HPV (human papillomavirus) peptide/DC (dendritic cell) mixed vaccine and preparation thereof
A hybrid vaccine, BM-DC technology, applied in the field of medical bioengineering, can solve the problems of clinical trials failing to achieve expected results, lack of anti-HPV drugs, limited induction and activation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of HPV11E7 polypeptide / DC mixed vaccine derived from mouse bone marrow
[0022] 1) Method:
[0023] The bone marrow of the mouse tibia and femur was blown into a cell suspension, and ammonium chloride was added to lyse the red blood cells. RPMI1640 culture solution 3ml / well was resuspended and cultured in six-well plate. After 48 hours, all the culture solution was discarded, and 2ml / well culture solution and cytokines were added. After 48 hours, all the cells were collected, resuspended and divided into 5 groups, each of which was incubated with different stimulants for 48 hours: (1) peptide (20 μg / ml) and imiquimod (2 μg / ml), (2) peptide (20μg / ml) and PIC (20μg / ml), (3) peptide (20μg / ml) and CpG (20μg / ml), (4) peptide (20μg / ml), (5)) blank control (without any Components), wherein the TLR ligands were added 2 hours after the addition of the peptide.
[0024] 2) DC morphology observation:
[0025] On the third day of DC isolation and culture,...
Embodiment 2
[0026] Example 2 Identification of DC surface molecular marker changes detected by flow cytometry:
[0027] 1) Method:
[0028] On day 7 of DC culture, DC (1×10 5 Each / 100ul) were co-incubated with FITC-labeled mouse CD40, CD80, CD11c and PE-labeled CD86, Iab antibodies for 30 minutes, and then flow cytometry detection was performed.
[0029] 2) Results:
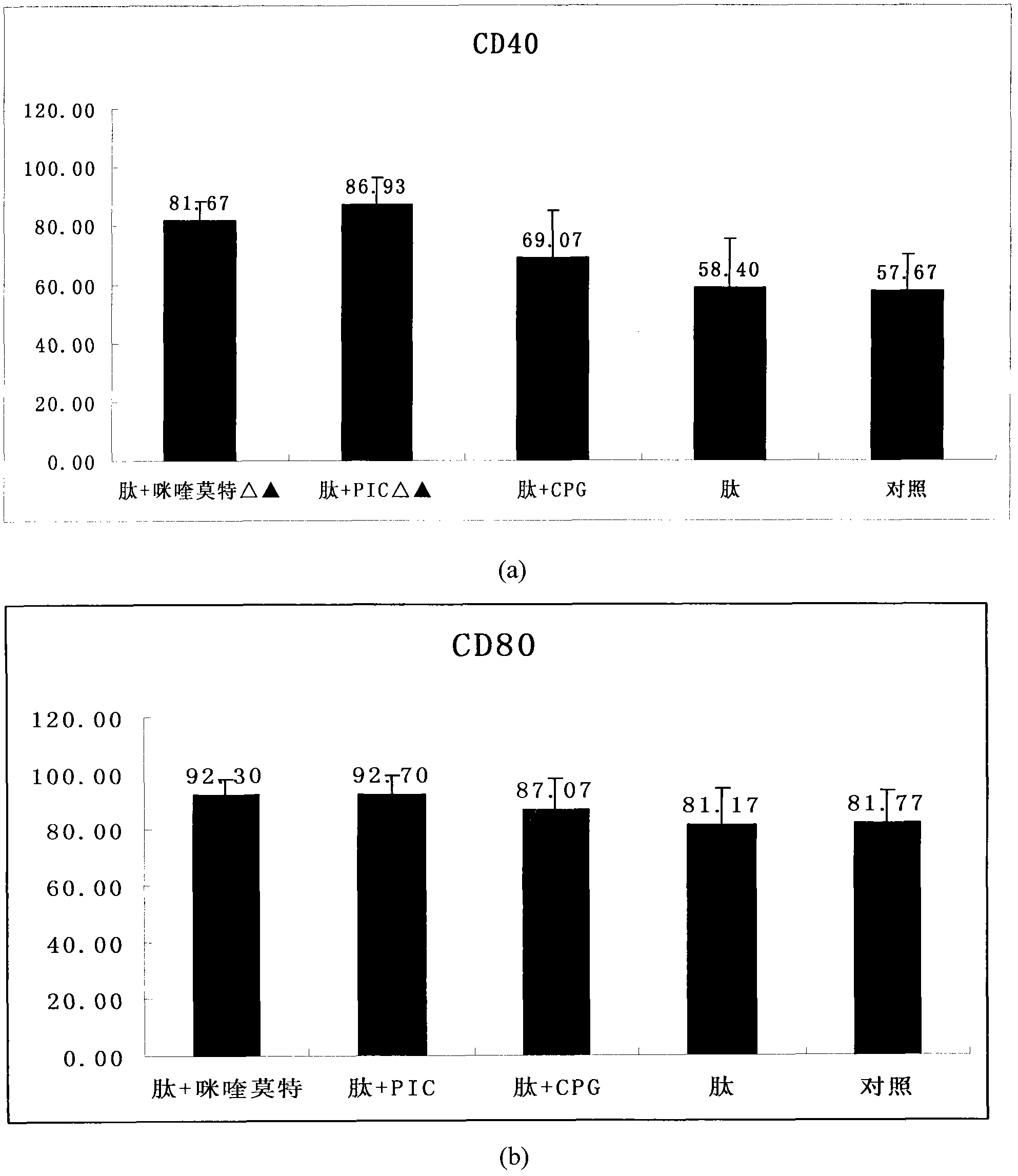
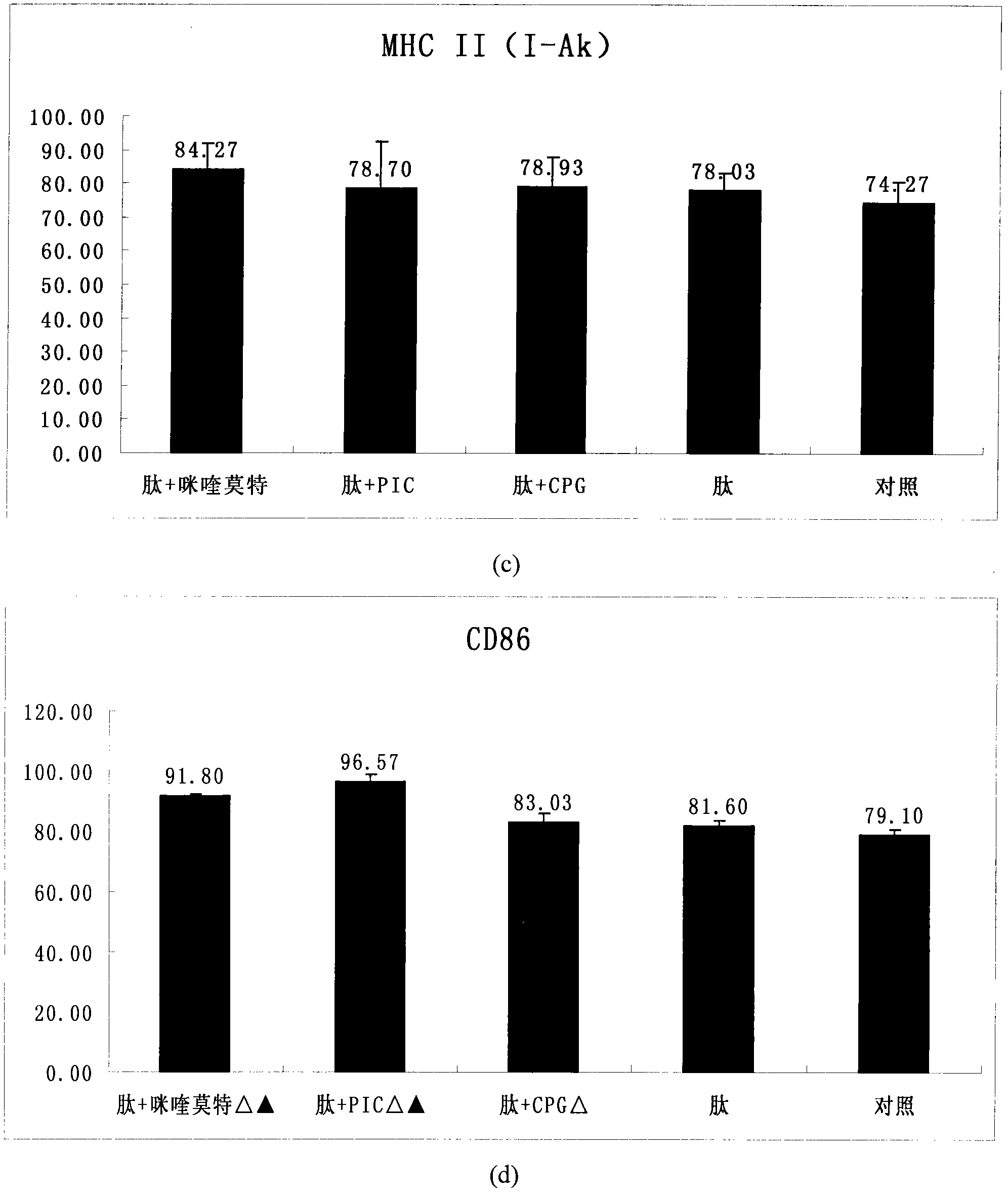
[0030] By flow cytometry analysis, CD11c positive cells accounted for 81.3%. DC phenotypes were analyzed by flow cytometry on the 5th and 7th day, the results can be found in figure 1 , figure 1 After BM-DCs were co-incubated with HPV11E7 polypeptide and TLR agonist for 48 hours, DC surface markers CD40, CD80, CD86 and MHCII (I-Ak) were detected by flow cytometry to determine their maturity.
