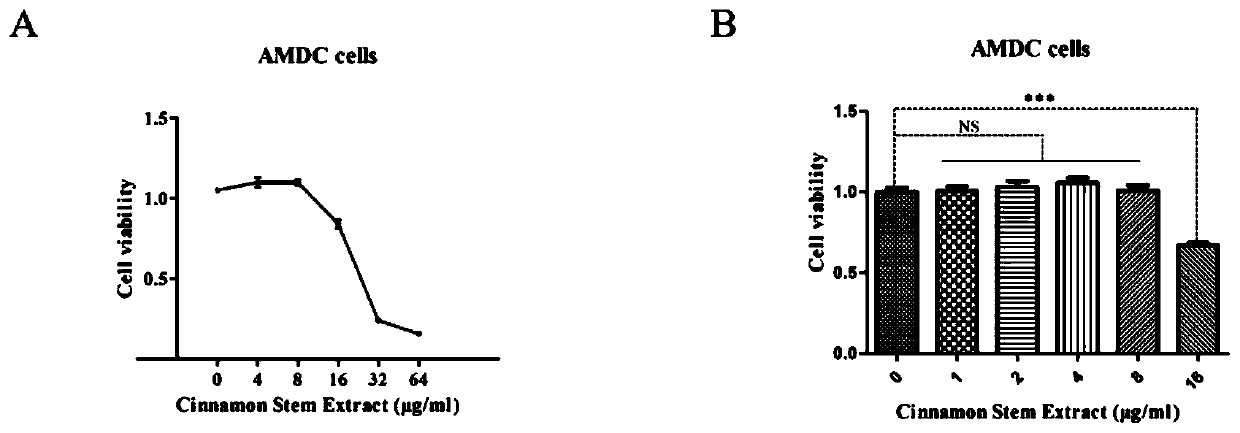
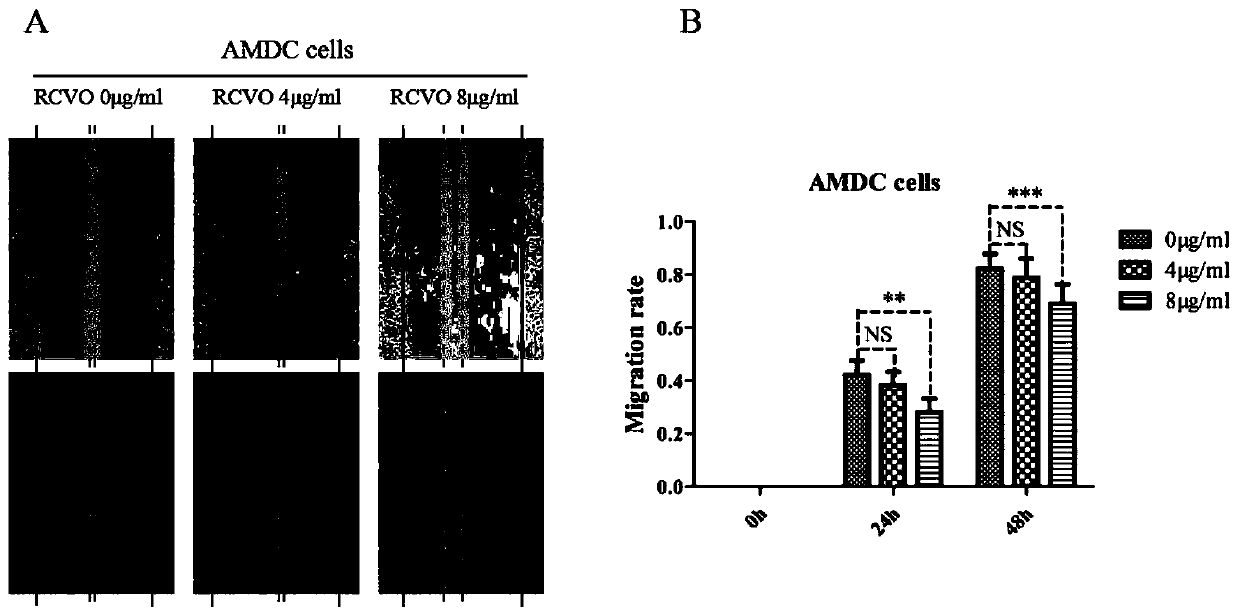
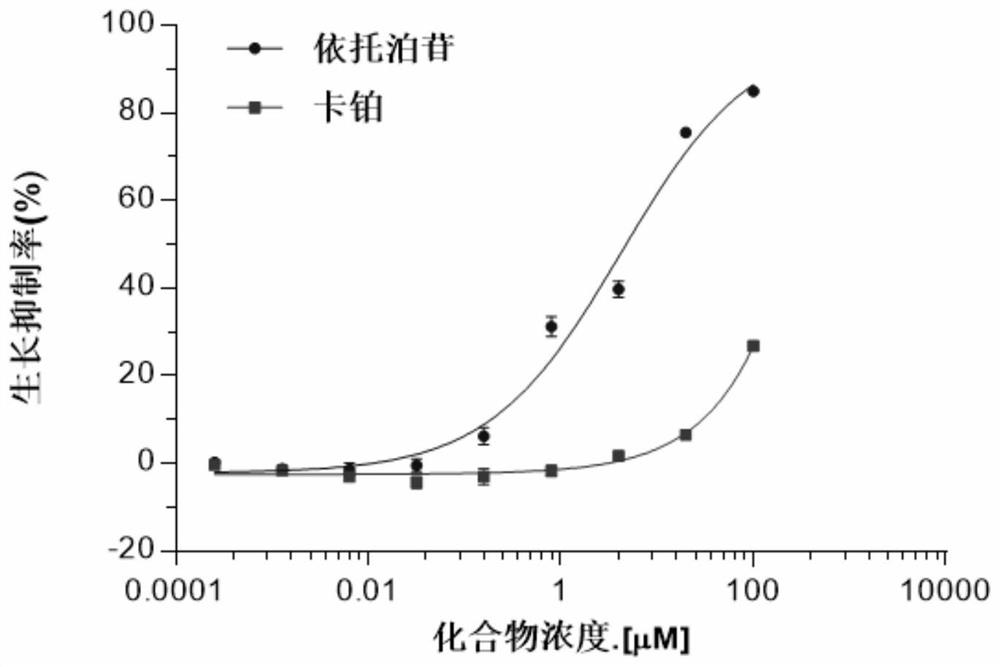
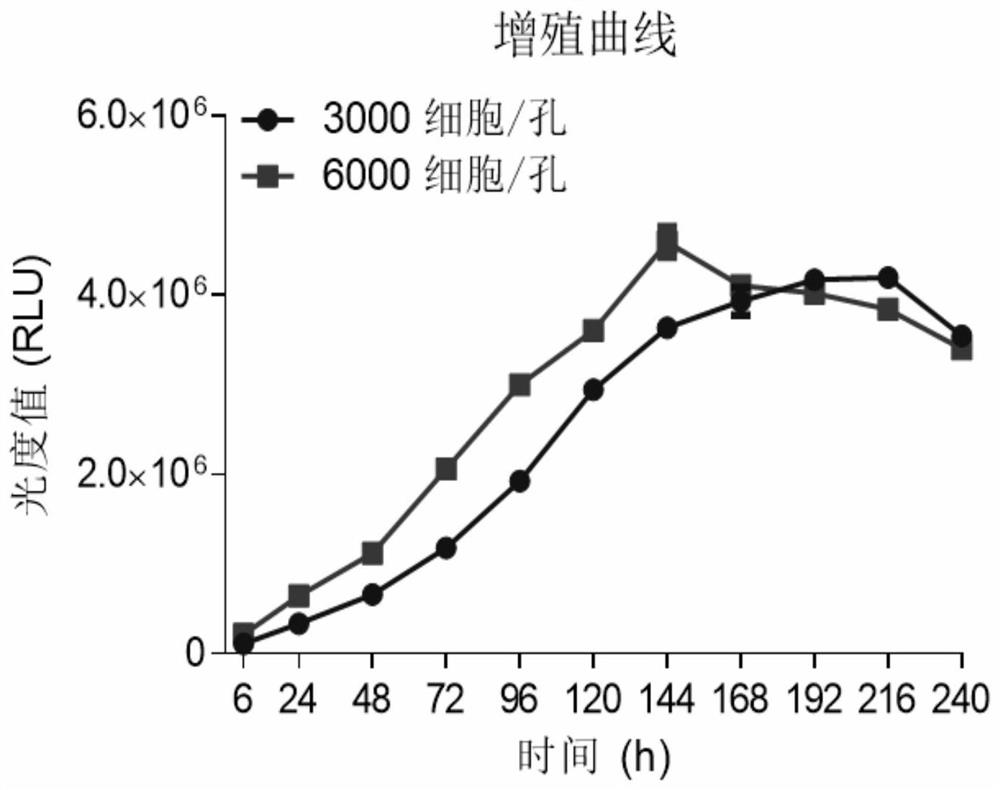
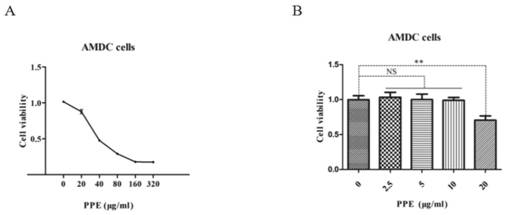
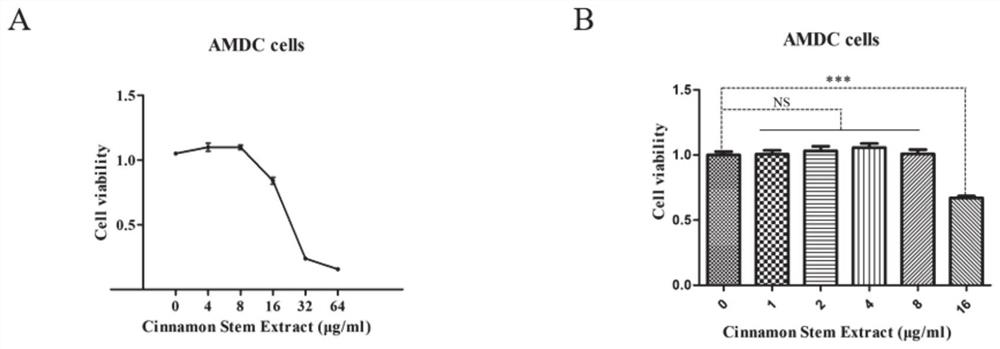
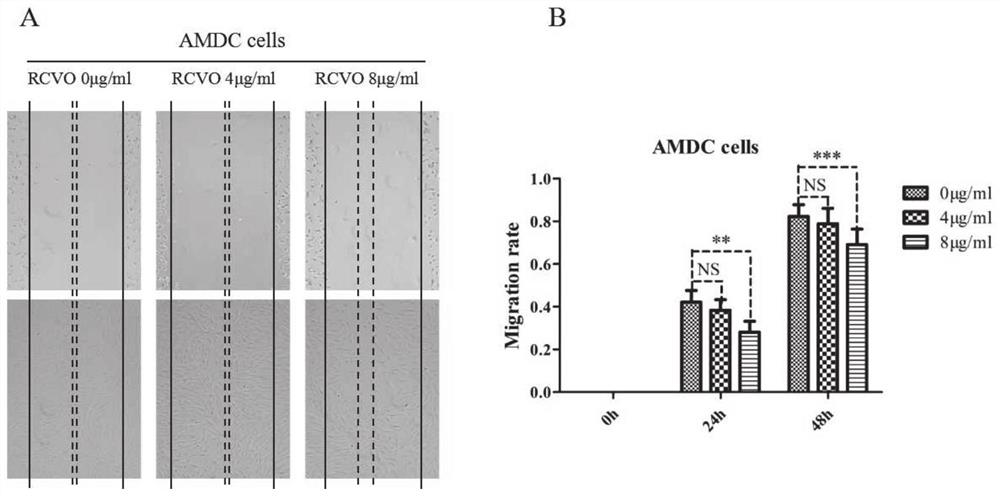
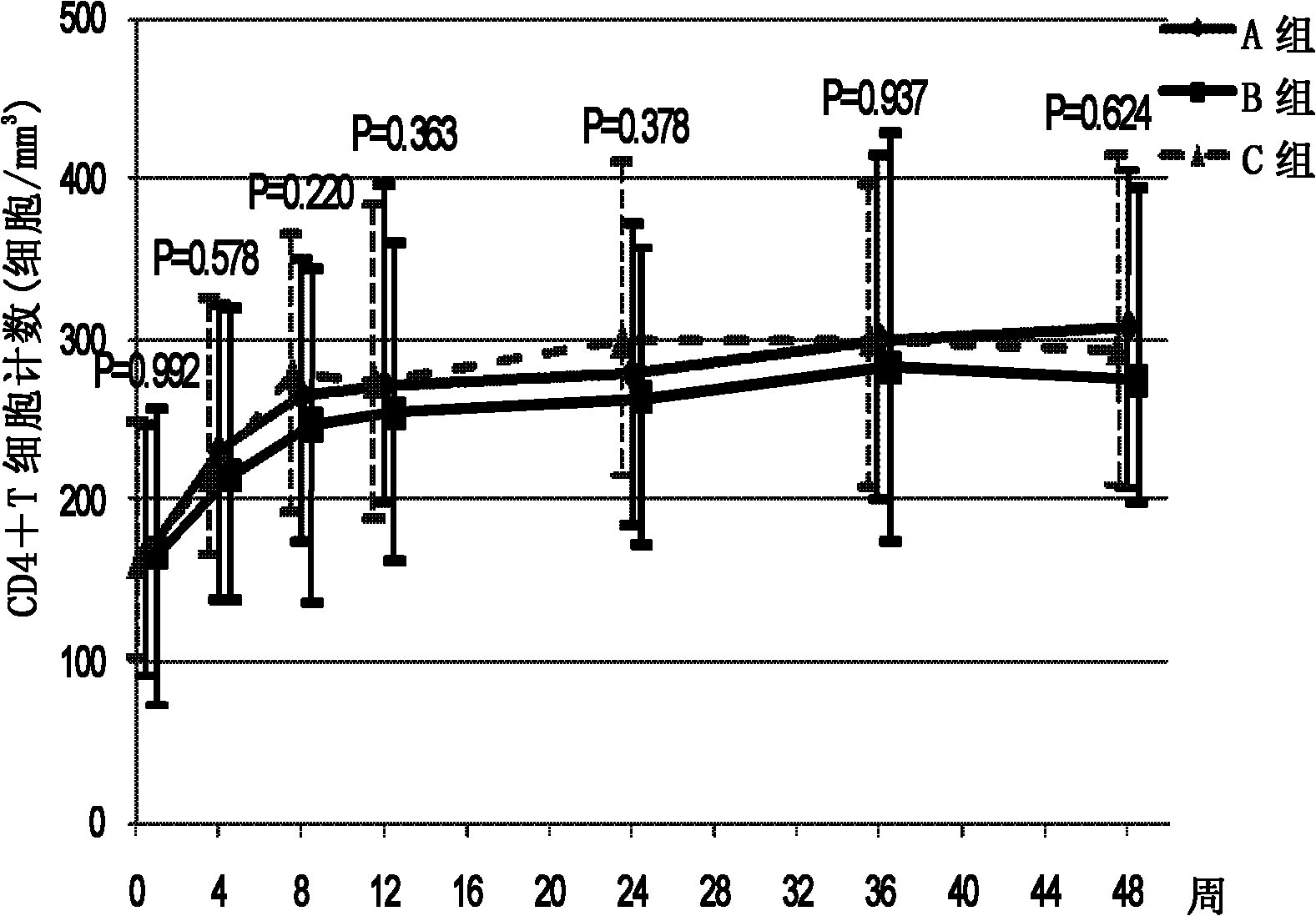
Patents
Literature
30 results about "First line therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
First line therapy is the treatment regimen or regimens that are generally accepted by the medical establishment for initial treatment of a given type and stage of cancer. It is also called primary treatment or therapy. The intent of first-line therapy is to cure the cancer if possible.
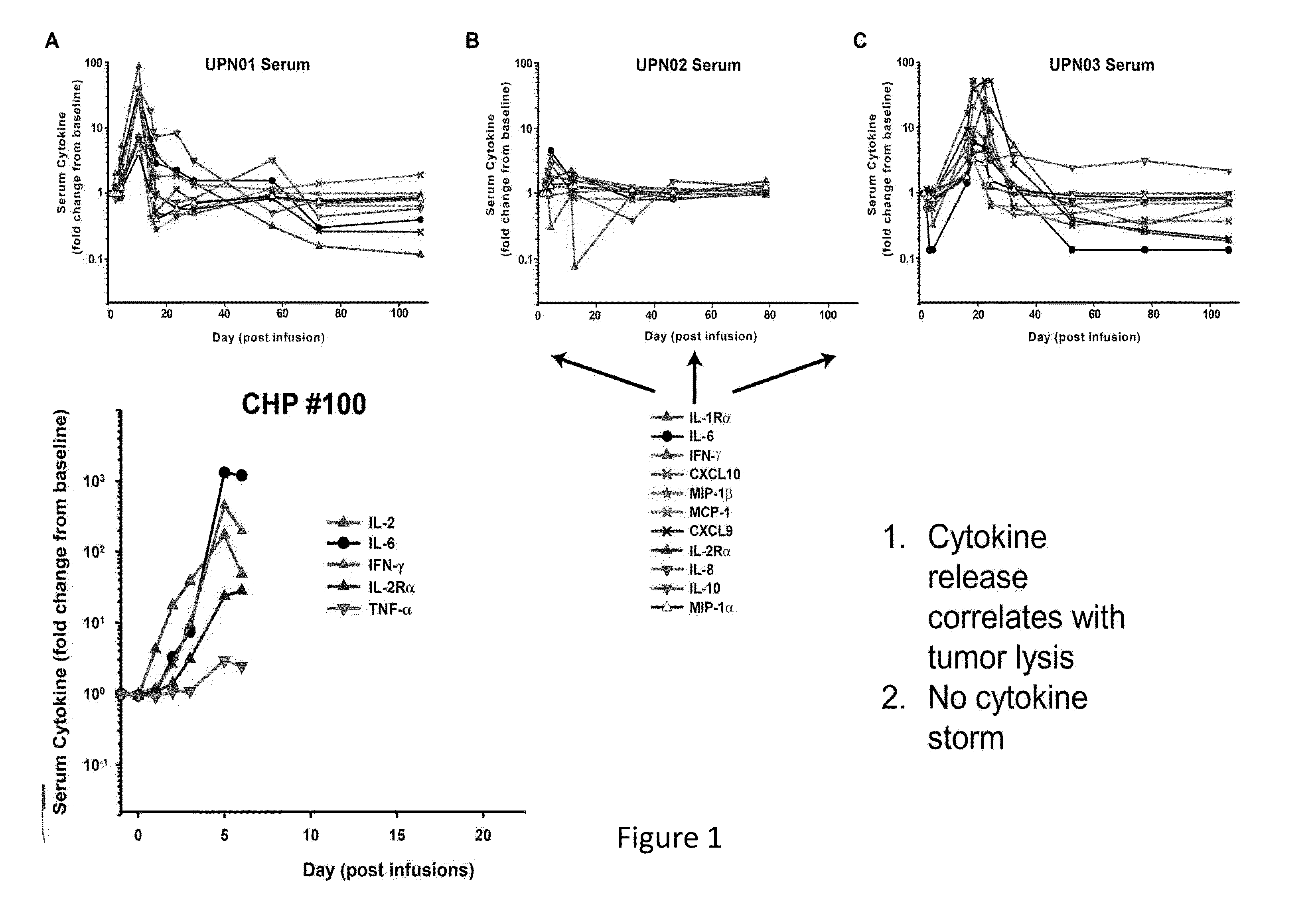
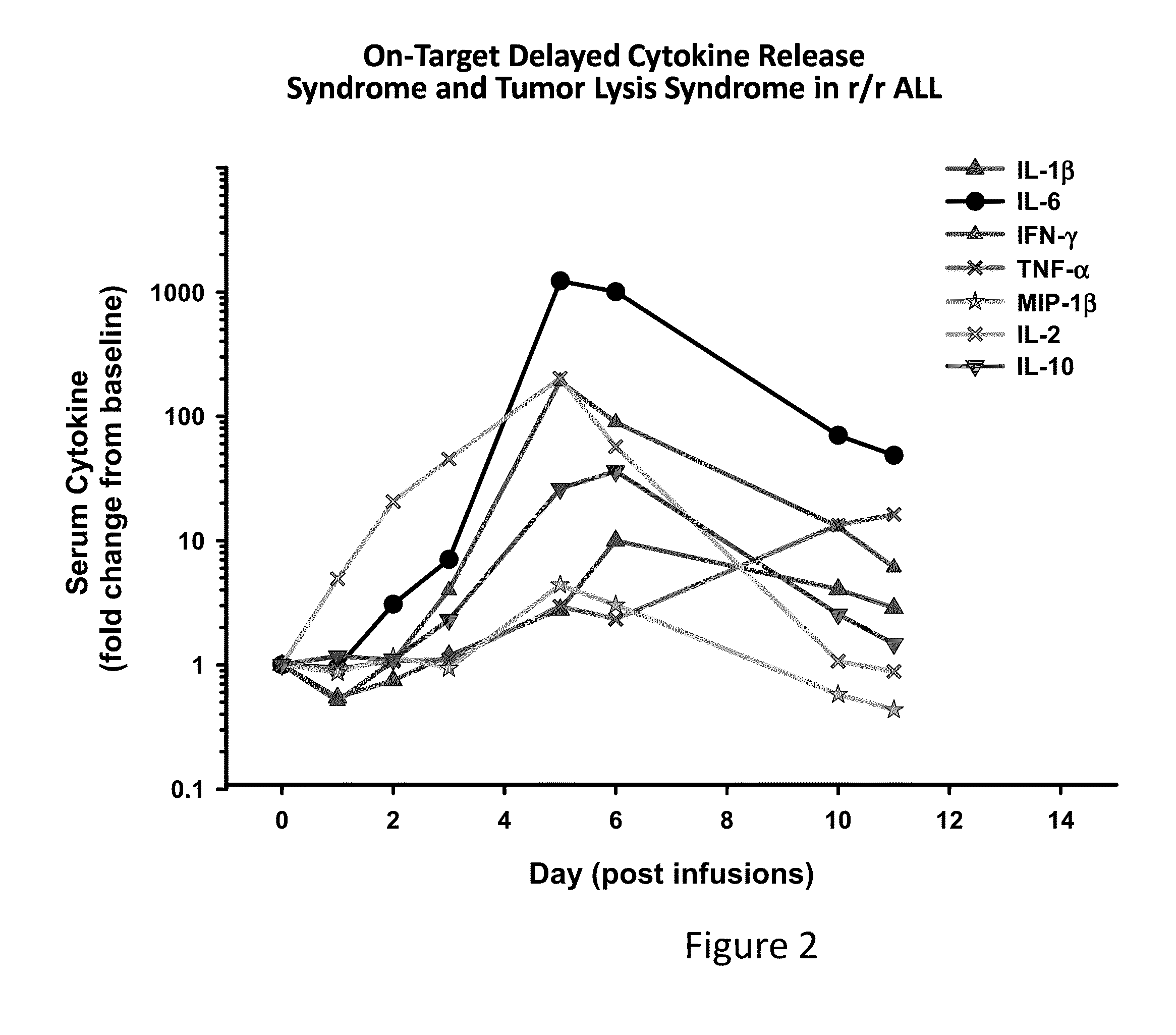
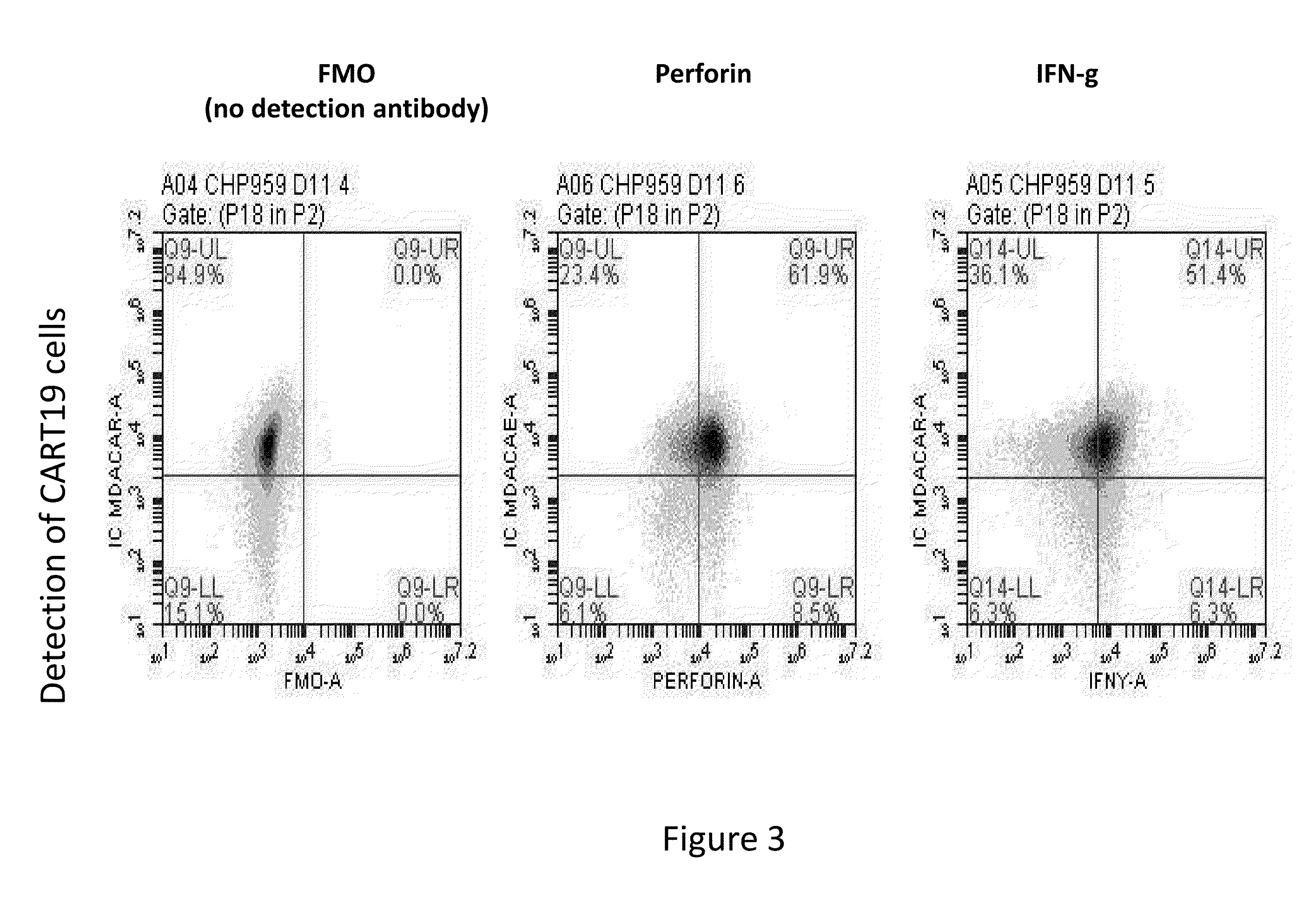
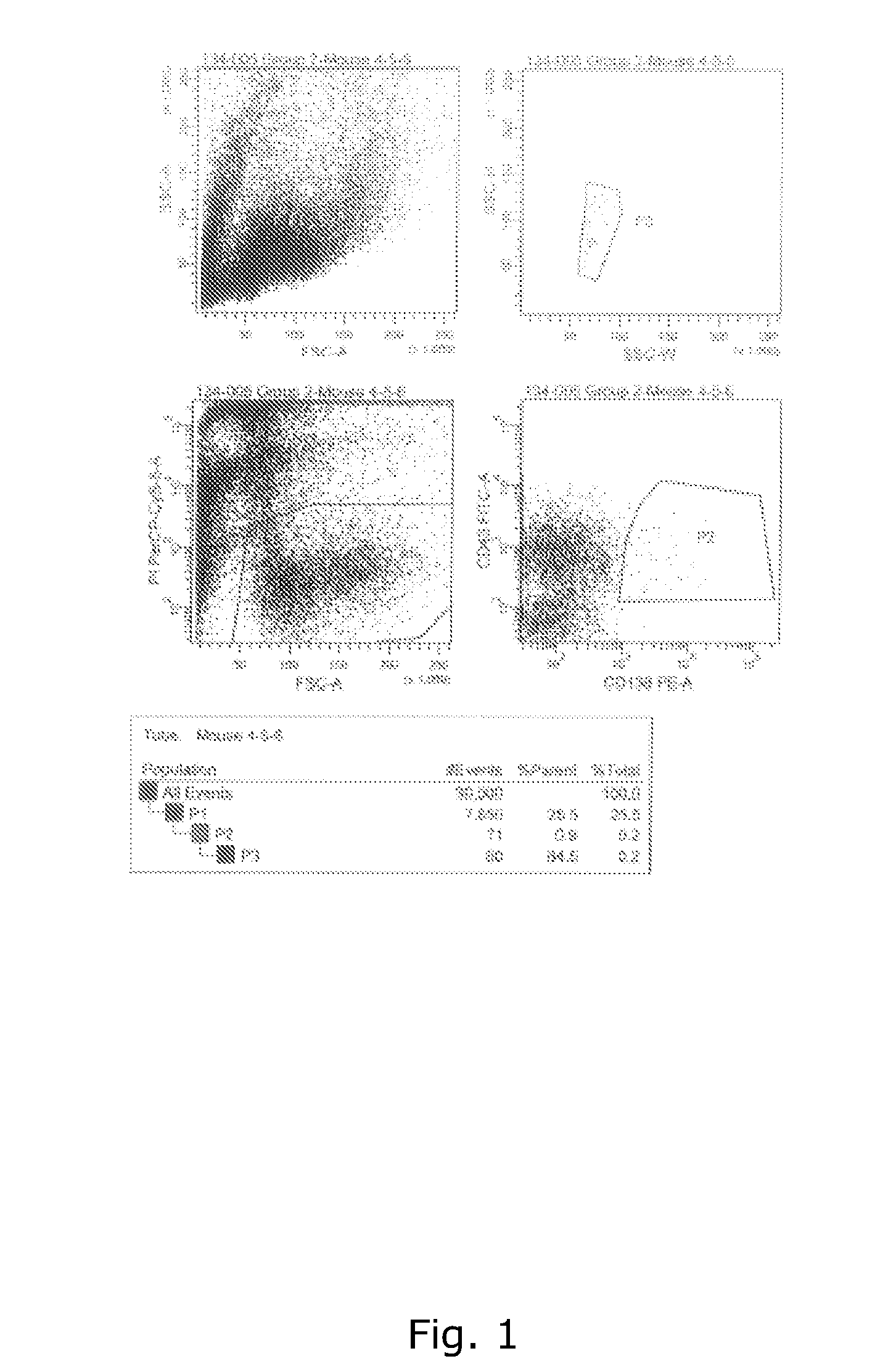
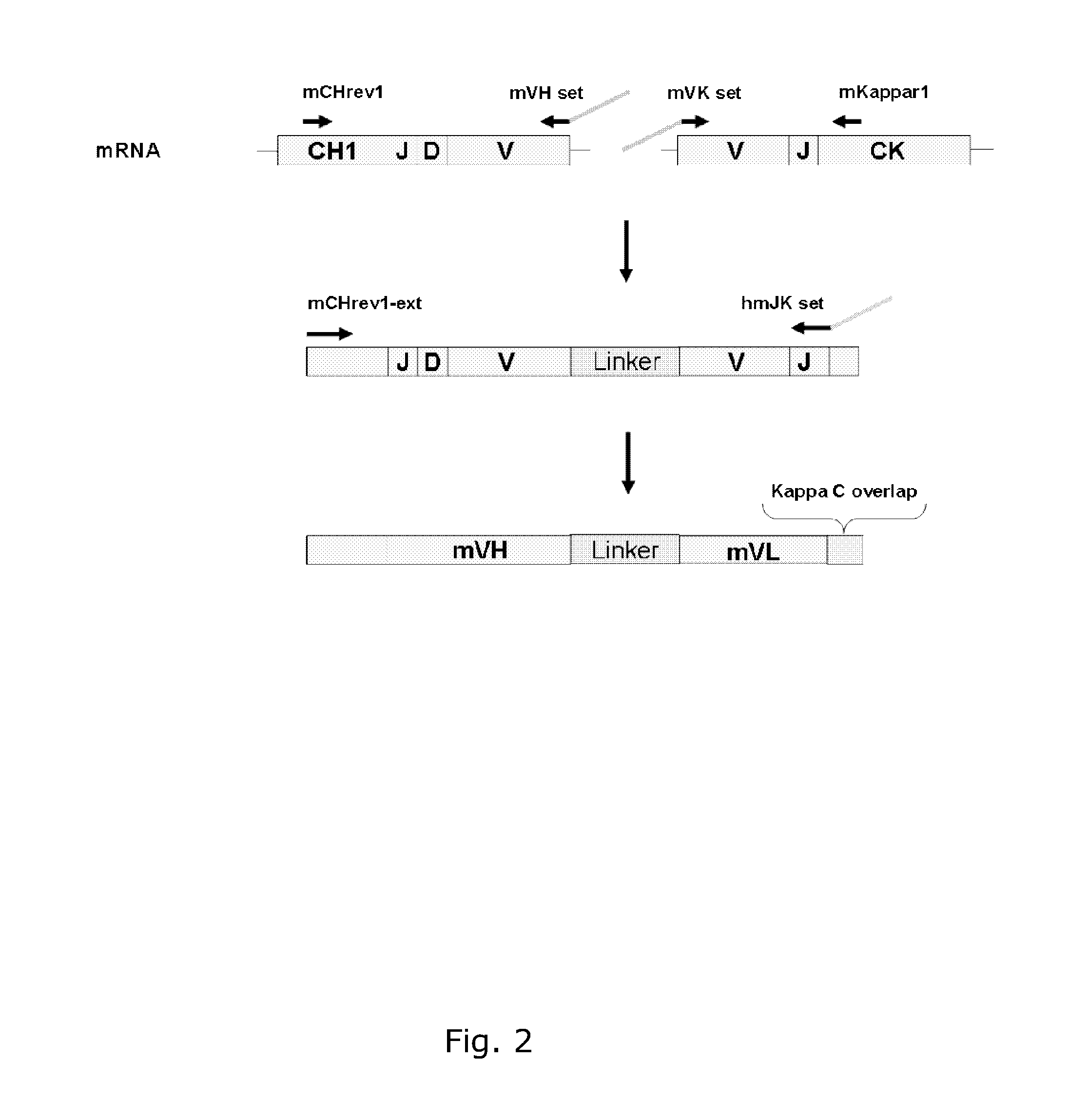
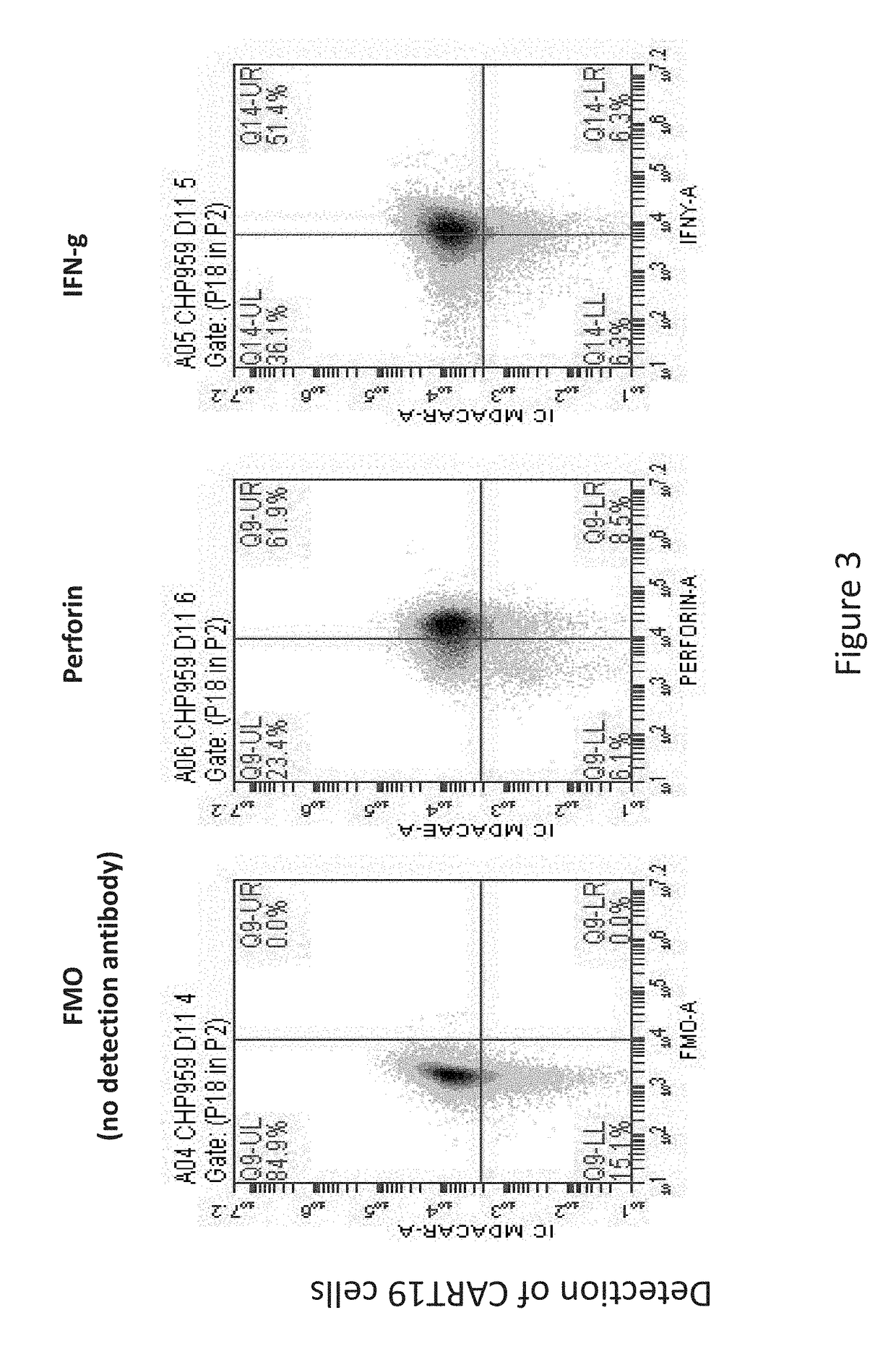
Toxicity Management for Anti-Tumor Activity of CARs
InactiveUS20150202286A1Reducing and avoiding adverse effectReducing and avoiding adverseOrganic active ingredientsBiocideAbnormal tissue growthAntigen binding
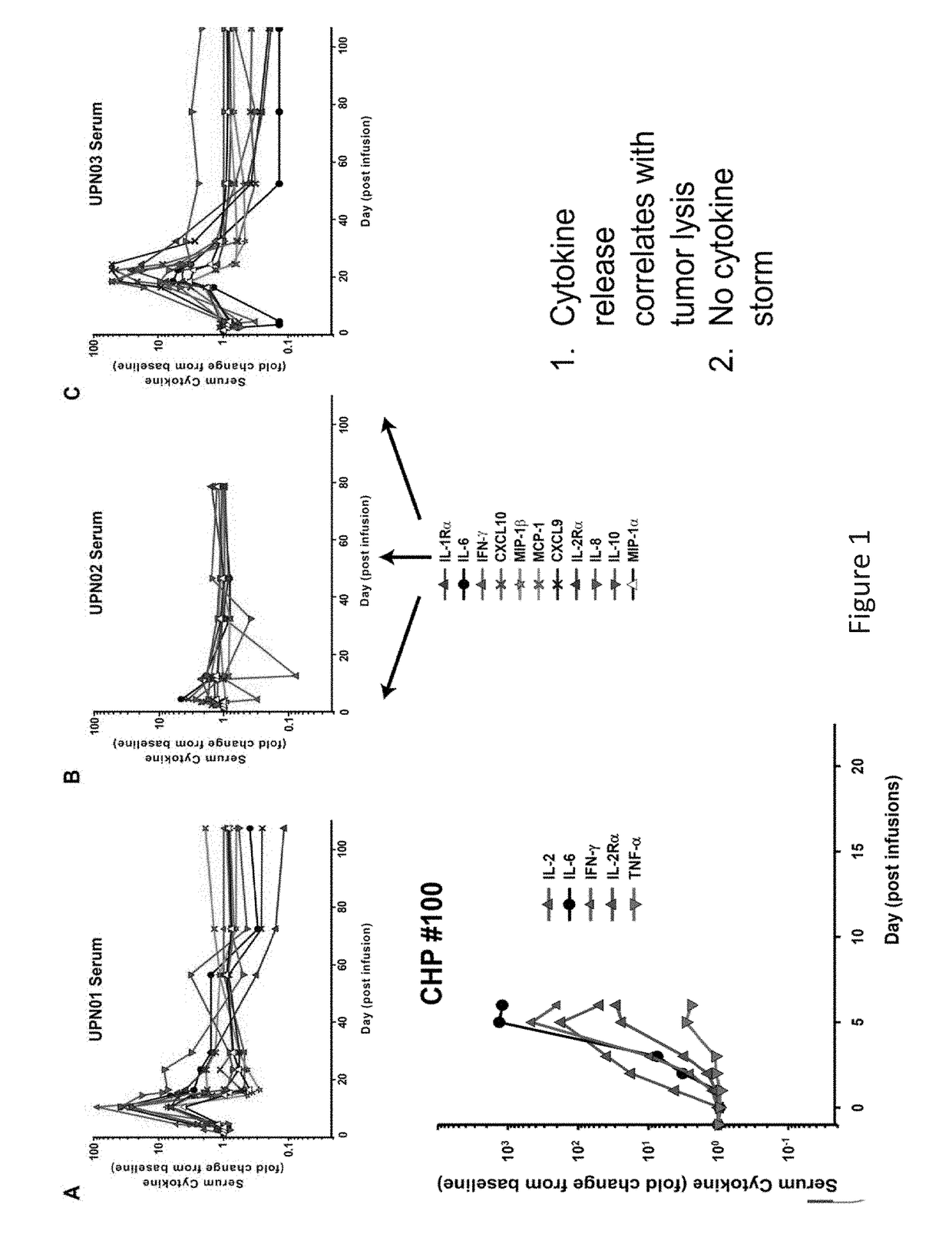
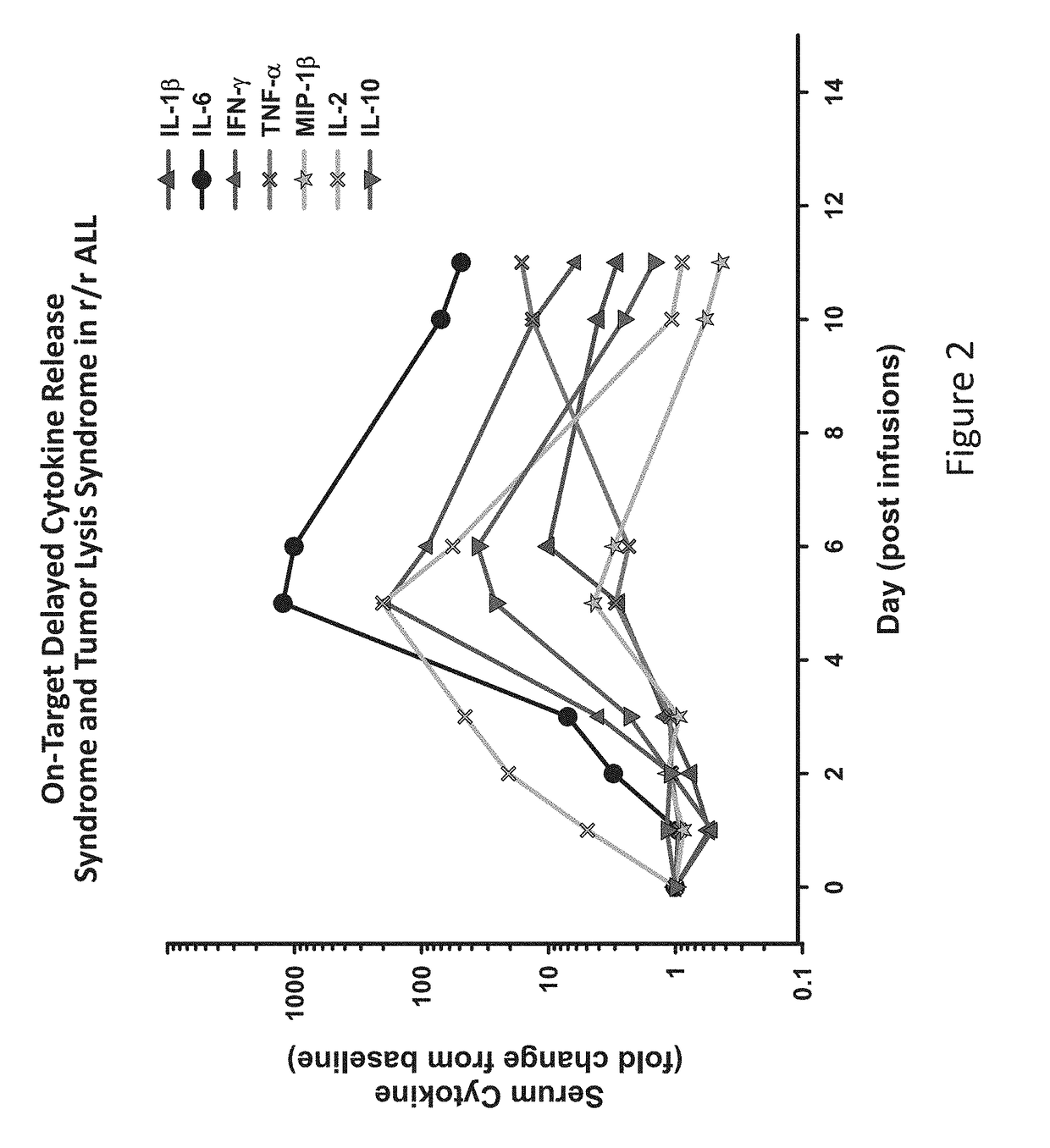
The present invention provides compositions and methods for treating cancer in a patient. In one embodiment, the method comprises a first-line therapy comprising administering to a patient in need thereof a genetically modified T cell expressing a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain and monitoring the levels of cytokines in the patient post T cell infusion to determine the type of second-line of therapy appropriate for treating the patient as a consequence of the presence of the CAR T cell in the patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA +1
