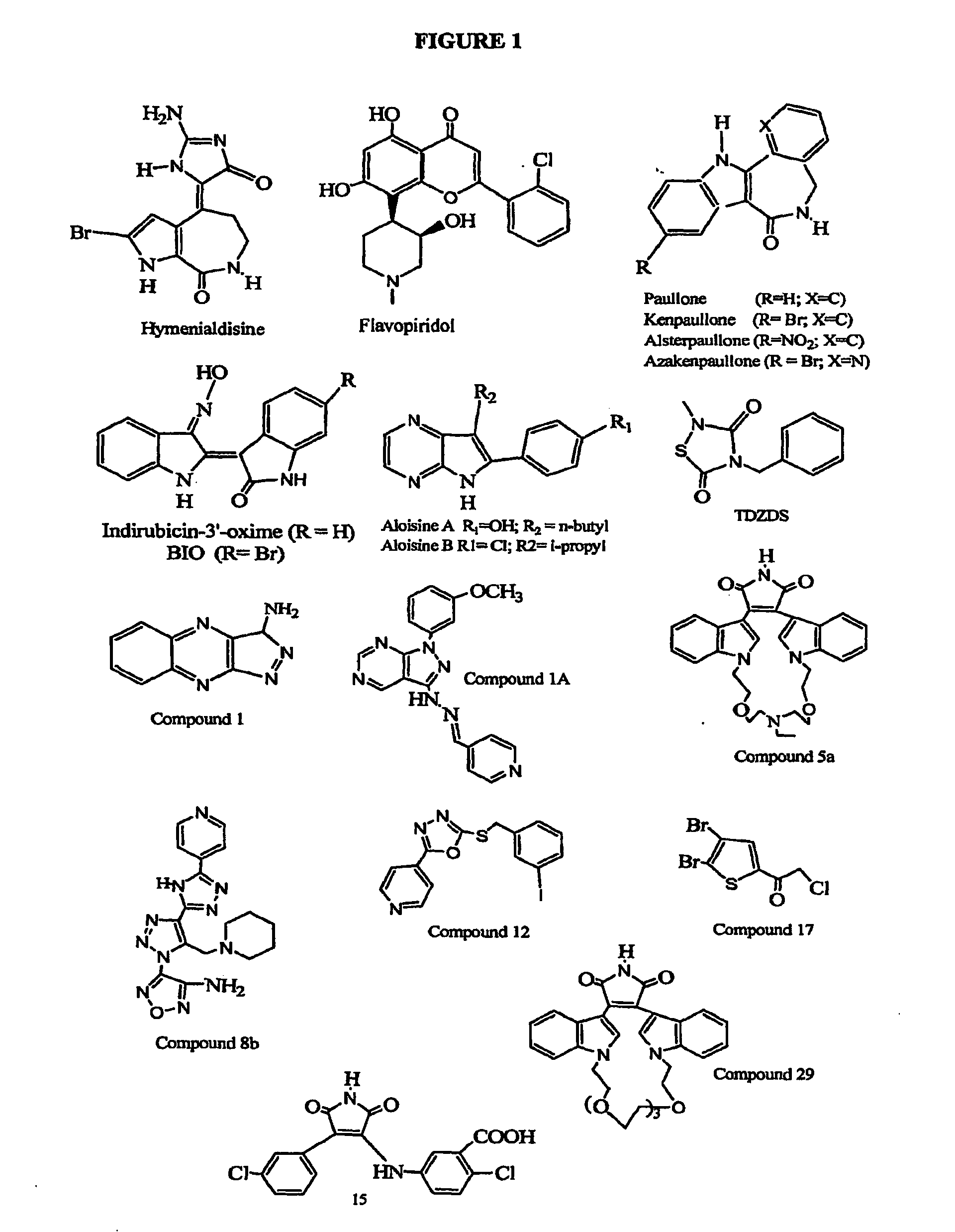
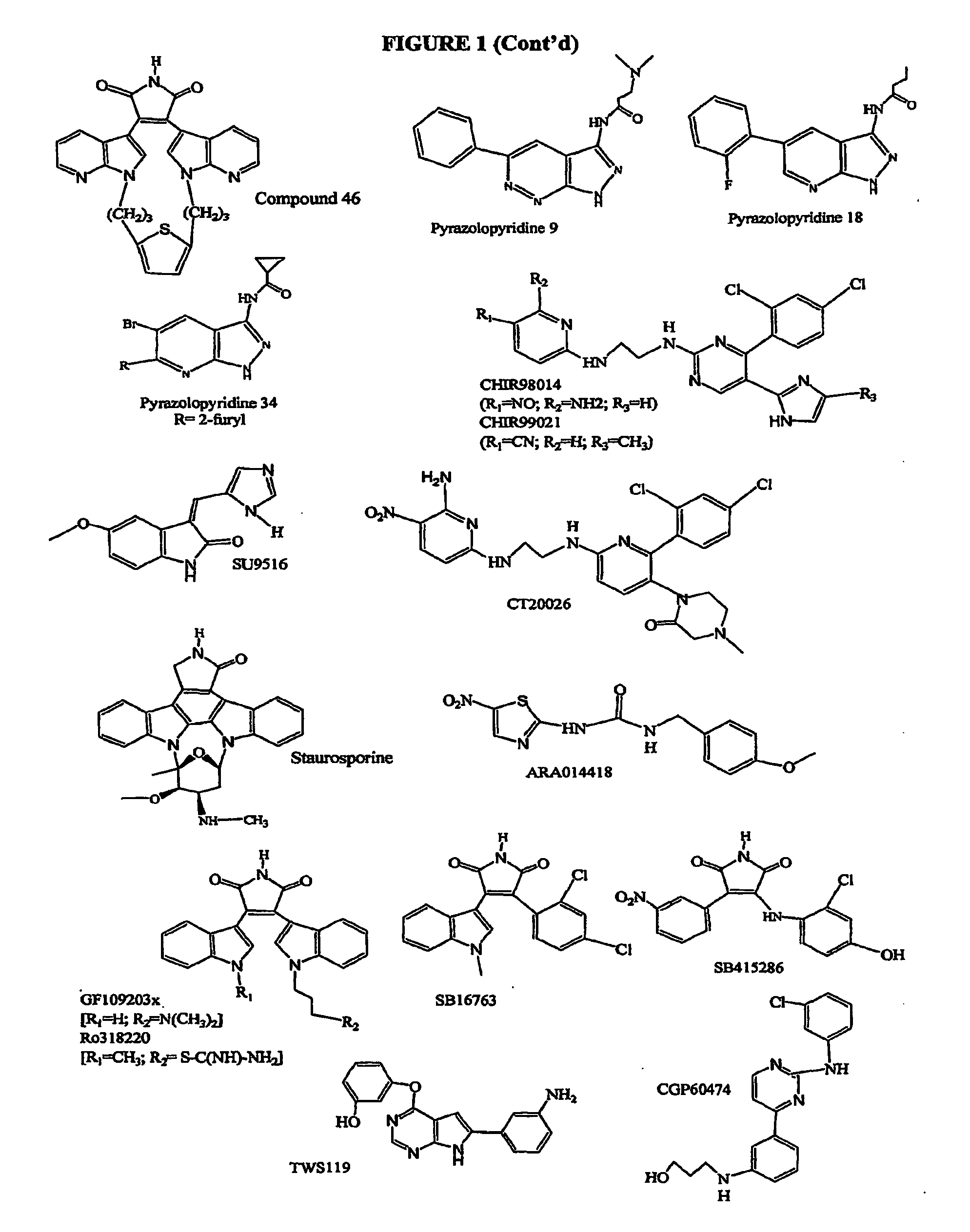
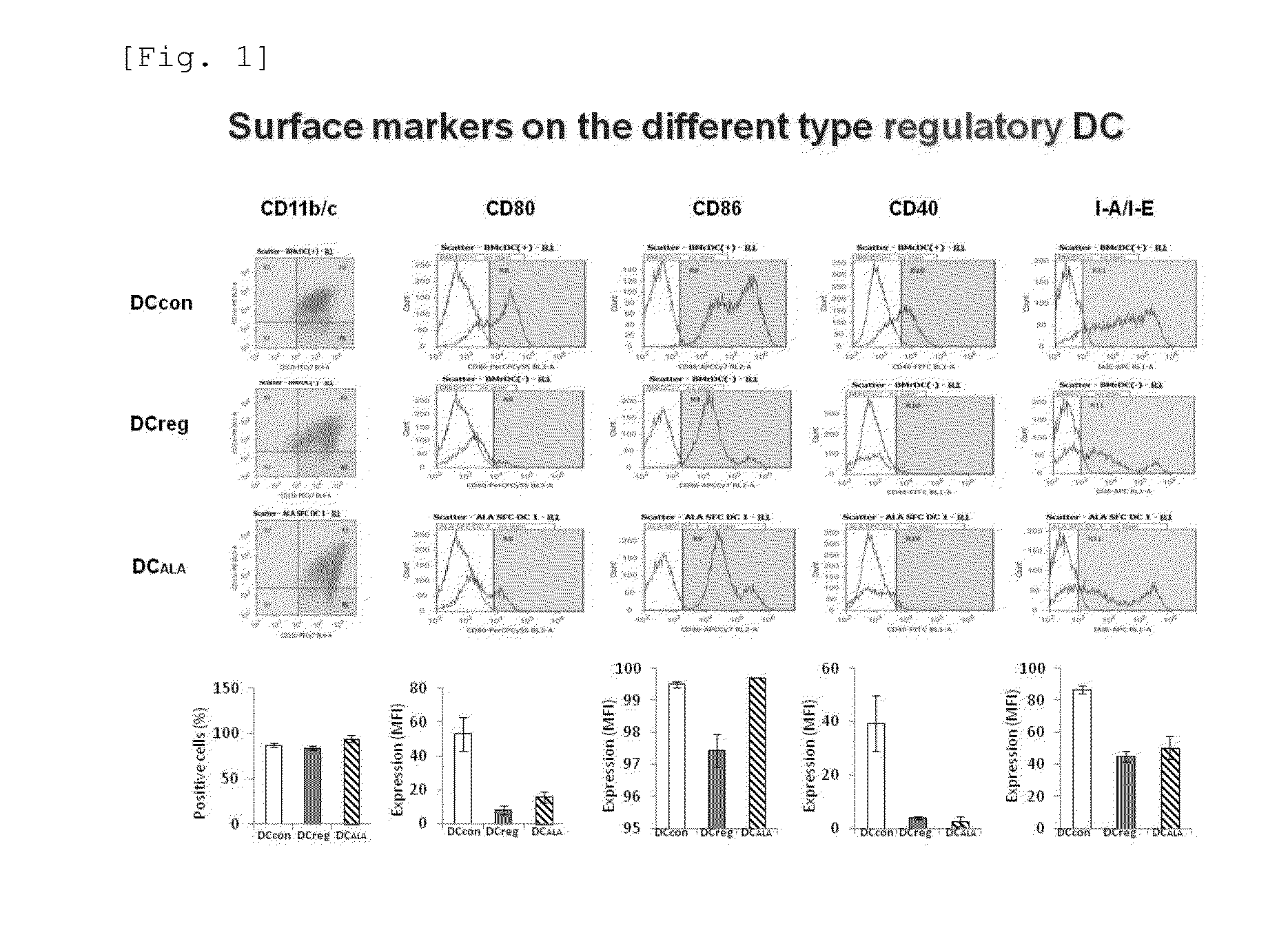
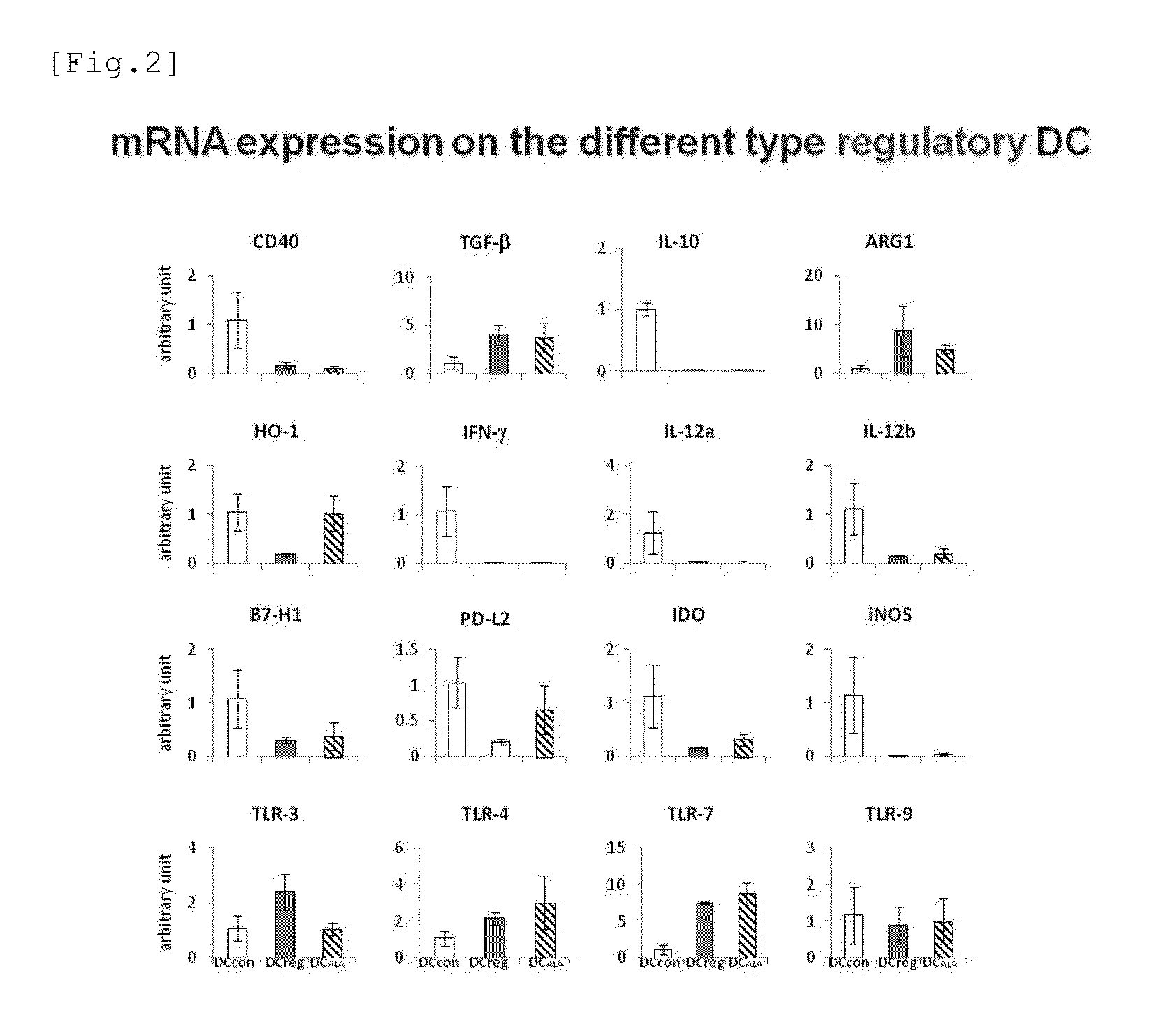
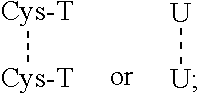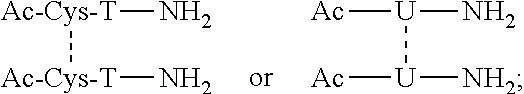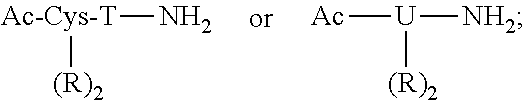Patents
Literature
151 results about "Immunologic Tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The failure of the immune system to respond to an antigen that previously caused an immune response.
Antisense oligomers and methods for inducing immune tolerance and immunosuppression
ActiveUS20060276425A1Reduce capacityIncrease productionBiocideOrganic active ingredientsExtracellularDendritic cell
A method and composition for inducing human dendritic cells to a condition of reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10 is disclosed. A population of dendritic cells is exposed to a substantially uncharged antisense compound, including partially positively charged, containing 12-40 subunits and a base sequence effective to hybridize to an expression-sensitive region of a preprocessed or processed human CD86 transcript identified, in its processed form, by SEQ ID NO:33, to form a duplex structure between said compound and transcript having a Tm of at least 45° C. Formation of the duplex blocks expression of full-length CD86 in said cells, which in turn leads to reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10.
Owner:SAREPTA THERAPEUTICS INC
Targeted/immunomodulatory fusion proteins and methods for making same
ActiveUS20130287802A1Effective compositionEfficient methodBacteriaPeptide/protein ingredientsCancer cellNucleotide
The present invention relates generally to the field of generating fusion proteins to be used in cancer therapy, and more specifically, to nucleotide sequences encoding the fusion proteins, wherein the chimeric fusion proteins comprises at least one targeting moiety and at least one immunomodulatory moiety that counteracts the immune tolerance of cancer cells.
