Method for performing reliability evaluation on result of quantitative detection item of detection system, and use
A detection system and quantitative detection technology, applied in the field of quality control in medical laboratories, can solve problems such as inability to evaluate reagents or calibrators, and achieve the effect of operational and efficient result reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
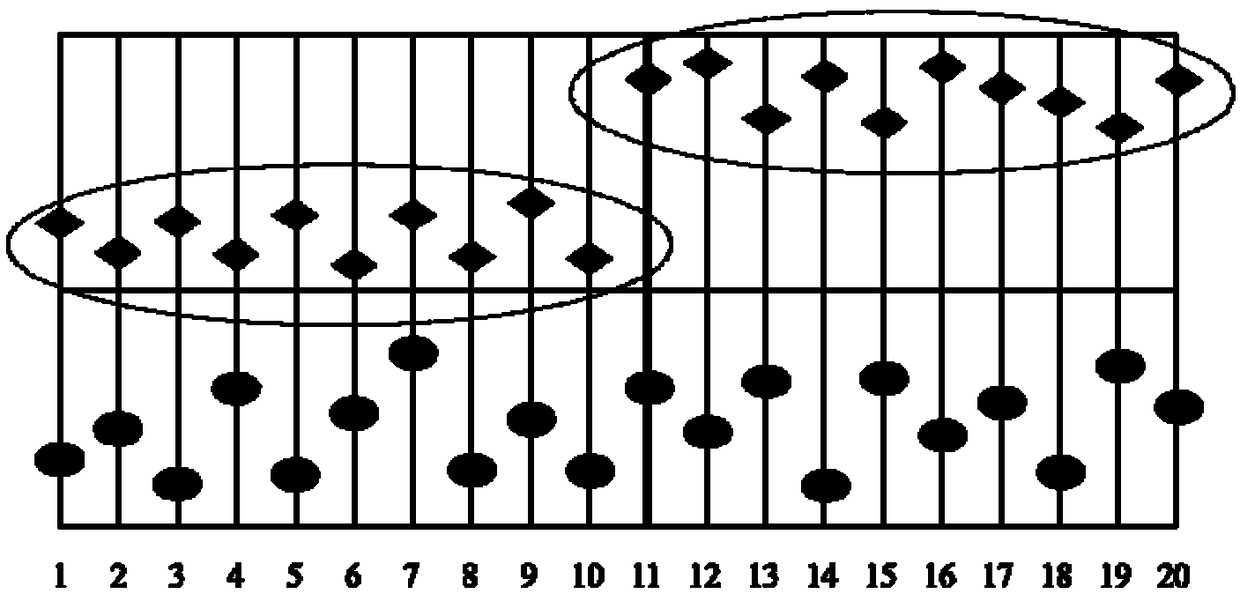
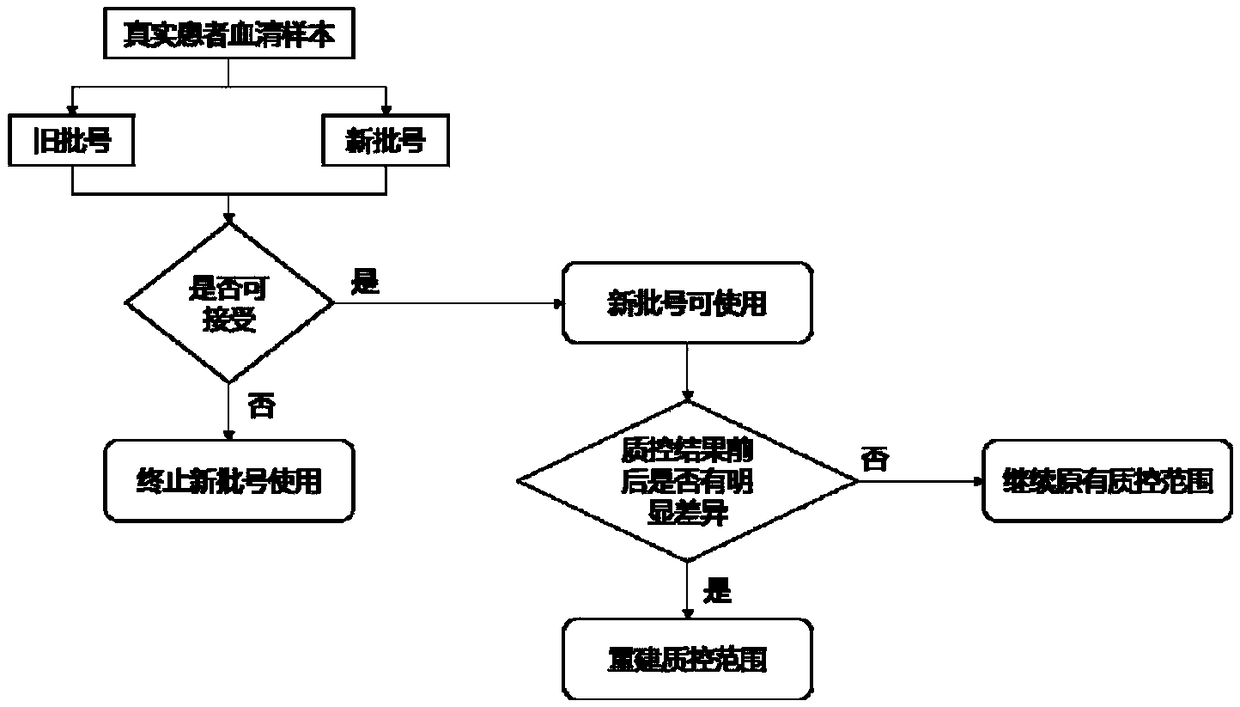
[0068] This embodiment provides a method for evaluating the reliability of the results of the quantitative detection items of the detection system. The schematic diagram of the changes in the results of different levels of quality control products before and after the batch number of the reagent or standard is changed is as follows: figure 1 As shown, the process of the method of this embodiment refers to figure 2 shown.
