Combined chimerism antigen receptor, expression vector, lentivirus and T-cell
A technology of chimeric antigen receptors and cells, applied in the field of biomedicine, can solve the problems of CD19, low effective rate of ALL, and small number of clinical studies, etc., and achieve the goal of increasing complete remission rate, preventing relapse, and increasing long-term survival rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Implementation example 1, construction of recombinant lentiviral vector pCAR19, pCAR22
[0074] By sequence 1 (CAR19, see Figure 13 , marked as CAR19, SEQ ID NO.1) and sequence 2 (CAR22, see Figure 14 , marked as CAR22, SEQ ID NO.2), was synthesized by Nanjing GenScript Biotechnology Co., Ltd., and the synthesized sequence was cloned into the T vector.
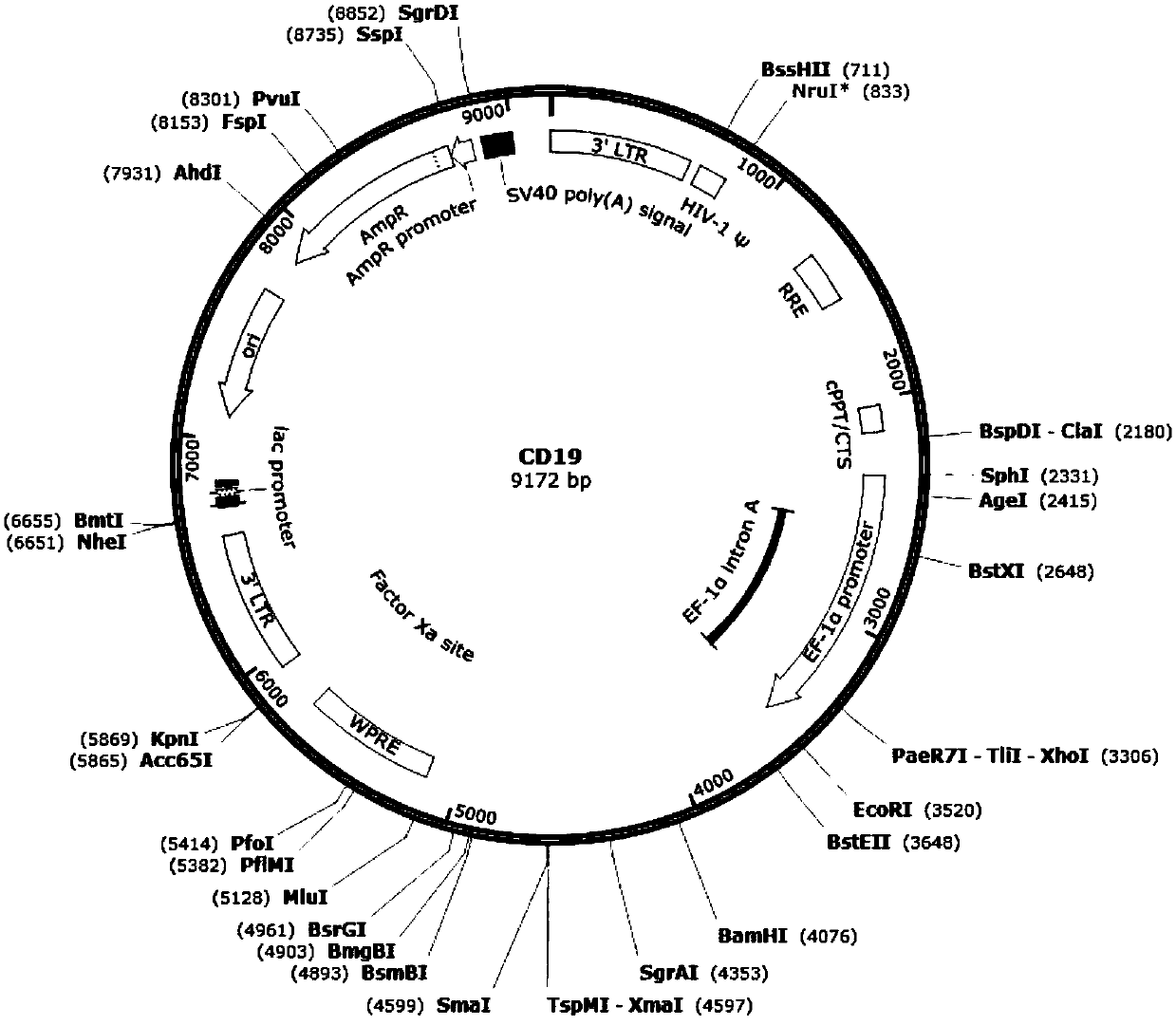
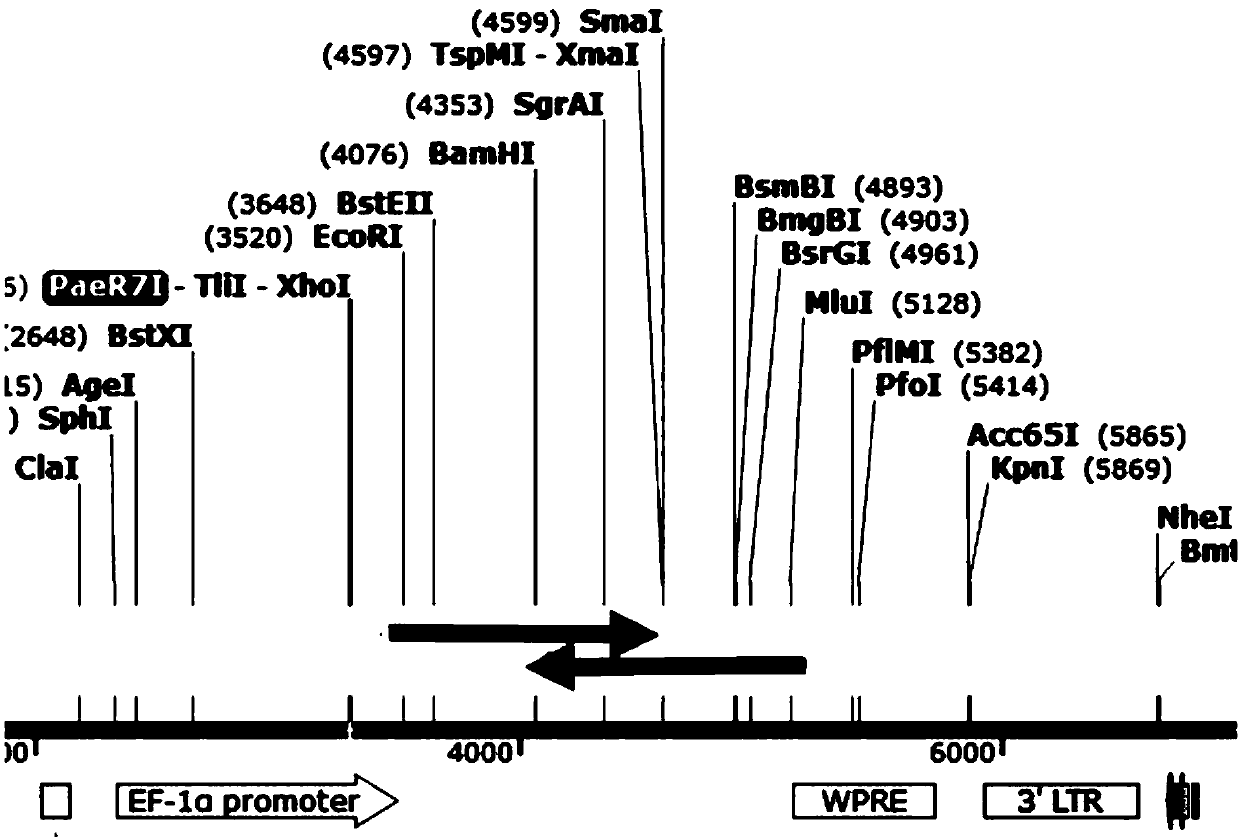
[0075] 1. The length of the CAR19 sequence fragment is 1596bp, with EcoRI and MluI restriction sites designed at both ends, and cloned into the multiple cloning site of the lentiviral backbone plasmid pLVX-EF1α-IRES-Puro to complete the construction of the vector. To build a complete graph see figure 1 .