Owner:BIOCON LTD
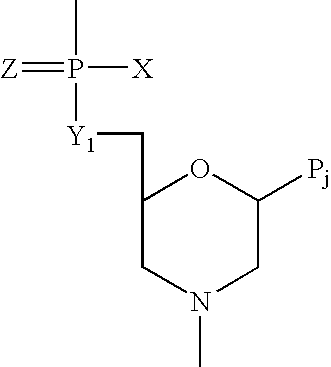
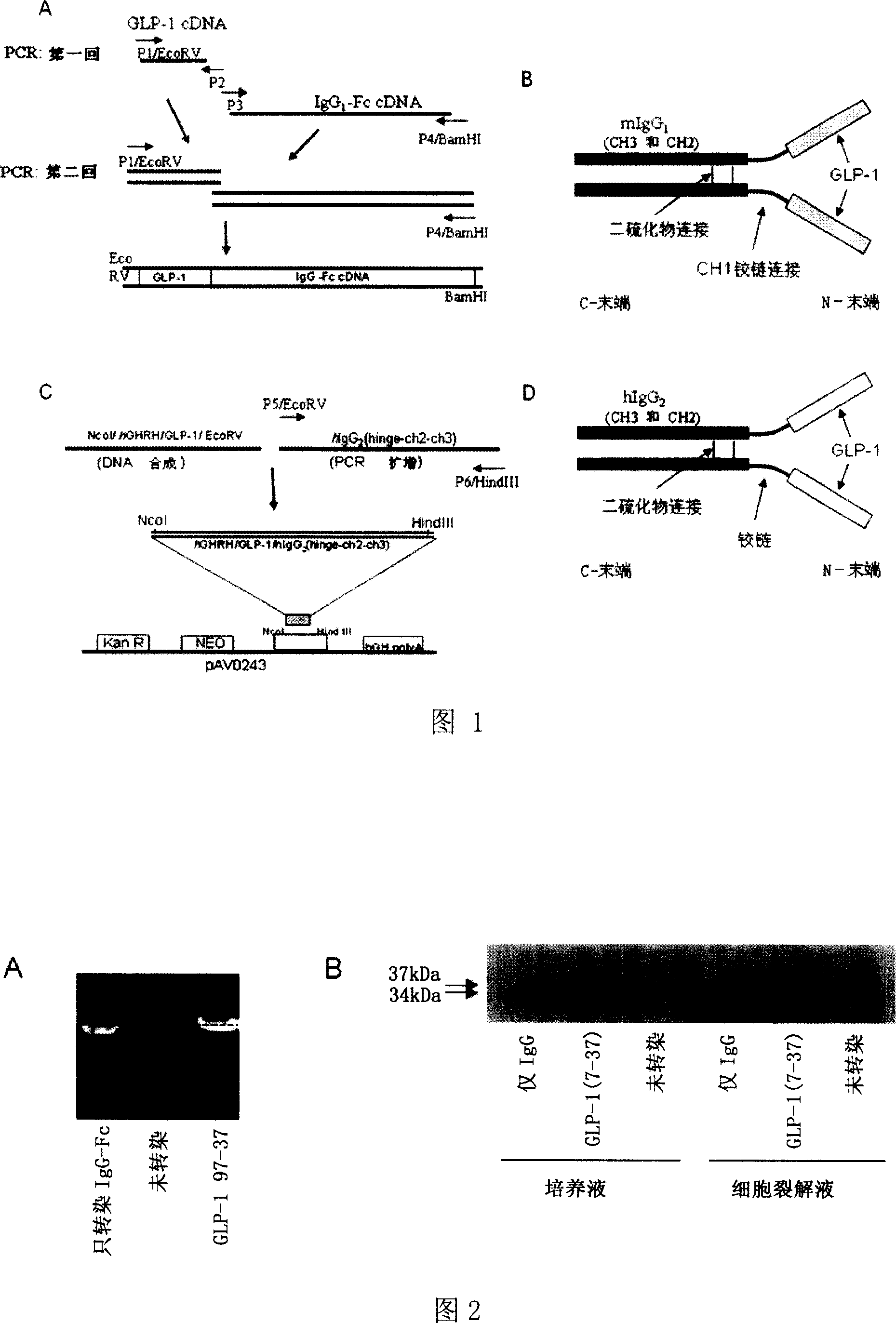
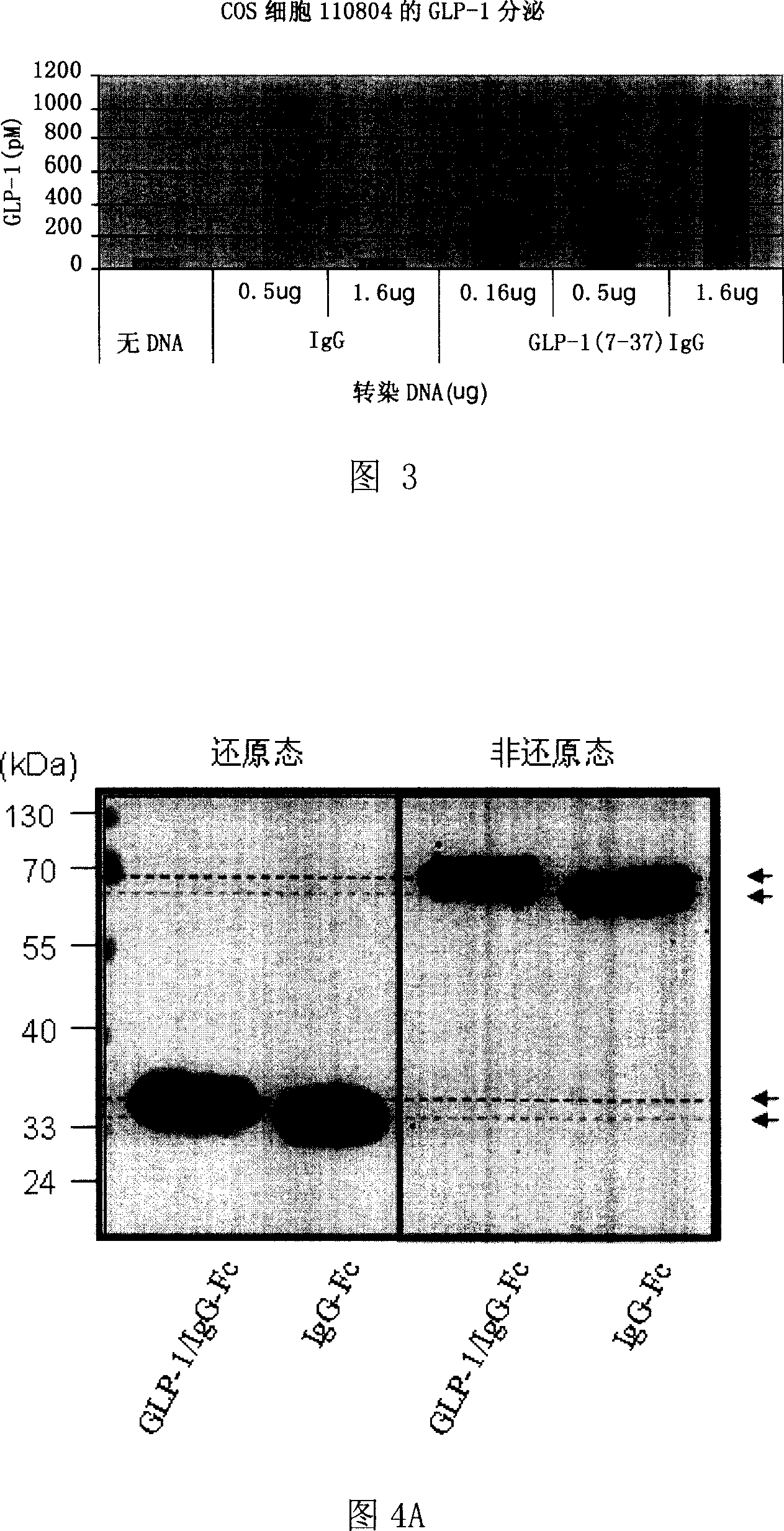
Fusion protein for treating diabetes, and its preparing method and use
InactiveCN1935846AImprove efficacyEasy to separate and purifyOrganic active ingredientsMetabolism disorderHalf-lifeFactor ii
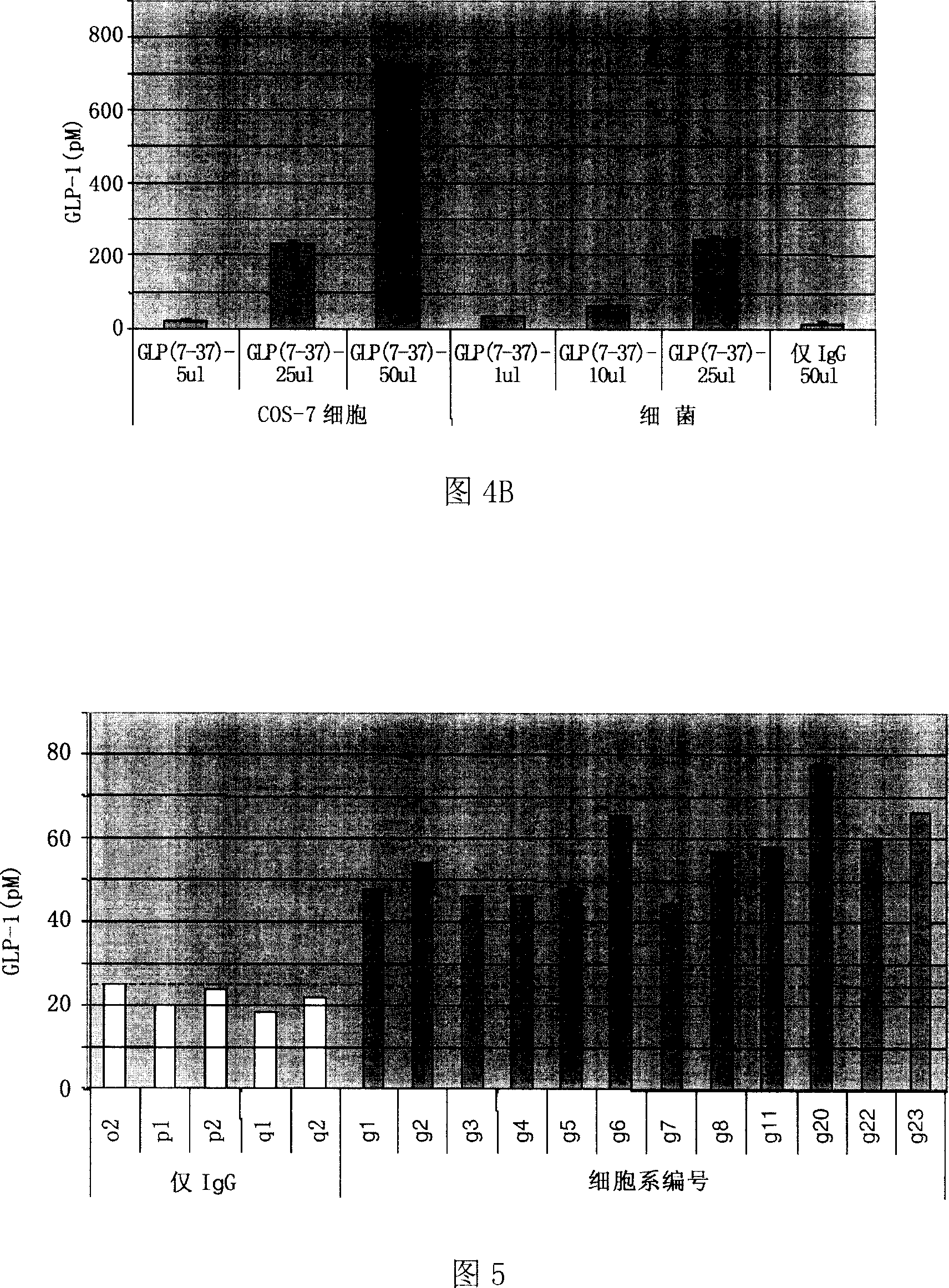
The invention relates to fusion protein used to prevent and cure I type and II type diabetes and its preparation method and application. It supplies the fusion protein which is formed by glucagon analogy peptide GLP-1, GLP-1 mutant and IgG-Fc, its analogy factor lizard salivary gland polypeptide extendin-4 and IgG-Fc, and the application of the above fusion protein and its DNA used in diabetes prevention and therapy. The fusion protein can increase not only GLP-1 effect, but also the affinity and immunological tolerance of ligand, which is excreted with the form of homogeneity dimmer to improve polypeptide drug effect, overcomes the defect of the GLP-1 for short half-life, and simplifies purification process.
Owner:王庆华 +1
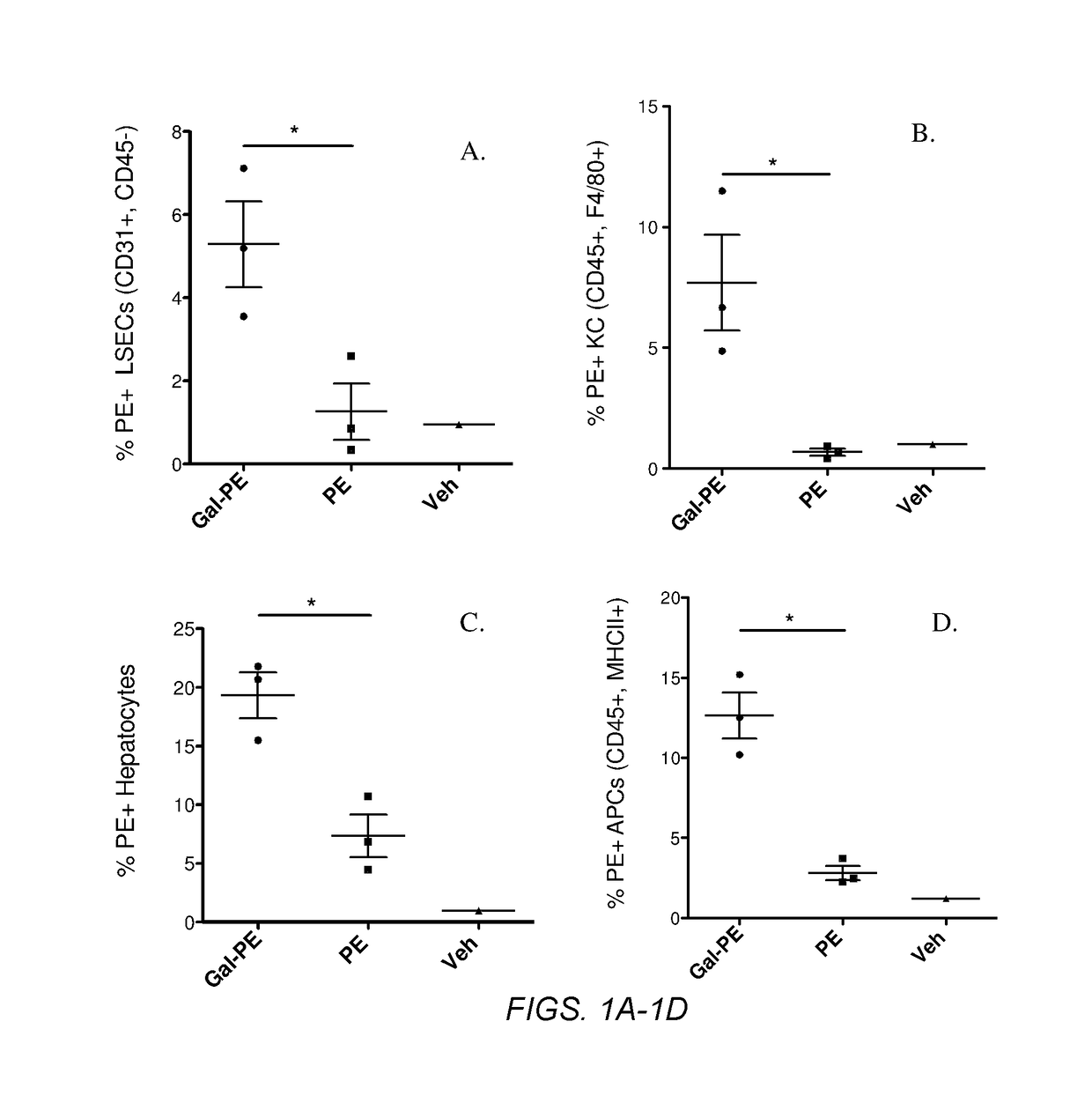
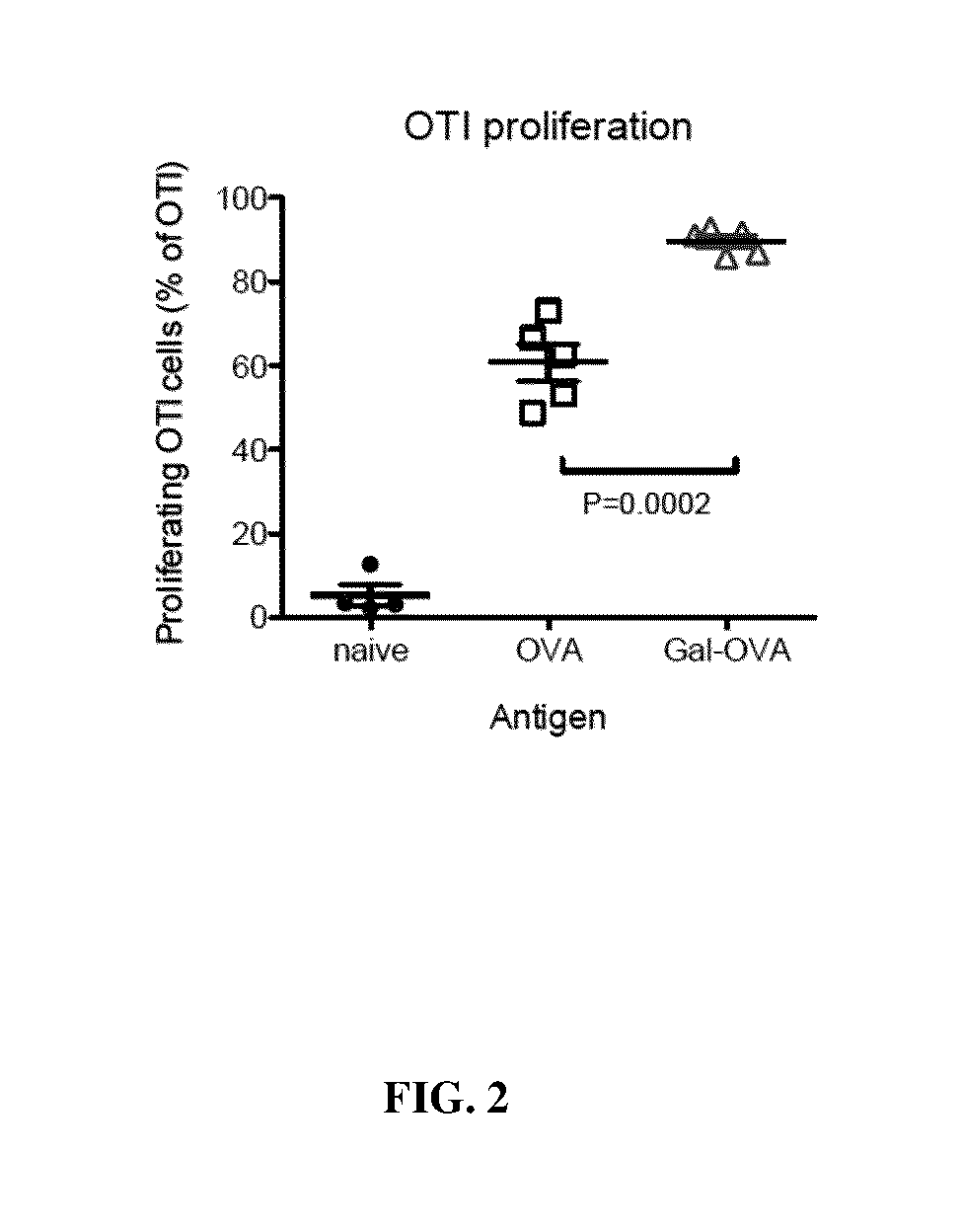
Glycotargeting therapeutics
ActiveUS20170007708A1Reduce immune responseReduce symptomPolypeptide with localisation/targeting motifPeptide/protein ingredientsAutoimmune diseaseTolerability
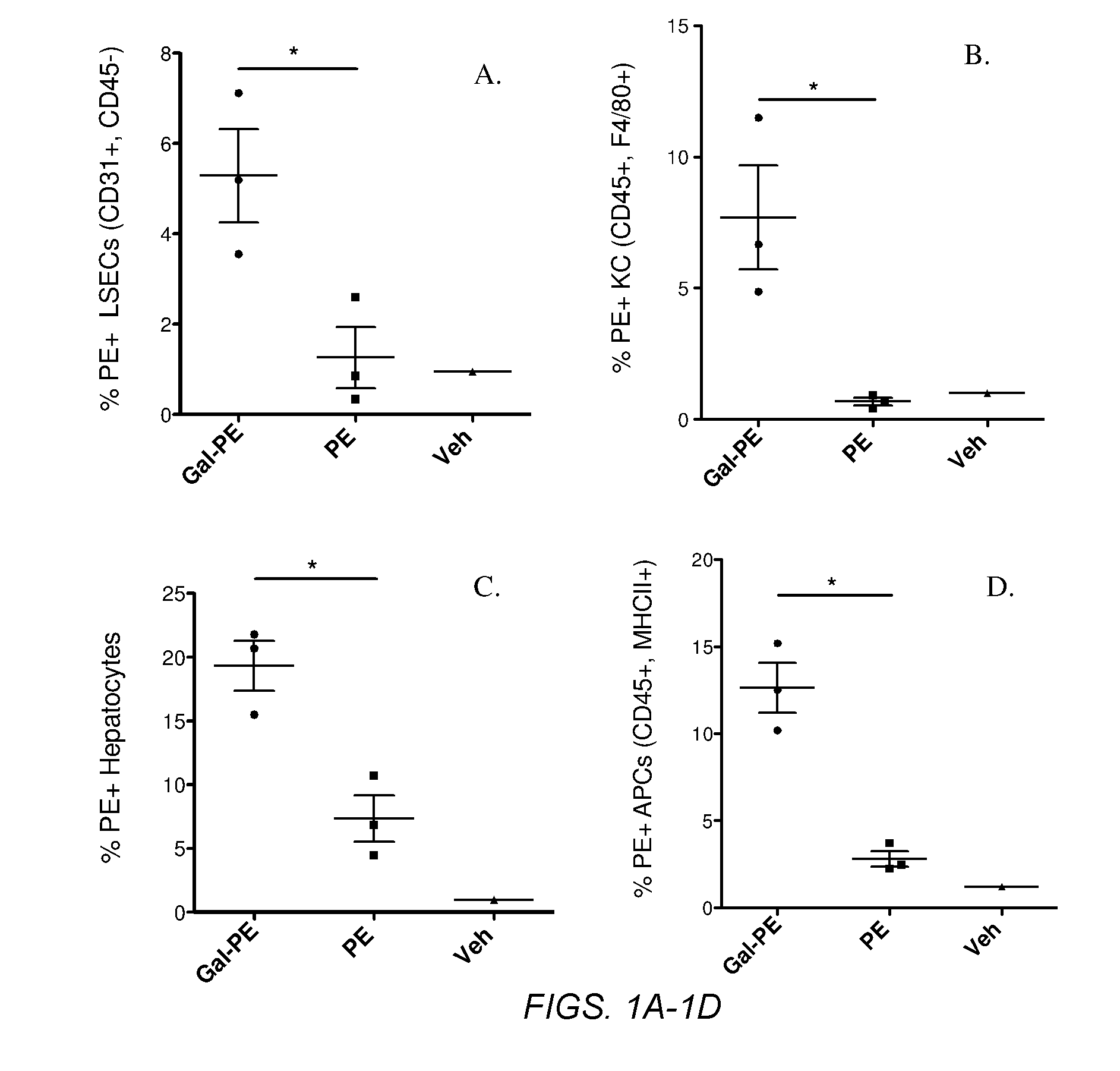
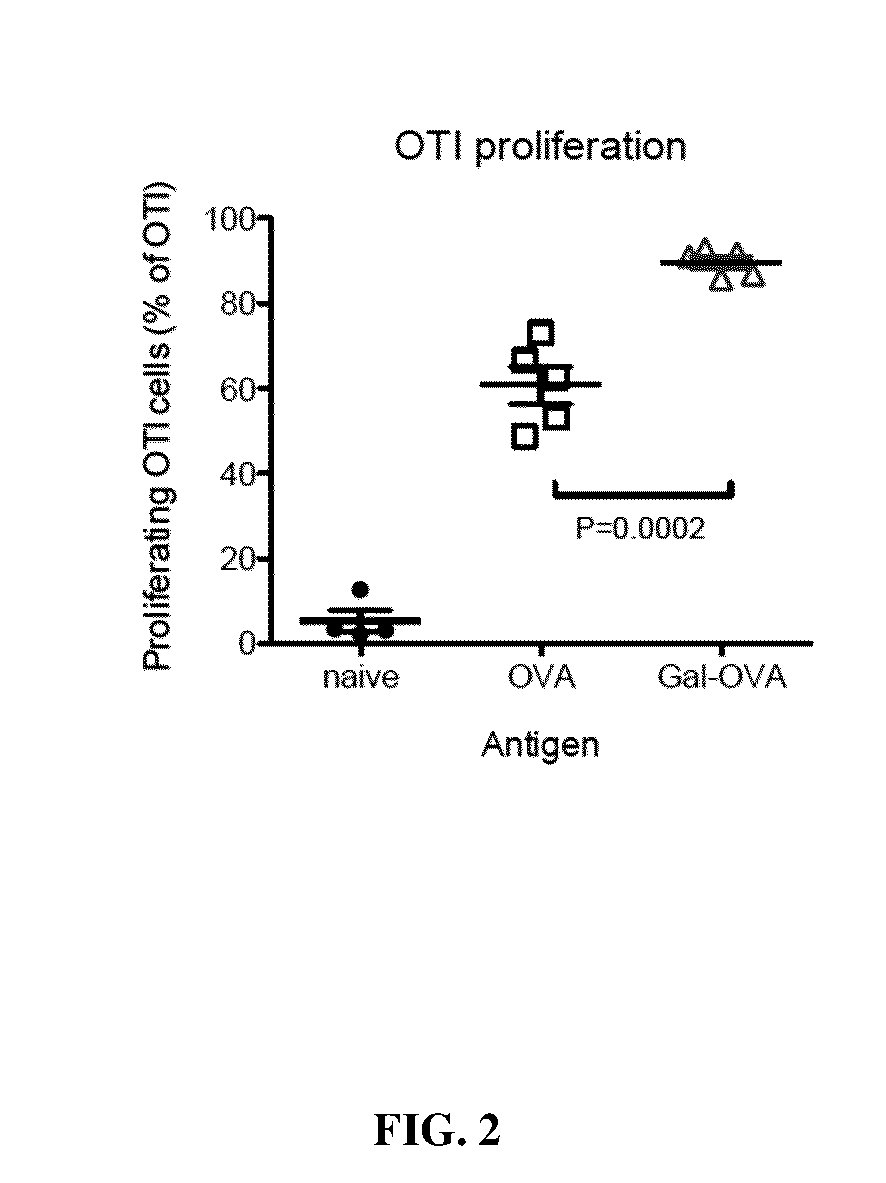
