[0004] 1. High cost of document control: the laboratory needs to invest a lot of human, financial and
material resources in the compilation, binding and management of documents
[0005] 2. The intensity and difficulty of document control work: document control vertically covers the
entire life cycle of documents from creation to destruction, and horizontally involves people, machines, materials, methods, environment and quality events related to laboratory quality management. The
workload is huge and Complicated, many tasks are difficult to complete by manual management alone
[0006] 3. Large waste of document control resources: There are many unavoidable mistakes in the
document preparation process, and there are duplication of labor and multiple copies of the same document, resulting in waste of resources
[0007] 4. Document control efficiency is low: When writing documents, identification information such as identification number and version number must be prepared, and the
layout of documents must be manually controlled; approval and release of documents must be repeatedly signed and stamped; it is not easy to obtain documents when using documents, and consulting document information takes up more time
[0008] 5. Document update is not timely: the document modification situation is complicated, the
response method is difficult to unify, the process of document approval and release takes a long time, and the document update is often not timely, which is also one of the reasons for the wrong use of documents
[0009] 6. Misuse of documents often occurs: due to the simultaneous preservation of electronic documents, multiple controlled documents and abolished documents, there are also "transitional" documents, which may easily cause
confusion and misuse
[0010] 7. Formalization of document training and review work: based on manually signed reading records and review records of record forms, it is difficult to track the learning and review of documents
[0011] 8. There are irregularities in record management: record files are generated by
filling in forms manually, and there are behaviors such as "early filling, supplementary filling, modification, forgery and counterfeit signatures" when
filling in records, making it difficult to ensure the requirements of "accurate, timely and complete" records
[0012] 9. Potential
biological safety hazard: documents are often used or stored interspersed in the experimental area and office area, and the risk is easily overlooked by the staff
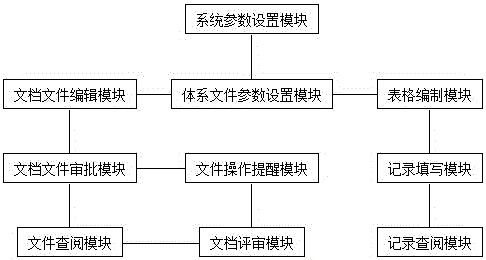
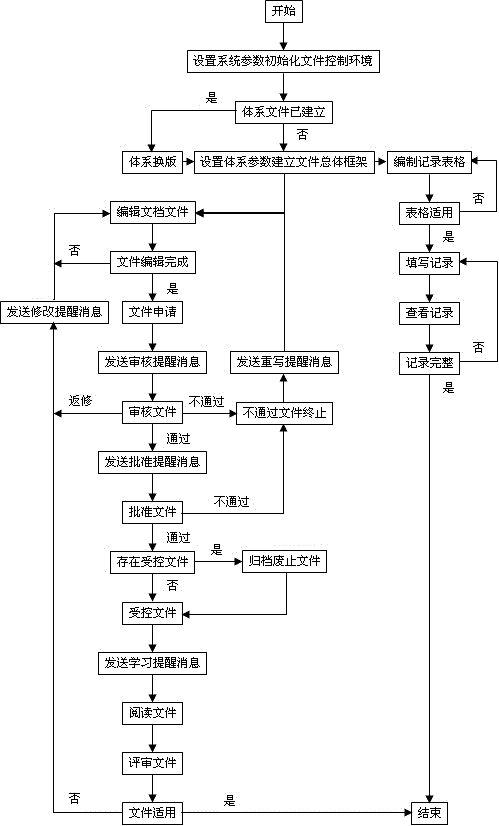
[0014] 1. The function is limited and does not meet the requirements of quality management standards: it mainly realizes the
centralized management of a single "complete
electronic document" written by external
software and has been "actually approved", that is, in a controlled state. It cannot uniformly control historical archive files, and does not involve file learning records and functions such as document review, and important functions such as writing or editing documents; the
software does not understand the "quality system documents" enough, and fails to realize the control of record documents, that is, it cannot fully realize the "compilation and management of record forms,
filling in records and consulting "
Record" 3 basic functions
[0015] 2. The approval of documents is not standardized: they only carry out "post-registration approval" for documents that have been "actually approved", that is, controlled
[0016] 3. It is inconvenient to use files: you need to install
client software or use third-party software to read files after downloading, and it does not support file search and file content search
[0017] 4. A small number of software has realized the file editing function, but there are: a. Using a mode similar to external Word editing to manually compile "complete electronic documents" containing various information, it is still necessary to additionally prepare file control information such as version number and identification number and control The
layout,
workload and difficulty of document writing have not improved compared with paper documents; b. There is no built-in document structure template, and the quality of document writing is uneven; c. The function of system version change is not supported, resulting in a huge amount of document transfer work
[0018] 5. A small number of software has realized the function of record file control, but there are: a. Compiling tables requires three complex operations: writing
database tables, creating form templates, and setting tables. It is necessary to consider table numbers and layouts. It is very difficult to update tables, directly modify or create new ones Replacement will introduce new problems such as changes in reference records or
numbering confusion; b. The preset
content format of the form record items is not flexible when filling in the records; the previous information can be modified when filling in the form that needs to be filled multiple times, and the record filling is not standardized; c .Inspection records must be browsed one by one and search is not supported, which is not convenient enough
[0019] It can be seen that for medical laboratories, there is no standardized and practical solution for effective control of quality system documents.
 Login to View More
Login to View More  Login to View More
Login to View More