Biomarker for predicting II-type diabetic vascular complication risk and application thereof
A technology for diabetes and complications, applied in cardiovascular system diseases, biological tests, biochemical equipment and methods, etc., can solve problems such as inability to choose treatment options, high mortality, and poor treatment effects of cardiovascular and cerebrovascular complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
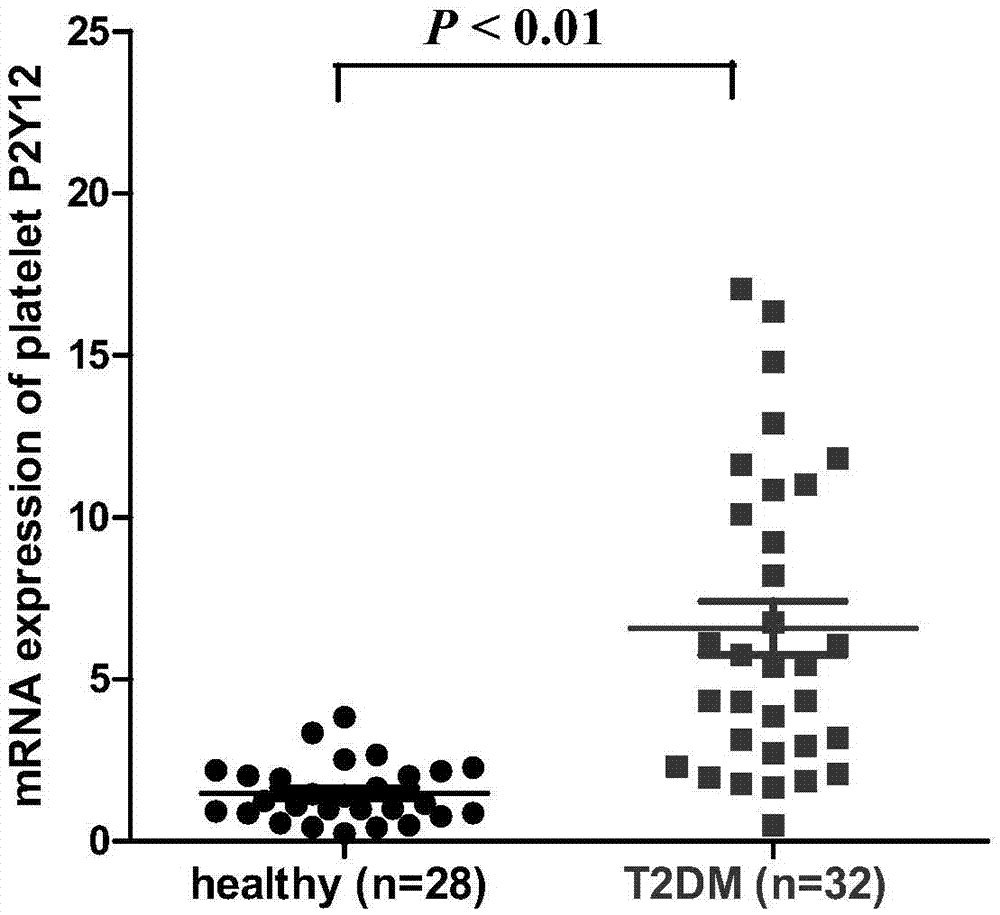
[0034] Example 1. Detection of P2Y12 receptor mRNA expression level in platelets of type II diabetic subjects by real-time fluorescent quantitative PCR method
[0035] The purpose of this example is to prove that the expression level of P2Y12 receptor mRNA in platelets of subjects with type II diabetes is significantly higher than that in platelets of healthy subjects. In this example, the preparation of platelets from patients with type II diabetes and healthy subjects: the platelets come from volunteers who have signed the informed consent form, and the volunteers have not taken any antiplatelet drugs such as aspirin or clopidogrel within 20 days before blood collection. drug. The anticoagulant was ACD (85mmol L-1 sodium citrate, 71.38 mmol L-1 citric acid, and 27.78mmol L-1 glucose). Centrifuge at 300g for 20 minutes to obtain platelet-rich plasma, and then centrifuge at 900g for 10 minutes to obtain platelets after taking the supernatant. The total mRNA was extracted by ...
