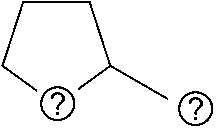
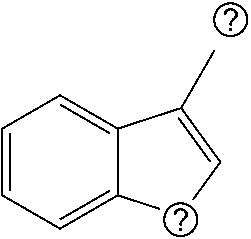
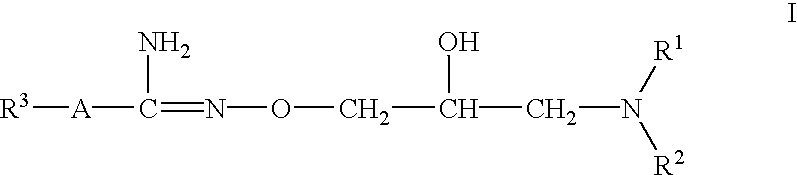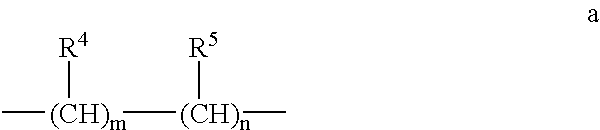Patents
Literature
96 results about "Actinic keratosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition which causes scaly patches on the skin from exposure to the sun over the years.
Dermal delivery
InactiveUS20110212157A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentOrganic active ingredientsPowder deliveryHypopigmentationChromhidrosis
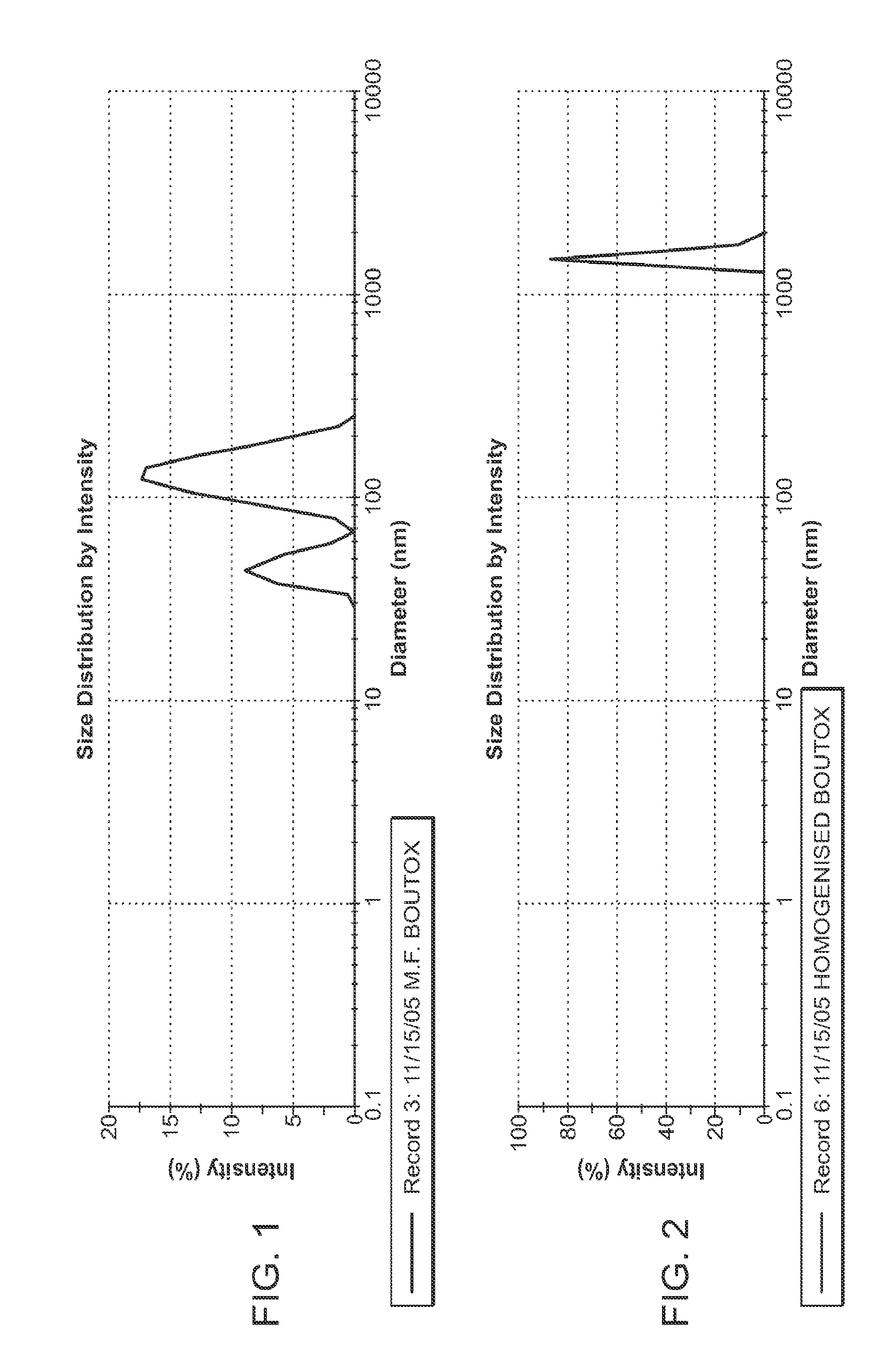
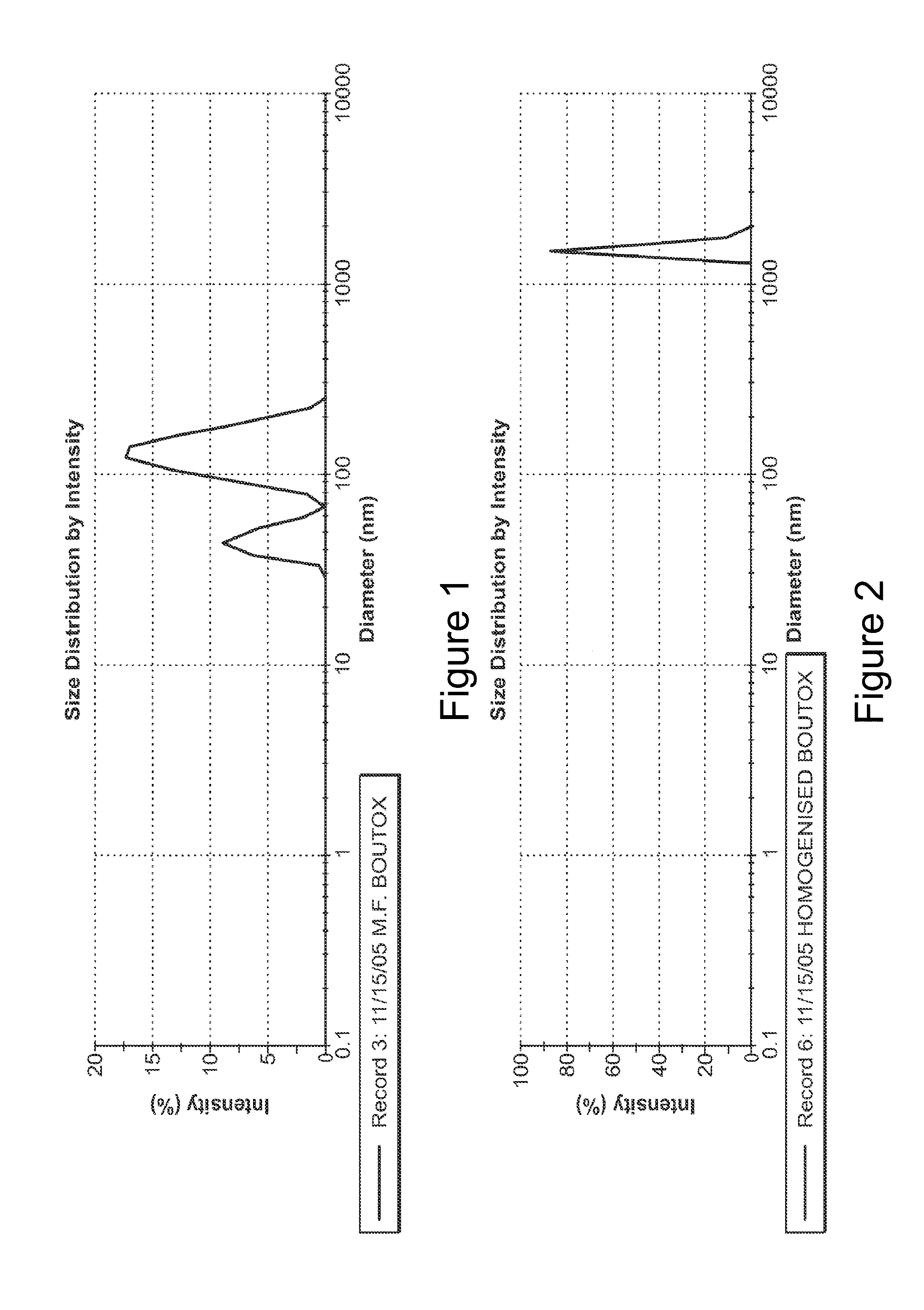
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Dermal Delivery
InactiveUS20160213757A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentCosmetic preparationsPeptide/protein ingredientsHypopigmentationTherapeutic effect
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Pharmaceutical formulations comprising an immune response modifier
Pharmaceutical formulations comprising an immune response modifier (IRM) chosen from imidazoquinoline amines, imidazotetrahydroquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, thiazolo-quinolineamines, oxazolo-quinolinamines, thiazolo-pyridinamines, oxazolo-pyridinamines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, and thiazolonaphthyridine amines; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid are useful for the treatment of dermal associated conditions. Novel topical formulations are provided. In one embodiment, the topical formulations are advantageous for treatment of actinic keratosis, postsurgical scars, basal cell carcinoma, atopic dermatitis, and warts.
Owner:3M INNOVATIVE PROPERTIES CO
Method of Treating Actinic Keratosis
InactiveUS20080262022A1High levelLow level of effectBiocideOintment deliveryActinic keratosisDermatology
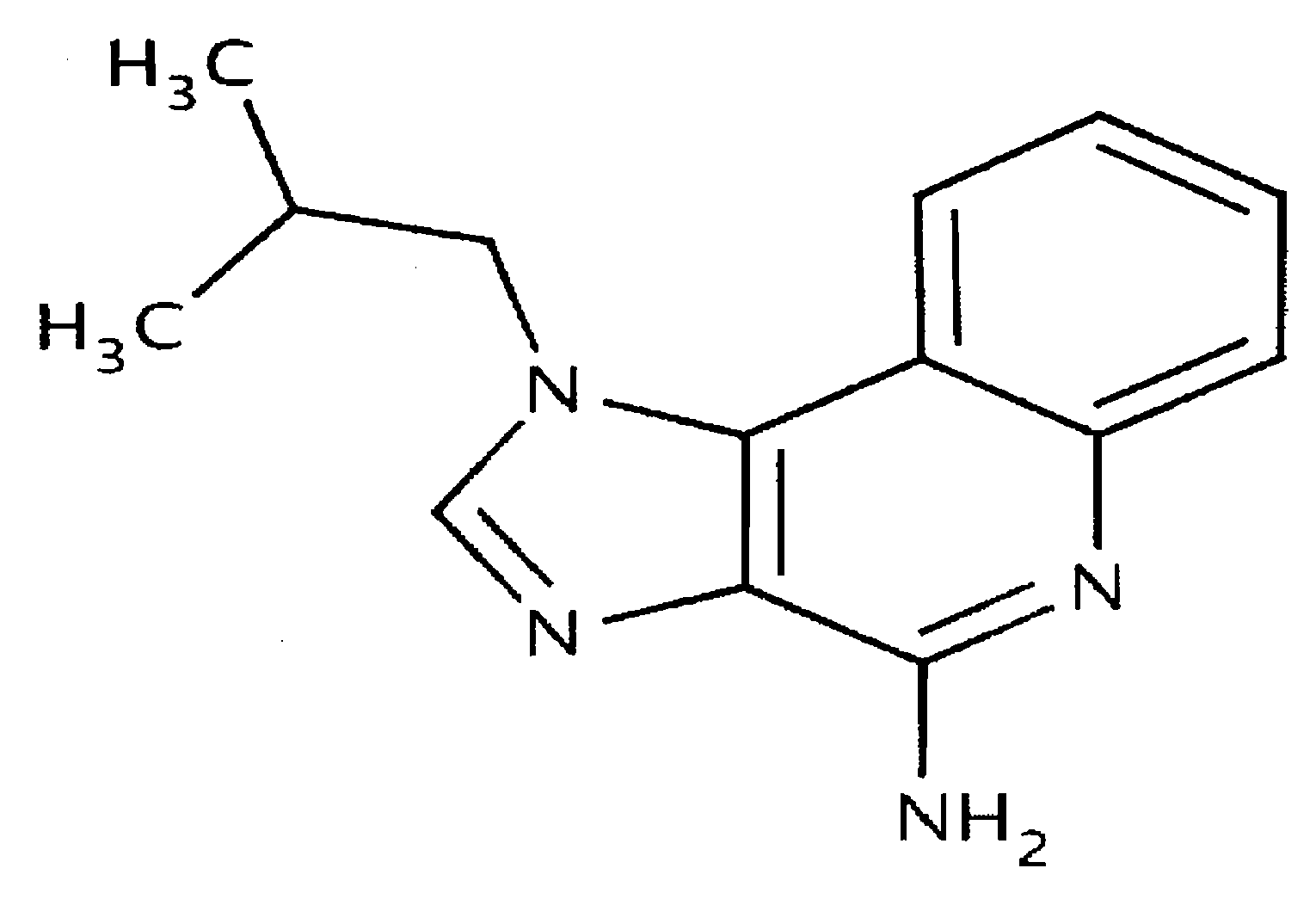
A method of treating actinic keratosis including applying topically to an actinic keratosis lesion twice per week for a duration of 8 weeks a formulation comprising 2-methyl-1-(2-methylpropyl)-1H-imidazo[4,5-c][1,5]naphthyridin-4-amine.
Owner:MEDICIS PHARMA CORP
Use of a polyphenol for the treatment of actinic keratosis
The present invention refers to a method for treating actinic keratosis by administering a pharmaceutically effective amount of a polyphenol to a patient as well as to the production of a medicament thereto.
Owner:MEDIGENE
Diclofenac compositions for the treatment of skin disorders
InactiveUS20050137164A1Readily availableSimilar and good performanceBiocideOrganic active ingredientsDiseaseActinic keratosis
Novel NSAID pharmaceutical compositions and methods for the treatment of skin disease and disorders such as actinic keratosis using same are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Nanoparticle compositions
InactiveUS20120328702A1Limit deliveryEffective for topical administrationAntibacterial agentsPowder deliveryDiseaseWrinkle skin
The present invention describes novel nanoparticle compositions, and systems and methods utilizing them for treating disorders and / or conditions associated with the epidermal and / or dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, actinic keratosis, facial wrinkles, muscle contracture, and headache. Methods generally involve administering nanoparticle compositions to the skin.
Owner:ANTERIOS INC
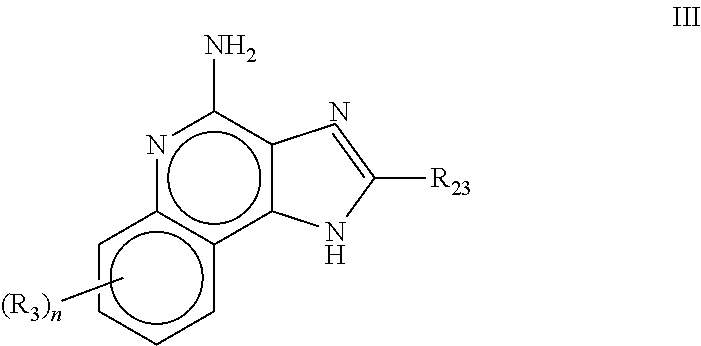
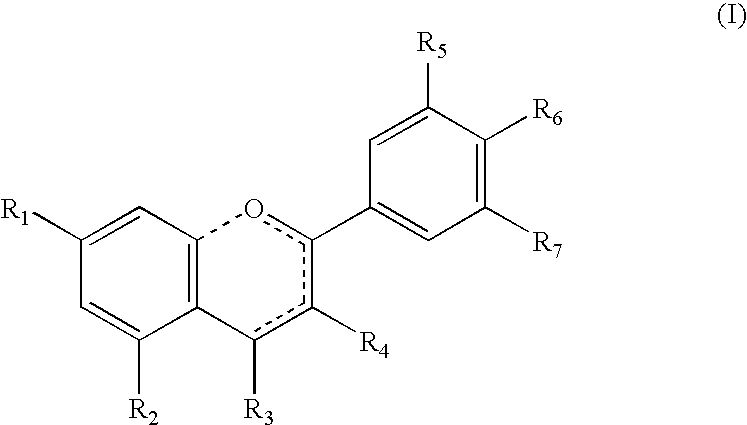
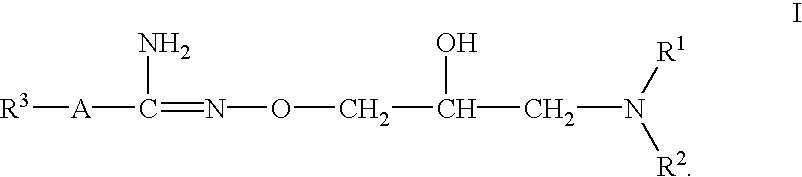
Use of 2,5-Dihydroxybenzene Derivatives for Treating Actinic Keratosis
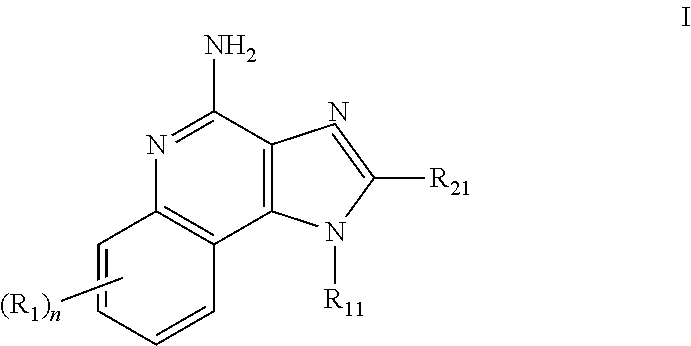
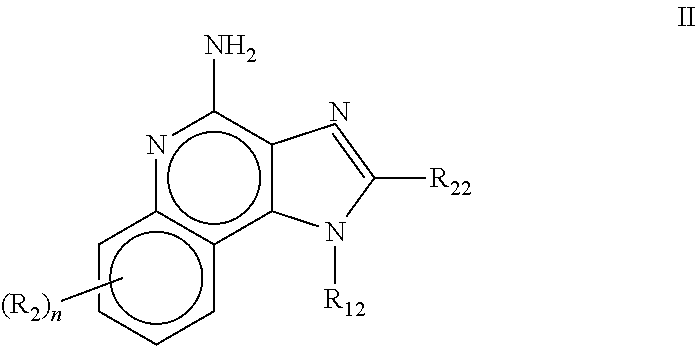
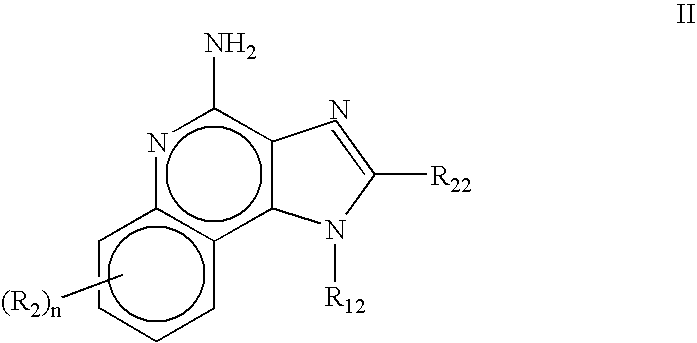
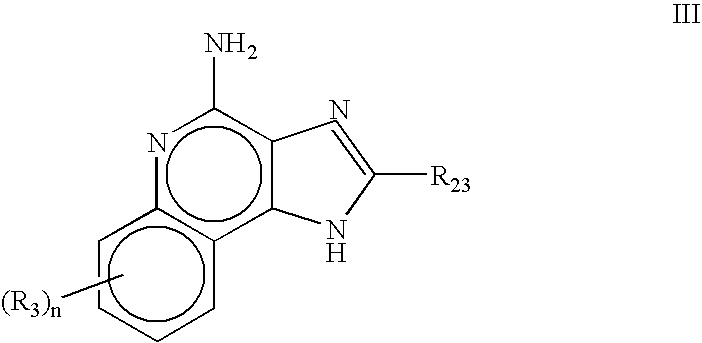
The present invention relates to the use of a 2,5-dihydroxybenzene derivative represented by Formula (I) or a pharmaceutically acceptable salt, solvate, isomer, or prodrug thereof for the therapeutic and / or phrophylactic treatment of, inter alia, actinic keratosis.
