Therapeutic agents - II
a technology of therapeutic agents and ocular irritation, applied in the field of chemical agents, can solve the problems of skin and ocular irritation, and achieve the effects of promoting the development of bipolar dendritic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PKC Activation: Kinase Activity of PKC as Measured by Enzyme Assay
Preparation of Chemical Fractions from E. peplus
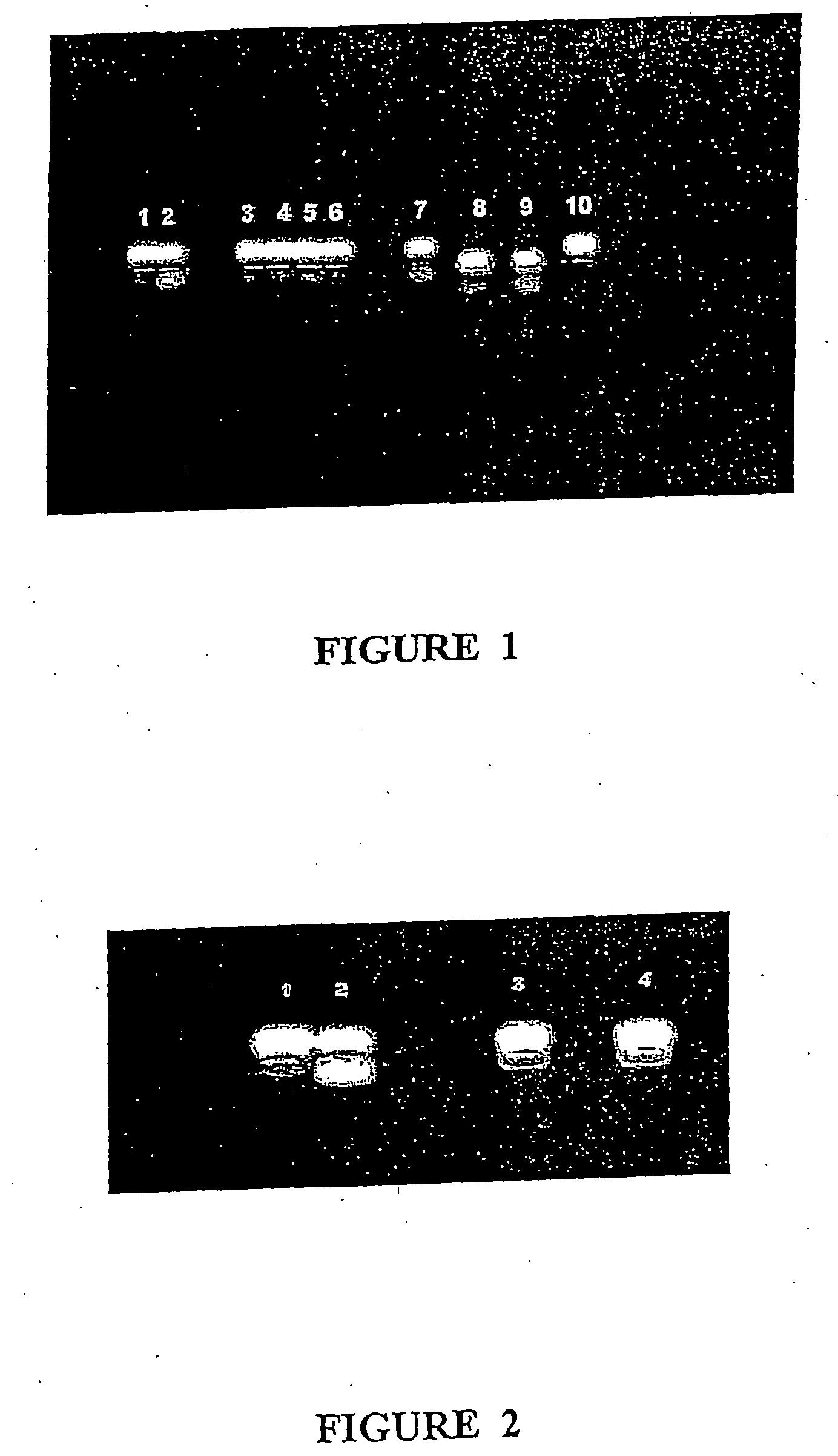
[0243] Sap from E. peplus plants was collected, stored at −20° C., thawed and stored at 4° C. for 1 week prior to use. The H fraction was prepared from frozen sap by thin layer chromatography (TLC) as described in International Patent Application No. PCT / AU98 / 00656 and was stored as dried silica-associated material at 4° C. This material was enriched in jatrophanes and pephanes. One to two months prior to use, the material was dissolved in ethylene glycol dimethyl ether (DME) and stored at 4° C. The concentrations were determined from the dry weight of the material. For PKC assays, crude sap (PEP001) and the PEP004 fraction was ether extracted twice to produce an ether-soluble fraction enriched in diterpenes, namely, ingenanes, jatrophanes and pepluanes. The remaining water soluble fraction was also used. An ingenane fraction was prepared from the ether-soluble extrac...
