Use of 2,5-Dihydroxybenzene Derivatives for Treating Actinic Keratosis
a technology of hydroxybenzene and derivatives, which is applied in the direction of drug compositions, metabolism disorders, therapy, etc., can solve the problems of no effective treatment for skin photoaging and no effective treatment for obesity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assay of 2,5-dihydroxybenzenesulfonic Acid on Photoaged Skin
[0277]In this study, 2,5-dihydroxybenzenesulfonic acid, potassium salt, formulated as 5% by weight in the form of cream has been used, since this type of formulation is a habitual procedure for topical treatments of the skin. Distilled water has been used as the aqueous phase of the cream. The oil phase thereof may comprise cetyl alcohol, stearyl alcohol or vaseline. Span (sorbitan monooleate) is an effective emulsifier to make the cream.
[0278]The following example illustrates the formulation of an effective cream for the topical treatment of skin photoaging and must not be construed as a limitation of the scope of the invention.
[0279]Active principle: 2,5-dihydroxybenzenesulfonic acid, potassium salt, 5% by weight. Excipients: cetyl alcohol (2.5%), stearyl alcohol (2.5%), liquid vaseline (30%), white soft vaseline (20%), sorbitan monooleate (5%) and distilled water (q.s. to 100 g).
[0280]Continuous topical treatment of huma...
example 2
Effect of 2,5-dihydroxybenzenesulfonate on Seborrheic Keratosis, Actinic Keratosis and Viral Warts
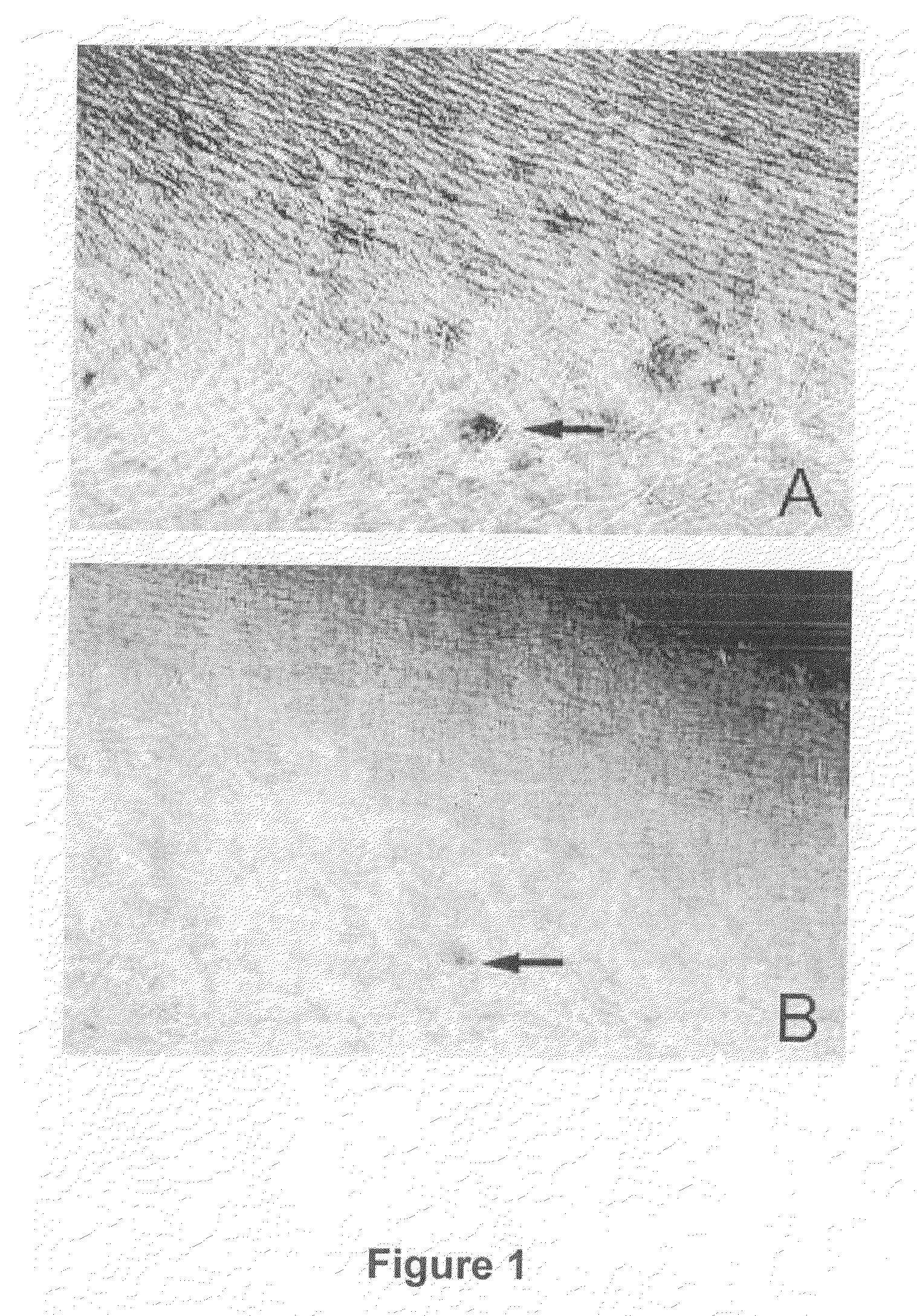
[0281]Topical treatment with the above described cream (twice a day for 11 days) of human skin (basal conditions shown in FIG. 2A) results in a reduction of seborrheic keratosis (big circle) as well as of viral warts (small circle), as shown in FIG. 2B.
