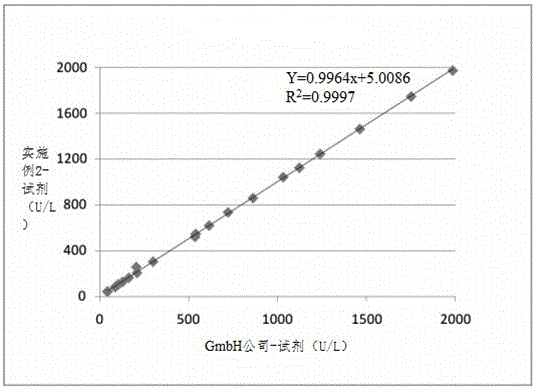
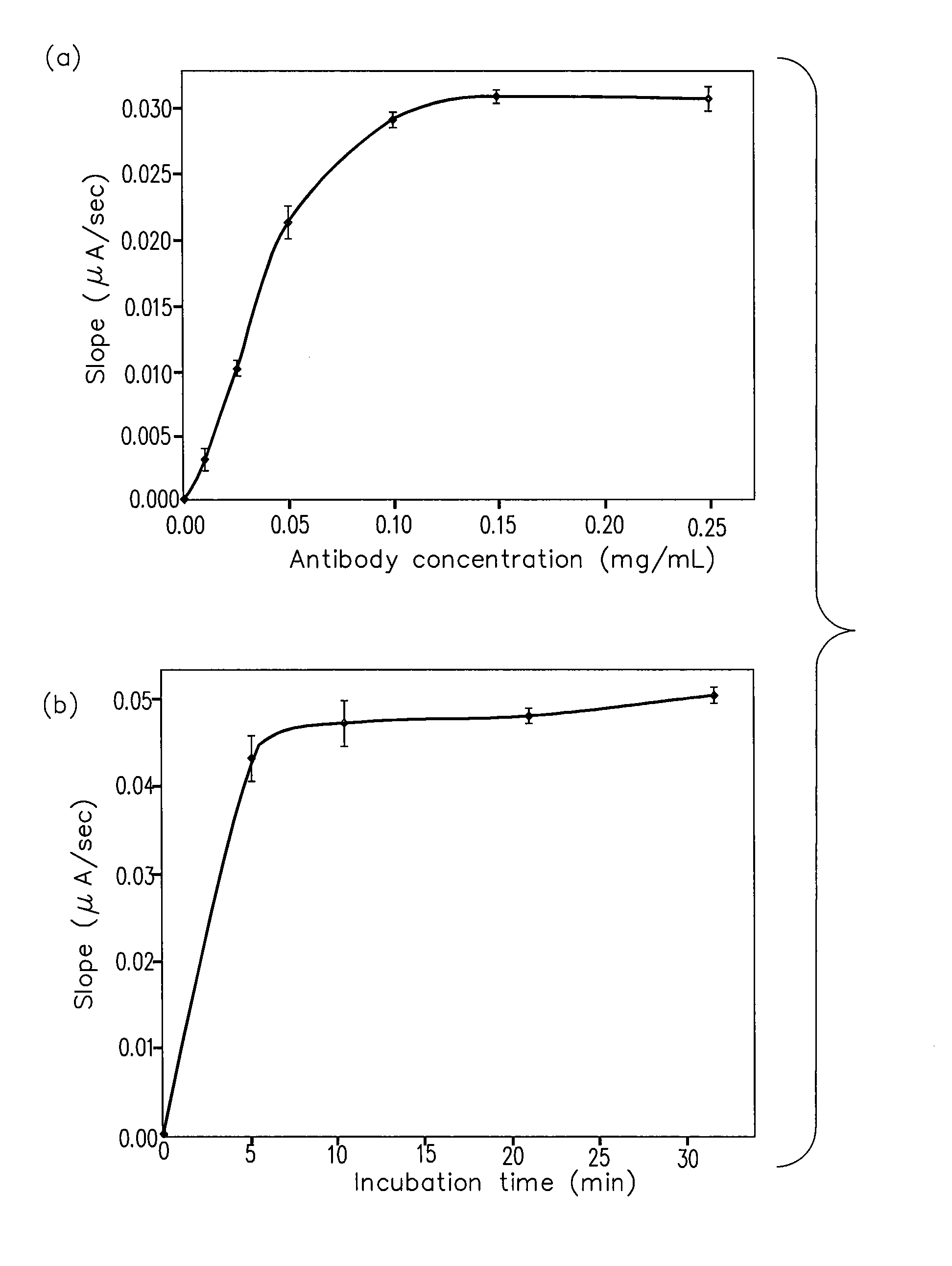
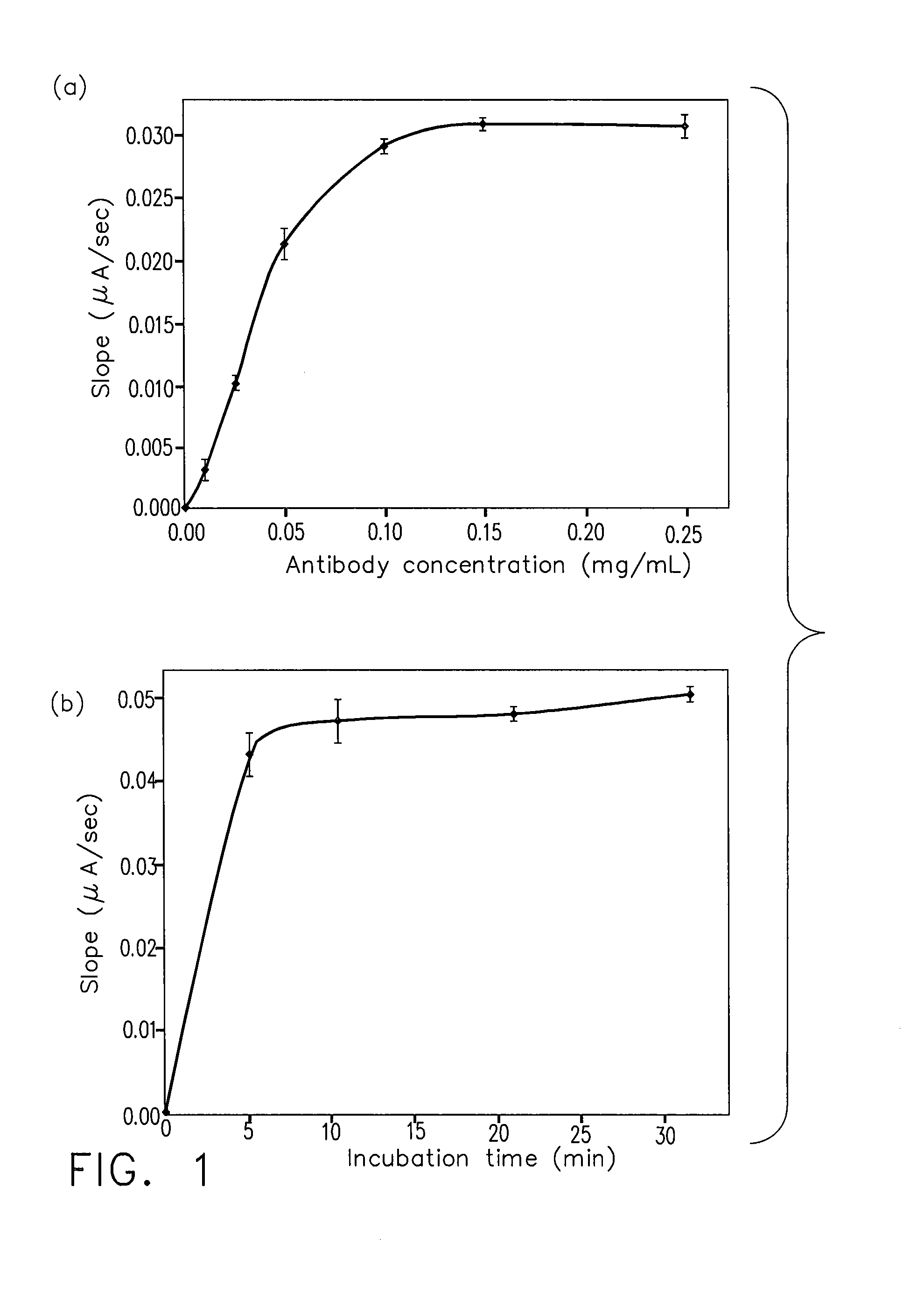
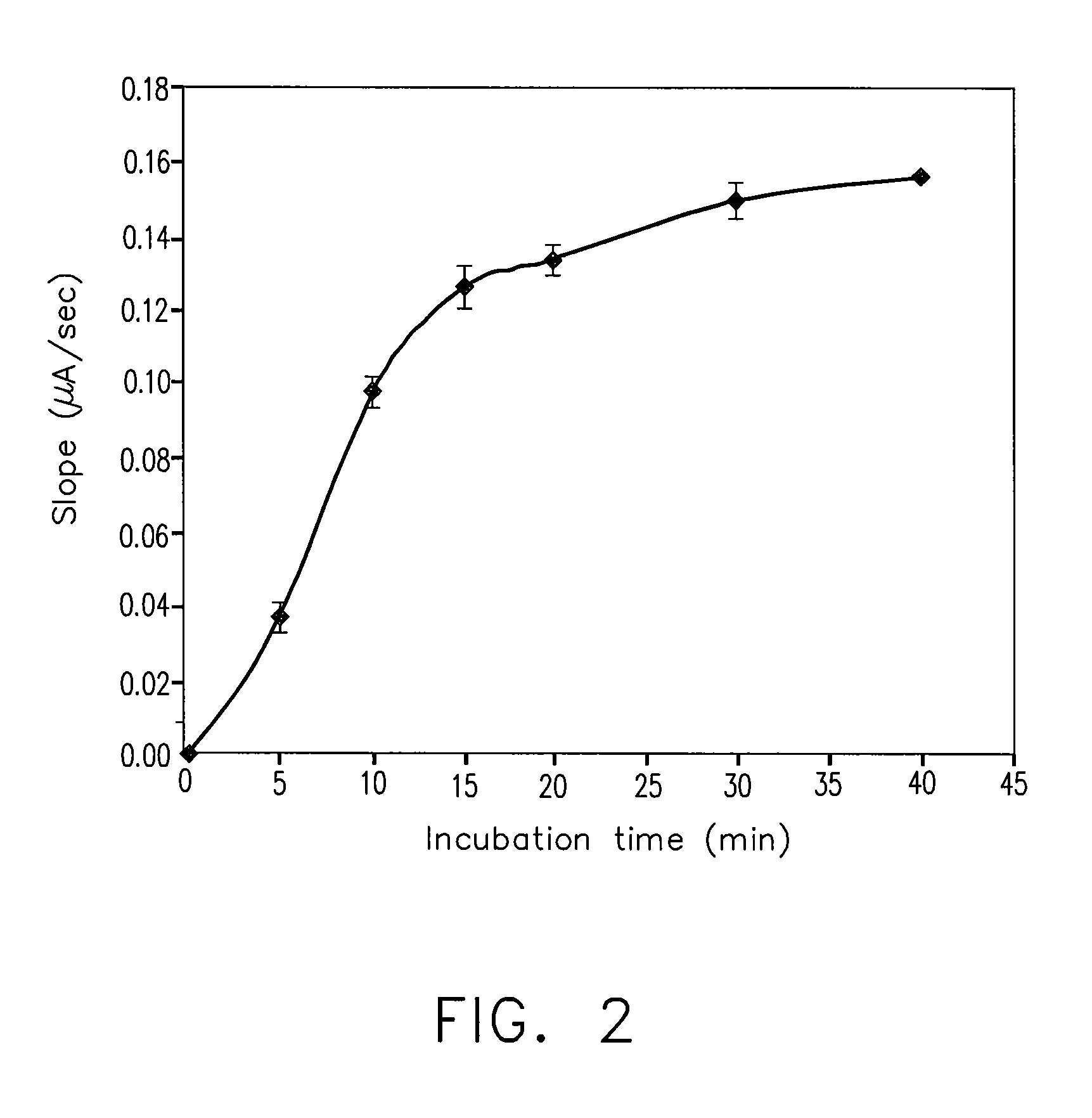
Patents
Literature
187 results about "Laboratory order" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical laboratory in a Hospital setting showing several automated analysers. A medical laboratory or clinical laboratory is a laboratory where tests are carried out on clinical specimens in order to obtain information about the health of a patient in order to provide diagnosis, treatment, and prevention of disease.
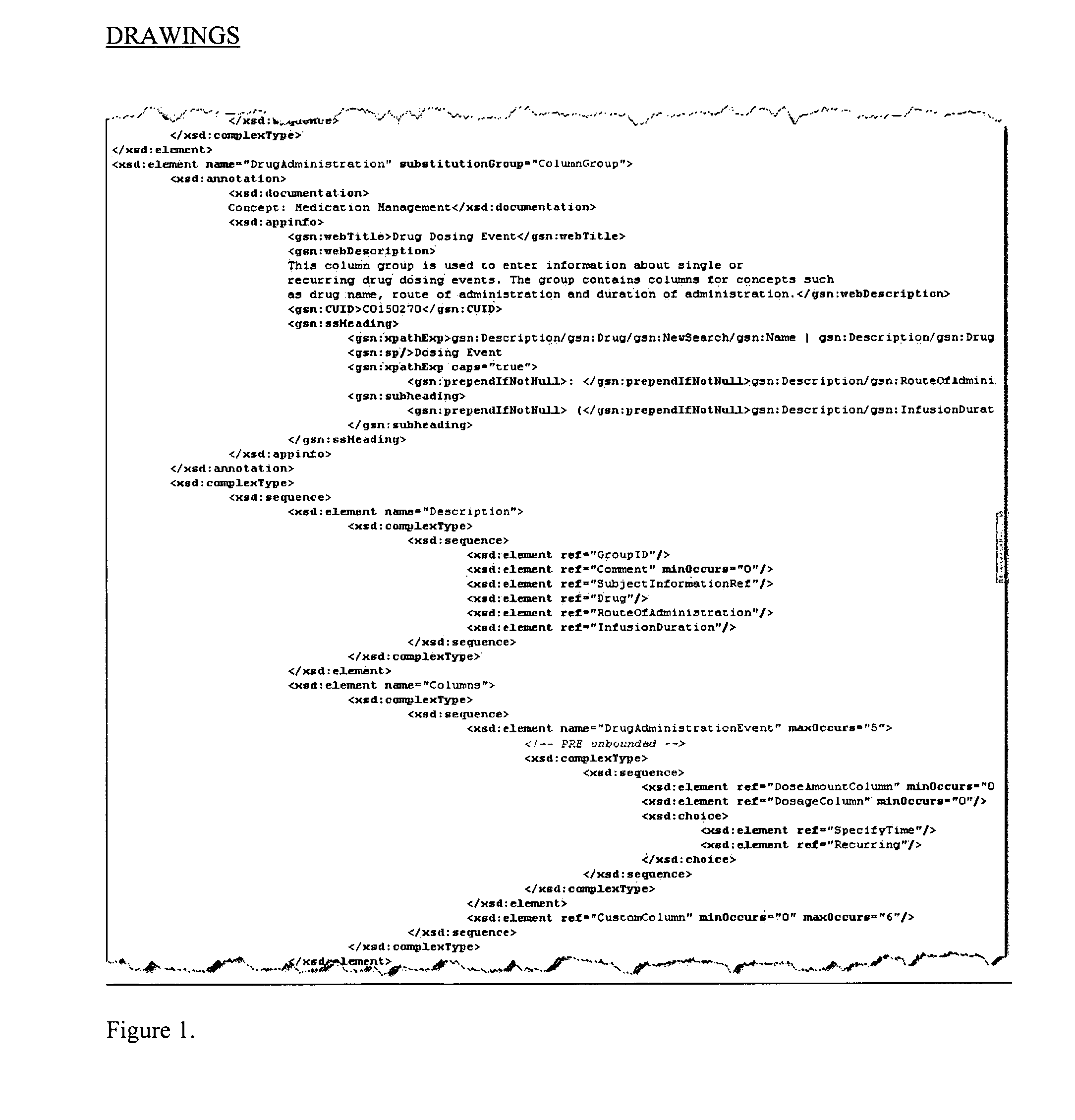
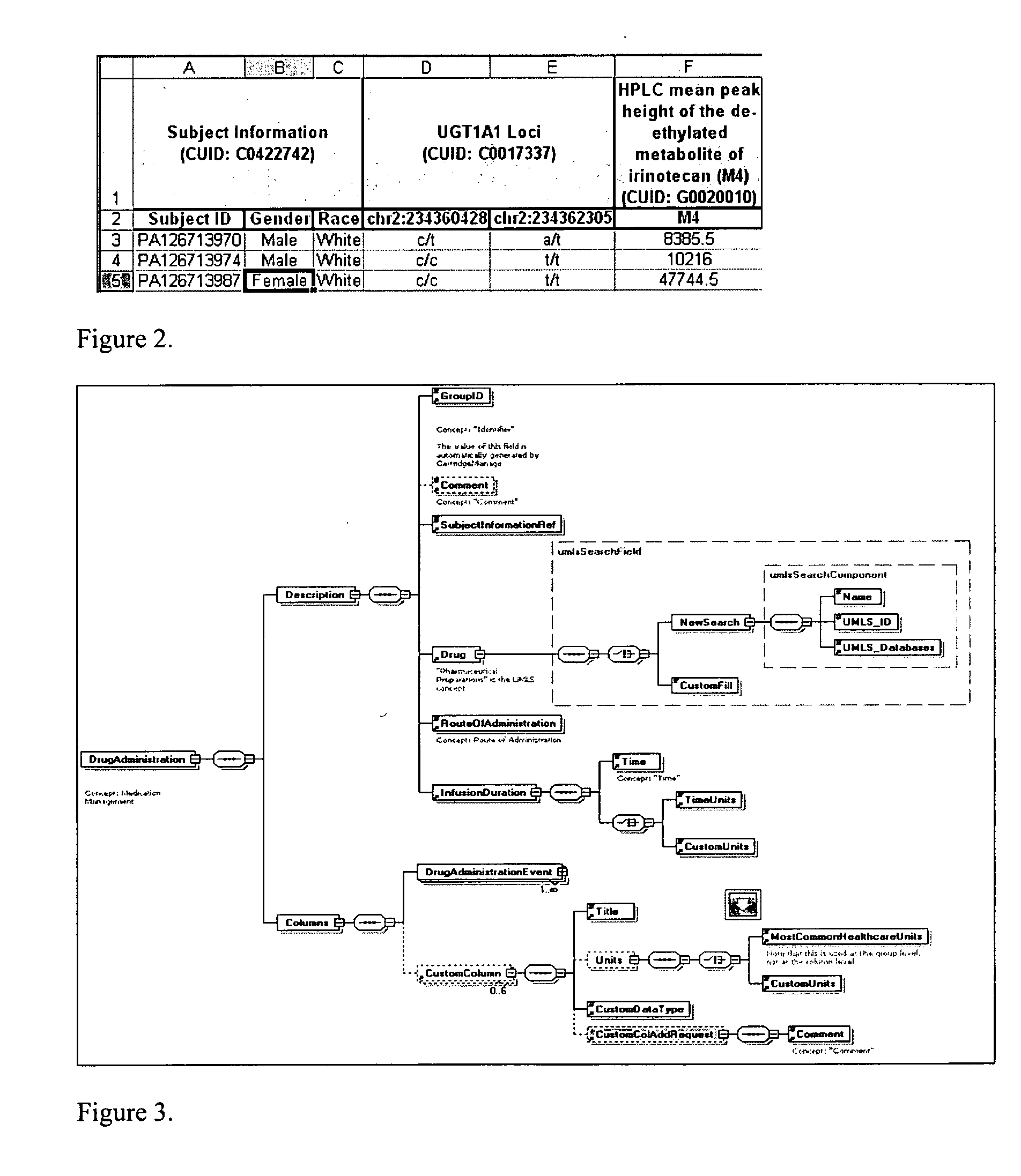
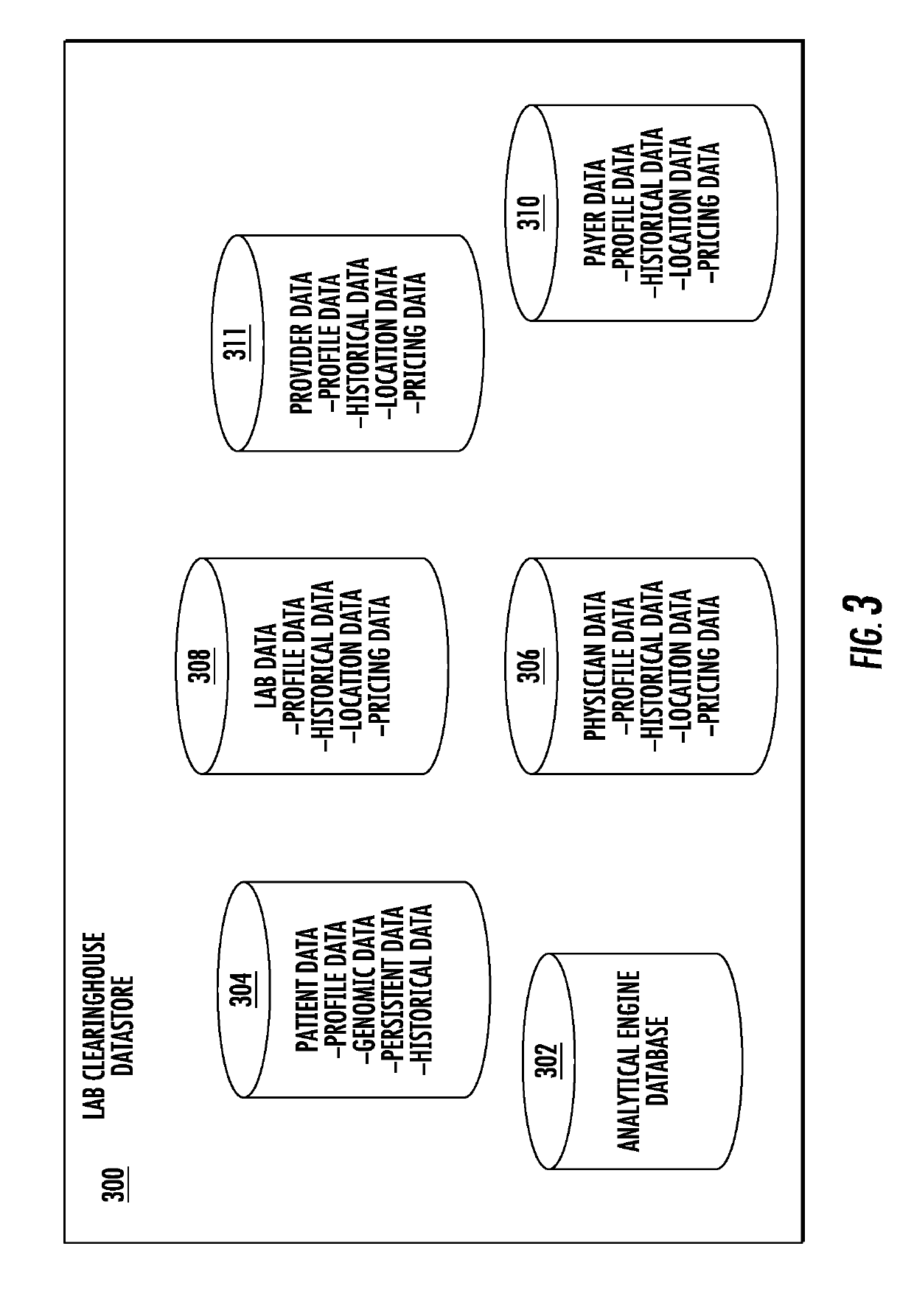
System and method for integrating and validating genotypic, phenotypic and medical information into a database according to a standardized ontology
InactiveUS20070178501A1Safest and most effective treatmentGood decisionData processing applicationsMicrobiological testing/measurementData validationMedical record
The system described herein enables clinicians and researchers to use aggregated genetic and phenotypic data from clinical trials and medical records to make the safest, most effective treatment decisions for each patient. This involves (i) the creation of a standardized ontology for genetic, phenotypic, clinical, pharmacokinetic, pharmacodynamic and other data sets, (ii) the creation of a translation engine to integrate heterogeneous data sets into a database using the standardized ontology, and (iii) the development of statistical methods to perform data validation and outcome prediction with the integrated data. The system is designed to interface with patient electronic medical records (EMRs) in hospitals and laboratories to extract a particular patient's relevant data. The system may also be used in the context of generating phenotypic predictions and enhanced medical laboratory reports for treating clinicians. The system may also be used in the context of leveraging the huge amount of data created in medical and pharmaceutical clinical trials. The ontology and validation rules are designed to be flexible so as to accommodate a disparate set of clients. The system is also designed to be flexible so that it can change to accommodate scientific progress and remain optimally configured.