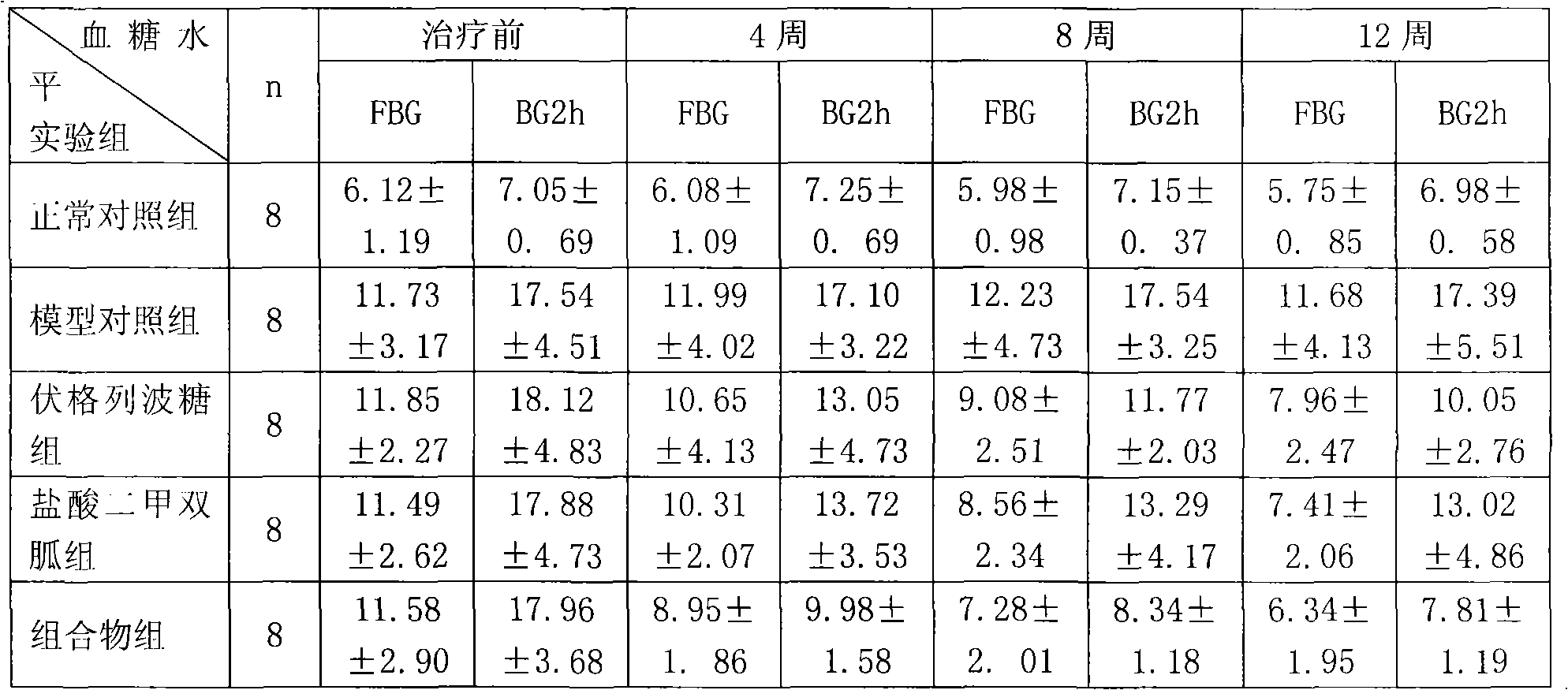
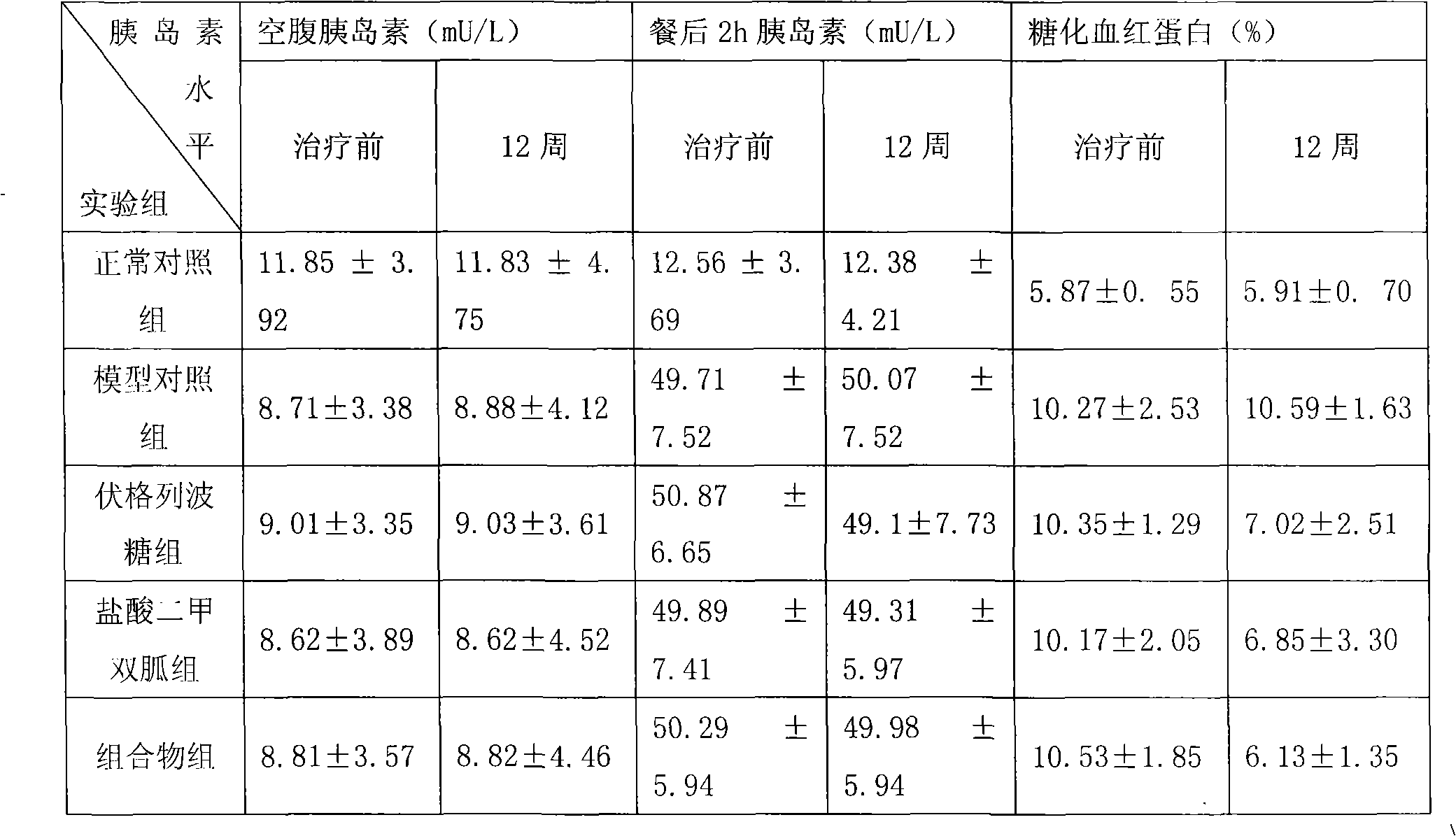
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
InactiveCN101590007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a metformin hydrochloride / voglibose sugar-lowering oral preparation composition and a preparation method thereof. The weight ratio of two main medicines is 8000:1-375:1, preferably 2500:1-625:1. Except for the main medicines, the composition also can further contain commonly used medicine accessories, such as a binder, a filling agent, a disintegrating agent, a lubricant, a flavoring agent, a wetting agent and a flow agent, and the obtained composition can be prepared into tablets, granules, soft and hard capsules, sustained and controlled release preparations, optimum enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules by conventional methods. The composition provided by the invention has action mechanism complementation of the main medicines, multiple target points, good compliance of patients, and the like. The sugar-lowering oral preparation composition can be used for the first-line therapy of type 2 diabetes, or can be used for second-line therapy under the condition that the metformin hydrochloride or sulfonylurea medicines fail to singly and effectively control blood sugar; and the sugar-lowering oral preparation composition is especially suitable for the therapy of diabetic patients suffering from latent autoimmune diabetes in adults (LADA) and hyperinsulinemia.