Owner:ACTION MEDICINES SL
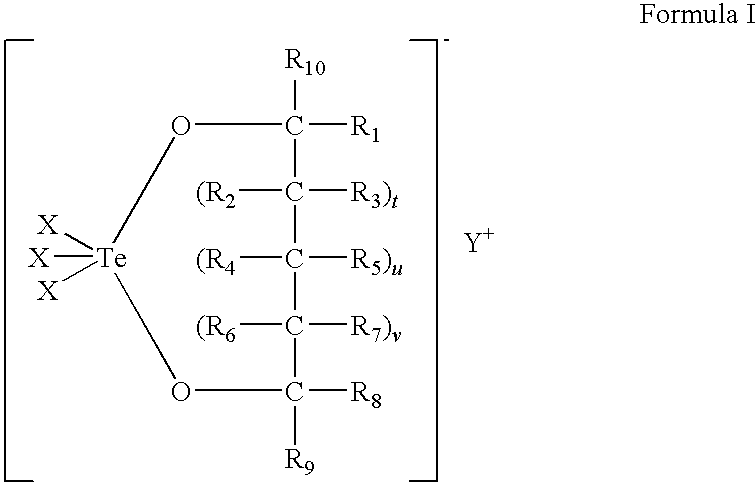
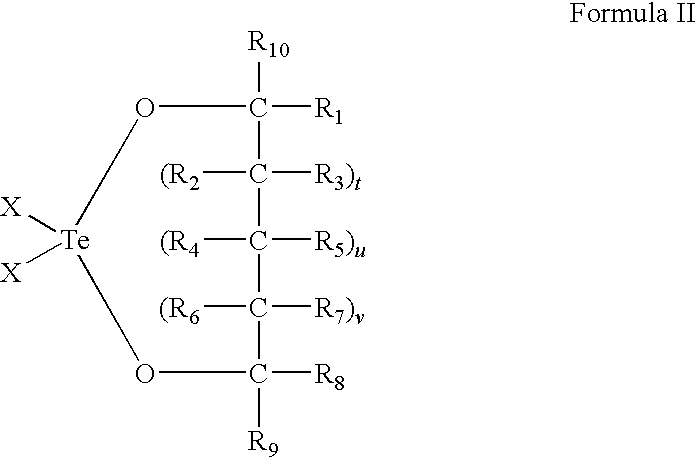
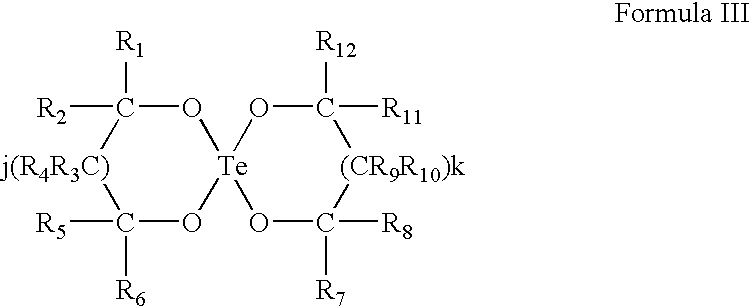
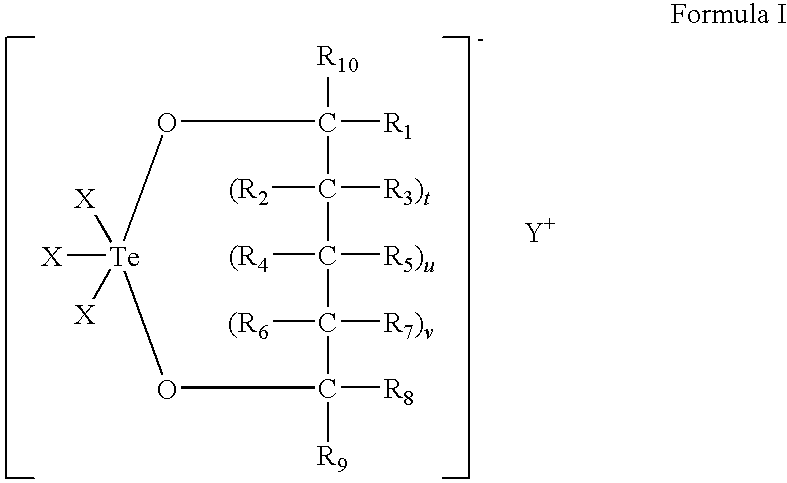
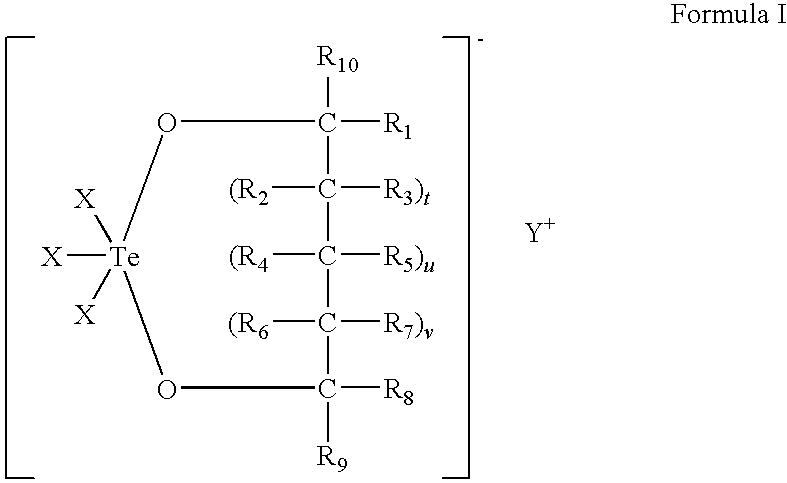
Use of tellurium compounds for treatment of basal cell carcinoma and/or actinic keratosis
Methods for treating skin conditions such as basal cell carcinoma and / or actinic keratosis are provided, which are effected by administering a pharmaceutically effective amount of a tellurium-containing compound. A pharmaceutical composition for treatment of skin conditions such as basal cell carcinoma an / or actinic keratosis is also provided.
Owner:CASSIOPEA SPA
Pharmaceutical formulations comprising an immune response modifier
Pharmaceutical formulations comprising an immune response modifier (IRM) chosen from imidazoquinoline amines, imidazotetrahydroquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, thiazolo-quinolineamines, oxazolo-quinolinamines, thiazolo-pyridinamines, oxazolo-pyridinamines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, and thiazolonaphthyridine amines; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid are useful for the treatment of dermal associated conditions. Novel topical formulations are provided. In one embodiment, the topical formulations are advantageous for treatment of actinic keratosis, postsurgical scars, basal cell carcinoma, atopic dermatitis, and warts.
