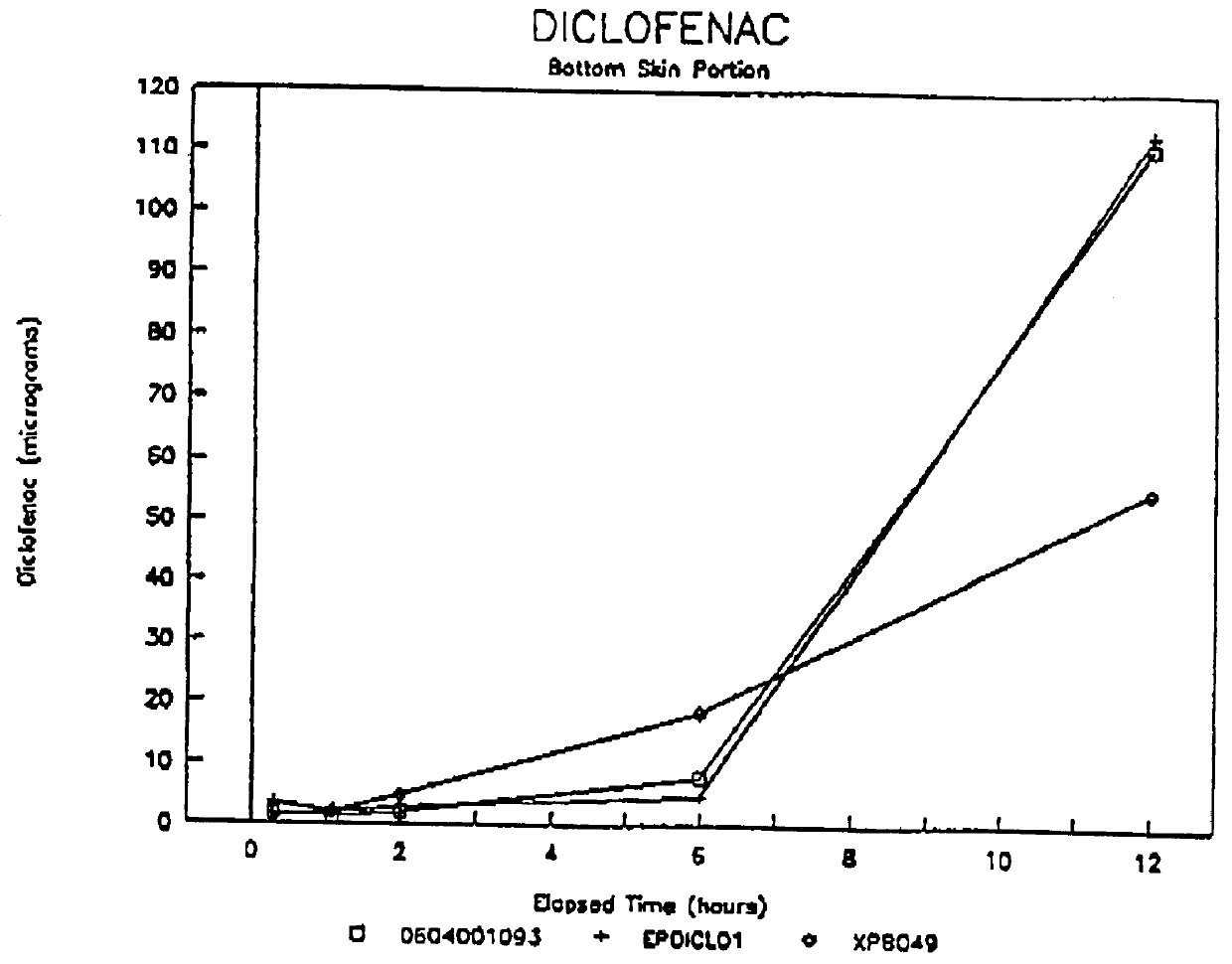
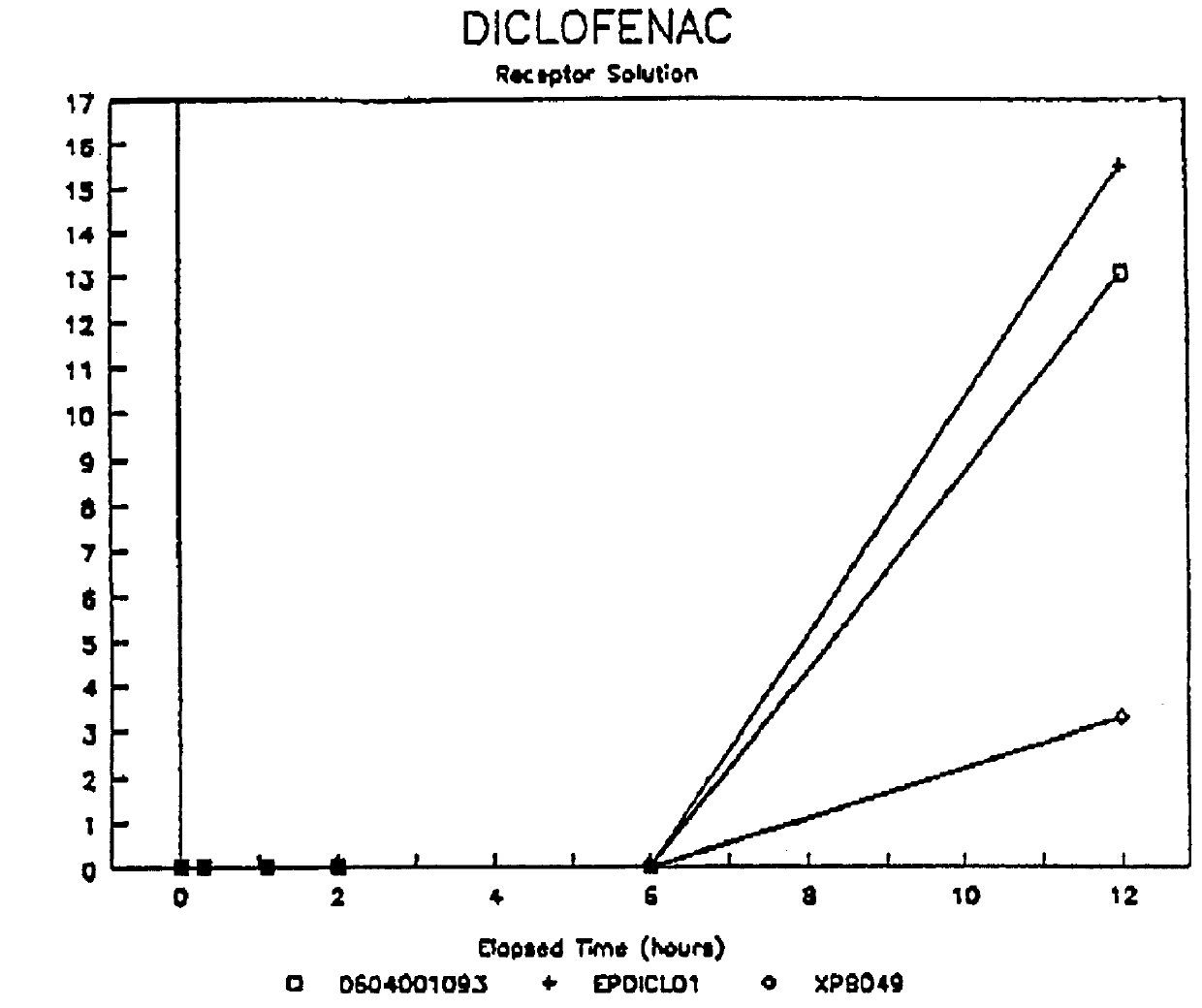
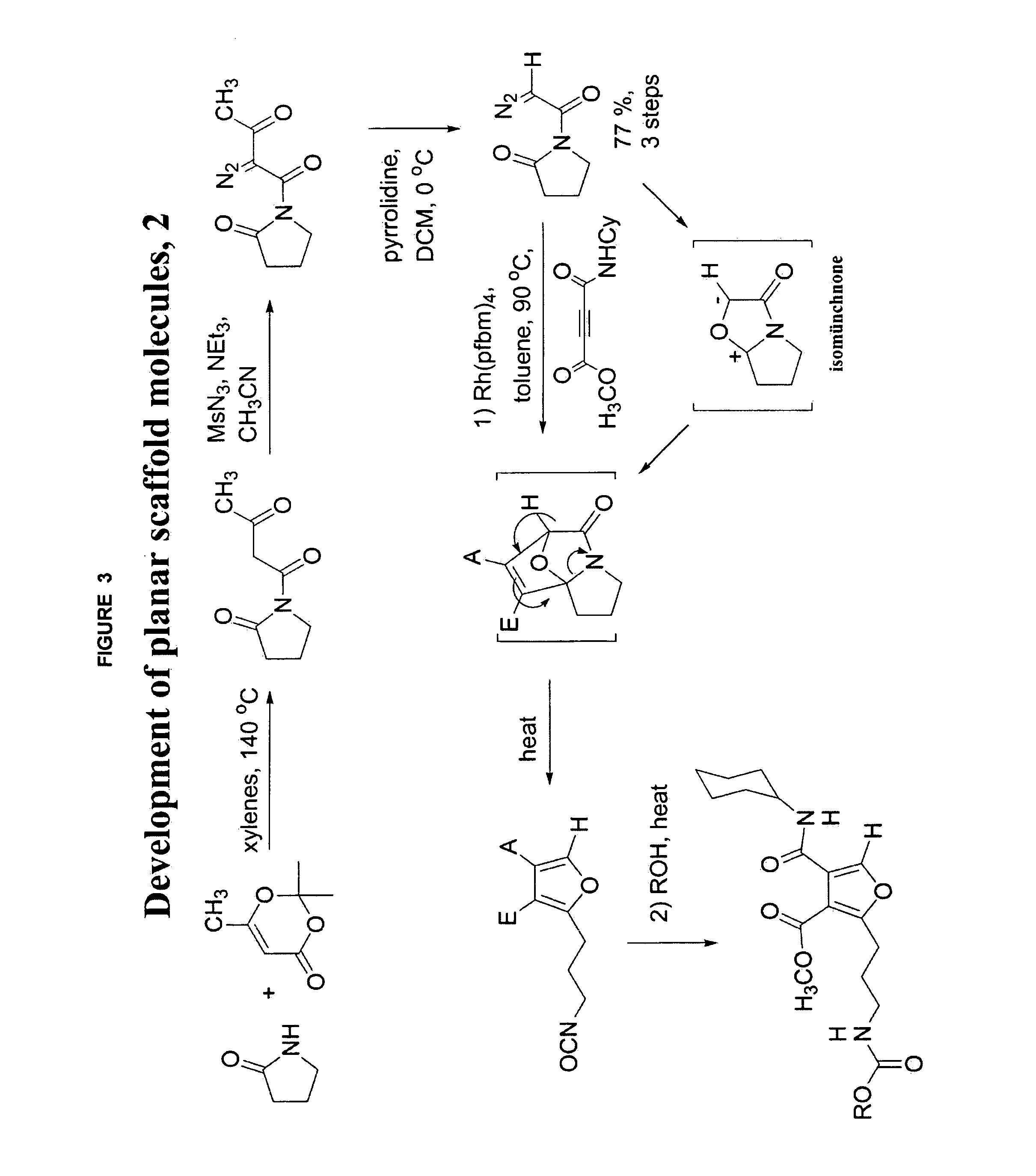
Patents
Literature
75 results about "Genital warts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A sexually transmitted disease resulting in a small lump or swelling on the genitals.
