Patents
Literature
42 results about "Tolerance induction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
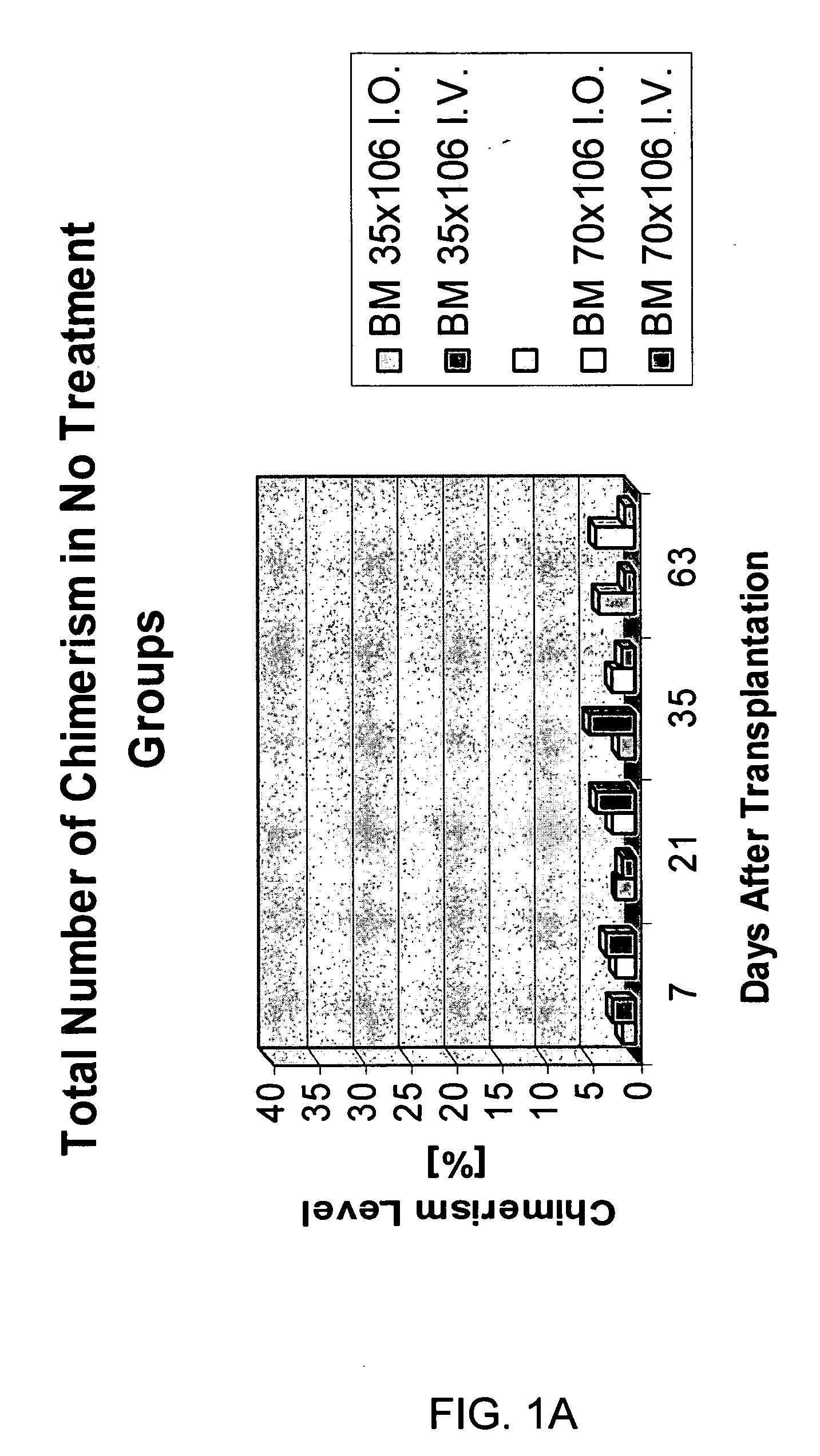
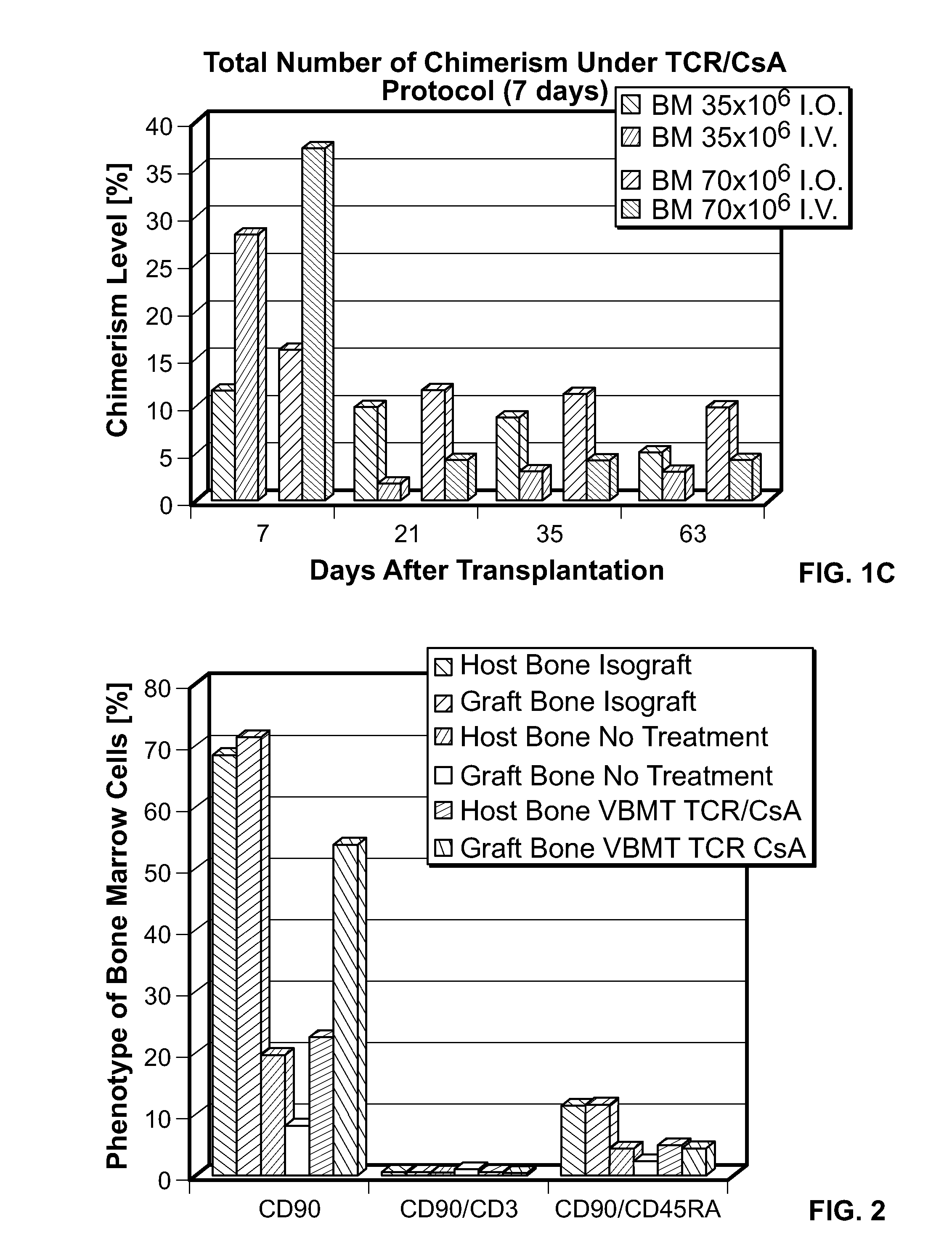
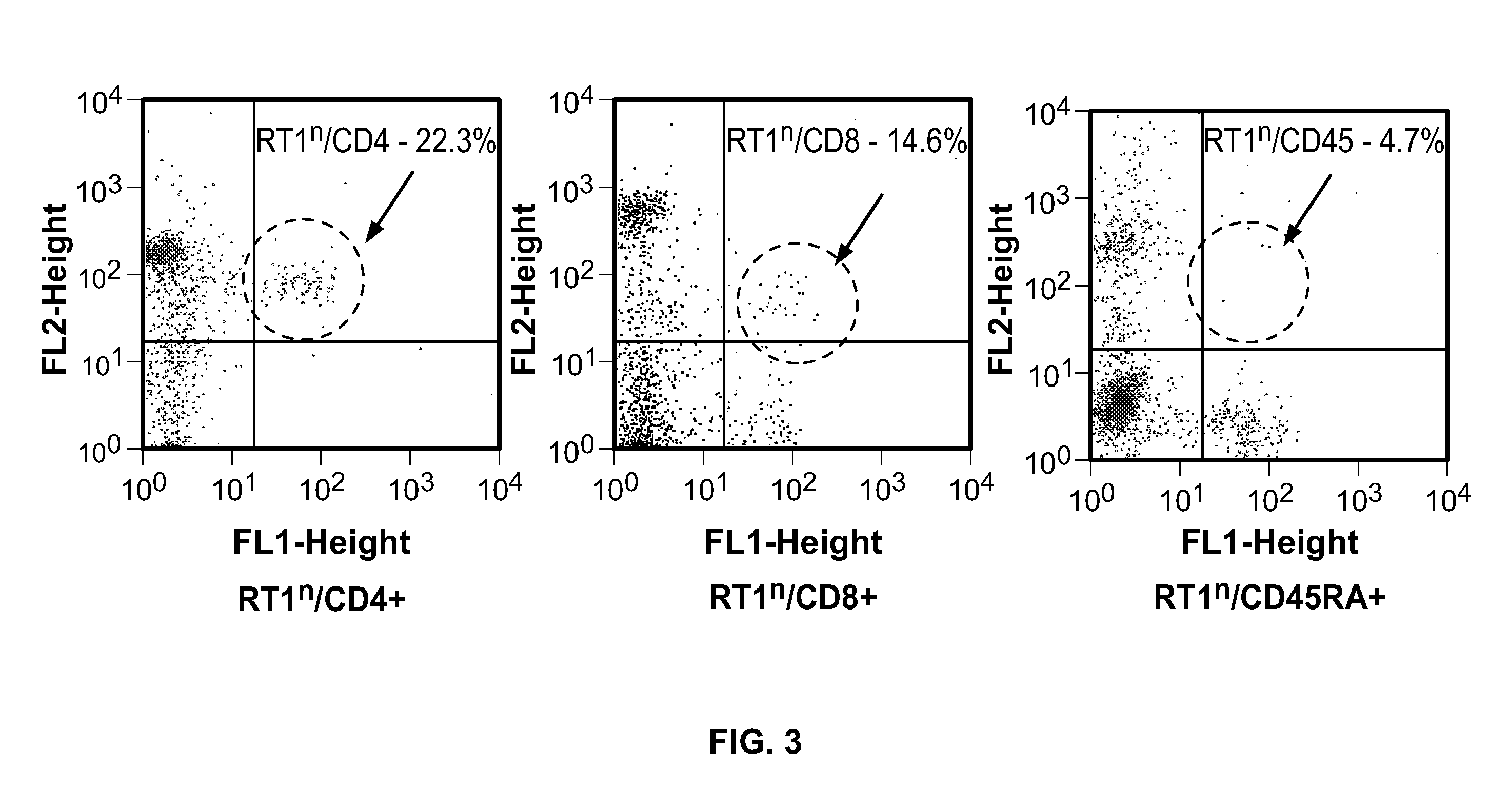
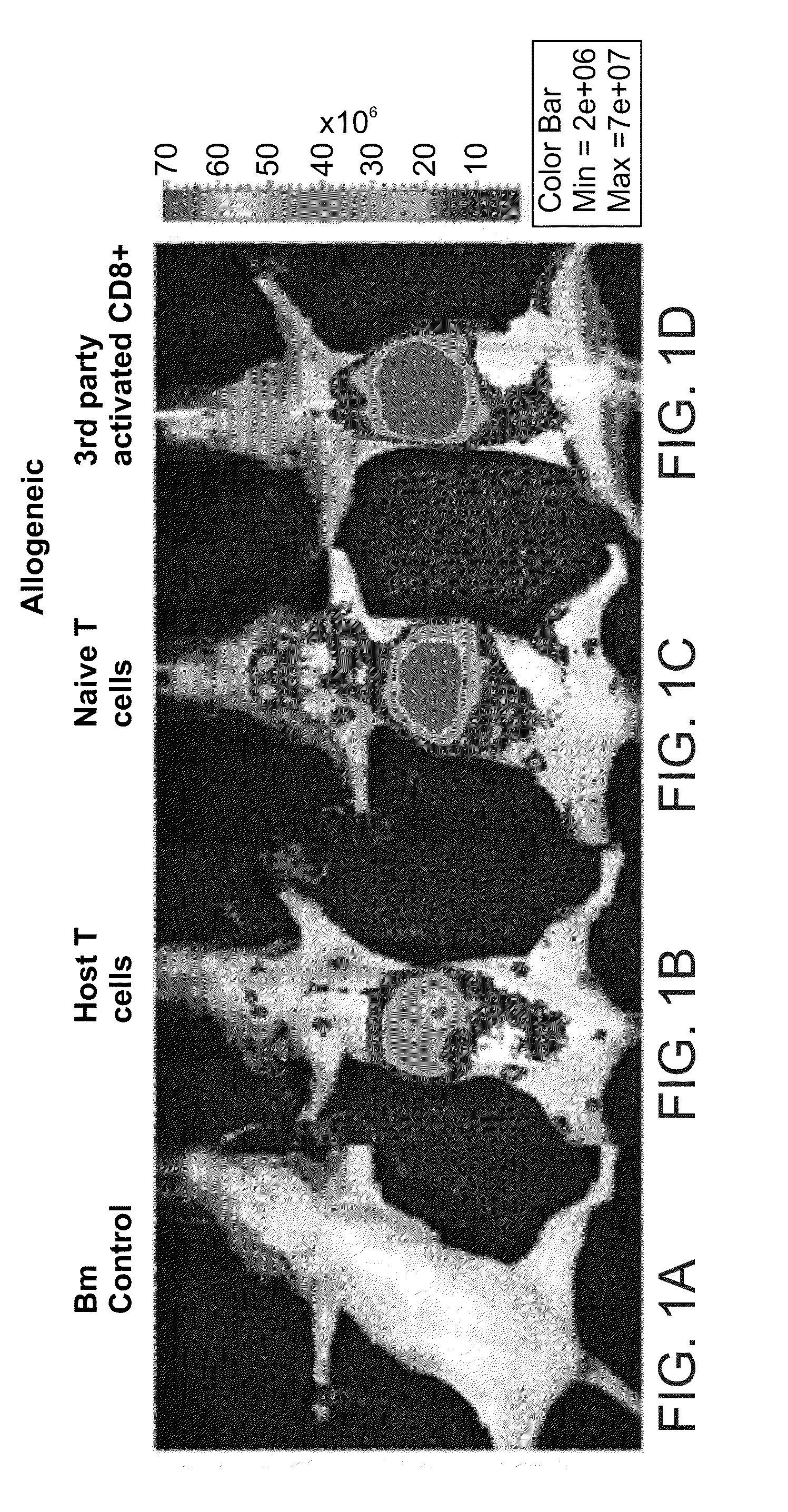
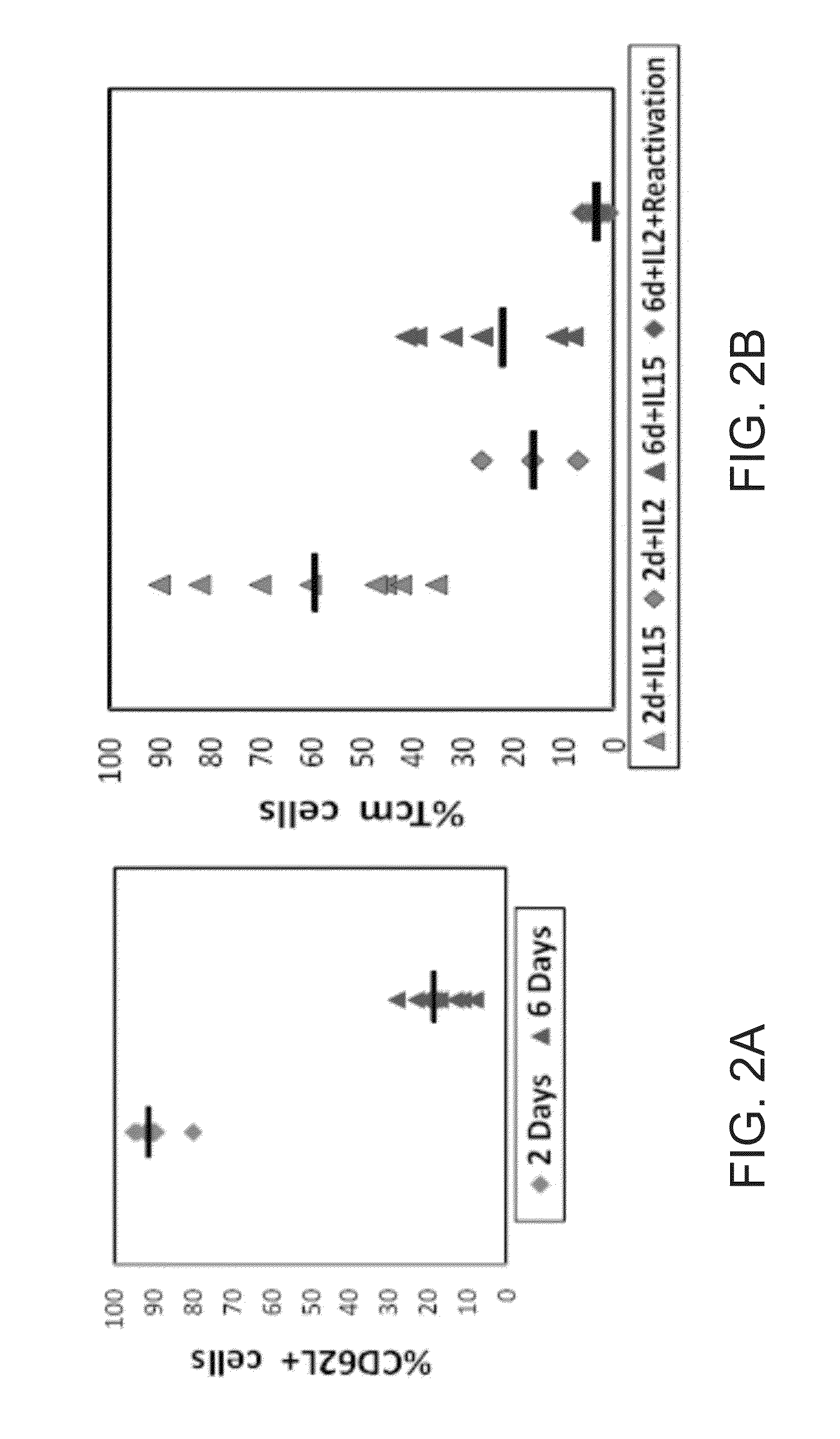
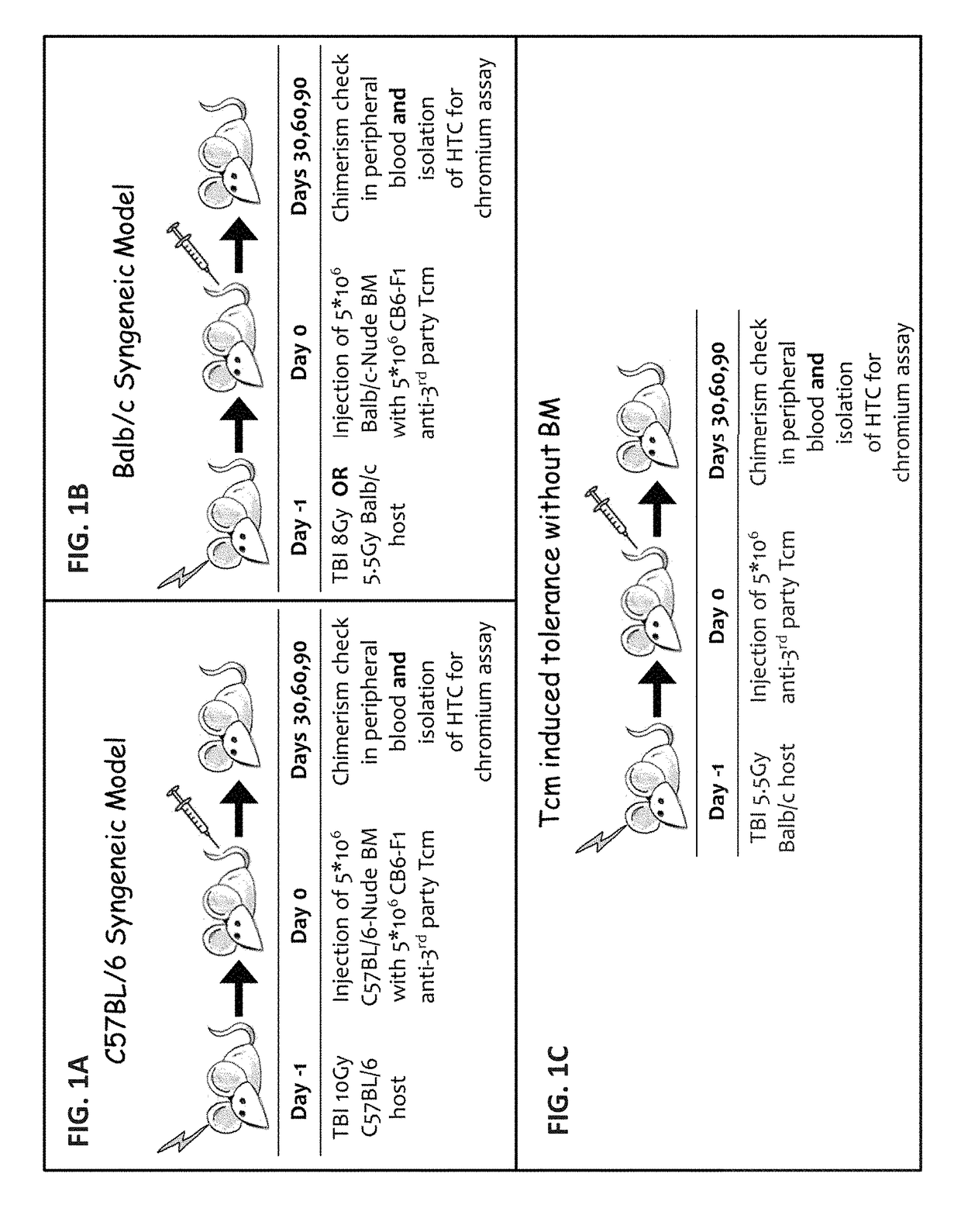
Induction of tolerance. The induction of donor hematopoietic macrochimerism is a promising approach for the induction of tolerance in the clinical setting of organ transplantation. Recent progress has resulted in substantially less toxic murine tolerance models that are based on macrochimerism.
