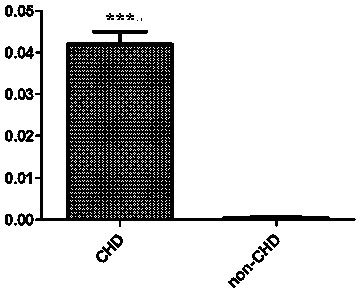
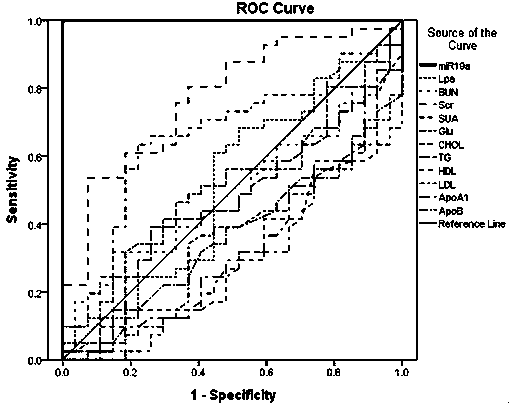
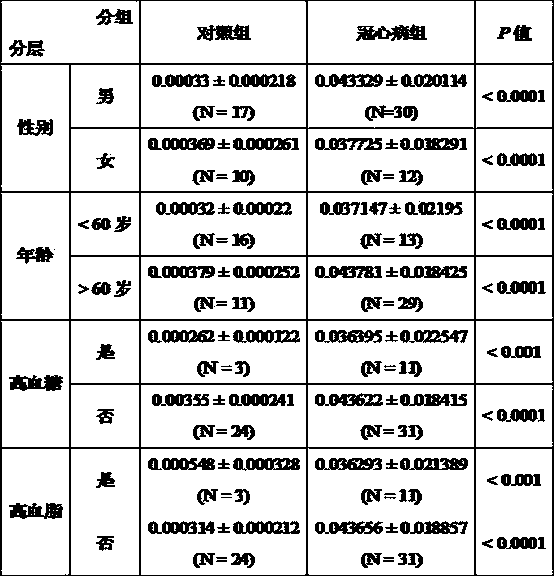
Serological biomarker miR-19a for detecting coronary heart disease and application of serological biomarker miR-19a
A mir-19a, biomarker technology, applied in the field of coronary heart disease biomarkers, can solve the problems of complex conditions, unclear pathogenesis, rare reports on the mechanism of coronary heart disease system, etc., and achieve the effect of accurate prediction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Subject group
[0021] The test subjects were patients diagnosed and treated in the hospital. Inclusion criteria for patients with coronary heart disease: previous history of myocardial infarction, or at least one coronary artery stenosis ≥ 50% in coronary angiography; age greater than 18 and less than 80 years old. Exclusion criteria: angina pectoris at rest; patients with severe heart failure (EF < 35%); patients with severe valvular disease or cardiomyopathy. A total of 180 cases in the coronary heart disease group and 169 cases in the non-coronary heart disease group were selected, and all subjects signed informed consent.
[0022] Table 1 Basic information of cases
[0023]
Embodiment 2
[0025] Plasma miRNA extraction
[0026] (1) Collect 1 ml of fresh blood from the patient, place it in an anticoagulant tube, centrifuge at 3000 rpm for 10 minutes at 4°C, and collect the upper plasma;
[0027] (2) Take 200 μl of plasma, add an equal volume of lysate MZ, shake and mix for 30 s;
[0028] (3) Leave at room temperature for 5 minutes to completely separate the protein complex;
[0029] (4) Centrifuge at 12,000 rpm for 10 min at room temperature, take the supernatant, and transfer it to a new RNase-free centrifuge tube;
[0030] (5) Add 200 μl of chloroform, shake vigorously for 15 s, and place at room temperature for 5 min;
[0031] (6) After centrifugation at 12,000 rpm at room temperature for 15 minutes, the sample will be divided into 3 layers: a yellow organic phase, an intermediate layer, and a colorless aqueous phase. RNA exists in the aqueous phase. Transfer the aqueous phase to a new tube;
[0032] (7) Measure the volume of the transfer solution, slow...
Embodiment 3
[0041] cDNA first-strand synthesis of miRNA by reverse transcription
[0042] (1) Add poly(A) to the 3' end of miRNA
[0043] 1) Put the 200 μl reaction tube without RNase on ice and add the following reagents (E.coliPoly(A)
[0044] Polymerase was added last).
[0045]
[0046] 2) Gently aspirate and mix the above reaction solution with a pipette, centrifuge briefly and react at 37°C for 60 min.
[0047]
[0048] (2) Reverse transcription reaction of poly(A) modified miRNA
[0049] 1) Prepare the reaction solution according to the following components.
[0050]
[0051] 2) Gently aspirate and mix the above reaction solution with a pipette, centrifuge briefly and react at 37°C for 60 min. The synthesized cDNA reaction solution was stored at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com