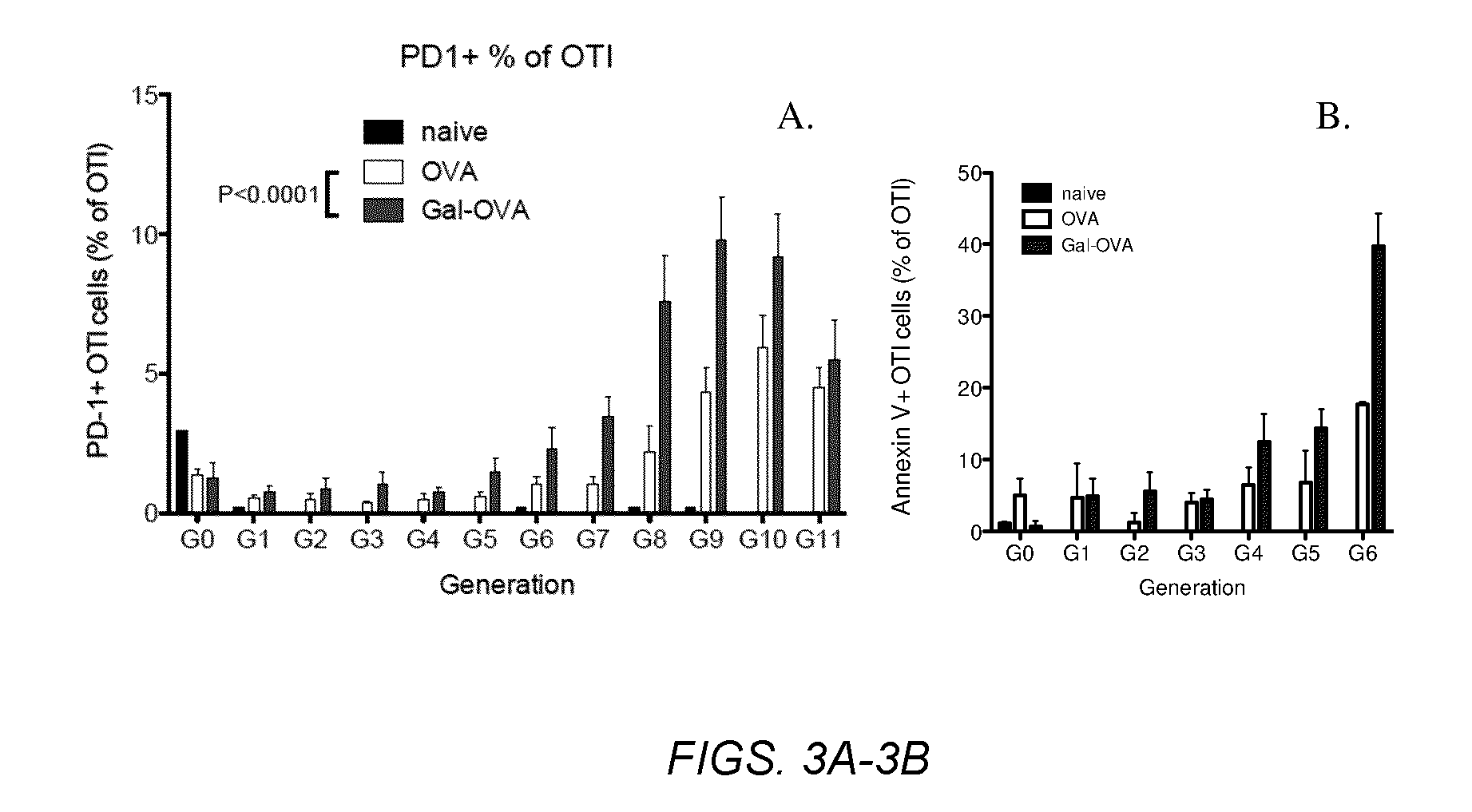
Several embodiments of the present disclosure relate to glycotargeting therapeutics that are useful in the treatment of transplant rejection, autoimmune disease, food allergy, and immune response against a therapeutic agent. In several embodiments, the compositions are configured to target the liver and deliver antigens to which tolerance is desired. Methods and uses of the compositions for induction of immune tolerance are also disclosed herein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Methods of reducing immunogenicity against factor viii in individuals undergoing factor viii therapy
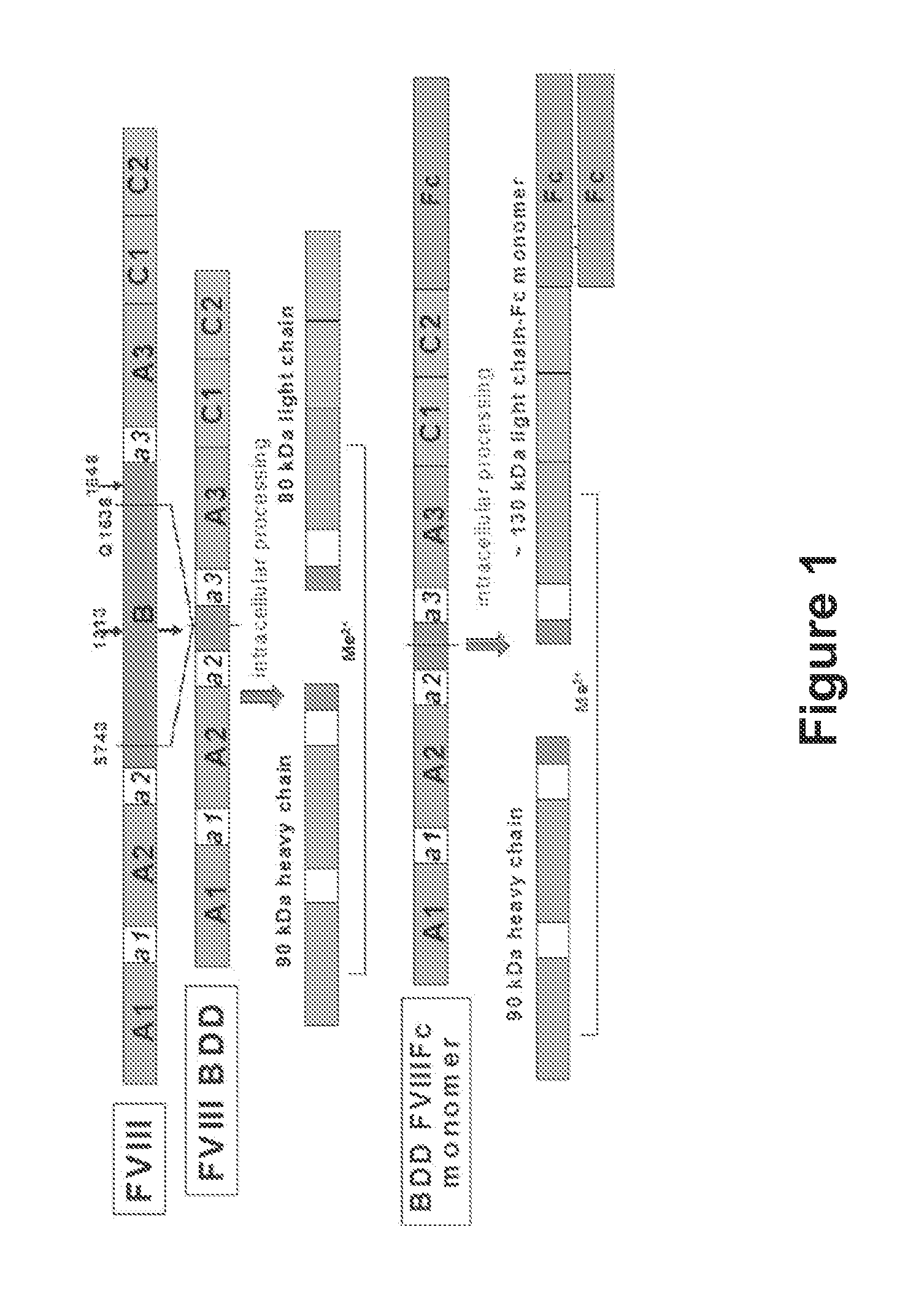
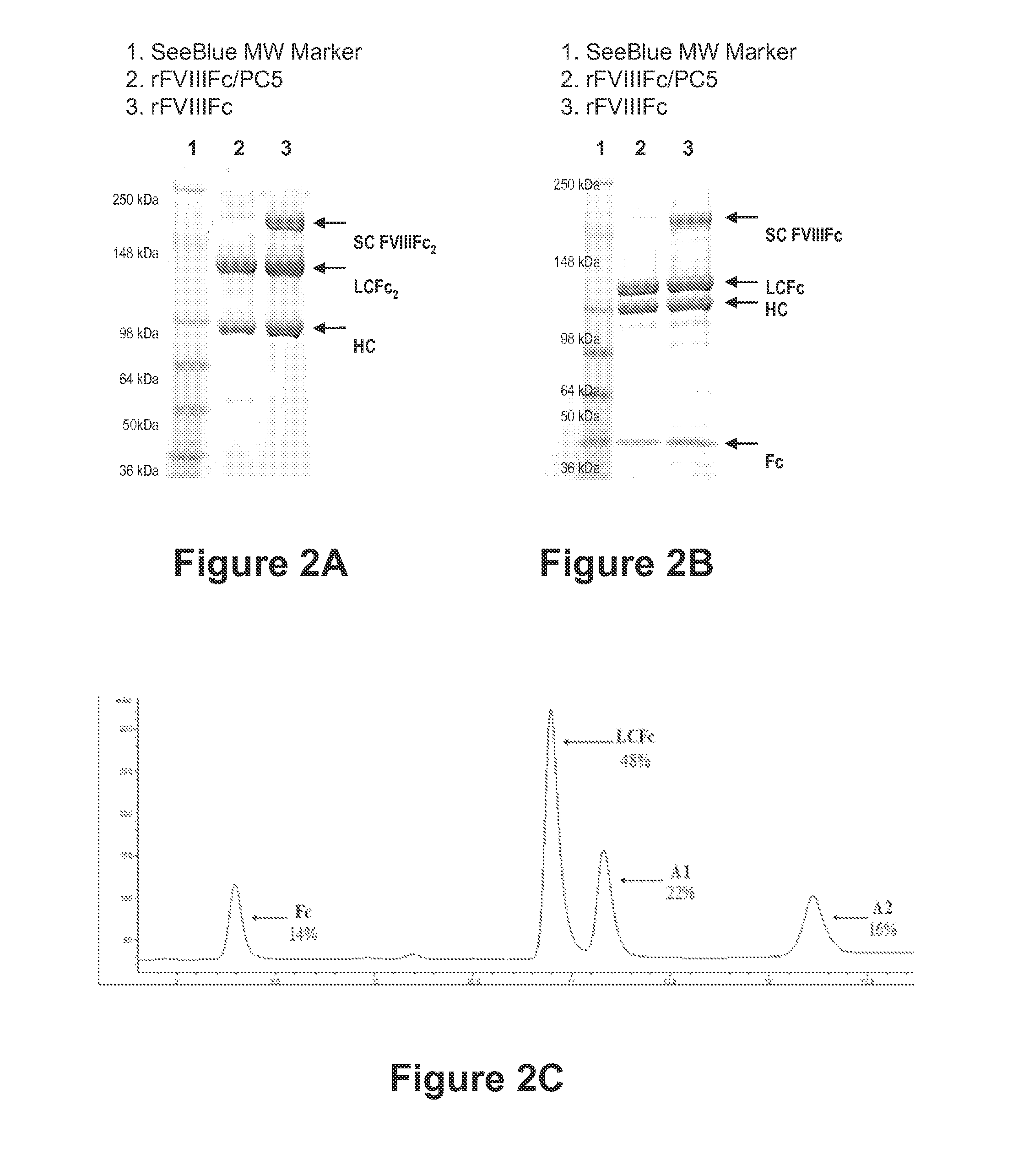
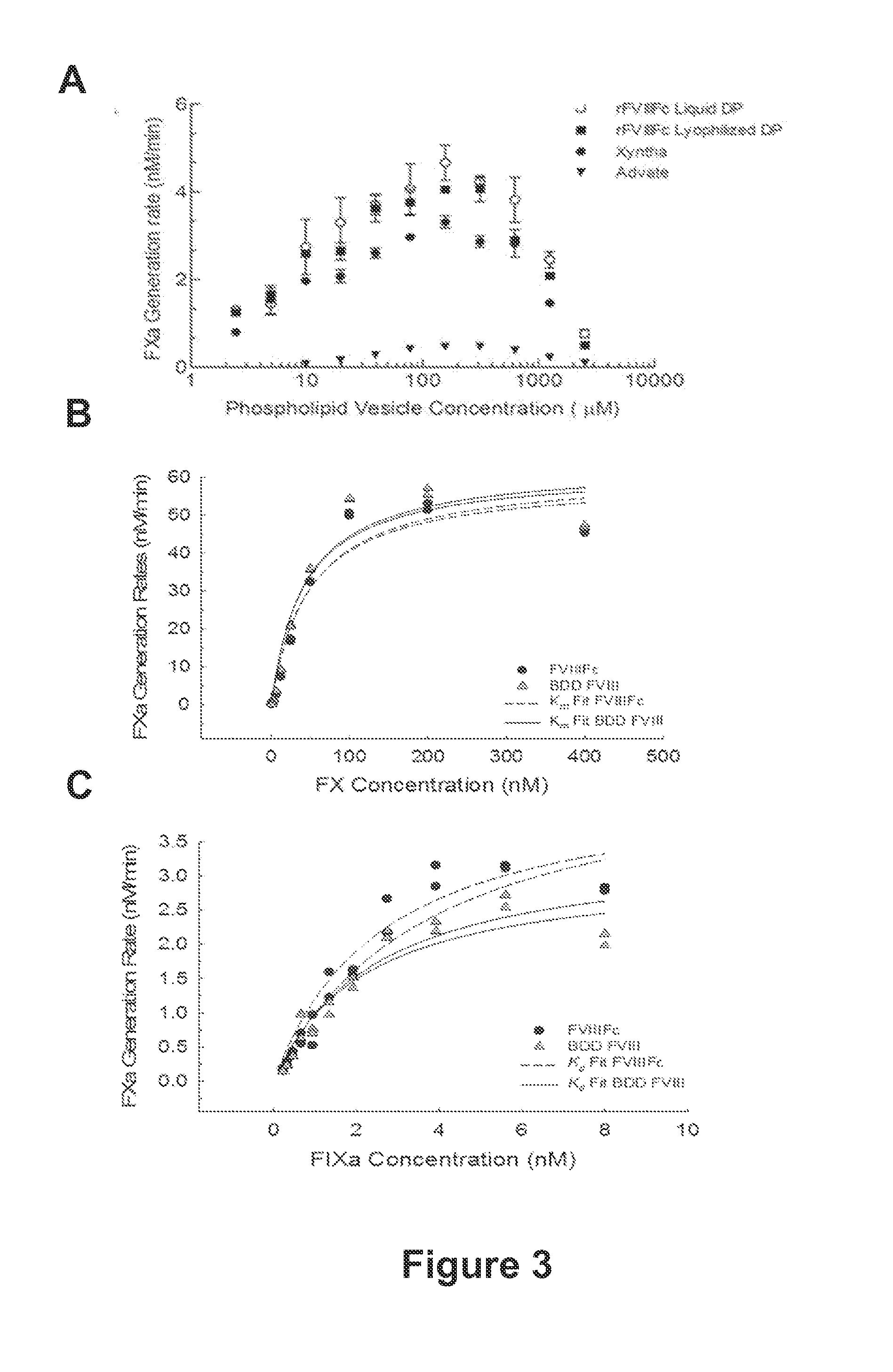
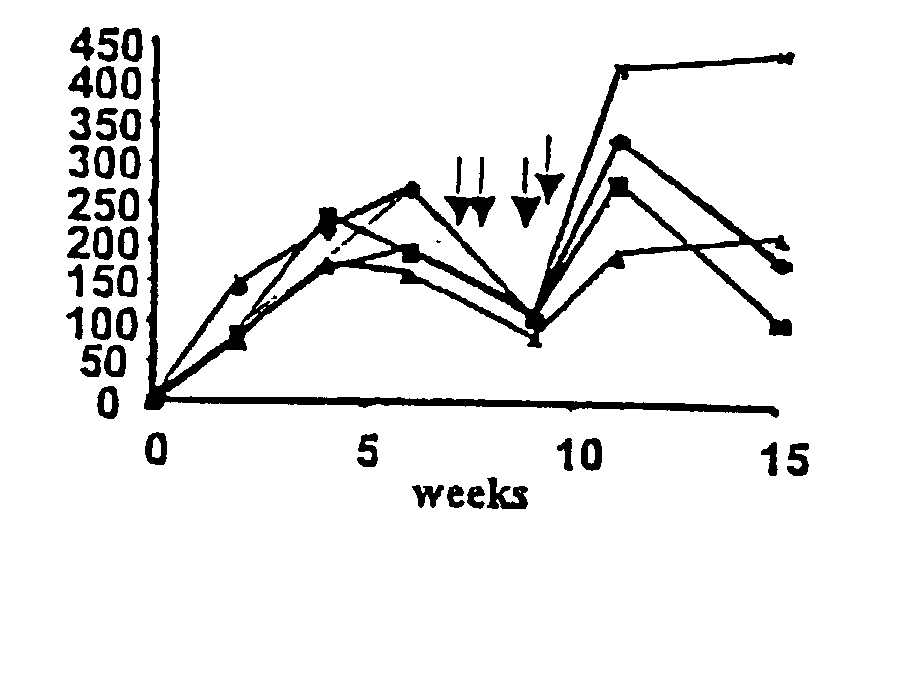
ActiveUS20140370035A1Improve immune toleranceReduce morbidityFactor VIINervous disorderFactor iiImmune tolerance
The present disclosure provides methods of administering chimeric and hybrid Factor VIII (FVIII) polypeptides comprising FVIII and Fc to subjects at risk of developing inhibitory FVIII immune responses, including anti-FVIII antibodies and / or cell-mediated immunity. The administration is sufficient to promote coagulation and to induce immune tolerance to FVIII. The chimeric polypeptide can comprise full-length FVIII or a FVIII polypeptide containing a deletion, e.g., a full or partial deletion of the B domain.
