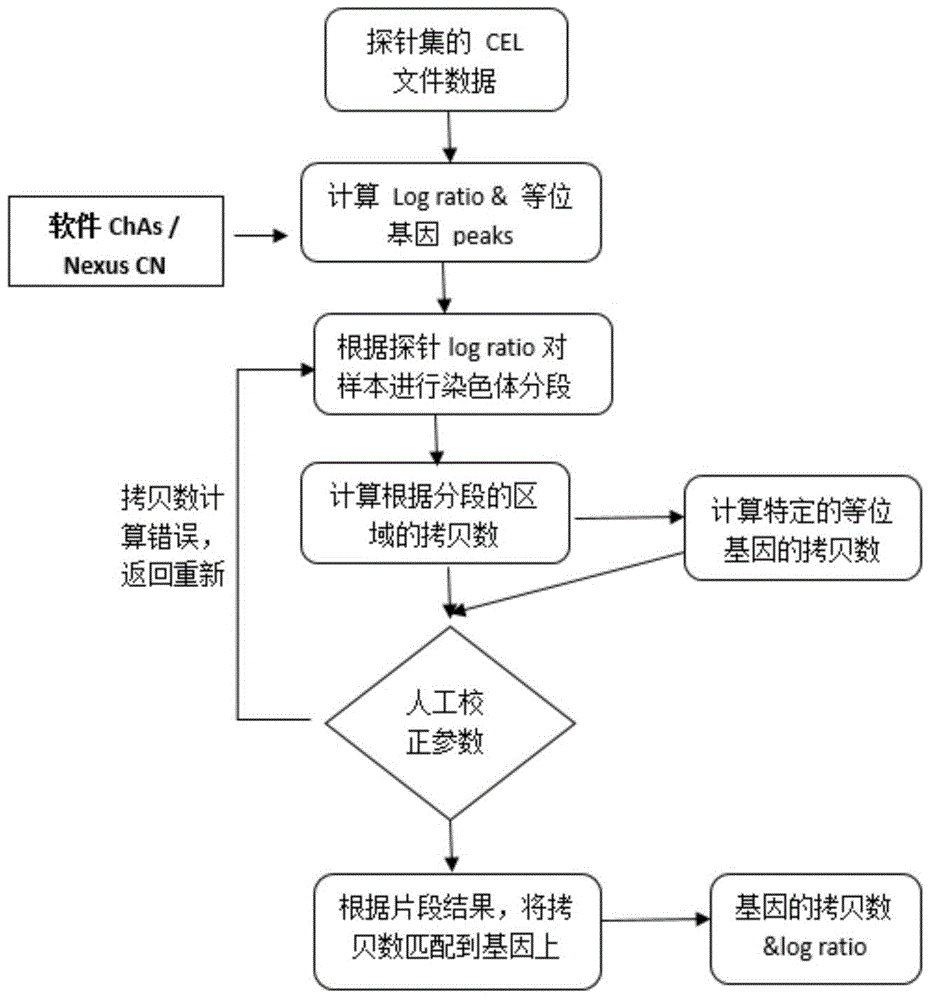
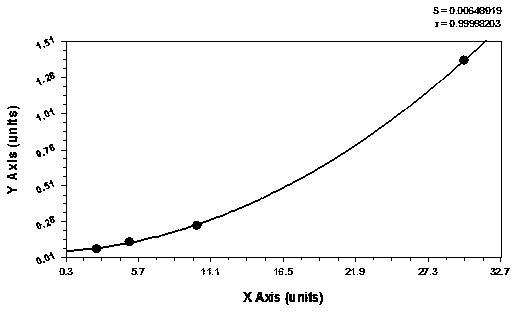
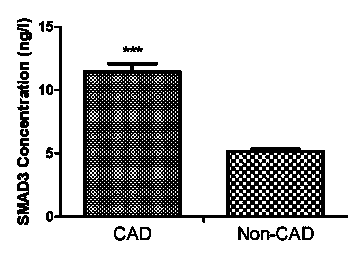
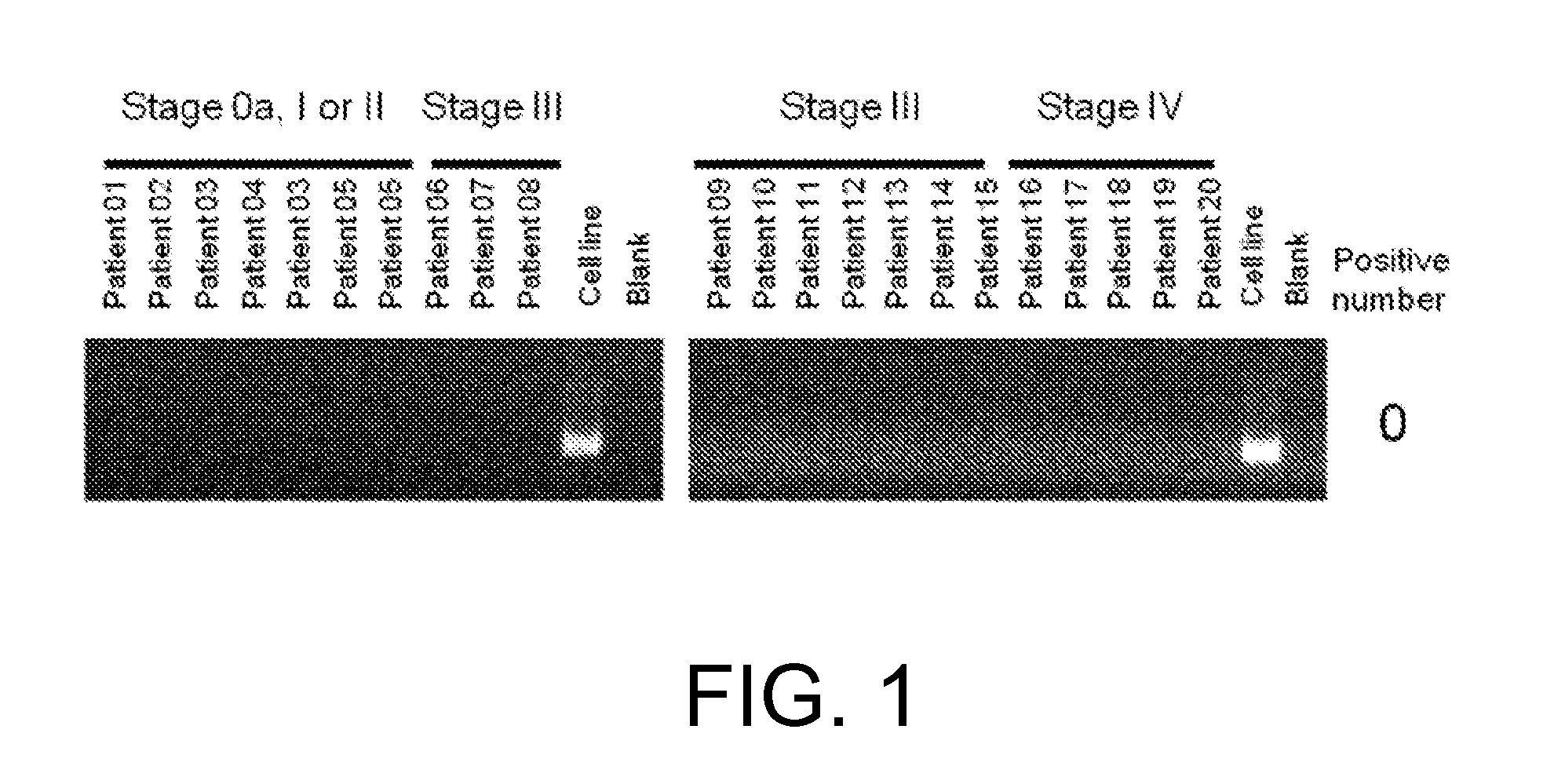
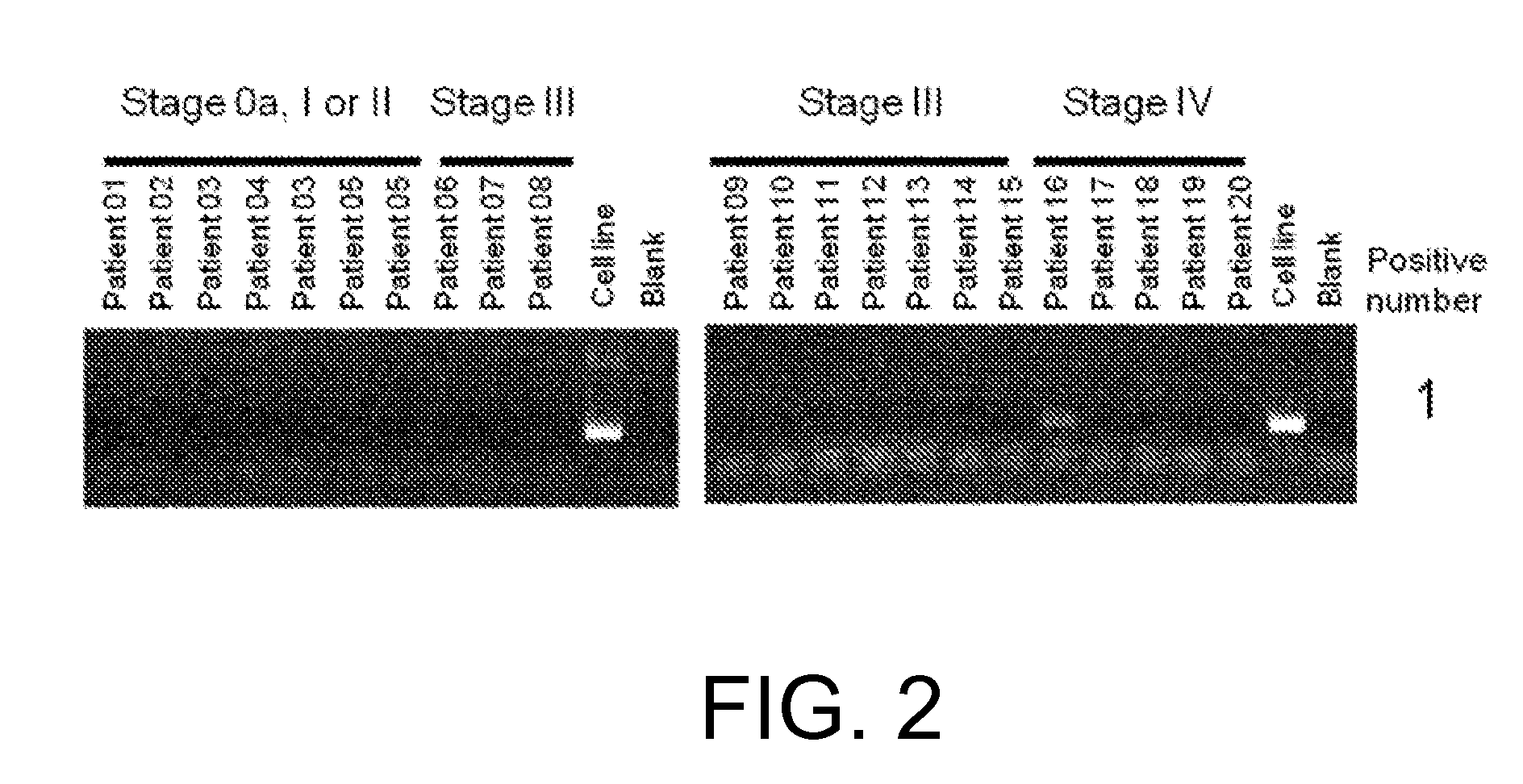
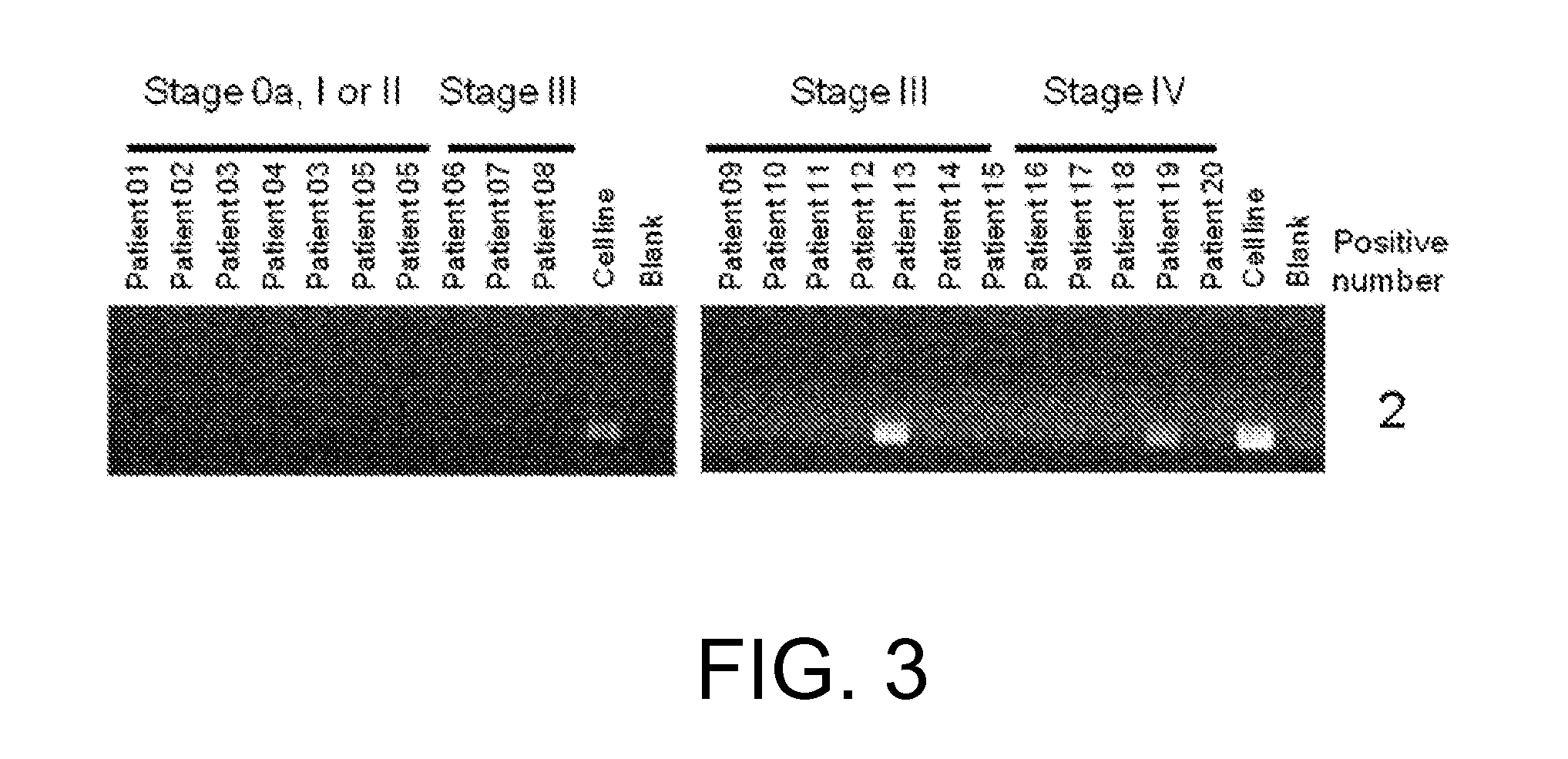
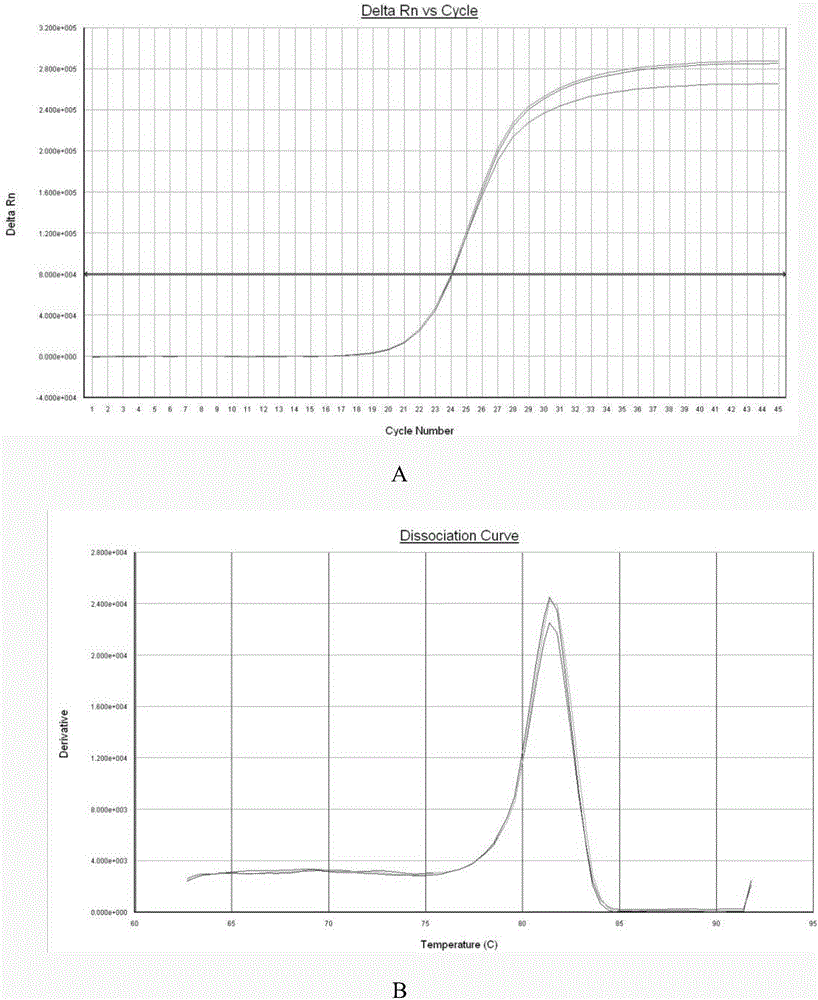
Patents
Literature
289 results about "Biomarker (medicine)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medicine, a biomarker is a measurable indicator of the severity or presence of some disease state. More generally a biomarker is anything that can be used as an indicator of a particular disease state or some other physiological state of an organism.