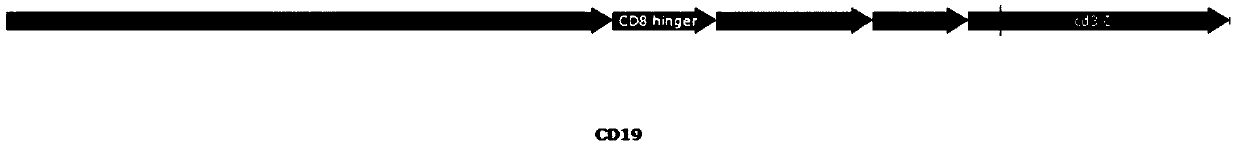
[0076] The part structure of CAR19 is figure 2 As shown, CD19 is a third-generation CAR that uses CD28 and 4-1BB as co-stimulatory signals.
[0077] Comparison chart of CAR19 sequence sequencing image 3 Shown: The black line at the bottom of the figure indicates the standard sequence, and the gray line indic...
Embodiment 2
[0084] Plasmid preparation of implementation example 2, pCAR19, pCAR22
[0085] Inoculate the DH5alpha strain of the pCAR19 or pCAR22 plasmid into 250 mL of LB culture medium containing 100 μg / mL ampicillin, and culture overnight at 37 °C and 220 rpm. The culture solution was centrifuged at 6000g for 20min at 4°C, and the supernatant was discarded.
[0086] Take out Buffers P1 from the EndoFree plasma mega kit (Qiagen), add 120mL pre-cooled Buffers P1 to the centrifuged E. coli pellet, cover the cap of the centrifuge bottle, shake the centrifuge bottle vigorously to completely disperse the E. coli pellet in Buffers P1 .
[0087] Add 120mL Buffers P2 to the centrifuge bottle, put the cap on the roller mixer, slowly increase the speed to 50rpm, mix thoroughly and place at room temperature for 5min.
[0088] Add 120mL Buffers P3 to the centrifuge bottle, put the cap on the roller mixer, slowly increase the speed to the maximum speed of the roller mixer 70rpm, and mix thoroughly...
Embodiment 3
[0109] Implementation example 3, preparation of recombinant lentivirus LVCAR19, LVCAR22
[0110] Insert 130.0~140.0×10 in multilayer cell culture bottle (Hyperflask) (Corning) 6 Number of 293T cells (Takara), a total of 560 mL DMEM complete medium (50 mL fetal bovine serum, 5 mL Antibiotic-Antimycotic (100×)), at 37 °C with 5% CO 2 Incubate for 24 hours in the incubator. Add DMEM complete medium mixed with 320 μg plasmid (pCAR19 or pCAR22: gag plasmid: vsvg plasmid = 6:3:2) into a 960 μg PEI tube, vortex, and equilibrate at room temperature for 10 minutes. Mix 35mL of the mixture of PEI and plasmid with 525mL of DMEM complete medium, and replace it into the above-mentioned multi-layer cell culture flask. Place the multilayer cell culture flask at 37 °C with 5% CO 2 After 3 days in the incubator, the cell culture supernatant was collected.
[0111] After the supernatant was centrifuged at 4000 rpm (or 3000 g) for 30 min, cryonase (Takara) was added to the centrifuged supern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com