Embodiment 2
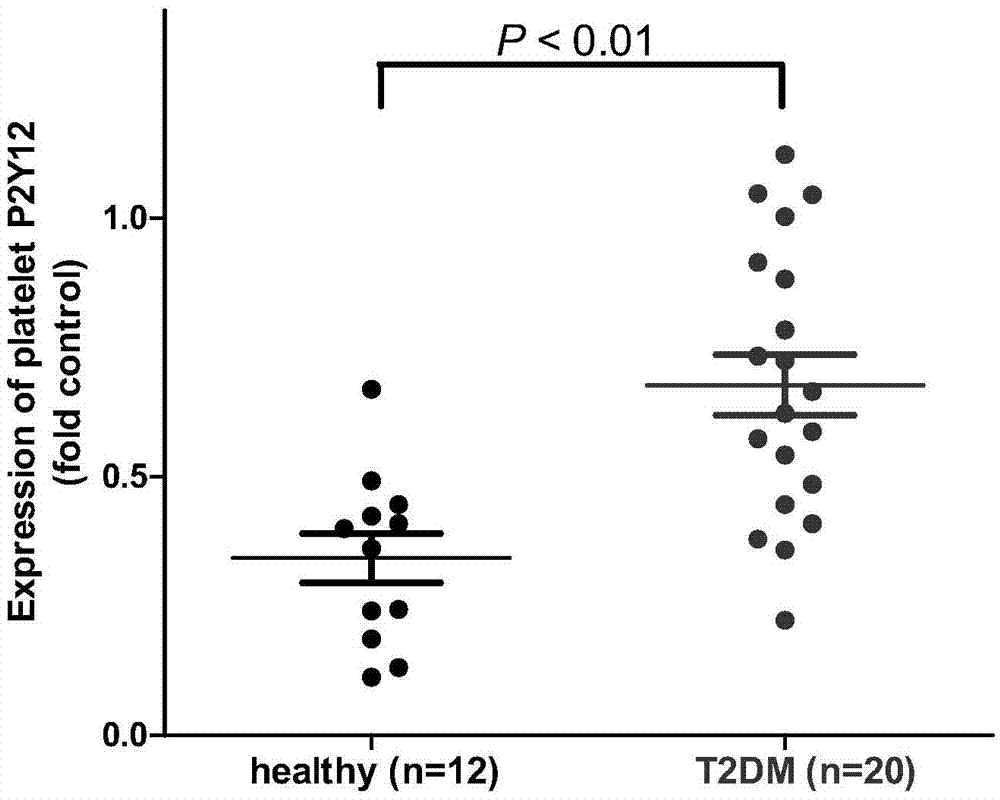
[0038] Example 2. Detection of P2Y12 receptor expression level in platelets of type II diabetic subjects by Western Blot method
[0039] The purpose of this example is to prove that the expression level of P2Y12 receptor protein in platelets of subjects with type II diabetes is significantly higher than that in platelets of healthy subjects.
[0040] In this example, the preparation method of platelets from type II diabetic subjects and healthy subjects is the same as that in Example 1. The platelets were lysed and resuspended by adding SDS lysis buffer, boiled for later use. The expression level of P2Y12 receptor protein was determined by Western Blot western blotting method, and the formula was:
[0041] P2Y12 receptor protein expression level=P2Y12 receptor protein band density / GAPDH protein band density
[0042] The results of the expression level of P2Y12 receptor protein in the subject's platelet samples are as follows: figure 2 As shown, the P2Y12 receptor protein e...
