Topical Use of Valproic Acid for the Prevention or Treatment of Skin Disorders
a technology of valproic acid and valproic acid, which is applied in the field of topical use of valproic acid for the prevention or treatment of skin disorders, can solve the problems of devastating and potentially fatal side effects, destabilisation of the interaction of histones with dna, and treatment is not immedia
Inactive Publication Date: 2006-07-20
TOPOTARGET GERMANY AG
View PDF2 Cites 40 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In the case of histone hyperacetylation, changes in electrostatic attraction for DNA and steric hindrance introduced by the hydrophobic acetyl group leads to destabilisation of the interaction of histones with DNA.
The great drawback to RA, however, is that it requires high levels of the medication in order to turn genes ‘on’ and ‘off,’ often triggering devastating and potentially fatal side effects (Altucci and Gronemeyer, 2001, Nature Reviews Cancer 1, 181-193).
The results of treatment are not immediate and may be visible only after weeks to months.
Discontinuation of treatment usually results in a loss of RA effects.
However, today more women are getting BCC's than in the past; nonetheless, men still outnumber them greatly.
Those whose occupations require long hours outdoors or who spend extensive leisure time in the sun are in particular jeopardy.
This neoplasm is locally invasive and can be very destructive causing the loss of an eye, ear, or a nose, and may be lethal if it invades the brain.
However, the possibility to combine individual treatments with each other in order to identify such combinations which are more effective than the individual approaches alone, requires extensive pre-clinical and clinical testing.
It is not possible to predict which combinations will show an additive or even synergistic effect.
However, inappropriate activation of inflammatory responses is the underlying cause of many common diseases and inflammatory reactions are, therefore, also an important target for drug development.
These cytokines are central to a damaging cascade, ultimately triggering the production of matrix metalloproteinases and osteoclasts, which results in irreversible damage to soft tissues and bones.
These secretions result in proliferation and decreased maturation of keratinocytes and associated vascular changes.
