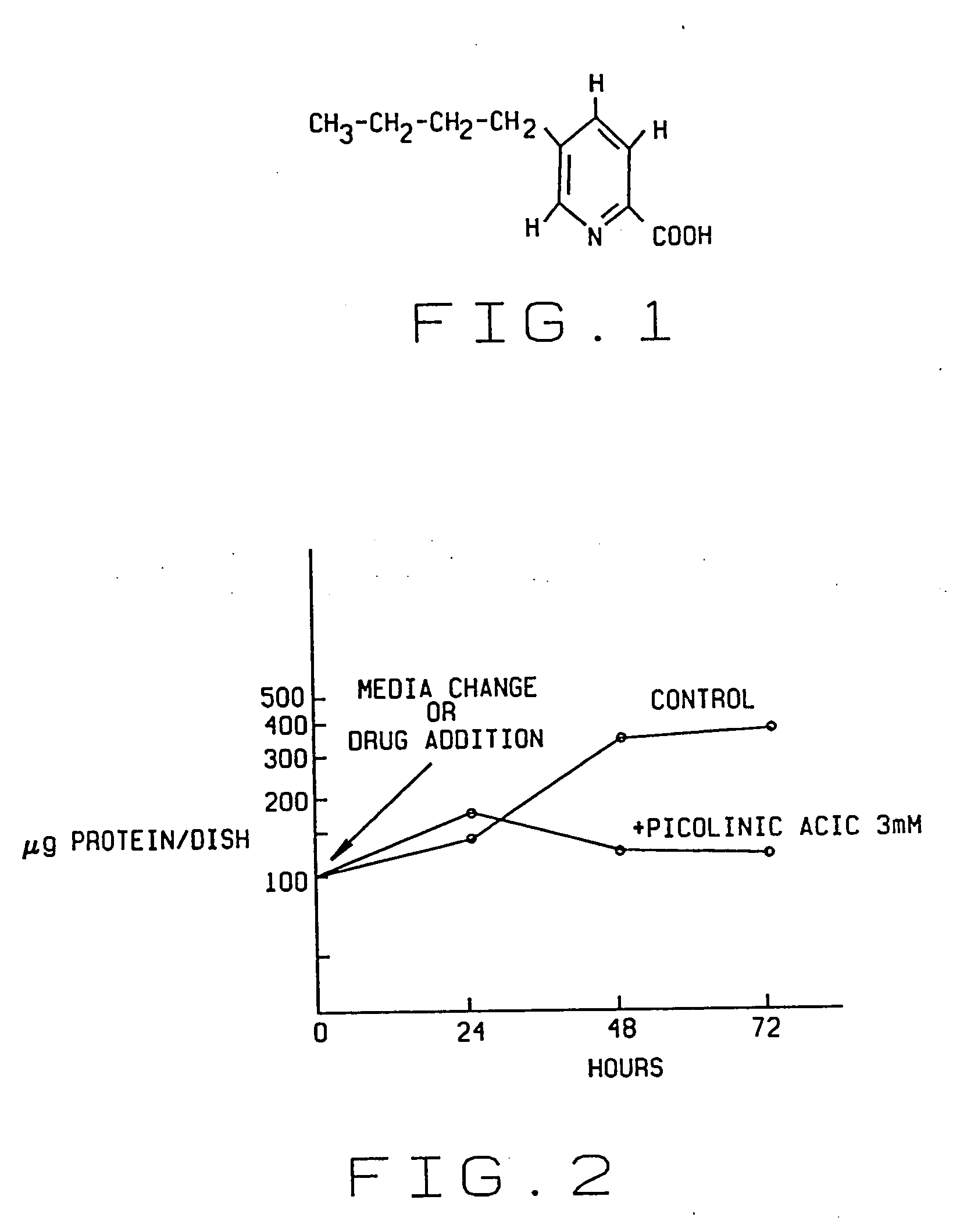
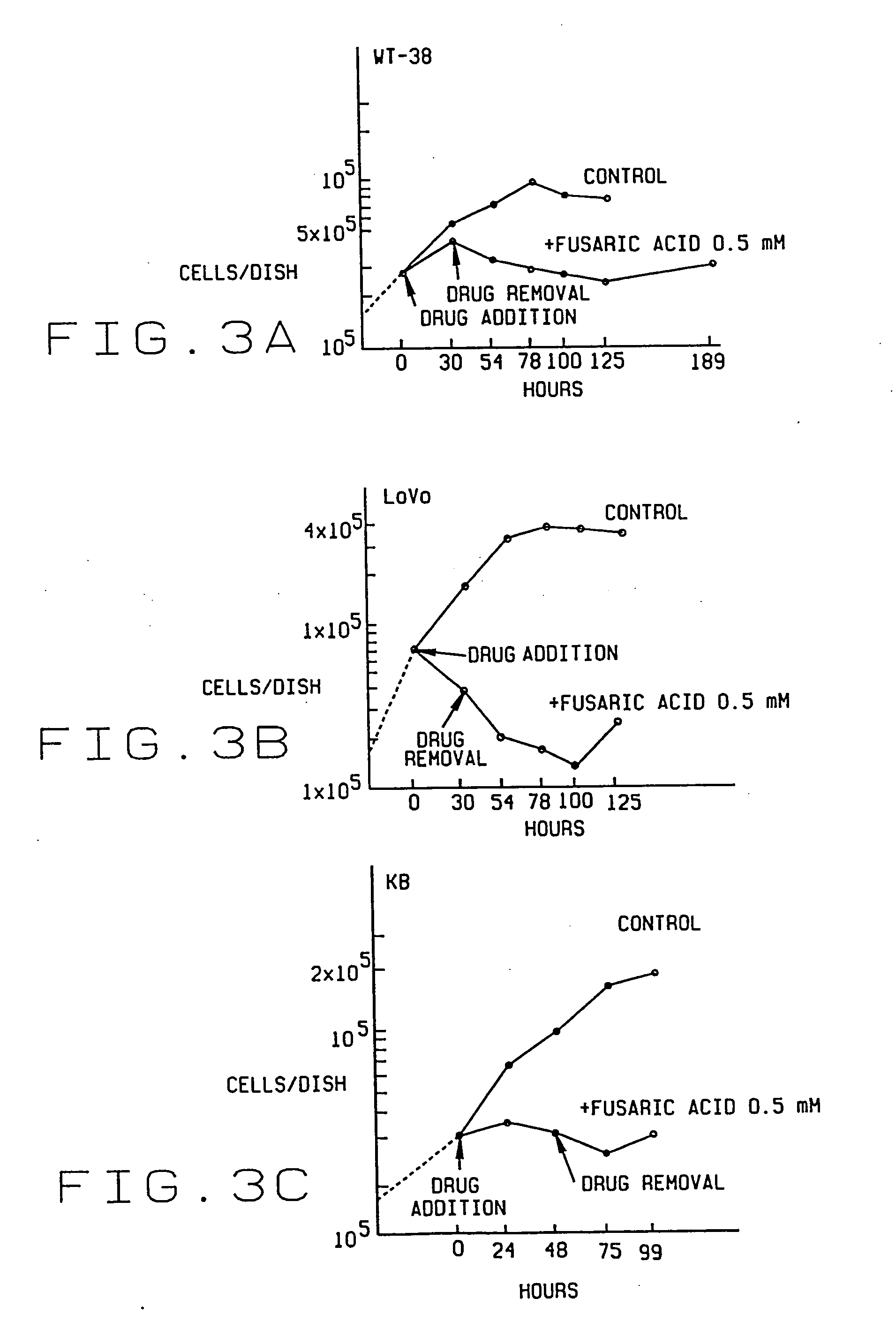
Pharmacological agent and method of treatment
a technology of a drug and a treatment method, applied in the direction of biocide, heterocyclic compound active ingredients, organic compounds of the group 3/13 element, etc., can solve the problems of plantar ulcers and plantar warts, vulnerable to specific antiviral attacks, etc., to achieve the effect of effectively eliminating chemotherapeutic agents, supplying the energy required for drug elimination, and high levels of p170 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 8
Treatment of Metastatic Cancer
[0116] The subject is a 62 year old Caucasian male with metastatic colon cancer. The subject presented with an enlarged lymph node in the neck. The lymph node was putting pressure on nerves causing a drooping of the subjects right eye lid. Subsequent testing such as CAT scan and MRI indicated that the lymph node was cancerous. Treatment was initiated with 500 mg of picolinic acid in capsule form, by mouth twice daily. Within 72 hours the lymph node was significantly reduced in size upon palpation, with an estimated reduction of over 50% in mass with concomitant lessening of the droop in the eyelid. An aspiration needle biopsy of the lymph node was attempted but had to be repeated due to the fact that the pathologist was withdrawing necrotic tissue from the lymph node. The subject remains on 500 mg picolinic acid twice daily and is tolerating treatment well. The protocol includes increasing the dosage up to 2000 mg per day, or more, if required.
example 9
Fusaric Acid Effect on Cells with Increased P-Protein Activity
