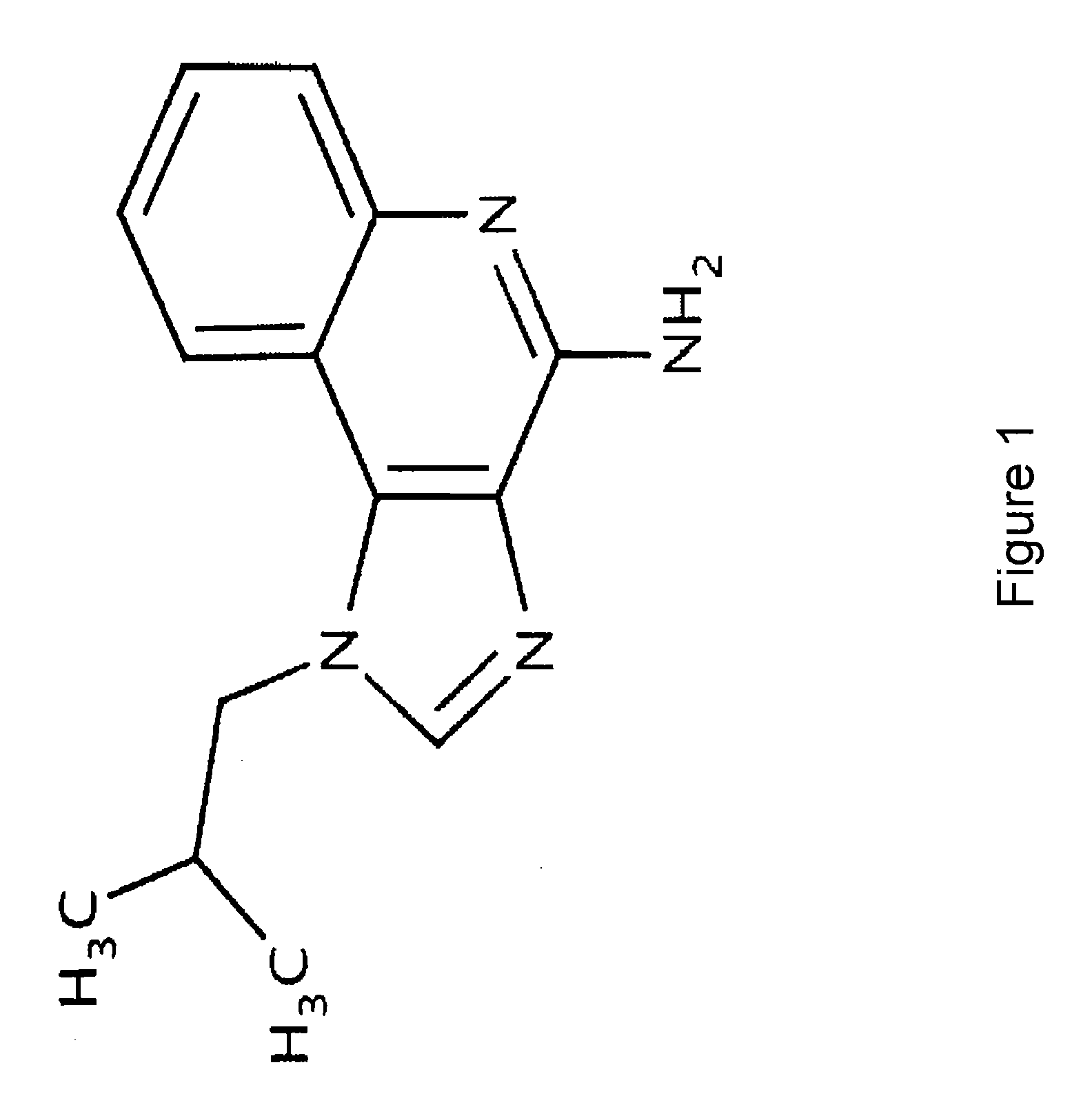
Itraconazole analogues and methods of use thereof
ActiveUS9650365B2Organic active ingredientsOrganic chemistryHedgehog signaling pathwayAngiogenesis growth factor
Disclosed herein are analogs of itraconazole that are both angiogenesis and hedgehog signaling pathway inhibitors. The compounds are expected to be useful in the treatment of cancer, particularly cancers that are dependent upon the hedgehog signaling pathway such as basal cell carcinoma and medulloblastoma.
