Synergistic combined medicine for treating advanced lung cancer by combining anlotinib with immune checkpoint inhibitor
An immune checkpoint and inhibitor technology, applied in the field of medicine, can solve problems such as synergistic drug compositions for advanced lung cancer, and achieve the effects of good safety, good effect and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Phase I clinical study of CITIC Dilimab combined with Anlotinib of the present invention
[0033] The clinical research design plan includes:
[0034] The subjects signed the informed consent form before carrying out the research-related procedures; central randomization was adopted, and the stratification factors included: histological type (non-squamous cell carcinoma vs squamous cell carcinoma), smoking history (yes vs no) and PD-L1 expression level (positive vs negative);
[0035] The subject's medical history will be collected by qualified designated personnel. Past medical history includes all previous and currently active diseases. Record autoimmune diseases at any time. Past medical history includes disease diagnosis and treatment. The subject's previous medical history of lung cancer will be recorded in detail separately, including chemotherapy, radiotherapy and surgical treatment, etc., which will not be listed as past medical history; The patient...
Embodiment 2
[0049] Example 2 Phase I clinical study of sintilimab combined with anlotinib
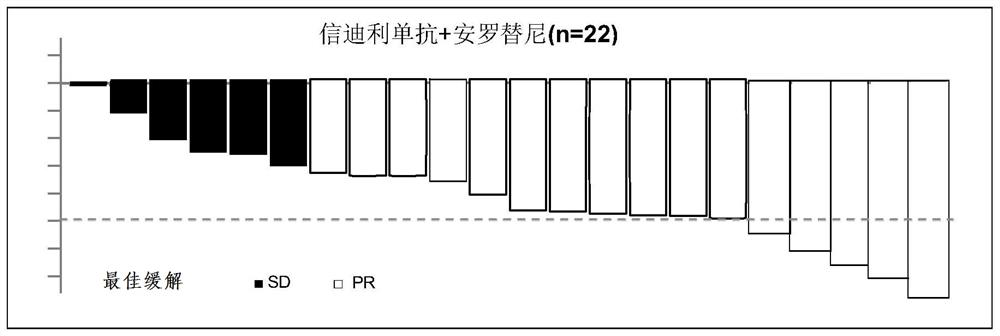
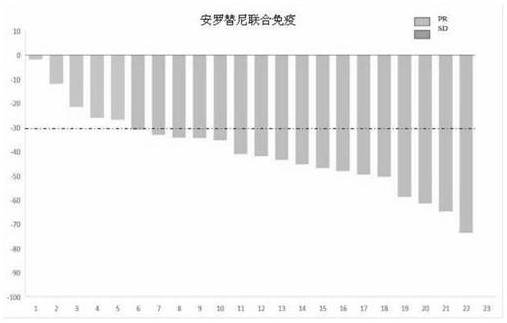
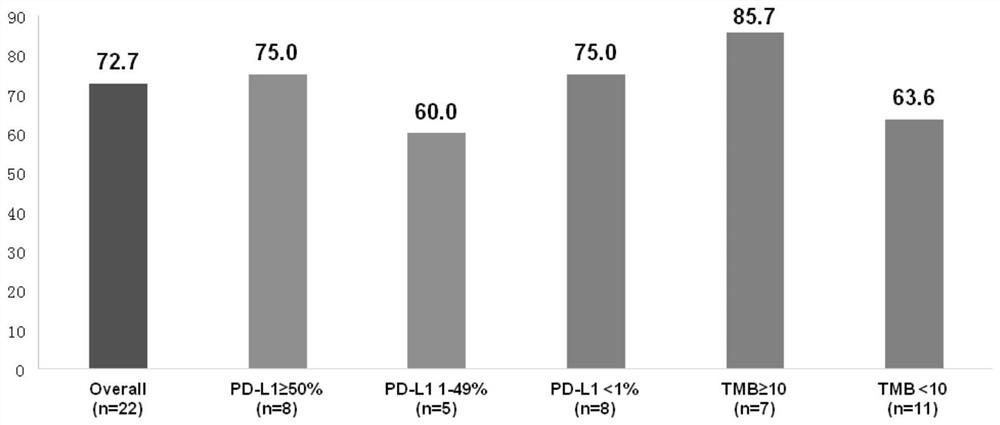
[0050] Based on the theoretical basis of synergy between immune and anti-angiogenic therapy, this example conducted a phase I exploratory study of small-sample combination therapy. From September 2018 to February 2019, 22 patients who had never received systemic anti-tumor therapy were enrolled. For stage IIIB / IV NSCLC with negative driver gene (EGFR / ALK / ROS1), the treatment plan is: sintilimab 200mg, intravenous infusion on the first day of each cycle; combined with anlotinib hydrochloride capsule 12mg once a day Oral, administered on days 1-14 of each cycle; every 3 weeks is a cycle; data as of the first efficacy analysis on April 8, 2019, all patients were under treatment and received at least one efficacy evaluation; 15 cases The patients achieved PR (7 of which were confirmed by secondary imaging evaluation), ORR was 68.2%, 7 patients were SD, and DCR was 100%. Of the 7 SD patients, 5 patients...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com