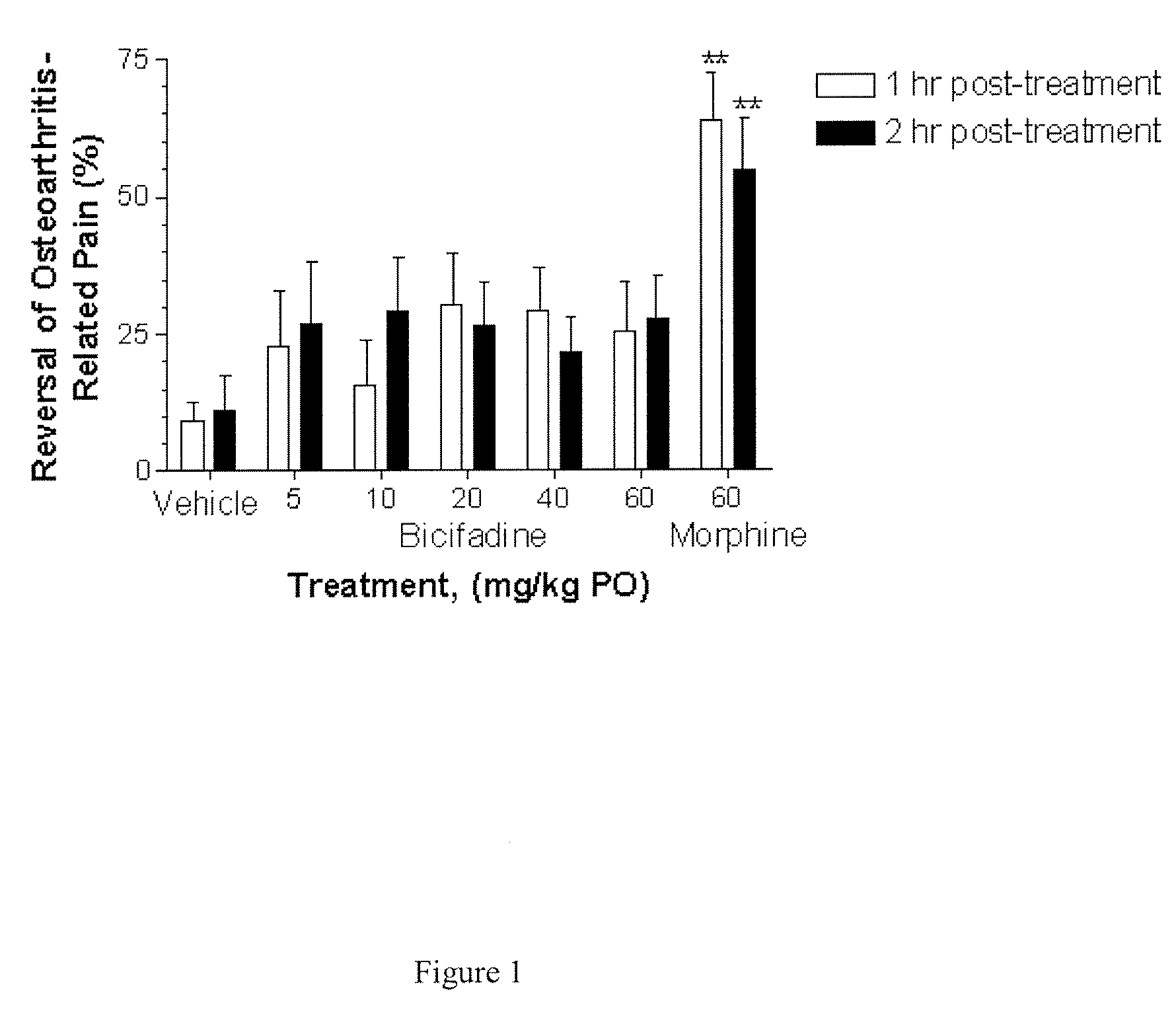Patents
Literature
88results about How to "Ensure adequate treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
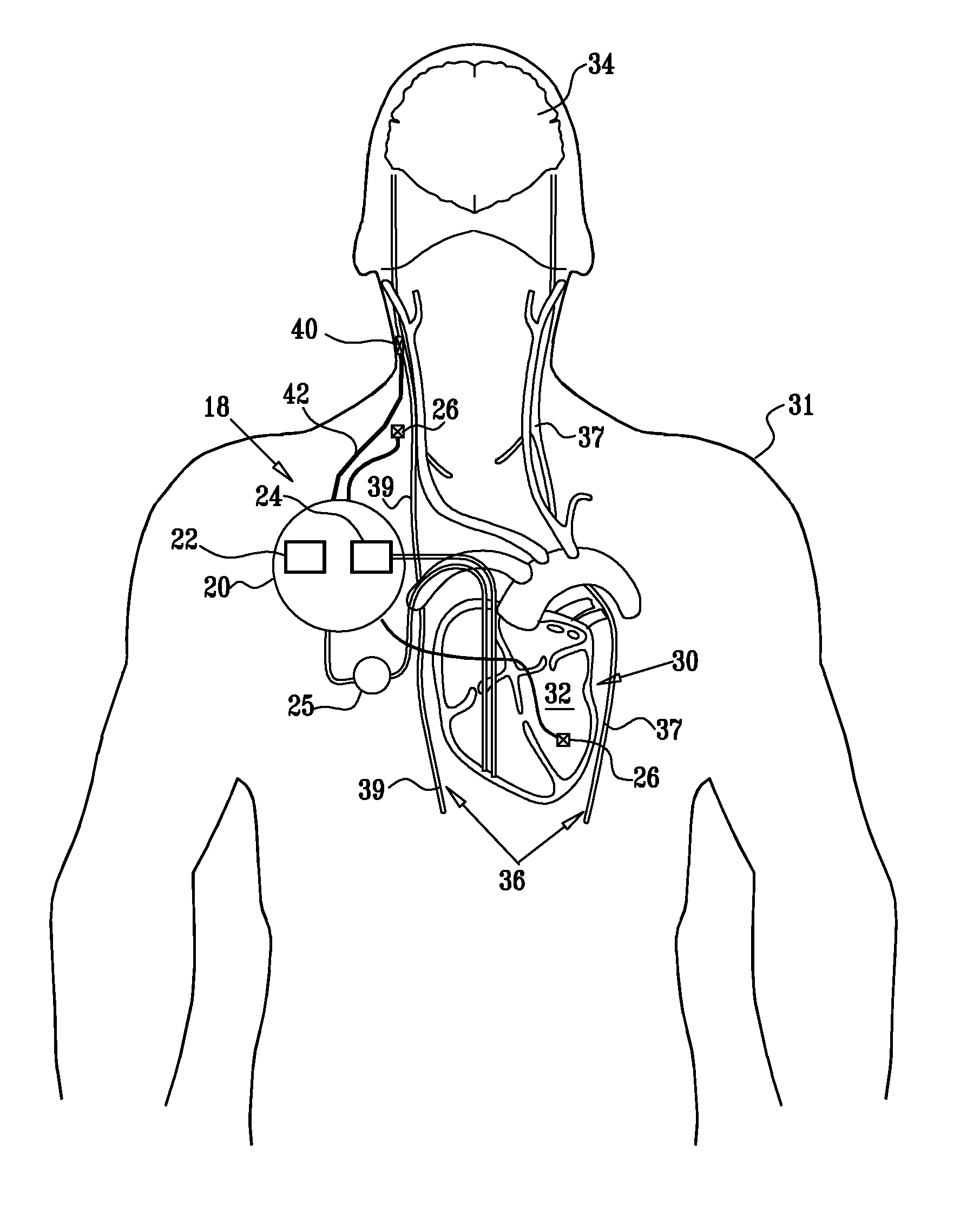
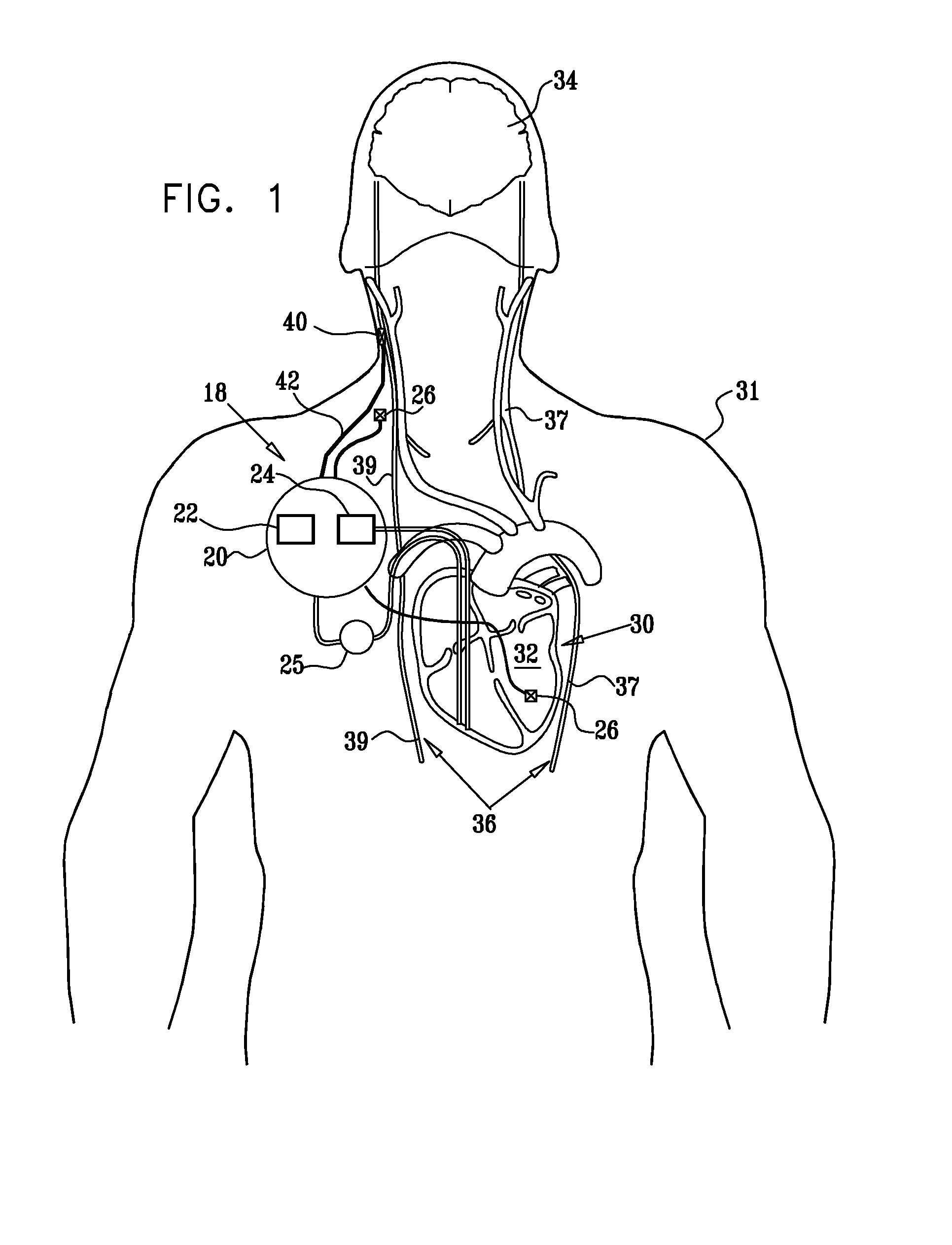
Nerve stimulation techniques
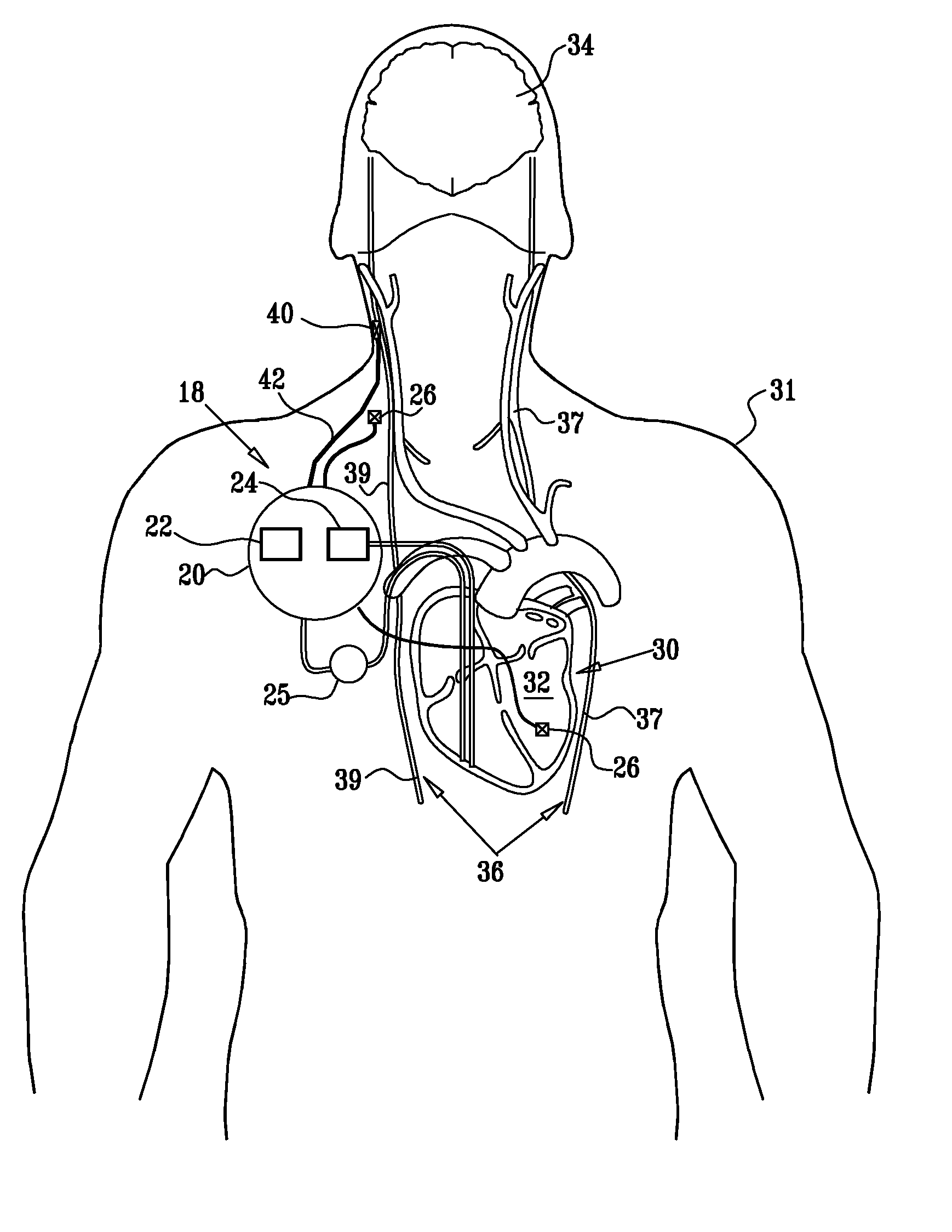
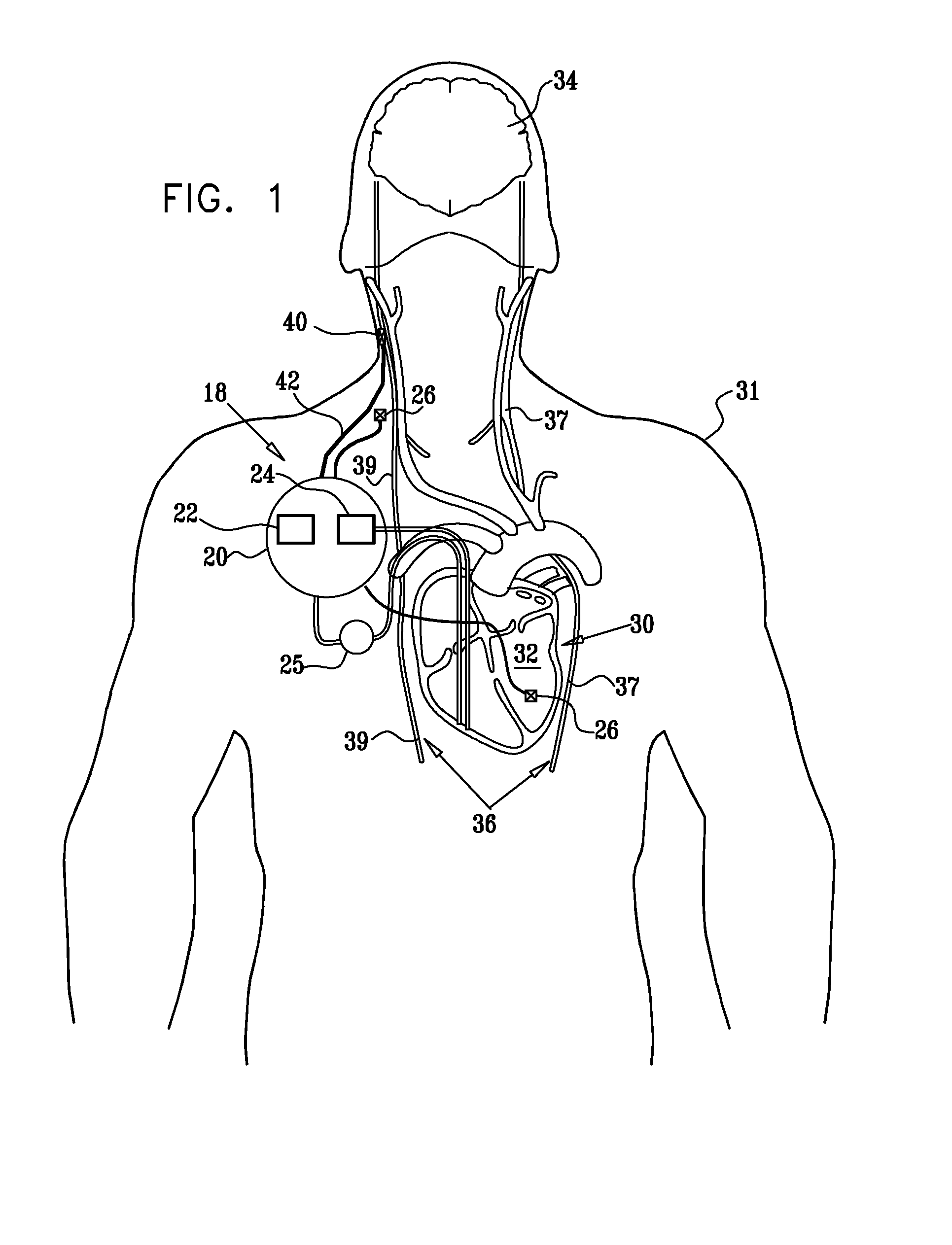
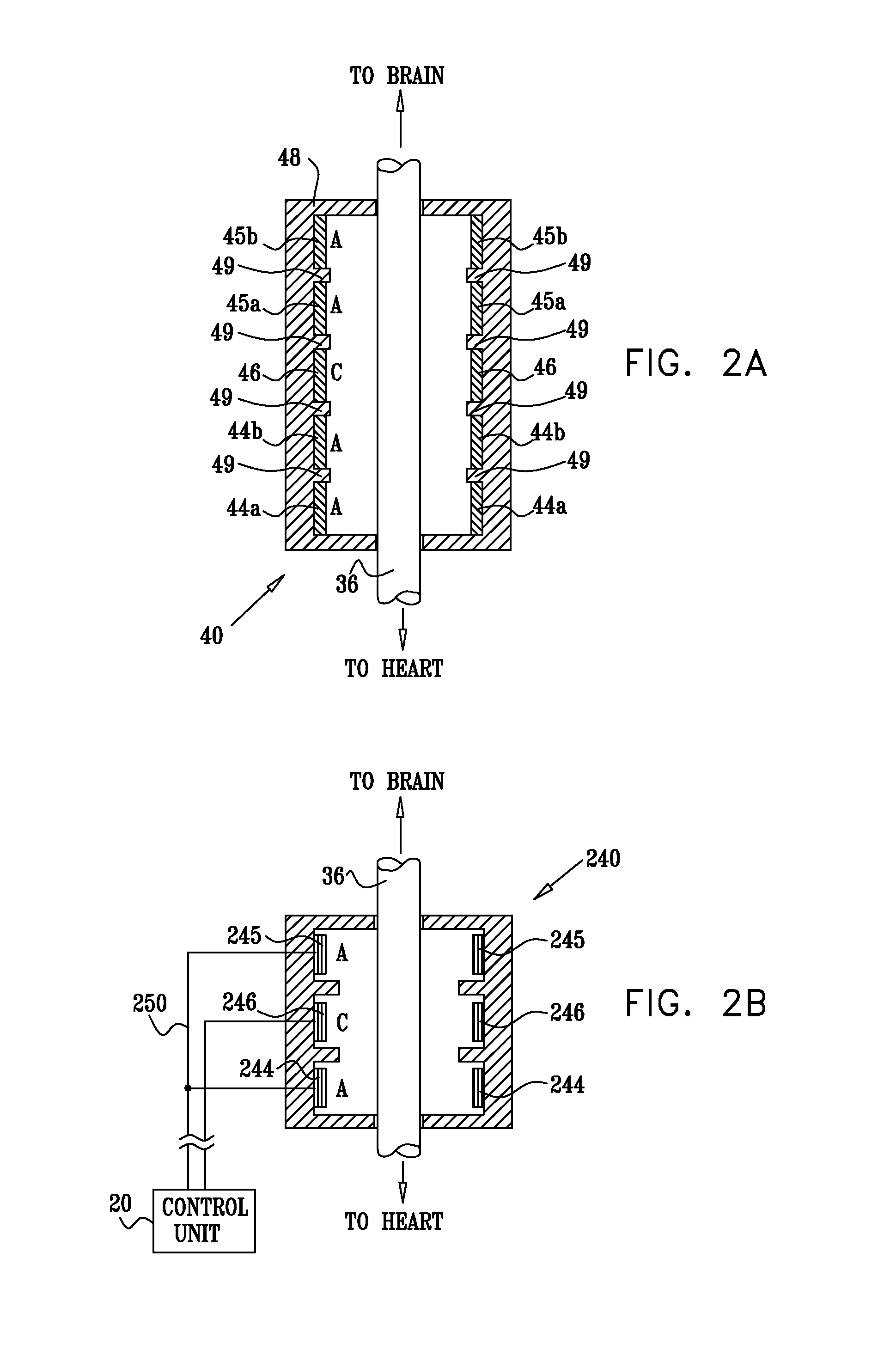
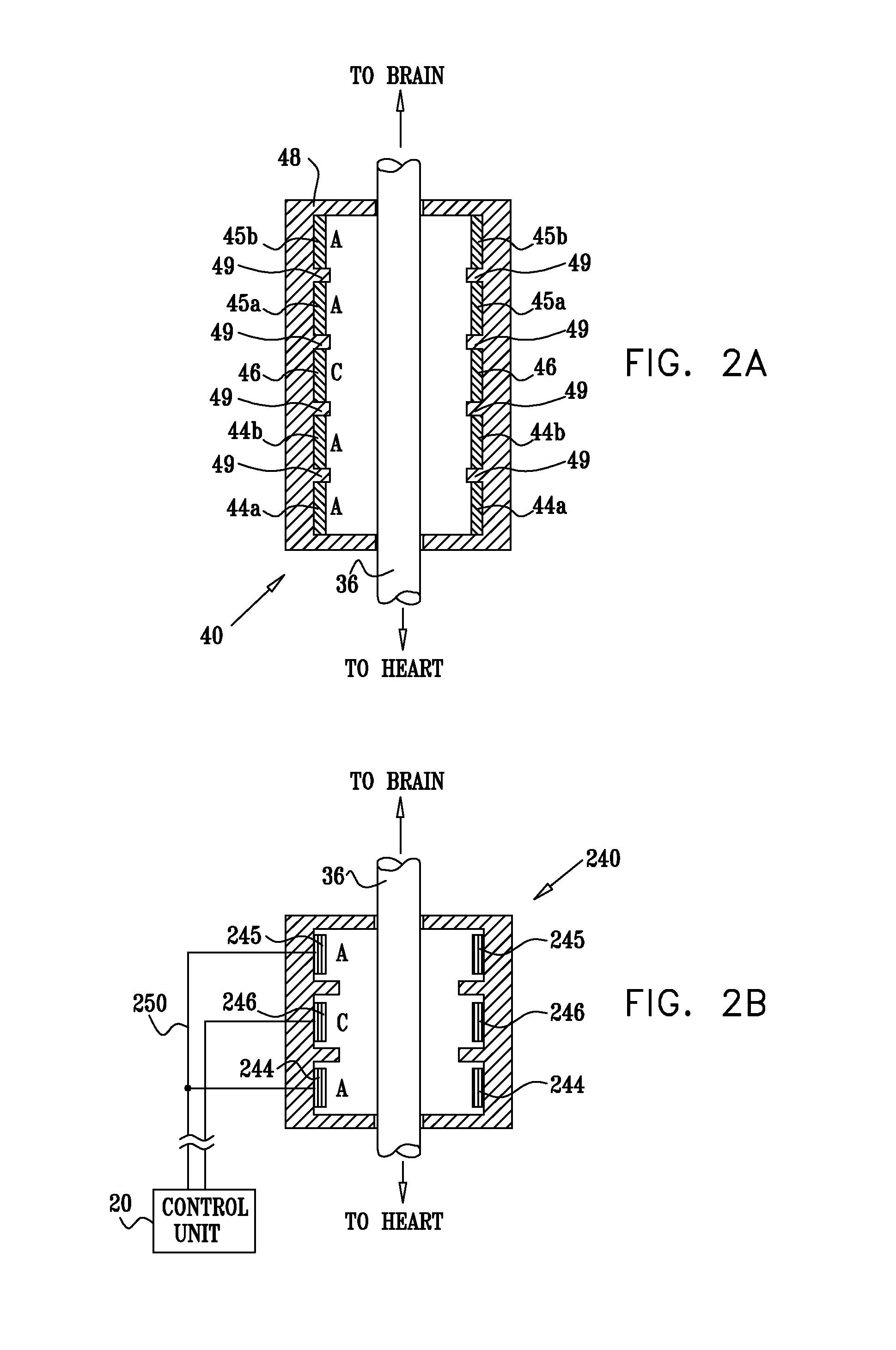
InactiveUS20110224749A1Minimize any unintended side effect of the signal applicationSuppresses afferent action potentialHeart stimulatorsMedicineCytokine
A method is provided for treating heart failure in a subject in need of such treatment, including applying a stimulating current to parasympathetic nervous tissue of the subject, selected from the group consisting of: a vagus nerve and an epicardial fat pad. The stimulating current is configured to inhibit release of at least one proinflammatory cytokine sufficiently to the treat heart failure of the subject. A level of the at least one proinflammatory cytokine is measured. Optionally, the stimulating current is configured to change a level of Connexin 43 of the subject, and the level of Connexin 43 is also measured. Other embodiments are also described.