Owner:UNIV OF CONNECTICUT
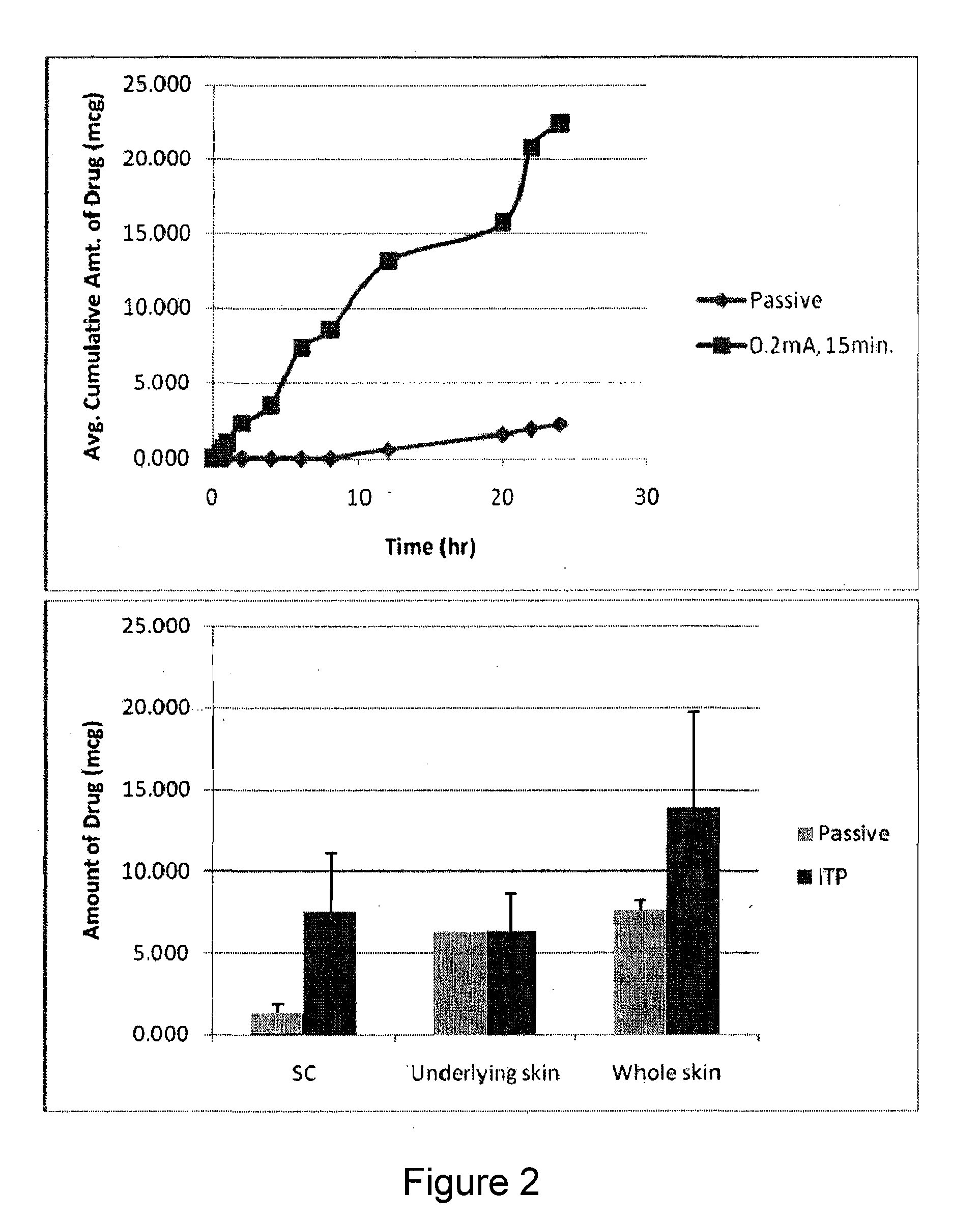
Pharmaceutical Formulations for Iontophoretic Delivery of an Immunomodulator
The present invention describes pharmaceutical formulations and methods suitable for iontophoretic delivery of the formulations to a subject. The formulations comprise an immunomodulator, such as imiquimod, and optionally include various agents and excipients. The formulations can be used as a treatment for skin diseases and conditions such as actinic keratosis, basal cell carcinoma and genital warts. The short term iontophoretic delivery of the formulations results in the creation of a depot effect in the skin of the subject, allowing for a sustained delivery. The shortened delivery time minimizes local side effects at the application site.