example 2
PKC Activation: Translocation of PKC
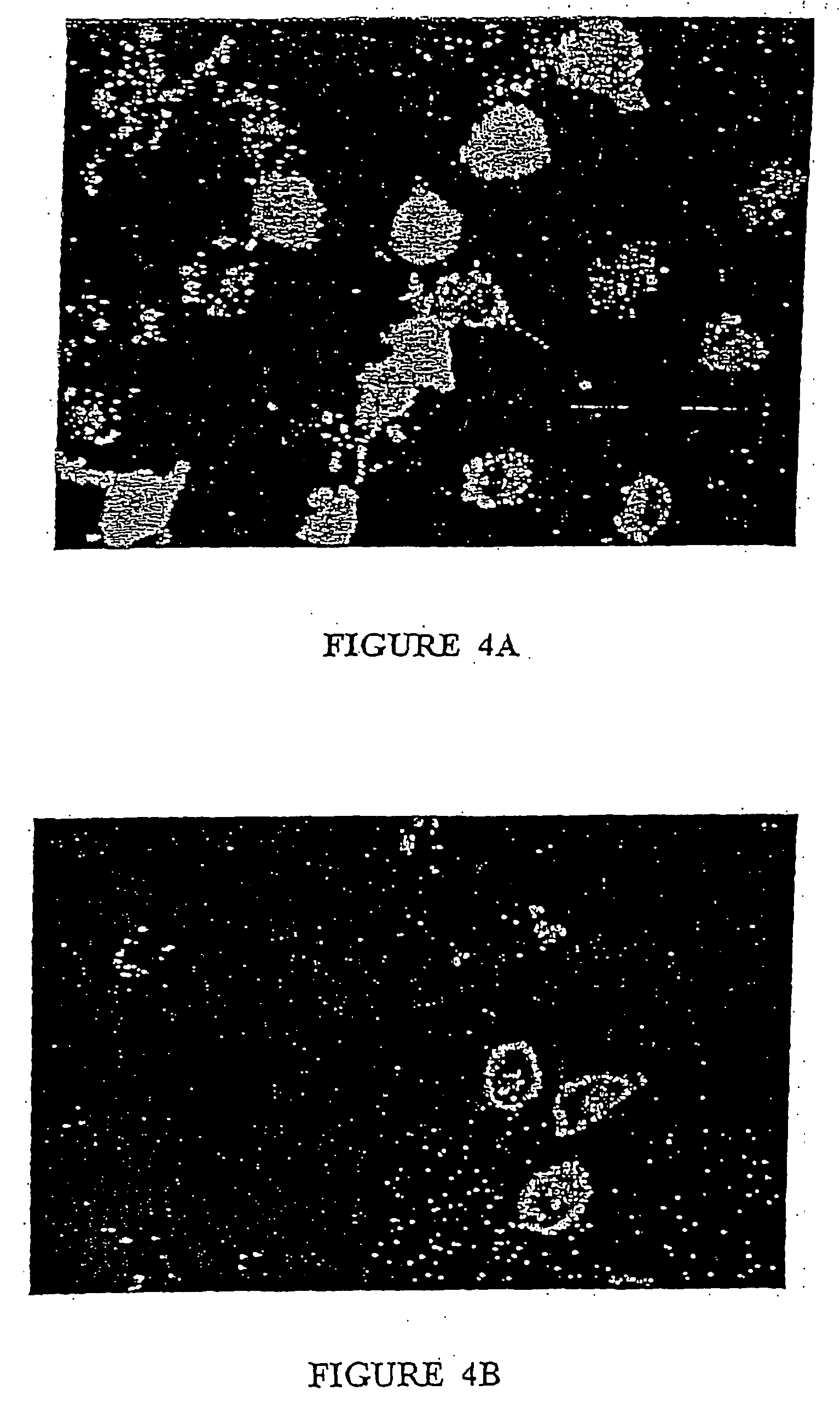
[0251] Activation of PKC can also be demonstrated by a simple fluorescence microscopy-based assay. Upon activation, PKC is known to translocate from the cytoplasm to the plasma membrane of the cell. By fusing PKC enzymes to the green fluorescent protein (GFP) or enhanced GFP (EGFP), activation of the PKC can be detected by the movement of diffuse cytoplasmic GFP to a ring of fluorescence associated with the plasma membrane. Using this assay, crude E. peplus extract has been shown to activate PKCβ and PKCγ.