Owner:MEDTRONIC INC
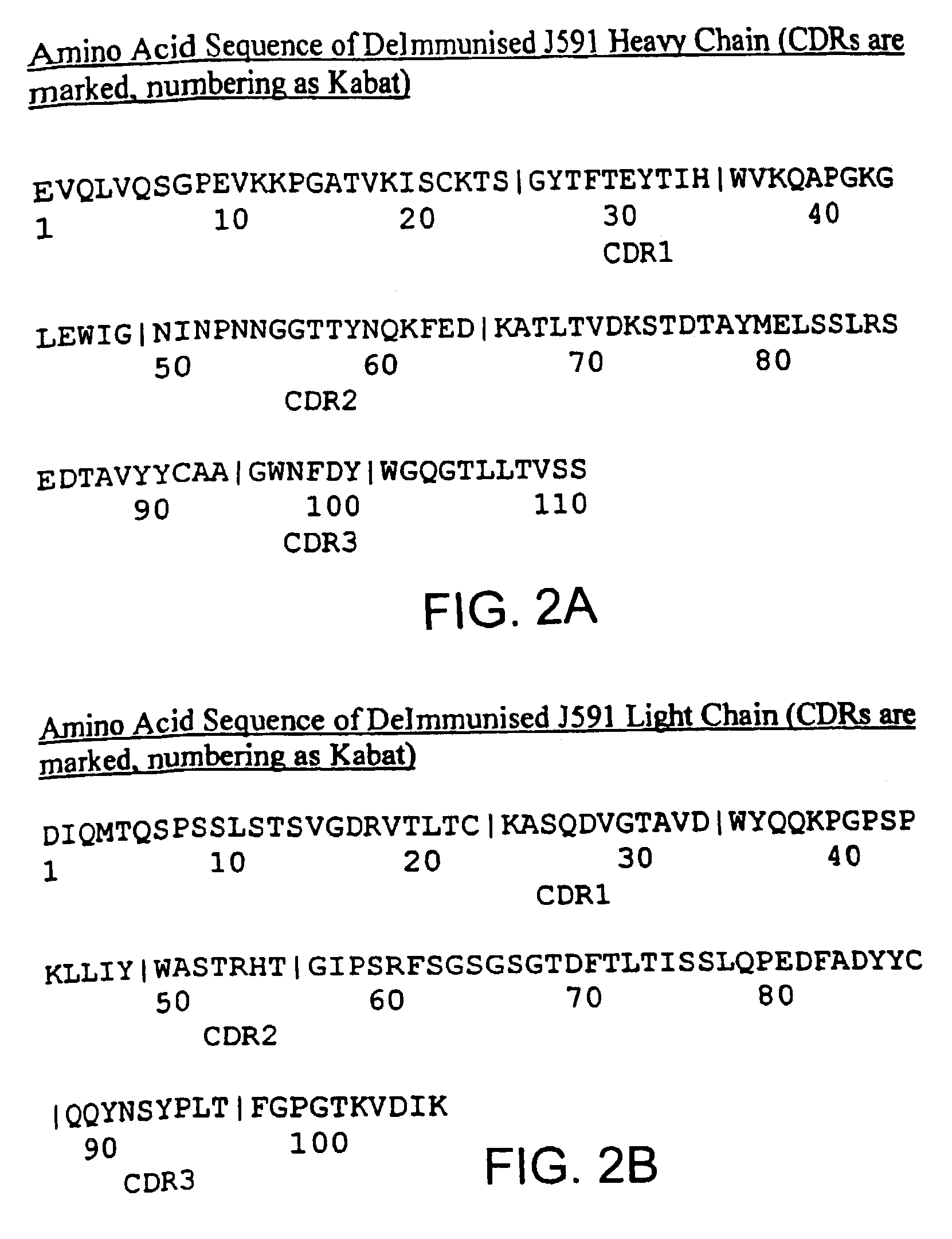
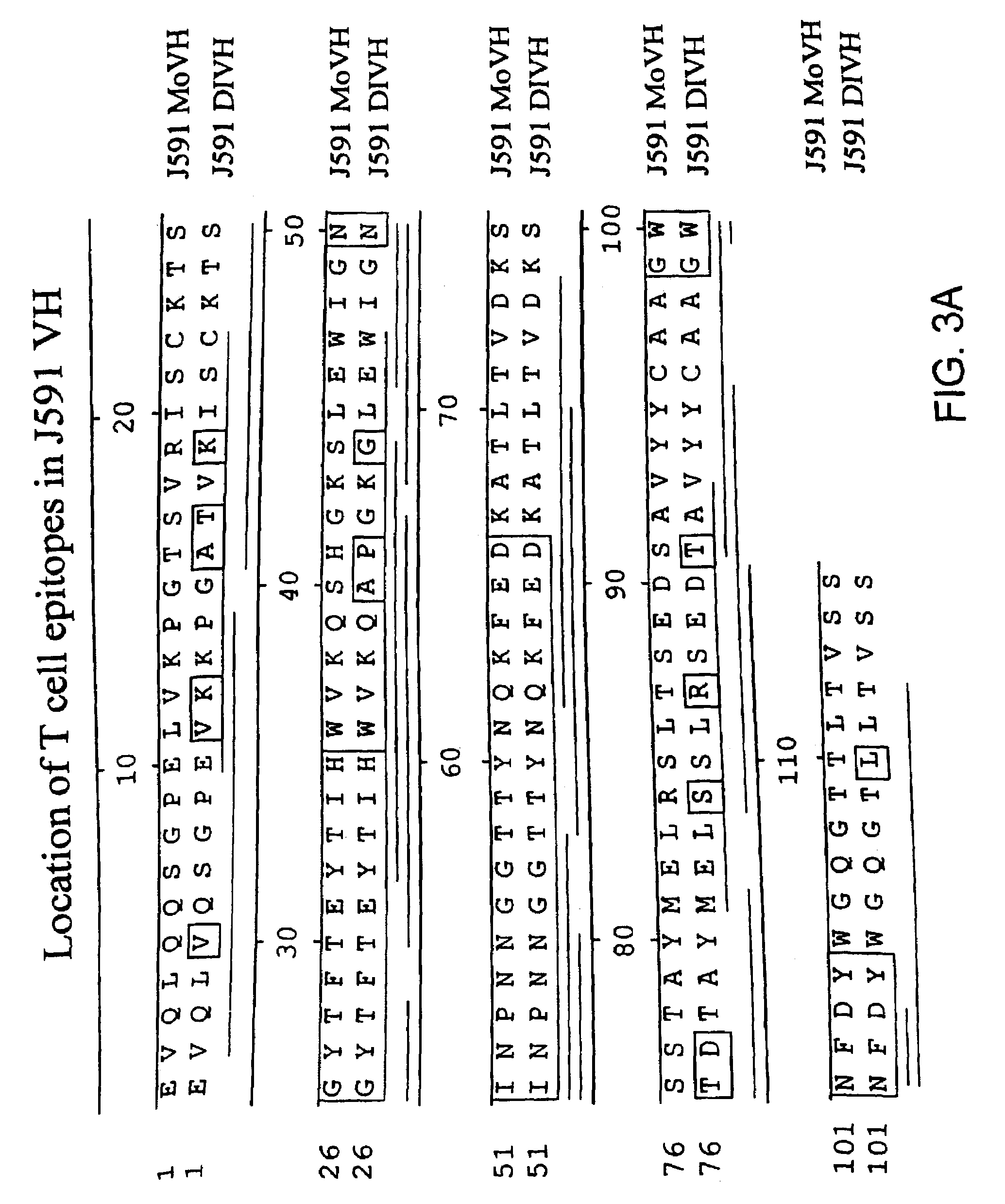
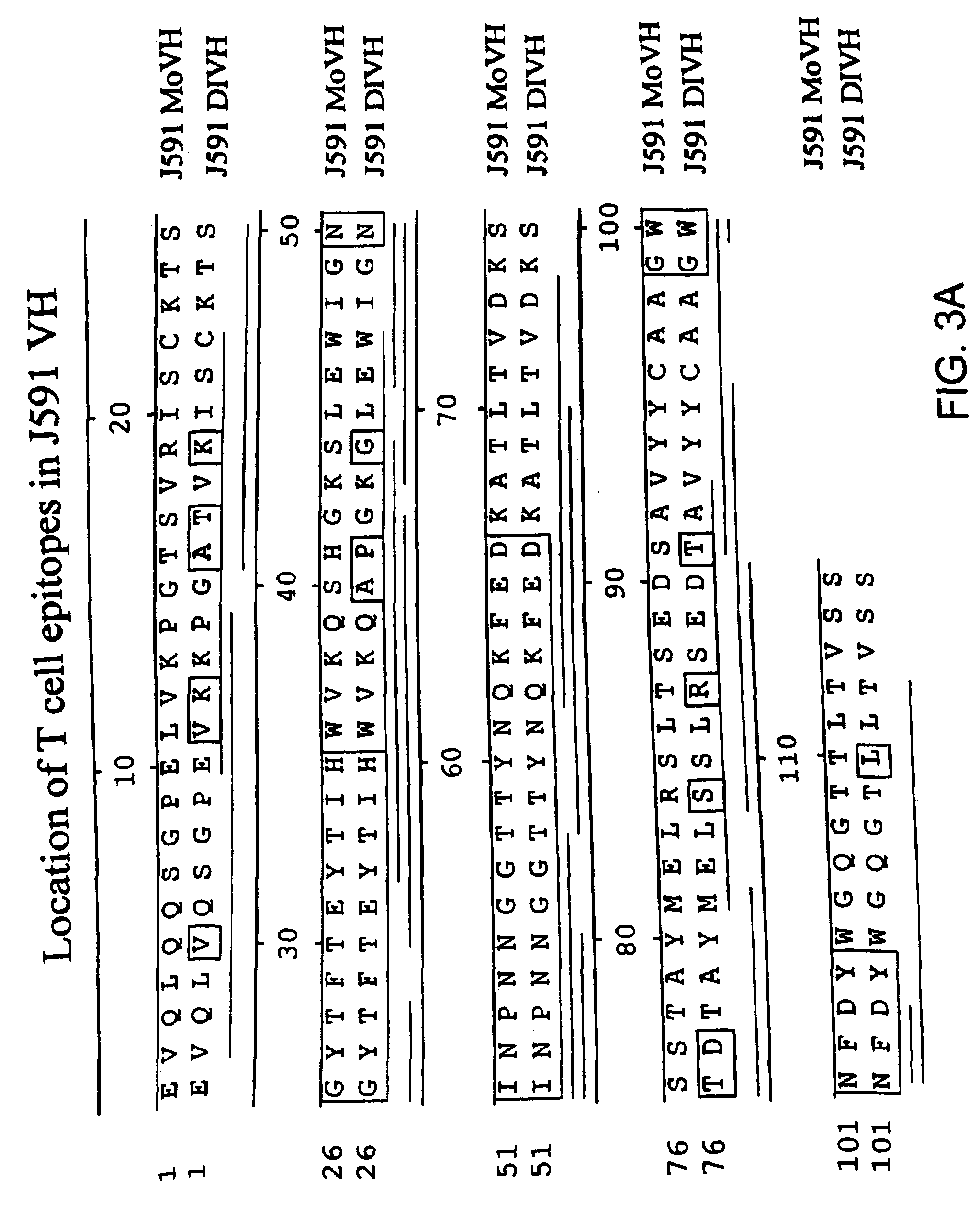
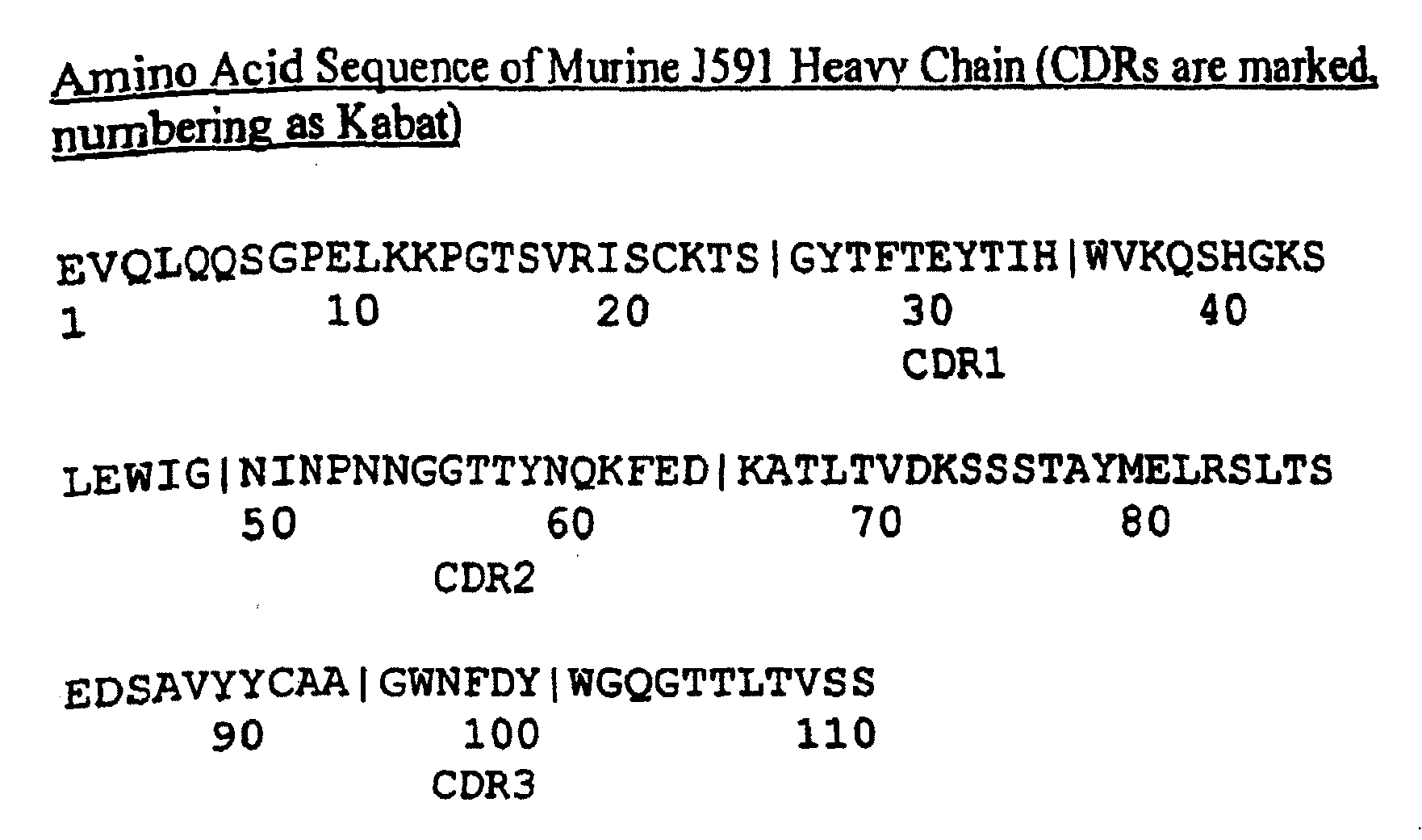
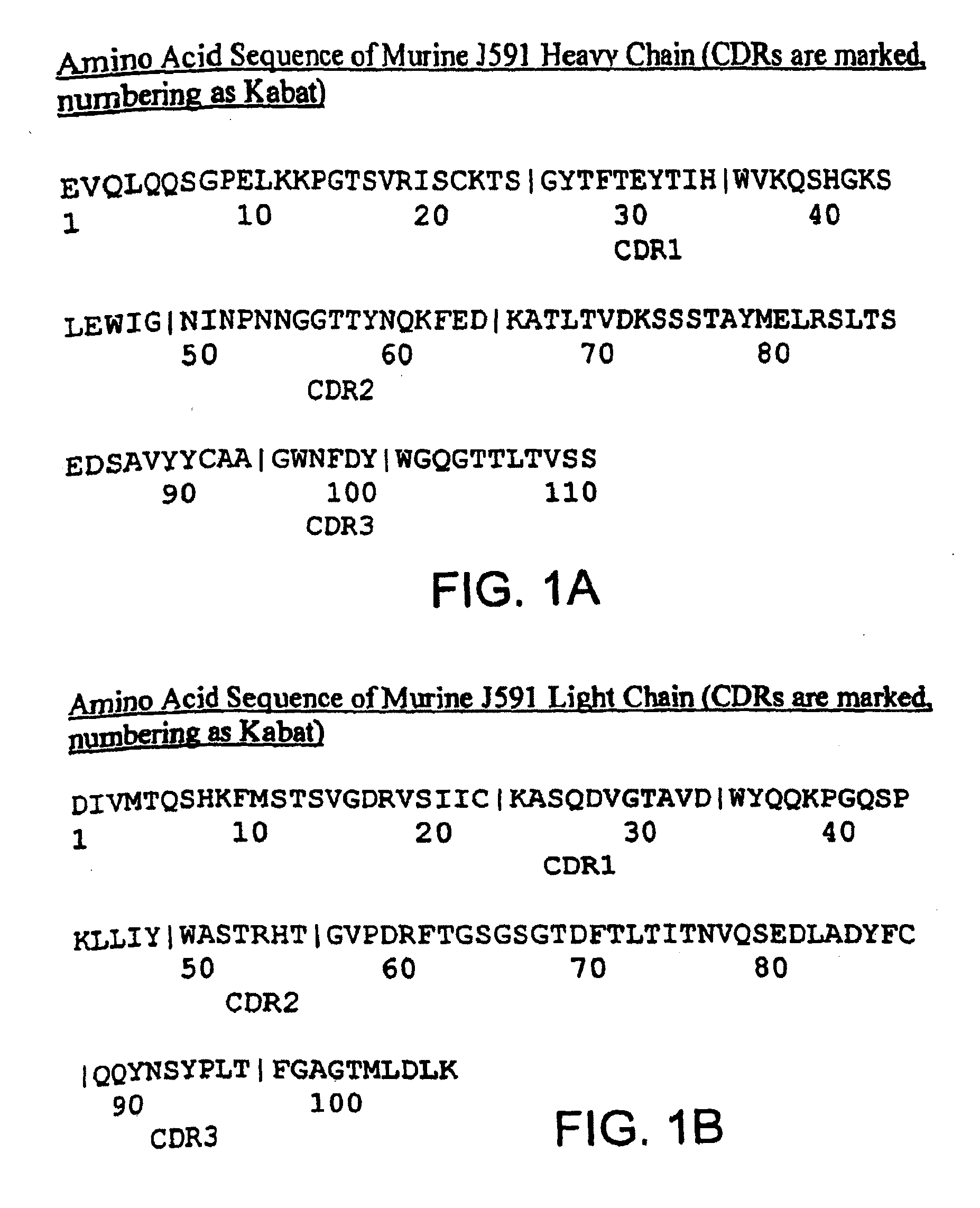
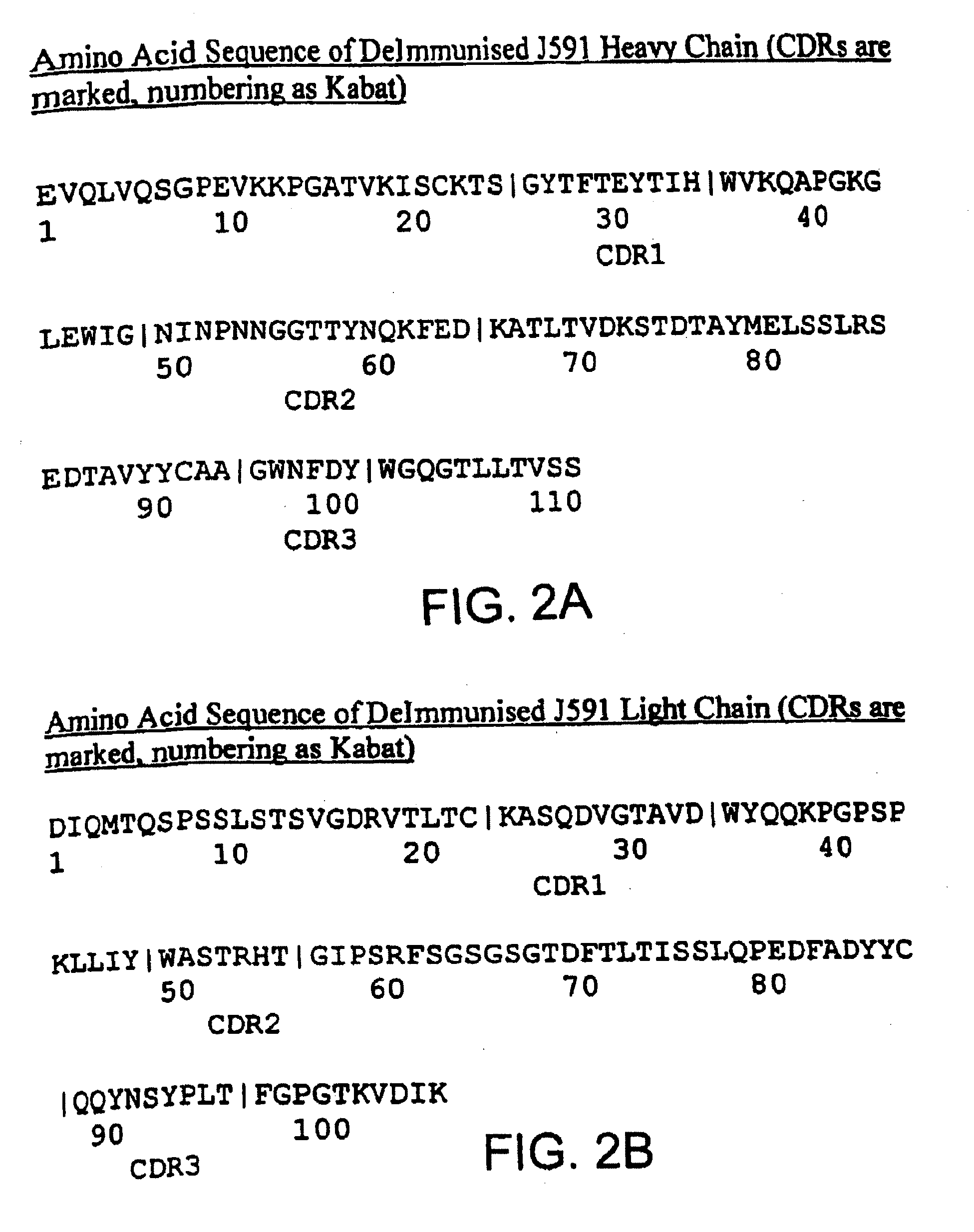
Modified antibodies to prostate-specific membrane antigen and uses thereof
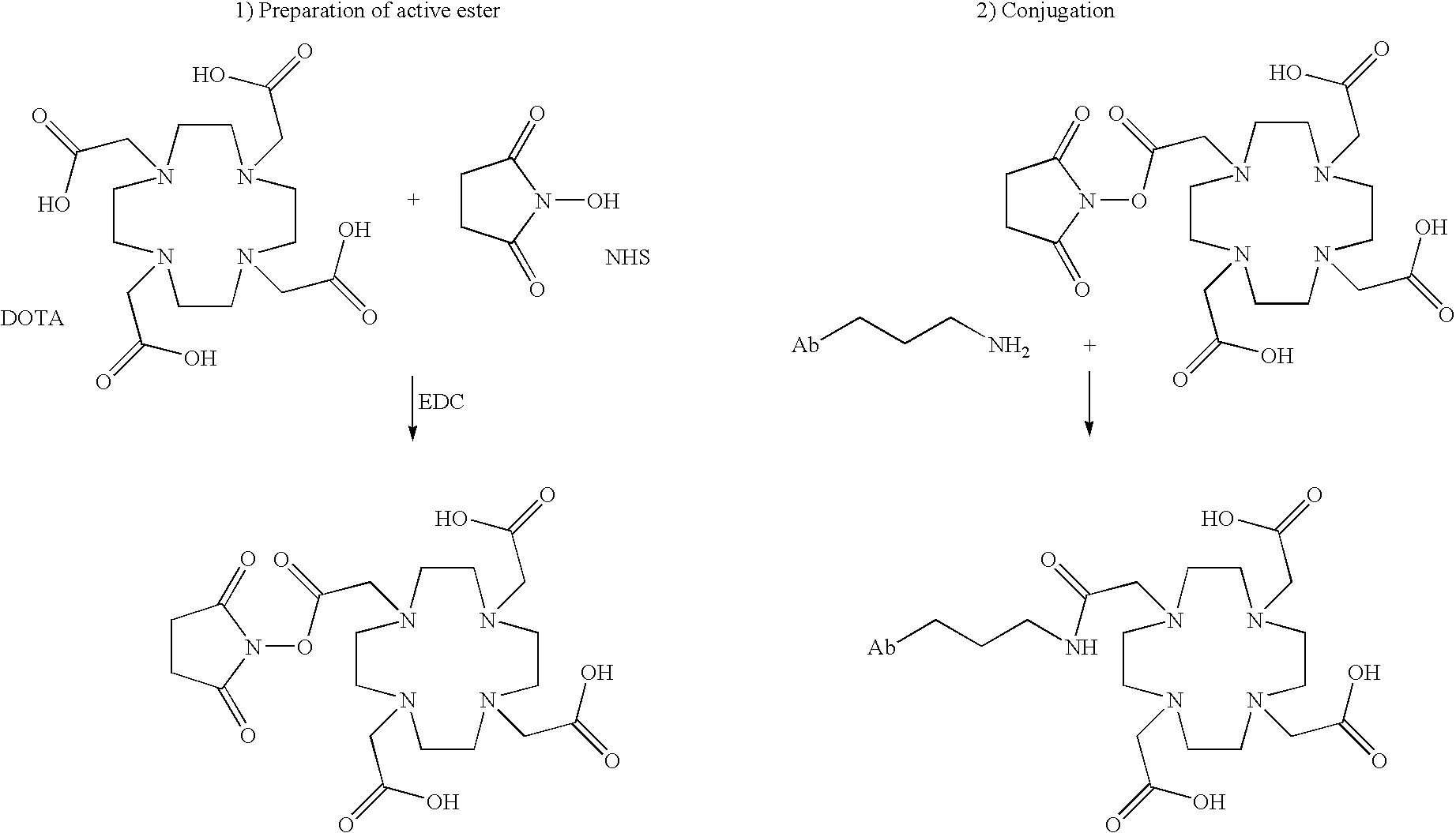
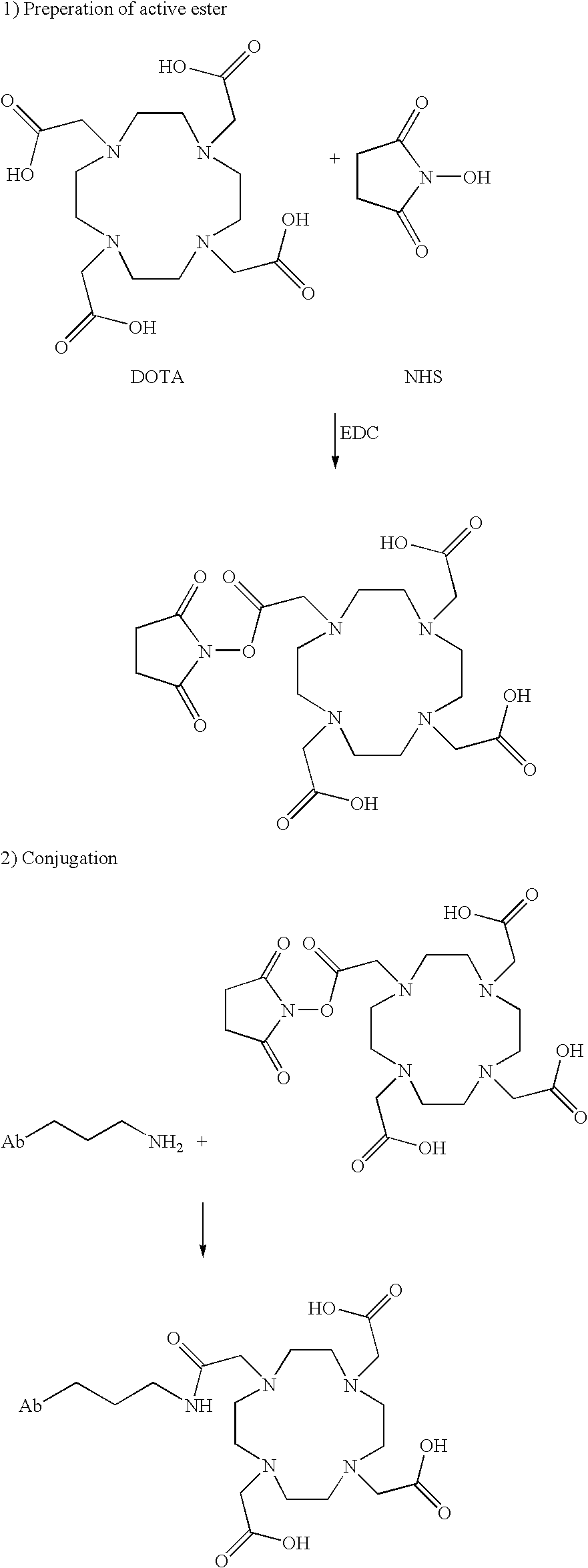
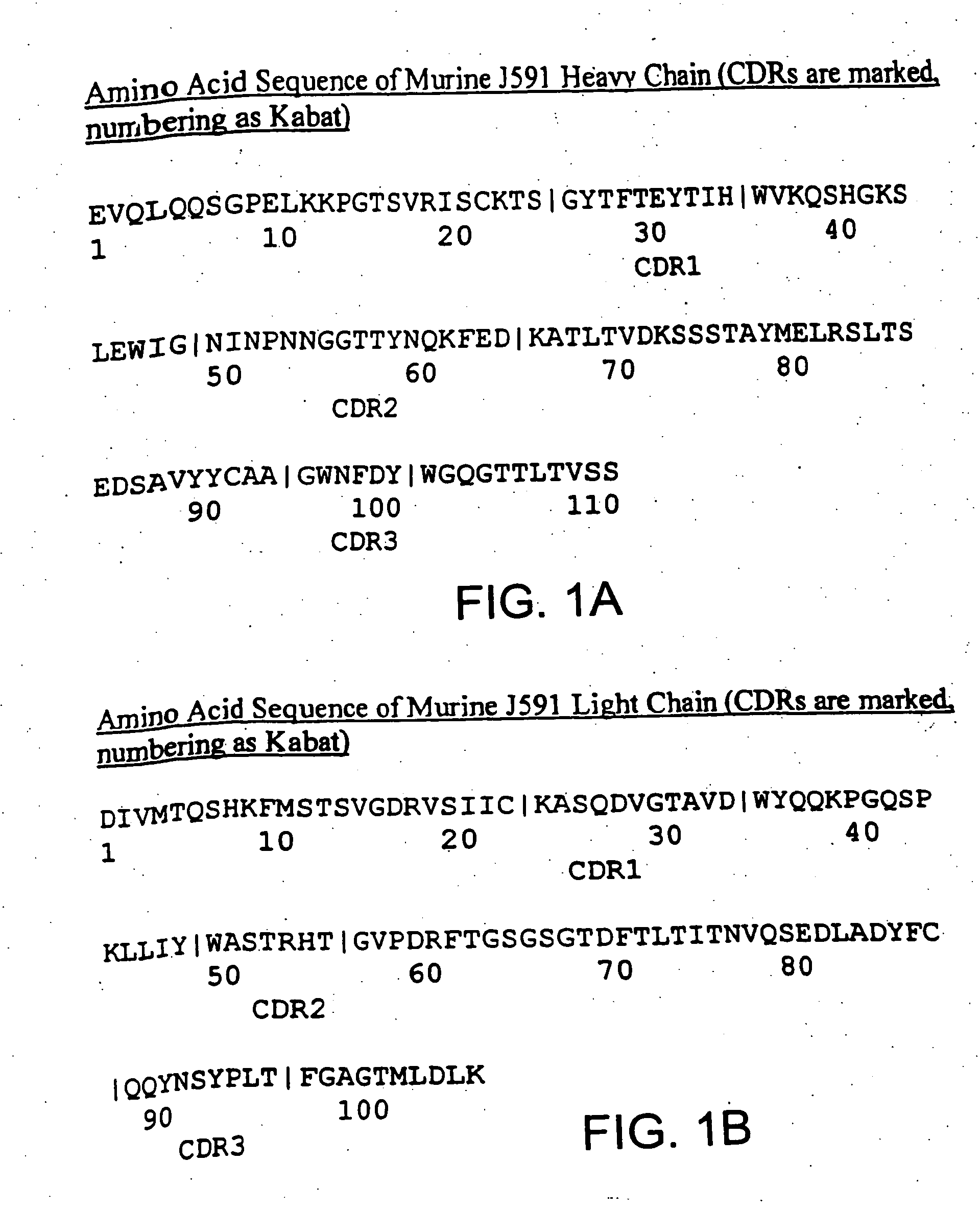
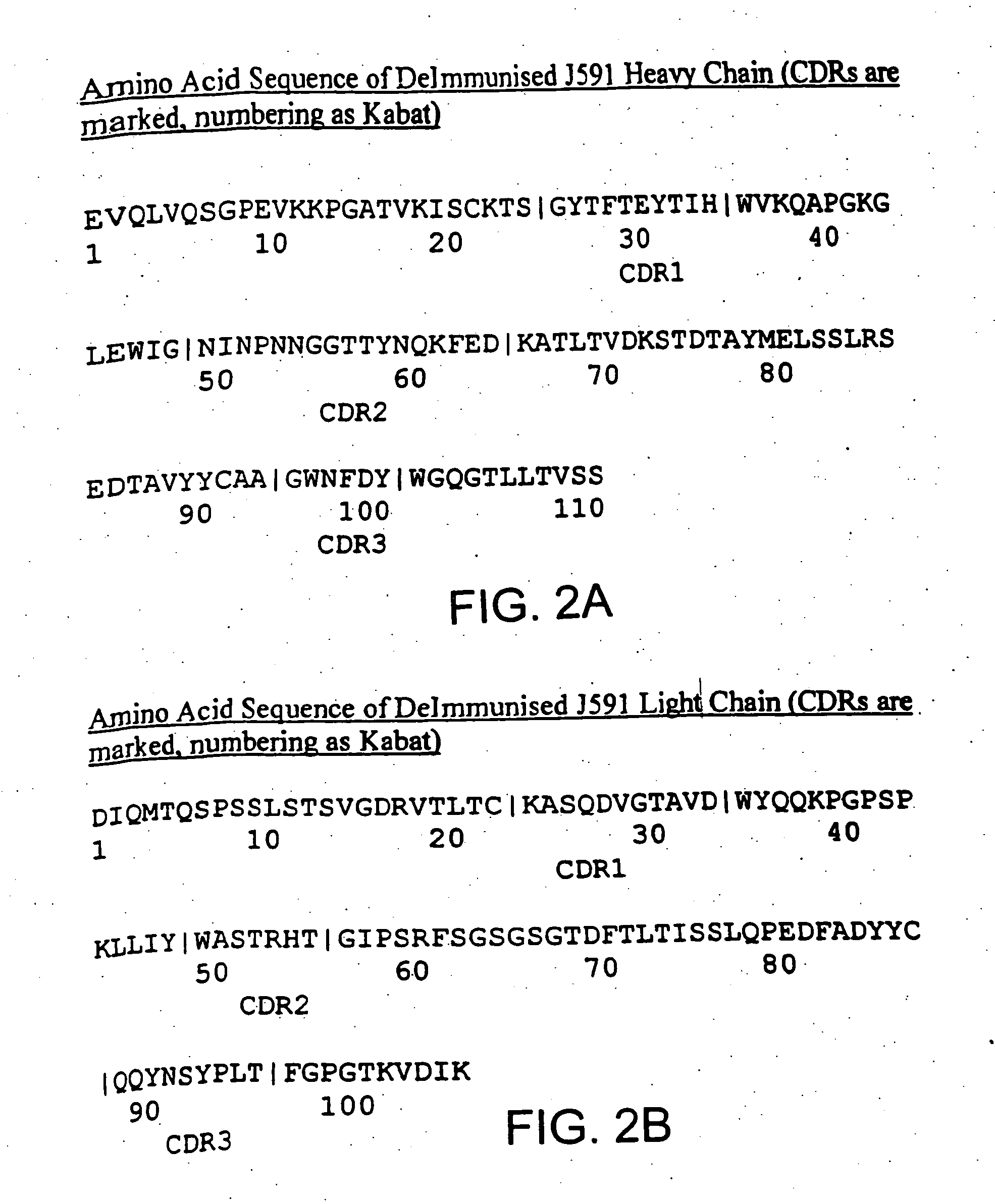
InactiveUS7045605B2Less immunogenicHigh affinityNervous disorderHybrid cell preparationAntigen Binding FragmentAntigen binding
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Methods of treating prostate cancer with anti-prostate specific membrane antigen antibodies
ActiveUS7514078B2Relieve painReduce needSugar derivativesHybrid cell preparationAntigen Binding FragmentAnti-PSMA Antibody
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Nerve stimulation techniques
InactiveUS8571653B2Minimize any unintended side effect of the signal applicationSuppresses afferent action potentialElectrotherapyPower flowMedicine
A method is provided for treating heart failure in a subject in need of such treatment, including applying a stimulating current to parasympathetic nervous tissue of the subject, selected from the group consisting of: a vagus nerve and an epicardial fat pad. The stimulating current is configured to inhibit release of at least one proinflammatory cytokine sufficiently to the treat heart failure of the subject. A level of the at least one proinflammatory cytokine is measured. Optionally, the stimulating current is configured to change a level of Connexin 43 of the subject, and the level of Connexin 43 is also measured. Other embodiments are also described.