[0117] Multidrug resistance (MDR) is a formidable obstacle to effective cancer chemotherapy. Studies have indicated that MDR is a phenomenon in which resistance to one drug is associated with resistance to a variety of unrelated drugs. Thus, even when a combination of chemotherapeutics is used, patients may exhibit concurrent resistance to some or all of the drugs, leading ultimately to failure of therapy.
[0118] One of the primary contributors to MDR is a glycoprotein denoted P-glycoprotein of molecular weight 170 Kdal, also know as P170. P-glycoprotein or P170 acts as a pump, effectively eliminating chemotherapeutic agents from the cell interior to the extracellular space. Although drug-sensitive cells are destroyed during the initial and subsequent courses of chemotherapy, drug resistance cells, containing elevated levels of P-glycoprotein, emerge, multiply and eventually lead to death of the host.
[0119] P-glycoprote...
example 10
Use of Fusaric Acid to Reduce the Expression of Retroviral mRNA Levels
[0121] By using Kirsten (K) sarcoma retrovirus-transformed NRK cells it was shown in preliminary experiments that fusaric acid reduces the expression of retroviral mRNA levels. Furthermore, it also may be shown that the combination of fusaric acid and interferon-gamma results in a potent inhibition of K sarcoma virus mRNA expression in K-NRK cells.
[0122] Identification of fusaric acid as a substance that can inhibit expression of mRNA controlled by a retroviral promoter is a great interest because of the importance of retroviruses, such as the human immunodeficiency virus (HIV), in animal and human disease. Although the biology of K-virus and HIV is different, fusaric acid may be effective in controlling HIV viral expression. Furthermore, the combination of fusaric acid plus interferon-gamma may be much more potent in inhibiting HIV expression in human monocytes and other infected cells. Thus, this inventions is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com