Stable Protein Formulations
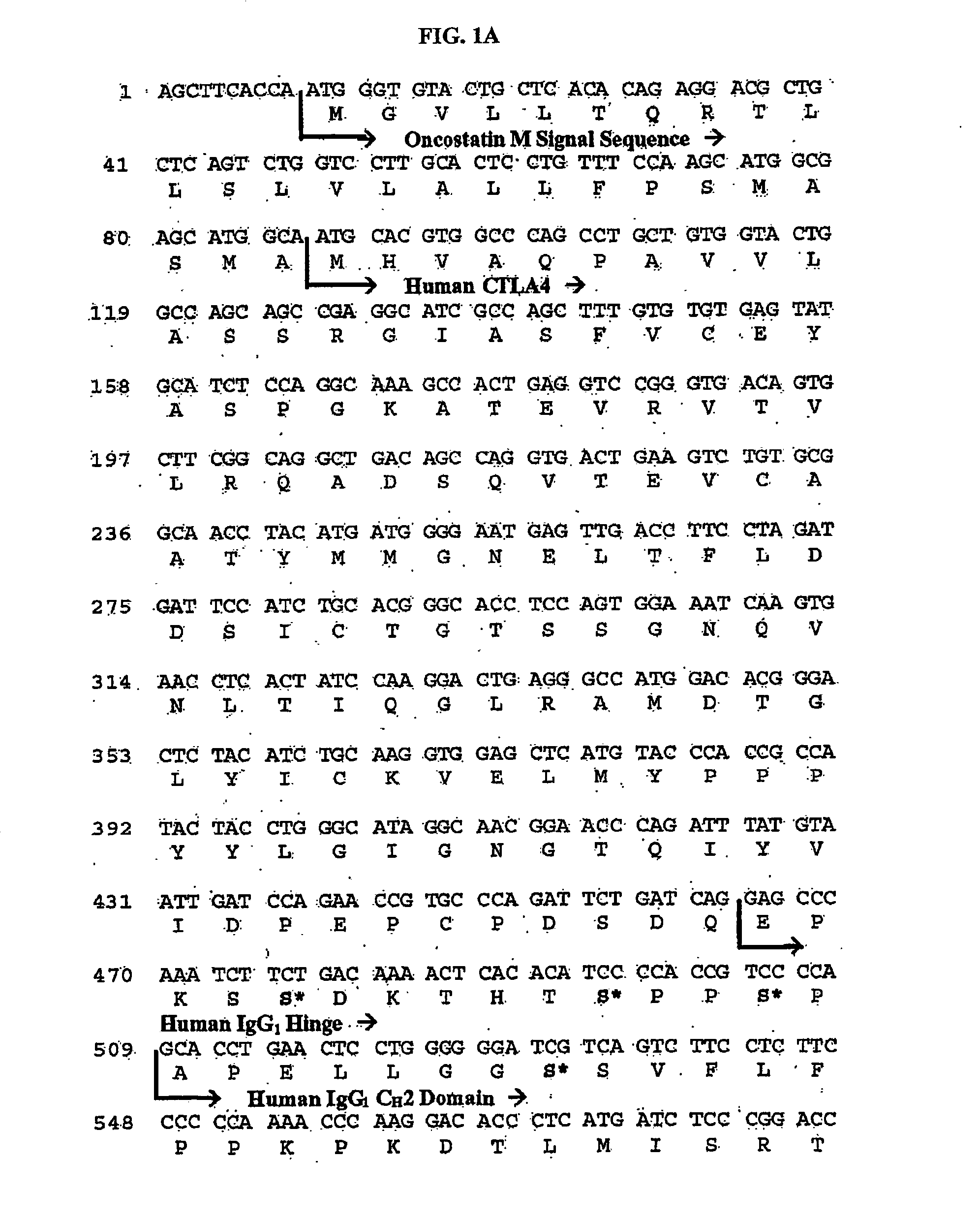
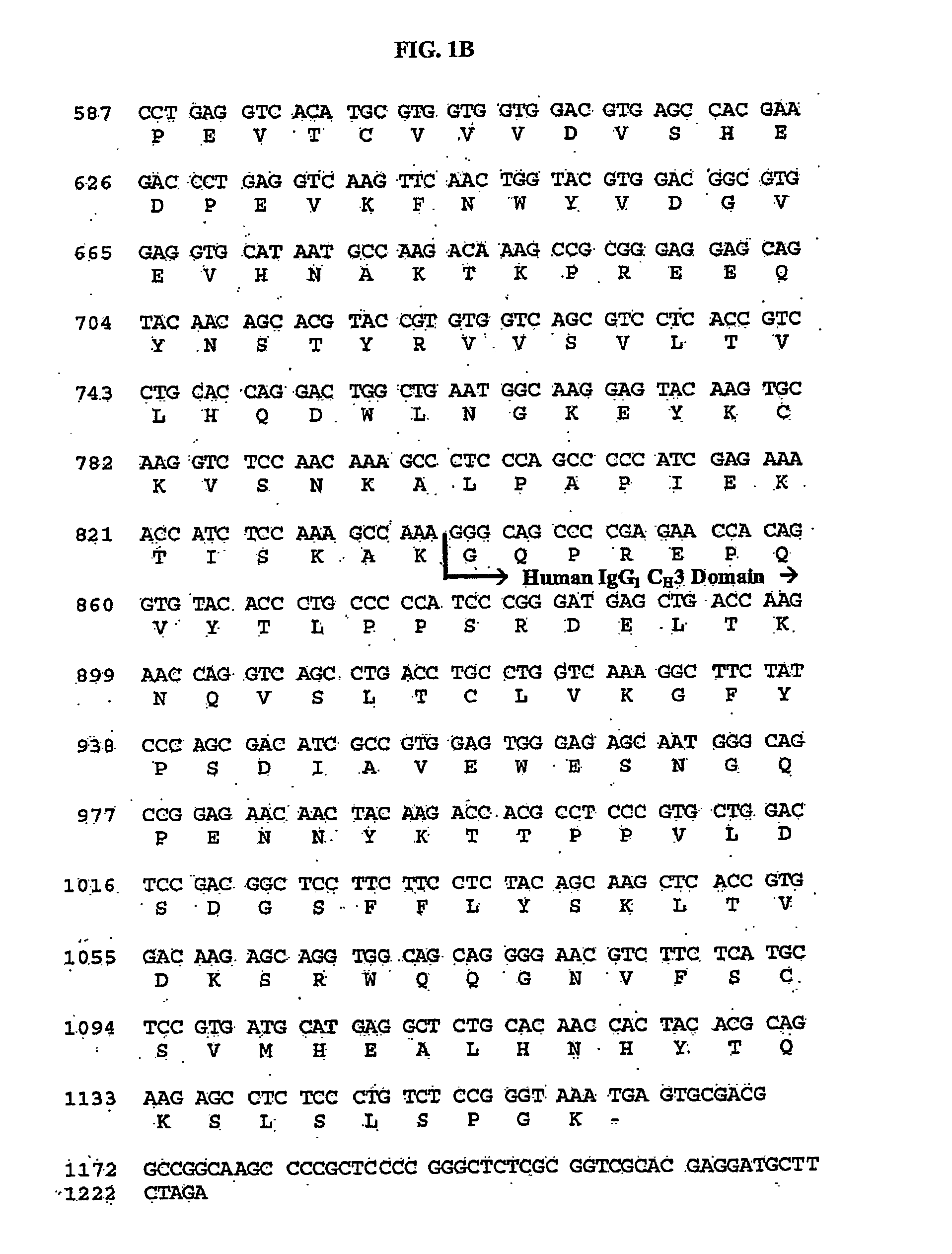
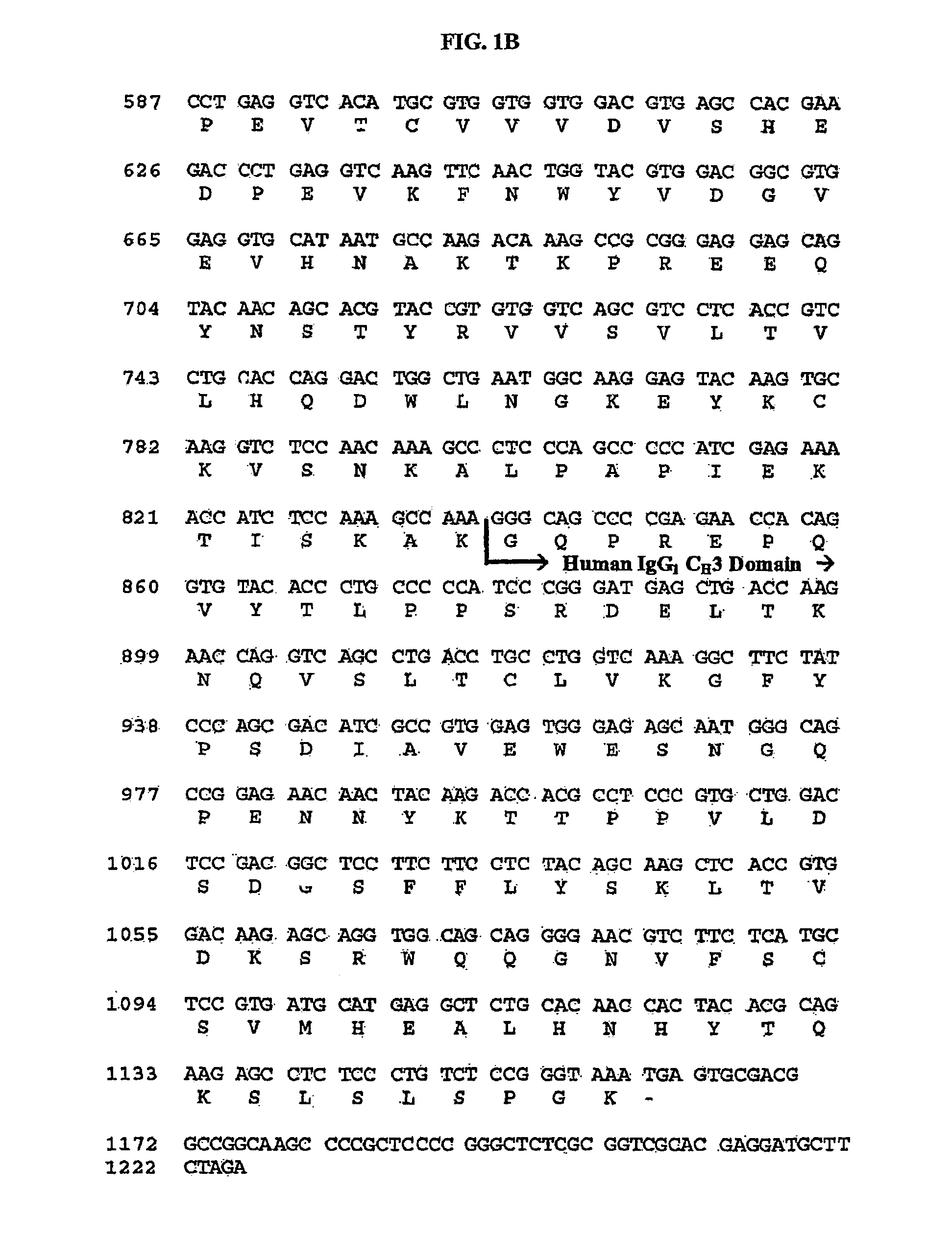
ActiveUS20100166774A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDiseaseTolerance induction
The present invention relates generally to stable formulations comprising CTLA4Ig molecules, including lyophilized, and liquid formulations for administration via various routes including, for example, routes such as intravenous (IV) and subcutaneous (SC) for treating immune system diseases and tolerance induction.
Owner:BRISTOL MYERS SQUIBB CO
Stable protein formulations
ActiveUS8476239B2Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsTolerance inductionDisease
The present invention relates generally to stable formulations comprising CTLA4Ig molecules, including lyophilized, and liquid formulations for administration via various routes including, for example, routes such as intravenous (IV) and subcutaneous (SC) for treating immune system diseases and tolerance induction.
Owner:BRISTOL MYERS SQUIBB CO
Tolerance induction and maintenance in hematopoietic stem cell allografts
InactiveUS20050058641A1Induce toleranceMaintain long-termBiocideGenetic material ingredientsTolerance inductionBiology
Provided are methods and compositions for the induction and maintenance of tolerance in hematopoietic stem cell allografts.
Owner:THE CLEVELAND CLINIC FOUND
Tolerance-induced targeted antibody production
InactiveUS20070036809A1Growth inhibitionOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthTolerance induction
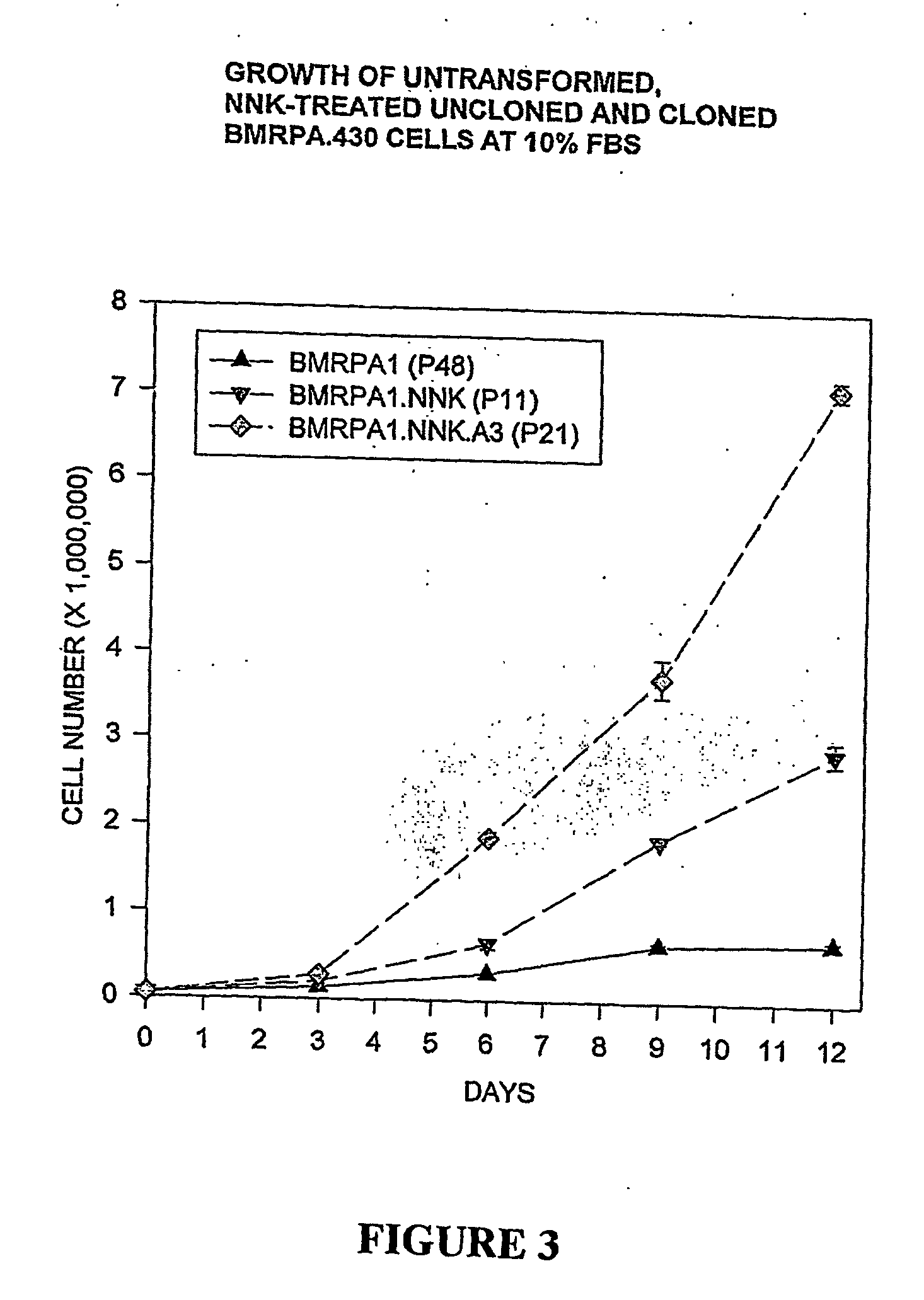
The present invention provides methods for directing the immune response of an animal towards immunologically weak or rare antigens such as tumor antigens. The methods combine subtractive immunization with hyperimmunization and result in the controlled or directed production of target-specific antibodies, helper T cells (CD4+-T lymphocytes) and cytotoxic T cells (CD8+-T lymphocytes). Also provided by the present invention are untransformed and transformed cell lines, and growth media necessary to grow the untransformed cell line in a differentiated state. Monoclonal antibodies which react with different neoplastic cell lines and hybridomas producing such antibodies are also provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Adoptive cell therapy with specific regulatory lymphocytes
InactiveUS20140356318A1BiocidePeptide/protein ingredientsTolerance inductionAdoptive cellular therapy
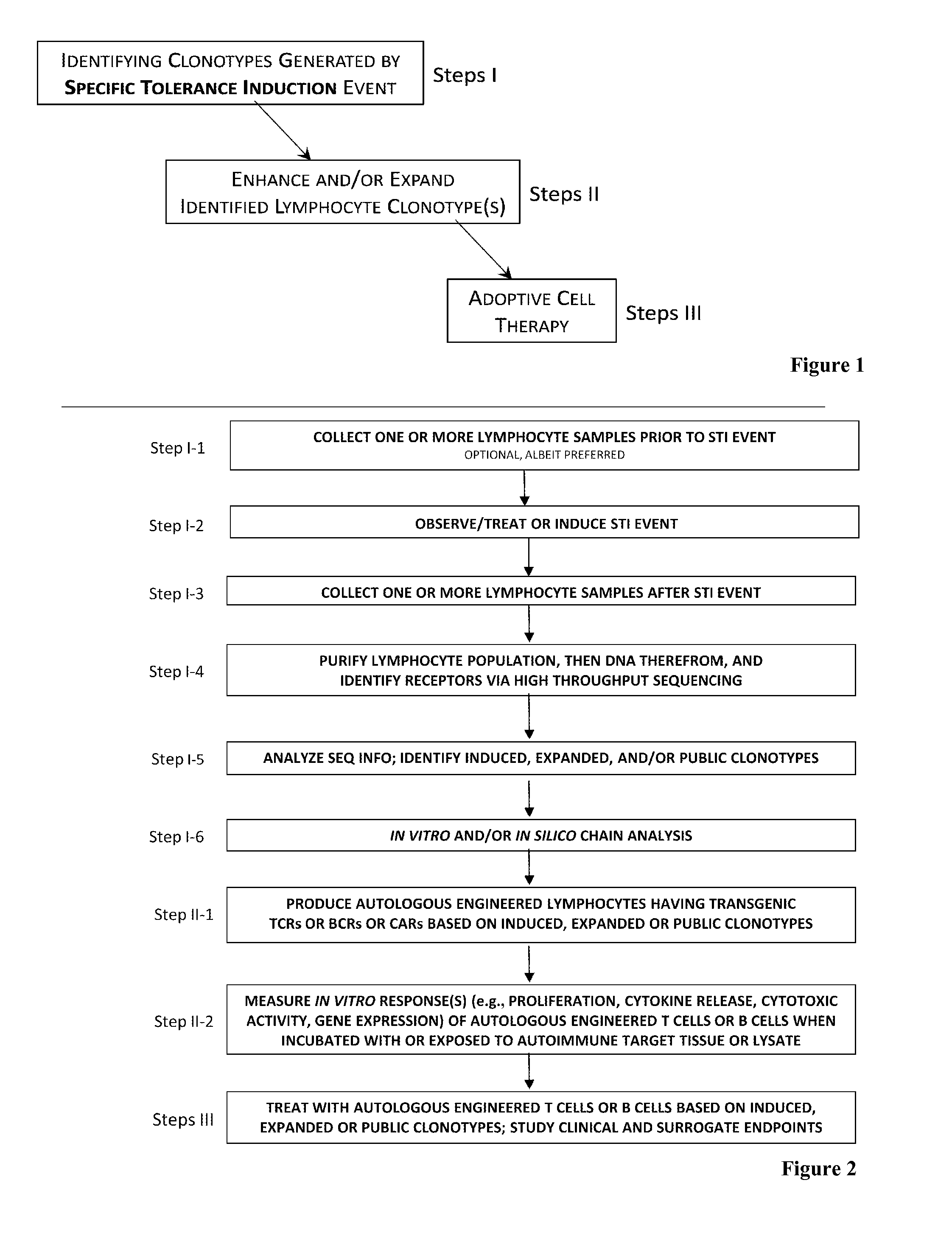
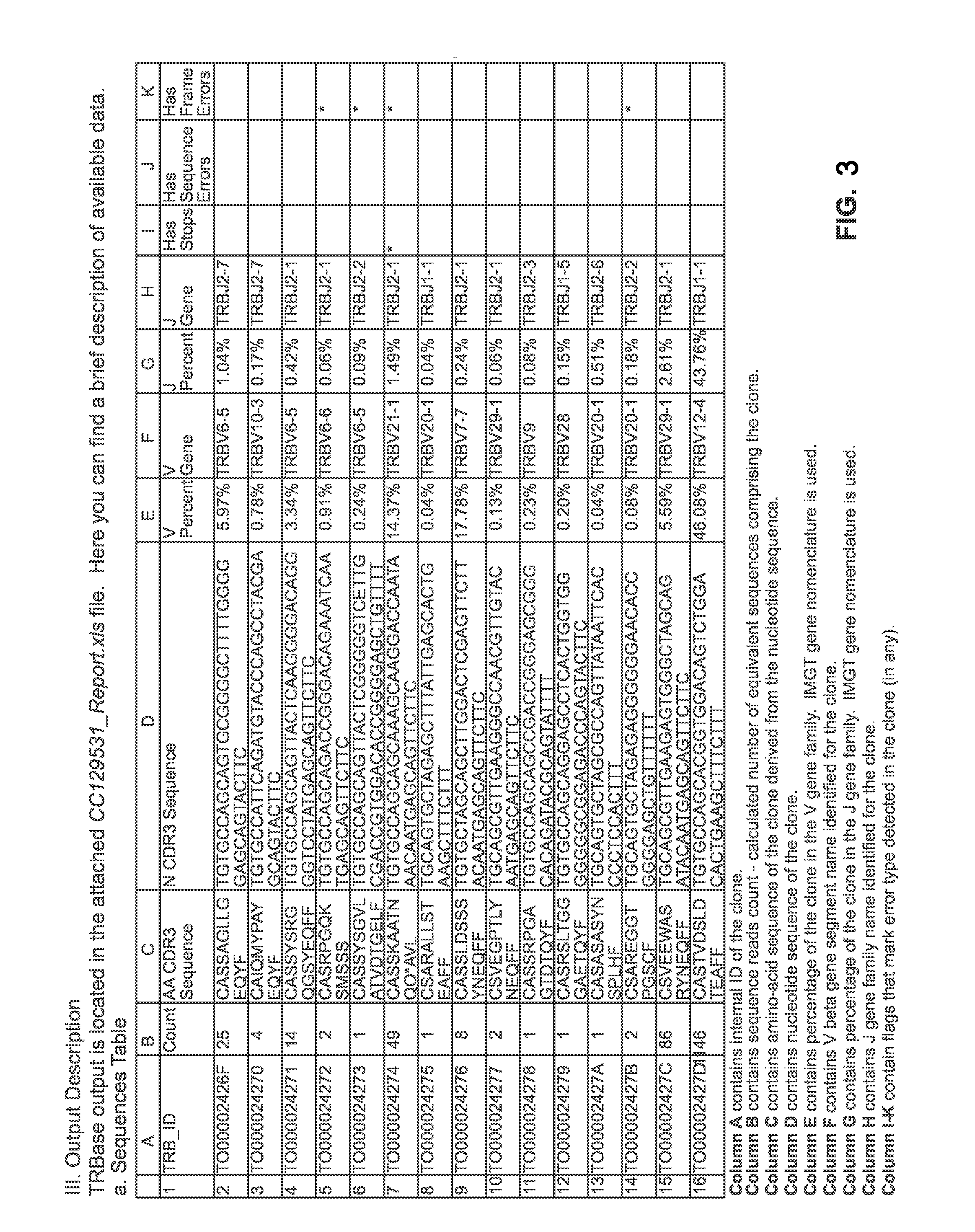
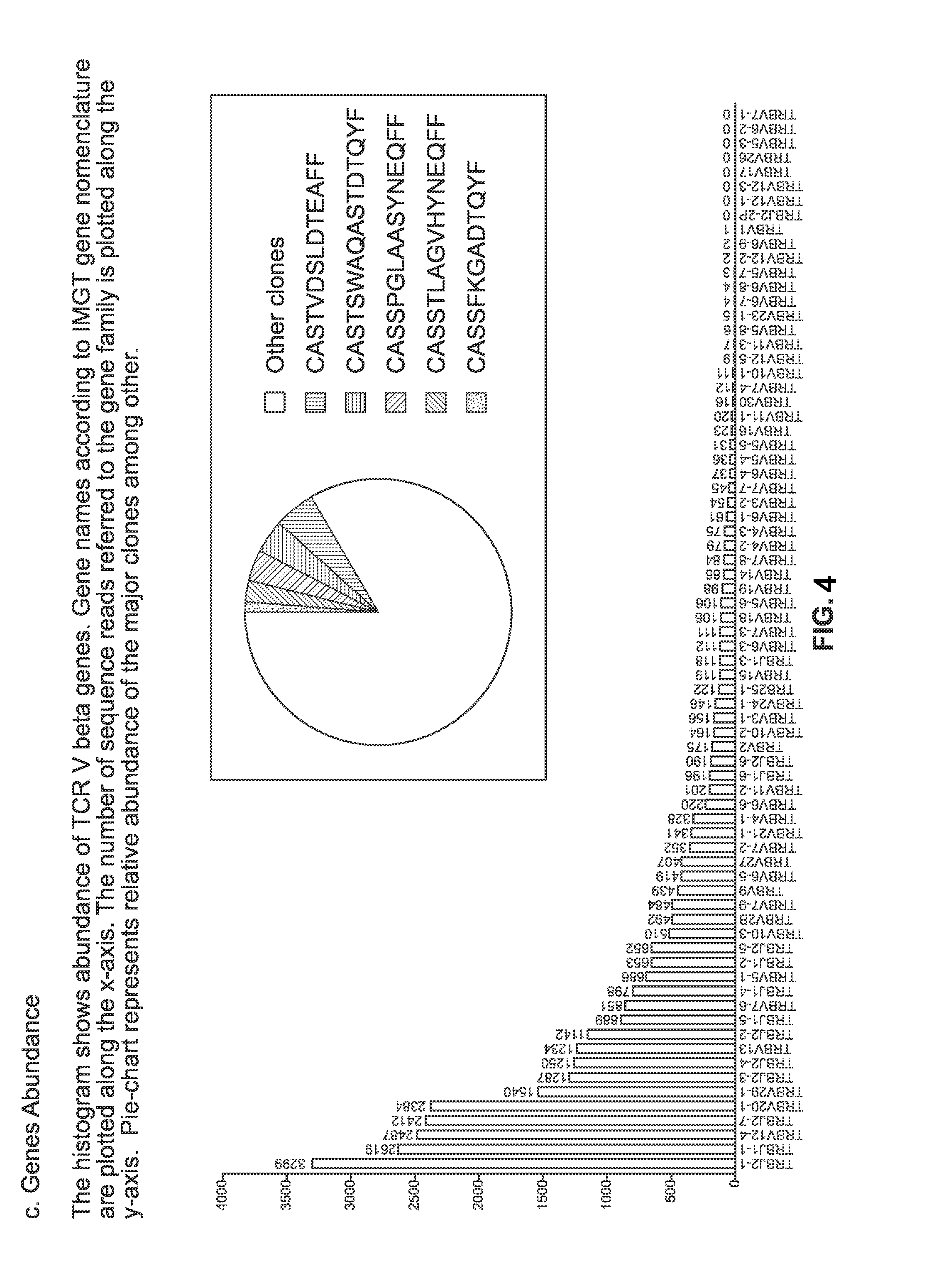
The present invention comprises a method of treatment of an autoimmune disease involving a specific tolerance induction (“STI”) event, wherein the method includes: collecting a first sample from the patient prior to the STI event; detecting an STI event or performing a procedure that correlates in time to an STI event; collecting a second sample from the patient after the STI event; preparing lymphocytes from the first and second samples; preparing and sequencing DNA or cDNA from the prepared lymphocytes; identifying sequences of prevalent T or B cell receptors (“prevalent receptor sequences”) among the lymphocytes of the second sample; selecting a regulatory lymphocyte that carries at least one prevalent receptor sequence, which selected regulatory lymphocyte (i) expresses at least one prevalent receptor sequence or (ii) is generated from an autologous or allogeneic naïve lymphocyte, which naïve lymphocyte is engineered and induced to become a regulatory lymphocyte that expresses at least one prevalent receptor sequence; culturing the selected regulatory lymphocyte, thereby generating daughter cells of said regulatory lymphocyte; and administering the daughter cells to the patient.