Owner:MEDTRONIC INC
Modified antibodies to prostate-specific membrane antigen and uses thereof
InactiveUS20060062793A1Less immunogenicHigh affinityIn-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellAntigen Binding Fragment
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:MILLENNIUM PHARMA INC
Dermal delivery
InactiveUS20110212157A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentOrganic active ingredientsPowder deliveryHypopigmentationChromhidrosis
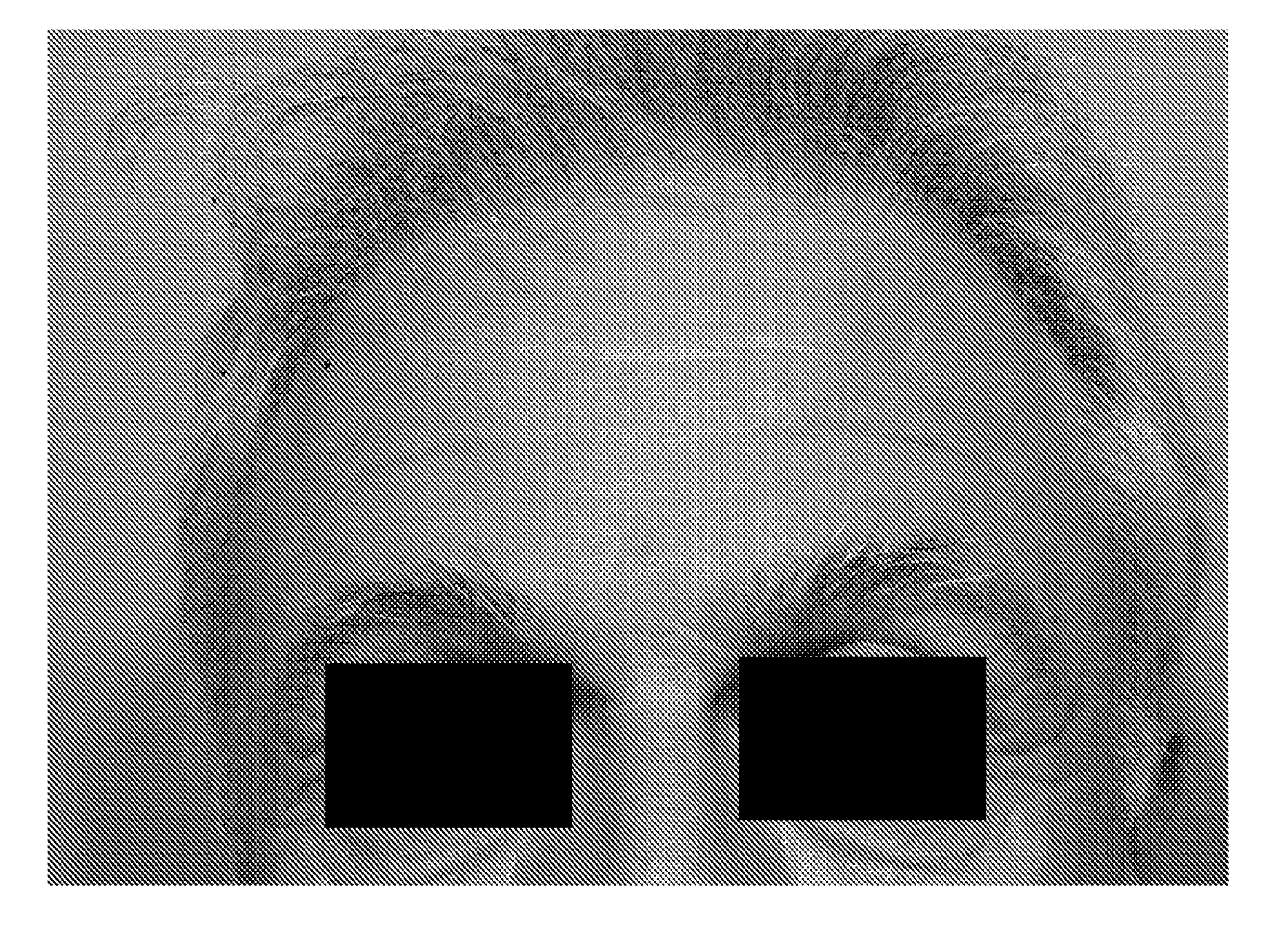
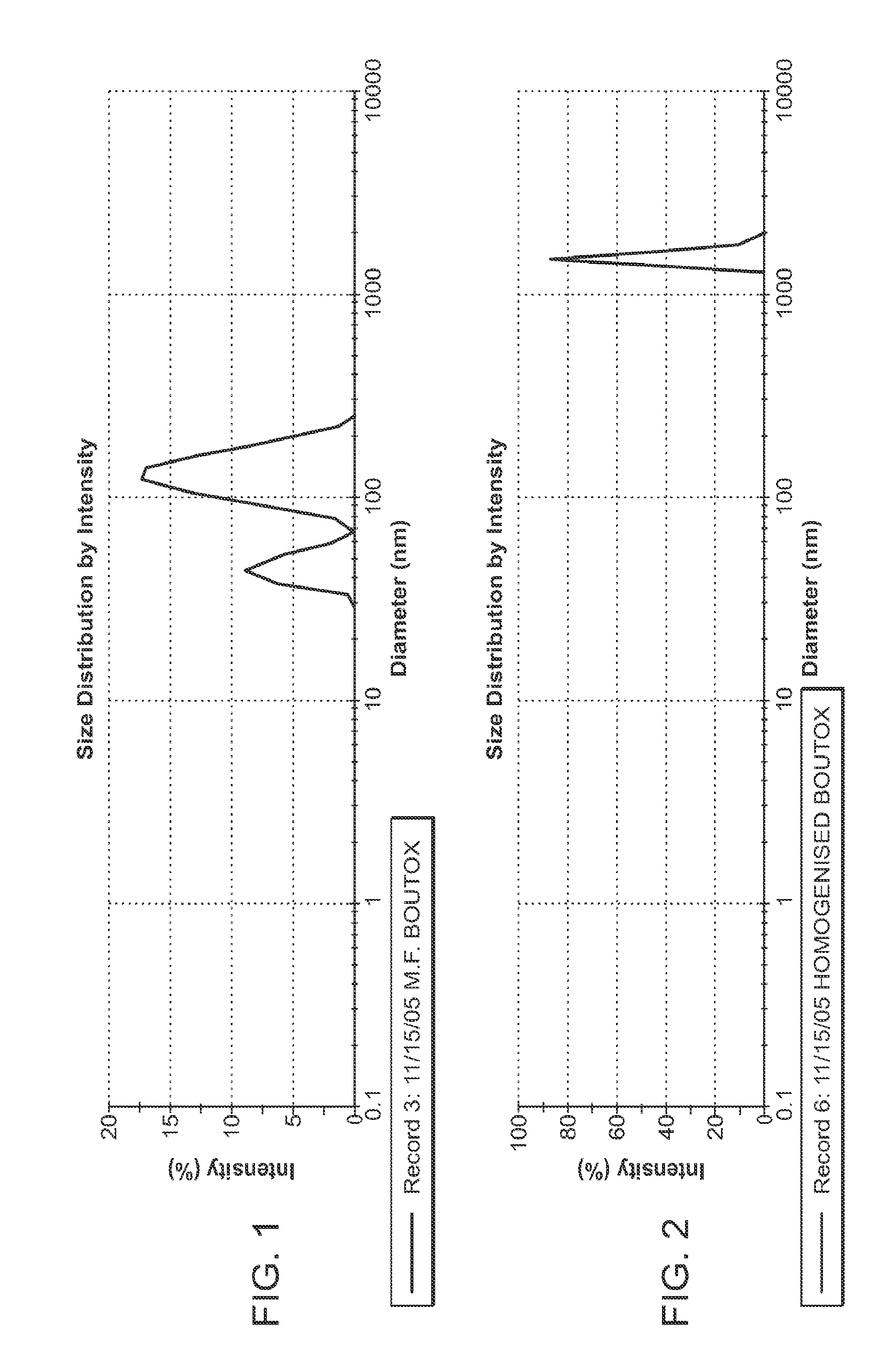
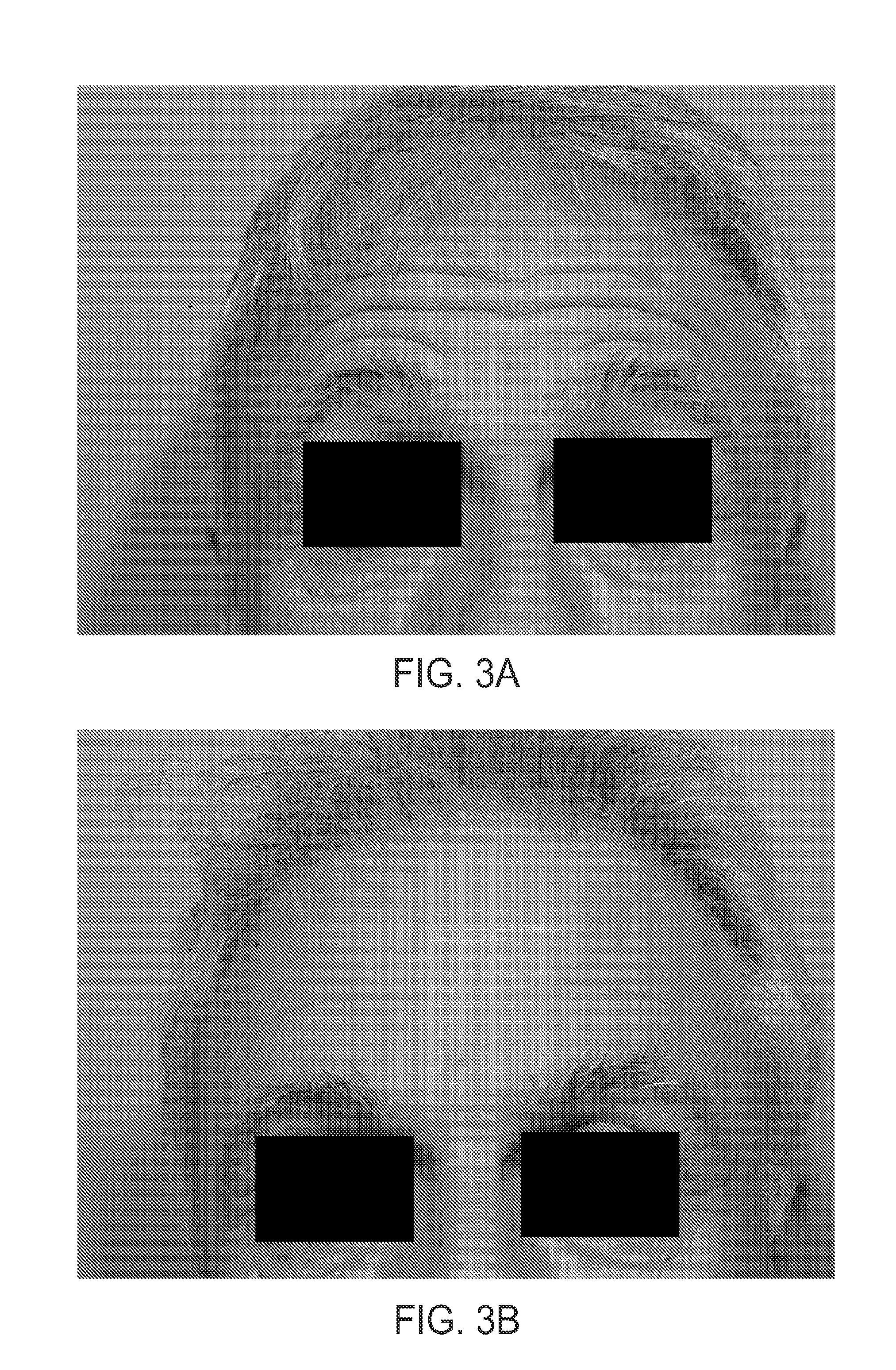
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Dermal Delivery
InactiveUS20160213757A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentCosmetic preparationsPeptide/protein ingredientsHypopigmentationTherapeutic effect
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Porous formed article and method for production thereof
ActiveUS20070128424A1High opening rateIncreasing treatment speedLayered productsSolid sorbent liquid separationIonFiber
A porous formed article which comprises an organic polymer resin and an inorganic ion absorbing material, and has communicating pores opening at an outer surface, wherein it has cavities in the interior of a fibril forming a communicating pore, at least a part of said cavities opens at the surface of the fibril, and the inorganic ion absorbing material is carried on the outer surface of said fibril and on the surface of inner cavities.
Owner:ASAHI KASEI CHEM CORP
Medical compositions for intravesical treatment of bladder cancer
InactiveUS6894071B2Characteristics of easeEase of reconstitutionBiocidePowder deliverySolubilityChemical composition
Anti-cancer coating compositions comprising 3-hydroxymethyl-5-aziridinyl-1-1-methyl-2-[1H-indole-4,7-dione]propenol (E09) are disclosed. More specifically, the coating compositions comprise EO9 and a formulation vehicle. The formulation vehicle improves the solubility and stability of EO9. Additionally, the coating compositions can include coating agents that provide better adhesion of the coating composition to the bladder wall during intravesical delivery of the coating composition.
Owner:SPECTRUM PHARMA INC
Methods for treating prostate cancer using modified antibodies to prostate-specific membrane antigen
InactiveUS7666414B2Relieve painReduce needIn-vivo radioactive preparationsSugar derivativesAntigen Binding FragmentAnti-PSMA Antibody
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Modified antibodies to prostate-specific membrane antigen and uses thereof
InactiveUS20060088539A1Relieve painReduce needIn-vivo radioactive preparationsAntipyreticCancer cellAntigen Binding Fragment
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
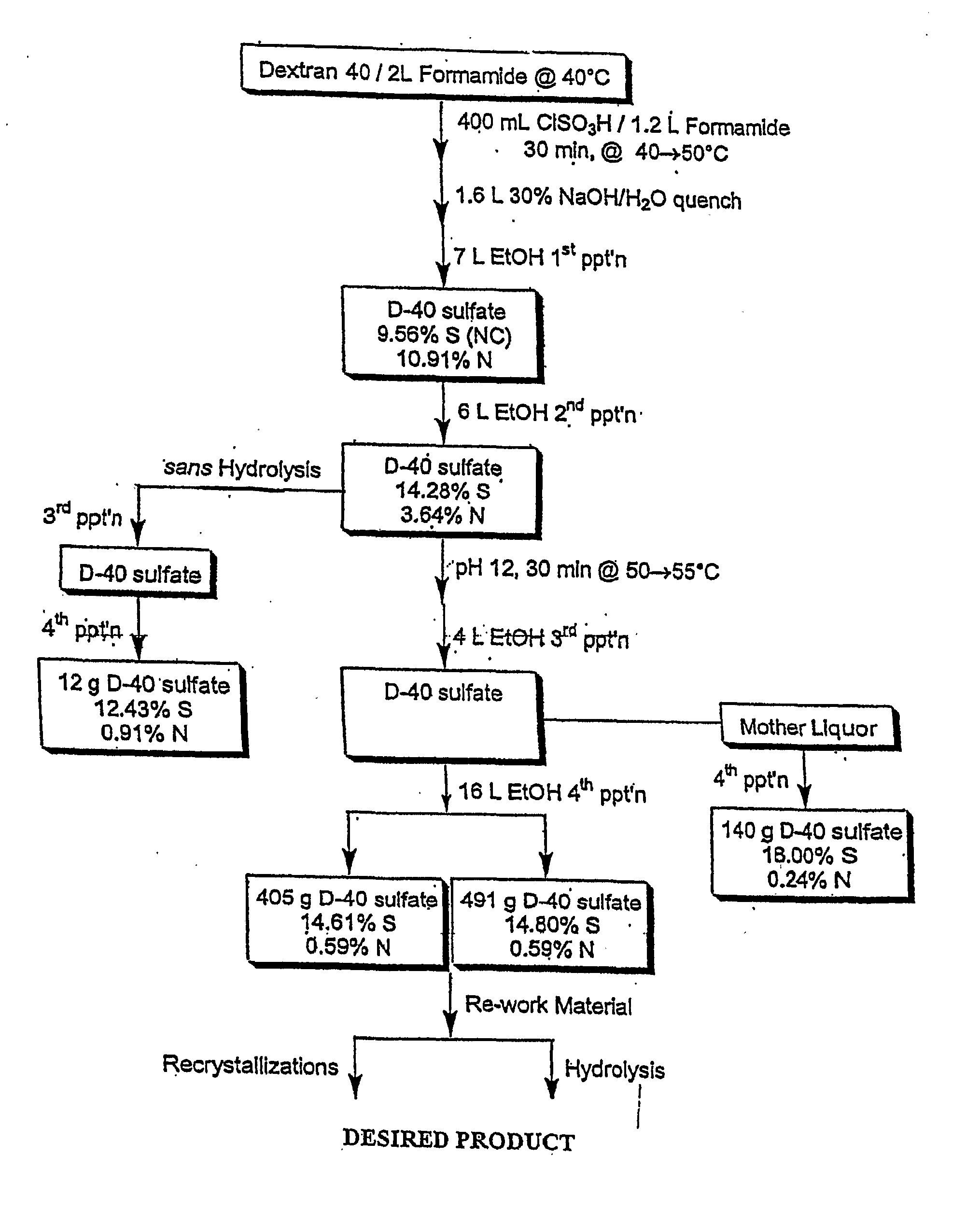
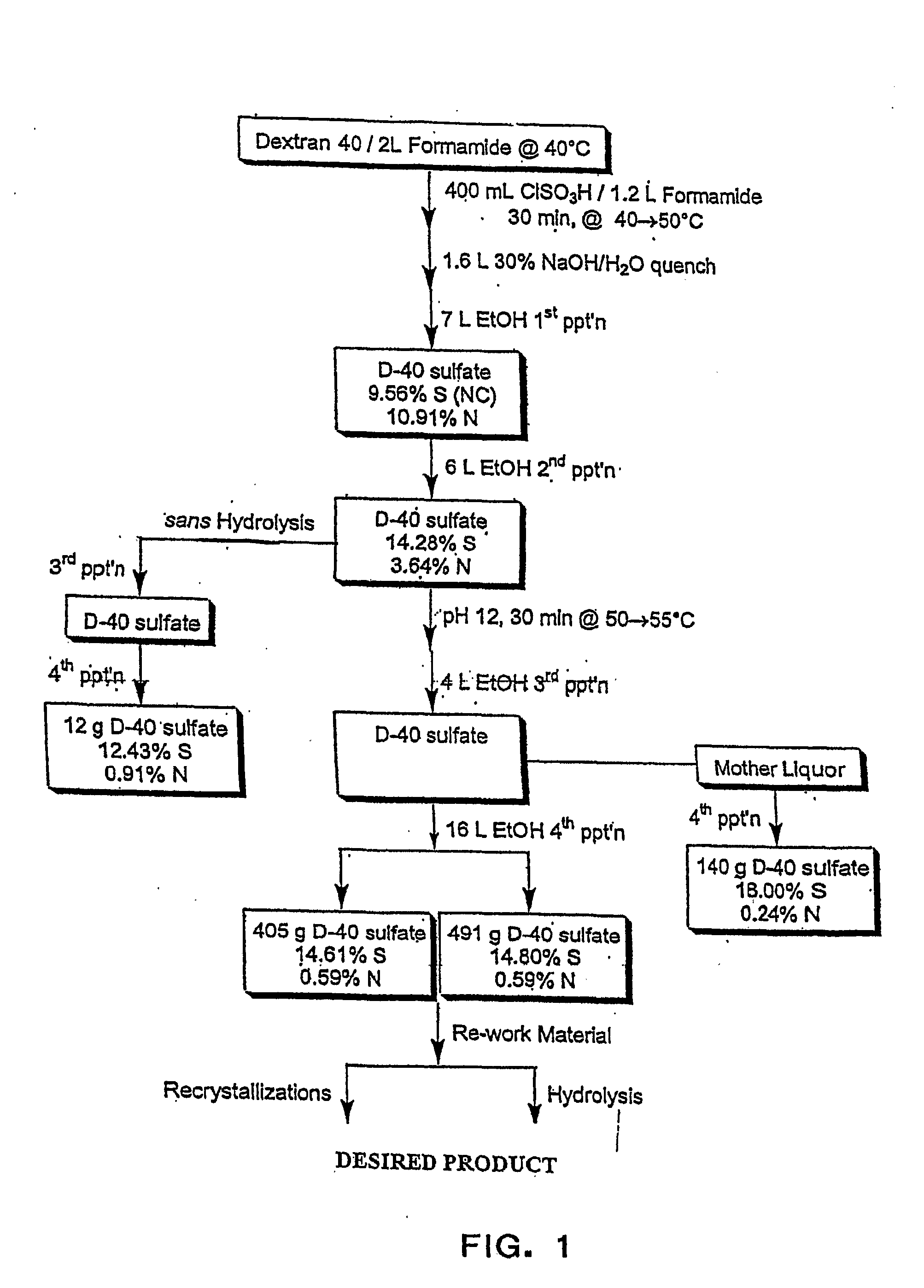
High Dose, Short Interval Use of Sulfated Polysaccharides for Treatment of Infections
InactiveUS20080004236A1Reducing and avoiding toxicityEnsure adequate treatmentOrganic active ingredientsBiocideMicroorganismSulfated polysaccharides
Methods and compositions for treating or preventing acute or chronic viral infection over a short time interval in mammals with sulfated polysaccharides wherein the polysaccharides have a percent of sulfur with respect to the sugar residue effective to enable maximal interaction of constituent sulfate groups with the microbe which causes the infection and wherein the sulfated polysaccharide is not substantially endocytosed or degraded by cell receptor binding in the mammal and thereby retains antiviral activity in vivo.