Embodiment 3
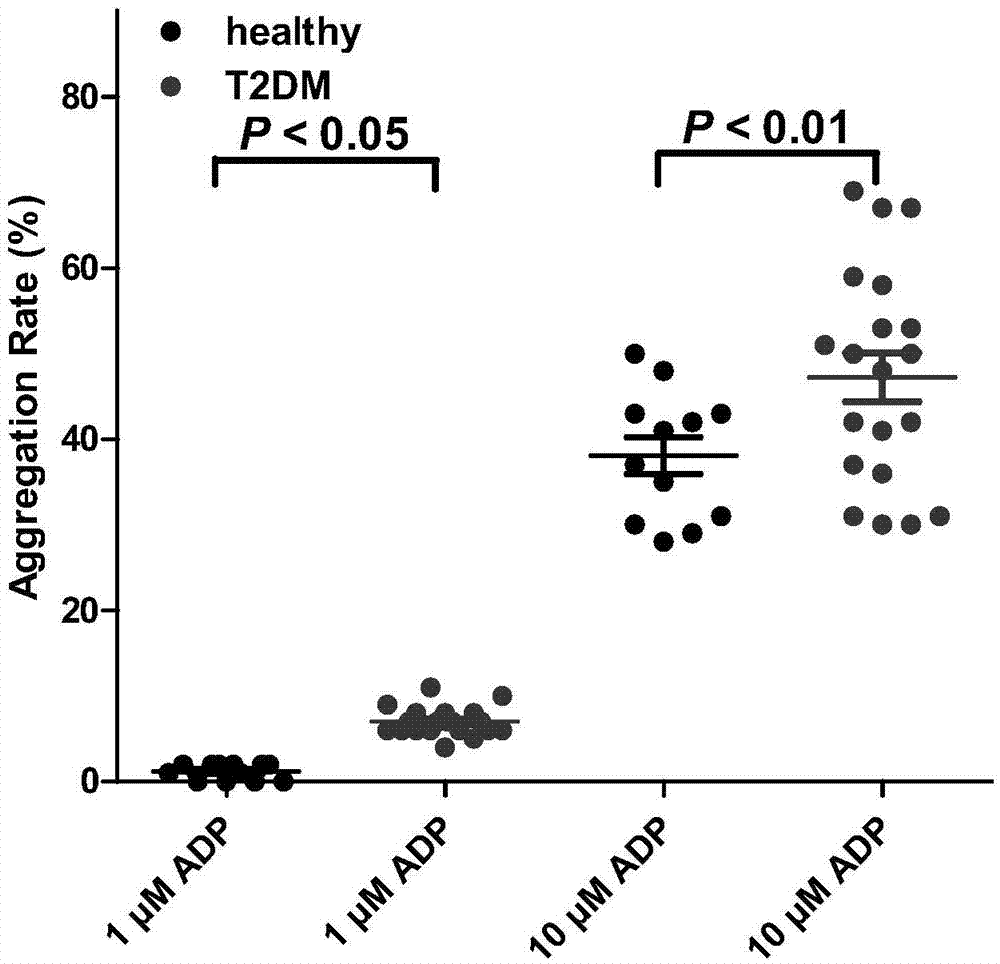
[0043] Example 3. ADP-induced platelet aggregation rate of platelet samples from type II diabetic subjects
[0044] The purpose of this example is to prove that the platelet aggregation rate of subjects with type II diabetes is significantly higher than that of healthy subjects.
[0045] In this example, the preparation method of platelets from type II diabetic subjects and healthy subjects is the same as that in Example 1. Platelets were resuspended in Tyrode buffer (138mmol L -1 NaCl, 2.7mmolL -1 KCl, 2 mmol L -1 MgCl 2 ,0.42mmol L -1 NaH 2 PO 4 ,5mmolL -1 Glucose, 10mmol L -1 HEPES, 0.2% bovine serum albumin, and 0.02 unit mL -1 apyrase, pH7.4), count platelets, and adjust the number of platelets to 2.5×10 8 platelets / mL;
[0046] Determination of platelet aggregation: Platelet aggregation was performed in a platelet aggregation instrument (Model 400VS, Chrono-Log, Haverston, PA), parameter settings: stirring speed 900rpm, temperature 37°C, before adding different ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com