Owner:3M INNOVATIVE PROPERTIES CO
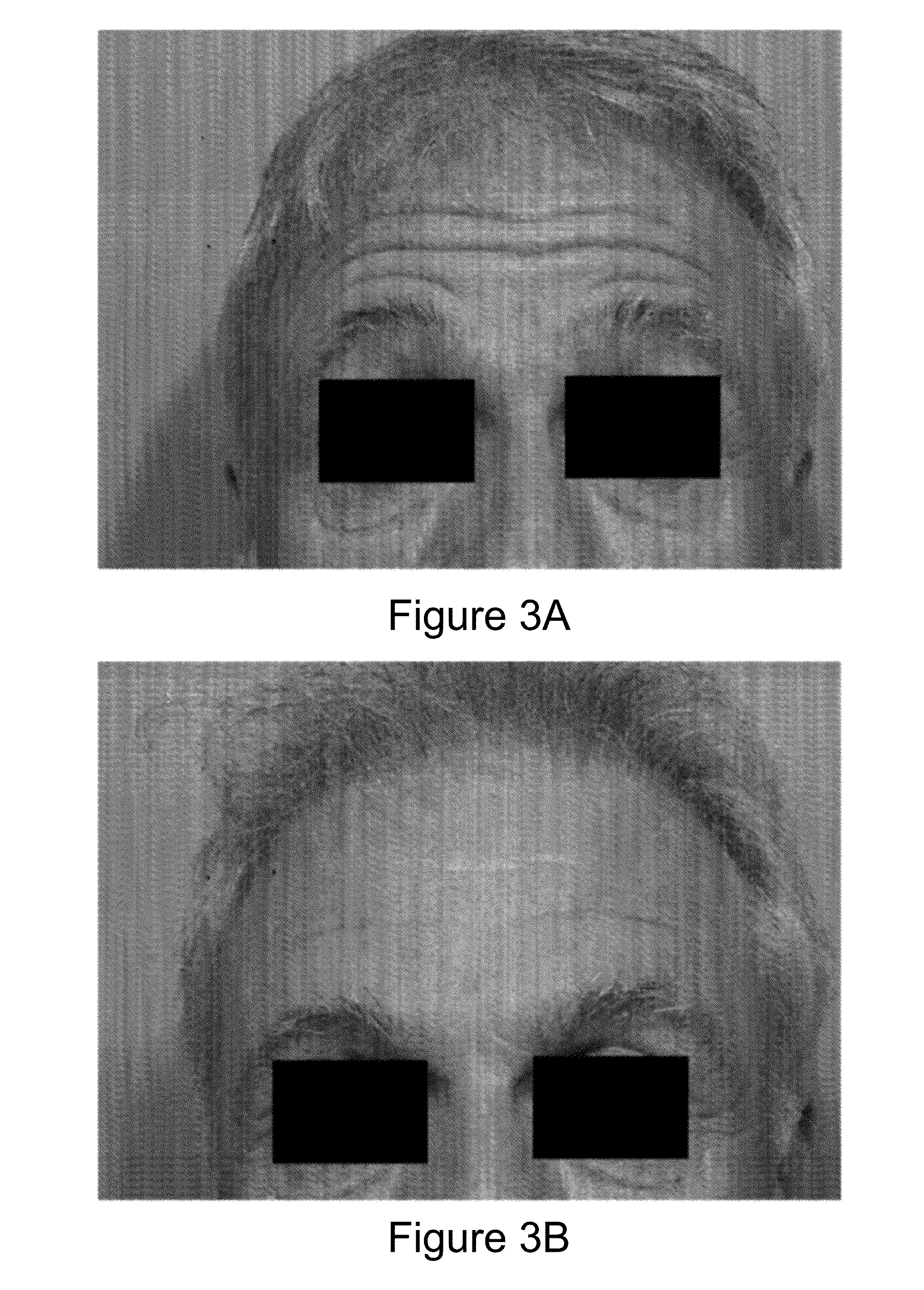
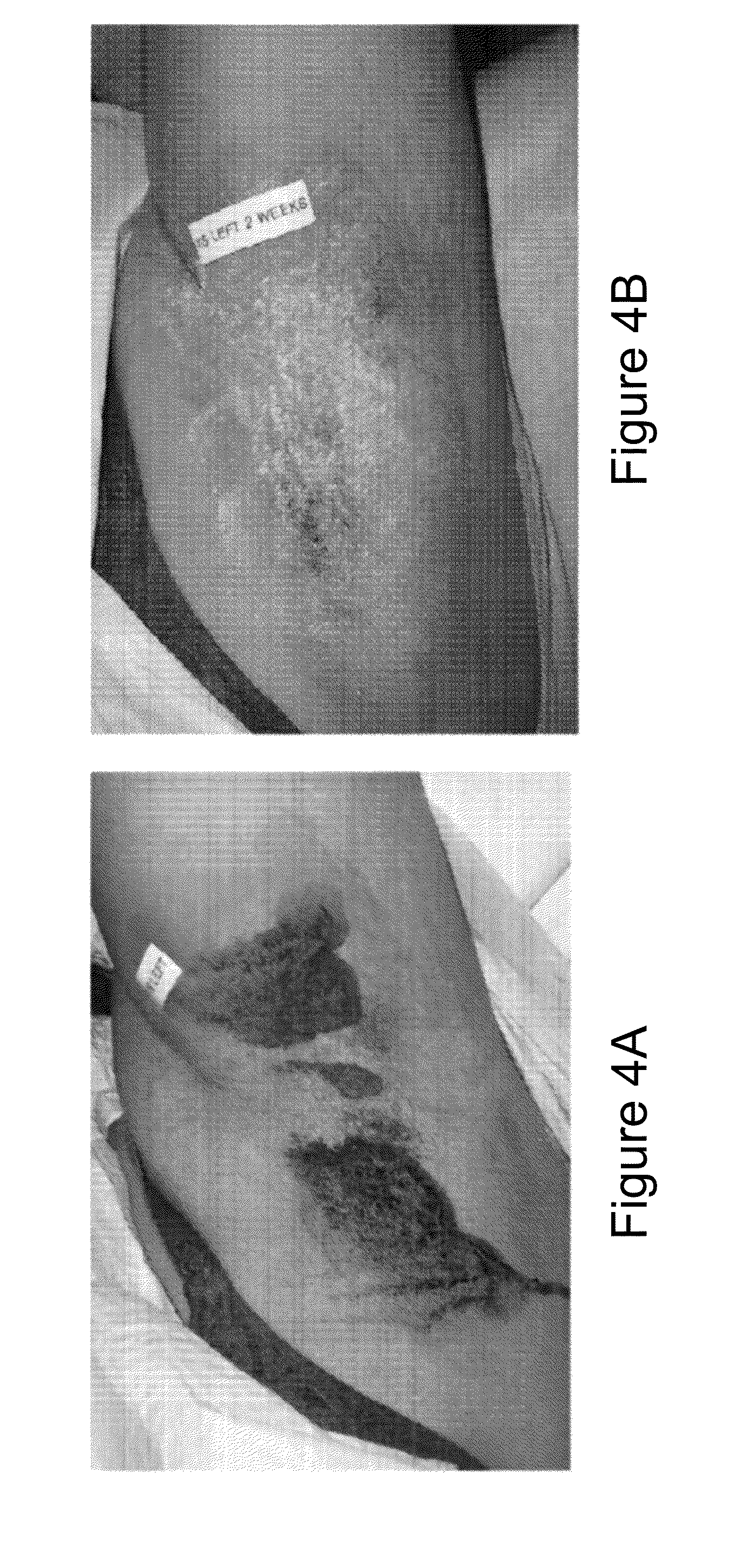
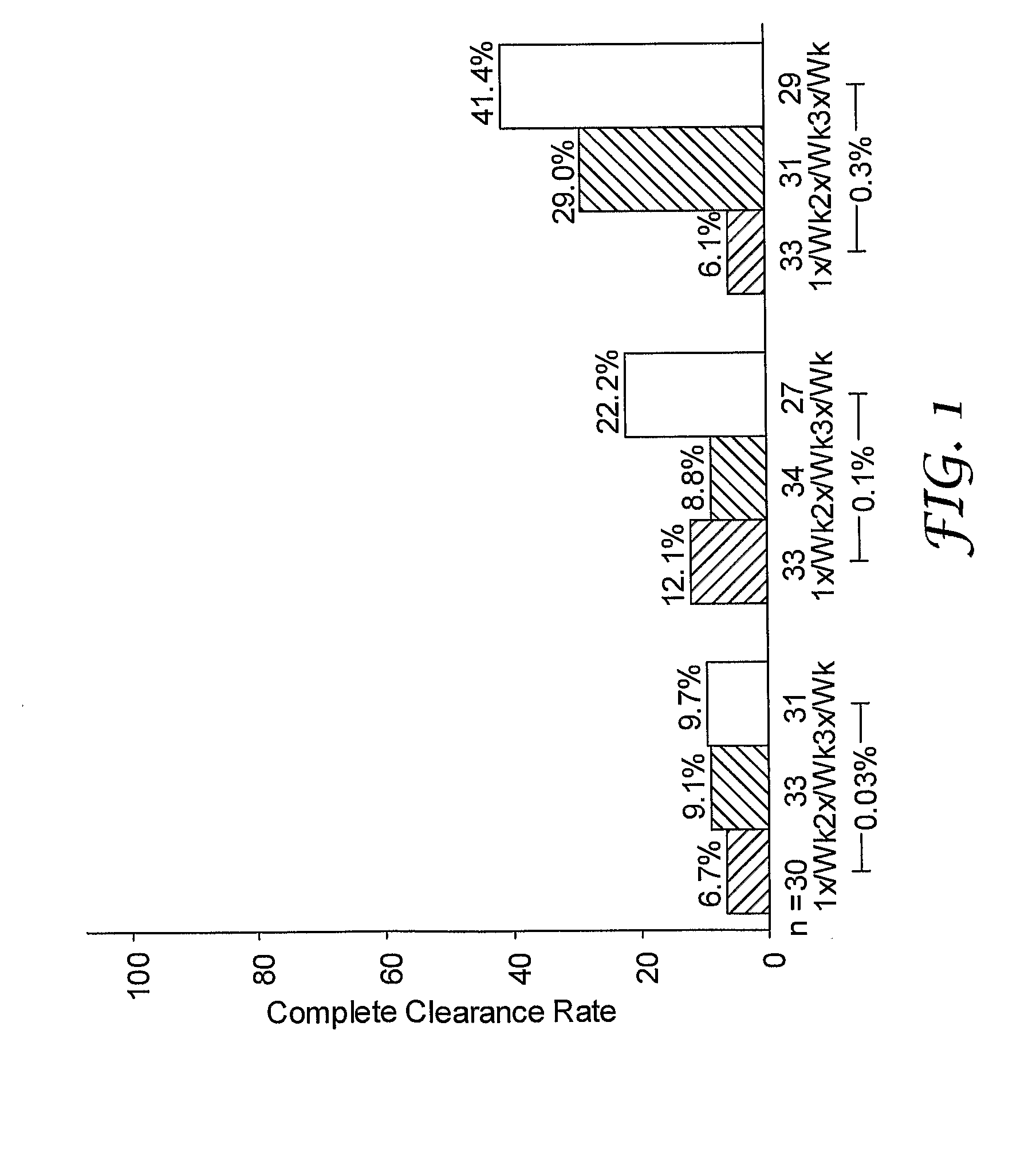
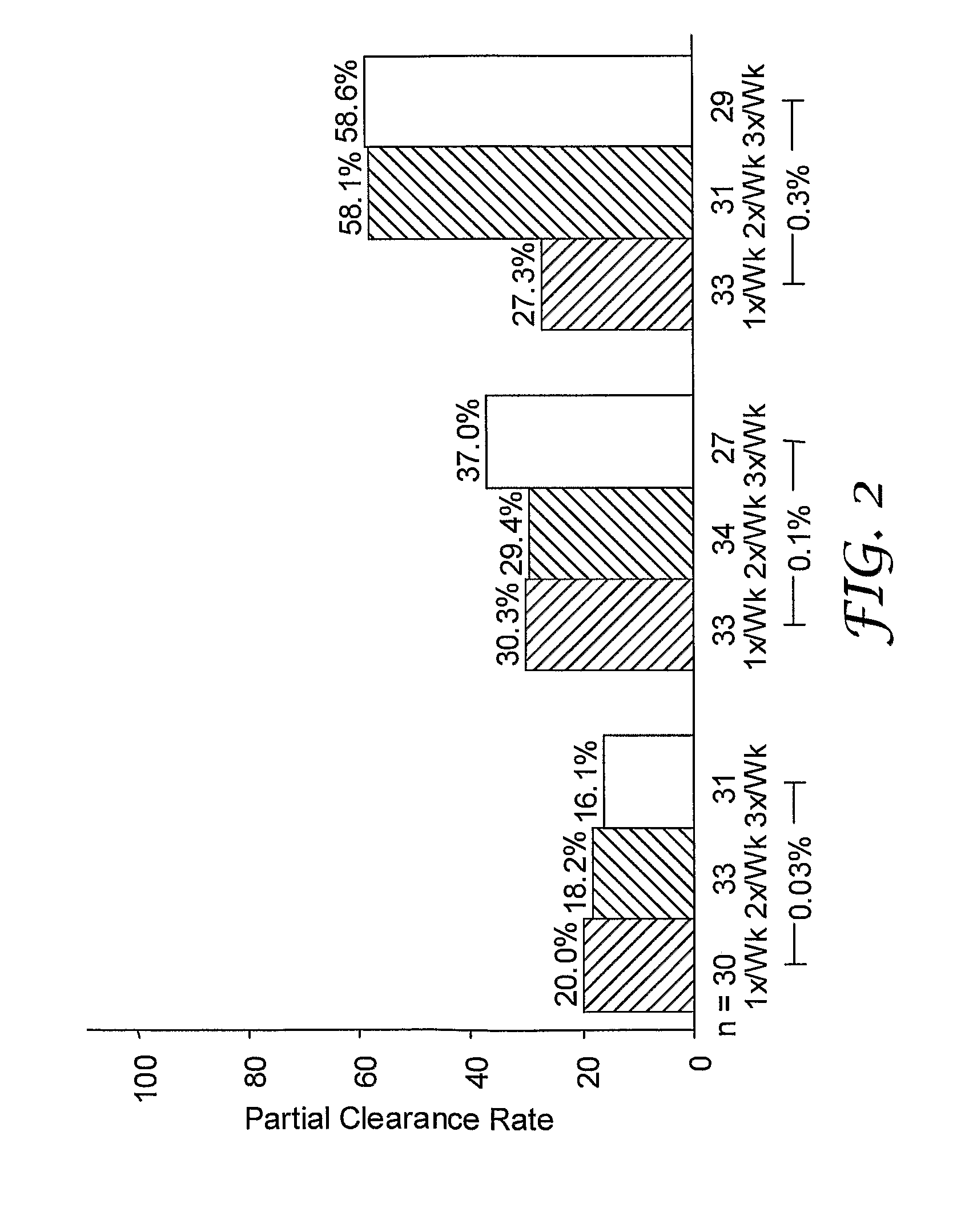
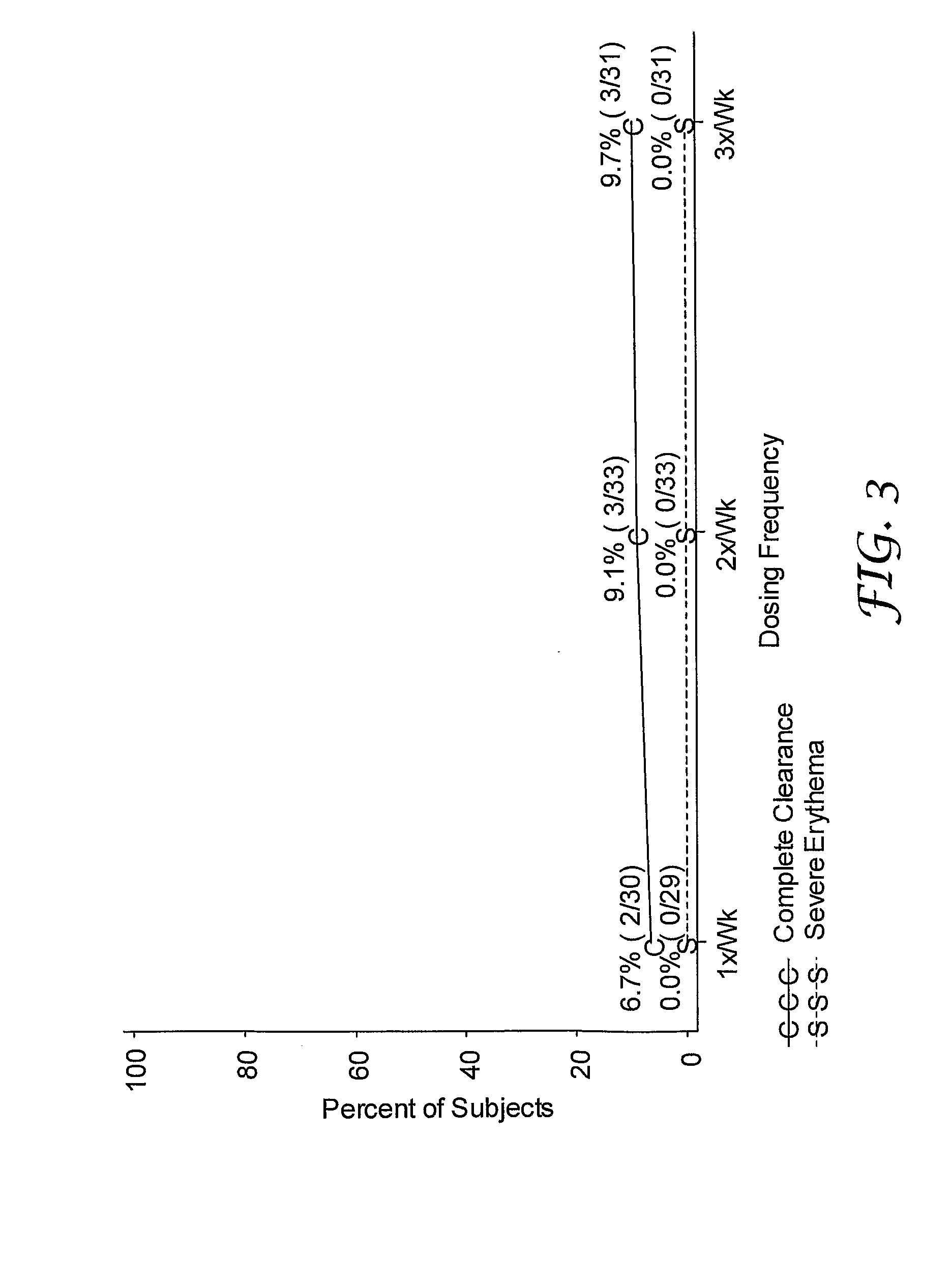
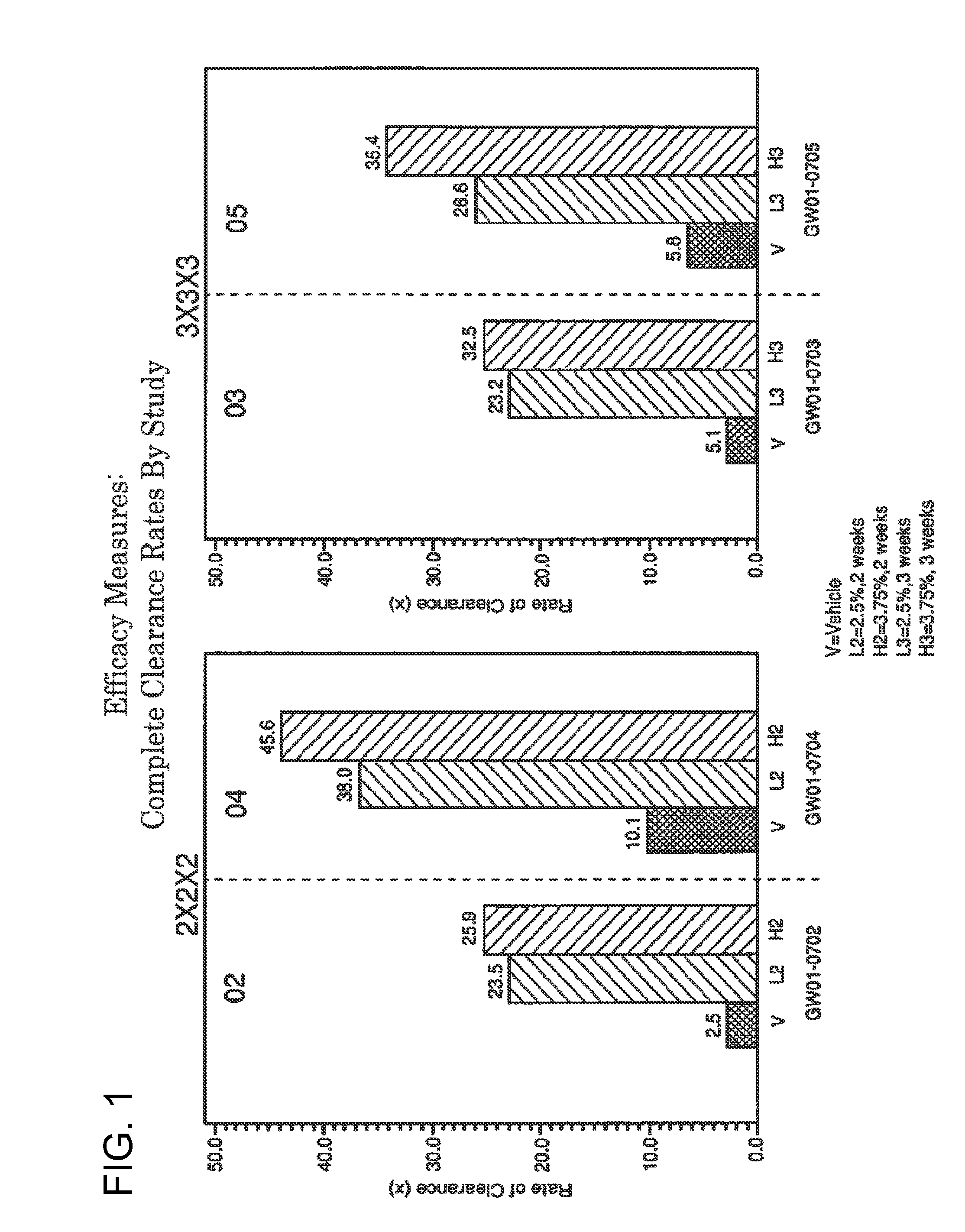
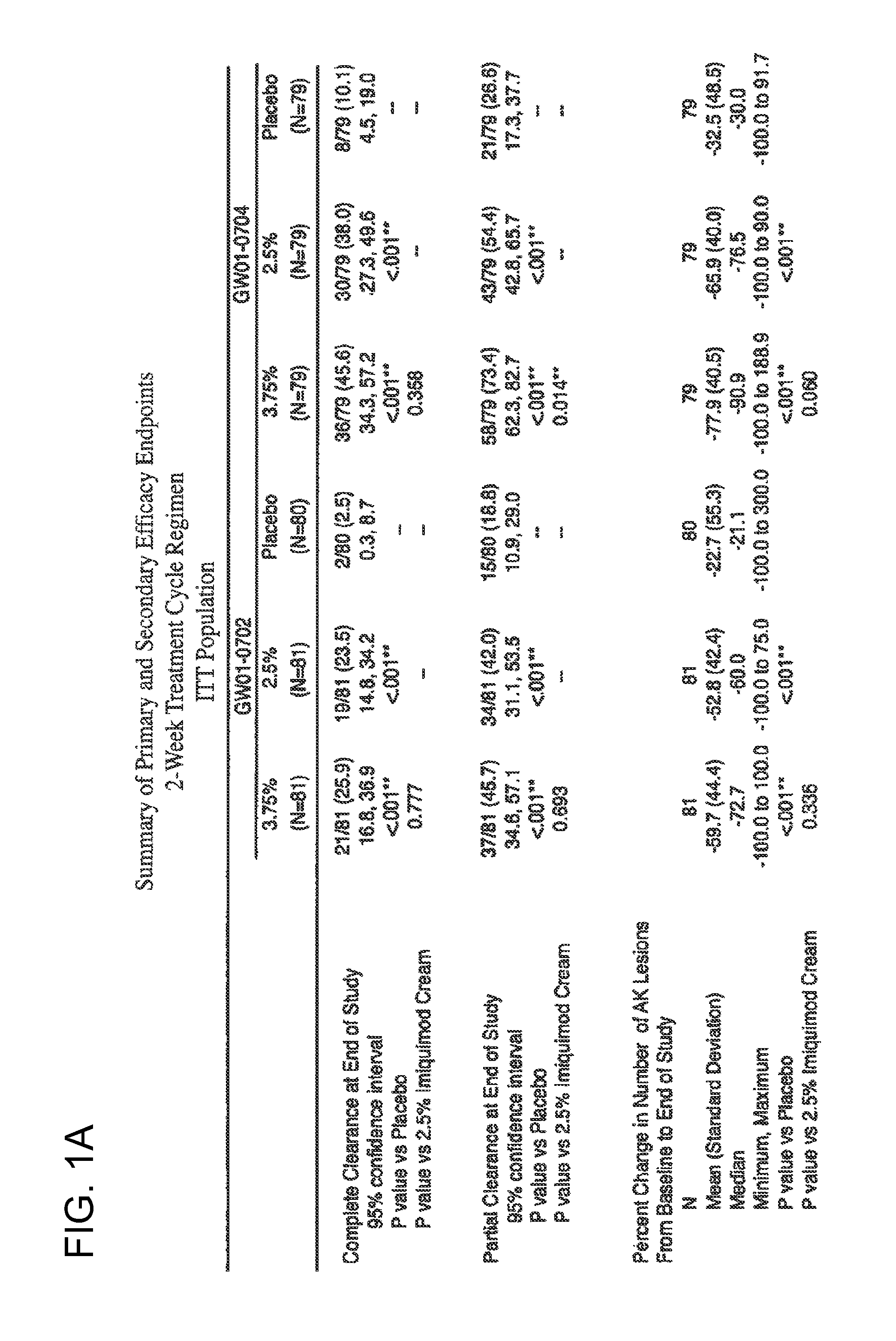
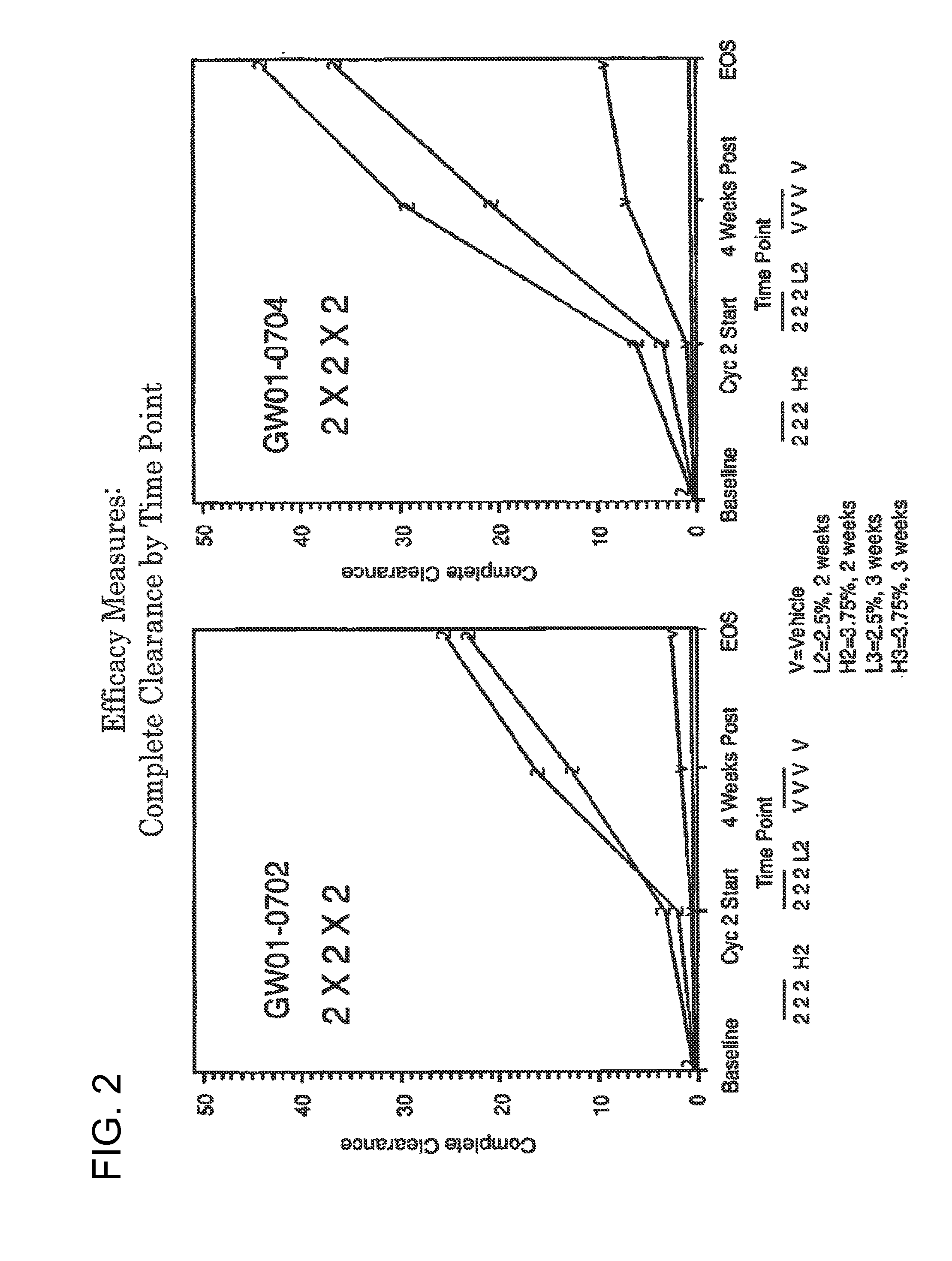
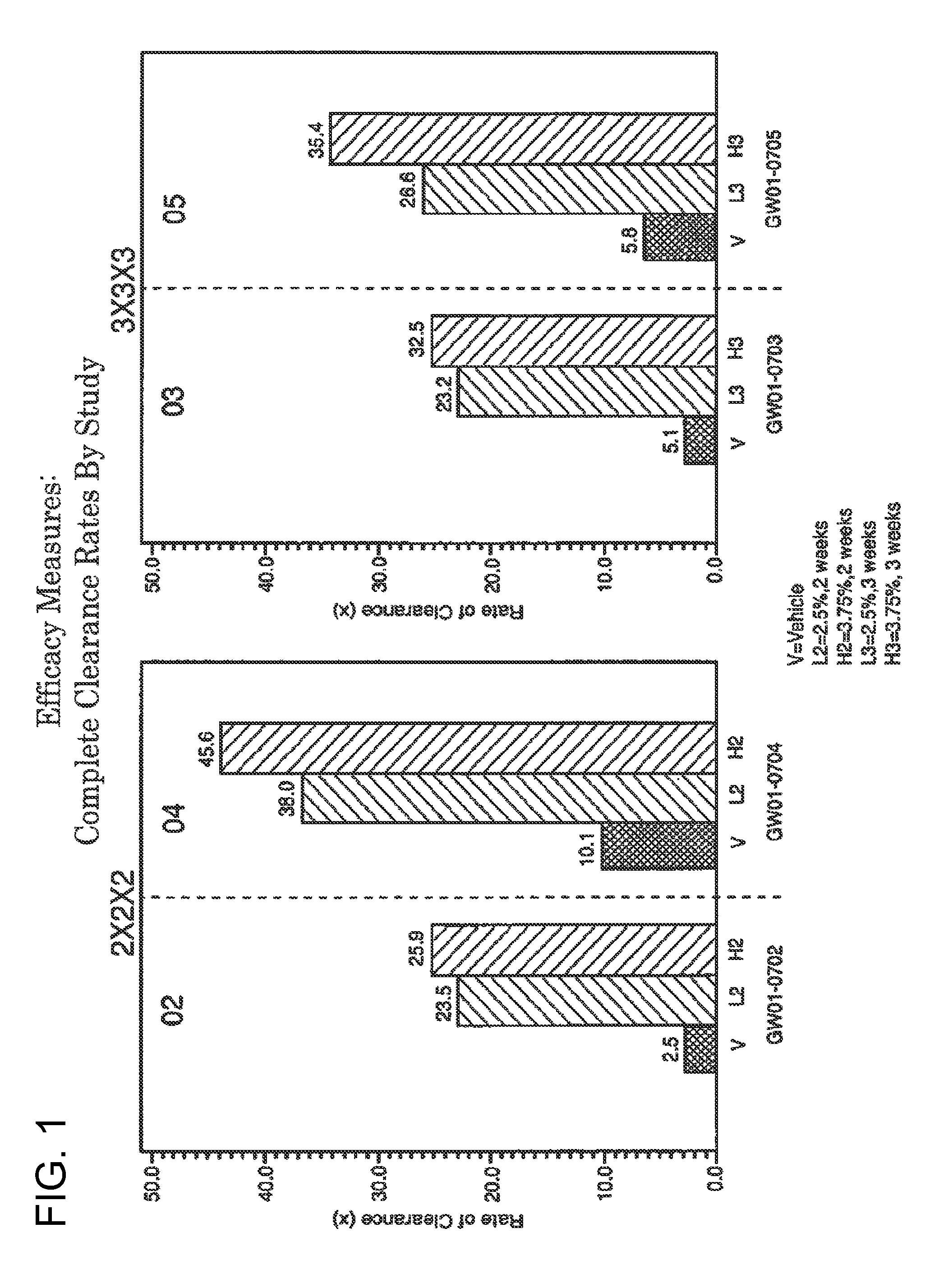
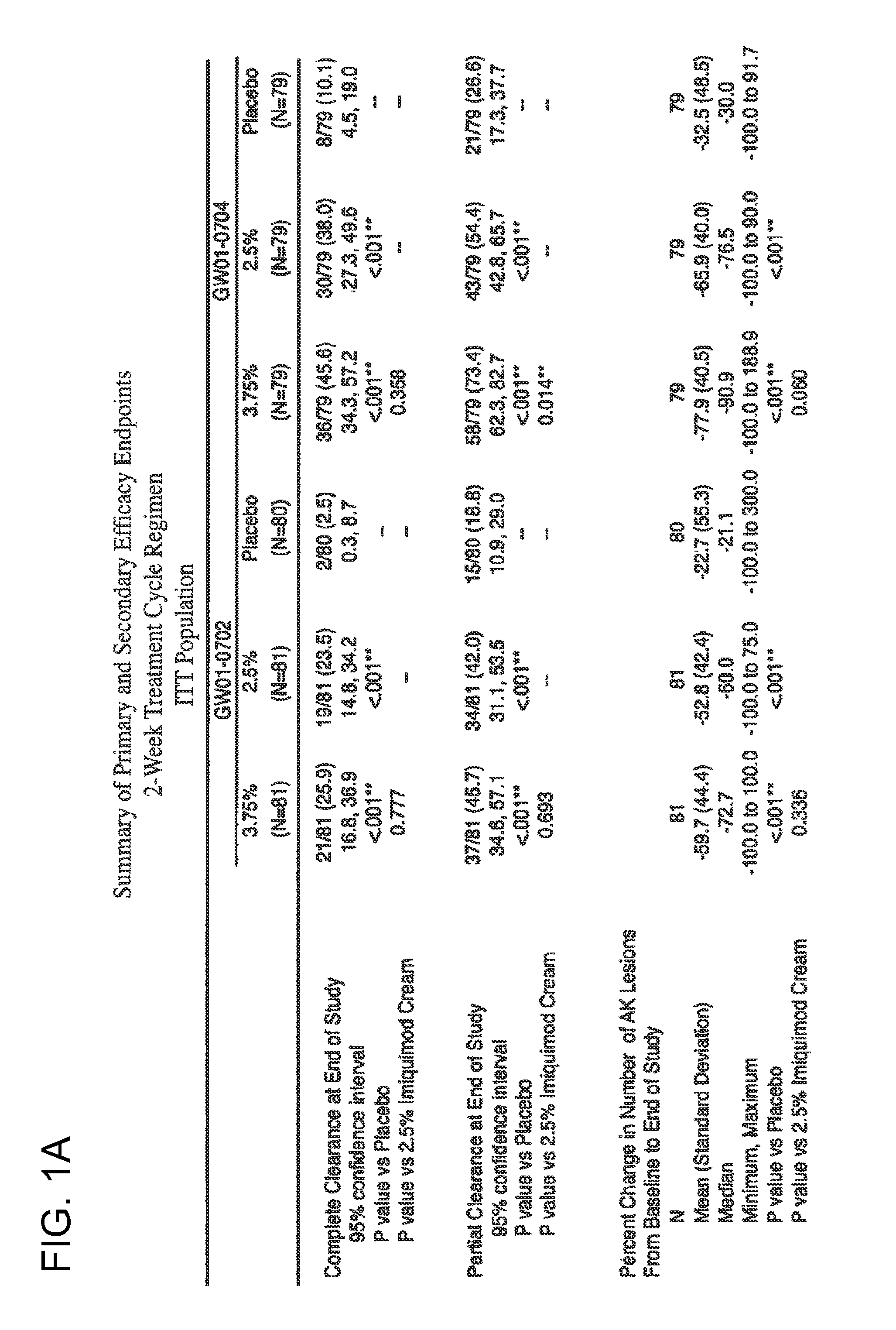
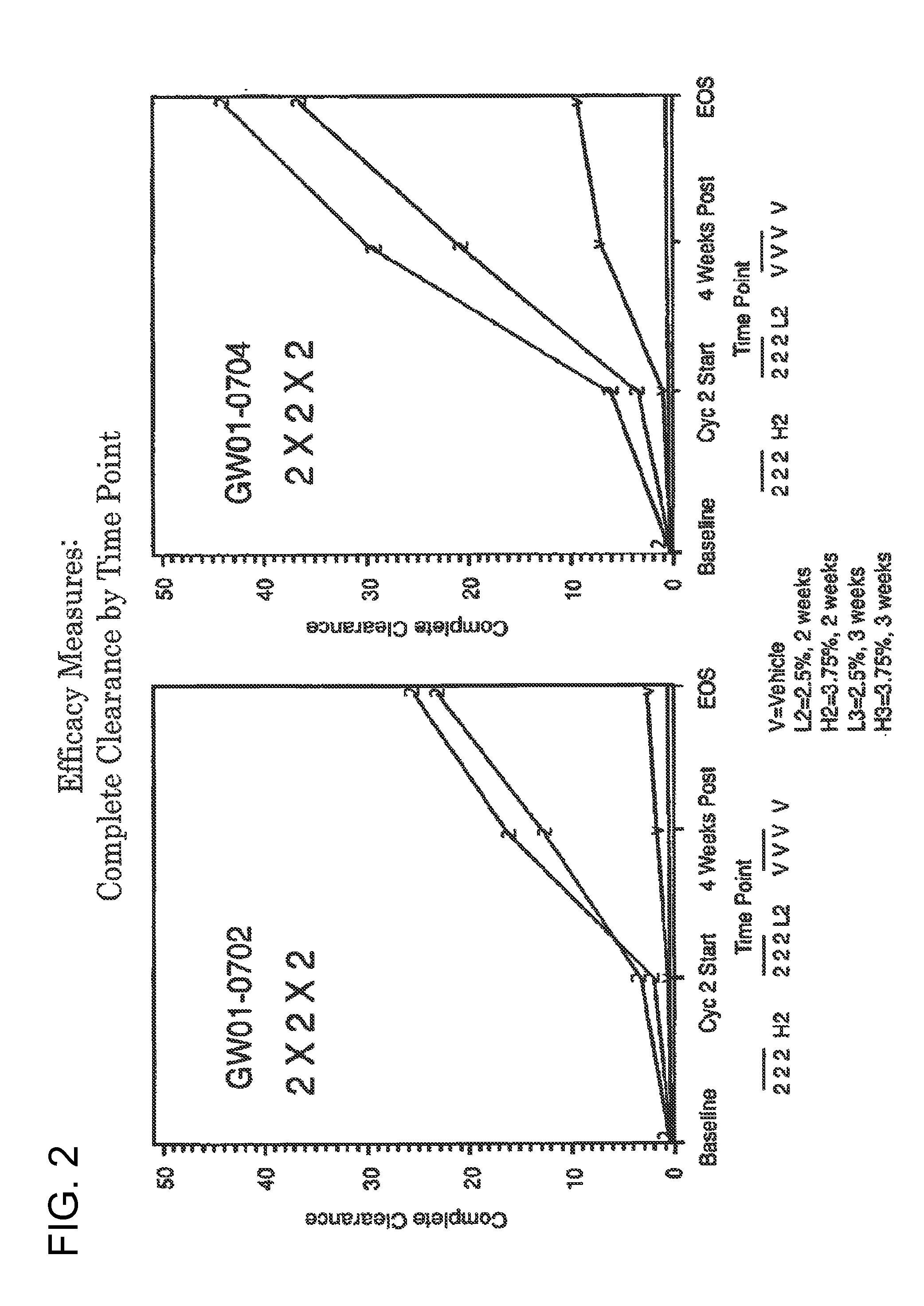
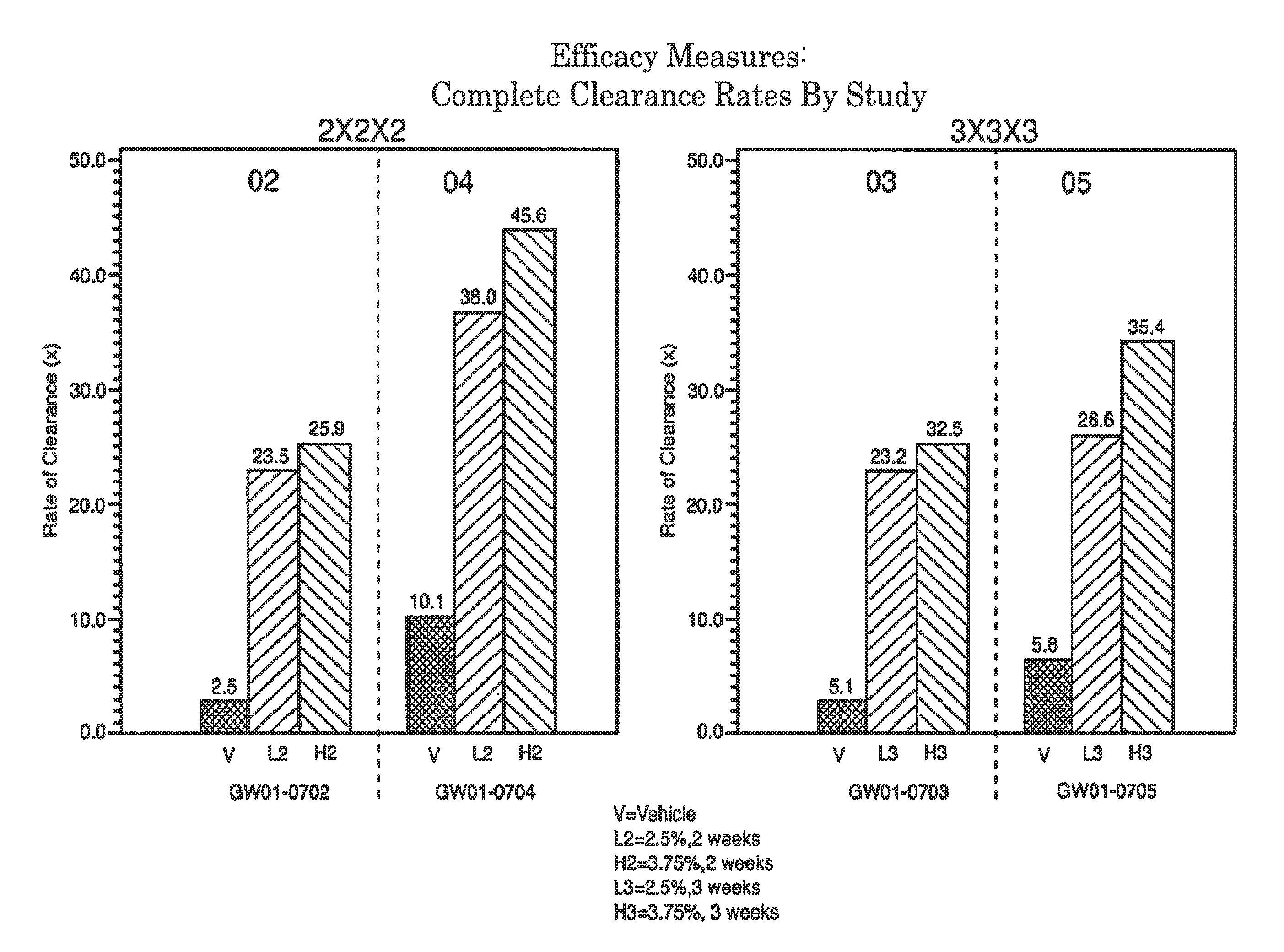
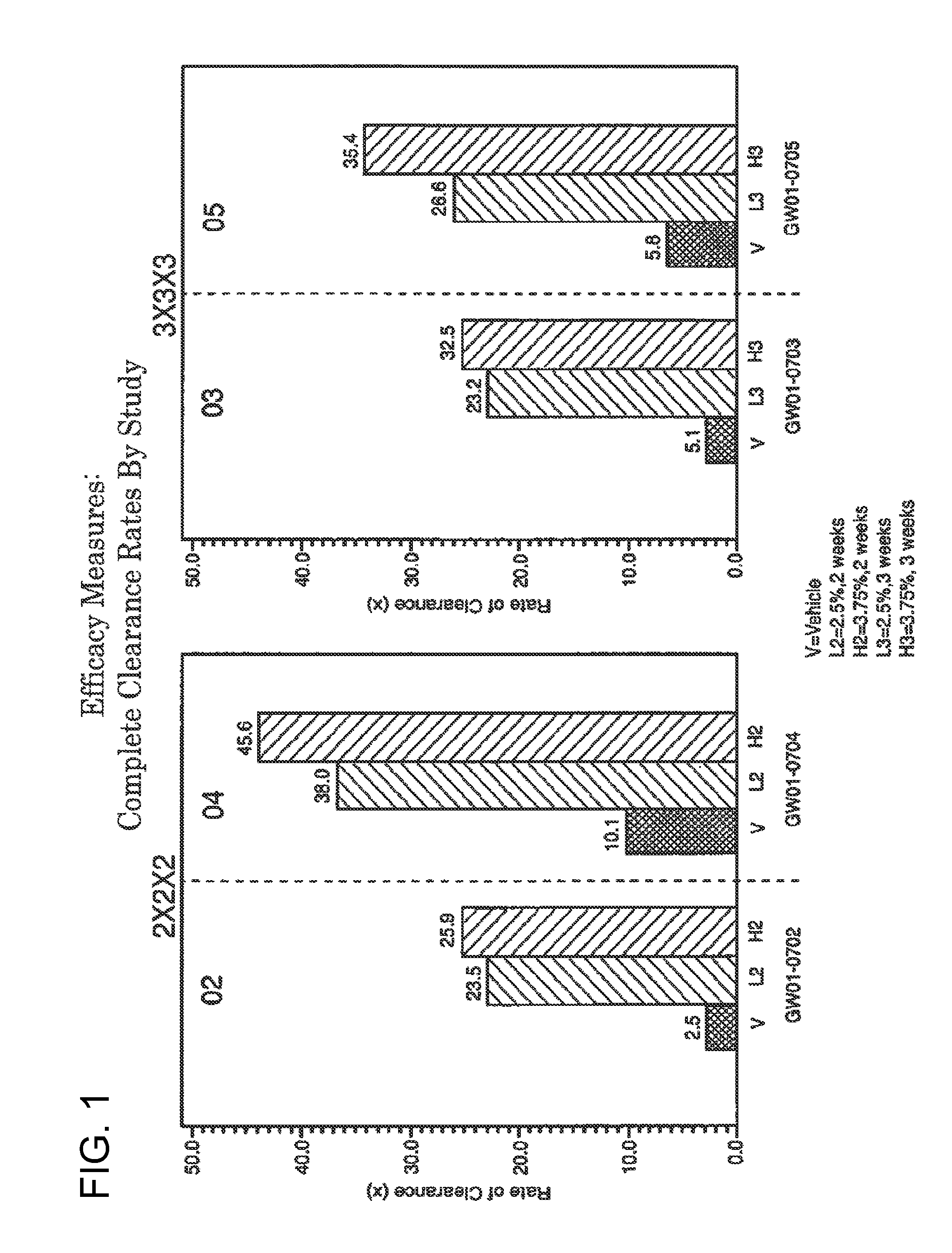
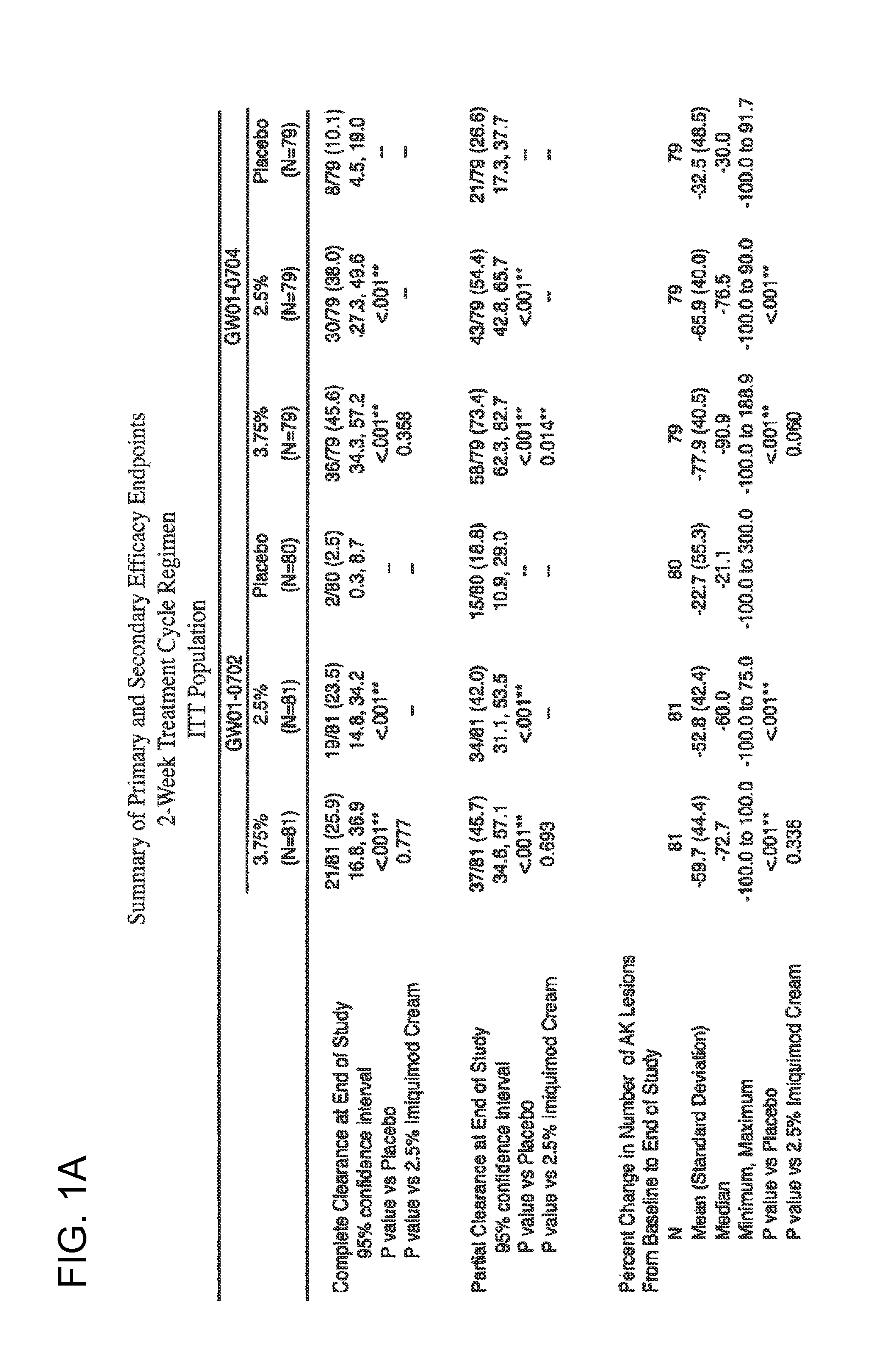
2 x 2 x 2 WEEK DOSING REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 3.75 % IMIQUIMOD
ActiveUS20110257218A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Owner:MEDICIS PHARMA CORP
Nanoparticle compositions
InactiveUS20150196490A1Effective for topical administrationLimit deliveryAntibacterial agentsPowder deliveryDiseaseCuticle
The present invention describes novel nanoparticle compositions, and systems and methods utilizing them for treating disorders and / or conditions associated with the epidermal and / or dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, actinic keratosis, facial wrinkles, muscle contracture, and headache. Methods generally involve administering nanoparticle compositions to the skin.
Owner:ANTERIOS INC
3 x 3 x 3 WEEK TREATMENT REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 2.5% IMIQUIMOD
InactiveUS20110257217A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara° 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Method for treating the pathological lesions of the skin that develop by ultraviolet radiation of the sunlight
The invention relates to methods for prevention and / or treatment of skin lesions caused by exposure to ultraviolet radiation. Exemplary condition that can be prevented or treated are actinic keratosis, dry skin, polymorphic light exanthema, photopathology, photo-allergy, solar atrophy, stria migrans, elastoma diffusum, X-ray dermatitis, gouty polychondritis and decubitis ulcer. The method employs application to the skin of a composition comprising a hydroximic acid derivative of the formula
Owner:N GENE RES LAB
Nanoparticle compositions and components thereof
InactiveUS20120328525A1Good effectReduce frequencyAntibacterial agentsPowder deliveryChromhidrosisNanoparticle
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis, among others. Methods generally involve administering provided compositions to the skin.