Owner:COMPER WAYNE D
Methods of reducing immunogenicity against factor viii in individuals undergoing factor viii therapy
ActiveUS20140370035A1Improve immune toleranceReduce morbidityFactor VIINervous disorderFactor iiImmune tolerance
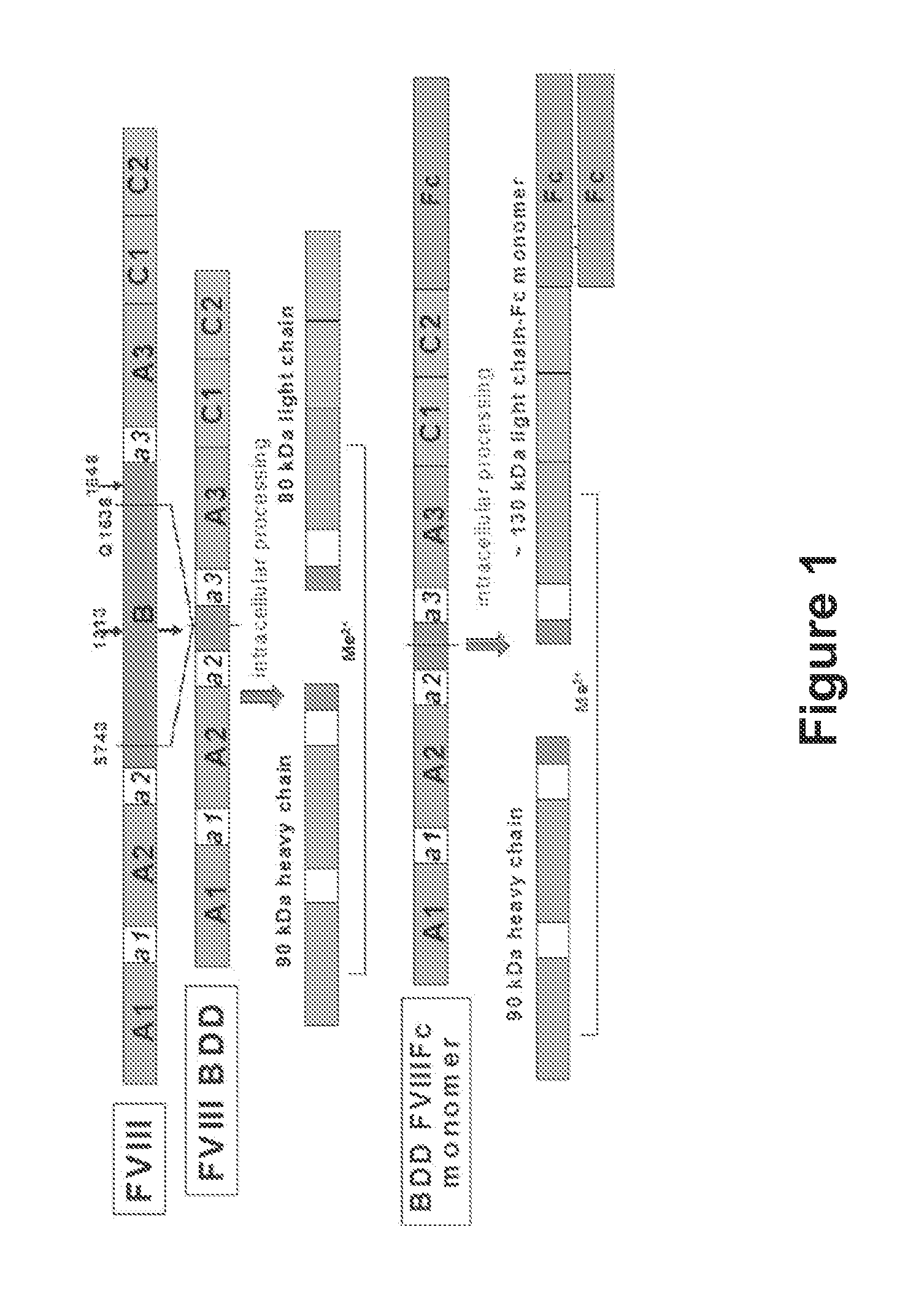
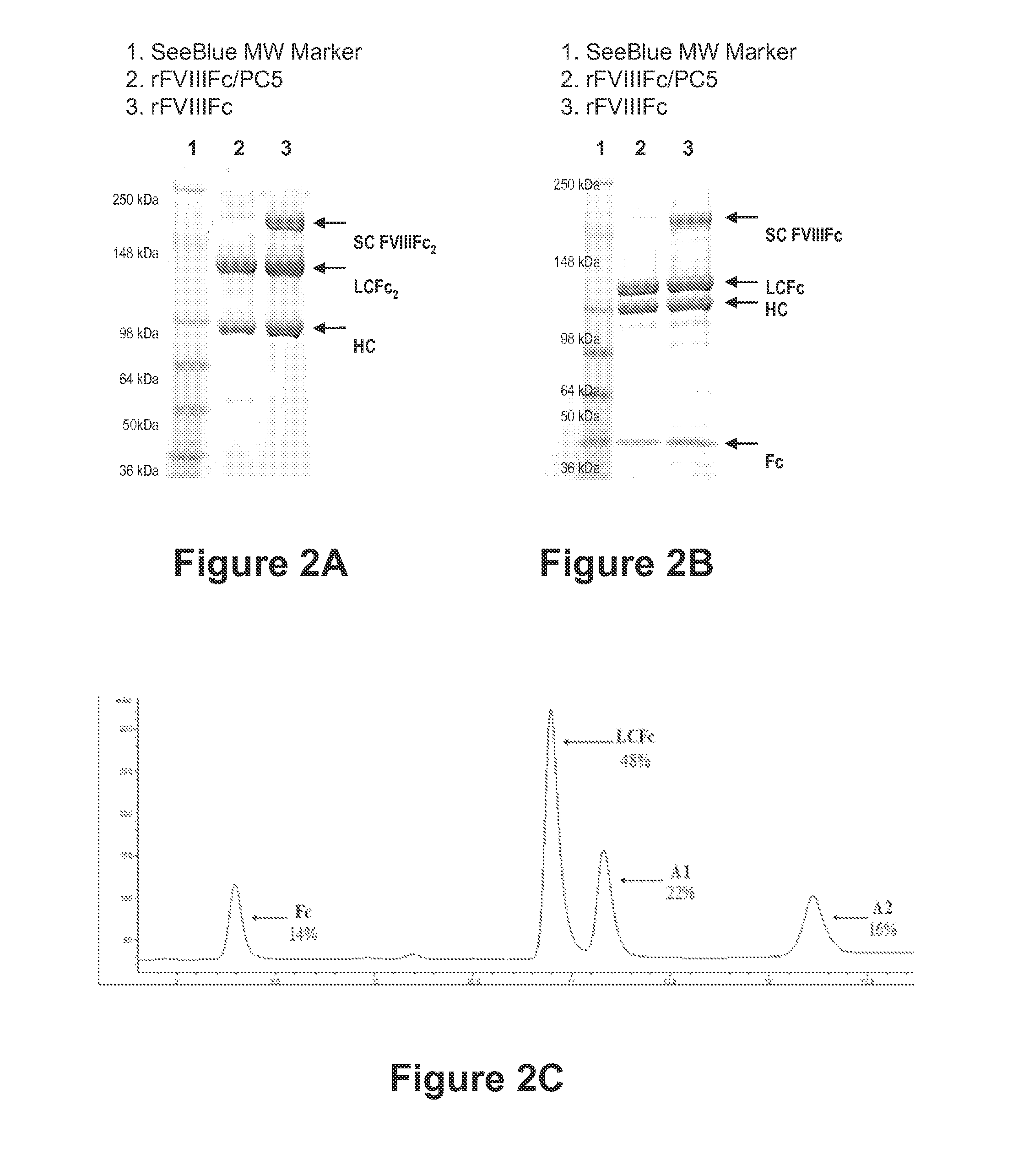
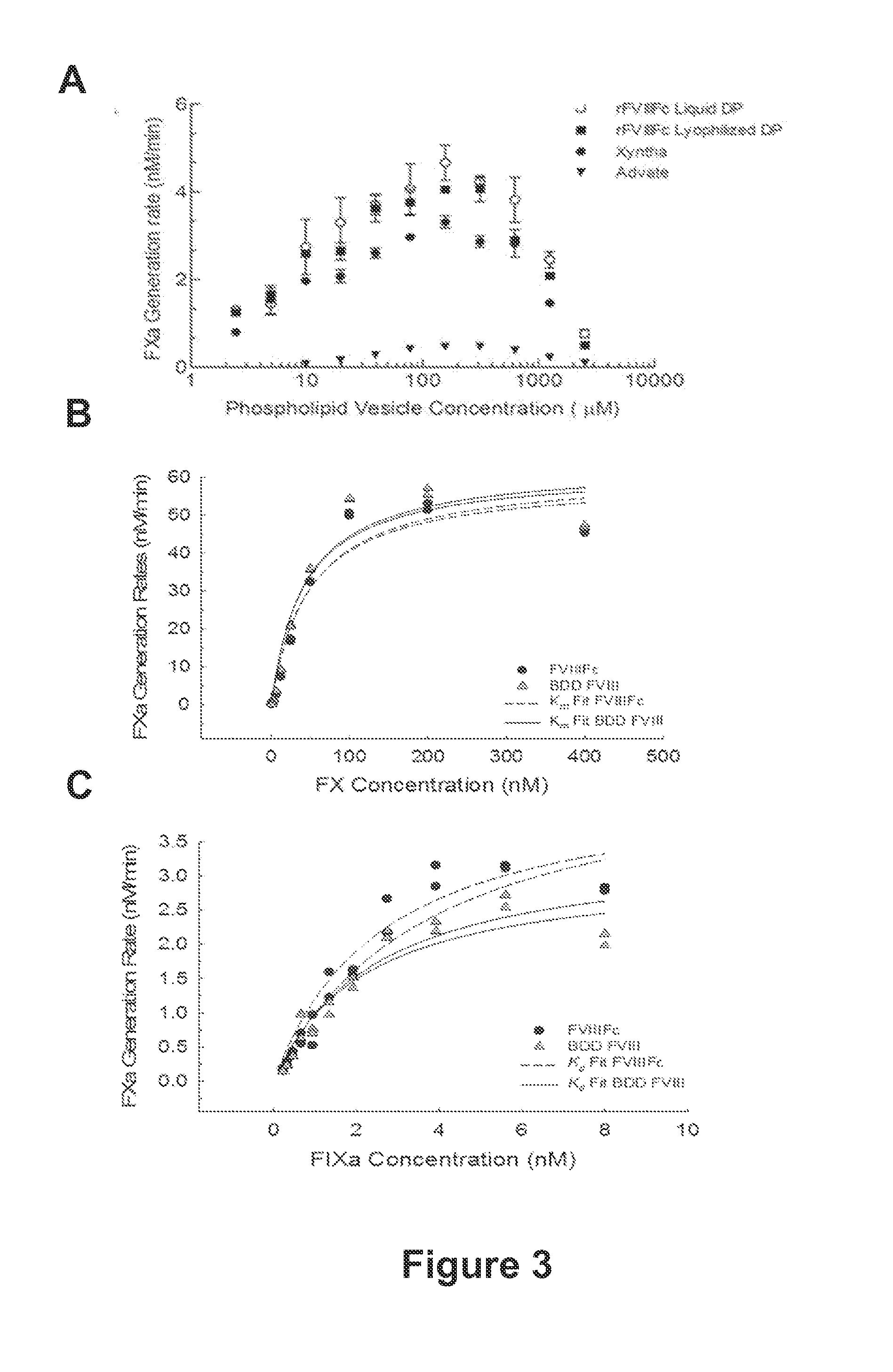
The present disclosure provides methods of administering chimeric and hybrid Factor VIII (FVIII) polypeptides comprising FVIII and Fc to subjects at risk of developing inhibitory FVIII immune responses, including anti-FVIII antibodies and / or cell-mediated immunity. The administration is sufficient to promote coagulation and to induce immune tolerance to FVIII. The chimeric polypeptide can comprise full-length FVIII or a FVIII polypeptide containing a deletion, e.g., a full or partial deletion of the B domain.
Owner:PUGET SOUND BLOOD CENT +1
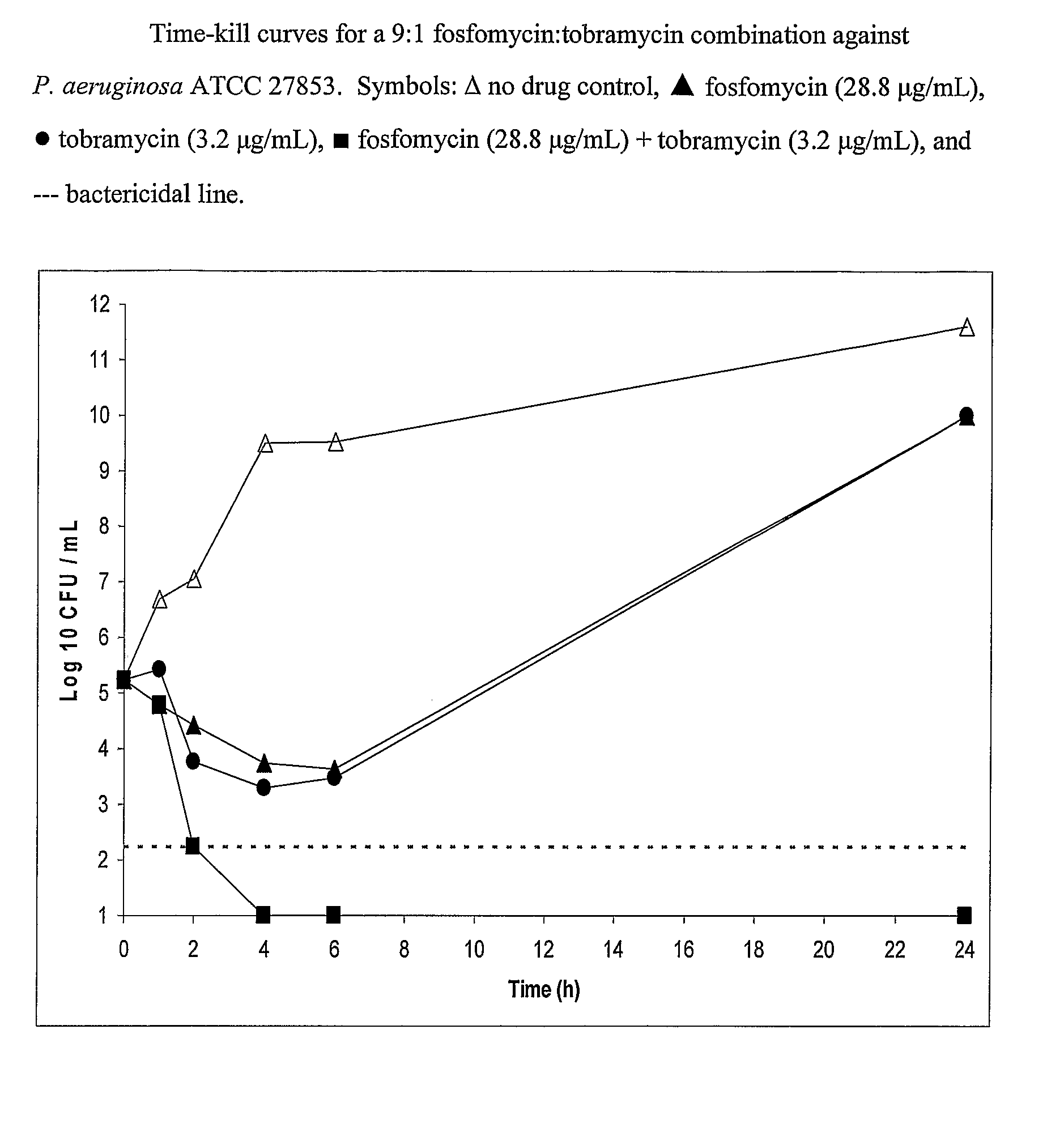
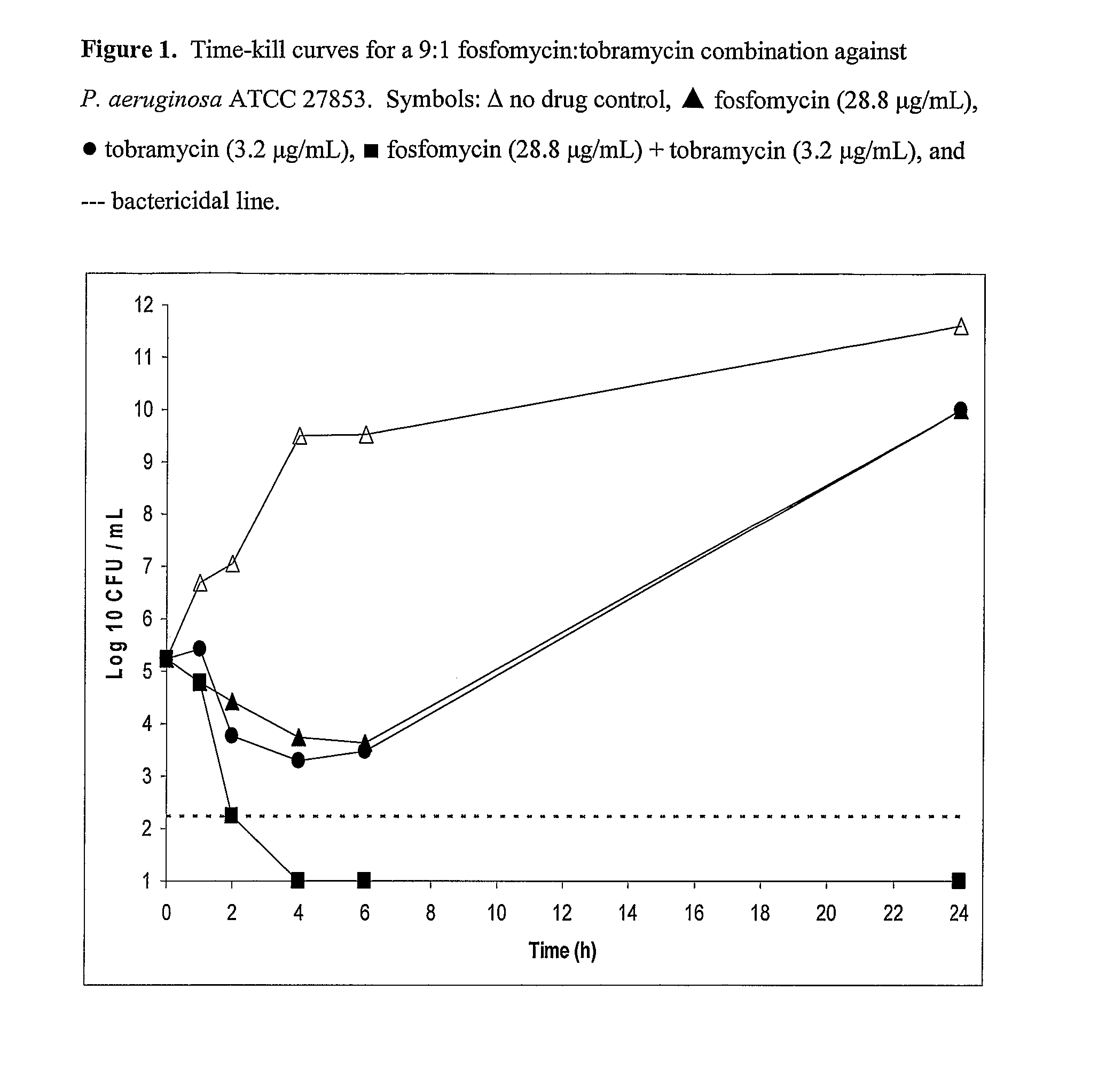
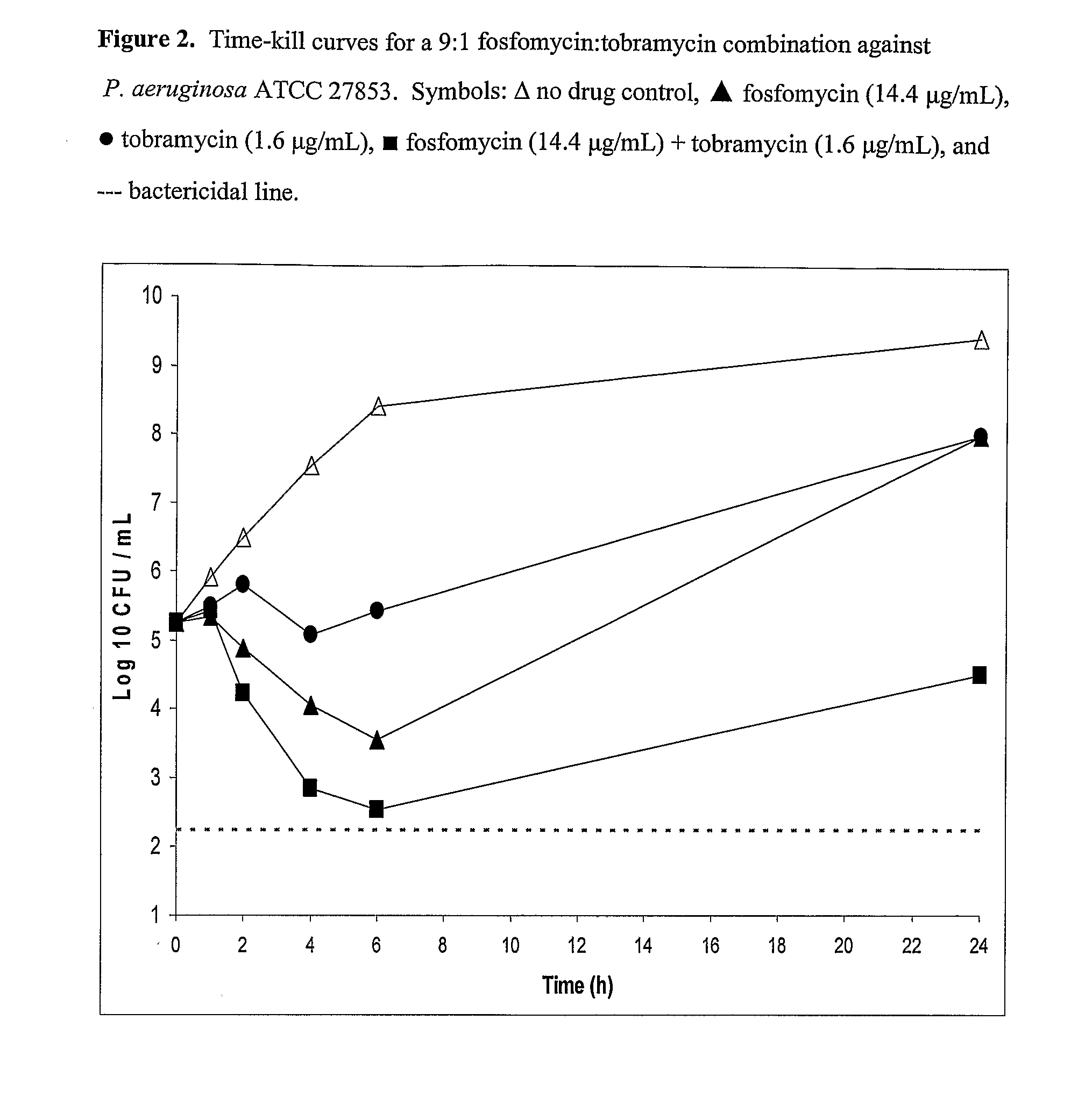
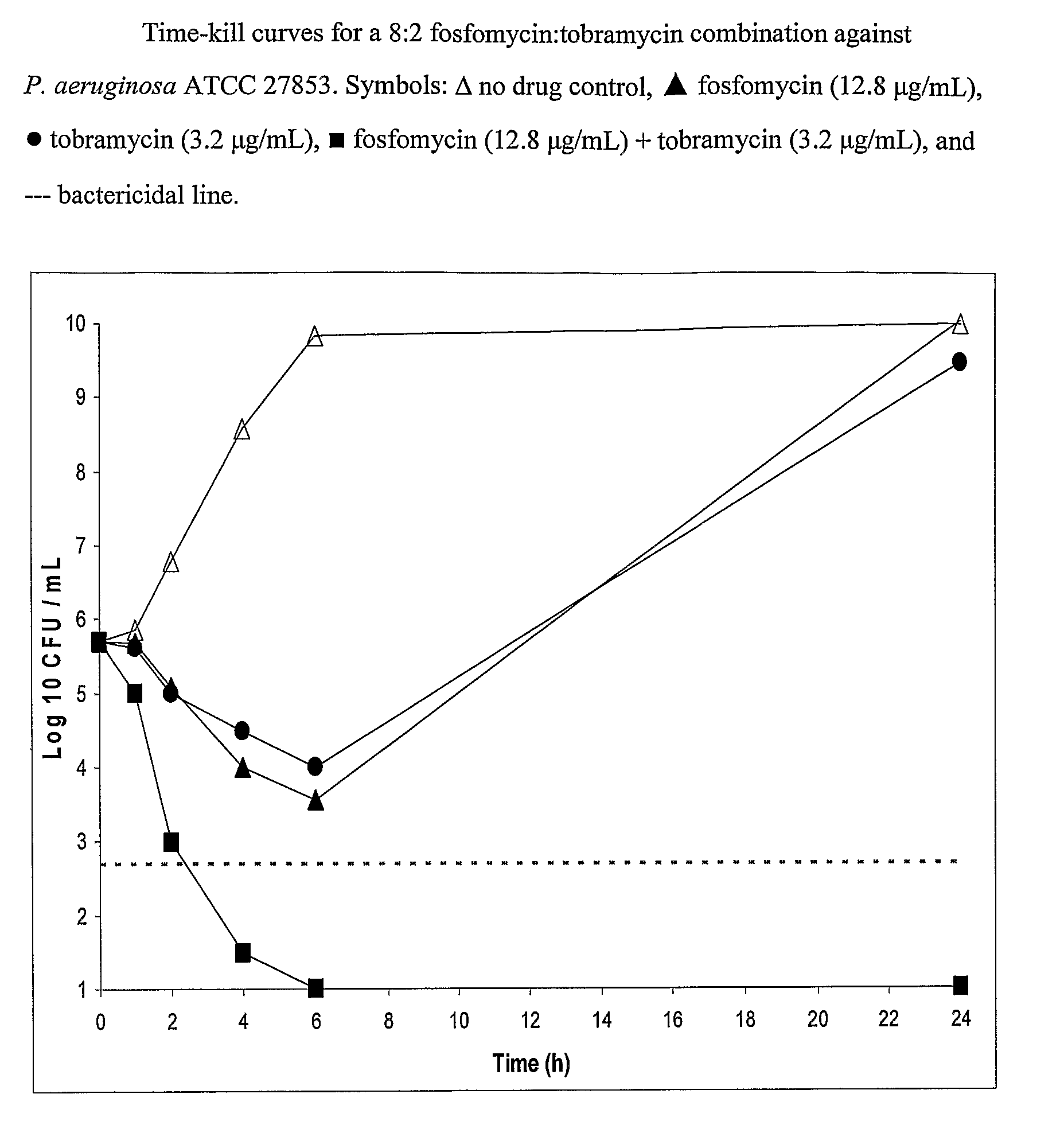
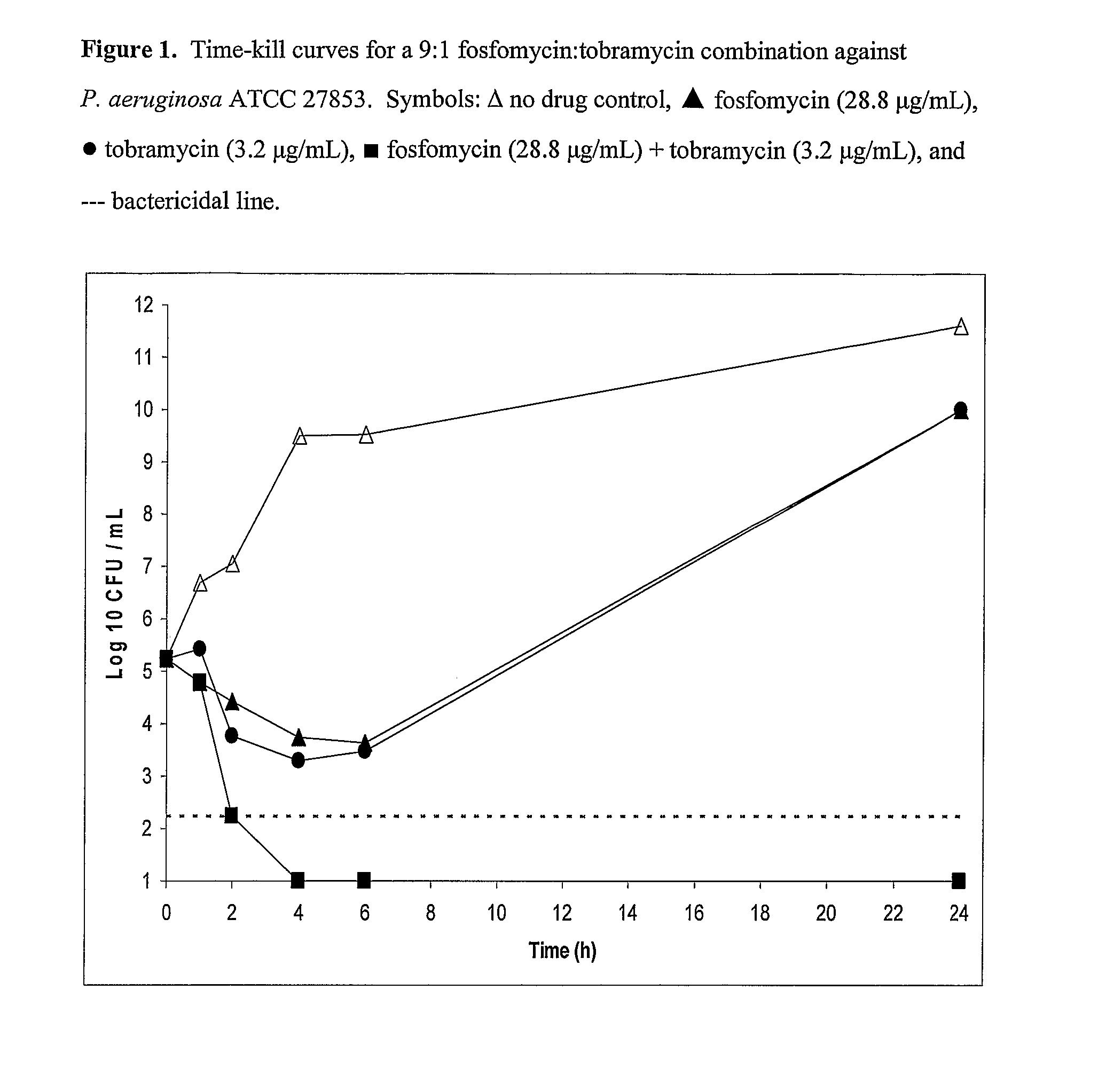
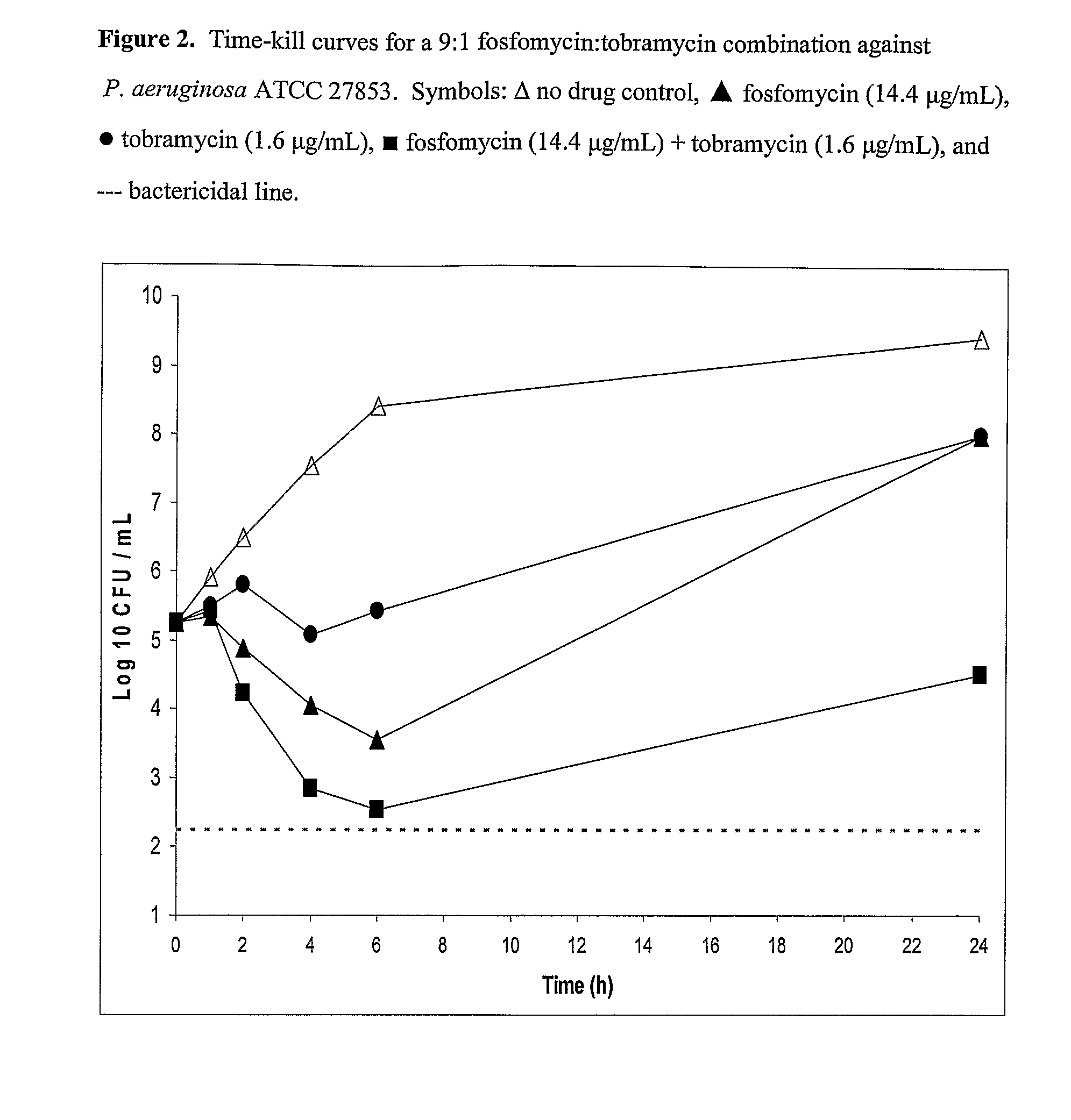
Aerosolized Fosfomycin/Aminoglycoside Combination for the Treatment of Bacterial Respiratory Infections
ActiveUS20070218013A1Reduce developmentIncreases the post antibiotic affect (PAE)Antibacterial agentsBiocideRespiratory tract infectionsAerosolization
A fosfomycin plus tobramycin combination formulation for delivery by aerosolization is described. The concentrated fosfomycin tobramycin combination formulation containing an efficacious amount of fosfomycin plus tobramycin is able to inhibit susceptible bacteria. Fosfomycin and tobramycin are formulated separately in a dual ampoule such that when reconstituted, the pH is between 4.5 and 8.0 or as a dry powder. The method for treatment of respiratory tract infections by a formulation delivered as an aerosol having mass medium average diameter predominantly between 1 to 5 μ, produced by a jet or ultrasonic nebulizer (or equivalent) or dry powder inhaler.