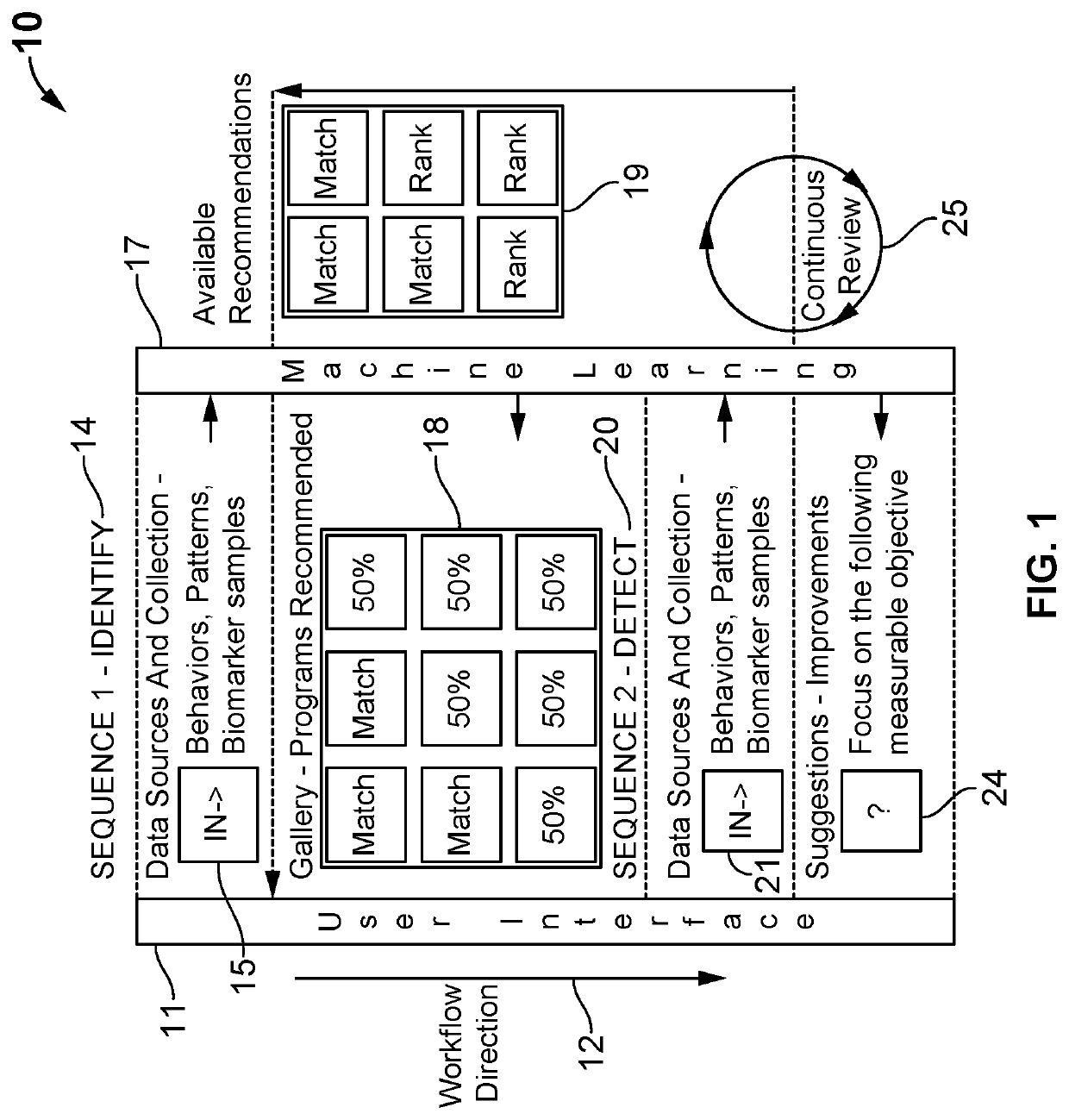
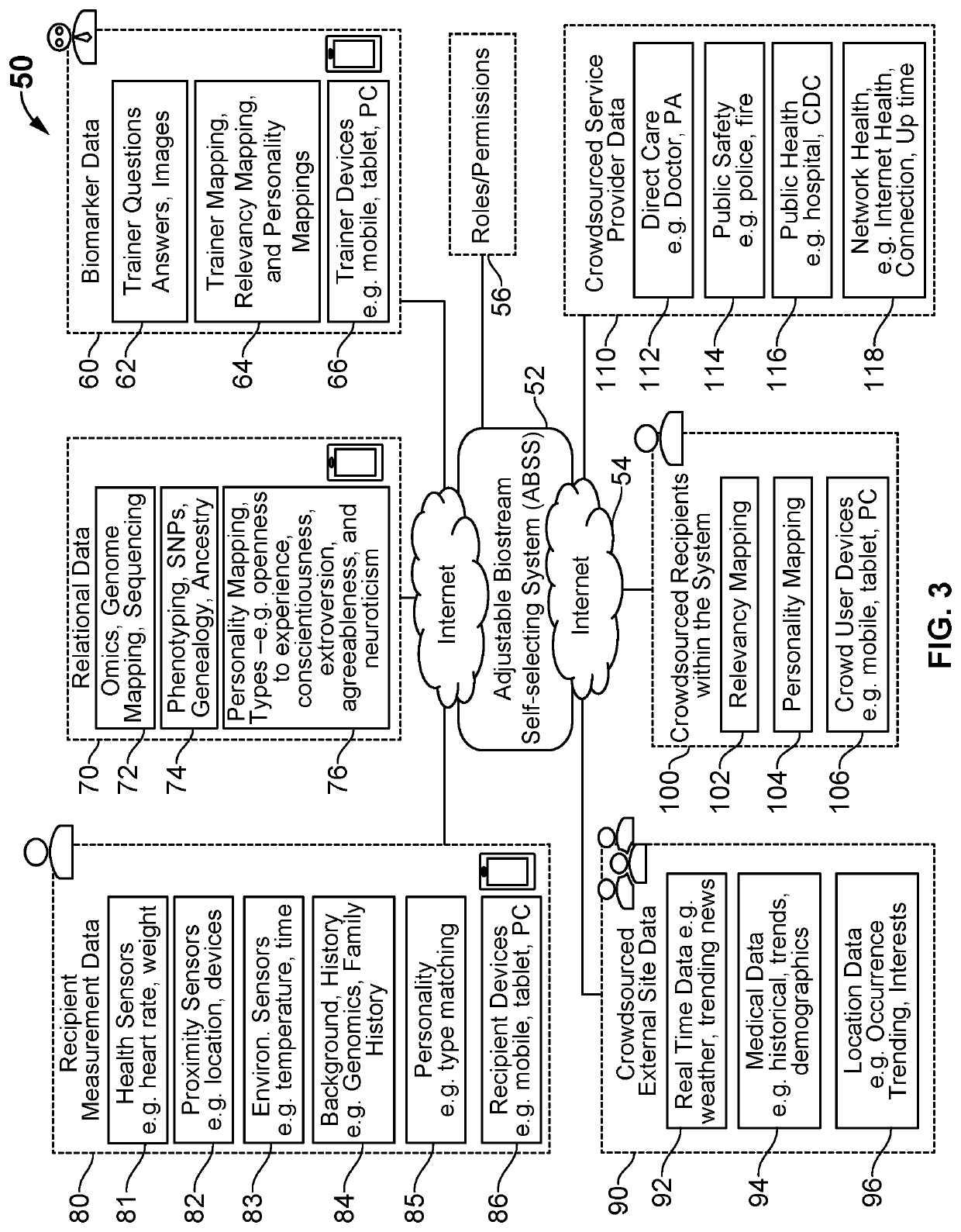
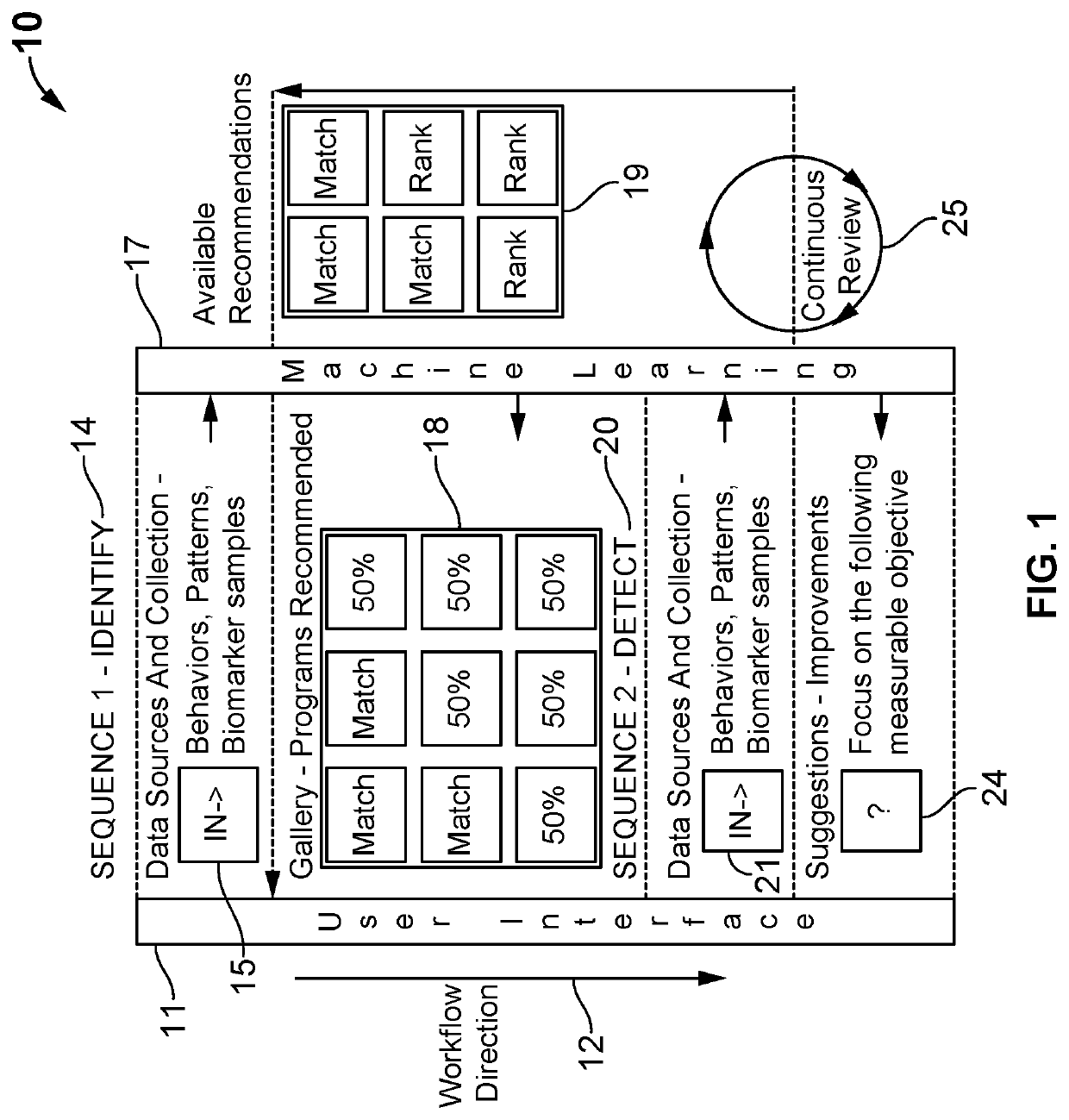
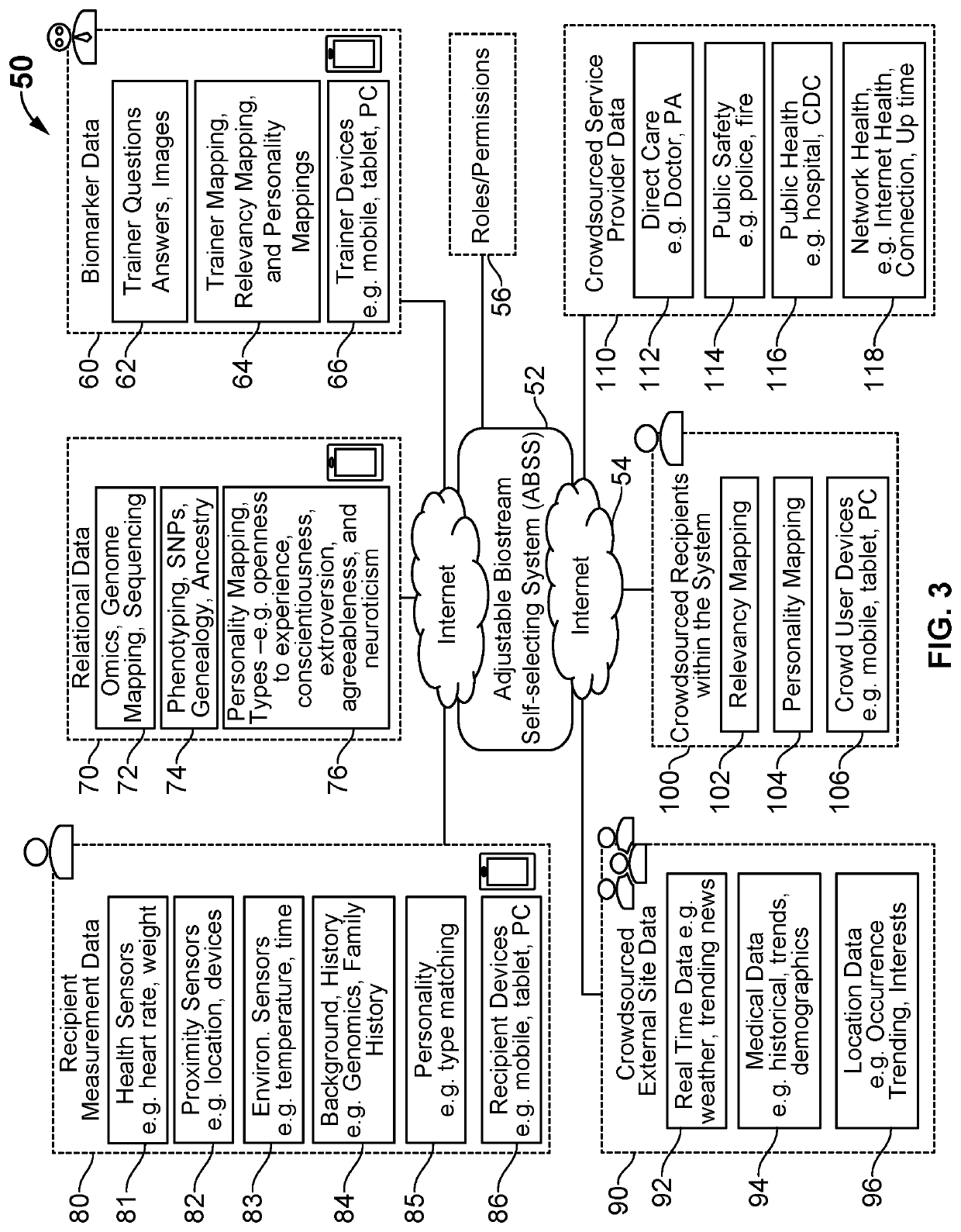
Digital therapeutics and biomarkers with adjustable biostream self-selecting system (ABSS)
ActiveUS20200131581A1Medical communicationMechanical/radiation/invasive therapiesFormularyPersonalization
A system and method for an adjustable bio-stream self-selecting system. Through a plethora of inputs, the system associates therapeutic recipes and associated biomarker in a personalized approach to recommending an individual to a specific therapeutic program. Therapeutic programs operate in accordance with personalized inputs suggested by the user and through digital markers and biomarkers, which trigger new recommendations by “knowing” the individual. Each bio-stream contains information utilized within these biomarkers to trigger additional therapy recommendations. Because of the complexity of the plurality of inputs, these biomarkers are managed in a way that enables low latency detections, low bandwidth needs, low processing needs, and less battery needs. The pre-processing of these biomarkers helps additional therapy management and precision medicine across larger global population needs of the system.
Owner:VIGNET INC
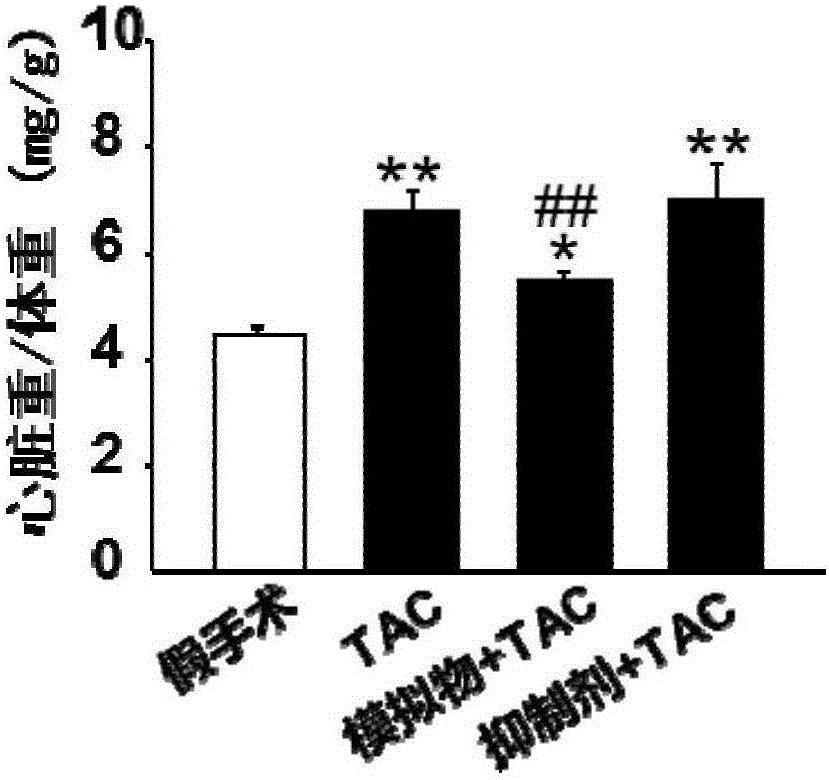
Application of miR-378 in inhibition of cardiac hypertrophy and myocardial fibrosis and diagnosis of heart failure
InactiveCN106729757AAids in diagnosisHelps reflect disease stateOrganic active ingredientsMicrobiological testing/measurementDiseaseCardiac muscle
The invention discloses an application of miR-378 in the preparation of a medicine or a kit for preventing or treating cardiac hypertrophy and myocardial fibrosis, and further discloses an application of the miR-378 as a heart failure biomarker. The miR-378 has a protection effect of resisting against myocardial remodeling on the heart through inhibiting cardiac hypertrophy and myocardial fibrosis, has potential prevention and treatment values on multiple heart diseases. The miR-378 is conducive to auxiliary diagnosis of the heart failure, meanwhile, due to expression difference in the blood of patients with mild and severe heart failures, the miR-378 is conducive to reflection of the disease state of the patients with heart failures, and provides a support for clinical doctors to quickly and accurately master the situations of the patients and timely take more personalized prevention and treatment schemes.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
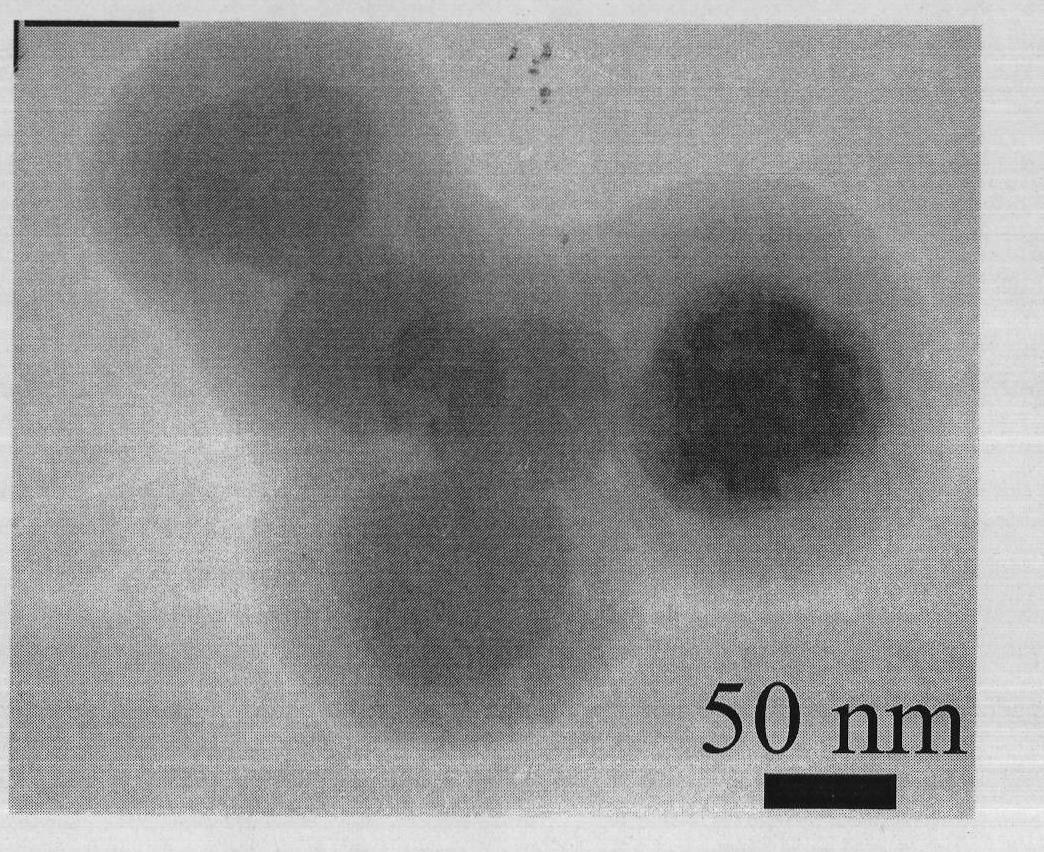
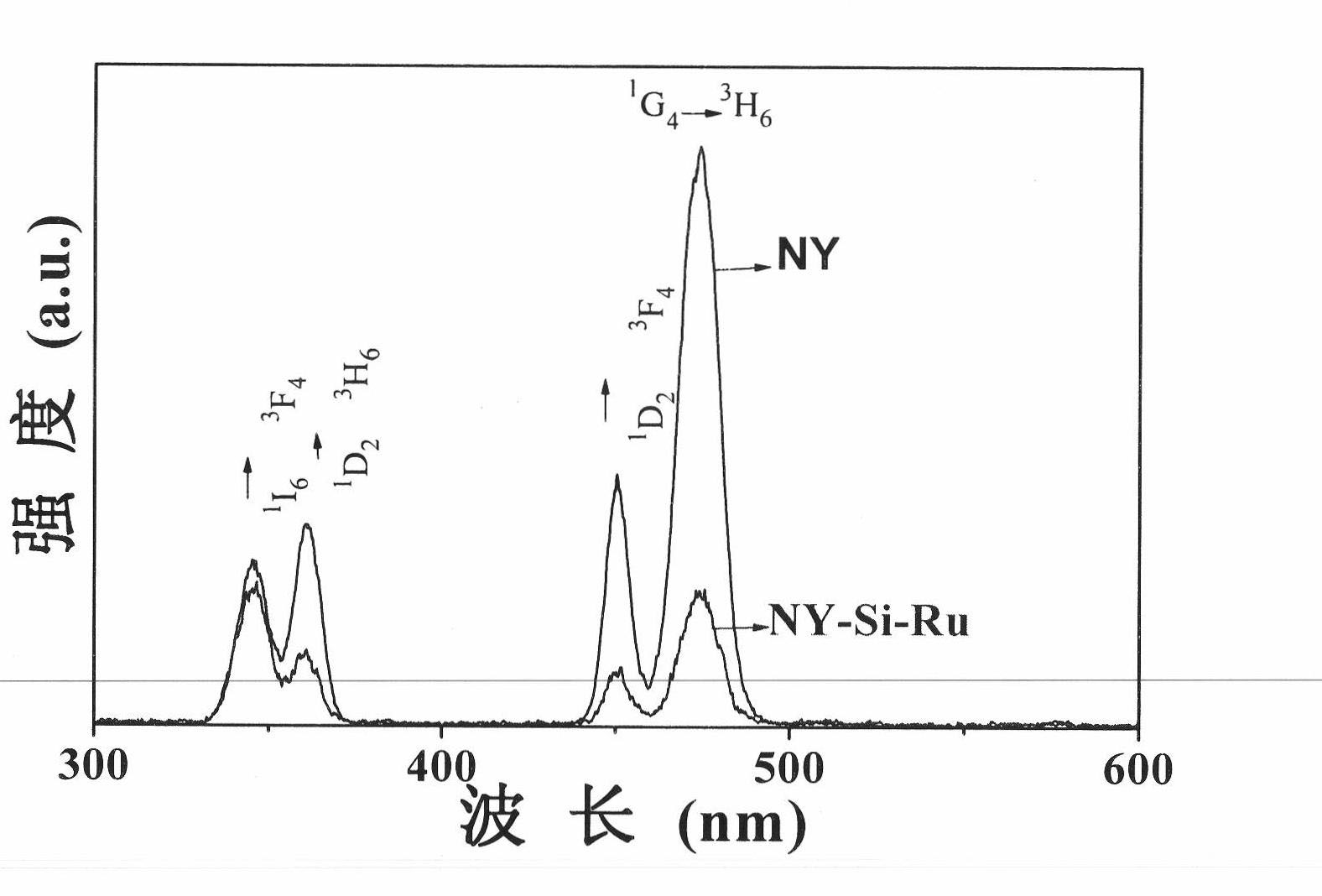
Multifunctional nano-composite having up-conversion luminescence, oxygen sensing and biological connectivity and preparation method thereof
InactiveCN101935529ARich functionalityEasy to prepareFluorescence/phosphorescenceLuminescent compositionsUpconversion luminescenceCore shell
The invention discloses a multifunctional nano-composite having up-conversion luminescence, oxygen sensing and biological connectivity and a preparation method thereof, in particular relates to a multifunctional nano-composite with a core-shell structure formed by connecting a [Ru(phen)2phen-Si]Cl2 complex with NaYF4:Yb<3+> and Tm<3+> nano-particles by using a SiO2 shell and preparation thereof. The nano-composite and the method solve the problems of the single structure of composites and a complex preparation process in the conventional fields of biomedicine and biochemistry. The composite comprises the [Ru(phen)2phen-Si]Cl2 complex, SiO2, and the NaYF4:Yb<3+> and Tm<3+> nano-particles in a mass ratio of 40-100:1.1-3.3:13.4-53.4. Due to the functions of the multifunctional composite, the application of the multifunctional composite in the fields of the biomedicine, such as biomarkers, oxygen concentration measurement in organisms and the like, is enhanced.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
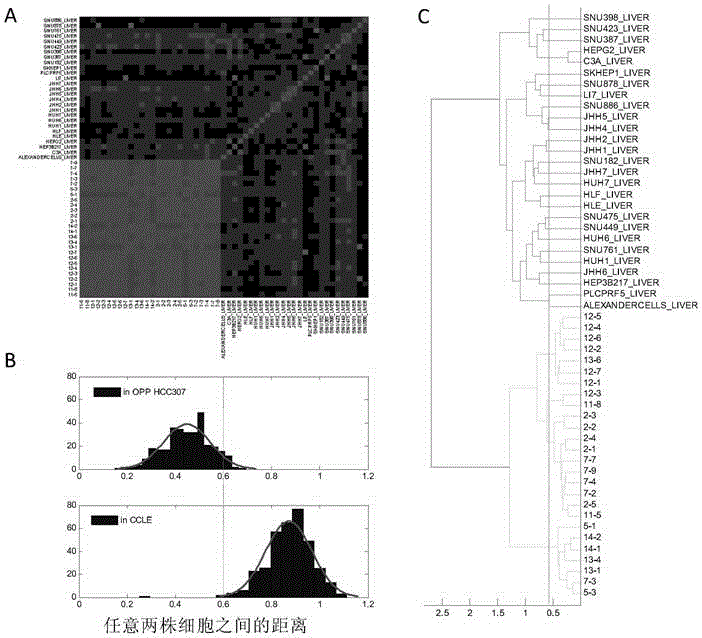
Screening method for anti-tumor medicine biomarker and application of anti-tumor medicine biomarker
ActiveCN104975063AReduce the differenceReduce background noiseMicrobiological testing/measurementTumor/cancer cellsBiomarker (medicine)Biologic marker
The invention discloses a screening method for an anti-tumor medicine biomarker and an application of the anti-tumor medicine biomarker. Particularly, the method comprises the following steps: (a) providing tumor cells as initial cells, wherein the tumor cells are from the same tumor tissues of the same object; (b) carrying out passage and establishment on the initial cells, and carrying out genome information detection on the obtained cell line, so as to obtain at least five tumor cell lines which are different in genome and highly homologous; (c) carrying out anti-tumor medicine sensitivity test on the tumor cell lines obtained from the step (b), and parting the tumor cell lines based on the sensitivity of the anti-tumor medicine; and (d) analyzing the genome information of the tumor cell lines based on the parting result, so as to determine the anti-tumor medicine biomarker. According to the method disclosed by the invention, the background noise between different tumor cell lines can be reduced to the maximal extent to contribute to efficient and accurate screening of the anti-tumor medicine biomarker.