Owner:ANTERIOS INC
Pump systems and methods for storing and dispensing a plurality of precisely measured unit-doses of imiquimod cream
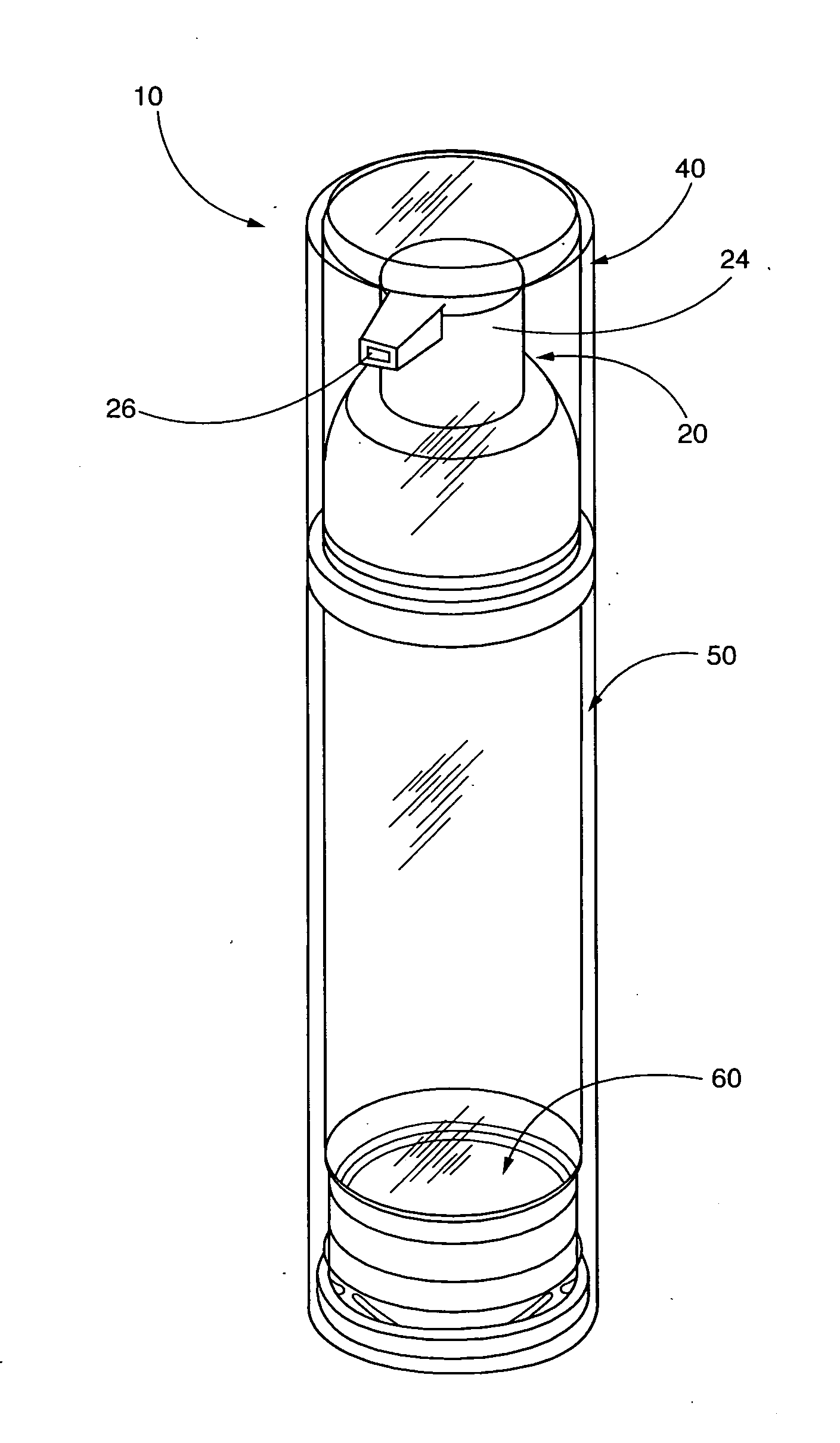
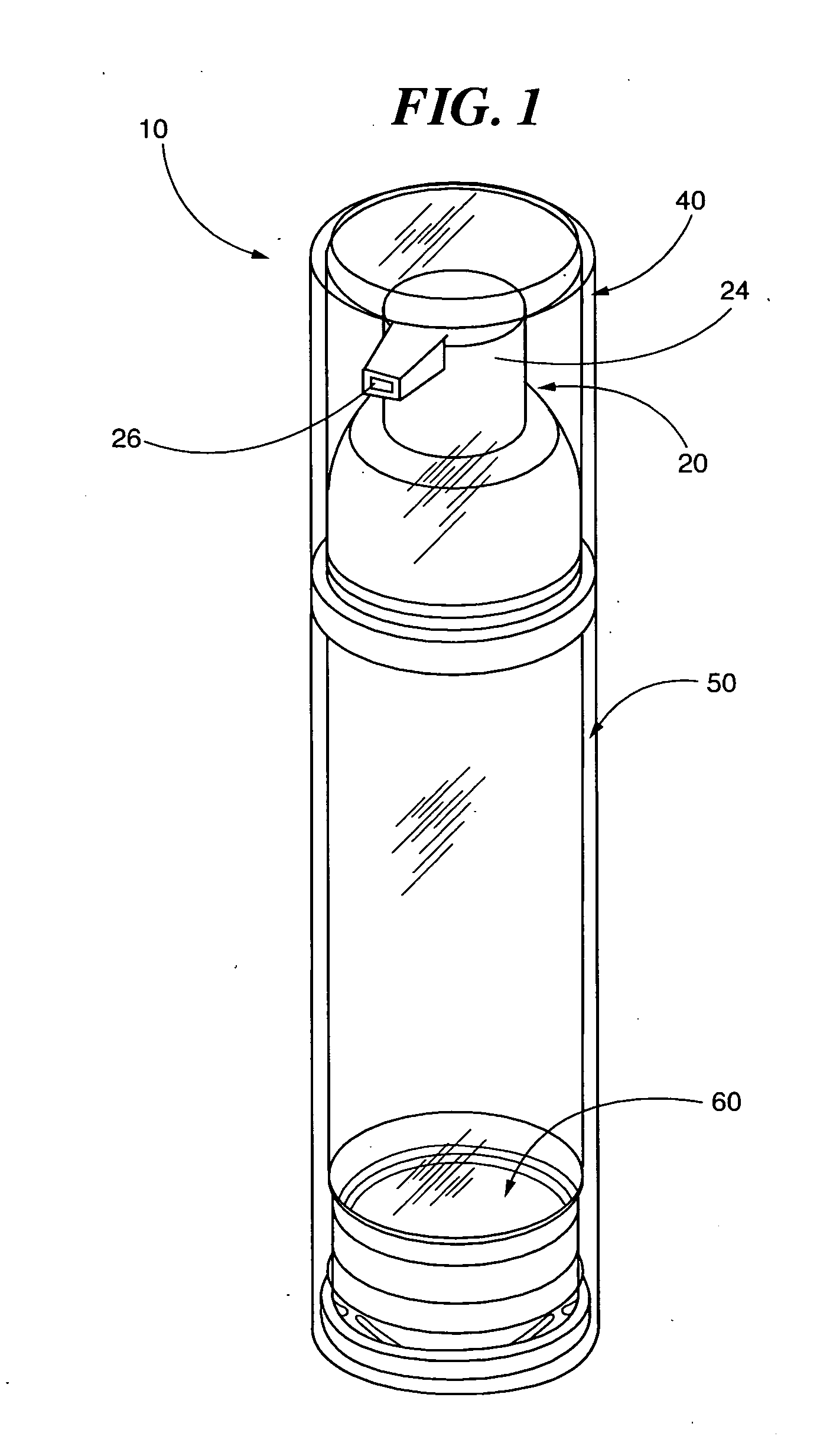
ActiveUS20120035556A1Effective treatmentEasy to storeOrganic active ingredientsOintment deliveryTopical treatmentActinic keratosis
The present invention is directed to airless storage and dispensing systems that include a pump or dispensing package pre-filled with a topical semi-solid imiquimod pharmaceutical formulation (“pump systems”) and methods for storing and dispensing from the pump systems a plurality of precisely measured and uniform unit doses of a topical semi-solid imiquimod pharmaceutical formulation, and more particularly to pump systems, pre-filled with a topical imiquimod pharmaceutical cream and methods for delivering multiple precisely measured unit doses of a topical imiquimod pharmaceutical cream, and methods for using a controlled delivery pump system to store and dispense a plurality of consistent and precisely measured unit doses of a topical imiquimod pharmaceutical cream for use in topically treating a dermal and mucosal-associated condition, such as, external genital warts and / or perianal warts (EGWs), actinic keratosis or actinic keratoses (AK or AKs) and superficial basal cell carcinoma (sBCC).
Owner:MEDICIS PHARMA CORP
Sulfonated precursors of thymidine for the treatment of epithelial hyperplasias
InactiveUS20110053965A1Facilitate subsequent penetrationOrganic active ingredientsBiocideKeloidEpithelial hyperplasia
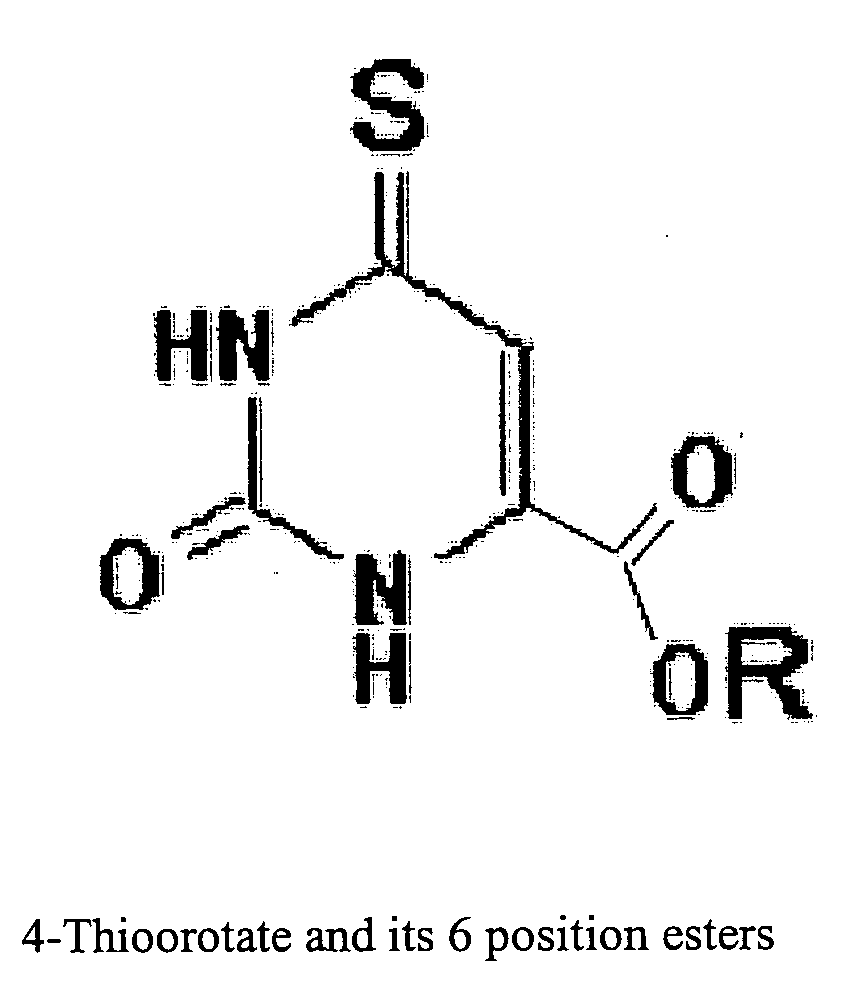
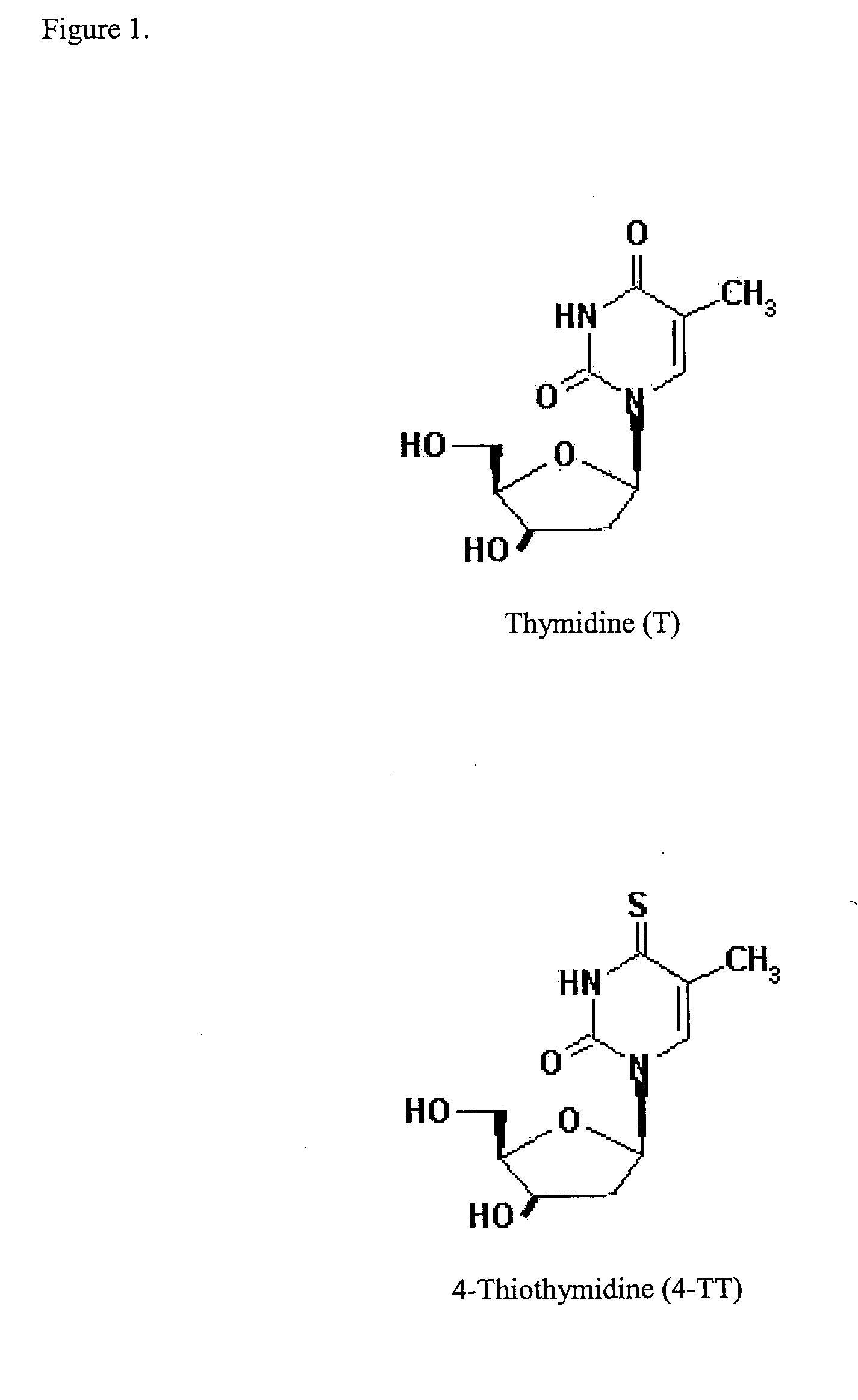
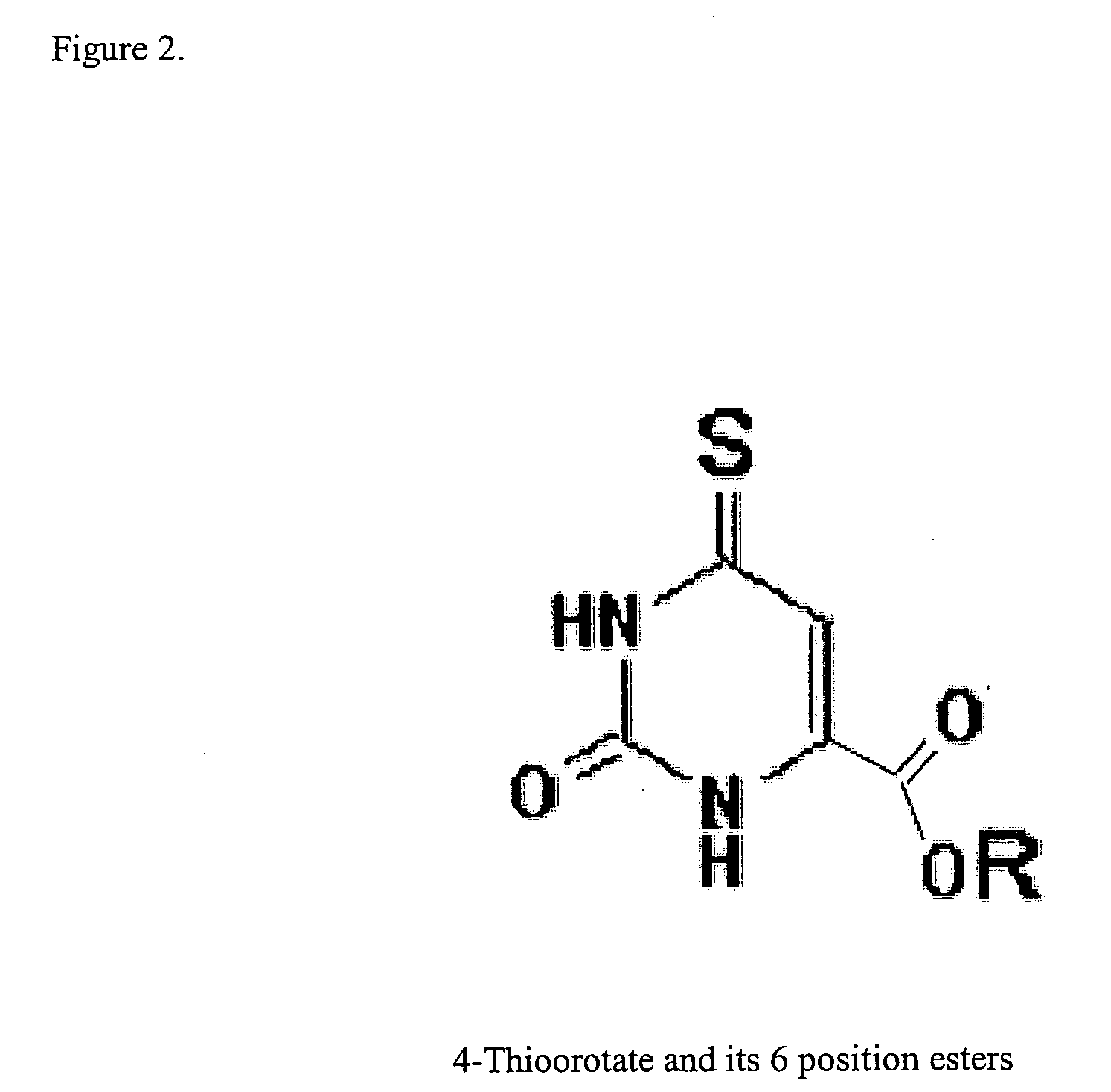
This invention relates to pharmaceutical compositions comprising a sulfonated biological precursor of thymidine, such as a precursor of 4-thiothymidine (4-TT), and their use in the photodynamic treatment of skin hyperplasias, including cancer, psoriasis, actinic keratosis and keloids, by topical or systemic administration.
