Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation and Optimization of Human Anti-Third Party T Central Memory (Tcm) Cells
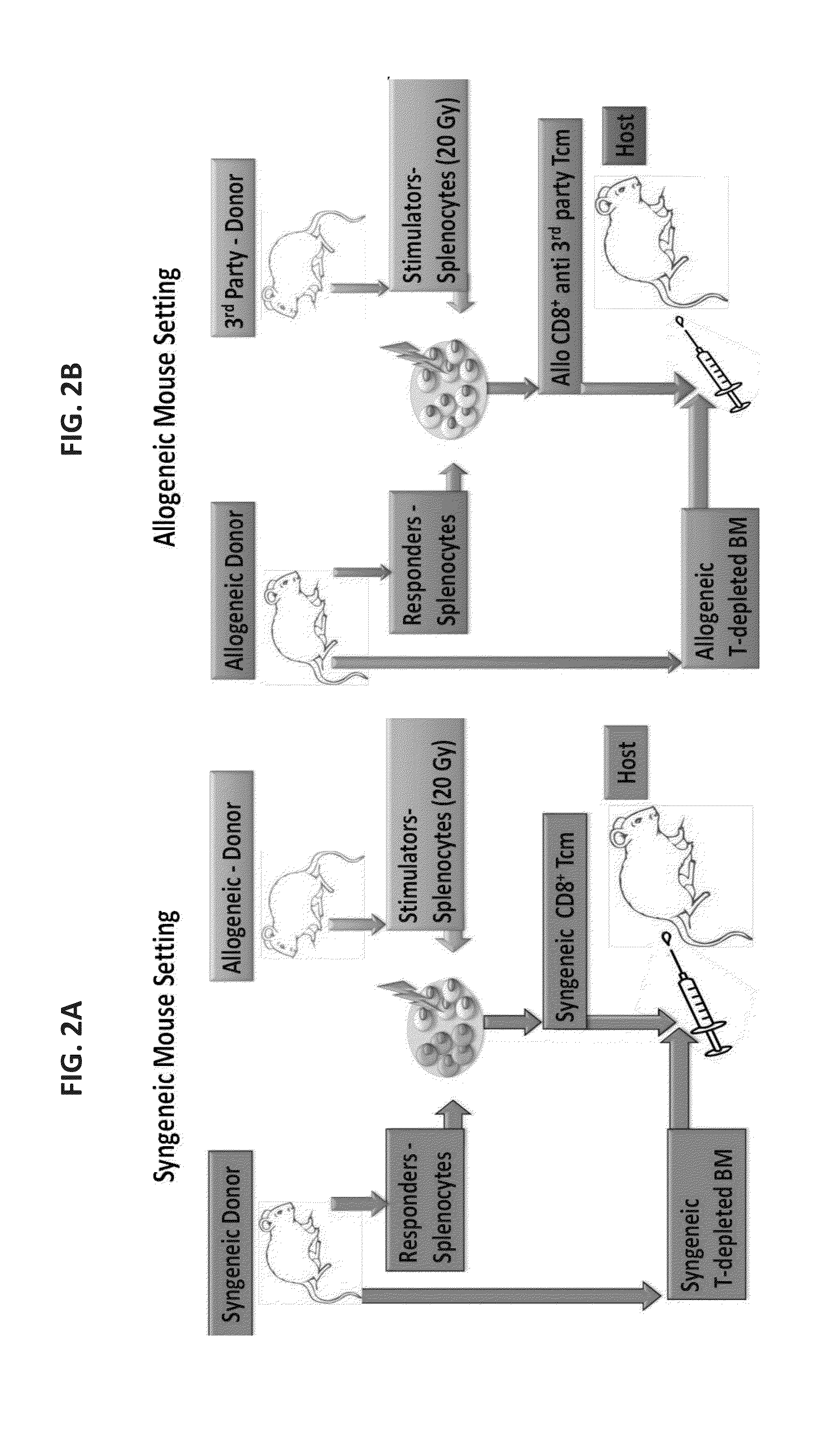
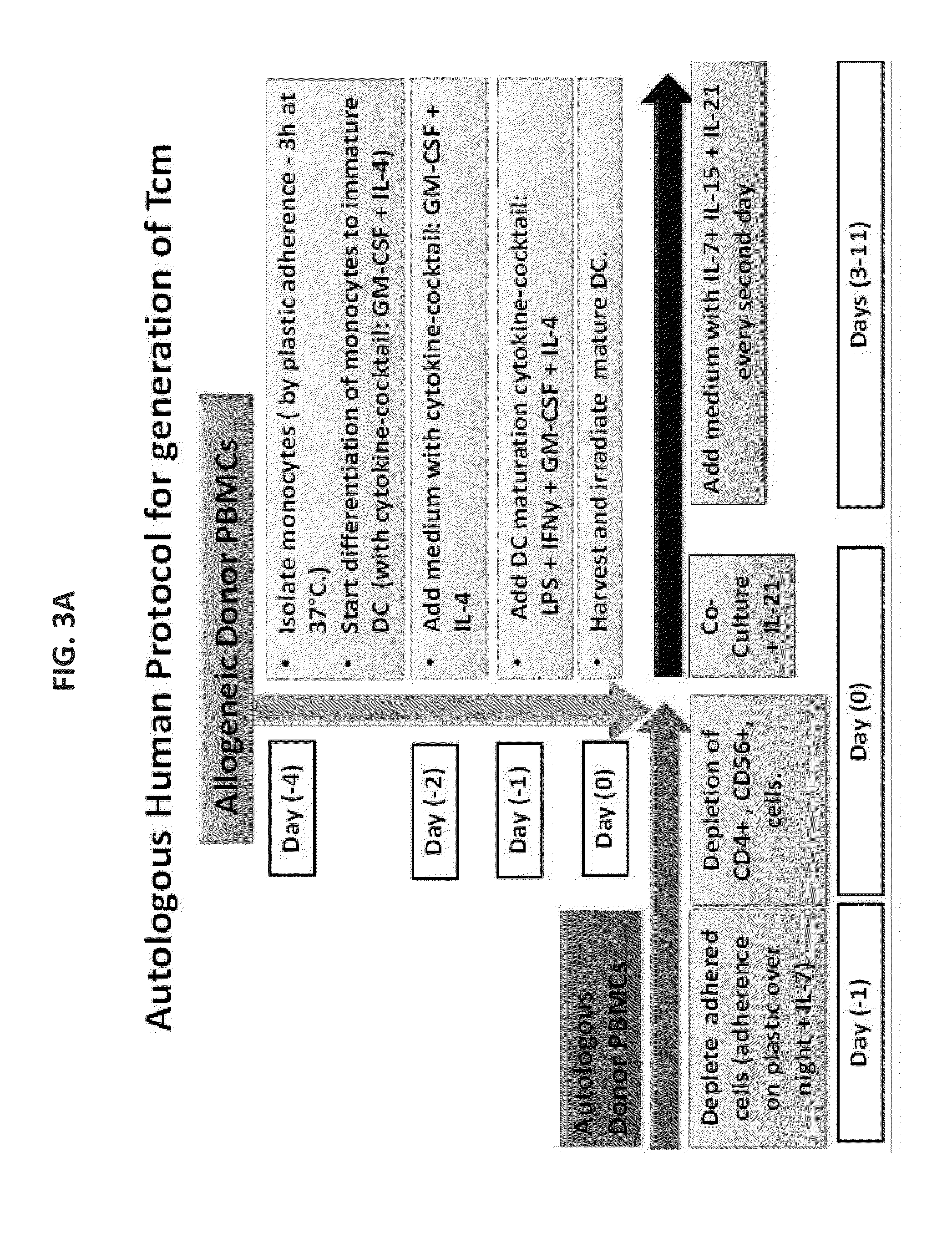
[0272]In order to translate the mouse studies previously presented to clinical application, the procedure was optimized for generating human anti-3rd party cytotoxic T lymphocytes (CTLs). To that end, different parameters were evaluated including different reagents for the isolation of CD8 responder cells, the composition of the stimulators and the cytokine milieu.
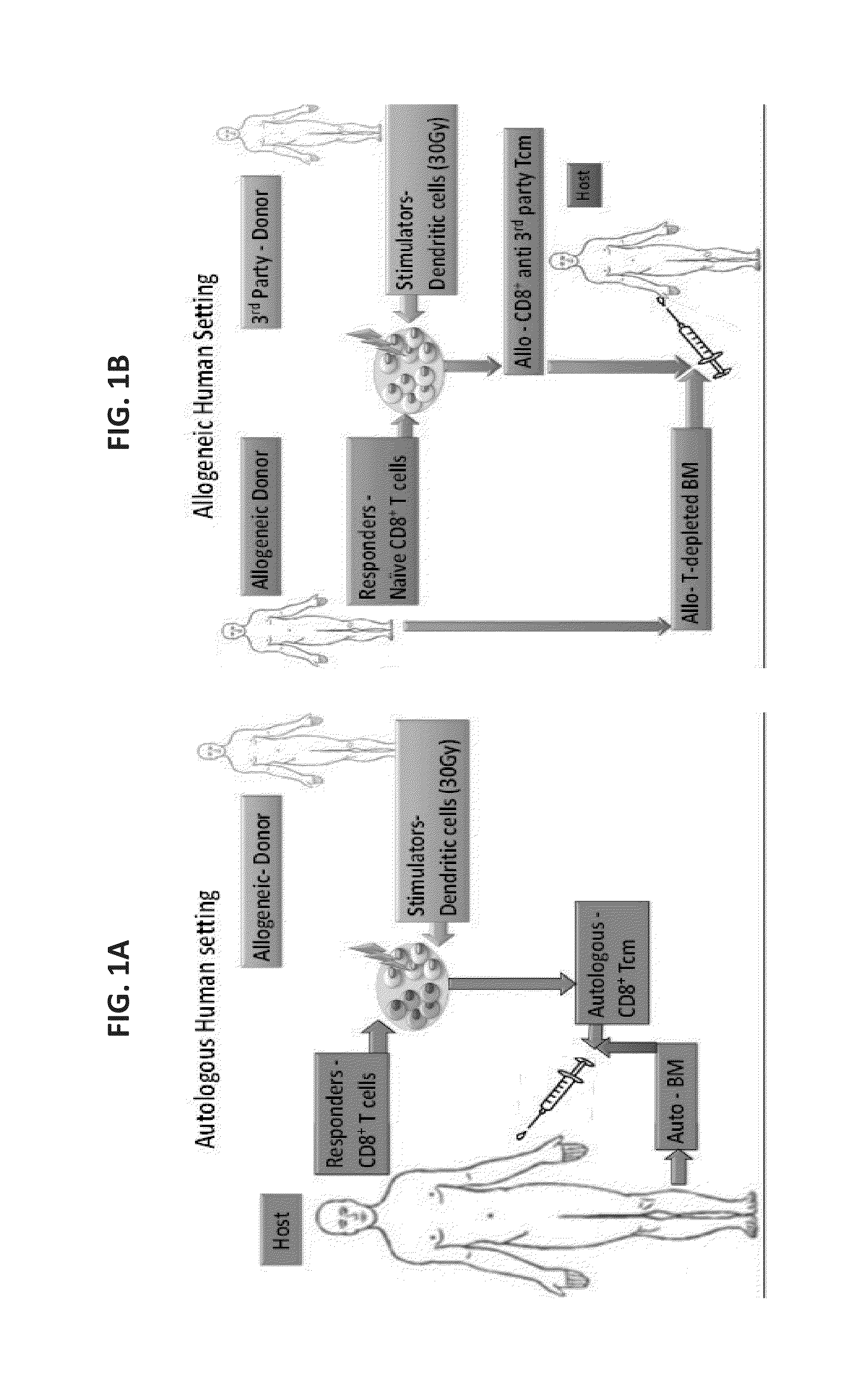
[0273]Potentially, as found in the previously presented mouse model, treatment with central memory T cells (Tcm) could be valuable either in the context of autologous [Lask A et al., Blood (ASH Annual Meeting Abstracts), (2010) 116: 424] or in allogeneic bone marrow transplant (BMT) [Ophir E et al., Blood. (2010) 115(10): 2095-104; Ophir E., 37th EBMT annual meeting, Apr. 3-6, 2011, Paris, France. Oral Presentation Abstract Nr: 662].