Owner:北京瑞伊人科技发展有限公司 +1
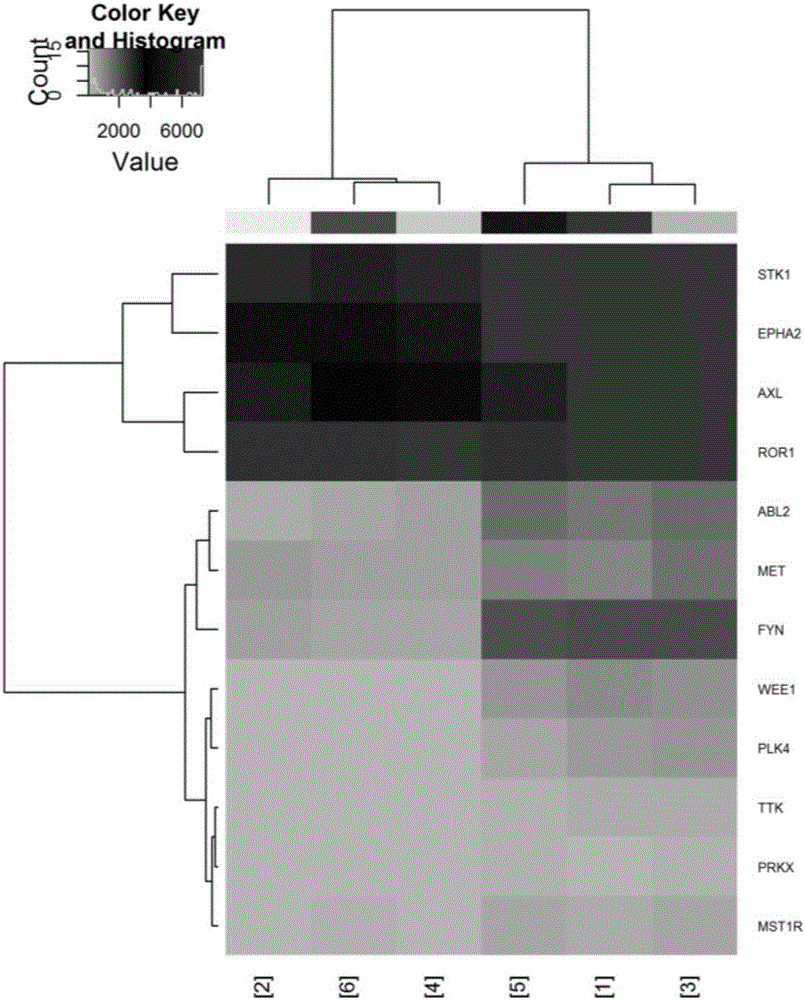
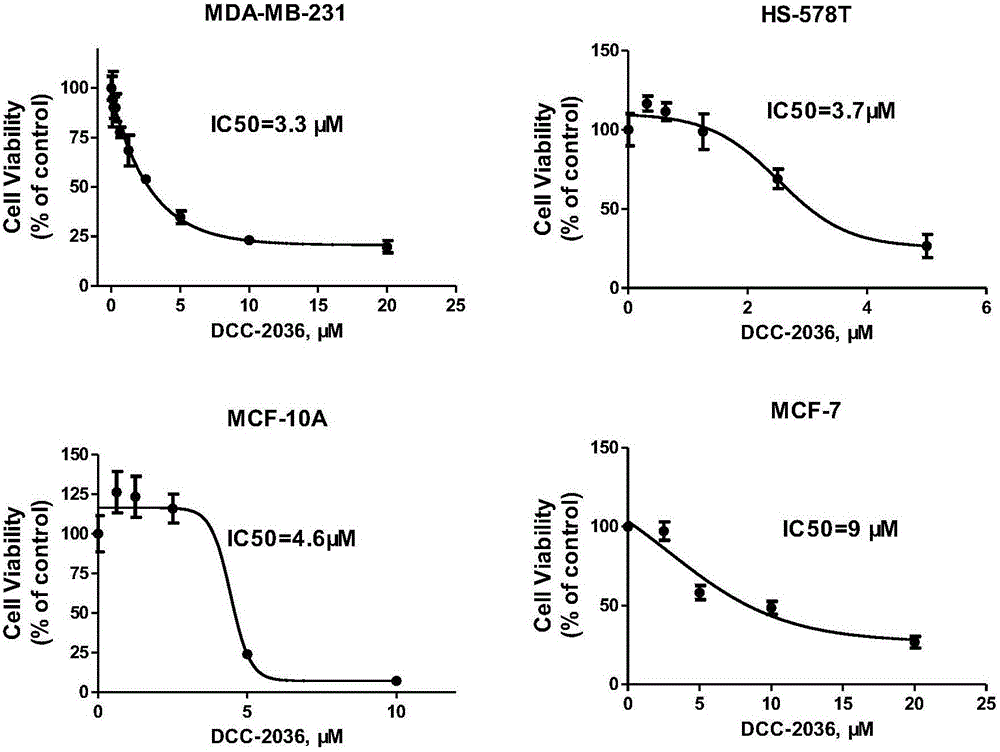
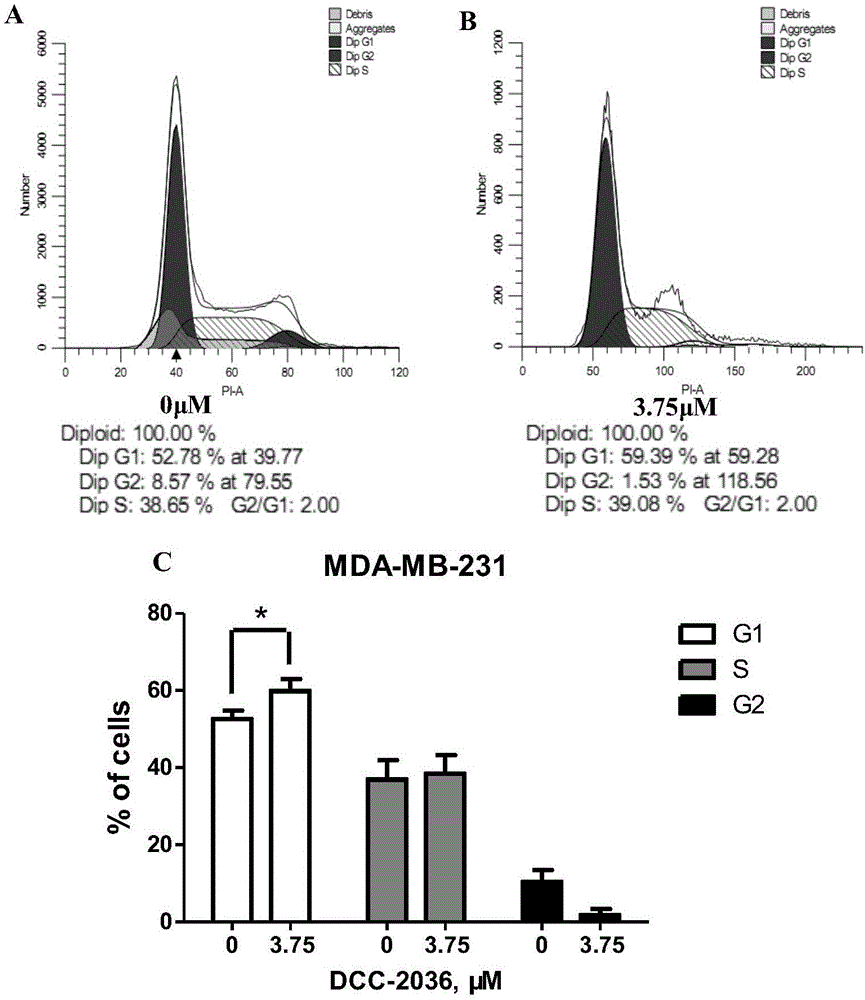
Novel application of tyrosine kinase inhibitor DCC-2036
InactiveCN106822128AEnhanced inhibitory effectTargeted therapy with strong specificityOrganic active ingredientsAntineoplastic agentsMda mb 231Tyrosine
The invention belongs to the field of medicinal chemistry, provides novel application of a tyrosine kinase inhibitor DCC-2036, and in particular discloses application of the tyrosine kinase inhibitor DCC-2036 in preparation ofmedicines for preventing or treating triple negative breast cancer. Tests show that the tyrosine kinase inhibitor DCC-2036 is capable of inhibiting proliferation and invasion and metastasis of TNBC(Triple Negative Breast Cancer) cell systems MDA-MB-231 and HS-578T, and moreover the inhibition function of the tyrosine kinase inhibitor DCC-2036 upon cell proliferation is prior to those of most TNBCclinicalfirst-line treatment medicines. Meanwhile, tests further show thatreceptor tyrosine kinaseAXL is a key target of DCC-2036 to take effects into play in TNBC cells, the tyrosine kinase inhibitor DCC-2036 targets AXL and further regulates downstream signal paths to take the anti-tumor function into play in the TNBC and is good in targeted therapy specificity and remarkable in effect, and normal tissue is generally not damaged.
Owner:THE FIRST AFFILIATED HOSPITAL HENGYANG MEDICAL SCHOOL UNIV OF SOUTH CHINA
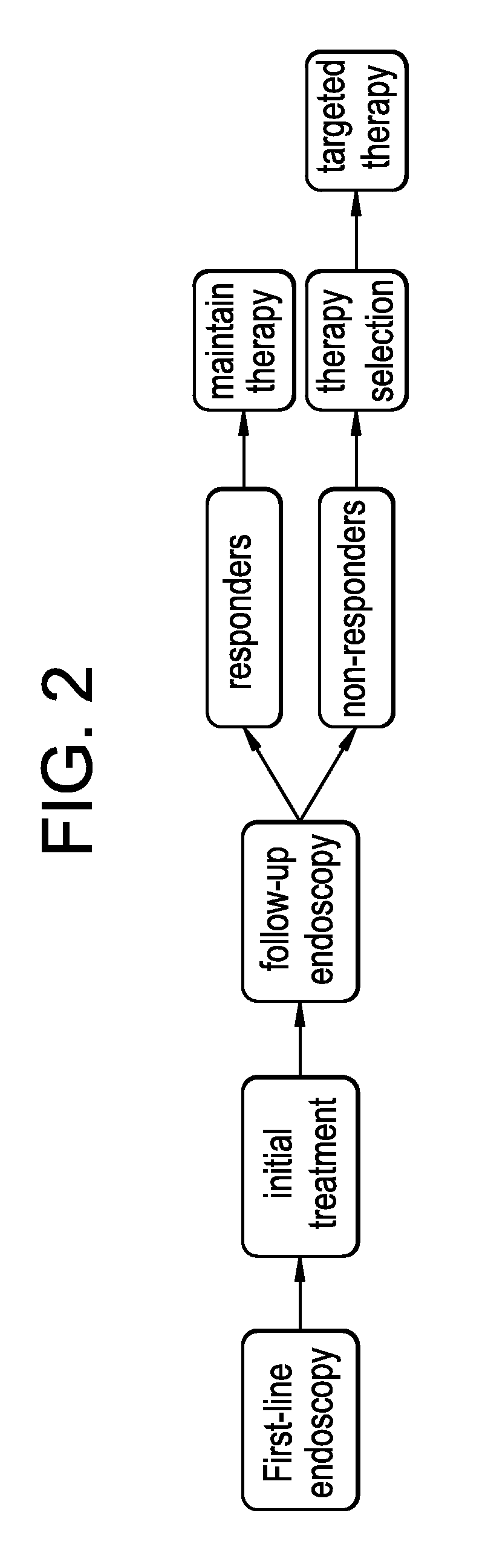
Therapy selection method
InactiveUS8568693B2Efficient use ofIn-vivo radioactive preparationsAntineoplastic agentsCyclaseRadio isotopes
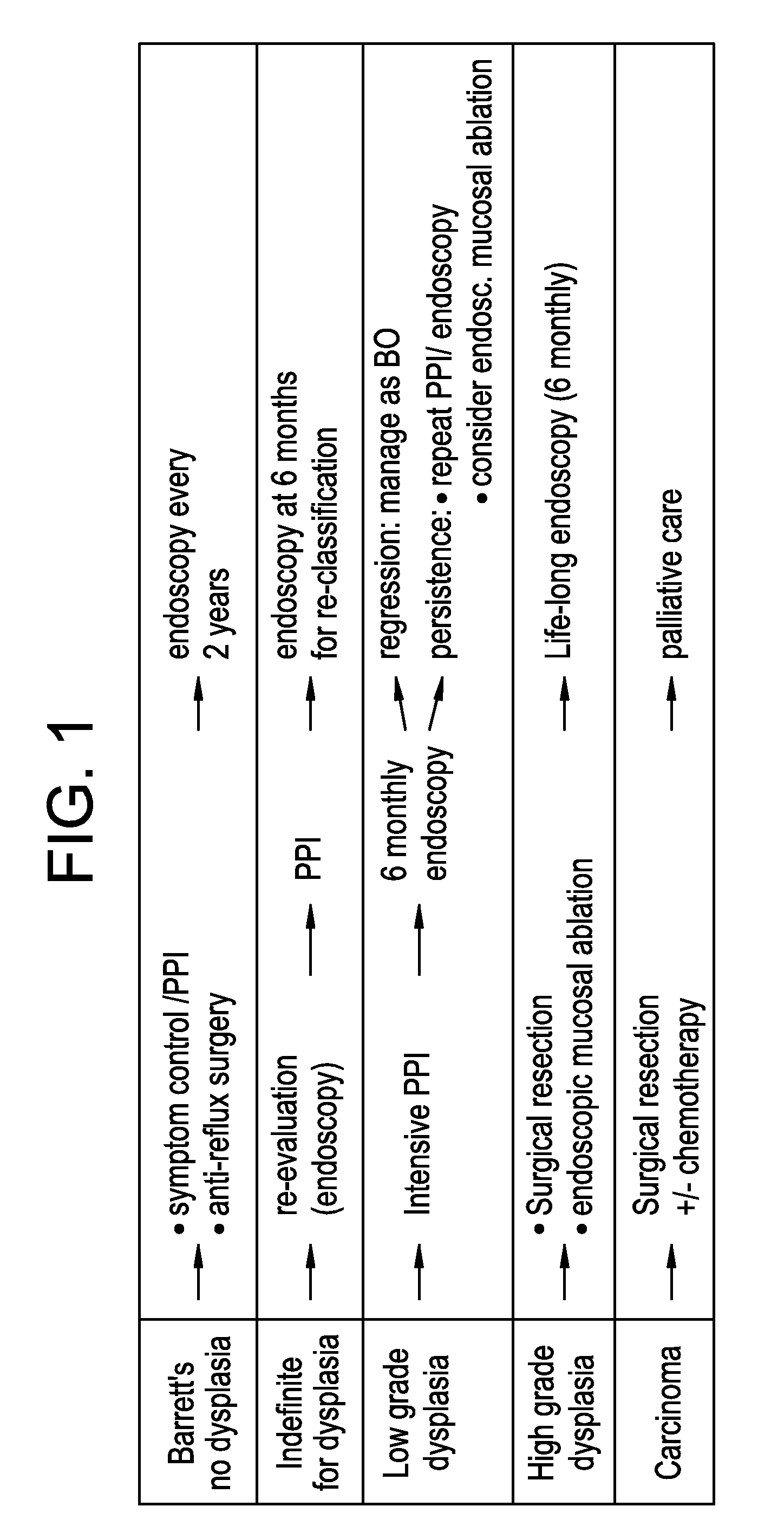
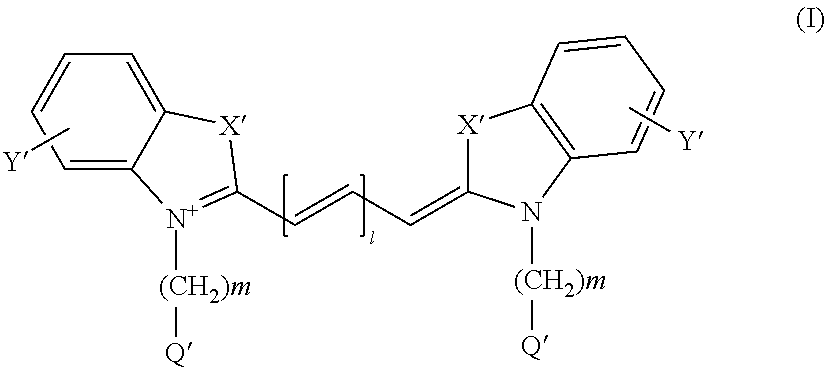
The present invention relates to a method to assist in the determination of therapy for a patient suffering from Barrett's oesophagus, especially where first-line therapy has been unsuccessful and when dysplasia has been diagnosed. The method comprises the use of an imaging agent comprising a vector which targets (a) Her2, (b) cMet, (c) guanylyl cyclase or (d) IGF1R. The imaging agent is suitable for radioisotope or optical imaging in vitro or preferably in vivo.
Owner:GE HEALTHCARE AS
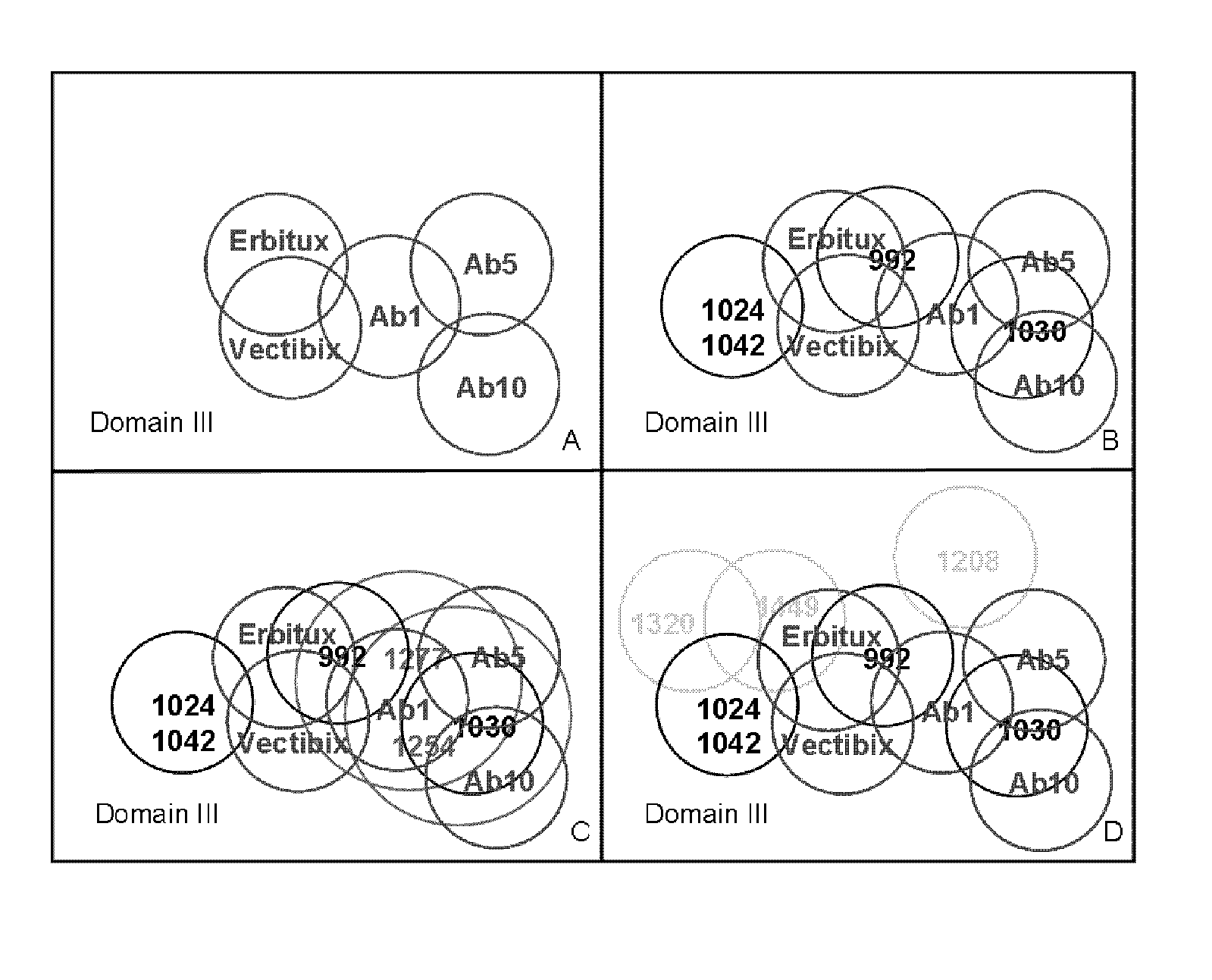
Methods using recombinant anti-epidermal growth factor receptor antibody compositions
ActiveUS8663640B2Rapid reduction in size of tumourLow efficacyImmunoglobulins against cell receptors/antigens/surface-determinantsVertebrate cellsAbnormal tissue growthHuman cancer
The invention relates to the field of recombinant antibodies for use in human cancer therapy. More specifically the invention provides the use of an antibody composition with two distinct non-overlapping binding specificities to human EGFR. The antibody composition is effecting in treating cancer following treatment with other anti-EGFR antibodies, whether the cancer shows progression during or following the prior treatment or not. The antibody composition can also be used for repeated treatment of recurrent tumors following first-line therapy with the antibody composition of the invention, as the composition does not lead to selection of resistant tumors. A further therapeutic use is the use of an antibody composition of the invention for treatment of cancer that is resistant to known anti-EGFR antibodies.
