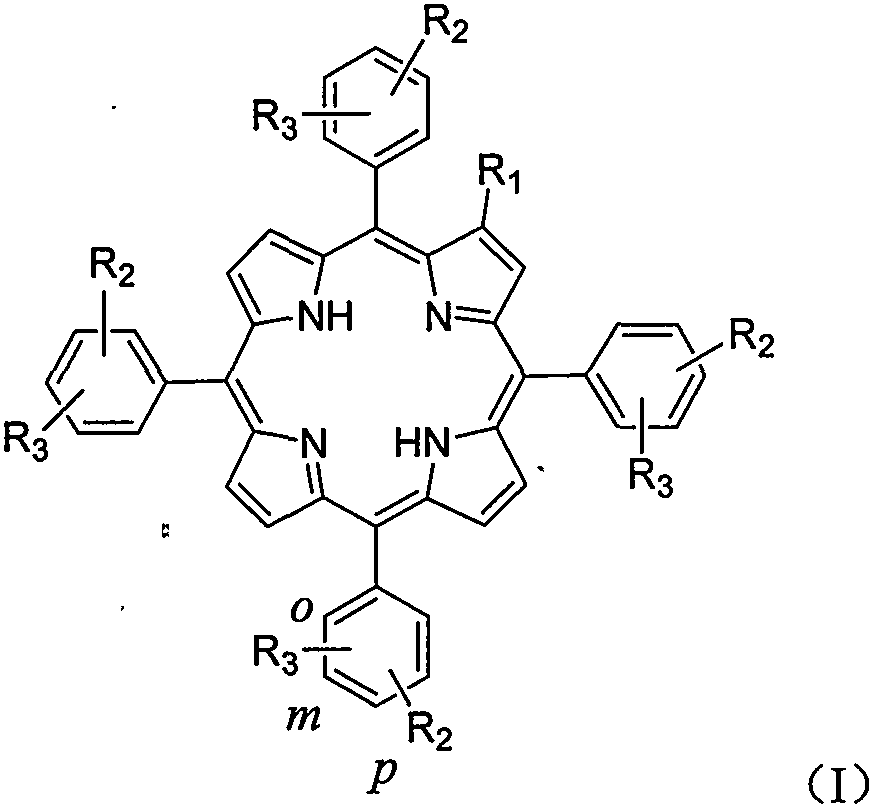
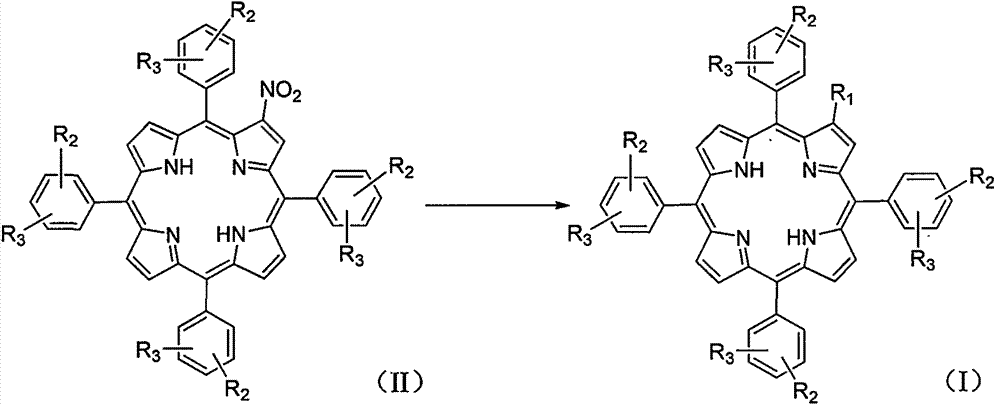
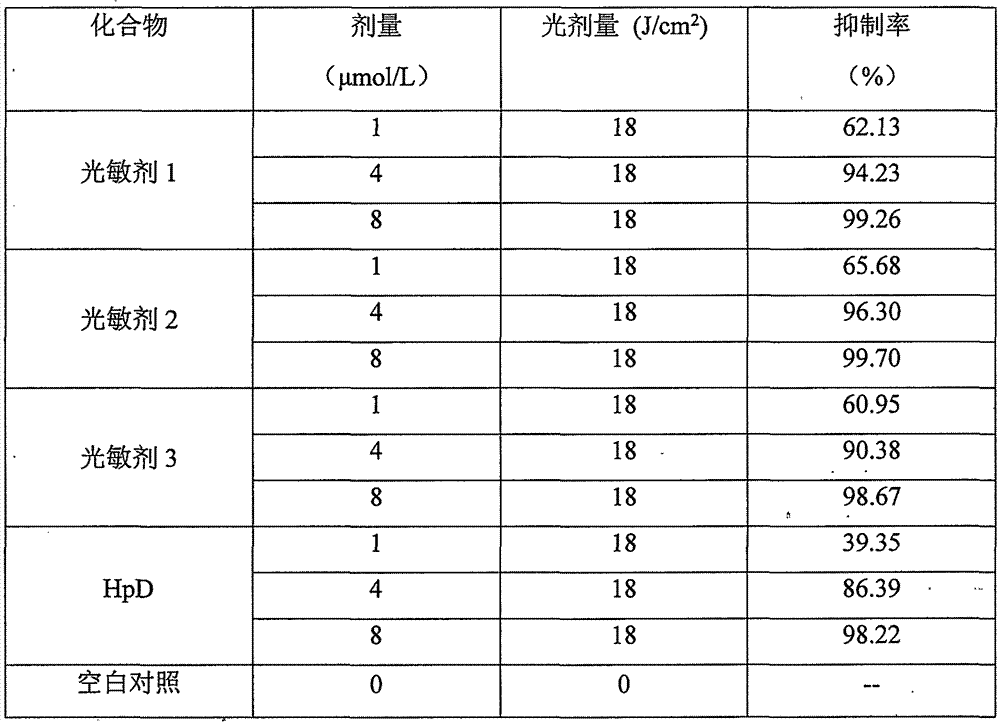
Amino-modified tetraphenylporphyrin compound as well as preparation method and application thereof
A technology of tetraphenylporphyrin and amino modification, which is applied in the field of photosensitive drugs and photodynamic therapy, and can solve the problems of strong dark toxicity of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of 2-morpholine-5,10,15,20-tetrakis(4-chlorophenyl)porphyrin (photosensitizer 1):
[0035] In a 100mL three-necked flask, 2-nitro-5,10,15,20-tetrakis(4-chlorophenyl)porphyrin (183mg, 0.23mmol) was dissolved in N,N-dimethylformamide (20mL) , adding potassium carbonate (360mg, 2.56mmol) and morpholine (0.2mL), heated to reflux for about 3h, and monitored by TLC until the reaction was complete. The reaction solution was evaporated to dryness, and dichloromethane (150 mL) was added for extraction. The organic phase was washed with water (100 mL×3), washed with saturated brine (100 mL×3), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure, and the resulting residue was subjected to column chromatography (eluent: petroleum ether:dichloromethane=10:1) to obtain a purple solid powder 2-morpholine-5,10,15,20-tetra( 111.4 mg of 4-chlorophenyl) porphyrin, the yield was 58%. 1 H NMR (400MHz, CDCl3): δ8.80-8.68(m, 5...
Embodiment 2
[0037] Preparation of 2-tetrahydropyrrole-5,10,15,20-tetrakis(4-chlorophenyl)porphyrin (photosensitizer 2):