Owner:PUGET SOUND BLOOD CENT +1
Induction of tolerance to a therapeutic polypeptide
Liver-directed gene transfer can induce immunological tolerance to a polypeptide associated with the expression of a therapeutic nucleic acid. Hepatic expression of a transgene induces tolerance to the expression product of the transgene, or to post-translational product related to transgene expression, thereby ameliorating or eliminating the immune responses associated with gene therapy and protein replacement, respectively, independent of the genetic background of the subject.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
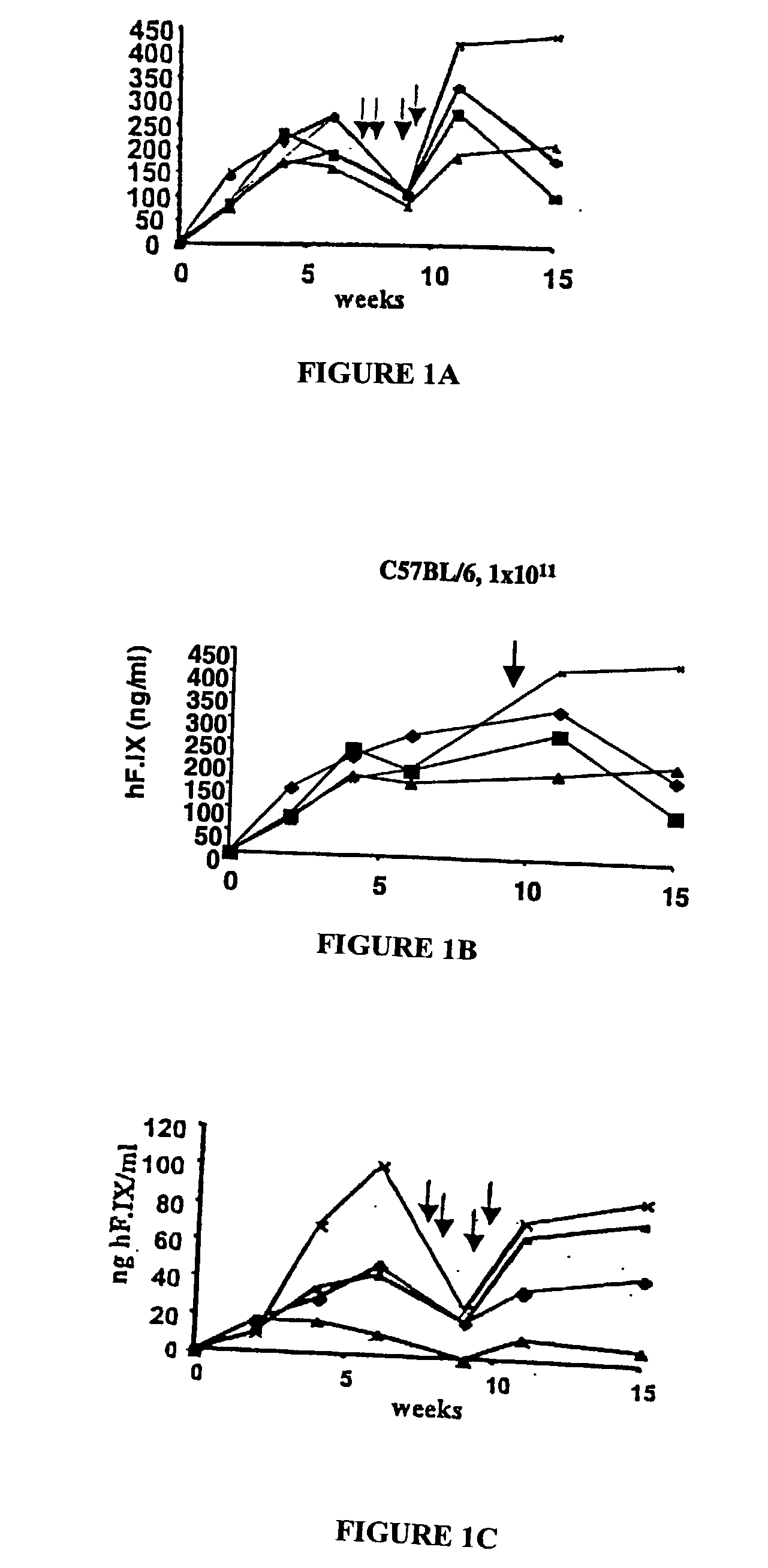
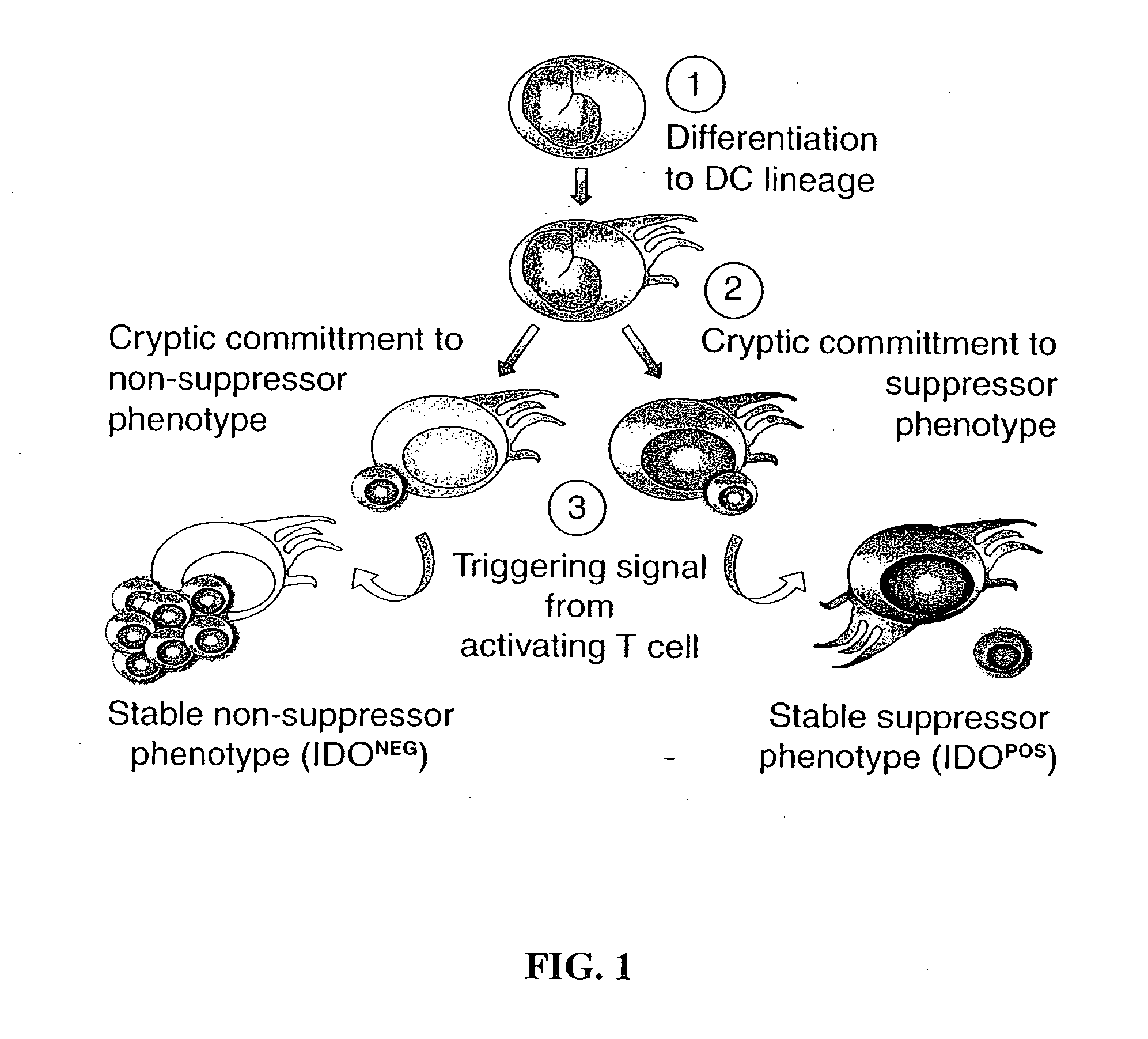
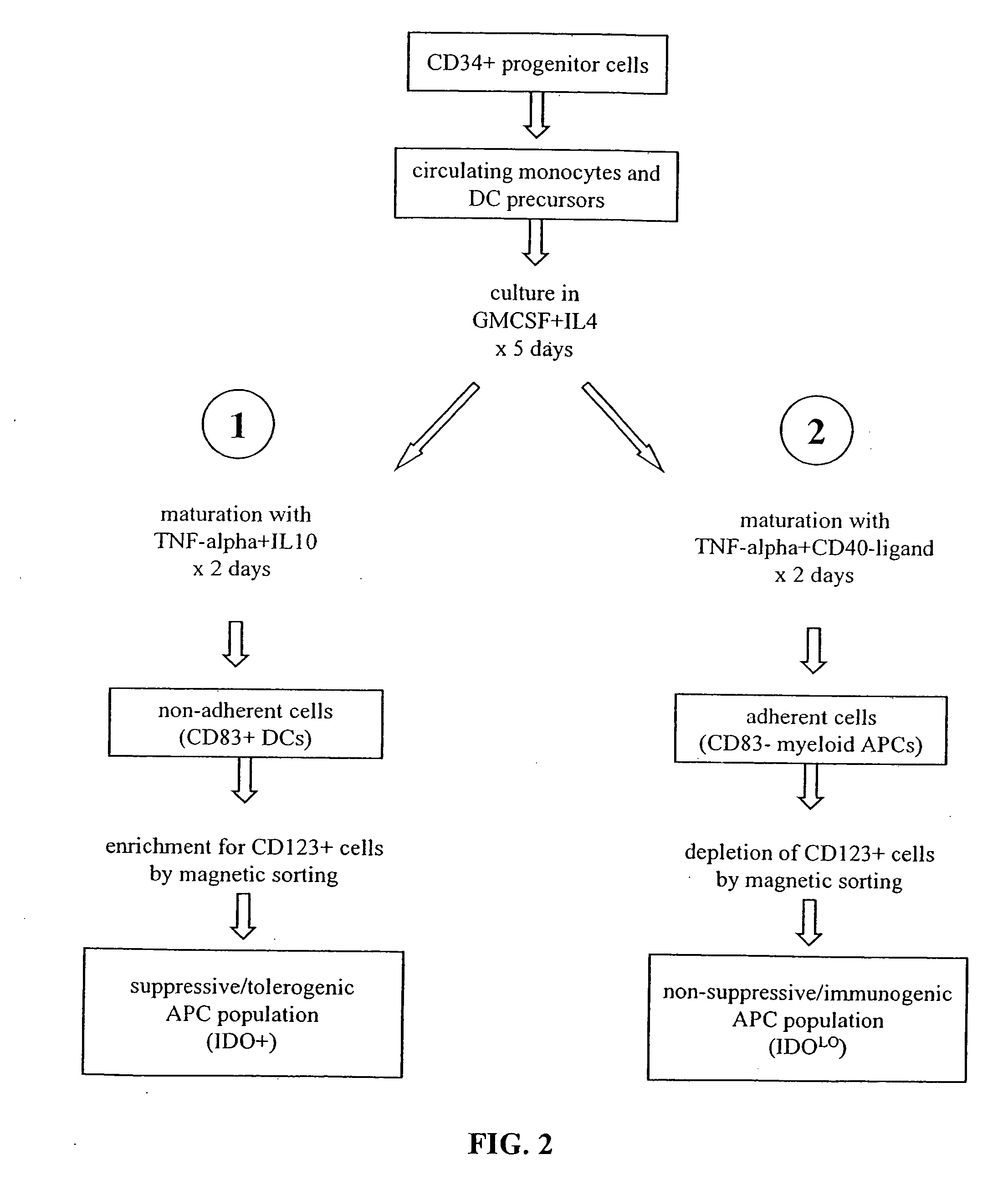
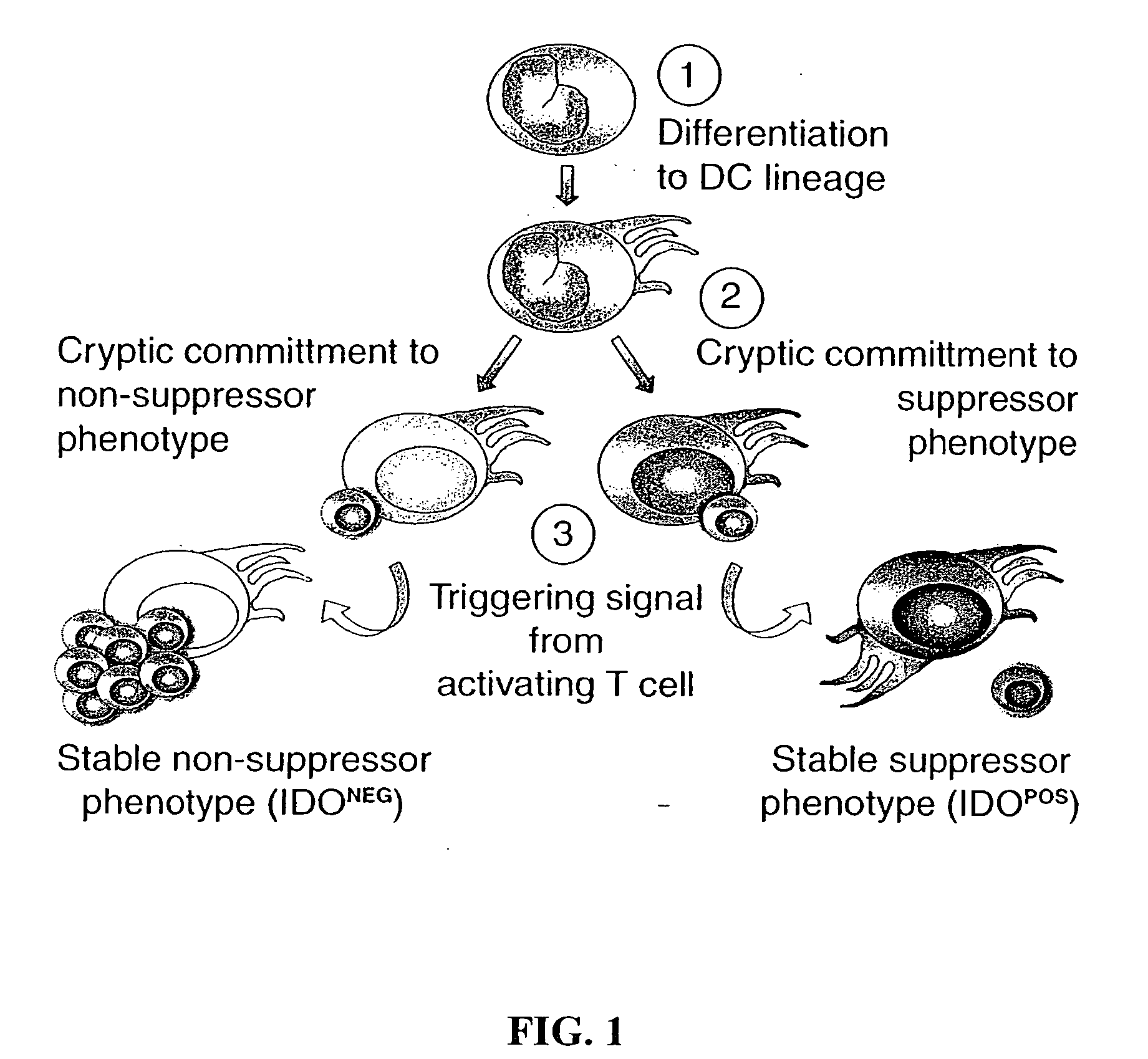
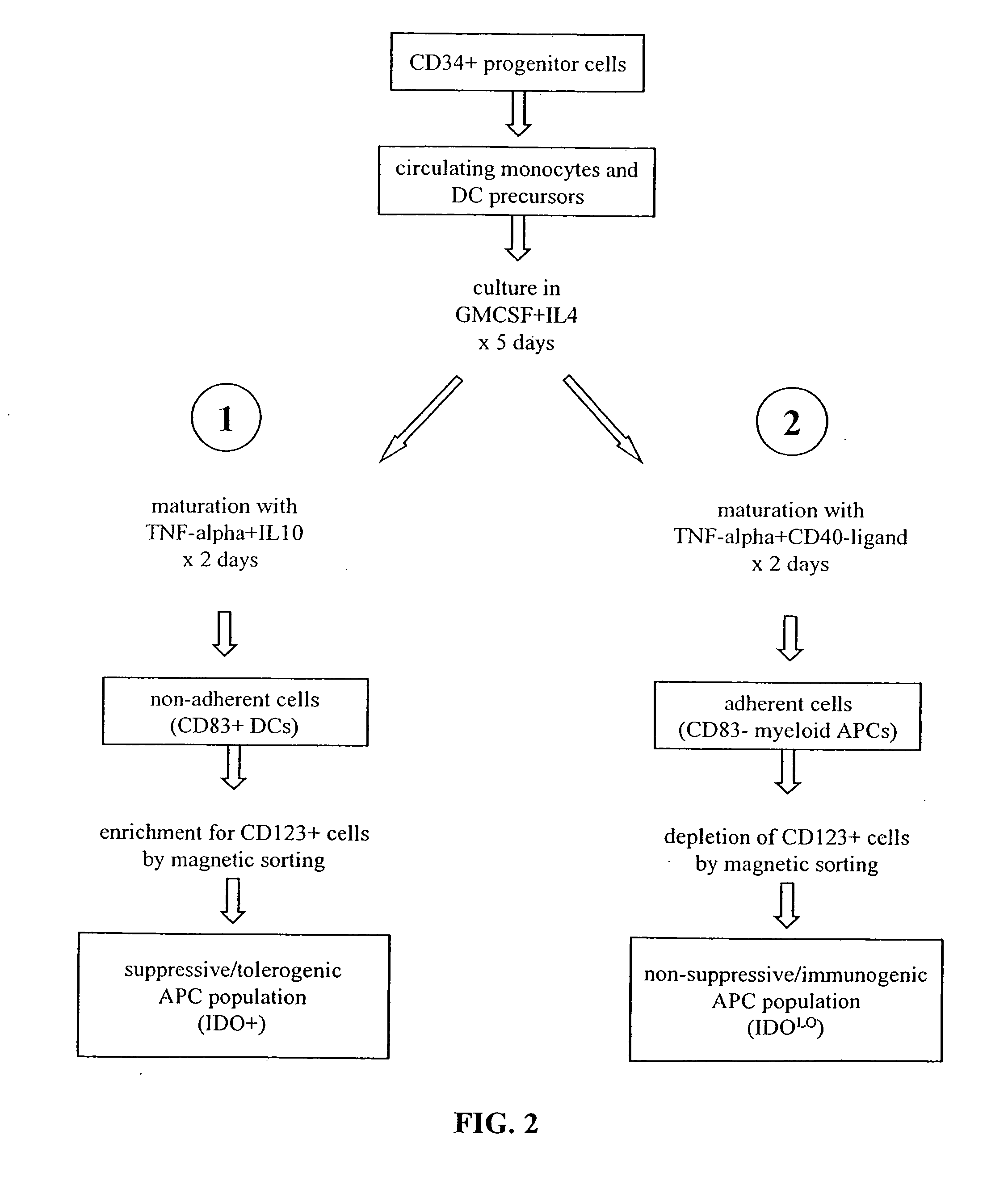
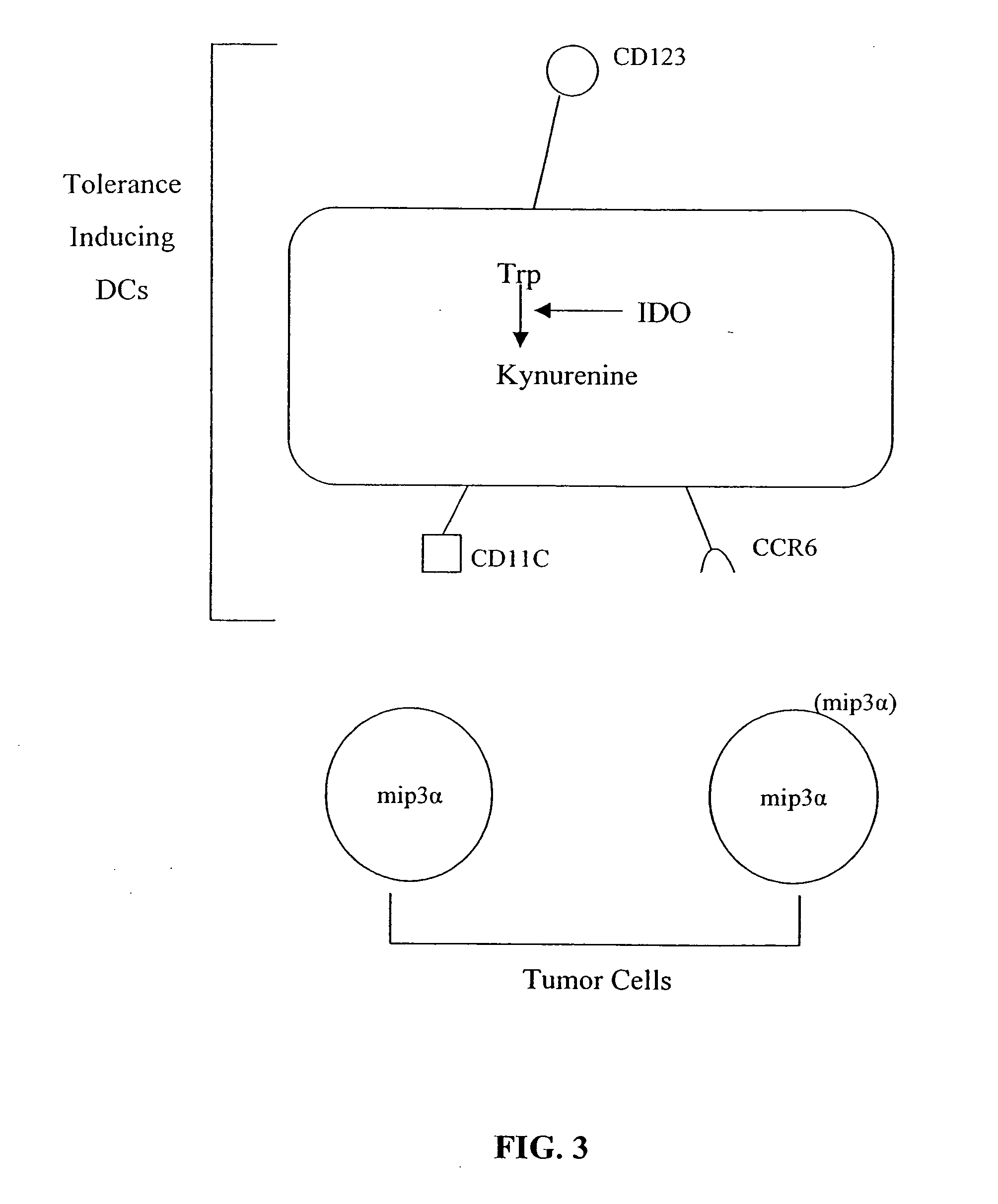
Antigen-presenting cell populations and their use as reagents for enhancing or reducing immune tolerance
InactiveUS20060292618A1Low levelImprove toleranceMicrobiological testing/measurementBlood/immune system cellsImmunologic disordersDisease
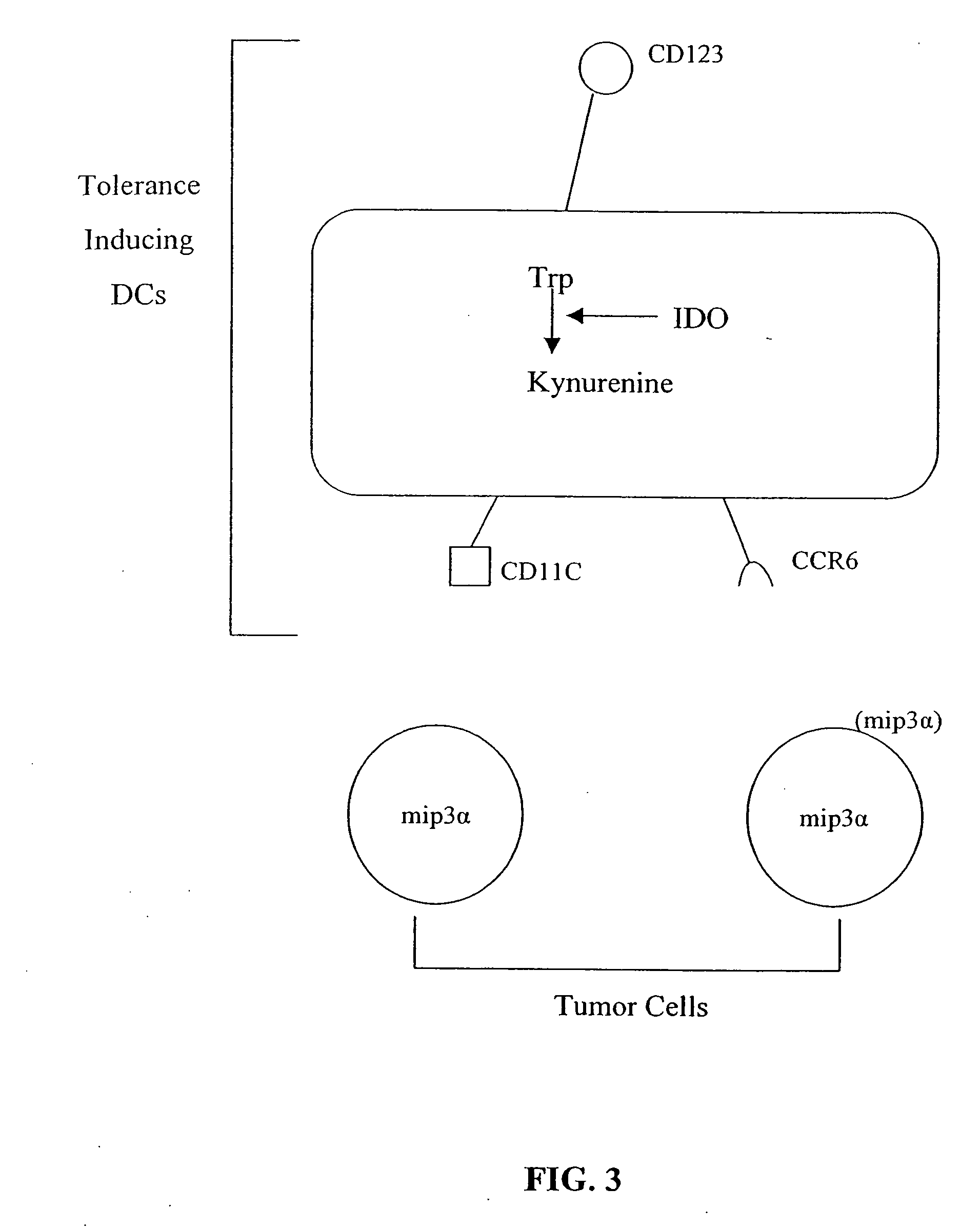
The present invention is based on the discovery antigen-presenting cells (APCs) may be generated to have predetermined levels of expression of the intracellular enzyme, indoleamine 2,3-dioxygenase (IDO). Because expression of high levels of IDO is correlated with a reduced ability to stimulate T cell responses and an enhanced ability to induce immunologic tolerance, APCs having high levels of IDO may be used to increase tolerance in the immune system, as for example in transplant therapy or treatment of autoimmune disorders. For example, APCs having high levels of IDO, and expressing or loaded with at least one antigen from a donor tissue may be used to increase tolerance of the recipient to the donor's tissue. Alternatively, APCs having reduced levels of IDO expression and expressing or loaded with at least one antigen from a cancer or infectious pathogen may be used as vaccines to promote T cell responses and increase immunity.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Methods for producing antibody
InactiveUS7750204B2Easy to getEfficient productionPeptide/protein ingredientsVirus peptidesImmune toleranceIndividual animal
This invention provides methods for producing antibodies, wherein the methods comprise the step of administering an immunogen comprising both a target antigen and a background antigen to transgenic animals, into which a gene coding for the background antigen has been introduced. Since immunotolerance to the background antigens have thus been induced in the transgenic animals, the animals efficiently produce antibodies to target antigens.