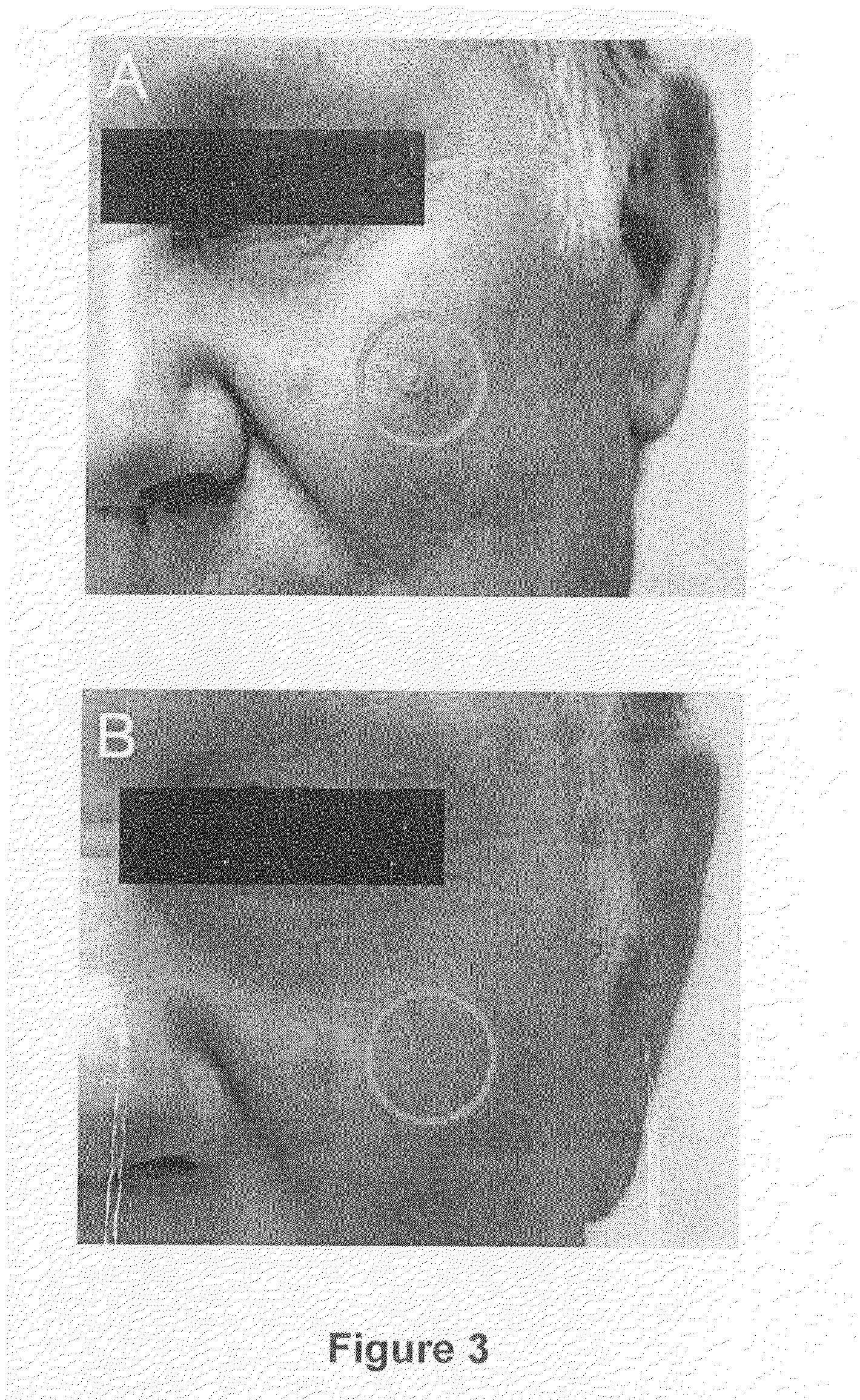
[0282]Another example of the effect of 2,5-dihydroxybenzenesulfonate on seborrheic keratosis is shown in FIG. 3, wherein the seborrheic keratosis is reduced due to the topical treatment on the affected area for 4 weeks, twice a day, with an emulsion containing the 2,5-dihydroxybenzenesulfonic acid, 2.5% by weight.
[0283]The emulsion containing 2.5% by weight of 2,5-dihydroxybenzenesulfonic acid was also used to treat two lesions of actinic keratosis in the same patient, as shown in FIGS. 4 and 5. After 15 days of treatment with said emulsion (twice a day), the actinic keratosis was clearly reduced, and the effect was even more evident afte...
example 3
Effect of an Emulsion of 2.5% 2,5-dihydroxybenzenesulfonate on Actinic Keratosis
[0284]An emulsion containing 2.5% by weight of 2,5-dihydroxybenzenesulfonic acid was used to treat two lesions of actinic keratosis in the same patient, as shown in FIGS. 4 and 5. After 15 days of treatment with said emulsion (twice a day), the actinic keratosis was clearly reduced, and the effect was even more evident after 4 weeks of treatment. Calcium salt of 2,5-dihydroxybenzenesulfonic acid was used.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com