Owner:LES LAB SERVIER
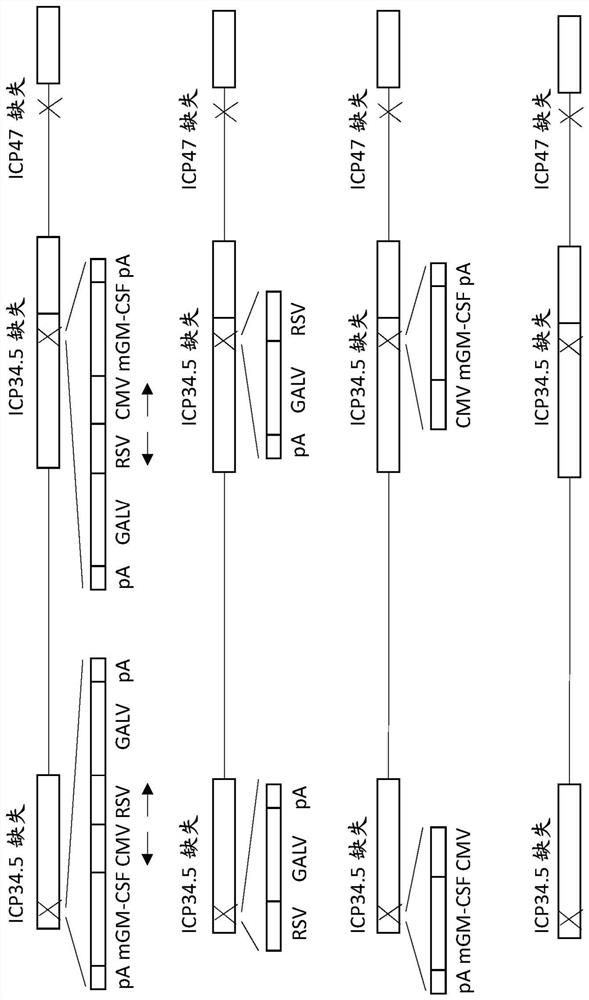
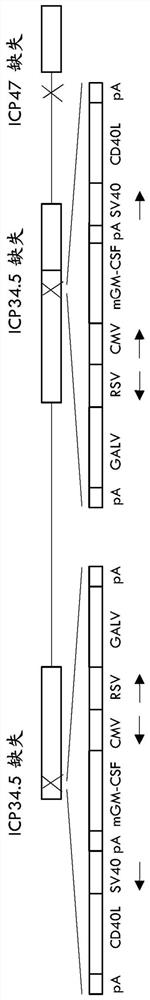
Treatment using oncolytic virus
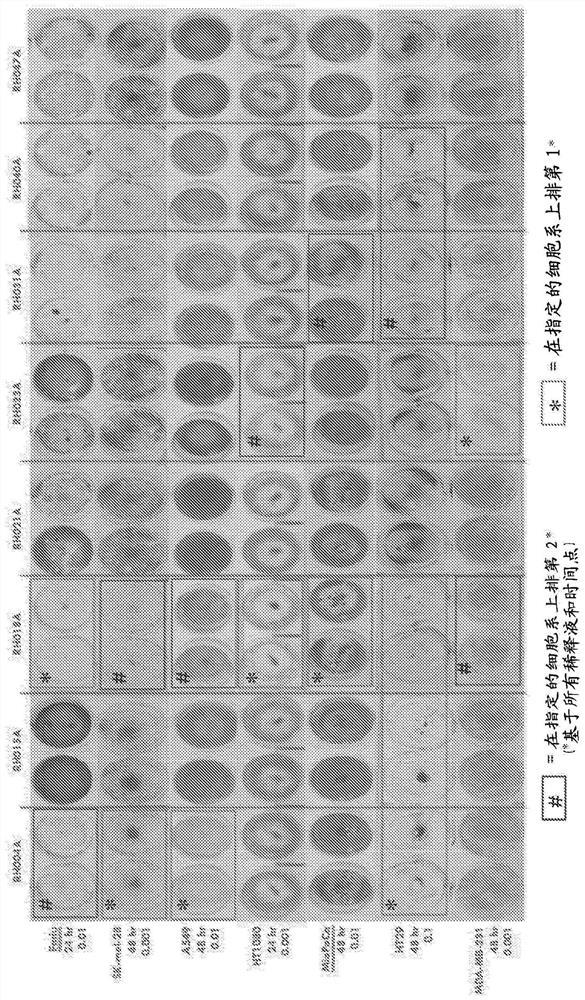
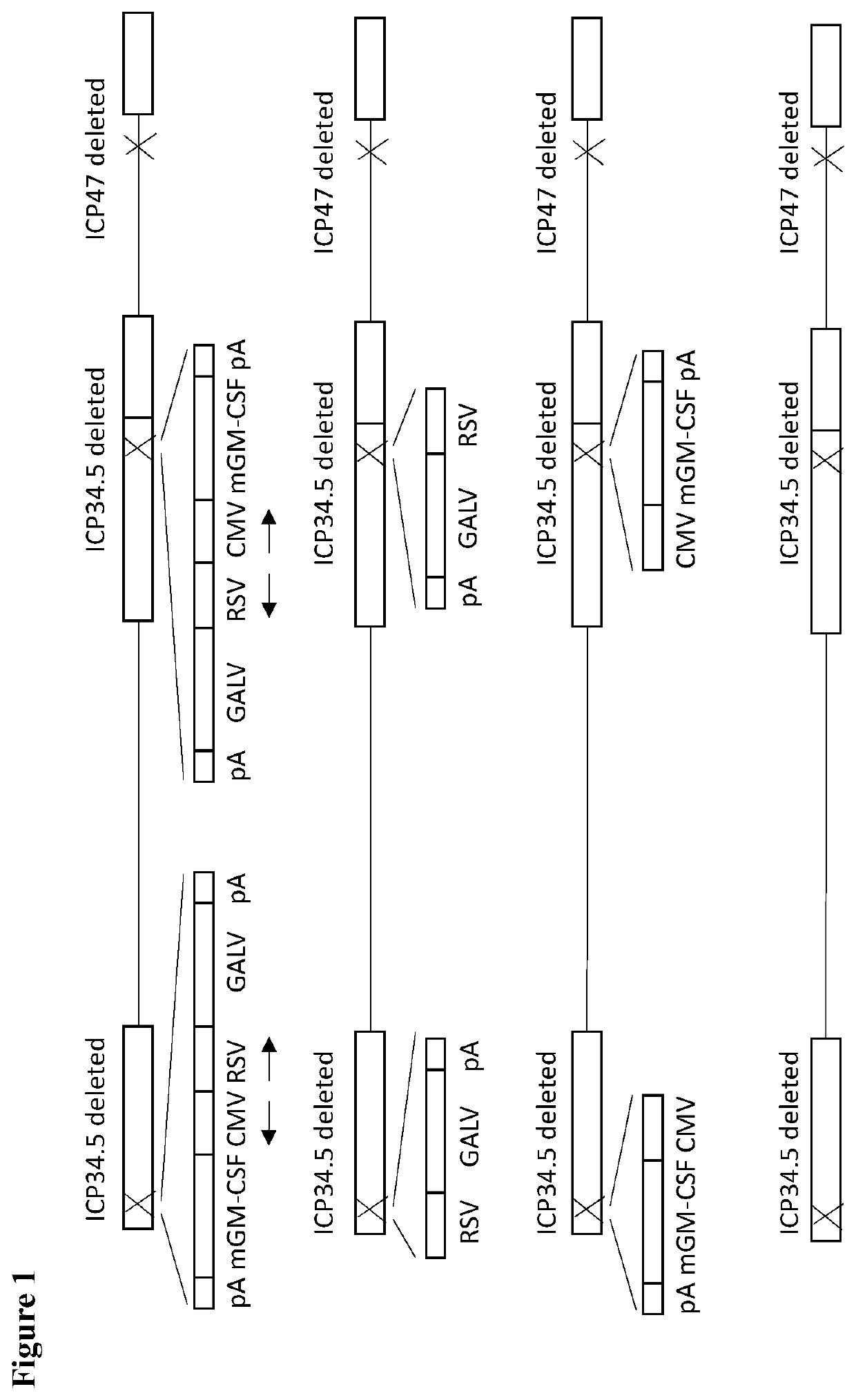
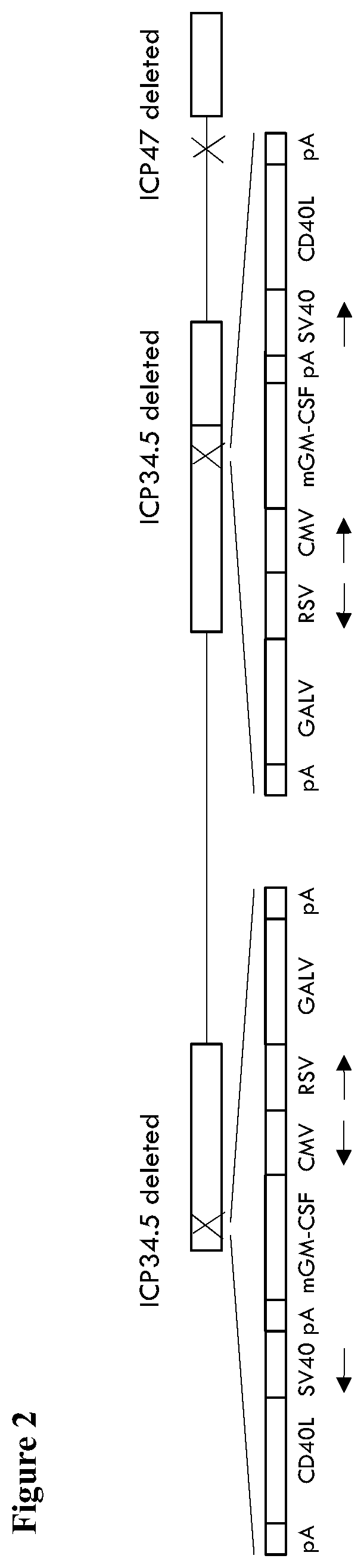
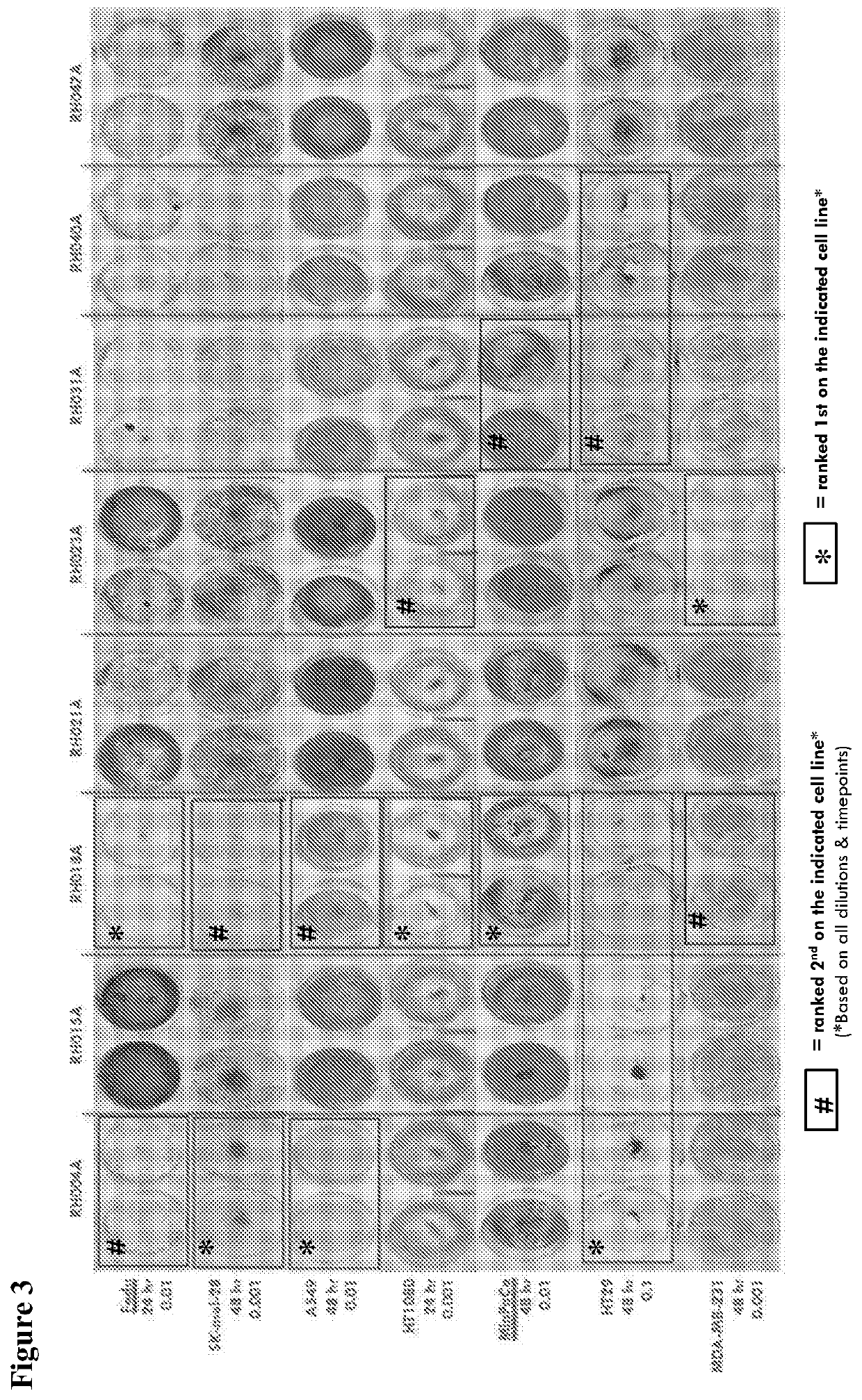
An oncolytic virus is for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelial carcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patientswith no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / orcancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panelof three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
Treatment using oncolytic virus
PendingUS20210252135A1Direct effectImprove immunityPeptide/protein ingredientsViral antigen ingredientsAppendiceal carcinomaSquamous Carcinomas
An oncolytic virus for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelialcarcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patients with no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / or cancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus: is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panel of three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
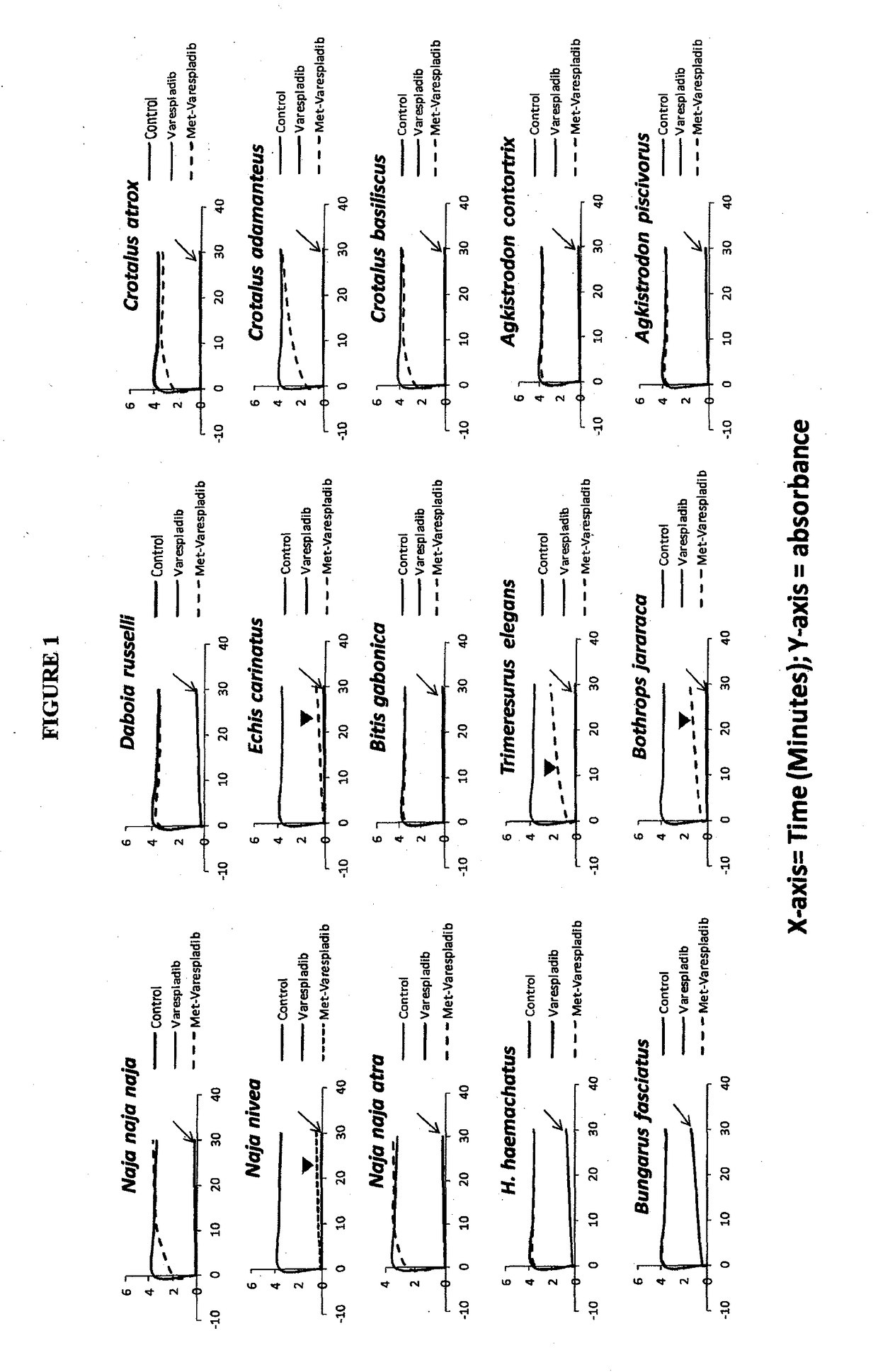
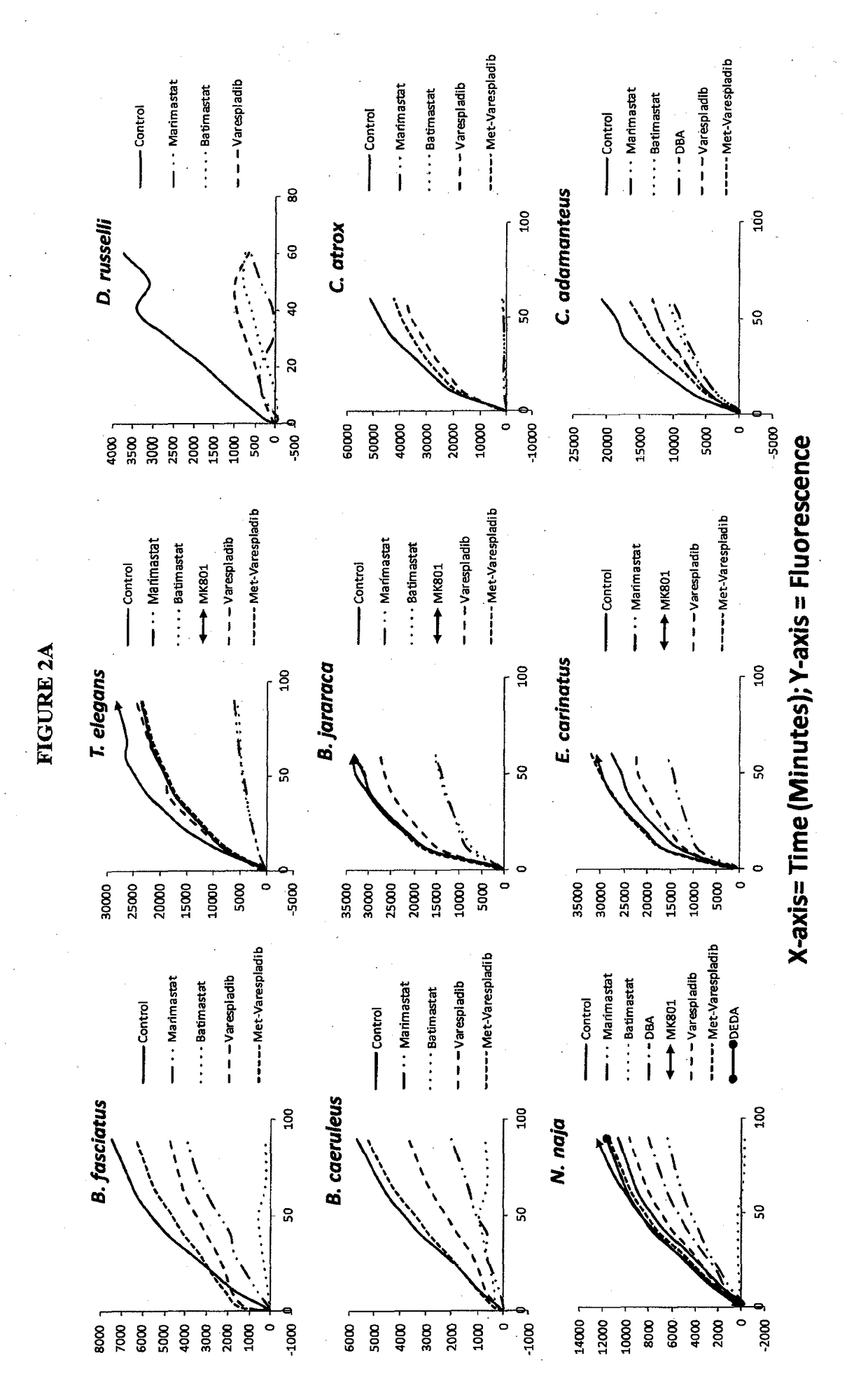
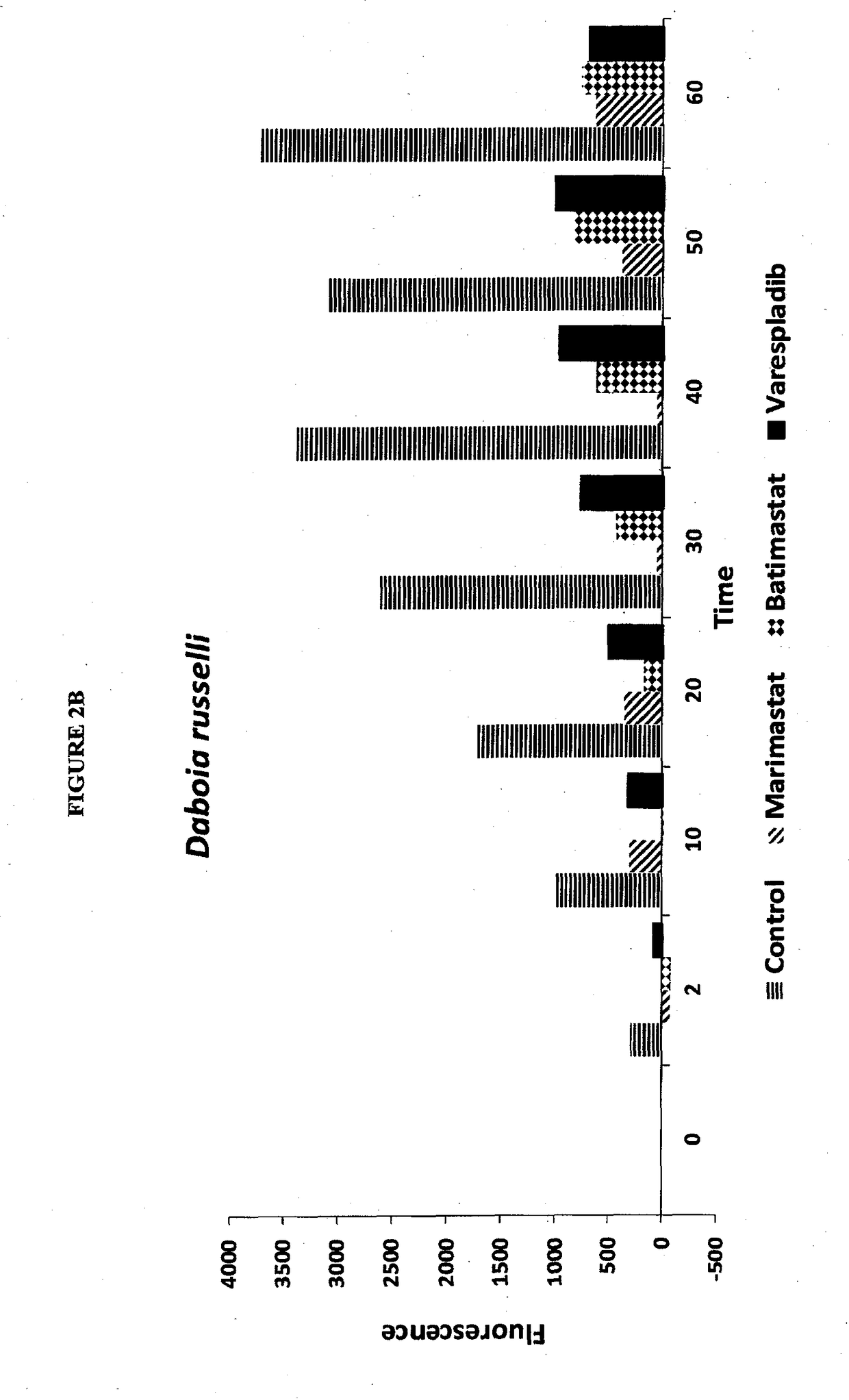
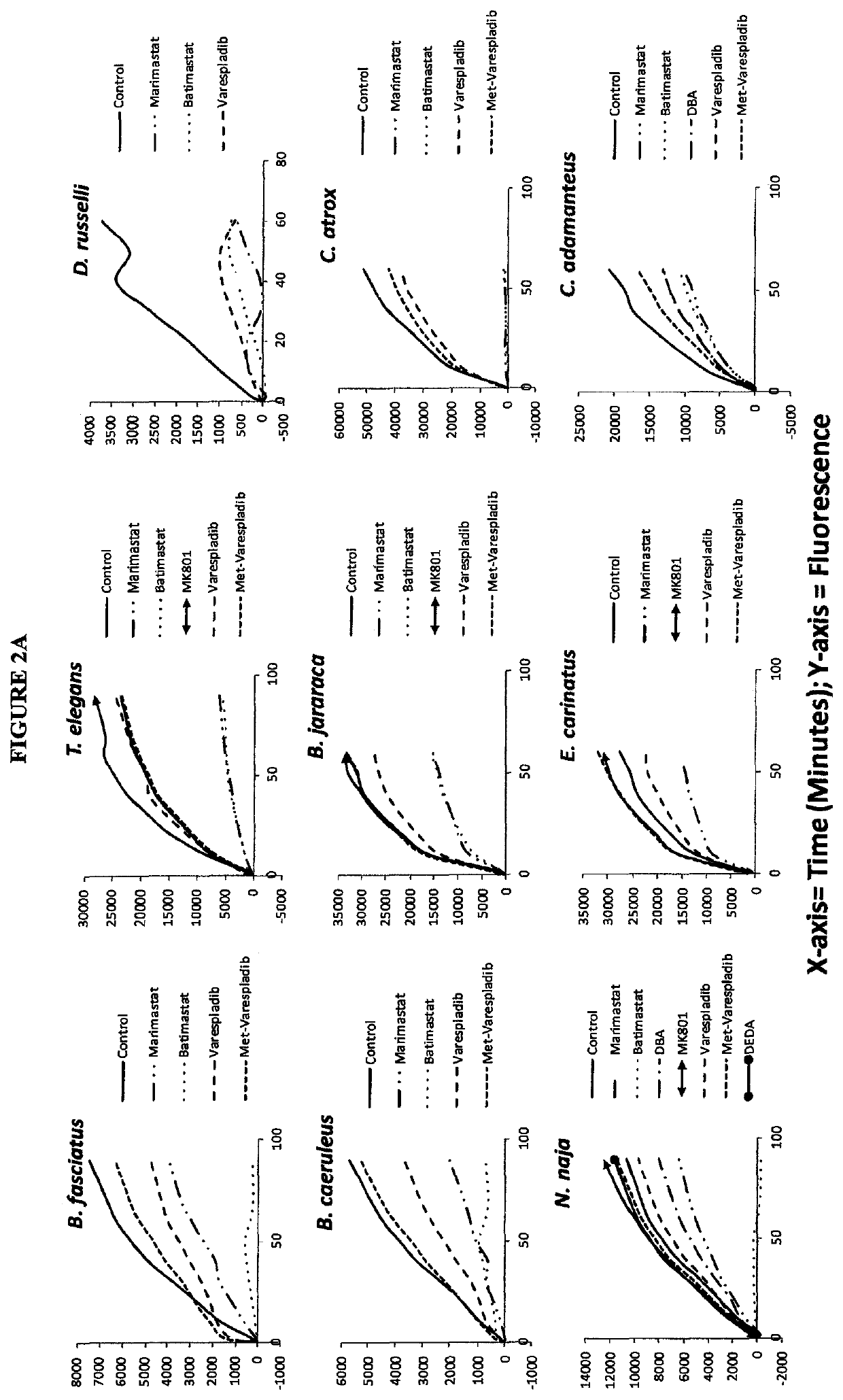
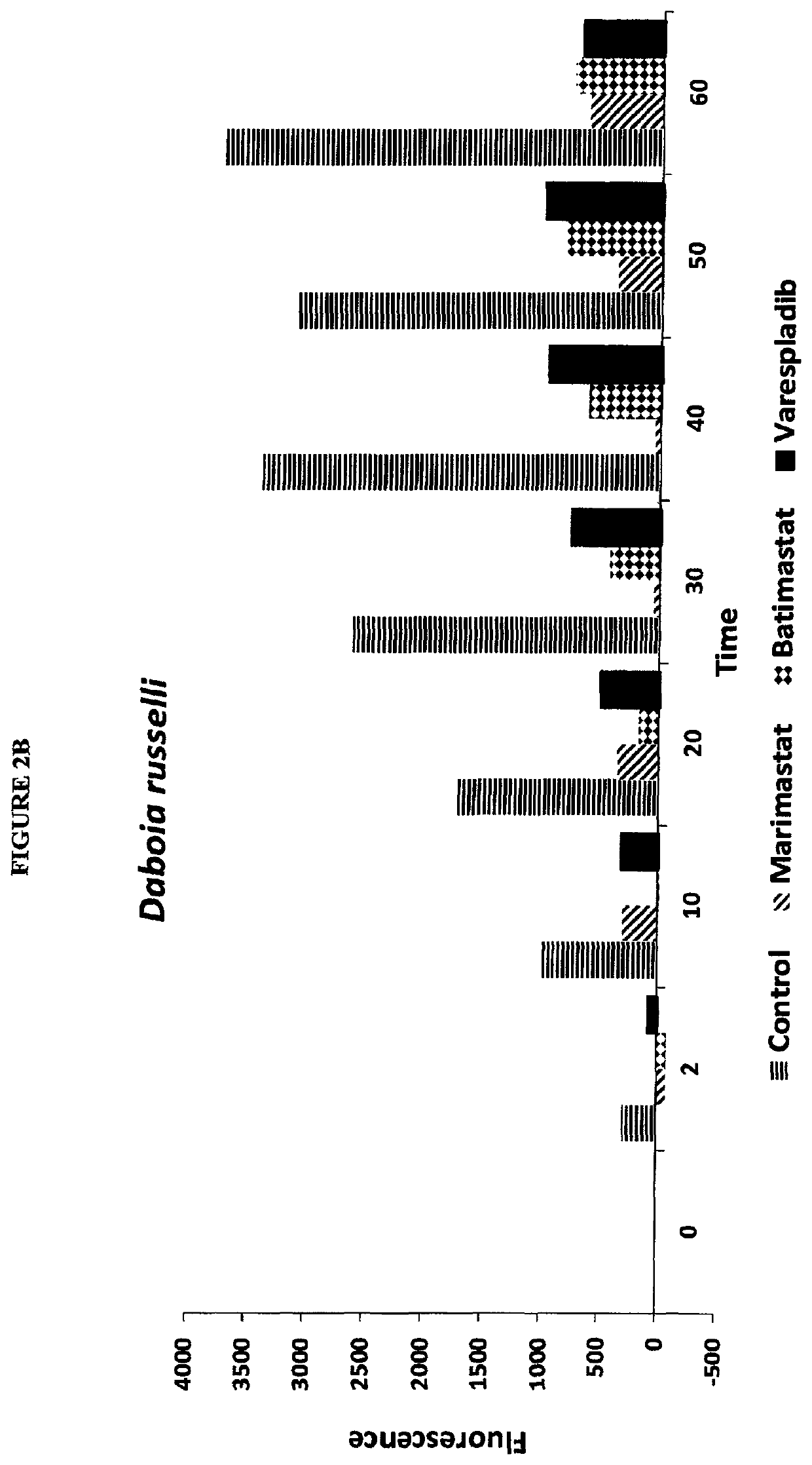
Envenomation therapies and related pharmaceutical compositions, systems and kits
ActiveUS20170354642A1Safe and cost-effectiveUnmet needPeptide/protein ingredientsImmunoglobulins against animals/humansNR1 NMDA receptorMoxisylyte
The invention provides methods of treatment, pharmaceutical compositions, systems and kits appropriate for first line and / or adjunct therapy with antivenom using at least one active component, in some instances at least two active components and in other instances no more than two active components selected from the group consisting of a selective secretory PLA2 inhibitor (sPLA2 or PLA2 inhibitor), a metalloproteinase inhibitor, a serine protease inhibitor, antivenom, one or more acetylcholinesterase inhibitors or a nAChR agonist paired with a mAChR antagonist, a NMDA receptor antagonist and a spreading factor inhibitor to treat a subject who suffers from an envenomation, preferably at the time of envenomation and often within a period of less than about an hour after an envenomation or 6 hours after an envenomation and throughout the course of treatment at time with or without antivenom as an adjunct therapy after an envenomation by, for example, a snake or invertebrate.