Owner:BARKEN ISRAEL
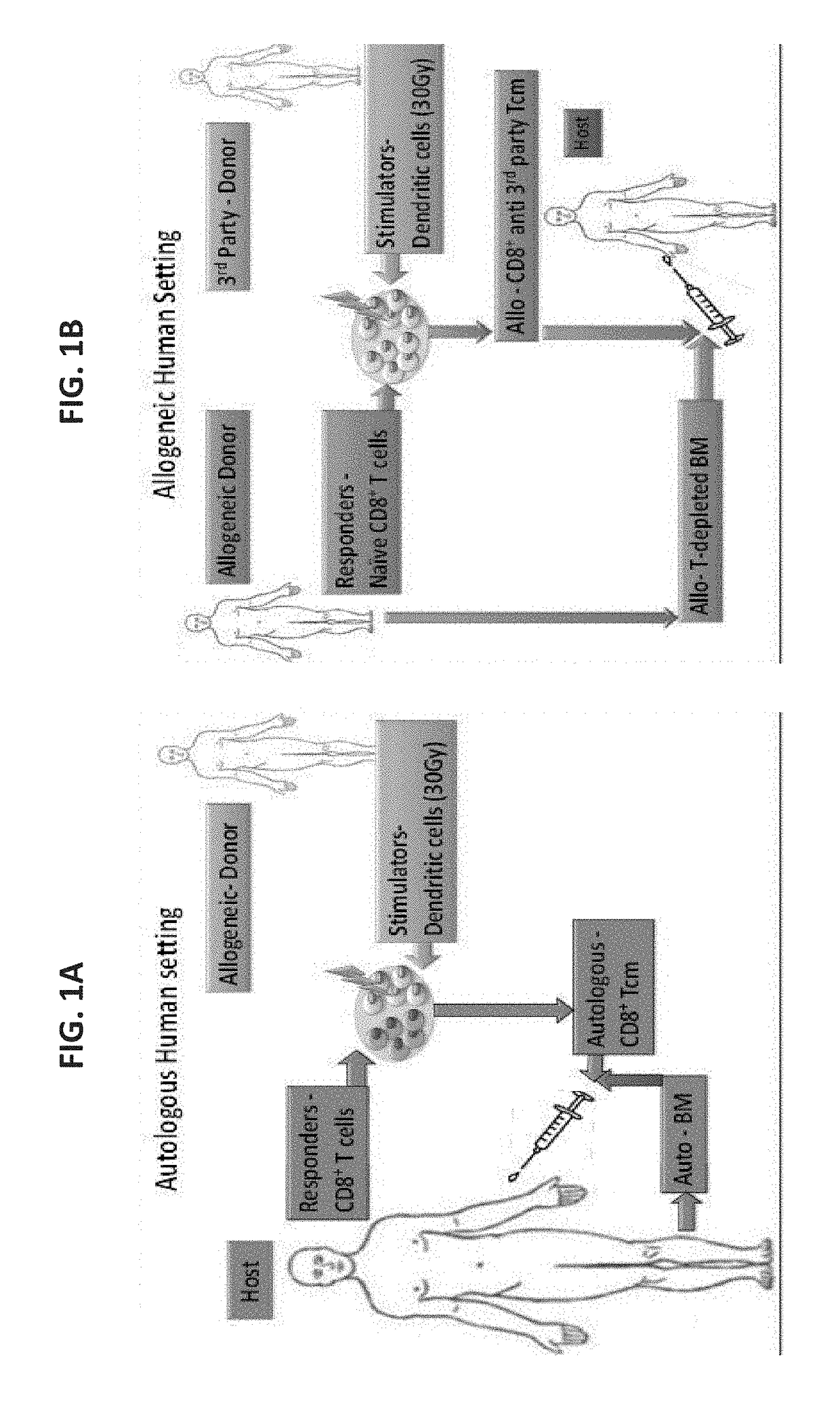
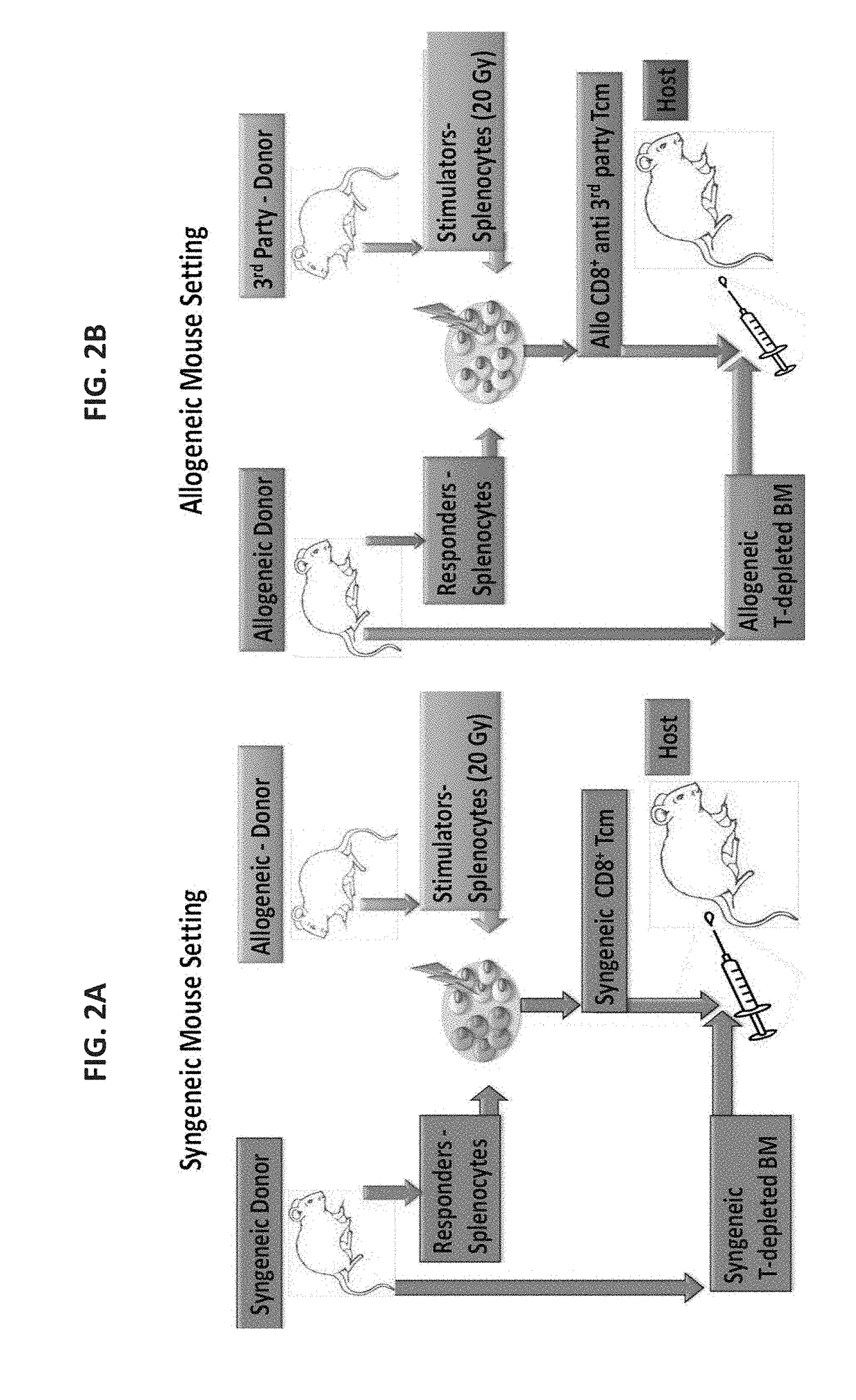
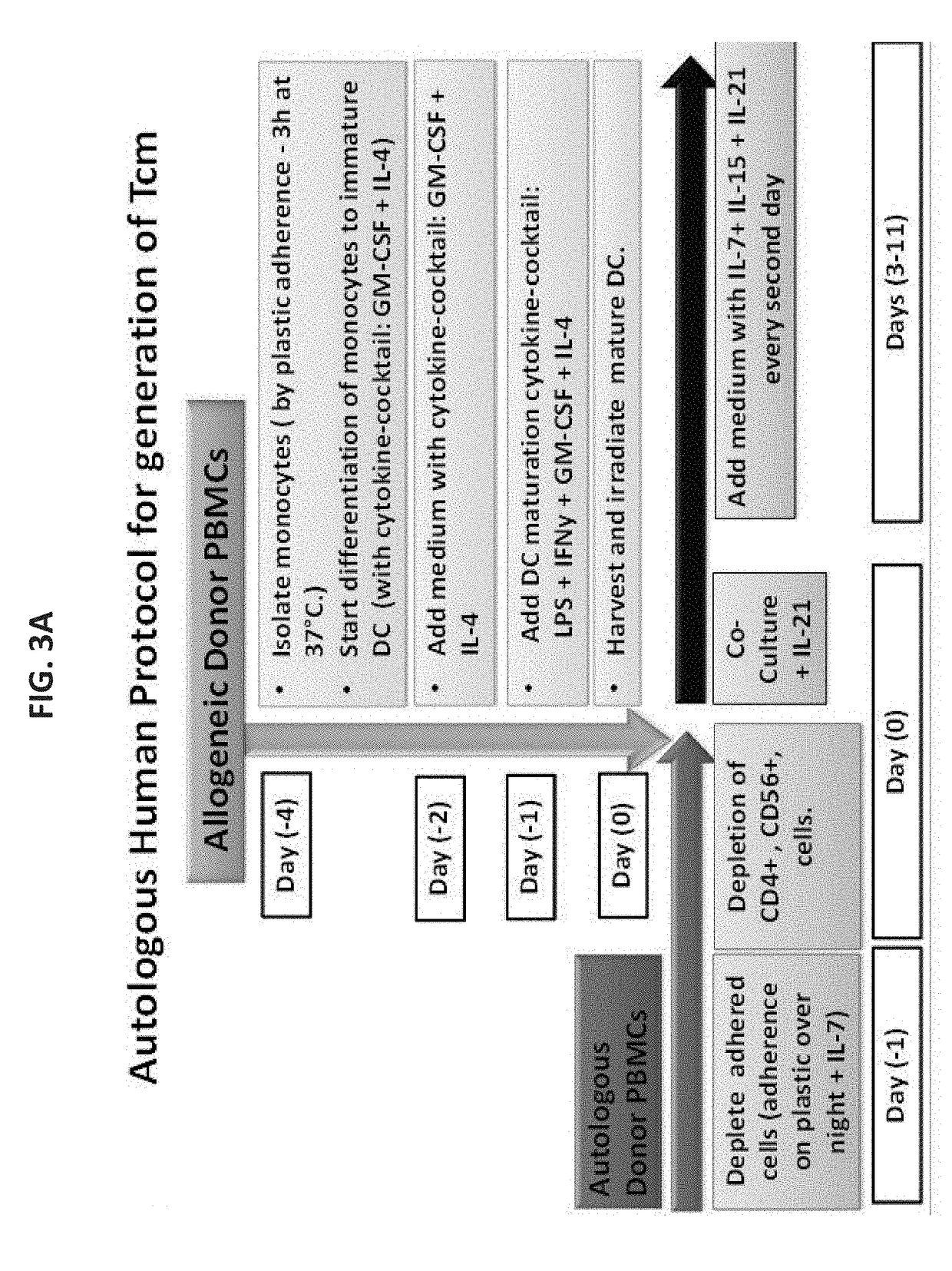
Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
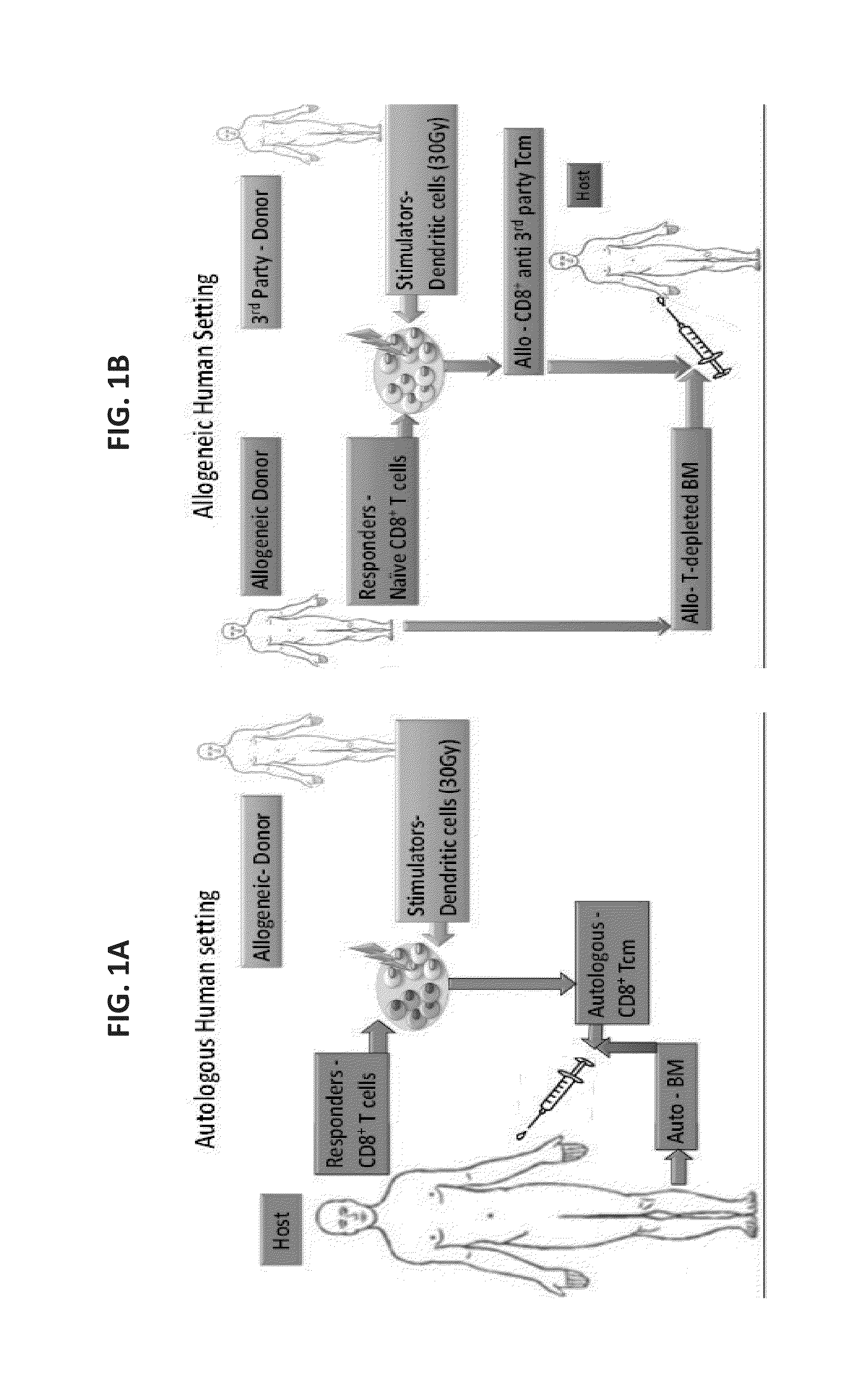
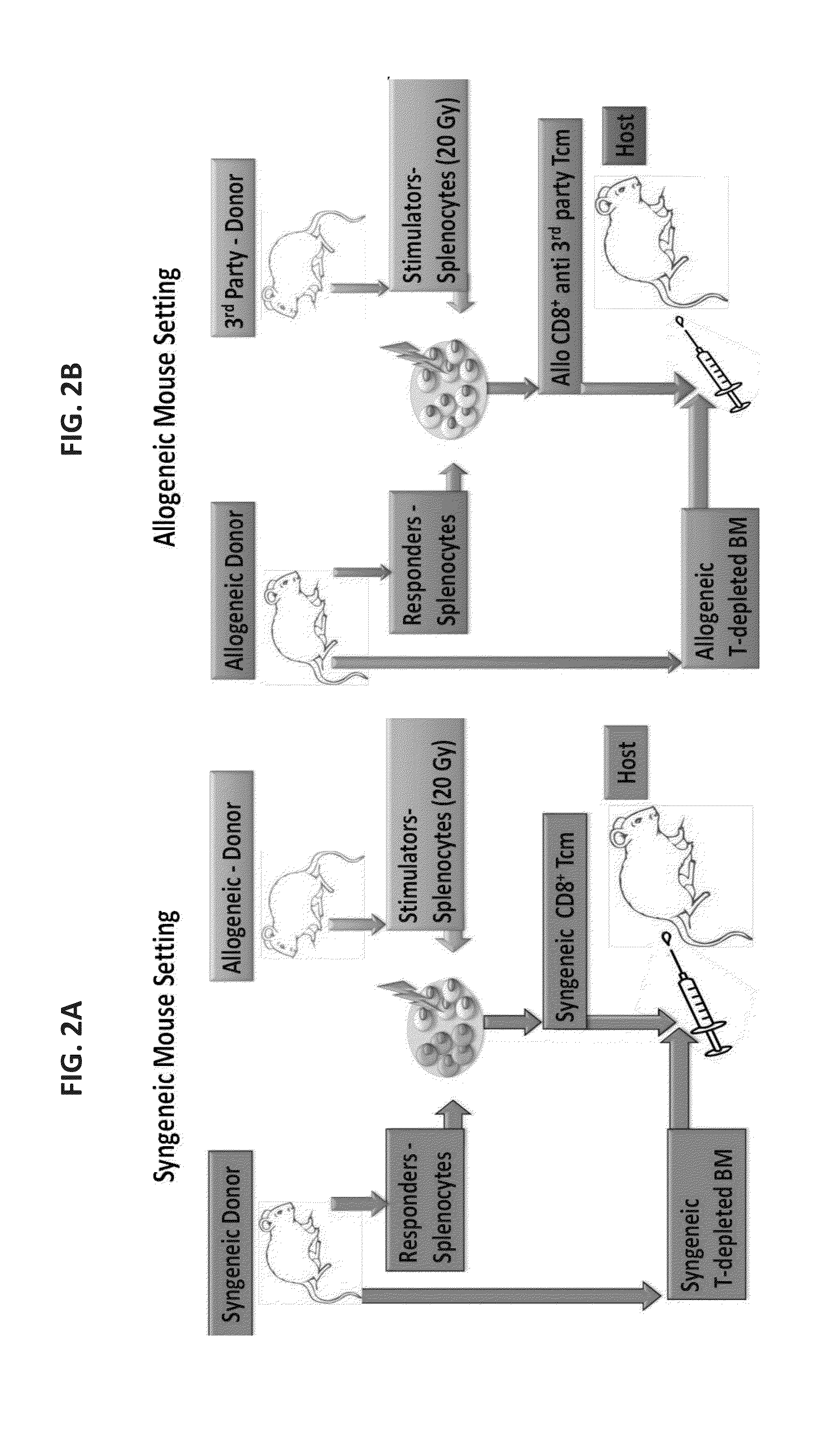
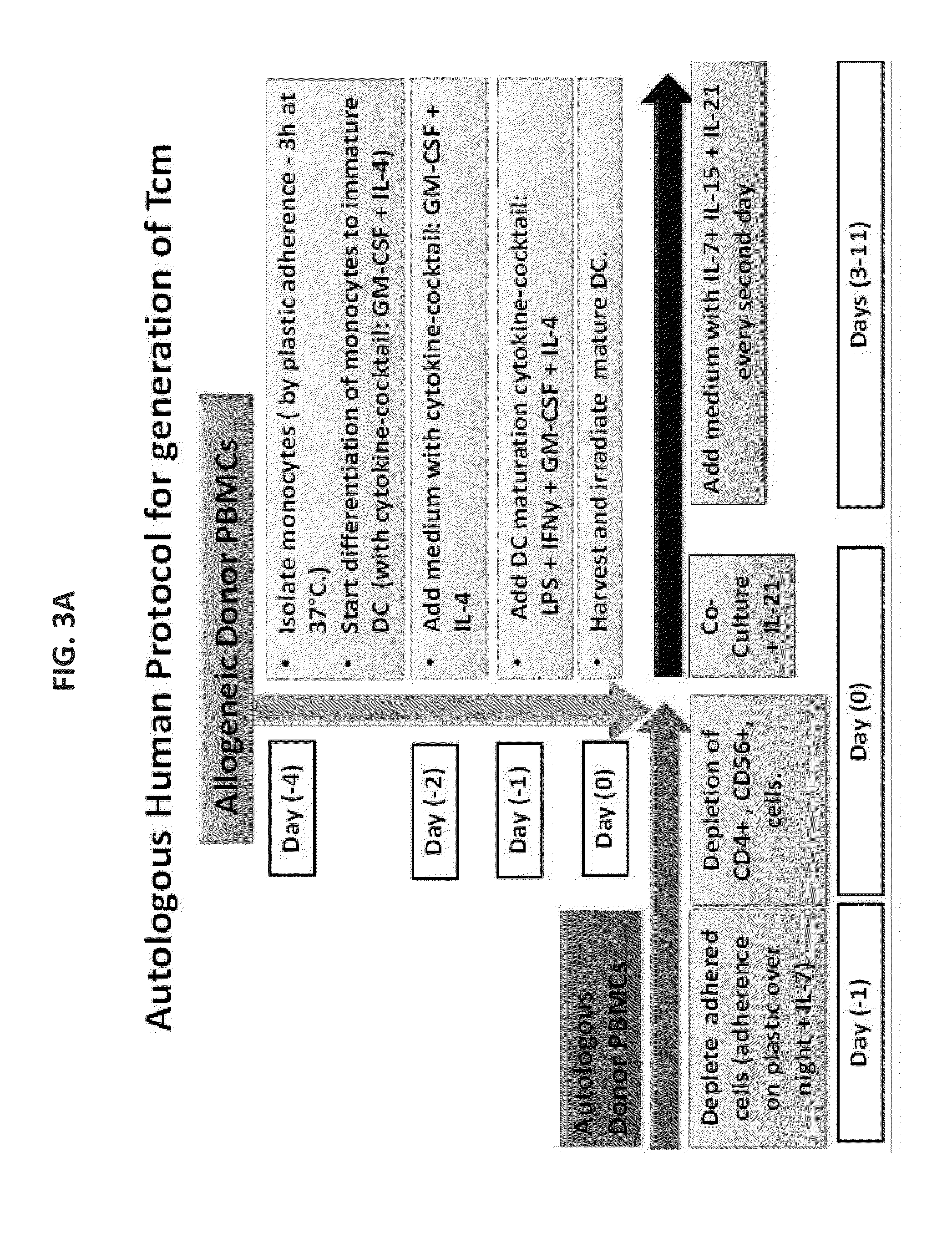
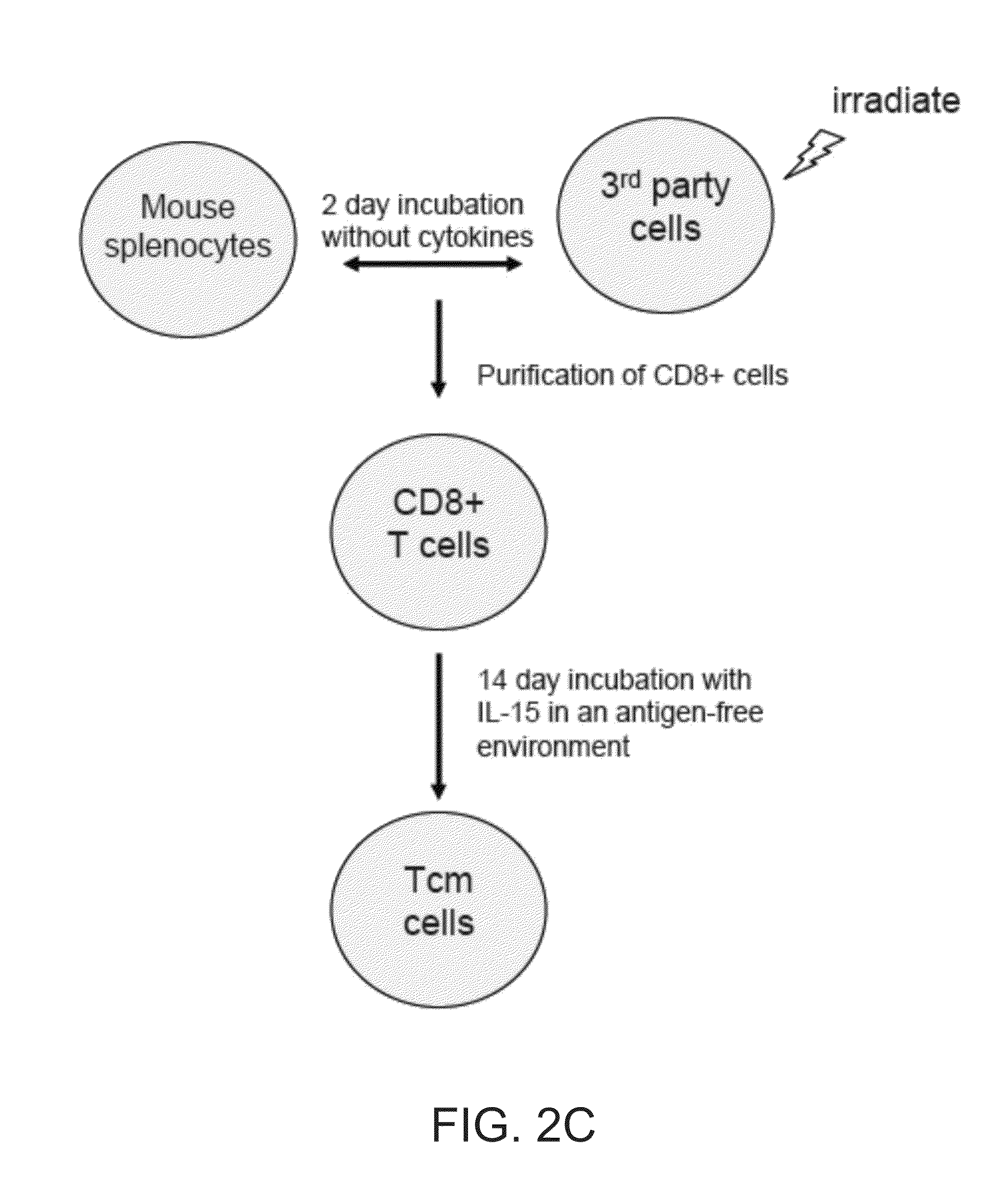
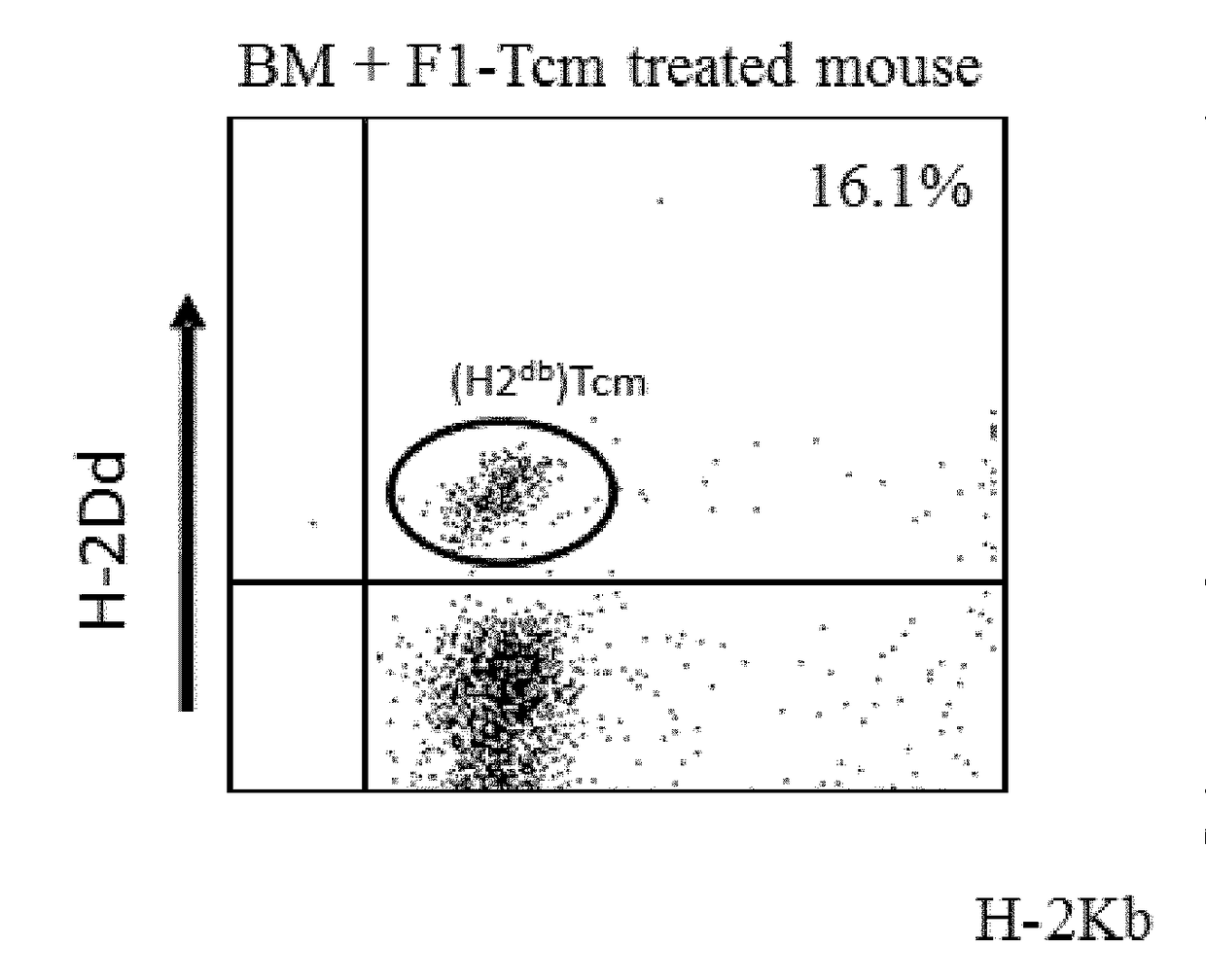
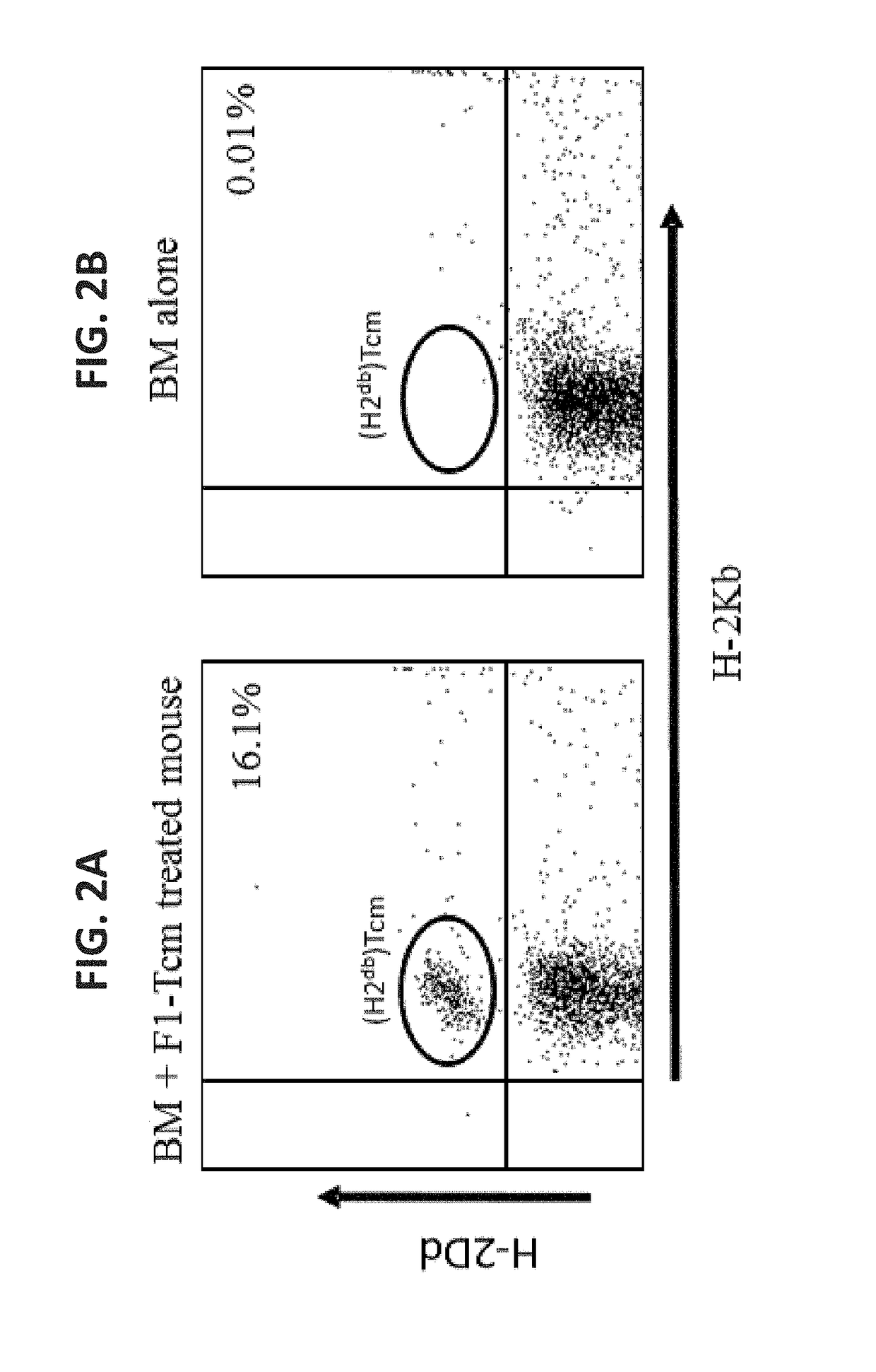
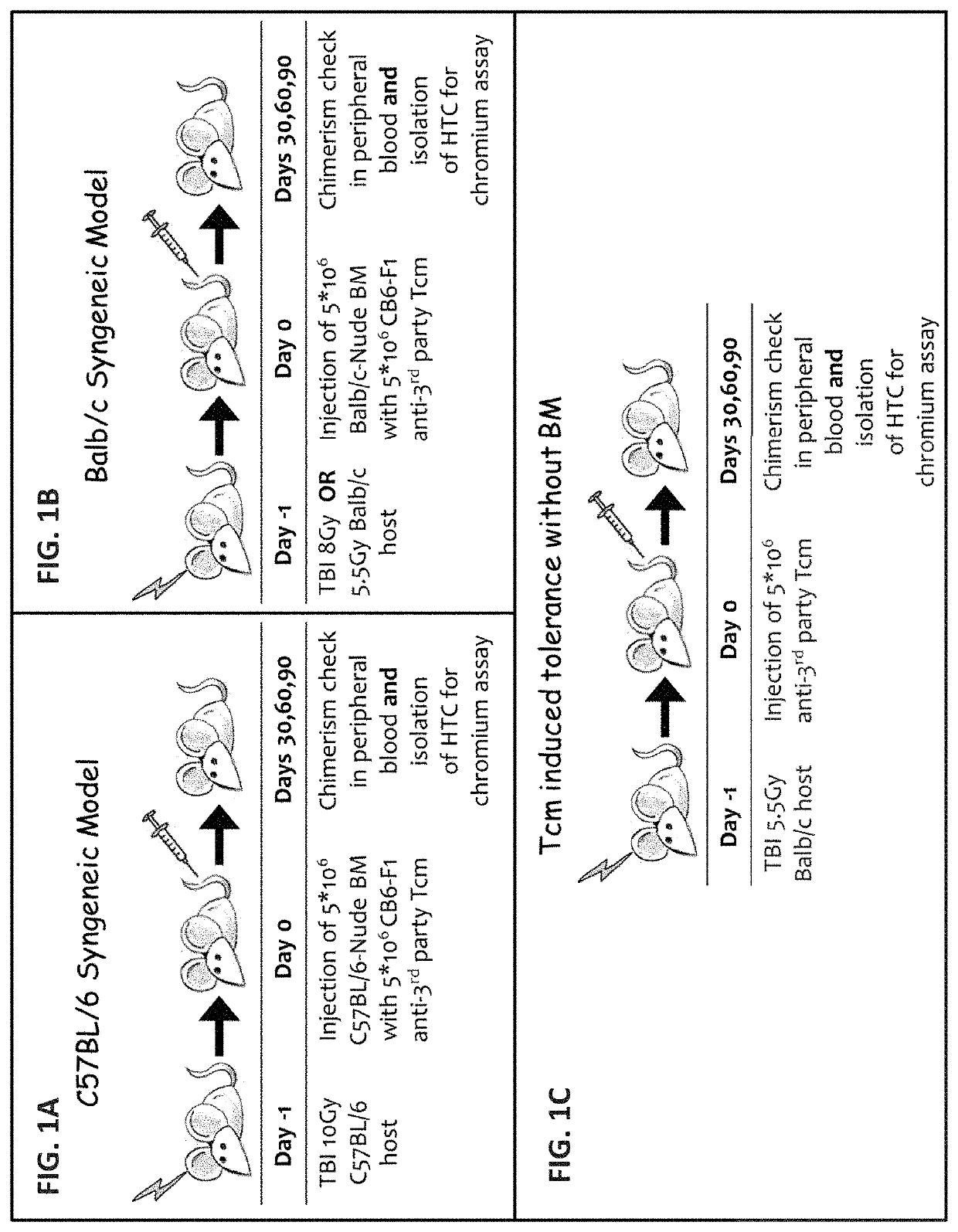
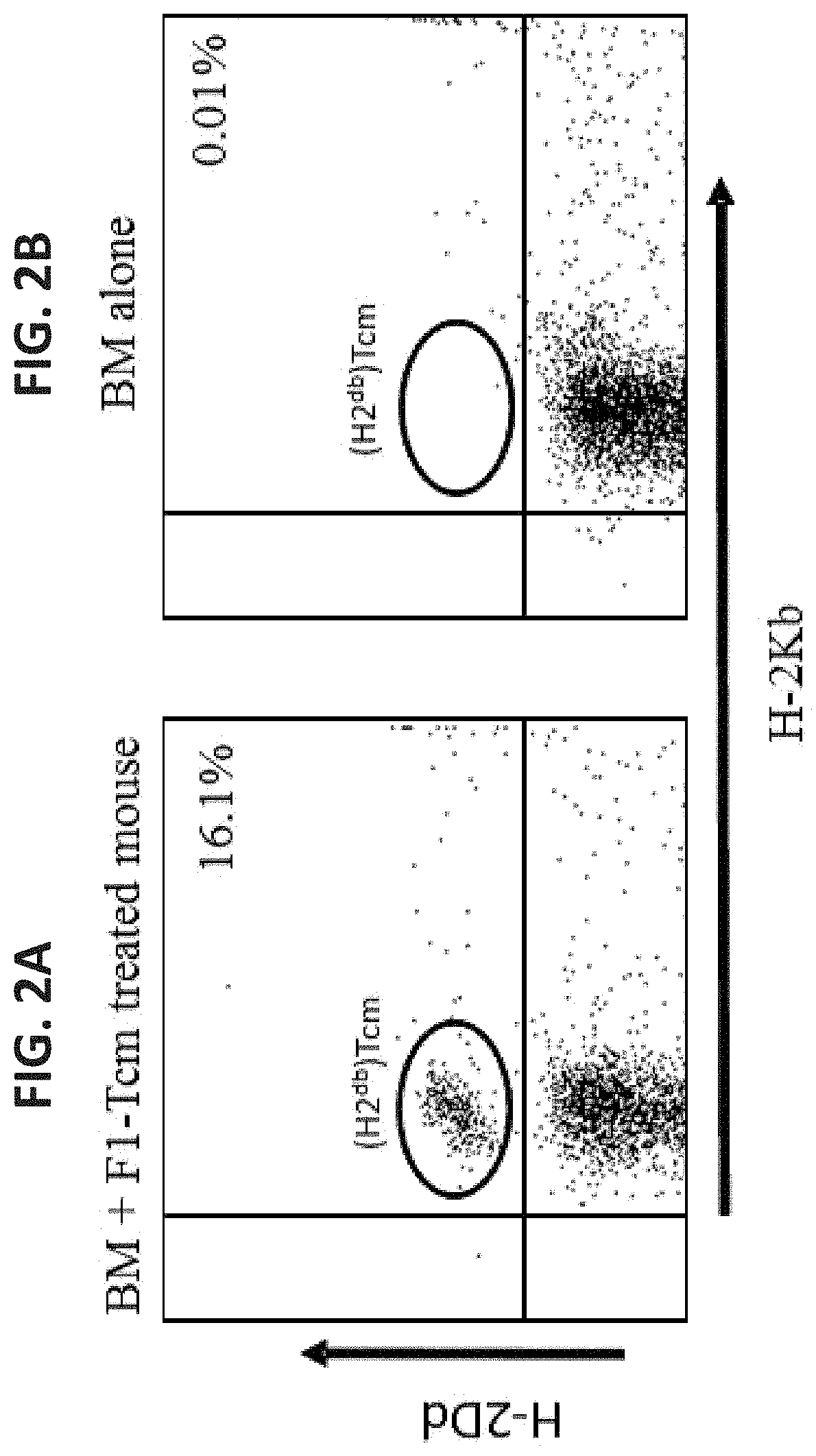
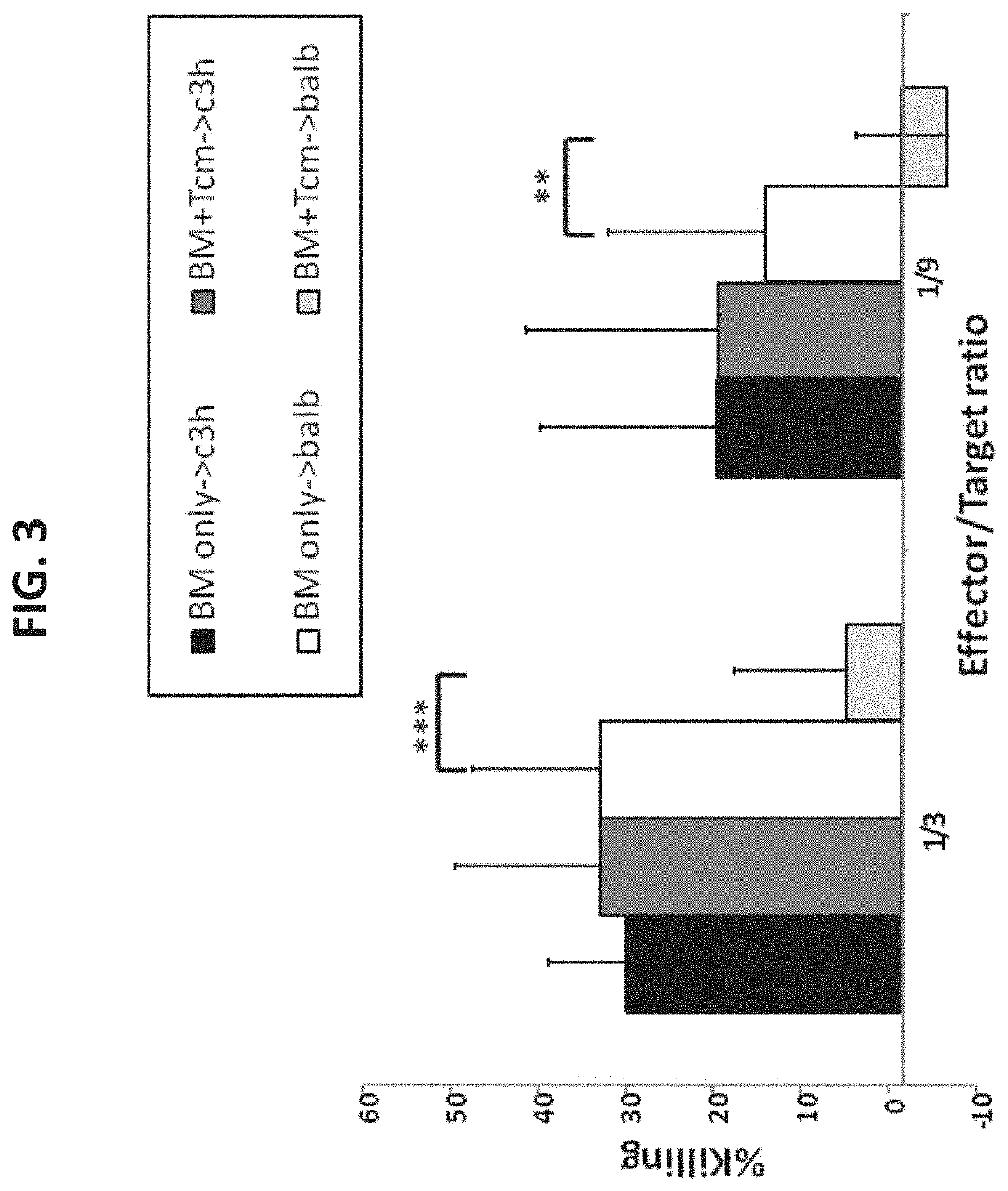
A method of generating an isolated population of cells comprising anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance-inducing cells and / or endowed with anti-disease activity, and capable of homing to the lymph nodes following transplantation is disclosed. The method comprising: (a) contacting peripheral blood mononuclear cells (PBMC) with a third party antigen or antigens in the presence of IL-21 so as to allow enrichment of antigen reactive cells; and (b) culturing the cells resulting from step (a) in the presence of IL-21, IL-15 and IL-7 in an antigen free environment so as to allow proliferation of cells comprising the central memory T-lymphocyte (Tcm) phenotype.
Owner:YEDA RES & DEV CO LTD
Optimized dosing with anti-CD4 antibodies for tolerance induction in primates
InactiveUS20060002921A1Lower immune responsePromote toleranceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTolerance induction