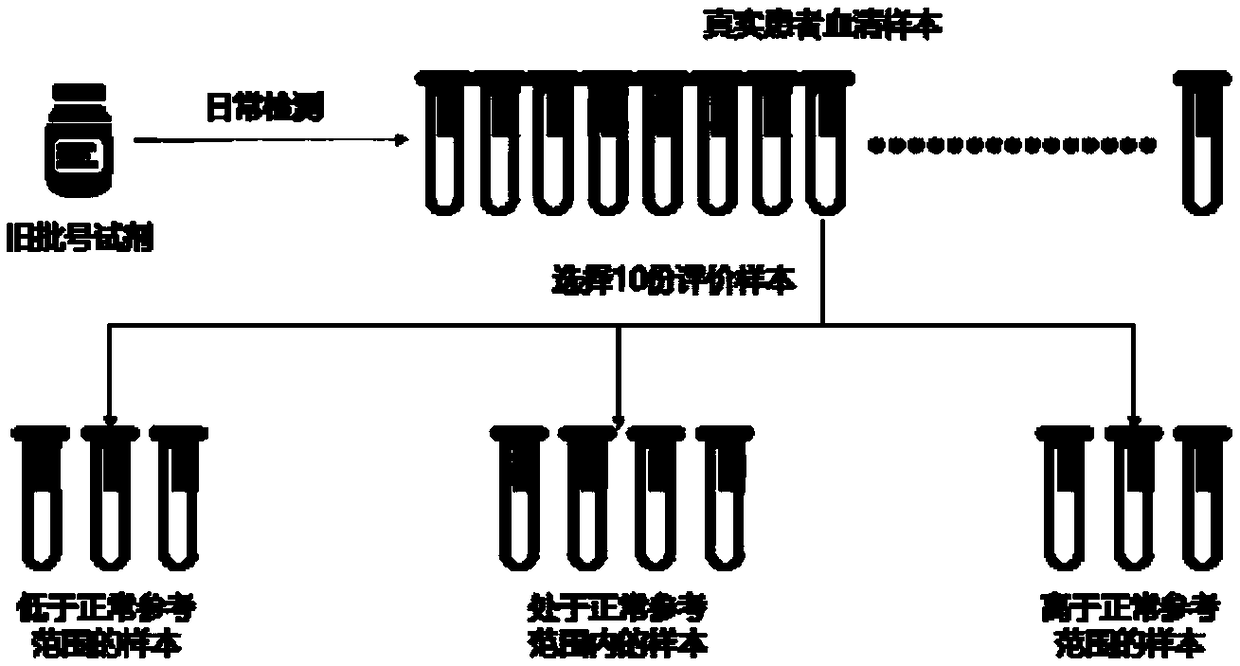
[0069] A. Selection of evaluation samples: select 10 real patient serum samples that have been tested before the batch number of the reagent / calibrator is changed as evaluation samples, and among these 10 samples, the detection values of 4 samples are within the normal reference range. The detection values of 3 samples were lower than the normal reference range, and the detection values of 3 samples were higher than the normal reference range. image 3 shown.
[0070] B. Detection of evaluation samples: test the above 10 evaluation samples with a ne...
Embodiment 2
[0076] A laboratory uses a brand A chemiluminescence instrument (supporting reagents) to detect thyroid-stimulating hormone (TSH), and the normal reference range of TSH is 0.3-3.0mIU / L. The laboratory also uses Kunlai Composite immunoassay non-fixed value quality control product (double level), when the reagent batch number is changed on the 15th, the quality control parameters and quality control graphics are as follows Figure 5 with Image 6 Shown, where the abscissa is the date, and the ordinate is the detection of thyroid-stimulating hormone (TSH), the unit is μIU / mL. The raw values of quality control results are shown in Table 1-Table 3.
[0077] Table 1 Raw values of quality control results
[0078]
[0079] Table 2 Raw values of quality control results
[0080]
[0081] Table 3 Raw values of quality control results
[0082]
[0083] Depend on Figure 5 with Image 6From the data in Table 1-Table 3, it can be clearly found that after changing th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com