Owner:NITRIC BIOTHERAPEUTICS INC
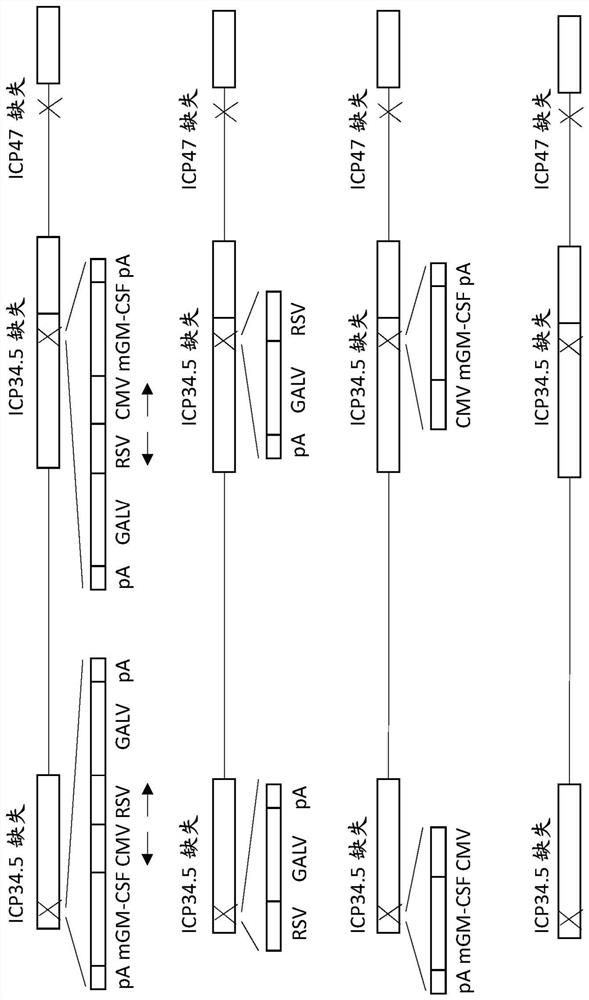
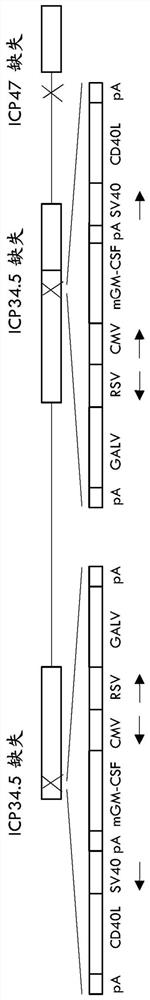
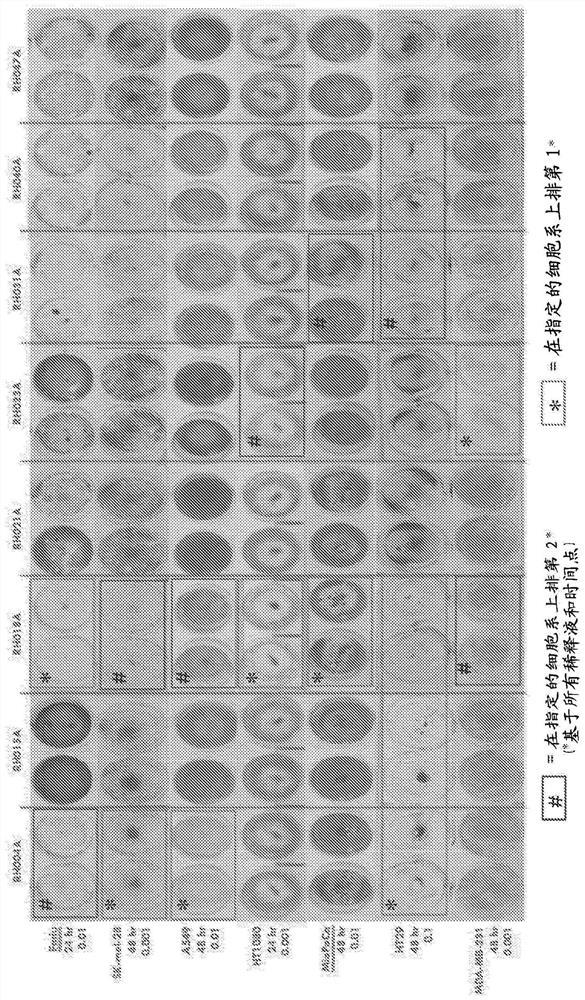
Treatment using oncolytic virus
An oncolytic virus is for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelial carcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patientswith no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / orcancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panelof three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com