Owner:思路迪科技(上海)有限公司
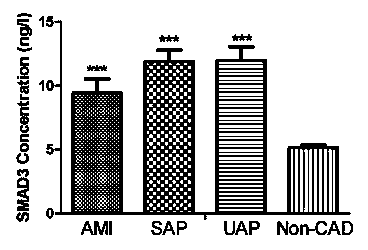
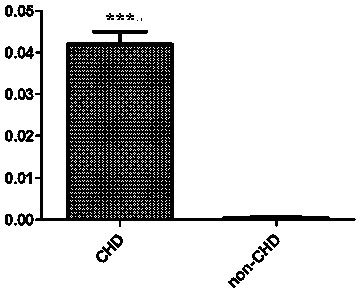
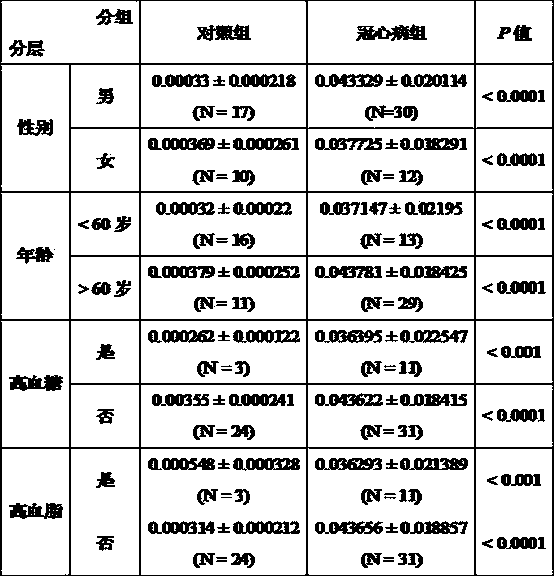
Serologic biomarker for coronary heart disease (CHD) detection, and application thereof
InactiveCN104311655AAccurate predictionBiological testingAnimals/human peptidesLaboratory orderSterile alpha motif
The invention belongs to the fields of laboratory medicine, clinical medicine, biotechnology and biochemistry, and relates to a serologic biomarker for coronary heart disease (CHD) detection, and application thereof. The biomarker is human SAMD3 (sterile alpha motif domain-containing protein 3). The biomarker is closely related to CHD incidence and development; SAMD3 is used as the biomarker for CHD incidence prediction and CHD development degree judgment, so that compared with the conventional pathological type diagnosis of CHD incidence and prognosis, the biomarker can more individually and accurately predict the early incidence situation and grade malignancy of CHD; moreover, the marker can be used for preparing a kit for screening CHD or assisting in pathological identification, clinical diagnosis and subtype discrimination of CHD.
Owner:雷桅
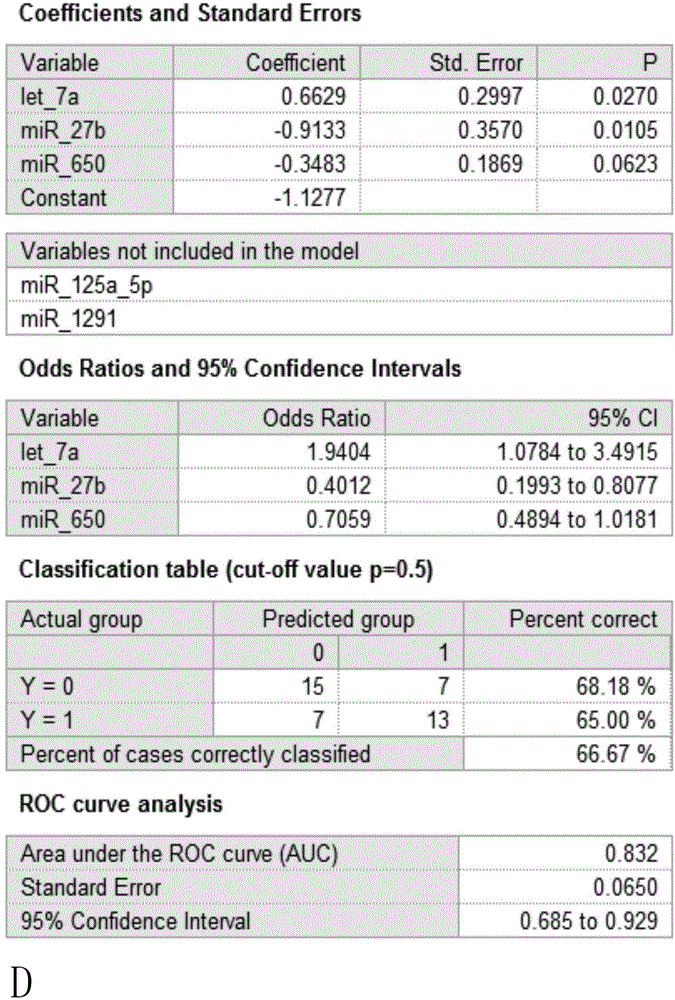
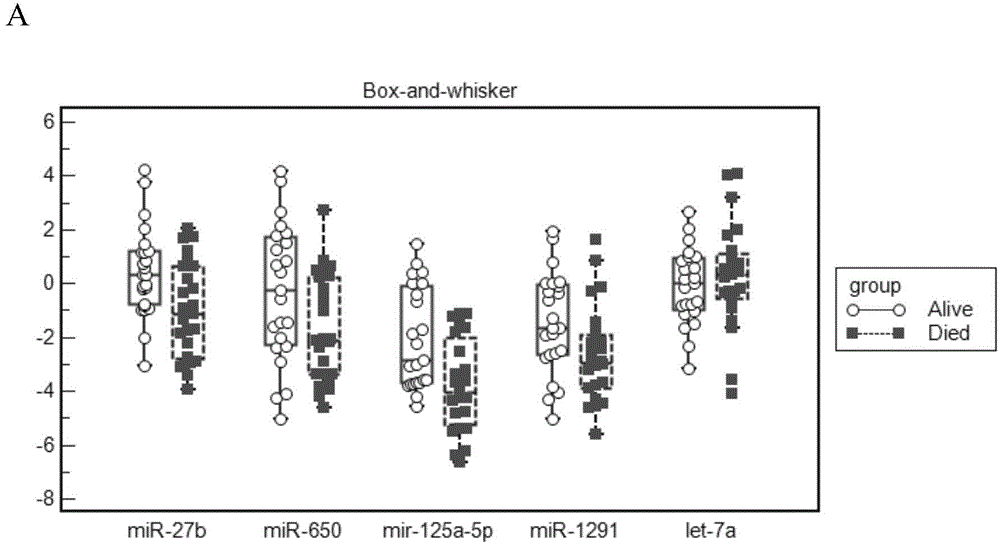
Micro RNA biomarker for predicting early non-metastatic colorectal cancer prognosis and detection method
The invention belongs to the technical field of biomedicine and relates to a micro RNA biomarker for predicting early non-metastatic colorectal cancer prognosis and a detection method. The early non-metastatic colorectal cancer refers to Dukes' A and Dukes' B glandular cancers. Particularly, the invention relates to a diagnostic kit for identifying molecular markers of one or more mammalian target cells for prognosis of different early non-metastatic colorectal cancers. The kit comprises multiple nucleic acid molecules, a miRNA sequence is encoded by each nucleic acid molecule, one or more in the multiple nucleic acid molecules have differential expressions in target cells and one or more control cells, and one or more nucleic acid molecules with differential expressions represent nucleic acid expression characteristics together, wherein the nucleic acid expression characteristics refer to indications for identifying prognosis of different early non-metastatic colorectal cancers. The invention further relates to a corresponding method for preventing or treating the disease by using the biomarker and detection method and a pharmaceutical composition.
Owner:上海兰卫医学检验所股份有限公司
Fgfr3 fusion gene and pharmaceutical drug targeting same
ActiveUS20150307945A1Avoid side effectsPrevent proliferationPolypeptide with localisation/targeting motifOrganic active ingredientsCancer cellSide effect
The FGFR-encoding gene was studied extensively with regard to its expression, hyperamplification, mutation, translocation, or such in various cancer cells. As a result, novel fusion polypeptides in which the FGFR3 polypeptide is fused with a different polypeptide were identified and isolated from several types of bladder cancer-derived cells and lung cancer cells. The use of a fusion polypeptide of the present invention as a biomarker in FGFR inhibitor-based cancer therapy enables one to avoid side effects in cancer therapy and control the therapeutic condition to produce the best therapeutic effect, thereby enabling individualized medicine.
Owner:CHUGAI PHARMA CO LTD
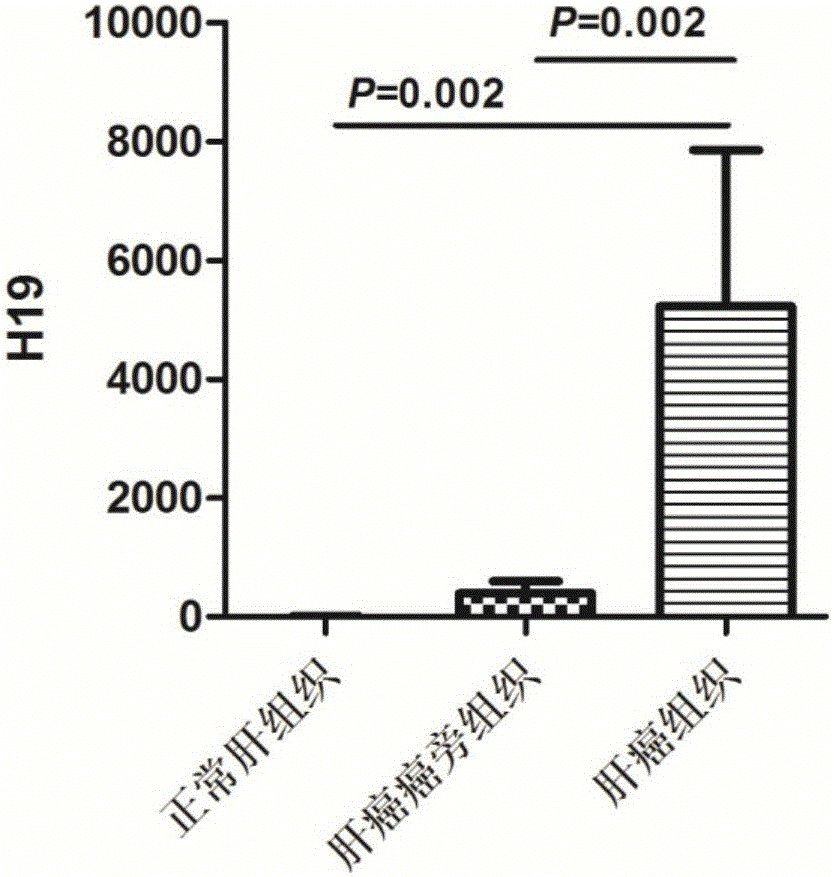
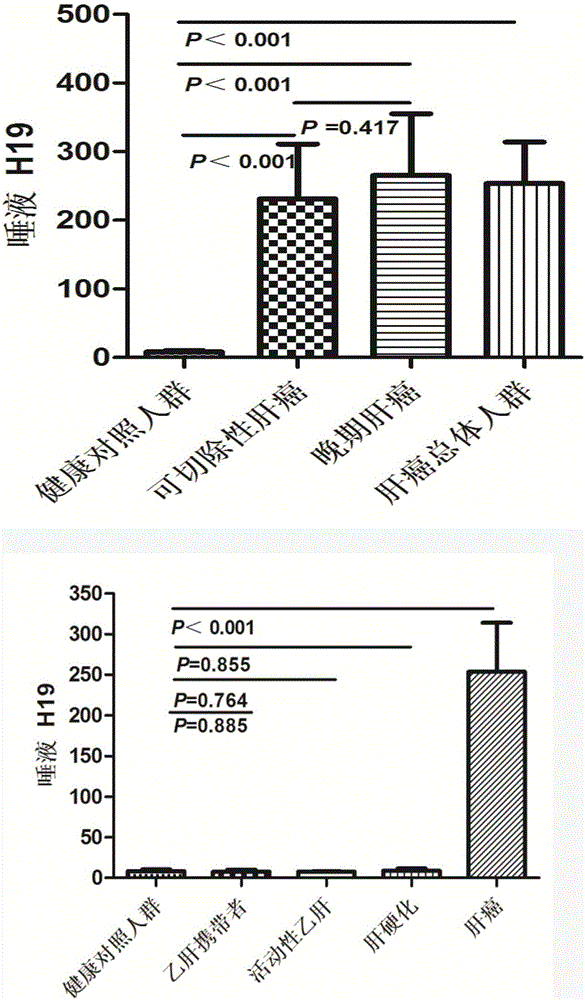
Liver cancer diagnosing reagent and kit using saliva as detection samples
PendingCN105132559ASampling method is simpleConvenient sampling methodMicrobiological testing/measurementDNA preparationSaliva sampleBiomarker (medicine)
The invention belongs to the technical field of biological medicine and relates to a detection reagent and kit for diagnosing liver cancer. The reagent is a long non-coding RNA (lncRNA) H19 detection reagent and used for detecting saliva samples. The kit using saliva as detection samples and used for diagnosing the liver cancer comprises protease and the lncRNA H19 detection reagent. The saliva detection reagent and kit has the advantages that the biomarker sensitive and specific in liver cancer diagnosing is found in the saliva for the first time, and great significance to early detection and treatment of the liver cancer, death rate lowering and China medical burden relieving is achieved.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
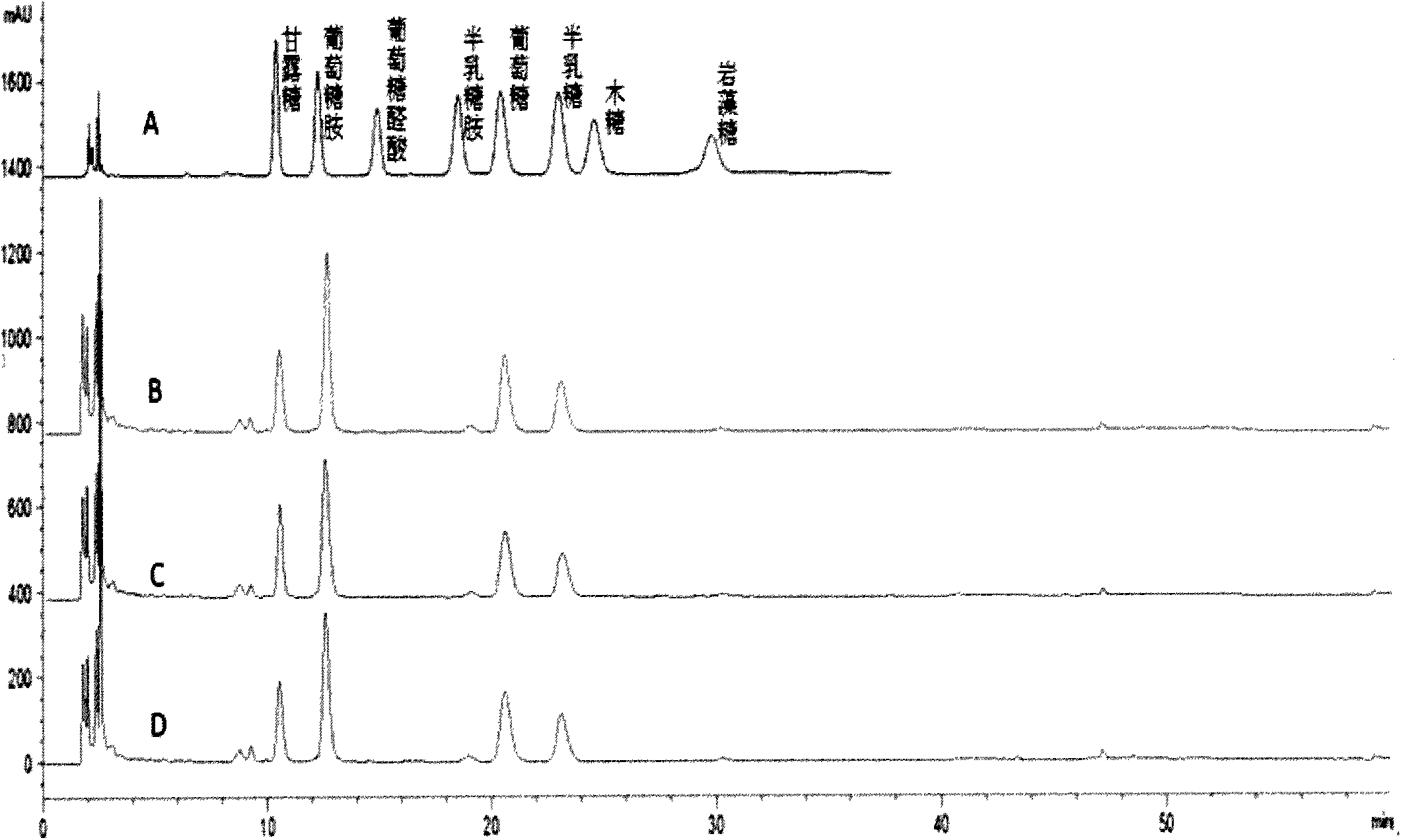
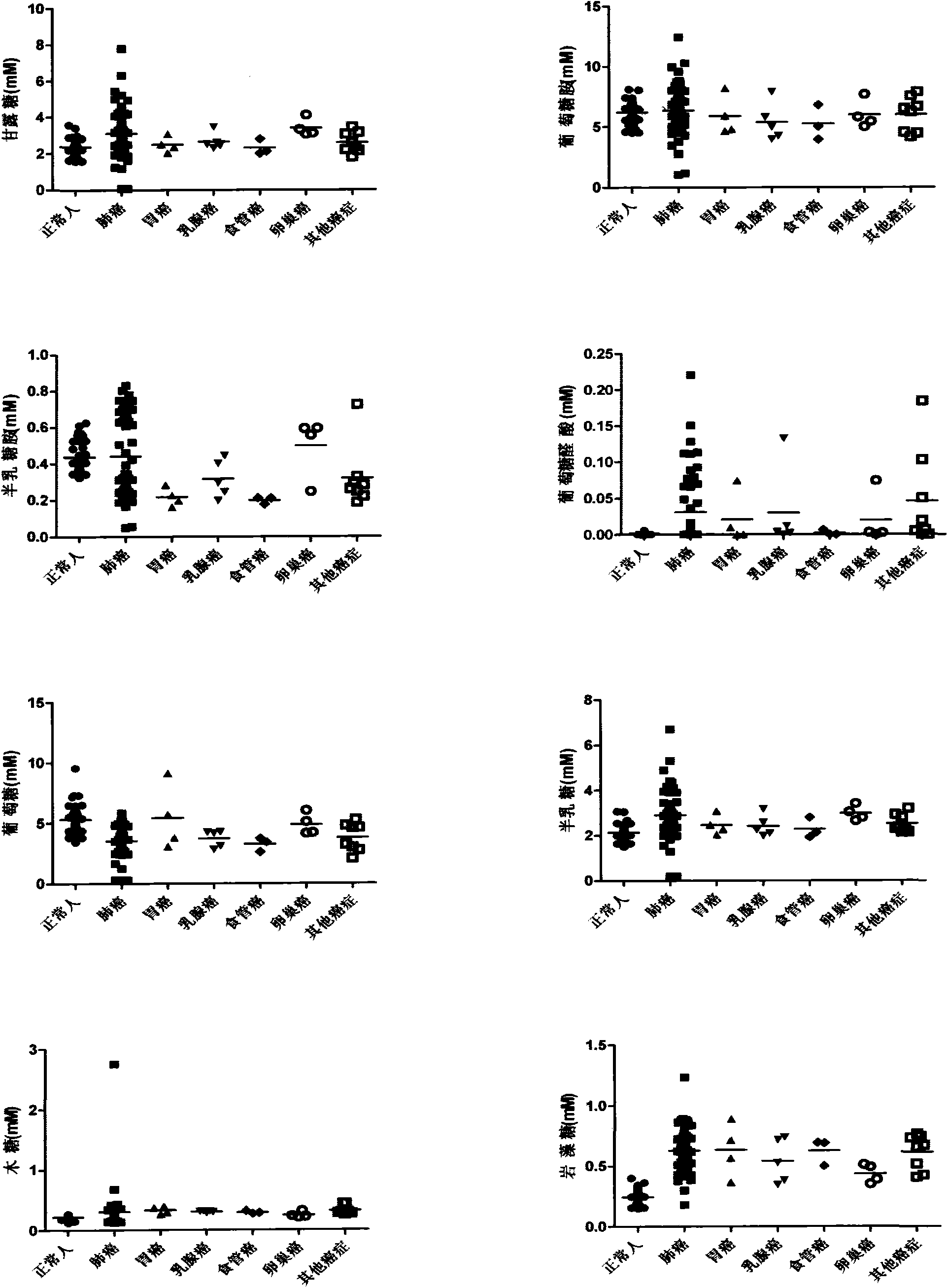
Application of method for degrading blood to obtain monosaccharide and detecting monosaccharide in cancer detection
ActiveCN103969371ASuitable for testingEasy to operateComponent separationOncologyBiomarker (petroleum)
The invention belongs to the field of medicines, and relates to an application of a method for degrading all carbohydrate chains in the blood to be monosaccharide and detecting the monosaccharide in cancer detection. A sample in the method for degrading the blood to obtain the monosaccharide and detecting the monosaccharide is the blood, and the cancer includes lung cancer, gastric cancer, ovarian cancer, penis cancer, esophagus cancer, oral cancer, biliary duct cancer, breast cancer, adenocarcinoma perampullaire, rectal cancer and bladder cancer. The method has the characteristics of simplicity and easiness in operation steps, easiness in popularization, short detection time, low requirement on the instrument, low detection cost, less consumption of blood and the like. The result displays that based on the content of eight monosaccharides in the blood, not only can a normal person and a cancer patient be distinguished, but also different cancers can be distinguished. The method for simplifying glycomics knowledge and applying in the detection of the biomarker of blood is first created in the world.
Owner:OCEAN UNIV OF CHINA
Biomarker combination and kit for predicting breast cancer and use method
ActiveCN106636430AHigh detection sensitivityImprove noise immunityMicrobiological testing/measurementDNA/RNA fragmentationBiologyTarget gene
The invention provides a biomarker combination and a kit for predicting breast cancer. Primer design genes are chosen from four types of telomere genes with the most obvious methylation rate difference in breast cancer tissues and normal tissues. According to the promoter regions and coding regions of the four types of telomere genes, methylation primers and qPCR (Quantitative Polymerase Chain Reaction) primers of a target gene are respectively designed, and thereby a methylation primer combination and a qPCR primer combination are formed. Since the biomarker combination provided by the invention is designed according to the telomere genes with the most obvious methylation rate difference in the breast cancer tissues and the normal tissues, when whether a sample is breast cancer is assayed, the biomarker combination can specifically capture and amplify specific DNA fragments in the assayed sample, so that the sensitivity of assay is increased, and the biomarker combination has good noise immunity, flexibility and accuracy, and can be used in the early prediction of the breast cancer, the evaluation and assay of medicines for treatment and the detection of prognosis recurrence. The method for predicting the breast cancer provided by the invention has the advantages of quickness in operation, simplicity, convenience and good feasibility and prediction effect.