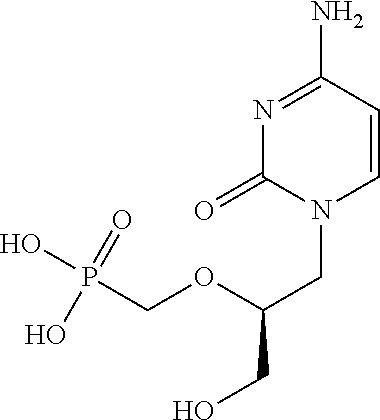
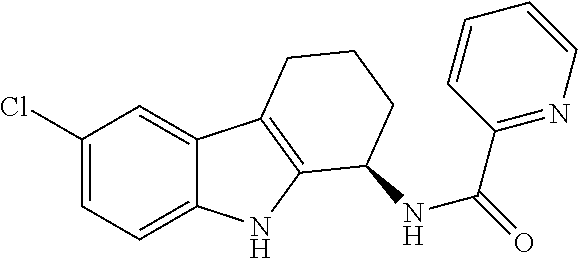
Pharmacological agent and method of treatment
InactiveUS20060074063A1Eliminate effectiveEliminate energyBiocideGroup 8/9/10/18 element organic compoundsWhole bodyVirus warts
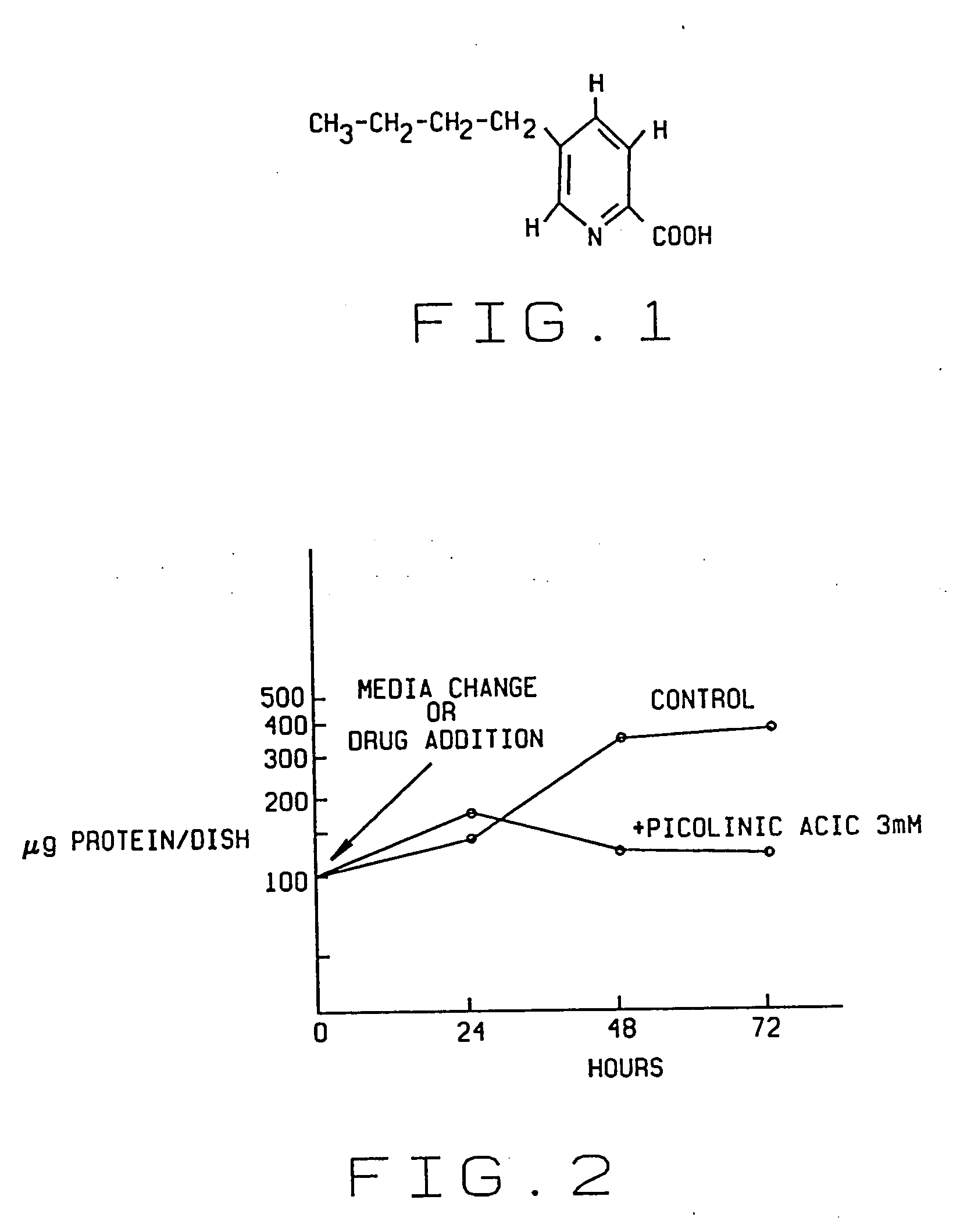
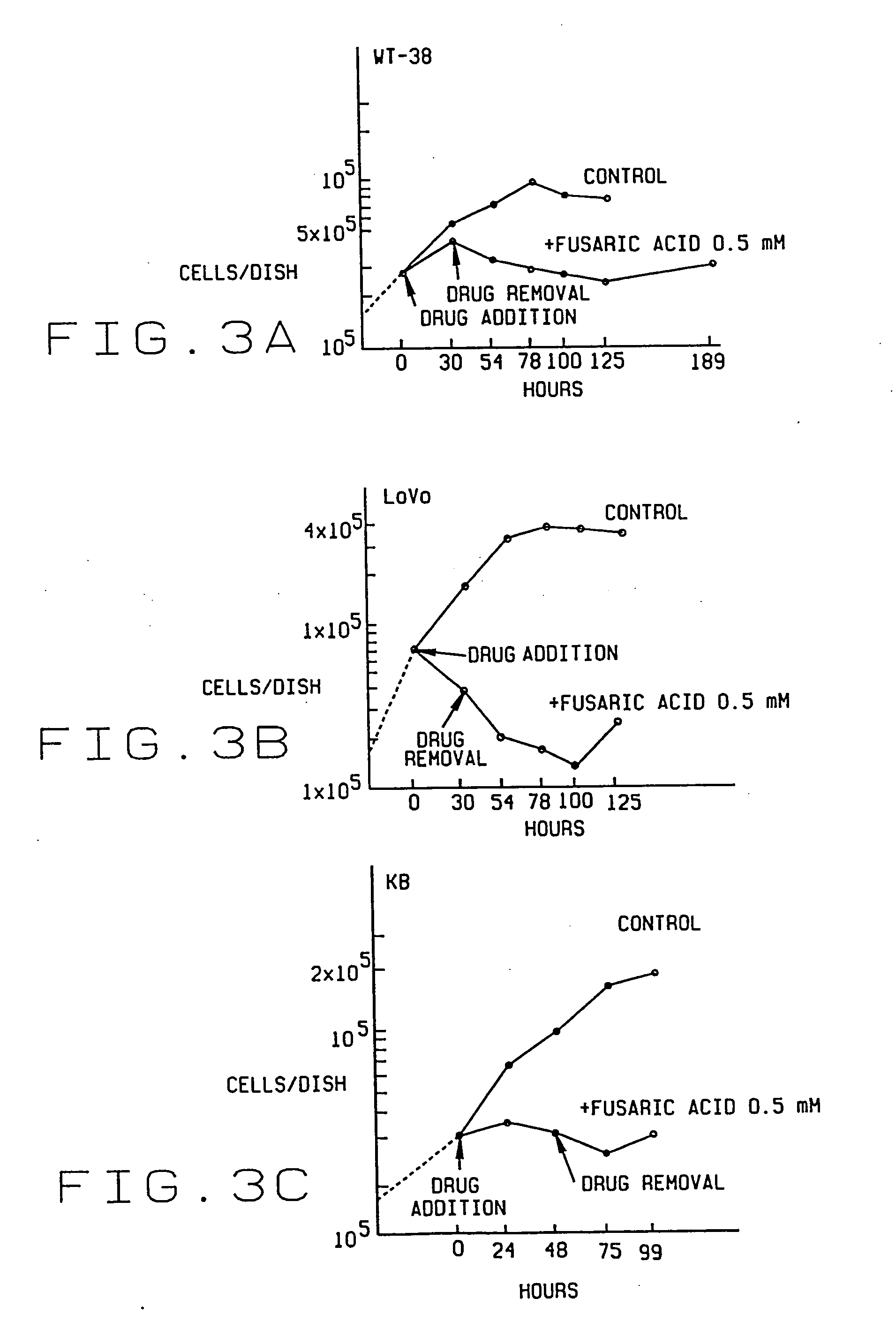
An antiproliferative, antiinflammatory, antiinfective, immunization agent of a metal ion chelating agent such as picolinic acid, analogs or derivatives thereof, and methods of using the same. The agents chelate metals in metal containing protein complexes and enzymes required for growth, replication or inflammatory response. The preparations can be administered systemically or topically. The products can be used to reduce systemic levels of metals in disease states such as Wilson's disease, iron or lead toxicity. The preparations have antineoplastic, antiviral, antiinflammatory, analgesic antiangiogenic and antiproliferative effects and are used in the treatment of warts, psoriasis, acne, cancers, sunburn, inflammatory responses, untoward angiogenesis and other diseases and in the prevention of sexually transmitted diseases such as genital warts, herpes and AIDS.
Owner:NOVACTYL
Formulations containing hyaluronic acid
InactiveUS6114314AInhibit synthesisQuick to penetrate into skinBiocideSugar derivativesDiseaseActinic keratoses
Topically applied transdermally quick penetrating (best targeting the epidermis and subsequently remaining there for a prolonged period of time) systemic independent acting, combinations and formulations which employ, combine, or incorporate a therapeutically effective non-toxic (to the patient) amount of a drug which inhibits prostaglandin synthesis together with an amount of hyaluronic acid and / or salts thereof (for example the sodium salt) and / or homologues, analogues, derivatives, complexes, esters, fragments, and / or sub units of hyaluronic acid to treat a disease and condition of the skin and exposed tissue for example, basal cell carcinoma, the precancerous, often recurrent, actinic keratoses lesions, fungal lesions, "liver" spots and like lesions (found for the most part in the epidermis), squamous cell tumours, metastatic cancer of the breast to the skin, primary and metastatic melanoma in the skin, genital warts cervical cancer, and HPV (Human Papilloma Virus) including HPV of the cervix, psoriasis (both plaque-type psoriasis and nail bed psoriasis), corns on the feet and hair loss on the head of pregnant women and remain in the skin for a prolonged period of time.