Owner:NATERA
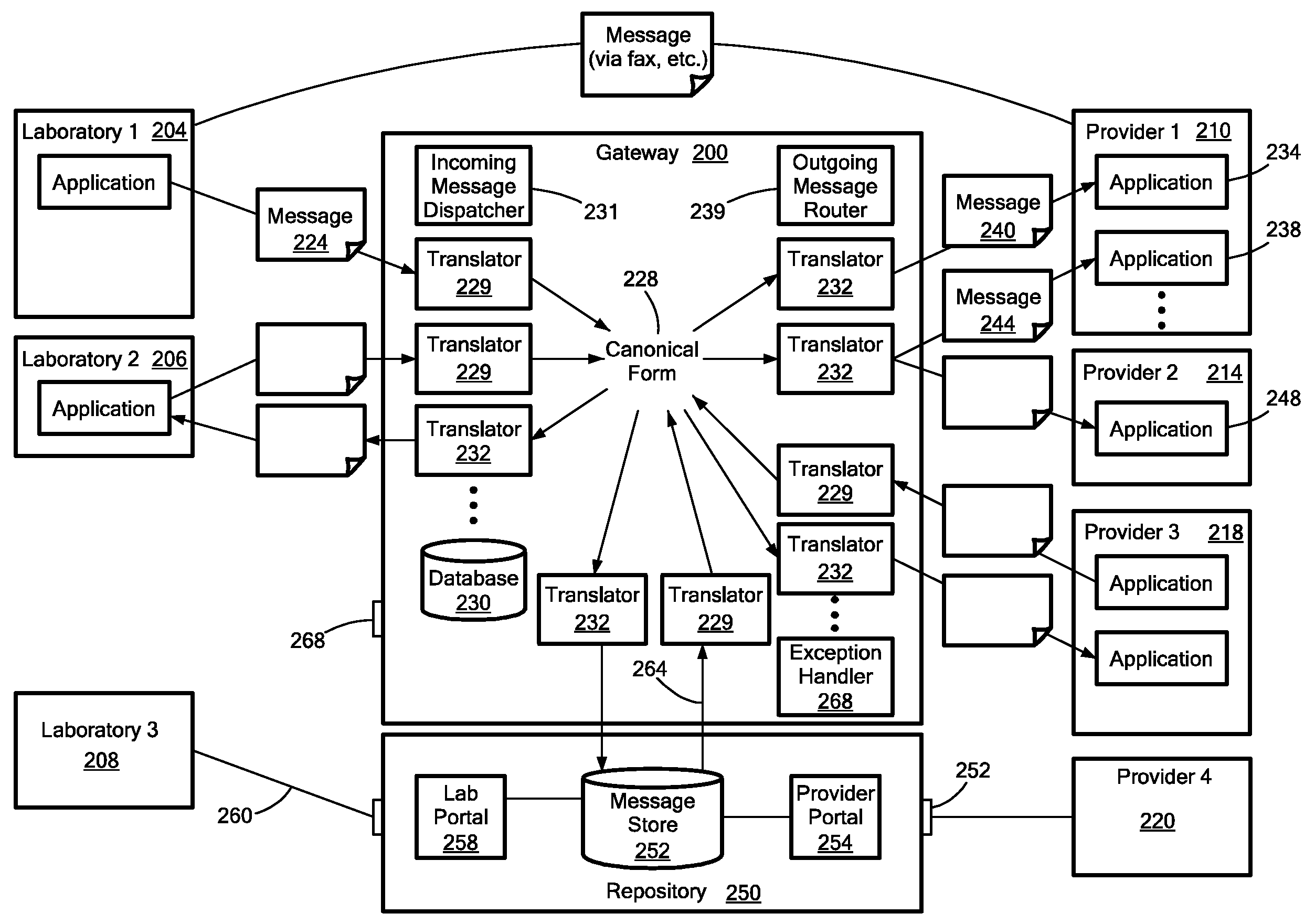
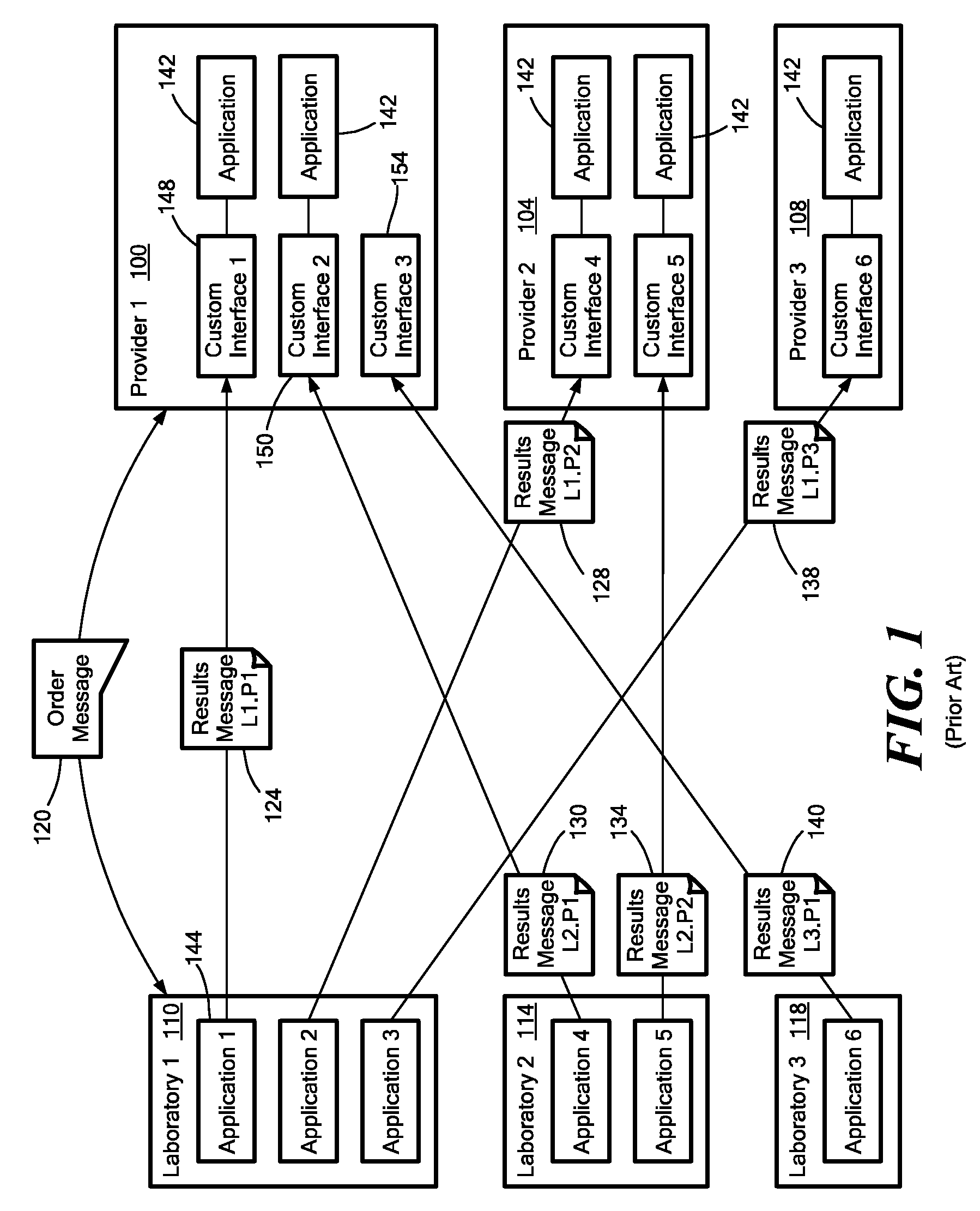
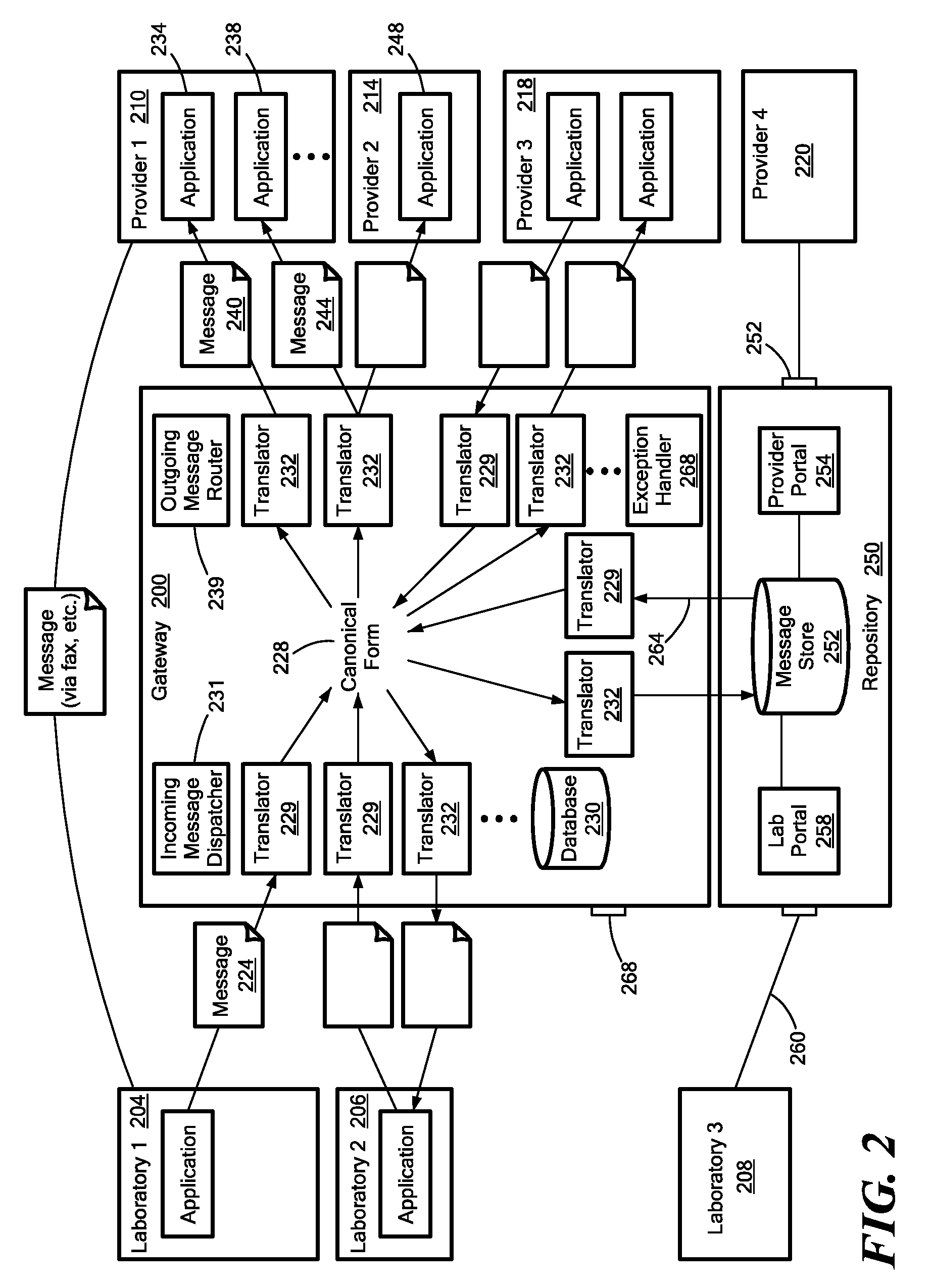
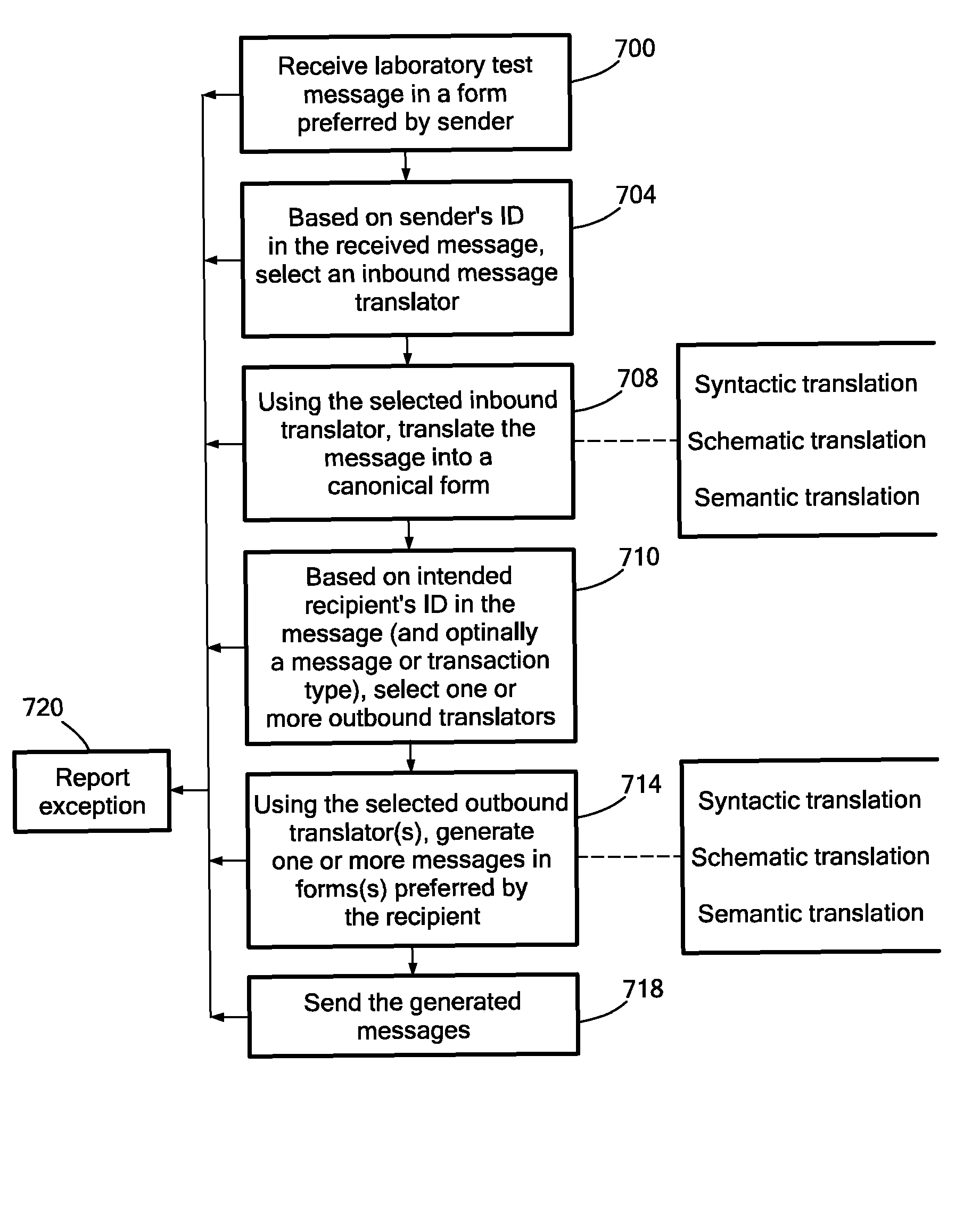
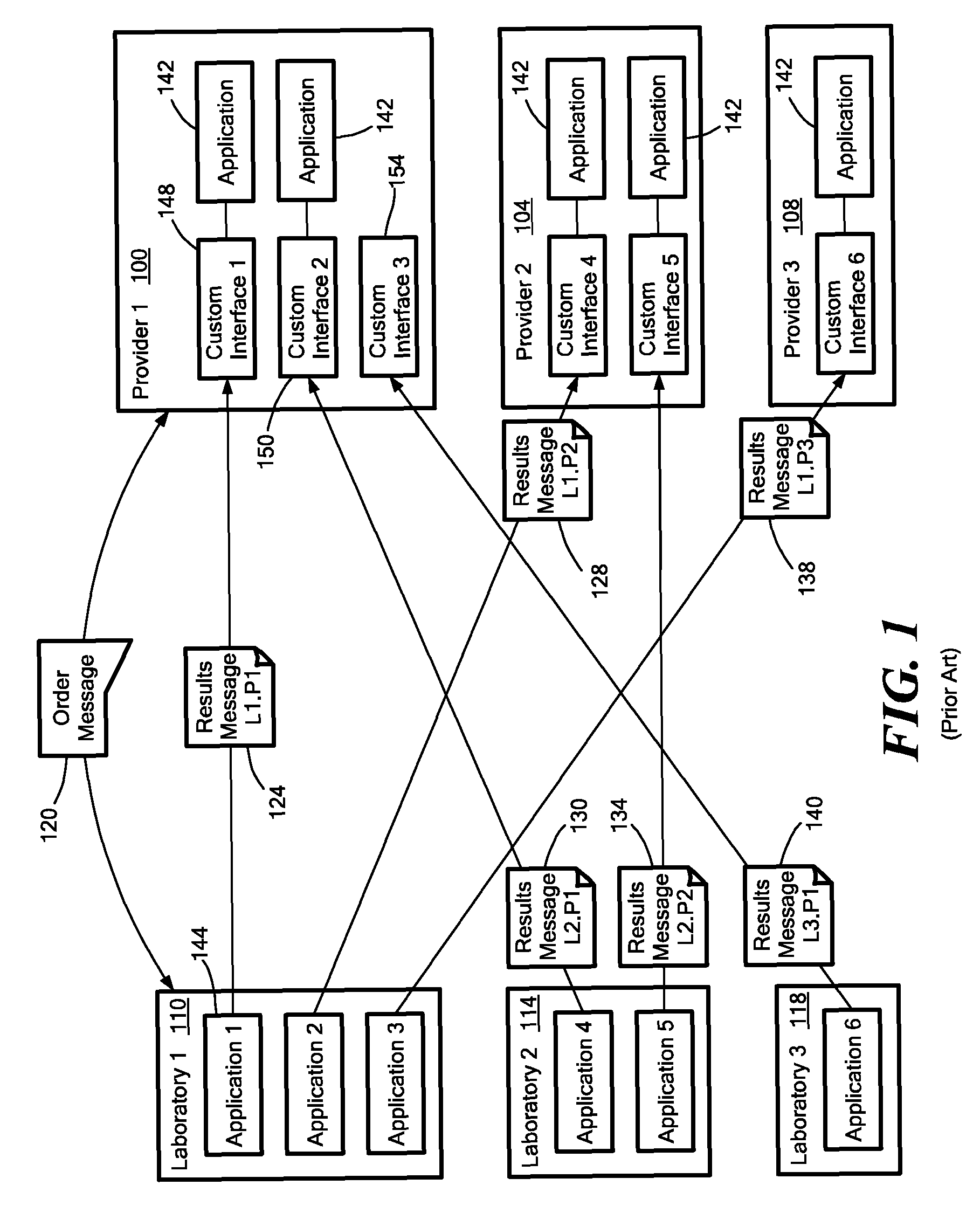
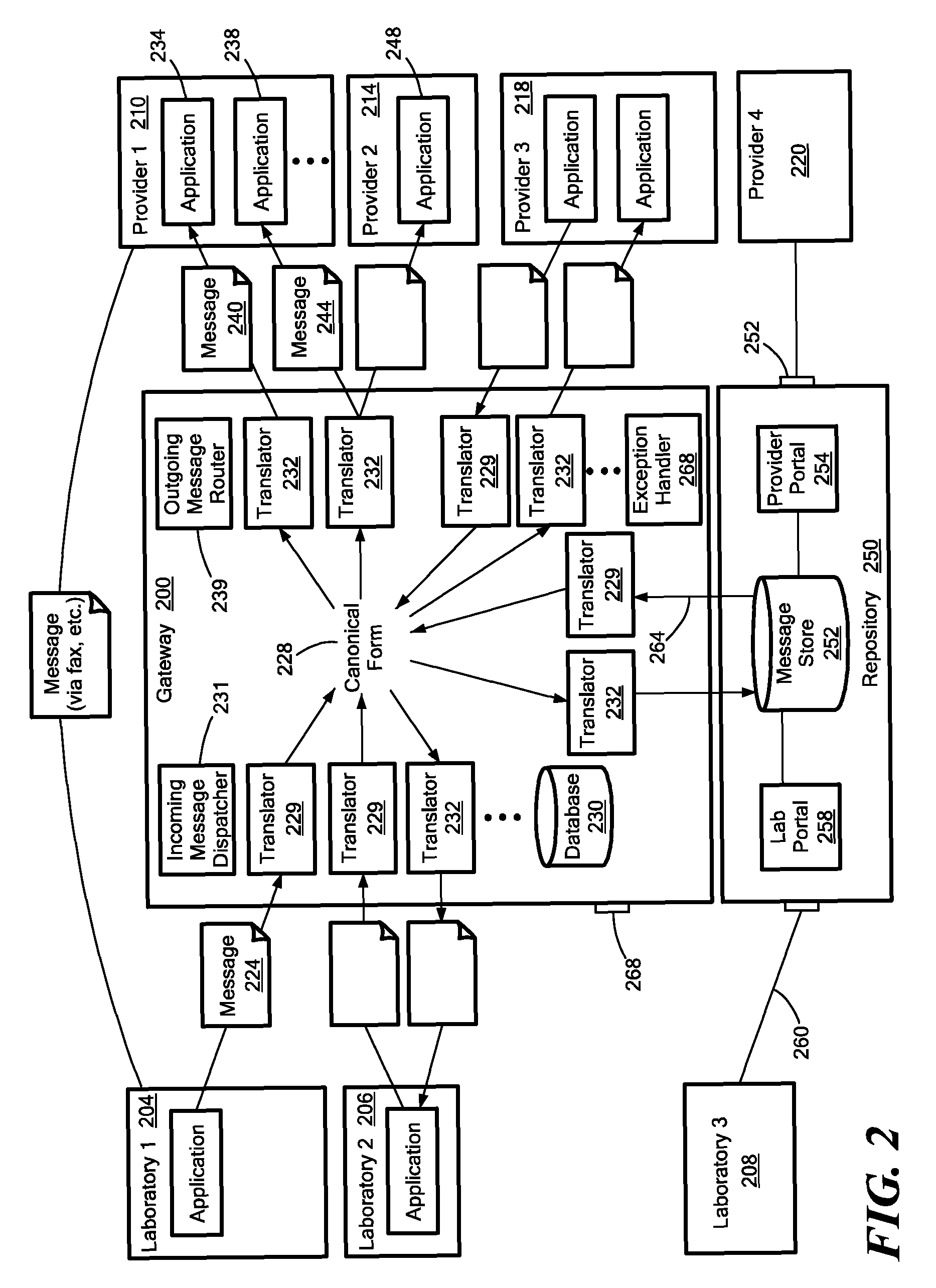
Medical laboratory report message gateway
ActiveUS20080270438A1Fault responseDigital data processing detailsMedical laboratoryBioinformatics databases
A gateway enables medical (including genetic and genomic) laboratories and health care providers (collectively “clients”) to communicate electronic messages with each other without developing and maintaining an interface for each peer. The gateway translates messages sent between the parties. The gateway receives messages from each sender in a form, and containing diagnostic codes, preferred by the sender. For each received message, the gateway ascertains an intended receiving client. Each client may specify one or more receivers (such as applications) that are to receive messages sent to the client, as well as a separate form, and optionally a set of codes, for each receiver. For each receiver, the gateway generates translated messages, according to the receiver's preferred form and / or codes. The gateway sends the translated messages to each of the designated receivers. The gateway may include a validation component to check incoming messages to ensure the messages include required information and that information values are valid or acceptable. The gateway may include an exception handler that notifies a sending client if a message from the client fails to be translated or sent correctly. The gateway may maintain a repository in which the gateway stores copies of messages the gateway sent or would have sent to clients. The gateway provides an interface, such as a secure web interface, to this repository. Clients may access messages or lists of messages, especially messages the clients are not otherwise capable of receiving, through this interface. The gateway may store copies of some of the data that flows through the gateway in a bioinformatics database, which may be automatically analyzed by the gateway or queried for research or patient care purposes.