Owner:HUNAN SHENGWEI GENE TECH
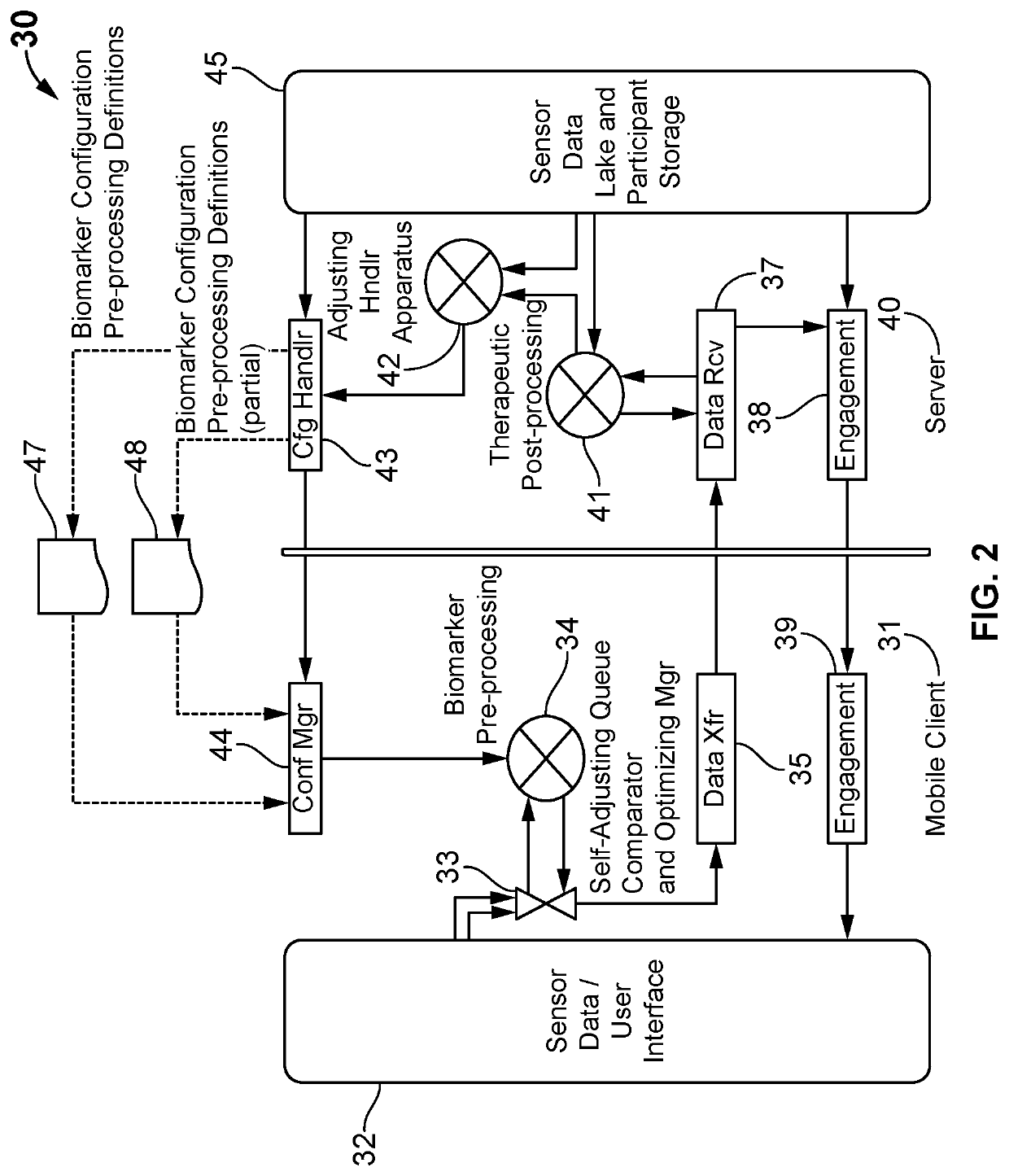
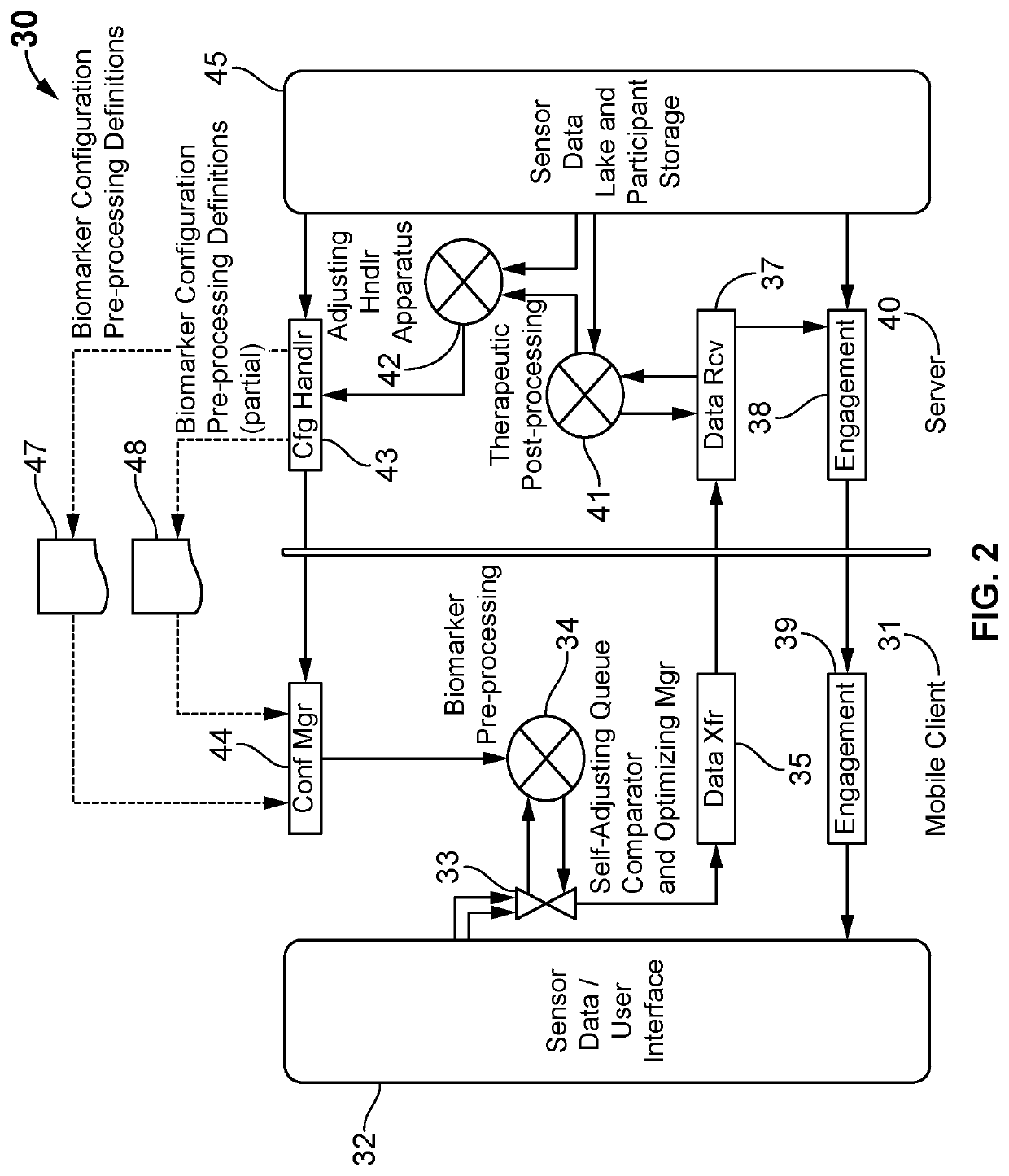
Adapted digital therapeutic plans based on biomarkers
ActiveUS11158423B2Medical communicationMechanical/radiation/invasive therapiesFormularyPersonalization
A system and method for an adjustable bio-stream self-selecting system. Through a plethora of inputs, the system associates therapeutic recipes and associated biomarker in a personalized approach to recommending an individual to a specific therapeutic program. Therapeutic programs operate in accordance with personalized inputs suggested by the user and through digital markers and biomarkers, which trigger new recommendations by “knowing” the individual. Each bio-stream contains information utilized within these biomarkers to trigger additional therapy recommendations. Because of the complexity of the plurality of inputs, these biomarkers are managed in a way that enables low latency detections, low bandwidth needs, low processing needs, and less battery needs. The pre-processing of these biomarkers helps additional therapy management and precision medicine across larger global population needs of the system.
Owner:VIGNET INC
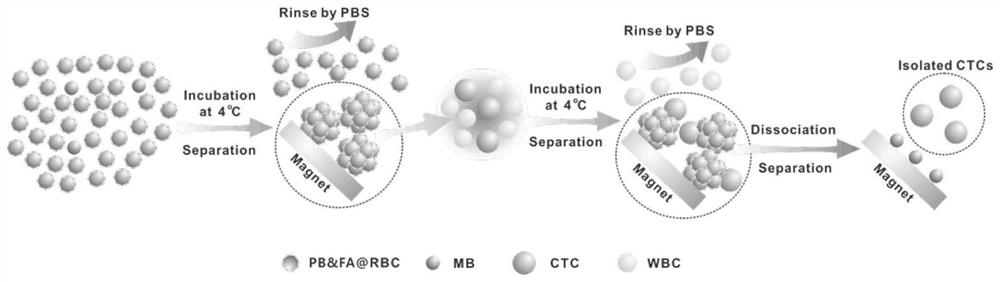
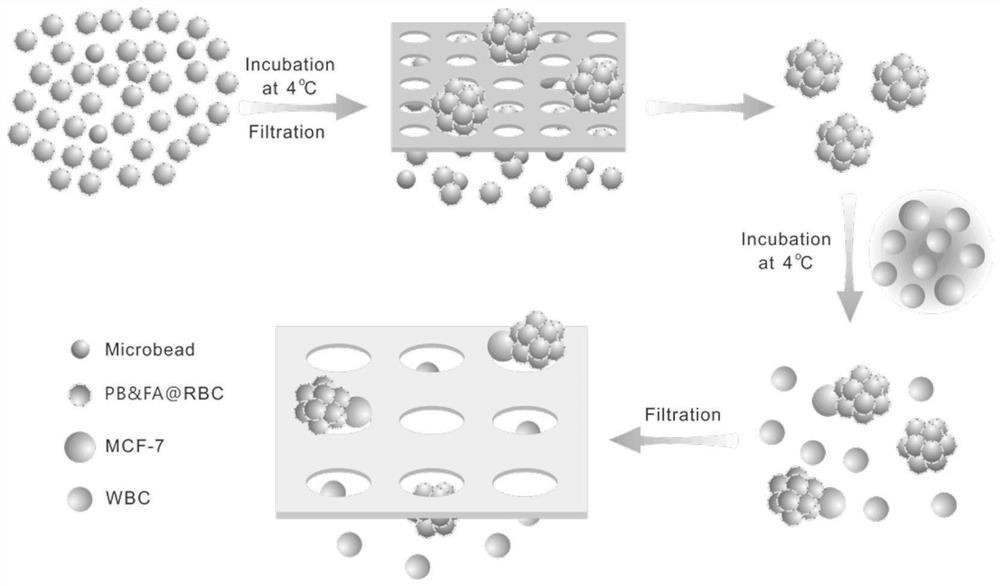
Magnetic red blood cell clusters for enriching circulating tumor cells based on magnetic separation method
ActiveCN111826351ACapture firmer and more efficientAvoid non-specific adsorptionCell dissociation methodsTumor/cancer cellsWhite blood cellRed blood cell
The invention discloses magnetic red blood cell clusters for enriching circulating tumor cells based on a magnetic separation method, and belongs to the technical field of biological medicines. Red blood cells are combined with nanometer-grade magnetic microspheres to obtain a red blood cell cluster type biomimetic material, and polybrene is modified on the surfaces of the red blood cells, so thatelectrostatic repulsion originally existing between the red blood cells can be eliminated, and further, the red blood cells can be tightly covered on the surfaces of the microspheres in the total layer. The red blood cells pack the nanometer-grade magnetic microspheres to prevent non-specific adsorption of white blood cells on the surfaces of the microspheres, and besides, folic acid (FA) modified on the surfaces of the red blood cell clusters or an antibody specially recognized or combined with a CTCs surface biomarker can realize direct targeting on CTCs, and finally, in a magnetic separating manner, the white blood cells in blood samples of a clinical patient can be removed, so that high-purity CTCs can be efficiently obtained.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
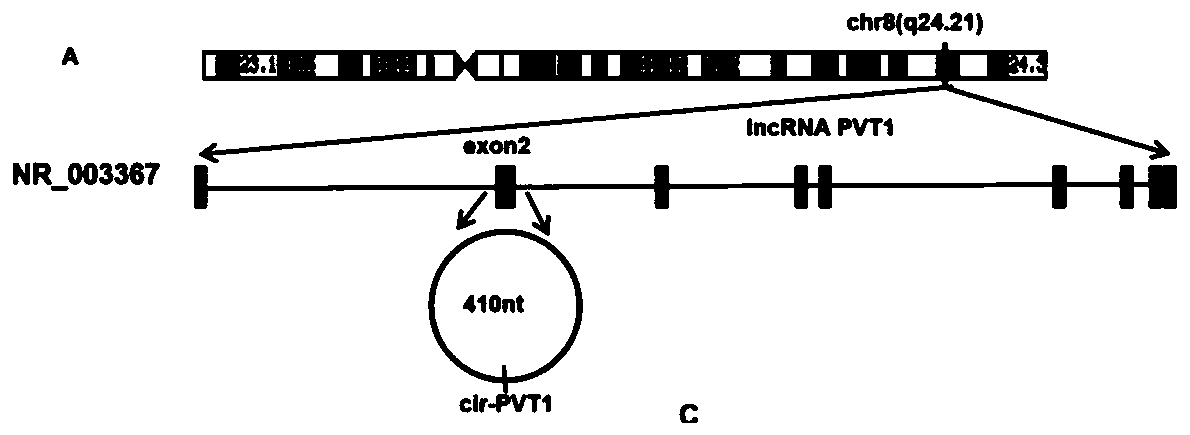
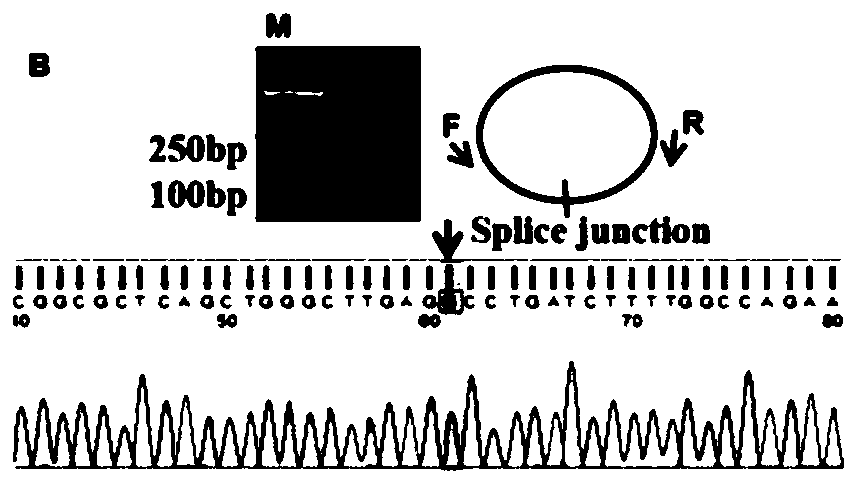
Application of CircRNA PVT1 and peptide fragment in tumor growth prediction, metastasis prediction, prognosis evaluation and treatment
PendingCN111057764AMicrobiological testing/measurementDisease diagnosisPharmaceutical drugBiomarker (medicine)
The invention belongs to the field of biotechnology and medicine and specifically relates to correlation and application of a CircRNA PVT1 (hsa _ circ_ 0001821) nucleic acid fragment and a CircRNA PVT1 translated peptide fragment PVT1-104aa in tumor growth prediction, metastasis prediction, prognosis evaluation and treatment. The CircRNAPVT1 and PVT1-104aa are used as drug targets for early diagnosis of tumors, treatment of tumors, alleviation or prevention of tumor metastasis and / or improvement of tumor prognosis. The tissue specificity and stability of CircRNA provided by the invention enable CircRNA to become a good biomarker, and tests prove that the CircRNA PVT1 nucleic acid fragment and the peptide fragment PVT1-104aa play a significant and stable role in tumor growth prediction, metastasis prediction, prognosis evaluation and treatment.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
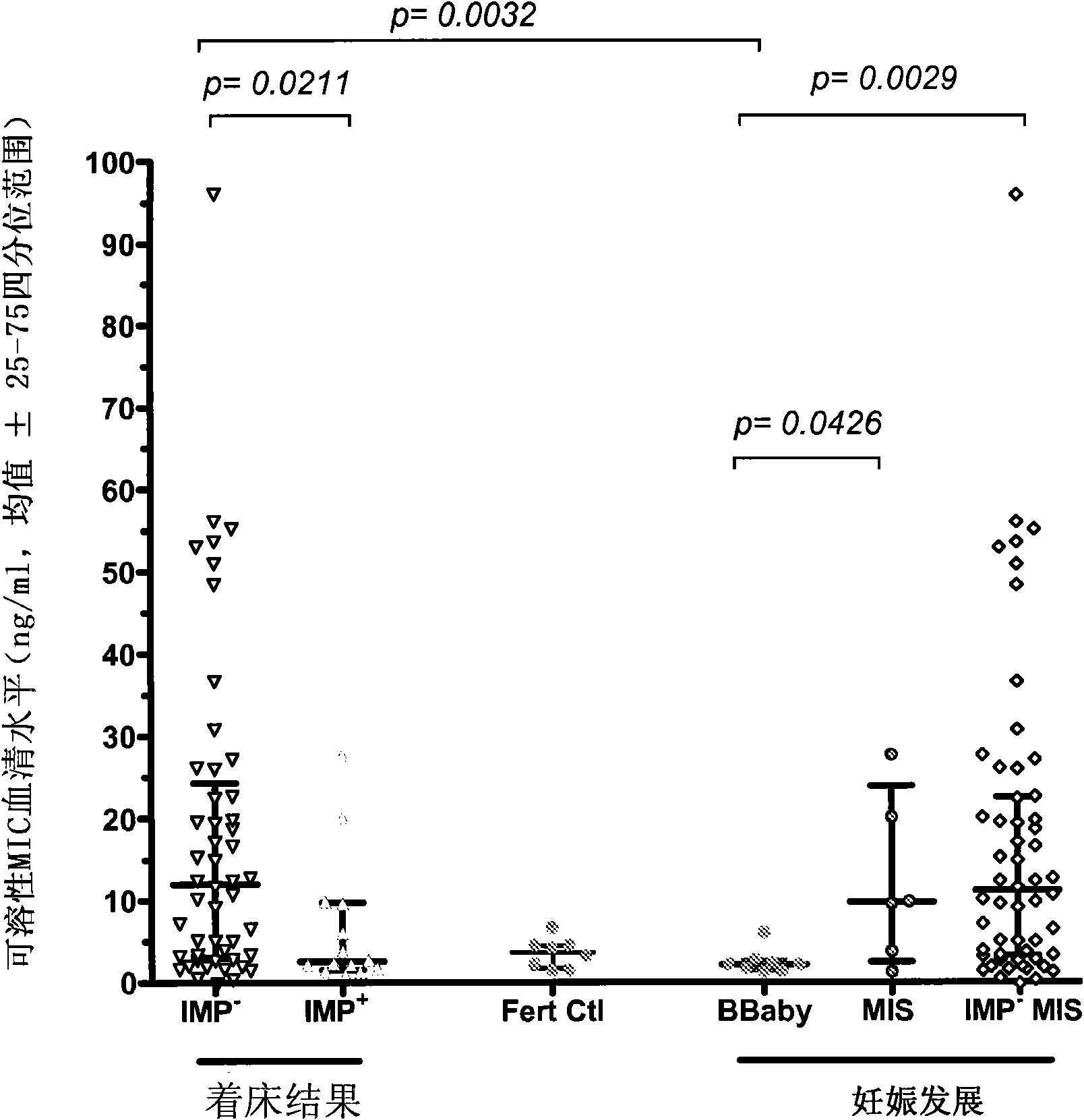
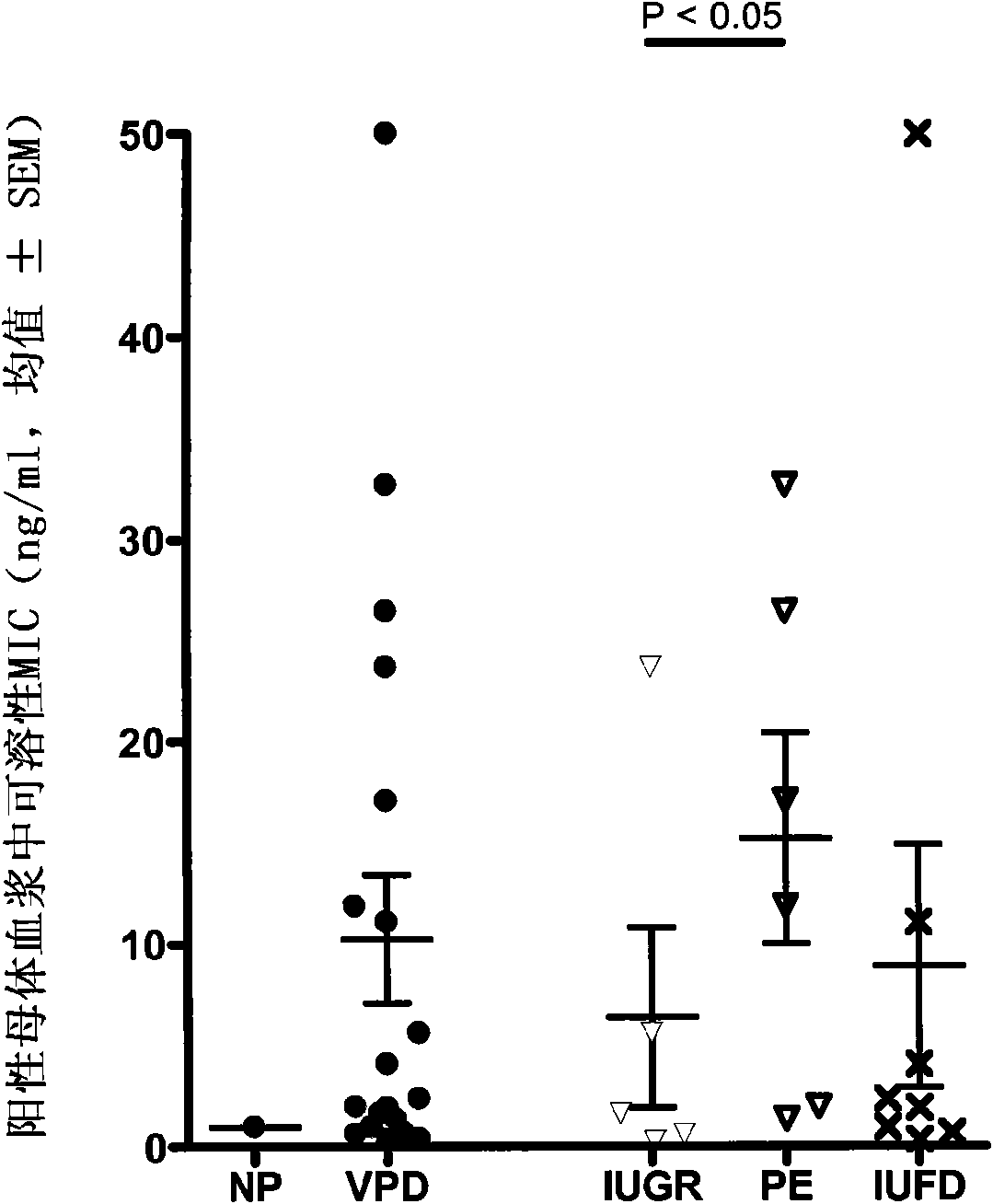
Biomarker for the medicine and the biology of the reproduction
The present invention relates to a new biomarker for the medicine and the biology of reproduction, in particular for in vitro fertilization (IVF) outcome. It relates to methods for predicting IVF outcome and for selecting the subject for IVF.