Owner:GILEAD SCI INC
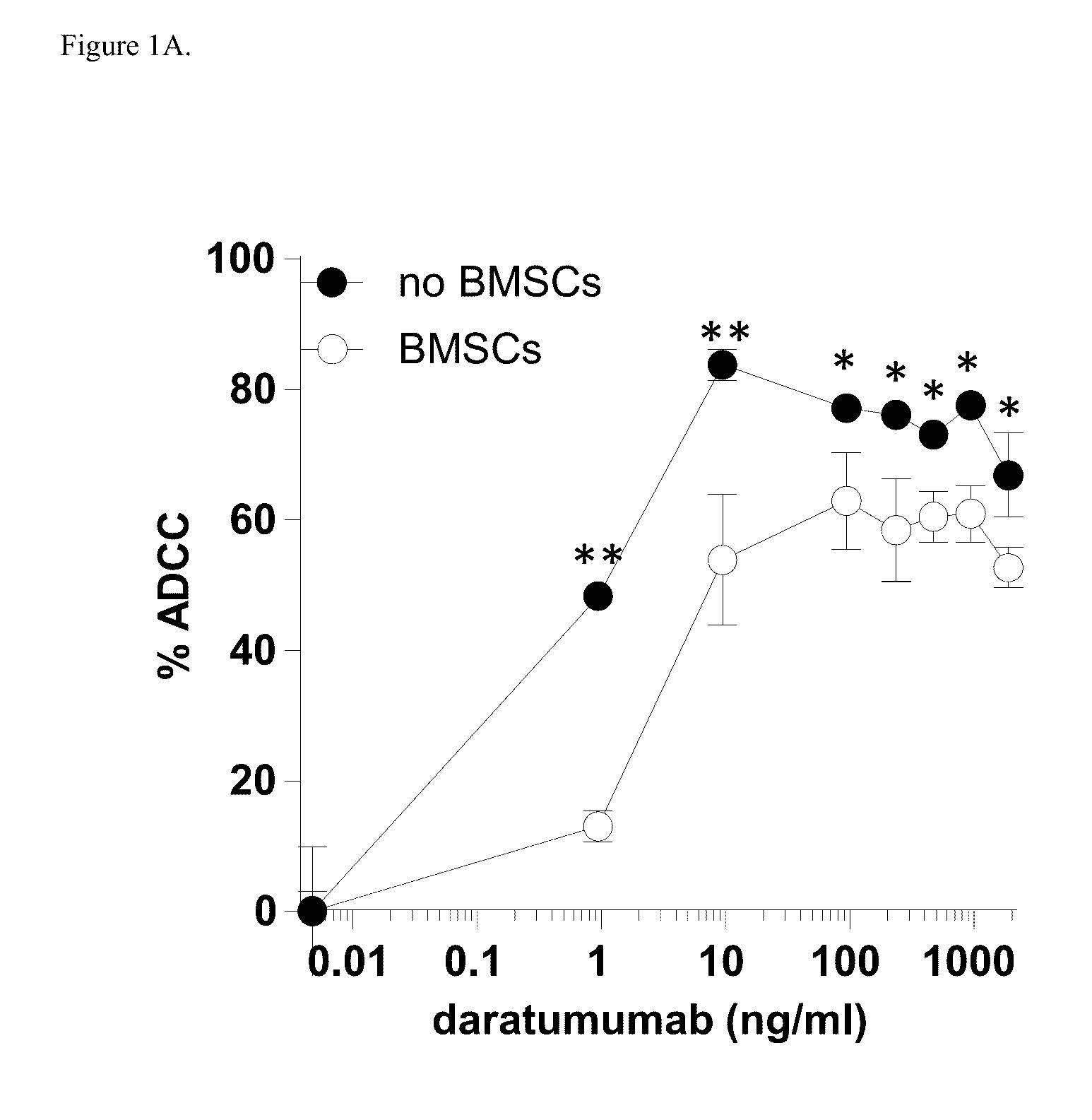
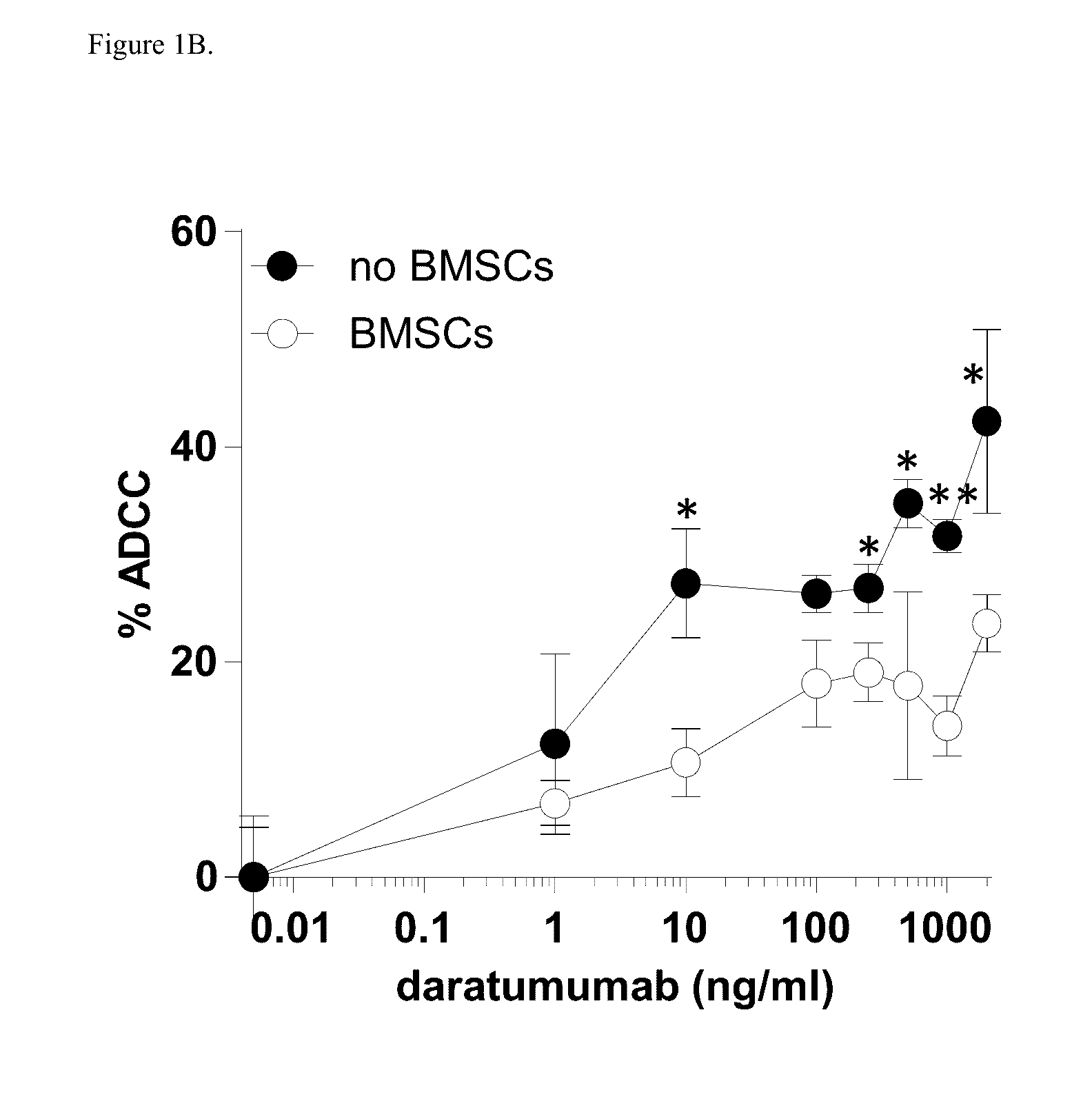
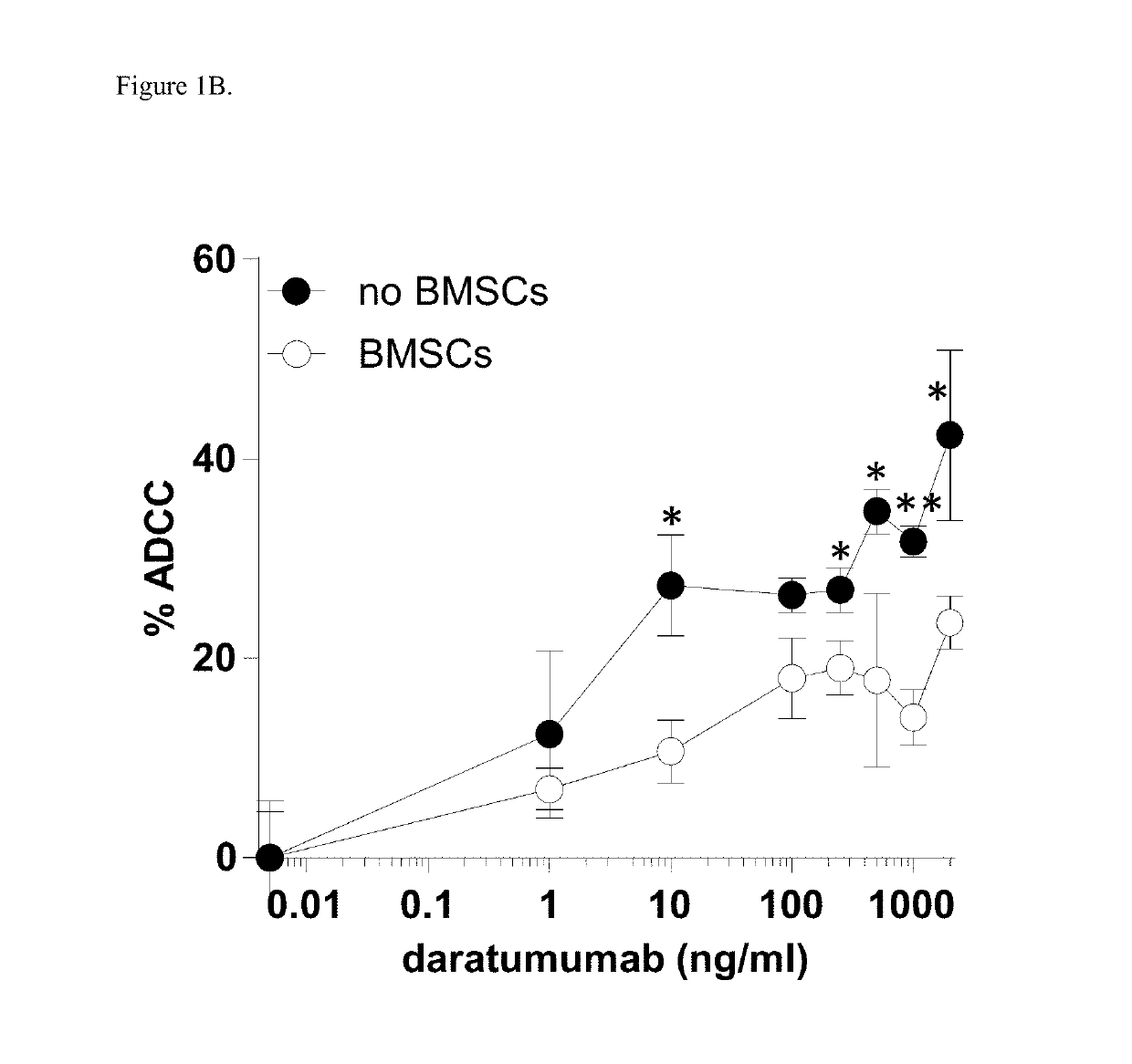
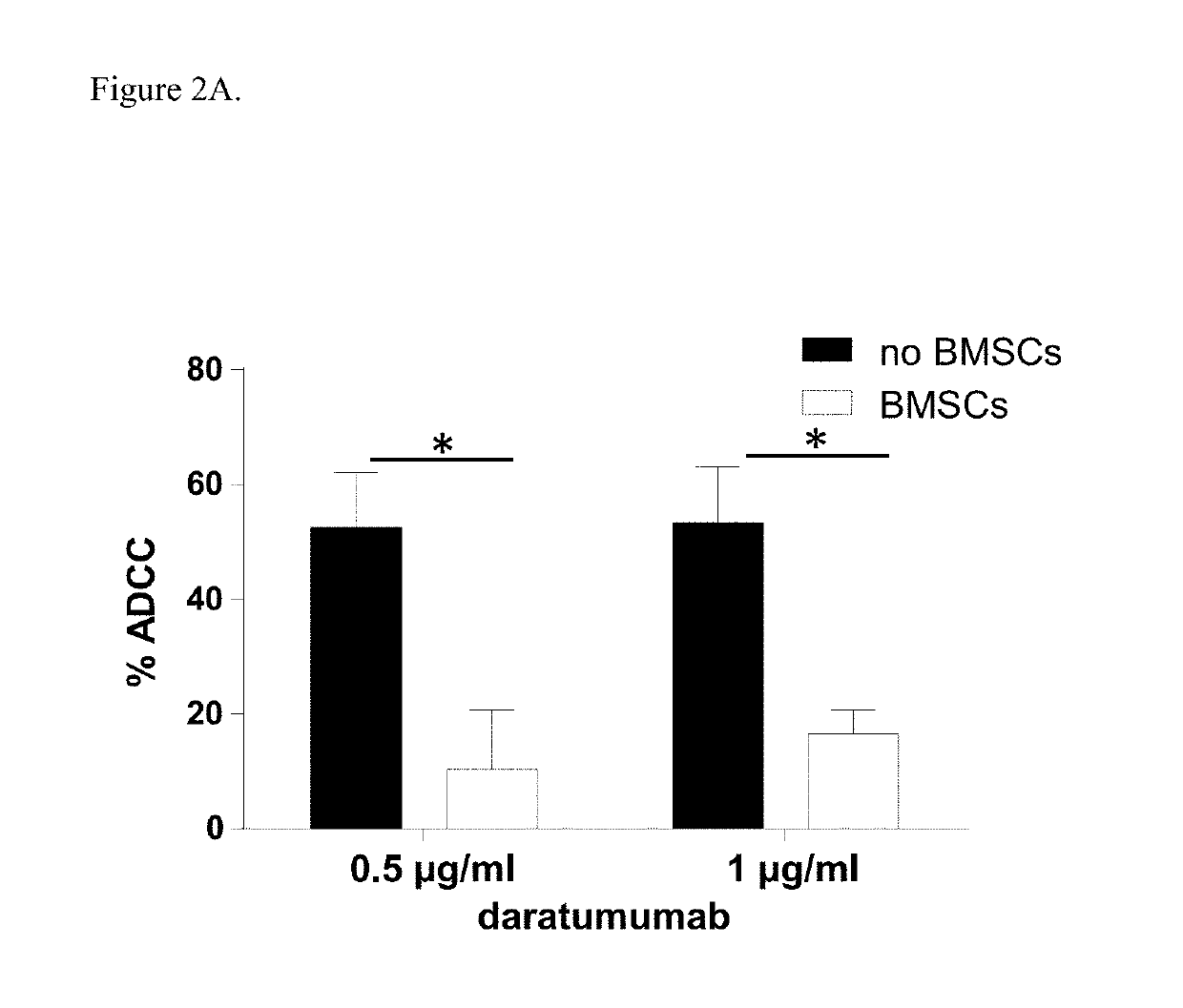
Combination therapies for heme malignancies with Anti-CD38 antibodies and survivin inhibitors
ActiveUS20160367663A1Ensure adequate treatmentOrganic active ingredientsPeptide/protein ingredientsSerum igeHeme
Owner:JANSSEN BIOTECH INC
Rainwater treatment process
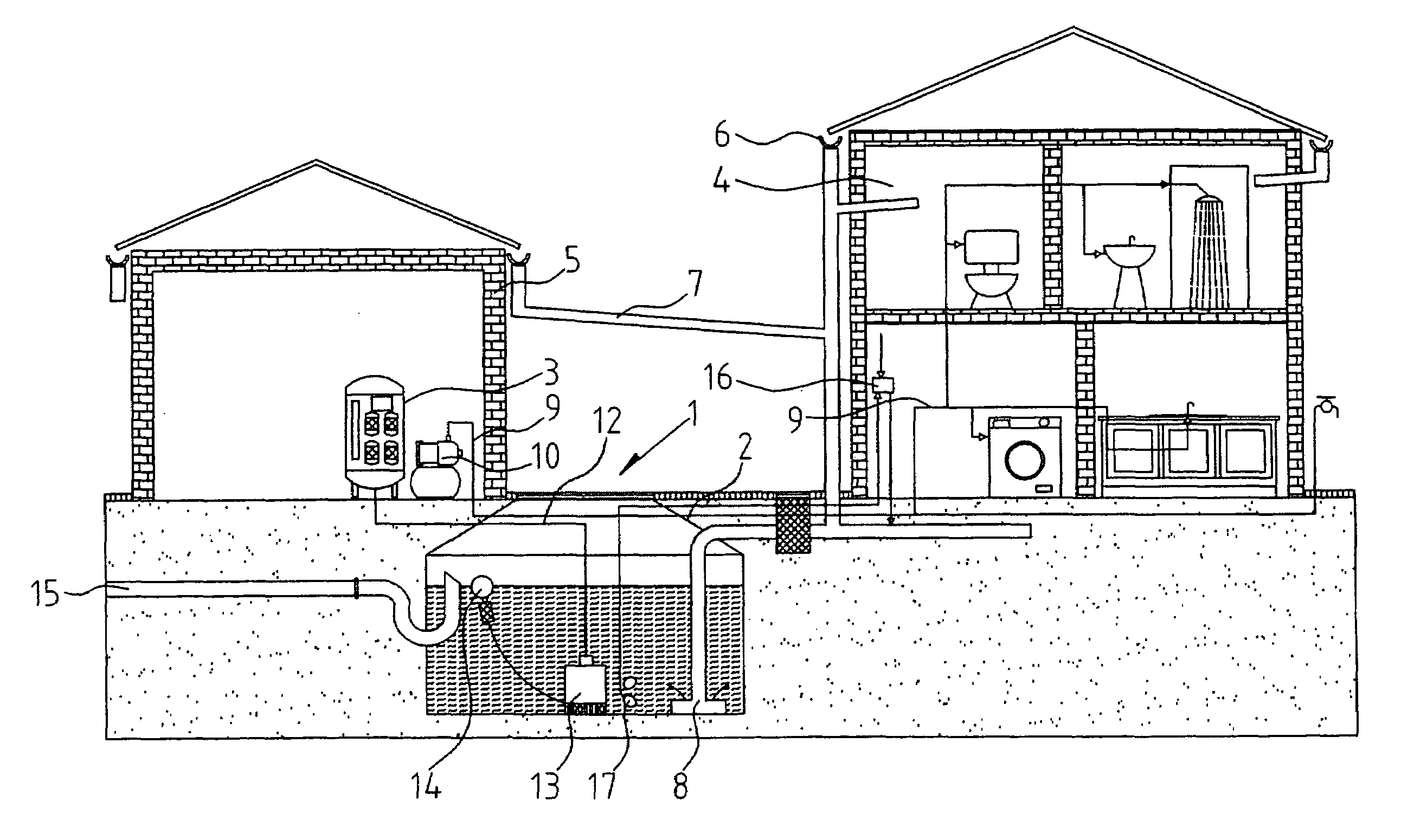
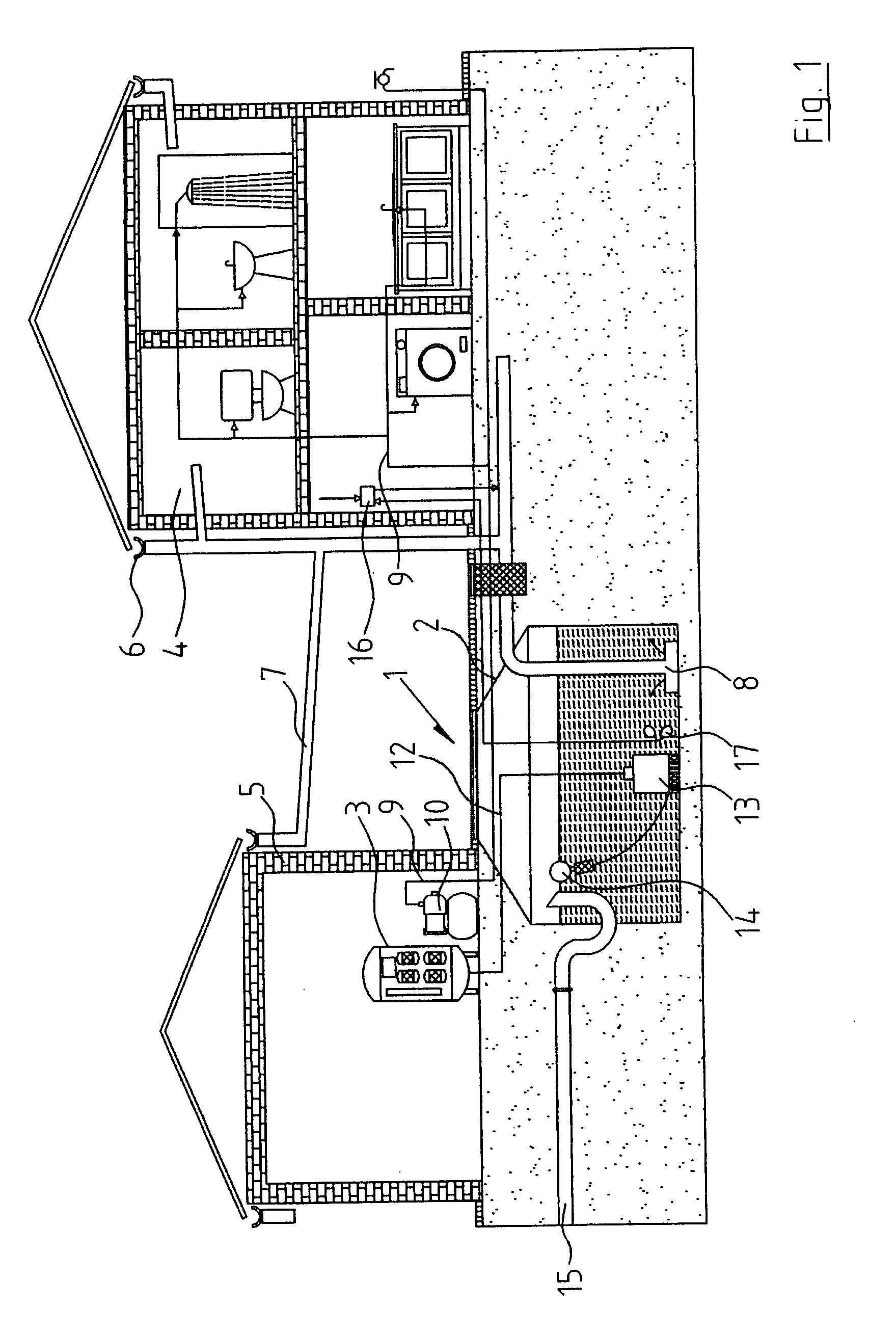
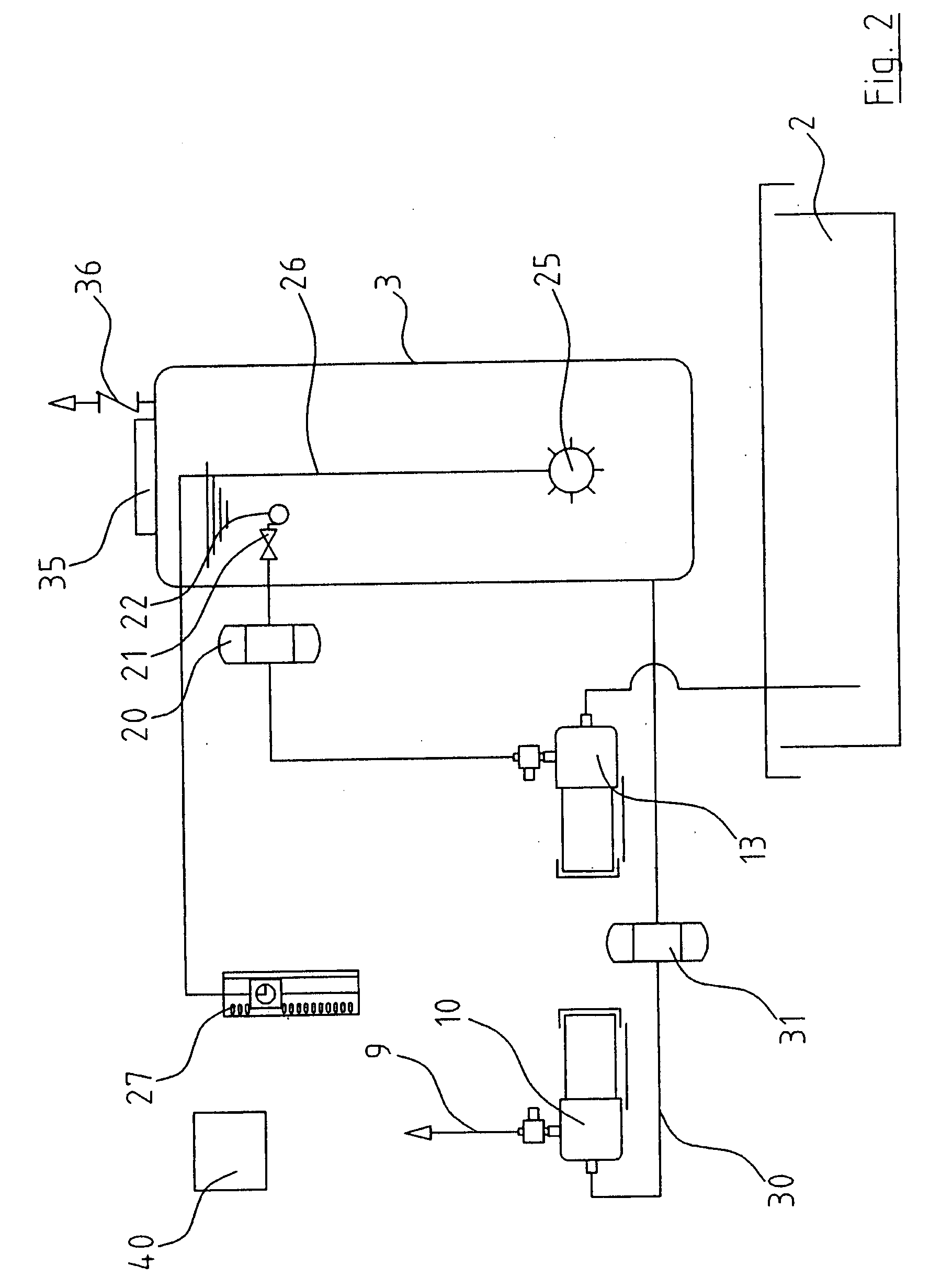
InactiveUS20080272064A1Maintain standardImprove the bactericidal effectWater treatment parameter controlTreatment involving filtrationEnvironmental engineeringEngineering
A rainwater treatment assembly (1) comprising a rainwater storage tank (2) and a treatment tank (3). Rainwater is delivered from the storage tank (3) where ozone is injected into it at regular time intervals. The ozone kills bacteria and oxidises transition metals.