Owner:CHUGAI PHARMA CO LTD
Antigen-presenting cell populations and their use as reagents for enhancing or reducing immune tolerance
InactiveUS20070048769A1Low levelImprove toleranceMicrobiological testing/measurementAntibody ingredientsAntigenDisease
The present invention is based on the discovery antigen-presenting cells (APCs) may be generated to have predetermined levels of expression of the intracellular enzyme, indoleamine 2,3-dioxygenase (IDO). Because expression of high levels of IDO is correlated with a reduced ability to stimulate T cell responses and an enhanced ability to induce immunologic tolerance, APCs having high levels of IDO may be used to increase tolerance in the immune system, as for example in transplant therapy or treatment of autoimmune disorders. For example, APCs having high levels of IDO, and expressing or loaded with at least one antigen from a donor tissue may be used to increase tolerance of the recipient to the donor's tissue. Alternatively, APCs having reduced levels of IDO expression and expressing or loaded with at least one antigen from a cancer or infectious pathogen may be used as vaccines to promote T cell responses and increase immunity.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Glycotargeting therapeutics
ActiveUS10046056B2Reduced responseRelieve symptomsPolypeptide with localisation/targeting motifPeptide/protein ingredientsAntigenAutoimmune condition
Several embodiments of the present disclosure relate to glycotargeting therapeutics that are useful in the treatment of transplant rejection, autoimmune disease, food allergy, and immune response against a therapeutic agent. In several embodiments, the compositions are configured to target the liver and deliver antigens to which tolerance is desired. Methods and uses of the compositions for induction of immune tolerance are also disclosed herein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Transfer factor solution for livestock and poultry, and preparation method thereof
InactiveCN101537169ARealize storage at room temperatureImprove disease resistanceAntibacterial agentsPeptide/protein ingredientsDiseaseGlycerol
The invention provides a transfer factor solution for livestock and poultry. Frozen pig spleens are washed clean by use of distilled water, cut into small pieces, added with distilled water free from heat source, subjected to cell disruption by use of a tissue bruiser and prepared into homogenate; the homogenate passes through a high-pressure homogenizer under the pressure of 60 kPa and then is added with a flocculating agent for flocculation; the flocculated homogenate is centrifuged at a centrifugation ambient temperature of 4 DEG C and at a speed of 7,000 r / min; precipitate is removed; supernatant is left on standby; the supernatant is filtered by use of a microporous filtering membrane 0.45 mu m in pore diameter, and then is ultrafiltered by use of a ultrafiltration membrane 5,000 Dalton in molecular weight cutoff so as to obtain a stock solution; after viruses are removed, nicotinamide is added as a stabilizer; the stock solution is subjected to aseptic filtration by use of the microporous filtering membrane; and the obtained product is added with glycerol as a heat-resisting protective agent and then is directly and separately packed. The transfer factor solution can be applied to livestock and poultry. The preparation method is characterized by directly using the pig spleen to extract the transfer factor solution so as to save manpower and materials and improve extraction efficiency. The preparation method combines a heat-resisting protection technique with transfer factors, thereby realizing the normal-temperature preservation of the transfer factors. The transfer factor solution can improve the disease-resisting capability of organisms, relieve immunologic suppression and immunologic tolerance, can play a role in emergency adjuvant treatment, and makes up for immunization blank after vaccination before antibody production.
Owner:TIANJIN RINGPU BIO TECH
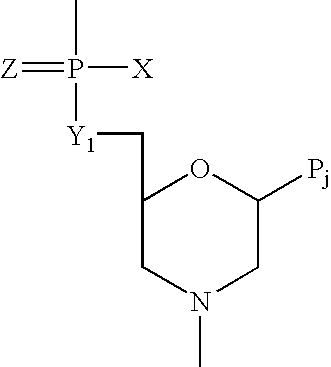
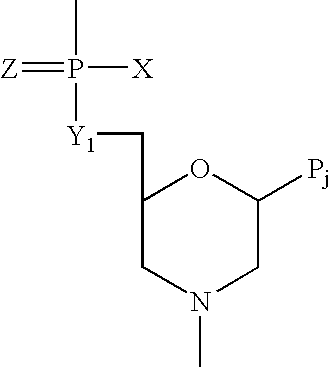
[1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof
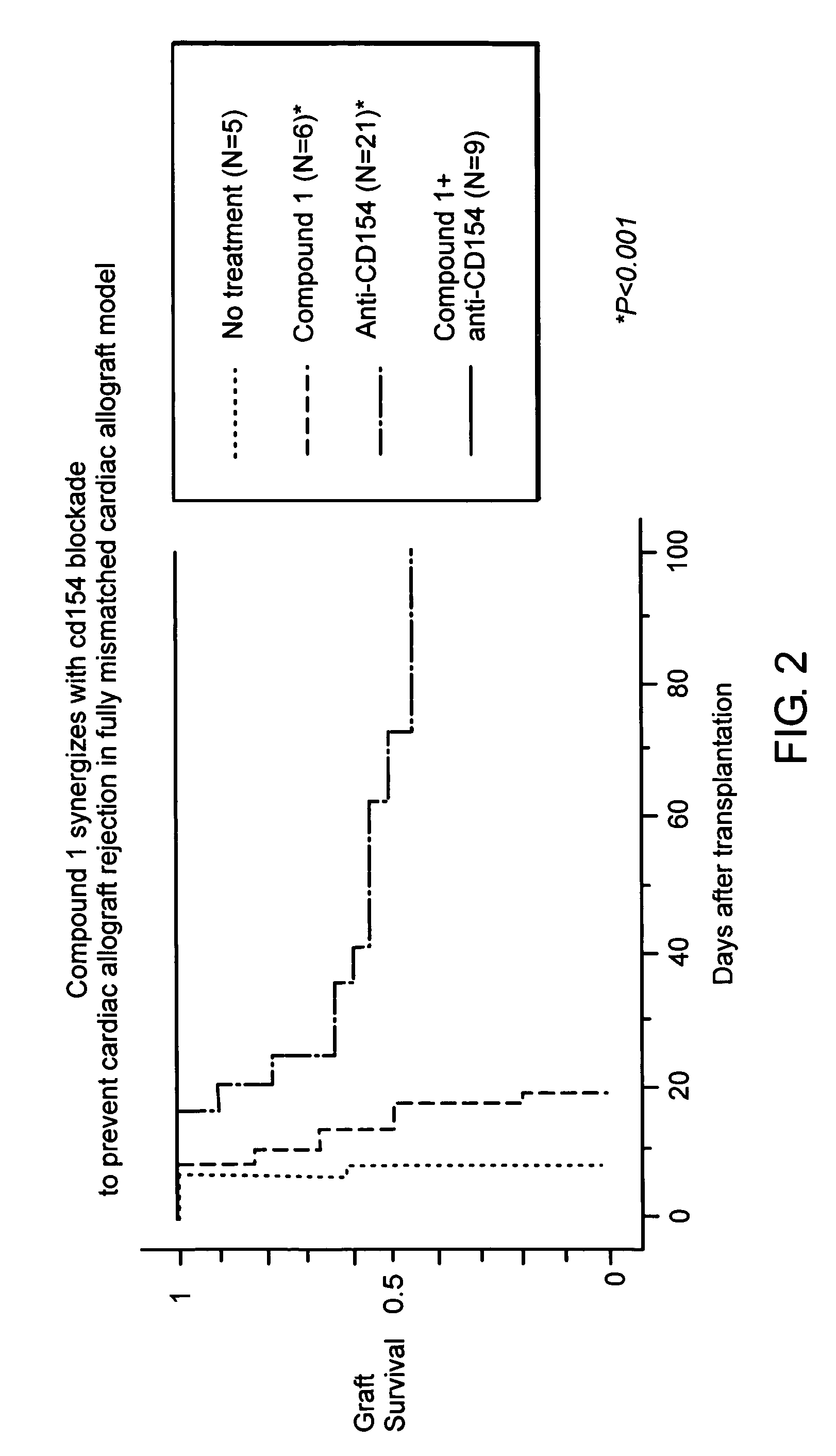
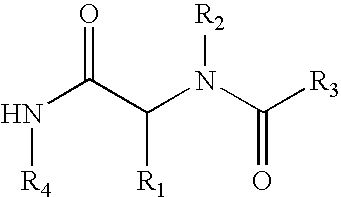
InactiveUS20070010515A1Suppression problemSuppresses lymphocyte proliferation responseOrganic active ingredientsBiocideGraft versus host reactionsAutoimmune condition
Novel [1,2,4]triazolo[1,5-a]pyrimidine derivative of the general formula (1): (1) its prodrug or a pharmaceutically acceptable salt thereof, which exhibits an antigen presentation inhibiting activity and is useful as a preventive and / or therapeutic agent for immunological rejection and / or graft versus host reaction in organ / bone marrow transplant, autoimmune disease, allergic disease and / or inflammatory disease and also useful as an anticancer drug or as an immunological tolerance inducer for transplanted organ / transplanted bone marrow.
Owner:NIPPON KAYAKU CO LTD +1
Insulin independence among patients with diabetes utilizing an optimized hamster reg3 gamma peptide
ActiveUS20160039875A1Less susceptible to protease cleavageImprove bioavailabilityMetabolism disorderPeptide sourcesHamsterRecent onset
Embodiments of the present invention provide for novel therapies, pharmaceutical compositions and methods for insulin independence utilizing a new optimized hamster Reg3 gamma peptide, which is new to the art and has not previously been considered for development in the 30 year history since its discovery. Methods, pharmaceutical compositions and therapies novel to the prior art are utilized in this invention to render patients with recent onset and existing type 1 diabetes insulin independent by an optimized hamster Reg3 gamma peptide and an immune tolerance agent for type 1 patients to become insulin independent and used alone without an immune tolerance agent for type 2 diabetes. While not wishing to be bound by theory, optimized Reg3 gamma peptides increases beta cell generation by its demonstrated properties shown within of transforming ductal pancreatic cells into new islets.
Owner:PERLE BIOSCI
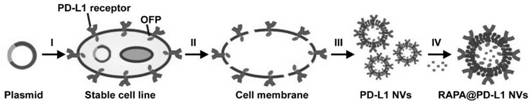
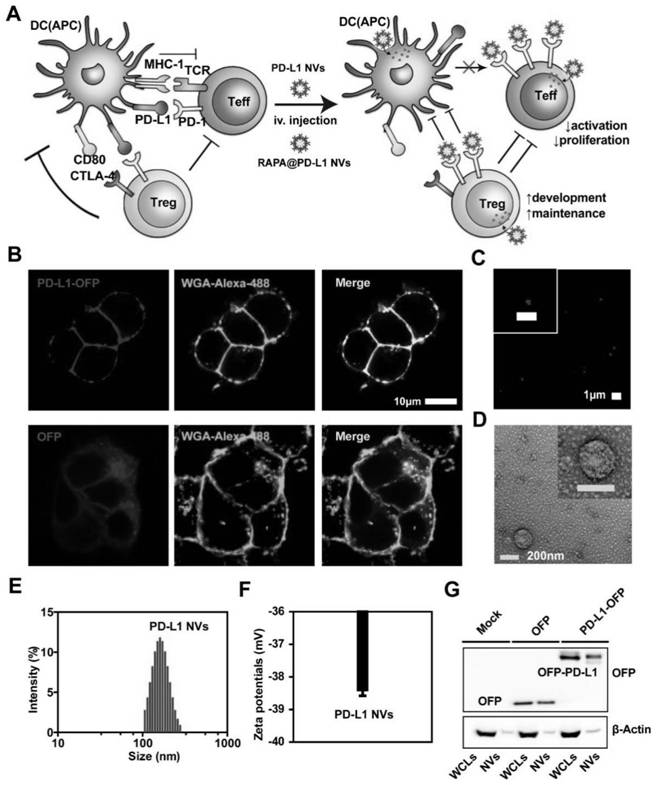
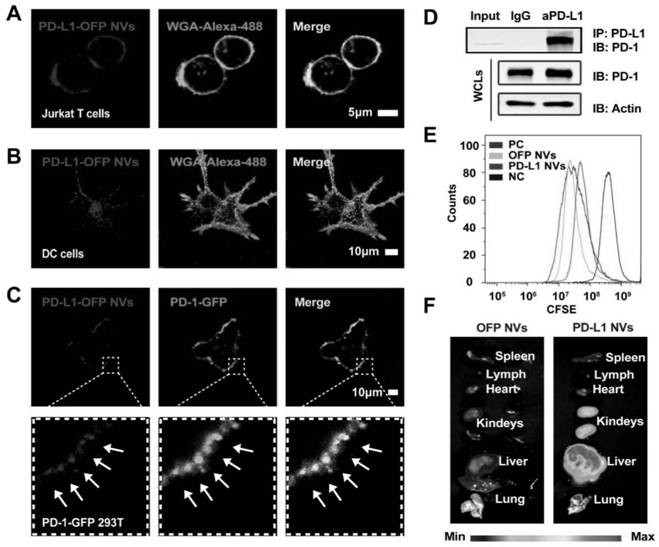
Cell membrane nano-vesicle wrapping immunosuppressant and over-expressing PD-L1 and preparation method and application thereof
ActiveCN111920769AReduce dosageLow toxicityOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsCell membraneT cell
The invention discloses a cell membrane nano-vesicle wrapping an immunosuppressant and overexpressing PD-L1 and a preparation method and application thereof. The cell membrane nano-vesicle is composedof a biological cell membrane, PD-L1 protein is expressed on the surface of the cell membrane, and the immunosuppressant is wrapped in the cell membrane nano-vesicle. PD-L1 protein can be expressed on the surface of a cell membrane; the PD-L1 protein can be combined with PD-1 on the surface of a T cell and activate a PD-1 / PD-L1 immune checkpoint signal axis; meanwhile, the PD-L1 cell membrane nano-vesicle is used as a targeted drug delivery system for loading the immunosuppressant, and the wrapped immunosuppressant is brought to effector T cells expressed by PD-1 to play a role so that the transmission of immunosuppressive drugs to target tissues is enhanced; through combined application of PD-1 / PD-L1 and an immunosuppressant double immune tolerance mechanism to T cells, in-vivo immunological rejection is significantly inhibited, the immunotherapy effect is improved, meanwhile, the dosage of the immunosuppressant is reduced, and the toxicity of the immunosuppressant is reduced.
Owner:中山大学深圳
Immunogen composition containing polypeptide derived from cytomegalovirus and use method thereof
InactiveCN101780277AEfficient activationHas anti-CMV viral activityAntiviralsTissue cultureCmv infectionsImmunosuppressive effect
The invention provides an immunogen composition containing polypeptide derived from cytomegalovirus (CMV) and a use method thereof. The composition comprises (a) at least one of CMV pp65 and polypeptide derived from CMV IE-1 polypeptide; and (b) polypeptide selected from groups formed by polypeptides derived from CMV VGLB, CMV VPAP and CMV p100 polypeptides, and the combination thereof. The immunogen composition of the invention can effectively activate CMV resistant immunoreaction after confirmation. Live virus, cells infected by CMV, and full-length CMV cells are not used in a polypeptide library so that no infection, mmunosuppression action, immunologic tolerance and the like are caused.