Owner:JAGOTEC AG +1
Chemoprevention and treatment of cervical or vaginal neoplasia
InactiveUS6399645B1Inhibit progressBiocideAnimal repellantsViral diseaseCervical intraepithelial neoplasia
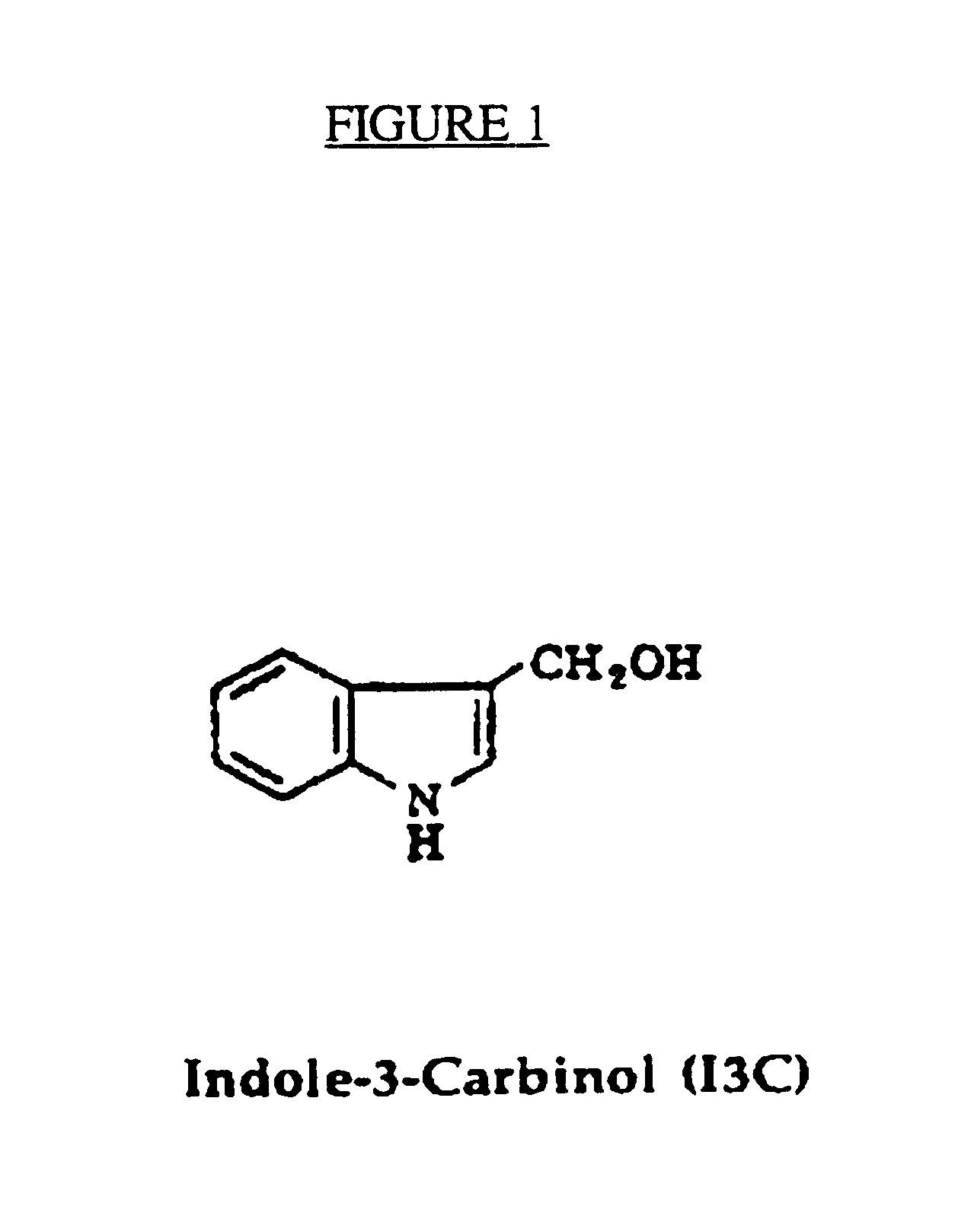
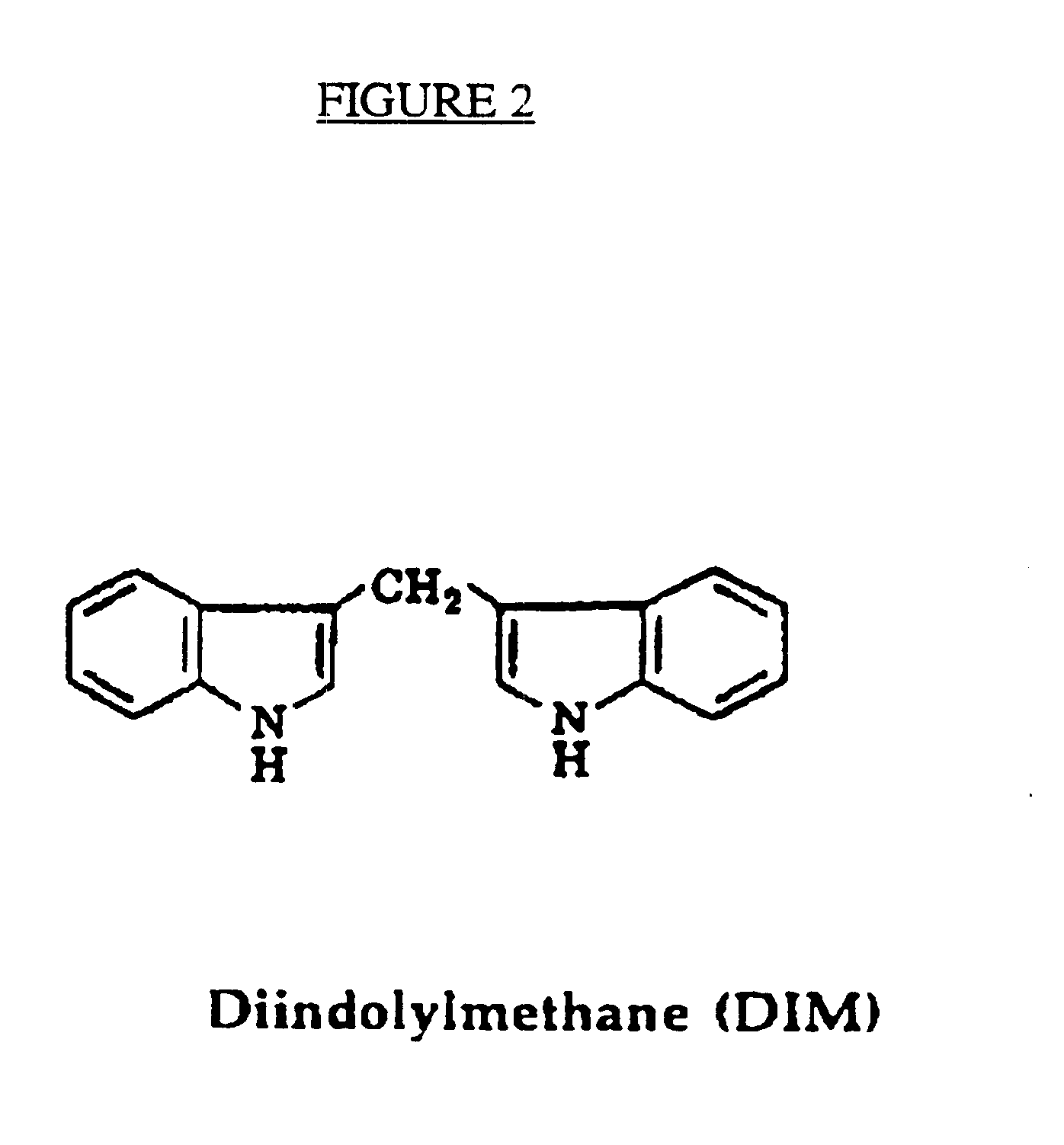
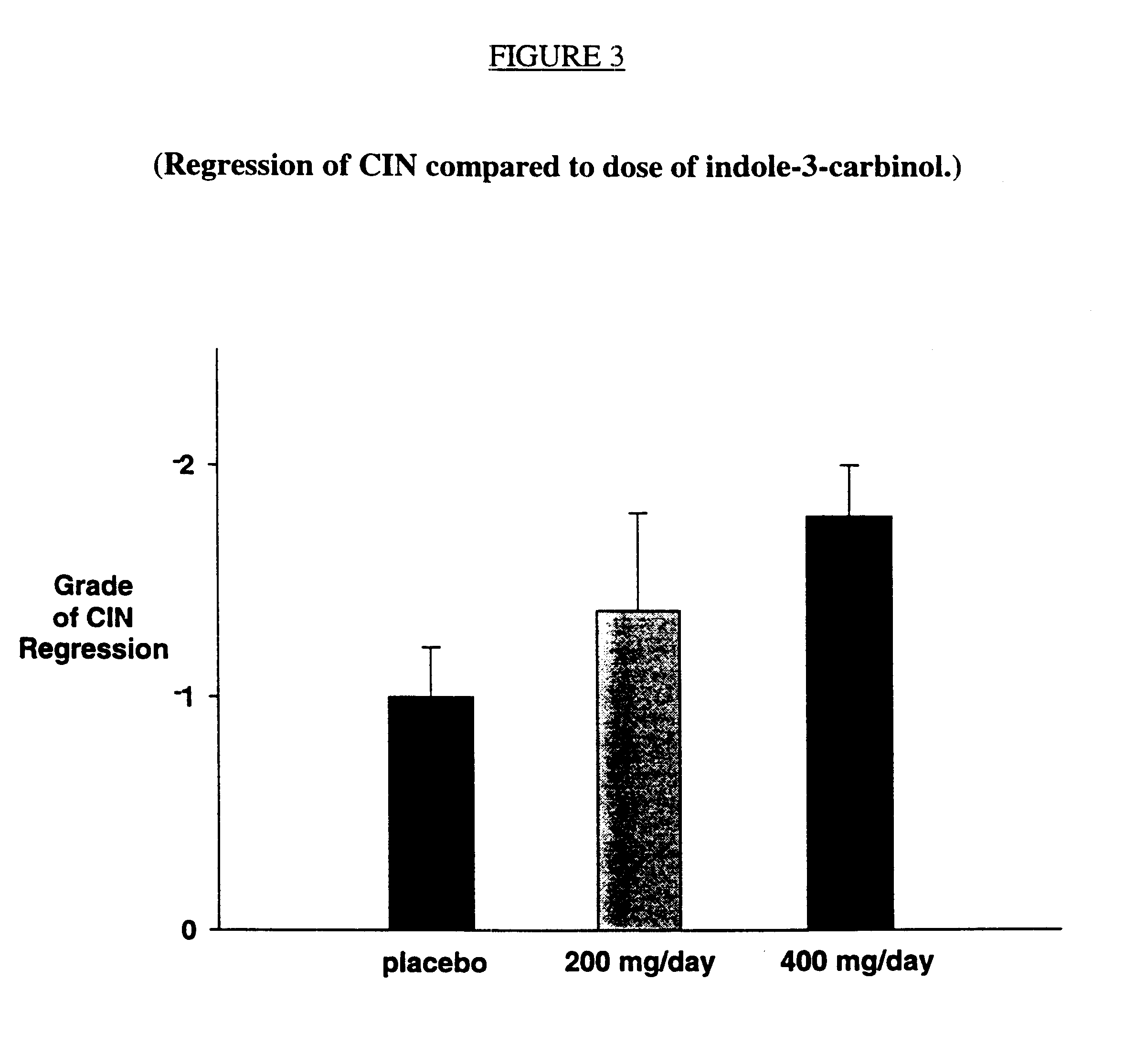
The invention relates to non-surgical methods for treating cervical or vaginal neoplasia including cervical intraepithelial neoplasia, intraepithelial neoplasia, vulvar intraepithelial neoplasia and ano-genital warts. The treatment, which utilizes an effective amount of indole-3-carbinol and / or diindolylmethane, is effective whether or not the patient is also infected with human papillomavirus, the most common sexually transmitted viral disease in the United States and a known risk factor for both cervical intraepithelial neoplasia and cervical cancer.
Owner:BELL MARIA +1
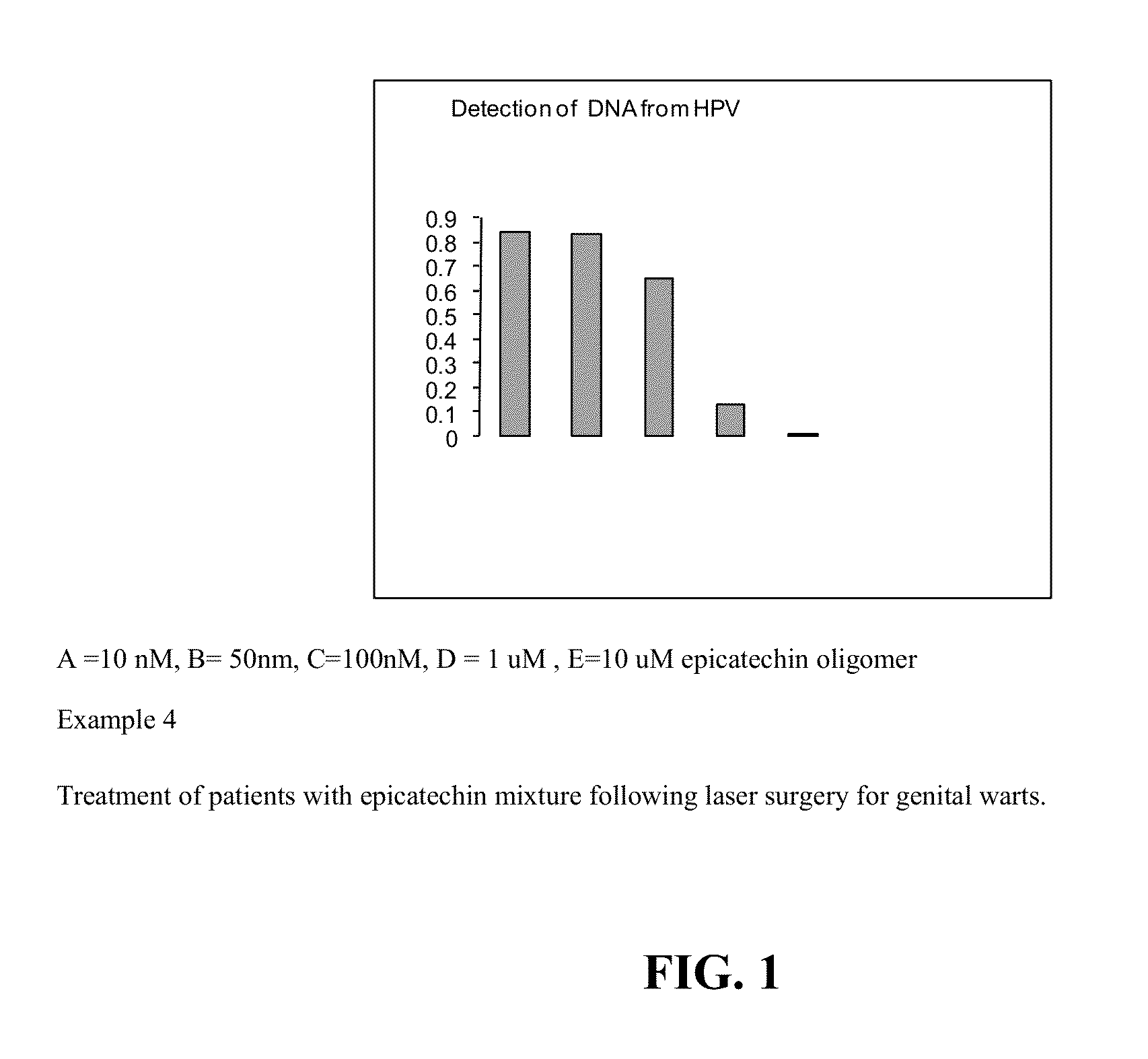
Antiviral epicatechins, epicatechin oligomers, or thiolated epicatechins from theobroma cacao for treatment of genital warts
Owner:CACAO BIO TECH
Antiviral epicatechins, epicatechin oligomers, or thiolated epicatechins from theobroma cacao for treatment of genital warts
InactiveUS20110218241A1Promote healingProvide activityBiocideAntiviralsBULK ACTIVE INGREDIENTPolymer
Epicatechins, Epicatechin Oligomers, or Thiolated Epicatechins are applied (A) directly to a genital wart in the form of a cream, ointment, paste or solution, (B) directly to the genital wart wherein such cream, ointment, paste or solution contains as an additional active ingredient a skin permeabilizing agent, (C) following electrosurgical resection or removal of the genital wart in such form of a cream, ointment, paste or solution, (D) following chemical resection or extirpation of the genital wart in such form, (E) following surgical resection or removal of the genital wart in such form, wherein said Epicatechins, Epicatechin Oligomers, or Thiolated Epicatechins both provide antiviral activity against multiple strains of human papilloma virus (HPV) and promote healing following resection polymers contained in a vehicle. Disclosed are the compositions, therapeutical kits containing such composition, methods of treatment using such composition, and methods of enhancing the stability of such composition.