Owner:LEONARD OWEN THOMAS
Treatment for multiple sclerosis
InactiveUS20090202527A1Improve the situationEnsure adequate treatmentNervous disorderPeptide/protein ingredientsDiseaseMedicine
Owner:BIOGEN MA INC
Therapeutic agents - II
InactiveUS20050209192A1Symptoms improvedEnsure adequate treatmentAntibacterial agentsOrganic active ingredientsChemical fractionChemical synthesis
The present invention relates generally to chemical agents useful in the treatment and prophylaxis of inflammatory conditions or in the amelioration of symptoms resulting from or facilitated by an inflammatory condition in a mammalian animal including human and primate, non-mammalian animal and avian species. More particularly, the present invention provides a chemical agent of the macrocyclic diterpene family obtaining from a member of the Euphorbiaceae family of plants or botanical or horticultural relatives thereof or derivatives or chemical analogues or chemically synthetic forms of the agents for use in the treatment or prophylaxis of an inflammatory condition or in the amelioration of symptoms resulting from or facilitated by an inflammatory condition in a mammal, animal or avian species. The present invention further contemplates a method for the prophylaxis or treatment of mammalian, animal or avian subjects for inflammatory conditions including chronic or transitory inflammatory conditions or for ameliorating. the symptoms of an inflammatory condition by the topical or systemic administration of a macrocyclic diterpene obtainable from a member of the Euphorbiaceae family or botanical or horticultural relatives thereof or a derivative, chemical analogue or chemically synthetic form of the agent. The chemical agent of the present invention may be in the form of a purified compound, mixture of compounds, a precursor form of one or more of the compounds capable of chemical transformation into a therapeutically active agent or be in the form of a chemical fraction, sub-fraction or preparation or extract of the plant.
Owner:PEPLIN RES
Aerosolized fosfomycin/aminoglycoside combination for the treatment of bacterial respiratory infections
InactiveUS7943118B2Reduce developmentIncreases the post antibiotic affect (PAE)Antibacterial agentsBiocideBacterial respiratory infectionTobramycin
Owner:GILEAD SCI INC
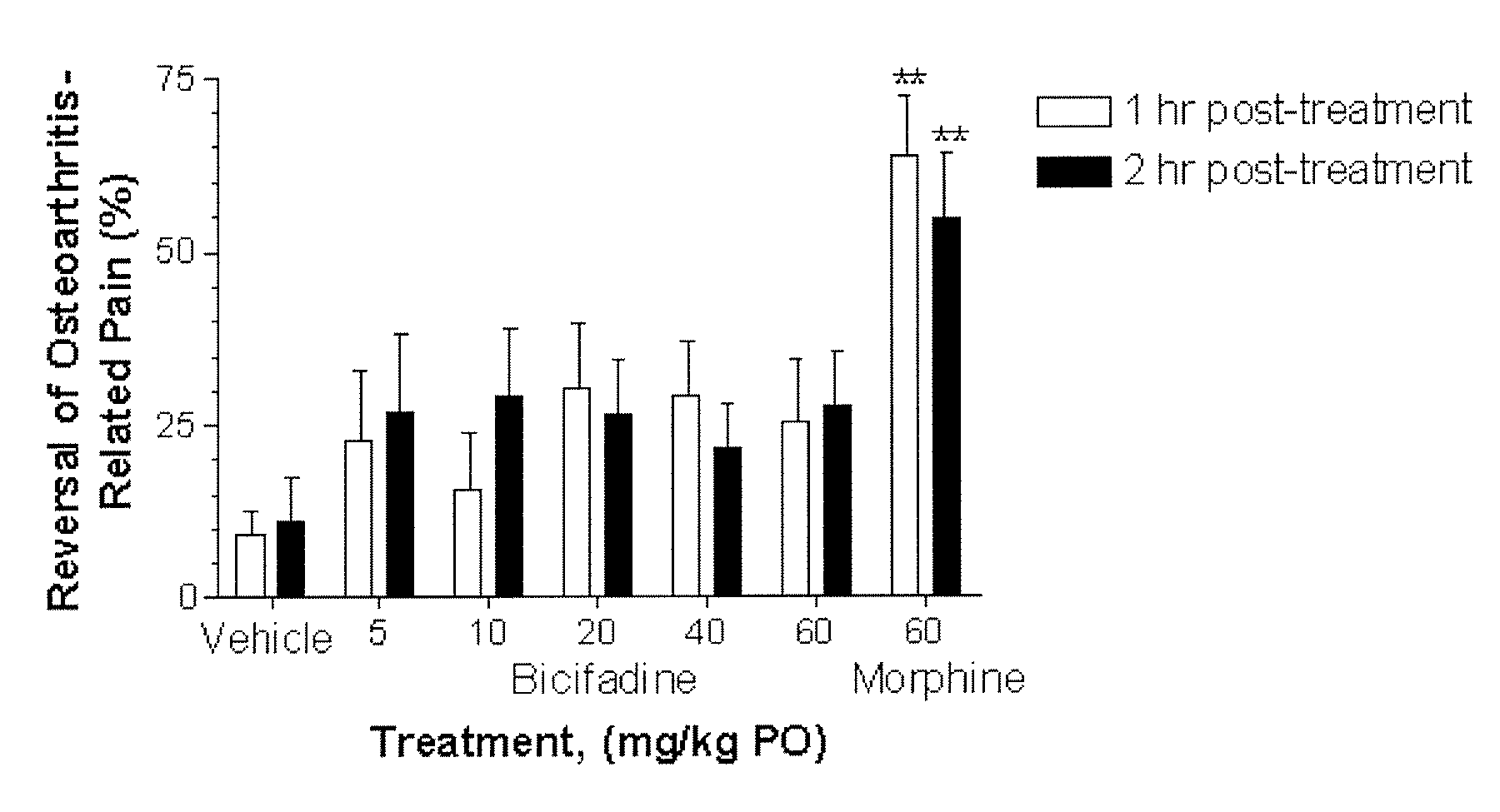
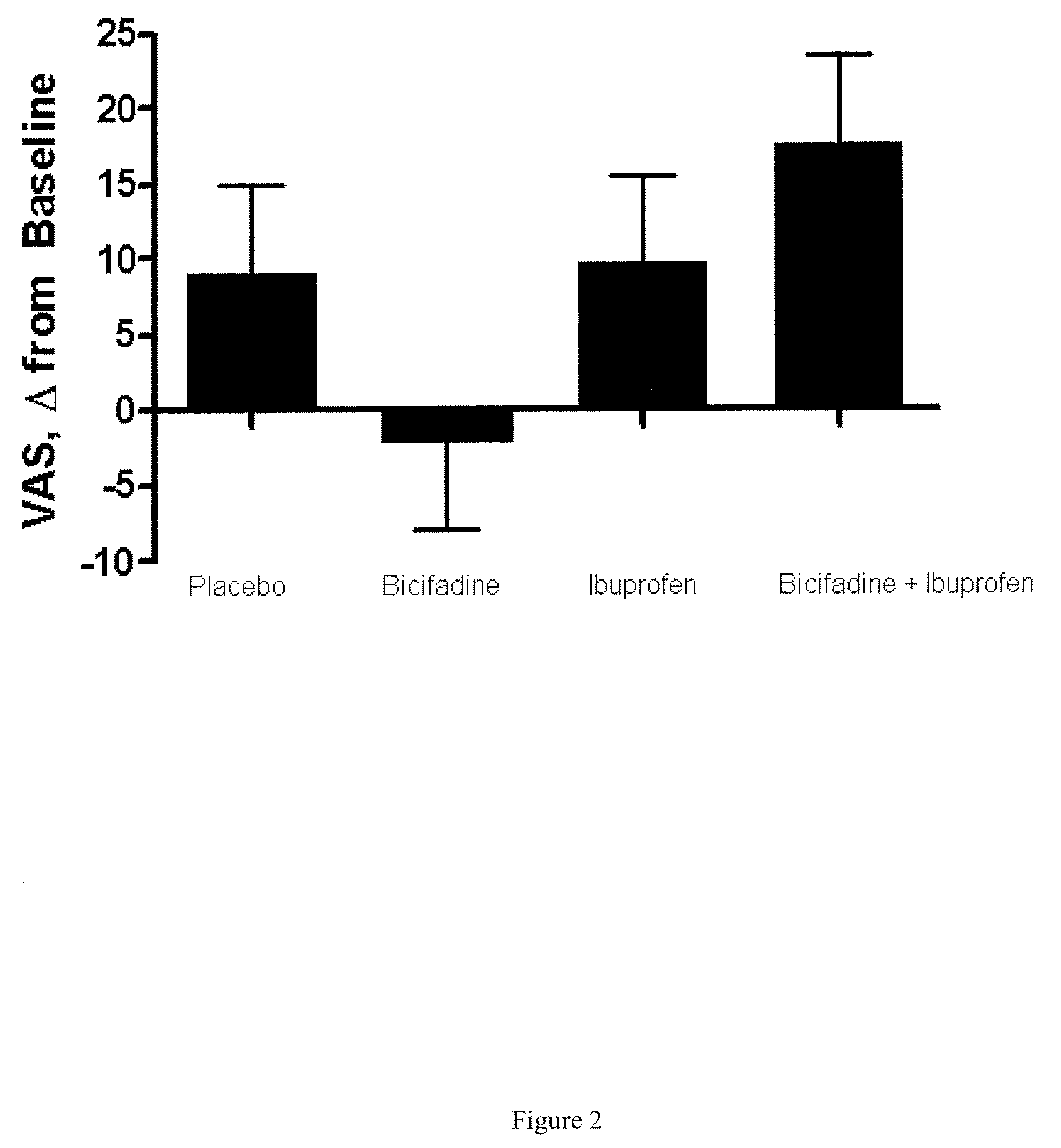
Compositions and Methods for Treatment of Chronic Pain Conditions
InactiveUS20080014272A1Effectively treated prophylacticallyEffectively therapeuticallyBiocidePowder deliveryBicifadineEnantiomer
The present invention relates to methods, pharmaceutical compositions and kits for treating osteoarthritis associated pain, inflammation and improving function in a patient comprising a first therapeutic agent which comprises bicifadine or a pharmaceutically acceptable salt, enantiomer, solvate, hydrate, polymorph or prodrug thereof and a second therapeutic agent which comprises a non-steroidal anti-inflammatory drug or derivative thereof.
Owner:DOV PHARMA
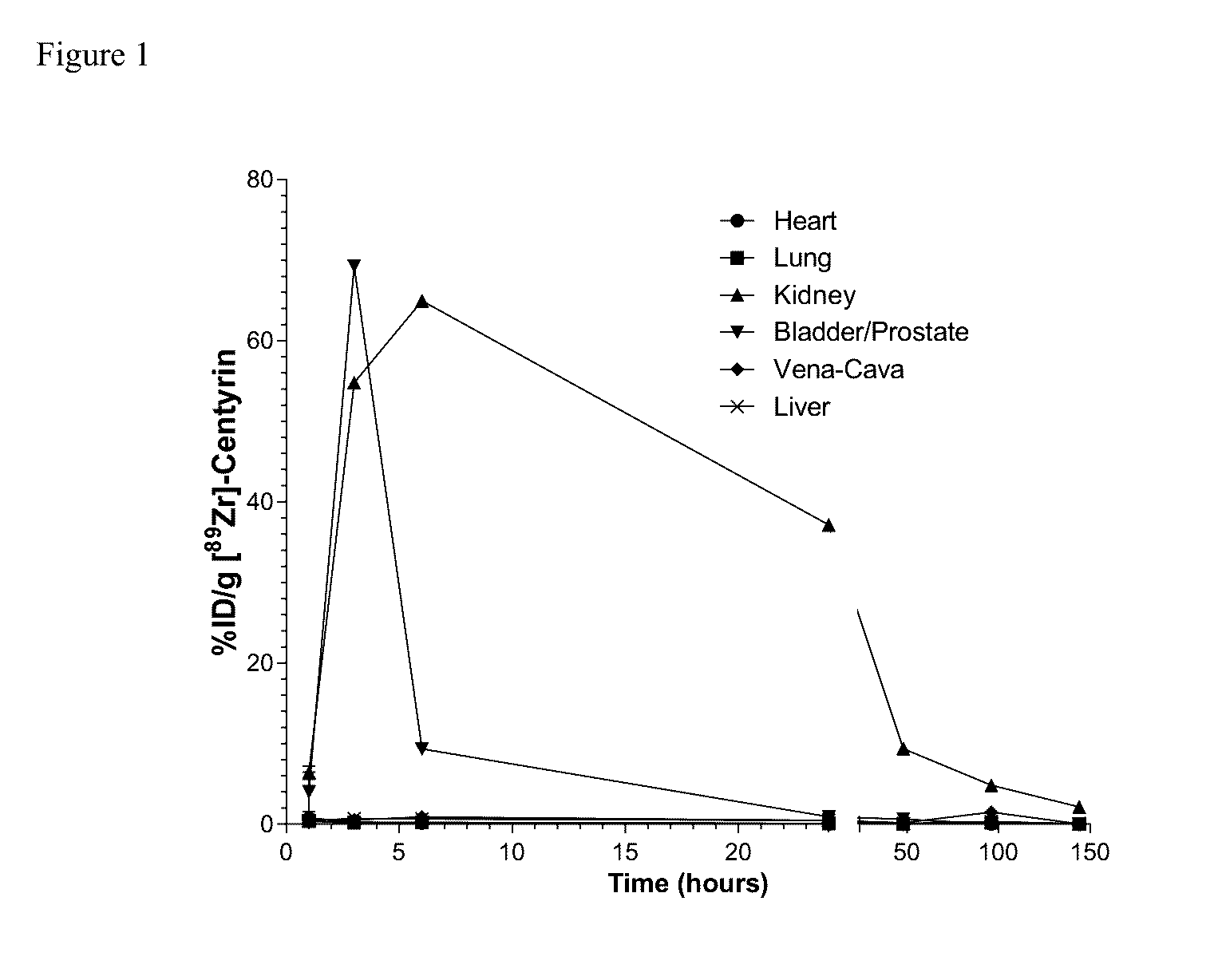
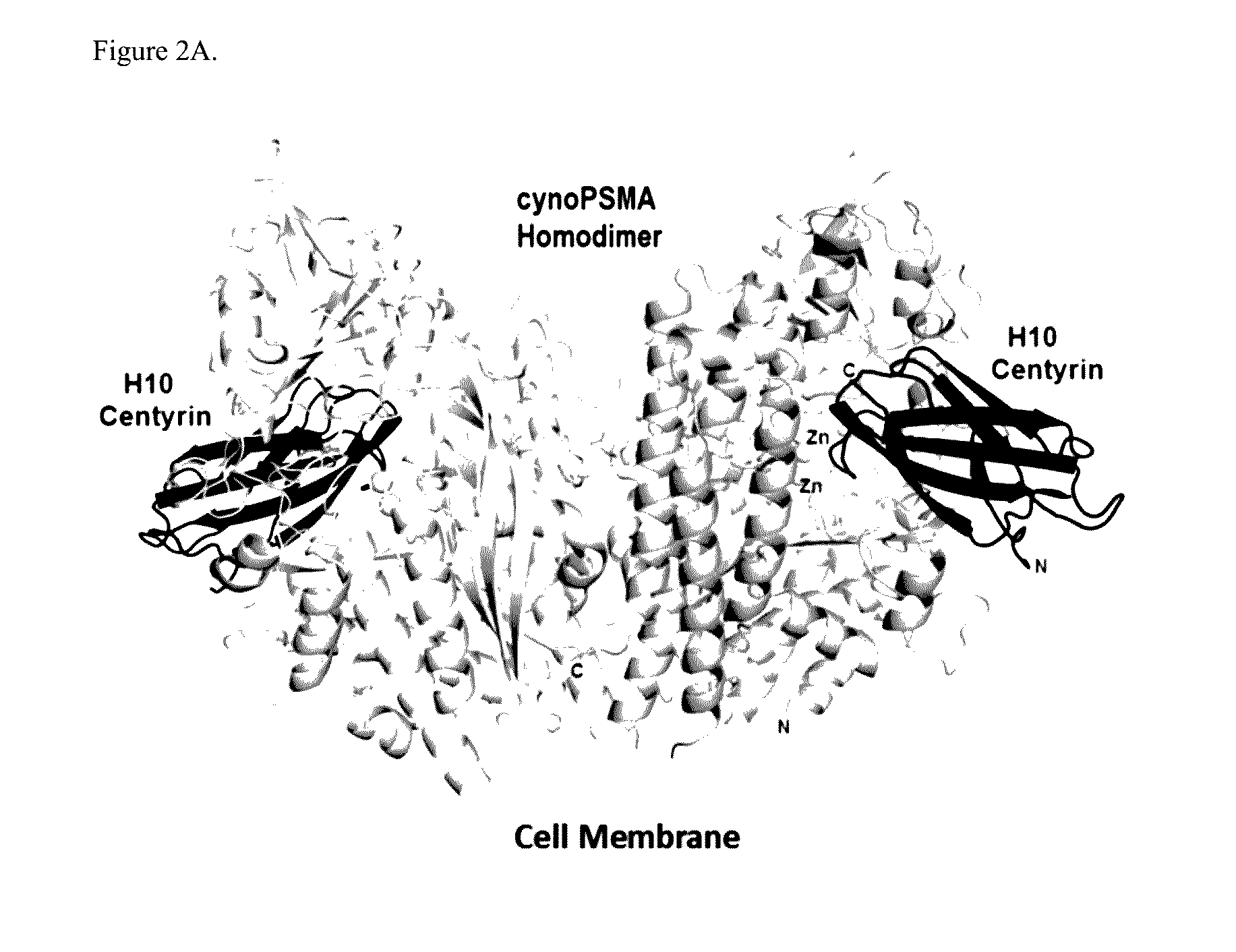
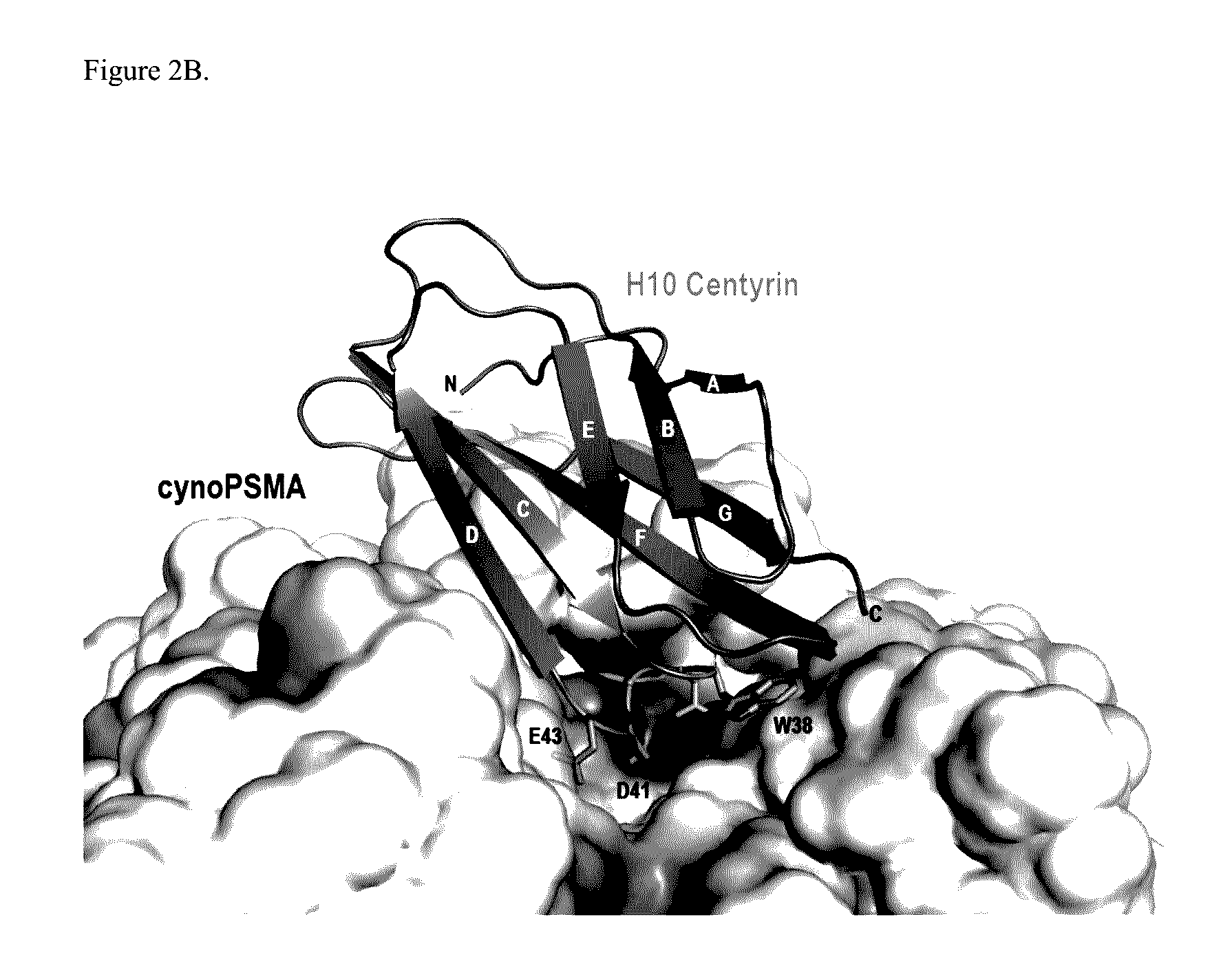
Prostate Specific Membrane Antigen Binding Fibronectin Type III Domains
ActiveUS20160326232A1Ensure adequate treatmentConnective tissue peptidesPeptide/protein ingredientsDiseaseProstate specific membrane
Owner:JANSSEN BIOTECH INC
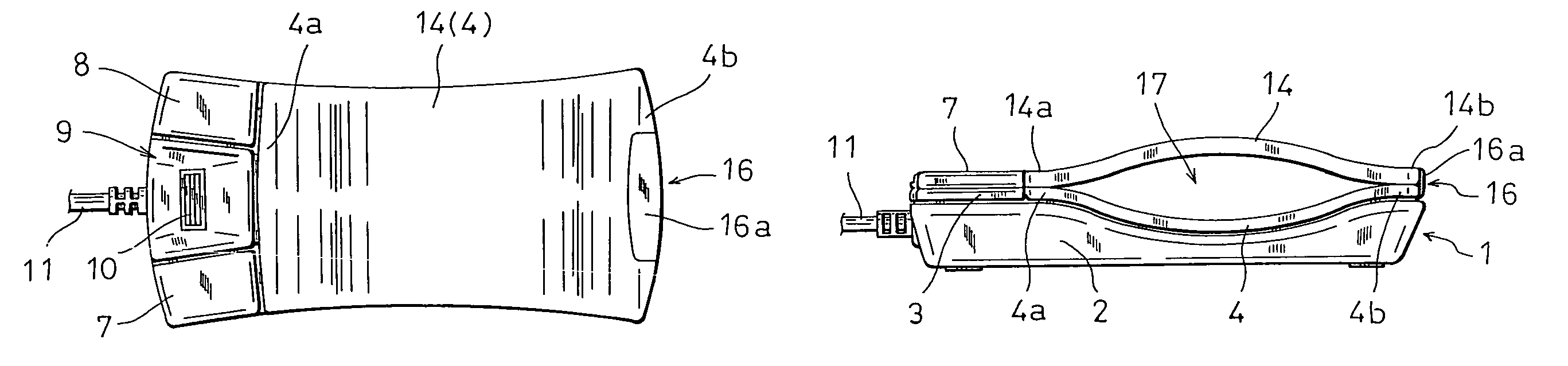
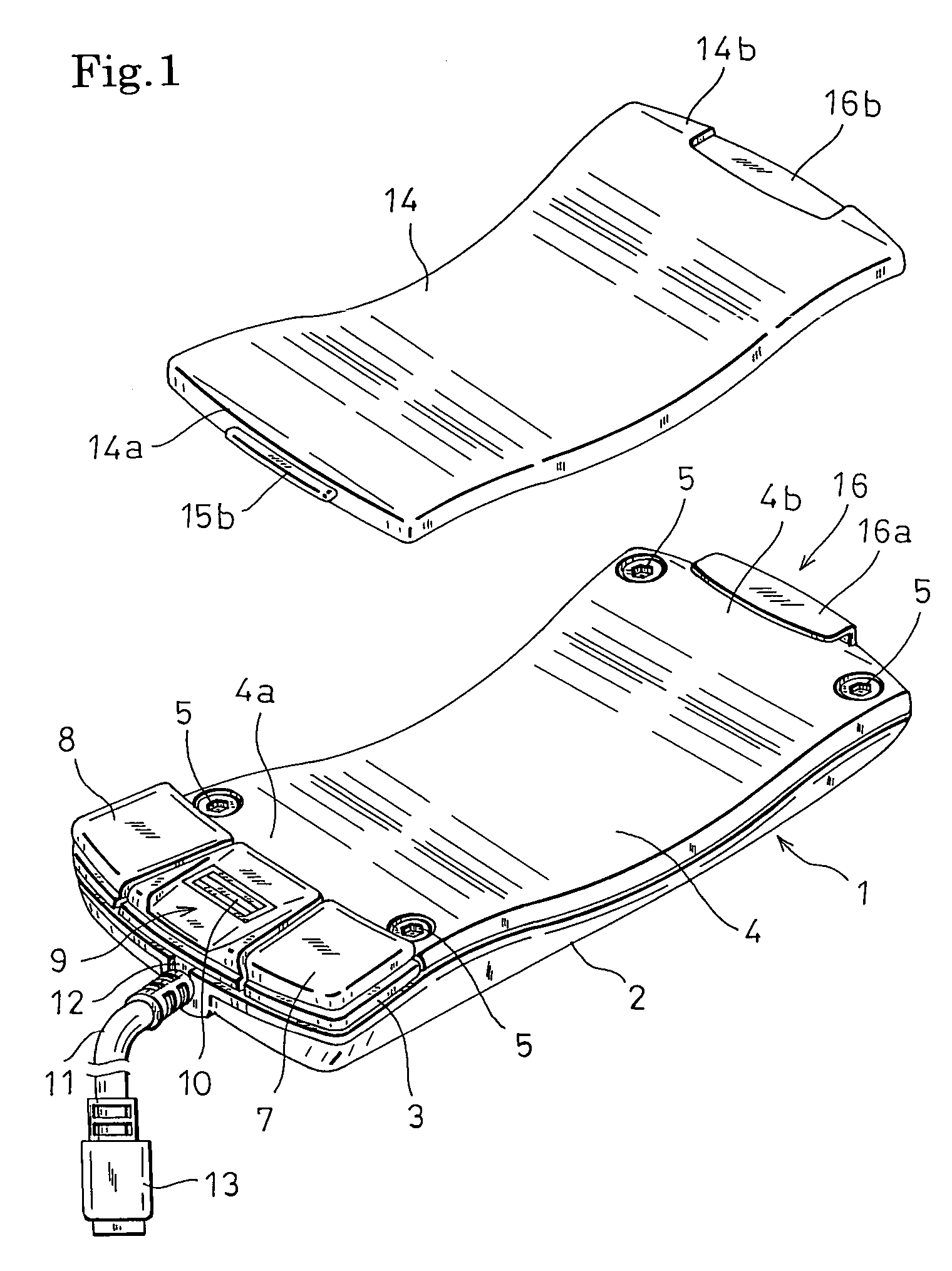
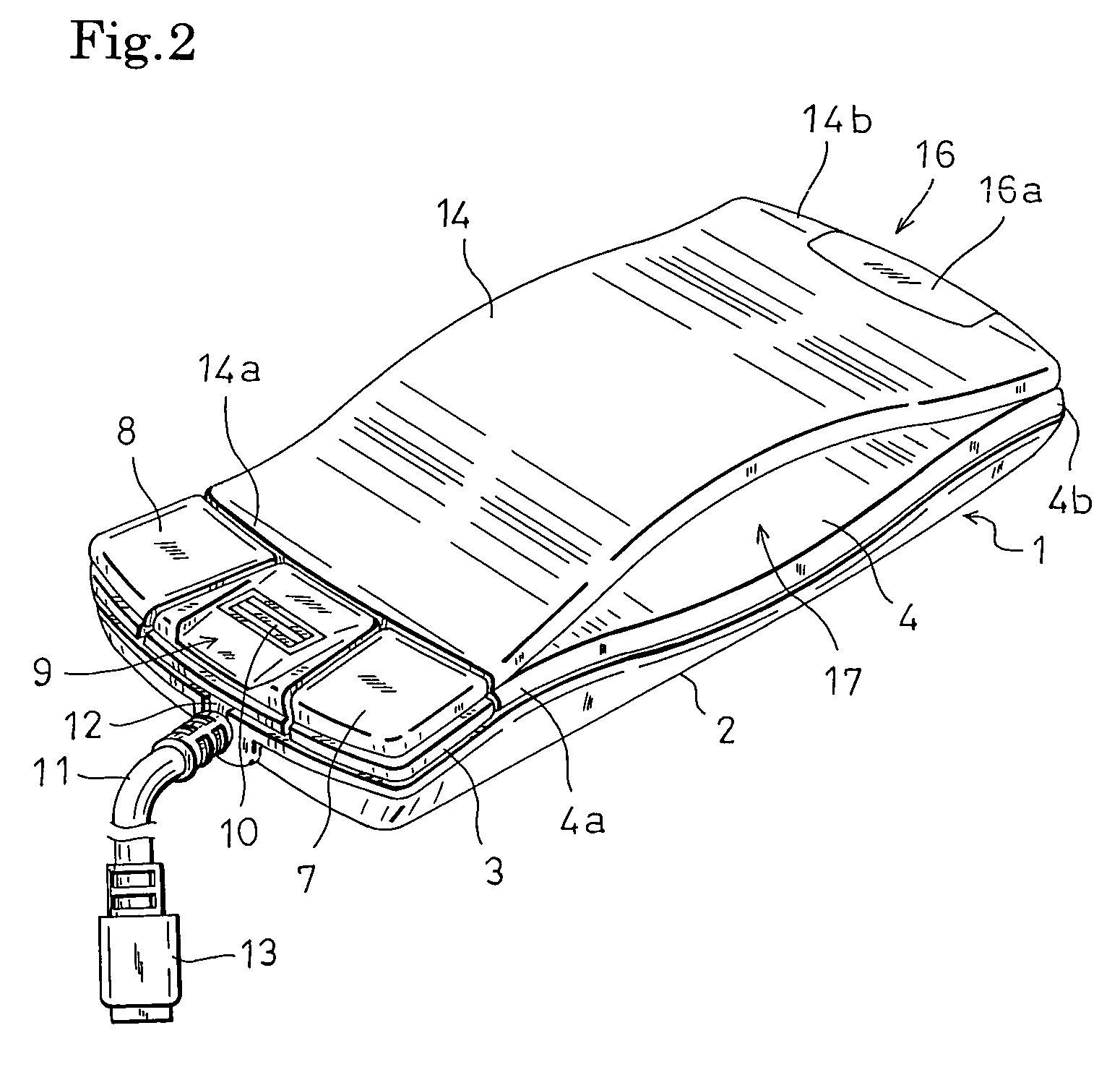
Computer mouse
InactiveUS7764270B2Improve the portability without impairing the operability of a mouseEasy to holdCathode-ray tube indicatorsInput/output processes for data processingComputer scienceComputer mouse
The invention relates to a computer mouse which is one kind of coordinate input devices for a computer, and more particularly to a computer mouse which is suitably used for a portable computer. The mouse has: a thin mouse case in which an upper face portion opposing a palm when the mouse is used is formed into a downward-concaved shape; and a plate-like member having a shape which extends along the downward-concaved upper face of the mouse case, and the plate-like member is attachable to the downward-concaved upper face of the mouse case in both an upward-convex direction and a downward-concave direction. When the mouse is to be used, the plate-like member is attached in the upward-convex manner to the downward-concaved upper face of the mouse case, and, when the mouse is to be carried, the plate-like member is attached in the downward-concave manner to the downward-concaved upper face of the mouse case, thereby realizing compaction of the mouse in which the original shape and size of the mouse are reproducible.