Owner:THE GENERAL HOSPITAL CORP
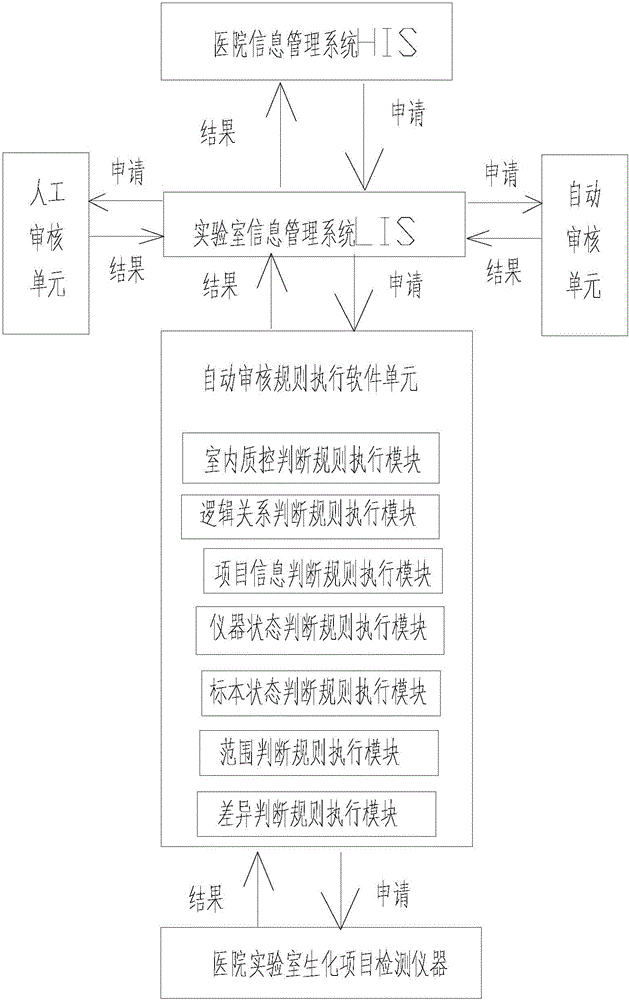
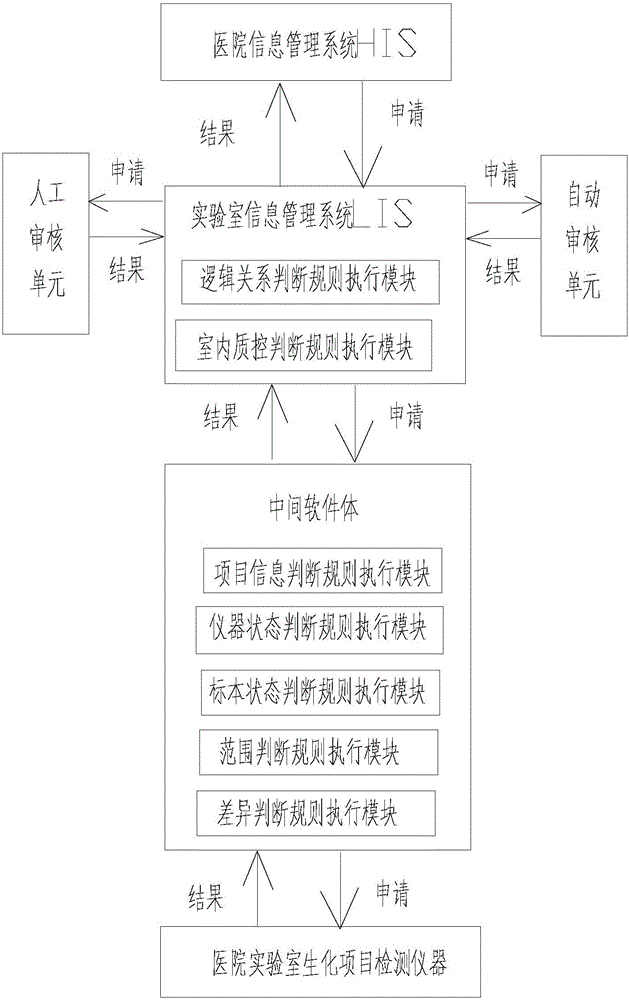
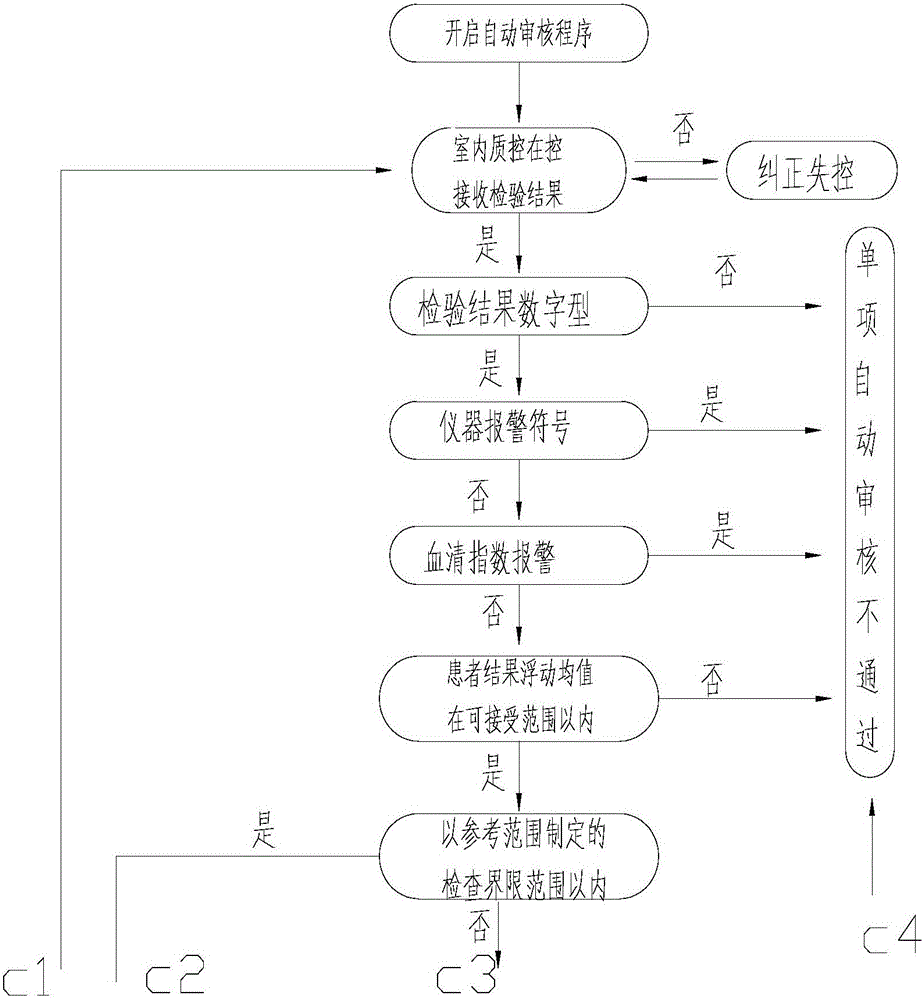
A medical laboratory clinical biochemical detection automatic checking method and system
ActiveCN106126958AImprove pass rateImprove accuracyMedical automated diagnosisMedical equipmentMedical laboratoryProcess quality
The invention provides a medical laboratory clinical biochemical detection automatic checking method and system. The method employs a computer software and hardware system and programs multiple checking rules to form a multiple checking rule execution module to automatically check the detection results of various biochemical immunity detection items. The detection results of the various biochemical immunity detection items cannot be sent to an automatic checking unit for automatic checking until they pass the multiple checking rules; if the detection results of the various biochemical immunity detection items cannot pass the above-mentioned checking rules, the detection results are transferred to a manual checking unit for checking; only after the detection results are checked by the manual checking unit or checked again after automatic treatment of dilution, reexamination, test item adding, unqualified sample return and the like by an assembly line can a detection report be issued; the multiple checking rules mainly include a clinical information judging rule, a sample state judging rule, an indoor quality control judging rule, an instrument state judging rule, a range judging rule, a difference judging rule, and a logical relationship judging rule. The checking rules are reasonable in design and cover the pre-analysis, analysis and post-analysis processes, thereby realizing whole-process quality control on a detection analysis process and guaranteeing the accuracy of detection results.
Owner:温冬梅
Kit and method for extracting and storing a skin tissue sample
InactiveUS20120323139A1Minimizing amount of delayEliminate needSurgical needlesVaccination/ovulation diagnosticsLaboratory orderMedical laboratory
A kit of components for extracting and storing a skin tissue sample includes an extraction cartridge with a plurality of blades, an actuator cartridge, and a cap with an aqueous preservative. The actuator cartridge is removeably attached to the extraction cartridge and causes the plurality of blades of the extraction cartridge to extract a skin tissue sample from the person. After the skin tissue sample is extracted, the cap is attached to the extraction cartridge to store and suspend the skin tissue sample in an aqueous preservative preloaded in a container in the cap. A liquid-tight seal prevents bodily fluids from contaminating the actuator cartridge and this enables the actuator cartridge to be removed from the extraction cartridge and to be reused. The extraction cartridge and the attached cap, which store the skin tissue sample, are sent to medical laboratory so that the skin tissue sample can be analyzed.
Owner:RICHARDSON WILL
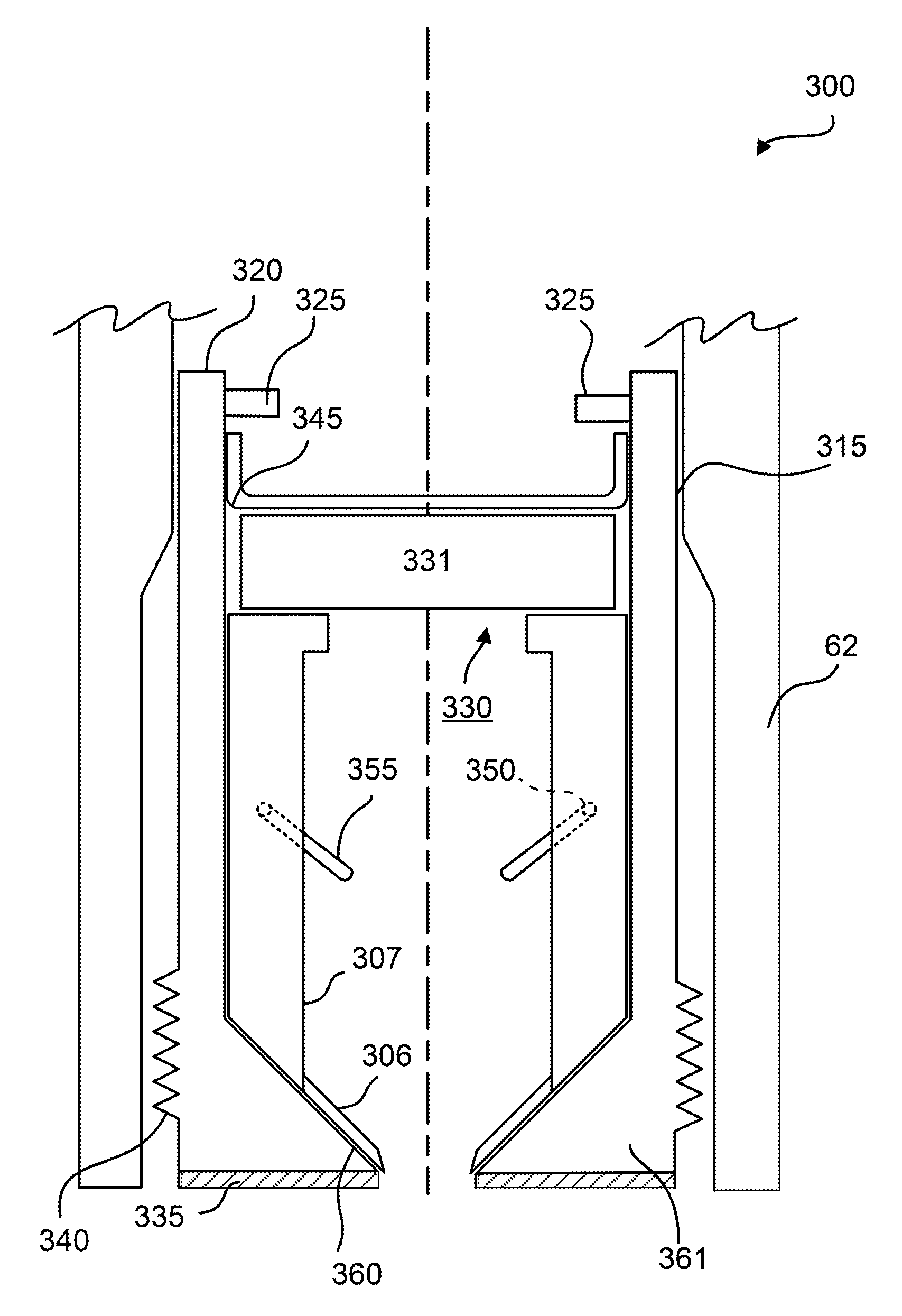
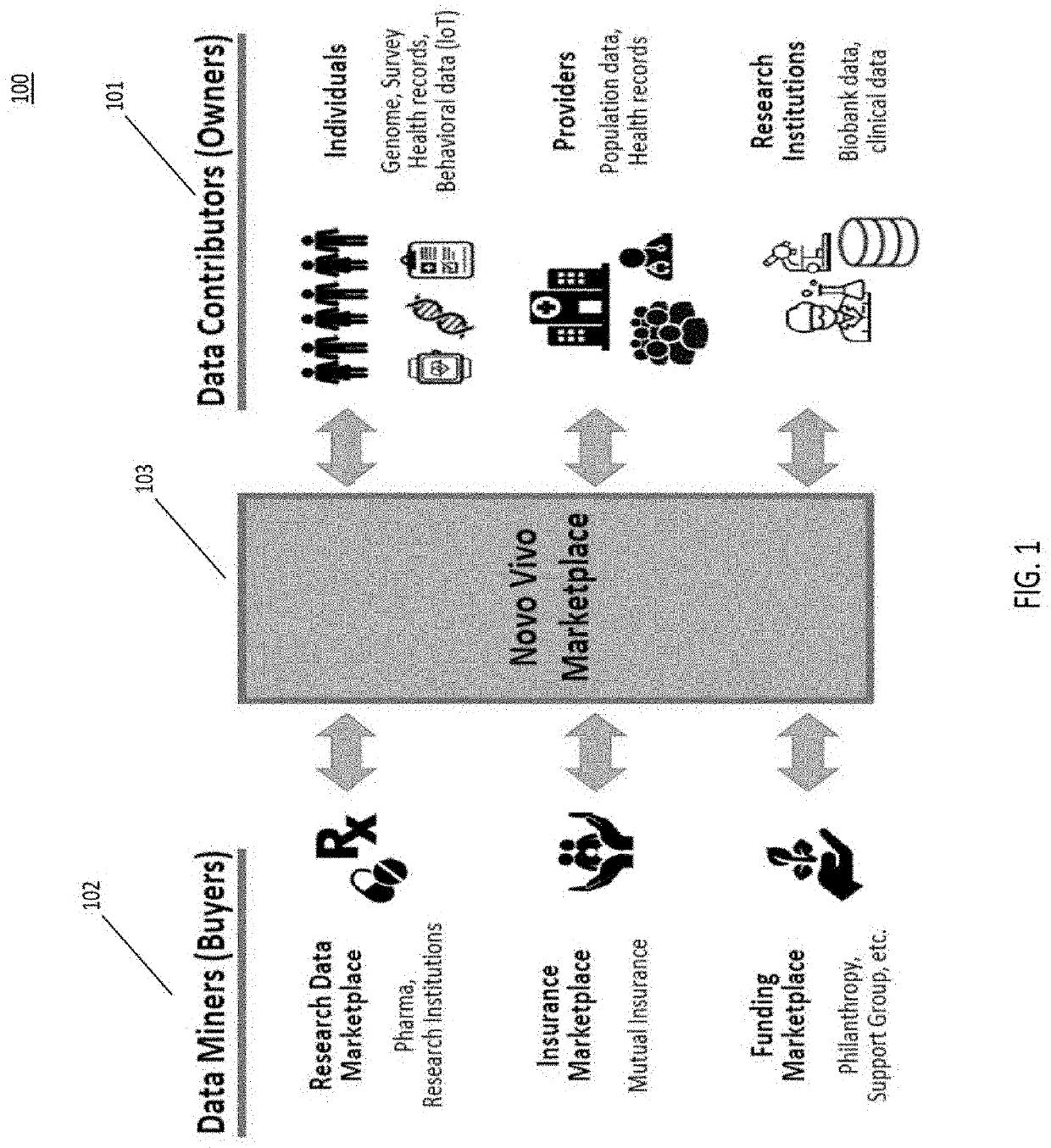
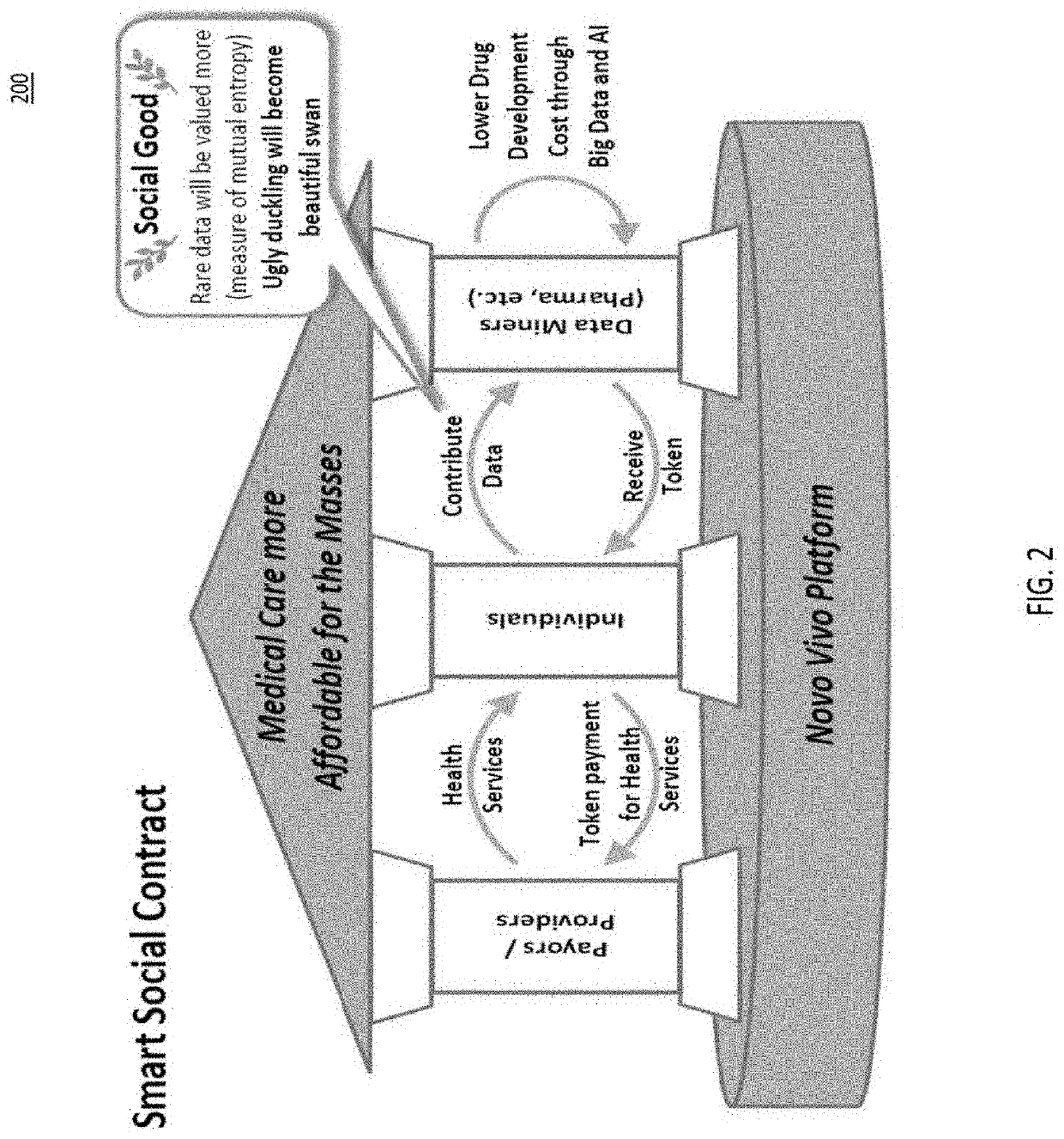
System for decentralized ownership and secure sharing of personalized health data
ActiveUS20200327250A1Maintain privacyMedical data miningEncryption apparatus with shift registers/memoriesLaboratory orderMedical laboratory
The system disclosed implements a secure method for facilitating secure exchange of health information among various stakeholders, including data owners or contributors, data requestors or miners, and medical providers, including hospitals, clinics, and research laboratories. Additional aspects of the system provide means for conducting secure research on health data collected from data contributors. Health information is exchanged using a decentralized system that incentivizes data contributors to provide health data to data miners. The data miners, which may be pharmaceutical companies, medical laboratories, or hospitals, use various methods in order to perform research on aggregated contributor data, while maintaining contributor privacy.