Owner:CACAO BIO TECH
Medicament for treating gynecology disease and dermatopathy and preparation thereof
The invention discloses a medicine for treating gynecological diseases and skin diseases and a preparation method thereof. The medicine is a Chinese patent drug which is prepared by the raw materials of phellodendron, radix sophorae flavescentis, common cnidium fruit, rhizoma atractylodis, honeysuckle flower, cortex dictamni, fructus kochiae and rehmanniae radix according to a certain preparation technique. By adopting the common local Chinese herbal materials as raw materials, the medicine of the invention has low cost; furthermore, the medicine of the invention has the efficacies of clearing away heat, eliminating dampness, dispelling wind, killing parasites, diminishing inflammation and relieving itching and can achieve good curative effect. Moreover, the medicine of the invention has the advantages of easy preparation, low cost and significant curative effect, thus being applicable to the treatment of pruritus vulvae, vaginitis, cervicitis, eczema scroti and verruca acuminate which are caused by various pathogenic bacteria and skin diseases caused by a variety of fungus.
Owner:陆荣政
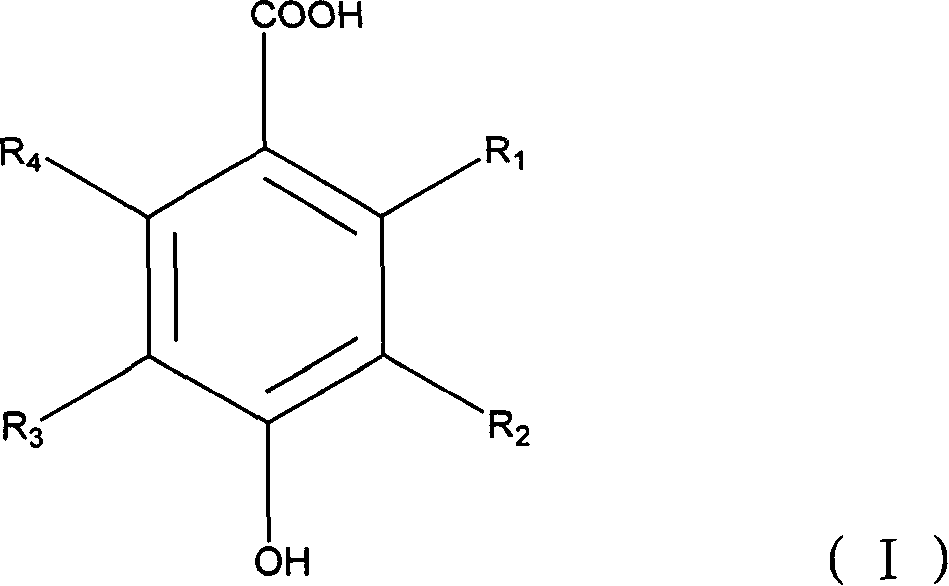
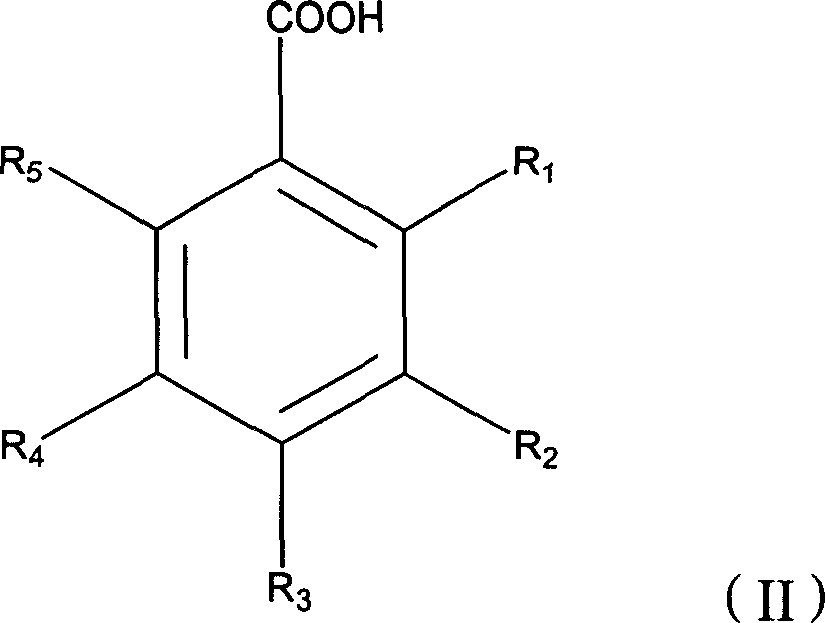
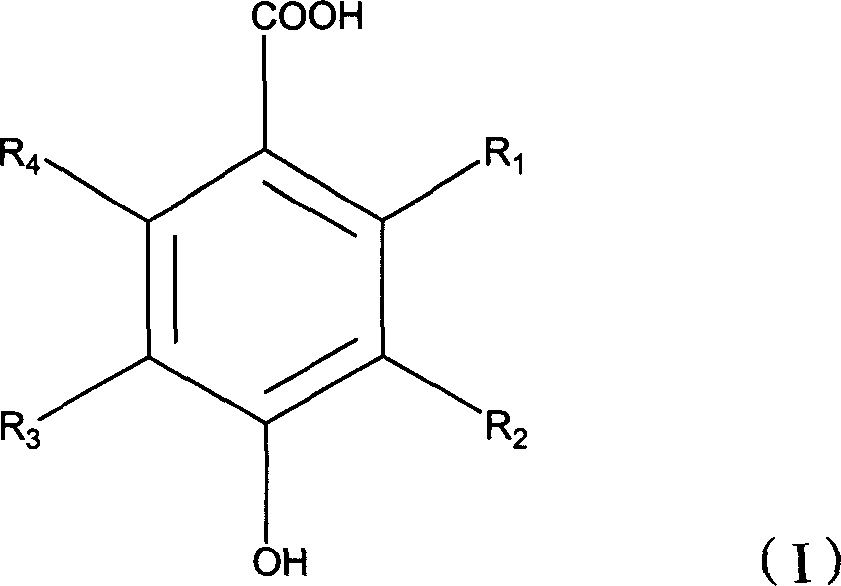
Compounds and methods for treating tumors, cancer and hyperproliferative diseases
The present invention relates to novel compounds, pharmaceutical compositions and methods for treating tumors, cancer and hyperproliferative diseases including psoriasis, genital warts and hyperproliferative cell growth diseases, including hyperproliferative keratinocyte diseases such as hyperkeratosis, ichthyosis, keratoderma or lichen planus. These compounds are described according to the chemical structure:where R1 is H, OH, F, Cl, Br, I, a C1-C6 optionally substituted alkyl or alkenyl group, an optionally substituted aryl group or a group;Ra is a H, OH, C1-C10, optionally substituted alkyl or alkenyl group, an optionally substituted O—(C1-C7 alkyl group) or O-aryl group, an amine group which is optionally substituted with at least one C1-C10 alkyl group which may be optionally substituted, or a single optionally substituted aryl group, biphenyl group, (C1-C6) alkylenearyl group, (C1-C6) alkylenebiphenyl group, heteroaryl group, heterocyclic group, (C1-C6) alkylene heteroaryl group or (C1-C6) alkylene heterocyclic group;R2 is a group;Rb is a H, OH, C1-C10, optionally substituted alkyl or alkenyl group, an optionally substituted O—(C1-C7 alkyl group) or O-aryl group, an amine group which is optionally substituted with at least one C1-C10 alkyl group which may be optionally substituted, or a single optionally substituted aryl group, biphenyl group, (C1-C6) alkylenearyl group, (C1-C6) alkylenebiphenyl group, heteroaryl group, heterocyclic group, (C1-C6) alkylene heteroaryl group or (C1-C6) alkylene heterocyclic group;R3 and R6 are each independently selected from H, OH, F, Cl, Br, I, a C1-C6 optionally substituted alkyl or alkenyl group, an optionally substituted aryl group, a carbamate, alkylene carbamate, urethane or alkylene urethane;R4 is a group, wherein Rb is as described above; andR5 is a group, wherein Rb is as described above,with the proviso that at least one of R1 and R2 or R4 and R5 contains an Ra or Rb group which is an amine group which is optionally substituted with at least one C1-C10 alkyl group which may be optionally substituted, or a single optionally substituted aryl group, biphenyl group, (C1-C6) alkylenearyl group, (C1-C6) alkylenebiphenyl group, heteroaryl group, heterocyclic group, (C1-C6) alkylene heteroaryl group or (C1-C6) alkylene heterocyclic group;or a stereoisomer, pharmaceutically acceptable salt, solvate, and polymorph thereof.
Owner:YALE UNIV
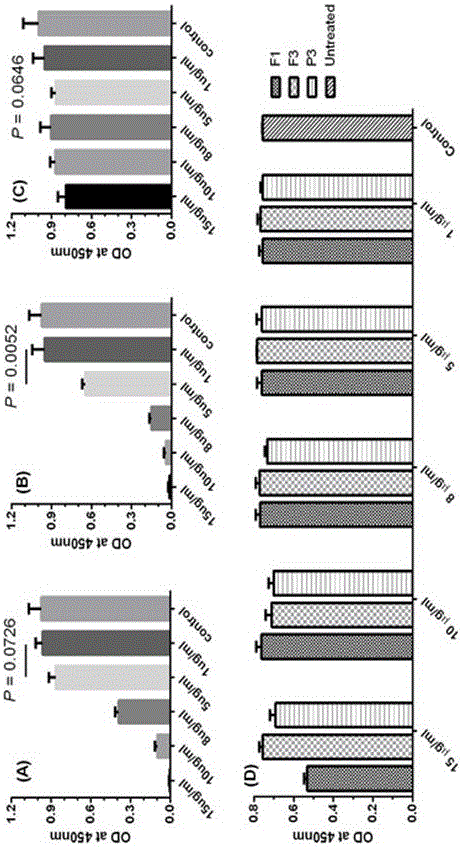
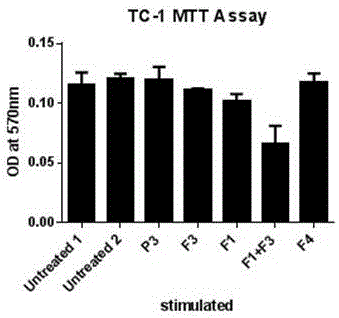
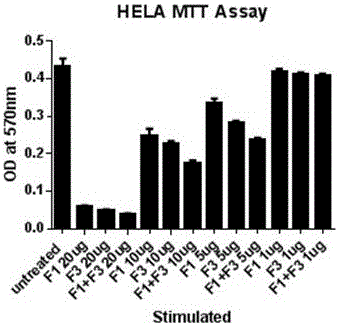
Pharmaceutical composition containing polypeptides F1 and F3 and application of pharmaceutical composition in treatment of HPV (Human Papilloma Virus) infected diseases
ActiveCN106749594AUnique mechanism of actionShort course of treatmentOrganic active ingredientsPeptide/protein ingredientsPenisSide effect
The invention relates to the technical field of polypeptide pharmaceutical preparations, and in particular relates to a pharmaceutical composition containing polypeptides F1 and F3 and application of the pharmaceutical composition in treatment of HPV infected diseases. The content of the polypeptides F1 and F3 or a mixture of the two polypeptides is 3-30 mu g in every part of the pharmaceutical composition, and the composition is prepared into multiple conventional dosage forms such as emulsion, ointment preparations and the like and is used for treating easily reoccurring diseases caused by the HPV infection, such as condyloma acuminate, cervical cancer, carcinoma of vulva, carcinoma of penis, anal carcinoma, carcinoma of mouth and the like. The pharmaceutical composition disclosed by the invention has the advantages of short usage course of treatment, small active ingredient amount, exact curative effects, low reoccurrence rate, small side effects, low treatment cost and the like, and brings an excellent therapy pathway to patients.
Owner:ZHONG AO BIOMEDICAL TECH (GUANGDONG) CO LTD
Ointment against virus of human papilloma
InactiveCN1456194ARich sources of medicineLow priceAnthropod material medical ingredientsAntiviralsDiseaseVomit black
A human papillomavirus (HPV)-resistant ointment for treating the wart of vulva, vagina and cervix, the black lesion of vulva, and pointed condylona caused by HPV is prepared from 12 raw materials including brucea fruit, Chinese gall, glycerine, monoglyceride, etc. Its advantages are high curative effect and no irritation to skin.