Owner:OPHIREX INC
Application of VEGFR inhibitor in preparation of anti-Alzheimer's disease medicine
PendingCN113750236AReduce penetrationImprove securityOrganic active ingredientsNervous disorderDiseaseDonepezil
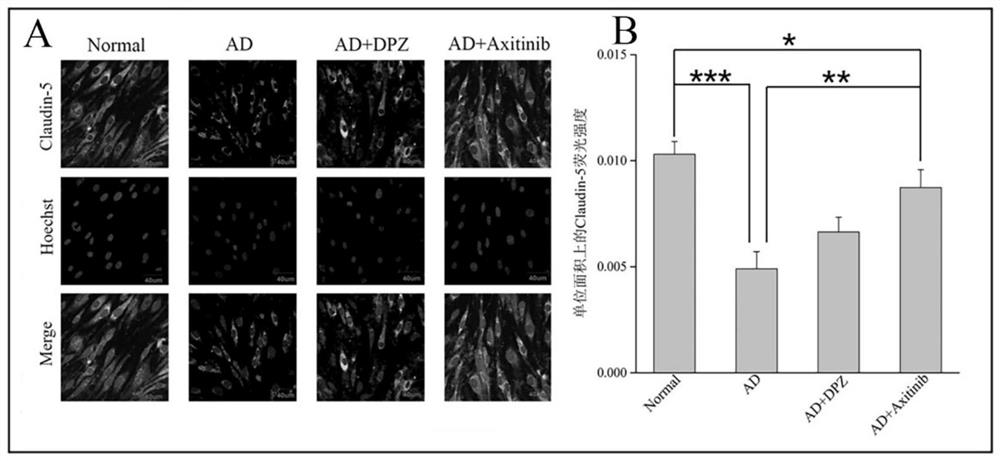
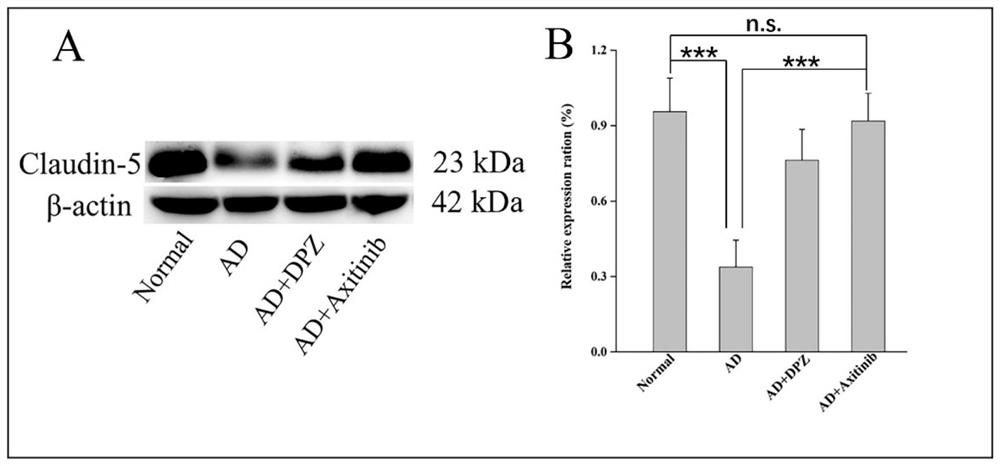
The invention discloses an application of a VEGFR inhibitor in preparation of an anti-Alzheimer's disease medicine. The VEGFR inhibitor is axitinib and an analogue thereof. It is found that axitinib has an unexpected curative effect on Alzheimer's disease, and Alzheimer's disease model animal in-vivo experiments prove that after administration of axitinib, beta-amyloid protein deposition in the brain of a model animal is significantly reduced, the content of acetylcholin esterase is reduced, the oxidative stress level and inflammatory response of the lesion part of the model animal are relieved, the learning and memory ability and learning and memory behaviors of the model animal on space and direction are improved, and the curative effect is obviously higher than that of a clinical first-line treatment drug donepezil. The axitinib and the analogue thereof provide a new means for clinical treatment of the Alzheimer's disease, and the axitinib and the analogue thereof are high in human body safety, low in price and easy to prepare. According to the invention, non-intracranial administration mode is adopted, safety is high, administration is convenient and fast, and patient compliance is high. The application of a VEGFR inhibitor in preparation of an anti-Alzheimer's disease medicine has wide market prospects and great social significance.
Owner:ZHEJIANG UNIV
Toxicity Management for Anti-Tumor Activity of CARs
ActiveUS20180243411A1Reducing and avoiding adverseOrganic active ingredientsMicrobiological testing/measurementAntigen bindingT cell
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Composition for treating liver cancer and application thereof
ActiveCN112870194AReduce synthesisReduce effluxOrganic active ingredientsDigestive systemCancer cellSide effect
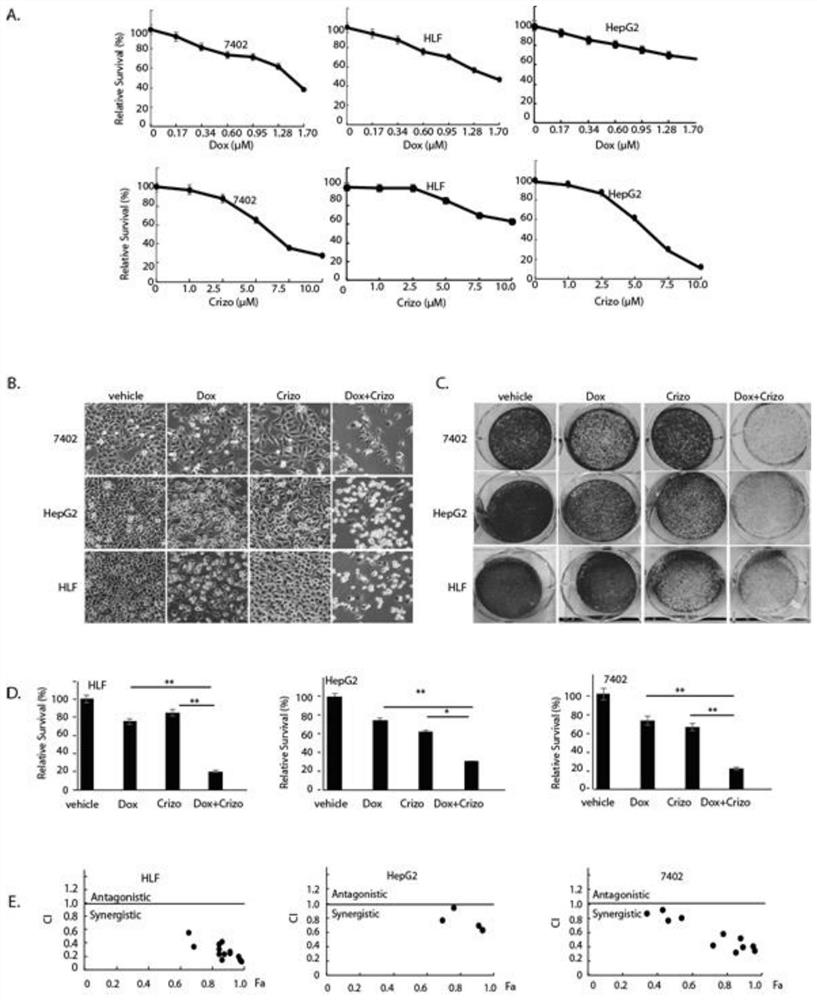
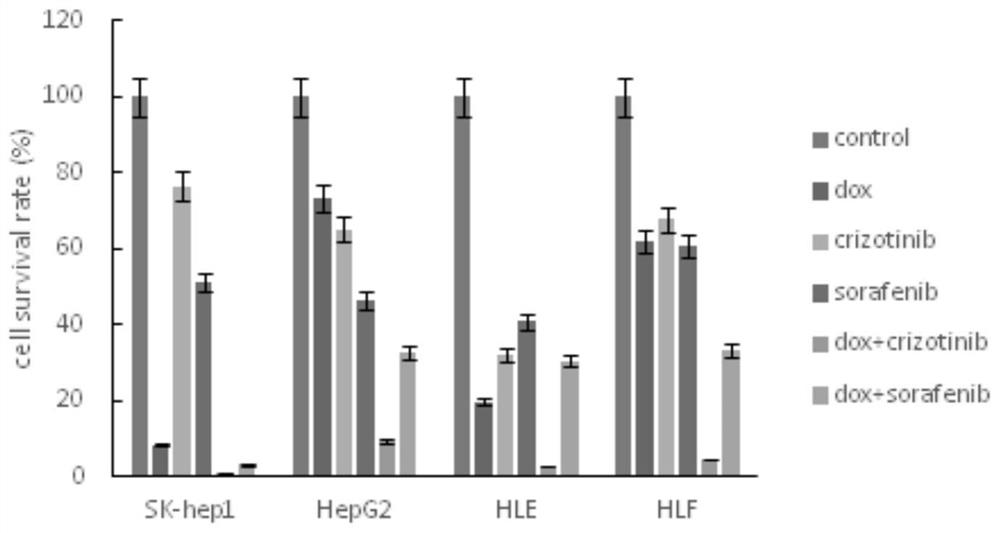
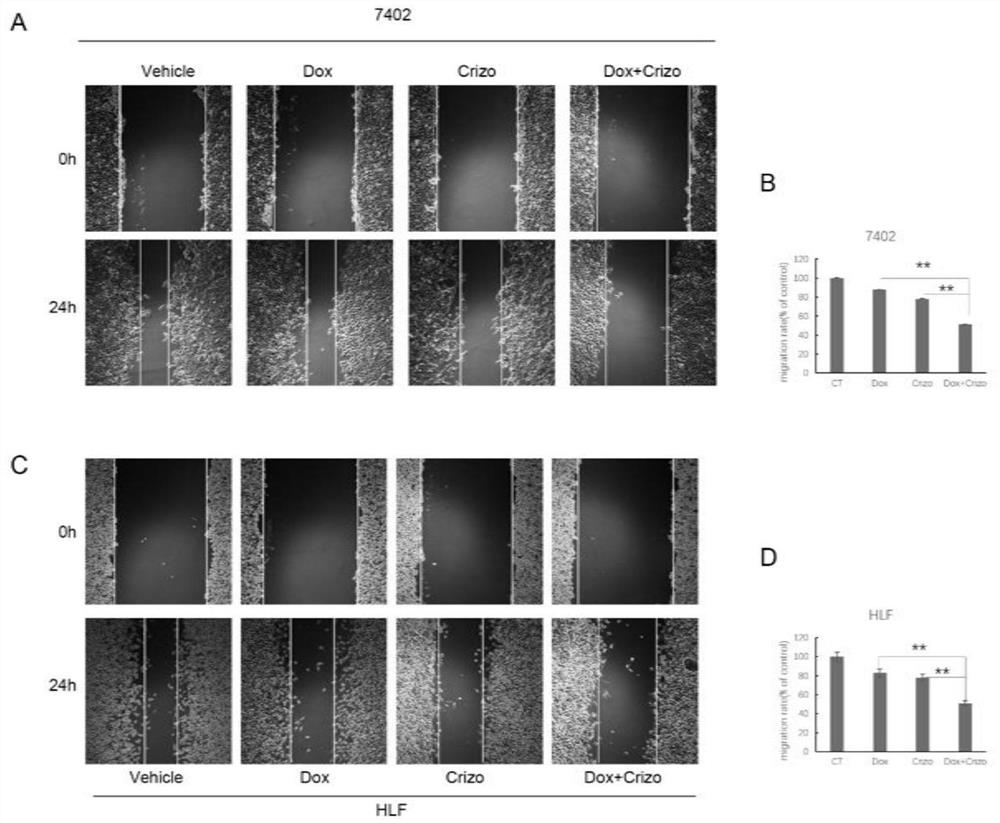
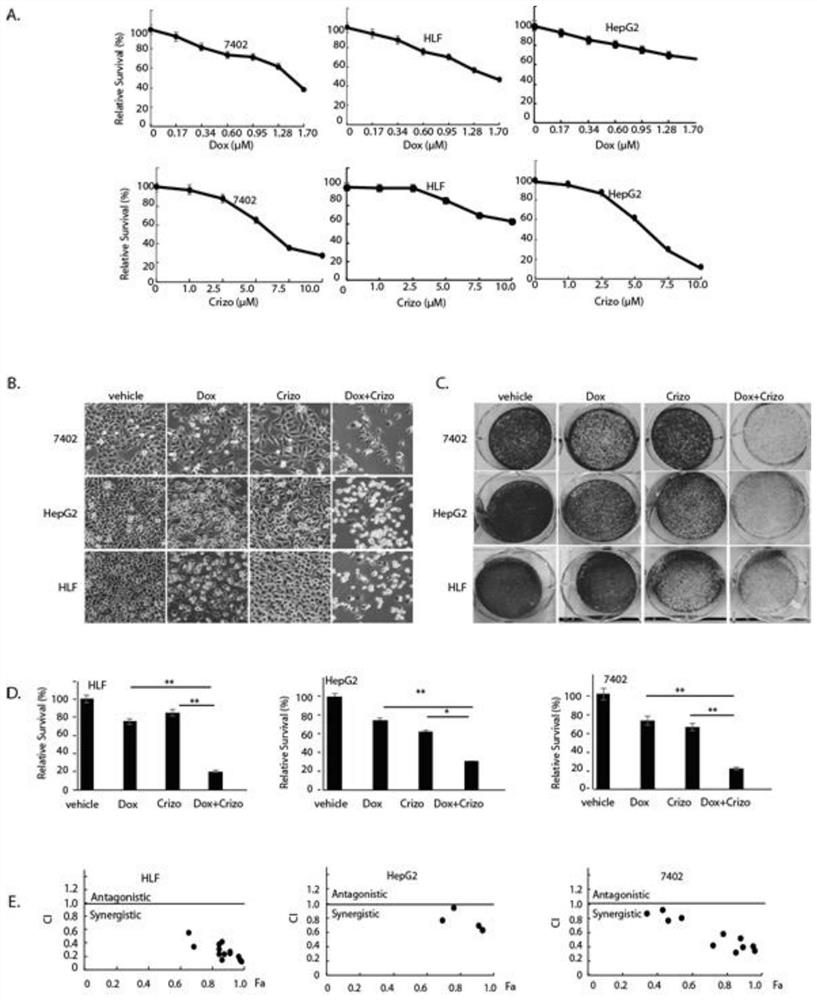
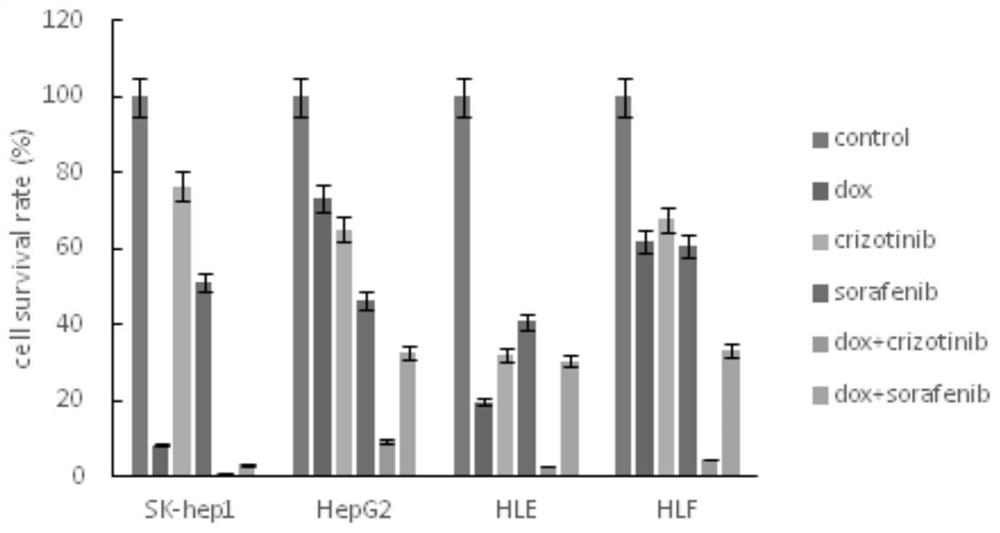
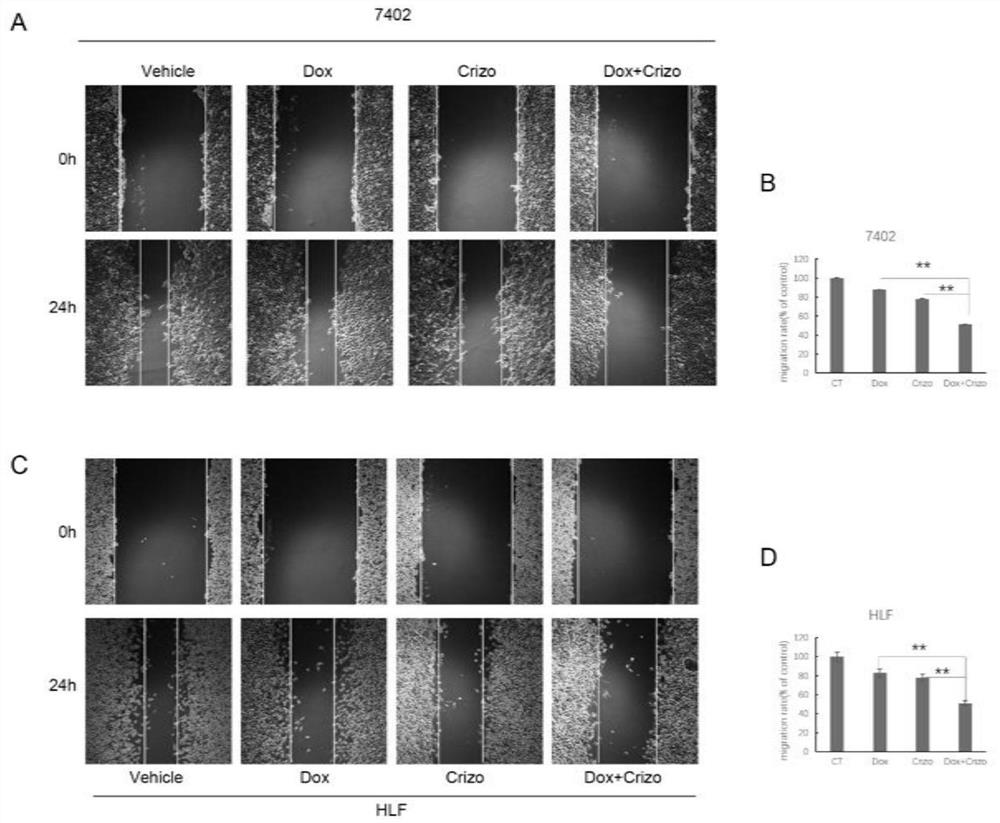
The invention belongs to the field of biological medicines, and particularly discloses a method for synergistically inducing cancer cell death through combination of Crizo and Dox. Compared with single-drug treatment, cell death is increased by at least 50% through combination of the two drugs, and the induction effect on liver cancer cell apoptosis is much higher than that of combination of first-line treatment drugs sorafenib and Dox for advanced liver cancer. The invention clarifies the mechanism of the action from the cellular level, and reveals that Crizo and Dox are combined to overcome the chemotherapeutic drug resistance by inhibiting the expression of MDR1 and inducing autophagic cell death. In-vivo experiments show that the combination of Crizo and Dox can effectively inhibit tumor growth on an animal model, has small toxic and side effects, and provides a potential treatment strategy for treating cancers, especially advanced liver cancer.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Anti-pd-l1 antibody treatment of bladder cancer
InactiveUS20190359715A1Effective treatmentLower Level RequirementsPharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAbnormal tissue growth
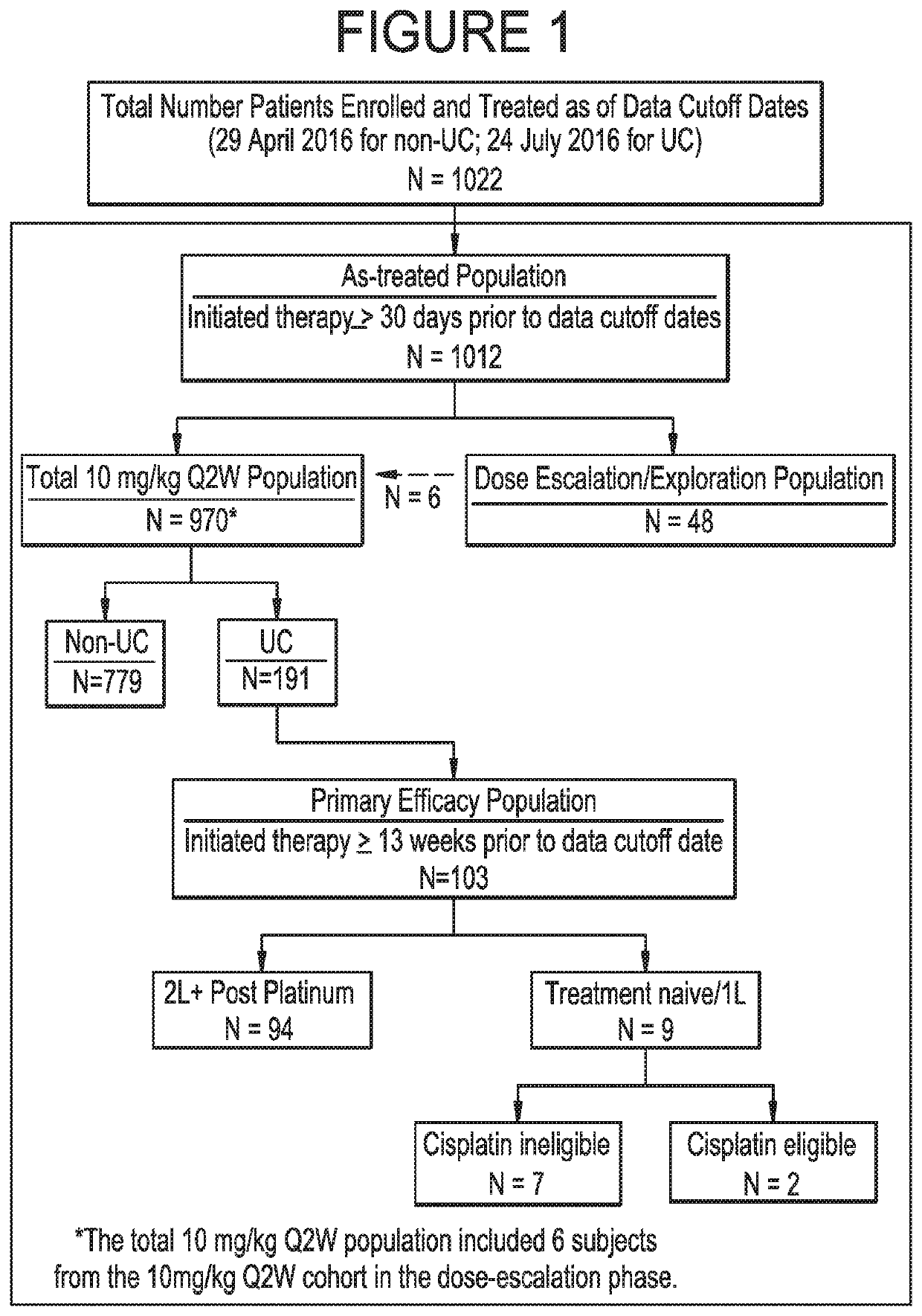
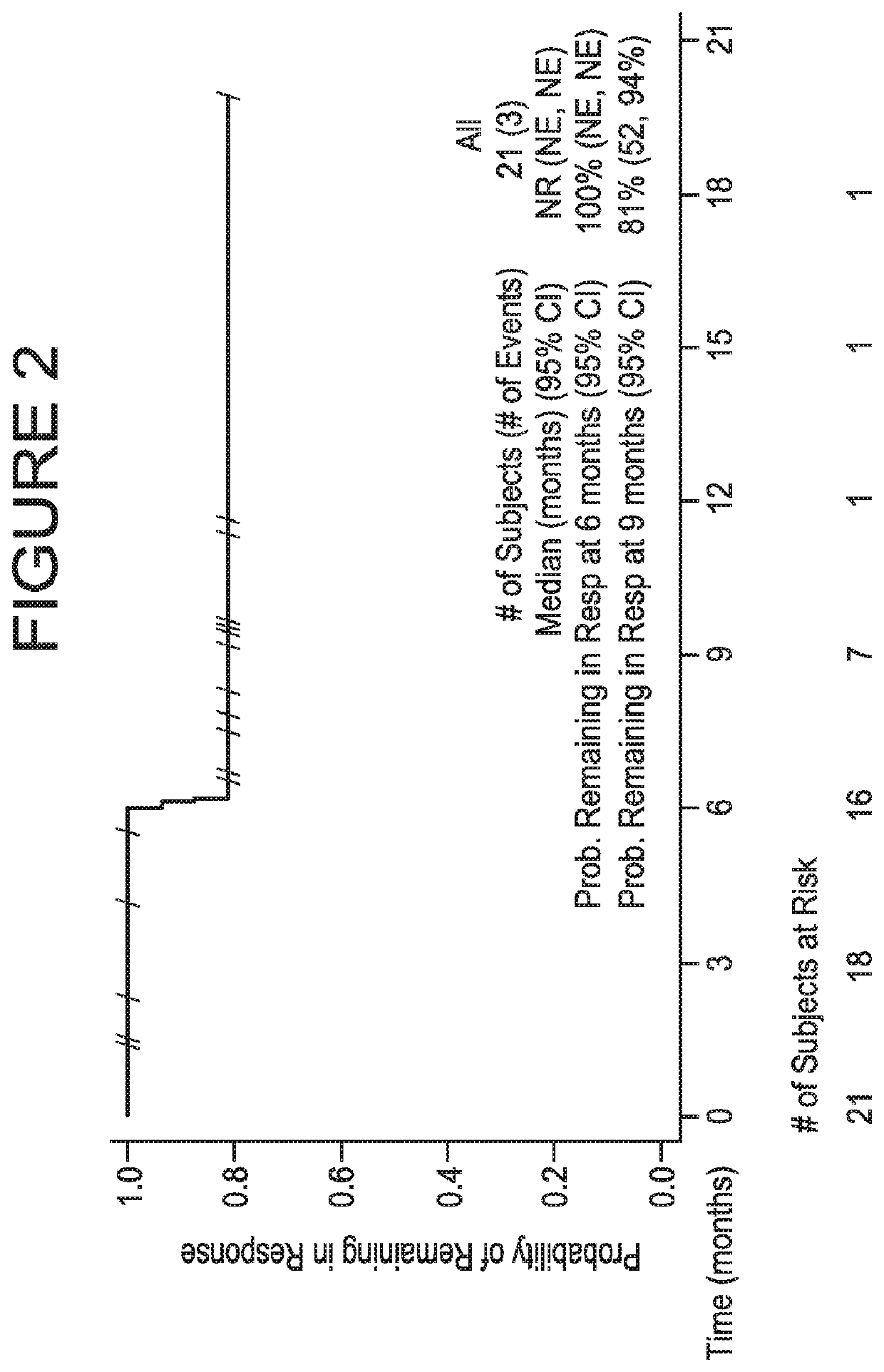
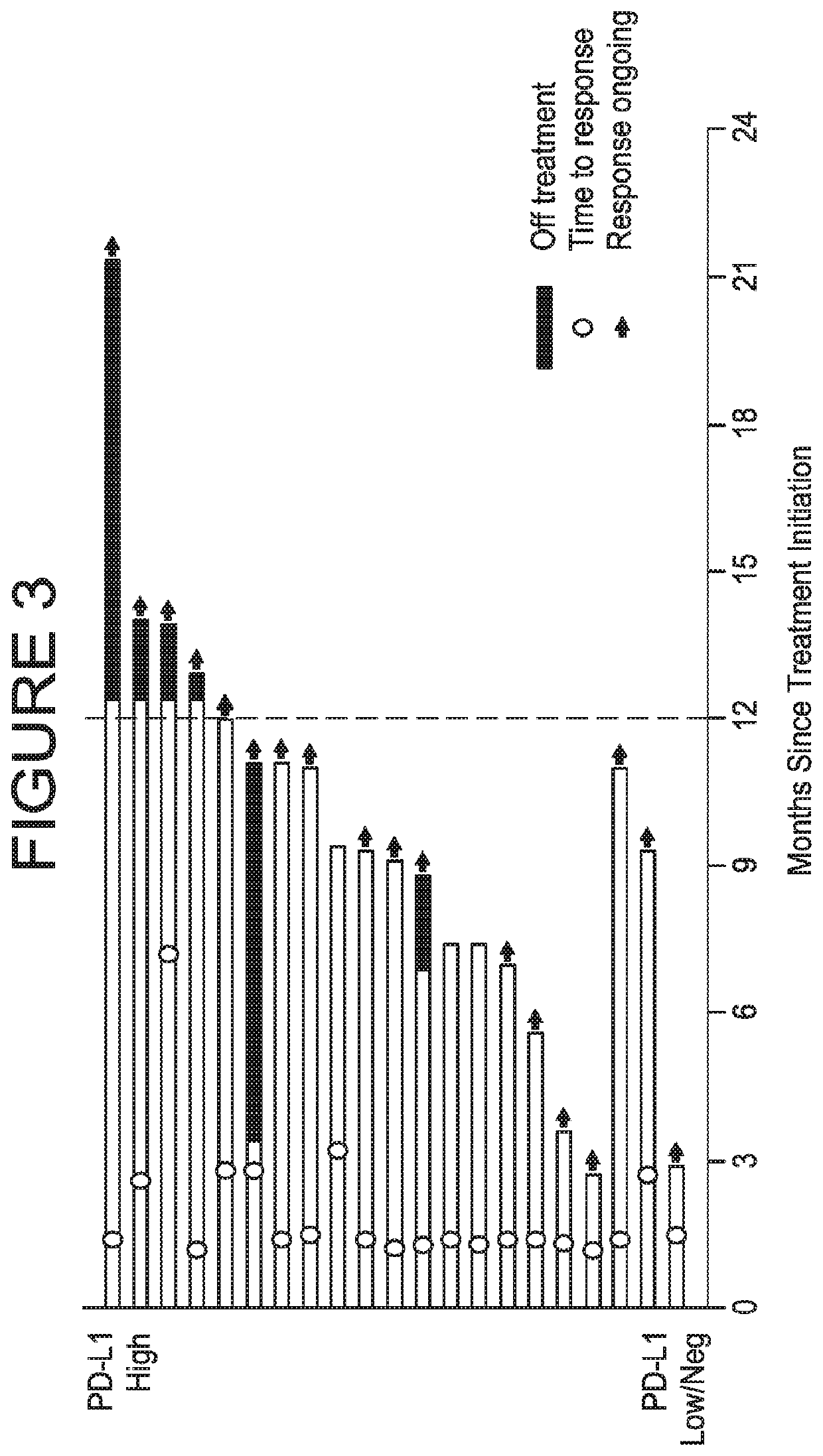
Provided are methods of treating bladder cancer (e.g., urothelial carcinoma, UC) in a subject having bladder cancer, e.g., UC, with an effective dose regimen of an anti-PD-L1 antibody, e.g., durvalumab, or an antigen binding fragment thereof. Also provided are methods in which an anti-PD-L1 antibody is used in combination with another immunotherapeutic agent, e.g., tremelimumab to treat a bladder cancer, e.g., UC, in a subject having bladder cancer. In some cases, the subject undergoing treatment is identified as having a bladder cancer or tumor that is PD-L1-low / neg, or PD-L1-high. Methods are also provided in which anti-PD-L1 antibody treatment of bladder cancer is used following a standard of care or first-line therapy in subjects who have progressed following such therapies or who have relapsed after a prior treatment regimen.