Owner:UNIV DAIX MARSEILLE
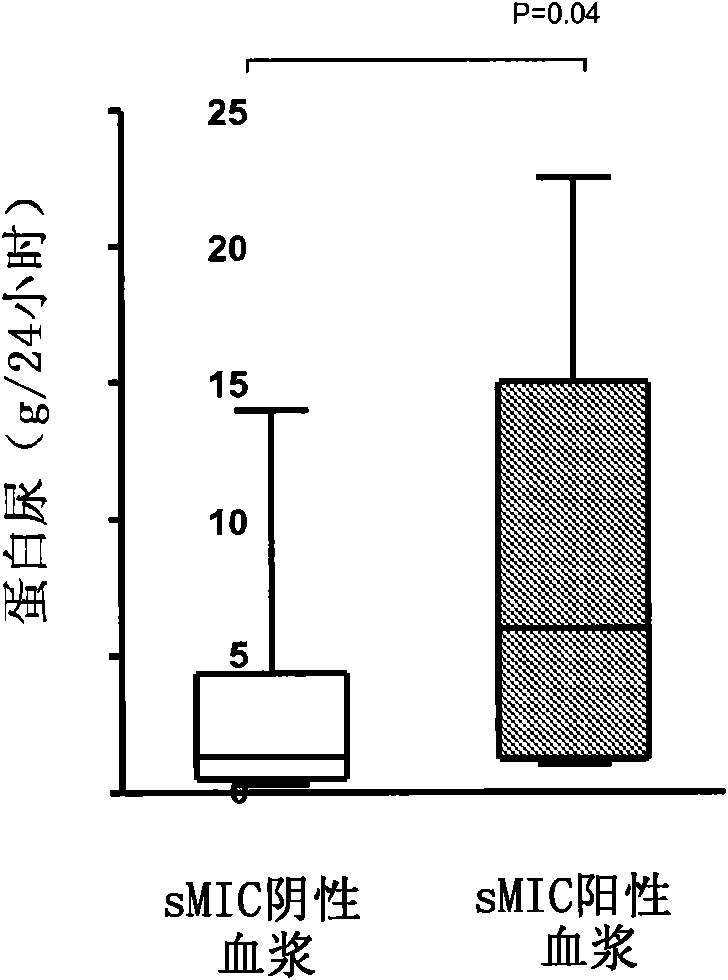
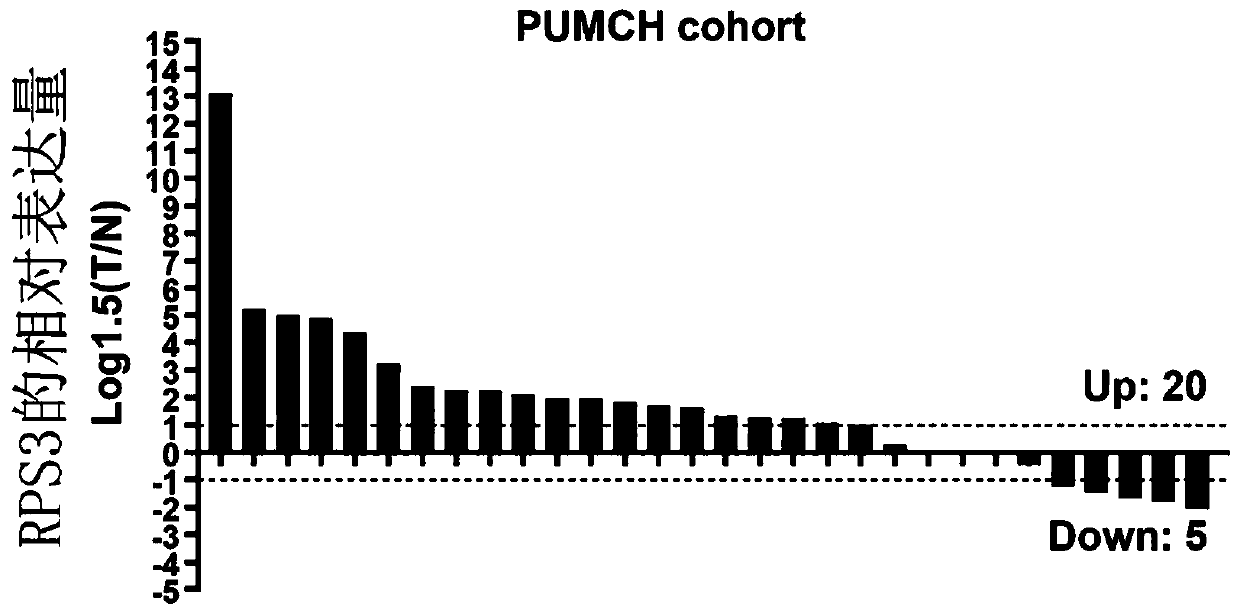
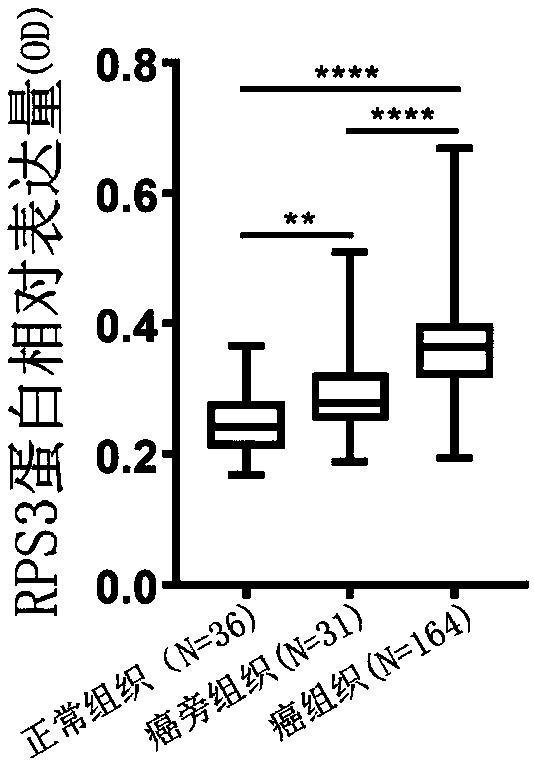
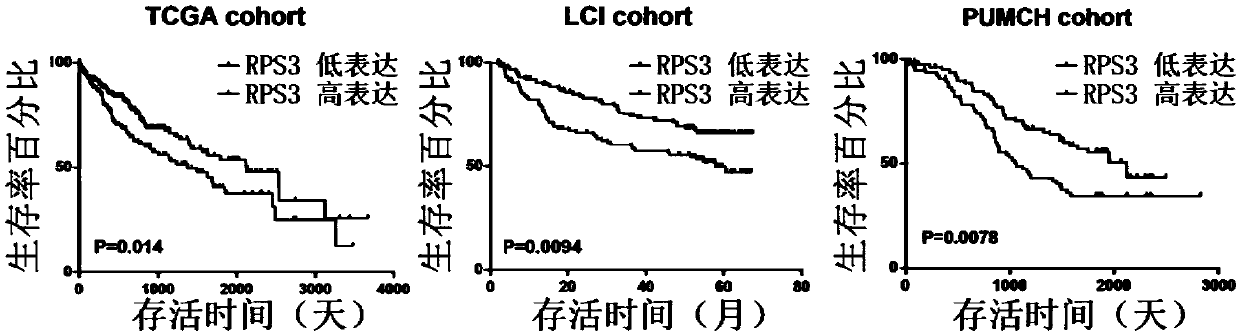
Biomarker in diagnosis of liver cancer and kit containing biomarker in diagnosis of liver cancer
ActiveCN111154869AMicrobiological testing/measurementBiological testingBiomarker (medicine)Cancer research
The invention provides a biomarker in diagnosis of liver cancer and application of the biomarker in diagnosis of liver cancer in production of a diagnostic reagent of liver cancer. It is found throughresearch that overexpression of RPS3 can promote liver cancer cell proliferation, clone formation, migration, invasion and the transformation capability of epithelial cells into mesenchymal cells, and on the contrary, knock-down of RPS3 can inhibit liver cancer cell proliferation, clone formation, migration, invasion and the transformation capability of epithelial cells into mesenchymal cells; after a liver cancer cell strain with knock-down RPS3 is subcutaneously injected into nude mice, the tumorigenicity is reduced; it is also found in people that compared with expression of RPS3 in para-carcinoma tissue, expression of RPS3 in liver cancer tissue is high, and high expression of the RPS3 affects the prognosis survival rate of patients to a certain extent; and on the basis, the biomarkerin diagnosis of liver cancer and application of the biomarker in diagnosis of liver cancer in production of the diagnostic reagent of liver cancer are provided. The biomarker is a RPS3 gene, mRNA ofthe RPS3 gene, or protein or a protein fragment encoded by the RPS3 gene, and the biomarker can be used as a medicine target for screening liver cancer medicines.
Owner:PEKING UNIV
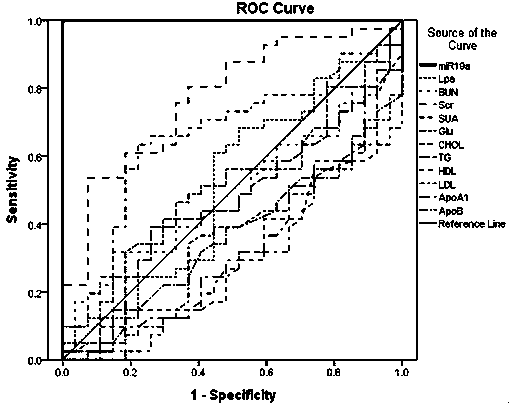
Serological biomarker miR-19a for detecting coronary heart disease and application of serological biomarker miR-19a
InactiveCN104278105AAccurate predictionMicrobiological testing/measurementDNA/RNA fragmentationCoronary heart diseaseBiomarker (medicine)
The invention relates to a serological biomarker miR-19a for detecting the coronary heart disease and application of the serological biomarker miR-19a, belonging to the fields of laboratory medicines, clinical medicines, biotechnology, biochemistry and molecular biology. The miRNA sequence is as follows: miR-19a: UGUGCAAAUCUAUGCAAAACUGA. The invention further discloses application of the miR-19a. The miR-19a is used for preparing a reagent product for screening the coronary heart disease or assisting coronary heart disease pathological authentication, biochemical detection and clinical diagnosis. The reagent product comprises a primer system and a primer probe system, wherein the primer system consists of cDNA amplification primers of the miR-19a; the primer probe system consists of the cDNA amplification primers of the miR-19a and a probe. The biomarker miR-19a molecule is closely related with the occurrence and the development of the coronary heart disease; and serving as the biomarker for predicating the occurrence of the coronary heart disease and judging the development degree of the coronary heart disease, compared with the existing normal pathological type diagnosis for an attack of the coronary heart disease and prognosis, the serological biomarker miR-19a can be used for predicating the early-stage morbidity and the malignancy degree of the coronary heart disease individually and accurately.
Owner:雷桅
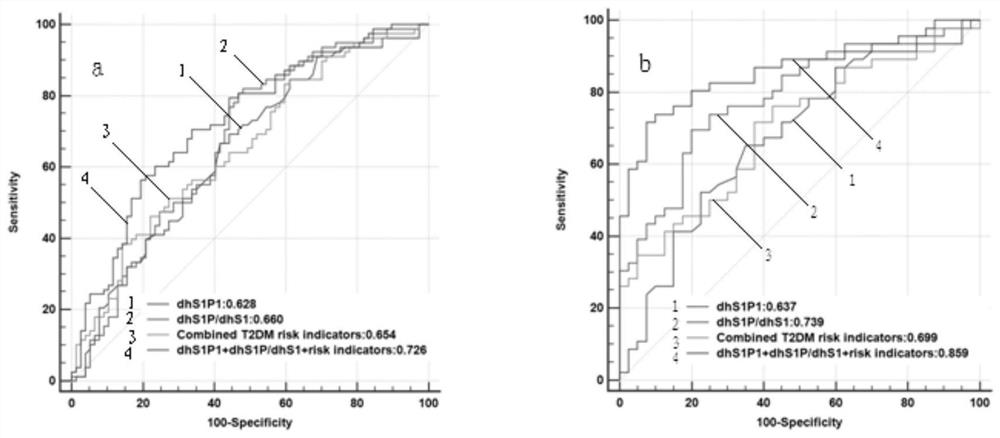
Biomarker for predicting early diabetes mellitus and diabetes mellitus occurrence and detection method and application of biomarker
ActiveCN113295793AImprove relevanceImprove accuracyComponent separationLow density lipoprotein cholesterolA lipoprotein
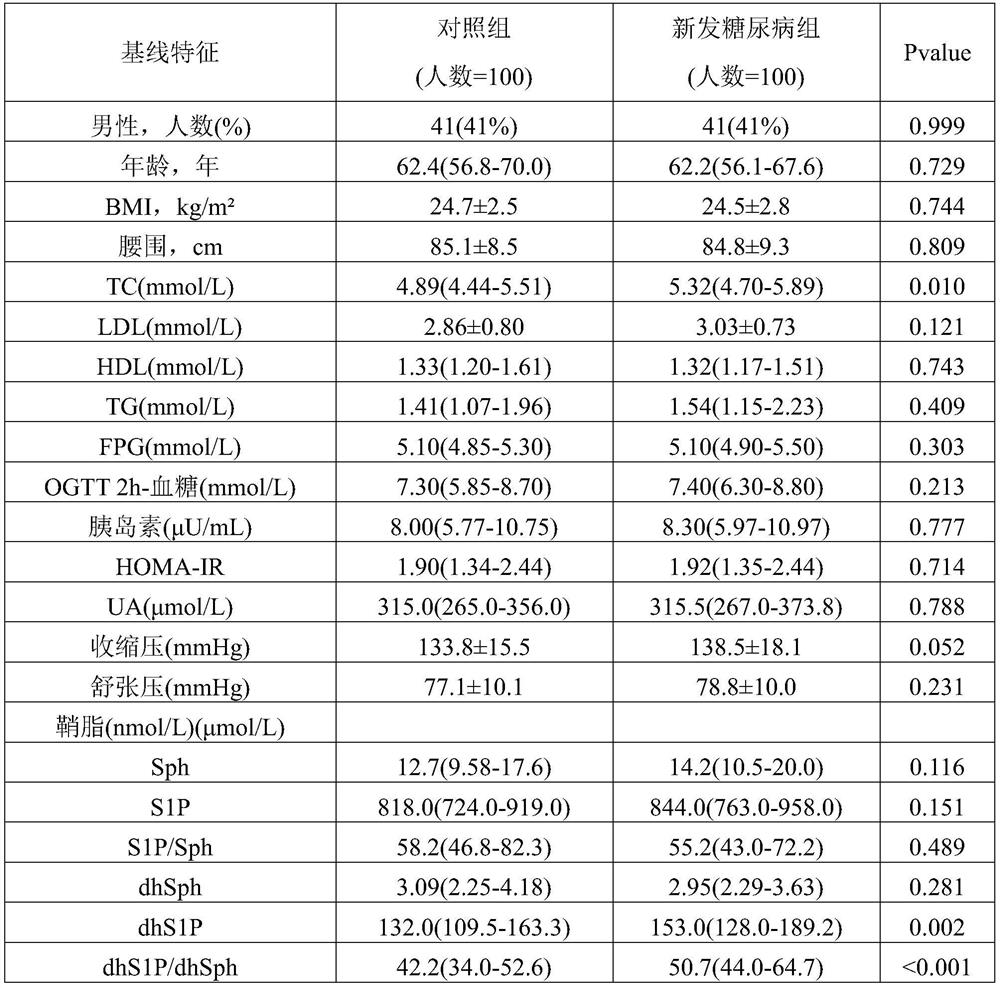
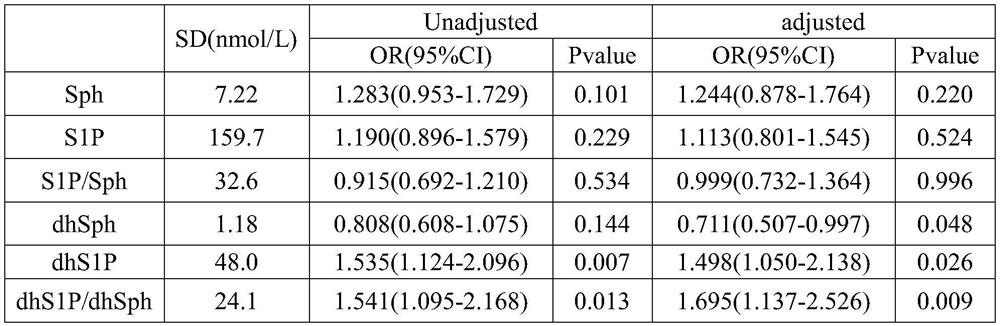
The invention discloses a biomarker for predicting early diabetes mellitus and diabetes mellitus occurrence and a detection method and application of the biomarker, and belongs to the technical field of biological medicines. The biomarkers are sphingosine dihydrogen (dhSph) and sphingosine 1-phosphate (dhS1P) in serum, the levels of the dhS1P and the dhSph in a serum sample of a subject are detected through high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS), and the ratio of the dhS1P to the dhSph is calculated; wherein the level of the ratio of dhS1P to dhS1P / dhSph in the serum sample is in positive correlation with the increase of the risk of diabetes; traditional diabetes risk factors including age, body mass index, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, fasting blood glucose, postprandial blood glucose, insulin resistance index and systolic pressure are combined to predict early diabetes, and the biomarker has high prediction value.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Recurrent spontaneous abortion biomarker and applications thereof
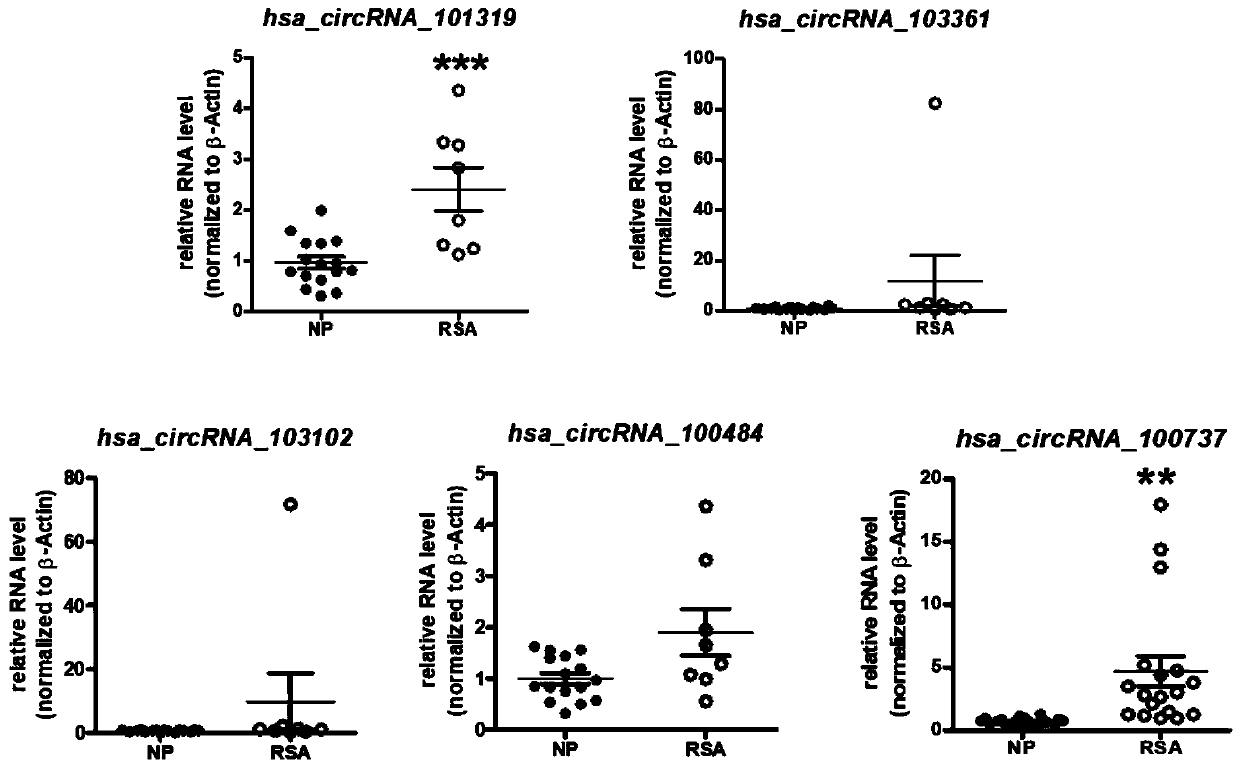
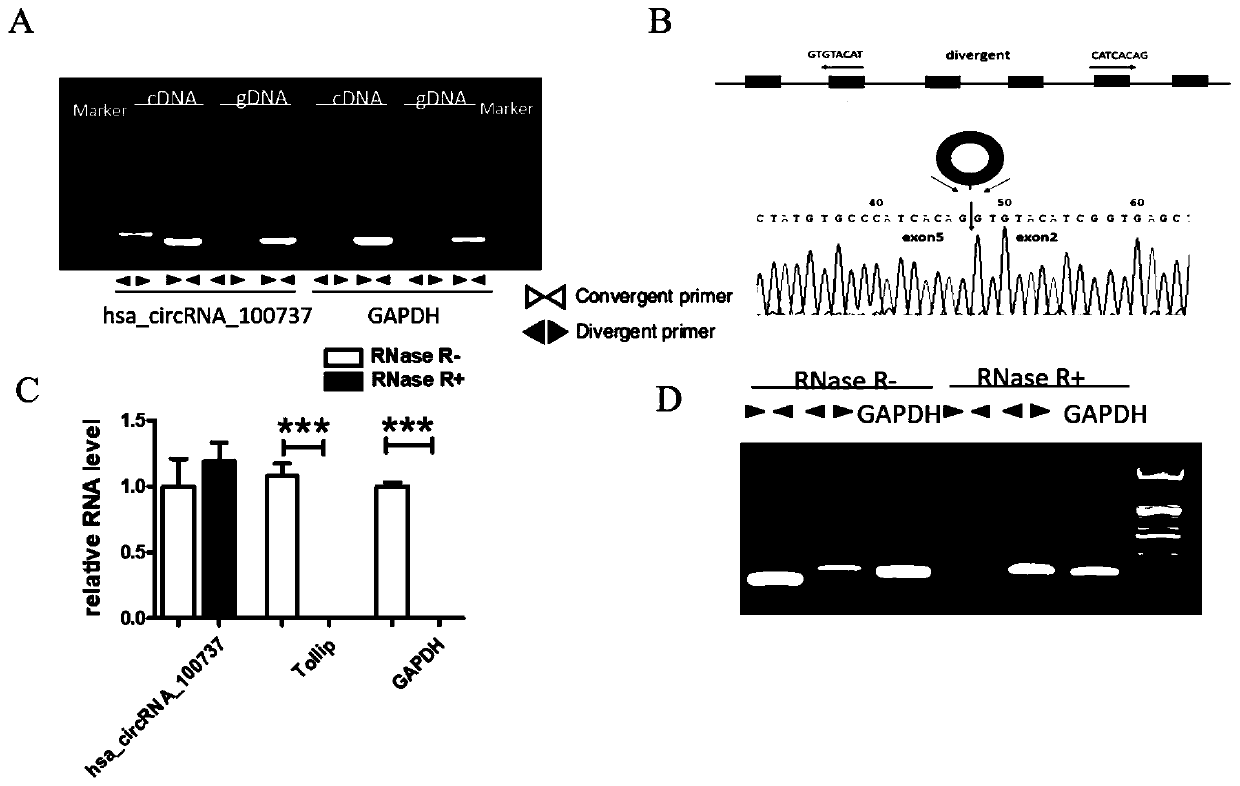
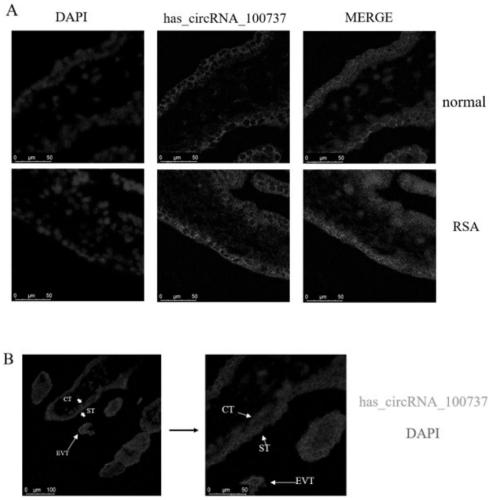
PendingCN111593116APrevent invasionPrevent proliferationMicrobiological testing/measurementSexual disorderDiseasePhysiology