Owner:HANGZHOU NUOWEI INFORMATION TECH CO LTD
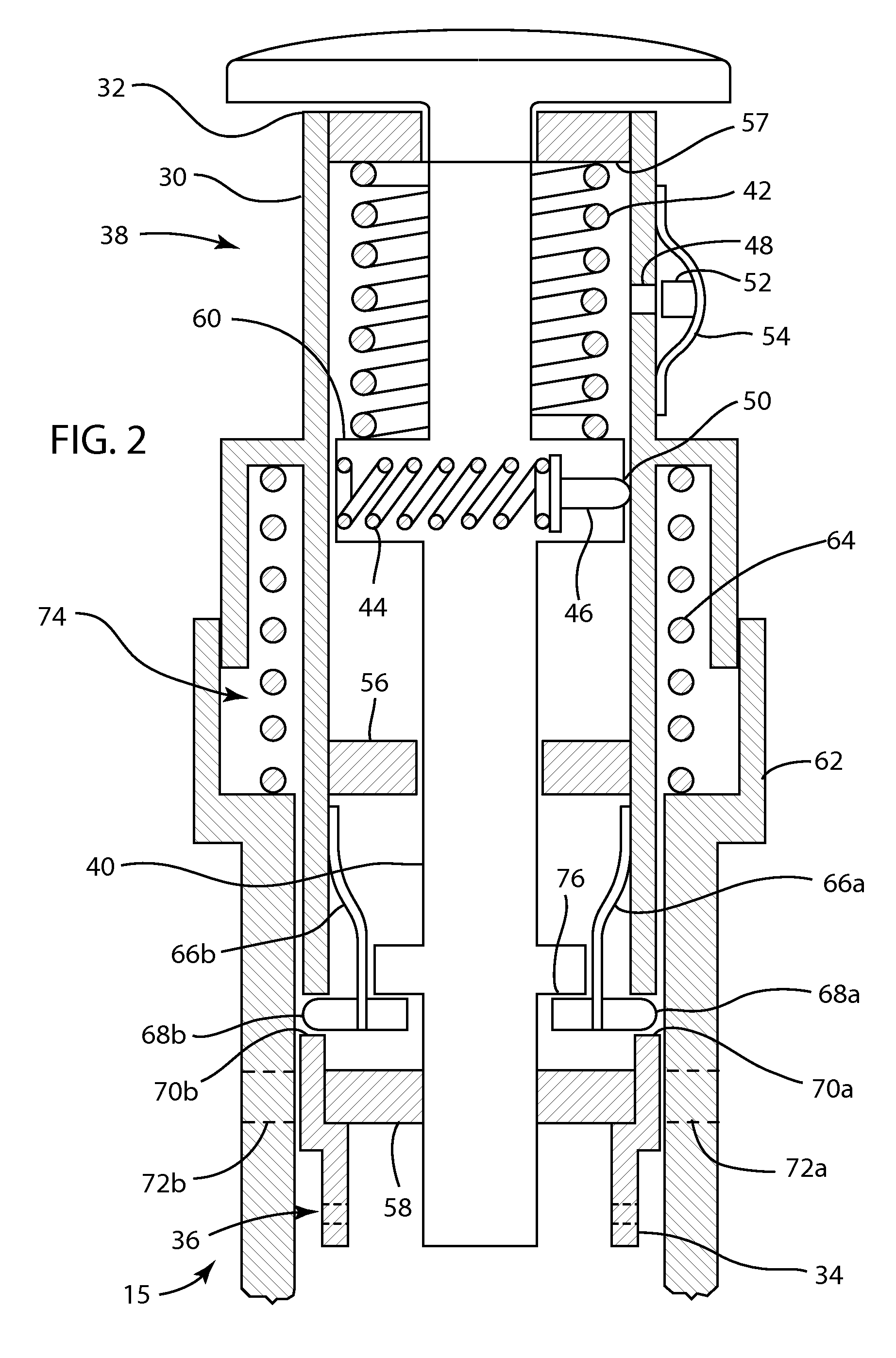
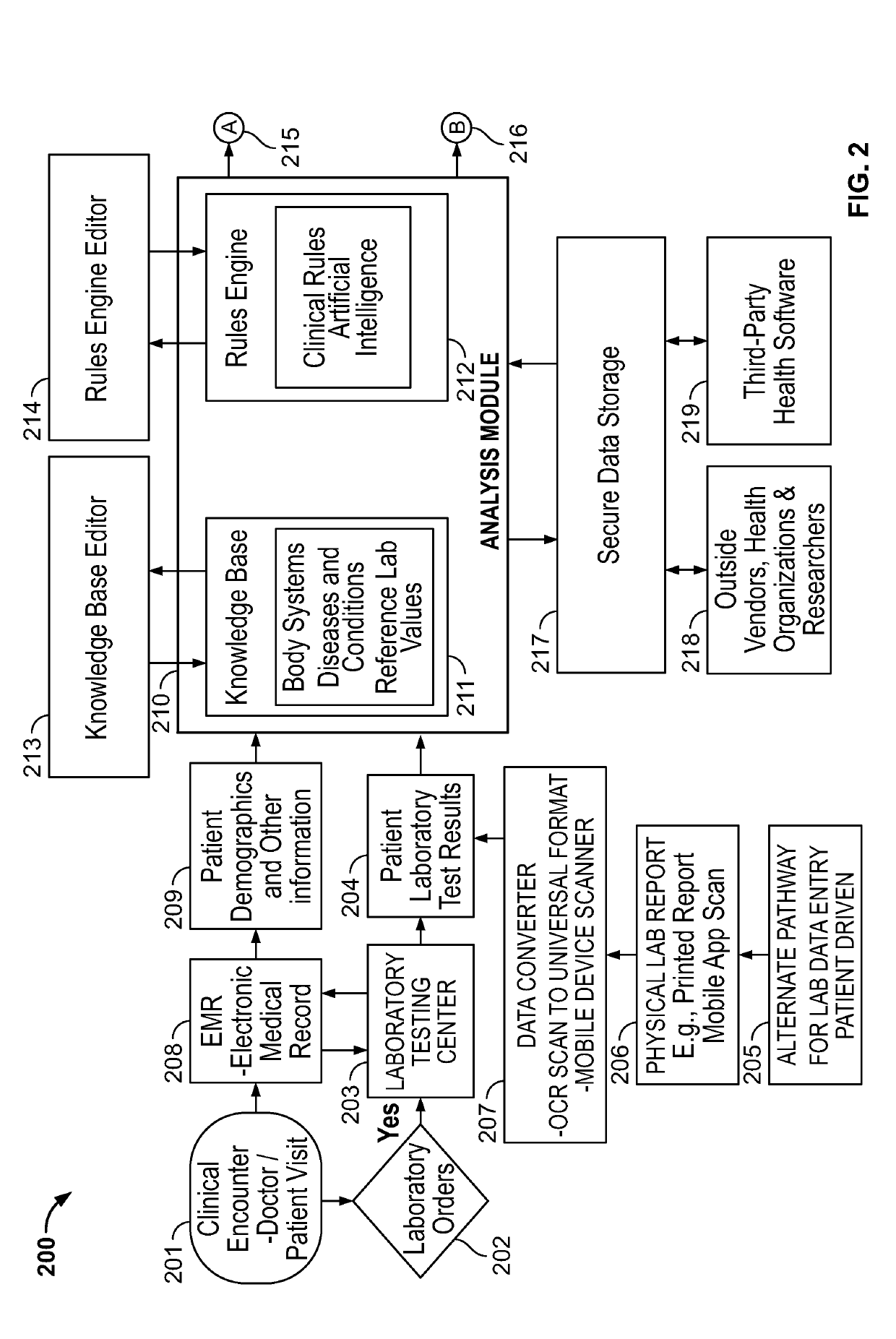
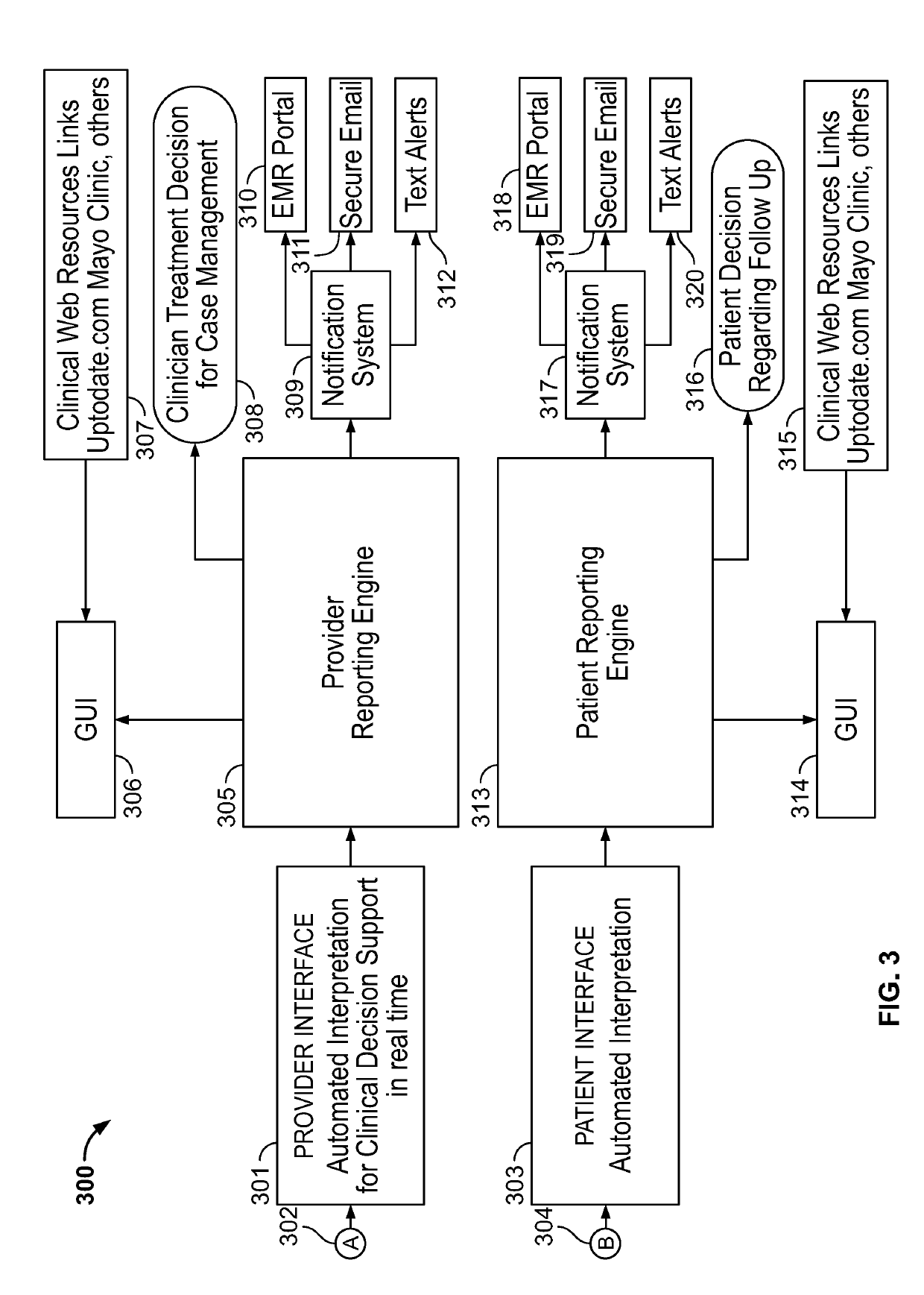
System and method for increasing efficiency of medical laboratory data interpretation, real time clinical decision support, and patient communications
ActiveUS20190108898A1Improve efficiencyEasy to compareMedical data miningMedical automated diagnosisLaboratory orderMedical laboratory
A system and method are disclosed for enhancing the efficiency and accuracy of analysis and interpretation of medical diagnostic laboratory test data for real-time clinical decision support, utilizing artificial intelligence techniques to automatically improve analytical performance and enhance provider and patient communications.
Owner:GULATI PETER
Medical laboratory report message gateway
ActiveUS7908293B2Digital data processing detailsComputer-assisted medical data acquisitionLaboratory orderMedical laboratory
A medical laboratory report communications gateway computer system is presented. The gateway is configured to receive medical laboratory reports from a plurality of clients. The gateway uses report form data stored in a database to perform an inbound translation on the medical laboratory report to transform the medical laboratory report to a canonical form. The gateway identifies a destination client for the medical laboratory report, and determines an outbound message form based on the destination client. The gateway performs the selected outbound translation on the medical laboratory report in the canonical form to transform the medical laboratory report in the canonical form into a form useable by the destination client. The gateway then transmits the translated medical laboratory report to the destination client.
Owner:THE GENERAL HOSPITAL CORP
Clinical medical care quality management system
InactiveCN105447655ASolve unified managementRealize interconnectionResourcesMedical recordMedical laboratory
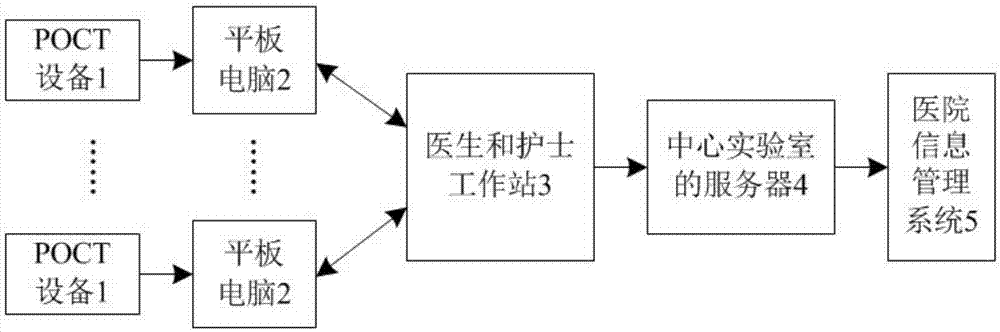
The invention relates to a clinical medical care quality management system, which comprises a hospital-wide POCT (Point-Of-Care Testing) network quality management subsystem, a regional center laboratory operation management subsystem and a regional medical examination quality management subsystem, wherein the hospital-wide POCT network quality management subsystem is used for managing all POCT equipment in a hospital; the regional center laboratory operation management subsystem takes a hospital with a highest regional popularity is taken as a top hospital, a medical examination and pathology diagnosis room is set as a regional medical diagnosis center laboratory or a third-party medial laboratory of a regional medical system, and the laboratory provides medical diagnosis service and large sample processing; and the regional medical examination quality management subsystem takes the hospital with the highest popularity as the top hospital, the medical laboratory of the hospital is set as a regional medical examination quality control center, and medical examination is taken as a basis to be connected with the hospital information management system, the laboratory information management system and the electronic medical record information system of the regional hospital.