Owner:VECTORITE BIOMEDICA
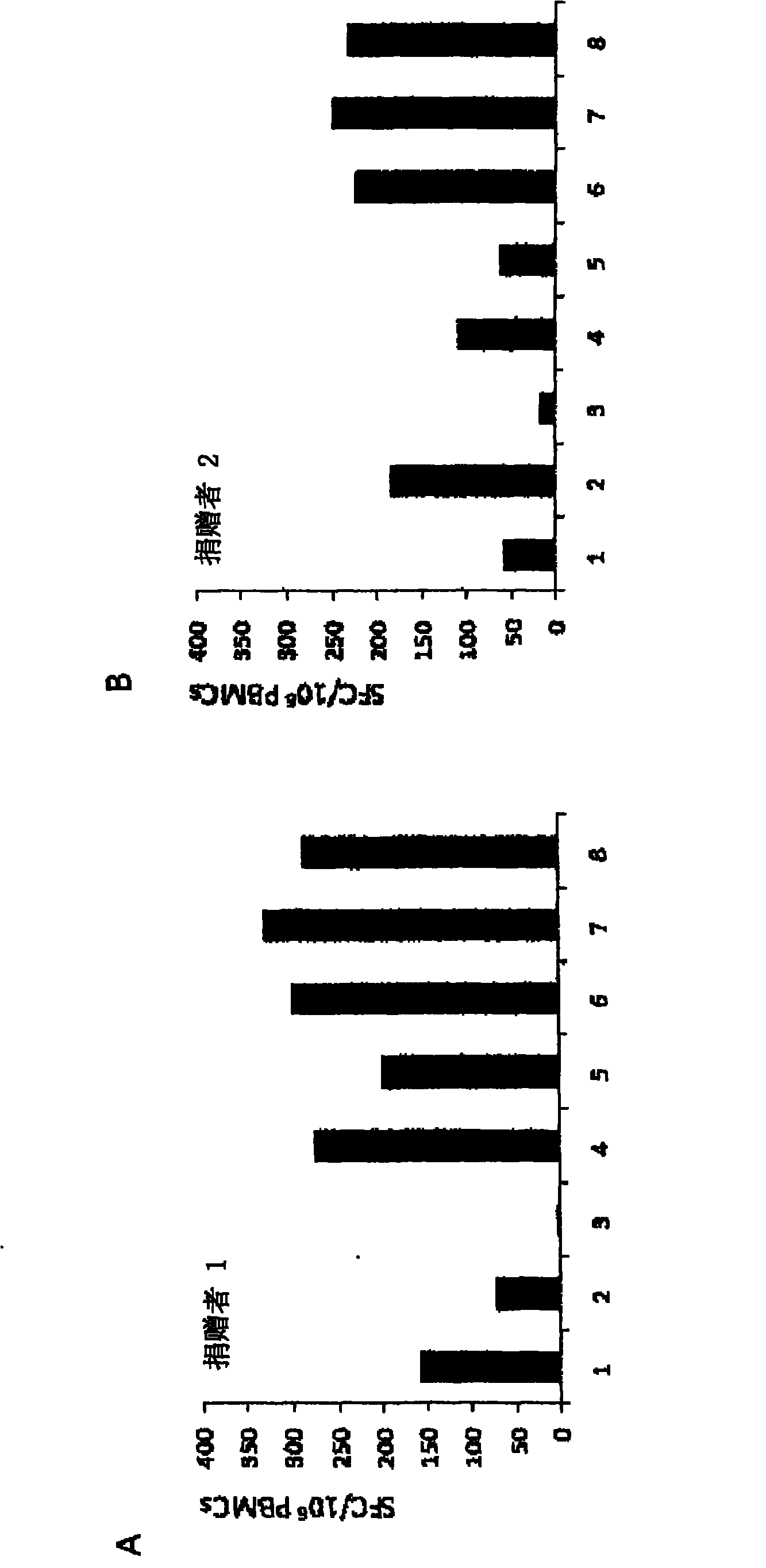
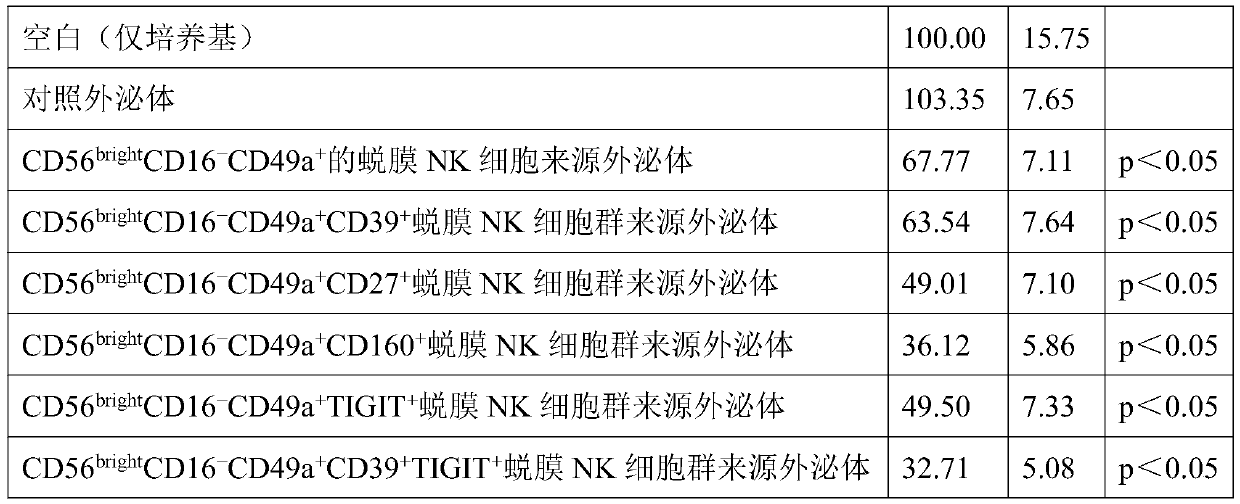
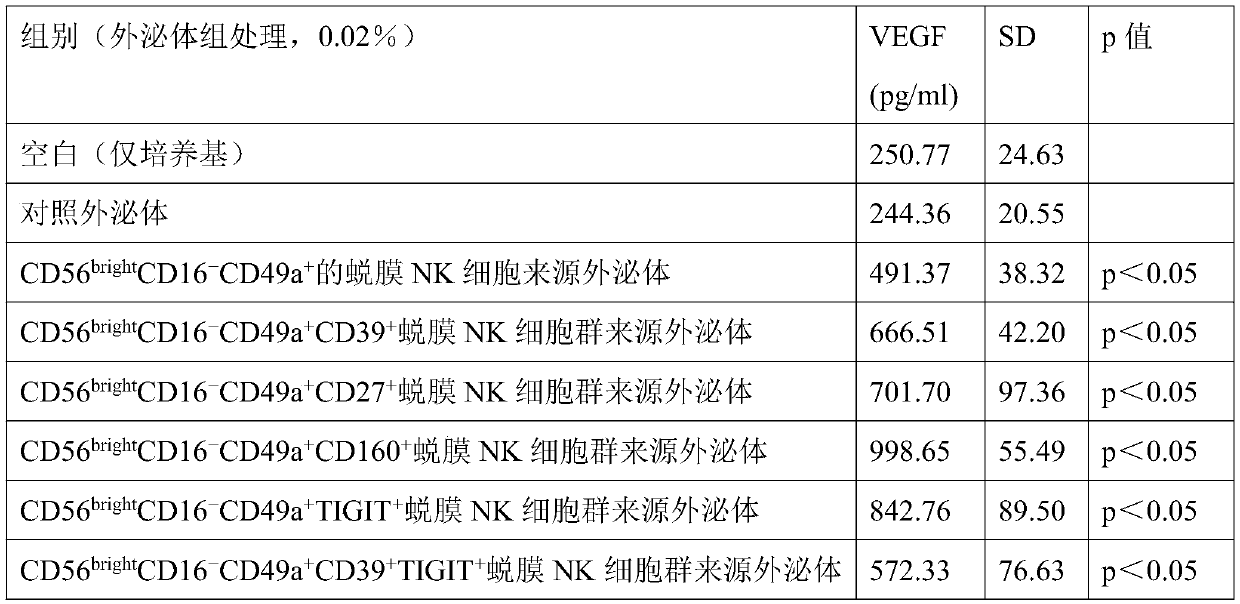
Application of decidua NK cells and decidua NK cell exosomes from cell subsets in preparing drugs and auxiliary therapeutic agents for treating diseases related to infertility
ActiveCN110721196AIncrease development rateImprove implantation rateCell dissociation methodsMammal material medical ingredientsDiseaseEndometrium thickness
The invention provides application of decidua NK cells and decidua NK cell exosomes from cell subsets in preparing drugs and auxiliary therapeutic agents for treating diseases related to infertility.Experiments prove that the exosomes treat endometrium growth disorders by promoting endometrial thickness increasing, improving endometrial cell activity, reducing endometrial cell damage, promoting VEGF expression, maintaining stem features of endometrium matrix cells and stimulating proliferation, so that the pregnancy success rate of endometrium damage model mice is increased to 50-70% from 20%; and the exosomes treat diseases related to maternal-fetal immunologic tolerance disorders by playing a role of immunologic tolerance, reducing a spontaneous abortion rate and improving a help T lymphocyte level. Besides, the exosomes can effectively increase the developmental rate of zygotes fertilized no matter in vitro or in vivo in a multiple mode, meanwhile, an in-vitro fertilization rate, an implantation rate of implantation and a birth rate are also increased, and positive assisting effects on infertility treatment are achieved.
Owner:PHARCHOICE THERAPEUTICS INC
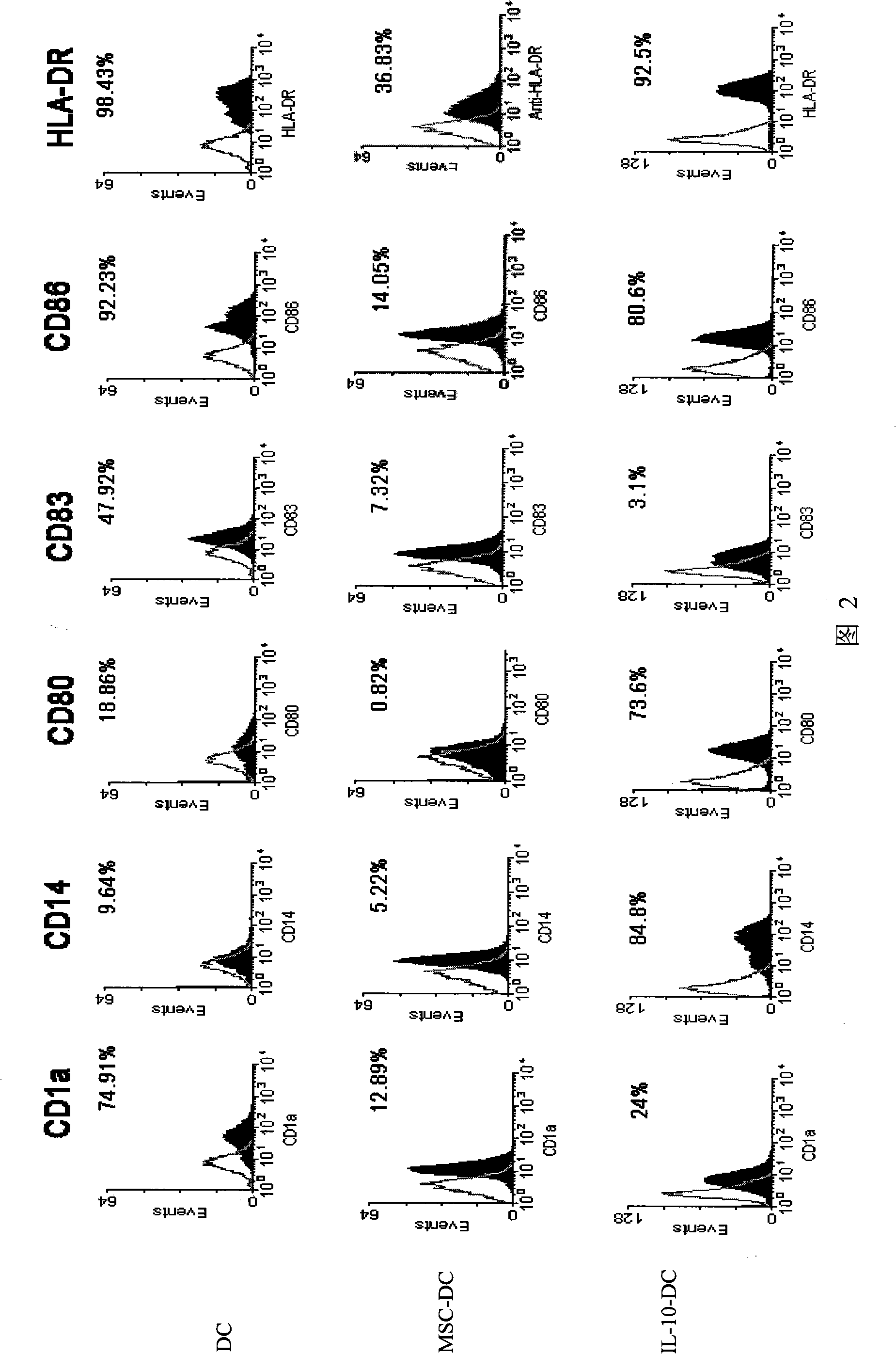
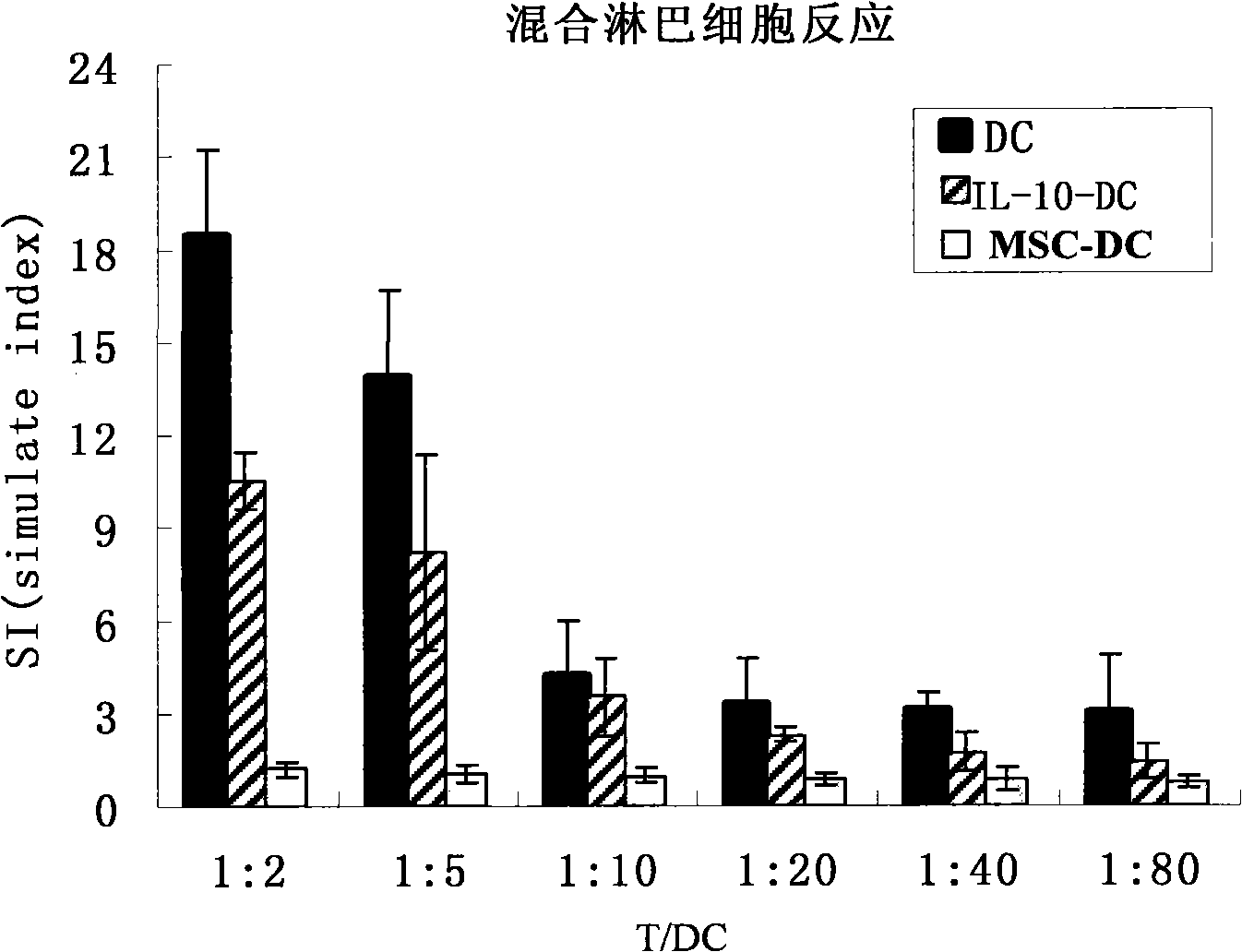
Immunologic tolerance dendritic cell, preparation method thereof and special culture medium
InactiveCN101514333APrevent proliferationReduce inflammatory factorsAntipyreticAnalgesicsInflammatory factorsDendritic cell
The invention discloses an immunologic tolerance dendritic cell, a preparation method thereof and a special culture medium. The culture medium is formed by the way that mesenchyma stem cells are added into a dendritic cell culture medium. The preparation method comprises: the precursors and the inducible factors of dendritic cells are added into the culture medium. The co-culture proportion of the mesenchyma stem cells to the dendritic cells is (1-2) to (1-10), preferably 1 to 1; the precursors of the dendritic cells are CD34 cells; and the inducible factors comprise GM-CSF, TNF- alpha, IL-4 and LPS. The invention provides the application of the immunologic tolerance dendritic cell during preparing immunologic tolerance medicine for organisms. The immunologic tolerance dendritic cell can lowly express co-stimulatory molecule, reduce inflammatory factors, increase the secretion of suppressor cytokines, have vasculogenesis activity, and inhibit the multiplication of variant T cells. The invention has the advantages of simple operation, convenience and practicality, and establishes a stable culture technical system for selective activation dendritic cells.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979AMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticL929 cellEscherichia coli
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Conjugate molecule
InactiveUS20130078243A1Surgical drugsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IHLA-G
This invention relates to a therapeutic molecule capable of suppressing an immune response against an organ or tissue transplantation in a patient. In particular, the invention relates to a conjugate comprising a first portion connected to a second portion, wherein the first portion binds to an MHC Class I molecule and the second portion has HLA-G activity. This conjugate may be used as a medicament to modulate immune responses and induce immunological tolerance specific to allogenic MHC complexes.
Owner:IBRAHIM MOHAMMAD
Inhibition of Glycogen Synthase Kinase and Methods of Treating Autoimmune or Immune Inflammatory Disease
The present invention relates to the use of glycogen synthase kinase 3(GSK3) inhibitors, especially inhibitors of GSK-3α, GSK-3β and GSK-3β2, preferably, inhibitors of GSK-3β, in patients having autoimmune diseases and / or immune dysfunction / dysregulation to induce immune tolerance. Inhibition of GSK leads to activation of a pathway of dendritic cell maturation which leads to a dendritic phenotype which attenuates, rather than induces, immune responses. The immune responses and mature dendritic cells produced by the method of the present invention redirect or attenuate the immune response in individuals, thus leading to effective therapies for a number of autoimmune diseases and / or diseases of immune dysfunction / dysregulation (immune inflammatory diseases), including systemic lupus erythematosus (SLE), autoimmune diabetes (type I diabetes mellitus), asthma, rheumatoid arthritis, inflammatory bowel disease, among numerous others.
Owner:MELLMAN IRA +1
Immune tolerance inducer
ActiveUS20150174090A1Good effectHeavy metal active ingredientsBiocideTolerance inductionAutoimmune condition
An object of the present invention is to provide a regulatory dendritic cell inducing agent applicable to the treatment of immune disease as well as an immune tolerance inducing agent such as a preventive and / or therapeutic agent for allergic disease and a preventive and / or therapeutic agent for autoimmune disease, both of which are safe and have the mechanism of action different from that of conventional drugs. As a means for achieving the above object, an immune tolerance inducing agent comprising 5-aminolevulinic acid (ALA) or a derivative thereof, or a salt of the 5-ALA or the derivative and an iron compound as active ingredients is prepared. Preferable examples of the ALAs can include ALA and various esters such as methyl ester, ethyl ester, propyl ester, butyl ester, and pentyl ester of ALA, and their hydrochlorides, phosphates, and sulfates. Preferable examples of the iron compound can include sodium ferrous citrate.