Owner:哈尔滨医科大学附属第一临床医学院 +3
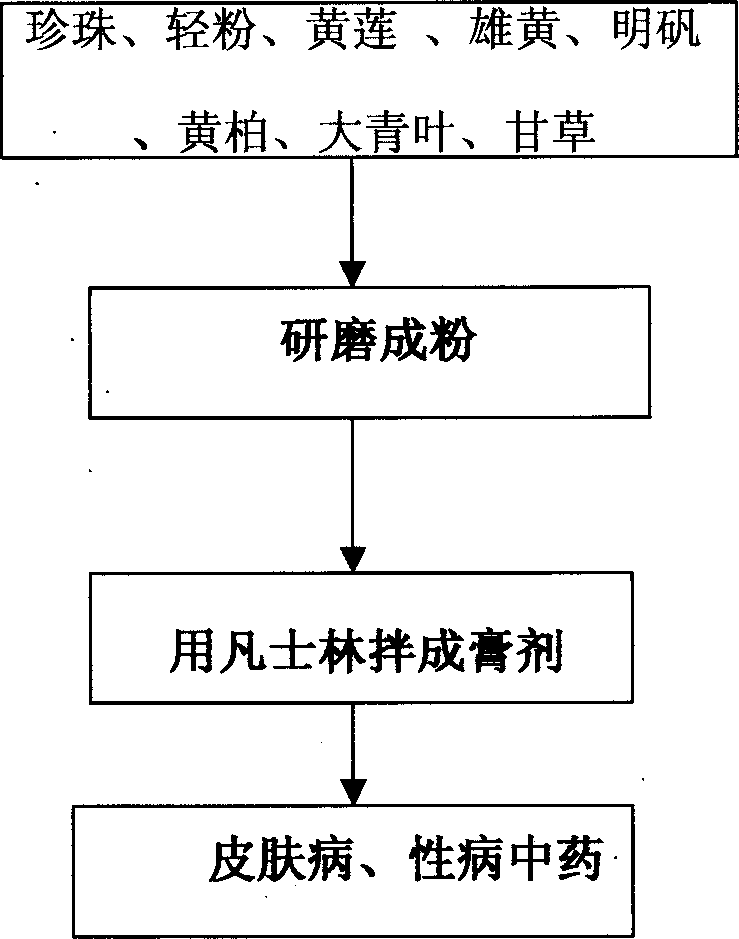
Chinese medicine for treating dermatopathy and venereal disease and its preparing process
InactiveCN1359710AHigh one-time cure rateNo side effectsUnknown materialsDermatological disorderDiseaseTreatment effect
A Chinese medicine for treating dermatopathy and venereal disease, including eczema, tinea, vulvitis, pointed condyloma, gonorrhea, syphilis etc is prepared from Chinese-medicinal materials including pearl, calomel, realgar, etc through pulverizing and mixing with vasuline. Its advantages are high curative effect, quickly taking its effect, no by-effect and no recurrence.
Owner:任泳橡
Application of p-hydroxybenzoic acid and its analogue in the preparing process of medicine for preventing and treating skin mucous membrane virus infection
The invention discloses an application of p-hydroxybenzoic acid and analogue in the skin virus infection drug, which is characterized by the following: fitting for infectious drug of people breast tumour virus and herpetoviridae virus; allocating each auxiliary material to prepare different agent or personal nursing product.
Owner:SHENGHUA GUANGZHOU PHARMA SCI & TECH
Pharmaceutical Formulations for Iontophoretic Delivery of an Immunomodulator
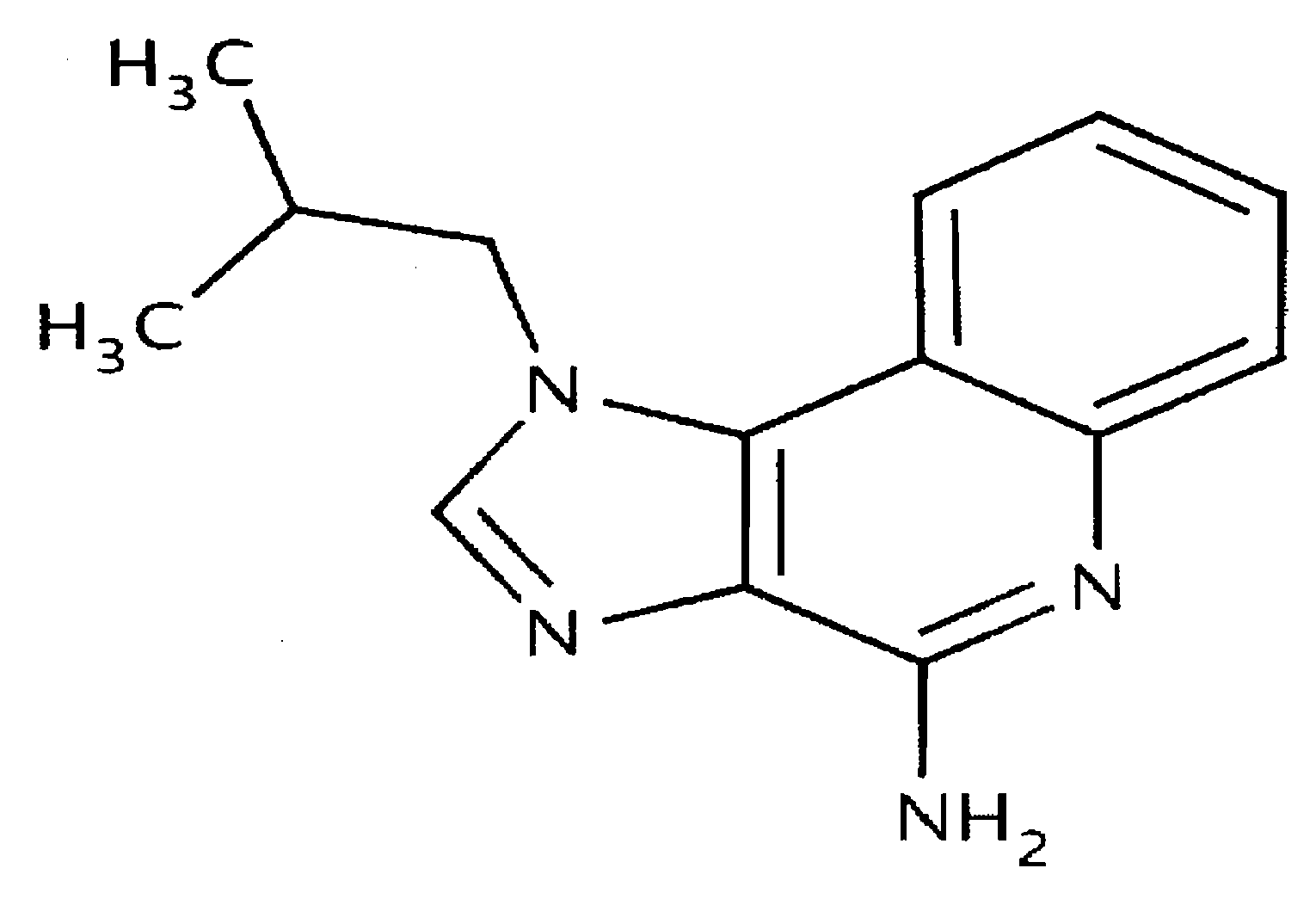
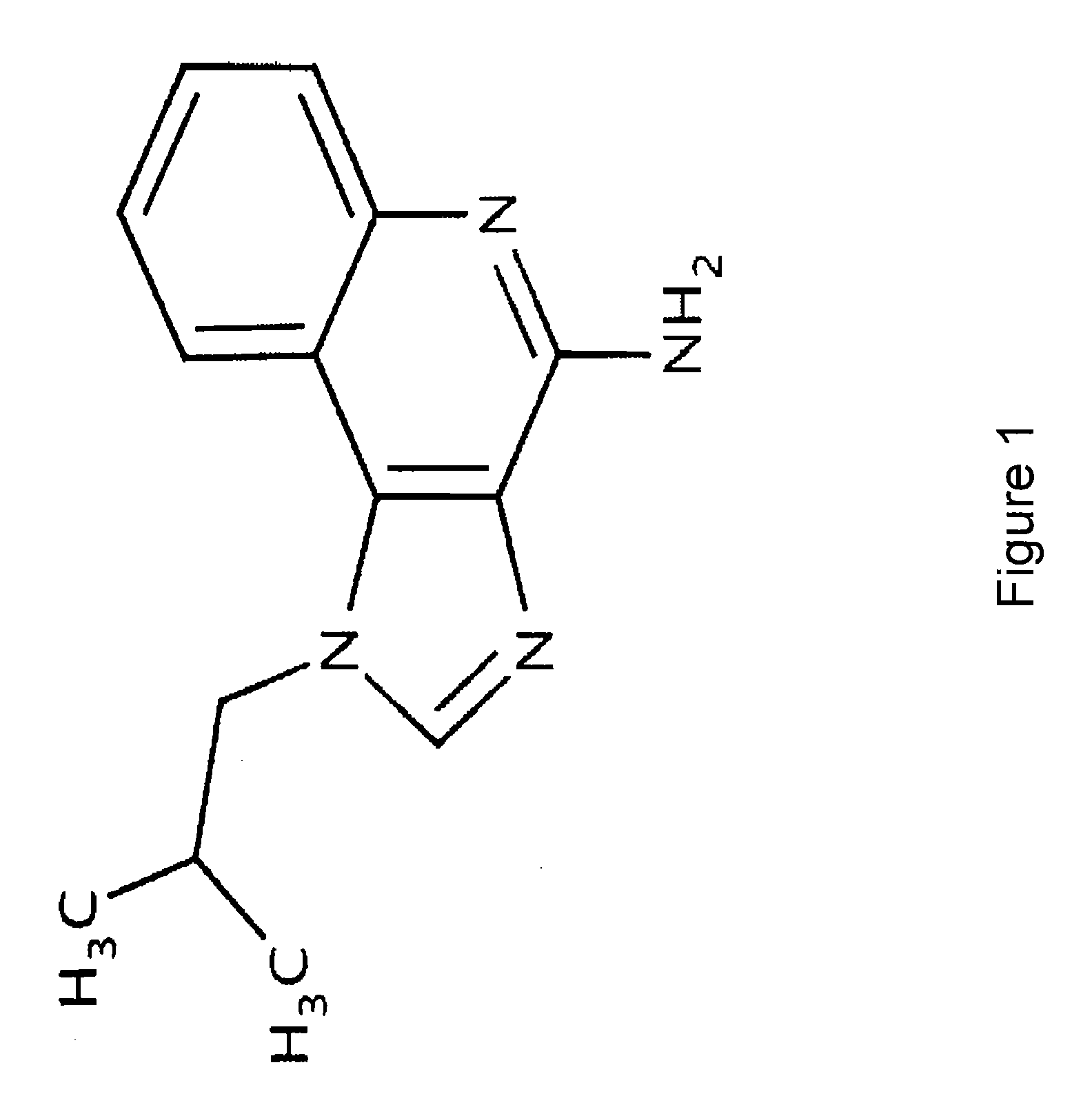
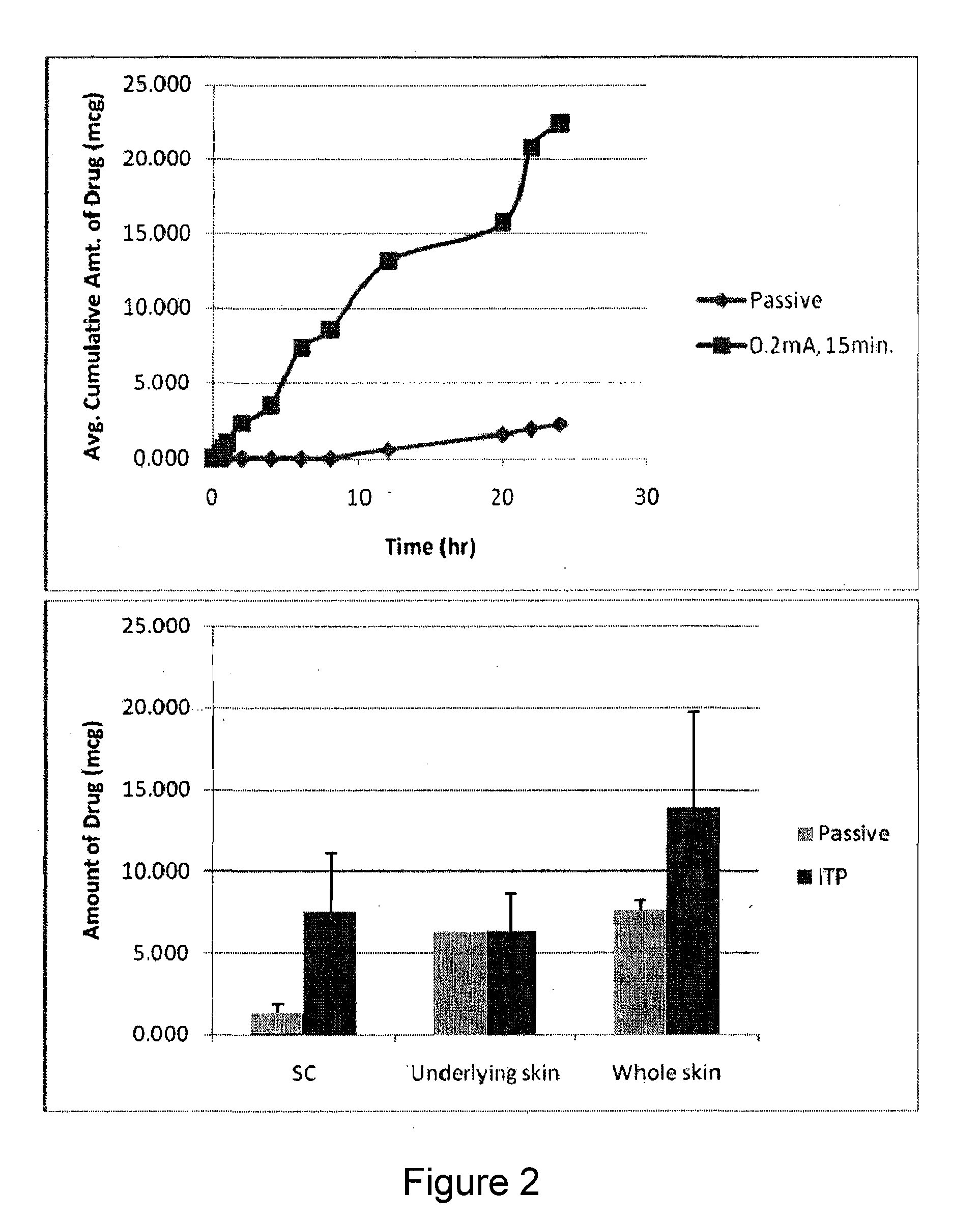
The present invention describes pharmaceutical formulations and methods suitable for iontophoretic delivery of the formulations to a subject. The formulations comprise an immunomodulator, such as imiquimod, and optionally include various agents and excipients. The formulations can be used as a treatment for skin diseases and conditions such as actinic keratosis, basal cell carcinoma and genital warts. The short term iontophoretic delivery of the formulations results in the creation of a depot effect in the skin of the subject, allowing for a sustained delivery. The shortened delivery time minimizes local side effects at the application site.