Owner:CHENGDU HUISHENGZHI MEDICAL TECH CO LTD
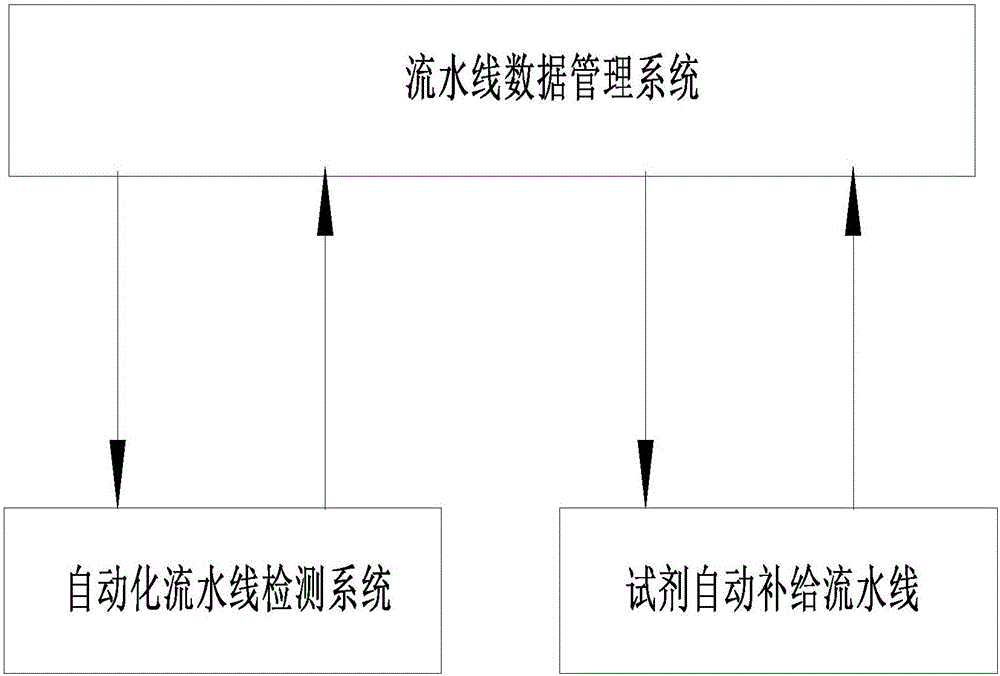
Medical laboratory automated assembly line reagent automatic supply method and system
ActiveCN105974894AReduce labor intensityGuaranteed accuracyProgramme total factory controlLaboratory orderMedical laboratory
The invention discloses a medical laboratory automated assembly line reagent automatic supply method and a system. According to the method, a reagent supply control module is added to an assembly line data management system; a reagent automatic supply assembly line is added beside an automated assembly line detection system; the assembly line data management system sets the minimum test value of reagent for each detection item of each analysis instrument; each analysis instrument transmits the test values of the detection items to the assembly line data management system through a reagent needle liquid level sensor during detection; and when the reagent of any detection item of any analysis instrument is lower than the minimum set value, the assembly line data management system calls the reagent supply control module, and the reagent supply control module gives an order automatically to make the reagent automatic supply assembly line work so as to automatically supply reagent to the detection item of the analysis instrument.
Owner:温冬梅
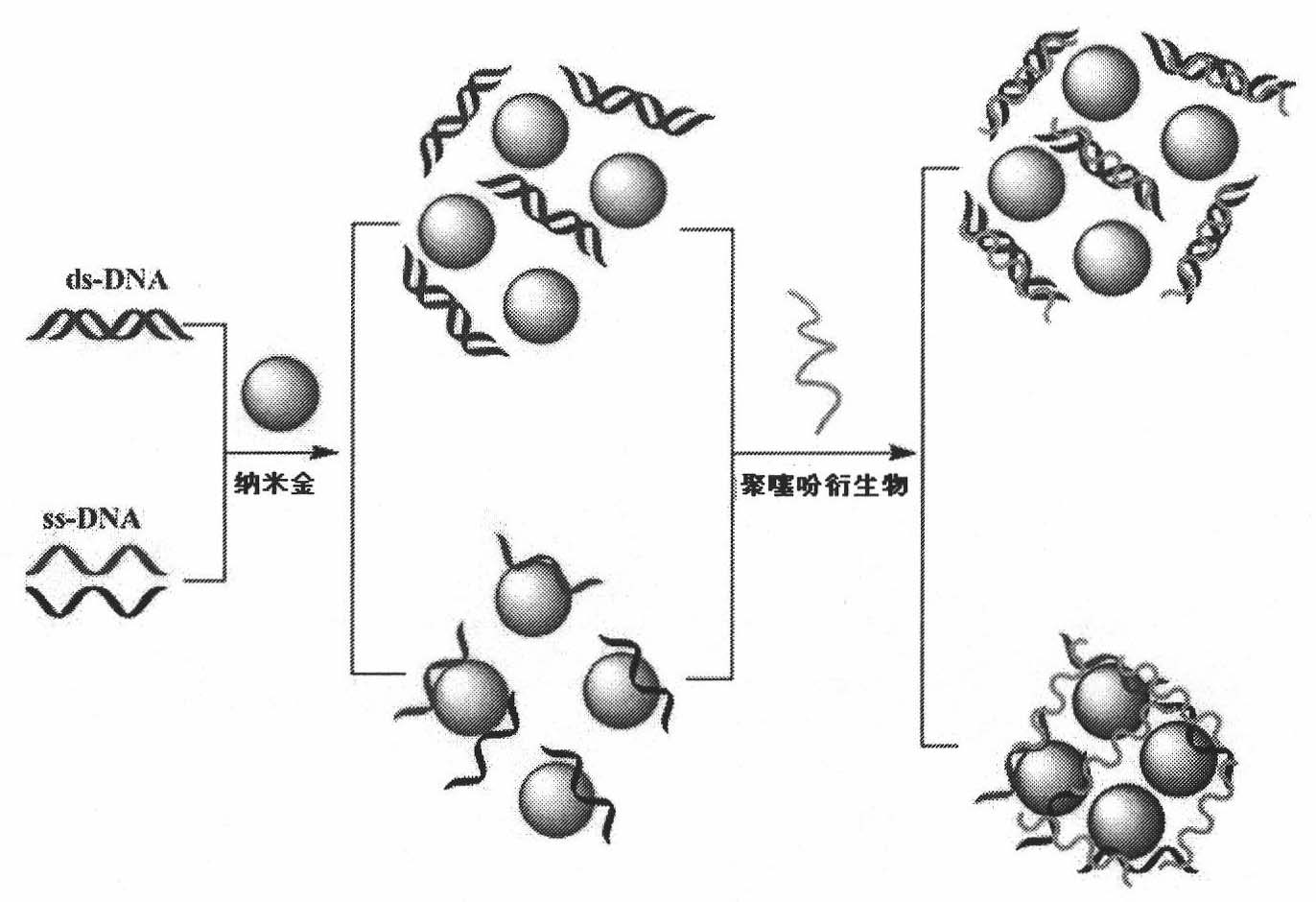
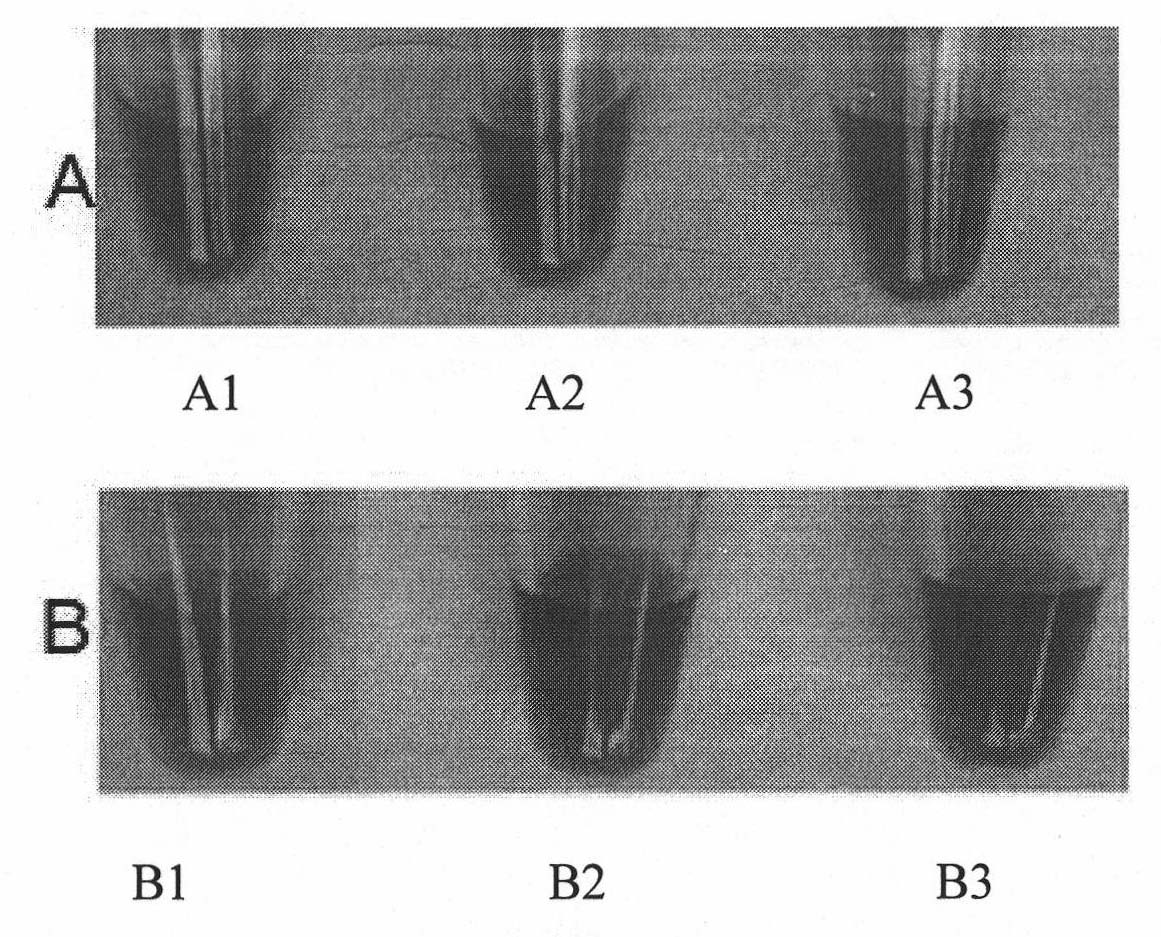
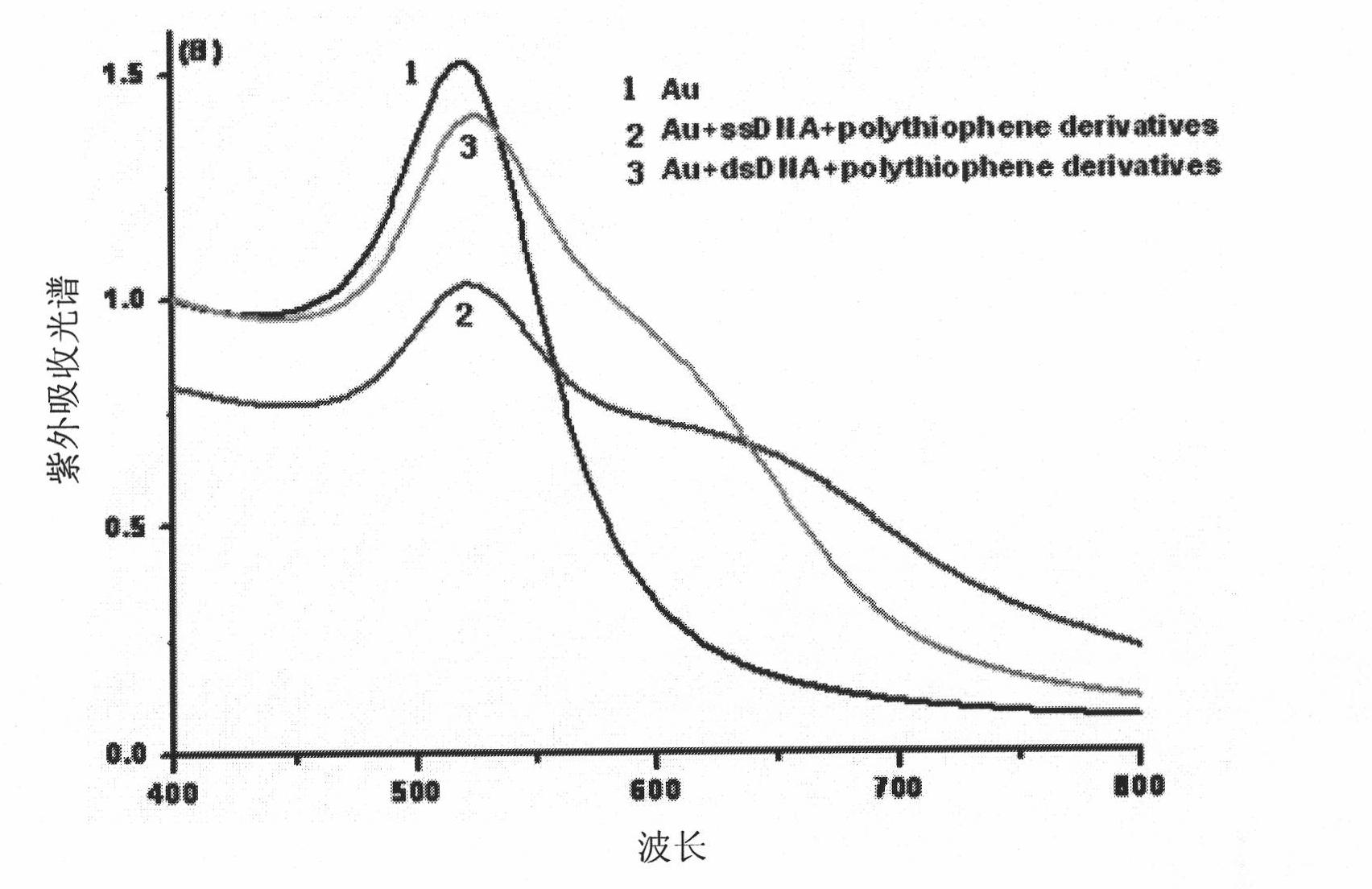
Method of colorimetric detection of target DNA by combining nanometer gold with polythiophene ramification
InactiveCN101818198AEasy to detectEasy to operateMicrobiological testing/measurementColor/spectral properties measurementsPolythiophene derivativeThiophene derivatives
The invention relates to a method of the colorimetric detection of target DNA by combining nanometer gold with polythiophene ramification, which comprises the following steps of: preparing a detection reagent (the nanometer gold and the polythiophene ramification) and a probe; and detecting the sequence of the target, etc. Based on the principle that a signal cascade is amplified due to the change of the color since the stability of the NDA-nanometer golden solution is changed when the polythiophene ramification is combined with the ssDNA / dDNA, the method realizes the high-sensitivity and fast detection of the DNA under the double action of a color converting developer and an amplified label, thereby building an analysis platform which directly detects the gene by combining the nanometer gold with the polythiophene ramification. The method has simple operation, does not need special instrument and equipment, has the characteristics of specificity, speediness and high sensitivity, and can be applied to the diagnosis and the detection of the target DNA in the clinical laboratory medicine and the environment.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Alpha-amylase detection reagent and application thereof
ActiveCN105021543AThe measurement result is accurateReduce mistakesColor/spectral properties measurementsLaboratory orderMedical laboratory
The present invention belongs to the field of medical laboratory technology, and in particular relates to an alpha-amylase detection reagent and application thereof. The reagent comprises a reagent R1 and a reagent R2; the reagent R1 is mainly composed of an MES buffer, NH4Cl, CaCl2, a surfactant and a preservative; the R2 reagent consists of an MES buffer, a 2-chloro-4-nitrobenzene-maltotriose and a preservative. The alpha-amylase detection reagent provided by the invention does not contain NaN3, does not lead to alpha-amylase allosterism in the determination process, and can reduce the error in measurement results; at the same time, the test does not need additional tool enzyme, and has short delay time; the test results have good repeatability and stability. Therefore, the invention is conducive to the clinical application of alpha-amylase detection reagent.
Owner:郑州金域临床检验中心有限公司
Determination of viable microorganisms using coated paramagnetic beads
InactiveUS20090170144A1Rapidly and accurately detectingRapidly and accurately and indicatingMicrobiological testing/measurementBiological material analysisMicroorganismLaboratory order
The present invention relates to methods for the detection of microorganisms. In one embodiment, the present invention provides methods for detecting live microorganisms in a culture by capturing and culting the microorganisms on para-tropic-coated paramagnetic beads. This technique is useful for any application in which it is necessary to monitor the biological contamination level, for example drinking water, recreational waters, food processing waters and medical laboratories. In one embodiment, the method for determining the concentration of viable microorganisms in a sample according to the invention further comprises an inducer reagent, wherein the inducer reagent includes an inducer compound that induces the activity of an enzyme unique to the microorganism of interest.
Owner:UNIVERSITY OF CINCINNATI
Serologic biomarker for coronary heart disease (CHD) detection, and application thereof
InactiveCN104311655AAccurate predictionBiological testingAnimals/human peptidesLaboratory orderSterile alpha motif
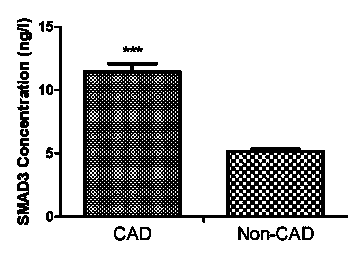
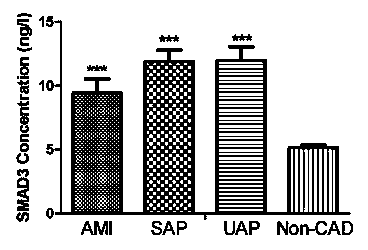
The invention belongs to the fields of laboratory medicine, clinical medicine, biotechnology and biochemistry, and relates to a serologic biomarker for coronary heart disease (CHD) detection, and application thereof. The biomarker is human SAMD3 (sterile alpha motif domain-containing protein 3). The biomarker is closely related to CHD incidence and development; SAMD3 is used as the biomarker for CHD incidence prediction and CHD development degree judgment, so that compared with the conventional pathological type diagnosis of CHD incidence and prognosis, the biomarker can more individually and accurately predict the early incidence situation and grade malignancy of CHD; moreover, the marker can be used for preparing a kit for screening CHD or assisting in pathological identification, clinical diagnosis and subtype discrimination of CHD.
Owner:雷桅
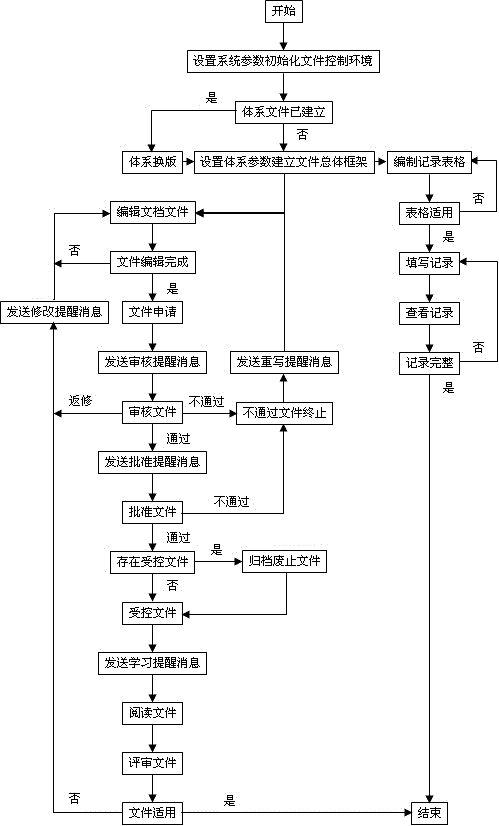
Control system for medical laboratory quality management system document and normalized control method thereof
InactiveCN106202568AGood effectAvoid wastingFile system administrationSpecial data processing applicationsLaboratory orderMedical laboratory
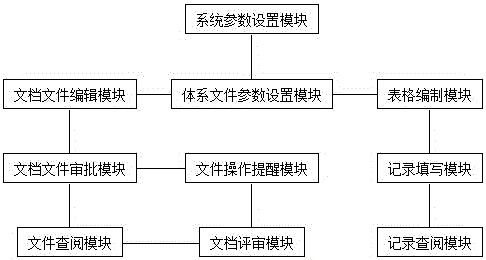
The invention discloses a control system for medical laboratory quality management system documents and a normalized control method thereof. The intelligent control system comprises a system parameter setting module, a system document parameter setting module, a file editing module, a file approving module, a document operation reminding module, a document referring module, a document reviewing module, a table compiling module, a record filling module and a record referring module. According to the control system for the medical laboratory quality management system documents and the normalized control method thereof provided by the invention, under the condition of meeting quality management standard, the compiling and alteration for the documents, the reviewing and approving for the documents, the referring and reviewing for the documents, the editing for record tables and the filling and reviewing for records can be realized in an informationized manner; simple operation is supported; the document conversion and the dynamic compiling of the record tables can be quickly realized; the quality system documents can be simply, efficiently and comprehensively controlled.