The invention discloses a recurrent spontaneous abortion biomarker and applications thereof, and belongs to the technical field of biomedicine. The circular RNA used for diagnosing recurrent spontaneous abortion is hsa_circRNA_100737, can be highly expressed in human RSA villi tissue in early pregnancy, can regulate the expression of IL-34 through miR-28-5p and can influence the proliferation, invasion and tube-forming functions of trophoblast through a miR-28-5p-IL-34 axis. The differential expression of the hsa_circRNA_100737 in normal early pregnancy and RSA villi tissue is taken as a breakthrough point to discuss the influences of the hsa_circRNA_100737 on the biological behavior of the trophoblast and mechanisms and to research whether the function of the hsa_circRNA_100737 of regulating the trophoblast can influence maternal-fetal immune tolerance, so that the pathogenesis of RSA can be hopefully analyzed, and new ideas can be provided for the prevention and treatment of diseasessuch as the RSA.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV
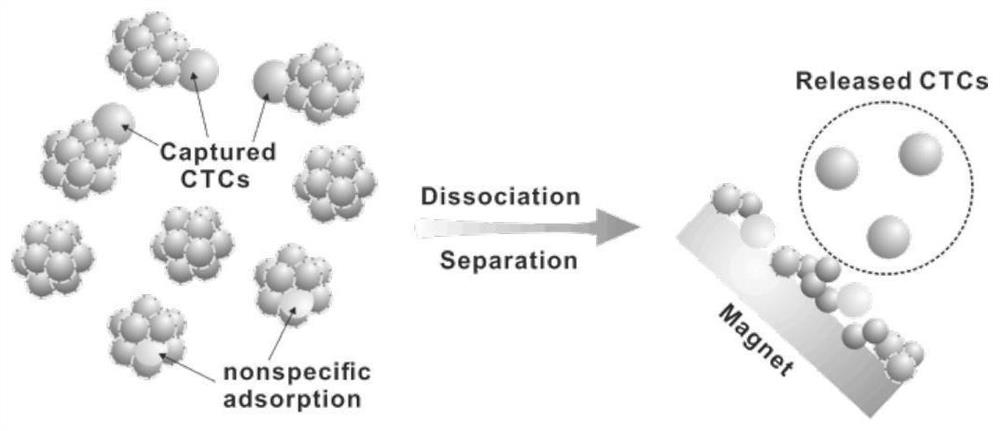
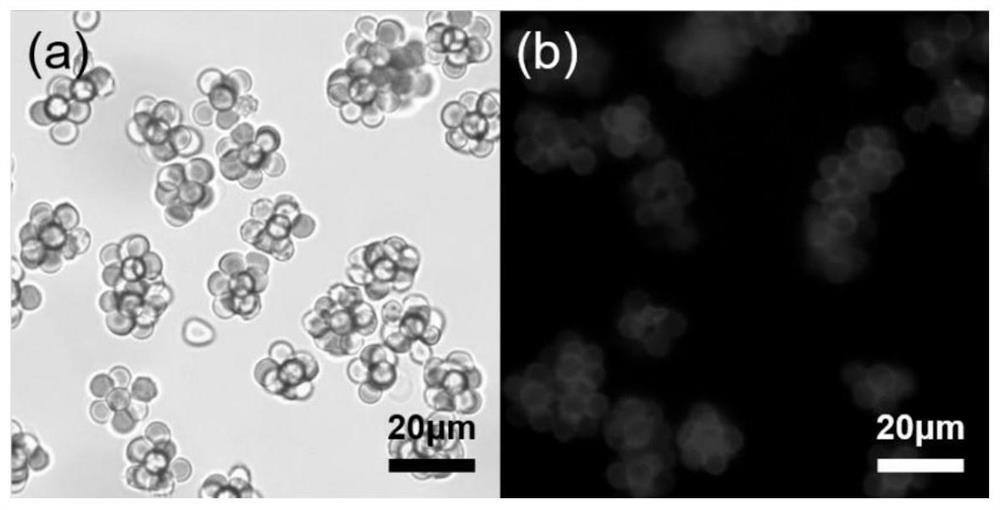
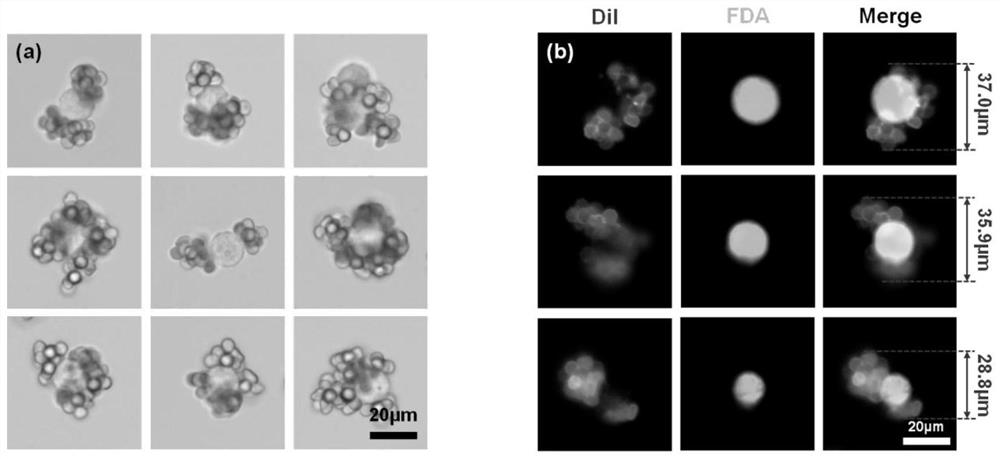
Erythrocyte cluster for enriching circulating tumor cells (CTCs) based on size filtering method
ActiveCN111826349ACapture firmer and more efficientAchieve capture purityBlood/immune system cellsMaterial analysisWhite blood cellBiomarker (medicine)
The invention discloses an erythrocyte cluster for enriching circulating tumor cells (CTCs) based on a size filtering method, and belongs to the technical field of biological medicine. By designing amicron-level CTCs targeting biomimetic material, erythrocytes are combined with a micro-sphere to obtain an erythrocyte cluster type biomimetic material; the surface of each erythrocyte is modified with polybrene, thereby eliminating original electrostatic repulsion between the erythrocytes, and then, dense full-layer coverage of the erythrocytes on the surface of the micro-sphere can be realized.Wrapping the micro-sphere with the erythrocytes can avoid non-specific adsorption of leukocytes on the surface of the microsphere; meanwhile, folic acid (FA) modifying the surface of erythrocyte cluster or an antibody which can specifically recognize and bind surface biomarkers of the CTCs can directly target the CTCs to achieve increase of sizes of the CTCs, and accordingly, leukocytes in a blood sample of a clinical patient can be finally removed in a manner of filtering, and the high-purity CTCs are obtained efficiently.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
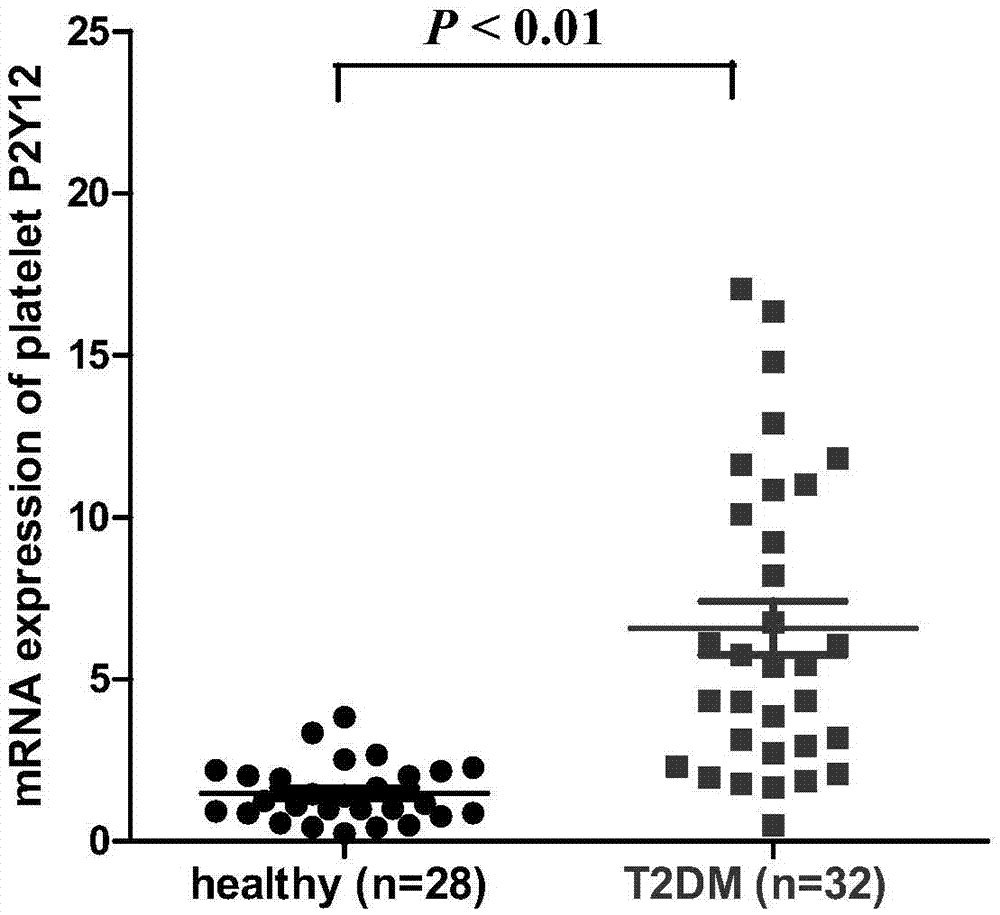
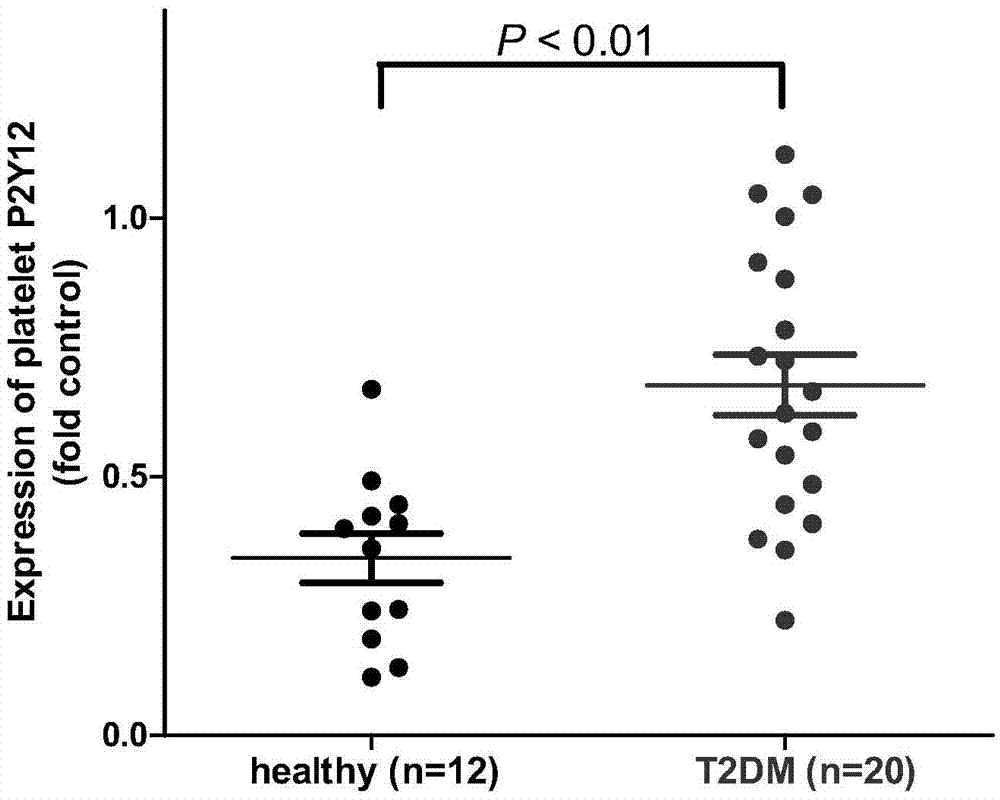
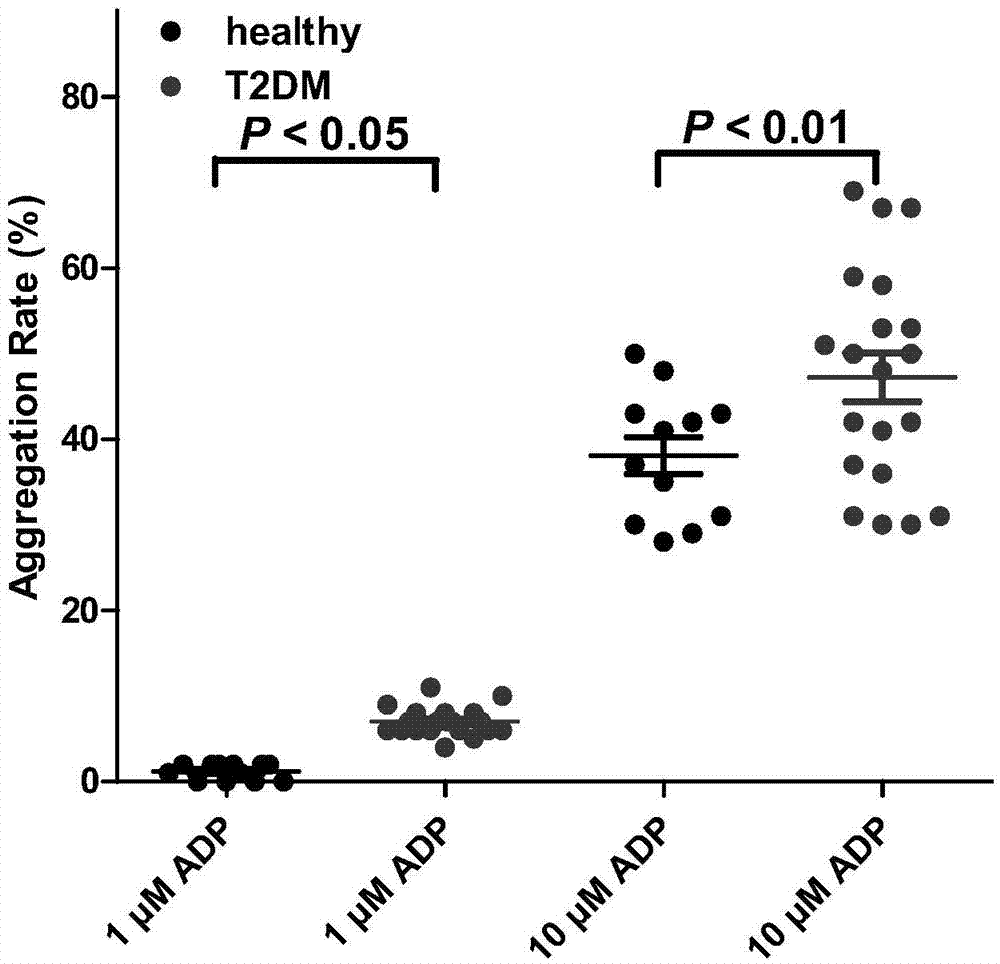
Biomarker for predicting II-type diabetic vascular complication risk and application thereof
InactiveCN106906278AImprove responsePersonalized Intervention TherapyOrganic active ingredientsMetabolism disorderScreening methodWestern blot
The invention belongs to the technical field of biology and diagnosis, and relates to a receptor of a definite, effective and visualized platelet reactivity biomarker P2Y12 and an application thereof for diagnosing and predicting the II-type diabetic vascular complication risk. The platelet activation biomarker P2Y12 can detect expression levels of platelet through fluorescent quantitative polymerase chain reaction (PCR), Western blot, enzyme linked immunosorbent assay (ELISA), flow cytometry, UV spectrophotometry-near infrared spectrometry, high performance liquid chromatography, colorimetry, a mass spectrum identification method and the like and detect content of P2Y12 in whole blood. The invention also provides a method of biomarker P2Y12 for diagnosing and predicting vascular complication of subjects as well as a medicine for treating II-type diabetic vascular complication and a screening method of the medicine. The invention further provides an intervene plane for individualizing P2Y12 inverse agonist and accurately treating the vascular complication of II-type diabetic patients.
Owner:FUDAN UNIV
Cuinse/zns nir-quantum dots (QDS) for biomedical imagiing
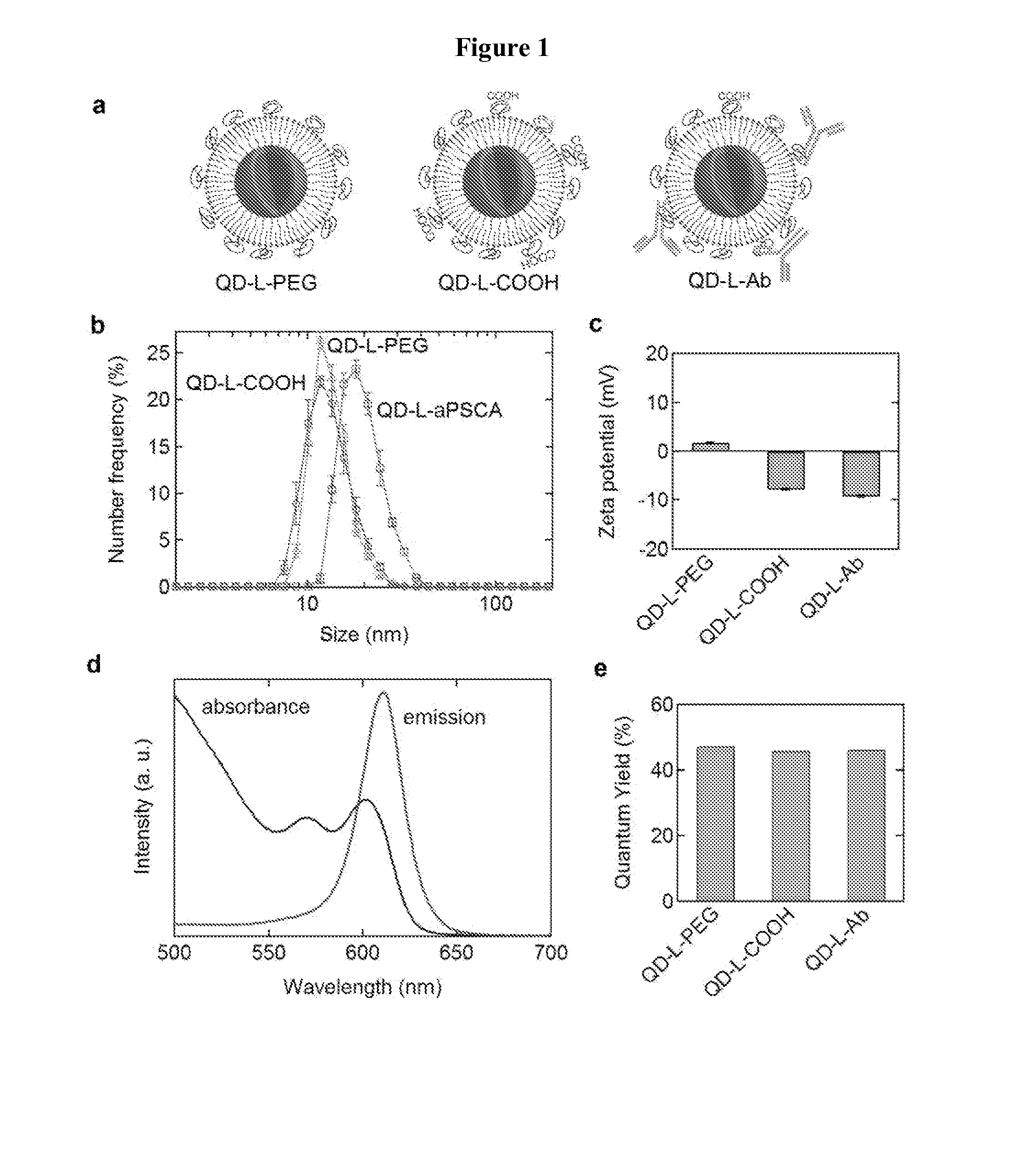
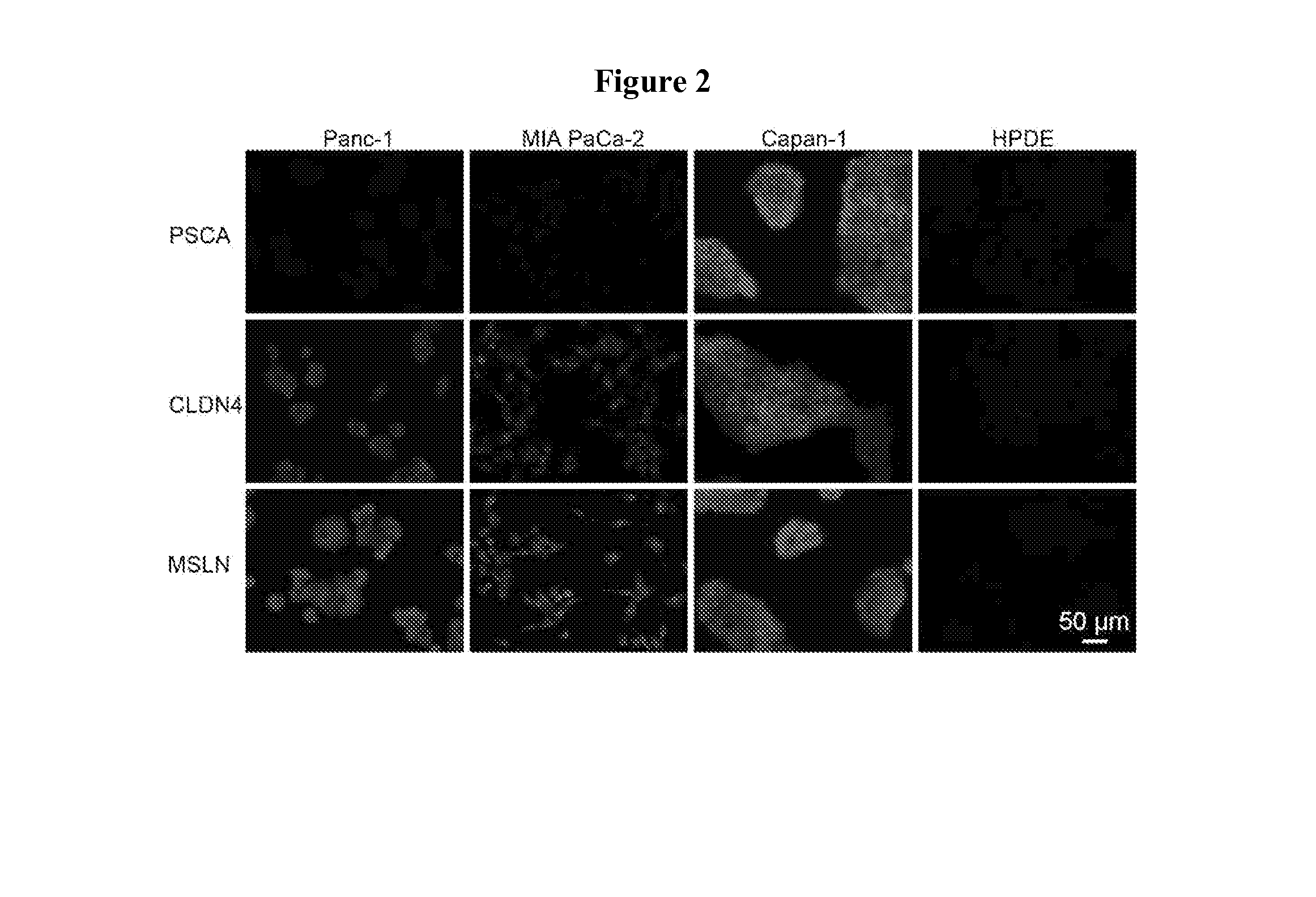
InactiveUS20140030193A1High quantum yieldImprove water stabilityUltrasonic/sonic/infrasonic diagnosticsSurgeryQuantum dotIn vivo
Applications in nanomedicine, such as diagnostics and targeted therapeutics, rely on the detection and targeting of membrane biomarkers. Disclosed herein are functionalized quantum dots exhibiting greater stability in water, methods of making the functionalized quantum dots and methods of in vivo imaging using the functionalized quantum dots.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Magnetic chitosan/doped rare earth compound particles and preparation method thereof
InactiveCN102329438AGood biocompatibilityLow toxicityInorganic non-active ingredientsEmulsion deliveryChemical compoundPharmaceutical drug
The invention relates to magnetic chitosan / doped rare earth compound particles and a preparation method thereof. The composition of the magnetic chitosan / doped rare earth compound particles is that: the weight ratio of chitosan to Fe3O4 (based on Fe) is 20:1-1:5; the weight ratio of doped rare earth compounds (based on La) to the chitosan is 2:100-50:100; and the doped rare earth compounds are LaF3-doped Eu as well as LaF3-doped Ce<3+> and Tb<3+> respectively. The preparation method comprises the following steps of: preparing magnetic chitosan (Fe3O4 / CS) and the doped rare earth compounds respectively; and preparing the magnetic chitosan / doped rare earth compound particles through ionic crosslinking by a reverse microemulsion method. The preparation method is simple. The obtained compound particles have better magnetic responsiveness and fluorescent characteristics, high biocompatibility, and low toxicity, can be used as a multi-functional biomarker probe in nuclear magnetic resonance imaging and fluorescence imaging, and are widely applied to the fields of targeted medicines, enzyme immobilization, biomolecular determination, cellular segregation and the like.