[0274]In the human autologous setting (FIG. 1A) anti-3rd party Tcm can be administrated together with autologou...
example 2
Large Scale Preparation of Human Anti-3Rd Party Tcm in Plastic Bags Using GMP Grade Reagents
[0316]To simulate the conditions anticipated when using patients own PBMC for the generation of autologous Tcm, initially two large scale leukaphersis procedures were performed, from two normal donors, and a large number of mononuclear cells were cryopreserved (divided into several batches). Each batch was used for one large scale experiment. In the first experiment, several technical problems were encountered including difficulty in the generation of DCs from the frozen bags according to the Wurzburg protocol and sourcing of a new GMP grade IL-15 for which the biological activity was unclear. These problems, which resulted in a very poor CD8 T cell expansion (around 3 folds), were corrected in the subsequent experiments by using the presently described protocol for DCs (as described above) and by using the appropriate concentration of IL-15 (i.e. 300 U / ml).
[0317]As can be seen in FIG. 15, wh...
example 3
GVL Potential of the Human Anti-3rd Party Tcm Against an Established Cell Line
[0319]Considering that in the autologous setting the potential use of anti-3rd party Tcm is solely for eradicating residual tumor cells (in the allogeneic setting it also serves to enhance engraftment of the BM cells) it is important to develop a straightforward assay for cytotoxic capacity ex-vivo, which could be used for quality control prior to infusion of the Tcm to the patient. To that end, a TCR independent assay was used based on the demonstration by Lask et al. [Lask A et al., J. Immunol. (2011) 187(4):2006-14] that a MHC mutated line not recognizable by TCR due to this mutation, can still be killed by anti-3rd party CTLs through their TCR independent killing mechanism. Clearly, if applicable also for human Tcm, such a killing modality could serve to distinguish the Tcm killing from that exhibited by NK cells.
[0320]To address this question, a mixed lymphocyte reaction (MLR) was carried out with ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com