Owner:HOSIDEN CORP
Combination therapies for heme malignancies with anti-CD38 antibodies and survivin inhibitors
ActiveUS10668149B2Ensure adequate treatmentOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesHeme
Owner:JANSSEN BIOTECH INC
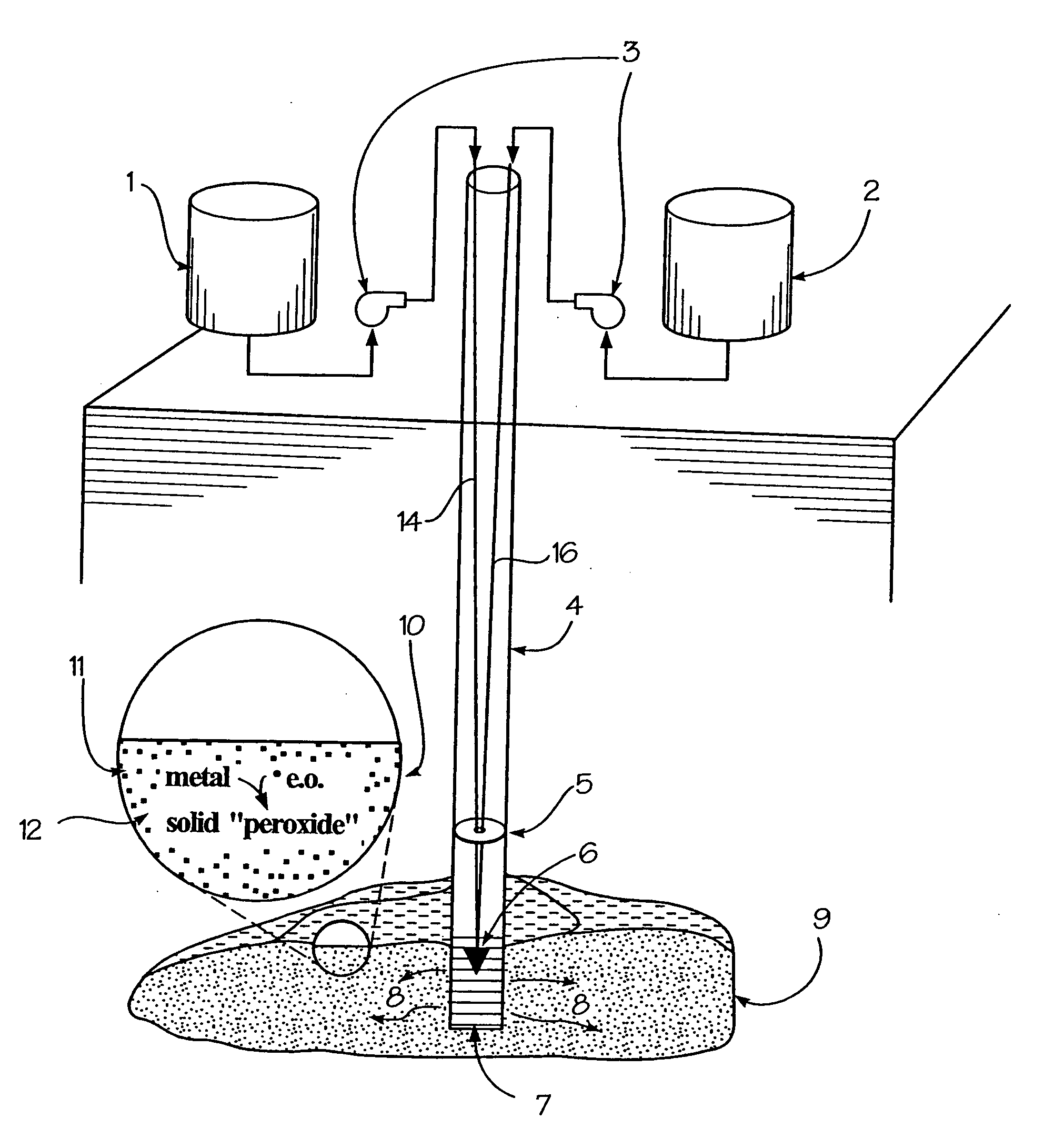
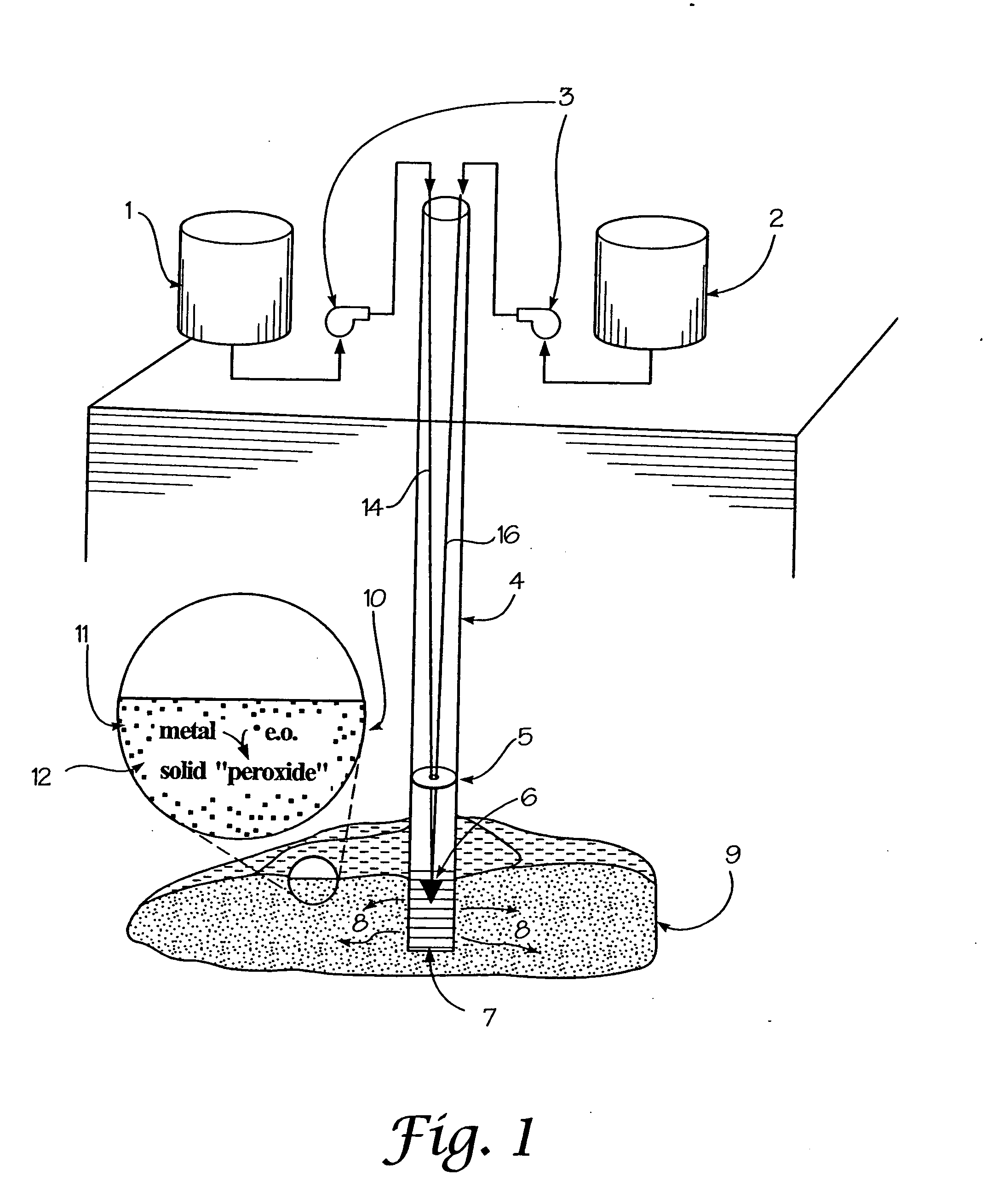
In-situ generation of oxygen-releasing metal peroxides
InactiveUS20050058512A1Ensure adequate treatmentEasy to useSolid waste disposalContaminated soil reclamationInjected materialAqueous solution
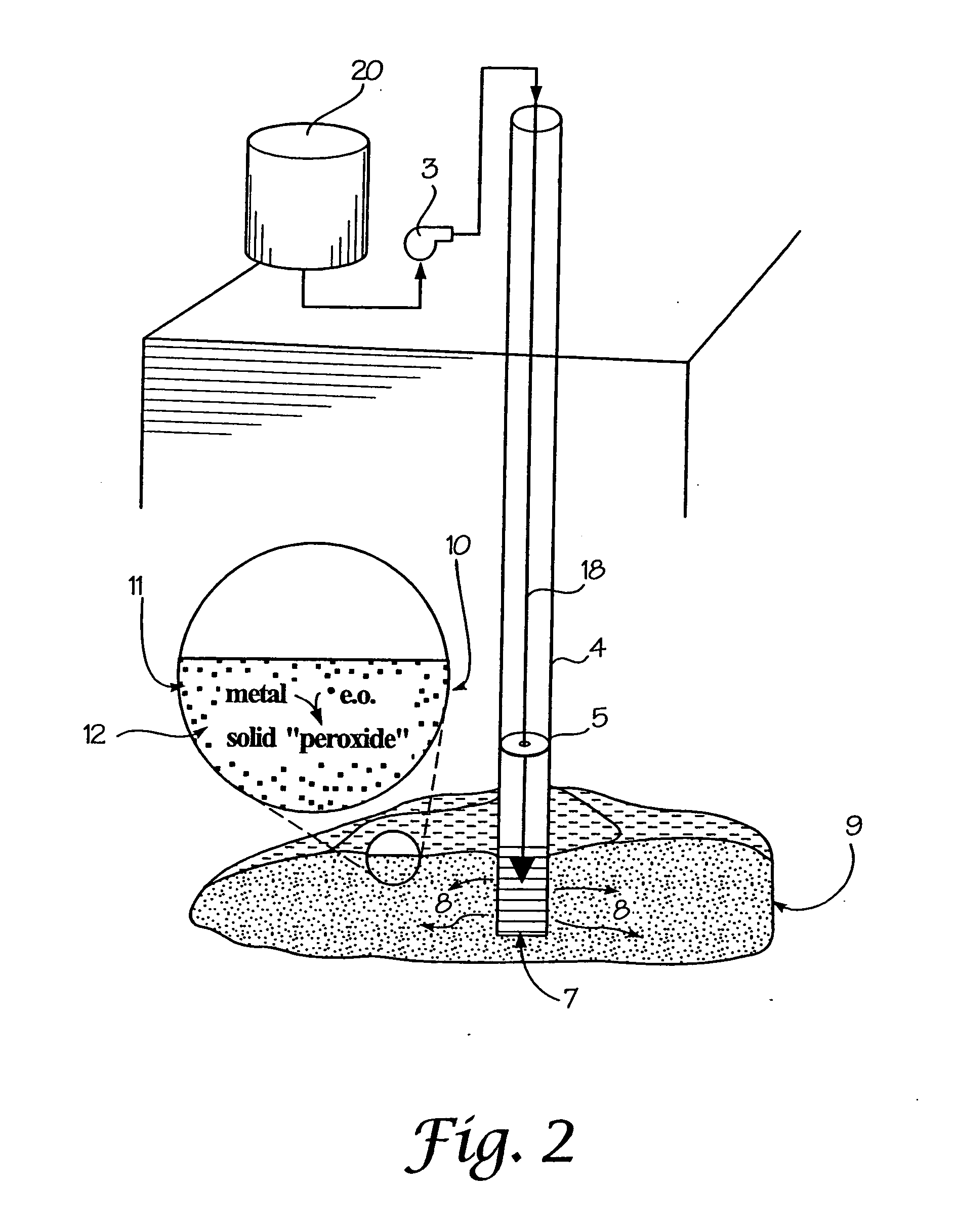
A method is provided for the remediation of contaminants in soil and groundwater. Via the injection of an aqueous energetic oxidant solution containing free radicals, oxidative conditions can be created within or ahead of a contaminant plume. Some contaminants may be remediated directly by reaction with the free radicals. Additionally and more importantly, the free radicals create an oxidative condition whereby native or injected materials, especially metals, are converted to peroxides. These peroxides provide a long-term oxygen reservoir, releasing oxygen relatively slowly over time. The oxygen can enhance microbial metabolism to remediate contaminants, can react with contaminant metals either to form immobile precipitants or to mobilize other metals to permit remediation through leaching techniques. Various injection strategies for injecting the energetic oxidant solution are also disclosed.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
Installation device
InactiveUS20030164394A1Shorten the timeEasy to cleanPrinted circuit assemblingWelding/cutting auxillary devicesMechanical engineering
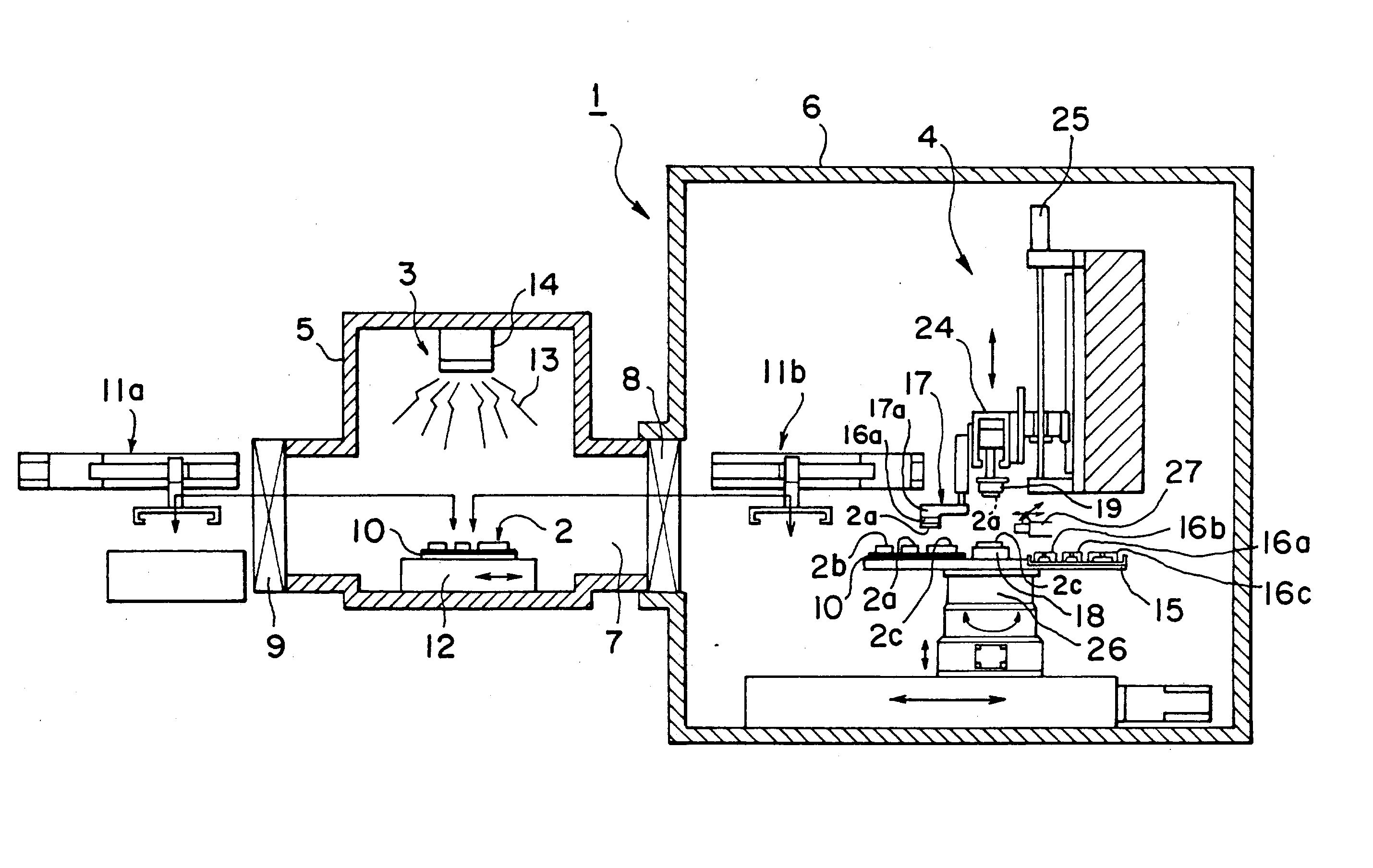
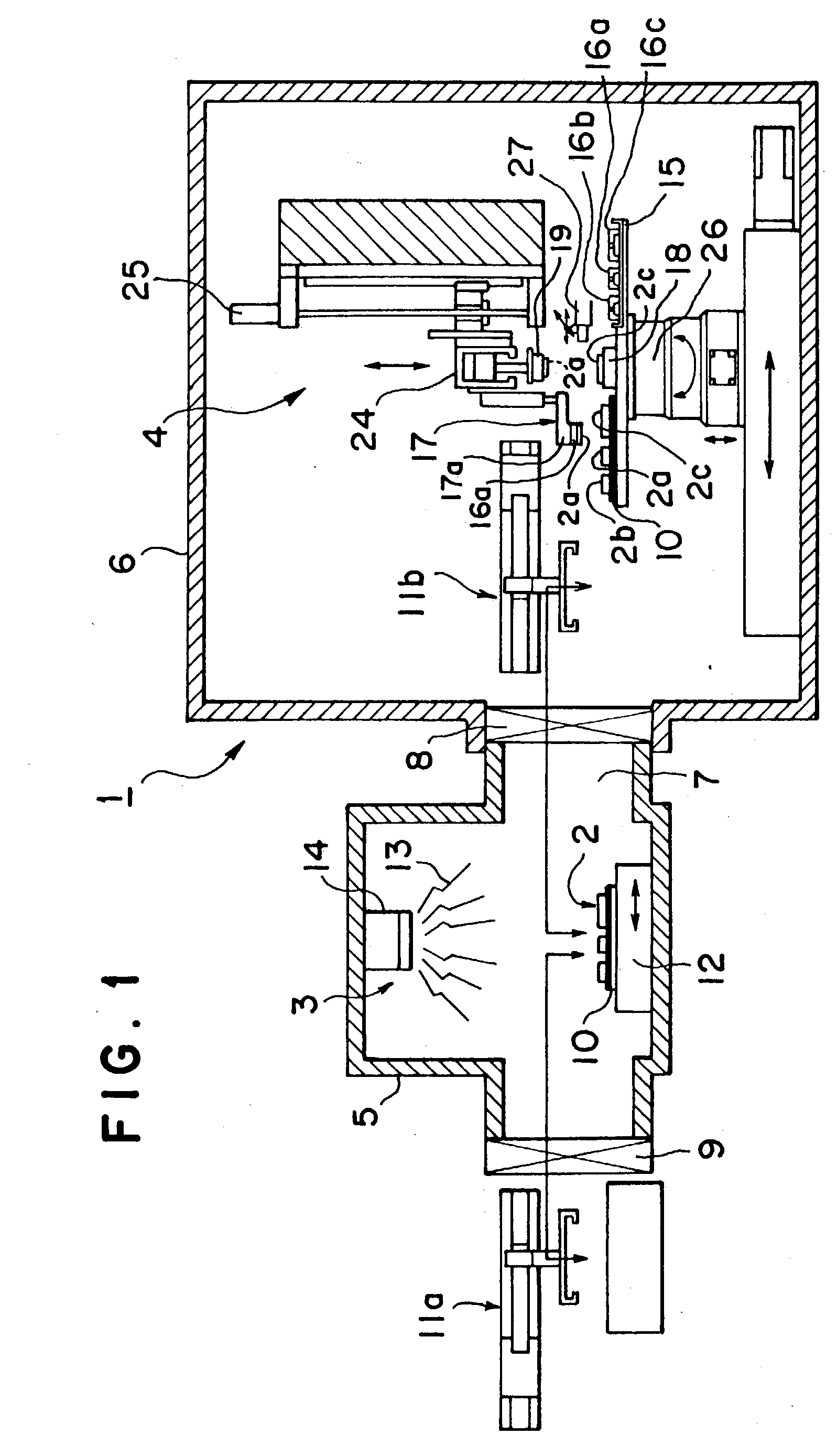
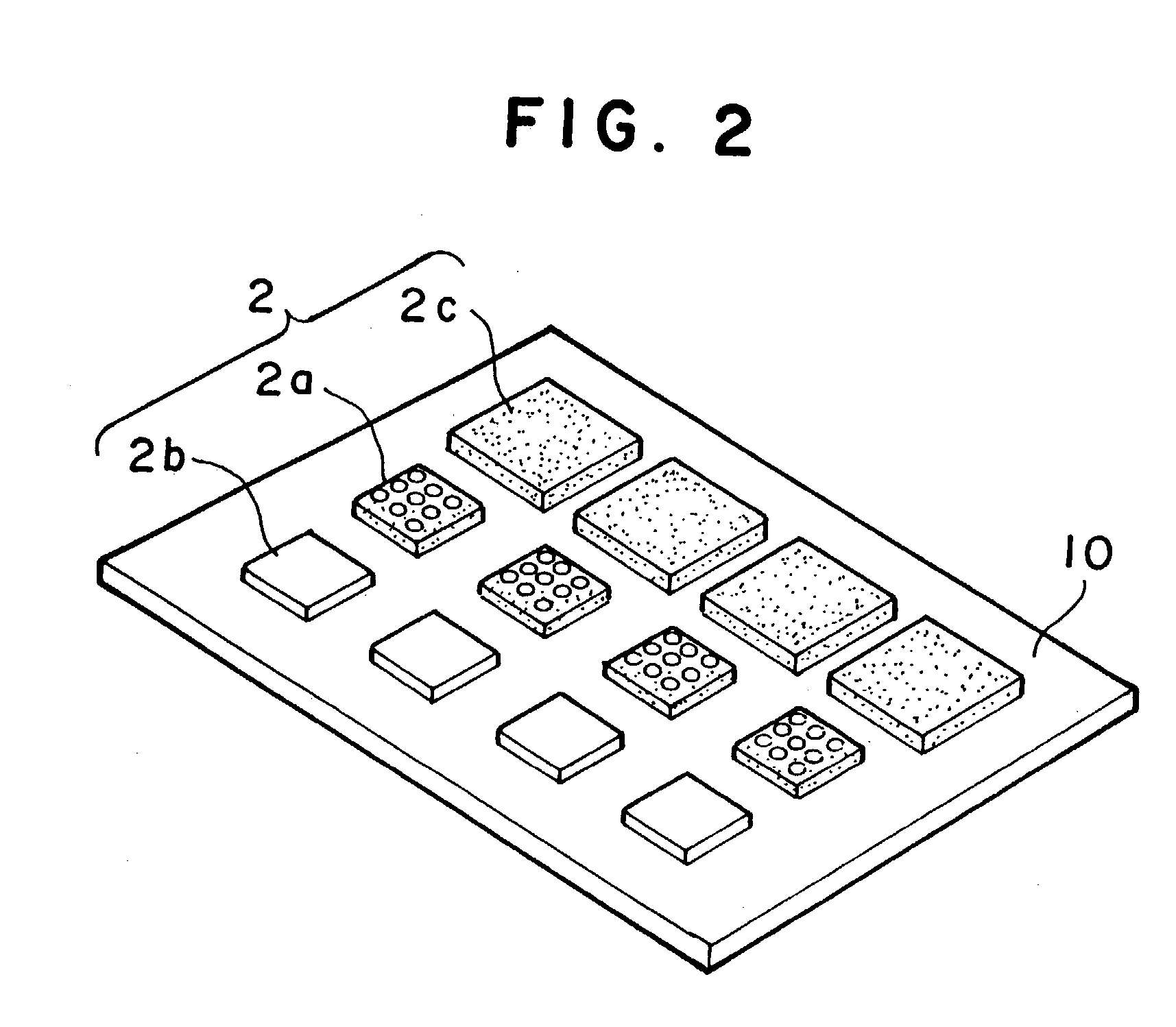
A mounting apparatus for bonding objects to each other, comprising a cleaning part (3) for cleaning at least the bonding surfaces of first objects (2a, 2b) and a bonding part (4) for bonding the cleaned first objects (2a, 2b) to a second object (2c), wherein both parts are connected to each other so that the objects can be conveyed between both parts, and an inverting mechanism (17) for turning over the first objects (2a, 2b) without touching the cleaned bonding surfaces is provided in the bonding part, whereby the cleaning of the bonding surfaces can be carried out efficiently, and a total time for a series of operations ranging from the cleaning to the completion of bonding can be shortened remarkably.