[0252] MM96L cells were first transfected using a commercially-available kit (Qiagen Effectine Transfection Kit) with a PKC-GFP expression vector (Clontech; http: / / www.clontech.com / gfp / ) and allowed to produce the PKC-GFP protein for 24 hr. The cells were then treated with crude E. peplus extract and TPA and observed under a fluorescent microscope (488 nm excitation). Two controls were used—no DNA, which allows for the identification of non-tra...
example 3
Binding of Compounds to PKC
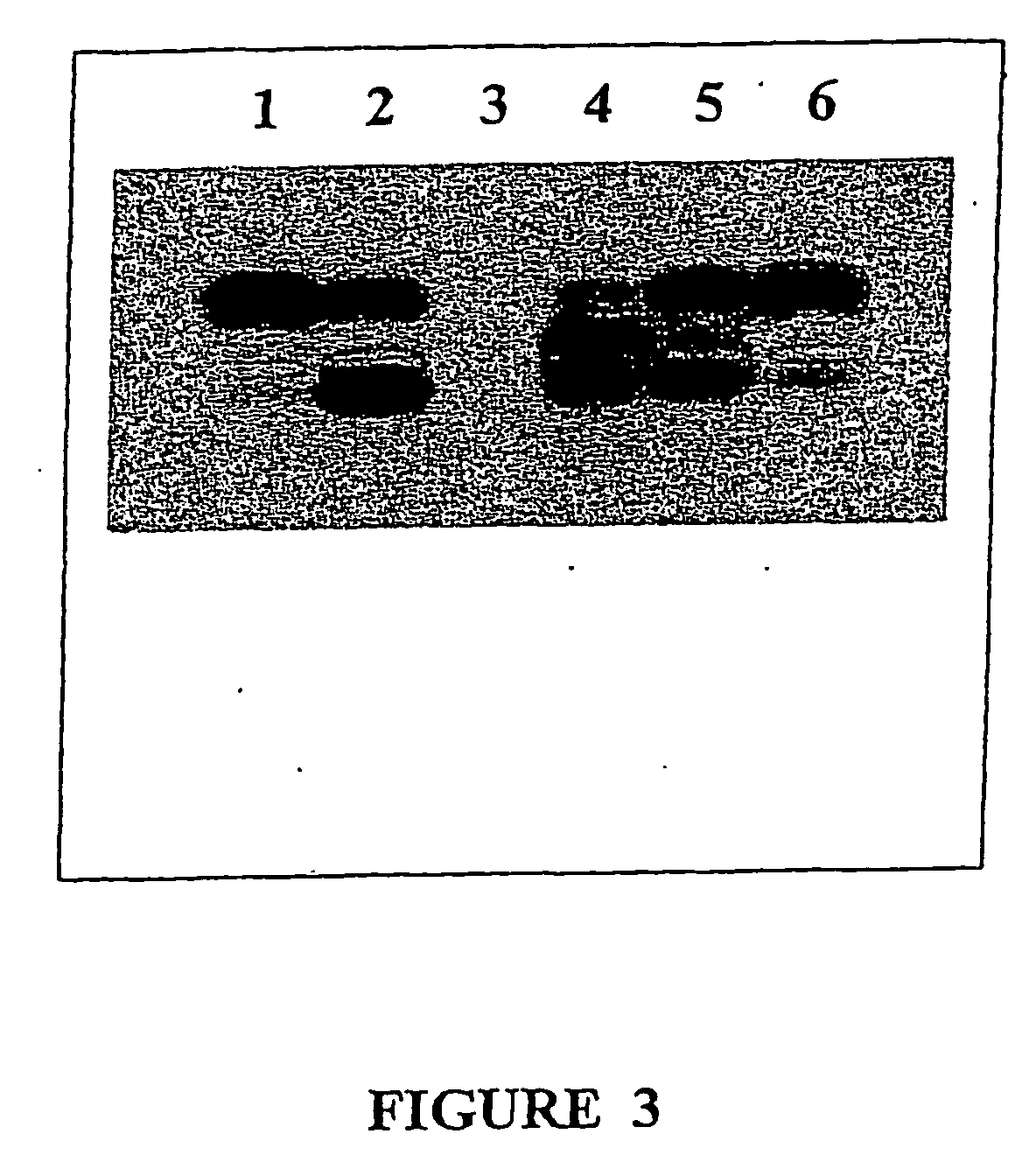
[0258] A competition assay was performed to determine whether the diterpene esters of the instant invention bind to the phorbol ester binding site of PKC. This competition assay showed that 23 μg / mL PEP003 displaced >90% [3H]-phorbol dibutyrate from binding to rat brain homogenate, used as a source of PKC (Gonzalez et al., 1999). This binding was not blocked by co-incubation with bisindolylmaleimide. These results show that PEP003 binds to the phorbol ester binding site of PKC, and bisindolylmaleimide does not.
PUM
| Property | Measurement | Unit |
|---|---|---|
| sizes | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com