Owner:欧阳能良
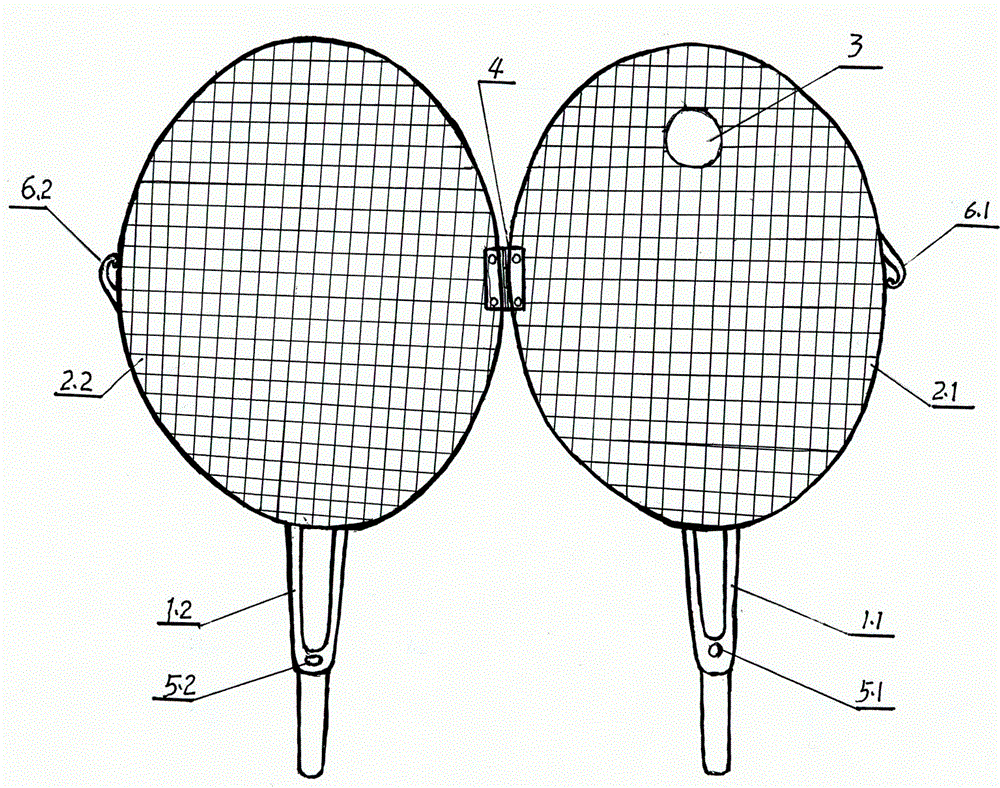
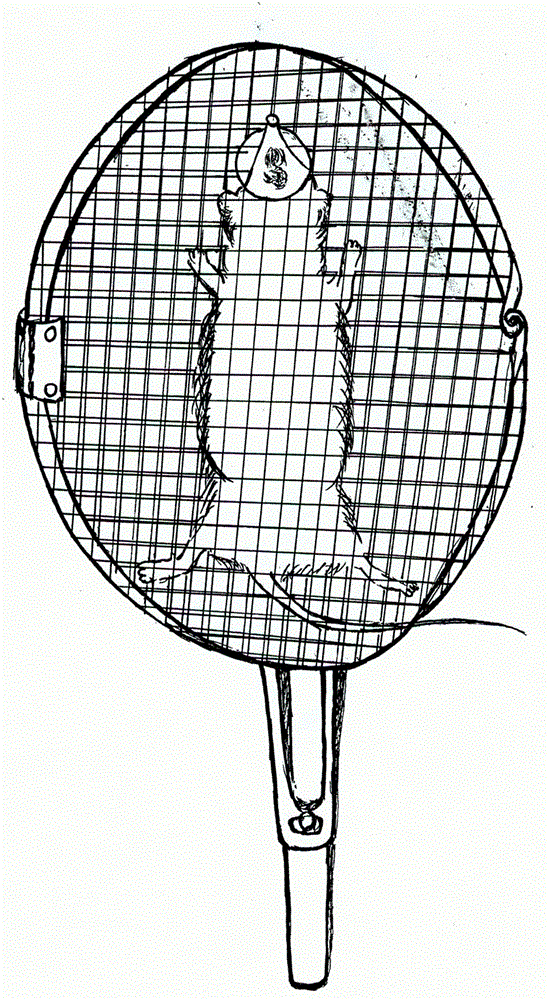
Noninvasive fixer for laboratory mouse
InactiveCN103142327ASo as not to damagePrevent being bittenAnimal fetteringLaboratory orderLaboratory mouse
The invention belongs to the field of medical laboratory apparatuses, and discloses a noninvasive fixer for a laboratory mouse. The noninvasive fixer is mainly characterized by structurally comprising a combined handle and clamping plates, wherein the plate surfaces of the clamping plates are mesh surfaces; a mouse mouth hole is formed in the mesh surface at one end of any clamping plate; a mouse mouth extension hole is formed outside the mouse mouth hole; and buckling parts are arranged on the handle. The conventional handholding mode is replaced, and the noninvasive fixer has the advantages of complete exposure of each part, fixing firmness, positioning accuracy, convenience for operation, adaptability to a plurality of experiments and capability of avoiding injuries to the laboratory mouse and an operator.
Owner:张蕾 +2
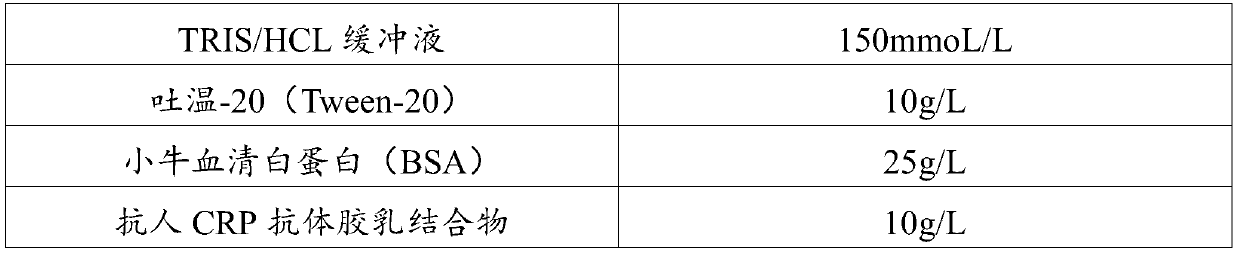
CRP (C-Reactive Protein) assay kit, and preparation method and application thereof
ActiveCN110646618ANo increase in R&D costsImprove detection accuracyMaterial analysis by observing effect on chemical indicatorDisease diagnosisActive agentSurface-active agents
The invention relates to a CRP (C-Reactive Protein) assay kit, and a preparation method and application thereof, and belongs to the technical field of IVD reagents (In Vitro Diagnostic Reagents). TheCRP assay kit provided by the invention comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises buffer solutions, inorganic salts, surfactants, stabilizing agents and preservatives;and the reagent R2 comprises buffer solutions, surfactants, sealing agents, anti-human CRP antibody latex conjugates and preservatives. The CRP assay kit provided by the invention has the advantages that on the basis of the existing latex immunoturbidimetry, a reaction system is optimized by a specific preparation method through an anti-freezing agent; the detection accuracy, the analysis sensitivity and precision of tested reagents are further improved; the research and development cost of the reagents are not improved; and the CRP assay kit can easily adapt to the development of the modern clinical laboratory medicine.
Owner:广州市伊川生物科技有限公司
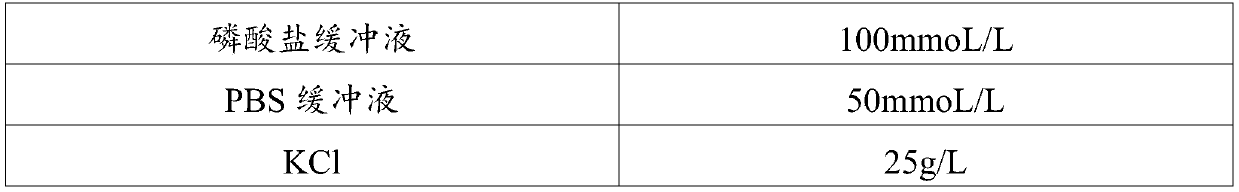
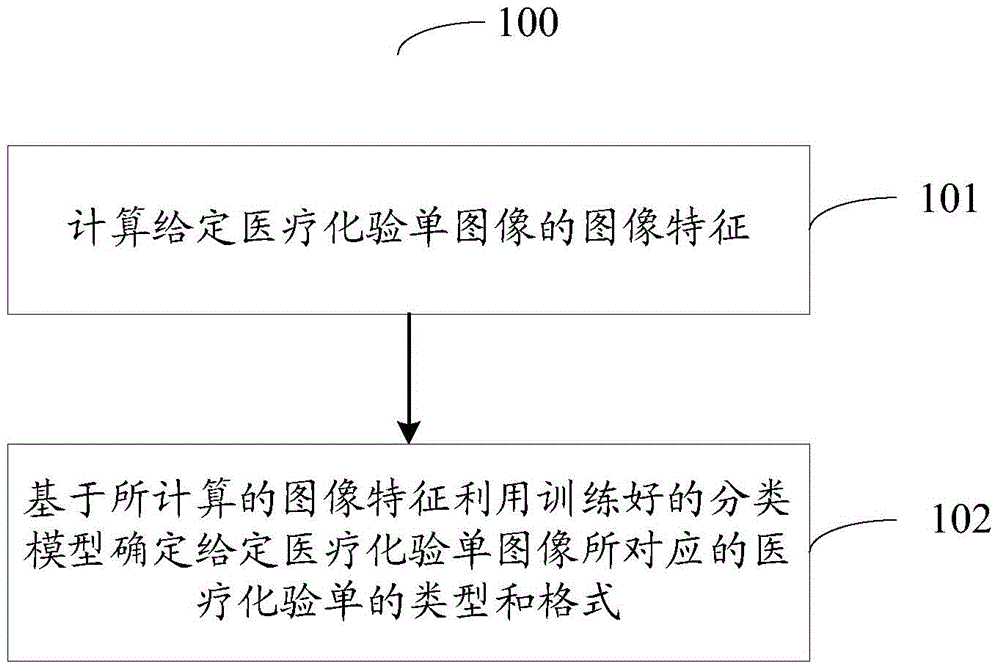
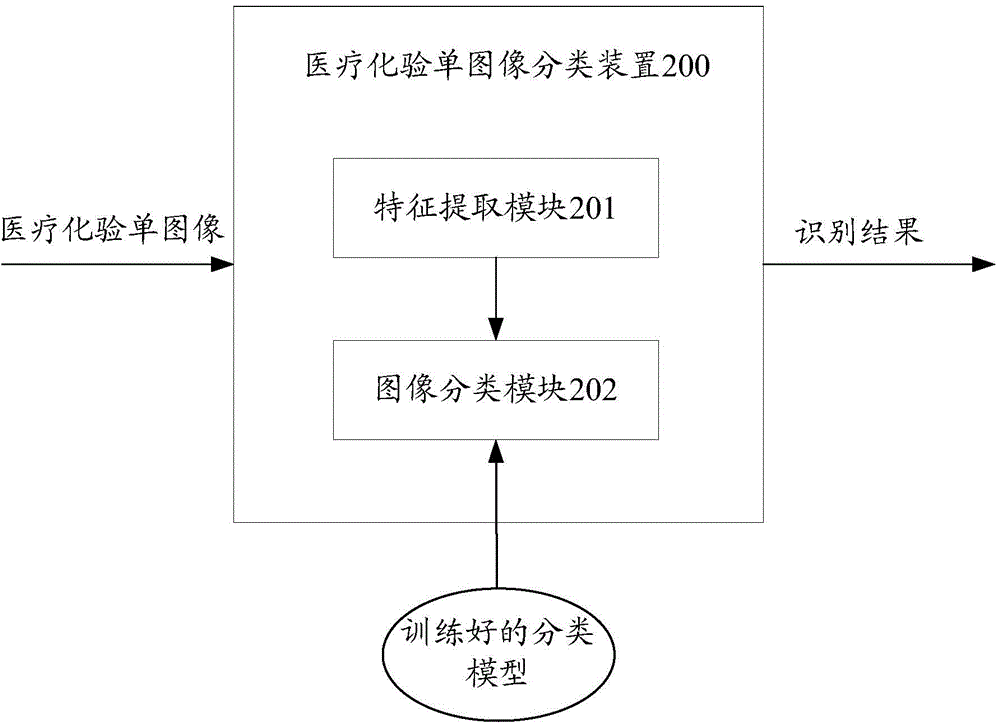
Medical laboratory report image classification method and apparatus
ActiveCN104966109AImprove recognition efficiencyCharacter and pattern recognitionLaboratory orderMedical laboratory
The present invention provides a medical laboratory report image classification method and apparatus. The medical laboratory report image classification method comprises: calculating an image feature of a given medical laboratory report image; and determining a type and a format of a medical laboratory report corresponding to the given medical laboratory report image based on the calculated image feature by using a trained classification model. According to the medical laboratory report image classification method and apparatus provided by the present invention, a type and a format of a medical laboratory report are automatically determined according to an image feature, thereby saving the process of manually identifying a type and a format of a medical laboratory report, and improving medical laboratory report identification efficiency.
Owner:BEIJING KUANGSHI TECH CO LTD +1
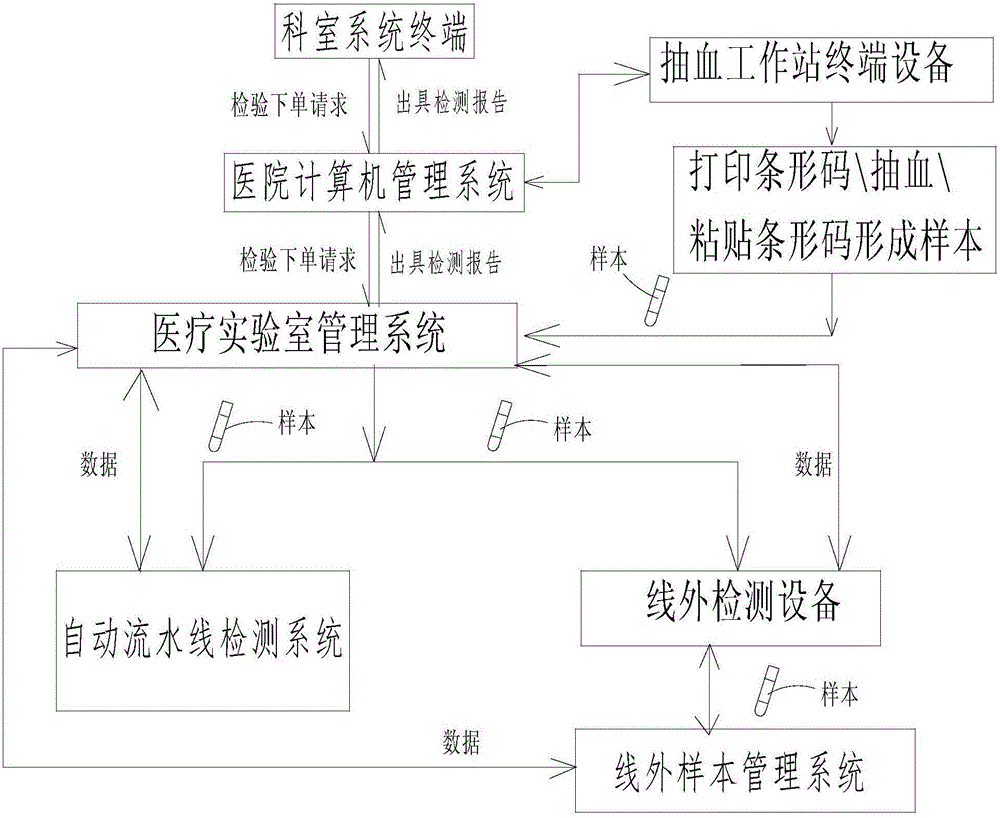
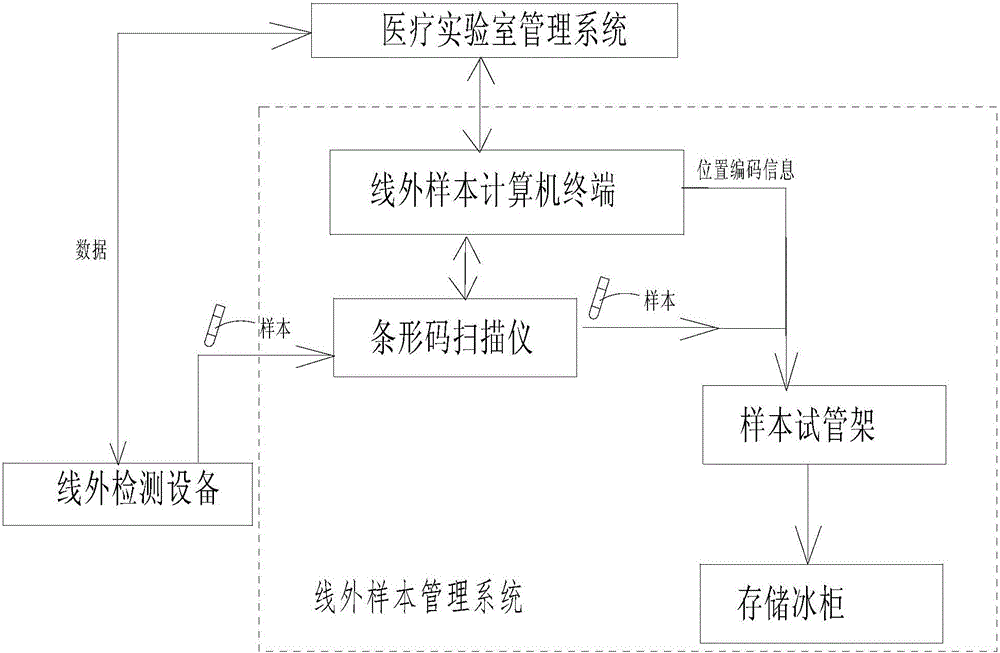
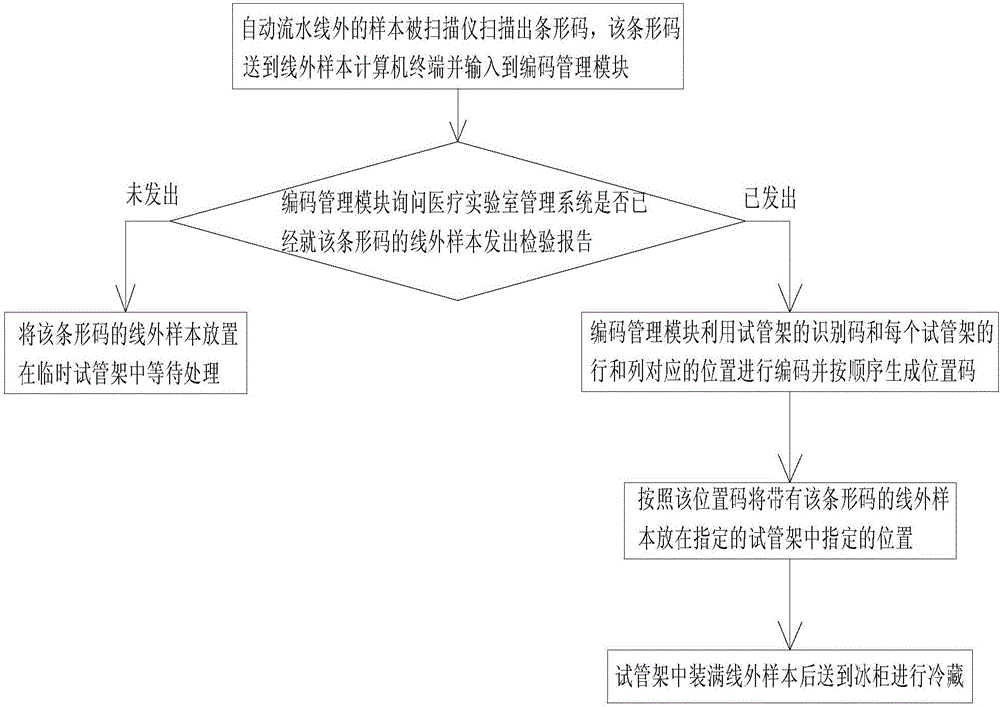
Sample management system for medical laboratory
InactiveCN105809601AEasy to classifyEasy to find samplesData processing applicationsCo-operative working arrangementsLaboratory orderMedical laboratory
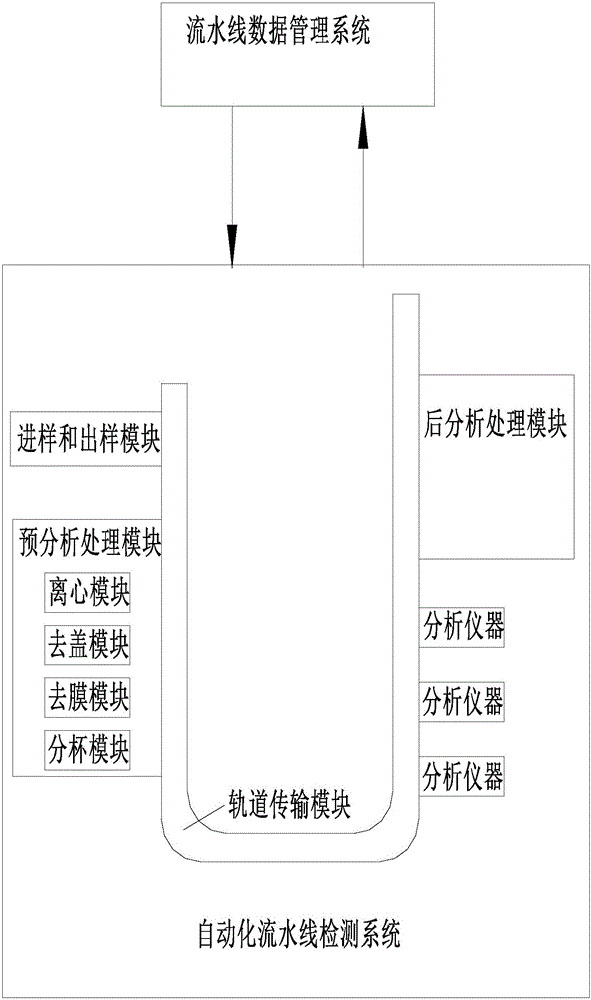
The invention discloses a sample management system for a medical laboratory.The sample management system comprises a medical laboratory management system, an automatic assembly line detection system and an off-line detection management system.The medical laboratory management system is in communication with the automatic assembly line detection system and the off-line detection management system.Samples with barcodes are classified after entering the medical laboratory management system, the classified samples enter the automatic assembly line detection system and the off-line detection management system respectively, the automatic assembly line detection system conducts uniform detection, management and storage on all the samples entering an automatic assembly line, and the off-line detection management system comprise off-line detection devices and an off-line sample management system and can change the disordered state of the samples out of the automatic assembly line into an ordered state, so that workers in the laboratory classify and find the samples conveniently.