[0038] In a 100mL three-necked flask, 2-nitro-5,10,15,20-tetrakis(4-chlorophenyl)porphyrin (183mg, 0.23mmol) was dissolved in N,N-dimethylformamide (20mL) , adding potassium carbonate (360mg, 2.56mmol) and tetrahydropyrrole (0.2mL), heated to reflux for about 3h, and monitored by TLC until the reaction was complete. The reaction solution was evaporated to dryness, and dichloromethane (150 mL) was added for extraction. The organic phase was washed with water (100 mL×3), washed with saturated brine (100 mL×3), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure, and the resulting residue was subjected to column chromatography (eluent: petroleum ether:dichloromethane=3:1) to obtain a purple solid powder 2-tetrahydropyrrole-5,10,15,20-tetrahydropyrrole (4-Chlorophenyl)porphyrin 90.4 mg, yield 48%. 1 H NMR (400MHz, CDCl...
Embodiment 3
[0040] Preparation of 2-piperidine-5,10,15,20-tetrakis(4-chlorophenyl)porphyrin (photosensitizer 3):
[0041] In a 100mL three-necked flask, 2-nitro-5,10,15,20-tetrakis(4-chlorophenyl)porphyrin (183mg, 0.23mmol) was dissolved in N,N-dimethylformamide (20mL) , adding potassium carbonate (360mg, 2.56mmol) and piperidine (0.3mL), heated to reflux for about 3h, monitored by TLC until the reaction was complete. The reaction solution was evaporated to dryness, and dichloromethane (150 mL) was added for extraction. The organic phase was washed with water (100 mL×3), washed with saturated brine (100 mL×3), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure, and the resulting residue was subjected to column chromatography (eluent: petroleum ether: dichloromethane = 3: 1) to obtain a purple solid powder 2-piperidine-5,10,15,20-tetra( 4-chlorophenyl) porphyrin 88.2 mg, yield 46%. 1 H NMR (400MHz, CDCl 3 ): δ8.83-8.81(m, 3H), 8.76-8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com