Owner:MEDIMMUNE LLC
Combination of cdk4/6 inhibitor combined with her2 inhibitor for gastric cancer treatment
ActiveCN109481687BGood benefitOrganic active ingredientsAntineoplastic agentsGastric carcinomaClinical research
The invention discloses CDK4 / 6 inhibitor and HER2 inhibitor combination for gastric cancer treatment and application of a CDK4 / 6 inhibitor and a HER2 inhibitor to preparation of a composition for treatment of HER2 positive gastric cancer patients with drug tolerance. In gastric cancer clinical research history, the patients with drug tolerance after first-line treatment are slightly benefited no matter which drug / scheme is adopted. Excellent benefits achieved by use of the treatment combination are rare, and the treatment combination is put forward for the first time on the basis of preclinical study and verified through clinical researches on patients and is a landmark major breakthrough in the field of gastric cancers.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL +1
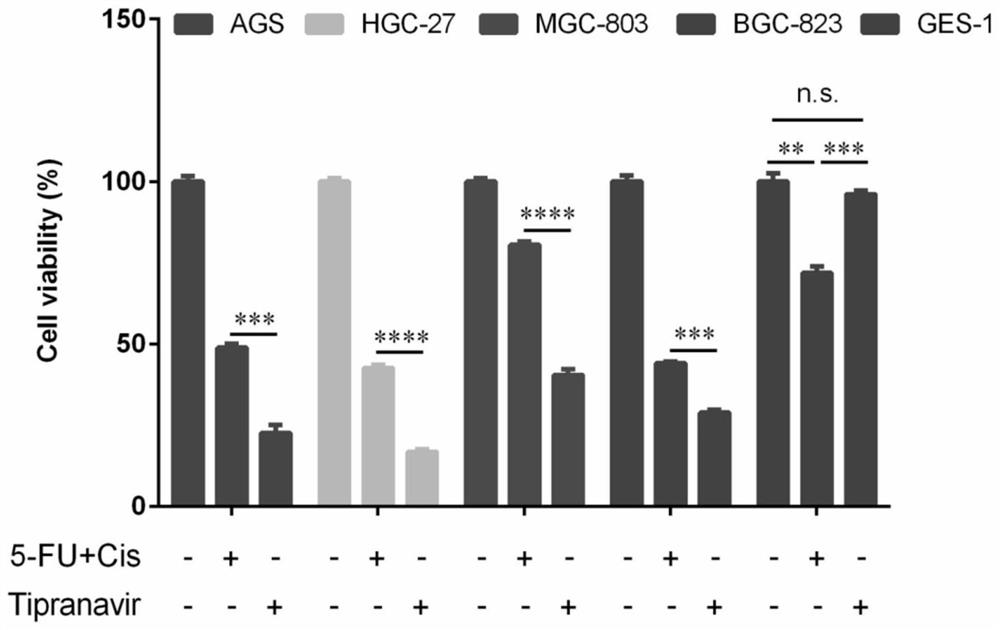
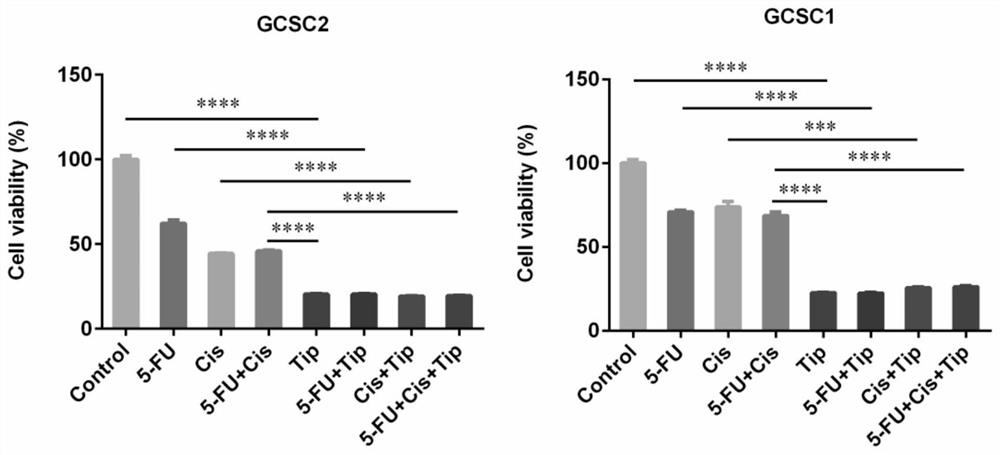
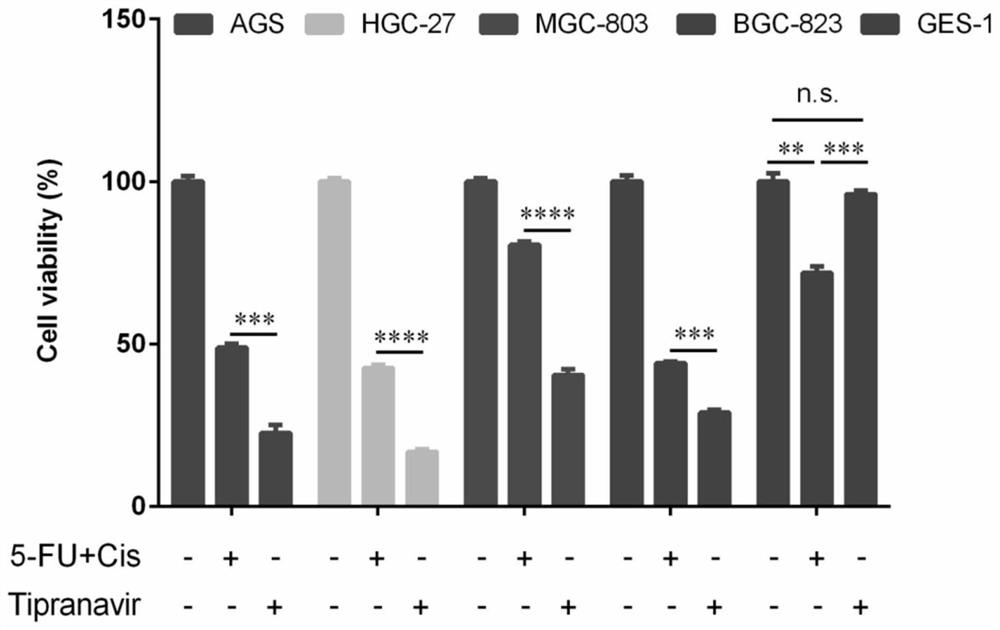
Application of Tipranavir in preparation of cancer treatment drug for killing tumor stem cells and tumor cells
ActiveCN112263578AImprove the quality of lifeExcellent anti-gastric cancer effectOrganic active ingredientsAntineoplastic agentsTumor therapyPharmaceutical medicine
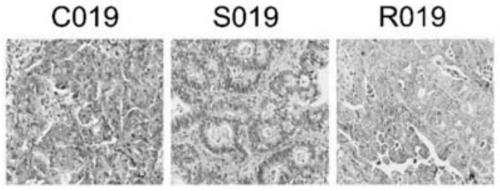
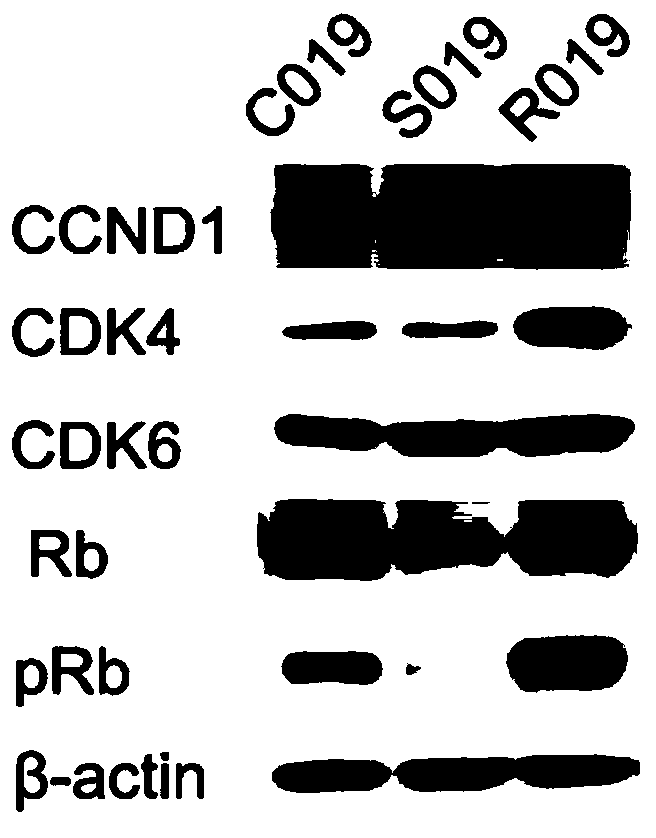
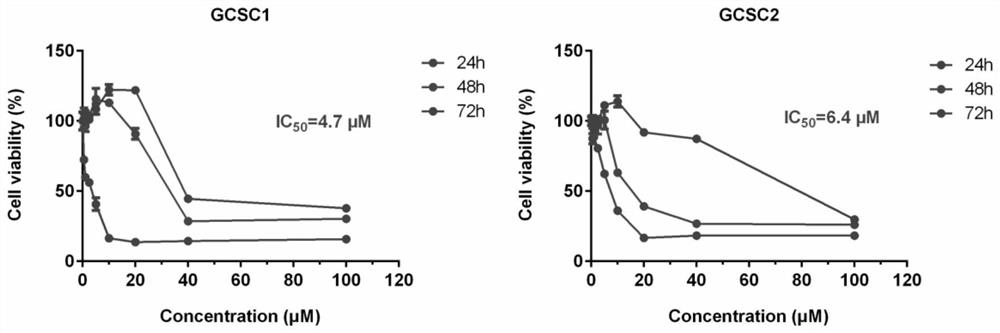
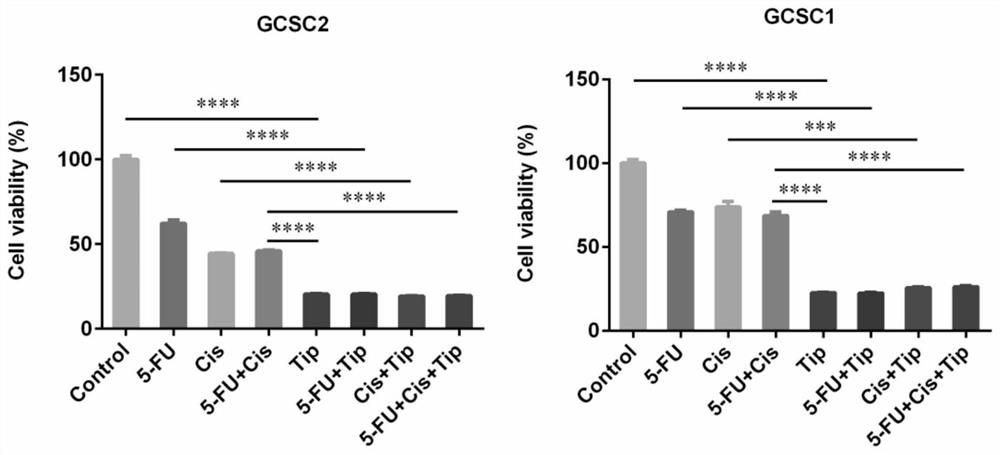
The invention discloses application of Tipranavir in preparation of a cancer treatment drug for killing tumor stem cells and tumor cells, and relates to the technical field of medicines. The inventionfurther discloses a cancer treatment drug. The drug can kill the tumor stem cells and the tumor cells; the drug takes the Tipranavir as a main active component and contains a pharmaceutically acceptable carrier. The invention provides the Tipranavir for the first time, and the Tipranavir can be used for preparing the anti-cancer drug for killing the tumor stem cells and the tumor cells; the anti-gastric cancer effect of the Tipranavir is obviously better than that of an existing gastric cancer first-line treatment drug (5-FU and cisplatin are combined); the Tipranavir has no obvious toxic side effect and the toxic side effect is obviously smaller than that of the gastric cancer first-line treatment drug (5-FU and cisplatin are combined); a novel drug and scheme are provided for tumor treatment of targeted tumor stem cells and healing of tumors; and the Tipranavir has important meanings on improvement of the tumor treatment effect and improvement of the living quality of patients.
Owner:SHENZHEN UNIV
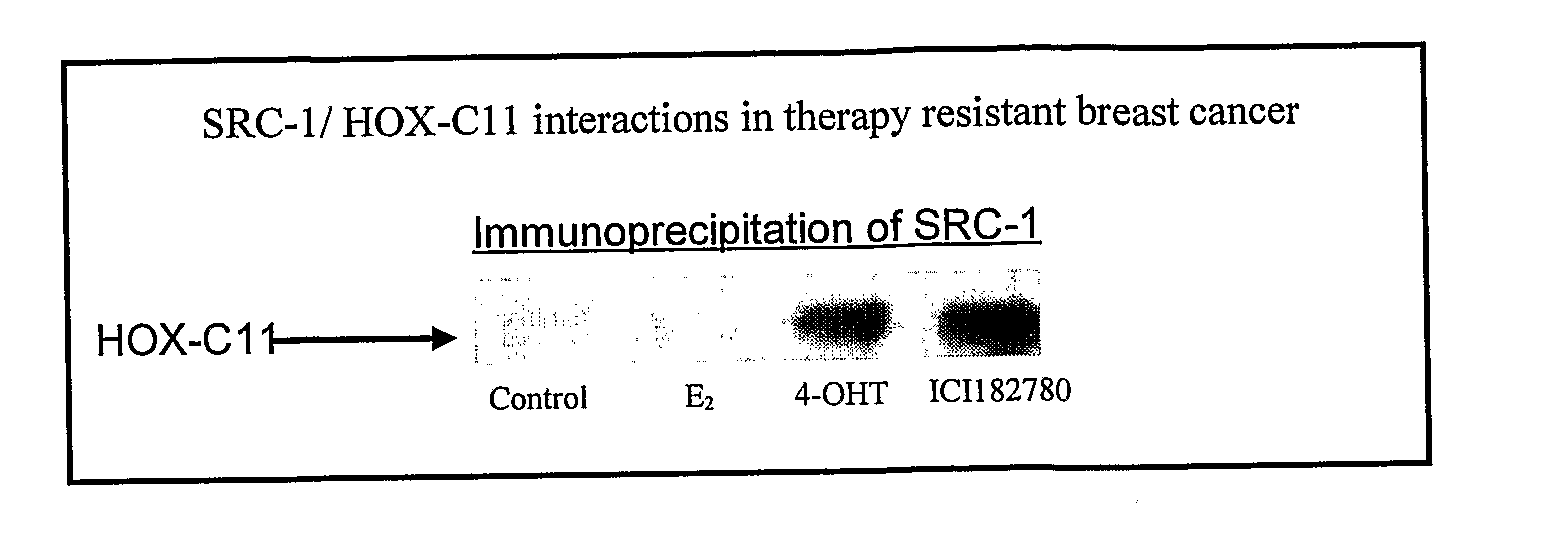
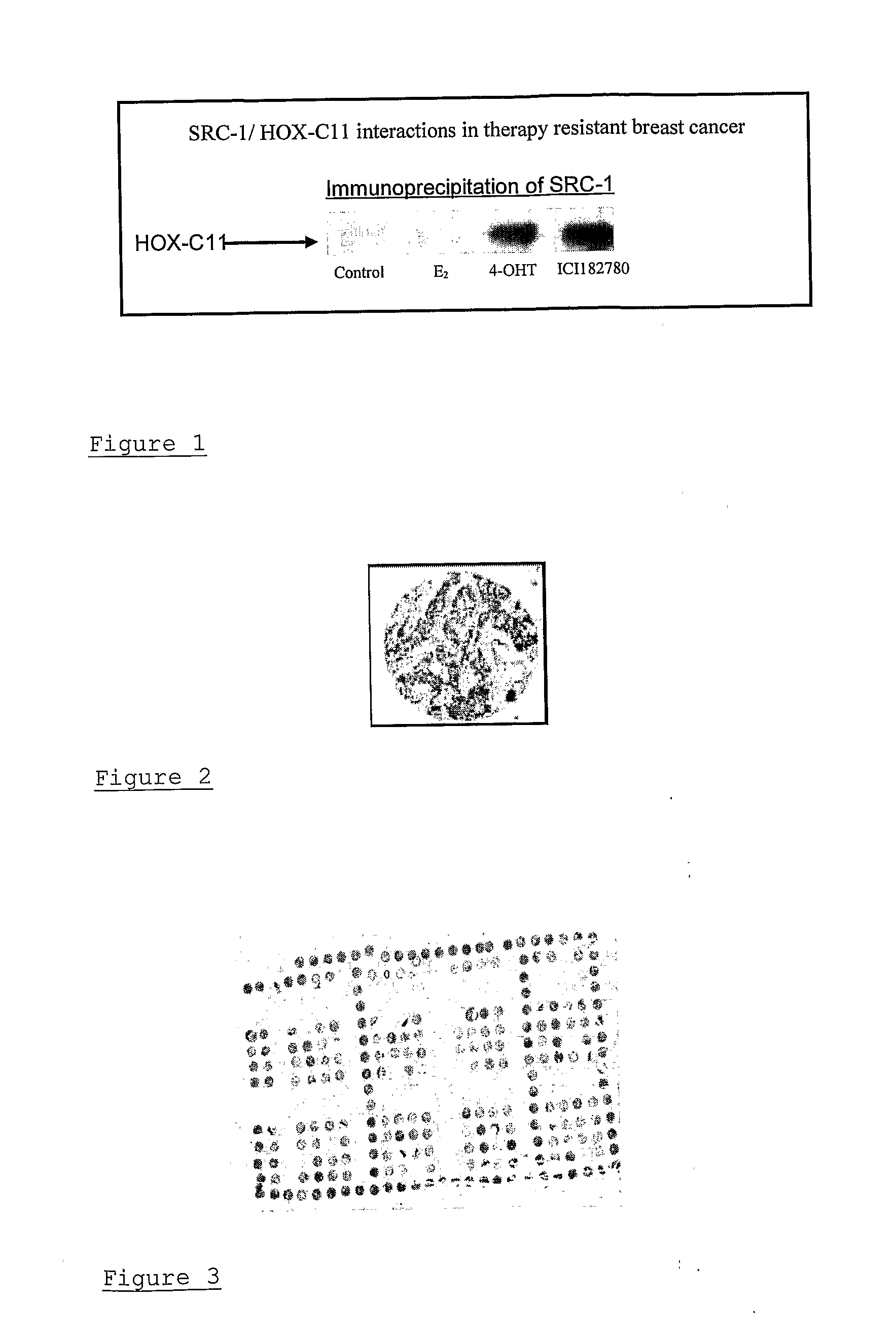
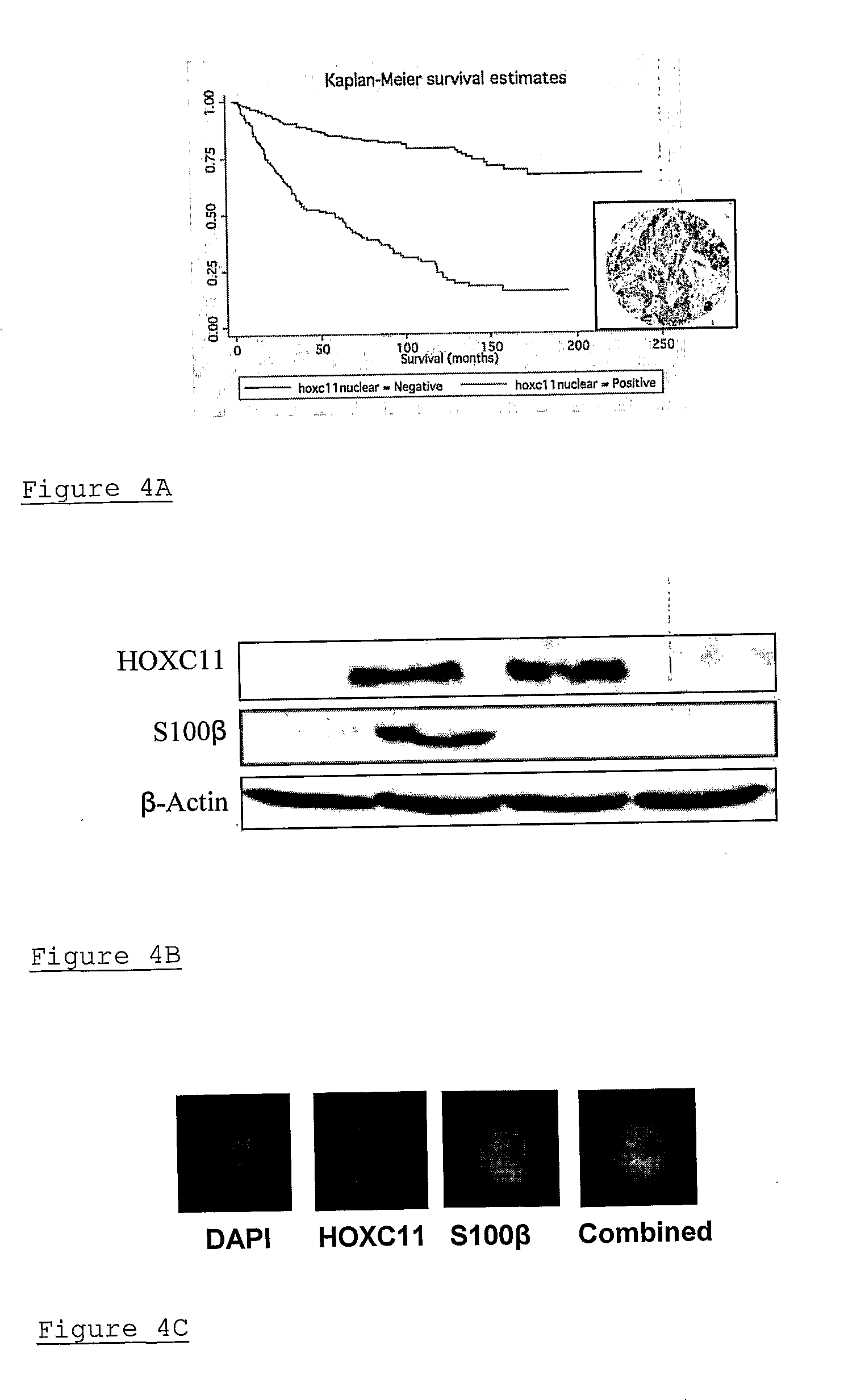
Method of assessing cancer status in a breast cancer patient
InactiveUS20110097806A1Suitable for detectionAvoid the needMicrobiological testing/measurementDisease diagnosisPositive recurrenceBiomarker (petroleum)
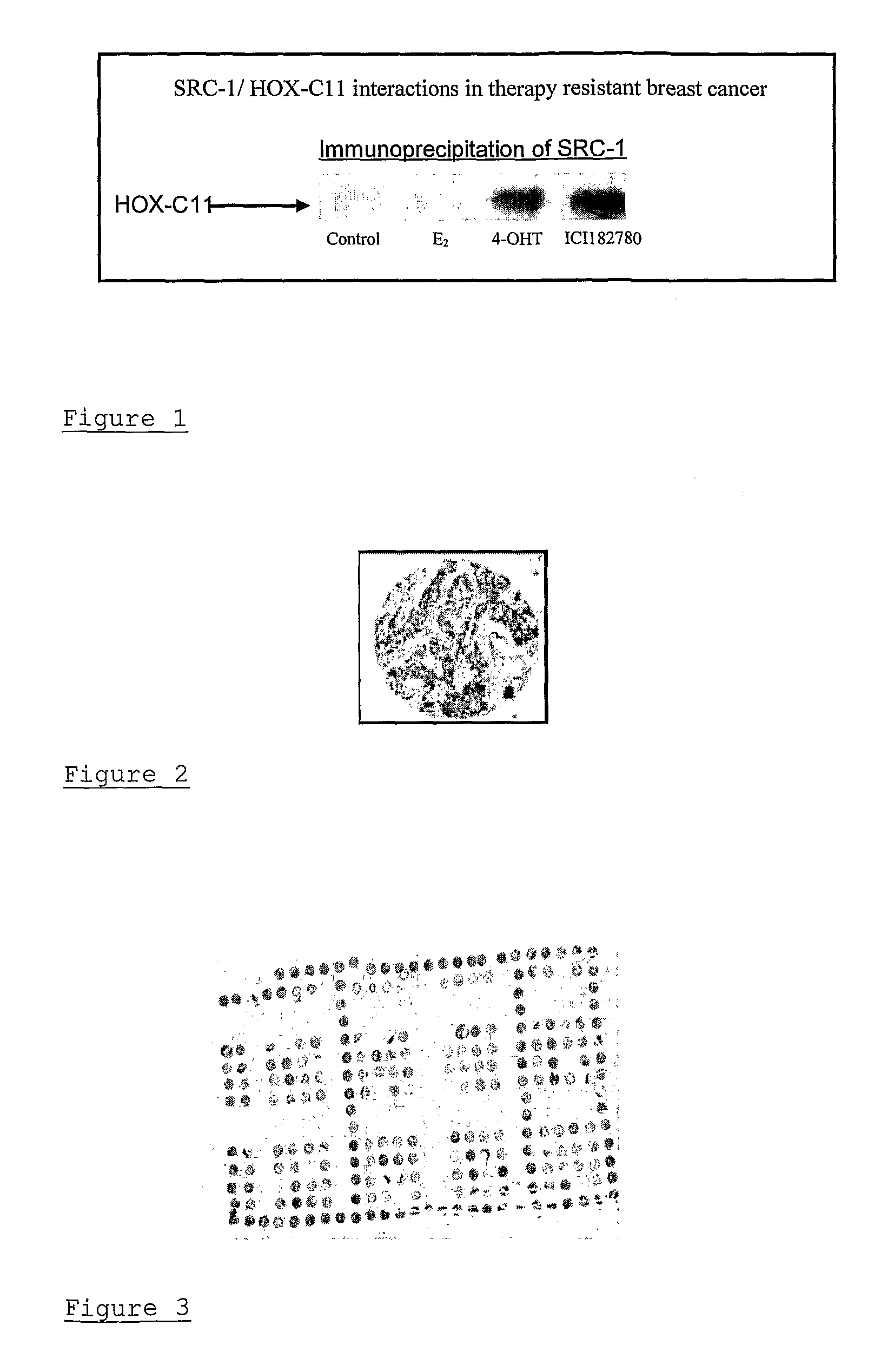
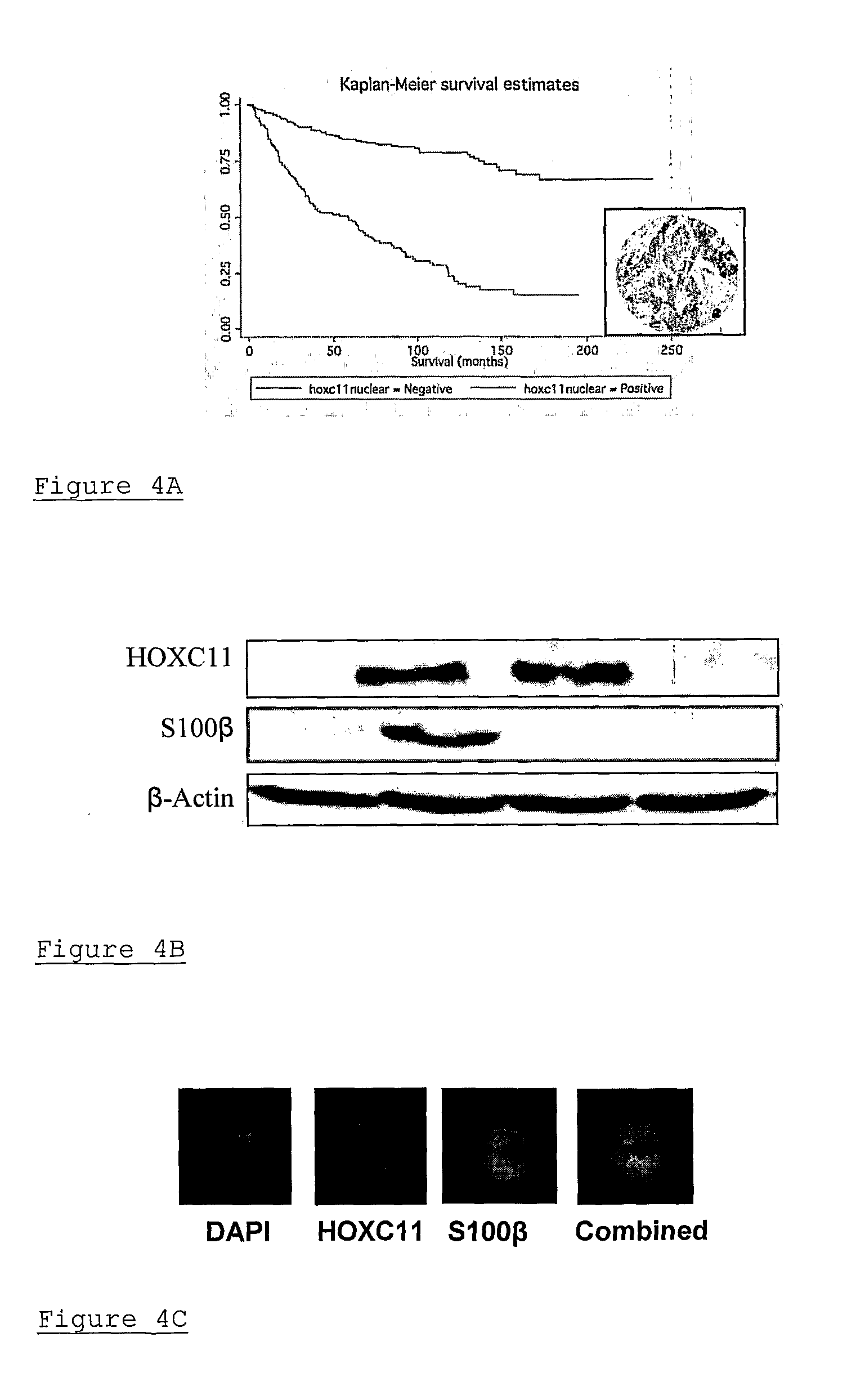
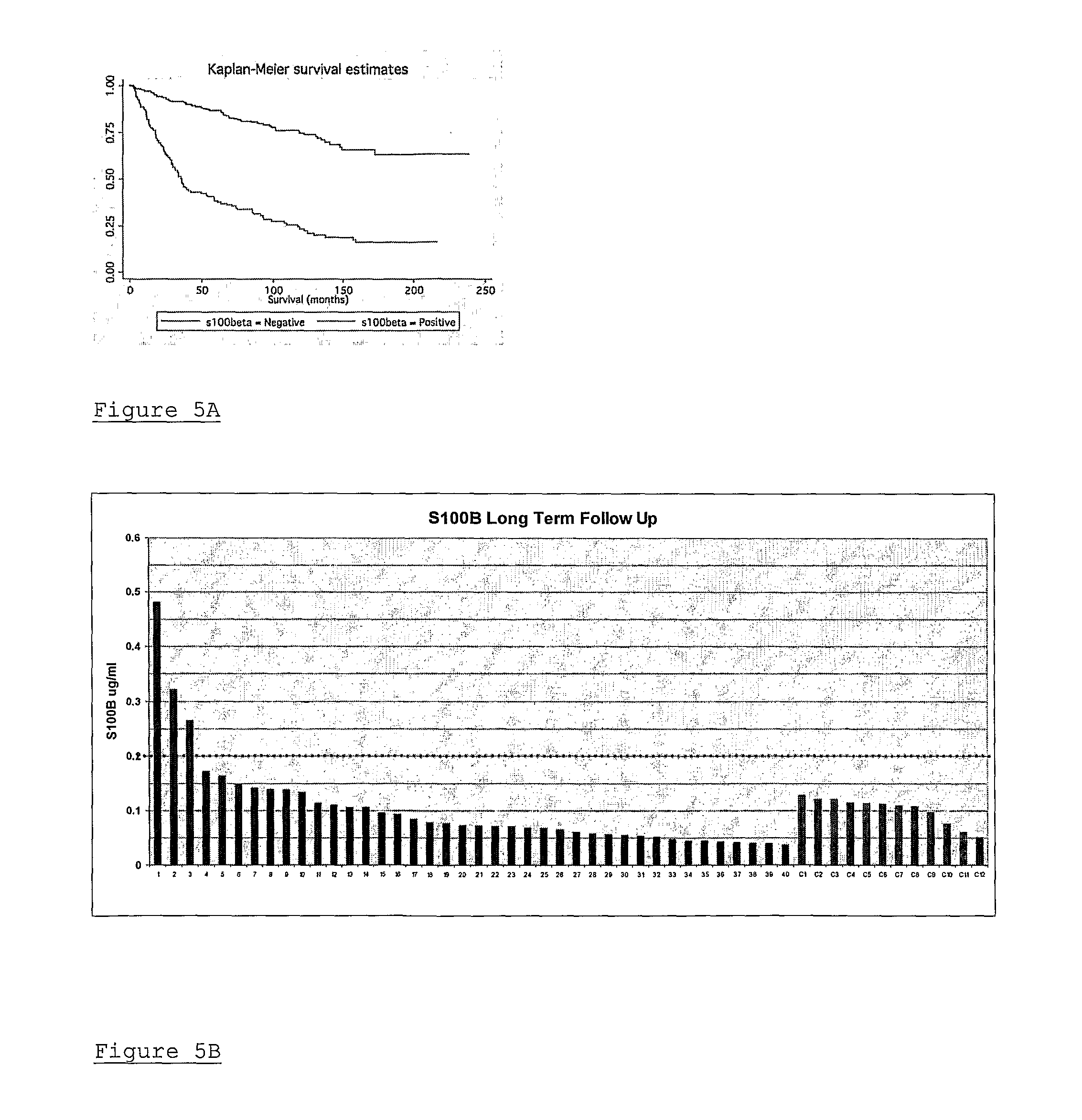
A method of assessing recurrence status in a breast cancer patient comprises a step of assaying a biological sample from the patient for a level of a biomarker selected from S100β or HOX-C1I, wherein positive detection of one or both of the biomarkers is indicative of a positive recurrence status. Suitably, the method is a method of prognosis of poor disease free survival in a breast cancer patient, wherein positive detection of one or both of the biomarkers is indicative of poor disease survival. The method may also be a method of diagnosis of recurrence, wherein positive detection of circulating S100β is a diagnostic variable of recurrence. The method of diagnosis is carried out on a patient who is undergoing first line therapy and / or a patient who has had surgery to remove a primary breast tumour.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
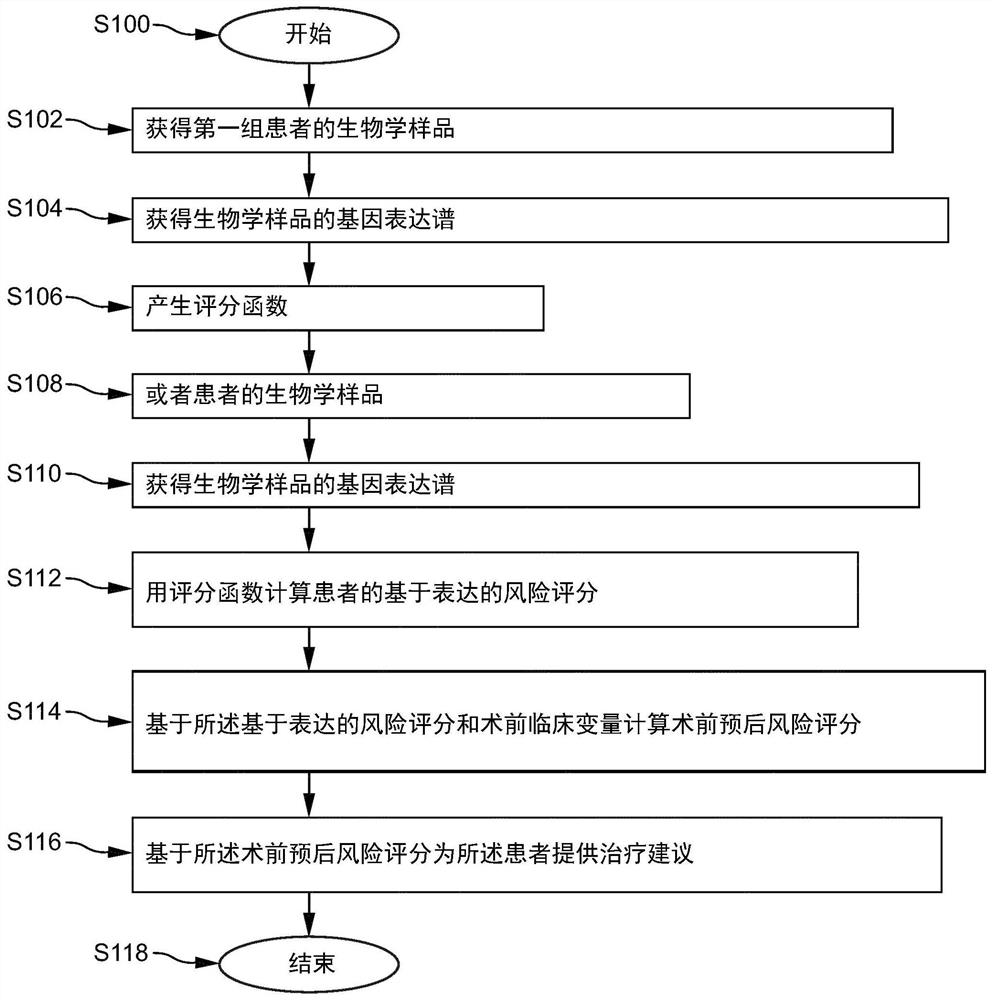
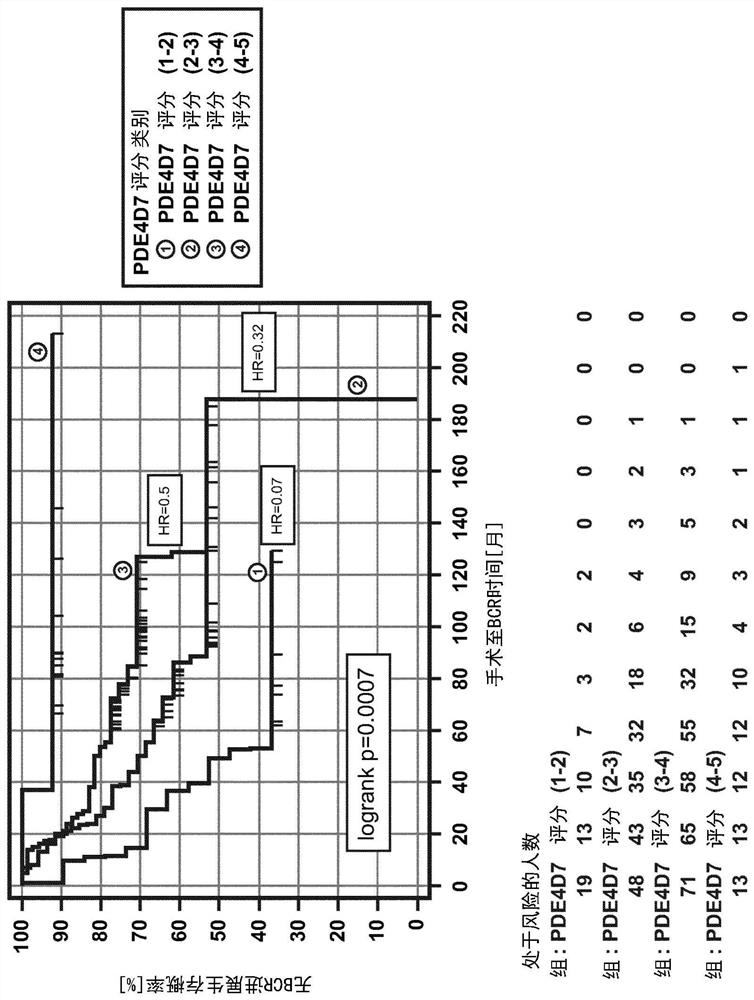
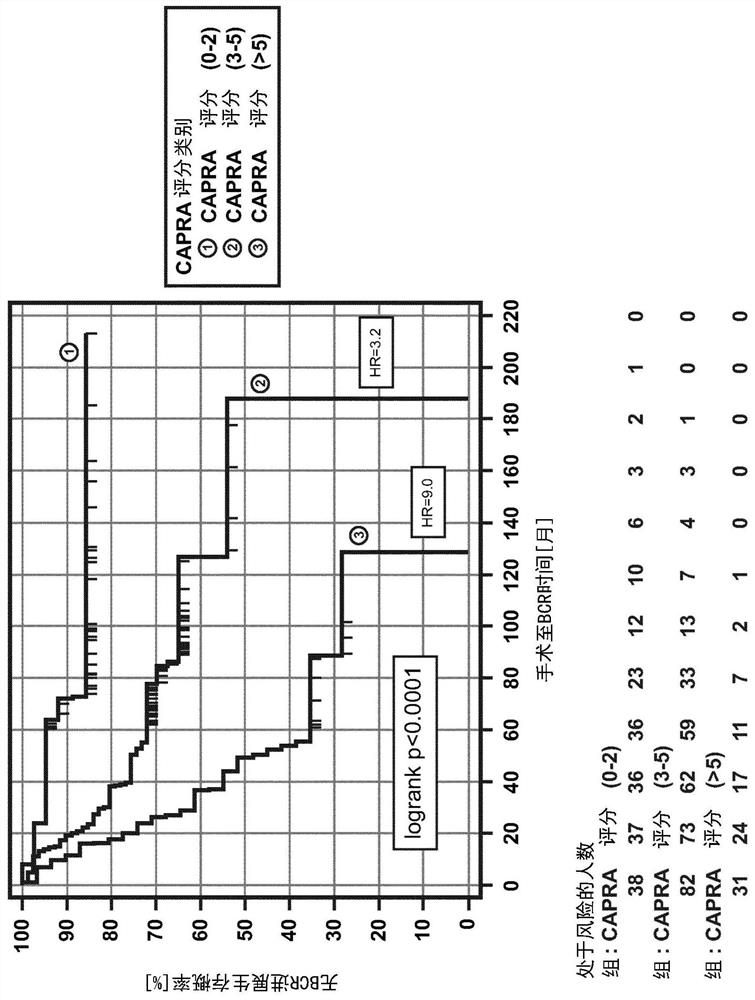
Pre-surgical risk stratification based on pde4d7 expression and pre-surgical clinical variables
PendingCN111742061AHealth-index calculationMicrobiological testing/measurementPhosphodiesteraseSurgical risk
Owner:KONINKLJIJKE PHILIPS NV
Application of cassia-twig rhizome-paridis resina-draconis extract in preparation of medicine for treating adenomyosis
ActiveCN111568989AEasy to prepareHas inhibitory effectSexual disorderPlant ingredientsCassiaPharmaceutical drug
The invention particularly relates to application of a cassia-twig rhizome-paridis resina-draconis extract in the preparation of a medicine for treating adenomyosis. In view of the current situation that no specific therapeutic medicine for the treatment of adenomyosis exists and the adenomyosis is relatively difficult to cure radically, extracts with anti- adenomyosis-activity are screened from the medicines commonly used clinically to promote blood circulation and remove blood stasis, soothe the liver and regulate qi, clarifying of the treatment mechanism of adenomyosis is facilitated, and corresponding medicines are provided for the treatment of clinical adenomyosis. The research of the invention respectively provides extracts of cassia twig, rhizoma paridis and resina draconis and combined extracts, and it is verified that the extracts have an inhibitory effect on cells of ectopic lesion of adenomyosis, and further, a mechanism of the extracts for inhibiting lesion tissues is further provided. The extracts of cassia twig, rhizoma paridis and resina draconis and the combined extracts are expected to become active ingredients for the treatment of clinical adenomyosis, and are ofgreat significance for the development of first-line therapeutic medicines.