Owner:SBI PHARMA CO LTD +1
Multifunctional antibody-ligand traps to modulate immune tolerance
InactiveUS20200140547A1Immunoglobulin superfamilyPeptide/protein ingredientsCancer preventionInfectious Disorder
The invention provides multifunctional antibody-ligand traps and methods of using them to counteract immune tolerance and / or immune dysfunction. The multifunctional antibody-ligand traps and fusion proteins of the invention can counteract immune dysfunction in order to restore and unleash antitumor or pathogen-directed immune responses. Provided here are promising immunotherapeutic agents for treatment and prevention of cancers, infectious diseases, and immuno-inflammatory disorders.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
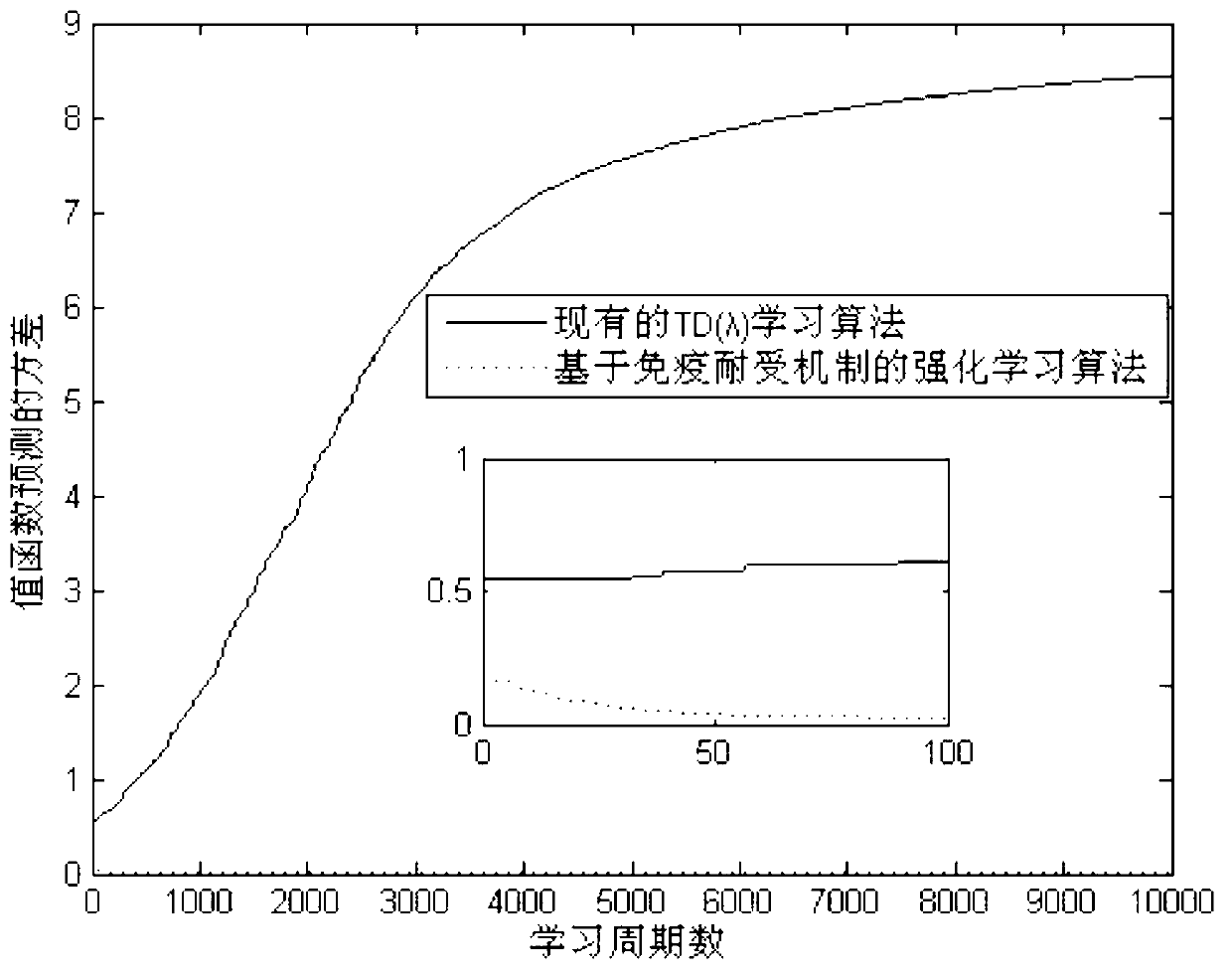
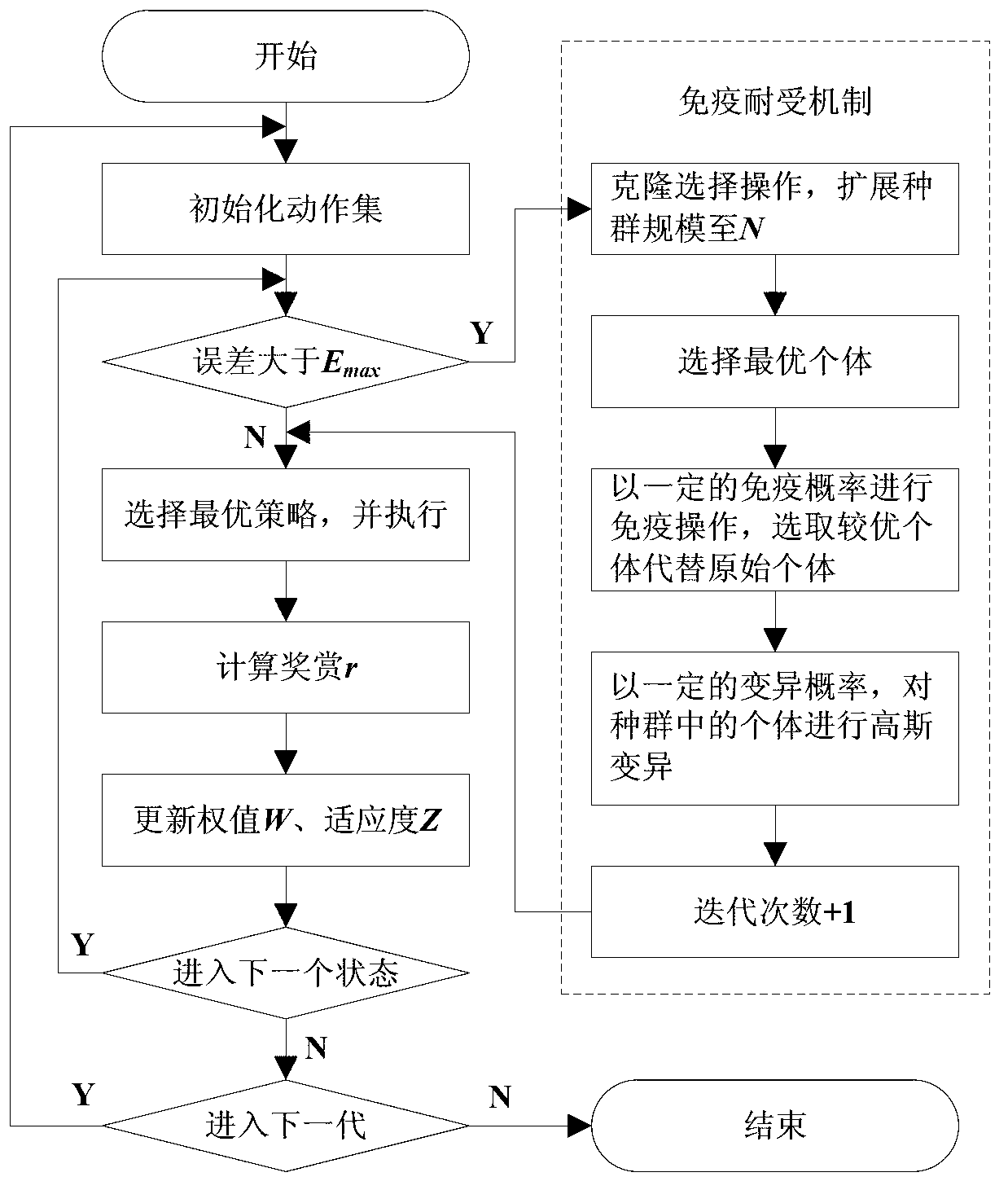
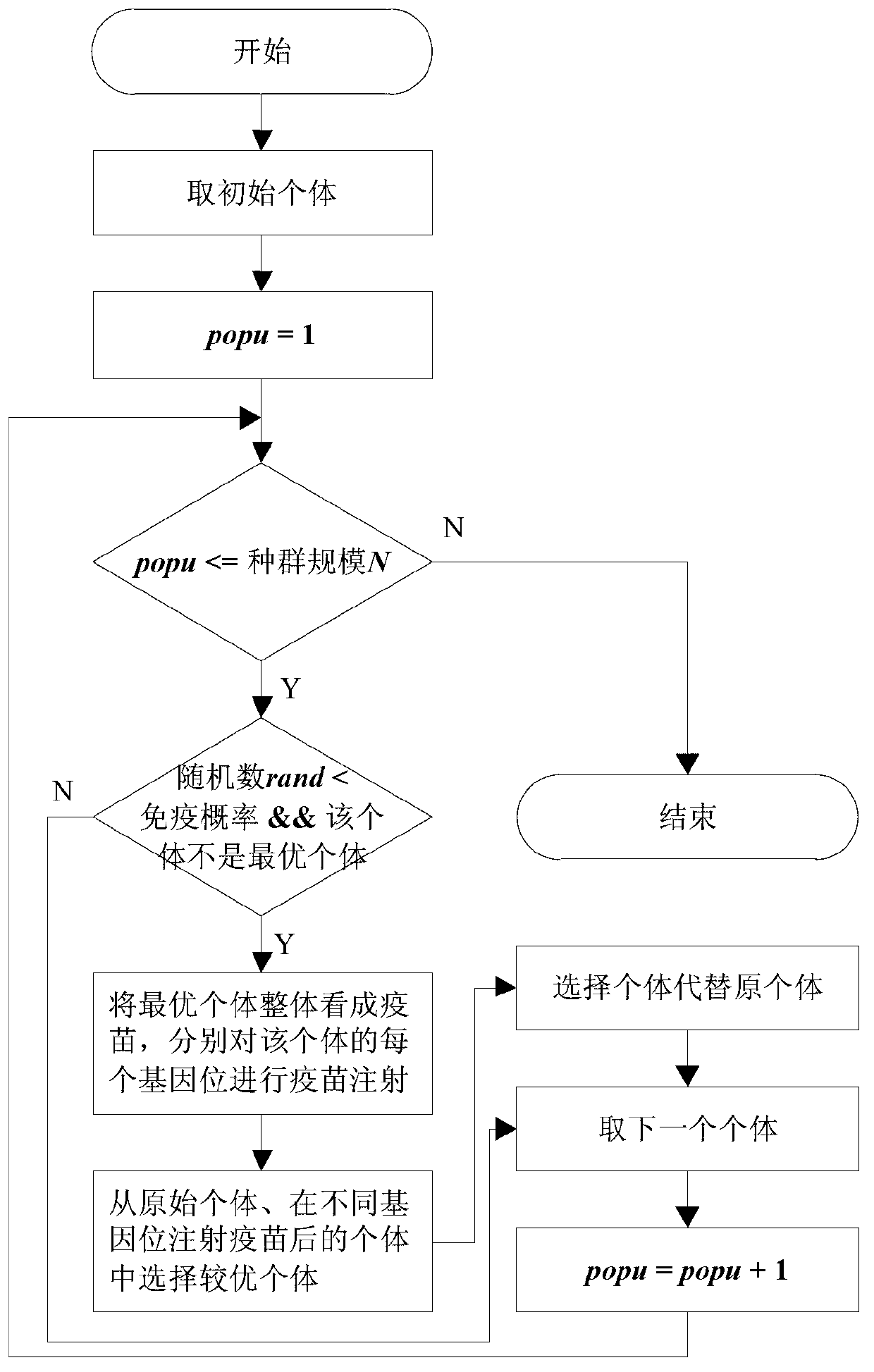
Reinforced learning algorithm based on immunologic tolerance mechanism
InactiveCN103218655AGuaranteed search performanceHave diversityNeural learning methodsArtificial immune systemPrimary response
The invention discloses a reinforced learning algorithm based on an immunologic tolerance mechanism. The reinforced learning algorithm based on the immunologic tolerance mechanism comprises the steps of firstly designing the vector quantity of a primary function and the vector quantity of a weight value of a TD (lambda), then encoding the vector quantity of the weight value according to the number of floating points, when the error between the environment of a system and the real environment is larger than a set threshold value, regarding the environment of the system as a primary response in an artificial immune system, when meeting the environment for the first time, optimizing the environment by the immunologic tolerance mechanism, memorizing environmental knowledge by a memory, namely an immune body, then selecting the optimal strategy according to parameters of a current system, updating the parameters of the system according to a feedback reward value (r), continuously carrying out an iteration for the next time, when the error between the environment of the system and the real environment is smaller than the threshold value, regarding the environment of the system as similar environment, regarding the similar environment as a secondary response in the artificial immune system, and directly selecting the optimal strategy according to parameters of the system through the fact that the system judges motion selection.
Owner:XIAN UNIV OF TECH
Induction of immune tolerance
ActiveUS7217718B2Highly effective in suppressingInduce toleranceBiocideNervous disorderDiseaseAutoimmune disease
Methods of inducing immune tolerance by administering an immunosuppressive agent and a compound represented by Formula (I) are disclosed:Additionally methods of suppressing an immune response by administering an immunosuppressive agent and a compound represented by Formula (I) are disclosed. Further disclosed are methods for treating autoimmune diseases by administering an immunosuppressive agent and a compound represented by Formula (I). The variables of Formula (I) are described herein.
Owner:GENZYME CORP
Method for enhancing humoral immune response
ActiveUS20190077872A1Enhance humoral immune responseEffective treatmentSerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDrugPeptide
The present invention provides methods for producing antibodies against peptides, proteins, or such to which immune tolerance is easily established, by using antigen-binding molecules comprising a domain that binds to a molecule expressed on the surface of a cell having an immune response-suppressing function and a T cell receptor (TCR) complex-binding domain. The present invention also provides pharmaceutical compositions for use in combination with therapeutic vaccines and agents for enhancing a humoral immune response, each comprising the antigen-binding molecules as active ingredients.
Owner:CHUGAI PHARMA CO LTD
Veterinary Chinese medicinal composition for enhancing nonspecific immunologic function and application thereof
InactiveCN101912452AEnhance non-specific immune functionDecreased growth performanceDigestive systemImmunological disordersDiseaseToxic material
The invention relates to a veterinary Chinese medicinal composition for improving growth performance and enhancing nonspecific immunologic function of an organism. The composition is prepared from root of Chinese pulsatilla, astragalus, bighead atractylodes rhizome, isatis root, baikal skullcap root, root of Chinese thorowax and rhubarb serving as raw materials. The invention also relates to a veterinary medicament prepared from the veterinary Chinese medicinal composition, a preparation method for the veterinary medicament and application of the veterinary Chinese medicinal composition. The veterinary Chinese medicinal composition of the invention has the effects of strengthening the body resistance to eliminate pathogenic factors, clearing away heat and toxic material and enhancing the nonspecific immunologic function of the organism, and can treat various atypical disease symptoms caused by low immunologic function of the organism, immunologic tolerance, endogenous toxin, stress and the like, the reduction in the growth performance and damp-heat disease symptoms such as wind-heat type cold, flu, bursa of fabricius, intestinal disease syndromes and the like.
Owner:四川华神农大动物保健药品有限公司
Method of reducing immunological tolerance to malignancy
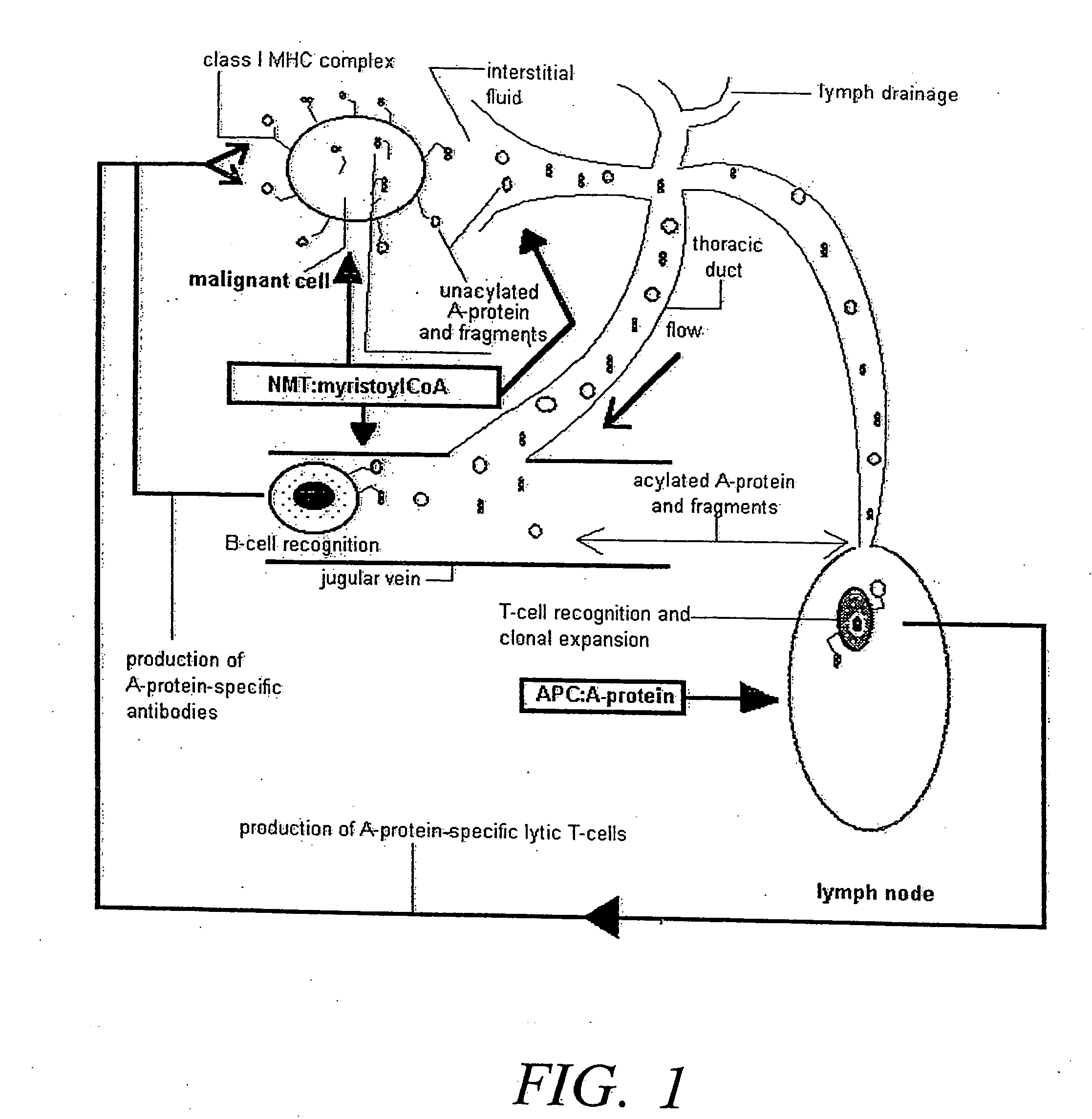
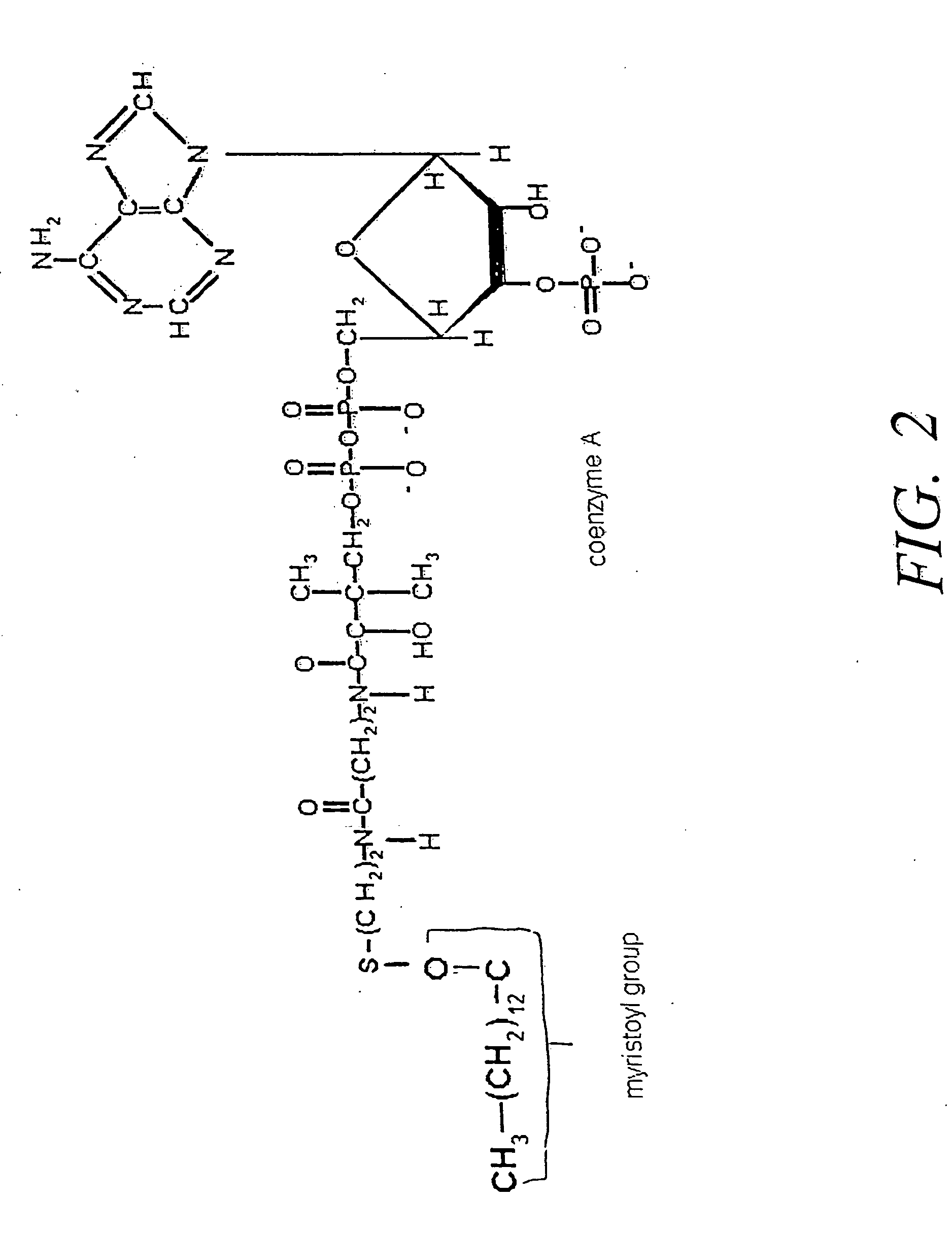
Disclosed are therapeutic formulations comprising myristoylCoA and N-myristoyl transferase and methods of using such formulations to stimulate the immune system of a mammal afflicted with carcinoma and having a detectable blood level of unacylated A-protein to produce an immune response thereto. Also disclosed are methods of treating an A-protein displaying carcinoma in a mammal.