Owner:NITRIC BIOTHERAPEUTICS INC
HPV (human papillomavirus) peptide/DC (dendritic cell) mixed vaccine and preparation thereof
InactiveCN102008721APromote maturityImprove the level ofViral antigen ingredientsAntiviralsAdjuvantHuman papillomavirus
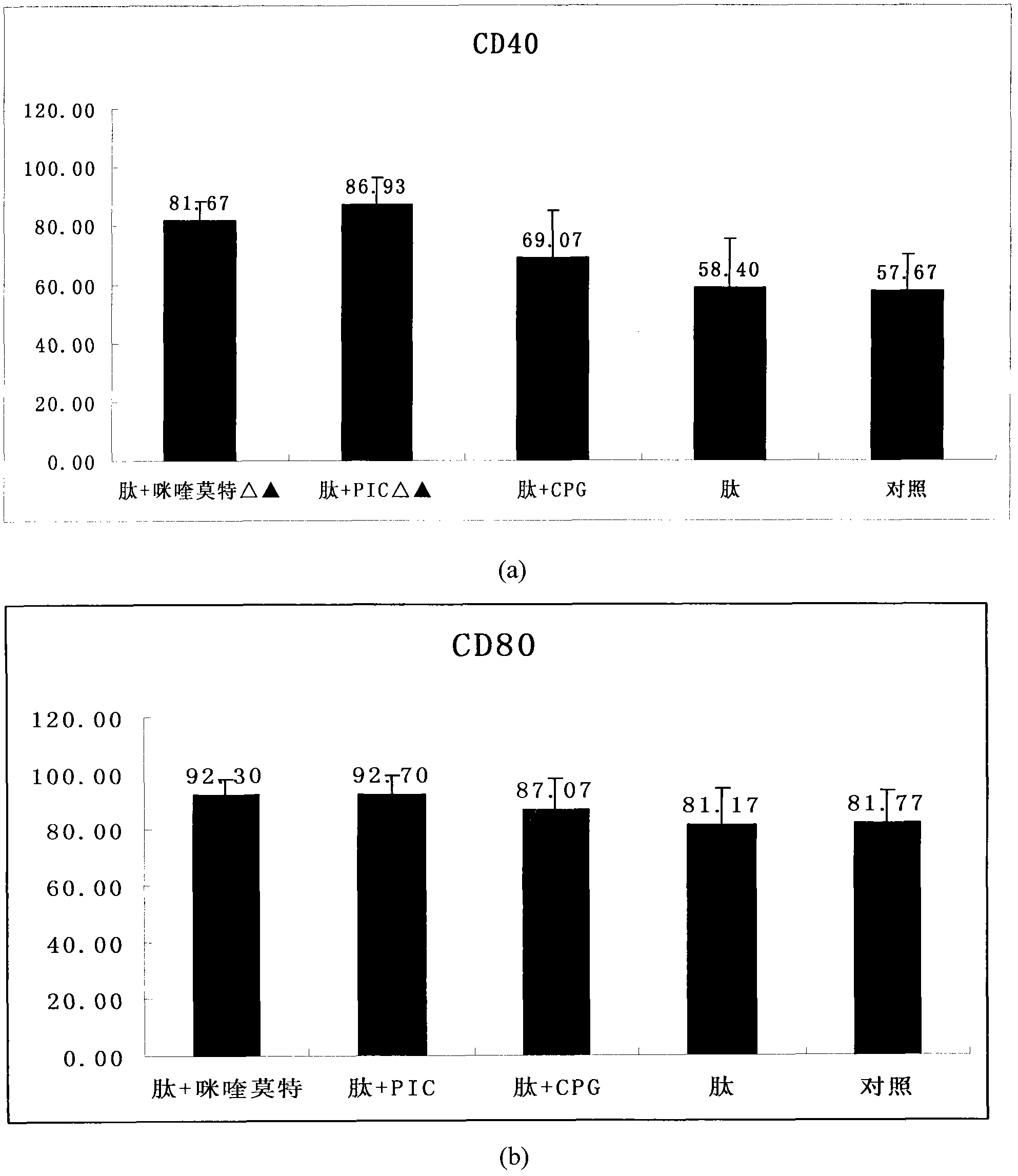
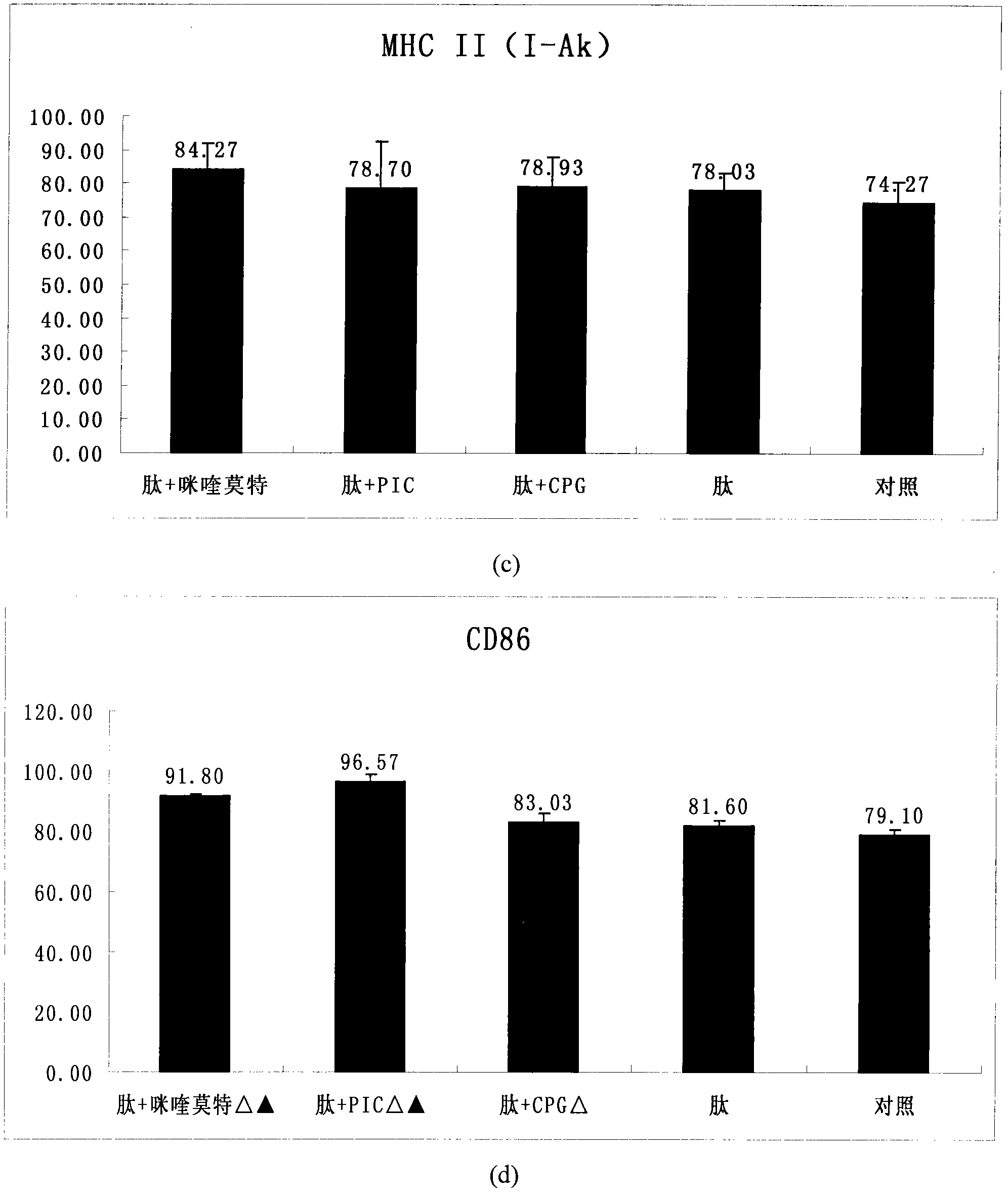
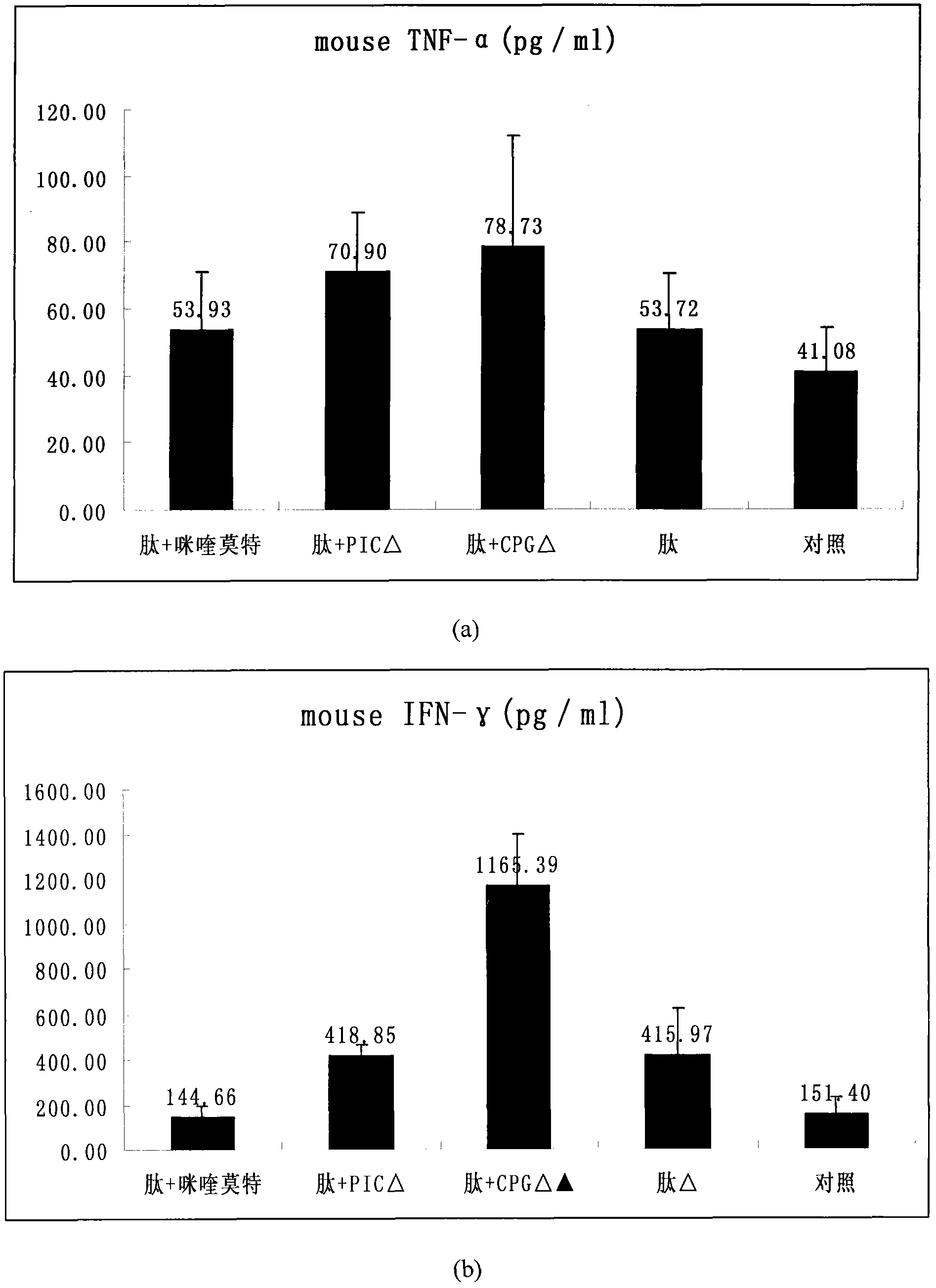
The invention provides an HPV (human papillomavirus) peptide / DC (dendritic cell) mixed vaccine prepared in the presence of a TLR (toll-like receptor) agonist. The mixed vaccine comprises one part of HPV11E77-15, one part of mouse bone marrow-derived dendritic cells which are cultured in vitro and one part of TLR9 agonist as an adjuvant, wherein the dendritic cells are extracted from mouse bone marrow and further cultured in vitro, the co-incubation with one section of HPV11E7CTL (cytotoxic T lymphocyte) epitope peptide with strongest immunogenicity is firstly carried out, the co-incubation with the TLR ligand CpG and the like is further respectively carried out for promoting the maturation of the DC, the degree of maturation can be confirmed by detecting the change of a marker on the surface of the DC after incubation, and the DC vaccine which is simulated to mature can be used for immunizing mice. The TLR ligand and the HPV11E7 peptide are utilized jointly to promote the degree of maturation of the DC, the level of TNF-alpha (tumor necrosis factor-alpha) and IFN-gamma (immunoreactive fibronectin-gamma) of factors of secretory cells of T cells can be particularly improved after combination of the CpG, and the HPV peptide / DC mixed vaccine can be used for preventing and treating genital warts and cervical cancer.