Owner:THE AFFILIATED HOSPITAL OF SHANDONG UNIV OF TCM
Human small cell lung cancer cell strain with combined drug resistance to etoposide and carboplatin as well as establishment method and application thereof
ActiveCN113234678ACell traits are stableStable traitsCompounds screening/testingCompound screeningCarboplatinPharmacology
The invention provides a human small cell lung cancer cell strain with combined drug resistance to etoposide and carboplatin as well as a preparation method and application thereof, wherein the human small cell lung cancer cell strain is preserved in the China Center for Type Culture Collection, and the preservation number is CCTCC No: C202168. The small cell lung cancer cell strain is stable in character, can be stably passed for multiple times, and can be used for generating human small cell lung cancer in mammals and preparing a human small cell lung cancer model; the cell is tolerant to combined medicine of etoposide and carboplatin which are first-line treatment medicines for small cell lung cancer, and can be used for researching a drug-resistant molecular mechanism of small cell lung cancer and candidating the drugs for treating drug-resistant human small cell lung cancer are screened. According to the invention, a new experimental material closer to clinical tumor biological characteristics is provided for small cell lung cancer research.
Owner:CENT SOUTH UNIV
Application of Guizhi Zhonglou Xuejie Extract in the Preparation of Drugs for Adenomyosis
ActiveCN111568989BEasy to prepareHas inhibitory effectSexual disorderPlant ingredientsBULK ACTIVE INGREDIENTBiology
The invention specifically relates to the application of the extract of cassia twigs and rhizomes in the preparation of medicines for treating adenomyosis. Aiming at the current situation that there is no specific drug for the treatment of adenomyosis and it is difficult to cure it, the purpose of the present invention is to screen the extracts with anti-adenomyosis activity from the drugs commonly used in clinical practice to promote blood circulation, remove blood stasis, soothe the liver and regulate qi. It helps to clarify the treatment mechanism of adenomyosis and provide corresponding drugs for the clinical treatment of adenomyosis. The research of the present invention provides the extracts of cinnamon twig, chrysanthemum, and jerky root, and combined extracts, and verifies that the above-mentioned extracts have an inhibitory effect on the ectopic lesion cells of adenomyosis. Further, it also provides the above-mentioned extracts that inhibit Mechanism of focal tissue. The extracts of cassia twig, chrysanthemum, japonicus and combined extracts provided by the present invention are expected to become active ingredients for the clinical treatment of adenomyosis, and are of great significance to the development of first-line therapeutic drugs.
Owner:THE AFFILIATED HOSPITAL OF SHANDONG UNIV OF TCM
Method of assessing cancer status in a breast cancer patient
InactiveUS8501483B2Avoid the needSuitable for detectionMicrobiological testing/measurementDisease diagnosisPositive recurrenceDisease
Described are methods for assessing recurrence status in a breast cancer patient that include assaying a biological sample from the patient for a level of a biomarker selected from such as S100β or HOX-C1I, where positive detection of one or both of the biomarkers indicates a positive recurrence status. The method can be used for prognosis of poor disease free survival in a breast cancer patient, where positive detection of one or both of the biomarkers indicates poor disease survival. The method may also be used for diagnosis of recurrence, where positive detection of circulating S100β is a diagnostic variable of recurrence. The method of diagnosis is carried out on a patient who is undergoing first line therapy and / or a patient who has had surgery to remove a primary breast tumour.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Use of tipranavir in the preparation of cancer therapeutic drugs that kill tumor stem cells and tumor cells
ActiveCN112263578BImprove the quality of lifeExcellent anti-gastric cancer effectOrganic active ingredientsAntineoplastic agentsTumor targetTumor therapy
The invention discloses the use of Tipranavir in the preparation of cancer treatment drugs for killing tumor stem cells and tumor cells, and relates to the technical field of medicine. The invention also discloses a cancer treatment drug, which can kill tumor stem cells and tumor cells; the drug uses Tipranavir as the main active ingredient and contains a pharmaceutically acceptable carrier. The present invention provides for the first time that Tipranavir can be used to prepare an anticancer drug that kills tumor stem cells and tumor cells. Its anti-gastric cancer effect is significantly better than the existing first-line treatment drug for gastric cancer (combined use of 5-FU and cisplatin), and has no obvious side effects. The toxic and side effects are significantly less than the first-line treatment of gastric cancer (5-FU and cisplatin combined), which will provide new drugs and solutions for tumor treatment targeting tumor stem cells and cure tumors, and have a positive effect on improving the effect of tumor treatment and the quality of life of patients. important meaning.