The present invention is based, at least in part, on the finding that tolerance can be induced by inhibition of CD4+ cells (and optionally CD8+ cells). Accordingly, the optimized dosing methods of the invention are useful in treating a primate, e.g., a human, by inhibiting CD4+ T cells to induce tolerance to at least one antigen, e.g., self or foreign, such as for inducting tolerance in a primate against a soluble or a cell bound antigen (e.g., an allogeneic or xenogeneic transplanted antigen).
Owner:TOLERX INC
Stable subcutaneous protein formulations and uses thereof
ActiveUS9309316B2Hybrid immunoglobulinsCell receptors/surface-antigens/surface-determinantsTolerance inductionDisease
The present invention relates generally to stable formulations comprising CTLA4Ig molecules, including lyophilized, and liquid formulations for administration via various routes including, for example, routes such as intravenous (IV) and subcutaneous (SC) for treating immune system diseases and tolerance induction.
Owner:BRISTOL MYERS SQUIBB CO
Tolerance induction and maintenance in hematopoietic stem cell allografts
InactiveUS20100150947A1Immunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsTolerance inductionBiology
Provided are methods and compositions for the induction and maintenance of tolerance in hematopoietic stem cell allografts.
Owner:THE CLEVELAND CLINIC FOUND
Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
An isolated population of cells comprising non-GVHD inducing anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype is provided. The cells being tolerance-inducing cells and capable of homing to the lymph nodes following transplantation. Methods of generating same, use of same and methods of treatment are also provided.
