Cell membrane nano-vesicle wrapping immunosuppressant and over-expressing PD-L1 and preparation method and application thereof
A PD-L1 and immunosuppressant technology, applied in the field of biomedicine, can solve problems such as gastrointestinal intolerance, achieve the effect of inhibiting immune rejection in vivo, improving efficacy, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
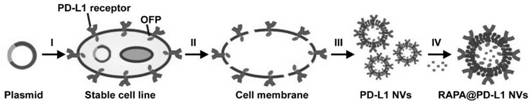
[0045] Example 1 Construction of Cell Membrane Nanovesicles Overexpressing PD-L1 Protein
[0046] 1. Construct a 293T cell line capable of stably overexpressing PD-L1 protein and OFP tagged protein on the cell membrane
[0047] (1) 950 μL of 1× HBS buffer, 10 μL of an aqueous solution of a mixed plasmid with a concentration of 1 μg / μL, the mixed plasmid is a second-generation lentiviral packaging vector pSPAX2 plasmid (geneseed ), GSF1) and pMD2.G plasmid (geneseed (geneseed), GSF2), 10 μg of human PDCD1 gene lentiviral ORFcDNA expression virus plasmid (Sino Biological Inc, HG10377-UT); mix well, and drop Inject 50 μL of CaCl with a concentration of 2.5 mol / L 2 Aqueous solution, mixed evenly, and placed at room temperature in the dark for 20-30 minutes to obtain the virus packaging mixture;
[0048] (2) will be cultured with 3×10 6 The 10% FBS DMEM medium of 293T cells was replaced with a new identical medium, and then the virus packaging mixture was added to the medium, an...
Embodiment 2
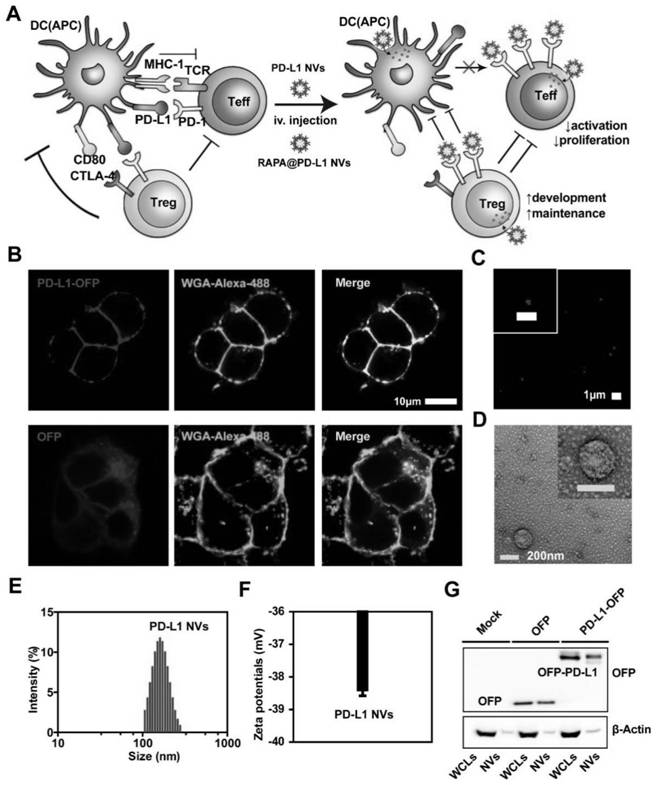
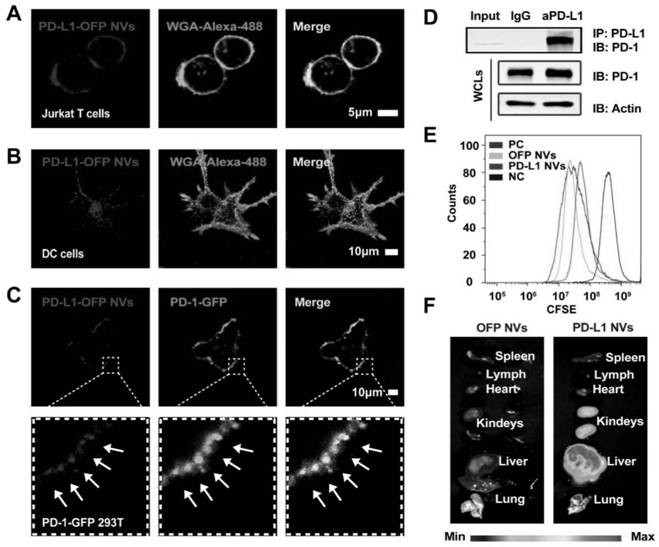
[0063] Example 2 In vitro biological behavior and in vivo distribution of PD-L1 NVs
[0064] Since hyperactivated T cells are negatively regulated through the PD-L1 / PD-1 pathway, we wanted to investigate whether the PD-L1 protein on NVs interacts with the corresponding ligand PD-1 on target cells in vitro. Here, we incubated PD-L1-OFPNVs with corresponding IL-2-stimulated Jurkat T cells or bone marrow-derived dendritic cells (dc), and the specific experimental steps are as follows:
[0065] Nanovesicle cell binding assay: Jurkat T cells were incubated with PD-L1 NVs (50 μg / ml, protein weight) for 30 minutes, and centrifuged with Ceoporep-4 to make slides (China Yingtai). Wheat germ agglutinin (WGA) and Alexa-Fluor 488 conjugate were added to stain the cell membrane for 10 min, and HEK 293T cells were incubated with PD-L1-OFP NVs (50 μg / mL, protein amount) for 30 min. On this basis, wheat germ agglutinin (WGA) and Alexa-Fluor 488 conjugate were added to stain the cell membrane...
Embodiment 3
[0068] Example 3 RAPA@PD-L1 NVs preparation and in vitro culture test
[0069] In this example, we verified whether PD-L1 NVs could serve as a targeted drug delivery system to deliver small doses of rapamycin (RAPA) to effector T cells to enhance immune suppression. According to the mechanism of rapamycin inhibition of mTOR feedback AKT / mTOR / p70S6K pathway and T cell proliferation ( Figure 4 A), rapamycin can inhibit the phosphorylation of mTOR upstream protein AKT and downstream S6 ribosomal protein. Therefore, we prepared rapamycin-enveloped vesicles (RAPA@PD-L1 NVs) by electroporation, carried out encapsulation experiments between rapamycin and PD-L1 NVs, and then determined the in vitro activity of rapamycin. Release. The specific test steps are as follows:
[0070] RAPA loading (RAPA@PD-L1 NVs preparation): 6 mg (protein weight) of purified vesicles (PD-L1 NVs) and 1 mg RAPA (100 mg / mL diluted in PBS, pH 10) were electroporated in 1 mL at 4 °C buffer (1.15mM potassiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com