Owner:WUHAN UNIV
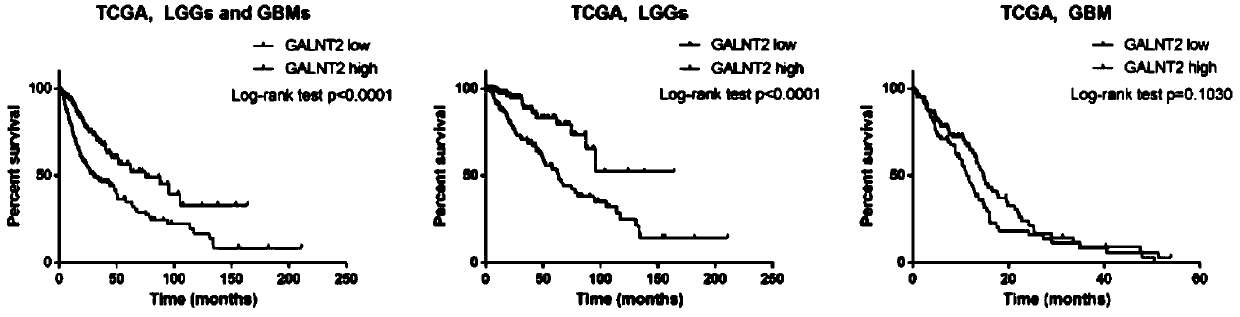
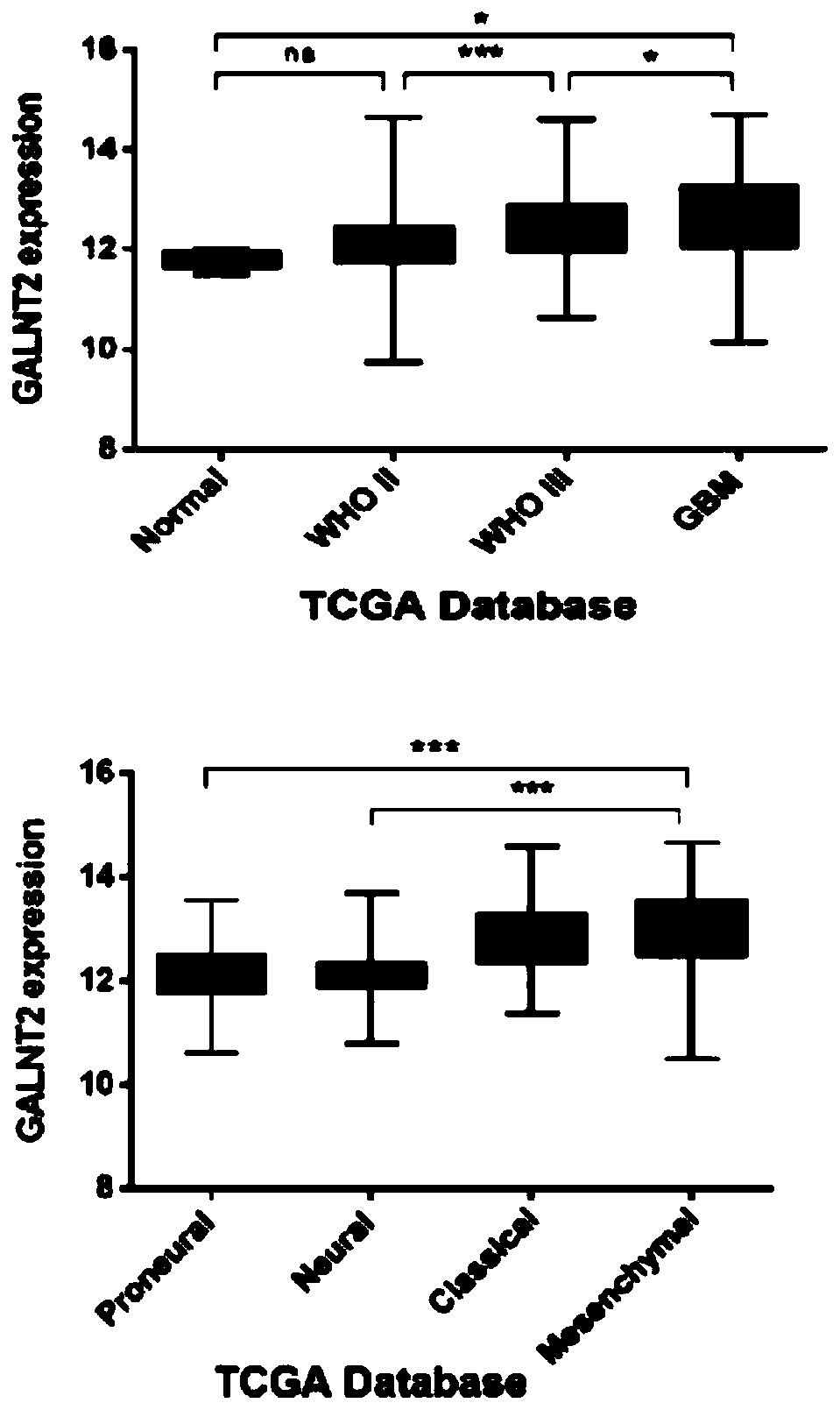
Application of GALNT2 as biomarker in diagnosis and/or treatment of glioma
ActiveCN109825579AStrong specificityHigh sensitivityOrganic active ingredientsMicrobiological testing/measurementPhosphorylationMalignant progression
The invention belongs to the field of medicine, and particularly relates to application of GALNT2 as a biomarker in the treatment and / or prognosis of glioma. GALNT2 promotes malignant progression of the glioma by affecting O-type glycosylation and phosphorylation of EGFR and affecting the downstream PI3K / Akt passage. Therefore, GALNT2 can serve as the novel biomarker and a potential target for future glioma treatment.
Owner:山东华链医疗科技有限公司
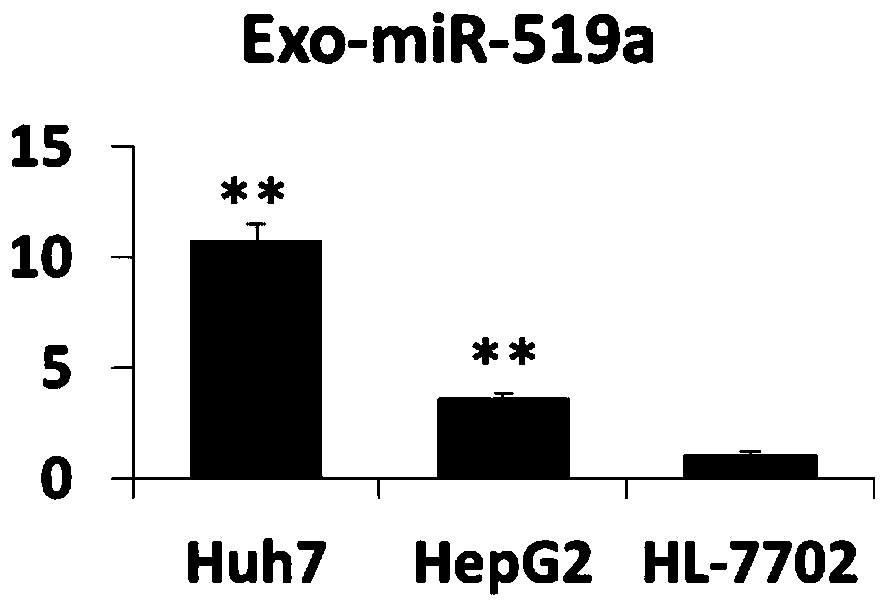
Blood exosome molecular marker and application thereof in preparation of liver cancer diagnostic products
ActiveCN110129440AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationLymphatic SpreadTrue positive rate
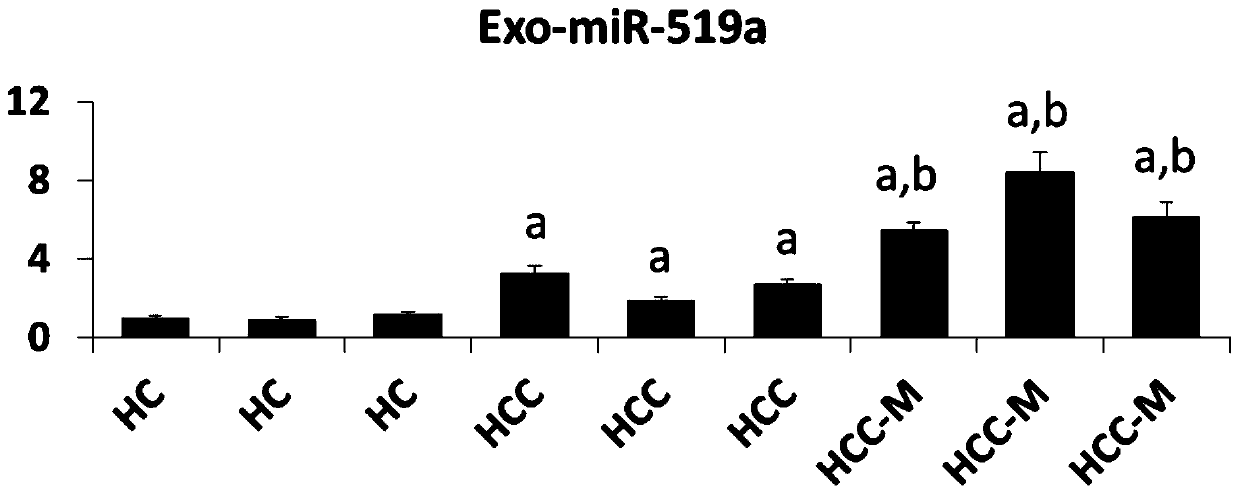
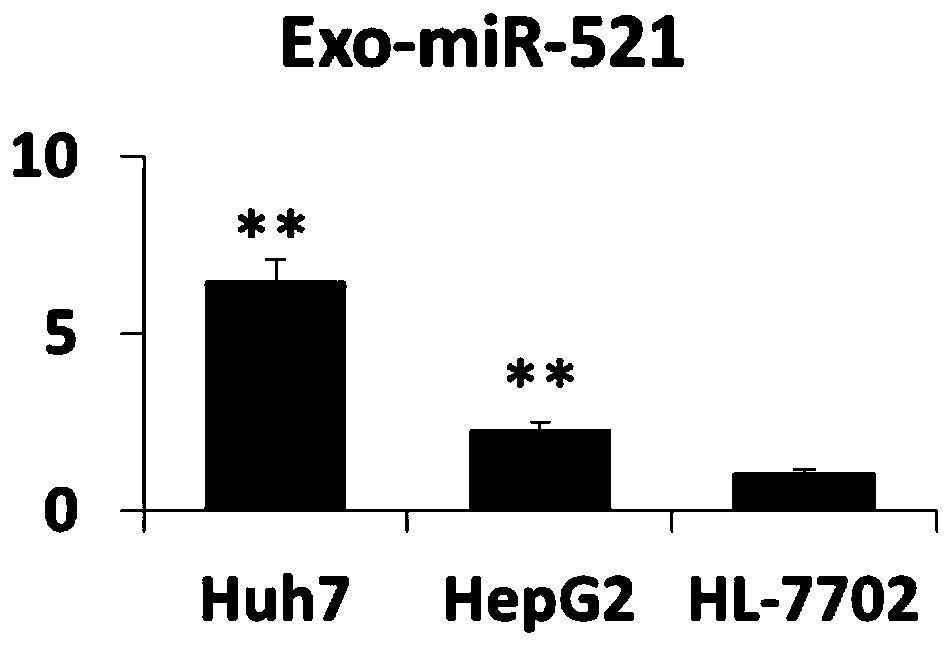
The invention belongs to the field of biomedicine and relates to a blood exosome molecular marker and application thereof in the preparation of liver cancer diagnostic products, in particular a bloodexosome (miR-519 and / or miR-521) molecular marker associated with liver cancer diagnosis and application thereof in the preparation of liver cancer diagnostic products, with miR-519a being CCUCUAGAGGGAAGCGCUUUCU and miR-521 being AACGCACUUCCCUUUAGAGUGU. The blood Exo-miR-519a and / or Exo-miR-521 is a novel biomarker, having good stability, causing minimal invasion, having good convenience of detection and good quantification precision; disease diagnostic sensitivity and specificity can be improved greatly; the development of the biomarker herein helps diagnose liver cancer and warn about metastasis risk, and references are provided for the research and development of other tumor like biomarkers.
Owner:ZHEJIANG UNIV
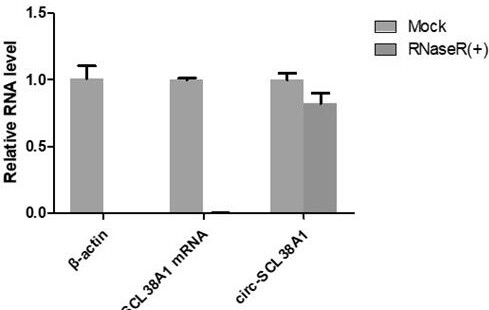
Application of circ-SLC38A1 as target point in medicine for inhibiting bladder cancer cells
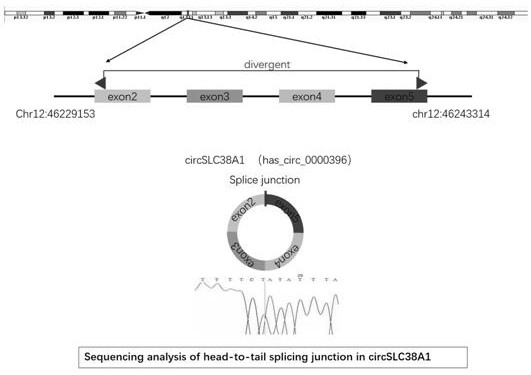
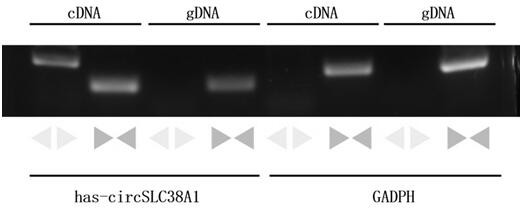
ActiveCN112011620AInhibit migrationAbility to inhibit invasionOrganic active ingredientsMicrobiological testing/measurementCancer cellBladder cancer patient
The invention belongs to the technical field of tumor biological gene therapy, and relates to a bladder cancer diagnosis and treatment biomarker and a target spot, in particular to application of circ-SLC38A1 as a target spot in a bladder cancer cell inhibition medicine. The invention discloses an expression condition of circ-SLC38A1 in tumor tissues of a bladder cancer patient and a function of circ-SLC38A1 in bladder cancer cells. It is found for the first time that circ-SLC38A1 can be used as a bladder cancer diagnosis biomarker and a drug therapy target. Compared with normal control, circ-SLC38A1 is significantly up-regulated in tumor tissues of bladder cancer patients. The result of the invention shows that interference on circ-SLC38A1 can inhibit the migration and invasion ability ofbladder cancer cells, and it is shown that the circ-SLC38A1 plays a role of a cancer-promoting gene in the occurrence and development of bladder cancer, and provides a new target for clinical treatment of bladder cancer.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
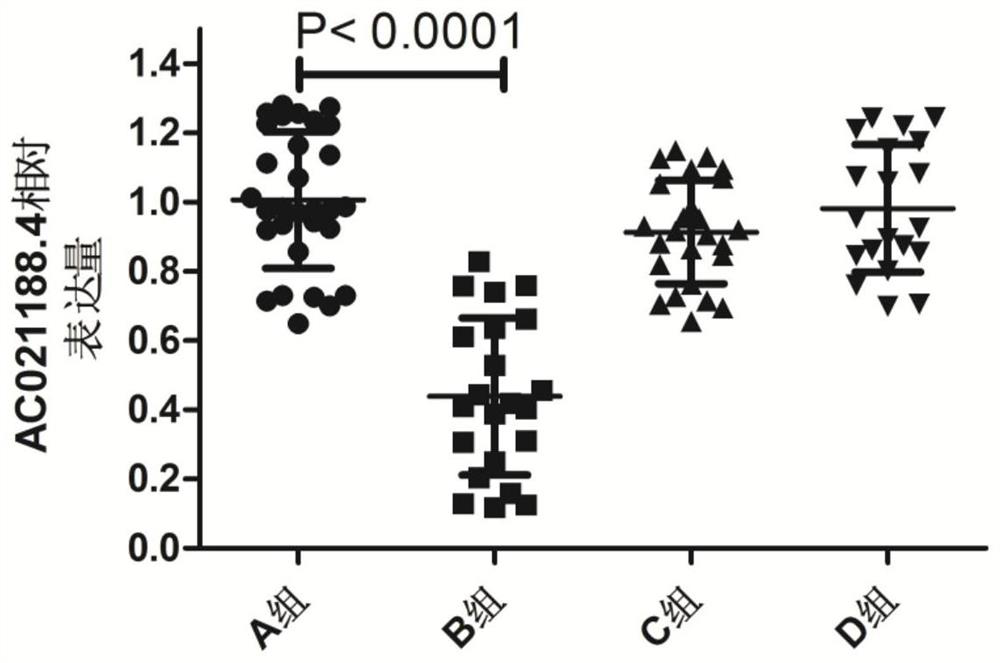
Biomarker for diabetes mellitus complicated with coronary heart disease
ActiveCN112522392AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationRNA extractionCoronary heart disease
The invention provides a biomarker for diabetes mellitus complicated with coronary heart disease, and belongs to the technical field of biomedicine. Through RNA extraction, it is found that compared with serum of a healthy human body, the expression quantity of the gene AC021188.4 in serum of a patient suffering from the diabetes mellitus complicated with the coronary heart disease is remarkably reduced, while the expression quantity of the gene AC021188.4 does not remarkably change in serum of a patient suffering from the simple coronary heart disease and serum of a patient suffering from thesimple diabetes mellitus, and therefore the AC021188.4 can serve as a gene marker for the mellitus complicated with coronary heart disease.