Owner:YEDA RES & DEV CO LTD
Genetically modified Anti-third party central memory t cells and use of same in immunotherapy
ActiveUS20180207272A1Immunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsReceptor degradationTransplantation
An isolated cell having a central memory T-lymphocyte (Tcm) phenotype, the cell being tolerance-inducing cell and capable of homing to the lymph nodes following transplantation, the cell being transduced to express a cell surface receptor comprising a T cell receptor signaling module is disclosed. Methods of generating same and using same are also disclosed.
Owner:YEDA RES & DEV CO LTD
Specific activation of a regulatory t cell and its use for treatment of asthma, allergic disease, autoimmune disease, graft rejection and for tolerance induction
ActiveUS20100074904A1Improve clinical pictureStrong proliferationSenses disorderVirusesTolerance inductionRegulatory T cell
The present invention relates specific activation of a regulatory T cell via a specific CD4 epitope and uses thereof, e.g. for the treatment of an autoimmune disease or an allergy or asthma or graft rejection or tolerance induction.
Owner:ACTITREXX GMBH
Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
A method of generating an isolated population of cells comprising anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance-inducing cells and / or endowed with anti-disease activity, and capable of homing to the lymph nodes following transplantation is disclosed. The method comprising: (a) contacting peripheral blood mononuclear cells (PBMC) with a third party antigen or antigens in the presence of IL-21 so as to allow enrichment of antigen reactive cells; and (b) culturing the cells resulting from step (a) in the presence of IL-21, IL-15 and IL-7 in an antigen free environment so as to allow proliferation of cells comprising the central memory T-lymphocyte (Tcm) phenotype.
Owner:YEDA RES & DEV CO LTD
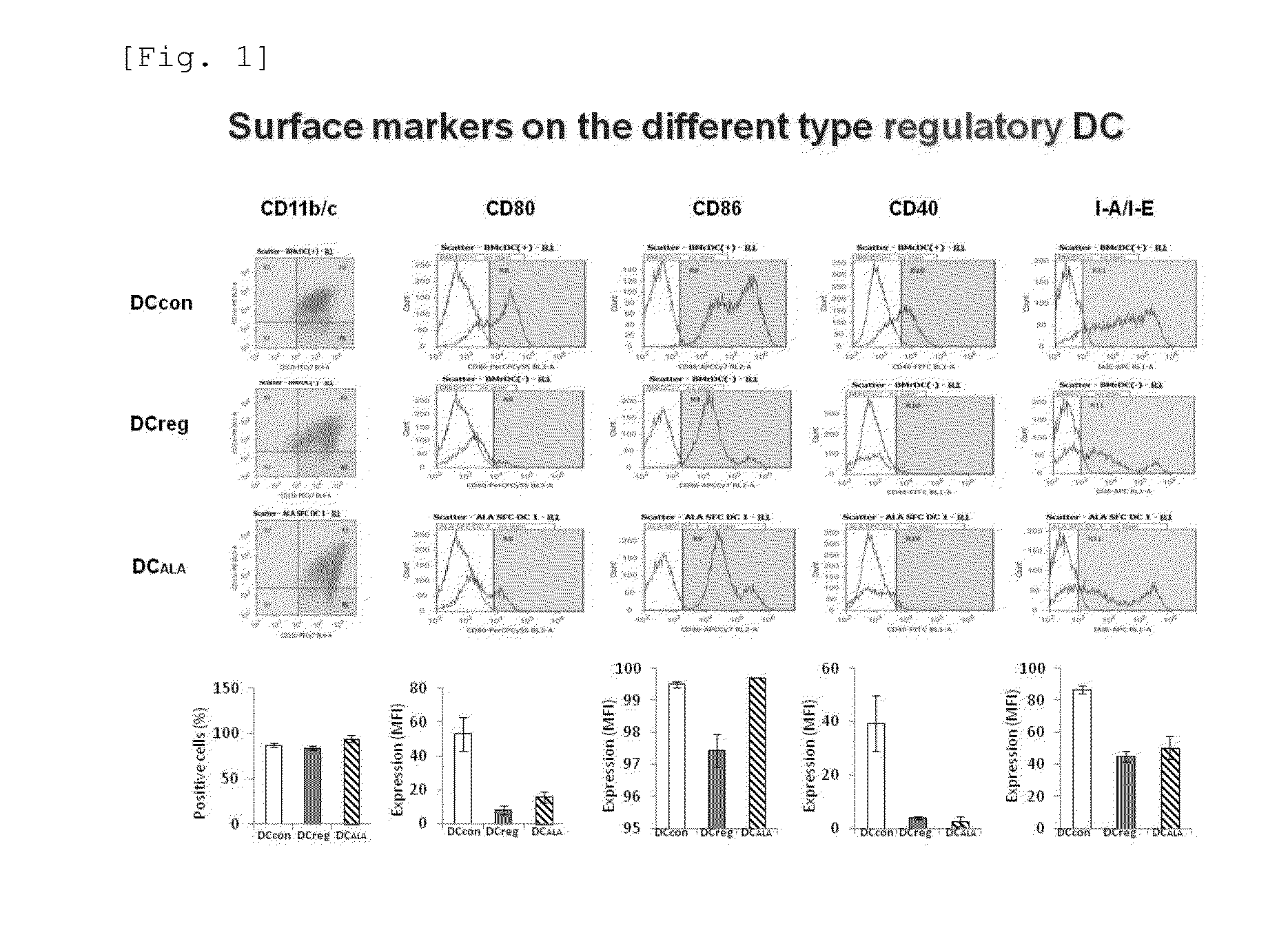
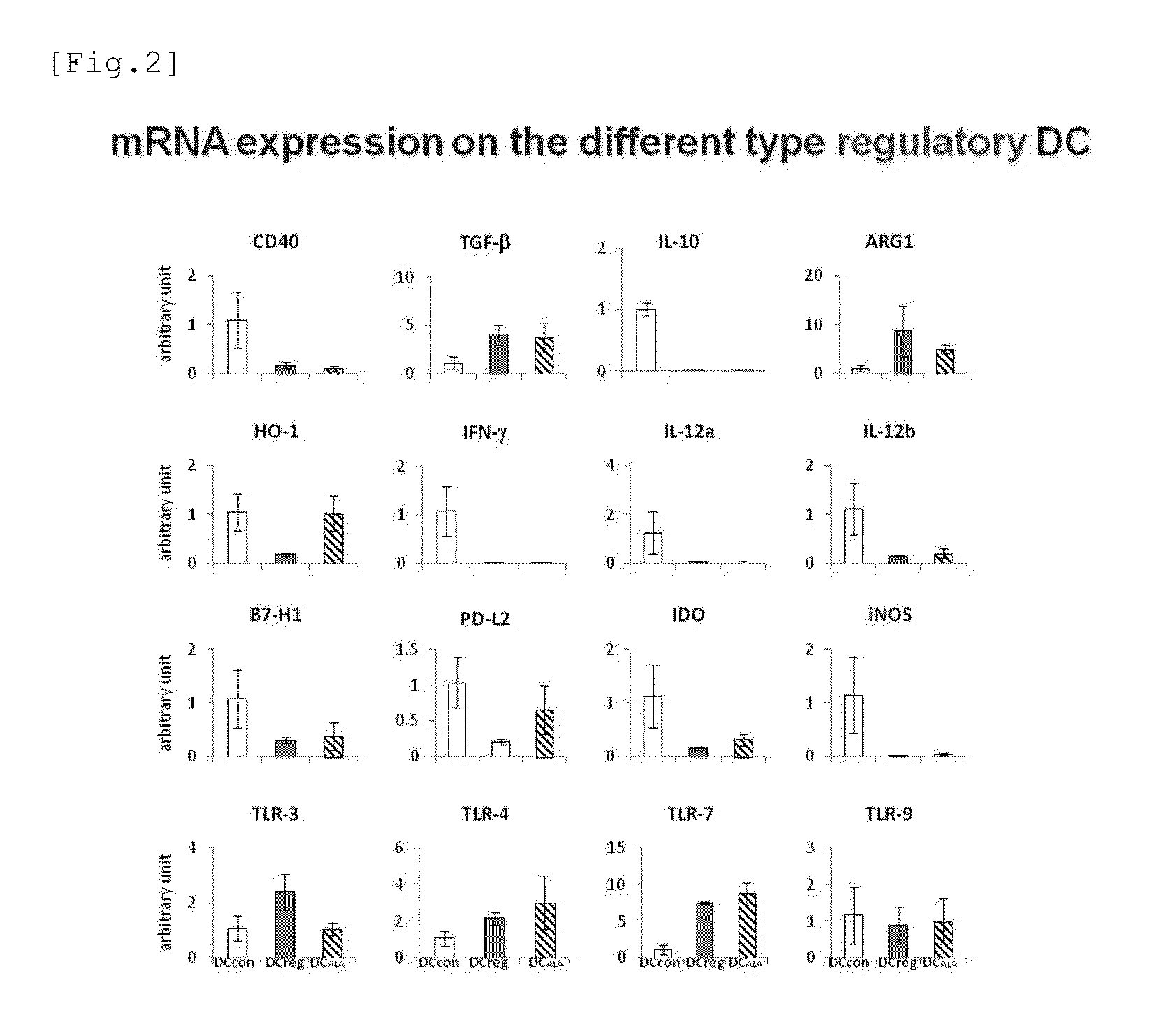
Immune tolerance inducer
ActiveUS20150174090A1Good effectHeavy metal active ingredientsBiocideTolerance inductionAutoimmune condition
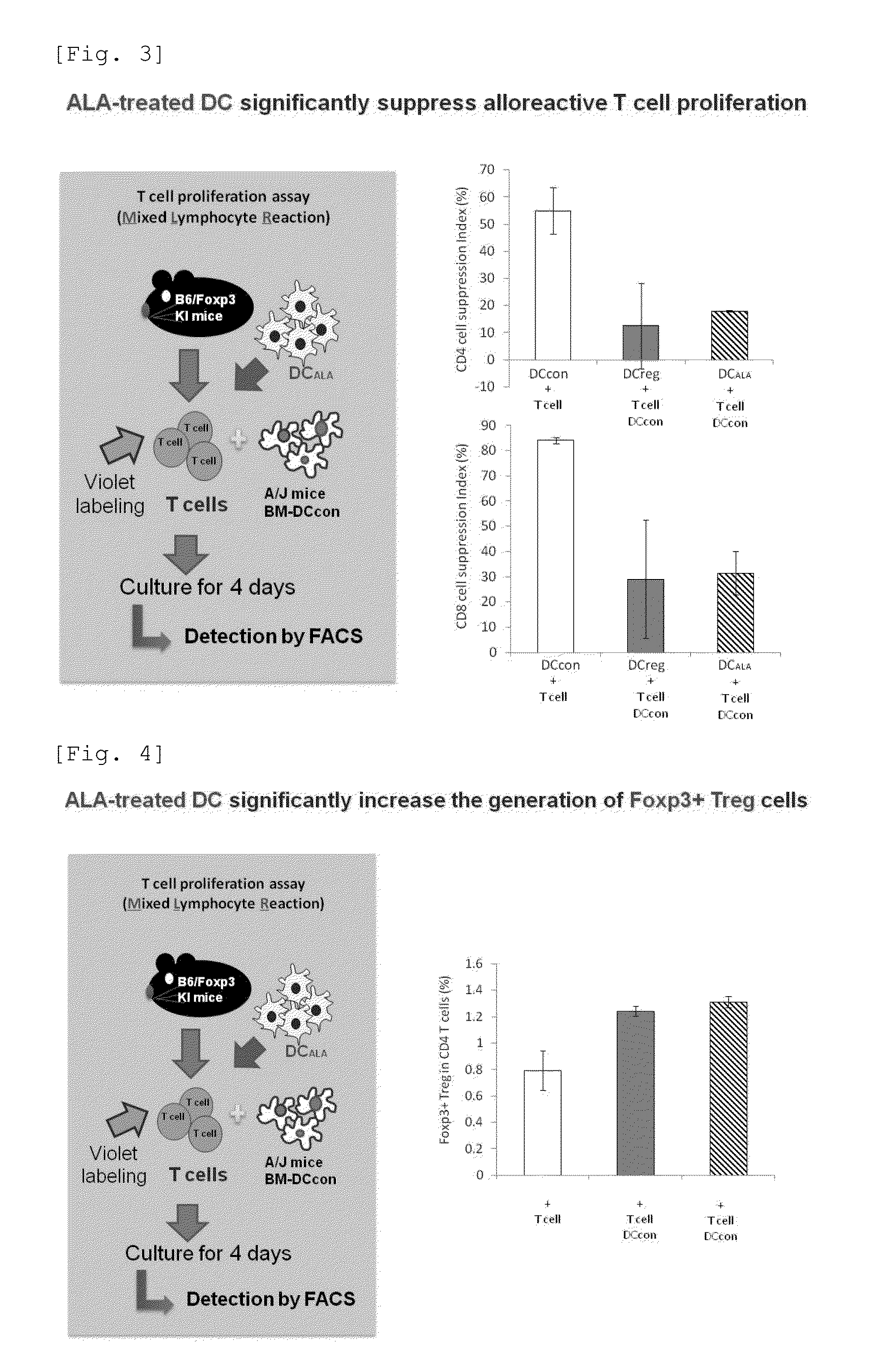
An object of the present invention is to provide a regulatory dendritic cell inducing agent applicable to the treatment of immune disease as well as an immune tolerance inducing agent such as a preventive and / or therapeutic agent for allergic disease and a preventive and / or therapeutic agent for autoimmune disease, both of which are safe and have the mechanism of action different from that of conventional drugs. As a means for achieving the above object, an immune tolerance inducing agent comprising 5-aminolevulinic acid (ALA) or a derivative thereof, or a salt of the 5-ALA or the derivative and an iron compound as active ingredients is prepared. Preferable examples of the ALAs can include ALA and various esters such as methyl ester, ethyl ester, propyl ester, butyl ester, and pentyl ester of ALA, and their hydrochlorides, phosphates, and sulfates. Preferable examples of the iron compound can include sodium ferrous citrate.
Owner:SBI PHARMA CO LTD +1
Compositions containing beta 2-glycoprotein I for the prevention and/or treatment of atherosclerosis
InactiveUS20050148499A1Induce tolerizationSaccharide peptide ingredientsDepsipeptidesGreek letter betaTolerance induction
A method for prevention and / or treatment of atherosclerosis in a subject, in need thereof is disclosed. The method is effected by orally administering to the subject an immunological oral tolerance-inducing composition which comprises an immunological oral tolerance-inducing amount of an active ingredient selected from the group consisting of beta2-glycoprotein-1 (β2GP-1) or a derivative of β2GP-1.
Owner:VASCULAR BIOGENICS
Optimized dosing with anti-CD4 antibodies for tolerance induction in primates
InactiveUS20080112949A1Lower immune responseInduce toleranceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTolerance induction
The present invention is based, at least in part, on the finding that tolerance can be induced by inhibition of CD4+ cells (and optionally CD8+ cells). Accordingly, the optimized dosing methods of the invention are useful in treating a primate, e.g., a human, by inhibiting CD4+ T cells to induce tolerance to at least one antigen, e.g., self or foreign, such as for inducting tolerance in a primate against a soluble or a cell bound antigen (e.g., an allogeneic or xenogeneic transplanted antigen).
Owner:TOLERX INC
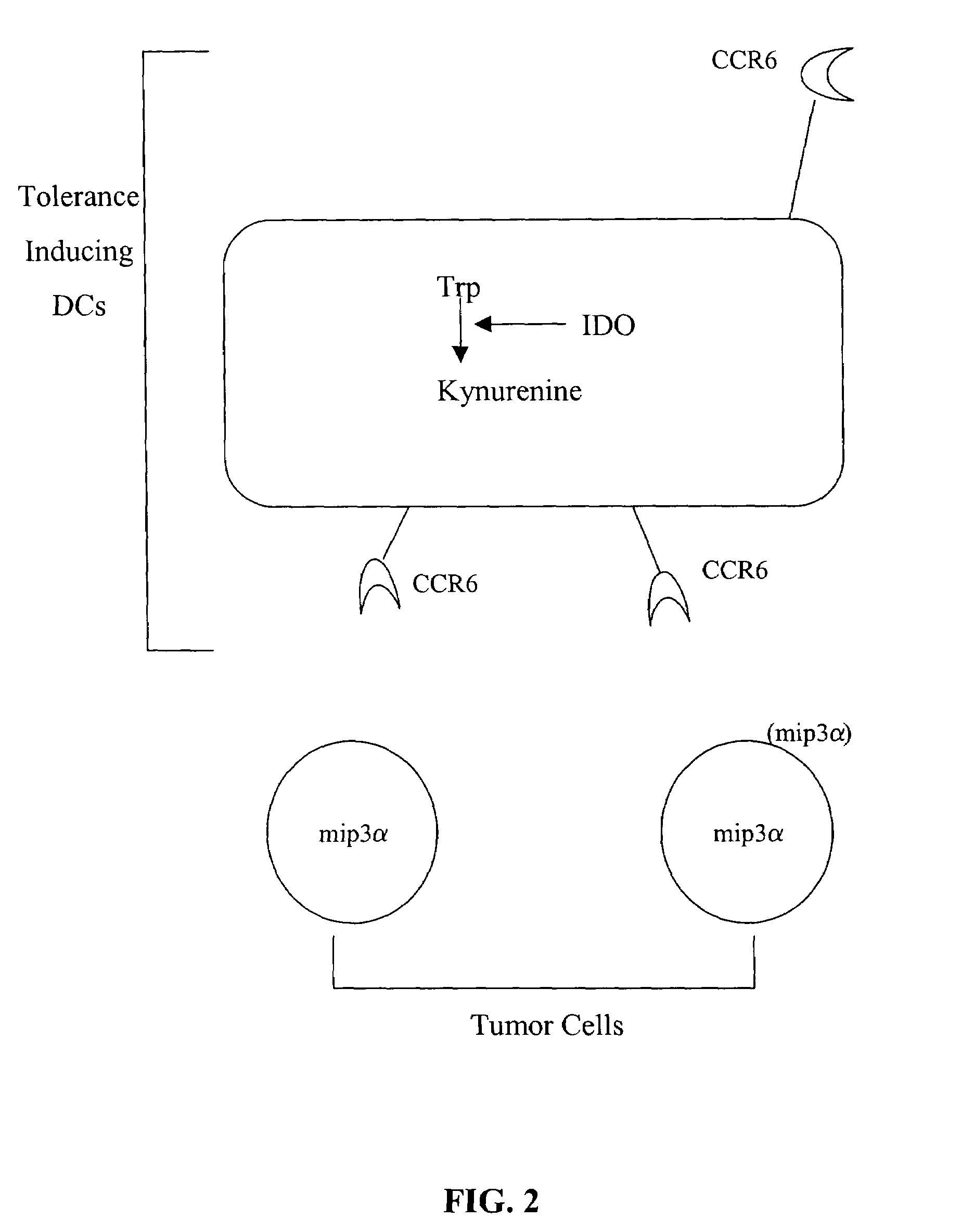
Chemokine receptor antagonists as therapeutic agents
InactiveUS7465448B2Reduce immune toleranceReduced Tolerance RequirementsPeptide/protein ingredientsBiological material analysisTolerance inductionAbnormal tissue growth
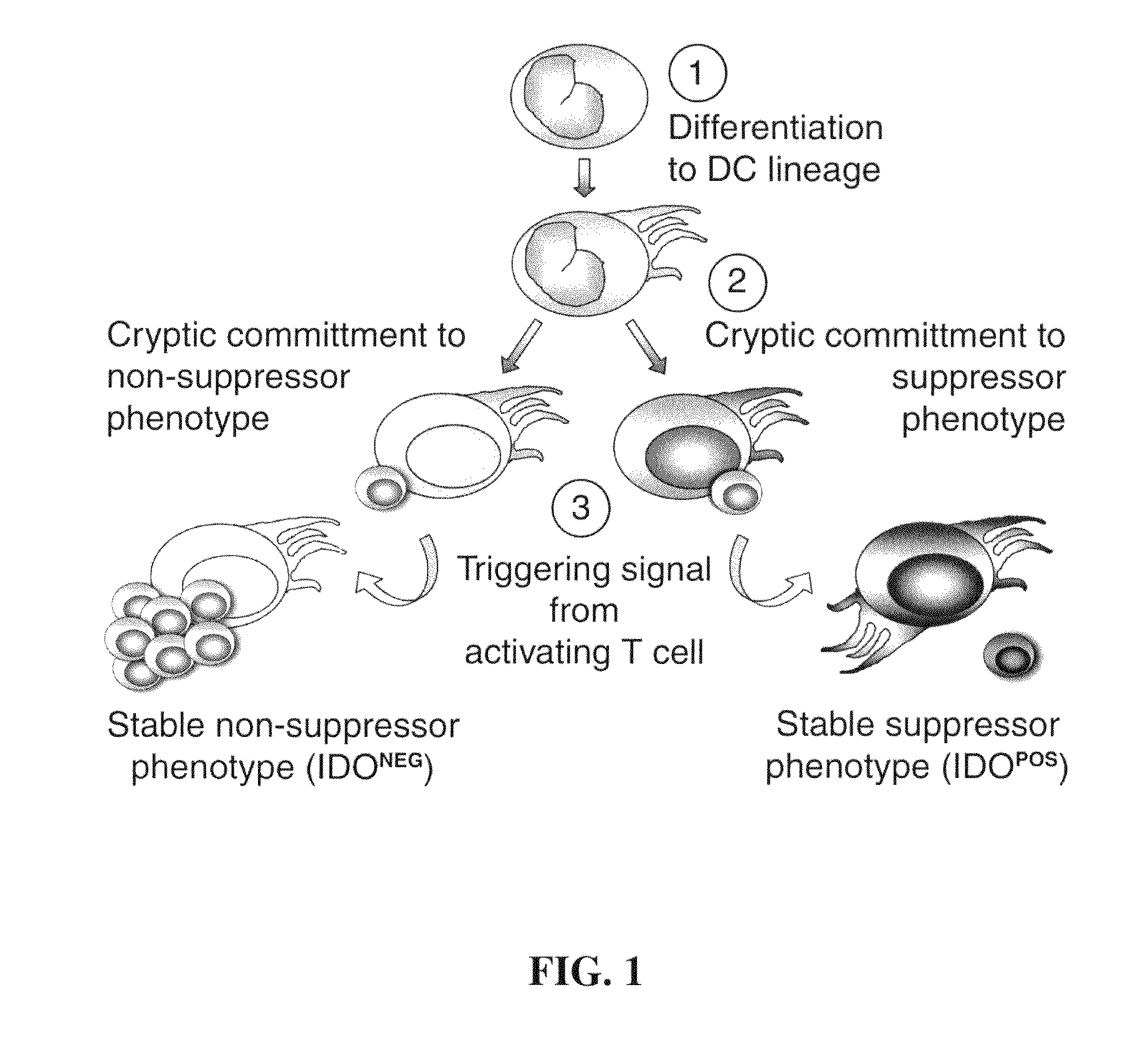
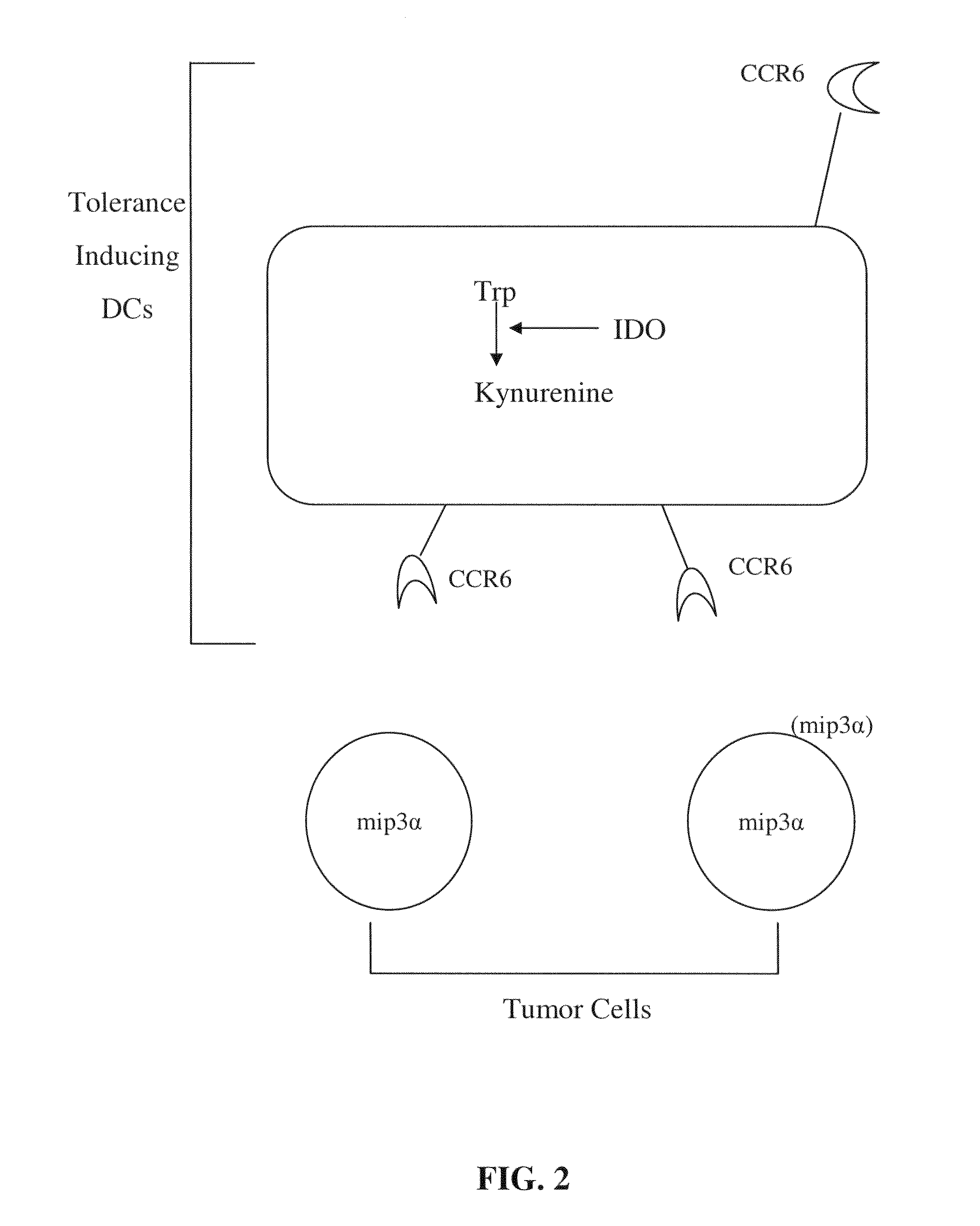
The present invention provides methods and compositions to reduce immune tolerance at specific sites. In one aspect, the present invention comprises methods and compositions to reduce tumorigenicity. In an embodiment, the present invention reduces recruitment of tolerance-inducing antigen presenting cells (APCs) or their precursors to a tumor and / or tumor draining lymph node by decreasing binding of at least one tumor-associated ligand to a chemokine receptor present on the tolerance-inducing APCs or APC precursors. In an embodiment, the chemokine receptor is CCR6 and the tumor-associated ligand is mip-3α. In another aspect, the present invention comprises methods and compositions to reduce immune tolerance to a virus. In an embodiment, the virus is HIV. The present invention further provides for the development of CCR6 antibodies and antagonists as therapeutic agents to prevent or reduce immune tolerance.
