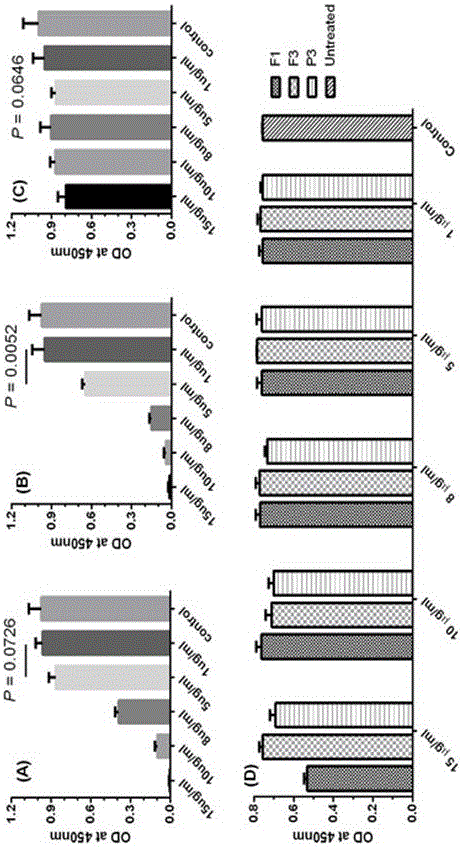
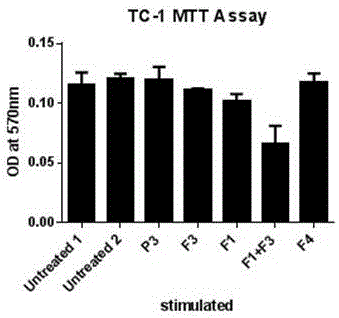
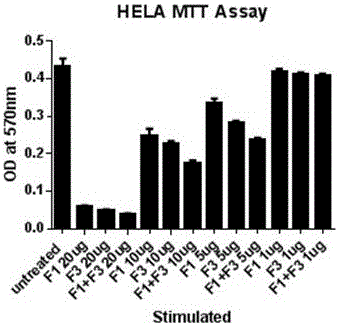
Pharmaceutical composition containing polypeptides F1 and F3 and application of pharmaceutical composition in treatment of HPV (Human Papilloma Virus) infected diseases
A composition and drug technology, applied in the field of polypeptide pharmaceutical preparations, can solve the problems of HPV infection that cannot be cured, many side effects, and high recurrence rate, and achieve the effects of unique drug action mechanism, low recurrence rate, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of F1, F3 polypeptides
[0050] 1) First, a 9-volt direct current stimulation is used to induce the tree frog to secrete a white viscous liquid. The tree frog is distributed in the coastal rainforest area of eastern Australia.
[0051] 2) Collect the liquid and transfer it to ultrapure water containing 20% methanol, shake it and filter it through a 0.45 μm PVDF membrane.
[0052] 3) The filtered supernatant was dried in a vacuum low-temperature centrifugal desiccant to form a white solid powder.
[0053] 4) Dissolve the white solid powder in 0.1% trifluoroacetic acid, and analyze the polypeptide sequence and post-translational modification therein by high-throughput high-performance liquid chromatography and mass spectrometry.
[0054] 5) Use the solid-phase peptide synthesis method to synthesize peptide F1 or F3 respectively. The specific steps are as follows:
[0055] a) On the CS336X peptide synthesizer, using chloromethyl polystyrene ...
Embodiment 2
[0061] Example 2: Preparation of an ointment composition comprising F1 and F3 polypeptides (prepared in 10 g amount)
[0062] formula:
[0063] F1 polypeptide: 10mg;
[0064] F3 polypeptide: 10mg;
[0065] PEG4000: 100 mg;
[0066] Arginine: 20 mg;
[0067] PEG400: Appropriate amount, add up to 10g.
[0068] According to the conventional method for preparing ointment compositions, the above-mentioned composition is divided into aluminum tubes for ointment packaging, and each tube contains 2 mg, 5 mg or 10 mg, which is an ointment.
Embodiment 3
[0069] Example 3: Preparation of an ointment composition comprising F1, F3 polypeptide and Imiquimod (prepared in 10 g amount)
[0070] formula:
[0071] F1 polypeptide: 2.5mg;
[0072] F3 polypeptide: 2.5mg;
[0073] Imiquimod: 30mg
[0074] PEG3000: 100 mg;
[0075] Lysine: 5 mg;
[0076] PEG200: Appropriate amount, add up to 10g.
[0077] According to the conventional method for preparing ointment compositions, the above composition is divided into aluminum tubes for ointment packaging, and each tube contains 2 mg, 5 mg or 10 mg, which is an ointment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com