Owner:SHENZHEN UNIV
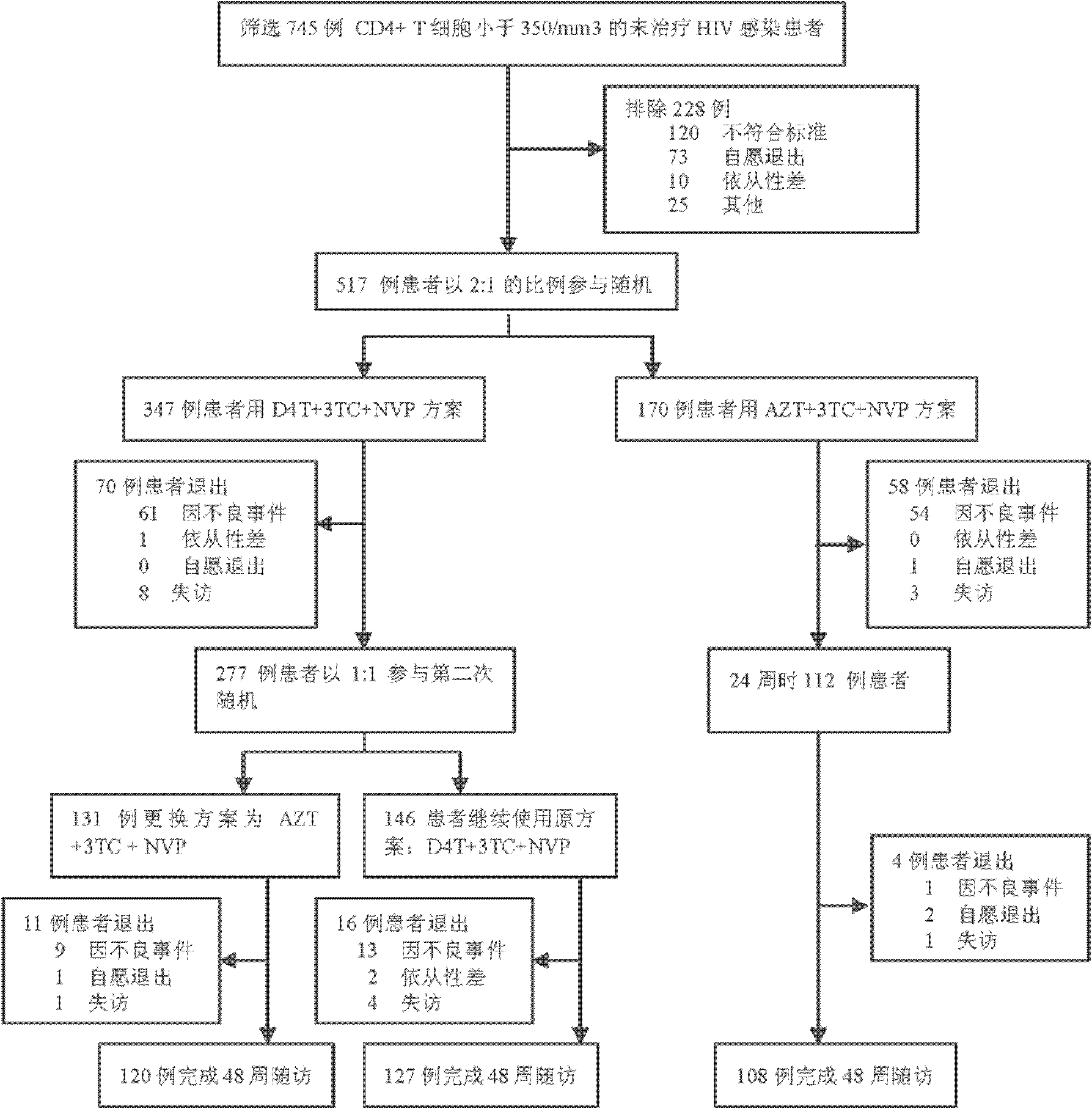
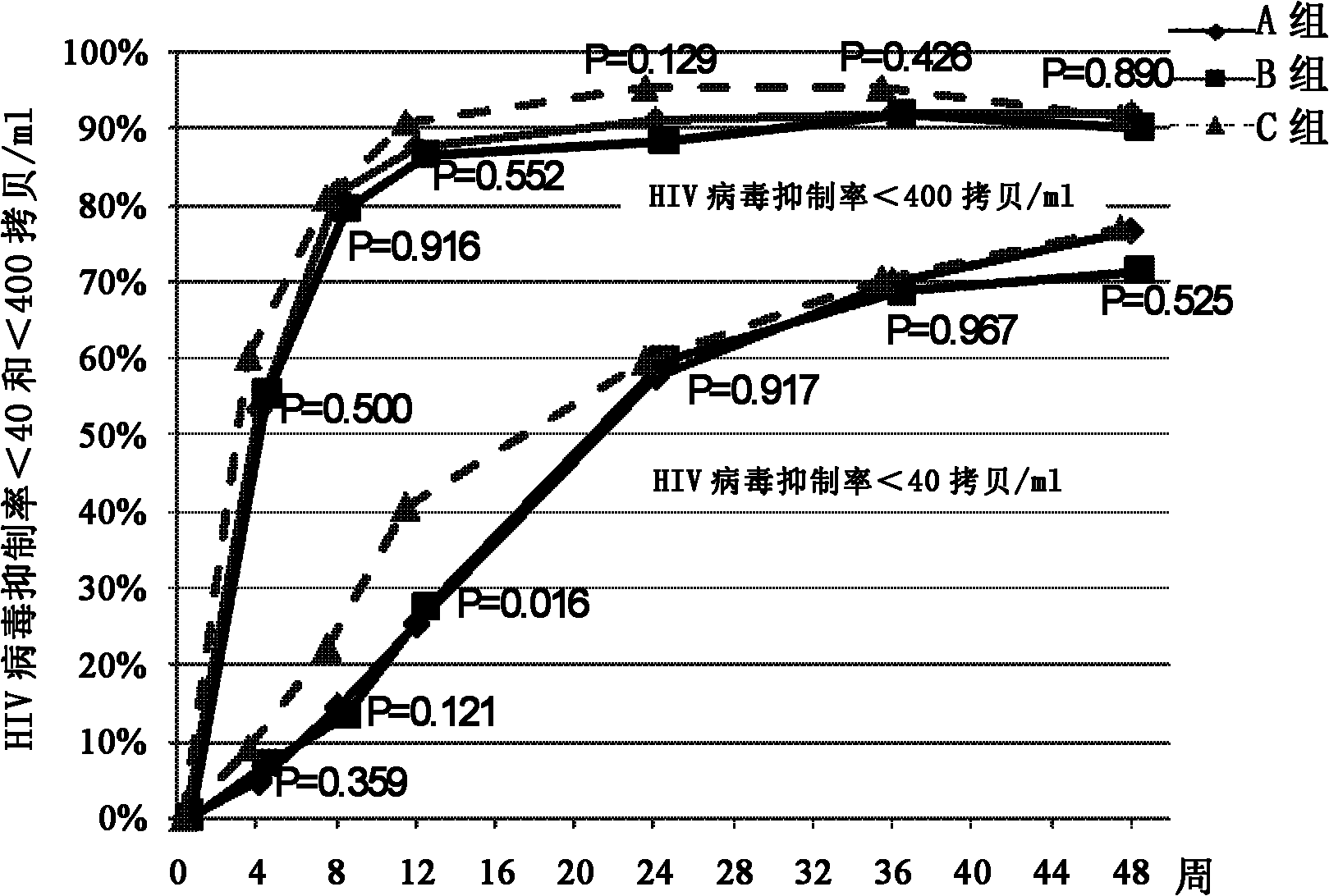
Optimized adult first-line AIDS antiviral therapy program
InactiveCN102727512AEffective treatmentLittle side effectsOrganic active ingredientsAntiviralsStage iibSide effect
The invention provides an optimized adult first-line AIDS (Acquired Immune Deficiency Syndrome) antiviral therapy program. Concretely, the therapy program of the invention includes (a) a first stage: employing the stavudine-containing (D4T) program to treat for a period of time (such as 4-12 months); and (b) a second stage: replacing stavudine with zidovudine (AZT) for treatment. The therapy program of the invention not only gets a good therapeutic effect, but also can reduce the toxic and side effects of D4T and AZT, and is an equivalent and relatively safe first-line therapy program.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
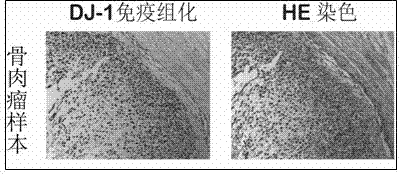
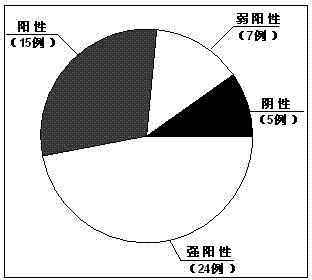
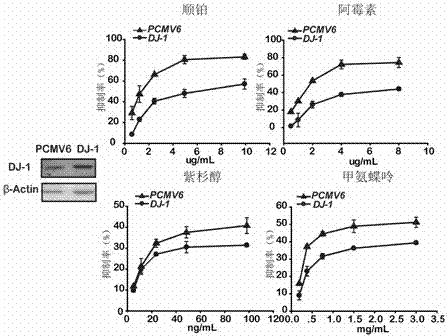
Application of DJ-1 protein in preparation of product for diagnosis and therapy of osteosarcoma
InactiveCN103585619AImprove accuracyReduce sensitivityPeptide/protein ingredientsIn-vivo testing preparationsNew medicationsPharmaceutical drug
The invention provides application of a DJ-1 protein in preparation of products for diagnosis and therapy of osteosarcoma, and the amino acid sequence of the DJ-1 protein is shown as SEQID No:1. Experiments show that the expression of the DJ-1 protein in an osteosarcoma tissue is significantly higher than that in a non tumor tissue, and over expression (knockout) of the DJ-1 protein in osteosarcoma cells can significantly reduce (increase) the sensitivity of the osteosarcoma cells to first-line treatment drugs, so that the DJ-1 protein can be used as a specific marker protein for diagnosis and therapy of the osteosarcoma, and can improve the accuracy of the diagnosis of the osteosarcoma and the effectiveness of the therapy of the osteosarcoma. The DJ-1 protein provides a new therapeutic target and effective new drugs for the prevention and therapy of the osteosarcoma.
Owner:ZHEJIANG UNIV
Synergistic combined medicine for treating advanced lung cancer by combining anlotinib with immune checkpoint inhibitor
PendingCN113925865AGood treatment toleranceHigh remission rateOrganic active ingredientsAntibody ingredientsDiseaseTolerability
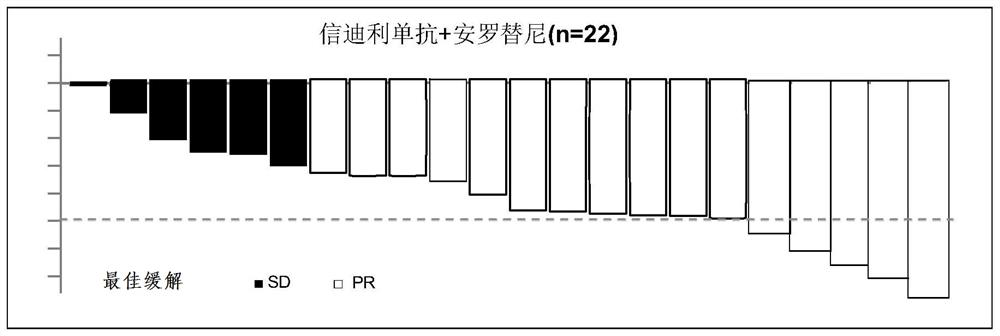
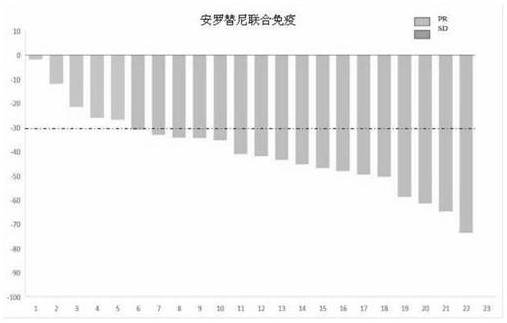
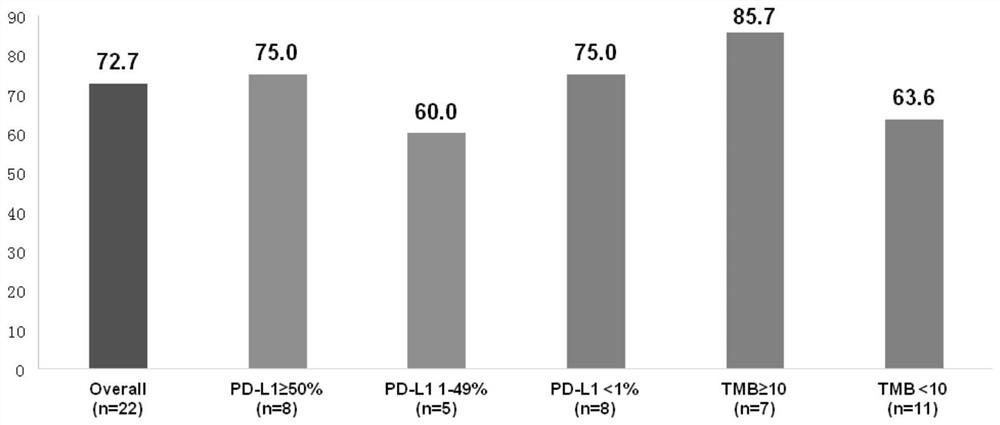
The invention belongs to the technical field of medicines, relates to a synergistic medicine of an anti-tumor medicine, and particularly relates to a synergistic combined medicine for treating the advanced lung cancer by combining anlotinib with an immune checkpoint inhibitor, in particular to a synergistic medicine composition for treating the advanced lung cancer, which is composed of the immune checkpoint inhibitor including Czodilithiumab (PD-1 and PD-L1) and the anlotinib. Random contrast research on first-line treatment of the advanced lung cancer shows that the synergistic combined medicine disclosed by the invention has a good effect on treatment of the advanced lung cancer and especially the anti-tumor activity, wherein the objective remission rate (ORR) reaches up to 72, 7%, the disease control rate (DCR) reaches 100%, all biomarker subgroups obtain high remission rates, and the survival-data pre-estimate 6-month progression-free survival (PFS) rate is 93.8%; and the synergistic combined medicine has good treatment tolerance, no unexpected toxicity is observed, no unexpected adverse event is increased, the synergistic combined medicine has good safety, and can be beneficial for being used as a first-line treatment intervention scheme for the advanced or metastatic lung cancer.
Owner:SHANGHAI CHEST HOSPITAL
Composition for treating liver cancer and its application
ActiveCN112870194BGrowth inhibitionPromote accumulationOrganic active ingredientsDigestive systemCancer cellSide effect
The invention belongs to the field of biomedicine and specifically discloses that Crizo combined with Dox synergistically induces cancer cell death. Compared with single-drug treatment, the combination of the two drugs increased cell death by at least 50%, which was much stronger than the combination of sorafenib and Dox, the first-line treatment drugs for advanced liver cancer, in inducing apoptosis of liver cancer cells. The present invention clarifies the mechanism of the above effects from the cellular level, and reveals that Crizo combined with Dox can overcome the resistance to chemotherapeutic drugs by inhibiting the expression of MDR1 and inducing autophagic cell death. Combined with in vivo experiments, it shows that Crizo combined with Dox can effectively inhibit tumor growth in animal models with less toxic side effects, which provides a potential therapeutic strategy for the treatment of cancer, especially advanced liver cancer.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Traditional Chinese medicine composition for treating allergic rhinitis and application thereof
ActiveCN112641822ADecreased secretory mucin MUC5ACImprove pathologyOrganic active ingredientsAerosol deliveryExperimental drugSide effect
The invention discloses a traditional Chinese medicine composition for treating allergic rhinitis and application thereof. The traditional Chinese medicine composition is prepared from eugenol and total saponins of radix astragali seu hedysari. A nasal spray prepared from the traditional Chinese medicine composition can be used for treating allergic rhinitis through local nasal administration, has an experimental drug effect superior to that of a first-line treatment drug, takes effect quickly, can take effect after being sprayed for 1-3 minutes, reduces nasal obstruction, running nose and the like, has no side effect, and can achieve a radical treatment effect on part of people. After the nasal spray is administrated to rats, the results show that the nasal spray for the corydalis bungeana group is superior to triamcinolone acetonide (positive control group) in the aspects of reducing a main allergic medium IgE, reducing nasal mucous membrane secretory mucoprotein MUC5AC and improving rhinitis pathology, the eugenol and the total saponins of radix astragali seu hedysari are matched to play a synergistic effect, and the effect exceeds that of single eugenol and single total saponins of radix astragali seu hedysari.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Application of gamboge and disulfiram composition in preparation of medicine for treating colon cancer
ActiveCN114306414AReduce dosageEasy dischargeOrganic active ingredientsDigestive systemMetaboliteSecond line treatment
The invention discloses application of a gamboge and disulfiram composition in preparation of a medicine for treating colon cancer, and relates to application of the gamboge and disulfiram composition in preparation of the medicine for treating the colon cancer. The invention aims to provide a low-toxicity second-line therapeutic drug after a first-line therapeutic drug for treating liver metastasis of intestinal cancer is resistant to drugs. A mouse colorectal cancer CT26.WT cell with a mutated KRAS gene is planted in the spleen of a mouse to establish a colorectal cancer liver metastasis model, the effect of the gamboge and disulfiram combined drug in treating colorectal cancer liver metastasis is studied, gambogic acid and disulfiram metabolite diethyl thiocarbamic acid can be subjected to a Michael addition reaction in gastric juice, a fat-soluble compound is formed, the lipid-soluble compound can be used for treating colorectal cancer liver metastasis, and the effect of treating colorectal cancer liver metastasis is achieved. The gambogic acid is not easy to be discharged out of a body, the pharmacokinetics of the gambogic acid is improved, the gambogic acid can be reduced into gambogic acid through in-vivo metabolism, and the effect of synergistically resisting intestinal cancer liver The gamboge and the disulfiram can achieve a synergistic effect, and the anti-tumor effect is remarkably improved. The method is applied to the field of medicine preparation.
Owner:NORTHEAST FORESTRY UNIVERSITY
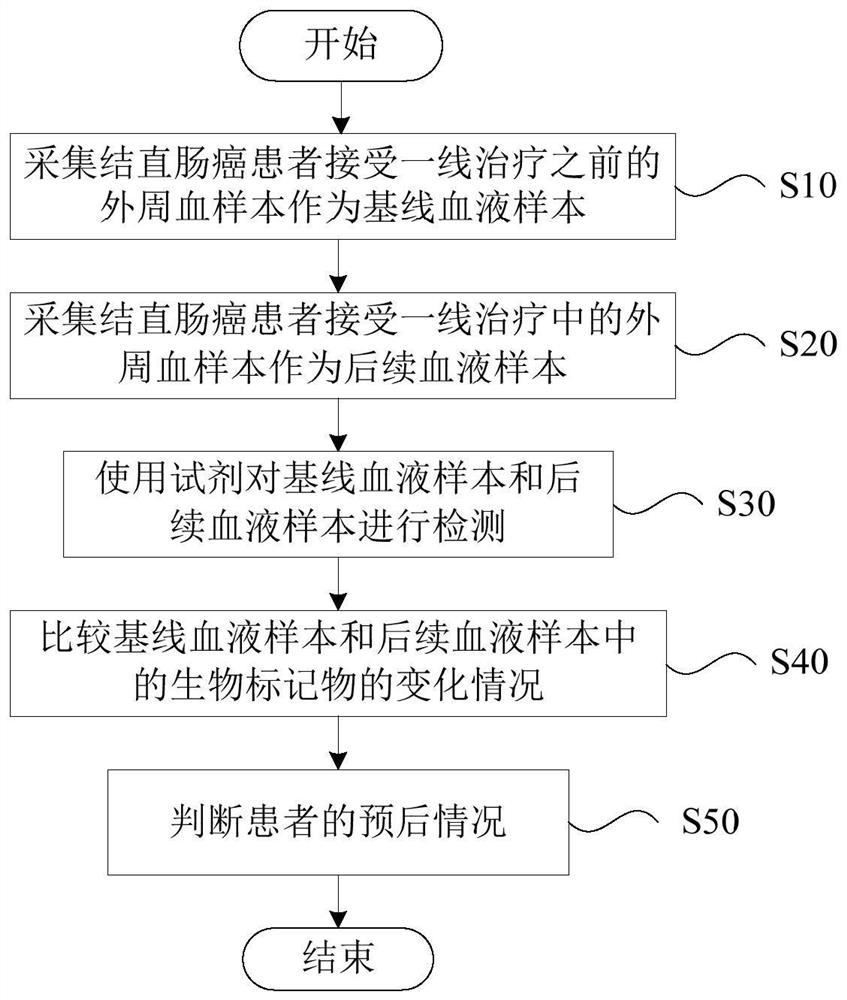
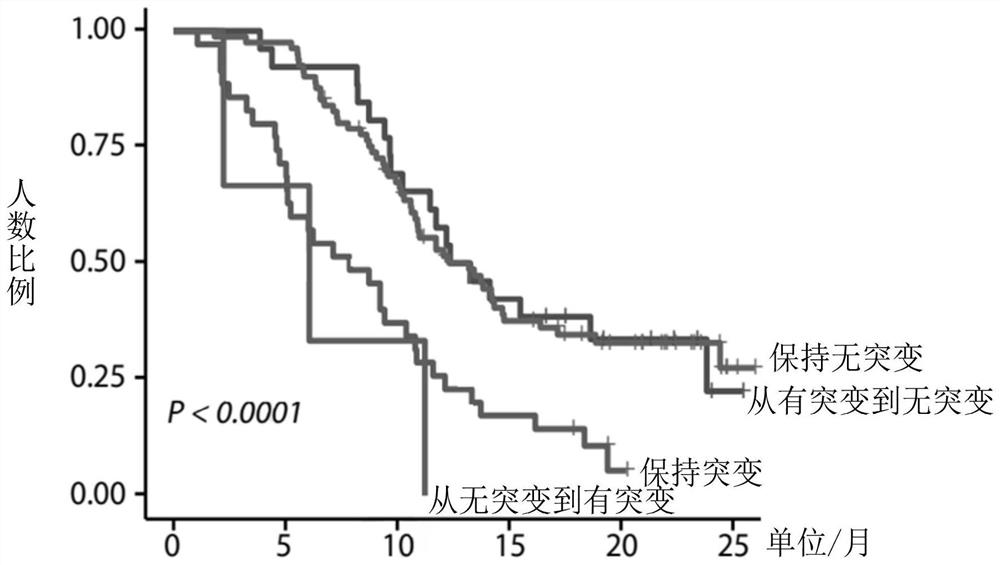
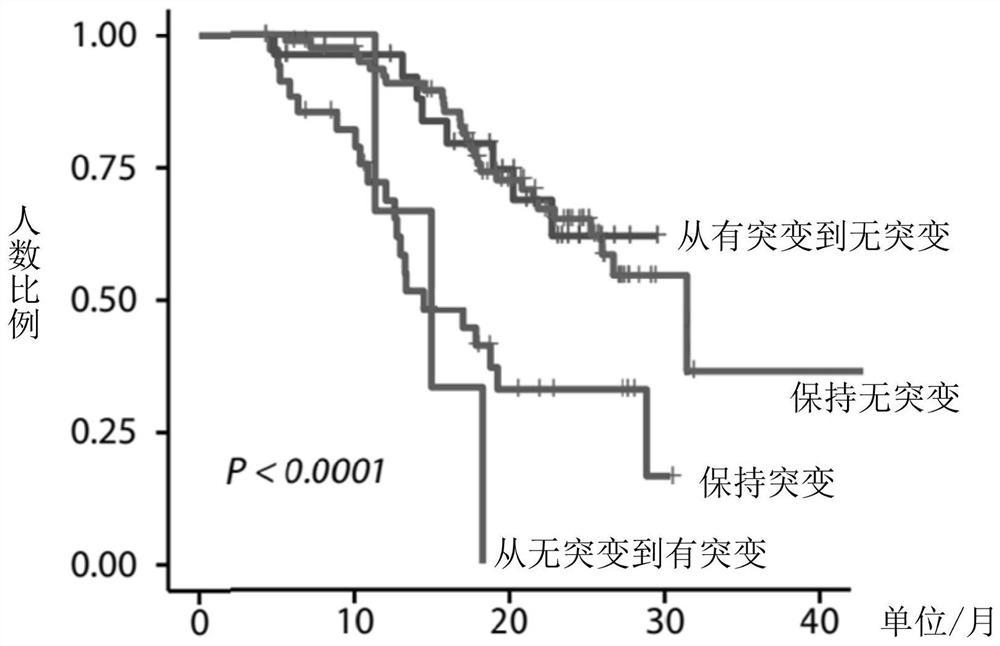
Application of biomarker in preparation of reagent for predicting prognosis of colorectal cancer patient
The invention discloses application of a biomarker in preparation of a reagent for predicting prognosis of a colorectal cancer patient. The biomarker related to the reagent comprises a KRAS gene, an NRAS gene, an HRAS gene and a BRAF gene, can be used for detecting the mutation state of the biomarker in circulating tumor DNA in peripheral blood, and is applied to predicting prognosis of a metastatic colorectal cancer patient receiving first-line treatment. According to the invention, the application of the biomarker in preparation of the reagent for predicting prognosis of the colorectal cancer patient can be provided.
Owner:SHENZHEN HAPLOX BIOTECH
Envenomation therapies and related pharmaceutical compositions, systems and kits
ActiveUS11000506B2Reduce the possibilityImprove permeabilityPeptide/protein ingredientsImmunoglobulins against animals/humansNR1 NMDA receptorNMDA receptor
The invention provides methods of treatment, pharmaceutical compositions, systems and kits appropriate for first line and / or adjunct therapy with antivenom using at least one active component, in some instances at least two active components and in other instances no more than two active components selected from the group consisting of a selective secretory PLA2 inhibitor (sPLA2 or PLA2 inhibitor), a metalloproteinase inhibitor, a serine protease inhibitor, antivenom, one or more acetylcholinesterase inhibitors or a nAChR agonist paired with a mAChR antagonist, a NMDA receptor antagonist and a spreading factor inhibitor to treat a subject who suffers from an envenomation, preferably at the time of envenomation and often within a period of less than about an hour after an envenomation or 6 hours after an envenomation and throughout the course of treatment at time with or without antivenom as an adjunct therapy after an envenomation by, for example, a snake or invertebrate.
Owner:OPHIREX INC
Preparation and method for treating diabetes
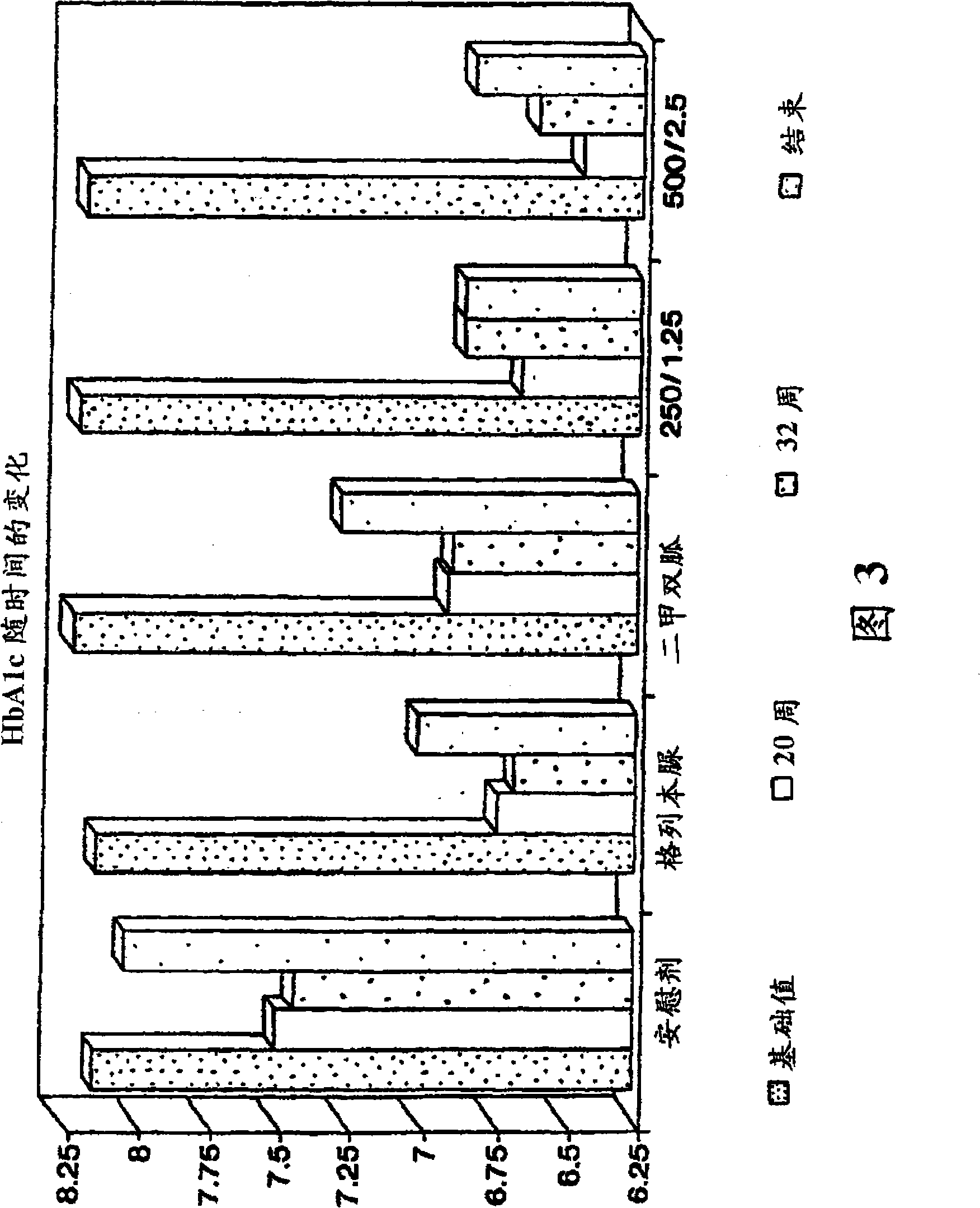
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com