Owner:GEORGIA HEALTH SCI UNIV RES INST
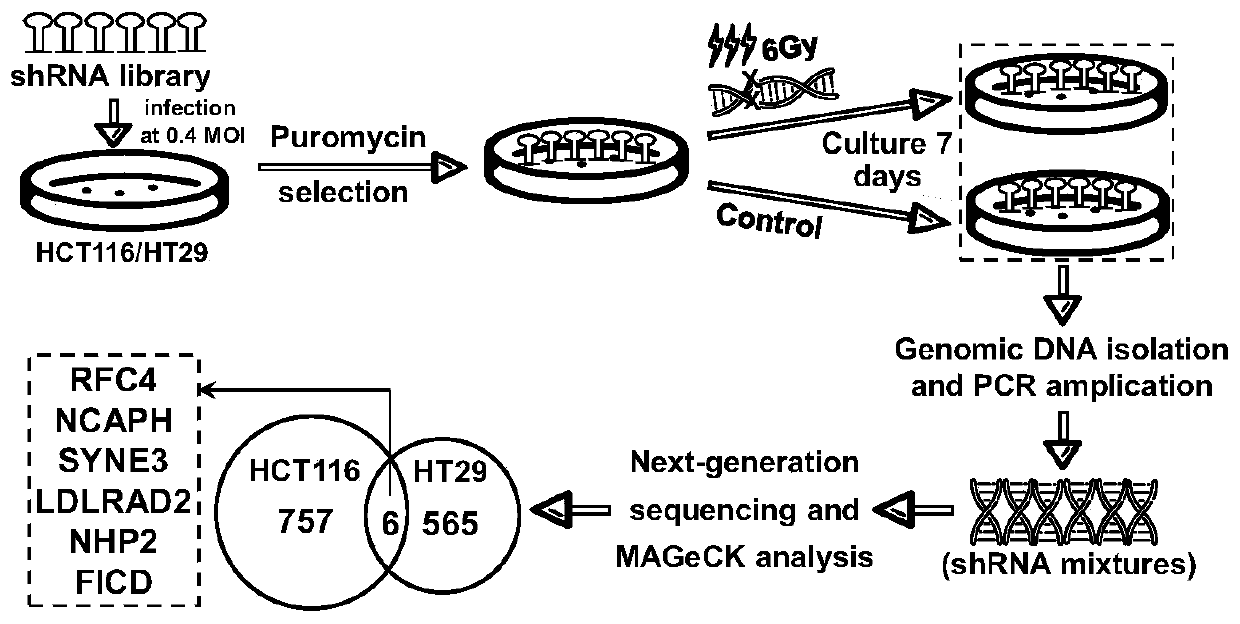
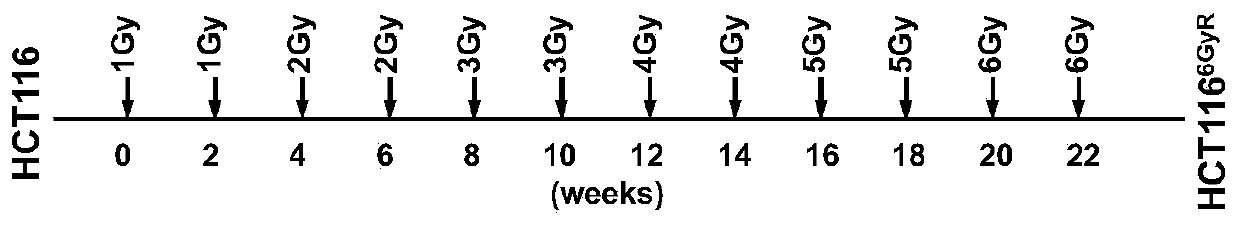
Kit for detecting cancer radiosensitivity and application of kit
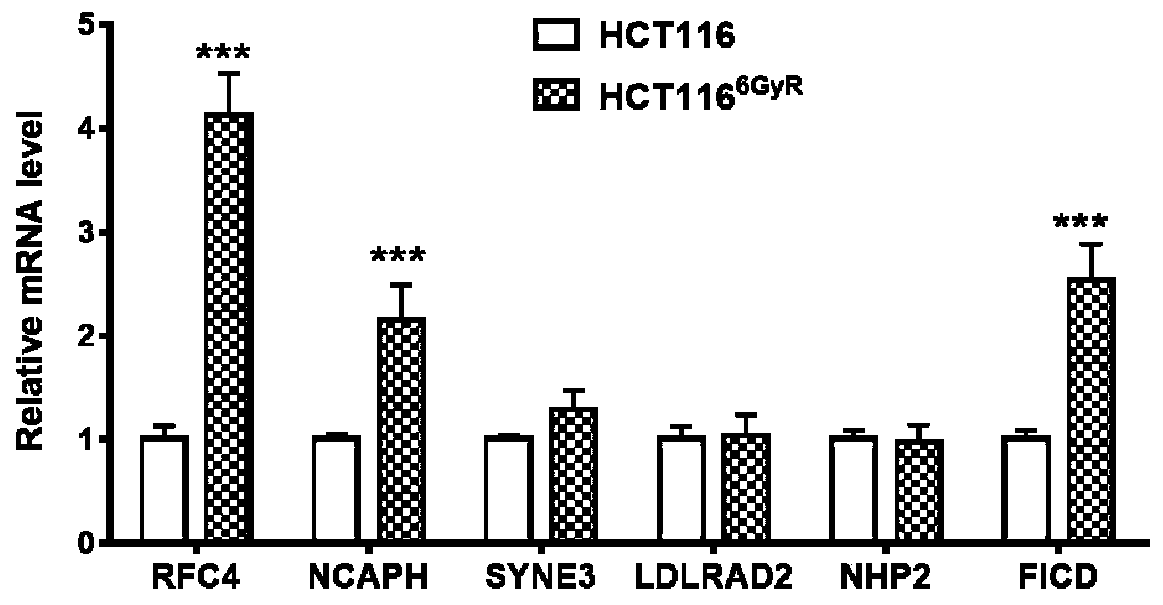
The invention relates to a kit for detecting cancer radiosensitivity and application of the kit to curative effect prediction. According to the kit of the invention, the screening of molecular markersis completely based on whole-genome RNAi library screening which is a more practical and effective screening mode in international research level. Identification is carried out step by step in vitroand vivo based on a radiotherapy tolerance induction process; it is finally found that RFC4 is a gene highly correlated with radiotherapy tolerance of colorectal cancer; clinical retrospective analysis and in-vitro and in-vivo functional analysis are performed on the identified RFC4 gene, it is revealed that the high expression levels of the RFC4 gene can be positively correlated with tumor regression bad performance, and the RFC4 gene can be used to predict radiotherapy tolerance / sensitivity. Based on the above technical schemes of the invention, the kit used for predicting the efficacy of local advanced neoadjuvant radiotherapy is obtained, and is of great realistic significance for solving the problem of differences between individuals in clinical efficacies and the prediction blanks ofregression / prognosis effects, and better achieving accurate treatment.
Owner:SUN YAT SEN UNIV CANCER CENT
Oral tolerance induction by collagen to prevent allograft rejection
InactiveUS7348005B2Avoid treatmentPrevent rejectionPeptide/protein ingredientsSnake antigen ingredientsTolerance inductionOral tolerance
The present invention relates to the use of collagen and MHC-like compounds to down regulate immune responses. Methods for administration of such compounds that induce immune tolerance are described. The invention is important in the context of allograft rejection, transplantation, graft rejection, pleural disease and immunotolerance.
Owner:INDIANA UNIV RES & TECH CORP
Autologous self-tolerance inducing cells of monocytic origin and their use in pharmaceutical preparations
InactiveUS20060188485A1Effect be loseEasy to handleBiocideNervous disorderTolerance inductionRegulatory T cell
Owner:BLASTICON BIOTECHNOLOGISCHE FORSCHUNG
Genetically modified veto cells and use of same in immunotherapy
ActiveUS20190338247A1Immunoglobulin superfamilyPeptide/protein ingredientsTolerance inductionTransgene
An isolated cytotoxic T-lymphocyte (CTL), said CTL being a tolerance inducing cell and substantially depleted of alloreactivity, and wherein said CTL does not comprise a central memory T-lymphocyte (Tcm) phenotype, the CTL being transduced to express a cell surface receptor comprising a T cell receptor signaling module, is disclosed. Methods of generating same and using same are also disclosed.
Owner:YEDA RES & DEV CO LTD
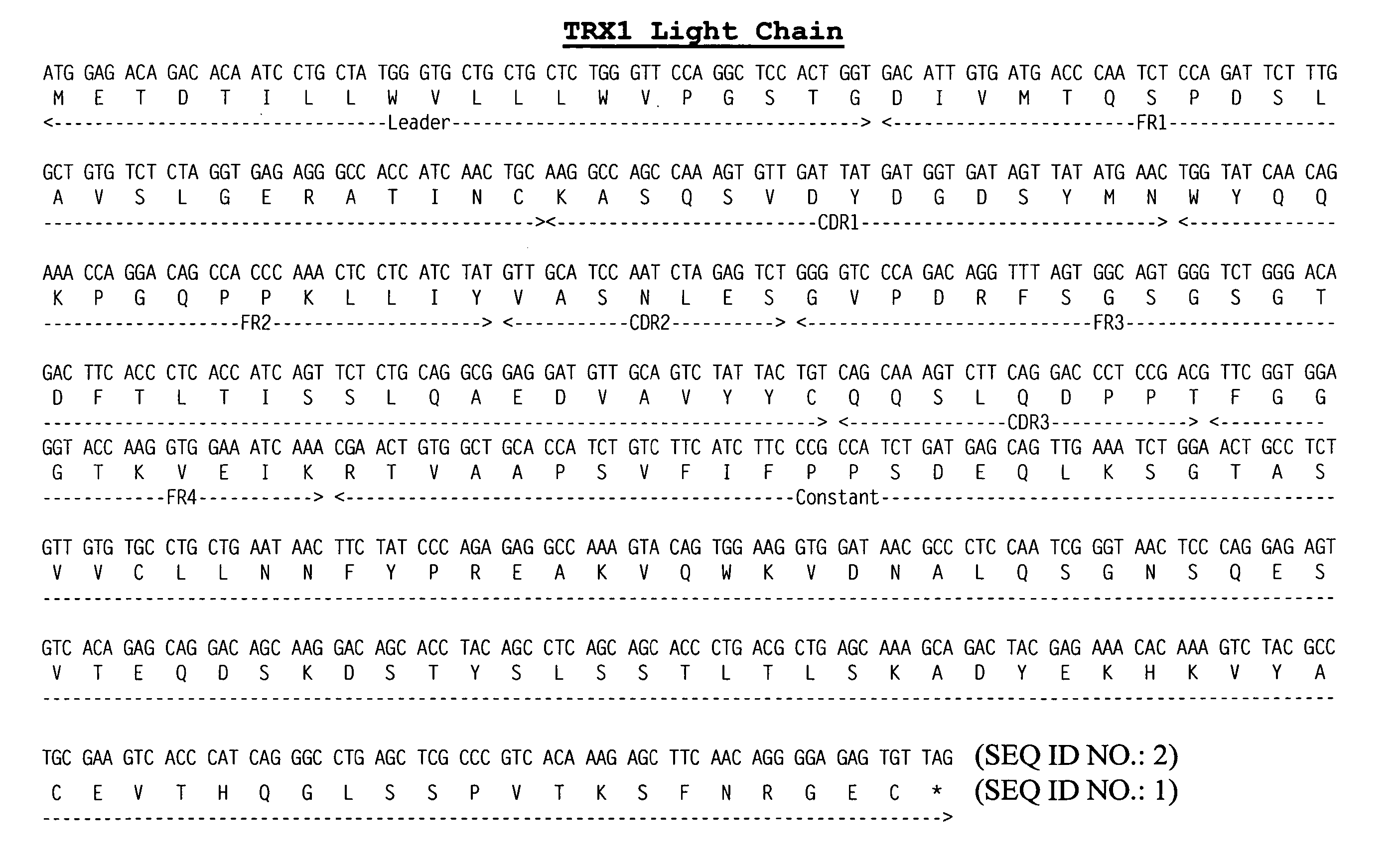
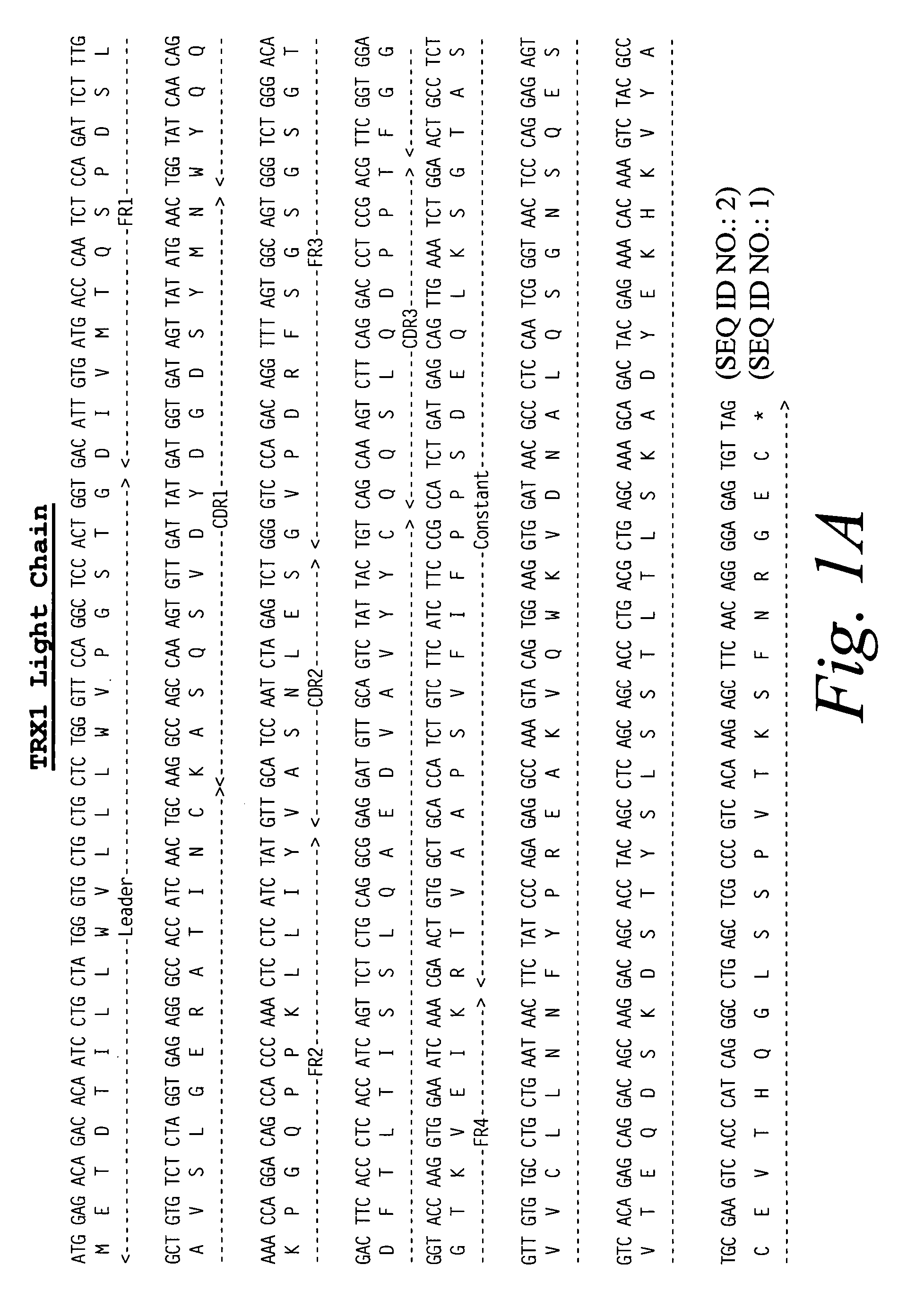
Compositions and methods for immune tolerance induction
ActiveUS10064922B2Low immunogenicityPeptide/protein ingredientsVertebrate antigen ingredientsTolerance inductionTherapeutic protein
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Medical application of ursolic acid-NF (Nuclear Factor)-kappaB inhibitor
InactiveCN102206243AMolecular smallStable structureOrganic active ingredientsMetabolism disorderReperfusion injuryUrsolic acid
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
APC-mediated tolerance induction for therapy of multiple sclerosis
The invention relates to transgene expression constructs—particularly self inactivating lentiviral vectors—comprising a dendritic cell specific promoter controlling the expression of autoantigen proteins, namely myelin basic protein, proteolipid protein and myelin oligodendrocyte glycoprotein, for use in the therapy of multiple sclerosis.
