Imiquimod vesicle gel and preparation method for same
A technology of imiquimod bubble and capsule gel, which is applied in the field of drug capsule gel and its preparation, can solve the problem of difficulty in increasing local drug concentration, achieve high-efficiency and low-toxicity treatment of related skin diseases, and reduce toxic and side effects , enhance the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
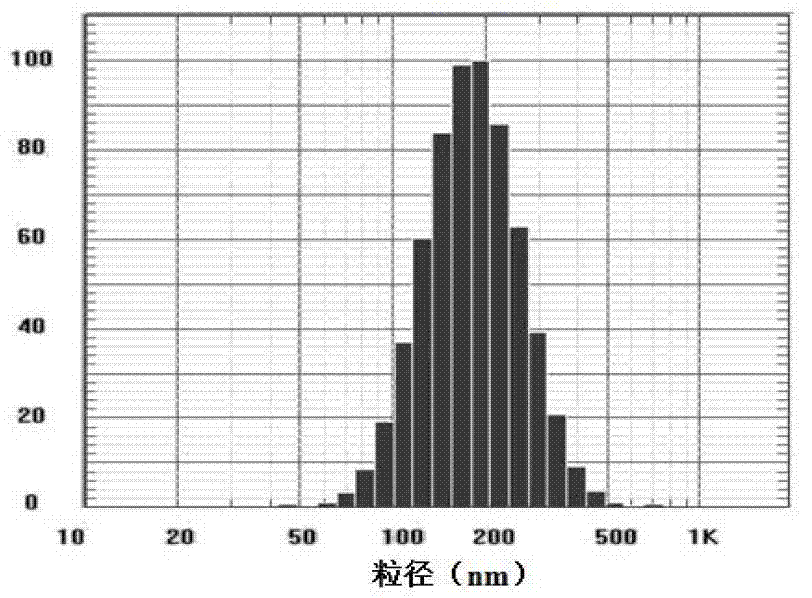
[0030] The preparation process of the vesicle suspension in the vesicle gel of the present invention is to dissolve the nonionic surfactant in the organic solvent earlier, then add the medicine of the prescribed amount, and after the ultrasound, adopt the injection method to inject the water phase (buffer Solution) is introduced into the organic phase and mixed, and the vesicle suspension is formed by self-assembly, and then mixed with the gel matrix prepared in advance. The imiquimod contained in the vesicle suspension prepared by the present invention is a poorly soluble drug. After comparing some investigation items, it was found that the preparation method of drug-loaded vesicles according to the present invention has no specific requirements for model drugs, and the same prescription and the same preparation method can be used to pack drugs of different properties, such as retinoic acid, 5-Fu. The prepared vesicle suspension can be further made into vesicle gel.
[0031...
Embodiment 1
[0033] 1. Preparation of imiquimod vesicle suspension
[0034] Take the prescribed amount of 10mg Brij78 (HLB value 15.3), 10mg Span85 (HLB value 1.8), 20mg Span40 (HLB value 6.7), 20mg Poloxamer188 (HLB value 29) in vials, add organic solvents, at 60 Magnetic stirring (2000r / min) in a water bath at ℃ to dissolve completely, add 10 mg of imiquimod (≤80 mesh), stir evenly, and sonicate for 3 minutes in a water bath at 60 ℃ to obtain an organic phase. Pour the PBS (pH 7.3) previously kept at 60°C into the organic phase under stirring state and stir it tightly for 10 minutes, then transfer it to a water bath at 37°C, stir at 2000r / min for 3 hours, and evaporate the ethanol and ethyl acetate. ester to obtain imiquimod vesicle suspension (abbreviation: vesicle suspension).
[0035] Using the same preparation method without adding drugs, a blank vesicle suspension was prepared for the preparation of the control preparation.
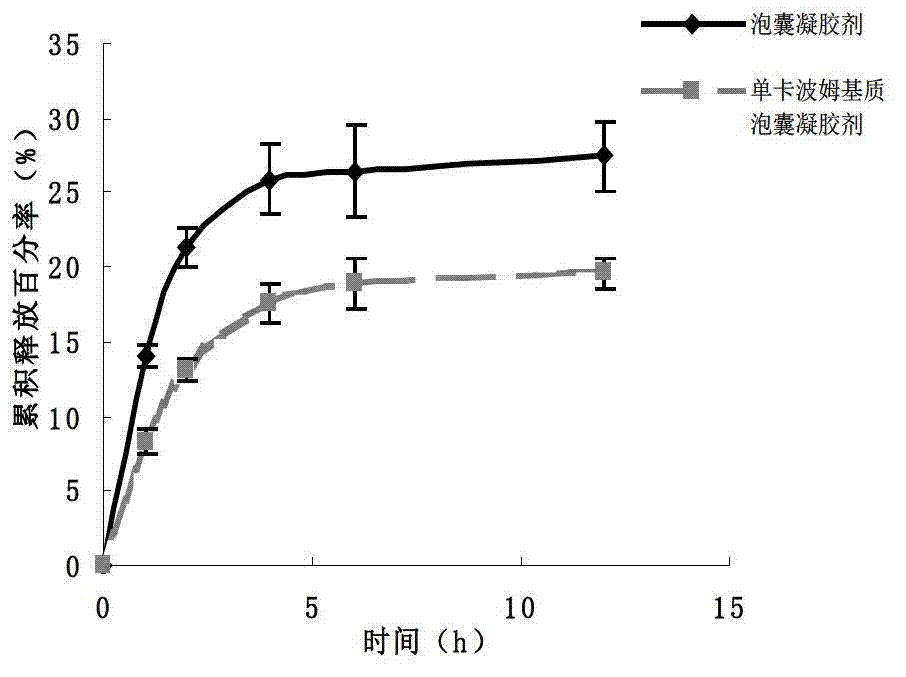
[0036] 2. Preparation of imiquimod vesicle gel
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com