Owner:SCHMIDT GEOFFREY J
Method for constructing chronic transfection model of hepatitis B virus
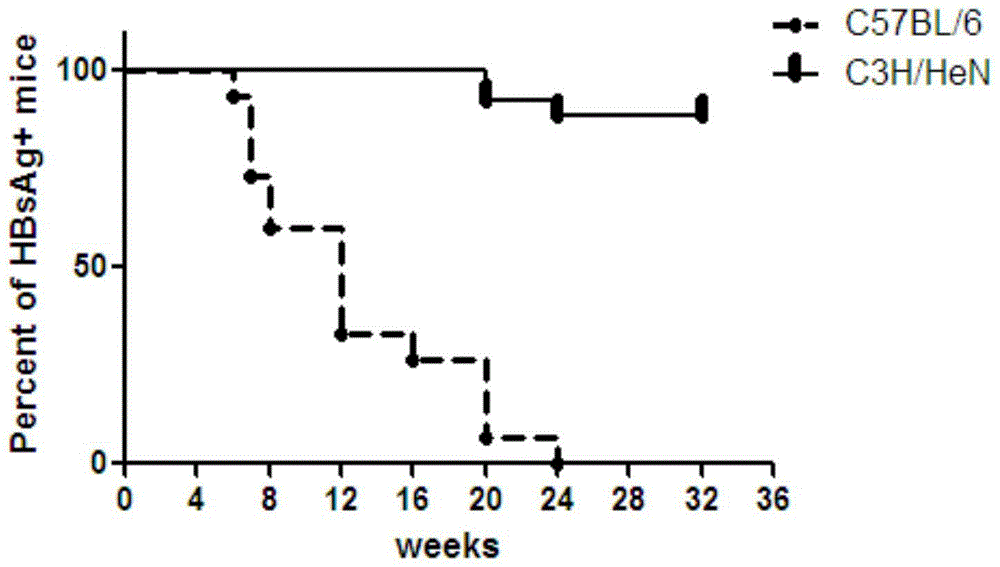
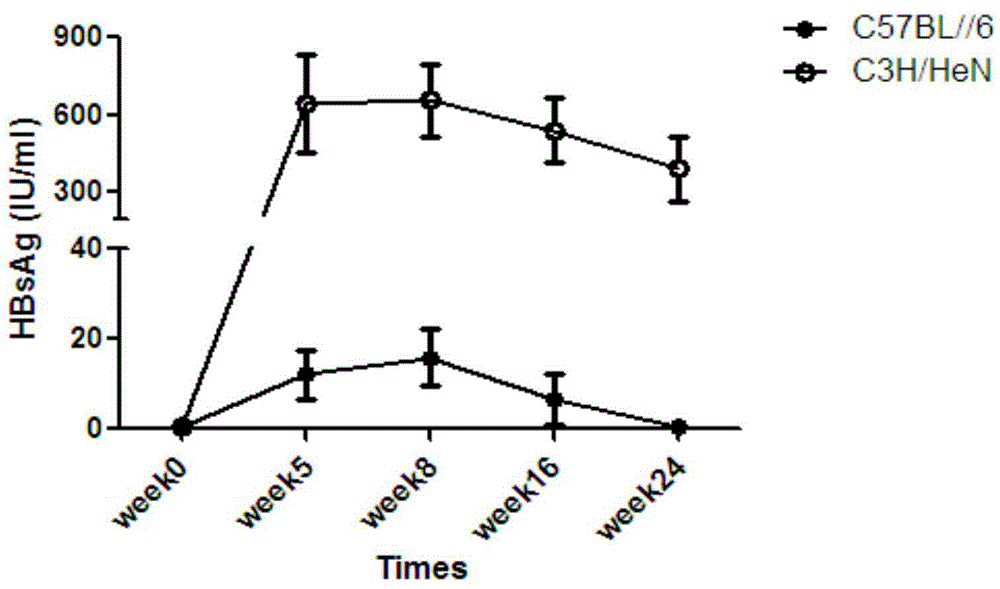
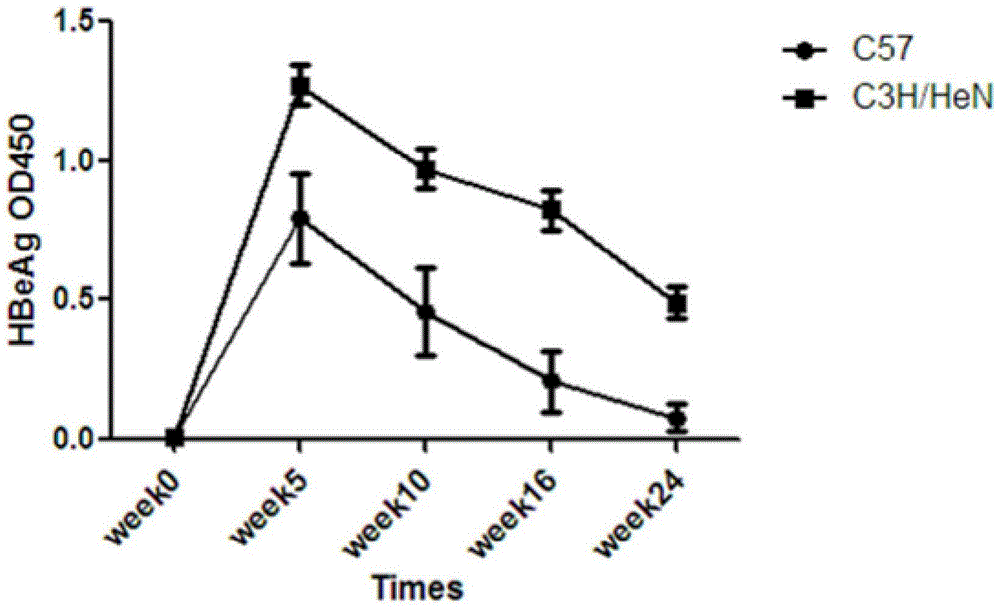
ActiveCN104673834AIncreased rate of chronic moldingFermentationGenetic engineeringMechanism of actionHepatitis B virus
The invention belongs to the field of biomedicine, and particularly relates to a method for constructing a chronic transfection model of a hepatitis B virus. The method disclosed by the invention comprises the following steps: transfecting HBV (hepatitis B virus) plasmids into a model-preparing experimental mouse (preferably adopting male C3H / HeN of 4-8-week old), and constructing the chronic transfection model of the HBV. The chronic transfection model of the hepatitis B virus constructed by the method can be used for researching specific action mechanism of single or multiple genes in an HBV pathogenic process; transgenic modification of the mouse is not needed; the method is free of limitations such as immunologic tolerance, easy to obtain, and low in cost; the problem of long-term shortage of an animal model suitable for the HBV in related technical fields can be solved; and a good animal model is provided for further research on life history and chronic mechanism of the HBV and screening of vaccines and new medicines.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Compositions and methods for immune tolerance induction
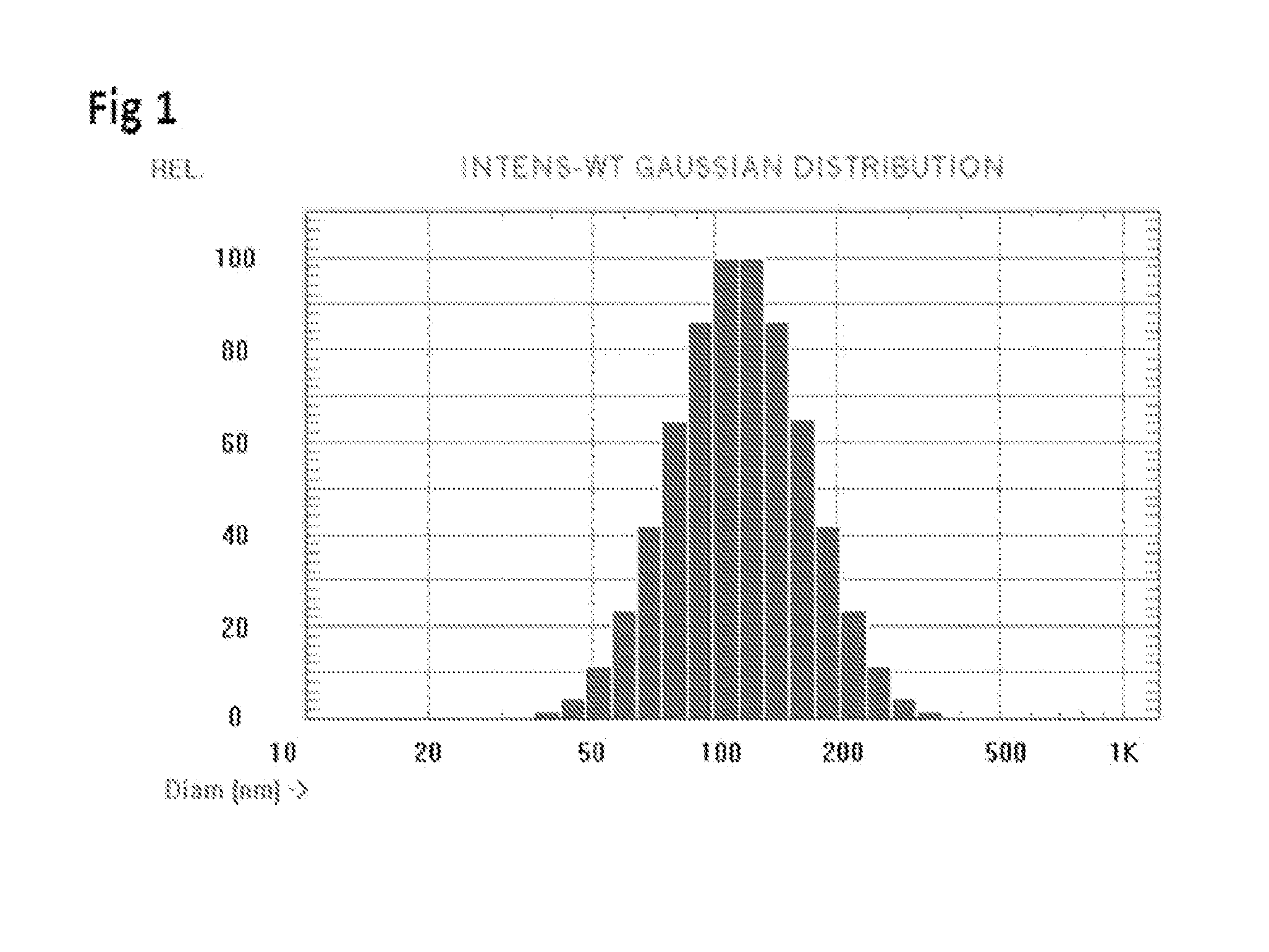
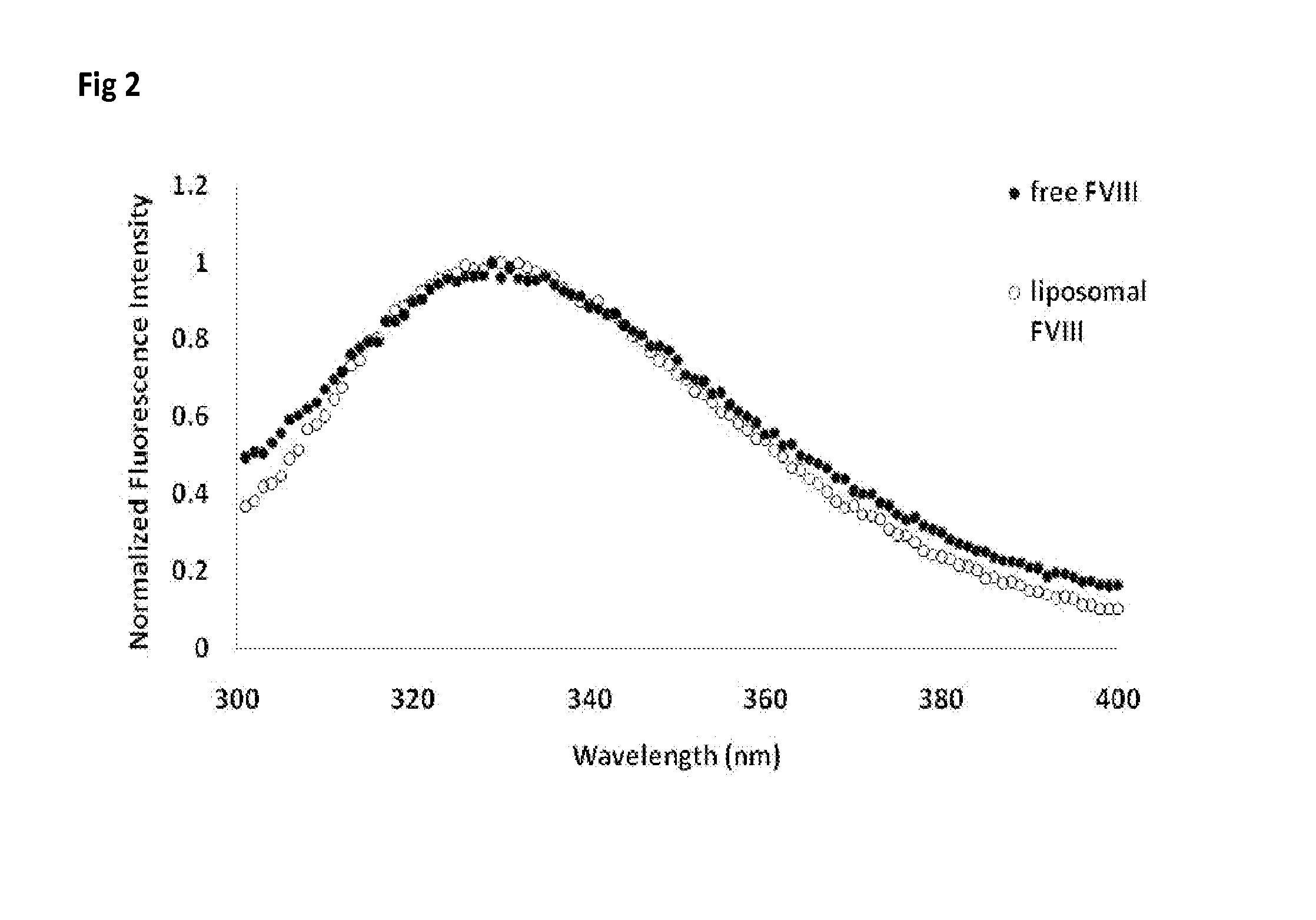
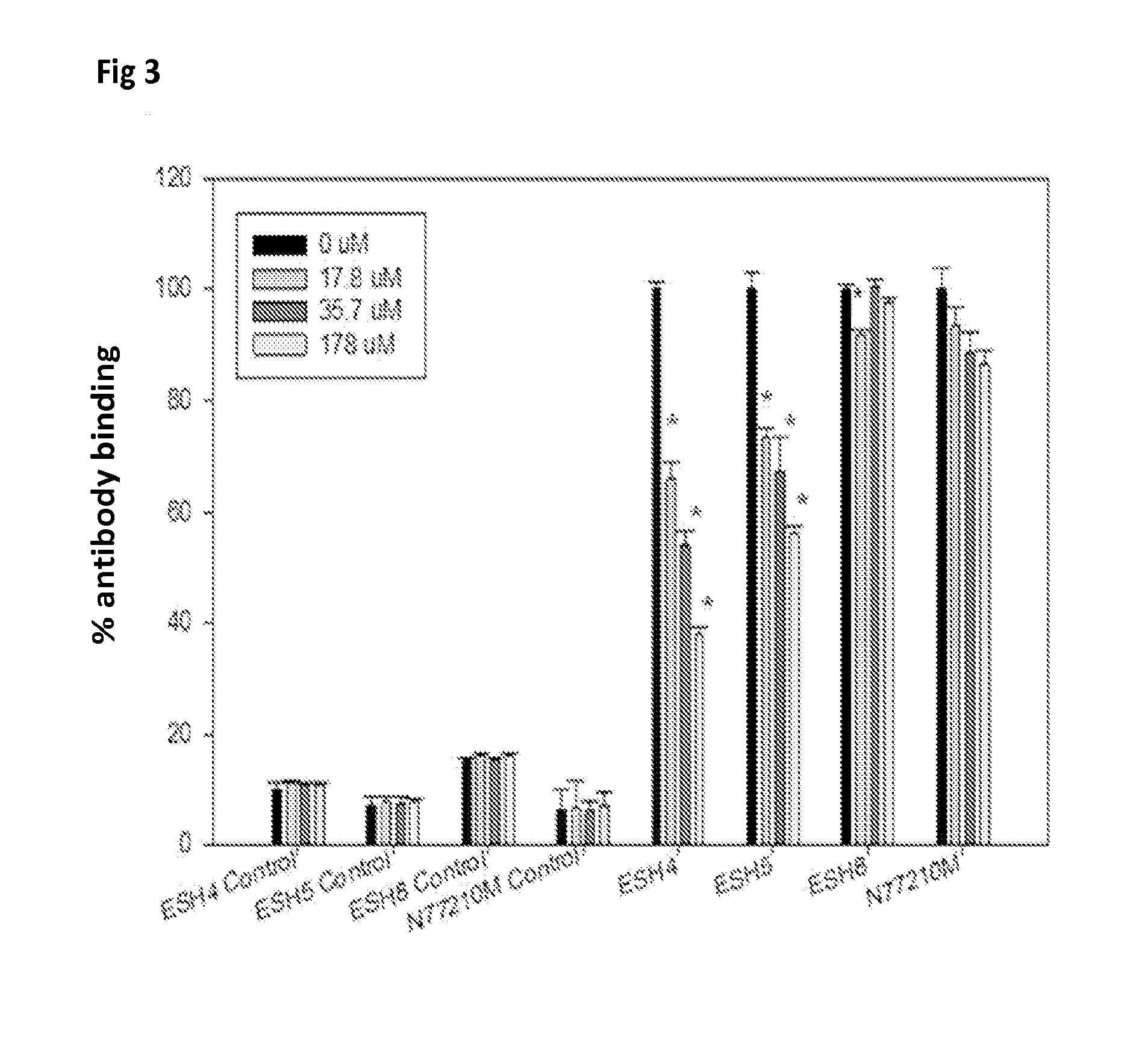
ActiveUS20150004218A1Low immunogenicityPeptide/protein ingredientsVertebrate antigen ingredientsTherapeutic proteinCholesterol
Methods and compositions to reduce immunogenicity of proteins are disclosed. Compositions comprising therapeutic proteins (such as Factor VIII or any other protein or peptide) complexed with liposomes comprising PS and PC (PS liposomes), or comprising PS, PI and PC and, optionally, cholesterol (PS / PI liposomes) may be used.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Immunomicrosphere for overcoming B cell immunological tolerance and application thereof
InactiveCN102008719APharmaceutical non-active ingredientsGranular deliveryMicrosphereCarrier protein
The invention relates to an immunomicrosphere for overcoming B cell immunological tolerance and application thereof. The immunomicrosphere comprises a microsphere medium, wherein the outside of the microsphere medium is coated with vaccine antigen, and the inside of the microsphere medium is coated with immune carrier protein for activating T cells. The invention also relates to application of the immunomicrosphere for overcoming the B cell immunological tolerance in preparing vaccines and immunological adjuvants. By using the technical scheme, the immunomicrosphere is combined with B cell receptor through the antigen and phagocytized by B cells, and the carrier protein is released and degraded in the cells; the immunomicrosphere is presented to Th cells through B cell MHC II to activate the TH cells to release cell factors, thereby promoting the activation and proliferation of the B cells; and high-titer antibody is generated through the affinity maturation process. The antigen protein and the immune carrier protein are respectively arranged on the surface of the microsphere and inside the microsphere, thereby preventing the antigen protein epitope and the Th cell activation epitope from competing with the B cell receptor.
Owner:SHANGHAI WEIQIU BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![[1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof [1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof](https://images-eureka.patsnap.com/patent_img/99341901-af93-48ea-8770-e981cf4bb912/US20070010515A1-20070111-C00001.png)
![[1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof [1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof](https://images-eureka.patsnap.com/patent_img/99341901-af93-48ea-8770-e981cf4bb912/US20070010515A1-20070111-C00002.png)
![[1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof [1,2,4] Triazolo [1,5, a] pyrimidin-2-ylurea derivative and use thereof](https://images-eureka.patsnap.com/patent_img/99341901-af93-48ea-8770-e981cf4bb912/US20070010515A1-20070111-C00003.png)