Owner:HUMANITARIAN SCI
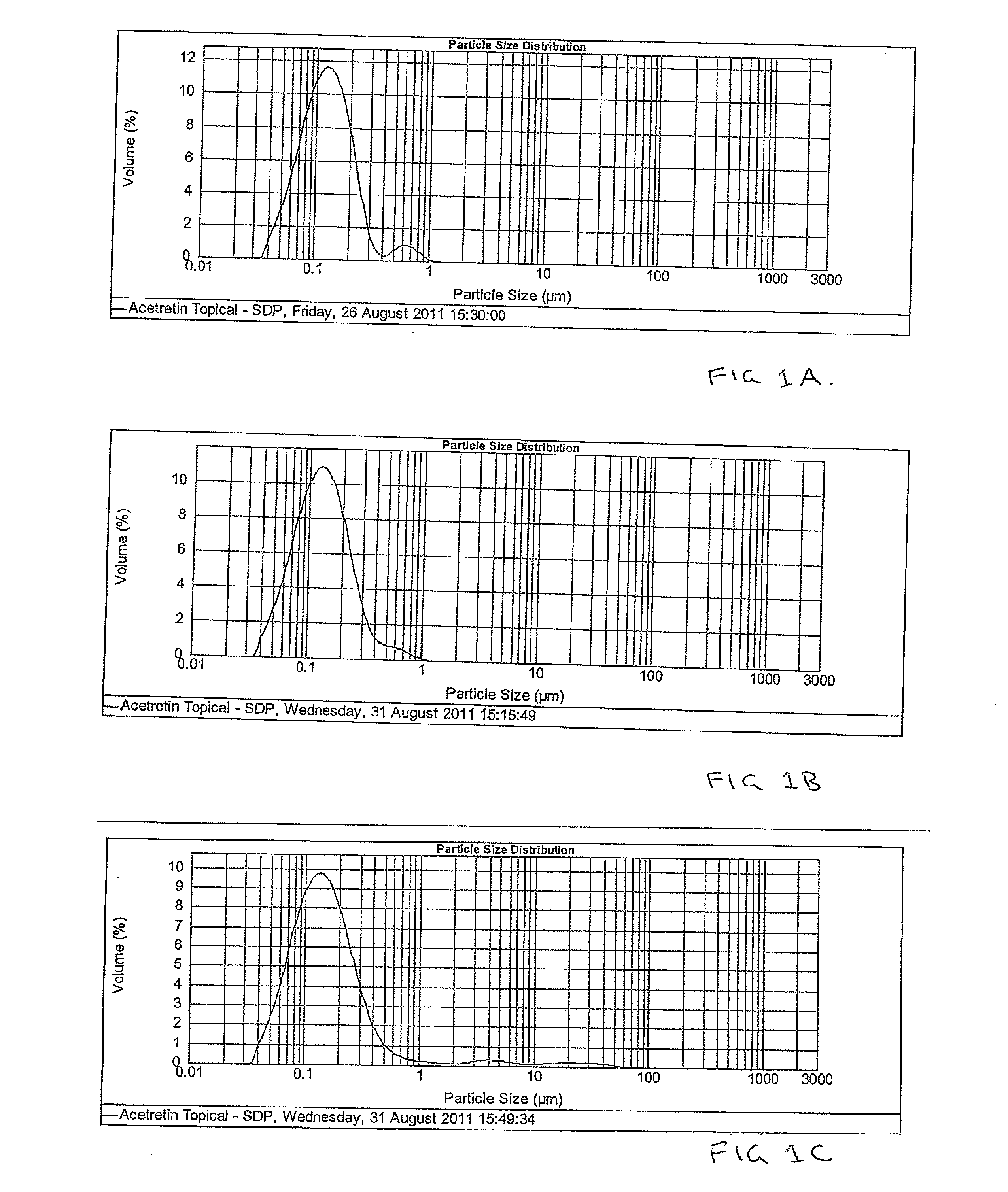
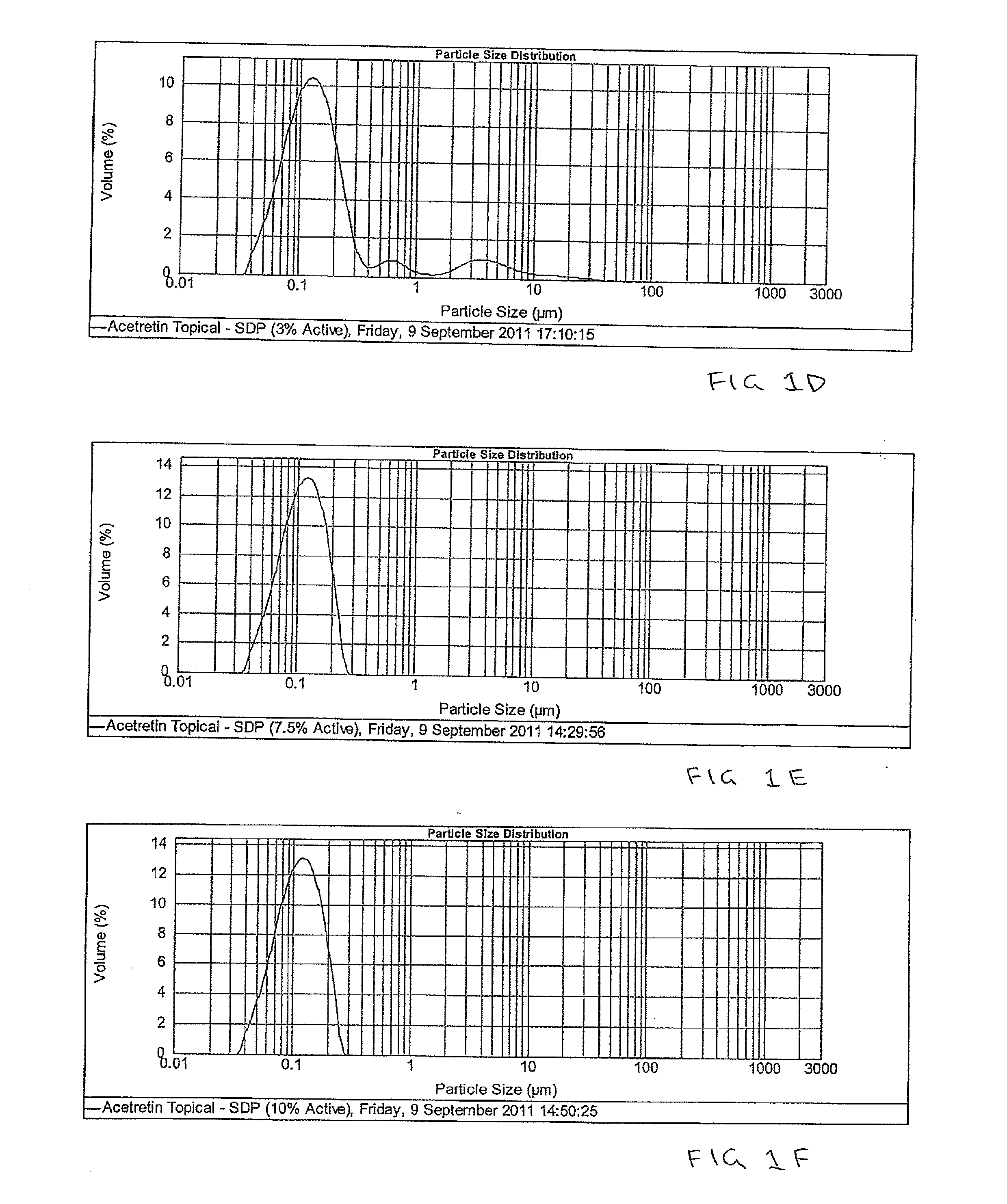
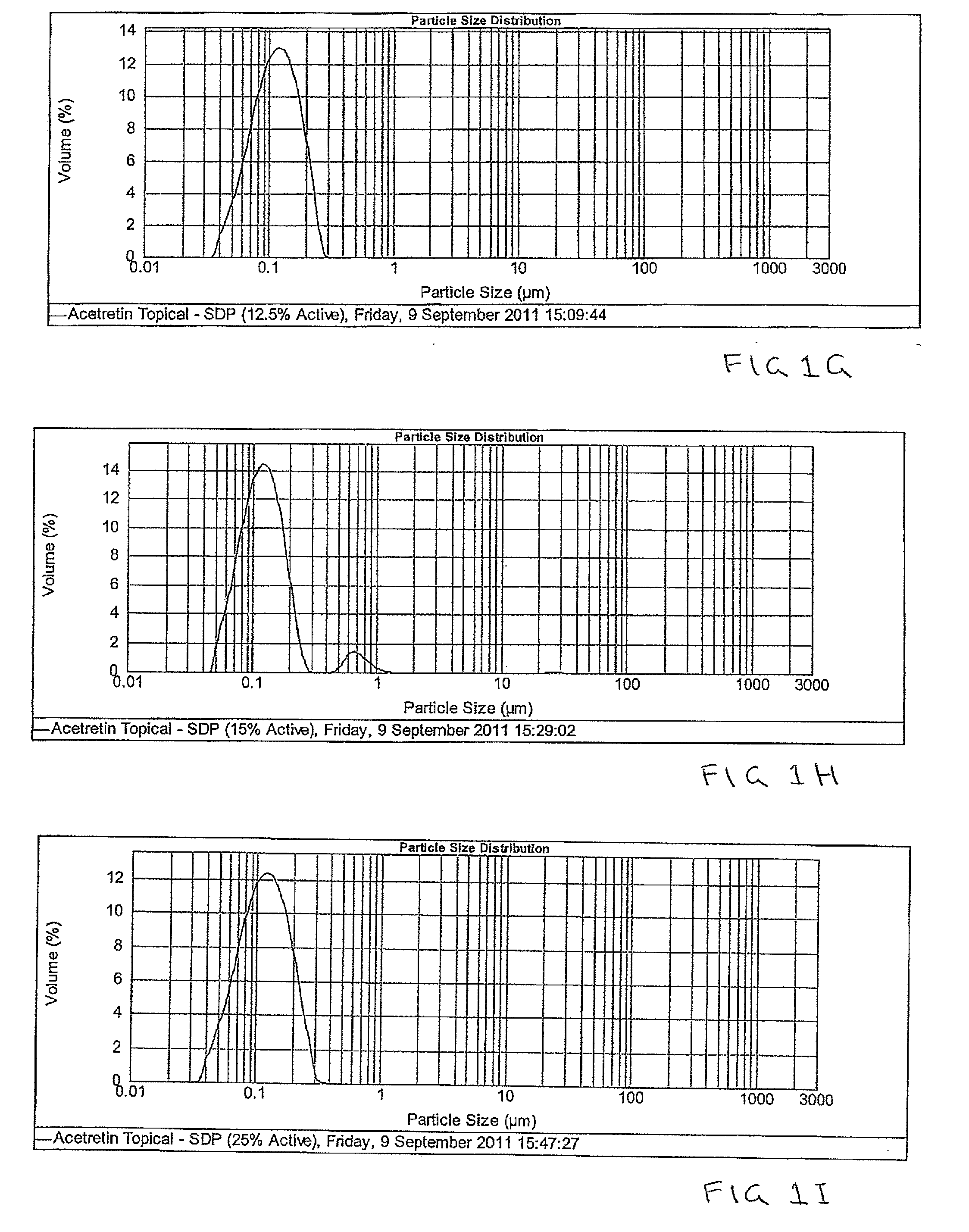
Pharmaceutical methods and topical compositions containing acitretin
The present invention is directed to methods and compositions for topical administration of acitretin. More specifically, the present invention is related to methods and compositions for the treatment or prevention or reduction of symptoms or signs of dermatological conditions using acitretin in a topical administration. More specifically, the present invention is related to methods and compositions containing acitretin which are effective for the treatment or prevention or reduction of symptoms or signs of keratoses, in particular actinic keratosis.
Owner:DOUGLAS PHARMA
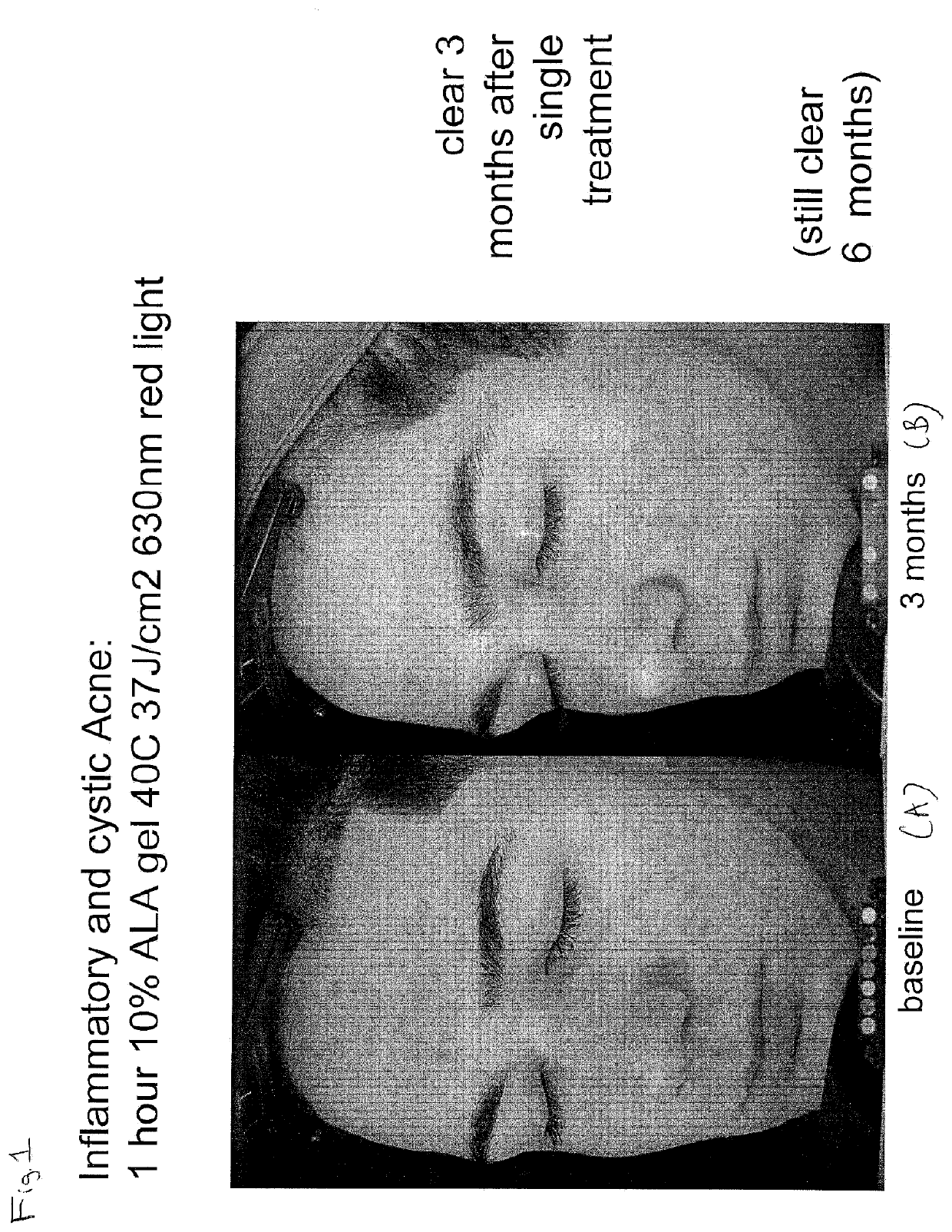
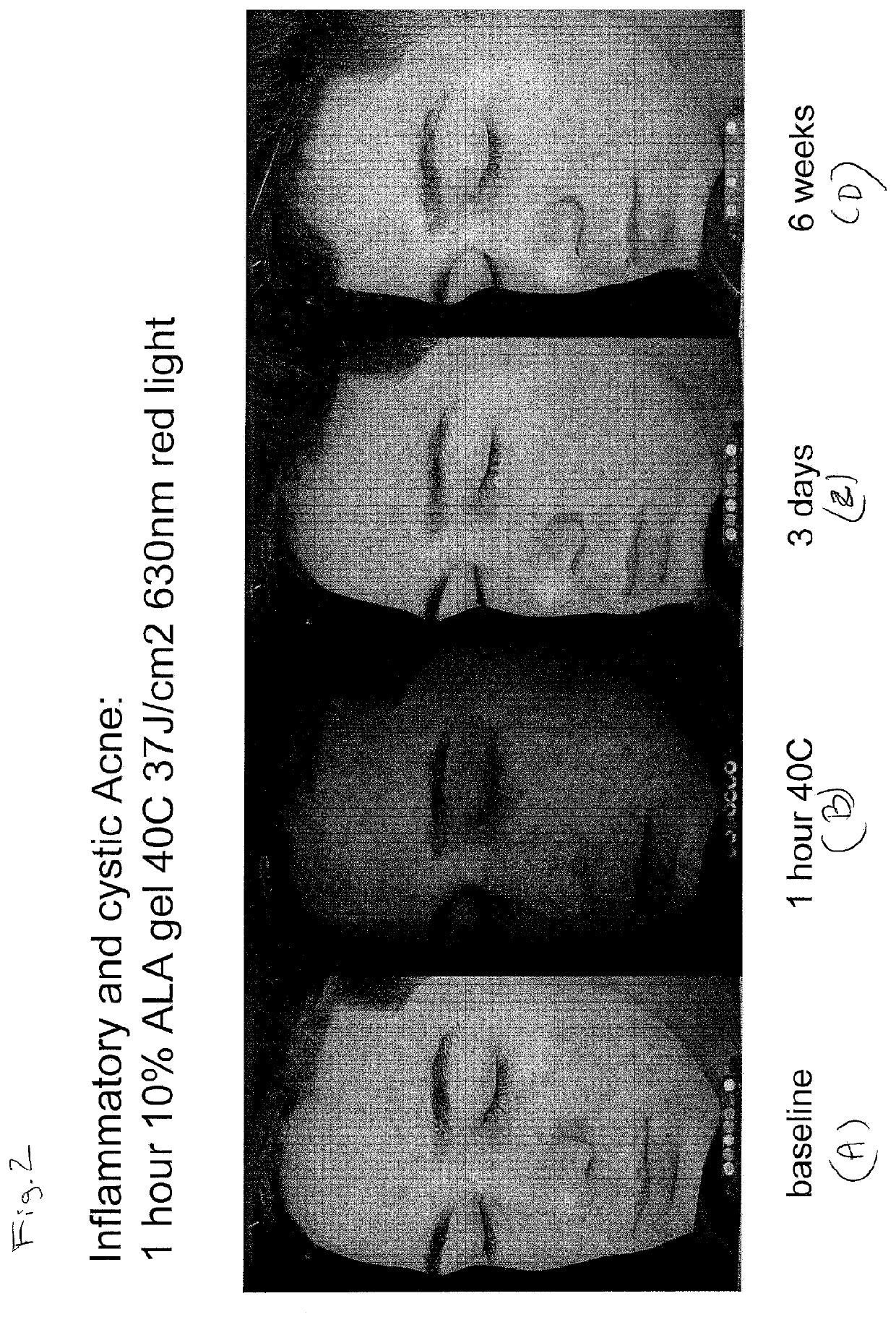
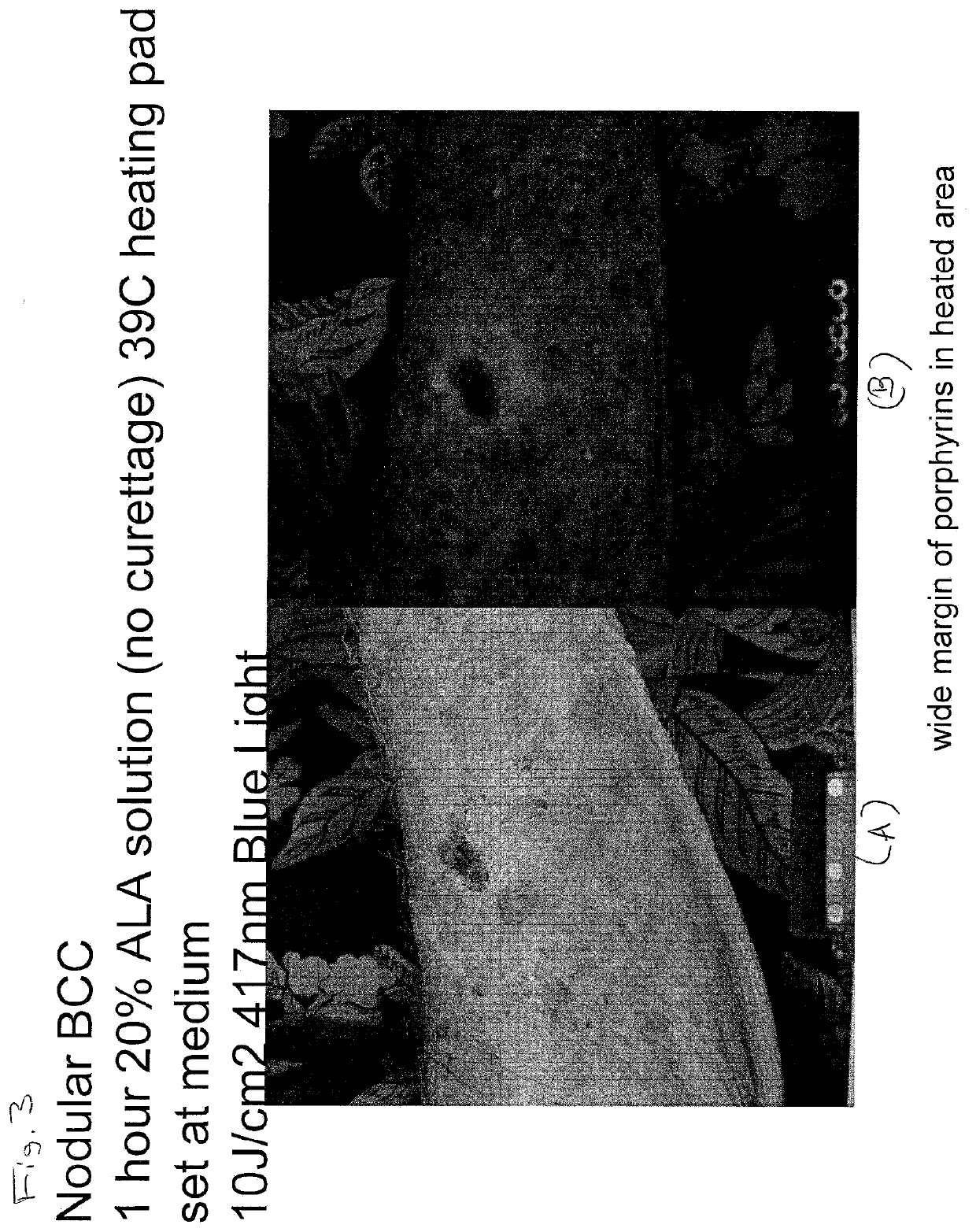
Photodynamic Therapy Method for Skin Disorders
InactiveUS20200261580A1Reduce healingEliminate side effectsOrganic active ingredientsEnergy modified materialsDiseasePhotodynamic therapy
The present invention is directed to methods of treating diseases and disorders of the skin (e.g., acne) with heat-enabled photodynamic therapy (HEPT). Methods of treating acne, non-melanoma skin cancer (NMSC), actinic keratosis (AK) or disseminated superficial actinic porokeratosis (DSAP) using red light photodynamic therapy on heat-treated skin.