Owner:苏黎世大学数学和自然科学部
Immune-tolerance inducer
InactiveUS20150283235A1Stable maintenanceFacilitated DiffusionOrganic active ingredientsAntibody ingredientsTolerance inductionHematopoietic cell
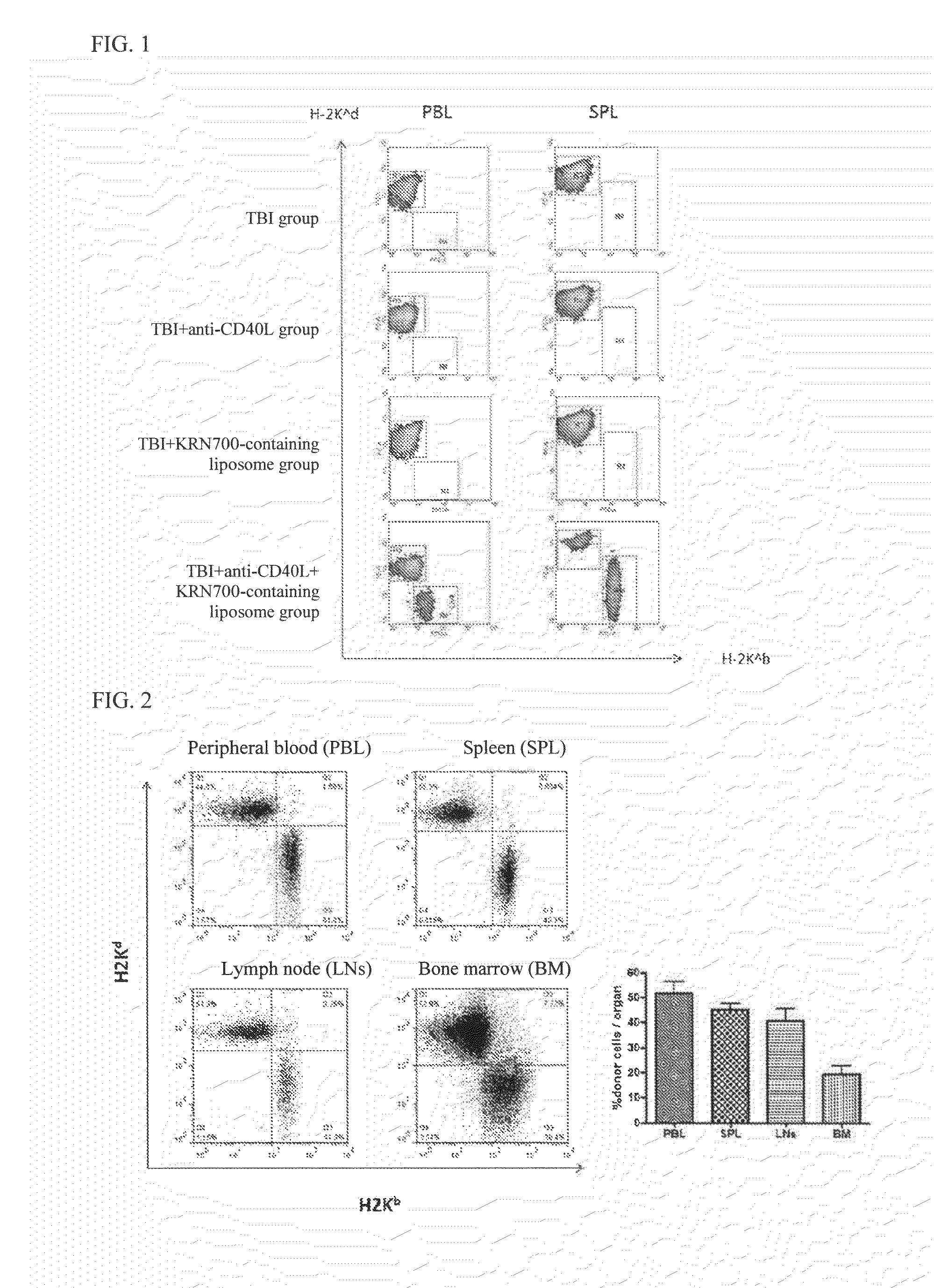
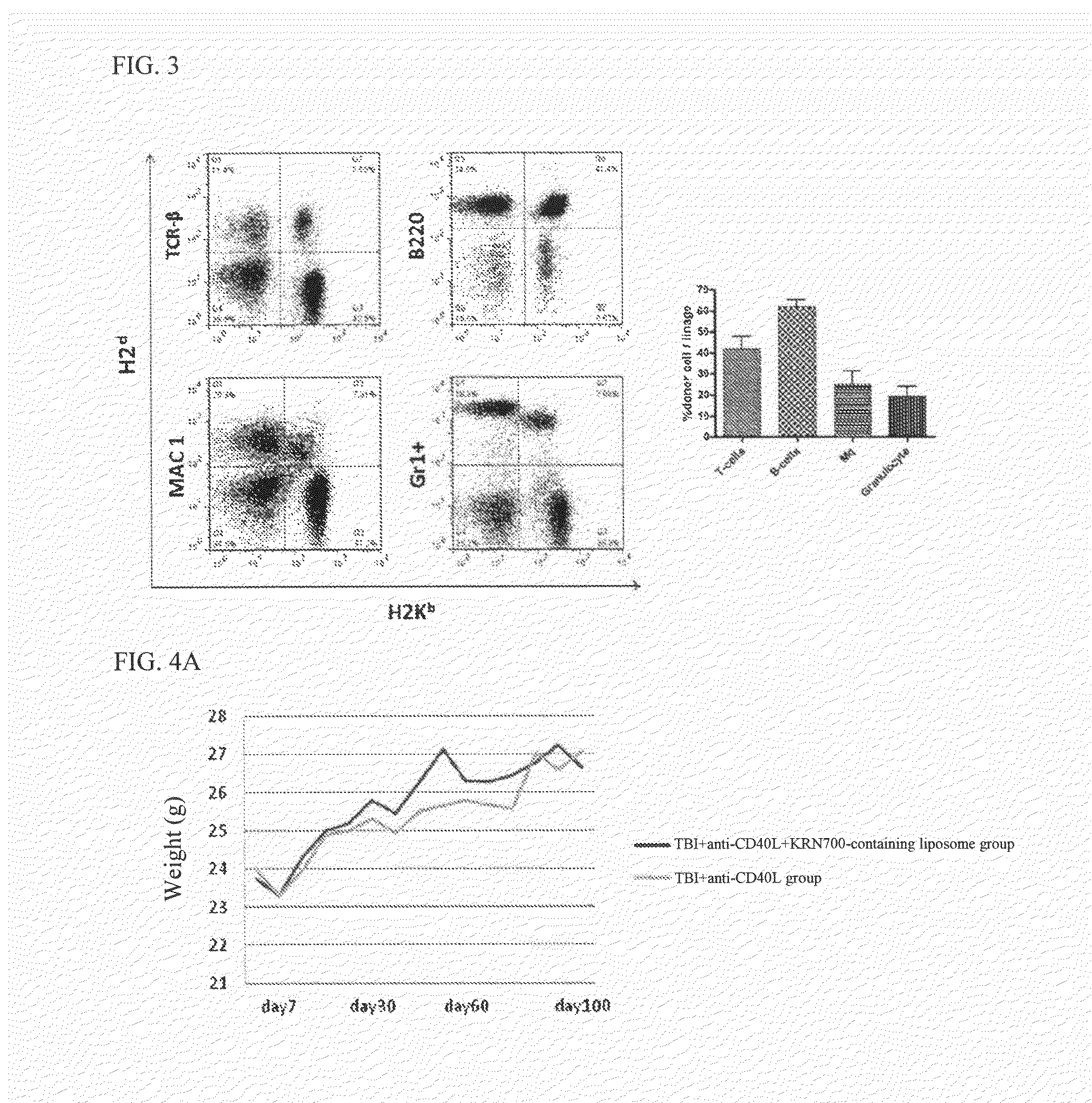
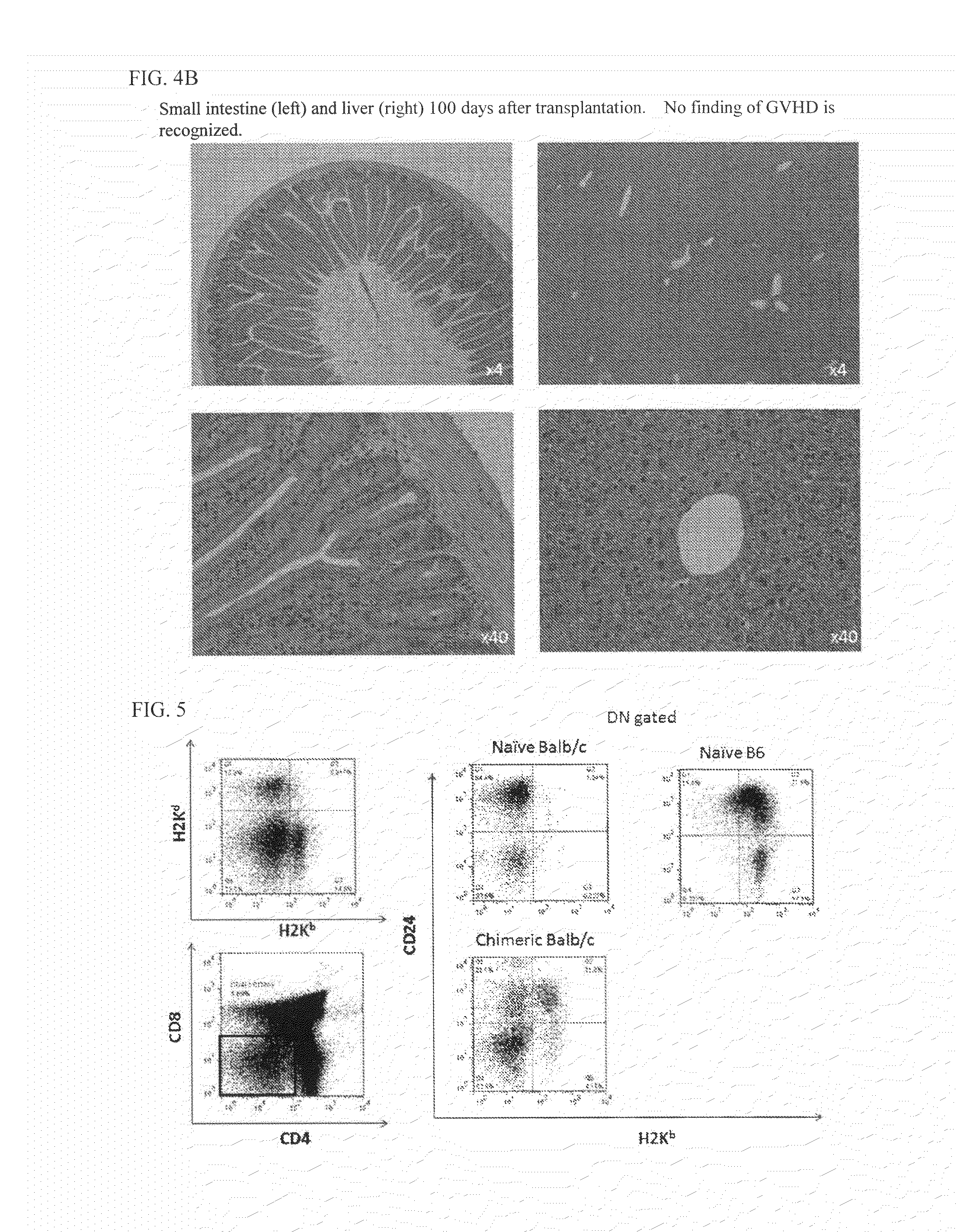
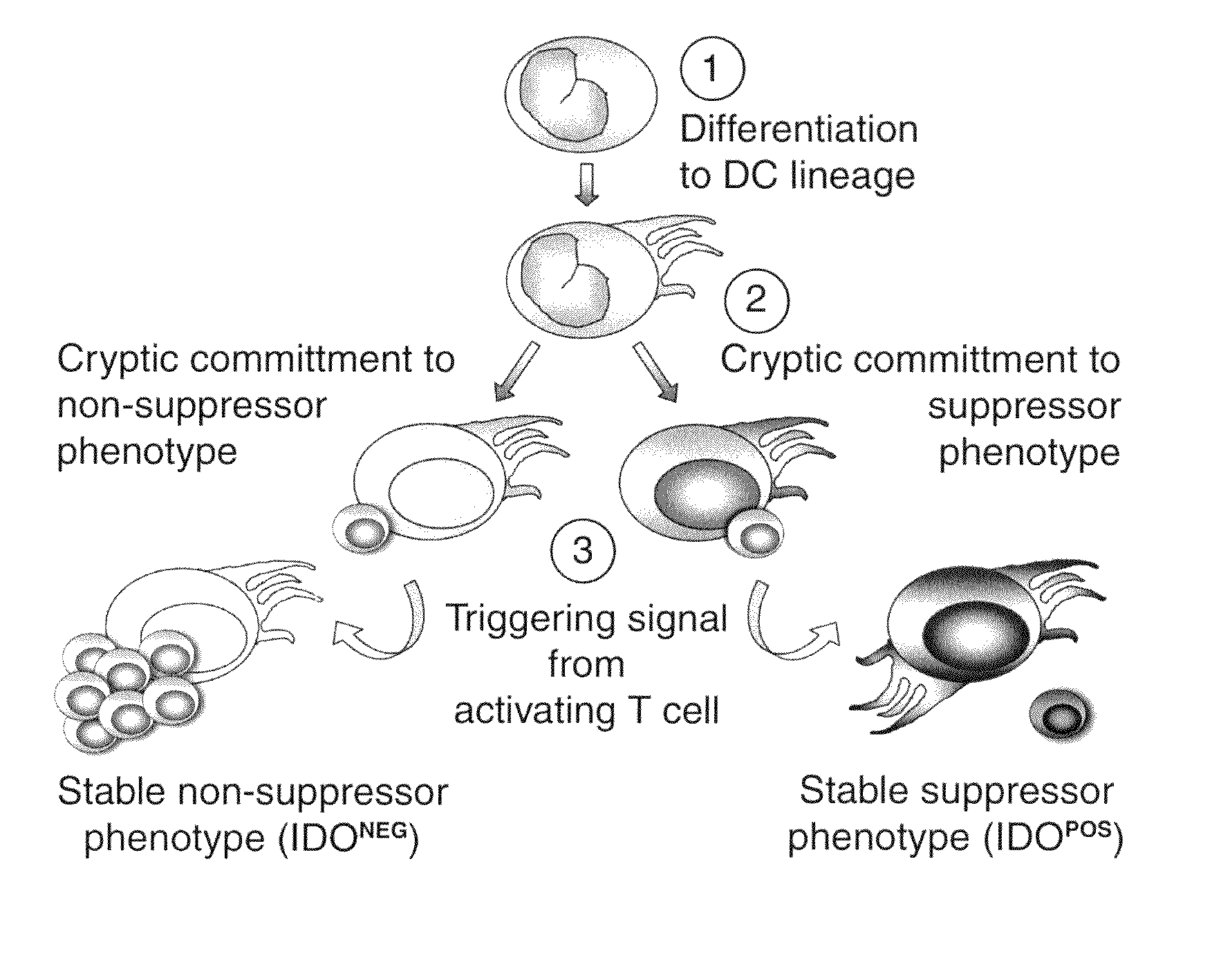
The purpose of the present invention is to create an immune tolerance-inducing agent used in therapy in which donor hematopoietic cells are transplanted into a recipient in order to induce immune tolerance in the recipient with respect to donor cells, tissue, or organs. By using an alpha-galactosylceramide-containing liposome in combination with a costimulatory-pathway-blocking substance, hematopoietic chimerism can be induced in the recipient by transplantation of donor hematopoietic cells, making it possible to induce immune tolerance in the recipient with respect to donor cells, tissue, or organs.
Owner:REGIMMUNE +1
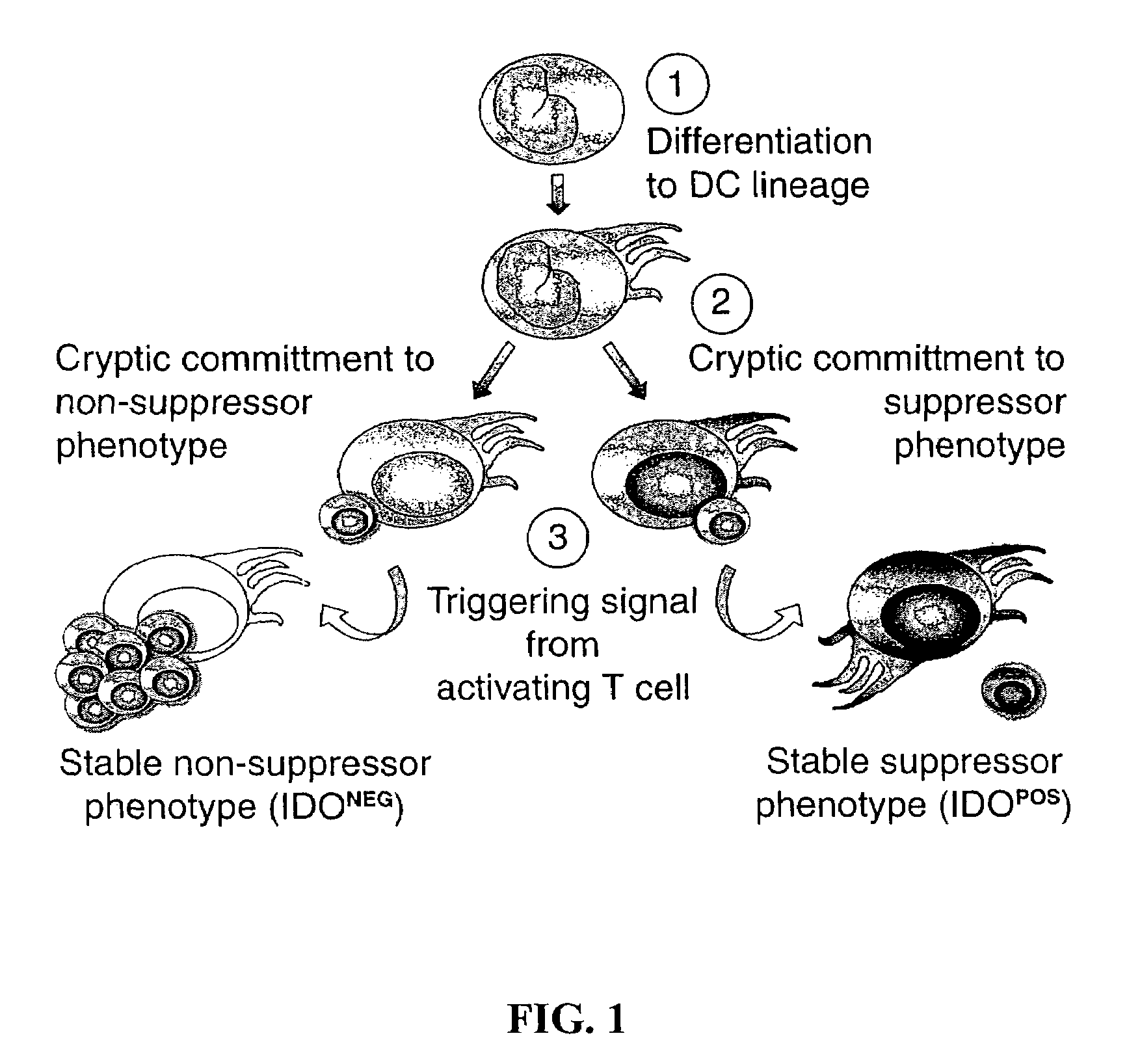
Chemokine receptor antagonists as therapeutic agents
InactiveUS20090041787A1Reduced Tolerance RequirementsReduce tumorgenicity in a subjectPeptide/protein ingredientsBiological material analysisTolerance inductionAbnormal tissue growth
The present invention provides methods and compositions to reduce immune tolerance at specific sites. In one aspect, the present invention comprises methods and compositions to reduce tumorigenicity. In an embodiment, the present invention reduces recruitment of tolerance-inducing antigen presenting cells (APCs) or their precursors to a tumor and / or tumor draining lymph node by decreasing binding of at least one tumor-associated ligand to a chemokine receptor present on the tolerance-inducing APCs or APC precursors. In an embodiment, the chemokine receptor is CCR6 and the tumor-associated ligand is mip-3α. In another aspect, the present invention comprises methods and compositions to reduce immune tolerance to a virus. In an embodiment, the virus is HIV. The present invention further provides for the development of CCR6 antibodies and antagonists as therapeutic agents to prevent or reduce immune tolerance.
Owner:MUNN DAVID H +2
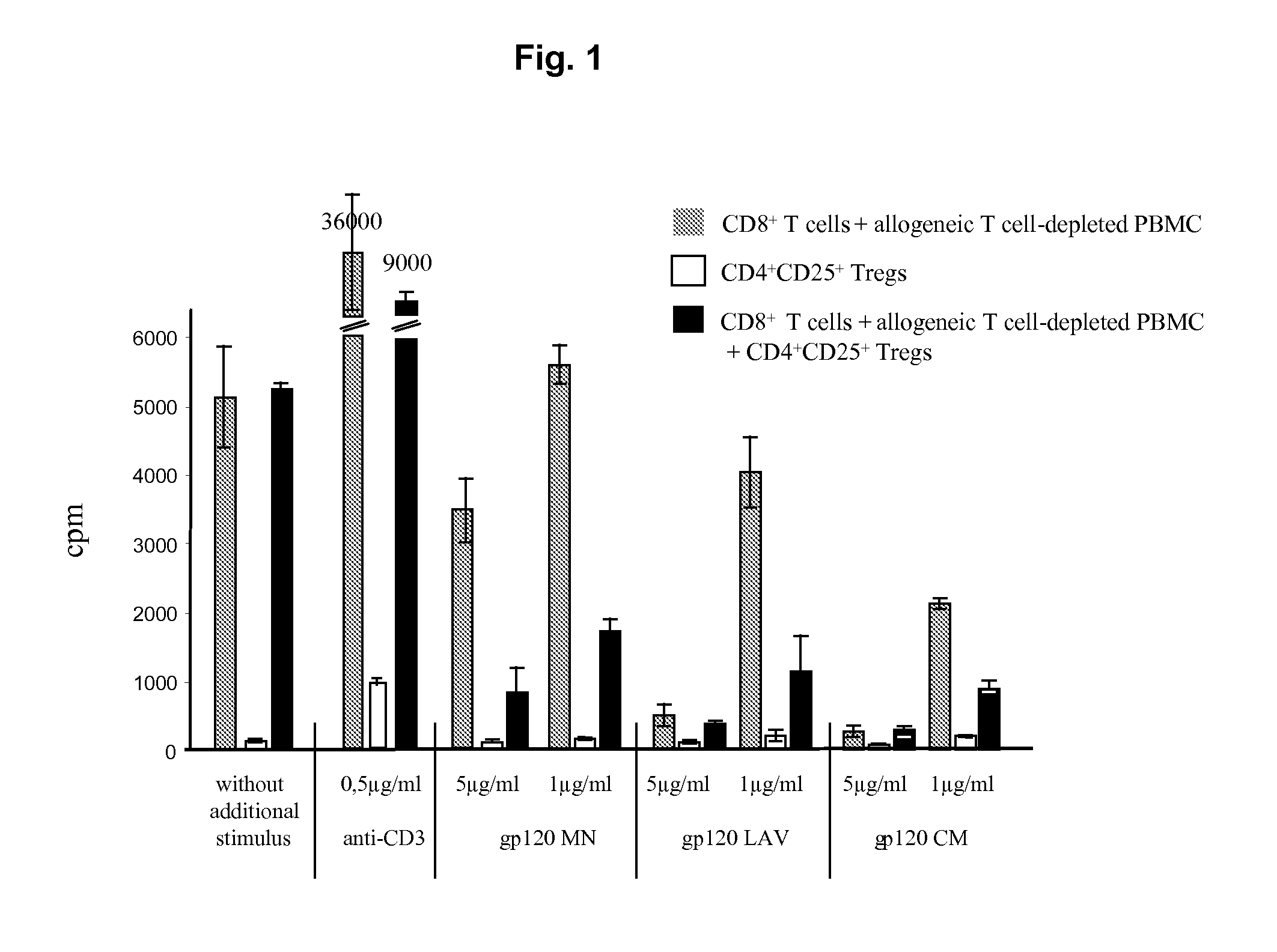
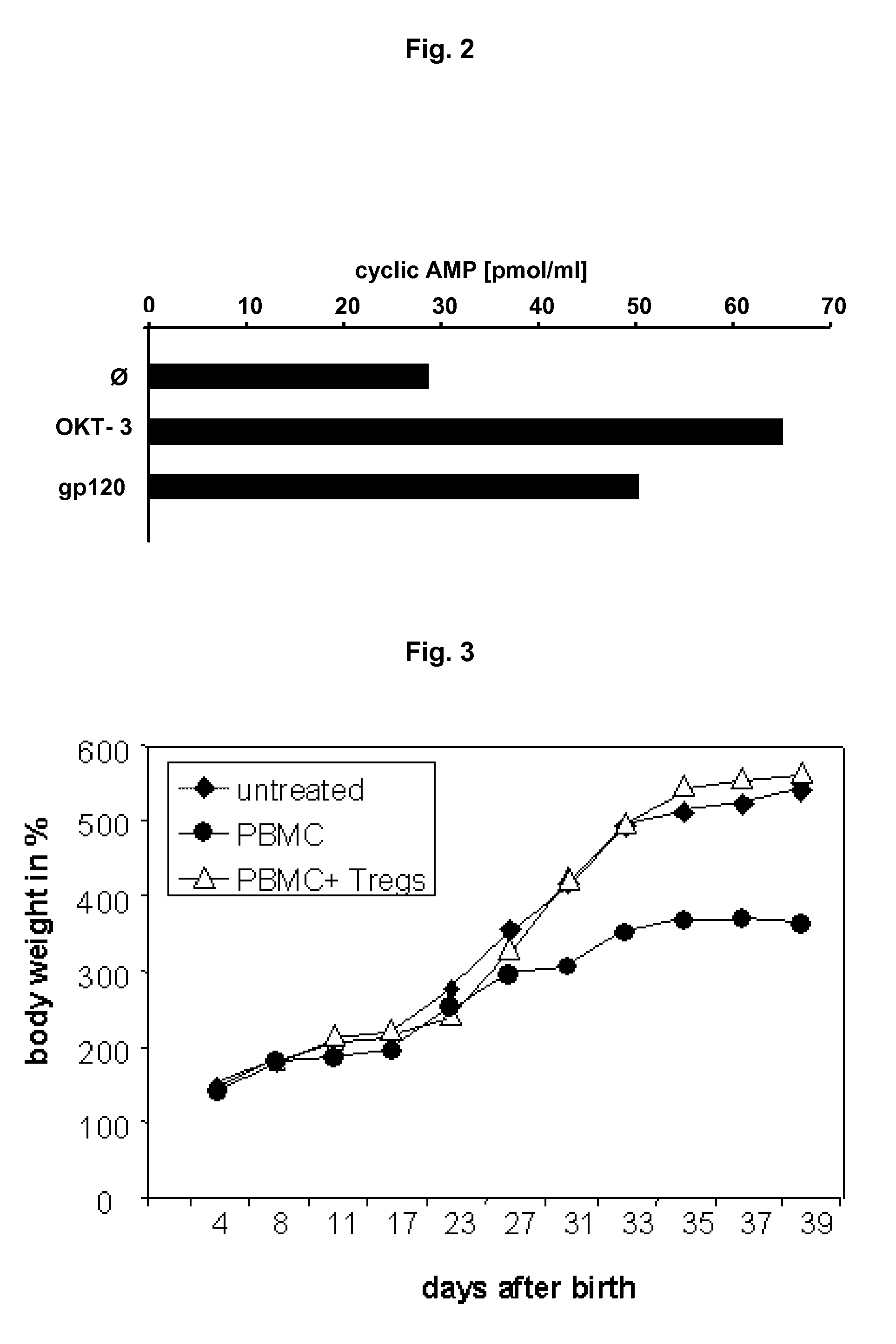
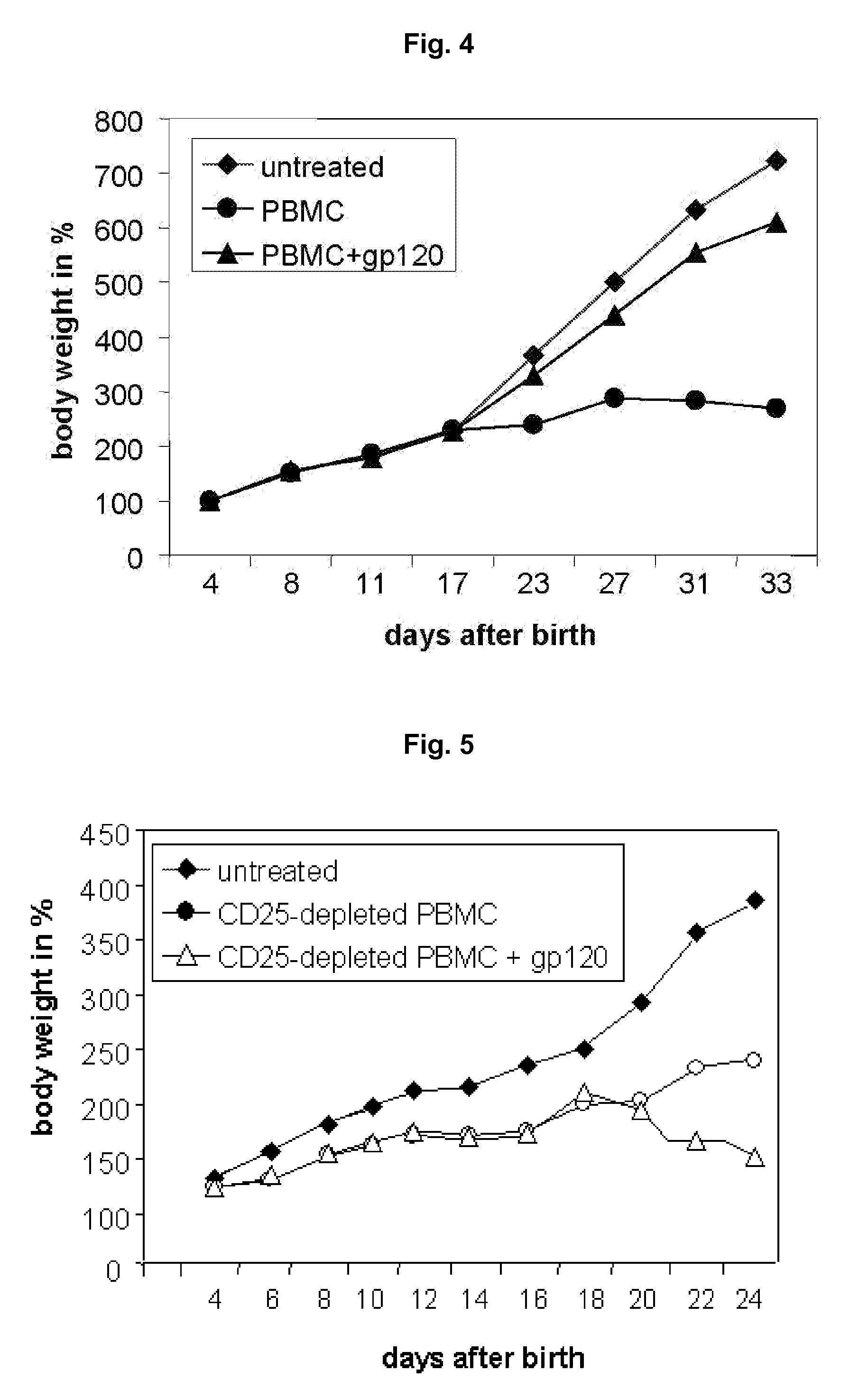
Screening method for the identification of agents capable of activating CD4+CD25+ regulatory T-cells through interactions with the HIV-1 GP120 binding site on CD4
ActiveUS8557533B2Improve clinical pictureStrong proliferationSenses disorderVirusesTolerance inductionDisease
The present invention relates specific activation of a regulatory T cell via a specific CD4 epitope and uses thereof, e.g. for the treatment of an autoimmune disease or an allergy or asthma or graft rejection or tolerance induction.