Interleukin 2 may potentiate a breakdown of immunologic tolerance to muscle-specific and tumor antigens, resulting in destruction of both tumor and muscle cells.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
formulation example 1
[0214]
White mineral oil20Cetyl Alcohol24Cetomacrogol 1000 6Valproic Acid>0.1, e.g., 0.1 to 6White vaselineadd to 100
formulation example 2
[0215]
Zinc Oxide25Starch25Valproic Acid>0.1, e.g., 0.1 to 6White vaselineadd to 100
formulation example 3
[0216]
White vaseline25Cetyl Alcohol10Tween 605Glycerin10EDTA0.2Parfume (optionally)2 dropsSodium Valproate>0.1, e.g., 0.1 to 6Bidistilled Wateradd to 10
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Login to View More
Abstract
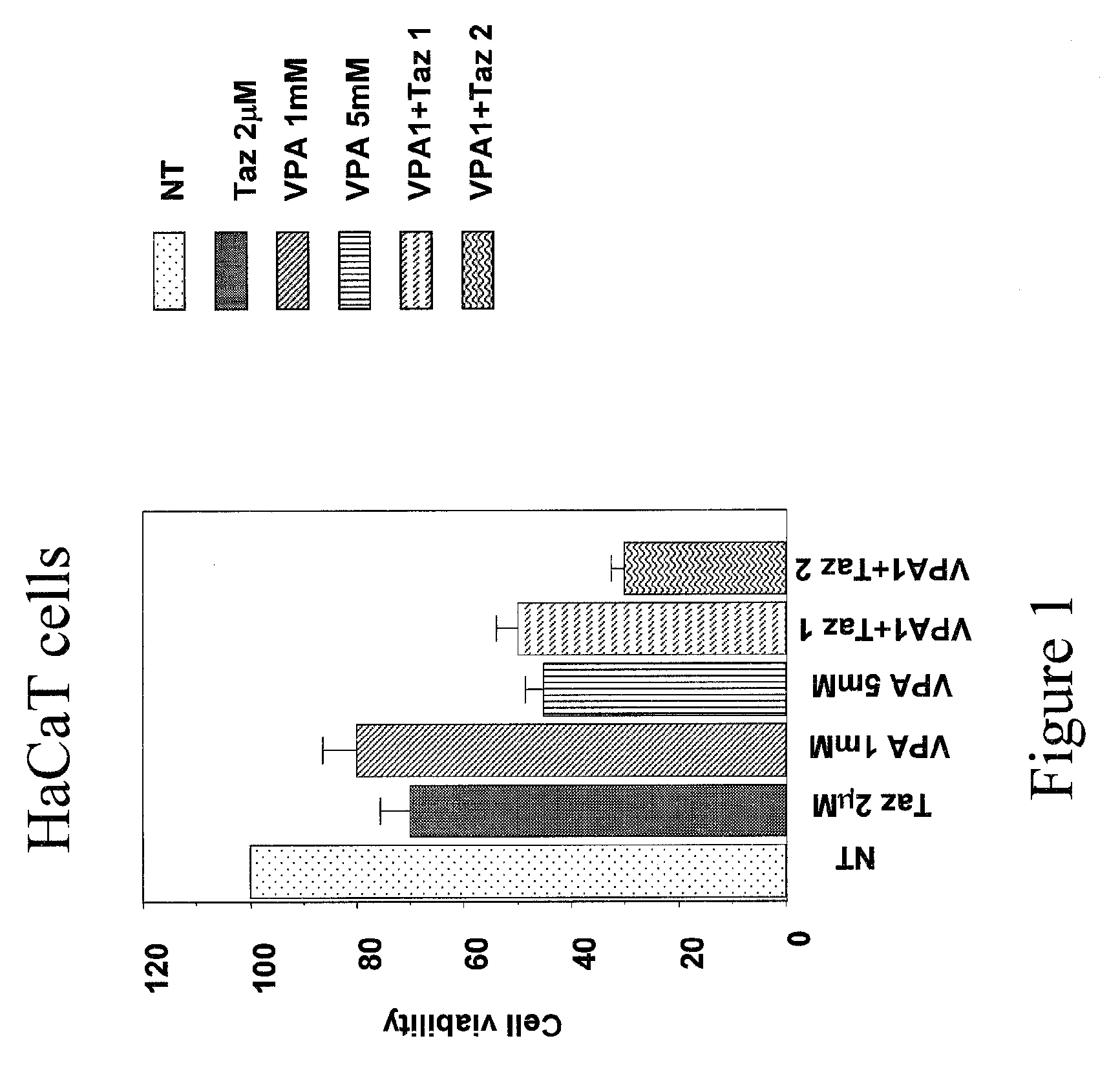
The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell Lymphoma and also for the topical treatment of pre-malignant lesions, and of inflammations of the skin and / or mucosa. The invention also relates to the use of this topically applicable formulation for the protection from UV light and for the treatment of sun burn. The invention includes the use of VPA for the manufacture of a clinically used medicament for the topical treatment of the human diseases listed above.
Description
[0001] This application claims priority under 35 U.S.C. §119 to European Patent Application No. 03014278.0, which was filed on Jun. 25, 2003, and under 35 U.S.C. §120 to PCT / EP2004 / 006789, which was filed Jun. 23, 2004, the entirety of which is hereby incorporated by reference. Also, the Sequence Listing on compact disk filed herewith is hereby incorporated by reference (File name: 033-005 Seq List; File size: 4 KB; Date recorded: Dec. 21, 2005).BACKGROUND OF THE INVENTION [0002] 1. Field of the Invention [0003] The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/19A61K31/20A61K31/4436A61K31/505A61P35/00
CPCA61K31/19A61K31/20A61K31/4436A61K31/505A61K2300/00A61P1/04A61P11/06A61P11/12A61P17/00A61P17/02A61P17/06A61P17/10A61P17/16A61P19/02A61P19/08A61P21/04A61P25/00A61P29/00A61P3/10A61P35/00A61P35/02A61P37/02A61P37/06A61P37/08A61P43/00A61P7/06A61P9/00A61P9/10
Inventor PELICCI, PIERCHIMENTI, SERGIOCOSTANZO, ANTONIONISTICO, STEVENPAOLINO, DONATELLAMINUCCI, SAVERIO
Owner TOPOTARGET GERMANY AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com