Owner:TORAY ENG CO LTD +1
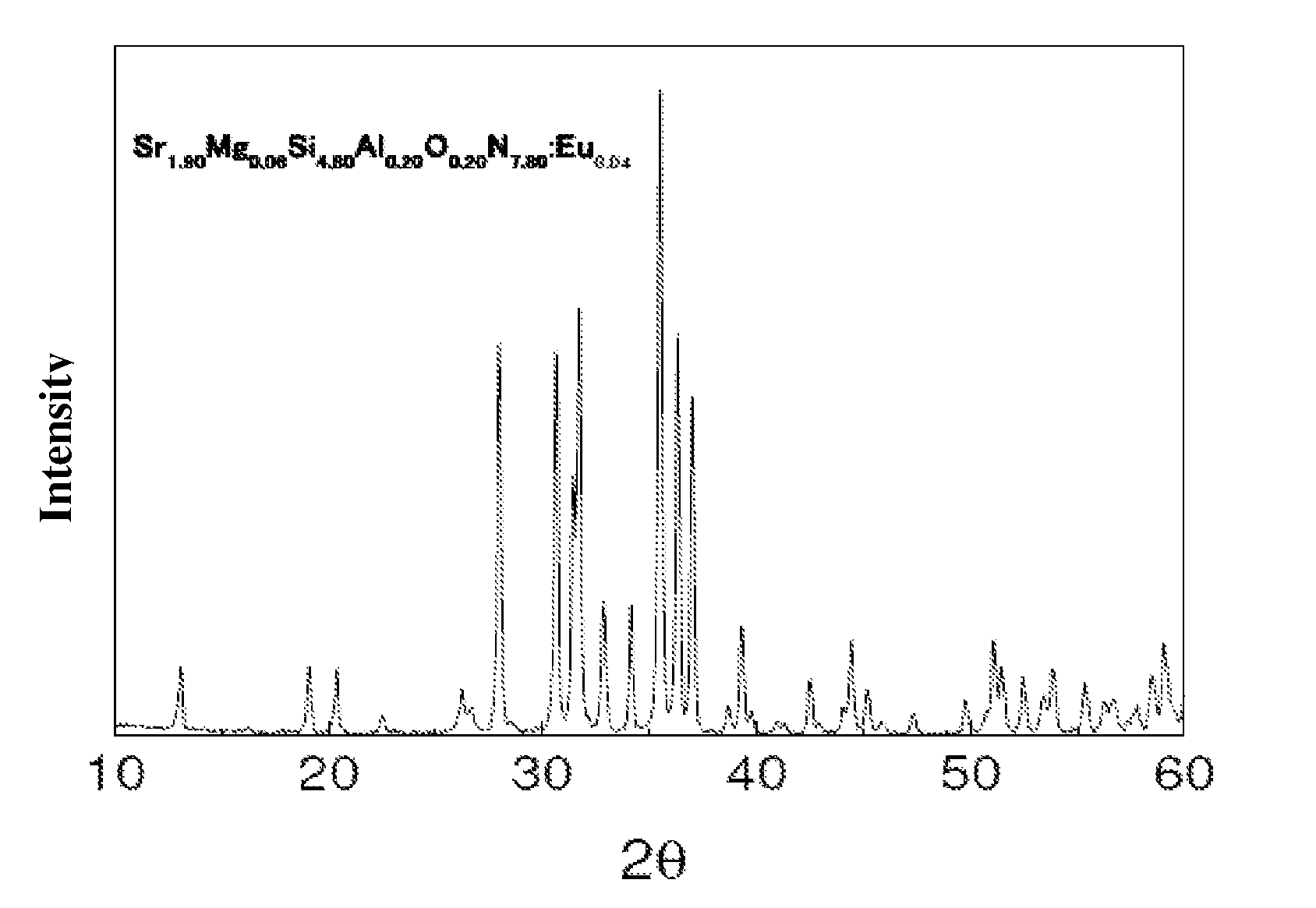
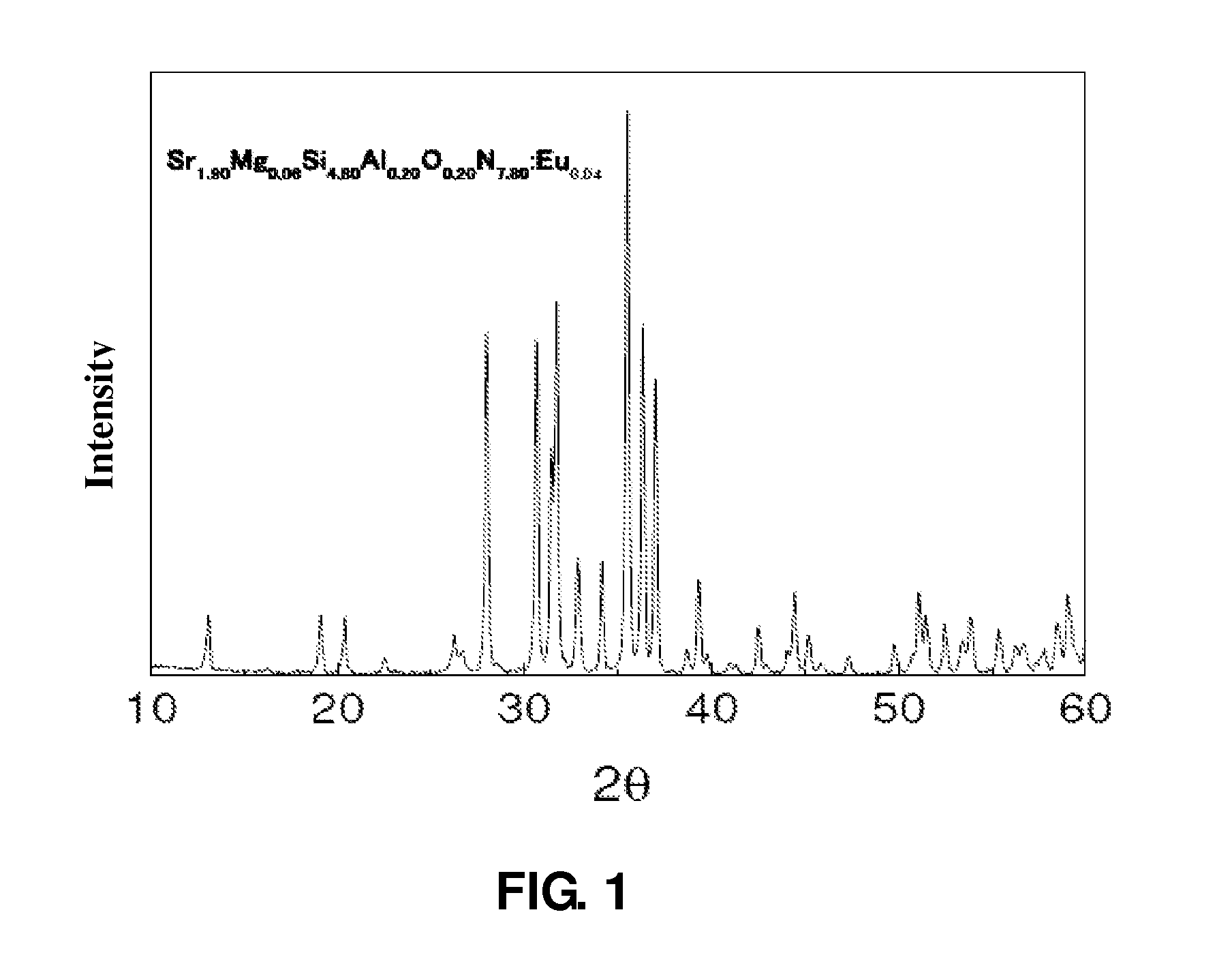
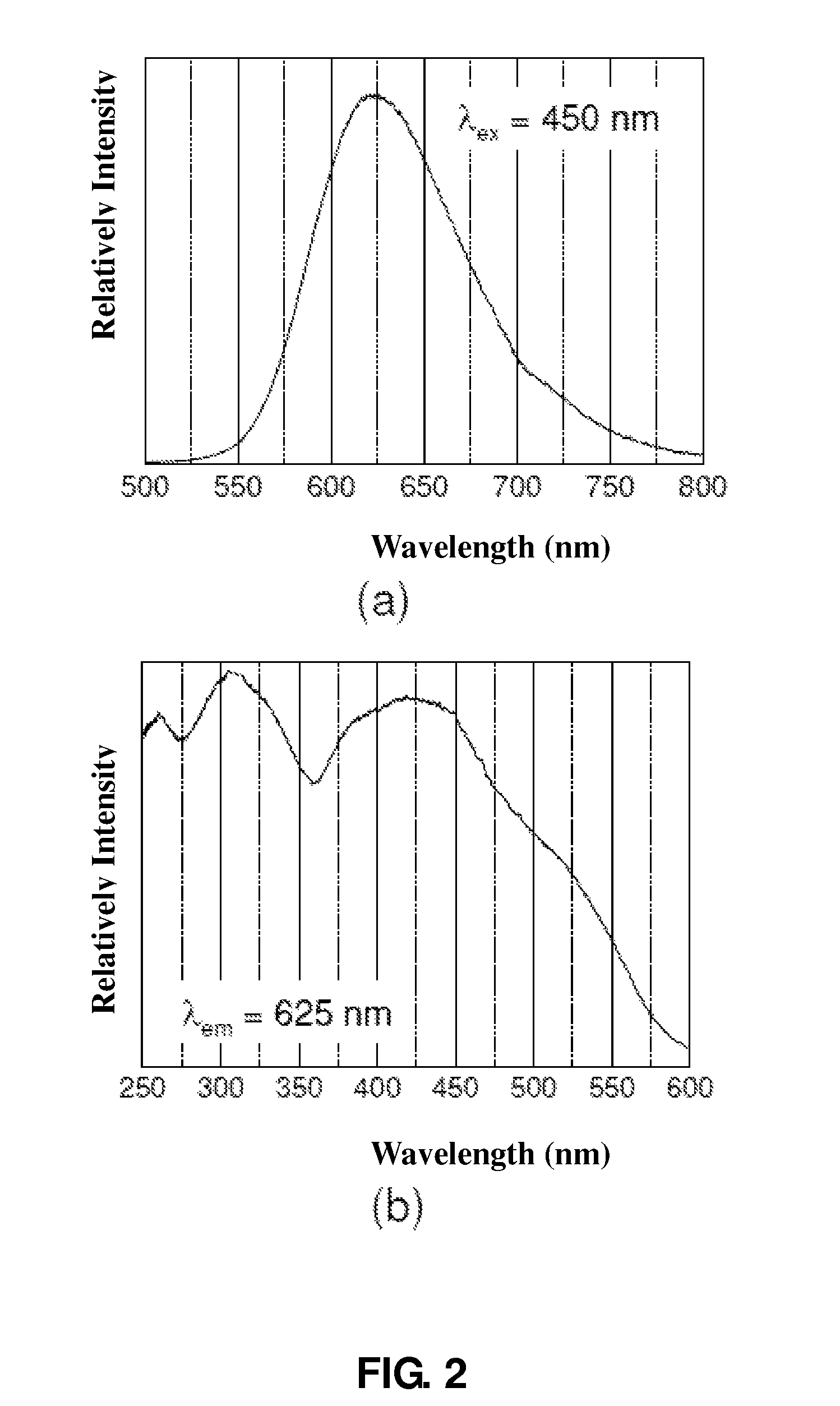
Oxynitride luminescent material, preparation method and its applications
ActiveUS8222805B2Easy to operateLow costDischarge tube luminescnet screensElectroluminescent light sourcesLength waveNear ultraviolet
Owner:BEIJING YUJI SCI & TECH
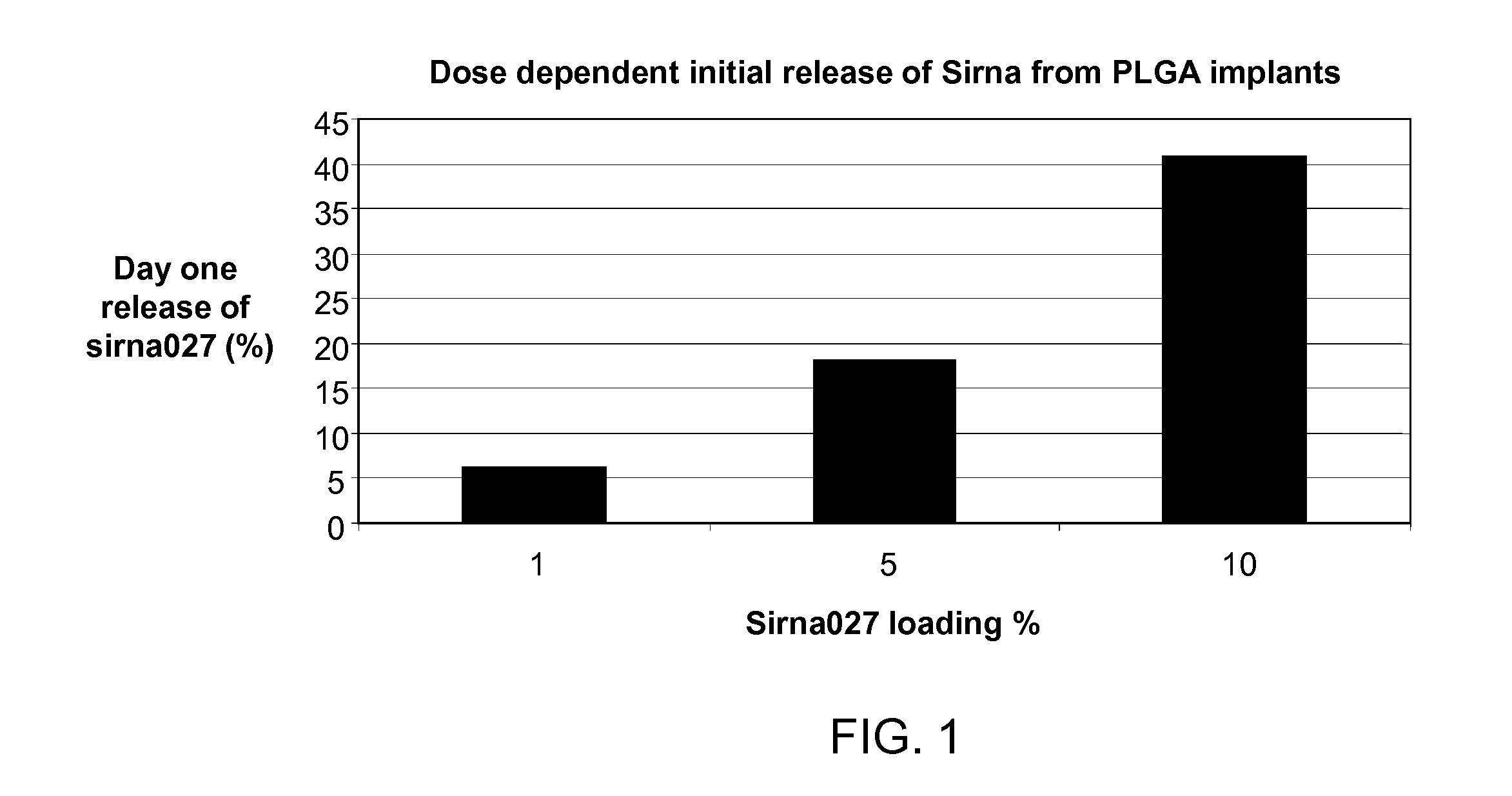
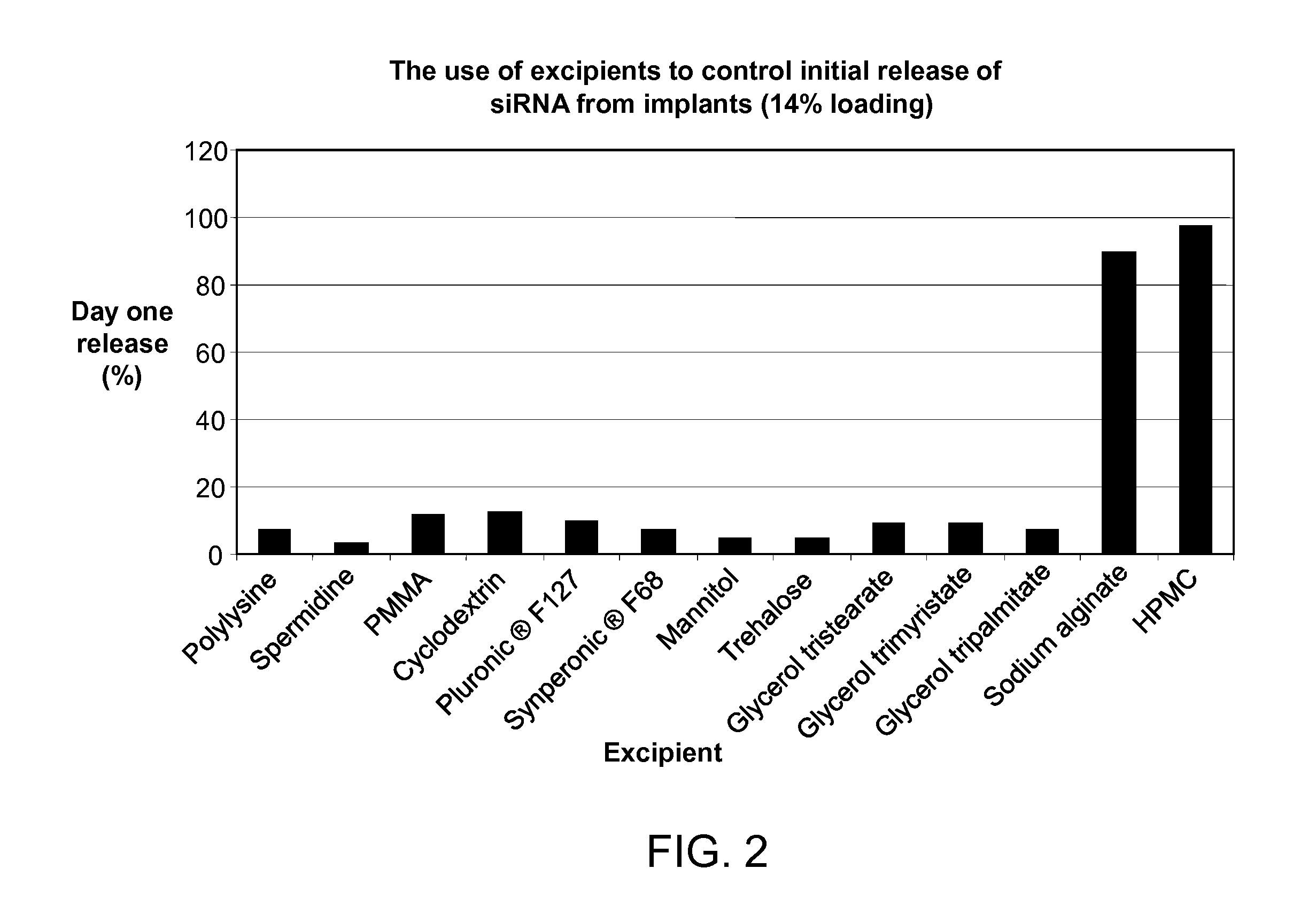
METHOD OF CONTROLLING INITIAL DRUG RELEASE OF siRNA FROM SUSTAINED-RELEASE IMPLANTS
InactiveUS20120022137A1Ensure adequate treatmentOrganic active ingredientsSenses disorderDiseaseOphthalmology
The present invention provides an intraocular implant comprising siRNA combined with a excipient effective to retard the initial release of the siRNA from an implant, wherein said siRNA and excipient is associated with a biocompatible polymer (e.g., a polymeric matrix), configured to release said siRNA into the eye of a patient at therapeutic levels for a time sufficient to treat an ocular condition or disease.
Owner:ALLERGAN INC
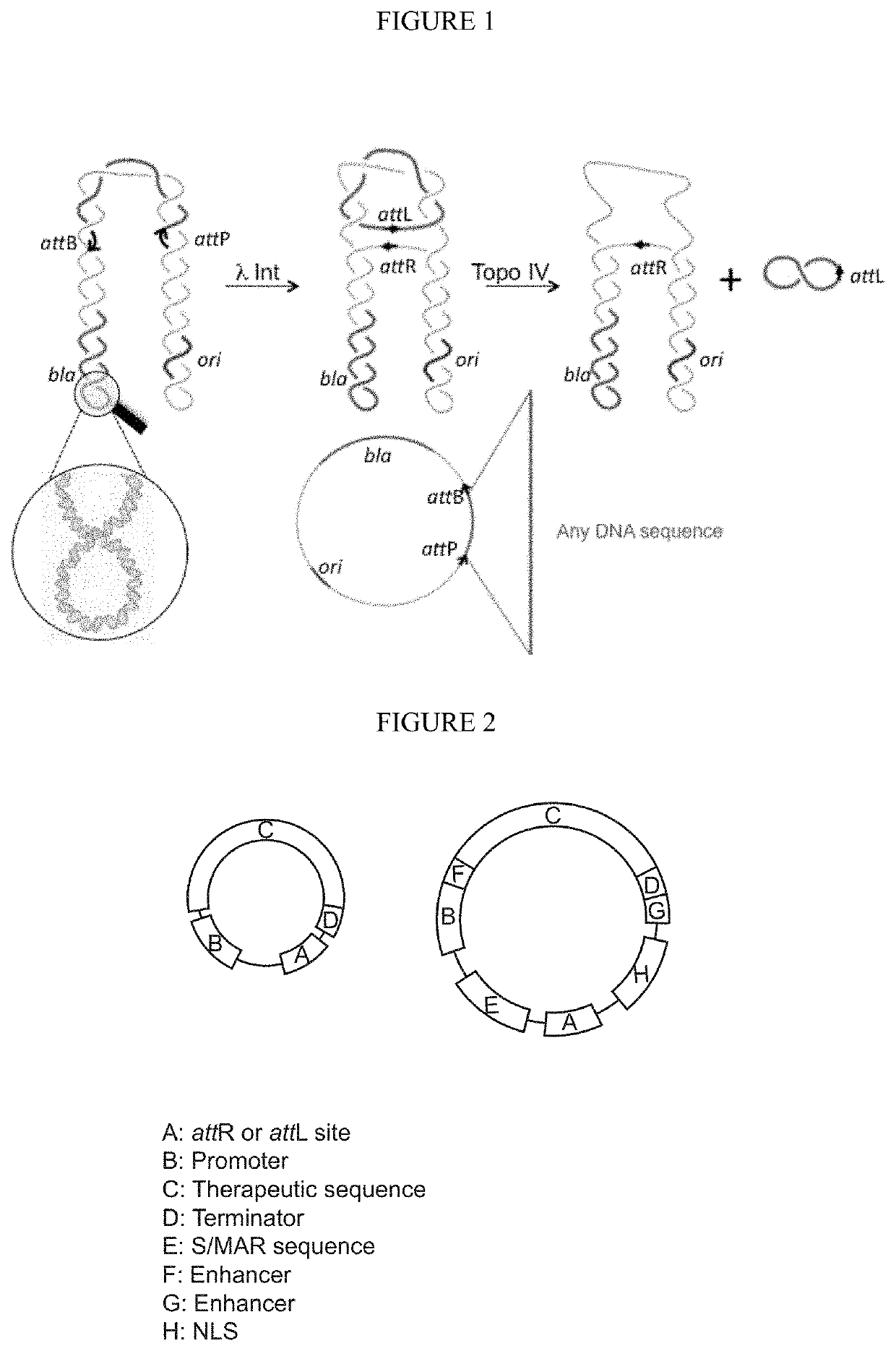
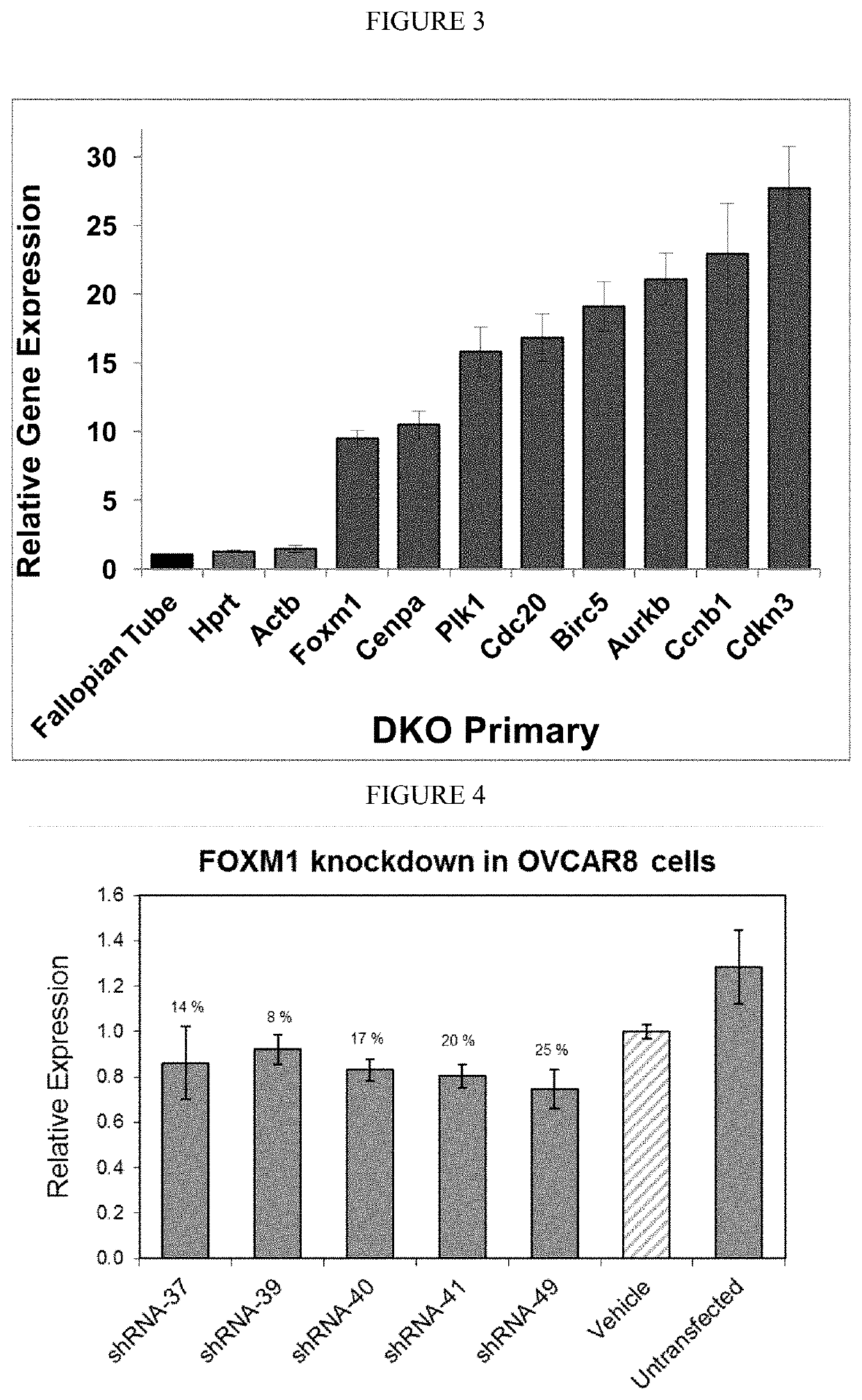
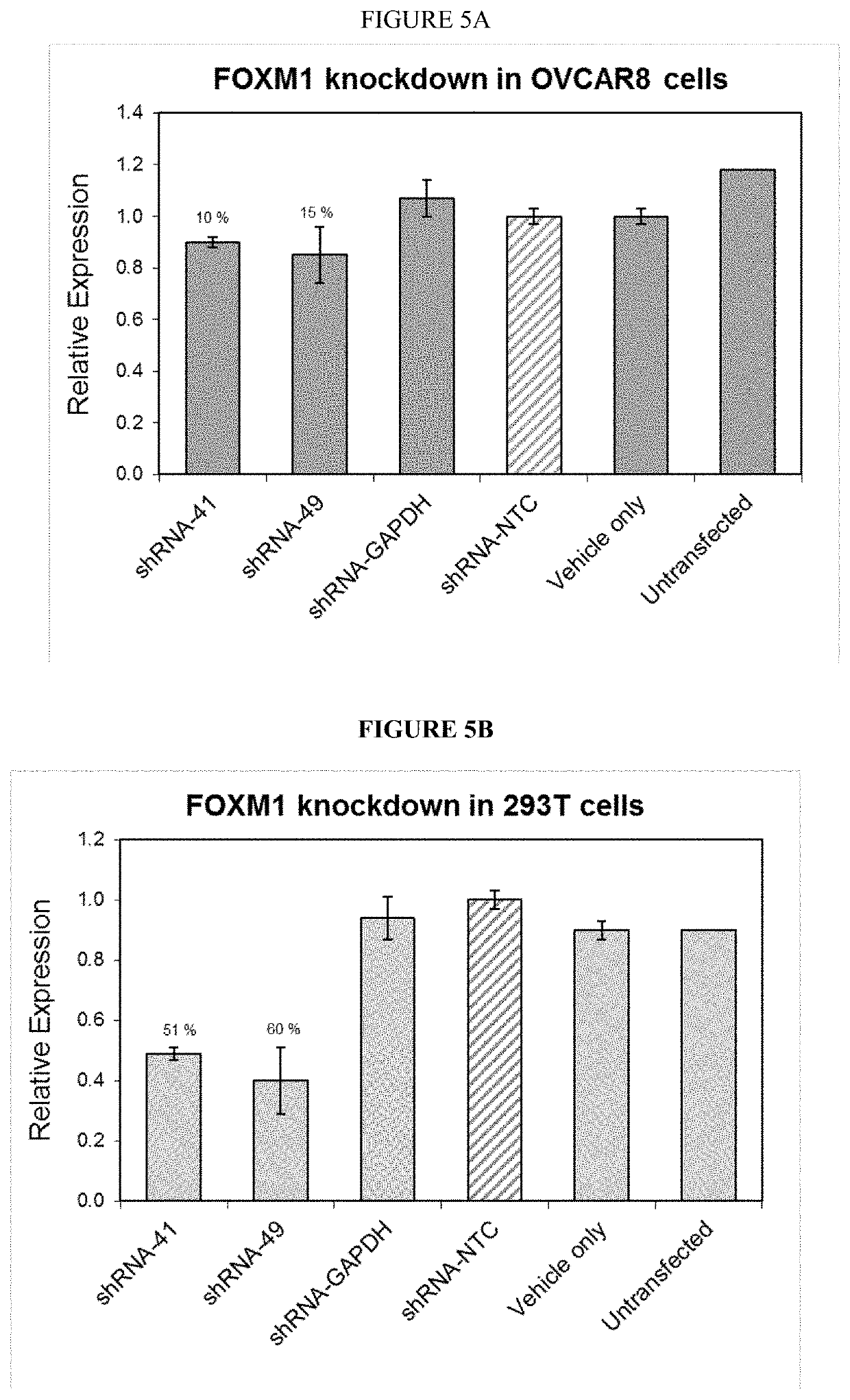
Using minivectors to treat ovarian cancer
PendingUS20200048716A1Improve survival rateEfficient transfectionOrganic active ingredientsSpecial deliveryCD44PRDM16
MiniVectors and compositions containing MiniVectors that target ovarian cancer genes selected from FOXM1, AKT, CENPA, PLK1, CDC20, BIRC5, AURKB, CCNB1, CDKN3, BCAM-AKT2, CDKN2D-WDFY2, SLC25A6, CIP2A, CD133, ALDH1A1, CD44, SALL4, and / or PRDM16, alone or in any combination, are provided, along with uses in the treatment of ovarian cancer.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Methods of treating prostate cancer with Anti-prostate specific membrane antigen antibodies
InactiveUS20090280120A1Relieve painReduce needImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntigen Binding FragmentOncology
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com