[0031] The results indicated that after only shocking with antigenic peptides, the maturity of DCs was slightly higher than that of the non-shocking group, but after adding TLR ligands, the maturity of DCs in each treatment group ...
Embodiment 3
[0035] Example 3 Inoculation of mice and observation of immunological characteristics
[0036] 1) Inoculation method:
[0037] After 5 groups of DCs with different treatments were washed at least twice with PBS, the cell concentration was adjusted to 3×10 5 / 200μl / mouse, injected into the peritoneal cavity of mice (group of 6×5). After that, inject once every other week for a total of 3 injections.
[0038] 2) Immunotherapy model protocol for tumor-bearing mice (in vivo experiment):
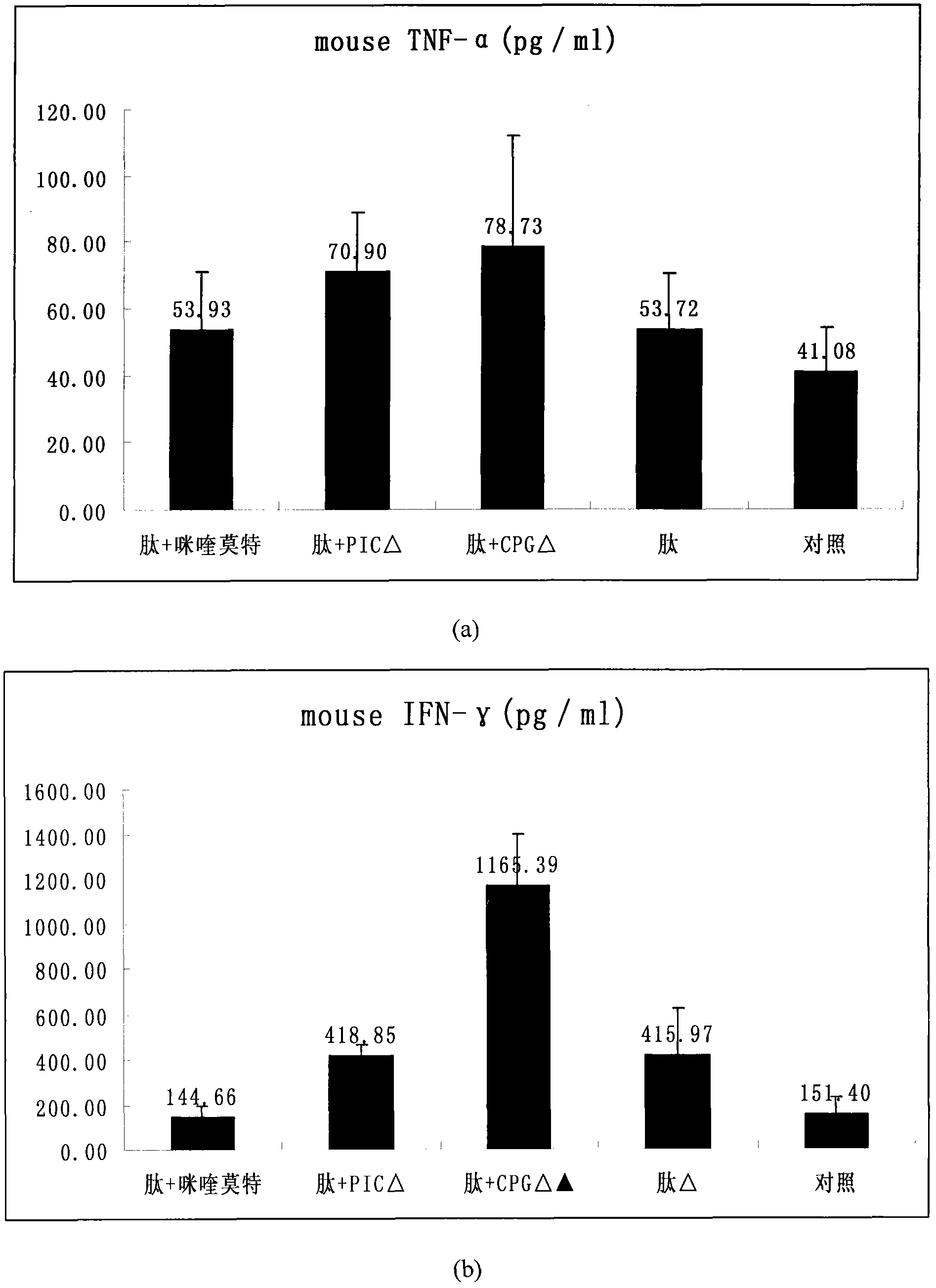
[0039] The mice were immunized with DC three times, and on the second day after the third immunization, B16 cells (1×10 5 / 200μl / piece). On the 19th day after tumor inoculation, all the mice were sacrificed, and splenocyte suspension was prepared. After 2 hours of attachment, the suspended cells were collected, and RPMI1640 culture medium containing muring IL-2 (20U / ml) and 10% fetal bovine serum After resuspension, culture them in a six-well plate (about 7×106 / ml), and add peptide (20 μg / ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com