Owner:吴剑杨
Device for approving medical tests across a plurality of medical laboratories, medical providers, and lab payers and methods for using the same
ActiveUS20190244688A1Medical communicationMedical practises/guidelinesLaboratory orderMedical laboratory
Owner:OPTUM
Preheated sterilizing box for medical laboratory equipment
InactiveUS20180289845A1Avoid breakingIncrease contact areaHeating or cooling apparatusLavatory sanitoryTemperature controlLaboratory order
The present invention relates to a preheated sterilizing box for medical laboratory equipment, comprising a body, a cover hinged to the body through a hinge, and an attachment device for preventing collision between preheated equipment. The cover comprises a cover housing, a UV lamp disposed on the cover and a transparent cover board. The body is separated by the separator and the heat guiding plate into a first chamber for holding preheated sterilizing equipment, a second chamber disposed with temperature control circuitry, a third chamber disposed with a heating material and a fourth chamber disposed with a battery. An insulating layer is disposed on the inside wall of the first chamber and the third chamber, a temperature sensor is embedded in the insulating layer disposed on the inside wall of the first chamber. A number of heat guiding poles and through holes are disposed on the heat guiding plate. The attachment device is disposed on the through holes. The sterilizing box has a sensible structure, having the advantages of simple, convenient to use, well portable, can effectively solve the problem of secondary contamination during preheating and sterilizing of medical laboratory equipment.
Owner:CHAN KA YAN
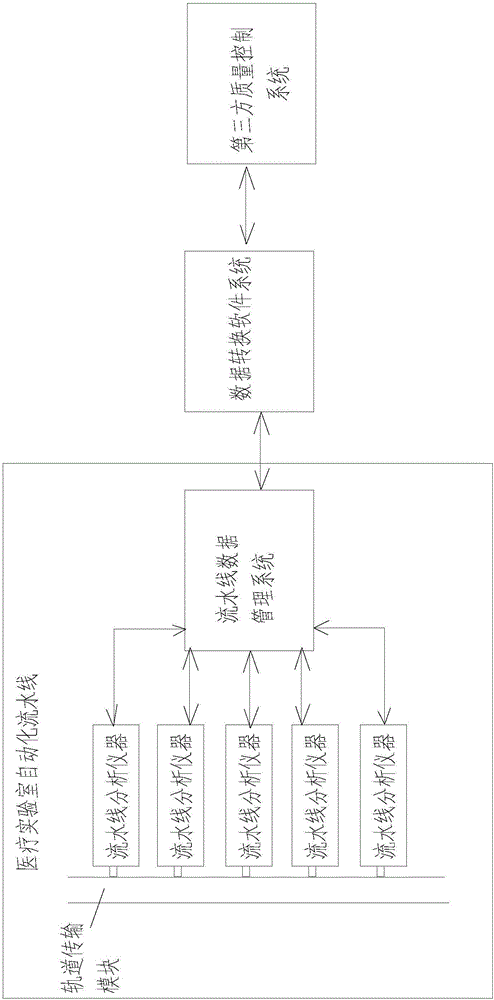
Method and system for controlling indoor quality of automatic assembly lines of medical laboratories
InactiveCN106203786AIncrease flexibilityAdapt to work needsResourcesMedical laboratoryIndoor air quality
The invention discloses a method and system for controlling indoor quality of automatic assembly lines of medical laboratories. The method comprises the following steps of: setting a corresponding quality control rule for each detection project by utilizing a third-party quality management system; carrying out data conversion through a data conversion system so as to transmit the detection projects and the corresponding quality control rules to an assembly line data management system and an assembly line analyzer, wherein the assembly line analyzer is provided with an indoor quality control detection module; receiving an instruction sent by the assembly line data management system by the indoor quality control detection module and acquiring corresponding IQC data by the indoor quality control detection module according to the corresponding quality control rules of the detection projects; transmitting the IQC data to the third-party quality management system through the assembly line data management system and the data conversion system; automatically analyzing the IQC data by the third-party quality management system so as to obtain a result of detecting whether the detection projects are out of control or not, and transmitting the result back to the assembly line data management system. The method and system are basically free of hand participation, high in accuracy and good in flexibility, and can dispatch resources to the greatest extent, thereby improving the efficiency and saving the human cost.
Owner:温冬梅
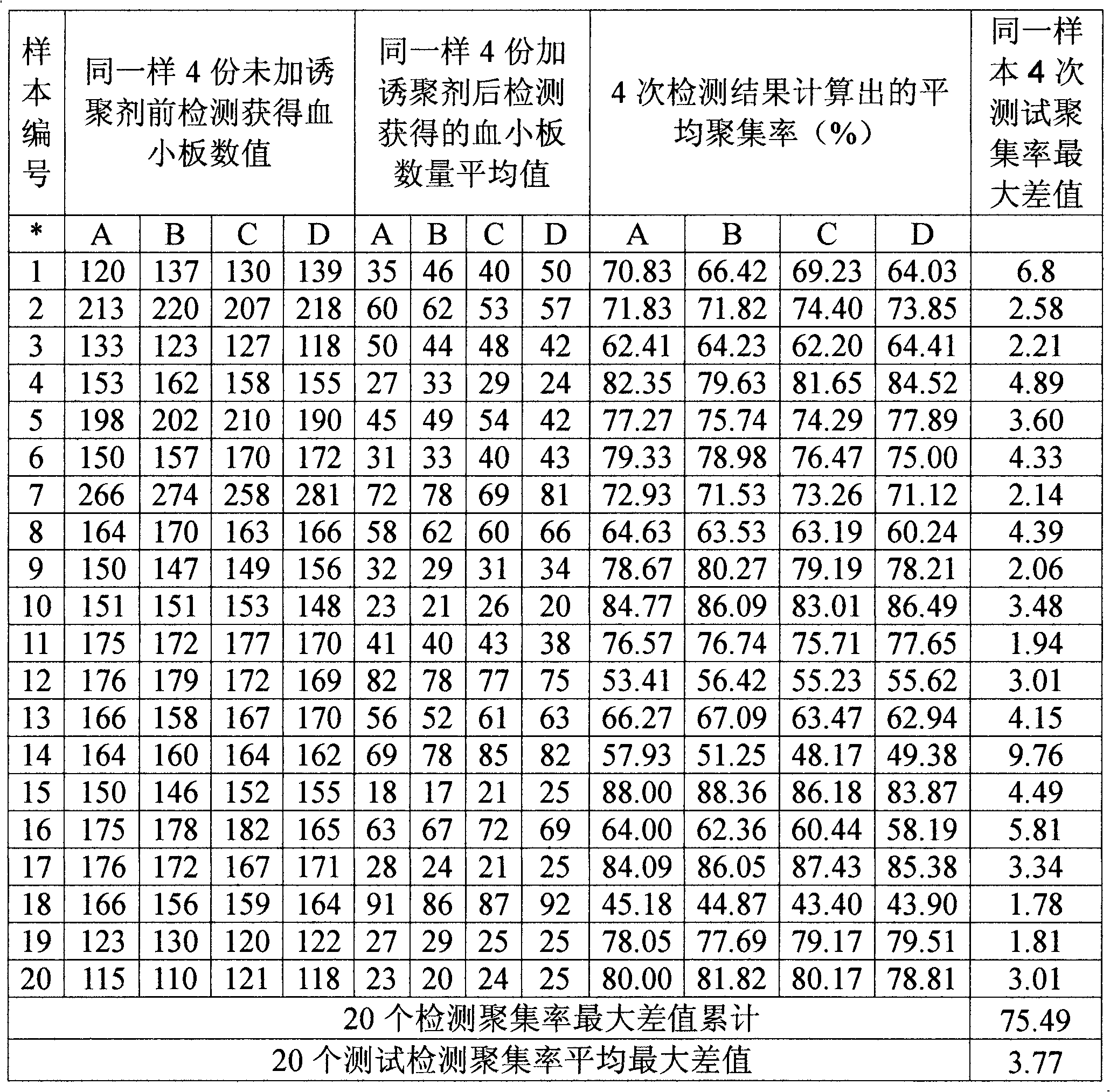
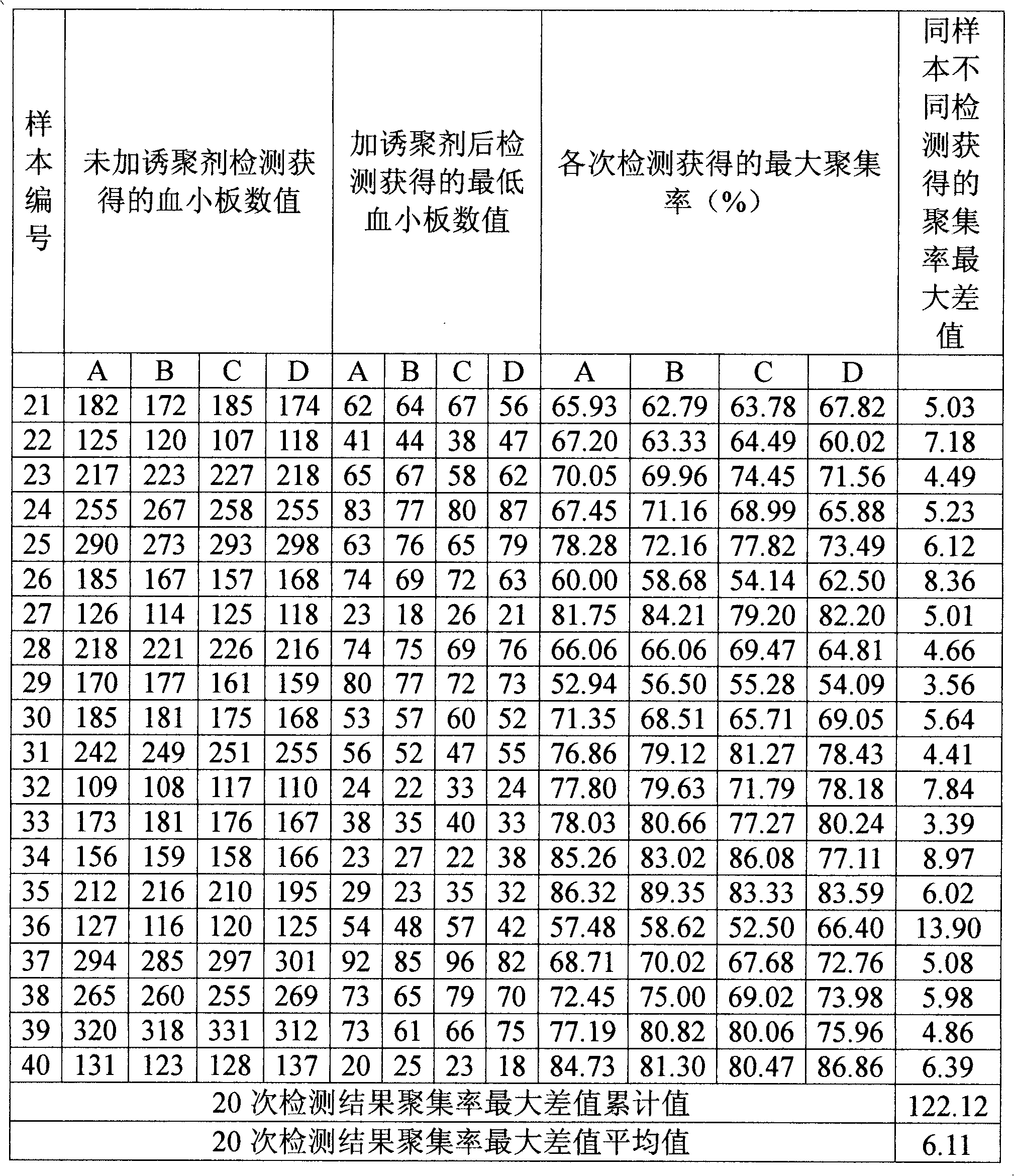
Optimized automation-adaptable platelet aggregation function inspection and analysis method
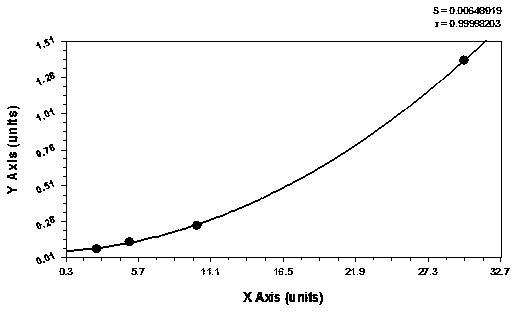
The invention provides an optimized automation-adaptable platelet aggregation function inspection and analysis method, and belongs to the field of clinical laboratory medicine. The method is an improvement and optimization of a platelet aggregation function inspection and analysis method using an automation counting method. The method is suitable for platelet aggregation function automatic analysis instruments using the counting method. According to the method, the number of platelets is inspected and recorded and an aggregation rate is calculated. A laboratory test result shows that the method is better in repeatability of the obtained result of platelet aggregation rate compared with a previous largest aggregation rate method.
Owner:SINNOWA MEDICAL SCI & TECH
Fully-automatic oxygen control animal tank
InactiveCN105662639AEliminate damageEliminate uneven distribution of oxygen concentrationBreathing protectionTreatment roomsAutomatic controlControl animal
A fully automatic oxygen-controlled animal cabin, belonging to the technical field of medical experiment equipment, which solves the problem of automatic control of low oxygen concentration in the animal cabin and the uniformity of the distribution of mixed gas in the animal cabin; the whole experimental equipment consists of nitrogen cylinders, Air pump, animal cabin, buffer tank, central processing unit, flow meter, distributor, several conduits, cables, etc.; by adjusting the concentration of nitrogen in the animal cabin, the effect of indirectly controlling the oxygen concentration is achieved; the size of the oxygen concentration and The time to maintain the hypoxic environment is automatically controlled by the central processing unit. The equipment is highly automated and does not require special personnel to wait; the use of flow meters, distributors and buffer tanks reduces or even eliminates the damage of small jets of gas to small animals. , has the advantages of uniform and gentle air supply, and eliminates the phenomenon of uneven oxygen concentration distribution in the animal cabin from the source.
Owner:THE FIRST HOSPITAL OF LANZHOU UNIV
Hand sanitizer applied to medical clinical laboratory and preparation method of hand sanitizer
InactiveCN107744495AImprove the bactericidal effectLong antibacterial timeCosmetic preparationsToilet preparationsChlorhexidine AcetateMedical laboratory
The invention discloses a hand sanitizer applied to medical clinical laboratory. The hand sanitizer consists of the following raw materials in parts by weight: 10-20 parts of scapharca subcrenata extract freeze-dried powder, 10-15 parts of fucosterol, 4-15 parts of carboxymethyl chitosan, 7-11 parts of juice of awned goosefoot, 5-10 parts of chitosan, 5-8 parts of polysaccharides of sargassum graminifolium, 30-68 parts of water of washing rice, 2-5 parts of chlorhexidine acetate, 5-10 parts of an extract of broadleaf holly leaves, 5-10 parts of cocamidopropyl betaine, 4-8 parts of an extract of fructus rubi, 4-6 parts of herba portulacae juice, 2-5 parts of jellyfish collagen, 2-5 parts of coconut shell activated carbon and 1-3 parts of guar gum. The hand sanitizer provided by the invention has a good sterilizing effect and is long in bacteriostasis duration; meanwhile, the hand sanitizer has moisturizing and nourishing effects; and the hand sanitizer, when used, is free from any sideeffects and discomfort; therefore, the hand sanitizer is safer.
Owner:吕凤鹏
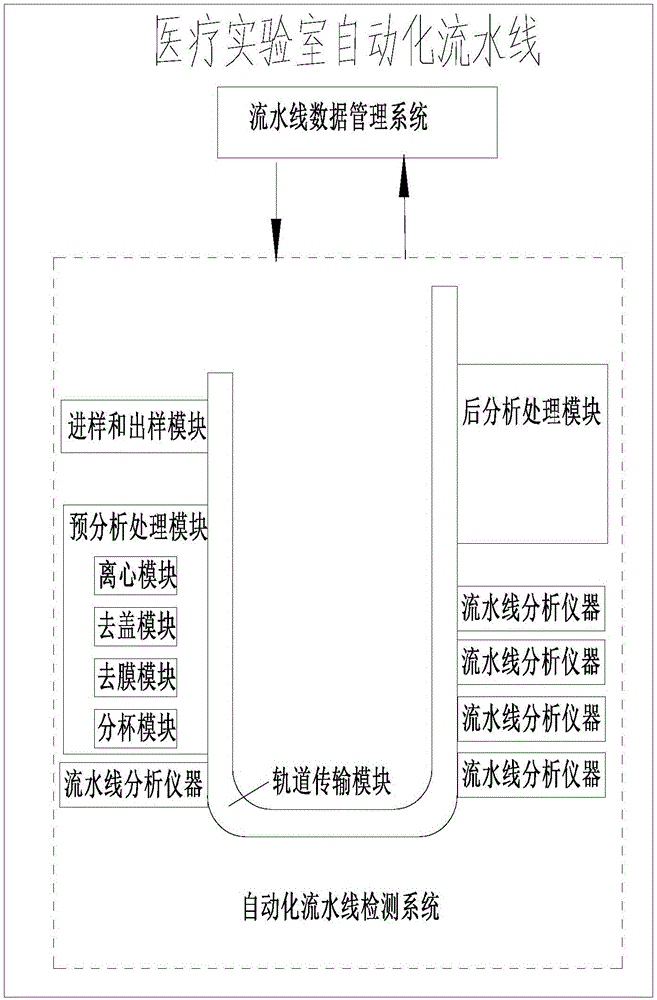
Medical laboratory automation flow line with mobile management system
ActiveCN105929800AReduce work intensityEasy to manageProgramme total factory controlMedical laboratoryOperational system
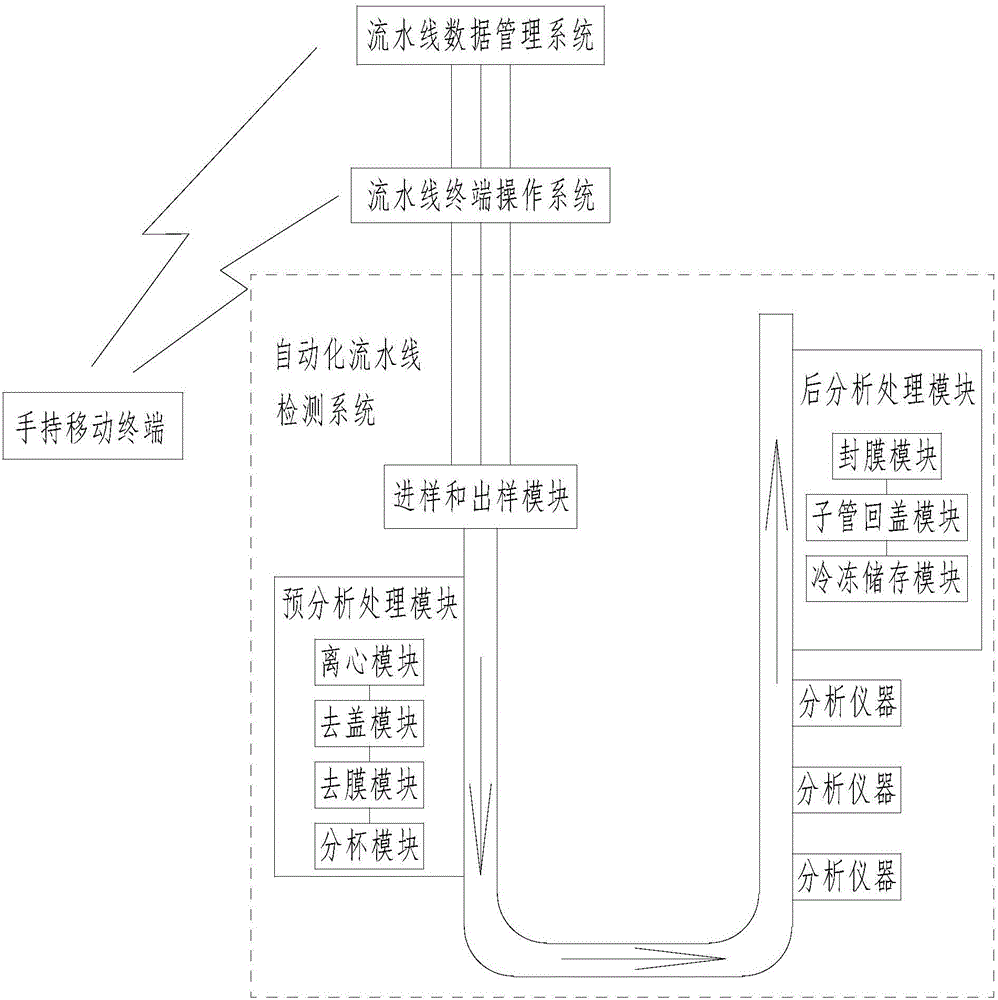
The invention discloses a medical laboratory automation flow line with a mobile management system. The medical laboratory automation flow line comprises an automation flow line detection system, a flow line terminal operation system, a flow line data management system Centralink and a hand-held mobile terminal. Wireless communication connection is established between the hand-held mobile terminal and the flow line terminal operation system. A client version of flow line management system software is installed in the hand-held mobile terminal. An operation commander is sent to the flow line terminal operation system by means of the client version of flow line management system software in the hand-held mobile terminal. The flow line terminal operation system automatically recognizes the operation commander and controls the automation flow line detection system to work. The invention makes it convenient for laboratory staffs to control the quality of patient samples in the whole course.
