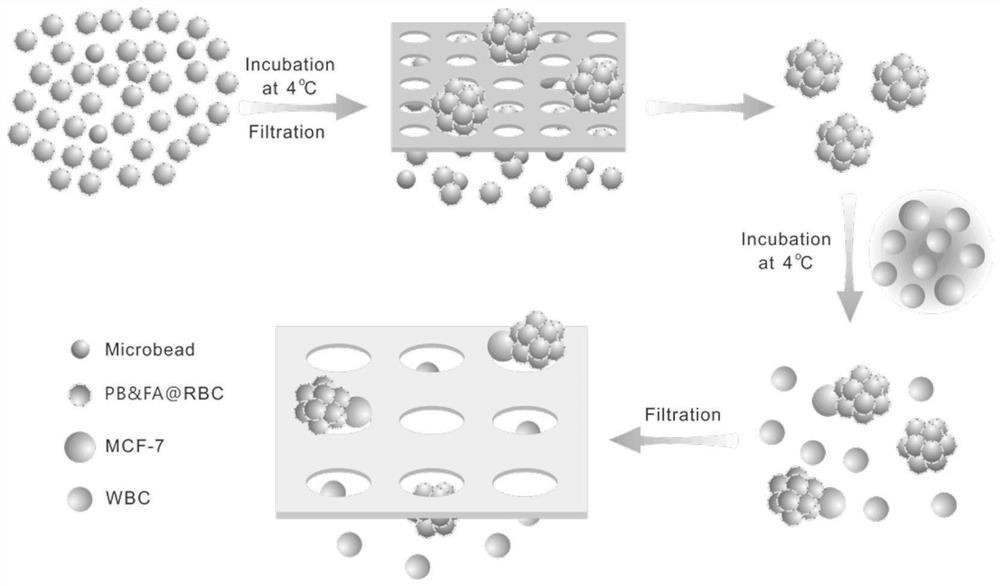
Erythrocyte cluster for enriching circulating tumor cells (CTCs) based on size filtering method
A tumor cell and red blood cell technology, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problems that the purity of CTCs needs to be improved, and the size difference between white blood cells and CTCs cannot be enlarged.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] [Example 1] Preparation of normal red blood cell clusters using 5-6μm polystyrene microspheres with surface modified carboxyl groups
[0055] Red blood cell modification process: mix 30μL of healthy human fresh red blood cells with 100μL of 5000-molecular-weight DSPE-PEG-FA (1mg / mL) PBS solution, incubate at 4℃ for 30min, centrifuge and wash with PBS three times to obtain folic acid Modified red blood cells. Then mix the surface-modified folic acid red blood cells with a 10mg / mL polybrene PBS solution, incubate at 4°C for 30 minutes, and wash three times with PBS to obtain surface-modified polybrene and folic acid red blood cells, and disperse them Put it in PBS solution for later use.
[0056] Preparation of red blood cell clusters: Wash the 5-6μm polystyrene microspheres with surface modified carboxyl groups three times with PBS, and then add more than 100 times the number of surface modified polybrene and folic acid red blood cells, and mix them evenly After centrifugat...
Embodiment 2
[0058] [Example 2] Red blood cell clusters or fluorescent red blood cell clusters capture MCF-7 cells
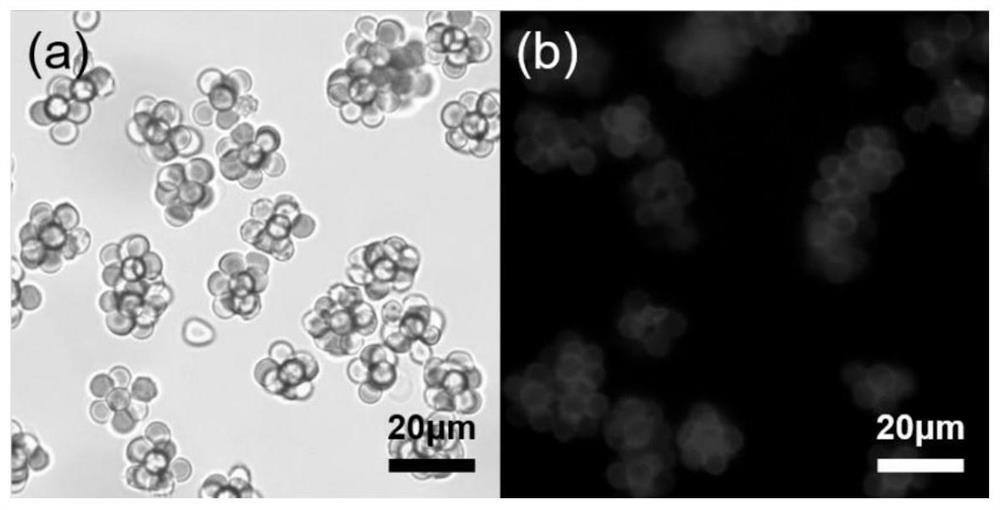
[0059] 1. Red blood cell clusters capture MCF-7 cells in PBS: take 50μL of red blood cell cluster suspension (density 1.2×10 7 Cells / mL), add 10uL of MCF-7 cell suspension (density 5×10 5 Pieces / mL), after mixing, place it at 4°C and incubate it for 120min, blow off the mixture at the bottom of the centrifuge tube after incubation, and filter it through a PDMS porous membrane with a pore size of 20μm to remove excess red blood cell clusters. That is, the MCF-7 cells captured by the red blood cell clusters can be obtained, and the bright field photos taken under the optical microscope are as image 3 (a) Shown.
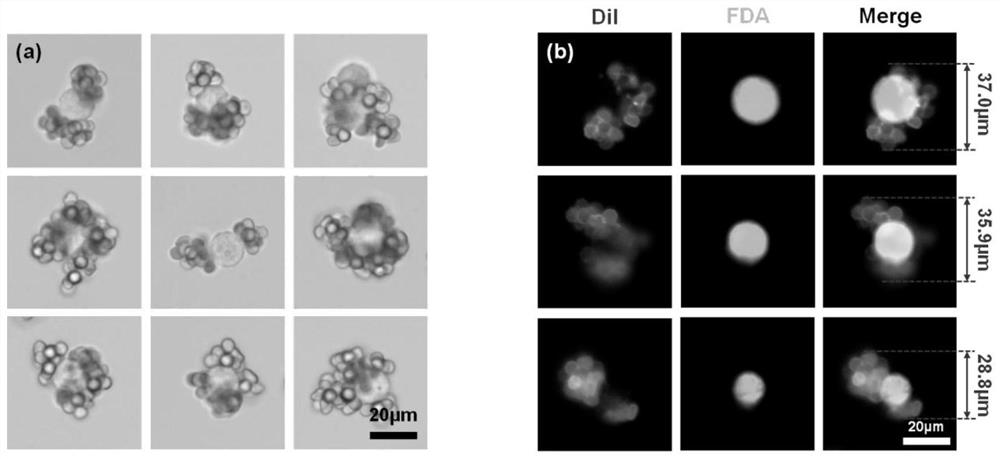
[0060] 2. Fluorescent red blood cell clusters were captured in PBS to capture MCF-7 cells: 50 μL of the fluorescent red blood cell cluster suspension prepared in Example 1 (density 1.2×10 7 Cells / mL), add 10μL of MCF-7 cell suspension (density 5×10 5 Pieces / mL), after mixi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com