Owner:ZHEJIANG UNIV
Medicine for treating pointed condyloma with pure Chinese medicine
InactiveCN1562138AEasy to useSignificant effectAnthropod material medical ingredientsDermatological disorderChinese GallChinese drug
An externally-applied Chinese medicine for treating pointed condyloma is prepared from 9 Chinese-medicinal materials including Chinese gall, stemona root, pangolin scales, alum, etc.
Owner:李兴春
Preparation method for toxin expelling and verruca dispelling pill
The invention relates to a preparation method for toxin expelling and verruca dispelling pill. Raw materials adopted by the pill comprise a dozen of Chinese herbals such as indigowoad root, indigowoad leaf, purslane, coix seed and the like. The toxin expelling and verruca dispelling pill is prepared according to a certain weight ratio. According to the present invention, the pure traditional Chinese drug materials are adopted for preparing the pill, such that the pill has no toxic and side-effect; the pill is mainly provided for treating flat wart, verruca vulgaris, plantar wart and condylomaacuminatum; the pill not only can provides a curative effect, but also can expel the toxin producing verruca diseases so as to effectively prevent the verruca from recurrence.
Owner:王金保
Anus lotion and preparation method thereof
ActiveCN103599229AWide range of pharmacological effectsEasy to useAnthropod material medical ingredientsAntipyreticDressing changeStephania
The invention discloses an anus lotion, which is prepared from the following raw materials by weight: 25-35g of radix sophorae flavescentis, 15-25g of cortex phellodendri, 10-20g of radix aconiti preparata, 10-20g of prepared kusnezoff monkshood root, 15-25g of radix angelicae, 25-35g of stephania terandra, 25-35g of dittany bark, 8-12g of dried alum, 12-18g of rheum officinale and 15-25g of gallnut in a manner of decocting after adding water. The anus lotion has the beneficial effects that the anus lotion has the main characteristics of convenience for use and wide pharmacological action, and simultaneously can treat anus diseases with a plurality of concurrent symptoms such as bleeding, pain, pruritus, secretion increase and the like. Clinical application finds that most of haemorrhoids can retract and dissipate as to internal hemorrhoids, inflammatory external hemorrhoids, external hemorrhoids and mixed hemorrhoids at each stage after a patient takes the anus lotion for 5-7 days. Anal eczema can scab and heal after the anus lotion is used for 1-2 weeks while the symptoms are obviously relieved after the patients with anus itching, condyloma acuminata and anal fissure take the anus lotion. Especially for the patient with an anus operation, the anus lotion can relieve swelling and pain, also can purify the wound, reduces the frequency of dressing change, and obviously shortens the wound healing time.
Owner:启东市第五人民医院
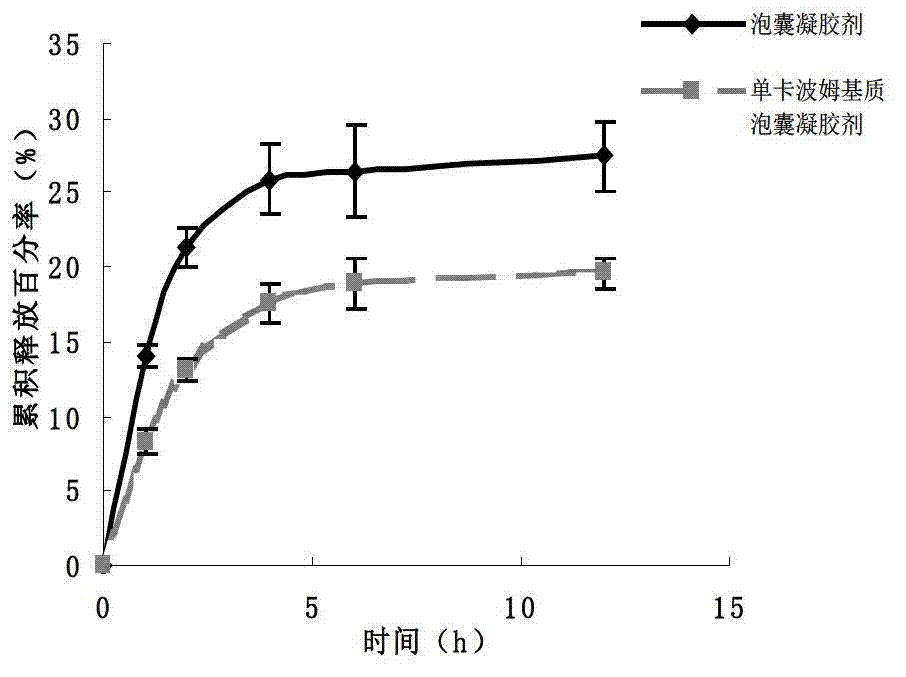
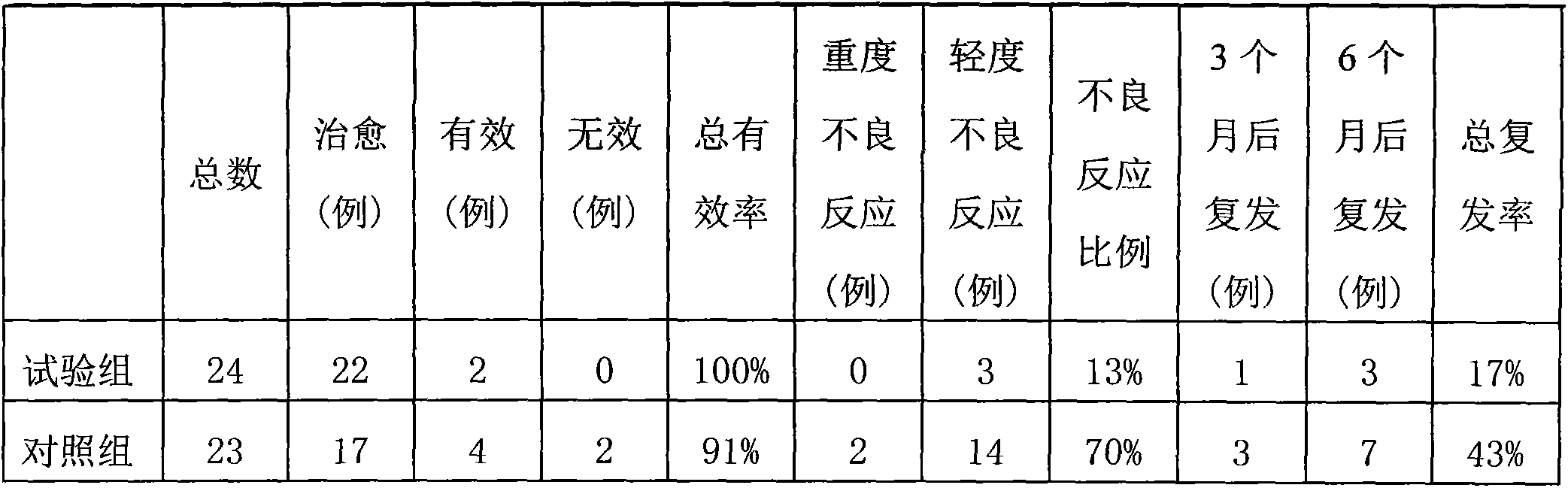
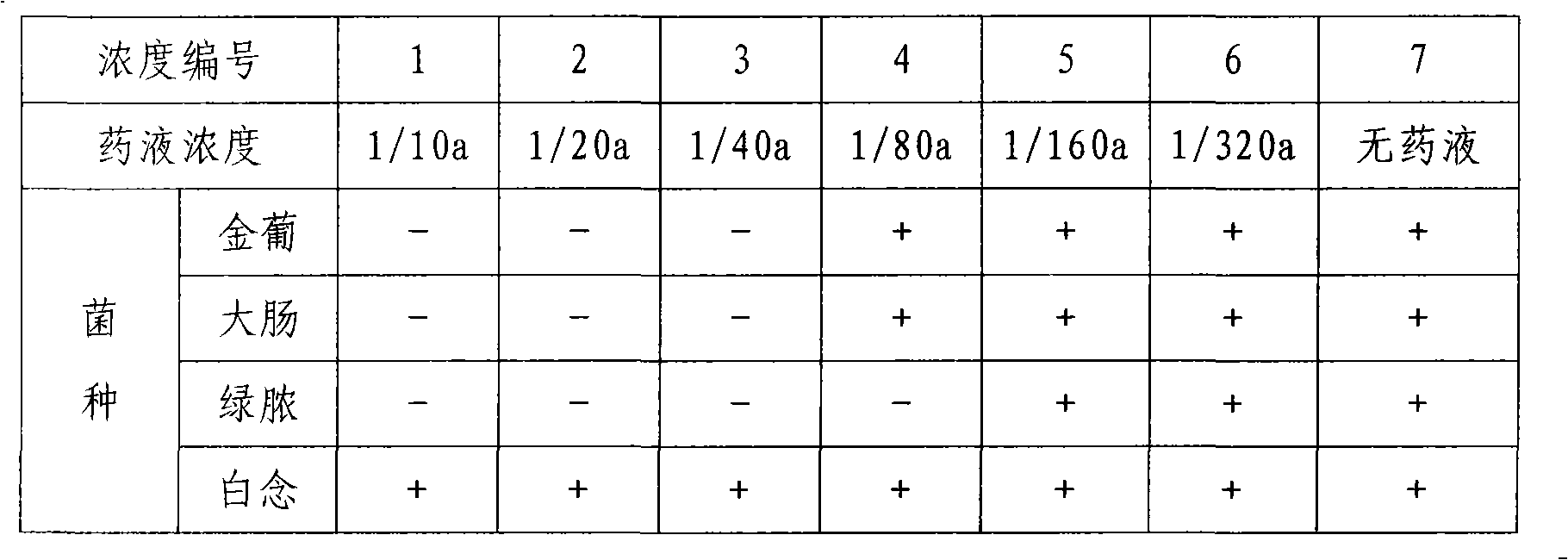
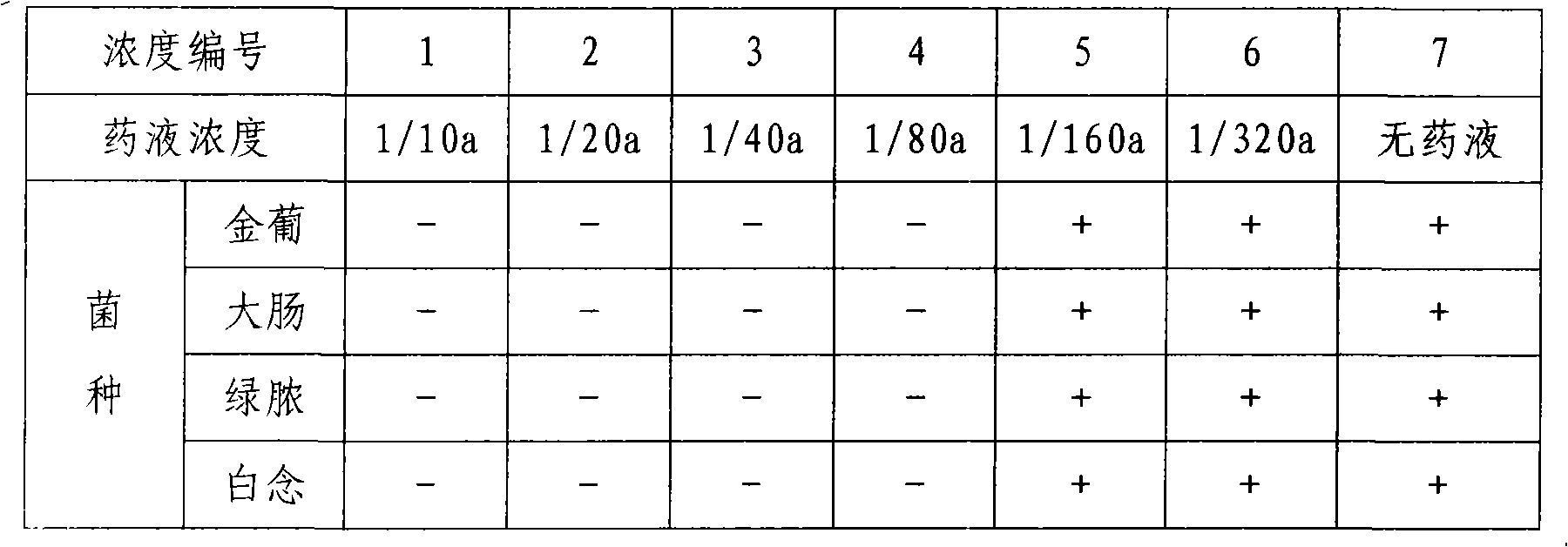
Imiquimod vesicle gel and preparation method for same
ActiveCN103202803AImprove retentionGood curative effectOrganic active ingredientsAerosol deliveryDiseaseMelanoma
The invention relates to an imiquimod vesicle gel and a preparation method for the same. The vesicle gel is obtained by preparing a vesicle suspension by using imiquimod and the nonionic surfactants of Brij, Span, Poloxamer and the like via self-assembly, then reacting with the mixed gel matrix of carbomer and povidone, and used for treating the diseases of exophytic genital warts, actinic keratosis, skin basal cancer, melanoma and the like via local application on the skin; and by adding povidone in the carbomer gel matrix, the in-vitro medicine release amount can be increased by 42.1%, thus being beneficial to promote the entrance of medicines in the skin to exert the functions. According to the imiquimod vesicle gel and the preparation method for the same disclosed by the invention, the intradermal retention volume of the medicines in 24 hours can be remarkably increased and is 2.1 times that of the commercially available emulsifiable pastes, and the dose of the medicines penetrating through the skin is reduced by 48.7%; and the equivalent intradermal retention volume can be achieved only by a half of the dose of the commercially available emulsifiable pastes, so that the effectiveness of the medicine effect of the medicated parts can be remarkably enhanced, the dose of the medicines entering in the body can be greatly reduced, and the toxic and side effects of the whole body can be reduced.