Owner:温冬梅
Fluid transfer apparatus
ActiveCN103917295AImprove convenienceRelieve pressureWithdrawing sample devicesBurettes/pipettesLaboratory orderElectrical control
The invention relates to a fluid transfer apparatus, in particular pipetting apparatus, for transferring at least one fluid laboratory sample, in particular a biochemical or medical laboratory sample, comprising a base body, which has a connecting section serving for connecting a container to the base body for the intake of the at least one fluid sample into the container, a movement device, which can bring about the intake and / or delivery of at least one fluid laboratory sample into the container, an electrical control device, which controls at least one function of the fluid transfer apparatus, a sensor device, which comprises at least one acceleration sensor which is signal-connected to the control device and by means of which at least one acceleration value can be measured during the movement of the fluid transfer apparatus, wherein the control device is designed such that it uses the at least one acceleration value during the control of the at least one function. The invention furthermore relates to a method for using at least one acceleration value in a fluid transfer apparatus.
Owner:EPPENDIRF AG
Closestool type female urine collector for medical clinical laboratory
PendingCN111870282AEasy to collectAvoid shootingSurgeryVaccination/ovulation diagnosticsLaboratory orderMedical laboratory
The invention relates to a collector, in particular to a closestool type female urine collector for a medical clinical laboratory. The invention aims to solve the technical problem of how to design the closestool type female urine collector for the medical laboratory department, which can conveniently collect urine for a female patient, can prevent the urine from being splashed on hands, and can prevent the urine can be prevented from falling onto the ground to influence the surrounding environment. The closestool type female urine collector for the medical laboratory department comprises a bottom plate and a support column, wherein support feet are fixedly connected to three sides of the bottom plate; and the support column is fixedly connected to the edge positions of one sides of the two support legs. Urination is realized for the patient sitting on a pedestal pan, the urine flows into a collecting container through a liquid outlet pipe, after the female patient finishes urination,a slope block is pushed to be separated from the collecting container, then the collecting container is taken down, and a proper amount of the urine is poured into a sampling test tube, so that the urine is more conveniently collected, and the urine is prevented from being splashed on hands.
Owner:熊志刚
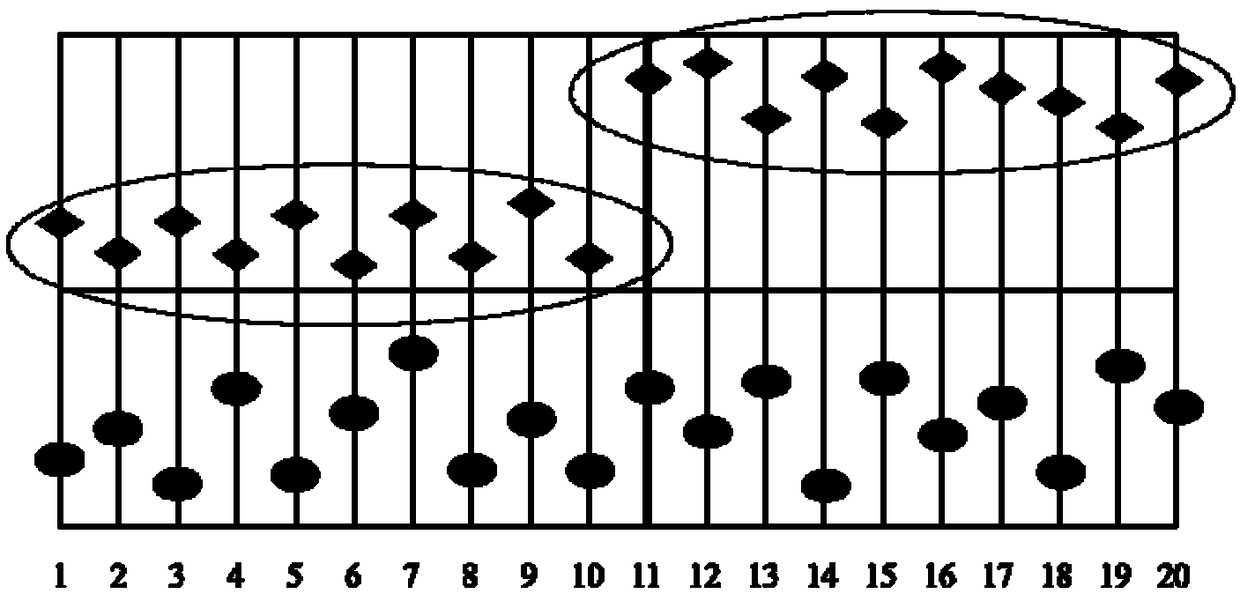
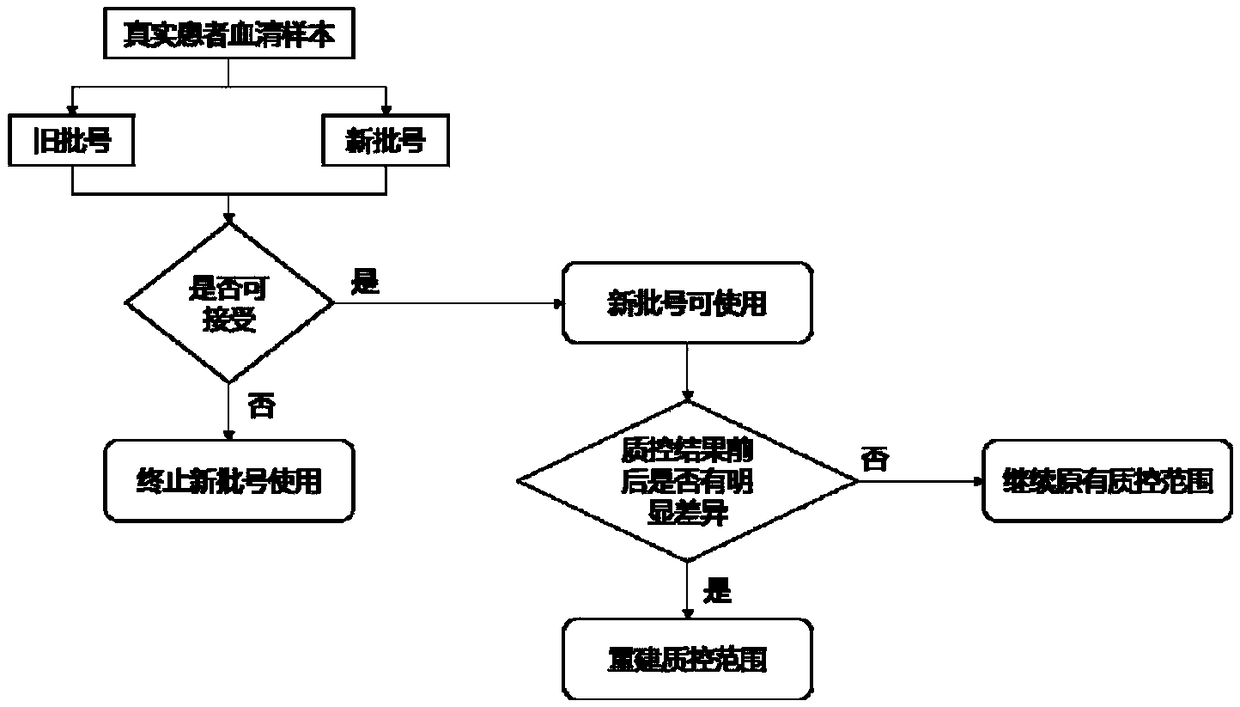
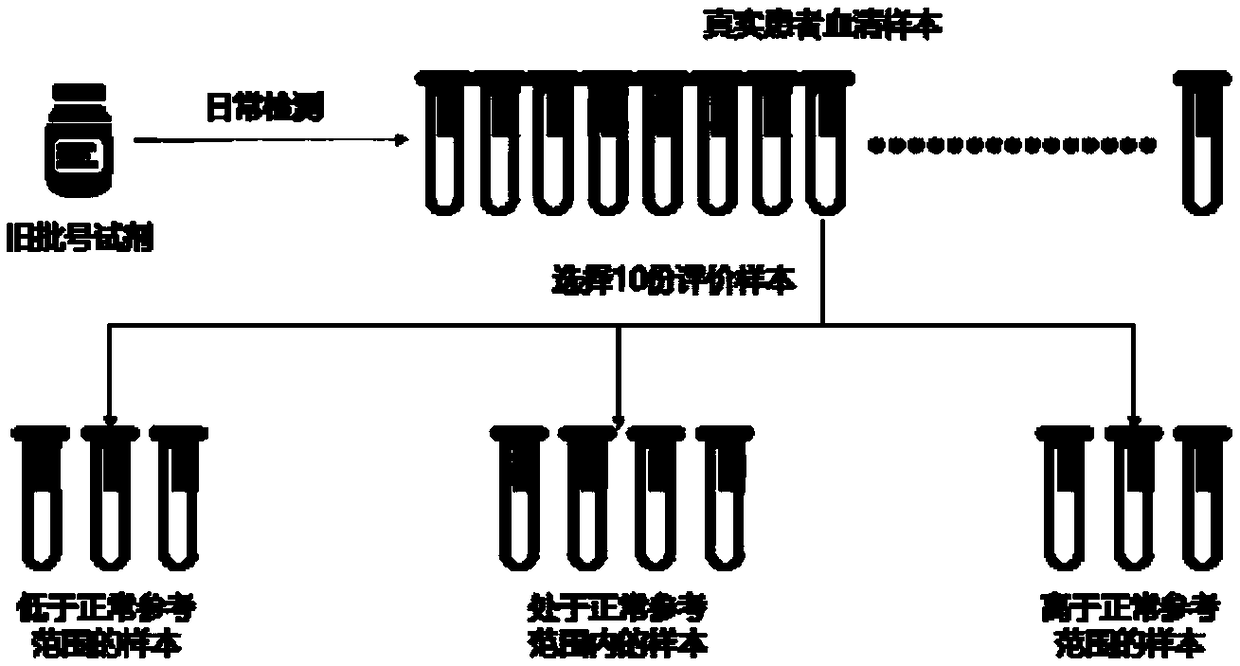
Method for performing reliability evaluation on result of quantitative detection item of detection system, and use
The invention provides a method for performing reliability evaluation on a result of a quantitative detection item of a detection system, and a use, and relates to the technical field of quality control of a medical laboratory. According to the method, actual experimental samples in the laboratory are used as evaluation samples, and there is no matrix effect compared with quality control. Representative samples are selected, that is, the experimental samples comprise an experimental sample in which a detection value of the quantitative detection item is less than a normal reference range, an experimental sample greater than the normal reference range, and an experimental sample within the normal reference range. In addition, the judging criteria of the method not only consider the requirements of quality objectives in the unique analysis of the detection item of the medical laboratory, but also consider the requirements of statistical linear regression. Therefore, the reliability of the result of the quantitative detection item of the detection system can be evaluated by the method in a simple and convenient, operable and efficient manner, and the method has an important practicalvalue on the quality control of the medical laboratory and the guarantee of the diagnosis and treatment of patients.
Owner:上海昆涞生物科技有限公司
Drug sustained-release implant agent and implanting device thereof
PendingCN107753418ALong release periodRelease stabilityOrganic active ingredientsPharmaceutical delivery mechanismLaboratory orderAdhesive
The invention discloses a drug sustained-release implant agent and an implanting device thereof. The drug sustained-release implant agent is in the form of a capsule and comprises a drug, a laboratory-grade silicone tube and a medical curing adhesive. The drug is put in the silicone tube, openings of the two ends of the silicone tube are sealed with the curing adhesive, and the drug is controlledto release by the surface micro-aperture of the silicone tube. The implanting device includes a hollow sleeve tube and a drug pushing needle core, and is used for implanting the drug sustained-releaseimplant in an animal body or subcutaneously. The drug sustained-release implant agent has the advantages of long sustained release period, stable release, and the like. The implanting device has theadvantages of simple and practical manufacturing and using methods, and has significant technical advantages and scientific value.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
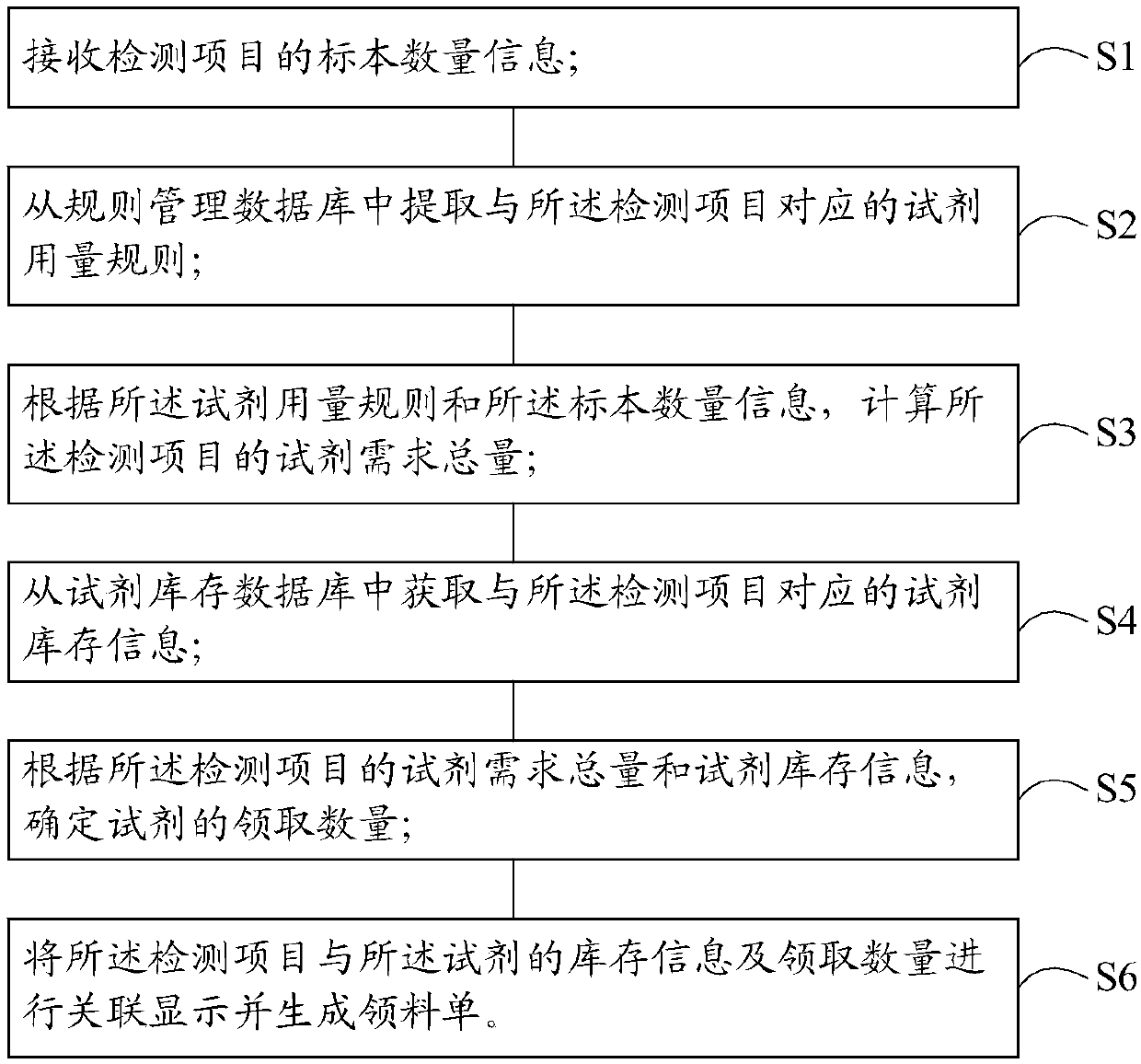
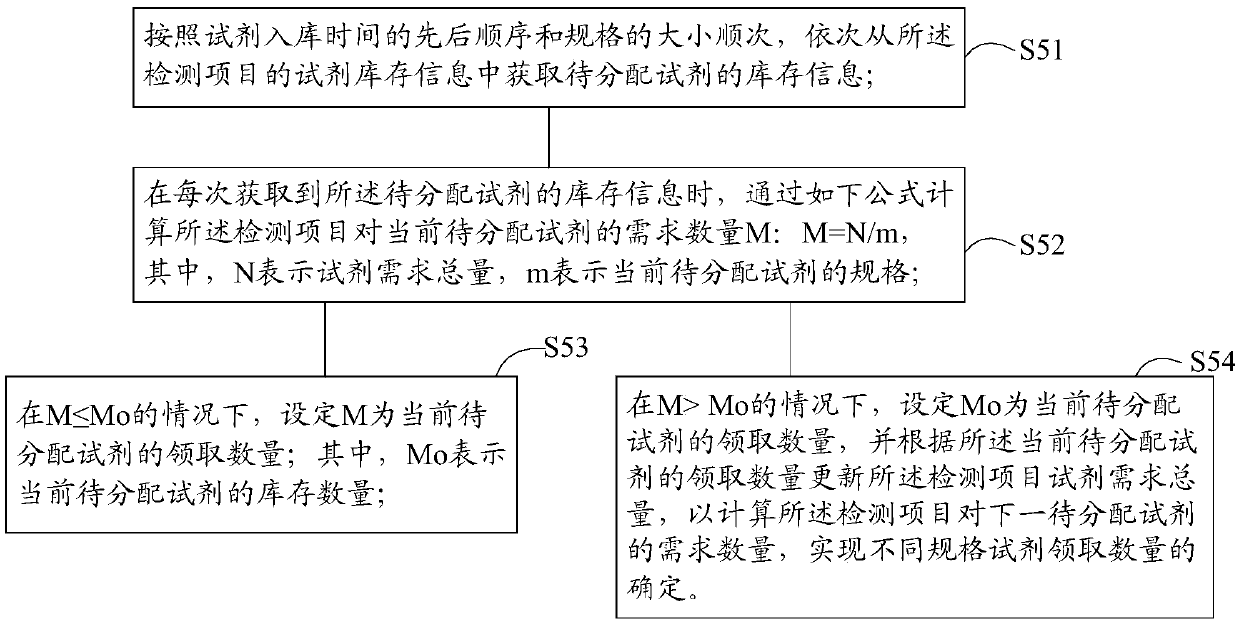
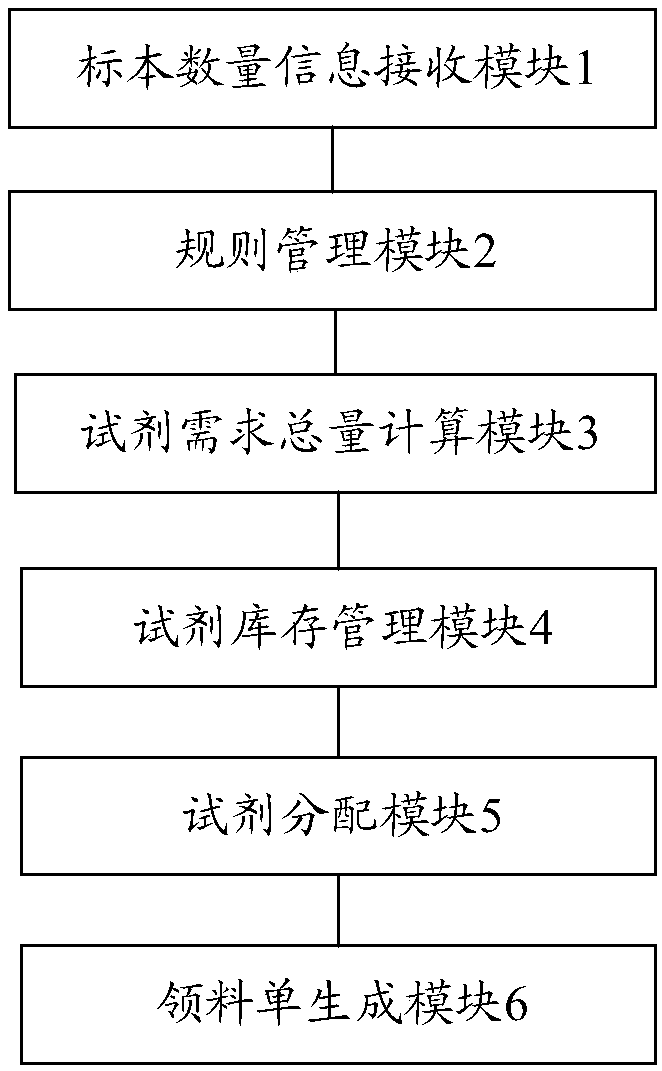
Reagent management method and system for medical laboratory
ActiveCN109545345AAvoid the problem of getting too muchImprove collection efficiencyResourcesHealthcare resources and facilitiesLaboratory orderMedical laboratory
The invention discloses a reagent management method for a medical laboratory. The management method comprises the steps: receiving the specimen quantity information of a detection item; extracting a reagent dosage rule corresponding to the detection item from a rule management database; calculating the total reagent demand of the detection item according to the reagent dosage rule and the specimenquantity information; obtaining reagent inventory information corresponding to the detection item from a reagent inventory database; determining the quantity of the reagent to be received according to the total reagent demand of the detection item and the reagent inventory information; correlating and displaying the detection item and the inventory information and receiving quantity of the reagent, and generating a requisition list. The invention also provides a reagent management system for the medical laboratory. By adopting the reagent management and system of the invention, it is possibleto effectively avoid insufficient reagent collection or excessive reagent collection, and improve the management efficiency of the reagent.
Owner:西安金域医学检验所有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com