Owner:ACTITREXX GMBH
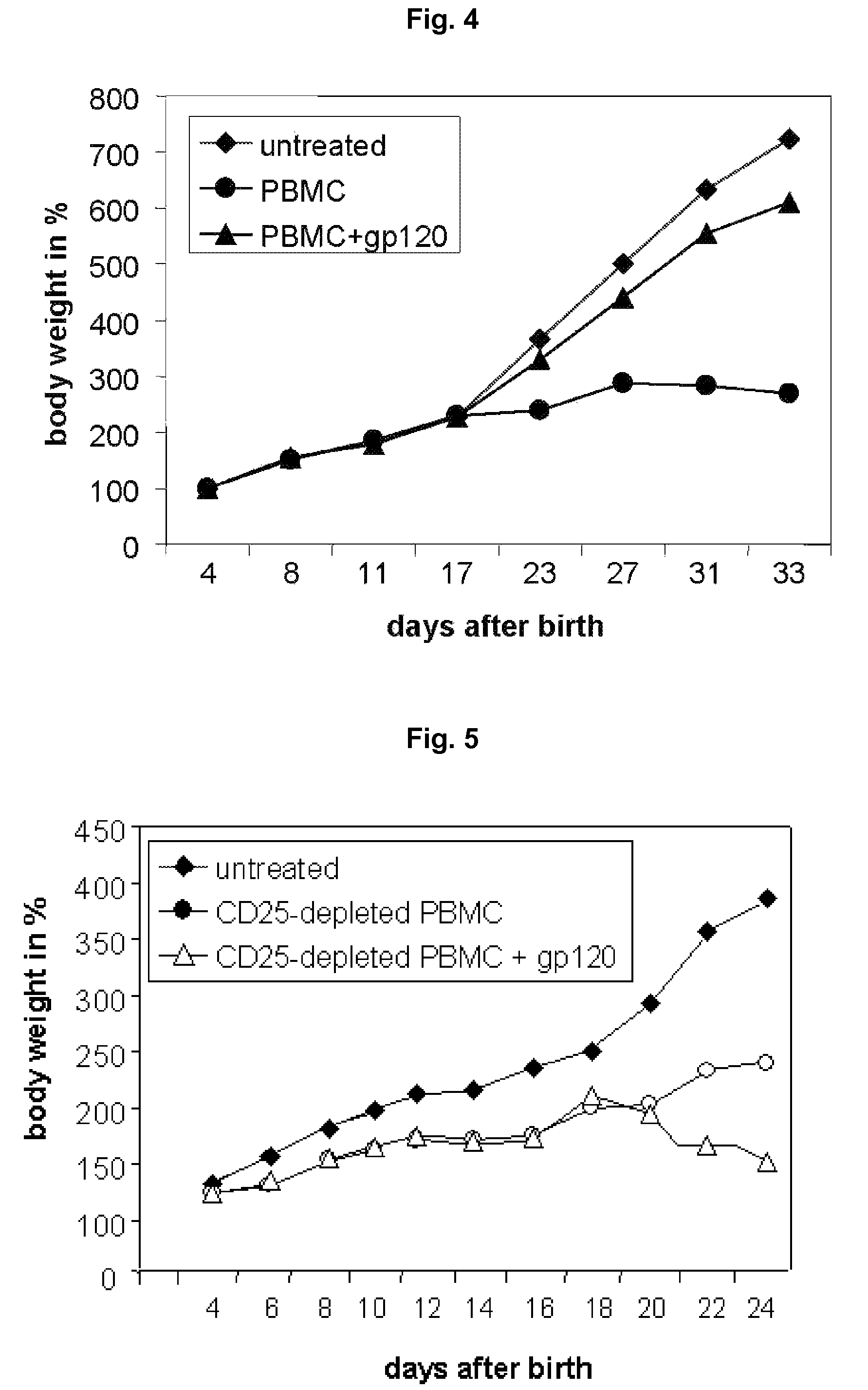
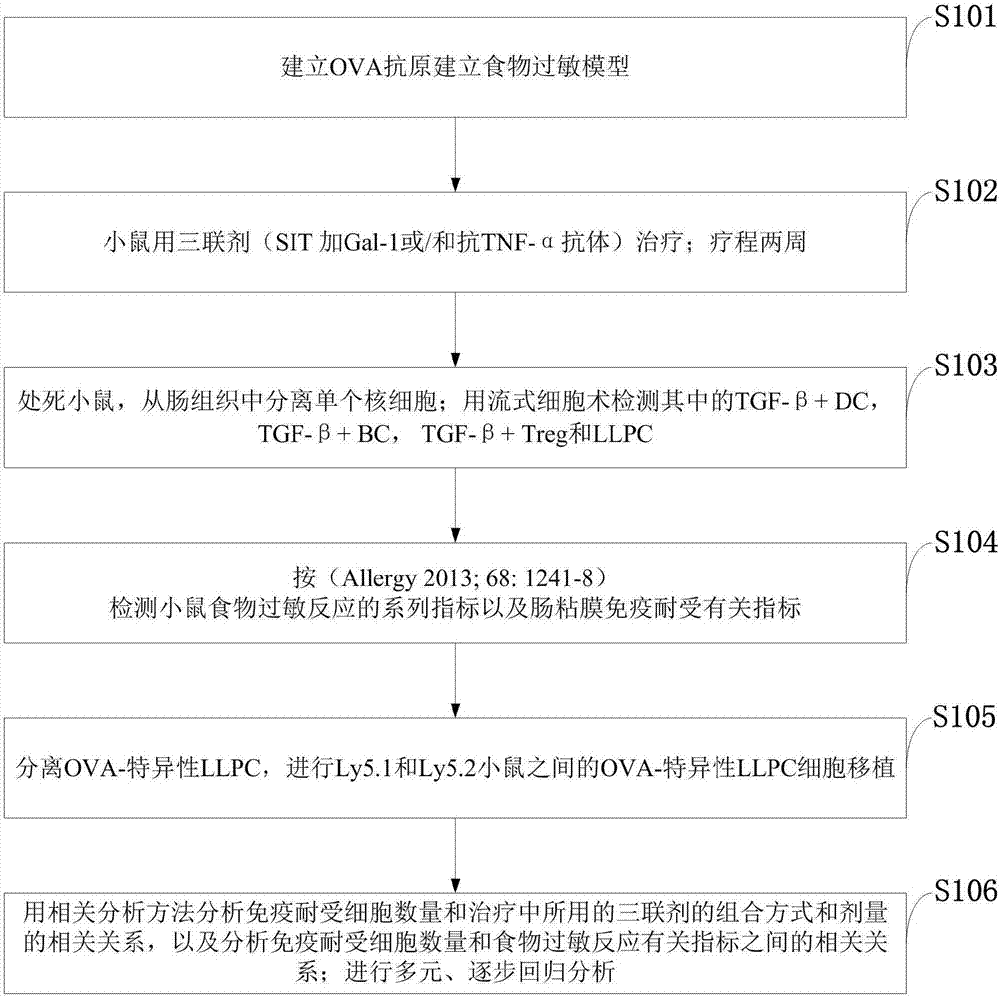
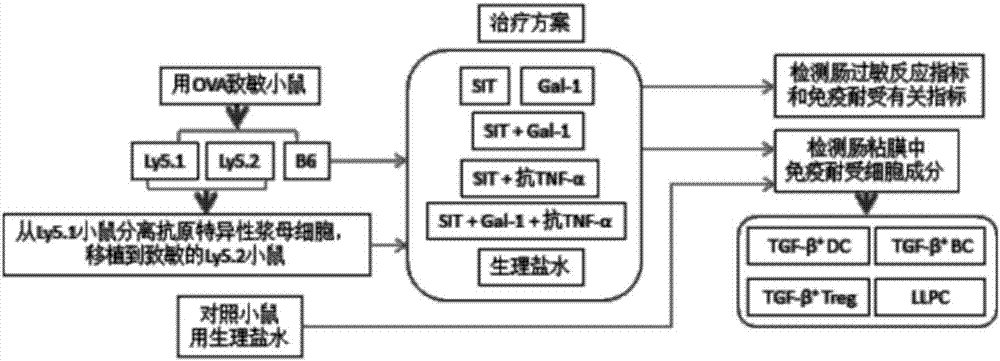
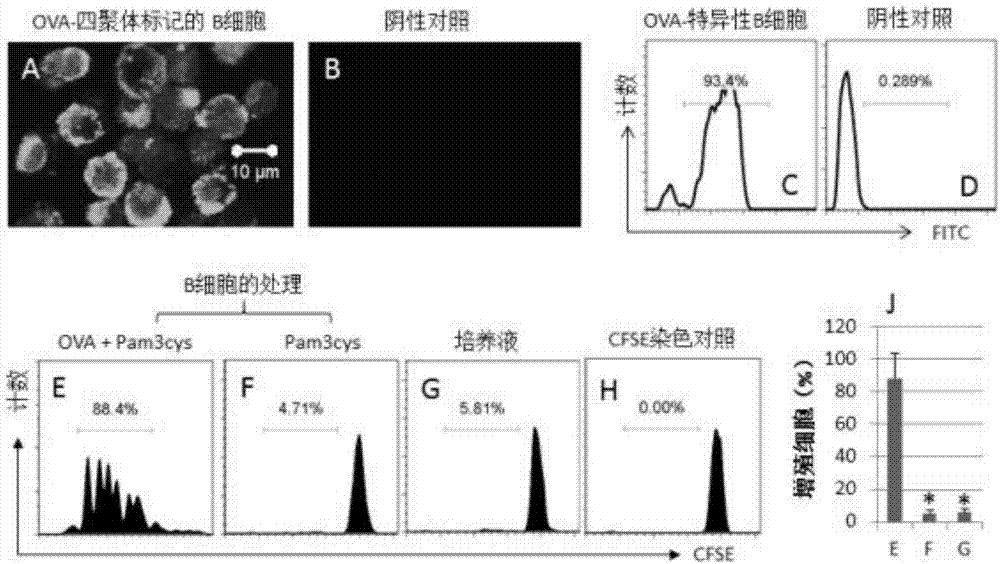
Recombinant Gal-1 allergy environment immunologic tolerance induction model and establishing method
InactiveCN107375950AInhibit expressionRestoration of antigen-specific immune toleranceCompounds screening/testingTolerance inductionRegulatory T cell
The invention belongs to the field of medical treatment and discloses a recombinant Gal-1 allergy environment immunologic tolerance induction model and an establishing method. The induction model can induce antigen-specificity regulatory T cells out and recover antigen-specificity immunologic tolerance in a body of a patient, so that allergy reaction is inhibited; under allergy reaction environment, a TNF-alpha content in the body is increased, Gal-1 expression is inhibited, and anti-TNF-alpha antibody is applied to improve Gal-1; the Gal-1 can promote LLPC to be differentiated into Breg, and the anti-TNF-alpha antibody, SIT and Gal-1 are jointly applied to promoting antigen-specificity immunologic tolerance to recover.
Owner:SHENZHEN UNIV
Methods of transplantation and disease treatment
ActiveUS10933124B2Immunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsTolerance inductionThird party
A method of transplantation is disclosed. The method comprising administering to a subject in need of transplantation of cells in suspension, a therapeutically effective amount of anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype, said anti-third party cells being tolerance-inducing cells and capable of homing to the lymph nodes following transplantation, wherein said cells in suspension comprise non-hematopoietic cells or hematopoietic cells which are not stem cells. Methods of treating and kits are also provided.
Owner:YEDA RES & DEV CO LTD
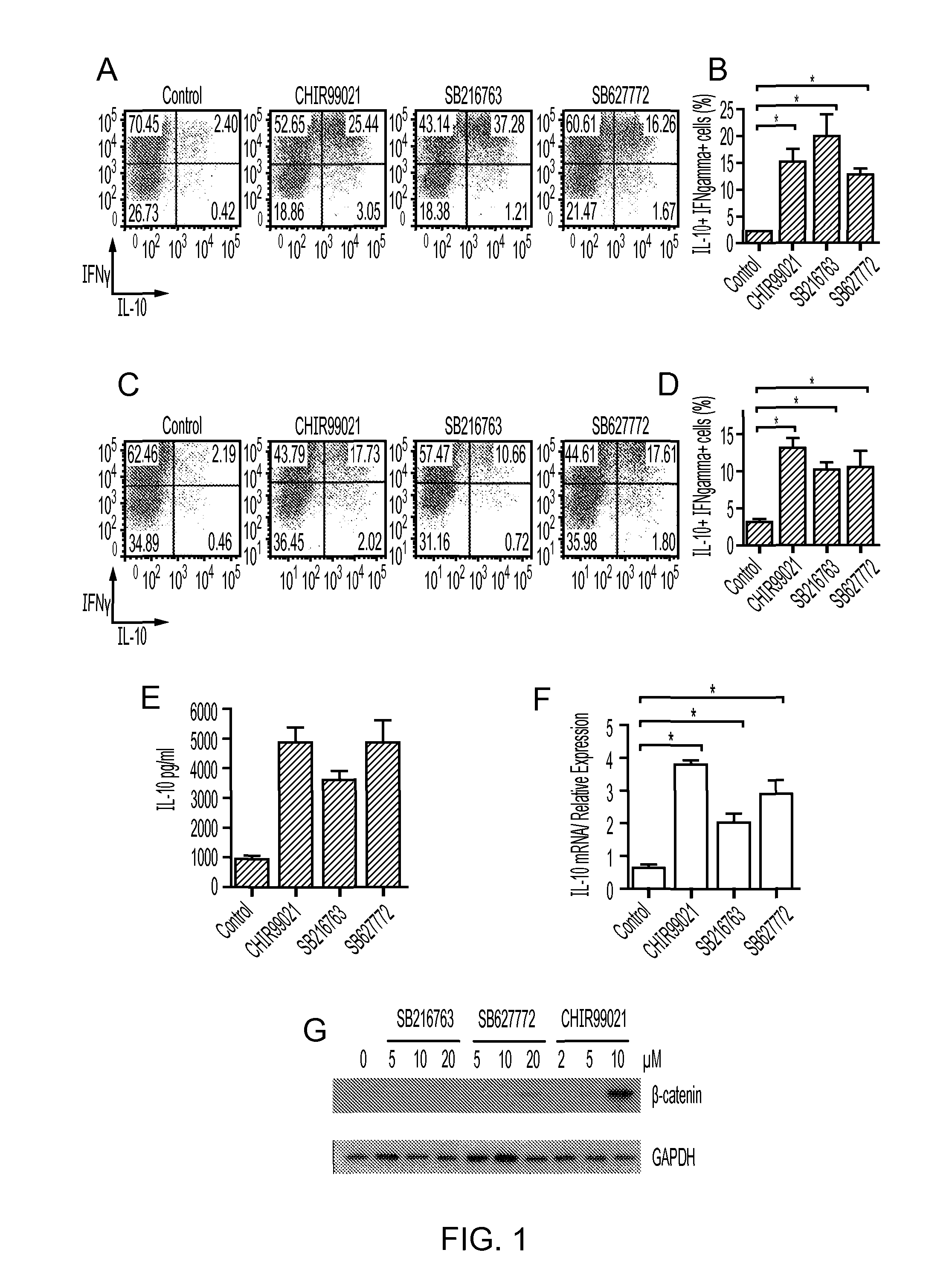
Tolerisation-Inducing Composition
InactiveUS20150071967A1Enhances tolerance inductionEnhance antigen-specific immunotherapyNervous disorderOrganic chemistryTolerance inductionLymphocyte
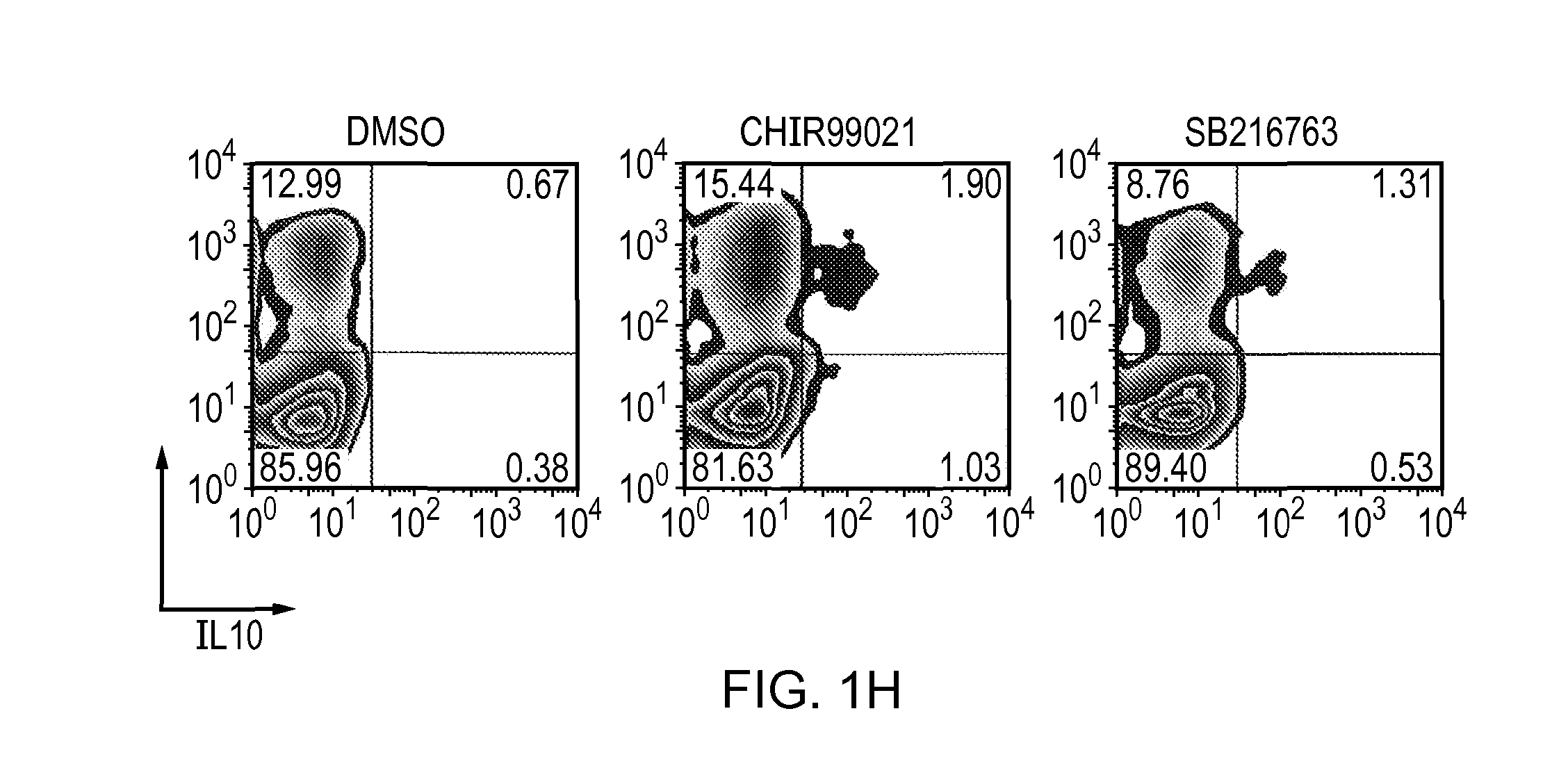
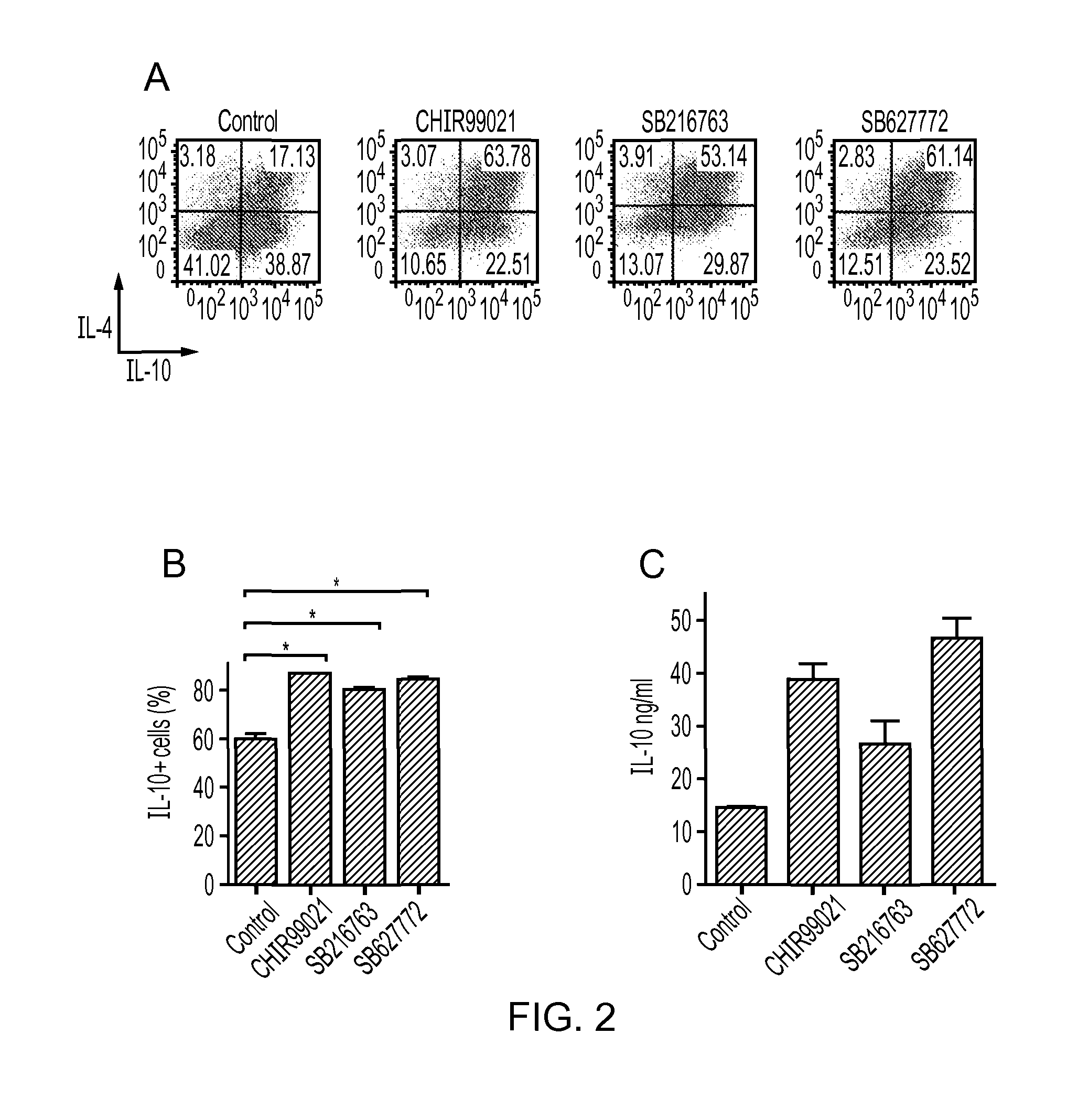
The present invention relates to a composition comprising a tolerogenic peptide and a GSK-3 inhibitor and uses thereof. The invention also relates to the use of a GSK-3 inhibitor to accelerate the peptide-mediated shift in secretion profile of lymphocytes from pro-inflammatory to anti-inflammatory cytokines. The GSK-3 inhibitor may be used to enhance antigen-specific immunotherapy.
Owner:UNIV OF BRISTOL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com