Owner:DUSA PHARMA INC
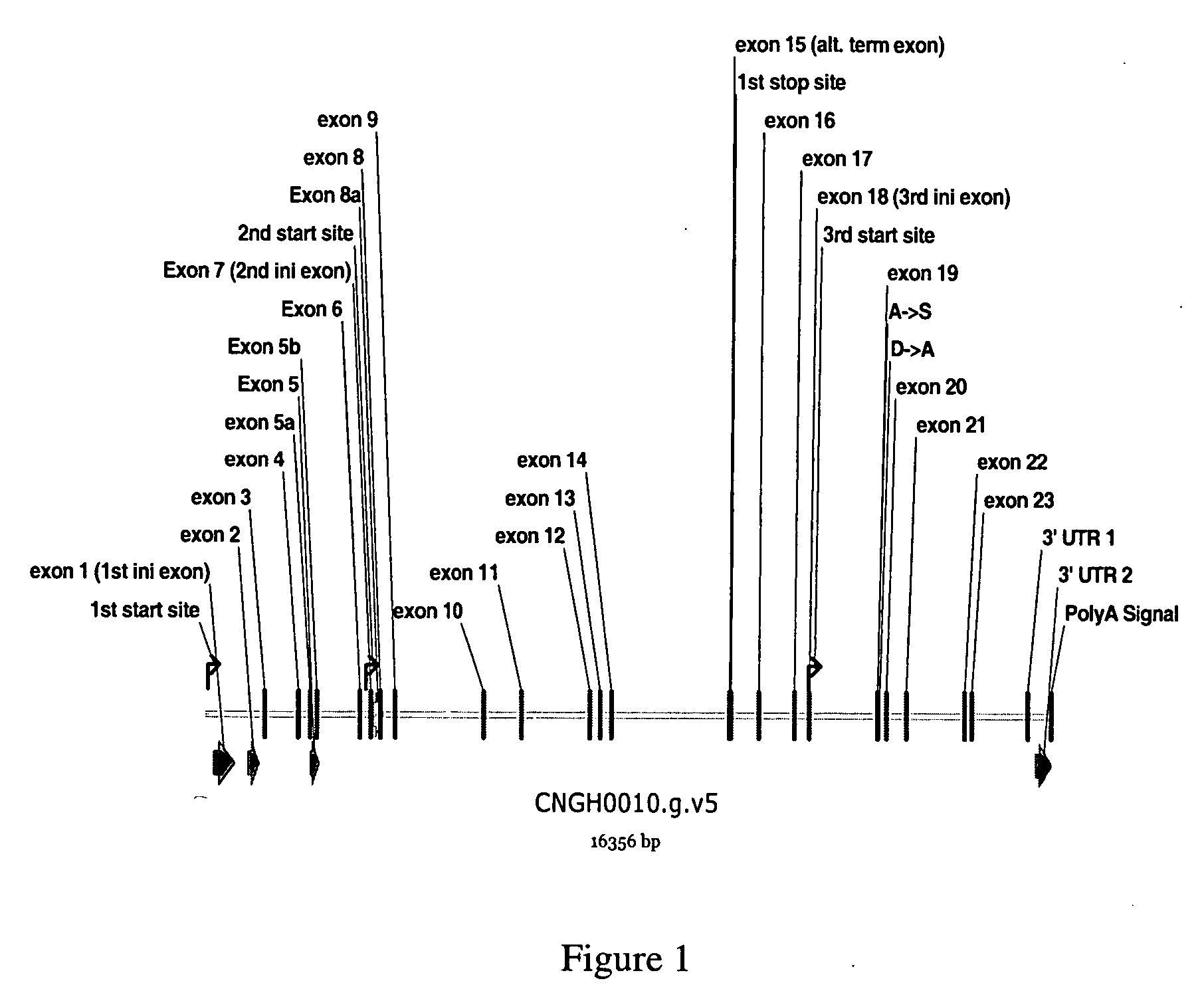
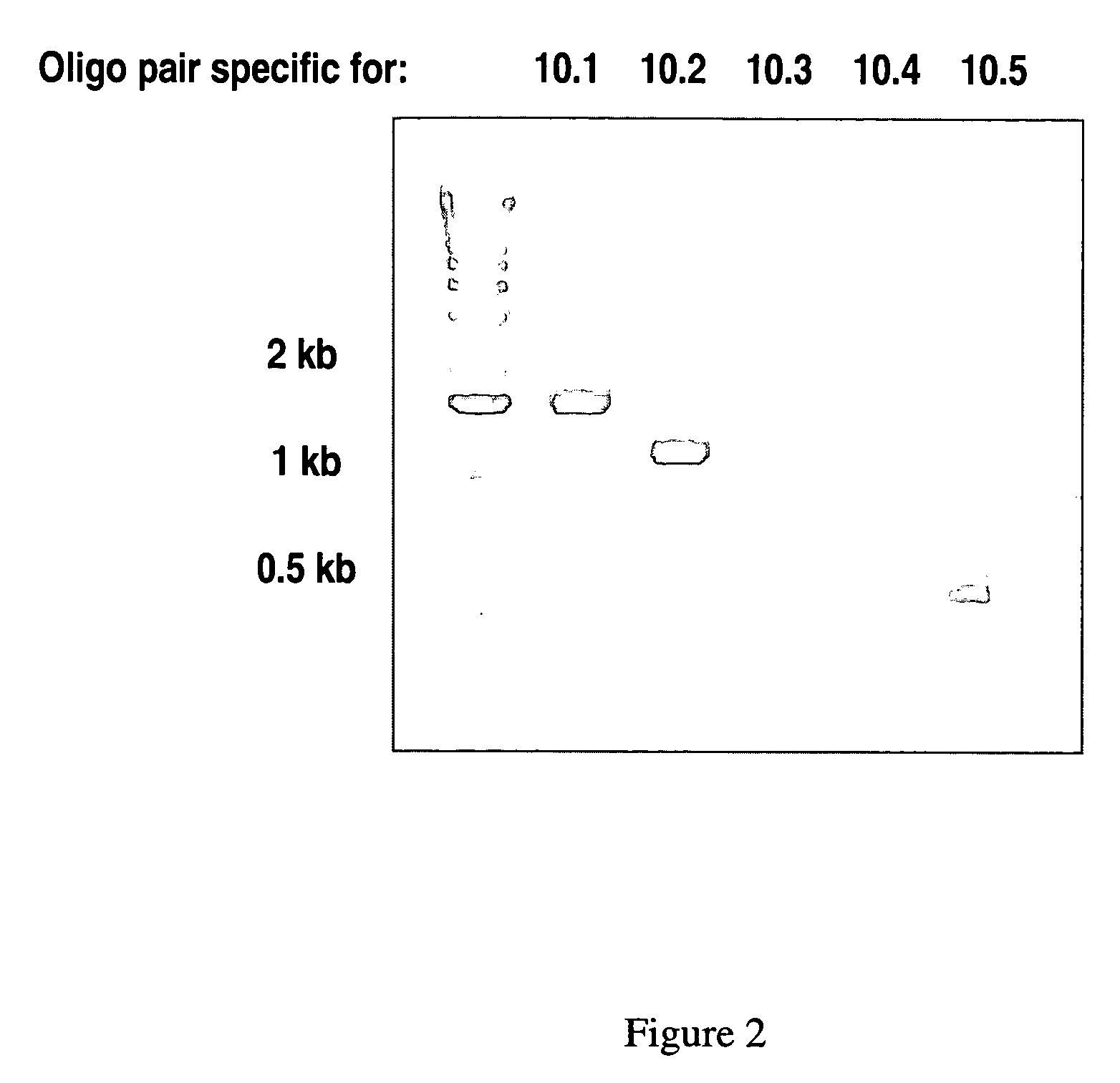
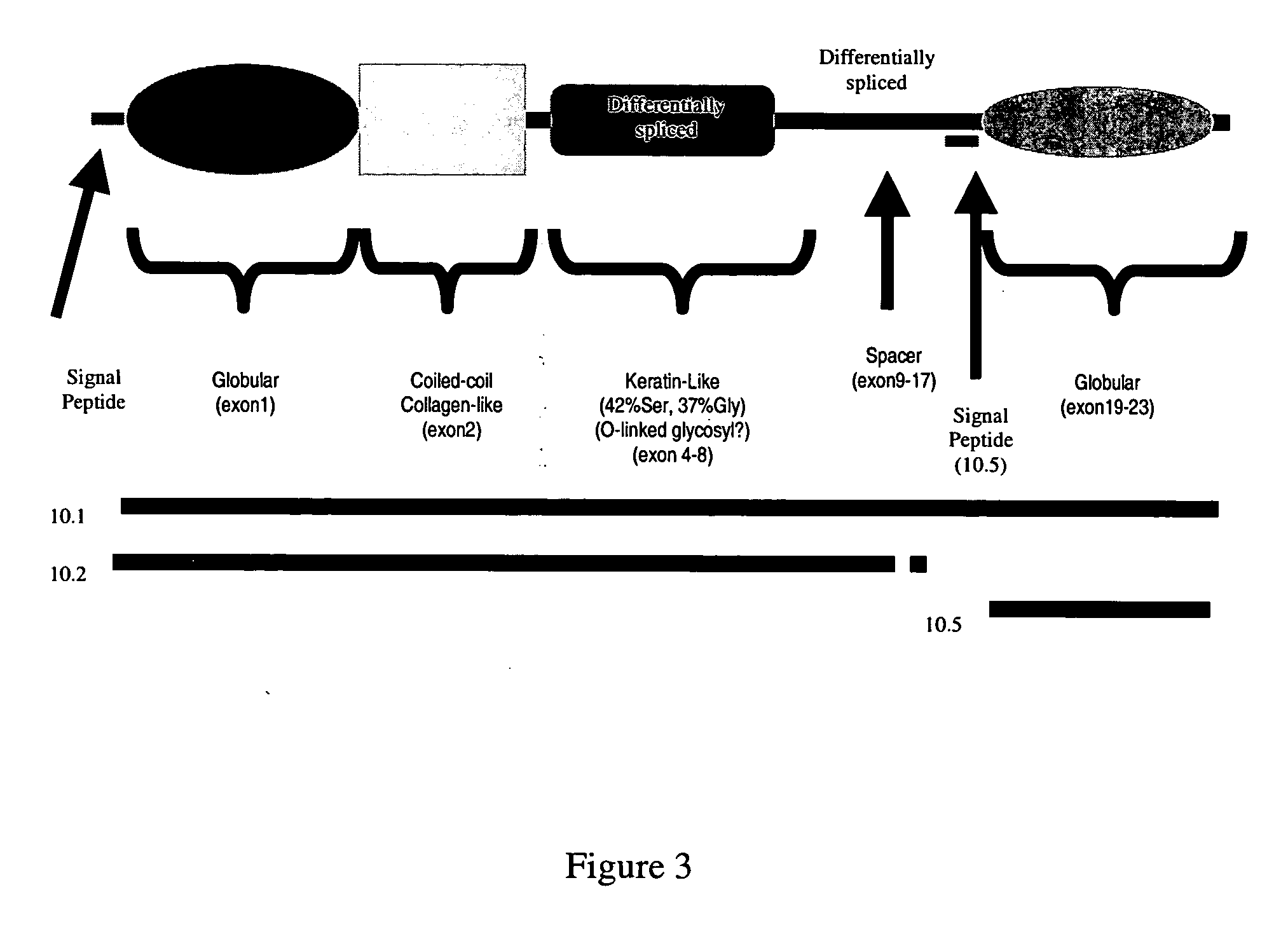
CNGH0010 specific polynucleotides, polypeptides, antibodies, compositions, methods and uses
ActiveUS20060141479A1Senses disorderPeptide/protein ingredientsAllergic dermatitisContact dermatitis
Novel polypeptides (CNGH0010) and antibodies, including specified portions or variants, specific for at least one such CNGH0010 polypeptide, variant, or fragment thereof, as well as nucleic acids encoding such CNGH0010 polypeptides and antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, are useful for therapeutic and diagnostic formulations, administration and devices. The aforesaid polypeptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-CNGH0010 antibodies. The CNGH0010 polypeptides and antibodies are used in modulating or treating at least one CNGH0010-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, psoriasis, rheumatoid arthritis, emphysema, asthma, diabetes, autoimmune thyroiditis, inflammatory bowel diseases, including Crohn's disease and ulcerative colitis, different types of dermatitis including allergic dermatitis, contact dermatitis, actinic keratosis, wound healing, scar formation, various renal diseases, various respiratory diseases, various diseases of reproductive organs, such as endometriosis, melanoma, squamous cell carcinoma, ovarian cancer, breast cancer, lung cancer, colon cancer, prostate cancer, renal cell carcinoma, Grave's diseases and other inflammatory and hyperproliferative diseases.
Owner:CENTOCOR
Topical composition for the treatment of actinic keratosis
ActiveUS8569320B2Improve usabilityAccurate doseBiocideSalicyclic acid active ingredientsOrganic solventActive agent
The invention relates to a topical gel composition for use in the treatment of actinic keratosis comprising (a) an active agent for treatment of actinic keratosis, (b) a keratolytically active agent, (c) a gel former, and (d) an organic solvent.
Owner:ALMIRALL HERMAL
Nanoparticle compositions and components thereof
InactiveUS20170087088A1Good effectReduce frequencyAntibacterial agentsSenses disorderChromhidrosisNanoparticle
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis, among others. Methods generally involve administering provided compositions to the skin.
Owner:ANTERIOS INC
Use of Tellurium Compounds for Protection from Ultra-Violet Radiation
Methods for treating skin conditions such as basal cell carcinoma and / or actinic keratosis are provided, which are effected by administering a pharmaceutically effective amount of a tellurium-containing compound. A pharmaceutical composition for treatment of skin conditions such as basal cell carcinoma an / or actinic keratosis is also provided.
Owner:SREDNI BENJAMIN
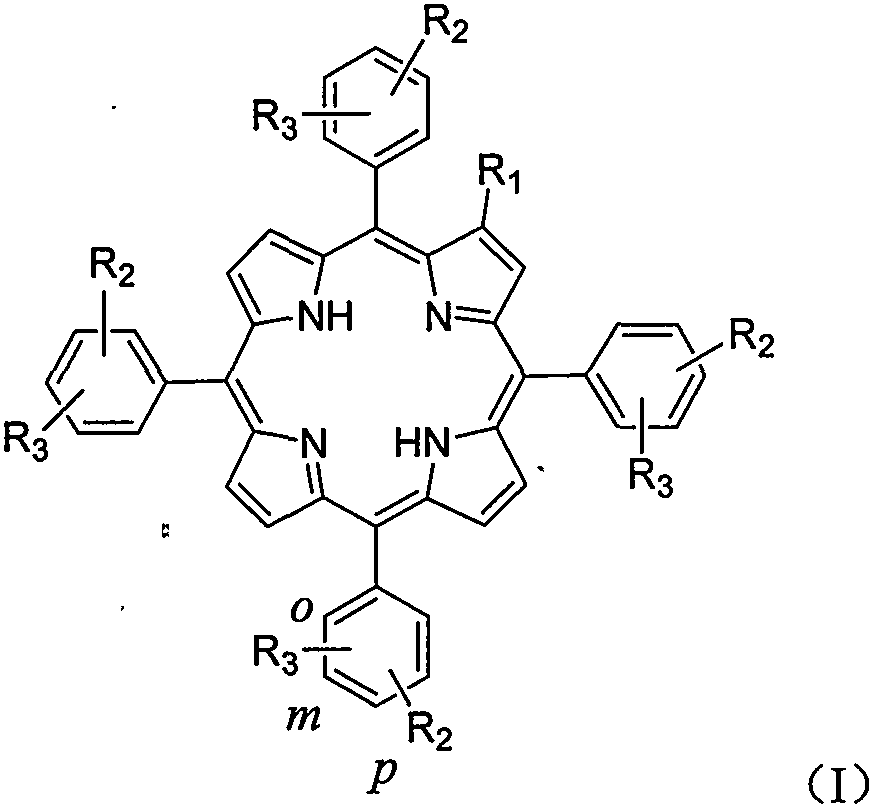
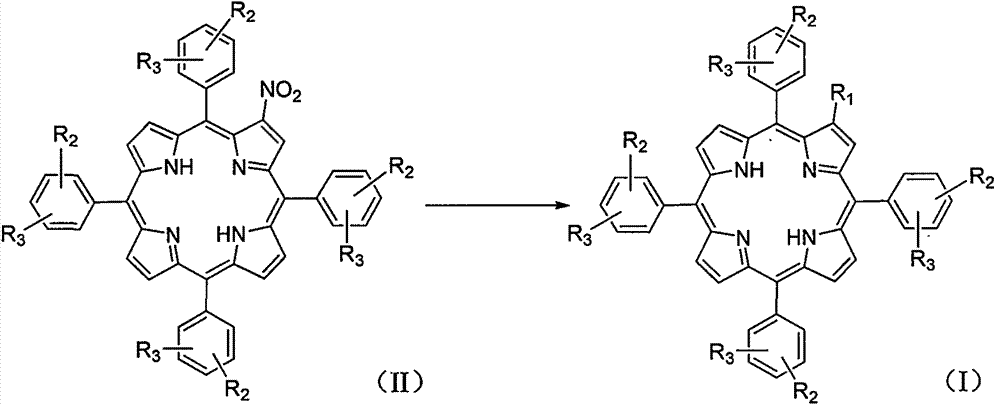
Amino-modified tetraphenylporphyrin compound as well as preparation method and application thereof
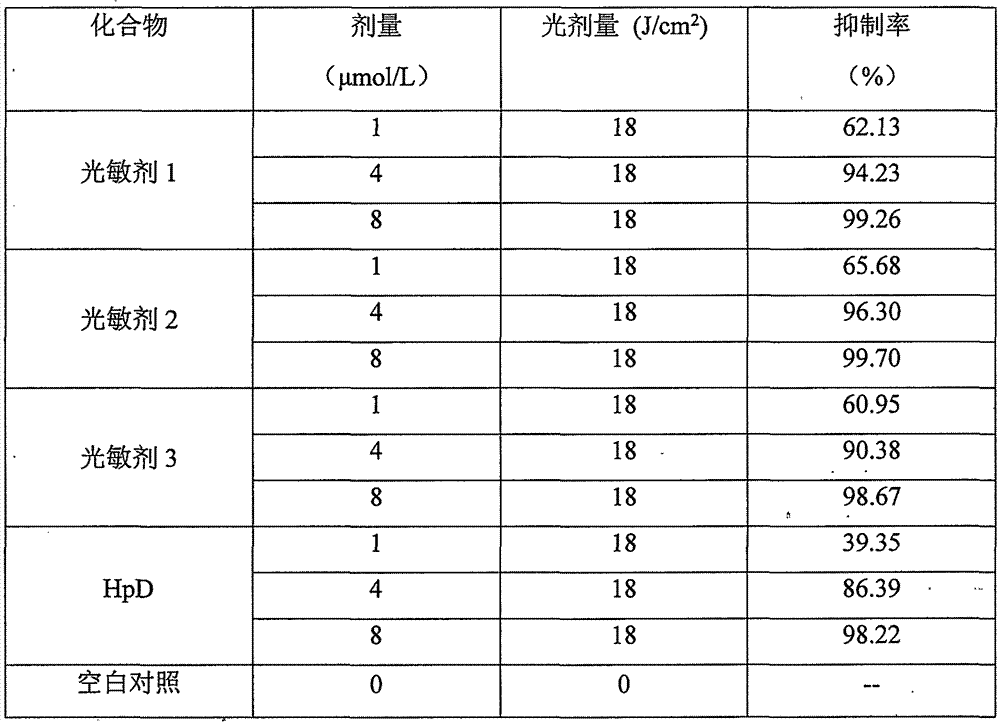
The invention relates to an amino-modified tetraphenylporphyrin compound as well as a preparation method and application thereof. The compound has a structure (I) shown in the description, wherein R2 and R3 are positioned at an ortho-position (o-) or meta-position (m-) or para-position (p-) of a benzene ring. The invention relates to the fields of photosensitive medicaments (also known as photosensitizers or photodynamic medicaments) and photodynamic therapy, in particular to a porphyrin photosensitizer, a preparation method thereof and application of the porphyrin photosensitizer in the field of medicine. The photosensitive medicament disclosed by the invention has the advantages of stable chemical property, single component and very strong photodynamic activity, and can be applied to photodynamic therapy of diseases such as tumors, retinal macular degeneration, actinic keratosis, port wine stains and condyloma acuminata.
Owner:陈志龙
Use of tellurium compounds for protection from ultra-violet radiation
Methods for treating skin conditions such as basal cell carcinoma and / or actinic keratosis are provided, which are effected by administering a pharmaceutically effective amount of a tellurium-containing compound. A pharmaceutical composition for treatment of skin conditions such as basal cell carcinoma an / or actinic keratosis is also provided.