Owner:青岛山大齐鲁医院(山东大学齐鲁医院(青岛))
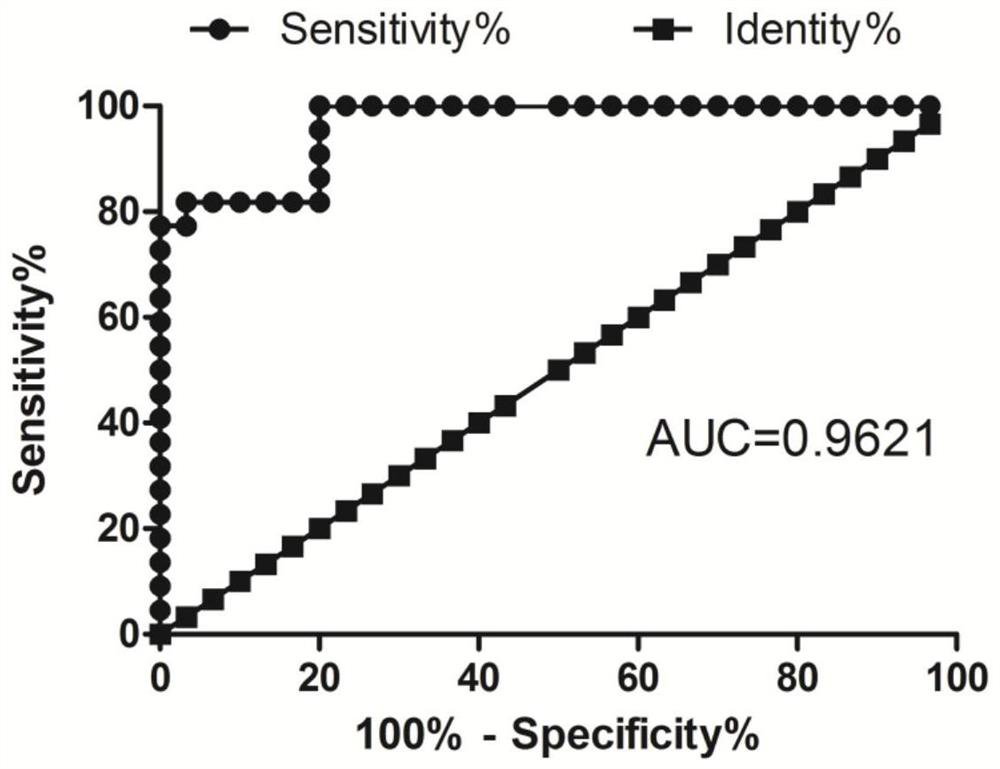
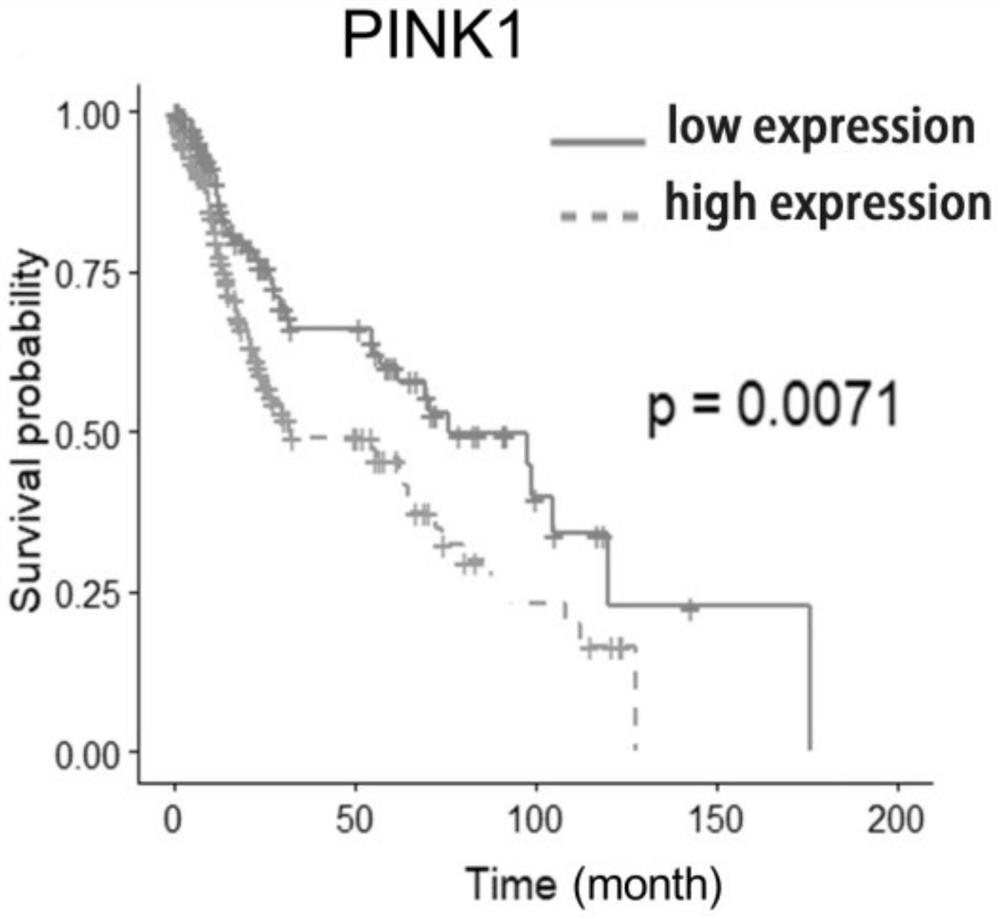
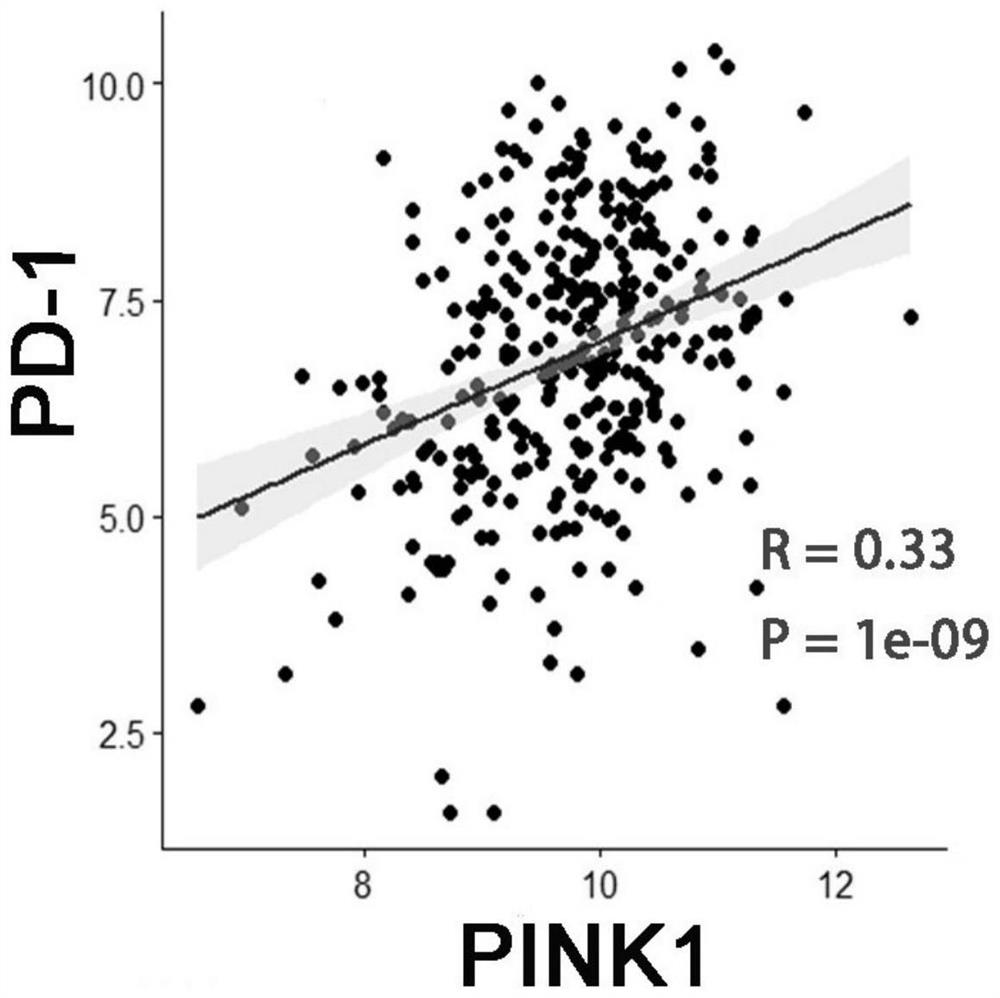
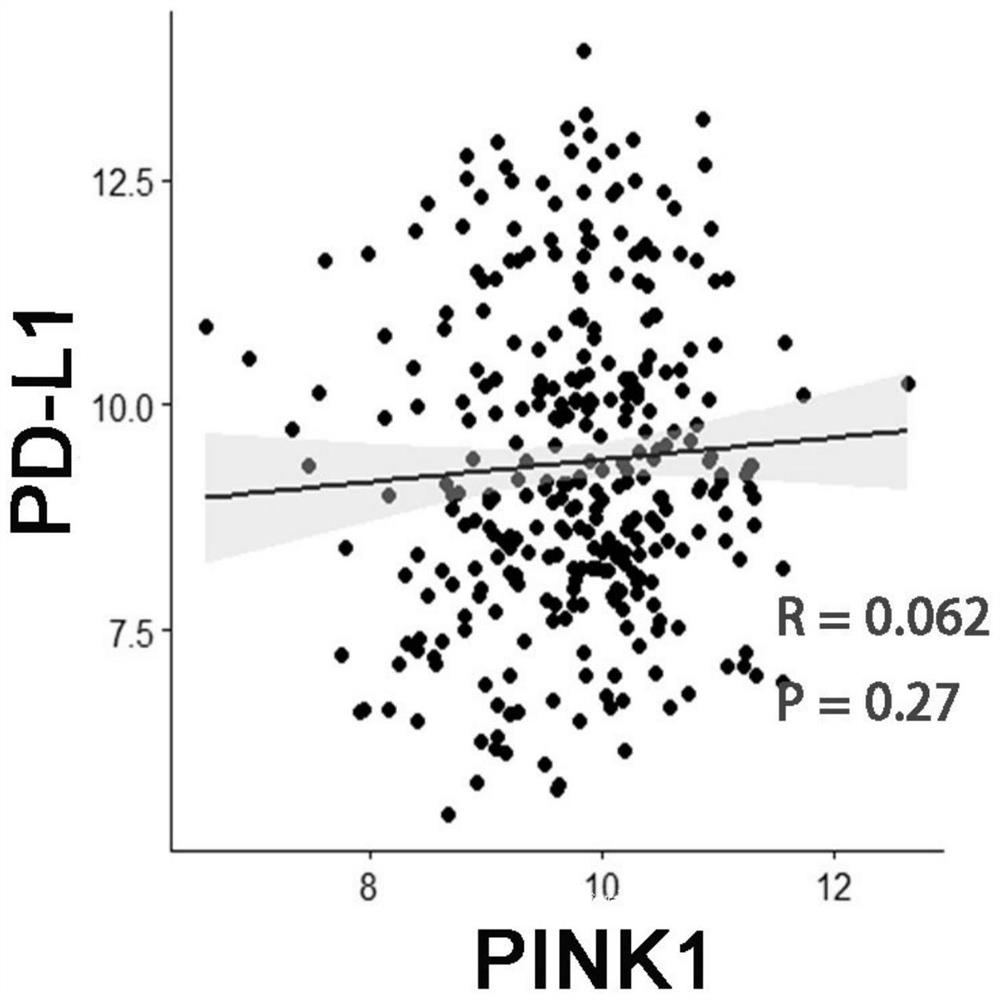
Application of PINK1 as diagnostic marker in construction of lung squamous cell carcinoma prognosis prediction model
PendingCN112908406AIncreased sensitivityStrong specificityMolecular designHealth-index calculationBiomarker (medicine)Biology
The invention relates to the technical field of bio-medicine, in particular to application of PINK1 serving as a diagnostic marker in construction of a lung squamous cell carcinoma prognosis prediction model. The biomarker PINK1 and other clinical indexes are used in a combined mode, and lung squamous cell carcinoma prognosis detection can be assisted. According to the invention, screening and construction are carried out after full transcriptome sequencing and machine learning of a lung squamous cell carcinoma specimen based on large-sample anti-tumor immuno-therapy, so that the prognosis condition of a lung squamous cell carcinoma patient can be efficiently and accurately predicted; and meanwhile, according to the correlation between the risk and different immune cell infiltration levels, immune-related pathways, key immune checkpoint inhibitor expression levels and the like, comprehensive evaluation of the tumor immune micro-environment is achieved, effective guidance is provided for clinicians to make a treatment decision on lung squamous cell carcinoma patients, invalid treatment is reduced, and therefore, the treatment cost and discomfort experience of the patients are reduced.
Owner:JINSHAN HOSPITAL FUDAN UNIV
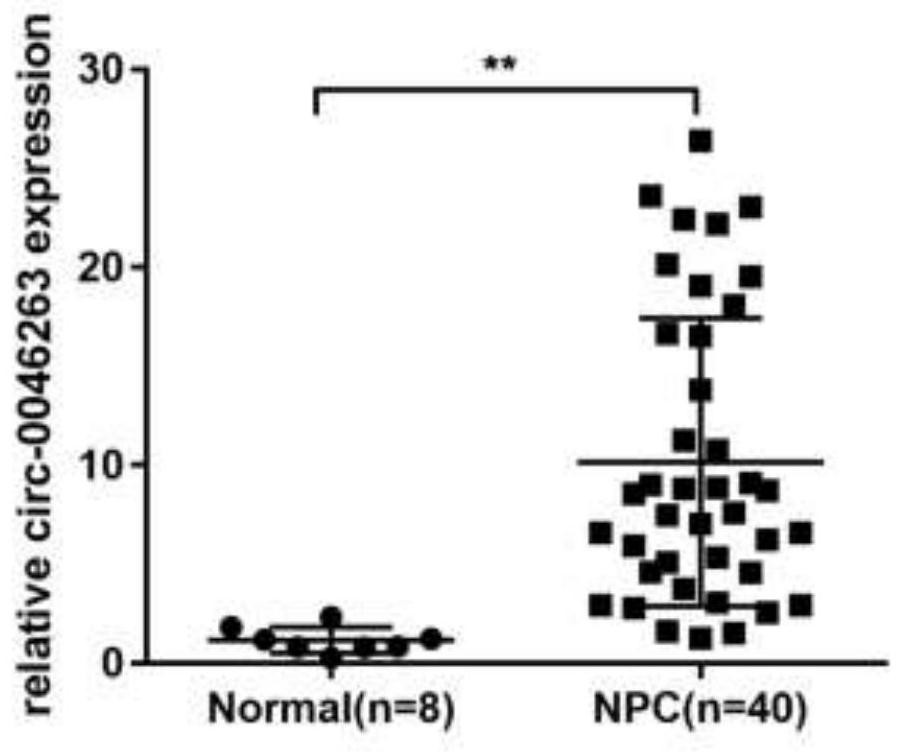
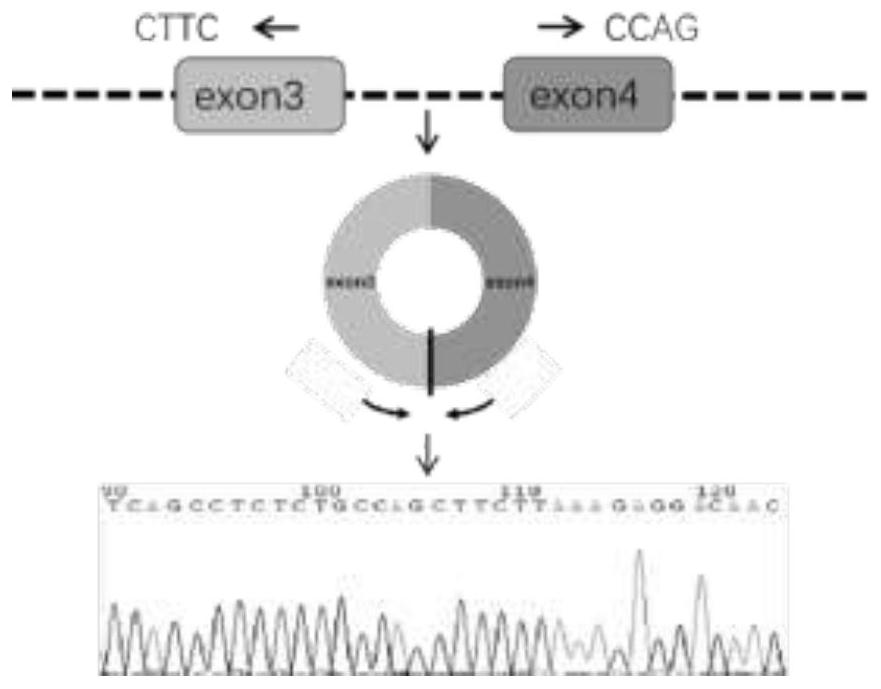
Biomarker for nasopharyngeal carcinoma metastasis diagnosis and/or prognosis evaluation
ActiveCN111718995APromote proliferationPromote migrationMicrobiological testing/measurementAntineoplastic agentsNasopharyngeal cancerTissue sample
The invention discloses a biomarker for nasopharyngeal carcinoma metastasis diagnosis and / or prognosis evaluation. In the invention, we prove that circ-0046263 is highly expressed in a nasopharyngealcarcinoma cell line compared with a normal nasopharyngeal epithelial cell NP69. Clinical tissue sample detection results show that the expression of circ-0046263 in nasopharyngeal carcinoma tissues isup-regulated and is in positive correlation with stages. In-vitro cell function experiments show that circ-0046263 can promote proliferation, migration and invasion of cells and promote epithelial-mesenchymal transition of the cells. Therefore, circ-0046263 can be used as a biomarker to be applied to preparation of a nasopharyngeal carcinoma metastasis diagnosis and / or prognosis evaluation reagent, a kit or a detection device, and also can be used as a target spot to be applied to screening of medicines for treating nasopharyngeal carcinoma or nasopharyngeal carcinoma metastasis; and a reagent for inhibiting circ-0046263 expression can be used for preparing a medicine for treating nasopharyngeal carcinoma or nasopharyngeal carcinoma metastasis.
Owner:JIANGSU CANCER HOSPITAL
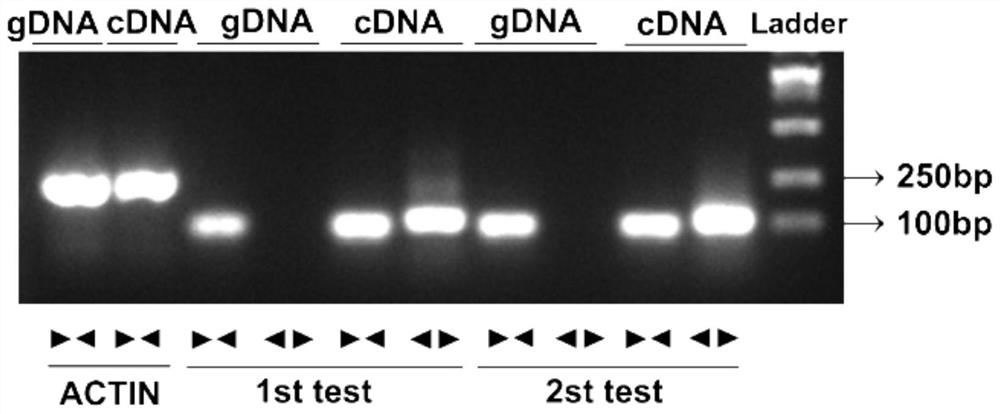
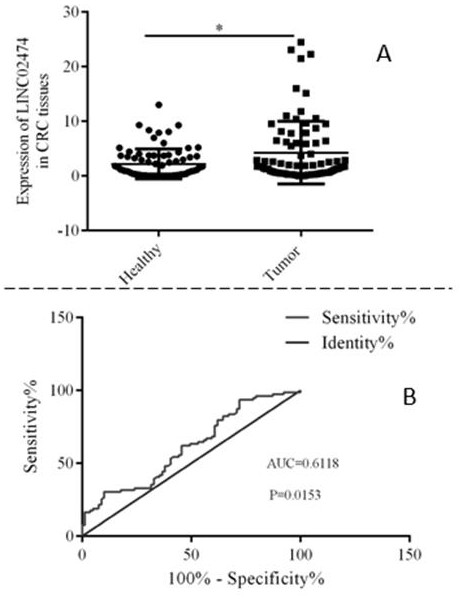
Application of LINC024724 as colorectal cancer diagnosis marker and treatment target
ActiveCN112410432AImprove accuracyInhibit migrationOrganic active ingredientsMicrobiological testing/measurementTreatment targetsCancer gene
The invention belongs to the technical field of biological medicines, and relates to a colorectal cancer diagnosis marker and a treatment target and application thereof. The invention firstly providesthat LINC02474 can be used as the colorectal cancer diagnosis marker and the treatment target, and provides a new thought for diagnosis and treatment of colorectal cancer. Researches show that compared with normal control, LINC02474 is remarkably up-regulated in tumor tissues of a patient with colorectal cancer, and the biomarker provided by the invention has relatively high accuracy. Results show that interfering LINC02474 can inhibit migration and invasion abilities of colorectal cancer cells, and prove that LINC02474 plays a role in promoting cancer genes in occurrence and development of colorectal cancer, provides a new thought for clinical treatment of colorectal cancer, and is expected to obtain a diagnosis and treatment scheme which is more acceptable to patients and is clinicallyfeasible.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
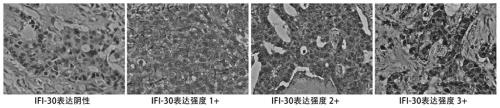
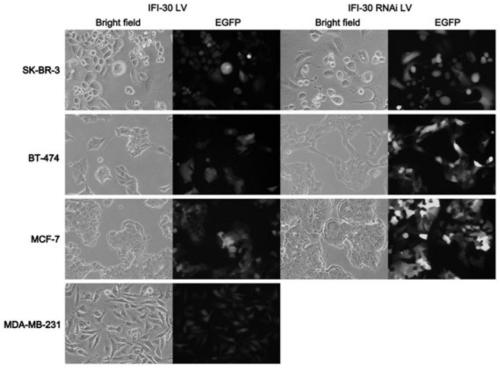
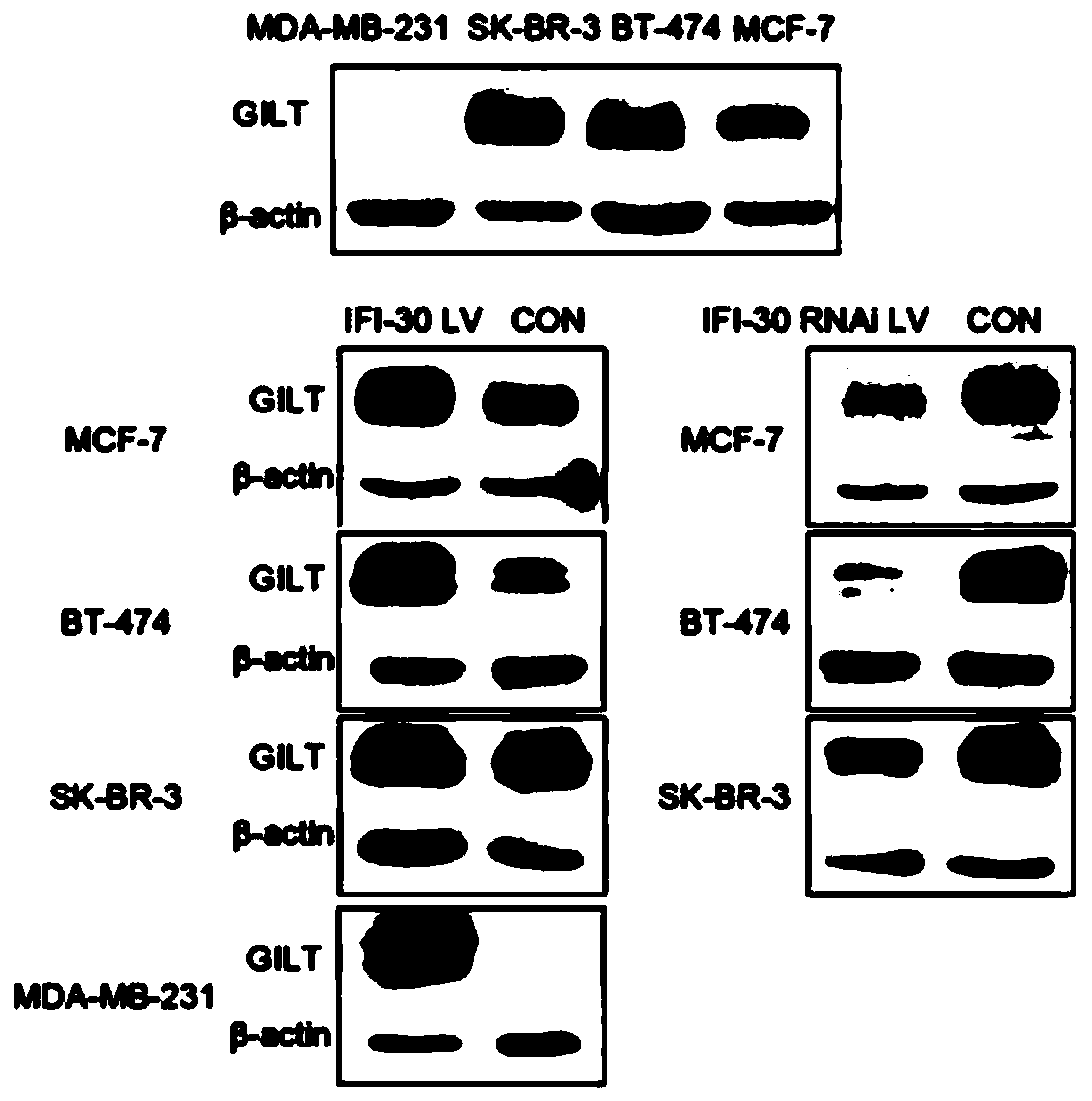
Application of protein/gene IFI30 related to breast cancer occurrence and development
InactiveCN111575382APromote proliferationReduce apoptosisPeptide/protein ingredientsMicrobiological testing/measurementPharmaceutical drugBiomarker (medicine)
The invention belongs to the technical field of biological medicines, particularly relates to application of protein / gene IFI30 related to breast cancer occurrence and development, and particularly provides application of the protein / gene IFI30 related to breast cancer occurrence and development, especially application value of the protein / gene IFI30 as a breast cancer molecular typing marker andapplication of the protein / gene IFI30 in preparation of a breast cancer molecular typing kit. The invention also provides a method for screening potential drugs for treating breast cancer. Whether thecandidate is a potential drug for treating breast cancer or not is judged by adjusting the expression level of the biomarker.
Owner:余之刚
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com