Owner:SUZHOU UNIV
Cream containing interferon encapsulated with liposome
InactiveUS20070077289A1Good curative effectImprove adhesionPeptide/protein ingredientsOrganic non-active ingredientsHerpetic stomatitisLiposome
This invention relates to a cream containing interferon encapsulated with liposome, which comprises IFN liposome and cream substrate. The advantage of the cream of the present invention is high efficiency of encapsulation, stable production technique, good homogeneity of products, stable efficacy and low rate of leakage. In order to improve the capability of encapsulation of liposome and the activity and efficacy of IFN, IFN liposome and substrate are utilized to prepare a form of cream which cures those skin disease infected by virus, such as herpes zoster, herpetic stomatitis, verruca, condyloma acuminata, mollusoum contagiosum, herpes genitalis, verrca planae, verruca vulgaris, genital ulcer, oral ulcer and pruritus.
Owner:SHENZHEN NEPTUNUS INTERLONG BIO TECHN HLDG
Pharmaceutical use of sophora alkaloids tanate for preventing and treating skin and mucosa infection
The present invention relates to an application of tannic acid and its matrine or their composition in preparationof medicine for preventing and curing mucocutaneous infection, including human papillomavirus infection, pointed condyloma, vaginitis, cervicitis and cervical erosion.
Owner:秦卫华
Lotion for treating condyloma acuminate and preparation method of lotion
InactiveCN103816437AOvercome tumorGood effectOrganic active ingredientsInorganic boron active ingredientsSide effectBrucea javanica
The invention provides a lotion for treating condyloma acuminate and a preparation method of the lotion. The lotion for treating condyloma acuminate is characterized by being prepared from the following components in parts by weight: 30 parts of isatis roots, 30 parts of folium isatidis, 20 parts of houttuynia cordata, 30 parts of honeysuckle, 30 parts of radix sophorae flavescentis, 30 parts of golden cypress, 30 parts of purslane, 20 parts of pearl barley, 20 parts of dried alum, 30 parts of brucea javanica, 10 parts of borax, 20 parts of mint, 0.75 part of fluorouracil and 1500 parts of water. By proportioning cold and cool medicines, the lotion can restrain sarcoma caused by damp and hot, mainly aims to condyloma acuminate, and is matched with fluorouracil. By combination of traditional Chinese and western medicines, the lotion is extremely excellent in effect, high in cure rate, extremely low in recurrence rate, free from oral administration, free from any general side effects, safe and non-toxic, convenient to use and worth clinically applying.
Owner:陈志刚
Chinese herbal compound formulation for treating vulvovaginitis and venereal disease, and preparation method thereof
InactiveCN101411838AGood effectTo achieve the purpose of treating vulvovaginitisHydroxy compound active ingredientsAntiinfectivesDiseasePatrinia
The invention discloses a Chinese medicine compound preparation for treating vulvovaginitis and venereal diseases and a method for preparing the same. The compound preparation is prepared from active ingredients including isatis root, patrinia, glabrous greenbrier rhizome, bugleweed, dyer woad leaves, radix sophorae flavescentis, cicada ecdysis, cnidium fruit, fructus kochiae, radix stemonae, borneol and the like. The method comprises the following steps: besides the borneol, decocting the active ingredients with water for two times, 1.5 hours for each time; filtering the mixture, and combining the filtrate; adding the borneol which is ground into fine powder into the mixture; and then adding 0.2 percent tween 80 for keeping stand, canning the mixture, and performing circulation steam sterilization at a temperature of 100 DEG C for 30 minutes to obtain the finished product. Through applying a mixed lotion of various antibacterial drugs, antiviral drugs and antipruritic drugs, the Chinese medicine compound preparation not only achieves the aim of treating the vulvovaginitis, but also has better effect on treating the venereal diseases such as verruca acuminata, gonococcal vaginitis and the like, and opens up a new way of conservative treatment of the venereal diseases.
Owner:GENERAL HOSPITAL OF THE SECOND ARTILLERY OF CHINESE PLA
Dermatosis eliminating medicine
InactiveCN100350921CGood curative effectLow costAmphibian material medical ingredientsAnthropod material medical ingredientsCurative effectLeukoderma
The present invention relates to medicine technology, and is one kind of dermatosis eliminating medicine. Long term clinical practice shows that the medicine has high and very fast curative effects on skin cancer, psoriasis, condyloma acuminata, verruca piana, verruca vulgasis, leukoderma and scar proliferation. The medicine is applied to the affected part to heal the said dermatosis fast without hemorrhage, pain and scar. The medicine has abundant material source and simple preparation process.
Owner:魏承堂
Fumigation agent for treating anal condyloma acuminata
InactiveCN105311547AGood effectPharmaceutical delivery mechanismAntiviralsIndigofera tinctoriaTherapeutic effect
The invention relates to a fumigation agent for treating anal condyloma acuminata, which is prepared from following components: 50 g of sophora flavescens, 20 g of radix indigofera tinctoria, 20 g of scouring rush, 15 g of peach kernel, 12 g of cortex moutan, 30 g of rhizoma sparganii and 30 g of curcuma zedoary. The fumigation agent has excellent treatment effects on the anal condyloma acuminata.
Owner:费俊
Externally-applied medicine for treating moist wart dermatosis and its making method
ActiveCN1939519AExtended treatment timeLower resistanceDermatological disorderPlant ingredientsCoptisCurative effect
An exterior-applied Chinese medicine for treating pointed condyloma and dermatopathy is prepared from 10 Chinese-medicinal materials including ginseng, longan aril, honeysuckle flower, coptis root, etc. Its preparing process is also disclosed.
Owner:孟平安
Sanitary hydrojet for cleaning vagina and preparation method
InactiveCN101502569AIncrease lethalityHas a killing effectOrganic active ingredientsDermatological disorderBacterial vaginosisWhole body
A vagina-cleaning sanitary spray liquid comprises vagina-cleaning spray liquid and a liquid spray bottle, and the vagina-cleaning spray liquid consists of the following pharmaceutical components by weight percentage: 0.146-0.15 wt percent of hexamethylene guanidine hydrochloride, 0.195-0.2 wt percent of glycerol, 0.097-0.1 wt percent of peony root extract, 0.029-0.03 wt percent of rosemary oil and 1.95-2.0 wt percent of diphenhydramine. The components are mixed and prepared into water solution, the vagina-cleaning spray liquid is filled in the liquid spray bottle, and a liquid spray head is sealed for preparing the vagina-cleaning sanitary spray liquid. The vagina-cleaning sanitary spray liquid can not only effectively kill bacteria and viruses of skin and mucous membrane parts of whole body, but also have better effect for killing adult male and female genital skin and mucous membrane infected bacteria, fungi and viruses, thereby having the effect of killing bacteria, fungi, gonorrhea coccus, human papilloma virus and HIV and being capable of effectively preventing female bacterial vaginosis, fungal vaginitis, male and female gonorrhea, genital warts, AIDS and so on.
Owner:LIAONING HUANREN PHARMA
Tinctura for eliminating tinea and relieving itching
The invention discloses a tincture against tinea and itching. More than twenty kinds of medicaments such as cortex phellodendri, indigo, rhubarb, pericarpium zanthoxyli, clove, peach kernel, pomegranate bark, Chinese gall are reasonably mixed and then subjected to crushing, extraction and separation, preparation, filtering, measuring and product finishing to obtain the tincture against tinea and itching. The tincture against tinea and itching is a transparent external use liquid preparation which has the smell of beetle fart and is brown yellow, and has obvious effects of treating chronic skin diseases such as neurodermitis, psoriasis, chronic eczema, lichen, ringworm of the foot, intractable prurigo, verruca acuminata, skin hyperkeratosis.
Owner:王洪斌
Traditional Chinese medicine preparation for treating anal pointed condyloma
InactiveCN104083681AGood curative effectHeavy metal active ingredientsDigestive systemMedicineWarm water
A traditional Chinese medicine preparation for treating anal pointed condyloma comprises the raw materials: 50 parts of folium isatidis , 50 parts of radix isatidis , 50 parts of spreading hedyotis herb , 50 parts of rhizoma smilacis glabrae, 50 parts of radix rehmanniae , 50 parts of magnetitum , 50 parts of concha ostreae , 50 parts of herba taraxaci, 12 parts of radix scutellariae and 12 parts of radix et rhizoma rhei. A preparation method of the ointment comprises: weighing 50 parts of folium isatidis , 50 parts of radix isatidis , 50 parts of spreading hedyotis herb , 50 parts of rhizoma smilacis glabrae, 50 parts of radix rehmanniae , 50 parts of magnetitum , 50 parts of concha ostreae , 50 parts of herba taraxaci, 12 parts of radix scutellariae and 12 parts of radix et rhizoma rhei, putting into a medicinal pot, immersing with warm water for 30 min, decocting with slow fire for about 40 min, so as to obtain a medicinal soup of about 200 ml, filtering the medicinal soup; and repeating decocting for one time. The traditional Chinese medicine preparation has the efficacy of clearing heat and detoxifying, harmonizing nutrient-blood, dispelling wind and moistening dry spleen, and is used to treat anal pointed condyloma.
Owner:冯振江
Traditional Chinese medicine preparation for treating anal pointed condyloma
InactiveCN103784666AHigh cure rateNot easy to relapseAnthropod material medical ingredientsAerosol deliverySmoked PlumInjury mouth
The invention discloses a traditional Chinese medicine preparation for treating anal pointed condyloma. The preparation is prepared from the following raw materials by weight: 25-35g of gallnuts, 8-12g of smoked plums, 8-12g of rhizoma cyperi, 8-12g of peach kernels, 1.8-2.2g of realgar and 8-12g of dried alum. The preparation method comprises the following steps: mixing, crushing and sieving the raw material medicines by a 100-mesh screen; and adding vinegar to prepare an ointment. The traditional Chinese medicine preparation for treating anal pointed condyloma mainly treats anal pointed condyloma. The use of the preparation comprises the following steps: smearing the traditional Chinese medicine preparation at the affected parts, wherein the smearing area is appropriately 1cm greater than the wart area; and then covering by an adhesive plaster. Large warts shall be excised by an electrotome or laser, and the smearing area after excision is appropriately 1cm greater than the wound area. The preparation is smeared once every two days, the wart is remarkably reduced after smearing for three times commonly, and the wart commonly fall after smearing for seven times, so that the effect of cure is realized.
Owner:邵飚
Medicament composition and use thereof in preparing antimicrobial medicament
The invention relates to an application of glycyrrhizic acid or a compound of a salt thereof and tannic acid and triclosan in the preparation of anti skin mucous membrane microorganism and acne drugs, in particular to the application in the preparation of drugs for preventing and treating bacteria, mycotic and virus infections of skin mucous membranes of human beings and animals, acne drugs, disinfectant and daily chemical products, wherein, the infections comprise verruca acuminate, verruca plana, common warts, colpitis, cervicitis, cervical erosion, herpes simplex, herpes zoster, herpes genitalis and aece. The application is to blend the glycyrrhizic acid or the compound of the salt thereof and the tannic acid and the riclosan with various auxiliary materials of skin mucous membrane dosage and prepare the mixture into different preparations.
Owner:GUANGZHOU JIECHENG BIOLOGICAL TECH
Medicine for increasing activity of human body cell and resisting HPV
PendingCN110201150APromote secretionIncrease secretionOrganic active ingredientsHeavy metal active ingredientsHuman papillomavirusLymphatic Spread
The invention belongs to the technical field of medicines, and provides a medicine for increasing the activity of human body cells and resisting HPV (human papillomavirus). The medicine comprises dioscorea villosa, liquid mineral substances, cordyceps sinensis, ganoderma lucidum, red ginseng, donkey-hide gelatin, saffron, ginseng, organic cranberry, Macfadyena unguis-cati (L.) A. Gentry, witch hazel, angelica archangelica roots, orange glycosides, aloe, sage, organic honeysuckle leaves, calendula and blackberry. The dioscorea villosa promotes female hormone secretion, increases female hormonesecretion amount and improves female ovarian function decline, nutrient substances in the liquid mineral substances are easily absorbed by a human body to stimulate cell activity and purifies tumor cells, the cordyceps sinensis inhibits tumor growth and metastasis, the red ginseng promotes repair of damaged mucosa, and problems that genital warts and cervical cancer which are caused by high-risk HPV infection and low-risk HPV infection of external genitalia damage human body skin, are contagious, and threaten life safety of patients and family members.
Owner:GUANGZHOU SHAMAN BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com