Owner:SREDNI BENJAMIN
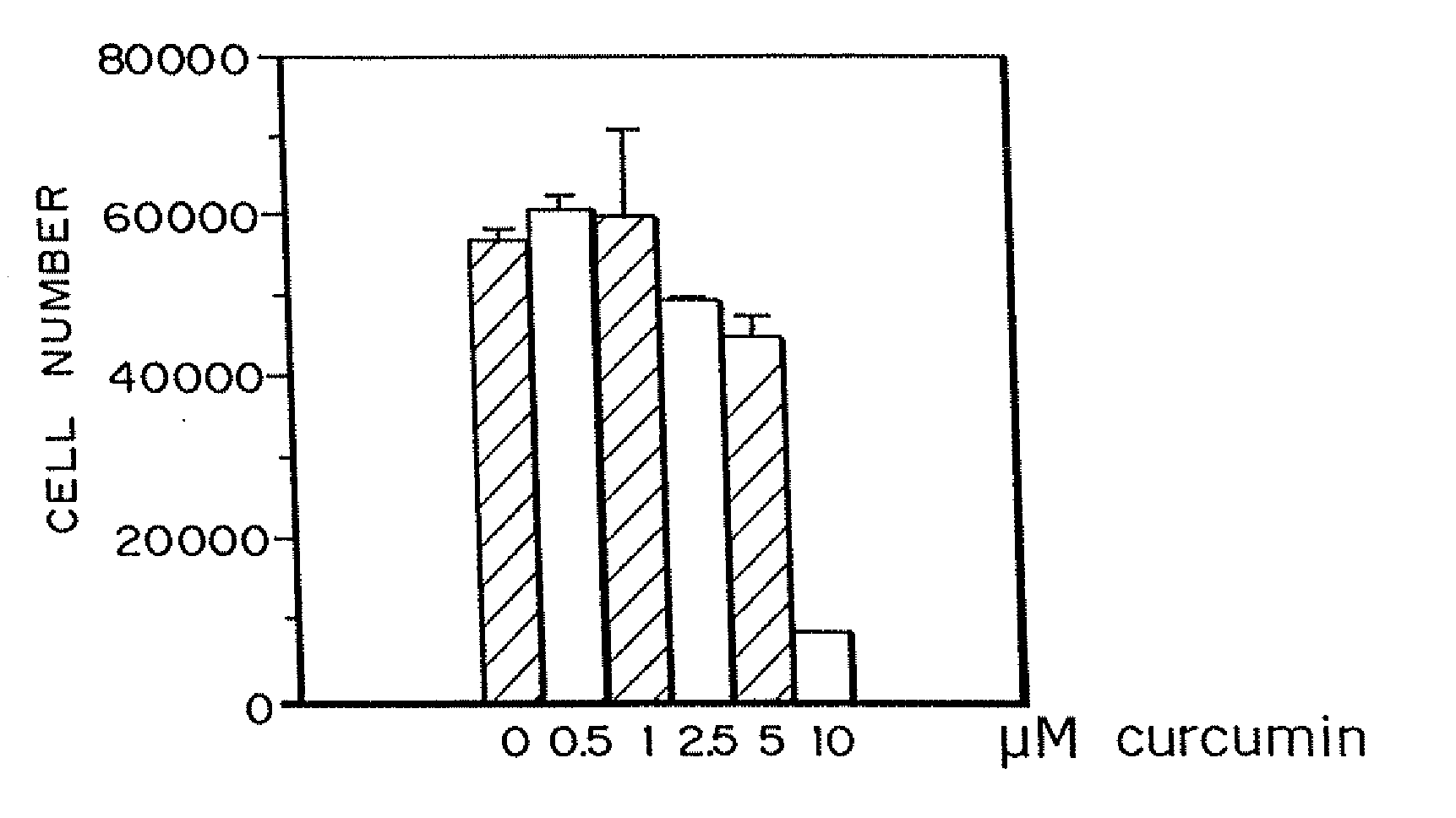
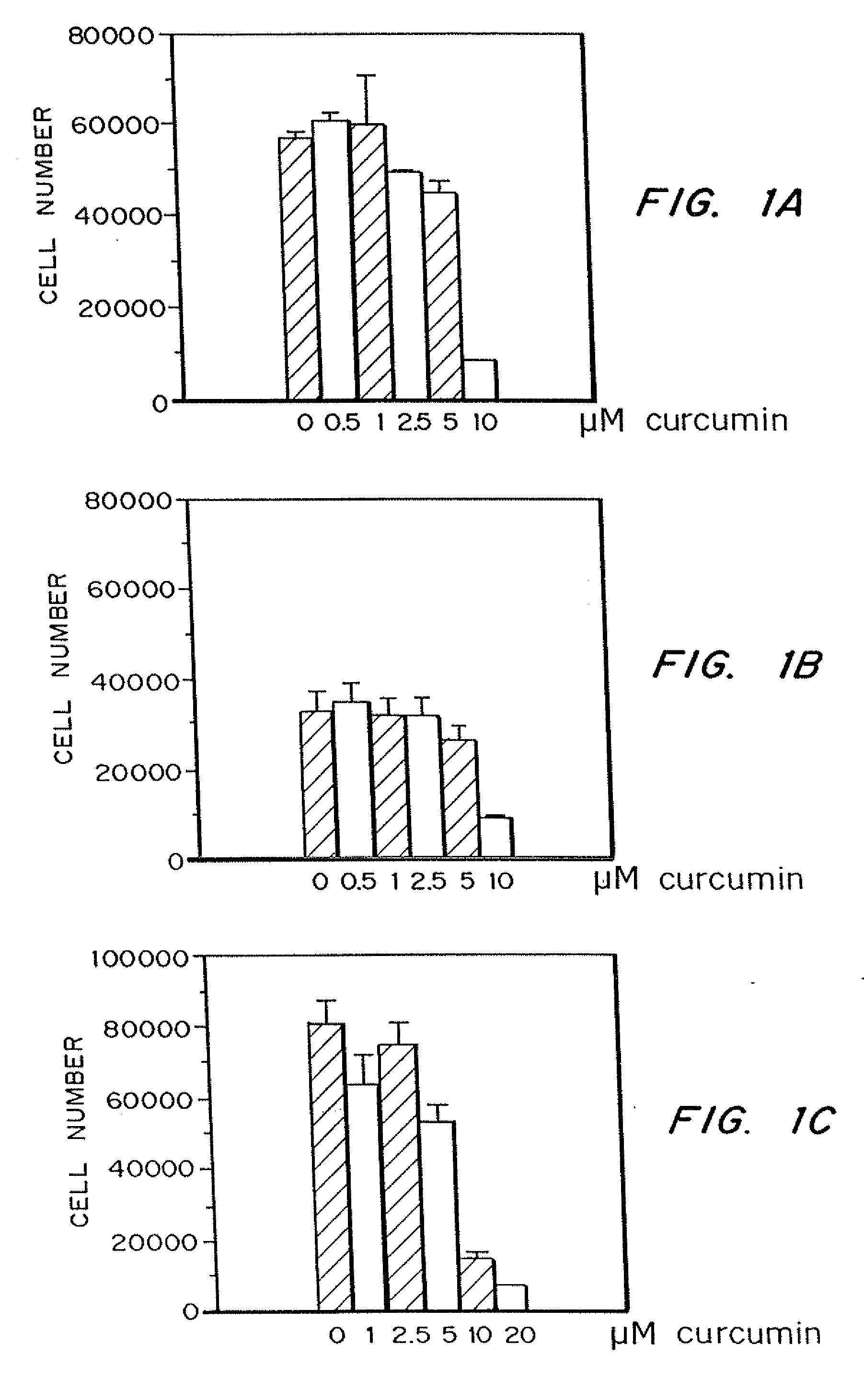
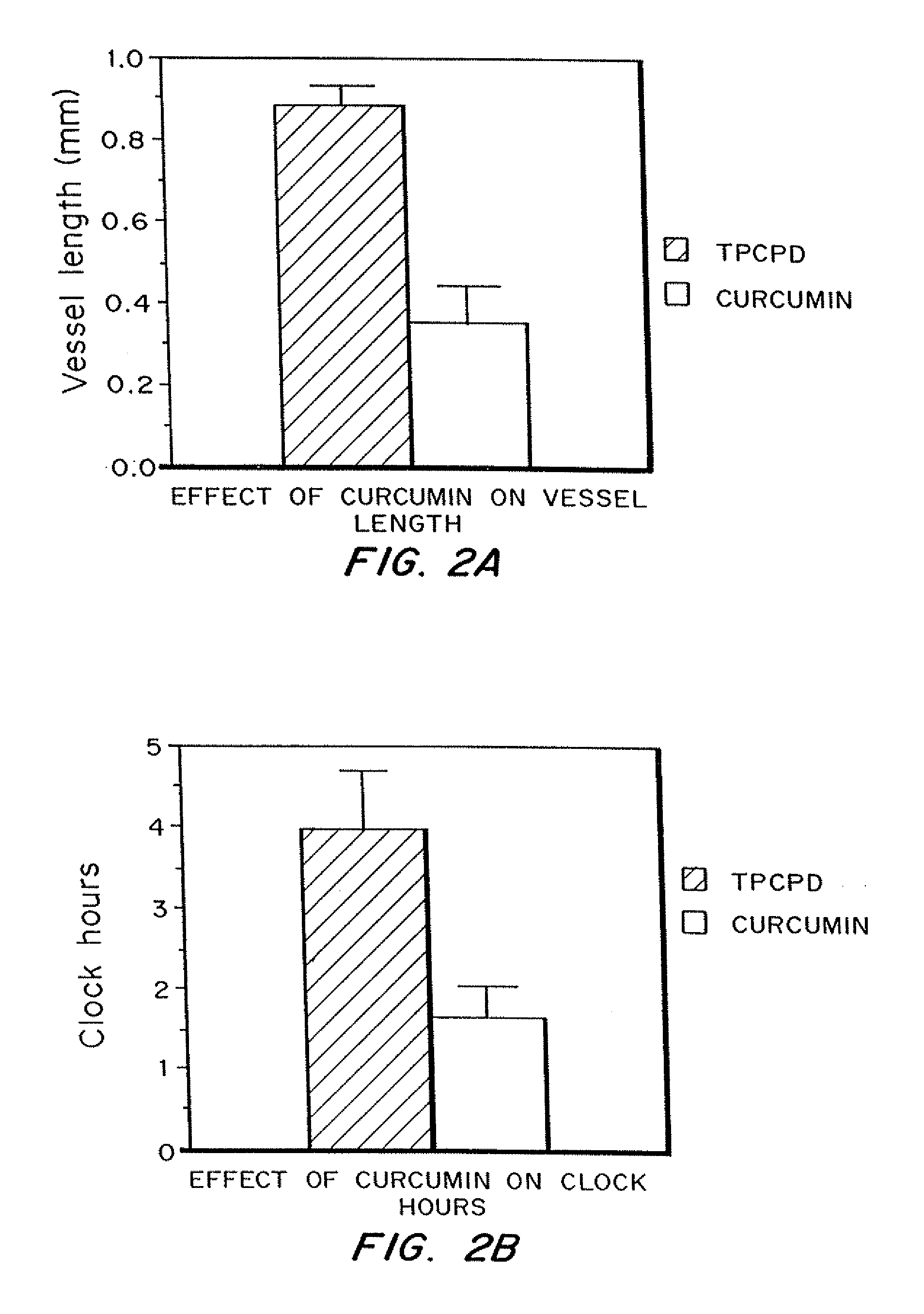
Curcumin and curcuminoid inhibition of angiogenesis
InactiveUS20090018209A1Inhibit angiogenesisImprove the level ofBiocideDigestive systemRecessive dystrophic epidermolysis bullosaSturge–Weber syndrome
Methods for treating diseases or disorders of the skin which are characterized by angiogenesis have been developed using curcumin and curcumin analogs. Based on the results obtained with curcumin, it has been determined that other angiogenesis inhibitors can also be used to treat these skin disorders. It has further been discovered that curcumin acts to inhibit angiogenesis in part by inhibition of basic fibroblast growth factor (bFGF), and thereby provides a means for treating other disorders characterized by elevated levels of bFGF, such as bladder cancer, using curcumin and other analogues which also inhibit bFGF. Representative skin disorders to be treated include the malignant diseases angiosarcoma, hemangioendothelioma, basal cell carcinoma, squamous cell carcinoma, malignant melanoma and Karposi's sarcoma, and the non-malignant diseases or conditions including psoriasis, lymphangiogenesis, hemangioma of childhood, Sturge-Weber syndrome, verruca vulgaris, neurofibromatosis, tuberous sclerosis, pyogenic granulomas, recessive dystrophic epidermolysis bullosa, venous ulcers, acne, rosacea, eczema, molluscum contagious, seborrheic keratosis, and actinic keratosis.
Owner:EMORY UNIVERSITY
Porifera-Based Therapeutic Compositions for Treating and Preventing Skin Diseases
Therapeutic compositions and methods for using same for treating skin conditions and diseases are disclosed. Treatable skin conditions and diseases include without limitation acne vulgaris, rosacea, seborrheic dermatitis, eczema (atopic dermatitis), psoriasis, photo-aging and actinic keratosisacne vulgaris, psoriasis, photo-aging and eczema. Therapeutic compositions disclosed are derived from Porifera species, specifically sponges, and more specifically fresh water sponges. One disclosed embodiment is derived from Spongilla species and is formulated with pharmaceutically acceptable excipients.
Owner:VILLANI INC
Pharmaceutical Formulations for Iontophoretic Delivery of an Immunomodulator
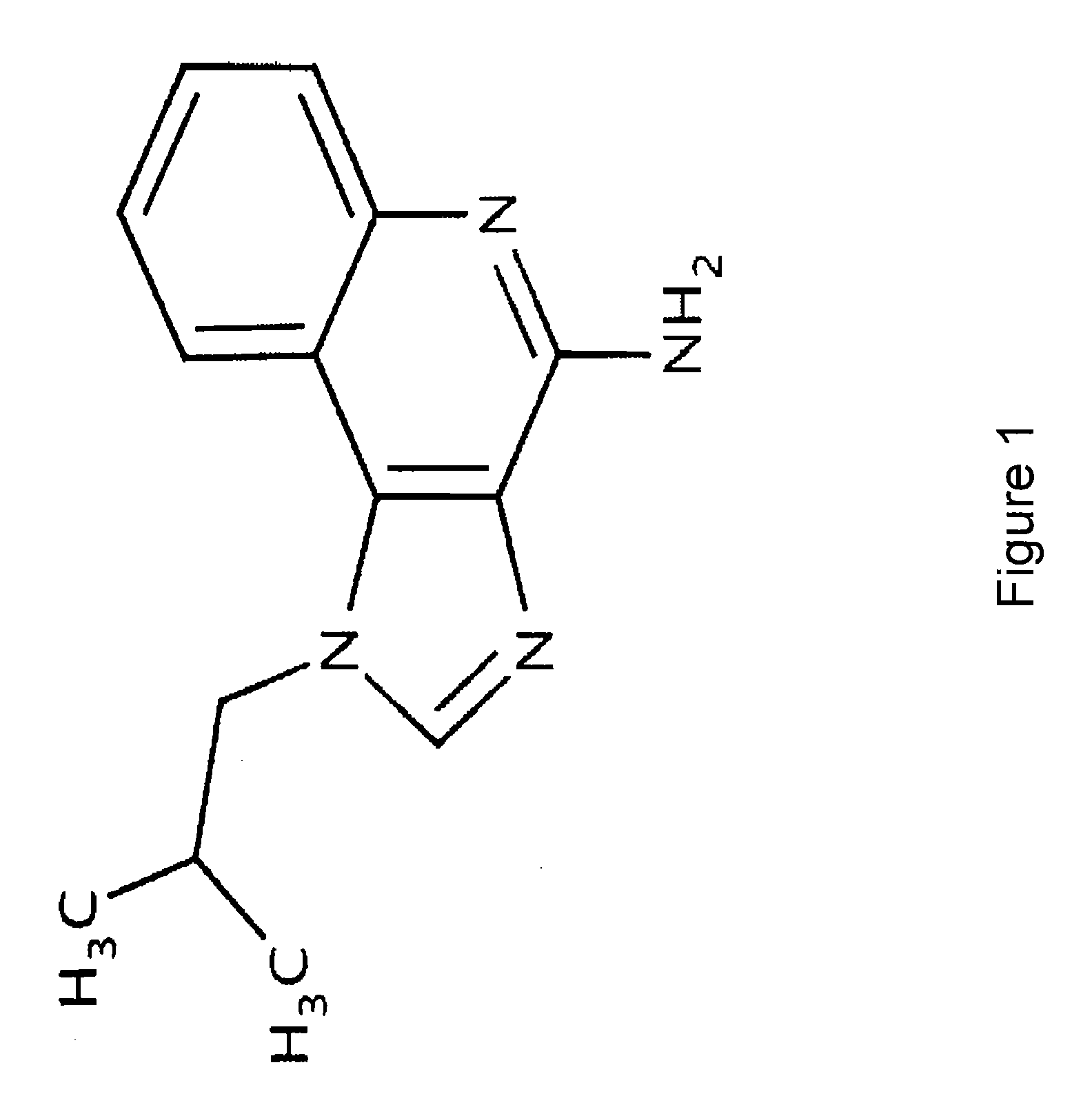
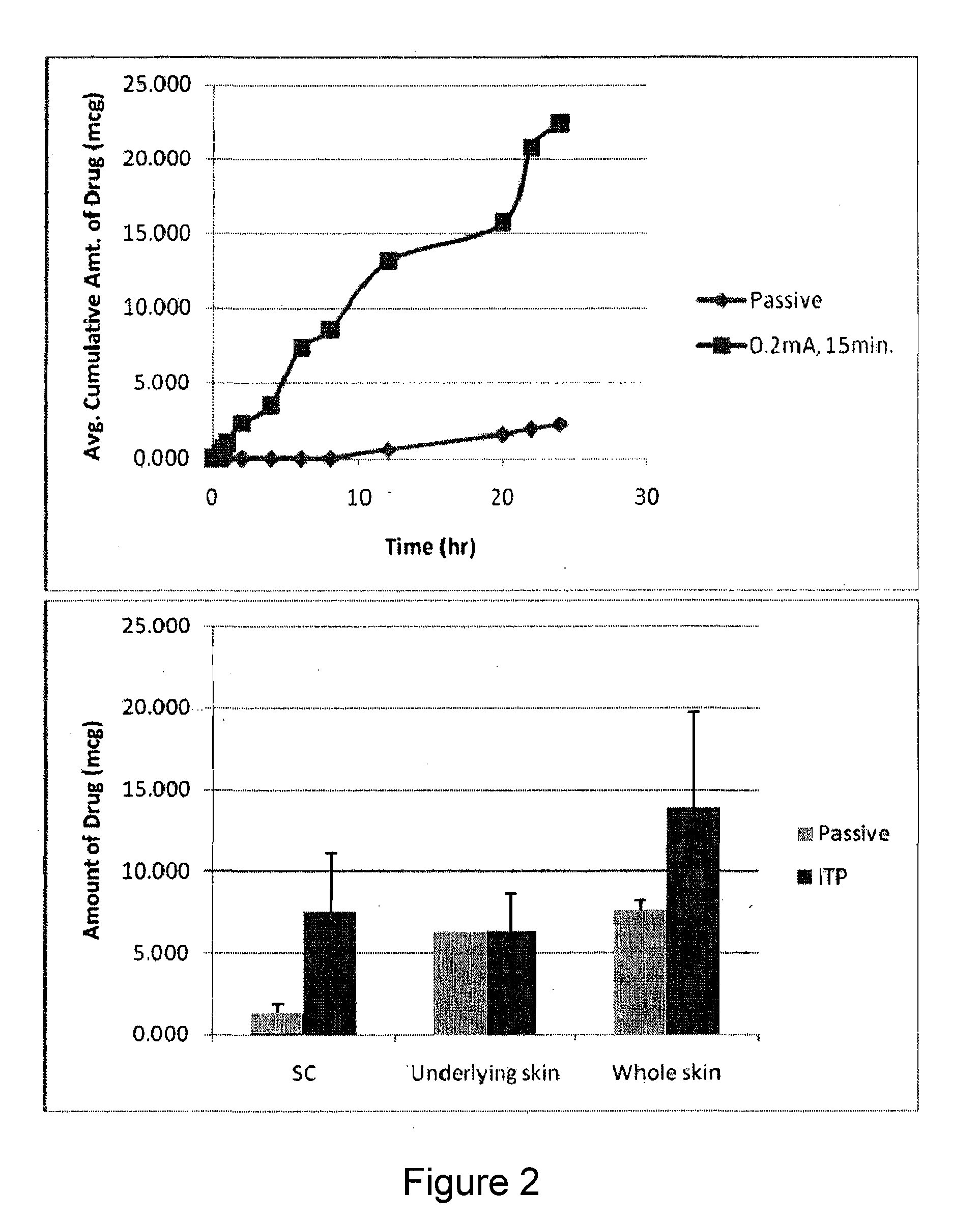
The present invention describes pharmaceutical formulations and methods suitable for iontophoretic delivery of the formulations to a subject. The formulations comprise an immunomodulator, such as imiquimod, and optionally include various agents and excipients. The formulations can be used as a treatment for skin diseases and conditions such as actinic keratosis, basal cell carcinoma and genital warts. The short term iontophoretic delivery of the formulations results in the creation of a depot effect in the skin of the subject, allowing for a sustained delivery. The shortened delivery time minimizes local side effects at the application site.
Owner:NITRIC BIOTHERAPEUTICS INC
Porifera-based therapeutic compositions for treating and preventing skin diseases
Therapeutic compositions and methods for using same for treating skin conditions and diseases are disclosed. Treatable skin conditions and diseases include, for example, acne vulgaris, rosacea, seborrheic dermatitis, eczema (atopic dermatitis), psoriasis, photo-aging and actinic keratosisacne vulgaris, psoriasis, photo-aging and eczema. Therapeutic compositions disclosed are derived from Porifera species, specifically sponges, and more specifically fresh water sponges. One disclosed embodiment is derived from Spongilla species and is formulated with pharmaceutically acceptable excipients.
Owner:VILLANI INC
Lower dosage strength pharmaceutical compositions forumlated with 3.75% imiquimod
InactiveUS20110257219A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com